-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Essentials of Protein Import in the Degenerate Mitochondrion of
Several essential biochemical processes are situated in mitochondria. The metabolic transformation of mitochondria in distinct lineages of eukaryotes created proteomes ranging from thousands of proteins to what appear to be a much simpler scenario. In the case of Entamoeba histolytica, tiny mitochondria known as mitosomes have undergone extreme reduction. Only recently a single complete metabolic pathway of sulfate activation has been identified in these organelles. The E. histolytica mitosomes do not produce ATP needed for the sulfate activation pathway and for three molecular chaperones, Cpn60, Cpn10 and mtHsp70. The already characterized ADP/ATP carrier would thus be essential to provide cytosolic ATP for these processes, but how the equilibrium of inorganic phosphate could be maintained was unknown. Finally, how the mitosomal proteins are translocated to the mitosomes had remained unclear. We used a hidden Markov model (HMM) based search of the E. histolytica genome sequence to discover candidate (i) mitosomal phosphate carrier complementing the activity of the ADP/ATP carrier and (ii) membrane-located components of the protein import machinery that includes the outer membrane translocation channel Tom40 and membrane assembly protein Sam50. Using in vitro and in vivo systems we show that E. histolytica contains a minimalist set up of the core import components in order to accommodate a handful of mitosomal proteins. The anaerobic and parasitic lifestyle of E. histolytica has produced one of the simplest known mitochondrial compartments of all eukaryotes. Comparisons with mitochondria of another amoeba, Dictystelium discoideum, emphasize just how dramatic the reduction of the protein import apparatus was after the loss of archetypal mitochondrial functions in the mitosomes of E. histolytica.
Published in the journal: The Essentials of Protein Import in the Degenerate Mitochondrion of. PLoS Pathog 6(3): e32767. doi:10.1371/journal.ppat.1000812
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000812Summary
Several essential biochemical processes are situated in mitochondria. The metabolic transformation of mitochondria in distinct lineages of eukaryotes created proteomes ranging from thousands of proteins to what appear to be a much simpler scenario. In the case of Entamoeba histolytica, tiny mitochondria known as mitosomes have undergone extreme reduction. Only recently a single complete metabolic pathway of sulfate activation has been identified in these organelles. The E. histolytica mitosomes do not produce ATP needed for the sulfate activation pathway and for three molecular chaperones, Cpn60, Cpn10 and mtHsp70. The already characterized ADP/ATP carrier would thus be essential to provide cytosolic ATP for these processes, but how the equilibrium of inorganic phosphate could be maintained was unknown. Finally, how the mitosomal proteins are translocated to the mitosomes had remained unclear. We used a hidden Markov model (HMM) based search of the E. histolytica genome sequence to discover candidate (i) mitosomal phosphate carrier complementing the activity of the ADP/ATP carrier and (ii) membrane-located components of the protein import machinery that includes the outer membrane translocation channel Tom40 and membrane assembly protein Sam50. Using in vitro and in vivo systems we show that E. histolytica contains a minimalist set up of the core import components in order to accommodate a handful of mitosomal proteins. The anaerobic and parasitic lifestyle of E. histolytica has produced one of the simplest known mitochondrial compartments of all eukaryotes. Comparisons with mitochondria of another amoeba, Dictystelium discoideum, emphasize just how dramatic the reduction of the protein import apparatus was after the loss of archetypal mitochondrial functions in the mitosomes of E. histolytica.
Introduction
Mitosomes and hydrogenosomes are metabolically-specialized forms of mitochondria, found in some of the unicellular pathogens which inhabit oxygen poor environments [1]. A lack of a recognizable mitochondrial compartment had led to the proposal of a group of primitive, primarily amitochondriate, eukaryotes [2]. However, recent evidence has shown the organelles referred to as hydrogenosomes and mitosomes in the ‘amitochondriate eukaryotes’ to be highly evolved mitochondria, having reduced their metabolic pathways as a response to their anaerobic and partly parasitic lifestyles in diverse eukaryotic lineages [1], [3]–[6]. There is no eukaryote known to be primarily amitochondriate, and even secondary loss of mitochondria has not been found.
The biogenesis of mitochondria is the defining aspect of the organelle and depends on the import of proteins from the cytosol, driven by a set of characteristic protein translocases installed in the outer and inner mitochondrial membranes. Mitochondrial precursor proteins are translated on ribosomes in the cytosol, and then recognized by a protein translocase in the outer mitochondrial membrane (the TOM complex). This TOM complex imports precursor proteins through a channel formed by the essential subunit Tom40. Subsequently, imported proteins are transferred to the sorting and assembly machinery (SAM complex) for assembly into the outer membrane, or one of two translocases in the inner mitochondrial membrane: the TIM22 complex for assembly into the inner membrane, or the TIM23 complex for translocation through the membrane and into the mitochondrial matrix [7],[8]. The presence of components of the TOM, TIM and SAM complexes in hydrogenosomes and mitosomes shows these organelles to be mitochondria, despite the impressive metabolic simplification that have taken place in these specialized compartments [9]–[13].
Mitosomes are the simplest form of mitochondria: they have lost their capacity for ATP synthesis, lost all vestiges of a mitochondrial genome and so far only limited set of proteins have been localized into these tiny double membrane-bound vesicles. This secondary reduction of function has occurred independently in distinct lineages of eukaryotes, being well characterized in the diplomonad Giardia intestinalis [14], the microsporidians (such as Encephalitozoon cuniculi [15], Antonospora locustae [12], Trachipleistophora hominis [16]) and the amoebozoan Entamoeba histolytica [17],[18]. The majority of known proteins found in the mitosomes of G. intestinalis and microsporidia are functional counterparts of mitochondrial proteins found in other organisms, and a unifying feature of all these organelles is their role in the synthesis of iron-sulfur clusters [14],[19],[20]. So far it is the sole metabolic process known to occur in mitosomes of G. intestinalis and microsporidia and conflicting data exist on the presence of the iron-sulfur clusters biosynthesis in E. histolytica mitosomes [21],[22]. In addition to being widespread in hydrogenosomes and mitosomes, the biogenesis of iron-sulfur centers is the only essential metabolic role of mitochondria in the model organism Saccharomyces cerevisiae [23].
Entamoeba histolytica, the causative agent of invasive amoebiasis in humans, seems to have taken further steps towards the extreme reduction of the mitochondrial compartment [24]. It represents the only known eukaryote in which the synthesis of iron-sulfur clusters is mediated by an NIF (nitrogen fixation) system acquired by horizontal gene transfer from an ε-proteobacterium [25]. According to prediction algorithms this biosynthetic pathway is predicted to be present in the cytosol instead of the mitochondrial compartment [26]. Consistently, Mi-Ichi et al, did not find either of Nif proteins in their mitosomal proteomic analysis [22] and also no iron-sulfur cluster containing protein is known to be present in the organelles as a candidate substrate for the NIF system [21]. However, Maralikova et al, presented data arguing for the dual localization of both Nif proteins with their specific enrichment in the mitosomes [21].
So far, the mitosomes of E. histolytica represent one of the simplest mitochondria known. With the presence of sulfate activation pathway in the mitosome the need for ATP in addition to the molecular chaperones within the organelle is obvious [17], [27]–[29]. Although the ADP/ATP carrier in the mitosomal membrane provides for ATP import [22],[27], two questions are left open. Firstly, how do mitosomes recycle inorganic phosphate (Pi) arising from ATP hydrolysis? Secondly, how are all the mitosomal proteins transported across the membranes of the organelle?
To address these questions, we performed hidden Markov model (HMM) searches of the E. histolytica genome and discovered candidate sequences for (i) a Pi carrier to complement the activity of the ATP/ADP carrier, (ii) Tom40, a channel for substrate protein transport across the outer mitosomal membrane, and (iii) Sam50, an assembly machine for Tom40 in the outer membrane. The mitosomal protein import pathway in E. histolytica is mitochondrial in nature. Analysis of the mitochondrial protein import machinery of the related amoeba Dictyostelium discoideum, suggests that E. histolytica has extensively stripped the mitochondrial protein import machines to their essentials. This remarkable degeneration of protein import is in keeping with the apparent paucity of proteins imported into mitosomes in E. histolytica.
Results
Substrate transport by mitochondrial carrier proteins in E. histolytica
The family of mitochondrial carrier proteins is well characterized in terms of primary sequence motives and crystal structure. According to the structure of the bovine ADP/ATP carrier, six transmembrane segments form an α-helical bundle, consisting of three homologous modules [30]. Each of these modules contains a signature motif in the odd-numbered helices [P]-x-[D/E]-x-x-[K/R]. The proline residue in the motif introduces a kink into three of the six transmembrane helices. In order to obtain a sensitive tool for analysis of E. histolytica genome, a hidden Markov model was built that describes the defining features of the mitochondrial carrier protein family.
Only two protein sequences were identified in the HMM search of the E. histolytica genome: 269.m00084 (E-value of 2.8×10−94) and 13.m00296 (E-value of 4.7×10−5) (Accession numbers XP_649800 and XP_656350, respectively). The first sequence corresponds to the ADP/ATP carrier [27] and the second to the novel, unannotated protein sequence. Additional analysis of the sequence length and predicted topology support the possibility that 13.m00296 could encode a mitochondrial carrier protein (Figure 1A), one of the most divergent of the carrier protein family. Based on the functional analyses that follow, we labeled the gene as ehpic and its translation product as EhPiC.
Fig. 1. Mitochondrial phosphate carrier in E. histolytica. 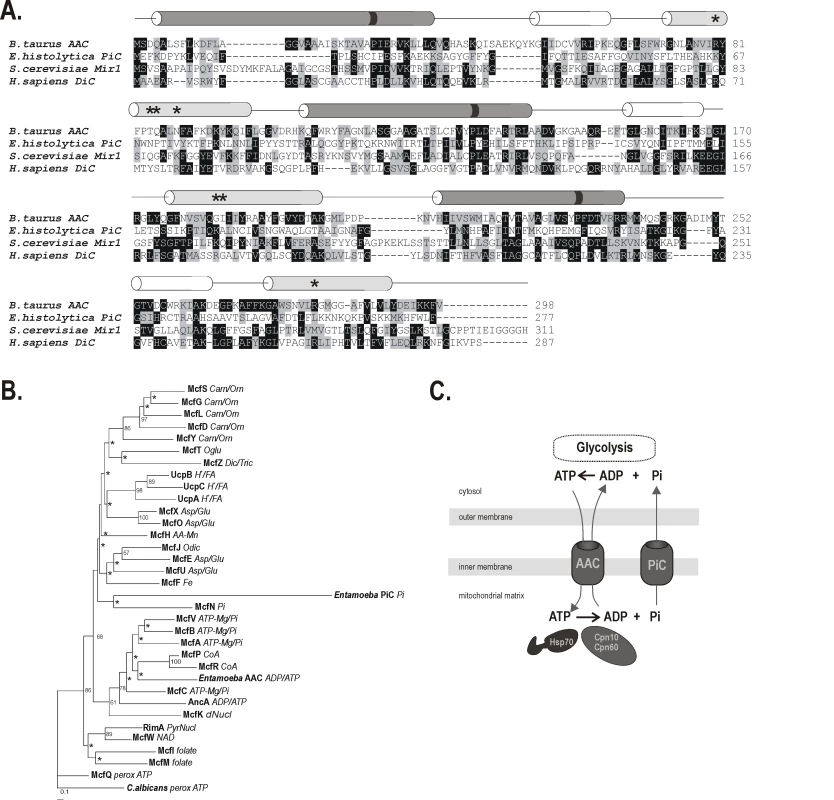
(A) Protein sequence alignment of EhPiC with Bos taurus ADP/ATP carrier, S. cerevisiae phosphate carrier and human dicarboxylate carrier. The secondary structure is schematically depicted by colored cylinders above the alignment according to [30]. The odd- and even-numbered transmembrane α-helices are shown in dark or light grey, respectively. The short helices exposed to the mitochondrial matrix are shown in white. The black stripes on the helices depict the presence/absence of conserved proline residues in the mitochondrial carriers signature motif. Asterisks indicate substrate contact sites. (B) Protein maximum likelihood tree of 34 mitochondrial carrier proteins from D. discoideum together with the ADP/ATP carrier and PiC carrier from E. histolytica constructed by Phyml [80]. C. albicans peroxisomal ADP/ATP carrier was used as the outgroup. The putative substrates of the carriers in D. discoideum are inferred from sequence similarity to S. cerevisiae and human carrier proteins [31] and indicated as follows: Carn/Orn – carnitine or ornithine, Asp/Glu – aspartate/glutamate, Oglu – 2-oxoglutarate, Dic/Tric – dicarboxylate/tricarboxylate, Pi – phosphate, PyrNucl – pyrimidine NTP/NMP, perox ATP – peroxisomal ATP carrier, H+ FA – H+ fatty acid, CoA – coenzyme A, dNucl – deoxynucleotide, AA-Mn – amino acid (Mn2+), Fe – iron (mitoferrin), asterisks denote nodes with boostrap value lower than 50. (C) A schematic representation of EhPiC and EhADP/ATP carrier in the mitosomes, depicting the role of EhPiC in recycling phosphate, which is released during ATP hydrolysis by the action of molecular chaperones. EhADP/ATP carrier mediates the exchange of cytosolic ATP for mitosomal ADP. The 31.7 kDa protein is predicted to have six transmembrane helices, although with the lack of signature motives in the odd-number helices. Only the third helix has a proline residue in the conserved position (Figure 1A). As is the case in fungi, animals and plants, D. discoideum has a diverse set of mitochondrial carrier proteins that can be compared in a phylogenetic analyses [31],[32]. When added to the dataset from D. discoideum, the E. histolytica ADP/ATP carrier clusters together with transporters of adenine nucleotides and coenzyme A, while EhPiC clusters with the Pi carrier McfN (Figure 1B). The overall sequence divergence of EhPiC is reflected by a long branch formed in the tree and also by low statistical support. However, a similar tree topology was obtained using different reconstruction methods (Supplementary information Figure S1), which indicated the affinity of EhPiC to McfN. The amino acid residues at three contact sites in the central pore are believed to determine the specificity of the transport channel and their composition can therefore serve for the estimation of the substrate nature [32]. Perhaps due to the high degree of sequence divergence, none of the three sites of EhPiC provided any leads towards the putative pore specificity (Figure 1A). The proposed function of a phosphate carrier in the mitosomes is summarized diagrammatically in Figure 1C.
To determine whether EhPiC could function as a phosphate carrier protein, we made use of S. cerevisiae as a cellular assay system. Fluorescence microscopy showed that EhPiC contains the targeting sequences necessary for the import into mitochondria in vivo (Figure 2A). The EhPiC protein can be translated in vitro and is imported by mitochondria isolated from S. cerevisiae as readily as the EhAAC (Figure 2B). Figure 2C shows that EhPiC behaves as a typical integral membrane protein, exclusively distributed in the pellet fraction after resisting sodium carbonate extraction of mitochondrial membranes. Soluble and peripheral membrane proteins, like mtHsp70, are extracted from membranes by sodium carbonate.
Fig. 2. EhPiC is a mitochondrial-type phosphate carrier. 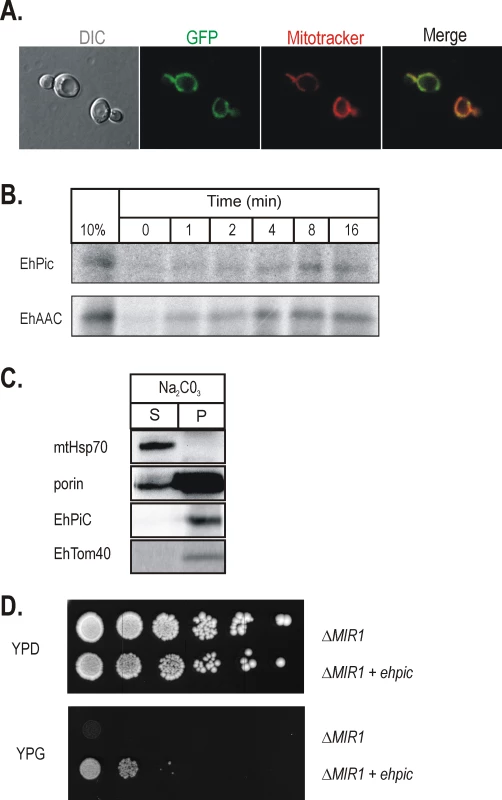
(A) The C-terminal GFP fusion of EhPiC expressed in yeast cells (green) co-stained with the mitochondria-specific stain Mitotracker red (red). The merged image demonstrates the co-localization of EhPiC with the mitochondrial compartment. DIC – differential contrast (Nomarski). (B) 35S-labelled EhPiC and EhAAC were incubated with yeast mitochondria, reactions were stopped on ice and the organelles subjected to treatment with proteinase K. 10%–10% of precursor amount used for the import reaction. (C) Yeast mitochondria with 35S-labelled precursors (after 15 minutes of import) were subjected to treatment with proteinase K and then extracted with sodium carbonate. The immunodecoration of mitochondrial Hsp70 and porin shows the distribution of the typical soluble and the integral membrane proteins, respectively. S-soluble fraction, P-pellet. (D) The Δmir1 deletion strain [33] was transformed by a plasmid carrying ehpic (Δmir1+ehpic) or with a plasmid without ehpic (Δmir1). Serial dilutions of transformed cells plated on fermentable (YPD) and non-fermentable (YPG) carbon source demonstrate the capability of EhPiC to restore the growth defect of the mutant strain. To test if EhPiC functions to transport phosphate, the coding sequence was cloned into a yeast expression vector and transformed into S.cerevisiae strain lacking the dominant phosphate carrier (Δmir1) [33],[34]. The Δmir1 mutants grow on glucose containing media but, due to failure to maintain phosphate transport to sustain oxidative phosphorylation, Δmir1 cells fail to grow on the non-fermentable carbon source glycerol (Figure 2D). EhPiC expression complements the growth defect of these cells, demonstrating that it functions as a phosphate carrier.
Protein import machinery encoded in the genome of E. histolytica
Both E. histolytica and the social amoeba D. discoideum are amoebozoans, one of the six super-groups of eukaryotes [35],[36]. As a sister clade to the animal and fungal lineages, it was not surprising that the core components of the TOM and TIM machinery, characterized in fungi and animals, have been identified in D. discoideum [37]–[40].
Given this conservation in protein import pathways between amoebozoans, fungi and animals, it was highly surprising when the only homologs of the mitochondrial protein import machinery identified in the genome of E. histolytica [41] were the chaperones mtHsp70, Cpn60 and Cpn10.
Hidden Markov models were built, representing search tools for 33 mitochondrial components known to participate during the import process either in fungal/animal or plant mitochondria. Each model was built from sequences clearly homologous to the functionally characterized import component. The models were assembled into a library and used to search the E. histolytica genome (see Materials and Methods). In parallel we analyzed the genome of D. discoideum (Figure 3), which is the most extensively studied species of Amebozoa super-group thus providing a referential dataset for our analysis of the E. histolytica genome.
Fig. 3. The minimalism of the protein import and assembly pathways in the mitosomes of E. histolytica. 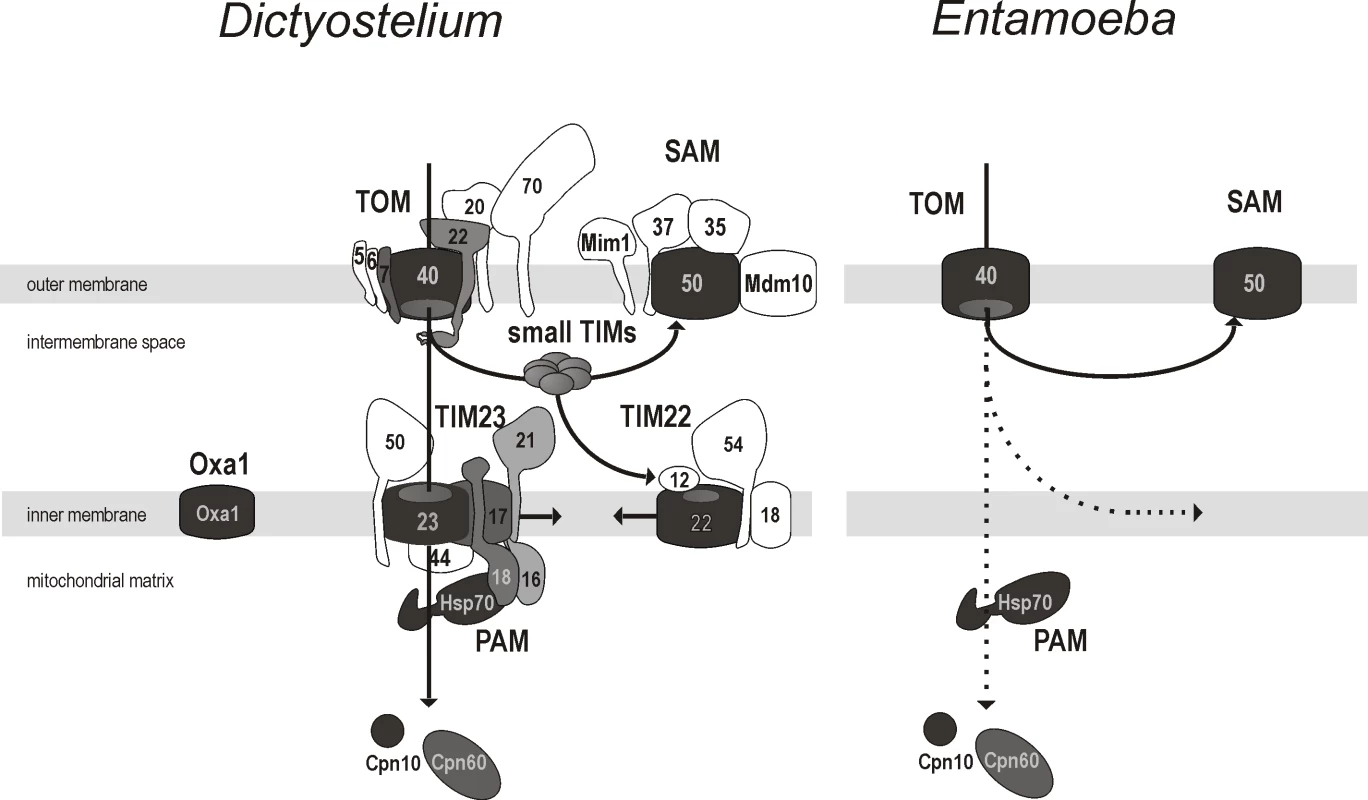
The schematic representation depicts the complex molecular apparatus in the amoebozoan mitochondria of D. discoideum and the mitosome of E. histolytica based on hidden Markov model analysis. Of the components present in animal and fungal mitochondria, those shown shaded were found in D. discoideum. The panel on the right shows the five components identified in E. histolytica. Some of the proteins found in the animals and fungi appear to be missing in Amoebozoa (shown in white). The dashed arrows highlight the unknown translocation pathway across or into the inner mitosomal membrane in E. histolytica. Two sequences 38.m00236 and 137.m00093 (accession numbers XP_655014 and XP_651988) were identified by the Tom40 - and Sam50-specific HMMs, respectively. Accordingly, these two open reading frames were named ehtom40 and ehsam50 and their putative protein products as EhTom40 and EhSam50. The third open reading frame identified in E. histolytica corresponds to the previously identified gene encoding for mitochondrial-type Hsp70 [42].
A core component of the TOM complex: EhTom40
The gene ehtom40 encodes a protein of 305 amino acids with a theoretical molecular weight of 34.4 kDa, similar in size to the Tom40 from Encephalizoon cuniculi [43]. EhTom40 is expressed in E. histolytica with the ehtom40 mRNA detected in extracts from amoebae by reverse transcription coupled with specific PCR amplification (Supplementary information Figure S2). The reverse BLASTP search with EhTom40 as a query provided hits to a ‘porin family protein’ from Arabidopsis thaliana (NP_175457.1), the Tom40 sequence from Trimastix pyriformis (ABW76113.1) and a hypothetical protein from Leishmania infantum (XP_001463193.1) representing an unidentified protein predicted to be a β-barrel. Such a structure is typical for Tom40 proteins. The mitochondrial porin 3 superfamily of proteins (Pfam01459) encompasses both the eukaryotic voltage-dependent anion channel (VDAC) proteins and Tom40s [44],[45]. Other mitochondrial β-barrel proteins like Sam50 do not fall within the porin 3 superfamily. Given the similarity of EhTom40 to β-barrel proteins predicted to be Tom40 (ABW76113.1) and VDAC (NP_175457.1), we performed a CLANS analysis using a sequence set containing 137 VDAC and 79 Tom40 sequences (including EhTom40). Membrane proteins are often difficult to resolve on phylogenetic trees but CLANS, that graphically depicts homology in large datasets of proteins, has been previously found to be very efficient in the classification of the β-barrel proteins from bacteria [46] and also in the characterization of a Tom40 homologue in G. intestinalis [47]. This approach utilizes all-against-all pairwise BLAST, clustering “vertices” (i.e. individual protein sequences) in three-dimensional space using an algorithm which applies a weak repulsive force to each vertex and an attractive force between each pair of vertices. The attractive and repulsive forces are proportional to the BLAST high scoring segment pair score. Figure 4A shows that cluster analysis performed on this superset of porin3 family proteins using a P-value cutoff of 10−82 results in the sequences clustering into two distinct groups. The clusters are either exclusively VDAC homologues or Tom40 homologues, corresponding to their independent phylogenies across eukaryotes. The EhTom40 is positioned on the periphery of Tom40 cluster, clearly separated from the compact cluster of VDAC sequences.
Fig. 4. A mitochondrial Tom40 in Entamoeba histolytica. 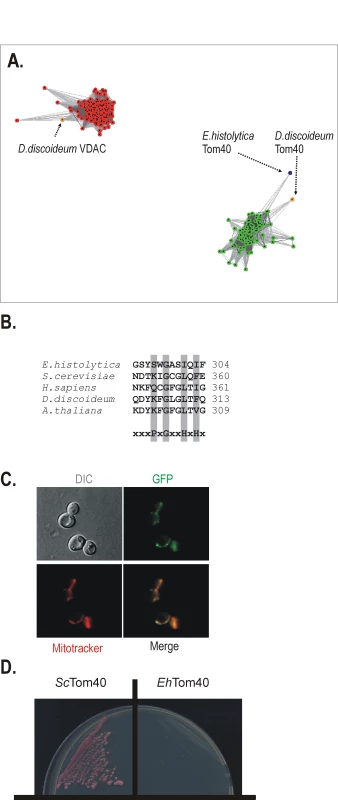
(A) A representative figure from the CLANS analysis of a data set containing 79 Tom40 sequences (green dots), 137 VDAC (red dots) sequences and the EhTom40 sequence (blue dot). A P-value threshold of 10−82 was used to give complete separation of the VDAC and Tom40 clusters. Tom40 and VDAC sequences from D. discoideum, as the closest relative in the dataset, are highlighted (orange). (B) The alignment shows the C-terminal β-strand from various Tom40 homologues. The motif for the general import signal for mitochondrial β-barrel proteins is highlighted, P-polar residue, G-glycine residue, H-hydrophobic residue [48]. (C) The C-terminal GFP fusion of EhTom40 in yeast cells (green), the yeast mitochondria stained with Mitotracker red (red). The merged image demonstrates the co-localization of EhTom40 with the mitochondrial compartment. DIC – differential contrast (Nomarski). (D) S. cerevisiae Δ tom40 mutants, carrying a counterselectable TOM40 gene and expressing either S. cerevisiae Tom40 (ScTom40) or EhTom40 were plated onto 5-FOA media to select against the covering plasmid. Cells were incubated for 4 days at 30°C and only strain expressing ScTom40 was viable. Mitochondrial β-barrel proteins contain a specific mitochondrial targeting signal (the β-signal, PxGxxHxH, where P stands for polar, G for glycine and H for hydrophobic residue) that sits in the last β-strand of the protein [48]. The β-signal is recognized and bound by the SAM complex, which then mediates folding and membrane insertion of the β-barrel. EhTom40 has a β-signal that strictly follows this rule (Figure 4B). When EhTom40 is expressed in S. cerevisiae, it is specifically targeted to mitochondria, as judged by fluorescence microscopy (Figure 4C). In order to test whether EhTom40 can functionally substitute for S. cerevisiae Tom40 we have transformed a haploid Δtom40 strain that carries the S.cerevisiae TOM40 on a counterselectable plasmid. Upon plating on 5-FOA media, to select against the covering plasmid, no viable transformants were obtained (Figure 4D), while the strain transformed with the homologous TOM40 remained viable. Apparently the sequence divergence between the fungal and amoebic Tom40 interferes with the correct docking and interaction with other TOM complex subunits and thus does not allow for functional complementation. Therefore we subsequently tested whether EhTom40 integrates into the native S. cerevisiae TOM complex using the in vitro import assays. Although the protein accumulated as a high molecular weight species in the mitochondrial membranes (Supplementary information Figure S3), the specific pull-down assay using the antibodies against TOM complex subunits did not recover any of EhTom40 protein (data not shown).
Given that TOM complex requires numerous binding sites on several distinct subunits to assemble properly, it is not surprising that these experiments failed. It was previously reported that even the dual point mutations in Tom40 of S.cerevisiae can result in the disassembly of the native TOM complex [49].
A core component of the SAM complex: EhSam50
The cDNA sequence of Sam50 homologue found in E. histolytica is deposited at NCBI under accession number XM_646896. It encodes for a protein of 388 amino acids with a theoretical molecular weight of 45.3 kDa. However, when aligned with the corresponding genomic sequence, the presence of an intervening sequence was revealed. The insertion of 72 bp starting at position 802 is limited by GAATGATT and TAG at the 5′ - and 3′-ends. These are conserved intron donor and acceptor splice sites identified in E. histolytica [50], suggesting that ehsam50 gene is interrupted by a single intron. According to the molecular weight of the protein when episomally expressed in E. histolytica and S. cerevisiae the mRNA is likely processed in vivo in both cellular systems and provides for EhSam50 translation.
As shown on phylogenetic reconstruction the protein sequence clusters with other mitochondrial Sam50 sequences [51],[52] with α-proteobacterial Omp85 sequences in a sister clade (Figure 5B) [53], implying that EhSam50 is of direct mitochondrial origin.
Fig. 5. Sam50 in Entamoeba histolytica. 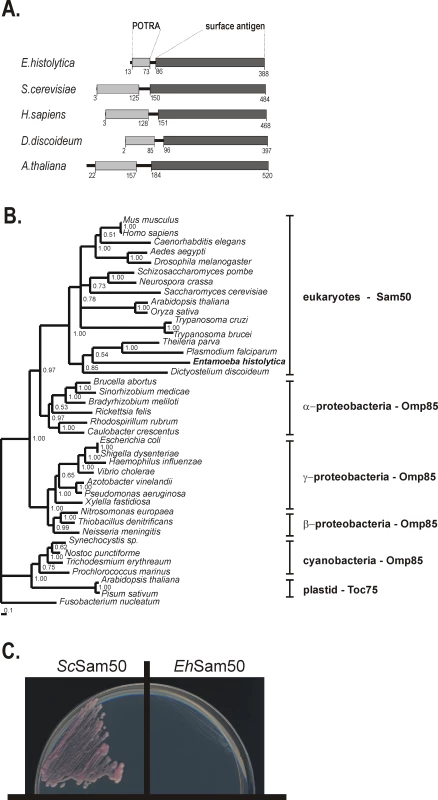
(A) Signature domains present in Sam50: A C-terminal surface antigen domain (dark grey) which corresponds to the membrane embedded β-barrel, and the POTRA domain (light grey) which is exposed to the intermembrane space. Both domains are present in EhSam50 as determined by Pfam search [45] and HHpred analyses [81]. (B) Protein maximum-likelihood phylogenetic tree was derived from a dataset of 407 aligned amino acids from 39 sequences using MrBayes [70]. Numbers at the individual nodes represent MrBayes posterior probabilities. (C) S. cerevisiae Δ sam50 mutants, carrying a counterselectable SAM50 gene and expressing either S. cerevisiae Sam50 (ScSam50) or EhSam50 were plated onto 5-FOA media to select against the covering plasmid. Cells were incubated for 4 days at 30°C and only strain expressing ScSam50 was viable. Omp85 proteins contain a C–terminal β-barrel domain (the ‘surface antigen domain’), which is integrated into the outer membrane. A characteristic N-terminal extension consists of five POTRA (polypeptide-transport-associated) domains [54]. In contrast, mitochondrial Sam50 proteins, including EhSam50, contain a single POTRA domain along a β-barrel domain (Figure 5A). Together with obtained phylogenetic data this provides additional support that EhSam50 is a genuine mitochondrial homologue, not a bacterial Omp85 acquired by a horizontal gene transfer. While the structural domains are well conserved in EhSam50, we could not obtain a viable S.cerevisiae Δsam50 strain expressing the E. histolytica protein (Figure 5C). As in the case of Tom40, the primary sequence was likely too divergent to support functional integration of EhSam50 into SAM complex of S.cerevisiae. So far, there is no record in the literature of successful heterologous replacement of SAM complex components, which suggests that tight protein-protein or protein-lipid interactions are involved in the SAM function.
Localization of proteins in E. histolytica
In order test EhSam50 in vivo, the expression of EhSam50 (and EhTom40 and EhPiC) in E. histolytica was confirmed by RT-PCR (Supplementary information Figure S2). As an experimental system, E. histolytica is challenging. In order to detect whether EhSam50, EhTom40 and EhPiC are localized to a subcellular compartment, we set about both raising antisera to the three proteins and creating HA-tagged versions of the proteins for expression in E. histolytica. Neither strategy allowed localization of EhPiC or EhTom40 in E. histolytica (recent data of Mi-Ichi et al. support the mitosomal distribution of EhTom40 [22]). However, an α-EhSam50 specific serum was raised and an E. histolytica strain expressing EhSam50 with a C-terminal HA-tag was cultivated. Fixed E. histolytica cells were probed with α-HA and with either α-EhSam50, α-mtHsp70 or α-Atg8 antibody and immunofluorescent labeling determined. Atg8 is a marker of autophagocytic vesicles, which might be of similar morphology to mitosomes [55]. EhSam50 was found in vesicles with a size and overall pattern similar to the cpn60-specific labeling by León-Avila and Tovar [56]. The co-distribution of HA - and EhSam50 - specific signal on the merged image shows that the antibodies specifically label the same cellular compartment (Figure 6A), distinct from the Atg8-specific labeling of autophagosomes [55]. More importantly, using a α-mtHsp70 raised against mitochondrial Hsp70 from Neocallimastix patriciarum [57] the specific co-localization with EhSam50 was found. This data strongly supports the mitosomal distribution of EhSam50 together with mitosomal Hsp70 [42].
Fig. 6. Sam50 is confined to the mitosomal vesicles uniformly distributed through the cytoplasm. 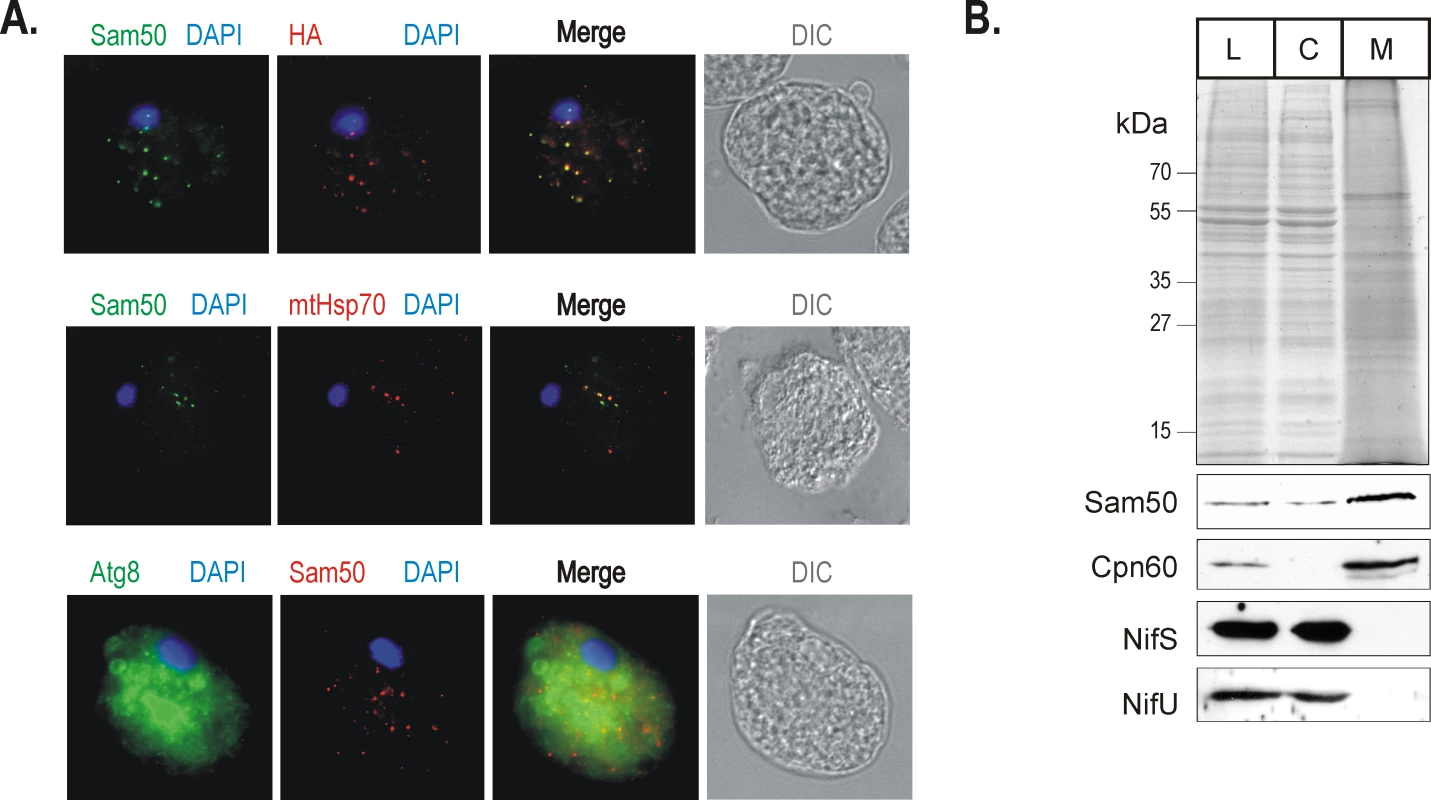
(A) The upper panel shows fixed E. histolytica cells expressing the HA-tagged version of EhSam50 were probed with α-HA (green) and α -rEhSam50 (red ) antibody for immnunofluorescent labeling. DIC – differential contrast (Nomarski). In the middle panel HA-tagged EhSam50 was labeled by α-HA (green) and mtHsp70 with polyclonal α-mtHsp70 (red) raised against Nyctotherus ovalis mtHsp70 [57]. The lower panel shows fixed E. histolytica cells expressing the HA-tagged version of EhSam50 were probed with α-Atg8, an autophagosome marker (green) and α-EhSam50 (red) antibody. (B) Cultured E. histolytica cells were disrupted and the cell lysate (L), cytosol (C) and mitosome-enriched fraction (M) and probed for NifS, NifU using homologous polyclonal antibodies [41] and Cpn60 using heterologous polyclonal antibody raised against Giardia intestinalis Cpn60. EhSam50 was decorated with α-HA monoclonal antibody. To further test the mitosomal distribution of the protein import machinery in E. histolytica, the cell fractions were probed for the mitosomal and the cytosolic marker proteins (Figure 6B). While our data suggested the cytosolic distribution of NifU and NifS proteins as found in the analysis of Mi-Ichi et al. [22], EhSam50 and mitosomal Cpn60 were distributed in the high-speed pellet fraction containing mitosomes.
Discussion
Hidden Markov models can be used for a highly sensitive search of sequence databases [58], and discovered three new mitosomal proteins in E. histolytica. Tom40 provides a channel for the import of mitochondrial proteins including Sam50 and Tom40 itself, whereas Sam50 is necessary for assembly of the Tom40 channel (and Sam50 itself). The characterization of homologs of Sam50 and Tom40 in E. histolytica suggests that mitosomes have a functional outer membrane as also recently shown by Maralikova et al. [21]. It is now clear, even with so few mitosomal proteins identified in E. histolytica, that we have in hand marker proteins for the outer membrane (Sam50, Tom40), the inner membrane (ADP/ATP carrier, PiC, sodium/sulfate symporter) and the mitosomal matrix (e.g. molecular chaperones, rubrerythrin, ATP sulfurylase).
The inner membrane of all mitochondria known so far is populated by the proteins from Tim17/22/23 family. These proteins contain four membrane spanning helices, by which they build up the core channels of the translocase of the inner membrane (TIM). In animals fungi, plants and diverse protists groups these proteins are present in three different forms as Tim17, Tim22 and Tim23, which play distinct roles in the import of matrix and/or inner membrane proteins. Some eukaryotes, notably trypanosomes and microsporidians, appear to make use of just a single Tim17/22/23 protein [13],[59], which might functionally substitute for the three distinct forms as a “jack of all trades”. Despite the high degree of similarity among the Tims, from these various eukaryotes including D. discoideum, the HMM models of the Tim17/22/23 family of proteins did not reveal any homologous sequences in E. histolytica. This could either mean that the putative E. histolytica Tims have diverged in sequence to such an extent as to be beyond the sensitivity of our HMM models, or that E. histolytica lacks any Tim17/22/23-related protein due to the overall mitochondrial reduction. Failure to detect a Tim17 family protein in mitosomes of G. intestinalis argues for such a common reductive step in mitochondrial adaptation [10],[60]. An interesting question is raised about the mechanism of carrier protein assembly in the mitosomal membranes of E. histolytica as these proteins normally require functional TIM22 translocase.
Owing to its extreme lifestyle, E. histolytica is believed to depend solely on cytosolic production of ATP through glycolysis [61]. The ADP/ATP carrier in E. histolytica mitosomes provides for retrograde transport of ATP into the mitosomal matrix, enabling the ATP-dependent activity of Hsp70 and Cpn60 as well as ATP sulfurylase and APS kinase [17],[22],[28],[29]. Our finding of the phosphate carrier EhPiC completes this metabolic shunt.
Targeting of the proteins into E. histolytica mitosomes
Most of soluble mitochondrial proteins contain cleavable N-terminal targeting sequence that usually forms an amphipathic helix, which is recognized by the receptor on the outer mitochondrial membrane and upon the translocation into mitochondrial matrix cleaved off by a matrix processing peptidase (MPP) [62]. The robust bioinformatic search for more proteins with putative mitosomal targeting sequences in E. histolytica is hindered by very poor training dataset available to date (Supplementary information Figure S4) [22]. However, in our additional HMM search for a putative MPP homologue we identified single candidate protein from M16 peptidase family and we are currently characterizing this protein. Although MPP is a heterodimer of two different but evolutionary related subunits, a single subunit minimalist version of the enzyme was reported recently in the mitosomes of G. intestinalis [60]. Given that mitosomes of E. histolytica represent even more reduced mitochondria one can anticipate analogous reduction occurring herein.
Protein import into amoebic mitochondria - a window into mitochondrial evolution
Phylogenetic reconstructions and the presence of flagellate cells in the life-cycles of some Amoebozoans suggested an affiliation of this supergroup with animals and fungi [36], so that studies on fundamental pathways in the Amoebozoa provide important additional information on the cell biology of the earliest animals and fungi.
Genome sequence analysis of the highly-studied amoebozoan, D. discoideum, revealed the presence of 14 different components taking part in the mitochondrial protein import in animals and fungi (see Figure 3). This analysis suggests that all four major membrane complexes: SAM, TOM, TIM22 and TIM23 are present in D. dictyostelium, as is the intermembrane space-located small TIM chaperones that shuttle substrates to the SAM or TIM22 complexes. The presence of these various complexes in D. discoideum is consistent with them having been installed in the earliest stages of eukaryote evolution. The components that are missing in D. discoideum are those that appear to be specific for fungi and animals, as has been previously discussed for receptor subunits Tom20 [63] and Tom70 [64]. That so many TOM and TIM components are missing from E. histolytica, is strong evidence for a secondary gene loss having occurred, as part of the reductive evolution impacting on the mitosomal organelle. The anaerobic lifestyle, combined with parasitism, of E. histolytica likely selected for a minimalist mitochondrial set up and the enormous reduction of the import machinery. This likely reflects the even more dramatic reduction of the overall mitochondrial metabolism. The anaerobic, free-living amoeba Mastigamoeba balamuthi looks to have traveled part way the same direction: 21 putative mitochondrial proteins have been identified in the limited EST dataset, while no TOM, TIM and SAM components were found so far [65].
The recent data on the function of E. histolytica mitosome have revealed remarkable divergence of the processes occurring within this mysterious mitochondrion. It now seems that while these organelles have lost vast majority of typical mitochondrial functions, they have accommodated several new unexpected roles not seen in other mitochondria [21],[22]. In this paper, we uncovered the essentials of the protein import into these organelles and it is very likely that the further research on the function and the biogenesis of E. histolytica mitosome will bring more of the unexpected.
Materials and Methods
Sequence search and analysis
The Hidden Markov models describing 33 mitochondrial components were constructed and compiled into a HMM library from manually prepared families of sequences (Oxa1, Tim10, Tim44, Tom20opistho, Tom20plants, Tom70, Pam16, Tim13, Tim50, Tom22opistho, Tom22plants, Hsp70, Pam18, Tim17, Tim54, Sam35, Tim18, Tim8, Tom40, Mdm10, Sam37, Tim21, Tim9, Tom5, Metaxin1, Sam50, Tim22, Tim9+10, Tom6, Metaxin2, Tim23, and Tom7). The HMM library was used to scan the genomes of E. histolytica (http://www.tigr.org/tdb/e2k1/eha1/) and D. discoideum (NCBI). In addition, a hidden Markov model based on 34 manually compiled MCP sequences was built and used to scan the two genomes. The program HMMER 2.3.2 was used in all calculations [66], and the search results were extracted with the programs prepared in-house.
The homology modeling of the mitochondrial carrier protein was performed with SwissModel at http://swissmodel.expasy.org//SWISS-MODEL.html [67]. The structure of bovine ATP/ADP carrier (PDB ID 2C3E) was used as a template [30]. The sequences were aligned using ClustalX [68] and edited manually in BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html).
For the neighbor joining analysis of mitochondrial carrier proteins amino acid sequences were aligned and the resulting alignment edited manually into a dataset of 33 sequences of 190 amino acid residues. SplitsTree4 [69] software was used to calculate the bootstrapping of 500 runs and to combine the results into a Neighbor-Net.
Protein maximum-likelihood phylogenetic tree of Omp85/Toc75/Sam50 proteins was derived from a dataset of 407 aligned amino acids from 39 sequences. The tree was obtained using the program MrBayes under the JTT substitution matrix with amino acid frequencies estimated from the dataset [70]. Site rate variation was modeled under a discrete approximation to the Γ distribution (one invariable and four variable rate categories). Four Monte Carlo Markov Chains, each with 2,000,000 generations, were performed with trees sampled every 100 generations. For compilation of Bayesian consensus topologies, a “burn-in” of 500 trees was used.
CLANS version 2 October 9, 2006 was obtained from http://bioinfoserver.rsbs.anu.edu.au/programs/clans/. The EhTom40 sequence was added to a sequence set of 137 VDAC and 79 Tom40 sequences); the sequence set was derived from both the results of BLAST and HMM searches using VDAC and Tom40 sequences. Cluster analysis was performed with a P-value cutoff (10−82) sufficient to observe complete separation of the VDAC and Tom40 sequence clusters. As the method is non-deterministic, the analysis was run until stable clusters formed (in excess of 200 iterations). Multiple runs were performed to ensure that the observed clusters formed using different starting positions for the sequences.
Entamoeba histolytica culture and preparation of RNA
Trophozoites of the E. histolytica isolate HM-1:IMSS were cultured axenically in TYI-S-33 medium in plastic tissue culture flasks [71]. For further experiments 1×106 trophozoites were cultivated for 24 h in 75 ml culture flasks. The trophozoites were then harvested after being chilled on ice for 5 min and sedimented at 430×g at 4°C for 5 min. The resulting pellet was washed once in phosphate-buffered saline pH 7.2 and once in 20 mM MOPS pH 7.2. The cell pellet was resuspended in 2 ml of 20 mM MOPS pH 7.2 with protease inhibitors and passed 6 times through a 25 G needle until most cell were broken. The cell lysate was diluted with 25 ml of 250 mM Sucrose, 20 mM MOPS pH 7.2 and spun down twice at 650×g for 10 min, resulting supernatant was spun down at 2,850×g for 10 min. The final high-speed pellet representing the mitosomal fraction was obtained after centrifugation at 100,000×g for 30 min. The high-speed supernatant corresponded to the cytosolic fraction.
For total RNA isolation 1×106 E. histolytica trophozoites were cultivated in 75 ml culture flasks for 24 h. The cells were harvested via chilling on ice for 5 min and sedimented at 200×g for 5 min at 4°C. The cell pellet was washed twice with PBS. The trophozoites were treated with TRIZOL reagent (Invitrogen) following the manufacturer's instructions. Extracted RNA was purified using the RNeasy mini kit (Qiagen) without β-mercaptoethanol and DNA was digested with DNase (Qiagen). cDNA synthesis was accomplished with SuperScriptIII Reverse Transcriptase (Invitrogen). In a final volume of 20 µl, 1 µg of RNase-free and DNase-treated total RNA was mixed with 5×First-Strand buffer, 500 µM dNTPs, 500 nM OdT-T71 (5′-GAG AGA GGA TCC AAG TAC TAA TAC GAC TCA CTA TAG GGA GAT24), 2 mM DTT, 40 U RnaseOut (Invitrogen) and SuperScriptIII (200 U/µl). cDNA was synthesised for 1 h at 42°C.
Yeast culture and cell fractionation
Saccharomyces cerevisiae strain W303a was grown in rich medium or selective medium as previously described [72]. The Δmir1 strain was a gift from Dr. Geneviève Dujardin (Centre de Génétique Moléculaire, CNRS, Paris, France) [33]. For the preparation of mitochondria for the in vitro study S. cerevisiae strain W303a was grown in lactate media at 25°C. The mitochondria were isolated by differential centrifugation as described previously [73]. For the growth assays the cells were grown to a mid-logarithmic phase in a complete media, diluted into OD600 = 0.2, spotted in the series of fivefold dilutions on the plates and incubated at 30°C for 3–6 days.
Cloning and expression of ehpic, ehtom40 and ehsam50
For GFP-tagging, the open reading frames were amplified by PCR using E. histolytica genomic DNA as template and primers containing 5′EcoRI and 3′BamHI or BglII sites (see Table S1) and cloned into p416MET25 vector [74]. To create the C-terminal HA-tag fusions the ORFs were cloned into a pYX143 vector with the use of 5′EcoRI and 3′ MluI restriction sites. For the expression of ehsam50 in E. histolytica, the ORF was amplified from pYX143 with the C-terminal HA-tag using the 5′KpnI and 3′ BglII restriction sites. The plasmid (pNC) used for transfection is a derivative of the expression vector EhNEO/CAT. The plasmid contains the neomycin phosphotransferase-coding sequence flanked by 480 bp of the 5′-untranslated sequence and 600 bp of the 3′-untranslated sequence of an E. histolytica actin gene. Transfections were performed by electroporation as described previously [75]. Drug selection started 48 h after transfection, using 10 µg/ml of the neomycin analogue G418. Two weeks later, the G418 concentration was increased to 50 µg/ml.
For the production of EhSam50-derived antigen the first 300 bp of ehsam50 were amplified by PCR and ligated into pET23a vector (Novagen) using 5′NdeI and 3′ XhoI restriction sites. E. coli strain BL21 (DE3) was used to produce the recombinant protein with C-terminal six histidine tag. The protein was purified to homogeneity under denaturing condition (8 M urea) on NTA-nickel column (Qiagen). For generation of polyclonal antibodies 100 µg recombinant EhSam50 (rEhSam50) was injected into a mouse, followed by two further injections.
In vitro protein import
Mitochondria were prepared according to the method of Daum et al [73]. For in vitro transcription the genes were amplified by PCR with the forward primers containing the SP6 promoter followed by a Kozak's sequence (ehpic, ehtom40, ehsam50) or cloned into pSP73 vector (ehAAC), which was linearized at a unique site downstream of the gene. [76],[77]. In vitro imports were assayed according to Gabriel and Pfanner [78].
Immunofluorescence microscopy and western blot analysis
E. histolytica cells were analyzed with α-EhSam50 antiserum, with an antiserum raised against the E. histolytica autophagosome marker Atg8 (autophagy related gene 8, a gift from Dr. Tomoyoshi Nozaki, National Institute of Infectious Diseases, Tokyo, Japan) or with a mouse monoclonal anti-HA antibody. Cells were fixed at room temperature for 30 min in PBS containing 3% paraformaldehyde and subsequently permeabilized with 0.05% saponin (PBSS). Samples were incubated at room temperature for 1 h with antisera against EhSam50 (1∶250 dilution), against Atg8 (1∶500 dilution) or against the HA-tag (1∶200, Roche). Secondary antibodies were Alexa-594 coupled α-mouse, Alexa-488 coupled α-rabbit, and Alexa-594 coupled α-mouse antibodies. Subsequently, cells were mounted on glass slides and examined under 6300× magnification. For deconvolution microscopy images of selected cells were captured with a 63× oil immersion lens in a UV equipped Leica DM RB microscope with 0.2-µm-diameter step Z-sections. Deconvoluted Z sections were examined for colocalisation of Atg8 and EhSam50 staining with the Openlab 4.0.4 program. Adobe Photoshop 7.0.5 was used for additional processing of the images. S. cerevisiae were analyzed as previously described (Beilharz et al. 2003). ImageJ software was used for additional image processing (http://rsbweb.nih.gov/ij/). In the western blot analysis cell fractions were probed with the mouse monoclonal anti-HA antibody (1∶500), rabbit polyclonal antibodies raised against Giardia intestinalis Cpn60 (1∶1000) (a kind gift from Dr. Robert Hirt, Newcastle University, UK), E. histolytica NifS and NifU (both 1∶1000) (a kind gift from Dr.Tomoyoshi Nozaki [26]).
The distribution of EhPiC, EhTom40 and EhSam50 in S. cerevisiae mitochondria extracted in fresh 100mM Na2CO3 was done as previously described [79].
Supporting Information
Zdroje
1. EmbleyTM
MartinW
2006 Eukaryotic evolution, changes and challenges. Nature 440 623 630
2. Cavalier-SmithT
1989 Molecular phylogeny. Archaebacteria and Archezoa. Nature 339 l00 l01
3. AkhmanovaA
VonckenF
van AlenT
vanHA
BoxmaB
1998 A hydrogenosome with a genome. Nature 396 527 528
4. HrdýI
HirtRP
DolezalP
BardonováL
FosterPG
2004 Trichomonas hydrogenosomes contain the NADH dehydrogenase module of mitochondrial complex I. Nature 432 618 622
5. DyallSD
YanW
Delgadillo-CorreaMG
LuncefordA
LooJA
2004 Non-mitochondrial complex I proteins in a hydrogenosomal oxidoreductase complex. Nature 431 1103 1107
6. van derGiezen
2009 Hydrogenosomes and mitosomes: conservation and evolution of functions. J Eukaryot Microbiol 56 221 231
7. MokranjacD
NeupertW
2005 Protein import into mitochondria. Biochem Soc Trans 33 1019 1023
8. KutikS
GuiardB
MeyerHE
WiedemannN
PfannerN
2007 Cooperation of translocase complexes in mitochondrial protein import. J Cell Biol 179 585 591
9. DolezalP
LikićV
TachezyJ
LithgowT
2006 Evolution of the molecular machines for protein import into mitochondria. Science 313 314 318
10. DolezalP
SmidO
RadaP
ZubacováZ
BursaćD
2005 Giardia mitosomes and trichomonad hydrogenosomes share a common mode of protein targeting. Proc Natl Acad Sci U S A 102 10924 10929
11. RegoesA
ZourmpanouD
León-AvilaG
van derGiezen
TovarJ
2005 Protein import, replication, and inheritance of a vestigial mitochondrion. J Biol Chem 280 30557 30563
12. BurriL
WilliamsBA
BursaćD
LithgowT
KeelingPJ
2006 Microsporidian mitosomes retain elements of the general mitochondrial targeting system. Proc Natl Acad Sci U S A 103 15916 15920
13. BurriL
KeelingPJ
2007 Protein targeting in parasites with cryptic mitochondria. Int J Parasitol 37 265 272
14. TovarJ
León-AvilaG
SanchezLB
SutakR
TachezyJ
2003 Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature 426 172 176
15. TsaousisAD
KunjiER
GoldbergAV
LucocqJM
HirtRP
2008 A novel route for ATP acquisition by the remnant mitochondria of Encephalitozoon cuniculi. Nature 453 553 556
16. WilliamsBA
HirtRP
LucocqJM
EmbleyTM
2002 A mitochondrial remnant in the microsporidian Trachipleistophora hominis. Nature 418 865 869
17. TovarJ
FischerA
ClarkCG
1999 The mitosome, a novel organelle related to mitochondria in the amitochondrial parasite Entamoeba histolytica. Mol Microbiol 32 1013 1021
18. MaiZ
GhoshS
FrisardiM
RosenthalB
RogersR
1999 Hsp60 is targeted to a cryptic mitochondrion-derived organelle (“crypton”) in the microaerophilic protozoan parasite Entamoeba histolytica. Mol Cell Biol 19 2198 2205
19. GoldbergAV
MolikS
TsaousisAD
NeumannK
KuhnkeG
2008 Localization and functionality of microsporidian iron-sulphur cluster assembly proteins. Nature 452 624 628
20. KatinkaMD
DupratS
CornillotE
MetenierG
ThomaratF
2001 Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414 450 453
21. MaralikovaB
AliV
Nakada-TsukuiK
NozakiT
van derGiezen
2009 Bacterial-type oxygen detoxification and iron-sulfur cluster assembly in amoebal relict mitochondria. Cell Microbiol
22. Mi-IchiF
YousufMA
Nakada-TsukuiK
NozakiT
2009 Mitosomes in Entamoeba histolytica contain a sulfate activation pathway. Proc Natl Acad Sci U S A
23. LillR
MühlenhoffU
2006 Iron-sulfur protein biogenesis in eukaryotes: components and mechanisms. Annu Rev Cell Dev Biol 22 457 486
24. ClarkCG
2000 The evolution of Entamoeba, a cautionary tale. Res Microbiol 151 599 603
25. van der GiezenM
CoxS
TovarJ
2004 The iron-sulfur cluster assembly genes iscS and iscU of Entamoeba histolytica were acquired by horizontal gene transfer. BMC Evol Biol 4 7
26. AliV
ShigetaY
TokumotoU
TakahashiY
NozakiT
2004 An intestinal parasitic protist, Entamoeba histolytica, possesses a non-redundant nitrogen fixation-like system for iron-sulfur cluster assembly under anaerobic conditions. J Biol Chem 279 16863 16874
27. ChanKW
SlotboomDJ
CoxS
EmbleyTM
FabreO
2005 A novel ADP/ATP transporter in the mitosome of the microaerophilic human parasite Entamoeba histolytica. Curr Biol 15 737 742
28. TovarJ
CoxSS
van der GiezenM
2007 A mitosome purification protocol based on percoll density gradients and its use in validating the mitosomal nature of Entamoeba histolytica mitochondrial Hsp70. Methods Mol Biol 390 167 177
29. van der GiezenM
León-AvilaG
TovarJ
2005 Characterization of chaperonin 10 (Cpn10) from the intestinal human pathogen Entamoeba histolytica. Microbiology 151 3107 3115
30. Pebay-PeyroulaE
Dahout-GonzalezC
KahnR
TrézéguetV
LauquinGJ
2003 Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426 39 44
31. SatreM
MatteiS
AubryL
GaudetP
PelosiL
2007 Mitochondrial carrier family: repertoire and peculiarities of the cellular slime mould Dictyostelium discoideum. Biochimie 89 1058 1069
32. KunjiER
RobinsonAJ
2006 The conserved substrate binding site of mitochondrial carriers. Biochim Biophys Acta 1757 1237 1248
33. HamelP
Saint-GeorgesY
de PintoB
LachacinskiN
AltamuraN
2004 Redundancy in the function of mitochondrial phosphate transport in Saccharomyces cerevisiae and Arabidopsis thaliana. Mol Microbiol 51 307 317
34. MurakamiH
BlobelG
PainD
1990 Isolation and characterization of the gene for a yeast mitochondrial import receptor. Nature 347 488 491
35. SimpsonAG
RogerAJ
2002 Eukaryotic evolution: getting to the root of the problem. Curr Biol 12 R691 R693
36. NikolaevSI
BerneyC
PetrovNB
MylnikovAP
FahrniJF
2006 Phylogenetic position of Multicilia marina and the evolution of Amoebozoa. Int J Syst Evol Microbiol 56 1449 1458
37. EichingerL
PachebatJA
GlocknerG
RajandreamMA
SucgangR
2005 The genome of the social amoeba Dictyostelium discoideum. Nature 435 43 57
38. MacasevD
WhelanJ
NewbiginE
Silva-FilhoMC
MulhernTD
2004 Tom22′, an 8-kDa trans-site receptor in plants and protozoans, is a conserved feature of the TOM complex that appeared early in the evolution of eukaryotes. Mol Biol Evol 21 1557 1564
39. BarthC
LeP
FisherPR
2007 Mitochondrial biology and disease in Dictyostelium. Int Rev Cytol 263 207 252
40. NagayamaK
ItonoS
YoshidaT
IshiguroS
OchiaiH
2008 Antisense RNA inhibition of the beta subunit of the Dictyostelium discoideum mitochondrial processing peptidase induces the expression of mitochondrial proteins. Biosci Biotechnol Biochem 72 1836 1846
41. LoftusB
AndersonI
DaviesR
AlsmarkUC
SamuelsonJ
2005 The genome of the protist parasite Entamoeba histolytica. Nature 433 865 868
42. BakatselouC
KidgellC
GrahamCC
2000 A mitochondrial-type hsp70 gene of Entamoeba histolytica. Mol Biochem Parasitol 110 177 182
43. WallerRF
JabbourC
ChanNC
CelikN
LikićVA
2009 Evidence of a reduced and modified mitochondrial protein import apparatus in microsporidian mitosomes. Eukaryot Cell 8 19 26
44. PusnikM
CharriereF
MaserP
WallerRF
DagleyMJ
2009 The single mitochondrial porin of Trypanosoma brucei is the main metabolite transporter in the outer mitochondrial membrane. Mol Biol Evol 26 671 680
45. FinnRD
TateJ
MistryJ
CoggillPC
SammutSJ
2008 The Pfam protein families database. Nucleic Acids Res 36 D281 D288
46. FrickeyT
LupasA
2004 CLANS: a Java application for visualizing protein families based on pairwise similarity. Bioinformatics 20 3702 3704
47. DagleyMJ
DolezalP
LikićVA
SmidO
PurcellAW
2009 The protein import channel in the outer mitosomal membrane of Giardia intestinalis. Mol Biol Evol 26 1941 1947
48. KutikS
StojanovskiD
BeckerL
BeckerT
MeineckeM
2008 Dissecting membrane insertion of mitochondrial beta-barrel proteins. Cell 132 1011 1024
49. GabrielK
EganB
LithgowT
2003 Tom40, the import channel of the mitochondrial outer membrane, plays an active role in sorting imported proteins. EMBO J 22 2380 2386
50. WilihoeftU
Campos-GongoraE
TouzniS
BruchhausI
TannichE
2001 Introns of Entamoeba histolytica and Entamoeba dispar. Protist 152 149 156
51. GentleIE
BurriL
LithgowT
2005 Molecular architecture and function of the Omp85 family of proteins. Mol Microbiol 58 1216 1225
52. VoulhouxR
TommassenJ
2004 Omp85, an evolutionarily conserved bacterial protein involved in outer-membrane-protein assembly. Res Microbiol 155 129 135
53. GentleI
GabrielK
BeechP
WallerR
LithgowT
2004 The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J Cell Biol 164 19 24
54. Sánchez-PulidoL
DevosD
GenevroisS
VicenteM
ValenciaA
2003 POTRA: a conserved domain in the FtsQ family and a class of beta-barrel outer membrane proteins. Trends Biochem Sci 28 523 526
55. PicazarriK
Nakada-TsukuiK
NozakiT
2008 Autophagy during proliferation and encystation in the protozoan parasite Entamoeba invadens. Infect Immun 76 278 288
56. León-AvilaG
TovarJ
2004 Mitosomes of Entamoeba histolytica are abundant mitochondrion-related remnant organelles that lack a detectable organellar genome. Microbiology 150 1245 1250
57. van derGiezen
BirdseyGM
HornerDS
LucocqJ
DyalPL
2003 Fungal hydrogenosomes contain mitochondrial heat-shock proteins. Mol Biol Evol 20 1051 1061
58. EddySR
1996 Hidden Markov models. Curr Opin Struct Biol 6 361 365
59. SchneiderA
BursaćD
LithgowT
2008 The direct route: a simplified pathway for protein import into the mitochondrion of trypanosomes. Trends Cell Biol 18 12 18
60. SmidO
MatuskovaA
HarrisSR
KuceraT
NovotnyM
2008 Reductive evolution of the mitochondrial processing peptidases of the unicellular parasites trichomonas vaginalis and Giardia intestinalis. PLoS Pathog 4 e1000243 doi:10.1371/journal.ppat.1000243
61. SaavedraE
EncaladaR
PinedaE
Jasso-ChavezR
Moreno-SánchezR
2005 Glycolysis in Entamoeba histolytica. Biochemical characterization of recombinant glycolytic enzymes and flux control analysis. FEBS J 272 1767 1783
62. ChacinskaA
KoehlerCM
MilenkovicD
LithgowT
PfannerN
2009 Importing mitochondrial proteins: machineries and mechanisms. Cell 138 628 644
63. LikićVA
PerryA
HulettJ
DerbyM
TravenA
2005 Patterns that define the four domains conserved in known and novel isoforms of the protein import receptor Tom20. J Mol Biol 347 81 93
64. ChanNC
LikićVA
WallerRF
MulhernTD
LithgowT
2006 The C-terminal TPR domain of Tom70 defines a family of mitochondrial protein import receptors found only in animals and fungi. J Mol Biol 358 1010 1022
65. GillEE
az-TrivinoS
BarberaMJ
SilbermanJD
StechmannA
2007 Novel mitochondrion-related organelles in the anaerobic amoeba Mastigamoeba balamuthi. Mol Microbiol 66 1306 1320
66. EddySR
1998 Profile hidden Markov models. Bioinformatics 14 755 763
67. ArnoldK
BordoliL
KoppJ
SchwedeT
2006 The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22 195 201
68. ThompsonJD
GibsonTJ
PlewniakF
JeanmouginF
HigginsDG
1997 The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25 4876 4882
69. HusonDH
BryantD
2006 Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23 254 267
70. HuelsenbeckJP
RonquistF
2001 MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17 754 755
71. DiamondLS
HarlowDR
CunnickCC
1978 A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg 72 431 432
72. LithgowT
JunneT
WachterC
SchatzG
1994 Yeast mitochondria lacking the two import receptors Mas20p and Mas70p can efficiently and specifically import precursor proteins. J Biol Chem 269 15325 15330
73. DaumG
BohniPC
SchatzG
1982 Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem 257 13028 13033
74. BeilharzT
EganB
SilverPA
HofmannK
LithgowT
2003 Bipartite signals mediate subcellular targeting of tail-anchored membrane proteins in Saccharomyces cerevisiae. J Biol Chem 278 8219 8223
75. HamannL
NickelR
TannichE
1995 Transfection and continuous expression of heterologous genes in the protozoan parasite Entamoeba histolytica. Proc Natl Acad Sci U S A 92 8975 8979
76. NijtmansLG
HendersonNS
HoltIJ
2002 Blue Native electrophoresis to study mitochondrial and other protein complexes. Methods 26 327 334
77. WittigI
BraunHP
SchaggerH
2006 Blue native PAGE. Nat Protoc 1 418 428
78. GabrielK
PfannerN
2007 The mitochondrial machinery for import of precursor proteins. Methods Mol Biol 390 99 117
79. YoukerRT
WalshP
BeilharzT
LithgowT
BrodskyJL
2004 Distinct roles for the Hsp40 and Hsp90 molecular chaperones during cystic fibrosis transmembrane conductance regulator degradation in yeast. Mol Biol Cell 15 4787 4797
80. GuindonS
GascuelO
2003 A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52 696 704
81. SodingJ
BiegertA
LupasAN
2005 The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res 33 W244 W248
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- All Mold Is Not Alike: The Importance of Intraspecific Diversity in Necrotrophic Plant Pathogens
- Tsetse EP Protein Protects the Fly Midgut from Trypanosome Establishment
- Perforin and IL-2 Upregulation Define Qualitative Differences among Highly Functional Virus-Specific Human CD8 T Cells
- N-Acetylglucosamine Induces White to Opaque Switching, a Mating Prerequisite in
- Origin and Evolution of Sulfadoxine Resistant
- Rapid Evolution of Pandemic Noroviruses of the GII.4 Lineage
- Natural Strain Variation and Antibody Neutralization of Dengue Serotype 3 Viruses
- Fine-Tuning Translation Kinetics Selection as the Driving Force of Codon Usage Bias in the Hepatitis A Virus Capsid
- Structural Basis of Cell Wall Cleavage by a Staphylococcal Autolysin
- Direct Visualization by Cryo-EM of the Mycobacterial Capsular Layer: A Labile Structure Containing ESX-1-Secreted Proteins
- Lipopolysaccharide Is Synthesized via a Novel Pathway with an Evolutionary Connection to Protein -Glycosylation
- MicroRNA Antagonism of the Picornaviral Life Cycle: Alternative Mechanisms of Interference
- Limited Trafficking of a Neurotropic Virus Through Inefficient Retrograde Axonal Transport and the Type I Interferon Response
- Direct Restriction of Virus Release and Incorporation of the Interferon-Induced Protein BST-2 into HIV-1 Particles
- RNAIII Binds to Two Distant Regions of mRNA to Arrest Translation and Promote mRNA Degradation
- Direct TLR2 Signaling Is Critical for NK Cell Activation and Function in Response to Vaccinia Viral Infection
- The Essentials of Protein Import in the Degenerate Mitochondrion of
- Dynamic Imaging of Experimental Induced Hepatic Granulomas Detects Kupffer Cell-Restricted Antigen Presentation to Antigen-Specific CD8 T Cells
- An Accessory to the ‘Trinity’: SR-As Are Essential Pathogen Sensors of Extracellular dsRNA, Mediating Entry and Leading to Subsequent Type I IFN Responses
- Innate Killing of by Macrophages of the Splenic Marginal Zone Requires IRF-7
- Exoerythrocytic Parasites Secrete a Cysteine Protease Inhibitor Involved in Sporozoite Invasion and Capable of Blocking Cell Death of Host Hepatocytes
- Inhibition of Macrophage Migration Inhibitory Factor Ameliorates Ocular -Induced Keratitis
- Membrane Damage Elicits an Immunomodulatory Program in
- Fatal Transmissible Amyloid Encephalopathy: A New Type of Prion Disease Associated with Lack of Prion Protein Membrane Anchoring
- Nucleophosmin Phosphorylation by v-Cyclin-CDK6 Controls KSHV Latency
- A Combination of Independent Transcriptional Regulators Shapes Bacterial Virulence Gene Expression during Infection
- Inhibition of Host Vacuolar H-ATPase Activity by a Effector
- Human Cytomegalovirus Protein pUL117 Targets the Mini-Chromosome Maintenance Complex and Suppresses Cellular DNA Synthesis
- Dispersion as an Important Step in the Biofilm Developmental Cycle
- Kaposi's Sarcoma-Associated Herpesvirus ORF57 Protein Binds and Protects a Nuclear Noncoding RNA from Cellular RNA Decay Pathways
- Differential Regulation of Effector- and Central-Memory Responses to Infection by IL-12 Revealed by Tracking of Tgd057-Specific CD8+ T Cells
- The Human Polyoma JC Virus Agnoprotein Acts as a Viroporin
- Expansion, Maintenance, and Memory in NK and T Cells during Viral Infections: Responding to Pressures for Defense and Regulation
- T Cell-Dependence of Lassa Fever Pathogenesis
- HIV and Mature Dendritic Cells: Trojan Exosomes Riding the Trojan Horse?
- Endocytosis of the Anthrax Toxin Is Mediated by Clathrin, Actin and Unconventional Adaptors
- A Capsid-Encoded PPxY-Motif Facilitates Adenovirus Entry
- Homeostatic Interplay between Bacterial Cell-Cell Signaling and Iron in Virulence
- Serological Profiling of a Protein Microarray Reveals Permanent Host-Pathogen Interplay and Stage-Specific Responses during Candidemia
- YfiBNR Mediates Cyclic di-GMP Dependent Small Colony Variant Formation and Persistence in
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Kaposi's Sarcoma-Associated Herpesvirus ORF57 Protein Binds and Protects a Nuclear Noncoding RNA from Cellular RNA Decay Pathways
- Endocytosis of the Anthrax Toxin Is Mediated by Clathrin, Actin and Unconventional Adaptors
- Perforin and IL-2 Upregulation Define Qualitative Differences among Highly Functional Virus-Specific Human CD8 T Cells
- Inhibition of Macrophage Migration Inhibitory Factor Ameliorates Ocular -Induced Keratitis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



