-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
LIGHT (TNFSF14) is a member of the TNF superfamily involved in inflammation and defence against infection. LIGHT signals via two cell-bound receptors; herpes virus entry mediator (HVEM) and lymphotoxin-beta receptor (LTβR). We found that LIGHT is critical for control of hepatic parasite growth in mice with visceral leishmaniasis (VL) caused by infection with the protozoan parasite Leishmania donovani. LIGHT-HVEM signalling is essential for early dendritic cell IL-12/IL-23p40 production, and the generation of IFNγ - and TNF-producing T cells that control hepatic infection. However, we also discovered that LIGHT-LTβR interactions suppress anti-parasitic immunity in the liver in the first 7 days of infection by mechanisms that restrict both CD4+ T cell function and TNF-dependent microbicidal mechanisms. Thus, we have identified distinct roles for LIGHT in infection, and show that manipulation of interactions between LIGHT and its receptors may be used for therapeutic advantage.
Published in the journal: Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection. PLoS Pathog 7(10): e32767. doi:10.1371/journal.ppat.1002279
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002279Summary
LIGHT (TNFSF14) is a member of the TNF superfamily involved in inflammation and defence against infection. LIGHT signals via two cell-bound receptors; herpes virus entry mediator (HVEM) and lymphotoxin-beta receptor (LTβR). We found that LIGHT is critical for control of hepatic parasite growth in mice with visceral leishmaniasis (VL) caused by infection with the protozoan parasite Leishmania donovani. LIGHT-HVEM signalling is essential for early dendritic cell IL-12/IL-23p40 production, and the generation of IFNγ - and TNF-producing T cells that control hepatic infection. However, we also discovered that LIGHT-LTβR interactions suppress anti-parasitic immunity in the liver in the first 7 days of infection by mechanisms that restrict both CD4+ T cell function and TNF-dependent microbicidal mechanisms. Thus, we have identified distinct roles for LIGHT in infection, and show that manipulation of interactions between LIGHT and its receptors may be used for therapeutic advantage.
Introduction
Tumour necrosis factor (TNF) superfamily members are involved in many biological functions, including cell growth and differentiation, apoptosis and organogenesis [1]. This broad range of activities is achieved by TNF family members interacting with functional receptors associated with distinct cell signalling pathways [2]. TNF, lymphotoxin (LT)α, LTβ and LIGHT (TNFSF14) comprise a closely related set of ligands in the TNF family [3], [4]. TNF exists as a cell-bound or soluble homotrimer that binds TNF receptor (TNFR)1 and TNFR2 [5], [6]. LTα can form a soluble homotrimer (LTα3) that binds TNFR1, TNFR2 and HVEM [5], [7], but can also form a cell-bound heterotrimer with LTβ (LTα1β2) that binds and signals through LTβR [8]. LIGHT exists in cell-bound and soluble forms that interact with both LTβR and herpes virus entry mediator (HVEM) [7], [9], [10]. HVEM also engages members of the immunoglobulin superfamily; B and T lymphocyte attenuator (BTLA) [11] and CD160 [12], as well as the envelope glycoprotein D of Herpes Simplex virus [13]. HVEM activates BTLA inhibitory signalling via SHP phosphatases suppressing T cell activation [14]. LIGHT, LTα and the Ig superfamily ligands can also activate HVEM-dependent cell survival signalling via NF-κB [15].
LIGHT has emerged as a key mediator of inflammation and immune homeostasis [4], [14]. There is broad expression of LIGHT and HVEM in the hematopoietic compartment [7], [9], [16], [17], [18], while LTβR expression is largely restricted to stromal and myeloid cells [7], [19], [20]. LTβR and HVEM are implicated as key host defence mechanisms against persistent viral [21] and bacterial pathogens [22]. However, little is known about the role of these receptors in infection with parasites that establish persistent infections in their hosts.
The protozoan parasite Leishmania donovani causes persistent infections in humans and experimental animals [23], [24]. We and others have defined important roles for TNF and LTα in host resistance in a mouse model of visceral leishmaniasis (VL) caused by L. donovani [25], [26], [27]. This disease model is characterised by an acute, resolving infection in the liver involving the formation of pro-inflammatory granulomas around infected Kupffer cells, and the establishment of a chronic infection in the spleen (reviewed in [24], [28], [29], [30]). Mice deficient in TNF are highly susceptible to L. donovani infection, and die in the second month of infection with unchecked parasite growth [25], [26], [31]. However, TNF also induces disease pathology in the spleen, including the loss of marginal zone macrophages and down-regulation of chemokine receptor expression by dendritic cells (DCs) [31], [32]. Mice lacking LTα display a less severe phenotype characterised by disrupted cellular trafficking into the liver and reduced control of hepatic parasite growth, although ultimately, infection is resolved in this organ [26].
Here we investigated the impact of L. donovani infection in LIGHT-deficient mice, as well as the roles of LIGHT binding each of its functional, cognate receptors during infection. We report a critical role for LIGHT in the resolution of hepatic infection, and more specifically, identify an important role for LIGHT-HVEM interactions in stimulating IL-12 production by DCs, and hence in the control of parasitic infections. Conversely, we also discovered that blockade of LIGHT-LTβR interactions dramatically enhanced early anti-parasitic immunity. Thus, we have identified distinct and opposing roles for LIGHT engagement of each of its receptors during infection.
Results
Organ-specific expression of LIGHT in response to L. donovani infection
Homeostatic levels of LIGHT mRNA in liver (Figure 1A) and spleen (Figure 1B) differed by an order of magnitude in naïve mice. Following L. donovani infection, LIGHT mRNA accumulation increased in the liver over the first 28 days, and remained elevated despite infection largely resolving (Figure 1C). In contrast, the initially high splenic LIGHT mRNA levels decreased over the first 28 days of infection (Figure 1B), and remained diminished as a persistent L. donovani infection became established (Figure S1A). Thus, an organ-specific pattern of LIGHT mRNA expression emerged in response to L. donovani infection.
Fig. 1. LIGHT is required for efficient parasite clearance in the liver. 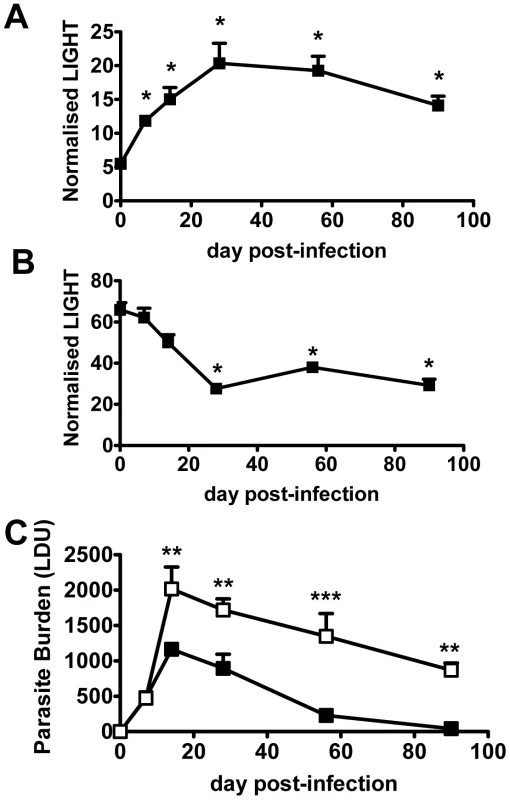
C57BL/6 mice were infected with L. donovani, and LIGHT mRNA accumulation was measured in liver (A) and spleen (B) at indicated times (expressed as the number of LIGHT mRNA molecules per 1000 HPRT mRNA molecules). (C) C57BL/6 (closed squares) and B6.LIGHT−/− (open squares) mice were infected with L. donovani, and hepatic parasite burdens were measured from day 7 p.i. to day 90 p.i. and data represent the mean +/−SEM. One representative experiment of at least 2 performed is shown (n = 4–5 mice per treatment group in each experiment). Statistical differences of p<0.05 (*), p<0.01 (**) or p<0.001 (***) are indicated. To establish whether LIGHT was required to control infection, we infected LIGHT-deficient and control C57BL/6 mice with L. donovani and followed the course of infection in the spleen and liver for 90 days. Despite no difference in hepatic parasite burdens in the first 7 days of infection, parasite growth was significantly greater in the livers of LIGHT-deficient mice from day 14 p.i. onwards. Furthermore, these mice failed to fully resolve hepatic infection in the time period studied (Figure 1C). TNF, IFNγ and nitric oxide (measured as the surrogate marker inducible nitric oxide synthase; NOS2) are all critical for control of L. donovani in the liver [26], [27], [31], [33], [34]. Serum TNF and IFNγ levels were reduced, and the accumulation of hepatic NOS2, IFNγ and TNF mRNA were all lower in LIGHT-deficient mice at 14 days, compared with control animals (Figure S1B–E). In the spleen, there were no significant differences in parasite burdens between C57BL/6 and B6.LIGHT−/− mice at any time point studied (data not shown). The accumulation of NOS2 mRNA was much lower in the spleen of C57BL/6 and B6.LIGHT−/− mice at day 14 p.i., compared with the liver, and no difference in IFNγ, TNF and NOS2 mRNA accumulation in the spleen between mouse strains was observed at this time point (Figure S1B–E). We therefore focused our attention on the liver.
The formation of pro-inflammatory granulomas around infected Kupffer cells is a critical step in host control of parasite growth in the liver [24], [28], [29], [30]. Liver immunohistochemistry revealed an increased number of inflammatory foci associated with increased parasite burden and impaired formation of inflammatory granulomas in B6.LIGHT−/− mice at day 14 p.i., relative to control mice, as indicated by a greater frequency of infected Kupffer cells with no surrounding leukocytes (KC), and a lower frequency of immature (IG) and mature granulomas (MG) (Figure 2A). To ensure that the failure to develop anti-parasitic immunity in the liver did not result from an as yet unidentified developmental defect in LIGHT-deficient mice, BM chimeras were made by engrafting LIGHT-deficient or control (C57BL/6) BM cells into lethally irradiated C57BL/6 mice. These mice were infected and parasite burdens measured 14 days later. BM chimeric mice responded to hepatic infection in accordance with their source of BM (Figure 2B). Hepatic parasite burdens were significantly increased in LIGHT-deficient BM chimeras compared to controls, indicating that LIGHT production by leukocytes was required for the efficient generation of anti-parasitic immunity in the liver at this early time point in infection. Additional experiments in T and B cell-deficient B6.RAG1−/− mice receiving LIGHT-deficient or wild-type T cells showed that LIGHT production by T cells was not required for the development of anti-parasitic immunity in the liver during the first 14 days of infection (Figure 2C). However, we cannot exclude a role for T cell-derived LIGHT in the generation of optimal early host immunity following L. donovani infection because we did find a small, but significant difference (p<0.001) in parasite burden at day 14 p.i. between B6.RAG1−/− mice that received LIGHT-deficient T cells and those that received wild-type T cells (Figure 2C). We also found that T cells per se were not a major source of hepatic LIGHT mRNA, although their presence was required for LIGHT expression to increase in the liver during this early period of infection (Figure 2D). Together, these data indicate that the generation of immune responses against L. donovani in the liver were impaired in the first 14 days of infection the absence of LIGHT.
Fig. 2. LIGHT from hematopoietic cells is required for efficient granuloma formation and control of L. donovani in the liver. 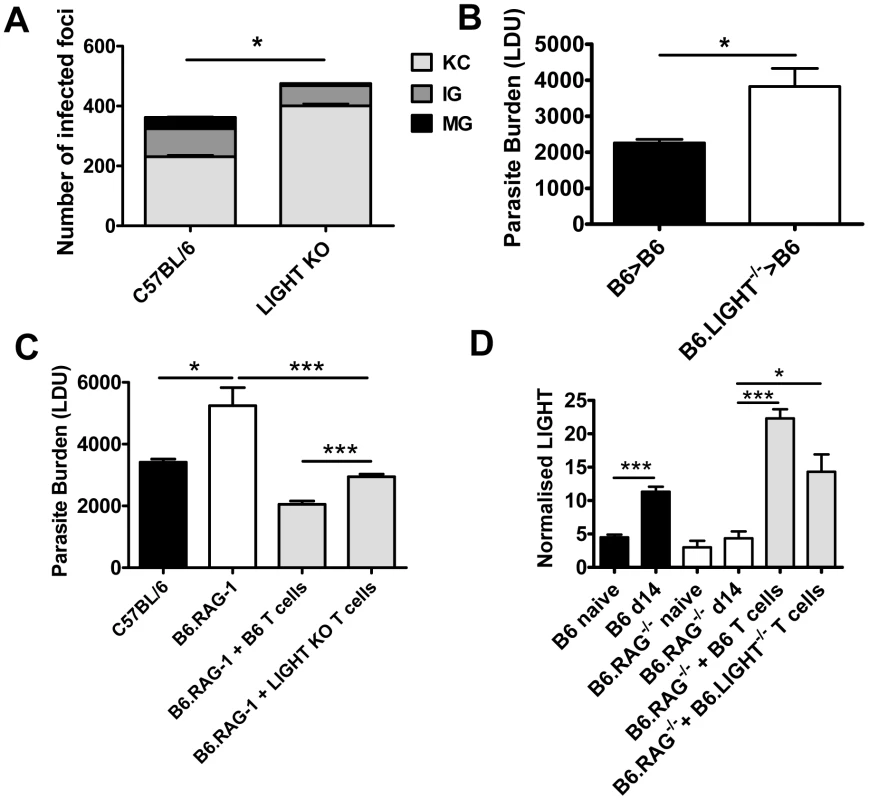
The number and maturity of hepatic granulomas were evaluated on liver sections taken at day 14 p.i. from C57BL/6 and B6.LIGHT−/− mice, as indicated (A), and data represent the mean frequency of infected Kupffer cells (KC), immature granulomas (IG) and mature granulomas (MG) per liver +/−SEM. (B) C57BL/6 mice engrafted with BM from C57BL/6 mice (B6>B6) (closed bars) and B6.LIGHT−/− mice (B6.LIGHT−/−>B6) (open bars) were infected with L. donovani and hepatic parasite burdens were measured at day 14 p.i. and are represented as the mean +/−SEM. Parasite burden (C) and LIGHT mRNA accumulation (D) in the livers of C57BL/6 and B6.RAG1−/− mice (plus or minus C57BL/6 or LIGHT-deficient CD4+ T cells and CD8+ T cells (equal numbers), as indicated) at day 14 p.i.. One representative experiment of at least 2 performed is shown (n = 4–5 mice per treatment group in each experiment). Statistical differences of p<0.05 (*), p<0.01 (**) or p<0.001 (***) are indicated. Treatment with anti-HVEM mAb impairs parasite clearance while treatment with anti-LTβR mAb promotes this process
Treatment with anti-HVEM mAb (LH1) that blocks LIGHT binding to HVEM, but not HVEM-BTLA interactions [35], significantly increased hepatic parasite load at day 14 p.i. in mice, similar to the increase in parasite burden observed in LIGHT-deficient mice (Figure 3A). Surprisingly, hepatic parasite burdens were significantly decreased by treatment of mice with anti-LTβR mAb (LLBT2) that blocks LIGHT binding to LTβR, but not LTα1β2-LTβR interactions [35] (Figure 3A). Antibody treatments had no significant effect on the low splenic parasite burden at this time point (Figure S2). The formation of granulomas was significantly impaired by anti-HVEM mAb, as indicated by a greater frequency of KC and a lower frequency of IG and MG (p<0.05, κ2 analysis; Figure 3B). In contrast, granuloma formation was significantly enhanced by anti-LTβR mAb, as indicated by a lower frequency of KC and a higher frequency of IG and MG (p<0.05, κ2 analysis; Figure 3B). Thus, these results indicate that HVEM and LTβR have distinct and opposing roles during the first 14 days of infection.
Fig. 3. Distinct effects of blocking LIGHT-HVEM and LIGHT-LTβR interactions on parasite growth in the liver. 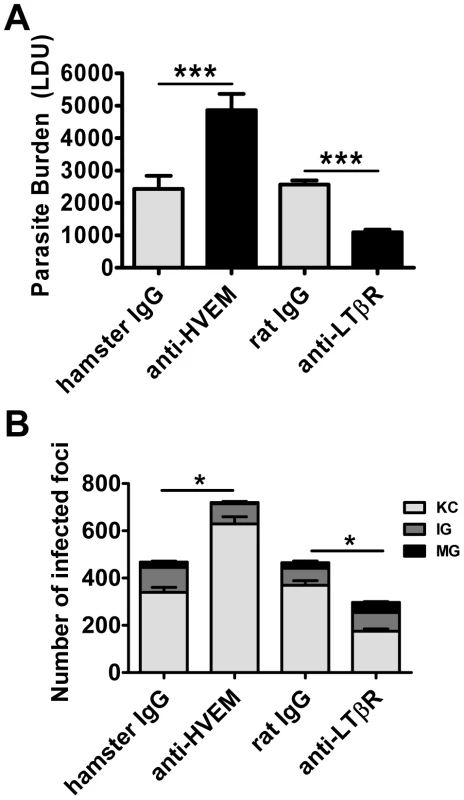
Parasite burdens were determined in the livers of L. donovani infected mice treated with anti-HVEM (LH1) mAb or control hamster IgG or anti-LTβR (LLBT2) mAb or control rat IgG (A). Data are represented as the mean +/− SEM at day 14 p.i.. The number and maturity of hepatic granulomas (B) were measured at day 14 p.i., as described in the legend of Figure 2. One representative experiment of 3 performed is shown (n = 5 mice per treatment group in each experiment). Statistical differences of p<0.05 (*) or p<0.001 (***) for control versus mAb-treated mice are shown. Stimulation of LTβR improves parasite clearance during an established infection
To further investigate the role of LTβR in VL, we treated mice with the agonist anti-LTβR antibody (3C8) which blocks binding of both LTα1β2 and LIGHT to LTβR, yet functions as an agonist directly activating LTβR signalling pathways [36], [37]. The anti-LTβR 3C8 enhanced parasite clearance in the liver during an established infection (Figure 4A), but had no anti-parasitic effect in the first 14 days of infection (data not shown), unlike the anti-LTβR mAb LLBT2 (Figure 3A). Importantly, 3C8 also prevented parasite growth in the spleen between days 14 to 28 p.i. (Figure 4B). In contrast, treatment with LLTB2 during established infection (days 14–28 p.i.) had no effect on parasite clearance in the liver or spleen (data not shown). Thus, treatment of L. donovani-infected mice with two different anti-LTβR mAbs had distinct effects on the course of infection, reflecting different functional properties of these mAbs.
Fig. 4. Agonistic stimulation of LTβR only enhances parasite clearance in an established infection. 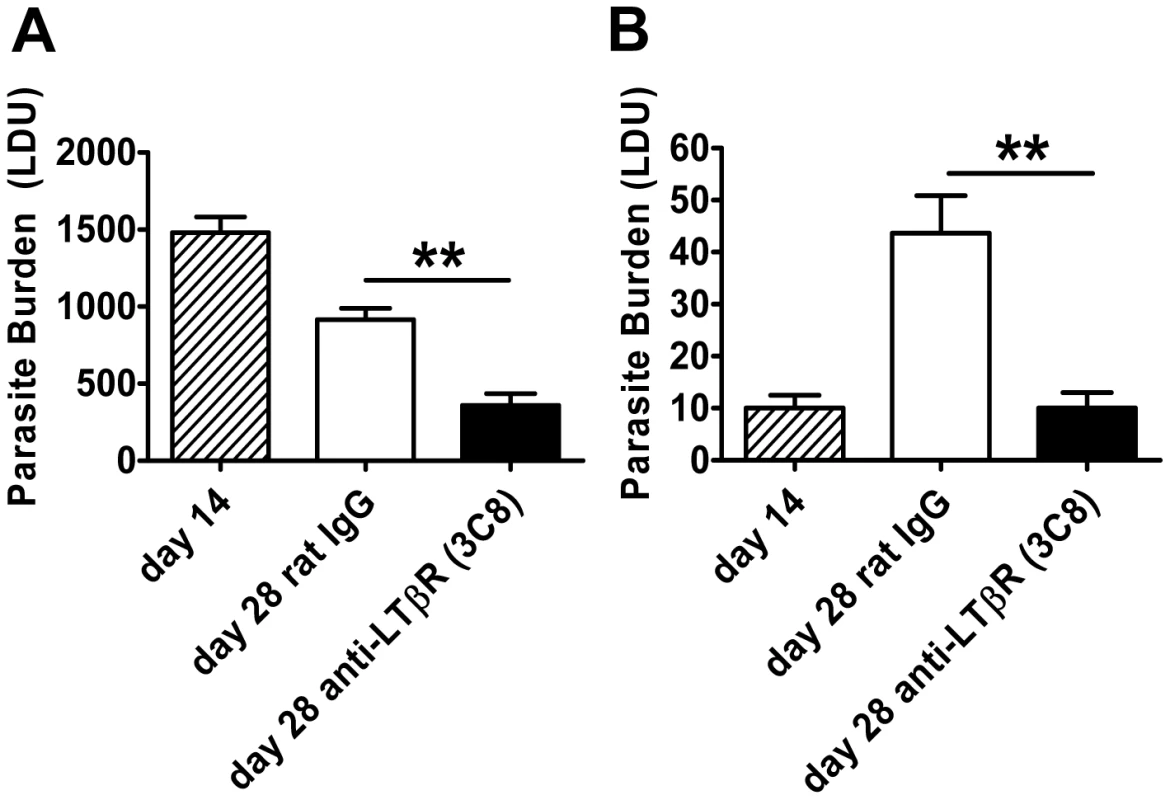
C57BL/6 mice with an established L. donovani infection were treated with control rat IgG or agonistic anti-LTβR mAb (3C8) from day 14 p.i. onwards and parasite burdens were determined in the liver (A) and spleen (B) at day 28 p.i.. One representative experiment of two performed is shown (n = 5 mice per treatment group in each experiment). Statistical differences of p<0.01 (**) for control versus treated mice are shown. Treatment with anti-HVEM mAb impairs Th1-mediated immunity
We next sought to identify anti-parasitic mechanisms dependent upon LIGHT-HVEM signalling. We previously showed that early splenic IL-12/IL-23p40 production by DC is critical for the efficient generation of immunity in the liver [38], [39]. Although no change in IL-12p35 mRNA accumulation was observed in any treatment group, the anti-HVEM mAb (LH1) inhibited splenic DC IL-12/IL-23p40 mRNA accumulation in response to L. donovani infection (Figure 5A). We next evaluated the importance of LIGHT-HVEM co-stimulatory signals for the development of L. donovani-specific CD4+ T cell priming and Th1 differentiation, the latter being a known IL-12-dependent process [39], [40], [41]. Mice were injected with CFSE-labelled OVA-specific CD4+ (OT-II) T cells, then infected with transgenic OVA-expressing L. donovani [42], and antigen-specific CD4+ T cell proliferation was assessed 4 days later by CFSE dilution. No antigen-specific CD4+ T cell proliferation was observed when mice were infected with wild-type parasites (Figure 5B), so no bystander activation had occurred, and OT-II cell proliferation occurred equally in control and anti-HVEM-treated mice (Figure 5B), indicating that LIGHT-HVEM interactions were not required for early priming of CD4+ T cell proliferation. In support of this result, proliferation of polyclonal antigen-specific, CD4+ T cells was similar between cells isolated from the spleens of control-treated and anti-HVEM treated mice (Figure 5C). However, production of IFNγ and TNF by these antigen-specific CD4+ T cells was inhibited by anti-HVEM mAb (Figure 5C). Furthermore, direct ex vivo production of IFNγ by hepatic CD4+ T cells (both total number and frequency) was significantly reduced by anti-HVEM mAb (Figure 5D), indicating that LIGHT-HVEM interactions play an important role in generating Th1 cell responses following L. donovani infection.
Fig. 5. LIGHT-HVEM interactions are required for early DC IL-12/IL-23p40 mRNA accumulation, and antigen-specific CD4+ T cell IFNγ and TNF production. 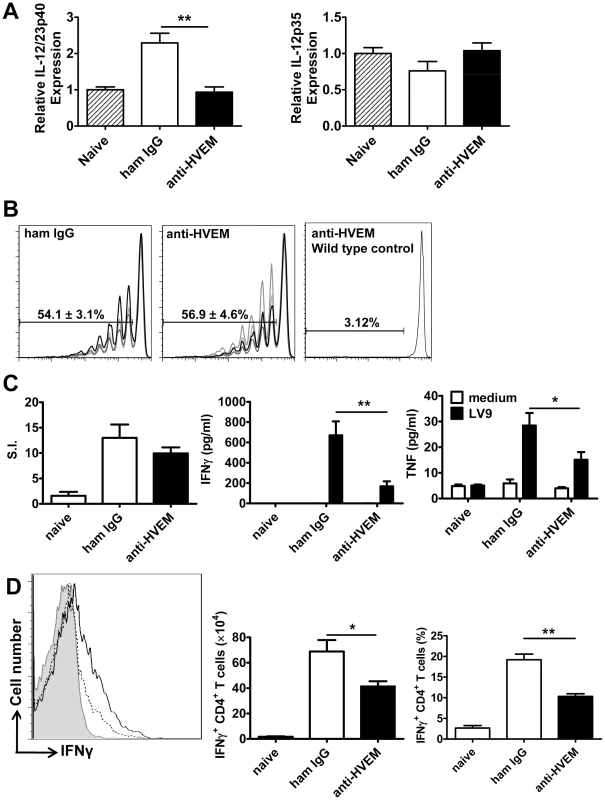
C57BL/6 mice were treated with anti-HVEM mAb or control hamster IgG or left untreated prior to infection with 1×108 L. donovani amastigotes. IL-12/IL-23p40 mRNA accumulation was measured in splenic CD11c+ DC that had been enriched by positive selection by MACS from naïve mice (hatched bars), untreated mice (grey bars), hamster IgG treated mice (open bars) or anti-HVEM treated mice (closed bars) at 5 hours p.i. (A). C57BL/6 mice treated with control hamster IgG or anti-HVEM mAb received CD45.1+ CFSE-labelled OVA-specific CD4+ T cells (OT-II) prior to infection with 2×107 OVA-expressing L. donovani. OT-II CFSE dilution in the spleen was assessed by flow cytometry on day 4 p.i., after gating on CD45.1+CD4+TCRβ+ cells (B) and % CFSE dilution +/− SEM is shown on each plot (CFSE dilution for 4 individual mice in each treatment group is shown, as well as CSFE dilution in a control animal that was infected with wild type parasites). Splenic CD4+ T cells were purified on day 14 p.i. from naïve mice or L. donovani-infected mice treated with control hamster IgG (open bars) or anti-HVEM mAb (closed bars) by positive MACS selection. CD4+ T cells were co-cultured with paraformaldehyde-fixed L. donovani amastigotes and irradiated spleen cells. Antigen-specific CD4+ T cell proliferation, as well as IFNγ and TNF levels in cell culture supernatants were measured by cytometric bead array at 72 h post-stimulation (C). Hepatic CD4+ T cell (CD4+TCRβ+) IFNγ production was measured in L. donovani-infected mice treated with either control hamster IgG (solid line) or anti-HVEM (dashed line) mAb on day 14 p.i.. Representative histograms showing IFNγ production by gated CD4+ TCRβ+ cells are shown and compared to isotype control antibody (solid gray) (D). Both the total number and frequency of IFNγ-producing CD4+ T cells are shown graphically. One representative experiment of 3 performed with similar outcome is shown (n = 4 mice per treatment group in each experiment). Statistical differences of p<0.05 (*), p<0.01 (**) or p<0.001 (***) for control versus mAb-treated mice are shown. Treatment with anti - LTβR mAb promotes Th1-mediated immunity
The anti-LTβR mAb (LLTB2) inhibited parasite growth in acute experimental VL (Figure 3A), but had no effect on splenic DC IL-12/IL-23p40 or IL-12p35 mRNA accumulation at 5 hours p.i. (Figure 6A), and no effect on the expansion of OVA-specific CD4+ T cells (OT-II cells) in mice infected with OVA-transgenic L. donovani (Figure 6B). We also found no differences in antigen-specific recall responses in splenic CD4+ T cells isolated from infected mice on day 14 p.i., yet the amount of TNF and IFNγ produced upon antigen-specific CD4+ T cell stimulation was greatly enhanced in these cells from mice treated with anti-LTβR mAb (Figure 6C). In addition, the number and frequency of IFNγ-producing hepatic CD4+ T cells measured directly ex vivo on day 14 p.i. was significantly increased in these mice (Figure 6D), suggesting LIGHT-LTβR binding suppresses the development of Th1 cell responses in VL.
Fig. 6. Treatment with anti-LTβR mAb LLTB2 leads to increased antigen-specific CD4+ T cell IFNγ and TNF production. 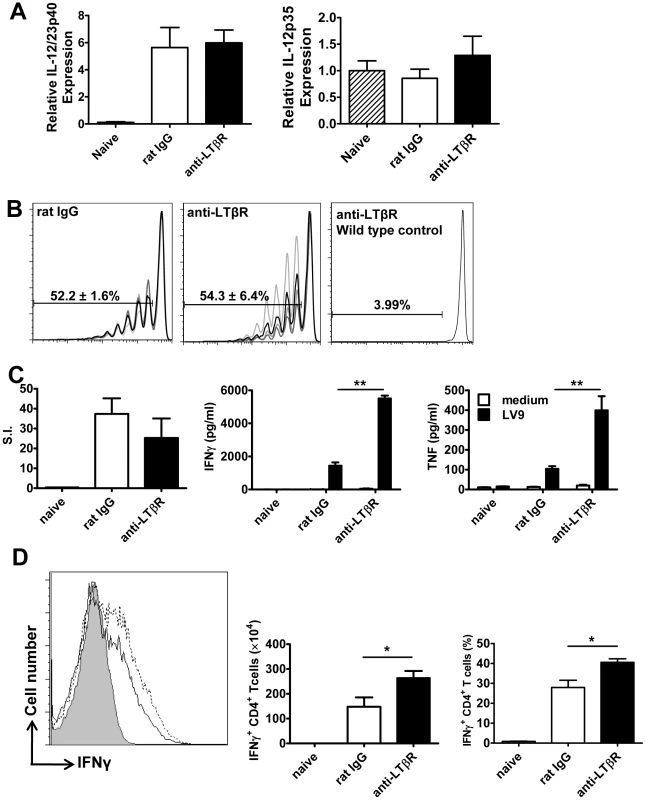
DC IL-12/IL-23p40 mRNA accumulation (A), splenic OT-II CFSE dilution (B), antigen-specific splenic CD4+ T cell proliferation, IFNγ and TNF production (C) and hepatic CD4+ T cell IFNγ production (D) were measured as described in the legend of Figure 5. One representative experiment of 3 performed with similar outcome is shown (n = 4 mice per treatment group in each experiment). Statistical differences of p<0.05 (*), p<0.01 (**) or p<0.001 (***) for control versus mAb-treated mice are shown. Treatment with anti - LTβR mAb promotes parasite clearance in the liver early during infection
We next investigated timing requirements for treatment with the anti-LTβR mAb (LLTB2) during acute infection with L. donovani. A single dose (100 µg) of anti-LTβR mAb at the time of infection was sufficient to reduce hepatic parasite burden as early as day 7 p.i. (Figure 7A). To test whether treatment with anti-LTβR mAb was simply shunting available LIGHT onto HVEM, we also co-treated mice with anti-LTβR (LLTB2) and anti-HVEM (LH1) mAbs (Figure 7B), and found no additional effect of co-administration over anti-LTβR alone by day 7 p.i., indicating that increased, early anti-parasitic immunity observed after anti-LTβR mAb (LLTB2) treatment was not caused by enhanced HVEM-mediated co-stimulation. Of note, there was no effect of anti-HVEM mAb treatment alone at day 7 p.i., indicating that the effect of this treatment on parasite burden only becomes apparent between days 7–14 p.i., similar to what was observed in LIGHT-deficient mice (Figure 1C). To test whether anti-LTβR mAb (LLTB2) agonist activity might account for the above effect, we treated LIGHT-deficient mice with this antibody and measured liver parasite burdens at day 7 p.i. (Figure 7C). Although a significant reduction in parasite burden was found in C57BL/6 mice treated with anti-LTβR mAb (LLTB2), no such effect was observed in LIGHT-deficient mice, indicating that the likely mechanism of action was via the blockade of LIGHT binding LTβR.
Fig. 7. Blockade of LIGHT-LTβR signalling has an anti-parasitic effect at the time of infection in the liver. 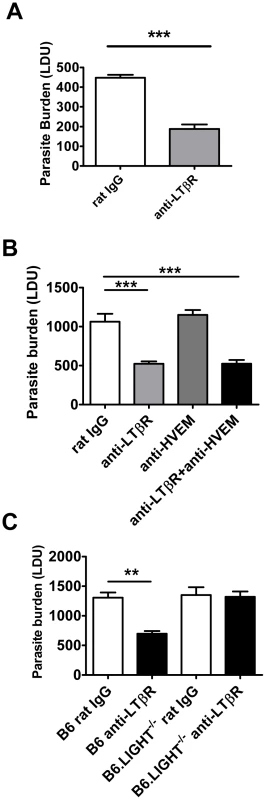
C57BL/6 mice were treated with anti-LTβR mAb (LLTB2) (light grey bars) or control rat IgG (open bars) and infected with L. donovani. Mice were injected with anti-LTβR mAb on the day of infection only and hepatic parasite burdens were determined in the liver at day 7 p.i. (A). C57BL/6 mice were treated with control rat IgG (open bars), anti-LTβR mAb (light grey bars), anti-HVEM mAb (dark grey bars) or both anti-LTβR and anti-HVEM mAbs (closed bars) on the day of L. donovani infection, and hepatic parasite burdens were determined in the liver at day 7 p.i. (B). C57BL/6 and B6.LIGHT−/− mice were treated with anti-LTβR mAb (closed bars) or control rat IgG (open bars) on the day of L. donovani infection, and hepatic parasite burdens were determined in the liver at day 7 p.i. (C). Data are representative of two experiments performed (n = 5 mice per treatment group in each experiment). Statistical differences of p<0.01 (**) and p<0.001 (***) for control versus treated mice are shown. Enhanced parasite clearance observed following treatment with anti-LTβR mAb requires CD4+ T cells and TNF
We investigated the cellular requirements for the early anti-parasitic effects of anti-LTβR mAb (LLTB2). Treatment with anti-LTβR mAb had no impact on hepatic parasite burdens in B6.RAG1−/− mice at day 7 p.i. (Figure S3), suggesting that B and/or T lymphocytes are required for the enhanced parasite clearance resulting from this treatment. We focused our attention on T cells because we have previously shown that B cells play a negative regulatory role in the liver during infection [43]. Depletion of CD4+ or CD8+ T cells alone during the first 7 days of infection had no effect on hepatic parasite burden (Figure 8A), despite T cells being required for the control of parasite growth at later stages of infection [26], [44], [45]. However, depletion of CD4+ T cells, but not CD8+ T cells, prevented the anti-parasitic effect mediated by anti-LTβR at day 7 p.i. (Figure 8A). Given that NKT cells comprise a significant proportion of hepatic CD4+ T cells, we also investigated whether this cell subset was required for the increased anti-parasitic activity. Treatment of NKT cell-deficient (B6.Jα18−/−) mice with the anti-LTβR mAb (Figure 8A) had no impact on the decreased liver parasite burden, indicating that conventional CD4+ T cells, but not NKT cells, are required for the enhanced parasite clearance following anti-LTβR mAb treatment.
Fig. 8. CD4+ T cells and TNF are required for enhanced parasite clearance following treatment with anti-LTβR mAb LLTB2. 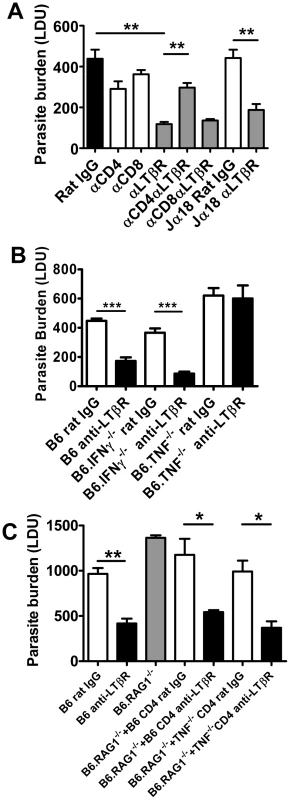
(A) C57BL/6 mice and B6.Jα18−/− mice were treated with control rat IgG or anti-LTβR mAb on the day of L. donovani infection, with or without CD4+ or CD8+ T cell depletion, as indicated. Hepatic parasite burdens were determined at day 7 p.i.. (B) C57BL6 mice, B6.TNF−/− mice and B6.IFNγ−/− mice were treated with control rat IgG (open bars) or anti-LTβR mAb (LLTB2) (closed bars) on the day of infection with L. donovani and hepatic parasite burdens were determined at day 7 p.i.. (C) C57BL/6 mice and B6.RAG1−/− mice were treated with control rat IgG (open bars) or anti-LTβR mAb on the day of L. donovani infection, as indicated. Prior to infection, B6.RAG1−/− mice also received splenic CD4+ T cells from C57BL/6 mice or B6.TNF−/− mice, as indicated. Hepatic parasite burdens were measured at day 7 p.i.. Data are from one of two experiments performed (n = 5 mice per treatment group in each experiment). Statistical differences of p<0.05 (*), p<0.01 (**) or p<0.001 (***) for control versus treated mice are shown. We observed increased CD4+ T cell TNF and IFNγ production was associated with improved control of parasite growth resulting from anti-LTβR mAb treatment (Figure 6). We next assessed whether these cytokines were required for the enhanced parasite clearance in mice receiving anti-LTβR (LLTB2) mAb. Hepatic parasite burdens were decreased similarly in anti-LTβR mAb treated control and IFNγ-deficient mice (Figure 8B). However, anti-LTβR mAb treatment in TNF-deficient mice had no impact on hepatic parasite burden (Figure 8B), indicating that TNF is critical for this enhanced parasite clearance. The failure of anti-LTβR mAb treatment in TNF-deficient animals was not caused by reduced expression of LTβR on the cells of these mice, as LTβR expression levels were no different to those on immune cells from C57BL/6 control mice (data not shown). Furthermore, adoptive transfer of wild type and TNF-deficient CD4+ T cells into B6.RAG1−/− mice and treatment with anti-LTβR mAb demonstrated that CD4+ T cells did not have to produce TNF (Figure 8C). Thus, anti-LTβR mAb treatment increased early hepatic anti-parasitic immunity by mechanisms requiring conventional CD4+ T cells and TNF, the latter potentially coming from a non-T cell source.
Discussion
We have identified distinct and opposing roles for LIGHT and its receptors during infection. LIGHT has important roles in T cell costimulation [3], [14]. Blockade of LIGHT impairs allogeneic T cell responses and graft versus host disease [46], [47], while over-expression of LIGHT by T cells causes inflammatory disease of the gut and reproductive tissues [48], [49]. Our results indicate that these effects could be mediated via the LIGHT-HVEM axis between T cells and DC. Early DC IL-12 production depends on the presence of T cells, and this IL-12 production is critical for generating anti-parasitic immune mechanisms that control L. donovani growth [24], [39], [40], [50]. Our finding that anti - HVEM mAb blocks IL-12/IL-23p40 mRNA accumulation during infection is consistent with a previous study that reported BM-derived DCs from LIGHT-deficient animals were impaired in their ability to produce IL-12 following activation in vitro [51]. This study also showed that blockade of LIGHT with soluble receptors in mice infected with L. major, a cause of cutaneous leishmaniasis, resulted in reduced IL-12 generation, associated with diminished CD4+ T cell IFNγ production and increased parasite growth and disease. Our finding that T cells did not have to produce LIGHT in order to promote anti-parasitic immunity, together with data from L. major infection in mice [51], support a model whereby DC-derived LIGHT interacts with T cell HVEM to promote DC IL-12 production.
The defect in anti-parasitic immunity observed in the absence of LIGHT was restricted to the liver, and not the spleen. The reason for this is unclear, but could relate to the requirement for cellular recruitment and granuloma development for control of parasite growth in the liver. Although increased tissue weight and cellular expansion are features of L. donovani infection in the spleen, organised inflammatory granulomas are rarely observed in this tissue [24], [28], [29], [30]. Importantly, parasite growth is contained in the spleen after 1–2 months of infection rather than efficiently controlled, as occurs in the liver. Therefore, it is possible that different anti-parasitic immune mechanisms operate in these two tissue sites during experimental VL with different requirements for LIGHT.
The LIGHT-specific blocking mAbs we have employed (LH1 and LLTB2) have previously been shown to selectively block interactions between LIGHT and its receptors (HVEM and LTβR, respectively) [35]. However, we cannot exclude the possibility that they may trigger some receptor activation following engagement, and that this may contribute to biological effects we have observed. In addition, because these mAbs cause the selective blockade of LIGHT binding to their respective receptors, we cannot rule out that they promote alternative receptor-ligand interactions (Figure 9A). For example, blocking LIGHT interacting with HVEM may allow HVEM to more readily engage BTLA on cells to increase inhibitory signals (Figure 9B), as well as increased CD160 signalling. Similarly, blockade of LIGHT binding LTβR may allow greater amounts of LIGHT to bind HVEM, thereby reducing negative signalling between HVEM and BTLA and potentially promoting LIGHT-HVEM-mediated T cell co-stimulation (Figure 9C). However, this latter possibility seems unlikely in the current study given that co-administration of LH1 and LLTB2 resulted in improved control of parasite growth (Figure 7B). Instead, LIGHT may send inhibitory signals via LTβR early during infection, although no such LTβR-mediated negative signalling pathway has yet been defined.
Fig. 9. Model of how manipulation of interactions between LIGHT and its receptors may impact on disease outcome. 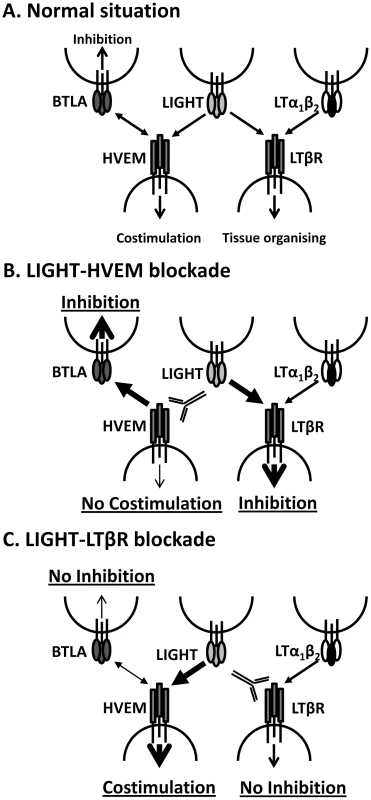
A. Under normal circumstances LIGHT will interact with HVEM and/or LTβR to promote anti-parasitic immunity, as well as with DCR3 (not shown). HVEM can also interact with BTLA to suppress immune responses, and can also interact with CD160 and HSV1 gD (not shown). LTα1β2 can also bind to LTβR and provide signals essential for tissue organising and homeostasis. B. Blockade of LIGHT-HVEM with antibody will prevent HVEM-mediated costimulation and increase HVEM-BTLA-mediated inhibitory signals. LTβR-mediated inhibitory signals may also be increased via greater interactions with LIGHT. Together, this results in suppression of anti-parasitic immunity. C. Blockade of LIGHT-LTβR with antibody will potentially prevent LTβR-mediated inhibitory signals, but will also promote LIGHT-HVEM co-stimulatory signalling and reduce HVEM-BLTA inhibitory signals. Together, this stimulates increased anti-parasitic immunity. The anti-parasitic effects of anti-LTβR mAb (LLTB2) were observed when it was administered at the time of infection, but not in mice with an established L. donovani infection, suggesting that a mAb with similar functional characteristics would have limited therapeutic potential for treatment of VL. However, our finding that the defined agonist anti-LTβR mAb (3C8) improved the rate of parasite clearance in the liver and reduced parasite load in the spleen, not only demonstrated fundamentally different biological activities for LLTB2 and 3C8 mAbs, but also shows that LTβR activation can promote beneficial immune mechanisms during established infection. This agonist antibody has previously been shown to promote DC development and maturation in vivo [20], [37], and this may explain the anti-parasitic effects observed after administration to L. donovani-infected mice because we have previously shown that DC adoptive transfer can improve control of parasite growth in infected mice [32]. Hence, the activation of anti-parasitic immune mechanisms by stimulation of LTβR represents a potential therapeutic strategy against chronic infectious diseases like VL. However, a better understanding of the functional characteristics of the different anti-LTβR mAbs will be required in order to better harness their therapeutic potential, including identification of the specific epitopes they recognise and signalling pathways they activate.
We previously reported increased monocyte recruitment into the spleen in an experimental model of cerebral malaria following treatment of mice with the anti-LTβR mAb (LLTB2), and that this treatment protected mice from disease [52]. Interestingly, no protection from experimental cerebral malaria was afforded by treatment with the anti-LTβR (3C8) mAb (Randall and Engwerda, unpublished), again emphasising the functional differences between LLTB2 and 3C8 anti-LTβR mAbs. An intriguing finding from our current studies was an increase in hepatic and splenic monocyte recruitment following anti-LTβR mAb LLTB2 treatment (CD11b+ Ly6Chi cells; Figure S4A and B). Flow cytometry analysis revealed that monocytes, along with DCs (both cDC and pDC), and neutrophils expressed the highest levels of LTβR in the liver, as previously reported [19], [20], [37], and furthermore, that expression of LTβR did not appear to change significantly on any of these cells during the first 5 days of infection with L. donovani (Figure S5). However, the increased monocyte recruitment was not necessary for improved early control of parasite growth in treated animals in the current study because mice lacking CCL2 that have an impairment in monocyte mobilisation [53], also had improved control of parasite growth following anti-LTβR (LLTB2) treatment at day 7 p.i. (Figure S4C). Although the early anti-parasitic effect of anti-LTβR (LLTB2) mAb appeared to involve blocking of LIGHT - LTβR interactions, as indicated by the failure of this antibody to improve parasite control in LIGHT-deficient mice (Figure 7C), we cannot exclude the possibility that some effects of this antibody, such as increased monocyte mobilisation, might involve agonist activities. Regardless, given the important role for monocyte infiltration into sites of infection and tumour growth [54], our results indicate that manipulation of the LIGHT-LTβR signalling axis offers a potential way to improve monocyte mobilisation for therapeutic applications. Furthermore, given the recent report that monocytes can migrate into secondary lymphoid tissues in response to interactions with gram negative bacteria and/or their products, and then develop into CD209a+, CD206+, CD14+, CD11chi DC capable of activating CD4+ T cells and cross-priming CD8+ T cells [55], our results suggest that manipulation of LIGHT signalling pathways may be one way to promote this process that may have applications in vaccination.
In summary, our findings further delineate the complex interactions between LIGHT and its receptors and demonstrate the therapeutic potential of modulating these immune regulatory pathways to improve disease outcomes. Our results provide mechanistic insight into the roles of LIGHT-HVEM interactions on DC function and CD4+ T cell priming, as well as anti-parasitic immune responses activated by blockade of LIGHT-LTβR interactions. Finally, we have identified two different mAbs that target LTβR with distinct functional outcomes on anti-parasitic immunity at different stages of infection.
Material and Methods
Mice
Inbred female C57BL/6 and B6.SJL.Ptprca (B6.CD45.1) mice were purchased from the Australian Resource Centre (Canning Vale, Western Australia), and maintained under conventional conditions. B6.RAG1−/− [56], B6.LIGHT−/− [57], B6.TNF−/− [58], B6.SJL.Ptprca×OT-II [59], B6.SJL.Ptprca×OT-I [60], B6.IFNγ−/− [61] and B6.Jα18−/− [62] were bred and maintained at the Queensland Institute of Medical Research. B6.CCL2−/− mice [53] were bred at Monash University and maintained at the Queensland Institute of Medical Research. All mice used were age - and sex-matched (6–10 weeks), and were housed under specific-pathogen free conditions. Chimeric mice were prepared by irradiating B6.SJL.Ptprca mice with 11Gy and then engrafting with 3×106 fresh bone marrow (BM) cells i.v. via the lateral tail vein. Mice were maintained on antibiotics for 2 weeks after engraftment and infected with L. donovani 8 weeks after receiving BM, as previously described [26]. Adoptive transfer of equal numbers (106) of purified CD4+ and CD8+ T cells (98% purity as determined by flow cytometry) into B6.RAG1−/− mice was performed as previously described [26].
Ethics statement
All animal procedures were approved and monitored by the Queensland Institute of Medical Research Animal Ethics Committee. This work was conducted under QIMR animal ethics approval number A02-634M, in accordance with the “Australian code of practice for the care and use of animals for scientific purposes” (Australian National Health & Medical Research Council).
Parasites and infections
L. donovani (LV9) and OVA-transgenic LV9 (PINK LV9) [42] were maintained by passage in B6.RAG1−/− mice and amastigotes were isolated from the spleens of chronically infected mice. Mice were infected by injecting 2×107 amastigotes i.v. via the lateral tail vein, killed at the times indicated in the text by CO2 asphyxiation and bled via cardiac puncture. In experiments examining DC IL-12/IL-23p40 production, mice were infected with 1×108 amastigotes intravenously, as previously described [63]. Spleens and perfused livers were removed at times indicated and parasite burdens were determined from Diff-Quik-stained impression smears (Lab Aids, Narrabeen, Australia) and expressed as Leishman-Donovan units (LDU) (the number of amastigotes per 1,000 host nuclei multiplied by the organ weight in grams) [64]. Liver and spleen tissue were also preserved in either RNAlater (Sigma-Aldrich, Castle Hill, Australia) or Tissue-Tek O.C.T. compound (Sakura, Torrence, USA). Hepatic mononuclear cells and splenocytes were isolated as previously described [65].
Antibodies
All antibody-producing hybridomas were grown in 5% (v/v) foetal calf serum, RPMI containing 10 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin. Purified antibody was prepared as previously described [63]. Mice were administered 100 µg of anti-LTβR mAb (LLTB2) or anti-HVEM mAb (LH1) [35] i.v. on the day of infection and every 5 days thereafter for 14 day experiments, or as a single dose on the day of infection for 7 day experiments.. The anti-LTβR mAb 3C8 was administered at 200 µg i.v. [36], [37] starting at the times indicated in the text and every 5 days thereafter. The anti-LTβR mAb (LLTB2) or anti-HVEM mAb (LH1) specifically block the binding of LIGHT to either LTβR or HVEM, respectively, but do not disrupt interactions between these receptors and other functional ligands (i.e., LTα1β2 for LTβR and BTLA for HVEM) [35]. The anti-LTβR mAb (3C8) blocks binding of both LTα1β2 and LIGHT, but is an agonist directly activating LTβR [66]. Mice were depleted of CD4+ or CD8+ T cells with anti-CD4 (YTS191.1) or anti-CD8β (53-5.8) mAbs, respectively, as previously described [64]. Depletion of T cell subsets was confirmed at completion of experiments by assessing T cell numbers in the spleen by flow cytometry. Greater than 95% of CD4+ and CD8+ T cells were depleted by antibody treatment. In all experiments, control mice received the same quantities of the appropriate control hamster IgG (UC8-1B9; ATCC, Manassas, VA) or control rat IgG (Sigma-Aldrich).
CD4+ T cell proliferation
To assess antigen-specific T cell proliferation in vivo, mice were infected with OVA-transgenic PINK LV9 [42]. Splenic OVA-specific OT-II T cells were isolated and labelled with CFSE, as previously described [64]. CFSE-labelled OT-II cells (1×106) were adoptively transferred into mice 2 h prior to infection with LV9 or PINK LV9. Expansion of CFSE+ cells in the spleen was monitored by FACS 4 days later. In all of these experiments, control animals were included that received the same number of CFSE-labelled OT-II cells, but were infected with wild-type parasites. No OT-II proliferation was ever observed in these animals. Re-stimulation assays for endogenous splenic CD4+ T cells were performed as previously described [63].
Assessment of granuloma formation
The maturation of granulomas was scored around infected Kupffer cells in acetone-fixed liver sections as previously described [64], [65].
Flow cytometry
Allophycocyanin (APC)-conjugated anti-TCRβ chain (H57-597), B220 (clone RA3-6B2), CD11c (clone N418), phycoerythrin (PE)-Cy5-conjugated anti-CD4 (GK1.5), PE-conjugated IFNγ (XMG1.2), CD8β (53-5.8), I-Ab (clone AF6-120.1), Ly6G (clone 1A8), CD45.1 (clone A20), CD45.2 (clone 104), rat IgG1 (RTK2071), fluorescein isothiocyanate (FITC)-conjugated CD19 (clone 6D5), BST2 (clone 120G8), Ly6C (clone AL-21) and biotinylated anti-NK1.1 (PK136), CD11b (clone M1/70), LTβR (3C8) were purchased from Biolegend (San Diego, CA) or BD Biosciences (Franklin Lakes, NJ). Biotinylated antibodies were detected with streptavidin conjugated alexa 488, PE or PE/Cy5 (Biolegend). Leukocyte populations were defined as follows; CD4+ T cells (CD4+, TCR+), CD8+ T cells (CD8+, TCR+), NKT cells (NK1.1+, TCR+), NK cells (NK1.1+, TCR−), B cells (B220+, CD19+), cDC (CD11chi, MHCIIhi, TCR−, B220−), pDC (CD11cint, MHCIIint, 120G8+), monocytes (CD11b+, Ly6C+) and neutrophils (CD11b+, Ly6G+). The staining of cell surface antigens and intracellular cytokine staining was carried out as described previously [63]. FACS was performed on a FACSCalibur or a FACS Canto II (BD Biosciences), and data were analysed using FlowJo software (TreeStar, Oregon, USA). Serum and/or tissue culture supernatants were assessed for the presence of soluble cytokines using flexset bead array kits and a FACSArray plate reader (BD Biosciences) according to the manufacturers' instructions.
Real Time PCR
RNA extraction and real-time RT-PCR was performed as previously described [63]. The number of IFNγ, TNF, NOS-2, LIGHT and hypoxanthine phosphoribosyltransferase (HPRT) cDNA molecules in each liver tissue sample were calculated using Platinum Sybr Green Master Mix (Invitrogen Life Technologies) [63]. Standard curves were generated with known amounts of cDNA for each gene, and the number of cytokine molecules per 1000 HPRT molecules in each sample was calculated. The number of IL-12/IL-23p40 and IL-12p35 cDNA molecules in each DC sample were calculated using Taqman Gene Expression Assays (Applied Biosystems). Relative quantitation of gene expression was performed using the relative standard curve method as described by Applied Biosystems. Briefly, standard curves were prepared for all target and endogenous control genes using an uninfected control sample. HPRT was used as the endogenous control. The amount of target gene or endogenous control in each sample was calculated from the appropriate standard curves. The target amount was then divided by the endogenous control amount to give the normalized target value. The average normalized values for the four naïve samples were used as the calibrator.
Statistical analysis
Statistical differences between groups was determined using the Mann-Whitney U test using GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego, CA) and p<0.05 was considered statistically significant. The distribution of hepatic histological responses was compared using X2 analysis with Microsoft Excel software. All data are presented as the mean values plus or minus standard error unless otherwise stated.
Supporting Information
Zdroje
1. LocksleyRMKilleenNLenardoMJ 2001 The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104 487 501
2. AggarwalBB 2003 Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3 745 756
3. WareCF 2005 Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol 23 787 819
4. WareCF 2008 Targeting lymphocyte activation through the lymphotoxin and LIGHT pathways. Immunol Rev 223 186 201
5. AggarwalBBEessaluTEHassPE 1985 Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature 318 665 667
6. RuulsSRHoekRMNgoVNMcNeilTLucianLA 2001 Membrane-bound TNF supports secondary lymphoid organ structure but is subservient to secreted TNF in driving autoimmune inflammation. Immunity 15 533 543
7. MauriDNEbnerRMontgomeryRIKochelKDCheungTC 1998 LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity 8 21 30
8. CrowePDVanArsdaleTLWalterBNWareCFHessionC 1994 A lymphotoxin-beta-specific receptor. Science 264 707 710
9. HarropJAMcDonnellPCBrigham-BurkeMLynSDMintonJ 1998 Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem 273 27548 27556
10. ZhaiYGuoRHsuTLYuGLNiJ 1998 LIGHT, a novel ligand for lymphotoxin beta receptor and TR2/HVEM induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest 102 1142 1151
11. SedyJRGavrieliMPotterKGHurchlaMALindsleyRC 2005 B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol 6 90 98
12. CaiGAnumanthanABrownJAGreenfieldEAZhuB 2008 CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol 9 176 185
13. WhitbeckJCPengCLouHXuRWillisSH 1997 Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol 71 6083 6093
14. MurphyTLMurphyKM 2010 Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol 28 389 411
15. CheungTCSteinbergMWOborneLMMacauleyMGFukuyamaS 2009 Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci U S A 106 6244 6249
16. OtterdalKSmithCOieEPedersenTMYndestadA 2006 Platelet-derived LIGHT induces inflammatory responses in endothelial cells and monocytes. Blood 108 928 935
17. KwonBSWangSUdagawaNHaridasVLeeZH 1998 TR1, a new member of the tumor necrosis factor receptor superfamily, induces fibroblast proliferation and inhibits osteoclastogenesis and bone resorption. Faseb J 12 845 854
18. MarstersSAAyresTMSkubatchMGrayCLRotheM 1997 Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem 272 14029 14032
19. BrowningJLSizingIDLawtonPBourdonPRRennertPD 1997 Characterization of lymphotoxin-alpha beta complexes on the surface of mouse lymphocytes. J Immunol 159 3288 3298
20. KabashimaKBanksTAAnselKMLuTTWareCF 2005 Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity 22 439 450
21. SchneiderKLoewendorfADe TrezCFultonJRhodeA 2008 Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe 3 67 76
22. EhlersSHolscherCScheuSTertiltCHehlgansT 2003 The lymphotoxin beta receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes. J Immunol 170 5210 5218
23. MurrayHWBermanJDDaviesCRSaraviaNG 2005 Advances in leishmaniasis. Lancet 366 1561 1577
24. StanleyACEngwerdaCR 2007 Balancing immunity and pathology in visceral leishmaniasis. Immunol Cell Biol 85 138 147
25. MurrayHWJungbluthARitterEMontelibanoCMarinoMW 2000 Visceral leishmaniasis in mice devoid of tumor necrosis factor and response to treatment. Infect Immun 68 6289 6293
26. EngwerdaCRAtoMStagerSAlexanderCEStanleyAC 2004 Distinct roles for lymphotoxin-alpha and tumor necrosis factor in the control of Leishmania donovani infection. Am J Pathol 165 2123 2133
27. TumangMCKeoghCMoldawerLLHelfgottDCTeitelbaumR 1994 Role and effect of TNF-alpha in experimental visceral leishmaniasis. J Immunol 153 768 775
28. EngwerdaCRKayePM 2000 Organ-specific immune responses associated with infectious disease. Immunol Today 21 73 78
29. EngwerdaCRAtoMKayePM 2004 Macrophages, pathology and parasite persistence in experimental visceral leishmaniasis. Trends Parasitol 20 524 530
30. KayePMSvenssonMAtoMMaroofAPolleyR 2004 The immunopathology of experimental visceral leishmaniasis. Immunol Rev 201 239 253
31. EngwerdaCRAtoMCotterellSEMynottTLTschannerlA 2002 A role for tumor necrosis factor-alpha in remodeling the splenic marginal zone during Leishmania donovani infection. Am J Pathol 161 429 437
32. AtoMStagerSEngwerdaCRKayePM 2002 Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat Immunol 3 1185 1191
33. SquiresKESchreiberRDMcElrathMJRubinBYAndersonSL 1989 Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J Immunol 143 4244 4249
34. MurrayHWNathanCF 1999 Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J Exp Med 189 741 746
35. AnandSWangPYoshimuraKChoiIHHilliardA 2006 Essential role of TNF family molecule LIGHT as a cytokine in the pathogenesis of hepatitis. J Clin Invest 116 1045 1051
36. DejardinEDroinNMDelhaseMHaasECaoY 2002 The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity 17 525 535
37. De TrezCSchneiderKPotterKDroinNFultonJ 2008 The inhibitory HVEM-BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J Immunol 180 238 248
38. GorakPMEngwerdaCRKayePM 1998 Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur J Immunol 28 687 695
39. EngwerdaCRMurphyMLCotterellSESmeltSCKayePM 1998 Neutralization of IL-12 demonstrates the existence of discrete organ - specific phases in the control of Leishmania donovani. Eur J Immunol 28 669 680
40. MurrayHW 1997 Endogenous interleukin-12 regulates acquired resistance in experimental visceral leishmaniasis. J Infect Dis 175 1477 1479
41. TrinchieriG 1995 Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol 13 251 276
42. PolleyRStagerSPrickettSMaroofAZubairiS 2006 Adoptive immunotherapy against experimental visceral leishmaniasis with CD8+ T cells requires the presence of cognate antigen. Infect Immun 74 773 776
43. SmeltSCCotterellSEEngwerdaCRKayePM 2000 B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol 164 3681 3688
44. McElrathMJMurrayHWCohnZA 1988 The dynamics of granuloma formation in experimental visceral leishmaniasis. J Exp Med 167 1927 1937
45. AlexanderCEKayePMEngwerdaCR 2001 CD95 is required for the early control of parasite burden in the liver of Leishmania donovani-infected mice. Eur J Immunol 31 1199 1210
46. TamadaKShimozakiKChapovalAIZhaiYSuJ 2000 LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol 164 4105 4110
47. TamadaKShimozakiKChapovalAIZhuGSicaG 2000 Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med 6 283 289
48. WangJLoJCFosterAYuPChenHM 2001 The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. J Clin Invest 108 1771 1780
49. ShaikhRBSanteeSGrangerSWButrovichKCheungT 2001 Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol 167 6330 6337
50. EngwerdaCRSmeltSCKayePM 1996 An in vivo analysis of cytokine production during Leishmania donovani infection in scid mice. Exp Parasitol 84 195 202
51. XuGLiuDOkworIWangYKornerH 2007 LIGHT Is critical for IL-12 production by dendritic cells, optimal CD4+ Th1 cell response, and resistance to Leishmania major. J Immunol 179 6901 6909
52. RandallLMAmanteFHZhouYStanleyACHaqueA 2008 Cutting edge: selective blockade of LIGHT-lymphotoxin beta receptor signaling protects mice from experimental cerebral malaria caused by Plasmodium berghei ANKA. J Immunol 181 7458 7462
53. LuBRutledgeBJGuLFiorilloJLukacsNW 1998 Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med 187 601 608
54. GeissmannFAuffrayCPalframanRWirrigCCioccaA 2008 Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol 86 398 408
55. CheongCMatosIChoiJHDandamudiDBShresthaE 2010 Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell 143 416 429
56. MombaertsPIacominiJJohnsonRSHerrupKTonegawaS 1992 RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68 869 877
57. ScheuSAlferinkJPotzelTBarchetWKalinkeU 2002 Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med 195 1613 1624
58. KornerHCookMRimintonDSLemckertFAHoekRM 1997 Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur J Immunol 27 2600 2609
59. BarndenMJAllisonJHeathWRCarboneFR 1998 Defective TCR expression in transgenic mice constructed using cDNA-based alpha - and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol 76 34 40
60. HogquistKAJamesonSCHeathWRHowardJLBevanMJ 1994 T cell receptor antagonist peptides induce positive selection. Cell 76 17 27
61. DaltonDKPitts-MeekSKeshavSFigariISBradleyA 1993 Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259 1739 1742
62. CuiJShinTKawanoTSatoHKondoE 1997 Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science 278 1623 1626
63. StanleyACDaltonJERossottiSHMacDonaldKPZhouY 2008 VCAM-1 and VLA-4 modulate dendritic cell IL-12p40 production in experimental visceral leishmaniasis. PLoS Pathog 4 e1000158
64. HaqueAStanleyACAmanteFHRivera FdeLZhouY 2010 Therapeutic glucocorticoid-induced TNF receptor-mediated amplification of CD4+ T cell responses enhances antiparasitic immunity. J Immunol 184 2583 2592
65. StanleyACZhouYAmanteFHRandallLMHaqueA 2008 Activation of invariant NKT cells exacerbates experimental visceral leishmaniasis. PLoS Pathog 4 e1000028
66. SanjoHZajoncDMBradenRNorrisPSWareCF 2010 Allosteric regulation of the ubiquitin:NIK and ubiquitin:TRAF3 E3 ligases by the lymphotoxin-beta receptor. J Biol Chem 285 17148 17155
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Quorum Sensing in Fungi: Q&AČlánek Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the MosquitoČlánek The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral GenomeČlánek A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,Článek A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of AlphaherpesvirusesČlánek The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate ofČlánek Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 10- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Quorum Sensing in Fungi: Q&A
- Discovery of an Ebolavirus-Like Filovirus in Europe
- Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response
- Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
- Herpesvirus Telomerase RNA (vTR) with a Mutated Template Sequence Abrogates Herpesvirus-Induced Lymphomagenesis
- Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
- Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus
- Novel Mouse Xenograft Models Reveal a Critical Role of CD4 T Cells in the Proliferation of EBV-Infected T and NK Cells
- Toll-8/Tollo Negatively Regulates Antimicrobial Response in the Respiratory Epithelium
- Exhausted Cytotoxic Control of Epstein-Barr Virus in Human Lupus
- Structural and Functional Analysis of Laninamivir and its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition
- Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
- Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
- HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
- Deep Molecular Characterization of HIV-1 Dynamics under Suppressive HAART
- Fitness Landscape of Antibiotic Tolerance in Biofilms
- The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral Genome
- Preventing Sepsis through the Inhibition of Its Agglutination in Blood
- A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,
- IFITM3 Inhibits Influenza A Virus Infection by Preventing Cytosolic Entry
- Targeting Cattle-Borne Zoonoses and Cattle Pathogens Using a Novel Trypanosomatid-Based Delivery System
- A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses
- Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1
- Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Histone Deacetylase 8 Is Required for Centrosome Cohesion and Influenza A Virus Entry
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- Co-opts the FGF2 Signaling Pathway to Enhance Infection
- IRAK-2 Regulates IL-1-Mediated Pathogenic Th17 Cell Development in Helminthic Infection
- Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly
- The Anti-interferon Activity of Conserved Viral dUTPase ORF54 is Essential for an Effective MHV-68 Infection
- A Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
- Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection
- ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation
- Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection
- RNA Polymerase II Stalling Promotes Nucleosome Occlusion and pTEFb Recruitment to Drive Immortalization by Epstein-Barr Virus
- Noninfectious Retrovirus Particles Drive the / Dependent Neutralizing Antibody Response
- Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont
- An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus
- as an Animal Model for the Study of Biofilm Infections
- Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
- The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate of
- Enhances Protective and Detrimental HLA Class I-Mediated Immunity in Chronic Viral Infection
- The Mouse IAPE Endogenous Retrovirus Can Infect Cells through Any of the Five GPI-Anchored EphrinA Proteins
- The Urgent Need for Robust Coral Disease Diagnostics
- HacA-Independent Functions of the ER Stress Sensor IreA Synergize with the Canonical UPR to Influence Virulence Traits in
- A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
- Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
- Mechanisms of Trafficking to the Brain
- Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
- The Role of Sialyl Glycan Recognition in Host Tissue Tropism of the Avian Parasite
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
- The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization
- Distinct Single Amino Acid Replacements in the Control of Virulence Regulator Protein Differentially Impact Streptococcal Pathogenesis
- Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge
- A Genetic Screen Reveals Arabidopsis Stomatal and/or Apoplastic Defenses against pv. DC3000
- Hepatitis C Virus Reveals a Novel Early Control in Acute Immune Response
- Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



