-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Inhibition of Both HIV-1 Reverse Transcription and Gene Expression by
a Cyclic Peptide that Binds the Tat-Transactivating Response Element (TAR)
RNA
The RNA response element TAR plays a critical role in HIV replication by
providing a binding site for the recruitment of the viral transactivator protein
Tat. Using a structure-guided approach, we have developed a series of
conformationally-constrained cyclic peptides that act as structural mimics of
the Tat RNA binding region and block Tat-TAR interactions at nanomolar
concentrations in vitro. Here we show that these compounds
block Tat-dependent transcription in cell-free systems and in cell-based
reporter assays. The compounds are also cell permeable, have low toxicity, and
inhibit replication of diverse HIV-1 strains, including both CXCR4-tropic and
CCR5-tropic primary HIV-1 isolates of the divergent subtypes A, B, C, D and
CRF01_AE. In human peripheral blood mononuclear cells, the cyclic peptidomimetic
L50 exhibited an IC50 ∼250 nM. Surprisingly, inhibition of
LTR-driven HIV-1 transcription could not account for the full antiviral
activity. Timed drug-addition experiments revealed that L-50 has a bi-phasic
inhibition curve with the first phase occurring after HIV-1 entry into the host
cell and during the initiation of HIV-1 reverse transcription. The second phase
coincides with inhibition of HIV-1 transcription. Reconstituted reverse
transcription assays confirm that HIV-1 (−) strand strong stop DNA
synthesis is blocked by L50-TAR RNA interactions in-vitro.
These findings are consistent with genetic evidence that TAR plays critical
roles both during reverse transcription and during HIV gene expression. Our
results suggest that antiviral drugs targeting TAR RNA might be highly effective
due to a dual inhibitory mechanism.
Published in the journal: Inhibition of Both HIV-1 Reverse Transcription and Gene Expression by a Cyclic Peptide that Binds the Tat-Transactivating Response Element (TAR) RNA. PLoS Pathog 7(5): e32767. doi:10.1371/journal.ppat.1002038
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002038Summary
The RNA response element TAR plays a critical role in HIV replication by
providing a binding site for the recruitment of the viral transactivator protein
Tat. Using a structure-guided approach, we have developed a series of
conformationally-constrained cyclic peptides that act as structural mimics of
the Tat RNA binding region and block Tat-TAR interactions at nanomolar
concentrations in vitro. Here we show that these compounds
block Tat-dependent transcription in cell-free systems and in cell-based
reporter assays. The compounds are also cell permeable, have low toxicity, and
inhibit replication of diverse HIV-1 strains, including both CXCR4-tropic and
CCR5-tropic primary HIV-1 isolates of the divergent subtypes A, B, C, D and
CRF01_AE. In human peripheral blood mononuclear cells, the cyclic peptidomimetic
L50 exhibited an IC50 ∼250 nM. Surprisingly, inhibition of
LTR-driven HIV-1 transcription could not account for the full antiviral
activity. Timed drug-addition experiments revealed that L-50 has a bi-phasic
inhibition curve with the first phase occurring after HIV-1 entry into the host
cell and during the initiation of HIV-1 reverse transcription. The second phase
coincides with inhibition of HIV-1 transcription. Reconstituted reverse
transcription assays confirm that HIV-1 (−) strand strong stop DNA
synthesis is blocked by L50-TAR RNA interactions in-vitro.
These findings are consistent with genetic evidence that TAR plays critical
roles both during reverse transcription and during HIV gene expression. Our
results suggest that antiviral drugs targeting TAR RNA might be highly effective
due to a dual inhibitory mechanism.Introduction
Highly active antiretroviral therapy (HAART) has led to a dramatic increase in the longevity of patients infected with HIV [1]. Unfortunately, even the most effective therapy does not completely eradicate the virus and active viral replication resumes immediately after treatment interruption [2]. The emergence of drug resistance further complicates antiviral therapy and can lead to treatment failure [3], underscoring the continuing need to develop new HIV antivirals with novel targets and mechanisms of action [4].
Tat, a viral encoded transcriptional activator, and its cellular co-factor, the transcription elongation factor-b (P-TEFb) are recruited to the elongating RNA polymerase II (RNAP II) through interactions with the trans-activation responsive element (TAR), a 59nucleotide RNA found at the 5′ end of all viral transcripts (for reviews, see [5], [6]). Assembly of this complex activates P-TEFb subunit CDK9 kinase activity [7]. CDK9-mediated phosphorylation of RNAP II and Spt5 (a subunit of the DRB-sensitivity inducing factor (DSIF)) directly enhance transcriptional elongation [8]–[10] as well as dissociation of repressive NELF factor(s) [11], allowing more efficient RNAP II promoter clearance. The Tat:P-TEFb crystal structure, reported by by Tahirov et al. [12], reveals how these proteins associate and the concomitant conformational changes this interaction induces in the CycT1:CDK-9 complex. While P-TEFb is utilized widely for transcription of many genes, the interaction between Tat and TAR is unique to lentiviruses. Drugs targeting Tat and/or TAR are expected to both block HIV-1 replication during the acute phase of HIV-1 infection and to prevent virus emergence from latency [13]. A variety of candidate small molecule inhibitors of either HIV transcription, or more specifically, the Tat-TAR interaction, have been identified during the last 15 years [9], [10], [14]–[19]. Unfortunately none of these compounds were sufficiently potent and/or selective to progress beyond phase I clinical trials. Linear polypeptide analogues have been shown to block the Tat-TAR interaction by binding to TAR RNA [16], [17], [20], [21], but their conformational flexibility also allowed promiscuous binding to host RNAs and non-specific blocks to viral infectivity [22], [23]. Using constrained cyclic peptidomimetics designed to mimic the antiparallel ß-sheet in the Tat RNA binding domain we identified a series of competitive inhibitors of the Tat-TAR interaction with improved affinity and selectivity compared to the linear peptides [24]–[27].
In this study, we investigated the antiviral mechanism(s) of these compounds. Peptidomimetics in this compound series were cell permeable, had low cytotoxicity, and inhibited viral replication of a diverse HIV-1 isolate panel in both primary human cells and immortalized cell lines with low micromolar half-maximal inhibitory concentration (IC50) values. In contrast to other flexible linear peptide inhibitors of TAR binding, constrained peptidomimetics had no effect on HIV-1 entry [22], [23]. Surprisingly, inhibition of viral transcription did not account for the full antiviral activity of these compounds. Timed drug addition experiments then demonstrated that the peptidomimetics have a dual mechanism of action, blocking both HIV-1 reverse transcription (an early infection event) and HIV-1 proviral RNA transcription (a later event).
Results
Inhibition of HIV-1 replication by Tat peptidomimetics
Linear peptide derivatives have been used previously to target TAR RNA and block Tat binding [16], [17], [20], [21] but their utility was limited by conformational flexibility which allowed non-specific RNA binding. To avoid this problem we have developed conformationally constrained mimics of HIV-1 Tat [24]–[27] based on the structure of the BIV (Bovine Immunodeficiency Virus) Tat-TAR complex [28], [29] (Figure 1). Specifically, we constructed a series of ß-hairpin mimics with 12-residue loops on a heterochiral D-Pro-L-Pro dipeptide scaffold. This D-Pro-L-Pro scaffold strongly favors a type-II' ß-turn backbone conformation, similar to that found in the Tar RNA binding domain [24]–[27]. Assayed in-vitro, several members of this series, L-50, L-51, and L-22, bound HIV-1 TAR with nanomolar affinity (Kd = 1, 5, and 30 nM, respectively) [25], [26].
Fig. 1. The structure and sequence of HIV-1 Tat/TAR RNA. 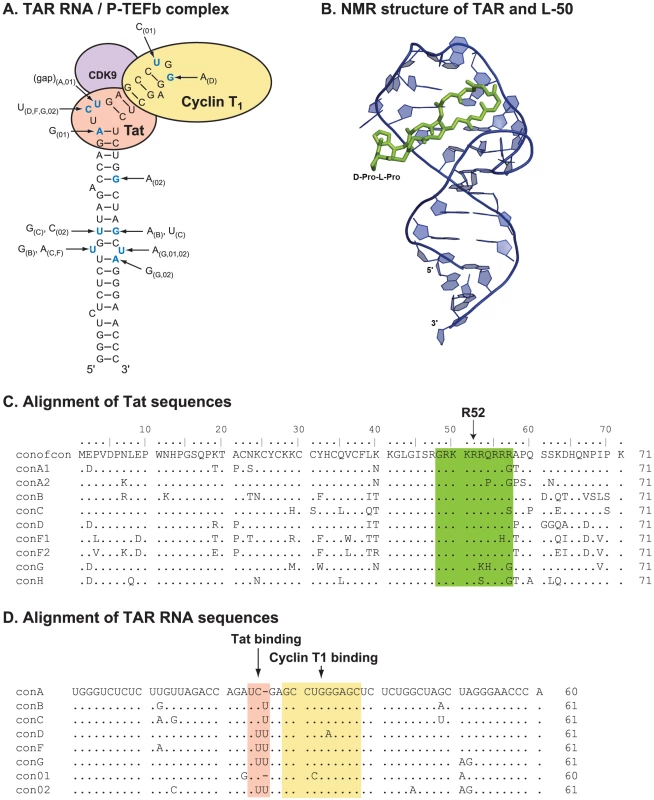
As shown in Figure 2A, the three Tat peptidomimetics L-50, L-51 and L-22, were able to inhibit replication of laboratory-adapted HIV-1 strain NL4-3 (HIV-1NL4-3) in U87 cells expressing the CD4 receptor and CXCR4 coreceptor (U87.CD4.CXCR4 cells). The relative potency of the compounds correlated with their TAR binding activity [26]. L-50, which had the highest affinity for TAR, inhibited HIV-1NL4-3replication with an IC50 value of 4.1 µM, which was about 10-fold more potent than either L-51 or L-22 (Figure 2B). The activity of L-50 was approximately 10-fold lower than the licensed antiretroviral drugs 3TC (IC50 = 0.12±0.03 µM on U87 cells; Figure 2B) as well as Enfuvirtide (IC50 = 0.1 to 0.5 µM in U87 cells) [30], a polypeptide retroviral entry inhibitor which does not require cellular uptake [31].
Cellular uptake
The sequences of the peptidomimetics are derived from the HIV-1 Tat basic domain (amino acids 48–57) which, in addition to RNA binding, permits the protein to transverse cellular membranes [32]–[35]. Since known cell-penetrating peptides have flexible structures, we tested whether the constrained peptides retained cell-penetrating properties. A fluorescein-labeled conjugate of polypeptide L-51, prepared by coupling a commercially available fluorescein-diacetate tag to a hydrazine-derivative of L-51, was rapidly internalized by living 293T fibroblasts (Figure 2C). The peptidomimetic accumulated in the nucleus with a substantial fraction in the nucleoli, resulting from their semblance to nuclear and nucleolar localization signals [36], [37].
Cytotoxicity
L-50 toxicity was compared to that of enfuvirtide (T20), another anti-HIV-1 inhibitor polypeptide. U87.CD4.CXCR4 cells were exposed to increasing concentrations of the peptides (maximal of 500 µM) for ten days and cell viability was measured using Alamar blue staining. Cell viability was similar to control conditions under all conditions, including the highest L-50 or enfuvirtide concentration tested (500 µM; data not shown), consistent with previous studies experiments with T20 [31].
Inhibition of multiple HIV-1 clades by the Tat peptidomimetics
Although pre-clinical development of antiviral drugs typically involves initial optimization with subtype B laboratory HIV-1 strains, HIV-1 subtypes A, C, D, and CRF01_AE account for over 85% of the global epidemic whereas subtype B comprises less than 10% of present infections [38]. Thus, the long-term utility of any antiretroviral lies in its ability to inhibit diverse primary HIV-1 isolates of all subtypes - particularly those strains that utilize the dominant co-receptor (CCR5) for entry. As shown in Figure 2D, L-50 effectively inhibited a panel of CCR5-tropic and CXCR4-tropic primary HIV-1 isolates including representatives of subtypes A, B, C, D, and CRF01_AE (Figure S1A, B). Aside from strain E6-CRF01_AE, the mean IC50 value for L-50 was 4.73±1.33 µM (Figure 2D). Cross-clade HIV-1 inhibition profiles are important attributes for potential Tat-TAR inhibitors, since there is considerable genetic diversity in both the R52 domain of the Tat protein (Figure 1C) as well as its TAR RNA target sequence in the HIV-1 LTR (Figure 1D). Furthermore, previous studies using a linear peptoid Tat-TAR interaction inhibitor in vitro showed that the principal antiviral activity was due to blocking the CXCR4 receptor, preventing virus entry rather than viral transcription [27]. The broad-spectrum of antiviral activity against multiple clades and viruses that utilize either CCR5 or CXCR4 co-receptors suggests that the constrained peptidomimetics do not act at viral entry.
Early-stage drug candidates often display efficient HIV-1 inhibition in cell lines but lack potency in primary CD4+ T lymphoctyes. In the case of L-50, the efficiency of HIV-1NL4-3 inhibition was actually increased in human PBMCs (IC50 0.25 µM) compared to U87.CD4.CXCR4 cells (IC50 4.1 µM) (Figure 2E).
Inhibition of Tat-dependent transactivation in cell-free transcription systems
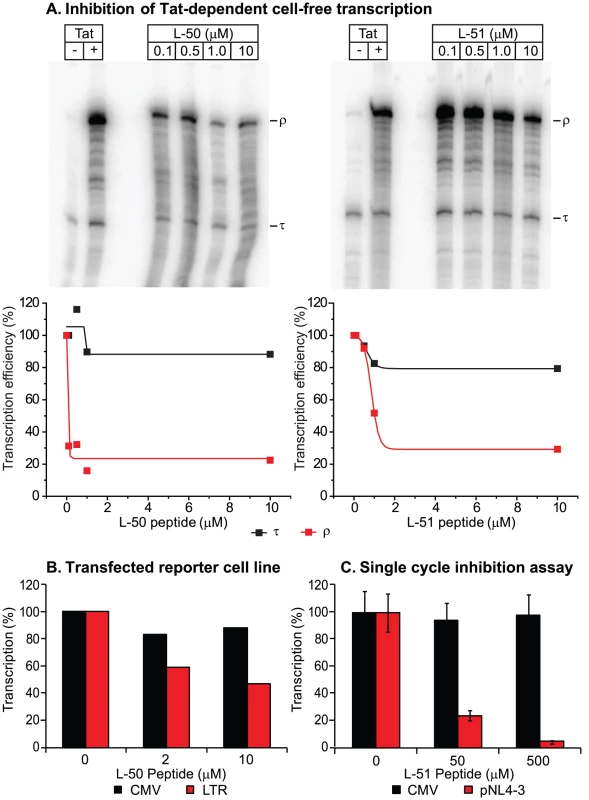
We next tested whether the peptidomimetics could inhibit Tat function in a cell-free transcription assay (Figure 3). Cell free transcription assays have been extensively used in previous studies of compounds capable of inhibiting Tat [8], [15], [39], [40]. Briefly, nuclear extracts are incubated with a DNA template harboring the HIV-1 long terminal repeat (LTR; contains the proviral promoter and the TAR region) either in the presence or absence of recombinant Tat protein (Figure 3A). A terminator sequence inserted downstream of the promoter provides a selective and effective block to RNAP II elongation in the absence of Tat. As shown in Figure 3A, transcripts that are unable to traverse the terminator (labeled τ) accumulated in the absence of Tat, whereas full-length transcript (labeled ρ) production increased over 20-fold when Tat was added. Since the levels of short transcripts (τ) should remain unaffected when Tat is inhibited, these transcripts provide an effective internal control for non-specific inhibition of transcription. As shown in Figures 3A, addition of increasing concentrations of the inhibitory peptides L-50 and L-51 decreased synthesis of full-length transcripts (ρ) with an IC50 of 50 nM and 500 nM, Under these conditions the production of short transcripts was largely unaffected. Thus, the Tat peptidomimetics were potent inhibitors of Tat-mediated transcriptional elongation in cellular extracts.
Inhibition of transactivation in vivo
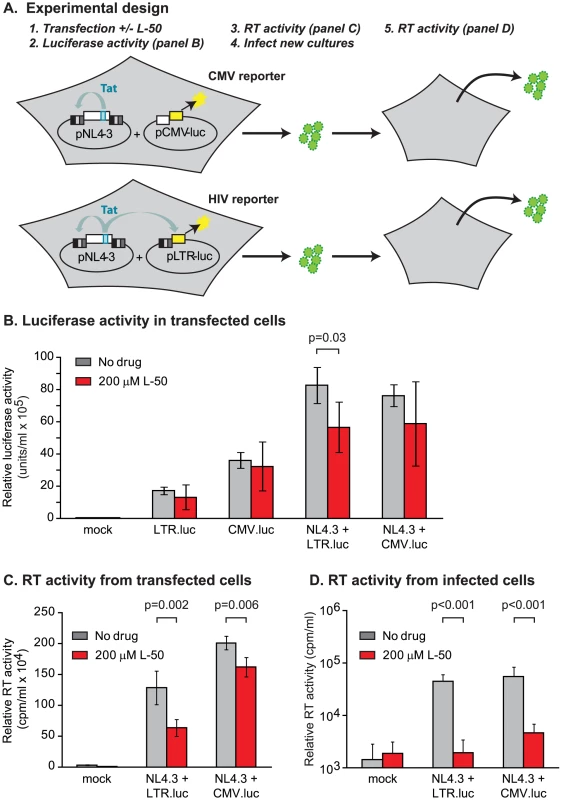
In order to evaluate the impact of the Tat peptidomimetics on HIV-1 transcription in vivo, we used three distinct reporter assay systems (Figures 3 & 4). First, we measured inhibition of transcription of stably transfected luciferase reporter genes under the control of the HIV-1 LTR and a control CMV promoter (Figure 3B). The addition of Tat increased the levels of the luciferase reporter gene expression by more than 30-fold in the case of LTR promoter while the transcription levels of the CMV promoter remained unaffected (data not shown). Treatment with 10 µM L-50 reduced Tat-mediated transactivation by 53%, but only reduced transcription from the control CMV promoter by 12% (Figure 3B).
Inhibition of HIV transcription was also measured in a single-cycle growth assay (Figure 3C). 293T cells were cotransfected with an infectious molecular clone HIVNL4-3 and a control vector carrying a luciferase reporter gene downstream of the CMV promoter. 293T cells support Tat-mediated HIV-1 transcription and virus production but are resistant to new rounds of virus production since they lack entry receptors. Addition of 50 µM L-50 peptide inhibited production of the viral RNA by 76% but as expected for a specific Tat-dependent transcription inhibitor, CMV promoter-driven luciferase activity reduced by less than 5% by L-50. Thus, inhibition of HIV-1 transcription by L-50 in both assays arises from blocks to transcription imposed by a disruption of the Tat-TAR interaction.
In the third assay, 293T cells were co-transfected with a reporter plasmid carrying a luciferase reporter downstream of the HIV-1 LTR promoter (pLTR.LUC) alone or in combination with the Tat-encoding infectious molecular clone HIV-1NL4-3. In control experiments, cells were co-transfected with a reporter plasmid carrying the luciferase gene downstream of the CMV promoter (pCMV.LUC) alone or in combination with HIV-1NL4-3 (Figure 4A). L-50 did not inhibit luciferase expression in cells transfected with either reporter plasmid alone (Figure 4B). Thus, although pLTR.luc produces the TAR RNA element as part of the luciferase mRNA, transcription is not blocked by L-50 in the absence of Tat. When cells were co-transfected with HIV-1NL4-3, Tat expression resulted in a ∼6.3-fold increase in luciferase expression. L-50 (200 µM) resulted in a comparatively modest (1.7-fold), but significant (p = 0.03, two-tailed T test, N = 5), decrease in luciferase expression (Figure 4B). As expected, CMV promoter-driven luciferase expression was not significantly inhibited by L-50.
In addition to measuring luciferase activity, we also measured viral production to provide an additional measurement of the antiviral activity of the compounds. It is important to note that, although luciferase expression is not LTR-driven in cells transfected by the pCMV.luc control plasmid, HIV-1NL4-3-encoded virion production is LTR-dependent in both sets of transfected cells. Treatment with 200 µM L-50 mediated a modest, but reproducible reduction in HIV-1 virion expression (Figure 4C). These results were consistent with measurements wherein L-50 inhibited transcription from LTR-driven cassettes (Figure 4B). The slightly greater inhibition of virus production from the pLTR.luc transfected cells by L-50 compared to the pCMV.luc transfected cells is likely due to competition between the viral and reporter expression cassettes for limited Tat protein (Figure 4C).
Thus we were able to demonstrate Tat-mediated transcriptional inhibition by L-50 in three distinct assays. However, in all these assays we were only able to detected a 2-fold reduction of RNA or virus production, which is considerably less than the 10 - to 100-fold HIV-1NL4-3 replication block in the multiple-cycle replication assays described above. Several possible explanations for this discrepancy might be: 1) virus released from the transfected cells were no longer replication-competent, 2) that L-50 has viricidial activity, or 3) that L-50 inhibited virus replication at additional steps in the viral life cycle prior to the onset of gene expression.
To test whether the virus released from transfected cells could be inhibited by L-50 under conditions of multiple-cycle growth, virus-containing supernatants from 293T transfections (containing L-50) were used to infect U87.CD4.CXCR4 cells (which support multiple rounds of HIV-1 replication) and viral progeny were measured 10 days post-infection. No measurable viral progeny were produced by viruses which were made in the presence L-50 (Figure 4D). Removal of L50 from virus was attempted by pelleting virus from supernatant of L50-treated cells transfected with NL4-3. It is important to note that virus production was already decreased by L50 at least 50% from transfected cells.
While the results of that experiment (Figure 4D) imply that L-50 inhibits viral egress through the target cells at some event prior to viral transcription, those data could not rule out virucidal activity. A cell-free preparation of HIV-1NL4-3 virions was incubated for 2 h with or without L-50 (50 µM or 500 µM). After pelleting virus to remove drug, we found similar viral infectivity with and without L-50 treatment suggesting that this drug was not a viricide (Figure S2). The strong implication of this experiment is that L-50 inhibited HIV replication by another mechanism during the replication cycle.
Time-dependent drug addition kinetics
The preceding experiments show that, although L-50 weakly inhibits HIV transcription in vivo, this activity does not account for the majority of its antiviral activity. To identify the key step(s) in the virus replication cycle that are inhibited by L-50, time-of-drug-inhibition experiments were performed (Figure 5). For each measurement, virus was added to cells at time 0 and HIV-1 gene expression (based on luciferase expression) was assayed at 72 h post infection. The HIV-1NL4-3virus employed in the experiment shown in Figure 5 carried the firefly luciferase gene inserted between the env and nef genes [41]. Luciferase expression was therefore dependent on LTR-mediated transcription and was used as a readout for viral replication. Comparable results were obtained in experiments when virus production was measured by RT activity (data not shown). Virus replication was limited to a single round by the addition of the HIV-1 protease inhibitor saquinavir, at 2 h post infection in each experiment.
The time of addition assay was calibrated using HIV inhibitors which selectively inhibit distinct steps in the viral replication cycle (Figure 5A). All drugs were added at intervals and, as the infection progressed, each drug became ineffective at a time consistent with the completion of their targeted infection event (Figure 5B). Additions of AMD3100, a CXCR4 antagonist which prevents HIV-1 co-receptor attachment, became ineffective very soon after infection (t1/2 = 0.77 h; Figure 5B & C). The viral fusion inhibitor Enfuvirtide (or T20) remained effective at slightly later additions (t1/2 = 1.0 h), consistent with a fusion event occurring after receptor binding. Inhibition by 3TC is slowly lost over 12 h (t1/2 = 6.6 h; Figure 5D), i.e. throughout the time required to complete reverse transcription (Figure 5B), consistent with previous results for this drug [42].
The integration inhibitor Raltegravir and the transcription inhibitor 5,6-dichloro-1-ß-D-ribobenzimidazole (DRB), blocked HIV-1 replication with significantly delayed kinetics compared to the entry and reverse transcription inhibitors (Raltegravir t1/2 = 11.5 h; DRB t1/2 = 31.1 h; Figure 5B & D). It is worth noting that data with DRB, which is a potent inhibitor of the CDK9 subunit of P-TEFb, can be considered to behave analogously to a potential Tat inhbititor, since P-TEFb is strictly required for Tat-dependent HIV transcription [43], [44].
When L-50 was added either prior to infection or at various time points following infection (every 30 minutes for the first 2 h, every hour for the next 13 hours, and then every 3–6 hours thereafter), we observed an unusual, biphasic inhibition profile over the 72 hour time course (Figure 5B). There was an initial inhibitory phase at approximately 2 h post infection (t1/2 = 1.8 h) when L-50 was able to block nearly 100% of viral replication. Thus, L-50 inhibits a step immediately following virus entry within the time window of the first stages of HIV-1 reverse transcription. When L-50 was added during the next 20 hours (t1/2 of 35.7 h), i.e. during the period after reverse transcription and subsequent to completion of proviral integration, there was a second phase of viral inhibition. The timing of this second phase was similar to the inhibitory kinetics of transcription inhibitor DRB (35.3 h; Figure 5E). Thus, L-50 blocks both an early post-entry step during HIV-1 replication as well as Tat-mediated transactivation of HIV-1 transcription.
L-50 does not block HIV-1 entry
The kinetic experiments shown in Figure 5, demonstrated that L-50 primarily inhibits HIV-1 replication at a step immediately following entry (t1/2 = 1.8 h) but prior to the inhibition of reverse transcription by 3TC (t1/2 = 6.6 h). The timing of these events is consistent with L-50 inhibiting either a very early step in reverse transcription or an entry step following formation of the gp41 pre-hairpin intermediate, i.e. the Enfuvirtide-sensitive state, prior to cell-viral membrane mixing and pore formation. To distinguish between these two possibilities, we performed a second time-of-drug inhibition experiment using cell-to-cell fusion to introduce Tat and activate an already integrated provirus (Figure 6A). In this assay, effector 293T cells were transfected with pREC.NFL, a CMV-driven expression plasmid carrying an HIV-1 provirus and lacks the HIV-1 5′-LTR sequence [45]. Transcription of the proviral construct produces all the HIV-1 proteins, including the gp120-gp41 envelope glycoprotein, which is expressed on the cell membrane and is required to induce cell fusion with the U87.CD4.CXCR4 target cells. Since the pREC.NFL vector lacks a5′-LTR, none of the transcripts produced in the effector cells can serve as templates for reverse transcription [45]. The U87.CD4.CXCR4 target cells were transfected with pDM128-LTR-fluc2 (see Materials and Methods) to provide a Tat - and Rev-dependent reporter. After fusion of the two cells, the HIV-1 Tat (and Rev) proteins migrate into the nucleus of the target cell, and stimulate luciferase production (Figure 6A). Thus, this assay is sensitive to inhibitors of membrane fusion and viral entry and inhibitors of viral transcription, but insensitive to inhibitors of reverse transcription.
Cell-to-cell fusion was initiated by pelleting the transfected 293T cells on to monolayers of U87.CD4.CXCR4 cells at 4°C. The drugs (L-50, 3TC, or DRB) to be tested were added at various time points, similar to the timed-drug-addition experiment described above (Figure 6A). Tat/Rev-dependent luciferase expression was measured in the cell lysates 72 h post fusion. This cell-to-cell fusion assay is highly sensitive to inhibition by both T20 and AMD3100 (data not shown) indicating that cell-to-cell fusion is both receptor-dependent and mechanistically identical to HIV-1 entry into a cell [46]. Because cell-to-cell fusion is not followed by reverse transcription or integration, 3TC was unable to inhibit reporter gene activation at any time (Figure 6B). In contrast to the time-of-drug addition experiment using free virus (Figure 5), L-50 exhibited a monophasic inhibition profile, with t1/2 of 8.2 h, similar to that exhibited by CDK-9 inhibitor DRB (t1/2 = 11.3 h; Figure 6B). Thus, this experiment provides strong and direct evidence that L50 does not inhibit the HIV-1 entry process and blocks Tat-dependent HIV-1 transcription, in contrast to the previously studied linear peptides derived from Tat [22], [23].
Inhibition of reverse transcription by L50 is independent of its effects on HIV transcription
In the experiment shown in Figure 6, we studied the late phase of L-50-mediated antiviral activity using an assay that was dependent on the Tat-TAR interaction but not on reverse transcription. To study the impact of L-50-on reverse transcription alone, we devised an assay that does not require HIV-1-dependent transcription for the readout (Figure 7A). For this experiment, we utilized an HIV-1 expression construct in which the env gene was replaced by a luciferase gene expressed under the control of the SV40 promoter (pNL.Luc.AM [47]). Using this reporter system, luciferase expression became dependent upon early infection events (i.e. entry, reverse transcription, integration), but the transcription of the reporter itself was independent of Tat-mediated transcriptional enhancement.
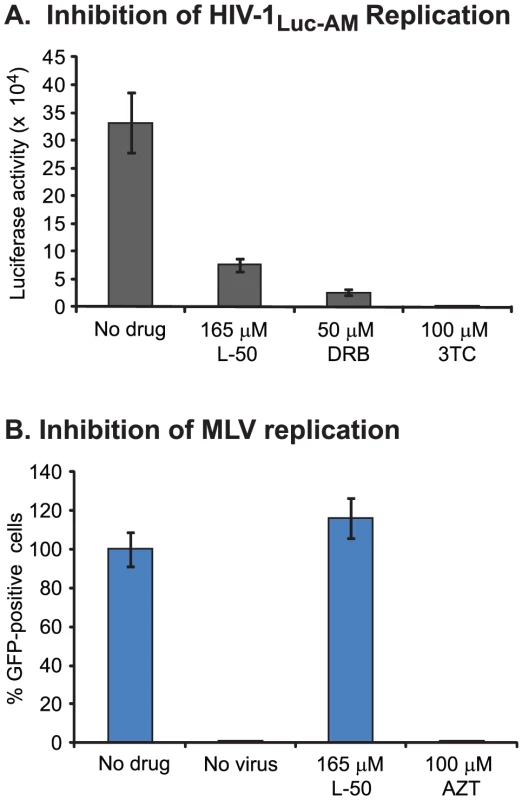
Pseudotyped HIV-1Luc-AM virus was produced by co-transfecting the pNL-Luc.AM vector with pSM.WT which expresses the HIV-1HXB2 env glycoproteins [47]. As shown in Figure 7A, L-50 efficiently blocked luciferase expression in HIV-1Luc-AM infected cells. As positive controls, both DRB and 3TC also efficiently inhibited luciferase expression from HIV-1Luc-AM. These findings are again consistent with a model in which L-50 blocks HIV-1 reverse transcription by binding TAR RNA.
L-50 does not inhibit MLV reverse transcription
Reverse transcription inhibitors, such as nucleoside analogues AZT, 3TC and ddI, generally block replication of all retroviruses as well as hepatitis B virus [48]. In contrast, non-nucleoside RT inhibitors, such as nevirapine and efavirenz, specifically bind to HIV-1 RT and not to polymerases from other lentiviruses or retroviruses [48]. Thus, it is possible that L-50, binds non-specifically to RNA structures present in other retroviruses. However, as shown in Figure 7B, L-50 (250 µM) did not inhibit murine leukemia virus (MLV) whereas AZT (100 µM) completely blocked MLV replication. These data suggest that L-50 is not a general inhibitor of retroviral reverse transcription, but is specific for the HIV-1 TAR RNA sequence and/or HIV-1 reverse transcriptase.
L-50 blocks HIV-1 reverse transcription in vitro
To test directly whether L-50 is able to inhibit HIV-1 reverse transcription, we used an in vitro assay reconstituting HIV-1 reverse transcription. This assay measures the synthesis of (-) strand strong stop DNA by recombinant HIV-1 RT (p66/p51) from an 18 nt DNA primer, pre-annealed to an HIV-1 RNA template encompassing the repeat (R), 5′ unique sequence (U5), and the primer binding sequence (PBS) [49]. Addition of increasing concentrations of L-50 strongly inhibited (−) strand strong stop DNA with an IC50 value of 5.5±0.57 µM (Figure 8A and B). This inhibitory concentration is only slightly greater than the RNA template concentration in the reaction mixture (∼2 µM) consistent with the very tight affinity of L-50 for TAR RNA [26].
Fig. 8. Inhibition of minus strand strong stop DNA synthesis by L50. 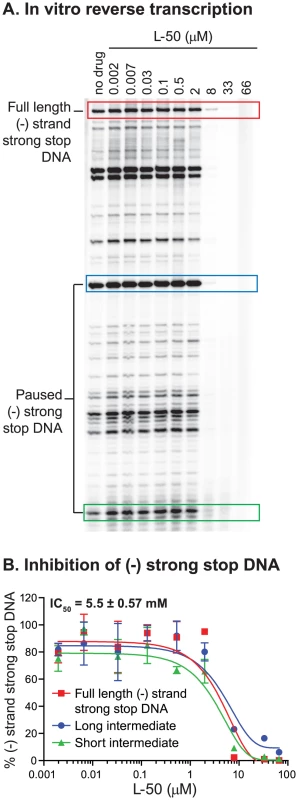
To determine if L-50 inhibition of reverse transcription was dependent on the high affinity Tat-binding site located at the TAR bulge, mutations that inactivate Tat-binding were introduced into the RNA template (Figure 9A). As expected, L-50 was less potent at inhibiting the (−) strand strong stop DNA on the mutant TAR RNA template (Figure 9B and C). For example, 8 µM L-50 inhibited 80% of (−) strong stop synthesis on the wild type template but the same drug concentration inhibited less than 10% of (−) strong stop DNA synthesis on a template with mutations in the TAR L-50 binding site.
Discussion
Design of peptidomimetic inhibitors with high affinity for TAR RNA
The Tat-TAR complex has long been the focus of drug discovery efforts because of its central and unique role in regulating HIV-1 transcription. Unfortunately, previous attempts to discover a potent, specific and stable anti-Tat-TAR compound with efficient cellular uptake have been unsuccessful. The majority of the efforts to design Tat inhibitors involved highly charged cationic drugs [14], [15], [21], [50]–[52], oligonucleotide analogues with poor bioavailability [53], [54], or proteolytically stable peptoids [16], [17] and oligo-carbamates [55]. These compounds were abandoned due to their comparatively non-specific interactions with RNA. Other lead molecules, such as ALX40-4C [18], [22], [56] and CGP64222 [17], were initially identified as Tat-inhibitors and progressed to early clinical studies but were withdrawn from further pharmaceutical development after their antiviral activity was identified as cell surface receptor ligands.
Our design of inhibitors of the TAR RNA-Tat protein interaction started with the assumption that constrained peptidomimetics would provide effective rationale to block the relatively large interfaces formed between the viral protein and its cognate RNA [24]–[27]. The D-Pro-L-Pro template restricts pharmacophores into planar ß-hairpin structures with side chains emerging from each side. Several lead peptides, including L-20, L-50, and L-51 had nanomolar affinities for HIV-1 TAR and effectively discriminated against the analogous BIV TAR RNA sequence. Specific and high affinity binding allowed these compounds to compete with Tat protein and displace it from preformed complexes with TAR in vitro. This potent inhibition of Tat is remarkable considering the 3-fold reduced size of the peptidomimetics compared to the protein [25].
NMR structures of the peptide-RNA complexes revealed that Tat peptidomimetics bind in the major groove near the UCU bulge, which forms an essential part of the Tat binding site, and induce specific conformational changes that are similar to those seen with Tat [27]. Specifically, the constrained Arg residues at positions 3 and 5 of the cyclic peptide structures are located near the bulge region of TAR RNA. The pharmacophore “locking” exemplified for these two critical residues leads to remarkable specificity in binding to TAR RNA. The larger peptidomimetics show enhanced affinity due to specific interactions with the TAR RNA apical loop sequence [26].
Cyclic peptidomimetics are potent inhibitors of HIV-1 replication
The three lead Tat peptidomimetics (L-20, L-50, and L-51) are potent inhibitors of HIV-1 replication. The lead molecule L-50 inhibits HIV-1 in primary human lymphocytes in the same concentration range as do the FDA-approved small molecule inhibitors nevirapine and 3TC. Additionally, these Tat peptidomimetics were not cytotoxic and did not inhibit cell growth up to 1 mM.
Direct addition of the peptidomimetics to cultured cells blocked HIV-1 replication, without requiring a carrier molecule to facilitate drug diffusion across plasma membranes. In contrast to previously characterized flexible peptides, which selectively inhibited HIV-1 replication by binding to the CXCR4 receptor [22], [23], L-50 inhibited a panel of both CXCR4 - and CCR5-using primary HIV-1 isolates, representing the major subtypes currently circulating (A, B, C, D, and CRF01_AE). This data was satisfying because it implied that L-50 recognized conserved RNA secondary structure elements in TAR RNA even though the nucleotide sequence in this region varies considerably between strains (Figure 1D).
Inhibition of multiple steps in the HIV-1 life cycle by the peptidomimetics
We demonstrated that the peptidomimetic L-50 inhibited Tat-mediated HIV-1 transcription using both cell free transcription assays and multiple independent in vivo assays using cell lines carrying reporter genes and integrated HIV-1 proviruses. In all of these assays, L-50 induced an approximately two-fold reduction in HIV-1 transcription, which was much less than the 10 - to 100-fold reduction in HIV-1 replication we observed in viral replication assays. Using a variety of HIV-1 inhibitors in a time-of-drug-addition experiment we identified two specific steps in the HIV-1 lifecycle that were inhibited by L-50. As expected, L-50 was identified as an inhibitor of HIV-1 transcription since it block viral replication with a time course that closely resembled the DRB, a drug which prevents RNAP II transcriptional elongation by selectively inhibiting the protein kinase component of P-TEFb, CDK9 [43]. Additionally, L-50 inhibited an early event in HIV-1 replication. L-50 retained antiviral activity after entry inhibitor Enfuvirtide became ineffective (t1/2 = 1.07 h), but lost some activity (L-50 t1/2 = 1.8 h) approximately 4.8 hours before nucleoside RT inhibitor 3TC (t1/2 = 6.6 h) lost activity.
To rule out non-specific HIV-1 entry inhibition, such as had been observed for linear Tat-derived peptidomimetic inhibitors [22], [23], we used a cell-to-cell fusion assay which is not dependent upon reverse transcription or integration but faithfully mimics the mechanism of virus-to-cell binding, fusion [31] and Tat-dependent transcription (of the luciferase reporter). L-50 inhibition kinetics again coincided with DRB inhibition but there was no measurable inhibition of cell-to-cell fusion by the compound.
Inhibition of HIV-1 reverse transcription
Both Tat and TAR RNA are thought to play an important role in HIV-1 reverse transcription [57]–[60]. TAR is a structure derived from the repeat (R) and 5′ unique sequence (U5) of the LTR, and located just upstream of the primer binding site (PBS), the initiation site of reverse transcription. There is evidence that TAR RNA structure contributes to the HIV-1 reverse transcription process [49], [61]–[64] by participating in a larger tertiary RNA secondary structure necessary for the efficient initiation of reverse transcription from tRNALys,3 [65], [66]. Nucleocapsid-mediated TAR RNA unwinding may also prevent self-priming and/or coordinate RNase H activity to free the end of single-stranded, minus strong-stop DNA. This strong-stop DNA product (complement of R and U5 RNA sequences) could then pair with the 3′ R region of RNA, adjacent to the U3 sequence, thereby facilitating the first template switch and eventual completion of minus DNA synthesis [63], [64]. This annealing process for template switching is also facilitated by the nucleocapsid protein (NC) chaperone activity [67]. Deleting or mutating TAR RNA reduces first template switch efficiency [49] and mutations that increase the stability of TAR RNA appear to block strand transfer [68].
There is also evidence that Tat plays a role in viral reverse transcription, although much of this work has been controversial. Loss of function Tat mutants have been reported to impair reverse transcription 3 - to 5-fold compared to wild type HIV-1 both in infected T cells and in endogeneous reverse transcription reactions [58], [69]. Tat-mediated RNA secondary structure remodeling may facilitate obligatory strand transfers during viral DNA synthesis by reverse transcriptase [60]. However, it is possible to create replication-competent viruses where the TAR RNA sequence is replaced by a short hairpin RNA structure suggesting that Tat is dispensible for reverse transcription [70]. Our kinetic studies demonstrate that the first phase of L-50 inhibition corresponds to reverse transcriptional initiation. HIV-1 reverse transcriptase encounters the TAR stem-loop within the first ∼150 nt of minus strong-stop DNA synthesis, so the L-50-TAR RNA complex might disrupt HIV-1 reverse transcription initiation, prevent elongation or block the first template switch. Synthesis of the proviral genome (nearly 20,000 nucleotides) is thought to occur within the first 12 hours of infection, consistent with the t1/2 (6.6 h) we measured for 3TC (and ∼7 h reported for non-nucleoside RT inhibitor nevirapine [42]). It is then not surprising that nucleoside RT inhibitor 3TC, which blocks polymerization opposite any guanosine/deoxyguanosine base during this process, exhibits prolonged activity compared to L-50. Thus, inhibition during minus-strand strong stop DNA synthesis would likely occur immediately following entry (∼1 hrs), within the first few minutes of reverse transcription, accounting for the narrow window of L-50 efficacy in the first phase (1.8 h). AZT and 3TC blocked MLV replication whereas L-50 did not, confirming that L-50 activity is specific to HIV-1. The exact inhibitory mechanism exerted by L-50 on HIV-1 reverse transcription is the subject of ongoing studies. The data presented in Figure 8 provides in vitro evidence that L-50 blocks (−) strand strong stop DNA synthesis in a TAR-dependent manner. However, we have not ruled out additional effects of L-50 on reverse transcription, including (1) disruption of tRNALys,3 binding to HIV-1 RNA, (2) inhibition of a transition from tRNALys,3 initiation (+1 to +5 nt) to (−) strong-stop DNA elongation, (3) block in proper RNase H digestion of 5′ LTR RNA, and finally, (4) prevention of the first strand switch necessary to complete (−) strand DNA synthesis.
Conclusions
We have shown that cyclic peptidomimetics derived from HIV-1 Tat are potent inhibitors of HIV-1 replication. Detailed characterization of the L-50 mechanism of action revealed that it is able to inhibit two important steps in the virus life cycle that involve TAR RNA: HIV-1 reverse transcription and Tat-mediated transcription. Although L-50 was more potent at inhibiting HIV-1 reverse transcription (IC50 = 1 to 10 µM) than HIV-1 mRNA transcription (IC50 >100 µM), this difference in potency might simply reflect the stoichometry necessary for inhibition. Relatively few virions enter a cell to establish infection which dramatically limits the number of TAR RNA sequences that need to be bound by L-50 (in theory, infection can be initiated by a single virion carrying only two viral genomes). By contrast, there are several thousand rounds of HIV-1 RNA transcripts produced during the later stages of the virus life cycle and each transcript carries TAR RNA as part of its leader sequence. Our unexpected discovery that L-50 has a dual inhibitory mechanism demonstrates that drugs can be designed that simultaneously inhibit reverse transcription and HIV-1 transcription by targeting the highly conserved TAR RNA element.
Methods
Peptide synthesis
The ADP-1 peptide containing the entire RNA-binding activity of Tat protein [71] was prepared on MBHA-Rink amide resin using Fmoc-chemistry on an Applied Biosystems 433A peptide synthesizer. The synthesis of the cyclic peptides has been described previously [25]. Peptide L-51 conjugated to a fluorescent dye was produced by coupling of commercially available 5(6)-carboxyfluorescein diacetate to an analogue of L-51, containing D-trans-4-hydrazinoproline (Hyd) in place of D-Proline (i.e. cyclo-(Arg-Thr-Arg-Thr-Arg-Gly-Lys-Arg-Arg-Ile-Arg-Val-Hyd-Pro).
Inhibition of Tat-dependent transactivation in cell-free transcription reactions
Plasmids carrying the wild type HIV-1 LTR [71] were linearized with XbaI and biotinylated at both the 5′ and 3′ ends by incorporation of biotin-16-dUTP (Roche) [72]. In order to form the elongation complexes, the DNA was linearized, biotinylated and immobilized on streptavidin-coated magnetic beads (Dynal) that were added to the reaction mixtures as described [8]. Twenty ng of Tat protein were added and the elongationcompetent complexes were assayed at increasing concentration of peptidomimetic inhibitors (usually from 0.1 to 20 µM). The reaction mixtures were incubated for 20 min at 30°C with occasional mixing and analyzed by fractionation of 6% polyacrylamide gels [8].
Microscopy
Human fibroblasts or HeLa cells (5×104) were plated on 35 mm glass bottom culture dishes in DMEM/10% FBS (Gibco, Invitrogen) and cultured for 12 to 48 hours to obtain a confluency of 40-60%. The medium was discarded and cells were washed with PBS followed by incubation with media containing 3 mM fluorescent L-51 at 37°C for 10 min. After incubation with the fluorescent peptide, the medium was discarded and the cells were washed five times with PBS and a final volume of PBS was added for observation of living cells. Cells were imaged at 40× magnification using an Olympus IX70 epifluorescent inverted microscope. Cell observation was done with a Delta Vision RT dencovolution system and images were captured using a CCD digital camera. Scattered light was computationally reassigned using Softworx software (Applied Precision Inc.).
Plasmids
Several previously reported eukaryotic expressioin constructs were used for this study. Plasmid pNL4.3 was originally reported by Adachi et al. [73] Plasmids pLTR.luc and pCMV.luc contains an HIV-1 LTR or CMV promoter cloned upstream of the firefly luciferase gene, respectively [74]. pDM128-LTR-fluc2 (a gift from David McDonald [75]) was produced by replacing the SV40 promoter with the HIV-1 LTR and replacing the exonic CAT coding sequence with the firefly luciferase gene. The resulting plasmid expresses firefly luciferase when the Tat dependent, LTR-driven unspliced RNA transcript is rescued by HIV-1 Rev. Plasmid pNL.luc.AM (a gift from Andre Marozsan [47]) is an HIV-1 expression construct wherein the Env ORF has been interrupted by an in-frame stop codon followed by SV40-promoted luciferase ORF. This construct produces luciferase constitutively in the transfected cells and, when co-expressed with a viral envelope supplied in trans, in target cells which have been infected by the resulting VLPs. Pseudotyped virus was produced using HIV-1 Env expression plasmid pSM.WT described elsewhere [76]. pREC-nfl (or pREC nfl_HIV-1) was cloned as previously described and lacks the 5′LTR of proviral NL4-3 DNA [45]. The near full length genomic (nfl) RNA is expressed from the CMV promoter and is spliced into all HIV-1 mRNA products to produce the full complement of HIV-1 proteins. The vector supports HIV-1 Env glycoprotein expression and cell fusion with U87.CD4.CXCR4 cells. The vector also produces virus particle that can de novo enter a susceptible cell but the core is incapable of supporting reverse transcription [45].
Cells and viruses
U87.CD4.CXCR4/CCR5 cells were obtained through the AIDS Research and Reference Reagent Program and were maintained in DMEM (Mediatech, Inc., Herndon, PA) supplemented FBS medium (15%, Life Technologies, Inc., Rockville, MD), penicillin/streptomycin, G418 and puromycin. 293T cells were maintained in DMEM supplemented with 10% FBS and pen/strep. PBMC from HIV-seronegative donors were prepared as previously described [77]. The pNL4-3 infectious molecular clone was obtained through the AIDS Research and Reference Reagent Program. Infectious virus was produced and titered as previously described [77].
Inhibition of Tat-dependent transactivation
293T cells were incubated with peptidomimetics and co-transfected with various combinations of pNL4-3 – an infectious molecular clone, pLTR.luc - a vector with the luciferase gene under control of the HIV-1 LTR, and pcDNA.LUC - a control plasmid expressing luciferase under control of the CMV promoter. Cells were transfected using a lipofectamine protocol as previously described [45]. Cell-free supernatant was collected 24 h post-transfection and assessed for RT activity, while the cells were lysed and assessed for luciferase activity. To assess inhibitor effects on integrated proviruses, 293T cells containing a Tat-deficient LTR reporter were pretreated with the peptides for 1 h and transfected with a Tat-expressing plasmid. The cells were incubated for 24 h, lysed, and assessed for luciferase activity.
Viral replication/inhibition assays
Drug sensitivity assays were performed on U87.CD4.CXCR4/CCR5 cells and in peripheral blood mononuclear cells (PBMC). Peptidomimetics or control drugs were added to cells to yield final concentrations between 100 µM and 10 nM. Cells were incubated with drug for 1 h and exposed to the virus at a multiplicity of infection of 0.001, incubated with the virus for 24 h, and input virus washed away. Supernatant aliquots were removed and virus production was quantified by reverse transcriptase assay [77]. Virus production at each drug concentration was normalized and the relative values were plotted versus drug concentration to determine 50% inhibitory concentrations (IC50). Variations on these drug susceptibility assays are defined in each figure and related text. For the time-of-drug-inhibition experiments, HIV-1 was spinonculated onto U87-CD4/CCR5 cells as previously described [42]. The cells were washed twice with cold phosphate buffered saline (PBS) to remove unbound virions. Cells were resuspended in cold medium and split into 96-well plates (50 µl/well). Virus-cell mixes were synchronized for entry by addition of 130 µl of 37°C medium, and then AMD3100 (10 µM), Enfuvirtide/T20 (10 µM), Lamivudine/3TC (100 µM), Raltegravir (10 µM), DRB (50 µM), and L50 (250 µM) was added at one of the various time points post synchronized infection (described in Figure 5) and maintained up to 72 h. Cells were incubated for 72 h and then treated with lysis buffer and luciferase activity was determined. The same procedure was utilized for the time-of-drug-addition experiment involving cell-to-cell fusion. However, in these analyses, 293T cells were transfected with pREC.nfl [45] and then added (like virus as the method above) to the U87.CD4.CXCR4 target cells. Cell-to-cell fusion was initiated by removing cold medium and adding 37°C medium.
In vitro reverse transcription assays
HIV-1 RNA representing the wild type R, U5, pbs, and uncoding sequence (270 nt) (wt R_U5_pbs RNA) was produced by in vitro T3 transcription (T3 MegaScript, Ambion) from a PCR product (with T3 extended primer) derived from NL4-3. The deleted R region transcript (ΔR_U5_pbs RNA) was produced using the same in vitro transcription method but from a truncated PCR production (i.e. lacking 41 nt of R region). The mutant TAR RNA transcript (with mutations 5′-UCUG-3′ to 5′-UGGU-3′ in the bulge) was generated by T3 transcription from a PCR product with the forward primer having the nt substitutions for site-directed mutagenesis. The primer sequences for PCR are available upon request. The DNA primer (18 nt), complementary to the primer binding sequence (3′ end of tRNALys,3), was annealed to the template as described [78]. The DNA primer was annealed to the various HIV-1 RNA templates by denaturation and annealing conditions previously described [78]. The primer:template annealed mixture (approximately 0.5 and 0.25 pmols) was added to 20 µl reaction mixture containing 50 ng of HIV-1 RT [49], [78] and in the absence or presence of L50 (0.002 to 66 µM). Reactions were incubated at 37°C for 45 min and then quenched with formamide EDTA loading buffer [78] for subsequent electrophoresis on a 8% denaturing polyacrylamide gel. Gels were autoradiographed and analyzed with a BioRad phosphorimager. The full length (−) strand strong stop DNA product is 181 nt on the HIV-1 wt_R_U5_pbs or mutant TAR RNA templates and 140 nt on the ΔR_U5_pbs RNA template.
Supporting Information
Zdroje
1. VolberdingPADeeksSG
2010
Antiretroviral therapy and management of HIV
infection.
Lancet
376
49
62
2. RichmanDDMargolisDMDelaneyMGreeneWCHazudaD
2009
The challenge of finding a cure for HIV
infection.
Science
323
1304
1307
3. Menendez-AriasL
2010
Molecular basis of human immunodeficiency virus drug resistance:
an update.
Antiviral Res
85
210
231
4. TaiwoBHicksCEronJ
2010
Unmet therapeutic needs in the new era of combination
antiretroviral therapy for HIV-1.
J Antimicrob Chemother
65
1100
1107
5. KarnJ
1999
Tackling Tat.
J Mol Biol
293
235
254
6. PeterlinBMPriceDH
2006
Controlling the elongation phase of transcription with
P-TEFb.
Mol Cell
23
297
305
7. WeiPGarberMEFangSMFischerWHJonesKA
1998
A novel CDK9-associated C-type cyclin interacts directly with
HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR
RNA.
Cell
92
451
462
8. KimYKBourgeoisCFIselCChurcherMJKarnJ
2002
Phosphorylation of the RNA polymerase II carboxyl-terminal domain
by CDK9 is directly responsible for human immunodeficiency virus type 1
Tat-activated transcriptional elongation.
Mol Cell Biol
22
4622
4637
9. CupelliLAHsuMC
1995
The human immunodeficiency virus type 1 Tat antagonist, Ro
5-3335, predominantly inhibits transcription initiation from the viral
promoter.
J Virol
69
2640
2643
10. HwangSTamilarasuNKiblerKCaoHAliA
2003
Discovery of a small molecule Tat-trans-activation-responsive RNA
antagonist that potently inhibits human immunodeficiency virus-1
replication.
J Biol Chem
278
39092
39103
11. FujinagaKIrwinDHuangYTaubeRKurosuT
2004
Dynamics of human immunodeficiency virus transcription: P-TEFb
phosphorylates RD and dissociates negative effectors from the
transactivation response element.
Mol Cell Biol
24
787
795
12. TahirovTHBabayevaNDVarzavandKCooperJJSedoreSC
2010
Crystal structure of HIV-1 Tat complexed with human
P-TEFb.
Nature
465
747
751
13. KarnJ
2011
The molecular biology of HIV latency: breaking and restoring the
Tat-dependent transcriptional circuit.
Curr Opin HIV AIDS
6
4
11
14. DavisBAfsharMVaraniGMurchieAIKarnJ
2004
Rational design of inhibitors of HIV-1 TAR RNA through the
stabilisation of electrostatic “hot spots”.
J Mol Biol
336
343
356
15. MurchieAIDavisBIselCAfsharMDrysdaleMJ
2004
Structure-based drug design targeting an inactive RNA
conformation: exploiting the flexibility of HIV-1 TAR RNA.
J Mol Biol
336
625
638
16. HamyFBrondaniVFlorsheimerAStarkWBlommersMJ
1998
A new class of HIV-1 Tat antagonist acting through Tat-TAR
inhibition.
Biochemistry
37
5086
5095
17. HamyFFelderERHeizmannGLazdinsJAboul-elaF
1997
An inhibitor of the Tat/TAR RNA interaction that effectively
suppresses HIV-1 replication.
Proc Natl Acad Sci U S A
94
3548
3553
18. O'BrienWASumner-SmithMMaoSHSadeghiSZhaoJQ
1996
Anti-human immunodeficiency virus type 1 activity of an
oligocationic compound mediated via gp120 V3 interactions.
J Virol
70
2825
2831
19. HsuMCSchuttADHollyMSliceLWShermanMI
1991
Inhibition of HIV replication in acute and chronic infections in
vitro by a Tat antagonist.
Science
254
1799
1802
20. HuqIPingYHTamilarasuNRanaTM
1999
Controlling human immunodeficiency virus type 1 gene expression
by unnatural peptides.
Biochemistry
38
5172
5177
21. LeeCWCaoHIchiyamaKRanaTM
2005
Design and synthesis of a novel peptidomimetic inhibitor of HIV-1
Tat-TAR interactions: squaryldiamide as a new potential bioisostere of
unsubstituted guanidine.
Bioorg Med Chem Lett
15
4243
4246
22. DoranzBJGrovit-FerbasKSharronMPMaoSHGoetzMB
1997
A small-molecule inhibitor directed against the chemokine
receptor CXCR4 prevents its use as an HIV-1 coreceptor.
J Exp Med
186
1395
1400
23. DaelemansDScholsDWitvrouwMPannecouqueCHatseS
2000
A second target for the peptoid Tat/transactivation response
element inhibitor CGP64222: inhibition of human immunodeficiency virus
replication by blocking CXC-chemokine receptor 4-mediated virus
entry.
Mol Pharmacol
57
116
124
24. AthanassiouZDiasRLMoehleKDobsonNVaraniG
2004
Structural mimicry of retroviral tat proteins by constrained
beta-hairpin peptidomimetics: ligands with high affinity and selectivity for
viral TAR RNA regulatory elements.
J Am Chem Soc
126
6906
6913
25. AthanassiouZPatoraKDiasRLMoehleKRobinsonJA
2007
Structure-guided peptidomimetic design leads to nanomolar
beta-hairpin inhibitors of the Tat-TAR interaction of bovine
immunodeficiency virus.
Biochemistry
46
741
751
26. DavidsonALeeperTCAthanassiouZPatora-KomisarskaKKarnJ
2009
Simultaneous recognition of HIV-1 TAR RNA bulge and loop
sequences by cyclic peptide mimics of Tat protein.
Proc Natl Acad Sci U S A
106
11931
11936
27. LeeperTCAthanassiouZDiasRLRobinsonJAVaraniG
2005
TAR RNA recognition by a cyclic peptidomimetic of Tat
protein.
Biochemistry
44
12362
12372
28. PuglisiJDChenLBlanchardSFrankelAD
1995
Solution structure of a bovine immunodeficiency virus Tat-TAR
peptide-RNA complex.
Science
270
1200
1203
29. YeXKumarRAPatelDJ
1995
Molecular recognition in the bovine immunodeficiency virus Tat
peptide-TAR RNA complex.
Chem Biol
2
827
840
30. DudleyDMWentzelJLLalondeMSVeazeyRSArtsEJ
2009
Selection of a simian-human immunodeficiency virus strain
resistant to a vaginal microbicide in macaques.
J Virol
83
5067
5076
31. WildCOasTMcDanalCBolognesiDMatthewsT
1992
A synthetic peptide inhibitor of human immunodeficiency virus
replication: correlation between solution structure and viral
inhibition.
Proc Natl Acad Sci U S A
89
10537
10541
32. RichardJPMelikovKVivesERamosCVerbeureB
2003
Cell-penetrating peptides. A reevaluation of the mechanism of
cellular uptake.
J Biol Chem
278
585
590
33. MannDAFrankelAD
1991
Endocytosis and targeting of exogenous HIV-1 Tat
protein.
EMBO J
10
1733
1739
34. ZieglerANerviPDurrenbergerMSeeligJ
2005
The cationic cell-penetrating peptide CPP(TAT) derived from the
HIV-1 protein TAT is rapidly transported into living fibroblasts: optical,
biophysical, and metabolic evidence.
Biochemistry
44
138
148
35. TakeuchiTKosugeMTadokoroASugiuraYNishiM
2006
Direct and rapid cytosolic delivery using cell-penetrating
peptides mediated by pyrenebutyrate.
ACS Chem Biol
1
299
303
36. MacaraIG
2001
Transport into and out of the nucleus.
Microbiol Mol Biol Rev
65
570
94, table
37. ScottMSBoisvertFMMcDowallMDLamondAIBartonGJ
2010
Characterization and prediction of protein nucleolar localization
sequences.
Nucleic Acids Res
38
7388
7399
38. ArienKKVanhamGArtsEJ
2007
Is HIV-1 evolving to a less virulent form in
humans?
Nat Rev Microbiol
5
141
151
39. BourgeoisCFKimYKChurcherMJWestMJKarnJ
2002
Spt5 cooperates with human immunodeficiency virus type 1 Tat by
preventing premature RNA release at terminator sequences.
Mol Cell Biol
22
1079
1093
40. GraebleMAChurcherMJLoweADGaitMJKarnJ
1993
Human immunodeficiency virus type 1 transactivator protein, tat,
stimulates transcriptional read-through of distal terminator sequences in
vitro.
Proc Natl Acad Sci U S A
90
6184
6188
41. WeberJWeberovaJCarobeneMMirzaMMartinez-PicadoJ
2006
Use of a novel assay based on intact recombinant viruses
expressing green (EGFP) or red (DsRed2) fluorescent proteins to examine the
contribution of pol and env genes to overall HIV-1 replicative
fitness.
J Virol Methods
136
102
117
42. LassenKGLobritzMABaileyJRJohnstonSNguyenS
2009
Elite suppressor-derived HIV-1 envelope glycoproteins exhibit
reduced entry efficiency and kinetics.
PLoS Pathog
5
e1000377
43. ManceboHSLeeGFlygareJTomassiniJLuuP
1997
P-TEFb kinase is required for HIV Tat transcriptional activation
in vivo and in vitro.
Genes Dev
11
2633
2644
44. WestMJLoweADKarnJ
2001
Activation of human immunodeficiency virus transcription in T
cells revisited: NF-kappaB p65 stimulates transcriptional
elongation.
J Virol
75
8524
8537
45. DudleyDMGaoYNelsonKNHenryKRNankyaI
2009
A novel yeast-based recombination method to clone and propagate
diverse HIV-1 isolates.
Biotechniques
46
458
467
46. FisherRABertonisJMMeierWJohnsonVACostopoulosDS
1988
HIV infection is blocked in vitro by recombinant soluble
CD4.
Nature
331
76
78
47. PugachPMarozsanAJKetasTJLandesELMooreJP
2007
HIV-1 clones resistant to a small molecule CCR5 inhibitor use the
inhibitor-bound form of CCR5 for entry.
Virology
361
212
228
48. de ClercqE
2010
In search of a selective therapy of viral
infections.
Antiviral Res
85
19
24
49. ArtsEJLiXGuZKleimanLParniakMA
1994
Comparison of deoxyoligonucleotide and tRNA(Lys-3) as primers in
an endogenous human immunodeficiency virus-1 in vitro reverse
transcription/template-switching reaction.
J Biol Chem
269
14672
14680
50. RennerSLudwigVBodenOSchefferUGobelM
2005
New inhibitors of the Tat-TAR RNA interaction found with a
“fuzzy” pharmacophore model.
Chembiochem
6
1119
1125
51. HeMYuanDLinWPangRYuX
2005
Synthesis and assay of isoquinoline derivatives as HIV-1 Tat-TAR
interaction inhibitors.
Bioorg Med Chem Lett
15
3978
3981
52. MeiHYCuiMHeldsingerALemrowSMLooJA
1998
Inhibitors of protein-RNA complexation that target the RNA:
specific recognition of human immunodeficiency virus type 1 TAR RNA by small
organic molecules.
Biochemistry
37
14204
14212
53. BranchAD
1998
A good antisense molecule is hard to find.
Trends Biochem Sci
23
45
50
54. JacqueJMTriquesKStevensonM
2002
Modulation of HIV-1 replication by RNA
interference.
Nature
418
435
438
55. TamilarasuNHuqIRanaTM
2001
Targeting RNA with peptidomimetic oligomers in human
cells.
Bioorg Med Chem Lett
11
505
507
56. Sumner-SmithMZhengYZhangYPTwistEMClimieSC
1995
Antiherpetic activities of N-alpha-acetyl-nona-D-arginine amide
acetate.
Drugs Exp Clin Res
21
1
6
57. ApolloniAMeredithLWSuhrbierAKiernanRHarrichD
2007
The HIV-1 Tat protein stimulates reverse transcription in
vitro.
Curr HIV Res
5
473
483
58. HarrichDUlichCGarcia-MartinezLFGaynorRB
1997
Tat is required for efficient HIV-1 reverse
transcription.
EMBO J
16
1224
1235
59. HarrichDUlichCGaynorRB
1996
A critical role for the TAR element in promoting efficient human
immunodeficiency virus type 1 reverse transcription.
J Virol
70
4017
4027
60. BoudierCStorchakRSharmaKKDidierPFollenius-WundA
2010
The mechanism of HIV-1 Tat-directed nucleic acid annealing
supports its role in reverse transcription.
J Mol Biol
400
487
501
61. ZengYLiuHWLandesCFKimYJMaX
2007
Probing nucleation, reverse annealing, and chaperone function
along the reaction path of HIV-1 single-strand transfer.
Proc Natl Acad Sci U S A
104
12651
12656
62. BerkhoutBVastenhouwNLKlasensBIHuthoffH
2001
Structural features in the HIV-1 repeat region facilitate strand
transfer during reverse transcription.
RNA
7
1097
1114
63. DriscollMDHughesSH
2000
Human immunodeficiency virus type 1 nucleocapsid protein can
prevent self-priming of minus-strand strong stop DNA by promoting the
annealing of short oligonucleotides to hairpin sequences.
J Virol
74
8785
8792
64. GuoJHendersonLEBessJKaneBLevinJG
1997
Human immunodeficiency virus type 1 nucleocapsid protein promotes
efficient strand transfer and specific viral DNA synthesis by inhibiting
TAR-dependent self-priming from minus-strand strong-stop
DNA.
J Virol
71
5178
5188
65. IselCWesthofEMassireCLe GriceSFEhresmannB
1999
Structural basis for the specificity of the initiation of HIV-1
reverse transcription.
EMBO J
18
1038
1048
66. BaudinFMarquetRIselCDarlixJLEhresmannB
1993
Functional sites in the 5′ region of human immunodeficiency
virus type 1 RNA form defined structural domains.
J Mol Biol
229
382
397
67. PanCMezeiMMujtabaSMullerMZengL
2007
Structure-guided optimization of small molecules inhibiting human
immunodeficiency virus 1 Tat association with the human coactivator
p300/CREB binding protein-associated factor.
J Med Chem
50
2285
2288
68. Heilman-MillerSLWuTLevinJG
2004
Alteration of nucleic acid structure and stability modulates the
efficiency of minus-strand transfer mediated by the HIV-1 nucleocapsid
protein.
J Biol Chem
279
44154
44165
69. MeredithLWSivakumaranHMajorLSuhrbierAHarrichD
2009
Potent inhibition of HIV-1 replication by a Tat
mutant.
PLoS One
4
e7769
70. DasATHarwigAVrolijkMMBerkhoutB
2007
The TAR hairpin of human immunodeficiency virus type 1 can be
deleted when not required for Tat-mediated activation of
transcription.
J Virol
81
7742
7748
71. ChurcherMJLamontCHamyFDingwallCGreenSM
1993
High affinity binding of TAR RNA by the human immunodeficiency
virus type-1 tat protein requires base-pairs in the RNA stem and amino acid
residues flanking the basic region.
J Mol Biol
230
90
110
72. KeenNJChurcherMJKarnJ
1997
Transfer of Tat and release of TAR RNA during the activation of
the human immunodeficiency virus type-1 transcription elongation
complex.
EMBO J
16
5260
5272
73. AdachiAGendelmanHEKoenigSFolksTWilleyR
1986
Production of acquired immunodeficiency syndrome-associated
retrovirus in human and nonhuman cells transfected with an infectious
molecular clone.
J Virol
59
284
291
74. MarozsanAJTorreVSJohnsonMBallSCCrossJV
2001
Mechanisms involved in stimulation of human immunodeficiency
virus type 1 replication by aminooxypentane RANTES.
J Virol
75
8624
8638
75. HopeTJHuangXJMcDonaldDParslowTG
1990
Steroid-receptor fusion of the human immunodeficiency virus type
1 Rev transactivator: mapping cryptic functions of the arginine-rich
motif.
Proc Natl Acad Sci U S A
87
7787
7791
76. PageKALandauNRLittmanDR
1990
Construction and use of a human immunodeficiency virus vector for
analysis of virus infectivity.
J Virol
64
5270
5276
77. TorreVSMarozsanAJAlbrightJLCollinsKRHartleyO
2000
Variable sensitivity of CCR5-tropic human immunodeficiency virus
type 1 isolates to inhibition by RANTES analogs.
J Virol
74
4868
4876
78. ArtsEJStetorSRLiXRauschJWHowardKJ
1996
Initiation of (-) strand DNA synthesis from tRNA(3Lys) on
lentiviral RNAs: implications of specific HIV-1 RNA-tRNA(3Lys) interactions
inhibiting primer utilization by retroviral reverse
transcriptases.
Proc Natl Acad Sci U S A
93
10063
10068
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Distribution of the Phenotypic Effects of Random Homologous Recombination between Two Virus SpeciesČlánek SIV Nef Proteins Recruit the AP-2 Complex to Antagonize Tetherin and Facilitate Virion ReleaseČlánek Dual Function of the NK Cell Receptor 2B4 (CD244) in the Regulation of HCV-Specific CD8+ T CellsČlánek A Large and Intact Viral Particle Penetrates the Endoplasmic Reticulum Membrane to Reach the CytosolČlánek Interleukin-13 Promotes Susceptibility to Chlamydial Infection of the Respiratory and Genital Tracts
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Lymphoadenopathy during Lyme Borreliosis Is Caused by Spirochete Migration-Induced Specific B Cell Activation
- Infections Are Virulent and Inhibit the Human Malaria Parasite in
- A Gamma Interferon Independent Mechanism of CD4 T Cell Mediated Control of Infection
- MDA5 and TLR3 Initiate Pro-Inflammatory Signaling Pathways Leading to Rhinovirus-Induced Airways Inflammation and Hyperresponsiveness
- The OXI1 Kinase Pathway Mediates -Induced Growth Promotion in Arabidopsis
- An E2F1-Mediated DNA Damage Response Contributes to the Replication of Human Cytomegalovirus
- Quantitative Subcellular Proteome and Secretome Profiling of Influenza A Virus-Infected Human Primary Macrophages
- Distribution of the Phenotypic Effects of Random Homologous Recombination between Two Virus Species
- Inhibition of Both HIV-1 Reverse Transcription and Gene Expression by a Cyclic Peptide that Binds the Tat-Transactivating Response Element (TAR) RNA
- A Viral Satellite RNA Induces Yellow Symptoms on Tobacco by Targeting a Gene Involved in Chlorophyll Biosynthesis using the RNA Silencing Machinery
- Misregulation of Underlies the Developmental Abnormalities Caused by Three Distinct Viral Silencing Suppressors in Arabidopsis
- Investigating the Host Binding Signature on the PfEMP1 Protein Family
- Human Neutrophil Clearance of Bacterial Pathogens Triggers Anti-Microbial γδ T Cell Responses in Early Infection
- Septation of Infectious Hyphae Is Critical for Appressoria Formation and Virulence in the Smut Fungus
- A Family of Helminth Molecules that Modulate Innate Cell Responses via Molecular Mimicry of Host Antimicrobial Peptides
- Phospholipids Trigger Capsular Enlargement during Interactions with Amoebae and Macrophages
- CTL Escape Mediated by Proteasomal Destruction of an HIV-1 Cryptic Epitope
- Evolution of Th2 Immunity: A Rapid Repair Response to Tissue Destructive Pathogens
- Extensive Genome-Wide Variability of Human Cytomegalovirus in Congenitally Infected Infants
- The Antiviral Efficacy of HIV-Specific CD8 T-Cells to a Conserved Epitope Is Heavily Dependent on the Infecting HIV-1 Isolate
- Epstein-Barr Virus Infection of Polarized Epithelial Cells via the Basolateral Surface by Memory B Cell-Mediated Transfer Infection
- Reactive Oxygen Species Hydrogen Peroxide Mediates Kaposi's Sarcoma-Associated Herpesvirus Reactivation from Latency
- Crystal Structure and Functional Analysis of the SARS-Coronavirus RNA Cap 2′-O-Methyltransferase nsp10/nsp16 Complex
- The Dot/Icm System Delivers a Unique Repertoire of Type IV Effectors into Host Cells and Is Required for Intracellular Replication
- AAV Exploits Subcellular Stress Associated with Inflammation, Endoplasmic Reticulum Expansion, and Misfolded Proteins in Models of Cystic Fibrosis
- Suboptimal Activation of Antigen-Specific CD4 Effector Cells Enables Persistence of In Vivo
- SIV Nef Proteins Recruit the AP-2 Complex to Antagonize Tetherin and Facilitate Virion Release
- Dual Function of the NK Cell Receptor 2B4 (CD244) in the Regulation of HCV-Specific CD8+ T Cells
- Transition of Sporozoites into Liver Stage-Like Forms Is Regulated by the RNA Binding Protein Pumilio
- A Large and Intact Viral Particle Penetrates the Endoplasmic Reticulum Membrane to Reach the Cytosol
- Transcriptome Analysis of in Human Whole Blood and Mutagenesis Studies Identify Virulence Factors Involved in Blood Survival
- Interleukin-13 Promotes Susceptibility to Chlamydial Infection of the Respiratory and Genital Tracts
- Structural Insights into Viral Determinants of Nematode Mediated Transmission
- Protective Efficacy of Serially Up-Ranked Subdominant CD8 T Cell Epitopes against Virus Challenges
- Viral CTL Escape Mutants Are Generated in Lymph Nodes and Subsequently Become Fixed in Plasma and Rectal Mucosa during Acute SIV Infection of Macaques
- Taking Some of the Mystery out of Host∶Virus Interactions
- Viral Small Interfering RNAs Target Host Genes to Mediate Disease Symptoms in Plants
- : An Emerging Cause of Sexually Transmitted Disease in Women
- Mitochondrial Ubiquitin Ligase MARCH5 Promotes TLR7 Signaling by Attenuating TANK Action
- The Hexamer Structure of the Rift Valley Fever Virus Nucleoprotein Suggests a Mechanism for its Assembly into Ribonucleoprotein Complexes
- Acquisition of Human-Type Receptor Binding Specificity by New H5N1 Influenza Virus Sublineages during Their Emergence in Birds in Egypt
- Stromal Down-Regulation of Macrophage CD4/CCR5 Expression and NF-κB Activation Mediates HIV-1 Non-Permissiveness in Intestinal Macrophages
- A Component of the Xanthomonadaceae Type IV Secretion System Combines a VirB7 Motif with a N0 Domain Found in Outer Membrane Transport Proteins
- Perturbs Iron Trafficking in the Epithelium to Grow on the Cell Surface
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Crystal Structure and Functional Analysis of the SARS-Coronavirus RNA Cap 2′-O-Methyltransferase nsp10/nsp16 Complex
- Lymphoadenopathy during Lyme Borreliosis Is Caused by Spirochete Migration-Induced Specific B Cell Activation
- The OXI1 Kinase Pathway Mediates -Induced Growth Promotion in Arabidopsis
- : An Emerging Cause of Sexually Transmitted Disease in Women
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy