-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The M3 Muscarinic Receptor Is Required for Optimal Adaptive Immunity to Helminth and Bacterial Infection
Recent data indicate that acetylcholine (ACh), a neurotransmitter which regulates a variety of physiological functions, also influences the immune system, and that lymphocytes have the capacity to synthesise and release ACh, controlling local innate immune responses and suppressing inflammation. Thus far however there has been little evidence to suggest that ACh influences adaptive immunity, characterised by activation and effector functions of lymphocytes. We show here that during the immune response to two different pathogens, ACh signals through muscarinic receptors, and the M3 receptor subtype specifically, resulting in enhanced activation and cytokine production by ‘helper’ T lymphocytes which protect the host against infection.
Published in the journal: The M3 Muscarinic Receptor Is Required for Optimal Adaptive Immunity to Helminth and Bacterial Infection. PLoS Pathog 11(1): e32767. doi:10.1371/journal.ppat.1004636
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004636Summary
Recent data indicate that acetylcholine (ACh), a neurotransmitter which regulates a variety of physiological functions, also influences the immune system, and that lymphocytes have the capacity to synthesise and release ACh, controlling local innate immune responses and suppressing inflammation. Thus far however there has been little evidence to suggest that ACh influences adaptive immunity, characterised by activation and effector functions of lymphocytes. We show here that during the immune response to two different pathogens, ACh signals through muscarinic receptors, and the M3 receptor subtype specifically, resulting in enhanced activation and cytokine production by ‘helper’ T lymphocytes which protect the host against infection.
Introduction
The role of acetylcholine (ACh) as a neurotransmitter is well established, both in the central nervous system and the periphery, where it regulates smooth muscle contraction and many other functions of the autonomic nervous system. Cholinergic signalling also influences the immune system, most notably in the cholinergic anti-inflammatory pathway, which results in the α7 nicotinic receptor subunit-dependent inhibition of macrophage TNF-α, IL-1β and IL-6 production [1,2]. The influence of cholinergic signalling on adaptive immunity however is largely unexplored, although there is evidence that nicotinic receptors influence B lymphocyte development and activation [3]. Expression of both nicotinic receptors (nAChRs) and muscarinic receptors (mAChRs) is affected by CD4 T cell activation in vitro [4], and mAChRs influence differentiation of CD8 T cells in vivo [5]. To our knowledge, nothing is known about the role of mAChRs in the adaptive response to infection.
Nippostrongylus brasiliensis is a common laboratory pathogen used to study T helper 2 immune response-mediated disease resolution, and biologically closely resembles the important human hookworms Ancylostoma duodenale and Necator americanus [6]. The Th2 response drives resolution of infection, and IL-13 signalling through IL-4Rα is an important component of the protective response [7]. This signalling pathway also enhances smooth muscle contractility, which is thought to contribute to parasite expulsion [8,9]. Previous studies in our laboratory showed delayed parasite expulsion in mice with smooth muscle cells deficient in IL-4Rα. Associated with this defect was reduced Th2 cytokine production, delayed goblet cell hyperplasia and lower expression of the M3 muscarinic receptor (M3R) in the intestine [10]. The mAChR family consists of five subtypes (M1-M5) of G protein-coupled receptors [11], which regulate a range of physiological activities including heart rate, smooth muscle contractility, and endocrine and exocrine gland secretion [12–14]. The M3R is the major mAChR expressed on smooth muscle, and drives contractile responses in the ileum [15]. Our previous investigation determined that upregulation of M3R expression induced by N. brasiliensis infection is related to IL-4Rα, sensitive to host immunity, and may therefore also contribute to the immune response [10].
In this study, we investigated the contribution of signalling through the M3R to protective immunity against N. brasiliensis, using both infection of M3R gene deficient mice (M3R−/− mice) and ex vivo CD4 T cell assays. M3R deficiency significantly abrogated the ability of BALB/c mice to launch an effective adaptive immune response to primary and secondary infection, and underlying this defect were reduced CD4 T cell-associated protective cytokine responses. Stimulation of CD4 T cells from N. brasiliensis-infected wild-type (WT) control mice with ACh and muscarinic agonists enhanced their secretion of Th2 cytokines, and this effect could be blocked by muscarinic and M3R-selective antagonists. A similar impairment in immunity was found in M3R−/− mice following systemic infection with Salmonella typhimurium. Immunity to S. typhimurium is dependent on a robust Th1 immune response [16], with production of IFN-γ by CD4 T cells critical for host protection and bacterial clearance [17]. In the absence of M3R expression, strikingly higher bacterial loads were observed, which again correlated with impaired CD4 T cell cytokine responses. Ex vivo stimulation of lymphocytes from S. typhimurium-infected WT mice with ACh and muscarinic agonists enhanced IFN-γ secretion, and this effect was blocked with an M3R-selective antagonist. Collectively, these results show for the first time that the magnitude and efficacy of the adaptive response to infection depends on cholinergic signalling via the M3R.
Results
M3R expression does not influence immune cell populations or the ability to polarise to Th1 and Th2 subsets in uninfected mice
To determine if expression of the M3R inherently influenced steady-state immune cell populations, we analysed the composition of leukocytes in secondary lymphoid tissues. There was no significant difference in the relative proportions of B cells, CD4 and CD8 T cells, dendritic cells or macrophages in spleens from naïve WT and M3R−/− mice (Fig. 1A), and equivalent proportions of B cells, CD4 and CD8 T cells were also recorded in the mesenteric lymph nodes (MLN) (Fig. 1A). M3R expression also did not appear to affect T cell activation status: WT and M3R−/− mice had equal proportions of naïve (CD3+CD4+CD44loCD62Lhi) and activated (CD3+CD4+CD44hiCD62Llo) CD4 T cells in spleen and MLN (Fig. 1B). Furthermore, in vitro differentiation of CD4 T cells into Th1 populations by addition of IFN-γ and anti-IL-4 antibody, or Th2 populations via IL-4 and anti-IFN-γ antibody, resulted in equivalent cytokine responses in WT and M3R−/− mice (Fig. 1C). However, anti-CD3 or PMA/ionomycin stimulation of either whole MLN cell suspensions or sorted CD4 T cells for 24 hours demonstrated an impaired ability of M3R−/ − CD4+ T cells to express markers of activation when compared to WT CD4+ cells (Fig. 1D). This finding shows that an absence of M3R expression in CD4 T cells results in an impaired ability to respond to a non-specific activating stimulus.
Fig. 1. Naïve M3R−/− mice have an equivalent composition of immune cells as WT BALB/c mice but M3R−/− CD4 T cells have an impaired ability to activate. 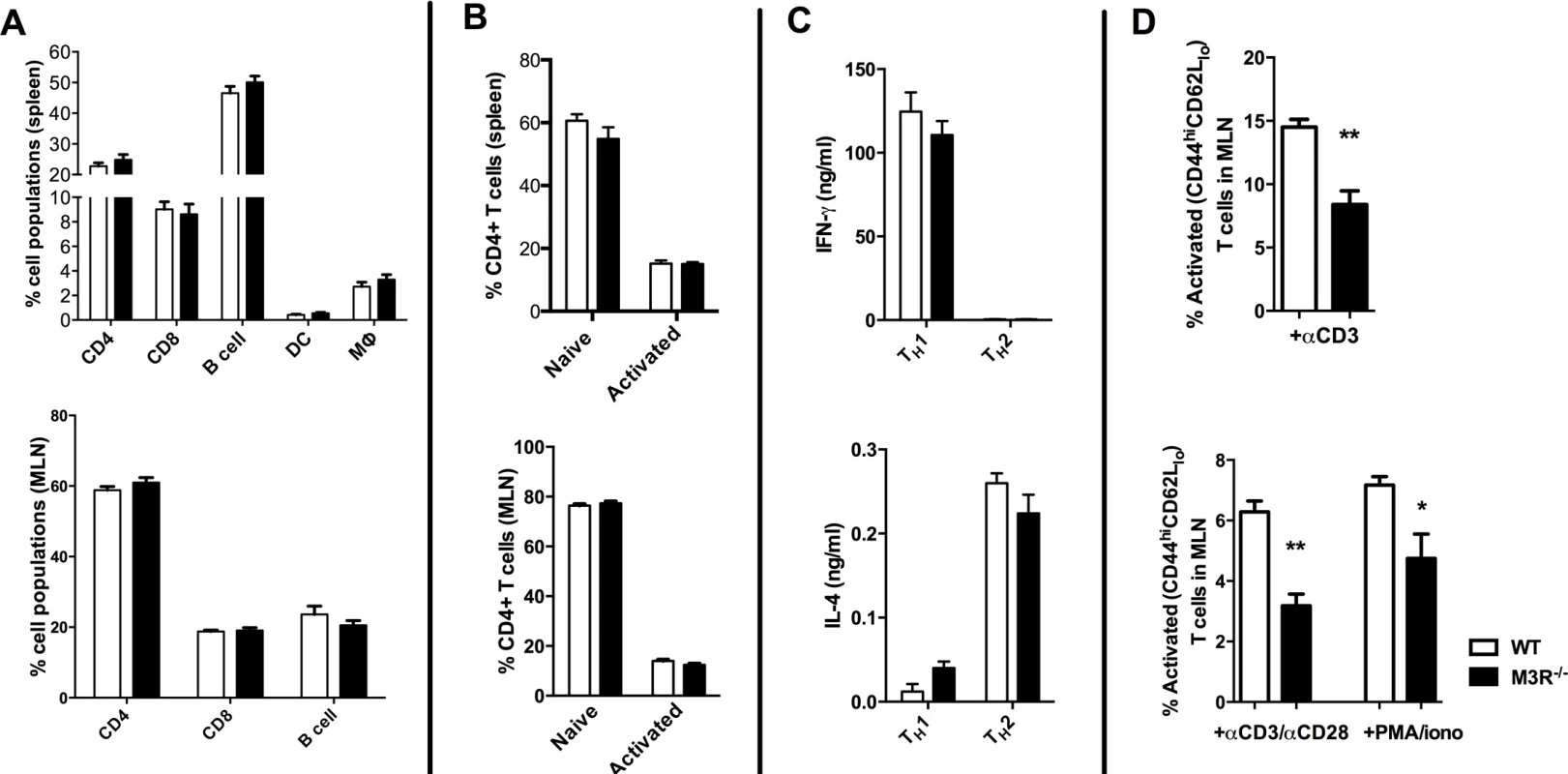
(A) Proportions of splenic immune cell populations (CD4, CD8, B cell, dendritic cell and macrophages) and MLN lymphoid populations (CD4, CD8 and B cells) in naïve mice. (B) Activation status (CD44 and CD62L) of splenic and MLN CD4 T cells in naïve animals. (C) The ability of naïve cells to be polarised ex vivo by priming to Th1 and Th2 phenotypes. (D) Ability of naïve CD4 T cells in whole MLN and CD4 only cell preparations to express activation markers in response to stimulation with anti-CD3 or PMA/ionomycin. White bar: WT BALB/c mice; black bar: M3R−/− mice. Data are shown as mean± SEM, n = 6; Representative of 2 separate experiments. M3R expression is required for optimal immunity to N. brasiliensis
We determined whether the M3R contributed to CD4+ T cell immune responses in an N. brasiliensis infection. Primary infection with N. brasiliensis was self-resolving in WT BALB/c control mice, with parasite clearance from the small intestine by day 9 post-infection (p.i.). M3R−/− mice exhibited delayed expulsion of worms, with significantly higher recovery of parasites from the intestine at days 7 and 9 p.i. (Fig. 2A). M3R−/− mice have previously been shown to be refractory to ACh-induced smooth muscle contraction [15], and intestinal smooth muscle hypercontraction is hypothesised to be a potential effector mechanism for expulsion of nematode parasites [9]. We observed increased ACh-driven smooth muscle contraction compared to naïve animals in infected WT mice but not infected M3R−/− mice (Fig. 2B), suggesting that M3R-mediated smooth muscle hypercontractility may indeed contribute to parasite expulsion. Surprisingly, in addition to this altered physiological response, levels of mRNA for IL-13 were reduced 4-fold in mesenteric lymph node (MLN) cells of M3R−/− mice compared to those of WT controls at day 7 p.i. (Fig. 2C). This was associated with reduced numbers of IL-13+ CD4 T cells (Fig. 2C). Additionally, M3R−/− mice had reduced CD3+CD4+CD44hiCD62Llo effector memory (activated) T cells in the MLN when compared to WT mice (Fig. 2D). Elevation of intracellular calcium (Ca2+)i is an essential event which is necessary for CD4 T cell activation and cytokine production [18]. We tested whether CD4 T cells from N. brasiliensis-infected M3R−/− mice had a reduced ability to mobilise Ca2+i. This was the case; elevation of Ca2+i by the calcium ionophore ionomycin was significantly impaired in M3R−/− CD4 T cells when compared to WT CD4 T cells. No difference was observed between M3R−/− and WT CD4 T cells when extracellular calcium was chelated by EGTA, although the response was much lower, suggesting that lack of the M3R impaired uptake of extracellular calcium across the plasma membrane. These data indicate that M3R expression is required for optimal T cell activation and protective immunity to primary infection by N. brasiliensis.
Fig. 2. M3R deficient mice exhibit delayed clearance of a primary N. brasiliensis infection and impaired T cell-associated protective responses. 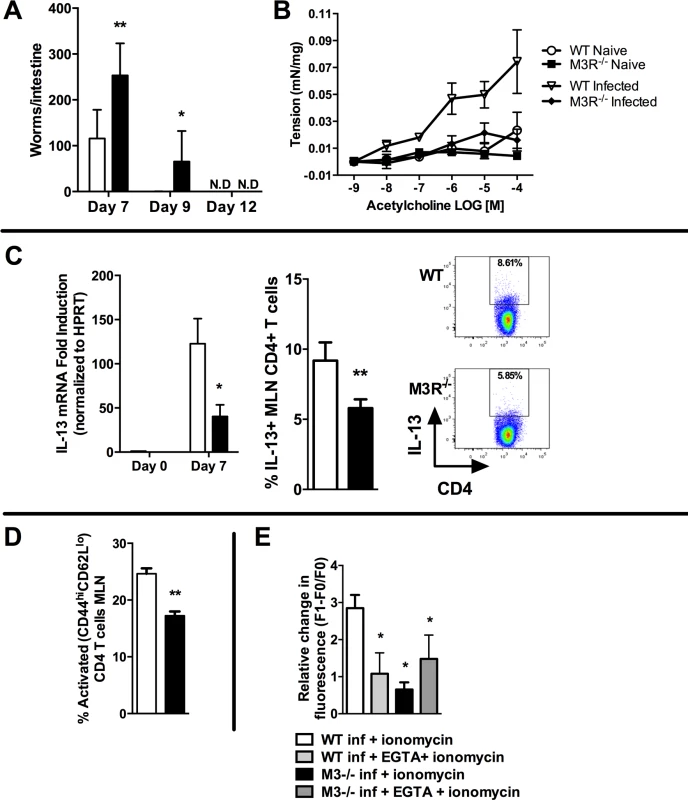
(A) Adult worm numbers in the small intestine were elevated in M3R−/− mice in comparison to WT BALB/c on day 7 and 9 p.i. (B) Hypercontractile responses of jejunum in response to varying doses of acetylcholine measured at day 9 p.i. were absent in M3R−/− mice. (C) Production of IL-13 by CD4 T cells is impaired in M3R−/− mice: IL-13 mRNA levels and proportion of IL-13+ CD4 T cells from the MLN at day 7 p.i. (D) Proportion of activated (CD44hi CD62Llo) CD4 T cells in MLN at day 7 p.i. (E) Ca2+ mobilisation in response to ionomycin in CD4 T cells in MLN at day 7 p.i. Data are shown as mean ± SEM, n = 4–6 and are representative of at least two independent experiments. Statistical significance was calculated using the Mann-Whitney two-tailed t test and denoted by * p<0.05, ** p<0.01. White bar: WT BALB/c mice; black bar: M3R−/− mice. Cholinergic stimulation of the M3R potentiates cytokine production in CD4 T cells
CD4 T cells isolated from the MLN of WT BALB/c mice 7 days p.i. with N. brasiliensis expressed mRNA for the M1, M3, M4 and M5 receptor subtypes (Fig. 3A). Cholinergic stimulation influenced several parameters of CD4 T cell function which are important in the immune response to infection. ACh enhanced expression of Ox40 on effector memory CD4 T cells either directly, or in cells stimulated with sub-maximal concentrations of anti-CD3 (Fig. 3B). When cells were treated with anti-CD3 in the presence or absence of ACh or the muscarinic agonists muscarine and oxotremorine-M (oxo-M) all agonists enhanced production of IL-4 and IL-13 approximately 2-fold, and potentiation of cytokine production by muscarine was demonstrated to be dose-dependent (Fig. 3C). Cholinergic enhancement of IL-13 secretion was blocked by the pan-specific muscarinic receptor antagonist atropine, and no enhancement was observed in cells from M3R−/− mice (Fig. 3D). Further confirmation that the cholinergic co-stimulatory signal acts through the M3R was provided by use of the M3R-selective antagonist J104129, which blocked potentiation of cytokine secretion by ACh (Fig. 3D).
Fig. 3. Signalling via the M3R potentiates Th2 cytokine production during N. brasiliensis infection. 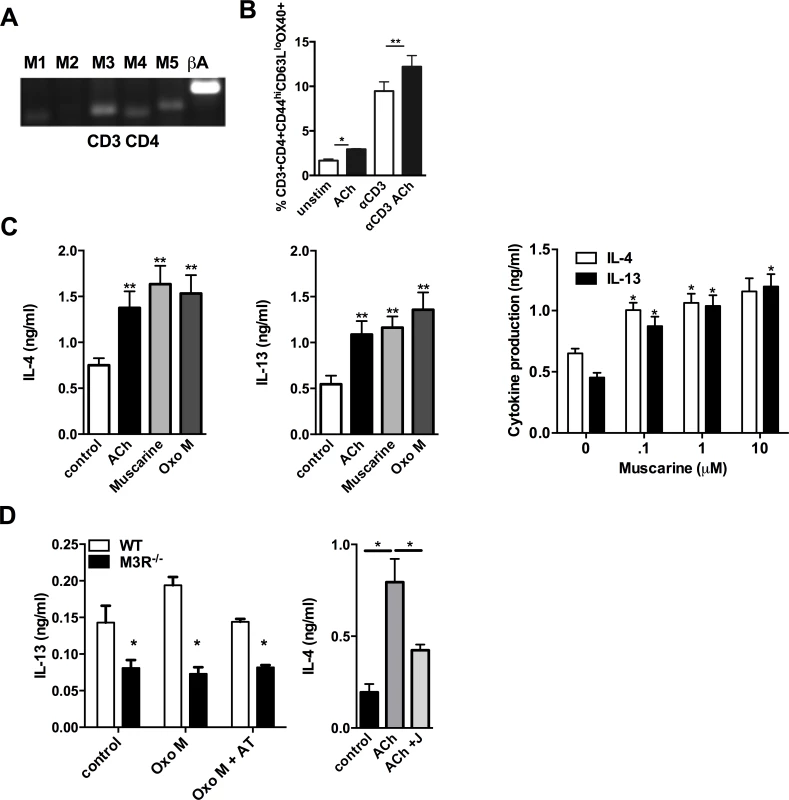
(A) Detection of mRNA for M1-M5 muscarinic receptors and β-actin (βA) in CD4 T cells isolated from MLN. (B) Ox40 expression on effector memory (CD44hiCD62Llo) subsets of CD4 T cells day 7 p.i. (C) Cytokine secretion by MLN cells day 7 p.i. after stimulation with sub-optimal anti-CD3 and muscarinic receptor agonists (10 μM, 48 hrs) and dose response to muscarine. Asterisks indicate significant differences between control and cholinergic stimulations. (D) IL-13 production from N. brasiliensis infected (day 7) WT and M3R−/− MLN cells after sub-optimal anti-CD3 stimulation plus agonists ACh and Oxotremorine M (Oxo M) at 10 μM and the muscarinic receptor antagonist atropine (AT, 100 μM) or the M3R-selective antagonist J104129 (J, 40 nM). Data are shown as mean ±SEM, n = 6 and are representative of at least two independent experiments. *p<0.05, **p<0.01 determined by the Mann-Whitney two-tailed t test. M3R receptor expression is required for effective recall immunity to N. brasiliensis
To test if cholinergic regulation of CD4 T cell cytokine responses influenced adaptive immunity, we next examined how this affected the outcome of secondary infection, as this requires a CD4 T cell-driven Th2 immune response in the lung [19,20]. Immunity to secondary infection was strikingly abrogated in M3R−/− mice, with higher numbers of larvae recovered from the lungs in comparison to WT controls (Fig. 4A). Levels of IL-13 and IL-4 in the lungs of M3R−/− mice were significantly decreased (Fig. 4B), and this was reflected by diminished numbers of IL-13+ and IL-4+ CD4 T cells (Fig. 4C), accompanied by reduced goblet cell hyperplasia and associated mucus production (Fig. 4D).
Fig. 4. M3R deficient mice have impaired memory immune responses during secondary infection with N. brasiliensis. 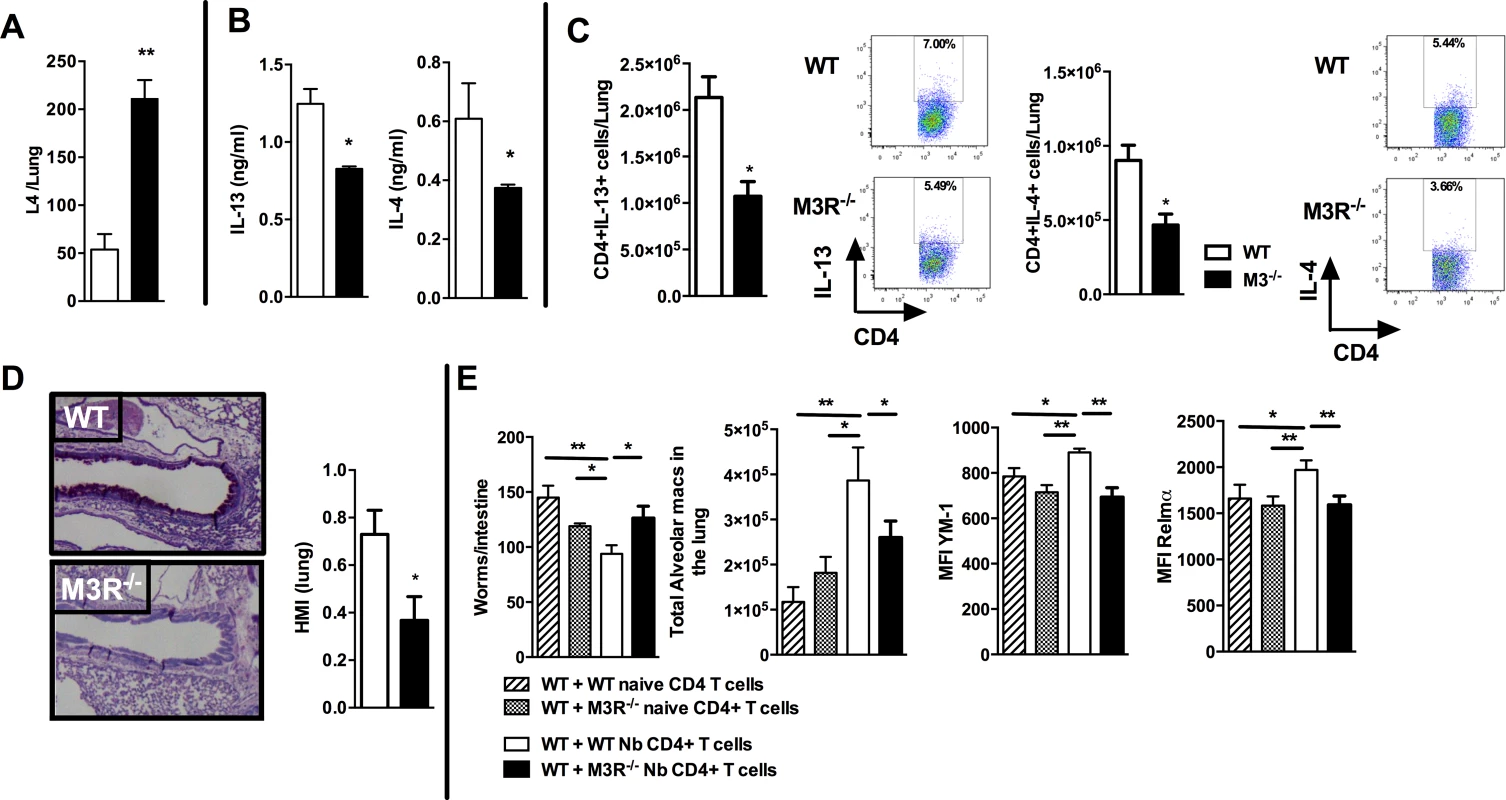
(A) Number of N. brasiliensis larvae recovered from the lungs at day 2 post-secondary infection. (B) Levels of IL-13 and IL-4 in total lung homogenates at day 2 p.i. (C) Intracellular IL-13 and IL-4 cytokine production in lung CD4 T cells at day 2 post secondary infection. (D) Goblet cells in the lungs visualised by Periodic Acid Schiff (PAS) staining and enumerated by Histological Mucus Index (HMI) at day 2 p.i. White bar = WT BALB/c mice, black bar = M3R−/− mice. (E) Recovery of adult worms from the intestines of WT BALB/c mice, alveolar macrophage numbers and expression of YM1 and RELMα after adoptive transfer of 5 × 105 CD4 cells from naïve WT mice, WT mice infected with N. brasiliensis, naïve M3R−/− mice and infected M3R−/− mice, and infection with N. brasiliensis thereafter. Data are shown as mean ± SEM, n = 6 and are representative of two independent experiments. Statistical significance was calculated using the Mann-Whitney two-tailed t test and denoted by *p<0.05, **p<0.01. Immunity to N. brasiliensis can be accelerated by adoptive transfer of CD4 T cells sensitised by primary infection into naïve mice [20]. We used this approach to test whether optimal adaptive immunity is dependent on signalling through the M3R on CD4 T cells. CD4 T cells from previously infected WT and M3R−/− mice were adoptively transferred i/v into naïve BALB/c mice and animals subsequently infected with N. brasiliensis. Recipients of WT CD4 T cells showed a significant reduction in the numbers of adult worms recovered from the small intestine at day 5 post infection, whereas recipients of M3R−/− CD4 T cells showed no reduction in worm burden (Fig. 4E). This defective ability to reduce worm burden in recipients of M3R−/− CD4 T cells was associated with an impaired induction of pulmonary alternatively activated macrophages (AAM) as demonstrated by reduced expression of RELMα and YM1 on alveolar macrophages (Fig. 4E). These data demonstrate that CD4 T cell responsiveness to cholinergic signalling via the M3R is essential for effective adaptive immunity to N. brasiliensis.
M3R expression is required for CD4-mediated Th1 protective immunity to S. typhimurium infection
Having established that cholinergic signalling via the M3R contributed directly to CD4 Th2-driven immunity to nematode infection, we next tested whether this influenced immunity in a Th1 setting. We therefore infected WT and M3R−/− mice with Salmonella typhimurium, control of which is dependent upon CD4 Th1 immune responses [16,17]. No difference in recovery of bacteria was observed early in infection (day 7), but at later time points M3R−/− mice harboured significantly higher bacterial loads in the spleen (Fig. 5A) and displayed significant weight loss compared to WT mice (Fig. 5A). This increased susceptibility to infection correlated with reduced IFN-γ secretion by splenocytes at days 18 and 27 p.i. (Fig. 5B). Underlying this reduction in the protective cytokine response was reduced antigen specific IFN-γ production by CD4 T cells as demonstrated by both real-time PCR and flow cytometry analysis (Fig. 5C). Additionally, fewer activated (CD4+CD44hiCD62Llo) T cells were found in M3R−/− mice in comparison to WT controls infected with S. typhimurium at day 18 p.i. (Fig. 5D).
Fig. 5. M3R deficient mice are more susceptible to S. typhimurium infection and exhibit impaired CD4 T cell responses. 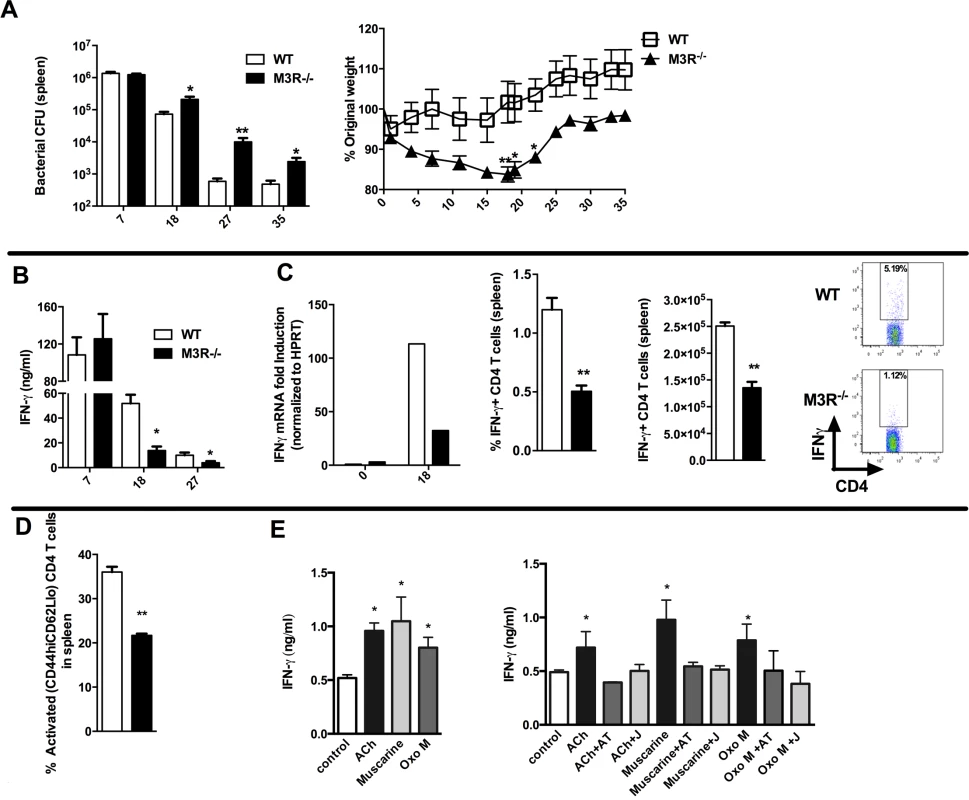
(A) Recovery of S. typhimurium from the spleens of infected mice at 7, 18, 27 and 35 days p.i. and weight loss at different days p.i. (B) IFN-γ secretion by antigen-restimulated splenocytes at different days p.i. (C) IFN-γ mRNA, proportion and number of IFN-γ+ CD4 splenic T cells assayed at day 18 p.i. (D) The proportion of activated CD4 T cells in spleens at day 18 p.i. (E) Secretion of IFN-γ by splenocytes stimulated with sub-optimal anti-CD3 after day 26 p.i. is enhanced by ACh and muscarinic agonists (10 μM). Enhancement of IFN-γ by ACh and muscarinic agonists is blocked by atropine (AT, 100 μM) and the M3R-selective antagonist J104129 (J, 40 nM). Data are shown as mean ± SEM, n = 5–6 and are representative of at least two independent experiments. Statistical significance was calculated using the Mann-Whitney two-tailed t test and denoted by *p<0.05, **p<0.01. Cholinergic signalling through the M3R augments IFN-γ production ex vivo in splenocytes from S. typhimurium infected mice
To confirm that cholinergic signalling influenced T cell cytokine responses during S. typhimurium infection, we carried out ex vivo restimulation experiments with splenocytes removed at day 26 p.i. Both ACh and muscarinic agonists enhanced production of IFN-γ in T cells stimulated sub-maximally with anti-CD3. Moreover, as with ex vivo stimulation of T cells from nematode-infected mice, the stimulatory effects were blocked by addition of atropine or the M3R-selective inhibitor J104127 (Fig. 5E). Collectively, these data indicate that cholinergic signalling via the M3R plays a common role in enhancing CD4 T cell activation and cytokine responses in both Th1 and Th2 settings.
Discussion
Understanding of the influence of cholinergic signalling on immune system function has thus far been largely restricted to innate immunity, principally focussed on dissection of the cholinergic anti-inflammatory pathway. The ultimate source of ACh in this reflex response is not neuronal, but a population of splenic noradrenaline-responsive CD4 T cells [21]. B cells also have the capacity of synthesizing and secreting ACh and, like T cells, this can regulate innate immunity, as B cell-derived ACh has been shown to inhibit neutrophil recruitment during sterile endotoxemia [22].
In contrast, little is known about cholinergic regulation of adaptive immunity, and the role of muscarinic receptors, despite numerous studies showing their expression on cells of the immune system [23]. A number of studies have observed that administration of muscarinic antagonists attenuates inflammation in vivo, suggesting a pro-inflammatory role for muscarinic receptors [24,25], although this conflicts with others which suggest that signalling through muscarinic receptors on vascular endothelium suppresses expression of cell adhesion molecules [22].
We demonstrate here that activation of the M3R is essential for optimal immune responses to two different pathogens; one which triggers Th2 cytokine production and another which triggers Th1 cytokines. In the case of primary infection with N. brasiliensis, absence of the M3R was associated with inhibition of smooth muscle contraction, reduced activation of CD4 T cells, lower IL-13 production and delayed expulsion of parasites from the small intestine. The impact of a lack of cholinergic signalling through the M3R was more evident during secondary infection, in which immunological memory in the lungs is particularly important [20] [26]. M3R deficient mice are highly susceptible to secondary infection, with a 4-fold higher recovery of parasites on day 2 p.i., lower levels of IL-4 and IL-13 in the lungs and reduced goblet cell hyperplasia in the airways. Mucus secretion by goblet cells is an important mediator of immunity to N. brasiliensis [27], and enhanced mucus production in the airways is correlated with reduced recovery of larvae following secondary infection [28]. Transfer of intact CD4 T cells from a prior infection confers protective pulmonary immunity to N. brasiliensis in naïve mice [20]. Using this approach we demonstrated that WT CD4 T cells from N. brasiliensis-infected mice were able to confer significant protection which was accompanied by expansion of AAMs in the lungs (which have recently been shown to be protective against lung stage N. brasiliensis infection [26]) of naive recipients, an effect which was not observed with CD4 T cells from M3R deficient mice.
The requirement of cholinergic signalling through the M3R for full CD4 T cell activation and cytokine production is not restricted to Th2 responses, but also extends to synthesis of IFN-γ and protection against bacterial infection. Our demonstration of an impaired response to the calcium ionophore ionomycin in M3R−/− mice (Fig. 2) may well underlie this common M3R-dependent effect on Th1 and Th2 driven immunity, as sustained elevation of Ca2+ is necessary for the action of NFAT which in turn is required for full cytokine transcription in T cells [18]. Muscarinic receptor signalling has been shown to cause the rapid release of Ca2+ from intracellular stores and a sustained influx of external Ca2+ in neuronal cells [29] as well as T and B cell lines [23]. Moreover, the use of antagonists suggested that this was most likely acting through the M3R in lymphocytes [30]. Our demonstration that M3R−/− CD4 T cells from N. brasiliensis-infected mice have an impaired ability to mobilize Ca2+ in response to ionomycin is indicative of a profound defect in cellular function.
No difference in recovery of S. typhimurium from the spleens of WT and M3R−/− mice was observed early in infection (day 7), when control is largely affected through innate myeloid cells [31]. However, eventual resolution of infection is dependent on CD4 T cell-coordinated responses [32], and higher bacterial loads in M3R−/− mice were associated with reduced IFN-γ from day 18 onwards (Fig. 5). Others have shown indirectly that cholinergic signalling can protect against bacterial infection. Thus, administration of paraoxon, a specific inhibitor of acetylcholinesterase, rendered mice more resistant to infection with S. typhimurium and enhanced production of IL-12. These protective effects were abrogated by co-administration of an oxime which reactivated acetylcholinesterase, implicating ACh as the agent driving potentiation of this response [33].
We show conclusively here that cholinergic signalling through the M3R is a major contributor to immunity to parasitic and bacterial infection, and that in its absence CD4 T cell responses are significantly impaired. Moreover, muscarinic agonists enhanced cytokine responses to both pathogens. The immunological defect in both scenarios may also be influenced by the lack of M3R expression on other cell types. However, the impaired immunity to S. typhimurium infection in M3R−/− mice was not related to altered macrophage function and was also independent of M3R influence on B cells [34]. Our data therefore suggest that M3R-dependent protection against infection may primarily be a function of altered CD4 T cell responsiveness. CD4 T cells also express the muscarinic receptors M1, M3 and M5. However, our experiments with M3R-deficient mice and use of specific antagonists strongly support the conclusion that cholinergic enhancement of cytokine responses by CD4 T cells is driven by the M3R. This is reinforced by pharmacological experiments which indicate that elevation of intracellular calcium in T cell lines stimulated with muscarinic agonists is most likely effected through the M3R [30].
The current study represents an important development in our understanding of cholinergic signalling in immunity, and may have implications for the use of functionally selective M3R antagonists such as tiotropium, widely used clinically as bronchodilators to treat chronic obstructive pulmonary disease [35] and more recently asthma [36]. Several studies have shown that tiotropium can inhibit allergen-induced eosinophilia and cytokine production [25], while others conclude that the M3R promotes allergen-induced tissue remodelling and goblet cell hyperplasia without directly affecting inflammation [37], perhaps indicative of varying effects in different animal models. As expression of the M3R has been demonstrated on other immune cell populations including macrophages and dendritic cells (DCs) [23], these cells may also respond directly to ACh. Studies of human DCs have shown that cholinergic stimulation in vitro enhanced expression of receptors involved in antigen presentation (CD86 and HLA-DR) and influenced mixed lymphocyte reactions to these agonists. Moreover, these effects could be blocked by atropine but not mecamylamine, suggesting that signalling was effected via muscarinic rather than nicotinic receptors. However, cholinergic stimulation of LPS-treated DCs led to contrasting effects; namely a reduction in antigen presentation. These findings indicate that complex muscarinic-dependent effects on myeloid cells exist which may differ depending on, for example, the maturation status of cells [38]. Furthermore, a study on human DC from nasal mucosa showed that addition of metacholine to DCs upregulated Ox40L, which can induce and interact with Ox40 on activated T cells [39]. Both these data and our demonstration that ACh upregulates expression of Ox40 on T cells provide a mechanism for cholinergic enhancement of T cell activation and lymphoid-myeloid cell interactions.
In summary, our data indicate that signalling through the M3R promotes CD4 T cell activation and cytokine production in two disparate murine models of infection, suggesting that there may be potential for the development of drugs aimed at enhancing immune function for immunoprophylaxis or other applications.
Materials and Methods
Ethics statement
All animal work was approved by the UCT Health Sciences Animal Ethics Committee (Project licence 012/054) to be in accordance with guidelines laid down by the South African Bureau of Standards. Research at Imperial College was approved and in accordance with regulations of the Home Office (PPL70/6957).
Mice
M3R−/− mice were backcrossed to BALB/c background for 10 generations for this study. Mice were bred under specific pathogen-free conditions and used aged 6–8 weeks. Protocols for all experiments were reviewed and approved by the UCT and ICL Animal Ethics committees.
Parasite and bacterial infection
For primary parasite infection, mice were infected subcutaneously with 500 N. brasiliensis infective larvae. To enumerate adult worms, mice were killed at various times post-infection (p.i.), intestines opened longitudinally, incubated in 10 ml saline for 3 hrs at 37°C and parasites counted under a dissecting microscope. For secondary infections, mice were treated at day 9 p.i. with ivermectin via drinking water to eliminate parasites, rested for 28 days and re-infected with 500 L3. Larvae were recovered from lungs by finely slicing the tissues, placing them in 5 ml saline for 3 hrs, and parasites subsequently enumerated.
Salmonella enterica serovar Typhimurium aroA− (SL3261) is an attenuated strain of STm SL1344 (44) and was maintained and used to infect mice intraperitoneally with an infectious dose of 5 × 105 CFU as described previously [40]. Tissue bacterial burdens were determined by direct culturing.
In vitro CD4 T cell differentiation into Th1 or Th2 phenotype
CD4 T cells were isolated from mesenteric lymph nodes of naïve mice using flow cytometry (>99% purity). Sorted CD4 T cells were plated at 1 × 105 cells/well on plates coated with 10 μg ml−1 anti-CD3 (BD Bioscience) and 5 μg ml−1 anti-CD28 (BD Bioscience). Th1 polarization conditions: 5 ng ml−1 rIL-12 (BD Bioscience) and 50 μg ml−1 anti-IL-4 (homemade Clone: 11B11); Th2 polarization: 50 ng ml−1 rIL-4 (BD Bioscience) and 50 μg ml−1 anti-IFN-γ. Cells were cultured in a final volume of 100 μl for 72 hrs, then transferred to fresh round-bottom 96 well plates and resuspended in appropriate antibody cocktails with the addition of 20 U ml−1 IL-2 (BD Bioscience) and cultured for another 48 hrs. Finally, cells were plated at 2 × 105 cells/well and incubated in 96 well plates coated with 20 μg ml−1 anti-CD3 (BD Bioscience). Supernatants were harvested after 48 hrs restimulation and used for ELISA.
Cytokine ELISA
Cytokine ELISAs were performed as previously described [41] using coating and biotinylated detection antibodies from R&D, with the exception of homemade coating antibodies for IL-4 (11B11) and IFN-γ (ANK18KL6). Streptavidin-conjugated HRP was used for detection with a commercially available substrate solution. MLN and lung cells were plated at 1 × 106 cells per well in 48 well plates pre-coated with 20 ug ml−1 anti-CD3 and restimulated for 72 hrs. Homogenates of lung and intestinal sections were prepared using a Polytron homogenizer and all samples standardized to 5 mg ml−1 protein prior to ELISA.
Calcium mobilisation assays
Changes in the cytosolic concentration of free Ca2+ were measured using the calcium indicator Fura-4-AM. Cellular suspensions of MLN were stained with anti-CD3 and anti-CD4 antibodies and then washed and resuspended to a concentration of 0.5 × 107 cells/ml in Ca2+ flux buffer (Hank’s balanced salt solution (HBSS) containing 1 mM CaCl2, 1 mM MgCl2, and 0.1% BSA), and labelled with 5 μM Fura-4-AM for 30 min at 37°C in the dark. Labelled cells were washed in Ca2+ flux buffer. Changes in Ca2+ in CD3+CD4+ cells following stimulation with 10 μM ionomycin in the presence or absence of 10 μM EGTA were monitored by flow cytometry.
Cholinergic stimulation
Cells were stimulated with 0.1 μg ml−1 (sub-optimal) anti-CD3, 10 μM ACh + 10 μM BW284C51, a specific inhibitor of acetylcholinesterases used to increase the half-life of acetylcholine (ACh), 10 μM Oxotremorine M (Oxo M), 10 μM muscarine or buffer controls for 48 hrs. The muscarinic receptor antagonist atropine (AT) was used at a concentration of 100 μM, and the M3R-selective antagonist J104129 [42] at a concentration of 40 nM. Supernatants were analysed for cytokines as described.
Measurement of intestinal contraction
Jejunum sections (1 cm) were obtained and hooked onto a force transducer, placed in PBS maintained at 37°C in an organ bath, and stimulated with ACh from 10−9 M to 10−3 M. In between stimulations, the intestinal segment was allowed to return to baseline contraction (at least 5 min). All measurements were recorded using the Powerlab acquisition unit and analysed using the Chart5 program. The amplitude was measured as the difference between the peak and trough of the contraction and reported in millinewtons (mN).
Flow cytometry
Single cell suspensions were prepared and 1 × 106 cells incubated in PBS + 0.1% BSA, 1% normal rat serum and appropriate antibody cocktails. Cell populations were determined and acquired on a BD FACS Fortessa (Becton Dickinson). Cell populations were identified by the following antibody staining strategies: CD4 T cells: CD3+CD4+; CD8 T cells: CD3+CD8+; B cells: CD19+B220+; Macrophages: CD11b+F4/80+; Dendritic cells: CD11c+. CD4 T cells populations were additionally stratified into naïve (CD44loCD62Lhi) and activated (CD44hiCD62Llo) T cell populations and stained for Ox40 (CD134). Alternatively activated macrophages were characterised by staining for YM1 and RELMα. Intra-cellular cytokine staining was carried on MLN, spleen or lung cells. Cells were re-suspended in complete media (IMDM (GIBCO/Invitrogen; Carlsbad, CA), 10% FCS, P/S) at 2.5×107/ml and stimulated with either10 μg/ml PMA/ionomycin or antigen and GolgiStop (as per manufacturer’s protocol; BD Pharmingen) at 37°C for 4 hours. After re-stimulation, cells were surface stained for CD3, CD4 then fixed and permeabilized with Cytofix/
Cytoperm Plus (as per manufacturer’s instructions; BD Pharmingen). Intracellular staining was performed by staining cells with either IL-13, IFN-γ or appropriately labeled isotype control. All analyses were performed with FlowJo software.
Adoptive transfer of CD4 T cells
CD4 T cells were purified from MLNs by positive selection using CD4 MACS beads (L3T4, MACS Miltenyi) according to the manufacturer’s protocol. Cells were further purified by flow cytometry to obtain purities above 95%, and 5 × 105 purified CD4 T cells from infected or naive animals transferred into naive WT or M3R−/− mice intravenously. Recipient mice were infected 24 hrs later with 500 L3 and killed 5 days post infection.
cDNA synthesis and RT-PCR
RNA was extracted using the Qiagen RNeasy Mini kit as per manufacturer’s protocols. RNA was converted to cDNA using random primers and Superscript II. The following primer pairs were used M1R: 5’-GGACAACAACACCAGAGGAGA-3’; 5’-GAGGTCACTTTAGGGTAGGG-3’; M2R: 5’-TGAAAACACGGTTTCCACTTC-3’, 5’-GATGGAGGAGGCTTCTTTTTG-3’; M3R: 5’-TTTACATGCCTGTCACCATCA-3’, 5’-ACAGCCACCATACTTCCTCCT-3’; M4R: 5’-TGCCTCTGTCATGAACCTTCT-3’, 5’-TGGTTATCAGGCACTGTCCTC-3’; M5R: 5’-CTCTGCTGGCAGTACTTGGTC-3’, 5’-GTGAGCCGGTTTTCTCTTCTT-3’; β-actin: 5’-TGGAATCCTGTGGCATCCATGAAAC-3’, 5’-TAAAACGCAGCTCAGTAACAGTCCG-3’; IL-13 : 5’-CTCCCTCTGACCCTTAAGGAG-3’; 5’-GAAGGGGCCGTGGCGAAACAG-3’
Histology
Lung and intestinal sections were fixed with 4% formalin in PBS solution, embedded in wax and cut into sections, then stained with Periodic Acid Schiff (PAS) stain to distinguish mucus-producing goblet cells. The histological mucus index (HMI) was used to quantify pulmonary goblet cell hyperplasia in individual mice, as described before (49).
Statistics
Values are expressed as mean ± standard deviation and significant differences were determined using either Mann-Whitney U test or ANOVA (GraphPad Prism4).
Zdroje
1. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, et al. (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421 : 384–388.
2. Tracey KJ (2009) Reflex control of immunity. Nat Rev Immunol 9 : 418–428. 19461672
3. Skok M, Grailhe R, Changeux JP (2005) Nicotinic receptors regulate B lymphocyte activation and immune response. Eur J Pharmacol 517 : 246–251. 15963492
4. Qian J, Galitovskiy V, Chernyavsky AI, Marchenko S, Grando SA (2011) Plasticity of the murine spleen T-cell cholinergic receptors and their role in in vitro differentiation of naive CD4 T cells toward the Th1, Th2 and Th17 lineages. Genes Immun 12 : 222–230. doi: 10.1038/gene.2010.72 21270829
5. Zimring JC, Kapp LM, Yamada M, Wess J, Kapp JA (2005) Regulation of CD8+ cytolytic T lymphocyte differentiation by a cholinergic pathway. J Neuroimmunol 164 : 66–75. 15913791
6. Gause WC, Urban JF Jr, Stadecker MJ (2003) The immune response to parasitic helminths: insights from murine models. Trends Immunol 24 : 269–277.
7. Akiho H, Lovato P, Deng Y, Ceponis PJ, Blennerhassett P, et al. (2005) Interleukin-4 - and -13-induced hypercontractility of human intestinal muscle cells-implication for motility changes in Crohn’s disease. Am J Physiol Gastrointest Liver Physiol 288: G609–615. 15528258
8. Akiho H, Deng Y, Blennerhassett P, Kanbayashi H, Collins SM (2005) Mechanisms underlying the maintenance of muscle hypercontractility in a model of postinfective gut dysfunction. Gastroenterology 129 : 131–141. 16012943
9. Zhao A, McDermott J, Urban JF Jr., Gause W, Madden KB, et al. (2003) Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol 171 : 948–954. 12847266
10. Horsnell WG, Cutler AJ, Hoving JC, Mearns H, Myburgh E, et al. (2007) Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Ralpha-deficient mice. PLoS Pathog 3: e1. 17222057
11. Felder CC (1995) Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB J 9 : 619–625. 7768353
12. Caulfield MP, Robbins J, Higashida H, Brown DA (1993) Postsynaptic actions of acetylcholine: the coupling of muscarinic receptor subtypes to neuronal ion channels. Prog Brain Res 98 : 293–301. 7504311
13. Eglen RM (2005) Muscarinic receptor subtype pharmacology and physiology. Prog Med Chem 43 : 105–136. 15850824
14. Wess J, Eglen RM, Gautam D (2007) Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov 6 : 721–733. 17762886
15. Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, et al. (2002) Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci 22 : 10627–10632. 12486155
16. Mittrucker HW, Kaufmann SH (2000) Immune response to infection with Salmonella typhimurium in mice. J Leukoc Biol 67 : 457–463.
17. Hess J, Ladel C, Miko D, Kaufmann SH (1996) Salmonella typhimurium aroA—infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol 156 : 3321–3326. 8617956
18. Guo L, Urban JF, Zhu J, Paul WE (2008) Elevating calcium in Th2 cells activates multiple pathways to induce IL-4 transcription and mRNA stabilization. J Immunol 181 : 3984–3993. 18768853
19. Ndlovu H, Darby M, Froelich M, Horsnell W, Luhder F, et al. (2014) Inducible Deletion of CD28 Prior to Secondary Nippostrongylus brasiliensis Infection Impairs Worm Expulsion and Recall of Protective Memory CD4(+) T Cell Responses. PLoS Pathog 10: e1003906. doi: 10.1371/journal.ppat.1003906 24516382
20. Thawer SG, Horsnell WG, Darby M, Hoving JC, Dewals B, et al. (2013) Lung-resident CD4 T cells are sufficient for IL-4Ralpha-dependent recall immunity to Nippostrongylus brasiliensis infection. Mucosal Immunol.
21. Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, et al. (2011) Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334 : 98–101. doi: 10.1126/science.1209985 21921156
22. Reardon C, Duncan GS, Brustle A, Brenner D, Tusche MW, et al. (2013) Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc Natl Acad Sci U S A 110 : 1410–1415. doi: 10.1073/pnas.1221655110 23297238
23. Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H (2007) Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci 80 : 2314–2319. 17383684
24. Razani-Boroujerdi S, Behl M, Hahn FF, Pena-Philippides JC, Hutt J, et al. (2008) Role of muscarinic receptors in the regulation of immune and inflammatory responses. J Neuroimmunol 194 : 83–88. 18190972
25. Bos IS, Gosens R, Zuidhof AB, Schaafsma D, Halayko AJ, et al. (2007) Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J 30 : 653–661. 17537779
26. Chen F, Wu W, Millman A, Craft JF, Chen E, et al. (2014) Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol 15 : 938–946. doi: 10.1038/ni.2984 25173346
27. Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, et al. (2011) Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med 208 : 893–900. doi: 10.1084/jem.20102057 21502330
28. Harvie M, Camberis M, Tang SC, Delahunt B, Paul W, et al. (2010) The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect Immun 78 : 3753–3762. doi: 10.1128/IAI.00502-09 20605978
29. Ebihara T, Guo F, Zhang L, Kim JY, Saffen D (2006) Muscarinic acetylcholine receptors stimulate Ca2+ influx in PC12D cells predominantly via activation of Ca2+ store-operated channels. J Biochem 139 : 449–458. 16567410
30. Fujii T, Kawashima K (2000) Calcium signaling and c-Fos gene expression via M3 muscarinic acetylcholine receptors in human T—and B-cells. Jpn J Pharmacol 84 : 124–132. 11128034
31. Wick MJ (2011) Innate immune control of Salmonella enterica serovar Typhimurium: mechanisms contributing to combating systemic Salmonella infection. J Innate Immun 3 : 543–549. doi: 10.1159/000330771 21912097
32. Buchan SL, Taraban VY, Slebioda TJ, James S, Cunningham AF, et al. (2012) Death receptor 3 is essential for generating optimal protective CD4(+) T-cell immunity against Salmonella. Eur J Immunol 42 : 580–588. 22259035
33. Fernandez-Cabezudo MJ, Lorke DE, Azimullah S, Mechkarska M, Hasan MY, et al. (2010) Cholinergic stimulation of the immune system protects against lethal infection by Salmonella enterica serovar Typhimurium. Immunology 130 : 388–398. doi: 10.1111/j.1365-2567.2009.03238.x 20408892
34. Vira A (2012) Role of M3 Muscarinic Receptor in Regulation of Immunity to Infectious Pathogens.: University of Cape Town.
35. Moulton BC, Fryer AD (2011) Muscarinic receptor antagonists, from folklore to pharmacology; finding drugs that actually work in asthma and COPD. Br J Pharmacol 163 : 44–52. doi: 10.1111/j.1476-5381.2010.01190.x 21198547
36. Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, et al. (2012) Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med 367 : 1198–1207. 22938706
37. Kistemaker LE, Bos ST, Mudde WM, Hylkema MN, Hiemstra PS, et al. (2014) Muscarinic M(3) receptors contribute to allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol 50 : 690–698. doi: 10.1165/rcmb.2013-0220OC 24156289
38. Salamone G, Lombardi G, Gori S, Nahmod K, Jancic C, et al. (2011) Cholinergic modulation of dendritic cell function. J Neuroimmunol 236 : 47–56. 21665296
39. Liu P, Yan J, Gong J, Wang C, Chen G (2011) Positive correlation between pregnancy-associated plasma protein-A level and OX40 ligand expression in patients with acute coronary syndromes. Biomed Pharmacother 65 : 193–197. doi: 10.1016/j.biopha.2010.10.011 21111564
40. Ross EA, Coughlan RE, Flores-Langarica A, Bobat S, Marshall JL, et al. (2011) CD31 is required on CD4+ T cells to promote T cell survival during Salmonella infection. J Immunol 187 : 1553–1565. doi: 10.4049/jimmunol.1000502 21734076
41. Horsnell WG, Vira A, Kirstein F, Mearns H, Hoving JC, et al. (2011) IL-4Ralpha-responsive smooth muscle cells contribute to initiation of TH2 immunity and pulmonary pathology in Nippostrongylus brasiliensis infections. Mucosal Immunol 4 : 83–92. doi: 10.1038/mi.2010.46 20737001
42. Mitsuya M, Mase T, Tsuchiya Y, Kawakami K, Hattori H, et al. (1999) J-104129, a novel muscarinic M3 receptor antagonist with high selectivity for M3 over M2 receptors. Bioorg Med Chem 7 : 2555–2567. 10632066
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and AdultsČlánek The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and HostČlánek Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for EntryČlánek Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 1- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- The Importance of Pathogen Load
- Implication of Gut Microbiota in Nonalcoholic Fatty Liver Disease
- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- Helminth-Induced Immune Regulation: Implications for Immune Responses to Tuberculosis
- The M3 Muscarinic Receptor Is Required for Optimal Adaptive Immunity to Helminth and Bacterial Infection
- An Iron-Mimicking, Trojan Horse-Entering Fungi—Has the Time Come for Molecular Imaging of Fungal Infections?
- Modulates the Unfolded Protein Response in during Infection
- Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and Adults
- Identification of HNRNPK as Regulator of Hepatitis C Virus Particle Production
- Parasite Biomass-Related Inflammation, Endothelial Activation, Microvascular Dysfunction and Disease Severity in Vivax Malaria
- : Trypanosomatids Adapted to Plant Environments
- Early Virus-Host Interactions Dictate the Course of a Persistent Infection
- TLR3 Signaling in Macrophages Is Indispensable for the Protective Immunity of Invariant Natural Killer T Cells against Enterovirus 71 Infection
- The Epstein-Barr Virus Encoded BART miRNAs Potentiate Tumor Growth
- Macrophage-Derived Human Resistin Is Induced in Multiple Helminth Infections and Promotes Inflammatory Monocytes and Increased Parasite Burden
- Dissemination of a Highly Virulent Pathogen: Tracking The Early Events That Define Infection
- Variability in Tuberculosis Granuloma T Cell Responses Exists, but a Balance of Pro- and Anti-inflammatory Cytokines Is Associated with Sterilization
- The Shear Stress of Host Cell Invasion: Exploring the Role of Biomolecular Complexes
- The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and Host
- Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for Entry
- Secreted Herpes Simplex Virus-2 Glycoprotein G Modifies NGF-TrkA Signaling to Attract Free Nerve Endings to the Site of Infection
- Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
- A New Family of Secreted Toxins in Pathogenic Neisseria Species
- A Human Type 5 Adenovirus-Based Therapeutic Vaccine Re-programs Immune Response and Reverses Chronic Cardiomyopathy
- Regulation of Oncogene Expression in T-DNA-Transformed Host Plant Cells
- GITR Intrinsically Sustains Early Type 1 and Late Follicular Helper CD4 T Cell Accumulation to Control a Chronic Viral Infection
- Cell Cycle-Independent Phospho-Regulation of Fkh2 during Hyphal Growth Regulates Pathogenesis
- Virus-Induced NETs – Critical Component of Host Defense or Pathogenic Mediator?
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Protective Efficacy of Centralized and Polyvalent Envelope Immunogens in an Attenuated Equine Lentivirus Vaccine
- Transmitted Virus Fitness and Host T Cell Responses Collectively Define Divergent Infection Outcomes in Two HIV-1 Recipients
- Systemic Expression of Kaposi Sarcoma Herpesvirus (KSHV) Vflip in Endothelial Cells Leads to a Profound Proinflammatory Phenotype and Myeloid Lineage Remodeling
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
- DNA Is an Antimicrobial Component of Neutrophil Extracellular Traps
- Uropathogenic Superinfection Enhances the Severity of Mouse Bladder Infection
- Well-Ordered Trimeric HIV-1 Subtype B and C Soluble Spike Mimetics Generated by Negative Selection Display Native-like Properties
- The Phylogenetically-Related Pattern Recognition Receptors EFR and XA21 Recruit Similar Immune Signaling Components in Monocots and Dicots
- Reprogramming of from Virulent to Persistent Mode Revealed by Complex RNA-seq Analysis
- Compartment-Specific and Sequential Role of MyD88 and CARD9 in Chemokine Induction and Innate Defense during Respiratory Fungal Infection
- Bacterial Flagella: Twist and Stick, or Dodge across the Kingdoms
- Elucidation of the RamA Regulon in Reveals a Role in LPS Regulation
- IL-1α Signaling Is Critical for Leukocyte Recruitment after Pulmonary Challenge
- Chronic Filarial Infection Provides Protection against Bacterial Sepsis by Functionally Reprogramming Macrophages
- Specificity and Dynamics of Effector and Memory CD8 T Cell Responses in Human Tick-Borne Encephalitis Virus Infection
- Promiscuous RNA Binding Ensures Effective Encapsidation of APOBEC3 Proteins by HIV-1
- Viral Activation of MK2-hsp27-p115RhoGEF-RhoA Signaling Axis Causes Cytoskeletal Rearrangements, P-body Disruption and ARE-mRNA Stabilization
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- : Trypanosomatids Adapted to Plant Environments
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



