-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Activation of Type I and III Interferon Response by Mitochondrial and Peroxisomal MAVS and Inhibition by Hepatitis C Virus
Mammalian cells developed several defense mechanisms against viral infection. One major strategy involves pattern recognition receptors (PRRs) recognizing non-self motifs in viral RNA and triggering the production of type I and III interferon (IFN) that induce an antiviral state. One central signaling molecule in this cascade is MAVS (Mitochondrial Antiviral Signaling protein), residing on mitochondria, mitochondria-associated membranes of the endoplasmic reticulum, and peroxisomes. Here we characterized the role of mitochondrial and peroxisomal MAVS for the activation of the IFN response and their counteraction by the hepatitis C virus (HCV), a major causative agent of chronic liver disease with a high propensity to establish persistence. By using various functional and genetic knock-out cell systems reconstituted to express exclusively mitochondrial or peroxisomal MAVS, we observed comparable activation of type I and III IFN response by either MAVS species. In addition, we found that the HCV protease residing in nonstructural protein 3 (NS3) efficiently cleaves MAVS independent from its subcellular localization. This cleavage is required for suppression of the IFN response and might contribute to HCV persistence. Our results indicate a largely localization-independent activation of the IFN response by MAVS in hepatocytes and its efficient counteraction by the HCV NS3 protease.
Published in the journal: Activation of Type I and III Interferon Response by Mitochondrial and Peroxisomal MAVS and Inhibition by Hepatitis C Virus. PLoS Pathog 11(11): e32767. doi:10.1371/journal.ppat.1005264
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005264Summary
Mammalian cells developed several defense mechanisms against viral infection. One major strategy involves pattern recognition receptors (PRRs) recognizing non-self motifs in viral RNA and triggering the production of type I and III interferon (IFN) that induce an antiviral state. One central signaling molecule in this cascade is MAVS (Mitochondrial Antiviral Signaling protein), residing on mitochondria, mitochondria-associated membranes of the endoplasmic reticulum, and peroxisomes. Here we characterized the role of mitochondrial and peroxisomal MAVS for the activation of the IFN response and their counteraction by the hepatitis C virus (HCV), a major causative agent of chronic liver disease with a high propensity to establish persistence. By using various functional and genetic knock-out cell systems reconstituted to express exclusively mitochondrial or peroxisomal MAVS, we observed comparable activation of type I and III IFN response by either MAVS species. In addition, we found that the HCV protease residing in nonstructural protein 3 (NS3) efficiently cleaves MAVS independent from its subcellular localization. This cleavage is required for suppression of the IFN response and might contribute to HCV persistence. Our results indicate a largely localization-independent activation of the IFN response by MAVS in hepatocytes and its efficient counteraction by the HCV NS3 protease.
Introduction
Vertebrates developed several defense mechanisms against invading pathogens. Upon viral infection foreign RNA or DNA is introduced into the host cell where it is detected by highly conserved pattern recognition receptors (PRRs) sensing distinct non-self motifs [1–7]. Well known examples of PRRs are RIG-I (Retinoic acid-Induced Gene I) and MDA5 (Melanoma Differentiation-Associated protein 5) that both are cytosolic RNA helicases recognizing primarily 5’-triphosphorylated and long (i.e. >2,000 nucleotides) double stranded (ds) RNA, respectively [1, 3, 8]. Upon interaction with RNA, both RIG-I-like receptors (RLR) induce a signaling cascade which leads to the production of type I and III Interferon (IFN) as well as IFN stimulated genes (ISGs). In the case of RIG-I, RNA interaction induces conformational changes, rendering the CARD (Caspase Activation and Recruitment Domain) accessible for ubiquitination by TRIM25 and subsequent interaction with the signal adaptor protein MAVS (Mitochondrial Antiviral-Signaling protein; also known as IPS-1, Cardif or VISA) [9–13]. MAVS is a ubiquitously expressed protein consisting of an N-terminal CARD, a proline-rich region and a single transmembrane domain at the very C-terminus. Activation of MAVS induces a prion-like oligomerization of the protein, forming large signaling platforms [14]. These platforms lead to the activation of NFκB and IFN regulatory factor 3 and 7 (IRF3, IRF7), which upon nuclear translocation drive the expression of IFNs and other cytokine genes [9–12, 15]. In principle the same pathway is utilized upon activation of MDA5 [14, 16, 17].
The IFN system consists of three classes that are grouped according to the receptor to which they bind. Type II IFN is primarily produced by T cells and natural killer cells in response to the recognition of infected cells. Type I IFNs include IFN-β, which is encoded by a single gene and synthesized by most cell types, especially fibroblasts, and IFN-α, which is encoded by a gene cluster of 13 genes and predominantly synthesized by leukocytes. Type III IFN has only been described recently and includes three members: IFN-λ1, IFN-λ2 and IFN-λ3 that are also known as IL-29, IL-28A and IL-28B, respectively [18, 19]. Recently a new member of this group was discovered, designated IFN-λ4, which is a frameshift variant arising from a peculiar polymorphism in the IL28 gene locus that is predictive for the outcome of therapy of chronic hepatitis C [20]. IFN-λ is produced by several cell types including hepatocytes [21, 22] where it contributes to the control of hepatitis C virus infection (HCV) [23, 24] Moreover, type I and III IFNs use different receptors, with type III receptors being composed of two chains: the ligand binding chain IFNλR1 and the accessory IL-10R2 subunit that is shared with the receptor for cytokines of the IL10 family [24]. Tissue distribution of the type III IFN receptor is by and large restricted to epithelial cells and hepatocytes in humans [25, 26]. Although type I and type III IFN use different receptors, it is believed that they both signal via the JAK/STAT pathway to induce a very similar set of more than 300 ISGs [23, 27].
HCV is a member of the genus Hepacivirus within the family Flaviviridae. A hallmark of HCV is its high propensity to persist. In fact, around 80% of HCV infections become persistent and persistently infected individuals have a high risk to develop chronic liver disease, including liver cirrhosis and hepatocellular carcinoma [28, 29]. The HCV particle is enveloped and contains a single-strand RNA genome of positive polarity. This ~9,600 nucleotides long RNA encodes a polyprotein that is cleaved into 10 products. Processing of the polyprotein is mediated, in part, by a viral serine-type protease that forms the N-terminal domain of nonstructural protein (NS) 3. Activation of this protease requires the viral cofactor NS4A that intercalates into the protease domain and, in addition, anchors the NS3/4A complex to intracellular membranes [30]. Apart from polyprotein cleavage, NS3/4A was found to counteract the activation of the IFN response by efficient cleavage of MAVS at amino acid 508 [10]. Consistent with the localization of the NS3 protease active site, the cleavage site in MAVS resides close to the membrane surface, thus liberating MAVS from the membrane and blocking RLR signaling function, both in vitro and in vivo [10, 31–33]. While these studies focused on MAVS localizing to mitochondria and mitochondria-associated membranes (MAMs), a distinct membrane compartment that links this organelle to the endoplasmic reticulum [32], MAVS was also reported to localize to peroxisomes to induce a rapid type I IFN-independent ISG response [34]. Moreover, it was reported that mitochondrial (mito) MAVS induces IFN-β and IFN-λ whereas peroxisomal (pex) MAVS only triggers an IFN-λ response [35]. However, the relative contribution of mito - and pexMAVS to the induction of the antiviral state and counteraction of the MAVS variants by viral infection remains largely unexplored.
In this study we evaluated the relative contribution of mito and pexMAVS to the activation of the type I and III IFN response in hepatocytes upon infection with various RNA viruses. In addition, we determined the control of either MAVS species by HCV. We found that mito - and pexMAVS activated type I and III IFN response with comparable efficiency and kinetics and both MAVS variants were potently counteracted by HCV.
Results
Determination of the subcellular localization of endogenous MAVS and establishment of cell lines with organelle-targeted MAVS variants
With the aim to characterize the activation of the interferon (IFN) response after viral infection by pex - and mitoMAVS we first determined the subcellular localization of endogenous MAVS on both organelles in the HCV-permissive human hepatoma cell line Huh7. Consistent with earlier reports endogenous MAVS primarily co-localized with mitochondria and, to a lesser extent, with peroxisomes as determined by co-staining with the marker protein PMP70 [9, 32, 34] (Fig 1A). Quantification of the MAVS distribution revealed that ~80% of the protein was localized on mitochondria and ~20% on peroxisomes (Fig 1B). However, ~17% of peroxisomes resided in close proximity to mitochondria, precluding the precise allocation of MAVS to either organelle. Thus, the total amount of pexMAVS might range between 3–20%. To study the impact of pex - and mitoMAVS separately, we utilized a strategy reported earlier [34] to generate MAVS variants exclusively localizing to either organelle. This was achieved by replacing the C-terminal transmembrane (TM) region of wild type MAVS (wtMAVS) with either the TM region of PEX13 (peroxisomal location; pexMAVS) or the TM region of Bcl-Xl (mitochondrial location; mitoMAVS). (Fig 1C). By using quantitative immunofluorescence, we confirmed the organelle-specific subcellular localization of these MAVS variants (Fig 1D).
Fig. 1. Subcellular localization of endogenous and engineered MAVS variants. 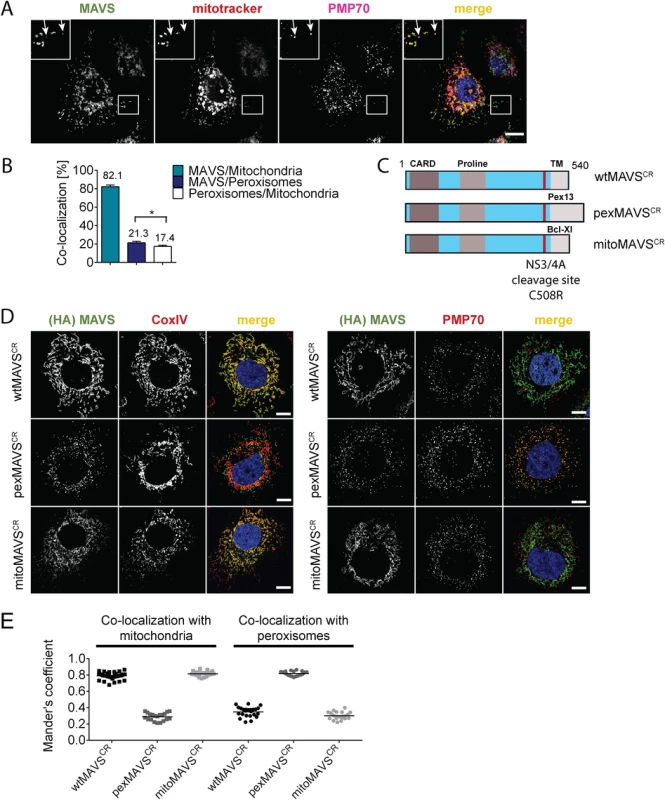
(A) Subcellular localization of MAVS in Huh7 cells was determined by immunofluorescence. White arrows indicate co-localization of MAVS (green) with the peroxisomal marker PMP70 (purple). Mitochondria were stained with mitotracker (red) while nuclei were stained with DAPI. Scale bar, 10 μm. (B) Quantification of subcellular localization of MAVS on mitochondria and peroxisomes as determined with the Mander’s overlap coefficient. In addition, co-localization of peroxisomes with mitochondria was determined. Bars indicate the standard error. *, P≤0.05. (C) Organelle-targeted MAVSCR (i.e. MAVS that is NS3/4A protease cleavage resistant) constructs were designed by replacing the transmembrane (TM)-domain (light grey) of wild type (wt) MAVS with either the TM-domain of Pex13 (pexMAVS) or Bcl-Xl (mitoMAVS). The HCV NS3/4A cleavage site was mutated to obtain a cleavage resistant (CR) MAVS protein (C508R). The MAVS coding sequence is indicated in light blue and includes the CARD as well as the proline-rich sequence (grey boxes). (D) Co-localization of MAVS variants with given marker proteins (red) was determined in Huh7-NS3/4A expressing cells transduced with wt-, pex- or mitoMAVSCR-HA (green) by using immunofluorescence. Scale bar, 20 μm. (E) The degree of co-localization was quantified by calculating the Mander’s overlap coefficient. Each dot represents a single cell; at least 20 cells were analyzed per condition. To avoid competition between endogenous and ectopically expressed MAVS variants we attempted to reduce the levels of endogenous MAVS by using siRNA - and shRNA-mediated knock-down approaches; however, expression levels were at best reduced by 70%, which we considered to be insufficient for functional assays. To overcome this limitation we stably expressed the HCV NS3/4A protease, which is known to efficiently cleave MAVS off the mitochondria [10, 36], thus rendering MAVS inactive and interrupting signaling. Cleavage of MAVS occurs at the membrane-proximal cysteine residue 508, which we replaced by an arginine residue to render MAVS cleavage resistant [10, 37]. Variants containing this C508R point mutation are designated MAVSCR throughout this report. Combining MAVSCR with the organelle-specific TMs thus offered a possibility to study pex - and mitoMAVS in diverse human cell lines expressing the NS3/4A protease. To this end, we utilized Huh7 and A549 cells where MAVS localization to the respective organelle was confirmed by quantitative immunofluorescence analyses (Fig 1D and 1E and S1 Fig, respectively). In both cell lines we observed highly significant co-localization of pexMAVSCR with peroxisomes and mitoMAVSCR with mitochondria, while wtMAVSCR mainly localized to mitochondria, with a small proportion localizing to peroxisomes, consistent with the subcellular distribution of endogenous MAVS (cf. Fig 1A and 1B).
Prior to conducting functional assays with these cell lines, we first determined whether wild type (i.e. C508) pexMAVS would be depleted from our cells by NS3/4A-mediated cleavage, because only then we would be able to measure IFN activation exclusively by exogenous organelle-specific MAVS. To allow measurement with high sensitivity we constructed a fusion protein composed of enhanced green fluorescent protein (eGFP) fused with a nuclear localization signal (NLS) and an organelle-specific targeting sequence (MAVS-C-terminal domain, MAVS-CTD) (S2A and S2B Fig). Analogous to an earlier report [38], upon cleavage of MAVS-eGFP the cleavage product is liberated from the membrane and translocated into the nucleus, which could be easily monitored by fluorescence imaging. Cells stably expressing NS3/4A or the non-functional protease mutant NS3/4A-S139A (S2C Fig) [39] were transfected with either one of the eGFP-NLS-MAVS-CTD constructs and subcellular eGFP distribution was determined. First, we confirmed localization of eGFP-NLS-tagged wt - and pexMAVS-CTD at peroxisomes in naïve Huh7 cells (S2B Fig). As expected, in cells expressing NS3/4A-S139A the eGFP distribution pattern of the MAVS variants was consistent with mitochondrial and peroxisomal localization (S2D Fig, left panel). In contrast, in NS3/4A-expressing cells transfected with eGFP-NLS-wtMAVS-CTD, the eGFP signal was exclusively detected in the nucleus reflecting highly efficient cleavage as reported earlier (S2D Fig, upper right panel) [10, 37, 38]. Importantly, analogous cleavage efficiency was also found with eGFP-NLS-pexMAVS-CTD (S2D Fig, lower right panel). These results demonstrate that HCV NS3/4A efficiently cleaves MAVS irrespective of its subcellular localization on mitochondria or peroxisomes.
Comparable activation of the type I and III IFN system by expression of MAVS proteins localizing to peroxisomes or mitochondria
Having confirmed that NS3/4A expressing cells are a valid model system for functional MAVS depletion and are suited to determine the capacity of organelle-specific MAVSCR variants to induce the IFN response, we transduced NS3/4A overexpressing Huh7 cells with lentiviral vectors encoding organelle-specific MAVSCR (Fig 2A). Consistent with earlier reports, we observed an intrinsic activation of the IFN response upon overexpression of MAVS as revealed by increased levels of IFN-λ in the supernatant of transduced cells [9, 40] (Fig 2B). This was not due to the lentiviral particles themselves since lentiviruses encoding the unrelated peroxisomal protein Pex14-HA did not induce IFN-λ expression. Apart from the activation of IFNL, we also observed an activation of IRF3 as determined by luciferase-based IFIT1, IFNB and IFNL1 promoter reporter assays (Fig 2C). Importantly, this activation was found with each MAVS variant and both, in the reporter assay and when measuring mRNA levels of endogenous ISG56 or various IFNs (Fig 2D). Moreover, analogous results were obtained with the IFN-competent cell line A549 (Fig 2E and S3 Fig) and with 293T cells (S4 Fig) demonstrating that the observed phenotypes are not specific to human hepatoma cells.
Fig. 2. Comparable activation of the IFN system by MAVS proteins localizing to peroxisomes or mitochondria in Huh7 and A549 cells. 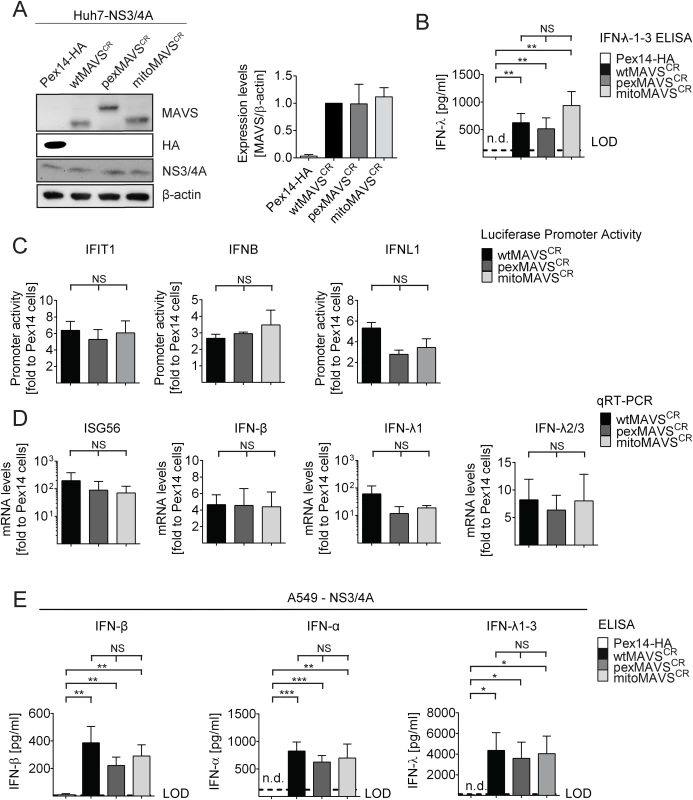
(A) Huh7-NS3/4A expressing cells were transduced with non-cleavable wt-, pex- or mitoMAVSCR or with Pex14-HA that served as control. Expression levels of MAVSCR variants were determined by Western blot. The quantification in the right panel displays MAVS abundance, normalized to β-actin, relative to wtMAVSCR expression levels (set to 1). Mean values from three independent experiments are shown. (B-D) Activation of the IFN response by overexpression of MAVS variants. (B) Huh7 cell culture supernatants were collected and IFN-λ1–3 protein levels were measured by ELISA. The dashed line indicates the limit of detection (LOD). N.d., not detectable. (C) For reporter-based analyses, firefly luciferase reporter plasmids were co-transfected with a SV40-based Renilla luciferase plasmid to normalize for transfection efficiency. Values for each reporter were normalized to those obtained for Pex14-HA transduced cells (set to 1). (D) Quantification of mRNA amounts of ISG56 and indicated IFNs as determined by qRT-PCR. Values were normalized to those obtained for Pex14-HA transduced cells (set to 1). All data were normalized to GAPDH using the ΔΔct method (E) A549 NS3/4A-expressing cells were transduced with lentiviruses containing non-cleavable wt-, pex- or mitoMAVSCR as well as Pex14-HA that served as control. Cells were stimulated by overexpressing MAVS variants, cell culture supernatants were collected and IFN protein levels were measured by ELISA. The dashed line indicates the limit of detection (LOD). N.d., not detectable. Data represent the mean from four independent experiments. Bars indicate the standard error. *, P≤0.05; **, P≤0.005; ***, P≤0.0005; NS, not significant. N.d., not detectable. To exclude possible confounding effects exerted by NS3/4A in a MAVS cleaveage-independent manner [41, 42], we transiently transduced naïve A549 cells and A549-MAVSKO cells with pex - or mitoMAVS variants. Of note, we observed a comparable induction of type I and type III IFN production, arguing that NS3/4A does not interfere with IFN activation, at least in this experimental setup (S5A and S5B Fig). We also determined the kinetics of IFN response activation by using microarrays to determine the transcriptome of wt-, mito - and pexMAVS-expressing cells. While most ISGs were upregulated to a similar degree, we observed subtle differences in induction kinetics for a few ISGs (S5C Fig). Although these differences were not consistent throughout the time course, it is possible that pex - and mitoMAVS have slightly different IFN induction capabilities.
Comparable activation of type I and III IFN response by peroxisomal or mitochondrial MAVS upon virus infection
Having shown that expression of pexMAVS and mitoMAVS were able to induce type I and III IFN responses, we next compared these MAVS variants for their capacity to induce the IFN response upon viral infection. We reconstituted MAVS activity in 293T cells overexpressing HCV NS3/4A (293T-NS3/4A) by lentiviral transduction of MAVSCR variants. As shown in Fig 3A, expression levels of the MAVS proteins in the cell pools were comparable, although wtMAVS abundance was ~20% higher as compared to the variants. Importantly, we only used cell pools that were not pre-activated by MAVS transduction, which we achieved by infecting the cells with MAVS-encoding lentiviruses at a very low MOI of 0.1 and passaging them under selective pressure. To investigate the IFN response we used the single stranded RNA virus Sendai Virus (SeV), which is specifically sensed by RIG-I [3, 7]. When we infected 293T-NS3/4A cells transduced with the empty vector with SeV or with other RNA viruses, we detected only a minor IFN response, which might be due to residual amounts of non-cleaved MAVS (0.2%) (Fig 3B, 3C and S6 Fig, respectively). However, in cells expressing mitochondrial or peroxisomal MAVS, IFN-λ expression was potently induced and amounts released into the culture supernatant of SeV-infected cells were well comparable between pexMAVSCR and mitoMAVSCR cells (Fig 3B). Consistent with this result we observed induction of endogenous ISG56, IFN-β and type III IFN expression upon SeV infection and these responses barely differed between the MAVS variants (Fig 3C). As reported earlier [43, 44], IFN-β expression was rapidly and very transiently induced and this also applied to IFN-λ1. In contrast, activation of IFN-λ2/3 expression was much more sustained and did not decline throughout the 48 h observation period. Activation of both type I and III IFN expression as well as ISG56 was also found when we infected the cells with other RNA viruses: Vesicular Stomatitis Virus (VSV) and Newcastle Disease Virus (NDV), which are both negative strand ssRNA viruses, and the dsRNA virus reovirus (S6 Fig). We note that kinetics and magnitudes of activation differed between these viruses, likely reflecting their diverse replication strategies. Nevertheless, comparable activation by mito - and pexMAVS was detected in all cases. Response levels were higher in case of wtMAVS, which might be due to the higher expression of this protein as compared to the MAVS variants (Fig 3A). Alternatively, highest IFN response might require both mito - and pexMAVS as suggested earlier [34]. Although we observed minor differences in ISG and IFN response in 293T cells between pex - and mitoMAVS we note that these differences were not consistent between different experiments. Taken together, we observed efficient MAVS-dependent activation of the type I and III IFN response upon virus infection, independent of the subcellular localization of MAVS.
Fig. 3. Comparable activation of type I and III IFN response by peroxisomal or mitochondrial MAVS upon virus infection. 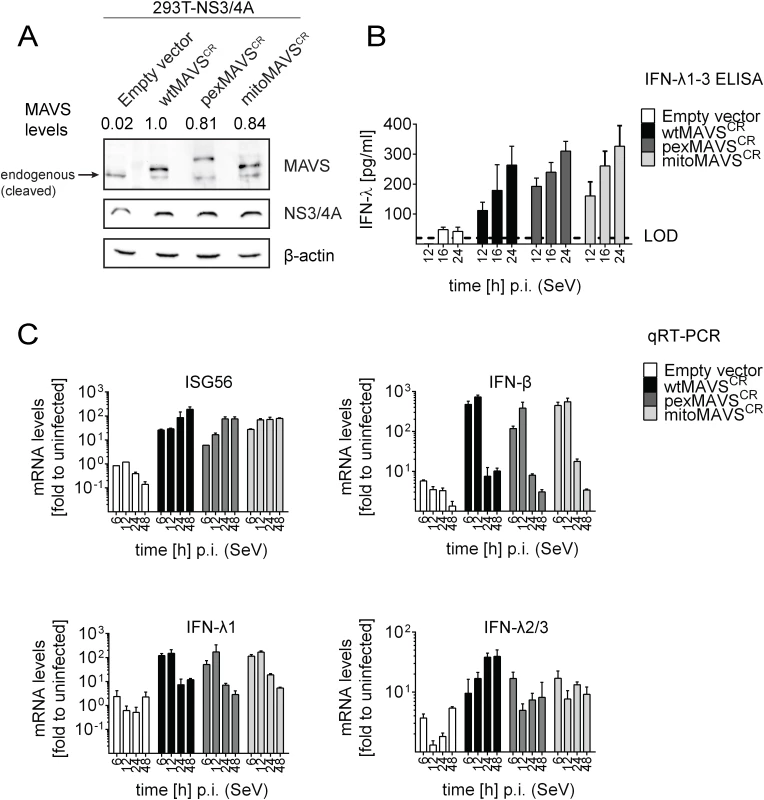
(A) Lysates of 293T cells stably expressing NS3/4A and various forms of non-cleavable MAVSCR or an empty vector were analyzed by Western blot. Numbers above each lane refer to MAVS expression levels normalized to β-actin and wtMAVSCR expression that was set to one. (B) Cells were infected with Sendai virus (SeV) with an MOI of 3. The amount of IFN-λ released at given time points into the cell culture supernatant was determined by ELISA. The dashed line represents the limit of detection (LOD). (C) Cells were infected with SeV and total RNA was extracted at time points specified in the bottom of each panel. mRNA amounts were quantified by qRT-PCR. All data were normalized to GAPDH using the ΔΔct method. A representative experiment of a total of three independent experiments is shown. Each value was measured in quadruplicates; mean values and standard errors are shown. Activation of the interferon response by mitochondrial and peroxisomal MAVS in reconstituted MAVS-/--mouse embryonic fibroblasts
In the experiments described so far, we degraded endogenous MAVS from the mitochondria and peroxisomes by using the HCV NS3/4A protease. Under these conditions we were not able to detect an IFN response in these cells unless we reconstituted with MAVSCR variants, demonstrating efficient blockade of MAVS-dependent signaling via mitochondria and peroxisomes. However, we could not exclude undesired effects exerted by either HCV NS3/4A overexpression or cleaved cytoplasmic MAVS. Therefore, we made use of mouse embryonic fibroblasts (MEFs) derived from a mouse line lacking endogenous MAVS (MAVS-/-) and reconstituted these cells by stable expression of wtMAVS, pexMAVS or mitoMAVS (Fig 4A). These cell pools were infected with SeV or reovirus, and analyzed for type I IFN production. At different time points after infection supernatants were harvested and secreted IFN-β was quantified by ELISA. While in MEFs lacking MAVS no IFN could be detected, in cells ectopically expressing organelle-specific MAVS similar levels of IFN-β were determined at 16 h post infection (Fig 4B). These results were further confirmed by the IFN reporter cell line L929-ISRE-Firefly-Luciferase, which is a reliable type I IFN bioassay as reported earlier [45]. Supernatants taken from virus-stimulated cells were UV-inactivated prior to adding to the reporter cell line. As expected, in MAVS-/- MEFs without reconstituted MAVS no type I IFN was produced upon infection with SeV or reovirus (Fig 4C). However, consistent with our results obtained in NS3/4A-expressing cells, we detected comparable amounts of type I IFN in the supernatants of MEFs expressing wt-, pex - or mitoMAVS. Analogous results were obtained when we quantified the mRNA amounts of endogenous IFN-β in infected and functionally reconstituted MAVS-/- MEFs (Fig 4D). A rapid and profound activation was observed in cells infected with either virus and no difference was detected between the MAVS variants. In contrast, induction of IFN-λ2/3 expression could not be detected in this cell system, arguing that the antiviral activity released into the culture supernatant was exclusively mediated by type I IFN (Fig 4E). In summary, these results corroborate the conclusion that mito and pexMAVS can induce the type I IFN response with comparable efficiency.
Fig. 4. No difference in virus-induced activation of type I and III IFN response in MAVS-/- mouse cells expressing peroxisomal and mitochondrial MAVS. 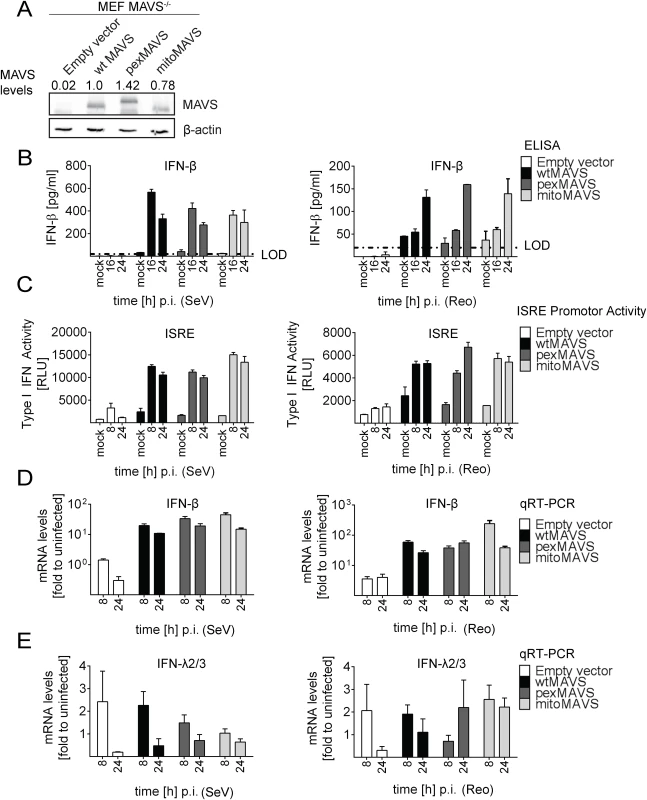
(A) Mouse Embryonic Fibroblasts (MEFs) from MAVS-/- knockout mice were transduced with lentiviral expression constructs encoding wt-, pex- or mitoMAVS or with the empty control vector. Expression of MAVS variants was validated by Western blot. Numbers above each lane refer to the expression level of the respective MAVS variant after normalization to β-actin and wtMAVSCR that was set to one. (B, C) Amounts of IFN-β released from MAVS-reconstituted MEFs at different time points after virus infection into the culture supernatant and antiviral activity contained therein. (B) Cells were infected with SeV (MOI = 5) or Reo virus (MOI = 50) and IFN-β protein levels were quantified by ELISA. Dashed line represents the limit of detection (LOD). (C) Culture supernatants of MAVS-reconstituted MEFs harvested at different time points after SeV or Reo virus infection were inactivated by UV irradiation and used to inoculate L929 reporter cells. Eight hours later, cells were lysed and luciferase activity was determined. (D, E) Cells treated as described in panel (B) were harvested at given time points and amounts of IFN-β (D) or IFN-λ2/3 (E) mRNA were determined by qRT-PCR. All data were normalized to the housekeeping gene GAPDH and to the respective uninfected control (set to 1). Each figure in panels B-D shows a representative experiment of four independent repetitions, each conducted in technical triplicates. Mean values and standard errors are shown. Peroxisomes are dispensable as signaling platforms for type I and III IFN response
The results described so far were based on the use of engineered MAVS variants with defined subcellular localization. Since we could not exclude that a small fraction of MAVS, undetectable by our assays, might have been mislocalized, we took advantage of cells lacking peroxisomes. By definition, these cells are unable to mount MAVS signaling platforms at peroxisomes, thus offering an alternative approach to study the activation of the IFN response in the absence of pexMAVS. This is the case with the patient-derived human fibroblast cell line ΔPex19 [46, 47], which naturally lacks the peroxisomal factor Pex19 required for budding of peroxisomes from the ER membrane [48]. These cells do not have peroxisomes but the peroxisomes can be restored by stably expressing recombinant Pex19 [47]. Indeed, upon ectopic expression of Pex19, we could detect peroxisomes, as revealed by staining with the peroxisomal marker PMP70 (cell line ΔPex19+Pex19), which were absent in non-reconstituted cells (Fig 5A).
Fig. 5. Activation of type I and III IFN response in peroxisome-deficient human cells. 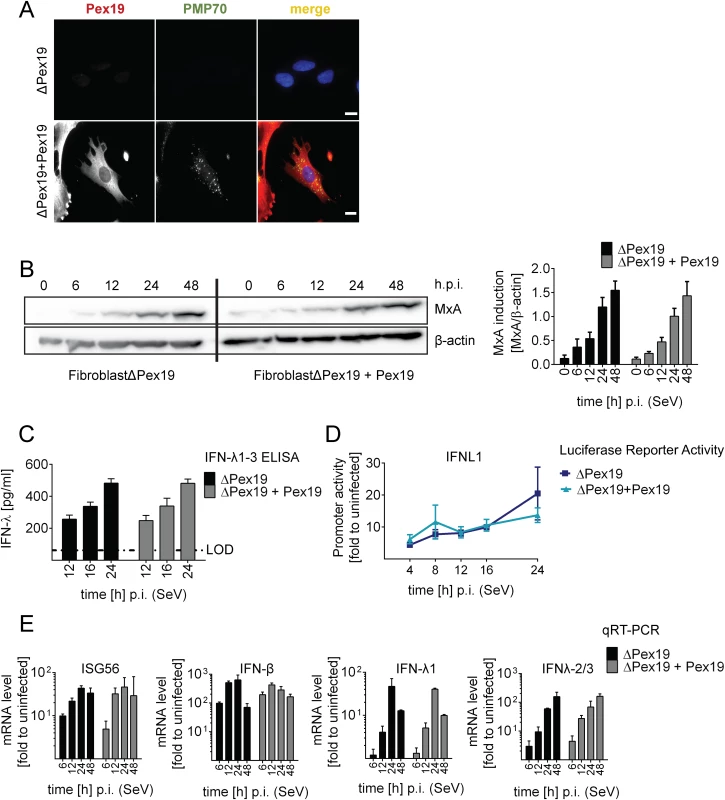
(A) Deficiency of peroxisome formation of the human fibroblast cell line ΔPex19 that does not contain peroxisomes was rescued by stable expression of Pex19 (ΔPex19+Pex19) as determined by immunofluorescence for Pex19 (red) and PMP70 (peroxisome marker, green). Nuclei were counterstained with DAPI. Scale bar, 40 μm. (B-D) Both cell lines were infected with SeV (MOI = 3) and samples were collected at indicated times points. (B) Total protein lysates of infected cells were analyzed for MxA abundance by Western blot. MxA-specific signals were quantified by using the Lab Image ID software package and values were normalized to β-actin that served as loading control. Values correspond to the mean of four independent experiments and standard errors. (C) Cell culture supernatants were harvested at given time points after SeV infection and amounts of IFN-λ1–3 were measured by ELISA. The dashed line indicates the limit of detection (LOD). (D) Fibroblasts were transfected with an IFN-λ-firefly luciferase reporter construct and 24 hours later infected with SeV. Cells were harvested at time points specified in the bottom and firefly luciferase activity was measured. Values were normalized to transfection efficiency as determined with a co-transfected SV40-based Renilla luciferase plasmid and are expressed relative to uninfected control cells (set to 1). (E) Total RNA was prepared from SeV-infected cells at time points specified in the bottom of each panel and amounts of mRNAs given on the top of each panel were determined by qRT-PCR. All data were normalized to GAPDH and uninfected control cells (set to 1). Mean values of a representative experiment conducted with technical triplicates are shown. Bars indicate the standard error. All experiments were performed at least three times. To determine activation of the type I and III IFN response in the absence or presence of peroxisomes we infected the two cell lines with SeV and monitored the kinetics of MxA protein accumulation that is induced by type I and III IFN. As shown in Fig 5B, activation of this ISG did not require peroxisomes and induction kinetics were virtually identical. Moreover, SeV infection also induced robust type III IFN production as measured by ELISA in culture supernatants and by a luciferase reporter assay based on the IFNL1 promoter (Fig 5C and 5D). Consistently, mRNA levels of ISG56 were induced in both cell lines already 6 h post infection and increased over time (Fig 5E). Importantly, both type III and type I IFN expression was induced with IFN-β showing a rapid response kinetic whereas activation of IFN-λ1 and IFN-λ2/3 was slower.
HCV triggers an IFN-λ response in Huh7 MAVSKO cells rescued with cleavage-resistant mitochondrial and peroxisomal MAVS
Having established the comparable activation of type I and III IFN response by mito - and pexMAVS, we wanted to clarify the relative impact of either MAVS species on the IFN response during infection with HCV. To this end we generated Huh7 MAVS knockout cell lines (Huh7 MAVSKO) by targeting the first exon of the coding sequence of MAVS with the CRISPR/Cas9 system [49]. Since in only a fraction of cells knock-out is achieved, we generated three independent knock-out cell clones, whereby each clone was generated by using each time the same sgRNA. For each of these cell clones cell pools were established expressing NS3/4A protease cleavage resistant MAVSCR variants localizing to the mitochondria or peroxisomes as well as wild type MAVSCR (Fig 6A; results obtained for only one cell clone are shown). In the initial set of experiments we determined HCV permissiveness of the cell lines by infecting them with an HCV reporter virus encoding Renilla luciferase (JcR2a). In all tested cell pools we observed robust HCV replication as determined by luciferase assay (Fig 6B, left panel) and analysis of cells by immunofluorescence staining revealed an infection efficiency of 60–80% (Fig 6B, right panel, and S7 Fig). Interestingly, after introducing the MAVSCR variants, the relative number of infected cells showed a minimal, yet statistically significant reduction, but there was no difference between organelle-specific MAVS variants (Fig 6B, right panel). Consistent with our earlier report [50], upon HCV and SeV infection there was no type I IFN production detectable in these Huh7 cells, (S8 Fig). In contrast, a robust type III IFN response was observed after infection with HCV. This response was first detected 48 h after infection (~55 pg/ml IFN-λ) and increased massively in the subsequent 24 h reaching ~250 pg/ml with pexMAVSCR cells and 305 pg/ml with mitoMAVSCR cells (Fig 6C). These quantitative differences corresponded well to the variations of expression levels of the MAVS variants (cf. Fig 6A). Consistently, mRNA levels of endogenous ISG56, IFN-λ1 and IFN-λ2/3 increased 48 h after HCV infection and reached maximum levels 72 h after infection (Fig 6D). These results were not a cell clone specific effect, because well comparable results were obtained with (a) two independent MAVSKO cell clones, each reconstituted with MAVSCR variants and analyzed in parallel and (b) Huh7 cells stably expressing NS3/4A to purge endogenous MAVS from intracellular membranes and reconstituted with MAVSCR variants (S9 Fig).
Fig. 6. HCV activates type III IFN response via peroxisomal and mitochondrial MAVS to a comparable extent. 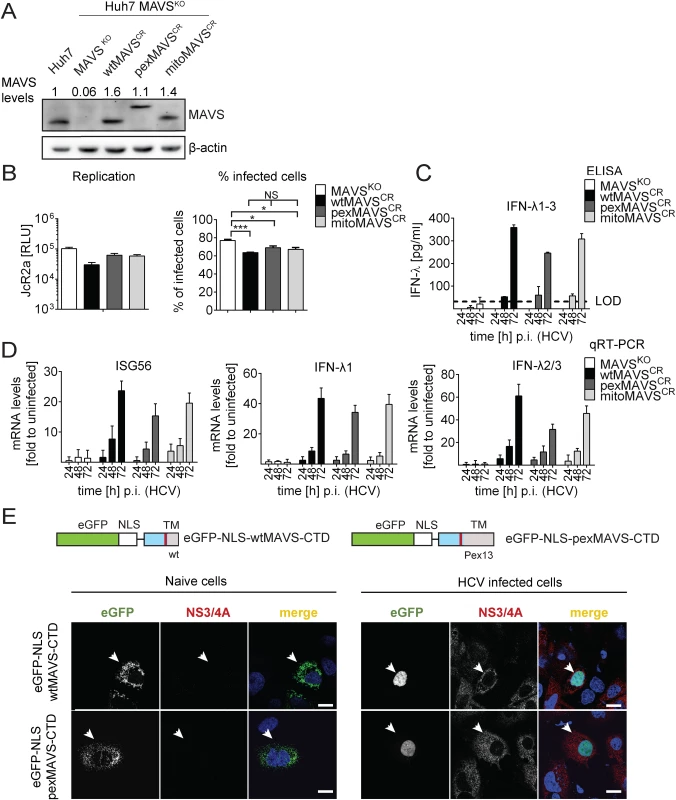
(A) Huh7 MAVSKO cell lines were generated using the CRISPR/Cas system. Cleavage resistant wt-, pex- and mitoMAVS were expressed in these cells by lentiviral transduction. Expression levels of the proteins were quantified by Western blot. Numbers above each lane refer to abundance of a given MAVS variant, normalized to β-actin and endogenous MAVS that was set to one. (B) Cell lines were infected with either the HCV reporter virus JcR2a (MOI = 1) or Jc1 (MOI = 1). Seventy two hours later HCV replication in JcR2a-infected cells was determined by luciferase assay whereas Jc1-infected cells were analyzed by NS5A-specific immunofluorescence. The percentage of infected cells was determined by counting at least 500 cells for each condition. (C) Cells were infected with the HCV isolate Jc1 (MOI = 5) and amounts of IFN-λ1–3 released into cell culture supernatants were determined by ELISA. The dashed line represents the limit of detection limit (LOD). N.d., not detectable. (D) Total RNA of infected cells was extracted and mRNA amounts of ISG56 and type III IFNs were determined by qRT-PCR. All data were normalized to GAPDH using the ΔΔct method and are expressed relative to uninfected control cells (set to 1). A representative experiment performed with technical triplicates is shown. Bars indicate the standard error. All experiments were conducted three times and with two additional Huh7 knockout cell clones. (E) Huh7 cells were infected with Jc1 (MOI = 5) and transfected 40 hours later with expression constructs encoding GFP-tagged CTD of wt- or pexMAVS (for construct design see Fig 2A). Note that HCV-mediated cleavage of the reporter results in nuclear accumulation of the eGFP signal. White arrow indicates an HCV infected cell. Scale bar, 20 μm. Efficient control of peroxisomal MAVS in hepatitis C virus infected cells
With the aim to characterize control of pexMAVS in HCV infected cells, we utilized the eGFP-NLS-MAVS-CTD fusion proteins described above (cf. S2A Fig), which were expressed in Huh7 cells 36 h after HCV infection by transient transfection. As expected, in uninfected cells we observed predominantly mitochondrial localization of the wtMAVS marker protein (Fig 6E, left panel). However, upon HCV infection cleaved wtMAVS-eGFP translocated into the nucleus, demonstrating efficient cleavage (Fig 6E, right panel). Importantly, pexMAVS was also cleaved quantitatively in HCV-infected Huh7 cells as revealed by the exclusive nuclear localization of the marker protein.
Taking advantage of this cell system, we next determined the impact of MAVS cleavage and MAVS subcellular localization on activation of the IFN response by HCV in an infection-based system. This was possible because the used cells were highly permissive for HCV, mounted a robust IFN response, but did not suppress HCV replication, thus excluding confounding effects on activation kinetics as a result of impaired virus replication. To this end we constructed Huh7 MAVSKO cells expressing exclusively HCV cleavable, i.e. wild type full-length MAVS variants localizing specifically to peroxisomes or mitochondria. All cell lines responded comparably to SeV infection by upregulation of ISG56 and IFN-λ1, thus validating their functionality (Fig 7A). Of note, the ISG response induced by SeV and Reo virus was only detectable at late time points after infection (> 4 hours; S10 Fig). As expected, upon HCV infection all MAVS variants were efficiently cleaved, yet also non-cleaved MAVS was detected by Western blot (Fig 7B). Since uncleaved MAVS might be produced by non-infected cells, we utilized an immunofluorescence-based single cell readout. We found that in HCV-infected cells MAVS was not detectable and this was independent from the subcellular localization of MAVS (S11 Fig). Consistent with this efficient MAVS cleavage in HCV-infected cells was the almost complete block of IFN-λ production and ISG56 response (Fig 7C and 7D). This inhibition was specific and not observed in cells reconstituted with the cleavage-resistant MAVS (wtMAVSCR).
Fig. 7. Blunting of the interferon response upon HCV infection occurs independent from MAVS subcellular localization. 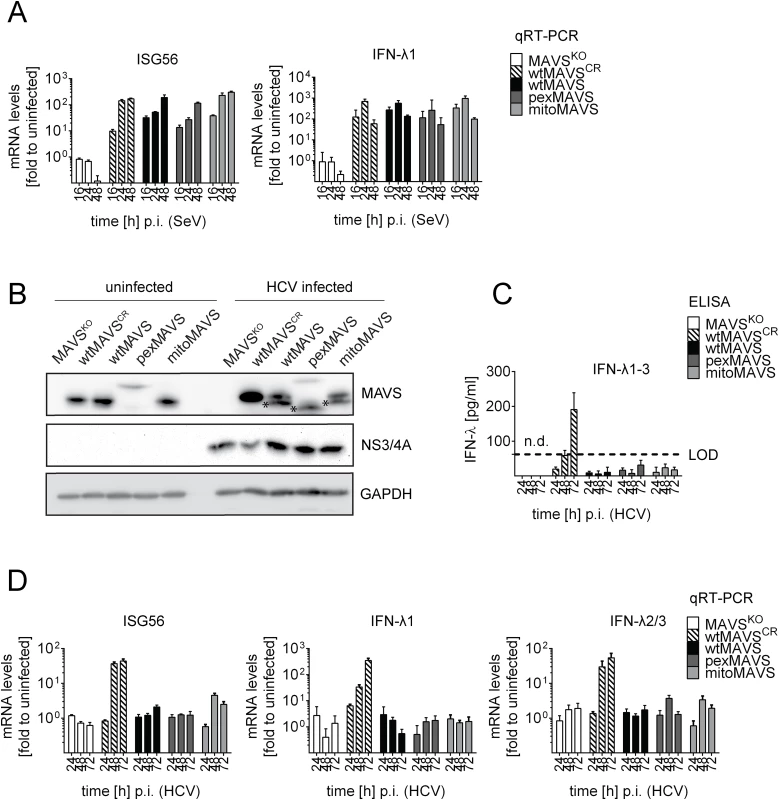
Huh7-MAVSKO cells were rescued with cleavable wt-, pex- or mitoMAVS or cleavage-resistant wtMAVSCR. (A) Cells were infected with SeV (MOI = 5) for periods specified in the bottom of each panel. Total RNA was isolated and amounts of ISG56 and IFN-λ1 mRNAs were determined by qRT-PCR. All data were normalized to GAPDH and uninfected control cells (set to 1). (B) Cells were infected with HCV and 48 hours later MAVS cleavage and HCV infection was determined by Western blot using antibodies specified in the right of each panel. Asterisks indicate cleavage products of MAVS variants. (C) Cell culture supernatants were harvested at given time points after HCV infection and amounts of IFN-λ1–3 were measured by ELISA. The dashed line indicates the limit of detection (LOD). (D) Amounts of ISG56 and IFN-λs mRNA were determined by qRT-PCR. Values were normalized to those obtained for uninfected cells. All data were normalized to GAPDH using the ΔΔct method. Taken together we demonstrate that both mitochondrially and peroxisomally localized MAVS induce a robust type III IFN response in Huh7 cells with comparable efficiency. Importantly, both MAVS variants are efficiently controlled in HCV-infected cells by the NS3/4A protease, thus blocking IFN response irrespective of MAVS localization.
Discussion
Activation of the IFN response by the RLRs RIG-I and MDA5 critically depends on MAVS that relays the signal via several protein kinases to NFκB and IRF3, which in turn promote the transcription of IFN-β, IFN-λ and several ISGs [9–12]. MAVS was originally found to localize to mitochondria, but more recent studies also described MAVS on MAMs and peroxisomes [9, 32, 34]. Although in this study we could confirm peroxisomal localization of MAVS in the human hepatoma cell line Huh7, it is still unclear how MAVS is targeted to this organelle. While the transmembrane region in the C-terminal part of MAVS appears to be responsible for targeting to mitochondria [9] and peroxisomes [34], the molecular details remain to be clarified. Three possibilities can be envisaged how MAVS is targeted to peroxisomes. First, regarding mitochondria, the majority of peroxisomal proteins is translated by free polyribosomes in the cytosol [51–53] and they are post-translationally incorporated into newly formed peroxisomes via Pex19p [53]. Second, MAVS is present on ER membranes [32]. Since peroxisomes are derived from the ER, MAVS might be incorporated into new peroxisomal membranes by chance when they bud off [54]. Third, MAVS might shuttle from mitochondria to peroxisomes via a specialized transport system called mitochondrial-derived vesicles as described for other proteins [55]. It remains to be determined by which route MAVS is targeted to peroxisomes or whether several of these routes are used. In any case, we observed efficient cleavage of pexMAVS by the HCV protease. This might occur at the ER, prior to MAVS “loading” onto peroxisomes. NS3/4A might also be recruited to peroxisomes where cleavage could occur. Consistent with the latter assumption it has been reported that a fraction of NS3/4A co-localizes with peroxisomes [32].
We and others observed that overexpression of MAVS per se was sufficient to activate the RLR pathway [9, 40]. However, there was no consistent difference between organelle-specific MAVS variants and this we observed with several cell lines and by using direct (ELISA, qRT-PCR and Western blotting) and indirect detection methods (luciferase-based promoter assays). Moreover, after RLR stimulation by viral infection, comparable responses were induced by pexMAVS or mitoMAVS. Of note, efficient antiviral signaling was also detected in the human fibroblast cell line ΔPex19, which lacks peroxisomes altogether, and no difference in activation kinetics or magnitude was found in this cell line after functional reconstitution of peroxisomes.
It has been reported that peroxisomal proteins, in the absence of peroxisomes, either stay in the cytosol, are degraded or mistargeted to other organelles such as mitochondria [56–59]. Consistently, immunofluorescence staining of MAVS in ΔPex19 cells revealed a typical mitochondrial distribution and the abundance of MAVS in cells with or without peroxisomes was well comparable. Therefore, we assume that mitochondrial MAVS might compensate for peroxisomal MAVS in cells lacking peroxisomes. In any case, the activation of type I and III IFN in ΔPex19 cells shows that peroxisomes are dispensable for induction of this antiviral response. To corroborate this assumption we attempted stable knock-down of Pex19 in Huh7 cells, but this was not possible because of cytotoxicity. Moreover, transient knock-down was limited because peroxisomes not only renew from the ER, but also renew from already existing peroxisomes (G. Dodt, Thübingen University, personal communication), thus requiring extended periods of Pex19 depletion which could not be achieved by transient knock-down.
Although we did not observe a difference in activation of the IFN response by mito - or pexMAVS in various cell lines and experimental approaches, Dixit and colleagues described a rapid, type I IFN-independent signaling of pexMAVS in MAVS-/- MEFs after reovirus infection [34]. Furthermore, the same group recently reported that upon viral infection pexMAVS induces IFN-λ1, but not IFN-β in Huh7 cells after knockdown of endogenous MAVS expression [35]. Consistent with this data, we could confirm IFN-λ1 activation by pexMAVS in MAVS knock-out Huh7 cells as well as IFN-λ2/3 production as determined by ELISA, luciferase reporter assay and qRT-PCR analysis. Moreover, for each condition and approach we detected comparable induction of type III IFNs by wt-, pex - and mitoMAVS. However, we did observe an induction of type I IFN by peroxisomally localized MAVS in i) MAVS-/- MEFs after stimulation with reovirus or SeV, ii) Huh7 and A549 cells after ectopic expression of MAVS variants and iii) 293T cells with a functional (NS3/4A-mediated) block of endogenous MAVS and expressing organelle-specific cleavage-resistant MAVS variants. These results are at odds with the earlier studies that concluded a selective activation of IFNL by pexMAVS. Currently, we can only speculate about possible reasons for the discrepancy. One possibility is the use of different experimental set ups, most notably the MAVS constructs. Common to the present and the earlier studies is the replacement of the C-terminal TM domain of MAVS by pex - and mito-targeting sequences (Pex13 and Bcl-XI, respectively; Fig 1C). However, while we fused MAVS with the targeting sequence at amino acid position 514 of MAVS, the MAVS fusion constructs employed in the earlier studies retained MAVS only up to amino acid residue 500, which might affect signaling capacity of the MAVS fusion proteins. Indeed, activation of the IFN response by pex - and mitoMAVS truncated at position 500 seems rather low as compared to wtMAVS [34, 35]. An alternative explanation for the discrepant results reported here and in the two earlier studies might be differences in sensitivities of used read-out systems to measure type I IFN. In fact, we were unable to detect IFN-β in Huh7 cells by ELISA, but could detect low endogenous IFN-β activation by qRT-PCR and by promoter assay. This appears to be a specific property of Huh7 cells, because in several other cell lines we could detect robust IFN-β by pex and mitoMAVS.
Currently, we cannot exclude that a very small fraction of pexMAVS is localized on mitochondria. To address this possibility we attempted to separate peroxisomes, MAMs and mitochondria biochemically, but consistent with an earlier report [32] we found that subcellular fractionation was not sufficient for that purpose. However, we did find that gradual knockdown of mitoMAVS to a level still detectable by fluorescence microscopy profoundly reduced the ISG response after SeV infection (S12 Fig). Given that pex - and mitoMAVS efficiently induced the IFN response to comparable levels, the degree of activation observed with pexMAVS cannot be ascribed to small amounts of pexMAVS mislocalized to mitochondria and not detectable by fluorescence microscopy. Thus we conclude that the activation observed with pexMAVS is indeed mediated by MAVS residing at peroxisomes. We also note that mitoMAVS might not only localize on mitochondria, but as reported earlier, Bcl-Xl also localizes to the ER [60–62]. However, ER or MAM localization of mitoMAVS would not affect our conclusions.
By using Huh7-MAVSKO cells expressing organelle-specific MAVSCR variants, we observed a profound activation and secretion of type III IFN upon HCV infection (Fig 6). Surprisingly, while this IFN is biologically active, HCV replication in infected cells was barely affected. This might be due on one hand to impaired responsiveness of Huh7-MAVSKO cells to IFN (S13 Fig), on the other hand to the limited IFN response of HCV in Huh7 cells [63]. Although this precludes studying the impact of IFN released from HCV-infected cells on viral replication and spread, it offers the possibility to characterize activation of the IFN response without confounding effects caused by a reduction in replication resulting from the antiviral state induced by IFN.
It has been proposed that HCV might induce type III IFN via non-cleaved pexMAVS [64]. However, we demonstrate that pexMAVS is efficiently cleaved in HCV-infected cells. While this result verifies the reported co-localization of NS3/4A with peroxisomes [32], we cannot exclude that a fraction of MAVS remains uncleaved. At the single cell level, despite Western Blot analysis indicating incomplete cleavage, MAVS was virtually non-detectable in HCV-infected cells. Thus, the apparent incomplete MAVS cleavage as detected by Western blot probably reflects incomplete infection of the used Huh7 cell cultures with uncleaved MAVS being produced by non-infected cells. The analogous might apply to MAVS cleavage as it was observed in the liver of HCV-infected patients [33]. Thus, the type III IFN response observed by our group and others [65–68] in primary human hepatocytes after HCV infection might result from a small fraction of non-cleaved MAVS or induction of other innate signaling pathways such as TLR3, rather than reflecting a specific role of peroxisomal MAVS.
The functional and biological differences of type I and III IFN remain poorly understood. Both classes of IFNs are produced after activation of RIG-I, MDA5 and TLR3 and both induce a very similar spectrum of ISGs via the JAK/STAT signaling pathway [69, 70]. However, the two IFN classes are produced in different tissues and bind to different receptors, IFNAR and IL28R, respectively, [18, 19] both of which also have distinct tissue distribution. Restriction of the IFN-λ receptor complex to epithelial cells and hepatocytes indicates a high importance of IFN-λ for control of infections with viruses of distinct tropism such as influenza or hepatitis viruses, respectively [65, 66, 71, 72]. Furthermore, stimulation of cells with IFN-β or IFN-λ1–3 induces a long-lasting ISG response, while IFN-α induces a much faster and less sustained ISG response [69]. Consistent with these reports we found that cells expressing only IFN-β and IFN-λ mount a similar ISG56 response, which was comparably induced by mitoMAVS and pexMAVS.
The observation that cleavage of MAVS by the NS3/4A protease completely blocks activation of the IFN response suggests that MAVS, which is not tethered to the membrane, loses its signaling capacity. Although purified MAVS protein forms prion-like structures even in solution in vitro and therefore independent of its transmembrane domain [15], we and others never detected an IFN response by cleaved MAVS inside cells. This might be due to the lower effective concentration of cleaved MAVS in the cytoplasm as compared to the in vitro conditions and to a rapid degradation of MAVS that has a half-life of only ~4 hours in HCV replicon-containing cells [9, 37].
In summary, our data indicate that peroxisomes appear to be dispensable in terms of signaling platforms in the type I and III IFN activation pathway, arguing for a redundant role of pex - and mitoMAVS. We observed comparable induction of type I and type III IFN by peroxisomal and mitochondrial MAVS upon viral infection and show that both, mitochondrial as well as peroxisomal MAVS are efficiently cleaved and inactivated in HCV-infected cells. Thus, HCV has evolved strategies to counteract this important IFN signaling molecule independent from its subcellular localization.
Materials and Methods
Cell culture
All cell lines were cultured in Dulbecco’s modified Eagle medium (DMEM, Life Technologies, Germany) supplemented with 10% fetal calf serum (GE Healthcare, Germany), 100 μg/ml penicillin, 100 μg/ml streptomycin (Sigma Aldrich, Germany), non-essential amino acids (Life Technologies, Germany) and maintained in a humidified incubator with 5% CO2 at 37°C. Stable cell lines were created by lentiviral transduction as described below and cultured in selection medium in the presence of 1 mg/ml G418 (Life Technologies, Germany), 5 μg/ml puromycin (Sigma Aldrich, Germany) and/or 5 μg/ml blasticidin (MP Biomedicals, USA). MAVS-/- mouse embryonic fibroblasts, human fibroblasts deficient for Pex19 (ΔPex19) and mouse L929-ISRE-Firefly-Luciferase cells were kindly provided by Ulrich Kalinke (Hannover, Germany), Gabrielle Dodt (Tübingen, Germany) and Steeve Boulant, (Heidelberg, Germany), respectively.
Virus production
Jc1 wild type HCV and the Renilla Luciferase reporter virus JcR2a were generated as described recently by using the plasmids pFKJFH1/J6/C846ΔG and pFKJFH1/J6/C846-JcR2a, respectively [73, 74]. Briefly, in vitro transcripts were purified by phenol/chloroform extraction and precipitated at room temperature using isopropanol. Ten microgram RNA was transfected by electroporation into 3.5x106 Huh7.5 cells using the GenePulser system (Bio-Rad, Germany). Cell culture supernatant was harvested 24, 48 and 72 hours post electroporation, pooled, filtered through a 0.45 μm SteriCap (Millipore, Germany) and precipitated with polyethylene glycol (PEG) 800 (Applichem, Germany) in PBS for 72 hours at 4°C. After centrifugation for two hours at 8,000x g, the pellet was resuspended in DMEM and the titer was determined by limiting dilution assay using the TCID50 method (http://www.klinikum.uni-heidelberg.de/Downloads.126386.0.html). Vesicular Stomatitis Virus (a kind gift from Gert Zimmer, Mittelhäusern, Switzerland) was produced by infecting DF1 chicken embryo fibroblasts (MOI = 0.0001) and harvesting culture supernatant 48 hours later. Virus stocks were titrated by standard plaque assay. Sendai virus (kindly provided by Rainer Zawatzki, Heidelberg, Germany) and New Castle Disease Virus (a kind gift from Georg Kochs, Freiburg, Germany) were propagated in LSL Valo SPF embryonic chicken eggs (Lohmann Tierzucht, Germany). Shortly, eggs were incubated at 37.8°C with 60% humidity and turned every 24 hours. Eggs were infected at embryonic development day 12 with 103 plaque forming units diluted in OptiMEM (Life Technologies, Germany). Allantoic fluid was harvested from 48 to 72 hours post infection, cellular debris was removed by centrifugation and the virus titer was determined by using the TCID50 method. Reo virus was kindly provided by Steeve Boulant (Heidelberg, Germany).
Antibodies
The mouse monoclonal antibody 9E10 detecting NS5A domain III of HCV was kindly provided by Charles Rice (New York, USA). The Pex19-specific monoclonal antibody was a kind gift of Gabrielle Dodt (Tübingen, Germany). The mouse monoclonal NS3/4A antibody recognizing the NS3 helicase (2E3) was generated in cooperation with Hengli Tang (San Diego, USA). The mouse anti-MxA antibody was purchased from Georg Kochs (Freiburg, Germany). Other commercially available antibodies used in this study were mouse monoclonal antibodies recognizing β-actin (Sigma Aldrich, Germany), GAPDH (Santa Cruz Biotechnology, USA) and the HA-tag (Sigma Aldrich, Germany) as well as rabbit polyclonal antibodies recognizing MAVS (Enzo Life science, Switzerland), PMP70 (Abcam, United Kingdom) and CoxIV (Cell Signaling, Netherlands). Goat-anti-rabbit-Alexa-488, goat-anti-mouse-Alexa-488, donkey-anti-mouse-Alexa-568, goat-anti-rabbit-Alexa-568, chicken-anti-rabbit-Alexa-647 (all from Life Technologies, Germany) were used as secondary antibodies. For Western blot analysis anti-mouse and anti-rabbit antibodies, each coupled with horseradish peroxidase were used (Sigma, Germany).
Plasmids and DNA cloning
Unless otherwise stated, plasmids were taken from the Orfeome cDNA library collection (Life Technologies, Germany) that is based on the pENTR221 vector. Organelle-specific targeting of MAVS was achieved by replacing the wild-type-transmembrane (TM) region of MAVS with the TM region of either Bcl-Xl (clone BC019307; aa 203–233) [75] or Pex13 (isolated from cDNA derived from Huh7 cells; aa 136–233) [76]) by using PCR-based mutagenesis. Cleavage resistant MAVS containing the C508R substitution was generated by QuickChange site directed mutagenesis (Agilent Technologies, Germany) as recommended by the manufacturer. To construct the organelle-specific MAVS-eGFP-NLS reporter, the C-terminal domain (CTD) of pex - and wtMAVS were inserted into the pCDNA6.2 vector (Life Technologies, Germany). All the other constructs were generated by inserting the respective gene into the pDONR207 vector and shuttling of the insert into the pWPI lentiviral transduction vector by using the Gateway cloning technology (Life Technologies, Germany) according to the instruction of the manufacturer. The type III IFN reporter pGL3-IFNλ1-Firefly-Lucifease was generated as previously described [77].
Lentivirus production and generation of stable cell lines
Stable cell lines were generated as described earlier [78]. In brief, 1.2x106 293T cells were seeded into a 6 cm-diameter dish one day before transfection. Plasmids pMD.G (encoding the VSV G glycoprotein), pCMVΔR8.91 (encoding HIV gag-pol) and pWPI (encoding the gene of interest and a selection marker) were transfected in a 1 : 3:3 ratio into the cells by using the CaPhos Mammalian Transfection kit (Clontech Laboratories, France) according the manufacturer´s protocol. Supernatant was harvested 36, 48 and 72 hours post transfection and passed through a 0.45 μm filter. Target cells were seeded at the density of 8x104 cells per well of a six-well plate and 24 hours later inoculated with different amounts of supernatant containing lentiviral particles The selection was started 36 hours post transduction. For MAVS transduction experiments lentiviral particles were purified by sedimentation onto a 20% sucrose cushion using a SW28 Ti rotor (Beckman Coulter, Germany) and two hours centrifugation at 20,000 rpm.
Luciferase based reporter systems
RIG-I signaling in Huh7, 293T and A549 cells with organelle-specific MAVS was measured indirectly by using dual luciferase reporter constructs as previously described [79]. Briefly, cells were seeded into 96 well plates 24 hours before co-transfection with either IFIT1, IFNB, or IFNL1 promotor constructs pGL3B or p125-Firefly-Luciferase (kindly provided by Ganes Sen, Cleveland and Takashi Fujita, Tokyo, respectively [79, 80]). The co-transfected Renilla-Luciferase reporter plasmid pRL-SV40 (Promega, Germany) served as transfection control. Plasmid transfection was conducted with the Effecten transfection reagent (Qiagen, Germany) according to the instructions of the manufacturer. Cells were infected with different viruses 24 hours post transfection. For some experiments specified in the results section, cells were transfected with reporter plasmids 24 hours after lentiviral transduction and lysed 16 hours later. For mouse type I IFN bioassays, MEFs expressing organelle-specific MAVS variants were infected with either Sendai or Reo virus. Supernatant was harvested at given time points and stored at -80°C. Mouse L929-ISRE-Firefly-luciferase reporter cells [45] were seeded 24 hours prior to treatment. Reporter cells were stimulated with UV-inactivated supernatant and eight hours later firefly luciferase activity contained in cell lysates was determined.
Quantitative RT-PCR
Total cellular RNA was isolated from single wells of a 24 well plate using the NucleoSpin RNA extraction kit (Macherey-Nagel, Germany) according to the manufacturer´s protocol. RNA analysis was carried out using the two-step qRT-PCR approach. Total RNA was reverse transcribed into cDNA and DNA amplicons were measured with SYBR Green (Bio-Rad, Germany). The following primers were used: human GAPDH: forward 5’-GAAGGTGAAGGTCGGAGTC-3’, reverse 5’ - GAAGATGGTGATGGGATTTC-3’; human IFN-β: forward 5’ - CGCCGCATTGACCATCTA-3’, reverse 5’-GACATTAGCCAGGAGGTTCTC-3’; human IFN-λ1: forward 5’-GCAGGTTCAAATCTCTGTCACC-3’, reverse 5’-AAGACAGGAGAGCTGCAACTC-3’; human IFN-λ2/3 forward 5’-CAGCTGCAGGTGAGGGA-3’, reverse 5’-GCGGTGGCCTCCAGAACCTT-3’; human ISG56: forward 5’-GAAGCAGGCAATCACAGAAA-3’, reverse 5’-TGAAACCGACCATAGTGGAA-3’; mouse GAPDH: forward 5’-AGGTCGGTGTGAACGGATTTG-3’, reverse 5’-TGTAGACCATGTAGTTGAGGTCA-3’; mouse IFN-β: forward 5’-CAGCTCCAAGAAAGGACGAAC-3’, reverse 5’-GGCAGTGTAACTCTTCTGCAT-3’; mouse IFN-λ2/3: forward 5’-AGGGTGCCATCGAGAAGAG-3’, reverse 5’-GTGGTCAGGGCTGAGTCATT-3’. Data were analyzed by using the ΔΔct method as described recently [81].
Enzyme-linked immunosorbent assay (ELISA)
IFN-α pan (Mabtech, Sweden), IFN-β as part of VeriPlex Human Cytokine 16-Plex ELISA Kit (PBL-Interferon source, USA), IFN-λ1–3 (PBL-Interferon source, USA) and mouse IFN-β (BioLegend, USA) contained in the supernatant of cells were quantified according to the instructions of the manufacturers.
Immunofluorescence
Cells were seeded on cover slips in a 24-well plate. In case of mitotracker staining (Life technologies, Germany), the dye was added 20 minutes before fixation with 4% paraformaldehyde (PFA) for 20 minutes. Cells were washed with PBS and permeabilized using 0.5% Triton X-100 for 5 minutes. After blocking of non-specific binding with 3% bovine serum albumin (BSA) in PBS for at least 20 minutes, cells were incubated with primary antibodies in 1% BSA for 60 minutes. After washing with PBS, cells were stained with secondary antibodies in 1% BSA. Nuclear DNA was stained with 4',6-diamidino-2-phenylindole (DAPI, MoBiTec, Germany). Cells were mounted on glass slides using Fluoromount-G (SouthernBiotech, USA). Pictures were taken on either a Leica SP2 confocal laser scanning microscope (Leica, Germany) or a Keyence BZ 9000 Imager (Keyence, Germany). Image processing including cropping, automatic contrast, merge, cell counting and Mander’s coefficient analysis were performed using the Fiji software package [82].
Creating human knockout cell line
Knock-out of MAVS in Huh7 cells was achieved by using the CRISPR/Cas9 system as previously described [49]. In brief, three different single-guide RNAs (sgRNAs) were designed targeting the first and second exon of the coding region of MAVS with the help of the open source tool [83] and inserted into the lentiviral vector lentiCRISPR v2 (Addgene #52961) also encoding the Cas9 nuclease. The following sgRNAs were used: 5’ TCAGCCCTCTGACCTCCAGCG 3’ (#1), 5’ CGCTGGAGGTCAGAGGGCTGG 3’ (#2), 5’ AGGGGCTGCAGAGGGTAAACG 3’ (#3). Lentiviruses were produced as described above and 8x104 cells were transduced three times using undiluted stocks of lentiviral particles encoding sgRNA #1, 2 or 3. All shown data were obtained by using a cell clone treated with sgRNA #1, but analogous results were obtained with cell clones generated with the two other sgRNAs. To establish MAVS knock-out cells, clonal selection was performed using single cell dilution in a 96-well plate. Knock-out was confirmed by Western blotting, immunofluorescence and functional tests.
Small interfering RNA (siRNA)-mediated knockdown
Cells were seeded with a density of 3x104 cells per well of a 24-well plate one day prior to transfection. Transfection was performed with Lipofectamine RNAiMax (Life technologies, Germany) according to the instruction of the manufacturer using a siRNA targeting MAVS (5’ CCCACAGGGUCAGUUGUAU 3’) and a control siRNA. Forty-eight hours post transfection cells were infected with Sendai virus. Knockdown efficiency was determined by Western blot analysis.
Western blot analysis
Cells were lysed in 2x Laemmli buffer (200 mM Tris-HCl [pH8.8], 5 mM EDTA, 0.1% (w/v) Bromophenol Blue, 10% (w/v) Sucrose, 3% (w/v) SDS, 2% β-Mercaptoethanol) and heated to 98°C for 10 minutes. Proteins were separated by SDS-PAGE and blotted onto a PVDF membrane by either semi-dry or wet-blot (Bio-Rad, Germany). Membranes were blocked with 5% milk in PBS containing 0.1% Tween 20 for one hour at room temperature or overnight at 4°C. Membranes were incubated with primary antibodies overnight at 4°C or for 90 minutes at room temperature. Secondary antibodies tagged with horseradish peroxidase were incubated for one hour at room temperature. After washing with PBS containing 0.1% Tween 20, proteins were visualized by using the Western Lightning Plus-ECL reagent (PerkinElmer, USA) and an INTAS Advanced Fluorescence and ECL imager (INTAS, Germany) or a Curix 60 Developer device (AGFA, Germany). The Lab Image 1D software package (Kapelan BioImaging Solutions, Germany) was used for quantification of specific signals that were normalized to β-actin or GAPDH or wild type MAVS expression as specified in the results section.
Microarray
Total RNA was purified as described above and analyzed using the Illumina HumanHT-12 v4 Expression BeadChip technology (Illuminia, USA) according to the protocols of the manufacturer. Data were processed using the software package R (http://www.r-project.org).
Statistics
Statistical analysis of obtained data was performed using the GraphPad Prism software package (GraphPad software, USA). Unpaired t-tests were performed as described in the results section. Asterisks indicate ***, P-value ≤0.0005; **, P-value ≤0.005; *, P-value ≤0.05; ns, non-significant.
Supporting Information
Zdroje
1. Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5'-phosphates. Science. 2006;314(5801):997–1001. doi: 10.1126/science.1132998 17038589.
2. Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature immunology. 2004;5(7):730–7. doi: 10.1038/ni1087 15208624.
3. Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–5. doi: 10.1038/nature04734 16625202.
4. Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–91. doi: 10.1126/science.1232458 23258413; PubMed Central PMCID: PMC3863629.
5. Wu J, Sun L, Chen X, Du F, Shi H, Chen C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–30. doi: 10.1126/science.1229963 23258412; PubMed Central PMCID: PMC3855410.
6. Pichlmair A, Schulz O, Tan CP, Rehwinkel J, Kato H, Takeuchi O, et al. Activation of MDA5 requires higher-order RNA structures generated during virus infection. Journal of virology. 2009;83(20):10761–9. doi: 10.1128/JVI.00770-09 19656871; PubMed Central PMCID: PMC2753146.
7. Baum A, Sachidanandam R, Garcia-Sastre A. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(37):16303–8. doi: 10.1073/pnas.1005077107 20805493; PubMed Central PMCID: PMC2941304.
8. Binder M, Eberle F, Seitz S, Mucke N, Huber CM, Kiani N, et al. Molecular mechanism of signal perception and integration by the innate immune sensor retinoic acid-inducible gene-I (RIG-I). The Journal of biological chemistry. 2011;286(31):27278–87. doi: 10.1074/jbc.M111.256974 21659521; PubMed Central PMCID: PMC3149321.
9. Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122(5):669–82. doi: 10.1016/j.cell.2005.08.012 16125763.
10. Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437(7062):1167–72. doi: 10.1038/nature04193 16177806.
11. Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Molecular cell. 2005;19(6):727–40. doi: 10.1016/j.molcel.2005.08.014 16153868.
12. Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, et al. IPS-1, an adaptor triggering RIG-I - and Mda5-mediated type I interferon induction. Nature immunology. 2005;6(10):981–8. doi: 10.1038/ni1243 16127453.
13. Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446(7138):916–20. doi: 10.1038/nature05732 17392790.
14. Wu B, Hur S. How RIG-I like receptors activate MAVS. Current opinion in virology. 2015;12 : 91–8. doi: 10.1016/j.coviro.2015.04.004 25942693.
15. Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146(3):448–61. doi: 10.1016/j.cell.2011.06.041 21782231; PubMed Central PMCID: PMC3179916.
16. Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. Journal of virology. 2008;82(1):335–45. doi: 10.1128/JVI.01080-07 17942531; PubMed Central PMCID: PMC2224404.
17. Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. Journal of immunology. 2005;175(5):2851–8. 16116171.
18. Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nature immunology. 2003;4(1):69–77. doi: 10.1038/ni875 12483210.
19. Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nature immunology. 2003;4(1):63–8. doi: 10.1038/ni873 12469119.
20. Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nature genetics. 2013;45(2):164–71. doi: 10.1038/ng.2521 23291588; PubMed Central PMCID: PMC3793390.
21. Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. Journal of virology. 2006;80(9):4501–9. doi: 10.1128/JVI.80.9.4501-4509.2006 16611910; PubMed Central PMCID: PMC1472004.
22. Park H, Serti E, Eke O, Muchmore B, Prokunina-Olsson L, Capone S, et al. IL-29 is the dominant type III interferon produced by hepatocytes during acute hepatitis C virus infection. Hepatology. 2012;56(6):2060–70. doi: 10.1002/hep.25897 22706965; PubMed Central PMCID: PMC3581145.
23. Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, MacDonald MR, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131(6):1887–98. doi: 10.1053/j.gastro.2006.09.052 17087946.
24. Laidlaw SM, Dustin LB. Interferon lambda: opportunities, risks, and uncertainties in the fight against HCV. Frontiers in immunology. 2014;5 : 545. doi: 10.3389/fimmu.2014.00545 25400636; PubMed Central PMCID: PMC4215632.
25. Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS pathogens. 2008;4(3):e1000017. doi: 10.1371/journal.ppat.1000017 18369468; PubMed Central PMCID: PMC2265414.
26. Dickensheets H, Sheikh F, Park O, Gao B, Donnelly RP. Interferon-lambda (IFN-lambda) induces signal transduction and gene expression in human hepatocytes, but not in lymphocytes or monocytes. Journal of leukocyte biology. 2013;93(3):377–85. doi: 10.1189/jlb.0812395 23258595; PubMed Central PMCID: PMC3579021.
27. Zhou Z, Hamming OJ, Ank N, Paludan SR, Nielsen AL, Hartmann R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. Journal of virology. 2007;81(14):7749–58. doi: 10.1128/JVI.02438-06 17507495; PubMed Central PMCID: PMC1933366.
28. Jacobson IM, Davis GL, El-Serag H, Negro F, Trepo C. Prevalence and challenges of liver diseases in patients with chronic hepatitis C virus infection. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2010;8(11):924–33; quiz e117. doi: 10.1016/j.cgh.2010.06.032 20713178.
29. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. Journal of hepatology. 2006;45(4):529–38. doi: 10.1016/j.jhep.2006.05.013 16879891.
30. Bartenschlager R, Lohmann V, Penin F. The molecular and structural basis of advanced antiviral therapy for hepatitis C virus infection. Nature reviews Microbiology. 2013;11(7):482–96. doi: 10.1038/nrmicro3046 23748342.
31. Foy E, Li K, Sumpter R Jr., Loo YM, Johnson CL, Wang C, et al. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(8):2986–91. doi: 10.1073/pnas.0408707102 15710892; PubMed Central PMCID: PMC549461.
32. Horner SM, Liu HM, Park HS, Briley J, Gale M Jr. Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(35):14590–5. doi: 10.1073/pnas.1110133108 21844353; PubMed Central PMCID: PMC3167523.
33. Bellecave P, Sarasin-Filipowicz M, Donze O, Kennel A, Gouttenoire J, Meylan E, et al. Cleavage of mitochondrial antiviral signaling protein in the liver of patients with chronic hepatitis C correlates with a reduced activation of the endogenous interferon system. Hepatology. 2010;51(4):1127–36. doi: 10.1002/hep.23426 20044805.
34. Dixit E, Boulant S, Zhang Y, Lee AS, Odendall C, Shum B, et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 2010;141(4):668–81. doi: 10.1016/j.cell.2010.04.018 20451243; PubMed Central PMCID: PMC3670185.
35. Odendall C, Dixit E, Stavru F, Bierne H, Franz KM, Durbin AF, et al. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nature immunology. 2014;15(8):717–26. doi: 10.1038/ni.2915 24952503; PubMed Central PMCID: PMC4106986.
36. Lin R, Lacoste J, Nakhaei P, Sun Q, Yang L, Paz S, et al. Dissociation of a MAVS/IPS-1/VISA/Cardif-IKKepsilon molecular complex from the mitochondrial outer membrane by hepatitis C virus NS3-4A proteolytic cleavage. Journal of virology. 2006;80(12):6072–83. doi: 10.1128/JVI.02495-05 16731946; PubMed Central PMCID: PMC1472616.
37. Loo YM, Owen DM, Li K, Erickson AK, Johnson CL, Fish PM, et al. Viral and therapeutic control of IFN-beta promoter stimulator 1 during hepatitis C virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(15):6001–6. doi: 10.1073/pnas.0601523103 16585524; PubMed Central PMCID: PMC1458687.
38. Jones CT, Catanese MT, Law LM, Khetani SR, Syder AJ, Ploss A, et al. Real-time imaging of hepatitis C virus infection using a fluorescent cell-based reporter system. Nature biotechnology. 2010;28(2):167–71. doi: 10.1038/nbt.1604 20118917; PubMed Central PMCID: PMC2828266.
39. Bartenschlager R, Ahlborn-Laake L, Mous J, Jacobsen H. Nonstructural protein 3 of the hepatitis C virus encodes a serine-type proteinase required for cleavage at the NS3/4 and NS4/5 junctions. Journal of virology. 1993;67(7):3835–44. 8389908; PubMed Central PMCID: PMC237748.
40. Cheng G, Zhong J, Chisari FV. Inhibition of dsRNA-induced signaling in hepatitis C virus-infected cells by NS3 protease-dependent and -independent mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(22):8499–504. doi: 10.1073/pnas.0602957103 16707574; PubMed Central PMCID: PMC1482521.
41. Oshiumi H, Miyashita M, Matsumoto M, Seya T. A distinct role of Riplet-mediated K63-Linked polyubiquitination of the RIG-I repressor domain in human antiviral innate immune responses. PLoS pathogens. 2013;9(8):e1003533. doi: 10.1371/journal.ppat.1003533 23950712; PubMed Central PMCID: PMC3738492.
42. Baril M, Racine ME, Penin F, Lamarre D. MAVS dimer is a crucial signaling component of innate immunity and the target of hepatitis C virus NS3/4A protease. Journal of virology. 2009;83(3):1299–311. doi: 10.1128/JVI.01659-08 19036819; PubMed Central PMCID: PMC2620913.
43. Mi Z, Ma Y, Tong Y. Avian influenza virus H5N1 induces rapid interferon-beta production but shows more potent inhibition to retinoic acid-inducible gene I expression than H1N1 in vitro. Virology journal. 2012;9 : 145. doi: 10.1186/1743-422X-9-145 22862800; PubMed Central PMCID: PMC3464129.
44. Abe K, Ishigami T, Shyu AB, Ohno S, Umemura S, Yamashita A. Analysis of interferon-beta mRNA stability control after poly(I:C) stimulation using RNA metabolic labeling by ethynyluridine. Biochemical and biophysical research communications. 2012;428(1):44–9. doi: 10.1016/j.bbrc.2012.09.144 23063848; PubMed Central PMCID: PMC3672056.
45. Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, et al. CD14 is required for MyD88-independent LPS signaling. Nature immunology. 2005;6(6):565–70. doi: 10.1038/ni1207 15895089.
46. Dodt G, Braverman N, Wong C, Moser A, Moser HW, Watkins P, et al. Mutations in the PTS1 receptor gene, PXR1, define complementation group 2 of the peroxisome biogenesis disorders. Nature genetics. 1995;9(2):115–25. doi: 10.1038/ng0295-115 7719337.
47. Muntau AC, Roscher AA, Kunau WH, Dodt G. The interaction between human PEX3 and PEX19 characterized by fluorescence resonance energy transfer (FRET) analysis. European journal of cell biology. 2003;82(7):333–42. doi: 10.1078/0171-9335-00325 12924628.
48. Agrawal G, Subramani S. Emerging role of the endoplasmic reticulum in peroxisome biogenesis. Frontiers in physiology. 2013;4 : 286. doi: 10.3389/fphys.2013.00286 24115935; PubMed Central PMCID: PMC3792350.
49. Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343(6166):84–7. doi: 10.1126/science.1247005 24336571; PubMed Central PMCID: PMC4089965.
50. Binder M, Kochs G, Bartenschlager R, Lohmann V. Hepatitis C virus escape from the interferon regulatory factor 3 pathway by a passive and active evasion strategy. Hepatology. 2007;46(5):1365–74. doi: 10.1002/hep.21829 17668876.
51. Fujiki Y, Rachubinski RA, Lazarow PB. Synthesis of a major integral membrane polypeptide of rat liver peroxisomes on free polysomes. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(22):7127–31. 6594687; PubMed Central PMCID: PMC392090.
52. Suzuki Y, Orii T, Takiguchi M, Mori M, Hijikata M, Hashimoto T. Biosynthesis of membrane polypeptides of rat liver peroxisomes. Journal of biochemistry. 1987;101(2):491–6. 3584097.
53. Imanaka T, Shiina Y, Takano T, Hashimoto T, Osumi T. Insertion of the 70-kDa peroxisomal membrane protein into peroxisomal membranes in vivo and in vitro. The Journal of biological chemistry. 1996;271(7):3706–13. 8631984.
54. Hoepfner D, Schildknegt D, Braakman I, Philippsen P, Tabak HF. Contribution of the endoplasmic reticulum to peroxisome formation. Cell. 2005;122(1):85–95. doi: 10.1016/j.cell.2005.04.025 16009135.
55. Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, et al. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Current biology: CB. 2008;18(2):102–8. doi: 10.1016/j.cub.2007.12.038 18207745.
56. Jones JM, Morrell JC, Gould SJ. PEX19 is a predominantly cytosolic chaperone and import receptor for class 1 peroxisomal membrane proteins. The Journal of cell biology. 2004;164(1):57–67. doi: 10.1083/jcb.200304111 14709540; PubMed Central PMCID: PMC2171958.
57. Kinoshita N, Ghaedi K, Shimozawa N, Wanders RJ, Matsuzono Y, Imanaka T, et al. Newly identified Chinese hamster ovary cell mutants are defective in biogenesis of peroxisomal membrane vesicles (Peroxisomal ghosts), representing a novel complementation group in mammals. The Journal of biological chemistry. 1998;273(37):24122–30. 9727033.
58. Muntau AC, Mayerhofer PU, Paton BC, Kammerer S, Roscher AA. Defective peroxisome membrane synthesis due to mutations in human PEX3 causes Zellweger syndrome, complementation group G. American journal of human genetics. 2000;67(4):967–75. doi: 10.1086/303071 10958759; PubMed Central PMCID: PMC1287898.
59. Ghaedi K, Honsho M, Shimozawa N, Suzuki Y, Kondo N, Fujiki Y. PEX3 is the causal gene responsible for peroxisome membrane assembly-defective Zellweger syndrome of complementation group G. American journal of human genetics. 2000;67(4):976–81. doi: 10.1086/303086 10968777; PubMed Central PMCID: PMC1287899.
60. Eno CO, Eckenrode EF, Olberding KE, Zhao G, White C, Li C. Distinct roles of mitochondria - and ER-localized Bcl-xL in apoptosis resistance and Ca2+ homeostasis. Molecular biology of the cell. 2012;23(13):2605–18. doi: 10.1091/mbc.E12-02-0090 22573883; PubMed Central PMCID: PMC3386223.
61. Tagami S, Eguchi Y, Kinoshita M, Takeda M, Tsujimoto Y. A novel protein, RTN-XS, interacts with both Bcl-XL and Bcl-2 on endoplasmic reticulum and reduces their anti-apoptotic activity. Oncogene. 2000;19(50):5736–46. doi: 10.1038/sj.onc.1203948 11126360.
62. Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(8):3668–72. 9108035; PubMed Central PMCID: PMC20498.
63. Bauhofer O, Ruggieri A, Schmid B, Schirmacher P, Bartenschlager R. Persistence of HCV in quiescent hepatic cells under conditions of an interferon-induced antiviral response. Gastroenterology. 2012;143(2):429–38 e8. Epub 2012/04/24. doi: 10.1053/j.gastro.2012.04.018 22522091.
64. Ding S, Robek MD. Peroxisomal MAVS activates IRF1-mediated IFN-lambda production. Nature immunology. 2014;15(8):700–1. doi: 10.1038/ni.2924 25045870.
65. Marukian S, Andrus L, Sheahan TP, Jones CT, Charles ED, Ploss A, et al. Hepatitis C virus induces interferon-lambda and interferon-stimulated genes in primary liver cultures. Hepatology. 2011;54(6):1913–23. doi: 10.1002/hep.24580 21800339; PubMed Central PMCID: PMC3219820.
66. Thomas E, Gonzalez VD, Li Q, Modi AA, Chen W, Noureddin M, et al. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology. 2012;142(4):978–88. doi: 10.1053/j.gastro.2011.12.055 22248663; PubMed Central PMCID: PMC3435150.
67. Hiet MS, Bauhofer O, Zayas M, Roth H, Tanaka Y, Schirmacher P, et al. Control of temporal activation of hepatitis C virus-induced interferon response by domain 2 of nonstructural protein 5A. Journal of hepatology. 2015. doi: 10.1016/j.jhep.2015.04.015 25908268.
68. Sheahan T, Imanaka N, Marukian S, Dorner M, Liu P, Ploss A, et al. Interferon lambda alleles predict innate antiviral immune responses and hepatitis C virus permissiveness. Cell host & microbe. 2014;15(2):190–202. Epub 2014/02/18. doi: 10.1016/j.chom.2014.01.007 24528865; PubMed Central PMCID: PMC4104123.
69. Bolen CR, Ding S, Robek MD, Kleinstein SH. Dynamic expression profiling of type I and type III interferon-stimulated hepatocytes reveals a stable hierarchy of gene expression. Hepatology. 2014;59(4):1262–72. doi: 10.1002/hep.26657 23929627; PubMed Central PMCID: PMC3938553.
70. Onoguchi K, Yoneyama M, Takemura A, Akira S, Taniguchi T, Namiki H, et al. Viral infections activate types I and III interferon genes through a common mechanism. The Journal of biological chemistry. 2007;282(10):7576–81. doi: 10.1074/jbc.M608618200 17204473.
71. Jewell NA, Cline T, Mertz SE, Smirnov SV, Flano E, Schindler C, et al. Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. Journal of virology. 2010;84(21):11515–22. doi: 10.1128/JVI.01703-09 20739515; PubMed Central PMCID: PMC2953143.
72. Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. Journal of virology. 2010;84(11):5670–7. doi: 10.1128/JVI.00272-10 20335250; PubMed Central PMCID: PMC2876583.
73. Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, Steinmann E, et al. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(19):7408–13. doi: 10.1073/pnas.0504877103 16651538; PubMed Central PMCID: PMC1455439.
74. Reiss S, Rebhan I, Backes P, Romero-Brey I, Erfle H, Matula P, et al. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell host & microbe. 2011;9(1):32–45. doi: 10.1016/j.chom.2010.12.002 21238945; PubMed Central PMCID: PMC3433060.
75. Kaufmann T, Schlipf S, Sanz J, Neubert K, Stein R, Borner C. Characterization of the signal that directs Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. The Journal of cell biology. 2003;160(1):53–64. doi: 10.1083/jcb.200210084 12515824; PubMed Central PMCID: PMC2172731.
76. Fransen M, Wylin T, Brees C, Mannaerts GP, Van Veldhoven PP. Human pex19p binds peroxisomal integral membrane proteins at regions distinct from their sorting sequences. Molecular and cellular biology. 2001;21(13):4413–24. doi: 10.1128/MCB.21.13.4413-4424.2001 11390669; PubMed Central PMCID: PMC87101.
77. Siegel R, Eskdale J, Gallagher G. Regulation of IFN-lambda1 promoter activity (IFN-lambda1/IL-29) in human airway epithelial cells. Journal of immunology. 2011;187(11):5636–44. doi: 10.4049/jimmunol.1003988 22058416.
78. Kaul A, Stauffer S, Berger C, Pertel T, Schmitt J, Kallis S, et al. Essential role of cyclophilin A for hepatitis C virus replication and virus production and possible link to polyprotein cleavage kinetics. PLoS pathogens. 2009;5(8):e1000546. doi: 10.1371/journal.ppat.1000546 19680534; PubMed Central PMCID: PMC2718831.
79. Grandvaux N, Servant MJ, tenOever B, Sen GC, Balachandran S, Barber GN, et al. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. Journal of virology. 2002;76(11):5532–9. 11991981; PubMed Central PMCID: PMC137057.
80. Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. The EMBO journal. 1998;17(4):1087–95. doi: 10.1093/emboj/17.4.1087 9463386; PubMed Central PMCID: PMC1170457.
81. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262 11846609.
82. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9(7):676–82. doi: 10.1038/nmeth.2019 22743772; PubMed Central PMCID: PMC3855844.
83. Heigwer F, Kerr G, Boutros M. E-CRISP: fast CRISPR target site identification. Nature methods. 2014;11(2):122–3. doi: 10.1038/nmeth.2812 24481216.
84. Friebe P, Boudet J, Simorre JP, Bartenschlager R. Kissing-loop interaction in the 3' end of the hepatitis C virus genome essential for RNA replication. Journal of virology. 2005;79(1):380–92. Epub 2004/12/15. doi: 10.1128/JVI.79.1.380-392.2005 15596831; PubMed Central PMCID: PMC538730.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin InfectionČlánek Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 11- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Parasite Glycobiology: A Bittersweet Symphony
- On the Discovery of TOR As the Target of Rapamycin
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
- PML/TRIM19-Dependent Inhibition of Retroviral Reverse-Transcription by Daxx
- Cleavage of a Neuroinvasive Human Respiratory Virus Spike Glycoprotein by Proprotein Convertases Modulates Neurovirulence and Virus Spread within the Central Nervous System
- Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Induces the Oncogenic miR-17-92 Cluster and Down-Regulates TGF-β Signaling
- Interferon-α Subtypes in an Model of Acute HIV-1 Infection: Expression, Potency and Effector Mechanisms
- Perivascular Arrest of CD8 T Cells Is a Signature of Experimental Cerebral Malaria
- Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells
- Evolution and Emergence of Enteroviruses through Intra- and Inter-species Recombination: Plasticity and Phenotypic Impact of Modular Genetic Exchanges in the 5’ Untranslated Region
- Interferon-γ Inhibits Ebola Virus Infection
- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- P-Type Cyclin CYC3 Modulates Endomitotic Growth during Oocyst Development in Mosquitoes
- Diversity of across Evolutionary Scales
- 50 Years of Disease in Humans: The Dramatic Emergence of a Cluster of Novel Fungal Pathogens
- Worse Comes to Worst: Bananas and Panama Disease—When Plant and Pathogen Clones Meet
- Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection
- Infection-Induced Retrotransposon-Derived Noncoding RNAs Enhance Herpesviral Gene Expression via the NF-κB Pathway
- Structural Insight into Archaic and Alternative Chaperone-Usher Pathways Reveals a Novel Mechanism of Pilus Biogenesis
- Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin Infection
- Global Analysis of the Fungal Microbiome in Cystic Fibrosis Patients Reveals Loss of Function of the Transcriptional Repressor Nrg1 as a Mechanism of Pathogen Adaptation
- The Transcription and Translation Landscapes during Human Cytomegalovirus Infection Reveal Novel Host-Pathogen Interactions
- The N-terminal Helical Region of the Hepatitis C Virus p7 Ion Channel Protein Is Critical for Infectious Virus Production
- Activation of Type I and III Interferon Response by Mitochondrial and Peroxisomal MAVS and Inhibition by Hepatitis C Virus
- Hsp70 Isoforms Are Essential for the Formation of Kaposi’s Sarcoma-Associated Herpesvirus Replication and Transcription Compartments
- Distinct Upstream Role of Type I IFN Signaling in Hematopoietic Stem Cell-Derived and Epithelial Resident Cells for Concerted Recruitment of Ly-6C Monocytes and NK Cells via CCL2-CCL3 Cascade
- and Bats: Story of an Emerging Friendship
- Emergence of Pathogenicity in Lagoviruses: Evolution from Pre-existing Nonpathogenic Strains or through a Species Jump?
- Ebolavirus Evolution: Past and Present
- Host and Symbiont Jointly Control Gut Microbiota during Complete Metamorphosis
- Non-Human Primates Harbor Diverse Mammalian and Avian Astroviruses Including Those Associated with Human Infections
- Lactate Dehydrogenase Is Associated with the Parasitophorous Vacuole Membrane and Is a Potential Target for Developing Therapeutics
- Five Questions about Mycoviruses
- Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
- Ethanolamine Signaling Promotes Niche Recognition and Adaptation during Infection
- Cross-Species Transmission and Differential Fate of an Endogenous Retrovirus in Three Mammal Lineages
- Memory Th1 Cells Are Protective in Invasive Infection
- Transcription Factor SomA Is Required for Adhesion, Development and Virulence of the Human Pathogen
- An -Methyltransferase Is Required for Infection of Tick Cells by
- RNA-seq Brings New Insights to the Intra-Macrophage Transcriptome of Typhimurium
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- On the Discovery of TOR As the Target of Rapamycin
- Parasite Glycobiology: A Bittersweet Symphony
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



