-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Ebolavirus Evolution: Past and Present
The past year has marked the most devastating Ebola outbreak the world has ever witnessed, with over 28,000 cases and over 11,000 deaths. Ebola virus (EBOV) has now been around for almost 50 years. In this review, we discuss past and present outbreaks of EBOV and how those variants evolved over time. We explore and discuss selective pressures that drive the evolution of different Ebola variants, and how they may modify the efficacy of therapeutic treatments and vaccines currently being developed. Finally, given the unprecedented size and spread of the outbreak, as well as the extended period of replication in human hosts, specific attention is given to the 2014–2015 West African outbreak variant (Makona).
Published in the journal: Ebolavirus Evolution: Past and Present. PLoS Pathog 11(11): e32767. doi:10.1371/journal.ppat.1005221
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1005221Summary
The past year has marked the most devastating Ebola outbreak the world has ever witnessed, with over 28,000 cases and over 11,000 deaths. Ebola virus (EBOV) has now been around for almost 50 years. In this review, we discuss past and present outbreaks of EBOV and how those variants evolved over time. We explore and discuss selective pressures that drive the evolution of different Ebola variants, and how they may modify the efficacy of therapeutic treatments and vaccines currently being developed. Finally, given the unprecedented size and spread of the outbreak, as well as the extended period of replication in human hosts, specific attention is given to the 2014–2015 West African outbreak variant (Makona).
Introduction
Filoviruses are negative-sense, single-stranded RNA viruses. Depending on the virus and the variant involved, case fatality rates in outbreak settings can vary between 25% and 90%. Almost 50 years ago, the first member of the Filoviridae family, Marburg virus (MARV), was identified following two simultaneous outbreaks in Marburg, Germany, and Frankfurt, Germany, as well as in Belgrade, Serbia (former Yugoslavia) [1]. It was not until 1976 that the first two outbreaks of Ebola virus (EBOV) occurred. Similarly to Marburg virus, EBOV caused two simultaneous, yet unrelated, outbreaks [2,3]. Even though the filovirus field has progressed tremendously in the last 50 years, there are still no licensed vaccines or treatments approved for human use. However, the situation is rapidly changing as a result of the current outbreak in West Africa. For the first time in the history of filovirus outbreaks, experimental treatments developed in laboratories have been used to treat Ebola-infected health care workers. The first therapeutic used in the 2014–2015 West African outbreak was ZMapp, a cocktail of three monoclonal antibodies developed at the Public Health Agency of Canada in collaboration with Defyrus, the United States Army Medical Research Institute of Infectious Diseases (USAMRIID), Kentucky BioProcessing, and Mapp Biopharmaceutical. ZMapp has been shown to be the most effective postexposure intervention to date in the nonhuman primate (NHP) model, with 100% protection up to five days after the challenge with EBOV [4]. Other therapeutic options that have been used on the field include MIL-77, an antibody-based cocktail closely related to ZMapp, and Favipiravir, a small molecule that interferes with the RNA-dependent RNA polymerase of a variety of viruses including EBOV, convalescent whole blood, and convalescent plasma [5]. Clinical trials for two vaccine candidates, the chimpanzee-adenovirus ChAd3-Zaire Ebola virus (ChAd3-ZEBOV) vaccine and the recombinant vesicular stomatitis virus-Zaire Ebola virus (rVSV-ZEBOV) vaccine, have also been accelerated with the support of the World Health Organization. It is interesting to note that these therapeutics and vaccines are among the few that made it so quickly from the laboratory to the field. WHO and its international partners have invested a considerable amount of effort to fast-track clinical trials for these two vaccines. These efforts were rewarded six months after the outbreak was officially declared, when Phase I clinical trials for the ChAd3-ZEBOV vaccine, developed in the United States, began in the United Kingdom and the US (September 2014), as well as in Mali and Switzerland (October 2014). The rVSV-ZEBOV vaccine, developed in Canada, also began trials in the US (October 2014) and Gabon, Germany, and Switzerland (November 2014), as well as Kenya and Canada (December 2014). Both the ChAd3-ZEBOV and the rVSV-ZEBOV vaccines began Phase III clinical trials at the beginning of 2015. Preliminary results from an open-label, cluster-randomised ring vaccination trial with rVSV-ZEBOV conducted in Guinea showed a vaccine efficacy of 100% [6]. While this number still requires further confirmation, it surely is encouraging. One of the key underlying problems with these therapies and vaccines is that they have been designed to treat or protect against only one virus of the Filoviridae family, which is currently composed of eight distinct viruses and many more variants. This review will focus on the evolution of filoviruses, with an in-depth look into the Ebolavirus genus and the impact that this evolution may have on current therapies and vaccines in development.
Ebolavirus
Ebolavirus was the second genus of the Filoviridae family to be discovered. It was first identified in 1976 following an outbreak in Zaïre (now known as the Democratic Republic of Congo, or DRC), located in Central Africa, resulting in 318 infections and 280 deaths with a case fatality rate (CFR) of 88% (Fig 1A) [3]. The outbreak was caused by what is now known to be the most lethal species of Ebolavirus, Zaire ebolavirus (EBOV). This species has caused 15 subsequent outbreaks (including the current West African outbreak and simultaneous outbreak in Boende, DRC) with an average CFR of 79% [7–14]. Simultaneously, an unrelated outbreak occurred in Sudan, located in the northeastern region of Africa, where 284 cases and 151 deaths were reported with a CFR of 53% [2]. This new species, Sudan ebolavirus (SUDV), ended up causing six additional outbreaks with an average CFR of 63% [15–19]. In 1994, a new Ebola species, Taï Forest ebolavirus (TAFV), was identified in West Africa [20], a considerable geographic distance from previous outbreaks. A scientist practicing an autopsy on a chimpanzee contracted the virus, most likely through handling infected fluids or organs from the infected animal. After receiving treatment in Switzerland, the patient fully recovered and is the only known case of TAFV disease in humans to date. In 2007, 13 years after the identification of the third species of Ebolavirus, Uganda became afflicted with another episode of viral hemorrhagic fever. There were 37 reported deaths over a total of 149 cases, for a CFR of 25%. This time, the causative agent was a genetically distinct species of Ebolavirus, Bundibugyo ebolavirus (BDBV) [21], the least lethal species of Ebolavirus in humans as of now, with an average CFR of 38% from documented outbreaks. There has been only one other outbreak caused by this species, which was in 2012 in the DRC [15]. The last species of the genus, Reston ebolavirus (RESTV), lies in a different category from the four species previously mentioned, due to its lack of pathogenicity in humans to date. This species has surfaced on seven different occasions, infecting NHPs, pigs, and humans [22–29]. RESTV is capable of inducing disease in NHPs, and more recently was shown to cause asymptomatic infection in pigs [30,31]. The 2014–2015 West African outbreak was caused by the EBOV variant Makona (Fig 2). This variant was found to belong to a different clade of EBOV; however, this clade is in a sister relationship with the other known EBOV sequences [32]. This would suggest a parallel evolution from a common ancestor, with variants from the DRC or Gabon. As to why fatality rates vary between species, that mystery remains at least partially unsolved to this day. Various outbreak settings and levels of clinical care over time make the analysis difficult. From an evolutionary point of view, the various levels of pathogenicity could be explained by various mutations in any of EBOV’s seven genes. Not enough is actually known about the precise mechanism of action of each protein of the virus and how variation in those proteins can attenuate or enhance pathogenicity, compared to other viruses such as influenza or the human immunodeficiency virus (HIV). However, many studies have linked different genes to functions associated with virulence [33–40]. For example, the glycoprotein (GP) gene has been linked to cytotoxicity and virulence, although not sufficiently to explain some of the important dichotomies between contrasting species such as Zaire and Reston [41–44]. Similarly, VP24 and VP35 are well-known inhibitors of innate immune signalling pathways, but the extent of the molecular disparities between the different species and variants has never been assessed directly [45]. The amount of clinical data collected during this outbreak may now provide some understanding as to the exact determinants of fatality rates and pathogenicity between species.
Fig. 1. Ebolavirus outbreaks past and present. 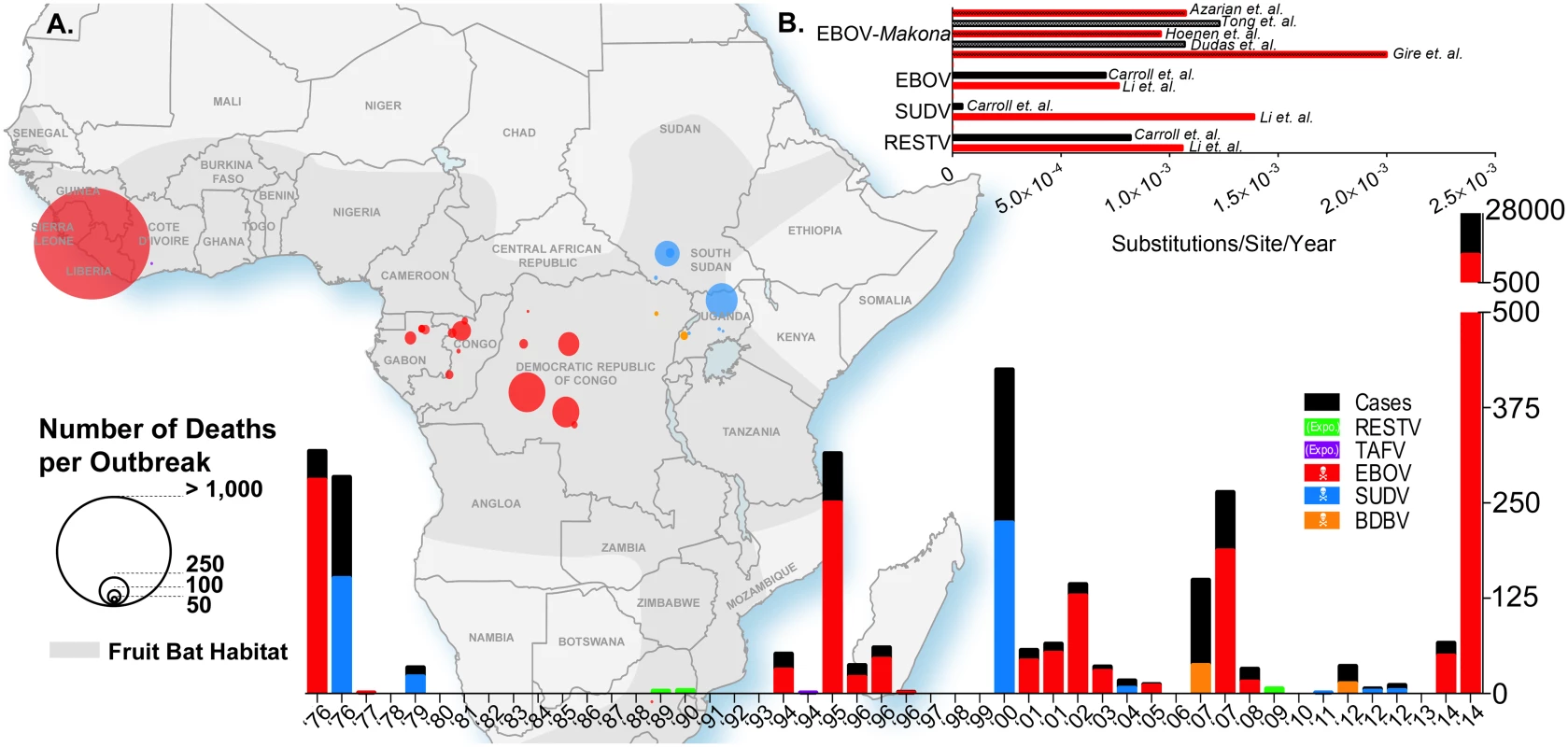
(A) The geographic map of Africa and the bottom histogram illustrate the number of cases, deaths, and the geographic distribution of several Ebola viruses including Reston (RESTV), Tai Forest (TAFV), Ebola (EBOV, formerly Zaire), Sudan (SUDV), and Bundibugyo (BDBV). The histogram in the top right (B) is a review of the calculated evolutionary rates available for EBOV, EBOV-Makona, SUDV, and RESTV from various publications. Fig. 2. Phylogenetic comparison of Filoviridae variants past and present. 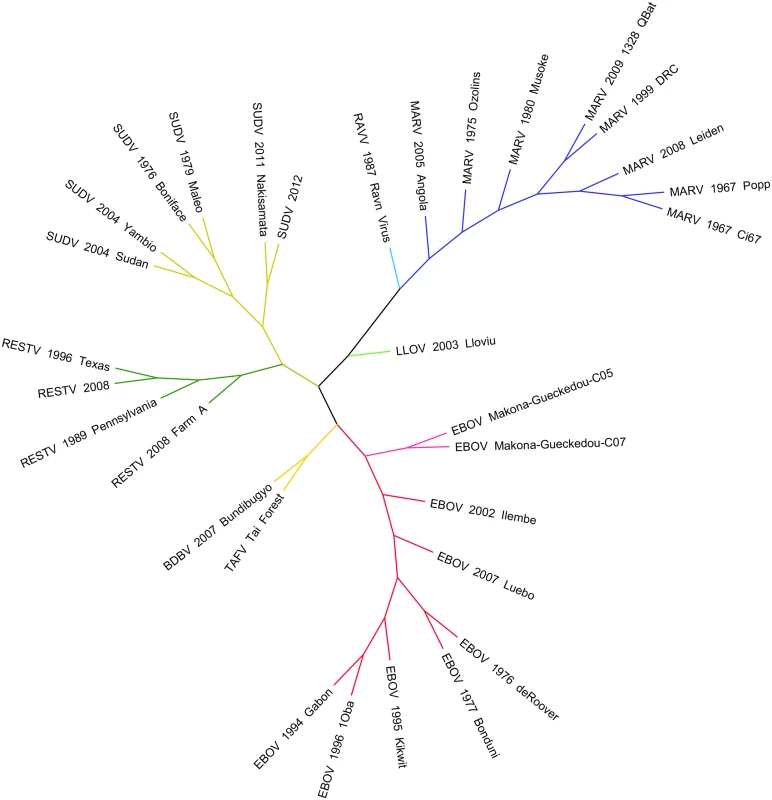
A phylogenetic analysis was undertaken of Filoviridae members from various historical outbreaks as well as the recent 2014–2015 West African outbreak. Thirty-one whole genome sequences were aligned using Clustal Omega 1.2 and visualized in Figtree 1.4. Evolutionary rate
The process through which various species of Ebolavirus have evolved remains unclear to this day. However, recent advances in next-generation sequencing (NGS) will allow for the unprecedented evolutionary analysis needed to understand Ebolavirus evolution. Early sequencing in the mid-1990s allowed for the establishment of the first phylogenetic tree to shed light on the relationship between different variants of the first four Ebolavirus species that had been discovered at that time (Bundibugyo ebolavirus had not been identified yet) as well as two viruses of the Marburg marburgvirus species [46]. This tree was based on the sequencing of the GP gene, which is the least conserved between Filoviruses [47]. Some reports suggest that the polymerase (L) gene should be used as a reference gene, as it is often thought to be the most conserved gene within most viruses. However, Sanchez et al. showed in 2005 that this was not the case for Ebolaviruses, and that VP40 and VP24 were conserved to an even greater extent between species than the L gene [47]. As mentioned by Li et al., it is unclear, however, if using GP as a reference sequence is the best option [48]. It was shown previously that the difference between the evolutionary rates of GP and L for EBOV was not statistically significant, with ~8.0 x 10−4 versus 6.2 x 10−4 substitutions per site per year, respectively [49,50]. In 2014, Li et al. established that the evolutionary rates for the same two genes were 13.94 x 10−4 and 23.14 x 10−4 substitutions per site per year for EBOV [48], a difference which was also not statistically significant. These studies establish that either gene could be suitable for phylogeny and evolutionary analysis of EBOV, even though GP is less conserved between species and variants and mutates at a similar rate as L. Based on that conclusion, it was estimated by Li et al. that the evolutionary rates were 7.66 x 10−4, 13.94 x 10−4, and 10.61 x 10−4 substitutions/site/year for EBOV, SUDV, and RESTV, respectively (Fig 1B) [48]. These rates are in keeping with those observed by Carroll et al. with the exception of SUDV (7.06 x 10−4, 0.46 x 10−4, and 8.21 x 10−4 substitutions/site/year for EBOV, SUDV, and RESTV, respectively) [51]. Some of the minor disparities in these studies could be explained by the use of only GP sequences by Li et al., whereas Carroll et al. based their findings on full genome sequences. One explanation for the drastic differences observed in evolutionary rates reported for SUDV could be the number of sequences included in the analyses. Carroll et al. included the sequence from the 2011 SUDV outbreak in Uganda, whereas Li et al. only included sequences up to the 2004 outbreak in Sudan. Of note, TAFV and BDBV were excluded from both analyses because of the lack of available sequences. These results are also in accordance with the analysis conducted by Walsh et al. that established the evolutionary rate of EBOV to be 9.50 x 10−4 substitutions/site/year [49].
Previous work conducted retrospectively after the 1995 outbreak in Kikwit, DRC, revealed that EBOV was genetically stable throughout the outbreak [52], based on the sequencing of a 249-nucleotide region in GP, suggesting that EBOV had been relatively constant in previous outbreak scenarios. Both the 1995 outbreak and the current 2014–2015 outbreak were caused by a single introduction of the virus into the community; however, the 1995 outbreak lasted only six months, which is in stark contrast to the current outbreak [53]. Initial studies established that the 2014–2015 outbreak strain (Makona) had an evolutionary rate between 1.07 x 10−3 [54] and ~2.0 x 10−3 substitutions/site/year [55]. The latter study by Gire et al. quite alarmingly revealed that the evolutionary rate for the Makona strain could be significantly higher than previous outbreaks. However, a recent study by Hoenen et al. established that the evolutionary rate for Makona, based on full-length genome sequencing of samples from the outbreak in Mali and the available sequences used by Gire et al., was 9.6 x 10−4 substitutions/site/year, a value that agrees with previously reported rates [56]. Interestingly, Hoenen et al. found that using the same dataset and analysis parameters used by Gire et al. yielded a rate of 6.9 x 10−4, while reanalysis of the sequences published by Gire et al. with a strict molecular clock model yielded a rate of 8.2 x 10−4 substitutions/site/year. This highlights the challenging nature of various predictive models for viral divergence and the fact that interpretations from these observations should be taken with caution. Molecular clock models are used to estimate the length of phylogeny divergence. In a strict molecular clock model, it is assumed that the rate of evolution for each branch of the phylogenetic tree is the same, while in a relaxed molecular clock model, that rate can vary among different sections of the phylogeny [57]. The most recent genetics analyses conducted by Tong et al. and Azarian et al. reported evolutionary rates of 1.23 x 10−3 (based on whole genome sequences) and 1.075 x 10−3 (based on GP sequences) substitutions/site/year, respectively [58,59]. Overall, these results indicate that the Makona variant is not evolving at a higher rate than previous outbreak variants, contrary to initial claims from Gire and colleagues.
Ancestor and selective pressure
The evolution of Ebolavirus, like any other virus, is complex, with many factors playing a role in the selection or elimination of particular variants. Phylogenetic analysis of the most extensively studied species of Ebolavirus, Zaire ebolavirus, reveals the complexity behind the evolution of this virus. It has been hypothesized for a while now that fruit bats could be the reservoir for Ebolavirus [60–62]. This hypothesis was considered because some specimens were found to be polymerase chain reaction (PCR)-positive and/or seropositive for antibodies against EBOV. It is important to note, however, that no live, replication-competent EBOV has ever been isolated from bats, unlike Marburg virus [63]. Interestingly, the sequencing of the L gene of bat-derived EBOV showed that it could have recently experienced a genetic bottleneck-type event [50], because the common ancestry of these sequences seems to be in opposition with the notion that EBOV and bats have been interacting together for an extended period of time. Based on the sequencing of EBOV GP, it was previously identified that all viruses causing outbreaks since 2001 had a most recent common ancestor circa 1999. Biek et al. have put forth several alternative scenarios that could explain this genetic bottleneck [50]. The first scenario that the authors raised to explain this bottleneck is that the bat population at this time decreased significantly, causing a reduction in the viral population size. Alternatively, infected bats could have introduced EBOV in the region near Congo and Gabon circa 1999. Another scenario is that the fruit bat species identified by Leroy et al. is not the primary reservoir, making it possible that EBOV was introduced by other species and emerged in the human population.
The evolutionary history of Ebolaviruses would not be complete without discussing the common ancestors of these viruses. In 1997, when there were only four species identified, Suzuki et al. estimated that EBOV and TAFV diverged about 700 to 1,300 years ago, that SUDV and RESTV diverged about 1,400 to 1,600 years ago, and that these two clusters diverged about 1,000 to 2,100 years ago [64]. The same analysis was conducted in 2014, but with the sequences of BDBV included. The results indicated that the common ancestor for these five species was about 1,257 years old [48], which is in accordance with the estimations previously made by Suzuki et al. Concerning the three main Ebolavirus species (EBOV, SUDV, and RESTV), Li et al. calculated that the times to most recent common ancestor (TMRCA) were 1,971, 1,969, and 1,970 years, respectively [48]. Again, similar results were obtained by Carroll et al. with the exception of SUDV. They estimated that the TMRCA was 1,960 years for EBOV, 1,173 years for SUDV, and 1,979 years for RESTV [51]. As discussed above, SUDV has a much slower evolutionary rate compared to the other species. The 800-year difference in TMRCA for EBOV and RESTV suggests that SUDV is much older. If we accept the fact that EBOV experienced a recent genetic bottleneck, it may just be that we are able to trace the most recent common ancestor further back in time for this species.
To assess the overall selection pressures EBOV, SUDV, and RESTV GP were under, the ratios (ω) between the number of nonsynonymous (dN) and synonymous (dS) substitutions per site were calculated for each species. A ratio greater than 1 indicates that an amino acid is under increased selection, whereas a ratio less than 1 indicates decreased selection [65,66]. While the results obtained for EBOV, SUDV, and RESTV were 0.229, 0.328, and 0.329, respectively, a secondary analysis by single likelihood ancestor counting (SLAC), fixed effects likelihood (FEL), internal FEL (IFEL), and random effects likelihood (REL) found several distinct amino acid positions in GP under positive selection. Positive results were recorded if they were identified by two of the four methods mentioned above in parentheses. The results varied from one species to another. For EBOV, the analysis revealed that amino acids 377 and 443 were under positive selection; for SUDV, the only hit was amino acid 503, which was observed by REL. For RESTV, the only positive results were for amino acid 229 [48]. Knowing that GP is involved in receptor binding and membrane fusion [67,68], it was hypothesized that the positive selection of certain amino acids on GP could explain why certain virus lineages gained a broader tropism and were able to infect primates following direct exposure to the virus, possibly through fruit bats [69]. However, considering the reduction of genetic diversity since 1970 in EBOV [48], the viruses that managed to survive in those animal reservoirs could potentially be the last remaining lineages of EBOV. It is possible that only a very short window is available for filoviruses to jump in and replicate within the human population. This window could be the result of extreme pressures, which could suggest an adaptation to a distant host that keeps these viruses unsuitable for a more symbiotic relationship in the human environment.
It is safe to say that because of the ongoing outbreak, the potential for increased evolution of EBOV and positive selection of epitopes (particularly within the EBOV GP) may lead to a reduced efficacy of the current therapies undergoing clinical testing. A recent analysis by Kugelman et al. identified 21 nonsynonymous mutations within GP comparing the EBOV Kikwit variant (used to generate ZMapp) and the EBOV Makona West African outbreak variant [70]. The ZMapp cocktail tolerates 18 of these mutations; however, three new mutations, and an additional one identified by Azarian et al., have evolved since the outbreak began and have yet to be evaluated for resistance [59]. In addition, there has only been one documented in vivo case of escape from ZMAb [71,72], the predecessor of ZMapp. Thus, monitoring the continued evolution of EBOV during the outbreak will be critical for the future efficacy of ZMapp. However, ZMapp targets several GP epitopes, which may cause EBOV to generate excessive GP mutations at the cost of viral fitness, reducing the likelihood of an EBOV variant completely circumventing treatment.
Concluding Remarks
While the current outbreak seems to be waning, there is always the possibility of resurgence. The apparition of a new epicenter in any of the affected countries could be due to a mutated variant of the original Makona or because of the introduction of an unrelated virus. In both scenarios, rapid identification of the virus as well as full genome sequencing will be vital for understanding what threat the international community is facing. Future outbreaks are also to be considered, opening the discussion of how new Ebola variants may affect current and future vaccines and therapies. Broader protective and curative options will need to be considered as new variants keep emerging. The discovery of a new filovirus in 2011 [73] is a reminder that this family of viruses still has a few tricks up its sleeve, and that targeted therapeutics and vaccines may not be enough in the eventuality that a new virus or variant ever spreads in humans. Alternatively, the current outbreak has not led to the emergence of a significantly divergent virus with obvious new characteristics, despite the extraordinary number of passages in thousands of humans. Taken together, these observations point at a relatively stable evolution of an emerging Ebola virus variant in the human population during the current West African outbreak.
Zdroje
1. Slenczka W, Klenk HD (2007) Forty years of marburg virus. J Infect Dis 196 Suppl: S131–S135.
2. WHO (1978) Ebola haemorrhagic fever in Sudan, 1976. Bull World Health Organ. 56(2):247–70. 307455
3. WHO (1978) Ebola heamorrhagic fever in Zaire, 1976. Bull World Health Organ. 56(2): 271–293. 307456
4. Qiu X, Wong G, Audet J, Bello A, Fernando L, et al. (2014) Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514(7520):47–53. doi: 10.1038/nature13777 25171469
5. Wong G, Kobinger GP (2015) Backs against the Wall: Novel and Existing Strategies Used during the 2014–2015 Ebola Virus Outbreak. Clin Microbiol Rev 28 : 593–601. doi: 10.1128/CMR.00014-15 25972518
6. Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, et al. (2015) Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 386 : 857–866. doi: 10.1016/S0140-6736(15)61117-5 26248676
7. Formenty P, Libama F, Epelboin A, Allarangar Y, Leroy EM, et al. (2003) Outbreak of Ebola hemorrhagic fever in the Republic of the Congo, 2003: a new strategy? Médecine Trop 63 : 291–295.
8. Georges a J, Leroy EM, Renaut a a, Benissan CT, Nabias RJ, et al. (1999) Ebola hemorrhagic fever outbreaks in Gabon, 1994–1997: epidemiologic and health control issues. J Infect Dis 179 Suppl: S65–S75.
9. Heymann DL, Weisfeld JS, Webb PA (1980) Ebola Hemorrhagic Fever: Tandala, Zaire, 1977–1978. J Infect Dis 142 : 1977–1978.
10. Khan a S, Tshioko FK, Heymann DL, Le Guenno B, Nabeth P, et al. (1999) The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis 179 Suppl: S76–S86.
11. MakwKaput V (2007) Déclaration fin d’épidémie de FHV à virus Ebola dans les zones de santé de Mweka, Bulape et Luebo, province du Kasai occidental, RD Congo. Memorandum.
12. WHO (2003) Outbreak(s) of Ebola haemorrhagic fever, Congo and Gabon, October 2001-July 2002. Wkly Epidemiol Rec 26 : 223–228.
13. WHO (2004) Ebola haemorrhagic fever in the Republic of the Congo—update 6. Dis Outbreak News.
14. WHO (2009) End of Ebola outbreak in the Democratic Republic of the Congo. Dis Outbreak News.
15. Albariño CG, Shoemaker T, Khristova ML, Wamala JF, Muyembe JJ, et al. (2013) Genomic analysis of filoviruses associated with four viral hemorrhagic fever outbreaks in Uganda and the Democratic Republic of the Congo in 2012. Virology 442 : 97–100. doi: 10.1016/j.virol.2013.04.014 23711383
16. MacNeil A, Shoemaker T, Balinandi S, Campbell S, Wamala JF, et al. (2012) Reemerging Sudan Ebola virus disease in Uganda, 2011. Emerg Infect Dis 18 : 1480–1483. doi: 10.3201/eid1809.111536 22931687
17. WHO (2005) Outbreak of Ebola haemorrhagic fever in Yambio, south Sudan, April—June 2004. Wkly Epidemiol Rec: 369–376.
18. Okware SI, Omaswa FG, Zaramba S, Opio A, Lutwama JJ, et al. (2002) An outbreak of Ebola in Uganda. Trop Med Int Heal 7 : 1068–1075.
19. Baron RC, McCormick JB, Zubeir OA (1983) Ebola virus disease in southern Sudan: hospital dissemination and intrafamilial spread. Bull World Health Organ 61(6):997–1003. 6370486
20. Le Guenno B, Formenty P, Wyers M, Gounon P, Walker F, et al. (1995) Isolation and partial characterisation of a new strain of Ebola virus. Lancet 345 : 1271–1274. 7746057
21. MacNeil A, Farnon EC, Morgan OW, Gould P, Boehmer TK, et al. (2011) Filovirus outbreak detection and surveillance: Lessons from bundibugyo. J Infect Dis 204 : 761–767.
22. Barrette RW, Metwally SA, Rowland JM, Xu L, Zaki SR, et al. (2008) Discovery of Swine as a Host for the Reston ebolavirus. Science (80-) 155 : 5–7.
23. Hayes CG, Burans JP, Ksiazek TG, Del Rosario R a., Miranda MEG, et al. (1992) Outbreak of fatal illness among captive macaques in the Philippines caused by an ebola-related filovirus. Am J Trop Med Hyg 46 : 664–671. 1621890
24. Jahrling PB, Geisbert TW, Dalgard DW, Johnson ED, Ksiazek TG, et al. (1990) Preliminary report: isolation of Ebola virus from monkeys imported to USA. Lancet 335 : 502–505. 1968529
25. Miranda MEG, White ME, Dayrit MM, Hayes CG, Ksiazek TG, et al. (1991)Seroepidemiological study of filovirus related to Ebola in the Philippines: 1985. Lancet 337(8738):425–6. 1671441
26. Miranda ME, Ksiazek TG, Retuya TJ, Khan a S, Sanchez a, et al. (1999) Epidemiology of Ebola (subtype Reston) virus in the Philippines, 1996. J Infect Dis 179 Suppl: S115–S119.
27. Center for Disease Control (1990) Filovirus infection in animal handlers. Morb Mortal Wkly 39 : 221.
28. WHO (1992) Viral haemorrhagic fever in imported monkeys. Wkly Epidemiol Rec 24 : 183.
29. WHO (2009) Ebola Reston in pigs and humans, Philippines. Wkly Epidemiol Rec 84 : 49–50. 19219963
30. Marsh G a, Haining J, Robinson R, Foord A, Yamada M, et al. (2011) Ebola Reston virus infection of pigs: clinical significance and transmission potential. J Infect Dis 204 Suppl: S804–S809.
31. Pan Y, Zhang W, Cui L, Hua X, Wang M, et al. (2014) Reston virus in domestic pigs in China. Arch Virol 159 : 1129–1132. doi: 10.1007/s00705-012-1477-6 22996641
32. Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, et al. (2014) Emergence of Zaire Ebola Virus Disease in Guinea—Preliminary Report. N Engl J Med 371(15):1418–25 doi: 10.1056/NEJMoa1404505 24738640
33. De La Vega M-A, Wong G, Kobinger GP, Qiu X (2014) The Multiple Roles of sGP in Ebola Pathogenesis. Viral Immunol 28 : 1–7.
34. Ebihara H, Takada A, Kobasa D, Jones S, Neumann G, et al. (2006) Molecular determinants of Ebola virus virulence in mice. PLoS Pathog 2 : 0705–0711.
35. Escudero-Pérez B, Volchkova V a, Dolnik O, Lawrence P, Volchkov VE (2014) Shed GP of Ebola Virus Triggers Immune Activation and Increased Vascular Permeability. PLoS Pathog 10: e1004509. doi: 10.1371/journal.ppat.1004509 25412102
36. García-Dorival I, Wu W, Dowall S, Armstrong S, Touzelet O, et al. (2014) Elucidation of the Ebola virus VP24 cellular interactome and disruption of virus biology through targeted inhibition of host cell protein function. J Proteome Res 13(11): 5120–35. doi: 10.1021/pr500556d 25158218
37. Liang H, Zhou Z, Zhang S, Zen K, Chen X, et al. (2014) Identification of Ebola virus microRNAs and their putative pathological function. Sci China Life Sci 57 : 973–981. doi: 10.1007/s11427-014-4759-2 25266153
38. Luthra P, Ramanan P, Mire CE, Weisend C, Tsuda Y, et al. (2013) Mutual antagonism between the Ebola virus VP35 protein and the RIG-I activator PACT determines infection outcome. Cell Host Microbe 14 : 74–84. doi: 10.1016/j.chom.2013.06.010 23870315
39. Xu W, Edwards MR, Borek DM, Feagins AR, Mittal A, et al. (2014) Ebola Virus VP24 Targets a Unique NLS Binding Site on Karyopherin Alpha 5 to Selectively Compete with Nuclear Import of Phosphorylated STAT1. Cell Host Microbe 16 : 187–200. doi: 10.1016/j.chom.2014.07.008 25121748
40. Yen B, Mulder LC, Martinez O, Basler CF (2014) Molecular Basis for Ebola Virus VP35 Suppression of Human Dendritic Cell Maturation. J Virol 88(21):12500–10. doi: 10.1128/JVI.02163-14 25142601
41. Yang ZY, Duckers HJ, Sullivan NJ, Sanchez A, Nabel EG, et al. (2000) Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat Med 6 : 886–889. 10932225
42. Chan S, Ma M, Goldsmith M (2000) Differential induction of cellular detachment by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J Gen Virol 81 : 2155–2159. 10950971
43. Takada a, Watanabe S, Ito H, Okazaki K, Kida H, et al. (2000) Downregulation of beta1 integrins by Ebola virus glycoprotein: implication for virus entry. Virology 278 : 20–26. 11112476
44. Morikawa S, Saijo M, Kurane I (2007) Current knowledge on lower virulence of Reston Ebola virus. Comp Immunol Microbiol Infect Dis 30 : 391–398. 17610952
45. Basler CF, Amarasinghe GK (2009) Evasion of interferon responses by Ebola and Marburg viruses. J Interf cytokine Res 29 : 511–520.
46. Sanchez a, Trappier SG, Mahy BW, Peters CJ, Nichol ST (1996) The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc Natl Acad Sci U S A 93 : 3602–3607. 8622982
47. Sanchez A, Rollin PE (2005) Complete genome sequence of an Ebola virus (Sudan species) responsible for a 2000 outbreak of human disease in Uganda. Virus Res 113 : 16–25. 16139097
48. Li YH, Chen SP (2014) Evolutionary history of Ebola virus. Epidemiol Infect 142 : 1138–1145. doi: 10.1017/S0950268813002215 24040779
49. Walsh PD, Biek R, Real L a (2005) Wave-like spread of Ebola Zaire. PLoS Biol 3: e371. 16231972
50. Biek R, Walsh PD, Leroy EM, Real L a (2006) Recent common ancestry of Ebola Zaire virus found in a bat reservoir. PLoS Pathog 2: e90. 17069458
51. Carroll S a, Towner JS, Sealy TK, McMullan LK, Khristova ML, et al. (2013) Molecular evolution of viruses of the family Filoviridae based on 97 whole-genome sequences. J Virol 87 : 2608–2616. doi: 10.1128/JVI.03118-12 23255795
52. Rodriguez LL, De Roo a, Guimard Y, Trappier SG, Sanchez a, et al. (1999) Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis 179 Suppl: S170–S176.
53. Muyembe-Tamfum JJ, Kipasa M, Kiyungu C, Colebunders R (1999) Ebola outbreak in Kikwit, Democratic Republic of the Congo: discovery and control measures. J Infect Dis 179 Suppl: S259–S262.
54. Dudas G, Rambaut A (2014) Phylogenetic Analysis of Guinea 2014 EBOV Ebolavirus Outbreak. PLoS Curr 6 : 1–11.
55. Gire SK, Goba A, Andersen KG, Sealfon RSG, Park DJ, et al. (2014) Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science (80-) 345 : 1369–1372.
56. Hoenen T, Safronetz D, Groseth A, Wollenberg KR, Koita A, et al. (2015) Mutation rate and genotype variation of Ebola virus from Mali case sequences. Science (80-) 348 : 117–119.
57. Pybus OG (2006) Model selection and the molecular clock. PLoS Biol 4 : 686–688.
58. Tong Y-G, Shi W-F, Di Liu, Qian J, Liang L, et al. (2015) Genetic diversity and evolutionary dynamics of Ebola virus in Sierra Leone. Nature. 524(7563):93–6. doi: 10.1038/nature14490 25970247
59. Azarian T, Lo Presti A, Giovanetti M, Cella E, Rife B, et al. (2015) Impact of spatial dispersion, evolution, and selection on Ebola Zaire Virus epidemic waves. Sci Rep 5 : 10170. doi: 10.1038/srep10170 25973685
60. Pourrut X, Souris M, Towner JS, Rollin PE, Nichol ST, et al. (2009) Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infect Dis 9 : 159. doi: 10.1186/1471-2334-9-159 19785757
61. Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, et al. (2005) Fruit bats as reservoirs of Ebola virus. Nature 438 : 575–576. 16319873
62. Olival K, Islam A, Yu M (2013) Ebola virus antibodies in fruit bats, Bangladesh. Emerg Infect Dis 19 : 270–273. doi: 10.3201/eid1902.120524 23343532
63. Towner JS, Amman BR, Sealy TK, Carroll S a R, Comer J a, et al. (2009) Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog 5: e1000536. doi: 10.1371/journal.ppat.1000536 19649327
64. Suzuki Y, Gojobori T (1997) The origin and evolution of Ebola and Marburg viruses. Mol Biol Evol: 800–806. 9254917
65. Hurst LD (2002) The Ka/Ks ratio: diagnosing the form of sequence evolution. Trends Genet 18 : 486–487. 12175810
66. Yang Z, Bielawski JP (2000) Statistical methods for detecting molecular adaptation. Trends Ecol Evol 15 : 496–503. 11114436
67. Lee J, Saphire E (2009) Ebolavirus glycoprotein structure and mechanism of entry. Future Virol 4 : 621–635. 20198110
68. Feldmann H, Volchkov VE, Volchkova V a, Ströher U, Klenk HD (2001) Biosynthesis and role of filoviral glycoproteins. J Gen Virol 82 : 2839–2848. 11714958
69. Leroy EM, Epelboin A, Mondonge V, Pourrut X, Gonzalez J-P, et al. (2009) Human Ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector borne zoonotic Dis 9 : 723–728. doi: 10.1089/vbz.2008.0167 19323614
70. Kugelman JR, Sanchez-lockhart M, Andersen KG, Gire S, Park DJ, et al. (2015) Evaluation of the Potential Impact of Ebola Virus Genomic Drift on the Efficacy of Sequence-Based Candidate Therapeutics. MBio 6 : 2013–2016.
71. Qiu X, Audet J, Wong G, Pillet S, Bello A, et al. (2012) Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci Transl Med 4 : 138ra81. doi: 10.1126/scitranslmed.3003876 22700957
72. Audet J, Wong G, Wang H, Lu G, Gao GF, et al. (2014) Molecular Characterization of the Monoclonal Antibodies Composing ZMAb: A Protective Cocktail Against Ebola Virus. Sci Rep 4 : 1–8.
73. Negredo A, Palacios G, Vázquez-Morón S, González F, Dopazo H, et al. (2011) Discovery of an ebolavirus-like filovirus in europe. PLoS Pathog 7: e1002304. doi: 10.1371/journal.ppat.1002304 22039362
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin InfectionČlánek Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 11- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Parasite Glycobiology: A Bittersweet Symphony
- On the Discovery of TOR As the Target of Rapamycin
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
- PML/TRIM19-Dependent Inhibition of Retroviral Reverse-Transcription by Daxx
- Cleavage of a Neuroinvasive Human Respiratory Virus Spike Glycoprotein by Proprotein Convertases Modulates Neurovirulence and Virus Spread within the Central Nervous System
- Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Induces the Oncogenic miR-17-92 Cluster and Down-Regulates TGF-β Signaling
- Interferon-α Subtypes in an Model of Acute HIV-1 Infection: Expression, Potency and Effector Mechanisms
- Perivascular Arrest of CD8 T Cells Is a Signature of Experimental Cerebral Malaria
- Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells
- Evolution and Emergence of Enteroviruses through Intra- and Inter-species Recombination: Plasticity and Phenotypic Impact of Modular Genetic Exchanges in the 5’ Untranslated Region
- Interferon-γ Inhibits Ebola Virus Infection
- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- P-Type Cyclin CYC3 Modulates Endomitotic Growth during Oocyst Development in Mosquitoes
- Diversity of across Evolutionary Scales
- 50 Years of Disease in Humans: The Dramatic Emergence of a Cluster of Novel Fungal Pathogens
- Worse Comes to Worst: Bananas and Panama Disease—When Plant and Pathogen Clones Meet
- Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection
- Infection-Induced Retrotransposon-Derived Noncoding RNAs Enhance Herpesviral Gene Expression via the NF-κB Pathway
- Structural Insight into Archaic and Alternative Chaperone-Usher Pathways Reveals a Novel Mechanism of Pilus Biogenesis
- Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin Infection
- Global Analysis of the Fungal Microbiome in Cystic Fibrosis Patients Reveals Loss of Function of the Transcriptional Repressor Nrg1 as a Mechanism of Pathogen Adaptation
- The Transcription and Translation Landscapes during Human Cytomegalovirus Infection Reveal Novel Host-Pathogen Interactions
- The N-terminal Helical Region of the Hepatitis C Virus p7 Ion Channel Protein Is Critical for Infectious Virus Production
- Activation of Type I and III Interferon Response by Mitochondrial and Peroxisomal MAVS and Inhibition by Hepatitis C Virus
- Hsp70 Isoforms Are Essential for the Formation of Kaposi’s Sarcoma-Associated Herpesvirus Replication and Transcription Compartments
- Distinct Upstream Role of Type I IFN Signaling in Hematopoietic Stem Cell-Derived and Epithelial Resident Cells for Concerted Recruitment of Ly-6C Monocytes and NK Cells via CCL2-CCL3 Cascade
- and Bats: Story of an Emerging Friendship
- Emergence of Pathogenicity in Lagoviruses: Evolution from Pre-existing Nonpathogenic Strains or through a Species Jump?
- Ebolavirus Evolution: Past and Present
- Host and Symbiont Jointly Control Gut Microbiota during Complete Metamorphosis
- Non-Human Primates Harbor Diverse Mammalian and Avian Astroviruses Including Those Associated with Human Infections
- Lactate Dehydrogenase Is Associated with the Parasitophorous Vacuole Membrane and Is a Potential Target for Developing Therapeutics
- Five Questions about Mycoviruses
- Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
- Ethanolamine Signaling Promotes Niche Recognition and Adaptation during Infection
- Cross-Species Transmission and Differential Fate of an Endogenous Retrovirus in Three Mammal Lineages
- Memory Th1 Cells Are Protective in Invasive Infection
- Transcription Factor SomA Is Required for Adhesion, Development and Virulence of the Human Pathogen
- An -Methyltransferase Is Required for Infection of Tick Cells by
- RNA-seq Brings New Insights to the Intra-Macrophage Transcriptome of Typhimurium
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- On the Discovery of TOR As the Target of Rapamycin
- Parasite Glycobiology: A Bittersweet Symphony
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



