-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Prothrombin gene 20210A mutation in Slovak population
Mutácia protrombínového génu 20210A v slovenskej populácii
Úvod:
Mutácia faktora V Leiden (FVL) spolu a mutácia G20210A v protrombínovom géne (PTM) patria medzi 2 najčastejšie genetické polymorfizmy, ktoré predispozíciou pre rozvoj prevej epizódy venózneho tromboemblizmu (VTE). PTM sa vyskytuje v 2 % belošskej populácie. Hlavným cieľom tejto práce bolo zistiť prevalenciu PTM v populácii pacientov s anamnézou trombotickej príhody vs. zdravých kontrolách.Materiál a metódy:
Za účelom posúdenie prítomnosti PTM bola realizovaná PCR analýza z DNA extrahovanej z periférnych leukocytov.Výsledky:
Do štúdie bolo zaradených 2 274 pacientov, z nich 157 (6,9 %) malo prítomnú PTM. PTM mutácia bola prítomná u 2,6 % kontrol z celkového počtu 303 dobrovoľníkov. Analyzovali sme klinickú manifestáciu PTM. Pozorovali sme 123 venóznych trombóz, 46 artériových trombóz a 14 opakovaných spontánnych potratov. V tomto článku sme ďalej analyzovali ďalšie možné rizikové faktory rozvoja trombózy u pacientov s prítomnou PTM.Záver:
Podľa našich vedomostí je toto najväčšia epidemiologická štúdia zameraná na výskyt PTM v strednej Európe. Za použitia štatistickej analýzy sme zistili relatívne vysoký výskyt PTM v populácii pacientov s anamnézou trombózy (6,9 %), ale aj u zdravých kontrol (2,6 %). Riziko trombózy je nezávislé od veku a pohlavia. Štúdia zároveň ukázala pomerne častý výskyt dvojitej prítomnosti PTM a FVL.Kľúčové slová:
mutácia – populácia – protrombín – trombóza
Authors: Juraj Chudej; Ivana Plameňová
Authors place of work: Department of Haematology and Transfusion Medicine, National Centre for Haemostasis and Thrombosis, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Slovakia
Published in the journal: Vnitř Lék 2016; 62(4): 281-286
Category: Původní práce
Summary
Introduction:
Factor V Leiden (FVL) and prothrombin G20210A mutation (PTM) are the two most common genetic polymorphisms known to predispose to a first episode of venous thromboembolism (VTE). PTM is present in 2 % Caucasian population. The main aim of this study was to identify the PTM in the patients with positive history of thrombotic events vs. control subjects.Materials and Methods:
The assessment of PTM was performed by the PCR analysis of the chromosomal DNA, which was isolated from the peripheral blood leukocytes.Results:
Of the 2 274 patients included, 157 (6.9 %) were carriers of the PTM. The mutation was present only in 2.6 % (n = 8) of the 303 controls. The following clinical manifestations of PTM were analysed. We observed 123 venous thrombotic events, 46 arterial thrombosis and 14 spontaneous abortions. In this article we analyse other possible risk factors for thromboembolic events in patients with carriage of PTM.Conclusions:
To our knowledge, this is the largest epidemiological study of PTM in Central Europe. Employing statistical analysis, we found relatively high prevalence of the PTM in both, the patients with positive thrombosis history (6.9 %), as well as in the control group (2.6 %). The risk of thrombosis by carriage of PTM is independent of age and gender. Study has shown relatively frequent presence of double carriership of PTM and factor V Leiden mutation (FVL).Key words:
mutation – population – prothrombin – thrombophilia – thrombosisIntroduction
The prothrombin G20210A mutation (PTM) is the second most common inherited risk factor for thrombosis [1]. The prothrombin gene, located on chromosome 11 (11p11-q12), is a 21-kb gene containing 14 exons [2]. The PTM is a single nucleotide substitution of adenine for guanine in the 3´-untranslated region of the gene, first described by Poort et al in 1996 [3]. Many studies have shown elevated prothrombin levels in patients with PTM [3–9]. In addition to this finding, prothrombin level itself is a risk factor for thrombosis [3]. The initial Poort et al study [3] found that the mutation was present in 18 % of patients with a personal and family history of VTE and 6.2 % of unselected patients with first time VTE, compared to 2.3 % of healthy controls. Subsequent studies show prevalence in healthy European and American individuals ranging from 1.2 to 4.6 % [10–15]. The mutation is less prevalent in non-Jewish, non-European populations [10,16–19].
The risk of VTE in women during pregnancy or oral contraceptive therapy appears to be increased in the presence of the PTM [20]. Martinelli et al [21] reported that the relative risk of deep vein thrombosis was increased 16.3-fold (95% CI, 3.4–79.1) in women with PTM who used oral contraceptives (OCPs) compared with non-carriers and non-users. And the relative risk of pregnancy and puerperal VTE in women with the PTM was 15.2 (95% CI, 4.2–52.6) [22].
Current research at our department is focused on platelet hyperaggregability and high activity of coagulation factor XI. We confirmed that these factors are associated with thrombosis and miscarriage [23–26].
The aim of the study was to detect the incidence of PTM in the patients with positive history of thrombotic events compared to the control group. In a more detailed value analysis, we evaluate the impact of this mutation on the clinical expression.
Material and Methods
Study population
The Ethical Committee of the Jessenius Faculty of Medicine, Comenius University approved the study. All study participants agreed to participate in the project and signed a written informed consent in accordance with the Declaration of Helsinki.
Data collection took place between September 2007 and September 2012. A detailed history regarding previous thrombotic episodes, a family history of thrombosis, and acquired risk factors such as cirrhosis, pregnancy, smoking, OCPs use, diabetes, hypertension, surgery, immobilization, injury, and malignant neoplasm was obtained from all patients and control subjects.
This study is comprised of patients (n = 2 274) with venous (n = 1 720) or arterial (n = 128) thrombosis and spontaneous abortion (n = 456). The miscarriage occurred in 220 patients during the first trimester of pregnancy (n = 74 recurrent pregnancy loss) and 102 patients during the second trimester (n = 31 recurrent pregnancy loss). 21 patients had recurrent pregnancy loss during the first and second trimester. The diagnosis of venous or arterial thrombosis was confirmed on ultrasonography, CT or angiography. Patients were initially examined and tested at the Department of Haematology and Transfusiology in Martin University Hospital. They were referred to undergo the thrombophilia screening as a part of differential diagnosis due to the thromboembolic event or spontaneous abortion. Most of the investigated parameters can be affected by acute thrombosis and anticoagulant therapy. Therefore, we used laboratory tests after discontinuation of the anticoagulant treatment. Patients were screened for Factor V Leiden (FVL), PTM, Protein C, Protein S, antithrombin III, factor VIII, factor IX, homocysteine, antiphospholipid syndrome and sticky platelet syndrome.
Furthermore, 303 randomly chosen healthy individuals were involved as control subjects. All control individuals were Caucasians of European origin, with negative personal and family history of the thrombosis without any chronic condition and any regular medication. The characteristics of the study population is presented in tab 1.
Tab. 1. Characteristics of the study population 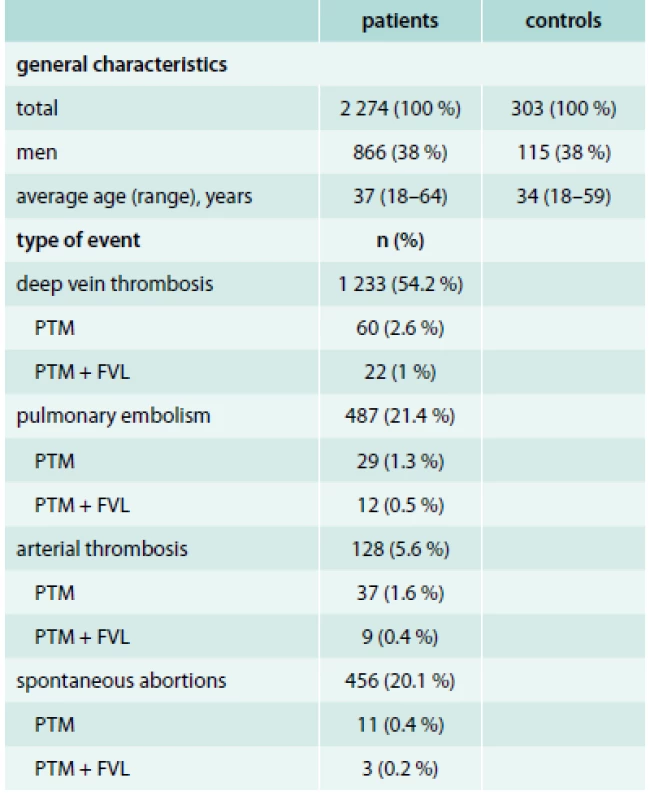
Analysis of prothrombin gene
The blood drawn from antecubital vein, collected into vials prefilled with 400 µL 0.5 molar EDTA was used for the DNA analysis. Genomic DNA was extracted from peripheral blood leukocytes by SiMaxTM Genomic DNA Extraction kit (SBS Genetech Co., Ltd., China) according to the manufacturer’s instructions. The strategy was direct detection of the 20210A allele in the prothrombin gene. A 345-bp fragment from exon 14 and the 3’-untranslated region of the prothrombin gene was amplified by polymerase chain reaction (PCR) using the primers 5’-TCTAGAAACAGTTGCCTGGC-3’ and a mutagenic primer 5´-ATAGCACTG GGAGCATTGAAG C-3´. Amplification was carried out in the following manner: initial denaturation at 95 °C for 3 minutes followed by 30 cycles of 95 °C for 30 seconds, 63 °C for 60 seconds, and 72 °C for 60 seconds. One microliter of HIND III restriction enzyme was added to the amplified DNA and incubated at 37 °C for 4 hours. The digested products (10 μL) were electrophoresed on 6% polyacrylamide gel until the blue dye front migrated at least 6 cm; 0.1% silver nitrate was used for staining. A heterozygous control was run with each batch of tests. An uncut amplified DNA and a wild-type DNA were also run with each batch of tests to ascertain the effectiveness of HIND III digestion.
Statistics
We used Microsoft Office Excel for Mac 2011 (version 14.3.0) to do data analysis. Differences in the incidence of parameters were evaluated by Chi-Square test. The Fisher exact test was used in case of low representation (less than 5). P values less than 0.05 were considered statistically significant.
Results
In summary, 2 274 patients with thrombotic events in the personal history and 303 control subjects were tested. The PTM was diagnosed in 157 patients (6.9 %) and 8 subjects (2.6 %) of the control group. According to the gender 92 (59 %) of patients were women, 65 (41 %) were men. In the control group, the ratio between men and women were proportional, see tab 1.
The average age of patients at the time of first thrombosis was 35.9 years and the median age was 34 years (interquartile range 18–64 years). The age distribution data are presented in graph 1, 2.
Graph 1. Age distribution of patients with evidence of PTM and history of thromboembolism or abortions 
Graph 2. Age distribution of patients with evidence of PTM according to the first thromboembolic event or abortion 
According to the PCR we discovered 156 heterozygotes and 1 homozygote in the group of patients. All subjects in control group were heterozygotes.
The most common clinical manifestation of PTM was the venous thrombosis – 123 events. In this case, the gender distribution was similar. The arterial thrombosis was presented less – 46 events. Interestingly, 1 patient had both, the arterial and venous thrombosis. The occurrence of PTM or PTM with FVL was relatively frequent in patients with arterial thrombosis, see tab. 1. The PTM was manifested by spontaneous abortions in 14 patients. Concomitant mutation of FVL gene was detected in 3 of these patients. Detailed analysis of data related to clinical manifestation of PTM is presented in graph 3, 4.
Graph 3. Clinical manifestations according to gender – male 
Graph 4. Clinical manifestations according to gender – female 
In relation with the use of hormonal contraception, PTM was manifested as the thromboembolic event in a subgroup of 11 women. As the additional acquired risk of thrombosis in women, we observed the presence of smoking. 10 women had a personal smoking history. 9 of them had a personal history of venous thrombosis. The combination of oral contraceptive pill use and smoking was present in one patient. In this case, the clinical manifestation of PTM was venous thrombosis.
Injury, surgery, immobilization, smoking and cardiovascular disease (CVD) were the most frequent acquired risk factors for thrombosis in patients with PTM. Diabetes and cancer represent only a small percentage of acquired risk factors. We demonstrated statistical significance of smoking and CVD compared to another acquired risk factors (graph 5).
Graph 5. The presence of acquired thrombophilia risks among patients with PTM 
Discussion
In conclusion, we tested 2 274 patients with thrombotic events in the personal history and 303 control subjects. The PTM was diagnosed in 157 patients (6.9 %) and in 8 controls (2.6 %). Using PCR we discovered 156 heterozygotes and 1 homozygote in the group of patients. All subjects in the control group were heterozygotes. Based on our results we can say that Slovakia is one of the European countries with high occurrence of the PTM in both groups. We believe it is caused by two main factors. On the one hand people are better informed about testing and prevention of thrombosis. On the other hand, a large group of medical specialists such as angiologists, neurologists, cardiologists, and gynaecologists refer their patients to a haematologist as any unprovoked episode of thrombosis that occurs in young adults < 50 years or any repeated thrombosis should be investigated for thrombophilia. Based on the above facts, the number of outpatient visits to haematologists has increased. Most of these patients are referred for differential diagnosis of the thromboembolic event or spontaneous abortion.
The geographical distribution of PTM in Europe is presented in tab. 2, [27–58]. In the presence of PTM, age and gender are independent risk factor for thrombosis [3]. In our study, there were 92 (59 %) women and 65 (41 %) men in the patient group. The PTM clinically manifested most often during the third and fourth decades of life. A large proportion of the female subjects in our sample can be explained by the fact that women of childbearing age are most often referred for the testing. Concerns about the use of OCs, spontaneous abortions, as well as their interest to find out the causes of thrombosis were the main characteristics of this age/gender group. Therefore, we can assume that the thrombophilia testing is indicated more often for females than males. Another part of the patients in this group was the subjects in whom the clinical manifestation of thrombosis could be explained by the presence of another congenital or acquired risk factors (FVL, injury, smoking, OCs, etc.).
Tab. 2. Geographical distribution of PTM in Europe 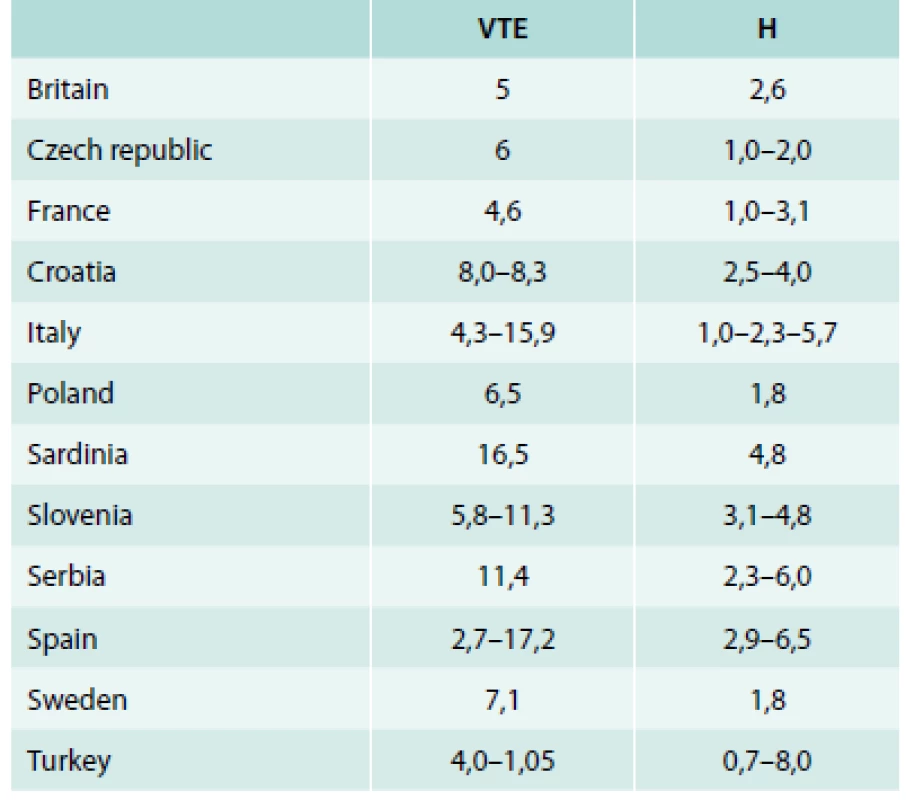
H – general healthy population % VTE – VTE patients % Among Caucasian population the prevalence of congenital thrombophilia associated with venous thromboembolism is between 24 and 37 % compared to 10 % for the control group [60,61]. Thrombosis in the patients with congenital thrombophilia is often initiated by external stimuli, which disrupts the labile haemostatic balance and starts a vicious cycle of thromboembolic complications. Among acquired thrombophilia risk factors, we demonstrated statistically significant difference in the incidence of smoking and CVD. Spontaneous thrombosis occurs rarely (0.4 % cases) [62].
Conclusions
Employing statistical analysis, we found relatively high prevalence of the PTM in both, the patients with positive thrombosis history (6.9 %), as well as in the control group (2.6 %). In the Slovak patients with clinical signs of thrombophilia we have found the PTM to be a fairly frequent risk factor contributing particularly to the occurrence of venous and arterial thrombosis. Therefore, we think that PTM (heterozygous and homozygous variant) is a risk factor for arterial and venous thrombosis. In our sample there were only a few spontaneous abortions; nevertheless, they represent a significant clinical problem. Our study can confirm that the risk of thrombosis in carriers of PTM is independent of age and gender. The results showed relatively frequent presence of double carriership of the PTM and FVL.
Juraj Chudej, M.D., PhD.
durochudej@pobox.sk
Department of Haematology and Transfusion Medicine, National Centre for Haemostasis and Thrombosis, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Slovakia
www.jfmed.uniba.sk
Doručeno do redakce 9. 7. 2015
Přijato po recenzi 1. 2. 2016
Zdroje
1. Chang MH, Lindegren ML, Butler MA et al. Prevalence in the United States of selected candidate gene variants: Third National Health and Nutrition Examination Survey, 1991–1994. Am J Epidemiol 2009; 169(1): 54–66.
2. Degen SJ, Davie EW. Nucleotide sequence of the gene for human prothrombin. Biochemistry 1987; 26(19): 6165–6177.
3. Poort SR, Rosendaal FR, Reitsma PH et al. A common genetic variation in the 3’-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood 1996; 88(10): 3698–3703.
4. Simioni P, Tormene D, Manfrin D et al. Prothrombin antigen levels in symptomatic and asymptomatic carriers of the 20210A prothrombin variant. Br J Haematol 1998; 103(4): 1045–1050.
5. Kyrle PA, Mannhalter C, Beguin S et al. Clinical studies and thrombin generation in patients homozygous or heterozygous for the G20210A mutation in the prothrombin gene. Arterioscler Thromb Vasc Biol 1998; 18(8):1287–1291.
6. Zawadzki C, Gaveriaux V, Trillot N et al. Homozygous G20210A transition in the prothrombin gene associated with severe venous thrombotic disease: two cases in a French family. Thromb Haemost 1998; 80(6): 1027–1028.
7. Morange PE, Barthet MC, Henry M et al. A three-generation family presenting five cases of homozygosity for the 20210 G to A prothrombin variant. Thromb Haemost 1998; 80(5): 859–860.
8. Eikelboom JW, Ivey L, Ivey J et al. Familial thrombophilia and the prothrombin 20210A mutation: association with increased thrombin generation and unusual thrombosis. Blood Coagul Fibrinolysis 1999; 10(1): 1–5.
9. Corral J, Zuazu-Jausoro I, Rivera J et al. Clinical and analytical relevance of the combination of prothrombin 20210A/A and factor V Leiden: results from a large family. Br J Haematol 1999; 105(2): 560–563.
10. Zivelin A, Rosenberg N, Faier S et al. A single genetic origin for the common prothrombotic G20210A polymorphism in the prothrombin gene. Blood 1998; 92(4): 1119–1124.
11. Ridker PM, Hennekens CH, Miletich JP. G20210A mutation in prothrombin gene and risk of myocardial infarction, stroke, and venous thrombosis in a large cohort of US men. Circulation 1999; 99(8): 999–1004.
12. Kapur RK, Mills LA, Spitzer SG et al. A prothrombin gene mutation is significantly associated with venous thrombosis. Arterioscler Thromb Vasc Biol 1997; 17(11): 2875–2879.
13. Margaglione M, Brancaccio V, Giuliani N et al. Increased risk for venous thrombosis in carriers of the prothrombin G–>A20210 gene variant. Ann Intern Med 1998; 129(2): 89–93.
14. Alhenc-Gelas M, Arnaud E, Nicaud V et al. Venous thromboembolic disease and the prothrombin, methylene tetrahydrofolate reductase and factor V genes. Thromb Haemost 1999; 81(4): 506–510.
15. Nowak-Gottl U, Junker R, Kreuz W et al. (Childhood Thrombophilia Study Group). Risk of recurrent venous thrombosis in children with combined prothrombotic risk factors. Blood 2001; 97(4): 858–862.
16. Arruda VR, Annichino-Bizzacchi JM, Goncalves MS et al. Prevalence of the prothrombin gene variant (nt20210A) in venous thrombosis and arterial disease. Thromb Haemost 1997; 78(6): 1430–1433.
17. Gurgey A, Kudayarov DK, Tuncer M et al. The factor V Leiden and prothrombin G20210A mutations in Kirghiz population. Thromb Haemost 2000; 84(2): 356.
18. Dilley A, Austin H, Hooper WC et al. Prevalence of the prothrombin 20210 G-to-A variant in blacks: infants, patients with venous thrombosis, patients with myocardial infarction, and control subjects. J Lab Clin Med 1998; 132(6): 452–455.
19. Ho CH. Prevalence of prothrombin 20210A allele and methylenetetrahydrofolate reductase C677T genetic mutations in the Chinese population. Ann Hematol 2000; 79(5): 239–242.
20. Vandenbroucke JP, Rosing J, Bloemenkamp KW et al. Oral contraceptives and the risk of venous thrombosis. N Engl J Med 2001; 344(20): 1527–1535.
21. Martinelli I, Taioli E, Bucciarelli P et al. Interaction between the G20210A mutation of the prothrombin gene and oral contraceptive use in deep vein thrombosis. Arterioscler Thromb Vasc Biol 1999; 19(3): 700–703.
22. Gerhardt A, Scharf RE, Beckmann MW et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000; 342(6): 374–380.
23. Sokol J, Biringer K, Skerenova M et al. Platelet aggregation abnormalities in patients with fetal losses: the GP6 gene polymorphism. Fertil Steril 2012; 98(5): 1170–1174.
24. Sokol J, Biringer K, Skerenova M et al. Different models of inheritance in selected genes in patients with sticky platelet syndrome and fetal loss. Semin Thromb Hemost 2015; 41(3): 330–335.
25. Sokol J, Biringer K, Skerenova M et al. Activity of coagulation factor XI in patients with spontaneous miscarriage: The presence of risk alleles. J Obstet Gynaecol 2015; 35(6): 621–624.
26. Šimonová R, Bartosová L, Chudy P et al. Nine kindreds of familial sticky platelet syndrome phenotype. Clin Appl Thromb Hemost 2013; 19(4): 395–401.
27. Francs F, Portols O, Gabriel F et al. Factor V Leiden (G1691A) and prothrombin-G20210A alleles among patients with deep venous thrombosis and in the general population from Spain. Rev Med Chil 2006; 134(1): 13–20.
28. Alvarez A, Barroso A, Robledo M et al. Prevalence of Factor V Leiden and the G20210A mutation of the prothrombin gene in a random group of patients with thrombotic episodes. Sangre (Barc) 1999; 44(1): 7–12.
29. Souto JC, Coll I, Llobet D et al. The prothrombin 20210A allele is the most prevalent genetic risk factor for venous thromboembolism in the Spanish population. Thromb Haemost 1998; 80(3): 366–369.
30. Leroyer C, Mercier B, Oger E et al. Prevalence of 20210 A allele of the prothrombin gene in venous thromboembolism patients. Thromb Haemost 1998; 80(1): 49–51.
31. Mazoyer E, Ripoll L, Gueguen R et al. (FITENAT Study Group). Prevalence of factor V Leiden and prothrombin G20210A mutation in a large French population selected for nonthrombotic history: geographical and age distribution. Blood Coagul Fibrinolysis 2009; 20(7): 503–510.
32. Reny JL, Alhenc-Gelas M, Fontana P et al. The factor II G20210A gene polymorphism, but not factor V Arg506Gln, is associated with peripheral arterial disease: results of a case-control study. J Thromb Haemost 2004; 2(8): 1334–1340.
33. Martinelli I, Bucciarelli P, Margaglione M et al. The risk of venous thromboembolism in family members with mutations in the genes of factor V or prothrombin or both. Br J Haematol 2000; 111(4): 1223–1239.
34. de Moerloose P, Reber G, Perrier A et al. Prevalence of factor V Leiden and prothrombin G20210A mutations in unselected patients with venous thromboembolism. Br J Haematol 2000; 110(1): 125–129.
35. Sottilotta G, Mamm C, Furl G et al. High incidence of factor V Leiden and prothrombin G20210A in healthy southern Italians. Clin Appl Thromb Hemost 2009; 15(3): 356–359.
36. Tosetto A, Missiaglia E, Frezzato M et al. The VITA project: prothrombin G20210A mutation and venous thromboembolism in the general population. Thromb Haemost 1999; 82(5): 1395–1398.
37. Bedencic M, Bozic M, Peternel P et al. Major and potential prothrombotic genotypes in patients with venous thrombosis and in healthy subjects from Slovenia. Pathophysiol Haemost Thromb 2008; 36(2): 58–63.
38. Zerjavic K, Zagradisnik B, Stangler Herodez S et al. Is the JAK2 V617F mutation a hallmark for different forms of thrombosis? Acta Haematol 2010; 124(1): 49–56.
39. Jukic I, Bingulac-Popovic J, Dogic V et al. ABO blood groups and genetic risk factors for thrombosis in Croatian population. Croat Med J 2009; 50(6): 550–558.
40. Herak DC, Antolic MR, Krleza JL et al. Inherited prothrombotic risk factors in children with stroke, transient ischemic attack, or migraine. Pediatrics 2009; 123(4): e653-e660. Dostupné z DOI: <http://dx.doi.org/10.1542/peds.2007–3737>.
41. Eterović D, Titlić M, Culić V et al. Lower contribution of factor V Leiden or G202104 mutations to ischemic stroke in patients with clinical risk factors: pair-matched case-control study. Clin Appl Thromb Hemost 2007; 13(2): 188–193.
42. Coen D, Zadro R, Honović L et al. Prevalence and association of the factor V Leiden and prothrombin G20210A in healthy subjects and patients with venous thromboembolism. Croat Med J 2001; 42(4): 488–492.
43. Djordjevic V, Rakicevic LJ, Mikovic D et al. Prevalence of factor V leiden, factor V cambridge, factor II G20210A and methylenetetrahydrofolate reductase C677T mutations in healthy and thrombophilic Serbian populations. Acta Haematol 2004; 112(4): 227–229.
44. Foka ZJ, Lambropoulos AF, Makris PE et al. High frequency of factor V Leiden and prothrombin G20210A mutations in Greek hemophiliacs. J Thromb Haemost 2003; 1(5): 1116–1167.
45. Hatzaki A, Anagnostopoulou E, Metaxa-Mariatou V et al. The impact of heterozygosity for the factor V Leiden and factor II G20210A mutations on the risk of thrombosis in Greek patients. Int Angiol 2003; 22(1): 79–82.
46. Zalavras CG, Giotopoulou S, Dokou E et al. Prevalence of the G20210A prothrombin gene mutation in Northwestern Greece and association with venous thromboembolism. Int Angiol 2003; 22(1): 55–57.
47. Antoniadi T, Hatzis T, Kroupis C et al. Prevalence of factor V Leiden, prothrombin G20210A, and MTHFR C677T mutations in a Greek population of blood donors. Am J Hematol 1999; 61(4): 265–267.
48. Tug E, Aydin H, Kaplan E et al. Frequency of genetic mutations associated with thromboembolism in the Western Black Sea Region. Intern Med 2011; 50(1): 17–21.
49. Altinisik J, Ates O, Ulutin T et al. Factor V Leiden, prothrombin G20210A, and protein C mutation frequency in Turkish venous thrombosis patients. Clin Appl Thromb Hemost 2008; 14(4): 415–420.
50. Irdem A, Devecioglu C, Batun S et al. Prevalence of factor V Leiden and prothrombin G20210A gene mutation. Saudi Med J 2005; 26(4): 580–583.
51. Xenophontos SL, Hadjivassiliou M, Ayrton N et al. Spectrum and prevalence of prothrombotic single nucleotide polymorphism profiles in the Greek Cypriot population. Int Angiol 2002; 21(4): 322–329.
52. Angelopoulou K, Nicolaides A, Constantinou DC. Prevalence of genetic mutations that predispose to thrombophilia in a Greek Cypriot population. Clin Appl Thromb Hemost 2000; 6(2): 104–107.
53. Barcellona D, Fenu L, Cauli C et al. Allele 4G of gene PAI-1 associated with prothrombin mutation G20210A increases the risk for venous thrombosis. Thromb Haemost. 2003; 90(6): 1061–1064.
54. Hillarp A, Zoller B, Svensson PJ et al. The 20210 A allele of the prothrombin gene is a common risk factor among Swedish outpatients with verified deep venous thrombosis. Thromb Haemost l997; 78(3): 990–992.
55. Kvasnička J. Doporučený postup pro indikaci molekulárně genetických vyšetření v rámci diagnostiky trombofilních stavů v žilním systému. Vnitř Lék 2010; 56(12): 1251.
56. Dulíček P. Trombofilní stavy. Vnitř Lék 2005; 91(7–8): 819–825.
57. Piťha J, Auzký O, Roztočil K. Co mají společného žilní a tepenná onemocnéní? Vnitř Lék 2014; 60(11): 985–989.
58. Dulíček P, Vodičková L, Malý J et al. ,,Nejasná” príčina vzniku recidívy venózneho tromboembolizmu. Vnitř Lék 2006; 52(1): 87–88.
59. Bauer KA. The thrombophilias: well-defined risk factors with uncertain therapeutic implications. Ann Int Med 2001; 135(5): 367–373.
60. Walker ID, Greaves M, Preston FE. Investigation and management of heritable thrombophilia. Br J Haematol 2001; 114(3): 512–528.
61. Rosendaal FR. Venous thrombosis: multicausal disease. Lancet 1999; 353(9159): 1167–1173.
62. Dahl OE. Mechanisms of hypercoagulability. Thromb Haemost 1999; 82(2): 902–906.
Štítky
Diabetológia Endokrinológia Interné lekárstvo
Článek Epikardiální tuk jako další kamínek v mozaice upřesnění kardiovaskulárního rizika? – editorialČlánek DRESS syndrom – editorialČlánek The role of epicardial fat and obesity parameters in the prediction of coronary heart diseaseČlánek Oznam/OznámeníČlánek DRESS syndromeČlánek 41. Angiologické dny
Článok vyšiel v časopiseVnitřní lékařství
Najčítanejšie tento týždeň
2016 Číslo 4- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Rizikové období v léčbě růstovým hormonem: přechod mladých pacientů k lékařům pro dospělé
- Statinová intolerance
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Epikardiální tuk jako další kamínek v mozaice upřesnění kardiovaskulárního rizika? – editorial
- Metformin u nemocných s 2. typem diabetes mellitus a laktátová acidóza – editorial
- Populace pacientů s diabetem se změnila, pacienti žijí déle a vykazují vyšší riziko srdečního selhání – editorial
- DRESS syndrom – editorial
- Effective bowel preparation before coloscopy – low-volume PEG in the divided dose regimen
- The role of epicardial fat and obesity parameters in the prediction of coronary heart disease
- Diuretic treatment in patients with acute pulmonary edema did not produces severe hyponatremia or hypokalemia
- Analysis of serum free light chains κ/λ ratio and heavy/light chain pairs of immunoglobulin to the stratification of multiple myeloma according to Mayo Stratification of Myeloma and Revised International Staging System
- Oznam/Oznámení
- Prothrombin gene 20210A mutation in Slovak population
- Treatment of 14 cases of Castleman’s disease: the experience of one centre and an overview of literature
- Chronic kidney diseases, metformin and lactic acidosis
- Beta-blockers and chronic obstructive pulmonary disease
- Overview of current modalities of colorectal cancer screening
- The effect of antihypertensive treatment on patients with diabetes depends on the values of blood pressure: a systematic survey and meta-analyses
- What are the effects of fixed-dose combination of candesartan and amlodipine
- Are some antidiabetic drugs also drugs useful for heart failure treatment?
-
PCSK9 inhibitors – new possibilities in the treatment of hypercholesterolemia: For which patients will be indicated?
Czech atherosclerosis society statement - DRESS syndrome
-
MUDr. Karel Roztočil, CSc.,
předseda České angiologické společnosti České lékařské společnosti JEP slaví 75. narozeniny - 41. Angiologické dny
-
Eva Sedláčková, Viera Bajčiová a kol.
Neuroendokrinní nádory
- Vnitřní lékařství
- Archív čísel
- Aktuálne číslo
- Iba online
- Informácie o časopise
Najčítanejšie v tomto čísle- DRESS syndrome
-
PCSK9 inhibitors – new possibilities in the treatment of hypercholesterolemia: For which patients will be indicated?
Czech atherosclerosis society statement - Beta-blockers and chronic obstructive pulmonary disease
- Chronic kidney diseases, metformin and lactic acidosis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



