-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
RNF12 Activates and Is Essential for X Chromosome Inactivation
In somatic cells of female placental mammals, one of the two X chromosomes is transcriptionally silenced to accomplish an equal dose of X-encoded gene products in males and females. Initiation of random X chromosome inactivation (XCI) is thought to be regulated by X-encoded activators and autosomally encoded suppressors controlling Xist. Spreading of Xist RNA leads to silencing of the X chromosome in cis. Here, we demonstrate that the dose dependent X-encoded XCI activator RNF12/RLIM acts in trans and activates Xist. We did not find evidence for RNF12-mediated regulation of XCI through Tsix or the Xist intron 1 region, which are both known to be involved in inhibition of Xist. In addition, we found that Xist intron 1, which contains a pluripotency factor binding site, is not required for suppression of Xist in undifferentiated ES cells. Analysis of female Rnf12−/− knockout ES cells showed that RNF12 is essential for initiation of XCI and is mainly involved in the regulation of Xist. We conclude that RNF12 is an indispensable factor in up-regulation of Xist transcription, thereby leading to initiation of random XCI.
Published in the journal: RNF12 Activates and Is Essential for X Chromosome Inactivation. PLoS Genet 7(1): e32767. doi:10.1371/journal.pgen.1002001
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002001Summary
In somatic cells of female placental mammals, one of the two X chromosomes is transcriptionally silenced to accomplish an equal dose of X-encoded gene products in males and females. Initiation of random X chromosome inactivation (XCI) is thought to be regulated by X-encoded activators and autosomally encoded suppressors controlling Xist. Spreading of Xist RNA leads to silencing of the X chromosome in cis. Here, we demonstrate that the dose dependent X-encoded XCI activator RNF12/RLIM acts in trans and activates Xist. We did not find evidence for RNF12-mediated regulation of XCI through Tsix or the Xist intron 1 region, which are both known to be involved in inhibition of Xist. In addition, we found that Xist intron 1, which contains a pluripotency factor binding site, is not required for suppression of Xist in undifferentiated ES cells. Analysis of female Rnf12−/− knockout ES cells showed that RNF12 is essential for initiation of XCI and is mainly involved in the regulation of Xist. We conclude that RNF12 is an indispensable factor in up-regulation of Xist transcription, thereby leading to initiation of random XCI.
Introduction
X chromosome inactivation (XCI) in placental mammals is random with respect to the parental origin of the X chromosome that undergoes inactivation, during early embryonic development [1]. In contrast, in marsupials and mouse extra-embryonic tissues XCI is imprinted. Imprinted XCI always targets the paternally inherited X chromosome (Xp), and is initiated during the early cleavage divisions [2], [3], [4]. In the inner cell mass (ICM) of the mouse blastocyst, the inactive X chromosome is reactivated, after which random XCI is initiated around 5.5 days of embryonic development.
In mouse, two non-coding X-linked genes, Xist and Tsix, play a central role in the random XCI mechanism. Upon initiation of XCI, Xist is up-regulated on the future inactive X chromosome (Xi), and the transcribed RNA spreads along the X in cis, directly and indirectly recruiting chromatin modifying enzymes acting to establish the Xi [5], [6], [7]. Tsix is a negative regulator of Xist; the Tsix gene overlaps with Xist but is transcribed in the anti-sense direction [8], [9].
Random XCI is a stochastic process in which each X chromosome has an independent probability to become inactivated [10], [11]. Initiation of XCI is thought to be regulated by X-encoded activators and autosomally encoded inhibitors [11], [12]. With two active X chromosomes, female cells will have a concentration of XCI activators two-fold higher than male cells, sufficiently different to drive XCI in female cells only. Rapid down-regulation of XCI activator genes in cis, after initiation of XCI on either one of the X chromosomes, prevents initiation of XCI on the second X chromosome.
XCI inhibitors are involved in maintaining a threshold for XCI to occur. So far, several XCI inhibitors have been identified, acting through different mechanisms, in mouse. YY1 and CTCF act as positive regulators of Tsix, by binding the DXpas34 Tsix regulatory element [13]. The pluripotency factors OCT4, SOX2 and NANOG were proposed to regulate XCI by binding to intron 1 of Xist and suppressing Xist expression directly [14]. OCT4 and SOX2 have also been implicated in the positive regulation of Tsix and Xite, the latter being an enhancer of Tsix [15]. These findings indicate that several proteins and pathways act in concert to suppress Xist transcription and to block Xist RNA spreading in cis.
XCI activators could act by activation of Xist, but also by suppression of negative regulators of Xist such as Tsix and the Xist intron 1 region. Recently, we identified RNF12 (RLIM) as the first X-linked activator of XCI [16]. This E3 ubiquitin ligase is involved in regulation of LIM-homeodomain transcription factors and telomere length homeostasis, through degradation of LDB1 and TRF1, respectively [17], [18]. Previously, we found that additional transgenic copies of the Rnf12 gene encoding this protein resulted in induction of XCI on the single X in transgenic male cells, and on both X chromosomes in a high percentage of female cells. XCI was also affected in Rnf12+/− ES cells supporting a dose-dependent role for RNF12 in activation of XCI. In the present study, we aimed to dissect the role of RNF12 in XCI, and we obtained evidence that RNF12 regulates XCI in trans, by activation of the Xist promoter. In addition, the generation and analysis of Rnf12−/− ES cells indicated that RNF12 is required for the XCI process and appears to be involved in XCI mainly by activation of Xist. The results reinforce that RNF12 is a key player in regulation of the XCI process.
Results
RNF12 acts in trans to activate XCI
XCI is regulated by several cis elements, and Rnf12 is located in close proximity to Xist (∼500 kb). Therefore, we aimed to test whether all the activity of RNF12 is mediated in trans. Our previous studies showed that Rnf12+/− female ES cells induce XCI in a reduced number of ES cells. Here, we rescued 129/Sv/Cast/Ei (129/Cas) polymorphic Rnf12+/− female ES cells by introducing a 129 BAC (RP24-240J16) construct covering Rnf12. RT-PCR analysis followed by RFLP detection confirmed expression of the transgenic copies of Rnf12 (Figure 1A). Xist RNA-FISH analysis, to detect the Xist coated inactive X chromosome (Xi) in day 3 differentiated transgenic ES cell lines with one additional copy of Rnf12, shows that XCI was restored to wild type level (Figure 1B). In line 20, with 5 transgenic copies of Rnf12 the percentage of cells with one or two Xi's is even more pronounced, supporting a dose dependent role of RNF12 in XCI (Figure 1B, 1C). XCI is skewed in wild type 129/Cas female ES cells towards inactivation of the 129 X. This is due to the presence of different X-linked cis elements (Xce) that affect random choice [19]. RT-PCR detecting a length polymorphism was used to distinguish Xist emanating from either the 129 or the Cas alleles. We observed that skewed XCI is more pronounced in the Rnf12+/− cells, as compared to XCI in wild type cells at day 3 of differentiation (Figure 1D). This could be caused by selection against cells inactivating the wild type X chromosome, which would result in complete loss of RNF12 from these cells. However, RNF12 possibly is not essential for cell survival, also of differentiated cells, so that selection against cells inactivating the wild type X chromosome might point to a role for RNF12 in maintaining Xist expression. In the rescued cell lines, Xist was up-regulated from both alleles at day 3 of differentiation (Figure 1D). This result demonstrates that RNF12 activates XCI in trans.
Fig. 1. RNF12 activates X chromosome inactivation in trans. 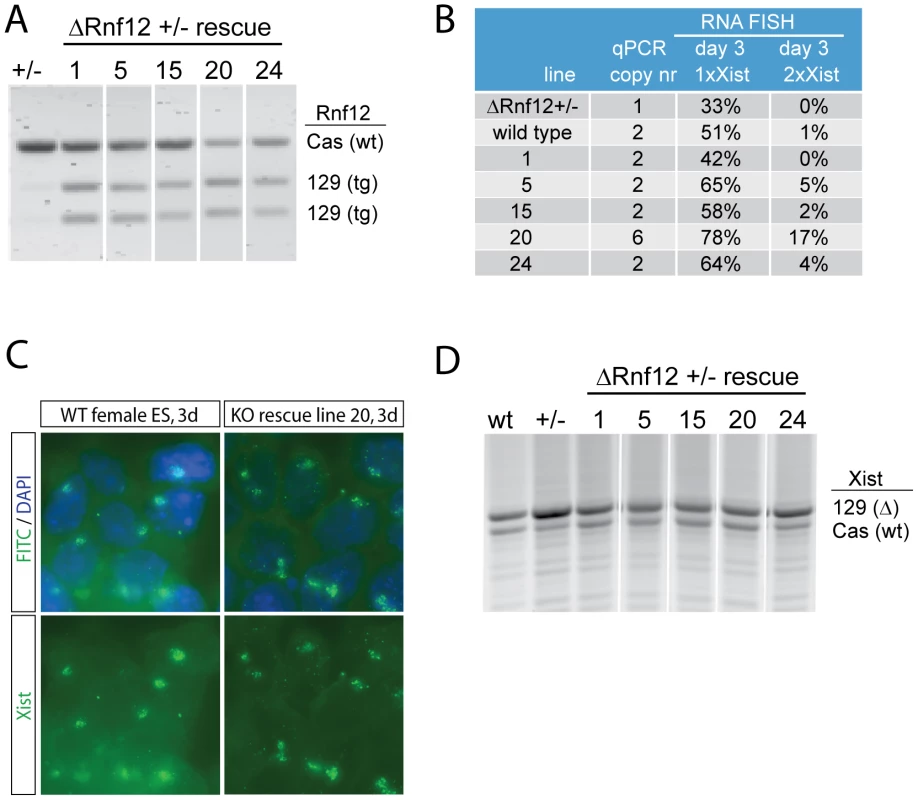
A) Allele specific RT-PCR analysis of Rnf12 expression with RNA isolated from day 3 differentiated female Rnf12 +/− ES cells (Cas/129, 129 Rnf12 targeted), and rescued cell lines obtained after stable integration of an 129 Rnf12 transgene. NheI digested 129 products were separated from undigested Cas products. B) Overview of RNA-FISH experiments detecting Xist expression in female wild type, Rnf12 +/− and Rnf12+/− rescued cell lines. qPCR copynumber analysis was performed on genomic DNA. RNA-FISH analysis was performed on day 3 differentiated ES cells, and the percentage of cells harbouring one Xist coated X chromosome (Xist cloud ( = Xi), 1x Xist) or two Xist coated X chromosomes (2x Xist) was determined. C) Representative pictures of RNA-FISH analysis, detecting Xist (FITC) in day 3 differentiated female wild type and Rnf12+/− rescued ES cells (line 20, Rnf12 overexpression). DNA is counterstained with DAPI in all RNA-FISH slides. D) Allele specific RT-PCR analysis of day 3 differentiated wild type, Rnf12+/− and Rnf12+/− rescued cell lines, detecting an Xist length polymorphism that discriminates 129 and Cas Xist. Counteracting roles for RNF12 and NANOG
One possible mechanism for regulation of XCI by RNF12, might be a direct interaction with Xist RNA to target chromatin components. However, examination of day 3 differentiated female cells by immunocytochemistry detecting RNF12, together with the Polycomb protein SUZ12 which accumulates on the Xi [20], [21], excludes this possibility (Figure 2A). Interestingly, we noticed that the RNF12 staining intensity was much more dynamic in female compared to male cells (Figure 2B, Figure S1). Also, in female cells, a SUZ12 coated Xi appeared mainly in cells with low RNF12 staining (Figure 2A, Figure S2, and data not shown). Immunostaining of differentiating female ES cells indicated a negative correlation between expression of RNF12 and NANOG, although expression was not completely mutually exclusive (Figure 2C). To analyze this in more detail, we targeted an Rnf12 promoter-mCherry construct into ES cells, also harboring a knock-in GFP transgene in the Nanog and Oct4 loci. We analyzed expanded individual clones and pooled clones and obtained similar results. FACS analysis, prior to differentiation and at different time points after differentiation of these double transgenic ES cell lines, showed a negative correlation between RNF12-mCherry and NANOG-GFP expression, but not for RNF12-mCherry and OCT4-GFP (Figure 2D, 2E, Figure S3). Our findings therefore suggest specific counteracting regulatory roles for RNF12 and NANOG in XCI, which might include an inhibitory effect of NANOG on Rnf12 transcription. Interestingly, NANOG has been implicated in the regulation XCI by direct suppression of Xist in ES cells, and Xist suppression in the ICM of the developing blastocyst corresponds with up-regulation of NANOG expression [22]. Therefore, mutual exclusive expression of RNF12 and NANOG may be required for initiation of XCI.
Fig. 2. Counteracting roles for RNF12 and NANOG in XCI. 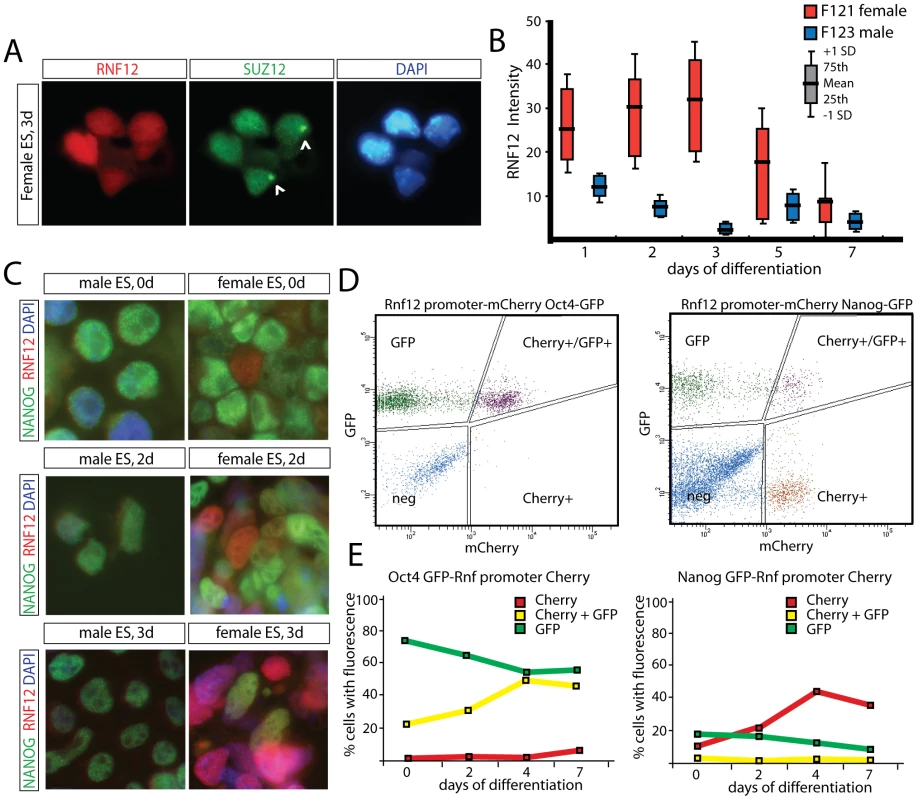
A) Immunocytochemistry detecting RNF12 (Alexa 546) and SUZ12 (Alexa 488) in day 3 differentiated female ES cells. Cells showing accumulation of SUZ12 on the X chromosome (Xi) show low levels of nuclear RNF12, suggesting that RNF12 is downregulated upon XCI. RNF12 does not accumulate on the SUZ12 coated Xi. B) Quantification of RNF12 staining intensities in female and male ES cells at different timepoints of differentiation. Red and blue box plots show results for female and male cells, respectively. Mean, interquartile range and standard deviation are indicated. N>100 cells per timepoint. Female cells show higher staining intensities and more fluctuation of RNF12 expression compared to male cells. C) Immunocytochemistry detecting RNF12 (rhodamine) and NANOG (FITC) in undifferentiated and day 2 and 3 differentiated male and female ES cells. D) FACS analysis of NANOG-GFP (right panel) and OCT4-GFP (left panel) ES cells transgenic for an Rnf12-mCherry promoter construct. FACS plots show results of undifferentiated ES cells. Cells are gated for GFP+, Cherry+, GFP+Cherry+ or negative. Results of a representative experiment are shown. E) Quantification of FACS analysis of NANOG-GFP (right panel) and OCT4-GFP (left panel) ES cells transgenic for an Rnf12 mCherry promoter construct. Cells were differentiated for up to 7 days, and the percentage of positive cells was determined (Cherry+, red line; GFP+, green line; Cherry+GFP+, yellow line). RNF12 does not regulate XCI through Xist intron 1
Recently, the first intron of Xist has been identified as a region involved in recruitment of three pluripotency factors, OCT4, NANOG and SOX2 [14]. It was shown that down-regulation of Nanog and Oct4, through gene ablation, resulted in an increase in Xist expression, and initiation of XCI in male cells. Interestingly, the intron 1-mediated suppression of XCI was suggested to directly act on Xist, without involvement of Tsix. To study if RNF12 might regulate XCI by interfering with binding of pluripotency factors to the intron 1 region of mouse Xist, we removed 1.2 kb of Xist intron 1 including all reported NANOG, OCT4 and SOX2 binding sites by homologous recombination with a BAC targeting construct, without disturbing the integrity of the Xist transcript. Targeted clones were screened by PCR amplification of a targeted RFLP (BsrgI) in female F1 2-1, 129/Cas polymorphic ES cells, which was confirmed by Southern blotting, followed by Cre mediated loop-out of the kanamycin/neomycin resistance cassette (Figure 3A, Figure S4). Xist RNA FISH at different time points of differentiation of several Xistintron1+/− ES cell lines indicated that XCI is initiated with the same kinetics as in wild type cells, and showed that the intron 1 region is not required for repression of Xist in undifferentiated ES cells or early during initiation of XCI (Figure 3B, 3C, and Figure S4G). Nevertheless, Xist specific RT-PCR, detecting a length polymorphism distinguishing 129 and Cas Xist, showed enhanced skewing at day 3 of differentiation towards 129 Xist expression, suggesting a role for the intron 1 region in suppressing Xist at later stages of differentiation, when NANOG, OCT4 and SOX2 are expressed at a lower level (Figure 3D). To test an involvement of the intron 1 region in RNF12-mediated activation of XCI, we introduced an Rnf12 BAC transgene into the Xistintron1+/− ES cell lines. Additional copies of Rnf12 resulted in induction of Xist, even in undifferentiated ES cells (Figure 3E, 3F, 3I), confirming our previous findings [16]. However, allele specific RT-PCR did not point to an increased preference for expression of the mutated or wild type allele, in undifferentiated ES cells (Figure 3G, 3H), indicating that RNF12-mediated action on XCI does not require the Xist intron 1 region (Figure 3J). At day 3 of differentiation, in several cell lines, we found higher expression of Cas Xist in Rnf12 transgenic Xistintron1+/− cells compared to Xistintron1+/− only cells. We attribute this finding to an increase in the percentage of cells with two Xist clouds. We conclude that the Xist intron 1 region is not essential for suppression of XCI in undifferentiated ES cells, but may play a role later during differentiation. Furthermore, RNF12-mediated activation of XCI is independent from the Xist intron 1 region.
Fig. 3. RNF12 initiates XCI independent of pluripotency factor binding to Xist intron 1. 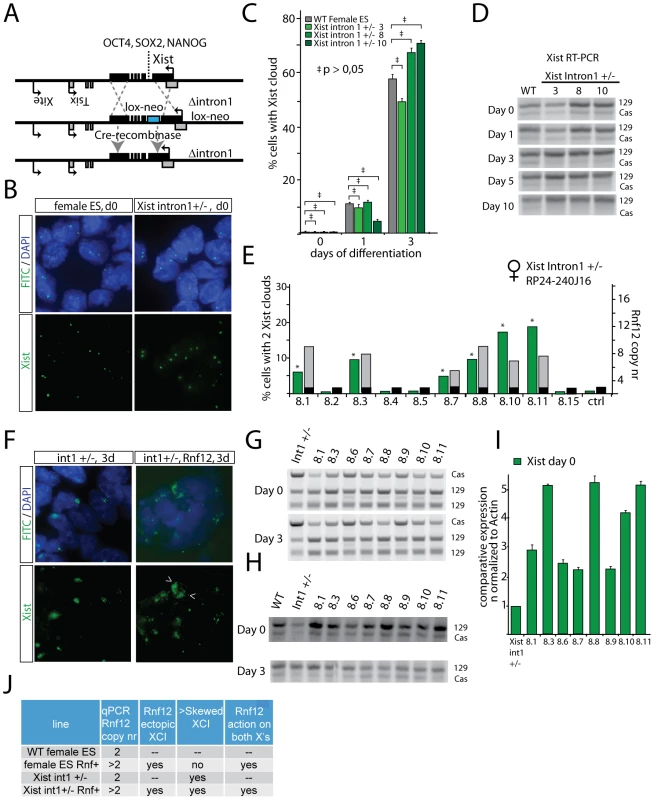
A) Schematic representation of part of the X chromosome and the strategy to target the Xist intron 1 pluripotency factor binding sites. A BAC targeting construct replacing Xist intron 1 by a floxed neomycin resistance cassette (Neo) was used to target specifically the 129 allele in Cas/129 female ES cells. The Neo cassette was looped out after transient expression of Cre recombinase. B) RNA-FISH analysis detecting Xist (FITC) in undifferentiated female wild type and Xist intron 1+/− ES cells. In both wild type and Xist intron 1 deleted cells, only pinpoint signals are visible, representing basal Xist and Tsix expression. C) Bar graph showing the percentage of wild type and Xist intron 1+/− ES cells that initiated XCI, detected by Xist RNA-FISH, at different time points of EB differentiation. No statistical significant differences were noticed between the wild type control and the cell lines harbouring a deletion of Xist intron 1 (95% confidence interval, N>100 cells per time point ‡ p>0.05). D) Allele specific RT-PCR analysis detecting Xist expression in female wild type and Xist intron 1+/− cell lines (clone 3, 8 and 10) during differentiation. E) qPCR analysis to determine the Rnf12 copy number in Xist intron 1+/− ES cells transgenic ES cell lines (transgenic, grey, and endogenous, black, copy number), and percentage of cells with two Xist clouds at day 3 of differentiation. F) RNA-FISH analysis detecting Xist (FITC) in day 3 differentiated Xist intron 1+/− ES cells, without (left panels) and with (right panels) an Rnf12 transgene. The Xist clusters in one cell with two Xist clusters are indicated with arrowheads. G) RFLP RT-PCR amplifying a NheI RFLP present on the endogenous129 Rnf12 allele, and the Rnf12 transgene. Relative expression analysis was performed with RNA isolated from undifferentiated and day 3 differentiated ES cell lines. H) RT-PCR amplifying a length polymorphism distinguishing Xist emanating from the mutated 129 allele and the wild type Cas allele, with RNA isolated from undifferentiated and day 3 differentiated ES cell lines. I) Xist expression in undifferentiated Rnf12 transgenic Xist intron 1+/− ES cells, and an Xist intron 1+/− control cell line was quantified qPCR. J) Table summarizing the results obtained with female wild type, Rnf12 transgenic, Xist intron 1+/− and Xist intron 1+/− Rnf12 transgenic ES cell lines. RNF12 regulates Xist
RNF12 could regulate XCI through activation of Xist or suppression of Tsix, or both. Previously, we analyzed Xist transgenic male ES cell lines with a BAC RP24-180B23 integration covering Xist only [16], or a BAC RP23-338B22 sequence containing both Xist and Tsix (Figure 4A). These male transgenic ES cell lines also contained 16 copies of an ms2 bacteriophage repeat sequence located in exon 7 of the endogenous Xist gene, allowing separate detection by RNA-FISH of autosomal versus endogenous Xist spreading [23]. Differentiation of transgenic male ES lines containing the Xist-Tsix transgene resulted in expression of Xist from the autosomal integration site in cell lines containing multicopy integrations. Autosomal spreading of Xist in these cell lines is most likely due to accumulation of enough Xist RNA to silence at least one copy of Tsix, allowing spreading of Xist in cis. Integration of truncated transgenes that lack Tsix would facilitate this process [16]. This also explained autosomal Xist spreading in BAC RP-24-180B23 single copy male transgenic ES cell lines upon differentiation, because Tsix is not covered by this BAC [16]. We used two of these, Xist only, BAC RP-24-180B23 ES cell lines to introduce 129 BAC RP24-240J16 transgenes covering Rnf12, and found Xist spreading on the single endogenous X (Figure 4B and 4C), confirming previous results. We also found a significant increase in the number of cells with autosomal Xist spreading, indicating that RNF12 activates XCI through Xist. Next, we introduced an Rnf12 transgene (BAC RP24-240J16) in a single copy Tsix male transgenic ES cell line that lacks transgenic Xist (BAC RP23-447O10). These double transgenic ES cell lines contain a Cas X chromosome which allowed RFLP mediated discrimination of endogenous (Cas) and transgenic (129) Tsix. Analysis of these cell lines indicated that transgenic over-expression of RNF12 does not lead to down-regulation of Tsix, as measured by qPCR and by RNA-FISH examining the relative number of Tsix pinpoint signals (Figure 4D, 4E, 4G). Interestingly, allele specific RT-PCR indicated that endogenous Tsix (Cas) is even down-regulated in samples with higher Xist expression, indicating Xist-mediated silencing of Tsix in cis (Figure 4F). Taken together, these results indicate that Xist and not Tsix is the functionally most important downstream target of RNF12.
Fig. 4. RNF12 activates Xist directly, but does not inhibit Tsix. 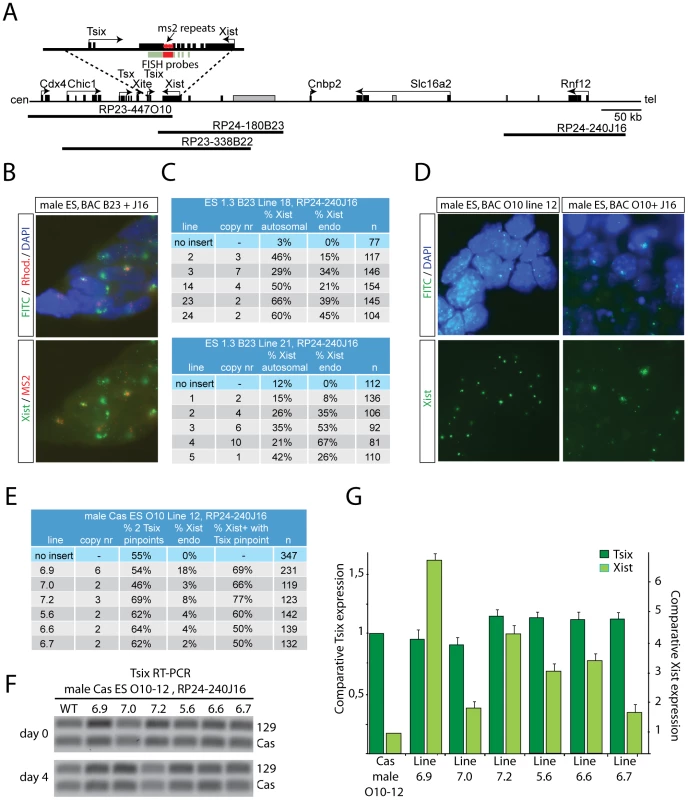
A) Map showing part of the mouse X chromosome, the location of the BAC sequences used, and the position of ms2 repeats within Xist. RNA-FISH probes are indicated in green and red, and non-annotated genes in grey. B) RNA-FISH analysis detecting endogenous Xist (ms2, rhodamine and FITC positive) and exogenous Xist (FITC) from the autosomally integrated Xist-only BAC RP24-180B23 in day 3 differentiated male ES cells transgenic for Rnf12 (BAC RP24-240J16). C) Table summarizing RNA-FISH results from B). Copy number of the Rnf12 transgene was determined by gDNA qPCR. Shown are the percentage of autosomal and endogenous Xist clouds; N, number of cells analyzed. D) RNA-FISH analysis detecting endogenous and transgenic Tsix (FITC, pinpoint signals) and endogenous Xist (FITC, clouds) in day 3 differentiated Tsix transgenic male cells (left panels) and day 4 differentiated Tsix transgenic male cells with additional copies of an Rnf12 transgene (right panels). E) Table summarizing results obtained with single copy Tsix transgenic male ES cell lines with a Rnf12 transgene, 4 days after differentiation. Shown are copy number of the Rnf12 transgene, percentage of cells with two Tsix signals, cells with an Xist cloud, and the percentage of cells with an Xist cloud and Tsix pinpoint signal (n is number of cells analyzed). F) Allele specific RT-PCR detecting transgenic (129) and endogenous (Cas) Tsix in undifferentiated and day 4 differentiated Tsix/Rnf12 double transgenic ES cells. G) qPCR analysis to quantify Xist and Tsix expression in day 4 differentiated Tsix/Rnf12 double transgenic ES cells, and a control cell line without an Rnf12 transgene. RNF12 is required for XCI
We previously found that the rate of initiation of XCI is reduced in differentiating female Rnf12+/− ES cells, compared to wild type ES cells [16]. The RNF12 protein level in these Rnf12+/− female cells is equal to that in male cells [16], but XCI is still occurring at a higher rate than in male cells. This indicated the presence of additional X-encoded XCI activators, but did not exclude the possibility that RNF12 is essential for XCI. To address this point, we generated Rnf12−/− female ES cells by targeting the wild type Cas Rnf12 allele in Rnf12+/− ES cells (Figure 5A). Correct targeting was confirmed by RT-PCR, showing loss of a targeted RFLP located in exon 5 of Rnf12 (Figure 5B). The presence of two X chromosomes in these Rnf12−/− female ES cells was ascertained by X chromosome DNA FISH analysis and amplification of an RFLP in the Xist gene (Figure 5C, and data not shown). Western blotting analysis confirmed the absence of RNF12 protein in the knockout cells (Figure 5D). RT-PCR and qRT-PCR of pluripotency associated genes and differentiation markers gave information that differentiation of the Rnf12−/− ES cells was not different from that of wild type ES cells (Figure 5E, 5F and Figure S5). However, Xist RNA FISH analysis showed that differentiating Rnf12−/− ES cells only sporadically initiate XCI (Figure 5G, 5H and 5I). QPCR analysis confirmed that Xist is not detectably up-regulated when measured for a population of Rnf12−/− cells upon differentiation. Moreover, DNA-FISH detecting a whole chromosome X paint probe at day 7 and 10 of differentiation excluded X chromosome loss (Figure S5). The few Rnf12−/− cells that initiated XCI appeared in clusters, suggesting clonal expansion of a few cells that initiated XCI (Figure S5). We therefore conclude that RNF12 is an essential factor in XCI.
Fig. 5. RNF12 is essential for XCI. 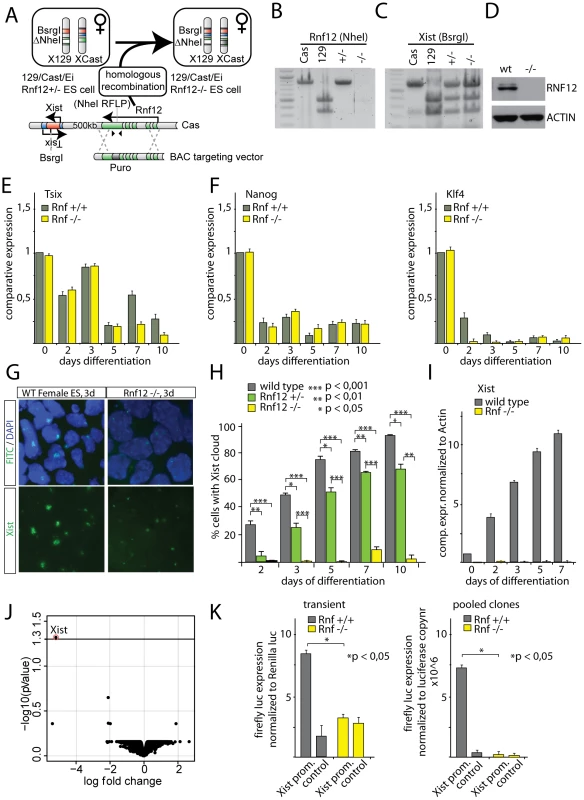
A) Targeting strategy to generate Rnf12−/− ES cells. The Cas Rnf12 allele of the previously generated heterozygous Rnf12+/− ES cells (Cas/129) was targeted with a BAC construct containing a puromycin selection cassette disrupting the open reading frame of Rnf12. B) PCR RFLP analysis with primers spanning a NheI RFLP descriminating the Cas (no NheI site) and the 129 (NheI site present) alleles, which was used to insert the targeting cassette. C) PCR RFLP analysis confirming the presence of two X chromosomes in Rnf12−/− ES cells. PCR primers span a BsrgI RFLP located in Xist. D) Western analysis of RNF12 protein and ACTIN in wild type and Rnf12−/− ES cells. E) qRT-PCR analysis detecting Tsix expression in female wild type and Rnf12−/− ES cells differentiated for up to 10 days. Results were normalized to Actin. F) qRT-PCR analysis as in (H), but now detecting Nanog (left graph) and Klf4 (right graph) expression. G) RNA-FISH analysis detecting Xist (FITC) in day 3 differentiated female wild type and Rnf12−/− ES cells. H) Bar graph showing the percentage of female wild type, Rnf12+/− and Rnf12−/− ES cells that initiated XCI, as determined by Xist RNA-FISH, at different time points of differentiation. *** p<0,001; ** p<0,01; * p<0,05, Student's T-test. I) qRT-PCR detecting Xist in female wild type and Rnf12−/− ES cells differentiated for up to 7 days. Results were normalized to Actin. J) Genome wide expression analysis comparing day 3 differentiated Rnf12−/− and wild type ES cells. Shown are the Log fold expression change and the adjusted P value. K) Luciferase assay detecting expression of an Xist-promoter-luciferase construct in female wild type and Rnf12−/− ES cells differentiated for 3 days. For transient experiments, cells were co-transfected at day 0 with the Xist-promoter-luciferase or control vector (empty luciferase vector) and a Renilla plasmid. Results were normalized to Renilla expression. For stable pooled clones, the promoter constructs were transfected, clones were pooled after selection and differentiated 3 days prior to analysis. RNF12 activates the Xist promoter
Evidently, the Rnf12−/− knockout cells present the possibility to study control of gene expression by RNF12. Therefore, we next performed micro-array expression analysis comparing day 3 differentiated Rnf12−/− and wild type cells. We found that Xist was the only gene that was subject to differential regulation, showing pronounced down-regulation (Figure 5J). Interestingly, none of the known downstream targets of RNF12 appeared affected in our analysis. This may be due to our ES cell differentiation system resulting in a mixed population of cells at different stages of differentiation. In addition, the 3-day-time span allowed in our studies for cell differentiation may have prevented detection of effects on downstream targets which are expressed at later stages of differentiation. Nevertheless, our results indicate that the main function of RNF12 at this early stage of differentiation concerns the regulation of XCI. The observed dependency of Xist transcription on RNF12 might be effectuated by RNF12 acting through the Xist promoter. To test this, we expressed Xist promoter luciferase reporter constructs, both transiently and stably, in wild type female and Rnf12−/− ES cell lines and differentiated these cells for 3 days. The results revealed an unequivocal correlation between RNF12 expression and luciferase expression (Figure 5K). Our results therefore demonstrate that RNF12 activates the Xist promoter, although this does not exclude a role for other cis regulatory sequences, further away from the Xist promoter, in RNF12-mediated activation of XCI.
Discussion
In ES cells, RNF12 exerts its main function in XCI
Here, we present evidence that RNF12 is an essential activator of random XCI. RNF12 acts in trans on the Xist promoter, in differentiating mouse ES cells, to activate Xist transcription, leading to Xist RNA cloud formation and spreading of the silencing complex over the future inactive X chromosome in cis. Although our results show that RNF12 acts in trans, it is to be expected that the close proximity of the Rnf12 gene to the Xist locus, taken together with the dose-dependent action of RNF12, is quite crucial for well-tuned regulation of XCI. Such proximity most likely facilitates rapid down-regulation of Rnf12 in cis upon initiation of XCI, leading to a lower nuclear RNF12 content, thereby preventing inactivation of the second X chromosome.
Whole genome expression analysis suggests that the major function of RNF12 in ES cells is its regulation of Xist RNA expression, hence XCI. This is a very surprising finding, as RNF12 has been implicated in many other biological pathways. Apparently, in the present cell differentiation system, loss of expression of RNF12 does not cause a deviation from the wild type differentiation process to such an extent that it affects gene expression other than that of Xist. However, also based on our studies we do not exclude a function for RNF12 at later stages of cell differentiation, or in mouse development. In addition, redundant pathways or proteins such as RNF6, a close homologue of RNF12, may prevent full phenotypic expression of loss of RNF12. However, RNF12 exerts a predominant role in targeting Xist, as evidenced by our observation that Xist is largely silenced in the RNF12 deficient cells.
While our manuscript was under review, Shin et al. (2010) published a paper suggesting that RNF12 might be required in particular for imprinted XCI in mice [24]. Remarkably, that study included the observation that RNF12 depletion did not prevent initiation of random XCI in a significant percentage of Rnf12−/− ES cells derived from mouse blastocysts. This discrepancy with our findings might be explained by experimental differences, such as differences concerning the design of the knockout, the genetic background of the ES cells, or the cell derivation and culture procedures. Differences in cell differentiation protocols have been shown to have a pronounced impact on the XCI process [25]. Also, ES cells derived from embryos with a different genetic background could express XCI activators and XCI inhibitors at different levels, allowing XCI in either a lower or a higher percentage of Rnf12−/− cells. Future studies comparing the two independently generated Rnf12−/− ES cell lines will yield useful information about these points.
Other XCI activators
Although our observations provide evidence that RNF12 is an essential factor for the XCI process to occur in differentiating ES cells, we anticipate that other XCI activators act in parallel, and might independently regulate Xist or Tsix, or both. Dosage compensation mechanisms in species such as D. melanogaster and C. elegans also involve multiple factors and pathways, possibly leading to increased fidelity of these mechanisms [26]. In such a mechanism involving multiple factors, RNF12 would be the dose-dependent factor that is required to exceed the cumulative threshold limit to proceed towards initiation of XCI. It is feasible that female Rnf12−/− cells sometimes do initiate XCI (Figure 6A), as a consequence of the stochasticity of the process. This would be compatible with a mechanism, in which the combined total activity of all putative XCI activators exclusive of RNF12 is just below or around the threshold to initiate XCI. Interestingly, Xist cloud formation is also sporadically found in male cells, but in contrast to female Rnf12−/− cells, this represents a lethal condition and will be selected against.
Fig. 6. Rnf12 and its role in the XCI regulatory network. 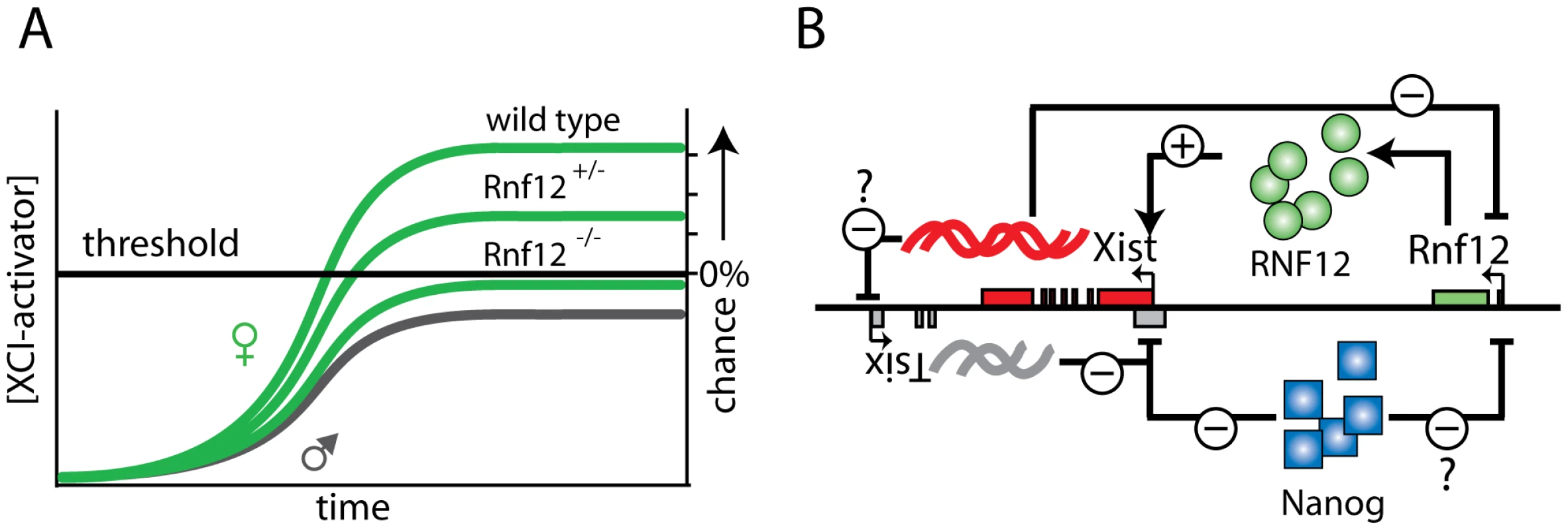
A) In wild type and Rnf12+/− cells the XCI activator concentration is above the threshold required to generate a probability to initiate XCI. In contrast, in most Rnf12−/− cells the XCI activator concentration is not sufficient to reach the threshold required to initiate XCI. B) The regulatory network of XCI. Xist is repressed in Tsix dependent and independent pathways (NANOG binding in intron 1). Activation of XCI is accomplished by RNF12 through activation of the Xist promoter, and possibly Xist mediated silencing of Tsix. Finally, Rnf12 is repressed by Xist and possibly NANOG. Our studies indicate that RNF12 participates in Xist promoter activation, through an action which requires the presence of the minimal promoter. Although the direct protein target(s) of RNF12 remain elusive, its reported E3 ubiquitin ligase activity [17] would be compatible with RNF12 targeting an inhibitor of Xist transcription through proteasome-mediated degradation. This does not exclude that RNF12 might be involved, in addition or alternatively, in activation of a transcription factor driving Xist expression through positive regulation of transcription. Furthermore, RNF12 could be involved in regulation of cis-regulatory sequences other than the Xist promoter, yet to be identified and further away from the Xist locus.
A function for RNF12 in maintaining Xist expression
Selection against cells inactivating the X chromosome containing the wild type allele of Rnf12 in the heterozygous Rnf12+/− ES cells could point to a continued requirement for Rnf12 in maintaining Xist expression, following the early stages of differentiation. From the fact that male Rnf12−/Y knockout male mice are viable [24], it can be concluded that RNF12 deficiency is compatible with survival of differentiated cells in which XCI does not play any role. Hence, it would be difficult to explain the observed selection against cells inactivating the wild type X chromosome in the heterozygous Rnf12+/− ES cells by loss of any possible function of RNF12 independent of XCI. If RNF12 would be required for maintaining Xist expression and XCI, the cells inactivating the wild type allele and becoming deficient in RNF12 can be expected to lose Xist expression and to reactivate the Xi. In contrast, cells inactivating the X chromosome containing the mutated allele, keeping one functional allele of Rnf12, will be able to maintain Xist expression and XCI. In a population of cells this will lead in skewed XCI of the mutated allele. In fact, such a mechanism might also be relevant to explain the reported defect in imprinted XCI resulting from an Rnf12 mutation [24].
Imprinted XCI involves activation of Xist on the Xp, and the observed phenotype concerns lack of this imprinted XCI of the Xp when the mutant Rnf12 allele is inherited from the mother. It was observed that no female embryos were born, inheriting a mutated Rnf12 allele from either a Rnf12−/− or a Rnf12+/− mother in crosses with wild type males. In contrast, the mutated allele was transmitted to male offspring. Maternal storage of RNF12 in the oocyte was proposed to play a crucial role in imprinted silencing of the Xp in the early embryo [24]. Rnf12 is at a 46 cM distance of the centromere, so that it can be expected that many haploid oocytes generated by the first meiotic division (the reduction division) of Rnf12+/− oocytes, which occurs at the time of ovulation, will contain both wild type and Rnf12 mutated alleles, as a consequence of meiotic recombination. Hence, we anticipate that there will be ongoing expression of Rnf12 in a high percentage of oocytes transmitting the mutated Rnf12 allele, until fertilization triggers meiotic division II. The recombined wild type and mutant alleles which are present within one haploid oocyte, will be exposed to the same maternal storage of RNF12. Taken together with the observation that Rnf12+/− oocytes did not give rise to female offspring carrying the mutant allele, whereas female offspring carrying the wild type allele were obtained at the expected mendelian ratio from these oocytes [24], this argues against a predominant role for maternal storage in imprinted XCI. Rather, we favor the hypothesis that continued transcription of Rnf12 throughout ovulation and after fertilization is required for sustained expression of RNF12, activation of Xist from the Xp, and maintenance of the inactive Xp. Future research will be required to address this hypothesis.
The link to pluripotency
Our results indicate a negative correlation between NANOG and RNF12 expression. NANOG and the other pluripotency factors OCT4 and SOX2 have been shown to be recruited to the Xist intron 1 region in undifferentiated ES cells, and were proposed to play a role in Tsix independent suppression of Xist [14]. In this regulatory mechanism, ablation of Tsix did not result in up-regulation of Xist in undifferentiated ES cells, and Tsix was not required for repression of Xist located on the inactivated paternal X chromosome in the inner cell mass. This pointed to an important role for recruitment of NANOG, OCT4 and SOX2 to Xist intron 1 in suppression of Xist in ES and ICM cells [14]. However, the present findings show that the intron 1 region is dispensable, in silencing the XCI process in undifferentiated ES cells. Deletion of Xist intron 1 caused an effect, but only in the form of skewing of XCI, which was notable at later stages of differentiation. Interestingly, a previous study analyzing an Xist mutant allele that lacks the intron 1 region but leaves the Xist promoter intact, also did not show up-regulation of the mutated allele in undifferentiated ES cells [27]. Although these latter results support our findings, they should be interpreted with caution because the selection cassette was still present in the cells analyzed by Marahrens et al. [27].
Like for the role of RNF12, this points to the presence of additional mechanisms, involved in suppression of XCI. Tsix and Xite are the most likely candidate genes taking part, and the combined action of these repressive mechanisms may be sufficient to suppress Xist. However, even with all the repressive elements in place RNF12 can induce Xist expression and XCI in undifferentiated ES cells [16]. This points towards another mechanism involved in Xist suppression, in which the nuclear concentration of the XCI activator may be too low in undifferentiated ES cells and ICM cells to allow Xist expression and initiation of XCI, even in the absence of repressive elements such as the intron 1 region. Future research should clarify whether these mechanisms indeed act synergistically in silencing the XCI process.
The negative correlation of RNF12 and NANOG expression that we report could reflect the differentiation state of the ES cells, and does not necessarily entail a cross-regulatory role for these proteins. Nevertheless, NANOG and other pluripotency factors are also recruited to the Rnf12 promoter in ES cells, where it might be involved in down-regulation of Rnf12 (Figure 6B) [28], which opens the intriguing possibility that NANOG might also be implicated in regulation of the initiation of XCI through suppression of Rnf12. This highlights the complexity of the overall mechanism and the interconnection of the different players involved in XCI, but also reinforces the predominant role of RNF12 in this process.
Methods
ES cell culture
ES cells were grown in standard ES medium containing DMEM, 15% foetal calf serum, 100 U ml−1 penicillin, 100 mg ml−1 streptomycin, non-essential amino acids, 0.1 mM β-mercaptoethanol, and 1000 U ml−1 LIF. To induce differentiation, ES cells were split, and pre-plated on non-gelatinised cell culture dishes for 60 minutes. ES cells were then seeded in non-gelatinised bacterial culture dishes containing differentiation medium to induce embryoid body (EB) formation. EB-medium consisted of IMDM-glutamax, 15% foetal calf serum, 100 U ml−1 penicillin, 100 mg ml−1 streptomycin, non-essential amino acids, 37.8 µl l−1 monothioglycerol and 50 µg/ml ascorbic acid. EBs were plated on coverslips 1 day prior to harvesting, and allowed to grow out.
Transgenesis and generation of knockout ES cell lines
For the Rnf12 rescue experiments, an Ampicilin-Puromycin resistance cassette was inserted in the backbone of BAC RP24-240J16 by homologous recombination in bacteria. The modified BAC was electroporated in to female heterozygous Rnf12+/− cells [16], and colonies were picked after 8–10 days of Puromycin selection, expanded and differentiated. BAC copynumber was determined by qPCR, and transgene specific expression was determined by allele specific RT-PCR, as described previously [16].
To generate the female homozygous Rnf12 −/− ES cell line, the previously generated Rnf12+/− ES cell line was targeted with an Rnf12 BAC targeting construct containing an Ampicilin-Puromycin cassette disrupting the open reading frame of Rnf12. To generate this targeting construct, targeting arms were PCR amplified using primers GCCTTCGAACATCTCTGAGC, GAGCCGGACTAATCCAAACA, cloned into pCR-BluntII-TOPO (Invitrogen), and linearized with NheI to introduce an Ampicilin-Puromycin cassette from pBluescript. The targeting cassette was inserted in a Cast/Ei Rnf12 BAC RP26-81P4 by homologous recombination in bacteria, and the resulting construct was used to target specifically the Cast/Ei X chromosome of the Rnf12 +/− ES cell line. Colonies were selected under Neomycin and Puromycin selection, and the absence of Rnf12 expression was confirmed by Western analysis.
To generate the Xist intron 1 deletion, a BAC targeting construct was generated by homologous recombination, replacing intron 1 by a floxed Neomycin cassette. Targetting arms were PCR amplified using primers 5′Forw:CATCAGGCTTGGCAGCAAGT, 5′R: CCTTGTTGGTCCAGACGACTATT and 3′Forw: CCAGACCAGGTCTTTGTATGCA, 3′Rev: GTGCTCCTGCCTCAAGAAGAA. Correctly targeted clones were identified by allele specific RFLP analysis using primers CAGTGGTAGCTCGAGCCTTT and CCAGAAGAGGGAGTCAGACG, followed by BsrGI digestion. The Neomycin cassette was removed by transient transfection with a CrePAC vector and selection with puromycin. The final cell lines were verified by Southern blotting.
Rnf12 and Xist reporter constructs
To generate the Rnf12 promoter cherry reporter cell lines, the Rnf12 promoter was PCR amplified using previous described primers [29], and cloned into pCR-BluntII-TOPO and sequence verified. The Rnf12 promoter was then released from pCR-BluntII-TOPO by digestion with SacI and KpnI, and blunt cloned into an AseI-BamHI fragment from pmCherry-N1 (Clonetech), thereby replacing the pCMV promoter of pmCherry-N1 with the Rnf12 promoter. The resulting construct was used to electroporate in Oct-GFP and Nanog-GFP ES cell lines. Both pooled cell lines and single colonies were expanded, and cherry expression was analysed by FACS analysis using a BD FACSAria apparatus.
The Xist promoter was amplified using primers: TCCCAAGGTATGGAGTCACC, and GGAGAGAAACCACGGAAGAA, and cloned into pGL3-basic vector. As a control, the promoter less pGL3-basic vector was transfected.
Stable pooled cell lines of wild type or Rnf12 −/− ES cells were generated by co-transfection with a puromycin or hygromycin selection vector. Expression of Luciferase was determined using the Bright-Glo luciferase assay system (Promega) and measured using a Promega luminometer. Results were normalized to the amount of protein present in the cell lysate measured by nanodrop, and copynumber of Xist promoter integration determined by qPCR. qRT-PCR using primers detecting luciferase (TCTAAGGAAGTCGGGGAAGC and CCCTCGGGTGTAATCAGAAT) confirmed the results obtained. For transient luciferase experiments, cells were co-transfected using the Xist reporter constructs and a control Renilla construct, using Lipofectamine 2000. Luciferase activity was measured using the Dual Glo luciferase system (Promega).
Xist RNA FISH, immunofluorescence, and Western analysis
Xist RNA-FISH was performed as described [11], [16]. Immunofluorescence was performed using standard procedures. RNF12 and NANOG were detected using a mouse anti - RNF12 antibody (1∶250, Abnova), and a rabbit anti-NANOG antibody (1∶100, SC1000, Calbiochem). ImageJ software was used to measure staining intensities; at least 100 cells were measured for each indicated time point, and background correction was performed. Western blotting was performed as previously described [16].
Expression analysis
RNA was isolated using Trizol reagent (Invitrogen) using manufacturers instructions. DNAse treatment was performed, and cDNA was prepared using SuperScriptII (Invitrogen), using random hexamers. qRT-PCR was performed using a Biorad thermocycler, using primers described in Table S1. Results were normalized to Actin, using the ΔCT method.
Whole genome wide expression analysis of female wild type and Rnf12−/− ES cells differentiated for 3 days was performed with Affymetrix Mouse Genome 430 2.0 Arrays. Differentially expressed genes were identified using Limma (Bioconductor package) in R software.
Supporting Information
Zdroje
1. LyonMF 1961 Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190 372 373
2. TakagiNSasakiM 1975 Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature 256 640 642
3. HuynhKDLeeJT 2003 Inheritance of a pre-inactivated paternal X chromosome in early mouse embryos. Nature 426 857 862
4. OkamotoIOtteAPAllisCDReinbergDHeardE 2004 Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303 644 649
5. BrockdorffNAshworthAKayGFMcCabeVMNorrisDP 1992 The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell 71 515 526
6. BrockdorffNAshworthAKayGFCooperPSmithS 1991 Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature 351 329 331
7. BrownCJBallabioARupertJLLafreniereRGGrompeM 1991 A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349 38 44
8. LeeJTDavidowLSWarshawskyD 1999 Tsix, a gene antisense to Xist at the X-inactivation centre. Nat Genet 21 400 404
9. LeeJTLuN 1999 Targeted mutagenesis of Tsix leads to nonrandom X inactivation. Cell 99 47 57
10. MonkhorstKde HoonBJonkersIMulugeta AchameEMonkhorstW 2009 The probability to initiate X chromosome inactivation is determined by the X to autosomal ratio and X chromosome specific allelic properties. PLoS ONE 4 e5616 doi:10.1371/journal.pone.0005616
11. MonkhorstKJonkersIRentmeesterEGrosveldFGribnauJ 2008 X inactivation counting and choice is a stochastic process: evidence for involvement of an X-linked activator. Cell 132 410 421
12. BarakatTSJonkersIMonkhorstKGribnauJ 2010 X-changing information on X inactivation. Exp Cell Res 316 679 687
13. DonohoeMEZhangLFXuNShiYLeeJT 2007 Identification of a Ctcf cofactor, Yy1, for the X chromosome binary switch. Mol Cell 25 43 56
14. NavarroPChambersIKarwacki-NeisiusVChureauCMoreyC 2008 Molecular coupling of Xist regulation and pluripotency. Science 321 1693 1695
15. DonohoeMESilvaSSPinterSFXuNLeeJT 2009 The pluripotency factor Oct4 interacts with Ctcf and also controls X-chromosome pairing and counting. Nature 460 128 132
16. JonkersIBarakatTSAchameEMMonkhorstKKenterA 2009 RNF12 is an X-Encoded dose-dependent activator of X chromosome inactivation. Cell 139 999 1011
17. BachIRodriguez-EstebanCCarriereCBhushanAKronesA 1999 RLIM inhibits functional activity of LIM homeodomain transcription factors via recruitment of the histone deacetylase complex. Nat Genet 22 394 399
18. HerYRChungIK 2009 Ubiquitin Ligase RLIM Modulates Telomere Length Homeostasis through a Proteolysis of TRF1. J Biol Chem 284 8557 8566
19. CattanachBMWilliamsCE 1972 Evidence of non-random X chromosome activity in the mouse. Genet Res 19 229 240
20. PlathKFangJMlynarczyk-EvansSKCaoRWorringerKA 2003 Role of histone H3 lysine 27 methylation in X inactivation. Science 300 131 135
21. SilvaJMakWZvetkovaIAppanahRNesterovaTB 2003 Establishment of histone h3 methylation on the inactive X chromosome requires transient recruitment of Eed-Enx1 polycomb group complexes. Dev Cell 4 481 495
22. SilvaJNicholsJTheunissenTWGuoGvan OostenAL 2009 Nanog is the gateway to the pluripotent ground state. Cell 138 722 737
23. JonkersIMonkhorstKRentmeesterEGrootegoedJAGrosveldF 2008 Xist RNA is confined to the nuclear territory of the silenced X chromosome throughout the cell cycle. Mol Cell Biol 28 5583 5594
24. ShinJBossenzMChungYMaHByronM 2010 Maternal Rnf12/RLIM is required for imprinted X-chromosome inactivation in mice. Nature 467 977 981
25. AhnJYLeeJT 2010 Retinoic acid accelerates downregulation of the Xist repressor, Oct4, and increases the likelihood of Xist activation when Tsix is deficient. BMC Dev Biol 10 90
26. ClineTWMeyerBJ 1996 Vive la difference: males vs females in flies vs worms. Annu Rev Genet 30 637 702
27. MarahrensYPanningBDausmanJStraussWJaenischR 1997 Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev 11 156 166
28. KimJChuJShenXWangJOrkinSH 2008 An extended transcriptional network for pluripotency of embryonic stem cells. Cell 132 1049 1061
29. OstendorffHPBossenzMMinchevaACopelandNGGilbertDJ 2000 Functional characterization of the gene encoding RLIM, the corepressor of LIM homeodomain factors. Genomics 69 120 130
Štítky
Genetika Reprodukčná medicína
Článek Composite Effects of Polymorphisms near Multiple Regulatory Elements Create a Major-Effect QTLČlánek Horizontal Transfer, Not Duplication, Drives the Expansion of Protein Families in ProkaryotesČlánek Segregating Variation in the Polycomb Group Gene Alters the Effect of Temperature on Multiple TraitsČlánek Global Analysis of the Impact of Environmental Perturbation on -Regulation of Gene ExpressionČlánek H3K9me-Independent Gene Silencing in Fission Yeast Heterochromatin by Clr5 and Histone DeacetylasesČlánek A Mutation in the Gene Encoding Mitochondrial Mg Channel MRS2 Results in Demyelination in the Rat
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2011 Číslo 1- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- A Meta-Analysis of Genome-Wide Association Scans Identifies IL18RAP, PTPN2, TAGAP, and PUS10 As Shared Risk Loci for Crohn's Disease and Celiac Disease
- Composite Effects of Polymorphisms near Multiple Regulatory Elements Create a Major-Effect QTL
- Horizontal Transfer, Not Duplication, Drives the Expansion of Protein Families in Prokaryotes
- Genome-Wide Association Study SNPs in the Human Genome Diversity Project Populations: Does Selection Affect Unlinked SNPs with Shared Trait Associations?
- Friedreich's Ataxia (GAA)•(TTC) Repeats Strongly Stimulate Mitotic Crossovers in
- Zebrafish Mutation Leads to mRNA Splicing Defect and Pituitary Lineage Expansion
- Histone H4 Lysine 12 Acetylation Regulates Telomeric Heterochromatin Plasticity in
- Bub1-Mediated Adaptation of the Spindle Checkpoint
- Segregating Variation in the Polycomb Group Gene Alters the Effect of Temperature on Multiple Traits
- Signaling Role of Fructose Mediated by FINS1/FBP in
- RNF12 Activates and Is Essential for X Chromosome Inactivation
- Comparative Study between Transcriptionally- and Translationally-Acting Adenine Riboswitches Reveals Key Differences in Riboswitch Regulatory Mechanisms
- Global Analysis of the Impact of Environmental Perturbation on -Regulation of Gene Expression
- Application of a New Method for GWAS in a Related Case/Control Sample with Known Pedigree Structure: Identification of New Loci for Nephrolithiasis
- H3K9me-Independent Gene Silencing in Fission Yeast Heterochromatin by Clr5 and Histone Deacetylases
- A Mutation in the Gene Encoding Mitochondrial Mg Channel MRS2 Results in Demyelination in the Rat
- Transcription Initiation Patterns Indicate Divergent Strategies for Gene Regulation at the Chromatin Level
- The Transposon-Like Correia Elements Encode Numerous Strong Promoters and Provide a Potential New Mechanism for Phase Variation in the Meningococcus
- Proteins Encoded in Genomic Regions Associated with Immune-Mediated Disease Physically Interact and Suggest Underlying Biology
- A Novel RNA-Recognition-Motif Protein Is Required for Premeiotic G/S-Phase Transition in Rice ( L.)
- The Mucin-Like Protein OSM-8 Negatively Regulates Osmosensitive Physiology Via the Transmembrane Protein PTR-23
- Genome Sequencing and Comparative Transcriptomics of the Model Entomopathogenic Fungi and
- Rnf12—A Jack of All Trades in X Inactivation?
- Joint Genetic Analysis of Gene Expression Data with Inferred Cellular Phenotypes
- Evolutionary Conserved Regulation of HIF-1β by NF-κB
- Quaking Regulates Expression through Its 3′ UTR in Oligodendrocyte Precursor Cells
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- H3K9me-Independent Gene Silencing in Fission Yeast Heterochromatin by Clr5 and Histone Deacetylases
- Evolutionary Conserved Regulation of HIF-1β by NF-κB
- Rnf12—A Jack of All Trades in X Inactivation?
- Joint Genetic Analysis of Gene Expression Data with Inferred Cellular Phenotypes
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



