Signaling Role of Fructose Mediated by FINS1/FBP in
Sugars are evolutionarily conserved signaling molecules that regulate the growth and development of both unicellular and multicellular organisms. As sugar-producing photosynthetic organisms, plants utilize glucose as one of their major signaling molecules. However, the details of other sugar signaling molecules and their regulatory factors have remained elusive, due to the complexity of the metabolite and hormone interactions that control physiological and developmental programs in plants. We combined information from a gain-of-function cell-based screen and a loss-of-function reverse-genetic analysis to demonstrate that fructose acts as a signaling molecule in Arabidopsis thaliana. Fructose signaling induced seedling developmental arrest and interacted with plant stress hormone signaling in a manner similar to that of glucose. For fructose signaling responses, the plant glucose sensor HEXOKINASE1 (HXK1) was dispensable, while FRUCTOSE INSENSITIVE1 (FINS1), a putative FRUCTOSE-1,6-BISPHOSPHATASE, played a crucial role. Interestingly, FINS1 function in fructose signaling appeared to be independent of its catalytic activity in sugar metabolism. Genetic analysis further indicated that FINS1–dependent fructose signaling may act downstream of the abscisic acid pathway, in spite of the fact that HXK1–dependent glucose signaling works upstream of hormone synthesis. Our findings revealed that multiple layers of controls by fructose, glucose, and abscisic acid finely tune the plant autotrophic transition and modulate early seedling establishment after seed germination.
Published in the journal:
Signaling Role of Fructose Mediated by FINS1/FBP in. PLoS Genet 7(1): e32767. doi:10.1371/journal.pgen.1001263
Category:
Research Article
doi:
https://doi.org/10.1371/journal.pgen.1001263
Summary
Sugars are evolutionarily conserved signaling molecules that regulate the growth and development of both unicellular and multicellular organisms. As sugar-producing photosynthetic organisms, plants utilize glucose as one of their major signaling molecules. However, the details of other sugar signaling molecules and their regulatory factors have remained elusive, due to the complexity of the metabolite and hormone interactions that control physiological and developmental programs in plants. We combined information from a gain-of-function cell-based screen and a loss-of-function reverse-genetic analysis to demonstrate that fructose acts as a signaling molecule in Arabidopsis thaliana. Fructose signaling induced seedling developmental arrest and interacted with plant stress hormone signaling in a manner similar to that of glucose. For fructose signaling responses, the plant glucose sensor HEXOKINASE1 (HXK1) was dispensable, while FRUCTOSE INSENSITIVE1 (FINS1), a putative FRUCTOSE-1,6-BISPHOSPHATASE, played a crucial role. Interestingly, FINS1 function in fructose signaling appeared to be independent of its catalytic activity in sugar metabolism. Genetic analysis further indicated that FINS1–dependent fructose signaling may act downstream of the abscisic acid pathway, in spite of the fact that HXK1–dependent glucose signaling works upstream of hormone synthesis. Our findings revealed that multiple layers of controls by fructose, glucose, and abscisic acid finely tune the plant autotrophic transition and modulate early seedling establishment after seed germination.
Introduction
Myriad metabolic pathways enable cells to sustain life with basic carbon and nitrogenous compounds. Thus, the integration of metabolite status, which reflects external and internal living conditions, into cellular activities (e.g., gene expression) is a pivotal process that equips organisms with the ability to survive and proliferate. For example, cellular metabolites often serve regulatory roles in modulating organism growth and development, from unicellular bacteria and yeasts to multicellular animals and plants [1]–[6]. To sense and transduce such metabolite signals, organisms have developed sophisticated biochemical and cellular mechanisms.
Glucose is an evolutionarily conserved regulatory sugar molecule in many different organisms [1]–[6]. It has multiple roles as an energy source, building block, and osmotic regulator, and also acts as a potent signaling molecule that regulates gene expression and controls organism growth and development. For example, in yeast, glucose is sensed by at least four different types of sensors, Hxk2, Snf3, Rgt2 and Gpr1, and regulates gene expression and cell growth [4]. In mammalian pancreatic islet β cells, glucose signaling may be a function of the total amount of ATP generated via catabolism [6].
In plants, glucose [7]–[9], sucrose [10]–[12], trehalose-6-phosphate [13], and low energy/high AMP concentrations [14], [15] function as cellular signaling molecules in specific regulatory pathways that modulate plant growth and development. Of these signaling metabolites, glucose has been studied the most comprehensively in plants. Glucose signaling modulates the gene expression of enzymes in the glyoxylate cycle [16] and the photosynthesis pathway [17], and is also involved in the developmental decision of whether to progress to normal seedling establishment after seed germination [18].
Glucose-mediated developmental repression is largely dependent on HEXOKINASE1 (HXK1) [7]–[9]. HXK1's function in glucose-mediated developmental repression is mostly independent of its catalytic activity and integrates glucose signaling with other plant hormone such as auxin and cytokinin. HXK1-independent glucose signaling has also been reported in plants. For instance, expression of the genes encoding chalcone synthase, phenylalanine ammonia-lyase, and asparagine synthase responds to glucose signaling, but their regulation is independent of HXK1 activity [3], [19]. A recent study further demonstrated that a refined low-glucose condition can uncouple HXK1-dependent and -independent glucose signaling responses during early A. thaliana seedling establishment [9], [20].
In both animals and plants, the developmental roles and regulatory functions of hexoses other than glucose have remained largely unknown. However, within the last few years, dietary fructose was implicated in mammalian cell signaling perturbation and metabolic syndromes such as insulin resistance, obesity, type 2 diabetes, and high blood pressure [21], [22].
Plant triose phosphates synthesized by photosynthetic activity are stored as transitory starch in chloroplasts or converted into sucrose in the cytoplasm through a series of enzymatic reactions carried out by fructose-1,6-bisphosphatase (FBP), UDP-glucose pyrophosphorylase, sucrose phosphate synthase, and sucrose phosphatase [2]. Sucrose is then stored in vacuoles or cleaved into glucose and fructose by invertases or UDP-glucose and fructose by sucrose synthases [23]. Thus, following sucrose hydrolysis, fructose becomes one of the prevalent hexoses in plants and has long been proposed as a possible signaling molecule [24]. Nevertheless, fructose signaling in plants has remained largely unexplored. Recently, Kato-Naguchi et al. [25] showed that the fructose analog psicose induced root growth inhibition in lettuce. Fructokinase (FRK), which performs the same catalytic function as HXK, but with fructose as the substrate rather than glucose, was the first fructose enzyme to be studied for a putative regulatory role in fructose signaling [24], [26], [27]. Although FRK is involved in modulation of plant growth, a regulatory role in fructose signaling was ruled out [28]; hence, little is known about fructose signaling and its regulatory pathways.
In this study, we used a cell-based functional screen and a reverse genetics assay to investigate the signaling role of fructose in A. thaliana. We identified FRUCTOSE INSENSITIVE1 as an indispensable regulatory factor in the signaling pathway. Here, we report the molecular and genetic characterization of fins1 in a fructose signaling context, and its close interactions with ABA signaling during early seedling development.
Results/Discussion
Fructose signaling modulates early seedling development and interacts with ABA and ethylene
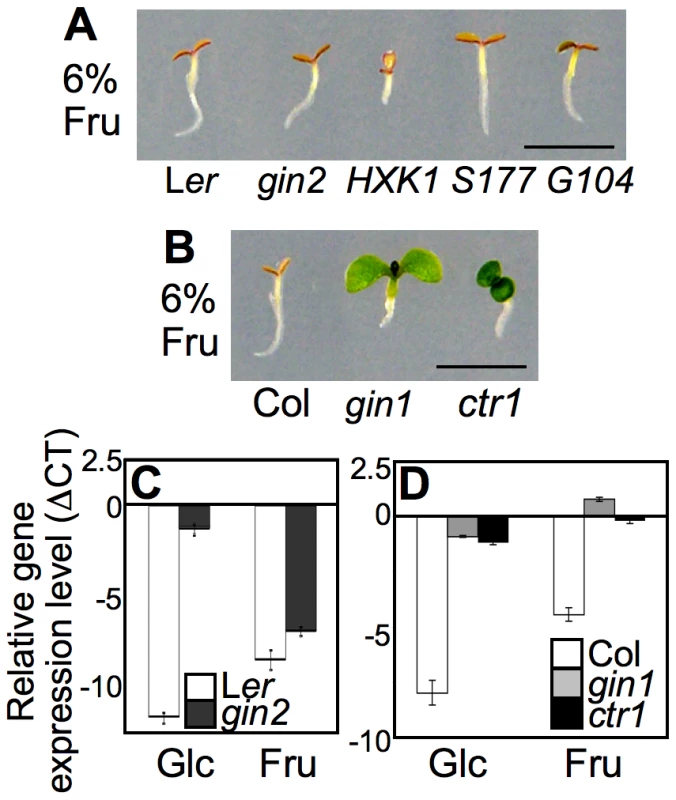
To evaluate the regulatory role of fructose signaling in plant developmental modulation, we examined A. thaliana seedling growth on 6% (w/v) fructose agar medium with full-strength Murashige and Skoog (MS) salts. Wild-type (WT; Ler and Col accession) seedlings grown on high-fructose medium exhibited a typical early developmental arrest, which was manifested by inhibition of hypocotyl and root growth and repression of cotyledon expansion and chlorophyll accumulation (Figure 1A and 1B). Although the seedling development repression pattern caused by high fructose was similar to that caused by high glucose (6%) [7], [20], fructose caused slightly more root growth inhibition than glucose (Figure S1). Mannitol, an osmotic control, did not induce the same seedling repression, suggesting that the observed phenotype was a developmental response to fructose signaling.
Recently, we refined glucose assay conditions for growing A. thaliana seedlings and showed that the high glucose requirement is due to the high nitrogen content in MS media [9], [20]. When MS salts were omitted, 2% glucose induced equivalent seedling growth repression to 6% glucose media including MS. However, decreasing the concentration of fructose in the absence of MS salts had little effect on seedling growth (Figure S2); this suggested that nitrogen had a different effect on fructose and glucose signaling.
Indeed, further experiments indicated that fructose and glucose signaling does rely on distinct sensors. The glucose-insensitive HXK1-null mutant gin2-1 (gin2) exhibited normal fructose sensitivity, as did transgenic gin2-expressing WT HXK1 and its catalytically inactive mutants HXK1S177A or HXK1G104D (Figure 1A). These data confirmed that the glucose sensor HXK1 was dispensable in fructose signaling. Although HXK1 carries out metabolic activities for both glucose and fructose, it does not appear to be involved in fructose signaling. This may reflect the fact that HXK1 has an approximately 100-fold higher affinity for glucose compared to fructose [28]–[30]. In a previous study, root growth inhibition in lettuce was reported in the presence of either the fructose analog psicose or the glucose analog mannose [25]. However, the HXK inhibitor mannoheptulose restored root growth in the presence of mannose, but not psicose. These results are further evidence that psicose/fructose signaling is independent of HXK function.
Plant sugar signaling, mainly glucose and sucrose, interacts with stress and defense hormone signaling pathways and coordinates seedling growth and development [1]–[3], [23], [31]. For glucose signaling, gin1, gin5, and gin6 were respectively identified as alleles of aba-deficient2 (aba2), aba3, and aba-insensitive4 (abi4) in the ABA pathway, and gin4 was found to be a new allele of constitutive triple response1 (ctr1) in the ethylene pathway [31]–[36]. These mutants have been selected repeatedly from various independent screens for sugar responses, further confirming that sugar signaling interacts with ABA and ethylene response pathways during early seedling development [37]–[40].
To test whether fructose signaling interacts with plant stress/defense hormones, we observed the early developmental response of ABA and ethylene mutants on a 6% fructose agar medium with MS salts. Unlike WT and gin2, both gin1-3 (gin1) and ctr1-1 (ctr1) seedlings were not only insensitive to high glucose, but also overcame fructose repression and developed green cotyledons (Figure 1B). GIN1/ABA2 encodes a short-chain dehydrogenase/reductase in ABA synthesis, and CTR1/GIN4 encodes a putative mitogen-activated protein kinase kinase kinase that functions as a negative regulator of ethylene signaling [31], [33]. Therefore, fructose signaling appears to interact positively with ABA signaling via hormone biosynthesis, whereas it is likely antagonized by ethylene signaling. Interestingly, in ABA-deficient gin1 mutants, cotyledon repression was de-repressed by fructose, but root repression was not (Figure 1B); however, glucose relieved both cotyledon and root growth repression in gin1 mutants (Figure S1) [20]. This indicated that fructose repression of root growth was independent of ABA biosynthesis, unlike cotyledon greening. This observation revealed differential seedling responses to fructose and glucose in an organ-specific manner.
We further monitored marker gene expression using real-time PCR with cDNA templates generated from mRNA of five-d-old seedlings grown on MS agar medium containing 6% glucose, fructose, or mannitol. Expression of the photosynthesis-related CHLOROPHYLL A/B BINDING PROTEIN2 (CAB2/AT1G29920) gene was markedly repressed in WT by both glucose and fructose (Figure 1C and 1D). Gene expression was similarly repressed in gin2 seedlings by fructose, but not by glucose (Figure 1C). However, CAB2 expression was de-repressed in both gin1 and ctr1 seedlings (Figure 1D). The CAB2 gene expression patterns in the mutants reflected the fructose resistance revealed by their phenotypes (Figure 1B). Taken together, these data indicated that fructose signaling was mediated through a unique/unknown sensor, but shared a downstream pathway with glucose signaling, which interacted with the plant stress and defense hormones ABA and ethylene to modulate early seedling development in A. thaliana.
Although the application of high sugar to A. thaliana growth media has been criticized because it is not a normal physiological condition, it is unclear how much sugar is actually taken up by roots, how fast it is metabolized or fluxed, and in which suborganelles the sugar is partitioned. These factors could affect developmental responses to high sugar levels. Glucose and sucrose nanosensors, which detect cytoplasmic levels of sugar content, have demonstrated that plant roots take up sugars supplied in growth media rather efficiently [41]. To comprehensively understand sugar uptake and allocation in plants, apoplasmic sugar levels, sugar distribution in subcellular organelles, and fluxes for specific sugars need to be monitored more closely. Further development of sugar nanosensors will hopefully lead to a better understanding of sugar sensing and signaling [42].
FINS1/FBP mediates fructose signaling
To learn more about the specific regulatory components involved in fructose signaling, we took advantage of a cell-based functional screen using transient expression of the A. thaliana mesophyll protoplast system [43]. Because fructose caused deficient chlorophyll accumulation in A. thaliana (Figure 1A and 1B), we reasoned that fructose signaling may affect photosynthetic gene expression in a manner similar to that of glucose signaling [7], [17]. To monitor the fructose signaling response in leaf mesophyll protoplasts, we generated a reporter construct with an approximately 0.5 kb CAB2 promoter fused to the firefly luciferase gene (CAB2-fLUC). In leaf mesophyll protoplasts, CAB2-fLUC activity was downregulated by fructose, but not by the osmotic control mannitol (Figure S3). We then screened several enzymes involved in fructose metabolism, including putative cytoplasmic FBP (AT1G43670), FRK1 (AT5G51830), and PFK1 (AT4G29220) for their potential roles in fructose signaling (Figure 2A). Of these enzymes, putative FBP (we tested two independent constructs, FBP_3 and FBP_4) had the greatest suppressive effect on CAB2-fLUC activity (Figure 2B). CAB2 promoter activity seemed to be suppressed even without high-fructose treatment, possibly because plant cells became hypersensitized to endogenous fructose when putative FBP was overexpressed. To test if putative FBP enzyme activity is required for CAB2 gene repression, we generated a catalytically inactive form, FBPS126AS127A (SSM), based on domain conservation in plant and animal FBPs (Figure S4). The dual mutation of S126A and S127A in FBP caused a loss of FBP enzymatic activity in protoplasts (Figure S5). This mutation probably distorted the local structure and prevented FBP121D from associating with a divalent ion that is necessary for the enzyme activity [45]. Interestingly, the catalytically inactive form FBPS126AS127A suppressed CAB2-fLUC activity in the same manner as the wild-type FBP (Figure 2B). This result indicates that the regulatory function of putative FBP in fructose signaling may be independent of its catalytic activity in sugar metabolism, similar to how HXK1 functions in glucose signaling [9], [19].
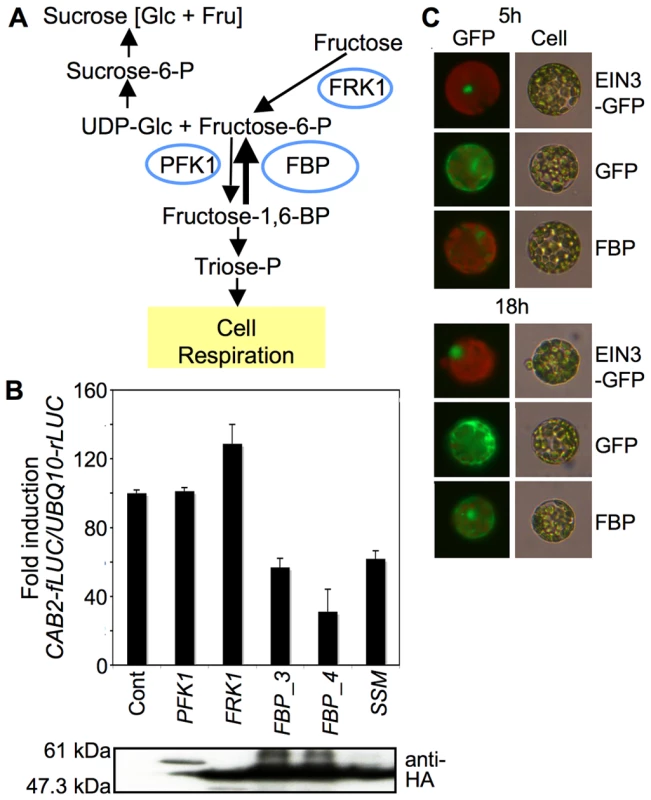
Surprisingly, we observed putative FBP in both the cytoplasm and nucleus (Figure 2C). We were not able to determine whether the nuclear localization of putative FBP depends on cellular fructose signaling (Figure S6), since it is almost impossible to generate zero-fructose conditions in plant cells. However, the nuclear localization of putative FBP certainly suggests that it could be directly involved in fructose-dependent gene regulation. Based on the initial functional screen and localization test in plant cells, we hypothesized that putative FBP was a regulatory factor in fructose signaling.
To study the role of putative FBP in fructose signaling in whole plants, we first obtained a T-DNA insertion mutant that did not accumulate full-length FBP transcript and genetically characterized FBP's function in fructose signaling (Figure 3A). The fins1 seedlings exhibited fructose-insensitive growth responses with progressive cotyledon greening with chlorophyll accumulation (Figure 3B) that was independent of osmotic effects (Figure S7), but displayed glucose-sensitive developmental arrest phenotypes. Since “fructose insensitivity” was the first phenotype that we encountered with this fbp mutant, we designated the allele fructose insensitive1 (fins1).
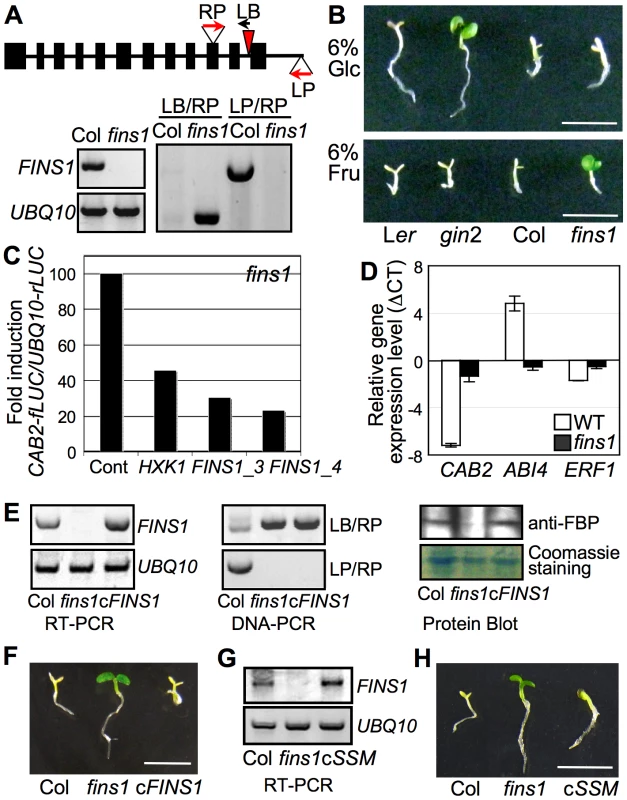
In fins1 protoplasts, FINS1 expression (using the two independent constructs FBP/FINS1_3 and FBP/FINS1_4) clearly suppressed CAB2-fLUC activity (Figure 3C), which was similar to the effect of HXK1 [7]. To investigate FINS1 function in fructose-mediated gene regulation, we examined marker gene expression in WT and fins1 seedlings grown on 6% fructose agar media with MS salts. Consistent with their growth phenotypes (Figure 3B), CAB2 expression was markedly repressed by fructose in WT, but not in fins1 seedlings (Figure 3D). A key transcription factor in ABA signaling, ABI4 (also known as GIN6/AT2G40220) [44] was induced by fructose in WT but not in fins1 seedlings. In contrast, ETHYLENE RESPONSE FACTOR1 (ERF1/AT3G23240), an ethylene response transcription factor [46], was repressed by fructose in WT, but de-repressed in fins1 seedlings. However, the change in ERF1 expression levels was relatively weak compared to other marker gene responses. These data showed that FINS1 had a central role in fructose-inducible gene regulation.
To verify that the fructose insensitivity exhibited by fins1 was due to the loss of FINS1, we complemented the fins1 mutant with FINS1 cDNA using an Agrobacterium system. Transgenic lines with FINS1 expression levels similar to that of WT were selected by gene transcript and protein levels using reverse transcriptase–dependent PCR and protein blot analysis, respectively (Figure 3E). The selected complementation lines had restored sensitivity to fructose and exhibited seedling developmental arrest similar to that of WT Col seedling (Figure 3F); this confirmed that loss of FINS1 function in fins1 seedling was responsible for fructose insensitivity. Furthermore, a fins1 mutant expressing catalytically inactive FBPS126AS127A also restored fructose sensitivity WT levels (Figure 3G and 3H and Figure S8A). The seedling response was specific to fructose, and did not occur in the presence of mannitol (Figure S8B). This response verified that the function of FINS1/FBP in fructose signaling was independent of its catalytic activity in sugar metabolism, as shown by the results of the cellular assay (Figure 2B).
As stated previously, unlike in the glucose assay, in which the high nitrogen levels of MS salts necessitated a high concentration of glucose, 2% fructose without MS salts did not cause the same phenotypic effect as 6% fructose with MS salts (Figure S2). Consequently, it was not clear whether fructose signaling was related to nitrogen signaling. To address this, we tested the effect of different concentrations of fructose on fructose-mediated seedling developmental responses without osmotic pressure, as well as the sugar-antagonistic effect of nitrate (Figure S9). At 3% fructose, fins1, FINS1-complemented fins1, gin2, HXK1-complemented gin2, and WT seedlings did not exhibit any obvious developmental phenotype (Figure S9), as was the case for 2% fructose (Figure S2). However, all of these seedlings exhibited severe growth repression at 5% fructose. Strikingly, at 4% fructose, fins1 showed a clear insensitivity, and FINS1-complemented fins1 restored seedling developmental arrest to a WT-like phenotype (Figure S9). The glucose-insensitive gin2 seedlings displayed consistent fructose-mediated developmental arrest phenotypes. Some of the extreme sensitivity of gin2 could have been due to its accession, because Ler was hypersensitive compared to Col at the same fructose concentration. These results confirmed that nitrogen affects fructose and glucose signaling in different ways [20]. Together with the initial cell-based functional screen, the reverse genetics analysis revealed the regulatory role of FINS1 in fructose signaling during early A. thaliana seedling establishment.
Regulatory role of FINS1/FBP in fructose signaling is independent of its sucrose metabolic activity
FBP isozymes have multiple roles in plant sugar metabolic pathways at different subcellular locales [47]. Chloroplast-localized FBP (AT3G54050) has 50% sequence homology to cytoplasmic FBP in A. thaliana and is mainly involved in starch biosynthesis [48]. Cytoplasmic FBP is involved in sucrose metabolism and is inactivated under dark conditions, mainly due to the increase in fructose-2,6-bisphosphate in some species [47], [49]. Consistent with these previous findings, etiolated WT, fins1, and FINS1-complemented fins1 seedlings did not show any striking phenotypic differences when they were grown on MS agar medium containing 6% glucose, fructose, or mannitol in completely dark conditions (Figure 4A–4C). This result suggested that FINS1 mainly mediated fructose signaling under light conditions (Figure 3B, 3F, and 3H).
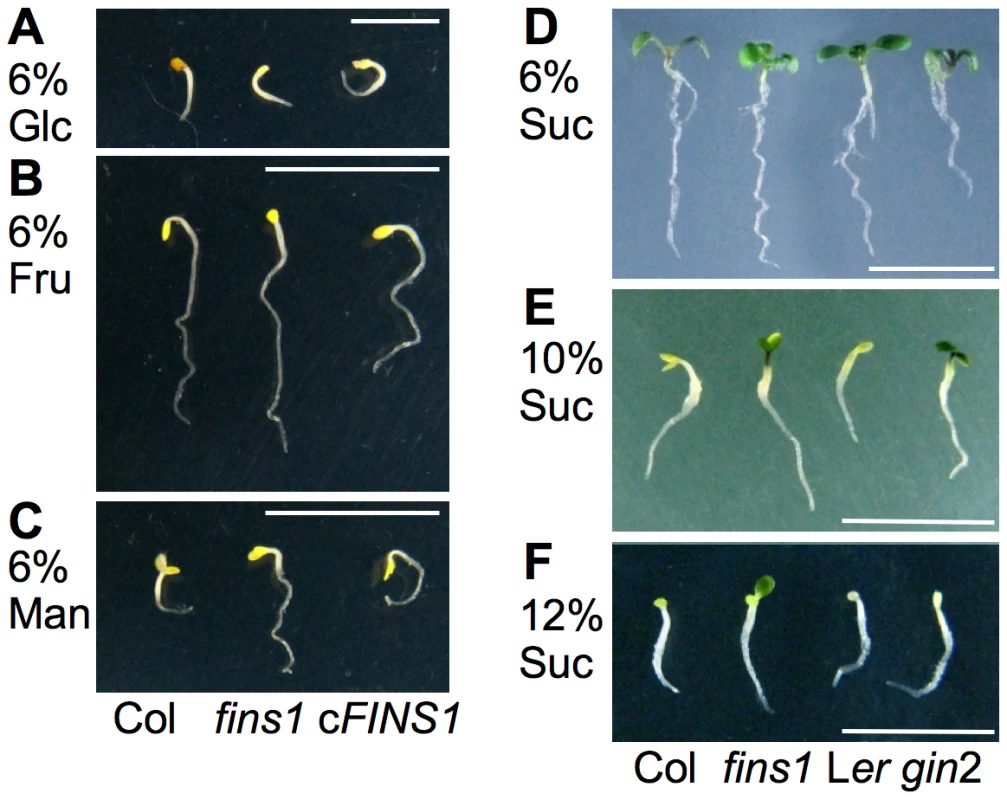
The genetic repression of FINS1 results in shifting sugar metabolism in favor of starch over sucrose synthesis, but does not affect A. thaliana growth [47]. To physiologically compensate for the decrease in sucrose content during the day, starch breakdown and sugar export are enhanced at night in A. thaliana [47] and tobacco [50], but not in rice [49]. Because FBP/FINS1 plays a central role in sucrose synthesis, we tested whether low sucrose in fins1 was a direct cause of its fructose insensitivity [47], [50], [51]. When we observed seedling growth phenotypes on MS agar media containing 6%, 10%, or 12% sucrose in the presence of light, fins1 seedlings were resistant to developmental arrest at high concentrations of sucrose. However, gin2 was resistant only up to 10% sucrose (Figure 4D–4F), indicating that sucrose levels were irrelevant to the fructose insensitivity of fins1 seedlings.
Sucrose is converted to fructose and glucose or UDP-glucose and fructose in plant cells and then is likely integrated into FINS1-dependent or HXK1-dependent signaling. Thus, the strong sucrose resistance of fins1 seedlings (Figure 4F) indicated that fructose became a predominant hexose after sucrose hydrolysis [2], [23], [24]. This finding was supported by a previous observation using a fluorescence resonance energy transfer–based nanosensor, which showed that a measurable cytoplasmic glucose level was induced within 10–20 s of sucrose application to A. thaliana roots [41]. To obtain further molecular insights into the interconnected nature of sugar signaling, we have currently performing a comprehensive analysis of transcriptome changes.
In summary, the fructose insensitivity of fins1 seedlings was most likely not caused by the loss of FBP catalytic activity or by lower sucrose in the mutant [47], because (1) the fructose-responsive CAB2 promoter activity was modulated by FINS1/FBP, but not by FRK1, which is also involved in the sucrose synthesis (Figure 2B); (2) the fructose signaling response was modulated similarly by catalytically active or inactive forms of FBP (Figure 2B and Figure 3H); and (3) high sucrose did not induce fins1 seedling developmental arrest (Figure 4F).
FINS1–dependent fructose signaling acts downstream of ABA signaling
Upon fructose treatment, we noted a slightly more inhibition of root growth (Figure 3B, Figure S1) and a marked ABA-dependent gene response (Figure 3D). These results led us to examine the interaction between fructose and ABA signaling. To do so, we generated transgenic gin1 seedlings that overexpress FINS1. We then analyzed the epistatic relationship between FINS1 in fructose signaling and GIN1 in the ABA pathway. FINS1-overexpressing gin1 seedlings exhibited a seedling developmental arrest phenotype like that observed in WT seedlings on 6% fructose agar medium with MS salts (Figure 5A). The fructose-dependent seedling response was not due to high osmotic effects, because seedlings grew similarly on 6% mannitol agar medium with MS salts (Figure S10). Thus, fructose signaling appears to be integrated into FINS1 downstream of GIN1, which is involved in ABA synthesis. Interestingly, gin1 seedlings that overexpress the plant glucose sensor AtHXK1 display glucose insensitivity, suggesting that glucose sensing by AtHXK1 occurs upstream of ABA synthesis [32]. Taken together, these findings indicate that although both fructose and glucose signaling crosstalk with ABA signaling during early seedling establishment, FINS1 and HXK1 function downstream and upstream of the ABA pathway, respectively.
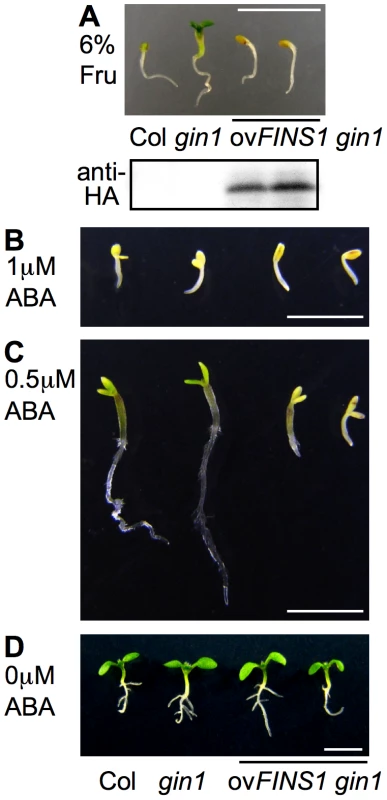
To further test whether FINS1 has a critical role in the ABA pathway, WT, gin1, and FINS1-overexpressing gin1 seedlings were grown on MS agar media containing different concentrations of ABA (Figure 5B–5D). All of the seedlings displayed characteristic developmental arrest phenotypes at a saturated level of 1 µM ABA (Figure 5B). Notably, FINS1-overexpressing gin1 seedlings, but not WT or gin1 seedlings, displayed similar growth inhibition at a sub-potent level of 0.5 µM ABA (Figure 5C). This result supports the notion that FINS1-dependent fructose signaling worked downstream of ABA synthesis (Figure 5A). Because these transgenic lines did not show any growth inhibition in the absence ABA (Figure 5D), it is unlikely that the growth response of the FINS1-overexpressing gin1 was caused by accelerated ABA synthesis rather than increased sensitivity to ABA.
Based on the results shown in Figure 5, we decided to investigate the definitive role of FINS1 in ABA signaling. When seedling growth was observed on MS agar media containing 1 µM ABA, fins1 and the constitutive ethylene signaling mutant ctr1 exhibited ABA insensitivity compared to WT, gin1, and gin2 (Figure 6A). Nevertheless, the fins1 phenotype clearly differed from that of ctr1 seedlings, suggesting that the ABA insensitivity of fins1 may not be directly related to an alteration in ethylene sensitivity.
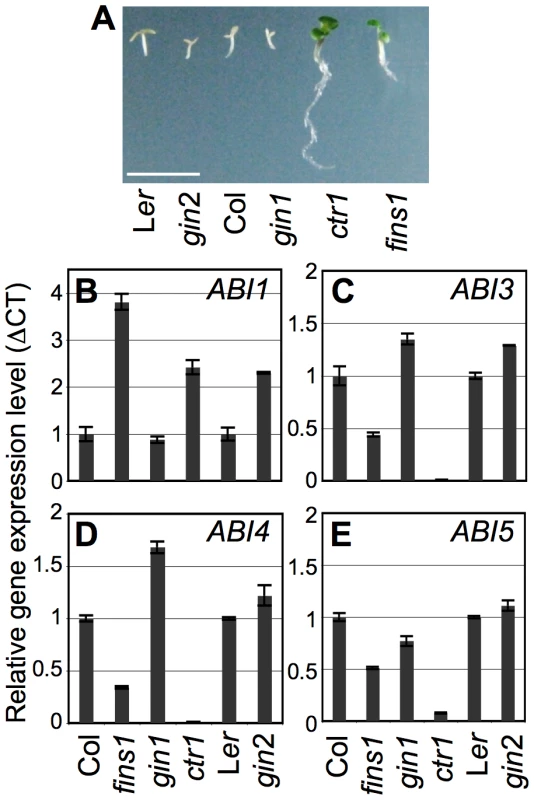
To elucidate the function of FINS1 in ABA-mediated gene regulation, we monitored the gene expression of ABI1 (an ABA negative regulator) and ABI3, ABI4, and ABI5 (ABA positive regulators) in fructose-insensitive fins1, fructose/glucose-insensitive gin1 and ctr1, and glucose-insensitive gin2 seedlings, and as well as in WT seedlings. ABI1 expression was higher in fins1, ctr1, and gin2 compared to its expression in WT and gin1 (Figure 6B). In contrast, expression of the ABA positive regulators (ABI3, ABI4, and ABI5) was suppressed in fins1, and suppressed to an even greater extent in ctr1 (Figure 6C–6E). The higher level of gene suppression in ctr1 correlated with its stronger ABA-insensitive response (Figure 6A). The ABA-dependent seedling phenotypes and gene expression patterns of fins1 further supported the idea that fructose signaling closely interacted with ABA signaling through FINS1. Unlike HXK1 in glucose signaling, FINS1 may not acts as a fructose sensor, because FINS1 binds more readily to fructose-1,6-bisphosphate than to fructose for its catalytic activity (Figure S5). However, it remains to be determined if fructose directly binds to putative FBP and acts as an allosteric regulator of the protein. Further elucidation of the biochemical and cellular processes underlying the interactions between GIN1 and FINS1 will provide a better mechanistic understanding of how fructose signaling controls early seedling establishment.
We have identified fructose as a novel hexose signal that modulates early establishment of A. thaliana seedlings via a pathway that is distinct from glucose signaling (Figure 1). Genetic analyses revealed that fructose signaling interacted positively with ABA and negatively with ethylene, similar to high glucose signaling. Using a cell-based functional screen and reverse genetic analysis, we uncovered a regulatory role for FINS1/FBP in fructose signaling that is independent of its catalytic activity (Figure 2 and Figure 3). fins1 seedlings also showed sucrose insensitivity, indicating that alteration of sucrose content by loss of FINS1 is irrelevant to the fructose insensitivity of fins1 (Figure 4).
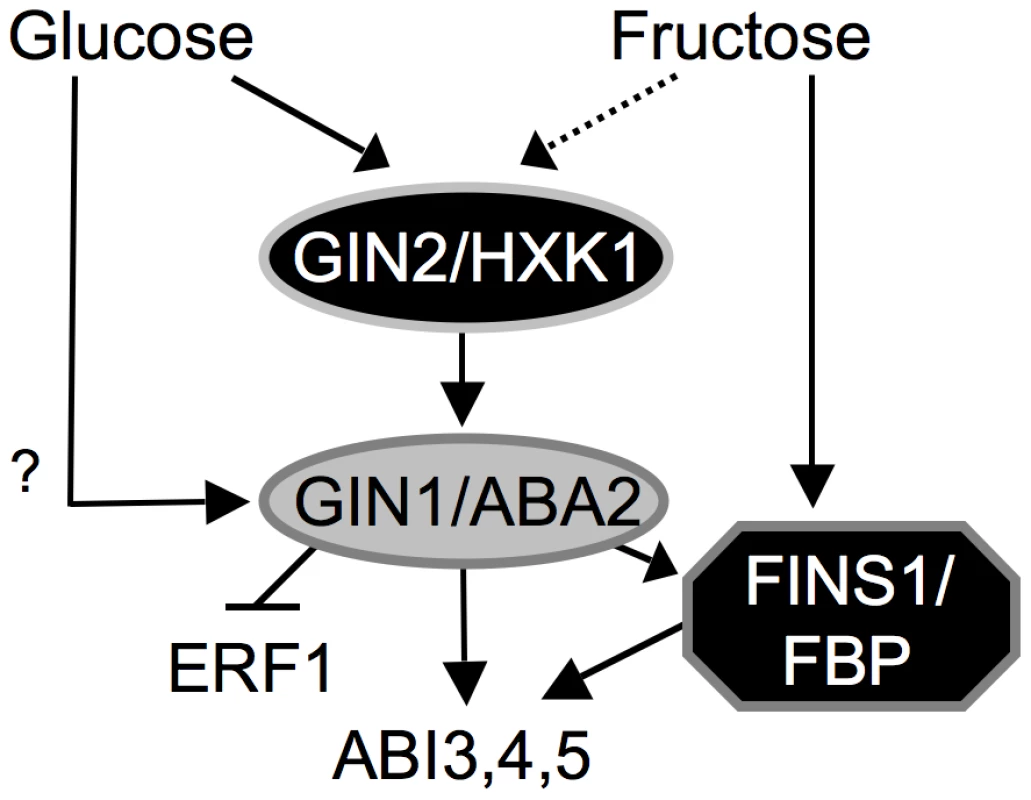
The growth response of transgenic gin1 seedlings expressing FINS1 to fructose and ABA indicated that fructose signaling was acting downstream of ABA synthesis (Figure 5). The ABA response was consistently compromised in fins1 seedlings (Figure 6). Further explorations of the biochemical connections among GIN1/ABA2, GIN2/HXK1, and FINS1/FBP within a sugar-signaling context will provide a better mechanistic understanding of hexose signaling processes during early seedling establishment (Figure 7). However, it is apparent that multiple layers of interactions/cross-talk among glucose, fructose, and ABA signaling pathways tightly modulate plant growth promotion and inhibition, and provide developmental plasticity during the plant autotrophic transition following seed germination.
Materials and Methods
Plasmid constructs
Approximately 0.5 kb of the CAB2 promoter was amplified by PCR and fused to LUC to create the CAB2-fLUC reporter construct [38]. All of the effector constructs were generated by inserting the cDNA between the 35SC4PPDK promoter and the NOS terminator in a plant expression vector for protoplast transient assays and then verifying by DNA sequencing.
A. thaliana mesophyll protoplast transient expression assay
Plants were grown in soil at 23°C for 20–22 d under 60 µmol/m2/s with a 13 h photoperiod. Protoplast isolation and transient expression assays were carried out as described previously [38]. All of the protoplasts transient assays were performed with UBQ10-renillaLUC (UBQ10-rLUC) as an internal control. The reporter activities were calculated based on the fLUC/rLUC ratio and normalized to the values obtained without treatment or effector expression.
Transgenic plants
Plasmid constructs for transgenic plants were generated by inserting the cDNA of FINS1 between the 35SC4PPDK promoter and the NOS terminator in a mini-binary vector pCB302 [8] and expressing it in fins1 or gin1 mutant plants. The transgenic lines expressing transgenes at levels similar to those of WT were selected and used for further analyses. We analyzed the phenotypes of transgenic plants/seedlings from at least two independent lines at the T2 or T3 generation, except for catalytically inactive FINS1_ssm-complemented fins1 (cSSM), which was used at the T1 generation. FINS1/FBP protein expression was analyzed using a cytoplasmic fructose-1,6-bisphosphatase–specific antibody (Agrisera, #AS04043) or HA antibody (Roche).
Sugar and ABA response assays
For sugar repression assays, seedlings were grown on MS (Caisson Laboratories) agar medium containing 6% glucose (Sigma), fructose (Sigma), or mannitol (Sigma) for 5 d under constant light (60 µmol/m2/s). A germination test was performed to determine the ABA sensitivity of each genotype grown on half-strength MS agar medium containing 1% sucrose and a designated amount of ABA under a photoperiod of 16 h light/8 h dark. For the sucrose assay, seedlings were grown on 6, 10, or 12% sucrose MS agar medium with a photoperiod of 16 h light/8 h dark until they showed a clear phenotype. For each experiment, seeds were stratified at 4°C for 4 d before plating. The results were confirmed through several replications.
RNA isolation and transcript measurement
For gene expression analysis, total RNA was isolated by the Trizol method (Invitrogen) and 1 µg of total RNA was used for cDNA synthesis [15]. We investigated glucose - and fructose-mediated gene regulation and their interactions with ABA and ethylene signaling by monitoring marker gene expression in WT and hormone mutants. Gene expression was quantitatively measured using real-time PCR with cDNA templates generated from the RNA of 5-d-old seedlings grown on MS media containing 6% glucose, fructose, or mannitol. Gene expression values in seedlings grown on mannitol served as osmotic controls. Real-time PCR was carried out with iQ SYBR Green dye-added PCR mix (Bio-Rad). Tubulin4 (AT1G04820) or elongation initiation factor4a (ELF4a, AT3G13920) transcript was used as a real-time PCR control with gene-specific primers. Detailed primer sequences are listed in Table S1. Each primer set was pretested by PCR for a single gene product. Experiments were repeated three times with consistent results.
Supporting Information
Zdroje
1. SmeekensS
2000 Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51 49 81
2. RollandF
Baena-GonzalezE
SheenJ
2006 Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu Rev Plant Biol 57 675 709
3. RamonM
RollandF
SheenJ
2008 Sugar sensing and signaling. The Arabidopsis book (TAB), ISSN: 1543–8120, 1–22
4. SantangeloGM
2006 Glucose signaling in Saccharomyces cerevisiae. Microbiology and Mol Biol Reviews 70 253 282
5. HermanMA
KahnBB
2006 Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J Clin Invest 116 1767 1775
6. SchuitFC
HuypensP
HeimbergH
PipeleersDG
2001 Glucose sensing in pancreatic β-cells: A model for the study of other glucose regulated cells in gut, pancrease, hypothalamus. Diabetes 50 1 11
7. MooreB
ZhouL
RollandF
HallQ
ChengWH
2003 Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300 332 336
8. ChoYH
YooSD
SheenJ
2006 Regulatory functions of neclear hexokinase1 complex in glucose signaling. Cell 127 579 589
9. ChoYH
YooSD
SheenJ
2007 Glucose signaling through nuclear hexokinase1 complex in Arabidopsis. Plant Signaling and Behavior 2 123 124
10. ChiouTJ
BushDR
1998 Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA 95 4784 4788
11. RookF
GerritsN
KortsteeA
vanKampenM
BorriasM
1998 Sucrose-specific signaling represses translation of the Arabidopsis ATB2 bZIP transcription factor gene. Plant J 15 253 263
12. VaughnMW
HarringtonGN
BushDR
2002 Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proc Natl Acad Sci USA 99 10876 10880
13. PaulMJ
PrimavesiLF
JhurreeaD
ZhangY
2008 Trehalose metabolism and signaling. Annu Rev Plant Biol 59 417 441
14. Baena-GonzálezE
SheenJ
2008 Convergent energy and stress signaling. Trends in Plant Sci 13 474 482
15. HalfordNG
HeySJ
2009 Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem J 419 247 259
16. GrahamIA
DenbyKJ
LeaverCJ
1994 Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell 6 761 772
17. JangJC
SheenJ
1994 Sugar sensing in higher plants. Plant Cell 6 1665 1679
18. JangJC
LeonP
ZhouL
SheenJ
1997 Hexokinase as a sugar sensor in higher plants. Plant Cell 9 5 19
19. Baena-GonzálezE
RollandF
TheveleinJM
SheenJ
2007 A central integrator of transcription networks in plant stress and energy signalling. Nature 448 938 943
20. ChoYH
SheenJ
YooSD
2010 Low glucose uncouples hexokinase1-dependent sugar signaling from stress and defense hormone abscisic acid and C2H4 responses in Arabidopsis. Plant Physiol 152 1180 1182
21. RutledgeAC
AdeliK
2007 Fructose and the metabolic syndrome: Pathophysiology and molecular mechanisms. Nutrition Reviews 65 S13 S23
22. WeiY
WangD
TopczewskiF
PagliassottiMJ
2007 Fructose-mediated stress signaling in the liver: implications for hepatic insulin resistance. J Nutr Biochem 18 1 9
23. KochK
2004 Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7 235 246
24. PegoJV
SmeekensS
2000 Plant fructokinases: A sweet family get together. Trends in Plant Sci 5 531 536
25. Kato-NoguchiH
TakaokaT
IzumoriK
2005 Psicose inhibits lettuce root growth via a hexokinase-independent pathway. Physiologia Plantarum 125 293 298
26. OdanakaS
BennettAB
KanayamaY
2002 Distinct physiological roles of fructokinase isozymes revealed by gene-specific suppression of Frk1 and Frk2 expression in tomato. Plant Physiol 129 1119 1126
27. GermanMA
DaiN
MatsevitzT
HanaelR
PetreikovM
2003 Suppression of fructokinase encoded by LeFRK2 in tomato stem inhibits growth and causes wilting of young leaves. Plant J 34 837 846
28. DaiN
Kandel-KfirM
PetreikovM
HanaelR
LevinI
2002 The tomato hexokinase LeHXK1 cloning, mapping, expression pattern and phylogenetic relationships. Plant Sci 163 581 590
29. GonzaliS
AlpiA
BlandoF
De BellisL
2002 Arabidopsis thaliana (HXK1 and HXK2) and yeast (HXK2) hexokinases overexpressed in transgenic lines are characterized by different catalytic properties. Plant Sci 163 943 954
30. GranotD
2007 Role of tomato hexose kinases. Functional Plant Biol 34 564 570
31. LeonP
SheenJ
2003 Sugar and hormone connections. Trends in Plant Sci 8 110 116
32. ZhouL
JangJC
JonesT
SheenJ
1998 Glucose and ethylene signal transduction cross-talk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95 10294 10299
33. ChengWH
EndoA
ZhouL
PenneyJ
ChenH
2002 A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14 2723 2743
34. LinPC
HwangSG
EndoA
OkamotoM
KoshibaT
2007 Ectopic expression of ABSCISIC ACID 2/GLUCOSE INSENSITIVE 1 in Arabidopsis promotes seed dormancy and stress tolerance. Plant Physiol 143 745 758
35. Arenas-HuerteroF
Arroyo-BecerraA
ZhouL
SheenJ
LeonP
2000 Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes and Dev 14 2085 2096
36. ArroyoA
BossiF
FinkelsteinRR
LeonP
2003 Three genes that affect sugar sensing abscisic acid insensitive 4, abscisic acid insensitive 5, and constitutive triple response 1 are differentially regulated by glucose in Arabidopsis. Plant Physiol 133 231 242
37. HuijserC
KortsteeA
PegoJ
WeisbeekP
WismanE
2000 The Arabidopsis SUCROSE UNCOUPLED-6 gene is identical to ABSCISIC ACID INSENSITIVE-4: Involvement of abscisic acid in sugar responses. Plant J 23 577 585
38. LabyRJ
KincaidMS
KimD
GibsonSI
2000 The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23 587 596
39. GibsonSI
LabyRJ
KimD
2001 The sugar-insensitive1 (sis1) mutant of Arabidopsis is allelic to ctr1. Biochem Biophys Res Commun 280 196 203
40. RookF
CorkeF
CardR
MunzG
SmithC
2001 Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J 26 421 433
41. ChaudhuriB
HörmannF
LalondeS
BradySM
OrlandoDA
2008 Protonophore - and pH-insensitive glucose and sucrose accumulation detected by FRET nanosensors in Arabidopsis root tips. Plant J 56 948 962
42. LalondeS
EhrhardtDW
FrommerWB
2005 Shining light on signaling and metabolic networks by genetically encoded biosensors. Curr Opin Plant Biol 8 574 581
43. YooSD
ChoYH
SheenJ
2007 Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nature Protocols 2 1565 1572
44. FinkelsteinRR
WangML
LynchTJ
RaoS
GoodmanHM
1998 The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10 1043 1054
45. WeeksCM
RoszakAW
ErmanM
KaiserR
JornvallH
1999 Structure of rabbit liver fructose1,6-bisphosphatase at 2.3A resolution. Acta Cryst D55 93 102
46. SolanoR
StepanovaA
ChaoQ
EckerJR
1998 Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12 3703 3714
47. StrandA
ZrennerR
TrevanionS
StittM
GustafssonP
2000 Decreased expression of two key enzymes in the sucrose biosynthesis pathway, cytoplasmic fructose-1-6-bisphosphatase and sucrose phosphate synthase, has remarkably different consequences for photosynthetic carbon metabolism in transgenic Arabidopsis thaliana. Plant J 23 759 770
48. SahrawyM
AvilaC
ChuecaA
CánovasFM
López-GorgéJ
2004 Increased sucrose level and altered nitrogen metabolism in Arabidopsis thaliana transgenic plants expressing antisense chloroplastic fructose-1,6-bisphosphatase. J Exp Bot 55 2495 2503
49. LeeSK
JeonJS
BörnkeF
VollL
ChoJI
2008 Loss of cytosolic fructose-1,6-bisphosphatase limits photosynthetic sucrose synthesis and causes severe growth retardations in rice (Oryza sativa). Plant Cell Environ 31 1851 1863
50. HäuslerRE
SchliebenNH
SchulzB
FlüggeUI
1998 Compensation of decreased triose phosphate/phosphate translocator activity by accelerated starch turnover and glucose transport in transgenic tobacco. Planta 204 366 376
51. LundmarkM
CavacoAM
TrevanionS
HurryV
2006 Carbon partitioning and export in transgenic Arabidopsis thaliana with altered capacity for sucrose synthesis grown at low temperature: a role for metabolite transporters. Plant Cell Environ 29 1703 1714
Štítky
Genetika Reprodukčná medicínaČlánok vyšiel v časopise
PLOS Genetics
2011 Číslo 1
- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
Najčítanejšie v tomto čísle
- H3K9me-Independent Gene Silencing in Fission Yeast Heterochromatin by Clr5 and Histone Deacetylases
- Evolutionary Conserved Regulation of HIF-1β by NF-κB
- Rnf12—A Jack of All Trades in X Inactivation?
- Joint Genetic Analysis of Gene Expression Data with Inferred Cellular Phenotypes
