-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Relationship Estimation from Whole-Genome Sequence Data
The determination of the relationship between a pair of individuals is a fundamental application of genetics. Previously, we and others have demonstrated that identity-by-descent (IBD) information generated from high-density single-nucleotide polymorphism (SNP) data can greatly improve the power and accuracy of genetic relationship detection. Whole-genome sequencing (WGS) marks the final step in increasing genetic marker density by assaying all single-nucleotide variants (SNVs), and thus has the potential to further improve relationship detection by enabling more accurate detection of IBD segments and more precise resolution of IBD segment boundaries. However, WGS introduces new complexities that must be addressed in order to achieve these improvements in relationship detection. To evaluate these complexities, we estimated genetic relationships from WGS data for 1490 known pairwise relationships among 258 individuals in 30 families along with 46 population samples as controls. We identified several genomic regions with excess pairwise IBD in both the pedigree and control datasets using three established IBD methods: GERMLINE, fastIBD, and ISCA. These spurious IBD segments produced a 10-fold increase in the rate of detected false-positive relationships among controls compared to high-density microarray datasets. To address this issue, we developed a new method to identify and mask genomic regions with excess IBD. This method, implemented in ERSA 2.0, fully resolved the inflated cryptic relationship detection rates while improving relationship estimation accuracy. ERSA 2.0 detected all 1st through 6th degree relationships, and 55% of 9th through 11th degree relationships in the 30 families. We estimate that WGS data provides a 5% to 15% increase in relationship detection power relative to high-density microarray data for distant relationships. Our results identify regions of the genome that are highly problematic for IBD mapping and introduce new software to accurately detect 1st through 9th degree relationships from whole-genome sequence data.
Published in the journal: Relationship Estimation from Whole-Genome Sequence Data. PLoS Genet 10(1): e32767. doi:10.1371/journal.pgen.1004144
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004144Summary
The determination of the relationship between a pair of individuals is a fundamental application of genetics. Previously, we and others have demonstrated that identity-by-descent (IBD) information generated from high-density single-nucleotide polymorphism (SNP) data can greatly improve the power and accuracy of genetic relationship detection. Whole-genome sequencing (WGS) marks the final step in increasing genetic marker density by assaying all single-nucleotide variants (SNVs), and thus has the potential to further improve relationship detection by enabling more accurate detection of IBD segments and more precise resolution of IBD segment boundaries. However, WGS introduces new complexities that must be addressed in order to achieve these improvements in relationship detection. To evaluate these complexities, we estimated genetic relationships from WGS data for 1490 known pairwise relationships among 258 individuals in 30 families along with 46 population samples as controls. We identified several genomic regions with excess pairwise IBD in both the pedigree and control datasets using three established IBD methods: GERMLINE, fastIBD, and ISCA. These spurious IBD segments produced a 10-fold increase in the rate of detected false-positive relationships among controls compared to high-density microarray datasets. To address this issue, we developed a new method to identify and mask genomic regions with excess IBD. This method, implemented in ERSA 2.0, fully resolved the inflated cryptic relationship detection rates while improving relationship estimation accuracy. ERSA 2.0 detected all 1st through 6th degree relationships, and 55% of 9th through 11th degree relationships in the 30 families. We estimate that WGS data provides a 5% to 15% increase in relationship detection power relative to high-density microarray data for distant relationships. Our results identify regions of the genome that are highly problematic for IBD mapping and introduce new software to accurately detect 1st through 9th degree relationships from whole-genome sequence data.
Introduction
The identification of related individuals from genetic data has a broad range of applications. The validation of known relationships in familial disease-gene studies ensures that pedigree errors or sample switches do not adversely affect power [1]. In case-control studies, the removal of related individuals is a standard quality control step to avoid spurious associations [2]. Population genetics studies typically must either explicitly account for familial relationships [3], or else exclude related individuals from analyses that rely on random mating and representative sampling assumptions [4]. Genetic relationship identification is also widely used in a number of forensic applications, including criminal investigations, identification of missing persons and victims of mass disasters [5], [6].
Methods applicable to the detection of close relationships have been available for decades [1], [7]. These methods typically rely on either genome-wide estimates of identity-by-descent (IBD) [8] or joint inference of IBD and relationships using sparse genetic markers [9]. With approximately 1,000 highly polymorphic markers, such methods are well powered to accurately identify relationships as distant as 3rd-degree relatives [9], but these methods do not benefit from further increases in marker density [10]. With the introduction of single-nucleotide polymorphism (SNP) microarrays, increased marker density enabled the accurate detection of local IBD segments. Newer relationship estimation methods take advantage of local IBD segment data to increase the range of detectable relationships [10], [11]. The relationship estimation software that we previously developed, Estimation of Recent Shared Ancestry (ERSA), has high power to detect relationships as distant as 8th-degree relatives (e.g., 3rd cousins once removed) from high-density SNP microarray data [10].
Whole-genome sequence (WGS) should represent the final step in increasing marker density, and thus, improved relationship detection accuracy. However, with the current complexity of WGS, which is based on high-throughput short-reads mapped to a legacy reference genome, a number of technical challenges must be overcome before potential improvements in relationship detection accuracy can be realized. To assess these challenges, we analyzed WGS data for 1490 distinct pairwise relationships from 258 individuals in 30 families (see Table 1). Our results highlight new issues specific to relationship estimation from WGS data and introduce new methods in ERSA 2.0 to mitigate these issues.
Tab. 1. Description of sequenced families. 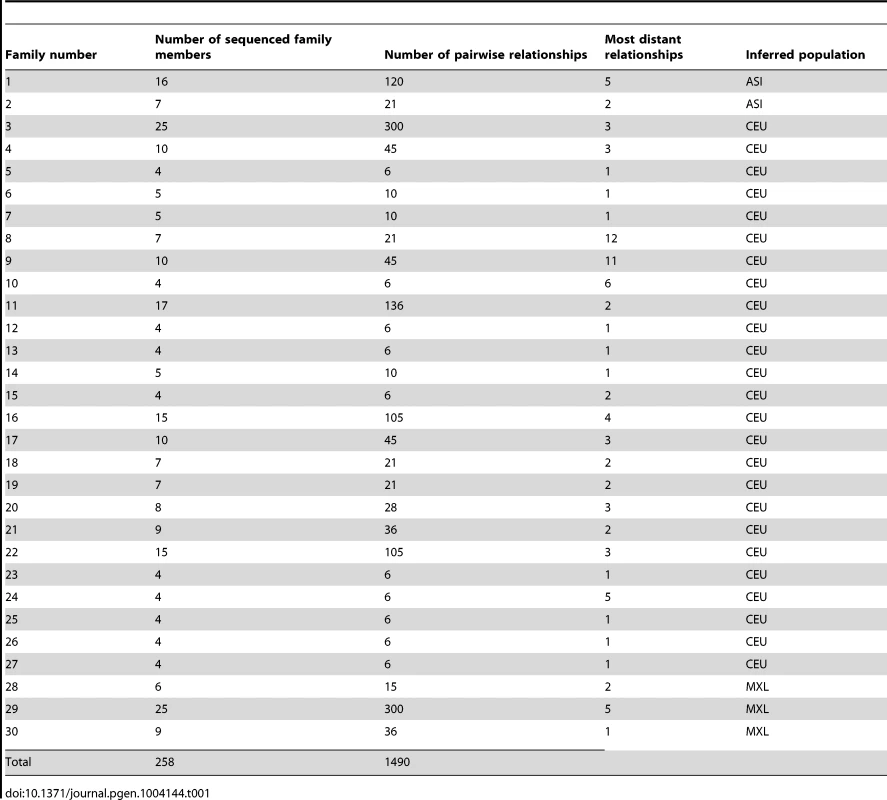
Results
To evaluate relationship-estimation accuracy on WGS data, we first inferred IBD segments between each pair of individuals with three different methods: Genetic Error-tolerant Regional Matching with Linear-time Extension (GERMLINE), Beagle's fastIBD, and Inheritance State Consistency Analysis (ISCA) [12]–[14]. We then applied ERSA separately to each of the three resulting IBD-segment datasets. For our initial analysis of control genomes from putatively unrelated individuals of European ancestry, we set the chance of falsely detecting a relationship between unrelated individuals to 0.1% (α = 0.001). With this threshold, we detected a significant relationship of 9th-degree or closer using GERMLINE in approximately 10% of all pairs of individuals. The estimated level of cryptic relatedness was 10-fold higher than we previously observed from high-density microarray data in this population [10], and thus was a strong indication of an elevated false-positive rate (Table 2). After further investigation, we identified several regions of the genome that were detected to be IBD far more often than would be expected by chance among pairs of controls (see Materials and Methods). Table 3 shows 14 regions of the genome greater than 5 cM with detected pairwise IBD identified in GERMLINE that exceeds the expected pairwise IBD by at least 4-fold between European controls. The regions of spurious IBD were largely consistent between the three IBD methods and among European, East Asian, and Mexican American populations (Figures 1 and S7, Tables 3 and S2, S3), which is a strong indication that the IBD segments in these regions are artifactual. To account for these spurious IBD segments, we developed a procedure within ERSA 2.0 to identify and mask regions of the genome with excess IBD in controls (see Materials and Methods). After applying this procedure, the rate of detected relationships among the European controls decreased from 10% to 1% at α = 0.001 using ERSA 2.0 and GERMLINE, which is the rate of cryptic relationships that we previously observed in this population [10]. In addition to region masking, we also implemented new models in ERSA 2.0 that improve the accuracy of relationship estimates for closely related individuals (see Materials and Methods). Although fastIBD detected many of the same regions as GERMLINE and ISCA, the rate of spurious IBD detection was generally much lower (Table 1 and Figure 1). For this reason, the rate of detected relationships among European controls was less than 0.002 at α = 0.001 using ERSA 2.0 and fastIBD, even without masking spurious IBD segments.
Fig. 1. Regions where excess IBD is detected by three IBD methods among the control populations. 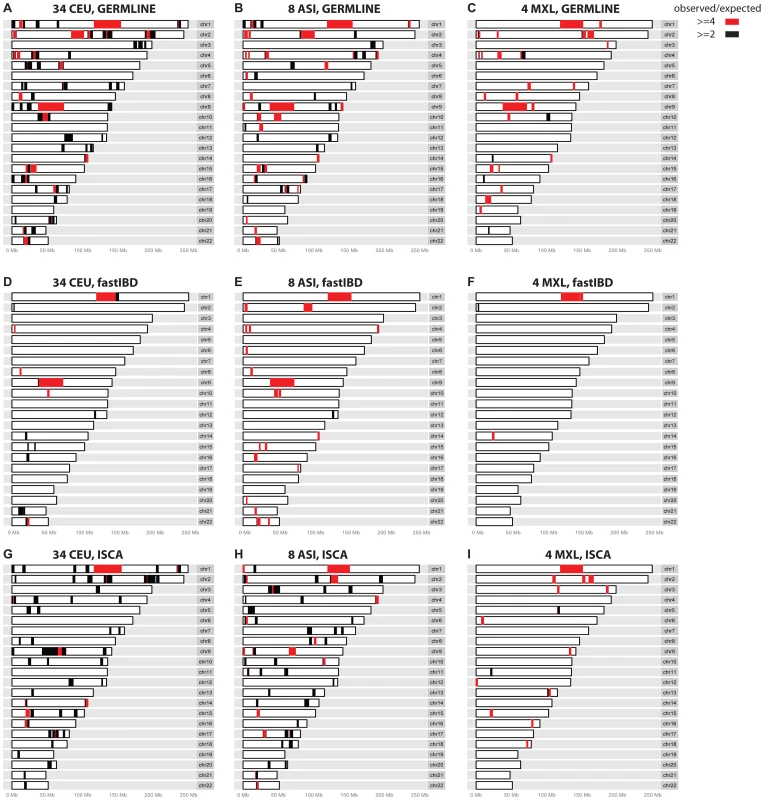
Regions that give rise to excess IBD inferences in GERMLINE (A–C), fastIBD (D–F), and ISCA (G–I) IBD. Black and red shading denotes degree of excess IBD detected (see legend). Tab. 2. Predicted relationships for 595 individual pairs in three groups of population controls: 561 pairs from 34 European controls (CEU), 28 pairs from 8 East Asian controls (ASI), and 6 pairs from 4 Mexican-American controls (MXL). 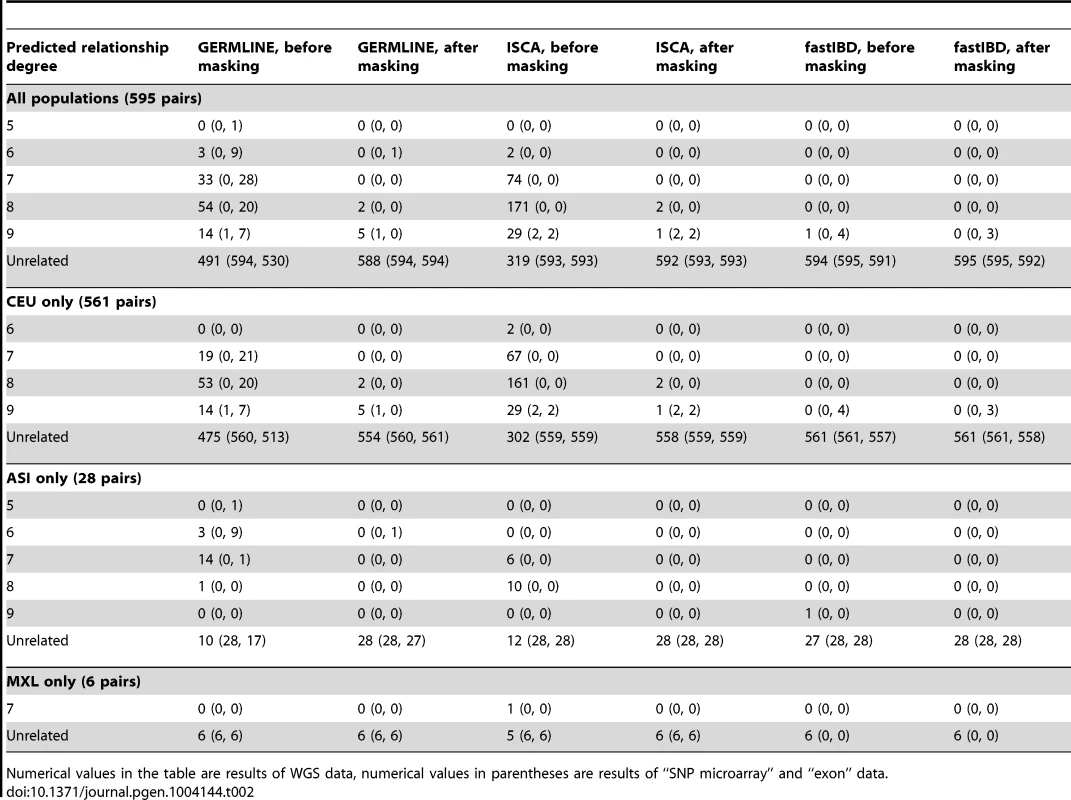
Numerical values in the table are results of WGS data, numerical values in parentheses are results of “SNP microarray” and “exon” data. Tab. 3. Genomic Regions in hg19 coordinates of at least 5-to-expected IBD of at least 4-fold. 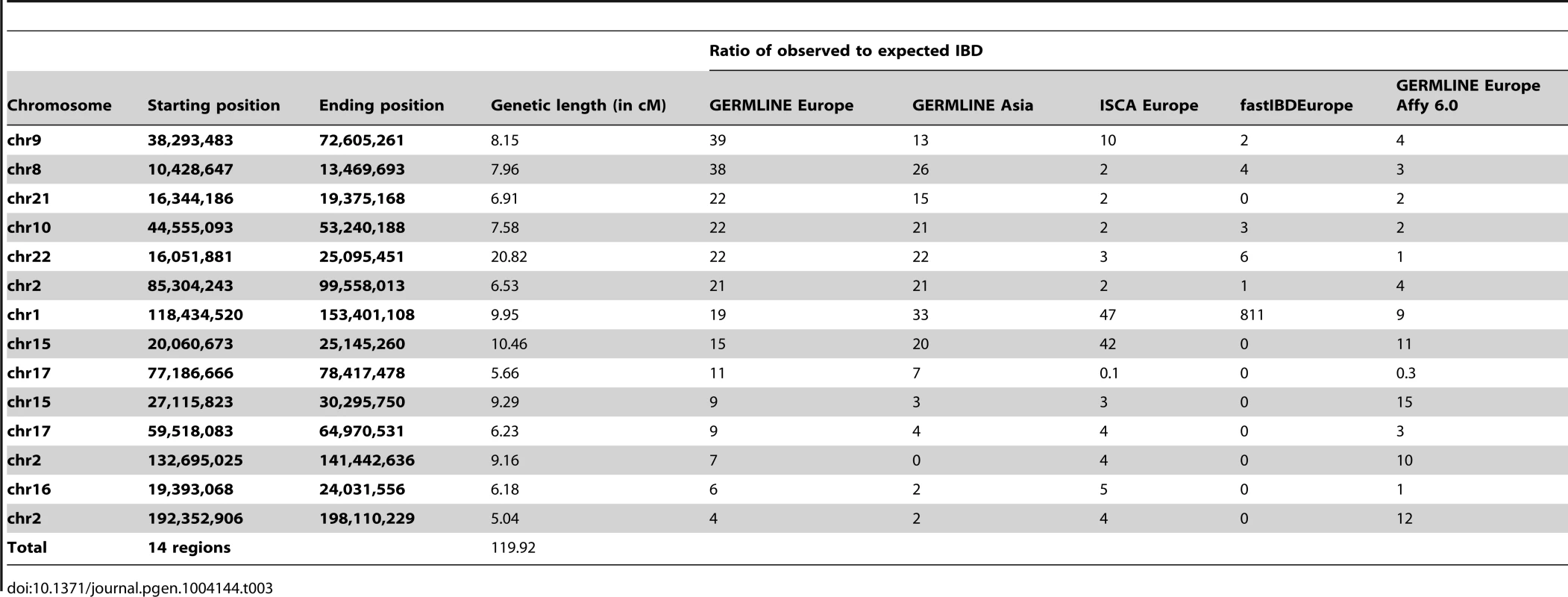
Figure 2 summarizes the ERSA 2.0 results from the 30 pedigrees (see also Table S1 and Figure S3). ERSA 2.0 detected all 1st through 6th degree relationships and 55% of 9th through 11th degree relationships in the 30 pedigrees. The performance of ERSA was very similar across the three IBD detection methods, with approximately a 5% difference in exact relationship prediction accuracy.
Fig. 2. Performance of relationship estimation in 30 sequenced families using (A) GERMLINE-ERSA2.0, (B) fastIBD-ERSA2.0, and (C) ISCA-ERSA2.0. 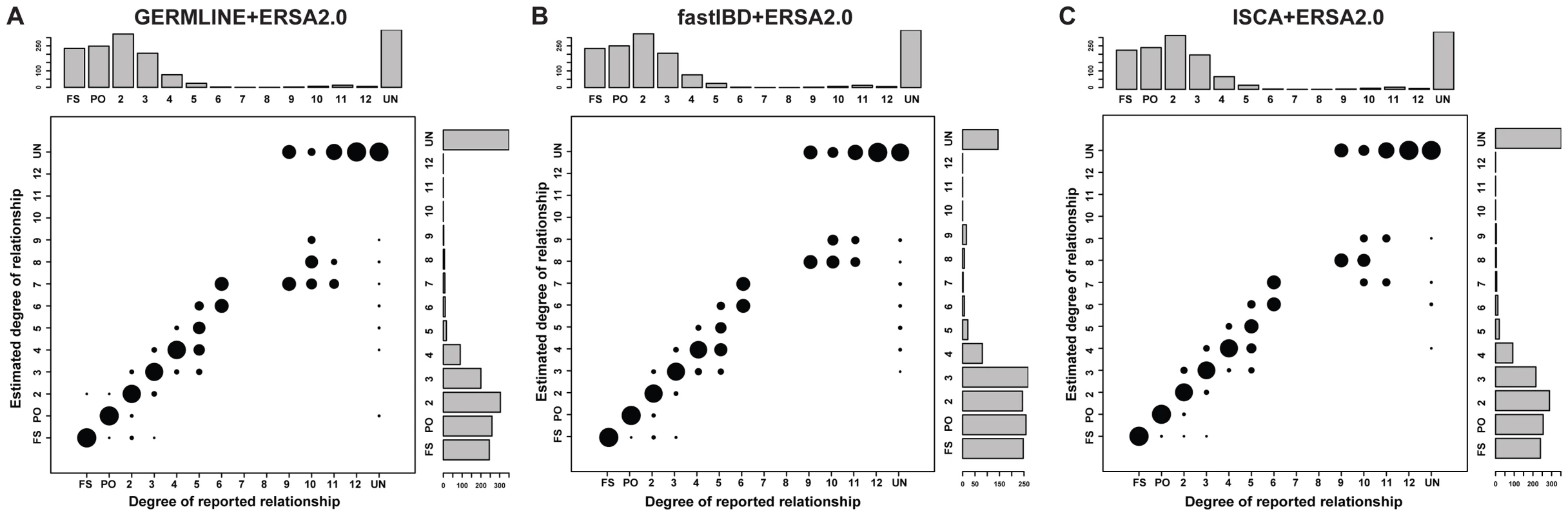
Area of the circles indicates the percentage of individual pairs whose estimated degrees of relationship are exactly the same as reported relationship. FS: full sibling. PO: parent offspring. UN: unrelated individuals. All ERSA analyses employed IBD masking. Histograms represent the number of pairs in each relationship category. Most of the pedigrees were ascertained on the basis of common, complex or rare, Mendelian diseases. As we have previously reported, this ascertainment can produce a downward bias in distant relationship estimates [10], which may account for the differences in relationship estimates between sequenced and simulated pedigrees for 10th through 12th degree relationships (see Figure S5). Although the 30 pedigrees included 1490 documented pairwise relationships, only 28 of these relationships were more distant than 6th degree. To evaluate performance of ERSA 2.0 and IBD detection methods for more-distant relationships, we simulated WGS data in 15-generation pedigrees (See Materials and Methods; Figures S1). ERSA 2.0 performed well with all three IBD detection methods (Figures 3, S5, and S10). For each method, we observed greater than 95% power to detect relationships as distant as 5th degree and greater than 50% power to identify relationships as distant as 8th degree (α = 0.001). We also performed IBD estimation using subsets of the data to represent SNP microarray data (using the set of positions from the Affymetrix 6.0 array) and whole-exome data. The increase in marker density from SNP microarray data to WGS data resulted in a 5% to 15% increase in power for distant relationships between 7th and 11th degree (Figure 3). Restricting markers to exonic regions reduced power relative to WGS data, with a 10% to 60% decrease in power for GERMLINE-ERSA and ISCA-ERSA and a 5% to 10% decrease in power for fastIBD-ERSA with 5th through 12th degree relationships (Figure 3). With exonic markers, we observed a modest increase in the rate of detected relationships among control populations of between 0.2 to 0.5% (Table 2). However, for exonic markers in simulated families, the power to detect distant relationships (10th–15th degree) increased by as much as 5%. This increase in power is very likely to be an artifact and is probably an indication that the increased difficulty of detecting IBD data from exonic markers may lead to improperly calibrated Type I error in ERSA 2.0 for some whole-exome datasets.
Fig. 3. Power of relationship estimation for simulated pedigrees using different methods and markers. 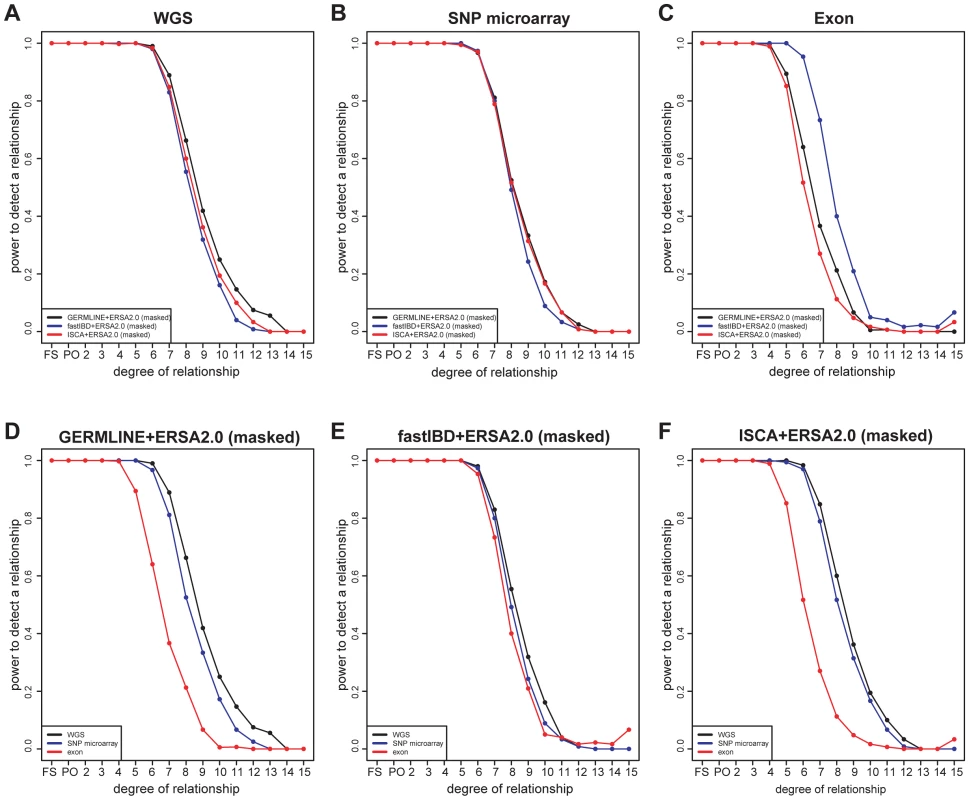
(A–C) Comparison of three methods: GERMLINE-ERSA2.0, fastIBD-ERSA2.0, and ISCA-ERSA2.0. (A) “WGS” represents simulated whole-genome data, (B) “SNP microarray” represents Affymetrix 6.0 microarray data, and (C) “exon” represents whole-exome data. (D–F) Comparison of different marker set: “WGS”, “SNP microarray”, and “exon”. (D) GERMLINE-ERSA2.0, (E) fastIBD-ERSA2.0, and (F) ISCA-ERSA2.0. To compare ERSA to an approach that does not rely on local IBD segment estimates, we also estimated pairwise relationships using RELPAIR, a method that jointly estimates IBD and relationships using sparse marker data. RELPAIR's performance was similar to ERSA for 1st and 2nd degree relationships. Both approaches accurately differentiate between parent-offspring and full-sibling relationships in over 96% of comparisons. RELPAIR had no ability to differentiate between 3rd through 5th degree relationships and had low power to detect relationships more distant than 5th degree (Figures S4 and S6), as previously reported [10].
Discussion
Our results demonstrate that several regions of the genome exhibit an excess of detected IBD with state-of-the-art WGS and IBD detection methods. These suspect IBD regions were typically characterized by an increase in departures from Hardy-Weinberg Equilibrium and were often near centromeric regions. Gaps in the recombination map and human reference assembly were also overrepresented. For example, although the regions in Table 3 represent less than 5% of the human genome, they represent 13% of the centromeric regions and 47% of the unmappable heterochromatic regions of the genome (“Gap” tracks in the UCSC Genome Browser). Notably, the IBD regions were not enriched for repetitive segments of the genome [15]. Because many of the regions were identified using three distinct IBD detection methods, the regions we identified with spurious IBD are unlikely to be the result of IBD detection algorithm errors. Although strong recent positive selection can produce this effect on a population scale, positive selection is unlikely to explain this result because the regions we identified were typically detected among Europeans, East Asians, and Mexican Americans and were far larger than any previous reported genomic signal of positive selection in humans (Figure 1 and Table 3). In addition, we observed very little overlap between the regions identified in Table 3 and a genome-wide search for genomic regions influenced by positive selection based on signals of excess IBD (Table S4) [16]. The regions identified using WGS data usually exhibited excess IBD in Affymetrix high-density microarray data as well, although at lower magnitudes and with smaller segment sizes (Table 3), suggesting that the excess IBD is not simply due to artifacts specific to high-throughput short-read resequencing. One potential explanation is that errors in published genetic maps in these regions overestimate the size of the IBD segments when measured by genetic distance. This hypothesis is supported by the gaps in the published recombination maps and relatively sparse high-density microarray marker density in these regions. Gaps in the human reference assembly may be another contributing factor, both directly due to the absence of markers and indirectly as a general indicator of mapping difficulty in flanking regions. The increased rate of deviations from Hardy-Weinberg equilibrium could also provide a partial explanation, given that erroneous heterozygote calls can result in false inferences of IBD segments. Some of the regions we have identified may be the result of long-range haplotypes with limited recombination between haplotypes [17], [18]. Of the 14 regions identified in Table 3, seven overlapped with regions previously identified in studies of long-range linkage disequilibrium (Tables S5, S6) [17], [18]. One of these regions, at position 10.5 to 13.5 Mb on chromosome 8, overlaps with a known inversion polymorphism that suppresses recombination between haplotypes [19].
Our analysis focused on three complementary pairwise IBD detection methods, GERMLINE, fastIBD, and ISCA (Figure 4). GERMLINE accepts phased genotype data and employs a haplotype hashing algorithm to reduce computation time [12]. Although GERMLINE is capable of analyzing unphased data, in our experience IBD segment identification and subsequent relationship estimation accuracy are both greatly reduced. Beagle fastIBD employs a similar approach to GERMLINE, but obtains multiple estimates of haplotype phase internally and evaluates each of these haplotypes [13]. The rate of spurious IBD detection in fastIBD was substantially lower than GERMLINE and ISCA, and we did not observe an excess of detected relationships among control populations with fastIBD and ERSA, even in the absence of masking (Table 2). However, the power of ERSA 2.0 to detect relationships was slightly reduced with fastIBD relative to the other two methods (Figure 3). Both GERMLINE and fastIBD are well optimized for large sample sizes, but neither distinguishes between haploid-identical regions (IBD1) and diploid-identical regions (IBD2). We originally described ISCA as a method for simultaneous detection of all blocks of identity throughout a pedigree [20], [21]. ISCA also performs well for detecting both IBD1 and IBD2 segments between pairs of individuals with an unknown relationship. ISCA employs a Hidden Markov Model that identifies both IBD1 and IBD2 segments [20]. Because ISCA is optimized for whole-genome data, the algorithm suppresses noise from segments of the genome that give rise to false positive IBD1 and IBD2 regions, such as compressions, centromeres, hemizygous regions, CNVs, reference gaps, and other irregularities [20]. Unlike GERMLINE and fastIBD, ISCA does not require phased data or population controls. However, because ISCA's execution time scales linearly with the number of individual pairs, it is slower than both GERMLINE and fastIBD for large sample sizes.
Fig. 4. Comparison of IBD inferred by GERMLINE, fastIBD, and ISCA. 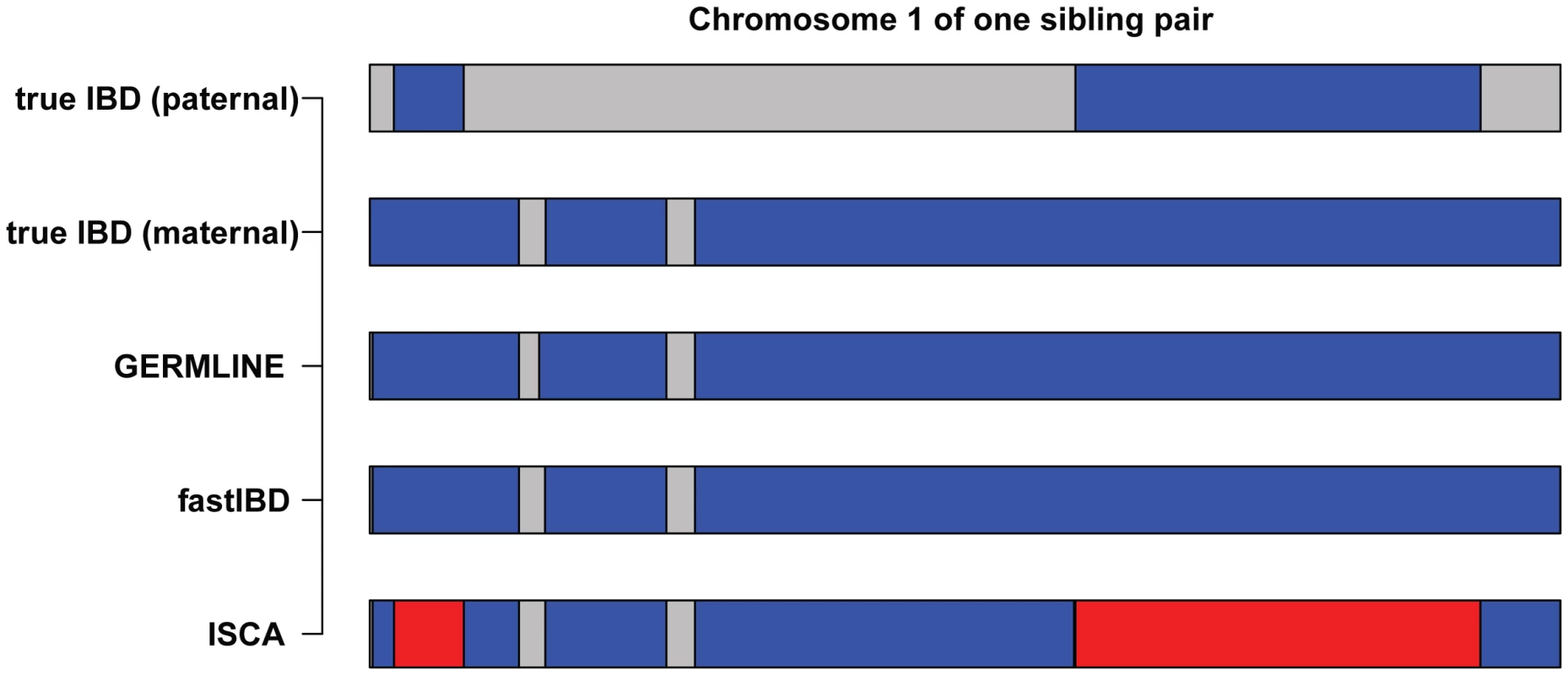
IBD between one simulated sibling pair was shown as an example (chromosome 1). Blue segments indicate haploid-identity (IBD1) and red segments indicate diploid-identity (IBD2). All of the datasets we evaluated included complete documentation of missing genotypes (i.e. no-calls). In our experience, missing genotype data are essential to accurate IBD estimation. Variant call data that does not report missing genotypes should not be used for relationship estimation.
WGS data present new challenges for IBD detection and relationship estimation. Using existing approaches, we observed a major increase in the detection of spurious IBD segments and false-positive relationships from WGS data of population controls. We provide a map of spurious IBD regions in the human reference sequence and present methods implemented in ERSA 2.0 that mask these regions to accurately detect pairwise relationships from IBD segment data. ERSA 2.0 also incorporates additional refinements to improve relationship detection accuracy for 1st - and 2nd-degree relationships. When error-prone IBD regions are masked, the relationship estimation methods in ERSA 2.0 perform well for a variety of IBD detection methods, including GERMLINE, fastIBD, and ISCA. Compared to high-density microarray data, WGS data provide a 5% to 15% increase in relationship detection power for 7th through 12th-degree relationships. Whole-exome data perform substantially worse than high-density microarray data for this purpose. Our results demonstrate that ERSA 2.0 can detect relationships as distant as 12th degree and has high power to detect relationships as distant as 8th degree from whole-genome sequence data.
Materials and Methods
Whole-Genome Sequence Data
We included 258 individuals from 30 families and 46 unrelated individuals (34 Europeans, 4 Mexican-Americans, and 8 East Asians) in this study. We evaluated population structure for each unrelated individual and for one member of each family by performing principal components analysis (PCA) that incorporated HapMap population samples [22]. Of the 30 pedigrees, 25 clustered with Europeans, 3 with Mexican-Americans, and 2 with East Asians (Figure S2). The 30 pedigrees include 1490 documented pairwise relationships (see Table 1). One of these pedigrees was CEPH Pedigree #1463, which consists of genomes of a seventeen-member, three-generation pedigree, with publically available data (ftp://ftp2.completegenomics.com/Pedigree_1463/). Complete Genomics performed all whole-genome sequencing.
Ethics Statement
With the exception of the publicly available CEPH Pedigree #1463, all other pedigree datasets are protected by human subjects protocols approved by the Western Institutional Review Board. Procedures followed were in accordance with institutional and national ethical standards of human experimentation. Proper informed consent was obtained. During subject recruitment, relationships were determined by interview and recorded.
Pedigree Simulations
We simulated non-founder whole-genome data from fifteen-generation families (Figure S1), selecting founders randomly from the unrelated individuals of European ancestry described above. The whole genomes of two offspring were simulated in each generation. Genotypes of non-founders were obtained by simulating meiosis (recombination points were randomly selected based on the recombination rate map in [23]) and de-novo mutation with an expected rate of 1e-7. Sequencing errors were added to all non-founder genomes with an error rate of 0.001 per polymorphic site. There were 1035 pairs of individuals in each family, containing 330 unrelated pairs, 75 first-degree relationships (60 parent-offspring and fifteen full sibling pairs), 84 second-degree relationships, 78 third-degree relationships, 72 fourth-degree relationships, and 66 fifth-degree relationships.
IBD Detection
We used ISCA to infer pairwise IBD1 and IBD2 segment estimates from unphased SNV data. We used Beagle and fastIBD to compute IBD estimates from unphased SNV data separately for each population. Each population combined European, Mexican-American, or East Asian control individuals with the pedigrees that clustered with those populations in PCA (Figure S2). We chose sequenced European genomes to serve as founders for each of the simulated pedigrees. The simulated pedigree genomes were phased with the European controls. Per the authors' recommendations, we ran fastIBD 10 times in each population and merged all segments within one megabase that overlapped between any of the 10 output files [13]; this additional step proved necessary for accurate relationship estimation in ERSA (Note that in our previous evaluation of fastIBD in ERSA we did not perform this step [10]). For GERMLINE, we first applied the grouping criteria above in three population analyses to phase each pedigree and each of the three control populations using Beagle [24], and then analyzed the phased data in GERMLINE.
We applied identical procedures for subsets of SNVs that lie within protein-coding exon boundaries or are Affymetrix 6.0 markers (Figure 3). For GERMLINE, we pruned the WGS datasets prior to phasing in Beagle. After generating IBD segments, we evaluated GERMLINE and fastIBD in ERSA 2.0. We estimated relationships for every pair of individuals within the pedigrees, using the appropriate control population identified in Figure S2.
ERSA
ERSA models the distribution of IBD segments between two individuals in a maximum likelihood framework. The null model assumes that the size and number of IBD segments follow an empirical distribution approximated from the control population. Under the alternative model, some IBD segments may follow the control population distribution, but one or more segments follow a theoretical distribution derived according to a hypothesized recent relationship. Let a equal the number of shared ancestors and d equal the total number of meioses that separate the two individuals for the proposed relationship. For each pair of individuals, ERSA calculates the maximum likelihood for each possible relationship to identify the most likely relationship for that pair. We use the chi-square approximation to the maximum likelihood ratio to establish confidence intervals and to test for significance. This test has two degrees of freedom. One degree of freedom results from a parameter describing the number of segments that are attributable to hypothesized relationship for the pair of individuals (the remaining segments are attributed to the population distribution). A second degree of freedom results from the parameters d and a, which act approximately as a single parameter for most values of d. For direct ancestor-descendant relationships, a = 0. In ERSA 1.0, we assume that, for most relationships, the length l of an IBD segment inherited from the proposed relationship is exponentially distributed with mean equal to
in cM [25]. This approximation assumes that only recombination can break up an IBD segment. Because IBD segments are also broken at chromosomal boundaries, where c is the number of autosomes and r is the expected number of recombination events per generation (r∼ = 35 in humans [23]). As d increases, Eq. 2 approaches Eq. 1, and thus Eq. 1 is a close approximation for distant relationships but is less accurate for close relationships. ERSA 2.0 uses Eq. 2 when a is equal to 1 or 0, resulting in an improvement in accuracy for closely related individuals (Figure S8). Empirically, we observed that Eq. 2 slightly reduced relationship estimation accuracy when a is equal to 2, perhaps due to minor biases in the estimated IBD lengths in GERMLINE and ISCA. Thus, we continue to use Eq. 1 for models where a is equal to 2. Both versions assume that the number of IBD segments, n, is Poisson distributed with mean equal toModifications of these formulas for specific relationships are described below. Hill and White have very recently employed simulations to derive precise estimates for the joint distribution of the number and length of shared segments for a wide range of relationships [26]. These distributions are likely to provide further improvement in relationship detection accuracy in the future.
Although related individuals may sometimes share short IBD segments, such segments can be difficult to distinguish from more distant IBD segments that would be shared by unrelated members of the population. Thus, we typically set a minimum IBD segment length, t, and ignore all segments smaller than this length. By default, t is equal to 2.5. Whenever t is greater than 0, all formulas are adjusted to condition on the probability that IBD segment lengths are greater than or equal to t [10].
All ERSA results were with confidence level = 0.999 and α = 0.001. Reported power estimates were the percentage of related pairs that were correctly predicted to be related at α = 0.001.
Genomic Region Masking
To mask genomic regions potentially prone to false-positive IBD, we first evaluate the distribution of IBD segments in a control population. Genomic regions are masked from the analysis if the ratio of observed to expected total IBD segment length exceeds a specified threshold, h. By default, h is equal to 4, but we observed similar results for values of h between 2 and 6 (Figure S9). The total IBD segment length equals the sum of all pairwise IBD segments that overlap a genomic region, with the segments truncated at the region boundaries. The expected total IBD segment length is calculated under the assumption that pairwise IBD segments in the population are distributed uniformly across the genome. Let m equal the summed length of all masked genomic regions, in cM. We subtract m/100 from r in all models to account for recombination events that cannot be observed. For each IBD segment, the length of the masked region is subtracted from the length of the IBD segment if the IBD segment wholly contains the region and extends at least b base pairs past the beginning and end of the region. By default, b is equal to 1 Mb. All other IBD segments that cross a masked region are truncated at the region boundary. Genomic region masking is an optional parameter in ERSA 2.0 (mask_common_shared_regions) that is inactive by default.
Parent-Offspring Relationships (a = 0, d = 1)
Because parents and offspring are IBD1 throughout the entire genome, there is no stochasticity in the number and lengths of IBD segments. Therefore, for both versions of ERSA, parent-offspring is reported as the most likely relationship if the total IBD segment length is at least z standard deviations above the expected total segment length of a full-sibling relationship (0.75). By default, z is equal to 2.33.
Other Direct Ancestor-Descendant Relationships (a = 0, d>1)
Other than parent-offspring, direct ancestor-descendant relationships (e.g. grandparent-grandchild) were not explicitly modeled in ERSA 1.0. The primary difference in IBD segment distribution between an ancestor-descendant relationship and a relationship with a shared ancestor is that recombination events in the first generation cannot be detected in a pairwise comparison unless complete phase information is available. ERSA 2.0 accounts for this difference with the following equations:
andFull-Sibling Relationships (a = 2, d = 2)
Because GERMLINE and fastIBD do not differentiate between IBD1 and IBD2, regions of IBD2 among full-siblings are merged with their flanking IBD1 segments and are reported as a single, larger IBD segment. Multiple IBD2 segments can be joined together in this manner. Let k equal the number IBD1 segments that have been bioinformatically merged. Conditioned on k and ignoring chromosomal boundaries, l follows a gamma distribution with shape parameter equal to k+1. ERSA 1.0 approximated the distribution of l using the maximum likelihood estimate of k with a single gamma distribution, which introduced an additional free parameter in the sibling model relative to other relationship models. To eliminate this free parameter, ERSA 2.0 assumes that l is distributed according to a mixture of gammas by summing over possible values of k. The likelihood of l is equal to:
The expected number of IBD segments for full sibling relationships in both versions is:
ERSA 2.0 now has the option of evaluating full-sibling models using IBD2 segment data. ERSA assumes that all overlapping IBD1 segments are merged into a single segment, and that each IBD2 segment is reported separately as an additional overlapping segment, which matches the output format that we generated from ISCA. Conditioned on the total length of IBD1 segments, T, the expected number of IBD2 segments under the null model is equal to the unconditional number of expected IBD1 segments multiplied by T/100r. The expected length of an IBD2 segment under the null model follows the empirical distribution for IBD1 segments. Under the alternative model, the number of IBD2 segments, n2, is approximately Poisson distributed with mean equal to
and the expected length of an IBD2 segment is approximately exponentially distributed with mean equal to 25 cM. The IBD2 option (‘use_ibd2_siblings’) was used for all ISCA analyses.Avuncular Relationships (a = 2, d = 3)
All segments that are IBD2 between siblings must be IBD1 in an avuncular relationship involving one sibling and the offspring of the other sibling. For each such segment, an additional IBD1 segment in the siblings may be inherited by the offspring with probability of 0.5 if the following two events occur: 1) a recombination event occurs within the segment in the offspring (probability of approximately 0.5) and 2) an IBD1 segment does not flank a second IBD2 segment (probability of 0.5). All IBD1 segments in the siblings that are not part of an IBD2 segment are broken into two segments by recombination in the offspring with probability of approximately 0.5, in which case one of the segments are shared, and are otherwise are inherited in the offspring with probability 0.5. This leads to the following expression:
Thus, expected number of segments in an avuncular relationship is equal to the expected number in a full-sibling relationship (Eq. 7). This correction was implemented in ERSA 2.0 (ERSA 1.0 erroneously applied Eq 3 to avuncular models). Both versions approximate the distribution of IBD segment lengths in an avuncular relationship using Eq. 1 and assuming an exponential distribution.
RELPAIR Analysis
We used RELPAIR to estimate relationships for all the pairs of individuals to which we applied ERSA 2.0. From the whole-genome data sets, we extracted 9999 well-spaced, relatively independent biallelic SNP loci with minor allele frequency >20%. Allele frequencies and linkage disequilibrium for all loci were assessed in the 34 unrelated CEU individuals using PLINK [27]. Linkage disequilibrium between loci was minimized by pruning correlated SNP loci (PLINK –indep, variance inflation factor up to 1.5 allowed, analysis conducted in windows of 400 SNPs, step size 10 SNPs.) Remaining closely-spaced SNP loci were removed until the target of 9999 SNPs was reached.
Software
The relationship identification methods described above are implemented in the software package ERSA 2.0, which is freely available for academic use (www.hufflab.org). The software for ISCA is available at http://familygenomics.systemsbiology.net/software. The pedigree simulation programs are available in http://caballero.github.io/FakeFamily/.
Supporting Information
Zdroje
1. BoehnkeM, CoxNJ (1997) Accurate inference of relationships in sib-pair linkage studies. Am J Hum Genet 61 : 423–429.
2. VoightBF, PritchardJK (2005) Confounding from cryptic relatedness in case-control association studies. PLoS Genet 1: e32.
3. KongA, ThorleifssonG, GudbjartssonDF, MassonG, SigurdssonA, et al. (2010) Fine-scale recombination rate differences between sexes, populations and individuals. Nature 467 : 1099–1103.
4. XingJ, WatkinsWS, ShlienA, WalkerE, HuffCD, et al. (2010) Toward a more uniform sampling of human genetic diversity: A survey of worldwide populations by high-density genotyping. Genomics 96 : 199–210.
5. LinTH, MyersEW, XingEP (2006) Interpreting anonymous DNA samples from mass disasters–probabilistic forensic inference using genetic markers. Bioinformatics 22: e298–306.
6. Alvarez-CuberoMJ, SaizM, Martinez-GonzalezLJ, AlvarezJC, EisenbergAJ, et al. (2012) Genetic identification of missing persons: DNA analysis of human remains and compromised samples. Pathobiology 79 : 228–238.
7. ThompsonEA (1975) The estimation of pairwise relationships. Ann Hum Genet 39 : 173–188.
8. Ehm MGWM (1996) Test statistic to detect errors in sib-pair relationships. Am J Hum Genet Suppl 69: A217.
9. EpsteinMP, DurenWL, BoehnkeM (2000) Improved inference of relationship for pairs of individuals. Am J Hum Genet 67 : 1219–1231.
10. HuffCD, WitherspoonDJ, SimonsonTS, XingJ, WatkinsWS, et al. (2011) Maximum-likelihood estimation of recent shared ancestry (ERSA). Genome Res 21 : 768–774.
11. HennBM, HonL, MacphersonJM, ErikssonN, SaxonovS, et al. (2012) Cryptic distant relatives are common in both isolated and cosmopolitan genetic samples. PLoS One 7: e34267.
12. GusevA, LoweJK, StoffelM, DalyMJ, AltshulerD, et al. (2009) Whole population, genome-wide mapping of hidden relatedness. Genome Res 19 : 318–326.
13. BrowningBL, BrowningSR (2011) A fast, powerful method for detecting identity by descent. Am J Hum Genet 88 : 173–182.
14. RoachJC, GlusmanG, SmitAF, HuffCD, HubleyR, et al. (2010) Analysis of Genetic Inheritance in a Family Quartet by Whole-Genome Sequencing. Science 328 : 636–9.
15. Smit AFA, Hubley R. (2008–2010) RepeatModeler Open-1.0.
16. AlbrechtsenA, MoltkeI, NielsenR (2010) Natural selection and the distribution of identity-by-descent in the human genome. Genetics 186 : 295–308.
17. GusevA, PalamaraPF, AponteG, ZhuangZ, DarvasiA, et al. (2012) The architecture of long-range haplotypes shared within and across populations. Mol Biol Evol 29 : 473–486.
18. PriceAL, WealeME, PattersonN, MyersSR, NeedAC, et al. (2008) Long-range LD can confound genome scans in admixed populations. Am J Hum Genet 83 : 132–135 author reply 135–139.
19. TianC, PlengeRM, RansomM, LeeA, VillosladaP, et al. (2008) Analysis and application of European genetic substructure using 300 K SNP information. PLoS Genet 4: e4.
20. RoachJC, GlusmanG, SmitAF, HuffCD, HubleyR, et al. (2010) Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science 328 : 636–639.
21. RoachJC, GlusmanG, HubleyR, MontsaroffSZ, HollowayAK, et al. (2011) Chromosomal haplotypes by genetic phasing of human families. Am J Hum Genet 89 : 382–397.
22. ConsortiumTIH (2007) A second generation human haplotype map of over 3.1 million SNPs. Nature 449 : 851–861.
23. McVeanGA, MyersSR, HuntS, DeloukasP, BentleyDR, et al. (2004) The fine-scale structure of recombination rate variation in the human genome. Science 304 : 581–584.
24. BrowningSR, BrowningBL (2007) Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am J Hum Genet 81 : 1084–1097.
25. ThomasA, SkolnickMH, LewisCM (1994) Genomic mismatch scanning in pedigrees. IMA J Math Appl Med Biol 11 : 1–16.
26. HillWG, WhiteIM (2013) Identification of pedigree relationship from genome sharing. G3 (Bethesda) 3 : 1553–1571.
27. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 : 559–575.
28. BrowningBL, BrowningSR (2013) Improving the Accuracy and Efficiency of Identity-By-Descent Detection in Population Data. Genetics 194(2): 459–471.
Štítky
Genetika Reprodukčná medicína
Článek Unwrapping BacteriaČlánek A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome StabilityČlánek The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory OrgansČlánek The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking inČlánek Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma DevelopmentČlánek Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 MiceČlánek High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 1- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- How Much Is That in Dog Years? The Advent of Canine Population Genomics
- The Sense and Sensibility of Strand Exchange in Recombination Homeostasis
- Unwrapping Bacteria
- DNA Methylation Changes Separate Allergic Patients from Healthy Controls and May Reflect Altered CD4 T-Cell Population Structure
- Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species
- Translation Enhancing ACA Motifs and Their Silencing by a Bacterial Small Regulatory RNA
- Relationship Estimation from Whole-Genome Sequence Data
- Genetic Models of Apoptosis-Induced Proliferation Decipher Activation of JNK and Identify a Requirement of EGFR Signaling for Tissue Regenerative Responses in
- ComEA Is Essential for the Transfer of External DNA into the Periplasm in Naturally Transformable Cells
- Loss and Recovery of Genetic Diversity in Adapting Populations of HIV
- Bioelectric Signaling Regulates Size in Zebrafish Fins
- Defining NELF-E RNA Binding in HIV-1 and Promoter-Proximal Pause Regions
- Loss of Histone H3 Methylation at Lysine 4 Triggers Apoptosis in
- Cell-Cycle Dependent Expression of a Translocation-Mediated Fusion Oncogene Mediates Checkpoint Adaptation in Rhabdomyosarcoma
- How a Retrotransposon Exploits the Plant's Heat Stress Response for Its Activation
- A Nonsense Mutation in Encoding a Nondescript Transmembrane Protein Causes Idiopathic Male Subfertility in Cattle
- Deletion of a Conserved -Element in the Locus Highlights the Role of Acute Histone Acetylation in Modulating Inducible Gene Transcription
- Developmental Link between Sex and Nutrition; Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles
- PP2A/B55 and Fcp1 Regulate Greatwall and Ensa Dephosphorylation during Mitotic Exit
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Comprehensive Functional Annotation of 77 Prostate Cancer Risk Loci
- Evolution of Chloroplast Transcript Processing in and Its Chromerid Algal Relatives
- A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome Stability
- New MicroRNAs in —Birth, Death and Cycles of Adaptive Evolution
- A Genome-Wide Screen for Bacterial Envelope Biogenesis Mutants Identifies a Novel Factor Involved in Cell Wall Precursor Metabolism
- FGFR1-Frs2/3 Signalling Maintains Sensory Progenitors during Inner Ear Hair Cell Formation
- Regulation of Synaptic /Neuroligin Abundance by the /Nrf Stress Response Pathway Protects against Oxidative Stress
- Intrasubtype Reassortments Cause Adaptive Amino Acid Replacements in H3N2 Influenza Genes
- Molecular Specificity, Convergence and Constraint Shape Adaptive Evolution in Nutrient-Poor Environments
- WNT7B Promotes Bone Formation in part through mTORC1
- Natural Selection Reduced Diversity on Human Y Chromosomes
- In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism
- The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory Organs
- The Acid Phosphatase-Encoding Gene Contributes to Soybean Tolerance to Low-Phosphorus Stress
- p53 and TAp63 Promote Keratinocyte Proliferation and Differentiation in Breeding Tubercles of the Zebrafish
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
- The SET Domain Proteins SUVH2 and SUVH9 Are Required for Pol V Occupancy at RNA-Directed DNA Methylation Loci
- Down-Regulation of Rad51 Activity during Meiosis in Yeast Prevents Competition with Dmc1 for Repair of Double-Strand Breaks
- Multi-tissue Analysis of Co-expression Networks by Higher-Order Generalized Singular Value Decomposition Identifies Functionally Coherent Transcriptional Modules
- A Neurotoxic Glycerophosphocholine Impacts PtdIns-4, 5-Bisphosphate and TORC2 Signaling by Altering Ceramide Biosynthesis in Yeast
- Subtle Changes in Motif Positioning Cause Tissue-Specific Effects on Robustness of an Enhancer's Activity
- C/EBPα Is Required for Long-Term Self-Renewal and Lineage Priming of Hematopoietic Stem Cells and for the Maintenance of Epigenetic Configurations in Multipotent Progenitors
- The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking in
- Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma Development
- Genome Sequencing Highlights the Dynamic Early History of Dogs
- Re-sequencing Expands Our Understanding of the Phenotypic Impact of Variants at GWAS Loci
- Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
- , a -Antisense Gene of , Encodes a Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas
- A Transcription Factor Is Wound-Induced at the Planarian Midline and Required for Anterior Pole Regeneration
- A Comprehensive tRNA Deletion Library Unravels the Genetic Architecture of the tRNA Pool
- A PNPase Dependent CRISPR System in
- Genomic Confirmation of Hybridisation and Recent Inbreeding in a Vector-Isolated Population
- Zinc Finger Transcription Factors Displaced SREBP Proteins as the Major Sterol Regulators during Saccharomycotina Evolution
- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Tissue Specific Roles for the Ribosome Biogenesis Factor Wdr43 in Zebrafish Development
- A Cell Cycle and Nutritional Checkpoint Controlling Bacterial Surface Adhesion
- High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
- E3 Ubiquitin Ligase CHIP and NBR1-Mediated Selective Autophagy Protect Additively against Proteotoxicity in Plant Stress Responses
- Evolutionary Rate Covariation Identifies New Members of a Protein Network Required for Female Post-Mating Responses
- 3′ Untranslated Regions Mediate Transcriptional Interference between Convergent Genes Both Locally and Ectopically in
- Single Nucleus Genome Sequencing Reveals High Similarity among Nuclei of an Endomycorrhizal Fungus
- Metabolic QTL Analysis Links Chloroquine Resistance in to Impaired Hemoglobin Catabolism
- Notch Controls Cell Adhesion in the Drosophila Eye
- AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes
- Genomes Reveal Evolution of Microalgal Oleaginous Traits
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Variation in Genome-Wide Levels of Meiotic Recombination Is Established at the Onset of Prophase in Mammalian Males
- Age, Gender, and Cancer but Not Neurodegenerative and Cardiovascular Diseases Strongly Modulate Systemic Effect of the Apolipoprotein E4 Allele on Lifespan
- Lifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response
- Is Non-Homologous End-Joining Really an Inherently Error-Prone Process?
- Vestigialization of an Allosteric Switch: Genetic and Structural Mechanisms for the Evolution of Constitutive Activity in a Steroid Hormone Receptor
- Functional Divergence and Evolutionary Turnover in Mammalian Phosphoproteomes
- A 660-Kb Deletion with Antagonistic Effects on Fertility and Milk Production Segregates at High Frequency in Nordic Red Cattle: Additional Evidence for the Common Occurrence of Balancing Selection in Livestock
- Comparative Evolutionary and Developmental Dynamics of the Cotton () Fiber Transcriptome
- The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen
- Crossover Patterning by the Beam-Film Model: Analysis and Implications
- Single Cell Genomics: Advances and Future Perspectives
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy












