-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Sense and Sensibility of Strand Exchange in Recombination Homeostasis
article has not abstract
Published in the journal: The Sense and Sensibility of Strand Exchange in Recombination Homeostasis. PLoS Genet 10(1): e32767. doi:10.1371/journal.pgen.1004104
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1004104Summary
article has not abstract
Upon entering a Regency-era ball, a Jane Austen heroine might ask herself, “Do I stay with my sister, or attempt to secure a partner?” The homologous recombination events that occur during meiosis need to make a similar decision, and how they do so is investigated in papers by Doug Bishop, Neil Hunter, and colleagues [1] and Nancy Hollingsworth and colleagues [2] in PLOS Genetics. The authors define the interplay between the strand exchange proteins Dmc1 and Rad51 in partner choice during meiotic double-strand break (DSB) repair and reveal that when the delicate balance between their activities is manipulated, partner choice is disrupted. However, like the plot twists that bring partners together to enforce happy endings in Austen's novels, robust buffering of recombination counteracts the deleterious effect of eschewing a partner in favor of your sister (chromatid).
For many organisms, accurate segregation of homologous chromosomes of different parental origin (homologs) in meiosis depends upon interhomolog crossovers. Crossovers are formed by the repair of programmed DSBs induced by Spo11 [3] and involve the exchange of genetic information flanking the initiating break. DSB ends are processed into 3′ single-stranded tails encased by a nucleoprotein filament, which contains strand exchange factors that are used to invade an intact homologous duplex to initiate recombination. Recombination intermediates can be resolved to form crossovers or noncrossovers, the latter of which do not involve exchange of flanking information. Failure to form crossovers leads to aneuploidy or gamete death; as such, crossover frequency and distribution is tightly controlled to ensure at least one crossover occurs between each pair of homologs (crossover assurance), that crossovers do not form in close juxtaposition (crossover interference), and that crossover numbers are maintained despite perturbations in the number of DSBs (crossover homeostasis) [4]. Recent studies of crossover control have revealed feedback regulation occurring at multiple steps during recombination progression—including DSB formation [5]–[7] and the crossover-noncrossover decision [8]—to provide robust homeostatic control. Critical questions remain: what intermediates are sensed by homeostatic mechanisms, and how do these mechanisms interact to execute crossover regulation?
Like DSB formation, the strand exchange reaction is a potential target of homeostatic control. The Rad51 strand exchange factor mediates recombination during the mitotic cell cycle, which preferentially utilizes the sister chromatid to template repair. While Rad51 also has a critical role in meiosis, its own strand exchange activity is not required. Instead, Rad51 functions as a cofactor for a meiosis-specific strand exchange factor, Dmc1 [9]. When Rad51 and Dmc1 act together, they exhibit homolog bias, directing strand exchange between homologs rather than sister chromatids. However, loss of Rad51 does not phenocopy loss of Dmc1 [3]. In the absence of Rad51, interhomolog recombination is reduced, and intersister recombination predominates. In the absence of Dmc1, all DSB repair is dramatically reduced, a recombination checkpoint is activated, and the strand exchange activity of Rad51 is inhibited by the Hed1 protein and by an effector kinase, Mek1. Removing this inhibition allows efficient recombination, although interhomolog crossovers are reduced compared to wild type.
Lao, Cloud, et al. [1] asked how well budding yeast dmc1 hed1 mutants, which use Rad51 alone, complete meiosis. Using assays that differentiate template choice in recombination intermediates, they observed a five-fold reduction in homolog bias in dmc1 hed1 and a two-fold reduction in hed1 alone. This implies that Dmc1 executes template choice by inhibiting the strand exchange activity of Rad51. In a complementary study, Liu et al. [2] took a different approach by generating a hypomorphic allele, dmc1-T159A. They then tweaked the balance of power between Rad51 and Dmc1 by introducing dmc1-T159A into strains lacking Hed1 and/or a bypass of Mek1 repression of Rad51. While dmc1-T159A showed no reduction in homolog bias on its own, coupling it with increased Rad51 activity led to a synergistic eight-fold reduction in homolog bias, inferring that inhibition of Rad51 strand exchange is required to favor interhomolog strand exchange by Dmc1.
Intriguingly, regardless of the extent of reduction in homolog bias, there was a much milder effect on ultimate crossover formation. Lao, Cloud, et al. found that dmc1 hed1 exhibited nearly wild-type crossover levels and distributions on chromosome III, suggesting that a highly effective compensatory mechanism is invoked. One such mechanism could be the maintenance of crossovers at the expense of noncrossovers [8]; indeed, the authors observed an increase in the crossover-to-noncrossover ratio in dmc1 hed1. To test the extent of this compensation, they coupled dmc1 hed1 with spo11 alleles that reduce global DSB numbers. Decreasing DSBs did not cause a further increase in the crossover-to-noncrossover ratio, implying that the dmc1 hed1 strain has maximized the buffering capacity afforded by the crossover-noncrossover decision. While this analysis by Lao, Cloud, et al. was limited to a single recombination hotspot, Liu et al. directly measured genome-wide recombination by whole-genome sequencing of tetrads from strains with altered interhomolog bias. Although crossover number in dmc1-T159A was equivalent to wild type, noncrossovers were significantly reduced. In hed1 alone or in hed1 dmc1-T159A double mutants, crossovers were significantly reduced, but noncrossovers were reduced to the same extent as that observed in dmc1-T159A alone. Despite the fact that the number of sequenced tetrads was low, these findings indicate that any compensatory mechanisms that work on the crossover-noncrossover decision are already maximized by the mild reduction in interhomolog bias observed in hed1. Remarkably, the spacing of crossovers was unaffected in all three strains, indicating that reduced interhomolog interactions do not alter crossover interference.
What mechanisms maintain crossover numbers genome-wide? Lao, Cloud, et al. argue that a second homeostatic mechanism—continued formation of DSBs—must account for the high levels of crossovers observed when interhomolog bias is disrupted (Figure 1). In support of this view, while crossovers were reduced locally in dmc1 hed1 at a hotspot that is saturated for DSB formation, crossovers increased in a larger adjacent chromosomal region. They propose that larger chromosomal regions retain a higher potential for receiving additional DSBs through this second homeostatic mechanism.
Fig. 1. Homeostatic regulation of meiotic recombination. 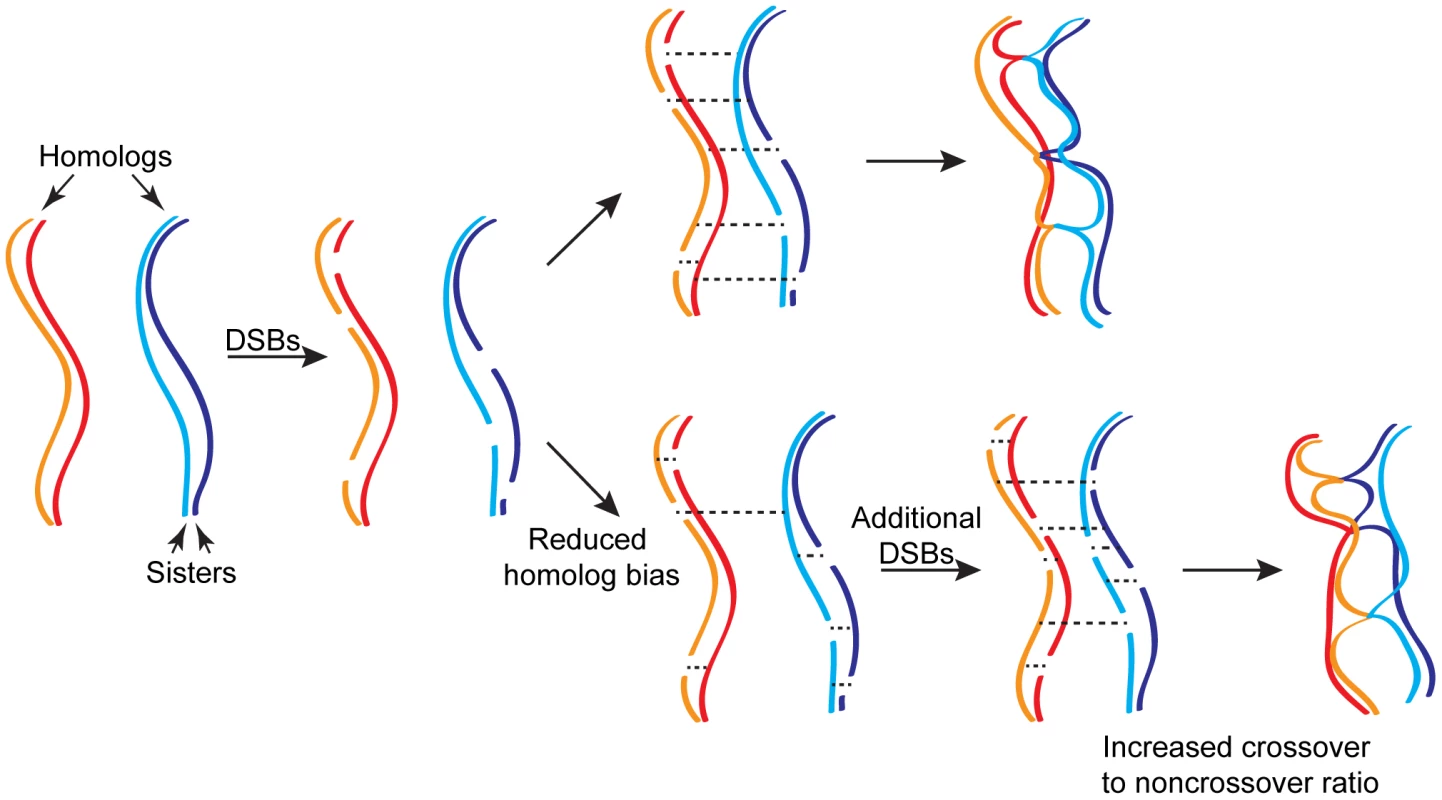
Wild-type meiosis, which has a marked preference for interhomolog recombination, is shown at the top. Dashed lines indicate recombination-dependent interactions that can occur either between sisters or homologs. A proportion of interhomolog interactions will become crossovers that exchange flanking genetic information between homologs and are required for accurate chromosome segregation. The remaining interhomolog interactions are likely to become noncrossovers, which constitute a patch-like repair at the site of the break and do not exchange flanking markers. Aberrant meiosis with reduced homolog bias is shown at the bottom. Additional DNA double-strand breaks (DSBs) are proposed to form in response to reduced interhomolog interactions. Further, when the frequency of successful interhomolog interactions is reduced, the ratio of crossover to noncrossover outcomes is increased. Together, the two types of homeostatic regulation work to maintain crossover number and distribution. An important insight gleaned from these studies is that successful interhomolog interactions are a critical gauge by which homeostatic regulation is effected. Thus, homeostatic sensing must occur between chromosomes (in trans) in response to interhomolog interactions, which may or may not be independent from sensing along a chromosome (in cis) in response to DSB formation [7]. Recent evidence of multiple types of feedback regulation of Spo11-mediated DSB formation will likely provide a key component to a synthetic model that can explain local, regional, and global regulation of meiotic recombination [5]–[7]. It is notable in this context that the mutants analyzed in the current studies show a delay in meiotic progression, suggesting that they activate the meiotic recombination checkpoint. One recently identified pathway for Spo11-mediated DSB feedback involves the recombination checkpoint preventing expression of proteins that shut off DSB formation [10], [11]. In the template choice mutants studied here, the prophase delay could lead to increased formation of DSBs, which eventually provoke enough interhomolog interactions to disengage the checkpoint and complete meiosis. Recent work in mouse spermatocytes indicates that DSB formation is significantly increased when chromosomes have failed to synapse in this organism, as well [12]. Taken together, these findings in yeast and in mice suggest the existence of highly tuned compensatory mechanisms able to target de novo DSB formation specifically to regions of the genome where more recombination is needed.
These two manuscripts have clarified the competitive relationship between Rad51 and Dmc1 during meiotic strand exchange and leveraged this relationship to investigate how interhomolog interactions are integrated into homeostatic regulation. Identifying all of the various components of the multilayered feedback mechanisms inherent to recombination homeostasis, and especially understanding how they crosstalk with one another, will continue to be a stimulating area of research.
Zdroje
1. LaoJP, CloudV, HuangC-C, GrubbJ, ThackerD, et al. (2013) Meiotic crossover control by concerted action of Rad51-Dmc1 in homolog template bias and robust homeostatic regulation. PLoS Genet 9: e1003978 doi:10.1371/journal.pgen.1003978
2. LiuY, GainesWA, CallenderT, BusyginaV, OkeA, et al. (2014) Down-regulation of Rad51 activity during meiosis in yeast prevents competition with Dmc1 for repair of double-strand breaks. PLoS Genet 10: e1004005 doi:10.1371/journal.pgen.1004005
3. Hunter N (2007) Meiotic recombination. In: Aguilera A, Rothstein R, editors. Topics in current genetics, molecular genetics of recombination. Heidelberg: Springer-Verlag. pp. 381–442.
4. GlobusST, KeeneyS (2012) The joy of six: how to control your crossovers. Cell 149 : 11–12.
5. JoyceEF, PedersenM, TiongS, White-BrownSK, PaulA, et al. (2011) Drosophila ATM and ATR have distinct activities in the regulation of meiotic DNA damage and repair. J Cell Biol 195 : 359–367.
6. LangeJ, PanJ, ColeF, ThelenMP, JasinM, et al. (2011) ATM controls meiotic double-strand-break formation. Nature 479 : 237–240.
7. ZhangL, KlecknerNE, StorlazziA, KimKP (2011) Meiotic double-strand breaks occur once per pair of (sister) chromatids and, via Mec1/ATR and Tel1/ATM, once per quartet of chromatids. Proc Natl Acad Sci U S A 108 : 20036–20041.
8. MartiniE, DiazRL, HunterN, KeeneyS (2006) Crossover homeostasis in yeast meiosis. Cell 126 : 285–295.
9. CloudV, ChanYL, GrubbJ, BudkeB, BishopDK (2012) Rad51 is an accessory factor for Dmc1-mediated joint molecule formation during meiosis. Science 337 : 1222–1225.
10. GrayS, AllisonRM, GarciaV, GoldmanAS, NealeMJ (2013) Positive regulation of meiotic DNA double-strand break formation by activation of the DNA damage checkpoint kinase Mec1(ATR). Open Biol 3 : 130019.
11. RockmillB, LefrançoisP, Voelkel-MeimanK, OkeA, RoederGS, et al. (2013) High throughput sequencing reveals alterations in the recombination signatures with diminishing spo11 activity. PLoS Genet 9: e1003932.
12. KauppiL, BarchiM, LangeJ, BaudatF, JasinM, et al. (2013) Numerical constraints and feedback control of double-strand breaks in mouse meiosis. Genes Dev 27 : 873–886.
Štítky
Genetika Reprodukčná medicína
Článek Unwrapping BacteriaČlánek A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome StabilityČlánek The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory OrgansČlánek The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking inČlánek Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma DevelopmentČlánek Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 MiceČlánek High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 1- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- How Much Is That in Dog Years? The Advent of Canine Population Genomics
- The Sense and Sensibility of Strand Exchange in Recombination Homeostasis
- Unwrapping Bacteria
- DNA Methylation Changes Separate Allergic Patients from Healthy Controls and May Reflect Altered CD4 T-Cell Population Structure
- Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species
- Translation Enhancing ACA Motifs and Their Silencing by a Bacterial Small Regulatory RNA
- Relationship Estimation from Whole-Genome Sequence Data
- Genetic Models of Apoptosis-Induced Proliferation Decipher Activation of JNK and Identify a Requirement of EGFR Signaling for Tissue Regenerative Responses in
- ComEA Is Essential for the Transfer of External DNA into the Periplasm in Naturally Transformable Cells
- Loss and Recovery of Genetic Diversity in Adapting Populations of HIV
- Bioelectric Signaling Regulates Size in Zebrafish Fins
- Defining NELF-E RNA Binding in HIV-1 and Promoter-Proximal Pause Regions
- Loss of Histone H3 Methylation at Lysine 4 Triggers Apoptosis in
- Cell-Cycle Dependent Expression of a Translocation-Mediated Fusion Oncogene Mediates Checkpoint Adaptation in Rhabdomyosarcoma
- How a Retrotransposon Exploits the Plant's Heat Stress Response for Its Activation
- A Nonsense Mutation in Encoding a Nondescript Transmembrane Protein Causes Idiopathic Male Subfertility in Cattle
- Deletion of a Conserved -Element in the Locus Highlights the Role of Acute Histone Acetylation in Modulating Inducible Gene Transcription
- Developmental Link between Sex and Nutrition; Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles
- PP2A/B55 and Fcp1 Regulate Greatwall and Ensa Dephosphorylation during Mitotic Exit
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Comprehensive Functional Annotation of 77 Prostate Cancer Risk Loci
- Evolution of Chloroplast Transcript Processing in and Its Chromerid Algal Relatives
- A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome Stability
- New MicroRNAs in —Birth, Death and Cycles of Adaptive Evolution
- A Genome-Wide Screen for Bacterial Envelope Biogenesis Mutants Identifies a Novel Factor Involved in Cell Wall Precursor Metabolism
- FGFR1-Frs2/3 Signalling Maintains Sensory Progenitors during Inner Ear Hair Cell Formation
- Regulation of Synaptic /Neuroligin Abundance by the /Nrf Stress Response Pathway Protects against Oxidative Stress
- Intrasubtype Reassortments Cause Adaptive Amino Acid Replacements in H3N2 Influenza Genes
- Molecular Specificity, Convergence and Constraint Shape Adaptive Evolution in Nutrient-Poor Environments
- WNT7B Promotes Bone Formation in part through mTORC1
- Natural Selection Reduced Diversity on Human Y Chromosomes
- In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism
- The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory Organs
- The Acid Phosphatase-Encoding Gene Contributes to Soybean Tolerance to Low-Phosphorus Stress
- p53 and TAp63 Promote Keratinocyte Proliferation and Differentiation in Breeding Tubercles of the Zebrafish
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
- The SET Domain Proteins SUVH2 and SUVH9 Are Required for Pol V Occupancy at RNA-Directed DNA Methylation Loci
- Down-Regulation of Rad51 Activity during Meiosis in Yeast Prevents Competition with Dmc1 for Repair of Double-Strand Breaks
- Multi-tissue Analysis of Co-expression Networks by Higher-Order Generalized Singular Value Decomposition Identifies Functionally Coherent Transcriptional Modules
- A Neurotoxic Glycerophosphocholine Impacts PtdIns-4, 5-Bisphosphate and TORC2 Signaling by Altering Ceramide Biosynthesis in Yeast
- Subtle Changes in Motif Positioning Cause Tissue-Specific Effects on Robustness of an Enhancer's Activity
- C/EBPα Is Required for Long-Term Self-Renewal and Lineage Priming of Hematopoietic Stem Cells and for the Maintenance of Epigenetic Configurations in Multipotent Progenitors
- The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking in
- Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma Development
- Genome Sequencing Highlights the Dynamic Early History of Dogs
- Re-sequencing Expands Our Understanding of the Phenotypic Impact of Variants at GWAS Loci
- Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
- , a -Antisense Gene of , Encodes a Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas
- A Transcription Factor Is Wound-Induced at the Planarian Midline and Required for Anterior Pole Regeneration
- A Comprehensive tRNA Deletion Library Unravels the Genetic Architecture of the tRNA Pool
- A PNPase Dependent CRISPR System in
- Genomic Confirmation of Hybridisation and Recent Inbreeding in a Vector-Isolated Population
- Zinc Finger Transcription Factors Displaced SREBP Proteins as the Major Sterol Regulators during Saccharomycotina Evolution
- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Tissue Specific Roles for the Ribosome Biogenesis Factor Wdr43 in Zebrafish Development
- A Cell Cycle and Nutritional Checkpoint Controlling Bacterial Surface Adhesion
- High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
- E3 Ubiquitin Ligase CHIP and NBR1-Mediated Selective Autophagy Protect Additively against Proteotoxicity in Plant Stress Responses
- Evolutionary Rate Covariation Identifies New Members of a Protein Network Required for Female Post-Mating Responses
- 3′ Untranslated Regions Mediate Transcriptional Interference between Convergent Genes Both Locally and Ectopically in
- Single Nucleus Genome Sequencing Reveals High Similarity among Nuclei of an Endomycorrhizal Fungus
- Metabolic QTL Analysis Links Chloroquine Resistance in to Impaired Hemoglobin Catabolism
- Notch Controls Cell Adhesion in the Drosophila Eye
- AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes
- Genomes Reveal Evolution of Microalgal Oleaginous Traits
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Variation in Genome-Wide Levels of Meiotic Recombination Is Established at the Onset of Prophase in Mammalian Males
- Age, Gender, and Cancer but Not Neurodegenerative and Cardiovascular Diseases Strongly Modulate Systemic Effect of the Apolipoprotein E4 Allele on Lifespan
- Lifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response
- Is Non-Homologous End-Joining Really an Inherently Error-Prone Process?
- Vestigialization of an Allosteric Switch: Genetic and Structural Mechanisms for the Evolution of Constitutive Activity in a Steroid Hormone Receptor
- Functional Divergence and Evolutionary Turnover in Mammalian Phosphoproteomes
- A 660-Kb Deletion with Antagonistic Effects on Fertility and Milk Production Segregates at High Frequency in Nordic Red Cattle: Additional Evidence for the Common Occurrence of Balancing Selection in Livestock
- Comparative Evolutionary and Developmental Dynamics of the Cotton () Fiber Transcriptome
- The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen
- Crossover Patterning by the Beam-Film Model: Analysis and Implications
- Single Cell Genomics: Advances and Future Perspectives
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



