-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
African Americans have a disproportionate risk for developing chronic kidney disease compared to European Americans. Previous studies have identified a region on chromosome 22 containing two genes, MYH9 and APOL1, which likely accounts for nearly all of this difference. Previous reports provided strong statistical evidence implicating APOL1 as the major contributor to nephropathy risk in African Americans, driven by two coding variants, termed G1 and G2. However, other groups still report statistical evidence for MYH9 association in kidney disease, and animal models have demonstrated biological relevance for MYH9 function in the kidney. Here, we show that suppressing apol1 in zebrafish embryos results in perturbed kidney function. Importantly, using this in vivo assay, we show that the G1 variant appears to cause a loss of APOL1 function, while the G2 variant results in an altered protein that may be acting antagonistically in the presence of normal APOL1. We also report a genetic interaction between apol1 and myh9 under anemic stress, which is consistent with our previous findings in sickle cell disease (SCD) nephropathy patients. Finally, we provide functional evidence in vivo that the G2-altered APOL1 may be interacting with MYH9 to confer nephropathy risk.
Published in the journal: Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress. PLoS Genet 11(7): e32767. doi:10.1371/journal.pgen.1005349
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005349Summary
African Americans have a disproportionate risk for developing chronic kidney disease compared to European Americans. Previous studies have identified a region on chromosome 22 containing two genes, MYH9 and APOL1, which likely accounts for nearly all of this difference. Previous reports provided strong statistical evidence implicating APOL1 as the major contributor to nephropathy risk in African Americans, driven by two coding variants, termed G1 and G2. However, other groups still report statistical evidence for MYH9 association in kidney disease, and animal models have demonstrated biological relevance for MYH9 function in the kidney. Here, we show that suppressing apol1 in zebrafish embryos results in perturbed kidney function. Importantly, using this in vivo assay, we show that the G1 variant appears to cause a loss of APOL1 function, while the G2 variant results in an altered protein that may be acting antagonistically in the presence of normal APOL1. We also report a genetic interaction between apol1 and myh9 under anemic stress, which is consistent with our previous findings in sickle cell disease (SCD) nephropathy patients. Finally, we provide functional evidence in vivo that the G2-altered APOL1 may be interacting with MYH9 to confer nephropathy risk.
Introduction
Chronic kidney disease (CKD) is an acute public health problem world-wide. Within the United States alone, it affects up to 14% of the adult population and is associated with both high costs and poor clinical outcomes[1]. Compared with European Americans, African Americans have a disproportionate risk for several forms of CKD, including human immunodeficiency virus (HIV)-associated nephropathy, focal segmental glomerulosclerosis (FSGS), hypertension-attributed CKD, and sickle cell disease nephropathy (SCDN), all of which contribute to a four-fold increased risk of the most severe stage of CKD, end-stage renal disease (ESRD)[1–5]. A genomic region on chromosome 22q12 likely accounts for almost all of this racial disparity. This region contains two genes, non-muscle myosin heavy chain IIA (MYH9; Entrez, 4627) and apolipoprotein L1 (APOL1; Entrez, 8542), both of which have been associated with increased risk among African American patients with nondiabetic nephropathy[5–11]. Initial admixture mapping and subsequent fine mapping studies focused on MYH9[8, 9, 11]. However, due to the inability to identify variants in MYH9 that alter protein sequence, the major source of genetic association has been attributed to APOL1, located 14 kb downstream of MYH9[6]. Two APOL1 alleles, G1 (encoding p.S342G and p.I384M in cis) and G2 (encoding p.N388del:Y389del), comprise one of the strongest genetic signals ever reported in complex human disease (odds ratios ranging from 10.5 to 16.9)[6, 7]. Additionally, these alleles alter the protein to confer resistance to Trypanosoma brucei rhodesiense, offering a potential evolutionary explanation for the increased occurrence observed among individuals of African ancestry[6].
Despite these genetic findings and the association of this locus with increased risk of multiple forms of CKD, there is a dearth of functional data to inform directly whether MYH9 or APOL1 is the driver of this genetic association. In mice, homozygous Myh9 knockouts die at an early embryonic stage[12], and heterozygotes appear viable without any detected abnormalities[13]. However, subsequent studies have demonstrated that knock-in mutants display renal glomerulosclerosis, while podocyte-specific deletion of Myh9 may predispose mice to glomerulopathy[14–16]. In zebrafish, myh9 is required for the normal development of the glomerulus; morpholino (MO)-induced myh9 suppression results in non-uniform podocyte foot processes and glomerular basement membrane thickening[17]. In contrast, the possible relevance of APOL1 to CKD is derived primarily from in vitro work: cellular localization studies of APOL1 in nondiabetic kidney disease patient biopsies suggest an implication in arteriopathy[18, 19], while overexpression of APOL1 and its risk alleles enhance podocyte necrosis in vitro [20].
Nephropathy is a major contributor to early mortality in patients with sickle cell disease (SCD)[21, 22]. SCDN is a clinically well-characterized pathology that includes glomerular hypertrophy, hyposthenuria, tubular dysfunction, proteinuria, and overall progressive renal failure[23]. We reported previously an association of both MYH9 and APOL1 variants as independent risk factors for proteinuria in a SCD study population[5]. Additionally, when glomerular filtration rate (GFR) in SCD patients was modeled as a function of the previously reported MYH9 risk haplotype and the APOL1 recessive model, we observed a significant interaction between the two genes, suggesting that APOL1 and MYH9 may act together to induce SCDN[5]. However, as with other forms of CKD, well-characterized in vivo model systems are needed to understand both the individual effects of APOL1 relevant to disease, and also the potential interaction of APOL1 with MYH9 in the context of anemic stress as observed in SCD.
Here, we used zebrafish as an in vivo model to study the consequences of gene perturbation and potential synergistic effects of APOL1 and MYH9 in kidney disease. Although the zebrafish pronephros is a simplified kidney, the structure and function of the larval glomerulus is similar to that of humans and represents a tractable model in which to study apol1 (RefSeq: NM_001030138) and myh9 (RefSeq: NM_001098177.2)[24, 25]. In this report, we provide insight into the role of apol1 in glomerular development and pronephric filtration in zebrafish embryos, as well as the effects of APOL1 G1 and G2 allelic expression. Moreover, we provide functional evidence for an interaction between myh9 and apol1 under anemic stress conditions. Overall, these data implicate both MYH9 and APOL1 as significant biological contributors to non-diabetic nephropathy and intimate context-dependent roles in disease pathology.
Results
Knockdown of zebrafish apol1 results in pericardial edema, compromised glomerular filtration, and disruption of the glomerular ultrastructure
The apolipoprotein L family of genes evolved rapidly in humans and some non-human primates[26, 27]. However, using BLAST and reciprocal BLAST searches against the D. rerio and H. sapiens genomes, we identified a single D. rerio locus encoding a protein of unknown function (chr2 : 37,674,122–37,676,731 Zv9; NCBI Ref: NP_001025309.1; 38% identity, 46% similarity on the amino acid level) as a possible unique functional ancestral ortholog to the human apolipoprotein L family (Fig 1A–1D). To explore the function of this transcript in developing zebrafish, we first asked whether the candidate apol1 ortholog is expressed in a temporal manner amenable to transient assays of renal development and function. RT-PCR analysis of cDNA generated from wild-type (WT) whole-larval total RNA collected at three days post-fertilization (dpf) and 5 dpf showed expression at time points corresponding to the formation of the pronephros. Additionally, we detected apol1 expression in flow-sorted podocyte fractions harvested from glomeruli of pod::NTR-mCherry adult zebrafish (Fig 1E) [28].
Fig. 1. Comparison of APOL1 human and zebrafish protein sequences and relevance to the zebrafish kidney. 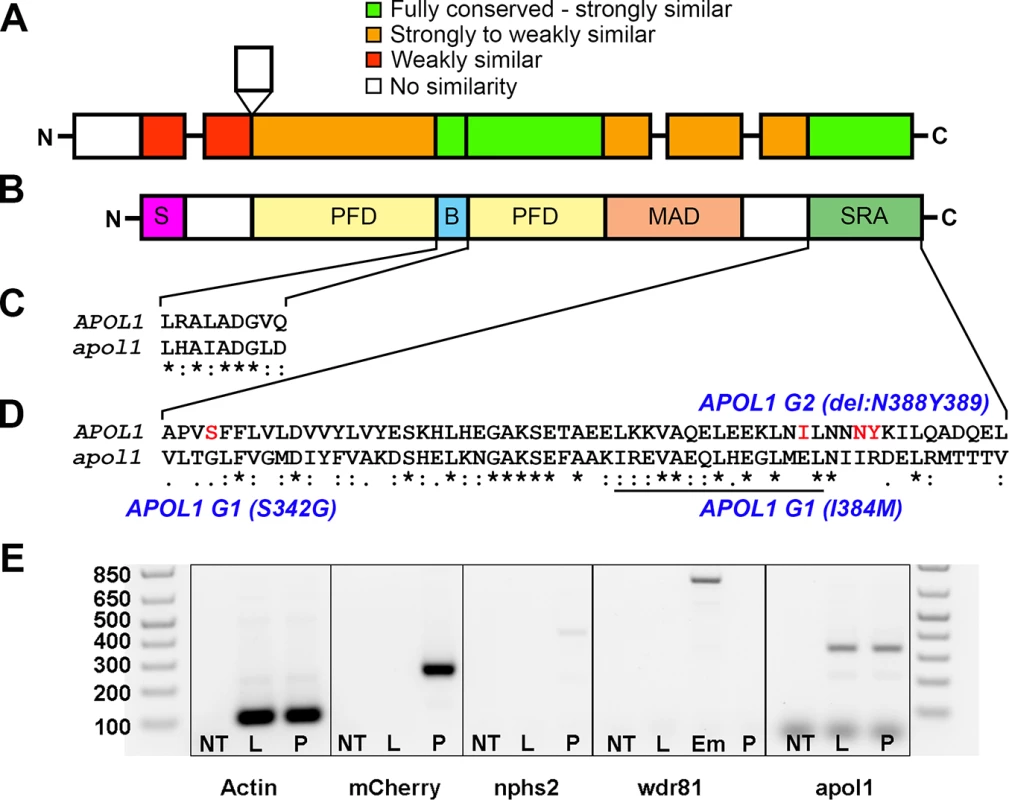
Protein domain schematic of (A) zebrafish APOL1 and (B) human APOL1 is shown, with zebrafish domains (NP_001025309) aligned to the human protein (NP_001130012) and coded based on summarized consensus scores (Gonnet PAM 250 matrix, Clustal Omega, Cambridge, UK; S, secretory domain, PFD, pore-forming domain, B, BH3 domain, MAD, membrane-addressing domain, SRA, serum resistance-associated binding domain). Prominent regions of the human and zebrafish alignments are expanded, including the (C) BH3 domain and (D) SRA binding domain, and consensus symbols are displayed (* (asterisk), fully conserved;: (colon), >0.5 in the Gonnet PAM 250 matrix;. (period), = <0.5 in the Gonnet PAM 250 matrix). The leucine zipper domain (codons 365–392 in APOL1, underline), and the location of the G1 and G2 risk alleles in CKD in African Americans (S342G/I384M and ΔN388Y389) are highlighted in red. (E) Podocytes from adult glomeruli of pod::NTR-mCherry zebrafish were flow-sorted and evaluated for apol1 RNA expression through RT-PCR. apol1 is expressed in fluorescence-activated cell sorted (FACS) podocytes and the adult liver. FACS podocytes also express zebrafish podocin (nphs2) but a purkinje-cell marker, wdr81[29], was undetectable. NT = non-template reverse transcription control; L = dissected adult liver cells from pod::NTR-mCherry zebrafish; P = fluorescence-activated cell sorted podocytes from dissected glomeruli of pod::NTR-mCherry zebrafish; Em = 5 dpf whole-zebrafish embryo cDNA. To test the effects of apol1 suppression, we designed a translation-blocking morpholino (MO; Gene Tools, LLC) targeting the candidate zebrafish apol1 locus (apol1-MO) and we injected increasing doses into embryos at the one to four cell stage (n = 49–65 embryos/injection; repeated three times). Masked scoring for morphological defects at 5 dpf revealed a dose-dependent increase of the percent of larvae displaying pericardial and yolk sac edema, a phenotype that has been implicated previously in glomerular filtration defects[24, 30] (Fig 2A–2C). Co-injection of WT APOL1 human mRNA (GenBank Accession: BC112943.1; 100 pg/nl) rescued significantly the edema caused by apol1 suppression (p<0.0001; Fig 2D), arguing not only that the phenotype was unlikely to be a non-specific toxic effect of the MO, but also that the zebrafish locus we targeted is the ortholog of the human transcript. Importantly, co-injection of human mRNA encoding other human apolipoprotein L members (APOL2, APOL3, APOL4, APOL5, and APOL6) with apol1 MO did not rescue the edema formation of apol1 morphants (S1 Fig). Additionally, we observed a significant decrease in endogenous APOL1 protein expression in apol1-MO injected zebrafish embryos (p = 0.026), which is restored to normal levels upon co-injection with wild-type human APOL1 mRNA (S2 Fig). Furthermore, as an additional test of the specificity of apol1 perturbation to edema formation, we induced microdeletions in exon 3 of apol1 using the CRISPR/Cas9 system[31, 32] (Fig 3A–3C). Injection of guide RNA and CAS9 protein into one-cell stage embryos reproduced the edema phenotype (scored in founders, F0) seen in apol1 morphants (n = 26–38 embryos/injection, repeated three times; p<0.001; Fig 3D).
Fig. 2. apol1 morphant zebrafish embryos display generalized edema and glomerular filtration defects indicative of nephropathy. 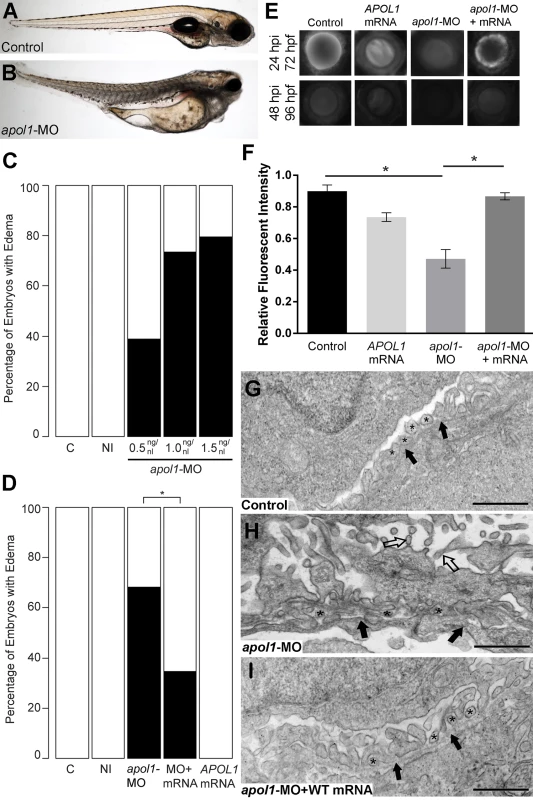
Representative live images of (A) sham-injected control larvae, and (B) apol1 morpholino (MO) injected larvae at 5 dpf. apol1 morphants display pericardial and yolk sac edema. (C) Injection of increasing doses of apol1-MO demonstrate dose-dependent effects when scored for generalized edema (n = 35–65 embryos/injection; repeated three times) compared to control larvae at 5 dpf. apol1 morpholino injected embryos were complemented with the respective human mRNA to APOL1 (100pg/nl) and scored for generalized edema at 5 dpf. (D) Ectopic expression of APOL1 rescues significantly the edema phenotype observed in apol1 morphants (1.0 ng/nl dose). We observed no significant phenotypes when APOL1 human mRNA is injected alone. 70kDa dextran-FITC conjugate was injected into the cardiac venous sinus of 48 hpf zebrafish larvae and fluorescence intensity in the eye vasculature was measured at 24 and 48 hpi. (E) Representative eye image series of zebrafish larvae for each injection group show a relatively stable or a decrease in fluorescence intensity over time compared to sham-injected controls. (F) Bar graphs summarize the fluorescence changes observed for each injection group for apol1 morphant larvae. Reduction in fluorescence intensity over the pupil was calculated relative to the 24 hpi time point; apol1 morphants display increased glomerular clearance of 70kDa dextran-FITC compared to control embryos over time, indicative of compromised glomerular filtration and proteinuria. These defects were rescued significantly when MO was co-injected with orthologous human mRNA. (G-I) Compared to (G) sham-injected controls, the glomerular ultrastructure of (H) apol1 morphant zebrafish display partial effacement of podocyte foot process (* asterisks), although the glomerular basement membrane (filled arrowheads) appears normal. Microvillus protrusions (open arrowheads) are also apparent in the urinary space. (I) Ultrastructure defects are rescued upon co-injection of human wild-type mRNA (100pg). Scale bar, 500nm. White bars, normal; black bars, edema. MO concentrations are in μg/μl, with 1nl injected into each embryo. C, sham-injected control; NI, non-injected control. Dextran values are in relative fluorescent intensity, mean ± SE. Control, sham-injected control (n = 29); MO, apol1 morpholino injected (n = 26); apol1-MO+mRNA (n = 28). h.p.f., hours post-fertilization; h.p.i., hours post-injection. *p<0.001. Fig. 3. apol1-CRISPR F0 zebrafish embryos reproduce phenotypes observed in apol1 morphants. 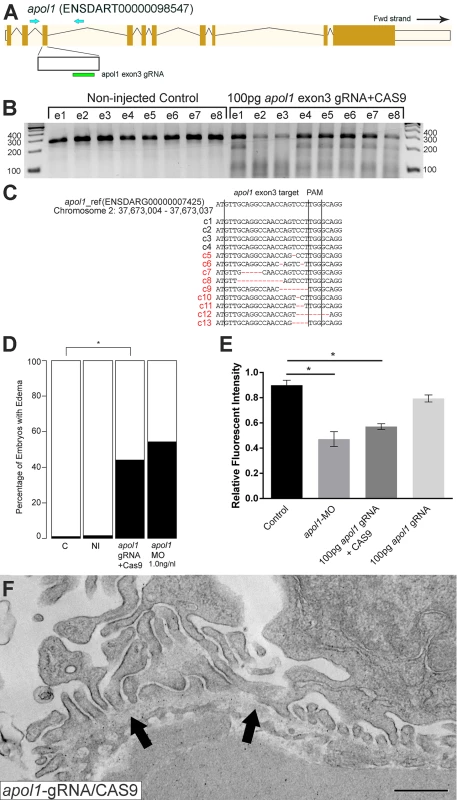
(A) Schematic of the zebrafish apol1 locus and location of the guide RNA (gRNA) target used for apol1-CRISPR experiments; the primers used to PCR-amplify the target region are shown (arrowheads). (B) At 1 dpf, a representative sampling of 8 founders and 8 non-injected controls were selected and subjected to T7 endonuclease 1 (T7E1) assay. The appearance of T7E1 fragments at ~180bp indicate positive gRNA targeting of exon 3 in the apol1 locus. No T7E1 fragments were detected in non-injected control embryos. In total, 25 out of 41 founders subjected to T7E1 assay showed the presence of T7E1 fragments, indicating that ~61% of founders have insertion/deletions (indels) in the exon 3 region of apol1. (C) Multiple sequence alignment of apol1 reference sequence (ENSDARG00000007425) to apol1-CRISPR variants generated from PCR amplification and subsequent TA cloning and sequencing of two representative apol1-gRNA/CAS9 injected founders. 13 PCR-cloned sequences are shown, representing four wild-type variants (c1-4) and all indel types detected among 50 PCR-clones (c5-13). Of 50 total PCR-clones sequenced, 31 showed detectable indels, representing an estimated 62% mosaicism in apol1-CRISPR/CAS9 injected founders. Lines mark the specific sequence targeted by the apol1-gRNA (exon3) and the location of the PAM recognition motif (i.e. TGG). (D) apol1-gRNA and CAS9 co-injected embryos were scored for edema formation at 5 dpf (n = 26–31 embryos/injection, repeated three times; *p<0.001). (E) apol1-gRNA and CAS9 co-injected embryos display increased glomerular clearance of 70kDa dextran-FITC compared to control embryos over time, similar to that of apol1-MO injected embryos (*p<0.001). Bar graphs summarize the changes for each injection group. Dextran values are in relative fluorescence intensity, mean ± SE. Control, sham-injected control (n = 19–21); apol1-gRNA+CAS9 (n = 11–17); apol1-gRNA alone (n = 13–14), repeated 2 times. (F) apol1-CRISPR/CAS9 injected embryos display podocyte foot process effacement at 5 dpf, similar to that of apol1 morphant larvae. Ultrastructural defects appear less severe when compared to apol1-MO injected embryos, however, including less foot process effacement and the absence of microvilli in the urinary space. Filled arrowheads, glomerular basement membrane. Scale bar, 500nm. To test whether the generalized edema phenotype was relevant to nephropathy, we assessed the integrity of the glomerular filtration barrier in apol1 morphants and F0 mutants as described[30]. First, we injected 70-kDa FITC-labeled dextran into the cardiac venous sinus of larvae at 48 hours post-fertilization (hpf). After injection, the eye vasculature was imaged at 24 and 48 hours post-injection (hpi; Fig 2E and 2F). We quantified the average fluorescence intensity (ImageJ) and calculated changes in intensity at 48 hpi relative to the 24 hpi measurements. apol1 morphant larvae display a significant reduction in circulating 70-kDa dextran compared to controls (n = 26; p = 4.44x10-4; MO vs. control; Fig 2E and 2F), consistent with the occurrence of proteinuria. Importantly, this phenotype was also reproduced in apol1 CRISPR/Cas9 larvae (Fig 3E). Upon co-injection of WT APOL1 human mRNA, the increased dextran clearance in apol1-MO larvae was rescued significantly and fluorescence intensity returned to levels indistinguishable from controls (n = 28; p = 7.75x10-4, MO vs. MO + mRNA; Fig 2E and 2F).
Next, we evaluated the cellular organization and patterning of the developing glomerulus in the context of apol1 suppression. We performed transmission electron microscopy (TEM) of ultrathin sections of zebrafish larvae at 5 dpf in WT and apol1 morphants and mutants, with myh9 morphants as a positive phenotypic control. In agreement with previous studies[17], myh9 morphant larvae exhibit focal bulges and glomerular basement membrane (GBM) thickening in comparison to controls, as well as the presence of microvillus protrusions, a defining characteristic of proteinuria (S3 and S4 Figs). Notably, apol1-MO injected larvae display a similar glomerular ultrastructure compared with myh9 morphants. Naked patches of GBM are apparent throughout the glomerulus, indicative of extensive podocyte effacement (Figs 2G, 2H, and S4). However, we did not observe GBM thickening as evident in myh9-MO injected larvae (S3 Fig). In areas in which we did observe foot process formation, podocyte protrusions were irregular and inhibited slit diaphragm development (Figs 2G, 2H, and S4). We also noted the formation of microvillus protrusion in the urinary space of apol1 morphants. Similarly, apol1-CRISPR/CAS9 injected embryos display an aberrant glomerular ultrastructure, as evident by podocyte foot process effacement (Fig 3F). Co-injection of orthologous WT human mRNA in apol1 morphants rescued these glomerular ultrastructure defects (Fig 2I). Together, these data represent compelling in vivo evidence implicating APOL1 in renal function.
Complementation of zebrafish apol1 morphants with human APOL1 risk alleles does not rescue kidney defects
Initial reports associating APOL1 variants with kidney disease in African Americans identified two independent sequence variants, termed G1 and G2, which reside in a 10-kb region in the last exon of the gene[5–7, 10]. The G1 allele consists of two nonsynonymous coding variants in perfect LD, rs73885319 and rs60910145, while the G2 variant consists of a six base pair deletion that removes amino acids N388 and Y389 (~21% and ~13% allele frequency in African Americans, G1 and G2 respectively; Fig 1D). Therefore, we evaluated the ability of each of the G1 and G2 alleles to rescue apol1-MO injected zebrafish larvae. APOL1 G1 (I384M/S342G) and G2 allelic constructs were generated from a WT APOL1 human cDNA clone, transcribed, and co-injected with apol1-MO in zebrafish embryos (100pg/nl). Importantly, each APOL1 allelic construct produces a stable protein detectable by immunoblotting when co-injected with apol1-MO (S2 Fig). apol1 morphants co-injected with either APOL1 G1 (I384M/S342G) or G2 human mRNA did not display significant rescue of edema formation in developing embryos compared to apol1-MO injected embryos alone (Fig 4A and 4B). In addition, we also co-injected each individual G1 variant (I384M and S342G) into apol1 morphant embryos. APOL1 message encoding either p.I384M or p.S342G were individually able to rescue significantly the edema caused by apol1 suppression (Fig 4C and 4D) suggesting that the cis effect of both variants in the same haplotype is required to confer pathogenicity. When APOL1 G2 mRNA was injected alone, a significant number of embryos developed edema in comparison to sham-injected controls (n = 52–63 embryos/injection; repeated three times; p = 0.012; Fig 4B); no edema was observed with injection of 100pg APOL1 G1 mRNA alone (Fig 4A). Additionally, dextran clearance assays demonstrated that neither APOL1 G1 or G2 mRNA were able to rescue glomerular filtration defects caused by apol1 suppression, while APOL1 G2 mRNA injected alone caused significant filtration defects compared to controls (n = 12–21; p = 0.003, Control vs. G2 mRNA; Fig 4E and 4F). Finally, when we injected embryos with APOL1 G2 titrated with increasing concentrations of APOL1 WT mRNA, we observed a significant reduction of edema formation in developing embryos (Fig 4G) suggesting that this allele is conferring a dominant negative effect on protein function.
Fig. 4. In vivo modeling of human APOL1 variants associated with disease. 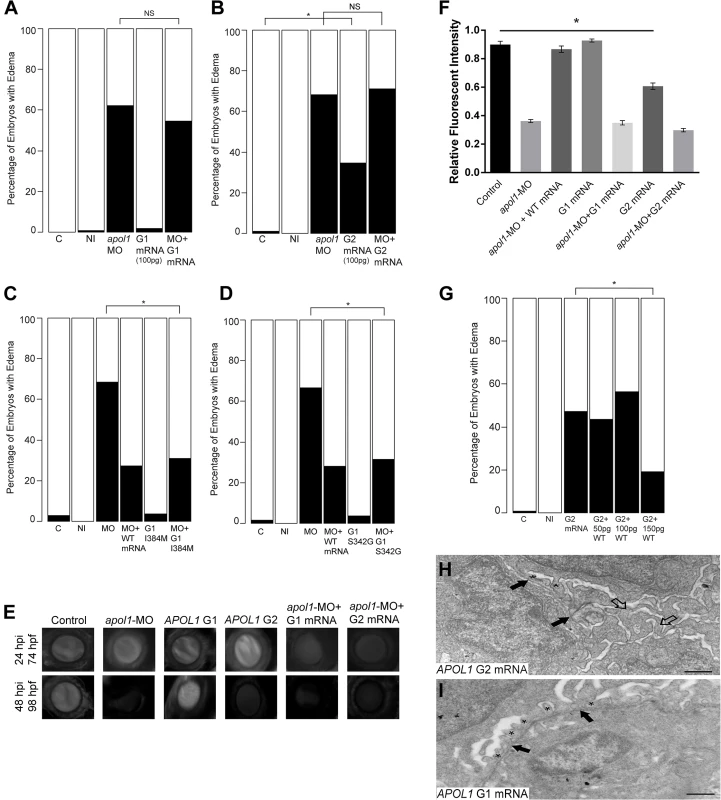
apol1 MO injected larvae were complemented with the respective human mRNA corresponding to APOL1 G1 (S342G/I384M) (100pg/nl) and G2 (100pg/nl) risk variants and scored for edema formation at 5 dpf (n = 26–65 embryos/injection; repeated three times). (A, B) Neither risk variant of APOL1 rescues significantly the edema phenotype observed in apol1 morphants. However, when human APOL1 G2 mRNA was injected alone (B), a significant number of embryos develop edema compared to sham-injected controls, suggesting a possible dominant-negative effect of the G2 altered protein. (C, D) apol1 morpholino injected larvae were complemented with human mRNA corresponding to either (C) APOL1 G1 I384M or (D) APOL1 G1 S342G and scored for edema formation at 5 dpf (n = 48–93 embryos/injection; repeated two times). Each individual variant comprising APOL1 G1 risk rescues significantly edema formation in apol1 morphant embryos, suggesting that both G1 variants must be present to confer loss of APOL1 function. (E-F) apol1 morphants co-injected with human APOL1 G1 or G2 mRNA fail to rescue filtration defects as indicated by dextran clearance, while larvae injected with G2 mRNA alone display increased clearance over time. (G) Titration of G2 injected embryos with increasing concentrations of human WT APOL1 mRNA show a significant reduction in edema formation of developing embryos at 5 dpf. (H) Zebrafish embryos injected with APOL1 G2 mRNA (100pg/nl) alone display glomerular aberrations similar to that of myh9 suppressed larvae, with microvillus protrusions present (open arrowheads), although the glomerular basement membrane appears normal (filled arrowheads). Podocyte foot processes (* asterisk) are apparent, although sparsely present. (I) Embryos injected with APOL1 G1 mRNA (100pg/nl) alone display normal glomerular ultrastructure. Scale bar, 500nm. White bars, normal; black bars, edema. C, sham-injected control; NI, non-injected control. *p<0.05. We also examined the glomerular ultrastructure of apol1 morphants co-injected with either APOL1 G1 or G2 human mRNA using TEM. However, we did not observe any noticeable improvement in glomerular ultrastructure abnormalities at 5 dpf (S5 Fig). In concurrence with our observations of gross morphological defects, embryos injected with G2 mRNA alone also display glomerular aberrations and microvillus protrusions (Fig 4H) similar to myh9 and apol1 morphants (Figs 2H and S4); no abnormalities were seen in larvae injected with G1 mRNA alone (Fig 4I). These data provide direct evidence for a functional consequence of the human APOL1 G1 and G2 risk alleles, and suggest that they confer loss-of-function and dominant negative effects, respectively.
myh9 and apol1 interact under anemic stress to exacerbate nephropathy phenotypes
Although recent studies have provided statistical evidence implicating APOL1 variation in nondiabetic nephropathies[7, 33, 34], MYH9 risk variants are still associated with chronic kidney disease (CKD) in non-African American populations[35] and in sickle cell disease nephropathy[5]. As such, our group and others have hypothesized that these genes may be co-regulated to induce nephropathy risk; in fact, when we modeled glomerular filtration rate in sickle cell patients as a function of the previously reported MYH9 risk haplotype and an APOL1 recessive model, we observed a significant interaction between the two genes[5]. Therefore, we tested for functional interaction effects between apol1 and myh9 in zebrafish, an experimentally tractable model for investigating additive and synergistic effects[36–40]. First, we co-injected both apol1-MO and myh9-MO into embryos and we scored for gross morphological defects at 5 dpf. Under this co-suppression model, we observed no significant differences in edema formation when compared to batches injected with either MO alone (Fig 5A), even when individual MO concentrations were reduced to subeffective doses (Fig 5B). Next, we tested the possibility that suppression of either apol1 or myh9 in zebrafish could be rescued significantly by the co-injection of the reciprocal human mRNA. myh9-MO was co-injected with human APOL1 WT mRNA (100pg/nl) and apol1-MO was co-injected with human MYH9 WT mRNA (100pg/nl). However, we were unable to rescue the suppression phenotypes of either apol1 or myh9 with the human mRNA of the reciprocal gene (S6 Fig).
Fig. 5. apol1 interacts with myh9 in an anemic context. 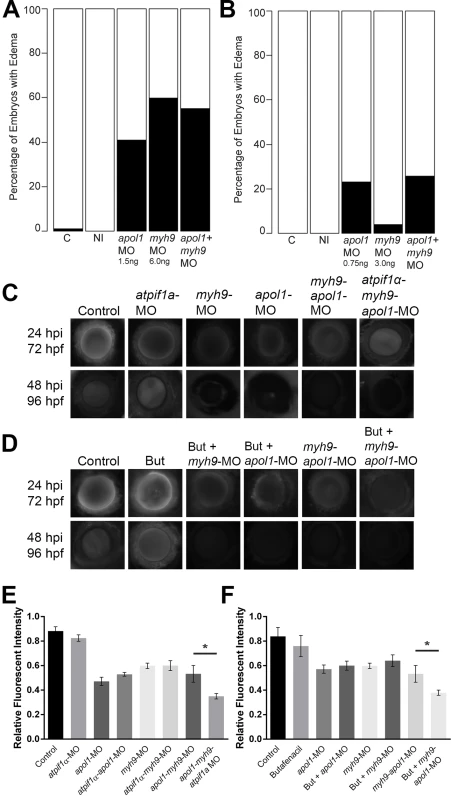
To test for epistatic effects of apol1 and myh9 in zebrafish, we first co-injected both apol1-MO (1.0ng/nl dose) and myh9-MO (6.0ng/nl dose) into zebrafish larvae and scored for edema formation at 5 dpf. (n = 39–89 embryos/injection; repeated three times). However, under this co-suppression model (A, B), we observed no significantly increased edema formation compared to each MO alone. We next tested for an interaction between apol1 and myh9 in the context of atpif1a suppression, predicting that the added stress of anemia would mimic our initial observations in sickle cell disease patients. 70kDa dextran-FITC conjugate was injected into the cardiac venous sinus of 48 hpf zebrafish larvae and fluorescence intensity in the eye vasculature was measured at 24 and 48 hours later. (C) Representative eye image series of zebrafish embryos for each injection group show relatively stable or decreased fluorescence intensity over time. (E) Bar graphs summarize the changes observed for each injection group. Zebrafish embryos injected with all three MOs show a significant increase in dextran clearance from the vasculature compared to co-suppression of apol1 and myh9. (D, F) These data are reproduced using butafenacil induced anemia (0.195 μM in embryo media, treated at 48 hpf). Dextran values are in relative fluorescence intensity, mean ± SE. Control, sham-injected control (n = 19); atpif1a MO injected (n = 14); apol1-MO+myh9-MO (n = 12); apol1-MO+myh9-MO+atpif1a-MO (n = 11); Butafenacil (n = 48); But+myh9-MO+apol1-MO (n = 18). hpf, hours post-fertilization; hpi, hours post-injection. *p<0.001. Our hypothesis for an interaction between APOL1 and MYH9 was based on data derived from SCD patients. Thus, we posited that myh9 and apol1 may only interact under additional biologic stress, such as anemia or hemolysis. Accumulating evidence suggests that both anemia and hemolysis, which are key features of SCD pathophysiology, impact renal function; in particular, hemolysis appears to be associated with both microalbuminuria and hyperfiltration[41, 42]. While a zebrafish model of SCD does not exist currently, suppression of ATPase inhibitory factor 1 (atpif1α), a mitochondrial protein, produces profound anemia in zebrafish by interfering with heme synthesis through decreased catalytic efficiency of ferrochelatase[43]. The resultant effect of low hemoglobin and hematocrit stresses the kidney because of the organ’s high oxygen consumption. Consistent with the original report[43], we observed a dose-dependent reduction in hemoglobin with increasing concentrations of the atpif1a MO (atpif1α-MO), as measured by o-dianisidine staining of whole MO-injected larvae at 4 dpf. Strikingly, we found a significantly more severe nephropathy phenotype in an anemic context as indicated by accelerated dextran clearance, with co-suppression of apol1 and myh9 under atpif1α-MO induced anemia (n = 12–19 embryos/injection; p<0.001 for myh9/apol1 MOs vs. myh9/apol1/atpif1a MOs; Fig 5C and 5E). Importantly, neither morphant alone resulted in a more severe phenotype under atpif1α-MO induced anemia (e.g. myh9-MO vs. myh9-atpif1α-MO; p = 0.78; or apol1-MO vs. apol1-atpif1α-MO; p = 0.90; Fig 5E). Furthermore, these observations were reproducible using an independent and non-genetic induction of anemia. Butafenacil, an inhibitor of protoporphyrinogen oxidase, causes loss of hemoglobin following exposure during early zebrafish development[44]. In a butafenacil-induced anemic context (0.195 μM treatment at 48 hpf), we observed a similar effect upon co-suppression of apol1 and myh9 (n = 17–23 embryos/injection; p<0.001 for myh9/apol1 MOs vs. myh9/apol1 + 0.195 μM butafenacil; Fig 5D and 5F).
APOL1 G2 (del:N388Y389) modulates myh9 expression in vivo
To dissect further the possible genetic interactions between myh9 and apol1, we tested whether suppression of endogenous apol1 or ectopic expression of mutant human APOL1 could alter expression of myh9 in zebrafish embryos. We monitored myh9 expression in zebrafish larvae using quantitative real-time PCR in the context of apol1 suppression, and G1 or G2 expression, as well as apol1/APOL1 modulation in conditions of anemia induced by atpif1α-MO injection at 5 dpf (Fig 6A) and 3 dpf (Fig 6B). We observed a significant decrease in myh9 expression when zebrafish embryos were injected with the proposed dominant-negative APOL1 G2 allele alone (21% reduction; p = 0.043; Fig 6B), suggesting that the mutant protein may be suppressing myh9, either directly or indirectly, to induce nephropathy. Furthermore, zebrafish embryos co-injected with APOL1 G2 mRNA and atpif1α-MO display an even greater reduction in myh9 expression compared to controls (46% reduction; p = 0.0013; Fig 6B), and a significant reduction of myh9 expression compared to APOL1 G2 mRNA alone (p = 0.0297; Fig 6B), suggesting that the altered APOL1 (p.Asn388_Tyr389del) protein has a more pronounced effect on myh9 expression in the context of anemic stress. We also observed a significant increase in myh9 expression in APOL1 G1/atpif1α-MO vs. APOL1 G1 injected embryos (Fig 6A), however, neither of these conditions induced nephropathy. To determine whether this effect was specific to myh9 or was a general effect on transcripts expressed in the glomerulus, we also assessed expression levels of other nephropathy-associated genes during apol1/APOL1 modulation and atpif1α induced anemia. We observed no significant differences in expression of genes implicated in familial focal segmented glomerulosclerosis, including anln[45], trpc6b[46], and wt1a[47] upon apol1/APOL1 modulation (S7 Fig), suggesting that APOL1 G2 regulation may be specific to myh9.
Fig. 6. myh9 expression in the context of apol1/APOL1 modulation. 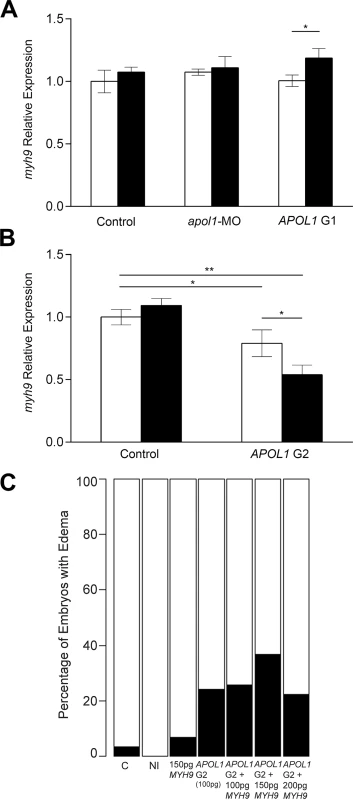
Zebrafish embryos were injected with either apol1-MO (1.0ng/nl dose), APOL1 G1 (S342G:I384M) mRNA (100pg), or APOL1 G2 (100pg) mRNA alone, in the absence (white bars) or presence (black bars) of atpif1α-MO. Total RNA at 5 dpf or 3 dpf (APOL1 G2/atpif1α-MO embryos did not survive to 5 dpf) was extracted and reverse-transcribed with random primers to obtain whole-embryo cDNA. myh9 expression was determined by quantitative real-time PCR and relative expression was calculated against actb1. (A) apol1-MO injected embryos do not display any significant changes in myh9 expression compared to sham-injected control embryos. Additionally, APOL1 G1 expression does not alter myh9 expression alone, however, under atpif1α-induced anemia, we observe an increase in myh9 expression. (B) APOL1 G2 expression results in a significant decrease in myh9 expression compared to sham-injected control embryos, suggesting that the altered APOL1 protein may regulate myh9 in vivo. (C) Co-injection of APOL1 G2 (100pg) and human WT MYH9 (n = 31–60; repeated two times), does not rescue edema formation caused by APOL1 G2 expression in 5 dpf larvae, suggesting that the interaction between APOL1 G2 and MYH9 may be indirect. Relative expression values are mean ± SE in triplicate with two biological replicates. * = p<0.05; ** = p<0.01. Based on the observations that APOL1 G2 expression has the ability to decrease myh9 expression in vivo, we next attempted to rescue APOL1 G2 defects by co-injecting human WT MYH9 mRNA. We injected a constant amount of APOL1 G2-encoding message (100pg) with increasing amounts of human MYH9 mRNA (100pg, 150pg, and 200pg) and scored larvae live for generalized edema at 5dpf. However, we did not observe a significant reduction of edema in APOL1 G2/MYH9 co-injected embryos (Fig 5C), suggesting that compensation with MYH9 message alone is not sufficient to account for the deleterious effects of the G2 variant, possibly because APOL1 G2 has a trans effect on other loci in the genome or is acting to perturb cellular pathways[20].
Discussion
In recent years, multiple lines of statistical evidence have implicated the MYH9/APOL1 locus on chromosome 22q12.3 with nondiabetic end-stage renal disease, focal segmental glomerulosclerosis, HIV-associated nephropathy, lupus nephritis, SCDN, and diabetic nephropathy in patients of recent African ancestry and European Americans[5–10, 33, 35, 48–50]. Additionally, APOL1 has been associated with an increased burden of cardiovascular disease in African Americans participating in the Jackson Heart Study[51]. Compelling statistical evidence in human cohorts points to the G1 and G2 alleles of APOL1, rather than MYH9 variation, as the most likely contributors to nephropathy risk. Nonetheless, functional studies of the MYH9 locus provide biological evidence for its role in the kidney, including perturbed glomerular development in myh9 morphant zebrafish[14–17]. Here, we have identified a functional ortholog of human APOL1 in zebrafish and, using transient genetic manipulation, provide functional evidence demonstrating apol1 involvement in both kidney development and filtration.
Although the human APOL gene cluster has undergone recent natural selection in primates[26, 27], we report the identification of a functional APOL1 ortholog in the zebrafish genome and its implication in renal function. Specific detection of the zebrafish apol1 protein product with the human APOL1 antibody, rescue of kidney defects in apol1 morphant embryos with human APOL1 mRNA, as well as recapitulation of renal phenotypes with an apol1-CRISPR/CAS9 F0 mutant, provide evidence that zebrafish apol1 is indeed functionally relevant to its human ortholog with respect to its role in the glomerulus. Furthermore, no other human mRNA in the human apolipoprotein L family ameliorated kidney defects induced by apol1 knockdown, supporting further its functional orthology to human APOL1. Nonetheless, it is unclear whether the zebrafish APOL1 protein serves all functions of its human counterpart, especially given the lack of a secretory domain in the zebrafish APOL1 peptide (Fig 1A).
Suppression and genome-editing of apol1 in zebrafish and three independent phenotypic scoring paradigms support a role for apol1 in nephropathy; we observed severe edema formation with concomitant glomerular filtration defects and severe podocyte loss. Complementation of apol1 suppression with APOL1 CKD risk alleles (G1 and G2) failed to ameliorate these observed defects. Notably, complementation of each individual variant of the G1 haplotype (I384M and S342G) rescued significantly nephropathy phenotypes caused by apol1 suppression, suggesting that both variants must be present in cis to confer risk. This is concordant with initial reports on the lytic potential of APOL1 recombinant proteins on T. b. rhodesiense, in which APOL1 variants with either S342G or I384M alone were less lytic than if both were present together[6].
Strikingly, injection of human APOL1 G2 mRNA alone resulted in significant edema formation in 5dpf zebrafish larvae as well as perturbed glomerular filtration and ultrastructural defects. Our expression data suggest that this could arise from myh9 suppression induced by the altered APOL1 protein harboring the G2 variant. The G2 deletion lies in the SRA-binding domain of APOL1 (Fig 1B and 1D). Therefore it is plausible that disruption of this region of the protein may either prohibit proper binding of APOL1 to its usual partners, or perhaps permit new interactions that induce nephropathy. Further studies are needed to elucidate the functional impacts of the altered APOL1 protein to nephropathy. We also report for the first time functional evidence of a genetic interaction between myh9 and apol1. Intriguingly, this interaction was only observed in the presence of anemic stress, consistent with our previous genetic association findings in human SCD patients[5].
An immediate question remains regarding the mechanism by which apol1 suppression is inducing kidney injury. Early studies revealed APOL1 mRNA expression in the placenta, lung, and liver, with specific cell-type expression found in endothelial cells and possibly macrophages[26]. More recent studies, however, have characterized the cellular localization of APOL1 in human kidney sections to podocytes, proximal tubules, and arteriolar endothelial cells[18]. These data are consistent with our observation of apol1 morphants and mutants exhibiting extensive podocyte loss and suggest that apol1 is necessary for the development and/or maintenance of glomerular podocytes. Interestingly, it has been shown that APOL1 may cause toxic renal effects through programmed cell death pathways leading to glomerulosclerosis[52, 53]. Thus, apol1 suppression could dysregulate autophagic pathways, causing podocyte malformation, thereby promoting the susceptibility of the pronephros to glomerular injury.
Initial studies implicating MYH9 in nondiabetic nephropathy failed to identify coding variants associated with renal outcome[8, 9], and since the nearby nonsynonymous variants identified in APOL1 provided stronger statistical association[5–7], it was hypothesized that APOL1 variation represents the true attribution to renal disease risk. In fact, it has been shown in multiple studies that controlling for the APOL1 risk alleles (G1-G2) attenuates significantly the effect of MYH9 SNPs[6, 33]. However, recent reports still demonstrate statistical association of MYH9 in nondiabetic nephropathy[5, 35] and previous in vivo modeling studies provide further evidence for the role of Myh9 in glomerular development and glomerulosclerosis[14–17]. As such, our group and others have postulated that complex genetic models may exist in this region, including the possibility of MYH9-APOL1 gene interaction[5, 10]. Our observation of exacerbated glomerular filtration in the context of anemic stress provides biological evidence in support of this hypothesis. Because knockdown of each of myh9 and apol1 independently impairs proper pronephric development and filtration, it is plausible that their encoded proteins are functioning in separate pathways to induce kidney dysfunction. However, these effects only appear to become additive under an additional stress (anemia). The associated variants alone may not be sufficient to induce nephropathy progression, while under low hemoglobin and hematocrit levels, additive effects between MYH9 and APOL1 may become apparent and result in a more drastic reduction in renal function, along with the observed significantly high early mortality rates among SCD nephropathy patients[21, 22, 41, 54].
Furthermore, we provide evidence suggesting that the functional consequences of APOL1 variation may not be acting in a strictly recessive manner as had been previously suggested[5–7, 55]. Our data demonstrate that APOL1 G1 (I384M/S342G) confers loss of proper APOL1 function in the developing zebrafish kidney, while APOL1 G2 is acting in a dominant-negative manner to induce nephropathy, possibly through suppression of myh9. These data indicate that the risk conferred by the APOL1/MYH9 locus is likely to be governed by a more complex model than recessive patterning as suggested previously.
In summary, our study demonstrates the essential role of both apol1 and myh9 in the development of the pronephric glomerulus and proper renal filtration in zebrafish. We report comprehensive in vivo causal evidence of apol1 involvement in kidney decline, and we provide the first in vivo evidence of a potential dominant-negative effect of the APOL1 G2 allele. Further, we have shown that the presence of the G2 allele decreases significantly the expression of myh9. Similar to the common haplotype on 10q26 that influences age-related macular degeneration underscored by complex regulatory events of neighboring genes ARMS2 and HTRA1, our data highlight further the importance of comprehensive evaluation of functional consequences at a susceptibility locus[56]. Taken together, these data provide essential biological insight into the mechanisms by which MYH9 and APOL1 confer disease risk and progression in human nondiabetic nephropathies.
Materials and Methods
Zebrafish stocks
We maintained WT zebrafish stocks (Ekkwill, Ekkwill x AB F1 outcross, or pod::NTR-mCherry[28] according to standard zebrafish husbandry procedures. Embryos were obtained from natural matings of adult fish.
Morpholino oligonucleotide-mediated knockdown and human mRNA complementation
Complementation assays were designed essentially as described[57]. Briefly, a MO was designed by Gene Tools, LLC (Philomath, OR) to target the translation initiation site of zebrafish apol1 (NM_001030138) (apol1-MO), (5’-AGTCGTCCAGCCATTCCATGAGGGT-3’). A translation-blocking morpholino (MO) targeting zebrafish myh9 and a splice-blocking MO targeting zebrafish atpif1a were described previously[17, 43]. APOL1 G1 and G2 allelic constructs were synthesized from a WT APOL1 human ORF clone (GenBank: BC112943) using site-directed mutagenesis (Stratagene, QuikChange II), subsequently transcribed (mMESSAGE mMACHINE, Life Technologies, Ambion) into capped mRNA and co-injected with apol1-MO into zebrafish embryos at the one-to-four cell stage (WT, 100pg/ nl; G1, 100pg/nl; G2, 100pg/nl). Controls were injected with phenol red. A WPI pneumatic pico pump microinjector was used for MO and mRNA injection to deliver 1 nl/embryo. After injection, embryos were maintained at 28°C in embryo medium.
Dextran microinjection and time-lapse filtration scoring
48 h.p.f. larvae were anesthetized in 1.0% tricaine and placed laterally in agarose wells. 70 kDa FITC-conjugated dextran (LifeTechnologies, 3.0nl/embryo) was injected into the cardiac venous sinus and larvae were transferred to embryo medium for recovery after injection. The eye vasculature of individual fish was imaged at 24, and 48 hours after dextran injection using a Nikon AZ100 fluorescent microscope and Nikon NIS Elements AR software. The average fluorescence intensity was measured across the eye (ImageJ) and changes in intensity relative to the 24 h.p.i measurements were calculated for comparison. GraphPad Prism version 6.03 (GraphPad Software, San Diego, CA) was used for statistical analysis of relative intensity.
Fluorescence-activated cell sorting (FACS)
Glomeruli from pod::NTR-mCherry adult zebrafish were manually dissected and dissociated in 0.5% trypsin/collagenase. Dissociated cells were then filtered through a 70μm strainer and filtered again through a 30μm strainer. Cell-sorting was done on a Beckman Coulter Astrios instrument for mCherry (610nm). Sorted cells were placed in RLT Buffer (Qiagen) and RNA was extracted using the RNeasy Micro Kit (Qiagen).
Reverse transcription and quantitative real-time PCR (qRT-PCR)
Total RNA from zebrafish embryos was extracted with TRIzol Reagent (Life Technologies) and cDNA was reverse transcribed using QuantiTect Reverse Transcription Kit (Qiagen). The following primers were used for amplification: actb1, Fwd: TTGTTGGACGACCCAGACAT, Rev: TGAGGGTCAGGATACCTCTCTT; nphs2, Fwd: CCTTCGCTAGCATTCCAGAC, Rev: GCAGCTCTGGAGGAAGATTG; wdr81, Fwd: ATGGAGAGAAAAACATGGAGGA, Rev: AAGGAGAAAACCTGGAAGAACC; apol1, Fwd: GACTTTCGATTAAGTGAAACTCAGAGAGA, Rev: GTTATGGTAGCTACACCTCCCACAGCGCTG; myh9 (qRT), Fwd: GGAAAAACCGAAAACACCAA, Rev: CAATATTGGCTCCAACGATGT; anln (qRT), Fwd: TTTGACCTTCACCACCACATT, Rev: TTTGGTGTGATTGCCTTTGA; wt1a (qRT), Fwd: ATGGCCAAACTGTCAGAAGAA, Rev: TTATTTCCTGCCGTTTCTGTG; trpc6b (qRT), Fwd: GGCACCATGAGCCAGAGCCCGGCGTTCGGG, Rev: CTAAGGTGGGCCCATTGGCACTTAAGAAAA. qRT-PCR was performed on a ABI Prism 7900HT instrument and cycle threshold values were computed using SDS 2.3 software (Applied Biosystems). Relative expression was calculated against actb1 in each sample and compared against sham-injected controls to determine significant differences in expression.
Transmission electron microscopy of glomerular ultrastructure
5 dpf embryos were anesthetized in 1.0% tricaine and then fixed in 4.0% gluteraldehyde in 0.1M Na2PO4 buffer containing 0.12mM CaCl2 at 4°C overnight. Fixed larvae were washed in 1X PBS, washed in 1X phosphate buffer, postfixed in 2% osmium tetroxide for 2 hours, and dehydrated through a graded acetone series. Embedding was performed with Epoxy 812. Sections were cut on a Leica-Reichert Ultracut E ultramicrotome and semithin sections (1.0μm) were collected and stained with toluidine blue. 90nm ultrathin sections were placed on copper grids and contrasted with 4.0% uranyl acetate for 10 minutes. Grids were incubated in lead citrate (Reynolds Lead) for 3 minutes and then examined on a Phillips CM12 electron microscope. Images were taken with an AMT XR61 camera.
Genome-editing of the apol1 locus using the CRISPR/CAS9 system
apol1 gRNA was produced by synthesizing and annealing two oligonucleotides, gRNA F: TAGGGTTGCAGGCCAACCAGTCCT and gRNA R: AAACAGGACTGGTTGGCCTGCAAC. The annealed oligos were then ligated to a T7cas9sgRNA2 vector by performing the ligation and digestion in a single step in a thermal cycler as described [31]. 2 μL of the reaction was used for transformation. Prior to transcription, the gRNA vector was linearized with BamHI. gRNA was transcribed using the MEGAshortscript T7 kit (Life Technologies, AM1354) and purified using alcohol precipitation. A total of 100pg of apol1 gRNA and 200pg of CAS9 protein (PNA Bio) was co-injected into individual cells of one-cell stage embryos. For T7 endonuclease I assay, genomic DNA was prepared from 1 dpf embryos as described [58]. A short stretch of the genomic region (~270–280 bp) flanking the apol1 gRNA target site was PCR amplified from the genomic DNA (Fwd: TGTGTGAAGGATGCATTTGTT, Rev: TGGGATAATGTATGGGAGAATG). The PCR amplicon was then denatured slowly and reannealed to facilitate heteroduplex formation. The reannealed amplicon was then digested with 5 units of T7 endonuclease I (New England Biolabs) at 37°C for 45 minutes. The samples were resolved by electrophoresis through a 3.0% agarose gel and visualized by ethidium bromide staining.
Western blot
Whole embryo protein lysates were collected at 2 dpf by homogenizing anesthetized embryos immersed in RIPA Buffer (50 mM Tris, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X 100, protease inhibitor (Roche, cat. no. 11697498001)). 100 mg protein was loaded into individual wells of a Mini-PROTEAN TGX Precast Gel (Bio-Rad) and a western blot was performed as described [59]. Blots were incubated overnight at 4°C with anti-APOL1 antibody (1 : 1000; Abcam, EPR2907, ab108315). The membranes were subsequently washed in PBST (0.1% Tween 20) and incubated for 1 hour at room temperature with anti-rabbit IgG conjugated to horseradish peroxidase (1 : 20,000; GE Healthcare, NA934V). ACTIN antibody (1 : 1000, Santa-Cruz, cat. no. sc-8432) was used as a loading control.
Ethics statement
All animal protocols were reviewed and approved by the Duke University Institutional Animal Care & Use Committee (IACUC; protocol A229-12-08).
Supporting Information
Zdroje
1. Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, et al. 'United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2012;59(1 Suppl 1):A7, e1–420. doi: 10.1053/j.ajkd.2011.11.015 22177944.
2. Byrne C, Nedelman J, Luke RG. Race, socioeconomic status, and the development of end-stage renal disease. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1994;23(1):16–22. 8285192.
3. Kopp JB, Winkler C. HIV-associated nephropathy in African Americans. Kidney international Supplement. 2003;(83):S43-9. 12864874.
4. Kitiyakara C, Eggers P, Kopp JB. Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2004;44(5):815–25. 15492947.
5. Ashley-Koch AE, Okocha EC, Garrett ME, Soldano K, De Castro LM, Jonassaint JC, et al. MYH9 and APOL1 are both associated with sickle cell disease nephropathy. British journal of haematology. 2011;155(3):386–94. doi: 10.1111/j.1365-2141.2011.08832.x 21910715.
6. Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–5. doi: 10.1126/science.1193032 20647424; PubMed Central PMCID: PMC2980843.
7. Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. Journal of the American Society of Nephrology: JASN. 2011;22(11):2129–37. doi: 10.1681/ASN.2011040388 21997394; PubMed Central PMCID: PMC3231787.
8. Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nature genetics. 2008;40(10):1175–84. doi: 10.1038/ng.226 18794856; PubMed Central PMCID: PMC2827354.
9. Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nature genetics. 2008;40(10):1185–92. doi: 10.1038/ng.232 18794854; PubMed Central PMCID: PMC2614692.
10. Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. Journal of the American Society of Nephrology: JASN. 2010;21(9):1422–6. doi: 10.1681/ASN.2010070730 20688934.
11. Grabhorn R, Kopp W, Gitzinger I, von Wietersheim J, Kaufhold J. [Differences between female and male patients with eating disorders—results of a multicenter study on eating disorders (MZ-Ess)]. Psychotherapie, Psychosomatik, medizinische Psychologie. 2003;53(1):15–22. doi: 10.1055/s-2003-36479 12514763.
12. Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. The Journal of biological chemistry. 2004;279(40):41263–6. doi: 10.1074/jbc.C400352200 15292239.
13. Matsushita T, Hayashi H, Kunishima S, Hayashi M, Ikejiri M, Takeshita K, et al. Targeted disruption of mouse ortholog of the human MYH9 responsible for macrothrombocytopenia with different organ involvement: hematological, nephrological, and otological studies of heterozygous KO mice. Biochemical and biophysical research communications. 2004;325(4):1163–71. doi: 10.1016/j.bbrc.2004.10.147 15555549.
14. Suzuki N, Kunishima S, Ikejiri M, Maruyama S, Sone M, Takagi A, et al. Establishment of mouse model of MYH9 disorders: heterozygous R702C mutation provokes macrothrombocytopenia with leukocyte inclusion bodies, renal glomerulosclerosis and hearing disability. PloS one. 2013;8(8):e71187. doi: 10.1371/journal.pone.0071187 23976996; PubMed Central PMCID: PMC3748045.
15. Zhang Y, Conti MA, Malide D, Dong F, Wang A, Shmist YA, et al. Mouse models of MYH9-related disease: mutations in nonmuscle myosin II-A. Blood. 2012;119(1):238–50. doi: 10.1182/blood-2011-06-358853 21908426; PubMed Central PMCID: PMC3251230.
16. Johnstone DB, Zhang J, George B, Leon C, Gachet C, Wong H, et al. Podocyte-specific deletion of Myh9 encoding nonmuscle myosin heavy chain 2A predisposes mice to glomerulopathy. Molecular and cellular biology. 2011;31(10):2162–70. doi: 10.1128/MCB.05234-11 21402784; PubMed Central PMCID: PMC3133349.
17. Muller T, Rumpel E, Hradetzky S, Bollig F, Wegner H, Blumenthal A, et al. Non-muscle myosin IIA is required for the development of the zebrafish glomerulus. Kidney international. 2011;80(10):1055–63. doi: 10.1038/ki.2011.256 21849970.
18. Madhavan SM O'Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR. APOL1 localization in normal kidney and nondiabetic kidney disease. Journal of the American Society of Nephrology: JASN. 2011;22(11):2119–28. doi: 10.1681/ASN.2011010069 21997392; PubMed Central PMCID: PMC3231786.
19. Ma L, Shelness GS, Snipes JA, Murea M, Antinozzi PA, Cheng D, et al. Localization of APOL1 Protein and mRNA in the Human Kidney: Nondiseased Tissue, Primary Cells, and Immortalized Cell Lines. Journal of the American Society of Nephrology: JASN. 2014. doi: 10.1681/ASN.2013091017 25012173.
20. Lan X, Jhaveri A, Cheng K, Wen H, Saleem MA, Mathieson PW, et al. APOL1 risk variants enhance podocyte necrosis through compromising lysosomal membrane permeability. American journal of physiology Renal physiology. 2014;307(3):F326–36. doi: 10.1152/ajprenal.00647.2013 24899058; PubMed Central PMCID: PMC4121568.
21. Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. The New England journal of medicine. 1994;330(23):1639–44. doi: 10.1056/NEJM199406093302303 7993409.
22. Elmariah H, Garrett ME, De Castro LM, Jonassaint JC, Ataga KI, Eckman JR, et al. Factors associated with survival in a contemporary adult sickle cell disease cohort. American journal of hematology. 2014;89(5):530–5. doi: 10.1002/ajh.23683 24478166; PubMed Central PMCID: PMC3988218.
23. Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364(9442):1343–60. doi: 10.1016/S0140-6736(04)17192-4 15474138.
24. Drummond IA. Zebrafish kidney development. Methods in cell biology. 2004;76 : 501–30. 15602890.
25. Ebarasi L, Oddsson A, Hultenby K, Betsholtz C, Tryggvason K. Zebrafish: a model system for the study of vertebrate renal development, function, and pathophysiology. Current opinion in nephrology and hypertension. 2011;20(4):416–24. doi: 10.1097/MNH.0b013e3283477797 21519251.
26. Monajemi H, Fontijn RD, Pannekoek H, Horrevoets AJ. The apolipoprotein L gene cluster has emerged recently in evolution and is expressed in human vascular tissue. Genomics. 2002;79(4):539–46. doi: 10.1006/geno.2002.6729 11944986.
27. Ko WY, Rajan P, Gomez F, Scheinfeldt L, An P, Winkler CA, et al. Identifying Darwinian selection acting on different human APOL1 variants among diverse African populations. American journal of human genetics. 2013;93(1):54–66. doi: 10.1016/j.ajhg.2013.05.014 23768513; PubMed Central PMCID: PMC3710747.
28. Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. Journal of the American Society of Nephrology: JASN. 2012;23(6):1039–47. doi: 10.1681/ASN.2011080776 22440901; PubMed Central PMCID: PMC3358760.
29. Traka M, Millen KJ, Collins D, Elbaz B, Kidd GJ, Gomez CM, et al. WDR81 is necessary for purkinje and photoreceptor cell survival. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(16):6834–44. doi: 10.1523/JNEUROSCI.2394-12.2013 23595742.
30. Hentschel DM, Mengel M, Boehme L, Liebsch F, Albertin C, Bonventre JV, et al. Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. American journal of physiology Renal physiology. 2007;293(5):F1746–50. doi: 10.1152/ajprenal.00009.2007 17699558.
31. Jao LE, Wente SR, Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(34):13904–9. doi: 10.1073/pnas.1308335110 23918387; PubMed Central PMCID: PMC3752207.
32. Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nature biotechnology. 2013;31(3):227–9. doi: 10.1038/nbt.2501 23360964; PubMed Central PMCID: PMC3686313.
33. Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, et al. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney international. 2013;83(1):114–20. doi: 10.1038/ki.2012.263 22832513; PubMed Central PMCID: PMC3484228.
34. Genovese G, Friedman DJ, Pollak MR. APOL1 variants and kidney disease in people of recent African ancestry. Nature reviews Nephrology. 2013;9(4):240–4. doi: 10.1038/nrneph.2013.34 23438974.
35. O'Seaghdha CM, Parekh RS, Hwang SJ, Li M, Kottgen A, Coresh J, et al. The MYH9/APOL1 region and chronic kidney disease in European-Americans. Human molecular genetics. 2011;20(12):2450–6. doi: 10.1093/hmg/ddr118 21429915; PubMed Central PMCID: PMC3098737.
36. Lindstrand A, Davis EE, Carvalho CM, Pehlivan D, Willer JR, Tsai IC, et al. Recurrent CNVs and SNVs at the NPHP1 locus contribute pathogenic alleles to Bardet-Biedl syndrome. American journal of human genetics. 2014;94(5):745–54. doi: 10.1016/j.ajhg.2014.03.017 24746959; PubMed Central PMCID: PMC4067552.
37. Margolin DH, Kousi M, Chan YM, Lim ET, Schmahmann JD, Hadjivassiliou M, et al. Ataxia, dementia, and hypogonadotropism caused by disordered ubiquitination. The New England journal of medicine. 2013;368(21):1992–2003. doi: 10.1056/NEJMoa1215993 23656588; PubMed Central PMCID: PMC3738065.
38. Davis EE, Zhang Q, Liu Q, Diplas BH, Davey LM, Hartley J, et al. TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nature genetics. 2011;43(3):189–96. doi: 10.1038/ng.756 21258341; PubMed Central PMCID: PMC3071301.
39. Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, et al. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nature genetics. 2009;41(6):739–45. doi: 10.1038/ng.366 19430481; PubMed Central PMCID: PMC2783476.
40. Chassaing N, Sorrentino S, Davis EE, Martin-Coignard D, Iacovelli A, Paznekas W, et al. OTX2 mutations contribute to the otocephaly-dysgnathia complex. Journal of medical genetics. 2012;49(6):373–9. doi: 10.1136/jmedgenet-2012-100892 22577225.
41. Guasch A, Navarrete J, Nass K, Zayas CF. Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. Journal of the American Society of Nephrology: JASN. 2006;17(8):2228–35. doi: 10.1681/ASN.2002010084 16837635.
42. Haymann JP, Stankovic K, Levy P, Avellino V, Tharaux PL, Letavernier E, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clinical journal of the American Society of Nephrology: CJASN. 2010;5(5):756–61. doi: 10.2215/CJN.08511109 20185605; PubMed Central PMCID: PMC2863976.
43. Shah DI, Takahashi-Makise N, Cooney JD, Li L, Schultz IJ, Pierce EL, et al. Mitochondrial Atpif1 regulates haem synthesis in developing erythroblasts. Nature. 2012;491(7425):608–12. doi: 10.1038/nature11536 23135403; PubMed Central PMCID: PMC3504625.
44. Leet JK, Lindberg CD, Bassett LA, Isales GM, Yozzo KL, Raftery TD, et al. High-content screening in zebrafish embryos identifies butafenacil as a potent inducer of anemia. PloS one. 2014;9(8):e104190. doi: 10.1371/journal.pone.0104190 25090246; PubMed Central PMCID: PMC4121296.
45. Gbadegesin RA, Hall G, Adeyemo A, Hanke N, Tossidou I, Burchette J, et al. Mutations in the gene that encodes the F-actin binding protein anillin cause FSGS. Journal of the American Society of Nephrology: JASN. 2014;25(9):1991–2002. doi: 10.1681/ASN.2013090976 24676636; PubMed Central PMCID: PMC4147982.
46. Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308(5729):1801–4. doi: 10.1126/science.1106215 15879175.
47. Hall G, Gbadegesin RA, Lavin P, Wu G, Liu Y, Oh EC, et al. A Novel Missense Mutation of Wilms' Tumor 1 Causes Autosomal Dominant FSGS. Journal of the American Society of Nephrology: JASN. 2015;26(4):831–43. doi: 10.1681/ASN.2013101053 25145932.
48. Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, et al. APOL1 risk variants, race, and progression of chronic kidney disease. The New England journal of medicine. 2013;369(23):2183–96. doi: 10.1056/NEJMoa1310345 24206458.
49. Foster MC, Coresh J, Fornage M, Astor BC, Grams M, Franceschini N, et al. APOL1 variants associate with increased risk of CKD among African Americans. Journal of the American Society of Nephrology: JASN. 2013;24(9):1484–91. doi: 10.1681/ASN.2013010113 23766536; PubMed Central PMCID: PMC3752955.
50. Cooke JN, Bostrom MA, Hicks PJ, Ng MC, Hellwege JN, Comeau ME, et al. Polymorphisms in MYH9 are associated with diabetic nephropathy in European Americans. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2012;27(4):1505–11. doi: 10.1093/ndt/gfr522 21968013; PubMed Central PMCID: PMC3315672.
51. Ito K, Bick AG, Flannick J, Friedman DJ, Genovese G, Parfenov MG, et al. Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circulation research. 2014;114(5):845–50. doi: 10.1161/CIRCRESAHA.114.302347 24379297; PubMed Central PMCID: PMC3982584.
52. Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA. Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. The Journal of biological chemistry. 2008;283(31):21540–9. doi: 10.1074/jbc.M800214200 18505729; PubMed Central PMCID: PMC2490785.
53. Hartleben B, Godel M, Meyer-Schwesinger C, Liu S, Ulrich T, Kobler S, et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. The Journal of clinical investigation. 2010;120(4):1084–96. doi: 10.1172/JCI39492 20200449; PubMed Central PMCID: PMC2846040.
54. Schmitt F, Martinez F, Brillet G, Giatras I, Choukroun G, Girot R, et al. Early glomerular dysfunction in patients with sickle cell anemia. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1998;32(2):208–14. 9708603.
55. Limou S, Nelson GW, Kopp JB, Winkler CA. APOL1 Kidney Risk Alleles: Population Genetics and Disease Associations. Advances in chronic kidney disease. 2014;21(5):426–33. doi: 10.1053/j.ackd.2014.06.005 25168832; PubMed Central PMCID: PMC4157456.
56. Yang Z, Tong Z, Chen Y, Zeng J, Lu F, Sun X, et al. Genetic and functional dissection of HTRA1 and LOC387715 in age-related macular degeneration. PLoS genetics. 2010;6(2):e1000836. doi: 10.1371/journal.pgen.1000836 20140183; PubMed Central PMCID: PMC2816682.
57. Niederriter AR, Davis EE, Golzio C, Oh EC, Tsai IC, Katsanis N. In vivo modeling of the morbid human genome using Danio rerio. Journal of visualized experiments: JoVE. 2013;(78):e50338. doi: 10.3791/50338 23995499; PubMed Central PMCID: PMC3856313.
58. Wang D, Jao LE, Zheng N, Dolan K, Ivey J, Zonies S, et al. Efficient genome-wide mutagenesis of zebrafish genes by retroviral insertions. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(30):12428–33. doi: 10.1073/pnas.0705502104 17640903; PubMed Central PMCID: PMC1924792.
59. Ma L, Murea M, Snipes JA, Marinelarena A, Kruger J, Hicks PJ, et al. An ACACB variant implicated in diabetic nephropathy associates with body mass index and gene expression in obese subjects. PloS one. 2013;8(2):e56193. doi: 10.1371/journal.pone.0056193 23460794; PubMed Central PMCID: PMC3584087.
Štítky
Genetika Reprodukčná medicína
Článek Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density ImputationČlánek AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct MechanismsČlánek A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seqČlánek TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive HematopoiesisČlánek Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 7- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- LINE-1 Retroelements Get ZAPped!
- /p23: A Small Protein Heating Up Lifespan Regulation
- Hairless Streaks in Cattle Implicate TSR2 in Early Hair Follicle Formation
- Ribosomal Protein Mutations Result in Constitutive p53 Protein Degradation through Impairment of the AKT Pathway
- Molecular Clock of Neutral Mutations in a Fitness-Increasing Evolutionary Process
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- The Alternative Sigma Factor SigX Controls Bacteriocin Synthesis and Competence, the Two Quorum Sensing Regulated Traits in
- BMP Inhibition in Seminomas Initiates Acquisition of Pluripotency via NODAL Signaling Resulting in Reprogramming to an Embryonal Carcinoma
- Comparative Study of Regulatory Circuits in Two Sea Urchin Species Reveals Tight Control of Timing and High Conservation of Expression Dynamics
- EIN3 and ORE1 Accelerate Degreening during Ethylene-Mediated Leaf Senescence by Directly Activating Chlorophyll Catabolic Genes in
- Genome Wide Binding Site Analysis Reveals Transcriptional Coactivation of Cytokinin-Responsive Genes by DELLA Proteins
- Sensory Neurons Arouse . Locomotion via Both Glutamate and Neuropeptide Release
- A Year of Infection in the Intensive Care Unit: Prospective Whole Genome Sequencing of Bacterial Clinical Isolates Reveals Cryptic Transmissions and Novel Microbiota
- Inference of Low and High-Grade Glioma Gene Regulatory Networks Delineates the Role of Rnd3 in Establishing Multiple Hallmarks of Cancer
- Novel Role for p110β PI 3-Kinase in Male Fertility through Regulation of Androgen Receptor Activity in Sertoli Cells
- A Novel Locus Harbouring a Functional Nonsense Mutation Identified in a Large Danish Family with Nonsyndromic Hearing Impairment
- Checkpoint Activation of an Unconventional DNA Replication Program in
- A Genetic Incompatibility Accelerates Adaptation in Yeast
- The SMC Loader Scc2 Promotes ncRNA Biogenesis and Translational Fidelity
- Blimp1/Prdm1 Functions in Opposition to Irf1 to Maintain Neonatal Tolerance during Postnatal Intestinal Maturation
- Discovery and Fine-Mapping of Glycaemic and Obesity-Related Trait Loci Using High-Density Imputation
- JAK/STAT and Hox Dynamic Interactions in an Organogenetic Gene Cascade
- Emergence, Retention and Selection: A Trilogy of Origination for Functional Proteins from Ancestral LncRNAs in Primates
- MoSET1 (Histone H3K4 Methyltransferase in ) Regulates Global Gene Expression during Infection-Related Morphogenesis
- Arabidopsis PCH2 Mediates Meiotic Chromosome Remodeling and Maturation of Crossovers
- AAA-ATPase FIDGETIN-LIKE 1 and Helicase FANCM Antagonize Meiotic Crossovers by Distinct Mechanisms
- A Conserved Pattern of Primer-Dependent Transcription Initiation in and Revealed by 5′ RNA-seq
- Tempo and Mode of Transposable Element Activity in Drosophila
- The Shelterin TIN2 Subunit Mediates Recruitment of Telomerase to Telomeres
- SAMHD1 Inhibits LINE-1 Retrotransposition by Promoting Stress Granule Formation
- A Genome Scan for Genes Underlying Microgeographic-Scale Local Adaptation in a Wild Species
- TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive Hematopoiesis
- Analysis of the Relationships between DNA Double-Strand Breaks, Synaptonemal Complex and Crossovers Using the Mutant
- Assessing Mitochondrial DNA Variation and Copy Number in Lymphocytes of ~2,000 Sardinians Using Tailored Sequencing Analysis Tools
- Allelic Spectra of Risk SNPs Are Different for Environment/Lifestyle Dependent versus Independent Diseases
- CSB-PGBD3 Mutations Cause Premature Ovarian Failure
- Irrepressible: An Interview with Mark Ptashne
- Genetic Evidence for Function of the bHLH-PAS Protein Gce/Met As a Juvenile Hormone Receptor
- Inactivation of Retinoblastoma Protein (Rb1) in the Oocyte: Evidence That Dysregulated Follicle Growth Drives Ovarian Teratoma Formation in Mice
- Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis
- Pyrimidine Pool Disequilibrium Induced by a Cytidine Deaminase Deficiency Inhibits PARP-1 Activity, Leading to the Under Replication of DNA
- Molecular Framework of a Regulatory Circuit Initiating Two-Dimensional Spatial Patterning of Stomatal Lineage
- RFX2 Is a Major Transcriptional Regulator of Spermiogenesis
- A Role for Macro-ER-Phagy in ER Quality Control
- Corp Regulates P53 in via a Negative Feedback Loop
- Common Cell Shape Evolution of Two Nasopharyngeal Pathogens
- Contact- and Protein Transfer-Dependent Stimulation of Assembly of the Gliding Motility Machinery in
- Endothelial Snail Regulates Capillary Branching Morphogenesis via Vascular Endothelial Growth Factor Receptor 3 Expression
- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Temporal Coordination of Carbohydrate Metabolism during Mosquito Reproduction
- mTOR Directs Breast Morphogenesis through the PKC-alpha-Rac1 Signaling Axis
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
- Cooperation between Paxillin-like Protein Pxl1 and Glucan Synthase Bgs1 Is Essential for Actomyosin Ring Stability and Septum Formation in Fission Yeast
- Encodes a Highly Conserved Protein Important to Neurological Function in Mice and Flies
- Identification of a Novel Regulatory Mechanism of Nutrient Transport Controlled by TORC1-Npr1-Amu1/Par32
- Aurora-A-Dependent Control of TACC3 Influences the Rate of Mitotic Spindle Assembly
- Large-Scale Phenomics Identifies Primary and Fine-Tuning Roles for CRKs in Responses Related to Oxidative Stress
- TFIIS-Dependent Non-coding Transcription Regulates Developmental Genome Rearrangements
- Genome-Wide Reprogramming of Transcript Architecture by Temperature Specifies the Developmental States of the Human Pathogen
- Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish
- The Catalytic and Non-catalytic Functions of the Chromatin-Remodeling Protein Collaborate to Fine-Tune Circadian Transcription in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Functional Constraint Profiling of a Viral Protein Reveals Discordance of Evolutionary Conservation and Functionality
- Reversible Oxidation of a Conserved Methionine in the Nuclear Export Sequence Determines Subcellular Distribution and Activity of the Fungal Nitrate Regulator NirA
- Modeling Implicates in Nephropathy: Evidence for Dominant Negative Effects and Epistasis under Anemic Stress
- Nutritional Control of DNA Replication Initiation through the Proteolysis and Regulated Translation of DnaA
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



