-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
article has not abstract
Published in the journal: Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?. PLoS Pathog 6(2): e32767. doi:10.1371/journal.ppat.1000795
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1000795Summary
article has not abstract
Caspase-1 Activation: More Common among Enteropathogens than We Thought?
Caspase-1 is an important converging point for danger signals initiating inflammation and defense. Recent work suggests that RhoGTPase activation and/or cytoskeletal disturbance may represent a novel pathway eliciting caspase-1 responses that are subverted by several enteropathogenic bacteria. The enteropathogen Salmonella Typhimurium employs the type III effector protein SopE, an activator (guanine nucleotide exchange factor [GEF]) for RhoGTPases, to elicit caspase-1 maturation and release of the pro-inflammatory cytokine IL-1β to trigger gut inflammation in vivo. Recently, a whole new family of pathogen-encoded RhoGTPase GEFs has been discovered, including Map, IpgB1, and IpgB2 from enteropathogenic Escherichia coli and Shigella spp.. We will discuss the evidence suggesting that these virulence factors may also activate caspase-1 signaling.
Caspase-1 Integrates Multiple Danger Signals
Mucosal surfaces are constantly exposed to a large number of commensal, non-pathogenic microorganisms towards which the gut immune system remains non-responsive [1]. In the case of an acute infection, this homeostasis is overruled and an inflammatory response is initiated. Detection of pathogen-derived molecules, bacterial growth, or other “danger signals” indicating infection or trauma occurs via “pattern recognition receptors” and other detectors on the surface and in the cytosol of mucosal cells and most other cells of the body [2],[3]. This detection process induces the production of pro-inflammatory cytokines, which initiate the innate immune defense. Caspase-1 represents an important converging point for processing danger signals: physical stress, pore-forming toxins, extracellular ATP, and the presence of conserved bacterial products (e.g., flagellin or peptidoglycan) in the cytosol are detected via danger-sensing molecules and adaptors [4]. These bind to caspase-1 and lead to the formation of multiprotein complexes called inflammasomes [5]. Thereby, caspase-1 is activated and catalyzes the maturation and release of pro-inflammatory cytokines like interleukin (IL)-1β and IL-18 (Figure 1A). Thus, caspase-1 activation is a central regulator of the innate immune defense. Recent work has indicated that the activation of RhoGTPases, in particular Rac1 and possibly Cdc42, might represent a novel type of signaling input that can activate caspase-1 signaling and which may represent a point of attack for bacterial virulence factors (Figure 1A, right, [6]–[8]).
Fig. 1. Danger-associated signaling via the inflammasome. 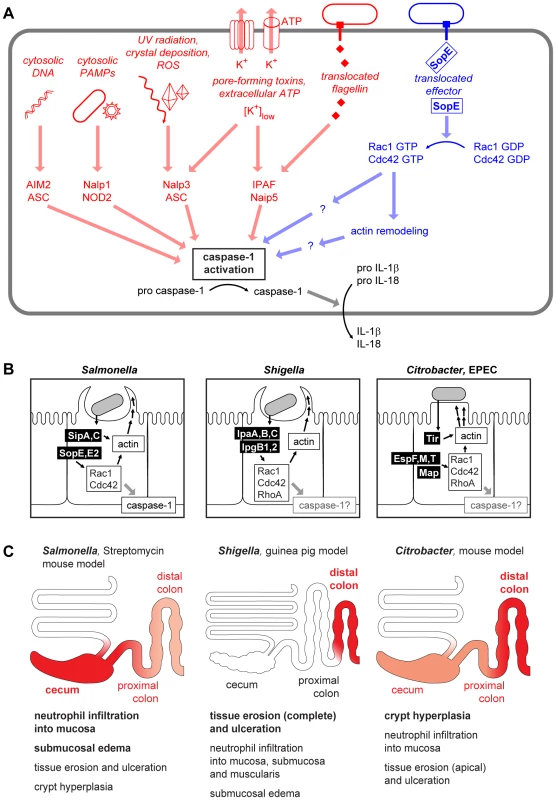
(A) Cytosolic microbe-associated molecular patterns and danger signals are detected and integrated by an array of inflammasome components converging at the activation of caspase-1 [5], [38]–[40]. In addition to the already known stimuli for inflammasome activation (left, red), a pathway involving S. Typhimurium SopE and Rho GTPases (right, blue) has been described recently [8]. (B) Comparison of the virulence mechanisms of Salmonella spp., Shigella spp., and Citrobacter/EPEC. The effector proteins injected can act both directly on the actin polymerization as well as via the activation of Rho GTPases [41]–[43]. (C) Comparison of pathologies in different animal models of Salmonella, Shigella, and Citrobacter/EPEC infection. The region of the strongest pathology and the most important pathological changes in each model is indicated in red. Representative images for the macroscopic and histological changes in the respective animal models can be found in references [44]–[48]. SopE, a Potent Activator of Host Cellular RhoGTPases
SopE from the enteropathogenic bacterium Salmonella enterica subspecies I serovar Typhimurium (S. Typhimurium) is a well-known functional mimic of mammalian GEFs [9]. SopE is delivered into host cells via the bacterial type III secretion system-1 (TTSS-1), binds to host cellular RhoGTPases, including Rac1 and Cdc42, and activates them by catalyzing rapid G-nucleotide exchange [9],[10].
The activity of cellular RhoGTPases depends on guanine nucleotide binding and is regulated by various cellular proteins. Rho-specific cellular GEFs stimulate the nucleotide exchange on RhoGTPases. Upon release of GDP and subsequent binding of GTP, the RhoGTPase switches to its active conformation and is now able to signal to downstream effectors [11]. By mimicking the function of endogenous GEFs, SopE disturbs the RhoGTPase activation cycle and initiates signaling downstream of Rac1 and Cdc42, respectively [9].
SopE is the prototype of a family of bacterial RhoGTPase GEFs that also includes SopE2 and BopE from Salmonella and Burkholderia spp. (Table 1, Figure 2A). In spite of the functional similarity, SopE-like GEFs have an entirely different three-dimensional structure than the Dbl-like mammalian GEFs for RhoGTPases (Figure 2B, [12]). SopE is composed of two three-helix bundles and a small GA-rich loop in the catalytic center. Nonetheless, several amino acid contacts with the RhoGTPase and the basic catalytic mechanism are shared with Dbl-like mammalian GEFs [12],[13]. Moreover, both GEF families bind to the same surface of the RhoGTPase, i.e., the “switch 1” and “switch 2” regions that facilitate G-nucleotide binding [12],[14]. Thus, SopE-like GEFs of pathogenic bacteria are “functional mimics” of host cellular Dbl-like GEFs.
Fig. 2. Sequence and structural comparison of GEFs from different origins. 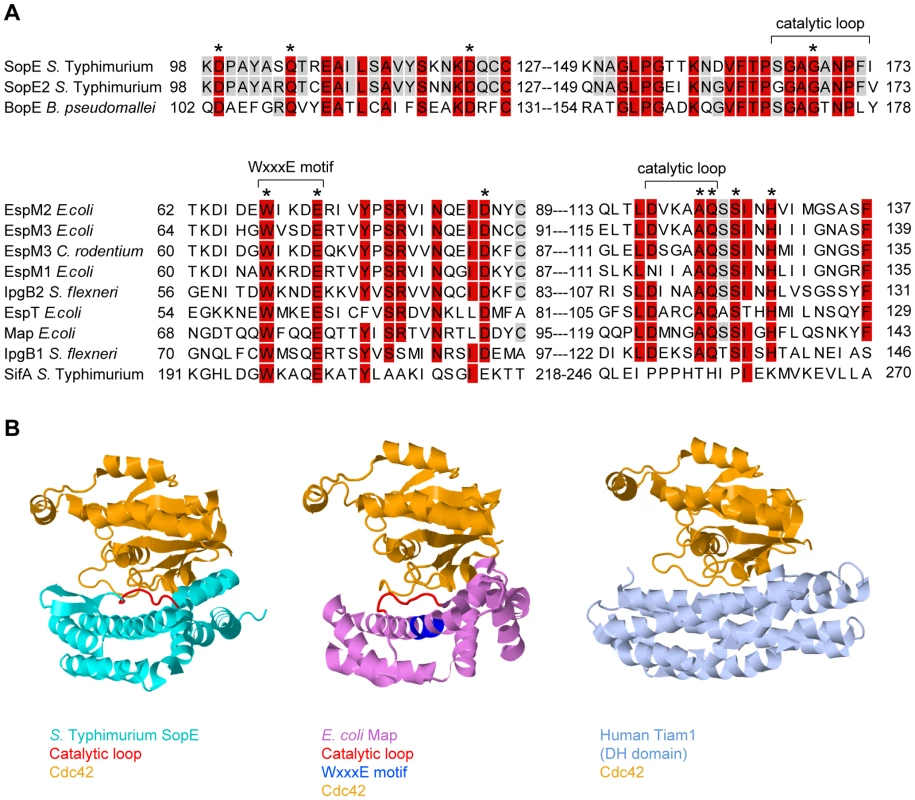
(A) Sequence alignment of SopE-type (upper alignment) and WxxxE-type (lower alignment) bacterial effector proteins with postulated Rho GTPase activity; >80% identity is shaded in red, >50% in grey. The residues shown to be important for catalytic GEF activity of SopE [13] or Map [19] are marked with asterisks. WxxxE motif and catalytic loops highlighted in (B) are marked with brackets. Note that SifA has a completely different amino acid sequence in its catalytic loop compared to the other proteins in the WxxxE family [19]. (B) Crystal structures of GEFs from different organisms in complex with Cdc42 (orange). Left panel: SopE (cyan) from S. Typhimurium [12]; the catalytic loop is highlighted in red. Middle panel: Map (pink) from enteropathogenic E. coli [19]; the WxxxE motif is highlighted in blue and the catalytic loop in red. Right panel: Human Tiam1 (pale blue); only the Rho GTPase binding DH domain is shown [14]. Structures were created using Jmol (http://www.jmol.org/). Tab. 1. Guanine Nucleotide Exchange Factors Involved in Pathogenesis and/or Caspase-1 Activation. 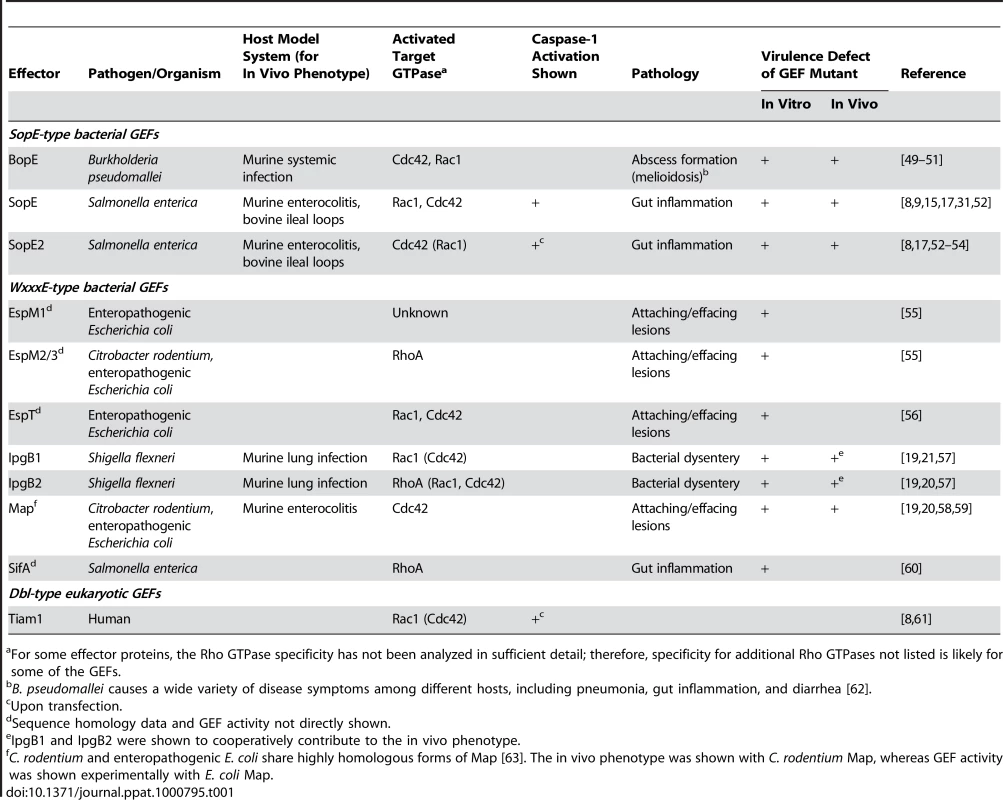
For some effector proteins, the Rho GTPase specificity has not been analyzed in sufficient detail; therefore, specificity for additional Rho GTPases not listed is likely for some of the GEFs. Caspase-1 Activation by SopE
SopE is one of the key TTSS-1 effectors driving host cell invasion and the induction of mucosal inflammation by S. Typhimurium [9], [15]–[17]. SopE was shown to induce a number of host cell responses, including the activation of JNK and PAK signaling and prominent actin cytoskeleton rearrangements [9],[18]. Therefore, it has been assumed that SopE - and RhoGTPase-mediated tissue invasion and/or JNK or PAK activation was sufficient to explain how SopE contributes to gut inflammation. However, recent work has identified a different biological mechanism: SopE-mediated RhoGTPase activation was found to drive not only tissue invasion, but also caspase-1 activation and the release of IL-1β (Figure 1A, right [8]). Caspase-1 was also activated in cells over-expressing constitutively active Rac1, constitutively active Cdc42, or an active mutant of the Dbl-like Rac1-GEF Tiam1 [6],[8]. Moreover, knockout mice lacking caspase-1/IL-1/IL-18 responses still allowed SopE-mediated tissue invasion, but failed to mount mucosal inflammation. In bone marrow chimaeric mice, caspase-1 was required primarily within stromal cells of the gut mucosa, most likely enterocytes [8]. Together, these data indicated that GEF-induced RhoGTPase activation in the mucosal epithelium can elicit caspase-1/IL-1β/IL-18-dependent gut inflammation.
A New Family of Bacterial GEFs
Recently, a second family of bacterial type III effector proteins, the WxxxE family, was found to possess GEF activity for host cellular RhoGTPases [19]. The WxxxE family includes Map from enteropathogenic E. coli (EPEC), IpgB1 and IpgB2 from Shigella, and SifA and SifB from Salmonella spp.. These proteins harbor a unique flexible loop in the catalytic site and an invariant WxxxE motif (Figure 2A). Although phylogenetically unrelated to SopE-like GEFs, the WxxxE family GEFs display a protein fold that is highly similar to that of SopE/E2 and employ the same catalytic mechanism (Figure 2B). Several WxxxE family members, e.g., Map, IpgB1, and IpgB2, have been shown to induce RhoGTPase activity [19]. Interestingly, they differ in their substrate specificities (Table 1, [19],[20]). For example, in vivo, IpgB1 activates Rac1 and Cdc42 [21], whereas IpgB2 preferentially activates RhoA, but also Cdc42 and Rac1 in vitro [19]. This observation suggests that, depending on the preferred target, at least a subset of WxxxE GEFs might serve similar functions as SopE in pro-inflammatory signaling.
Caspase-1: An Achilles' Heel Subverted by Mucosal Pathogens?
The vast majority of SopE or WxxxE family type III effector proteins have been detected in enteropathogenic bacteria (Table 1). In the case of SopE, GEF-induced RhoGTPase activation was shown to elicit caspase-1 responses and the release of caspase-1-dependent cytokines, i.e., IL-1β and IL-18, thus leading to gut inflammation [8]. Based on these data, we hypothesize that the WxxxE family members found in enteropathogenic E. coli and Shigella spp. may have the same biological function, i.e., activating caspase-1 in the host's intestine. Among these effector proteins, GEFs with specificity for Rac1 and Cdc42 are especially good candidates to activate caspase-1, since activated Rac1 (and to a lesser extent Cdc42) has been shown to activate caspase-1 [6]. Thus, GEF-mediated caspase-1 activation might be a common strategy of enteropathogenic bacteria for enhancing mucosal inflammation (Figure 1B). This is of special significance in light of earlier work on Salmonella and Citrobacter infection models showing that enteropathogenic bacteria can benefit from eliciting gut inflammation: although some aspects of the pathology differ between the model systems (e.g., proximal/distal location of the most severe lesions in the large intestine Figure 1C), the host's inflammatory response is thought to suppress growth of the competing commensal microflora in either case [22]–[24]. Therefore, it is tempting to speculate that virulence factors with Rac1 - and/or Cdc42-specific GEF activity might allow enteropathogens to subvert Rho GTPase-mediated caspase-1 activation in order to gain an edge against the commensal microflora.
Analysis of Caspase-1 Activation by Bacterial Effector Proteins—A Tricky Task
In principle, knockout mice deficient in caspase-1, IL-18, or IL-1 signaling allow the analysis of caspase-1 activation by candidate virulence factors. However, this analysis is tricky for two different reasons.
First, caspase-1 activation and IL-1/IL-18 signaling represent an important arm of the innate immune defense (Figure 1A) which helps to clear pathogens and limits further pathogen-inflicted damage [25]–[29]. Therefore, caspase-1, IL-1, or IL-18 deficiency tends to increase pathogen loads in infected tissues, can trigger additional (caspase-1/IL-1/IL-18 independent) pro-inflammatory pathways and amplify disease symptoms. This phenotype has been observed in Shigella and Citrobacter infection experiments and may have masked WxxxE protein-induced effects [25],[30].
Second, there is appreciable functional overlap between the Shigella, Salmonella, and EPEC virulence factors inducing mucosal inflammation. Not all of them require caspase-1 [8],[31]. Therefore, the contribution of caspase-1-dependent virulence factors may be “masked” in wild-type infections, as was shown to be the case in Salmonella infections [8],[28]. The investigation of virulence factors activating caspase-1 thus requires knowledge about functionally overlapping pro-inflammatory mechanisms. These would have to be disrupted in order to unequivocally identify (or refute) a caspase-1-mediated virulence mechanism.
Thus, in the case of pathogens employing SopE or WxxxE family effector proteins, caspase-1, IL-1, or IL-18 deficiency should have two opposing effects: reducing the responsiveness towards the caspase-1-activating virulence factor versus increasing pathogen loads (and possibly damage) in the host's tissue. Therefore, the experiments need to be designed carefully in order to discern these opposing effects. For example, SopE-mediated caspase-1 activation was clearly observed in S. Typhimurium strains lacking other virulence factors, but it was masked in the context of the wild-type pathogen [8],[28]. A similar balance between caspase-1-mediated innate defense and pathogen-induced pathology may have prohibited unequivocal detection of WxxxE protein-mediated caspase-1 activation in the cases of Shigella spp., Citrobacter spp., or enteropathogenic E. coli [30]. Pathogen mutants stripped of all but the caspase-1-dependent virulence factor protein, effector protein expression in a Salmonella mutant stripped of all relevant effector proteins, or tissue culture transfection experiments might help to circumvent this technical problem.
The RhoGTPase–Caspase-1 Connection: What's in Between?
In HEK293 cells, Rac1 and Cdc42 activation is sufficient for activating caspase-1 [6],[8]. However, it has remained unclear whether a specific downstream effector protein of Rac1 and/or Cdc42 or disturbance of the actin cytoskeleton inflicted by the activated RhoGTPases transmits the danger signal towards caspase-1. The Rac1 effector kinase LIMK was suggested to be involved in signaling to caspase-1 [6], and direct interaction of caspase-1 with and phosphorylation by PAK-1 was shown [32]. Other reports support a role for actin and actin-binding proteins. The gelsolin family protein flightless-I binds and inhibits caspase-1, thereby possibly linking caspase-1 activity to the actin cytoskeleton [33]. In macrophages, inflammasome components might be sequestered by perinuclear F-actin structures that form upon certain inhibitory stimuli [34]. The molecular links between Rac1/Cdc42 and inflammasome activation remain to be elucidated.
An Evolutionary Role for Linking Actin Polymerization to Caspase-1 Activation
The detection of microbe-associated molecular patterns alone is not sufficient to induce an inflammatory response in the gut mucosa. Additional signals characteristic of pathogen growth within a tissue or other trauma are thought to be required before the innate immune system responds. A prominent example of such a two-layered control mechanism is the pro-inflammatory cytokine IL-1β. Pro-IL-1β expression is induced upon TLR4 stimulation by bacterial lipopolysaccharide (LPS). However, the pro-IL-1β has to be cleaved and activated by caspase-1 before it is secreted [35]. Caspase-1 in turn is activated in response to numerous danger signals [36]. Manipulation or disruption of the cell cytoskeleton has been proposed recently as a “pattern of pathogenicity” signal that feeds into caspase-1 activation and pro-IL-1β processing [37]. Based on this, it is tempting to speculate that Rac1/Cdc42 activation or actin disturbance itself might represent a pathogen-associated danger signal sensed by the innate immune system. If this held true, the SopE and WxxxE families of type III effector proteins might subvert a pathogen-sensing mechanism for eliciting mucosal inflammation. With knockout mice deficient in caspase-1 and several inflammasome components and a growing number of useful infection models at hand, investigating the involvement of WxxxE GEFs in caspase-1 activation will be an interesting task for future research.
Accession Numbers
The UniProt (http://www.uniprot.org/uniprot/) accession numbers for the protein sequences used for alignment in Figure 2A are O52623 (S. enterica sv. Typhimurium SopE), Q7CQD4 (S. enterica sv. Typhimurium SopE2), Q63K41 (B. pseudomallei BopE), C6URF9 (E. coli EspM2), B5YYM1 (E. coli EspM3), B1GVN9 (C. rodentium EspM3), Q8VQ34 (E. coli EspM1), Q9AJW7 (S. flexneri IpgB2), B9WN88 (E. coli EspT), Q7DB76 (E. coli Map), Q6XVY7 (S. flexneri IpgB1), and Q56061 (S. enterica sv. Typhimurium SifA).
The PDB (http://www.rcsb.org/pdb/) accession codes for the structures shown in Figure 2B are 1gzs for SopE [12], 3cgc for Map [19], and 1foe for Tiam1 [14].
Zdroje
1. LotzM
GutleD
WaltherS
MenardS
BogdanC
2006 Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med 203 973 984
2. KawaiT
AkiraS
2006 TLR signaling. Cell Death Differ 13 816 825
3. ShawMH
ReimerT
KimYG
NunezG
2008 NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol 20 377 382
4. BenkoS
PhilpottDJ
GirardinSE
2008 The microbial and danger signals that activate Nod-like receptors. Cytokine 43 368 373
5. FranchiL
EigenbrodT
Munoz-PlanilloR
NunezG
2009 The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 10 241 247
6. SchotteP
DeneckerG
Van Den BroekeA
VandenabeeleP
CornelisGR
2004 Targeting Rac1 by the Yersinia effector protein YopE inhibits caspase-1-mediated maturation and release of interleukin-1beta. J Biol Chem 279 25134 25142
7. GalleM
SchotteP
HaegmanM
WullaertA
YangHJ
2008 The Pseudomonas aeruginosa Type III secretion system plays a dual role in the regulation of caspase-1 mediated IL-1beta maturation. J Cell Mol Med 12 1767 1776
8. MullerAJ
HoffmannC
GalleM
Van Den BroekeA
HeikenwalderM
2009 The S. Typhimurium Effector SopE Induces Caspase-1 Activation in Stromal Cells to Initiate Gut Inflammation. Cell Host Microbe 6 125 136
9. HardtWD
ChenLM
SchuebelKE
BusteloXR
GalanJE
1998 S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93 815 826
10. RudolphMG
WeiseC
MiroldS
HillenbrandB
BaderB
1999 Biochemical analysis of SopE from Salmonella typhimurium, a highly efficient guanosine nucleotide exchange factor for RhoGTPases. J Biol Chem 274 30501 30509
11. HallA
1994 Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol 10 31 54
12. BuchwaldG
FriebelA
GalanJE
HardtWD
WittinghoferA
2002 Structural basis for the reversible activation of a Rho protein by the bacterial toxin SopE. Embo J 21 3286 3295
13. SchlumbergerMC
FriebelA
BuchwaldG
ScheffzekK
WittinghoferA
2003 Amino acids of the bacterial toxin SopE involved in G-nucleotide exchange on Cdc42. J Biol Chem 278 27149 27159
14. WorthylakeDK
RossmanKL
SondekJ
2000 Crystal structure of Rac1 in complex with the guanine nucleotide exchange region of Tiam1. Nature 408 682 688
15. WoodMW
RosqvistR
MullanPB
EdwardsMH
GalyovEE
1996 SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol Microbiol 22 327 338
16. MiroldS
EhrbarK
WeissmüllerA
PragerR
TschäpeH
2001 Salmonella host cell invasion emerged by acquisition of a mosaic of separate genetic elements, including Salmonella Pathogenicity island 1 (SPI1), SPI5, and sopE2. J Bacteriol 183 2348 2358
17. ZhangS
SantosRL
TsolisRM
StenderS
HardtWD
2002 The Salmonella enterica Serotype Typhimurium Effector Proteins SipA, SopA, SopB, SopD, and SopE2 Act in Concert To Induce Diarrhea in Calves. Infect Immun 70 3843 3855
18. ChenLM
BagrodiaS
CerioneRA
GalanJE
1999 Requirement of p21-activated kinase (PAK) for Salmonella typhimurium-induced nuclear responses. J Exp Med 189 1479 1488
19. HuangZ
SuttonSE
WallenfangAJ
OrchardRC
WuX
2009 Structural insights into host GTPase isoform selection by a family of bacterial GEF mimics. Nat Struct Mol Biol 16 853 860
20. AltoNM
ShaoF
LazarCS
BrostRL
ChuaG
2006 Identification of a bacterial type III effector family with G protein mimicry functions. Cell 124 133 145
21. OhyaK
HandaY
OgawaM
SuzukiM
SasakawaC
2005 IpgB1 is a novel Shigella effector protein involved in bacterial invasion of host cells. Journal of Biological Chemistry 280 24022 24034
22. StecherB
RobbianiR
WalkerAW
WestendorfAM
BarthelM
2007 Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol 5 e244 doi:10.1371/journal.pbio.0050244
23. LuppC
RobertsonML
WickhamME
SekirovI
ChampionOL
2007 Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2 119 129
24. RaffatelluM
GeorgeMD
AkiyamaY
HornsbyMJ
NuccioSP
2009 Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5 476 486
25. SansonettiPJ
PhaliponA
ArondelJ
ThirumalaiK
BanerjeeS
2000 Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12 581 590
26. TsujiNM
TsutsuiH
SekiE
KuidaK
OkamuraH
2004 Roles of caspase-1 in Listeria infection in mice. Int Immunol 16 335 343
27. MariathasanS
WeissDS
DixitVM
MonackDM
2005 Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med 202 1043 1049
28. Lara-TejeroM
SutterwalaFS
OguraY
GrantEP
BertinJ
2006 Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med 203 1407 1412
29. RaupachB
PeuschelSK
MonackDM
ZychlinskyA
2006 Caspase-1-mediated activation of interleukin-1beta (IL-1beta) and IL-18 contributes to innate immune defenses against Salmonella enterica serovar Typhimurium infection. Infect Immun 74 4922 4926
30. LebeisSL
PowellKR
MerlinD
ShermanMA
KalmanD
2009 Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun 77 604 614
31. HapfelmeierS
EhrbarK
StecherB
BarthelM
KremerM
2004 Role of the Salmonella Pathogenicity Island 1 Effector Proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica Subspecies 1 Serovar Typhimurium Colitis in Streptomycin-Pretreated Mice. Infect Immun 72 795 809
32. BasakC
PathakSK
BhattacharyyaA
MandalD
PathakS
2005 NF-kappaB - and C/EBPbeta-driven interleukin-1beta gene expression and PAK1-mediated caspase-1 activation play essential roles in interleukin-1beta release from Helicobacter pylori lipopolysaccharide-stimulated macrophages. J Biol Chem 280 4279 4288
33. LiJ
YinHL
YuanJ
2008 Flightless-I regulates proinflammatory caspases by selectively modulating intracellular localization and caspase activity. J Cell Biol 181 321 333
34. PelegrinP
SurprenantA
2009 Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J 28 2114 2127
35. MariathasanS
NewtonK
MonackDM
VucicD
FrenchDM
2004 Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430 213 218
36. PetrilliV
DostertC
MuruveDA
TschoppJ
2007 The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol 19 615 622
37. VanceRE
IsbergRR
PortnoyDA
2009 Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 6 10 21
38. GurcelL
AbramiL
GirardinS
TschoppJ
van der GootFG
2006 Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126 1135 1145
39. SutterwalaFS
MijaresLA
LiL
OguraY
KazmierczakBI
2007 Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med 204 3235 3245
40. MariathasanS
MonackDM
2007 Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol 7 31 40
41. Bourdet-SicardR
EgileC
SansonettiPJ
Tran Van NhieuG
2000 Diversion of cytoskeletal processes by Shigella during invasion of epithelial cells. Microbes Infect 2 813 819
42. HaragaA
OhlsonMB
MillerSI
2008 Salmonellae interplay with host cells. Nat Rev Microbiol 6 53 66
43. DeanP
KennyB
2009 The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr Opin Microbiol 12 101 109
44. StecherB
PaesoldG
BarthelM
KremerM
JantschJ
2006 Chronic Salmonella enterica Serovar Typhimurium-Induced Colitis and Cholangitis in Streptomycin-Pretreated Nramp1+/+ Mice. Infect Immun 74 5047 5057
45. MundyR
MacDonaldTT
DouganG
FrankelG
WilesS
2005 Citrobacter rodentium of mice and man. Cell Microbiol 7 1697 1706
46. ValdezY
GrasslGA
GuttmanJA
CoburnB
GrosP
2009 Nramp1 drives an accelerated inflammatory response during Salmonella-induced colitis in mice. Cell Microbiol 11 351 362
47. ShimDH
SuzukiT
ChangSY
ParkSM
SansonettiPJ
2007 New animal model of shigellosis in the Guinea pig: its usefulness for protective efficacy studies. J Immunol 178 2476 2482
48. ManganPR
HarringtonLE
O'QuinnDB
HelmsWS
BullardDC
2006 Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441 231 234
49. StevensMP
FriebelA
TaylorLA
WoodMW
BrownPJ
2003 A Burkholderia pseudomallei type III secreted protein, BopE, facilitates bacterial invasion of epithelial cells and exhibits guanine nucleotide exchange factor activity. J Bacteriol 185 4992 4996
50. StevensMP
HaqueA
AtkinsT
HillJ
WoodMW
2004 Attenuated virulence and protective efficacy of a Burkholderia pseudomallei bsa type III secretion mutant in murine models of melioidosis. Microbiology 150 2669 2676
51. UpadhyayA
WuHL
WilliamsC
FieldT
GalyovEE
2008 The guanine-nucleotide-exchange factor BopE from Burkholderia pseudomallei adopts a compact version of the Salmonella SopE/SopE2 fold and undergoes a closed-to-open conformational change upon interaction with Cdc42. Biochem J 411 485 493
52. FriebelA
IlchmannH
AelpfelbacherM
EhrbarK
MachleidtW
2001 SopE and SopE2 from Salmonella typhimurium activate different sets of RhoGTPases of the host cell. Journal of Biological Chemistry 276 34035 34040
53. StenderS
FriebelA
LinderS
RohdeM
MiroldS
2000 Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol Microbiol 36 1206 1221
54. HapfelmeierS
StecherB
BarthelM
KremerM
MüllerA
2005 The Salmonella Pathogenicity Island (SPI)-1 and SPI-2 Type III Secretion Systems Allow Salmonella Serovar Typhimurium to trigger Colitis via MyD88-Dependent and MyD88-Independent Mechanisms. J Immunol 174 1675 1685
55. ArbeloaA
BulginRR
MacKenzieG
ShawRK
PallenMJ
2008 Subversion of actin dynamics by EspM effectors of attaching and effacing bacterial pathogens. Cell Microbiol 10 1429 1441
56. BulginRR
ArbeloaA
ChungJC
FrankelG
2009 EspT triggers formation of lamellipodia and membrane ruffles through activation of Rac-1 and Cdc42. Cell Microbiol 11 217 229
57. HachaniA
BiskriL
RossiG
MartyA
MenardR
2008 IpgB1 and IpgB2, two homologous effectors secreted via the Mxi-Spa type III secretion apparatus, cooperate to mediate polarized cell invasion and inflammatory potential of Shigella flexenri. Microbes Infect 10 260 268
58. KennyB
EllisS
LeardAD
WarawaJ
MellorH
2002 Co-ordinate regulation of distinct host cell signalling pathways by multifunctional enteropathogenic Escherichia coli effector molecules. Mol Microbiol 44 1095 1107
59. MaC
WickhamME
GuttmanJA
DengW
WalkerJ
2006 Citrobacter rodentium infection causes both mitochondrial dysfunction and intestinal epithelial barrier disruption in vivo: role of mitochondrial associated protein (Map). Cell Microbiol 8 1669 1686
60. OhlsonMB
HuangZ
AltoNM
BlancMP
DixonJE
2008 Structure and function of Salmonella SifA indicate that its interactions with SKIP, SseJ, and RhoA family GTPases induce endosomal tubulation. Cell Host Microbe 4 434 446
61. MichielsF
HabetsGG
StamJC
van der KammenRA
CollardJG
1995 A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 375 338 340
62. WhiteNJ
2003 Melioidosis. Lancet 361 1715 1722
63. DengW
LiY
VallanceBA
FinlayBB
2001 Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect Immun 69 6323 6335
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR AvidityČlánek Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 2- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Pathogen Entrapment by Transglutaminase—A Conserved Early Innate Immune Mechanism
- Broadly Protective Monoclonal Antibodies against H3 Influenza Viruses following Sequential Immunization with Different Hemagglutinins
- Neutrophil-Derived CCL3 Is Essential for the Rapid Recruitment of Dendritic Cells to the Site of Inoculation in Resistant Mice
- Differentiation, Distribution and γδ T Cell-Driven Regulation of IL-22-Producing T Cells in Tuberculosis
- IFN-α-Induced Upregulation of CCR5 Leads to Expanded HIV Tropism In Vivo
- An Extensive Circuitry for Cell Wall Regulation in
- TgMORN1 Is a Key Organizer for the Basal Complex of
- Direct Presentation Is Sufficient for an Efficient Anti-Viral CD8 T Cell Response
- Immunoelectron Microscopic Evidence for Tetherin/BST2 as the Physical Bridge between HIV-1 Virions and the Plasma Membrane
- A New Nuclear Function of the Glycolytic Enzyme Enolase: The Metabolic Regulation of Cytosine-5 Methyltransferase 2 (Dnmt2) Activity
- Genome-Wide mRNA Expression Correlates of Viral Control in CD4+ T-Cells from HIV-1-Infected Individuals
- Structural and Biochemical Characterization of SrcA, a Multi-Cargo Type III Secretion Chaperone in Required for Pathogenic Association with a Host
- A Major Role for the ApiAP2 Protein PfSIP2 in Chromosome End Biology
- HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR Avidity
- Fis Is Essential for Capsule Production in and Regulates Expression of Other Important Virulence Factors
- Vaccinia Protein F12 Has Structural Similarity to Kinesin Light Chain and Contains a Motor Binding Motif Required for Virion Export
- A Novel Pseudopodial Component of the Dendritic Cell Anti-Fungal Response: The Fungipod
- Efficacy of the New Neuraminidase Inhibitor CS-8958 against H5N1 Influenza Viruses
- Long-Lived Antibody and B Cell Memory Responses to the Human Malaria Parasites, and
- IPS-1 Is Essential for the Control of West Nile Virus Infection and Immunity
- Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
- Ats-1 Is Imported into Host Cell Mitochondria and Interferes with Apoptosis Induction
- Six RNA Viruses and Forty-One Hosts: Viral Small RNAs and Modulation of Small RNA Repertoires in Vertebrate and Invertebrate Systems
- The Syk Kinase SmTK4 of Is Involved in the Regulation of Spermatogenesis and Oogenesis
- Optineurin Negatively Regulates the Induction of IFNβ in Response to RNA Virus Infection
- On the Diversity of Malaria Parasites in African Apes and the Origin of from Bonobos
- Five Questions about Viruses and MicroRNAs
- A Broad Distribution of the Alternative Oxidase in Microsporidian Parasites
- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Peptides Presented by HLA-E Molecules Are Targets for Human CD8 T-Cells with Cytotoxic as well as Regulatory Activity
- Interaction of Rim101 and Protein Kinase A Regulates Capsule
- Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites
- Exacerbated Innate Host Response to SARS-CoV in Aged Non-Human Primates
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
- Universal Features of Post-Transcriptional Gene Regulation Are Critical for Zygote Development
- Highly Differentiated, Resting Gn-Specific Memory CD8 T Cells Persist Years after Infection by Andes Hantavirus
- Arterivirus Nsp1 Modulates the Accumulation of Minus-Strand Templates to Control the Relative Abundance of Viral mRNAs
- Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification
- Quantitative Comparison of HTLV-1 and HIV-1 Cell-to-Cell Infection with New Replication Dependent Vectors
- The Disulfide Bonds in Glycoprotein E2 of Hepatitis C Virus Reveal the Tertiary Organization of the Molecule
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



