-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
Due to the lack of fossil evidence, the timescales of bacterial evolution are largely unknown. The speed with which genetic change accumulates in populations of pathogenic bacteria, however, is a key parameter that is crucial for understanding the emergence of traits such as increased virulence or antibiotic resistance, together with the forces driving pathogen spread. Methicillin-resistant Staphylococcus aureus (MRSA) is a common cause of hospital-acquired infections. We have investigated an MRSA strain (ST225) that is highly prevalent in hospitals in Central Europe. By using mutation discovery at 269 genetic loci (118,804 basepairs) within an international isolate collection, we ascertained extremely low diversity among European ST225 isolates, indicating that a recent population bottleneck had preceded the expansion of this clone. In contrast, US isolates were more divergent, suggesting they represent the ancestral population. While diversity was low, however, our results demonstrate that the short-term evolutionary rate in this natural population of MRSA resulted in the accumulation of measurable DNA sequence variation within two decades, which we could exploit to reconstruct its recent demographic history and the spatiotemporal dynamics of spread. By applying Bayesian coalescent methods on DNA sequences serially sampled through time, we estimated that ST225 had diverged since approximately 1990 (1987 to 1994), and that expansion of the European clade began in 1995 (1991 to 1999), several years before the new clone was recognized. Demographic analysis based on DNA sequence variation indicated a sharp increase of bacterial population size from 2001 to 2004, which is concordant with the reported prevalence of this strain in several European countries. A detailed ancestry-based reconstruction of the spatiotemporal dispersal dynamics suggested a pattern of frequent transmission of the ST225 clone among hospitals within Central Europe. In addition, comparative genomics indicated complex bacteriophage dynamics.
Published in the journal: A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant. PLoS Pathog 6(4): e32767. doi:10.1371/journal.ppat.1000855
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000855Summary
Due to the lack of fossil evidence, the timescales of bacterial evolution are largely unknown. The speed with which genetic change accumulates in populations of pathogenic bacteria, however, is a key parameter that is crucial for understanding the emergence of traits such as increased virulence or antibiotic resistance, together with the forces driving pathogen spread. Methicillin-resistant Staphylococcus aureus (MRSA) is a common cause of hospital-acquired infections. We have investigated an MRSA strain (ST225) that is highly prevalent in hospitals in Central Europe. By using mutation discovery at 269 genetic loci (118,804 basepairs) within an international isolate collection, we ascertained extremely low diversity among European ST225 isolates, indicating that a recent population bottleneck had preceded the expansion of this clone. In contrast, US isolates were more divergent, suggesting they represent the ancestral population. While diversity was low, however, our results demonstrate that the short-term evolutionary rate in this natural population of MRSA resulted in the accumulation of measurable DNA sequence variation within two decades, which we could exploit to reconstruct its recent demographic history and the spatiotemporal dynamics of spread. By applying Bayesian coalescent methods on DNA sequences serially sampled through time, we estimated that ST225 had diverged since approximately 1990 (1987 to 1994), and that expansion of the European clade began in 1995 (1991 to 1999), several years before the new clone was recognized. Demographic analysis based on DNA sequence variation indicated a sharp increase of bacterial population size from 2001 to 2004, which is concordant with the reported prevalence of this strain in several European countries. A detailed ancestry-based reconstruction of the spatiotemporal dispersal dynamics suggested a pattern of frequent transmission of the ST225 clone among hospitals within Central Europe. In addition, comparative genomics indicated complex bacteriophage dynamics.
Introduction
Clinical microbiologists have frequently been astonished by the impressive capability of pathogenic bacteria to acquire novel traits such as antimicrobial resistance. However, the actual speed at which nucleotide substitutions, entire genes, or complex mobile genetic elements are gained and lost in bacterial populations has rarely been determined [1],[2],[3],[4]. A measure of the real-time nucleotide substitution rate in natural populations of pathogenic bacteria would enable the dating of evolutionary events and the reconstruction of a pathogen's demographic history based on DNA sequence variation, which ultimately could provide fundamental insights into the forces driving pathogen emergence and spread [2],[5].
Methicillin-resistant Staphylococcus aureus (MRSA) are a common cause of hospital-acquired infections, imposing a heavy burden on patients and health care resources [6]. The prevention and treatment of such infections has become increasingly difficult due to this bacterium's ability to acquire resistance against all classes of antibiotics. Staphylococcus aureus has long been known to cause local outbreaks and regional epidemics of hospital infections, where the causative strains – identified through bacterial typing – may spread both within and across hospital wards, and among different hospitals [7]. Contemporary typing of S. aureus is performed by using molecular techniques, including DNA macrorestriction (pulsed field gel electrophoresis) and DNA sequence-based methods. Among the latter, multilocus sequence typing (MLST), which indexes variation at seven slowly evolving genetic loci, has been extremely useful to gain a basic understanding of the population structure of S. aureus [8]. While more than 1,400 MLST-based sequence types (ST) have been reported for S. aureus to date, most of this diversity is clustered in a limited number of clonal complexes [8]. The worldwide predominance of a few clonal lineages among MRSA has resulted in the conception that MRSA strains may spread globally very rapidly [9],[10]. However, by investigating the diversity and phylogeography of one such clone (ST5) in greater detail, we have recently detected considerable spatial subdivision among populations from different localities, indicating that the dispersal of this clone over long distances happens rarely in comparison to the frequency at which novel MRSA arise through acquisition of the genetic methicillin-resistance island SCCmec [11].
In the present study, we have investigated the evolutionary history of an MRSA strain that recently emerged in Central Europe. By MLST, this strain is identified as sequence type ST225 (allelic profile, 1-4-1-4-12-25-10), which is a single locus variant of ST5, the presumed ancestor of clonal complex CC5 [8]. While ST225 had been discovered first among isolates collected during the 1990s in the USA [8],[12], it was not detected in any European country before the year 2000 [13],[14],[15],[16],[17],[18]. Since 2001, however, its reported proportional abundance in Germany increased very rapidly [14], and it was also reported from hospitals in neighboring countries [19],[20]. Hence, this strain has a demonstrated ability to spread rapidly and to become predominant in the hospital environment, thereby replacing other MRSA strains that heretofore had been established for years [14]. At the same time, ST225 seems almost entirely restricted to the hospital environment, since it has not been reported from asymptomatic S. aureus carriage outside of hospitals and it is very rarely found among isolates from community-associated MRSA infections; in the latter, sporadic cases, close contacts to hospital patients or staff could not be excluded [21],[22].
We analyzed an international sample of MRSA type ST225 sequenced at 118,804 basepairs per isolate. Based on serial, time-structured samples of DNA sequences, we observed the accumulation of genetic diversity over a few years. By using coalescent (i. e., genealogy-based) methods, we calculated divergence times and reconstructed the pathogen's past demography. Our results are consistent with a scenario of a recent reduction in population size that has caused losses of genetic variation, and a subsequent population expansion of ST225 within Central Europe.
Results/Discussion
Variation within ST225
Isolates affiliated to ST225 – including both, MRSA and methicillin-susceptible S. aureus (MSSA) – display very limited genotypic and phenotypic variability based on contemporary, molecular typing techniques and antimicrobial resistance (Table S2). We used denaturing high-perfomance liquid chromatography (dHPLC) to screen for sequence polymorphisms at 269 genetic loci (predominantly randomly chosen housekeeping genes) from each of 73 S. aureus isolates (Tables S2, S3). Genome fragments investigated were scattered along the S. aureus chromosome and altogether comprised 4.2% (118,804 basepairs) of the genome (Table S3). Polymorphisms were ascertained through subsequent sequence analysis (Table S4a). All isolates belonged to sequence type ST225 or a single locus variant thereof (ST710) and had been isolated between 1994 and 2007 in the USA, the Czech Republic, Denmark, Switzerland, and Germany (Table S2). These analyses revealed 48 bi-allelic polymorphisms (BiPs; i. e., polymorphic sites at which exactly two alleles were observed), including 11 synonymous base substitutions in protein-coding regions, 26 non-synonymous substitutions, 10 substitutions in intergenic regions, and one insertion of a single nucleotide (Tables S4a, S5). The nucleotide diversity, π (the average number of nucleotide differences per site between sequences from two isolates), was 0.00001 for coding regions and 0.00003 for non-coding regions (Table S1). This level of diversity is extremely low; in a similar study on a global sample of S. aureus sequence type ST5 (the founder of clonal complex CC5), we recently discovered ten-fold higher diversity in both, protein-coding and intergenic regions [11]. In 70 ST225 isolates from Europe, we found 41 BiPs, which corresponds to 0.6 BiPs per isolate or 28 differences between any two 2.8 Mbp genomes. A similar level of divergence was recently reported for community-associated MRSA strain ‘USA300’, which, on average, displayed 35 differences between any two out of eight re-sequenced genomes [23]. The dN/dS value (the ratio of changes at non-synonymous sites to changes at synonymous sites) for protein-coding genes in ST225 was 0.77, hence, similar to the value found for ST5 [11]. This high proportion of non-synonymous substitutions is unlikely to represent a signal of selective pressures, but is a consequence of the dynamics of short-term evolution (i. e., evolution which occurs within a few years, see below) [11],[24],[25].
The 48 BiPs enabled the discrimination of 36 haplotypes (i. e., unique combinations of BiP alleles) among the 73 isolates investigated (Table S2). There were only five parsimony informative sites (where derived alleles occurred in >1 haplotype), and four of these were found in isolates from the USA. Consequently, most of the variation was unique to individual haplotypes, and little phylogenetic structure was discerned among European ST225 isolates (Figure 1). The minimum spanning tree based on these BiPs shows a star-like radiation that is rooted at a hypothetical node representing the most recent common ancestor of ST225 and the JH strain (ST105; Figure 1a). This ancestor is affiliated to lineage ST5-K within the ST5 radiation (Figure 1b). It carries a number of derived alleles (listed in Table S4b) that distinguish it from ST5 haplotypes, in agreement with the previous presumption that ST5 was the ancestral genotype within the clonal complex CC5 [8].
Fig. 1. Radiation of ST225. 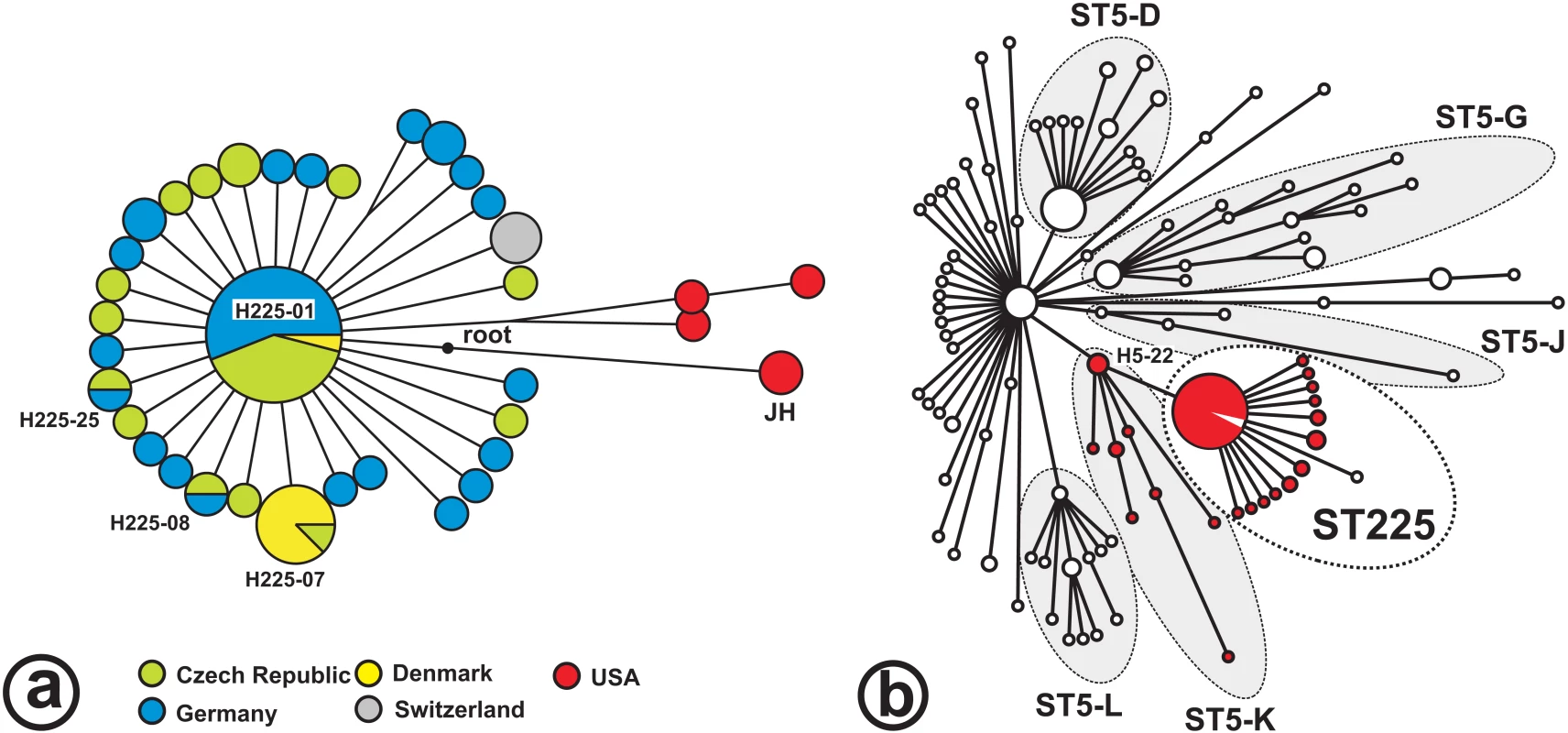
Minimum spanning trees; the tree in Figure 1a is based on 269 loci investigated in 73 ST225 isolates, and the position of the JH strain was resolved based on published genome sequences for isolates JH1 and JH9 [28]. The ancestral node (‘root’) was determined by comparison to genome sequences from more distantly related isolates, including N315 (GenBank accession number BA000018), COL (CP000046), and MW2 (BA000033). Colours indicate the isolates' countries of origin. The tree in Figure 1b is based on 108 loci to show the relationship of ST225 to the previously reported ST5 radiation [11]. Haplotype H5-22 represents two ST5 isolates [11] and the JH strain. Red colour labels isolates harboring prophage ΦSaST5K. Recent origin and long-distance dissemination of ST225-MRSA
All ST225 MRSA isolates that we have investigated, including those from the US, carry a unique 997-basepair deletion in their SCCmec cassettes, which encompasses a 0.3-kb open reading frame (N315-SA0035) and the adjacent direct repeat unit (dru) locus. Deletions of the dru locus have rarely been reported [26],[27]. The presence of this characteristic feature in SCCmec indicates that the most recent common ancestor of the ST225 radiation had already been methicillin-resistant, which suggests that the entire radiation is younger than a few decades. The same dru deletion was present in the genome of the closely related JH strain (ST105, represented by isolates JH1 and JH9 [28], Figure 1), indicating it also existed in the common ancestor of ST225 and ST105, which, hence, already was methicillin-resistant. In addition, we found identical recombinase (ccrB) and helicase (cch) gene sequences in SCCmec from all ST225 MRSA isolates and from the JH genome (not shown), supporting the notion of a common origin. The dru deletion in international isolates also indicates a history of long-distance dissemination of MRSA, since sequence identity in this region would be unlikely if SCCmec elements had been imported repeatedly into locally endemic, methicillin-susceptible ST225 strains. Notably, our methicillin-susceptible isolates could not be distinguished from MRSA based on BiPs (Table S2), lending support to the presumption that they represent strains that have lost methicillin resistance together with parts of their SCCmec elements. Three of these MSSA carried SCCmec remnants in their chromosomes which we detected by PCR and sequencing, including the region with the dru deletion (Table S2). Even those isolates with no detectable traces of SCCmec may be secondary MSSA, however, since spontaneous, precise excision of SCCmec from the staphylococcal chromosome has been reported [29],[30].
There are several arguments why our American isolates of ST225 represent the ancestral population of the European clade. First, US ST225 isolates have been observed as early as 1994 (Table S2), whereas this clone was not encountered before 2000 in Europe. Second, considerable genetic diversity is observed among US isolates even from a single federal state (Wisconsin), with seven SNPs including four parsimony informative sites observed in only three isolates (Figure 1a). This is in stark contrast with the extremely low genetic diversity in European isolates, which suggests a recent population bottleneck (i. e., a brief reduction in population size) associated with the introduction of ST225 into Europe. A population bottleneck occurs, for example, when a small number of individuals founds a new population (‘founder effect’), and may result in a significant loss of genetic variation. Third, American ST225 carry a spa sequence (spa type t002) that is presumably ancestral to spa from European ST225 (t003, t045, t456, t1107; Tables S2a, S2b); the latter spa sequences may have arisen from t002 through deletions of individual repeat units, a frequent phenomenon during DNA replication, whereas the opposite (regain of unique repeats) appears less likely. Spa type t002 was also previously considered ancestral to other spa types based on the presence of a large number of single-repeat variants [31]. Finally, the ST225 radiation branches off from the ST5-K lineage (Figure 1b), to which the majority of ST5 isolates from the USA had been affiliated as reported in our previous study [11].
Taken together, we conclude that ST225 evolved from an MRSA that already carried the dru deletion in its SCCmec element. The novel clone spread to Europe somewhat later, where it rapidly became highly prevalent. The hypothesis of a single transmission event from the US is further supported by the low diversity and the monophyletic structure of the European ST225 radiation (Figure 1). However, current data do not preclude the existence of an ancestral ST225 population outside the US, although no such isolate has been observed so far.
Temporal signal in DNA sequences and dates of divergence
A plot of genetic distance from a common ancestor against sampling time gave a first indication of a measurable accumulation of DNA sequence variation over the sampling time interval (Figures 2a, 2b). Such sets of temporally spaced molecular sequences with a statistically significant number of genetic differences can be used to simultaneously estimate divergence times, temporal changes of population size, and nucleotide substitution rates by applying suitable statistical methods [32]. Based on the sequence variation ascertained, we calculated the age (divergence time) of ST225 by applying a Bayesian coalescent method of phylogenetic inference that incorporated a strict molecular clock model [33]. The relaxed molecular clock model was ruled out as it yielded a posterior distribution of clock rates showing negligible variation (with the standard deviation abutting zero), and was not statistically supported (likelihood ratio test, P = 0.99). Based on our dataset of 73 sequences, the most recent common ancestor of ST225 was estimated to 1990 (95% confidence intervals, 1987 to 1994) (Table 1). The age of the American ST225 clade coincides with the age of the entire ST225 radiation, and the European clade was estimated to have diverged since 1995 (95% confidence intervals, 1991 to 1999) (Table 1). Alternative tree priors (i. e., prior probability distributions) for the Bayesian analysis resulted in very similar time spans (Table 1). Sampling from the prior distribution, in contrast, resulted in hugely inflated divergence times (Table 1), suggesting our results are not mere artefacts reflecting the priors. While it may seem surprising that the little sequence variation discovered may suffice to calculate divergence times with such tight confidence intervals, a test based on random permutation of sampling times across isolates resulted in much older dates and much larger credible intervals (Figure 3), indicating our age calculations were based on a genuine signal in the data [34].
Fig. 2. Increase of DNA sequence variation over the sampling time interval. 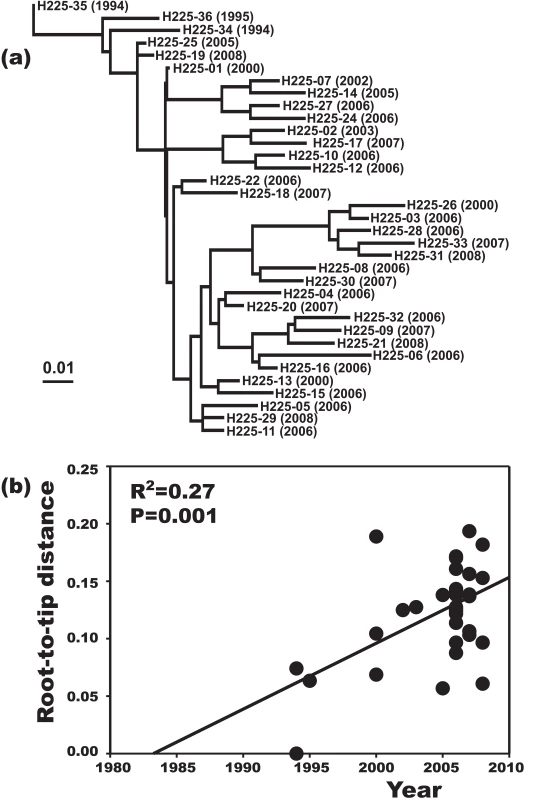
Maximum-likelihood phylogenetic tree based on sequence variation among haplotypes (a). In this tree, each haplotype has a particular distance to the root. In (b), these root-to-tip genetic distances are plotted against sampling dates. The figure illustrates a positive correlation of divergence with sampling date, and, hence, a significant increase of DNA sequence variation over the sampling time interval. Fig. 3. Effect of date permutation on the estimate of divergence time. 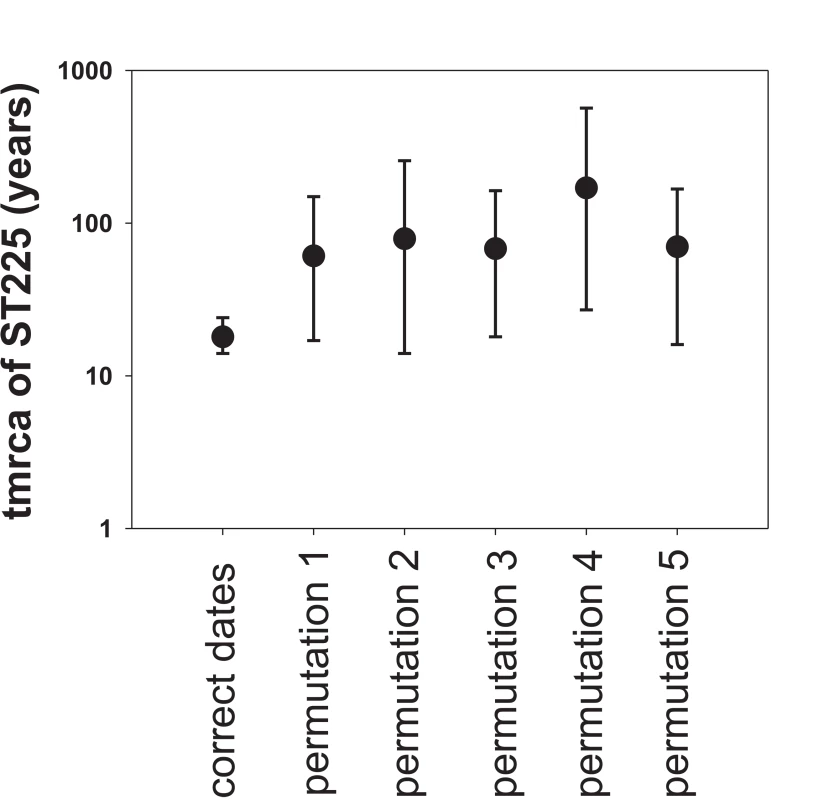
Age of the ST225 clade (tmrca, time since the most recent common ancestor) and 95% confidence intervals on a log scale, determined by Bayesian phylogenetics analysis with the tips constrained by the correct dates and with the dates switched across isolates (permutations 1 to 5). Tab. 1. Results of Bayesian analyses. 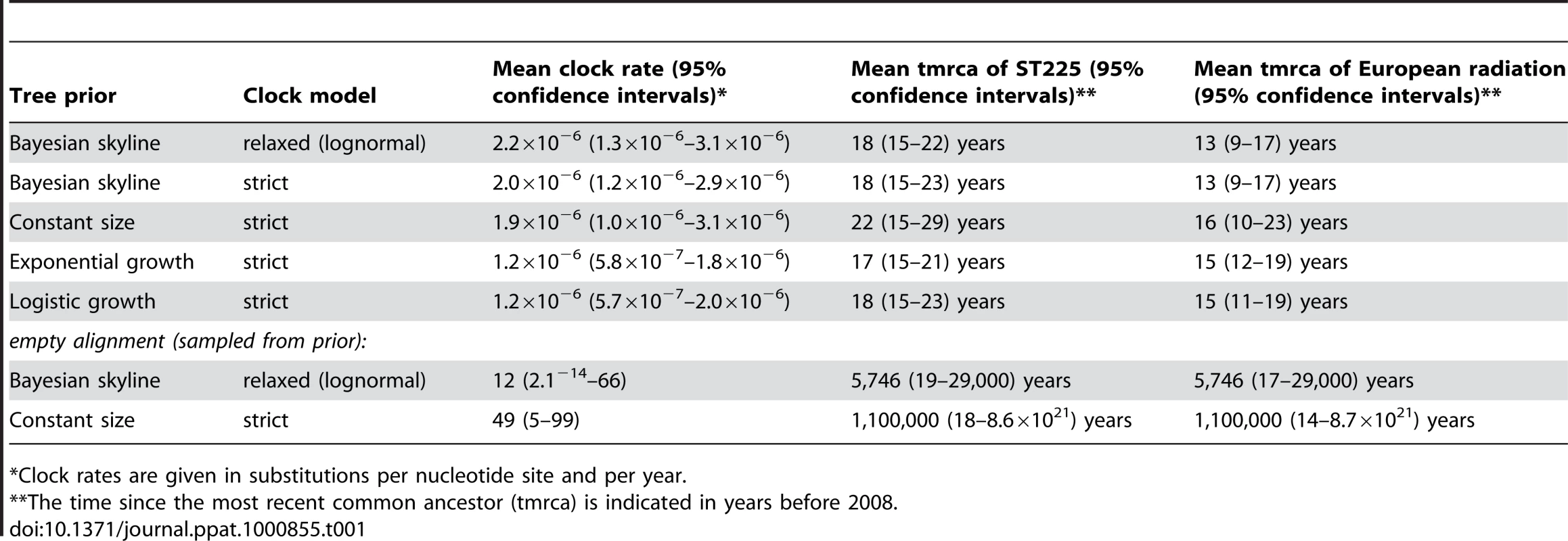
*Clock rates are given in substitutions per nucleotide site and per year. Demographic expansion
The Bayesian skyline plot indicates a very sharp increase of the effective population size starting in 2001, with strong growth continuing for about three years and levelling off thereafter (Figure 4a). This demographic expansion, including the timing of events, is in full agreement with our observation of ST225 abundance in Central Europe (Figure 4b). This scenario is also consistent with a rampant expansion of the clone after its trans-Atlantic spread. The skyline plot (Figure 4a) was not unduly affected by heterogeneity in sample size per year, as indicated by the analyses of ten random subsamples of sequences from each year (Figure S1). However, we cannot exclude that population growth may have been more stochastic during the 1990s than is suggested by the current skyline plot (Figure 4a). To gain more detailed insights into the population structure during this time period, it would be particularly useful to investigate additional American ST225 isolates collected between 1990 and today, which are unfortunately not available at present. The composition of our sample seems to reflect the worldwide population structure of ST225 quite well, since many thousands of MRSA isolates have been genotyped to date in many countries, but no ST225 has ever been found outside Central Europe or the US. In a recent survey based on MLST typing of over 2,000 MRSA isolates sampled from Wisconsin, we did not find a single additional ST225 isolate (unpublished results of SKS). To probe the abundance of ST225 in Germany during the 1990s, we randomly chose 200 isolates from 1997 from the culture archive of the German national reference center for staphylococci and characterized them by spa typing and MLST. None of them was affiliated to ST225, suggesting that, at the time, the strain had been either absent or very rare in Germany.
Fig. 4. Effective population size through time in comparison to surveillance data. 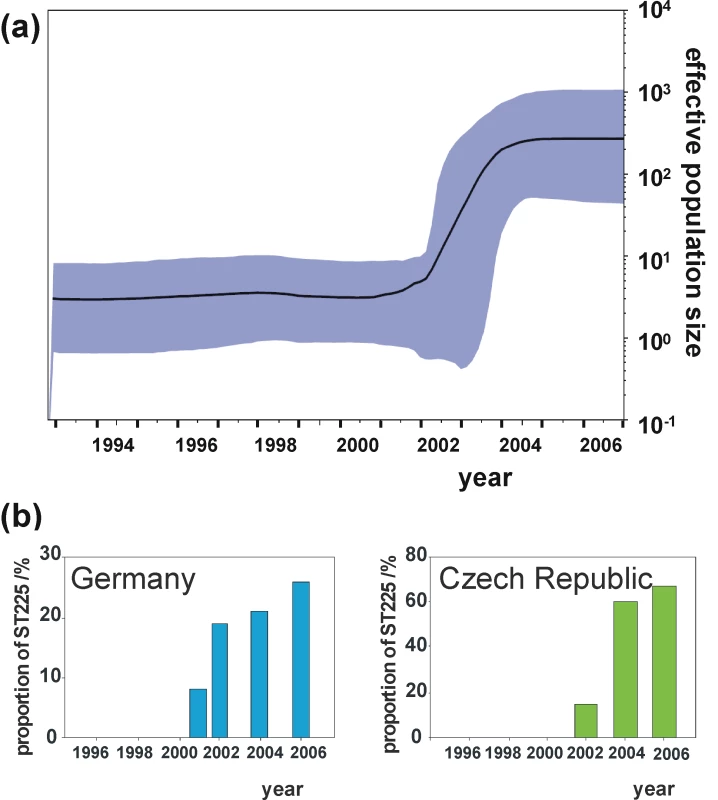
Bayesian skyline plot (Figure 4a), showing the effective population size of ST225 through time (black line), estimated from the concatenated dataset. The shaded area represents 95% confidence intervals. Proportional abundance of ST225 among MRSA in Germany and the Czech Republic (Figure 4b). Data for Germany are based on 2,000 MRSA isolates on average typed per year at the national reference centre for staphylococci. These isolates were received from all over the country and were associated with approximately 10% of all MRSA infections in Germany [66]. Data for the Czech Republic are based on 142 MRSA isolates recovered from blood samples in 13 different hospitals throughout the country. High rates of short-term evolution
The mean nucleotide substitution rate within ST225 was estimated at 2.0×10−6 substitutions per nucleotide site and year (95% confidence intervals, 1.2×10−6 to 2.9×10−6) (Table 1). This short-term evolutionary rate varied only slightly depending on clock model and choice of priors (Table 1), and was also largely confirmed by an alternative method based on a full likelihood model assuming a perfect star genealogy, which gave a rate of 1.1×10−6 (95% confidence intervals, 7.5×10−7 to 1.4×10−6). Even higher upper limits of substitution rates in bacteria have previously been estimated for Neisseria gonorrhoeae (4.6×10−5; [2]), Helicobacter pylori (4.1×10−5; [4]), and Campylobacter jejuni (6.6×10−5; [3]). In contrast to S. aureus, however, these three species are characterized by extremely high rates of homologous recombination, and, hence, part of the polymorphisms observed might have resulted from recombination rather than mutation [2],[3],[4]. Therefore, those reported rates had been considered maximal estimates; in the case of H. pylori, 100-fold lower rates were equally likely [2],[4].
Our rate for MRSA ST225 exceeds an evolutionary rate estimate that had been proposed for Escherichia coli in the past (3×10−8 substitutions per nucleotide site and year) by almost two orders of magnitude [35]. That previous estimate had been based on a laboratory mutation rate of 10−10 per nucleotide site and generation, and the assumption of approximately 300 generations elapsing per year [35]. Mutation frequencies measured in vitro (i. e., the average fraction of individuals carrying a particular resistance mutation in a laboratory culture) are very similar in E. coli and S. aureus [36],[37], suggesting comparable underlying mutation rates (the probability of a mutation to occur in each generation). While ‘mutator’ strains with elevated mutation frequencies have been described, they seem to be uncommon among clinical isolates [38]. A mutator phenotype for ST225 is also not supported by a comparison of whole genome sequences from 04-02981 (ST225, accession number CP001844, see below) and related isolates, including N315, JH1 and JH9 (Figure S2), and additional isolates (our unpublished data). In the genome from 04-02981, we detected no inactivating mutations in any genes involved in DNA replication fidelity, DNA repair mechanisms, or recombination, which are commonly associated with mutator phenotypes [38],[39]. Instead, it seems likely that the massive clonal expansion of ST225 was associated with short bacterial generation times and frequent transmission to new hosts. During rapid demographic expansions, both genetic drift and natural selection will be reduced, thus leading to an increase in the number of mutations segregating in a population, at least transiently [40].
Our results pointing to a rapid clonal evolution of S. aureus suggest that other bacteria may evolve faster than previously acknowledged. It must be considered, however, that observed molecular clock rates are time-dependent [41]. Generally, clock rates decline from initial mutation rates to long-term substitution rates, because the majority of mutations get eliminated with time due to genetic drift and selection [41]. Such rate curves have not yet been determined for bacteria. However, our results imply that recent divergence times of bacteria were possibly overestimated with dating based on the molecular clock rate suggested by Guttman and Dykhuizen [35],[42],[43]. It will be interesting to investigate short-term evolutionary rates in additional clones of S. aureus and other bacterial species. The time dependency of these rates may be established by comparing radiations at different levels of divergence.
Interestingly, the high rates of evolutionary change we found in MRSA caused the accumulation of DNA sequence variation within a few years, a feature that heretofore had been found only in highly recombinant (panmictic) gonococcus [2] and in rapidly evolving viruses [44]. Importantly, the time-structured sampling of DNA sequences within evolutionary timescales enables the application of sophisticated analytical methods, which opens up exciting prospects for investigations of the recent evolutionary history of bacterial pathogens, together with the forces that have shaped their spatial distribution.
Dispersal among hospitals within Central Europe
We have investigated ST225 isolates from four European countries (Table S2, Figure 1a) by reconstructing the most likely ancestry path between isolates to reveal the spatiotemporal dynamic of ST225 spread by applying the SeqTrack algorithm [45]. Interestingly, our results indicate that multiple haplotypes have been introduced into several countries (Figure 1a). Figure 5 represents the cumulative number of isolates from any location (bubbles) and the inferred ancestries (arrows) for successive time windows. Note that while Figure 5 represents the best-supported ancestry path given the sampled isolates, some ancestries might not correspond to actual transmission events, as the true ancestral population might not have been sampled. To avoid any overinterpretation of the results, we restrict our interpretation to the global pattern and some specific unambiguous features of the inferred ancestries.
Fig. 5. Ancestry-based scenario for the spatial spread of ST225. 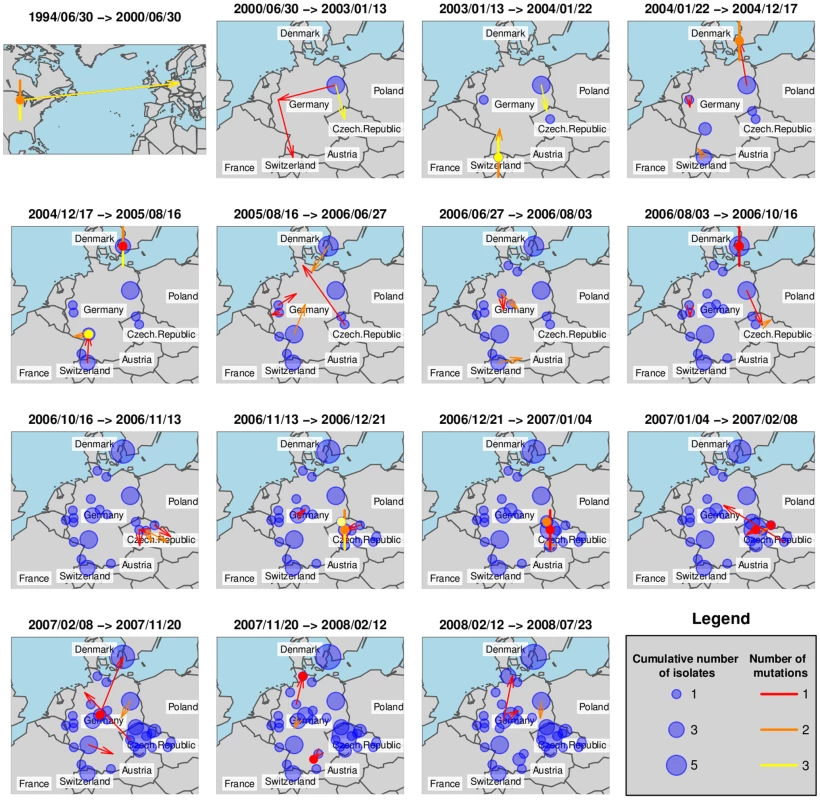
This figure shows the spatial spread of ST225, inferred for successive time windows by applying the SeqTrack algorithm. Each arrow represents an inferred ancestry, pointing from the ancestor to its descendent. Local ancestries are represented by colored dots for single isolates and dots with additional segments representing multiple ancestry events (one segment per isolate). Cumulative numbers of isolates are indicated with blue bubbles. After initial seeding into Europe, ST225 was transmitted to other locations in Germany and to additional European countries (the Czech Republic, Switzerland and Denmark). Some local ancestries (i. e., within the same city) are characterized by a relatively large genetic differentiation (Figure 5, colored dots) suggesting long-term persistence of ST225 within the same location. Another interesting feature of the reconstruction of the spatiotemporal dynamics of ST225 lies in the repeated transmission events between countries. For instance, one isolate from Denmark is assigned an ancestor from Germany with high likelihood (same genotype) at least three years after the first transmission from Germany to Denmark. The first transmission (Figure 5, time window 2004/01/22–2004/12/17) could be traced back epidemiologically to an index patient that had been transferred from a hospital in Germany into a hospital in Copenhagen, Denmark, in 2004, where the carried MRSA strain (haplotype H225-07; Table S2, Figure 1a) later caused an outbreak involving multiple patients and staff. Two additional isolates collected from the same hospital in 2006 and 2007 were affiliated to the same haplotype (Table S2), indicating the clone was still present three years after the initial outbreak. However, a second haplotype (H225-01) was indicated to have been introduced from Germany into Denmark (Figure 5, time window 2007/02/08–2007/11/20), and this is unlikely to be an artefact due to insufficient sampling within Denmark, as several local ancestry events were identified earlier within Denmark.
The SeqTrack results indicated 15 transfers among different countries within Europe (Figure 5). Considering the low informative diversity discovered, the limited number of isolates and countries investigated, and the short time span since emergence of ST225 has started in Europe, this number of detected international transfers of clones is very high. It indicates that cross-border spread of MRSA between the countries considered must have occurred frequently, and, more generally, that the turnover of hospital-associated MRSA is quite rapid even within a larger geographic region (Central Europe). Hence, the question arises how efficient geographic dissemination may be mediated. Abundant international travel will result in occasional hospitalization outside the country of residency, and potential subsequent cross-border patient transfers into the respective home countries. This route is exemplified by the introduction of haplotype H225-07 from Germany into Denmark, with the subsequent establishment of this clone in the hospital for several years. In addition, it is well documented that colonized health-care personnel may promote the spread of MRSA [46]. It is also possible that some spread of ST225 occurs outside of hospitals, even though the lack of community-associated isolates suggests the prevalence to be low [47]. Efficient containment of MRSA spread requires pro-active surveillance and eradication of colonization [46],[48].
Clues from the genome sequence
It is unclear at present, if the success of particular MRSA strains such as ST225 may be due to fortuitous stochastic events or adaptive genetic changes. To reveal any genetic traits that distinguish ST225 from other strains of MRSA and may enable its massive expansion within short time, we sequenced the genome from one representative isolate, MRSA 04-02981 (haplotype H225-01, sequence accession number CP001844). We used both 454 (Roche) and Solexa (Illumina) technology, and closed the genome sequence by using long-PCR and Sanger-sequencing. The final genome sequence likely contains very few sequencing errors, if any, since the application of two independent sequencing approaches resulted in only six conflicting SNP calls. The genome from isolate 04-02981 was found to be co-linear with previously sequenced genomes from related isolates N315 (ST5) and JH1 (ST105) [28],[49]. There was no indication for the presence of any plasmids in isolate 04-02981.
Base substitutions were distributed evenly among genes of different functional categories (not shown). The effects that individual missense mutations may have on protein function are hard to predict in most cases. In the genomes from both, 04-02981 and the JH strain (including isolates JH1 and JH9), two open reading frames were truncated, one of which encodes an unknown, hypothetical protein (N315-SAS092) and another (N315-SA1092) encodes Smf, a protein that has been suspected to be associated with transformation competence. In addition, two open reading frames were uniquely truncated in the genome from 04-02981, encoding an adhesion factor (N315-SA1267) and the transcription regulator norG (N315-SA0104). The latter pseudogene initially appeared particularly interesting, because experimental disruption of this gene had been shown previously to result in a fourfold increase of in vitro resistance to beta-lactam antibiotics [50]. However, after applying a deletion-specific PCR (Table S6), we found that none of the other ST225 isolates in our collection had this deletion. Hence, truncation of norG is not a common trait of ST225, but rather is an idiosyncrasy of isolate 04-02981, which just happened to be the one we had chosen for genome sequencing.
Complex phage dynamics
The genome of isolate 04-02981 contains a stretch of 44 kilobases of DNA that is inserted in a non-coding region downstream of the sufB gene (N315-SA0778), resulting in a duplication of the 67-basepair sequence upstream of the integration site. The inserted sequence is highly similar (sequence identity, 99.5%) to an as yet unnamed prophage previously found in the JH strain at the same genomic position [28]. It shares 50% or less overall sequence similarity to other phage genomes sequenced previously, including Φ11 from S. aureus NCTC8325 [28]. The prophage contains 68 predicted open reading frames, 19 of which encode proteins for basic phage functionality, and 49 of which have unknown functions. None of them has similarities to any known or presumed virulence factors.
By using PCRs targeting five specific regions (Table S6), we detected the presence of this prophage in all European ST225 isolates investigated and in other isolates affiliated to lineage ST5-K, but not in any other ST5 strains (Figure 1b). Thus, this particular prophage is specific to lineage ST5-K and its descendants, and we thus named it ΦSaST5K. Of note, prophage ΦSaST5K was not detected in any of our three ST225 isolates from the US, and, hence, it must have been lost by their common ancestor. There is a second phage – ΦN315 – in the genome of 04-02981, which it shares with isolate N315, an MRSA from Japan that is affiliated to lineage ST5-G [11]. In the JH strain, however, ΦN315 has been replaced apparently by another, dissimilar phage [28], and JH1 and JH9 harbor two additional prophages that have as yet not been seen in any other sequenced S. aureus genomes (Figure S2, Table S8b). This comparison of only three closely related MRSA genomes already points to the existence of complex phage dynamics, with varying apparent half-lives of prophages in their respective bacterial host chromosomes.
Our data indicates that several phages are associated to ST225 and its ancestral lineage, and may have played a role for its evolution. Bacteriophages have been suspected to promote the spread of pathogenic bacteria, by using various potential mechanisms. For example, phage genes may be directly implicated in immune evasion or virulence [51], or indirectly by affecting in trans the activity of bacterial genes outside the prophage, which in turn may enhance transmission or affect other fitness-related traits [52]. Alternatively, phages may possibly impact on competition between strains of staphylococci by driving lysis of bacterial cells that do not carry a related lysogenic phage.
Prospects
We have shown that a strain of MRSA has accumulated measurable genetic change within an epidemiological timescale. The high short-term evolutionary rate in this MRSA enabled the estimation of divergence times and analyses of past changes in population size based on time-structured, serial DNA sequence samples, which heretofore had been possible only for highly recombinant gonococci and viruses. Moreover, ancestry reconstruction revealed the history of geographic spread of this MRSA at unprecedented detail. Confirmation of higher than expected short-term substitution rates in a wider range of bacterial pathogens, together with the tangible prospect of whole-genome sequences for large numbers of related isolates [53],[54] could prefigure a golden age for bacterial epidemiology. Presumably, bacterial pathogens will soon be amenable to detailed investigation of their recent evolutionary history and spread. At the same time, abundant polymorphisms will be discovered that will be useful for bacterial typing in epidemiological surveillance [55],[56],[57].
Methods
Bacterial isolates
Sources and properties of 73 isolates of S. aureus are listed in Table S2a. Susceptibilty to antibiotics was tested by using the broth microdilution method according to the DIN58940 instructions [58] and bacterial typing was performed as described previously [31].
Genome sequencing
Draft genome sequences were generated and assembled commercially. 454 sequencing was performed on a GS FLX machine at 454/Roche in Branford, CT, USA, providing 32-fold average coverage of the staphylococcal chromosome and resulting in 42 initial contigs with >500 basepairs. Solexa sequencing was performed on a Genome Analyzer System at GATC in Konstanz, Germany, generating paired-end reads that were mapped onto the N315 genome sequence at 49-fold average coverage. Remaining gaps between contigs were closed by PCR using Hot Taq DNA polymerase (Peqlab, Germany) or long PCR using the Expand Long Template PCR System (Roche), respectively, and subsequent Sanger sequencing (primers in Table S7). Comparisons of contigs and genomes were performed by using Kodon software (Applied Maths, Belgium). After correcting sequences at contig ends and within repetitive elements, there were 468 sequence differences to N315, including base substitutions, insertions, and deletions (Tables S8A–S8D, Figure S2). Sequence differences to N315 that were shared between ST225 and the JH strain were considered correct since matching data had been generated in an independent study [28]. For insertions in the sequenced genome, we relied on 454 data, since they could not be detected among Solexa reads mapped against the N315 genome (Tables S8A–S8D). Gene annotation was performed automatically using the RAST server [59] and corrected manually using Kodon and Artemis software [60]. The annotated genome sequence from isolate 04-02981 was submitted to GenBank (accession number CP001844).
Mutation discovery by dHPLC
Mutation discovery was performed as described previously [11]. PCR primers used for amplification and sequencing are listed in Table S3. A minimum spanning tree based on BiPs was constructed with Bionumerics 5.1. The ancestral node was determined by comparison to genome sequences from isolates N315 and JH1.
PCR
PCR amplification of regions including the dru deletion, the four-basepair deletion within norG, SCCmec remnants, and prophage-specific fragments, respectively, were performed by using Hot Taq DNA polymerase (Peqlab, Germany) according to the manufacturer's instructions and by using the primers listed in Table S6.
Regression of root-to-tip distances against sampling dates
Based on an alignment of polymorphic sites in protein-coding sequences, a maximum likelihood tree was calculated by using Treefinder software (available at www.treefinder.de), applying the HKY model of DNA substitution. Rooting of the tree and linear regression of root-to-tip distances against dates of first haplotype appearance was performed by using Path-O-Gen software (available at http://tree.bio.ed.ac.uk/software/pathogen/), and the significance of the correlation was determined with SigmaPlot 11.0 (SPSS).
Likelihood ratio test
To assess whether nucleotide substitution rates in protein-coding sequences departed significantly from expectations under a strict molecular clock, we used a likelihood ratio test, based on a comparison of likelihood scores for maximum-likelihood trees calculated by using PAUP, with and without a molecular clock enforced. The statistical significance of the difference between likelihood scores was determined by assuming a chi-square distribution and s-2 degrees of freedom, where s was the number of sequences [61].
Bayesian analyses
Evolutionary rates, divergence times, and Bayesian skyline plots were computed with the BEAST software (available at http://beast.bio.ed.ac.uk/) [62], using the HKY model of nucleotide substitution and a strict clock model (unless stated otherwise), with concatenated protein-coding sequences (108,261 basepairs) dated based on the year of isolate sampling, and with 108 iterations after a burn-in phase of 106 iterations. Markov chain Monte Carlo samples from three independent analyses were combined for estimation of posteriors, resulting in effective sample size values greater than 1,000 for all parameters. Various prior sets were used as indicated (Table 1). To test if date estimates were unduly influenced by prior assumptions, analyses were re-run (5×107 iterations) on each of five datasets generated by randomly switching sampling dates across isolates. To sample from the prior distributions, analyses were run on an empty alignment. Further, to test if the resulting Bayesian skyline plot was confounded by temporal variation in sample size, we generated and analysed (107 iterations) a series of datasets by subsampling from time classes and randomly drawing four isolates from each year.
Nucleotide substitution-rate estimate assuming a star genealogy
For an alternative rate estimate, we used a full likelihood model assuming that demographic expansion was strong enough to result in a perfect star genealogy (i.e., without any coalescent events). To avoid violation of this assumption, we analysed protein-encoding loci (108,261 basepairs) from 58 European isolates exclusively, including only one isolate from each haplotype, except for the ancestral haplotype H225-01. Likelihood of the model for each locus was then given by the binomial probability of the number of mutations observed in all isolates, given the sum of the genealogical branch lengths for all isolates (i. e., date of isolate collection - date of expansion start) and a substitution rate parameter per locus and per year. A point multilocus substitution rate estimate (per nucleotide site and per year) and its 95% confidence interval were inferred based on the product of the above-described likelihood function for all loci, considering that all loci had a specific number of sites, were independent, and had a single, constant mutation rate. The procedure was written in R [63] and is available upon request to R. Leblois.
Analysis of spatiotemporal dynamics of spread
The SeqTrack algorithm [45] was used to reconstruct the most plausible scenario for the spatiotemporal spread of the ST225 clone. This new method has been developed to study the dispersal and transmission of emerging pathogens during disease outbreaks, such as the 2009 swine-origin influenza A/H1N1 pandemic [45]. SeqTrack reconstructs the most likely ancestries among sampled strains using their genotype and sampling dates. This method differs fundamentally from phylogenetics in that it does not attempt to infer hypothetical (and unobserved) common ancestors, but rather seeks to reconstruct ancestries directly from the sampled isolates. Because of the low level of genetic variability in ST225 (most strains differ by a single nucleotide from each other), we used a maximum parsimony approach to infer ancestries. Thus, the most likely ancestry path was searched for by minimizing the number of mutations between ancestors and descendents. Whenever several strains were equally likely ancestors of the isolate under consideration, we retained the one that was geographically closest. All analyses were performed using the R software [63]. Raw genetic distances between isolates (in terms of number of point mutations) were computed using the ape package [64]. SeqTrack analysis was then run using the seqtrack function implemented in the adegenet package [65].
Supporting Information
Zdroje
1. AchtmanM
WagnerM
2008 Microbial diversity and the genetic nature of microbial species. Nature Reviews Microbiology 6 431 440
2. Pérez-LosadaM
CrandallKA
ZenilmanJ
ViscidiRP
2007 Temporal trends in gonococcal population genetics in a high prevalence urban community. Infect Genet Evol 7 271 278
3. WilsonDJ
GabrielE
LeatherbarrowAJ
CheesbroughJ
GeeS
2009 Rapid evolution and the importance of recombination to the gastroenteric pathogen Campylobacter jejuni. Mol Biol Evol 26 385 397
4. FalushD
KraftC
TaylorNS
CorreaP
FoxJG
2001 Recombination and mutation during long-term gastric colonization by Helicobacter pylori: estimates of clock rates, recombination size, and minimal age. Proc Natl Acad Sci U S A 98 15056 15061
5. GilbertMT
RambautA
WlasiukG
SpiraTJ
PitchenikAE
2007 The emergence of HIV/AIDS in the Americas and beyond. Proc Natl Acad Sci U S A 104 18566 18570
6. KleinE
SmithDL
LaxminarayanR
2007 Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis 13 1840 1846
7. WilliamsREO
1959 Epidemic staphylococci. Lancet 1 190 195
8. EnrightMC
RobinsonDA
RandleG
FeilEJ
GrundmannH
2002 The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci U S A 99 7687 7692
9. HiramatsuK
CuiL
KurodaM
ItoT
2001 The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol 9 486 493
10. CrisóstomoMI
WesthH
TomaszA
ChungM
OliveiraDC
2001 The evolution of methicillin resistance in Staphylococcus aureus: similarity of genetic backgrounds in historically early methicillin-susceptible and -resistant isolates and contemporary epidemic clones. Proc Natl Acad Sci U S A 98 9865 9870
11. NübelU
RoumagnacP
FeldkampM
SongJH
KoKS
2008 Frequent emergence and limited geographic dispersal of methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 105 14130 14135
12. ShuklaSK
StemperME
RamaswamySV
ConradtJM
ReichR
2004 Molecular characteristics of nosocomial and Native American community-associated methicillin-resistant Staphylococcus aureus clones from rural Wisconsin. J Clin Microbiol 42 3752 3757
13. MelterO
Aires de SousaM
UrbaskovaP
JakubuV
ZemlickovaH
2003 Update on the major clonal types of methicillin-resistant Staphylococcus aureus in the Czech Republic. J Clin Microbiol 41 4998 5005
14. WitteW
KlareI
NübelU
StrommengerB
WernerG
2008 Emergence and spread of antibiotic resistant Gram positive bacterial pathogens. International Journal of Medical Microbiology 298 365 377
15. JohnsonAP
PearsonA
DuckworthG
2005 Surveillance and epidemiology of MRSA bacteraemia in the UK. J Antimicrob Chemother 56 455 462
16. DauwalderO
LinaG
DurandG
BesM
MeugnierH
2008 Epidemiology of invasive methicillin-resistant Staphylococcus aureus clones collected in France in 2006 and 2007. J Clin Microbiol 46 3454 3458
17. ConceicaoM
Aires de SousaM
FüziM
TóthÁ
PásztiJ
2007 Replacement of methicilln-resistant Staphylococcus aureus clones in Hungary over time: a 10-year surveillance study. Clin Microbiol Infect 13 971 979
18. DeurenbergRH
VinkC
OudhuisGJ
MooijJE
DriessenC
2005 Different clonal complexes of methicillin-resistant Staphylococcus aureus are disseminated in the Euregio Meuse-Rhine regions. Antimicrob Agents Chemother 49 4263 4271
19. BartelsMD
BoyeK
LarsenAR
SkovR
WesthH
2007 Rapid increase of genetically diverse methicillin-resistant Staphylococcus aureus, Copenhagen, Denmark. Emerg Infect Dis 13 1533 1540
20. QiW
EnderM
O'BrienF
ImhofA
RuefC
2005 Molecular epidemiology of methicillin-resistant Staphylococcus aureus in Zürich, Switzerland (2003): prevalence of type IV SCCmec and a new SCCmec element associated with isolates from intravenous drug users. J Clin Microbiol 43 5164 5170
21. FariaNA
OliveiraDC
WesthH
MonnetDL
LarsenAR
2005 Epidemiology of emerging methicillin-resistant Staphylococcus aureus (MRSA) in Denmark: a nationwide survey in a country with low prevalence of MRSA infection. J Clin Microbiol 43 1836 1842
22. HanssenAM
FossumA
MikalsenJ
HalvorsenDS
BukholmG
2005 Dissemination of communiy-acquired methicillin-resistant Staphylococcus aureus clones in northern Norway: sequence types 8 and 80 predeominate. J Clin Microbiol 43 2118 2124
23. KennedyAD
OttoM
BraughtonKR
WhitneyAR
ChenL
2008 Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci U S A 105 1327 1332
24. RochaEP
SmithJM
HurstLD
HoldenMT
CooperJE
2006 Comparisons of dN/dS are time dependent for closely related bacterial genomes. J Theor Biol 239 226 235
25. KryazhimskiyS
PlotkinJB
2008 The population genetics of dN/dS. PLoS Genetics 4 e1000304 doi:10.1371/journal.pgen.1000304
26. GoeringRV
MorrisonD
Al-DooriZ
EdwardsGFS
GemmellCG
2008 Usefulness of mec-associated direct repeat unit (dru) typing in the epidemiolological analysis of highly clonal methicillin-resistant Staphylococcus aureus in Scotland. Clin Microbiol Infect 14 964 969
27. OliveiraDC
WuSW
De LencastreH
2000 Genetic organization of the downstream region of the mecA element in methicillin-resistant Staphylococcus aureus isolates carrying different polymorphisms of this region. Antimicrob Agents Chemother 44 1906 1910
28. MwangiMM
WuSW
ZhouY
SieradzkiK
de LencastreH
2007 Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci U S A 104 9451 9456
29. KatayamaY
ItoT
HiramatsuK
2000 A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 44 1549 1555
30. NotoMJ
FoxPM
ArcherGL
2008 Spontaneous deletion of the methicillin resistance determinant, mecA, partially compensates for the fitness cost associated with high-level vancomycin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 52 1221 1229
31. StrommengerB
BraulkeC
HeuckD
SchmidtC
PasemannB
2008 Spa-typing of Staphylococcus aureus as a frontline tool in epidemiological typing. J Clin Microbiol 46 574 581
32. DrummondAJ
PybusOG
RambautA
ForsbergR
RodrigoAG
2003 Measurably evolving populations. Trends Ecol Evol 18 481
33. DrummondAJ
HoSYW
PhillipsMJ
RambautA
2006 Relaxed phylogenetics and dating with confidence. PLoS Biol 4 e88 doi:10.1371/journal.pbio.0040088
34. LowderB
GuinaneCM
Ben ZakourNL
WeinertL
Conway-MorrisA
2009 Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc Natl Acad Sci U S A 106 19545 19550
35. GuttmanDS
DykhuizenDE
1994 Clonal divergence in Escherichia coli as a result of recombination, not mutation. Science 266 1380 1383
36. O'NeillAJ
CoveJH
ChopraI
2001 Mutation frequencies for resistance to fusidic acid and rifampicin in Staphylococcus aureus. J Antimicrob Chemother 47 647 650
37. Al MamunAAM
2007 Elevated expression of DNA polymerase II increases spontaneous mutagenesis in Escherichia coli. Mutat Res 625 29 39
38. O'NeillAJ
ChopraI
2002 Insertional inactivation of mutS in Staphylococcus aureus reveals potential for elevated mutation frequencies, although the prevalence of mutators in clinical isolates is low. J Antimicrob Chemother 50 161 169
39. HorstJ-P
WuT
MarinusMG
1999 Escherichia coli mutator genes. Trends Microbiol 7 29 36
40. HahnMW
RausherMD
CunninghamCW
2002 Distinguishing between selection and population expansion in an experimental lineage of bacteriophage T7. Genetics 161 11 20
41. HoSYW
PhillipsMJ
CooperA
DrummondAJ
2005 Time dependency of molecular rate estimates and systematic overestimation of recent divergence times. Mol Biol Evol 22 1561 1568
42. AchtmanM
ZurthK
MorelliG
TorreaG
GuiyouleA
1999 Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proceedings of the National Academy of Sciences of the United States of America 96 14043 14048
43. ZhangW
QiW
AlbertTJ
MotiwalaAS
AllandD
2006 Probing genomic diversity and evolution of Escherichia coli O157 by single nucleotide polymorphisms. Genome Res 16 757 767
44. HolmesEC
2008 Evolutionary history and phylogeography of human viruses. Annu Rev Microbiol 62 307 328
45. JombartT
EggoRM
DoddPJ
BallouxF
2009 Spatiotemporal dynamics in the early stages of the 2009 A/H1N1 influenza pandemic. PLoS Curr Influenza: RRN1026. Available: http://knol.google.com/k/thibaut-jombart/spatiotemporal-dynamics-in-the-early/11igg07td5uji/1
46. AlbrichWC
HarbarthS
2008 Health-care workers: source, vector, or victim of MRSA? The Lancet 8 289 301
47. WitteW
StrommengerB
CunyC
HeuckD
NübelU
2007 Methicillin-resistant Staphylococcus aureus containing the Panton-Valentine leukocidin gene in Germany in 2005 and 2006. J Antimicrob Chemother 60 1258 1263
48. BootsmaMCJ
DiekmannO
BontenMJM
2006 Controlling methicillin-resistant Staphylococcus aureus: quantifying the effects of interventions and rapid diagnostic testing. Proc Natl Acad Sci U S A 103 5620 5625
49. KurodaM
OhtaT
UchiyamaI
BabaT
YuzawaH
2001 Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 357 1225 1240
50. Truong-BolducQC
HooperDC
2007 The transcriptional regulators NorG and MgrA modulate resistance to both quinolones and β-lactams in Staphylococcus aureus. J Bacteriol 189 2996 3005
51. BaeT
BabaT
HiramatsuK
SchneewindO
2006 Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol Microbiol 62 1035 1047
52. MoxonER
JansenVA
2005 Phage variation: understanding the behaviour of an accidental pathogen. Trends Microbiol 13 563 565
53. MaidenMC
2008 Population genomics: diversity and virulence in the Neisseria. Current Opinion in Microbiology 11 467 471
54. ParkhillJ
2008 Time to remove the model organism blinkers. Trends Microbiol 16 510 511
55. KurtK
AlderbornA
NilssonM
StrommengerB
WitteW
2009 Multiplexed genotyping of methicillin-resistant Staphylococcus aureus isolates by use of padlock probes and tag microarrays. J Clin Microbiol 47 577 585
56. BakerS
HoltK
van de VosseE
RoumagnacP
WhiteheadS
2008 High-throughput genotyping of Salmonella Typhi allows geographical assignment of haplotypes and pathotypes within an urban district of Jakarta, Indonesia. J Clin Microbiol
57. KeimP
Van ErtMN
PearsonT
VoglerAJ
HuynhLY
2004 Anthrax molecular epidemiology and forensics: using the appropriate marker for different evolutionary scales. Infect Genet Evol 4 205 213
58. Anonymous 2004 DIN 58940. Medical microbiology – susceptibility testing of pathogens to antimicrobial agents. Part 8 – microdilution. DIN-Taschenbuch 222: medizinische Mikrobiologie und Immunologie – diagnostische Verfahren Berlin, Germany Deutsches Institut für Normung, Beuth-Verlag
59. AzizRK
BartelsD
BestAA
DeJonghM
DiszT
2008 The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9 75
60. RutherfordK
ParkhillJ
CrookJ
HorsnellT
RiceP
2000 Artemis: sequence visualization and annotation. Bioinformatics 16 944 945
61. FelsensteinJ
1981 Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17 368 376
62. DrummondAJ
RambautA
2007 BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7 214
63. R Development Core Team 2009 R: a language and environment for statistical computing Vienna, Austria R Foundation for Statistical Computing
64. ParadisE
ClaudeJ
StrimmerK
2004 APE: Analysis of phylogenetics and evolution in R language. Bioinformatics 20 289 290
65. JombartT
2008 adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24 1403 1405
66. GastmeierP
GeffersC
2008 [Nosocomial infections in Germany. What are the numbers, based on the estimates for 2006?]. Dtsch Med Wochenschr 133 1111 1115
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument TurnoverČlánek Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic PathwaysČlánek The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-DependentČlánek Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 4- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Innate Recognition of Fungal Cell Walls
- Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument Turnover
- Junín Virus Infection of Human Hematopoietic Progenitors Impairs Proplatelet Formation and Platelet Release via a Bystander Effect Involving Type I IFN Signaling
- The Endosymbiotic Bacterium Induces Resistance to Dengue Virus in
- Natural Regulatory T Cells in Malaria: Host or Parasite Allies?
- Keratinocytes Determine Th1 Immunity during Early Experimental Leishmaniasis
- Spatial and Temporal Association of Outbreaks of H5N1 Influenza Virus Infection in Wild Birds with the 0°C Isotherm
- Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic Pathways
- RNA Polymerase Activity and Specific RNA Structure Are Required for Efficient HCV Replication in Cultured Cells
- The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
- Inadequate Clearance of Translocated Bacterial Products in HIV-Infected Humanized Mice
- Topology and Organization of the Type III Secretion Needle Complex Components
- Temperature Modulates Plant Defense Responses through NB-LRR Proteins
- Peptide Inhibitors of Dengue-Virus Entry Target a Late-Stage Fusion Intermediate
- Identification of Host-Dependent Survival Factors for Intracellular through an siRNA Screen
- Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation
- Increased Asymmetric Dimethylarginine in Severe Falciparum Malaria: Association with Impaired Nitric Oxide Bioavailability and Fatal Outcome
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Induces Brain Microvascular Endothelial Cell Detachment from the Matrix and Cleavage of Occludin: A Role for MMP-8
- Two Coregulated Efflux Transporters Modulate Intracellular Heme and Protoporphyrin IX Availability in
- The Type I NADH Dehydrogenase of Counters Phagosomal NOX2 Activity to Inhibit TNF-α-Mediated Host Cell Apoptosis
- Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
- Increased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma
- The RING-CH Ligase K5 Antagonizes Restriction of KSHV and HIV-1 Particle Release by Mediating Ubiquitin-Dependent Endosomal Degradation of Tetherin
- Molecular Mechanisms of Ethanol-Induced Pathogenesis Revealed by RNA-Sequencing
- Highly Frequent Mutations in Negative Regulators of Multiple Virulence Genes in Group A Streptococcal Toxic Shock Syndrome Isolates
- Emergence and Pathogenicity of Highly Virulent Genotypes in the Northwest United States
- Structural and Functional Analysis of Viral siRNAs
- Prion Shedding from Olfactory Neurons into Nasal Secretions
- a GATA Transcription Factor That Directs Disparate Fates in Including Morphogenesis and Siderophore Biosynthesis
- Three Members of the 6-cys Protein Family of Play a Role in Gamete Fertility
- Complement as an Endogenous Adjuvant for Dendritic Cell-Mediated Induction of Retrovirus-Specific CTLs
- A Genomic Survey of Positive Selection in Provides Insights into the Evolution of Accidental Virulence
- Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes
- Blood Fluke Exploitation of Non-Cognate CD4 T Cell Help to Facilitate Parasite Development
- Antagonism of Tetherin Restriction of HIV-1 Release by Vpu Involves Binding and Sequestration of the Restriction Factor in a Perinuclear Compartment
- The Development of Therapeutic Antibodies That Neutralize Homologous and Heterologous Genotypes of Dengue Virus Type 1
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- Interactions with Bacteria in the Context of Human Health and Disease
- Viral Capsid Is a Pathogen-Associated Molecular Pattern in Adenovirus Keratitis
- Electron Tomography Reveals the Steps in Filovirus Budding
- Selective Condensation Drives Partitioning and Sequential Secretion of Cyst Wall Proteins in Differentiating
- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
- VacA Toxin/Subunit p34: Targeting of an Anion Channel to the Inner Mitochondrial Membrane
- Production of Extracellular Traps against and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA
- A Differential Role for Macropinocytosis in Mediating Entry of the Two Forms of Vaccinia Virus into Dendritic Cells
- Impaired Innate Immunity in Mice but Preserved CD8 T Cell Responses against in -, -, - or -Deficient Mice
- SARS-CoV Pathogenesis Is Regulated by a STAT1 Dependent but a Type I, II and III Interferon Receptor Independent Mechanism
- Proteolysis of Human Thrombin Generates Novel Host Defense Peptides
- Multilayered Mechanism of CD4 Downregulation by HIV-1 Vpu Involving Distinct ER Retention and ERAD Targeting Steps
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



