-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
Despite the described central role of jasmonate signaling in plant defense against necrotrophic pathogens, the existence of intraspecific variation in pathogen capacity to activate or evade plant jasmonate-mediated defenses is rarely considered. Experimental infection of jasmonate-deficient and jasmonate-insensitive Arabidopsis thaliana with diverse isolates of the necrotrophic fungal pathogen Botrytis cinerea revealed pathogen variation for virulence inhibition by jasmonate-mediated plant defenses and induction of plant defense metabolites. Comparison of the transcriptional effects of infection by two distinct B. cinerea isolates showed only minor differences in transcriptional responses of wild-type plants, but notable isolate-specific transcript differences in jasmonate-insensitive plants. These transcriptional differences suggest B. cinerea activation of plant defenses that require plant jasmonate signaling for activity in response to only one of the two B. cinerea isolates tested. Thus, similar infection phenotypes observed in wild-type plants result from different signaling interactions with the plant that are likely integrated by jasmonate signaling.
Published in the journal: Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis. PLoS Pathog 6(4): e32767. doi:10.1371/journal.ppat.1000861
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000861Summary
Despite the described central role of jasmonate signaling in plant defense against necrotrophic pathogens, the existence of intraspecific variation in pathogen capacity to activate or evade plant jasmonate-mediated defenses is rarely considered. Experimental infection of jasmonate-deficient and jasmonate-insensitive Arabidopsis thaliana with diverse isolates of the necrotrophic fungal pathogen Botrytis cinerea revealed pathogen variation for virulence inhibition by jasmonate-mediated plant defenses and induction of plant defense metabolites. Comparison of the transcriptional effects of infection by two distinct B. cinerea isolates showed only minor differences in transcriptional responses of wild-type plants, but notable isolate-specific transcript differences in jasmonate-insensitive plants. These transcriptional differences suggest B. cinerea activation of plant defenses that require plant jasmonate signaling for activity in response to only one of the two B. cinerea isolates tested. Thus, similar infection phenotypes observed in wild-type plants result from different signaling interactions with the plant that are likely integrated by jasmonate signaling.
Introduction
Jasmonate-mediated signaling controls diverse aspects of plant growth and defense. In particular, jasmonate signaling exerts a major influence on plant response to wounding, chewing insects, and necrotrophic pathogens such as Botrytis cinerea, Alternaria brassicicola, Plectosphaerella cucumerina, and Sclerotinia sclerotiorum [1]–[6]. Appropriate plant responses to these diverse stimuli are believed to be tailored by cross-talk between jasmonate and other hormone signals, such as salicylic acid (SA), ethylene, and abscisic acid (ABA) [7]–[14]. Jasmonate signaling therefore does not mediate plant defense in isolation, but as part of a network of signals with the potential for positive and negative interactions. These signals include inputs from the pathogen that may influence the plant's defense response with positive or negative outcomes for the plant.
Two major pathogen classes are roughly delineated by the pathogen's “lifestyle”: biotrophic pathogens infect living host cells and necrotrophic pathogens kill cells prior to consuming them [15]–[17]. This difference in the pathogen's mode of attack strongly influences which signaling networks mediate the plant response. Plant responses to biotrophic pathogens are largely mediated by salicylate signaling with an emphasis on specific recognition of pathogen effectors by the products of plant resistance (R) genes, often characterized by nucleotide binding sites and leucine-rich repeats [18], [19]. Plant responses to necrotrophic pathogens appear to be mediated by a complex web of signaling dominated by jasmonates and ethylene [20]–[22]. Specific recognition of necrotrophic pathogens by the products of plant R genes is currently unknown, although recent identification of a gene possessing structural similarities to R-genes as the molecular basis of a quantitative trait locus (QTL) affecting resistance of Arabidopsis thaliana to multiple necrotrophic and hemibiotrophic pathogens has been suggested to link mechanisms of defense against biotrophic and necrotrophic pathogens [23]. While plants respond to biotrophic and necrotrophic pathogens via different signaling systems, these systems activate common defense responses, such as the production of the A. thaliana defense metabolite, camalexin. Thus, common responses may be controlled by distinct regulatory networks.
The simplified statement that biotrophic and necrotrophic pathogens activate distinct, but overlapping, defense signaling pathways is largely based on observation of single genotypes of the respective pathogens. Yet biotrophic pathogen species exhibit considerable variation in activation of plant defense signaling. This biotroph variation is largely associated with diversity in the R-gene mediated specificity of plant-pathogen recognition, a phenomenon not documented for necrotrophic pathogens [24]–[26]. Examples of naturally occurring intraspecific pathogen variation affecting plant defense against necrotrophs include variation in toxin production by pathogens and variation in pathogen tolerance or detoxification of plant-produced defense compounds [27]–[31].
While activating plant defense signaling should logically hinder infection, pathogens may manipulate plant defense signaling to improve pathogenesis by diverting plant resources toward defense strategies that are less effective against, or actually increase sensitivity to, the pathogen. Pathogens are known to produce plant hormones or analogues such as coronatine, gibberellins or ABA, and the production of these compounds has been associated with virulence [32]–[34]. Interestingly, the ability to produce these compounds may vary among isolates of the same pathogen species as shown by a survey of 95 strains of Pseudomonas syringae where only 15% assayed positively for coronatine production [35]. While production of ABA by pathogenic fungi has not been as extensively assayed, ABA-overproducing and ABA-deficient B. cinerea strains have been described [36]. In addition, some B. cinerea isolates produce ethylene [37]. Thus, while elements of plant defense signaling may be associated with resistance to particular pathogens, pathogen variation in activation, manipulation, and response to plant defense signaling may alter these associations. Despite available literature suggesting that B. cinerea natural diversity could impact plant defense signaling, this diversity has not been routinely integrated into studies of plant—pathogen interaction.
Unlike many pathogens that possess shorter or longer biotrophic stages, B. cinerea is identified as an unambiguously necrotrophic pathogen [17], [20]. This ascomycete fungus occupies broad geographic and host ranges and exhibits a high degree of genetic and phenotypic variability [38]–[40]. However, this variation has been little explored in the context of plant defense signaling. Testing the interaction between a collection of B. cinerea isolates and A. thaliana mutant genotypes with defined deficiencies in jasmonate signaling revealed significant variation in plant response to B. cinerea isolates that was not apparent in wild-type plants. This included variation in lesion phenotype, altered mRNA transcript accumulation responses, and variation in accumulation of the A. thaliana defense metabolite camalexin. An unexpected dependency of camalexin accumulation in response to B. cinerea infection on intact jasmonate signaling was also revealed. The results presented here, while not contradicting the accepted view that jasmonate-mediated defense is vital for plant resistance to B. cinerea, suggest that additional pathways modulate A. thaliana—B. cinerea interactions. Finally, the architecture of plant defense signaling networks that provide resistance to necrotrophic pathogens is not static, and will vary with the pathogen genotype investigated.
Results
Pathogen variation in jasmonate-dependent infection phenotypes
To test effects of jasmonate-mediated plant defense on diverse B. cinerea isolates, A. thaliana leaves of the aos genotype (deficient in jasmonate biosynthesis) and its corresponding wild-type were inoculated with 10 diverse B. cinerea isolates, two abiotic elicitors (acifluorfen and AgNO3), or a mock inoculation (Table 1) [41]. Visible initiation of leaf necrotic lesions was observed between 24 and 48 hours post inoculation with B. cinerea. While tissue necrosis of aos plants initiated within a time frame similar to wild-type plants, lesions expanded more rapidly in aos plants, with near total consumption of the leaf by B. cinerea between 72 and 96hpi. aos mutant leaves failed to develop the zone of chlorosis surrounding the developing lesion that is often observed in B. cinerea infections (Figure 1A).
Fig. 1. A. thaliana leaves showing necrotic lesions formed by B. cinerea infection at 72hpi. 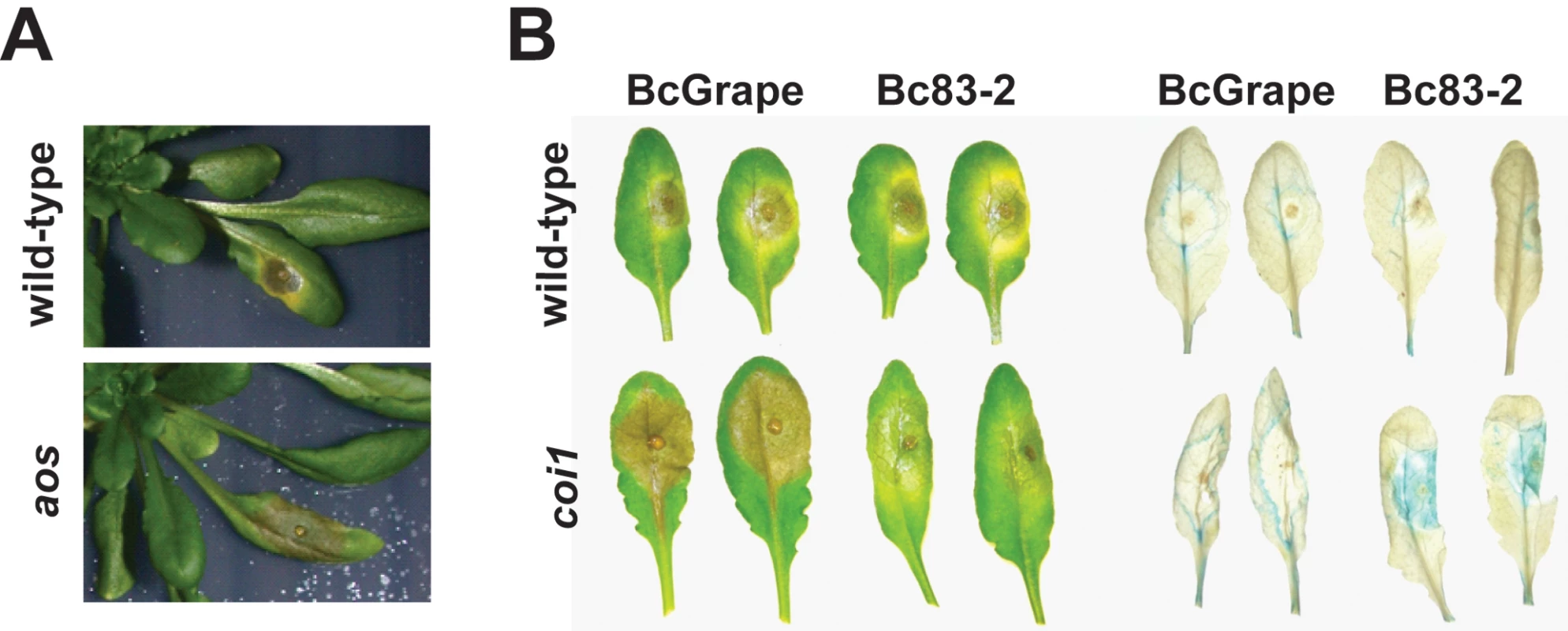
Horizontal labels indicate the B. cinerea isolate used for inoculum. Vertical labels show plant genotypes. A) BcGrape lesions on wild-type and aos plants; B) lesions on wild-type and coi1 detached leaves (left); ProCYP79B2:GUS, COI1 (WT) and coi1 leaves additionally containing a transgenically-introduced fusion of the CYP79B2 promoter region to a GUS (uidA) reporter infected with BcGrape or Bc83-2 and subsequently stained for the presence of GUS activity (right). Tab. 1. B. cinerea isolates and abiotic treatments. 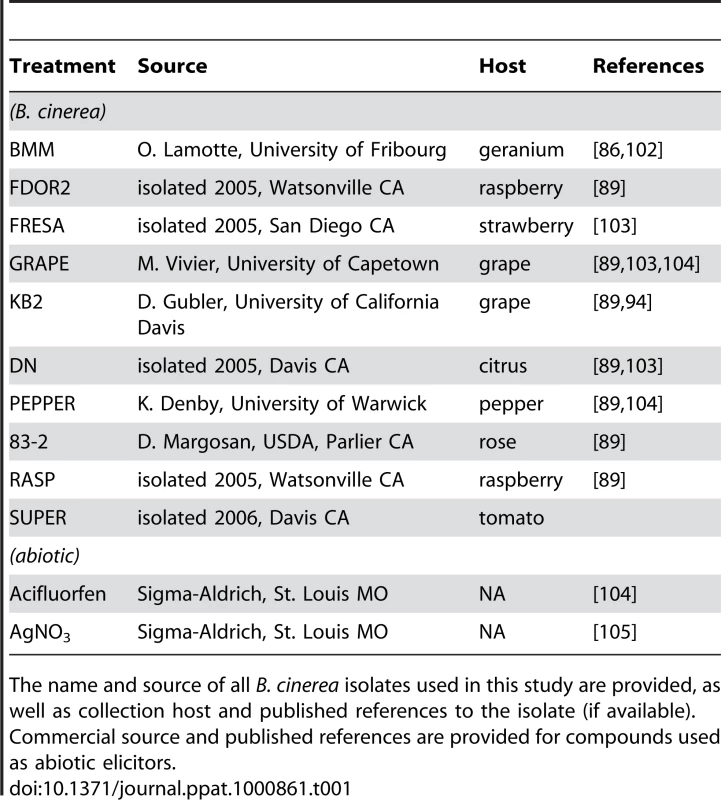
The name and source of all B. cinerea isolates used in this study are provided, as well as collection host and published references to the isolate (if available). Commercial source and published references are provided for compounds used as abiotic elicitors. A comparison of camalexin accumulation in wild-type versus aos leaves induced by 10 B. cinerea isolates revealed significant diversity (Figure 2). Among the B. cinerea isolate treatments tested, camalexin accumulation in aos leaves ranged from 5% to 50% of camalexin accumulation in wild-type leaves, with a median camalexin accumulation among B. cinerea infections of 14% wild-type levels. Mock treatment, acifluorfen, and AgNO3 induced camalexin in aos leaves at 5–7% wild-type levels. In no case was the absence of jasmonate synthesis associated with increased camalexin accumulation. To explore the observed pathogen variation in interaction with jasmonate-deficient genotypes and activation of metabolic defense, the two B. cinerea isolates inducing camalexin accumulation in the aos leaves at the highest and lowest levels relative to wild-type leaves, BcGrape (5%) and Bc83-2 (50%) were used for further experiments.
Fig. 2. Variation in camalexin accumulation in jasmonate-deficient A. thaliana. 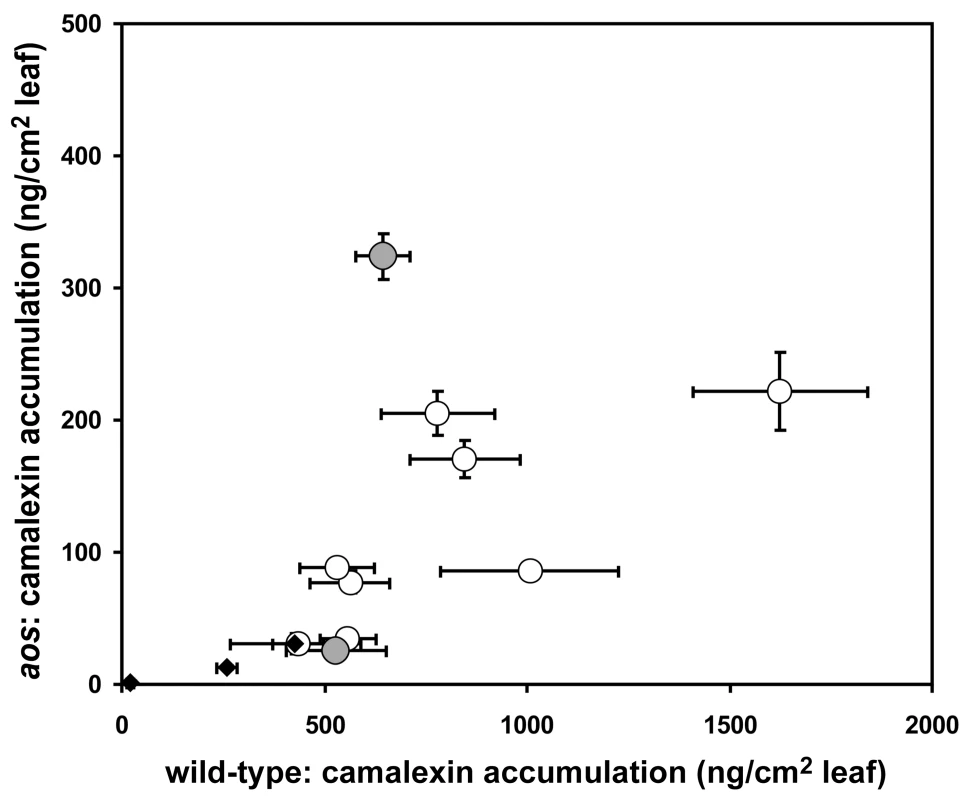
Mean (± SE) camalexin accumulation in detached leaves of wild-type and jasmonate-deficient (aos) A. thaliana treated with 10 different isolates of B. cinerea (circles) or abiotic treatments (mock inoculation, AgNO3, and acifluorfen) (diamonds). 10 leaves per genotype×isolate combination were measured. Vertical error bars not visible are contained within the boundary of the data point marker. Filled circles highlight B. cinerea isolates selected as inducing high (Bc83-2) and low (BcGrape) relative levels of camalexin accumulation in aos plants. One hypothesis that could explain the differential accumulation of camalexin in BcGrape and Bc83-2 infected jasmonate-deficient plants is that one of the B. cinerea isolates produces a molecule that stimulates the intact jasmonate perception in the A. thaliana aos mutant. To determine whether plant deficiencies in jasmonate synthesis and jasmonate perception create similar infection phenotypes and show fully overlapping effects on plant defense signaling, we generated a double mutant containing both aos and the coronatine-insensitive 1 (coi1) mutation that confers deficiency in jasmonate perception [42]. A population segregating both coi1 and aos mutations was experimentally infected with BcGrape and Bc83-2. coi1 aos double mutant plants displayed infection phenotypes for both tested isolates that did not differ significantly from those observed in either the single mutant coi1 or aos plants (Figure 3). Both the coi1 and aos mutations appear recessive for these phenotypes, as infection phenotypes of plants heterozygous for either or both mutations tested did not differ significantly from homozygous wild-type plants (data not shown). The similarity of coi1 and aos phenotypes suggested that camalexin accumulation in jasmonate-deficient plant genotypes infected with Bc83-2 is not likely mediated by isolate-specific production of a metabolite with jasmonate-like coi1 dependent activity similar to coronatine [43].
Fig. 3. Lesion size and camalexin accumulation in A. thaliana deficient in both synthesis and perception of jasmonates. 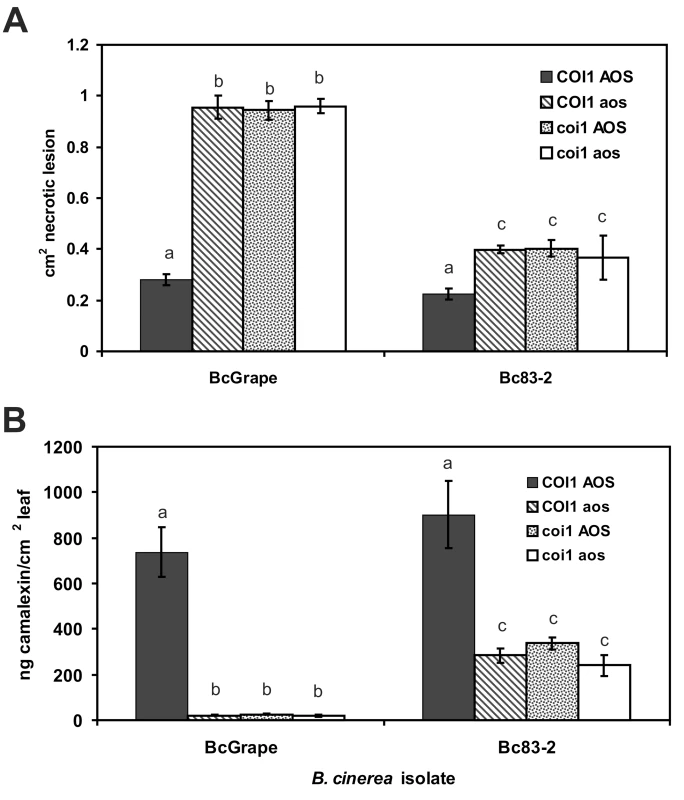
A) Mean (± SE) area of necrotic lesions formed by B. cinerea isolates BcGrape or Bc83-2 on wild-type, coi1, aos, and coi1 aos double mutant plants at 72 hours post-inoculation B) Mean (± SE) camalexin accumulation in wild-type (Col-0), coi1, aos, and coi1 aos double mutants plants infected with B. cinerea isolates BcGrape or Bc83-2. Within each figure, letters above bars indicate statistical significance; bars not sharing letters represent significant mean differences at p<0.05. Testing this segregating population also showed that the glabrous (gl1) mutation, present in the aos mutant background and thus segregating in the aos×coi1 F2 population, had no significant effect on lesion size or camalexin accumulation [44]. We additionally tested a downstream component of the JA pathway, utilizing JAZ1Δ3 mutant plants. These plants produce a modified version of the JAZ1 protein that confers a dominant jasmonate-insensitive phenotype. The JAZ1Δ3 mutant plants showed defects in B. cinerea mediated camalexin induction similar to aos and coi1 plants, but with a less-dramatic increase in lesion size (Figure S1). These defense responses showed similar B. cinerea isolate dependency to that observed in aos and coi1. Thus, B. cinerea isolates vary in their stimulation of signaling networks within A. thaliana as demonstrated by the ability of Bc83-2 to induce moderate camalexin levels in the absence of a functional jasmonate signaling pathway (Figure 3).
The interaction of jasmonate-mediated defense with camalexin biosynthesis
The A. thaliana Phytoalexin Deficient 3 (PAD3) locus encodes a cytochrome P450 enzyme catalyzing the final steps of camalexin biosynthesis [45]. The increased susceptibility of pad3 mutants to necrotrophic pathogens has supported the conclusion that camalexin is an important defense against these pathogens [28], [46]. We showed that camalexin accumulation depends in part upon an intact jasmonate signaling pathway (Figures 2 and 3). To evaluate the extent that increased susceptibility of jasmonate-insensitive A. thaliana genotypes is due to decreased camalexin accumulation in these mutants, we measured development of necrotic lesions and camalexin accumulation in experimentally-infected Col-0 (wild-type), coi1, pad3, and coi1 pad3 double mutant plants (Figure 4). Lesion size at 72hpi did not differ between coi1 and coi1 pad3 plants, but both of these genotypes developed significantly larger lesions than pad3 single mutants, indicating that camalexin deficiency explains a significant fraction of, but not the entire increase in, susceptibility of jasmonate mutants to B. cinerea (Figure 4A). As anticipated, pad3 and coi1 pad3 plants did not accumulate measurable amounts of camalexin (Figure 4B). This observation shows that camalexin accumulation in jasmonate mutants infected with Bc83-2 is not due to a previously-undescribed camalexin biosynthetic capacity in B. cinerea. Further, the similarity in lesion size between pad3 mutant plants infected with BcGrape and Bc83-2 suggests that the difference in susceptibility of jasmonate mutants to these two isolates is not explained by camalexin accumulation in jasmonate mutants infected with Bc83-2.
Fig. 4. Response to B. cinerea infection in jasmonate-insensitive and camalexin-deficient A. thaliana. 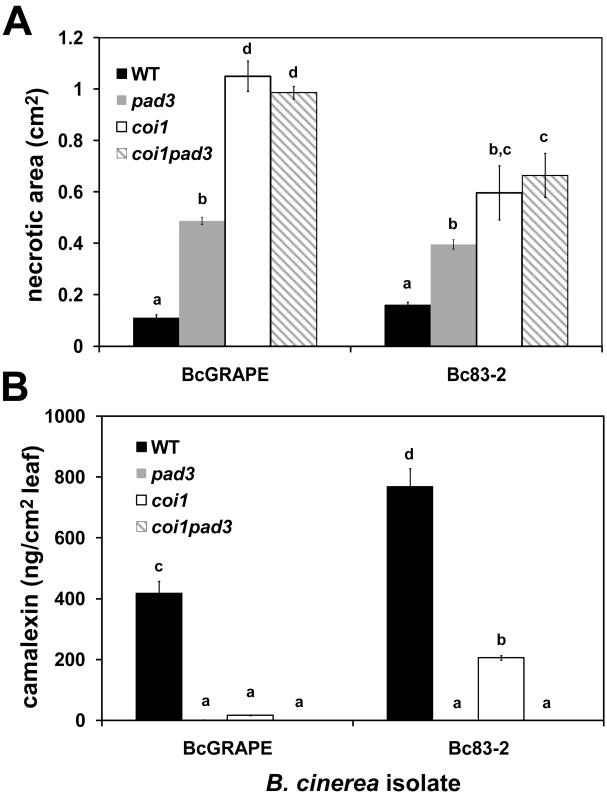
Necrotic area and camalexin accumulation induced by B. cinerea isolates BcGrape and Bc83-2 in A. thaliana genotypes WT (wild-type Col-0 produced as seed from heterozygous COI1/coi1), pad3, coi1, and coi1 pad3. Measurements were taken at 72 hours post inoculation. Within each figure, letters above bars indicate statistical significance; bars not sharing letters represent significant mean differences at p<0.05. A) necrotic area (cm2 ± SE) B) camalexin (ng/cm2 leaf area ± SE). While BcGrape and Bc83-2 induced similar levels of necrosis on wild-type and pad3 plants, lesions produced by Bc83-2 on coi1 and coi1 pad3 plants were significantly smaller than those produced by BcGrape, supporting our observations that jasmonate deficiency had comparatively less impact on plant susceptibility to Bc83-2 (Figures 2 and 4). Consistent with previous experiments, coi1 plants infected with BcGrape accumulated extremely low levels of camalexin that did not significantly differ from levels accumulated in pad3 mutants, and coi1 plants infected with Bc83-2 accumulated camalexin at levels significantly lower than wild-type but significantly greater than pad3 mutant plants (Figure 4). In combination, this shows that while camalexin is a large component of the jasmonate-mediated defense against B. cinerea, its accumulation does not explain the differential virulence of Bc83-2 and BcGrape on jasmonate-deficient A. thaliana.
Camalexin accumulation in wild-type and jasmonate-insensitive leaves over a time course of B. cinerea infection
To determine whether observed differences in camalexin accumulation and lesion growth between B. cinerea treatments were associated with differences in the timing of plant response, time course experiments were conducted using wild-type (COI1/COI1) and coi1 mutant plants (Figure 5). B. cinerea isolates BcGrape and Bc83-2 produced similarly-sized necrotic lesions on wild-type leaves at 48 hpi, but lesions produced by BcGrape infection of coi1 leaves rapidly expanded starting at 40–48 hpi. Bc83-2 showed an increase in induced necrosis on coi1 leaves that was less dramatic than shown by BcGrape but still significantly larger than necroses formed on wild-type leaves. By 32 hpi, camalexin was significantly induced in wild-type but not coi1 leaves (Figure 5B). Camalexin accumulation at all time points after 24 hpi was highest in wild-type leaves infected with Bc83-2. coi1 infected with Bc83-2 showed consistently higher levels of camalexin than coi1 infected with BcGrape. Thus, the difference in camalexin response or virulence between Bc83-2 and BcGrape does not appear to be solely an issue of infection timing but rather variation in pathogen interaction with the plant.
Fig. 5. Development of B. cinerea-induced necrotic lesions and accumulation of camalexin in wild-type and jasmonate-deficient A. thaliana leaves over a four-day infection period. 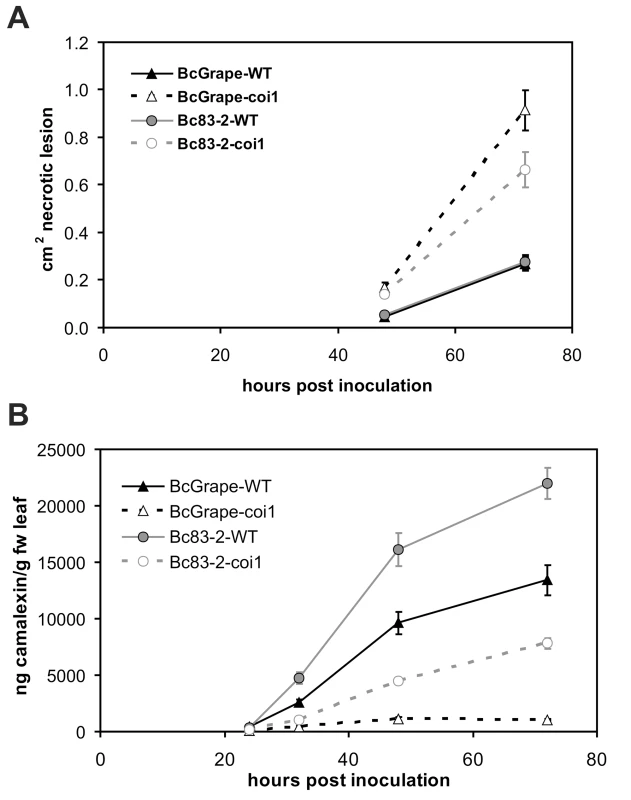
Infection time course for wild-type (solid lines) and coi1 (dashed lines) A. thaliana leaves inoculated with B. cinerea isolates BcGrape (triangles) or Bc83-2 (circles), with measurements taken at 24-hour intervals following inoculation. Values presented are the mean (± SE) of three independent time course experiments, with 10 leaves per isolate×genotype×time point within each experiment. Points with non-overlapping error bars represent significant mean differences at p<0.05. A) lesion development (cm2 necrotic lesion ± SE) B) camalexin accumulation (ng camalexin/g leaf tissue ± SE). Transcription of camalexin biosynthetic genes
To explore mechanisms controlling altered accumulation of camalexin in jasmonate deficient plants as well as differences between B. cinerea treatments, we examined transcript levels of PAD3 and CYP71A13. These genes encode enzymes which catalyze respectively the first committed step and the final steps in camalexin biosynthesis [45], [47], [48]. Relative levels of PAD3 and CYP71A13 transcripts were measured at 24 and 48 hours post-inoculation, time points flanking the observed onset of camalexin accumulation (Figure 5B). PAD3 transcript levels were low but detectable at 24 hours post inoculation (Figure 6A). At 48 hpi, all B. cinerea treated samples showed significantly increased PAD3 transcript accumulation compared to mock treatments. Samples from coi1 mutants showed less induction of PAD3 than wild-type samples but the reduction was not commensurate with the observed decrease in metabolite accumulation. While camalexin accumulation was nearly abolished in coi1 infected with BcGrape, PAD3 transcript was reduced by only half. Further, Bc83-2 infection is associated with relatively higher camalexin accumulation in coi1, but significantly lower PAD3 transcript accumulation in coi1 compared to BcGrape infected coi1. CYP71A13 transcript accumulation showed a similar pattern (Figures S2 and S4). Lack of correlation between PAD3 transcript accumulation and camalexin accumulation measured from the same tissue pool contrasts with previous reports that PAD3 transcript and camalexin accumulation are highly correlated (Figure 6B) [45]. B. cinerea infection with diverse isolates thus reveals evidence of additional regulation of camalexin biosynthesis, beyond transcriptional regulation of known biosynthetic genes.
Fig. 6. Directed measurement of camalexin biosynthetic transcript as compared to camalexin accumulation in the same tissues. 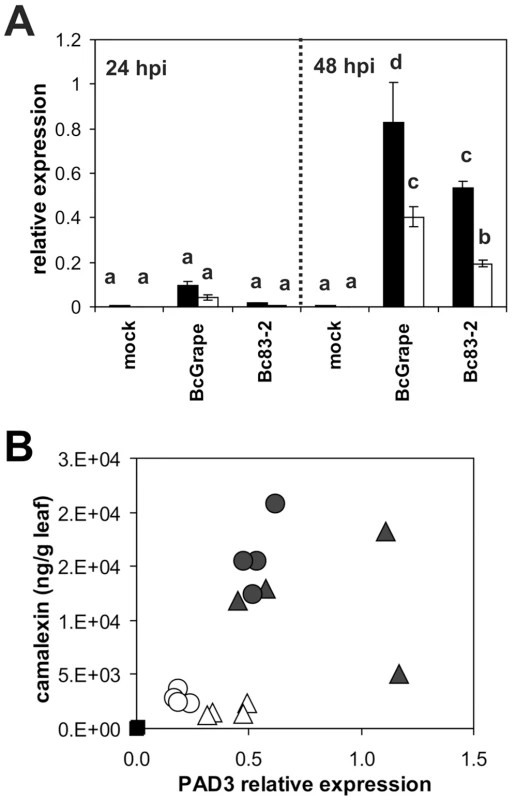
A) Relative transcript levels of PAD3 (At3g26830) in leaves of wild-type (filled bars) and coi1 (open bars) A. thaliana leaves at 24 and 48 hours post-inoculation with B. cinerea isolates BcGrape, Bc83-2, or a mock inoculation. Data are means of four independent biological replicates with bars indicating standard error. Within each figure, letters above bars indicate statistical significance; bars not sharing letters represent significant mean differences at p<0.05. B) Relative transcript level of PAD3 at 48 hpi (x-axis) in relation to camalexin accumulated (ng/g leaf tissue). Each point represents a single sample. Filled symbols represent samples from wild-type plants and open symbols represent samples from coi1 plants. Treatments are indicated as: square = mock, triangle = BcGrape, circle = Bc83-2. PAD3 transcript levels were normalized to transcript levels of reference genes At4g26410 and At4g34270 measured in the same samples. As camalexin accumulation during B. cinerea infection occurs primarily within the plant tissue immediately bordering the developing lesion, it is possible that the spatial distribution of camalexin biosynthetic transcript within an infected leaf may be more relevant to camalexin accumulation than total transcript accumulation within a leaf [30]. To visualize effects of jasmonate insensitivity and B. cinerea isolate differences on the pattern of transcript accumulation of the camalexin biosynthetic enzyme CYP79B2, we crossed a CYP79B2 promoter-GUS fusion transgene into a coi1 background. CYP79B2 catalyzes the conversion of tryptophan to indole-3-acetaldoxime during camalexin biosynthesis in planta [45], [49]. Leaves from homozygous wild-type and coi1 plants showing GUS activity were inoculated with B. cinerea isolates BcGrape and Bc83-2. Wild-type leaves infected with either B. cinerea isolate showed blue staining indicative of GUS activity in a narrow zone bordering the lesion, consistent with previous studies showing that camalexin accumulates primarily within this zone (Figure 1) [30]. coi1 leaves showed a dramatic difference in staining pattern between BcGrape and Bc83-2 infections, with BcGrape-infected coi1 leaves showing patterns of GUS activity similar to those seen in wild-type plants, and Bc83-2 infected coi1 leaves showing intense blue staining within the area visually defined as the necrotic lesion. This intense staining was not associated with increased accumulation of CYP79B2 transcript in coi1 leaves infected with Bc83-2 (Figure S3). The presence of the ProCYP79B2:GUS transgene did not significantly affect camalexin accumulation compared to plants without the transgene from the same segregating F2 population.
A possible explanation for the above observation is that there is less cell death within the Bc83-2 lesion in comparison to BcGrape. We stained infected leaves with a vital stain, Trypan Blue, to compare patterns of cell death associated with infection by the two isolates on wild-type and coi1 leaves. This showed similarly sized halos of plant cell death surrounding the BcGrape and Bc83-2 lesions on both wild-type and coi1 leaves that was a lighter color in the coi1 lesions (Figure 7). Interestingly, these areas contained no detectable fungal cells, suggesting that plant cell death can be caused by mobile plant or fungal signals. No living or dead plant cells were visible within the hyphal mass, suggesting that B. cinerea rapidly consumes material in this region and that the observed difference in camalexin accumulation is not due to differential presence of plant cells. These results suggest that the observed GUS staining pattern is caused by persistence of plant-produced protein within the Bc83-2 lesion, rather than active transcription and translation from the plant genome within the Bc83-2 lesion, implying that the absence of a functional jasmonate signaling network alters the ability of Bc83-2 to degrade or disperse proteins. Trypan Blue staining also showed that the two isolates have different growth habits independent of the plant genotypes tested. Bc83-2 hyphae grew at higher density with a well-defined boundary to the hyphal mass, while BcGrape hyphae grew more sparsely with isolated probing hyphae that grow into the surrounding plant issue.
Fig. 7. Cellular responses in wild-type and jasmonate-deficient A. thaliana. 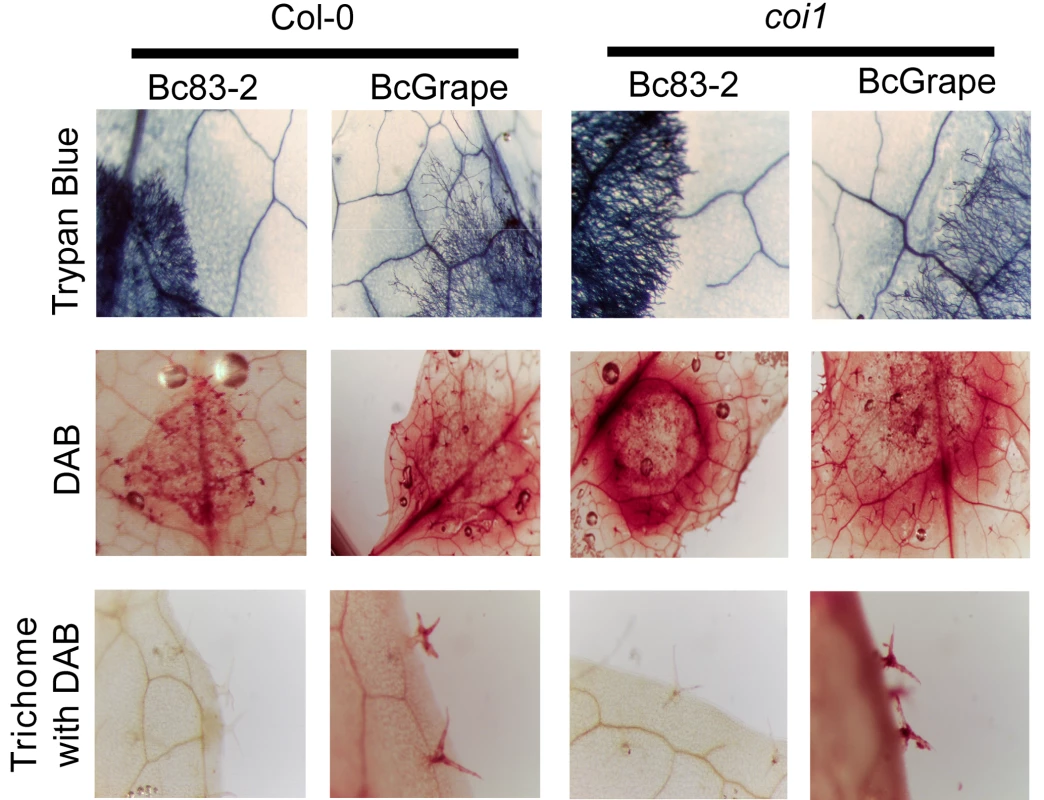
Horizontal labels indicate B. cinerea isolate and A. thaliana genotypes. Vertical labels indicate the stains applied to the leaves shown; Trypan Blue stains dead plant cells and living fungal hyphae, DAB stains H2O2 accumulation sites. The bottom row shows H2O2 accumulation in trichomes located at least 1 cm from the developing necrotic lesion. We further compared the infection phenotypes of BcGrape and Bc83-2 using staining for H2O2 accumulation (DAB). On wild-type A. thaliana leaves, infection by either tested B. cinerea isolate was associated with diffuse H2O2 generation within and around the lesion, suggesting that both the plant and fungus generate H2O2. In contrast, Bc83-2 caused a strong halo of H2O2 surrounding the developing lesion on coi1 whereas the BcGrape lesions were associated with a H2O2 accumulation pattern similar to that observed in wild-type leaves (Figure 7). As generation of reactive oxygen species, including H2O2, is associated with production of camalexin, the observed pattern of H202 accumulation supports our earlier observation that Bc83-2 induces camalexin via a jasmonate-independent mechanism that is lacking in BcGrape infections. Interestingly, this staining also showed that infection by BcGrape is associated with a systemic accumulation of H2O2 in trichomes that was independent of plant jasmonate perception and not seen in leaves infected with Bc83-2 (Figure 7). These B. cinerea isolates elicit distinct defense responses from plants that include both jasmonate-dependent and jasmonate-independent phenotypes, suggesting both the danger of oversimplifying models of plant—“B. cinerea” interaction and the rich potential of intraspecific studies of this pathogen.
Transcriptional profiling
To identify additional differences in plant transcriptional response to these two B. cinerea isolates and build hypotheses regarding the molecular basis of differences in infection phenotype, whole-genome transcriptional profiles of A. thaliana leaves inoculated with B. cinerea isolates BcGrape or Bc83-2 were compared to each other and to control leaves using both wild-type and jasmonate-insensitive (coi1) plants. Based on directed transcript measurements, where induction of camalexin biosynthetic and other defense-associated transcripts was not detected until 48hpi, transcriptional profiling was performed on samples from this 48hpi time point (Figures 6 and S2). Additionally, both B. cinerea isolates had initiated lesions by 48hpi, but lesions at this time point, arising from a single inoculation droplet per leaf, occupy only a small portion of the total leaf area and do not show the large differences in lesion size observed on coi1 leaves at later time points (Figure 5). Estimates of transcript accumulation obtained from arrays were highly consistent with targeted transcript measures obtained via quantitative RT-PCR, with significant Pearson correlation coefficients ranging from 0.76 to 0.91 (Figure S4). Array data are provided as Dataset S1.
A. thaliana transcriptional responses to B. cinerea infection
Of 22810 transcripts represented on the arrays, over half (12,999) showed significant effects for the model transcript = genotype + treatment + (genotype × treatment) even after false-discovery adjustments. The majority (11,989) of these statistically significant transcript changes were associated with treatment where most of these transcripts differed between B. cinerea-infected and control leaves, rather than between leaves infected with the two B. cinerea isolates. We therefore describe statistically significant plant responses consistent between both pathogen isolates as responsive to “B. cinerea”. Transcript accumulation from 1458 genes of the B. cinerea-responsive loci identified above showed greater than 2-fold increase in response to B. cinerea infection, while transcripts from 1602 genes showed more than 2-fold decrease relative to control samples. Differences in transcript abundance between wild-type and coi1 plants as well as between B. cinerea-inoculated and control plants showed overlap with previous studies [50], [51].
All known enzymes of the camalexin biosynthetic pathway were upregulated by B. cinerea infection, with CYP71A13 and PAD3 respectively showing 124-fold and 67-fold increases in B. cinerea infected leaves. An additional five transcripts contributing to biosynthesis of the camalexin precursor, tryptophan, were also upregulated in response to B. cinerea, but less dramatically than camalexin biosynthetic genes (Table S1). Other transcripts showing greater than 2-fold transcriptional effects of B. cinerea infection that have been previously identified as contributing to plant defense against fungal pathogens included a camalexin regulator (PAD4), the MYB transcription factor botrytis-susceptible 1 (BOS1), phenylalanine ammonia lyase (PAL1), polygalacturonase-inhibiting protein (PGIP1), and pathogenesis response proteins (PR1, PR4, and PR5) (Table S1). Transcripts of PDF1.2a and VSP2, considered markers for jasmonate signaling, were detected only at extremely low levels in both B. cinerea-infected and control leaves from coi1 plants, further supporting our conclusion that camalexin accumulation in jasmonate mutants infected with Bc83-2 is not attributable to isolate-specific pathogen-mediated jasmonate signaling independent of coi1 and aos (Figures S2 and S4) [52], [53].
Pathway responses
To associate biological activities with the numerous transcriptional changes caused by B. cinerea infection, genes showing >2-fold transcriptional changes in B. cinerea-infected leaves were grouped by annotated associations with metabolic pathways [54]. In addition to upregulation of camalexin and tryptophan biosynthetic genes, described above, transcripts associated with ascorbate-glutathione metabolism, including 10 glutathione transferases, were strongly upregulated by B. cinerea infection (Table S1). Genes associated with lignin biosynthesis and jasmonate synthesis and response, including six genes encoding JAZ proteins, also showed positive transcriptional responses to B. cinerea infection. Pathways downregulated in B. cinerea-infected leaves primarily control core metabolic functions such as biosynthesis of chlorophyll and starch, but transcripts linked with the biosynthesis of aliphatic glucosinolates, metabolites primarily associated with plant defense against insect herbivores, were an exception to this pattern. Aliphatic glucosinolate-associated transcripts, including three regulatory MYB transcription factors, were strongly decreased in B. cinerea-infected leaves (Table S1). This is consistent with previously documented local repression of aliphatic glucosinolate biosynthesis by B. cinerea infection [55].
Identification of putative response networks
We used the A. thaliana co-expression database ATTED-II to investigate patterns of co-expression for genes transcriptionally affected by B. cinerea infection that are not currently associated with described metabolic pathways. Microarray data have successfully identified genes controlling A. thaliana—B. cinerea interactions [50], [56]–[59]. Among the transcripts lacking prior pathway associations, we identified three groups of co-regulated loci that may represent undescribed B. cinerea responsive networks (Table 2). These proposed groups, described below, represent hypothesized contributions of these genes to A. thaliana—B. cinerea interaction, requiring experimental validation.
Tab. 2. Co-expressed gene networks highly altered by B. cinerea treatment. 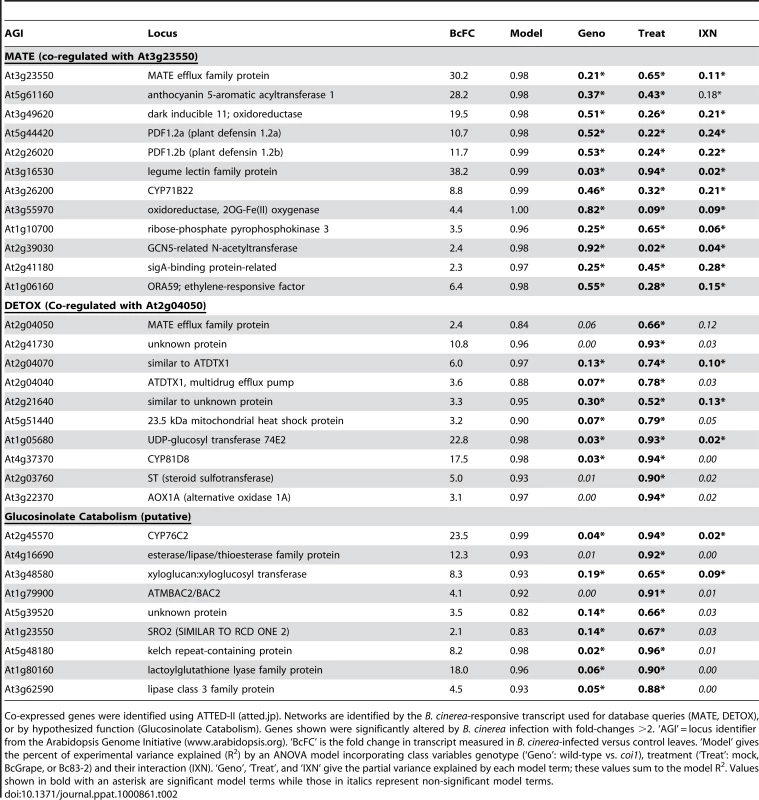
Co-expressed genes were identified using ATTED-II (atted.jp). Networks are identified by the B. cinerea-responsive transcript used for database queries (MATE, DETOX), or by hypothesized function (Glucosinolate Catabolism). Genes shown were significantly altered by B. cinerea infection with fold-changes >2. ‘AGI’ = locus identifier from the Arabidopsis Genome Initiative (www.arabidopsis.org). ‘BcFC’ is the fold change in transcript measured in B. cinerea-infected versus control leaves. ‘Model’ gives the percent of experimental variance explained (R2) by an ANOVA model incorporating class variables genotype (‘Geno’: wild-type vs. coi1), treatment (‘Treat’: mock, BcGrape, or Bc83-2) and their interaction (IXN). ‘Geno’, ‘Treat’, and ‘IXN’ give the partial variance explained by each model term; these values sum to the model R2. Values shown in bold with an asterisk are significant model terms while those in italics represent non-significant model terms. DETOX: Transcript from the At2g04050 locus, encoding a Multidrug and Toxin Extrusion (MATE) efflux family protein, has been previously documented to increase in response to elevated soil concentrations of boron, tri-nitro toluene, and NaCl [60]–[62]. 16 B. cinerea responsive transcripts were identified as co-regulated with At2g04050 These transcripts were induced by both B. cinerea isolates, but accumulated to lower levels in coi1 leaves infected with BcGrape than wild-type leaves infected with BcGrape, while Bc83-2 infected leaves showed an opposite pattern (Figure 8). This suggests that jasmonate signaling activates this putative network in response to BcGrape but represses it in response to the Bc83-2 isolate, indicating that intraspecific pathogen diversity can affect the outcome of jasmonate signaling. The genes in this network included several multidrug transporters that may act in response to fungal toxins, and UGT74E2, a glucosyltransferase implicated in detoxification (Table 2) [60], [63]. This network showed an overrepresentation of ABA response elements (ABRE) suggesting a possible influence of ABA [64], [65].
Fig. 8. Genotype and B. cinerea effects on transcription of co-regulated genes and biosynthetic pathways. 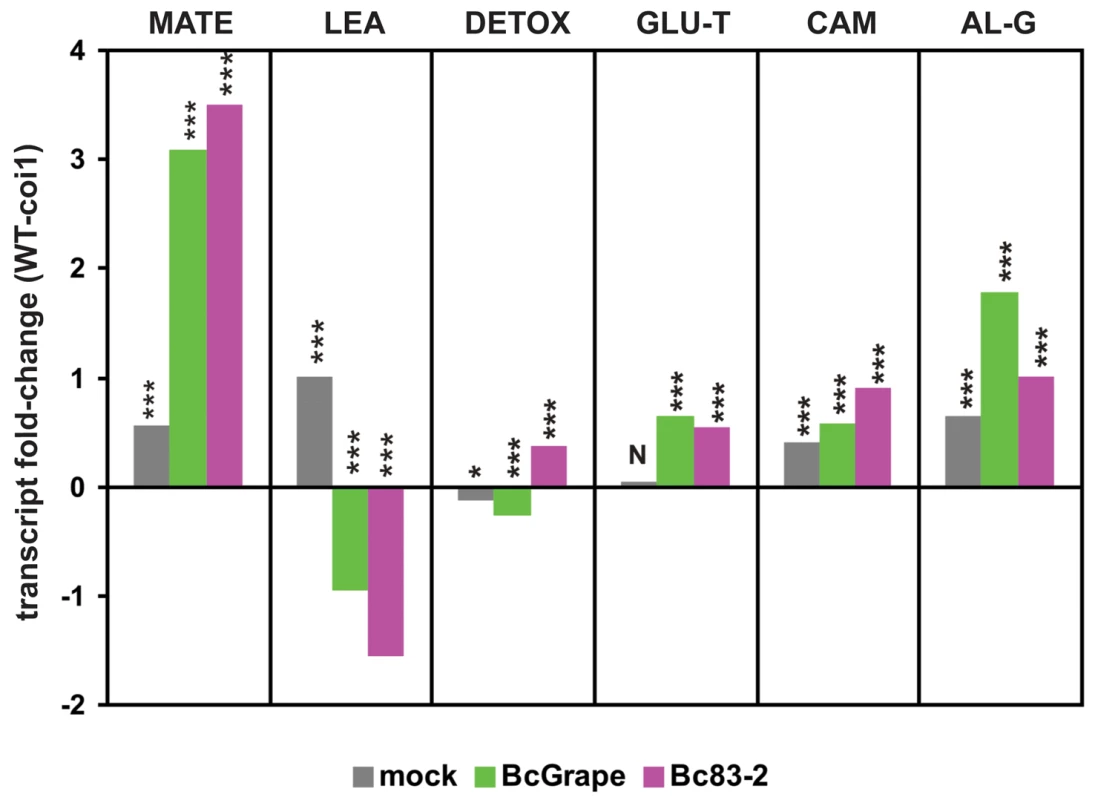
The y-axis displays the log2 fold-difference in mean expression values between wild-type and coi1 leaves for groups of transcripts clustered by similarity of expression. Each unit on the vertical axis is equivalent to a 2-fold difference in transcript level. Significant differences between wild-type and coi1 transcript levels within each treatment are indicated above the bars (‘***’ = p<0.0001, ‘*’ = p<0.05, ‘N’ = p>0.05). MATE (n = 40) and LEA (n = 16) represent groups of coregulated transcripts showing the greatest magnitude of transcript difference between leaves infected with B. cinerea isolates BcGrape and Bc83-2 (Table S2). DETOX (n = 16) and GLU-T (n = 15) are transcript clusters upregulated by B. cinerea infection. CAM (n = 5) and AL-G (n = 23) are groups of transcripts empirically associated with biosynthesis of camalexin and aliphatic glucosinolates, respectively. Lists of loci associated with these transcripts are provided in Tables 2 and S1. Glucosinolate turnover: Another co-expressed cluster of 15 B. cinerea-induced transcripts includes loci encoding enzymes hypothesized to function in catabolism of glucosinolates (Table 2). Glucosinolate turnover may play a role in fungal defense by allowing redistribution of cellular resources stored in glucosinolates to antifungal metabolites [55]. Alternatively, accumulation of these transcripts in response to B. cinerea may relate to the function of glucosinolate activation products in pathogen defense and signaling [66], [67]. This hypothesized upregulation of glucosinolate catabolism contrasts with downregulation of transcripts involved in the biosynthesis and activation of aliphatic glucosinolates in B. cinerea-infected leaves. Transcripts involved in both the synthesis of aliphatic glucosinolates and their hypothesized catabolism were detected at higher levels in wild-type leaves than coi1 leaves (Figure 8).
MATE: A group of 40 transcripts induced by B. cinerea infection were identified by association with the highly B. cinerea-responsive locus At3g23550, encoding another MATE transporter (Table 2 and Table S2). MATE proteins are associated with resistance to toxins, but may also be involved in transport of plant-produced metabolites required for defense [68]–[70]. This group of transcripts also contains several likely biosynthetic genes, such as acyltransferases, oxidoreductases, and cytochromes P450. Promoter analysis showing an over-representation of two elements, ABRE and GC box, supports coordinated transcriptional response of these genes [64], [65]. Considering inclusion of transcripts previously associated with plant defense against necrotrophic pathogens, such as PDF1.2 defensins and the ethylene and jasmonate responsive transcription factor ORA59 (a member of a secondary metabolite regulatory gene family), in this group we hypothesize that these genes contribute to biosynthesis and transport of a currently unknown defense-associated metabolite [71].
Comparison of transcriptional effects: BcGrape vs. Bc83-2
Differences in transcript accumulation after infection by BcGrape or Bc83-2 were generally similar in direction of effect between wild-type and coi1 leaves but of greater magnitude in coi1 leaves. Of 824 transcripts showing differential accumulation in response to the two tested B. cinerea isolates, 787 show larger differences in coi1 leaves than wild-type (Table S2). While this correlates with lesion development at later time points, lesion sizes at 48 hours do not significantly differ among genotype×isolate combinations (Figure 5). To identify patterns in these transcript differences that might enhance our understanding of the biology of A. thaliana response to B. cinerea, we clustered these transcripts by similarity of normalized transcript levels. This identified two large groups of transcripts, those showing relative increases in transcript level in response to B. cinerea (clusters 1–3) and those relatively decreased in B. cinerea-infected leaves (clusters 4–6) (Figure 9, Table S2). Subsequent clustering of transcript profiles for these loci by genotype and treatment suggested that BcGrape and Bc83-2 infections exert similar transcriptional effects on wild-type leaves. This contrasts with a dramatic difference in transcript patterns observed in coi1 samples, where Bc83-2 infected coi1 leaves showed transcript patterns similar to mock-inoculated samples while BcGrape infected coi1 were more transcriptionally similar to infected wild-type samples. This echoes the pattern observed for the putative DETOX network for A. thaliana response to B. cinerea, and suggests that jasmonate has opposing transcriptional effects on a set of genes in response to these two B. cinerea isolates (Figure 8).
Fig. 9. Normalized transcript levels from A. thaliana loci showing significant differences in transcript level between B. cinerea isolate treatments. 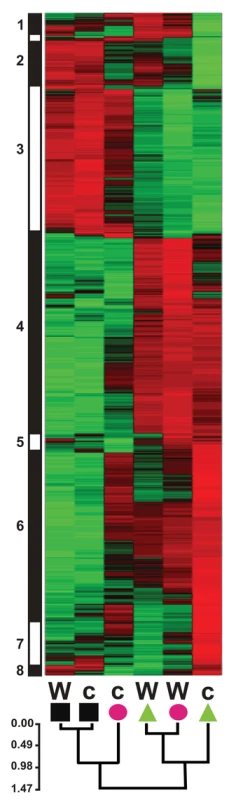
Z-score normalized genotype×treatment means for 824 transcripts are clustered vertically by transcriptional similarity among loci using Pearson correlation coefficients and WPGMA. The vertical bar to the left shows rough grouping of transcripts by similarity of normalized expression values; numbers correspond to transcript groups listed in Table S2. Green coloring indicates relatively higher transcript levels; red indicates lower transcription. Horizontal clustering shows similarity among genotype×treatment effects on relative transcript level. Genotype (W = wild-type, c = coi1) and treatment (square = mock, triangle = BcGrape, circle = Bc83-2) are indicated at the base of each column. The scale bar shows Pearson correlation distances. Differential transcriptional response to BcGrape
In wild-type plants, the transcript with the greatest increase in accumulation in leaves infected with BcGrape relative to Bc83-2 differed only by 1.75-fold. In coi1 leaves, however, a greater than 10-fold difference was observed between the two B. cinerea treatments. This transcript, At3g23550 from the above MATE network, and associated transcripts (Table 2), showed greater accumulation in leaves infected with BcGrape relative to leaves infected with Bc83-2 with a differential coi1 dependence between the two isolates (Table S2, Figure 9 (clusters 3, 4, and 6)).
Differential transcriptional response to Bc83-2
The locus associated with the largest transcript difference where Bc83-2 infected leaves showed higher transcript levels than BcGrape infected leaves was At1g52690, encoding a late embryogenesis abundant (LEA) protein. LEA proteins are associated with seed maturation, but are also suggested to play important roles in stress tolerance in vegetative tissues [72]. This transcript has not been previously described as pathogen-responsive, but is upregulated in response to exogenous application of ABA and osmotic stress (Genevestigator Response Viewer; www.genevestigator.ethz.ch). In Bc83-2 inoculated wild-type leaves, this transcript accumulated to levels 3-fold greater than those observed in BcGrape-infected wild-type leaves, and more than 11-fold greater than observed in BcGrape-infected leaves from coi1 plants. However, in the initial analysis of B. cinerea effects on transcript abundance, At1g52690 transcript was identified as downregulated by B. cinerea infection, suggesting that this difference represents a failure of Bc83-2 infection or associated plant defense response to reduce transcript accumulated from this locus.
16 associated transcripts showed expression patterns similar to At1g52690. All of these loci showed higher relative transcript accumulation in Bc83-2 infected leaves compared to those infected with BcGrape (Table S2). These transcripts were also detected at higher levels in coi1 leaves infected with either B. cinerea isolate than similarly-treated wild-type leaves, suggesting that these transcripts are repressed by jasmonate-mediated response to B. cinerea infection (Figure 8). The expression pattern displayed by these transcripts in Bc83-2 infected leaves is similar to the pattern observed in uninfected leaves, further supporting the hypothesis that BcGrape infection represses accumulation of these transcripts, but that this does not occur during a similar stage of infection with Bc83-2 (Figure 9). As such, BcGrape and Bc83-2 differ in induction of both positive and negative transcriptional responses in planta.
Discussion
Jasmonate signaling plays a vital role in plant defense against the highly variable necrotrophic fungal pathogen B. cinerea but its molecular effects may differ with pathogen diversity. We show that the genetic diversity contained within B. cinerea generates quantitative variation in plant response to the pathogen in the absence of jasmonate synthesis or perception. This variation in plant response was most readily observed as differential accumulation of the A. thaliana defense metabolite camalexin, which did not directly correspond with changes in associated biosynthetic transcripts. Analyses of A. thaliana transcriptional responses to B. cinerea isolates BcGrape and Bc83-2 revealed highly similar changes in transcript levels induced by infection of wild-type plants, yet dramatic differences in transcript profiles between A. thaliana infected with these two pathogen isolates when jasmonate signaling is impaired by mutation of COI1.
Regulation of camalexin accumulation
Jasmonate signaling controls a substantial portion of camalexin accumulation
Camalexin and jasmonate-mediated defenses have been presented as separate elements of A. thaliana resistance to necrotrophic pathogens because jasmonate mutants had no detected effect on the accumulation of camalexin biosynthetic transcripts [51], [73]. We show that, in response to B. cinerea, camalexin accumulation was substantially decreased by deficiencies in jasmonate signaling. A. thaliana mutants deficient in jasmonate synthesis or jasmonate perception showed significantly lower camalexin accumulation than wild-type plants for all 10 B. cinerea isolates tested. Observation of similarly dramatic decreases in camalexin accumulation for jasmonate deficient plants treated with the abiotic elicitors AgNO3 and acifluorfen suggests that decreased camalexin accumulation observed in response to B. cinerea infection in these genotypes is not due to an active pathogen repression of camalexin biosynthesis or accumulation in the absence of jasmonate signaling (Figure 2). The similarity of responses shown by three mutants deficient in distinct aspects of jasmonate signaling, aos, coi1, and JAZ1Δ3, suggests that decreased camalexin induction represents a requirement for the entire jasmonate pathway. Yet previous study suggested that camalexin accumulation in response to B. cinerea infection does not require MYC2 [74], a transcriptional regulator of jasmonate signaling, which is repressed by physical interaction with JAZ proteins [75]–[77]. This suggests that jasmonate signaling controls B. cinerea-induced camalexin production via an unidentified transcription factor that is repressed by JAZ proteins in a manner similar to MYC2.
Camalexin biosynthesis is regulated at multiple functional levels
Previous studies report accumulation of camalexin biosynthetic transcripts in coi1 mutant plants at levels greater than or equal to wild-type in response to B. cinerea and oligogalacturonides, leading to the conclusion that jasmonate and camalexin responses are not connected [50], [51]. In light of the common assumption that camalexin transcript levels predict the level of metabolite accumulation, the metabolite is infrequently measured. Our data showed a dramatic decrease in camalexin metabolite accumulation in the coi1 mutant without an equivalent decrease in transcript levels for the first and last enzymatic steps, PAD3 and CYP71A13 (Figures 3 and 6). In addition, variance in camalexin-associated transcripts measured in transcriptional profiling experiments was explained primarily by treatment (B. cinerea infection), rather than genotype, despite an obvious effect of plant genotype on camalexin accumulation (Table S1, Figures 3-5). Thus, observed accumulation of these camalexin biosynthetic transcripts is not a reliable surrogate for measurement of metabolite accumulation. These results suggest that additional regulation of camalexin biosynthesis exists, either post-transcriptional regulation or transcriptional regulation involving an unidentified pathway intermediate. While an alternative explanation, that B. cinerea degrades camalexin, remains plausible, this is not supported by the observation that camalexin accumulation was also lower in jasmonate-deficient leaves treated with abiotic elicitors of camalexin. The relative performance of BcGrape and Bc83-2 on camalexin deficient pad3 plants suggests that these isolates are not camalexin-insensitive, and camalexin insensitivity documented in B. cinerea is associated with export, rather than degradation, of the metabolite [78]. These data support a deficiency in camalexin biosynthesis by the plant, rather than active degradation by the pathogen that functions only in the absence of jasmonate-mediated plant defense (Figure 2). Thus, jasmonate signaling likely plays a complex regulatory role in camalexin synthesis.
Similarity of response of wild-type A. thaliana to distinct B. cinerea isolates
Wild-type (Col-0) A. thaliana leaves showed similar responses to the two B. cinerea isolates used in these experiments (Figures 1 and 3). These included not only visual and biochemical symptoms (leaf necrosis and camalexin accumulation), but also transcriptional responses to infection (Figures 6 and S2). Comparison with an earlier transcriptional profiling dataset revealed that an unnamed B. cinerea isolate showed similar effects to this experiment: of 7718 transcripts described as significantly responding to B. cinerea treatment, 6465 showed a significant effect of B. cinerea treatment in the experiments described here [51]. Of these, 6107 transcripts showed the same directionality of B. cinerea effect. Where the effects of the three B. cinerea isolates represented in these two datasets disagree, no single isolate appears to be an outlier. This suggests that, while B. cinerea isolates elicit different transcriptional responses from wild-type A. thaliana, comparison among datasets reveals a consistent transcriptional signature of B. cinerea infection.
Differential A. thaliana response to B. cinerea isolates in the absence of jasmonate signaling
While infection of wild-type plants with genetically and phenotypically distinct B. cinerea isolates elicited very similar plant responses, infection phenotypes displayed by jasmonate-deficient plants indicate that the phenotypic similarity observed in wild-type plants must be produced by different mechanisms. In particular, the isolate Bc83-2 induces camalexin accumulation both via jasmonate signaling and an additional pathway that is either not induced or specifically blocked by BcGrape infection. Examining differences in transcription between A. thaliana leaves infected with these B. cinerea isolates revealed that transcriptional responses to these isolates differed more dramatically in jasmonate-insensitive coi1 plants than in the wild-type background (Figure 9, Table S2). This suggests that jasmonates are not only important signaling components but also integrators of signals from diverse pathogen genotypes into consistent plant defense responses.
The visually distinctive lesion phenotype produced by Bc83-2 infection of jasmonate-deficient A. thaliana genotypes, coupled with the persistence of plant-produced GUS activity within the lesion produced by Bc83-2 on coi1 mutants, initially suggested that the mechanisms by which this isolate induces plant death may be jasmonate-dependent (Figure 1). However, vital staining indicated that the B. cinerea isolates caused similar patterns of plant cell death in leaves of both wild-type and jasmonate-insensitive plants (Figure 7). Thus, the observed differences in plant transcriptional response to these pathogen isolates are not likely linked to simple differences in the number of living cells in the leaf, but instead result from differences in plant—pathogen communications, potentially including plant perception of pathogen-induced damage and pathogen metabolism of dead plant tissues.
Jasmonate deficiency reveals plant differences in transcriptional regulation that suggest hypotheses regarding the mechanisms controlling plant response to B. cinerea variation
The A. thaliana transcripts showing the greatest magnitude of differential response to infection of jasmonate-insensitive plants by B. cinerea isolates BcGrape or Bc83-2 were an extrusion transporter (elevated in BcGrape treatments compared to Bc83-2) and an ABA-responsive transcript (elevated in Bc83-2 treatments compared to BcGrape). An extrusion transporter might function in plant resistance to B. cinerea-produced necrotic toxins, for which isolate differences in biosynthetic capacity have been documented [73]–[75]. Analyses of the secondary metabolic output of BcGrape and Bc83-2 may provide evidence of differential production of candidate phytotoxic compounds to guide future study. Alternatively, At3g23550 may play a role in plant defense processes independent of export of pathogen-produced toxins, as similar plant MATE transporters are implicated in the synthesis and transport of plant-produced compounds such as anthocyanins, nicotine, and salicylic acid [79]–[81].
The ABA-responsive transcript that showed the greatest magnitude of differential transcription favoring Bc83-2 infection, late embryogenesis abundant protein At1g52690, also showed an isolate specific interaction consistent with observed differences in lesion development on jasmonate-insensitive plants, where similar transcript levels were observed between BcGrape-infected coi1 and wild type leaves while Bc83-2 infected coi1 leaves showed elevated transcript levels in comparison with wild-type. The group of transcripts identified as co-regulated with this gene (LEA) contains a set of genes that are annotated as ABA-responsive and show enrichment for promoter motifs associated with ABA regulation (Table S2). Transcript accumulation from this group of genes was generally decreased in B. cinerea-infected wild-type plants (Figure 8). This suggests that ABA signaling contributes to differentiation of these two isolates in planta. While ABA antagonism of both salicylate and jasmonate-mediated plant defenses has been described, the observed increase in accumulation of these transcripts in the absence of functional jasmonate signaling suggests that jasmonate signaling also antagonizes ABA [8], [9], [13], [82], [83]. B. cinerea as a species can produce ABA, and blocking activation of ABA signaling via use of the competitive inhibitor beta-aminobutyric acid has been shown to increase plant resistance to B. cinerea [36], [84]–[86]. Both of these B. cinerea isolates are able to produce ABA, but quantitative analysis of ABA biosynthesis by both the plant and the pathogen during the process of plant infection is necessary to determine the contribution of ABA to differences in infection phenotypes observed in these B. cinerea isolates in the absence of intact jasmonate signaling.
Conclusion
Despite similarities in lesion development and transcriptional effects on wild-type plants, the two B. cinerea isolates tested in this study, BcGrape and Bc83-2, show differing interactions with plant response networks that are masked by the response of an intact plant jasmonate signaling pathway. These differences are revealed in mutants deficient in jasmonate biosynthesis and several aspects of jasmonate signaling, most strikingly by quantitative differences in camalexin accumulation in jasmonate-deficient A. thaliana leaves infected with these pathogen isolates. Examination of transcriptional response to B. cinerea infection in plants with impaired jasmonate signaling has revealed the involvement of at least two groups of co-regulated loci not previously associated with plant defense responses. Exploration of the function of these putative networks in A. thaliana defense against B. cinerea and other pathogens may provide novel insight into mechanisms of plant defense.
Methods
Plant materials
A. thaliana mutants deficient in jasmonate biosynthesis, allene oxide synthase (aos), and biosynthesis of camalexin, phytoalexin deficient 3 (pad3-1), were obtained from the Arabidopsis Biological Resource Center (www.biosci.ohio-state.edu/pcmb/Facilities/abrc/abrchome.htm) [41], [45]. All mutant lines were in the Col-0 genetic background, with aos mutants additionally containing the visible marker gl1. The presence of a mutant aos allele was determined by PCR using gene-specific and insert-specific primers [41]. A. thaliana segregating the coronatine insensitive 1 (coi1-1) mutation, conferring deficiency in jasmonate perception, was obtained from J. Glazebrook, University of Minnesota [42]. Homozygous coi1-1 plants were identified using a CAPS marker; a 531bp fragment of At2g39940 (COI1) contains an Xcm1 restriction site that is abolished by the coi1-1 mutation [42]. Plants with coi1 aos double mutant genotypes were generated by fertilizing aos plants with pollen from COI1/coi1 heterozygous plants. F1 progeny were genotyped to select COI1/coi1-1 heterozygotes; these were allowed to self-pollinate and B. cinerea lesion growth and camalexin accumulation phenotypes were determined for a segregating F2 population. ProCYP79B2:GUS contains a transgenic fusion of the CYP79B2 promoter to a β-glucuronidase reporter [87]. ProCYP79B2:GUS coi1-1 plants were generated by fertilizing male-sterile coi1-1 flowers with ProCYP79B2:GUS pollen, allowing F1 plants to self-pollinate, and selecting appropriate genotypes from the F2 segregants. A. thaliana containing the JAZ1Δ3::GUS transgene, conferring a dominant jasmonate-insensitive phenotype, was obtained from G. Howe, Michigan State University [88].
Plant growth conditions
Plants for all experiments were grown in 36-cell flats (approximately 120cm3 soil per cell) in a growth chamber at 12h∶12h light∶dark, 22°C, 50–60% RH, and ∼150µE light intensity. Seed was sown on soil (Sunshine Mix #1, Sun Gro Horticulture Ltd., Bellevue WA) and thinned to one plant per cell at three days post-germination. Genotypes compared within an experiment were systematically interspersed within flats. Plants were sub-irrigated twice weekly with deionized water. Experiments were conducted with mature, non-bolting rosette plants at 5–6 weeks post-planting.
Treatments
Source and reference data for B. cinerea isolates used in this study are provided in Table 1. Preliminary experiments compared infection phenotypes of whole rosettes (detached from the root approximately 0.5cm below the soil surface and placed on agar) with observations of detached single leaves; no differences in measured phenotypes were observed (Figure 1). Further experiments used detached rosette leaves, inoculated with B. cinerea spores as previously described [89]. Inoculum was freshly prepared for each experiment from concentrated spore stocks stored at −20°C in 25% glycerol. Leaves were inoculated with 5µl droplets of spore suspension (5×105 spores/ml in half-strength filtered organic grape juice) (Santa Cruz Organics, California USA). Digital photographs were analyzed using Image J to measure lesion area [89], [90]. Control leaves (mock) were inoculated with half-strength grape juice. Abiotic elicitors of camalexin were 5mM AgNO3 and 10µM acifluorfen (Sigma-Aldrich, St. Louis, MO USA), applied as four 5µl droplets per leaf to one side of the midvein.
Staining of ProCYP79B2:GUS leaves for GUS activity at 72 hours post-inoculation was performed as described [91]. Staining of wild-type and coi1 leaves for cell death (Trypan Blue) and H2O2 accumulation (DAB) at 72 hours post-inoculation was performed as described [92], [93].
Camalexin measurements
Camalexin was extracted in 90% MeOH and quantified via HPLC as previously described [30]. Whole leaves were collected in 500µl 90% MeOH in 96 - deep-well plates and stored at −20°C until extraction and analysis, except tissue samples used for transcript measurements where fresh tissue was frozen in liquid nitrogen, ground without solvent, and separate aliquots of frozen tissue were removed for RNA isolation and camalexin extraction. Camalexin measurements are standardized by tissue weight (g) or leaf area (cm2); leaf weight and area are highly correlated within the A. thaliana genotypes used for these experiments.
Time course experiments
Seed from heterozygous COI1/coi1 A. thaliana was grown as described (“Plant Growth Conditions”) and genotyped 2–3 days prior to experiments. DNA was isolated from the first true leaves to minimize stress to the plant and maximize leaf tissue available for experiments. Eight leaves were detached from each homozygous wild-type or coi1 plant, such that each plant contributed one leaf per B. cinerea isolate (BcGrape vs. Bc83-2)×time point (24, 32, 48, and 72 hours post-inoculation) combination. At each time point, leaves were photographed and six to eight leaves per plant genotype×B. cinerea isolate combination were collected individually into 90% MeOH and processed as described (“Camalexin measurements”).
Data analysis
Comparisons of lesion and camalexin data for the experiments described above were performed using a 2-way factorial ANOVA model with classes plant genotype and treatment (Table 1). A genotype×treatment interaction term was included in the model. Specific comparisons of least-squares means were evaluated for significance using Tukey's HSD adjusted p-values. Time course experiments were analyzed similarly, but including time point as an additional class variable. These analyses were conducted in SAS (Version 9.1, SAS Systems, Cary NC USA).
Directed transcript measurements and transcript profiling
Plant growth and treatments
Seed from COI1/coi1 heterozygote plants was grown to 5 weeks old as a segregating population. Leaves harvested from plants into trays of 1% phytagar were inoculated with B. cinerea isolate Grape, 83-2, or a mock treatment. After inoculation, additional tissue was removed from plants for genotyping. Leaves from homozygous wild-type and coi1 mutant plants were collected at 24 and 48 hours post-inoculation. Five to seven leaves were pooled for each sample, with each genotype×treatment×time point combination represented by four independent samples. Leaves were collected in 15ml tubes, frozen in liquid nitrogen, and stored at −80°C. Total RNA was isolated from frozen tissue ground in liquid nitrogen by TRIzol extraction (Life Technologies, Grand Island, NY USA) and further purified using the Qiagen RNeasy kit with on-column DNase treatment (Qiagen Inc, Valencia, CA).
Directed transcript measurements
Selected defense-related transcripts were measured both to guide experimental design for array transcript profiling experiments and to provide corroborative measurement of transcripts of particular interest. Isolated mRNA was reverse transcribed using Superscript III (Invitrogen, Carlsbad, California). Quantitative RT-PCR was conducted in 50 µl reactions containing 10 ng cDNA, 1× iQ SYBR Green supermix (Bio-Rad Laboratories, Hercules, CA, USA), and 200 or 250 nM of each primer as previously described [74]. Amplification and analysis of cDNA were as described [94]. We analyzed transcripts encoding camalexin biosynthetic enzymes PAD3 (At3g26830; primer sequences (5′ to 3′) GCAAGAGAACGATGGAGATG and TCTTGTCCCCAAGTGTTGTC) and CYP71A13 (At2g30770, primer sequences TCGGTTGCATCCTTCTCTTC and ATATCGCAGTGTCTCGTTGG), jasmonate-responsive proteins PDF1.2a (At5g44420) and VSP2 (At5g24770), and wound-responsive transcripts GST1 (At1g02930) and PR5 (At1g75040, primer sequences CGATAAGCCGGAAACTTGTC and AAGTGAAGGTGCTCGTTTCG). Primer sequences used for PDF1.2a and VSP2 were as previously published [74]. The reference genes At4g34270 and At4g26410 were used for transcript normalization [95].
Significant differences in the mean relative expression of each target transcript were evaluated using an ANOVA model incorporating GENOTYPE (wild-type versus coi1), TREATMENT (mock inoculation, B. cinerea isolates BcGrape or Bc83-2), and TIMEPOINT (24 or 48hpi) as class variables and including all interaction terms. Specific comparisons between genotypes for each treatment by time point combination were evaluated using pairwise comparisons of least squares means.
Genome-wide transcriptional profiling
Transcript profiling of samples collected at 48hpi was performed using A. thaliana ATH1 arrays (Affymetrix, Santa Clara, CA USA) and the same RNA samples used for RT-PCR experiments. Reverse transcription of mRNA, hybridization, washing and scanning of arrays were performed by the UC Berkeley Functional Genomics Laboratory (http://microarrays.berkeley.edu/). Four independent biological replicate samples from each treatment group were separately assayed (four chips per treatment × genotype combination). RMA-corrected and quantile normalized individual probe intensities were summarized by probeset using the median-polish algorithm [96]; data are provided as Dataset S1. All preliminary analyses were conducted using the “affy” package within Bioconductor (www.bioconductor.org) [97].
Summary values for each transcript-associated probeset represented on the ATH1 array were analyzed in R using a generalized linear model procedure with a model including the class variables GENOTYPE and TREATMENT as described for directed transcript analyses, as well as a GENOTYPE×TREATMENT interaction term (http://www.r-project.org/)[98]. P-values were estimated from an F-distribution, and adjusted for false discovery due to multiple comparisons using the q-value algorithm within R/QVALUE [99]. A similar analysis excluding transcript values for mock-inoculated samples was performed to explicitly identify transcript differences between the two B. cinerea isolate treatments.
Transcripts with full model q-values under 0.001 (equivalent to a false discovery rate of one transcript in 1000) were retained for further analysis. Specific model effects genotype, treatment, and their interaction were then considered significant at a threshold of q≤0.01. RMA median-polish values for transcripts showing a significant treatment effect in the model specifically comparing BcGrape to Bc83-2 infected leaves were normalized using a z-score transformation, where the overall mean value for each transcript is subtracted from the mean for each genotype×treatment combination and the result is divided by that transcript's overall standard deviation. Pearson correlation coefficients of these z-score normalized transcripts were used to cluster transcripts by the weighted pair-group method with averaging (WPGMA) (www.bioinf.ebc.ee/EP/EP/EPCLUST/). Gene ontology and annotation descriptions for these transcripts were obtained from The Arabidopsis Information Resource (www.arabidopsis.org). In addition, average per-locus transcript values from leaves infected with BcGrape or Bc83-2 were compared within each plant genotype. Transcripts showing the largest magnitude of difference between BcGrape and Bc83-2 infected leaves within each plant genotype were selected for further exploratory analyses. Lists of genes co-regulated with these transcripts were generated using ATTED-II (atted.jp) [100]. These lists were compared with the list of transcripts showing significant isolate differences to identify previously undescribed gene networks associated with differential plant responses to these B. cinerea isolates. Analysis of promoter elements was conducted using the Athena package with elements considered significantly overrepresented at a P value of <0.001 [101].
Supporting Information
Zdroje
1. HoweGA
2004 Jasmonates as signals in the wound response. J Plant Growth Regul 23 223 237
2. KodaY
1997 Possible involvement of jasmonates in various morphogenic events. Physiol Plant 100 639 646
3. Pena-CortesH
BarriosP
DortaF
PolancoV
SanchezC
2004 Involvement of jasmonic acid and derivatives in plant responses to pathogens and insects and in fruit ripening. J Plant Growth Regul 23 246 260
4. ThalerJS
OwenB
HigginsVJ
2004 The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol 135 530 538
5. ThommaB
EggermontK
BroekaertWF
CammueBPA
2000 Disease development of several fungi on Arabidopsis can be reduced by treatment with methyl jasmonate. Plant Physiol Biochem 38 421 427
6. TrusovY
RookesJE
ChakravortyD
ArmourD
SchenkPM
2006 Heterotrimeric G proteins facilitate Arabidopsis resistance to necrotrophic pathogens and are involved in jasmonate signaling. Plant Physiol 140 210 220
7. AdieB
ChicoJM
Rubio-SomozaI
SolanoR
2007 Modulation of plant defenses by ethylene. J Plant Growth Regul 26 160 177
8. AdieB
Perez-PerezJ
Perez-PerezMM
GodoyM
Sanchez-SerranoJJ
2007 ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 19 1665 1681
9. AndersonJP
BadruzsaufariE
SchenkPM
MannersJM
DesmondOJ
2004 Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16 3460 3479
10. BeckersGJM
SpoelSH
2006 Fine-tuning plant defence signalling: Salicylate versus jasmonate. Plant Biol 8 1 10
11. de BruxellesGL
RobertsMR
2001 Signals regulating multiple responses to wounding and herbivores. Crit Rev Plant Sci 20 487 521
12. DevotoA
TurnerJG
2005 Jasmonate-regulated Arabidopsis stress signalling network. Physiol Plant 123 161 172
13. Robert-SeilaniantzA
NavarroL
BariR
JonesJD
2007 Pathological hormone imbalances. Curr Opin Plant Biol 10 372 379
14. ThalerJS
BostockRM
2004 Interactions between abscisic-acid-mediated responses and plant resistance to pathogens and insects. Ecology 85 48 58
15. Hammond-KosackKE
ParkerJE
2003 Deciphering plant-pathogen communication: fresh perspectives for molecular resistance breeding. Curr Opin Biotechnol 14 177 193
16. LewisDH
1973 Concepts in fungal nutrition and the origin of biotrophy. Biol Rev 48 261 278
17. OliverRP
IpchoSVS
2004 Arabidopsis pathology breathes new life into the necrotrophs-vs.-biotrophs classification of fungal pathogens. Mol Plant Pathol 5 347 352
18. JonesJDG
DanglJL
2006 The plant immune system. Nature 444 323 329
19. WitPJGM
2007 Visions & reflections (minireview) - How plants recognize pathogens and defend themselves. Cellu Mol Life Sci 64 2726 2732
20. GlazebrookJ
2005 Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43 205 227
21. ThommaBPHJ
EggermontK
TierensKFMJ
BroekaertWF
1999 Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol 121 1093 1101
22. ThommaBPHJ
EggermontK
PenninckxIAMA
Mauch-ManiB
VogelsangR
1998 Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95 15107 15111
23. StaalJ
KaliffM
DewaeleE
PerssonM
DixeliusC
2008 RLM3, a TIR domain encoding gene involved in broad-range immunity of Arabidopsis to necrotrophic fungal pathogens. Plant J 55 188 200
24. BentAF
MackeyD
2007 Elicitors, effectors, and R genes: The new paradigm and a lifetime supply of questions. Annu Rev Phytopathol 45 399 436
25. EllisJG
DoddsPN
LawrenceGJ
2007 Flax rust resistance gene specificity is based on direct resistance-avirulence protein interactions. Annu Rev Phytopathol 45 289 306
26. van OoijenG
van den BurgHA
CornelissenBJC
TakkenFLW
2007 Structure and function of resistance proteins in solanaceous plants. Annu Rev Phytopathol 45 43 72
27. DerckelJP
BaillieulF
ManteauS
AudranJC
HayeB
1999 Differential induction of grapevine defenses by two strains of Botrytis cinerea. Phytopathology 89 197 203
28. FerrariS
PlotnikovaJM
De LorenzoG
AusubelFM
2003 Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2 but not SID2, EDS5, or PAD4. Plant J 35 193 205
29. JeandetP
Douillt-BreuilAC
BessisR
DebordS
SbaghiM
2002 Phytoalexins from the vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J Agric Food Chem 50 2731 2741
30. KliebensteinDJ
RoweHC
DenbyKJ
2005 Secondary metabolites influence Arabidopsis/Botrytis interactions: variation in host production and pathogen sensitivity. Plant J 44 25 36
31. QuiddeT
ButtnerP
TudzynskiP
1999 Evidence for three different specific saponin-detoxifying activities in Botrytis cinerea and cloning and functional analysis of a gene coding for a putative avenacinase. Eur J Plant Pathol 105 273 283
32. BrooksDM
BenderCL
KunkelBN
2005 The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid-dependent defences in Arabidopsis thaliana. Mol Plant Pathol 6 629 639
33. CumagunCJR
BowdenRL
JurgensonJE
LeslieJF
MiedanerT
2004 Genetic mapping of pathogenicity and aggressiveness of Gibberella zeae (Fusarium graminearum) toward wheat. Phytopathology 94 520 526
34. UppalapatiSR
IshigaY
WangdiT
KunkelBN
AnandA
2007 The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol Plant-Microbe Interact 20 955 965
35. HwangMSH
MorganRL
SarkarSF
WangPW
GuttmanDS
2005 Phylogenetic characterization of virulence and resistance phenotypes of Pseudomonas syringae. Applied and Environmental Microbiology 71 5182 5191
36. SiewersV
SmedsgaardJ
TudzynskiP
2004 The p450 monooxygenase BcABA1 is essential for abscisic acid biosynthesis in Botrytis cinerea. Appl Environ Microbiol 70 3868 3876
37. CristescuSM
De MartinisD
HekkertStL
ParkerDH
HarrenFJM
2002 Ethylene production by Botrytis cinerea in vitro and in tomatoes. Appl Environ Microbiol 68 5342 5350
38. EladY
WilliamsonB
TudzynskiP
DelenN
2004 Botrytis: Biology, Pathology and Control Dordrecht Kluwer Academic Publishers 428
39. PrinsTW
TudzynskiP
von TiedemannA
TudzynskiB
ten HaveA
2000 Infection strategies of Botrytis cinerea and related necrotrophic pathogens.
KronstadJ
Fungal Pathology Dordrecht, The Netherlands Kluwer Academic Publishers 32 64
40. WilliamsonB
TudzynskB
TudzynskiP
van KanJAL
2007 Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol 8 561 580
41. ParkJH
HalitschkeR
KimHB
BaldwinIT
FeldmannKA
2002 A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31 1 12
42. XieD-X
FeysBF
JamesS
Nieto-RostroM
TurnerJG
1998 COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280 1091 1094
43. KatsirL
ChungHS
KooAJK
HoweGA
2008 Jasmonate signaling: a conserved mechanism of hormone sensing. Curr Opin Plant Biol 11 428 435
44. CaloL
GarciaI
GotorC
RomeroLC
2006 Leaf hairs influence phytopathogenic fungus infection and confer an increased resistance when expressing a Trichoderma alpha-1,3-glucanase. J Exper Bot 57 3911 3920
45. ZhouN
TootleTL
GlazebrookJ
1999 Arabidopsis PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monooxygenase. Plant Cell 11 2419 2428
46. ThommaB
NelissenI
EggermontK
BroekaertWF
1999 Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J 19 163 171
47. BottcherC
WestphalL
SchmotzC
PradeE
ScheelD
2009 The multifunctional enzyme CYP71B15 (PHYTOALEXIN DEFICIENT3) converts cysteine-idole-3-acetonitrile to camalexin in the indole-3-acetonitrile metabolic network of Arabidopsis thaliana. Plant Cell 21 1830 1845
48. NafisiM
GoregaokerS
BotangaCJ
GlawischnigE
OlsenCE
2007 Arabidopsis cytochrome P450 monooxygenase 71A13 catalyzes the conversion of indole-3-acetaldoxime in camalexin synthesis. Plant Cell 19 2039 2052
49. GlawischnigE
2007 Camalexin. Phytochemistry 68 401 406
50. AbuQamarS
ChenX
DhawanR
BluhmB
SalmeronJ
2006 Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. Plant J 48 28 44
51. FerrariS
GallettiR
DenouxC
De LorenzoG
AusubelFM
2007 Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol 144 367 379
52. BergerS
Mitchell-OldsT
StotzHU
2002 Local and differential control of vegetative storage protein expression in response to herbivore damage in Arabidopsis thaliana. Physiol Plant 114 85 91
53. MannersJM
PenninckxI
VermaereK
KazanK
BrownRL
1998 The promoter of the plant defensin gene PDF1.2 from Arabidopsis is systemically activated by fungal pathogens and responds to methyl jasmonate but not to salicylic acid. Plant Mol Biol 38 1071 1080
54. ZhangP
FoersterH
TissierCP
MuellerL
PaleyS
2005 MetaCyc and AraCyc. Metabolic Pathway Databases for Plant Research. Plant Physiol 138 27 37
55. KliebensteinDJ
RoweHC
DenbyKJ
2005 Secondary metabolites influence Arabidopsis/Botrytis interactions: variation in host production and pathogen sensitivity. Plant J 44 25 36
56. DhawanR
LuoH
FoersterAM
AbuqamarS
DuHN
2009 HISTONE MONOUBIQUITINATION1 interacts with a subunit of the mediator complex and regulates defense against necrotrophic fungal pathogens in Arabidopsis. Plant Cell 21 1000 1019
57. MengisteT
ChenX
SalmeronJ
DietrichR
2003 The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 15 2551 2565
58. VeroneseP
NakagamiH
BluhmB
AbuQamarS
ChenX
2006 The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell 18 257 273
59. ZhengZY
Abu QamarS
ChenZX
MengisteT
2006 Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J 48 592 605
60. Gandia-HerreroF
LorenzA
LarsonT
GrahamIA
BowlesDJ
2008 Detoxification of the explosive 2,4,6-trinitrotoluene in Arabidopsis: discovery of bifunctional O - and C-glucosyltransferases. Plant J 56 963 974
61. KasajimaI
IdeY
HiraiMY
FujiwaraT
2007 Boron transcriptome analysis - profiles of boron nutrition-regulated genes and role of WRKY6 in regulation. 18th International Conference on Arabidopsis Research. Beijing, China
62. MaSS
GongQQ
BohnertHJ
2006 Dissecting salt stress pathways. J Exper Bot 57 1097 1107
63. ColemanJ
Blake-KalffM
DaviesE
1997 Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends Plant Sci 2 144 151
64. TutejaN
2007 Abscisic Acid and abiotic stress signaling. Plant Signal Behav 2 135 138
65. Yamaguchi-ShinozakiK
ShinozakiK
2005 Organization of cis-acting regulatory elements in osmotic - and cold-stress-responsive promoters. Trends Plant Sci 10 88 94
66. BednarekP
Pislewska-BednarekM
SvatosA
SchneiderB
DoubskyJ
2009 A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323 101 106
67. ClayNK
AdioAM
DenouxC
JanderG
AusubelFM
2009 Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323 95 101
68. DienerAC
GaxiolaRA
FinkGR
2001 Arabidopsis ALF5, a Multidrug Efflux Transporter gene family member, confers resistance to toxins. Plant Cell 13 1625 1638
69. MagalhaesJV
LiuJ
GuimaraesCT
LanaUGP
AlvesVMC
2007 A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39 1156 1161
70. OmoteH
HiasaM
MatsumotoT
OtsukaM
MoriyamaY
2006 The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends in Pharmacological Sciences 27 587 593
71. PreM
AtallahM
ChampionA
De VosM
PieterseCMJ
2008 The AP2/ERF Domain Transcription Factor ORA59 Integrates Jasmonic Acid and Ethylene Signals in Plant Defense. Plant Physiol 147 1347 1357
72. Bies-EtheveN
Gaubier-ComellaP
DeburesA
LasserreE
JobetE
2008 Inventory, evolution and expression profiling diversity of the LEA (late embryogenesis abundant) protein gene family in Arabidopsis thaliana. Plant Mol Biol 67 107 124
73. SpoelSH
JohnsonJS
DongX
2007 Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc Natl Acad Sci USA 104 18842 18847
74. WalleyJW
RoweHC
XiaoYM
ChehabEW
KliebensteinDJ
2008 The chromatin remodeler SPLAYED regulates specific stress signaling pathways. PLoS Path 4 e1000237 doi:10.1371/journal.ppat.1000237
75. BrowseJ
2009 Jasmonate passes muster: A receptor and targets for the defense hormone. Annu Rev Plant Biol 60 183 205
76. ChicoJM
ChiniA
FonsecaS
SolanoR
2008 JAZ repressors set the rhythm in jasmonate signaling. Curr Opin Plant Biol 11 486 494
77. ChiniA
FonsecaS
FernandezG
AdieB
ChicoJM
2007 The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448 666 U664
78. StefanatoFL
Abou-MansourE
BuchalaA
KretschmerM
MosbachA
2009 The ABC transporter BcatrB from Botrytis cinerea exports camalexin and is a virulence factor on Arabidopsis thaliana. Plant J 58 499 510
79. GomezC
TerrierN
TorregrosaL
VialetS
Fournier-LevelA
2009 Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters. Plant Physiol 150 402 415
80. NawrathC
HeckS
ParinthawongN
MetrauxJP
2002 EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14 275 286
81. ShojiT
InaiK
YazakiY
SatoY
TakaseH
2009 Multidrug and Toxic Compound Extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots. Plant Physiol 149 708 718
82. AudenaertK
De MeyerGB
HofteMM
2002 Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol 128 491 501
83. de Torres-ZabalaM
TrumanW
BennettMH
LafforgueG
MansfieldJW
2007 Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J 26 1434 1443
84. JakabG
CottierV
ToquinV
RigoliG
ZimmerliL
2001 beta-Aminobutyric acid-induced resistance in plants. Eur J Plant Pathol 107 29 37
85. OritaniT
KiyotaH
2003 Biosynthesis and metabolism of abscisic acid and related compounds. Nat Prod Rep 20 414 425
86. ZimmerliL
MetrauxJP
Mauch-ManiB
2001 beta-aminobutyric acid-induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinerea. Plant Physiol 126 517 523
87. MikkelsenMD
HansenCH
WittstockU
HalkierBA
2000 Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J Biol Chem 275 33712 33717
88. ThinesB
KatsirL
MelottoM
NiuY
MandaokarA
2007 JAZ repressor proteins are targets of the SCFCO11 complex during jasmonate signalling. Nature 448 661 U662
89. RoweHC
KliebensteinDJ
2007 Elevated genetic variation within virulence-associated Botrytis cinerea polygalacturonase loci. Mol Plant-Microbe Interact 20 1126 1137
90. AbramoffMD
MagelhaesPJ
RamSJ
2004 Image Processing with ImageJ. Biophoton Internatl 11 36 42
91. WeigelD
GlazebrookJ
2002 Arabidopsis, a Laboratory Manual New York Cold Spring Harbour Press
92. ClarkeJD
2009 Phenotypic Analysis of Arabidopsis Mutants: Diaminobenzidine Stain for Hydrogen Peroxide. Cold Spring Harbor Protocols Cold Spring Harbor, NY, USA Cold Spring Harbor Press pp. 10.1101/pdb.prot4981
93. RateDN
CuencaJV
BowmanGR
GuttmanDS
GreenbergJT
1999 The Gain-of-Function Arabidopsis acd6 Mutant Reveals Novel Regulation and Function of the Salicylic Acid Signaling Pathway in Controlling Cell Death, Defenses, and Cell Growth. Plant Cell 11 1695 1708
94. WalleyJW
CoughlanS
HudsonME
CovingtonMF
KaspiR
2007 Mechanical stress induces biotic and abiotic stress responses via a novel cis-element. PLoS Genet 3 e172 doi:10.1371/journal.pgen.0030172
95. CzechowskiT
StittM
AltmannT
UdvardiMK
ScheibleW-R
2005 Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139 5 17
96. BolstadBM
IrizarryRA
AstrandM
SpeedTP
2003 A comparison of normalization methods for high density oligonucleotide array data based on bias and variance. Bioinformatics 19 185 193
97. GentlemanRC
CareyVJ
BatesDM
BolstadBM
DettlingM
2004 Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5 R80
98. R_Development_Core_Team 2009 R: Language and Environment for Statistical Computing Vienna R Foundation for Statistical Computing
99. StoreyJD
2002 A direct approach to false discovery rates. J Royal Stat Soc Ser B 64 479 498
100. ObayashiT
HayashiS
SaekiM
OhtaH
KinoshitaK
2008 ATTED-II provides coexpressed gene networks for Arabidopsis. Nucl Acids Res DOI:10.1093/nar/gkn1807
101. O'ConnorTR
DyresonC
WyrickJJ
2005 Athena: a resource for rapid visualization and systematic analysis of Arabidopsis promoter sequences. Bioinformatics 21 4411 4413
102. BessireM
ChassotC
JacquatAC
HumphryM
BorelS
2007 A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J 26 2158 2168
103. RoweHC
KliebensteinDJ
2008 Complex genetics control natural variation in Arabidopsis thaliana resistance to Botrytis cinerea. Genetics 180 2237 2250
104. DenbyKJ
KumarP
KliebensteinDJ
2004 Identification of Botrytis cinerea susceptibility loci in Arabidopsis thaliana. Plant J 38 473 486
105. SchuheggerR
RauhutT
GlawischnigE
2007 Regulatory variability of camalexin biosynthesis. J Plant Physiol 164 636 644
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument TurnoverČlánek Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic PathwaysČlánek The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 4- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Innate Recognition of Fungal Cell Walls
- Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument Turnover
- Junín Virus Infection of Human Hematopoietic Progenitors Impairs Proplatelet Formation and Platelet Release via a Bystander Effect Involving Type I IFN Signaling
- The Endosymbiotic Bacterium Induces Resistance to Dengue Virus in
- Natural Regulatory T Cells in Malaria: Host or Parasite Allies?
- Keratinocytes Determine Th1 Immunity during Early Experimental Leishmaniasis
- Spatial and Temporal Association of Outbreaks of H5N1 Influenza Virus Infection in Wild Birds with the 0°C Isotherm
- Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic Pathways
- RNA Polymerase Activity and Specific RNA Structure Are Required for Efficient HCV Replication in Cultured Cells
- The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
- Inadequate Clearance of Translocated Bacterial Products in HIV-Infected Humanized Mice
- Topology and Organization of the Type III Secretion Needle Complex Components
- Temperature Modulates Plant Defense Responses through NB-LRR Proteins
- Peptide Inhibitors of Dengue-Virus Entry Target a Late-Stage Fusion Intermediate
- Identification of Host-Dependent Survival Factors for Intracellular through an siRNA Screen
- Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation
- Increased Asymmetric Dimethylarginine in Severe Falciparum Malaria: Association with Impaired Nitric Oxide Bioavailability and Fatal Outcome
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Induces Brain Microvascular Endothelial Cell Detachment from the Matrix and Cleavage of Occludin: A Role for MMP-8
- Two Coregulated Efflux Transporters Modulate Intracellular Heme and Protoporphyrin IX Availability in
- The Type I NADH Dehydrogenase of Counters Phagosomal NOX2 Activity to Inhibit TNF-α-Mediated Host Cell Apoptosis
- Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
- Increased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma
- The RING-CH Ligase K5 Antagonizes Restriction of KSHV and HIV-1 Particle Release by Mediating Ubiquitin-Dependent Endosomal Degradation of Tetherin
- Molecular Mechanisms of Ethanol-Induced Pathogenesis Revealed by RNA-Sequencing
- Highly Frequent Mutations in Negative Regulators of Multiple Virulence Genes in Group A Streptococcal Toxic Shock Syndrome Isolates
- Emergence and Pathogenicity of Highly Virulent Genotypes in the Northwest United States
- Structural and Functional Analysis of Viral siRNAs
- Prion Shedding from Olfactory Neurons into Nasal Secretions
- a GATA Transcription Factor That Directs Disparate Fates in Including Morphogenesis and Siderophore Biosynthesis
- Three Members of the 6-cys Protein Family of Play a Role in Gamete Fertility
- Complement as an Endogenous Adjuvant for Dendritic Cell-Mediated Induction of Retrovirus-Specific CTLs
- A Genomic Survey of Positive Selection in Provides Insights into the Evolution of Accidental Virulence
- Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes
- Blood Fluke Exploitation of Non-Cognate CD4 T Cell Help to Facilitate Parasite Development
- Antagonism of Tetherin Restriction of HIV-1 Release by Vpu Involves Binding and Sequestration of the Restriction Factor in a Perinuclear Compartment
- The Development of Therapeutic Antibodies That Neutralize Homologous and Heterologous Genotypes of Dengue Virus Type 1
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- Interactions with Bacteria in the Context of Human Health and Disease
- Viral Capsid Is a Pathogen-Associated Molecular Pattern in Adenovirus Keratitis
- Electron Tomography Reveals the Steps in Filovirus Budding
- Selective Condensation Drives Partitioning and Sequential Secretion of Cyst Wall Proteins in Differentiating
- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
- VacA Toxin/Subunit p34: Targeting of an Anion Channel to the Inner Mitochondrial Membrane
- Production of Extracellular Traps against and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA
- A Differential Role for Macropinocytosis in Mediating Entry of the Two Forms of Vaccinia Virus into Dendritic Cells
- Impaired Innate Immunity in Mice but Preserved CD8 T Cell Responses against in -, -, - or -Deficient Mice
- SARS-CoV Pathogenesis Is Regulated by a STAT1 Dependent but a Type I, II and III Interferon Receptor Independent Mechanism
- Proteolysis of Human Thrombin Generates Novel Host Defense Peptides
- Multilayered Mechanism of CD4 Downregulation by HIV-1 Vpu Involving Distinct ER Retention and ERAD Targeting Steps
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



