-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Innate Recognition of Fungal Cell Walls
article has not abstract
Published in the journal: Innate Recognition of Fungal Cell Walls. PLoS Pathog 6(4): e32767. doi:10.1371/journal.ppat.1000758
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1000758Summary
article has not abstract
The emergence of fungal infections as major causes of morbidity and mortality in immunosuppressed individuals has prompted studies into how the host recognizes fungal pathogens. Fungi are eukaryotes and as such share many similarities with mammalian cells. The most striking difference, though, is the presence of a cell wall that serves to protect the fungus from environmental stresses, particularly osmotic changes [1]. This task is made challenging because the fungus must remodel itself to allow for cell growth and division, including the conversion to different morphotypes, such as occurs during germination of spherical spores into filamentous hyphae. The cell wall also connects the fungus with its environment by triggering intracellular signaling pathways and mediating adhesion to other cells and extracellular matrices. Here, important facts and concepts critical for understanding innate sensing of the fungal cell wall by mammalian pathogens are reviewed.
There Are Intra - and Interspecies Similarities and Differences in Fungal Cell Wall Composition
The fungal cell wall is predominantly composed of carbohydrate polymers interspersed with glycoproteins. The three major components, found in all medically important fungi studied to date, are β-glucans (polymers of glucose), chitin (polymer of N-acetylglucosamine), and mannans. While these three components are intermingled throughout the cell wall, chitin tends to predominate near the plasma membrane, whereas the mannans have a propensity for the outer cell wall [1], [2]. β-1,3-glucans form the main structural scaffold of the cell wall and have varying amounts of β-1,6 branches. Chitin is thought to mostly add structural strength to the cell wall. Mannans are chains of up to several hundred mannoses that are added to fungal proteins via N - or O-linkages [3]. Mannoproteins can covalently attach to glucans or chitin via either their sugar residues or via glycosylphosphatidylinositol (GPI) links. GPI anchors may also attach mannoproteins to the plasma membrane. Finally, proteins that are normally found in intracellular compartments, such as heat shock proteins, have been found cross-linked in the cell wall in a manner allowing interaction with immune cells [1], [4], [5].
While the above provides a general overview of the cell wall, it is important to emphasize that extensive differences may be found when comparing different fungal species and even when comparing strains within a species. Examples include α-glucans in addition to β-glucans in some (but not all) strains of Histoplasma capsulatum, chitosan in Cryptococcus neoformans, and galactomannans in species of Aspergillus [6], [7]. Many fungi also have melanin in their cell walls. More subtle differences exist too, such as variations in the length and type of linkages in mannans [3], [8].
There Are Multiple Receptors for Fungal Cell Wall Components
Considering their surface exposure and universal features, it is not surprising that components of the fungal cell wall are recognized by the innate immune system. The ability of animals to sense β-glucans is found in primitive invertebrates, most notably the horseshoe crab. In mammals, many receptors that recognize β-glucans have been described, including dectin-1, complement receptor 3 (CR3, which binds β-glucans at a site distinct from its complement binding site), and three members of the scavenger receptor family, CD5, CD36, and SCARF1 [8]–[10]. The relative contribution of each remains to be fully determined. Dectin-1, a transmembrane C-type lectin receptor highly expressed on myeloid cells, has specificity for β-1,3-glucans [8], [10]. Receptor engagement leads to phosphorylation by Src family kinases of a tyrosine-based activation-like motif (ITAM) located on dectin-1′s cytoplasmic tail and the initiation of Syk-and CARD9-dependent signaling cascades [10]. This results in phagocytosis, the respiratory burst, and activation of the transcription factors NF-κB and NFAT, leading to cytokine/chemokine gene induction. Recently, susceptibility to fungal infections has been associated with mutations in the genes encoding for dectin-1 and CARD9 [11], [12].
Two C-type lectin receptors, the mannose receptor (CD206, also known as the macrophage mannose receptor) and DC-SIGN (CD209), appear to be the major receptors on human myeloid cells that recognize mannans [3]. These two receptors have cytoplasmic motifs directing mannosylated antigens to the endocytic pathway of dendritic cells, where they can be processed and subsequently presented to T cells. The mannose receptor, which is expressed at high levels on alternatively activated macrophages, has no known cytoplasmic signaling motifs. Stimulation of the mannose receptor can lead to either proinflammatory or anti-inflammatory responses, depending upon the ligand and host cell studied [13]. Other receptors that recognize mannose residues include langerin and dectin-2. Dectin-2 and the mannose receptor also have affinity for α-glucans and chitin, respectively, although other chitin receptors undoubtedly exist [14], [15]. Thus, there is redundancy with regards to the number of host receptors that recognize glucans, mannans, and chitin. Moreover, some receptors recognize more than one fungal cell wall carbohydrate.
Surface components on fungi stimulate the toll-like receptors (TLRs) TLR2 and TLR4. With few exceptions, such as phospholipomannan of Candida albicans, the ligands responsible for stimulating the TLRs remain undefined. Some pathogenic fungi gain entry into the cell by displaying cell surface ligands for phagocytic receptors. The chitin-linked protein, BAD1, facilitates access of Blastomyces dermatitidis into macrophages via CR3, which triggers an anti-inflammatory program that fosters pathogen survival [16]. Heat shock protein 60, located on the cell wall of H. capsulatum, is recognized by CD18 on macrophages [4]. Commensal fungi express adhesions, many of which are mannoproteins, that facilitate colonization of epithelial surfaces [5].
Opsonization Provides Further Means for Host Recognition of Fungi
Fungi are potent activators of the complement system, resulting in opsonization due to deposition of C3b and iC3b on the fungal surface and recruitment of inflammatory cells as a result of C3a and C5a generation. However, fungi are resistant to complement-mediated lysis, presumably due to their thick cell wall. Fungi can activate the classical, alternative, and lectin complement pathways. Normal human serum contains antibodies to fungal cell wall components, particularly mannans, that can initiate classical pathway activation upon binding. Such antibodies may also directly opsonize fungi for recognition by phagocytic Fc receptors (FcRs). Activation of the lectin pathway occurs when recognition of exposed mannans by mannose-binding lectin (MBL) triggers MBL-associated serine proteases. Recent work has emphasized the potential contribution of the ficolins and long pentraxin 3 (PTX3) to activation of the lectin complement pathway by fungi [17]. In most models, complement deficiency makes mice more susceptible to experimental mycoses.
Fungal Masking of Ligands Can Influence Host Recognition and Pathogenicity
Most medically important fungi are opportunistic pathogens, generally attaining importance only when the individual is immunocompromised. Nevertheless, many fungi “mask” ligands, with the end result being reduced stimulation of innate immunity. The most extreme example is the capsule of C. neoformans, which completely eclipses the cell wall and imparts virulence on the fungus. In the absence of opsonization, encapsulated C. neoformans are not phagocytosed. The outer layer of mannans mostly mask β-glucans on C. albicans and other fungi, leaving only small amounts of β-glucans exposed [2]. α-glucans, present on some strains of H. capsulatum, form a layer that covers the β-glucans, thus preventing recognition by dectin-1 [6]. Recently, it was demonstrated that RodA, a surface hydrophobin that forms a rodlet layer on the surface of Aspergillus fumigatus conidia by covalent attachment to the cell wall, masks immunogenic determinants on the spores, resulting in a lack of dendritic cell and alveolar macrophage activation and maturation [18]. However, the rodlet layer is shed when conidia swell and germinate into hyphae. While hyphae are avidly recognized by phagocytes, they attain sizes that preclude phagocytosis. Indeed, essential to the pathogenicity of many fungi is their ability to undergo phase transition and display multiple morphotypes with differing surface properties.
Synergism or Antagonism May Be Seen When Fungi Stimulate Multiple Receptors
Studying responses following stimulation of an individual receptor with its cognate ligand provides important insights into host responses and pathogenicity. However, during in vivo infection, a panoply of fungal ligands is displayed in variable concentrations, resulting in stimulation of multiple host cell receptors. Additionally, as fungi generally activate complement and may be recognized by antibody, both opsonic and non-opsonic recognition of fungi typically transpires. It is becoming increasingly clear that ligand combinations elicit complex patterns of inflammatory responses [8]. For example, cryptococcal mannoproteins are weak stimulators of cytokine responses. However, when TLR ligands are combined with the mannoproteins, then synergistic stimulation is observed [13]. In contrast, when dendritic cells are incubated with TLR ligands and β-glucans, synergistic production of TNFα is observed, but IL-12p70 is suppressed [19].
Immune sensing of unopsonized C. albicans monocytes/macrophages is mediated by at least three recognition systems composed of mannose receptors binding N-linked mannosyl residues, TLR4 binding O-linked mannoses, and dectin-1 recognizing β-glucans [8]. Adding to the complexity, following incubation with human serum, CR3 recognizes complement deposited on β-1,6-glucan branches and FcRs recognize bound IgG antibody [20].
In conclusion, common and distinct features are found when comparing cell walls of different fungi. A large number of host receptors sense cell wall components and deposited opsonins (Table 1), although some potential ligands may be masked from their cognate receptors. The nature of the innate and subsequent acquired immune response to fungal pathogens is profoundly influenced by which receptors are stimulated and to what extent.
Tab. 1. Examples of Fungal Cell Wall Ligands and Their Cognate Phagocytic Receptors. 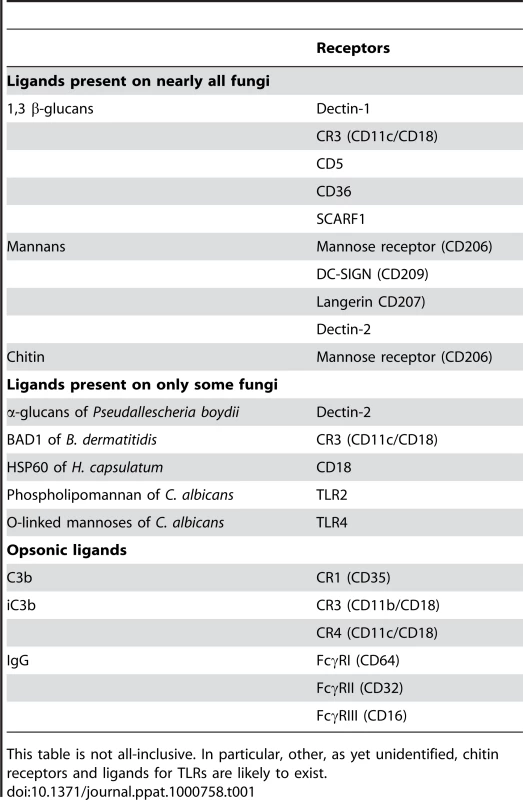
This table is not all-inclusive. In particular, other, as yet unidentified, chitin receptors and ligands for TLRs are likely to exist.
Zdroje
1. BowmanSM
FreeSJ
2006 The structure and synthesis of the fungal cell wall. Bioessays 28 799 808
2. WheelerRT
FinkGR
2006 A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog 2 e35 doi:10.1371/journal.ppat.0020035
3. LevitzSM
SpechtCA
2006 The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res 6 513 524
4. LongKH
GomezFJ
MorrisRE
NewmanSL
2003 Identification of heat shock protein 60 as the ligand on Histoplasma capsulatum that mediates binding to CD18 receptors on human macrophages. J Immunol 170 487 494
5. ChaffinWL
2008 Candida albicans cell wall proteins. Microbiol Mol Biol Rev 72 495 544
6. RappleyeCA
EissenbergLG
GoldmanWE
2007 Histoplasma capsulatum α-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc Natl Acad Sci U S A 104 1366 1370
7. BanksIR
SpechtCA
DonlinMJ
GerikKJ
LevitzSM
2005 A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot Cell 4 1902 1912
8. NeteaMG
GowNA
MunroCA
BatesS
CollinsC
2006 Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest 116 1642 1650
9. MeansTK
MylonakisE
TampakakisE
ColvinRA
SeungE
2009 Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med 206 637 653
10. GoodridgeHS
WolfAJ
UnderhillDM
2009 Beta-glucan recognition by the innate immune system. Immunol Rev 230 38 50
11. FerwerdaB
FerwerdaG
PlantingaTS
WillmentJA
van SprielAB
2009 Human Dectin-1 Deficiency and Mucocutaneous Fungal Infections. N Engl J Med 361 1760 1767
12. GlockerE-O
HennigsA
NabaviM
SchafferAA
WoellnerC
2009 A Homozygous CARD9 Mutation in a Family with Susceptibility to Fungal Infections. N Engl J Med 361 1727 1735
13. DanJM
WangJP
LeeCK
LevitzSM
2008 Cooperative stimulation of dendritic cells by Cryptococcus neoformans mannoproteins and CpG oligodeoxynucleotides. PLoS ONE 3 e2046 doi:10.1371/journal.pone.0002046
14. BittencourtVC
FigueiredoRT
da SilvaRB
Mourao-SaDS
FernandezPL
2006 An alpha-glucan of Pseudallescheria boydii is involved in fungal phagocytosis and Toll-like receptor activation. J Biol Chem 281 22614 22623
15. LeeCG
Da SilvaCA
LeeJY
HartlD
EliasJA
2008 Chitin regulation of immune responses: an old molecule with new roles. Curr Opin Immunol
16. BrandhorstTT
WuthrichM
Finkel-JimenezB
WarnerT
KleinBS
2004 Exploiting type 3 complement receptor for TNF-alpha suppression, immune evasion, and progressive pulmonary fungal infection. J Immunol 173 7444 7453
17. MaYJ
DoniA
HummelshojT
HonoreC
BastoneA
2009 Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. J Biol Chem 284 28263 28275
18. AimaniandaV
BayryJ
BozzaS
KniemeyerO
PerruccioK
2009 Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460 1117 1121
19. HuangH
OstroffGR
LeeCK
WangJP
SpechtCA
2009 Distinct patterns of dendritic cell cytokine release stimulated by fungal beta-glucans and toll-like receptor agonists. Infect Immun 77 1774 1781
20. Rubin-BejeranoI
AbeijonC
MagnelliP
GrisafiP
FinkGR
2007 Phagocytosis by human neutrophils is stimulated by a unique fungal cell wall component. Cell Host Microbe 2 55 67
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument TurnoverČlánek Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic PathwaysČlánek The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-DependentČlánek Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 4- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Innate Recognition of Fungal Cell Walls
- Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument Turnover
- Junín Virus Infection of Human Hematopoietic Progenitors Impairs Proplatelet Formation and Platelet Release via a Bystander Effect Involving Type I IFN Signaling
- The Endosymbiotic Bacterium Induces Resistance to Dengue Virus in
- Natural Regulatory T Cells in Malaria: Host or Parasite Allies?
- Keratinocytes Determine Th1 Immunity during Early Experimental Leishmaniasis
- Spatial and Temporal Association of Outbreaks of H5N1 Influenza Virus Infection in Wild Birds with the 0°C Isotherm
- Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic Pathways
- RNA Polymerase Activity and Specific RNA Structure Are Required for Efficient HCV Replication in Cultured Cells
- The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
- Inadequate Clearance of Translocated Bacterial Products in HIV-Infected Humanized Mice
- Topology and Organization of the Type III Secretion Needle Complex Components
- Temperature Modulates Plant Defense Responses through NB-LRR Proteins
- Peptide Inhibitors of Dengue-Virus Entry Target a Late-Stage Fusion Intermediate
- Identification of Host-Dependent Survival Factors for Intracellular through an siRNA Screen
- Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation
- Increased Asymmetric Dimethylarginine in Severe Falciparum Malaria: Association with Impaired Nitric Oxide Bioavailability and Fatal Outcome
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Induces Brain Microvascular Endothelial Cell Detachment from the Matrix and Cleavage of Occludin: A Role for MMP-8
- Two Coregulated Efflux Transporters Modulate Intracellular Heme and Protoporphyrin IX Availability in
- The Type I NADH Dehydrogenase of Counters Phagosomal NOX2 Activity to Inhibit TNF-α-Mediated Host Cell Apoptosis
- Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
- Increased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma
- The RING-CH Ligase K5 Antagonizes Restriction of KSHV and HIV-1 Particle Release by Mediating Ubiquitin-Dependent Endosomal Degradation of Tetherin
- Molecular Mechanisms of Ethanol-Induced Pathogenesis Revealed by RNA-Sequencing
- Highly Frequent Mutations in Negative Regulators of Multiple Virulence Genes in Group A Streptococcal Toxic Shock Syndrome Isolates
- Emergence and Pathogenicity of Highly Virulent Genotypes in the Northwest United States
- Structural and Functional Analysis of Viral siRNAs
- Prion Shedding from Olfactory Neurons into Nasal Secretions
- a GATA Transcription Factor That Directs Disparate Fates in Including Morphogenesis and Siderophore Biosynthesis
- Three Members of the 6-cys Protein Family of Play a Role in Gamete Fertility
- Complement as an Endogenous Adjuvant for Dendritic Cell-Mediated Induction of Retrovirus-Specific CTLs
- A Genomic Survey of Positive Selection in Provides Insights into the Evolution of Accidental Virulence
- Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes
- Blood Fluke Exploitation of Non-Cognate CD4 T Cell Help to Facilitate Parasite Development
- Antagonism of Tetherin Restriction of HIV-1 Release by Vpu Involves Binding and Sequestration of the Restriction Factor in a Perinuclear Compartment
- The Development of Therapeutic Antibodies That Neutralize Homologous and Heterologous Genotypes of Dengue Virus Type 1
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- Interactions with Bacteria in the Context of Human Health and Disease
- Viral Capsid Is a Pathogen-Associated Molecular Pattern in Adenovirus Keratitis
- Electron Tomography Reveals the Steps in Filovirus Budding
- Selective Condensation Drives Partitioning and Sequential Secretion of Cyst Wall Proteins in Differentiating
- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
- VacA Toxin/Subunit p34: Targeting of an Anion Channel to the Inner Mitochondrial Membrane
- Production of Extracellular Traps against and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA
- A Differential Role for Macropinocytosis in Mediating Entry of the Two Forms of Vaccinia Virus into Dendritic Cells
- Impaired Innate Immunity in Mice but Preserved CD8 T Cell Responses against in -, -, - or -Deficient Mice
- SARS-CoV Pathogenesis Is Regulated by a STAT1 Dependent but a Type I, II and III Interferon Receptor Independent Mechanism
- Proteolysis of Human Thrombin Generates Novel Host Defense Peptides
- Multilayered Mechanism of CD4 Downregulation by HIV-1 Vpu Involving Distinct ER Retention and ERAD Targeting Steps
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



