-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
Bacterial superantigens (SAg) stimulate T-cell hyper-activation resulting in immune modulation and severe systemic illnesses such as Staphylococcus aureus toxic shock syndrome. However, all known S. aureus SAgs are encoded by mobile genetic elements and are made by only a proportion of strains. Here, we report the discovery of a novel SAg staphylococcal enterotoxin-like toxin X (SElX) encoded in the core genome of 95% of phylogenetically diverse S. aureus strains from human and animal infections, including the epidemic community-associated methicillin-resistant S. aureus (CA-MRSA) USA300 clone. SElX has a unique predicted structure characterized by a truncated SAg B-domain, but exhibits the characteristic biological activities of a SAg including Vβ-specific T-cell mitogenicity, pyrogenicity and endotoxin enhancement. In addition, SElX is expressed by clinical isolates in vitro, and during human, bovine, and ovine infections, consistent with a broad role in S. aureus infections of multiple host species. Phylogenetic analysis suggests that the selx gene was acquired horizontally by a progenitor of the S. aureus species, followed by allelic diversification by point mutation and assortative recombination resulting in at least 17 different alleles among the major pathogenic clones. Of note, SElX variants made by human - or ruminant-specific S. aureus clones demonstrated overlapping but distinct Vβ activation profiles for human and bovine lymphocytes, indicating functional diversification of SElX in different host species. Importantly, SElX made by CA-MRSA USA300 contributed to lethality in a rabbit model of necrotizing pneumonia revealing a novel virulence determinant of CA-MRSA disease pathogenesis. Taken together, we report the discovery and characterization of a unique core genome-encoded superantigen, providing new insights into the evolution of pathogenic S. aureus and the molecular basis for severe infections caused by the CA-MRSA USA300 epidemic clone.
Published in the journal: A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia. PLoS Pathog 7(10): e32767. doi:10.1371/journal.ppat.1002271
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002271Summary
Bacterial superantigens (SAg) stimulate T-cell hyper-activation resulting in immune modulation and severe systemic illnesses such as Staphylococcus aureus toxic shock syndrome. However, all known S. aureus SAgs are encoded by mobile genetic elements and are made by only a proportion of strains. Here, we report the discovery of a novel SAg staphylococcal enterotoxin-like toxin X (SElX) encoded in the core genome of 95% of phylogenetically diverse S. aureus strains from human and animal infections, including the epidemic community-associated methicillin-resistant S. aureus (CA-MRSA) USA300 clone. SElX has a unique predicted structure characterized by a truncated SAg B-domain, but exhibits the characteristic biological activities of a SAg including Vβ-specific T-cell mitogenicity, pyrogenicity and endotoxin enhancement. In addition, SElX is expressed by clinical isolates in vitro, and during human, bovine, and ovine infections, consistent with a broad role in S. aureus infections of multiple host species. Phylogenetic analysis suggests that the selx gene was acquired horizontally by a progenitor of the S. aureus species, followed by allelic diversification by point mutation and assortative recombination resulting in at least 17 different alleles among the major pathogenic clones. Of note, SElX variants made by human - or ruminant-specific S. aureus clones demonstrated overlapping but distinct Vβ activation profiles for human and bovine lymphocytes, indicating functional diversification of SElX in different host species. Importantly, SElX made by CA-MRSA USA300 contributed to lethality in a rabbit model of necrotizing pneumonia revealing a novel virulence determinant of CA-MRSA disease pathogenesis. Taken together, we report the discovery and characterization of a unique core genome-encoded superantigen, providing new insights into the evolution of pathogenic S. aureus and the molecular basis for severe infections caused by the CA-MRSA USA300 epidemic clone.
Introduction
Staphylococcus aureus is responsible for an array of diseases including life-threatening toxinoses such as toxic shock syndrome (TSS) and necrotizing pneumonia. Many strains of S. aureus have been shown to produce members of a family of more than 20 serologically distinct superantigenic (SAg) toxins, which contribute to disease pathogenesis via modulation of the host immune response [1], [2]. Previously characterized SAgs are small secreted proteins of 20 to 28 kDa in size, which share similar biochemical, structural, and immunobiological properties [1], [2], but can be differentiated into 4 distinct subgroups according to their phylogenetic relatedness [3]–[5]. They share a compact 2-domain protein structure consisting of domain A which contains a long central α-helix and a C-terminal β-grasp motif, and the smaller domain B which contains an N-terminal oligonucleotide-oligosaccharide binding (OB) fold [1], [2], [5], [6]. SAgs bypass the conventional antigen processing pathway, by binding simultaneously to MHC class II molecules of antigen-presenting cells, outside of the antigen-binding groove, and the variable region of the T-cell receptor (TCR) β-chain (Vβ) [7], [8]. In so doing, SAgs can react with all T-cells expressing reactive Vβ-TCR regions (up to 50%), instead of the normal responding frequency to an antigen of approximately 0.01%, resulting in massive inflammatory cytokine release and consequent toxic shock [9]. SAgs also have the capacity to cause immune suppression by inducing T-cell anergy, and may contribute to bacterial persistence during chronic infection [10].
All staphylococcal SAgs identified to date are encoded by mobile genetic elements (MGE) such as plasmids, phages, transposons and S. aureus pathogenicity islands (SaPIs), or the highly variable genomic region vSaβ [4], [11]–[14]. Accordingly, the distribution of SAg genes is S. aureus strain-dependent. For example, Omoe et al. reported that 80% of human isolates contain at least one SAg gene, including 50% which contain the enterotoxin gene cluster (egc) locus, and Smyth et al. demonstrated that 57% of animal strains examined contained at least one SAg gene with the egc found in 30% of isolates [15], [16]. These data collectively suggest that no single SAg is encoded by more than 50% of strains and that some strains may not have superantigenic capacity at all. The USA300 S. aureus clone which is responsible for the current CA-MRSA epidemic affecting the USA and other countries is typically associated with skin and soft tissue infections and has the capacity to cause lethal toxinoses such as necrotizing pneumonia and extreme pyrexia [17]–[19]. However, USA300 strains do not typically produce TSST-1 or staphylococcal enterotoxins B and C, the SAgs most often associated with toxic shock [20], [21]. Here, we report the discovery of a unique core genome-encoded SAg (designated SElX), encoded by the great majority of S. aureus strains, determine its immunobiological function and examine its molecular evolution. Our data suggest that SElX contributes to immune modulation in both human and animal disease pathogenesis, and demonstrate a role for SElX in the development CA-MRSA USA300 necrotizing pneumonia in a rabbit model of infection.
Results
Identification of a novel putative SAg gene encoded by the great majority of S. aureus strains
In order to examine the superantigenic capacity of the epidemic CA-MRSA clone, USA300, we carried out a genome-wide screen of USA300 strain FPR3757 to identify genes encoding proteins with homology to known SAgs [21]. In addition to characterized SAgs, SElK and SElQ, we discovered a SAg gene homolog, SAUSA300_0370 which encodes a protein with 24% amino acid identity to the major virulence factor, TSST-1 and 27% identity with the SAg-like protein, SSL7. The gene, designated selx encoding staphylococcal enterotoxin-like toxin X (SElX), is 612 bp in length and is most closely-related to the phylogenetic group IV of staphylococcal SAgs represented by TSST-1, closely allied to the staphylococcal SAg-like (SSL) protein family (Fig. 1a). The mature form of SElX is 168 amino acids in length with a predicted molecular weight of 19343 Da, smaller than any known staphylococcal SAg [1], [4]. In order to examine the distribution of selx among S. aureus strains, we screened a total of 114 isolates by a combination of bioinformatic interrogation of 53 publicly available S. aureus genome sequences (Table S1), and PCR analysis using selx-specific oligonucleotide primers (Table S2) of a further 61 S. aureus isolates selected to represent the full breadth of species diversity and to include the most important human and animal pathogenic clones (Table S3). Remarkably, 108 of the 114 isolates (95%) contained the selx gene which included all strains examined except isolates of the CC30 clonal complex (Fig. 1b; Table S1 and S3).
Fig. 1. Phylogenetic analysis of selx and its species-wide distribution. 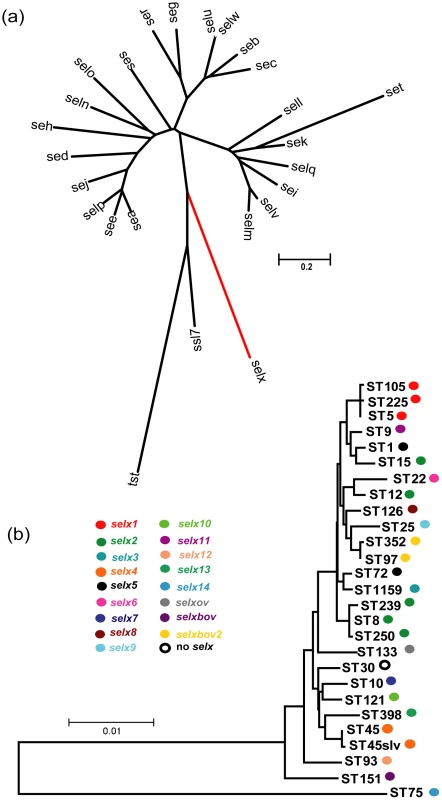
a) Neighbor-joining tree based on the sequences of all known staphylococcal SAgs and the SAg-like protein, SSL7 b) Neighbor-joining tree of concatenated MLST sequences of representative S. aureus STs representing the breadth of species diversity. Coloured circles denote the presence of specific selx alleles. Analysis of the location of selx in the genomes of strains representing diverse multi-locus sequence types (ST)s, including MSSA476 (ST1), N315 (ST5), USA300 TCH3757 (ST8), H19 (ST10), A9635 (ST45slv), TCH130 (ST72), JKD6159 (ST93), JH1 (ST105), ED133 (ST133), RF122 (ST151), 04–02891 (ST225), JKD6008 (ST239), COL (ST250) and TCH959 (ST1159), revealed that selx is located at an identical chromosomal site in all strains examined (Fig. S1). Specifically, it is located ∼400 kb from the origin of replication in the oriC environ among a cluster of 4 genes specific for the S. aureus species including 2 genes encoding hypothetical proteins of unknown function, and a predicted integrase pseudogene which contains partial homology to an integrase encoded by the S. aureus bacteriophage, PT1028 [22]. The gene cluster is flanked by conserved genes encoding ribosomal proteins and a DNA-binding protein involved in DNA replication, which are located at the same genomic location in other staphylococcal species such as Staphylococcus epidermidis (Fig. S1). The genetic linkage of selx with an integrase pseudogene, its wide distribution across the full breadth of S. aureus species diversity, and its absence in the genomes of other staphylococcal species indicates an ancient horizontal acquisition which may have occurred during S. aureus speciation. The existence of a single S. aureus clonal complex (CC30), which does not contain selx but contains the adjacent integrase pseudogene at the same chromosomal site, is consistent with a deletion event in a progenitor of the clonal lineage which contained the selx gene (Fig. S1).
selx exhibits considerable species-wide allelic variation and evidence for assortative recombination
In order to examine the allelic variation of selx among S. aureus strains representing the breadth of species diversity, DNA sequencing of selx was carried out for 11 S. aureus strains representative of ST 9, 12, 15, 25, 45, 97, 121, and 126 and compared to the sequence of selx for 49 strains available in Genbank (Table S1). selx was represented by 17 distinct allelic variants encoded by human and animal strains, designated selx1-14, selxbov1-2, and selxov, respectively. Novel nucleotide sequences for selx8-11 and selxbov2 have been deposited in GenBank under accession numbers HQ850970, HQ850971, HQ850968, HQ850969 and HQ85097, respectively. Of note, the human ST398 strain OS385 contains selx13, which is a 315 bp truncated C-terminal fragment of the gene predicted to be non-functional. With the exception of the truncated selx13 there are 85.9% non-variable nucleotide sites among the selx alleles identified. This corresponds to a total of 40 (13%) variable amino acid residues among the 204 amino acid sites of SElX (Fig. S2). Pairwise amino acid alignment reveals 89.5 to 98.5% amino acid identity among the different allelic variants made by the major S. aureus pathogenic clones (Fig. S2).
In general, each clonal complex (consisting of closely-related STs) contains a unique selx allele (Fig. 1b) consistent with point mutation being the major driving force for evolution within individual lineages. However, allele selx2 was identified in ST12, ST15 and CC8 strains, and selx5 was encoded by both ST1 and ST72 strains, respectively, indicating that assortative recombination of the selx gene has contributed to its distribution among some lineages which have not shared a recent common ancestor (Fig. 1b). In addition, examination of the contribution of recombination to selx diversity employing the RDP3 suite of programs [23], revealed evidence for at least 3 distinct recombination events leading to hybrid alleles of selx (Fig. S3). Furthermore, a phylogenetic tree based on selx gene sequences had a topology distinct from the phylogenetic tree derived from concatenated MLST loci (Fig. S4). Taken together, these data suggest that recombination has contributed to the diversification and distribution of selx among S. aureus clones.
SElX is expressed by clinical isolates in vitro and during human, bovine and ovine infection
In order to determine whether selx was expressed by clinical isolates we examined by quantitative real-time PCR (qRT-PCR), the transcription of selx by human S. aureus CA-MRSA USA300 strain LAC, bovine strain RF122, and ovine strain ED133 during exponential and stationary phases of growth in vitro. selx was transcribed by each strain in a growth-phase dependent manner, and the highest relative expression level was demonstrated by USA300 strain LAC (Fig. 2). To investigate the in vitro expression of SElX we carried out Western blot analysis with stationary phase culture supernates of 15 representative clinical isolates of S. aureus of human, bovine, and ovine origin, with rat anti-serum specific for SElX. Expression of SElX was detected in 13 of 15 isolates including 5 of 5 human isolates from sepsis, scalded skin syndrome and infective endocarditis patients, 4 of 4 bovine mastitis isolates, 3 of 4 ovine mastitis isolates, and a single caprine mastitis isolate. A single avian isolate ED98 did not express SElX in detectable quantities. These data indicate that SElX is made by the majority of clinical isolates at detectable levels in nutrient replete conditions in vitro (Table S3).
Fig. 2. SElX is expressed by clinical isolates in vitro and during infection. 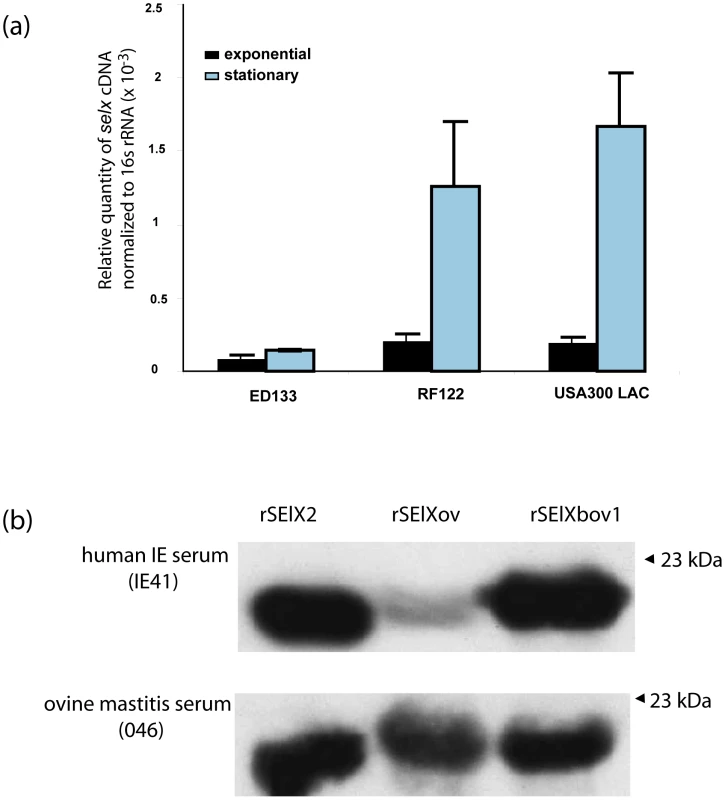
a) Growth-phase dependent transcription of selx determined by qRT-PCR. b). Representative Western blot analysis of rSElX proteins with human infective endocarditis (IE) or ovine mastitis sera samples. To determine whether SElX is expressed during human and animal colonization or infection, Western immunoblot analysis was carried out using recombinant SElX human (SElX2), bovine (SElXbov1) and ovine (SElXov) variants, with sera from humans, cows and sheep recovering from S. aureus infections, and from healthy human volunteers. Specific SElX antisera did not cross-react with TSST-1 or SSL7, its closest homologs (Fig. S5). In addition, we identified individuals who were seroconverted for SElX but not for SSL7 or TSST-1, and an individual who was seroconverted for SSL7 and TSST-1 but not for SElX (Fig. S5). Taken together, these data indicate a lack of cross-reactivity of antibodies specific for TSST-1, SSL7 and SElX. All 5 human, 4 bovine and 5 ovine serum samples from infected individuals, and 20 of 23 serum samples from healthy humans contained antibody that recognized the SElX recombinant proteins, whereas only one serum sample (from a healthy human) out of the total of 47 contained antibody that recognized recombinant SElO SAg (Table 1). These data indicate that SElX is made during S. aureus colonization or infection of humans and animals and stimulates a humoral immune response. Of note, densitometric analysis of SElX immuno-reactive bands indicated approximately 2-fold lower reactivity of human IgG with recombinant SElXov, in comparison to IgG from S. aureus-infected sheep, suggesting that SElXov made by ovine S. aureus may vary antigenically (Fig. 2 b). Overall, the expression of SElX during infection of humans and animals suggests an important general role during S. aureus infection of multiple host species.
Tab. 1. Immunoreactivity of recombinant SAg variants with sera from healthy humans, and bovine, ovine and human patients recovering from S. aureus infections. 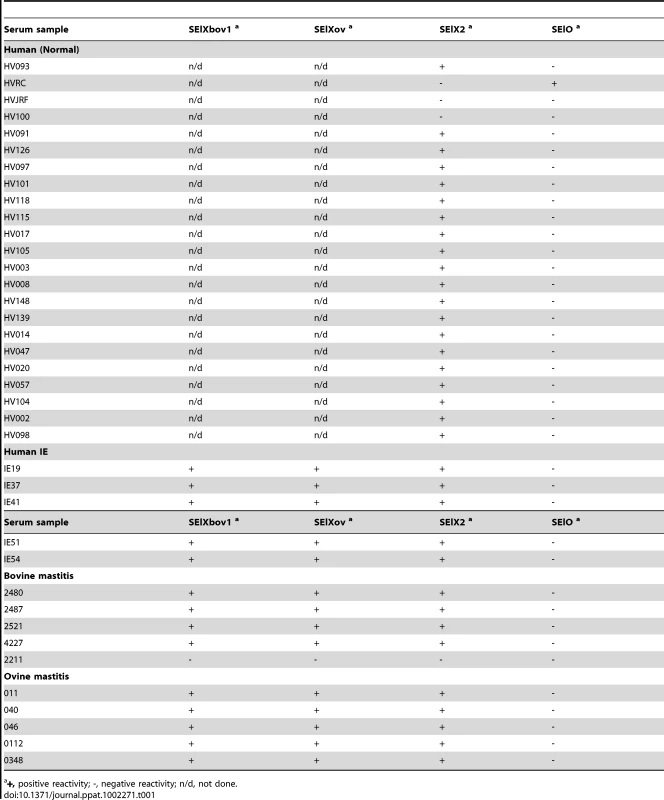
+, positive reactivity; -, negative reactivity; n/d, not done. SElX has a unique predicted SAg structure
The SElX2 variant encoded by S. aureus USA300 strain LAC, contains 27% and 24% amino acid identity with its nearest protein homologs, superantigen-like protein 7 (SSL7) and TSST-1, respectively. In order to investigate the predicted structure of the novel SAg, we carried out structural modeling using the program Phyre [24] with SSL7 and TSST-1 crystal structures from the PDB database as templates. Considering, the low sequence homology of SElX with its closest homologs SSL7 and TSST-1, used as templates for modeling, the results should be considered to be speculative. The hypothetical predicted structures of SElX variants imply a potential to form a characteristic 2 domain SAg structure with some unusual features (Fig. 3). The predicted A-domain containing the C-terminus has a long central α-helix, positioned along one side of a 4-stranded β-sheet, forming a structure typical of the β-grasp motif [25]. However the B-domain which contains the N-terminus, is considerably smaller than the B-domain of previously characterized SAgs and lacks an OB fold (Fig. 3). The OB fold is the site typically involved in SAg binding to the α-chain of the MHC class II complex on antigen presenting cells [2], [26]. The functional implications of this unique predicted B-domain structure are currently unknown.
Fig. 3. SElX is predicted to have a truncated B-domain. 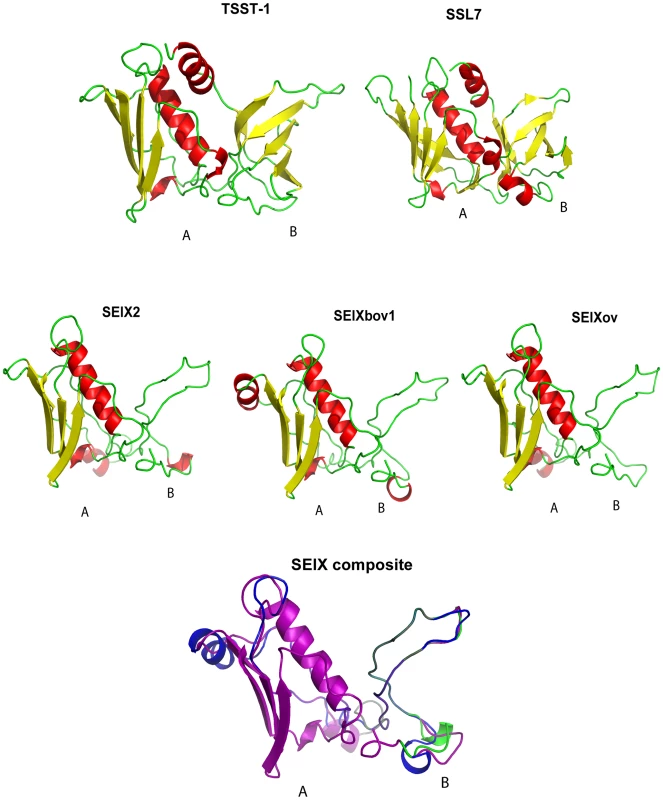
Schematic diagram of the solved structures of TSST-1, SSL7 and modelled 2-domain structures of human, bovine and ovine variants of SElX. SElX composite structure was obtained by superimposition of protein variants in PyMol. SElX is mitogenic and demonstrates Vβ-dependent T-cell proliferation
We examined the function of representative allelic variants of SElX from human, bovine and ovine isolates. Recombinant human (rSElX2), bovine (rSElXbov1), and ovine (rSElXov) variants were purified and used to stimulate human PBMC in a thymidine incorporation assay. All 3 rSElX variants were mitogenic for human T-cells at concentrations above 0.1 ng/µl (Fig. 4a). Further, in order to examine the mitogenicity of SElX expressed in a native S. aureus background, selxbov1 was cloned into the pALC2073 vector and expressed in a SAg-deficient strain RF122-8. The supernatant from cultures of RF122-8 + pALC2073:SElXbov1 had mitogenic activity for bovine lymphocytes, but supernatant from strain RF122-8 containing the pALC2073 plasmid only had none (data not shown). SElX was also mitogenic for rabbit lymphocytes and importantly, the superantigenic activity of SElX for both human and rabbit T-cells is comparable or higher to the activity of TSST-1, the SAg responsible for the toxinoses toxic shock syndrome (TSS) (Fig. 4b, c).
Fig. 4. SElX stimulates proliferation of human and rabbit lymphocytes. 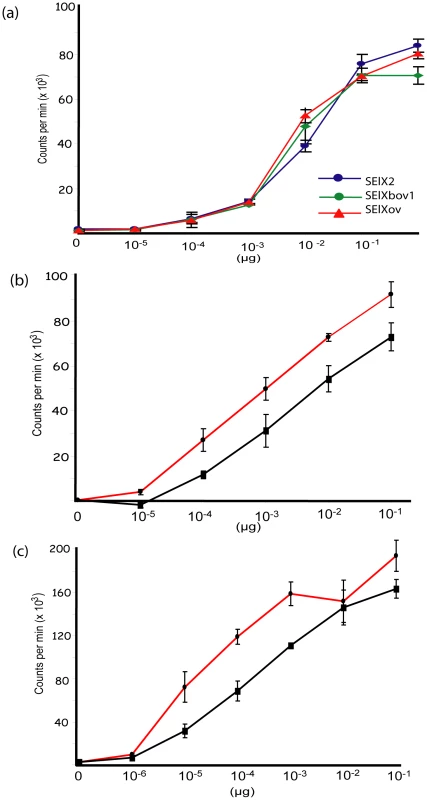
a) Proliferation of human PBMC cultures with recombinant SElX2, SElXbov1, and SElXov variants measured by [3H]-thymidine incorporation. Proliferation of human (b) or rabbit (c) PBMC cultures with recombinant SElX2 (red line) or TSST-1 (black line) measured by [3H]-thymidine incorporation. We recently designed a novel assay for quantifying human Vβ (humVβ) gene expression including all of the known Vβ subgroups [27]. Previous studies of the bovine Vβ (bovVβ)-dependent expansion capacity of staphylococcal SAgs have been restricted by the number of identified bovine Vβ subfamilies [11], [28]. The recent bovine genome sequencing project has facilitated a comprehensive description of the bovine Vβ subgroups and the repertoire of functional Vβ genes [29], [30]. In the current study, design of a novel bovVβ-dependent expansion assay has allowed us for the first time to evaluate the response of 23 human and 18 bovine Vβ subfamilies to stimulation with a staphylococcal SAg (Fig. 5a, b). rSElX2 and rSElXbov1 activated humVβ subfamiles 1, 6, 18 and 21, whereas rSElXov activated 1, 6 and 18 but not 21 (Table 2). All SElX variants stimulated bovVβ subfamily X. In addition SElXov activated bovVβ 16, SElXbov1 activated bovVβ 3, 5, 11, 16 and 17, and SElX2 activated bovVβ 5, 6, 17 and 24. Different human and bovine Vβ subfamilies are activated in response to stimulation with SElX. This is in part due to the activation of humVβ subfamilies 18 and 21, for which there are no bovine orthologs, and bovVβ X which has no human ortholog. However, the orthologous humVβ 6 and bovVβ 6 are both activated by SElX2. In addition, bovVβ subfamily 16 which is activated by SElXbov1 and SElXov is phylogenetically related to humVβ 6. Of note, the humVβ 1 subfamily and the closely related bovVβ 5 but not the orthologous bovVβ 1 were activated by SElX variants. These data indicate a unique pattern of Vβ gene activation for SElX in comparison to other previously characterized SAgs [3], [27]. Importantly, the results demonstrate differences in superantigenic activity and distinct Vβ activation profiles for different SElX variants made by S. aureus strains associated with different host species.
Fig. 5. SElX activates Vβ-specific human and bovine Tcells. 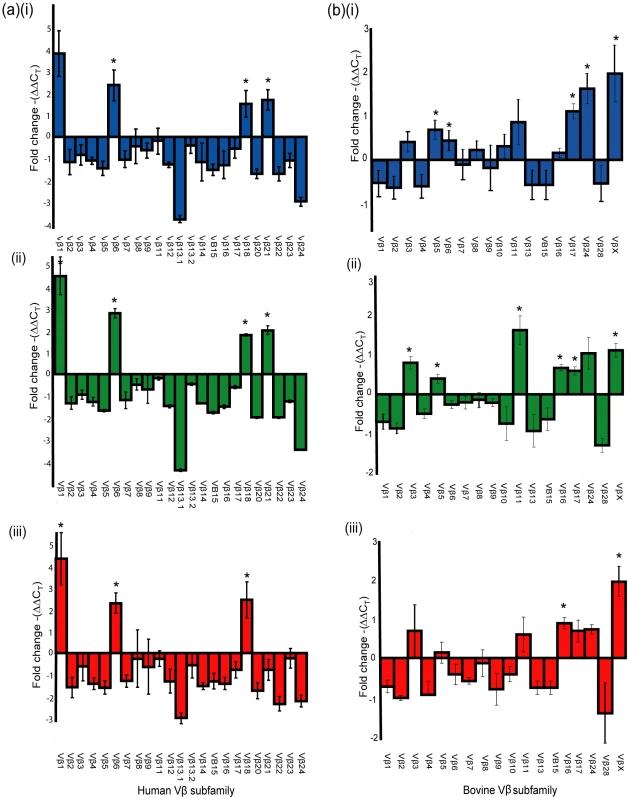
(ii) Relative fold-change in Vβ expression (mean ± S.E.M.) for human (A) or bovine (B) T cells after stimulation with (i) SElX2, (ii) SElXbov1, and (iii) SElXov. *indicates statistical significance (p<0.05). Tab. 2. Human and bovine Vβ subfamilies activated in response to rSElX variants. 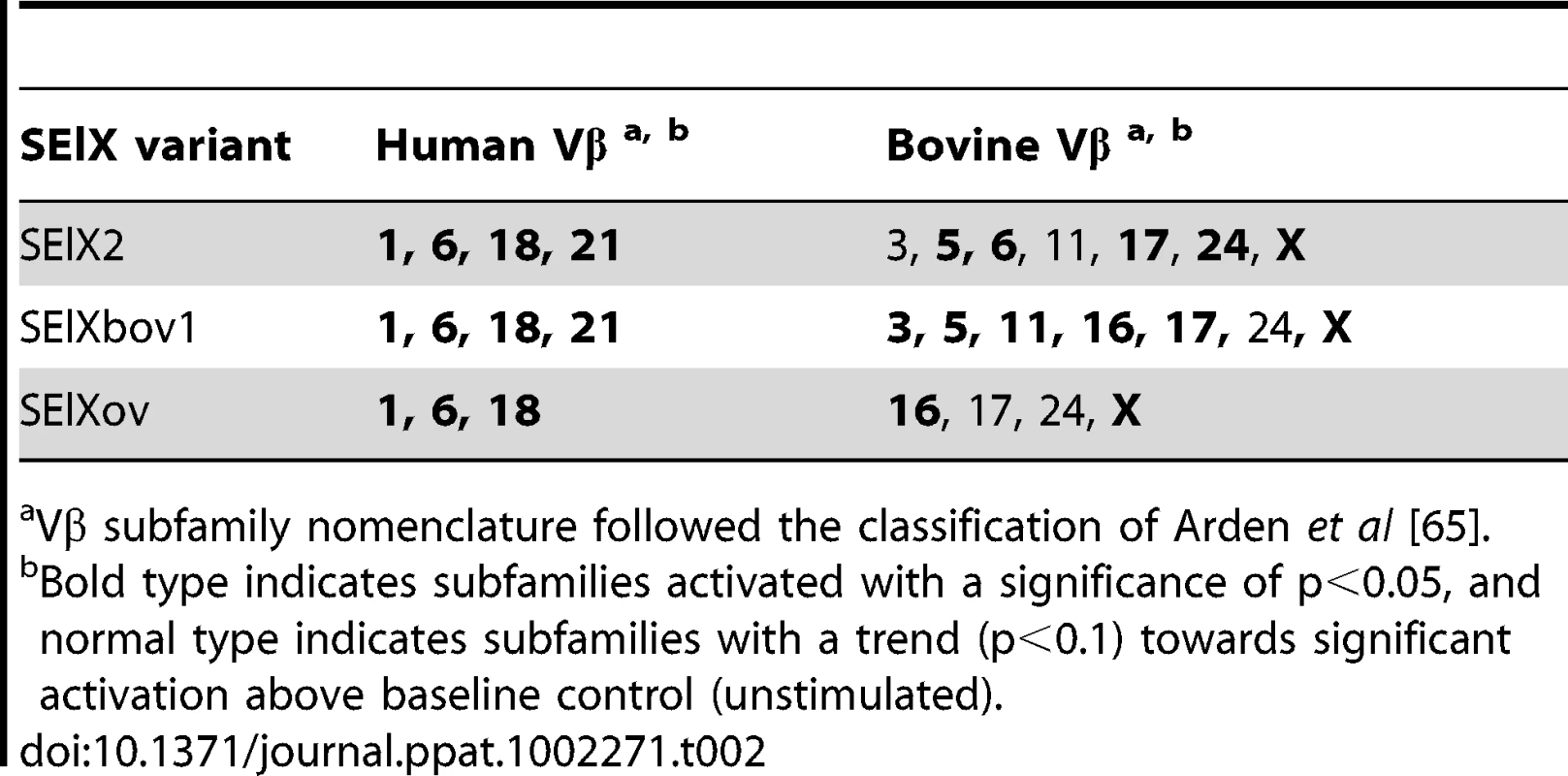
Vβ subfamily nomenclature followed the classification of Arden et al [65]. SElX has functional activities characteristic of SAgs
We examined the ability of rSElX2 to cause TSS in rabbits using a standard mini-osmotic pump model of TSS at a dose of 200 µg/kg. Of the 5 rabbits given rSElX2, 4 succumbed within 7 d, compared to 0 of 5 treated with PBS (p<0.05) (Fig. 6a). The positive control TSST-1 was lethal for 3 of 3 animals in the 7 d test period (Fig. 6a). Changes in temperature of the rabbits were recorded before and 24 h post-SAg challenge. Rabbits treated with rSElX2 developed fever with a 2.0°C increase in body temperature over the 24 h test period, compared to 0.4°C for PBS treated animals (p<0.001) (Fig. 6a).
Fig. 6. SElX causes TSS and contributes to the severity and lethality of necrotizing pneumonia caused by CA-MRSA USA300. 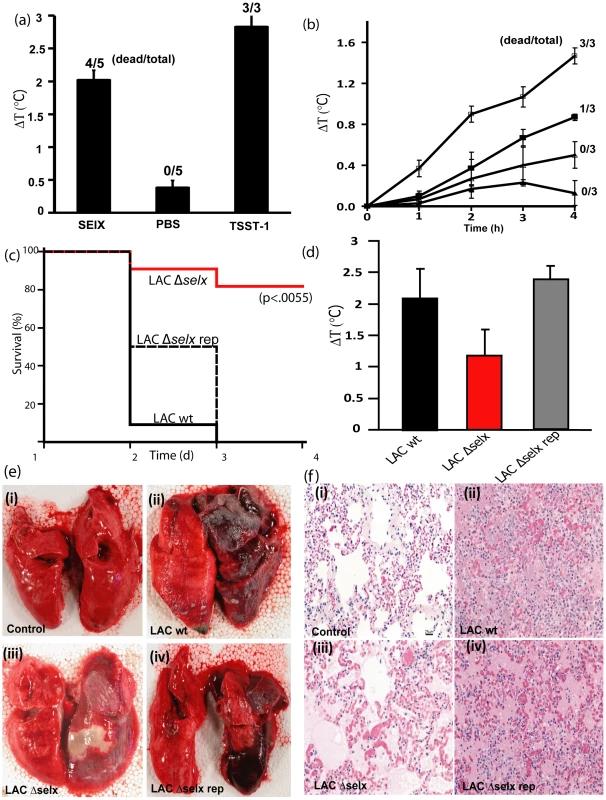
A) The ability of rSElX2, administered at a dose of 200 µg/kg, to cause TSS was measured using a standard miniosmotic pump model of TSS. TSST-1 administration was used as the positive control and PBS the negative control. B) Fever in response to intravenous injection of rSElX2 at concentrations of 10 µg/kg (□), 1 µg/kg (▪), 0.1 µg/kg (Δ), of body weight per ml, or PBS (▴), was evaluated over a 4 h test period, followed by the ability to enhance the lethality of LPS over 48 h. C) Kaplan-Meier Curves of % survival of rabbits infected with S. aureus LAC, S. aureus LACΔselx, and LACΔselx rep. D). Increase in rabbit core temperature (ΔT °C) 2 d after pulmonary infection with LAC wt, LACΔselx, and LACΔselx rep. E). Gross pathology of lungs from uninfected rabbit (i) or from rabbits infected with LAC wt (ii), LACΔselx (iii), and LACΔselx rep (iv). F) Haematoxylin Eosin-stained tissue sections from (i) uninfected rabbit lung, or from rabbit lungs infected with (ii) LAC wt, (iii) LACΔselx and (iv) LACΔselx rep isogenic strains. Rabbits were also used to evaluate fever responses over 4 h after intravenous injection, followed by the ability to enhance lethality of LPS over a 48 h test period. The capacity to cause fever that peaks 4 h after injection, and to amplify the lethal effects of LPS by up to 106-fold are defining activities of SAgs. rSElX2 caused dose-dependent fever responses in rabbits that peaked 4 h after injection and the 4 h fever response of animals receiving 10 µg/kg of rSElX2, compared to PBS, was significantly different (p value of 0.001) (Fig. 6b). The minimum pyrogenic dose of SAg is defined as the dose per kg required to cause an average 0.5°C rise in rabbit body temperature in 3 animals; the minimum pyrogenic dose of rSElX2 was calculated to be 1.0 µg/kg. rSElX2 also enhanced rabbit susceptibility to lethal shock by LPS (Fig. 6b). Taken together, rSElX2 demonstrated the functional characteristics required for classification as a SAg, including mitogenicity, pyrogenicity, enhancement of endotoxin shock, and lethality when administered by mini-osmotic pump.
SElX contributes to lethality of CA MRSA USA300 in a rabbit model of necrotizing pneumonia
CA-MRSA is notorious for causing skin and soft tissue infections, and severe necrotizing pneumonia [20], [31]. We found that CA-MRSA USA300 strains expressed elevated levels of selx in vitro relative to other clinical isolates (Fig. 2a). In order to investigate the hypothesis that SElX contributes to the pathogenesis of necrotizing pneumonia, we constructed an SElX-deficient mutant of CA-MRSA USA300 strain LAC (LAC Δselx), and a repaired derivative with an intact functional selx gene (LAC Δselx rep). To rule out the possibility of spurious mutations acquired during the construction of LAC Δselx in loci which could influence virulence, such as the accessory gene regulator (agr) [32], we compared the isogenic LAC wild type, LAC Δselx and LAC Δselx rep strains for hemolytic titre, secreted and cell wall-associated protein profiles, and in vitro growth rate. In each case we found that the strains were indistinguishable (Fig. S6 and data not shown). In addition, we compared LAC wild type and LAC Δselx for α-toxin and PVL expression levels by ELISA and found identical levels of expression (data not shown). We then compared the ability of wild type, mutant and repaired strains to cause lethal necrotizing pneumonia in a rabbit model [33]. For animals receiving wild type LAC, all 11 rabbits succumbed within 4 d, compared to 2 of 11 receiving LAC Δselx (p<0.002) (Fig. 6c). Importantly, 4 of 4 rabbits infected with the repaired strain LAC Δselx rep succumbed within 4 d (Fig. 6c). Body temperatures were recorded in the first 24 h of the experiment, and animals receiving strain LAC demonstrated significantly higher body temperatures than animals receiving the SElX-deficient strain LAC Δselx (p<0.002) and LAC Δselx rep had wild type levels of pyrogenicity (Fig. 6d). On gross examination, regionally extensive to lobar areas of lung from rabbits infected with wild type USA300 LAC and the repaired strain USA300 LAC Δselx rep were dark red to purple, heavy and oozed blood on the cut surface (Fig. 6e). In contrast, lungs from rabbits infected with the LAC Δselx mutant strain were reddened with well demarcated, focal white areas (abscess), but did not show signs of haemorrhage (Fig. 6e). Histological examination of tissues from both the wild type USA300 LAC and the repaired strain USA300 LAC Δselx rep revealed severe haemorrhagic and necrotising pneumonia (Fig. 6f). Tissue sections from rabbits infected with the LAC Δselx mutant strain were only moderately affected (Fig. 6f). Taken together, these data collectively indicate that SElX made by USA300 LAC causes high fever and contributes to severity of infection and lethality in a rabbit model of necrotizing pneumonia.
Discussion
We have identified a novel SAg which is encoded by the great majority of strains, and which causes lethality in a model of severe S. aureus human disease. The distribution of selx across the full breadth of S. aureus diversity, its absence among other staphylococcal species, and its genetic linkage with an integrase pseudogene suggests an ancient horizontal acquisition event which happened prior to the most recent common ancestor of the S. aureus species. The gene is absent from only a single clone examined (CC30) but the associated integrase pseudogene is retained suggesting that a deletion event has resulted in the loss of selx during the evolution of the CC30 lineage. Of note, previous studies have discovered that the majority of CC30 isolates contain a SaPI which encodes TSST-1, the most closely-related SAg to SElX [34], [35]. These data imply that virtually all S. aureus isolates have the capacity to produce either TSST-1 or SElX suggesting an important role for this sub-group of toxins in S. aureus pathogenesis.
The selx gene has undergone diversification leading to at least 17 different allelic variants identified among the major clonal lineages (Fig. 1 b). Although SElX is the first SAg identified which is encoded in the core genome of S. aureus, the SAgs, SMEZ and SPEG, are made by the majority of Group A Streptococcal (GAS) isolates [36]. Extensive allelic variation of SMEZ contributes to antigenic variation, but does not influence Vβ-specificity or mitogenicity [36]. Whereas GAS is specific for human hosts, S. aureus is also represented by strains which are specialized for ruminant or avian host species [37], [38]. In contrast to SMEZ, SElX bovine - and ovine-specific variants displayed distinct Vβ subgroup proliferation profiles for bovine lymphocytes in comparison to a human-specific SElX variant suggesting that they have undergone adaptive diversification leading to enhanced activity in ruminants. Previously, it was shown that the proliferation of human and bovine lymphocytes varied in response to stimulation with allelic variants of the SAg staphylococcal enterotoxin C (SEC). The SEC bovine variant (SECbov) requires between 10 - and 1000-fold more toxin than SEC1 and the SEC ovine variant (SECov) to induce proliferation of human or bovine PBMCs, and results in a distinct Vβ-dependent T-cell activation profile [28], [39].
Although most of the allelic variation in selx was clonal and due to point mutation, we found evidence of a role for recombination in the diversification and distribution of SElX, including whole gene transfer of the selx allele between pathogenic clones. Recombination rates are predicted to be low in S. aureus in general [40], but some recombination, particularly among genes involved in virulence has been observed [41], [42]. Such rapid evolution may facilitate antigenic or functional diversification of proteins which are critical for bacterial survival during infection.
Analysis of selx transcription levels revealed a growth phase-dependent expression analogous to numerous other staphylococcal virulence proteins which are under the control of agr. Of note, the human CA-MRSA USA300 strain LAC had relatively high levels of expression of SElX which correlates with the previously reported up-regulation of RNAIII and secreted virulence proteins by the USA300 epidemic clone [43]. In addition to demonstrating the in vitro expression of SElX by S. aureus strains, we also identified SElX-specific antibodies in human and ruminant convalescent serum samples, and in healthy individuals. The high frequency of sero-conversion to SElX among human and livestock populations is consistent with the expression of SElX by most commensal and disease-associated isolates of S. aureus, and imply a broad role for SElX in S. aureus colonization or infection of multiple host species.
Of particular importance, the USA300 epidemic clone is currently the primary infectious cause of human mortality in the USA, and unlike most strains of hospital-associated MRSA can cause disease in otherwise healthy individuals [44]. The molecular basis for the increased virulence of USA300 strains has been the subject of intensive research efforts. Several reports have highlighted the high levels of expression of secreted proteins such as Panton-Valentine leukocidin, α-toxin, and phenol-soluble modulins and evidence exists for a role for each of these toxins in the pathogenesis of CA-MRSA severe infection [43], [45]–[50]. Recently, Assimacopoulos et al. described an S. aureus extreme pyrexia syndrome that is associated with CA-MRSA USA300 strains [51]. Our studies suggest that SElX contributes to the pathogenesis of pulmonary illness caused by these strains, and its induction of high fever during infection is consistent with a role in extreme pyrexia syndrome. While most studies on the pathogenesis of severe S. aureus pulmonary infections have focused on the role of cytolysins [43], [45], [49], [50], our data suggest that the novel SAg SElX may influence the outcome of severe infection caused by CA-MRSA USA300. This is in agreement with a recent study that shows that TSST-1, SEB and SEC contribute to lethal pneumonia of rabbits caused by CA-MRSA USA200 and USA400 strains [33]. Of note, rabbits are much more similar to humans than mice in their sensitivity to both SAgs and cytolytic toxins [52]. Importantly, we have shown that SElX is mitogenic for both rabbit and human T cells. As the majority of previous studies into the virulence of S. aureus infections have been carried out in mice, the importance of SAgs in the pathogenesis of severe infections may have been underestimated.
Because of the role of SAgs in S. aureus pathogenesis and their potential as an agent of biological warfare, therapeutic strategies which target these toxins have been the focus of considerable research efforts [6]. Structural modeling revealed the potential of SElX to form the characteristic 2 domain SAg structure joined by a central α-helix. However domain B of SElX is predicted to be much smaller than that of other SAgs identified to date (Fig. 4). Ongoing crystallography analyses should result in important insights into the molecular interaction of the uniquely structured SElX with its ligands which may facilitate the design of molecules to inhibit its function.
CA-MRSA USA300 strains are characterized by high expression of a number of secreted virulence factors which through direct activity or immunopathology result in the severe symptoms associated with infections such as necrotizing pneumonia [43], [45]–[50]. Here, we report the discovery of a novel secreted virulence determinant made by most strains of S. aureus that may contribute to the severity of some human diseases caused by highly-virulent clones such as CA-MRSA USA300. Furthermore, the phylogenetic and immunobiological characterization of a unique S. aureus core genome-encoded SAg provides new insights into the evolution of pathogenic S. aureus and its capacity to cause disease in multiple host species.
Materials and Methods
Ethics statement
All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies. Animal experimentation was performed under a University of Minnesota approved Institutional Animal Care and Use Committee (IACUC) protocol (0908A71722). University of Minnesota is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AALAC). Animals are maintained in accordance with the applicable portions of the Animal Welfare Act and the DHHS “Guide for the Care and Use of Laboratory Animals”. In agreement with the University of Minnesota IACUC, animals that failed to exhibit escape behaviour and at the same time could not right themselves were prematurely euthanized. Animals were euthanized with intravenous injection of 1 ml of Beuthanasia D, whether prematurely or at the end of experimentation. Human peripheral blood mononuclear cells were isolated from heparinized venous blood of four different healthy donors in accordance with a human subject protocol approved by the University of Idaho Institutional Review Board for Human Subjects (approval number 05–056), in accordance with the principles of the Declaration of Helsinki. Donors were informed the procedure risks and provided a written consent prior to participation. The University of Idaho is fully accredited by the AALAC.
S. aureus strains
S. aureus strains were selected to represent clonal genotypes which represent the breadth of species diversity, different host-associations and geographic origins (Table S3). S. aureus strains were grown in tryptone soya broth (TSB) or brain heart infusion (BHI) broth with shaking at 200 rpm, or on tryptone soya agar (TSA) plates. For use in animal studies, organisms were cultured in Todd Hewitt broth or on Todd Hewitt agar. Media was supplemented where appropriate with chloramphenicol (Sigma-Aldrich) at 12.5 µg/ml and 150 µg/ml X-gal (5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside) (Melford).
Bioinformatic analyses
The novel putative SAg gene, selx was first identified by BLAST analysis of the genome of USA300 FPR3757 with the gene sequence specific for the SAg TSST-1. The sequences of distinct selx alleles were identified by BLASTn analysis of S. aureus whole genome sequences deposited in GenBank (Table S1). Additional selx alleles were sequenced with selxseq primers (Table S2) by Genepool Sequencing Service (University of Edinburgh, UK), aligned by ClustalW using MEGA 4.0.1 software [53] and Neighbor-Joining phylogenetic trees constructed using the Nucleotide Maximum Composite Likelihood model. At least 500 bootstrap trees were generated to examine the robustness of the inferred phylogenetic relationships. Recombination detection program RDP v3.44 was used to identify putative end points of recombination events [23]. Unique recombination events were detected by at least 3 of the programs employed by the RDP suite, p<0.05. Protein structural modeling of the derived amino acid sequences of selx2, selxbov1 and selxov was carried out. Predicted 3D structures were obtained for SElX2, SElXbov1 and SElXov with the program Phyre using the crystal structure of TSST-1 as a template, (PDB file c5tssA) [24]. PDB files were generated for each predicted structure and analyzed using Pymol software [54]. Amino acid sequence alignment was carried out using ClustalW and viewed with Jalview [55].
Transcriptional analysis of selx
Total RNA was extracted from S. aureus strains LAC, RF122, and ED133 exponential (OD600 = 0.6) and stationary phase (16 h) cultures using the RNeasy miniprep kit (QIAgen) as described in the manufacturer's instructions except for re-suspension in TE buffer with 100 µg/ml Lysostaphin and incubation at 37°C for 20 min. RNA was treated with Turbo DNase (Ambion Inc) and. 0.5 µg mRNA from at least 3 independent total RNA extractions were reverse-transcribed to cDNA with the Power SYBR Green RNA-to-CT 2-Step Kit (Applied Biosystems). To quantify cDNA generated by reverse transcription from target RNA, qRT-PCR reactions were carried out in 25 µl reactions containing 50 ng of cDNA, 300 nM selxq or 16S rRNA primers (Table S2), and SYBR Green I dye master mix (AB), using a Mx3000P light cycler (Strategene). 16S rRNA primers have been described elsewhere [56]. Relative values of transcription of selx were determined by comparative quantification to the internal control 16SrRNA. The thermal conditions were: 10 min at 95°C for 1 cycle, 20 s at 95°C, 20 s at 60°C and 20 s at 72°C for 40 cycles. RNA samples were processed in triplicate with no template (NTC), no Reverse Transcriptase (no RT) and positive genomic DNA controls. Fluorescence was measured at the end of the annealing phase of each cycle and a threshold value for the fluorescence set by the MxPro qPCR software version 4.1.
Cloning and purification of recombinant SAgs
5′ primers for cloning selx2, selxbov1, and selxov into the pET15b plasmid (Novagen), were designed to anneal immediately after the signal peptide coding region, as predicted by Signal P 3.0 Server (http://www.cbs.dtu.dk/services/SignalP/), and 3′ primers were designed to include the stop codon of the gene (Table S2). PCR reactions were carried out using Vent polymerase (NEB) and contained 100 nM selxpET forward and reverse primers designed to incorporate restriction sites NdeI and BamHI (Table S2) and 10 ng of template DNA. PCR products were restriction digested with NdeI and BamHI (NEB), purified, and ligated with T4 DNA ligase (NEB), and transformed into E. coli DH5α cells. pET constructs were isolated from DH5α using the QIAprep Spin Miniprep kit (QIAgen), and transformed into E. coli BL21. BL21 cells containing the pET plasmid constructs were cultured in Luria-broth containing 50 µg/ml ampicillin (Sigma-Aldrich) and induced in mid-exponential phase of growth (OD600 = 0.6), with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) (ForMedium Ltd.) for 4 h. Cells were recovered by centrifugation at 8000 xg, disrupted using a French Press, and His-tagged recombinant proteins were purified by affinity chromatography on a Ni-NTA nickel affinity column (Invitrogen). Proteins were dialysed using Spectra/Por Float-A-Lyzer tubing with a 8000 to 10000 molecular weight cut off (MWCO) (Spectrum Laboratories), and LPS was removed using ProteoSpin endotoxin removal kit (Norgen Biotek).
Western immunoblot analysis
Supernates from stationary phase cultures of S. aureus strains were concentrated with Amicon Ultra-15 Centrifugal Filter units (10000 MWCO) (Millipore). Recombinant proteins and concentrated secreted proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Amersham Hybond ECL, GE Healthcare) in Towbin transfer buffer. The membrane was incubated in 1 x PBS (pH 7.3) containing 8% powdered milk (Fluka), at 4°C overnight, washed 3 times with washing buffer, 1 x PBS (pH 7.3) containing 1% powdered milk and 0.05% Tween 20 (Sigma-Aldrich). The membrane was incubated for 1 h with primary antibody, which was either a 1∶2500 (bovine and ovine) or 1∶5000 (human) dilution of serum, or 2 h with 1∶1500 dilution of rat anti-sera raised against rSElXbov1. The membrane was then incubated with horse radish perixodase conjugated (HRP) secondary antibodies for 1 h, which was 1∶2500 goat anti-bovine IgG, 1∶2500 rabbit anti-sheep IgG, (Santa Cruz Biotechnology), 1∶5000 rabbit anti-human IgG (Dako), or 1∶1500 goat anti - rat IgG (Abcam), depending on the experiment. Human sera samples were obtained from infectious endocarditis patients [57], ovine serum samples were obtained from experimentally-infected sheep and provided by E. Vautor [58] and from cows with bovine mastitis by C. Smyth. Semi-quantitative spot densitometry was carried out with the ChemiImager 4000i.V4 program, using a MultiImager light cabinet (Alpha Innotech).
Construction of selx-deficient and selx-repaired derivatives of S. aureus USA300 LAC
An selx-deficient strain of USA300 LAC was obtained by allele replacement using the pMAD-CM plasmid [59]. PCR products of 578 bp and 592 bp, flanking the left (AB) and right (CD) regions of selx, respectively were amplified with primers listed in Table S2. PCR amplification with primers A and D was carried out with 100 ng of purified AB and CD template DNA to produce a single spliced AD fragment by overlapping PCR, facilitated by sequence complementary to primer B incorporated into the CD fragment by primer C. AD products were then purified and cloned into the Strataclone pSC-B plasmid (Stratagene). pSC-B plasmid containing the AD insert was digested with EcoRI for 3 h at 37°C. The AD fragment was then purified, ligated into the dephosphorylated gene replacement plasmid, pMAD-CM , to create pMAD::selx, which was transformed by electroporation into LAC. LAC containing pMAD::selx was shaken overnight at 30°C in TSB containing chloramphenicol. To select for integration of the plasmid into the chromosome through homologous recombination, 10-fold dilutions were incubated on TSA containing chloramphenicol at 44°C, a temperature non-permissive for plasmid replication. Double cross-over excisants were selected for by growing integrants in TSB without chloramphenicol at 30°C for 24 h. 10-fold dilutions were plated onto TSA containing X-gal and incubated on TSA at 30°C overnight. White colonies were screened for antibiotic sensitivity on chloramphenicol plates (inferring loss of the plasmid) and screened for WT or mutated forms of the gene by PCR with primers upstream (E) and downstream (Z) of selx (Table S2). The resulting selx-deficient strain, LACΔselx was sequenced using E and Z primers in order to confirm the in-frame deletion event. The selx deletion in LACΔselx was repaired using an identical allelic exchange approach employing pMAD-CM. Oligonucleotide primers (Table S2) designed to incorporate a single synonymous substitution into the selx gene were used to amplify 2 PCR products specific for left and right regions of the selx gene (rep AB and rep CD primers). The resulting PCR products were spliced to produce an intact selx gene, and cloned into pMAD as previously described resulting in pMAD::selx rep. Allelic exchange was carried out as described for selx mutant construction and the repaired selx gene sequenced. Western blot analysis confirmed the restoration of SElX expression (Fig. S6a).
Pyrogenicity, endotoxin enhancement and miniosmotic pump lethality studies
American Dutch Belted rabbits were injected with rSElX2 at doses of 10, 1, and 0.1 µg/kg of body weight per ml intravenously. Three rabbits were injected with each dose and temperature was measured hourly for 4 h. After 4 h, each rabbit was injected intravenously with 1 µg of lipopolysaccharide (LPS) from Salmonella enterica serovar typhimurium (1/500 of the 50% lethal dose of endotoxin alone). SAgs characteristically amplify the lethal effects of LPS by up to 106-fold. Lethality was assessed over a 48 h period [60]. In agreement with the University of Minnesota IACUC, animals that failed to exhibit escape behaviour and at the same time could not right themselves were prematurely euthanized. It is our experience in over 30 years of similar experimentation that this point is 100% predictive of SAg lethality. Animals were euthanized with intravenous injection of 1 ml of Beuthanasia D, whether prematurely or at the end of experimentation. Miniosmotic pumps, containing 200 µg of rSElX2 or TSST-1, or PBS, were implanted subcutaneously into 5 American Dutch Belted rabbits per group (3 for TSST-1) [61]. Fever was assessed after 24 h, and lethality of the toxins over a period of 7 d. The same criteria as above were used for assessing need for premature euthanasia.
Necrotizing pneumonia rabbit model
Wild-type LAC and the selx knock-out strain were cultured in Todd Hewitt broth for 16 h and washed once in Todd Hewitt broth to remove exoproteins. The bacteria were re-suspended in Todd Hewitt broth at 1×1010 colony-forming units (CFU)/ml for use in injections. American Dutch Belted rabbits (11 per group for LAC wt and LAC Δselx, and 4 for LAC Δselx rep) were anesthetized with ketamine and xylazine. Their tracheas were exposed and 2×109 USA300 CA-MRSA strain LAC, the isogenic selx-deficient LAC strain or its repaired derivative were administered intra-tracheally through catheters in 0.2 ml volumes. The animals were closed and monitored for 4 d for development of fatal necrotizing pneumonia.
Preparation and stimulation of lymphocytes
Blood was obtained from 2 Holstein–Friesian cattle aged 18–36 m via jugular vein puncture. Animals were reared indoors and maintained on a ration of hay and concentrates. Peripheral blood mononuclear cells (PBMC) were isolated from blood by density gradient centrifugation using Ficoll Paque Plus (GE Healthcare) as described previously [27], [62]. Human PBMC were isolated from venous blood of 3 healthy donors as described previously [27]. Splenocytes were obtained from American Dutch Belted rabbits as previously described [63]. Bovine PBMC were adjusted to a concentration of 1 × 106 cells/ml in complete cell culture medium (RPMI 1640, Gibco) supplemented with 10% heat-inactivated FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, 292 µg/ml L-glutamine (PSG) and 50 µM 2-Mercaptoethanol (Sigma-Aldrich), and cultured at 37°C, 5% CO2. Human PBMC (1 × 106) were suspended in RPMI 1640 medium (Life technologies) supplemented with 2% FBS, 100 U/ml penicillin G, and 100 µg/ml streptomycin. The cultures were co-incubated with 10-fold dilutions of rSElX proteins (1 to 10−6 µg/ml) for 72 h at 37°C, 5% CO2. After adding [3H] thymidine (1 µCi), Cells were cultured for a further 18 h and cellular DNA was harvested on glass fiber filters. [3H]-thymidine incorporation was quantified by liquid scintillation counting as described previously [27].
Analysis of Vβ-dependent T-cell activation
Total RNA was extracted from human PBMC prior to and after stimulation with rSElX proteins (1 µg/ml) for 96 h. Expansion of cells expressing different humVβ gene subfamilies was determined using qRT-PCR as described previously [27]. Total RNA was extracted from bovine PBMC before and after stimulation with rSElX variants (1 µg/ml) using Tri-reagent (Sigma–Aldrich). First-strand cDNA was generated from 0.5 µg of RNA using Power SYBR Green RNA-to-CT 2-Step Kit (AB). BovVβ subfamily-specific qRT-PCR primers were designed with Primer3 [64], based on an alignment of bovVβ sequences. cDNA sequences for bovine TRBV genes were derived from the bovine genome and cDNA analyses described by Connelley et al [29] (Table S2). To quantify bovVβ subfamily gene expression, qRT-PCR reactions were carried out in 25 µl reactions containing 100ng cDNA, 100 nM primers (Table S2), and SYBR Green I dye master mix (AB) using a Stratagene Mx3000P light cycler. The thermal conditions were 1 cycle at 50°C for 10 min, 1 cycle at 95°C for 10 min, 15 s at 95°C and 1 min at 60°C for 40 cycles. RNA samples were processed in triplicate with NTC and noRT controls. The threshold cycle (CT) was determined using MxPro software version 4.1 and normalized to internal controls, β-actin and Constant β (Cβ), by calculating ΔCT [CT target - CT β-actin - CT (β-actin – Cβ)]. Normalized ΔCT data were then compared by calculating -ΔΔCT = -(ΔCT stimulated - ΔCT of unstimulated). Values >0 indicate expansion of particular subset in response to stimulation.
Statistical analysis
Student's t-test analysis was used to assess differences in fever responses in rabbits and differences in humVβ and bovVβ gene expression. Fisher's exact test was used to assess differences in rabbit survival.
Supporting Information
Zdroje
1. ThomasDChouSDauwalderOLinaG 2007 Diversity in Staphylococcus aureus enterotoxins. Chem Immunol Allergy 93 24 41
2. DingesMMOrwinPMSchlievertPM 2000 Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 13 16 34
3. ThomasDDauwalderOBrunVBadiouCFerryT 2009 Staphylococcus aureus superantigens elicit redundant and extensive human Vbeta patterns. Infect Immun 77 2043 2050
4. OnoHKOmoeKImanishiKiIwakabeYHuD-L 2008 Identification and Characterization of Two Novel Staphylococcal Enterotoxins, Types S and T. Infect Immun 76 4999 5005
5. SeoKSBohachGA 2007 Staphylococcus aureus; DoyleMPLBMontvilleTJ Washington D.C ASM Press
6. FraserJDProftT 2008 The bacterial superantigen and superantigen-like proteins. Immunol Rev 225 226 243
7. ChoiYWKotzinBHerronLCallahanJMarrackP 1989 Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc Natl Acad Sci U S A 86 8941 8945
8. DellabonaPPeccoudJKapplerJMarrackPBenoistC 1990 Superantigens interact with MHC class II molecules outside of the antigen groove. Cell 62 1115 1121
9. DrakeCGKotzinBL 1992 Superantigens: biology, immunology, and potential role in disease. J Clin Immunol 12 149 162
10. KawabeYOchiA 1990 Selective anergy of V beta 8+,CD4+ T cells in Staphylococcus enterotoxin B-primed mice. J Exp Med 172 1065 1070
11. FitzgeraldJRMondaySRFosterTJBohachGAHartiganPJ 2001 Characterization of a putative pathogenicity island from bovine Staphylococcus aureus encoding multiple superantigens. J Bacteriol 183 63 70
12. JarraudSPeyratMALimATristanABesM 2001 egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J Immunol 166 669 677
13. JohnsMBJrKhanSA 1988 Staphylococcal enterotoxin B gene is associated with a discrete genetic element. J Bacteriol 170 4033 4039
14. Ben ZakourNLGuinaneCMFitzgeraldJR 2008 Pathogenomics of the staphylococci: insights into niche adaptation and the emergence of new virulent strains. FEMS Microbiol Lett 289 1 12
15. SmythDSHartiganPJMeaneyWJFitzgeraldJRDeobaldCF 2005 Superantigen genes encoded by the egc cluster and SaPIbov are predominant among Staphylococcus aureus isolates from cows, goats, sheep, rabbits and poultry. J Med Microbiol 54 401 411
16. OmoeKHuDLTakahashi-OmoeHNakaneAShinagawaK 2005 Comprehensive analysis of classical and newly described staphylococcal superantigenic toxin genes in Staphylococcus aureus isolates. FEMS Microbiol Lett 246 191 198
17. ChambersHF 2005 Community-associated MRSA--resistance and virulence converge. N Engl J Med 352 1485 1487
18. DaumRSItoTHiramatsuKHussainFMongkolrattanothaiK 2002 A novel methicillin-resistance cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis 186 1344 1347
19. KlevensRMMorrisonMANadleJPetitSGershmanK 2007 Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298 1763 1771
20. FrancisJSDohertyMCLopatinUJohnstonCPSinhaG 2005 Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis 40 100 107
21. DiepBAGillSRChangRFPhanTHChenJH 2006 Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367 731 739
22. KwanTLiuJDuBowMGrosPPelletierJ 2005 The complete genomes and proteomes of 27 Staphylococcus aureus bacteriophages. Proc Natl Acad Sci U S A 102 5174 5179
23. HeathLvan der WaltEVarsaniAMartinDP 2006 Recombination Patterns in Aphthoviruses Mirror Those Found in Other Picornaviruses. J Virol 80 11827 11832
24. KelleyLASternbergMJ 2009 Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4 363 371
25. BurroughsAMBalajiSIyerLMAravindL 2007 Small but versatile: the extraordinary functional and structural diversity of the beta-grasp fold. Biol Direct 2 18
26. McCormickJKTrippTJLleraASSundbergEJDingesMM 2003 Functional Analysis of the TCR Binding Domain of Toxic Shock Syndrome Toxin-1 Predicts Further Diversity in MHC Class II/Superantigen/TCR Ternary Complexes. J Immunol 171 1385 1392
27. SeoKSParkJYTermanDSBohachGA 2010 A quantitative real time PCR method to analyze T cell receptor Vbeta subgroup expansion by staphylococcal superantigens. J Transl Med 8 2
28. DeringerJRElyRJMondaySRStauffacherCVBohachGA 1997 Vbeta-dependent stimulation of bovine and human T cells by host-specific staphylococcal enterotoxins. Infect Immun 65 4048 4054
29. ConnelleyTAertsJLawAMorrisonWI 2009 Genomic analysis reveals extensive gene duplication within the bovine TRB locus. BMC Genomics 10 192
30. ElsikCGTellamRLWorleyKCGibbsRAMuznyDM 2009 The genome sequence of taurine cattle: a window to ruminant biology and evolution. Science 324 522 528
31. HeroldBCImmergluckLCMarananMCLauderdaleDSGaskinRE 1998 Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279 593 598
32. SomervilleGABeresSBFitzgeraldJRDeLeoFRColeRL 2002 In Vitro Serial Passage of Staphylococcus aureus: Changes in Physiology, Virulence Factor Production, and agr Nucleotide Sequence. J Bacteriol 184 1430 1437
33. StrandbergKLRotschaferJHVetterSMBuonpaneRAKranzDM 2010 Staphylococcal superantigens cause lethal pulmonary disease in rabbits. J Infect Dis 202 1690 1697
34. LindsayJAMooreCEDayNPPeacockSJWitneyAA 2006 Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. J Bacteriol 188 669 676
35. HoldenMTFeilEJLindsayJAPeacockSJDayNP 2004 Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U S A 101 9786 9791
36. ProftTMoffattSLWellerKDPatersonAMartinD 2000 The streptococcal superantigen SMEZ exhibits wide allelic variation, mosaic structure, and significant antigenic variation. J Exp Med 191 1765 1776
37. LowderBVGuinaneCMBen ZakourNLWeinertLAConway-MorrisA 2009 Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc Natl Acad Sci U S A 106 19545 19550
38. GuinaneCMBen ZakourNLTormo-MasMAWeinertLALowderBV 2010 Evolutionary Genomics of Staphylococcus aureus Reveals Insights into the Origin and Molecular Basis of Ruminant Host Adaptation. Genome Biol Evol 2 454 466
39. MarrJCLyonJDRobersonJRLupherMDavisWC 1993 Characterization of novel type C staphylococcal enterotoxins: biological and evolutionary implications. Infect Immun 61 4254 4262
40. FeilEJCooperJEGrundmannHRobinsonDAEnrightMC 2003 How clonal is Staphylococcus aureus? J Bacteriol 185 3307 3316
41. RobinsonDAMonkABCooperJEFeilEJEnrightMC 2005 Evolutionary Genetics of the Accessory Gene Regulator (agr) Locus in Staphylococcus aureus. J Bacteriol 187 8312 8321
42. WatanabeSItoTSasakiTLiSUchiyamaI 2009 Genetic diversity of staphylocoagulase genes (coa): insight into the evolution of variable chromosomal virulence factors in Staphylococcus aureus. PLoS One 4 e5714
43. LiMDiepBAVillaruzAEBraughtonKRJiangX 2009 Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 106 5883 5888
44. OttoM 2010 Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol 64 143 162
45. LiMCheungGYHuJWangDJooHS 2010 Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis 202 1866 1876
46. SchlievertPM 2009 Cytolysins, superantigens, and pneumonia due to community-associated methicillin-resistant Staphylococcus aureus. J Infect Dis 200 676 678
47. MontgomeryCPBoyle-VavraSAdemPVLeeJCHusainAN 2008 Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J Infect Dis 198 561 570
48. Labandeira-ReyMCouzonFBoissetSBrownELBesM 2007 Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 315 1130 1133
49. DiepBAChanLTattevinPKajikawaOMartinTR 2010 Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A 107 5587 5592
50. Bubeck WardenburgJBaeTOttoMDeleoFRSchneewindO 2007 Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 13 1405 1406
51. AssimacopoulosAPStrandbergKLRotschaferJHSchlievertPM 2009 Extreme pyrexia and rapid death due to Staphylococcus aureus infection: analysis of 2 cases. Clin Infect Dis 48 612 614
52. DingesMMSchlievertPM 2001 Comparative analysis of lipopolysaccharide-induced tumor necrosis factor alpha activity in serum and lethality in mice and rabbits pretreated with the staphylococcal superantigen toxic shock syndrome toxin 1. Infect Immun 69 7169 7172
53. TamuraKDudleyJNeiMKumarS 2007 MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol Biol Evol 24 1596 1599
54. DeLanoWL 2002 The PyMOL Molecular Graphics System. ScientificD San Carlos, CA, USA
55. WaterhouseAMProcterJBMartinDMClampMBartonGJ 2009 Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics 25 1189 1191
56. SterCGilbertFBCochardTPoutrelB 2005 Transcriptional profiles of regulatory and virulence factors of Staphylococcus aureus of bovine origin: oxygen impact and strain-to-strain variations. Mol Cell Probes 19 227 235
57. DagaSShepherdJCallaghanGHungRDawsonD 2011 Platelet receptor polymorphisms do not influence Staphylococcus aureus-platelet interactions or infective endocarditis. Microbes Infect 13 216 225
58. Le MaréchalCJanGEvenSMcCullochJAAzevedoV 2009 Development of serological proteome analysis of mastitis by Staphylococcus aureus in ewes. J Microbiol Methods 79 131 136
59. MemmiGFilipeSRPinhoMGFuZCheungA 2008 Staphylococcus aureus PBP4 is essential for beta-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob Agents Chemother 52 3955 3966
60. SchlievertPM 1982 Enhancement of host susceptibility to lethal endotoxin shock by staphylococcal pyrogenic exotoxin type C. Infect Immun 36 123 128
61. ParsonnetJGillisZARichterAGPierGB 1987 A rabbit model of toxic shock syndrome that uses a constant, subcutaneous infusion of toxic shock syndrome toxin 1. Infect Immun 55 1070 1076
62. GoddeerisBMMorrisonWI 1988 Techniques for the generation, cloning, and characterization of bovine cytotoxic T cells specific for the protozoan Theileria parva. Methods Cell Sci 11 101 110
63. BarsumianELSchlievertPMWatsonDW 1978 Nonspecific and specific immunological mitogenicity by group A streptococcal pyrogenic exotoxins. Infect Immun 22 681 688
64. RozenS 2000 Primer3 on the WWW for general users and for biologist programmers. Krawetz SMSe Bioinformatics Methods and Protocols: Methods in Molecular Biology Totowa, NJ Humana Press 365 386
65. ArdenBClarkSPKabelitzDMakTW 1995 Human T-cell receptor variable gene segment families. Immunogenetics 42 455 500
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Quorum Sensing in Fungi: Q&AČlánek Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the MosquitoČlánek The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral GenomeČlánek A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,Článek A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of AlphaherpesvirusesČlánek The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate ofČlánek Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during InfectionČlánek Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 10- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Quorum Sensing in Fungi: Q&A
- Discovery of an Ebolavirus-Like Filovirus in Europe
- Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response
- Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
- Herpesvirus Telomerase RNA (vTR) with a Mutated Template Sequence Abrogates Herpesvirus-Induced Lymphomagenesis
- Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
- Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus
- Novel Mouse Xenograft Models Reveal a Critical Role of CD4 T Cells in the Proliferation of EBV-Infected T and NK Cells
- Toll-8/Tollo Negatively Regulates Antimicrobial Response in the Respiratory Epithelium
- Exhausted Cytotoxic Control of Epstein-Barr Virus in Human Lupus
- Structural and Functional Analysis of Laninamivir and its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition
- Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
- Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
- HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
- Deep Molecular Characterization of HIV-1 Dynamics under Suppressive HAART
- Fitness Landscape of Antibiotic Tolerance in Biofilms
- The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral Genome
- Preventing Sepsis through the Inhibition of Its Agglutination in Blood
- A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,
- IFITM3 Inhibits Influenza A Virus Infection by Preventing Cytosolic Entry
- Targeting Cattle-Borne Zoonoses and Cattle Pathogens Using a Novel Trypanosomatid-Based Delivery System
- A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses
- Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1
- Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Histone Deacetylase 8 Is Required for Centrosome Cohesion and Influenza A Virus Entry
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- Co-opts the FGF2 Signaling Pathway to Enhance Infection
- IRAK-2 Regulates IL-1-Mediated Pathogenic Th17 Cell Development in Helminthic Infection
- Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly
- The Anti-interferon Activity of Conserved Viral dUTPase ORF54 is Essential for an Effective MHV-68 Infection
- A Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
- Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection
- ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation
- Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection
- RNA Polymerase II Stalling Promotes Nucleosome Occlusion and pTEFb Recruitment to Drive Immortalization by Epstein-Barr Virus
- Noninfectious Retrovirus Particles Drive the / Dependent Neutralizing Antibody Response
- Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont
- An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus
- as an Animal Model for the Study of Biofilm Infections
- Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
- The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate of
- Enhances Protective and Detrimental HLA Class I-Mediated Immunity in Chronic Viral Infection
- The Mouse IAPE Endogenous Retrovirus Can Infect Cells through Any of the Five GPI-Anchored EphrinA Proteins
- The Urgent Need for Robust Coral Disease Diagnostics
- HacA-Independent Functions of the ER Stress Sensor IreA Synergize with the Canonical UPR to Influence Virulence Traits in
- A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
- Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
- Mechanisms of Trafficking to the Brain
- Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
- The Role of Sialyl Glycan Recognition in Host Tissue Tropism of the Avian Parasite
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
- The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization
- Distinct Single Amino Acid Replacements in the Control of Virulence Regulator Protein Differentially Impact Streptococcal Pathogenesis
- Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge
- A Genetic Screen Reveals Arabidopsis Stomatal and/or Apoplastic Defenses against pv. DC3000
- Hepatitis C Virus Reveals a Novel Early Control in Acute Immune Response
- Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



