-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Serovar Copenhageni
Leptospirosis, a global disease caused by the unusual bacterium Leptospira, is transmitted from animals to humans. Pathogenic species of Leptospira are excreted in urine from infected animals and can continue to survive in suitable environments before coming into contact with a new reservoir or accidental host. Leptospires have an inherent ability to survive a wide range of conditions encountered in nature during transmission and within mammals. However, we know very little about the regulatory pathways and gene products that promote mammalian host adaptation and enable leptospires to establish infection. In this study, we used a novel system whereby leptospires are cultivated in dialysis membrane chambers implanted into the peritoneal cavities of rats to compare the gene expression profiles of mammalian host-adapted and in vitro-cultivated organisms. In addition to providing a facile system for studying the transcriptional and physiologic changes leptospires undergo during mammalian infection, our data provide a rational basis for selecting new targets for mutagenesis.
Published in the journal: A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Serovar Copenhageni. PLoS Pathog 10(3): e32767. doi:10.1371/journal.ppat.1004004
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004004Summary
Leptospirosis, a global disease caused by the unusual bacterium Leptospira, is transmitted from animals to humans. Pathogenic species of Leptospira are excreted in urine from infected animals and can continue to survive in suitable environments before coming into contact with a new reservoir or accidental host. Leptospires have an inherent ability to survive a wide range of conditions encountered in nature during transmission and within mammals. However, we know very little about the regulatory pathways and gene products that promote mammalian host adaptation and enable leptospires to establish infection. In this study, we used a novel system whereby leptospires are cultivated in dialysis membrane chambers implanted into the peritoneal cavities of rats to compare the gene expression profiles of mammalian host-adapted and in vitro-cultivated organisms. In addition to providing a facile system for studying the transcriptional and physiologic changes leptospires undergo during mammalian infection, our data provide a rational basis for selecting new targets for mutagenesis.
Introduction
Leptospirosis is a neglected disease of global significance [1], [2]. Pathogenic leptospires, shed in animal urine or free-living within contaminated water, enter the host through small abrasions in the skin or contact with mucous membranes of the eyes, nose or throat. Organisms disseminate almost immediately following acquisition, travelling via the bloodstream to multiple tissues [3]. L. interrogans, an extracellular pathogen, is thought to penetrate host tissues by intercellular migration [4]. In immunocompetent hosts, the majority of leptospires are thought to be cleared by opsonophagocytosis following the appearance of specific antibodies [5]. However, organisms that reach the kidneys, an immunoprivileged site [1], adhere to and colonize the proximal convoluted renal tubules, where they replicate exponentially. The majority of human disease is caused by Leptospira interrogans serovar (sv.) Copenhageni for which Rattus norvegicus serves as a reservoir host [3], [6], [7]. Experimentally-infected rats can excrete up to 107 leptospires/ml of urine for months without clinical signs of infection, thus exemplifying the unique biological equilibrium that can exist between pathogen and reservoir host [8], [9], [10].
The genome sequences of several pathogenic and saprophytic Leptospira spp., including L. interrogans sv. Copenhageni, are now complete [6], [11], [12], [13], [14], [15], [16]. L. interrogans sv. Copenhageni Fiocruz L1-130 harbors 3728 protein-encoding genes [11], [12]. By comparative genomics, Picardeau et al. [14] identified 1431 “pathogen-specific” genes that are present within either or both of the pathogenic species, L. interrogans and L. borgpetersenii, but are absent from the free-living saprophyte L. biflexa. Although the majority (62%) of these pathogen-specific genes encode proteins of unknown function, it is possible that some are required by Leptospira to respond to unique environmental cues encountered within the mammalian host. Along these lines, the genome of L. interrogans contains >200 protein-coding sequences potentially involved in gene regulation, including gene products associated with two component signal transduction systems, alternate sigma factors, anti-sigma factors, and anti-sigma factor antagonists [11], [12]. Not surprisingly, the pathogen-specific group also includes numerous gene products whose annotated functions or cellular location suggest a potential role in virulence-related processes such as adherence, digestion of host tissues and extracellular matrix, and evading the host's innate and adaptive immune responses [14], [17].
To identify novel leptospiral virulence determinants, investigators have manipulated in vitro growth conditions to simulate those encountered within the mammalian host, including increased temperature and/or osmolarity, iron starvation, and the presence of serum [18], [19], [20], [21], [22]. However, the extent to which these in vitro conditions faithfully reproduce those encountered by Leptospira in vivo is unclear. In an effort to characterize leptospires in a truly mammalian host-adapted state, we cultivated virulent low-passage L. interrogans sv. Copenhageni within the peritoneal cavities of rats using a modification of our dialysis membrane chamber (DMC) model [23], [24]. Given that rats are a natural reservoir host for this species of Leptospira [2], [25], [26], we reasoned that this model would be ideal for this purpose. Originally developed to study host adaption by Lyme disease spirochetes (Borrelia burgdorferi) [23], [24], this technique, which uses dialysis membrane tubing with an 8000 Da molecular weight cut-off, provides bacteria with access to host nutrients while protecting them from the host's cellular immune response. The DMC model has been instrumental in studying the contribution of mammalian host-specific signals to differential gene expression in B. burgdorferi on a genome-wide scale as well as enabling us to characterize the transcriptional and physiological changes integral to the mammalian host-adaptation process [23], [24], [27], [28], [29].
In recent years, high-throughput RNA sequencing (RNA-Seq) has replaced microarrays as the method of choice for genome-wide transcriptional profiling in bacteria [30], [31]. Unlike microarrays, RNA-Seq allows transcription to be understood at the single-nucleotide level. Here, we used an RNA-Seq approach to compare the transcriptome of virulent low passage Leptospira interrogans sv. Copenhageni cultivated within DMCs with that of leptospires grown under standard in vitro conditions (30°C in EMJH). Using this approach, we determined the relative expression levels of “core” housekeeping genes under both growth conditions, and, more importantly, we identified 166 genes that are differentially-expressed by leptospires within the mammalian host, the majority of which are pathogen-specific (i.e., not present within saprophytic Leptospira). Most notably, our analyses highlight novel physiological aspects of mammalian-host adaptation by leptospires with respect to heme uptake and utilization. Moreover, we identified 11 novel non-coding (ncRNAs) transcripts which represent candidate small regulatory RNAs. In addition to providing a facile system for studying the transcriptional and physiologic changes leptospires undergo during mammalian infection, our data provide a rational basis for selecting new targets for mutagenesis.
Results
Virulent leptospires become mammalian host-adapted during growth within dialysis membrane chambers
Our extensive experience with cultivation of Lyme disease spirochetes in DMCs implanted into rats [23], [24], [27], [32], a natural reservoir for L. interrogans, led us to ask whether the DMC model could be used to generate mammalian host-adapted Leptospira. In preliminary experiments, we determined that virulent low-passage L. interrogans sv. Copenhageni strain Fiocruz L1-130, diluted to low density (1×104 leptospires/ml) in EMJH medium, undergoes exponential replication within DMCs, reaching a maximal density of ∼7×107 leptospires/ml within 8 days post-implantation (data not shown). Importantly, leptospires recovered from DMCs explanted daily between 8 and 12 days post-implantation were vigorously motile by dark-field microscopy. The polypeptide profiles of leptospires in DMCs explanted between 9 and 12 days were highly similar (data not shown). On the basis of these studies, we chose 10 days as our standard period for intraperitoneal implantation. As shown in Figure 1A, under these conditions, we noted numerous polypeptides whose expression was either increased or decreased in response to mammalian host-derived signals compared to in vitro-grown bacteria. The polypeptide differences between in vitro - and DMC-cultivated organisms were even more apparent by two-dimensional SDS-PAGE (Figure S1). While a comprehensive quantitative analysis of these differentially-expressed polypeptides is necessary to identify the corresponding leptospiral proteins, these data support our contention that virulent leptospires substantially alter their proteome in response to mammalian host-specific signals.
Fig. 1. Virulent leptospires become mammalian host-adapted during growth within dialysis membrane chambers. 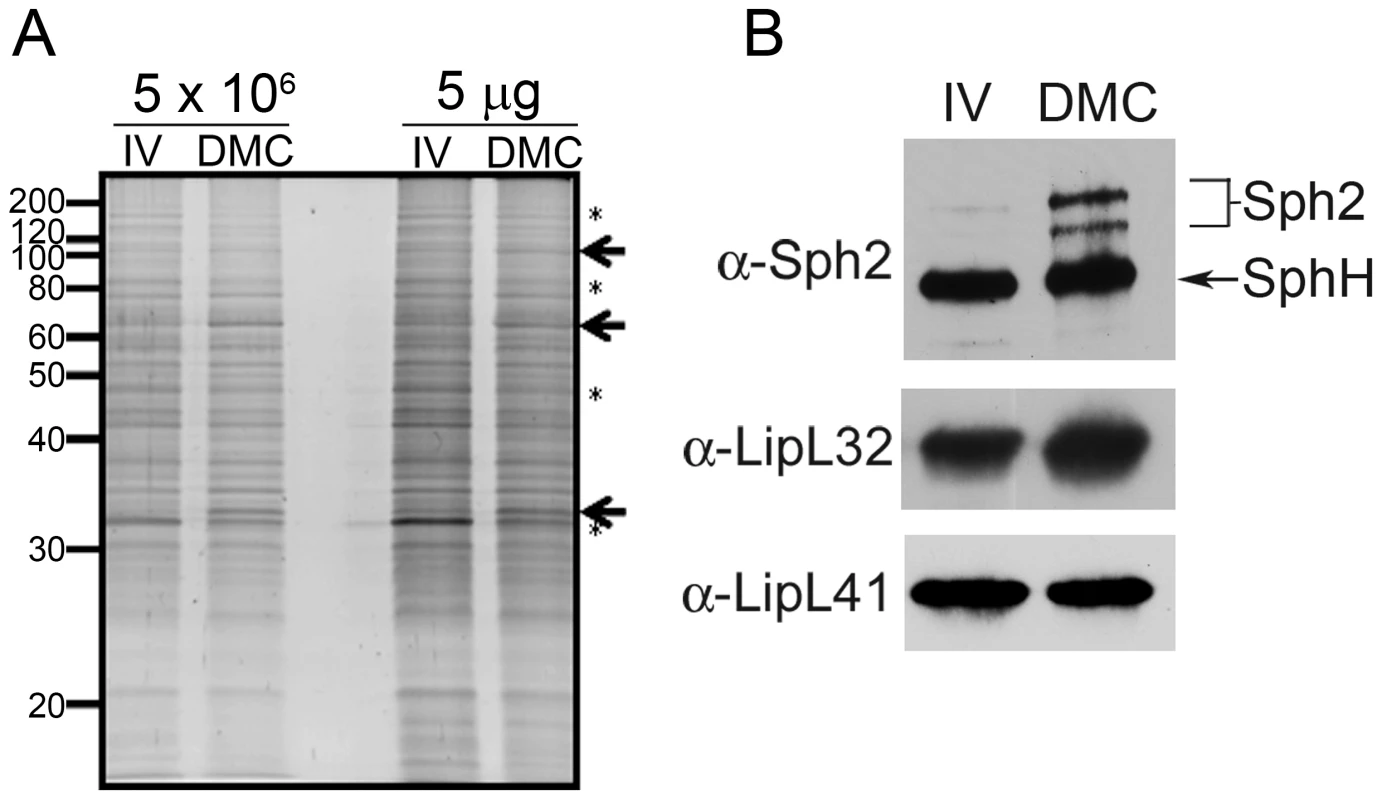
Representative whole cell lysates of leptospires cultivated to late-logarithmic phase in EMJH medium at 30°C in vitro (IV) and within dialysis membrane chambers (DMC) implanted into the peritoneal cavities of female Sprague-Dawley rats. (A) Lysates were loaded according to the numbers of leptospires (5×106 per lane) or total protein (5 µg per lane) and stained with SYPRO Ruby gel stain. Arrows and asterisks are used to highlight examples of polypeptides whose expression appears to be increased or decreased, respectively, within DMCs compared to in vitro. Molecular mass markers are indicated on the left. (B) Immunoblot analyses using rabbit polyclonal antisera directed against Sph2 [34], LipL32 [38] and LipL41 [39]. An arrow is used to indicate a band of the predicted molecular mass for SphH, a second, closely-related sphingomyelinase in L. interrogans recognized by antiserum directed against Sph2 [34], [37]. With B. burgdorferi, successful mammalian host-adaptation within DMCs is determined by the reciprocal expression of the outer surface lipoprotein (Osp) A and OspC lipoproteins, which are OFF and ON, respectively, within the mammal [23]. However, no expression profile associated with host-adapted L. interrogans has been reported and only a handful of leptospiral genes/proteins have been shown to be reproducibly upregulated during mammalian infection. Among these is Sph2, one of four sphingomyelinase-like proteins encoded by L. interrogans sv. Copenhageni [12]. Although most strains of L. interrogans encodes at least 3 distinct sphingomyelinase-like proteins (Sph1, Sph2 and Sph3), only Sph2 is thought to be a “true” (i.e., enzymatically active) sphingomyelinase [33]. Expression of Sph2 is upregulated in vitro in response to serum [21] and/or increased osmolarity [34] and during mammalian infection [35]. On the other hand, SphH, a closely-related pore-forming protein without sphingomyelinase activity [33], [36], is expressed constitutively in vitro [34], [37] and by leptospires colonizing the renal tubules of infected hamsters [37]. Consistent with these previous studies, the level of Sph2 was substantially higher in DMC-cultivated leptospires compared to in vitro-grown organisms, whereas SphH was expressed at similar levels under both conditions (Figure 1B). Immunoblots using antisera against LipL32 and LipL41, two leptospiral lipoproteins expressed constitutively in vitro and during mammalian infection [38], [39], [40], were performed as loading controls (Figure 1B). We considered these data as strong indication that DMC-cultivated leptospires are in a mammalian host-adapted state.
RNA-Seq analysis of Leptospira cultivated in vitro and within DMCs
Having established the feasibility of using DMCs to generate mammalian host-adapted L. interrogans, we compared the transcriptional profiles of DMC - and in vitro-cultivated leptospires by RNA-Seq. To ensure that our data would be robust and reproducible, we generated Illumina TruSeq libraries from three biologically-independent samples for each growth condition. The sequence statistics and numbers of mapped reads for each biological replicate are summarized in Table 1 and displayed graphically in Figure 2. The total number of reads ranged from ∼8–14 million per library, of which 79–94% of reads mapped to the L. interrogans sv. Copenhageni Fiocruz L1-130 reference genome [11], [12]; only those reads that mapped to a single location on either Chromosome 1 or 2 were used to assess gene expression. The majority (43–55%) of unique sequence reads mapped to protein-coding mRNAs annotated on Chromosome 1, while ∼3–5% mapped to predicted ORFs on Chromosome 2; this 12∶1 ratio is consistent with the relative coding capacities of the two chromosomes [11], [12]. As discussed below, a considerable number of reads (13–20%) in both chromosomes mapped to non-coding regions that represent candidate small regulatory RNAs (sRNAs) (Table 1).
Fig. 2. Mapping of RNA-Seq reads. 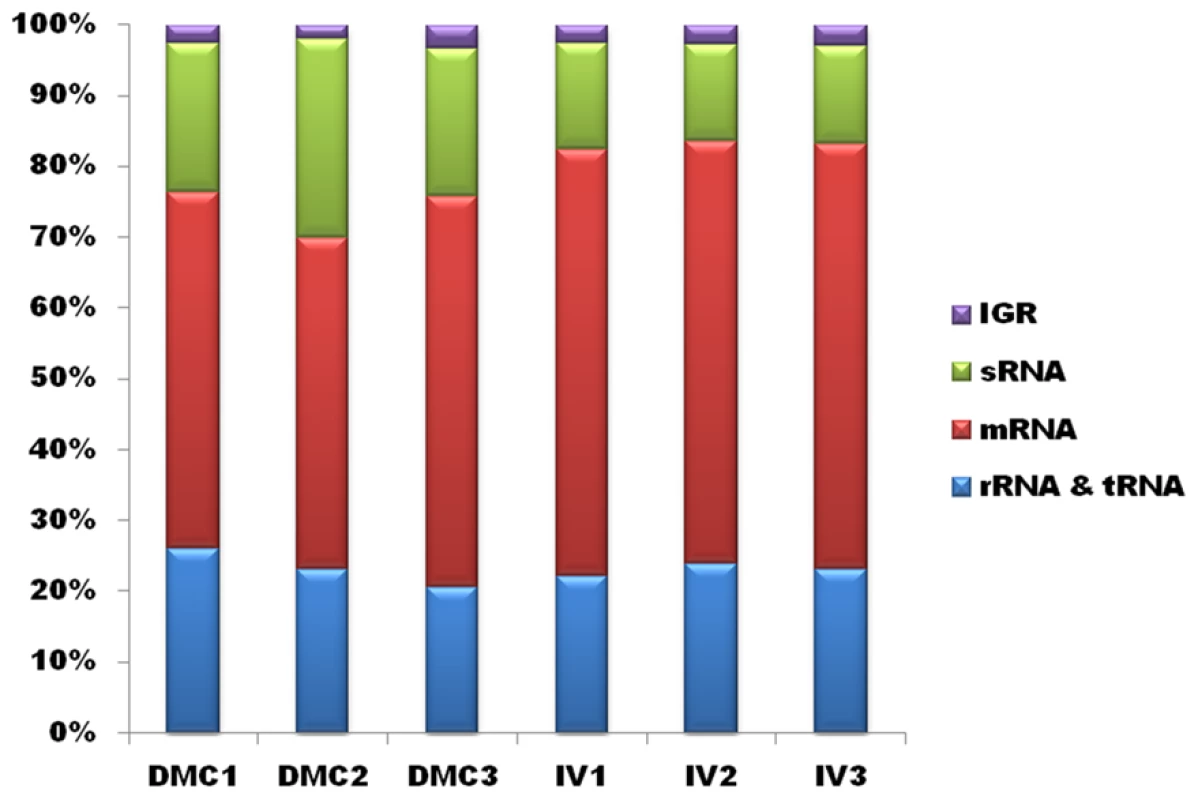
Percentage of uniquely mapping reads from each biological replicate of leptospires cultivated in DMCs or under standard in vitro growth conditions (30°C in EMJH). Tab. 1. Summary of RNA-Seq mapping data. 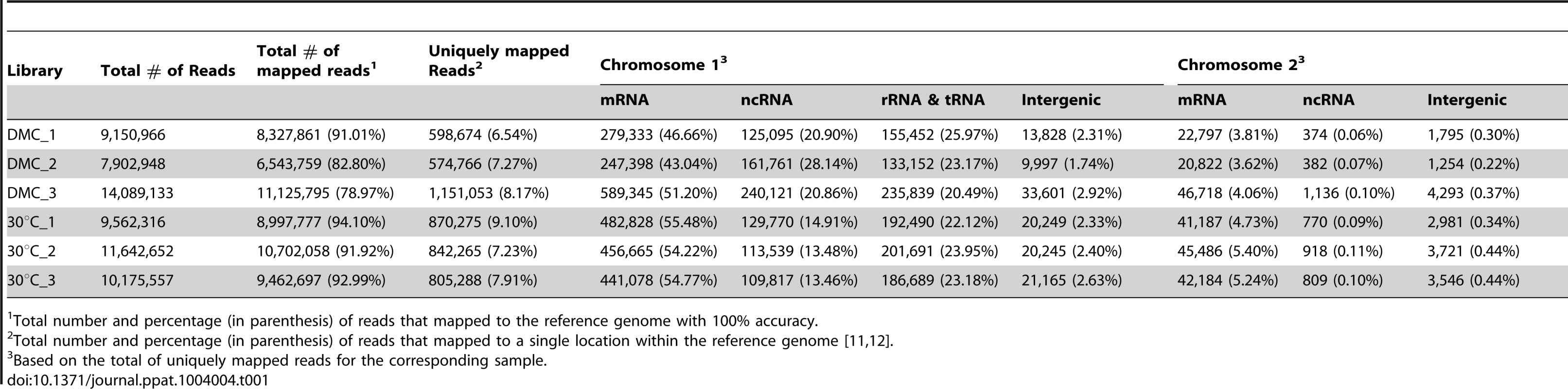
Total number and percentage (in parenthesis) of reads that mapped to the reference genome with 100% accuracy. RNA-Seq provides comprehensive coverage of the leptospiral transcriptome under in vivo and in vitro growth conditions
The genome of L. interrogans sv. Copenhageni Fiocruz L1-130 harbors 3728 protein-encoding genes [11], [12]. The vast majority (∼94%) of these (3489 and 3499 in DMC - and in vitro-cultivated leptospires, respectively), were represented in our RNA-Seq data by a mean expression value of ≥1 (Table S2). We observed average mean expression values of 67.2 and 60.5 per gene in DMC - and in vitro-cultivated organisms, respectively (data not shown).
By comparative genomics, Picardeau et al. [14] identified 2052 “core” protein-coding genes that are shared between pathogenic (L. interrogans and L. borgpetersenii) and saprophytic (L. biflexa) Leptospira species. Not surprisingly, many of these core gene products are associated with housekeeping functions, such as motility, energetics and intermediary metabolism, DNA and RNA metabolism, and cell division [14]. Analysis of the protein-coding sequences for the 100 most highly-expressed genes (i.e., Top 100) in DMC-cultivated leptospires revealed that 66 are conserved (i.e., ≥40% amino acid identity over ≥80% of the coding region) between pathogenic and saprophytic Leptospira spp. and, therefore, part of the core group (Table S3); of note, the percentage (66%) of core genes within our Top100 is similar to the overall percentage (55%) of core genes within the entire L. interrogans sv. Copenhageni genome [14]. Consistent with their proposed housekeeping functions, 62 (94%) of the 66 core genes within the Top 100 were expressed at similar levels in vitro and within DMCs (Table S3). Thirty-four of the Top 100 genes are pathogen-specific (i.e., no orthologous gene identified in L. biflexa), two of which (LIC10465/ligA and LIC12653) are found only in L. interrogans (i.e., absent in L. borgpetersensii, L. licerasiae and L. santarosai). Eight of the 34 pathogen-specific genes within the Top 100 were upregulated by L. interrogans sv. Copenhageni within DMCs (see Table S3 and below).
We also surveyed both DMC - and in vitro-derived datasets for genes associated with key metabolic pathways. One unusual metabolic feature of pathogenic leptospires, compared to other spirochetes, is that they are unable to utilize glucose despite encoding a seemingly complete glycolytic pathway, relying instead on β-oxidation of long-chain fatty acids as sources of both carbon and energy [11], [41]. By RNA-Seq, we detected uniquely mapped reads for all of the genes thought to be involved in glucose uptake and utilization (KEGG pathway lic00010), each of which was expressed at similar levels in DMCs and in vitro (Table S4). However, two genes, LIC13358 and LIC20119, both encoding putative phosphoglucomutases, and LIC12908, encoding the only glucose transporter identified in L. interrogans [11], [12], [42], were expressed at extremely low levels, both in DMCs and in vitro (Table S2). These data support the findings of Zhang et al. [42], who proposed that the inability of pathogenic leptospires to utilize glucose stems from insufficient glucose uptake and/or catalysis rather than an incomplete glycolytic pathway. As one might predict, we detected significant numbers of sequence reads for genes involved in the uptake and β-oxidation of medium and long-chain fatty acids (KEGG pathway lic00071), the citric acid cycle (KEGG lic00020), generation of NAD/NADP (KEGG lic00760), and oxidative phosphorylation (KEGG lic00190). All of the individual genes involved in these energetic pathways were expressed at similar levels under both growth conditions (Table S4).
Genes whose expression was significantly upregulated by leptospires in DMCs compared to in vitro-grown bacteria
Using DESeq [43], we identified 166 genes whose expression was either positively - or negatively-regulated by ≥2-fold (adjusted p-value≤0.05) within the mammal (Tables 2 and 3). Although some variance was observed between biological replicates (Table S2), a heat map representing the expression data for all 166 differentially-expressed genes confirmed that each biological replicate clustered with its respective sample source (Figure S2). Of the 110 genes upregulated by L. interrogans within DMCs, 106 are on Chromosome 1 while only 4 are on Chromosome 2 (Table 2). All but 3 of the upregulated genes appear to be pathogen-specific (i.e., a paralogous gene/protein could not be identified in L. biflexa; 54 of these are unique to L. interrogans and an additional 7 are unique to serovar Copenhageni (Figure 3). Almost half (49/110) of the genes upregulated in DMCs encode hypothetical proteins (Figure 4 and Table 3), which is consistent with the overall percentage (40%) of hypothetical genes annotated within L. interrogans [6], [11]. Based on searches performed using the Conserved Domain Database [44], [45], none of the hypothetical proteins encoded by these genes contained readily identifiable functional domains (data not shown). However, one gene (LIC12986) recently was shown to be required for leptospires to survive within hamsters and to colonize the renal tubules of mice [46].
Fig. 3. Conservation of L. interrogans sv. Copenhageni Fiocruz L1-130 differentially-expressed genes among virulent and saprophytic Leptospira spp. Protein sequence similarities were determined using GLSEARCH (v. 34.05). 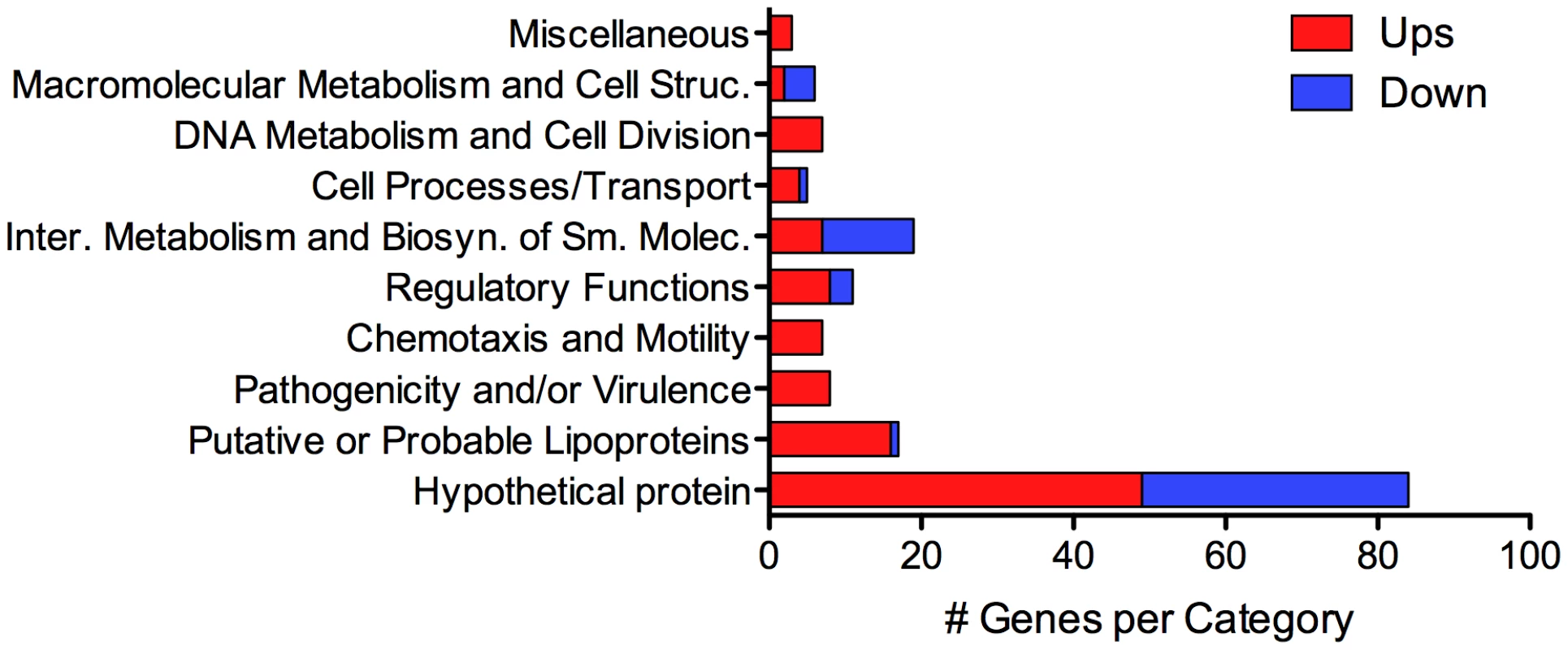
Genomes used for analysis: L. interrogans sv. Lai strain 56601, L. borgpetersenii sv. Hardjo strain L550, L. santarosai sv. Shermani strain LT821; L. licerasiae sv. Varillal strain VAR010; and L. biflexa sv. Patoc strain Patoc1 Ames, respectively. The color coding used in the heat map is as follows: blue, 95–100% identity; green, 90–94% identity; orange, 85–89%; and yellow, 80–84%. Fig. 4. Functional categories of genes differentially-expressed by L. interrogans sv Copenhageni strain Fiocruz L1-130 within DMCs. 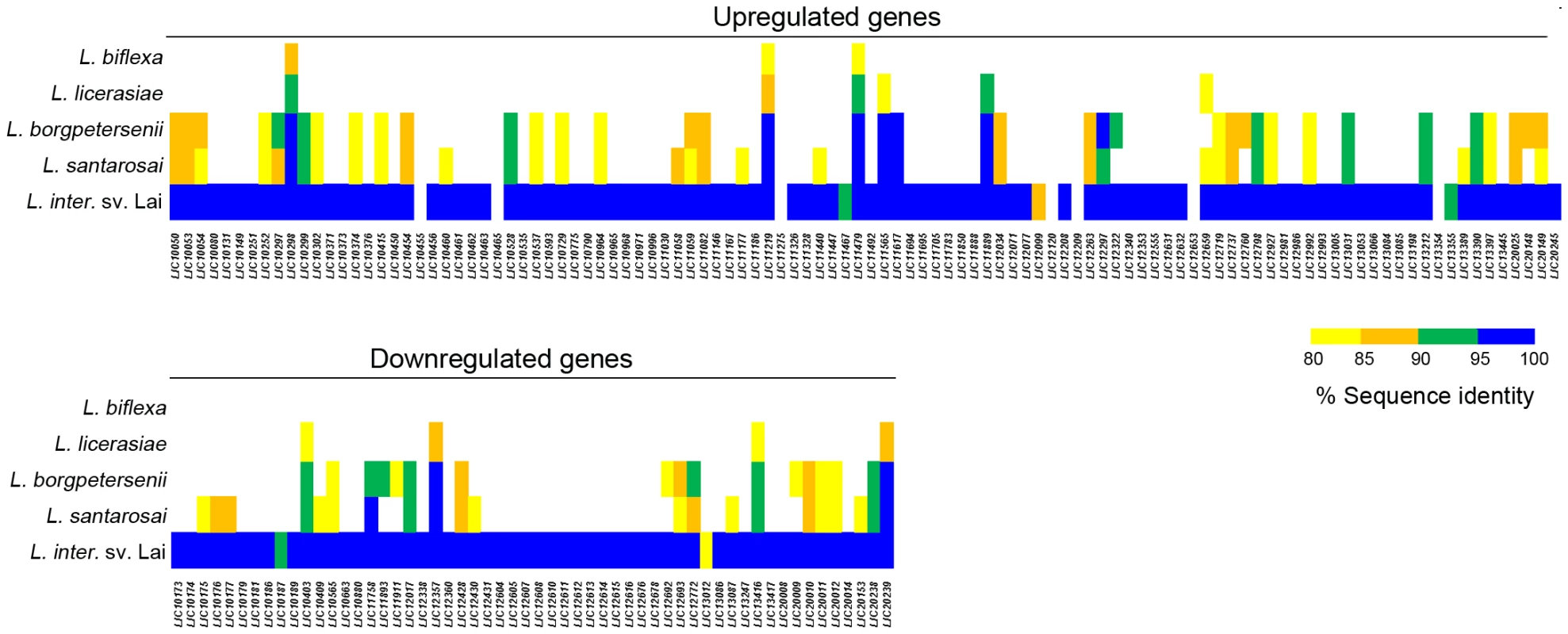
Functional categories are based on those of [11], [12] and the Leptospira interrogans sv. Copenhageni Genome Project database (http://aeg.lbi.ic.unicamp.br/world/lic/). The number of upregulated (Ups) and downregulated (Down) genes within each category are indicated in red and blue, respectively. Tab. 2. L. interrogans sv. Copenhageni genes upregulated in DMCs compared to in vitro. 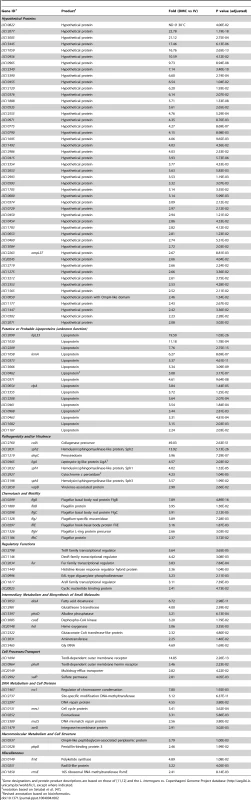
Gene designations and protein product descriptions are based on those of [11], [12] and the L. interrogans sv. Copenhageni Genome Project database (http://aeg.lbi.ic.unicamp.br/world/lic/), except where indicated. Tab. 3. Leptospiral genes differentially-expressed within DMCs compared to in vitro. 
Percentage of genes based on the total number of genes in upregulated or downregulated category. Of the remaining upregulated genes, 16 encode putative lipoproteins of unknown function [47] (Figure 4 and Table 3). Surface-exposed spirochetal lipoproteins have been implicated in a wide range of pathogenesis-related functions, including adherence to extracellular matrix components and nutrient acquisition [48]. However, because the mechanism(s) responsible for sorting individual spirochetal lipoproteins remain poorly understood, it is not possible to predict based on amino acid sequence alone which, if any, might function at the pathogen-host interface.
Virulence-associated genes
Eight DMC-upregulated genes encode proteins implicated in pathogenicity and/or virulence. LIC12760/colA, the most significantly upregulated gene (49-fold) in our studies, encodes a collagenase precursor. Degradation of host tissues by this enzyme is thought to promote bacterial colonization and/or dissemination as well as provide an additional source of nutrients (e.g., amino acids) [49]. LIC12631, LIC12632 and LIC13198, respectively, encode Sph2, Sph1 and Sph3. Lysis of host erythrocytes by Sph2 may enhance acquisition of fatty acids and heme/iron from the host. Narayanavari et al. [33] also raised the possibility that the non-catalytic Sphs (Sph1 and Sph3) function as adhesins via their interaction with host sphingomyelin. LIC10465 encodes leptospiral immunoglobulin-like (Lig) protein A; this multifunctional, outer membrane-associated lipoprotein has been shown to promote binding to host molecules, including fibronectin, fibrinogen and extracellular matrix [50], [51], [52]. Moreover, antibodies against LigA are protective in a hamster model of acute infection [53]. LIC12659/vapB encodes a putative virulence-associated protein with similarity to the AbrB-like family of transcriptional regulators [54]; ArbB-like transcription factors, also referred to as transition state regulator proteins, have been identified in diverse bacteria but only orthologs from Bacillus have been characterized with respect to function and DNA-binding capabilities [55], [56]. LIC11219 and LIC12927, encoding a peroxiredoxin (AhpC) and cytochrome c peroxidase, respectively, are discussed below.
Motility-related genes
Consistent with the highly invasive nature of leptospiral infection, seven motility-related genes were upregulated within DMCs (Table 2), including three (LIC10299/flgB, LIC10298/flgC and LIC10297/fliE) involved in flagellar basal body formation (Figure 5). The L. interrogans genome contains five copies of flaB (LIC11889, LIC11890, LIC11531, LIC11532 and LIC12947), which encode the flagellar core subunit flagellin. Of these, only LIC11889 was differentially-expressed within DMCs. Interestingly, based on the number of uniquely mapped reads determined by DESeq, LIC12947 was expressed at substantially lower levels than the other four flaB paralogs under both growth conditions (Table S2), suggesting that this gene product may not contribute significantly to the formation of flagella in vitro or in vivo.
Fig. 5. IGB viewer of normalized gene expression data for the flagellar genes fliE, flgB and flgC. 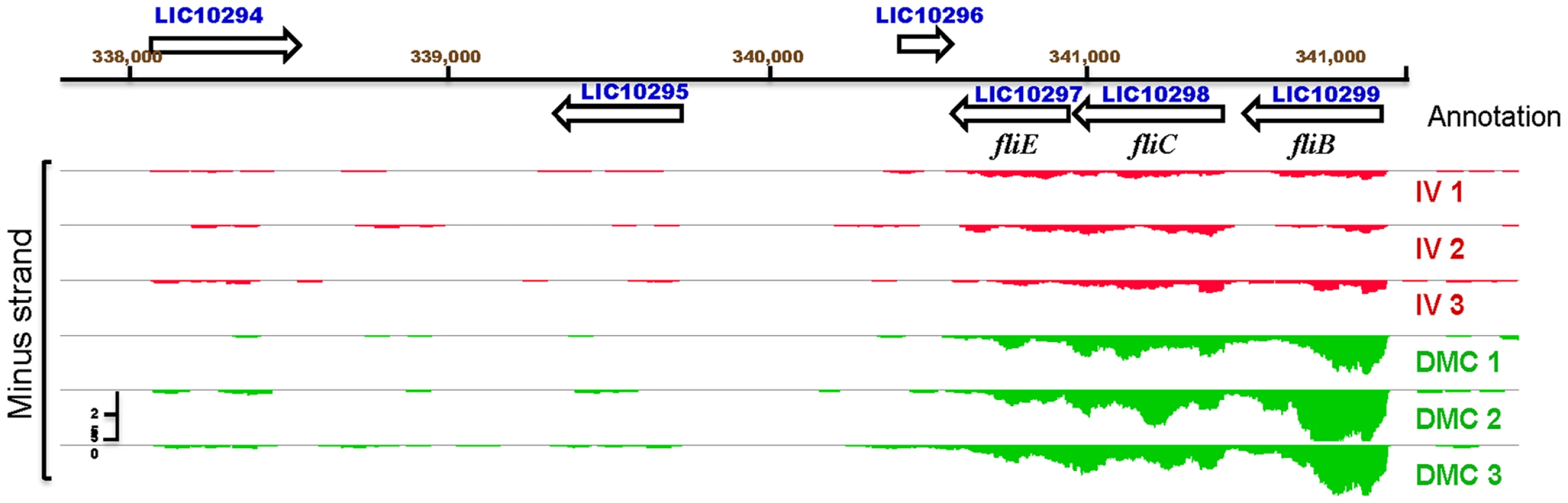
Visualization of normalized mapped reads for minus (-) strand of an operon encoding genes fliE, flgB and flgC of the flagellar proximal rod shows increased expression by leptospires cultivated in dialysis membrane chambers (DMC, green) compared to those cultivated in vitro (IV, red). Annotated genes on Chromosome 1 are in blue. The vertical “read count” scale is 0–50. Uptake and utilization of iron/heme
Unlike B. burgdorferi [57], L. interrogans requires iron for growth in vitro and, presumably, within the mammalian host. In EMJH medium, leptospires obtain iron from Fe(II) sulphate, while organisms in the mammal acquire iron from heme and/or heme-containing proteins [58]. Heme (free or complexed with hemoglobin) is appropriated from the host by high-affinity TonB-dependent outer membrane receptor (TB-DR) proteins. Based on bioinformatic analysis, L. interrogans encodes at least 13 putative TB-DRs [58], however, only two (LIC10964 and LIC11694) were upregulated within DMCs (Table 3 and Table S4). While most often associated with iron uptake, TB-DRs also may bind vitamin B12, a nutrient essential for leptospires in vitro and, presumably, in vivo [59]. Only one TB-DR in L. interrogans sv. Copenhageni (LIC12374/btuB) is annotated as being specific for vitamin B12, and the gene encoding this transporter component was not differentially-expressed in DMCs compared to in vitro (Table S4). Transport of heme and/or iron across the outer membrane requires energy produced by an inner membrane complex of the energy transduction protein TonB and two accessory proteins, ExbB and ExbD [59]. L. interrogans encodes at least two TonB-ExbB-ExbD complexes, arranged in separate operons, one on each chromosome. Interestingly, the transporter on Chromosome 2 (LIC20216-20218) was expressed at much higher levels (>14-fold) than its counterpart on Chromosome 1 (LIC10889-10892) under both growth conditions. Neither operon, however, was differentially-expressed in DMCs.
Consistent with an increased requirement for iron and/or heme in vivo, we detected increased expression (3.27-fold) of heme oxygenase (LIC20148/hol) [60] within DMCs. Once released, iron would be stored in the cytoplasm by bacterioferritin (LIC11310) and/or ferredoxin (LIC13258 and LIC13209) gene products, all of which were well expressed by leptospires in vitro and in DMCS (Table S5).
When in excess, iron can lead to toxicity via the production of reactive oxygen species (ROS). As such, bacterial genes associated with iron homeostasis often are regulated by the ferric uptake regulator protein Fur, a global iron-responsive transcriptional repressor [61]. L. interrogans encodes at least four putative Fur paralogs (LIC11006, LIC11158, LIC12034 and LIC20147). We used SLiMSearch [62] to survey the L. interrogans genome for “fur boxes” ([GC]AT[AT]AT[GC]AT[AT]AT[GC]AT[AT]AT[GC]) [61], and were unable to identify any obvious Fur-regulated genes (data not shown). Fur proteins, including those encoded by Leptospira spp. [58], share significant sequence similarity with orthologs for Zur, a zinc uptake regulator, and Per, an oxidative stress response regulator [63]. Based on bioinformatics and/or experimental evidence, two of Furs identified in L. interrogans sv. Copenhageni (LIC12034 and LIC20147) appear to encode Per orthologs [22], [58]. One of these (LIC12034) was upregulated in DMCs (Table 2), suggesting that leptospires within DMCs are under some degree of oxidative stress.
Oxidative and thermal stress-related genes
Leptospires must cope with numerous stressors within the host, most notably, oxidative stress. Incomplete reduction of oxygen by iron-containing cytochromes is one potential source of endogenous ROS [64]. Leptospires likely encounter exogenously-derived ROS within the proximal renal tubules, a highly oxygenated tissue niche. Not surprisingly, L. interrogans encodes a more diverse repertoire of antioxidant proteins than either Treponema pallidum or B. burgdorferi (Table S5). Although L. interrogans encodes a functional catalase [65], it lacks superoxide dismutase (the enzyme typically associated with detoxification of O2•−) and the regulatory proteins OxyR and SoxR. Interestingly, only two oxidative stress-associated genes (LIC12927 and LIC11219) were upregulated within DMCs (Table 2). The former encodes a cytochrome c peroxidase while the latter encodes an AhpC-type peroxiredoxin. In E. coli, AhpC scavenges basal levels of endogenous peroxide generated as a metabolic by-product [66]. Increased expression of AhpC within DMCs is consistent with increased uptake of exogenously-derived heme (see above) and increased potential for Fenton chemistry within the cytoplasm. Like T. pallidum [67], L. interrogans does not encode an AhpF, the usual reducing partner for AhpC, and most likely uses thioredoxin/thioredoxin reductase and/or glutaredoxin for this purpose, all of which were well expressed by DMC-cultivated leptospires (Table S5).
In addition to oxidative stress, increased temperature within the host might induce a stress response by leptospires in vivo [18], [19]. However, consistent with previous reports [19], [68], [69], none of the classical heat shock response genes encoded by L. interrogans were upregulated within DMCs, compared to in vitro growth at 30°C (Table S4).
Regulators of transcription
The leptospiral genome encodes >200 gene products with the potential to directly regulate transcription (Figure 3), including numerous two-component sensor histidine kinases (HKs) and/or response regulators (RRs), alternate sigma factors, sigma factor regulators, anti-sigma factor antagonists, and trans-acting factors [11], [12]. Only a few of these were upregulated within DMCs. One (LIC11440) encodes a hybrid sensor kinase/response regulator (HK/RR) protein; the sensor for this HK/RR contains a PAS-type sensor domain, which typically recognize small molecules, including heme [70]. Three additional genes (LIC12798, LIC11146 and LIC11617) were DMC-upregulated and encode putative transcriptional regulators belonging to the TetR [71], DeoR [72], and ArsC [73] families of repressor proteins.
Genes whose expression was significantly downregulated in DMCs compared to in vitro
By RNA-Seq, we identified 56 genes (47 on Chromosome 1 and 9 on Chromosome 2) that were downregulated in DMCs (Tables 3 and 4). All of the downregulated genes are pathogen-specific (i.e., not found in L. biflexa); almost half (26/56) are unique to L. interrogans (i.e., not in L. borgpetersenii, L. santarosai or L. licerasiae) (Figure 3). As with the upregulated gene subset, more than half (35/56) of the DMC-downregulated genes encode hypothetical proteins (Figure 4 and Table 3); of note, almost half (43%) of these appear to be transcribed in two polycistronic operons (LIC10173-10177 and LIC12604-12616). Interestingly, all of the genes within these two putative operons are pathogen-specific. Only one lipoprotein (LIC20153) was expressed at lower levels in DMCs (compared to 16 upregulated).
Tab. 4. L. interrogans sv. Copenhageni genes downregulated in DMCs compared to in vitro. 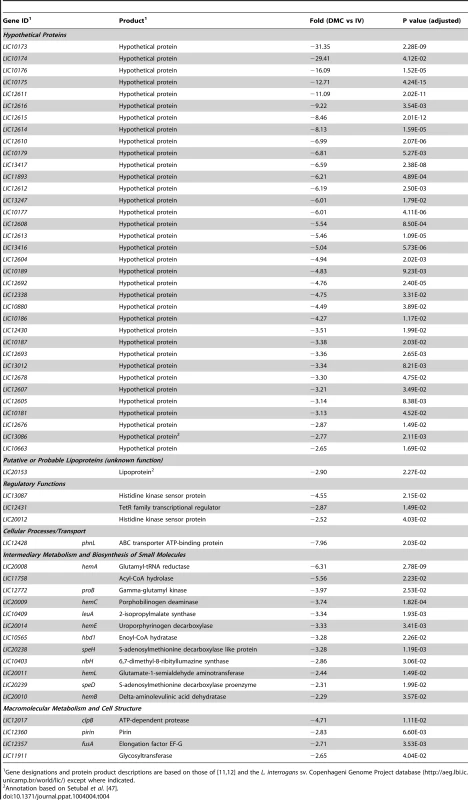
Gene designations and protein product descriptions are based on those of [11], [12] and the L. interrogans sv. Copenhageni Genome Project database (http://aeg.lbi.ic.unicamp.br/world/lic/) except where indicated. Five genes related to de novo heme biosynthesis (LIC20008/hemA, LIC20009/hemCD, LIC20010/hemB, LIC20011/hemL and LIC20014/hemE) [74] were DMC-downregulated these findings imply that leptospires can scavenge heme from the mammalian host. The heme biosynthetic operon also contains genes encoding a two component system (TCS). Signal transduction by the orthologous TCS in L. biflexa is required for regulation of heme biosynthesis [75]. Although both the histidine kinase (HK; LIC20012) and the response regulator (RR; LIC20013) were downregulated (2.50 - and 2.22-fold; respectively) in DMCs, the fold-change for the RR was not significant (p = 0.097). Based on their tandem arrangement and similar expression profiles, these heme biosynthetic genes appear to be transcribed as a single operon. LIC20017/hemG and LIC20018/hemH, encoding enzymes responsible for the last two steps in heme biosynthesis, respectively, are located downstream of the larger biosynthetic operon; both of these genes appear to be transcribed as monocistronic messages at similar levels in vitro and in DMCs (Table 4 and data not shown).
Identification of novel candidate small RNAs
One of the advantages of RNA-Seq is that it allows visualization of uniquely mapped reads within non-annotated regions of the genome. Using the IGB browser, we detected at least 11 regions that were transcriptionally-active but not protein coding; these non-coding RNA (ncRNA) transcripts are novel candidate small regulatory RNAs (sRNAs) within L. interrogans (Table 5 and Figure S3). Five of these are homologous to known sRNA families (tmRNA, RNaseP, PyrR binding site and two cobalamin sRNAs) (http://rfam.sanger.ac.uk/) [76], [77], [78], [79]. The expression of 8 of the 11 putative sRNAs was validated by reverse-transcriptase PCR in L interrogans sv. Copenhageni strain RJ16441 (Table 5) and all predicted sRNAs were highly conserved in the closely-related virulent serovar type strain Lai [80]. One of the predicted sRNAs, LIC1nc80 (Figure 6), was significantly DMC-upregulated (4.39-fold) compared to in vitro-cultivated leptospires (Table S2). Further characterization of these candidate sRNAs (i.e., by Northern blot) is required to understand their function(s) and relationships to the surrounding genes (i.e., 5′ UTR verses bone fide sRNA).
Fig. 6. IGB viewer of candidate sRNA LIC1nc80. 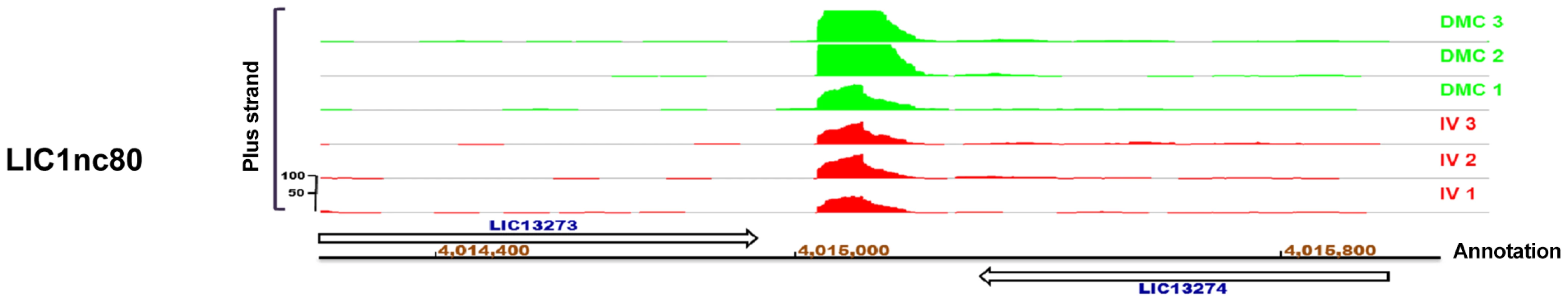
LICnc80 was identified as an area of high transcriptional activity within an intergenic region of the genome of L. interrogans sv. Copenhageni Fiocruz L1-130. Expression data for leptospires cultivated in DMCs (green) compared to those cultivated in vitro (IV, red) are indicated on the plus strand of the genome. Annotated genes on the relevant chromosome and nucleotide co-ordinates are indicated. The vertical “read count” scale is 0–100. Tab. 5. Candidate small non-coding RNAs identified by RNA-Seq. 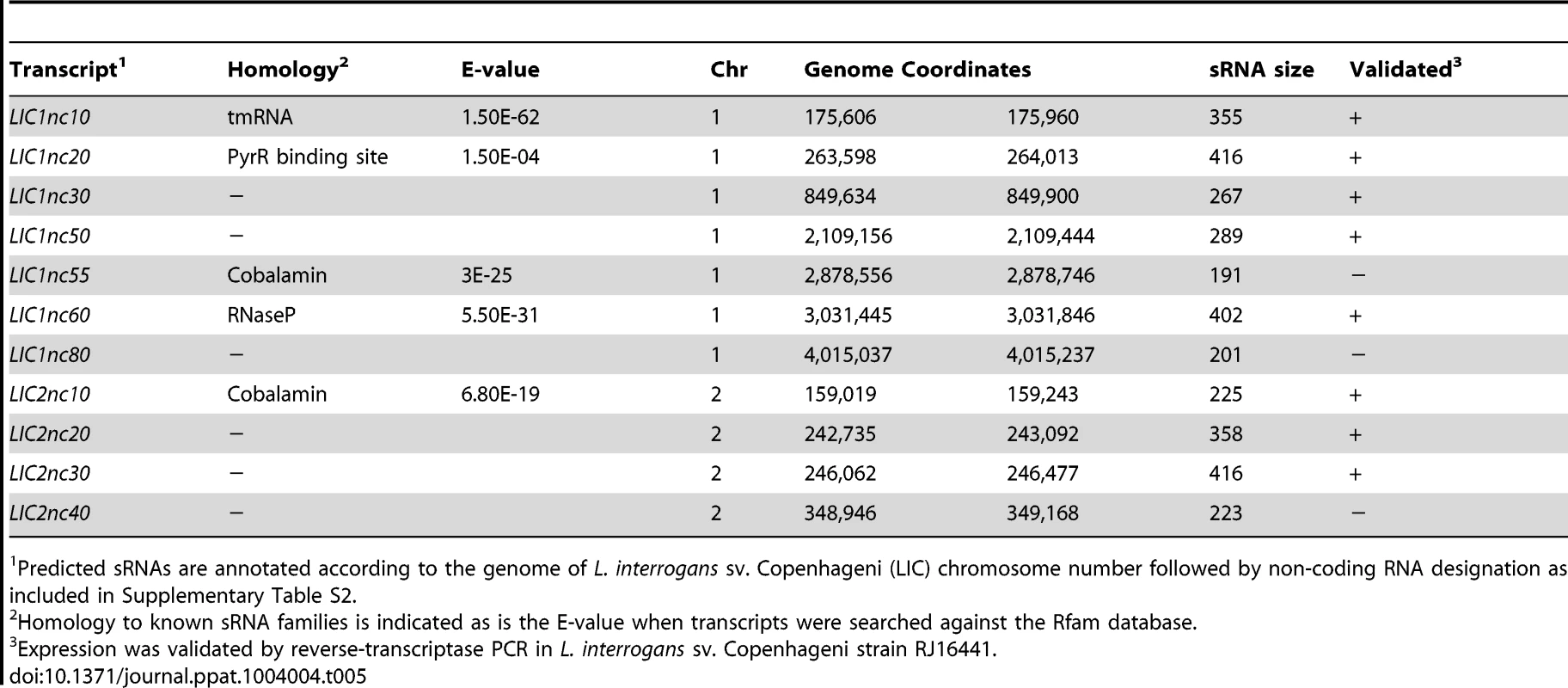
Predicted sRNAs are annotated according to the genome of L. interrogans sv. Copenhageni (LIC) chromosome number followed by non-coding RNA designation as included in Supplementary Table S2. Validation of RNA-Seq data by quantitative RT-PCR
To validate our RNA-Seq data, we performed quantitative reverse transcription-PCR (qRT-PCR) on a panel of 14 genes that were, according to DESeq analysis, upregulated (LIC12631/sph2, LIC11888 and LIC11889/flaB), downregulated (LIC10175, LIC10179 and LIC12615), or unchanged (LIC10191/loa22, LIC12966/lipL41, LIC13166/ompL36, LIC10787/flaA-2, LIC10068, LIC10421, LIC12339, and LIC20001) in DMCs compared to in vitro.While there is some debate regarding the most appropriate leptospiral gene to use for normalization [21], [81], we selected LIC11352/lipL32 based on studies demonstrating that its expression was relatively unchanged under a wide-range of growth conditions, including increased temperature, increased osmolarity, and/or exposure to serum [21], [38], . Representative results are shown in Figure 7A; data for the entire panel are presented in Figure S4. Overall, we saw strong agreement between our RNA-Seq and qRT-PCR datasets; the correlation coefficient (R2) between RNA-Seq and qRT-PCR data across the entire panel was 0.8881 (Figure 7B). We also used qRT-PCR to confirm the relative expression for two (LIC1nc60/RNase P and LIC2nc10/cobalamin) of the putative sRNAs (Figure S4); of these, only LIC1nc60/RNaseP was upregulated (2.65-fold; p = 0.0054) within DMCs.
Fig. 7. Validation of comparative RNA-Seq analysis. 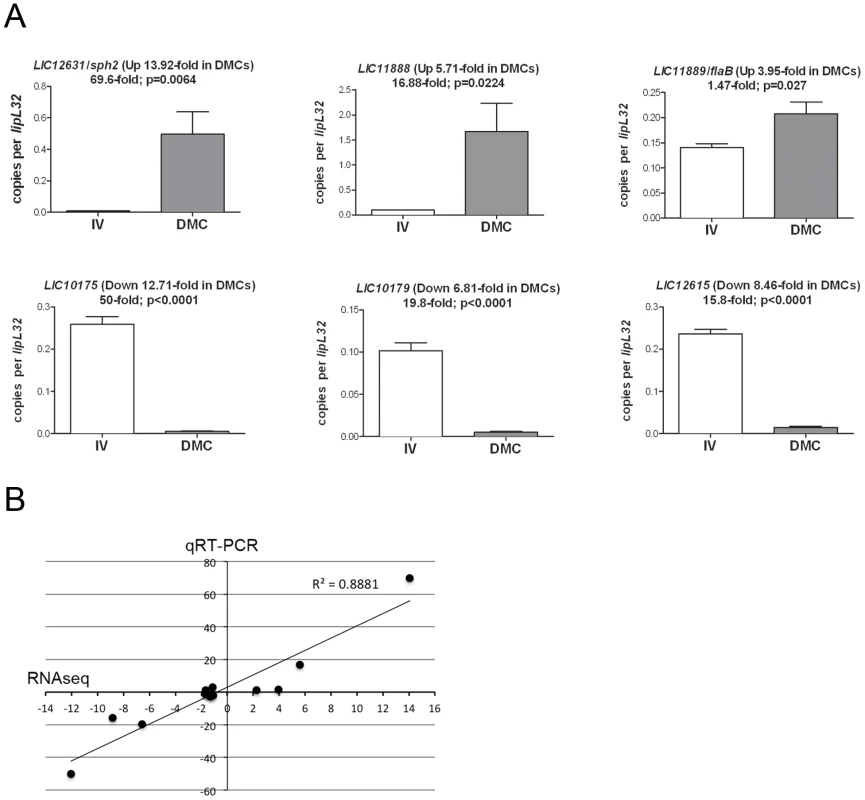
(A) qRT-pCR analysis of representative genes identified by RNA-Seq. Values represent the average transcript copy numbers for each gene normalized per lipL32 transcript. Bars indicate the standard error of the mean (SEM). Results presented are mean values from at least 3 biologically-independent samples of leptospires for each growth condition. The fold-regulation for each gene determined by RNA-Seq is indicated in parentheses. The fold-regulation between in vitro- (IV) and DMC-cultivated leptospires determined by qRT-PCR are indicated. P values were calculated using an unpaired t-test. (B) Correlation coefficient (R2) between RNA-Seq and qRT-PCR data. Discussion
The identification of genes/proteins that are differentially-expressed by microorganisms only during infection and/or within specific host niches often provides insight into the parasitic strategies of pathogens. During natural and experimental infection in rats, L. interrogans rapidly disseminate hematogenously to all tissues but are cleared by 7 days post-inoculation from all sites except the kidneys [7], [82]. The ability of leptospires to colonize and persist within renal tubules almost certainly involves unique virulent determinants [1]; however, the paucilbacillary nature of leptospiral infection, even within this preferred niche, hinders our ability to perform global gene expression studies on L. interrogans within host tissues. Prior studies, including several using microarray-based approaches [18], [19], [21], [22], [83], have manipulated in vitro growth conditions to simulate the environmental signals encountered by leptospires within the mammal. Based on extensive studies with B. burgdorferi, another pathogenic spirochete, we and others have demonstrated that bone fide mammalian host adaptation is a complex and dynamic process that cannot be fully reproduced ex vivo [23], [27], [32]. We therefore used a rat peritoneal dialysis membrane chamber (DMC) model to generate sufficient L. interrogans in a mammalian host-adapted state to perform global transcriptional studies. Cultivation of leptospires within DMCs, in conjunction with next generation sequencing, enabled us to define for the first time the transcriptome of L. interrogans within the mammalian host.
In order to transition from a free living to infectious state, leptospires must adjust their metabolism to utilize nutrients available within the mammalian host. Quite surprisingly, we found that the majority of genes implicated in central and intermediary metabolism were expressed by leptospires at similar levels in DMCs and in vitro. We interpret these data to suggest that EMJH, the medium commonly used to cultivate pathogenic and saprophytic leptospires in vitro, reflects the overall composition of nutrients available within mammalian host fairly well. Nonetheless, leptospires cultivated within DMCs differentially-regulated a handful of genes whose products are involved in metabolic and biosynthetic pathways, most notably, heme uptake and utilization (see below). Although increased temperature often is implicated as an important stimulus for host adaptation, we observed very little overlap (<10%) between the cohort of genes that were upregulated in DMCs and those previously identified as being temperature-regulated in vitro [18], [19], [21], [68]. Thus, differential gene regulation by leptospires within DMCs appears to be driven primarily by non-thermal mammalian host-specific stimuli. The relatively small pore size of the dialysis tubing used to cultivate leptospires within rat peritoneal cavities would exclude macromolecules and most serum proteins but allow for efficient exchange of nutrients (i.e., glucose, ions, and free amino acids) present within serum. These are the same types of small molecules that leptospires likely encounter within proximal convoluted tubules, where the composition of the glomerular ultrafiltrate most closely resembles that of interstitial fluid [84]. Further experimentation is required to assess how closely DMC-cultivated leptospires resemble their counterparts within host tissues during acute and/or chronic infection. The DMC model does have some limitations. For instance, virulence genes associated with pulmonary haemorrhage may be expressed only within the context of lung tissue. Because bacteria within DMCs are prevented from interacting with host immune cells and immunoglobulin [85], this model does not enable us to identify genes that are differentially-regulated in response to specific pathogen-host interactions and/or immune evasion.
Although increased temperature often is implicated as an important stimulus for host adaptation, we observed very little overlap (<10%) between the cohort of genes that were upregulated in DMCs and those previously identified as being temperature-regulated in vitro [18], [19], [21], [68]. We observed a similarly limited overlap between our RNA-Seq data and genes found to be differentially regulated in vitro in response to exposure to serum [21] and low iron [22]. We observed a somewhat higher, but nonetheless small, degree of overlap (16%) between our RNA-seq dataset and genes identified by Matsunaga et al. [20] as being upregulated by physiologic osmolarity (EMJH supplemented with 120 mM NaCl); included in this overlap are lipL53 (LIC12099), sph2 (LIC12631), a putative CoA-transferase (LIC12322), phoD (LIC13397) and hol (LIC20148; see below). Thus, differential gene regulation by leptospires within DMCs appears to be driven by mammalian host-specific stimuli that are not readily reproduced in vitro.
The relatively small pore size of the dialysis tubing used to cultivate leptospires within rat peritoneal cavities would exclude macromolecules and most serum proteins but allows for efficient exchange of nutrients (i.e., glucose, ions, and free amino acids) present within serum. These are the same types of small molecules that leptospires likely encounter within proximal convoluted tubules, where the composition of the glomerular ultrafiltrate most closely resembles that of interstitial fluid [84]. Further experimentation is required to assess how closely DMC-cultivated leptospires resemble their counterparts within host tissues during acute and/or chronic infection. The DMC model does have some limitations. For instance, virulence genes associated with pulmonary haemorrhage may be expressed only within the context of lung tissue. Because bacteria within DMCs are prevented from interacting with host immune cells and immunoglobulin [85], this model does not enable us to identify genes that are differentially-regulated in response to specific pathogen-host interactions and/or immune evasion.
To date, >20 named species of Leptospira have been identified based on molecular taxonomic analyses [86]. Leptospira spp. can be further divided into three major groups based on pathogenicity: pathogenic (9 species), intermediate virulence (5 species) and free-living saprophytes (6 species). The vast majority (69%) of genes upregulated by leptospires in response to mammalian host signals are found only in pathogenic and intermediate virulence species (i.e., absent in L. biflexa), suggesting that their gene products may help promote infection and/or colonization within mammal. However, more than half (64/110) of these upregulated genes encode either hypothetical proteins or lipoproteins of unknown function without any obvious conserved/functional domains. While their functions remain to be determined, our finding that these protein-coding genes are differentially-regulated in response to mammalian host-specific signals make them attractive candidates for further experimentation in animals model and, in particular, their potential use as part of a mono - or multi-valent protein-based vaccine. Thirty-five of the 56 genes downregulated in DMCs encode hypothetical proteins. Interestingly, all but 7 of these are unique to pathogenic and intermediate virulence species, raising the possibility that these genes products, while not required for survival within the host, facilitate the transition from a free-living to infective state.
Heme is the major source of iron in L. interrogans and also serves as a cofactor for proteins essential for respiration (i.e., cytochromes), biosynthesis of vitamin B12, and detoxification of reactive oxygen intermediates (i.e., catalase). Unlike B. burgdorferi [87] and T. pallidum [88], L. interrogans possess a complete set of genes required for de novo heme biosynthesis as well as the uptake and utilization of exogenous heme [58], [74], [89]. By RNA-Seq, expression of 6 heme biosynthesis genes was significantly downregulated in DMCs compared to in vitro, while heme oxygenase (LIC20148/hol) and phuR, encoding a TonB-dependent heme receptor, were upregulated; these data support the notion that pathogenic leptospires preferentially use exogenously derived heme within the mammal. Of the four putative fur orthologs encodes by L. interrogans, only one (LIC12034) was upregulated in DMCs. Recently, Marcsisin et al. [46] demonstrated that inactivation of this gene had no effect on virulence in a hamster acute infection, implying that this Fur paralog is not responsible for downregulation of the heme operon within DMCs. Alternatively, downregulation of heme biosynthesis is not a prerequisite for survival in vivo. Because heme is highly toxic [90], there is relatively little, if any, free heme within plasma [91]. In the glomerulus, the molecular weight cut-off for ultrafiltration is ∼70 kDa [92]. Thus, while L. interrogans is able to use haemoglobin (64 kDa) as a source of heme in vitro [60], this micronutrient is likely present in only minute amounts within the proximal tubules. Smaller molecules (≤20 kDa), only the other hand, easily pass through the glomerulus into Bowmen's capsule; it is worth noting that this molecular weight cut-off is essentially equivalent to that of the dialysis tubing used for our DMCs (8 kDa MWCO). Instead, leptospires may be using myoglobin (16.7 kDa), which is present in human plasma at concentrations similar to that of haemoglobin [93]. Both hemoglobin and myoglobin, released by red blood cell turnover and muscle tissue damage, respectively, are filtered by the kidneys and would be available to leptospires within the renal tubules.
Small non-coding RNAs (sRNAs) are increasingly recognized as essential post-transcriptional gene expression regulators that enable bacteria to adjust their physiology in response to environmental cues [94]. Bacterial sRNAs range from 50 to 500 nucleotides and frequently are located within intergenic regions [95]. By diverse mechanisms, including changes in RNA conformation, interactions with DNA, other RNAs and proteins, sRNAs can modulate transcription, translation, mRNA stability and DNA maintenance or silencing [96], [97]. Five of the 11 candidate sRNAs identified as part of this study are conserved in bacteria and known to carry out specific housekeeping functions, including RNase P (LIC1nc60), responsible for processing of tRNAs and other RNAs, and tmRNA (LIC1nc10), which acts as both a transfer RNA (tRNA) and mRNA to tag incompletely-translated proteins for degradation and to release stalled proteins [76], [77]. We also identified two cobalamin riboswitches (LIC1nc55 and LIC2nc10), which act as cis-regulatory elements in 5′ untranslated regions of vitamin B12-related genes; allosteric rearrangement of mRNA structure is mediated by ligand binding resulting in modulation of gene expression or translation of mRNA [78]. LIC1nc55 lies upstream of LIC121374/btuB, which encodes a constitutively-expressed TonB-dependent outer membrane cobalamin receptor protein [98]. We also identified a candidate sRNA (LIC2nc10) upstream of LIC20135; although annotated as a ferredoxin, LIC20135 contains a domain conserved within sirohydrochlorinin cobalt chelatases, an important enzyme involved in biosynthesis of vitamin B12. Finally, LIC1nc20 contains a conserved PyrR binding site; this RNA element is found upstream of genes involved in pyrimidine biosynthesis and transport in Bacillus subtilis [79]. In L. interrogans, this sRNA was found downstream of genes encoding hypothetical proteins. In addition to these known sRNAs, we identified six transcriptionally-active, non-coding regions that encode novel candidate regulatory sRNAs. LIC1nc30, LIC1nc50, LIC2nc30 and LIC2nc40 were all identified in the 5′ untranslated regions for LIC14007, LIC10702, LIC20192 and LIC20276, respectively, all of which encode proteins of unknown function. The remaining two putative sRNAs (LIC1nc80 and LIC2nc20) are located in the 3′ untranslated region of genes, which are known to be a repository of sRNAs in other bacterial species [99].
The L. interrogans genome encodes >200 proteins whose annotations suggest a role in transcriptional regulation (i.e., sigma factors, anti-sigma factors and trans-acting factors), two-component signal transduction and the synthesis/degradation of cyclic nucleotides [11], [12]. By RNA-Seq, the vast majority of these putative regulatory proteins were expressed at similar levels in vitro and in DMCs; this finding is not unexpected given that these types of regulatory factors typically are activated at the protein level by endogenously - or exogenously-derived small molecules and environmental stimuli [100], [101], [102].
Recent advances in Leptospira molecular genetics, including the development of site-directed [103] and transposon-mediated [104], [105], [106] mutagenesis techniques, now make it possible to determine the contribution(s) of genes that are regulated within DMCs. We anticipate that this approach will identify proteins involved in environmental sensing, mammalian host adaptation and/or the expression of specific virulence determinants in vivo.
Materials and Methods
Ethics statement
All animal experimentation was conducted following the Guide for the Care and Use of Laboratory Animals (Eighth Edition) and in accordance with protocol (ACC# 100570-0116) reviewed and approved by the University of Connecticut Health Center Institutional Animal Care and Use Committee. The UCHC laboratory animal care program is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The USDA Site ID: Customer Number 44, Certificate Number 16-R-0025, PHS Assurance Number A3471-01.
Bacteria
Virulent low-passage Leptospira interrogans sv. Copenhageni strains Fiocruz L1-130, kindly provided by Dr. David Haake (UCLA), and RJ16441 were cultivated in vitro under standard conditions at 30°C in EMJH medium [107] supplemented with 1% rabbit serum (Pel-Freez Biologicals, Rogers, AR) with 100 µg/ml 5-fluorouracil. Cultures were passaged in vitro no more than 3 times before being used for experimentation.
Cultivation of virulent L. interrogans within dialysis membrane chambers
To obtain L. interrogans in a mammalian host-adapted state, organisms were cultivated in dialysis membrane chambers (DMCs) as previously described [23], [24]. Briefly, DMCs were constructed using standard dialysis membrane tubing (Spectra-Por; 8000 MWCO). Prior to use, 8-inch strips of dialysis tubing were tied off at one end and then sterilized by boiling for 20 min in sterile water containing 5 mM EDTA, followed by two successive boiling washes in water alone. Dialysis bags were cooled to room temperature and then filled with ∼8–9 mls of EMJH medium (supplemented with 10% vaccine-grade bovine serum albumin to maintain osmotic pressure) containing 104 organisms per ml. Once filled, the tubing was tied and excess membrane removed from both ends. For implantation, female Sprague-Dawley rats (150–174 g) were anesthetized by intramuscular injection of a mixture of ketamine (50 mg/kg), xylazine (5 mg/kg), and acepromazine (1 mg/kg). Using strict aseptic technique, a DMC was implanted into the peritoneal cavity of each rat. Analgesia (carprofen; 5 mg/kg) was administered on the day of surgery and once the following day. At designated time points (typically 9–10 days post-implantation), rats were euthanized by CO2 narcosis and DMCs recovered. The contents of each chamber were removed by gentle syringe aspiration with an 18G needle; the needle was removed prior to expelling the DMC dialysate into a sterile 15 ml conical bottom tube. Bacteria were enumerated by dark field microscopy immediately following explant using a Petroff-Hausser counting chamber (Hausser Scientific Co., Horsham, PA).
Gel electrophoresis and immunoblotting
In vitro-cultivated L. interrogans, harvested at late-log phase (5×108–1×109 per ml) and leptospires explanted from DMCs were processed for one - and two-dimensional SDS-polyacrylamide gel electrophoresis (1D and 2D SDS-PAGE, respectively) as previously described [8]. Protein concentrations were determined using the DC protein assay kit (Bio-Rad). Total protein separated by 1D SDS-PAGE was detected by SYPRO Ruby protein gel stain (Sigma-Aldrich Inc, Ireland) as per manufacturer's instructions. Images were visualized with the BioSpectrum AC Imaging System (Ultra-Violet Products Ltd, UK). For immunoblotting, proteins were transferred to nylon-supported nitrocellulose, incubated with rabbit polyclonal antiserum directed against Sph2 [34], LipL32 [38] and LipL41 [39] followed by goat anti-rabbit secondary antibody (Southern Biotechnology Associates, Birmingham, Ala.). Blots were developed using the SuperSignal West Pico chemiluminescence substrate according to the manufacturer's instructions (Pierce, Rockford, Ill.). 2D gels were loaded with 500 µg total protein and stained with silver as previously described [8].
RNA isolation, library preparation and RNA-Seq
Total RNA was extracted using TRIzol reagent (Invitrogen) from three biologically-independent samples of (i) in vitro-cultivated leptospires or (ii) leptospires cultivated in DMCs (2 rats per sample) for 10 days as described above. Purified RNA was treated with Turbo DNAfree (Ambion, Inc. Austin, TX) as previously described [108] to remove contaminating genomic DNA. The integrity of DNase-treated RNAs use for RNA-Seq were assessed using the Agilent Bioanalyzer RNA NanoChip (Agilent Technologies, Wilmington, DE) to ensure that each had an RNA integrity (RIN) value ≥8. One-hundred ng of total RNA was used for library generation according to Illumina standard protocols (TruSeq RNA Sample Preparation Guide, Low-Throughput Protocol, Part # 15008136 Rev. A). cDNAs were normalized using a duplex-specific nuclease (DSN) approach according to the DSN Normalization Sample Preparation Guide, Early Access Protocol, Part # 15014673 Rev. C, which decreases the prevalence of highly abundant transcripts, such as rRNAs. 76-bp paired-end sequencing was carried out by Sequensys (Prognosys Biosciences, La Jolla, USA) on an Illumina Genome Analyzer IIx according to the manufacturer's instructions.
RNA-Seq data analysis
Mapping of sequenced reads to Chromosome 1 and 2 of the reference genome of Leptospira interrogans sv. Copenhageni strain Fiocuz L1-130 (NCBI Reference Sequence: NC_005823.1 and NC_005824.1 respectively) [11] was carried out using the software tool segemehl [109] with accuracy set to 100%. To increase coverage, mismatched nucleotides at the lower-quality 3′ end were removed from the reads and the mapping was repeated until a match was found or the read length decreased below 20 nucleotides (see [110]). Reads that mapped to (i) ribosomal or transfer RNAs or (ii) more than one reference genome location (e.g., paralogous genes) were discarded. Uniquely mapped reads (i.e., mapped to a single genomic location) were selected for further analysis, such as data visualisation and determination of differential gene expression. Normalization, differentially-expressed genes, regulatory fold-changes and statistical significance were determined using DESeq [43]. Read coverage used for graphical display was normalized as follows to compensate for different library sizes: the number of reads covering each nucleotide position was divided by the total number of mapped reads in the library and then multiplied with the number of mapped reads from the smallest library. Mapped unique reads were visualised with the Integrated Genome Browser (IGB, version 5.5) (http://bioviz.org/igb/releases.html) [111].
Bioinformatics
Putative orthologous relations between proteins in other Leptospira serovars and/or species were determined using BlastP alignment (≥40% amino acid identify over ≥80% of the length of the smallest protein) as previously described [14]. Protein sequence similarity between differentially-expressed genes identified in L. interrogans sv. Copenhageni and other Leptospira spp. (L. interrogans sv. Lai strain 56601 [80]; L. borgpetersenii sv. Hardjo strain L550 [13]; L. santarosai sv. Shermani strain LT821 [15]; L. licerasiae sv. Varillal strain VAR010 [16]; and L. biflexa sv. Patoc strain Patoc1 Ames [14]) was determined using GLSEARCH (version 35.04) from the FASTA package [112]. GLSEARCH identifies the optimal alignment across the entire genome of each strain, translated into all six reading frames, and calculates the percent identity across the whole length of the corresponding sequence. Conserved domain searches were performed on full length protein coding sequences using the NCBI Conserved Domain Database interface [44], [45]. The presence of fur boxes was investigated using the predictive computational tool SLiMSearch [62]. SLiMSearch, which can be used to determine the occurrences of a predefined motif in DNA and protein sequences, makes use of disorder and conservation masking to reduce the number of false positives. The fur box consensus sequence ([GC]AT[AT]AT[GC]AT[AT]AT[GC]AT[AT]AT[GC]) used to search the genome of Leptospira interrogans sv. Copenhageni was based on that of [61]. Putative functions of candidate sRNAs were identified by BLAST using the Rfam database, Wellcome Trust Sanger Institute (http://rfam.sanger.ac.uk/).
Quantitative RT-PCR
DNase-treated RNAs (∼1 µg per sample), isolated from leptospires grown to late-logarithmic phase at 30°C in vitro and within DMC, were prepared as described above and converted to cDNA using SuperScript III (Invitrogen) in the presence and absence of reverse transcriptase (RT) according to the manufacturer's instructions. cDNAs were assayed in quadruplicate using iQ Supermix (Bio-Rad) using the primer pairs described in Table S1. For relative quantitation of transcript levels, amplicons corresponding to each gene of interest were cloned into the pCR2.1-TOPO cloning vector (Invitrogen), then purified recombinant plasmid DNAs for each amplicon were diluted (107–102 copies/µl) to generate a standard curve. Reaction conditions for each primer pair were optimized to ensure that each had an amplification efficiency of >90%. Transcript copy numbers for each gene of interest were calculated using the iCycler post-run analysis software based on internal standard curves then normalized against copies of lipL32 (LIC11352) present in the same cDNA. Normalized copy number values were compared within Prism v5.00 (GraphPad Software, San Diego, CA) using an unpaired t-test with two-tailed p values and a 95% confidence interval.
Supporting Information
Zdroje
1. KoAI, GoarantC, PicardeauM (2009) Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol 7 : 736–747.
2. BhartiAR, NallyJE, RicaldiJN, MatthiasMA, DiazMM, et al. (2003) Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 3 : 757–771.
3. AthanazioDA, SilvaEF, SantosCS, RochaGM, Vannier-SantosMA, et al. (2008) Rattus norvegicus as a model for persistent renal colonization by pathogenic Leptospira interrogans. Acta Trop 105 : 176–180.
4. MarshallRB (1976) The route of entry of leptospires into the kidney tubule. J Med Microbiol 9 : 149–152.
5. FaineS (1957) Virulence in Leptospira. I. Reactions of guinea-pigs to experimental infection with Leptospira icterohaemorrhagiae. British Journal of Experimental Pathology 38 : 1–7.
6. AdlerB, LoM, SeemannT, MurrayGL (2011) Pathogenesis of leptospirosis: the influence of genomics. Vet Microbiol 153 : 73–81.
7. Faine S, Adler B., Bolin C. and Perolat P. (1999) Leptospira and Leptospirosis. Melbourne, Australia: MediSci.
8. MonahanAM, CallananJJ, NallyJE (2008) Proteomic analysis of Leptospira interrogans shed in urine of chronically infected hosts. Infect Immun 76 : 4952–4958.
9. IdoY, HokiR, ItoH, WaniH (1917) The rat as a carrier of Spirocheta icterohaemorrhagiae, the causative agent of Weil's disease (spirochaetosis icterohaemorrhagica. Journal of Experimental Medicine 26 : 341–353.
10. Bonilla-SantiagoR, NallyJE (2011) Rat model of chronic leptospirosis. Curr Protoc Microbiol Chapter 12: Unit12E 13.
11. NascimentoAL, KoAI, MartinsEA, Monteiro-VitorelloCB, HoPL, et al. (2004) Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J Bacteriol 186 : 2164–2172.
12. NascimentoAL, Verjovski-AlmeidaS, Van SluysMA, Monteiro-VitorelloCB, CamargoLE, et al. (2004) Genome features of Leptospira interrogans serovar Copenhageni. Braz J Med Biol Res 37 : 459–477.
13. BulachDM, ZuernerRL, WilsonP, SeemannT, McGrathA, et al. (2006) Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc Natl Acad Sci U S A 103 : 14560–14565.
14. PicardeauM, BulachDM, BouchierC, ZuernerRL, ZidaneN, et al. (2008) Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS One 3: e1607.
15. ChouLF, ChenYT, LuCW, KoYC, TangCY, et al. (2012) Sequence of Leptospira santarosai serovar Shermani genome and prediction of virulence-associated genes. Gene 511 : 364–370.
16. RicaldiJN, FoutsDE, SelengutJD, HarkinsDM, PatraKP, et al. (2012) Whole genome analysis of Leptospira licerasiae provides insight into leptospiral evolution and pathogenicity. PLoS Negl Trop Dis 6: e1853.
17. XueF, YanJ, PicardeauM (2009) Evolution and pathogenesis of Leptospira spp.: lessons learned from the genomes. Microbes Infect 11 : 328–333.
18. LoM, BulachDM, PowellDR, HaakeDA, MatsunagaJ, et al. (2006) Effects of temperature on gene expression patterns in Leptospira interrogans serovar Lai as assessed by whole-genome microarrays. Infect Immun 74 : 5848–5859.
19. QinJH, ShengYY, ZhangZM, ShiYZ, HeP, et al. (2006) Genome-wide transcriptional analysis of temperature shift in L. interrogans serovar Lai strain 56601. BMC Microbiol 6 : 51.
20. MatsunagaJ, LoM, BulachDM, ZuernerRL, AdlerB, et al. (2007) Response of Leptospira interrogans to physiologic osmolarity: relevance in signaling the environment-to-host transition. Infect Immun 75 : 2864–2874.
21. PatarakulK, LoM, AdlerB (2010) Global transcriptomic response of Leptospira interrogans serovar Copenhageni upon exposure to serum. BMC Microbiol 10 : 31.
22. LoM, MurrayGL, KhooCA, HaakeDA, ZuernerRL, et al. (2010) Transcriptional response of Leptospira interrogans to iron limitation and characterization of a PerR homolog. Infect Immun 78 : 4850–4859.
23. AkinsDR, BourellKW, CaimanoMJ, NorgardMV, RadolfJD (1998) A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. Journal of Clinical Investigation 101 : 2240–2250.
24. CaimanoMJ (2005) Cultivation of Borrelia burgdorferi in dialysis membrane chambers in rat peritonea. Curr Protoc Microbiol Chapter 12: Unit 12C 13.
25. ThiermannAB (1981) The Norway rat as a selective chronic carrier of Leptospira icterohaemorrhagiae. J Wildl Dis 17 : 39–43.
26. LevettPN (2001) Leptospirosis. Clin Microbiol Rev 14 : 296–326.
27. CaimanoMJ, IyerR, EggersCH, GonzalezC, MortonEA, et al. (2007) Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Molecular Microbiology 65 : 1193–1217.
28. RevelAT, TalaatAM, NorgardMV (2002) DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proceedings of the National Academy of Sciences 99 : 1562–1567.
29. BrooksCS, HeftyPS, JolliffSE, AkinsDR (2003) Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect Immun 71 : 3371–3383.
30. CroucherNJ, ThomsonNR (2010) Studying bacterial transcriptomes using RNA-seq. Current Opinion in Microbiology 13 : 619–624.
31. FiliatraultMJ (2011) Progress in prokaryotic transcriptomics. Current Opinion in Microbiology 14 : 579–586.
32. CaimanoMJ, EggersCH, GonzalezCA, RadolfJD (2005) Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. Journal of Bacteriology 187 : 7845–7852.
33. NarayanavariSA, SritharanM, HaakeDA, MatsunagaJ (2012) Multiple leptospiral sphingomyelinases (or are there?). Microbiology 158 : 1137–1146.
34. MatsunagaJ, MedeirosMA, SanchezY, WerneidKF, KoAI (2007) Osmotic regulation of expression of two extracellular matrix-binding proteins and a haemolysin of Leptospira interrogans: differential effects on LigA and Sph2 extracellular release. Microbiology 153 : 3390–3398.
35. ArtiushinS, TimoneyJF, NallyJ, VermaA (2004) Host-inducible immunogenic sphingomyelinase-like protein, Lk73.5, of Leptospira interrogans. Infect Immun 72 : 742–749.
36. ZhangYX, GengY, BiB, HeJY, WuCF, et al. (2005) Identification and classification of all potential hemolysin encoding genes and their products from Leptospira interrogans serogroup Icterohae-morrhagiae serovar Lai. Acta Pharmacol Sin 26 : 453–461.
37. CarvalhoE, BarbosaAS, GomezRM, OliveiraML, RomeroEC, et al. (2010) Evaluation of the expression and protective potential of Leptospiral sphingomyelinases. Curr Microbiol 60 : 134–142.
38. HaakeDA, ChaoG, ZuernerRL, BarnettJK, BarnettD, et al. (2000) The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect Immun 68 : 2276–2285.
39. ShangES, SummersTA, HaakeDA (1996) Molecular cloning and sequence analysis of the gene encoding LipL41, a surface-exposed lipoprotein of pathogenic Leptospira species. Infect Immun 64 : 2322–2330.
40. NallyJE, MonahanAM, MillerIS, Bonilla-SantiagoR, SoudaP, et al. (2011) Comparative proteomic analysis of differentially expressed proteins in the urine of reservoir hosts of leptospirosis. PLoS One 6: e26046.
41. BasemanJB, CoxCD (1969) Intermediate energy metabolism of Leptospira. J Bacteriol 97 : 992–1000.
42. ZhangQ, ZhangY, ZhongY, MaJ, PengN, et al. (2011) Leptospira interrogans encodes an ROK family glucokinase involved in a cryptic glucose utilization pathway. Acta Biochim Biophys Sin (Shanghai) 43 : 618–629.
43. AndersS, HuberW (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106.
44. Marchler-BauerA, ZhengC, ChitsazF, DerbyshireMK, GeerLY, et al. (2013) CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res 41: D348–352.
45. Marchler-BauerA, BryantSH (2004) CD-Search: protein domain annotations on the fly. Nucleic Acids Research 32: W327–W331.
46. MarcsisinRA, BartphoT, BulachDM, SrikramA, SermswanRW, et al. (2013) Use of a high-throughput screen to identify Leptospira mutants unable to colonise the carrier host or cause disease in the acute model of infection. J Med Microbiol 62(Pt 10): 1601–8.
47. SetubalJC, ReisM, MatsunagaJ, HaakeDA (2006) Lipoprotein computational prediction in spirochaetal genomes. Microbiology 152 : 113–121.
48. HaakeDA (2000) Spirochaetal lipoproteins and pathogenesis. Microbiology 146(Pt 7): 1491–1504.
49. JanwitthayananW, KeelawatS, PayungpornS, LowanitchapatA, SuwancharoenD, et al. (2013) In vivo gene expression and immunoreactivity of Leptospira collagenase. Microbiol Res 168 : 268–272.
50. ChoyHA, KelleyMM, CrodaJ, MatsunagaJ, BabbittJT, et al. (2011) The multifunctional LigB adhesin binds homeostatic proteins with potential roles in cutaneous infection by pathogenic Leptospira interrogans. PLoS One 6: e16879.
51. LinY-P, McDonoughSP, SharmaY, ChangY-F (2011) Leptospira immunoglobulin-like protein B (LigB) binding to the C-terminal fibrinogen αC domain inhibits fibrin clot formation, platelet adhesion and aggregation. Molecular Microbiology 79 : 1063–1076.
52. LinY-P, McDonoughSP, SharmaY, ChangY-F (2010) The terminal immunoglobulin-like repeats of LigA and LigB of Leptospira enhance their binding to gelatin binding domain of fibronectin and host cells. PLoS One 5: e11301.
53. CoutinhoML, ChoyHA, KelleyMM, MatsunagaJ, BabbittJT, et al. (2011) A LigA three-domain region protects hamsters from lethal infection by Leptospira interrogans. PLoS Negl Trop Dis 5: e1422.
54. VaughnJL, FeherV, NaylorS, StrauchMA, CavanaghJ (2000) Novel DNA binding domain and genetic regulation model of Bacillus subtilis transition state regulator AbrB. Nature Structural Biology 7 : 1139–1146.
55. BobayBG, MuellerGA, ThompsonRJ, MurzinAG, VentersRA, et al. (2006) NMR structure of AbhN and comparison with AbrBN: FIRST insights into the DNA binding promiscuity and specificity of AbrB-like transition state regulator proteins. J Biol Chem 281 : 21399–21409.
56. SullivanDM, BobayBG, KojetinDJ, ThompsonRJ, RanceM, et al. (2008) Insights into the nature of DNA binding of AbrB-like transcription factors. Structure 16 : 1702–1713.
57. PoseyJE, GherardiniFC (2000) Lack of a role for iron in the Lyme disease pathogen. Science 288 : 1651–1653.
58. LouvelH, BommezzadriS, ZidaneN, Boursaux-EudeC, CrenoS, et al. (2006) Comparative and functional genomic analyses of iron transport and regulation in Leptospira spp. J Bacteriol 188 : 7893–7904.
59. NoinajN, GuillierM, BarnardTJ, BuchananSK (2010) TonB-dependent transporters: regulation, structure, and function. Annu Rev Microbiol 64 : 43–60.
60. MurrayGL, EllisKM, LoM, AdlerB (2008) Leptospira interrogans requires a functional heme oxygenase to scavenge iron from hemoglobin. Microbes Infect 10 : 791–797.
61. EscolarL, Perez-MartinJ, de LorenzoV (1999) Opening the iron box: transcriptional metalloregulation by the Fur protein. J Bacteriol 181 : 6223–6229.
62. DaveyNE, HaslamNJ, ShieldsDC, EdwardsRJ (2011) SLiMSearch 2.0: biological context for short linear motifs in proteins. Nucleic Acids Research 39: W56–W60.
63. HantkeK (2001) Iron and metal regulation in bacteria. Curr Opin Microbiol 4 : 172–177.
64. ImlayJA (2008) Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77 : 755–776.
65. EshghiA, LourdaultK, MurrayGL, BartphoT, SermswanRW, et al. (2012) Leptospira interrogans catalase is required for resistance to H2O2 and for virulence. Infect Immun 80 : 3892–3899.
66. SeaverLC, ImlayJA (2001) Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol 183 : 7173–7181.
67. ParsonageD, DesrosiersDC, HazlettKR, SunY, NelsonKJ, et al. (2010) Broad specificity AhpC-like peroxiredoxin and its thioredoxin reductant in the sparse antioxidant defense system of Treponema pallidum. Proc Natl Acad Sci U S A 107 : 6240–6245.
68. LoM, CordwellSJ, BulachDM, AdlerB (2009) Comparative transcriptional and translational analysis of leptospiral outer membrane protein expression in response to temperature. PLoS Negl Trop Dis 3: e560.
69. NallyJE, TimoneyJF, StevensonB (2001) Temperature-regulated protein synthesis by Leptospira interrogans. Infect Immun 69 : 400–404.
70. GalperinMY, NikolskayaAN, KooninEV (2001) Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol Lett 203 : 11–21.
71. RamosJL, Martínez-BuenoM, Molina-HenaresAJ, TeránW, WatanabeK, et al. (2005) The TetR family of transcriptional repressors. Microbiology and Molecular Biology Reviews 69 : 326–356.
72. Hammer-JespersenK, Munch-PtersenA (1975) Multiple regulation of nucleoside catabolizing enzymes: regulation of the deo operon by the cytR and deoR gene products. Mol Gen Genet 137 : 327–335.
73. XuC, ShiW, RosenBP (1996) The chromosomal arsR gene of Escherichia coli encodes a trans-acting metalloregulatory protein. Journal of Biological Chemistry 271 : 2427–2432.
74. GueganR, CamadroJM, Saint GironsI, PicardeauM (2003) Leptospira spp. possess a complete haem biosynthetic pathway and are able to use exogenous haem sources. Mol Microbiol 49 : 745–754.
75. LouvelH, BettonJM, PicardeauM (2008) Heme rescues a two-component system Leptospira biflexa mutant. BMC Microbiol 8 : 25.
76. KazantsevAV, PaceNR (2006) Bacterial RNase P: a new view of an ancient enzyme. Nat Rev Microbiol 4 : 729–740.
77. KeilerKC (2007) Physiology of tmRNA: what gets tagged and why? Curr Opin Microbiol 10 : 169–175.
78. FranklundCV, KadnerRJ (1997) Multiple transcribed elements control expression of the Escherichia coli btuB gene. J Bacteriol 179 : 4039–4042.
79. BonnerER, D'EliaJN, BillipsBK, SwitzerRL (2001) Molecular recognition of pyr mRNA by the Bacillus subtilis attenuation regulatory protein PyrR. Nucleic Acids Res 29 : 4851–4865.
80. RenSX, FuG, JiangXG, ZengR, MiaoYG, et al. (2003) Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature 422 : 888–893.
81. MatsuiM, RouleauV, Bruyere-OstellsL, GoarantC (2011) Gene expression profiles of immune mediators and histopathological findings in animal models of leptospirosis: comparison between susceptible hamsters and resistant mice. Infect Immun 79 : 4480–4492.
82. MonahanAM, CallananJJ, NallyJE (2009) Review paper: Host-pathogen interactions in the kidney during chronic leptospirosis. Vet Pathol 46 : 792–799.
83. XueF, DongH, WuJ, WuZ, HuW, et al. (2010) Transcriptional responses of Leptospira interrogans to host innate immunity: significant changes in metabolism, oxygen tolerance, and outer membrane. PLoS Negl Trop Dis 4: e857.
84. TaalMW, ChertowGM, MarsdenPA, SkoreckiK, UYuASL, et al. (2012) Brenner and Rector's The Kidney: Elsevier Mosby Saunders.
85. GrimmD, EggersCH, CaimanoMJ, TillyK, StewartPE, et al. (2004) Experimental assessment of the roles of linear plasmids lp25 and lp28-1 of Borrelia burgdorferi throughout the infectious cycle. Infection and Immunity 72 : 5938–5946.
86. SmytheL, AdlerB, HartskeerlRA, GallowayRL, TurenneCY, et al. (2013) Classification of Leptospira genomospecies 1, 3, 4 and 5 as Leptospira alstonii sp. nov., Leptospira vanthielii sp. nov., Leptospira terpstrae sp. nov. and Leptospira yanagawae sp. nov., respectively. Int J Syst Evol Microbiol 63 : 1859–1862.
87. FraserCM, CasjensS, HuangWM, SuttonGG, ClaytonR, et al. (1997) Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390 : 580–586.
88. FraserCM, NorrisSJ, WeinstockGM, WhiteO, SuttonGG, et al. (1998) Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281 : 375–388.
89. MurrayGL, SrikramA, HenryR, PuapairojA, SermswanRW, et al. (2009) Leptospira interrogans requires heme oxygenase for disease pathogenesis. Microbes Infect 11 : 311–314.
90. KumarS, BandyopadhyayU (2005) Free heme toxicity and its detoxification systems in human. Toxicol Lett 157 : 175–188.
91. TongY, GuoM (2009) Bacterial heme-transport proteins and their heme-coordination modes. Arch Biochem Biophys 481 : 1–15.
92. LoteCJ (2012) Principles of Renal Physiology: Springer.
93. StoneMJ, WatermanMR, HarimotoD, MurrayG, WillsonN, et al. (1977) Serum myoglobin level as diagnostic test in patients with acute myocardial infarction. Br Heart J 39 : 375–380.
94. RepoilaF, DarfeuilleF (2009) Small regulatory non-coding RNAs in bacteria: physiology and mechanistic aspects. Biol Cell 101 : 117–131.
95. AltuviaS (2007) Identification of bacterial small non-coding RNAs: experimental approaches. Curr Opin Microbiol 10 : 257–261.
96. StorzG, VogelJ, WassarmanKM (2011) Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell 43 : 880–891.
97. WatersLS, StorzG (2009) Regulatory RNAs in bacteria. Cell 136 : 615–628.
98. SchauerK, RodionovDA, de ReuseH (2008) New substrates for TonB-dependent transport: do we only see the ‘tip of the iceberg’? Trends Biochem Sci 33 : 330–338.
99. ChaoY, PapenfortK, ReinhardtR, SharmaCM, VogelJ (2012) An atlas of Hfq-bound transcripts reveals 3′ UTRs as a genomic reservoir of regulatory small RNAs. EMBO J 31 : 4005–4019.
100. ÖsterbergS, Peso-SantosTd, ShinglerV (2011) Regulation of alternative sigma factor use. Annual Review of Microbiology 65 : 37–55.
101. CapraEJ, LaubMT (2012) Evolution of two-component signal transduction systems. Annual Review of Microbiology 66 : 325–347.
102. HenggeR (2009) Principles of c-di-GMP signalling in bacteria. Nat Rev Micro 7 : 263–273.
103. CrodaJ, FigueiraCP, WunderEAJr, SantosCS, ReisMG, et al. (2008) Targeted mutagenesis in pathogenic Leptospira species: disruption of the LigB gene does not affect virulence in animal models of leptospirosis. Infect Immun 76 : 5826–5833.
104. MurrayGL, MorelV, CerqueiraGM, CrodaJ, SrikramA, et al. (2009) Genome-wide transposon mutagenesis in pathogenic Leptospira species. Infect Immun 77 : 810–816.
105. BourhyP, LouvelH, Saint GironsI, PicardeauM (2005) Random insertional mutagenesis of Leptospira interrogans, the agent of leptospirosis, using a mariner transposon. J Bacteriol 187 : 3255–3258.
106. RistowP, BourhyP, da Cruz McBrideFW, FigueiraCP, HuerreM, et al. (2007) The OmpA-like protein Loa22 is essential for leptospiral virulence. PLoS Pathog 3: e97.
107. JohnsonRC, WalbyJ, HenryRA, AuranNE (1973) Cultivation of parasitic leptospires: effect of pyruvate. Appl Microbiol 26 : 118–119.
108. MulayVB, CaimanoMJ, IyerR, Dunham-EmsS, LiverisD, et al. (2009) Borrelia burgdorferi bba74 is expressed exclusively during tick feeding and is regulated by both arthropod - and mammalian host-specific signals. Journal of Bacteriology 191 : 2783–2794.
109. HoffmannS, OttoC, KurtzS, SharmaCM, KhaitovichP, et al. (2009) Fast Mapping of short sequences with mismatches, insertions and deletions using index structures. PLoS Comput Biol 5: e1000502.
110. KrogerC, DillonSC, CameronAD, PapenfortK, SivasankaranSK, et al. (2012) The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. Proc Natl Acad Sci U S A 109: E1277–1286.
111. NicolJW, HeltGA, BlanchardSGJr, RajaA, LoraineAE (2009) The Integrated Genome Browser: free software for distribution and exploration of genome-scale datasets. Bioinformatics 25 : 2730–2731.
112. PearsonWR (2000) Flexible sequence similarity searching with the FASTA3 program package. Methods Mol Biol 132 : 185–219.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule FormationČlánek Oral Bacteria and CancerČlánek A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
- Putting Fungi to Work: Harvesting a Cornucopia of Drugs, Toxins, and Antibiotics
- Mycobacteriophages: Windows into Tuberculosis
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- Five Things to Know about Genetically Modified (GM) Insects for Vector Control
- A Missing Dimension in Measures of Vaccination Impacts
- Eosinophils Are Important for Protection, Immunoregulation and Pathology during Infection with Nematode Microfilariae
- Clonality of HTLV-2 in Natural Infection
- Production, Fate and Pathogenicity of Plasma Microparticles in Murine Cerebral Malaria
- Group B Streptococcal Infection of the Choriodecidua Induces Dysfunction of the Cytokeratin Network in Amniotic Epithelium: A Pathway to Membrane Weakening
- New Insights into How Adapts to Its Mammalian Host during Bubonic Plague
- Foodborne Transmission of Nipah Virus in Syrian Hamsters
- A Polysaccharide Virulence Factor from Elicits Anti-inflammatory Effects through Induction of Interleukin-1 Receptor Antagonist
- Structural and Functional Characterization of a Complex between the Acidic Transactivation Domain of EBNA2 and the Tfb1/p62 Subunit of TFIIH
- Adaptive Gene Amplification As an Intermediate Step in the Expansion of Virus Host Range
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Hepatitis B Virus Infection and Immunopathogenesis in a Humanized Mouse Model: Induction of Human-Specific Liver Fibrosis and M2-Like Macrophages
- Crk Adaptors Negatively Regulate Actin Polymerization in Pedestals Formed by Enteropathogenic (EPEC) by Binding to Tir Effector
- Fatty Acid Biosynthesis Contributes Significantly to Establishment of a Bioenergetically Favorable Environment for Vaccinia Virus Infection
- A Cytosolic Chaperone Complexes with Dynamic Membrane J-Proteins and Mobilizes a Nonenveloped Virus out of the Endoplasmic Reticulum
- Intracellular Promote Invasive Cell Motility through Kinase Regulation of the Host Actin Cytoskeleton
- MAVS-MKK7-JNK2 Defines a Novel Apoptotic Signaling Pathway during Viral Infection
- RON5 Is Critical for Organization and Function of the Moving Junction Complex
- Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway
- and Exhibit Metabolic Symbioses
- The Herpes Virus Fc Receptor gE-gI Mediates Antibody Bipolar Bridging to Clear Viral Antigens from the Cell Surface
- Target Cell Availability, Rather than Breast Milk Factors, Dictates Mother-to-Infant Transmission of SIV in Sooty Mangabeys and Rhesus Macaques
- Evolution of the Retroviral Restriction Gene : Inhibition of Non-MLV Retroviruses
- Infection of Adult Thymus with Murine Retrovirus Induces Virus-Specific Central Tolerance That Prevents Functional Memory CD8 T Cell Differentiation
- Fha Interaction with Phosphothreonine of TssL Activates Type VI Secretion in
- In Vivo Administration of a JAK3 Inhibitor during Acute SIV Infection Leads to Significant Increases in Viral Load during Chronic Infection
- Lack of Detectable HIV-1 Molecular Evolution during Suppressive Antiretroviral Therapy
- Activation of HIV-1 from Latent Infection via Synergy of RUNX1 Inhibitor Ro5-3335 and SAHA
- A Compact, Multifunctional Fusion Module Directs Cholesterol-Dependent Homomultimerization and Syncytiogenic Efficiency of Reovirus p10 FAST Proteins
- The Role of Host and Microbial Factors in the Pathogenesis of Pneumococcal Bacteraemia Arising from a Single Bacterial Cell Bottleneck
- Genetic Dissection of Gut Epithelial Responses to
- Two-Component System Cross-Regulation Integrates Response to Heme and Cell Envelope Stress
- Oral Mycobiome Analysis of HIV-Infected Patients: Identification of as an Antagonist of Opportunistic Fungi
- A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Serovar Copenhageni
- Inflammasome Sensor NLRP1 Controls Rat Macrophage Susceptibility to
- ChIP-Seq and RNA-Seq Reveal an AmrZ-Mediated Mechanism for Cyclic di-GMP Synthesis and Biofilm Development by
- The Hypervariable Amino-Terminus of P1 Protease Modulates Potyviral Replication and Host Defense Responses
- Caspase-1-Dependent and -Independent Cell Death Pathways in Infection of Macrophages
- The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression
- Different Mutagenic Potential of HIV-1 Restriction Factors APOBEC3G and APOBEC3F Is Determined by Distinct Single-Stranded DNA Scanning Mechanisms
- Oral Bacteria and Cancer
- Identification of OmpA, a Protein Involved in Host Cell Invasion, by Multi-Phenotypic High-Content Screening
- Transovarial Transmission of a Plant Virus Is Mediated by Vitellogenin of Its Insect Vector
- VE-Cadherin Cleavage by LasB Protease from Facilitates Type III Secretion System Toxicity in Endothelial Cells
- Dimerization of VirD2 Binding Protein Is Essential for Induced Tumor Formation in Plants
- Crystal Structure of the Vaccinia Virus DNA Polymerase Holoenzyme Subunit D4 in Complex with the A20 N-Terminal Domain
- Post-Translational Regulation via Clp Protease Is Critical for Survival of
- Modulation of Phagosomal pH by Promotes Hyphal Morphogenesis and Requires Stp2p, a Regulator of Amino Acid Transport
- Rotavirus Activates Lymphocytes from Non-Obese Diabetic Mice by Triggering Toll-Like Receptor 7 Signaling and Interferon Production in Plasmacytoid Dendritic Cells
- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Interferon Regulatory Factor-1 Protects from Fatal Neurotropic Infection with Vesicular Stomatitis Virus by Specific Inhibition of Viral Replication in Neurons
- HMGB1-Promoted and TLR2/4-Dependent NK Cell Maturation and Activation Take Part in Rotavirus-Induced Murine Biliary Atresia
- An Immunomics Approach to Schistosome Antigen Discovery: Antibody Signatures of Naturally Resistant and Chronically Infected Individuals from Endemic Areas
- PPARγ Agonists Improve Survival and Neurocognitive Outcomes in Experimental Cerebral Malaria and Induce Neuroprotective Pathways in Human Malaria
- A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
- Viral OTU Deubiquitinases: A Structural and Functional Comparison
- Heterogeneity and Breadth of Host Antibody Response to KSHV Infection Demonstrated by Systematic Analysis of the KSHV Proteome
- Influenza A Virus Assembly Intermediates Fuse in the Cytoplasm
- Broadly Reactive Human CD8 T Cells that Recognize an Epitope Conserved between VZV, HSV and EBV
- Oncogenic Human Papillomaviruses Activate the Tumor-Associated Lens Epithelial-Derived Growth Factor (LEDGF) Gene
- Erythrocyte Invasion: Combining Function with Immune Evasion
- IL-1α and Complement Cooperate in Triggering Local Neutrophilic Inflammation in Response to Adenovirus and Eliminating Virus-Containing Cells
- Chronic Exposure to Type-I IFN under Lymphopenic Conditions Alters CD4 T Cell Homeostasis
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



