-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression
Flagellar-mediated motility is fundamental to Salmonella pathogenesis, which takes the lives of hundreds of thousands of people each year. The genes of the Salmonella pathogenicity island 1 and those of the flagellar regulon are part of the same transcriptional hierarchy. We report the novel finding where the key control of this network takes place at the flhDC promoter region. We followed the transcription from the two “live” flhDC promoters as a function of the cell growth phase. P1 comes on early in the cell cycle, while P5 comes on late. Transcription of P5 is HilD dependent, which represents a totally new finding and Salmonella specific: there is no HilD in E. coli flhDC control, no P5 transcription. P1 & P5 can express flhDC to equivalent levels, yet only P1 - dependent expression produces motility UNLESS we artificially induce P5 EARLY in the cell cycle. This work is the foundation for the cell cycle stages a Salmonella bacterium experiences during host infection. This is a significant conceptual advance in Salmonella pathogenesis: one can no longer consider gene regulation at 37°C and OD 0.6 as a reflection of the Salmonella infection cycle; the whole cell growth cycle must be considered in understanding this complex biological processes.
Published in the journal: The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression. PLoS Pathog 10(3): e32767. doi:10.1371/journal.ppat.1003987
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003987Summary
Flagellar-mediated motility is fundamental to Salmonella pathogenesis, which takes the lives of hundreds of thousands of people each year. The genes of the Salmonella pathogenicity island 1 and those of the flagellar regulon are part of the same transcriptional hierarchy. We report the novel finding where the key control of this network takes place at the flhDC promoter region. We followed the transcription from the two “live” flhDC promoters as a function of the cell growth phase. P1 comes on early in the cell cycle, while P5 comes on late. Transcription of P5 is HilD dependent, which represents a totally new finding and Salmonella specific: there is no HilD in E. coli flhDC control, no P5 transcription. P1 & P5 can express flhDC to equivalent levels, yet only P1 - dependent expression produces motility UNLESS we artificially induce P5 EARLY in the cell cycle. This work is the foundation for the cell cycle stages a Salmonella bacterium experiences during host infection. This is a significant conceptual advance in Salmonella pathogenesis: one can no longer consider gene regulation at 37°C and OD 0.6 as a reflection of the Salmonella infection cycle; the whole cell growth cycle must be considered in understanding this complex biological processes.
Introduction
Tens of millions of human cases of Salmonellosis, a foodborne gastroenteritis caused by Salmonella enterica, occur worldwide every year killing more than a hundred thousand people annually (World Health Organization Fact sheet N°139, August 2013). Typhoid fever caused by Salmonella Typhi kills an equivalent number of people each year. A prominent player in Salmonella pathogenesis is the bacterial flagellum. The bacterial flagellum is an ion-powered, complex motor organelle that endows bacterial cells, such as Escherichia coli and Salmonella enterica, with the ability to propel themselves through liquid medium and across hydrated surfaces [1]. Motility also plays an important role in biofilm formation and in the ability of many pathogens to reach their sites of infection and establish disease [2], [3].
Early work on the discovery of Salmonella virulence genes identified a transposon insertion in the flagellar filament cap gene, fliD, as defective for survival of cells in macrophages [4]. However, fliD is in an operon with the fliT gene whose product is a regulator of the flagellar and Spi1 virulence genes master regulatory complex FlhD4C2 [5], [6]. The transposon insertion in fliD was polar on fliT gene expression and thus identified regulation of FlhD4C2 activity as critical for Salmonella virulence. The two proteins that make up the FlhD4C2 transcriptional regulatory complex are co-expressed from the flhDC operon, class 1 promoter, which is at the top of a complex transcriptional hierarchy for both flagellar and Spi1 virulence genes expression. The decision whether or not to produce flagella is regulated at the levels of flhDC transcription, translation, FlhD4C2 assembly and stability [7]. Positive regulators of flhDC operon transcription include cAMP-CRP, Fis, Fur, H-NS and QseB [8]–[14]. A large number of regulatory factors are also reported to inhibit flhDC transcription. These factors include, LrhA, RcsB, RtsB, SlyA, DskA, PefI-SrgD, FimZ, HdfR, OmpR and RflM [15]–[20]. The FlhD4C2 activity generates an auto-regulatory loop by activating transcription of the rflM gene encoding a LysR-type DNA binding protein RflM, which in turn inhibits the transcription of flhDC [21]. The post-transcriptional factors regulating flhDC include, CsrA [22], [23], Hsp70 chaperone DnaK [24] and ClpXP protease [25]. Recently an FlhD4C2 repressed gene, ydiV [26], was shown to code for a protein (YdiV) that will bind to FlhD4C2, in its free or DNA-bound form, remove FlhD4C2 from DNA and serves as an adapter that targets FlhD4C2 for ClpXP-dependent degradation [27], [28].
In Salmonella, an initial characterization of the flhDC promoter region identified six transcriptional start sites (TSSs) [13]. In a recent study, only four of the original six TSSs were detected [29]. The presence of six TSSs in the Salmonella flhDC regulatory region combined with the presence of DNA binding sites of CRP, LrhA, RtsB, HilD, RcsB, HNS and others indicated a complex level of the flhDC transcriptional regulation.
Salmonella enterica is an intracellular facultative pathogen causing a range of diseases in a variety of hosts [30]. Important virulence factors required for Salmonella invasion of epithelial cells and development of Salmonellosis are encoded within the Salmonella pathogenicity island 1 (Spi1) genes. Spi1 encodes a virulence-associated type III secretion system (T3SS) as part of an injectisome structure required for the secretion and injection of multiple effector proteins into the cytoplasm of host cells [31]–[36]. Expression of Spi1 genes is controlled in response to specific combinations of environmental signals in a complex hierarchical process with multiple transcriptional regulators. These include, HilA, a member of the OmpR/ToxR family of transcriptional regulators, which promotes transcription of genes encoding the necessary components for a functional Spi1 injectisome system [32], [35], [37], [38]. Also included are the hilC and hilD genes whose products are members of the Ara/XylS family of transcriptional regulators that control hilA gene transcription. HilD is at the top of the regulatory network controlling Spi1 expression because most regulators controlling hilA transcription appears to be HilD-dependent [39], [40].
It is noteworthy to mention that many protein components of the Spi1 and flagella T3SS exhibit a significant degree of amino acid identity, leading to the production of remarkably similar T3SS structures [16], [33], [34], [41], [42]. Furthermore, many of the transcriptional and posttranslational regulatory factors of flhDC also target the main transcriptional regulators of Spi1, such as HilA and HilD [11], [43]–[52]. In addition, the ATP-dependent Lon protease was shown to degrade both FlhD4C2 and HilD [24], [25]. Coordinated expression of Spi1 and flagellar genes has been recently demonstrated [53]. In Salmonella, expression of Spi1 genes is activated by FliZ [54]–[57], which is encoded within the flagellar fliAZY operon. FilZ activates the hilD gene expression at the posttranslational level and HilD in turn promotes transcription of the rtsAB operon, which encodes a pathogenesis-related DNA-binding regulatory proteins. RtsA and RtsB reciprocally regulate both the Spi1 and flagellar genes [17]. The direct binding of RtsB to the flhDC promoter region inhibits flhDC transcription and motility [17].
We decided to investigate how input from different regulatory factors might integrate multiple environmental or cell cycle signals into the control of flhDC expression in Salmonella enterica. We explored how and when positive and negative regulators affect flhDC expression throughout the cell growth cycle. We measured the effect of RcsB, LrhA, RflM, SlyA, RtsB and HilD regulatory factors on flhDC operon transcription at different cell growth phases. We characterized the specific TSSs within the flhDC promoter region and their involvement in the positive and negative control of flhDC cell-cycle dependent transcription. Finally, we examined how the individual TSSs and protein regulatory factors controlled the interconnection between the flagellar and Spi1 regulons.
Results
Dynamics of flhDC operon transcription in liquid culture after induction from stationary phase
To investigate flhDC operon transcription at different phases of the cell growth, we constructed a transcriptional fusion of the flhDC promoter region to the luciferase operon of Photorhabdus luminescence (luxCDBAE operon). Because the flhDC operon is autoregulated negatively by RflM and positively by HilD, we designed strains harboring an intact copy of the flhDC operon under the control of its native promoter (PflhDC) and an in-frame fusion of a second copy of the promoter region of flhDC (through the first 272 nucleotides of flhD coding sequence) to the luciferase operon: DUP[(PwtflhDC-luxCDBAE)*Km*(PwtflhDC-flhD+C+)] (Figure 1A). Thus, individual PflhDC promoter regions transcribe both the luminescence operon reporter and the flhDC operon. This results in a strain with luminescence readout for the level of transcriptional activation of flhDC under conditions that also preserves the wild-type expression of the flagellar regulon including flhDC autoregulation through FlhD4C2-dependent expression of rflM and hilD genes. For simplicity, we will refer to the DUP[(PwtflhDC-luxCDBAE)*Km*(PwtflhDC-flhD+C+)] construct as PwtflhDC.
Fig. 1. Growth phase dependent transcription of the flhDC operon promoter in Salmonella enterica serovar Typhimurium is controlled by LrhA, RcsB, RflM, HilD, SlyA and RtsB. 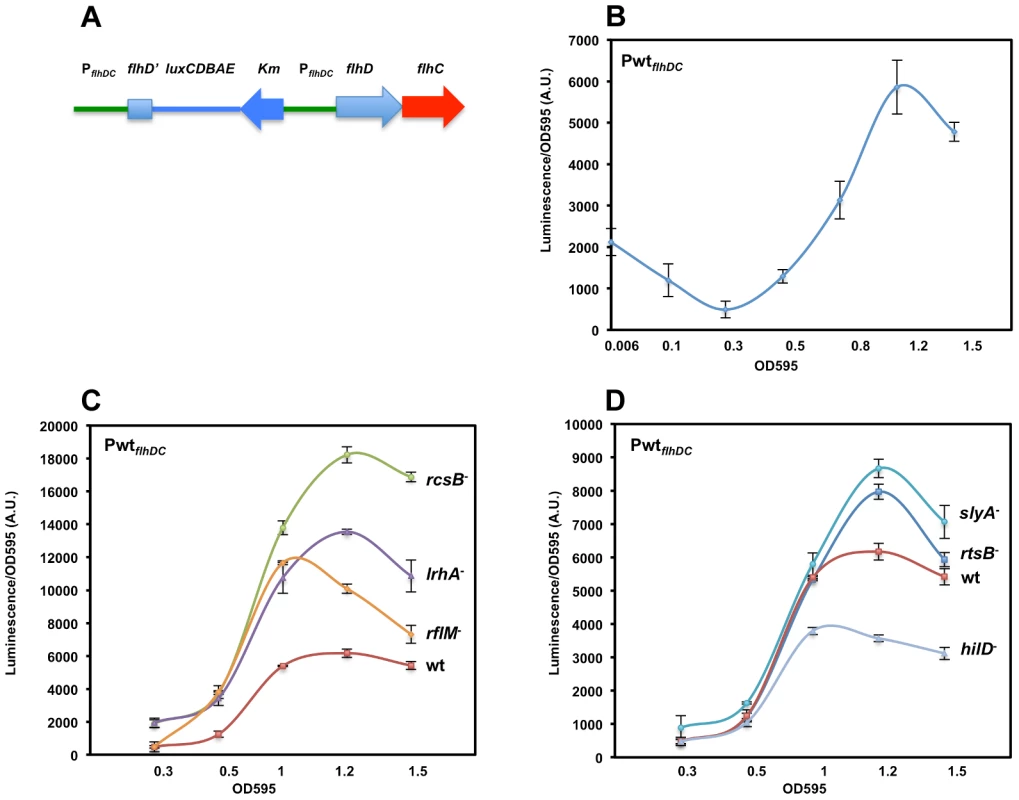
(A) Diagram depicting a duplicated chromosomal region that includes fusion of the flhDC promoter region (PflhDC, a 728 bp upstream of the start codon of flhD and the first 272 nucleotides of flhD coding region) to the luciferase operon of Photorhabdus luminescens in addition to a wild-type flhDC promoter-operon region. (B) A time course plot showing PflhDC-lux expression at increasing cell density of strain PwtflhDC-luxCDBAE-PwtflhDCflhD+C+ (TH18684) grown in LB media at 30°C with shaking. Luciferase activity was measured along with the OD595. Plots represent the recorded luciferase activity divided by the OD595. (C & D) Time course plots showing PwtflhDC-lux expression at increasing cell density in the absence of flhDC regulators. Individual regulators of flhDC promoter (PwtflhDC) transcription were removed by deletion in the PwtflhDC-luxCDBAE-PwtflhDCflhD+C+ background. Plots for specific individual strains are identified at the right of their corresponding plots (wt = wild-type (TH18684), rcsB− = ΔrcsB::tetRA (TH19230), lrhA− = ΔlrhA::tetRA (TH18722), rflM− = ΔrflM::FCF (TH18716), rtsB− = rtsB::T-POP (TH18724), slyA− = slyA::T-POP (TH18720) and hilD− = ΔhilD::tetRA (TH19654)). (C) Loss of RcsB, LrhA or RflM resulted in increased transcription of the flhDC operon at early growth phase. (D) Effect of removal of virulence-related genes slyA, rtsB or hilD differentially affected flhDC operon transcription. Deletion of either the rtsB or slyA gene resulted in increased flhDC operon transcription once cells reach stationary phase contrary to a deletion in the hilD gene, which resulted in increased flhDC transcription once bacterial cells enter mid exponential phase. The OD595 values are shown at the bottom of the chart. Values are the average of three independent experiments done in duplicate. Error bars represent standard deviation. Following batch inoculation of an overnight culture of the PwtflhDC strain into fresh media with shaking at 30°C, transcription of the flhDC genes declined 4-fold during the initial lag phase transition to log phase growth to a minimal value (Figure 1B). This observation is consistent with that reported in an earlier study [11]. After the transition to log phase growth, transcription of flhDC increased more than 10-fold between OD 0.3 and 1.2, followed by a decline in flhDC transcription as cells enter late log and stationary phase growth (Figure 1B).
Dynamics of flhDC operon transcription during cell cycle growth in liquid culture in the absence of transcriptional regulators
In Salmonella enterica, flagellar regulon transcription is highest during the exponential phase of growth and decays in late stationary phase [58]. Transcription of the flagellar master regulatory operon, flhDC, is under both negative and positive control by multiple regulatory factors. Null mutations in any one of the rcsB, rflM, lrhA, slyA, and rtsB genes result in increased transcription of the flhDC operon, which is consistent with an inhibitory activity on flhDC expression. HilD is an activator of flhDC transcription such that over-expression of the hilD gene increases flhDC expression (Singer et al. submitted). The diversity of transcription factors controlling expression of flhDC reflects the complexity of flhDC transcriptional regulation and suggests that flhDC transcription is controlled when Salmonella cells are experiencing different metabolic or environmental states, or different growth conditions under which these transcriptional factors are active. We examined both the timing and magnitude of individual regulatory proteins on flhDC transcriptional control throughout the cell's growth phase. We tested flhDC transcriptional levels as a function of the cell's growth phase in strains missing the individual negative regulators RcsB, LrhA, RflM, RtsB, SlyA and the positive regulator HilD (Figures 1C & D). As was presented above for the wild-type strain, this was done by growing PwtflhDC cells in liquid culture at 30°C using luciferase as the reporter for flhDC transcription levels. Luciferase levels were determined at specific optical densities shown in Figure 1. As expected, removal of individual inhibitors resulted in an increase in flhDC transcription levels while removal of HilD decreased flhDC transcription. Importantly, our assay revealed a growth phase-dependent hierarchy of the effect of these regulators. At OD 0.3, basal flhDC transcription was elevated in the absence of LrhA and RcsB, while removal of RflM, RtsB, SlyA or HilD exhibited the same basal level of transcription as wild type (Figures 1C & 1D). This suggests that RcsB and LrhA act earlier, during lag phase, to inhibit flhDC transcription. This effect could also represent a carry-over of repression from stationary phase that keep flhDC transcription low during the transition to log growth. In the absence of RflM we observed an earlier transition to flhDC activation than in the other mutant strains. Since FlhD4C2 transcribes the rflM gene and RflM protein inhibits flhDC transcription (flhDC auto-inhibition), this result suggests that flhDC auto-inhibition through RflM occurs during early exponential phase to control when full FlhD4C2-dependent gene expression occurs at log phase. The negative effect of RtsB and SlyA on flhDC transcription was detected as cells enter early stationary phase. We also observed that the maximum flhDC transcription level peaked earlier for both the hilD and rflM mutants at OD 1, while the wild type and mutants in rcsB, lrhA, slyA and rtsB peaked around OD 1.2.
The data presented in Figure 1C demonstrate that initial flhDC transcription is kept low by a combination of repressors including at least RcsB and LrhA. Initial FlhD4C2 expression during the stationary to log phase transition produces enough RflM to maintain a low level of flhDC transcription until an OD of ∼0.3 is reached. After OD 0.3, flhDC transcription increased significantly, but RflM, RcsB and LrhA reduce the overall level. Interestingly, the wild-type level is balanced by the presence of the HilD activator of flhDC transcription, the hilD-activated inhibitor of flhDC transcription RtsB and by the virulence associated factor SlyA (Figure 1D).
The effect of growth conditions on flhDC transcription as a function of cell growth
In order to obtain more detailed information relating the effect of specific regulatory proteins on flhDC transcription as a function of the cell's growth phase, we determined luciferase levels for the PwtflhDC grown in liquid culture at 30°C in 96 well plates with a microplate reader. Using this assay system, we could measure the activity of flhDC transcription at shorter times intervals (6 min) with continuous shaking at 150 rpm. We observed the same trend of regulation of the flhDC operon as seen in batch cultures for lrhA, rcsB (Figure 2A), rflM (Figure 2B), slyA and rtsB (Figure 2C), and hilD mutants (Figure 2D). However, the pattern observed in 96 well plates was somewhat different compared to the batch growth. We observed that activation of flhDC transcription took place earlier at OD∼0.2 rather than OD∼0.3. Consistent with this observation, the differences between the activity of flhDC in wild-type versus mutant strains also occurred at an earlier OD measurement in microtiter plate growth compared to growth in batch culture. The cells in 96 well plates reached maximum expression at OD∼0.6 compared to OD∼1.2 in the batch culture. We attribute these differences to the mode of growth in 96 well plates (150 rpm) where bacterial cells are grown in much lower volumes and likely to be subjected to different oxygen levels in the medium compared to batch cultures. It has been shown that activation of flgA, a gene under the control of flhDC, under static conditions (no shaking of 96 well plates) occurred immediately after dilution of an overnight culture into LB-1% Salt [53]. When we tested the activation of flhDC operon in standing batch culture in LB, we observed that flhDC transcription increased at OD∼0.12 (Figure S1), which is earlier compared to what we observed either in batch shaking (OD∼0.3) or 96 well grown cultures (OD∼0.2). Moreover, the shutdown of flhDC transcription observed in standing cultures took place after cells reach an OD∼0.6 compared to shaking batch culture where the shutdown started at an OD∼1.2.
Fig. 2. Precise transcriptional regulation of the flhDC operon is growth phase dependent. 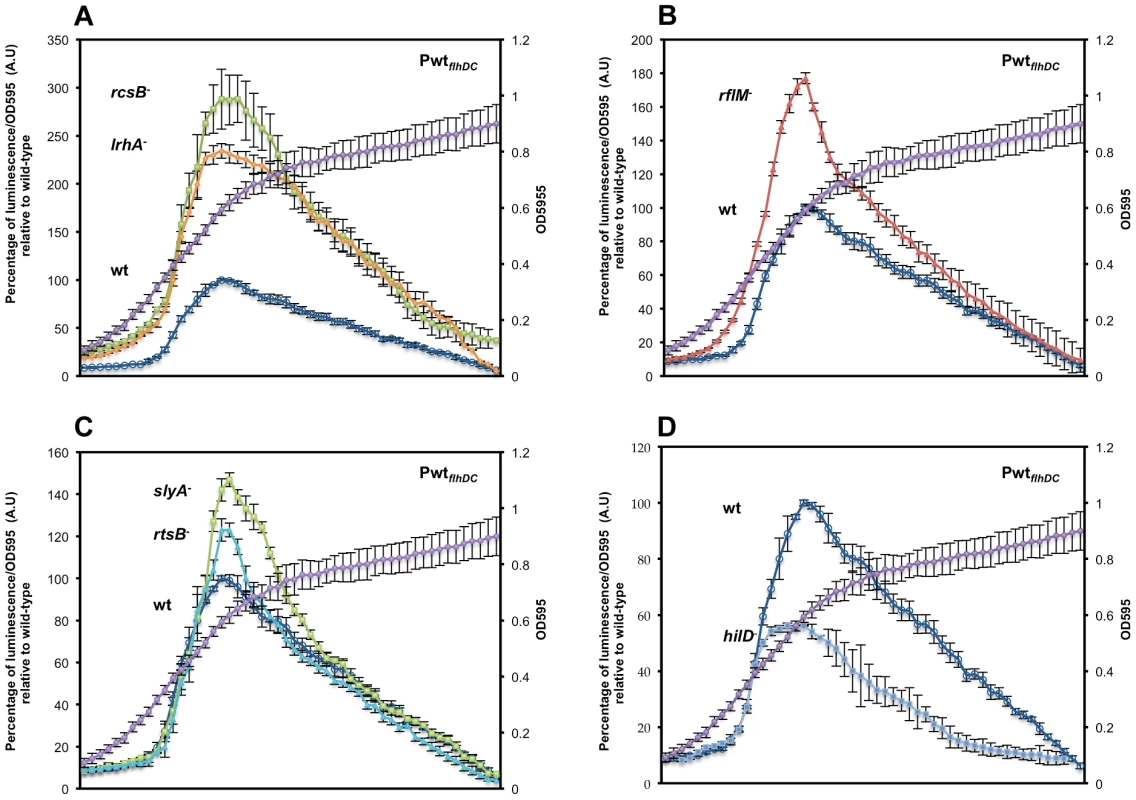
Transcription kinetics for the flhDC operon in various mutant backgrounds with the PwtflhDC-luxCDBAE-PwtflhDCflhD+C+ reporter construct measured in a 96 well plate growth format. The luciferase activity was investigated in seven genetic backgrounds: (A) wild-type (TH18684) empty circles, ΔrcsB::tetRA (TH19230) filled squares, ΔlrhA::tetRA (TH18722) filled diamonds, (B) ΔrflM::FCF (TH18716) filled triangle (C) rtsB::T-POP (TH18724) filled circles, slyA::T-POP (TH18720) filled squares and (D) ΔhilD::tetRA (TH19654) filled diamonds. The genotypes of the strains are indicated in the left of their plots at the level of their maximum A.U's. Cells from overnight cultures were diluted 1 to 500 in LB and 200 µl was inoculated into 96 well dark plates that were sealed with a breath easy membrane and incubated at 30°C in a plate reader with 5 min orbital shaking at 150 rpm. After a pause of 5 second following shaking, luminescence and OD595 of the inoculated wells were read during 95 second. The luminescence was recorded with a 0.1 s integration time for normalization. Arbitrary units (A.U.) were calculated as luminescence reading divided by OD595. The average at each time point was normalized to the maximum A.U. of the wild-type strain. Each data point represents six experiments performed in triplicate in different days. Error-bars indicate standard deviations. A representative growth curve is shown in the second axis of the plots. Growth phase transcriptional dynamics of flhDC transcriptional regulators
Because flhDC transcription is differentially regulated by different transcription factors in a growth phase dependent manner, we hypothesized that the effect of each of these regulators is observed at the time when they are produced during the cell growth cycle. To investigate this possibility we placed the luxCDBAE operon reporter under control of the promoters of the six regulatory genes lrhA, rcsB, rflM, slyA, rtsB and hilD, whose products have been demonstrated to bind directly to the flhDC promoter region and monitored their expression profile at different optical densities (binding of RflM or SlyA to the flhDC promoter region has not been reported). We monitored the activities of these constructs in 96 well plates over time. We observed that the transcription of the autoregulated gene lrhA is immediately activated following dilution from an overnight culture, and before the activation of flhDC (Figure S2.A). Transcription of rcsD (which is the first gene of the rcsDB operon transcribed from the rcsD promoter) also initiated before flhDC (Figure S2.B), whereas transcription of rflM overlapped with that of flhDC (Figure S2.B). Since rflM transcription is dependent on FlhD4C2, these results suggest that low basal levels of FlhD4C2 are sufficient to promote rflM gene transcription. In addition, transcription of rflM reached a maximum at OD∼0.35 and decayed very quickly (Figure S2.B) compared to the rest of the regulators tested in this study. The transcription of hilD gene is under positive autoregulatory control by HilD itself [59] and by HilD-activated RtsA [17]. In addition, the product of an flhDC activated gene, FliZ controls HilD at a posttranslational level [54], [57]. We observed that transcription of hilD increased at OD of ∼0.4 (Figure S2.C), at the same time when HilD promoted transcription of flhDC (Figure 2D). The expression of the HilD-activated rtsA gene (the first gene of the rtsAB operon) appeared to be activated at the same time as hilD (Figure S2.C). Transcription of the slyA gene was activated just after flhDC transcription started and before initiation of hilD and rtsA transcription, with a peak of expression at entry into stationary phase (Figure S2.D). These results suggest that there is a hierarchy of transcription of the factors regulating flhDC transcription that mirrors their effect on the transcriptional regulation of the flhDC operon.
We next asked if the protein levels of the regulatory factors controlling flhDC transcription were also growth phase dependent. We performed Western blot analysis of whole cell lysates of Salmonella strains (LrhA-HA, RcsB-3×Flag, RflM-HA, SlyA-HA, RtsB-HA and HilD-Flag) at different optical densities (Figure 3A). We established that LrhA is present at an early time point during cell growth (OD∼0.2) with maximum expression at OD∼0.6 followed by a decay at late stationary phase (note that both the N-terminal and C-terminal HA-tag fusion to LrhA are made but not functional and therefore there is no positive feedback regulation of lrhA transcription by LrhA protein [18]). The level of RcsB protein, the transcriptional regulator of the phosphorelay system RcsDBC, also appeared to be growth phase dependent because RcsB protein was detected early in the growth phase (OD∼0.2) and increased at the stationary phase of cell growth. The FlhD4C2 activated RflM, was produced early in the growth phase (OD∼0.2), and increased at OD∼0.4 followed by a quick decay during the rest of the cell's growth phase. HilD protein, the positive activator of flhDC transcription, was detected at OD∼0.4 and increased at stationary phase (Figure 3A). RtsB, whose gene is under the transcriptional control by HilD, was not detected early in the growth phase and was present at OD∼1.3. The absence of RtsB at an early time point in the blot might be due to the detection limits for low protein levels in our experiment (See CHIP, Figure 3B, where RtsB was already associated with the promoter of flhDC at OD∼1). In contrast, the negative regulator SlyA was produced during all the phases of cell growth, with a sharp increase at OD∼1. These results demonstrate a hierarchy at the level of expression of flhDC regulators that specifically mimics the differential dynamics of flhDC operon transcription.
Fig. 3. The expression levels and the in-vivo binding of regulatory factors controlling flhDC operon transcription during cell growth phases. 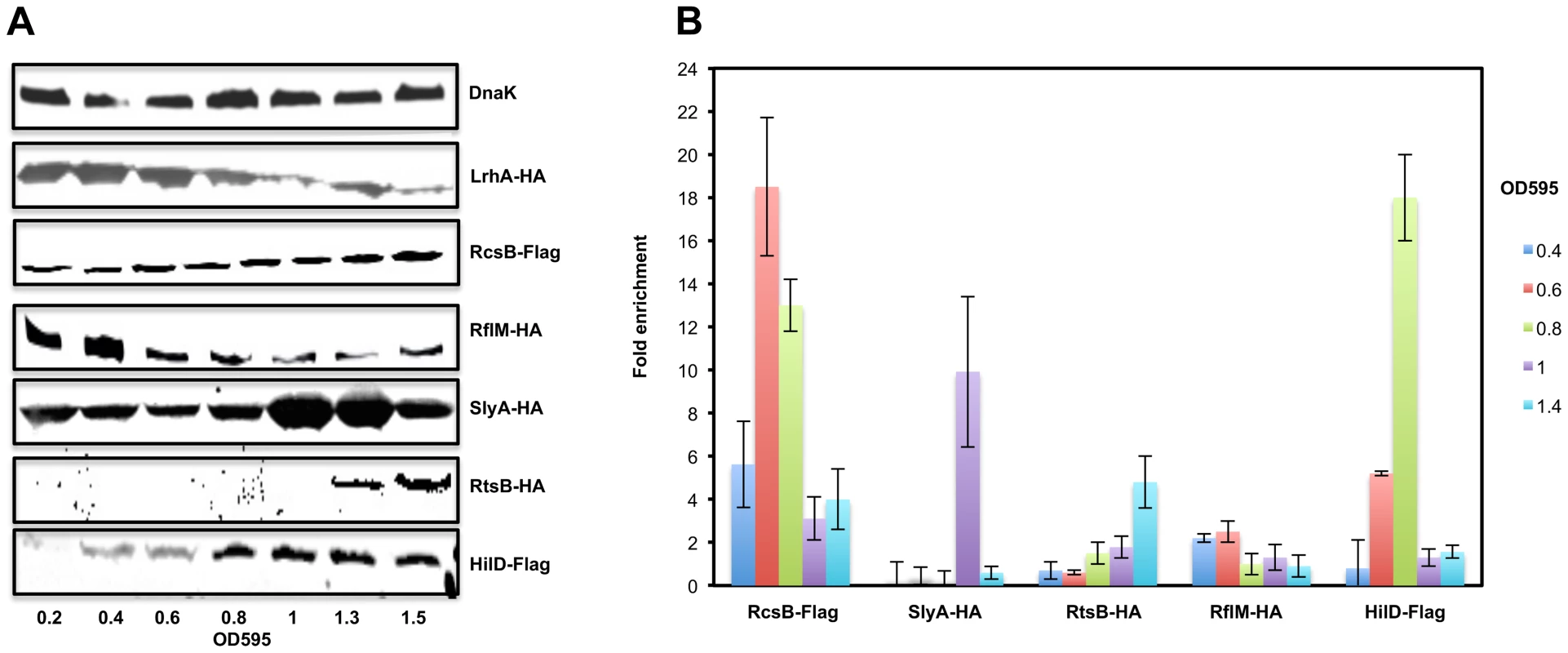
(A) Expression of the RcsB, LrhA, RflM, SlyA, HilD and RtsB proteins in LB during growth after dilution from overnight culture. Immunoblots of whole-cell lysates of S. typhimurium strains carrying one of the following: a FLAG-tag in either the rcsB (TH18628) or hilD (TH20451) gene, an HA-tag in rflM (TH19853), slyA (TH19855), or rtsB (TH19854) gene. Growth of an individual tagged strain was monitored at OD595 and total proteins were extracted. Blotting was performed using a monoclonal anti-HA or anti-Flag antibody. Equivalent amounts of proteins (50 µg per lane) were analyzed at each time point. As a loading control, DnaK was detected using a monoclonal antibody against DnaK. The OD595 are indicated at the bottom of the figure. (B) In vivo binding of regulatory factors controlling flhDC transcription to the promoter region of flhDC. Chart represents the fold enrichment of the flhDC regulatory region DNA bound by different transcriptional factors at different ODs. Cells were grown at 30°C until they reach the ODs shown in the left of the chart. Pull-down of the DNA-protein complexes and RT-PCRs were conducted as described in Material and Methods. Fold enrichment was calculated relative to a no-antibody control as described in Material and Methods. Bars represent the average of two independent experiments of a Chromatin Immuno-precipitation assay (CHIP). In vivo binding by regulators of flhDC transcription to the flhDC promoter region
We examined the in vivo binding dynamics within the flhDC promoter region by these regulatory factors. At different optical densities (0.4 to 1.4), chromatin immunoprecipitations (ChIP) were conducted using strains with individually tagged transcriptional factors, RcsB, RflM, HilD, RtsB, LrhA and SlyA (Figure 3B). Expression of RcsB and binding of RscB to its target DNA at the flhDC promoter was detected throughout the entire growth phase. However, RcsB bound levels increased as cells progressed to exponential phase (OD 0.4 to 0.6) followed by decreased binding at latter stages of growth. The transcriptional regulator RflM binding to DNA was detected at OD∼0.4 with maximal binding at OD∼0.6, but was no longer bound the flhDC promoter region beyond OD∼0.6. HilD, a transcriptional activator of flhDC, was bound to the flhDC promoter region at OD∼0.4 increasing to a maximum bound level at OD∼0.8 and followed by absence of bound HilD at OD∼1. SlyA was not physically associated with the flhDC promoter at OD∼0.4 and ∼0.6, but was bound to the flhDC promoter region at OD∼0.8. There was no binding of RtsB to the flhDC promoter at an early time point of cell growth OD∼0.4 to 0.6. Binding by RtsB had initiated by OD 0.8 and increased through OD 1.4. We were unable to immunoprecipitate LrhA tagged protein because C-terminal or N-terminal tagged LrhA behaved like lrhA null mutant (Figure S5; ). These results highlight the binding dynamics of different regulators to the flhDC promoter region resulting in a dynamic of flhDC operon transcription.
Molecular analysis of the individual flhDC transcriptional start-sites
Six transcriptional start sites, designated P1flhDC, P2flhDC, P3flhDC, P4flhDC, P5flhDC and P6flhDC, within Salmonella flhDC promoter region were obtained by primer extension [13]. However only P1flhDC, P3flhDC, P4flhDC and P5flhDC were detected by RNA-Seq based approach [29]. Each of these TSSs was preceded by a hexamer motif (−10 box) with the consensus invariant residues adenine at position 2 (A2) and thymine at position 6 (T6), except for P4 (Figure 4A). To investigate the authenticity of these TSSs, we made alterations of the −10 sequences targeting the conserved residues A2 and T6 by changing them to a cytosine residue (C) and also by totally changing the −10 box to a GTTGGT sequence (Figure 4B). As controls, additional mutations were made in each −10 box, in a nucleotide other than A2 or T6 (Figures 4B & S3A) that supposedly should not alter significantly the effect of RNAP on transcription [60]. Because flhDC is subjected to negative and positive transcriptional feedback, mutations of the promoters responsible for transcription of flhDC operon in the wild-type strain might affect the positive and negative auto-regulation of flhDC transcription. We thus monitored the activities of the promoters mutants fused to luciferase operon in an flhD+C+ background (described above). Mutations of wild-type sequence P1flhDC (TATAGT) to GTTGGT (P1−.1flhDC); TCTAGC (P1−.2flhDC) or TCTAGT (P1−.3flhDC) but not TACAGT (P1−.4flhDC) were associated with a significant reduction of flhDC transcription (Figure 4C). Mutations of the wild-type P5flhDC (TATGCT) to TCTGCC (P5−.2flhDC) or TCTGCT (P5−.3flhDC) but not to TACGCT (P5−.4flhDC) reduced significantly the transcription of flhDC to the same extent as the mutation of −10 to GTTGGT (P5−.1flhDC) (Figure 4D). These results indicated that P1flhDC and P5flhDC are bona-fide promoters.
Fig. 4. Effects of mutations in putative transcriptional start-sites within the flhDC promoter region on flhDC operon transcription. 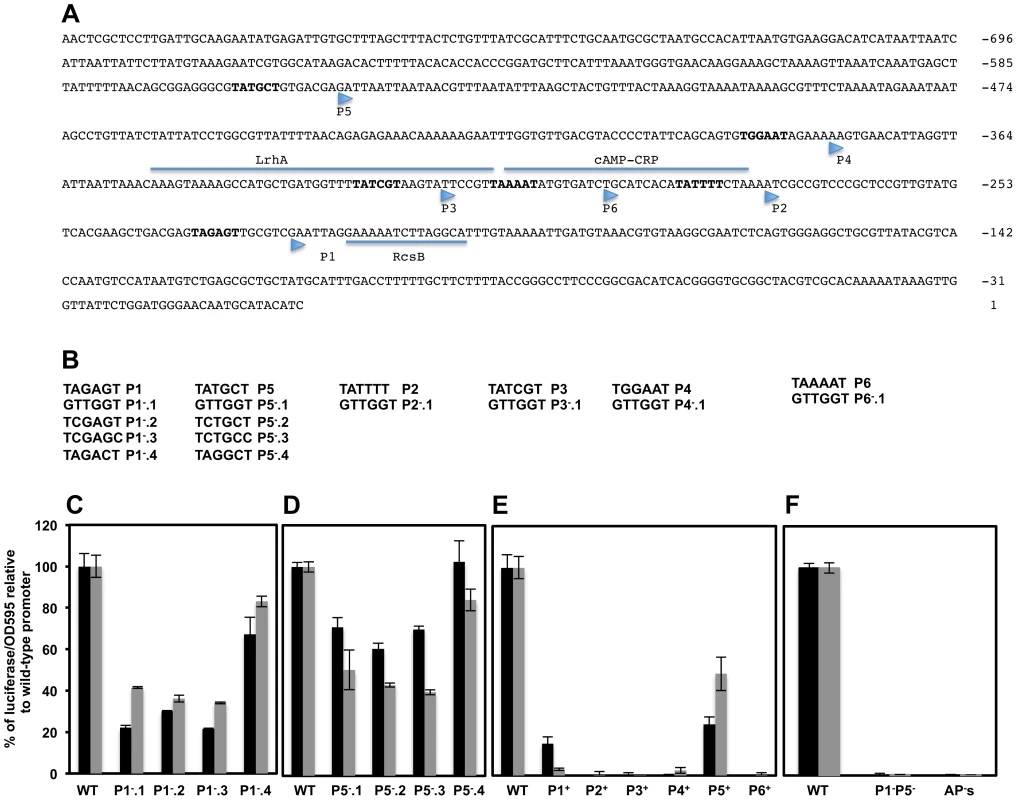
(A) DNA sequence and regulatory elements of the upstream regulatory region of S. typhimurium. Nucleotides are labeled respective to the start of the open reading frame of FlhD. The −10 box of the putative promoters are in bold and their respective transcription start site are indicated by arrowheads as determined by primer extension [13]. The transcriptional factors LrhA, RcsB, RtsB and CRP have been shown to bind directly to the flhDC promoter regulatory region. Experimental evidence and mutations analysis have been performed to delineate the exact binding of LrhA, RcsB and CRP (underlined). The exact binding of the RtsB has not been defined but it has been shown that RtsB binds directly to flhDC promoter region corresponding to a DNA fragment covering from +4 to +104 nucleotides respective to the P1 transcription start site [17]. The direct and exact binding site for SlyA, RflM and HilD transcriptional factors have not been defined yet. (B) DNA sequences of the −10 boxes [13] of putative transcriptional start-sites (shown as P1−, P2−, P3−, P4−, P5− and P6−) and mutant constructs that were made in each of these −10 boxes. The individual transcriptional start-sites promoter mutants were made separately at each single −10 box or were combined together leaving only one functional −10 box out of the six described promoter start-sites (shown as P1+, P2+, P3+, P4+, P5+ and P6+). Charts represent the luciferase activities of the PwtflhDC-luxCDBAE-PwtflhDCflhD+C+ reporter construct in wild-type and isogenic strains carrying mutations in individual start-site −10 boxes. Cells were grown overnight in LB and diluted 1 to 500 in fresh media, and grown at 30°C with shaking and luciferase activities were recorded at two optical densities (0.5, black bars and 1, grey bars). Charts of luciferase activity in strains with mutations in the P1 (C) and P5 (D) promoters of flhDC operon compared to the wild-type flhDC promoter activity that was set at 100%. Each specific mutation is indicated under their corresponding bars. (E) Graph of luciferase activity in strains harboring only one single wild-type −10 box of the indicated putative promoter. P1+ represents a strain that has only a functional P1 promoter while the rest of the promoters are mutated, etc (F) Luciferase activity of a strain with mutations in both P1 and P5 (P1−P5−), compared to wild type promoter and to a construct with mutations in all six promoters: AP's. Results are the average of three independent experiments done in duplicate. Error bars represent standard deviation. Analysis of mutations of −10 sequences of the P2flhDC and P6flhDC (overlapping with the CRP binding site which is required for the transcription of flhDC from P1flhDC promoter) and P3flhDC (overlapping with the LrhA binding site) and P4flhDC were not conclusive (Supplementary Text S1 & Figure S3).
We further investigated the authenticity of the six putative TSSs of the flhDC operon, by engineering strains with combined mutations in the promoter region of flhDC leaving only one wild-type −10 sequence from the six described promoters. Thus, P1+ designates a strain that has only a functional P1 promoter, etc. We also constructed a control strain with combined mutations in all the six promoters, AP−flhDC (All Promoters mutated). We established that P1+flhDC and P5+flhDC were able to promote flhDC operon transcription but to a lesser extent to what is observed in the wild-type strain (Figure 4E). The transcription of flhDC was totally abolished in strains harboring P2+flhDC, P3+flhDC, P6+flhDC and APs−flhDC, while P4+flhDC mutants showed very low level of flhDC transcription (1.8% relative to the wild-type strain) (Figure 4E). These results suggested that in the wild-type background P1flhDC and P5flhDC are the main promoters driving flhDC operon transcription with a marginal activity from the P4flhDC promoter. Yanagihara et al., 1999; have demonstrated that P6flhDC is only active in the absence of CRP, we confirmed that P6+flhDC (only P6 is functional) is inhibited by CRP, because in a crp null mutant there was an increase of transcription of P6+flhDC compared to wild-type (Figure S3H).
Since only mutations in P1flhDC and P5flhDC promoters significantly affected the expression of flhDC, we would expect the level of transcription of flhDC operon in the absence of both P1 and P5 promoters to be similar to the level of transcription of flhDC operon in the absence of all flhDC promoters (P1 through P6). To investigate this hypothesis, we measured the luciferase activity in a strain with combined mutations in P1flhDC and P5flhDC promoters (P1−P5−flhDC) and compared it to the luciferase activity of a wild-type strain and to a strain with all six promoters mutated (strain AP's). We observed that transcription of flhDC operon in strain P1−P5−flhDC was totally abolished to the same levels observed in a strain with all flhDC promoters mutated (Figure 4F). These results demonstrated that in a wild-type background P1flhDC and P5flhDC are the major promoters driving transcription of flhDC operon. We concluded that transcription of the flhDC operon in strain P1−flhDC (harboring mutations of the −10 box of P1flhDC) is driven from the P5flhDC promoter and that transcription of the flhDC operon in strain P5−flhDC (harboring mutations of −10 box of P5flhDC) is driven from P1flhDC.
Dynamics of flhDC transcription from P1flhDC and P5flhDC promoters
Once we established that P1flhDC and P5flhDC are the main promoters driving transcription of the flhDC operon, we monitored the expression of the P1flhDC and P5flhDC promoters at different optical densities using PwtflhDC, P1−flhDC and P5−flhDC constructs (Figure 5A). The transcription profile of flhDC operon in strains P1−flhDC (P5-expressed) and P5−flhDC (P1-expressed) demonstrated that both promoters are required for transcription of flhDC because the expression of flhDC operon in constructs P1−flhDC (P5-expressed) and P5−flhDC (P1-expressed) did not reach the expression levels of the wild-type strain, PwtflhDC (both P1 and P5 are expressed) (Figure 5A). Moreover, transcription of flhDC operon from P1flhDC is activated earlier than P5flhDC because (i) the transcription profile of the flhDC operon in construct P5−flhDC (P1-expressed) overlapped with that of the wild-type strain from OD 0.1 to OD 0.4, (Figure 5A) and (ii) there was a delay in the transcription of flhDC operon in construct P1−flhDC (P5-expressed) where the transcription started taking place at OD∼0.35 (Figure 5A) compared to the wild-type PwtflhDC and P5−flhDC (P1-expressed) strains (OD∼0.2). The same hierarchy of expression of P1 and P5 was observed in batch culture (Figure 6A & B). The transcription of flhDC operon in P5−flhDC (P1-expressed) started declining at OD∼0.4–0.5, meanwhile, transcription of flhDC operon in strain P1−flhDC (P5-expressed) was more pronounced at a later growth stage accounting for ∼60% relative to the wild-type at OD∼0.6 (Figure 5A). It is apparent from the dynamic profile of flhDC operon transcription, that P5flhDC promoter transcription occurs concomitantly with a cessation or decline in the transcription from P1flhDC (Figure 5A). These results indicate that P1flhDC is an early promoter, whose activation drives the transcription of flhDC operon synthesis at early growth phase followed by a cessation or decline once P5flhDC promoter is activated.
Fig. 5. Transcription levels of the P1flhDC and the P5flhDC promoters during the cell growth phase and their regulation by HilD and RflM. 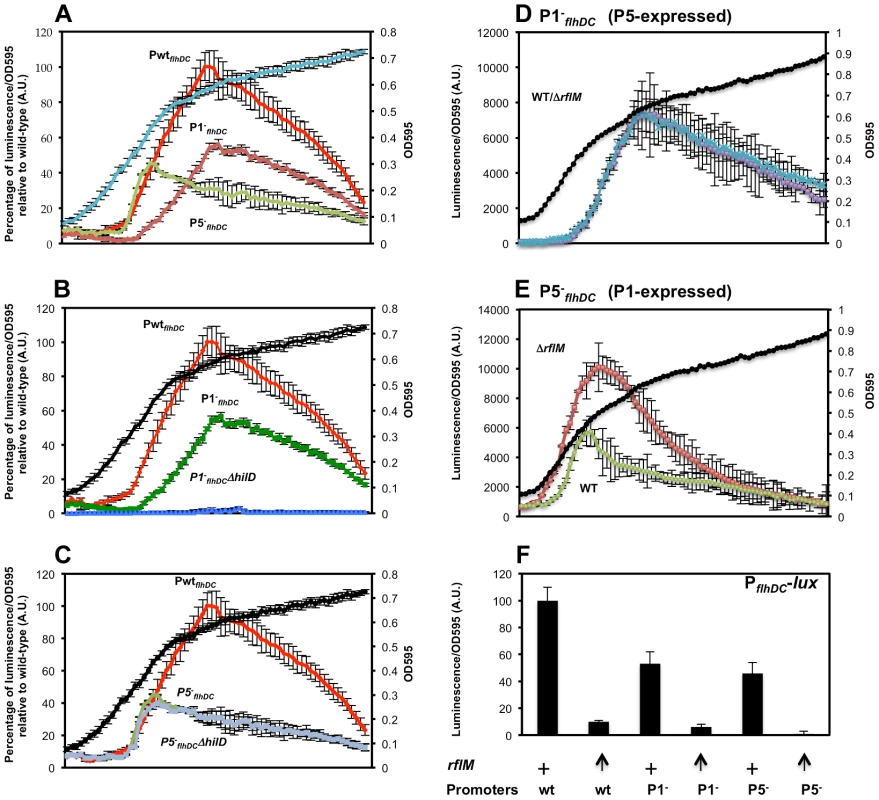
(A) Luciferase activity was measured in three genetic backgrounds: PwtflhDC-luxCDBAE-PwtflhDCflhD+C+ (TH18684) filled square, P1−fhlDC-luxCDBAE-PwtflhDCflhDC+ (TH18889) filled circle and P5−fhlDC-luxCDBAE- PwtflhDCflhDC+ (TH18895) filled triangle. Luciferase activity relative to the wild-type strain is shown (first axis) along with the OD 595 (Second axis). In the absence of the P5 promoter (P5−flhDC) the flhDC operon (transcribed from P1) was activated earlier than the isogenic strain that transcribed flhDC from the P5 promoter (P1−flhDC). Transcription of the flhDC operon from the P1 promoter (P5−flhDC) was activated at the same time as with the wild-type promoter (PwtflhDC) at OD∼0.2. When cells reach an OD of 0.4, P1flhDC promoter activity (P5−flhDC) ceased and declined afterwards. Transcription from the P5flhDC start-site (P1−flhDC) took place at an OD of ∼0.35. (B & C) HilD promotes transcription of the flhDC operon from the P5flhDC promoter. Luciferase activity relative to the wild-type strain is shown (First axis) along with the OD 595 (Second axis). Luciferase activity was investigated in five strains: PwtflhDC-luxCDBAE-PwtflhDCflhD+C+ (TH18684), P1−fhlDC-luxCDBAE-PwtflhDCflhD+C+ (TH18889), P1−fhlDC-luxCDBAE-PwtflhDCflhD+C+ ΔhilD::TetRA (TH19965), P5−fhlDC-luxCDBAE-PwtflhDCflhD+C+ (TH18895) and P5−fhlDC-luxCDBAE-PwtflhD+C+ ΔhilD::tetRA (TH19966). Transcription of flhDC operon from the P5flhDC promoter (P1−flhDC) was totally abolished in the absence of HilD. The absence of HilD had no effect on the transcription of flhDC operon from the P1flhDC promoter (P5−flhDC). Each data point of the plots represents the average of five independent replicates performed in different days of six measurements for wild-type flhDC promoter and three measurements for the rest of strains. (D & E) RflM feedback inhibits transcription of the flhDC operon. Luciferase activity of P1−flhDC and P5−flhDC flhDC promoters expressing the luxCDBAE reporter is presented as a function of the cell growth phase in isogenic strains in the presence (WT) and absence (ΔrflM) of RflM. Luciferase levels at different points during the cell growth phase were measured for the (D) P1−fhlDC-luxCDBAE-PwtflhDCflhD+C+, and (E) P5−fhlDC-luxCDBAE-PwtflhDCflhD+C+ duplication constructs. Growth conditions and luciferase activity were analyzed as described in Figure 2. A representative growth curve is shown in each plot. Plots represent the average of five independent replicates performed in different days of six measurements for each data point. Error bars represent standard deviation. (F) Overproduction of RflM inhibits transcription from the P1flhDC and P5flhDC promoters. Luciferase activity for PwtfhlDC-luxCDBAE-PwtflhDCflhD+C+, P1−fhlDC-luxCDBAE-PwtflhDCflhD+C+ and P5−fhlDC-luxCDBAE-PwtflhDCflhD+C+ was investigated, when cells reached an OD 1, in two genetic backgrounds: ParaBADFCF, and ParaBADrflM+. Growth conditions and luciferase activities were analyzed as described in Figure 4. To induce expression of rflM from the arabinose promoter (ParaBADrflM+), arabinose was added at 0.2% (indicated by an arrow). (+) Indicates wild-type level of rflM. Chart represents the average of three independent experiments. Fig. 6. Effects of RcsB, LrhA, RtsB and SlyA on transcription of P1flhDC and P5flhDC. 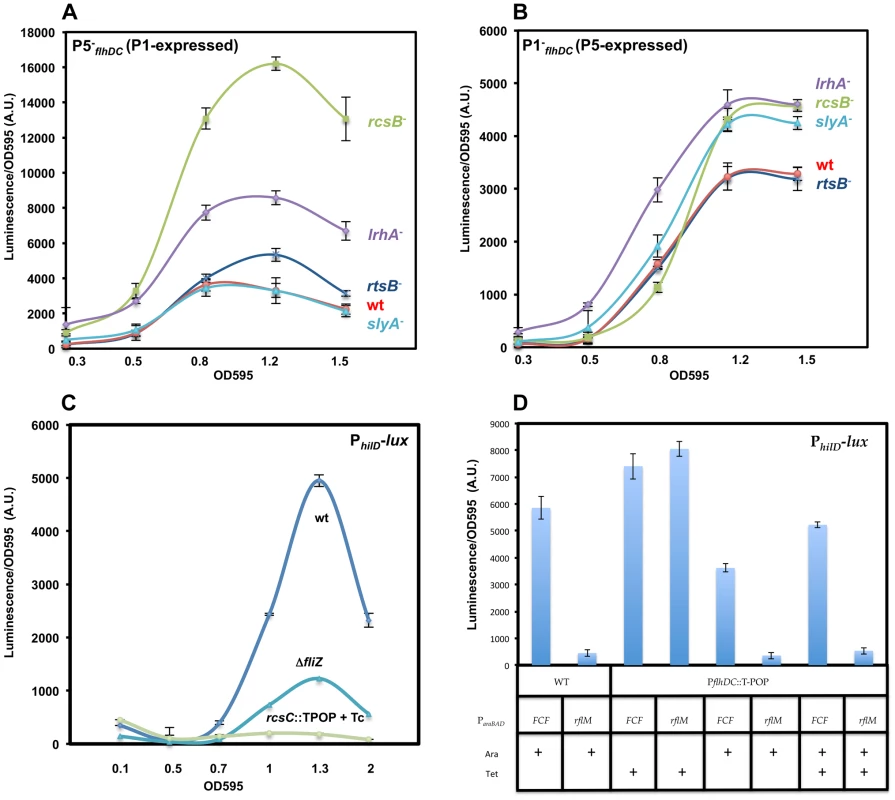
For these assays, we compared the transcription from the P1−flhDC (defective in the P1 start-site) and the P5−flhDC (defective in the P5 start-site) promoter constructs. Plots represent luciferase activity divided by the OD595 plotted against the OD595 values shown at the bottom of the chart. (A) RcsB, LrhA and RtsB but not SlyA repressed transcription from the P1flhDC promoter. Luciferase activity of P5−fhlDC-luxCDBAE-PwtflhDCflhD+C+ transcriptional fusion (P1-expressed) was investigated in five genetic backgrounds: wild-type (TH18895), ΔrcsB::tetRA (TH20237), rtsB::T-POP (TH19976), ΔlrhA::tetRA (TH19974), slyA::T-POP (TH19975). (B) RcsB, LrhA and SlyA but not RtsB are negative regulators of P5flhDC promoter. Luciferase activity of P1−fhlDC-luxCDBAE-PwtflhD+C+ transcriptional fusion (P5-expressed) was measured in wild-type (TH18889), ΔrcsB::tetRA (TH20236), rtsB::T-POP (TH19972), ΔlrhA::tetRA (TH19970) and slyA::T-POP (TH19971)., (C) RcsB inhibits hilD transcription in an flhDC independent manner. Luciferase activities of the PhilD-luxCDBAE transcriptional fusion in wild-type (rcsC+) (TH19425), rcsC::T-POP (TH19687) and ΔfliZ::FCF (TH19690) backgrounds were recorded as described in Figure 1. FliZ, a post-translational activator of HilD, promotes transcription of the auto-regulated hilD gene. Tetracycline (Tc) was used at 3 µg/ml to induce rcsC transcription in the rcsC::T-POP background resulting in activation of RcsB. Upon RscB acticvation (rcsC::T-POP +Tc), transcription of hilD was abolished. The inhibitory effect of RcsB on hilD transcrption (40-fold) is more dramatic than the four-fold decrease in the absence of FliZ. Results are the average of two independent experiments performed in duplicate. Error bars represent standard deviation. (D) RflM inhibits hilD transcription in an flhDC independent manner. Luciferase activity of strains harboring a hilD transcriptional fusion, PhilD-luxCDBAE, was measured in four genetic backgrounds, ParaBAD::FCF (TH20541) (Column 1), ParaBAD::rflM+ (TH20542) (Column 2) and ParaBAD::FCF PflhDC::T-POP (TH20543) (Column 3, 5 and 7) and ParaBAD::rflM+ PflhDC::T-POP (TH20544) (Column 4, 6 and 8). ParaBAD::rflM+ strains, in the presence of arabinose (Ara and +) leads to the overexpression of rflM and ParaBAD::FCF serves as a control. Addition of tetracycline (Tet and +) to PflhDC::T-POP strains allows the overexpression of flhDC and in the absence of tetracycline the cells are flhDC−. Cells were diluted 1 to 500 from an overnight culture into LB in the presence arabinose, tetracycline or arabinose and tetracycline. 0.2% arabinose (Ara) was added to induce transcription of rflM and 3 µg/ml tetracycline (Tet) to induce transcription of flhDC. At an OD595∼1, the luciferase activity was recorded as described in Figure 4. HilD specifically activates transcription from the P5flhDC promoter
We have demonstrated that HilD is a positive regulator of flhDC transcription (Figure 1D & 2D). As shown (Figure 2D), when cells are grown in the 96 well plate format, the effect of HilD on the transcription of flhDC takes place starting at OD∼0.4. In order to determine which of the two promoters, P1flhDC or P5flhDC, is the target of the positive regulation by HilD, we compared the dynamic profile of flhDC transcription in PwtflhDC, P1−flhDC and P5−flhDC constructs in a wild-type and its isogenic strain hilD null mutant (Figures 5B & C). We established that, relative to the wild-type strain background, a deletion of hilD (i) reduced PwtflhDC promoter transcription; (ii) abolished the transcription of flhDC operon in construct P1−flhDC (P5-expressed) (Figure 5B) and (iii) did not affect the transcription of flhDC operon in construct P5−flhDC (P1-expressed) (Figure 5C). These results indicate that HilD promotes transcription from P5flhDC and has no apparent effect on P1flhDC promoter transcription.
The negative autoregulation of flhDC transcription via RflM is at the P1flhDC promoter
Transcription of the flhDC operon is subjected to negative feedback by RflM, which is activated at the transcriptional level by FlhD4C2 [21]. To further study the effect of the negative autoregulation on flhDC operon transcription kinetics, we monitored the transcription profile, over time, in the three strains PwtflhDC, P1−flhDC and P5−flhDC in the absence and presence of RflM. We established that there was an increase in the transcription from PwtflhDC in the absence of RflM (Figure 2B). We demonstrated that the P1flhDC promoter is under negative autoregulation by RflM because the expression of flhDC operon in strain P1−flhDC (P5-expressed) was similar between the wild-type and its isogenic rflM null mutant (Figure 5D). Additionally, we found that RflM did not appear to regulate P5flhDC because flhDC transcription in strain P5−flhDC (P1-expressed) increased in the absence of RflM (Figure 5E). These results demonstrated that in the wild-type background the P1flhDC promoter is subjected to negative autoregulation through RflM, while transcription from P5flhDC appeared to be RflM independent.
We employed an alternative approach to confirm which of the flhDC promoters is specifically inhibited by the transcriptional factor RflM. We monitored the transcription of flhDC in a strain that overproduces RflM under control of the arabinose promoter, ParaBAD::rflM+. In the presence of arabinose, used to induce overexpression of rflM, we observed an inhibition of transcription of flhDC operon in the three strains tested, PwtflhDC, P1−flhDC and P5−flhDC (Figure 5F). These results suggest that RflM is able to inhibit transcription of flhDC operon from both promoters, P1 and P5, which is in contradiction to the specific inhibition of the P1flhDC but not the P5flhDC promoter by RflM observed in Figures 5D & E. RflM protein production or stability appears to decline in function of cell growth cycle (Figure 3A), suggested that continuous production of RflM might affect indirectly the expression of P5flhDC. Because HilD is an activator of the P5flhDC promoter, we hypothesized that overexpression of RflM inhibits transcription of hilD gene. In order to test this hypothesis, we monitored the activity of a luciferase transcriptional fusion of the hilD promoter, PhilD, in two genetic backgrounds: (i) ParaBAD::FCF (ii) ParaBAD::rflM+. We observed that under conditions that overproduce RflM, presence of arabinose, there was an inhibition of transcription of the autoregulated gene hilD (Figure 6, compare column 1 to column 2). Note that the strains used to determine luciferase activity are all flhD+C+, and overexpression of RflM inhibits flhDC transcription required for production of the posttranslational regulator of HilD. Thus, the effect of RflM, on hilD transcription could be indirect through inhibiting flhDC. To test if the effect of RflM on hilD, is direct or indirect we used two additional strains (i) ParaBAD::FCF PflhDC::T-POP and (ii) ParaBAD::rflM+ PflhDC::T-POP. For the PflhDC::T-POP backgrounds the flhDC operon is transcribed from the tetracycline(Tc)-inducible tetA promoter, and as such are flhDC− in the absence of tetracycline and flhDC+ in the presence of tetracycline. First, we observed that flhDC controlled transcription of the hilD gene, because in the absence of Tc, there was a 2-fold decrease in the PhilD transcription level in the PflhDC::TPOP strain background (Figure 6D, compare column 3 to column 5). Moreover, we demonstrated that under condition of RflM overexpression, there was a higher level of inhibition of hilD transcription compared to the reduction observed in the PflhDC::T-POP background (Figure 6D, compare column 5 to column 6). The overproduction effect of RflM was not rescued by addition of Tc to induce flhDC transcription, an activator of hilD transcription (Figure 6D, compare column 6 to column 8). These results demonstrated that RflM could inhibit transcription of the hilD gene in an flhDC independent manner. Thus flhDC and rflM have opposite effects on the transcription of hilD, where flhDC is an indirect positive regulator of HilD, yet high levels of RflM inhibit hilD transcription. Since HilD is an activator of P5flhDC transcription, we conclude that the negative effect of RflM overproduction on transcription of P5flhDC is indirect and through inhibition of hilD gene transcription,
Targeting of the flhDC promoter region by RcsB, LrhA, SlyA and RtsB
The presence of two principal TSSs within the flhDC operon promoter region combined with the hierarchical regulation by different transcriptional factors, suggests that there is differential regulation at the promoter by different transcriptional regulators at different cell growth phases. We investigated which of the specific regulators: RcsB, LrhA, SlyA and RtsB control transcription of flhDC through the P1flhDC and P5flhDC promoters start-sites.
1. P1flhDC is negatively regulated by RcsB, RtsB and LrhA but not by SlyA
To determine if RcsB, RtsB, LrhA and SlyA regulate P1flhDC, we monitored the transcription of flhDC operon of the construct P5−flhDC (P1-expressed) in strains defective in either the rcsB, lrhA, slyA or rtsB genes. We observed increased transcription in the P5−flhDC background, in either rcsB, rtsB or lrhA null mutants compared to the wild-type strain (Figure 6A). The transcription of P1flhDC increased 5-fold, 2-fold and 1.6-fold in rcsB, lrhA and rtsB mutant strains, respectively. These results demonstrated that RcsB, LrhA and RtsB repress transcription from P1flhDC. However, a null mutation in slyA gene did not affect transcription from P1flhDC, because there were no differences in the transcription levels for the P5−flhDC mutant promoter at any point of time during all the growth phases between the wild-type and the slyA mutant. The same effect of RcsB, LrhA and SlyA was also observed in strain P1+flhDC (this strain is (P5P4P3P6P2)−) (Figure S4.A). However, there was no effect of rtsB mutation on the expression of P1+flhDC, which could be attributed to either the low level of expression flhDC in construct P1+flhDC or to the additional mutations (P5P4P3P6P2)− present in the P1+flhDC construct (See supplementary Text S1).
2. P5flhDC is negatively regulated by RcsB, LrhA, SlyA but not by RtsB
We monitored the transcription of the flhDC operon in construct P1−flhDC (P5-expressed) in strains lacking either the rcsB, lrhA, slyA or rtsB genes. We demonstrate that the transcription from P5flhDC promoter is regulated by RcsB, LrhA and SlyA proteins, because transcription of flhDC in construct P1−flhDC (P5-expressed) increased in rcsB (2-fold), lrhA (2-fold) and slyA (1.8-fold) mutant strains (Figure 6B). We also demonstrated that transcription of the P5flhDC promoter is not regulated by RtsB protein because transcription of flhDC in construct P1-flhDC (P5-expressed) was independent of RtsB (Figure 6B). In strain P5+flhDC, (this strain is (P4P3P6P2P1)−), we observed the same regulation as with P1−flhDC (Figure S4.B).
The transcription kinetics of the P1−flhDC (P5-expressed) in the ΔrcsB mutant was different than that of the PwtflhDC or the P5−flhDC (P1-expressed) in the absence of RcsB. While, there was a relief in the inhibition of transcription for the PwtflhDC or P5−flhDC (P1-expressed) constructs at earlier time points of cell growth, the rcsB mutation resulted in increased transcription in the construct P1−flhDC (P5-expressed) only at later stage of growth (Figure 6B). It has been demonstrated that RcsB regulates flhDC transcription by direct binding to an RcsB binding sequence located 11 bp downstream of P1flhDC. Inspection of the DNA upstream region of flhDC operon did not reveal the presence of any additional consensus RcsB-binding site, suggesting that the inhibitory effect of RcsB on transcription from the P5flhDC promoter might be indirect and through the repression of an activator or activation of a repressor. It has been shown that RcsB inhibits hilA transcription [61], whose activation is under the control of hilD. Because hilD is an activator of transcription from the P5flhDC promoter, we hypothesized that the effect of RcsB on P5flhDC transcription was due to derepression of hilD transcription in an rcsB mutant background. To test this hypothesis, we monitored the expression of a transcriptional fusion of hilD to luciferase, PhilD-lux, in wild-type, ΔfliZ::FCF and rcsC::T-POP strains (Figure 6C). We used an rcsC::T-POP allele that results in tetracycline(Tc)-dependent transcription of the rcsC gene [62] and thus activation of the transcription factor RcsB, to monitor the effect of RcsB on hilD transcription. We used a fliZ null mutant to detect if the effect of RcsB on hilD is through the flhDC regulated gene fliZ, which is the post-translational activator of the autoregulated hilD gene. We observed that FliZ regulated transcription of hilD, because there was a 2-fold reduction of PhilD-dependent transcription in the strain lacking fliZ compared to the fliZ+ background (Figure 6C). However, under conditions that over-express RcsC (addition of Tc in the presence of the rcsC::T-POP allele to induce transcription of rcsC), the transcription of hilD was abolished (Figure 6C). Compared to the effect of deleting fliZ, overexpression of RcsC exerted a more pronounced inhibitory effect on hilD transcription. These results demonstrate that RcsB inhibits transcription of hilD in both flhDC dependent and independent manners and suggested that the P5flhDC promoter is indirectly regulated by the RcsB transcriptional factor.
The timing of transcription of flhDC as a prerequisite for motility
There appears to be five stages of flhDC transcription that are controlled by three clusters of response regulators. Deletion of either, rcsB, lrhA or rflM resulted in increased motility compared to the wild-type strain [18], [19], [62]. We observed that null mutations in any of the late regulators: hilD, rtsB or slyA, did not affect motility (Figure 7A). Based on the expression profiles of the flhDC operon in these mutant strains, these results establish that Salmonella wild-type motility will only need to reach a threshold of flhDC expression for motility, while increased flhDC expression later in the growth phase has no further effect on motility. It is noteworthy to mention that factors that affected the early transcription of the P1flhDC promoter: LrhA, RcsB (Figure 6A) and RflM (Figure 5E) affected motility while transcriptional factors, HilD and SlyA, that regulate P5flhDC promoter late in the growth phase (Figure 5B & 6B) did not affect motility (Figure 7A). Moreover, RtsB, by inhibiting transcription from P1flhDC at later stages of growth (Figure 6B), did not inhibit motility suggesting that the growth phase combined with activation of flhDC promoters is important for motility (Figure 7A). It is noteworthy to mention that the factors that affected transcription of P5 flhDC but not motility are bona fide virulence factors.
Fig. 7. Time-dependent transcription of flhDC operon controls motility of Salmonella. 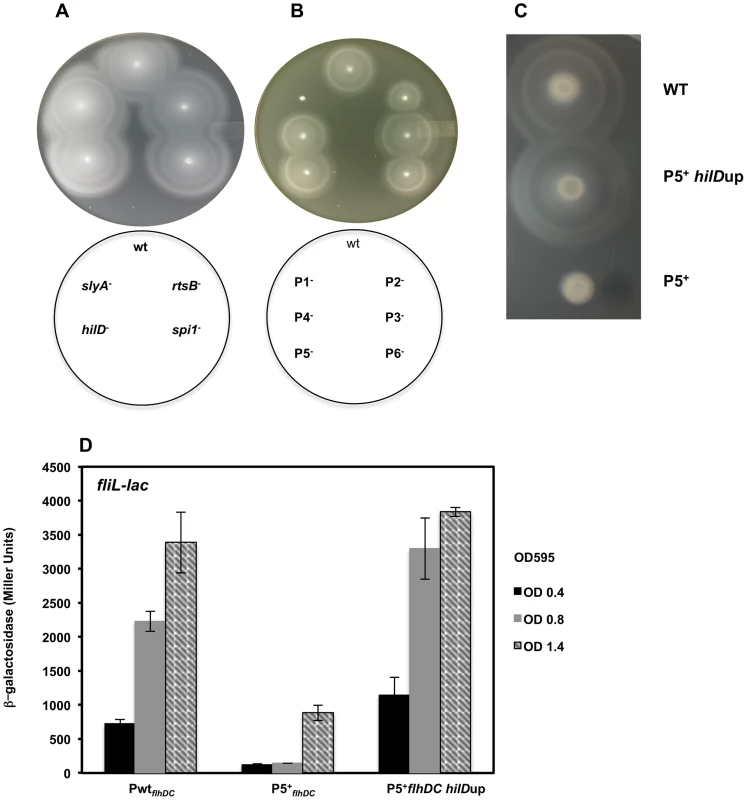
(A) A representative image of motility of the wild-type strain compared to (A) slyA, rtsB, spi1 and hilD null mutants. Null mutations in slyA, rtsB, spi1 or hilD does not affect motility compared to the wild-type strain (B) A representative image of motility of the wild-type strain compared to constructs harboring single promoters mutations in the flhDC regulatory region. (C &D) Early transcription of P5flhDC promotes motility (C) the motility defect of P5+ construct (only P5 is active and the promoters P1, P2, P3, P4 and P6 are mutated) was rescued by a mutation that overexpresses hilD (P5+ hilDup) and (D) transcription of class 2 gene, fliL, of the P5+ construct in a wild-type strain compared to its isogenic strain hilDup (mutation that overexpress HilD). ß-galactosidase activity (Miller Units) of a lac fusion to fliL gene was investigated in three genetic backgrounds: wild-type, P5+flhDC and P5+flhDC hilDup strains. Values are average of two experiments done in duplicate at different ODs. We decided to study the effect of the flhDC promoter mutations on the motility of Salmonella. We constructed strains harboring single mutation in each of the promoters separately. Thus P1− refers to a strain that has a mutation in P1 promoter, etc. Note that these strains in contrast to strains harboring the luciferase constructs do not harbor a duplication of the flhDC operon. We demonstrated that strains defective in P1flhDC start-site transcription (only P1 is mutated) were non-motile while P5flhDC defective strains (only P5 is mutated) exhibited no apparent reduction of motility (Figure 7B). There was a motility defect of the strains P2− and P6− that is related to the effect of CRP (as discussed earlier and in Supplementary material). The motility of P3− and P4− were not significantly different from the wild-type strain. These results confirmed that in the wild-type background transcription from P1flhDC is a prerequisite for motility while P5flhDC is not required for motility. These results also suggested that the right timing of expression of flhDC is essential for motility. If this hypothesis is correct, we could expect that if flhDC is expressed from P5flhDC promoter at an early time point it should confer a motility phenotype. To test this hypothesis we used the non-motile strain P5+ (only P5 is functional and the other promoters are mutated) (Figure 7C) to isolate suppressors of motility inhibition. This strain was used in order to limit isolating mutations in the other promoters of flhDC that would otherwise suppress motility [16]. We isolated a spontaneous suppressor that restores motility to the P5+ strain (Figure 7C) and mapped the mutation to the promoter region of hilD gene (addition of a thymine residue at position −51 from the start codon of HilD and resulting in higher expression of hilD (labeled hilDup)). The isolation of this mutation confirmed that HilD regulates the P5flhDC promoter. If the hypothesis that the timing of expression of flhDC as a prerequisite for motility is correct, then a hilD-up mutation should promote transcription of flhDC operon from P5 promoter at early growth phase. To test this hypothesis we used a transcriptional lac fusion to fliL, a class 2 promoter that is positively regulated by FlhD4C2, as readout to determine the expression of the P5 promoter transcription. The transcription of fliL indicates the presence of FlhD4C2-dependent transcription. Transcription of fliL in the P5+ strain was very low during early growth phases and increased when cells reached an OD of 1.4 (Figure 7D). These results suggest that P5+ cells are able to express flagellar genes at later stage of cell's growth phase yet they are not motile. Interestingly, overexpression of hilD, hilDup mutant resulted in a premature activation of P5flhDC, leading to the transcription of fliL at early growth phase and similar to the timing and levels of the wild-type strain (Figure 7D). These results suggested that the timing of FlhD4C2 production during an early growth phase is critical for motility.
Discussion
The complex networks and the number of factors necessary for the production of functional flagella and the resulting motility, though beneficial for the bacteria, represent a significant requirement on the cell's resources [63], [64]. At the top of this cascade sits the flhDC operon [7]. We established now that Salmonella flhDC operon is primarily transcribed from two promoters, P1flhDC and P5flhDC. The activities of these two promoters are coupled to five stages controlling flhDC transcription and each stage is differentially controlled by a set of transcriptional factors: (1) repression of transcription of flhDC during the initial growth phase by LrhA and RcsB (2) repression by RflM at early exponential phase (3) activation through the action of HilD at mid exponential phase (4) repression by SlyA and RtsB at the onset of stationary phase, and finally (5) shut down at late stationary phase.
Dynamics of flhDC operon transcription in a growth phase dependent manner
The pre-log steady state transcription of flhDC regulation is controlled by two transcription factors, RcsB and LrhA. Null mutation in any of these transcriptional regulators, promoted flhDC transcription early in the growth phase and this inhibition was maintained throughout the rest of the growth phase (exponential and stationary). We found that the effect of LrhA and RcsB was coincident with activation of transcription of their respective genes. As cell densities reached an OD of 0.2–0.3, transcription of flhDC increased. The increased flhDC transcription resulted in transcription of rflM, which in turn resulted in the feedback inhibition of flhDC transcription. This effect was consistent with the concurrent transcriptional activation of flhDC and rflM, where a surge of transcription of rflM mimicked that of flhDC and decayed quickly compared to the rest of the regulators controlling flhDC transcription. At the protein level, RflM appeared to follow the same early production and a quick decay as observed at the transcriptional level. We conclude that RflM limits flhDC transcription perhaps to efficiently control the kinetic expression of the middle and late flagellar class genes to facilitate flagellum assembly. Class 2 promoters respond differently to FlhD4C2 levels allowing the cell to control the timing of an individual class 2 operon transcription with respect to the other class 2 operons. Auto-repression at the transcriptional level has been shown to reduce relative variance and duration of fluctuations, and consequently limits noise in downstream expression [65], [66]. Expression of fliC, encoding the filament component of the flagellum, has been demonstrated to be bistable [67], [68]. We suggest that RflM would fulfill the noise reduction of flagellar class 2 and class 3 promoters transcription during exponential growth phase, by controlling class 1 flhDC operon transcription. In support of this hypothesis, a null mutation of rflM gene has been shown to increase heterogeneity of fliC expression in a cell population when compared to wild-type [21].
Once bacteria reach mid-exponential phase growth, there is a second layer of control on flhDC operon transcription. This control is positive, and is brought on by the effect of a virulence-associated transcription factor, HilD. There was a delay in the positive effect of HilD compared to the negative control exerted by RcsB, LrhA and RflM. This delayed HilD effect on flhDC operon transcription was due to the time required to activate HilD expression through FlhD4C2-dependent FliZ production. FlhD4C2 activates fliZ gene transcription from a flagellar class 2 promoter and FliZ, in turn, activates hilD expression at the post-translational level [57]. Finally, a third layer of flhDC transcription takes place and, unexpectedly, is also controlled by HilD. HilD activates the transcription of two regulatory factor genes, rtsB [17] and slyA (data not shown). RtsB and SlyA are two DNA binding regulators, which then act to inhibit flhDC transcription.
There is no doubt that flagellar motility provides a significant survival advantage over non-motile bacteria in many environmental situations. Furthermore, the link between production of flagella and other regulatory networks [69]–[72] would be affected if an unchecked production of flagella occurs. The overexpression of the flagellar regulon also attenuates Salmonella virulence [73]. These observations could explain the array of negative regulators controlling transcription of flhDC operon and keeping a check on the flagellar synthesis as well as FlhD4C2 production.
P1flhD and P5flhD are the main promoters driving flhDC transcription
While the literature reports the presence of either four or six transcription start-sites in the flhDC promoter region [13], [29], our work suggests that only the P1flhDC and P5flhDC promoters are functional in a wild-type strain under laboratory growth conditions. First, we demonstrated that there was a reduction in flhDC operon transcription in the absence of P1flhDC or P5flhDC compared to the wild-type strain (Figure 4C & D). Second, we showed that flhDC operon transcription was totally abolished in P1−P5−flhDC double mutant (Figure 4F). We confirmed that the P6flhDC promoter is active only in the absence of CRP [13]. Moreover, there was no apparent effect of P4flhDC, P3flhDC and P2flhDC promoters on flhDC transcription. In E. coli, CsrA, a carbon storage global regulator, activates flhDC expression in an RNaseE-dependent manner through protection of 5′end cleavage [23]. The 5′-UTR of the P5flhDC start-site transcript is 534 bases in length. We suspect that the presumed P3flhDC and P2flhDC start-sites resulted from RNAseE-dependent RNA-processing and/or degradation of the P5flhDC transcript. The P4flhDC start-site might also result from RNA processing; however, the isolation of mutants in the −10 region of P4flhDC that result in increased flhDC transcription suggests there might be unknown conditions where transcription from P4flhDC occurs [16].
Activation of flhDC operon transcription from the P1flhDC promoter establish two disparate regulatory loops
Genes with multiple transcription start-sites combined with auto-regulatory networks have been described in other systems. These include, Salmonella phoP, Bordetela pertussis bvgA, E.coli rrnA, and Salmonella fliAZ operon [27], [74]–[78]. These four cases bear similarity with flhDC operon transcription from P1flhDC and P5flhDC promoters. However, the case of flhDC is more elaborate, where two disparate pathways are used as feedback control. First, we demonstrated a sequential activation of P1flhDC and P5flhD transcripts that are growth phase dependent (Figure 5A). The P1flhDC promoter activating two regulatory pathways resulting in both a negative and a positive regulatory loop and each of these loops has a specific effect on the flhDC operon promoters. The negative loop starts with P1flhDC, leading to the production of FlhD4C2 that activates rflM, which in turn feedback inhibits the P1flhDC promoter (Figure 5E). The positive feedback loop is also generated from P1flhDC, where transcription of flhDC operon from P1flhDC leads to fliZ gene transcription followed by FliZ activation of hilD. HilD then activates the second flhDC transcriptional cycle from P5flhDC (Figure 5B). Paradoxically, HilD controls transcription of rtsB and slyA genes, whose products binds to the flhDC promoter region (Figure 3B) and inhibit transcription, from P1flhDC and P5flhDC, respectively (Figures 6A & B).
Importance of timing of flhDC transcription activation on motility and virulence
The three promoter classes of the flagellar regulon, class 1, class 2 and class 3; are expressed in a temporal cascade that coincides with flagellum assembly [79]. The control of flagella production is ultimately determined through the production of FlhD4C2. However, when flhDC is highly over-expressed the cells are not motile for reasons that are not understood. Thus, an intricate temporal control of gene expression and specific quantities of a functional FlhD4C2 master regulator are essential for motility. For example, the activator of type I fimbriae gene expression, FimZ, represses flhDC transcription suggesting that adherence is impeded in the presence of functional flagella. Neither deletion of flhDC nor over-expression of flhDC affect type I fimbriae gene expression suggesting that the presence of fimbriae (at wild-type levels) does not impede swimming. FlhD4C2 activity is also required in other cell processes such as Spi1 gene expression and other genes less characterized such as the srfABC operon [80], which is implicated in surfactin production and the modABC operon [80], which is involved an anaerobic respiration. This leads us to speculate that P1flhDC is required for flagella production and P5flhDC is required for growth in various environmental conditions such as in biofilms or in host cells. One possibility is that the activation of flhDC transcription from P5flhDC might represent a mechanism of protein amplification by a surge of transcription, when it is necessary to turn on the Spi1 regulatory network, even under conditions where flagella synthesis is inhibited at the level of fliA and fliC. This scenario can be very useful after infection when the bacteria requires expression of virulence factors to survive the physical and immune clearance of the eukaryotic host.
Flagella appear to be required for reaching and selecting point of entry of bacteria into host cells [81]. The low pH of the stomach will cause flagella already present to depolymerize [82]. In the intestine, the early transcription of flhDC operon from the P1 promoter provides the transcription factor, FlhD4C2 for expression of functional flagellar machinery to reassemble filaments and allow bacterial cells to swim to selected points of entry into epithelia cells. At the time of invasion, expression of both T3SS1 and flagella has been shown to be required. Thus, in the second step, the already expressed flhDC from P1flhDC promoter activates transcription of fliZ, the posttranslational regulator of HilD. In turn, HilD promotes transcription of Spi1 genes, leading to invasion. Thus P1-expressed flhDC fulfills two functions: driving the cells near the point of entry and also boosting the expression of Spi1, necessary for invasion, through its effect on HilD. It is noteworthy to mention that invasion of epithelial cells is a rapid process occurring within 10 to 15 minutes after introduction of S. typhimurium into the intestinal lumen [83]. Translocation of bacteria across the epithelial barrier and into the underlying tissue is observed within 2 hours after infection of ligated ileal loops [83], [84]. Interestingly Salmonella can replicate within two distinct intracellular environments: intravacuolar and cytosolic [85]. Once inside the host, the expression of both flagella and Spi1 appear to be downregulated but not abolished with most of the cytosolic population expressing both flagella and Spi1 at latter stage of infection. In addition, only a subset of T3SS1-induced cytosolic bacteria was motile [85]. We speculate that once bacteria invade epithelial cells, HilD activates P5flhDC and down-regulates the transcription of P1flhDC in an RtsB-dependent manner. The transcription from P5flhDC is bistable leading to two populations of cells, one is flagellated and the other is not (∼10% of cells being flagellated). This bistable expression of P5flhDC is reminiscent with the bistable expression of Spi1. We suggest that the presence of two populations inside epithelial cells could be explained by the bistability from P5flhDC promoter and the consequent downregulation of P1flhDC might represent a mechanism to limit the number of flagellated cells. The cytosolic growth of Salmonella leads to the extrusion of epithelial cells as a host defense mechanism [85]. The consequent release of the invasion-prone flagellated cells bacteria back into the mucus rich and inflamed gut endows Salmonella with a fitness advantage to use the energy-taxis mechanism to benefit from inflammation [86]. We speculate that the different timing of expression of flagellar promoters P1 and P5 and the bistable expression of P5flhDC represent a mechanism by which bacteria can disseminate and increase transmission by fecal shedding. These hypotheses are under investigation.
An additional scenario is that the transcription from P5flhDC has no effect on the synthesis of flagella but rather leads to the production of single subunits of the active transcriptional complex FlhD4C2. It has been shown that the inhibition of FlhD4C2-dependent transcription inside host cells is due to the effect of YdiV-mediated ClpXP degradation of the FlhD4C2 complex. The expression from P5flhDC late during cell growth will not allow for motility because the activation of the ClpXP leads to the degradation of the complex. However, ClpXP in addition to degrading the FlhD4C2complex also degrades the FlhC single subunit but not FlhD. This leads to the hypothesis that single FlhD or FlhC subunits might activate transcription of other genes required for virulence [87]
Conclusions
Our finding can be rationalized in terms of a model (Figure 8). Two regulatory factors, LrhA and RcsB regulate flhDC by inhibiting transcription from P1flhDC and P5flhDC. The effect of RcsB is more dominant on P1flhDC then on P5flhDC, whereas LrhA represses more strongly P5flhDC than P1flhDC. Transcription activation of P1flhDC by CRP leads to a rapid transcription of rflM, which in turn reduces transcription of P1flhDC, and limits a rapid class 2 and class 3 genes expression. The FlhD4C2 complex, already produced, allows motility to proceed and also promotes activation of HilD at the posttranslational level through FliZ, ultimately leading to activation of transcription from the P5flhDC promoter. This positive autoregulation also generates a subsequent inhibition of flhDC operon transcription, of both P1flhDC and P5flhDC promoters, by two HilD-induced regulatory factors SlyA and RtsB, themselves regulated by different environmental cues. The activation of transcription from P5flhDC would lead to higher expression of FlhD4C2. Though not necessary for motility, it could affect expression of HilD. Because, HilD is required for Salmonella survival inside host cells, this positive circle of activation might be well suited for virulence.
Fig. 8. Model depicting the flagellar and Spi1 regulatory circuitry. 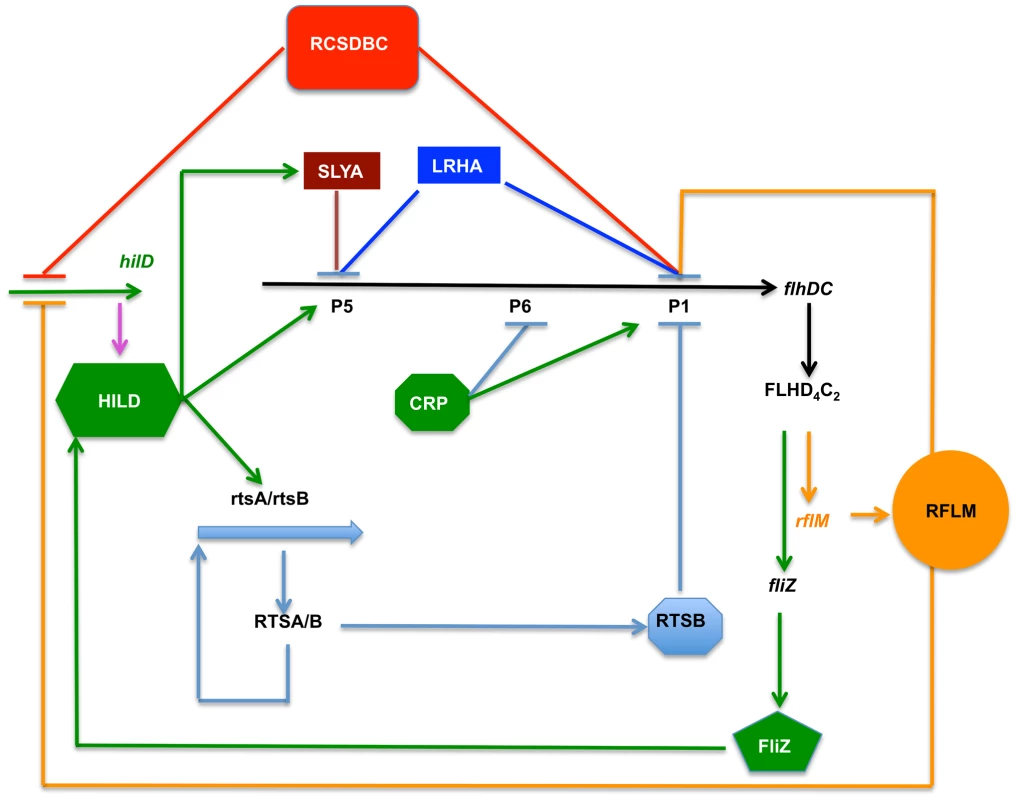
RcsB and LrhA inhibit transcription of flhDC at early cell's growth phase. These two regulatory factors inhibit transcription from the P1flhDC and P5flhDC promoters. Under proper conditions CRP activates transcription from P1flhDC. This activation produces enough FlhD4C2 to promote synthesis of flagellar proteins required for flagellum assembly and motility of Salmonella. There is a simultaneous activation of the FlhD4C2-dependent rflM gene. RflM feedback inhibits any further surge of transcription from P1flhDC. This effect limits the flhDC expression resulting in differential expression of flagellar class 2 genes. RflM transcription appears to be short-lived as there is a quick decay of rflM transcription and RflM production. The mechanism by which transcription inhibition of rflM happens is unclear. It appears that if RflM expression is maintained, P5flhDC transcription is not activated. On the other hand, FlhD4C2 promotes fliZ transcription, whose product activates HilD at the posttranslational level. HilD positively activates transcription from P5flhDC and inhibits P1flhDC transcription through the activation of rtsB. HilD also activates transcription of slyA, whose product inhibits transcription from P5flhDC. In the wild-type strain, flhDC transcription from P5flhDC does not affect motility, where only a threshold of flhDC transcription is required to promote motility. The timing at which transcription of flhDC takes place appears to be a signal for FlhDC regulation of motility. However, P5flhDC also is able to promote motility in the absence of P1flhDC and when appropriate conditions are met such overexpression of HilD that allows for an early transcription of flhDC operon from P5flhDC. Materials and Methods
Bacterial strains, primers and standard genetic manipulations
Bacterial strains and primers used in this study are listed in Table S1 and Table S2, respectively (Supplementary Information). Bacterial cells were routinely grown in Luria-Bertani (LB) broth and, when necessary, supplemented with appropriate antibiotics at the following concentrations: Kanamycin (5–10 µg/ml), tetracycline (15 µg/ml) in agar plates and for induction of T-POP 3.5 µg/ml). L-arabinose was used at 0.2% (w/v) when needed. Motility agar plates were prepared as described earlier [62]. The generalized transducing phage of S. typhimurium P22HT105/1 int-201 was used in all transductional crosses [88]
Construction of transcriptional fusions to a luciferase reporter
For the construction of strain TH18684 DUP[(PwtflhDC8093-luxCDBAE)*Km*(PwtflhDC-flhD+C+)] primers 5104 and 5103 [designed to delete the replication origin and tetracycline resistance (TcR) cassette of the plasmid pRG38 [89]] were used to amplify the kanamycin cassette of pKD3. The PCR product was electroporated into TH18710 (LT2/pKD46/pRG38) followed by selection for kanamycin resistance (KmR). KmR colonies were pooled and infected with P22 to produce a transducing lysate. This lysate was used to transduce LT2 selecting KmR. The KmR transductants were replica-plated in LB+Km and LB+Tc. Tc-sensitive (TcS) and KmR colonies should have resulted from integration of PflhDC-luxCDBAE into the chromosome generating a duplication of the promoter region of the flhDC operon. To check the integration of a single copy of PflhDC-luxCDBAE-Km and to screen for the presence of any duplication of the luxCDBAE upon integration, a set of primers [1401 (reverse for luxC) - 3091 (forward in upstream of PwtflhDC promoter region not present in the plasmid pRG38)] demonstrated the correct integration of the plasmid at the flhDC promoter region. A second PCR reaction using [Primers 267 (Km) and 1403 (luxE)] demonstrated the correct placement of Km cassette after the luciferase operon. Amplification with primers, 1403 and 1401, indicated a single copy integration of the plasmid without its origin of replication. Five candidates were obtained having a single integration of PwtflhDC-luciferase into the chromosome. One of the five candidates was sequenced and used in this study (TH18684). The Duplication of PflhDC was maintained in the presence of 5–10 µg/ml Km.
Mutations in the promoter region of PflhDC-lux were constructed using the λ-Red recombinase system, as reported previously [90], using the primers listed in Table S2. All transcriptional fusion constructs using the luciferase operon reporter used the strain TH18727: (DUP[(PflhDC8093::tetRA-luxCDBAE*Km*(PflhDCflhD+flhC+)]/pKD46) as the electroporation recipient. Individual fusion constructs with specific promoter regions were designed as follows: the rcsB promoter region included 400 bp upstream of the start codon through 230 bp of coding region, the rcsD promoter region included 466 bp upstream of the start codon through 260 bp of coding region, the slyA promoter region included 258 bp upstream of the start codon and 290 bp of the coding region, the hilD promoter region included 300 bp upstream of the start codon through 240 bp of coding region, the rtsA promoter region included 264 bp upstream of the start codon through 290 bp of coding region, the lrhA promoter region included 880 bp upstream of the start codon through 200 bp of coding region and the rflM promoter region included 460 bp upstream of the start codon through 284 bp of coding region. The promoter regions defined above were amplified by PCR using the respective primers listed in Table S2, and electroporated into strain TH18727, using the Lambda-Red recombinase system selecting for replacement of tetRA element with a PCR-amplified DNA fragment [90].
Construction of tagged proteins
Chromosomal FLAG-tagged HilD, RcsB and chromosomal HA-tagged RtsB, SlyA, RflM, HilD and LrhA were generated by the Lambda-Red recombinase system, as described previously [91] using gene-specific primer pairs, as shown in Table S2. All strains were verified by PCR amplification and DNA sequence analysis.
Growth conditions and luciferase assays
LB+Km medium containing 1% tryptone, 0.5% yeast extract, and 0.5% NaCl was used for growth of all bacterial cultures to determine the transcription activities of luciferase. Overnight cultures in LB+Km cultures were adjusted to the same OD 595 nm, then, 8-ml glass tubes containing 2 ml of LB+Km were inoculated with a 500-fold dilution of the bacterial suspensions and incubated at 30°C in a water bath with shaking at 250 rpm. For determination of luciferase activity in batch cultures, samples (200 µl) were taken at different time point and the light production along with the OD595 were measured in 96 well plates in a microplate reader (PolarStar Optima). For the determination of luciferase activity in 96 well plates, adjusted OD595 of overnight bacterial cultures at 37°C were diluted 500-fold in LB+Km and 200 µl of diluted bacteria were added to 96 well dark plates (Greiner). The plates were sealed with breathe easy membrane (to minimize evaporation and to allow growth in semi-aerobic conditions) and incubated in a chamber/shaker of a PolarStar Optima microplate reader (BMG labtech) set at 30°C. The conditions of the plate reader to determine the light production and OD 595 nm were as follow: orbital Shaking for 300 s at 150 rpm, 5 s stop and 95 s for luciferase light reading of the wells. For normalization of results a 0.1 s integration time was used. The OD 595 nm and light production (luciferase) was measured over time using a PolarStar Optima microplate reader (BMG labtech). For the background, we took the average measurements of the strain (TH18402) harboring mutations in all the promoters of flhDC. After background correction, relative light units (Arbitrary Units) were calculated by dividing the lights reading with its corresponding OD 595 nm. The OD 595 nm in our setting of the PolarStar Optima reader corresponds to ∼1.69 factor of the OD 595 nm read with 1 ml spectrophotometer.
Protein extraction and western blotting
Whole-cell extracts were prepared from samples of cultures grown in LB. 500-ml flasks containing 100 ml of LB were inoculated with a 500-fold dilution of the bacterial suspensions and incubated at 30°C in an orbital shaker at 150 rpm. Cells were collected at different optical densities (0.25, 0.4, 0.6, 0.8, 1 and 1.3) and washed twice with ice cold PBS. Pellets were lysed, at room temperature for 15 minutes, using B-PER reagent (Fisher, product #78243) with freshly added lysozyme (1 mg/ml) and protease inhibitors (Roche). The lysates were clarified by centrifugation at 4°C for 10 minutes. Supernatants were transferred to new eppendorfs and the extracted proteins were quantified using the BSA assay (BioRad). Samples, containing 50 µg of total protein per lane, were electrophoresed onto 12% to 14% Tris/SDS gels. To detect RtsA-HA a 15% Tricine-SDS gel was used as described [92]. Following transfer onto a 0.45 µm pore size polyvinylidene difluoride (PDVF) membrane (Immobilon P, Millipore) using a semidry transfer apparatus (Bio-Rad), membrane were blocked for 1 hour at room temperature with freshly prepared non-fat dry milk (5% w/v) in PBS. For detection of HA-tagged or Flag-tagged proteins, membrane blots were incubated overnight at 4°C with anti-HA (Covance) or anti-Flag M2 (Sigma) mouse monoclonal antibodies at 1∶1,000 and 1∶2,000 dilutions respectively. DnaK was detected using Anti-DnaK (Covance) diluted 1∶10,000. The blots were washed three times with PBS-T (PBS+0.1% tween) and incubated protected from light with green or red infrared dye-conjugated secondary antibody in non-fat dry milk (3% w/v) in PBS-T for 45 minutes at room temperature. Following three washes in PBS-T and one wash in PBS. Labeled proteins bands were detected using the Odyssey Infrared Imaging System (Li-COR Biosciences, Lincoln, NE, USA).
Chromatin-Immunoprecipitation (CHIP)
CHIP was performed as in [93] with modifications. Bacterial batch cultures were grown at 30°C to different ODs, at which point formaldehyde (final concentration of 1%) was added to cells. After 20 min at room temperature in an orbital shaker, cross-linking was quenched by the addition of glycine (500 mM) for 10 minutes. Samples were then placed on ice for an additional 10 minutes to complete quenching. Cells were collected by centrifugation, and washed twice with cold phosphate-buffer saline (pH 7.5). Cells pellets were resuspended in 1 ml of lysis buffer (10 mM Tris, pH 8.0, 20% sucrose, 50 mM NaCl, 10 mM EDTA, 10 µg/ml of lysozyme) and incubated at 37°C for 30 min. Following lysis, 1 ml of immunoprecipitation buffer (50 mM HEPES-KOH, pH 7.5, 150 mM NaCl, 10 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulfate) and phenylmethylsulfonyl fluoride (final concentration of 1 mM) were added. To shear cellular DNA to an average size of 500 to 1,000 bp, the cell extracts were sonicated on ice using Misonix Sonicator 3000 with a microtip at power 2 for three 10 s pulses, with 30 s rests on ice between pulses. The lysates were clarified by centrifugation and the supernatant were treated with 5 µl RNaseA (10 µg/ml) at 37°C for 30 minutes. The treated supernatant was retained for use as the input sample in the immunoprecipitation experiments. Aliquots of sheared samples were uncross-linked by incubation for 2 h at 42°C and 6 h at 65°C in 0.5× elution buffer containing freshly added 0.8 mg/ml of Proteinase K. DNA was purified using a PCR purification Kit (Bioline). An aliquot of purified DNA was run in a 1.25% agarose gel to confirm the shearing of DNA to 500–1000 bp fragments and DNA was quantified using Nanodrop spectrophotometer. An Aliquot of the input sample (2 µg) was used for each immunoprecipitation experiment. The sample was incubated with 50 µl of proteinPlus A/G beads (Santa Cruz) and 4 µl of HA monoclonal antibody (Covance) or Flag M2 antibody (Sigma) for 90 min at room temperature on a rotating wheel. An immunoprecipitation experiment without antibody was also set up as a negative control. The beads were collected by centrifugation and subsequently washed three time with immunoprecipitation buffer and once with immunoprecipitation buffer plus 300 mM NaCl, once with wash buffer (10 mM Tris-HCl, pH 8.0, 250 mM LiCl, 1 mM EDTA, 0.5% Nonidet-P40, 0.5% sodium deoxycholate) and finally with PBS buffer (pH 7.5). Immunoprecipitated complexes were then removed from the beads by treatment with elution buffer (50 mM Tris-HCl [pH 7.5], 10 mM EDTA, 1% SDS). Crosslinking of immunoprecipitated samples was reversed by incubation for 2 h at 42°C and 6 h at 65°C in 0.5× elution buffer with 0.8 mg/ml of Pronase (Roche). Prior to analysis, DNA was purified from the immunoprecipitate by using a PCR purification kit (Bioline) and resuspended in 30 µl of TE and quantified using a Nanodrop spectrophotometer. Two micrograms of the fragmented DNA, isolated from DNA-protein complexes, was used as the input in all ChIP assays. Following purification, Real-time PCRs were run on a C1000 thermal cycler (BioRad) to analyze immunoprecipitated DNA. DNA samples were used in a 20 µl reaction mix containing a 1 µM concentration of each oligonucleotide and 10 µl of 2× SYBR-Green Reaction mix. Two pairs of primers, 3569-3477 and 3753-3090 covering the promoter region of flhDC were used (Table S2). PCR conditions were as follow: Initial denaturation at 95°C for 3 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min, followed by the default melting curve program of the PCR machine. Fold-enrichments were determined by the 2−ΔCT method described in SA Biosciences User manual. To account for chromatin sample preparation differences, CHIP DNA fractions Ct values (Mean threshold cycles) were normalized (ΔCt(normalized ChIP) to the Input DNA fraction Ct values by substracting the Ct-values of the sample from the corresponding no antibody control. The percentage input of each ChIP fraction was calculated using 2(−ΔCt(normalized ChIP) and adjusted to the normalized background (No antibody) using the following formula: ΔΔCt(Chip) = ΔCt(normalized ChIP)−ΔCt(normalized NoAb). The IP fold enrichment was then calculated using 2(−ΔΔCt(ChIP/NAC)) to evaluate the fold amount of starting material of the sample applied in the real-time PCR.
Supporting Information
Zdroje
1. KojimaS, BlairDF (2004) The bacterial flagellar motor: structure and function of a complex molecular machine. Int Rev Cytol 233 : 93–134.
2. StecherB, HapfelmeierS, MullerC, KremerM, StallmachT, et al. (2004) Flagella and chemotaxis are required for efficient induction of Salmonella enterica serovar Typhimurium colitis in streptomycin-pretreated mice. Infect Immun 72 : 4138–4150.
3. StecherB, RobbianiR, WalkerAW, WestendorfAM, BarthelM, et al. (2007) Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol 5 : 2177–2189.
4. BaumlerAJ, KustersJG, StojiljkovicI, HeffronF (1994) Salmonella typhimurium loci involved in survival within macrophages. Infect Immun 62 : 1623–1630.
5. YamamotoS, KutsukakeK (2006) FliT acts as an anti-FlhD2C2 factor in the transcriptional control of the flagellar regulon in Salmonella enterica serovar typhimurium. J Bacteriol 188 : 6703–6708.
6. HungCC, HainesL, AltierC (2012) The flagellar regulator fliT represses Salmonella pathogenicity island 1 through flhDC and fliZ. PLoS One 7: e34220.
7. ChilcottGS, HughesKT (2000) Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar typhimurium and Escherichia coli. Microbiol Mol Biol Rev 64 : 694–708.
8. KomedaY, SuzukiH, IshidsuJI, IinoT (1976) The role of cAMP in flagellation of Salmonella typhimurium. Mol Gen Genet 142 : 289–298.
9. SoutourinaO, KolbA, KrinE, Laurent-WinterC, RimskyS, et al. (1999) Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J Bacteriol 181 : 7500–7508.
10. CampoyS, JaraM, BusquetsN, de RozasAM, BadiolaI, et al. (2002) Intracellular cyclic AMP concentration is decreased in Salmonella typhimurium fur mutants. Microbiology 148 : 1039–1048.
11. KellyA, GoldbergMD, CarrollRK, DaninoV, HintonJC, et al. (2004) A global role for Fis in the transcriptional control of metabolism and type III secretion in Salmonella enterica serovar Typhimurium. Microbiology 150 : 2037–2053.
12. StojiljkovicI, BaumlerAJ, HantkeK (1994) Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a fur titration assay. J Mol Biol 236 : 531–545.
13. YanagiharaS, IyodaS, OhnishiK, IinoT, KutsukakeK (1999) Structure and transcriptional control of the flagellar master operon of Salmonella typhimurium. Genes Genet Syst 74 : 105–111.
14. SperandioV, TorresAG, KaperJB (2002) Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol 43 : 809–821.
15. KoM, ParkC (2000) H-NS-Dependent regulation of flagellar synthesis is mediated by a LysR family protein. J Bacteriol 182 : 4670–4672.
16. ErhardtM, HughesKT (2010) C-ring requirement in flagellar type III secretion is bypassed by FlhDC upregulation. Mol Microbiol 75 : 376–393.
17. EllermeierCD, SlauchJM (2003) RtsA and RtsB coordinately regulate expression of the invasion and flagellar genes in Salmonella enterica serovar Typhimurium. J Bacteriol 185 : 5096–5108.
18. LehnenD, BlumerC, PolenT, WackwitzB, WendischVF, et al. (2002) LrhA as a new transcriptional key regulator of flagella, motility and chemotaxis genes in Escherichia coli. Mol Microbiol 45 : 521–532.
19. WangQ, ZhaoY, McClellandM, HarsheyRM (2007) The RcsCDB signaling system and swarming motility in Salmonella enterica serovar typhimurium: dual regulation of flagellar and SPI-2 virulence genes. J Bacteriol 189 : 8447–8457.
20. MouslimC, GroismanEA (2003) Control of the Salmonella ugd gene by three two-component regulatory systems. Mol Microbiol 47 : 335–344.
21. SingerHM, ErhardtM, HughesKT (2013) RflM Functions as a Transcriptional Repressor in the Autogenous Control of the Salmonella Flagellar Master Operon flhDC. J Bacteriol 195 : 4274–4282.
22. WeiBL, Brun-ZinkernagelAM, SimeckaJW, PrussBM, BabitzkeP, et al. (2001) Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol Microbiol 40 : 245–256.
23. YakhninAV, BakerCS, VakulskasCA, YakhninH, BerezinI, et al. (2013) CsrA activates flhDC expression by protecting flhDC mRNA from RNase E-mediated cleavage. Mol Microbiol 87 : 851–866.
24. TakayaA, MatsuiM, TomoyasuT, KayaM, YamamotoT (2006) The DnaK chaperone machinery converts the native FlhD2C2 hetero-tetramer into a functional transcriptional regulator of flagellar regulon expression in Salmonella. Mol Microbiol 59 : 1327–1340.
25. TomoyasuT, TakayaA, IsogaiE, YamamotoT (2003) Turnover of FlhD and FlhC, master regulator proteins for Salmonella flagellum biogenesis, by the ATP-dependent ClpXP protease. Mol Microbiol 48 : 443–452.
26. WadaT, TanabeY, KutsukakeK (2011) FliZ acts as a repressor of the ydiV gene, which encodes an anti-FlhD4C2 factor of the flagellar regulon in Salmonella enterica serovar typhimurium. J Bacteriol 193 : 5191–5198.
27. TakayaA, ErhardtM, KarataK, WinterbergK, YamamotoT, et al. (2012) YdiV: a dual function protein that targets FlhDC for ClpXP-dependent degradation by promoting release of DNA-bound FlhDC complex. Mol Microbiol 83 : 1268–1284.
28. WadaT, MorizaneT, AboT, TominagaA, Inoue-TanakaK, et al. (2011) EAL domain protein YdiV acts as an anti-FlhD4C2 factor responsible for nutritional control of the flagellar regulon in Salmonella enterica Serovar Typhimurium. J Bacteriol 193 : 1600–1611.
29. KrogerC, DillonSC, CameronAD, PapenfortK, SivasankaranSK, et al. (2012) The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. Proc Natl Acad Sci U S A 109: E1277–1286.
30. OhlME, MillerSI (2001) Salmonella: a model for bacterial pathogenesis. Annu Rev Med 52 : 259–274.
31. GalanJE, CurtissR3rd (1989) Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A 86 : 6383–6387.
32. EichelbergK, GalanJE (1999) Differential regulation of Salmonella typhimurium type III secreted proteins by pathogenicity island 1 (SPI-1)-encoded transcriptional activators InvF and hilA. Infect Immun 67 : 4099–4105.
33. HueckCJ (1998) Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev 62 : 379–433.
34. KimbroughTG, MillerSI (2000) Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc Natl Acad Sci U S A 97 : 11008–11013.
35. LeeCA, JonesBD, FalkowS (1992) Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci U S A 89 : 1847–1851.
36. MillsDM, BajajV, LeeCA (1995) A 40 kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol Microbiol 15 : 749–759.
37. BajajV, HwangC, LeeCA (1995) hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol 18 : 715–727.
38. DarwinKH, MillerVL (2001) Type III secretion chaperone-dependent regulation: activation of virulence genes by SicA and InvF in Salmonella typhimurium. EMBO J 20 : 1850–1862.
39. EllermeierJR, SlauchJM (2007) Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol 10 : 24–29.
40. SchechterLM, LeeCA (2001) AraC/XylS family members, HilC and HilD, directly bind and derepress the Salmonella typhimurium hilA promoter. Mol Microbiol 40 : 1289–1299.
41. MacnabRM (2004) Type III flagellar protein export and flagellar assembly. Biochim Biophys Acta 1694 : 207–217.
42. CornelisGR (2006) The type III secretion injectisome. Nat Rev Microbiol 4 : 811–825.
43. SittkaA, LucchiniS, PapenfortK, SharmaCM, RolleK, et al. (2008) Deep sequencing analysis of small noncoding RNA and mRNA targets of the global post-transcriptional regulator, Hfq. PLoS Genet 4: e1000163.
44. AltierC, SuyemotoM, LawhonSD (2000) Regulation of Salmonella enterica serovar typhimurium invasion genes by csrA. Infect Immun 68 : 6790–6797.
45. WilsonRL, LibbySJ, FreetAM, BoddickerJD, FahlenTF, et al. (2001) Fis, a DNA nucleoid-associated protein, is involved in Salmonella typhimurium SPI-1 invasion gene expression. Mol Microbiol 39 : 79–88.
46. TeplitskiM, GoodierRI, AhmerBM (2003) Pathways leading from BarA/SirA to motility and virulence gene expression in Salmonella. J Bacteriol 185 : 7257–7265.
47. JonasK, EdwardsAN, AhmadI, RomeoT, RomlingU, et al. (2010) Complex regulatory network encompassing the Csr, c-di-GMP and motility systems of Salmonella Typhimurium. Environ Microbiol 12 : 524–540.
48. BaxterMA, JonesBD (2005) The fimYZ genes regulate Salmonella enterica Serovar Typhimurium invasion in addition to type 1 fimbrial expression and bacterial motility. Infect Immun 73 : 1377–1385.
49. FortuneDR, SuyemotoM, AltierC (2006) Identification of CsrC and characterization of its role in epithelial cell invasion in Salmonella enterica serovar Typhimurium. Infect Immun 74 : 331–339.
50. EllermeierJR, SlauchJM (2008) Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J Bacteriol 190 : 476–486.
51. TeixidoL, CarrascoB, AlonsoJC, BarbeJ, CampoyS (2011) Fur activates the expression of Salmonella enterica pathogenicity island 1 by directly interacting with the hilD operator in vivo and in vitro. PLoS One 6: e19711.
52. TroxellB, SikesML, FinkRC, Vazquez-TorresA, Jones-CarsonJ, et al. (2011) Fur negatively regulates hns and is required for the expression of HilA and virulence in Salmonella enterica serovar Typhimurium. J Bacteriol 193 : 497–505.
53. SainiS, SlauchJM, AldridgePD, RaoCV (2010) Role of cross talk in regulating the dynamic expression of the flagellar Salmonella pathogenicity island 1 and type 1 fimbrial genes. J Bacteriol 192 : 5767–5777.
54. LinD, RaoCV, SlauchJM (2008) The Salmonella SPI1 type three secretion system responds to periplasmic disulfide bond status via the flagellar apparatus and the RcsCDB system. J Bacteriol 190 : 87–97.
55. KageH, TakayaA, OhyaM, YamamotoT (2008) Coordinated regulation of expression of Salmonella pathogenicity island 1 and flagellar type III secretion systems by ATP-dependent ClpXP protease. J Bacteriol 190 : 2470–2478.
56. IyodaS, KamidoiT, HiroseK, KutsukakeK, WatanabeH (2001) A flagellar gene fliZ regulates the expression of invasion genes and virulence phenotype in Salmonella enterica serovar Typhimurium. Microb Pathog 30 : 81–90.
57. ChubizJE, GolubevaYA, LinD, MillerLD, SlauchJM (2010) FliZ regulates expression of the Salmonella pathogenicity island 1 invasion locus by controlling HilD protein activity in Salmonella enterica serovar typhimurium. J Bacteriol 192 : 6261–6270.
58. WangQ, FryeJG, McClellandM, HarsheyRM (2004) Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Mol Microbiol 52 : 169–187.
59. EllermeierCD, EllermeierJR, SlauchJM (2005) HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol 57 : 691–705.
60. LaishramRS, GowrishankarJ (2007) Environmental regulation operating at the promoter clearance step of bacterial transcription. Genes Dev 21 : 1258–1272.
61. MouslimC, DelgadoM, GroismanEA (2004) Activation of the RcsC/YojN/RcsB phosphorelay system attenuates Salmonella virulence. Mol Microbiol 54 : 386–395.
62. WozniakCE, LeeC, HughesKT (2009) T-POP array identifies EcnR and PefI-SrgD as novel regulators of flagellar gene expression. J Bacteriol 191 : 1498–1508.
63. FontaineF, StewartEJ, LindnerAB, TaddeiF (2008) Mutations in two global regulators lower individual mortality in Escherichia coli. Mol Microbiol 67 : 2–14.
64. MacnabRM (2003) How bacteria assemble flagella. Annu Rev Microbiol 57 : 77–100.
65. LestasI, VinnicombeG, PaulssonJ (2010) Fundamental limits on the suppression of molecular fluctuations. Nature 467 : 174–178.
66. AlonU (2007) Network motifs: theory and experimental approaches. Nat Rev Genet 8 : 450–461.
67. CummingsLA, WilkersonWD, BergsbakenT, CooksonBT (2006) In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol Microbiol 61 : 795–809.
68. StewartMK, CummingsLA, JohnsonML, BerezowAB, CooksonBT (2011) Regulation of phenotypic heterogeneity permits Salmonella evasion of the host caspase-1 inflammatory response. Proc Natl Acad Sci U S A 108 : 20742–20747.
69. SainiS, EllermeierJR, SlauchJM, RaoCV (2010) The role of coupled positive feedback in the expression of the SPI1 type three secretion system in Salmonella. PLoS Pathog 6: e1001025.
70. CleggS, HughesKT (2002) FimZ is a molecular link between sticking and swimming in Salmonella enterica serovar Typhimurium. J Bacteriol 184 : 1209–1213.
71. GolubevaYA, SadikAY, EllermeierJR, SlauchJM (2012) Integrating global regulatory input into the Salmonella pathogenicity island 1 type III secretion system. Genetics 190 : 79–90.
72. PesaventoC, BeckerG, SommerfeldtN, PosslingA, TschowriN, et al. (2008) Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli. Genes Dev 22 : 2434–2446.
73. YangX, ThornburgT, SuoZ, JunS, RobisonA, et al. (2012) Flagella overexpression attenuates Salmonella pathogenesis. PLoS One 7: e46828.
74. ShinD, LeeEJ, HuangH, GroismanEA (2006) A positive feedback loop promotes transcription surge that jump-starts Salmonella virulence circuit. Science 314 : 1607–1609.
75. ScarlatoV, PrugnolaA, AricoB, RappuoliR (1990) Positive transcriptional feedback at the bvg locus controls expression of virulence factors in Bordetella pertussis. Proc Natl Acad Sci U S A 87 : 10067.
76. MurrayHD, ApplemanJA, GourseRL (2003) Regulation of the Escherichia coli rrnB P2 promoter. J Bacteriol 185 : 28–34.
77. WozniakCE, ChevanceFF, HughesKT (2010) Multiple promoters contribute to swarming and the coordination of transcription with flagellar assembly in Salmonella. J Bacteriol 192 : 4752–4762.
78. TanabeY, WadaT, OnoK, AboT, KutsukakeK (2011) The transcript from the sigma(28)-dependent promoter is translationally inert in the expression of the sigma(28)-encoding gene fliA in the fliAZ operon of Salmonella enterica serovar Typhimurium. J Bacteriol 193 : 6132–6141.
79. KarlinseyJE, TanakaS, BettenworthV, YamaguchiS, BoosW, et al. (2000) Completion of the hook-basal body complex of the Salmonella typhimurium flagellum is coupled to FlgM secretion and fliC transcription. Mol Microbiol 37 : 1220–1231.
80. FryeJ, KarlinseyJE, FeliseHR, MarzolfB, DowidarN, et al. (2006) Identification of new flagellar genes of Salmonella enterica serovar Typhimurium. J Bacteriol 188 : 2233–2243.
81. MisselwitzB, BarrettN, KreibichS, VonaeschP, AndritschkeD, et al. (2012) Near surface swimming of Salmonella Typhimurium explains target-site selection and cooperative invasion. PLoS Pathog 8: e1002810.
82. VegotskyA, LimF, FosterJF, KofflerH (1965) Disintegration of flagella by acid. Arch Biochem Biophys 111 : 296–307.
83. SantosRL, ZhangS, TsolisRM, BaumlerAJ, AdamsLG (2002) Morphologic and molecular characterization of Salmonella typhimurium infection in neonatal calves. Vet Pathol 39 : 200–215.
84. ReisBP, ZhangS, TsolisRM, BaumlerAJ, AdamsLG, et al. (2003) The attenuated sopB mutant of Salmonella enterica serovar Typhimurium has the same tissue distribution and host chemokine response as the wild type in bovine Peyer's patches. Vet Microbiol 97 : 269–277.
85. KnodlerLA, VallanceBA, CelliJ, WinfreeS, HansenB, et al. (2010) Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc Natl Acad Sci U S A 107 : 17733–17738.
86. Rivera-ChavezF, WinterSE, LopezCA, XavierMN, WinterMG, et al. (2013) Salmonella uses energy taxis to benefit from intestinal inflammation. PLoS Pathog 9: e1003267.
87. PrussBM, CampbellJW, Van DykTK, ZhuC, KoganY, et al. (2003) FlhD/FlhC is a regulator of anaerobic respiration and the Entner-Doudoroff pathway through induction of the methyl-accepting chemotaxis protein Aer. J Bacteriol 185 : 534–543.
88. Davis RW, DBotstein, J. RRoth and Cold Spring Harbor N.Y. Laboratory of Quantitative Biology. (1980) Advanced bacterial genetics: a manual for genetic engineering. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
89. GoodierRI, AhmerBM (2001) SirA orthologs affect both motility and virulence. J Bacteriol 183 : 2249–2258.
90. KarlinseyJE, HughesKT (2006) Genetic transplantation: Salmonella enterica serovar Typhimurium as a host to study sigma factor and anti-sigma factor interactions in genetically intractable systems. J Bacteriol 188 : 103–114.
91. UzzauS, Figueroa-BossiN, RubinoS, BossiL (2001) Epitope tagging of chromosomal genes in Salmonella. Proc Natl Acad Sci U S A 98 : 15264–15269.
92. SchaggerH (2006) Tricine-SDS-PAGE. Nat Protoc 1 : 16–22.
93. GraingerDC, OvertonTW, ReppasN, WadeJT, TamaiE, et al. (2004) Genomic studies with Escherichia coli MelR protein: applications of chromatin immunoprecipitation and microarrays. J Bacteriol 186 : 6938–6943.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule FormationČlánek Oral Bacteria and CancerČlánek A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
- Putting Fungi to Work: Harvesting a Cornucopia of Drugs, Toxins, and Antibiotics
- Mycobacteriophages: Windows into Tuberculosis
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- Five Things to Know about Genetically Modified (GM) Insects for Vector Control
- A Missing Dimension in Measures of Vaccination Impacts
- Eosinophils Are Important for Protection, Immunoregulation and Pathology during Infection with Nematode Microfilariae
- Clonality of HTLV-2 in Natural Infection
- Production, Fate and Pathogenicity of Plasma Microparticles in Murine Cerebral Malaria
- Group B Streptococcal Infection of the Choriodecidua Induces Dysfunction of the Cytokeratin Network in Amniotic Epithelium: A Pathway to Membrane Weakening
- New Insights into How Adapts to Its Mammalian Host during Bubonic Plague
- Foodborne Transmission of Nipah Virus in Syrian Hamsters
- A Polysaccharide Virulence Factor from Elicits Anti-inflammatory Effects through Induction of Interleukin-1 Receptor Antagonist
- Structural and Functional Characterization of a Complex between the Acidic Transactivation Domain of EBNA2 and the Tfb1/p62 Subunit of TFIIH
- Adaptive Gene Amplification As an Intermediate Step in the Expansion of Virus Host Range
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Hepatitis B Virus Infection and Immunopathogenesis in a Humanized Mouse Model: Induction of Human-Specific Liver Fibrosis and M2-Like Macrophages
- Crk Adaptors Negatively Regulate Actin Polymerization in Pedestals Formed by Enteropathogenic (EPEC) by Binding to Tir Effector
- Fatty Acid Biosynthesis Contributes Significantly to Establishment of a Bioenergetically Favorable Environment for Vaccinia Virus Infection
- A Cytosolic Chaperone Complexes with Dynamic Membrane J-Proteins and Mobilizes a Nonenveloped Virus out of the Endoplasmic Reticulum
- Intracellular Promote Invasive Cell Motility through Kinase Regulation of the Host Actin Cytoskeleton
- MAVS-MKK7-JNK2 Defines a Novel Apoptotic Signaling Pathway during Viral Infection
- RON5 Is Critical for Organization and Function of the Moving Junction Complex
- Immune Suppression by Neutrophils in HIV-1 Infection: Role of PD-L1/PD-1 Pathway
- and Exhibit Metabolic Symbioses
- The Herpes Virus Fc Receptor gE-gI Mediates Antibody Bipolar Bridging to Clear Viral Antigens from the Cell Surface
- Target Cell Availability, Rather than Breast Milk Factors, Dictates Mother-to-Infant Transmission of SIV in Sooty Mangabeys and Rhesus Macaques
- Evolution of the Retroviral Restriction Gene : Inhibition of Non-MLV Retroviruses
- Infection of Adult Thymus with Murine Retrovirus Induces Virus-Specific Central Tolerance That Prevents Functional Memory CD8 T Cell Differentiation
- Fha Interaction with Phosphothreonine of TssL Activates Type VI Secretion in
- In Vivo Administration of a JAK3 Inhibitor during Acute SIV Infection Leads to Significant Increases in Viral Load during Chronic Infection
- Lack of Detectable HIV-1 Molecular Evolution during Suppressive Antiretroviral Therapy
- Activation of HIV-1 from Latent Infection via Synergy of RUNX1 Inhibitor Ro5-3335 and SAHA
- A Compact, Multifunctional Fusion Module Directs Cholesterol-Dependent Homomultimerization and Syncytiogenic Efficiency of Reovirus p10 FAST Proteins
- The Role of Host and Microbial Factors in the Pathogenesis of Pneumococcal Bacteraemia Arising from a Single Bacterial Cell Bottleneck
- Genetic Dissection of Gut Epithelial Responses to
- Two-Component System Cross-Regulation Integrates Response to Heme and Cell Envelope Stress
- Oral Mycobiome Analysis of HIV-Infected Patients: Identification of as an Antagonist of Opportunistic Fungi
- A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Serovar Copenhageni
- Inflammasome Sensor NLRP1 Controls Rat Macrophage Susceptibility to
- ChIP-Seq and RNA-Seq Reveal an AmrZ-Mediated Mechanism for Cyclic di-GMP Synthesis and Biofilm Development by
- The Hypervariable Amino-Terminus of P1 Protease Modulates Potyviral Replication and Host Defense Responses
- Caspase-1-Dependent and -Independent Cell Death Pathways in Infection of Macrophages
- The Effect of Cell Growth Phase on the Regulatory Cross-Talk between Flagellar and Spi1 Virulence Gene Expression
- Different Mutagenic Potential of HIV-1 Restriction Factors APOBEC3G and APOBEC3F Is Determined by Distinct Single-Stranded DNA Scanning Mechanisms
- Oral Bacteria and Cancer
- Identification of OmpA, a Protein Involved in Host Cell Invasion, by Multi-Phenotypic High-Content Screening
- Transovarial Transmission of a Plant Virus Is Mediated by Vitellogenin of Its Insect Vector
- VE-Cadherin Cleavage by LasB Protease from Facilitates Type III Secretion System Toxicity in Endothelial Cells
- Dimerization of VirD2 Binding Protein Is Essential for Induced Tumor Formation in Plants
- Crystal Structure of the Vaccinia Virus DNA Polymerase Holoenzyme Subunit D4 in Complex with the A20 N-Terminal Domain
- Post-Translational Regulation via Clp Protease Is Critical for Survival of
- Modulation of Phagosomal pH by Promotes Hyphal Morphogenesis and Requires Stp2p, a Regulator of Amino Acid Transport
- Rotavirus Activates Lymphocytes from Non-Obese Diabetic Mice by Triggering Toll-Like Receptor 7 Signaling and Interferon Production in Plasmacytoid Dendritic Cells
- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Interferon Regulatory Factor-1 Protects from Fatal Neurotropic Infection with Vesicular Stomatitis Virus by Specific Inhibition of Viral Replication in Neurons
- HMGB1-Promoted and TLR2/4-Dependent NK Cell Maturation and Activation Take Part in Rotavirus-Induced Murine Biliary Atresia
- An Immunomics Approach to Schistosome Antigen Discovery: Antibody Signatures of Naturally Resistant and Chronically Infected Individuals from Endemic Areas
- PPARγ Agonists Improve Survival and Neurocognitive Outcomes in Experimental Cerebral Malaria and Induce Neuroprotective Pathways in Human Malaria
- A Non-Coding RNA Promotes Bacterial Persistence and Decreases Virulence by Regulating a Regulator in
- Viral OTU Deubiquitinases: A Structural and Functional Comparison
- Heterogeneity and Breadth of Host Antibody Response to KSHV Infection Demonstrated by Systematic Analysis of the KSHV Proteome
- Influenza A Virus Assembly Intermediates Fuse in the Cytoplasm
- Broadly Reactive Human CD8 T Cells that Recognize an Epitope Conserved between VZV, HSV and EBV
- Oncogenic Human Papillomaviruses Activate the Tumor-Associated Lens Epithelial-Derived Growth Factor (LEDGF) Gene
- Erythrocyte Invasion: Combining Function with Immune Evasion
- IL-1α and Complement Cooperate in Triggering Local Neutrophilic Inflammation in Response to Adenovirus and Eliminating Virus-Containing Cells
- Chronic Exposure to Type-I IFN under Lymphopenic Conditions Alters CD4 T Cell Homeostasis
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Cytomegalovirus m154 Hinders CD48 Cell-Surface Expression and Promotes Viral Escape from Host Natural Killer Cell Control
- Human African Trypanosomiasis and Immunological Memory: Effect on Phenotypic Lymphocyte Profiles and Humoral Immunity
- DHX36 Enhances RIG-I Signaling by Facilitating PKR-Mediated Antiviral Stress Granule Formation
- Conflicting Interests in the Pathogen–Host Tug of War: Fungal Micronutrient Scavenging Versus Mammalian Nutritional Immunity
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



