-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Neutrophil Elastase Causes Tissue Damage That Decreases Host Tolerance to Lung Infection with Species
Two distinct defense strategies can protect the host from infection:
resistance is the ability to destroy the infectious agent, and tolerance is the ability to withstand infection by minimizing the negative impact it has on the host's health without directly affecting pathogen burden. Burkholderia pseudomallei, the causative agent of melioidosis, is a Gram-negative intracellular bacteria that is categorized as a potential bioterrorism agent. Using murine models, we previously demonstrated that during B. pseudomallei infection, production of IL-1β is deleterious as it recruited excessive neutrophils to the site of infection. In the present work, we focused on the detrimental role of neutrophils during infection with B. pseudomallei and B. thailandensis. Here, we demonstrate that the excessive recruitment of neutrophils to the site of infection causes tissue damage because of release of the protease elastase. Mice lacking neutrophil elastase have increased survival even though they carry an equal amount of bacteria in their organs as compared to the wild-type C57BL/6J. Thus, neutrophil elastase is a host defense mechanism that causes tissue damage and reduces host tolerance to infection.
Published in the journal: Neutrophil Elastase Causes Tissue Damage That Decreases Host Tolerance to Lung Infection with Species. PLoS Pathog 10(8): e32767. doi:10.1371/journal.ppat.1004327
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004327Summary
Two distinct defense strategies can protect the host from infection:
resistance is the ability to destroy the infectious agent, and tolerance is the ability to withstand infection by minimizing the negative impact it has on the host's health without directly affecting pathogen burden. Burkholderia pseudomallei, the causative agent of melioidosis, is a Gram-negative intracellular bacteria that is categorized as a potential bioterrorism agent. Using murine models, we previously demonstrated that during B. pseudomallei infection, production of IL-1β is deleterious as it recruited excessive neutrophils to the site of infection. In the present work, we focused on the detrimental role of neutrophils during infection with B. pseudomallei and B. thailandensis. Here, we demonstrate that the excessive recruitment of neutrophils to the site of infection causes tissue damage because of release of the protease elastase. Mice lacking neutrophil elastase have increased survival even though they carry an equal amount of bacteria in their organs as compared to the wild-type C57BL/6J. Thus, neutrophil elastase is a host defense mechanism that causes tissue damage and reduces host tolerance to infection.Introduction
Although the inflammatory response elicited by infection is highly effective against microbes, it is also greatly pathogenic and, in extreme cases, the damage caused to host tissues becomes the main source of morbidity and mortality associated with the disease [1]. After infection has occurred, the host can protect itself using mainly two distinct strategies: resistance or tolerance (reviewed in [2]–[4]. Resistance relates to the host's ability to destroy and eliminate the infectious agent. In contrast, tolerance does not directly affect pathogen burden, but rather it is a measure of the host's ability to withstand the presence of the infectious agent by minimizing the negative impact it has on the host's health. Much of what is known about the protective response to infection concerns resistance mechanisms and clearly falls in the realm of immunology. In contrast, the phenomenon of tolerance to infection in animals has remained largely unexplored, and only recently has begun to attract the attention of immunologists and microbiologists.
Burkholderia pseudomallei is a Gram-negative flagellate bacterium that causes melioidosis, a disease endemic to South-East Asia and other tropical regions [5]. Because melioidosis carries a high fatality rate, B. pseudomallei is classified as category B potential bioterrorism agent by the CDC and NIAID. B. pseudomallei infection can be contracted through ingestion, inhalation, or subcutaneous inoculation, and can lead to a broad-spectrum of disease forms including pneumonia, septicemia, and organ abscesses. Following infection of macrophages and other non-phagocytic cell types, B. pseudomallei is able to escape the phagosome and replicate in the host cell cytoplasm. Using a murine model of melioidosis, we have recently shown that the inflammasome component NLRP3 mediates caspase-1-dependent production of IL-1β and IL-18 while NLRC4 mediates pyroptosis [6]. While IL-18 and pyroptosis were equally important for protection from melioidosis, IL-1β was found to have a deleterious role. Recent studies have confirmed the deleterious role of IL-1β in melioidosis [7], [8].
To understand the reason for the detrimental effect of IL-1β during melioidosis, we have now focused our attention on the role of neutrophils and the potentially damaging enzyme neutrophil elastase (NE). Our results show that NE-deficient mice had an improved survival compared to C57BL/6J mice infected with B. thailandensis or B. pseudomallei. Surprisingly, bacterial burdens were comparable between C57BL/6J and NE-deficient mice, but lung tissue damage was reduced in NE-deficient mice. Taken together, our results suggest that NE is a host effector mechanism that negatively impacts host tolerance to infection with Burkholderia species.
Results
IL-1β Is Deleterious during Infection with B. thailandensis and B. pseudomallei
Although not pathogenic to humans, B. thailandensis causes morbidity and mortality in mice, shares several of the virulence factors of B. pseudomallei, and is often used as a model for melioidosis [9], [10]. Confirming what we observed during infection with B. pseudomallei [6], production of IL-1β following infection with B. thailandensis was dependent on NLRP3, ASC, and caspase-1 while pyropotosis was dependent on NLRC4 (supplementary figure S1A–D).
Similar to our [6] and others [11] studies with B. pseudomallei, IL-18-deficient mice were more susceptible than C57BL/6J wild type mice to B. thailandensis infection (fig. 1A) and had higher bacteria burdens in organs (fig. 1C). In contrast, mice deficient in IL-1 receptor (Il-1r1−/−) were more resistant to infection with B. thailandensis (fig. 1B) and showed reduced organ bacteria burdens (fig. 1C). Mice deficient in both IL-18 and IL-1RI (DKO) were as susceptible as C57BL/6J, suggesting that the deleterious role of IL-1β counteracts the protective role of IL-18. As shown in figure 1B and D, the IL-1RI-mediated deleterious role during B. thailandensis infection was due to the action of IL-1β, not IL-1α, although both cytokines are produced during infection [6]. The increased survival and lower bacterial burden observed in Il1-r1−/− mice correlated with reduced neutrophil influx in the alveolar spaces, reduced levels of myeloperoxidase and elastase in lung homogenates (fig. 1E), and lower inflammation in the lung (fig. 1F), suggesting that neutrophil recruitment to the lungs is as deleterious in this infection model as it is during B. pseudomallei infection [6]. These results support the validity of B. thailandensis as a model to study the role of IL-1 and neutrophils in melioidosis. It is well established that activated neutrophils release proteases and toxic radicals that may cause immunopathology and damage to host tissue. In fact, reduced numbers of neutrophils in the BALF of Il1-r1−/− mice correlated with decreased host tissue damage as measured by lower amount of collagen and IgM in the BALF of these mice (fig. 1G).
Fig. 1. Deleterious role of IL-1β during infection with B. thailandensis. 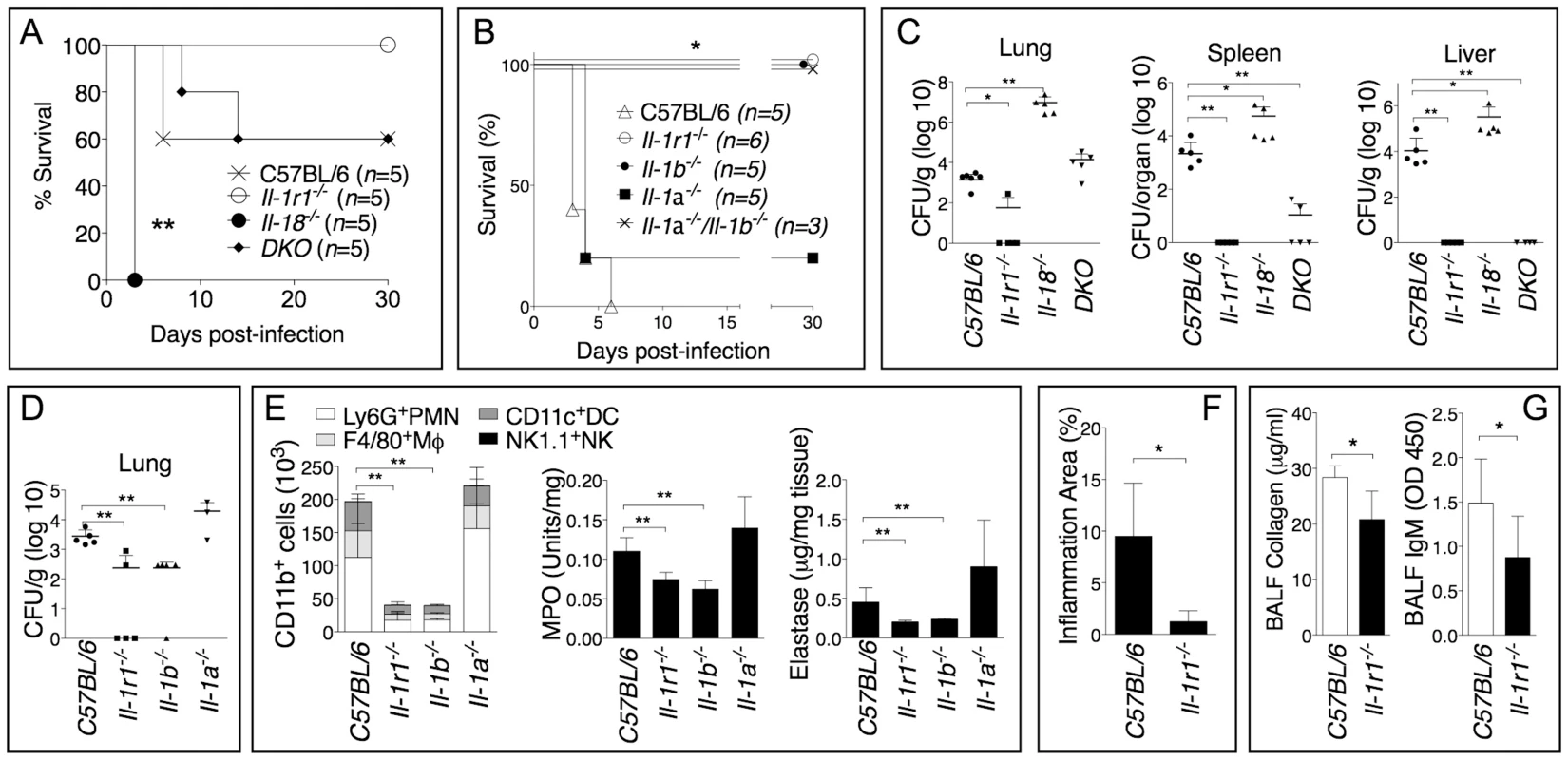
Mice were infected intranasaly with B. thailandensis (A, 5×104 CFU or B, 5×105 CFU) and their survival was monitored. (C, D) Mice infected with B. thailandensis (5×105 CFU) were sacrificed 72 h post-infection and the organs bacterial burden in the indicated tissues was measured. (E) Flow cytometric analysis of BAL cells and MPO and elastase activity levels in the lung homogenates obtained from the indicated mouse strains at 72 h p.i. (n = 5 for all except Il-1a−/− n = 3). (F) The total area of the inflammatory nodules of lung sections stained with H&E was measured and expressed as percentage of the total lung lobe area (n = 5). (G) IgM and collagen were measured in the BALF collected 72 h p.i. from mice (n = 5) infected with B. thailandensis (5×105 CFU). Data are expressed as mean + S.D. *p<0.05, **p<0.01. (A, B) log rank Kaplan-Meier test, (C–G) Mann-Whitney U test. DKO is Il-1r1−/−/Il-18−/−double deficient mouse. One representative experiment of three (A–C, F, G) or one (D–E) is shown. Neutrophil Elastase Decreases Host Tolerance to Infection with B. thailandensis and B. pseudomallei
To determine which of the potentially damaging effector mechanisms account for the deleterious role of neutrophils during infection with B. thailandensis we analyzed mice that lack expression of the ROS-generating enzyme NADPH oxidase (gp91phox), the inducible nitric oxidase (Nos2), or the NE (Elane). Previous works have shown that gp91phox−/− mice are more susceptible while Nos2−/− mice are more resistant to melioidosis [12], [13]. In order to detect a protective effect in these mouse strains, we infected them with a dose of B. thailandensis that was lethal to C57BL/6J mice (fig. 2A). At this dose, the survival of gp91phox−/− and Nos2−/− mice was not significantly different from that of C57BL/6J mice. In contrast, Elane−/− mice were completely protected from mortality. Although some level of protection was observed in Nos2−/− mice, in agreement with previous work [12], their survival was significantly lower (p<0.05) than that of Elane−/− mice suggesting that the deleterious role of NE is much more significant than that of iNOS. Bacteria could not be recovered from organs of Elane−/− mice that survived infection. Combined administration of sivelestat, a selective NE inhibitor, and the serine protease inhibitor BBI to infected C57BL/6J mice rescued their survival (fig. 2B), reinforcing the notion that NE activity is detrimental in melioidosis. Neutrophil recruitment to the lung and alveolar spaces and the overall extent of inflammation were not affected by the absence of NE (fig. 2C and supplementary figure S2). Quite surprisingly, C57BL/6J and Elane−/− mice had comparable bacterial burdens in their organs (fig. 2D). This was true even when mice were infected with a dose of bacteria that was lethal for both strains (fig. 2E). Thus, the higher health of Elane−/− mice, despite bacteria burdens similar to those of C57BL/6J mice, indicates that the absence of NE increases tolerance rather than resistance to B. thailandensis. It is worth noting that the previously reported protection of Nos2−/− mice was associated with decreased bacterial burdens [12] suggesting a resistance mechanism. In mammals, several genes encode for elastases, and the matrix metalloprotease MMP12 is the main elastase expressed in macrophages, a cell type that is infected by B. thailandensis and plays a prominent role in protection from melioidosis [14]. Interestingly, Mmp12−/− mice were not protected from infection with B. thailandensis (supplementary fig. S3).
Fig. 2. Neutrophil elastase reduces host tolerance to infection with B. thailandensis. 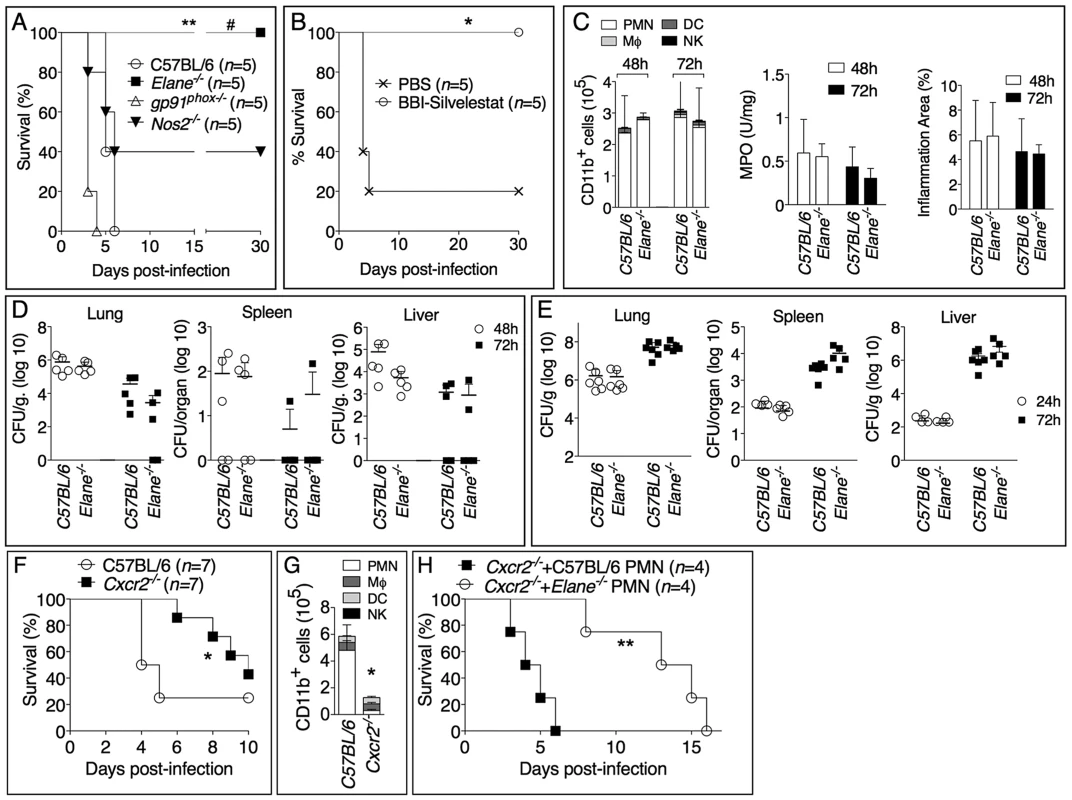
(A) Mice were intranasaly infected with 5×105 CFU B. thailandensis and their survival was monitored. (B) Elastase inhibitor sivelestat (125 µg/mouse, i.n.) and serine protease inhibitor BBI (3 mg/mouse, oral gavage) were administered on day 1, 2, 3 p.i. to C57BL/6 intranasaly infected with 1×105 CFU B. thailandensis. (C) Flow cytometric analysis of BAL cells, levels of MPO activity in lung homogenates, and total area of the inflammatory nodules for mice infected with 5×105 CFU B. thailandensis (n = 5). (D, E) Organs bacterial burden in the indicated tissues of mice infected with 5×105 CFU (D) or 106 CFU (E) B. thailandensis. (F) Wild type and Cxcr2−/− mice (n = 7, pool of two experiments) were intranasaly infected with 2×105 CFU B. thailandensis and survival and BALF myeloid cells composition (G) (n = 4 C57BL/6J, n = 5 Cxcr2−/−) were measured. (H) Cxcr2−/− mice (n = 4) were intranasaly infected with 2×105 CFU B. thailandensis and their survival was monitored. C57BL/6J or Elane−/− neutrophils (1.5×106) were adoptively transferred by intravenous injection 24 and 96 hours p.i. Data are expressed as mean +S.D.*p<0.05, **p<0.01. # p<0.05 Elane−/− respect to Nos2−/−.(A, B, F, H) log rank Kaplan-Meier test, (C, D, E, G) Mann-Whitney U test. One representative experiment of three (A, C, D, E, G) or two (B, F, H) is shown. To confirm the deleterious role of neutrophils during B. thailandensis infection, we performed parallel studies in mice deficient in expression of CXCR2, the chemokine receptor most critical for neutrophil recruitment to infection sites. As expected, neutrophil recruitment to the alveolar spaces was markedly reduced in Cxcr2−/− mice compared to C57BL/6J mice following infection with B. thailandensis (fig. 2G). Cxcr2−/− mice were also significantly less susceptible than C57BL/6J mice when infected with a dose that killed 72% of C57BL/6J mice (fig. 2F). This is a remarkable result considering that absence of CXCR2 is known to negatively impact the overall health status of the mice, which in fact appear runt and are more susceptible to several bacterial infections [15]–[17]. These findings were in agreement with our and other's previous observations that neutrophils are not particularly effective at controlling B. thailandensis or B. pseudomallei [6], [18]–[20], although a protective effect for these cells has also been reported [21]. Because of the defective neutrophil chemotaxis, Cxcr2−/− mice provided a fitting model to test the role of NE with the prediction that C57BL/6J or Elane−/− neutrophils, adoptively transferred into Cxcr2−/− mice, would be able to be recruited to the lung, following infection with B. thailandensis, and exert their deleterious effects. Indeed, as shown in figure 2H, transfer of C57BL/6J neutrophils significantly shortened the mean time to death of the recipient Cxcr2−/− mice, a result consistent with a deleterious role for NE. In contrast, mice that received NE-deficient neutrophils showed a significantly longer survival. The fact that their survival was even longer than that of Cxcr2−/− mice that did not receive neutrophils may suggest that, in absence of elastase, neutrophils may play a protective role in melioidosis. This notion is in agreement with published works [21] and is supported by the observation that a single intranasal transfer of either wild type or NE-deficient neutrophils into C57BL/6 mice 18 hours p.i. significantly decreased organ bacterial burdens (supplementary figure S4A). However, multiple transfers of wild type neutrophils into infected Elane−/− mice increased lung tissue damage as well as bacteria burden (supplementary figure S4B, C). Collectively, these experiments support the notion that, in the early phase of the infection, and in limited number, neutrophils play a protective role against lung infection with B. thailandensis. In contrast, excessive and sustained neutrophil recruitment to the lung becomes deleterious due to the damaging action of NE and because of neutrophil's inability to restrain intracellular bacteria replication [6], [18]–[20].
To further confirm our findings, we examined the role of NE during infection with the significantly more virulent agent B. pseudomallei and observed an identical scenario. The majority of C57BL/6J mice intranasaly infected with B. pseudomallei succumbed to infection whereas Elane−/− mice showed complete protection (fig. 3A). Both groups of mice lost weight to the same extent during the first 72–96 hours post infection, but only the Elane−/− mice (and a single C57B/L6J survivor) started to gain their weight back thereafter, suggesting that the initial wasting response to infection is not responsible for the susceptibility of C57BL/6J mice. Interestingly, weight loss in Il-1r1−/− mice infected with B. pseudomallei is much less prominent than in C57BL/6 mice (supplementary figure S5), suggesting that IL-1 deleterious role is not exclusively mediated by NE. Neutrophil recruitment and overall inflammation were comparable between C57BL/6J and Elane−/− mice (fig. 3B, C). Remarkably, C57BL/6J and Elane−/− mice had similar bacteria burdens in the lung, spleen, and liver (fig. 3D).
Fig. 3. Neutrophil elastase reduces host tolerance to infection with B. pseudomallei. 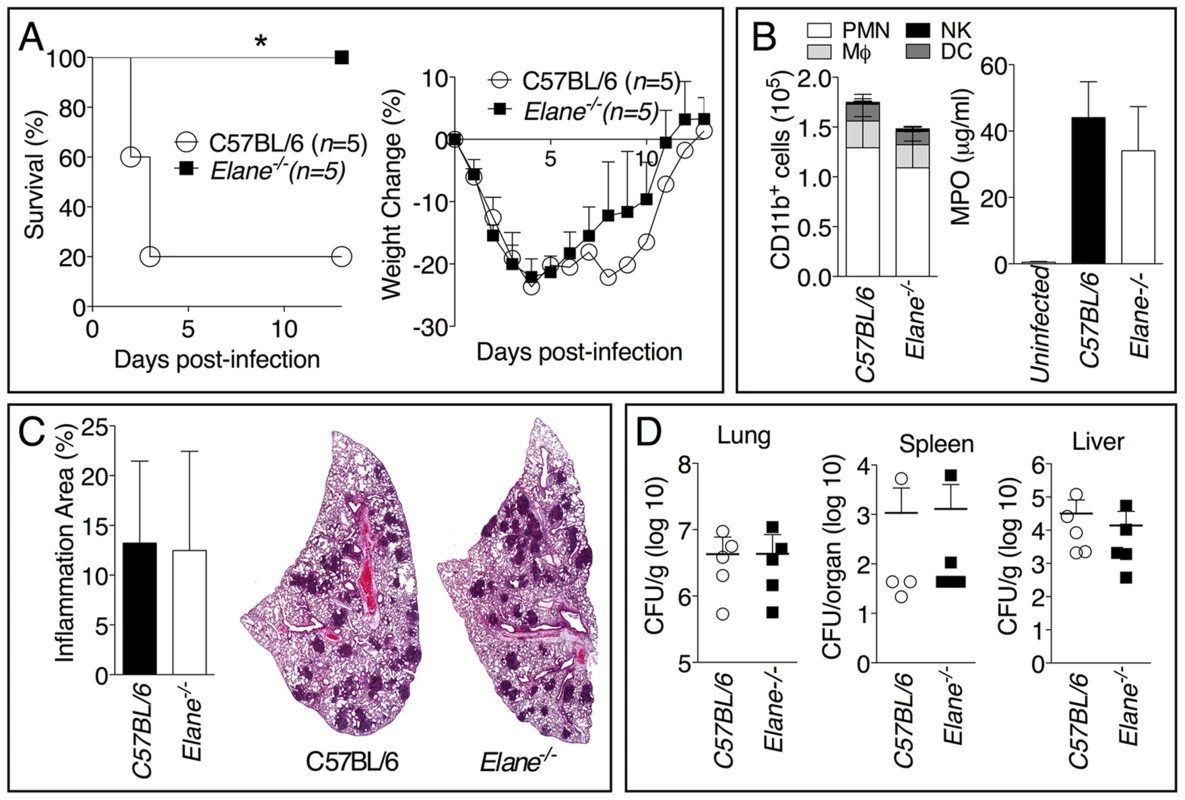
Mice (n = 5) were intranasaly infected with 400 CFU B. pseudomallei and their survival and weight were monitored (A). (B) Flow cytometric analysis of BAL cells and levels of MPO in BALF collected 72 h p.i. from mice (n = 5) infected as in A. (C) Representative lung sections stained with H&E and the total area of the inflammatory nodules was measured and expressed as percentage of the total lung lobe area (n = 5). (D) Tissue burdens of B. pseudomallei at 72 h p.i. Data are expressed as mean + S.D. *p<0.05. (A) log rank Kaplan-Meier test, (B–D) Mann-Whitney U test. It has been shown that during highly neutrophilic inflammation, proteases released by neutrophils, including NE, are able to process pro-IL-1β [22]–[24]. It is therefore conceivable that NE deficiency may protect mice from melioidosis because of decreased processing of IL-1β. However, C57BL/6J and Elane−/− neutrophils released equal amount of IL-1β when infected in vitro with B. thailandensis (fig. 4A) and mature IL-1β and IL-18 were present in equal amount in the BALF of Elane−/− or C57BL/6J mice infected with B. thailandensis or B. pseudomallei (fig. 4B, C). Absence of NE did not affect pyropoptosis (fig. 4D), ruling out modulation of this protective mechanism as the reason for the protective effect. The level of other pro-inflammatory cytokines and acute phase proteins in BALF or serum of Elane−/− mice were reduced, though not significantly, a possible reflection of increased release of endogenous alarmins (fig. 5).
Fig. 4. Role of elastase in IL-1β and IL-18 processing and pyroptosis. 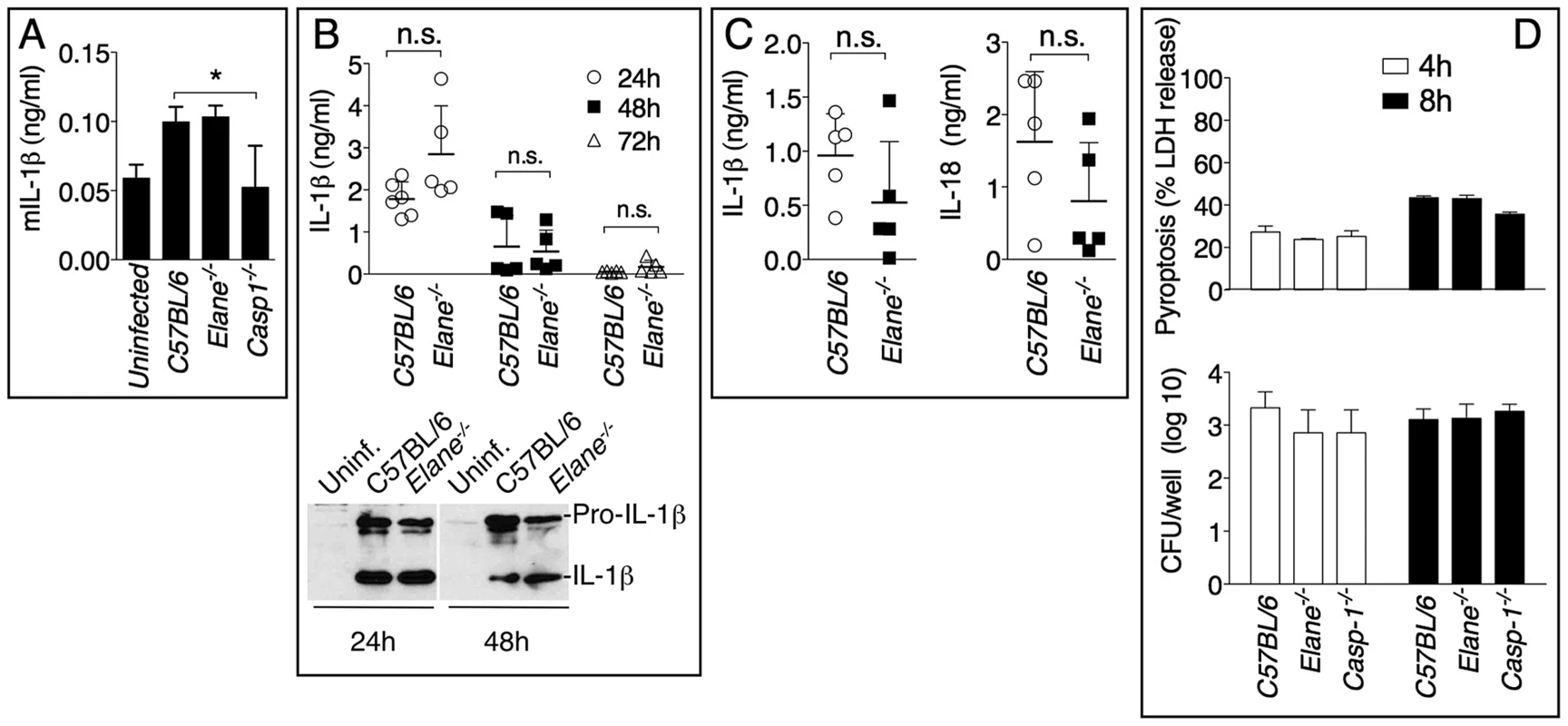
(A) Neutrophils were purified from bone marrow and infected in vitro with B. thailandensis 1∶100 m.o.i., and release of IL-1β was measured. (B, C) IL-1β and IL-18 were measured in BALF of mice infected with 5×105 CFU B. thailandensis (B) or 400 CFU B. pseudomallei (C). (D) Pyropoptosis and intracellular bacteria replication in neutrophils infected in vitro as in (A). Data are expressed as mean + S.D. *p<0.05. (A, D) One way ANOVA Tukey Post-test, (B, C) Mann-Whitney U test. One representative experiment of three (A, B, D) or one (C) is shown. Fig. 5. Inflammatory cytokines and acute phase proteins in BALF and sera of infected mice. 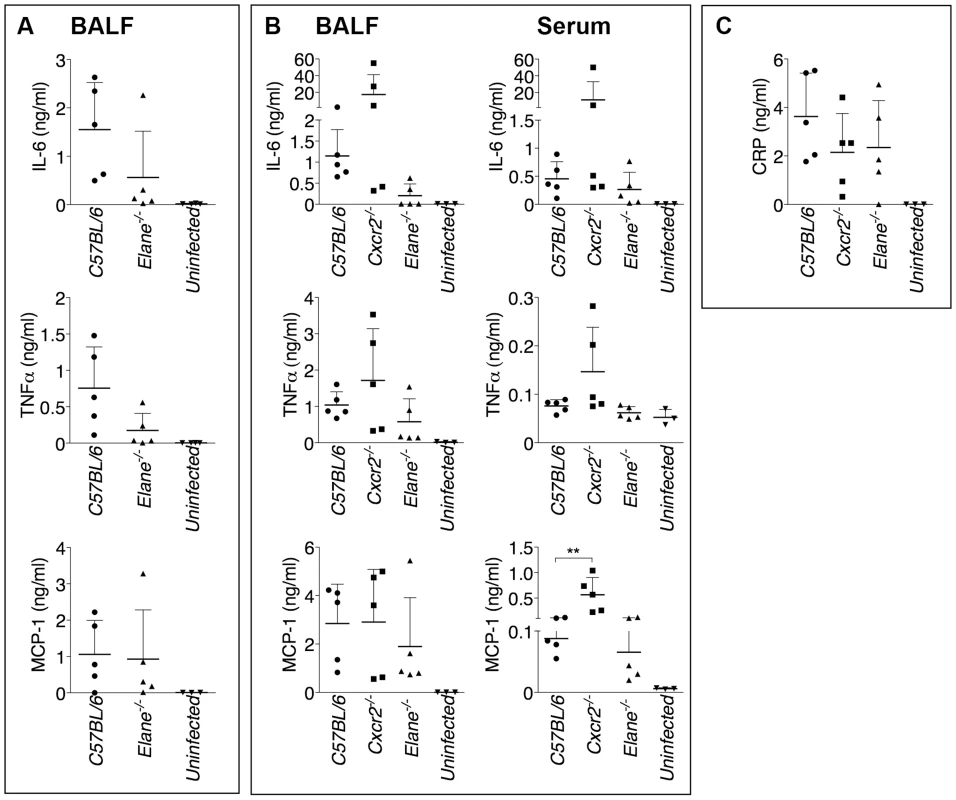
Cytokines were measured in BALF of mice infected with 400 CFU B. pseudomallei (A) or in BALF and serum of mice infected with 5×105 CFU B. thailandensis (B) 72 hours p.i. (C) Levels of C Reactive Protein in BALF of mice infected with 5×105 CFU B. thailandensis. Data are expressed as mean + S.D. **p<0.01. Mann-Whitney U test. One representative experiment of two is shown. Neutrophil Elastase Causes Lung Tissue Damage and Increases Vascular Leakage
Minimization of immune-mediated tissue damage is likely among the most effective strategies to increase host tolerance to infection. In agreement with this notion, significantly reduced tissue damage was measured in Elane−/− mice infected with B. thailandensis or B. pseudomallei compared to C57BL/6J mice as demonstrated by lower levels of IgM, collagen, and total protein in BALF (fig. 6A, B) and decreased extravascular leakage of intravenously administered Evans blue (fig. 6C). Elane−/− mice also had decreased BALF levels of surfactant protein D, another marker of tissue damage (supplementary figure S6) [25]–[27]. Reg3γ, a protein with antibacterial and regenerative functions released by epithelial cells in response to infection and damage [28], and the alarmin HMGB1 were also detected in higher amounts in the BALF of C57BL/6J than Elane−/− mice, consistent with the increased tissue damage. These findings were consistent with the conclusion that C57BL/6J mice experienced more tissue damage than the Elane−/− mice as a consequence of Burkholderia infection. Although the overall extent of inflammation was similar in infected C57BL/6J and Elane−/− mice (fig. 2 and 3), examination of H&E stained lung sections at high magnification revealed extensive hemorrhage indicating injury to the alveolar capillary endothelium and increased interstitial edema in C57BL/6J mice compared to Elane−/− or Il-1r1−/− mice. Image processing analysis was used to measure interstitial edema revealing that in C57BL/6J mice the alveolar septa are significantly thicker than in Elane−/− or Il-1r1−/− mice (fig. 6D, E arrow heads). Reduced elastic fiber staining was also observed in C57BL/6J mice but not in Elane−/− or Il-1r1−/− mice (fig. 6F), suggesting that NE-mediated degradation of alveolar basement membrane contributes to lung tissue damage.
Fig. 6. Neutrophil elastase causes lung tissue damage during infection with Burkholderia species. 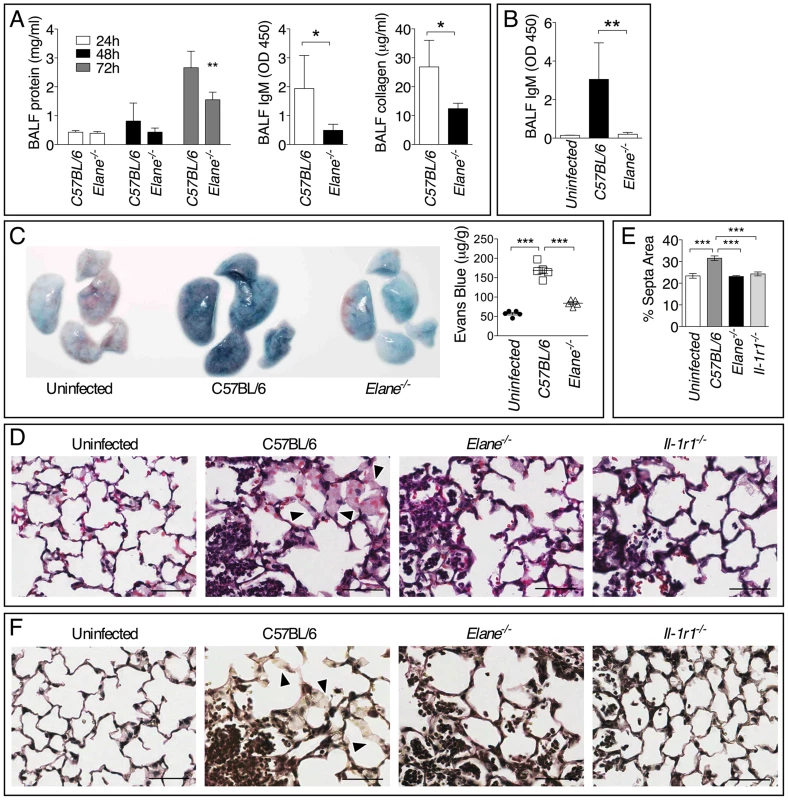
Total protein, IgM, and collagen were measured in the BALF collected at 72 h p.i. from mice infected with 5×105 CFU B. thailandensis (n = 5) (A) or 400 CFU B. pseudomallei (n = 5) (B). (C) Extravascular leakage of intravenously administered Evans blue in mice infected with B. thailandensis as in (A) was measured 72 h p.i in the lung tissue (µg/g tissue). (D) Representative photographs of histological sections of lung tissues stained with H&E. Alveolar septa are significantly thicker in C57BL/6J than in Elane−/− or Il-1r1−/− mice (arrow heads). (E) Area of alveolar septa was measured by Image J software. (F) Verhoeff-van Gieson stain for elastic fibers. Reduced elastic fiber staining was observed in C57BL/6J mice but not in Elane−/− or Il-1r1−/− mice (arrow heads). Bar is 50 µm. Data are expressed as mean + S.D. *p<0.05, **p<0.01, ***p<0.001. Mann-Whitney U test. One representative experiment of three (A, C–F) or one (B) is shown. Bradykinin Contributes to NE-Mediated Lung Damage and Decreased Host Tolerance
Consistent with the decreased vascular leakage observed in Elane−/− mice, their BALF contained reduced levels of bradykinin (fig. 7A), a peptide that is critically involved in regulating vascular permeability and leakage. Treatment of infected mice with the bradykinin antagonist HOE-140 significantly increased mice survival (fig. 7B) and decreased vascular leakage (fig. 7C) but, more importantly, did not affect bacteria burden (fig. 7D). These results suggest that one of the mechanisms through which NE decreases tolerance to Burkholderia infections is by increasing generation of bradykinin and consequent vascular leakage.
Fig. 7. Bradykinin contributes to NE-mediated lung damage and decreased host tolerance. 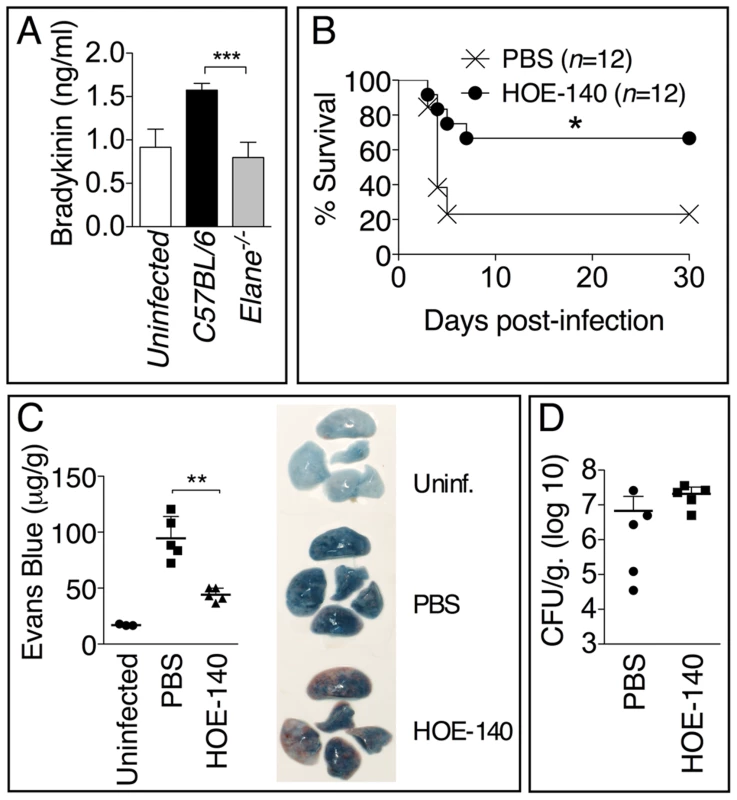
(A) Levels of bradykinin were measured 72 h p.i. in the BALF of mice (n = 5) infected with 5×105 CFU B. thailandensis. (B) Mice (n = 12) infected with 5×105 CFU B. thailandensis were administered bradykinin antagonist HOE-140 (10 µg i.n., daily) and their survival monitored. Results pooled from three experiments are shown. (C) Extravascular leakage of intravenously administered Evans blue (µg/g tissue) and bacteria burdens (D) were measured at 72 h p.i. Data are expressed as mean + S.D. *p<0.05, **p<0.01, ***p<0.001. (A, C, D) Mann-Whitney U test. (B) log rank Kaplan-Meier test. One representative experiment of three (A) or two (C, D) is shown. Discussion
The ability of a host to survive an infection depends on resistance mechanisms that eliminate the infectious agent, and on tolerance mechanisms that minimize the negative impact the infection has on the host's health without directly affecting pathogen burden [2], [3]. Our understanding of the mechanisms that regulate host tolerance to infection is still rudimentary. Early evidence that tolerance mechanisms existed in mammals was obtained by studying Plasmodium-infected mice with the observation that disease severity does not directly correlate with the parasite burden [29]. Using a similar mouse model of malaria, it has also been demonstrated that sickle hemoglobin, whose prevalence is elevated in areas of high malaria incidence, increases host tolerance to Plasmodium and, therefore, protects those who carry this mutation from severe malaria [30]. Sickle hemoglobin was shown to increase expression of heme oxygenase-1, an enzyme that converts the free heme into antioxidant molecules that are protective against oxidative damage caused by severe malaria. Heme oxygenase-1 also prevented a pathological immune response without affecting the parasite burden. A recent study has shown that promotion of tissue repair through administration of amphiregulin, an EGF family member that plays a role in maintaining lung homeostasis, can protect mice co-infected with influenza A virus and Legionella pneumophila [31]. The observed protective effect was not due to changes in pathogen burden, suggesting it affected tolerance rather than resistance mechanisms. Interestingly, in this model excessive inflammation and immunopathology did not appear to be the cause of morbidity and mortality. Analysis of resistance and tolerance mechanisms is often complicated because some host factors may control and participate simultaneously in both types of responses, as it has been proposed for the enzyme Chi3l1 during Streptococcus pneumoniae infection [32].
While neutrophils are critical for protection against several bacterial and fungal infections, their role in melioidosis remains unclear. We and others have shown that neutrophils are not very effective at containing B. pseudomallei infection and, in fact, may foster its systemic dissemination [6], [18]–[20]. Our results show that excessive PMN recruitment to the lung decrease host tolerance to melioidosis because of the damaging action of NE on lung tissue. However, our results also suggest that, in limited amounts and in absence of NE, neutrophils may play a protective role in melioidosis, in agreement with published work [21].
Our observation that NE plays a detrimental role in melioidosis is surprising because several reports have shown that this protease is crucially involved in defense against bacterial pathogens through various mechanisms including direct killing [33], degradation of virulence factors [34], or regulation of the inflammatory response by targeting cytokines, chemokines and their receptors [35], [36]. NE has also been shown to be crucial for deployment of neutrophil extracellular traps [37], an important antimicrobial effector mechanism [38]. NE-deficiency however, can also be beneficial during infections, as demonstrated by the fact that Elane−/− mice are protected from anthrax toxin [39] and that NE deficiency did not affect infection with B. cepacia complex [40], again suggesting that neutrophils and NE are not effective antimicrobial agents against Burkholderia species. Whether NE's effects on host's tolerance are due to direct action on the bacteria remains to be determined, but we were unable to obtain data supporting this possibility. It should be mentioned that any direct action of NE on bacteria would likely affect bacteria's proliferation/virulence, a scenario that contrasts with our observation that the protection conferred by NE deficiency is not associated with reduced bacteria burdens suggesting that NE is not targeting the bacterium but rather the host.
Although our results support a model where IL-1β is deleterious because of excessive recruitment of neutrophils and the subsequent NE release, it was surprising to find out that different defense mechanisms account for the protection in Il-1r1−/− mice compared to Elane−/− mice. Thus, despite bacteria burdens similar to those of C57BL/6J mice, Elane−/− mice have higher health indicating that the absence of NE increases tolerance rather than resistance to B. thailandensis and B. pseudomallei. In contrast, the higher health of Il-1r1−/− mice may involve both resistance and tolerance mechanisms; the decreased organ bacterial burdens observed in Il-1r1−/− mice is consistent with increased resistance while the decreased neutrophil influx, NE levels, and tissue damage are consistent with increased tolerance. Taken together, these results suggest that the deleterious role of IL-1β is not solely mediated by NE, even though NE absence is sufficient to confer protection by enhancing tolerance.
NE is known to cause host tissue damage leading to functional impairment of multiple organs, including the lungs [41], [42]. The deleterious action of NE has been best documented during experimental models of acute lung injury [43], [44] where this enzyme has been shown to inflict tissue damage by direct degradation of extracellular matrix components, adhesion molecules, antimicrobial and protective host factors such as the protein SPLUNC1 [45], mucins [46], surfactant protein A and D [47], and various cytokines. NE is a promiscuous protease, which suggests that multiple mechanisms may account for its negative impact on host tolerance to melioidosis. In our experiments we observed decreased collagen degradation in the BALF of Elane−/− mice compared to controls and decreased elastic fiber staining suggesting that NE may directly damage the lung connective tissues. The decreased level of bradykinin and the consequent diminished vascular leakage observed in Elane−/− mice suggests that generation of bradykinin may be an additional mechanism through which NE can exert its deleterious effect. NE and elastases of bacterial origin have been shown to be able to process kininogen and generate bradykinin [48], [49]. Alternatively, the damage caused by NE to the endothelium of alveolar capillaries may lead to activation of the contact system and generation of bradykinin [50].
Our attempts to obtain evidence that NE may degrade factors important for lung physiology, such as surfactants, were inconclusive. However, we found that proteins that exert antimicrobial and protective functions such as SP-D and Reg3γ were present in higher amounts in the BALF of C57BL/6J compared to Elane−/− mice. Induction of these factors is part of the physiologic response to lung damage and likely reflects the increased tissue damage inflicted by NE in C57BL/6J mice. Our study shows that neutrophil recruitment to the lung is not affected by NE deficiency, as previously shown [51]. Similarly, the overall extent of inflammation and the level of several proinflammatory cytokines, including IL-1β and IL-18, were not significantly different in presence or absence of NE. Production of mature IL-1β by infected Elane−/− mice or in vitro-infected Elane−/− neutrophils was comparable to the controls suggesting that during melioidosis the majority of IL-1β is generated in a caspase-1-dependent fashion, as we and others previously showed [6], [52].
Considering that defense mechanisms such as IL-1 and NE that are protective in a majority of bacterial and viral infections become deleterious in melioidosis clearly sets Burkholderia apart from most Gram-negative bacteria. This raises the possibility that other infectious models where excessive inflammation is known to be deleterious [53], [54] can become useful to separate and identify the relative contribution of resistance and tolerance to host survival. Bacterially expressed elastases have been shown to act as virulence factors [55]. Future studies should examine whether Burkholderia species adopt this strategy.
In conclusion, our study reveals that NE is a host effector mechanism that can negatively impact the host's tolerance to infection with Burkholderia species by causing lung tissue damage and increasing bradykinin-mediated alveolar microvascular permeability. Counteracting NE activity through the use of clinically tested protease inhibitors has proven to be beneficial in a number of human inflammatory conditions and mouse models [56], [57]. Our study shows that this may be an effective therapeutic intervention for melioidosis.
Materials and Methods
Ethics Statement
All the animal experiments described in the present study were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal studies were conducted under protocols approved by the Rosalind Franklin University of Medicine and Science Institutional Animal Care and Use Committee (IACUC) (protocol # B12-07), and the University of Tennessee Health Science Center IACUC (protocol #1854). All efforts were made to minimize suffering and ensure the highest ethical and humane standards.
Mice
C57BL/6J, Il-1r1−/−, Il-18−/−, Elane−/−, Mmp12−/−, Nos2−/−, gp91phox−/−, and Cxcr2+/− mice were purchased from Jackson lab. Il-1a−/−and Il-1a−/−/Il-1b−/− were from I. Iwakura, Il-1b−/− from D. Chaplin, Asc−/−, Nlrp3−/−, Nlrc4−/− from V. Dixit, and Casp1−/− from F. Sutterwala. Il-18−/−-Il-1r1−/− double deficient mice (DKO) were obtained by crossing the parental single knock-out mice. Cxcr2−/− mice were obtained by crossing heterozygotes. All mouse strains were on C57BL/6J genetic background and were bred under specific pathogen-free conditions. Age-(8–12 weeks old) and sex-matched animals were used in all experiments. Experimental groups were composed of at least 5 mice, unless stated otherwise.
Bacteria Strains and Intranasal Infections
B. thailandensis E64 was obtained from ATCC. B. pseudomallei 1026b strain is a clinical virulent isolate. Bacteria were grown in Luria broth to mid-logarithmic phase, their titer was determined by plating serial dilutions on LB agar, and stocks were maintained frozen at −80°C in 20% glycerol. For infections, frozen stocks were diluted in sterile PBS to the desired titer. Mice were anesthetized using isoflurane and the infectious doses were applied to the nares in 50 µl total volume. All work with B. pseudomallei was performed under biosafety level-3 (BSL3/ABSL3) containment according to policies and standard operating procedures approved via the UTHSC Committee on Biocontainment and Restricted Entities, a subcommittee of the UTHSC Institutional Biosafety Committee. The UTHSC has been approved for select agent work by the Centers for Disease Control.
Determination of Bacteria Growth in Organs
Organs aseptically collected were weighted and homogenized in 1 ml PBS using 1 mm zirconium beads and the Mini16 bead beater. Serial dilutions were plated as described above on LB agar plates containing Streptomycin (100 µg/ml).
BALF Collection, Cytokines, IgM, Collagen, Myeloperoxidase, and Elastase Measurements
BALF were collected from euthanized mice by intratracheal injection and aspiration of 1 ml PBS. Cytokines levels in tissue culture conditioned supernatants, BALF, or sera were measured by ELISA using the following kits: mIL-1β , mIL-6, mTNFα, mMCP (eBioscience), mIL-18 (MBL Nagoya, Japan), mCRP (Abcam), Bradykinin (Enzo), IgM (Southern Biotech). Collagen in BALF was measured using the hydroxyproline assay kit (Condrex Inc). Myeloperoxidase or elastase activity were measured in lung homogenates using the MPO fluorometric detection kit (Enzo) or as previously described [58]. In Figure 3B, MPO was measured in the BALF by ELISA (Hycult Biotech).
Evans Blue
Two hours before euthanasia, infected mice were intravenously injected with 0.312 mg Evans Blue in a volume of 0.2 ml PBS. After BALF collection, lungs were perfused with PBS, incubated in formamide for 18 hours at 37°C to extract Evans blue, and absorbance was measured at 620 nm.
Flow Cytometry
Cells obtained from BALF were counted and stained with CD11b, CD11c, F4/80, Ly6G, NK1.1 and acquired with a LSRII BD flow cytometer. Analysis was performed with FACS DIVA software.
Histology and Measurement of Area of Inflammatory Foci and Interstitial Edema
For lung fixation, the trachea was cannulated and the lung was inflated by instillation of 4% paraformaldehyde at the pressure of 25 cm/H2O. Formalin-fixed paraffin-embedded lung sections were stained with H&E or Verhoeff-van Gieson and scanned using the Aperio Scanscope XT. The Aperio ImageScope software was used to quantitate the area of the inflammatory foci compared to the total lung lobe area. ImageJ software was used to determine interstitial edema as the area of the alveolar septa. For each lung sections, five random fields (20× magnification, 793×794 pixels∼160,000 µm2) at that did not contain inflammatory foci or bronchioles or blood vessels were selected. Using “magic wand” tool of ImageJ, the empty alveolar spaces were deleted; the threshold color was adjusted to select only the eosin staining (septa); the image was converted to 8-bit and the threshold adjusted. ImageJ was used to measure the percentage of eosin stained area over the total area in each image. Results from lungs of 5 animals (H&E).
Western Blot
BALF were separated by SDS-PAGE, transferred to PVDF membranes, and probed with rabbit anti-mSP-D (Abcam), rabbit anti-HMGB1 (Abcam), rabbit anti-mReg3γ (Abgent), or goat anti-mIL-1β (R&D Systems).
Mouse Neutrophil Isolation and Adoptive Transfer
Neutrophils were isolated from bone marrow of C57BL/6J or Elane−/− mice using Miltenyi Ly6G microbeads. For adoptive transfer, Cxcr2−/− mice were intranasaly infected with 2×105 CFU B. thailandensis and 1.5×106 neutrophils of either genotype were transferred into recipient mice 24 and 96 hr after infection by retro-orbital intravenous injection in a volume of 0.15 ml.
Pyroptosis and Intracellular Bacteria Growth
Release of LDH in tissue culture media, a reflection of pyroptosis, was measured using the Roche Cytotox detection kit. Neutrophils (5×105 cells) were plated in 24 well plates. Bacteria were added to the cell culture and the plates were centrifuged at 1500 rpm for 10 minutes to maximize and synchronize infection and incubated for 30 minutes at 37°C. Cells were washed with PBS to remove extracellular bacteria and medium containing kanamycin (200 µg/ml) was added to inhibit extracellular bacteria growth. Media were collected at 4 and 8 hours post infection for LDH measurement. Cells were lysed in PBS-2% saponin-15% BSA and serial dilutions of the lysates were plated on LB agar plates containing streptomycin (100 µg/ml) using the Eddy Jet Spiral Plater (Neutec). Bacterial colonies were counted 48 hours later using the Flash & Grow Automated Bacterial Colony Counter (Neutec).
Statistical Analysis
All data were expressed as mean + S.D. Survival curves were compared using the log rank Kaplan-Meier test. Mann-Whitney U test or One way ANOVA Tukey Post-test were used for analysis of the rest of data as specified in the figure legends. Significance was set at p<0.05.
Accession Numbers
Mouse Elane - Q3UP87
Mouse NOS2 - P29477
Mouse gp91phox - Q61093
Mouse CXCR2 - P35343
Mouse MMP12 - P34960
Mouse IL1alpha - P01582
Mouse Il-1beta - p10749
Mouse IL-1RI - P13504
Mouse IL-18 - P70380
Mouse NLRP3 - Q8R4B8
Mouse NLRC4 - Q3UP24
Mouse ASC - Q9EPB4
Mouse Casp-1 - P29452
Mouse SP-D - P50404
Mouse Reg3gamma - O09049
Mouse HMGB1 - P63158
Supporting Information
Zdroje
1. NathanC, DingA (2010) Nonresolving inflammation. Cell 140 : 871–882.
2. SchneiderDS, AyresJS (2008) Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol 8 : 889–895.
3. MedzhitovR, SchneiderDS, SoaresMP (2012) Disease tolerance as a defense strategy. Science 335 : 936–941.
4. AyresJS (2013) Inflammasome-microbiota interplay in host physiologies. Cell Host Microbe 14 : 491–497.
5. WiersingaWJ, van der PollT, WhiteNJ, DayNP, PeacockSJ (2006) Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol 4 : 272–282.
6. Ceballos-OlveraI, SahooM, MillerMA, Del BarrioL, ReF (2011) Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1beta is deleterious. PLoS Pathog 7: e1002452.
7. KohGC, WeehuizenTA, BreitbachK, KrauseK, de JongHK, et al. (2013) Glyburide reduces bacterial dissemination in a mouse model of melioidosis. PLoS Negl Trop Dis 7: e2500.
8. KewcharoenwongC, RinchaiD, UtispanK, SuwannasaenD, BancroftGJ, et al. (2013) Glibenclamide reduces pro-inflammatory cytokine production by neutrophils of diabetes patients in response to bacterial infection. Sci Rep 3 : 3363.
9. HaragaA, WestTE, BrittnacherMJ, SkerrettSJ, MillerSI (2008) Burkholderia thailandensis as a model system for the study of the virulence-associated type III secretion system of Burkholderia pseudomallei. Infect Immun 76 : 5402–5411.
10. BrettPJ, DeShazerD, WoodsDE (1998) Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int J Syst Bacteriol 48 (Pt 1) 317–320.
11. WiersingaWJ, WielandCW, van der WindtGJ, de BoerA, FlorquinS, et al. (2007) Endogenous interleukin-18 improves the early antimicrobial host response in severe melioidosis. Infect Immun 75 : 3739–3746.
12. BreitbachK, WongprompitakP, SteinmetzI (2011) Distinct roles for nitric oxide in resistant C57BL/6 and susceptible BALB/c mice to control Burkholderia pseudomallei infection. BMC Immunol 12 : 20.
13. BreitbachK, KlockeS, TschernigT, van RooijenN, BaumannU, et al. (2006) Role of inducible nitric oxide synthase and NADPH oxidase in early control of Burkholderia pseudomallei infection in mice. Infect Immun 74 : 6300–6309.
14. MiyagiK, KawakamiK, SaitoA (1997) Role of reactive nitrogen and oxygen intermediates in gamma interferon-stimulated murine macrophage bactericidal activity against Burkholderia pseudomallei. Infect Immun 65 : 4108–4113.
15. HerboldW, MausR, HahnI, DingN, SrivastavaM, et al. (2010) Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect Immun 78 : 2620–2630.
16. SpehlmannME, DannSM, HruzP, HansonE, McColeDF, et al. (2009) CXCR2-dependent mucosal neutrophil influx protects against colitis-associated diarrhea caused by an attaching/effacing lesion-forming bacterial pathogen. J Immunol 183 : 3332–3343.
17. Del RioL, BennounaS, SalinasJ, DenkersEY (2001) CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J Immunol 167 : 6503–6509.
18. ChanchamroenS, KewcharoenwongC, SusaengratW, AtoM, LertmemongkolchaiG (2009) Human polymorphonuclear neutrophil responses to Burkholderia pseudomallei in healthy and diabetic subjects. Infect Immun 77 : 456–463.
19. EganAM, GordonDL (1996) Burkholderia pseudomallei activates complement and is ingested but not killed by polymorphonuclear leukocytes. Infect Immun 64 : 4952–4959.
20. LiuPJ, ChenYS, LinHH, NiWF, HsiehTH, et al. (2013) Induction of mouse melioidosis with meningitis by CD11b+ phagocytic cells harboring intracellular B. pseudomallei as a Trojan horse. PLoS Negl Trop Dis 7: e2363.
21. EastonA, HaqueA, ChuK, LukaszewskiR, BancroftGJ (2007) A critical role for neutrophils in resistance to experimental infection with Burkholderia pseudomallei. J Infect Dis 195 : 99–107.
22. CoeshottC, OhnemusC, PilyavskayaA, RossS, WieczorekM, et al. (1999) Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci U S A 96 : 6261–6266.
23. GretenFR, ArkanMC, BollrathJ, HsuLC, GoodeJ, et al. (2007) NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell 130 : 918–931.
24. GumaM, RonacherL, Liu-BryanR, TakaiS, KarinM, et al. (2009) Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum 60 : 3642–3650.
25. CrouchEC (1998) Structure, biologic properties, and expression of surfactant protein D (SP-D). Biochim Biophys Acta 1408 : 278–289.
26. TaylorMD, Van DykeK, BowmanLL, MilesPR, HubbsAF, et al. (2000) A characterization of amiodarone-induced pulmonary toxicity in F344 rats and identification of surfactant protein-D as a potential biomarker for the development of the toxicity. Toxicol Appl Pharmacol 167 : 182–190.
27. KunitakeR, KuwanoK, YoshidaK, MaeyamaT, KawasakiM, et al. (2001) KL-6, surfactant protein A and D in bronchoalveolar lavage fluid from patients with pulmonary sarcoidosis. Respiration 68 : 488–495.
28. ChoiSM, McAleerJP, ZhengM, PociaskDA, KaplanMH, et al. (2013) Innate Stat3-mediated induction of the antimicrobial protein Reg3gamma is required for host defense against MRSA pneumonia. J Exp Med 210 : 551–561.
29. RabergL, SimD, ReadAF (2007) Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318 : 812–814.
30. FerreiraA, MargutiI, BechmannI, JeneyV, ChoraA, et al. (2011) Sickle hemoglobin confers tolerance to Plasmodium infection. Cell 145 : 398–409.
31. JamiesonAM, PasmanL, YuS, GamradtP, HomerRJ, et al. (2013) Role of tissue protection in lethal respiratory viral-bacterial coinfection. Science 340 : 1230–1234.
32. Dela CruzCS, LiuW, HeCH, JacobyA, GornitzkyA, et al. (2012) Chitinase 3-like-1 promotes Streptococcus pneumoniae killing and augments host tolerance to lung antibacterial responses. Cell Host Microbe 12 : 34–46.
33. BelaaouajA (2002) Neutrophil elastase-mediated killing of bacteria: lessons from targeted mutagenesis. Microbes Infect 4 : 1259–1264.
34. WeinrauchY, DrujanD, ShapiroSD, WeissJ, ZychlinskyA (2002) Neutrophil elastase targets virulence factors of enterobacteria. Nature 417 : 91–94.
35. KessenbrockK, DauT, JenneDE (2011) Tailor-made inflammation: how neutrophil serine proteases modulate the inflammatory response. J Mol Med (Berl) 89 : 23–28.
36. PhamCT (2006) Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 6 : 541–550.
37. BrinkmannV, ReichardU, GoosmannC, FaulerB, UhlemannY, et al. (2004) Neutrophil extracellular traps kill bacteria. Science 303 : 1532–1535.
38. BrinkmannV, ZychlinskyA (2012) Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol 198 : 773–783.
39. FangH, SunC, XuL, OwenRJ, AuthRD, et al. (2010) Neutrophil elastase mediates pathogenic effects of anthrax lethal toxin in the murine intestinal tract. J Immunol 185 : 5463–5467.
40. VethanayagamRR, AlmyroudisNG, GrimmMJ, LewandowskiDC, PhamCT, et al. (2011) Role of NADPH oxidase versus neutrophil proteases in antimicrobial host defense. PLoS One 6: e28149.
41. AbrahamE (2003) Neutrophils and acute lung injury. Crit Care Med 31: S195–199.
42. ZemansRL, ColganSP, DowneyGP (2009) Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol 40 : 519–535.
43. MoraesTJ, ZurawskaJH, DowneyGP (2006) Neutrophil granule contents in the pathogenesis of lung injury. Curr Opin Hematol 13 : 21–27.
44. GrommesJ, SoehnleinO (2011) Contribution of neutrophils to acute lung injury. Mol Med 17 : 293–307.
45. JiangD, WenzelSE, WuQ, BowlerRP, SchnellC, et al. (2013) Human neutrophil elastase degrades SPLUNC1 and impairs airway epithelial defense against bacteria. PLoS One 8: e64689.
46. HenkeMO, JohnG, RheineckC, ChillappagariS, NaehrlichL, et al. (2011) Serine proteases degrade airway mucins in cystic fibrosis. Infect Immun 79 : 3438–3444.
47. von BredowC, WiesenerA, GrieseM (2003) Proteolysis of surfactant protein D by cystic fibrosis relevant proteases. Lung 181 : 79–88.
48. KozikA, MooreRB, PotempaJ, ImamuraT, Rapala-KozikM, et al. (1998) A novel mechanism for bradykinin production at inflammatory sites. Diverse effects of a mixture of neutrophil elastase and mast cell tryptase versus tissue and plasma kallikreins on native and oxidized kininogens. J Biol Chem 273 : 33224–33229.
49. SakataY, AkaikeT, SugaM, IjiriS, AndoM, et al. (1996) Bradykinin generation triggered by Pseudomonas proteases facilitates invasion of the systemic circulation by Pseudomonas aeruginosa. Microbiol Immunol 40 : 415–423.
50. OehmckeS, HerwaldH (2010) Contact system activation in severe infectious diseases. J Mol Med (Berl) 88 : 121–126.
51. HircheTO, AtkinsonJJ, BahrS, BelaaouajA (2004) Deficiency in neutrophil elastase does not impair neutrophil recruitment to inflamed sites. Am J Respir Cell Mol Biol 30 : 576–584.
52. MiaoEA, LeafIA, TreutingPM, MaoDP, DorsM, et al. (2010) Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11 : 1136–1142.
53. WangT, TownT, AlexopoulouL, AndersonJF, FikrigE, et al. (2004) Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med 10 : 1366–1373.
54. Le GofficR, BalloyV, LagranderieM, AlexopoulouL, EscriouN, et al. (2006) Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog 2: e53.
55. HaseCC, FinkelsteinRA (1993) Bacterial extracellular zinc-containing metalloproteases. Microbiol Rev 57 : 823–837.
56. ShawL, WiedowO (2011) Therapeutic potential of human elafin. Biochem Soc Trans 39 : 1450–1454.
57. MottaJP, Bermudez-HumaranLG, DeraisonC, MartinL, RollandC, et al. (2012) Food-grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci Transl Med 4 : 158ra144.
58. CortelingR, WyssD, TrifilieffA (2002) In vivo models of lung neutrophil activation. Comparison of mice and hamsters. BMC Pharmacol 2 : 1.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial PathogensČlánek A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during TransmissionČlánek The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil ChemotaxisČlánek Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 8- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Regulatory RNAs Involved in Bacterial Antibiotic Resistance
- From Dandruff to Deep-Sea Vents: -like Fungi Are Ecologically Hyper-diverse
- Pathogenicity and Epithelial Immunity
- Mother–Infant HIV Transmission: Do Maternal HIV-Specific Antibodies Protect the Infant?
- Hell's BELs: acterial 3 igases That Exploit the Eukaryotic Ubiquitin Machinery
- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Primary Seronegative but Molecularly Evident Hepadnaviral Infection Engages Liver and Induces Hepatocarcinoma in the Woodchuck Model of Hepatitis B
- TLR2 Signaling Decreases Transmission of by Limiting Bacterial Shedding in an Infant Mouse Influenza A Co-infection Model
- Production of an Attenuated Phenol-Soluble Modulin Variant Unique to the MRSA Clonal Complex 30 Increases Severity of Bloodstream Infection
- Inhibition of the TRAIL Death Receptor by CMV Reveals Its Importance in NK Cell-Mediated Antiviral Defense
- Early Mucosal Sensing of SIV Infection by Paneth Cells Induces IL-1β Production and Initiates Gut Epithelial Disruption
- Limited HIV Infection of Central Memory and Stem Cell Memory CD4+ T Cells Is Associated with Lack of Progression in Viremic Individuals
- Virus-Specific Regulatory T Cells Ameliorate Encephalitis by Repressing Effector T Cell Functions from Priming to Effector Stages
- A Tick Gut Protein with Fibronectin III Domains Aids Congregation to the Gut during Transmission
- The HIV-1 Envelope Transmembrane Domain Binds TLR2 through a Distinct Dimerization Motif and Inhibits TLR2-Mediated Responses
- Infection with MERS-CoV Causes Lethal Pneumonia in the Common Marmoset
- VGIII Isolates Causing Infections in HIV/AIDS Patients in Southern California: Identification of the Local Environmental Source as Arboreal
- Diverse Host-Seeking Behaviors of Skin-Penetrating Nematodes
- Capsid Protein VP4 of Human Rhinovirus Induces Membrane Permeability by the Formation of a Size-Selective Multimeric Pore
- The Murine Gammaherpesvirus Immediate-Early Rta Synergizes with IRF4, Targeting Expression of the Viral M1 Superantigen to Plasma Cells
- Characterization of an Insecticidal Toxin and Pathogenicity of against Insects
- The Vi Capsular Polysaccharide Enables Serovar Typhi to Evade Microbe-Guided Neutrophil Chemotaxis
- Histone Deacetylase Inhibitors Impair the Elimination of HIV-Infected Cells by Cytotoxic T-Lymphocytes
- A Locus Encompassing the Epstein-Barr Virus Kinase Regulates Expression of Genes Encoding Viral Structural Proteins
- Distinct APC Subtypes Drive Spatially Segregated CD4 and CD8 T-Cell Effector Activity during Skin Infection with HSV-1
- Structure of CfaA Suggests a New Family of Chaperones Essential for Assembly of Class 5 Fimbriae
- Adoptive Transfer of EBV Specific CD8 T Cell Clones Can Transiently Control EBV Infection in Humanized Mice
- Schistosome Feeding and Regurgitation
- EVM005: An Ectromelia-Encoded Protein with Dual Roles in NF-κB Inhibition and Virulence
- Rabies Virus Hijacks and Accelerates the p75NTR Retrograde Axonal Transport Machinery
- Why HIV Virions Have Low Numbers of Envelope Spikes: Implications for Vaccine Development
- Identification of Anti-virulence Compounds That Disrupt Quorum-Sensing Regulated Acute and Persistent Pathogenicity
- HIV-1 Receptor Binding Site-Directed Antibodies Using a VH1-2 Gene Segment Orthologue Are Activated by Env Trimer Immunization
- Cooperation between Epstein-Barr Virus Immune Evasion Proteins Spreads Protection from CD8 T Cell Recognition across All Three Phases of the Lytic Cycle
- Parasite Extracellular Vesicles: Mediators of Intercellular Communication
- RC1339/APRc from Is a Novel Aspartic Protease with Properties of Retropepsin-Like Enzymes
- Cyclic di-GMP-dependent Signaling Pathways in the Pathogenic Firmicute
- Non-random Escape Pathways from a Broadly Neutralizing Human Monoclonal Antibody Map to a Highly Conserved Region on the Hepatitis C Virus E2 Glycoprotein Encompassing Amino Acids 412–423
- Neutrophil Elastase Causes Tissue Damage That Decreases Host Tolerance to Lung Infection with Species
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- SGNH Hydrolase-Like Proteins AlgJ and AlgX Have Similar Topology but Separate and Distinct Roles in Alginate Acetylation
- Why Sexually Transmitted Infections Tend to Cause Infertility: An Evolutionary Hypothesis
- Late Engagement of CD86 after Influenza Virus Clearance Promotes Recovery in a FoxP3 Regulatory T Cell Dependent Manner
- Determinants of Influenza Transmission in South East Asia: Insights from a Household Cohort Study in Vietnam
- A Novel Signal Transduction Pathway that Modulates Quorum Sensing and Bacterial Virulence in
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- A Cysteine Protease Inhibitor of Is Essential for Exo-erythrocytic Development
- EBNA3C Augments Pim-1 Mediated Phosphorylation and Degradation of p21 to Promote B-Cell Proliferation
- On the Front Line: Quantitative Virus Dynamics in Honeybee ( L.) Colonies along a New Expansion Front of the Parasite
- Assembly and Architecture of the EBV B Cell Entry Triggering Complex
- NLR-Associating Transcription Factor bHLH84 and Its Paralogs Function Redundantly in Plant Immunity
- The PDZ-Binding Motif of Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Is a Determinant of Viral Pathogenesis
- Strain-Specific Properties and T Cells Regulate the Susceptibility to Papilloma Induction by Papillomavirus 1
- Human Cytomegalovirus pUL79 Is an Elongation Factor of RNA Polymerase II for Viral Gene Transcription
- The GAP Activity of Type III Effector YopE Triggers Killing of in Macrophages
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Disruption of Fas-Fas Ligand Signaling, Apoptosis, and Innate Immunity by Bacterial Pathogens
- Ly6C Monocyte Recruitment Is Responsible for Th2 Associated Host-Protective Macrophage Accumulation in Liver Inflammation due to Schistosomiasis
- Host Responses to Group A Streptococcus: Cell Death and Inflammation
- Pathogenicity and Epithelial Immunity
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



