-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
Listeria monocytogenes is a foodborne bacterial pathogen that preferentially infects immunocompromised hosts, eliciting a severe and often lethal disease. In humans, clinical manifestations range from asymptomatic intestinal carriage and gastroenteritis to harsher systemic states of the disease such as sepsis, meningitis or encephalitis, and fetal infections. The surface of L. monocytogenes is decorated with wall teichoic acids (WTAs), a class of carbohydrate-based polymers that contributes to cell surface-related events with implications in physiological processes, such as bacterial division or resistance to antimicrobial peptides (AMPs). The addition of other molecules to the backbone of WTAs modulates their chemical properties and consequently their functionality. In this context, we studied the role of WTA tailoring mechanisms in L. monocytogenes, whose WTAs are strictly decorated with monosaccharides. For the first time, we link WTA glycosylation with AMP resistance by showing that the decoration of L. monocytogenes WTAs with l-rhamnose confers resistance to host defense peptides. We suggest that this resistance is based on changes in the permeability of the cell wall that delay its crossing by AMPs and therefore promote the protection of the bacterial membrane integrity. Importantly, we also demonstrate the significance of this WTA modification in L. monocytogenes virulence.
Published in the journal: L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane. PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004919
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004919Summary
Listeria monocytogenes is a foodborne bacterial pathogen that preferentially infects immunocompromised hosts, eliciting a severe and often lethal disease. In humans, clinical manifestations range from asymptomatic intestinal carriage and gastroenteritis to harsher systemic states of the disease such as sepsis, meningitis or encephalitis, and fetal infections. The surface of L. monocytogenes is decorated with wall teichoic acids (WTAs), a class of carbohydrate-based polymers that contributes to cell surface-related events with implications in physiological processes, such as bacterial division or resistance to antimicrobial peptides (AMPs). The addition of other molecules to the backbone of WTAs modulates their chemical properties and consequently their functionality. In this context, we studied the role of WTA tailoring mechanisms in L. monocytogenes, whose WTAs are strictly decorated with monosaccharides. For the first time, we link WTA glycosylation with AMP resistance by showing that the decoration of L. monocytogenes WTAs with l-rhamnose confers resistance to host defense peptides. We suggest that this resistance is based on changes in the permeability of the cell wall that delay its crossing by AMPs and therefore promote the protection of the bacterial membrane integrity. Importantly, we also demonstrate the significance of this WTA modification in L. monocytogenes virulence.
Introduction
Listeria monocytogenes (Lm) is a ubiquitous Gram-positive bacterium and the causative agent of listeriosis, a human foodborne disease with high incidence and morbidity in immunocompromised hosts and other risk groups, such as pregnant women, neonates and the elderly. Clinical manifestations range from febrile gastroenteritis to septicemia, meningitis and encephalitis, as well as fetal infections that can result in abortion or postnatal health complications [1]. The most invasive and severe forms of the disease are a consequence of the ability of this pathogen to overcome important physiological barriers (intestinal epithelium, blood-brain barrier and placenta) by triggering its internalization and promoting its intracellular survival into phagocytic and non-phagocytic cells. Once inside a host cell, a tightly coordinated life cycle, whose progression is mediated by several specialized bacterial factors, enables Lm to proliferate and spread to neighboring cells and tissues [2, 3].
The Lm cell wall is composed of a thick peptidoglycan multilayer that serves as a scaffold for the anchoring of proteins, among which are several virulence factors [4], and of glycopolymers such as teichoic acids, which account for up to 70% of the protein-free cell wall mass [5, 6]. These anionic polymers are divided into membrane-anchored teichoic acids (lipoteichoic acids, LTAs) and peptidoglycan-attached teichoic acids (wall teichoic acids, WTAs). In Listeria, WTAs are mainly composed of repeated ribitol-phosphate subunits, whose hydroxyl groups can be substituted with a diversity of monosaccharides [5]. While the polymer structure and the chemical identity of the substituent groups of LTAs are rather conserved across listeriae [7, 8], they display a high variability in WTAs, even within the same species [9]. Specific WTA substitution patterns are characteristic of particular Lm serotypes: N-acetylglucosamine is common to serogroups 1/2 and 3, and to serotype 4b, but serogroup 1/2 also contains l-rhamnose, whereas serotype 4b displays d-glucose and d-galactose [10]. The broad structural and chemical similarity of LTAs and WTAs results in a considerable degree of functional redundancy, which has complicated the characterization of these macromolecules and the assignment of specific biological roles. However, studies on Gram-positive bacteria have revealed their contribution to important physiological functions (e.g. cell envelope cationic homeostasis [11], regulation of autolysin activity [12], assembly of cell elongation and division machineries [13], defense against antimicrobial peptides [14]) and to virulence-promoting processes, such as adhesion and colonization of host tissues [15, 16].
Antimicrobial peptides (AMPs) are a large family of small peptides (<10 kDa) produced by all forms of living organisms [17], which constitute a major player of the innate immune response against microbial pathogens. Despite their structural diversity, the majority of AMPs share both cationic and amphipathic properties that favor respectively their interaction with the negatively charged prokaryotic surface and insertion into the plasma membrane [17, 18]. Subsequent pore formation or other AMP-mediated membrane-disrupting mechanisms induce bacterial death through direct cell lysis or deleterious interaction with intracellular targets [19]. Bacteria have evolved multiple strategies to avert killing by AMPs [20, 21]. One strategy consists in the modification of their cell surface charge, a process achieved mainly by masking anionic glycopolymers with positively charged groups, thus decreasing their affinity to AMPs. In Gram-positive pathogens, d-alanylation of teichoic acids is a well-characterized mechanism and was demonstrated to be important for bacterial resistance to host-secreted AMPs [22, 23]. In contrast, the contribution of WTA glycosylation mechanisms in AMP resistance has not yet been investigated.
We have previously reported genome-wide transcriptional changes occurring in Lm strain EGD-e during mouse infection [24]. Our analysis revealed an elevated in vivo expression of the lmo1081-1084 genes, here renamed as rmlACBD because of the high homology of the corresponding proteins with enzymes of the l-rhamnose biosynthesis pathway. In this work, we show that the decoration of Lm WTAs with l-rhamnose requires the expression of not only the rmlACBD locus but also of rmlT, an upstream-flanking gene encoding a putative rhamnosyltransferase. We also demonstrate that Lm becomes more susceptible to AMPs in the absence of WTA l-rhamnosylation and predict that this effect is due to an increase of the Lm cell wall permeability to these bactericides, which results in a faster disruption of the plasma membrane integrity with lethal consequences for the bacterial cell. Importantly, we present evidence that this WTA tailoring process is required for full-scale Lm virulence in the mouse model of infection.
Results
The rmlACBD locus is required for the presence of l-rhamnose in Lm WTAs
To identify new Lm genes potentially critical for the infectious process, we previously performed the first in vivo transcriptional profiling of Lm EGD-e. Among the Lm genes displaying the largest increase in transcription throughout infection, we identified a set of previously uncharacterized genes that are included in a pentacistronic operon (lmo1080 to lmo1084) [25]. This operon is found in L. monocytogenes strains belonging to serogroups 1/2, 3 and 7, and is absent from serogroup 4 strains [26] (Fig 1). Interestingly, aside from Listeria seeligeri 1/2b strains, this locus is not found in any other Listeria spp., such as the nonpathogenic Listeria innocua or the ruminant pathogen Listeria ivanovii, which pinpoints it as a genetic feature of a particular subset of pathogenic Listeria strains and suggests that its expression may be important to Listeria pathogenesis in humans.
Fig. 1. Genes encoding the l-rhamnose biosynthesis pathway are distributed in listeriae and other bacterial species. 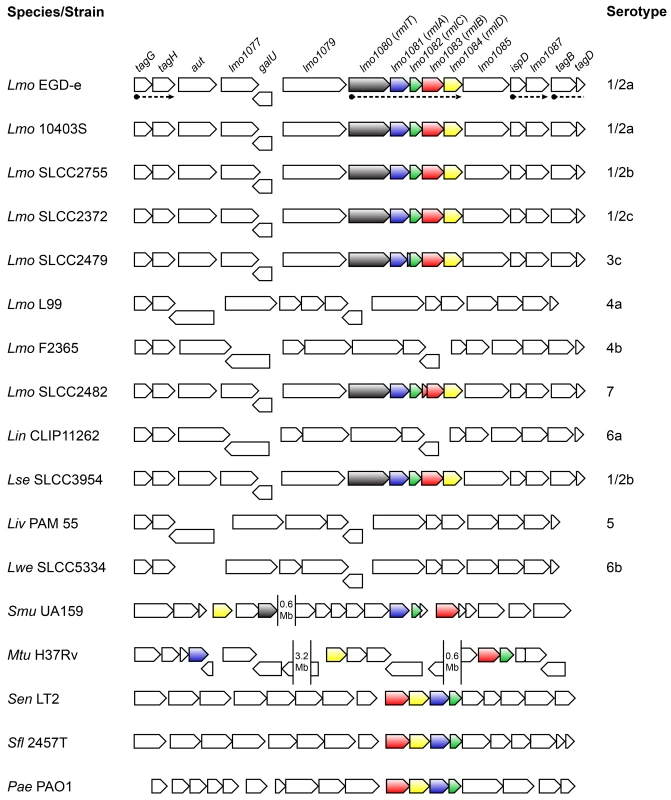
Comparison of the genomic organization of the l-rhamnose pathway genes in the genus Listeria and other bacteria. The corresponding species and strains are indicated on the left (Lmo, Listeria monocytogenes; Lin, Listeria innocua; Lse, Listeria seeligeri; Liv, Listeria ivanovii; Lwe, Listeria welshimeri; Smu, Streptococcus mutans; Mtu, Mycobacterium tuberculosis; Sen, Salmonella enterica serovar Typhimurium; Sfl, Shigella flexneri; Pae, Pseudomonas aeruginosa) and listerial serotypes are indicated on the right. Genes are represented by boxed arrows and their names are provided for strain EGD-e. Operons are underlined by dashed arrows and homologs of the rml genes are shown with identical colors. Numbered gaps indicate the genetic distance (Mb, mega base pairs) between rml genes located far apart in the chromosome. Bacterial genomic sequences were obtained from NCBI database and chromosomal alignments assembled using Microbial Genomic context Viewer and Adobe Illustrator. The four proteins encoded by the lmo1081-lmo1084 genes share a high amino acid sequence homology with the products of the rmlABCD gene cluster. These genes are widely distributed among Gram-negative (e.g. Salmonella enterica [27], Shigella flexneri [28], Vibrio cholerae [29], Pseudomonas aeruginosa [30]) and Gram-positive species (e.g. Mycobacterium tuberculosis [31], Streptococcus mutans [32], Geobacillus tepidamans [33], Lactobacillus rhamnosus [34]) (Fig 1), the majority of which being known pathogens or potentially pathogenic. Despite the inter-species variability observed in the genetic organization of the rml genes, the respective proteins exhibit a remarkable degree of conservation (S1 Table in S1 Text). In light of this, we renamed the lmo1081-lmo1084 genes to rmlACBD, respectively (Fig 1).
The RmlABCD proteins catalyze the conversion of glucose-1-phosphate to a thymidine-diphosphate (dTDP)-linked form of l-rhamnose [35] (S1A Fig in S1 Text), which is a component of the WTAs from most Listeria strains possessing the rml genes [6]. To address the role of rmlACBD in Lm WTA glycosylation with l-rhamnose, we constructed an Lm EGD-e derivative mutant strain lacking the rmlACBD locus (ΔrmlACBD) (S2A Fig in S1 Text) and investigated if the absence of these genes could affect the WTA l-rhamnosylation status. We prepared WTA hydrolysates from exponential phase cultures of wild type (EGD-e), ΔrmlACBD and a complemented ΔrmlACBD strain expressing rmlACBD from its native promoter within an integrative plasmid (ΔrmlACBD+rmlACBD). Samples were resolved by native PAGE and the gel stained with Alcian blue to visualize WTA polymer species. A mutant strain unable to synthesize WTAs (ΔtagO1ΔtagO2) [36] was used to confirm that the detected signal corresponds to WTAs. Compared to the wild type sample, the ΔrmlACBD WTAs displayed a shift in migration, which was reverted to a wild type-like profile in WTAs from the ΔrmlACBD+rmlACBD sample (Fig 2A), indicating that the native WTA composition requires the presence of the rmlACBD genes. To confirm this, we investigated the WTA carbohydrate composition from these strains. WTA polymers were isolated from cell walls purified from bacteria in exponential growth phase, hydrolyzed and analyzed by high-performance anion exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD) to detect monosaccharide species. WTA extracts obtained from ΔrmlACBD bacteria completely lacked l-rhamnose, in contrast to those isolated from the parental wild type strain (Fig 2B). The role of rmlACBD in Lm WTA l-rhamnosylation was definitely confirmed by the analysis of WTAs from ΔrmlACBD+rmlACBD bacteria, in which l-rhamnose was detected at levels similar to those observed in the wild type sample (Fig 2B). Similar observations were made with purified cell wall samples that contain WTAs still attached to the peptidoglycan matrix (S3A Fig in S1 Text). The absence of muramic acid, one of the peptidoglycan building blocks, from WTA extracts (Fig 2B) indicates that l-rhamnose is specifically associated with WTAs and is not a putative peptidoglycan contaminant. This is corroborated by the absence of l-rhamnose in purified peptidoglycan samples (Fig 2C).
Fig. 2. A functional rml operon is required for glycosylation of Lm WTAs with l-rhamnose. 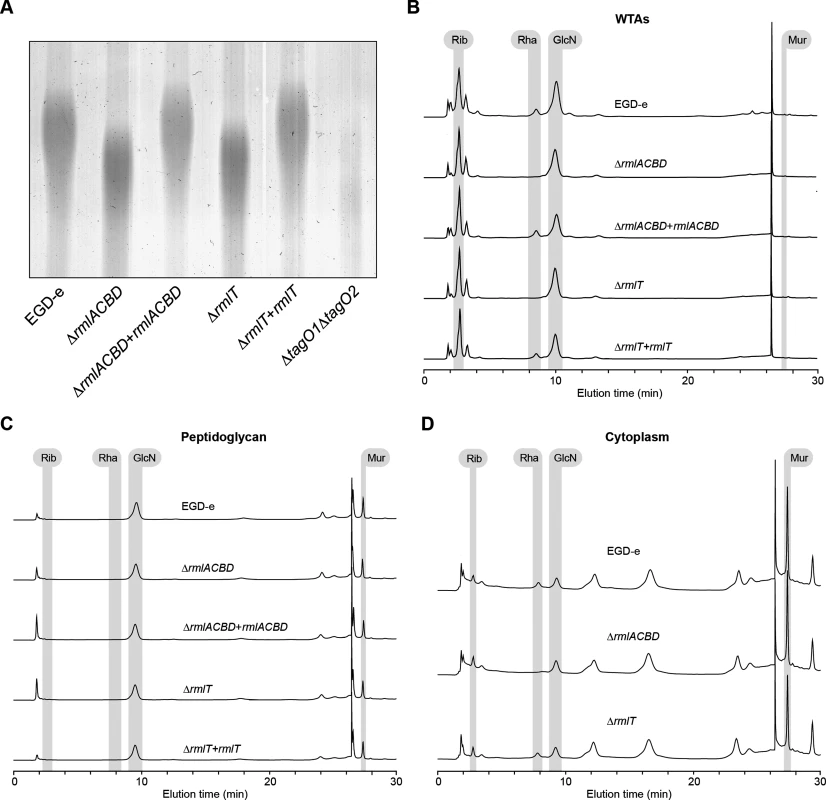
(A) Alcian blue-stained 20% polyacrylamide gel containing WTA extracts from logarithmic-phase cultures of different Lm strains. (B–D) HPAEC-PAD analyses of the sugar composition of the (B) WTA, (C) peptidoglycan and (D) cytoplasmic fractions isolated from the indicated Lm strains. Samples were hydrolyzed in 3 M HCl (2 h, 95°C), diluted with water and lyophilized before injection into the HPLC equipment. Standards for ribitol (Rib), l-rhamnose (Rha), glucosamine (GlcN), and muramic acid (Mur) were eluted under identical conditions to allow peak identification. WTAs have been identified as important regulators of peptidoglycan cross-linking and maturation [37]. To investigate if l-rhamnose decoration of WTAs has any involvement in the maturation of the Lm peptidoglycan, we performed HPLC analysis of the muropeptide composition of mutanolysin-digested peptidoglycan samples from wild type, ΔrmlACBD and ΔrmlACBD+rmlACBD bacteria. No differences in the nature and relative amount of muropeptide species were observed between strains (S3B Fig in S1 Text), ruling out a role for WTA l-rhamnosylation in the consolidation of the peptidoglycan architecture. Overall, these results confirm that a functional rmlACBD locus is required for the association of l-rhamnose with Lm WTAs, likely by providing the molecular machinery responsible for the synthesis of l-rhamnose.
RmlT is required for the incorporation of l-rhamnose into Lm WTAs
The rml operon in Lm includes a fifth gene, lmo1080, located upstream of rmlA (Fig 1), which codes for a protein similar to the B. subtilis minor teichoic acid biosynthesis protein GgaB, shown to possess sugar transferase activity [38]. Conserved domain analysis of the translated Lmo1080 amino acid sequence revealed that its N-terminal region is highly similar (e-value 10–22) to a GT-A family glycosyltransferase domain (S1B Fig in S1 Text). In GT-A enzymes, this domain forms a pocket that accommodates the nucleotide donor substrate for the glycosyl transfer reaction, and contains a signature DxD motif necessary to coordinate a catalytic divalent cation [39]. This motif is also found within the predicted glycosyltransferase domain sequence of Lmo1080 as a DHD tripeptide (S1B Fig in S1 Text). For these reasons, we investigated whether Lmo1080, which we renamed here RmlT (for l-rhamnose transferase), was involved in the l-rhamnosylation of Lm WTAs. We constructed an Lm EGD-e mutant strain lacking rmlT (S2A Fig in S1 Text) and analyzed the structure and sugar composition of its WTAs as described above. WTAs isolated from ΔrmlT bacteria displayed a faster migration in gel (Fig 2A) and did not contain any trace of l-rhamnose (Fig 2B), fully recapitulating the ΔrmlACBD phenotype. Reintroduction of a wild type copy of rmlT into the mutant strain (ΔrmlT+rmlT) resulted in a phenotype that resembles that of the wild type strain, with regards to WTA gel migration profile (Fig 2A) and presence of l-rhamnose in the WTA fraction (Fig 2B).
To discard the possibility that the deletion of rmlT exerted a negative polar effect on the downstream expression of rmlACBD, potentially disrupting the synthesis of l-rhamnose used for WTA glycosylation, we compared the transcription of the rmlACBD genes in the wild type and ΔrmlT Lm strains by quantitative real-time PCR. Transcript levels were unchanged in the ΔrmlT background as compared to the wild type strain (S2B Fig in S1 Text), indicating that the deletion of rmlT did not interfere with the transcription of rmlACBD. To definitely confirm that Lm ΔrmlT still holds the capacity to synthesize l-rhamnose, being only incapable to incorporate it in nascent WTA polymers, we evaluated the presence of l-rhamnose in the cytoplasmic compartment of this strain. The intracellular content of early exponential-phase bacteria from the wild type, ΔrmlACBD and ΔrmlT strains was extracted, hydrolyzed and analyzed by HPAEC-PAD to compare the sugar composition of cytoplasmic extracts. As shown in Fig 2D, a peak corresponding to l-rhamnose was detected in the cytoplasmic samples from the wild type and ΔrmlT strains, but not from the ΔrmlACBD strain, clearly demonstrating that, as opposed to ΔrmlACBD bacteria, ΔrmlT bacteria retain a functional l-rhamnose biosynthesis pathway. These results indicate that the depletion of l-rhamnose observed in ΔrmlT WTAs is a consequence of the absence of the WTA l-rhamnosyltransferase activity performed by RmlT. Therefore, we propose RmlT as the glycosyltransferase in charge of decorating Lm WTAs with l-rhamnose.
WTA l-rhamnosylation promotes Lm resistance to AMPs
WTAs were previously associated with bacterial resistance against salt stress [40] and host defense effectors, such as lysozyme [37, 41]. We thus investigated the potential involvement of WTA l-rhamnosylation in these processes by assessing the growth of the ΔrmlACBD and ΔrmlT strains in the presence of high concentrations of either NaCl or lysozyme. As shown in Fig 3A, no significant difference was observed between the growth of the wild type and the two mutant strains in BHI broth containing 5% NaCl. Similarly, no difference was detected between the growth behavior of these strains after the addition of different concentrations of lysozyme (50 μg/ml and 1 mg/ml) to bacterial cultures in the exponential phase (Fig 3B). As expected, we observed an immediate and significant decrease in the survival of the lysozyme-hypersensitive ΔpgdA mutant [42] (Fig 3B). These data demonstrate that Lm does not require l-rhamnosylated WTAs to grow under conditions of high osmolarity nor to resist the cell wall-degrading activity of lysozyme.
Fig. 3. WTA l-rhamnosylation promotes Lm resistance against AMPs. 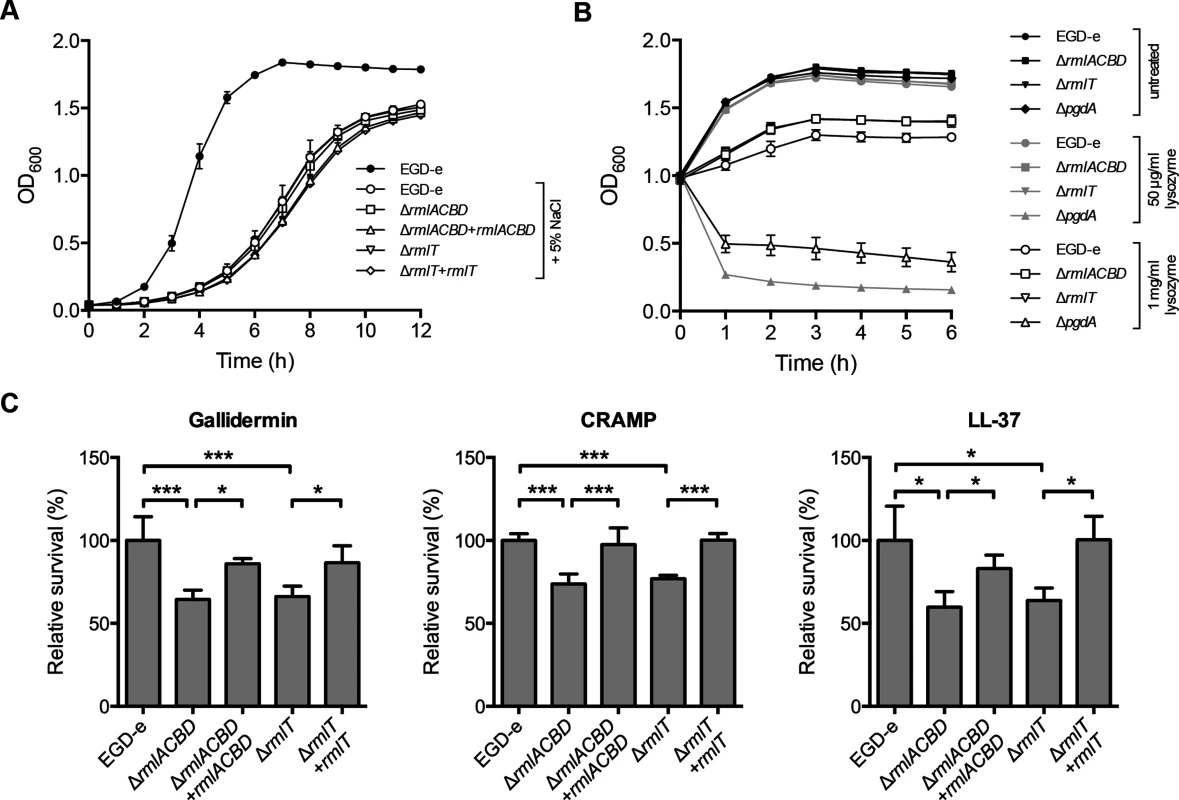
(A) Growth of Lm strains in BHI broth supplemented with 5% NaCl. A growth curve of wild type EGD-e in the absence of 5% NaCl was included as a control for optimal growth. (B) Growth of mid-exponential-phase Lm strains untreated (black symbols) or challenged with 50 μg/ml (gray symbols) or 1 mg/ml (white symbols) of lysozyme. Optical density of the shaking cultures was monitored spectrophotometrically at 600 nm. (C) Quantification of viable bacteria after treatment of mid-exponential-phase Lm strains (2 h, 37°C) with gallidermin (1 μg/ml), CRAMP or LL-37 (5 μg/ml). Averaged replicate values from AMP-treated samples were normalized to untreated control samples and the transformed data expressed as the percentage of surviving bacteria relative to wild type Lm (set at 100). Data represent mean±SD of three independent experiments. *, p≤0.05; ***, p≤0.001. WTAs were also found to be involved in bacterial resistance to host-secreted defense peptides [14, 43]. To investigate the role of WTA l-rhamnosylation in Lm resistance to AMPs, we evaluated the in vitro survival of wild type, ΔrmlACBD and ΔrmlT Lm, as well as of the respective complemented strains, in the presence of biologically active synthetic forms of AMPs produced by distinct organisms: gallidermin, a bacteriocin from the Gram-positive bacterium Staphylococcus gallinarum [44]; CRAMP, a mouse cathelicidin [45], or its human homolog LL-37 [46]. After two hours of co-incubation with different AMP concentrations, surviving bacteria were enumerated by plating in solid media. The overall survival levels of Lm varied with each AMP, evidencing their distinct antimicrobial effectiveness (S4 Fig in S1 Text). However, when compared to the wild type strain, the ΔrmlACBD and ΔrmlT mutants displayed a consistent decrease in their survival levels in the presence of any of the three AMPs (Fig 3C), in a dose-dependent manner (S4 Fig in S1 Text). Restoring WTA l-rhamnosylation through genetic complementation of the mutant strains resulted in an increase of the survival rate to wild type levels. This result demonstrated the important contribution of l-rhamnosylated WTAs towards Lm resistance against AMPs, pointing to a role for WTA glycosylation in bacterial immune evasion mechanisms.
WTA l-rhamnosylation interferes with Lm cell wall crossing by AMPs
The increased AMP susceptibility of Lm strains defective in WTA l-rhamnosylation suggests that this process is required to hinder the bactericidal activity of AMPs. Since AMPs generally induce bacterial death by disrupting the integrity of the plasma membrane, we hypothesized that the higher susceptibility of the ΔrmlACBD and ΔrmlT mutant strains resulted from an increased AMP-mediated destabilization of the Lm membrane. In this context, two scenarios were envisioned: i) AMPs could be binding with higher affinity to the l-rhamnose-deficient Lm cell wall, or ii) they could be crossing it at a faster pace, thus reaching the membrane more quickly than in wild type Lm. To explore these possibilities, we first investigated the binding affinity of the mouse cathelicidin CRAMP towards Lm cell walls depleted of l-rhamnose. For this, we incubated the different Lm strains with CRAMP for a short period and analyzed by flow cytometry the amount of Lm-bound peptide exposed at the cell surface and accessible for antibody recognition. We detected fluorescence associated with surface-exposed CRAMP in all strains (Fig 4A). However, the mean fluorescence intensity (MFI) values were significantly reduced in both ΔrmlACBD and ΔrmlT mutants, in comparison to wild type Lm and the complemented strains (Fig 4A and 4B). This suggests that CRAMP was less accessible to immunolabeling at the cell surface of Lm lacking l-rhamnosylated WTAs.
Fig. 4. WTA l-rhamnosylation interferes with the Lm cell wall crossing by AMPs. 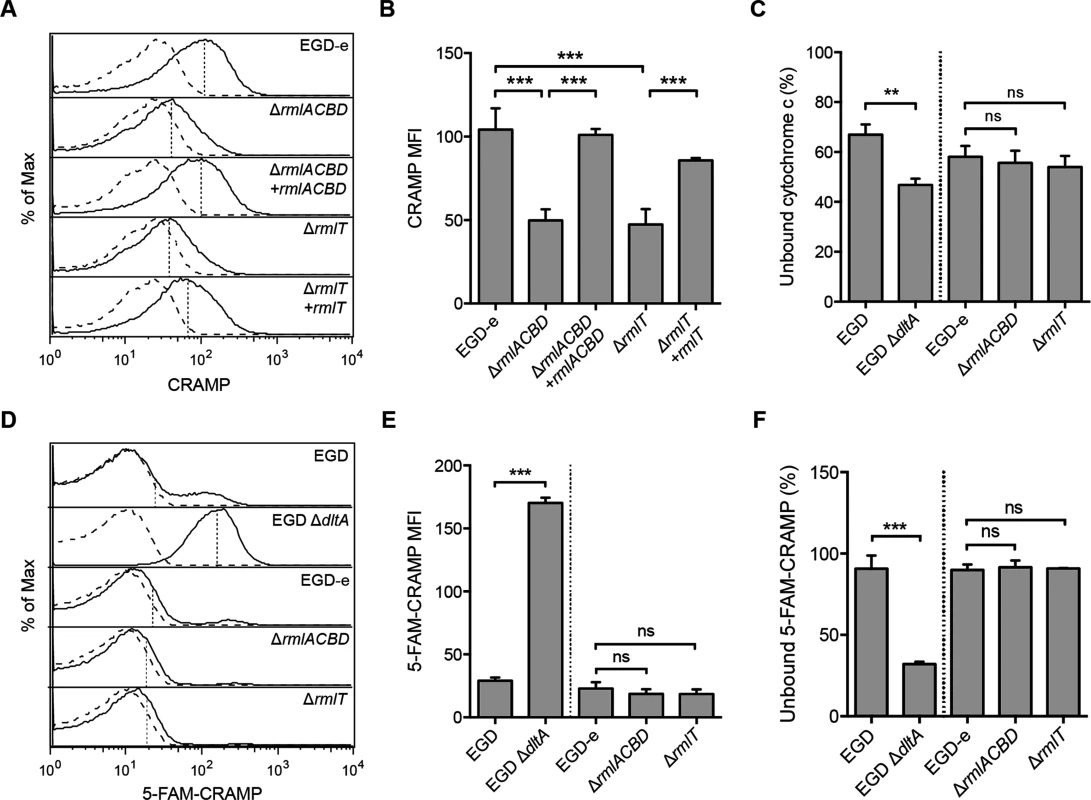
(A and B) Flow cytometry analysis of Lm surface-exposed CRAMP levels in mid-exponential-phase Lm strains, following incubation (5 min) in a 5-μg/ml solution of the peptide and immunolabeling with anti-CRAMP and Alexa Fluor 488-conjugated antibodies. (A) Representative experiment showing overlaid histograms of CRAMP-treated (solid line) and untreated (dashed line) samples, with mean fluorescence intensity (MFI) values from treated samples indicated by vertical dashed lines. (B) Mean±SD of the MFI values of CRAMP-treated samples from three independent experiments. (C) Cell surface charge analysis of Lm strains deficient for WTA l-rhamnosylation as determined by cytochrome c binding assays. Mid-exponential-phase bacteria were incubated with equine cytochrome c (0.5 mg/ml), centrifuged and the supernatant was recovered for spectrophotometric quantification of the unbound protein fraction. Values from Lm-containing samples are expressed as the percentage of unbound cytochrome c relative to control samples lacking bacteria. Data represent the mean±SD of three independent experiments. (D and E) Flow cytometry analysis of total Lm-associated CRAMP levels in mid-exponential-phase Lm strains, following incubation (5 min) with a 5-μg/ml solution of fluorescently labeled peptide (5-FAM-CRAMP). (D) Representative experiment showing overlaid histograms of FAM-CRAMP-treated (solid line) and untreated (dashed line) samples, with MFI values from treated samples indicated by vertical dashed lines. (E) Mean±SD of the MFI values of 5-FAM-CRAMP-treated samples from three independent experiments. (F) Fluorometric quantification of the unbound CRAMP fraction in the supernatant of suspensions of mid-exponential-phase Lm strains, following incubation (5 min) with a 5-μg/ml solution of 5-FAM-CRAMP. Data are expressed as the percentage of unbound fluorescent peptide relative to control samples lacking bacteria, and represent the mean±SD of three independent experiments performed in triplicates. ns = not significant, p>0.05; **, p≤0.01; ***, p≤0.001. The affinity of AMPs towards the bacterial surface is driven by electrostatic forces between positively charged peptides and the anionic cell envelope [23]. To determine if variations of the Lm surface charge contributed to the reduced amount of CRAMP exposed at the surface of ΔrmlACBD and ΔrmlT bacteria, we compared the surface charge of Lm with or without l-rhamnosylated WTAs. For this, we analyzed the binding of cytochrome c, a small protein with positive charge at physiological conditions (isoelectric point ~10), to the wild type and mutant Lm strains. As positive control, we used a mutant strain that cannot modify its LTAs with d-alanine (ΔdltA) and, as a result, displays a higher surface electronegativity and a concomitant higher affinity for positively charged compounds [14, 47]. As expected, the level of cytochrome c binding was higher with the ΔdltA strain than with the respective wild type strain, as illustrated by a decreased percentage of unbound cytochrome c (Fig 4C). However, no significant difference in cytochrome c binding levels was observed between ΔrmlACBD, ΔrmlT and wild type EGD-e strains (Fig 4C), indicating that the absence of l-rhamnose in WTAs does not affect the Lm surface charge. This was further corroborated by zeta potential measurements showing similar pH-dependent variations for both wild type and mutant strains (S5 Fig in S1 Text). Overall, these results allowed us to discard electrostatic changes as a reason behind the difference in the levels of CRAMP detected at the Lm cell surface.
To further explore the decreased levels of surface-exposed CRAMP in Lm strains lacking l-rhamnosylated WTAs, we compared total levels of bacterium-associated CRAMP in the different strains by flow cytometry, following a short incubation with a fluorescently labeled form of this AMP. The intensity of Lm-associated CRAMP fluorescence was comparable for the wild type EGD-e, ΔrmlACBD and ΔrmlT strains (Fig 4D and 4E), indicating that the overall peptide levels associated to Lm cells were similar between the different strains. Accordingly, the residual fluorescence in the supernatants obtained by centrifugation of the bacteria-peptide suspensions was also similar (Fig 4F). As positive control we used the ΔdltA strain, which displayed a significantly stronger peptide binding than its parental wild type strain (Fig 4D–4F). These data strongly suggest that the increased CRAMP susceptibility of Lm strains lacking l-rhamnosylated WTAs results from an improved penetration of CRAMP through their cell walls. Altogether, these results showed that l-rhamnosylated WTAs do not interfere with the Lm surface charge or with the binding efficiency of AMPs, but likely promote Lm survival by hindering the crossing of its cell wall by these bactericidal molecules.
WTA l-rhamnosylation delays AMP interaction with the Lm plasma membrane
In light of these results, we then examined whether WTA l-rhamnosylation interfered with the dynamics of AMP interaction with the Lm plasma membrane. We performed a time-course study to follow Lm membrane potential changes induced by CRAMP. In live bacteria, the membrane potential is an electric potential generated across the plasma membrane by the concentration gradients of sodium, potassium and chloride ions. Physical or chemical disruption of the plasma membrane integrity leads to the suppression of this potential (depolarization) [48]. Lm strains were incubated with DiOC2(3), a green fluorescent voltage-sensitive dye that readily enters into bacterial cells. As the intracellular dye concentration increases with higher membrane potential, it favors the formation of dye aggregates that shift the fluorescence emission to red. After stabilization of the DiOC2(3) fluorescence, CRAMP was added to bacterial samples and the rate of Lm depolarization was immediately analyzed by measuring the red fluorescence emission decline in a flow cytometer. The decrease in the membrane potential was consistently greater in the ΔrmlACBD and ΔrmlT strains as compared to wild type Lm, particularly in the first 10–15 min (Fig 5A), indicating that the Lm plasma membrane integrity is compromised faster by the action of CRAMP in the absence of l-rhamnosylated WTAs. To investigate if increased CRAMP-mediated disruption of the Lm membrane integrity was associated with increased permeabilization, we monitored in real time the entry of the fluorescent probe SYTOX Green into the different Lm strains, following the addition of CRAMP. This probe only enters into bacterial cells with a compromised membrane and displays a strong green fluorescence emission after binding to nucleic acids. As expected, when CRAMP was omitted from the bacterial suspensions, any increase in SYTOX Green-associated fluorescence was detected (Fig 5B). However, in the presence of the peptide, the green fluorescence intensity of samples containing the ΔrmlACBD or ΔrmlT mutants increased earlier than in samples containing wild type Lm (Fig 5B), eventually reaching similar steady-state levels at later time points (S7 Fig in S1 Text). These observations indicate that the CRAMP-mediated permeability increase of the Lm membrane to SYTOX Green occurs faster in strains lacking l-rhamnosylated WTAs.
Fig. 5. WTA l-rhamnosylation delays AMP interaction with the Lm plasma membrane. 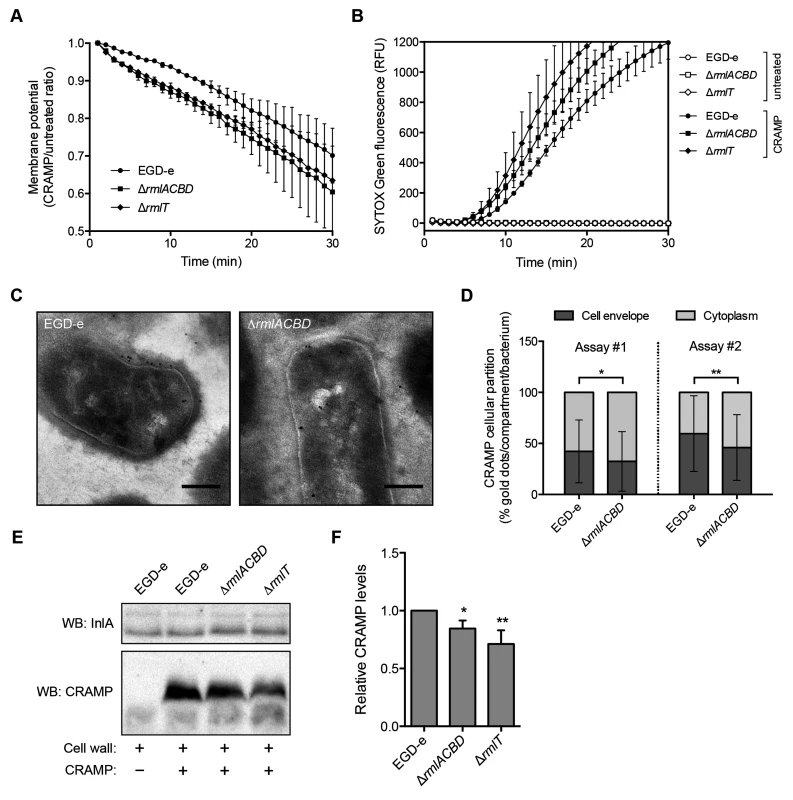
(A) Depolarization rate of Lm strains in response to CRAMP. Mid-exponential-phase bacteria pre-stained (15 min) with 30 μM DiOC2(3) were challenged with 50 μg/ml CRAMP and changes in the membrane potential, expressed as the ratio of CRAMP-treated versus untreated samples, were monitored during 30 min. Data represent the mean±SD of three independent experiments. (B) SYTOX Green uptake kinetics of Lm strains in response to CRAMP-mediated membrane permeabilization. Exponential-phase bacteria were incubated (37°C) with PBS (white symbols) or 50 μg/ml CRAMP (black symbols), in the presence of 1 μM SYTOX Green, and the increase in green fluorescence emission was recorded over time. (C and D) Transmission electron microscopy analysis of the subcellular distribution of CRAMP in immunogold-labeled sections of mid-exponential-phase wild type and ΔrmlACBD Lm strains treated with 50 μg/ml CRAMP (15 min, 37°C). (C) Representative images of contrasted sections of Lm cells showing CRAMP-specific gold labeling (10-nm black dots). Scale bar: 0.2 μm. (D) Quantification of the subcellular partition of CRAMP labeling in wild type and ΔrmlACBD Lm strains, for two independent assays. The percentages of cell envelope- and cytoplasm-associated gold dots per bacterium were quantified (at least 90 cells per strain) and the results expressed for each strain as mean±SD. (E and F) Western blot analysis of levels of CRAMP bound to purified cell wall of different Lm strains. Purified cell wall (100 μg) was incubated with CRAMP (5 min), washed and digested overnight with mutanolysin. (E) Supernatants from mutanolysin-treated samples were resolved in 16% Tris-tricine SDS-PAGE and immunoblotted for CRAMP. The Lm cell wall-anchored protein InlA was used as loading control. (F) Quantification of the relative CRAMP levels represented as the mean±SD of four independent blots. *, p≤0.05; **, p≤0.01. To investigate the ultrastructural localization of the peptide, we performed immunoelectron microscopy on CRAMP-treated wild type and ΔrmlACBD Lm strains. Interestingly, CRAMP-specific labeling was not only detected in the Lm cell envelope, as expected, but also in the cytoplasm (Fig 5C), suggesting that this AMP may additionally target components or processes inside Lm. Comparison of the subcellular distribution of CRAMP between these two bacterial compartments revealed a preferential cell envelope localization in wild type Lm, which contrasted with the slight but significantly higher cytoplasmic localization of the peptide in the ΔrmlACBD strain (Fig 5D). These observations are in agreement with a model in which CRAMP crosses the Lm cell wall more efficiently in the absence of WTA l-rhamnosylation, therefore reaching the bacterial membrane and the cytoplasm comparatively faster.
Finally, to confirm that the presence of l-rhamnosylated WTAs hinders the capacity of AMPs to flow through the Lm cell wall, we assessed levels of CRAMP retained in purified cell wall samples from the wild type, ΔrmlACBD and ΔrmlT strains by Western blot. After incubation with CRAMP, peptides trapped within the peptidoglycan matrix were released by mutanolysin treatment of the cell wall and quantitatively resolved by SDS-PAGE. Immunoblotting revealed a small but consistent decrease in the amount of peptide associated with the cell wall from the two mutant strains in comparison with wild type Lm (Fig 5E and 5F). This result indicates that the lack of l-rhamnose in WTAs results in a partial loss of the AMP retention capacity of the Lm cell wall, which induces an enhanced AMP targeting of the Lm plasma membrane and consequent bacterial killing.
All combined, these data support a model where the l-rhamnosylation of WTAs alters the Lm cell wall permeability to favor the entrapment of AMPs. This obstructive effect hinders AMP progression through the cell wall and delays their lethal interaction with the plasma membrane.
WTA l-rhamnosylation is crucial for AMP resistance in vivo and Lm virulence
To evaluate the importance of WTA l-rhamnosylation in Lm pathogenicity, we assessed the in vivo virulence of Lm strains lacking l-rhamnosylated WTAs. BALB/c mice were inoculated orally with wild type, ΔrmlACBD or ΔrmlT strains, and the bacterial load in the spleen and liver of each animal was quantified three days later. The proliferative capacity of both ΔrmlACBD and ΔrmlT mutant strains was similarly reduced in both organs, although more significantly in the liver (Fig 6A and 6B). To determine if the decreased virulence of the mutant strains was due to a specific defect in the crossing of the intestinal epithelium, BALB/c mice were challenged intravenously, bypassing the intestinal barrier. Three days post-infection, the differences between mutant and wild type strains, in both organs, were similar to those observed in orally infected animals (Fig 6C and 6D), thus discarding any sieving effect of the intestinal epithelium on the decreased splenic and hepatic colonization by both ΔrmlACBD and ΔrmlT. Importantly, organs of mice infected intravenously with the complemented strains (ΔrmlACBD+rmlACBD and ΔrmlT+rmlT) displayed bacterial loads comparable to wild type Lm-infected organs (Fig 6C and 6D). The attenuated in vivo phenotype of the ΔrmlACBD and ΔrmlT strains was not caused by an intrinsic growth defect, as demonstrated by their wild type-like growth profiles in broth or inside eukaryotic cells (S8 Fig in S1 Text). These results confirmed the involvement of the rml operon in virulence, revealing a significant contribution of WTA l-rhamnosylation to Lm pathogenesis. Importantly, the in vivo attenuation of the ΔrmlT strain, which is unable to append l-rhamnose to its WTAs but is able to synthesize the l-rhamnose precursor, showed that although l-rhamnose biosynthesis is required to achieve optimal levels of virulence it is its covalent linkage to the WTA backbone that is crucial for the successful Lm host infection.
Fig. 6. WTA l-rhamnosylation is necessary for AMP resistance in vivo and Lm virulence. 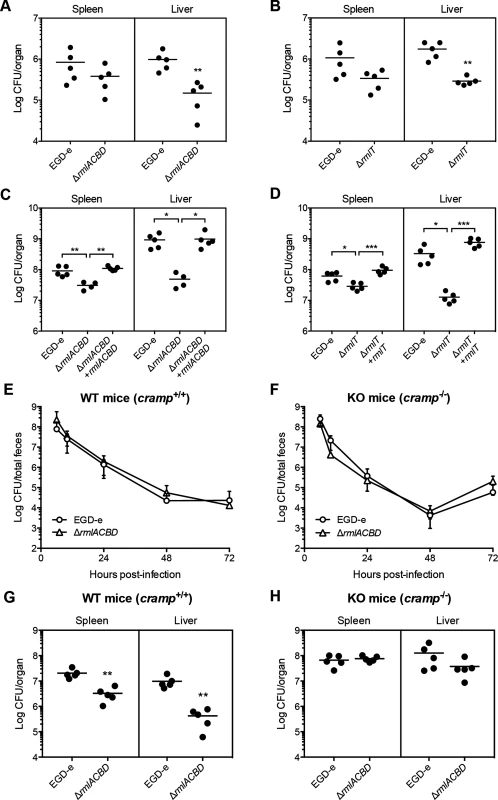
(A–D) Quantification of viable bacteria in the spleen and liver recovered from BALB/c mice (n = 5), three days after (A and B) oral or (C and D) intravenous infection with sub-lethal doses of indicated Lm strains. Data are presented as scatter plots, with each animal indicated by a dot and the mean indicated by a horizontal line. (E and F) Quantification of the fecal shedding of wild type or ΔrmlACBD Lm strains after oral infection of (E) wild type (WT, cramp+/+) and (F) CRAMP knockout (KO, cramp-/-) 129/SvJ mice (n = 5). Total feces produced by each animal at specific time points were collected and processed for bacterial enumeration in Listeria-selective agar media. Data are expressed as mean±SD. (G and H) Quantification of viable bacteria in spleens and livers recovered from (G) wild type (WT, cramp+/+) and (H) CRAMP knockout (KO, cramp-/-) 129/Sv mice (n = 5), three days after intravenous infection with sub-lethal doses of wild type or ΔrmlACBD Lm strains. Data are presented as scatter plots, with each animal represented by a dot and the mean indicated by a horizontal line. *, p≤0.05; **, p≤0.01; ***, p≤0.001. To evaluate the protective role of WTA l-rhamnosylation against AMPs in vivo, we performed virulence studies in a CRAMP-deficient mouse model. To determine the influence of WTA l-rhamnosylation in Lm intestinal persistence, we performed oral infections of adult CRAMP knockout 129/SvJ mice (cramp-/-, KO) [49] and of age - and background-matched wild type mice (cramp+/+, WT), with the wild type or ΔrmlACBD Lm strains and monitored the respective fecal carriage. In both WT and KO mice, we observed comparable dynamics of fecal shedding of the wild type and ΔrmlACBD strains (Fig 6E and 6F). In agreement with the comparable virulence defects observed for WTA l-rhamnosylation-deficient bacteria, following oral or intravenous inoculation of BALB/c mice (Fig 6A–6D), these results suggest a minor role for CRAMP in the control of Lm during the intestinal phase of the infection.
We then inoculated WT and KO mice intravenously and quantified bacterial numbers in the spleen and liver, three days post-infection. In line with what was observed in BALB/c mice (Fig 6C), the ΔrmlACBD strain showed significant virulence attenuation in both organs of WT mice (Fig 6G). Interestingly, this virulence defect was nearly abolished in KO animals, with the ΔrmlACBD strain displaying an organ-colonizing capacity similar to wild type bacteria (Fig 6H). In addition, bacterial loads were higher in the organs of KO mice than in those of WT animals (Fig 6G and 6H). These data indicate that, in comparison to their WT congeners, KO mice are more susceptible to Lm infection, and confirm the in vivo listericidal activity of CRAMP.
Altogether, these results highlight a key role for host-produced CRAMP in restraining Lm infection and demonstrate that WTA l-rhamnosylation also promotes resistance to AMPs in an in vivo context.
Discussion
Teichoic acids are key players in the maintenance of the Gram-positive cell envelope integrity and functionality. They are typically decorated with d-alanine and/or a variety of glycosyl groups, which influence the overall properties of these polymers [9]. Whereas d-alanylation of WTAs has been demonstrated to contribute towards bacterial defense against AMPs [14, 23], the involvement of glycosylation in this process has never been investigated. In this study, we show for the first time that the glycosylation of Lm WTAs with l-rhamnose is mediated by the WTA l-rhamnosyltransferase RmlT and confers protection against AMPs in vitro and during mouse infection. Based on our data, we propose that this protection results from a delayed traversal of the Lm cell envelope by AMPs in the presence of l-rhamnose-decorated WTAs. Most importantly, we reveal a key role for l-rhamnosylated WTAs in the processes underlying Lm pathogenesis.
Unlike S. aureus or B. subtilis [22], WTAs in Listeria are not decorated with d-alanine, undergoing only glycosylation with a small pool of monosaccharides [6, 10]. Among these is l-rhamnose, which is the product of a remarkably conserved biosynthetic pathway that is encoded by the rmlABCD genes [35]. Interestingly, a significant number of bacteria harboring these genes are commonly pathogenic [27–32] and have l-rhamnose in close association with surface components [50, 51]. In Listeria, the rmlACBD locus is only found in certain serotypes of Lm (1/2a, 1/2b, 1/2c, 3c and 7) and L. seeligeri (1/2b). These serotypes were all shown to have l-rhamnose in their WTAs, except for Lm serotypes 3c and 7 [6], which appear to be unable to produce this sugar because of mutations within rmlA and rmlB, respectively (Fig 1). Our results confirmed that the appendage of l-rhamnose to Lm WTAs requires the products of the rmlACBD locus. Ultimately, WTA glycosylation is catalyzed by glycosyltransferases, a class of enzymes that recognize nucleotide-sugar substrates and transfer the glycosyl moiety to a WTA subunit [52]. In silico analysis of lmo1080, the first gene of the operon including rmlACBD (Fig 1) showed that it encodes a protein with putative glycosyltransferase activity. The genomic location and predicted protein function were strong indicators that this gene might encode the transferase involved in the l-rhamnosylation of Lm WTAs. Our data demonstrated that whereas lmo1080, that we renamed rmlT, is dispensable for rhamnose biosynthesis, it is required for the addition of l-rhamnose to WTAs in Lm strains with a functional l-rhamnose pathway, thus validating RmlT as the l-rhamnose-specific WTA glycosyltransferase in Lm.
WTAs are associated with the natural resistance of S. aureus to peptidoglycan-degrading enzymes, such as lysozyme [37, 41]. In contrast, absence of WTA decoration, but not of the polymers, was shown to induce an increase of the staphylococcal susceptibility to lysostaphin [53]. Modifications of the Lm peptidoglycan, such as N-deacetylation [42], were found to contribute to protection against lysozyme, but the role of WTAs and in particular their decoration, was never addressed. Our results discard WTA l-rhamnosylation as a component of the Lm resistance mechanism to this host immune defense protein, as well as its involvement in the promotion of growth under osmotic conditions. Other innate immune effectors, such as antimicrobial peptides (AMPs), also target bacterial organisms [54] that in turn have developed resistance strategies to avoid injury and killing induced by AMPs. Among these strategies is the reshaping and fine-tuning of cell envelope components to lower AMP affinity to the bacterial surface [21]. Previous studies showed a clear link between the d-alanylation of WTAs and AMP resistance [14, 43]. In this context, we found here a similar role for WTA l-rhamnosylation, showing that, in the absence of l-rhamnosylated WTAs, bacteria exhibit an increased susceptibility to AMPs produced by bacteria, mice and importantly by humans. Although from such distinct sources, AMPs used here share a cationic nature that supports their activity. However, while teichoic acid d-alanylation is known to reduce the cell wall electronegativity [14], glycosyl substituents of Lm WTAs are neutrally charged and WTA glycosylation should thus promote AMP resistance through a different mechanism.
It is well established that AMPs induce bacterial death mainly by tampering with the integrity of the plasma membrane. This can be achieved through multiple ways, all of which are driven by the intrinsic amphipathic properties of this class of peptides [55]. Nonetheless, the initial interaction of AMPs with bacterial surfaces is mediated by electrostatic forces between their positive net charge and the anionic cell envelope [23]. Our data show that, unlike d-alanylation [56], WTA l-rhamnosylation does not interfere with the Lm cell surface charge, in agreement with l-rhamnose being an electrostatically neutral monosaccharide. Importantly, the reduced levels of surface-exposed CRAMP in Lm strains lacking l-rhamnosylated WTAs suggested instead that their increased susceptibility to this peptide was correlated with its improved penetration of the l-rhamnose-depleted Lm cell wall. We confirmed this premise with data showing that CRAMP-mediated cell depolarization and plasma membrane permeabilization events occur earlier in WTA l-rhamnosylation-deficient Lm strains. In addition, we also observed a predominant cytoplasmic presence of CRAMP in these mutant strains, in contrast to the preferential cell envelope localization in wild type Lm, further suggesting a WTA l-rhamnosylation-dependent kinetic discrepancy in the progression of CRAMP through the Lm cell envelope. Saar-Dover et al. demonstrated in the WTA-lacking Streptococcus agalactiae (GBS) that LTA d-alanylation promoted resistance to the human cathelicidin LL-37 by hindering cell wall crossing and plasma membrane disturbance [57]. They proposed that the underlying mechanism does not rely on modulation of the surface charge but on LTA conformation-associated alterations of the cell wall packing density [57]. Our data are in line with these observations and although we did not detect changes in the cell wall cross-linking status, we cannot ignore a possible impact of l-rhamnosylation on WTA polymer conformation accounting for changes in cell wall permeability. If one considers that the peptidoglycan, a multi-layered and compact structure, is densely populated with WTA polymers decorated with multiple units of the rather bulky l-rhamnose molecule, spatial constraints and increased cell wall density need to be accounted. In fact, we showed that purified Lm cell wall depleted of l-rhamnose does not retain CRAMP in its peptidoglycan matrix as effectively as cell wall containing l-rhamnosylated WTAs. In addition, we have indications that soluble l-rhamnose interferes with CRAMP activity, improving the survival of WTA l-rhamnosylation mutants of Lm. These observations suggest a potential interaction between l-rhamnose and AMPs, which could favor the “retardation effect” that ultimately promotes Lm survival.
We previously reported a significantly increased transcription of rmlACBD during mouse spleen infection [24], which suggested that WTA l-rhamnosylation is highly activated by Lm to successfully infect this host organ. Our infection studies in mice confirmed the importance of this mechanism for Lm pathogenesis by revealing a significant virulence attenuation of WTA l-rhamnosylation-deficient Lm strains. Surprisingly, the expression of rmlT appeared unchanged during mouse spleen infection as compared to growth in BHI [24], suggesting that an increased L-rhamnose biosynthesis could be sufficient to induce an increased WTA l-rhamnosylation and AMP resistance. Faith et al. also observed a decreased bacterial burden of a serotype 4b Lm strain lacking the gtcA gene [58], a mutation that resulted in complete loss of galactose decoration of its WTAs [59]. Interestingly, gtcA is also present in Lm EGD-e, where it appears to be involved in WTA substitution with N-acetylglucosamine [60], and was shown to contribute to the colonization of the mouse spleen, liver and brain [61]. However the mechanism through which this occurs remains unclear.
Virulence studies in mice lacking the CRAMP gene corroborated our in vitro susceptibility data and revealed the importance of WTA l-rhamnosylation-promoted resistance to AMPs for Listeria virulence. In vivo data also provided a strong insight into the protective role of CRAMP against systemic infection by Lm, as had been previously observed with other bacterial pathogens [49, 62, 63]. Our results on fecal shedding dynamics suggest that the contribution of CRAMP to the control of Lm during the intestinal phase of infection is minimal. A previous report showed a negligible enteric secretion of CRAMP in normal adult mice [64], which may explain the similar shedding behavior of the wild type and ΔrmlACBD strains that were observed in both mouse strains. In this scenario, infection studies in newborn animals, whose enterocytes actively express CRAMP [45, 64], may provide conclusive information regarding the role of WTA l-rhamnosylation in the Lm resistance to CRAMP during the intestinal phase of the infection. Notwithstanding, CRAMP is actively produced by phagocytes in adult mice [65]. As a major target for Lm colonization, the spleen is also an important reservoir of phagocytic cells. We can speculate that WTA l-rhamnosylation is particularly important to increase the chances of Lm surviving CRAMP-mediated killing during spleen infection. Considering our data on the Lm susceptibility to LL-37, the human homolog of CRAMP, we can also envisage this scenario in the context of human infection.
In conclusion, our work has unveiled for the first time a role for WTA glycosylation in bacterial resistance to AMPs. We propose that WTA l-rhamnosylation reduces the cell wall permeability to AMPs, promoting a delay in the crossing of this barrier and in the disruption of the plasma membrane, thus favoring Lm survival and virulence in vivo. Our findings reveal a novel facet in the contribution of WTA modifications towards AMP resistance, reinforcing the crucial role of these Gram-positive surface glycopolymers in host defense evasion.
Materials and Methods
Bacterial strains and growth conditions
Bacterial strains used in this study are listed in Table 1. Lm and E. coli strains were routinely cultured aerobically at 37°C in brain heart infusion (BHI, Difco) and Lysogeny Broth (LB) media, respectively, with shaking. For experiments involving the Lm ΔtagO1ΔtagO2 strain, bacteria were first cultured overnight at 30°C with shaking in the presence of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), washed and diluted (1 : 100) in fresh BHI and cultured overnight at 30°C with shaking [36]. When appropriate, the following antibiotics were included in culture media as selective agents: ampicilin (Amp), 100 μg/ml; chloramphenicol (Cm), 7 μg/ml (Lm) or 20 μg/ml (E. coli); erythromycin (Ery), 5 μg/ml. For genetic complementation purposes, colistin sulfate (Col) and nalidixic acid (Nax) were used at 10 and 50 μg/ml, respectively.
Tab. 1. Plasmids and bacterial strains. 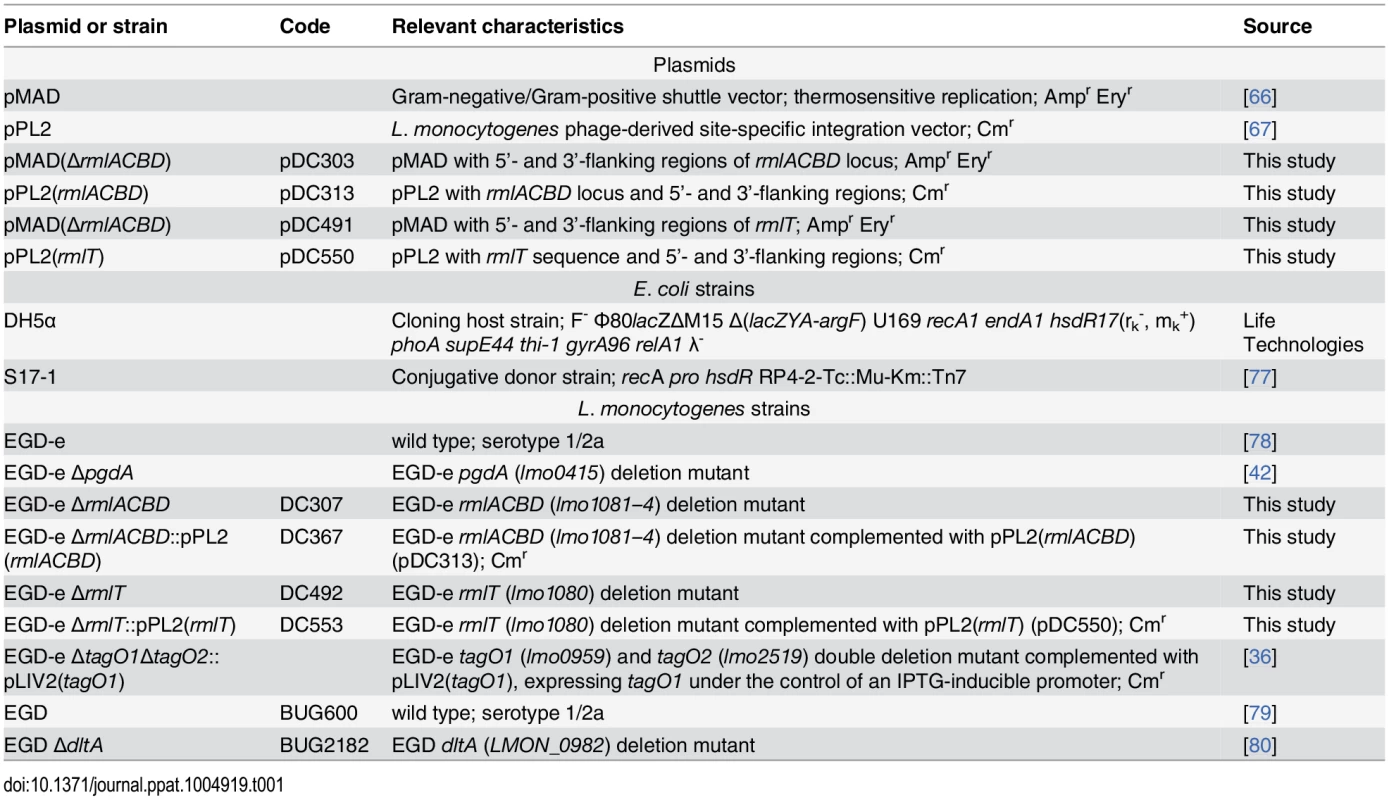
Construction and complementation of mutant strains
Lm mutant strains were constructed in the EGD-e background through a process of double homologous recombination mediated by the suicide plasmid pMAD [66]. DNA fragments corresponding to the 5’ - and 3’-flanking regions of the rmlACBD locus (lmo1081—4) were amplified by PCR from Lm EGD-e chromosomal DNA with primers 1–2 and 3–4 (S2 Table in S1 Text), and cloned between the SalI—MluI and MluI—BglII sites of pMAD, yielding pDC303. Similarly, DNA fragments corresponding to the 5’ - and 3’-flanking regions of rmlT (lmo1080) were amplified with primers 15–16 and 17–18 (S2 Table in S1 Text), and cloned between the SalI—EcoRI and EcoRI—BglII sites of pMAD, yielding pDC491. The plasmid constructs were introduced in Lm EGD-e by electroporation and transformants selected at 30°C in BHI—Ery. Positive clones were re-isolated in the same medium and grown overnight at 43°C. Integrant clones were inoculated in BHI broth and grown overnight at 30°C, after which the cultures were serially diluted, plated in BHI agar and incubated overnight at 37°C. Individual colonies were tested for growth in BHI—Ery at 30°C and antibiotic-sensitive clones were screened by PCR for deletion of rmlACBD (primers 5–6, 7–8, 9–10 and 11–12) and rmlT (primers 19–20) (S2 Table in S1 Text). Genetic complementation of the deletion mutant strains was performed as described [24]. DNA fragments containing either the rmlACBD or rmlT loci were amplified from Lm EGD-e chromosomal DNA with primers 13–14 and 21–22 (S2 Table in S1 Text), respectively, and cloned between the SalI—PstI sites of the phage-derived integrative plasmid pPL2 [67], generating pDC313 and pDC550. The plasmid constructs were introduced in the E. coli strain S17-1 and transferred, respectively, to the ΔrmlACBD and ΔrmlT strains by conjugation on BHI agar. Transconjugant clones were selected in BHI—Cm/Col/Nax and chromosomal integration of the plasmids confirmed by PCR with primers 23 and 24 (S2 Table in S1 Text). All plasmid constructs and gene deletions were confirmed by DNA sequencing.
Gene expression analyses
Total bacterial RNA was isolated from 10 ml of exponential cultures (OD600 = 0.6) by the phenol-chloroform extraction method, as previously described [68], and treated with DNase I (Turbo DNA-free, Ambion), as recommended by the manufacturer. Purified RNAs (1 μg) were reverse-transcribed with random hexamers, using iScript cDNA Synthesis kit (Bio-Rad Laboratories). Quantitative real-time PCR (qPCR) was performed in 20-μl reactions containing 2 μl of cDNA, 10 μl of SYBR Green Supermix (Bio-Rad Laboratories) and 0.25 μM of forward and reverse primers (S2 Table in S1 Text), using the following cycling protocol: 1cycle at 95°C (3 min) and 40 cycles at 95°C (30 s), 55°C (30 s) and 72°C (30 s). Each target gene was analyzed in triplicate and blank (water) and DNA contamination controls (unconverted DNase I-treated RNA) were included for each primer pair. Amplification data were analyzed by the comparative threshold (ΔΔCt) method, after normalization of the test and control sample expression values to a housekeeping gene (16S rRNA). For qualitative analysis, PCR was performed in 20-μl reactions containing 2 μl of cDNA, 10 μl of MangoMix 2× reaction mix (Bioline) and 0.5 μM of forward and reverse qPCR primers, using the following protocol: 1 cycle at 95°C (5 min), 25 cycles at 95°C (30 s), 55°C (30 s) and 72°C (20 s), and 1 cycle at 72°C (5 min). Amplification products were resolved in 1% (w/v) agarose gel and analyzed in a GelDoc XR+ System (Bio-Rad Laboratories).
WTA PAGE analysis
Extraction and analysis of Lm WTAs by polyacrylamide gel electrophoresis was performed essentially as described [69], with the exception that WTAs extracts were obtained from exponential-phase cultures. Sedimented bacteria were washed (buffer 1 : 50 mM MES buffer, pH 6.5) and boiled for 1 h (buffer 2 : 4% SDS in buffer 1). After centrifugation, the pellet was serially washed with buffer 2, buffer 3 (2% NaCl in buffer 1) and buffer 1, before treatment with 20 μg/ml proteinase K (20 mM Tris-HCl, pH 8; 0.5% SDS) at 50°C for 4 h. The digested samples were thoroughly washed with buffer 3 and distilled water and incubated overnight (16 h) with 0.1 M NaOH, under vigorous agitation. Cell wall debris were removed by centrifugation (10,000 rpm, 10 min) and the hydrolyzed WTAs present in the supernatant were directly analyzed by native PAGE in a Tris-tricine buffer system. WTA extracts were resolved through a vertical (20 cm) polyacrylamide (20%) gel at 20 mA for 18 h (4°C). To visualize WTAs, the gel was stained in 0.1% Alcian blue (40% ethanol; 5% acetic acid) for 30 min and washed (40% ethanol; 10% acetic acid) until the background is fully cleared. Optionally, for increased contrasting, silver staining can be performed on top of the Alcian blue staining.
Purification of cell wall components
Cell walls of Lm strains were purified as described before [70], with modifications. Overnight cultures were subcultured into 1–2 liters of BHI broth (initial OD600 = 0.005) and bacteria grown until exponential phase (OD600 = 1.0–1.5). Cultures were rapidly cooled in an ice/ethanol bath and bacteria harvested by centrifugation (7,500 rpm, 15 min, 4°C). The pellet was resuspended in cold ultrapure water and boiled for 30 min with 4% SDS to kill bacteria and inactivate cell wall-modifying enzymes. The samples were cleared of SDS by successive cycles of centrifugation (12,000 rpm, 10 min) and washing with warm ultrapure water until no detergent was detected [71]. SDS-free samples were resuspended in 2 ml of ultrapure water and cell walls disrupted with glass beads in a homogenizer (FastPrep, Thermo Savant). Fully broken cell walls were separated from glass beads by filtration (glass filters, pore size: 16–40 μm) and from unbroken cell walls and other debris by low-speed centrifugation (2,000 rpm, 15 min). Nucleic acids were degraded after incubation (2 h) at 37°C with DNase (10 μg/ml) and RNase (50 μg/ml) in a buffer containing 50 mM Tris-HCl, pH 7.0, and 20 mM MgSO4. Proteins were then digested overnight at 37°C with trypsin (100 μg/ml) in the presence of 10 mM CaCl2. Nuclease and proteases were inactivated by boiling in 1% SDS, and samples were centrifuged (17,000 rpm, 15 min) and washed twice with ultrapure water. Cell walls were resuspended and incubated (37°C, 15 min) in 8 M LiCl and then in 100 mM EDTA, pH 7.0, after which they were washed twice with water. After resuspension in acetone and sonication (15 min), cell walls were washed and resuspended in ultrapure water before undergoing lyophilization.
To obtain purified peptidoglycan, cell walls (20 mg) were incubated for 48 h with 4 ml of 46% hydrofluoric acid (HF), under agitation at 4°C. Samples were washed with 100 mM Tris-HCl, pH 7.0, and centrifuged (17,000 rpm, 30 min, 4°C) as many times as necessary to neutralize the pH. The pellet was finally washed twice with water prior to lyophilization. WTA extracts were obtained by incubating 1 mg of cell wall with 300 μl of 46% HF (18 h, 4°C). After centrifugation (13,200 rpm, 15 min, 4°C), the supernatant was recovered and evaporated under a stream of compressed air. The dried WTA residue was resuspended in water and lyophilized.
Extraction of bacterial cytoplasmic content
The intracellular content of Lm strains was isolated according to a modified version of the protocol by Ornelas-Soares et al. [72]. Bacterial cultures (200 ml) were grown until early exponential phase (OD600 = 0.3), and vancomycin was added at 7.5 μg/ml (5×MIC value [73]) to induce the cytoplasmic accumulation of the peptidoglycan precursor UDP-MurNAc-pentapeptide. Cultures were grown for another 45 min and chilled in an ice-ethanol bath for 10 min. Bacteria were then harvested by centrifugation (12,000 rpm, 10 min, 4°C), washed with cold 0.9% NaCl, resuspended in 5 ml of cold 5% trichloroacetic acid and incubated for 30 min on ice. Cells and other debris were separated by centrifugation (4,000 rpm, 15 min, 4°C) and the supernatant was extracted with 1–2 volumes of diethyl ether as many times as necessary to remove TCA (sample pH should rise to at least 6.0). The aqueous fraction containing the cytoplasmic material was lyophilized and the dried residue resuspended in ultrapure water.
HPLC analyses
To analyze their sugar composition, purified cell wall and peptidoglycan (200 μg each), as well as cytoplasmic (500 μg) and WTA extracts were hydrolyzed in 3 M HCl for 2 h at 95°C. After vacuum evaporation, the samples were washed with water and lyophilized. The hydrolyzed material was then resuspended in 150 μl of water and resolved by high-performance anion-exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD). Ten microliters were injected into a CarboPac PA10 column (Dionex, Thermo Fisher Scientific) and eluted at 1 ml/min (30°C) with 18 mM NaOH, followed by a gradient of NaCH3COO: 0–20 mM (t = 25–30 min), 20–80 mM (t = 30–35 min), 80–0 mM (t = 40–45 min). Standards for glucosamine, muramic acid, l-rhamnose and ribitol (Sigma-Aldrich) were eluted under the same conditions to enable identification of chromatogram peaks. Data were acquired and analyzed with the Chromeleon software (Dionex, Thermo Fisher Scientific).
Muropeptide samples were prepared and analyzed as described [74], with minor changes. Purified peptidoglycan was digested with 200 μg/ml mutanolysin (Sigma-Aldrich) in 12.5 mM sodium phosphate, pH 5.5, for 16 h at 37°C. Enzymatic activity was halted by heating at 100°C for 5 min, after which the digested sample was reduced for 2 h with 2.5 mg/ml of sodium borohydride (NaBH4) in 0.25 M borate buffer, pH 9.0. The reaction was stopped by lowering the sample pH to 2 with ortho-phosphoric acid. After centrifugation, the supernatant was analyzed by reverse phase HPLC. Fifty microliters were injected into a Hypersil ODS (C18) column (Thermo Fisher Scientific) and muropeptide species eluted (0.5 ml/min, 52°C) in 0.1 M sodium phosphate, pH 2.0, with a gradient of 5–30% methanol and detected at 206 nm.
Intracellular multiplication
Mouse macrophage-like J774A.1 cells (ATCC, TIB-67) were propagated in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and infection assays were performed as described [24]. Briefly, cells (~2×105/well) were infected for 45 min with exponential-phase bacteria at a multiplicity of infection of ~10 and treated afterwards with 20 μg/ml gentamicin for 75 min. At several time-points post-infection, cells were washed with PBS and lysed in cold 0.2% Triton X-100 for quantification of viable intracellular bacteria in BHI agar. One experiment was performed with triplicates for each strain and time-point.
Resistance to salt stress and lysozyme
Lm cultures grown overnight were appropriately diluted in BHI broth and their growth under the presence of stressful stimuli was monitored by optical density measurement at 600 nm (OD600). For comparative analysis of Lm resistance to salt stress, bacterial cultures were diluted 100-fold in BHI alone (control) or BHI containing 5% NaCl. To assess the Lm resistance to lysozyme, exponential-phase cultures (OD600 ≈ 1.0) were challenged with different doses of chicken egg white lysozyme (Sigma). A mutant Lm strain hypersensitive to lysozyme (ΔpgdA) was used as a positive control for susceptibility.
AMP susceptibility
Bacteria in the exponential phase of growth (OD600 = 0.7–0.8) were diluted (104 CFU/ml) in sterile PB medium (10 mM phosphate buffer, pH 7.4; 1% BHI) and mixed in a 96-well microplate with increasing concentrations of gallidermin (Santa Cruz Biotechnology), CRAMP or LL-37 (AnaSpec). Bacterial suspensions without AMPs were used as reference controls for optimal growth/survival. After incubation for 2 h at 37°C, the mixtures were serially diluted in sterile PBS and plated in BHI agar for quantification of viable bacteria. Each condition was analyzed in duplicate in three independent assays.
Cytochrome c binding
Cytochrome c binding assays were performed as described [56]. Bacteria from mid-exponential-phase cultures (OD600 = 0.6–0.7) were washed in 20 mM MOPS buffer, pH 7.0, and resuspended in ½ volume of 0.5 mg/ml equine cytochrome c (Sigma-Aldrich) in 20 mM MOPS buffer, pH 7.0. After 10 min of incubation, bacteria were pelleted and the supernatant collected for quantification of the absorbance at 530 nm. The mean absorbance values from replicate samples containing bacteria were subtracted to the mean value of a reference sample lacking bacteria, and the results were presented for each strain as percentage of unbound cytochrome c.
Zeta potential measurements
Bacteria (1 ml) from mid-exponential-phase cultures were washed twice with deionized water and diluted (107 CFU/ml) in 15 mM NaCl solutions adjusted to different pH values (1 to 7) with nitric acid. Bacterial suspensions (750 μl) were injected into a disposable capillary cell cuvette (DTS1061, Malvern Instruments) and the zeta potential was measured at 37°C in a ZetaSizer Nano ZS (Malvern Instruments), under an automated field voltage. Samples were measured in triplicate in three independent assays.
Flow cytometry analyses
Bacteria from 500 μl of mid-exponential-phase cultures were washed twice with PBS and treated for 5 min with 5 μg/ml CRAMP or PBS (untreated control). After centrifugation, the supernatant was removed and PBS-washed bacteria were incubated for 1 h with rabbit anti-CRAMP (1 : 100, Innovagen), followed by 1 h with Alexa Fluor 488-conjugated anti-rabbit IgG (1 : 200, Molecular Probes). Finally, bacteria were fixed with 3% paraformaldehyde for 15 min, washed and resuspended in PBS. Alternatively, bacteria were similarly treated with an N-terminally 5-FAM-labeled synthetic form of CRAMP (95% purity, Innovagen), washed and resuspended in PBS. Samples were acquired in a FACSCalibur flow cytometer equipped with CellQuest software (BD Biosciences) and data were analyzed with FlowJo (TreeStar Inc.). Green fluorescence was collected from at least 50,000 FSC/SSC-gated bacterial events in the FL1 channel (530 nm/20 nm bandpass filter). Fluorescence intensities were plotted in single-parameter histograms and results were presented as the average mean fluorescence intensity (MFI) value from three independent analyses.
For bacterial membrane potential studies, the lipophilic fluorescent probe DiOC2(3) (3,3-diethyloxacarbocyanine, Santa Cruz Biotechnology) was used as a membrane potential indicator [48, 75]. Mid-logarithmic phase bacteria were diluted (106 CFU/ml) in PBS with 30 μM DiOC2(3) and incubated for 15 min in the dark. CRAMP was added to a final concentration of 50 μg/ml and the sample was immediately injected in the flow cytometer. Control samples treated with PBS or with 1.5 mM sodium azide (uncoupling agent) were analyzed to determine the fluorescence values corresponding to basal (100%) and null (0%) membrane potential (S6 Fig in S1 Text). Green and red (FL3, 670 nm/long bandpass filter) fluorescence emissions were continuously collected from FSC/SSC-gated bacteria for 30 min. After acquisition, a ratio of red over green fluorescence (R/G) was calculated per event and plotted in the y-axis versus time. A series of consecutive one-minute-wide gates was applied to the plot and the mean R/G value per gate was determined. The mean R/G values from uncoupler-treated samples were deducted from the corresponding values from the untreated and CRAMP-treated samples, and the resulting values for each condition were normalized as percentage of the initial value (t = 1 min). Finally, the temporal variation of the Lm membrane potential was represented graphically as the ratio of the normalized values from CRAMP-treated over untreated samples.
SYTOX Green uptake
Bacterial uptake of the cell-impermeable SYTOX Green dye was used to study membrane permeabilization induced by CRAMP [57]. Exponential-phase bacteria were washed and resuspended (107 CFU/ml) in sterile PBS containing 1 μM SYTOX Green (Molecular Probes). After 20 min of incubation in the dark, bacterial suspensions were mixed in PCR microplate wells with 50 μg/ml CRAMP or PBS (negative control) for a total volume of 100 μl. The mixtures were immediately placed at 37°C in a real-time PCR detection system (iQ5, Bio-Rad Laboratories) and fluorescence emission at 530 nm was recorded every minute following excitation at 488 nm.
Binding of AMP to purified cell walls
One-hundred micrograms of purified cell wall were resuspended in 50 μl of 5 μg/ml CRAMP or PBS (negative control) and gently shaken for 5 min. Samples were centrifuged (16,000 × g, 1 min), washed in PBS and in TM buffer (10 mM Tris-HCl, 10 mM MgCl2, pH 7.4) before overnight incubation at 37°C with mutanolysin (400 U/ml) in TM buffer (50 μl). Supernatants were resolved by tricine-SDS-PAGE in a 16% gel, transferred onto nitrocellulose membrane and blotted with rabbit anti-CRAMP (1 : 1000) or mouse anti-InlA (L7.7; 1 : 1000), followed by HRP-conjugated goat anti-rabbit or anti-mouse IgG (1 : 2000, P.A.R.I.S). Immunolabeled bands were visualized using SuperSignal West Dura Extended Duration Substrate (Pierce) and digitally acquired in a ChemiDoc XRS+ system (Bio-Rad Laboratories).
Immunoelectron microscopy
Exponential-phase bacteria treated with 50 μg/ml CRAMP for 15 min at 37°C were fixed for 1 h at room temperature (4% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M sodium cacodylate, pH 7.2), stained with 1% osmium tetroxide for 2 h and resuspended in 30% BSA (high-purity grade). Bacterial pellets obtained after centrifugation in microhematocrit tubes were fixed overnight in 1% glutaraldehyde, dehydrated in increasing ethanol concentrations, and embedded in Epon 812. Ultrathin sections (40–50 nm) were placed on 400-mesh Formvar-coated copper grids and treated with 4% sodium metaperiodate and 1% periodic acid (10 min each) for antigen retrieval. For immunogold labeling of CRAMP, sections were blocked for 10 min with 1% BSA and incubated overnight (4°C) with rabbit anti-CRAMP (1 : 100 in 1% BSA). After extensive washing, sections were labeled with 10-nm gold complex-conjugated anti-rabbit IgG (1 : 200 in 1% BSA) for 2 h, washed and contrasted with 4% uranyl acetate and 1% lead citrate. Images were acquired in a Jeol JEM-1400 transmission electron microscope equipped with a Gatan Orius SC1000 CCD camera and analyzed using ImageJ software.
Animal infections
Virulence studies were done in mouse models of the following strains: wild type BALB/c and 129/SvJ (Charles River Laboratories); and CRAMP-deficient (cramp-/-) 129/SvJ, which was bred in our facilities from a breeding pair provided by Dr. Richard L. Gallo (University of California, USA) [49]. Infections were performed in six-to-eight week-old specific-pathogen-free females as described [76]. Briefly, for oral infections, 12-h starved animals were inoculated by gavage with 109 CFU in PBS containing 150 mg/ml CaCO3, while intravenous infections were performed through the tail vein with 104 CFU in PBS. In both cases, the infection was carried out for 72 h, at which point the animals were euthanatized by general anesthesia. The spleen and liver were aseptically collected, homogenized in sterile PBS, and serial dilutions of the organ homogenates plated in BHI agar. For analysis of Lm fecal carriage, total feces produced by each infected animal (n = 5 per strain) up to a given time-point were collected, homogenized in PBS and serial dilutions plated in Listeria selective media (Oxoid) for bacterial enumeration. Mice were maintained at the IBMC animal facilities, in high efficiency particulate air (HEPA) filter-bearing cages under 12 h light cycles, and were given sterile chow and autoclaved water ad libitum.
Ethics statement
All the animal procedures were in agreement with the guidelines of the European Commission for the handling of laboratory animals (directive 2010/63/EU), with the Portuguese legislation for the use of animals for scientific purposes (Decreto-Lei 113/2013), and were approved by the IBMC Animal Ethics Committee, as well as by the Direcção Geral de Veterinária, the Portuguese authority for animal protection, under license PTDC/SAU-MIC/111581/2009.
Statistical analyses
Statistical analyses were performed with Prism 6 (GraphPad Software). Unpaired two-tailed Student’s t-test was used to compare the means of two groups; one-way ANOVA was used with Tukey’s post-hoc test for pairwise comparison of means from more than two groups, or with Dunnett’s post-hoc test for comparison of means relative to the mean of a control group. Mean differences were considered statistically non-significant (ns) when p value was above 0.05. For statistically significant differences: *, p≤0.05; **, p≤0.01; ***, p≤0.001.
Supporting Information
Zdroje
1. Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes and Infection. 2007;9 : 1236–43. doi: 10.1016/j.micinf.2007.05.011 1906370.
2. Cossart P, Toledo-Arana A. Listeria monocytogenes, a unique model in infection biology: an overview. Microbes and infection / Institut Pasteur. 2008;10 : 1041–50. doi: 10.1016/j.micinf.2008.07.043 18775788.
3. Camejo A, Carvalho F, Reis O, Leitão E, Sousa S, Cabanes D. The arsenal of virulence factors deployed by Listeria monocytogenes to promote its cell infection cycle. Virulence. 2011;2 : 379–94. doi: 10.4161/viru.2.5.17703 21921683.
4. Carvalho F, Sousa S, Cabanes D. How Listeria monocytogenes organizes its surface for virulence. Frontiers in cellular and infection microbiology. 2014;4 : 48. doi: 10.3389/fcimb.2014.00048 24809022.
5. Fiedler F, Seger J, Schrettenbrunner A, Seeliger H. The biochemistry of murein and cell wall teichoic acids in the genus Listeria. Systematic and Applied Microbiology. 1984;5 : 360–76. doi: 10.1016/S0723-2020(84)80038-7
6. Fiedler F. Biochemistry of the cell surface of Listeria strains: a locating general view. Infection. 1988;16 Suppl 2:S92–7. 3417357.
7. Uchikawa K-i, Sekikawa I, Azuma I. Structural studies on lipoteichoic acids from four Listeria strains. Journal of bacteriology. 1986;168 : 115–22. 3093460.
8. Ruhland GJ, Fiedler F. Occurrence and biochemistry of lipoteichoic acids in the genus Listeria. Systematic and Applied Microbiology. 1987;9 : 40–6. doi: 10.1016/S0723-2020(87)80054-1
9. Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nature reviews Microbiology. 2008;6 : 276–87. doi: 10.1038/nrmicro1861 18327271.
10. Uchikawa K, Sekikawa I, Azuma I. Structural studies on teichoic acids in cell walls of several serotypes of Listeria monocytogenes. Journal of biochemistry. 1986;99 : 315–27. 3084460.
11. Marquis RE, Mayzel K, Carstensen EL. Cation exchange in cell walls of gram-positive bacteria. Canadian journal of microbiology. 1976;22 : 975–82. 822931.
12. Peschel A, Vuong C, Otto M, Götz F. The D-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrobial agents and chemotherapy. 2000;44 : 2845–7. doi: 10.1128/AAC.44.10.2845-2847.2000.Updated 10991869.
13. Schirner K, Marles-Wright J, Lewis RJ, Errington J. Distinct and essential morphogenic functions for wall - and lipo-teichoic acids in Bacillus subtilis. The EMBO journal. 2009;28 : 830–42. doi: 10.1038/emboj.2009.25 19229300.
14. Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Götz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. The Journal of Biological Chemistry. 1999;274 : 8405–10. doi: 10.1074/jbc.274.13.8405 10085071.
15. Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, Gross M, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nature medicine. 2004;10 : 243–5. doi: 10.1038/nm991 14758355.
16. Weidenmaier C, Peschel A, Xiong Y-Q, Kristian Sa, Dietz K, Yeaman MR, et al. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. The Journal of infectious diseases. 2005;191 : 1771–7. doi: 10.1086/429692 15838806.
17. Cederlund A, Gudmundsson GH, Agerberth B. Antimicrobial peptides important in innate immunity. The FEBS journal. 2011;278 : 3942–51. doi: 10.1111/j.1742-4658.2011.08302.x 21848912.
18. Peters BM, Shirtliff ME, Jabra-Rizk MA. Antimicrobial peptides: primeval molecules or future drugs? PLoS pathogens. 2010;6:e1001067. doi: 10.1371/journal.ppat.1001067 21060861.
19. Brogden Ka. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature reviews Microbiology. 2005;3 : 238–50. doi: 10.1038/nrmicro1098 15703760.
20. Peschel A, Sahl H-G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nature reviews Microbiology. 2006;4 : 529–36. doi: 10.1038/nrmicro1441 16778838.
21. Koprivnjak T, Peschel A. Bacterial resistance mechanisms against host defense peptides. Cellular and molecular life sciences: CMLS. 2011;68 : 2243–54. doi: 10.1007/s00018-011-0716-4 21560069.
22. Neuhaus FC, Baddiley J. A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiology and molecular biology reviews: MMBR. 2003;67 : 686–723. doi: 10.1128/MMBR.67.4.686 14665680.
23. Koprivnjak T, Peschel A, Gelb MH, Liang NS, Weiss JP. Role of charge properties of bacterial envelope in bactericidal action of human group IIA phospholipase A2 against Staphylococcus aureus. The Journal of biological chemistry. 2002;277 : 47636–44. doi: 10.1074/jbc.M205104200 12359734.
24. Camejo A, Buchrieser C, Couvé E, Carvalho F, Reis O, Ferreira P, et al. In vivo transcriptional profiling of Listeria monocytogenes and mutagenesis identify new virulence factors involved in infection. PLoS Pathogens. 2009;5:e1000449. doi: 10.1371/journal.ppat.1000449 19478867.
25. Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, Balestrino D, et al. The Listeria transcriptional landscape from saprophytism to virulence. Nature. 2009;459 : 950–6. doi: 10.1038/nature08080 19448609.
26. Doumith M, Cazalet C, Simoes N, Frangeul L, Jacquet C, Kunst F, et al. New aspects regarding evolution and virulence of Listeria monocytogenes revealed by comparative genomics and DNA arrays. Infection and immunity. 2004;72 : 1072–83. doi: 10.1128/IAI.72.2.1072 14742555.
27. Li Q, Reeves PR. Genetic variation of dTDP-L-rhamnose pathway genes in Salmonella enterica. Microbiology. 2000;146 (Pt 9):2291–307. 10974117.
28. Macpherson DF, Manning PA, Morona R. Characterization of the dTDP-rhamnose biosynthetic genes encoded in the rfb locus of Shigella flexneri. Molecular microbiology. 1994;11 : 281–92. 8170390.
29. Li Q, Hobbs M, Reeves PR. The variation of dTDP-L-rhamnose pathway genes in Vibrio cholerae. Microbiology (Reading, England). 2003;149 : 2463–74. doi: 10.1099/mic.0.26382-0 12949172.
30. Aguirre-Ramírez M, Medina G, González-Valdez A, Grosso-Becerra V, Soberón-Chávez G. The Pseudomonas aeruginosa rmlBDAC operon, encoding dTDP-L-rhamnose biosynthetic enzymes, is regulated by the quorum-sensing transcriptional regulator RhlR and the alternative sigma factor σS. Microbiology (Reading, England). 2012;158 : 908–16. doi: 10.1099/mic.0.054726-0 22262098.
31. Li W, Xin Y, McNeil MR, Ma Y. rmlB and rmlC genes are essential for growth of mycobacteria. Biochemical and biophysical research communications. 2006;342 : 170–8. doi: 10.1016/j.bbrc.2006.01.130 16472764.
32. Tsukioka Y, Yamashita Y, Oho T, Nakano Y, Koga T. Biological function of the dTDP-rhamnose synthesis pathway in Streptococcus mutans. Journal of bacteriology. 1997;179 : 1126–34. 9023194.
33. Zayni S, Steiner K, Pföstl A, Hofinger A, Kosma P, Schäffer C, et al. The dTDP-4-dehydro-6-deoxyglucose reductase encoding fcd gene is part of the surface layer glycoprotein glycosylation gene cluster of Geobacillus tepidamans GS5-97T. Glycobiology. 2007;17 : 433–43. doi: 10.1093/glycob/cwl084 17202151.
34. Péant B, LaPointe G, Gilbert C, Atlan D, Ward P, Roy D. Comparative analysis of the exopolysaccharide biosynthesis gene clusters from four strains of Lactobacillus rhamnosus. Microbiology (Reading, England). 2005;151 : 1839–51. doi: 10.1099/mic.0.27852-0 15941992.
35. Giraud MF, Naismith JH. The rhamnose pathway. Current opinion in structural biology. 2000;10 : 687–96. 11114506.
36. Eugster MR, Loessner MJ. Wall teichoic acids restrict access of bacteriophage endolysin Ply118, Ply511, and PlyP40 cell wall binding domains to the Listeria monocytogenes peptidoglycan. Journal of bacteriology. 2012;194 : 6498–506. doi: 10.1128/JB.00808-12 23002226.
37. Atilano ML, Pereira PM, Yates J, Reed P, Veiga H, Pinho MG, et al. Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107 : 18991–6. doi: 10.1073/pnas.1004304107 20944066.
38. Freymond P-P, Lazarevic V, Soldo B, Karamata D. Poly(glucosyl-N-acetylgalactosamine 1-phosphate), a wall teichoic acid of Bacillus subtilis 168: its biosynthetic pathway and mode of attachment to peptidoglycan. Microbiology (Reading, England). 2006;152 : 1709–18. doi: 10.1099/mic.0.28814-0 16735734.
39. Breton C, Snajdrová L, Jeanneau C, Koca J, Imberty A. Structures and mechanisms of glycosyltransferases. Glycobiology. 2006;16 : 29R–37R. doi: 10.1093/glycob/cwj016 16037492.
40. Chassaing D, Auvray F. The lmo1078 gene encoding a putative UDP-glucose pyrophosphorylase is involved in growth of Listeria monocytogenes at low temperature. FEMS microbiology letters. 2007;275 : 31–7. doi: 10.1111/j.1574-6968.2007.00840.x 17666069.
41. Bera A, Biswas R, Herbert S, Kulauzovic E, Weidenmaier C, Peschel A, et al. Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. Journal of bacteriology. 2007;189 : 280–3. doi: 10.1128/JB.01221-06 17085565.
42. Boneca IG, Dussurget O, Cabanes D, Nahori M-A, Sousa S, Lecuit M, et al. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proceedings of the National Academy of Sciences of the United States of America. 2007;104 : 997–1002. doi: 10.1073/pnas.0609672104 17215377.
43. Kovacs M, Halfmann A, Fedtke I, Heintz M, Peschel A, Vollmer W, et al. A Functional dlt Operon, Encoding Proteins Required for Incorporation of D-Alanine in Teichoic Acids in Gram-Positive Bacteria, Confers Resistance to Cationic Antimicrobial Peptides in Streptococcus pneumoniae. Journal of Bacteriology. 2006;188 : 5797–805. doi: 10.1128/JB.00336-06 16885447.
44. Kellner R, Jung G, Hörner T, Zähner H, Schnell N, Entian KD, et al. Gallidermin: a new lanthionine-containing polypeptide antibiotic. European journal of biochemistry / FEBS. 1988;177 : 53–9. 3181159.
45. Gallo RL, Kim KJ, Bernfield M, Kozak Ca, Zanetti M, Merluzzi L, et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. The Journal of biological chemistry. 1997;272 : 13088–93. 9148921.
46. Vandamme D, Landuyt B, Luyten W, Schoofs L. A comprehensive summary of LL-37, the factoctum human cathelicidin peptide. Cellular immunology. 2012;280 : 22–35. doi: 10.1016/j.cellimm.2012.11.009 23246832.
47. Abachin E, Poyart C, Pellegrini E, Milohanic E, Fiedler F, Berche P, et al. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Molecular microbiology. 2002;43 : 1–14. 11849532.
48. Shapiro HM. Membrane potential estimation by flow cytometry. Methods (San Diego, Calif). 2000;21 : 271–9. doi: 10.1006/meth.2000.1007 10873481.
49. Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner Ra, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414 : 454–7. doi: 10.1038/35106587 11719807.
50. Frirdich E, Whitfield C. Lipopolysaccharide inner core oligosaccharide structure and outer membrane stability in human pathogens belonging to the Enterobacteriaceae. Journal of endotoxin research. 2005;11 : 133–44. doi: 10.1179/096805105X46592 15949142.
51. Chatterjee D. The mycobacterial cell wall: structure, biosynthesis and sites of drug action. Current opinion in chemical biology. 1997;1 : 579–88. 9667898.
52. Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyltransferases: structures, functions, and mechanisms. Annual review of biochemistry. 2008;77 : 521–55. doi: 10.1146/annurev.biochem.76.061005.092322 18518825.
53. Brown S, Xia G, Luhachack LG, Campbell J, Meredith TC, Chen C, et al. Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proceedings of the National Academy of Sciences of the United States of America. 2012;109 : 18909–14. doi: 10.1073/pnas.1209126109 23027967.
54. Guilhelmelli F, Vilela N, Albuquerque P, Derengowski LDS, Silva-Pereira I, Kyaw CM. Antibiotic development challenges: the various mechanisms of action of antimicrobial peptides and of bacterial resistance. Frontiers in microbiology. 2013;4 : 353. doi: 10.3389/fmicb.2013.00353 24367355.
55. Nguyen LT, Haney EF, Vogel HJ. The expanding scope of antimicrobial peptide structures and their modes of action. Trends in biotechnology. 2011;29 : 464–72. doi: 10.1016/j.tibtech.2011.05.001 21680034.
56. Vadyvaloo V, Arous S, Gravesen A, Héchard Y, Chauhan-Haubrock R, Hastings JW, et al. Cell-surface alterations in class IIa bacteriocin-resistant Listeria monocytogenes strains. Microbiology (Reading, England). 2004;150 : 3025–33. doi: 10.1099/mic.0.27059-0 15347760.
57. Saar-Dover R, Bitler A, Nezer R, Shmuel-Galia L, Firon A, Shimoni E, et al. D-alanylation of lipoteichoic acids confers resistance to cationic peptides in group B streptococcus by increasing the cell wall density. PLoS pathogens. 2012;8:e1002891. doi: 10.1371/journal.ppat.1002891 22969424.
58. Faith N, Kathariou S, Cheng Y, Promadej N, Neudeck BL, Zhang Q, et al. The role of L. monocytogenes serotype 4b gtcA in gastrointestinal listeriosis in A/J mice. Foodborne pathogens and disease. 2009;6 : 39–48. doi: 10.1089/fpd.2008.0154 18991548.
59. Promadej N, Fiedler F, Cossart P, Dramsi S, Kathariou S. Cell wall teichoic acid glycosylation in Listeria monocytogenes serotype 4b requires gtcA, a novel, serogroup-specific gene. Journal of bacteriology. 1999;181 : 418–25. 9882654.
60. Eugster MR, Haug MC, Huwiler SG, Loessner MJ. The cell wall binding domain of Listeria bacteriophage endolysin PlyP35 recognizes terminal GlcNAc residues in cell wall teichoic acid. Molecular microbiology. 2011;81 : 1419–32. doi: 10.1111/j.1365-2958.2011.07774.x 21790805.
61. Autret N, Dubail I, Trieu-Cuot P, Berche P, Charbit A. Identification of new genes involved in the virulence of Listeria monocytogenes by signature-tagged transposon mutagenesis. Infection and immunity. 2001;69 : 2054–65. doi: 10.1128/IAI.69.4.2054-2065.2001 11254558.
62. Huang LC, Reins RY, Gallo RL, McDermott AM. Cathelicidin-deficient (Cnlp-/-) mice show increased susceptibility to Pseudomonas aeruginosa keratitis. Investigative ophthalmology & visual science. 2007;48 : 4498–508. doi: 10.1167/iovs.07-0274 17898271.
63. Chromek M, Arvidsson I, Karpman D. The antimicrobial peptide cathelicidin protects mice from Escherichia coli O157:H7-mediated disease. PloS one. 2012;7:e46476. doi: 10.1371/journal.pone.0046476 23077510.
64. Ménard S, Förster V, Lotz M, Gütle D, Duerr CU, Gallo RL, et al. Developmental switch of intestinal antimicrobial peptide expression. The Journal of experimental medicine. 2008;205 : 183–93. doi: 10.1084/jem.20071022 18180308.
65. Rosenberger CM, Gallo RL, Finlay BB. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proceedings of the National Academy of Sciences of the United States of America. 2004;101 : 2422–7. 14983025.
66. Arnaud M, Chastanet A, Débarbouillé M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Applied and environmental microbiology. 2004;70 : 6887–91. doi: 10.1128/AEM.70.11.6887-6891.2004 15528558.
67. Lauer P, Chow MYN, Loessner MJ, Portnoy DA, Calendar R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. Journal of bacteriology. 2002;184 : 4177–86. doi: 10.1128/JB.184.15.4177 12107135.
68. Milohanic E, Glaser P, Coppée J-Y, Frangeul L, Vega Y, Vázquez-Boland Ja, et al. Transcriptome analysis of Listeria monocytogenes identifies three groups of genes differently regulated by PrfA. Molecular microbiology. 2003;47 : 1613–25. 12622816.
69. Carvalho F, Pucciarelli MG, Portillo FG-d, Cabanes D, Cossart P. Extraction of cell wall-bound teichoic acids and surface proteins from Listeria monocytogenes. In: Delcour AH, editor. Methods in molecular biology (Clifton, NJ). Totowa, NJ: Humana Press; 2013. p. 289–308.
70. Filipe SR, Tomasz A, Ligoxygakis P. Requirements of peptidoglycan structure that allow detection by the Drosophila Toll pathway. EMBO reports. 2005;6 : 327–33. doi: 10.1038/sj.embor.7400371 15791270.
71. Hayashi K. A rapid determination of sodium dodecyl sulfate with methylene blue. Analytical biochemistry. 1975;67 : 503–6. 1163770.
72. Ornelas-Soares A, de Lencastre H, de Jonge BL, Tomasz A. Reduced methicillin resistance in a new Staphylococcus aureus transposon mutant that incorporates muramyl dipeptides into the cell wall peptidoglycan. The Journal of Biological Chemistry. 1994;269(44):27246–50. Epub 1994/11/04. 7961632.
73. Blanot S, Boumaila C, Berche P. Intracerebral activity of antibiotics against Listeria monocytogenes during experimental rhombencephalitis. The Journal of antimicrobial chemotherapy. 1999;44(4):565–8. Epub 1999/12/10. 10588323.
74. de Jonge BL, Chang YS, Gage D, Tomasz A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. The Journal of biological chemistry. 1992;267 : 11248–54. 1597460.
75. Novo D, Perlmutter NG, Hunt RH, Shapiro HM. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry. 1999;35 : 55–63. 10554181.
76. Cabanes D, Lecuit M, Cossart P. Animal models of Listeria infection. Current protocols in microbiology. 2008;Chapter 9:Unit9B.1. doi: 10.1002/9780471729259.mc09b01s10 18729060.
77. Simon R, Priefer U, Pühler A. A Broad Host Range Mobilization System for In Vivo Genetic Engineering: Transposon Mutagenesis in Gram Negative Bacteria. Bio/Technology. 1983;1 : 784–91. doi: 10.1038/nbt1183-784
78. Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, Baquero F, et al. Comparative genomics of Listeria species. Science (New York, NY). 2001;294 : 849–52. doi: 10.1126/science.1063447 11679669.
79. Murray EGD, Webb RA, Swann MBR. A disease of Rabbits characterised by a large mononuclear Leucocytosis, caused by a hitherto undescribed bacillus Bacterium monocytogenes. The Journal of Pathology and Bacteriology. 1926;29 : 407–39. doi: 10.1002/path.1700290409 188406400008.
80. Mandin P, Fsihi H, Dussurget O, Vergassola M, Milohanic E, Toledo-Arana A, et al. VirR, a response regulator critical for Listeria monocytogenes virulence. Molecular microbiology. 2005;57 : 1367–80. doi: 10.1111/j.1365-2958.2005.04776.x 16102006.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR SelectionČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



