Determination of CMC of cationic tenside in aqueous and mixed water-alcohol solutions
Authors:
Andrea Gálusová; Fils Andriamainty; Roman Mikláš
Authors‘ workplace:
Comenius University in Bratislava, Faculty of Pharmacy, Department of Pharmaceutical Chemistry, Bratislava, Slovak Republic
Published in:
Čes. slov. Farm., 2015; 64, 206-208
Category:
44<sup>th</sup> Conference drug synthesis and analysis
Introduction
The critical micelle concentration (CMC) indicates a narrow concentration range in which the creation of aggregates of the so-called micelles occurs. The process of self-assembly of surfactants into micelles is important in many areas from biological systems to technical application1). The studied compound belongs to the group of quaternary ammonium compounds, cationic surfactants, with potentially antimicrobial effect. Short-chain alcohols are organic additives which can act as co-solvents and co-surfactants depending on their concentration in the solution, therefore their influence on CMC may vary2, 3).
The aim of the work was to determine the influence of alcohol on CMC of the substance derived from the derivative of camphoric acid N,N-dimethyl-2-oxo-2-(tetradecyloxy)-N-{2-[1,8,8-trimethyl-2,4-dioxo-3-azabicyclo[3.2.1.]octan-3-yl]ethyl}ethanaminium bromide with the working label 1182-RM-12-14 in aqueous solutions.
Experimental methods
Material and methods
Methanol, ethanol and propanol used in the experiment were obtained from centralCHEM. Pyrene was purchased from Sigma-Aldrich, Switzerland. A spectrophotometer Spekol 1300 Analytic Jena AG (Germany) was used for absorbance measurement.
Preparation of pyrene solution
0.0012 mol/l pyrene stock solution was prepared by dissolving the calculated amount of the compound under study in ethanol for UV (99%) and sonicated to clarity. 2 μmol/l pyrene solutions of the studied compound were used to prevent excimer formation and were prepared by adding the calculated amount of pyrene stock solution into the aqueous or mixed water-alcohol solution of the studied compound5).
Absorbance study
The spectra were recorded in 200–400 nm wavelength. Laboratory temperature throughout the curse of experiment was 25 °C. CMC of compound 1182-RM-12-14 was determined by absorption spectrophotometry with pyrene in UV/VIS region in aqueous; methanol, ethanol and propanol solutions with concentration 0.1 M, 0.2M and 0.5 M. The CMC was determined according to the method of Basu Ray et al.4).
Results and discussion
The pyrene absorption spectrum shows eight strong (s) and weak (w) peaks at 232w, 242s, 252w, 260w, 272s, 308w, 320s, 336s nm6) (Fig. 1).
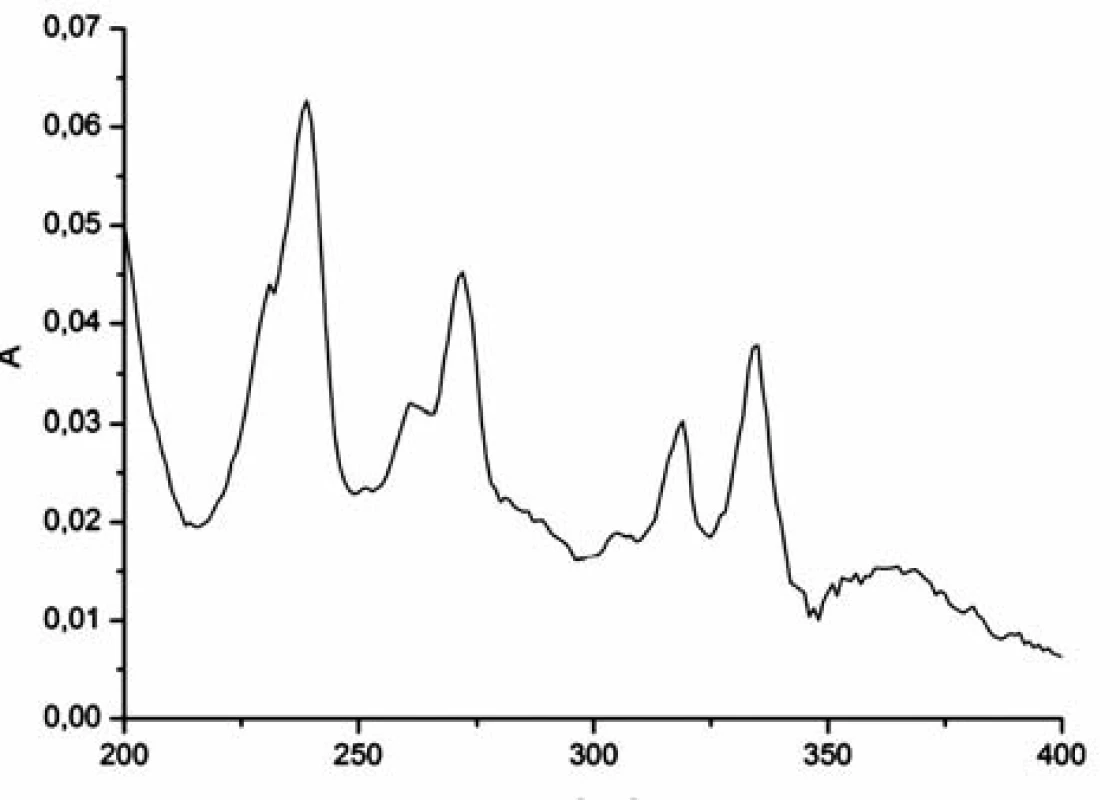
In the aqueous solution, pyrene peaks were present at 308, 322 and 336 nm (Fig. 2) and in mixed water-alcohol solutions at 322 and 336 nm (Fig. 3). The rest of the typical pyrene peaks were masked due to strong absorption of compound 1182-RM-12-14 in the near UV region.
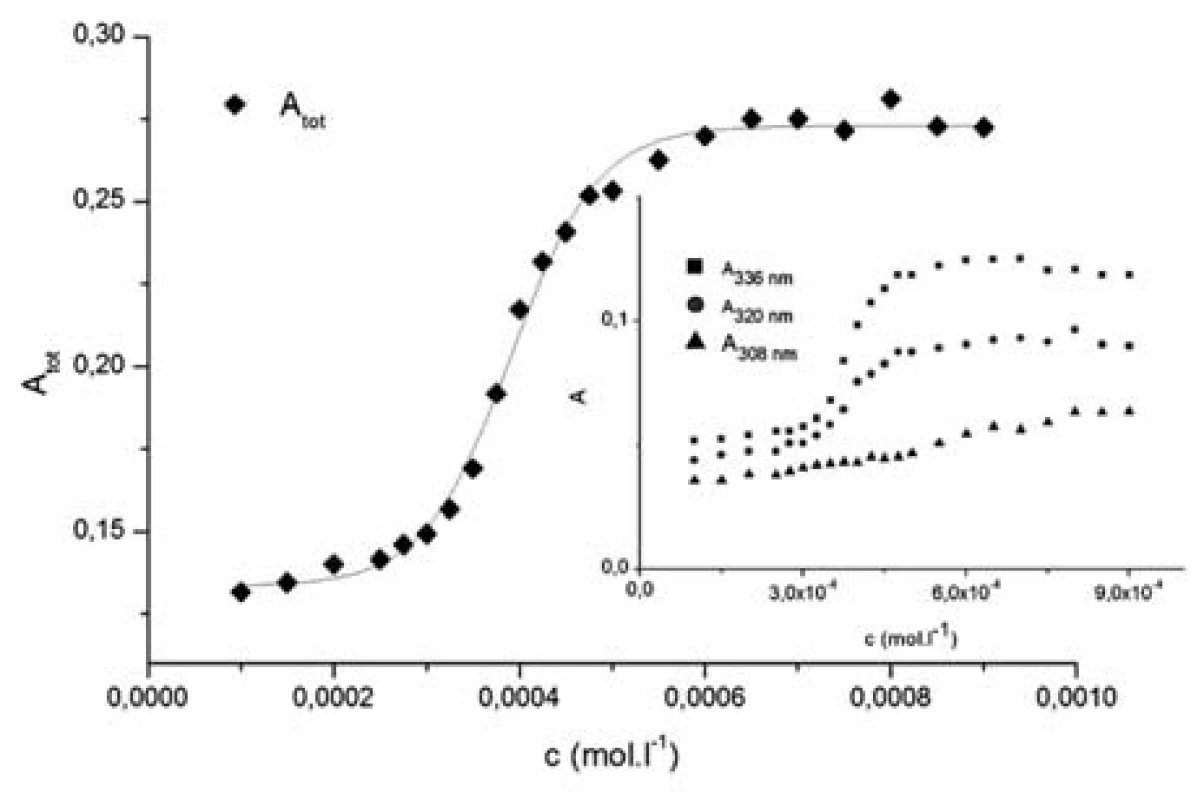
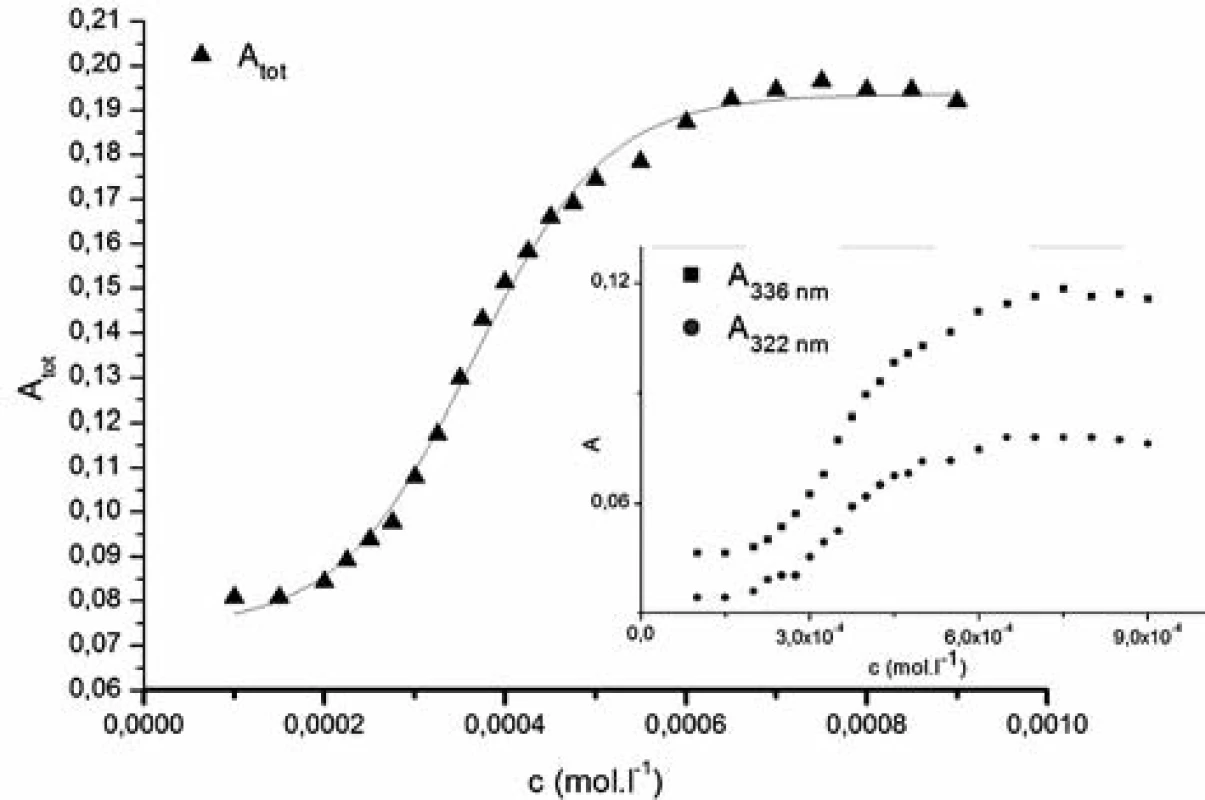
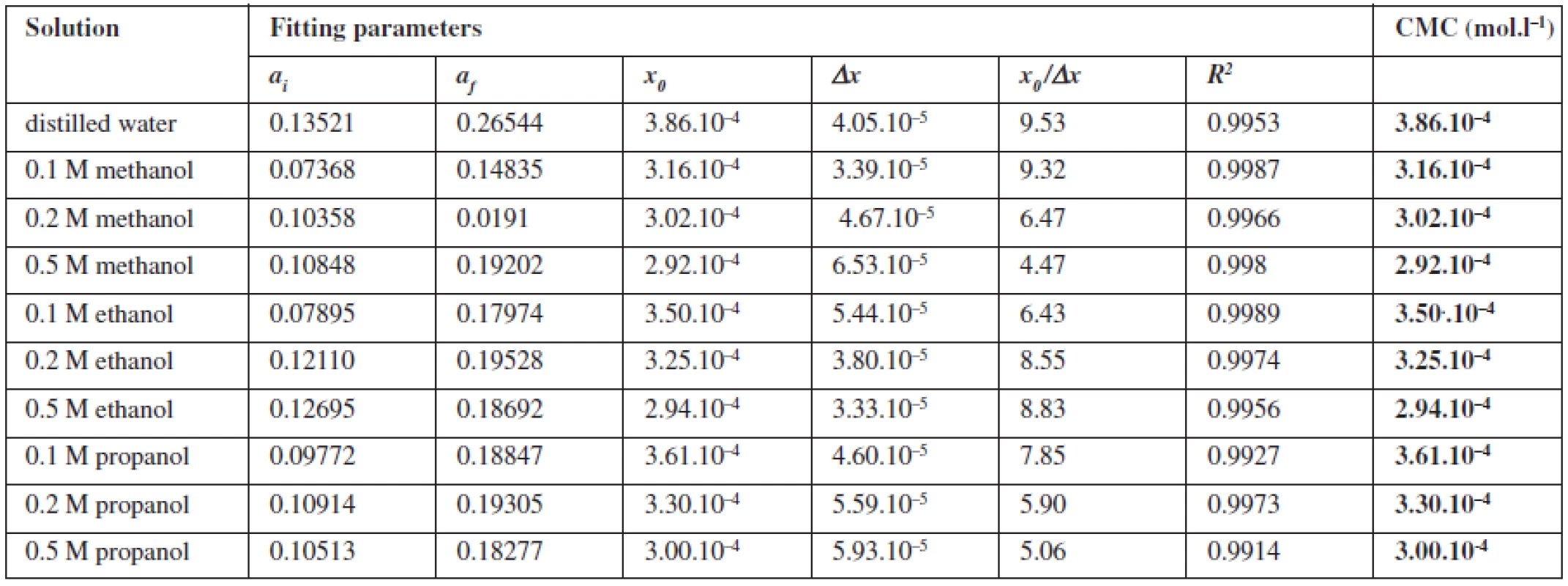
The pyrene plots of dependence of the sum of absorbances of the unmasked pyrene peaks Atot versus the tenside concentration (1182-RM-12-14) in the solvent system show a typical sigmoidal shape which was used to determine the CMC by fitting with Sigmoidal-Boltzmann equation [1]:
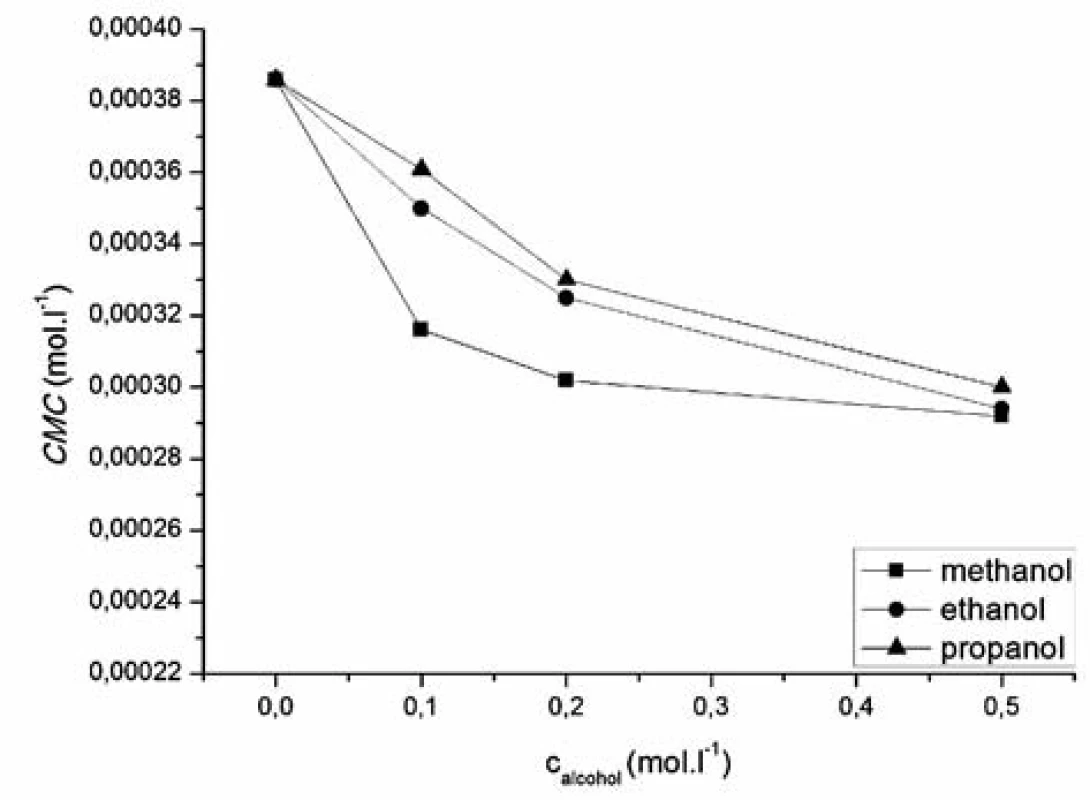
where x is the concentration of the surfactant, ai and af are the initial and final asymptotes of the sigmoid, x0 is the centre of the sigmoid and Δx is the interval of the independent variable x. The sigmoidal plot shows two potential values of CMC that can be found using equation [1], CMC1 at x0 and CMC2 at (x0 + 2Δx). The ratio x0/Δx is used to determine if CMC is represented by CMC1 or CMC2. If ratio (x0/Δx) Δ 10, CMC = CMC1 = x0, and if (x0/Δx) Δ 10, CMC = CMC2 = (x0 + 2Δx). According to Aguiar et al., our results were analyzed by equation [1]. Ratios (x0/Δx) for all observed solutions were lower than 10, thus the CMC is represented by x04, 7). The most significant fitting parameters for the studied systems, CMC and regression-square (R2), are presented in Table 1. Experimental results show that in the studied concentration range, CMC of the studied compound decreases with increasing concentration of alcohol as shown in Figure 4. The CMC was decreased the most by methanol, less by ethanol and the least by propanol. The observed decrease in CMC can be the result of entering the polar area of the micelle by short-chain alcohol molecules, which decreases the electrostatic repelling force between the ionic heads of surfactants in the micelle and results in a decrease in CMC. Therefore, when short-chain alcohols are present in a solution in a low concentration, they behave like co-surfactants. We assume that if the concentration of a mixed water-alcohol solution is higher, we would observe an increase in CMC values due to the fact that in higher concentrations alcohols may self-aggregate, which may disrupt the water structure, dielectric constants of the mixtures decrease, as a result, the electrostatic force of the ionic head groups in micelle increases, which results in an increase of CMC values. In this second scenario, short-chain alcohols in higher concentrations would act as co-solvents2, 3).
Conclusions
In the studied concentration range, our results showed a decrease in CMC by the influence of short-chain alcohols, wherein the higher the concentration of alcohol was, the bigger decrease in the critical micelle concentration was observed. The critical micelle concentration was decreased the most by methanol, less by ethanol and the least by propanol.
The work was supported by the grant No. UK/265/2015.
Conflicts of interest: none.
PharmDr. Andrea Gálusová
Comenius University in Bratislava, Faculty of Pharmacy, Department of Pharmaceutical Chemistry
Odbojárov 10, 832 32 Bratislava, Slovak Republic
e-mail: andrea.galusova@gmail.com
Sources
1. Tanford C. The Hydrophobic Effect: Formulation of Micelles and Biological Membranes. 2nd edition. New York: Wiley-Interscience Publication 1980.
2. Huang J., Mao M., Zhu B. The surface physico-chemical properties of surfactants in ethanol-water mixtures. Colloids and surfaces A: Physicochemical and Engineering aspects 1999; 155, 339–348.
3. Bielawska M., Chodzinska A., Janczuk B., Zdziennicka, A. Determination of CTAB CMC in mixed water + short-chain alcohol solvent by surface tension, conductivity, density and viscosity measurements. Colloids and Surfaces A: Physiochemical and Engineering aspects 2013; 424, 81–88.
4. Basu Ray G., Chakraborty I., Moulik S. Pyrene absorption can be a convenient method for probing critical micellar concentration (cmc) and indexing micellar polarity. J. Colloid Interface Sci. 2006; 294, 248–254.
5. Kalyansundaram K., Thomas J. K. Environmental effects on vibronic band ingtensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977; 99, 2039–2044.
6. Khan Z. H., Hhanna B. N. Electronic absorption spectra of pyrene and its monopositive ion. J. Chem. Phys. 1973; 59, 3015.
7. Aguiar J., Carpena P., Molina-Bolivar J., Carmero Ruiz C. On the determination of the critical micelle concentration by the pyrene 1 : 3 ratio method. J. Colloid Interface Sci. 2003; 258, 116–122.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2015 Issue 5
-
All articles in this issue
- Methods used in pharmaceutical technology to increase bioavailability of poorly soluble drugs after oral administration
- Level and factors influencing the patients’ satisfaction with the pharmaceutical care in Slovakia
- Drugs and health care expenditure on the aging population
- Drug bioavailability increasing by formulation of liquisolid systems
- Evaluation of compressibility of tableting mixtures using the compaction equation
- Branched polyesters as mucoadhesive carriers of drugs
- Evaluation of water absorption rate of tablets by using an Enslin-Neff device
- Evaluation of the influence of lubricants on the viscoelastic properties of tablets using the stress relaxation test
-
44th Conference drug synthesis and analysis
(Brno, 2nd to 4th September 2015) – Part 1 - Determination of biologically active compounds in the fungi of the genus Cordyceps sinensis by HPLC and NMR
- Determination of CMC of cationic tenside in aqueous and mixed water-alcohol solutions
- A comparison of SiO2-, Cu-, and Ni-supported Au nanoparticles for selective glycerol oxidation to acetic acid*
- Determination of acid-base dissociation constants of newly synthesized arylethanolamine derivatives using capillary zone electrophoresis
- HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
- The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate
- The utilization of radionuclide X-ray spectrometry in the determination of elements in medicinal plants and medicinal products used as antianemics
- On-line hyphenated capillary electrophoresis and tandem mass spectrometry used for the analysis of selected biogenic amines in grape leaves
- Validation of spectrophotometric methods of assaying metronidazole in capsules
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Methods used in pharmaceutical technology to increase bioavailability of poorly soluble drugs after oral administration
- Level and factors influencing the patients’ satisfaction with the pharmaceutical care in Slovakia
- HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
- The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate

