The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate
Authors:
Oleksandr Kryvanych; Nataliia Bevz; Nataliia Harna; Olena Bevz
Authors‘ workplace:
National university of Pharmacy, Kharkiv, Ukraine
Published in:
Čes. slov. Farm., 2015; 64, 216-219
Category:
44<sup>th</sup> Conference drug synthesis and analysis
Introduction
Phenylephrine hydrochloride (mesaton) belongs to the group of α-agonists – α-adrenergic stimulants; it has a little effect on β-adrenergic receptors of the heart. Due to its vasoconstrictive action it reduces edema and hyperemia of the mucous membranes of the upper respiratory tract and paranasal sinuses, increases blood pressure, but it has almost no effect on the cardiac output value. Thanks to these properties mesaton has found application in medicine with paracetamol and chlorpheniramine maleate in the symptomatic treatment of influenza, acute respiratory viral infections and cold in order to reduce temperature, eliminate headaches, pain in muscles and joints, edema of the mucous membranes of the respiratory tract.
The literature describes methods for quantitative determination of phenylephrine hydrochloride in combined dosage forms by HPLC1, 2); they are characterized by high efficiency, selectivity, but are not always suitable for routine analysis.
The given method is based on the chemical properties of phenols. Phenols with the unsubstituted para-position are characterized by formation of indophenols with Gibbs reagent (2,6-dichloroquinone-4-chlorimide) in the alkaline medium3).
As the object of research, Antiflu tablets containing 325 mg of paracetamol, 5 mg of phenylephrine hydrochloride, 2 mg of chlorpheniramine maleate and such excipients as microscopic cellulose, croscarmellose sodium, colloidal anhydrous silica, stearic acid, magnesium silicate, magnesium stearate, hypromellose, polyethylene glycol, mineral oil, D & C yellow varnish dye No. 10 (E 104) were chosen.
The presence of a large amount of paracetamol in the composition of the dosage form impedes determination of a small amount of phenylephrine hydrochloride by direct spectrophotometry. Therefore, it is advisable to use other accurate and selective methods in routine analysis.
Experimental methods
When developing the method for quantitative determination of phenylephrine hydrochloride, reagents meeting the requirements of the State Pharmacopoeia of Ukraine4, 5) were used: the standard samples of phenylephrine hydrochloride (Unichem Laboratories Ltd, India, batch number РРРРН/1104), paracetamol (Anqui Lu’an Pharmaceutical Co. Ltd, China, batch number PPPH 1410746) and chlorpheniramine maleate (Anqui Lu’an Pharmaceutical Co. Ltd, China, batch number SLL/P/1113052), Antiflu tablets (Contract Pharmacal Corporation, USA, batch number 0280111213).
The following analytical equipment was used: an Evolution 60s spectrophotometer, an «AXIS» ANG 200 laboratory balance (Poland), and measuring glassware of class A.
Assay
(absorption spectrophotometry)
Test solution
Weigh and powder 20 tablets. Shake the quantity of the powder containing the equivalent of 0.025 g of phenylephrine hydrochloride with 30 ml of 0.1 M hydrochloric acid for 15 minutes, dilute the same solvent to 50 ml and filter. Add 1 ml of the filtrate, 7 ml of 0.1 M hydrochloric acid, 1 ml of water, 5 ml of isopropyl alcohol, 2 ml of ammonia buffer with pH 10 and 1 ml 0.25% solution of 2,6-dichloroquinone-4-chlorimide and dilute to 25 ml with 0.1 M hydrochloric acid and mix.
Reference solution
Dissolve 50 mg of phenylephrine hydrochloride RS in 30 ml of 0.1 M hydrochloric acid and dilute the solution to the volume of 50 ml with the same solvent. To 0.5 ml of the solution obtained add 7 ml of 0.1 M hydrochloric acid, 1 ml of water, 5 ml of isopropyl alcohol, 2 ml of ammonia buffer with pH 10 and 1 ml of 0.25% solution of 2,6-dichloroquinone-4-chlorimide, dilute the solution to the volume of 25 ml with 0.1 M hydrochloric acid and mix.
Compensation solution
Dilute 7 ml of 0.1 M hydrochloric acid, 1 ml of water, 5 ml of isopropyl alcohol, 2 ml of ammonia buffer with pH 10 and 1 ml of 0.25% solution of 2,6-dichloroquinone-4-chlorimide with 0.1 M hydrochloric acid to the volume of 25 ml.
Measure the absorbance at the maximum at 615 nm.
Preparation 0.25% solution of 2,6-dichloroquinone-4-chlorimide
Dissolve 0.125 g of 2,6-dichloroquinone-4-chlorimide in 30 ml of isopropyl alcohol and dilute the solution to the volume of 50 ml with the same solvent.
Results and discussion
A method based on the properties of phenylephrine hydrochloride to form a blue colour with 2,6-dichloroquinone-4-chlorimide in the alkaline medium has been proposed.
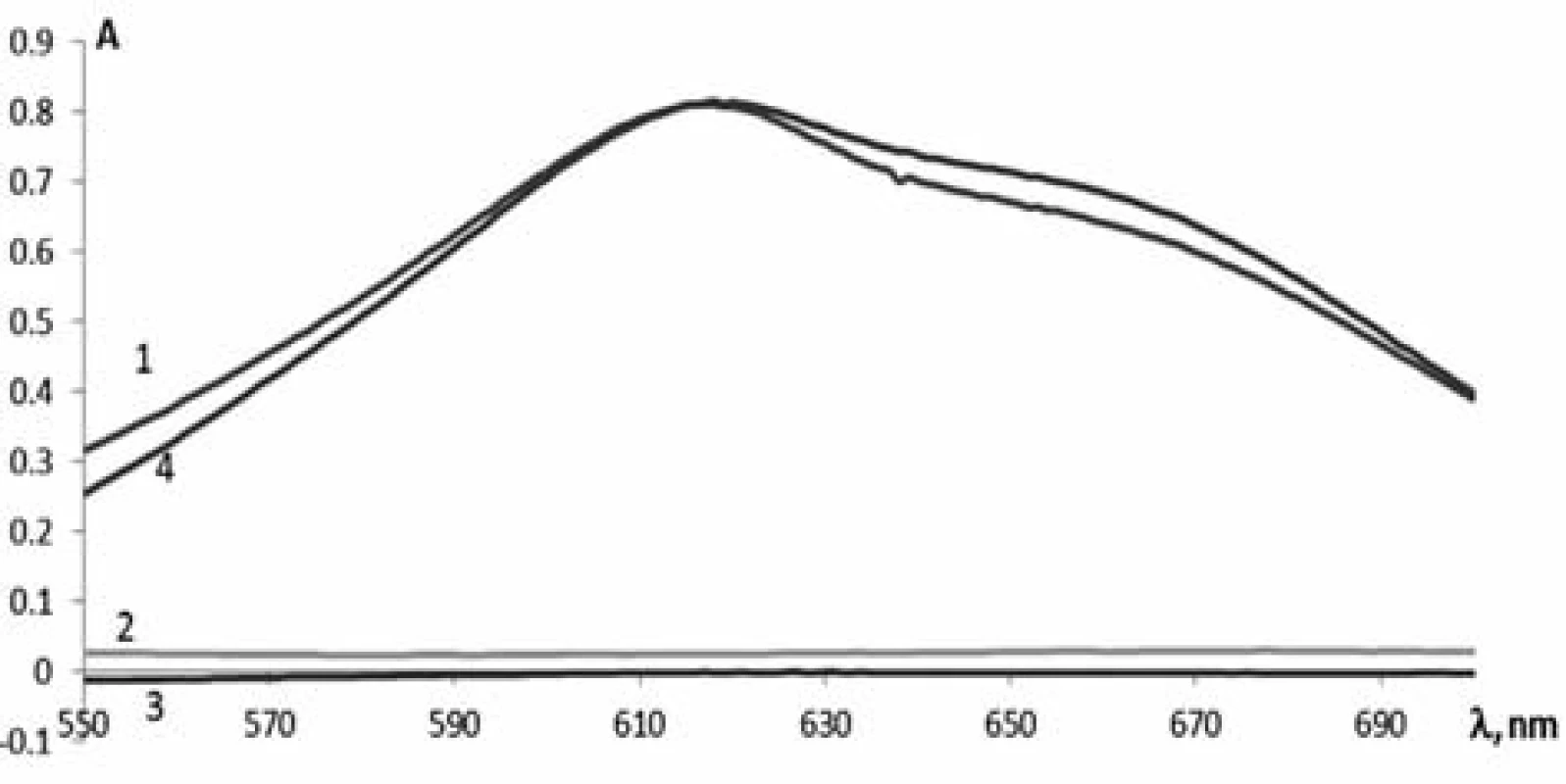
To develop the method for the quantitative determination of phenylephrine hydrochloride by absorption spectrophotometry, the solutions of phenylephrine hydrochloride, paracetamol, chlorpheniramine maleate and the test solution of Antiflu tablets were prepared dissolving them in acid and adding the necessary reagents specified above. Their absorption was studied in the range from 550 nm to 650 nm (Fig. 1). As can be seen from the spectra obtained, the maximum of phenylephrine hydrochloride (Curve 1) is observed at 615 nm. The absorption spectrum of the tablet weight of Antiflu (Curve 4) after the reaction with 2,6-dichloroquinone-4--chlorimide almost coincides with the absorption spectrum of the reference solution, but it is slightly more intense. This suggests the effect of other APIs (Curve 2 and 3). When calculating the effect of paracetamol and chlorpheniramine maleate it has been found that this value is about 3%, and only slightly affects the results of the quantitative determination of phenylephrine hydrochloride with tolerances of ± 10%.
According to standardized procedures, such validation characteristics of the spectrophotometric method as robustness, linearity, accuracy and precision were studied6, 7).
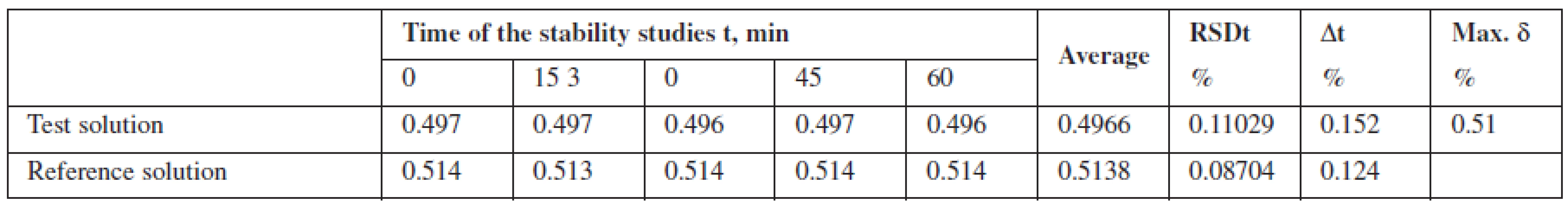
Robustness was investigated by studying stability of the solution of phenylephrine hydrochloride standard sample relative to the test solution for one hour. It was found that the test solution of Antiflu tablets was stable within the time studied.
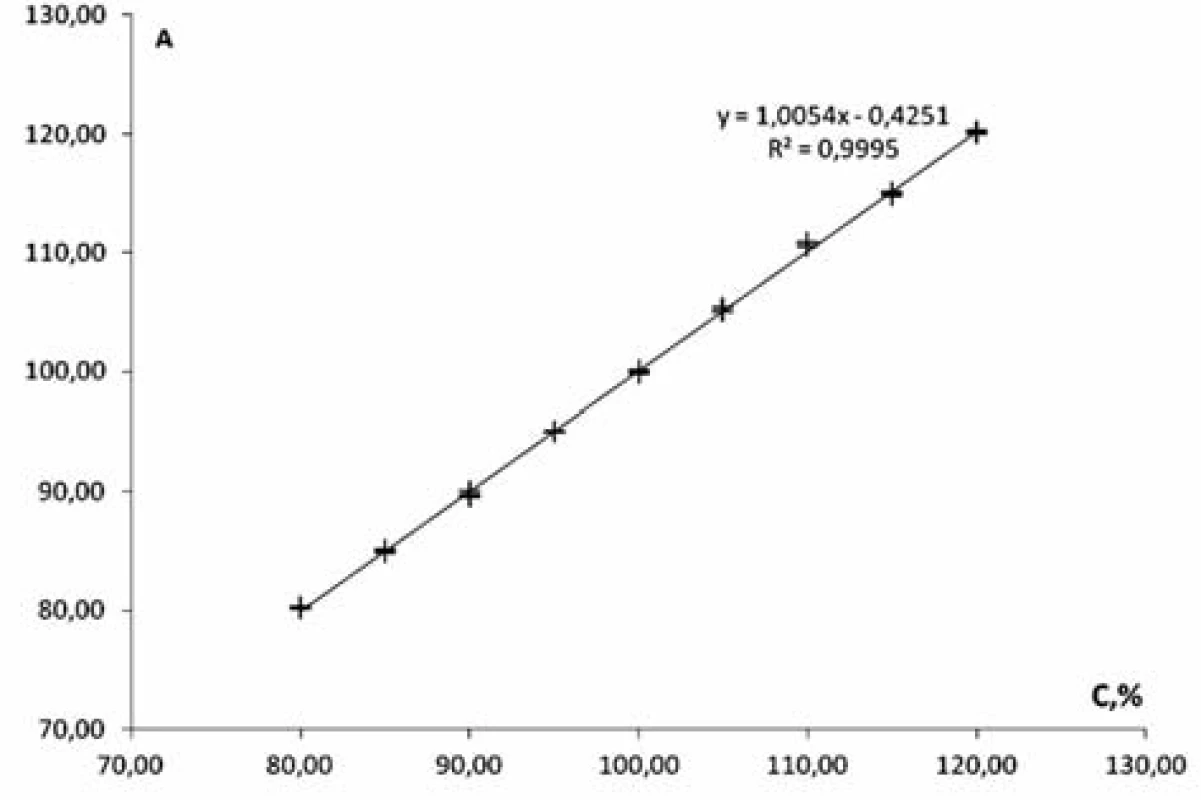
Linearity of the method was studied within the range of concentrations from 80% to 120% in relation to the nominal value of mesaton in Antiflu tablets. Nine solutions with the known concentration were prepared by taking the desired aliquot of the model solution. The results are given on the graph in the normalized coordinates (Figure 2).
The calculation of the linear dependence parameters (Table 2) was performed by the least square method (according to the data of Table 3); its results were compared with the acceptance criteria given in the State Pharmacopoeia of Ukraine.
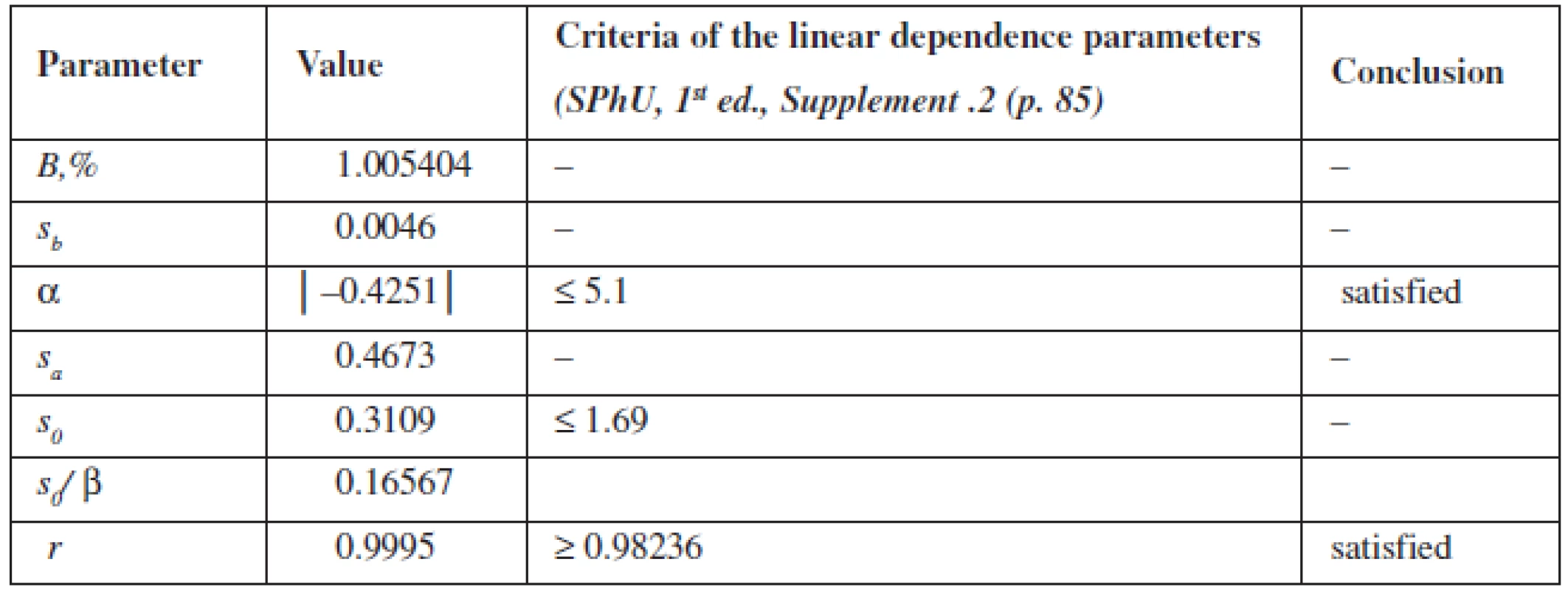
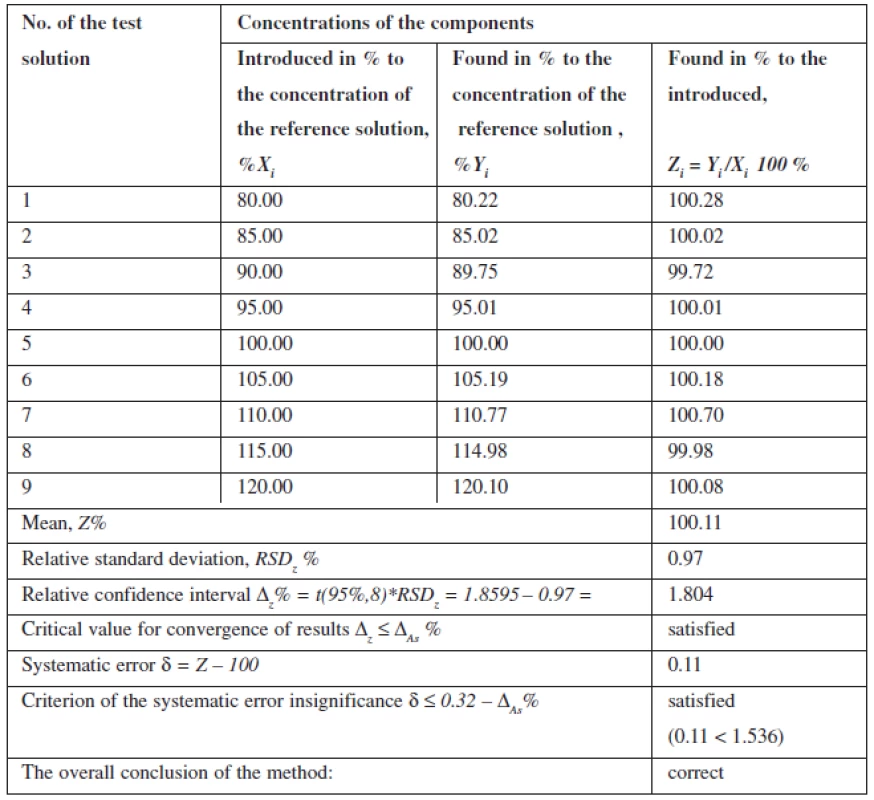
The data obtained satisfy all requirements.
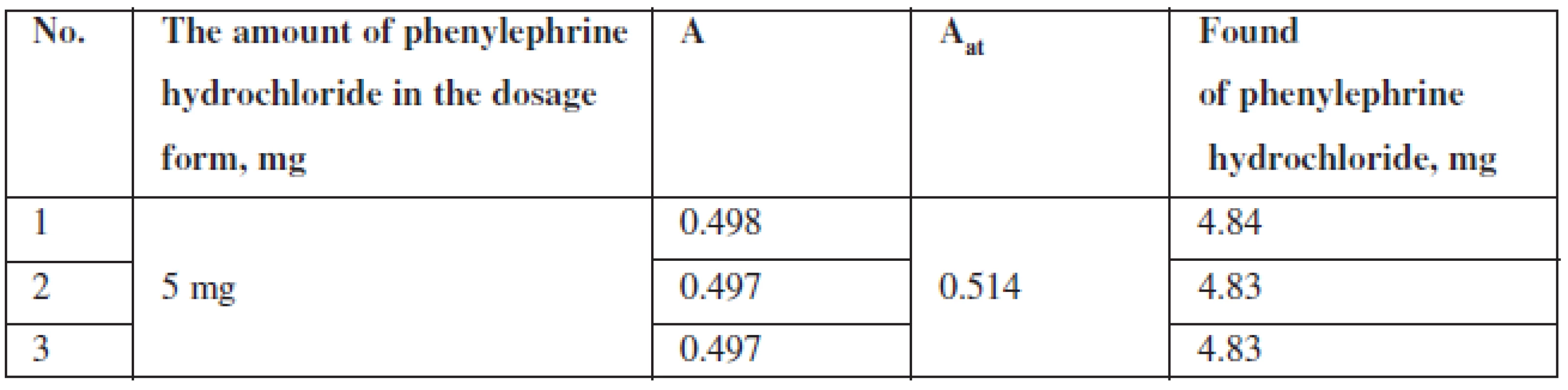
The method proposed was used for quantitative determination of phenylephrine hydrochloride Antiflu tablets.
The content of phenylephrine hydrochloride (mg) in tablets was calculated according to the formula:
where: msample – is the sample weight of the tablet powder, maverage – is the average weight of one tablet, A – is the optical density of the test solution, Ast – is the optical density of the reference solution.
The results of quantitative spectrophotometric determination of phenylephrine hydrochloride in Antiflu tablets are shown in Table 4.
Conclusions
- The method for quantitative determination of phenylephrine hydrochloride in tablets in the presence of other APIs has been developed by visible absorption spectrophotometry after the reaction with the solution of 2,6-dichloroquinone-4-chlorimide.
- The validation characteristics of the method (linearity, accuracy, precision, convergence) have been studied. The results obtained indicate the possibility for using this method for analysis of multicomponent dosage forms with phenylephrine hydrochloride.
Conflicts of interest: none.
Nataliya Bevz
National university of Pharmacy
4 Blukhera str., Kharkov, Ukraine
e-mail: Natali.chek@mail.ru
Sources
1. Pirol O., Sukuroglu M., Ozden T. Simultaneous Determination of Paracetamol, Phenylephrine Hydrochloride, Oxolamine Citrate and Chlorpheniramine Maleate by HPLC in Pharmaceutical Dosage Forms. E-Journal of Chemistry 2011; 8, 1275–1279.
2. Hamide S., Tuncel O. Simultaneous High-Performance Liquid Chromatographic Determination of Paracetamol, Phenylephrine HCl, and Chlorpheniramine Maleate in Pharmaceutical Dosage Forms. Journal of Chromatographic Science 2002; 40, 97–100.
3. Государственная Фармакопея СССР X. М: Медицина 1968.
4. Державна фармакопея України – 1-е вид. – Х.: РІРЕГ, Держ. п-во «Науково-експертний фармакопейний центр» 2001.
5. European Pharmacopoeia. http://online6.edqm.eu/ep803
6. Державна фармакопея України / Державне підприємство «Науково-експертний фармакопейний центр». – 1-е вид. – Доповнення 2. – Харків: Державне підприємство «Науково-експертний фармакопейний центр» 2008.
7. Гризодуб А. И., Леонтьев Д. А., Денисенко Н. В., Подпружников Ю. В. Стандартизованная процедура валидации методик количественного анализа лекарственных средств методом стандарта. Фармаком 2004; 3, 1–15.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2015 Issue 5
-
All articles in this issue
- Methods used in pharmaceutical technology to increase bioavailability of poorly soluble drugs after oral administration
- Level and factors influencing the patients’ satisfaction with the pharmaceutical care in Slovakia
- Drugs and health care expenditure on the aging population
- Drug bioavailability increasing by formulation of liquisolid systems
- Evaluation of compressibility of tableting mixtures using the compaction equation
- Branched polyesters as mucoadhesive carriers of drugs
- Evaluation of water absorption rate of tablets by using an Enslin-Neff device
- Evaluation of the influence of lubricants on the viscoelastic properties of tablets using the stress relaxation test
-
44th Conference drug synthesis and analysis
(Brno, 2nd to 4th September 2015) – Part 1 - Determination of biologically active compounds in the fungi of the genus Cordyceps sinensis by HPLC and NMR
- Determination of CMC of cationic tenside in aqueous and mixed water-alcohol solutions
- A comparison of SiO2-, Cu-, and Ni-supported Au nanoparticles for selective glycerol oxidation to acetic acid*
- Determination of acid-base dissociation constants of newly synthesized arylethanolamine derivatives using capillary zone electrophoresis
- HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
- The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate
- The utilization of radionuclide X-ray spectrometry in the determination of elements in medicinal plants and medicinal products used as antianemics
- On-line hyphenated capillary electrophoresis and tandem mass spectrometry used for the analysis of selected biogenic amines in grape leaves
- Validation of spectrophotometric methods of assaying metronidazole in capsules
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Methods used in pharmaceutical technology to increase bioavailability of poorly soluble drugs after oral administration
- Level and factors influencing the patients’ satisfaction with the pharmaceutical care in Slovakia
- HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
- The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate




