The utilization of radionuclide X-ray spectrometry in the determination of elements in medicinal plants and medicinal products used as antianemics
The work was based on the identification and determination of selected elements in teas, plants and medicinal products for the treatment of anemia. To evaluate the quality of medicinal plants Urtica dioica L., Papaver somniferum, L., leguminous plant Lens culinaris, M. and also in medicaments Aktiferrin® gtt., Ferronat® retard pot.tbl and Sorbifer® Durulues® por. Tbl. Flm. was used nuclear analytical method Radionuclide X-ray Fluorescence Spectrometry. This method is suitable for the analysis of samples in the solid state and a liquid state. Solid samples were homogenized and compressed into tablets of defined shape and weight. Liquid samples were filtered through a chelating membrane 3M EmporeTM, which are used to selectively capture the polyvalent metal cations and are preconcentrating the elements from sample. Samples were analysed using radiation radionuclide 238 Pu and evaluated by means of a semiconductor detector and a multichannel analyser.
Authors:
Oľga Lukačovičová; Emil Havránek
Authors‘ workplace:
Comenius University in Bratislava. Faculty of Pharmacy, Department of Pharmaceutical Analysis and Nuclear Pharmacy, Bratislava, Slovak Republic
Published in:
Čes. slov. Farm., 2015; 64, 220-222
Category:
44<sup>th</sup> Conference drug synthesis and analysis
Overview
The work was based on the identification and determination of selected elements in teas, plants and medicinal products for the treatment of anemia. To evaluate the quality of medicinal plants Urtica dioica L., Papaver somniferum, L., leguminous plant Lens culinaris, M. and also in medicaments Aktiferrin® gtt., Ferronat® retard pot.tbl and Sorbifer® Durulues® por. Tbl. Flm. was used nuclear analytical method Radionuclide X-ray Fluorescence Spectrometry. This method is suitable for the analysis of samples in the solid state and a liquid state. Solid samples were homogenized and compressed into tablets of defined shape and weight. Liquid samples were filtered through a chelating membrane 3M EmporeTM, which are used to selectively capture the polyvalent metal cations and are preconcentrating the elements from sample. Samples were analysed using radiation radionuclide 238 Pu and evaluated by means of a semiconductor detector and a multichannel analyser.
Introduction
Anemia is a disease in which decreases in haemoglobin and red blood cells below the normal level take place. Sideropenic anemia (due to iron deficiency) is the most common type of anemia. Manifestation of iron deficiency includes fatigue, sleepiness, headache, distractibility, poor blood circulation, heart palpitations, brittle nails and hair, nausea, irritability. Oral treatment with iron compounds cause stomach and intestinal disorders1). Therefore, researchers are looking for alternative sources, such as food and medicinal plants, characterized by high levels of iron. An effort to streamline the treatment of disease, tea or preparations are utilized in large volumes. Some toxic elements may be present, which in large volumes cause serious health problems. It is necessary to check the quality of the products used. There it is possible to use different analytical methods. X-ray Fluorescence Spectrometry is a technique used in a wide range of interdisciplinary applications2, 3). This method is based on the interaction low-energy radiation gamma ray and X with the sample. Analytically significant is the fact that the energy of the resulting fluorescence radiation is characteristic of the emitting element. The intensity of this radiation is proportional to the amount of the analyte in the sample. It is suitable for a simultaneous and rapid determination of elements (Z = 14 to 92). It is used for the determination of trace elements in environmental samples (soil, aerosols are deposited on the filter), the composition of metal alloys and thin films with technological applications as well as non-destructive analysis of archaeological objects and art work.
Experimental methods
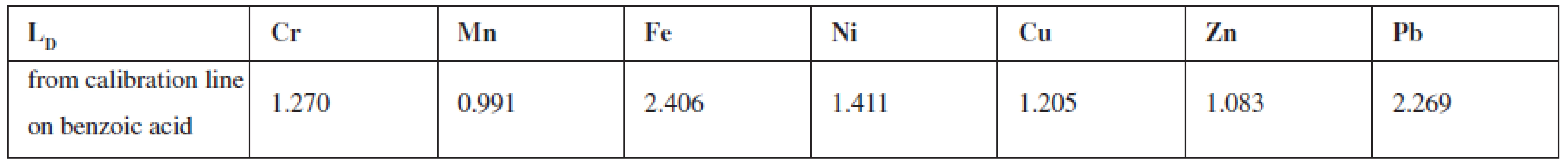
To assess the content of selected elements in samples of biological origin (plants) and organic nature (medicaments) by the nuclear analytical method, Radionuclide X-ray Fluorescence Spectrometry, it was necessary to choose a suitable radionuclide radiation source. The condition was that this source of energy was higher than the energy of the final determined element in the matrix. The analysis employed the excitation source 238Pu; detection, a semiconductor detector Si/Li for detection (operating voltage 500V); signal processing used a multichannel analyser ORTEC® with the software MAESTRO® – 32. To eliminate penetration of primary radiation and to ensure shielding the source and the percentage of registering fluorescence, reflective side geometrical arrangement of the sample-source-detector was used. Solid samples included the medicinal plants Urtica dioica L., Papaver somniferum, L., the leguminous plant Lens culinaris, M. and the solid medicaments Ferronat® retard pot. tbl and Sorbifer® Durulues® por. Tbl. Flm. After homogenization they were compressed into tablets of a defined shape (20 mm) and weight (app. 0.3g) and subjected to analysis. In the measured spectra energy maxima (peaks) were presented, corresponding to specific elements (after energetic calibration). For the determination of the selected elements in solid samples standards were prepared which differed in the content of added elements one to each other. Peak areas (with a precise addition of a particular element) in the standard were compared with the peaks areas in the sample. Analytical lines were also prepared, the parameters of which were used in the calculation of the various elements. All standards to evaluate plants were prepared on one matrix of Urtica dioica L. The same standards were prepared for the evaluation of medicinal products, but because of the fundamentally different nature (organic character), the selected matrix was benzoic acid. Samples of medicinal products contain a high content of iron, and therefore require the development of standards from the compounds of iron which were mixed in different proportions to benzoic acid. From these samples a calibration line was prepared that was used for the evaluation of iron.
Liquid samples of the medicaments Aktiferrin® gtt., Ferronat® retard pot. tbl and Sorbifer® Durulues® por. Tbl. Flm. were dissolved, and were filtered through a chelating membrane 3 M EmporeTM, which is used to selectively capture the polyvalent metal cations and preconcentrates the elements from the sample4). The uptake of iron on the disk depends on the pH of the filtered solution. To evaluate the effective pH to determine the samples, a calibration line was constructed, depending on the measured signal from the element content. The best response was observed at pH 1, so the sample preparation used water adjusted to pH 1.
Results and discussion
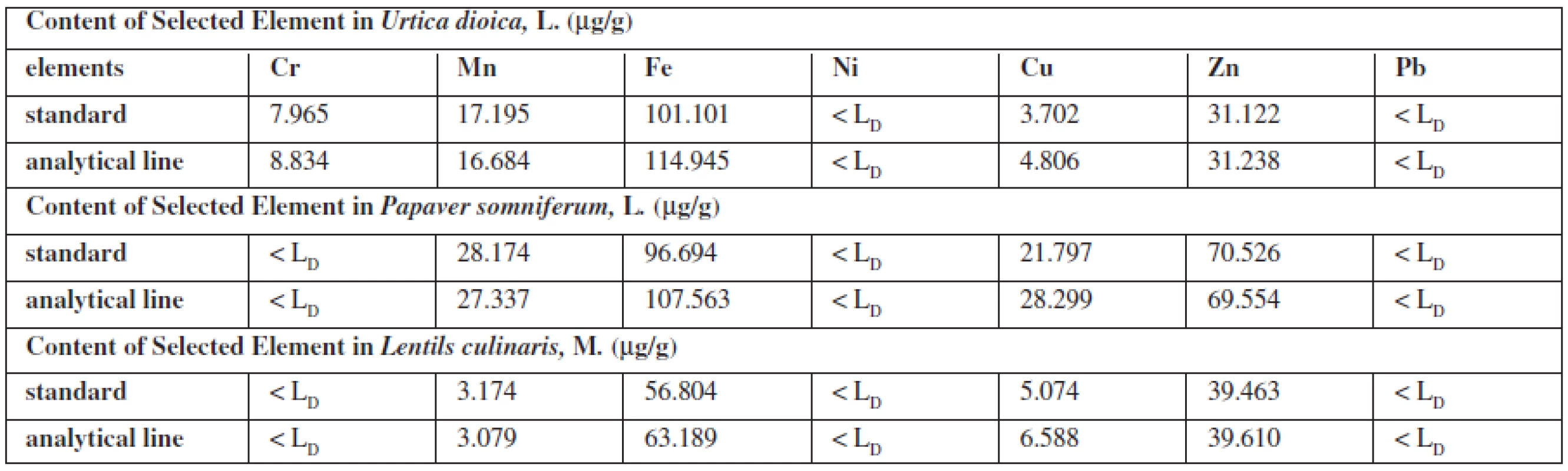
Standards for the evaluation of the elements in the plants were prepared on Urtica dioica L., which contains all the elements evaluated in a proportional rate. As the sample of the poppy plant had a problem with a high oil content, which depreciated the objectivity of assessment and sample the lentils culinary, M. were difficult to achieve the uniformity and thickness of the grain. The standard on Urtica dioica L. contained 25 μμg Cr, 50 μμg Mn, 100 μμg Fe, 25 μμg Ni, 25 μμg Cu, 50 μμg Zn, 25 μμg Pb on 1 μg of plant. The obtained signals (the area of the peak round the energy maximum of each element) were adjusted from non-analytical signals from the original matrix. This area was compared with the area measured under the peak of the corresponding element. On the same matrix analytical lines were prepared – dependence measured signal on the content of each element. The linearity was confirmed by calculated correlation factors in the content range of 0–100 μμg. Parameters of lines were used to calculate the content of a certain element.
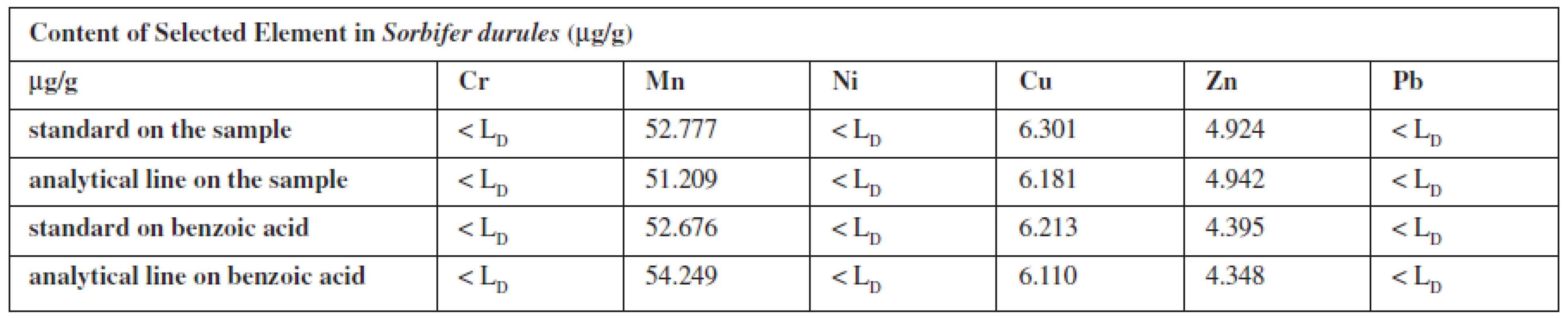
The contents of the element in medicaments were calculated from standards (the same composition standards as the biological one) and analytical line prepared on own sample or benzoic acid.
Iron was at high concentrations, and therefore the sample was diluted 1 : 10, the measurement time was shortened to 100 s, and especially for the preparation of the standard the measurement of solid Fe2O3 was used, which was prepared by weighing the calculated amount of a compound of the content corresponding to the exact amount of iron. After calculation (with parameters of calibration line constructed from solid Fe2O3 in benzoic acid), 106.54 mg of Fe was found.
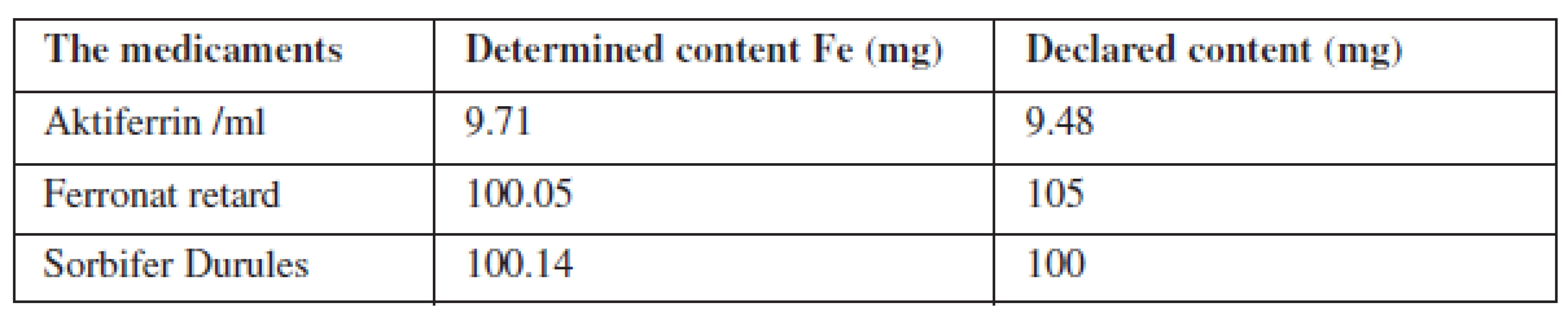
The liquid samples were analysed by a thin membrane 3 M EmporeTM, that selective uptake of polyvalent metals from solutions. The optimum pH of the solution to be shed through the membrane was found. Dissolved samples flowed through the conditioned membranes and the results were compared with the measured signals from the calibration line. The determined content of iron was compared with that declared by the factory.
Conclusions
Radionuclide X-ray Fluorescence Spectrometry has proven very useful to evaluate the polycomponent biological and organic samples of solid and liquid state. Solid samples were analyzed chemically unmodified, homogenized, and compressed into tablets of the same parameters. It is time-consuming to evaluate the elements in liquid samples because here it was necessary to find the right conditions for the use of a chelating membrane (pH of liquid medium). To determine the high content of the element showed that the preferred dilution of the sample, the reduction of the measurement time and the preparation of solid mixtures of standards. This process was quite demanding, but appears most appropriate. This method is non-destructive and fast.
Financial support this study was provided by the Slovak Grant Agency for Science under the project VEGA No.1/0873/15 and UK/195/2015.
Conflicts of interest: none.
Ing. Oľga Lukačovičová, PhD.
Katedra farmaceutickej analýzy a nukleárnej farmácie, Farmaceutická fakulta UK
Odbojárov 10, 832 32 Bratislava, Slovak Republic
e-mail: lukacovicova@fpharm.uniba.sk
Sources
1. Alleyne M., Horne K. D., Miller J. L. Individualized treatment for iron deficiency anemia in adults. Am. J. Med. 2008; 121, 943–948
2. Guerra M. B. B, Almeida E., Carvalho G. G. A., Souza P. F., Nunez L. C., Júnior D. S., Krug F. J. Comparison of analytical performance of benchtop and handheld energy dispersive X-ray fluorescence systems for the direct analysis of plant materials.
J. Anal. At. Spectrom., 2014; 29, 1667–1674.
3. Dezideri D., Meli M. A., Roselli C. Determination of essential and non-essential elements in some medicinal plants by polarized X-ray fluorescence spectrometer (EDPXRF). Microchem. J. 2010; 95, 174–180.
4. Kocot K., Sitko R. Trace and ultratrace determination of heavy metal ions by energy-dispersive X-rayfluorescence spectrometry using grapheme as solid sorbent in dispersive micro solid-phase extraction. Spectrochim. Acta, Part B. 2014; 94/95, 7–13.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2015 Issue 5
-
All articles in this issue
- Methods used in pharmaceutical technology to increase bioavailability of poorly soluble drugs after oral administration
- Level and factors influencing the patients’ satisfaction with the pharmaceutical care in Slovakia
- Drugs and health care expenditure on the aging population
- Drug bioavailability increasing by formulation of liquisolid systems
- Evaluation of compressibility of tableting mixtures using the compaction equation
- Branched polyesters as mucoadhesive carriers of drugs
- Evaluation of water absorption rate of tablets by using an Enslin-Neff device
- Evaluation of the influence of lubricants on the viscoelastic properties of tablets using the stress relaxation test
-
44th Conference drug synthesis and analysis
(Brno, 2nd to 4th September 2015) – Part 1 - Determination of biologically active compounds in the fungi of the genus Cordyceps sinensis by HPLC and NMR
- Determination of CMC of cationic tenside in aqueous and mixed water-alcohol solutions
- A comparison of SiO2-, Cu-, and Ni-supported Au nanoparticles for selective glycerol oxidation to acetic acid*
- Determination of acid-base dissociation constants of newly synthesized arylethanolamine derivatives using capillary zone electrophoresis
- HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
- The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate
- The utilization of radionuclide X-ray spectrometry in the determination of elements in medicinal plants and medicinal products used as antianemics
- On-line hyphenated capillary electrophoresis and tandem mass spectrometry used for the analysis of selected biogenic amines in grape leaves
- Validation of spectrophotometric methods of assaying metronidazole in capsules
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Methods used in pharmaceutical technology to increase bioavailability of poorly soluble drugs after oral administration
- Level and factors influencing the patients’ satisfaction with the pharmaceutical care in Slovakia
- HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
- The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate