Validation of spectrophotometric methods of assaying metronidazole in capsules
Authors:
Artem Myhal; Anna Dobrova; Olga Golovchenko; Victoriya Georgiyants
Authors‘ workplace:
The National University of Pharmacy, Kharkiv, Ukraine
Published in:
Čes. slov. Farm., 2015; 64, 225-227
Category:
44<sup>th</sup> Conference drug synthesis and analysis
Introduction
The international community imposes stringent requirements for the quality and safety of products. Nevertheless, counterfeiting of medicines is a special social danger and an urgent problem. Therefore, the suggested methods of quality control must comply with regulatory requirements and fully confirm the quality of goods.
Validation of quality control methods is recommended by the State Pharmacopoeia of Ukraine (SPhU) and the world’s leading Pharmacopoeias during the registration of medicines. This procedure aims to experimentally verify the correctness and accuracy of the above methods1–3, 6, 7, 10–13).
The guarantee of the safety and effectiveness of drugs is also their stability and absence of side effects of interaction that can take place in the organism, with different active ingredients. This applies to simultaneously appointed drugs and components of food, drinks, mineral water, food additives that people use independently in their daily diet. Patients rarely adhere to the recommended diet, without changing their eating habits. Therefore, the study of influence of the most common cases of interaction on bioavailability and pharmacological activity of prescribed drugs is relevant.
In order to experimentally prove the expediency or inadmissibility of the combined use of metronidazole as a helicobacter drug with others drugs, food, mineral water and other beverages rich in metal cations, we carried out validation of analytical method of assaying metronidazole in capsules by the standard method within the UV-spectrophotometric method according to the SPhU requirements, which is planned for use in studying the bioavailability of products of metronidazole interaction with metal salts.
Experimental methods
The object of study is metronidazole capsules “TRIKACIDE” with the content of the active substance constituting 500 mg (manufactured by PHARMASCIENCE INC., Quebec, Canada, Series: 6452653).
The standard sample substance of metronidazole (manufactured by LUOTIAN HONGYUAN BIOCHEMICAL CO., LTD, China Series: 08111803) was used to prepare the metronidazole reference solution.
An “Evolution 60S” Spectrophotometer (USA), a “Specord 200” Spectrophotometer (Germany), a AB 204 S/A METTLER TOLEDO analytical balance as well as a class A measuring vessel and reagents that conform to the SPhU were used in the study.
Methods of assaying metronidazole in capsules
The exact weight amount of the contents of 20 capsules, equivalent to 0.1000 g of metronidazole (approximately 0.1209 g) is placed in a 100.0 ml volumetric flask, 50 ml of 0.1 M hydrochloric acid solution is added, shaken for 15 minutes, brought to the mark with the same solvent. The obtained solution is then filtered, the first and the last portions of the filtrate are rejected. The aliquot of 1.0 ml of the resulting solution is taken and placed in a 100.0 ml volumetric flask and adjusted to the mark with the same solvent. The measurement of absorbance is carried out on a spectrophotometer at 277 nm wavelength in a ditch with a layer thickness of 10 mm. The measurement of the metronidazole standard sample solution absorbance was carried out at the same time10–13).
Compensation solution is 0.1 M hydrochloric acid solution.
The content of the active ingredient, according to the SPhU should be between 90–110% of the nominal content per average weight of the capsules contents (0.450–0.550)13).
Linearity, accuracy, precision and reproducibility were studied in model mixtures of a series of samples containing the known amounts of active substances and excipients. The study was conducted in the application range of 80–120% method with 5% increments. Absorbance was measured three times with the cell removed. The average value was used for the results calculation 1–6, 8–9, 14).
Results and discussion
The graph (Figure 1) confirms the linear dependence of absorbance on the concentration of the metronidazole solution1, 4, 5, 12).
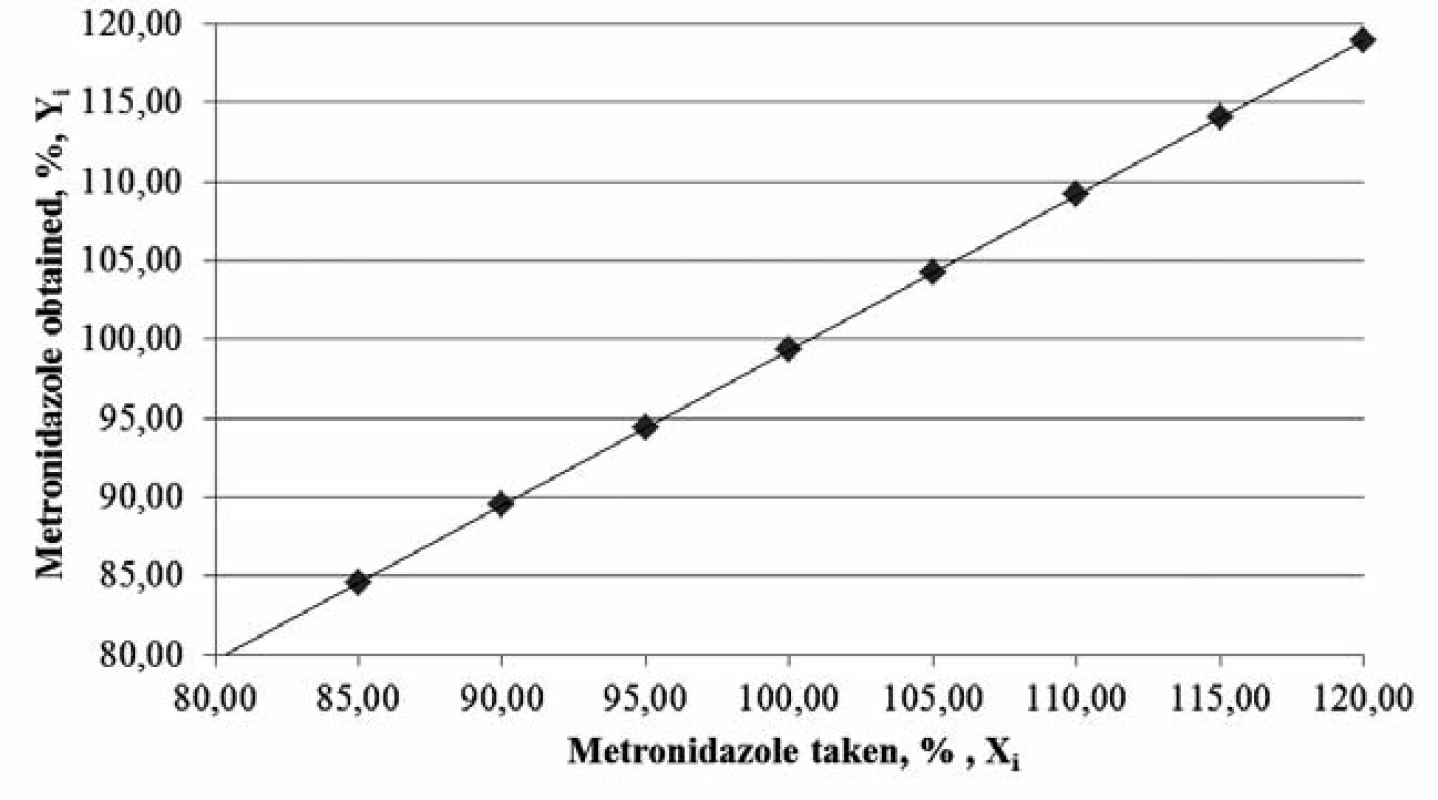
The calculation of the linear dependence for metronidazole was performed by the least squares method (Table 1).
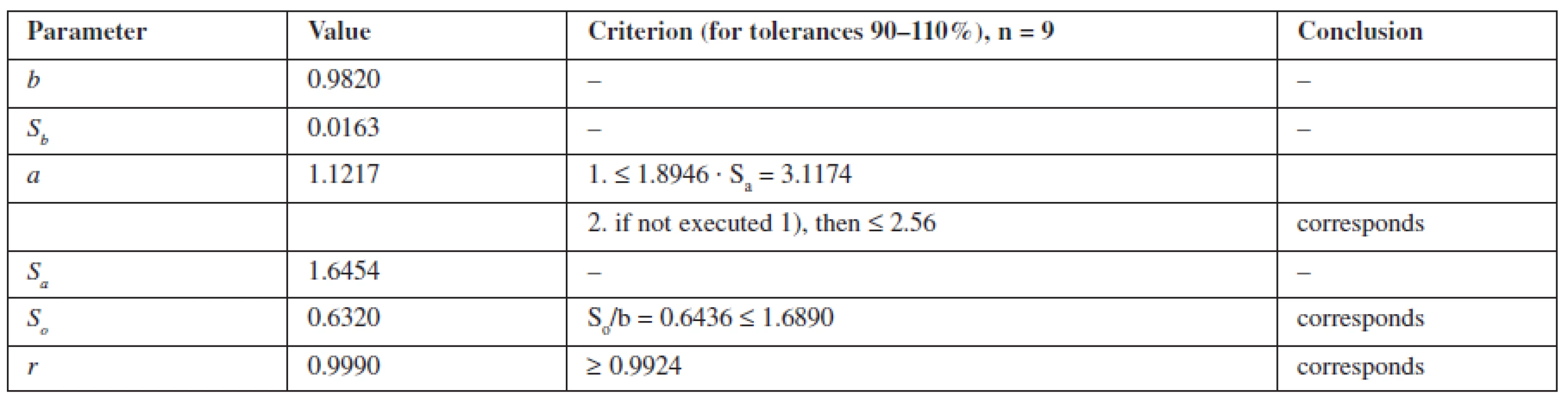
According to Table 1, the parameters of linear dependence correspond to the requirements, confirming the linearity of the proposed methods.
The criterion of practical uncertainty of methods for systematic error was 0.66%, executed at γ, % = 0.66 ≤ 1.024, characterizing the accuracy of the proposed spectrophotometric method of assaying in the range of applying the 80–120% method.
This method is characterized by convergence, as the relative confidence interval, ΔZ, % = t(95%,8) · Sz = 1.0957 is below the critical value for convergence of results, which is in this case 3.20% (Table 2).
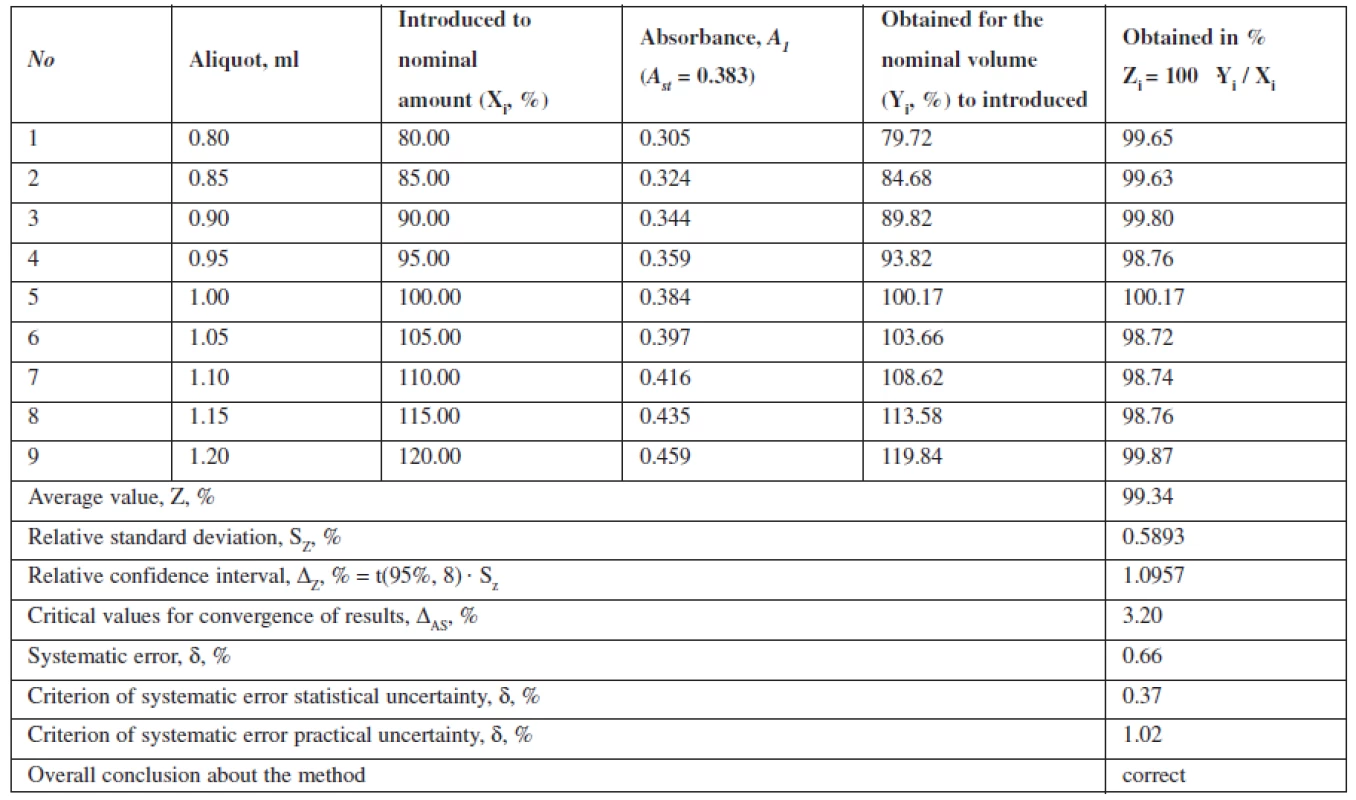
The research of reproducibility is a necessary condition if the method is planned to be included to the pharmacopeia and to be transferred to another laboratory. This parameter characterizes the accuracy of interlaboratory study methods on a series of test solutions1–2, 4, 5, 12, 15). The reproducibility was studied by measuring the optical absorption of the series of analytical solutions in different laboratories, with different equipment, by different analysts.
The data show that the proposed method can be correctly reproduced in other laboratories and characterized by the relative confidence interval of 100 ± 0.58% with a probability of 95%. Value Δx,r, % = 0.58 ≤ 3,2. The interlaboratory systematic error constituted 0.54%.
The projected total uncertainty of results of assaying methods ΔAS is 1.16% and not greater than the critical value (3.20%), which is insignificant.
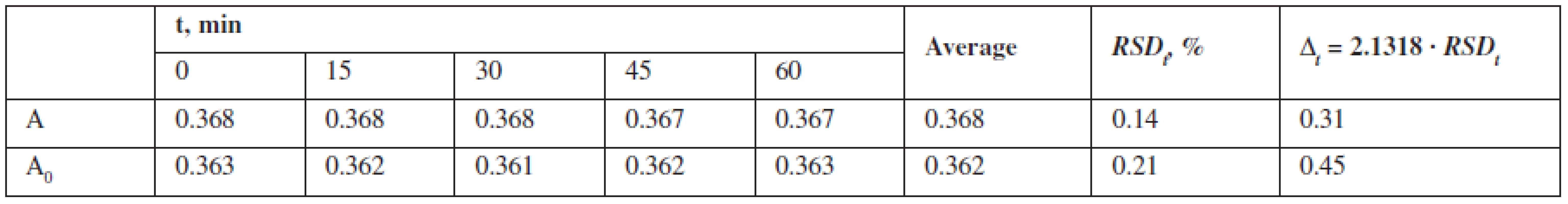
The data confirm that the solutions are stable during the research time. The stability of solutions confirms an insignificant impact of reagents, equipment, and the subjective factor if the method is transferred.
To assess the “robustness“, the stability of the test solutions was studied by measuring their absorbance every 15 minutes during an hour 1, 4–6) (Table 3).
Conclusions
Validation characteristics for the proposed assaying method were defined. Eligibility criteria for content tolerances of ± 10% were used.
The researched validation characteristics confirm linearity, precision (convergence, reproducibility), as well as the accuracy of the proposed method.
The prediction of total uncertainty of the proposed methods, determined with the use of the methods transferred, meets the eligibility criteria, which confirms the possibility of using the method of assaying the metronidazole capsules in other laboratories.
Conflicts of interest: none.
Artem Myhal
The National University of Pharmacy
53, Pushkinska str., 61002 Kharkiv, Ukraine
e-mail: artem.migal@yandex.ua
Sources
1. Bagirov V. L., Grizodub A. I., Chibilyaev T. H., et. al .Manual in validation of methods of drugs analysis. Moscow: Pharm. Ind. 2007.
2. Bezugly P. O., Georgiyants V. A., Grytsenko I. S., et al. Pharmaseutical analisis: textbook for students of higher pharmaseut. educ.estebl. III-IV level accreditation. Kharkiv: ed. NUPh, Golden pages 2013.
3. Chung Chow Chan, et al. Analytical method validation and instrument performance verification. John Wiley & Sons, Inc. 2004, 11–51.
4. Grizodub O. I. Standard procedures for the validation of methods of quality control of medicines. Kharkiv: Farmakom 2006; 1/2, 35–44.
5. Grizodub O. I. Validation of spectrophotometric methods of quantitative analysis of drugs in accordance with the requirements of SPhU. Kharkiv: Pharmakom 2002; 3, 42–50.
6. Grizodub O. I., Leontiev D. A., Denisenko N. V. A standardized procedure for validation of methods of quantitative analysis by standard medicines. Kharkiv: Pharmakom 2004; 3, 3–17.
7. Grizodub O. I., Zvolinsky N. N., Arkhipov N. N., et al. Reproducibility of pharmacopoeial spectrophotometric techniques quantify drugs in different laboratories Kharkiv: Pharmakom 2004; 2, 20–34.
8. European Pharmacopoeia. Sixth edition. Volume 2.2. Council of Europe: Strasbourg 2007; 2414–2415.
9. Salgado H. R. N., Oliveira C. L. C. G. Development and validation of an UV spectrophotometric method for determination of gatifloxacin in tablets. Pharmazie 2005; 4, 263–264.
10. State Pharmacopoeia of Ukraine. State Enterprise “Scientific and expert pharmacopoeia center”. 1st ed. Kharkiv: RIREH 2001.
11. State Pharmacopoeia of Ukraine. State Enterprise “Scientific and expert pharmacopoeia center”. App. 1., 1st ed. Kharkiv: RIREH 2004.
12. State Pharmacopoeia of Ukraine. State Enterprise “Scientific and expert pharmacopoeia center”. App. 2., 1st ed. Kharkiv: RIREH 2008.
13. State Pharmacopoeia of Ukraine. State Enterprise “Scientific and expert pharmacopoeia center”. App. 4., 1st ed. Kharkiv: RIREH 2011.
14. United States Pharmacopeia 26. – USP Convention Inc. – Rockville, 2007 [Electronic version].
15. Vishnevska L. I., Yevtifyeyeva O. A., Garna S. V. Validation characteristic of quantitative determination method of flavonoids by UV-spectrophotometry in tincture difficult “Bronhofit”. Ukrainian medical almanac 2010; 13(1), 33–35.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2015 Issue 5
-
All articles in this issue
- Methods used in pharmaceutical technology to increase bioavailability of poorly soluble drugs after oral administration
- Level and factors influencing the patients’ satisfaction with the pharmaceutical care in Slovakia
- Drugs and health care expenditure on the aging population
- Drug bioavailability increasing by formulation of liquisolid systems
- Evaluation of compressibility of tableting mixtures using the compaction equation
- Branched polyesters as mucoadhesive carriers of drugs
- Evaluation of water absorption rate of tablets by using an Enslin-Neff device
- Evaluation of the influence of lubricants on the viscoelastic properties of tablets using the stress relaxation test
-
44th Conference drug synthesis and analysis
(Brno, 2nd to 4th September 2015) – Part 1 - Determination of biologically active compounds in the fungi of the genus Cordyceps sinensis by HPLC and NMR
- Determination of CMC of cationic tenside in aqueous and mixed water-alcohol solutions
- A comparison of SiO2-, Cu-, and Ni-supported Au nanoparticles for selective glycerol oxidation to acetic acid*
- Determination of acid-base dissociation constants of newly synthesized arylethanolamine derivatives using capillary zone electrophoresis
- HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
- The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate
- The utilization of radionuclide X-ray spectrometry in the determination of elements in medicinal plants and medicinal products used as antianemics
- On-line hyphenated capillary electrophoresis and tandem mass spectrometry used for the analysis of selected biogenic amines in grape leaves
- Validation of spectrophotometric methods of assaying metronidazole in capsules
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Methods used in pharmaceutical technology to increase bioavailability of poorly soluble drugs after oral administration
- Level and factors influencing the patients’ satisfaction with the pharmaceutical care in Slovakia
- HPLC method for stability evaluation of pharmaceutical preparations containing sodium picosulfate
- The use of 2,6-dichloroquinone-4-chlorimide for quantitative determination of phenylephrine hydrochloride in combined tablets with paracetamol and chlorpheniramine maleate
