-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Multiple Independent Loci at Chromosome 15q25.1 Affect Smoking Quantity: a Meta-Analysis and Comparison with Lung Cancer and COPD
Recently, genetic association findings for nicotine dependence, smoking behavior, and smoking-related diseases converged to implicate the chromosome 15q25.1 region, which includes the CHRNA5-CHRNA3-CHRNB4 cholinergic nicotinic receptor subunit genes. In particular, association with the nonsynonymous CHRNA5 SNP rs16969968 and correlates has been replicated in several independent studies. Extensive genotyping of this region has suggested additional statistically distinct signals for nicotine dependence, tagged by rs578776 and rs588765. One goal of the Consortium for the Genetic Analysis of Smoking Phenotypes (CGASP) is to elucidate the associations among these markers and dichotomous smoking quantity (heavy versus light smoking), lung cancer, and chronic obstructive pulmonary disease (COPD). We performed a meta-analysis across 34 datasets of European-ancestry subjects, including 38,617 smokers who were assessed for cigarettes-per-day, 7,700 lung cancer cases and 5,914 lung-cancer-free controls (all smokers), and 2,614 COPD cases and 3,568 COPD-free controls (all smokers). We demonstrate statistically independent associations of rs16969968 and rs588765 with smoking (mutually adjusted p-values<10−35 and <10−8 respectively). Because the risk alleles at these loci are negatively correlated, their association with smoking is stronger in the joint model than when each SNP is analyzed alone. Rs578776 also demonstrates association with smoking after adjustment for rs16969968 (p<10−6). In models adjusting for cigarettes-per-day, we confirm the association between rs16969968 and lung cancer (p<10−20) and observe a nominally significant association with COPD (p = 0.01); the other loci are not significantly associated with either lung cancer or COPD after adjusting for rs16969968. This study provides strong evidence that multiple statistically distinct loci in this region affect smoking behavior. This study is also the first report of association between rs588765 (and correlates) and smoking that achieves genome-wide significance; these SNPs have previously been associated with mRNA levels of CHRNA5 in brain and lung tissue.
Published in the journal: Multiple Independent Loci at Chromosome 15q25.1 Affect Smoking Quantity: a Meta-Analysis and Comparison with Lung Cancer and COPD. PLoS Genet 6(8): e32767. doi:10.1371/journal.pgen.1001053
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001053Summary
Recently, genetic association findings for nicotine dependence, smoking behavior, and smoking-related diseases converged to implicate the chromosome 15q25.1 region, which includes the CHRNA5-CHRNA3-CHRNB4 cholinergic nicotinic receptor subunit genes. In particular, association with the nonsynonymous CHRNA5 SNP rs16969968 and correlates has been replicated in several independent studies. Extensive genotyping of this region has suggested additional statistically distinct signals for nicotine dependence, tagged by rs578776 and rs588765. One goal of the Consortium for the Genetic Analysis of Smoking Phenotypes (CGASP) is to elucidate the associations among these markers and dichotomous smoking quantity (heavy versus light smoking), lung cancer, and chronic obstructive pulmonary disease (COPD). We performed a meta-analysis across 34 datasets of European-ancestry subjects, including 38,617 smokers who were assessed for cigarettes-per-day, 7,700 lung cancer cases and 5,914 lung-cancer-free controls (all smokers), and 2,614 COPD cases and 3,568 COPD-free controls (all smokers). We demonstrate statistically independent associations of rs16969968 and rs588765 with smoking (mutually adjusted p-values<10−35 and <10−8 respectively). Because the risk alleles at these loci are negatively correlated, their association with smoking is stronger in the joint model than when each SNP is analyzed alone. Rs578776 also demonstrates association with smoking after adjustment for rs16969968 (p<10−6). In models adjusting for cigarettes-per-day, we confirm the association between rs16969968 and lung cancer (p<10−20) and observe a nominally significant association with COPD (p = 0.01); the other loci are not significantly associated with either lung cancer or COPD after adjusting for rs16969968. This study provides strong evidence that multiple statistically distinct loci in this region affect smoking behavior. This study is also the first report of association between rs588765 (and correlates) and smoking that achieves genome-wide significance; these SNPs have previously been associated with mRNA levels of CHRNA5 in brain and lung tissue.
Introduction
Smoking is associated with many different diseases. Lung cancer is the illness most identified with smoking, and its prevalence over time mirrors per capita tobacco consumption [1]. There has been a reduction in smoking in the United States, and a concomitant decline in the incidence of lung cancer is beginning to emerge. Nonetheless more people die from lung cancer each year than from any other cancer [2]. Chronic obstructive pulmonary disease (COPD), another serious lung disease largely attributable to smoking, is also among the leading causes of death.
Recently, genetic findings for nicotine dependence and smoking related diseases converged to implicate the chromosome 15q25.1 region, which includes the CHRNA5-CHRNA3-CHRNB4 cluster of cholinergic nicotinic receptor subunit genes. The nicotine dependence locus tagged by the single nucleotide polymorphism (SNP) rs16969968 and correlates has been replicated for smoking related traits including cigarettes-per-day and heavy smoking [3]–[11], and has been reported as the most significant association genome-wide in very recent meta-analyses [12]–[14]. This locus has also been associated with risk for lung cancer and COPD in several genome-wide association studies (GWAS) [6], [15]–[18]. This represents an exciting overlap of genetic findings for nicotine dependence and smoking related diseases. Though different SNPs may be reported by each study, the high correlation between the associated SNPs (r2>0.8 with rs16969968) implies that these statistical signals tag the same locus in European-ancestry populations. The SNP rs16969968 results in an amino acid change (D398N) in the alpha5 receptor subunit protein and has been shown to affect receptor function [19].
Extensive genotyping of the CHRNA5-CHRNA3-CHRNB4 region has provided potential evidence for at least two additional distinct signals for nicotine dependence [4], [7], [8], [20]. A second locus, tagged by rs578776, is associated with nicotine dependence and smoking in several samples of European-ancestry, with the minor allele protective in the sense that it is elevated in controls; rs578776 has only low correlation with rs16969968 in European-ancestry populations (r2 = 0.24 in the HapMap CEU panel), though the linkage disequilibrium (LD) coefficient |D'| is 1. A third important locus in this region is a group of highly correlated SNPs, tagged by rs588765, which are associated with mRNA levels of CHRNA5 in brain tissue [21], [22] and lung tissue [23]–[25] from European-ancestry subjects. When rs16969968 and rs588765 (or correlates) are studied together, three common haplotypes are observed, each with distinct effects on risk [7], [22]. There are hints that other, less common variants (minor allele frequency (MAF)≤5%) also contribute to nicotine dependence in this region, including a fourth locus represented by rs12914008 which has shown a relatively strong odds ratio of 0.73 in European-American subjects [4].
With the support of the National Institute on Drug Abuse (NIDA), we formed the Consortium for the Genetic Analysis of Smoking Phenotypes (CGASP), which includes smoking, lung cancer, and COPD researchers, to enable the pursuit of several research goals. For this first analysis project we focused on the chromosome 15q25.1 region containing CHRNA5-CHRNA3-CHRNB4. Specifically, we focused on the four distinct loci discussed above, which have low correlation with each other and have demonstrated evidence for involvement in nicotine dependence. Analyses were undertaken to investigate two questions: first, are there multiple statistically distinct genetic loci in this region that exert independent effects on smoking, and second, are similar patterns of genetic risk shared across smoking, lung cancer, and COPD.
Methods
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki and obtained informed consent from participants and approval from the appropriate institutional review boards.
Samples and study design
All subjects included in these meta-analyses were current or former smokers of European ancestry. Results from 34 datasets, which include a total of 38,617 unrelated subjects who were assessed for cigarettes-per-day, contributed to the meta-analyses. Eight of the datasets were drawn from family-based studies and contributed only a subset of unrelated individuals to these analyses. Table 1 gives sample sizes and demographics of each participating study sample. Text S7.describes additional details for each dataset, including ascertainment criteria and genotyping methods, and documents that four datasets are also members of other consortia. All datasets contributed to the analyses of smoking. A subset of these 34 datasets also had information on lung cancer cases and lung-cancer-free smoker controls (6 datasets, N = 13,614 smokers) and/or COPD cases and COPD-free smoker controls (4 datasets, N = 6,182 smokers). The data for these traits are described in Table 2 and Table 3 respectively.
Tab. 1. Description of contributing datasets for CPD. 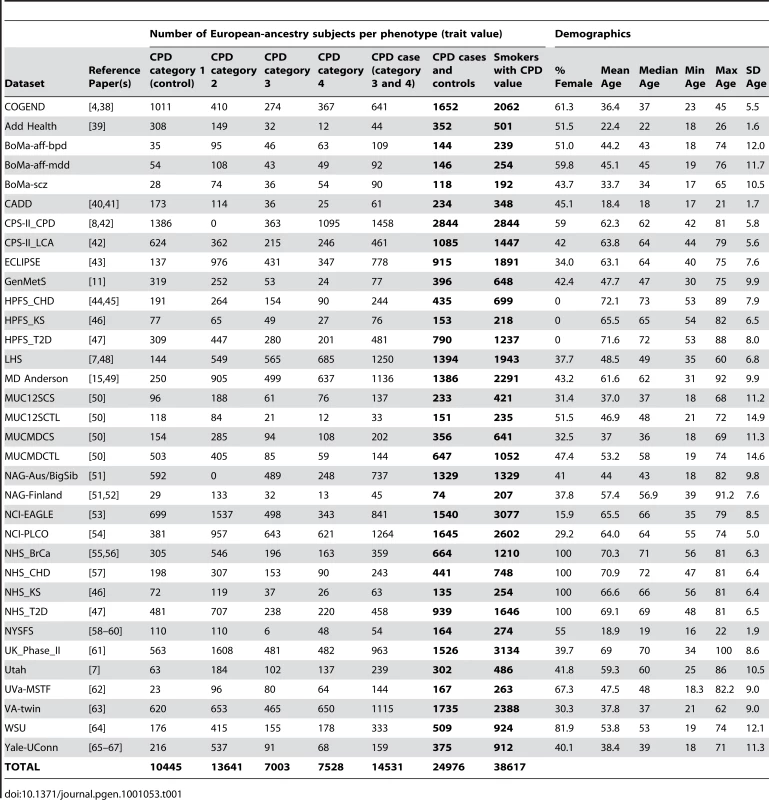
Tab. 2. Description of contributing datasets for lung cancer. 
Tab. 3. Description of contributing datasets for COPD. 
Traits for analysis
The traits examined were smoking quantity, lung cancer, and COPD. Two smoking traits were derived from measurements of cigarettes smoked per day (CPD): a 4-level categorical trait (CPD≤10, 10<CPD≤20, 20<CPD≤30, and CPD>30) and a dichotomous trait contrasting subjects from the lowest smoking category (CPD≤10: light-smoking “controls”) to those in the two highest categories combined (CPD>20: heavy smoking cases). The dichotomous trait of heavy versus light smoking was our primary trait for analysis. For one study (NAG-Finland), which used different boundaries to record CPD as detailed in the supplemental material, the distribution of CPD was examined to harmonize the phenotypes and select alternative boundaries. The numbers of subjects in each smoking category, total and by study, are given in Table 1. Lung cancer and COPD were analyzed as dichotomous traits. COPD cases were defined to have COPD as determined by post-bronchodilator spirometry as GOLD Stage II or worse (N = 1,719), or self-reported COPD, emphysema or chronic bronchitis.
SNPs for analysis
In European-ancestry populations, each of the four loci of interest can be represented by various highly correlated SNPs (SNPs having high r2 with each other). For each locus, we chose one target SNP for analysis: rs16969968 (locus 1), rs578776 (locus 2), rs588765 (locus 3), and rs12914008 (locus 4); the pairwise correlations between any two of these loci are r2<0.5 (Table S1). In samples for which a given target SNP was not available, we chose a highly correlated proxy SNP based on r2 computed with Haploview [26] using downloaded HapMap CEU genotype data, Release 23 [27]. Table S2 lists the proxy SNPs used and their r2 with the corresponding target SNPs. Figure S1 displays the SNPs for each of the 4 loci in relation to the CHRNA5-CHRNA3-CHRNB4 cluster.
Statistical analyses and meta-analysis
To ensure uniform analyses, SAS (SAS Institute, Cary, NC) and R [28] scripts for genetic association analyses were developed centrally and then distributed. The scripts were executed by each participating site, and the results returned to the coordinating group.
In each dataset, associations between the loci and the traits were evaluated using logistic regression. Our primary analysis model coded genotypes additively as the number of copies of the minor allele according to the HapMap CEU reference population. This allele is referred to as the “coded allele” (C) and the major allele is referred to as the “reference allele” (R). To confirm the appropriateness of the additive model, for each locus a 2 degree of freedom model including the additive term and a heterozygote deviation term was evaluated. The analyses of the 4-level CPD trait used generalized logistic regression to obtain separate effect estimates (beta coefficients) for each category with respect to the lowest smoking category as the referent. All these association analyses included sex and age as covariates. In addition, lung cancer and COPD analyses included categorical cigarettes-per-day as an unordered covariate.
Association results from each dataset, including the beta coefficient and standard error, were provided to the coordinating team. Meta-analysis was carried out using PLINK [29] to obtain overall summary odds ratios (ORs) and statistics. The R package rmeta [30] was used to verify results and create plots. There was no evidence of significant heterogeneity across datasets for these analyses (minimum heterogeneity p = 0.21 for dichotomous CPD, 0.07 for lung cancer, 0.24 for COPD; for categorical CPD a nominally significant p was seen only for category 3 and locus 1 (p = 0.007)). Because of varying study designs, ascertainment strategies, and representative SNPs, we nevertheless report results from random effects meta-analyses.
As noted earlier, locus 1 (representing rs16969968) is a highly replicated association finding and furthermore rs16969968 has been shown to have functional effects on the resulting alpha5-containing receptor [19]. Therefore an important question is whether the remaining loci demonstrate additional independent effects on disease risk. Although loci 2, 3 and 4 are not highly correlated with rs16969968, |D'| is high. A high |D'| can correspond to a low r2 if the alleles that tend to co-occur on the same haplotype have very different allele frequencies. Previous results in the COGEND data suggest that there may be independent or synergistic effects on nicotine dependence between locus 1 and locus 3 [4], and haplotype analyses in the Utah and LHS samples [7], and in the COGEND and CPS-II-CPD samples [22], also indicate effects of haplotypes containing loci 1, 2 and 3.
To test whether additional loci contribute to dichotomous smoking quantity over and above the effect of rs16969968, we included both locus 1 and each of the other loci in the logistic regression models adjusting for sex and age, with and without a SNP×SNP interaction term. For lung cancer and COPD the models also included categorical cigarettes-per-day as an unordered covariate. These results were then meta-analyzed as described above. The SNP×SNP interaction term was never significant in the meta-analysis (p>0.3), so we report results from the joint models without interactions. To allow comparison between single-SNP and joint results on comparable data, for each locus pair we also repeated the univariate single-SNP meta-analyses on the subset of datasets that had genotypes available at both loci. For dichotomous smoking quantity we also tabulated pair-wise joint genotype by case status counts for locus 1 (rs16969968) versus each of the other three loci across the contributing datasets that had both loci.
Multiple test correction
Across the four target loci, multiple traits (4), the multiple models (additive and additive+heterozygote deviation), and the 2-SNP joint analyses (3 loci), our study was designed to perform fewer than 80 tests. A conservative Bonferroni correction would result in an uncorrected p-value threshold of 6.25×10−4 corresponding to an experiment-wide alpha of 0.05. The results tables report uncorrected p-values which we compared to this threshold to determine statistical significance.
Results
We calculated allele frequencies within each sample to confirm that the coded allele (minor allele in HapMap CEU) was indeed the minor allele as expected in these European-ancestry subjects. Table S3 shows allele frequencies in each sample for the SNPs used. For each locus, frequencies are similar across studies and proxy SNPs, and similar to the frequencies in the HapMap CEU reference population.
All reported results are based on additive models. The additive model is appropriate because none of the tests for deviation from the additive assumption were significant. For each analysis, the tables and figures report the number of individuals successfully genotyped for the relevant SNP or SNPs.
Dichotomous CPD, single-SNP analysis
Table 4 summarizes the meta-analysis results of dichotomous CPD (heavy/light smoking) in single-SNP analysis. Meta-analysis across all 34 samples clearly shows a highly significant association between dichotomous CPD and locus 1 (tagging rs16969968). Figure 1 displays a forest plot of the summary meta-analysis results for locus 1 (p = 5.96×10−31, OR = 1.33, 95% confidence interval (1.26–1.39)), and also the ORs in each contributing dataset.
Fig. 1. Forest plot for dichotomous CPD at locus 1 (tagging rs16969968). 
The ORs and 95% CIs are for the effect per allele using additive coding in the logistic regression with age and sex as covariates. The box size indicates the precision of the OR estimate. The case and control totals include only individuals with a genotype call for locus 1. The heterogeneity p-value is 0.21. Tab. 4. Meta-analysis results for dichotomous CPD cases/controls. 
Logistic regression with sex and age as covariates. The same analysis of locus 2 (tagging rs578776) yields a meta-analysis p-value of 1.38×10−25 and an OR of 0.78 (0.74–0.81), indicating a protective association for the minor allele as has previously been reported (Figure 2). Locus 3 (tagging rs588765) under the same model gives a p-value of 0.00027 and OR of 0.93 (0.89–0.97), which meets our threshold for multiple-test corrected significance but, unlike locus 1 and locus 2, does not surpass genome-wide significance (Figure 3). Locus 4 (tagging rs12914008) does not show a main effect on dichotomous CPD (p = 0.45, OR = 1.05 (0.93–1.17). The forest plot for locus 4 is given in Figure S2.
Fig. 2. Forest plot for dichotomous CPD at locus 2 (tagging rs578776). 
The ORs and 95% CIs are for the effect per allele using additive coding in the logistic regression with age and sex as covariates. The heterogeneity p-value is 0.69. Fig. 3. Forest plot for dichotomous CPD at locus 3 (tagging rs588765). 
The ORs and 95% CIs are for the effect per allele using additive coding in the logistic regression with age and sex as covariates. The heterogeneity p-value is 0.59. Categorical CPD, single-SNP analysis
The categorical CPD analysis, which includes all 4 CPD levels in a generalized logit model, allows us to evaluate genetic effects for each CPD category with respect to the lowest smoking class (CPD≤10). Table 5 shows the results.
Tab. 5. Meta-analysis results for categorical CPD. 
Generalized logistic regression, additive model. For locus 1 (rs16969968), we see an ordinal effect with increasing CPD; that is, the odds ratio increases from 1.15 to 1.29 to 1.40 for categories 2, 3 and 4, with a corresponding decrease in p-value from 3.17×10−8 to 2.12×10−12 to 5.47×10−40. A similar ordinal effect is seen for locus 2 (rs578776), with the odds ratio decreasing from 0.88 to 0.79 to 0.77. For locus 3 (rs588765) we see an effect only with the highest smoking category (CPD>30). For locus 4 no effect is seen across smoking categories, consistent with the dichotomous CPD results.
Joint analysis for dichotomous CPD
To dissect the potential distinct effects of these loci on heavy versus light smoking, we carried out meta-analyses of joint SNP models that included sex, age, locus 1 and each of the other loci, coded additively.
In the joint analysis of locus 1 and locus 2, there is suggestive evidence of distinct effects, but the association at locus 2 is no longer genome-wide significant in the presence of locus 1. Both SNPs become less significant compared to their single locus models: in the joint model, locus 1 gives p = 2.15×10−22, OR = 1.27 (1.21–1.33) and locus 2 gives p = 4.50×10−7, OR = 0.87 (0.83–0.92). When each SNP is placed individually in the model and meta-analyzed across the 32 datasets that provided data for both loci, locus 1 gives p = 1.41×10−32, OR = 1.34 while locus 2 gives p = 1.38×10−25, OR = 0.76. The risk-increasing alleles at locus 1 (C) and locus 2 (R) are positively correlated, even though the minor alleles are negatively correlated.
In joint analysis of locus 1 and locus 3, locus 1 (rs16969968) yields a p-value of 3.52×10−36, OR = 1.47 (1.38–1.56); locus 3 (rs588765) gives p = 6.03×10−9, OR = 1.17 (1.11–1.23). Thus locus 3 attains genome-wide significance (p<5×10−8) after adjusting for the effect of locus 1. Note that adjusting for locus 1 changes the direction of effect for locus 3 (OR>1) compared to the single-SNP results. In the 33 datasets that have both loci genotyped, we obtain p = 5.39×10−29, OR = 1.32 for locus 1 alone, and p = 0.00027, OR = 0.93 (0.89–0.97) for locus 3 alone. The evidence for association in the joint model is stronger than when each SNP is analyzed alone. In fact, when locus 1 is not taken into account, the effect of locus 3 is potentially masked, and the effect of the minor allele is in an opposite direction (protective versus risk).
To further examine these interesting results for locus 1 and locus 3, we show the number of heavy and light smokers in each joint genotype class, and corresponding odds ratios using the genotype that is homozygous for both reference (major) alleles as the reference group (Table 6). The reference alleles (major in HapMap CEU) are labeled “R” and the coded alleles (minor in HapMap CEU) are labeled “C”.
Tab. 6. Joint genotype table for locus 1 versus locus 3 in CPD cases (heavy smokers) and controls (light smokers). 
R = reference allele. The first important observation is that there are very few subjects in certain cells, namely the cells corresponding to RC/CC at locus 1/locus 3, CC/RC, and CC at both loci. This table therefore reveals that the risk alleles at locus 1 (C) and locus 3 (C) are negatively correlated, and explains why the effect of rs588765 is seen only after adjusting for rs16969968. This pattern also reflects the high |D'| between the loci.
The second observation is that for the remaining, well populated cells, the coded allele at locus 3 increases risk on the background of a fixed genotype at locus 1 (e.g. row 1 of the table, corresponding to the stratum of RR homozygotes at locus 1). Similarly, for a fixed genotype at locus 3, the coded allele at locus 1 increases risk (e.g. column 1 of the table, corresponding to the stratum of RR homozygotes at locus 3). Thus for each locus, the effect seen in the joint, 2-SNP logistic regression is confirmed in the most informative stratum at the other locus.
For locus 1 and locus 4 in the joint model, locus 1 gives p = 1.01×10−38, OR = 1.35 (1.29–1.41) and locus 4 gives p = 5.55×10−3, OR = 1.17 (1.05–1.31). While the effect for locus 4 is stronger than was seen in single-SNP analysis, it does not meet our multiple test threshold for significance. In single-SNP analysis of the 25 datasets that have genotypes at both loci, locus 1 alone gives p = 7.56×10−35, OR = 1.33; locus 4 is non-significant (p = 0.45, OR = 1.05).
Lung cancer controlled for CPD
In Table 7 we report the single-SNP meta-analysis results for the six lung cancer datasets; recall that all subjects were smokers, and sex, age and categorical CPD were included as covariates. As with the CPD traits, locus 1 (rs16969968) shows highly significant evidence for association with lung cancer (p = 1.99×10−21). The summary odds ratio of 1.31 (1.24–1.38) closely matches the dichotomous CPD odds ratio of 1.33 (1.26–1.39). Figure 4 shows the association results for locus 1 by dataset and the overall meta-analysis results.
Fig. 4. Forest plot for lung cancer at locus 1 (tagging rs16969968). 
The ORs and 95% CIs are for the effect per allele using additive coding in the logistic regression with age, sex and categorical CPD as covariates. The heterogeneity p-value is 0.86. Tab. 7. Meta-analysis results for lung cancer. 
Logistic regression with sex, age and categorical CPD as covariates. Locus 2 (rs578776) also shows evidence of association with lung cancer in single-SNP analysis (p = 9.74×10−10; OR = 0.82 (0.77–0.87)) (Figure 5). Locus 3 results in a p-value of 0.0004 (OR = 0.90 (0.86–0.96)) (Figure 6); as with categorical CPD, this meets our multiple-test-corrected threshold but is not genome-wide significant. Locus 4 shows no evidence for association with lung cancer; the forest plot is given in Figure S3.
Fig. 5. Forest plot for lung cancer at locus 2 (tagging rs578776). 
The ORs and 95% CIs are for the effect per allele using additive coding in the logistic regression with age, sex and categorical CPD as covariates. The heterogeneity p-value is 0.44. Fig. 6. Forest plot for lung cancer at locus 3 (tagging rs588765). 
The ORs and 95% CIs are for the effect per allele using additive coding in the logistic regression with age, sex and categorical CPD as covariates. The heterogeneity p-value is 0.99. Joint analyses for lung cancer controlled for CPD
Similar to our analyses of categorical CPD, we carried out joint analyses of locus 1 with each of the other 3 loci, with covariates for sex, age and dummy-coded CPD. After adjusting for the effect of locus 1, none of the other loci reached our multiple-test-corrected significance threshold.
For locus 1 and locus 2 jointly in the model, locus 1 gave p = 2.68×10−13, OR = 1.26 (1.19–1.34) and locus 2 gave p = 0.012, OR = 0.91 (0.85–0.98). In joint analysis of locus 1 and locus 3, locus 1 yields p = 2.24×10−19, OR = 1.39 (1.30–1.50) and locus 3 gives p = 0.0050, OR = 1.11 (1.03–1.19), showing the same change from protective to risk for the minor allele as was observed in the dichotomous CPD analysis. Finally, in the last pairing, locus 1 gives p = 2.66×10−22 OR = 1.33 (1.26–1.41) and locus 4 gives p = 0.028, OR = 1.26 (1.02–1.55).
COPD controlled for CPD
Table 8 summarizes the meta-analysis results for the 3 datasets with the COPD trait; as with lung cancer, all subjects were smokers and sex, age, and categorical CPD were included as covariates. In these analyses, only locus 1 provides even suggestive evidence for association though it does not survive multiple test correction (uncorrected p = 0.01). The locus 1 odds ratio is 1.12 (1.02–1.23), a point estimate lower than that for CPD (1.33) and lung cancer (1.31) (Figure 7).
Fig. 7. Forest plot for COPD at locus 1 (tagging rs16969968). 
The ORs and 95% CIs are for the effect per allele using additive coding in the logistic regression with age, sex and categorical CPD as covariates. The heterogeneity p-value is 0.88. Tab. 8. Meta-analysis results for COPD. 
Logistic regression with sex, age and categorical CPD as covariates. Discussion
The first goal of this meta-analysis project was to test whether distinct loci in the CHRNA5-CHRNA3-CHRNB4 gene cluster demonstrate independent effects on smoking behavior (heavy (CPD>20) versus light (CPD≤10) smoking). We selected loci for study based on prior statistical and/or functional evidence for involvement. The second goal was to test whether similar patterns of association are seen across these loci in the smoking-related diseases of lung cancer and COPD. This meta-analysis marks the first large-scale effort to line up association results for these related traits – smoking, lung cancer, and COPD – using a uniform analysis protocol. Our results contribute important new insights about genetic risk for these traits. In particular, we demonstrate strong evidence that smoking behavior is influenced by multiple distinct loci in this region, including two loci that are associated with relevant biological effects in functional studies.
First, our results show that locus 1, representing the CHRNA5 amino acid change rs16969968 and correlates, demonstrates highly significant association with smoking behavior (OR = 1.33, p = 5.96×10−31). Our strong evidence for the involvement of locus 1 with smoking across these samples marks the robustness of its genetic effect. The contributing datasets for the smoking analyses range from samples ascertained for nicotine dependence, lung cancer, or COPD, to adolescent samples, to populations ascertained for a variety of diseases including schizophrenia, alcohol or other substance dependence, breast cancer, type 2 diabetes, and heart disease. This meta-analysis represents a very diverse group, and yet the association between rs16969968 and smoking behavior is consistent.
The second, and novel, finding from this meta-analysis is the evidence for an additional, distinct, locus in this region that is associated with heavy/light smoking and is genome-wide significant. We demonstrated that locus 3, representing rs588765 and correlates, attains a p-value of p = 6.03×10−9 (OR = 1.17) when we adjust for locus 1 in a logistic regression model. It is notable that the association between locus 3 and CPD is not as apparent in the single-SNP analysis that does not control for locus 1 (e.g. meta-analysis p = 0.0003, OR = 0.93, which does not reach genome-wide significance). The negative correlation between the risk alleles at locus 1 and locus 3 (r = −0.64) masks the effect at the latter locus in single-SNP analysis, a phenomenon known as suppression [31], [32]. The association evidence for both SNPs is strengthened in the joint analysis, with a reversal of the direction of effect for locus 3. This evidence of statistically independent association for locus 3 with smoking in our analysis is compelling given that these SNPs have also been implicated in altered mRNA levels for CHRNA5 in brain and lung tissue from European-ancestry subjects [21], [22], [24]. Thus, both statistical and functional evidence indicate that at least one SNP correlated with CHRNA5 mRNA levels is involved in risk, and highlight locus 3 as an important group of SNPs for further investigation.
A third observation from this study is that locus 2 (rs578776 and correlates) shows evidence for involvement in heavy/light smoking. Locus 2 is genome-wide significant in the single-SNP analysis of dichotomous CPD without adjustment for locus 1, with the minor allele elevated in controls (meta-analysis p = 1.38×10−25, OR = 0.78). However the association is much weaker (p = 4.50×10−7, OR = 0.87) in the joint logistic regression model that includes locus 1 and locus 2. One interpretation is that part of the single-SNP association at locus 2 is driven by the effect of locus 1 (perhaps related to the high |D'|). Nevertheless, there is evidence for residual signal at locus 2.
We tested a fourth locus representing rs12914008, a relatively uncommon (MAF ∼5%) non-synonymous SNP in CHRNB4 that has previously shown suggestive evidence for association in European-Americans [4]. In both the univariate analysis and the joint analysis with locus 1, locus 4 is not associated with smoking behavior after multiple test correction. Because of the low allele frequency of this variant, the power to detect an effect is lower than for the other three loci.
This meta-analysis therefore highlights locus 1, locus 2, and locus 3, and indicates dependencies in their effects on risk for heavy smoking. Haplotypes based on these three loci have been described [7], [22] and are seen in HapMap CEU, where the observed haplotype patterns for rs16969968 (locus 1), rs578776 (locus 2), and rs588765 (locus 3) are: A-G-C (frequency 0.425), G-G-T (0.333), G-A-C (0.207), G-A-T (0.035). Only four of the eight possible haplotypes are observed. This is consistent with the correlation structure between the loci. Locus 2 and locus 3 have low correlation with each other (e.g. r2 = 0.07 between rs578776 and rs588765 in HapMap CEU release 23); however their correlation sharply increases when locus 1 is taken into account (e.g. in GG homozygotes at rs16969968, r2 = 0.74 in HapMap CEU).
Our association results together with the correlation patterns of these three loci suggest that future haplotype or diplotype analyses across large datasets could clarify the relative contributions of these loci. Our evidence that multiple distinct genetic loci affect smoking quantity is consistent with previous reports of risk and protective haplotypes for nicotine dependence in the Utah and LHS samples [7], and in the COGEND and CPS-II-CPD samples [22]. The Utah/LHS study haplotype included 5 SNPs: two that represent locus 1 (rs16969968 and rs1051730), two that represent locus 2 (rs569207 and rs578776), and one that represents locus 3 (rs680244). The COGEND and CPS-II-CPD haplotype analyses included up to 3 loci, one each for locus 1, 2 and 3. Across all these published studies, the high-risk haplotype carries the risk allele at rs16969968 (locus 1); because of the high |D'| between loci, only one haplotype carries that allele. Among the remaining haplotypes, a low risk haplotype is obtained when the minor allele at locus 2 or the major allele at locus 3, or both, is paired with the non-risk allele at rs16969968.
Taken together, our meta-analysis results argue strongly for the existence of at least two statistically distinct loci in this region that affect risk for heavy smoking. In particular, both locus 1 and locus 3, which have known functional effects, are genome-wide significant in joint, mutually-adjusted analysis. The minor allele at locus 3 shifts from a marginally significant protective factor when considered alone to a robust risk factor when considered in combination with locus 1. The statistical evidence and negatively correlated alleles at locus 1 and locus 3 are consistent with at least two mechanistic models: distinct effects of two loci where the minor allele at each locus increases risk across a constant background at the other locus, or a haplotype dose effect where alleles at the two loci act in concert on the same haplotype strand. In the latter model, the minor-major and major-minor haplotypes each increase risk relative to the major-major haplotype, as can be seen in Table 6 once it is recognized that the rarity of the minor-minor haplotype implies that the double-heterozygote cell essentially represents the minor-major and major-minor diplotype. It is also possible that multiple rare variants underlie these findings, as has been suggested in general for disease associations with common SNPs [33]. It remains possible that these associations with locus 1, locus 2 and locus 3 are reflecting correlation with yet another underlying, untyped variant that alone explains the altered biology leading to risk. However, biological involvement of multiple loci appears more likely given that two of these loci represent two distinct, relevant functional consequences: namely, locus 1 (the amino acid change at rs16969968) is associated with altered receptor response to a nicotine agonist in vitro [19], and locus 3 (rs588765 and correlates) is associated with altered mRNA levels of CHRNA5 in brain and lung tissue [22], [24]. Further investigation via resequencing, biological/functional assays, and animal models is needed to dissect the causal biology that underlies the statistical evidence.
An important open question is the degree to which the associations between chr15q25 variants and lung cancer are due to their effects on smoking. When comparing smoking and lung cancer single-SNP results, the patterns of association (odds ratios and directions of effect) were similar across the loci studied. Locus 1 is associated with lung cancer even when controlling for amount smoked per day (p = 1.99×10−21, OR = 1.31). This result suggests possible direct genetic effects of locus 1 on this cancer, at least in the presence of smoking. However, CPD is not a sufficient proxy for carcinogen exposure [34], and in never-smokers there is a lack of association between locus 1 and lung cancer [35]–[37], so it is possible that more refined adjustment for smoking will reduce or abolish this association.
For lung cancer, after controlling for categorical CPD and effects of locus 1, we were not able to definitively demonstrate association at either locus 2 or locus 3 after correction for multiple tests. For the mutually adjusted analysis of locus 1 and locus 3 for lung cancer, we observed the same change in the direction for the locus 3 odds ratio that we observed in the joint-SNP analysis of smoking. However, unlike what was seen for smoking, for lung cancer the magnitude (and significance) of the effects did not increase. There are several possible reasons for this, including: chance, the smaller sample size for lung cancer, or qualitative differences in the relationship between these loci and smoking behavior versus the relationship between these loci and lung cancer (after adjusting for smoking quantity). This highlights the challenges posed when attempting to dissect the contributions of multiple loci of modest effect on complex, correlated traits. Further studies, and larger sample sizes, are needed.
For COPD, when controlling for cigarettes-per-day we did not find evidence for association with any of the loci after correction for multiple tests. For locus 1, the odds ratio of 1.12 (1.01–1.23) is lower than for smoking and lung cancer. The COPD analyses were based on smaller samples than those available for CPD or for lung cancer.
Very recently, three other large smoking genetics consortia published their meta-analysis findings that confirm locus 1 (representing not only rs16969918 but also rs1051730 and other SNPs) as the locus most associated with smoking quantity, genome-wide [12]–[14]. All three studies used linear regression to test for association with either quantitative CPD value [14] or categorical CPD (1–10, 11–20, 21–30, and 31+) [12], [13]. Those consortia also report results from conditional analyses in which a locus 1 SNP was included as a covariate, paralleling our joint analyses.
In contrast to our novel finding in CGASP of genome-wide significance for locus 3 when analyzed jointly with locus 1, none of the other consortia report strong evidence for locus 3 when paired with locus 1. In the Oxford-GSK study [13], imputation using 1000 Genomes data detected the most significant single-SNP association for CPD at the locus 1 SNP rs55853698 (r2>0.96 with rs16969968). After conditioning on rs55853698, the strongest residual signal was detected at a locus 2 SNP, rs6495308 (p = 3.96×10−5; r2 = 0.825 with rs578776 in HapMap CEU); they do not report the association result for rs588765 in the conditioned analysis, although it must have been less significant than 3.96×10−5. In their single-SNP analysis, rs6495308 (locus 2) gave a p-value of 2.2×10−10. Their results for locus 2 are therefore consistent with our observation that in joint analysis of locus 1 and locus 2, the significance at locus 2 is reduced compared to the single-SNP analysis. They do not report on whether the evidence for locus 1 and locus 3 strengthens in the joint analysis compared to single-SNP analysis, as we observed in the CGASP datasets. They do note that there is no obvious residual association with a third SNP after conditioning on either the pairing of locus 1 (rs16969968) and locus 3 (rs588765), or the pairing of locus 1 (rs55853698) and locus 2 (rs6495308). That result is consistent with the correlation and haplotype structure of these three loci discussed previously.
In the ENGAGE study [12], conditioning on the locus 1 SNP rs1051730 identified residual evidence at rs2869046 (p = 4.8×10−5) and rs2036534 (p = 9.1×10−5), neither of which is genome-wide significant. Rs2036534 tags locus 2 (r2 = 0.74 with rs578776 in HapMap CEU) while rs2869046 is only weakly correlated with locus 3 (r2 = 0.46).
In TAG [14], the conditional analyses indicated residual association at rs684513 (p = 6.3×10−9), rs9788682 (p = 1.06×10−8), and rs7163730 (p = 1.22×10−8), which attain genome-wide significance. These SNPs are each correlated with locus 2, and much less correlated with locus 3 (r2 = 0.7, 0.55 and 0.56 respectively with rs578776 in HapMap CEU; r2<0.11 with rs588765). It is possible that differences in samples, phenotype definitions, or analysis methods may be contributing to the differences between our strong findings for locus 3 and the three other consortium reports. To further understand the genetic contributions in this region, more work is needed, and not only statistical evidence but also biological evidence will be important.
In summary, our meta-analysis demonstrates significant, robust association of locus 1, representing the non-synonymous CHRNA5 SNP rs16969968 as well as rs1051730 and rs55853698, with smoking heaviness across very diverse datasets. Our study also demonstrates strong evidence that at least one additional distinct locus in this region affects risk for heavy smoking. In particular, we have identified for the first time that locus 3 – representing the CHRNA5 expression-associated SNPs rs588765 and correlates – surpasses GWAS-level significance for association with heavy smoking in European-ancestry subjects; this effect is detectable after adjusting for the effect of rs16969968. This new result for locus 3 raises the corresponding SNPs (rs588765 and correlates) to the level of interest already accorded to the two loci which have previously been detected at GWAS-level significance in single-SNP analyses: locus 1 (rs16969968 and correlates) and locus 2 (rs578776 and correlates). Our result also has implications for all genetic association studies, as it illustrates that joint analysis of SNPs is an important tool for identifying genome-wide significant effects that, soberingly, may be obscured in single SNP analyses.
Our study used multiple highly correlated SNPs to represent each of the 4 tested loci, depending on availability in each dataset, and all subjects were of European ancestry. Hence this study is not designed to determine which SNP(s), among the highly correlated SNPs for each locus, are most likely to be biologically involved. Future work, involving large-scale meta-analysis of other populations (e.g. Asian or African ancestry) to capitalize on LD differences between populations, comprehensive functional annotation of genetic variants, DNA re-sequencing and variant discovery, and functional and animal studies may help narrow down these large sets of correlated SNPs to the most promising causal alleles.
Supporting Information
Zdroje
1. MackayJ
EriksenM
ShafeyO
2006 The Tobacco Atlas. 2 ed Atlanta, GA American Cancer Society
2. American Cancer Society 2009 Cancer Facts and Figures Atlanta American Cancer Society
3. SacconeSF
HinrichsAL
SacconeNL
ChaseGA
KonvickaK
2007 Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Human Molecular Genetics 16 36 49
4. SacconeNL
WangJC
BreslauN
JohnsonEO
HatsukamiD
2009 The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res 69 6848 6856
5. BerrettiniW
YuanX
TozziF
SongK
FrancksC
2008 alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Molecular Psychiatry 13 368 373
6. ThorgeirssonTE
GellerF
SulemP
RafnarT
WisteA
2008 A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452 638 642
7. WeissRB
BakerTB
CannonDS
von NierderhausernA
DunnDM
2008 A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet 4 e1000125 doi:10.1371/journal.pgen.1000125
8. StevensVL
BierutLJ
TalbotJT
WangJC
SunJ
2008 Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev 17 3517 3525
9. ShervaR
WilhelmsenK
PomerleauCS
ChasseSA
RiceJP
2008 Association of a SNP in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with “pleasurable buzz” during early experimentation with smoking. Addiction 103 1544 1552
10. BakerTB
WeissRB
BoltD
von NiederhausernA
FioreMC
2009 Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res 11 785 796
11. KeskitaloK
BromsU
HeliovaaraM
RipattiS
SurakkaI
2009 Association of serum cotinine level with a cluster of three nicotinic acetylcholine receptor genes (CHRNA3/CHRNA5/CHRNB4) on chromosome 15. Hum Mol Genet 18 4007 4012
12. ThorgeirssonTE
GudbjartssonDF
SurakkaI
VinkJM
AminN
2010 Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet 42 448 453
13. LiuJZ
TozziF
WaterworthDM
PillaiSG
MugliaP
2010 Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet 42 436 440
14. The Tobacco and Genetics Consortium 2010 Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet 42 441 447
15. AmosCI
WuX
BroderickP
GorlovIP
GuJ
2008 Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet 40 616 622
16. HungRJ
McKayJD
GaborieauV
BoffettaP
HashibeM
2008 A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 452 633 637
17. LiuP
VikisHG
WangD
LuY
WangY
2008 Familial aggregation of common sequence variants on 15q24-25.1 in lung cancer. J Natl Cancer Inst 100 1326 1330
18. PillaiSG
GeD
ZhuG
KongX
ShiannaKV
2009 A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet 5 e1000421 doi:10.1371/journal.pgen.1000421
19. BierutLJ
StitzelJA
WangJC
HinrichsAL
GruczaRA
2008 Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 165 1163 1171
20. SacconeNL
SacconeSF
HinrichsAL
StitzelJA
DuanW
2009 Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet 150B 453 466
21. WangJC
GruczaR
CruchagaC
HinrichsAL
BertelsenS
2009 Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry 14 501 510
22. WangJC
CruchagaC
SacconeNL
BertelsenS
LiuP
2009 Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet 18 3125 3135
23. WangJC
BierutLJ
GoateAM
2009 Variants weakly correlated with CHRNA5 D398N polymorphism should be considered in transcriptional deregulation at the 15q25 locus associated with lung cancer risk. Clin Cancer Res 15 5599; author reply 5599
24. FalvellaFS
GalvanA
FrullantiE
DraganiTA
2009 Reply to the Letter to the Editor from Wang. Clin Cancer Res 15 5599
25. FalvellaFS
GalvanA
FrullantiE
SpinolaM
CalabroE
2009 Transcription deregulation at the 15q25 locus in association with lung adenocarcinoma risk. Clin Cancer Res 15 1837 1842
26. BarrettJC
FryB
MallerJ
DalyMJ
2005 Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21 263 265
27. The International HapMap Consortium 2005 A haplotype map of the human genome. Nature 437 1299 1320
28. R Development Core Team 2008 R: A language and environment for statistical computing Vienna, Austria R Foundation for Statistical Computing
29. PurcellS
NealeB
Todd-BrownK
ThomasL
FerreiraMA
2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
30. LumleyT
rmeta: Meta-analysis, R package version 2.15
31. CongerAJ
1974 A revised definition for suppressor variables: A guide to their identification and interpretation. Educational and Psychological Measurement 34 35 46
32. MacKinnonDP
KrullJL
LockwoodCM
2000 Equivalence of the mediation, confounding, and suppression effect. Prevention Science 1 173 181
33. DicksonSP
WangK
KrantzI
HakonarsonH
GoldsteinDB
2010 Rare variants create synthetic genome-wide associations. PLoS Biol 8 e1000294 doi:10.1371/journal.pbio.1000294
34. Le MarchandL
DerbyKS
MurphySE
HechtSS
HatsukamiD
2008 Smokers with the CHRNA lung cancer-associated variants are exposed to higher levels of nicotine equivalents and a carcinogenic tobacco-specific nitrosamine. Cancer Res 68 9137 9140
35. WangY
BroderickP
MatakidouA
EisenT
HoulstonRS
2010 Role of 5p15.33 (TERT-CLPTM1L), 6p21.33 and 15q25.1 (CHRNA5-CHRNA3) variation and lung cancer risk in never-smokers. Carcinogenesis 31 234 238
36. SpitzMR
AmosCI
DongQ
LinJ
WuX
2008 The CHRNA5-A3 region on chromosome 15q24-25.1 is a risk factor both for nicotine dependence and for lung cancer. J Natl Cancer Inst 100 1552 1556
37. LandiMT
ChatterjeeN
YuK
GoldinLR
GoldsteinAM
2009 A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet 85 679 691
38. BierutLJ
MaddenPA
BreslauN
JohnsonEO
HatsukamiD
2007 Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet 16 24 35
39. HarrisKM
HalpernCT
SmolenA
HaberstickBC
2006 The National Longitudinal Study of Adolescent Health (Add Health) twin data. Twin Res Hum Genet 9 988 997
40. StallingsMC
CorleyRP
HewittJK
KrauterKS
LessemJM
2003 A genome-wide search for quantitative trait loci influencing substance dependence vulnerability in adolescence. Drug Alcohol Depend 70 295 307
41. StallingsMC
CorleyRP
DenneheyB
HewittJK
KrauterKS
2005 A genome-wide search for quantitative trait Loci that influence antisocial drug dependence in adolescence. Arch Gen Psychiatry 62 1042 1051
42. CalleEE
RodriguezC
JacobsEJ
AlmonML
ChaoA
2002 The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer 94 2490 2501
43. VestboJ
AndersonW
CoxsonHO
CrimC
DawberF
2008 Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J 31 869 873
44. RimmEB
GiovannucciEL
WillettWC
ColditzGA
AscherioA
1991 Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 338 464 468
45. PaiJK
MukamalKJ
RexrodeKM
RimmEB
2008 C-reactive protein (CRP) gene polymorphisms, CRP levels, and risk of incident coronary heart disease in two nested case-control studies. PLoS One 3 e1395 doi:10.1371/journal.pone.0001395
46. CurhanGC
TaylorEN
2008 24-h uric acid excretion and the risk of kidney stones. Kidney Int 73 489 496
47. QiL
CornelisMC
KraftP
StanyaKJ
KaoWH
2010 Genetic variants at 2q24 are associated with susceptibility to type 2 diabetes. Hum Mol Genet 19 2706 2715
48. AnthonisenNR
ConnettJE
KileyJP
AltoseMD
BaileyWC
1994 Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 272 1497 1505
49. SpitzMR
WeiQ
DongQ
AmosCI
WuX
2003 Genetic susceptibility to lung cancer: the role of DNA damage and repair. Cancer Epidemiol Biomarkers Prev 12 689 698
50. Van den OordEJ
RujescuD
RoblesJR
GieglingI
BirrellC
2006 Factor structure and external validity of the PANSS revisited. Schizophr Res 82 213 223
51. SacconeSF
PergadiaML
LoukolaA
BromsU
MontgomeryGW
2007 Genetic linkage to chromosome 22q12 for a heavy-smoking quantitative trait in two independent samples. Am J Hum Genet 80 856 866
52. LoukolaA
BromsU
MaunuH
WidenE
HeikkilaK
2008 Linkage of nicotine dependence and smoking behavior on 10q, 7q and 11p in twins with homogeneous genetic background. Pharmacogenomics J 8 209 219
53. LandiMT
ConsonniD
RotunnoM
BergenAW
GoldsteinAM
2008 Environment And Genetics in Lung cancer Etiology (EAGLE) study: an integrative population-based case-control study of lung cancer. BMC Public Health 8 203
54. HayesRB
RedingD
KoppW
SubarAF
BhatN
2000 Etiologic and early marker studies in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials 21 349S 355S
55. CaporasoN
GuF
ChatterjeeN
Sheng-ChihJ
YuK
2009 Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS ONE 4 e4653 doi:10.1371/journal.pone.0004653
56. HunterDJ
KraftP
JacobsKB
CoxDG
YeagerM
2007 A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet 39 870 874
57. ColditzGA
RimmEB
GiovannucciE
StampferMJ
RosnerB
1991 A prospective study of parental history of myocardial infarction and coronary artery disease in men. Am J Cardiol 67 933 938
58. ElliottDS
HuizingaD
AgetonSS
1985 Explaining delinquency and drug use Beverly Hills, CA Sage Publications
59. ElliottDS
HuizingaD
MenardS
1989 Multiple Problem Youth: Delinquency, Drugs and Mental Health Problems New York, NY Springer
60. HoftNR
CorleyRP
McQueenMB
SchlaepferIR
HuizingaD
2009 Genetic association of the CHRNA6 and CHRNB3 genes with tobacco dependence in a nationally representative sample. Neuropsychopharmacology 34 698 706
61. BroderickP
WangY
VijayakrishnanJ
MatakidouA
SpitzMR
2009 Deciphering the impact of common genetic variation on lung cancer risk: A genome-wide association study. Cancer Res 69 6633 6641
62. LiMD
XuQ
LouXY
PayneTJ
NiuT
2010 Association and interaction analysis of variants in CHRNA5/CHRNA3/CHRNB4 gene cluster with nicotine dependence in African and European Americans. Am J Med Genet B Neuropsychiatr Genet 153B 745 756
63. ChenX
ChenJ
WilliamsonVS
AnSS
HettemaJM
2009 Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet
64. SchwartzAG
CoteML
WenzlaffAS
LandS
AmosCI
2009 Racial differences in the association between SNPs on 15q25.1, smoking behavior, and risk of non-small cell lung cancer. J Thorac Oncol 4 1195 1201
65. FarrerLA
KranzlerHR
YuY
WeissRD
BradyKT
2009 Association of variants in MANEA with cocaine-related behaviors. Arch Gen Psychiatry 66 267 274
66. ZhangH
KranzlerHR
WeissRD
LuoX
BradyKT
2009 Pro-opiomelanocortin gene variation related to alcohol or drug dependence: evidence and replications across family - and population-based studies. Biol Psychiatry 66 128 136
67. GelernterJ
YuY
WeissR
BradyK
PanhuysenC
2006 Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet 15 3498 3507
Štítky
Genetika Reprodukčná medicína
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2010 Číslo 8- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Mutation in the Gene Encoding Ubiquitin Ligase LRSAM1 in Patients with Charcot-Marie-Tooth Disease
- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Did Genetic Drift Drive Increases in Genome Complexity?
- The 5p15.33 Locus Is Associated with Risk of Lung Adenocarcinoma in Never-Smoking Females in Asia
- An Alpha-Catulin Homologue Controls Neuromuscular Function through Localization of the Dystrophin Complex and BK Channels in
- Epigenetically-Inherited Centromere and Neocentromere DNA Replicates Earliest in S-Phase
- Survival and Growth of Yeast without Telomere Capping by Cdc13 in the Absence of Sgs1, Exo1, and Rad9
- Tuberous Sclerosis Complex 1 Regulates dE2F1 Expression during Development and Cooperates with RBF1 to Control Proliferation and Survival
- Disease-Associated Mutations That Alter the RNA Structural Ensemble
- The Transcriptomes of Two Heritable Cell Types Illuminate the Circuit Governing Their Differentiation
- Inactivation of VCP/ter94 Suppresses Retinal Pathology Caused by Misfolded Rhodopsin in
- Multiple Independent Loci at Chromosome 15q25.1 Affect Smoking Quantity: a Meta-Analysis and Comparison with Lung Cancer and COPD
- Transcriptional Regulation by CHIP/LDB Complexes
- Conserved Role of in Ethanol Responses in Mutant Mice
- A Global Overview of the Genetic and Functional Diversity in the Pathogenicity Island
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- Extracellular Dopamine Potentiates Mn-Induced Oxidative Stress, Lifespan Reduction, and Dopaminergic Neurodegeneration in a BLI-3–Dependent Manner in
- Genetic Analysis of Baker's Yeast Msh4-Msh5 Reveals a Threshold Crossover Level for Meiotic Viability
- Genome-Wide Association Studies of Serum Magnesium, Potassium, and Sodium Concentrations Identify Six Loci Influencing Serum Magnesium Levels
- Something New: An Interview with Radoje Drmanac
- The Extinction Dynamics of Bacterial Pseudogenes
- Microtubule Actin Crosslinking Factor 1 Regulates the Balbiani Body and Animal-Vegetal Polarity of the Zebrafish Oocyte
- Consistent Association of Type 2 Diabetes Risk Variants Found in Europeans in Diverse Racial and Ethnic Groups
- Transmission of Mitochondrial DNA Diseases and Ways to Prevent Them
- Telomere Disruption Results in Non-Random Formation of Dicentric Chromosomes Involving Acrocentric Human Chromosomes
- Chromosome Axis Defects Induce a Checkpoint-Mediated Delay and Interchromosomal Effect on Crossing Over during Drosophila Meiosis
- Dynamic Chromatin Organization during Foregut Development Mediated by the Organ Selector Gene PHA-4/FoxA
- Ancient Protostome Origin of Chemosensory Ionotropic Glutamate Receptors and the Evolution of Insect Taste and Olfaction
- A Wnt-Frz/Ror-Dsh Pathway Regulates Neurite Outgrowth in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Identification of the Bovine Arachnomelia Mutation by Massively Parallel Sequencing Implicates Sulfite Oxidase (SUOX) in Bone Development
- Common Inherited Variation in Mitochondrial Genes Is Not Enriched for Associations with Type 2 Diabetes or Related Glycemic Traits
- A Model for Damage Load and Its Implications for the Evolution of Bacterial Aging
- Did Genetic Drift Drive Increases in Genome Complexity?
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



