-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Expression in Planarian Neoblasts after Injury Controls Anterior Pole Regeneration
Some animals are capable of regenerating organs damaged or removed by injury, and this ability likely requires precise control of secreted proteins that promote growth. Planarians are flatworms that can regenerate any missing tissues by regulating the activity of adult stem cells that can produce any specialized cell type. We identify the zic-1 gene as activated in planarian stem cells by injury and needed for head regeneration after decapitation. This gene's product likely acts as a transcription factor to produce cells that secrete a growth-promoting protein, NOTUM, at the tip of the regenerating tissue outgrowth to organize and enable head regeneration. These results suggest that regeneration requires specialized uses of stem cell descendants to orchestrate new tissue production following injury.
Published in the journal: Expression in Planarian Neoblasts after Injury Controls Anterior Pole Regeneration. PLoS Genet 10(7): e32767. doi:10.1371/journal.pgen.1004452
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004452Summary
Some animals are capable of regenerating organs damaged or removed by injury, and this ability likely requires precise control of secreted proteins that promote growth. Planarians are flatworms that can regenerate any missing tissues by regulating the activity of adult stem cells that can produce any specialized cell type. We identify the zic-1 gene as activated in planarian stem cells by injury and needed for head regeneration after decapitation. This gene's product likely acts as a transcription factor to produce cells that secrete a growth-promoting protein, NOTUM, at the tip of the regenerating tissue outgrowth to organize and enable head regeneration. These results suggest that regeneration requires specialized uses of stem cell descendants to orchestrate new tissue production following injury.
Introduction
Regeneration is widespread in animals but still poorly understood. In animals with this ability, injury responses and positional cues likely interact to signal the production of missing structures. Across vertebrates and invertebrates, regeneration can involve either stem cells of high potency or cells with more limited potential [1]. Therefore, rather than the presence or absence of a single regenerative cell type, patterning systems that directly or indirectly control such cells may instead be the critical determinate for regenerative abilities. Injuries can alter tissue variously, so regenerating animals must have robust cell signaling mechanisms that enable appropriate restoration of structures damaged or lost by wounding. An important unresolved issue in understanding regeneration mechanisms is whether stem cells function only in production of differentiated cell types that comprise fully regenerated structures, or whether they could have additional functions in directing outgrowth by controlling tissue patterning.
Planarians can regenerate nearly any tissue damaged by injury [2], and the processes of head or tail regeneration serve as simple models for study of mechanisms that relate wounding to stem cell activation and growth signaling. Planarians rely on adult pluripotent stem cells of the neoblast population for producing all differentiated cell types needed for whole-body regeneration and for viability through ongoing tissue homeostasis [3]. Neoblasts express the PIWI homolog smedwi-1 [4] and are the only known proliferating cells in planarians [5], so FACS isolation of G2/S/M cells labeled with DNA-binding vital dyes can purify this cell population [4], [6]. Neoblasts respond to injury through changes in proliferation [7], localization [8] and gene expression [9]. RNAi and small molecule treatments have implicated several signaling pathways and processes in head and tail regeneration that therefore directly or indirectly control the function of neoblasts or their differentiating progeny, including Wnt [10]–[18], BMP [19]–[23], Activin [24], [25], Hedgehog [15], [26], FGF, and calcium signaling [27], as well as communication through gap-junctions [28], [29] and bioelectric signaling [30], [31].
Neoblast-dependent processes likely provide input into the control of canonical Wnt signaling that directs head and tail regeneration and polarized responses on the anteroposterior (A/P) axis [10]–[12]. wnt1 is required for tail regeneration and is activated early by 6 hours after injury [13], [32] in body-wall muscle cells [33] in a neoblast-independent manner. Subsequently, by 48–72 hours, in fragments regenerating a new tail, wnt1 becomes expressed in a focus of cells at the posterior pole in a neoblast-dependent manner. By contrast, notum encodes a secreted hydrolase that can inhibit Wnt signaling in planarians [14], Drosophila [34], [35], and zebrafish [36], and is required for head regeneration in planarians [14]. Injury activates notum expression preferentially at anterior-facing injury sites within body-wall muscle cells [33] with kinetics similar to wound-induced wnt1 expression. Subsequently, by 48–72 hours, in fragments regenerating a new head, notum is expressed in a focus of cells at the newly forming anterior pole. Temporal RNAi experiments found that wnt1 and notum are required during tail and head regeneration [13], [14], indicating the importance of these expression behaviors for their growth promoting functions.
The involvement of additional genes in head and tail regeneration also points to the importance of neoblast-dependent processes in pole formation. pbx is a TALE class homeodomain transcription factor expressed broadly, including within neoblasts, and is required for head and tail regeneration as well as pole expression, but not wound-induced expression, of wnt1 and notum [37], [38]. Tail regeneration and expression of wnt1 in the tail requires the transcription factor pitx [39], [40]. Additionally, the forkhead transcription factor foxD is activated early by 6 hours in body-wall muscle cells at injury sites that disrupt the midline, including anterior and posterior amputation sites, is subsequently expressed in neoblasts and the anterior pole, and is needed for notum anterior pole expression and head regeneration [41]. Therefore, these studies indicate that pole formation is either a consequence of neoblast-dependent growth or alternatively might require specialized differentiation activities of neoblasts.
We used transcriptional profiling to identify a Zic transcription factor, Smed-zic-1, as a likely candidate for control of neoblasts to produce an anterior pole in head regeneration. Zinc fingers of the cerebellum (Zic) proteins, homologous to Drosophila odd-paired, are conserved in animals, can act as transcription factors, and participate in axis formation, neurogenesis, and mesoderm formation [42]–[49]. However, pleiotropy and redundancy have complicated the identification of specific shared functions for vertebrate Zic genes, and loss-of-function studies have not yet pointed to their common use in only a single signaling pathway [50]. Additionally, functions for Zic proteins in injury responses have not been described. The injury-dependent program of adult organ formation in planarians provides a simple system to identify and clarify highly conserved, and therefore central, zic gene functions used in stem cell control, tissue patterning and growth. Our studies indicate that Zic proteins can participate in organ regeneration through stem cell-dependent production of signaling centers that direct subsequent tissue outgrowth.
Results
Identification of zic-1 as induced in neoblasts early in head regeneration
To identify genes involved in putative stem cell-dependent patterning processes, we first examined neoblast requirements for injury-induced expression of notum, a Wnt inhibitor required for head regeneration [14]. In head regeneration, notum expression is activated in a number of cells near the anterior-facing amputation site by 18 hours and subsequently by 48–72 hours its expression occurs prominently at the regenerating anterior pole. Animals treated with lethal doses of gamma irradiation were depleted of smedwi-1-positive neoblasts, as described previously [4], and succeeded in expressing notum at 18 hours but not at the anterior pole by 72 hours (Fig. 1A, Fig. S1). These results confirm previous observations that anterior pole formation is neoblast-dependent [41].
Fig. 1. Strategy for identifying genes required for neoblast-dependent formation of the anterior pole. 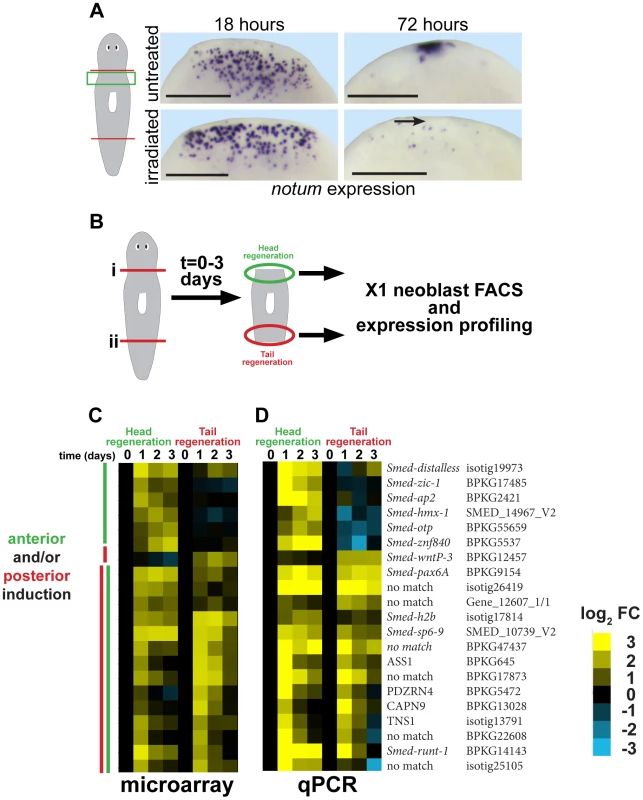
(A) in situ hybridizations to detect expression of notum after amputation in untreated animals or animals exposed to gamma irradiation (6000 Rads) 48 hours prior to surgery. Late (72 hours) expression of notum at the anterior pole is irradiation sensitive (arrow), but not early (18 hours) expression of notum in disperse cells near the wound site. Cartoon shows surgery (red lines) and enlarged region (green box). Bars, 300 microns. (B) Cartoon showing strategy to identify genes expressed differentially within neoblasts in head and tail regeneration. Animals were amputated transversely at either planes (i) or (ii), and X1 neoblasts purified by FACS from macerated tissue fragments from regions near injury sites engaged in either head or tail regeneration in a time series. Expression profiling was performed to identify genes whose expression significantly changed at 1, 2, or 3 days after amputation as compared to neoblasts isolated from regionally matched non-regenerating tissue. (C) Heat map showing only genes upregulated as detected by microarray (yellow, log2 fold-change (FC), chosen with false-discovery rate <10%) in head and/or tail regeneration. (D) qPCR experiment showing expression of 21/24 candidate genes upregulated in neoblasts due to regeneration whose expression behavior was confirmed upregulated. (C–D) Heat maps show microarray and qPCR data for genes ordered using hierarchical clustering with both microarray and qPCR expression values as inputs and broadly categorize into those upregulated primarily in anterior or posterior regeneration, or both (yellow, log2 fold-change versus 0 hour timepoint for each anterior or posterior series). We further analyzed a subset of genes that reproducibly increased in expression specific to anterior regeneration. We reasoned that genes important for a neoblast-dependent step in anterior pole formation might be expressed in neoblasts specifically during head regeneration. To identify such genes, we used Hoechst staining and FACS to isolate X1 neoblasts (G2/S/M cells) from tissues near anterior - and posterior-facing wound sites over three days of regeneration and transcriptionally profiled these cells using custom microarrays (Agilent) performed in biological triplicates (Fig. 1B, Fig. S2A). 66 genes had expression changes predicted with a false discovery rate of 10% (Benjamini-Hochberg method for correction of multiple hypothesis testing, see Methods), of which 24 were detected as upregulated in neoblasts during regeneration with various kinetics and specificities (Fig. 1C, Fig. S2B, Table S1). We used qPCR to validate the expression behavior of these genes. Expression upregulation was confirmed for 21 of these genes (Fig. 1D), and with broadly similar kinetics as measured by microarray. Hierarchical clustering classified genes as having induction broadly polarized to anterior regeneration (distalless, zic-1, ap2, hmx-1, otp, znf840), posterior regeneration (wntP-3), or that occurred in both anterior and posterior regeneration in either analysis. Nine of the 21 confirmed injury-induced genes encoded predicted transcription factors with six previously identified as expressed in neoblast subpopulations (runt-1, hmx-1, ap-2, pax-6, dlx-3, and sp6-9) [9], [51], [52] and three not yet identified as neoblast-expressed (zic-1, znf840, and otp). Of these nine genes, expression profiles from the microarray indicated four were induced in early head regeneration (24 hours) much greater than in tail regeneration: zic-1, distalless (dlx), ap-2, and hmx-1. dlx, ap2, and hmx-1 have reported expression patterns and functions related to regeneration of eye cells and specific neuronal subpopulations rather than pole regionalized expression or functions in head formation [9], [51], [52]. Therefore, we focused subsequent analysis on zic-1. A previous phylogenetic study identified this gene as one of two planarian Zic family members (previously designated as zicA and zicB), with representatives in all animals [45]. We renamed the zicA gene Smed-zic-1 (hereafter referred to as zic-1) in keeping with the Schmidtea mediterranea gene nomenclature guidelines [53].
We first used in situ hybridizations to confirm zic-1's injury-induced expression behavior. In uninjured animals, zic-1 was expressed in the head region and at the anterior pole (Fig. 2A). In animal fragments fixed during regeneration of a head and/or a tail, zic-1 was expressed near anterior-facing injury sites by 18 hours (Fig. 2B). zic-1 was expressed preferentially at anterior - versus posterior-facing amputation sites made at similar axial positions, although it was initially expressed more strongly at injury sites derived from the anterior (prepharyngeal) versus the posterior (postpharyngeal) of the amputated animal. By 42 hours, zic-1 expression in posterior-facing wound sites was reduced but expression at anterior-facing sites persisted, and by 72 hours zic-1 was expressed strongly in the anterior pole and in surrounding regions. Injury induced zic-1 expression was not exclusive to the anterior, as expression at posterior-facing wound site of head fragments was observed by 18 hours and later near but not coincident with the posterior pole of regenerating head fragments by 72 hours. However, expression at anterior-facing injury sites was prominent and persistent. We conclude that zic-1 expression is activated early following injury, marks the anterior pole, and occurs differentially in head versus tail regeneration.
Fig. 2. Injury induces expression of zic-1 in neoblasts and in the regenerating anterior pole. 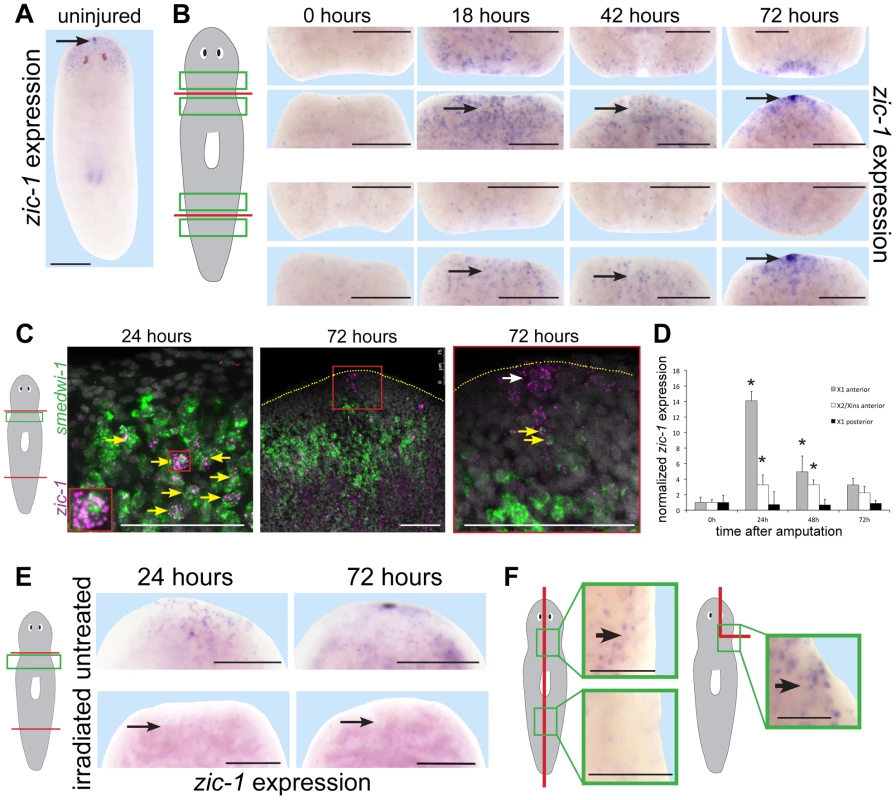
(A–B) in situ hybridizations to detect expression of zic-1 in uninjured or regenerating animals as indicated. (A) zic-1 was expressed in the head region and head tip in uninjured animals (arrow). (B) zic-1 was expressed preferentially near anterior-facing versus posterior-facing amputation sites by 18 hours and is expressed at the regenerating anterior pole by 72 hours after surgery (arrows). (C) Double fluorescence in situ hybridizations (FISH) to detect co-expression of zic-1 (magenta) with smedwi-1 (green) mRNA in tissue regions near anterior-facing amputation sites. Hoechst staining shows nuclei (gray). By 24 hours after injury, numbers of zic-1+/smedwi-1+ cells increased substantially (13/29 of zic-1+ cells were smedwi-1+, n = 3 animals at 0 hours to 506/530 of zic-1+ cells were smedwi-1+, n = 4 animals at 24 hours) and by 72 hours zic-1 was expressed at the anterior pole in non-neoblast cells lacking smedwi-1 mRNA expression (10/10 cells, n = 3 animals). Arrows indicate cells co-expressing (yellow) and not singly-expressing the two genes (white). Red box indicates enlarged region of a zic-1+/smedwi-1+ cell (24 hours, inset) or anterior pole region (72 hours, right). Yellow dotted line shows anterior edge (72 hours). (D) qPCR to measure zic-1 expression versus gapdh in FACS-sorted X1 or X2/Xins cells purified as in Fig. S2A from tissue near anterior or posterior-facing injury sites as in Fig. 1B in a time series relative to expression at 5 minutes post surgery. Error bars are standard deviations. (E) Injury-induced zic-1 expression was eliminated in animals lethally irradiated with 6000 Rads immediately prior to amputation (arrows). (F) Early wound-induced expression of zic-1 occurred independently of anterior removal or midline disruption. Left, laterally amputated animals activated zic-1 expression near the injury site preferentially in animal anterior versus posterior (11/12 animals probed). Right, asymmetric anterior wedge removed tissue without midline or anterior pole disruption and resulted in expression of zic-1 by 18 hours after surgery (6/7 animals probed). Unless otherwise noted, all panels represent at least 4 of 5 animals probed. Cartoons show surgeries and enlarged regions. Anterior, top. Bars, 250 microns (A, B, E) and 75 microns (C, F). We next performed experiments to verify expression of zic-1 in neoblasts. We used double fluorescence in situ hybridization (FISH) to detect injury-induced zic-1 expression in smedwi-1-positive neoblasts (Fig. 2C). In freshly amputated animals, a small number of zic-1-positive cells were identifiable, and some co-expressed smedwi-1 (13/29 cells, n = 3 animals). By 24 hours of anterior regeneration, the number of zic-1/smedwi-1 double-positive cells near the injury site greatly increased (506/530 cells, n = 4 animals). Additionally, there were a large number of smedwi-1+ cells that lacked zic-1 expression, suggesting that zic-1 expression marks a neoblast subpopulation near injury sites. The entire neoblast population increases in number due to amputation by 24 hours [5], [7] but does so significantly less than the ∼10-fold increase in zic-1+/smedwi-1+ cells we observed. Anterior pole cells expressing zic-1 at 72 hours lacked expression of smedwi-1 (10/10 cells, n = 3 animals). All non-neoblast cells are descended from neoblasts [3], so these results indicated that injury activates expression of zic-1 in a subpopulation of neoblasts at anterior-facing injury sites and subsequently in neoblast descendants located at the regenerating anterior pole.
We performed two additional tests to confirm expression of zic-1 in the neoblast population due to injury. First, we quantified zic-1 expression in X1 cells by realtime PCR, and observed a 14-fold induction of zic-1 expression during head regeneration by 24 hours that was specific to anterior-facing injury sites (Fig. 2D). X2/Xins cells representing G1 cells of the animal also increased their expression of zic-1 due to amputation but to a lesser extent than did X1 cells. Second, we examined zic-1 expression in animals exposed to lethal doses of gamma irradiation the day of head and tail amputation (Fig. 2E). This dose was sufficient to eliminate neoblasts, and abolished both early wound-induced and anterior pole expression of zic-1 (8/8 animals). These experiments support the conclusion that zic-1 expression is induced by injury in neoblasts.
Planarian genes induced transcriptionally by injury vary in their responsiveness to wound site orientation, extent and location [9], [13]–[15], [25], [32]. To examine requirements for injury-induced zic-1 expression, we made symmetric lateral amputations or asymmetric wedges that removed part of the brain without pole removal or injuring through the animal midline (Fig. 2F). In both cases, zic-1 was expressed in cells near the injury sites by 18 hours after wounding (lateral amputation, 12/12 worms had expression; wedge incision, 6/7 worms had expression) in irradiation-sensitive cells (lateral amputation, 0/8 worms had expression; wedge, 0/5 had expression). Additionally, wound-site proximal zic-1 expression was preferentially anterior and not posterior in laterally amputated animals at 18 hours (11/12 animals assayed). These results indicate that zic-1 activation in neoblasts occurs with anterior bias and independently of midline disruption, pole removal and head removal.
zic-1 is co-expressed with anterior pole genes in recently neoblast-derived cells
We examined the relationship between injury-induced neoblast expression and anterior pole expression of zic-1. First, we tested whether zic-1 co-expressed with other genes that together mark cells the anterior pole: notum, follistatin (fst), and foxD. Double FISH experiments performed on uninjured animals detected co-expression of zic-1 and notum, zic-1 and foxD, and zic-1 and follistatin at the anterior pole (Fig. 3A). Additionally, we performed similar experiments to analyze the relationship between these four genes during both early (18 hours) and late (48–72 hours) regeneration phases (Fig. 3B). At 18 hours, notum is expressed primarily in the body wall musculature [33] whereas zic-1 is expressed primarily in neoblasts (Fig. 2C). Consistent with these observations, the majority of notum-expressing cells did not express zic-1 at 24 hours of regeneration (142/151 notum+ cells were zic-1−, n = 3 animals), only a small fraction of zic-1+/notum+ cells were identifiable (9/151 notum+ cells were zic-1+, n = 3 animals). Subsequently, at 72 hours, notum and zic-1 were co-expressed in many cells at the regenerating anterior pole (34/39 notum+ cells were zic-1+, n = 7 animals). Similarly, at early times in regeneration (6–18 hours), foxD and follistatin are primarily expressed in collagen+ cells of the body wall musculature near injury sites that disrupt the midline, and at generic injury types, respectively [25], [33], [41]. By 72 hours, however, both genes were expressed in cells of the anterior pole that co-express zic-1 (32/36 zic-1+ cells were foxD+, n = 5 animals; 36/69 zic-1+ cells were fst+, n = 6 animals). Therefore, zic-1 and notum, foxD, and follistatin are not co-expressed early after head amputation, but later at the regenerating anterior pole.
Fig. 3. zic-1 is co-expressed with notum, foxD and follistatin in neoblast descendent cells at the anterior pole. 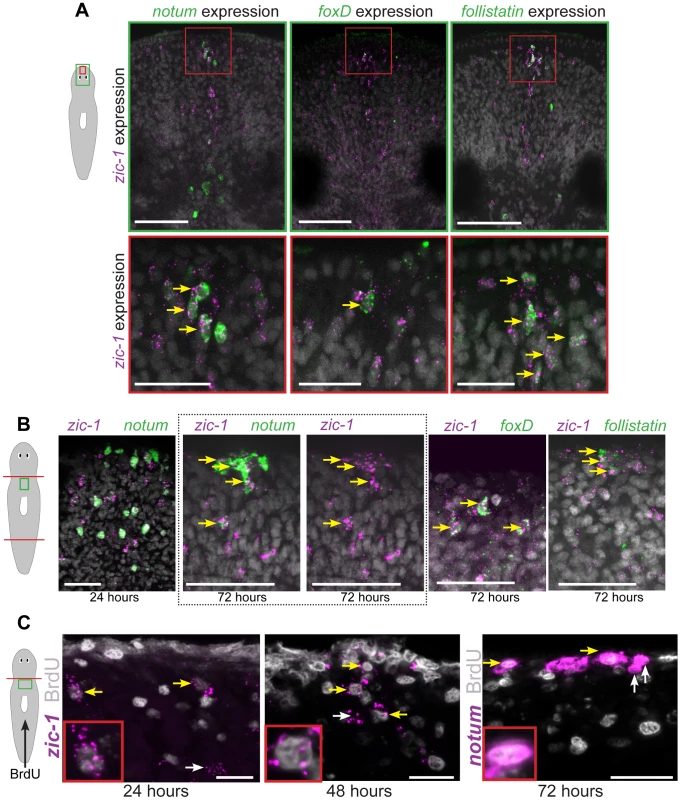
(A) Double FISH to detect expression of zic-1 (magenta) and either notum, foxD, or follistatin (green) in at the anterior pole of uninjured animals with nuclei stained using Hoechst (gray). Cartoon depicts fields of view in green (top) or red (bottom) borders. (B) Double FISH as in (A) detecting expression in regenerating fragments at the indicated times after decapitation. Dotted box, contains image of anterior pole showing zic-1 and notum staining (left) and corresponding image of only zic-1 staining (right). zic-1 is not highly co-expressed with notum at 24 hours (9/142 notum+ cells were zic-1+, n = 3 worms), but is co-expressed with notum at the anterior pole by 72 hours after surgery. At 72 hours in the regenerating anterior pole, 62.1±5.3% zic-1+ cells co-expressed notum (n = 3 worms), 91.7±11.8% zic-1+ cells co-expressed foxD (n = 5 worms), and 56.1±28.6% zic-1+ cells co-expressed follistatin (n = 6 worms). (C) Animals were injected with bromodeoxyuridine (BrdU) two days prior to decapitation, then fixed at the indicated times and probed by in situ hybridization and immunostaining to detect BrdU and either zic-1 or notum. This pulse of BrdU labeled 35.2±16.6% of zic-1+ cells at 24 hours in the parenchyma (n = 5 worms, 440 zic-1+ cells counted), and 80.4±20% of zic-1+ cells at the anterior pole by 48 hours (n = 5/6 worms with anterior poles, n = 49 zic-1+ cells counted), and labeled notum+ cells at the anterior pole by 72 hours. Yellow arrows indicate co-labeled cells, white arrows indicate single-labeled cells. Cartoons show surgeries and enlarged region. Anterior top. Bars, 20 microns (C), 30 microns(A bottom panels, B), 75 microns (A top panels). We further used multiplex histology and labeling experiments to characterize the cell composition of the regenerating anterior pole using notum as a marker. Some 48-hour anterior pole cells expressing notum also expressed SMEDWI-1 protein (86.5±8.3% notum+ cells co-expressed SMEDWI-1, n = 5 animals) (Fig. S3), suggesting some pole cells are recently differentiated from smedwi-1+ neoblasts [54]. notum+ anterior pole cells did not express prog-1, which marks a postmitotic population of neoblast descendants [55]. A fraction of notum+ cells at the anterior pole of 72-hour regenerating animals expressed collagen (17.3±16.8% notum+ cells co-expressed collagen, n = 5 animals), which marks body-wall muscle cells that express secreted regulators of regeneration [33]. Together, these results suggest that anterior pole cells include cells of the body-wall musculature formed by neoblast differentiation during regeneration.
To further examine the hypothesis that cells of the regenerating anterior pole are descended recently from neoblasts, we performed bromodeoxyuridine (BrdU) labeling experiments. As neoblasts are believed to be the only proliferating cell population in planarians, BrdU first marks neoblasts and subsequently their differentiating progeny and differentiated cell types [52], [55]. We administered a pulse of BrdU to label neoblasts two days prior to head amputation, fixed animals in a time series and detected zic-1 mRNA expression by FISH and BrdU incorporation by immunostaining (Fig. 3C). BrdU+zic-1+ cells were observed at 24 hours after amputation in the parenchyma near the injury site (35.2±16.6% of zic-1+ cells were BrdU+, n = 6 worms), and at 48 hours near the anterior pole (80.4±20% of zic-1+ cells were BrdU+, n = 5 worms). Furthermore, we detected BrdU+notum+ cells at the anterior pole of 72-hour regenerating fragments. Therefore, these results indicate that the anterior pole contains cells recently differentiated from neoblasts and are consistent with the hypothesis that some zic-1+ neoblasts form zic-1+ anterior pole cells.
zic-1 is needed for head regeneration and production of an anterior signaling center
We next used RNAi to investigate functions for zic-1 in regeneration. Animals treated with zic-1 double-stranded RNA (dsRNA) were amputated to remove heads and tails and scored for phenotypes 8 days after surgeries. Inhibition of zic-1 caused overt defects in head but not tail regeneration, including cyclopia (25% of 74 animals assayed), absence of eyes (12% of 74 animals assayed) and head regeneration failure (63% of 90 animals assayed) (Fig. 4A). Two dsRNAs targeting non-overlapping regions of the zic-1 gene individually caused identical defects in anterior regeneration, suggesting these effects are likely due to zic-1 inhibition and not an off-target effect of RNAi (5′ dsRNA: 7/14 animals were headless; 3′ dsRNA: 3/6 animals were headless). Similar anterior regeneration defects have been observed due to inhibition of notum [14], foxD [41], follistatin [24], [25], patched [15], prep [56], pbx [37], [38], and H+,K+-ATPase [30]. We used in situ hybridizations to analyze the tissue content and regional expression status of headless zic-1(RNAi) animals. Headless zic-1(RNAi) animals lacked a brain (gpas, chat) and also lacked expression of markers of the head tip (sFRP-1), head region (prep), and anterior pole (notum, follistatin and foxD) (Fig. 4B–C). Headless (Fig. 4B) and eyeless (Fig. S4A) zic-1(RNAi) animals had normal expression of posterior markers fzd-4 and wnt1, suggesting that posterior regeneration occurred normally and that such animals did not undergo a polarity transformation to form a tail at the anterior-facing wound sites. To further test whether zic-1 has functions in midline formation, we analyzed expression of slit, a marker of the midline [57]. zic-1(RNAi) animals had laterally expanded anterior put not posterior expression of slit (Fig. 4D). zic-1(RNAi) animals maintained broad expression of pbx (Fig. S4B), indicating zic-1 likely does not function in head regeneration by regulating pbx expression. Together, these results indicate zic-1 participates in head formation rather than pole identity determination.
Fig. 4. zic-1 is required for anterior pole formation and head regeneration. 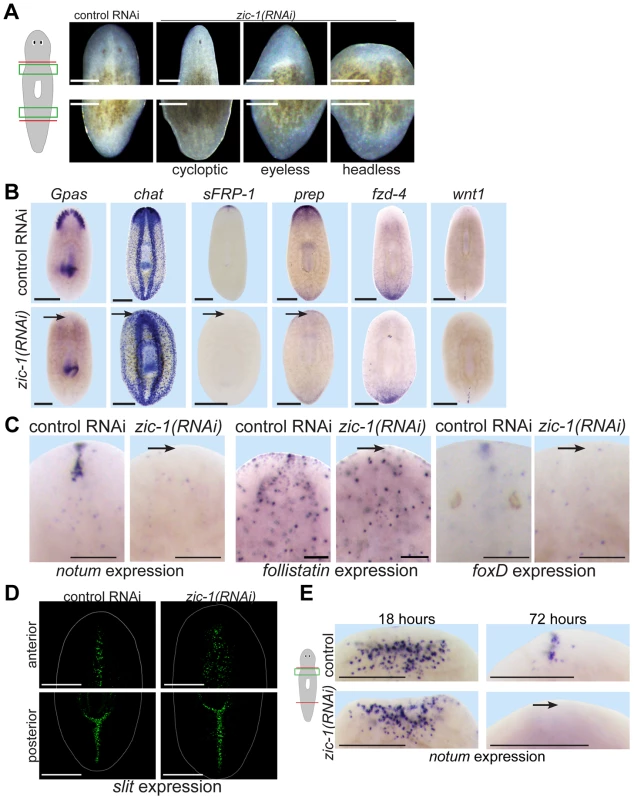
(A) Animals 8 days after amputation of heads and tails after treatment with control dsRNA or zic-1 dsRNA, cartoon shows enlarged regions of the anterior and posterior blastemas. Inhibition of zic-1 function caused anterior regeneration defects such as cyclopia (24%, n = 74 animals scored), lack of eyes (12%, n = 74 animals scored), and head regeneration failure (63%, n = 90 animals scored). (B–D) in situ hybridizations of animals fixed 8 days after amputation. (B) The anterior region of headless zic-1(RNAi) regenerating animals lacked a brain (gpas, 2/2 animals probed; chat, 4/4 animals probed) and expression of head tip marker (sFRP-1, 11/11 animals probed) and head region marker (prep, 8/8 animals probed) and did not express posterior markers (fzd-4, 7/7 animals probed; wnt1, 7/7 animals probed). (C) zic-1(RNAi) animals failed to express notum (6/6 animals probed), follistatin (4/5 animals probed), and foxD (14/14 animals probed) at the anterior pole after 8 days of regeneration. (D) zic-1(RNAi) animals had a laterally expanded midline in their anterior but not their posterior, as assayed by slit expression (4/4 animals). (E) zic-1 is required for anterior pole formation by 72 hours and not for early wound-induced notum expression at 18 hours of regeneration. In situ hybridizations to detect notum expression after treatment with control or zic-1 dsRNA, amputation of heads and tails, and fixation at 18 or 72 hours. zic-1 inhibition prevented expression of notum at the anterior pole by 72 hours (8/8 animals) but had no effect on its expression at 18 hours (122±53 notum+ cells in control and 108±35 notum+ cells in zic-1(RNAi) animals, 8 animals probed, p = 0.59 t-test). Cartoon shows surgery and enlarged regions. Bars, 100 microns (C), 250 microns (A), 300 microns (B, D, E). zic-1 inhibition resembled some defects due to inhibition of notum, so we determined the stage(s) of notum expression affected by zic-1 RNAi. zic-1 inhibition prevented anterior pole expression of notum by 72 hours, but not its early wound-induced expression (Fig. 4E), similar to the impact of irradiation on notum expression (Fig. 1A). Together, these experiments indicate zic-1 is required for head regeneration, midline patterning, and anterior identity.
zic-1 inhibition produced multiple regeneration phenotypes, so we sought to understand their relationships by performing RNAi attenuation experiments and additional histological analysis. Dilution of zic-1 dsRNA with an equal amount of control dsRNA increased the penetrance of cyclopia and decreased the penetrance of headlessness (Fig. S4C). In addition, cycloptic zic-1(RNAi) animals had a low level of notum and sFRP-1 expression at the anterior pole (Fig. S4D). We conclude that cyclopia is likely a hypomorphic phenotype due to zic-1 RNAi and we did not investigate it further.
An additional Zic-family gene in the S. mediterranea genome, formerly referred to as zicB, we renamed Smed-zic-2 (here after referred to as zic-2) in keeping with the S. mediterranea guidelines for gene names [53]. zic-2 was expressed in the anterior of uninjured animals (Fig. S5A), and we speculated that it could have redundant functions with zic-1. Double fluorescence in situ hybridizations revealed that zic-2 was expressed in neoblasts at 24-hours and in cells of the anterior region at 72-hours in an irradiation sensitive manner (Fig. S5B). Inhibition of zic-2 by RNAi resulted in weakly penetrant cyclopia after amputation and regeneration (Fig. S5C). However, simultaneous inhibition of zic-1 and zic-2 increased the frequency of headless animals versus inhibition of zic-1 or zic-2 alone (p-value<0.001, Fisher's exact test). zic-2 RNAi reduced expression of injury-induced zic-1 (p = 0.07), as measured by manual cell scoring and realtime PCR (Fig. S5D–E), providing a possible explanation of these effects given that dsRNA treatments in general likely reduce rather than eliminate gene function. By contrast, zic-1 RNAi caused a reduction in zic-2 expression (p = 0.07) (Fig. S5E). Taken together, these results suggest zic-1 and zic-2 function together in head regeneration. Because head regeneration strongly required zic-1, we focused subsequent analysis on that gene.
zic-1 has injury-independent functions in anterior pole maintenance
Given the prominent injury-induced behavior of zic-1 expression, we hypothesized that it may have functions specific to regeneration as opposed to those tissue maintenance in the absence of injury, similar to follistatin [25], runt-1 [9], and foxD [41]. To test whether zic-1 functions only in regeneration, we fed animals bacteria expressing dsRNA for 10 weeks. No overt abnormalities became apparent in these animals (Fig. S6A), and sFRP-1 expression was normal (Fig. S6B). However, prolonged zic-1 RNAi without injury caused a reduction in the number of notum+ cells near the anterior pole (11±1 notum+ cells in control animals versus 5.8±0.5 cells in zic-1(RNAi) animals, p<0.005 by t-test) (Fig. S6B). This defect was specific to expression of notum at the anterior pole, as notum expression at the brain commissure was normal (10.7±2.3 notum+ cells in control animals versus 13±1.6 cells in zic-1(RNAi) animals, p>0.05 by t-test). By contrast, amputation of animals undergoing RNAi in parallel treatments for only three weeks resulted in head regeneration failure (40%, n = 20 anterior-facing wounds). These results point to the importance of injury-induced zic-1 expression for regeneration and indicate injury-independent functions in anterior pole maintenance.
zic-1 allows sustained Wnt signaling inhibition needed for head regeneration
We next examined determinants of zic-1 expression and function. Expression of some Wnt signaling ligands and secreted inhibitors occurs independently of neoblasts and also with A/P polarization at injury sites [13], [32]. Therefore, we tested canonical Wnt signaling as a candidate pathway controlling early zic-1 activation (Fig. 5A–B). Inhibition of beta-catenin-1 by RNAi resulted in ectopic zic-1 expression at posterior-facing wounds in trunk fragments by 24 hours after injury. Conversely, inhibition of APC, an intracellular inhibitor of beta-catenin-1, reduced zic-1 expression at anterior-facing wound sites in trunk fragments. Because zic-1 expression behavior depends on A/P axis position (Fig. 2A), we further investigated the impact of beta-catenin-1 or APC inhibition in anterior-facing wound sites from anterior - and posterior-facing injury amputation sites at different A/P axial positions (see cartoons to show surgeries in Fig. 5A–B, S7A–B). APC inhibition decreased zic-1 expression at anterior-facing injury sites in both the anterior and posterior of the body, and also in posterior-facing injury sites from the anterior of the body (Fig. 5A–B, S7A–B). By contrast, beta-catenin-1 inhibition increased zic-1 expression at anterior - and posterior-facing amputation sites located in posterior regions of the animal but not in the anterior of the animal (Fig. 5A–B, S7A–B). Taken together, these results indicate that perturbation of beta-catenin or APC function has opposite effects on zic-1 expression that are independent of polarity transformations caused by these treatments. Animal-wide gradients of beta-catenin activity have been proposed based on phenotypic evidence and expression domains of Wnt ligands [58]. We speculate that anterior regions may normally have sufficiently low beta-catenin-1 activity that beta-catenin-1 RNAi does not further increase zic-1 expression after injury. Furthermore, in uninjured animals, beta-catenin-1 or APC RNAi did not simply result in constitutive activation or inhibition of zic-1 in the regions assessed in the regeneration assay (4/4 animals scored, Fig. S7C), indicating these effects were specific to an injury context.
Fig. 5. Polarized expression of zic-1 early after injury is controlled by notum's inhibition of canonical Wnt signaling. 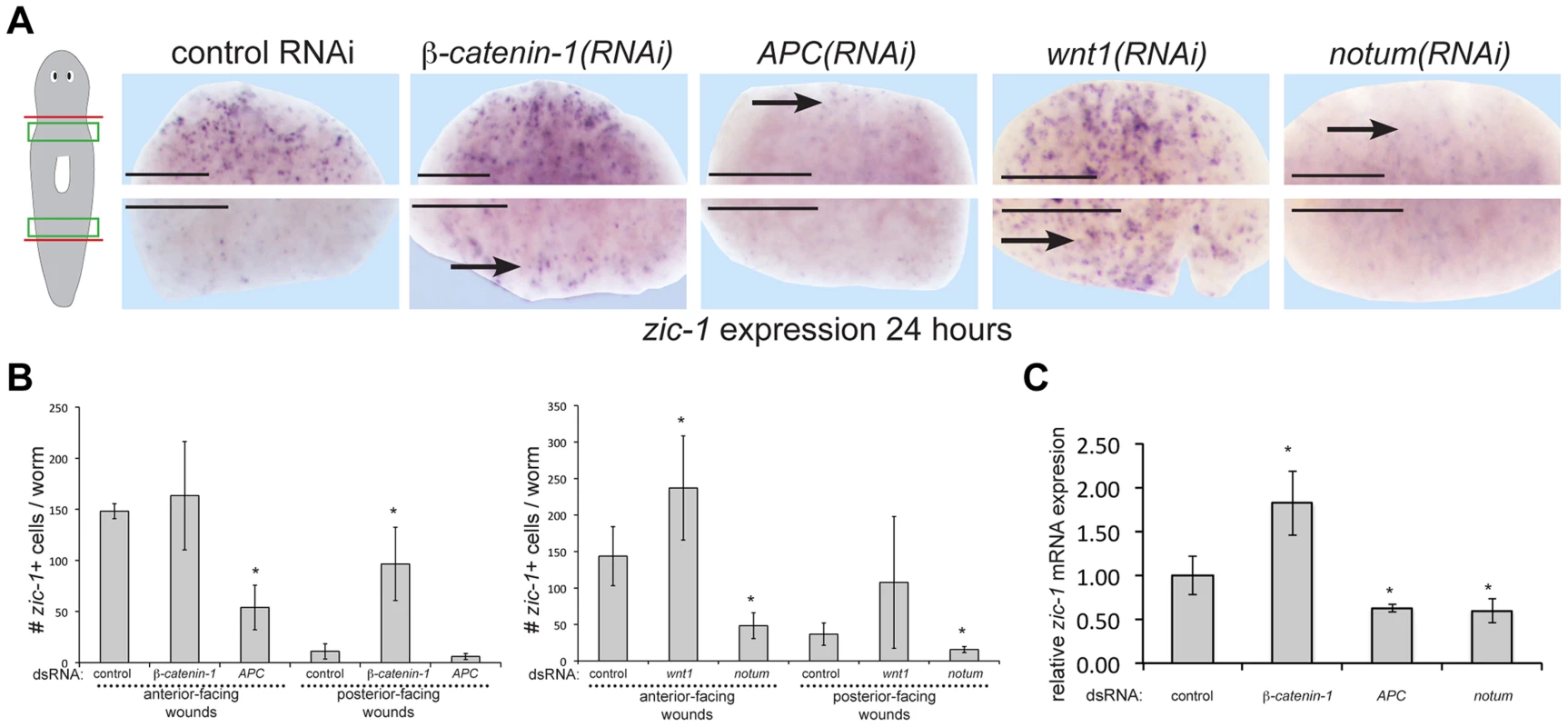
(A) beta-catenin-1 or APC were inhibited by feeding animals bacteria expressing dsRNA four times over two weeks and notum or wnt1 were inhibited by injection of dsRNA. Animals were fixed 24 hours after amputation of heads and tails and stained with a zic-1 riboprobe. Cartoon shows surgery and enlarged region. beta-catenin-1 inhibition caused ectopic expression of zic-1 at posterior-facing wound sites, whereas APC inhibition caused a reduction of zic-1 expression at anterior-facing wound sites. Similarly, wnt1 inhibition increased zic-1 expression at anterior- and posterior-facing wound sites, whereas notum inhibition decreased its expression at both wound sites. (B) Quantitation of number of cells expressing zic-1 under each treatment. Error bars standard deviations, asterisks p-value<0.05 by t-test. (C) qPCR assays were performed on total RNA isolated with 4 biological replicates of three worms each to measure zic-1 expression relative to gapdh after the indicated RNAi treatments. Error bars standard deviations, asterisks p-value<0.05 by t-test. Scale bars, 250 microns. wnt1 and notum are expressed prior to zic-1 activation in response to injury, so we tested their requirements for zic-1 expression. wnt1 RNAi resulted in ectopic zic-1 expression at posterior-facing amputation sites, and notum RNAi reduced expression at anterior-facing amputation sites. We confirmed the effects of Wnt signaling perturbation on zic-1 expression using manual cell counting (Fig. 5B) and qPCR (Fig. 5C). Therefore, taken together with previous observations, these results indicate that Wnt signaling inhibition by early injury-induced notum is necessary and sufficient to activate early zic-1 expression by 24 hours.
We examined additional genes involved in head and tail regeneration for their requirements for 24-hour zic-1 expression. Inhibition of hedgehog and patched had no apparent effect on zic-1 expression as measured by qPCR and did not alter numbers of zic-1+ cells as measured by manual counting (Fig. S8A). hedgehog and patched oppositely regulate wnt-1 expression, suggesting that transcriptional activation of zic-1 could have additional inputs other than Wnt signaling. However, patched(RNAi) animals of the same cohort regenerated with anterior defects, including anterior tail formation (3/7 animals) and headlessness (2/7), suggesting that Wnt signaling inhibition rather than axis polarization or regionalization is a driver of zic-1 expression due to injury. Additionally, pbx inhibition reduced but did not eliminate zic-1 expression 24 hours after amputation, as measured by reduced numbers of zic-1+ cells and reduced zic-1 mRNA levels (Fig. S8B–C). follistatin inhibition reduced zic-1+ cell numbers at 24 hours after amputation and reduced zic-1 mRNA levels, although not significantly as determined by a t-test, consistent with follistatin participating in early signaling due to any injury that removes tissue [25]. pitx inhibition reduced the numbers of zic-1+ levels, although not significantly as determined by a t-test, and reduced zic-1 mRNA expression. foxD inhibition did not visibly alter zic-1 expression or numbers of zic-1+ cells at 24 hours, though reduced mRNA expression of zic-1 but not significantly (as determined by t-test). Additionally, prep RNAi did not alter numbers of zic-1+ cells nor mRNA levels due to injury expression. Finally, pitx RNAi reduced the numbers of zic-1+ cells and reduced zic-1 mRNA levels. We conclude that pbx, follistatin are likely required for maximal levels of zic-1 expression, and that zic-1+ cells are still abundant after inhibition of hedgehog, patched, foxD, pitx and prep.
Given the central function of Wnt signaling in head regeneration [10], [11], [59], we next tested candidate functional interactions with zic-1. Whereas zic-1 promotes head regeneration (Fig. 4A), beta-catenin-1 suppresses it [10], [11], [59], so we examined the outcome of simultaneous inhibition of both genes. zic-1(RNAi);beta-catenin-1(RNAi) animals all regenerated heads at anterior-facing wounds and posterior-facing wounds, identical to beta-catenin-1(RNAi) animals (Fig. 6A–B). qPCR performed on RNA from animals of the same cohort harvested 24 hours after amputation in biological triplicate verified zic-1 transcript reduction by zic-1 dsRNA treatments (Fig. 6C). These experiments indicate the suppressive effect of beta-catenin-1 dsRNA was not simply due to reduction in zic-1 RNAi efficiency. Additionally, we used in situ hybridizations to measure notum expression in animals from this experiment fixed at 72 hours and 14 days of regeneration (Fig. S9A). beta-catenin-1 inhibition reduced anterior pole expression of notum at 72 hours, consistent with notum's described property as a feedback inhibitor of Wnt signaling. Such animals regenerate an anterior head with reduced numbers of notum+ cells at the anterior pole by 14 days. zic-1 inhibition eliminated notum expression at the pole by 72-hours. Simultaneous inhibition of beta-catenin-1 and zic-1 resulted in head regeneration but in the absence of notum expression at 72 hours.
Fig. 6. zic-1 inhibits Wnt signaling to promote head outgrowth. 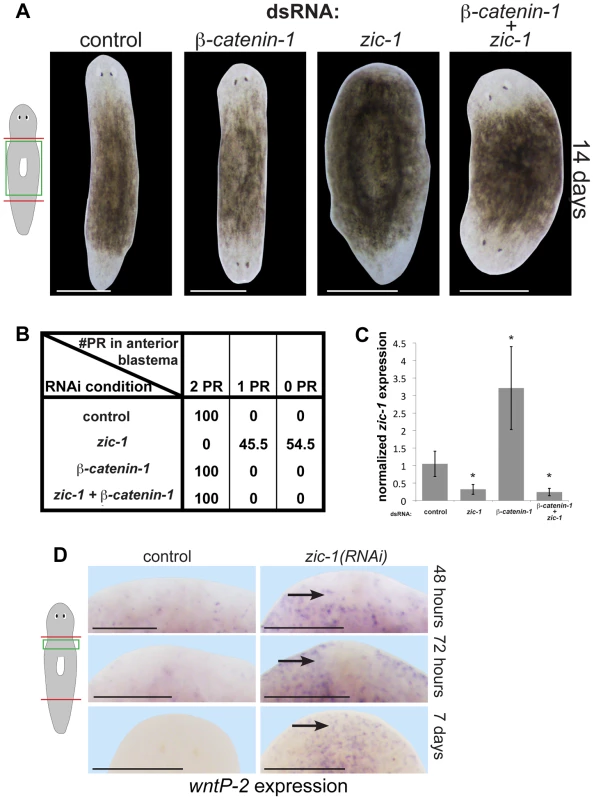
(A) Single and double-RNAi as indicated to examine interactions between zic-1 and beta-catenin-1. Total concentrations of dsRNA were normalized by control dsRNA so that the absolute amount of each utilized dsRNA was equivalent across treatments. zic-1(RNAi);beta-catenin-1(RNAi) animals all regenerated heads after decapitation suggesting that beta-catenin-1 inhibition is required for the zic-1's head-promoting function. (B) Scoring percent of anterior regeneration blastema with 0, 1, or 2 photoreceptors (PR) (n>9 animals). (C) qPCR analysis of zic-1 expression versus gapdh in animals treated with dsRNA as indicated. zic-1 dsRNA reduced zic-1 mRNA levels (p<0.05, t-test), beta-catenin-1 dsRNA increased zic-1 mRNA (p<0.05, t-test), and simultaneous treatment with zic-1 and beta-catenin-1 dsRNA decreased zic-1 mRNA (p<0.01, t-test). (D) wntP-2, a gene normally expressed in the posterior by beta-catenin-1 activity, was ectopically expressed in zic-1(RNAi) animals by 48–72 hours after decapitation, and maintained 7 days after amputation. Taken together, zic-1 inhibits beta-catenin-1 to promote head formation. Scale bars, 300 microns (D) or 500 microns (A). These results indicate that experimental inhibition of beta-catenin-1 can promote head regeneration in the absence of zic-1 and notum, likely by fulfilling the normal function of anterior pole-expressed notum as a Wnt inhibitor. In support of the first model, wntP-2/wnt11-5, a gene whose expression is activated by Wnt signaling in the posterior [13], is inappropriately expressed in the anterior of zic-1(RNAi) animals by 48/72 hours and even 7 days after decapitation (Fig. 6D), suggesting zic-1 inhibits beta-catenin-1 signaling. notum is required for anterior polarity and head regeneration whereas zic-1 is required only for head regeneration, and this distinction may account for the difference in extent of ectopic anterior wntP-2 expression observed after inhibition of the two genes [14]. We additionally found that zic-1 RNAi had no effect on beta-catenin-1 mRNA levels (Fig. S9B). beta-catenin-1 shares a similar epistatic relationship to zic-1 and notum, and zic-1 is required for notum expression, so we propose that injury-induced zic-1 promotes notum expression at the anterior pole which in turn represses Wnt signaling to allow head formation. We suggest that early, zic-1-independent, wound-induced notum expression is required for anterior polarity, whereas later zic-1-dependent notum expression at the pole orchestrates head patterning and outgrowth.
zic-1 controls a neoblast specification program that forms the anterior pole as an early step in head regeneration
zic-1's expression in neoblasts suggested it might function to control neoblast behaviors relevant for pole formation and/or specification of anterior cell types. Notably, however, tail regeneration and wnt1 expression at the posterior pole, both neoblast-dependent processes, occurred normally in zic-1(RNAi) animals (Fig. 4A–B), indicating zic-1 is not required for generic neoblast function. Additionally, zic-1(RNAi) animals had abundant smedwi-1 expression in both the anterior and posterior (Fig. S10A), indicating this gene is not required for regional maintenance of neoblasts in the anterior.
We next tested for possible involvement of zic-1 in neoblast specification programs. We purified total RNA from X1 neoblasts isolated by FACS from animals treated with zic-1 or control dsRNA in a time series and analyzed expression of 15 transcription factors and signaling molecules described to be activated in neoblast subpopulations due to regeneration (heat map shown in Fig. 7A with fold-changes and p-values shown in Table S2). zic-1 RNAi caused a significant (t-test p-value<0.05) reduction greater than 2-fold in expression of ovo (0, 24 and 48 hours), zic-1 (0, 24, 48 hours), notum (48 hours) and distalless (dlx) (24 hours). zic-1 RNAi reduced hmx-1 expression although not significantly (p = 0.11 at 24 hours), and significantly elevated expression of ap2 by 1.7-fold (48 hours). Several genes did not have significant fold-changes greater than 1.7-fold (p>0.05, t-test): hmx-1, sp6/9, OtxA, pax6A, soxB, runt-1, six1-2, six3-1, sim, and coe. We confirmed the observations of this expression analysis for selected genes using in situ hybridizations. zic-1 inhibition reduced ovo expression by 48 hours (Fig. S10B) and had minimal effects on runt-1 by 24 hours (Fig. S10C). Because runt-1 is also activated in neoblasts early (by 3 hours) in response to injury, we examined candidate reciprocal interactions with zic-1. However, runt-1 RNAi did not alter expression of wound-induced zic-1 (Fig. S10D). Therefore, zic-1 and runt-1 likely do not regulate each other transcriptionally. Finally, ap2 was induced by injury and expressed highly in the anterior of zic-1 RNAi animals (Fig. S10E), indicating that zic-1 RNAi does not simply result in a generic failure of neoblast functions in the anterior. These results indicate zic-1 is needed for expression of notum, ovo and distalless within the neoblast population and highlight the specificity of these functions.
Fig. 7. zic-1 controls utilization of foxD+smedwi-1+ progenitors for notum expression and anterior pole formation. 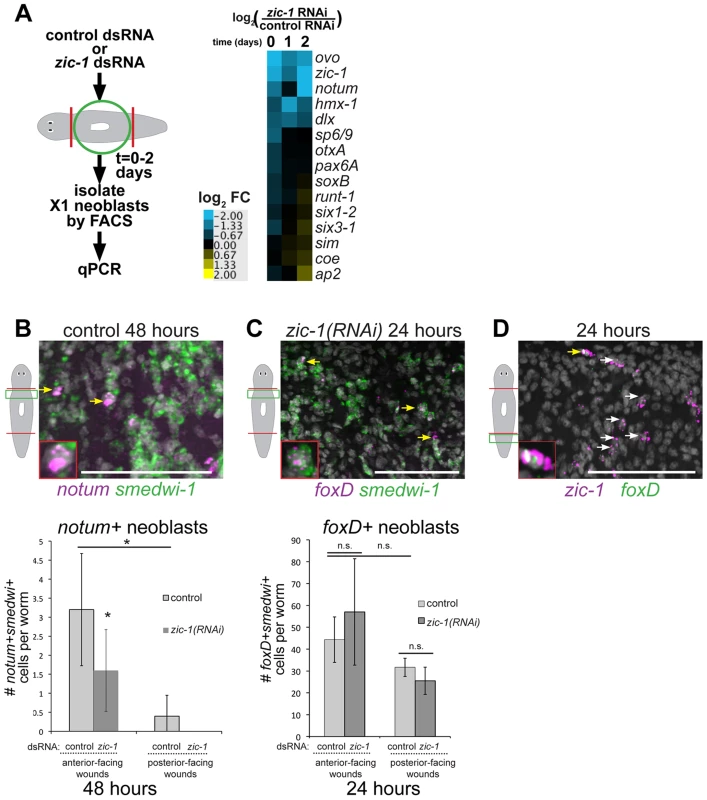
(A) Measurement of expression changes in X1 neoblasts due to treatment with zic-1 versus control dsRNA. Trunk fragments were macerated 5 minutes (0 days), 24 hours (1 day) and 48 hours (2 days) after head and tail amputation, and X1 cells were isolated from 3 independent biological replicates of 10 worms each. qPCR examined putative expression changes in a cohort of 15 transcription factors and signaling molecules that mark neoblast subpopulations induced by regeneration. Cartoon depicts experimental design. Heat map shows log2 fold-changes in gene expression for the indicated genes in zic-1(RNAi) X1 neoblasts versus controls averaged from three biological replicates for each condition. Blue color indicates downregulation and yellow indicates upregulation, due to zic-1 RNAi. (B–D) Double FISH to detect expression of zic-1, foxD, smedwi-1 and/or notum with nuclei counterstained with Hoechst (gray). (B) In situ hybridization probing to detect notum (magenta) and smedwi-1 (green) to detect notum+smedwi-1+ cells. (B, top) Representative image of notum+smedwi-1+ cells, (B, bottom) quantification of notum+smedwi-1+ cells. zic-1(RNAi) reduced numbers of notum+ neoblasts by 48 hours and smedwi-1+ cells were more numerous in anterior than posterior. (C) foxD (magenta) expression in smedwi-1+ (green) neoblasts 24 hours after injury was not eliminated due to zic-1 RNAi. Bottom, quantification of foxD+smedwi-1+ cells from control and zic-1(RNAi) animals fixed 24 hours after head and tail amputation and probed by double FISH. foxD+smedwi-1+ cells were produced in equal numbers at anterior- versus posterior-facing wound sites and were not reduced by zic-1 inhibition (control animals, 29±1 foxD+smedwi-1+ cells in anterior- versus 32±4 in posterior-facing wounds; zic-1 RNAi animals, 19±5 foxD+smedwi-1+ cells in anterior- versus 26±6 in posterior-facing wounds, n = 3 worms each). (D) foxD (green) is expressed in a subpopulation of zic-1+ cells (magenta, 3.4±1.8% of 420 zic-1+ cells were foxD+, n = 5 worms) by 24 hours. Yellow arrow indicates double positive cell (also inset), and white arrows indicate zic-1+ cells. Cartoons show surgeries and zoomed areas. Error bars standard deviations, asterisks p-value<0.05 by t-test. Bars, 75 microns. To identify neoblast responses regulated by zic-1 and specific for anterior pole formation, we turned our attention to notum and foxD, which are both needed for head formation and expressed in subpopulations of neoblasts during regeneration. notum expression was reduced by 48 hours in the analysis of X1 cells from zic-1(RNAi) animals (Fig. 7A), suggesting that zic-1 may control anterior pole formation by regulating specification of notum in neoblasts. To confirm this prediction, we analyzed zic-1(RNAi) versus control animals at 48 hours of regeneration using double FISH and counting of notum+/smedwi-1+ neoblasts. zic-1(RNAi) animals had reduced numbers of notum+/smedwi-1+ cells, indicating zic-1 is required for specification of notum+smedwi-1+ cells in addition to expression of notum in smedwi-1− cells of the anterior region (Fig. 7B, Fig. 4E). We next examined the relationship between zic-1 and foxD. foxD expression is irradiation sensitive by 24 hours and co-expressed in a neoblast subpopulation believed to participate in pole formation [41]. zic-1 inhibition did not alter the abundance of foxD+/smedwi-1+ neoblasts at anterior-facing wound sites by 24 hours (Fig. 7C). In addition, foxD is expressed in a subpopulation of zic-1+ cells at 24 hours, with 66% of foxD+ cells co-expressing zic-1 (14/21 cells, n = 5 worms), and 3% of zic-1+ cells co-expressing foxD (14/420 cells, n = 5) (Fig. 7D). We did not observe a general requirement of foxD for 24-hour zic-1 expression (Fig. S8B–C), but we cannot rule out the possibility that foxD is required for zic-1 expression in this small minority of zic-1+ cells that co-express foxD. Taken together, these results suggest zic-1 is not likely needed for specification of foxD+ neoblasts but rather for their utilization or further specification to form notum+ neoblasts that regenerate the anterior pole.
We further noted differences in the abundance of these progenitor cells at anterior - and posterior-facing wounds important for considering their relationships. foxD+ neoblasts were present at both anterior - and posterior-facing wounds in similar numbers (p-value>0.05, t-test) (Fig. 7C). By contrast, zic-1 expression in neoblasts was polarized for anterior-facing injury sites at 24 hours (Fig. 1C–D), and notum+ neoblasts were enriched near anterior-facing wounds versus posterior-facing wound sites (Fig. 7B). These results suggest that zic-1 may act as a polarizing cue to promote foxD progenitors to undergo anterior pole cell specification only at anterior-facing injury sites.
Discussion
Tissue organizers orchestrate growth and patterning in embryonic development, but it is not clear whether or how such signaling centers could contribute to coordinated growth in adulthood. In adult regeneration, stem cells or other progenitor cell types provide a supply of new differentiating cells necessary to replace missing structures, but such cells likely require instructive information prompted by injury in order to construct complex tissues. Here we describe the function of a Zic family transcription factor that couples injury signaling and polarized Wnt signaling cues [10], [11], [13], [14], [32], [59] for activation of stem cells to produce an anterior signaling center needed for head regeneration (Fig. 8).
Fig. 8. zic-1 function couples injury signaling and polarized Wnt signaling cues for activation of stem cells to produce the anterior pole in early head regeneration. 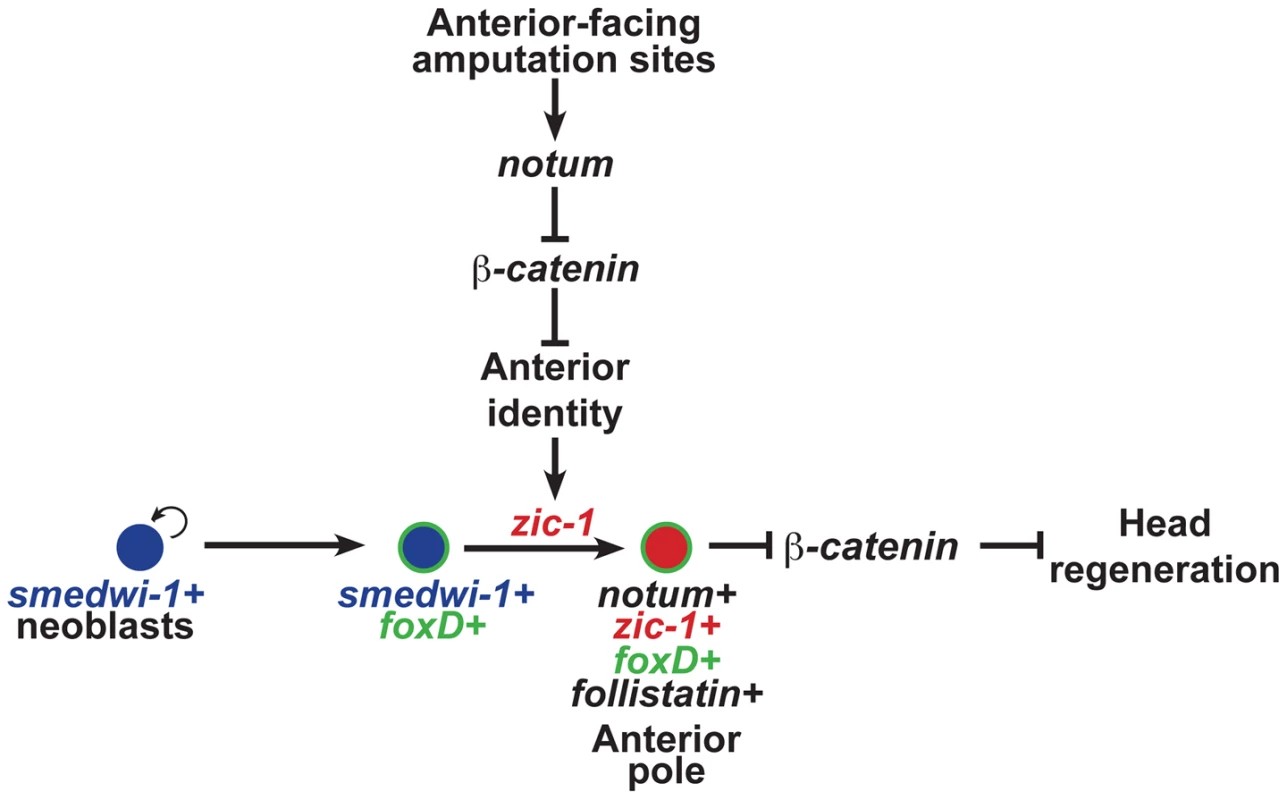
Anterior pole formation is neoblast-dependent, specific to anterior-facing amputations and midline cues, and dependent on foxD and zic-1. Prior to 24 hours, injury-induced expression of notum and wnt1 (not depicted) in collagen+ body wall muscle cells engages a decision process that determines anterior versus posterior identity at an amputation site. At anterior-facing amputation sites, this results in a low canonical Wnt signaling environment which promotes anterior identity and, directly or indirectly, expression of zic-1 in a population of smedwi-1+ neoblasts, some of which are foxD+. By 48 hours, zic-1 promotes specification of notum+smedwi-1+ progenitors that form an anterior pole signaling center that expresses zic-1, foxD, follistatin and notum. Signaling from the anterior pole, likely including notum activity, suppresses Wnt signaling to promote head outgrowth and patterning. Experimental inhibition of beta-catenin-1 can fulfill the signal inhibitory function of notum necessary for head formation in the absence of zic-1 or the anterior pole. zic-1 expression preferential to anterior-facing amputation sites ensures anterior-specific utilization of foxD+smedwi-1+ progenitors that are formed at both anterior and posterior injury sites. The functional and expression data support a model for head regeneration in which an initial anterior polarity decision, controlled by injury-induced notum, activates zic-1 expression in neoblasts, which enables head regeneration by production of notum+ cells of the anterior pole. Before 24 hours at anterior-facing amputation sites, notum is activated in cells of the body-wide musculature where it inhibits injury-induced wnt1 to create a low Wnt signaling environment, anterior identity, and subsequently zic-1 expression in neoblasts. By contrast, posterior-facing injury sites do not abundantly express notum, allowing injury-induced wnt1 signaling to predominate and enforce high levels of beta-catenin-1 signaling that ultimately suppress zic-1 expression. Anterior - or posterior-facing injury sites have different zic-1 expression responses according to their position along the A-P axis. We suggest that the initial expression of zic-1 at the posterior-facing wound sites of head fragments reflects a low Wnt signaling environment present at the time of injury, but in such fragments zic-1 expression is ultimately suppressed as the region takes on posterior polarity and identity through wnt1 signaling. foxD is expressed in neoblast subpopulations at both anterior - and posterior-facing amputation sites. zic-1 activity at anterior-facing wound sites triggers further specification or utilization of foxD-expressing neoblasts for expression of notum and subsequent anterior pole formation by 48 hours. zic-1 is neoblast-expressed early and co-expresses with foxD in pole progenitors at the anterior pole, so the most likely scenario is that it acts autonomously to control this specification process, but we cannot rule out possible indirect functions for zic-1 in head regeneration or patterning. The regenerating anterior pole produces NOTUM protein, which likely sustains a low Wnt signaling environment, possibly through positive feedback by specification of additional zic-1+ neoblasts that continue to contribute new cells to the anterior pole to promote head regeneration.
Injuries can alter tissue content in numerous ways, so regenerating animals must have mechanisms that robustly allow production of appropriate missing structures. It is difficult to envision a complex contingency system that continuously probes each tissue type for its presence or absence to address this need. Alternatively, a relatively simple system that utilizes information about the position, orientation, and extent of a wound site could be sufficient for achieving this goal. zic-1 expression activation after wounding depends both on the injury's A/P axial location (Fig. 2F) as well as its A/P orientation (Fig. 2B), suggesting the existence of such systems. It is also possible that zic-1 expression is activated at other sites or tissues requiring maintenance of low Wnt signaling, as we observed expression of zic-1 in some non-anterior regions during regeneration, similar to notum [14]. However, sustained and abundant zic-1 expression is enriched at anterior-facing amputation sites (Fig. 2B). Wound-induced signaling through 6–24 hour expression of wnt1 and notum may have a particular importance in regeneration scenarios that require significant alteration of A/P tissue identity. Examining the relationship between pre-existing regional cues and wound-activated signaling will be important for understanding how regenerative outgrowth is achieved.
Planarian transcription factors have been identified that function in either neoblast subpopulations for specification of specific differentiated cell types or function broadly in the process of axis formation. Several lineage-specific transcription factors are expressed within subpopulations of neoblasts for forming cells of the eye, protonephridia, intestine, brain [3], [51], [52], [60]–[62] and additional post-mitotic progeny [55], [63]–[65]. For example, runt-1 is expressed within neoblasts [9] and is required for eye formation [66]. By contrast, transcriptional regulators have also been implicated in axis formation. Tail regeneration and expression of wnt1 in the tail requires islet1, a transcription factor expressed in the posterior [67], as well as pitx, expressed at the anterior and posterior poles [39], [40]. The TALE homeobox gene prep is needed for head formation [56], and pbx, another TALE transcription factor, is needed for both head and tail formation [37], [38]. Our results identify zic-1 as an injury-induced neoblast-expressed gene with possible related functions in both cell specification and axis formation.
The participation of vertebrate Zic proteins in multiple developmental processes and the existence of several Zic family members have complicated efforts to identify their common use in only a single signaling pathway, and interactions with Hedgehog, Wnt, Activin, and retinoic acid signaling have been reported [50], [68]. Early wound-induced wnt1 expression that occurs by 18 hours after injury is dependent on hedgehog signaling, and still occurs in irradiated animals [13], [32] that lack zic-1 expression (Fig. 2E). Therefore, neoblast-expressed zic-1 is unlikely to be a general regulator of Hedgehog signaling. Our results indicate repression of Wnt signaling, possibly through expression of signaling inhibitors, could be an ancient and conserved function for Zic proteins. Additionally, Activin signaling repression by follistatin is needed for both head and tail regeneration in planarians [25], suggesting that zic-1 is not a general regulator of that pathway in planarians. Vertebrate Zic2 and Zic3 regulate organizer function by inhibition of Wnt signaling [42], [69]. Additionally, mutations in human zic2 lead to holoprosencephaly [70], a midline defect of the anterior due to perturbed organizer function [43]. Our studies of planarian zic-1 suggest Zic proteins may have had ancient functions and conserved functions in Wnt signaling inhibition involved in injury responses or control of tissue organizing regions.
In principle, the positional information necessary for regeneration could either be dependent or independent of stem cell function. Planarians depleted of neoblasts maintain the ability to undergo some generic injury-induced signaling and also signaling polarized along the body axes [9], [13], [32]. By contrast, our analysis of zic-1 suggests that a critical step in blastema formation is the production of new differentiated cells to perform signaling functions that subsequently direct the activity of additional cells for tissue outgrowth. Programs that use stem cells or specialized progenitors to produce signaling centers after injury may be utilized in general for normal regenerative growth. The use of stem cells to construct tissue organizers post-embryonically could facilitate the synthesis or repair of non-regenerative organs.
Materials and Methods
Animals and irradiation treatments
Animals of the asexual strain of the planarian Schmidtea mediterranea were maintained in planarian water (1× Montjuic salts) at ∼19°C as previously described [14]. Planarians were fed a liver paste and starved for at least seven days before experiments. Where indicated, animals were gamma-irradiated with a lethal dose of 6,000 Rads using a Cesium source irradiator 48 hours before surgeries (Fig. 1A, S1, S2A and S5B) or the day of amputation (Fig. 2E).
FACS sorting, RNA extraction and microarray analysis
For gene expression profiling by microarray (Fig. 1C) and realtime PCR (Fig. 1D), neoblasts were isolated from tissue near wound site by FACS in a time series (0, 24, 48 and 72 hours post amputation). For each biological replicate, tissue from anterior - or posterior-facing injury sites from 8 animals was collected, macerated, labeled with Hoechst 33342 and propidium iodide and sorted as previously described [64]. For each biological replicate, 40,000–100,000 cells were collected into Trizol-LS, and RNA was extracted using RNeasy Plus Mini Kit (Qiagen, 74134). Libraries were prepared using a WTA2 amplification kit (Sigma), and arrays were hybridized and scanned according to the manufacturer's instructions at the Washington University Genome Technology Access Center. Custom oligonucleotide arrays from Agilent had 140,000 probes intended to represent the majority of S. mediterranea genes identified through previous EST sequencing, RNA-seq and transcriptome assembly, and gene predictions [53], [64], [66], [71], [72]. The limma Bioconductor software package (Linear Models for Microarray Data) implemented in R was used for analysis of single-channel microarray data [73]. Briefly, array intensities were background subtracted (method = “normexp”, offset = 16), quantile-normalized between arrays, and differential gene expression was evaluated using eBayes which produces for each gene a t-statistic of the ratio of the log2-fold change to the standard error moderated across all genes in the experimental series to obtain a p-value that was used to compute a false-discovery rate by correcting for multiple hypothesis testing using the Benjamini-Hochberg method. Genes with at least one time point having a with a false-discovery rate of <0.10 as compared to the 0 hour expression for either anterior - or posterior-facing wound sites, as appropriate, were selected for further investigation. GeneCluster3.0 and Java TreeView were used for hierarchical clustering and visualization. Microarray data have been deposited at NCBI with accession number GSE56178. For analysis of progenitor genes in zic-1 RNAi worms (Fig. 7A), neoblasts were isolated in similar way from animal fragments generated by head and tail removal (“trunks”) by FACS in a time series using a BD FacsAria SORP 5-Laser. RNA was extracted using RNeasy Mini Kit (Qiagen, 74104) and gene expression analyzed by RT-qPCR.
Cloning
Array oligonucleotide sequences matching clone BPKG17485 [74] were blasted to a planarian transcriptome database (PlanMine, http://planmine.mpi-cbg.de) identifying exact matches for dd_Smed_v4_22585_0_1 and uc_Smed_v1_Contig48469. The predicted ORF encoded a protein of 529 amino acids with 87% identity to Dugesia japonica zic-A and identical to Schmidtea mediterranea zicA as named previously in a phylogenetic study [45], and we renamed this gene zic-1. zic-2 was identified through a transcriptome search of Zic-family orthologs as dd_Smed_v4_15531_0_1 (PlanMine). The predicted ORF encoded a protein of 478 amino acids with 86% identity to Dugesia japonica zicB and identical to Schmidtea mediterranea zicB in a previous phylogenetic study [45].
Riboprobes for zic-1 were made from a PCR product cloned by RT-PCR into pGEM vector using the primers 5′-CACTGCATGTATCAACACCAAG-3′ and 5′-AAGCAATTCTCCCACCGTTA-3′.
Double-stranded RNA (dsRNA) was generated by in vitro transcription or expression from a bacterial dsRNA expression vector (pPR244) as previously described [75]. Unless otherwise noted, all zic-1 RNAi experiments used dsRNA derived from a 1611-bp zic-1 fragment cloned using the primers 5′-CACTGCATGTATCAACACCAAG-3′ and 5′-AAGCAATTCTCCCACCGTTA-3′. Additionally, non-overlapping zic-1 fragments were cloned to create a ∼700 bp 5′ fragment (using primers 5′-AAGCAATTCTCCCACCGTTA-3′ and 5′-AACCATTTCAATGCCCTTTC-3′) and a ∼900 bp 3′ fragment (using primers 5′-CGCGGACAATCTTTCCAATA-3′ and 5′-CACTGCATGTATCAACACCAAG-3′). zic-2 was cloned using the primers 5′-TCACGGAATCTGAATGTGGA-3′ and 5′-TGAAACCGAGAGGTTTTCGT-3′.
Other riboprobes and/or dsRNAs (smedwi-1, notum, beta-catenin-1, APC, wnt1, wntP-2, gpas, chat, sFRP-1, fzd-4, prep, pbx, pitx, follistatin, foxD, ovo, ap2, runt-1, slit, collagen, prog-1, ap2) were as previously described [9], [14], [37]–[39], [41], [51], [56].
Fixations and whole-mount in situ hybridization
Animals were fixed and stained as previously described [76]. In brief, animals were killed in 5% N-acetyl-cysteine in 1×PBS for 5 minutes and then fixed in formaldehyde for 17 minutes at room temperature. Subsequently, animals were bleached overnight (∼16 hours) in 6% hydrogen peroxide in methanol on a light box. Riboprobes were synthesized as previously described as digoxigenin - or fluorescein-labeled [76]. Colorimetric (NBT/BCIP) or fluorescence in situ hybridizations were performed as previously described [76]. For FISH, blocking solution was modified to MABT with 10% horse serum and 10% western blot blocking reagent (Roche) [77]. Anti-Dig-HRP and anti-FL-HRP antibodies were used at a 1∶2000 dilution, and anti-Dig-AP was used at a 1∶4000 dilution. Hoechst 33342 (Invitrogen) was used 1∶500 as counterstain. Images of colorimetric staining were acquired using a Leica M210F scope with a Leica DFC295 camera and adjusted for brightness and contrast. Fluorescence imaging was performed on either a Leica DM5500B compound microscope with Optigrid structured illumination system for optical sectioning or a Leica laser scanning SP5 confocal microscope at 40× or 63×. Images are maximum projections of a z-series with adjustments to brightness and contrast using ImageJ and Photoshop.
In FISH staining, cells expressing one or two genes of interest were manually counted from z-stacks using a ∼300 micron region near injury sites, each cell was labeled with color-coded marks and double checked by comparing with neighbor planes using ImageJ and Metamorph. For enumeration of WISH staining, NBT-BCIP precipitated material with the size and shape of cells were counted as the sum of ventral and dorsal views using a ∼300 micron region near injury sites using a dissecting microscope at 12× and additional magnification with a 1.7× turret dorsal and a manual counter. As a measure of the reproducibility of this assay, the signal to noise ratio (average/standard deviation) from 5 technical replicates scored on 3 specimens each was determined to be 9.8+/−2.3%.
BrdU labeling and immunofluorescence
Uninjured worms were injected a solution of 5 mg/ml BrdU (Sigma-Aldrich/Fluka 16880) dissolved in water, two days prior to amputation of heads. Animals were fixed using NAC/FA solutions and rehydrated in a methanol series before FISH staining. Post-fixation in 4% formaldehyde and acid hydrolyzation in 2N HCl were performed after FISH detection, followed by antibody labeling of incorporated BrdU. Animals were blocked in PBSTB (PBSTx+0.25% BSA) for 4–6 hours at room temperature and incubated overnight in rat anti-BrdU (Oxford Biotechnology OBT0030S) antibody diluted 1∶1000 in PBSTB. Animals were rinsed in PBSTB and incubated overnight in goat anti-rat-HRP (AbCam ab7097) antibody diluted 1∶1000 in PBSTB blocking solution. Tyramide development was performed at room temperature for 1 hour in 1∶150 red tyramide (Alexa Fluor 568 T20914) in amplification buffer. BrdU imaging was performed on a Leica SP5 Confocal microscope. Polyclonal rabbit anti-SMEDWI-1 antibody (a kind gift of P. Reddien) was diluted 1∶2000 in PBSTB and detected by tyramide amplification as above [7].
RNAi protocols
RNAi treatment was performed either by injection or by feeding. For RNAi by injection, dsRNA was synthesized from in vitro transcription reactions (Promega) using PCR templates with flanking T7 sequences (Denville), purified by phenol extraction and ethanol precipitation, resuspended in RNase-free water and annealed at 65°C for 10 minutes, 37°C for 20–30 minutes, then on ice for at least 10 minutes. dsRNA corresponding to C. elegans unc-22, a gene not present in the planarian genome, served as a negative control.
RNAi by injection was performed similarly to that described previously [37] and was performed as follows. Unless otherwise noted, animal fragments generated by head and tail removal (“trunks”) were injected 2–3 times with 30 nL of 3 µg/µl dsRNA and injected again the following two days, the animals were amputated anteriorly and posteriorly to remove ∼300 microns of tissue near the prior injury site, allowed to regenerate 6–8 days prior to another dsRNA injection and amputation of heads and tails for subsequent assaying of regeneration phenotypes and histological analysis.
For double-RNAi experiments, a similar injection strategy was used that involved three consecutive days of dsRNA injection, amputation and regeneration for 8 days, another three consecutive days of injections, then amputation of heads and tails and scoring of regeneration phenotypes 8 days later. dsRNA concentrations were normalized using control dsRNA so that every animal received an equivalent dose of zic-1 and/or beta-catenin-1or zic-2 dsRNA. For inhibition of beta-catenin-1, animals were given control dsRNA in the first set of dsRNA injections and beta-catenin-1 dsRNA during the second set of injections. To generate zic-1(RNAi);beta-catenin-1(RNAi) animals, worms were given zic-1 dsRNA during the first set of injections and a 1∶1 mixture of beta-catenin-1 and zic-1 dsRNA during the second set of injections. For inhibition of zic-2, animals were given a 1∶1 mixture of zic-2 and control dsRNA in both sets of dsRNA injections. To generate zic-1(RNAi);zic-2(RNAi) animals, worms were given a 1∶1 mixture of both zic-1 and zic-2 dsRNA.
For experiments involving RNAi by bacterial feeding, animals were fed a mixture of liver paste and E. coli expressing dsRNA, as described previously [14], two times a week (every 3–4 days) for either 10 weeks without injury (Fig. S6), or 2 weeks (Fig. 5 and S7) prior to amputation of heads and tails.
Realtime PCR
mRNA of zic-1 was detected by realtime PCR using SYBR Green PCR Master Mix (Applied Biosystems). Total RNA was extracted by mechanical homogenization in Trizol (Invitrogen) from three regenerating fragments in three (Fig. 6C, S5E, and S9B) or four (Fig. 5C, S5E, and S8) biological replicates. RNA samples were DNase-treated (TURBO DNase, Ambion) and cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen). zic-1 mRNA was detected using the following primers: 5′-TGGAAATAGAAATCTTGGTGGATT-3′ and 5′-AATCGGTTGTAATAGATTCGATGG-3′, zic-2 mRNA was detected using 5′-CCTATGGTTGGATAAACACATGAA-3′ and 5′-CGACATGATCAAGTGTTAAGTGGT-3′, and gapdh mRNA was detected using previously described primers [14]. Primer sequences used in Fig. 1D and Fig. 7A are described in Table S3. Relative mRNA abundance was calculated using the delta-Ct method after verification of primer amplification efficiency. p-values below 0.05 were considered as significant differences.
Supporting Information
Zdroje
1. TanakaEM, ReddienPW (2011) The cellular basis for animal regeneration. Dev Cell 21 : 172–185.
2. RinkJC (2013) Stem cell systems and regeneration in planaria. Dev Genes Evol 223 : 67–84.
3. WagnerDE, WangIE, ReddienPW (2011) Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 332 : 811–816.
4. ReddienPW, OviedoNJ, JenningsJR, JenkinJC, Sánchez AlvaradoA (2005) SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science 310 : 1327–1330.
5. NewmarkPA, Sánchez AlvaradoA (2000) Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Dev Biol 220 : 142–153.
6. HayashiT, AsamiM, HiguchiS, ShibataN, AgataK (2006) Isolation of planarian X-ray-sensitive stem cells by fluorescence-activated cell sorting. Dev Growth Differ 48 : 371–380.
7. WenemoserD, ReddienPW (2010) Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Dev Biol 344 : 979–991.
8. GuedelhoeferOCt, Sánchez AlvaradoA (2012) Amputation induces stem cell mobilization to sites of injury during planarian regeneration. Development 139 : 3510–3520.
9. WenemoserD, LapanSW, WilkinsonAW, BellGW, ReddienPW (2012) A molecular wound response program associated with regeneration initiation in planarians. Genes Dev 26 : 988–1002.
10. GurleyKA, RinkJC, Sánchez AlvaradoA (2008) Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science 319 : 323–327.
11. PetersenCP, ReddienPW (2008) Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science 319 : 327–330.
12. AdellT, SalóE, BoutrosM, BartschererK (2009) Smed-Evi/Wntless is required for beta-catenin-dependent and -independent processes during planarian regeneration. Development 136 : 905–910.
13. PetersenCP, ReddienPW (2009) A wound-induced Wnt expression program controls planarian regeneration polarity. Proc Natl Acad Sci U S A 106 : 17061–17066.
14. PetersenCP, ReddienPW (2011) Polarized notum activation at wounds inhibits Wnt function to promote planarian head regeneration. Science 332 : 852–855.
15. RinkJC, GurleyKA, ElliottSA, Sánchez AlvaradoA (2009) Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science 326 : 1406–1410.
16. LiuSY, SelckC, FriedrichB, LutzR, Vila-FarreM, et al. (2013) Reactivating head regrowth in a regeneration-deficient planarian species. Nature 500 : 81–84.
17. SikesJM, NewmarkPA (2013) Restoration of anterior regeneration in a planarian with limited regenerative ability. Nature 500 : 77–80.
18. UmesonoY, TasakiJ, NishimuraY, HroudaM, KawaguchiE, et al. (2013) The molecular logic for planarian regeneration along the anterior-posterior axis. Nature 500 : 73–76.
19. GavinoMA, ReddienPW (2011) A Bmp/Admp regulatory circuit controls maintenance and regeneration of dorsal-ventral polarity in planarians. Curr Biol 21 : 294–299.
20. ReddienPW, BermangeAL, KiczaAM, Sánchez AlvaradoA (2007) BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development 134 : 4043–4051.
21. Gonzalez-SastreA, MolinaMD, SalóE (2012) Inhibitory Smads and bone morphogenetic protein (BMP) modulate anterior photoreceptor cell number during planarian eye regeneration. Int J Dev Biol 56 : 155–163.
22. MolinaMD, NetoA, MaesoI, Gomez-SkarmetaJL, SalóE, et al. (2011) Noggin and noggin-like genes control dorsoventral axis regeneration in planarians. Curr Biol 21 : 300–305.
23. MolinaMD, SalóE, CebriaF (2007) The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Dev Biol 311 : 79–94.
24. Roberts-GalbraithRH, NewmarkPA (2013) Follistatin antagonizes activin signaling and acts with notum to direct planarian head regeneration. Proc Natl Acad Sci U S A 110 : 1363–1368.
25. GavinoMA, WenemoserD, WangIE, ReddienPW (2013) Tissue absence initiates regeneration through Follistatin-mediated inhibition of Activin signaling. Elife 2: e00247.
26. YazawaS, UmesonoY, HayashiT, TaruiH, AgataK (2009) Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. Proc Natl Acad Sci U S A 106 : 22329–22334.
27. ZhangD, ChanJD, NogiT, MarchantJS (2011) Opposing roles of voltage-gated Ca2+ channels in neuronal control of regenerative patterning. J Neurosci 31 : 15983–15995.
28. OviedoNJ, MorokumaJ, WalentekP, KemaIP, GuMB, et al. (2010) Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol 339 : 188–199.
29. OviedoNJ, LevinM (2007) smedinx-11 is a planarian stem cell gap junction gene required for regeneration and homeostasis. Development 134 : 3121–3131.
30. BeaneWS, MorokumaJ, LemireJM, LevinM (2013) Bioelectric signaling regulates head and organ size during planarian regeneration. Development 140 : 313–322.
31. BeaneWS, MorokumaJ, AdamsDS, LevinM (2011) A chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration. Chem Biol 18 : 77–89.
32. GurleyKA, ElliottSA, SimakovO, SchmidtHA, HolsteinTW, et al. (2010) Expression of secreted Wnt pathway components reveals unexpected complexity of the planarian amputation response. Dev Biol 347 : 24–39.
33. WitchleyJN, MayerM, WagnerDE, OwenJH, ReddienPW (2013) Muscle cells provide instructions for planarian regeneration. Cell Rep 4 : 633–641.
34. GiraldezAJ, CopleyRR, CohenSM (2002) HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev Cell 2 : 667–676.
35. GerlitzO, BaslerK (2002) Wingful, an extracellular feedback inhibitor of Wingless. Genes Dev 16 : 1055–1059.
36. FlowersGP, TopczewskaJM, TopczewskiJ (2012) A zebrafish Notum homolog specifically blocks the Wnt/beta-catenin signaling pathway. Development 139 : 2416–2425.
37. ChenCC, WangIE, ReddienPW (2013) pbx is required for pole and eye regeneration in planarians. Development 140 : 719–729.
38. BlassbergRA, FelixDA, Tejada-RomeroB, AboobakerAA (2013) PBX/extradenticle is required to re-establish axial structures and polarity during planarian regeneration. Development 140 : 730–739.
39. MarzM, SeebeckF, BartschererK (2013) A Pitx transcription factor controls the establishment and maintenance of the serotonergic lineage in planarians. Development 140 : 4499–4509.
40. CurrieKW, PearsonBJ (2013) Transcription factors lhx1/5-1 and pitx are required for the maintenance and regeneration of serotonergic neurons in planarians. Development 140 : 3577–3588.
41. ScimoneML, LapanSW, ReddienPW (2014) A forkhead transcription factor is wound-induced at the planarian midline and required for anterior pole regeneration. PLoS Genet 10: e1003999.
42. FujimiTJ, HatayamaM, ArugaJ (2012) Xenopus Zic3 controls notochord and organizer development through suppression of the Wnt/beta-catenin signaling pathway. Dev Biol 361 : 220–231.
43. WarrN, Powles-GloverN, ChappellA, RobsonJ, NorrisD, et al. (2008) Zic2-associated holoprosencephaly is caused by a transient defect in the organizer region during gastrulation. Hum Mol Genet 17 : 2986–2996.
44. LeeH, StultzBG, HurshDA (2007) The Zic family member, odd-paired, regulates the Drosophila BMP, decapentaplegic, during adult head development. Development 134 : 1301–1310.
45. ArugaJ, KamiyaA, TakahashiH, FujimiTJ, ShimizuY, et al. (2006) A wide-range phylogenetic analysis of Zic proteins: implications for correlations between protein structure conservation and body plan complexity. Genomics 87 : 783–792.
46. KuoJS, PatelM, GamseJ, MerzdorfC, LiuX, et al. (1998) Opl: a zinc finger protein that regulates neural determination and patterning in Xenopus. Development 125 : 2867–2882.
47. NakataK, NagaiT, ArugaJ, MikoshibaK (1997) Xenopus Zic3, a primary regulator both in neural and neural crest development. Proc Natl Acad Sci U S A 94 : 11980–11985.
48. MizusekiK, KishiM, MatsuiM, NakanishiS, SasaiY (1998) Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development 125 : 579–587.
49. DiNardoS, O'FarrellPH (1987) Establishment and refinement of segmental pattern in the Drosophila embryo: spatial control of engrailed expression by pair-rule genes. Genes Dev 1 : 1212–1225.
50. HoutmeyersR, SouopguiJ, TejparS, ArkellR (2013) The ZIC gene family encodes multi-functional proteins essential for patterning and morphogenesis. Cell Mol Life Sci 70 : 3791–3811.
51. LapanSW, ReddienPW (2012) Transcriptome Analysis of the Planarian Eye Identifies ovo as a Specific Regulator of Eye Regeneration. Cell Rep 2 : 294–307.
52. LapanSW, ReddienPW (2011) dlx and sp6-9 Control optic cup regeneration in a prototypic eye. PLoS Genet 7: e1002226.
53. ReddienPW, NewmarkPA, Sánchez AlvaradoA (2008) Gene nomenclature guidelines for the planarian Schmidtea mediterranea. Dev Dyn 237 : 3099–3101.
54. GuoT, PetersAH, NewmarkPA (2006) A Bruno-like gene is required for stem cell maintenance in planarians. Dev Cell 11 : 159–169.
55. EisenhofferGT, KangH, Sánchez AlvaradoA (2008) Molecular analysis of stem cells and their descendants during cell turnover and regeneration in the planarian Schmidtea mediterranea. Cell Stem Cell 3 : 327–339.
56. FelixDA, AboobakerAA (2010) The TALE class homeobox gene Smed-prep defines the anterior compartment for head regeneration. PLoS Genet 6: e1000915.
57. CebriaF, GuoT, JopekJ, NewmarkPA (2007) Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Dev Biol 307 : 394–406.
58. AdellT, CebriaF, SalóE (2010) Gradients in planarian regeneration and homeostasis. Cold Spring Harb Perspect Biol 2: a000505.
59. IglesiasM, Gomez-SkarmetaJL, SalóE, AdellT (2008) Silencing of Smed-betacatenin1 generates radial-like hypercephalized planarians. Development 135 : 1215–1221.
60. ScimoneML, SrivastavaM, BellGW, ReddienPW (2011) A regulatory program for excretory system regeneration in planarians. Development 138 : 4387–4398.
61. ForsthoefelDJ, ParkAE, NewmarkPA (2011) Stem cell-based growth, regeneration, and remodeling of the planarian intestine. Dev Biol 356 : 445–459.
62. CowlesMW, BrownDD, NisperosSV, StanleyBN, PearsonBJ, et al. (2013) Genome-wide analysis of the bHLH gene family in planarians identifies factors required for adult neurogenesis and neuronal regeneration. Development 140 : 4691–4702.
63. WagnerDE, HoJJ, ReddienPW (2012) Genetic regulators of a pluripotent adult stem cell system in planarians identified by RNAi and clonal analysis. Cell Stem Cell 10 : 299–311.
64. ScimoneML, MeiselJ, ReddienPW (2010) The Mi-2-like Smed-CHD4 gene is required for stem cell differentiation in the planarian Schmidtea mediterranea. Development 137 : 1231–1241.
65. PearsonBJ, Sánchez AlvaradoA (2008) Regeneration, stem cells, and the evolution of tumor suppression. Cold Spring Harb Symp Quant Biol 73 : 565–572.
66. SandmannT, VoggMC, OwlarnS, BoutrosM, BartschererK (2011) The head-regeneration transcriptome of the planarian Schmidtea mediterranea. Genome Biol 12: R76.
67. HayashiT, MotoishiM, YazawaS, ItomiK, TanegashimaC, et al. (2011) A LIM-homeobox gene is required for differentiation of Wnt-expressing cells at the posterior end of the planarian body. Development 138 : 3679–3688.
68. MaurusD, HarrisWA (2009) Zic-associated holoprosencephaly: zebrafish Zic1 controls midline formation and forebrain patterning by regulating Nodal, Hedgehog, and retinoic acid signaling. Genes Dev 23 : 1461–1473.
69. PourebrahimR, HoutmeyersR, GhogomuS, JanssensS, ThelieA, et al. (2011) Transcription factor Zic2 inhibits Wnt/beta-catenin protein signaling. J Biol Chem 286 : 37732–37740.
70. BrownSA, WarburtonD, BrownLY, YuCY, RoederER, et al. (1998) Holoprosencephaly due to mutations in ZIC2, a homologue of Drosophila odd-paired. Nat Genet 20 : 180–183.
71. AdamidiC, WangY, GruenD, MastrobuoniG, YouX, et al. (2011) De novo assembly and validation of planaria transcriptome by massive parallel sequencing and shotgun proteomics. Genome Res 21 : 1193–1200.
72. ReschAM, PalakodetiD, LuYC, HorowitzM, GraveleyBR (2012) Transcriptome analysis reveals strain-specific and conserved stemness genes in Schmidtea mediterranea. PLoS One 7: e34447.
73. SmythGK (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article3.
74. LabbeRM, IrimiaM, CurrieKW, LinA, ZhuSJ, et al. (2012) A comparative transcriptomic analysis reveals conserved features of stem cell pluripotency in planarians and mammals. Stem Cells 30 : 1734–1745.
75. ReddienPW, BermangeAL, MurfittKJ, JenningsJR, Sánchez AlvaradoA (2005) Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev Cell 8 : 635–649.
76. PearsonBJ, EisenhofferGT, GurleyKA, RinkJC, MillerDE, et al. (2009) Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev Dyn 238 : 443–450.
77. KingRS, NewmarkPA (2013) In situ hybridization protocol for enhanced detection of gene expression in the planarian Schmidtea mediterranea. BMC Dev Biol 13 : 8.
Štítky
Genetika Reprodukčná medicína
Článek Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome EvolutionČlánek Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder PopulationČlánek Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among PancrustaceansČlánek Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery DiseaseČlánek An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation inČlánek Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 7- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Cuba: Exploring the History of Admixture and the Genetic Basis of Pigmentation Using Autosomal and Uniparental Markers
- Clonal Architecture of Secondary Acute Myeloid Leukemia Defined by Single-Cell Sequencing
- Mechanisms of Functional Variants That Impair Regulated Bicarbonate Permeation and Increase Risk for Pancreatitis but Not for Cystic Fibrosis
- Nucleosomes Shape DNA Polymorphism and Divergence
- Functional Diversification of Hsp40: Distinct J-Protein Functional Requirements for Two Prions Allow for Chaperone-Dependent Prion Selection
- Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome Evolution
- Activation of the Immune System by Combinations of Common Alleles
- Age-Associated Sperm DNA Methylation Alterations: Possible Implications in Offspring Disease Susceptibility
- Muscle-Specific SIRT1 Gain-of-Function Increases Slow-Twitch Fibers and Ameliorates Pathophysiology in a Mouse Model of Duchenne Muscular Dystrophy
- MDRL lncRNA Regulates the Processing of miR-484 Primary Transcript by Targeting miR-361
- Hypersensitivity of Primordial Germ Cells to Compromised Replication-Associated DNA Repair Involves ATM-p53-p21 Signaling
- Intrapopulation Genome Size Variation in Reflects Life History Variation and Plasticity
- SlmA Antagonism of FtsZ Assembly Employs a Two-pronged Mechanism like MinCD
- Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder Population
- Determinative Developmental Cell Lineages Are Robust to Cell Deaths
- DELLA Protein Degradation Is Controlled by a Type-One Protein Phosphatase, TOPP4
- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among Pancrustaceans
- UVB Induces a Genome-Wide Acting Negative Regulatory Mechanism That Operates at the Level of Transcription Initiation in Human Cells
- The Nesprin Family Member ANC-1 Regulates Synapse Formation and Axon Termination by Functioning in a Pathway with RPM-1 and β-Catenin
- Combinatorial Interactions Are Required for the Efficient Recruitment of Pho Repressive Complex (PhoRC) to Polycomb Response Elements
- Recombination in the Human Pseudoautosomal Region PAR1
- Microsatellite Interruptions Stabilize Primate Genomes and Exist as Population-Specific Single Nucleotide Polymorphisms within Individual Human Genomes
- An Intronic microRNA Links Rb/E2F and EGFR Signaling
- An Essential Nonredundant Role for Mycobacterial DnaK in Native Protein Folding
- Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery Disease
- The Genomic Landscape of the Ewing Sarcoma Family of Tumors Reveals Recurrent Mutation
- Evolution and Genetic Architecture of Chromatin Accessibility and Function in Yeast
- An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation in
- Stage-Dependent and Locus-Specific Role of Histone Demethylase Jumonji D3 (JMJD3) in the Embryonic Stages of Lung Development
- Genome Wide Association Identifies Common Variants at the Locus Influencing Plasma Cortisol and Corticosteroid Binding Globulin
- Regulation of Feto-Maternal Barrier by Matriptase- and PAR-2-Mediated Signaling Is Required for Placental Morphogenesis and Mouse Embryonic Survival
- Apomictic and Sexual Germline Development Differ with Respect to Cell Cycle, Transcriptional, Hormonal and Epigenetic Regulation
- Functional EF-Hands in Neuronal Calcium Sensor GCAP2 Determine Its Phosphorylation State and Subcellular Distribution , and Are Essential for Photoreceptor Cell Integrity
- Comparison of Methods to Account for Relatedness in Genome-Wide Association Studies with Family-Based Data
- Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
- Cis and Trans Effects of Human Genomic Variants on Gene Expression
- 8.2% of the Human Genome Is Constrained: Variation in Rates of Turnover across Functional Element Classes in the Human Lineage
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- A Loss of Function Screen of Identified Genome-Wide Association Study Loci Reveals New Genes Controlling Hematopoiesis
- Unraveling Genetic Modifiers in the Mouse Model of Absence Epilepsy
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
- The Coding and Noncoding Architecture of the Genome
- A Novel Locus Is Associated with Large Artery Atherosclerotic Stroke Using a Genome-Wide Age-at-Onset Informed Approach
- Brg1 Loss Attenuates Aberrant Wnt-Signalling and Prevents Wnt-Dependent Tumourigenesis in the Murine Small Intestine
- The PTK7-Related Transmembrane Proteins Off-track and Off-track 2 Are Co-receptors for Wnt2 Required for Male Fertility
- The Co-factor of LIM Domains (CLIM/LDB/NLI) Maintains Basal Mammary Epithelial Stem Cells and Promotes Breast Tumorigenesis
- Essential Genetic Interactors of Required for Spatial Sequestration and Asymmetrical Inheritance of Protein Aggregates
- Meiosis-Specific Cohesin Component, Is Essential for Maintaining Centromere Chromatid Cohesion, and Required for DNA Repair and Synapsis between Homologous Chromosomes
- Silencing Is Noisy: Population and Cell Level Noise in Telomere-Adjacent Genes Is Dependent on Telomere Position and Sir2
- The Two Cis-Acting Sites, and , Contribute to the Longitudinal Organisation of Chromosome I
- A Broadly Conserved G-Protein-Coupled Receptor Kinase Phosphorylation Mechanism Controls Smoothened Activity
- Requirements for Acute Burn and Chronic Surgical Wound Infection
- LIN-42, the PERIOD homolog, Negatively Regulates MicroRNA Transcription
- WAPL Is Essential for the Prophase Removal of Cohesin during Meiosis
- Expression in Planarian Neoblasts after Injury Controls Anterior Pole Regeneration
- Sox11 Is Required to Maintain Proper Levels of Hedgehog Signaling during Vertebrate Ocular Morphogenesis
- Accumulation of a Threonine Biosynthetic Intermediate Attenuates General Amino Acid Control by Accelerating Degradation of Gcn4 via Pho85 and Cdk8
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



