-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
Craniofacial abnormalities are among the most common birth defects. Understanding the molecular mechanisms underlying craniofacial disorders is crucial for developing treatment strategies. Much of the craniofacial skeleton arises from specialized embryonic structures known as pharyngeal arches. Patterning of these arches requires precise spatial and temporal expression of multiple genes, which is coordinated between tissues by secreted signals. Wnts are secreted ligands expressed throughout the pharyngeal arches yet their role in craniofacial patterning remains unclear. In this study we examine the role of Wnts in craniofacial patterning using transgenic zebrafish to inhibit downstream Wnt signaling. We show that Wnt signaling deficient embryos have lower jaw specific defects, which strongly resembles loss-of-function phenotypes in both the Bmp and Edn1 signaling pathways. Through rescue experiments we find that Wnts are upstream regulators of both Bmp and Edn1 signaling. We thus have uncovered a crucial requirement for Wnt signaling in craniofacial patterning.
Published in the journal: Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton. PLoS Genet 10(7): e32767. doi:10.1371/journal.pgen.1004479
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004479Summary
Craniofacial abnormalities are among the most common birth defects. Understanding the molecular mechanisms underlying craniofacial disorders is crucial for developing treatment strategies. Much of the craniofacial skeleton arises from specialized embryonic structures known as pharyngeal arches. Patterning of these arches requires precise spatial and temporal expression of multiple genes, which is coordinated between tissues by secreted signals. Wnts are secreted ligands expressed throughout the pharyngeal arches yet their role in craniofacial patterning remains unclear. In this study we examine the role of Wnts in craniofacial patterning using transgenic zebrafish to inhibit downstream Wnt signaling. We show that Wnt signaling deficient embryos have lower jaw specific defects, which strongly resembles loss-of-function phenotypes in both the Bmp and Edn1 signaling pathways. Through rescue experiments we find that Wnts are upstream regulators of both Bmp and Edn1 signaling. We thus have uncovered a crucial requirement for Wnt signaling in craniofacial patterning.
Introduction
A fundamental question in skeletal biology is how cartilages and bones with distinct shapes arise from skeletogenic precursor cells. Much of the craniofacial skeleton derives from neural crest (NC) cells that migrate in streams into the pharyngeal arches and contain anterior-posterior (A-P) patterning information obtained prior to migration [1]–[3]. However, these NC cells also become intimately associated with epithelia, including surface ectoderm and pharyngeal endoderm, which produce signals important for skeletal patterning. For example, Fgf8 from the facial ectoderm regulates A-P polarity of the mandibular arch as well as NC proliferation/survival [4]–[6]. Surgical disruption of the pharyngeal endoderm in chick [7], or mutations that disrupt endoderm in zebrafish, lead to severe cartilage malformations [8]–[10]. Endoderm-derived Fgf3 induces cartilage formation [9] and sphingosine phosphate-1 from endoderm modulates Shh signaling to promote mandibular growth and patterning [11]–[13]. Ectoderm-derived Shh induces upper jaw and neurocranial structures [14], [15]. Thus, craniofacial skeletal shapes reflect interplay between epithelial signals and intrinsic properties of mesenchyme, but the mechanisms underlying these interactions remain unclear.
One well-studied example of such epithelial-mesenchymal interactions in the pharyngeal skeleton is the induction of ventral skeletal fates along the dorsal-ventral (D-V) axis of the mandibular arch by ectodermal Endothelin 1 (Edn1) and Bone morphogenetic proteins (Bmps) [16]–[22]. Conditional loss of Bmp4 in the facial ectoderm in mice inhibits ventral mandibular growth and patterning [23]. Loss of Edn1 and/or any of several components of its signal transduction pathway leads to severe jaw truncations, both in mice and in humans, and in some cases transformations of ventral tissues (the lower jaw) to a more dorsal identity [22], [24]–[27]. Initially both Edn1 and Bmps induce similar subsets of ventral/intermediate genes as well as restricting Jag1 signaling to the dorsal domain [16], [19], [20], [28]. But once arch primordia are established, effects of Bmps become more ventrally restricted to domains that no longer depend on Edn1, particularly the transcription factor Hand2. Uniform application of Bmp or Edn1 proteins can restore many aspects of D-V patterning in Bmp - and Edn1-deficient zebrafish embryos, suggesting that other ventralizing signals must interact to control D-V patterning [22].
Wnts are good candidates for additional signals involved in D-V patterning based on their localized expression and known requirements in craniofacial development. Several Wnt ligands show restricted expression in facial epithelia (ectoderm, endoderm) in zebrafish, chick and mouse embryos [29]–[32] and Frizzled (Fzd) receptors are expressed throughout arch NC cells and endoderm [31], [33], [34]. In addition, expression of Lef1, Tcf1, β-catenin (βcat), [35], and transgenic Wnt signaling reporter lines (Lef/Tcf promoters driving β-gal or LacZ expression) in mice all are ventrally (distally) restricted in the mandibular as well as distal maxillary prominences [36], [37]. Like Bmps, Wnt signaling is necessary for early NC cell induction [38], [39] and also plays later roles in NC migration, fate specification, and proliferation [40]. In zebrafish, conditional overexpression of a dominant negative Tcf3 (dntcf3) during NC cell specification dramatically reduces NC cell numbers [41], similar to depletion of Fzd3 receptors in Xenopus [42]. Wnt1−/−/Wnt3−/− double mutant mice show reduced proliferation of pre-migratory NC cells [43]. Conditional loss of βcat in the pharyngeal ectoderm impairs growth of the facial prominences [44], while conditional loss of βcat in cranial NC cells leads to apoptosis and a nearly complete loss of NC-derived craniofacial structures [45]. Finally, loss of Tcf4/Lef1 function or overexpression of the Wnt inhibitor Dickkopff-1 (Dkk1) results in smaller facial structures and clefting between the frontonasal and maxillary prominences [36]. Similarly, Wnt signaling is important for facial midline development in humans as incidences of cleft lip and palate have been mapped to genetic variations in Wnt ligands [46].
In this study, we examine temporal requirements for Wnt signaling in zebrafish D-V craniofacial development. We utilize two transgenic lines, Tg(hsp701:dkk1-GFP)(dkk1+) and Tg(hsp70I:tcf3-GFP) (dntcf3+), to interfere with Wnt signaling conditionally, in a stage-specific manner. Tg(hsp701:dkk1-GFP) embryos overexpress dkk1, a secreted negative regulator, while Tg(hsp70I:tcf3-GFP) embryos overexpress a dominant negative form of the Tcf3 transcription factor. Both methods of inhibiting Wnt signaling after NC cell migration result in proliferation and ventral patterning defects in the mandibular and hyoid arches. Interestingly, dkk1+ embryos also show unique clefting of the lower jaw. Defects in ventral-intermediate specific gene expression and expansion of the dorsal specific jag1b resemble loss of Bmp and Edn1 signaling [19], [20]. We show that Wnt signaling promotes Bmp signaling through regulation of expression of two specific Bmp receptors, bmpr1ab and bmpr1ba, in the pharyngeal arches. Ectopic Bmp protein can rescue msxe, dlx3b, dlx5a but not hand2 in the absence of Wnt signaling, demonstrating that Wnts participate in a regulatory network with Bmp and Edn1 signaling, but separately in hand2 regulation, to control D-V pharyngeal patterning. Chimeric analyses reveal that dntcf3 acts cell autonomously in pharyngeal endoderm, which also expresses dkk1. This suggests that Wnts regulate patterning in the endoderm, which through some as yet unknown signal imparts D-V patterning upon neighboring skeletal progenitors in the NC.
Results
Wnt responses are highest in the ventral pharyngeal arches
Numerous Wnt ligands (Wnt2, Wnt4, Wnt5a/b, Wnt 6, and Wnt7a/b) and receptors (Fzd1, Fzd3, Fzd4, Fzd6, Fzd7, Fzd8, and Fzd10) are expressed broadly in the pharyngeal ectoderm, endoderm, neural crest (NC), and mesoderm [29], [32], [34], [35]. To determine which regions of the pharyngeal arches respond directly to Wnt signaling we used in situ hybridization (ISH) to examine expression of the direct downstream Wnt target mycn (Fig. 1A,B), an oncogene with roles in regulating Wnt-dependent morphogenesis and proliferation [47], [48]. mycn mRNA was detected throughout the arches but at higher levels in the ventral domain, primarily within the NC mesenchyme (arrowheads in Fig. 1A,B). To further address which pharyngeal tissues respond directly, we examined expression of a transgenic Wnt reporter zebrafish Tg(7xTCF-Xla.Siam:GFP)ia4 (7xTCF:GFP) [49], which contains seven TCF response elements driving expression of GFP, thus acting as a live reporter in cells where stabilized β-catenin (βcat) interacts with Tcf transcription factors. ISH for GFP mRNA at 28 hours postfertilization (hpf) revealed regions of 7xTCF:GFP expression in the ventral first and second arches (Fig. 1C), which in transverse sections appeared localized both to arch NC cells and pharyngeal endoderm, but not pharyngeal ectoderm (Fig. 1D).
Fig. 1. Wnt signaling in the pharyngeal arches. 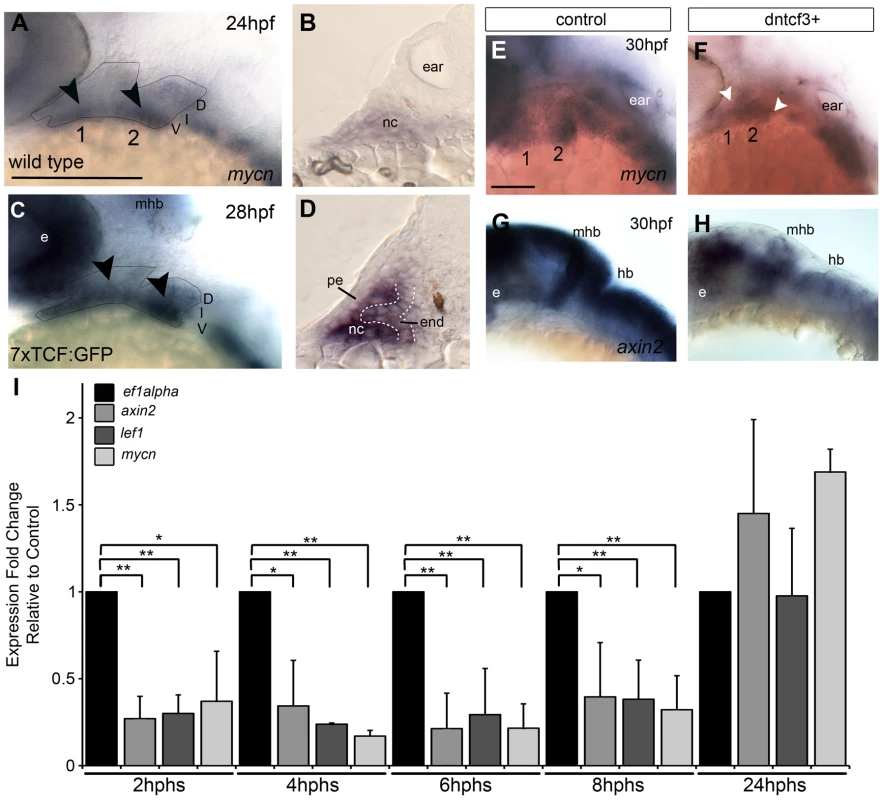
(A–H) In situ hybridization (ISH) and (I) quantitative, real-time PCR (qPCR) analysis of Wnt target gene expression; (A, C, E–H) lateral views, anterior to the left; (B, D) transverse sections through arch 2. (A, B) mycn mRNA is ventrally restricted (black arrowheads) in wild type (WT) embryos. Arches 1 and 2 are outlined by dotted lines. (C, D) GFP mRNA is ventrally restricted (arrowheads) in Tg(7xTCF-Xla.Siam:GFP)ia4 transgenics. (B, D) Both mycn (B) and 7xTCF;GFP (D) are expressed in ventral nc cells and pharyngeal endoderm, and excluded from pharyngeal ectoderm. (E–H) mycn and axin2 expression in controls (E, G), and dntcf3+ embryos at 26 hpf (F, H; heat shocked at 22 hpf). (I) qPCR analysis of axin2, lef1 and mycn expression in dntcf3+ embryos, normalized to nontransgenic, heat-shocked controls, with ef1alpha as an internal control. * P<0.05, ** P<0.001. Abbreviations: e, eye; D, dorsal arch; end, pharyngeal endoderm; hb, hindbrain; I, intermediate arch; mhb, mid-hindbrain boundary; nc, neural crest; pe, pharyngeal ectoderm; V, ventral arch. Scale bars: 100 µm. Blocking Wnt signaling after NC migration disrupts the ventral arch skeleton
To bypass earlier requirements for Wnts in embryogenesis we took a conditional loss-of-function approach using heat shock-inducible transgenic zebrafish lines to inhibit Wnt signaling in a temporally-controlled manner. Tg(hsp70I:tcf3-GFP) (hs-dntcf3) embryos overexpress a truncated form of the transcription factor tcf3 with GFP replacing the βcat-interacting domain, under control of heat shock promoter 70 [41]. With a similar hsp70 promoter, Tg(hsp701:dkk1-GFP) (hs-dkk1) embryos overexpress full length dkk1b tagged with a GFP [50], which prevents Fzd-Lrp co-receptor binding [51], [52].
To verify that Wnt-βcat signaling was affected in hs-dkk1+ and hs-dntcf3+ embryos we used ISH to examine the expression of mycn and axin2, both direct Wnt targets, after heat shocking during stages of craniofacial patterning. At 4 hours post heat shock (hphs) hs-dntcf3+ embryos heat shocked at 22 hpf showed severe reductions in expression of mycn in the arches, eyes, and brain and axin2 (which shows only very weak or no expression in pharyngeal arches), in the eyes and brain (Fig. 1E–H). Similarly, compared with controls (Fig. S1A, C) at 2 hphs hs-dkk1+ embryos heat shocked at 24 hpf showed reduced mycn in the arches, eye, and brain and axin2 expression in the brain but to a lesser extent than in hs-dntcf3+ embryos (Fig. S1B, D).
To determine stage-specific defects caused by disrupting Wnt signaling in hs-dntcf3+ and hs-dkk1+ embryos we performed quantitative real time PCR (qPCR) analysis of direct Wnt targets. In hs-dntcf3+ embryos, axin2 and mycn expression was reduced significantly from 2–8 hphs (Fig. 1I), but recovered and slightly increased by 24 hphs (Fig. 1I). Lef1, a transcriptional cofactor in the Wnt pathway [53], [54], was also significantly reduced in hs-dntcf3+ embryos from 2–8 hphs (Fig. 1I). In hs-dkk1+ embryos, axin2 expression was severely reduced from 1–8 hphs, while Lef1 downregulation started later, from 4–8 hphs (Fig. S1E). Thus both hs-dntcf3 and hs-dkk1 lines deplete Wnt signaling almost immediately after heat shock and repress Wnt signaling for up to 8 hphs.
Alcian blue staining of cartilage in larvae at 96 hpf revealed that, compared with controls, hs-dkk1+/ − heterozygotes heat shocked for 30 min at 16–28 hpf developed mandibular clefting and reduced Meckel's cartilages (Mc), as well as mild reductions in other craniofacial cartilages (Fig. 2A, B, D). Homozygous hs-dkk1+/+ larvae displayed dramatic shortening of Mc ventrally in the first arch, as well as the symplectic (Sy), a more intermediate/dorsal element of the second arch (Fig. 2C, E; [19], [20]). Thus, hereafter “dkk1+” refers to homozygous hs-dkk1 embryos/larvae heat shocked for 30 min between 20–22 hpf.
Fig. 2. Requirements for Wnt signaling in craniofacial cartilage development. 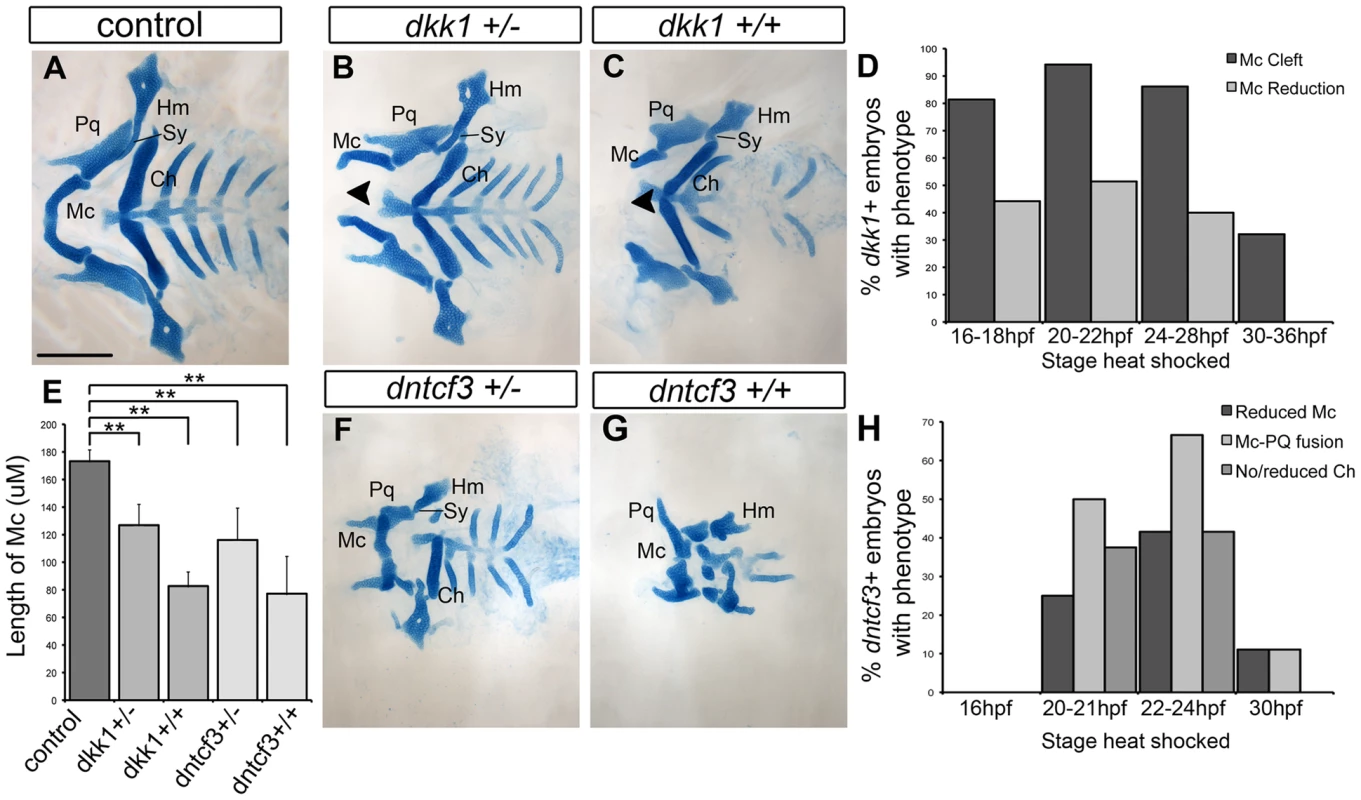
(A–C, F, G) Alcian blue stained cartilage at 96 hpf, dissected and flat mounted, ventral views, anterior to the left. (A) Wild-type control, (B) dkk1+/− heterozygote, (C) dkk1+/+ homozygote, (F) dntcf3+/− heterozygote, (G) dntcf3+/+ homozygote, heat shocked as described (see Results). Arrowheads indicate clefting of Meckel's cartilage (Mc). (D) Histogram quantifying the frequency of Mc defects in dkk1+ embryos heat shocked at different stages from 16–36 hpf. (E) Histogram quantifying average Mc length, p<0.01. (H) Histogram quantifying the frequency of cartilage defects in arches 1 and 2 in dntcf3+ embryos heat shocked at different stages from 16–30 hpf. Abbreviations: Ch, ceratohyal; Hm, hyomandibular; Mc, Meckels; Pq, palatoquadrate; Sy, symplectic. Scale bar: 100 µm. Heterozygous hs-dntcf3+ larvae heat shocked slightly later (22–24 hpf) also showed mild reductions in Mc, but in this case Mc was fused to the more dorsal palatoquadrate (Pq) in arch 1 (Fig. 2F, H). The ceratohyal (Ch) in ventral arch 2 was also variably reduced, but more posterior cartilages appeared largely unaffected. Cartilage defects in homozygous hs-dntcf3 larvae heat shocked similarly were much more severe (Fig. 2E, G) – 41% showed reduced Mc and Ch reduction/loss, while 66% showed joint fusion between Mc and Pq (Fig. 2H). Thus, hereafter “dntcf3+” refers to heterozygous hs-dntcf3+/ − embryos/larvae heat shocked for 12 min between 22–24 hpf.
To determine tissue-specific requirements for Wnt signaling in the pharyngeal arches, we transplanted dntcf3+ cells at gastrula stages either into the fate map position that gives rise to NC or co-injected with Taram-A mRNA to drive them to an endodermal fate, into non-transgenic WT hosts [9]. While dntcf3+ NC cells in chimeras that virtually filled the entire mandibular arch caused no discernable cartilage defects (not shown), large grafts of dntcf3+ endodermal cells into the pharyngeal region induced D-V patterning defects that resembled dntcf+ embryos, including reduced Mc and fused Mc-Pq (Fig. S2A). These results suggest that the critical direct response to Wnt occurs in the endoderm (which expresses 7XTCF:GFP) and is indirectly relayed to surrounding NC cells.
To verify that cartilage defects in dkk1+ and dntcf3+ larvae reflect specific requirements for Wnt signaling we attempted to rescue them using the compound 6-bromoindirubin-3′-oxime (BIO), which stabilizes Wnt signaling by inhibiting GSK-3 [55]. BIO treatments of 7xTCF:GFP embryos at 24 hpf caused ectopic gfp expression and direct Wnt targets were upregulated in a dose-dependent manner as determined by ISH and qPCR analysis at 30 hpf (Fig. S3). Treatment of wild type embryos with BIO resulted in an overall reduction of cartilages in a dose-dependent manner, which correlated with reduced proliferating cell nuclear antigen (pcna) expression (which marks cells in mitosis [56]) in the arches at 30 hpf, indicating reduced proliferation of cartilage precursors (Fig. S4J–L). Despite their smaller sizes, dorsal cartilages acquired more rod-like morphologies similar to ventral Mc and Ch, suggesting partial ventralization (Fig. S4A–C). BIO treatments of both heat shocked dntcf3+ and dkk1+ transgenics partially rescued cartilage defects, including Mc-Pq joint fusions and Ch was consistently restored in dntcf3+ larvae (Fig. S4D–I, M). BIO also rescued Mc clefting in dkk1+ larvae at higher concentrations (Fig. S4G–I). Therefore loss of canonical Wnt signaling in dntcf3+ and dkk1+ embryos accounts for the majority of craniofacial defects.
Blocking Wnt signaling disrupts proliferation in ventral arch NC cells
Cartilages in dntcf3+ and dkk1+ larvae were 30–50% smaller than controls (Fig. 2). This reduced cartilage size was not due to increased cell death as we could detect no differences in the number of acridine orange stained cells in the arches between dntcf3+, dkk1+ and control embryos at 6 hphs (Fig. S5). To examine proliferation in the arches we performed ISH for pcna. Pcna mRNA was detected throughout the pharyngeal arches from 3–22 hphs (25–44 hpf) in controls (Fig. 3A–D), but somewhat reduced at 3 hphs (25 hpf) in both dkk1+ and dntcf3+ embryos (Fig. 3A, E, I, M). By 6 hphs (28 hpf) pcna expression was nearly undetectable in the arches in both dkk1+ (66%, n = 6) and dntcf3+ (60%, n = 10) (Fig. 3B, F, J, M). By 8 hphs (30 hpf), pcna expression had recovered slightly in dntcf3+ embryos (36%, n = 11) (Fig. 3K, M) but not in dkk1+ embryos (80%, n = 5) (Fig. 3G, M). Both recovered completely by 22–28 hphs (44 hpf) (Fig. 3D, H, L, M).
Fig. 3. Requirements for Wnt signaling in pharyngeal arch cell proliferation. 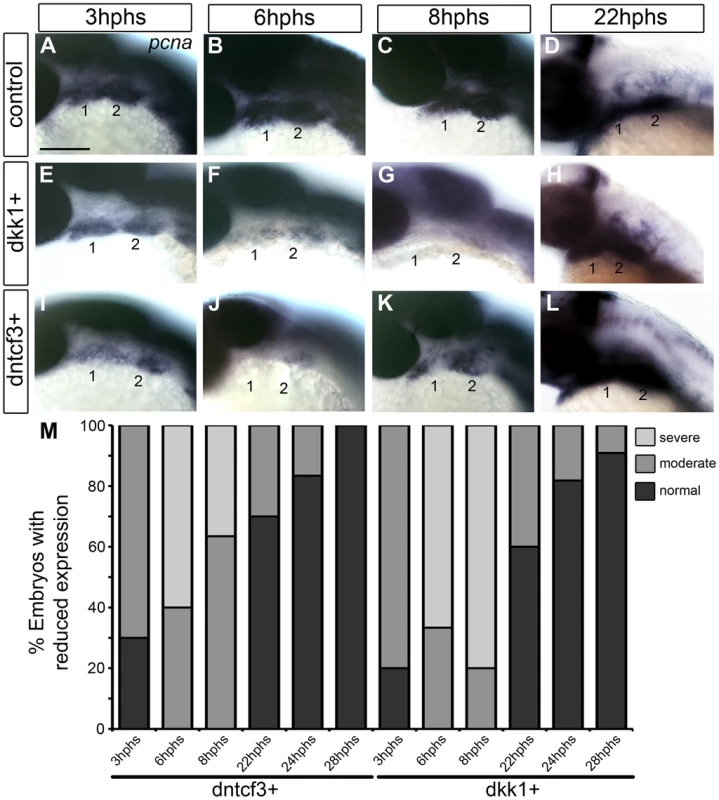
(A–L) Whole mount ISH for pcna in embryos fixed at 3–22 hours post-heat shock (hphs), lateral views, anterior to the left (all heat shocked at 22 hpf). (A–D) pcna is expressed throughout arches, brain and eyes. (E–H) dkk1+ embryos have reduced pcna expression at 3 hphs (E), severe reductions at 6 hphs (F) and virtually no arch expression at 8 hphs (G), before expression returns at 22 hphs (H). (I–L) dntcf3+ embryos show reduced pcna expression at 3 hphs (I), and virtually no expression at 6 hphs (J, K) before expression rebounds between 8–22 hphs (L). (M) Histogram quantifying percentages of dkk1+ and dntcf3+ embryos with moderate versus severe reductions in pcna expression. Scale bar: 100 µm. To confirm these apparent defects in proliferation we used an antibody that recognizes phosphoHistone3 (pH 3), a protein involved in chromosome condensation in mitotic cells [57], which marks a subset of pcna+ dividing cells. In dkk1+ and dntcf3+ embryos pH 3 staining was reduced throughout the eye and brain (Fig. S6A–B, D–E). At 4 hphs, dkk1+ embryos had a 75% reduction in pH 3+ cells in the arches compared to controls (Fig. S6A′, B′, C). Similarly, at 3 hphs dntcf3+ embryos had approximately 50% fewer pH 3+ cells in the arches than controls (Fig. S6D′, E′, F). Thus depleting Wnt signaling in embryos between 24–30 hpf severely impairs proliferation in the arches, which correlates with reductions in cartilage and in Wnt target gene expression (Figs. 1, 2).
Blocking Wnt signaling disrupts expression of ventral arch patterning genes
To investigate roles for Wnt signaling in D-V patterning within the arches, we examined expression of genes that mark distinct ventral, intermediate and dorsal regions of the arch primordia in dntcf3+ and dkk1+ embryos with ISH [19]. hand2 expression in the ventral-most domains of each arch was severely reduced in both dkk1+ (53%, n = 15) and dntcf3+ (59%, n = 17) embryos (Fig. 4A–C, Q), with a small domain of expression remaining at the arch 1–2 boundary in dntcf3+ embryos (Fig. 4C). Similarly, expression of dlx3b and dlx6a in the intermediate domains of each arch were mildly reduced in dkk1+ (44%, n = 18, 23%, n = 21) and severely reduced in dntcf3+ embryos (83%, n = 12; 90%, n = 21) (Fig. 4D–I, Q). Finally, expression of the Notch ligand, jag1b in the dorsal-most domains of each arch [28], was variably expanded ventrally in dkk1+ (10.5%, n = 57) embryos and consistently expanded in dntcf3+ embryos (55.5%, n = 9) as well as chimeras in which dntcf3+ cells were transplanted into the pharyngeal endoderm (Fig. 4J–L, Q; Fig. S2B–C). These gene expression changes were not simply due to an overall loss of arches or NC cells, since dlx2a expression (Fig. 4N, P) as well as sox10:lynTdtom expression throughout the D-V extent of the arch NC were unaffected in the arches of both dkk1+ and dntcf3+ embryos (Fig. S7). Additionally, BIO treatments of wild type embryos caused dorsal expansion of expression of the ventral-intermediate gene msxe, mild expansion of dlx3b and hand2 expression, and reduced jag1b expression in the dorsal domain (Fig. S8). Therefore, Wnt signaling promotes ventral and intermediate-cell fates in the arches. Dntcf3+ embryos in particular, with residual hand2 expression at the arch 1–2 boundary, closely resemble mutants in Bmp and Edn1 signaling [19], [20].
Fig. 4. Requirements for Wnt signaling in dorsal-ventral arch patterning. 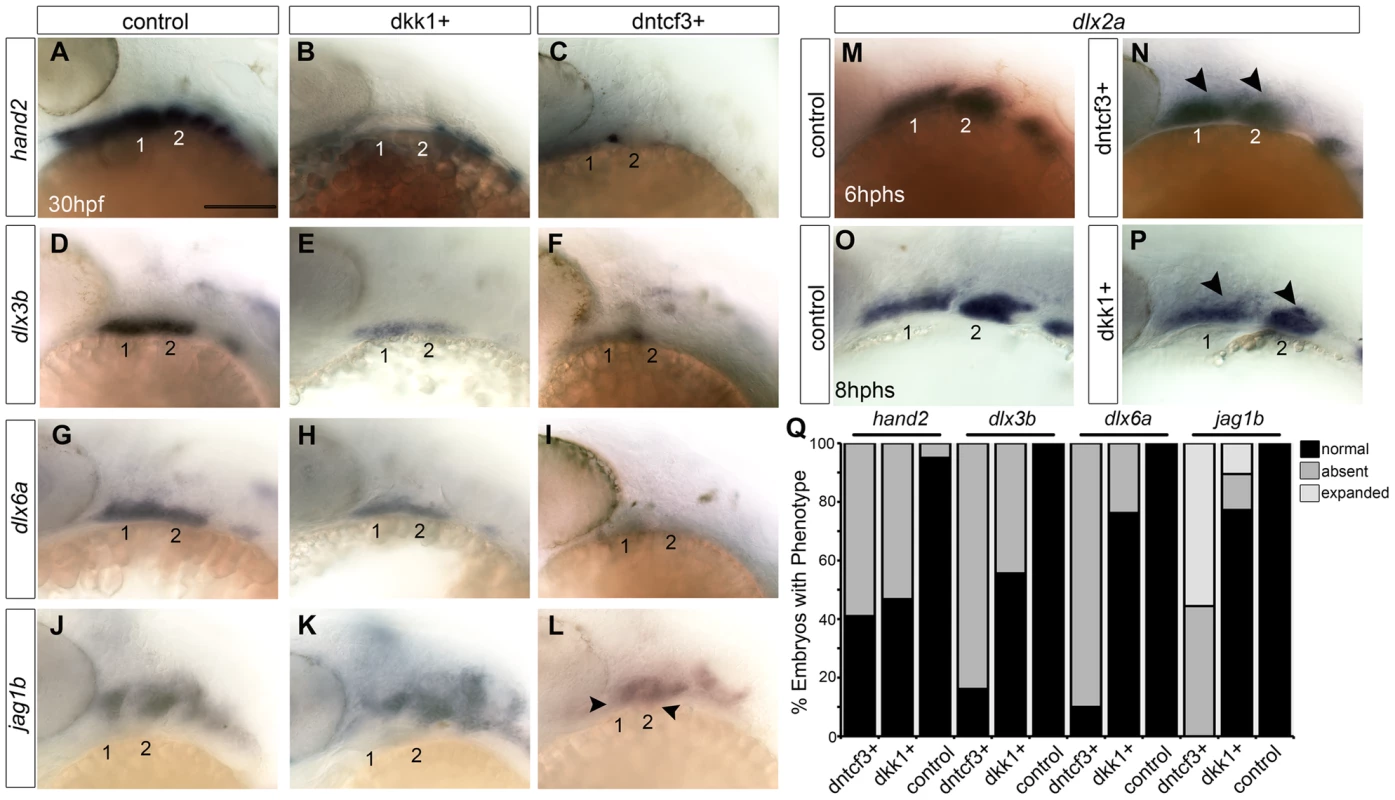
(A–P) Whole mount ISH for genes involved in dorsal-ventral (D-V) patterning in control (A, D, G, J), dkk1+ embryos heat shocked at 20–22 hpf (B, E, H, K), and dntcf3+ embryos heat shocked at 22–24 hpf (C, F, I, L), lateral views, anterior to the left. hand2 (B, C), dlx3b (E, F), and dlx6a (H, I) expression is reduced, while jag1b (K, L) expression expands ventrally (arrowheads) particularly in dntcf3+ embryos, compared with controls. (M–P) dlx2a expression in control (M,O), dntcf3+ (N), and dkk1+ (P) embryos. The dorsal boundary of dlx2a expression (arrowheads) appears to shift ventrally in some cases, (N,P) compared to the control (M,O). (Q) Histogram quantifying percentages of dkk1+ and dntcf3+ embryos with defects in D-V patterning. Scale bar: 100 µm. Blocking Wnt signaling disrupts Bmp and Edn1 signaling in the arches
Because dntcf3+ embryos showed D-V defects in cartilage morphology and gene expression that more closely resembled Bmp - and Edn1-deficient embryos than dkk1+ we focused on dntcf3+. To examine interactions between Wnt and Bmp signaling in the arches we used an antibody that recognizes phosphorylated Smad1/5/8 (pSmad1/5/8) in dntcf3+ embryos. In controls pSmad1/5/8 localized to ventral arches 1 and 2 where levels of Bmp signaling have been shown to be highest at 24 hpf (Fig. 5A–C; [19]). Anti-pSmad1/5/8 staining was slightly reduced in the first arch at 2 hphs (24–26 hpf) in dntcf3+ embryos (Fig. 5D), in both arches by 4 hphs (Fig. 5E), and virtually lost altogether at 6 hphs (Fig. 5F). Western blots confirmed that pSmad1/5/8 levels were much lower than controls at 6 hphs (Fig. 5G, H). To examine potential interactions between Wnt and Edn1 signaling in the arches we performed qPCR for Edn1 in dntcf3+ embryos at 6 hphs. Edn1 expression was significantly reduced relative to control (Fig. 5I). These results reveal an indirect role for Wnts in D-V patterning through regulation of both Bmp and Edn1 signaling.
Fig. 5. Wnt signaling regulates Bmp and Edn1 signaling in the arches. 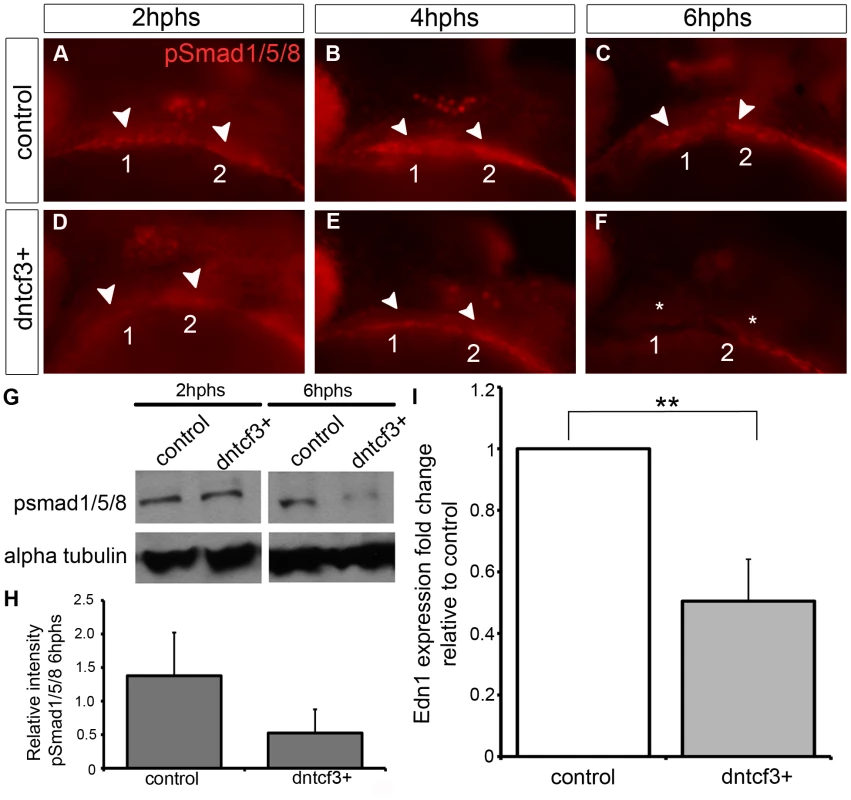
(A–F) Anti-pSmad1/5/8 staining in control (A–C) and dntcf3+ (D–F) embryos heat shocked at 22–24 hpf, lateral views, anterior to the left. pSmad1/5/8 expression is localized ventrally in the arches (arrowheads), reduced at 2 hphs (D), severely reduced at 4 hphs (E), and absent (asterisk) by 6 hphs (F). (G) Western blot analysis of anti-pSmad1/5/8 in control and dntcf3+ embryos. Protein was extracted at 2 hphs or 6 hphs and alpha tubulin was used as a loading control. (H) Histogram quantifying pSmad1/5/8 expression at 6 hphs, based on Western blots, in control and dntcf3+ embryos, levels normalized to alpha tubulin. (I) Histogram quantifying Edn1 expression by qPCR, in control and dntcf3+ embryos, p<0.01. Exogenous Bmp or Edn1 proteins partially rescue ventral patterning in Wnt-deficient arches
Bmps act together with Edn1 to promote ventral-intermediate cell fates in the arches [16], [17], [21], [22], [58]. Therefore we examined the ability of Bmp and Edn1 to restore ventral-intermediate gene expression in Wnt signaling-deficient embryos. Beads coated in human recombinant BMP4/7 heterodimers effectively induce Bmp target genes in zebrafish pharyngeal arches [19]. Similarly, microinjection of a 25 ng/nl BMP4/7 solution extracellularly on one side of the head induced Bmp signaling, as measured by expression of the transgenic Bmp-response element reporter (Bre:Gfp; [19]) at 8 hours post injection (hpi) (Fig. 6A, B). Unilateral injections of BMP4/7 protein into dntcf3+ embryos at 4 hphs partially rescued cartilage defects on the injected side (Fig. 6C–J). Typically this restored Mc length and Ch, but not the Mc-Pq joint, and rescue was dose-dependent (Fig. 6C, D). These results suggest that Wnt signaling acts upstream of, or possibly in parallel to, Bmp signaling to promote ventral cartilage cell fates in the arches. EDN1 protein injections have previously been shown to rescue an Edn1 mutant phenotype and partially rescue a Bmp loss of function phenotype [16], [19]. EDN1 injections into dntcf3+ embryos also partially rescued Mc length, but notably were more proficient at rescuing Ch and joint development (Fig. 6C–D, L).
Fig. 6. BMP protein rescues craniofacial phenotypes in dntcf+ embryos. 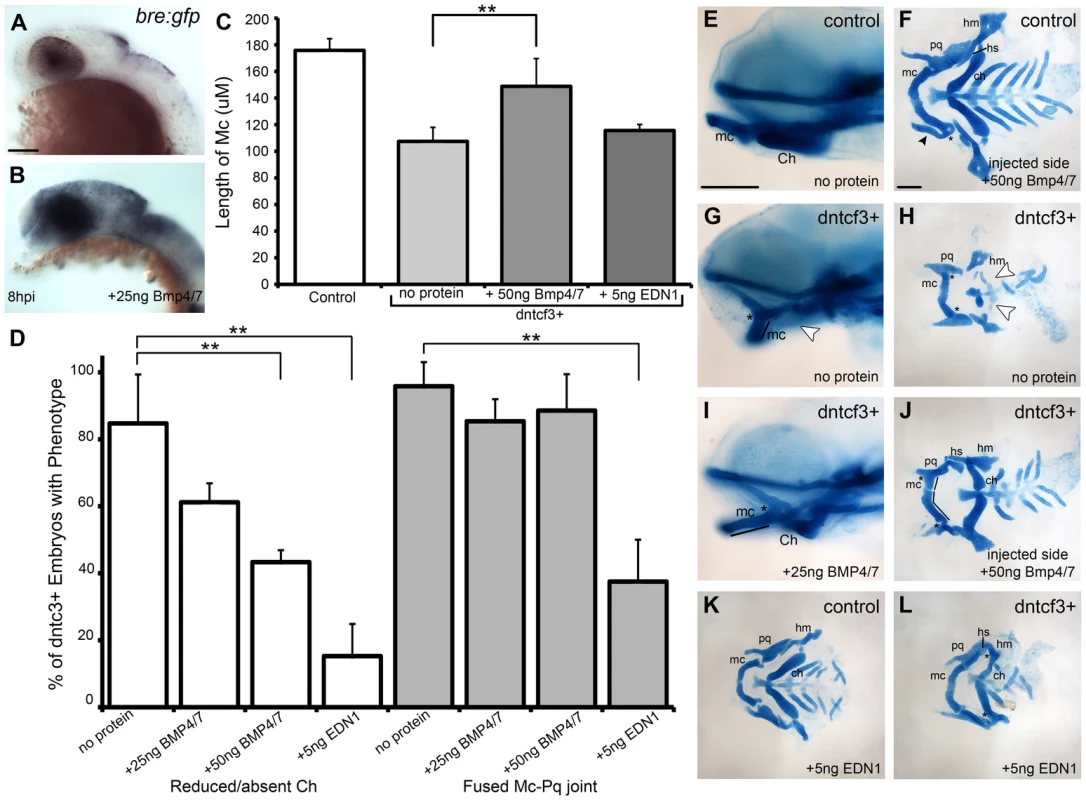
(A,B) Whole mount ISH for gfp in Tg(Bre:gfp) embryos, lateral views, anterior to the left. (A) Wildtype and (B) following injection with 25 ng/embryo BMP4/7 into the arch region at 20 hpf. (C) Histogram quantifying Mc length (µM) in controls, dntcf3+ embryos, and dntcf3+ embryos injected with either 50 ng of BMP4/7 or 5 ng EDN1. (D) Histograms quantifying numbers of dntcf3+ embryos, alone or injected with BMP4/7 or EDN1 proteins, with reductions in Ch (left) and joint fusions between Mc and Pq (right). Numbers of embryos with rescue of Ch or Mc-Pq were averaged from three independent experiments (minimum of 10 embryos per protein injection experiment) and samples compared using a paired student t-test. (E, G, I) Whole mounted, alcian-stained 4 dpf larvae; control (E), dntcf3+ (G), and dntcf3+ injected with 25 ug BMP4/7 (I), lateral views, anterior to the left. Arrowhead in G indicates Ch loss. Black lines in G and I indicate Mc length and asterisks indicate Mc-Pq joint fusion. (F, H, J–L) Dissected, flat-mounted alcian-stained cartilages at 4 dpf, ventral views, anterior to the left; control with unilateral injection of 50 ng BMP4/7 (F), dntcf3+ (H), dntcf3+ with 25 ng BMP4/7 (J), control with 5 ng EDN1 (K) and dntcf3+ with 5 ng EDN1 (L). Arrowhead in F indicates duplicate Mc. Black lines in J indicate Mc length. Arrowheads in H indicate Sy and Ch. Asterisks in H, J and L indicate Mc-Pq joint fusions. **P<0.001. Abbreviations: Ch, ceratohyal; mc, Meckel's; mc', duplicated Meckel's; Sy, symplectic. Scale bar: 100 µm. While both Bmp and Edn1 signaling induce many of the same genes that specify ventral-intermediate NC cell fates in the early arches, by later stages Bmps become much stronger inducers of hand2 (ventral) and msxe (ventral-intermediate) [19], [20]. Strikingly, neither BMP4/7 nor EDN1 protein injections at 4 hphs were sufficient to rescue hand2 expression in dntcf3+ embryos (Fig. 7A–D, Q). BMP4/7 but not EDN1 restored msxe expression (Fig. 7E–H), particularly in the mandibular arch near the injection site (Fig. 7G). In contrast, both BMP4/7 and EDN1 injections restored dlx3b and dlx5a expression in the intermediate domain (Fig. 7I–P, Q). These results suggest that hand2 expression absolutely requires Wnt signaling to respond to Bmps, while other signals can partially substitute for Wnts in induction of more intermediate-dorsal NC cell fates.
Fig. 7. Bmp and Edn1 restore ventral-intermediate gene expression, but not hand2, in dntcf3+ embryos. 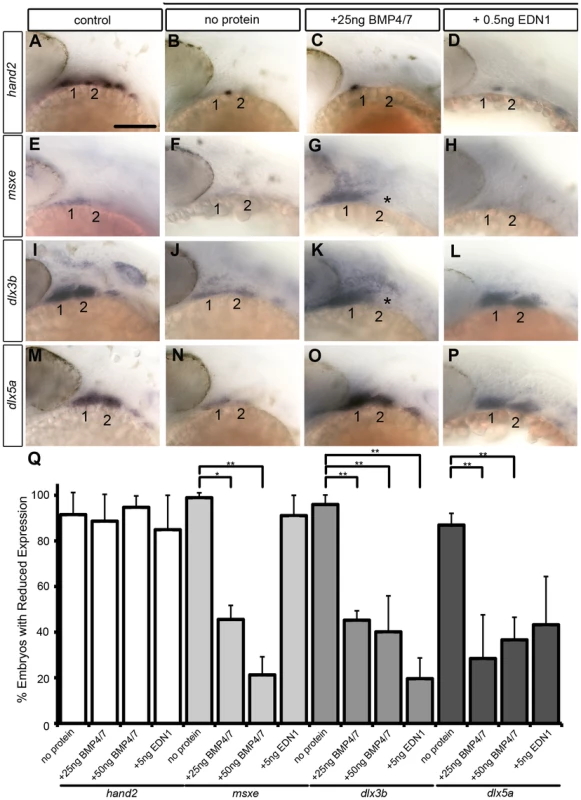
(A–P) Whole mount ISH for ventral-intermediate patterning genes in heat shocked controls (A, E, I, M), dntcf3+ embryos (B, F, J, N), and dntcf3+ embryos with either 25 ng BMP4/7 (C, G, K, O), or 5 ng EDN1 (D, H, L, P), lateral views, anterior to the left. Asterisks in G and K indicate cases in which rescue occurred in arch 1 but not arch 2. (Q) Histogram quantifying the number of embryos with reductions in arch expression for each gene. Quantification obtained by counting number of embryos with rescue of gene expression in three independent experiments (minimum of 10 embryos per protein injection experiment). Statistical method used is a paired student t-test. * P<0.05, ** P<0.001. Scale bar: 100 µm. Wnt signaling regulates expression of Bmp receptors
To further investigate how Wnts might regulate the ability of NC cells to respond to Bmp signaling, we examined whether or not dntcf3+ embryos show any changes in expression of Bmp receptors. Zebrafish have four type 1 receptors (Bmpr1aa, ab, ba, bb) and two type II receptors (Bmpr2a and b). Whole mount ISH for all six receptors revealed that only bmpr1ab, bmpr1ba, and bmpr1bb are expressed strongly in the arches at 24 hpf (Fig. S9C–F, I–J, M–R). bmpr1aa, bmpr2a, and bmpr2b were detected much more broadly throughout the embryo at this stage (Fig. S9A–B, G–H, K–L). Bmpr1ab expression extended throughout arches 1 and 2, while bmpr1ba and bmpr1bb expression was restricted to more intermediate and ventral domains (Fig. S9M–O). Transverse sections additionally showed that bmpr1ab and bmpr1ba expression is limited to arch NC cells and not surrounding epithelia (Fig. S9P–R). In dntcf3+ embryos bmpr1ab was severely reduced (57% n = 35) (Fig. 8A–B, G), while bmpr1ba was slightly reduced (bmpr1ab: 46% n = 57) and bmpr1bb expression was largely unaffected (bmpr1bb: 31% n = 29) (Fig. 8C–F, G).
Fig. 8. Bmp receptor expression in dntcf3+ embryos. 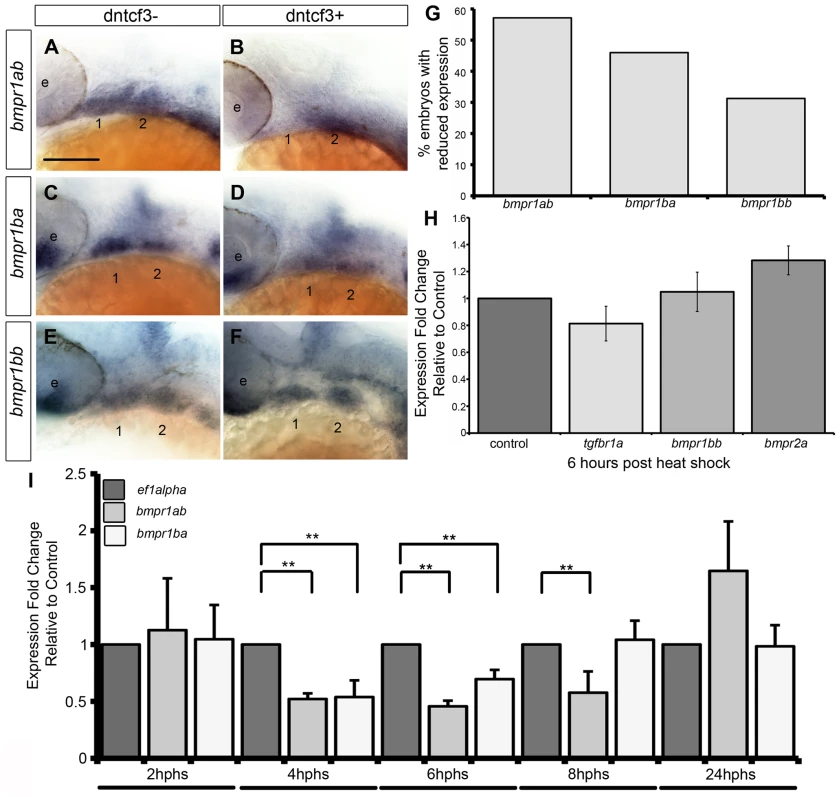
(A–F) Whole mount ISH for bmpr1ab (A, B), bmpr1ba (C, D), and bmpr1bb (E, F) expression, lateral views, anterior to the left. (G) Histogram quantifying the number of dntcf3+ embryos with reduced expression. (H,I) qPCR analysis of Bmp receptor expression levels in dntcf3+ embryos at different times post heat shock, normalized to nontransgenic, heat-shocked controls, with ef1alpha as an internal control. * P<0.05, ** P<0.001. Abbreviations: e, eye; nc, neural crest. Scale bar: 100 µm. Changes in Bmp receptor expression in dntcf3+ embryos were further quantified by qPCR analysis. At 6 hphs we compared the relative expression of arch specific Bmp receptors (bmpr1ab, bmpr1ba, bmpr1bb) with ubiquitously expressed bmpr2a and tgfbr1a, a TGF-B receptor expressed in the arches unrelated to Bmp signaling [59]. There was no detectable reduction in tgfbr1a or bmpr2a expression in dntcf3+ embryos (Fig. 8H). At 6 hphs, both bmpr1ab and bmpr1ba expression were reduced (Fig. 8I) but bmpr1bb expression showed no difference from controls (Fig. 8H). A time series analysis revealed no change in bmpr1ab and bmpr1ba expression at 2 hphs, despite reduced Wnt signaling (see Fig. 1), but levels dropped dramatically by 4 hphs. bmpr1ba but not bmpr1ab expression recovered substantially by 8 hphs. This suggests differential requirements for Wnt signaling in induction of Bmp receptors.
Dkk1b functions in the pharyngeal endoderm
dkk1+ embryos exhibit a unique clefting of the mandible not seen with dntcf3+. Although primarily known as a repressor of Wnt signaling, Dkk1 has also been reported to positively regulate the Wnt-PCP pathway [60]. To gain further insights into its tissue-specific functions, we examined dkk1b expression in pharyngeal arch primordia. Of the five known dkk genes in zebrafish, only dkk1b is expressed in the embryonic arches [61]. We found that between 28–48 hpf dkk1b expression localized to the pharyngeal endoderm, particularly the pouches between arches (Fig. S10A–C). Consistent with this, expression was lost in van gogh (vgo) mutants, which lack pouches [10] (Fig. S10D–E). dkk1b expression was also detected in the stomodeum (oral ectoderm) at 28 hpf (Fig. S10A) and later in the ectoderm of the mouth at 48 hpf (Fig. S10F).
Signals from the pharyngeal endoderm and oral ectoderm are necessary for craniofacial patterning and chondrogenesis [9], [10], [13]–[15]. To determine if there were gross defects in these epithelial layers in dntcf embryos, we examined nkx2.3, and found that its expression in the pharyngeal endoderm was disorganized in the first two pouches (Fig. S11B,F) and severely reduced in the more posterior pouches (Fig. S11B). In contrast, anterior pouches appeared unaffected in dkk1+ embryos, while the more posterior pouches were occasionally disorganized (Fig. S11D,H). Expression of pitx2ca in the oral ectoderm (Fig. S11I–R) was delayed in dkk1+ embryos until 26 hphs (Fig. S11O), by 30 hphs the mouth opening was abnormally elongated laterally and by 51 hphs showed a ventral midline fold (Fig.S11P–R). Thus pharyngeal pouch and mouth defects differ between dntcf and dkk1 embryos, which could account for some of the differences in their effects on growth and morphogenesis of the lower jaw.
Discussion
We show that Wnt signaling promotes proliferation and provides ventral-intermediate patterning cues to NC cells in the pharyngeal arches by participating in a regulatory network with Edn1 and Bmp (Fig. 9). By overexpressing Dkk1 or dnTcf3 to disrupt Wnt signaling, we show that Wnt promotes expression of ventral (hand2) and ventral-intermediate genes (dlx3b, dlx5a, msxe) and their corresponding skeletal derivatives, and acts upstream or in parallel to the ventralizing activities of Edn1 and Bmp. Unlike Edn1 and Bmp, however, our chimeric analyses suggest that direct responses to Wnt signaling occur in the pharyngeal endoderm, which also expresses dkk1. This endoderm must secondarily produce as yet unknown signals important for D-V patterning, which regulate the competence of NC cells to respond to Bmp signaling, in part by transcriptionally regulating Bmp receptors. Overexpression of dkk1 also causes a unique midline clefting of the mandible, which we suggest reflects a role in formation of the mouth.
Fig. 9. Model for the role of Wnt in dorsal-ventral arch patterning. 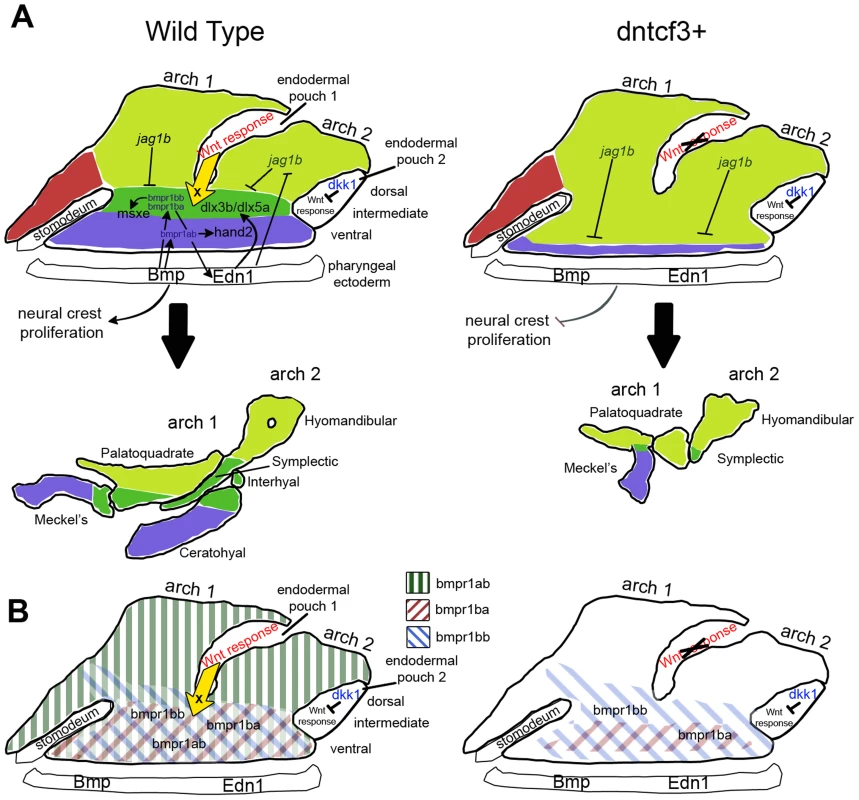
(A) Diagrams illustrating regulation of expression of dorsal-ventral (D-V) arch patterning genes in the skeletogenic neural crest at 24 hpf (colored regions) by Bmp and Edn1 signals from the pharyngeal ectoderm and Wnt responses in the pharyngeal endoderm. Each arch is subdivided into ventral (blue), intermediate (dark green), and dorsal (light green) domains, which correspond to eventual dorsal-intermediate-ventral cartilage identities. Arrows indicate inductive influences by each signal on target genes. The yellow arrow indicates an unknown ventralizing signal X from the endoderm induced in response to Wnt. (B) Diagrams illustrating regulation of Bmp receptor expression along the D-V axis at 24 hpf (colored diagonal bars) by Wnt, Bmp and Edn1. Wnt-mediated patterning and growth in craniofacial development
Direct Wnt responses in the ventral first and second arches resemble the pattern of TOP:Gal expression in mice, including distal (ventral) arch 1 [36], [37]. Mycn, a direct transcriptional Wnt target, is also expressed in both fish and mouse arch NC cells, where it is likely to inhibit Wnt-β-catenin signaling [62], and provide negative feedback. Murine Mycn is expressed in highly proliferative cells and mutants show hypoplasia of the mandibular arch [63], [64]. Similarly, we find reduced proliferation in the pharyngeal arches in Wnt-deficient zebrafish and smaller craniofacial cartilages (Fig. 3; Fig. S5). Thus, Wnt signaling may promote growth of the ventral arches through induction of mycn expression.
We show a critical requirement for Wnt signaling in arch growth and patterning that is distinct from its earlier roles in NC induction and migration. Earlier heat shocks of dntcf3 or dkk1 zebrafish (10–20 hpf) disrupt premigratory NC formation [41], [65] similar to Wnt1/Wnt3a mutant mice [43]. Unlike recent conditional loss - and gain-of-function studies of βcat in the pharyngeal ectoderm in mice [44], we find no changes in cell survival in the arches in dntcf3+ embryos.
D-V defects in gene expression in the arches caused by overexpressing dntcf3 or dkk1 at these later stages point to a problem with canonical Wnt signaling. Both reduce expression of canonical Wnt target genes up to 8 hphs (Fig. 1I; Fig. S1), and both lead to ventral cartilage and joint defects. However, dkk1 overexpression has more subtle effects (restricted primarily to Mc and the jaw joint) than dntcf3. Defects in dkk1+ embryos are also stronger when heat shocked at slightly earlier stages (15–22 hpf), than dntcf3+ (22–24 hpf). These differences may reflect distinct functions for the two transgenes, or a delay due to the time required for Dkk1 to competitively bind with the Lrp5/6 co-receptor, whereas Tcf3 directly binds βcat and Wnt target genes. Heat shocking dntcf3 or dkk1 at earlier stages eliminates cartilage, consistent with requirements for canonical Wnt signaling in NC induction. Both mycn and axin2 expression recover by 24 hphs in heat shocked embryos indicating a transient requirement for canonical Wnt signaling prior to skeletal cell differentiation.
Wnt and the D-V signaling network in pharyngeal arch development
Bmps and Edn1 secreted by the pharyngeal ectoderm both promote ventral-intermediate skeletal cell fates in the arches [19], [20] and our results implicate Wnt as an additional ventralizing factor. Overexpression of dntcf3 leads to reduced hand2, dlx3b, and dlx5a ventrally and expansion of dorsal jag1b expression (Fig. 4), and similar but less severe changes in gene expression result from dkk1 overexpression. Loss of the positive Wnt regulator, R-spondin, in mice also disrupts expression of Hand2, Dlx5, Dlx6, and Msxe [66], suggesting a conserved requirement for Wnt signaling in promoting ventral-intermediate cell fates.
How are these different ventralizing signals integrated during D-V arch patterning? Wnts can either activate or inhibit Bmp signaling in different developmental contexts [67]–[71]. pSmad1/5/8 expression is reduced in the pharyngeal arches of dntcf3+ embryos (Fig. 5), suggesting a novel role for Wnts upstream of Bmp signaling during arch development. Consistent with this model, microinjection of Bmp4/7 protein directly into the arch primordia at 4 hphs rescues ventral cartilages (Mc, Ch) in dntcf3+ embryos, but not joint fusions (Fig. 6C, F). Similar injections of BMP4/7 protein into edn1−/− mutant zebrafish rescues ventral cartilages but not joint fusions, while ectopic Edn1 can rescue joint defects caused by a loss of Bmp [19]. We show that injection of EDN1 protein rescues joint fusions in Wnt deficient embryos (Fig. 6D,L). Wnts also induce Edn1 expression in the pharyngeal ectoderm [66]. Taken together, these results suggest that Wnt signaling influences ventral cell fates in the arches through both Bmps and Edn1, and joint patterning specifically through Edn1.
Another clue to specificity in the D-V arch patterning system comes from the fact that msxe expression (a direct BMP target and marker of more intermediate identities) is induced by Bmp4/7 in edn1−/− mutants [20], and by Edn1 protein in the absence of Bmp signaling [19]. Surprisingly, however, msxe expression in dntcf3+ embryos is only rescued by BMP4/7, and not EDN1, while dlx3b and dlx5a expression is rescued in both. This suggests that Edn1 can only induce msxe expression in the arches in the presence of Wnt signaling. Thus Wnt controls the competence for arch cells to respond to Edn1 in addition to inducing expression of Edn1 itself. Mice mutant in the essential Wnt receptor co-factor, Lrp6, also lack expression of Msx1 and Msx2, in the arches [72], possibly as a result of defects in Bmp signaling.
Neither BMP4/7 nor EDN1 overexpression rescues hand2 expression in dntcf3+ embryos, revealing a critical requirement for Wnt in induction of hand2 [17], [23], [73]–[75]. Both Bmp and Edn1 induce hand2 expression and specify the ventral arch domain initially, but later Bmps maintain hand2 in the ventral domain while Edn1 promotes expression of more ventral-intermediate genes. We pinpoint a critical period for Wnt signaling in D-V patterning between 24–30 hpf, when hand2 expression is unresponsive to Edn1. Our results suggest that Bmp signaling requires Wnt signaling to induce hand2 expression in the arches. Consistent with this model, Wnt signaling directly regulates Hand2 transcription in chondrocytes [76]. Failure of hand2 induction is not simply due to loss of cells, since ventral NC cells are still present in the arches of dntcf3+ embryos (Fig. 4N–O; Fig. S7). BMP protein can also induce hand2 throughout the D-V extent of the arch [20]. Our results suggest that Wnt signaling plays a critical role in regulating the competence of cells to respond to BMPs and to express hand2.
We propose that Wnt signaling activates a signal (factor X) from the pharyngeal endoderm that primes the ability of NC cells to respond to Bmp signaling, in part through the transcriptional regulation of Bmp receptors (Fig. 9). Similarly in D-V patterning of the mouse limb Wnt signaling is thought to act upstream of Bmpr1a [71]. Three type I Bmp receptors, bmpr1ab, bmpr1ba, bmpr1bb, have arch-specific expression in zebrafish, similar to mice [77]. These are expressed in nested patterns within the arches: bmpr1ab throughout and bmpr1ba/bb only in intermediate-ventral domains. Thus Bmpr1 receptors may have distinct roles in different spatial domains (in addition to their cell-type specific roles [78], [79]), but this has been difficult to test due to early lethality in traditional Bmpr knockouts [80], [81]. We show that overexpression of dntcf3+ inhibits bmpr1ab and bmpr1ba, but not bmpr1bb and bmpr2a, expression in the arches. This reduction in Bmpr expression occurs later than most direct Wnt targets (Fig. 8I; 4–8 hphs) suggesting that it is indirect, consistent with our model that Wnt activates an unknown signal from the endoderm important in this process. bmpr1ba expression also recovers by 8 hphs, before bmpr1ab expression and within the period during which Wnt signaling is significantly reduced (Fig. 1I) indicating that bmpr1ab is particularly sensitive. This could help explain the inability of Bmp protein to rescue hand2 expression in dntcf3+ embryos if bmpr1ab plays a specific role in hand2 induction. In contrast, intermediate-ventral genes such as msxe and dlx3b, may be rescued by Bmp protein because other Bmp receptors are sufficient for their induction. Such distinct transcriptional roles for Bmp receptors could help fine-tune D-V domains within an arch despite the relatively broad expression of Bmp ligands.
Distinct roles for Wnt signaling in pharyngeal endodermal development
Both dntcf3+ and dkk1+ embryos appear to function in the pharyngeal endoderm, which is an important signaling center in craniofacial development [7], [9]. Our chimeric analyses demonstrate a cell autonomous requirement for dntcf3 in endoderm (Fig. S2) and dkk1 expression is restricted to this epithelium. Interestingly, we do not detect dkk1 expression in the first pharyngeal pouch, which lies between arches 1 and 2 (Fig. S10). This could help explain why Wnt signaling only appears to be required for D-V patterning in these arches; the more posterior ceratobranchials (arches 3–7) are largely unaffected in heterozygous dkk1+ and dntcf3+ embryos (Fig. 2B,F). These results suggest a previously unrecognized role for Wnts and Wnt antagonists in endoderm and the existence of an as yet unknown factor X produced by endoderm that is important in D-V patterning of the NC (see Fig. 9).
The distinct and highly penetrant clefting of the mandible observed in dkk1+ embryos is never observed in dntcf3+ embryos. Disruption of the canonical Wnt pathway can cause clefting of the palate in mice [36], [46], [67], but such midline clefts in the lower jaw are rare. Midline facial defects, particularly of the frontonasal process, have been reported in Wnt signaling mutants in mice [36], [66], [67]. Mice mutant for Dlx5/6 have cleft mandibles and Wnt9b mutants have cleft lip [82], [83]. Humans with Richieri-Costa-Perieira syndrome also exhibit clefting of the lower jaw similar to what we describe in dkk1+ embryos [68], [84]. Dkk1 not only inhibits canonical Wnt signaling [52] but can also activate the non-canonical Wnt-PCP pathway during zebrafish gastrulation [60]. Non-canonical Wnt signaling has also been implicated in craniofacial midline development as Wnt5a mutant mice have clefting of the secondary palate [66]. Thus, overexpression of Dkk1 may lead to both a canonical Wnt/βcat loss-of-function and a non-canonical Wnt-PCP gain-of-function to cause lower jaw clefting.
Overexpression of dkk1 also leads to elongation and ventral clefting of the mouth, which is not observed in dntcf3+ embryos. Both loss - and gain-of-function mutations in mammalian Dkk1 result in midline clefts in the frontonasal and maxillary prominences [36]. NC cells fated to form the lower jaw lie adjacent to the oral ectoderm (stomodeum), which secretes important skeletogenic signals such as Shh [14], [15] and Bmps [19]. dkk1b transcripts are normally restricted to the anterior ectoderm of the mouth opening (Fig. S10F) where both fgf8 (distal) and shh (medial) are expressed. Thus, misexpressing dkk1b throughout the oral ectoderm may disrupt one of these other signals. Future experiments are needed to determine the roles of Wnt signaling in mouth development and the causes of mandibular clefts.
Materials and Methods
Ethics statement
All zebrafish work was performed using protocols approved by the University of California, Irvine Institutional Animal Care and Use Committee (Protocol # 2000-2149-4).
Animals
Adult Tg(hsp701:dkk1-GFP) (dkk1) [50] and Tg(hsp70I:tcf3-GFP) (dntcf3) [41] fish were genotyped by performing PCR analysis for gfp (sense, GTGATGCAACATACGGAAAAC; antisense, GCCATGTGTAATCCCAGCAGC) using genomic DNA extracted from fin clips as template. Zygosity of fish was determined by outcrossing genotyped transgenic adults with wild type adults and scoring the Mendelian ratio of GFP positive to GFP negative after heat shocking as described below. To observe NC, we outcrossed adult homozygous Tg(hsp70I:tcf3-GFP) fish with sox10;LynTdtomato fish to make a stable double transgenic line. Adult heterozygous Tg(7xTCF-Xla.Siam:GFP)ia4 (7xTCF;GFP) transgenics [49] were in-crossed and sorted by GFP expression for downstream phenotypic analysis. Tg(BRE:gfp) (bre:gfp) [19] heterozygous adults were in-crossed, selected for strong GFP expression, and used for protein injection (see below).
Heat shock conditions
Heat shocks were performed in a thermal cycler at 39°C for either 12 min (dntcf3) or 30 min (dkk1). Fluorescence was checked 1-hour post heat shock and GFP-negative embryos were separated and used as controls. Embryos were then raised in a 28.8°C incubator until they were fixed for in situ analysis, RNA extraction, or protein extraction at various time points after heat shock or 4 dpf for skeletal staining.
Phenotypic analysis
Alcian blue staining of cartilage was performed on 96 hpf embryos as previously described [19]. In situ hybridization was performed on embryos fixed in 4% PFA for one hour at room temperature. Probes used include gfp [19], dlx2a, dlx3b, dlx6a [85], mycn [86], hand2 [87], jag1b [28], msxe, dkk1b, nkx2.3 [17], ednrA1, ednrA2 (Nair et al, 2007), and pitx2ca [88]. The axin2a probe was synthesized directly from a PCR product with a T7 promoter site added at the 3′ end (sense, AGAAGATGACCCACGTCCAC; antisense, TAATACGACTCACTATAGGGGACTGTGACCTTGTGCTGAGAC). The Bmp receptor probes were synthesized directly from PCR products with a T7 promoter site added at the 3′ end: Bmpr1aa (sense, TAGCCAACCCCAATGCTTAC; antisense, TAATACGACTCACTATAGGGCCCATTTGTCTCGCAGGTAT), Bmpr1ab (sense, GATGCCACAAACAACACCTG; antisense, TAATACGACTCACTATAGGGACTTTCACCGCCACATTTTC), Bmpr1ba (sense, AGAATCTCTGCGGGATCTCA; antisense, TAATACGACTCACTATAGGGGCTCCGTTTCTCTTGACCAG), Bmpr1bb (sense, TCACGGATTATCACGAGAGCG; antisense, TAATACGACTCACTATAGGGATTATGAGCCCAGCACTCGC), Bmpr2a (sense, CCACAATGACACCTCAGTGG; antisense, TAATACGACTCACTATAGGGTTAGGGACGTTCTGCTGCTT), Bmpr2b (sense, TATTGTCGCGCTGTTCTTTG; antisense, TAATACGACTCACTATAGGGGCAGATAGGCCAGTCCTCTG). A pcna probe was generated by a T7 promoter from a clone of the ORF (Open Biosystems, clone ID:7000501) in pExpress1 after linearization with EcoRI. Immunolabeling was performed using 1∶500 rabbit anti-phosphoHistodone3 (Upstate Biotechnology), 1∶1000 rabbit anti-pSmad1/5/8 (Millipore), or 1∶500 chick anti-gfp (AbCam) antibodies diluted in 1% DMSO, 0.5% Triton ×100 in PBS and detected using 1∶1000 dylight donkey anti-rabbit 564 (Jackson ImmunoResearch Laboratories) or 1∶1000 donkey anti-chick dylight 488 (Jackson ImmunoResearch Laboratories).
Protein injection
Tg(hsp70I:tcf3-GFP) embryos were heat shocked as described, anesthetized, and then embedded in 1% low melt agarose in embryo medium. Human EDN1 (Sigma-Aldrich) was diluted to 10 µg/µl and human recombinant BMP4/7 (R&D Systems) was diluted to either 50 ng/nl or 10 ng/nl. A 0.5 nl droplet of protein solution was pressure injected into the arch region 4 hours post heat shock (∼26 hpf) using a glass needle. Embryos were then carefully removed from the agarose using forceps and fixed for phenotypic analysis 4 hours later (∼30 hpf) or 4 dpf for alcian stain using 4% PFA at room temperature for 1 hour.
6-bromoindirubin-3′-oxime (BIO) treatment
Dechorionated control, dkk1+, and dntcf3+ embryos were placed in dishes containing 50 µm or 100 µm of BIO (30 mm stock in DMSO) (Sigma) diluted in embryo medium (EM) at 2 hours post heat shock [55]. The dishes were placed in a 28.8° incubator for 6 hours and then washed several times with EM. Embryos were fixed for in situ hybridization, harvested for RNA, or allowed to develop to 4 dpf and then fixed for alcian blue staining.
Western blot
Protein was extracted from dechorionated embryos by adding 2 µl/embryo of sample buffer (60 mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.01% bromophenol blue) and homogenizing with a pestle. The sample was boiled in a 95°C heat block for 10 min and then immediately placed on ice. Before loading into a 10% SDS gel the sample was spun down at 1300 rpm for 5 min. The membrane was blocked in 3% BSA and 3% Donkey Serum in TBST (1XTBS −20 mM Tris-HCl, 150 mM NaCl, 0.1% Tween) for one hour at room temperature and incubated overnight at 4°C with 1∶1000 rabbit anti-pSmad1/5/8 (Cell Signaling). The next day the membrane was washed several times in TBST and then incubated with 1∶5000 donkey anti-rabbit HRP (GeneTex) for 1 hour at room temperature.
Quantitative real time PCR analysis
Total RNA was extracted from control, dkk1+, and dntcf3+ embryos at various time points past heat shock using Trizol reagent (Ambion). cDNA synthesis was performed with 1 µg of RNA using Protoscript M-MuLV First Strand cDNA synthesis kit (New England Biosystems). qPCR was performed using Light Cycler 480 SYBR Green Master (Roche Applied Science) in a Light Cycler 480 Real Time PCR machine. Q-PCR primer sets used were for mycn (Sense-AACAAGAGGGAGAATGCCA; Antisense-TAGAAGTCATCCTCGTCCG), axin2 (Sense - CAATGGACGAAAGGAAAGATCC; Antisense-AGAAGTACGTGACTACCGTC), lef1 (Sense-CCAGACATTCCCAATTTCTATCC; Antisense-GTGATGTGAGAACCAACCC), ef1alpha (Sense - CAAGGGATGGAAGATTGAGC; Antisense - AACCATACCAGGCTTGAGGA), bmpr1ab (Sense - CATGAGGGAAGTGGTATGTG; Antisense - ATGACTCGTAAGCACTCGT), and bmpr1ba (Sense - GACAATATACTGGGATTTATAGCGG; Antisense – ATGATAGTCTGTGATCAGGTAGAG), bmpr1bb (Sense-AACATACTGGGCTTCATCG; Antisense - CTCGTGATAATCCGTGATCAG), bmpr2a (Sense-TTTCCCAGGTGAAACAGTG; Antisense-TGCATGTCCTCTATGGTAGG), tgfbr1a (Sense-GCATGATCAAGCTGTCTCTG; Antisense-CAGGCTTACCCTGAGTACC), and edn1 (Sense-TATGGGTGAACACACCAGAGCGAA; Antisense-CGCTTGGCAGAATGAAGAGCATGT).
Chimeric analyses
Tg(hsp70I:tcf3-GFP) donor embryos were injected at the 1-cell stage with 15 pg Taram-A (Tar*) mRNA, which drives cells to an endodermal fate, combined with a 1∶1 mixture of 6% biotin-dextran and 6% rhodamine-dextran. Cells were transplanted into WT hosts at the 30–50% epiboly stage (5 hpf) to generate chimeras, as described previously [9]. Host embryos with red fluorescent cells in the pharyngeal endoderm were sorted at 22 hpf, heat-shocked and raised to either 30 hpf for ISH or 5 dpf for skeletal staining. Grafts were labeled at either time-point using a peroxidase-coupled streptavidin and diaminobenzidine.
Supporting Information
Zdroje
1. SchillingTF, KnightRD (2001) Origins of anteroposterior patterning and Hox gene regulation during chordate evolution. Philos Trans R Soc Lond B Biol Sci 356 : 1599–1613.
2. HuntP, GulisanoM, CookM, ShamMH, FaiellaA, et al. (1991) A distinct Hox code for the branchial region of the vertebrate head. Nature 353 : 861–864.
3. OlssonL, EricssonR, CernyR (2005) Vertebrate head development: segmentation, novelties, and homology. Theory Biosci 124 : 145–163.
4. TuckerAS, YamadaG, GrigoriouM, PachnisV, SharpePT (1999) Fgf-8 determines rostral-caudal polarity in the first branchial arch. Development 126 : 51–61.
5. TrumppA, DepewMJ, RubensteinJL, BishopJM, MartinGR (1999) Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev 13 : 3136–3148.
6. CreuzetS, SchulerB, CoulyG, Le DouarinNM (2004) Reciprocal relationships between Fgf8 and neural crest cells in facial and forebrain development. Proc Natl Acad Sci U S A 101 : 4843–4847.
7. CoulyG, CreuzetS, BennaceurS, VincentC, Le DouarinNM (2002) Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development 129 : 1061–1073.
8. AlexanderJ, RothenbergM, HenryGL, StainierDY (1999) casanova plays an early and essential role in endoderm formation in zebrafish. Dev Biol 215 : 343–357.
9. DavidNB, Saint-EtienneL, TsangM, SchillingTF, RosaFM (2002) Requirement for endoderm and FGF3 in ventral head skeleton formation. Development 129 : 4457–4468.
10. PiotrowskiT, Nusslein-VolhardC (2000) The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio). Dev Biol 225 : 339–356.
11. BritoJM, TeilletMA, Le DouarinNM (2006) An early role for sonic hedgehog from foregut endoderm in jaw development: ensuring neural crest cell survival. Proc Natl Acad Sci U S A 103 : 11607–11612.
12. SwartzME, NguyenV, McCarthyNQ, EberhartJK (2012) Hh signaling regulates patterning and morphogenesis of the pharyngeal arch-derived skeleton. Dev Biol 369 : 65–75.
13. BalczerskiB, MatsutaniM, CastilloP, OsborneN, StainierDY, et al. (2012) Analysis of sphingosine-1-phosphate signaling mutants reveals endodermal requirements for the growth but not dorsoventral patterning of jaw skeletal precursors. Dev Biol 362 : 230–241.
14. WadaN, JavidanY, NelsonS, CarneyTJ, KelshRN, et al. (2005) Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development 132 : 3977–3988.
15. Eberhart JK, Swartz ME, Crump JG, Kimmel CB (2006) Early Hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development. England. pp. 1069–1077.
16. MillerCT, SchillingTF, LeeK, ParkerJ, KimmelCB (2000) sucker encodes a zebrafish Endothelin-1 required for ventral pharyngeal arch development. Development 127 : 3815–3828.
17. MillerCT, YelonD, StainierDY, KimmelCB (2003) Two endothelin 1 effectors, hand2 and bapx1, pattern ventral pharyngeal cartilage and the jaw joint. Development 130 : 1353–1365.
18. NairS, LiW, CornellR, SchillingTF (2007) Requirements for Endothelin type-A receptors and Endothelin-1 signaling in the facial ectoderm for the patterning of skeletogenic neural crest cells in zebrafish. Development 134 : 335–345.
19. AlexanderC, ZunigaE, BlitzIL, WadaN, Le PabicP, et al. (2011) Combinatorial roles for BMPs and Endothelin 1 in patterning the dorsal-ventral axis of the craniofacial skeleton. Development 138 : 5135–5146.
20. ZunigaE, RippenM, AlexanderC, SchillingTF, CrumpJG (2011) Gremlin 2 regulates distinct roles of BMP and Endothelin 1 signaling in dorsoventral patterning of the facial skeleton. Development 138 : 5147–5156.
21. MedeirosDM, CrumpJG (2012) New perspectives on pharyngeal dorsoventral patterning in development and evolution of the vertebrate jaw. Dev Biol 371 : 121–135.
22. ClouthierDE, GarciaE, SchillingTF (2010) Regulation of facial morphogenesis by endothelin signaling: Insights from mice and fish. Am J Med Genet A 152A: 2962–2973.
23. LiuW, SunX, BrautA, MishinaY, BehringerRR, et al. (2005) Distinct functions for Bmp signaling in lip and palate fusion in mice. Development 132 : 1453–1461.
24. RiederMJ, GreenGE, ParkSS, StamperBD, GordonCT, et al. (2012) A human homeotic transformation resulting from mutations in PLCB4 and GNAI3 causes auriculocondylar syndrome. Am J Hum Genet 90 : 907–914.
25. ClouthierDE, HosodaK, RichardsonJA, WilliamsSC, YanagisawaH, et al. (1998) Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 125 : 813–824.
26. ClouthierDE, WilliamsSC, YanagisawaH, WieduwiltM, RichardsonJA, et al. (2000) Signaling pathways crucial for craniofacial development revealed by endothelin-A receptor-deficient mice. Dev Biol 217 : 10–24.
27. YanagisawaH, YanagisawaM, KapurRP, RichardsonJA, WilliamsSC, et al. (1998) Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development 125 : 825–836.
28. ZunigaE, StellabotteF, CrumpJG (2010) Jagged-Notch signaling ensures dorsal skeletal identity in the vertebrate face. Development 137 : 1843–1852.
29. SummerhurstK, StarkM, SharpeJ, DavidsonD, MurphyP (2008) 3D representation of Wnt and Frizzled gene expression patterns in the mouse embryo at embryonic day 11.5 (Ts19). Gene Expr Patterns 8 : 331–348.
30. JezewskiPA, FangPK, Payne-FerreiraTL, YelickPC (2008) Zebrafish Wnt9b synteny and expression during first and second arch, heart, and pectoral fin bud morphogenesis. Zebrafish 5 : 169–177.
31. Geetha-LoganathanP, NimmagaddaS, AntoniL, FuK, WhitingCJ, et al. (2009) Expression of WNT signalling pathway genes during chicken craniofacial development. Dev Dyn 238 : 1150–1165.
32. CurtinE, HickeyG, KamelG, DavidsonAJ, LiaoEC (2011) Zebrafish wnt9a is expressed in pharyngeal ectoderm and is required for palate and lower jaw development. Mech Dev 128 : 104–115.
33. NikaidoM, LawEW, KelshRN (2013) A systematic survey of expression and function of zebrafish frizzled genes. PLoS One 8: e54833.
34. SissonBE, TopczewskiJ (2009) Expression of five frizzleds during zebrafish craniofacial development. Gene Expr Patterns 9 : 520–527.
35. VendrellV, SummerhurstK, SharpeJ, DavidsonD, MurphyP (2009) Gene expression analysis of canonical Wnt pathway transcriptional regulators during early morphogenesis of the facial region in the mouse embryo. Gene Expr Patterns 9 : 296–305.
36. BrugmannSA, GoodnoughLH, GregorieffA, LeuchtP, ten BergeD, et al. (2007) Wnt signaling mediates regional specification in the vertebrate face. Development 134 : 3283–3295.
37. ManiP, JarrellA, MyersJ, AtitR (2010) Visualizing canonical Wnt signaling during mouse craniofacial development. Dev Dyn 239 : 354–363.
38. LaBonneC, Bronner-FraserM (1999) Molecular mechanisms of neural crest formation. Annu Rev Cell Dev Biol 15 : 81–112.
39. Garcia-CastroMI, MarcelleC, Bronner-FraserM (2002) Ectodermal Wnt function as a neural crest inducer. Science 297 : 848–851.
40. AybarMJ, MayorR (2002) Early induction of neural crest cells: lessons learned from frog, fish and chick. Curr Opin Genet Dev 12 : 452–458.
41. LewisJL, BonnerJ, ModrellM, RaglandJW, MoonRT, et al. (2004) Reiterated Wnt signaling during zebrafish neural crest development. Development 131 : 1299–1308.
42. DeardorffMA, TanC, Saint-JeannetJP, KleinPS (2001) A role for frizzled 3 in neural crest development. Development 128 : 3655–3663.
43. IkeyaM, LeeSM, JohnsonJE, McMahonAP, TakadaS (1997) Wnt signalling required for expansion of neural crest and CNS progenitors. Nature 389 : 966–970.
44. ReidBS, YangH, MelvinVS, TaketoMM, WilliamsT (2011) Ectodermal Wnt/beta-catenin signaling shapes the mouse face. Dev Biol 349 : 261–269.
45. BraultV, MooreR, KutschS, IshibashiM, RowitchDH, et al. (2001) Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128 : 1253–1264.
46. ChiquetBT, BlantonSH, BurtA, MaD, StalS, et al. (2008) Variation in WNT genes is associated with non-syndromic cleft lip with or without cleft palate. Hum Mol Genet 17 : 2212–2218.
47. ShuW, GuttentagS, WangZ, AndlT, BallardP, et al. (2005) Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol 283 : 226–239.
48. ten BergeD, BrugmannSA, HelmsJA, NusseR (2008) Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development 135 : 3247–3257.
49. MoroE, Ozhan-KizilG, MongeraA, BeisD, WierzbickiC, et al. (2012) In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Dev Biol 366 : 327–340.
50. Stoick-CooperCL, WeidingerG, RiehleKJ, HubbertC, MajorMB, et al. (2007) Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development 134 : 479–489.
51. GlinkaA, WuW, DeliusH, MonaghanAP, BlumenstockC, et al. (1998) Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391 : 357–362.
52. SemenovMV, TamaiK, BrottBK, KuhlM, SokolS, et al. (2001) Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol 11 : 951–961.
53. FilaliM, ChengN, AbbottD, LeontievV, EngelhardtJF (2002) Wnt-3A/beta-catenin signaling induces transcription from the LEF-1 promoter. J Biol Chem 277 : 33398–33410.
54. VadlamudiU, EspinozaHM, GangaM, MartinDM, LiuX, et al. (2005) PITX2, beta-catenin and LEF-1 interact to synergistically regulate the LEF-1 promoter. J Cell Sci 118 : 1129–1137.
55. MeijerL, SkaltsounisAL, MagiatisP, PolychronopoulosP, KnockaertM, et al. (2003) GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol 10 : 1255–1266.
56. MagaG, HubscherU (2003) Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci 116 : 3051–3060.
57. HendzelMJ, WeiY, ManciniMA, Van HooserA, RanalliT, et al. (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106 : 348–360.
58. ThomasT, KuriharaH, YamagishiH, KuriharaY, YazakiY, et al. (1998) A signaling cascade involving endothelin-1, dHAND and msx1 regulates development of neural-crest-derived branchial arch mesenchyme. Development 125 : 3005–3014.
59. ParkSO, LeeYJ, SekiT, HongKH, FliessN, et al. (2008) ALK5 - and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood 111 : 633–642.
60. CaneparoL, HuangYL, StaudtN, TadaM, AhrendtR, et al. (2007) Dickkopf-1 regulates gastrulation movements by coordinated modulation of Wnt/beta catenin and Wnt/PCP activities, through interaction with the Dally-like homolog Knypek. Genes Dev 21 : 465–480.
61. UntergasserG, MartowiczA, HermannM, TochterleS, MeyerD (2011) Distinct expression patterns of dickkopf genes during late embryonic development of Danio rerio. Gene Expr Patterns 11 : 491–500.
62. JhoEH, ZhangT, DomonC, JooCK, FreundJN, et al. (2002) Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22 : 1172–1183.
63. StantonBR, PerkinsAS, TessarolloL, SassoonDA, ParadaLF (1992) Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev 6 : 2235–2247.
64. HirningU, SchmidP, SchulzWA, RettenbergerG, HameisterH (1991) A comparative analysis of N-myc and c-myc expression and cellular proliferation in mouse organogenesis. Mech Dev 33 : 119–125.
65. DorskyRI, MoonRT, RaibleDW (1998) Control of neural crest cell fate by the Wnt signalling pathway. Nature 396 : 370–373.
66. JinYR, TurcotteTJ, CrockerAL, HanXH, YoonJK (2011) The canonical Wnt signaling activator, R-spondin2, regulates craniofacial patterning and morphogenesis within the branchial arch through ectodermal-mesenchymal interaction. Dev Biol 352 : 1–13.
67. HeF, XiongW, YuX, Espinoza-LewisR, LiuC, et al. (2008) Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development 135 : 3871–3879.
68. LancasterMA, GopalDJ, KimJ, SaleemSN, SilhavyJL, et al. (2011) Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nat Med 17 : 726–731.
69. TzahorE, KempfH, MootoosamyRC, PoonAC, AbzhanovA, et al. (2003) Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev 17 : 3087–3099.
70. NakashimaA, KatagiriT, TamuraM (2005) Cross-talk between Wnt and bone morphogenetic protein 2 (BMP-2) signaling in differentiation pathway of C2C12 myoblasts. J Biol Chem 280 : 37660–37668.
71. SoshnikovaN, ZechnerD, HuelskenJ, MishinaY, BehringerRR, et al. (2003) Genetic interaction between Wnt/beta-catenin and BMP receptor signaling during formation of the AER and the dorsal-ventral axis in the limb. Genes Dev 17 : 1963–1968.
72. SongL, LiY, WangK, WangYZ, MolotkovA, et al. (2009) Lrp6-mediated canonical Wnt signaling is required for lip formation and fusion. Development 136 : 3161–3171.
73. ChariteJ, McFaddenDG, MerloG, LeviG, ClouthierDE, et al. (2001) Role of Dlx6 in regulation of an endothelin-1-dependent, dHAND branchial arch enhancer. Genes Dev 15 : 3039–3049.
74. HowardMJ, StankeM, SchneiderC, WuX, RohrerH (2000) The transcription factor dHAND is a downstream effector of BMPs in sympathetic neuron specification. Development 127 : 4073–4081.
75. XiongW, HeF, MorikawaY, YuX, ZhangZ, et al. (2009) Hand2 is required in the epithelium for palatogenesis in mice. Dev Biol 330 : 131–141.
76. AbeM, MichikamiI, FukushiT, AbeA, MaedaY, et al. (2010) Hand2 regulates chondrogenesis in vitro and in vivo. Bone 46 : 1359–1368.
77. DaneshSM, VillasenorA, ChongD, SoukupC, CleaverO (2009) BMP and BMP receptor expression during murine organogenesis. Gene Expr Patterns 9 : 255–265.
78. ChenD, JiX, HarrisMA, FengJQ, KarsentyG, et al. (1998) Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol 142 : 295–305.
79. KapsC, HoffmannA, ZilbermanY, PelledG, HauplT, et al. (2004) Distinct roles of BMP receptors Type IA and IB in osteo-/chondrogenic differentiation in mesenchymal progenitors (C3H10T1/2). Biofactors 20 : 71–84.
80. MishinaY, SuzukiA, UenoN, BehringerRR (1995) Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev 9 : 3027–3037.
81. GuZ, ReynoldsEM, SongJ, LeiH, FeijenA, et al. (1999) The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development 126 : 2551–2561.
82. JuriloffDM, HarrisMJ (2008) Mouse genetic models of cleft lip with or without cleft palate. Birth Defects Res A Clin Mol Teratol 82 : 63–77.
83. DepewMJ, SimpsonCA, MorassoM, RubensteinJL (2005) Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J Anat 207 : 501–561.
84. FavaroFP, Zechi-CeideRM, AlvarezCW, MaximinoLP, AntunesLF, et al. (2011) Richieri-Costa-Pereira syndrome: a unique acrofacial dysostosis type. An overview of the Brazilian cases. Am J Med Genet A 155A: 322–331.
85. AkimenkoMA, EkkerM, WegnerJ, LinW, WesterfieldM (1994) Combinatorial expression of three zebrafish genes related to distal-less: part of a homeobox gene code for the head. J Neurosci 14 : 3475–3486.
86. Loeb-HennardC, KremmerE, Bally-CuifL (2005) Prominent transcription of zebrafish N-myc (nmyc1) in tectal and retinal growth zones during embryonic and early larval development. Gene Expr Patterns 5 : 341–347.
87. YelonD, TichoB, HalpernME, RuvinskyI, HoRK, et al. (2000) The bHLH transcription factor hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development 127 : 2573–2582.
88. EssnerJJ, BranfordWW, ZhangJ, YostHJ (2000) Mesendoderm and left-right brain, heart and gut development are differentially regulated by pitx2 isoforms. Development 127 : 1081–1093.
Štítky
Genetika Reprodukčná medicína
Článek Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome EvolutionČlánek Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder PopulationČlánek Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among PancrustaceansČlánek Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery DiseaseČlánek An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation inČlánek Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 7- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Cuba: Exploring the History of Admixture and the Genetic Basis of Pigmentation Using Autosomal and Uniparental Markers
- Clonal Architecture of Secondary Acute Myeloid Leukemia Defined by Single-Cell Sequencing
- Mechanisms of Functional Variants That Impair Regulated Bicarbonate Permeation and Increase Risk for Pancreatitis but Not for Cystic Fibrosis
- Nucleosomes Shape DNA Polymorphism and Divergence
- Functional Diversification of Hsp40: Distinct J-Protein Functional Requirements for Two Prions Allow for Chaperone-Dependent Prion Selection
- Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome Evolution
- Activation of the Immune System by Combinations of Common Alleles
- Age-Associated Sperm DNA Methylation Alterations: Possible Implications in Offspring Disease Susceptibility
- Muscle-Specific SIRT1 Gain-of-Function Increases Slow-Twitch Fibers and Ameliorates Pathophysiology in a Mouse Model of Duchenne Muscular Dystrophy
- MDRL lncRNA Regulates the Processing of miR-484 Primary Transcript by Targeting miR-361
- Hypersensitivity of Primordial Germ Cells to Compromised Replication-Associated DNA Repair Involves ATM-p53-p21 Signaling
- Intrapopulation Genome Size Variation in Reflects Life History Variation and Plasticity
- SlmA Antagonism of FtsZ Assembly Employs a Two-pronged Mechanism like MinCD
- Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder Population
- Determinative Developmental Cell Lineages Are Robust to Cell Deaths
- DELLA Protein Degradation Is Controlled by a Type-One Protein Phosphatase, TOPP4
- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among Pancrustaceans
- UVB Induces a Genome-Wide Acting Negative Regulatory Mechanism That Operates at the Level of Transcription Initiation in Human Cells
- The Nesprin Family Member ANC-1 Regulates Synapse Formation and Axon Termination by Functioning in a Pathway with RPM-1 and β-Catenin
- Combinatorial Interactions Are Required for the Efficient Recruitment of Pho Repressive Complex (PhoRC) to Polycomb Response Elements
- Recombination in the Human Pseudoautosomal Region PAR1
- Microsatellite Interruptions Stabilize Primate Genomes and Exist as Population-Specific Single Nucleotide Polymorphisms within Individual Human Genomes
- An Intronic microRNA Links Rb/E2F and EGFR Signaling
- An Essential Nonredundant Role for Mycobacterial DnaK in Native Protein Folding
- Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery Disease
- The Genomic Landscape of the Ewing Sarcoma Family of Tumors Reveals Recurrent Mutation
- Evolution and Genetic Architecture of Chromatin Accessibility and Function in Yeast
- An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation in
- Stage-Dependent and Locus-Specific Role of Histone Demethylase Jumonji D3 (JMJD3) in the Embryonic Stages of Lung Development
- Genome Wide Association Identifies Common Variants at the Locus Influencing Plasma Cortisol and Corticosteroid Binding Globulin
- Regulation of Feto-Maternal Barrier by Matriptase- and PAR-2-Mediated Signaling Is Required for Placental Morphogenesis and Mouse Embryonic Survival
- Apomictic and Sexual Germline Development Differ with Respect to Cell Cycle, Transcriptional, Hormonal and Epigenetic Regulation
- Functional EF-Hands in Neuronal Calcium Sensor GCAP2 Determine Its Phosphorylation State and Subcellular Distribution , and Are Essential for Photoreceptor Cell Integrity
- Comparison of Methods to Account for Relatedness in Genome-Wide Association Studies with Family-Based Data
- Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
- Cis and Trans Effects of Human Genomic Variants on Gene Expression
- 8.2% of the Human Genome Is Constrained: Variation in Rates of Turnover across Functional Element Classes in the Human Lineage
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- A Loss of Function Screen of Identified Genome-Wide Association Study Loci Reveals New Genes Controlling Hematopoiesis
- Unraveling Genetic Modifiers in the Mouse Model of Absence Epilepsy
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
- The Coding and Noncoding Architecture of the Genome
- A Novel Locus Is Associated with Large Artery Atherosclerotic Stroke Using a Genome-Wide Age-at-Onset Informed Approach
- Brg1 Loss Attenuates Aberrant Wnt-Signalling and Prevents Wnt-Dependent Tumourigenesis in the Murine Small Intestine
- The PTK7-Related Transmembrane Proteins Off-track and Off-track 2 Are Co-receptors for Wnt2 Required for Male Fertility
- The Co-factor of LIM Domains (CLIM/LDB/NLI) Maintains Basal Mammary Epithelial Stem Cells and Promotes Breast Tumorigenesis
- Essential Genetic Interactors of Required for Spatial Sequestration and Asymmetrical Inheritance of Protein Aggregates
- Meiosis-Specific Cohesin Component, Is Essential for Maintaining Centromere Chromatid Cohesion, and Required for DNA Repair and Synapsis between Homologous Chromosomes
- Silencing Is Noisy: Population and Cell Level Noise in Telomere-Adjacent Genes Is Dependent on Telomere Position and Sir2
- The Two Cis-Acting Sites, and , Contribute to the Longitudinal Organisation of Chromosome I
- A Broadly Conserved G-Protein-Coupled Receptor Kinase Phosphorylation Mechanism Controls Smoothened Activity
- Requirements for Acute Burn and Chronic Surgical Wound Infection
- LIN-42, the PERIOD homolog, Negatively Regulates MicroRNA Transcription
- WAPL Is Essential for the Prophase Removal of Cohesin during Meiosis
- Expression in Planarian Neoblasts after Injury Controls Anterior Pole Regeneration
- Sox11 Is Required to Maintain Proper Levels of Hedgehog Signaling during Vertebrate Ocular Morphogenesis
- Accumulation of a Threonine Biosynthetic Intermediate Attenuates General Amino Acid Control by Accelerating Degradation of Gcn4 via Pho85 and Cdk8
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



