-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Retinoic Acid Activates Two Pathways Required for Meiosis in Mice
The transition from mitosis to meiosis is a defining feature of germ cells, the precursors of eggs and sperm. In mice, retinoic acid (RA), a vitamin A derivative, induces expression of the gene Stra8, which in turn is required for the first critical steps of meiosis. The timing of Stra8 expression in mammalian germ cells is influenced by an RA-degrading enzyme, CYP26B1, that is normally expressed in fetal testes to delay meiosis in males. It is unknown if Stra8 is RA's only meiosis-inducing target in germ cells or if other such genes are regulated by RA independently of Stra8. To investigate this question, we generated two lines of mice: Cyp26b1 mutants and Stra8 mutants. Our genetic experiments comparing germ cell development in these two mutants revealed a new RA target, Rec8. We demonstrate that Rec8 upregulation by RA occurs in the same temporal and spatial manner as Stra8, but Rec8 expression is independent of Stra8. Rec8, like Stra8, plays a critical role during early meiotic processes, suggesting that RA induces meiosis in at least two independent pathways. These findings expand our understanding of the gene regulatory network involved in meiotic initiation in mammals.
Published in the journal: Retinoic Acid Activates Two Pathways Required for Meiosis in Mice. PLoS Genet 10(8): e32767. doi:10.1371/journal.pgen.1004541
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004541Summary
The transition from mitosis to meiosis is a defining feature of germ cells, the precursors of eggs and sperm. In mice, retinoic acid (RA), a vitamin A derivative, induces expression of the gene Stra8, which in turn is required for the first critical steps of meiosis. The timing of Stra8 expression in mammalian germ cells is influenced by an RA-degrading enzyme, CYP26B1, that is normally expressed in fetal testes to delay meiosis in males. It is unknown if Stra8 is RA's only meiosis-inducing target in germ cells or if other such genes are regulated by RA independently of Stra8. To investigate this question, we generated two lines of mice: Cyp26b1 mutants and Stra8 mutants. Our genetic experiments comparing germ cell development in these two mutants revealed a new RA target, Rec8. We demonstrate that Rec8 upregulation by RA occurs in the same temporal and spatial manner as Stra8, but Rec8 expression is independent of Stra8. Rec8, like Stra8, plays a critical role during early meiotic processes, suggesting that RA induces meiosis in at least two independent pathways. These findings expand our understanding of the gene regulatory network involved in meiotic initiation in mammals.
Introduction
Most eukaryotes reproduce sexually, with life cycles that display an alternation of diploid and haploid phases. The generation of haploid cells from diploid cells is achieved through meiosis, featuring a single round of DNA replication (meiotic S) followed by two rounds of division (meiosis I and meiosis II).
In all sexually reproducing organisms, including fungi, plants, and animals, cells of the germ line activate the meiotic program when conditions are opportune and appropriate to the species' reproductive strategies. In yeast, for example, the meiotic program is initiated only when diploid cells are starved for nutrients and cannot proliferate. In mammals, the meiotic program is initiated only after the specialized cells of the germ line have migrated to the gonad. The timing of mammalian meiotic initiation differs dramatically between the sexes. In males, meiotic initiation does not commence until a spermatogonial stem cell population has been established, well after birth. In females, meiosis is initiated shortly after the germ cells have entered the gonad, during fetal development.
In mice, the published data are consistent with a model whereby an extrinsic meiosis-initiating signal – retinoic acid (RA) – induces transcription and expression of a single meiotic factor – Stra8 – which in turn governs the meiotic program [1]–[4]. In the ovary, induction of Stra8 in fetal germ cells expressing Dazl, an intrinsic factor, is required for meiotic DNA replication and the subsequent events of meiotic prophase [2], [5], [6]. In fetal testes, this process is temporarily blocked: CYP26B1, a cytochrome p450 enzyme, degrades RA, preventing expression of Stra8 and thus precluding meiotic initiation [1], [3], [7]. After birth, RA induces Stra8 in testicular germ cells, leading to meiotic initiation [3], [4].
Although the currently accepted model in mice postulates that RA induction of Stra8 may be necessary and sufficient for meiotic initiation [8], evidence suggests that other, independent factors are also at play: germ cells in Stra8-deficient fetal ovaries express Rec8 [2], encoding a meiosis-specific component of the cohesin complex. Rec8 is required for completion of sister chromatid cohesion, proper synapsis, and chiasmata formation [9], [10]. We decided to examine how Rec8 expression is regulated during the meiotic transition and whether RA plays a role in its expression. Our investigation proceeded by first comparing the patterns and regulation of Rec8 and Stra8 expression and then exploring important differences with respect to their roles in driving meiotic initiation. We discovered that RA activates meiosis in two independent ways, both of which require Dazl expression in the germ cells.
Results
Rec8, like Stra8, is expressed in an anterior-to-posterior wave in fetal ovaries
We first sought to investigate how Rec8 expression is initiated in the germ cells of fetal ovaries. If Rec8 is regulated like Stra8 and other early meiotic markers, it should initiate expression in an anterior-to-posterior pattern between E12.5 and E16.5 [5], [11], [12]. Using whole mount in situ hybridization, we discovered that Rec8 expression does unfold this way from E13.0 to E16.0 (Figure 1A). These findings suggested that Rec8, like Stra8, could be a target of RA signaling. Furthermore, since Dazl expression is required for ovarian germ cells to respond to RA signaling, perhaps, as with Stra8 expression, expression of Rec8 requires both DAZL and RA. We tested this new model (Figure 1B) in fetal ovaries, fetal testes and adult testes.
Fig. 1. In fetal ovaries, Rec8 is expressed in an anterior-to-posterior wave. 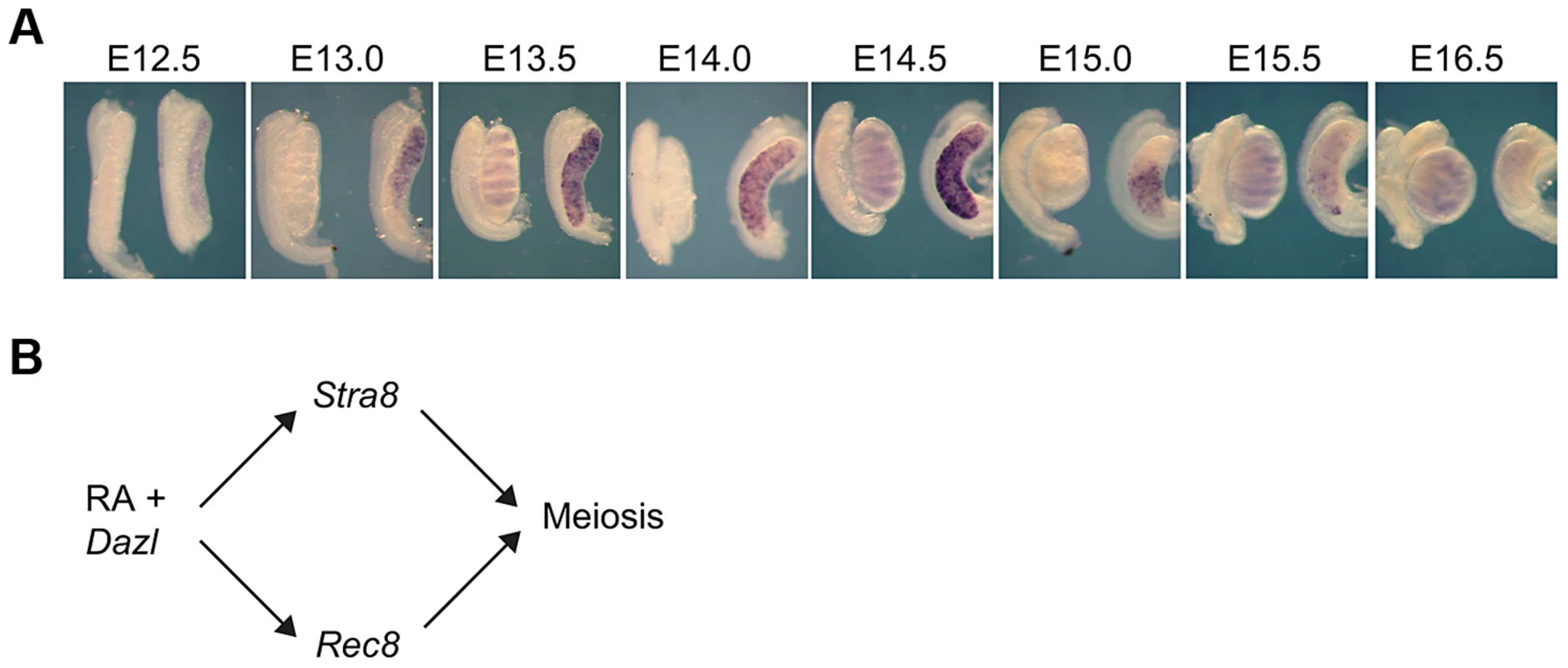
A) Rec8 expression pattern from E12.5–E16.0 in fetal gonads. B) Proposed model: RA signaling regulates meiotic initiation in mouse germ cells in parallel pathways through Stra8 and Rec8. In all panels, testes are at left and ovaries at right. RA induces Rec8 in fetal ovaries
We examined if RA signaling was required for Rec8 expression in the germ cells of fetal ovaries. We harvested ovaries at E12.5 and cultured them for two days in the presence of the RA receptor pan-antagonist BMS-204493 and then evaluated expression of both Stra8 and Rec8 using quantitative RT-PCR. BMS-204493 antagonizes all three RAR isotypes [13] and prevents RA signaling in fetal ovaries without killing the germ cells. We discovered that BMS-204493 dramatically lowered Rec8 expression, similar to Stra8 (Figure 2A), indicating that, in wild-type fetal ovaries, RA signaling is required for the germ cells to express Rec8. Taking these results together with our laboratory's previous finding that Stra8-deficient fetal ovaries express Rec8 [2], we conclude that RA induces Rec8 in fetal ovaries independently of Stra8.
Fig. 2. In fetal ovaries and postnatal testes, Rec8 is a target of RA signaling. 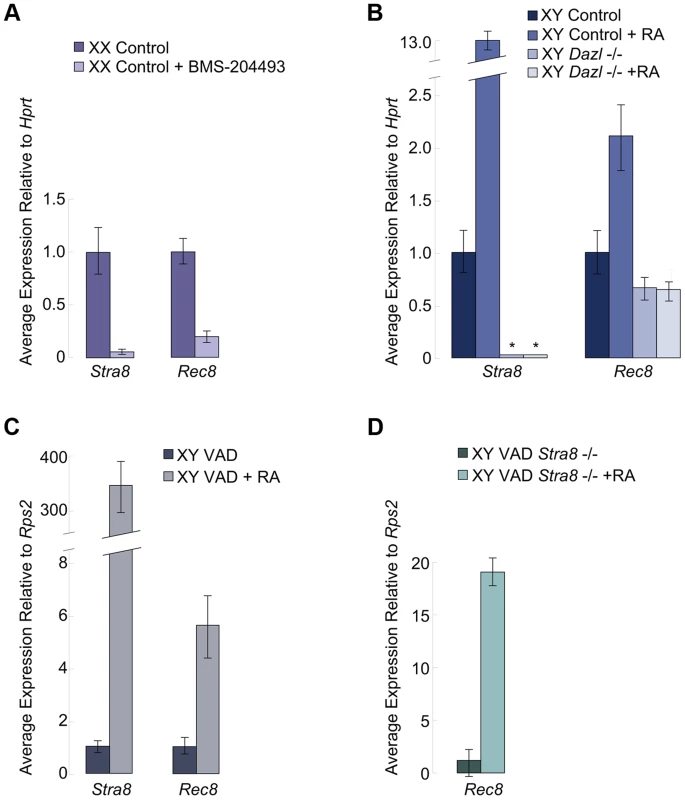
Quantitative RT-PCR analyses of A) Stra8 and Rec8 transcription in E12.5 ovaries cultured in control medium or with pan-RAR inhibitor added, B) Stra8 and Rec8 transcription in E14.5 control and Dazl-deficient testes cultured in control medium or with RA added (Stra8 is undetectable in Dazl−/−; indicated by asterisks), C) Stra8 and Rec8 expression in RA-restored or control adult VAD testes compared to pre-injection, contralateral testes, and D) Rec8 expression in Stra8-deficient VAD adult testes, without and with RA restoration. In fetal testes, RA-mediated upregulation of Rec8 requires Dazl
We next considered whether RA regulation of Rec8 expression resembles that of Stra8 in other respects. Germ cells in wild-type fetal testes express Stra8 when exposed to high levels of exogenous RA [3], but germ cells in Dazl-deficient testes do not [6]. Thus, during meiotic initiation, the germ cells must express Dazl in order to respond to RA signaling. We tested whether RA-mediated upregulation of Rec8 expression similarly requires Dazl. We used quantitative RT-PCR to compare Rec8 expression levels in E12.5 Dazl-deficient testes cultured for two days with or without RA added to the medium (Figure 2B). We found that, unlike Stra8, Rec8 is expressed, albeit at very low levels, in wild-type and Dazl-deficient testes. However, similarly to Stra8, Rec8 expression was significantly upregulated by RA treatment in wild-type but not Dazl-deficient testes (Figure 2B). Thus RA-induced upregulation of Rec8 in embryonic testes depends on Dazl.
RA induces Rec8 expression in adult testes independently of Stra8
RA also regulates Stra8 expression and meiotic initiation in germ cells of postnatal testes [3], [4]. We examined whether Rec8 followed a similar pattern to Stra8 here as well. Since retinoic acid is a metabolite of vitamin A, vitamin A-deficient (VAD) mice can be used to evaluate the effects of dramatically reduced RA signaling on postnatal testes. We removed testes from several vitamin A-deficient adult males and VAD males with restored RA signaling (24 hours post RA injection) and evaluated Rec8 and Stra8 transcripts by quantitative RT-PCR. Like Stra8, Rec8 transcription was dramatically increased 24 hours after injection of RA (Figure 2C). These results demonstrate that RA regulates Rec8 transcription in adult testes in vivo, as in fetal ovaries; this signaling event is shared between the sexes.
To test whether this Rec8 upregulation in postnatal testes was Stra8-dependent, we examined Rec8 expression in Stra8-deficient, VAD testes before and after injection of RA. While the Stra8-deficient, RA-deficient VAD testes expressed very little Rec8, restoration of RA resulted in dramatically increased expression of Rec8 (Figure 2D). Thus, as in fetal ovaries, RA induces Rec8 expression in postnatal testes independently of Stra8.
RA induces Rec8 expression in Cyp26b1-deficient fetal testes independently of Stra8
Germ cells in Cyp26b1-deficient fetal testes express Stra8 and several other early meiotic factors at the same time as they do in fetal ovaries because of uninhibited RA signaling [1], [7], [14](Figure S1). However, whether STRA8 protein is expressed and, if so, whether it influences other early meiotic factors has not been determined. We developed a system of single - and double-mutant mice with which to analyze in vivo the effects of RA signaling on germ cells in the presence and absence of STRA8. We found that STRA8 protein is expressed in Cyp26b1-deficient fetal testes but not in double-mutant Cyp26b1-deficient/Stra8-deficient testes (Figure 3A). We then assayed Rec8 expression in single - and double-mutant fetal testes using quantitative RT-PCR. In both cases, Rec8 expression is higher than in wild type, achieving similar levels in single - and double-mutant samples (Figure 3B). High expression levels in the double mutant indicate that RA induction of Rec8 in Cyp26b1-deficient fetal testes is independent of Stra8.
Fig. 3. In Cyp26b1-deficient/Stra8-deficient fetal testes, Rec8 is induced by RA signaling. 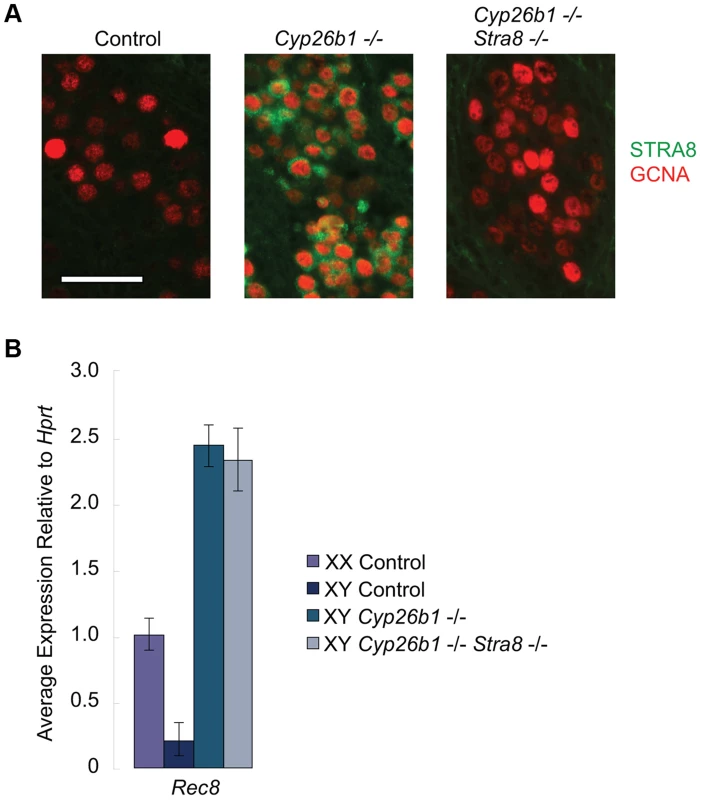
A) Fluorescent immunohistochemical staining for STRA8 protein (green) and GCNA (red) in E15.5 testes of the indicated genotypes (400×). Scale bar: 50 µm. B) Quantitative RT-PCR analysis of Rec8 transcription in E14.5 gonads of the indicated genotypes. DNA replication, DNA double-strand break formation, and upregulation of Dmc1 are all dependent on STRA8 induction in Cyp26b1-deficient fetal testes
In our studies above, we have established that RA regulates Rec8, and that it does so in parallel to its other known target, Stra8, in fetal ovaries, adult testes and in Cyp26b1-deficient fetal testes (Figure 1B). Drawing on the comparative model we used to examine Rec8 expression in fetal testes, we explored whether RA regulates other early meiotic factors in parallel to Stra8.
We first tested whether ectopic RA signaling is sufficient to drive DNA replication in germ cells of fetal testes, and, if so, whether this effect is also mediated through STRA8. The thymidine analog 5-bromo-2-deoxyuridine (BrdU) can be incorporated into newly synthesized DNA during S phase. We injected BrdU into pregnant females, dissected E16.5 fetal gonads and immunostained them with anti-GCNA (a germ cell marker) and anti-BrdU antibodies. In wild-type animals, testicular germ cells have arrested in G0/G1 by E16.5. We can therefore detect ectopic germ cell proliferation in response to STRA8 upregulation by assaying for ongoing DNA replication in E16.5 fetal gonads. BrdU incorporation was evident in germ cells of Cyp26b1-deficient testes (Figure 4A), consistent with transition towards meiosis. In contrast, GCNA-positive germ cells of double-mutant Cyp26b1-deficient/Stra8-deficient testes were uniformly negative for BrdU at E16.5. We conclude that the DNA replication observed in germ cells of Cyp26b1-deficient fetal testes at E16.5 depends on and is mediated through STRA8 (Figure 4A).
Fig. 4. In Cyp26b1-deficient testes, STRA8 induces Dmc1 expression, DNA replication and DNA double-strand break formation. 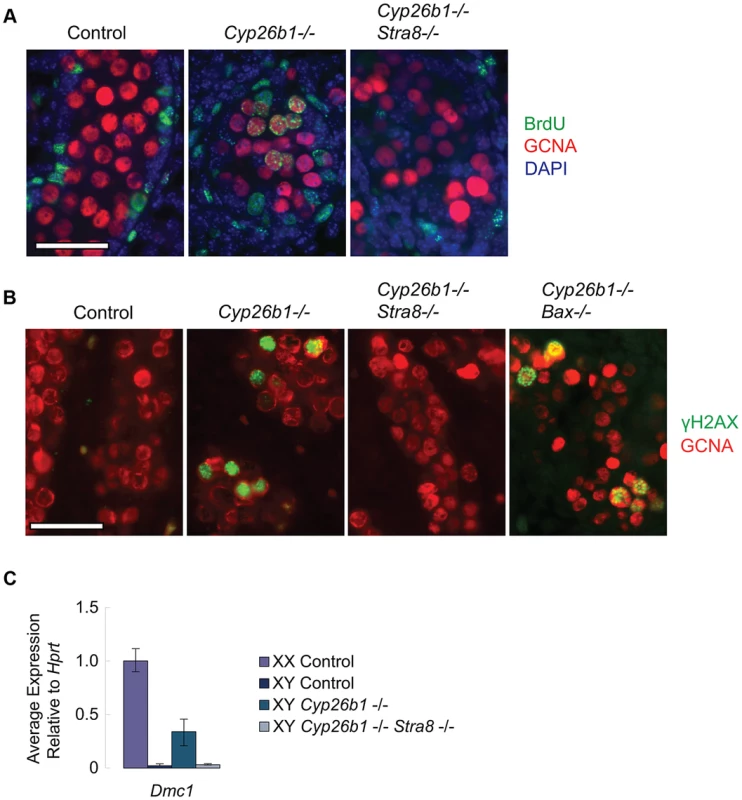
A) Fluorescent immunohistochemical staining for BrdU (green) and GCNA (red) in E16.5 testes of the indicated genotypes (400×). Scale bar: 50 µm. B) Fluorescent immunohistochemical staining for γH2AX protein (green) in E15.5 testes of the indicated genotypes (400×). Scale bar: 50 µm. C) Quantitative RT-PCR analysis of Dmc1 transcription in E14.5 gonads of the indicated genotypes. We then determined if RA is sufficient in fetal testes to induce DNA double strand breaks (DSBs), which are required for meiotic recombination [15]–[19], and if the generation of these DSBs is mediated through STRA8 induction. We assayed for the presence of γH2AX, a phosphorylated histone variant that localizes to DSBs, by immunostaining at E15.5, when DSBs are first observed [20]. Cyp26b1-deficient testes displayed many germ cells positive for γH2AX, suggesting that DSBs are induced by RA (Figure 4B). In contrast, we rarely observed γH2AX-positive germ cells in double-mutant Cyp26b1-deficient/Stra8-deficient testes (Figure 4B). This result suggests that the induction of DSBs in Cyp26b1-deficient testes is driven by ectopic RA and STRA8.
Since DSBs arise not only during meiotic recombination but also during apoptosis [21], and apoptosis has been reported in Cyp26b1-deficient testes [7], we tested whether γH2AX-positive germ cells observed in Cyp26b1-deficient testes represent meiotic and not simply apoptotic events. We first generated double mutant Cyp26b1-deficient/Bax-deficient embryos. Bax is a proapoptotic gene, and its deletion has been shown to suppress apoptosis in germ cells [14], [22], [23](Figure S2). Staining in double-mutant Cyp26b1-deficient/Bax-deficient testes revealed many γH2AX-positive germ cells (Figure 4B), confirming that most γH2AX-positive germ cells observed in Cyp26b1-deficient testes represent meiotic rather than apoptotic DNA DSBs. Formation of meiotic DNA DSBs thus represents another portion of the meiotic pathway that is STRA8-mediated.
Meiotic DSBs are processed by DMC1, an ortholog of the bacterial strand exchange protein RecA, which commences expression early during meiotic initiation. We compared the effects of RA on Dmc1 expression in Cyp26b1-deficient testes and in double-mutant (Cyp26b1-deficient/Stra8-deficient) testes. The Cyp26b1-deficient testes displayed increased levels of Dmc1, while levels of Dmc1 in double-mutant testes were similar to controls (Figure 4C). Thus, RA is sufficient to drive Dmc1 expression in fetal testes in vivo, but this induction requires mediation by STRA8.
In summary, it appears that RA induction of STRA8 in fetal testes is required for all of the above-tested markers/processes during early meiosis, with the notable exception of RA-regulated Rec8 expression.
Stra8 and Dmc1 are expressed independently of Rec8
To exclude the possibility that induction of Stra8 and its downstream target Dmc1 depends on Rec8 function, we examined Stra8 and Dmc1 expression in Rec8-deficient (Rec8mei8/mei8) ovaries and testes [9]. As expected, we found no significant difference in Stra8 and Dmc1 expression levels between control and Rec8-deficient E13.5 ovaries (Figure 5A). Similarly, we detected STRA8 and DMC1 proteins in both control and Rec8-deficient adult testes (Figure 5B). We conclude that RA induction of Stra8, and its downstream targets, is independent of and occurs in parallel with RA induction of Rec8.
Fig. 5. Stra8 and Dmc1 expression in male and female germ cells is independent of Rec8. 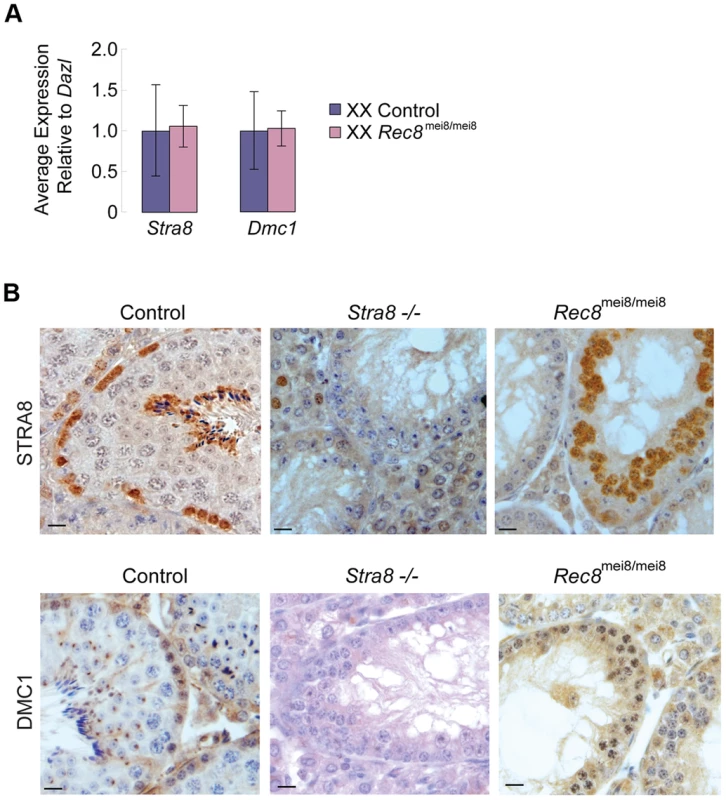
A) Quantitative RT-PCR analysis of Stra8 and Dmc1 transcription in E13.5 Rec8-deficient and control ovaries. B) Colorimetric immunohistochemical staining for STRA8 and DMC1 proteins in control, Stra8−/−, and Rec8mei8/mei8 adult testes. Scale bar: 10 µm. Discussion
Our findings lead us to conclude that RA plays a broad and encompassing role in regulating and coordinating the transition from mitosis to meiosis in mouse germ cells, in both fetal ovaries and postnatal testes. Surprisingly, RA accomplishes this by independently inducing both Stra8 and Rec8, which both play critical roles in the earliest stages of meiosis. The discovery that RA induction of Stra8 in Cyp26b1-deficient fetal testes mediates DNA replication, DSB formation, and the expression of recombinase Dmc1 provides critical details about the Stra8 pathway. Moreover, Stra8 induction was recently shown to be required for SYCP3 expression in Cyp26b1-deficient testes [24]. Rec8 induction is the first component of the molecular program of meiotic initiation shown to be Stra8-independent in mice. Now that Rec8's independent induction has been established, its expression pattern and function invite deeper investigation.
How Rec8 expression is induced by RA remains elusive. Stra8's promoter region contains two putative RA Response Elements (RAREs), suggesting that RA could be turning on this gene directly [25]. A chromatin immunoprecipitation-sequencing (ChIP-Seq) study in embryonic stem cells identified RAR binding sites in both Stra8 and Rec8 promoter regions, suggesting that Rec8 may also be regulated by RA directly [26]. Intriguingly, in the same study, Dmc1, which is dependent on STRA8, does not show such RAR binding sites, consistent with Stra8 and Rec8 being regulated directly, unlike Stra8's downstream targets.
What purpose does RA upregulation of REC8 serve? It may ensure that Rec8 is expressed during pre-meiotic S phase so that its product can be incorporated into the meiotic cohesin complex that joins sister chromatids. Indeed, germ cells in Rec8-deficient mice later show defects that can be traced to its cohesion function – incorrect synapsis topology and failure at chromosome segregation and chiasmata formation [9], [10]. Recent studies also suggest a role for cohesins in direct regulation of gene expression by novel mechanisms involving DNA looping [27], [28]. It is presently unknown if Rec8 is a direct transcriptional regulator. However, Rec8 null animals exhibit partial embryonic lethality and fail to thrive [10], phenotypes hard to reconcile with an exclusive role in germ cell meiotic cohesion.
The mechanisms that govern meiotic initiation have been explored most thoroughly in yeast, and these studies offer interesting parallels to our findings in mice. In both yeast and mice, the decision to initiate the meiotic program is taken prior to pre-meiotic DNA replication [2], [29]. Our finding that RA regulates Rec8 is consistent with an early role of RA in this transition, since at least in budding yeast, REC8 associates with chromosomes from late G1 phase [30]. In addition, in both yeast and mice, the decision to initiate meiosis requires an extrinsic signal and an intrinsic competence factor [1], [3], [6], [31], [32]. In yeast, the extrinsic signal – nutrient depletion – activates multiple molecular pathways in parallel, and these converge on IME1, which is required for upregulating the expression of meiosis-specific transcripts. However, IME1 is not sufficient to induce meiosis in yeast [33], [34]. Our studies show that, analogously, RA activates at least two pathways by regulating Stra8 and Rec8 independently. While many early meiotic processes described so far hinge on STRA8, STRA8 may not be sufficient for meiosis in mice. The search for additional RA targets will likely yield further insights into the networks governing transition from mitosis to meiosis in mammals.
Materials and Methods
Ethics statement
All experiments involving mice were approved by the Committee on Animal Care at the Massachusetts Institute of Technology.
Targeted disruption of the Cyp26b1 gene
Cyp26b1-deficient mice were generated by deleting a 2.9-kb portion of the gene (including exons 4, 5, 6, and the coding region of exon 7) by homologous recombination in embryonic stem (ES) cells (Figure S1). A Cyp26b1/PGK-Neo targeting construct was assembled using PCR products amplified with Advantage HF2 polymerase (Clontech) using mouse (C57BL/6J) genomic BAC RP24-470O13 (GenBank Accession AC159337) as template. The targeting construct was linearized and electroporated into v6.5 ES cells [35]. Cells harboring the construct were selected using neomycin (Invitrogen). ES cell colonies were screened by PCR for homologous integration at both the 5′ and 3′ arms of the construct. Clones that tested positive by both PCR assays were confirmed by Southern blot analysis using EcoRV and Nde1 restriction endonucleases.
Correctly targeted ES cell clones were injected into Balb/c or C57Bl/6N blastocysts and transferred to pseudopregnant Swiss Webster females. Germline transmission was obtained with one clone, and the resulting homozygous embryos displayed anomalies of limb, eye, and facial development and died at birth, as previously described [7], [36]. Embryos were genotyped by PCR, (primer sequences available in Note S1).
Additional mutant mouse strains
Mice carrying the DazlTM1Hgu allele [37] were generously provided by Howard Cooke, MRC Human Genetics Unit, Western General Hospital, Edinburgh, UK, and Dazl-deficient mice were generated as described previously [6], [38]. Stra8-deficient mice were generated as described previously [2], [4]. Bax-deficient mice were generated by mating Baxtm1Sjk/+ mice obtained from The Jackson Laboratory (Bar Harbor, ME). Rec8-deficient mice were generated by mating Rec8mei8/+ mice [9], which were generously provided by John Schimenti, Cornell University, Ithaca, New York.
Mouse embryo collection and in situ hybridization
Mouse embryos used in whole mount in situ hybridizations and gonad cultures were obtained from matings between CD1 random bred mice (Charles River Labs). Noon of the day when vaginal plug was recorded was considered E0.5. Whole mount in situ hybridizations with the Stra8 probe were performed as previously described [3], [39]. Digoxigenin riboprobe for Rec8 was generated by amplifying cDNA fragments by RT-PCR from Rec8 (NM_020002.2: bases 274–865), and inserting them into TA cloning vector pCR4-TOPO (Invitrogen). Plasmid was linearized with Spe1 or Not1 and transcribed with T7 or T3 respectively to make the antisense and sense probes.
RT-PCR
For experiments involving Rec8-deficient mice, total RNAs were prepared from gonads using the RNeasy plus Micro RNA isolation kit (QIAGEN), and reverse transcription was carried out using the high-capacity cDNA reverse transcription kit (Applied Biosystems). For all other experiments, total RNAs were prepared using TRIzol (Invitrogen) extraction followed by DNase (Ambion) treatment, and reverse transcription was carried out using the RETROscript reverse transcription kit (Life Technologies). The resulting total cDNAs were analyzed quantitatively using SYBR Green PCR reagents (Applied Biosystems) with primers for Dmc1, Rec8, Stra8, or Dazl. Expression profiles were tested in triplicate on at least two litters of embryos on an ABI 7500 instrument (Applied Biosystems). Data were analyzed using the comparative Ct (ΔΔCt) method and one-tail, unpaired student T test (significance cutoff p<0.01). Results were normalized to Rps2 (VAD experiments on adult testis), Dazl (Rec8-mutant experiments on embryonic ovary), and Hprt (all other experiments). Primers were selected from PrimerBank [40] (Note S1).
Immunofluorescent studies of tissue sections
Fetal gonads were dissected in phosphate buffered saline (PBS), fixed in 4% paraformaldehyde overnight at 4°C, embedded in paraffin and sectioned. Slides were incubated with anti-GCNA IgM (courtesy of G. Enders, undiluted supernatant), anti-STRA8 (Abcam. 1∶100), and anti-phosphoH2A.X (Upstate Cell Signaling Solutions, 1∶250 dilution). Colorimetric staining was performed using ABC reagents (Vector Laboratories) and developed with DAB peroxidase substrate (Vector Laboratories).
Sections were mounted in Vectashield Medium with DAPI (Vector Laboratories), and fluorescent staining was obtained using Texas-Red or FITC-conjugated secondary antibodies (Jackson Immunoresearch Laboratories, 1∶500 dilution).
Immunohistochemical studies of tissue sections
Adult testes were fixed in Bouin's solution overnight at 4°C, washed with PBS and 70% ethanol, embedded in paraffin, and sectioned at 5 µm thickness. Slides were matured overnight, de-waxed, rehydrated, and heated in 10 mM sodium citrate buffer (pH 6.0) for antigen retrieval. Sections were incubated in 3% hydrogen peroxide for 5 min and blocked in 2.5% normal horse serum (Vector Laboratories) for 80 minutes at room temperature. Later, slides were incubated overnight with anti-STRA8 (Abcam, 1∶500) or anti-DMC1 (Santa Cruz Biotechnology, 1∶50 dilution). The following day, slides were washed three times in PBS and incubated with anti-rabbit ImmPRESS peroxidase reagent (Vector Laboratories) for 30 minutes. The slides were later developed using a DAB substrate kit (Vector Laboratories) for 1 minute. The slides were counterstained with Mayer's hematoxylin for 5 minutes and washed in running water, dehydrated, and mounted with Permount (Fisher Scientific).
TUNEL analysis
Apoptotic cells were detected in paraffin sections of fetal testes using the Fluorescein in situ Cell Death Detection Kit (Roche Applied Science) and mounted in Vectashield Medium with DAPI (Vector Laboratories).
BrdU incorporation
Pregnant females were injected with 5-bromo-2-deoxyuridine (BrdU) solution (50 mg/kg) at 18.5 days post coitum. Six hours later, fetal gonads were dissected. Gonads were then fixed in 4% paraformaldehyde overnight at 4°C, embedded in paraffin, and sectioned. Prior to antibody application, sections were treated with denaturing reagent (3.5N HCl) for 2 min. Incorporated BrdU was detected using anti-BrdU (Accurate Chemical & Scientific Corp., 1∶500 dilution) in anti-GCNA IgM supernatant.
Mouse fetal gonad culture
Pregnant female mice were sacrificed by cervical dislocation and embryos were removed into PBS solution. After determining tail somite number, fetal ovaries and mesonephroi were dissected. One gonad from each embryo was then placed in a 35 µl droplet of culture media (DME +10% FBS) supplemented with either 5 µM pan-RAR inhibitor BMS-204493 (Bristol-Myers Squibb) or all trans RA (Sigma) dissolved in ethanol in a Petri plate. Control media contained vehicle (ethanol) alone. Petri plates were then inverted and placed within larger plates containing water and incubated at 37°C with 5% CO2. Media was replaced after 24 hours. After 48 hours, tissue was removed from media, mesonephroi were dissected off and ovaries were placed individually into TRIzol reagent (Invitrogen). Samples were then processed for quantitative RT-PCR as described above.
Analysis of Rec8 expression in vitamin-A-deficient testes
Adult female mice (129/SvJ) were fed a Vitamin-A-Deficient (VAD) diet (Harlan Teklad, Indianapolis) for at least 2 weeks before mating and throughout pregnancy. Their male offspring were fed a VAD diet for 13–14 weeks. In the first experiment with wild-type animals, one testis was removed from each animal and cut into two pieces; one fixed in Bouin's solution for histological assessment of spermatogenesis and the other placed in TRIzol (Invitrogen) for RNA extraction to serve as a pre-injection control in RT-PCR analysis. Incisions were sutured and the animals recovered for 24 h. Three animals with similarly deficient spermatogenesis (as judged by pre-injection testicular histology) were injected with 100 µl of 7.5 mg/ml all-trans retinoic acid (Sigma) in 10% ethanol/90%sesame oil solution. The animals' remaining testes were harvested 24 h after injection. In contrast, both testes were harvested from two Stra8-deficient VAD animals at the same time (one was analyzed histologically to confirm depletion) and compared to testes harvested from two RA-restored Stra8-deficient animals. Quantitative RT-PCR analysis was performed, in triplicate, using Stra8 and Rec8 primers, and Rps2 was used as a normalization control (primer sequences in Note S1).
Supporting Information
Zdroje
1. BowlesJ, KnightD, SmithC, WilhelmD, RichmanJ, et al. (2006) retinoid signaling determines germ cell fate in mice. Science 312 : 596–600.
2. BaltusAE, MenkeDB, HuYC, GoodheartML, CarpenterAE, et al. (2006) in germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic dna replication. Nat Genet 38 : 1430–1434.
3. KoubovaJ, MenkeDB, ZhouQ, CapelB, GriswoldMD, et al. (2006) retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 103 : 2474–2479.
4. AndersonEL, BaltusAE, Roepers-GajadienHL, HassoldTJ, de RooijDG, et al. (2008) stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A 105 : 14976–14980.
5. MenkeDB, KoubovaJ, PageDC (2003) sexual differentiation of germ cells in xx mouse gonads occurs in an anterior-to-posterior wave. Dev biol 262 : 303–312.
6. LinY, GillME, KoubovaJ, PageDC (2008) germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science 322 : 1685–1687.
7. MacleanG, LiH, MetzgerD, ChambonP, PetkovichM (2007) apoptotic extinction of germ cells in testes of cyp26b1 knockout mice. Endocrinology 148 : 4560–4567.
8. GriswoldMD, HogarthCA, BowlesJ, KoopmanP (2012) initiating meiosis: the case for retinoic acid. Biol reprod 86 : 35.
9. BannisterLA, ReinholdtLG, MunroeRJ, SchimentiJC (2004) positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin rec8. Genesis 40 : 184–194.
10. XuH, BeasleyMD, WarrenWD, van der HorstGT, MckayMJ (2005) absence of mouse rec8 cohesin promotes synapsis of sister chromatids in meiosis. Dev cell 8 : 949–961.
11. YaoHH, DinapoliL, CapelB (2003) meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads. Development 130 : 5895–5902.
12. BullejosM, KoopmanP (2004) germ cells enter meiosis in a rostro-caudal wave during development of the mouse ovary. Mol reprod dev 68 : 422–428.
13. GermainP, IyerJ, ZechelC, GronemeyerH (2002) co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature 415 : 187–192.
14. SuzukiA, SagaY (2008) nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes dev 22 : 430–435.
15. BaudatF, ManovaK, YuenJP, JasinM, KeeneyS (2000) chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking spo11. Mol cell 6 : 989–998.
16. RomanienkoPJ, Camerini-OteroRD (2000) the mouse spo11 gene is required for meiotic chromosome synapsis. Mol cell 6 : 975–987.
17. YoshidaK, KondohG, MatsudaY, HabuT, NishimuneY, et al. (1998) the mouse reca-like gene dmc1 is required for homologous chromosome synapsis during meiosis. Mol cell 1 : 707–718.
18. PittmanDL, CobbJ, SchimentiKJ, WilsonLA, CooperDM, et al. (1998) meiotic prophase arrest with failure of chromosome synapsis in mice deficient for dmc1, a germline-specific reca homolog. Mol cell 1 : 697–705.
19. RogakouEP, PilchDR, OrrAH, IvanovaVS, BonnerWM (1998) dna double-stranded breaks induce histone h2ax phosphorylation on serine 139. J biol chem 273 : 5858–5868.
20. MahadevaiahSK, TurnerJM, BaudatF, RogakouEP, de BoerP, et al. (2001) recombinational dna double-strand breaks in mice precede synapsis. Nat genet 27 : 271–276.
21. RogakouEP, Nieves-NeiraW, BoonC, PommierY, BonnerWM (2000) initiation of dna fragmentation during apoptosis induces phosphorylation of h2ax histone at serine 139. J biol chem 275 : 9390–9395.
22. KnudsonCM, TungKS, TourtellotteWG, BrownGA, KorsmeyerSJ (1995) bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270 : 96–99.
23. StallockJ, MolyneauxK, SchaibleK, KnudsonCM, WylieC (2003) the pro-apoptotic gene bax is required for the death of ectopic primordial germ cells during their migration in the mouse embryo. Development 130 : 6589–6597.
24. SabaR, WuQ, SagaY (2014) cyp26b1 promotes male germ cell differentiation by suppressing stra8-dependent meiotic and stra8-independent mitotic pathways. Dev biol 389 : 173–181.
25. Oulad-AbdelghaniM, BouilletP, DecimoD, GansmullerA, HeybergerS, et al. (1996) characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by stra8, a novel retinoic acid-responsive gene. J cell biol 135 : 469–477.
26. MahonyS, MazzoniEO, MccuineS, YoungRA, WichterleH, et al. (2011) ligand-dependent dynamics of retinoic acid receptor binding during early neurogenesis. Genome biol 12: r2.
27. DorsettD (2011) cohesin: genomic insights into controlling gene transcription and development. Curr opin genet dev 21 : 199–206.
28. KageyMH, NewmanJJ, BilodeauS, ZhanY, OrlandoDA, et al. (2010) mediator and cohesin connect gene expression and chromatin architecture. Nature 467 : 430–435.
29. MarstonAL, AmonA (2004) meiosis: cell-cycle controls shuffle and deal. Nat rev mol cell biol 5 : 983–997.
30. MichaelisC, CioskR, NasmythK (1997) cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 91 : 35–45.
31. HopperAK, HallBD (1975) mating type and sporulation in yeast. I. Mutations which alter mating-type control over sporulation. Genetics 80 : 41–59.
32. HopperAK, KirschJ, HallBD (1975) mating type and sporulation in yeast. Ii. Meiosis, recombination, and radiation sensitivity in an alpha-alpha diploid with altered sporulation control. Genetics 80 : 61–76.
33. ColominaN, LiuY, AldeaM, GariE (2003) tor regulates the subcellular localization of ime1, a transcriptional activator of meiotic development in budding yeast. Mol cell biol 23 : 7415–7424.
34. SmithHE, SuSS, NeigebornL, DriscollSE, MitchellAP (1990) role of ime1 expression in regulation of meiosis in saccharomyces cerevisiae. Mol cell biol 10 : 6103–6113.
35. RideoutWM3rd, WakayamaT, WutzA, EgganK, Jackson-GrusbyL, et al. (2000) generation of mice from wild-type and targeted es cells by nuclear cloning. Nat genet 24 : 109–110.
36. YashiroK, ZhaoX, UeharaM, YamashitaK, NishijimaM, et al. (2004) regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev cell 6 : 411–422.
37. RuggiuM, SpeedR, TaggartM, MckaySJ, KilanowskiF, et al. (1997) the mouse dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 389 : 73–77.
38. LinY, PageDC (2005) dazl deficiency leads to embryonic arrest of germ cell development in xy c57bl/6 mice. Dev biol 288 : 309–316.
39. WilkinsonDG, NietoMA (1993) detection of messenger rna by in situ hybridization to tissue sections and whole mounts. Methods enzymol 225 : 361–373.
40. WangX, SeedB (2003) a pcr primer bank for quantitative gene expression analysis. Nucleic acids res 31: e154.
Štítky
Genetika Reprodukčná medicína
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 8- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- DNA Double Strand Break Repair in Mitosis Is Suppressed by Phosphorylation of XRCC4
- Inference of Transposable Element Ancestry
- The Population Genetics of Evolutionary Rescue
- Retinoic Acid Activates Two Pathways Required for Meiosis in Mice
- Pooled Segregant Sequencing Reveals Genetic Determinants of Yeast Pseudohyphal Growth
- Comprehensive Identification of Single Nucleotide Polymorphisms Associated with Beta-lactam Resistance within Pneumococcal Mosaic Genes
- SMA-Causing Missense Mutations in Display a Wide Range of Phenotypes When Modeled in
- Branch Migration Prevents DNA Loss during Double-Strand Break Repair
- Transcriptome Sequencing from Diverse Human Populations Reveals Differentiated Regulatory Architecture
- Genetic Deletion of SEPT7 Reveals a Cell Type-Specific Role of Septins in Microtubule Destabilization for the Completion of Cytokinesis
- Tethering Sister Centromeres to Each Other Suggests the Spindle Checkpoint Detects Stretch within the Kinetochore
- Global Genetic Variations Predict Brain Response to Faces
- Demography and the Age of Rare Variants
- The Response to High CO Levels Requires the Neuropeptide Secretion Component HID-1 to Promote Pumping Inhibition
- Sp6 and Sp8 Transcription Factors Control AER Formation and Dorsal-Ventral Patterning in Limb Development
- The Groucho Co-repressor Is Primarily Recruited to Local Target Sites in Active Chromatin to Attenuate Transcription
- A Transposable Element Insertion Confers Xenobiotic Resistance in Drosophila
- The Genomic Architecture of Population Divergence between Subspecies of the European Rabbit
- Human Social Genomics
- Gene Expansion Shapes Genome Architecture in the Human Pathogen : An Evolutionary Genomics Analysis in the Ancient Terrestrial Mucorales (Mucoromycotina)
- Canonical Non-Homologous End Joining in Mitosis Induces Genome Instability and Is Suppressed by M-phase-Specific Phosphorylation of XRCC4
- Roles of Type 1A Topoisomerases in Genome Maintenance in
- The TRIM-NHL Protein LIN-41 Controls the Onset of Developmental Plasticity in
- Wnt-Mediated Repression via Bipartite DNA Recognition by TCF in the Hematopoietic System
- KDM6 Demethylase Independent Loss of Histone H3 Lysine 27 Trimethylation during Early Embryonic Development
- Integration of UPR and Oxidative Stress Signaling in the Control of Intestinal Stem Cell Proliferation
- miR171-Targeted Scarecrow-Like Proteins Bind to GT -Elements and Mediate Gibberellin-Regulated Chlorophyll Biosynthesis under Light Conditions
- Syndecan-1 Is Required to Maintain Intradermal Fat and Prevent Cold Stress
- LIN-3/EGF Promotes the Programmed Cell Death of Specific Cells in by Transcriptional Activation of the Pro-apoptotic Gene
- A System for Genome-Wide Histone Variant Dynamics In ES Cells Reveals Dynamic MacroH2A2 Replacement at Promoters
- Multiple Regulation of Rad51-Mediated Homologous Recombination by Fission Yeast Fbh1
- A Genome-Wide Association Study of the Maize Hypersensitive Defense Response Identifies Genes That Cluster in Related Pathways
- The RNA Helicases AtMTR4 and HEN2 Target Specific Subsets of Nuclear Transcripts for Degradation by the Nuclear Exosome in
- Asymmetric Division and Differential Gene Expression during a Bacterial Developmental Program Requires DivIVA
- A Model-Based Approach for Identifying Signatures of Ancient Balancing Selection in Genetic Data
- Chromatin Insulator Factors Involved in Long-Range DNA Interactions and Their Role in the Folding of the Drosophila Genome
- Conditional Inactivation of Upstream Binding Factor Reveals Its Epigenetic Functions and the Existence of a Somatic Nucleolar Precursor Body
- Evidence for Divisome Localization Mechanisms Independent of the Min System and SlmA in
- Patterns of Admixture and Population Structure in Native Populations of Northwest North America
- Response Regulator Heterodimer Formation Controls a Key Stage in S Development
- A Genetic Strategy to Measure Circulating Insulin Reveals Genes Regulating Insulin Production and Secretion
- EVA-1 Functions as an UNC-40 Co-receptor to Enhance Attraction to the MADD-4 Guidance Cue in
- Dysfunction of the CNS-Heart Axis in Mouse Models of Huntington's Disease
- An Otx/Nodal Regulatory Signature for Posterior Neural Development in Ascidians
- Phosphorylation of a Central Clock Transcription Factor Is Required for Thermal but Not Photic Entrainment
- Genome-Wide Patterns of Genetic Variation within and among Alternative Selective Regimes
- EF-P Dependent Pauses Integrate Proximal and Distal Signals during Translation
- Ku-Mediated Coupling of DNA Cleavage and Repair during Programmed Genome Rearrangements in the Ciliate
- Functional Specialization Among Members Of Knickkopf Family Of Proteins In Insect Cuticle Organization
- Playing RNase P Evolution: Swapping the RNA Catalyst for a Protein Reveals Functional Uniformity of Highly Divergent Enzyme Forms
- The Translational Regulators GCN-1 and ABCF-3 Act Together to Promote Apoptosis in
- Meta-Analysis of Genome-Wide Association Studies in African Americans Provides Insights into the Genetic Architecture of Type 2 Diabetes
- A -Regulatory Mutation of Causes Silky-Feather in Chickens
- VIB1, a Link between Glucose Signaling and Carbon Catabolite Repression, Is Essential for Plant Cell Wall Degradation by
- A Population Genetic Signal of Polygenic Adaptation
- A Conserved Dopamine-Cholecystokinin Signaling Pathway Shapes Context–Dependent Behavior
- The MAP Kinase p38 Is Part of Circadian Clock
- The Cohesin Subunit Rad21 Is Required for Synaptonemal Complex Maintenance, but Not Sister Chromatid Cohesion, during Drosophila Female Meiosis
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Meta-Analysis of Genome-Wide Association Studies in African Americans Provides Insights into the Genetic Architecture of Type 2 Diabetes
- KDM6 Demethylase Independent Loss of Histone H3 Lysine 27 Trimethylation during Early Embryonic Development
- The RNA Helicases AtMTR4 and HEN2 Target Specific Subsets of Nuclear Transcripts for Degradation by the Nuclear Exosome in
- EF-P Dependent Pauses Integrate Proximal and Distal Signals during Translation
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



