-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Emerging Role of Urease as a General Microbial Virulence Factor
article has not abstract
Published in the journal: The Emerging Role of Urease as a General Microbial Virulence Factor. PLoS Pathog 10(5): e32767. doi:10.1371/journal.ppat.1004062
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004062Summary
article has not abstract
Urea is generated in humans following the breakdown of amino acids and is evenly distributed throughout the body, including in the central nervous system, subcutaneous adipose tissue, blood serum, and epithelial lining fluid [1], [2]. Various pathogenic microbes are able to utilise urea as a nitrogen source through the activity of the enzyme urease that converts urea into ammonia and carbamic acid, with the spontaneous hydrolysis of carbamic acid to carbonic acid generating a further ammonia molecule.
CH4N2O+H2O→NH3+CH3NO2
CH3NO2+H2O→NH3+H2CO3
Under physiological conditions the proton of carbonic acid dissociates, and the ammonia molecules become protonated to form ammonium, causing an increase in local pH that can interfere with host function. The role of urease in the virulence of some bacterial pathogens is well established; however, more recent studies are beginning to highlight the function of urease during human fungal infections, suggesting that this enzyme has a wide role during microbial infection.
Established Roles for Urease during Microbial Infection
Urease activity is the basis of acid acclimation that enables Helicobacter pylori to colonise the acidic environment of the human stomach. In response to the acidification of the periplasm of H. pylori, a proton gated urea channel (UreI) imports urea into the bacterial cytosol, where it is hydrolysed by urease [3]. Ammonia and bicarbonate (following the conversion of carbonic acid to bicarbonate) are then utilised to buffer protons within the periplasm [4]. Without this mechanism, H. pylori is unable to establish infection in the stomach [5], [6]. The urease-derived ammonia is also toxic to host epithelial cells, resulting in cell damage at the sites of H. pylori infection [7].
A distinct urease-dependent process is associated with bacterial urinary tract infections, including those caused by Proteus and Klebsiella species. Infection by these urease-positive bacteria can result in the development of infection stones that surround and protect the pathogen. Stones form due to the precipitation of the minerals struvite and carbonate apatite, which are produced by the binding of ammonium to magnesium ions and bicarbonate to calcium ions, respectively [8], [9]. As with H. pylori infection in the stomach, ammonia has an additional role in urinary tract infections by causing damage to the glycosaminoglycan surface of the urothelium that protects epithelial cells from bacterial infection [10], [11]. Therefore, urease-mediated pH changes and damage to host epithelial cells are associated with the promotion of some bacterial infections.
Emerging Pathogenic Roles for Urease during Fungal Infections
Studies of two evolutionarily diverse fungi, Cryptococcus neoformans (a basidiomycete) and Coccidioides posadasii (an ascomycete), suggest that urease has conserved roles in promoting bacterial and fungal infections. The inhalation and subsequent germination of fungal spores is the route of entry into the body for many fungal pathogens. Successful infection of the lung requires the pathogen to evade the host immune system, and subsequent dissemination is dependent on the ability of the fungus to move from the lungs to other organs via the bloodstream. C. neoformans and Co. posadasii are saprophytic yeast that infect humans via the lungs and cause disease, with the severity of infection correlating with a loss of host immune function, and in both cases, the disseminated form of infection is potentially life threatening [12], [13]. Studies of both C. neoformans and Co. posadasii suggest that urease-dependent pH changes are involved in immune system evasion and that ammonia toxicity to host cells promotes systemic disease.
Urease is required for the full virulence of C. neoformans and Co. posadasii in animal models of disease [14], [15]. Urease-positive strains of both yeasts promote host responses that are consistent with a nonprotective Type 2 (Th2) rather than a fungicidal Type 1 (Th1) immune response [15], [16]. Consequently, mice infected with urease-positive C. neoformans have higher levels of serum IgE, Th2 cytokines, and alternatively activated macrophages as compared to control mice infected with a urease-negative strain [16]. Furthermore, a Th2 host response may also provide the pathogen with additional host-derived urea as alternatively activated macrophages convert arginine to ornithine and urea [17]. The detection of urease-dependent increases in arginase expression and higher levels of urea at sites of Co. posadasii infection support this positive feedback model [15], [16].
One mechanism to account for the urease-dependent Th2 polarisation of the host immune system is that the pH changes associated with urease activity cause a reduction in the acidification and maturation of phagolysosomes in phagocytic cells, resulting in a loss of pathogen killing and antigen presentation. A range of studies are consistent with this model. Fungi excrete ammonia as part of their internal pH control, and localised increases in pH are associated with Co. posadasii infection [15]. Ammonia prevents phagosome and lysosomal fusion and maturation in mouse peritoneal macrophages [18]. Immature dendritic cells promote Th2 polarisation, and higher levels of this class of antigen-presenting cells are found in mice infected with urease-positive rather than urease-negative C. neoformans [16]. Consistent with these findings are bacterial studies of urease-deficient Mycobacterium bovis, which localise more efficiently with lysosomes than a parental wild-type strain [19]. Furthermore, M. bovis-derived ammonia accumulates in macrophages and correlates with the reduction in the cell surface trafficking of the major histocompatibility complex class II [20].
The dissemination of a fungal pathogen from the lungs to other organs will be facilitated by damage to epithelial cells that allow pathogen access to and from the bloodstream. Ammonia is toxic to mammalian cells, and the urease-dependent damage to human epithelial cells by bacterial pathogens suggests that the ammonia produced by urease-positive fungi may also promote fungal dispersal [7], [10], [11], [21]. Evidence to support this comes from two studies involving C. neoformans. Urease activity negatively influences the integrity of endothelial junctions in vitro, as assayed using human brain microvascular endothelial cells [22]. Urease also promotes the crossing of C. neoformans across the blood-brain barrier in vivo after the pathogen becomes trapped in the small capillaries of the mouse brain [23]. Therefore, a plausible model is that circulating host urea is metabolised by yeast trapped in the small capillaries of the brain, resulting in the export of ammonia. The localised death of host epithelium cells then results in a loss of integrity of the blood-brain barrier, allowing C. neoformans to migrate into the brain parenchyma. A prediction is that pathogen-derived ammonia has the potential to kill host cells throughout the body.
The studies relating to the ammonia repression of phagocyte function and localised tissue damage suggest a general model for the role of urease during fungal infections (Figure 1). Initially inhaled fungal spores germinate and fungal cells are able to proliferate in imunocompromised individuals due to a reduction in host immune function. Metabolism of host-derived urea results in the excretion of ammonia from fungal cells, which contributes to host tissue damage and further inhibition of the host immune system by repressing phagocyte function. Fungal cell access to the bloodstream is facilitated by damage to lung tissue allowing distribution of fungal cells throughout the body, which become trapped in small capillary beds. Urease-dependent ammonia secretion then causes damage to capillary epithelial cells, enabling fungal cells to traverse from the bloodstream into organs where continued urease activity promotes infection.
Fig. 1. Model of urease function during fungal infection via the lungs. 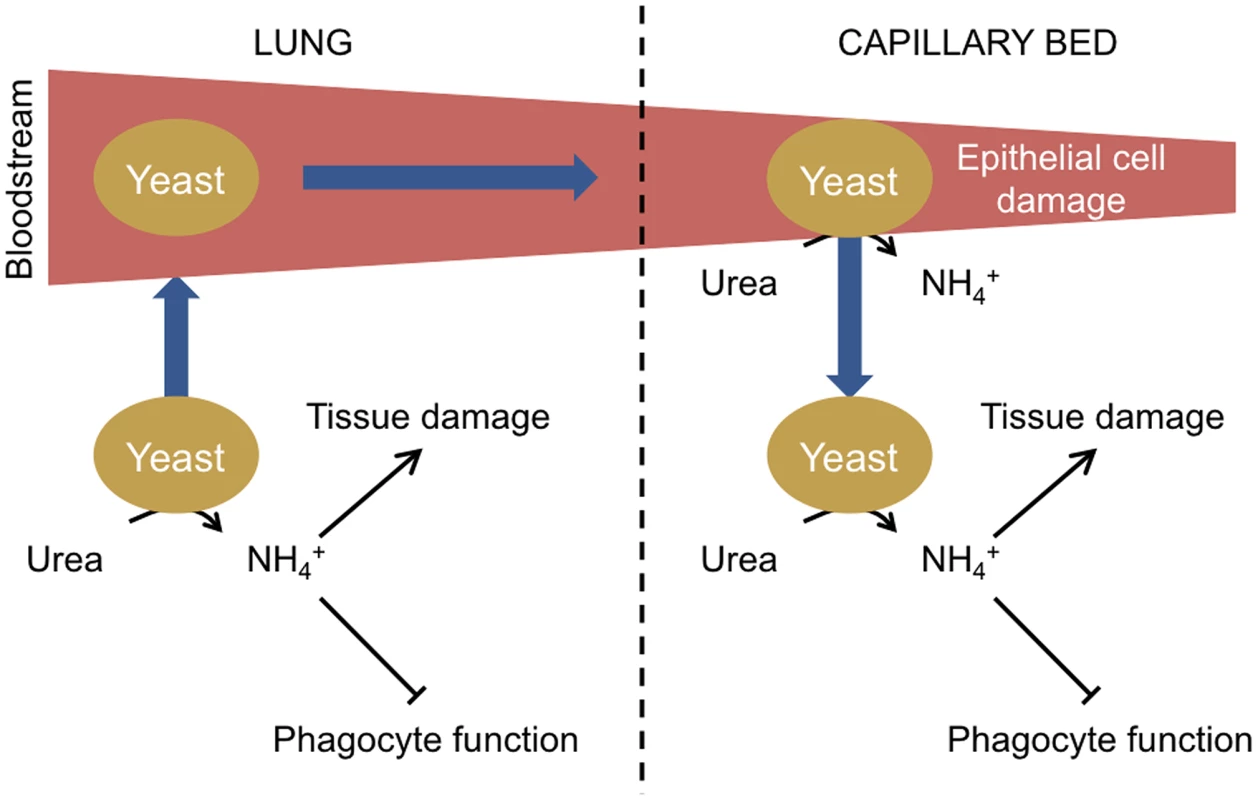
Following the inhalation of spores, yeasts germinate in the lungs. If the initial infection is not cleared due to a defective host immune response, yeast cells proliferate. Urea is present in the epithelial lining fluid of the lungs, and the urease-dependent secretion of ammonia from yeast cells further inhibits immune function by impairing phagocyte function and contributes to lung tissue damage. Individual yeast cells gain access to the bloodstream in damaged areas and circulate in the body until becoming trapped in small capillary beds. Circulating urea is then metabolised by the trapped yeast, resulting in ammonia secretion and damage to the epithelial cells of the capillary beds, enabling yeast cells to cross and proliferate in host organs. Urease as a Therapeutic Target
Urease was the first protein to be identified as a nickel enzyme [24]. Humans do not contain urease, and no human nickel enzymes are known, making urease a potential therapeutic target. Notably, the maturation of urease is a complex process involving a number of accessory proteins that are involved in the delivery and insertion of nickel into the active site of the enzyme, which contains two nickel atoms bridged by a carbamylated lysine residue. An impressive range of in vivo and in vitro studies of urease maturation in different bacteria, including H. pylori, Sporosarcina pasteurii, and Klebsiella aerogenes, have culminated in the following model of urease activation [25], [26]. Urease initially folds in an apo-form to which a complex of three additional accessory proteins (UreD, UreF, and UreG) binds. UreG is a GTPase that forms a dimer and contains a nickel-binding site at its dimer interface. The accessory protein complex increases exposure of the apo-active site, resulting in carbamylation of the active site lysine and nickel insertion by a process that requires carbon dioxide and GTP hydrolysis. Nickel is delivered to UreG by the nickel-binding chaperone UreE, which acquires the metal from an imported pool of nickel. It is presently not clear if UreE delivers nickel to the accessory protein complex before or after it binds to apo-urease. Once the active enzyme is formed, the accessory protein complex dissociates.
The bacterial mechanism of urease maturation is predicted to occur in fungal cells. Homologues of the bacterial UreDFG proteins (Ure467) and a nickel importer (Nic1) exist in C. neoformans and are essential for full urease activity [22]. It appears that fungi lack a nickel chaperone with the Ure7 GTPase potentially having a dual GTPase/nickel chaperone role, as it contains a histidine-rich nickel-binding domain [22]. As with other metal chaperones, an intriguing question is where in the cell Ure7 initially binds nickel and whether this involves a direct interaction with the Nic1 nickel importer. The complexity of urease maturation therefore suggests that this pathway is a potential target for chemical intervention, in addition to the development of inhibitors of the mature enzyme, including nickel chelators and substrate analogues [27].
Perspectives
The model of urease function during fungal infections is based on studies of two pathogenic fungi and the extrapolation of work with bacterial pathogens. However, nothing is known about the role of this enzyme during infection by other urease-positive fungi that infect the lungs, such as Aspergillus fumigatus, Histoplasma capsulatum, Blastomyces dermatitidis, and Paracoccidioides brasiliensis [28], [29]. It would also be interesting to determine why some but not all dermatophyte species that cause skin infections are urease positive [30]. Future work needs to address whether and to what extent urease is required for the virulence of these organisms, and if it is required, whether this simply relates to the provision of a nitrogen source for the pathogen or involves more complex pH-mediated damage to host cells and immunity. Potentially, such studies will enable similar strategies to be developed to treat a wide range of disparate bacterial and fungal infections.
Zdroje
1. Ronne-EngströmE, CesariniKG, EnbladP, HesselagerG, MarklundN, et al. (2001) Intracerebral microdialysis in neurointensive care: the use of urea as an endogenous reference compound. J Neurosurg 94 : 397–402.
2. TyvoldSS, SolligårdE, LyngO, SteinshamnSL, GunnesS, et al. (2007) Continuous monitoring of the bronchial epithelial lining fluid by microdialysis. Respir Res 8 : 78.
3. WeeksDL, EskandariS, ScottDR, SachsG (2000) A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science 287 : 482–485.
4. MarcusEA, MoshfeghAP, SachsG, ScottDR (2005) The periplasmic alpha-carbonic anhydrase activity of Helicobacter pylori is essential for acid acclimation. J Bacteriol 187 : 729–738.
5. Mollenhauer-RektorschekM, HanauerG, SachsG, MelchersK (2002) Expression of UreI is required for intragastric transit and colonization of gerbil gastric mucosa by Helicobacter pylori. Res Microbiol 153 : 659–666.
6. SkouloubrisS, ThibergeJM, LabigneA, De ReuseH (1998) The Helicobacter pylori UreI protein is not involved in urease activity but is essential for bacterial survival in vivo. Infect Immun 66 : 4517–4521.
7. SmootDT, MobleyHL, ChippendaleGR, LewisonJF, ResauJH (1990) Helicobacter pylori urease activity is toxic to human gastric epithelial cells. Infect Immun 58 : 1992–1994.
8. GriffithDP, MusherDM, ItinC (1976) Urease, the primary cause of infection-induced urinary stones. Invest Urol 13 : 346–350.
9. HedelinH, GrenaboL, PetterssonS (1985) Urease-induced crystallization in synthetic urine. J Urol 133 : 529–532.
10. ParsonsCL, StaufferC, MulhollandSG, GriffithDP (1984) Effect of ammonium on bacterial adherence to bladder transitional epithelium. J Urol 132 : 365–366.
11. MusherDM, GriffithDP, YawnD, RossenRD (1975) Role of urease in pyelonephritis resulting from urinary tract infection with Proteus. J Infect Dis 131 : 177–181.
12. IdnurmA, BahnYS, NielsenK, LinX, FraserJA, et al. (2005) Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat Rev Microbiol 3 : 753–764.
13. NguyenC, BarkerBM, HooverS, NixDE, AmpelNM, et al. (2013) Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev 26 : 505–525 doi:10.1128/CMR.00005-13
14. CoxGM, MukherjeeJ, ColeGT, CasadevallA, PerfectJR (2000) Urease as a virulence factor in experimental cryptococcosis. Infect Immun 68 : 443–448.
15. Mirbod-DonovanF, SchallerR, HungCY, XueJ, ReichardU, et al. (2006) Urease produced by Coccidioides posadasii contributes to the virulence of this respiratory pathogen. Infect Immun 74 : 504–515.
16. OsterholzerJJ, SuranaR, MilamJE, MontanoGT, ChenGH, et al. (2009) Cryptococcal urease promotes the accumulation of immature dendritic cells and a non-protective T2 immune response within the lung. Am J Pathol 174 : 932–943 doi:10.2353/ajpath.2009.080673
17. GordonS (2003) Alternative activation of macrophages. Nat Rev Immunol 3 : 23–35.
18. GordonAH, HartPD, YoungMR (1980) Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature 286 : 79–80.
19. MukaiT, MaedaY, TamuraT, MiyamotoY, MakinoM (2008) CD4+T-cell activation by antigen-presenting cells infected with urease-deficient recombinant Mycobacterium bovis bacillus Calmette-Guérin. FEMS Immunol Med Microbiol 53 : 96–106.
20. SendideK, DeghmaneAE, ReyratJM, TalalA, HmamaZ (2004) Mycobacterium bovis BCG urease attenuates major histocompatibility complex class II trafficking to the macrophage cell surface. Infect Immun 72 : 4200–4209.
21. SchneiderM, MarisonIW, von StockarU (1996) The importance of ammonia in mammalian cell culture. J Biotechnol 46 : 161–185.
22. SinghA, PantingRJ, VarmaA, SaijoT, WaldronKJ, et al. (2013) Factors required for activation of urease as a virulence determinant in Cryptococcus neoformans. MBio 4: e00220–13 doi:10.1128/mBio.00220-13
23. ShiM, LiSS, ZhengC, JonesGJ, KimKS, et al. (2010) Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest 120 : 1683–16793 doi:10.1172/JCI41963
24. DixonNE, GazzolaTC, blakeleyRL, ZermerB (1975) Letter: Jack bean urease (EC 3.5.1.5). A metalloenzyme. A simple biological role for nickel? J Am Chem Soc 97 : 4131–4133.
25. FarrugiaMA, MacomberL, HausingerRP (2013) Biosynthesis of the urease metallocenter. J Biol Chem 288 : 13178–13185 doi:10.1074/jbc.R112.446526
26. FongYH, WongHC, YuenMH, LauPH, ChenYW, et al. (2013) Structure of UreG/UreF/UreH complex reveals how urease accessory proteins facilitate maturation of Helicobacter pylori urease. PLoS Biol 11: e1001678 doi:10.1371/journal.pbio.1001678
27. FollmerC (2010) Ureases as a target for the treatment of gastric and urinary infections. J Clin Pathol 63 : 424–430 doi:10.1136/jcp.2009.072595
28. Cerqueira GC, Arnaud MB, Inglis DO, Skrzypek MS, Binkley G, et al.. (2013) Aspergillus Genome Database. Available: http://www.aspgd.org. Accessed 16 April 2014.
29. RappleyeCA, GoldmanWE (2006) Defining virulence genes in the dimorphic fungi. Annu Rev Microbiol 60 : 281–303.
30. SummerbellRC, RosenthalSA, KaneJ (1988) Rapid method for differentiation of Trichophyton rubrum, Trichophyton mentagrophytes, and related dermatophyte species. J Clin Microbiol 26 : 2279–2282.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge inČlánek Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Surveillance for Emerging Biodiversity Diseases of Wildlife
- The Emerging Role of Urease as a General Microbial Virulence Factor
- PARV4: An Emerging Tetraparvovirus
- Epigenetic Changes Modulate Schistosome Egg Formation and Are a Novel Target for Reducing Transmission of Schistosomiasis
- The Human Adenovirus E4-ORF1 Protein Subverts Discs Large 1 to Mediate Membrane Recruitment and Dysregulation of Phosphatidylinositol 3-Kinase
- A Multifactorial Role for Malaria in Endemic Burkitt's Lymphoma Pathogenesis
- Structural Basis for the Ubiquitin-Linkage Specificity and deISGylating Activity of SARS-CoV Papain-Like Protease
- Cathepsin-L Can Resist Lysis by Human Serum in
- Epstein-Barr Virus Down-Regulates Tumor Suppressor Expression
- BCA2/Rabring7 Targets HIV-1 Gag for Lysosomal Degradation in a Tetherin-Independent Manner
- The Evolutionarily Conserved Mediator Subunit MDT-15/MED15 Links Protective Innate Immune Responses and Xenobiotic Detoxification
- Suppressor of Cytokine Signaling 4 (SOCS4) Protects against Severe Cytokine Storm and Enhances Viral Clearance during Influenza Infection
- T Cell Inactivation by Poxviral B22 Family Proteins Increases Viral Virulence
- Dynamics of HIV Latency and Reactivation in a Primary CD4+ T Cell Model
- HIV and HCV Activate the Inflammasome in Monocytes and Macrophages via Endosomal Toll-Like Receptors without Induction of Type 1 Interferon
- Virus and Autoantigen-Specific CD4+ T Cells Are Key Effectors in a SCID Mouse Model of EBV-Associated Post-Transplant Lymphoproliferative Disorders
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- Squalene Synthase As a Target for Chagas Disease Therapeutics
- The Contribution of Viral Genotype to Plasma Viral Set-Point in HIV Infection
- Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge in
- Anthrax Lethal Factor as an Immune Target in Humans and Transgenic Mice and the Impact of HLA Polymorphism on CD4 T Cell Immunity
- Ly49C-Dependent Control of MCMV Infection by NK Cells Is -Regulated by MHC Class I Molecules
- Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
- A Large Family of Antivirulence Regulators Modulates the Effects of Transcriptional Activators in Gram-negative Pathogenic Bacteria
- Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response
- Malaria Parasite Infection Compromises Control of Concurrent Systemic Non-typhoidal Infection via IL-10-Mediated Alteration of Myeloid Cell Function
- A Role for in Higher Order Structure and Complement Binding of the Capsule
- Hip1 Modulates Macrophage Responses through Proteolysis of GroEL2
- CD8 T Cells from a Novel T Cell Receptor Transgenic Mouse Induce Liver-Stage Immunity That Can Be Boosted by Blood-Stage Infection in Rodent Malaria
- Phosphorylation of KasB Regulates Virulence and Acid-Fastness in
- HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality
- A Novel Mechanism Inducing Genome Instability in Kaposi's Sarcoma-Associated Herpesvirus Infected Cells
- Structural and Biochemical Characterization Reveals LysGH15 as an Unprecedented “EF-Hand-Like” Calcium-Binding Phage Lysin
- Hepatitis C Virus Cell-Cell Transmission and Resistance to Direct-Acting Antiviral Agents
- Different Modes of Retrovirus Restriction by Human APOBEC3A and APOBEC3G
- TNFα and IFNγ but Not Perforin Are Critical for CD8 T Cell-Mediated Protection against Pulmonary Infection
- Large Scale RNAi Reveals the Requirement of Nuclear Envelope Breakdown for Nuclear Import of Human Papillomaviruses
- The Cytoplasmic Domain of Varicella-Zoster Virus Glycoprotein H Regulates Syncytia Formation and Skin Pathogenesis
- A New Class of Multimerization Selective Inhibitors of HIV-1 Integrase
- Are We There Yet? The Smallpox Research Agenda Using Variola Virus
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
- Dynamic Functional Modulation of CD4 T Cell Recall Responses Is Dependent on the Inflammatory Environment of the Secondary Stimulus
- Bacterial Superantigens Promote Acute Nasopharyngeal Infection by in a Human MHC Class II-Dependent Manner
- Follicular Helper T Cells Promote Liver Pathology in Mice during Infection
- A Nasal Epithelial Receptor for WTA Governs Adhesion to Epithelial Cells and Modulates Nasal Colonization
- Unexpected Role for IL-17 in Protective Immunity against Hypervirulent HN878 Infection
- Human Cytomegalovirus Fcγ Binding Proteins gp34 and gp68 Antagonize Fcγ Receptors I, II and III
- Expansion of Murine Gammaherpesvirus Latently Infected B Cells Requires T Follicular Help
- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Molecular Signatures of Hemagglutinin Stem-Directed Heterosubtypic Human Neutralizing Antibodies against Influenza A Viruses
- The Downregulation of GFI1 by the EZH2-NDY1/KDM2B-JARID2 Axis and by Human Cytomegalovirus (HCMV) Associated Factors Allows the Activation of the HCMV Major IE Promoter and the Transition to Productive Infection
- Inactivation of Fructose-1,6-Bisphosphate Aldolase Prevents Optimal Co-catabolism of Glycolytic and Gluconeogenic Carbon Substrates in
- New Insights into Rotavirus Entry Machinery: Stabilization of Rotavirus Spike Conformation Is Independent of Trypsin Cleavage
- Prophenoloxidase Activation Is Required for Survival to Microbial Infections in
- SslE Elicits Functional Antibodies That Impair Mucinase Activity and Colonization by Both Intestinal and Extraintestinal Strains
- Timed Action of IL-27 Protects from Immunopathology while Preserving Defense in Influenza
- HIV-1 Envelope gp41 Broadly Neutralizing Antibodies: Hurdles for Vaccine Development
- The PhoP-Dependent ncRNA Mcr7 Modulates the TAT Secretion System in
- Cellular Superspreaders: An Epidemiological Perspective on HIV Infection inside the Body
- The Inflammasome Pyrin Contributes to Pertussis Toxin-Induced IL-1β Synthesis, Neutrophil Intravascular Crawling and Autoimmune Encephalomyelitis
- Papillomavirus Genomes Associate with BRD4 to Replicate at Fragile Sites in the Host Genome
- Integrative Functional Genomics of Hepatitis C Virus Infection Identifies Host Dependencies in Complete Viral Replication Cycle
- Co-assembly of Viral Envelope Glycoproteins Regulates Their Polarized Sorting in Neurons
- Targeting Membrane-Bound Viral RNA Synthesis Reveals Potent Inhibition of Diverse Coronaviruses Including the Middle East Respiratory Syndrome Virus
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



