-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
article has not abstract
Published in the journal: Dust Devil: The Life and Times of the Fungus That Causes Valley Fever. PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004762
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004762Summary
article has not abstract
Coccidioides Biology
Coccidioides immitis and C. posadasii are pathogenic, dimorphic, soil-dwelling Ascomycetes in the Onygenales order. On average, both Coccidioides species have 29 Mb haploid genomes, containing approximately 10,000 open reading frames (ORFs) on five chromosomes [1]. Coccidioides’ most recent common ancestor underwent gene family expansions for proteases and keratinases, membrane biology genes, and toxin production, all likely utilized for survival in animal tissues and morphological changes; and a loss of genes associated with degradation of plant tissue, such as tannases, cellulases, and cutinases [1]. Coccidioides and other fungi in the family Onygenaceae are able to degrade keratin and may cause skin disease in humans and animals. Both species of Coccidioides are distantly related to other dimorphic human pathogens, such as Histoplasma (Ajellomyces) capusulatum, in the new family Ajellomycetaceae [2].
Both Coccidioides species have similar biology, with a well-characterized asexual life cycle with distinct saprobic and parasitic stages, and only molecular evidence of a sexual cycle (Fig 1). In the saprobic phase, Coccidioides cycles between mycelial and arthroconidial stages. Arthroconidia are abscised and become airborne by soil disturbance. Inhalation of arthroconidia by a potential host can lead to coccidioidomycosis, commonly known as (San Joaquin) Valley fever. In an infected host, Coccidioides cycles between uninucleate endospores and multinucleate spherules (Fig 1). Differential phenotypes between the species, including temperature sensitivity and salt tolerance, have been described [3] (personal communication, Marc Orbach to B. Barker). No differential disease phenotypes have been investigated, although extreme variation in virulence among strains is documented [4]. The most pathogenic strains can cause fatal disease within eight days with as few as 50 arthroconidia administered intranasally in immunocompetent mice, and some cause much later onset of disease symptoms and death [4–6]. For humans, minimum dosage is not known, but it has been stated that the infectious dose is a single arthroconidium [7]. Both species have been shown to infect a wide variety of mammals, with varying levels of disease [8].
Fig. 1. Life cycle of Coccidioides. 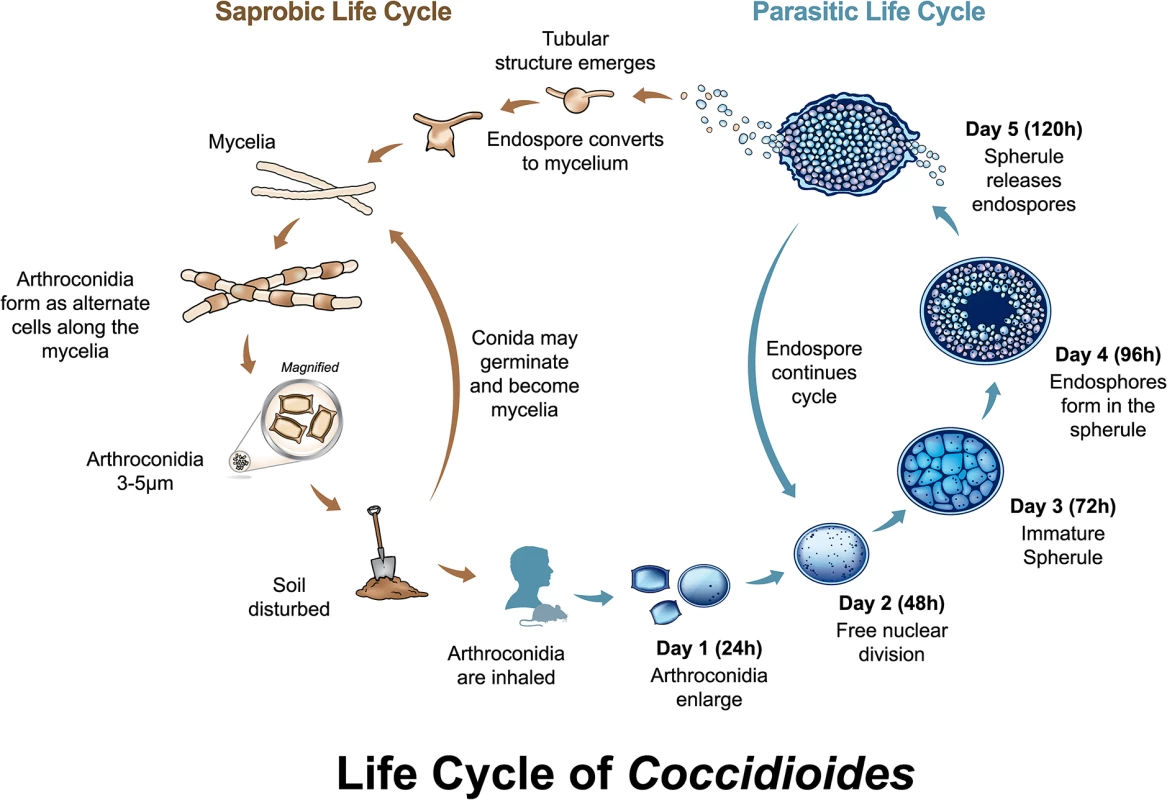
Both Coccidioides species share the same asexual life cycle, switching between saprobic (on left) and parasitic (on right) life stages. The saprobic cycle is found in the environment, and produces the infectious arthroconidia. The conidia may be inhaled by a susceptible host, or may return to the environment to continue the saprobic life cycle. The parasitic life cycle is initiated when arthroconidia enlarge and transform into immature spherules, either in vivo or under specific in vitro conditions. From 24 to 72 hours, spherules undergo free nuclear division and begin developing endospores. From 72 to 120 hours, the mature spherules rupture to release endospores. Each endospore can initiate a new spherule, or, under particular atmospheric conditions, nutrient changes, and/or lower temperature, the endospore can convert to a mycelium and initiate the saprobic phase. This occurs in rare circumstances in the living host [45], but is found most commonly in the environment. Molecular evidence of mating includes identification and characterization of mating type loci, recombination, and distinct gene genealogies [9–11]. In addition to intra-species sexual recombination, population genomics revealed signatures of hybridization and gene introgression between the two species [12]. At this time, no laboratory-controlled genetic recombination or sexual structures have been described.
Coccidioides Ecology and Population Genetics
The recognition that the genus Coccidioides contains two species is a relatively recent discovery [3]. C. immitis is the name of the first species described, with isolates initially being categorized as California and non-California C. immitis. Non-California C. immitis was later found to be another species, C. posadasii [3]. C. immitis is found primarily in the San Joaquin Valley of California. However, recent work has also identified this species in Utah and eastern Washington state [13,14]. Whether the fungus has always been present, or if this reflects a more recent migration, is unknown. C. posadasii is found from Arizona to Texas, and throughout Mexico into Central and South America. Similarity of genotypes from Texas and South America indicates a more recent introduction of C. posadasii into this region [15]. However, a more complete study of patient and environmental isolates is needed before strong conclusions can be drawn [16]. Indeed, the majority of analyses to date have relied on fungal isolates obtained from human patients, which likely does not represent the overall diversity that occurs in nature.
Multiple analyses of many strains from several geographic locations reveal a high degree of diversity, with little to no clonal structure [3,11,17]. The only nearly identical genome sequences are multiple isolations from the same patient, tracking organ transplant from donor to recipient, and a match of a soil isolate to a patient [13,17,18]. In fact, analysis at a single 10-meter square area in Tucson, Arizona, revealed multiple genotypes present in a single environmental location [11]. Genetic diversity supports the idea that the recent increase in human coccidioidomycosis in the endemic regions is not due to an emerging hyper-virulent strain, but rather an overall increase in exposure of susceptible hosts to environmentally occurring arthroconidia [16,17].
The small number of studies focused on identifying the natural host makes it difficult at this time to make a general statement regarding the ecological niche of Coccidioides. As a pathogen infecting mainly mammals, the suggestion has been made that desert rodents, specifically the heteromyids, are the primary host species for Coccidioides [1,11,19]. Many North American soils that test positive for Coccidioides are associated with rodent burrows or rodent activity [11,20,21]. Studies in South America also found Coccidioides associated with armadillos and bats [22,23]. At this time, the natural host and ecology of the organism is not well understood.
Coccidioidomycosis
Coccidioidomycosis is an endemic disease and Coccidioides fungi are biosafety level 3 organisms, just recently removed from the Federal Select Agent list (1995–2013). Based on early studies in which a skin test measured a delayed type hypersensitivity (DTH) to Coccidioides antigen to indicate previous infection, approximately 60% of natural human infections are asymptomatic [20]. In the 1990s, the United States discontinued the DTH skin test for clinical use, but recently it has received new U.S. Food and Drug Administration (FDA) approval (FDA approval letter 07/29/2011). As of yet, a comprehensive study of infection rates in the general population in all endemic areas has not been conducted. The primary clinical presentation of coccidioidomycosis is pneumonia, which generally resolves without treatment. Some hosts will carry lung nodules or cavities of viable Coccidioides; however, incidence and long-term consequences of asymptomatic carriage are unknown. Others suffer chronic disease, sometimes requiring life-long antifungal treatment. Infections disseminate in fewer than 1% of cases, and create lesions at a single body site or potentially affect multiple organ systems. Central nervous system involvement is often fatal if left untreated [20]. Coccidioidomycosis is diagnosed by serology, microscopy, antigen detection, and/or culture, all of which have limitations [24,25].
Cellular immunity, generated by a Th1 response and manifested by DTH, is essential to defense against coccidioidomycosis and long-lived protection from reinfection [26]. Several studies show that response to Coccidioides is more effective in the presence of immune factors such as interferon gamma (IFNγ) released by Th1 cells [27]. Recent data substantiate an essential role for Th17 pathway induction in mice for long-term immunity as well [28]. Patients with chronic disease appear to mount a non-protective Th2-type humoral immune response [27]. Immunodeficiency, either genetic or acquired, is a major risk factor for disseminated disease [29,30].
Many known and unknown factors shape the immune response to Coccidioides and manifestation of disease. For reasons that are not well understood, African Americans and Filipinos appear at a greater risk for disseminated coccidioidomycosis than other ethnicities [27,31]. Underlying health issues and elder age are also known risk factors for more severe disease [20]. Different strains of Coccidioides affect the magnitude of the immediate immune response [27,32]. Few studies have elucidated roles of other important cell types, such as natural killer cells or dendritic cells, which produce IFNγ to activate macrophages and IL-12 to activate T cells. The current data suggest that a complex combination of host immune factors determines the advancement or clearance of infection [26].
Initial Stages of Infection
The largest gap in our knowledge of host immunity is the first five days of the innate response. The initial encounter of inhaled arthroconidia with the host lung is not well understood. It is believed that arthroconidia reach the alveoli where mucociliary clearance factors are encountered. Lung epithelial cells are equipped with pattern recognition receptors (PRRs), such as toll-like receptors (TLRs) and Dectin-1, which are capable of inducing immediate effector responses [33], and these cells influence alveolar macrophage regulation [34]. Yet, the role of epithelial cells in response to Coccidioides inhalation is unknown.
In the lungs, innate immune cells recognize fungal components using multiple receptors, inducing phagocytosis and production of reactive oxygen species (ROS). Resident alveolar macrophages are naturally tolerant to prevent overreaction, with low phagocytic activity and respiratory burst [34]. Upon encounter with arthroconidia, non-fungicidal macrophage engulfment occurs. Within hours, there is a Coccidioides-antigen activated influx of polymorphonuclear neutrophils (PMNs) [35], which may enhance spherule formation [36]. PMNs respond in a similar manner as macrophages, engulfing arthroconidia without killing them [37]. Direct observation implicates failure of phagosome-lysosome fusion, relieving the arthroconidia from contact with lytic enzymes. When activated in vitro with IFNγ or T cells from an immune host, the phagocytes’ ability to kill arthroconidia increases [37,38]. This may be a contributing factor to the success of IFNγ as a therapy for disseminated coccidioidomycosis [39].
Based on in vitro and in vivo observations, arthroconidia enlarge and transform into immature spherules (Fig 1, parasitic life cycle). In addition to the induction of phagocytosis and ROS production, TLR-2 and Dectin-1, when bound by spherules, collaborate to trigger cascades mediated by MyD88 and Card-9 intracellular adaptors [40]. Subsequent activation of transcription factor NFκB produces proinflammatory cytokines TNFα, MIP-2, and IL-6, which are essential effectors of Th1 and Th17 cellular responses [40,41]. Dectin-1 also mediates production of critical Th1 cytokines IL-12 and IFNγ, and Th17 cytokines IL-23, IL-17a, IL-22, and IL-1β [41]. Spherules increase mRNA expression and other factors for evasion of host Dectin-1, as well as resistance to, and suppression of, host oxidative defenses [42].
For the next few days (days two to three), spherules undergo free nuclear division, and mature into large (30 to 80μm) septate cells containing developing endospores. Some literature suggests that growing intracellular spherules lyse their phagocytes, but direct evidence is lacking. Regardless, spherules are probably too large for phagocytosis. Additionally, spherules produce an alkaline extracellular matrix (ECM) that prevents PMN contact and degranulation-induced damage [32,43].
On day four or five, the mature spherules rupture to release endospores, although this process may take longer in vitro. A renewed host response comprises another influx of PMNs. PMNs and macrophages readily engulf the endospores. However, phagocytosis is still impeded as endospores can remain in large clusters tied by fibrillar structures originating from the spherule outer wall [37] and are protected by the spherule ECM [32,43]. From this point, the Coccidioides parasitic cycle continues (Fig 1). At this time, host and/or pathogen mechanisms terminating this cycle in the majority of hosts are unknown.
Future Directions
Coccidioides species and the mycoses they cause have been studied for over a hundred years. With this review, we attempt to summarize some of the more recent findings that have taken place in this field of research. Nevertheless, there is still a dearth of information about these fungal organisms and how they survive within the environment and inside host organisms.
Multiple research aims can greatly advance this research field. Analyzing environmental Coccidioides isolates would increase our understanding of the ecology of these fungi. Further characterizing host and pathogen genetics that influence the course of disease could lead to a better understanding of mycosis and development of immunotherapies for disease. Screening Coccidioides for susceptibility to novel therapeutics, particularly those that are being developed for other fungi, will aid in developing new treatments to eliminate the fungus from the patient to prevent reactivation of disease.
Many of the previous immunological studies employed various in vitro methodologies to answer scientific questions while adhering to the biosafety regulations that governed Coccidioides research at the time. Though these studies provided the vital foundation of our current knowledge of Coccidioides immunology, the answers provided by those studies may not entirely capture in vivo or in situ events. Previous studies that examined potential vaccine candidates in murine models of coccidioidomycosis were also very beneficial to our understanding of coccidioidomycosis and vaccinology [44]; however, our knowledge of a naïve host immune response to infection remains limited. Now that Coccidioides is no longer a Select Agent, and-omic technologies are cheaper and more efficient than ever, the time has come to study the in vivo and in situ host immune response to coccidioidomycosis.
Zdroje
1. Sharpton TJ, Stajich JE, Rounsley SD, Gardner MJ, Wortman JR, Jordar VS, et al. (2009) Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Research 19 : 1722–1731. doi: 10.1101/gr.087551.108 19717792
2. Untereiner WA, Scott JA, Naveau FA, Sigler L, Bachewich J, Angus A. (2004) The Ajellomycetaceae, a new family of vertebrate-associated Onygenales. Mycologia 96 : 812–821. 21148901
3. Fisher MC, Koenig GL, White TJ, Taylor JW (2002) Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 94 : 73–84. 21156479
4. Friedman L, Smith CE, Gordon LE (1955) The assay of virulence of Coccidioides in white mice. The Journal of Infectious Diseases 97 : 311–316. 13286489
5. Muhammed M, Feldmesser M, Shubitz LF, Lionakis MS, Sil A, Wang Y, et al. (2012) Mouse models for the study of fungal pneumonia: a collection of detailed experimental protocols for the study of Coccidioides, Cryptococcus, Fusarium, Histoplasma and combined infection due to Aspergillus-Rhizopus. Virulence 3 : 329–338. doi: 10.4161/viru.20142 22546902
6. Shubitz L, Peng T, Perrill R, Simons J, Orsborn K, Galgiani JN. (2002) Protection of mice against Coccidioides immitis intranasal infection by vaccination with recombinant antigen 2/PRA. Infection and Immunity 70 : 3287–3289. 12011027
7. Nicas M, Hubbard A (2002) A risk analysis for airborne pathogens with low infectious doses: application to respirator selection against Coccidioides immitis spores. Risk Analysis 22 : 1153–1163. 12530785
8. Shubitz LF (2007) Comparative aspects of coccidioidomycosis in animals and humans. Annals of the New York Academy of Sciences 1111 : 395–403. 17332082
9. Burt A, Dechairo BM, Koenig GL, Carter DA, White TJ, Taylor JW. (1997) Molecular markers reveal differentiation among isolates of Coccidioides immitis from California, Arizona and Texas. Molecular Ecology 6 : 781–786. 9262014
10. Koufopanou V, Burt A, Taylor JW (1997) Concordance of gene genealogies reveals reproductive isolation in the pathogenic fungus Coccidioides immitis. Proceedings of the National Academy of Science 94 : 5478–5482. 9144263
11. Barker BM, Tabor JA, Shubitz LF, Perrill R, Orbach MJ (2012) Detection and phylogenetic analysis of Coccidioides posadasii in Arizona soil samples. Fungal Ecology 5 : 163–176.
12. Neafsey DE, Barker BM, Sharpton TJ, Stajich JE, Park DJ, Whiston E, et al. (2010) Population genomic sequencing of Coccidioides fungi reveals recent hybridization and transposon control. Genome Research 20 : 938–946. doi: 10.1101/gr.103911.109 20516208
13. Litvintseva AP, Marsden-Haug N, Hurst S, Hill H, Gade L, Driebe EM, et al. (2014) Valley fever: Finding new places for an old disease: Coccidioides immitis found in Washington state soil associated with recent human infection. Clinical Infectious Disease 60(1) e1–e3.
14. Johnson SM, Carlson EL, Fisher FS, Pappagianis D (2014) Demonstration of Coccidioides immitis and Coccidioides posadasii DNA in soil samples collected from Dinosaur National Monument, Utah. Medical Mycology 52 : 610–617. doi: 10.1093/mmy/myu004 24847036
15. Fisher MC, Koenig GL, White TJ, San-Blas G, Negroni R, Alvarez IG, et al. (2001) Biogeographic range expansion into South America by Coccidioides immitis mirrors New World patterns of human migration. Proceedings of the National Academy of Science 98 : 4558–4562. 11287648
16. Barker BM, Jewell KA, Kroken S, Orbach MJ (2007) The population biology of Coccidioides: epidemiologic implications for disease outbreaks. Annals of the New York Academy of Sciences 1111 : 147–163. 17344537
17. Jewell K, Cheshier R, Cage GD (2008) Genetic diversity among clinical Coccidioides spp. isolates in Arizona. Medical Mycology 46 : 449–455. doi: 10.1080/13693780801961337 18608919
18. Engelthaler DM, Chiller T, Schupp JA, Colvin J, Beckstrom-Sternberg SM, Driebe EM, et al. (2011) Next-generation sequencing of Coccidioides immitis isolated during cluster investigation. Emerging Infectious Disease 17 : 227–232. doi: 10.3201/eid1702.100620 21291593
19. Catalán-Dibene J, Johnson SM, Eaton R, Romero-Olivares AL, Baptista-Rosas RC, Pappagianis D, et al. (2014) Detection of coccidioidal antibodies in serum of a small rodent community in Baja California, Mexico. Fungal Biology 118 : 330–339. doi: 10.1016/j.funbio.2014.01.006 24607357
20. Nguyen C, Barker BM, Hoover S, Nix DE, Ampel NM, Frelinger JA, et al. (2013) Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clinical Microbiology Reviews 26 : 505–525. doi: 10.1128/CMR.00005-13 23824371
21. Baptista-Rosas RC, Catalán-Dibene J, Romero-Olivares AL, Hinojosa A, Cavazos T, Riquelme M (2012) Molecular detection of Coccidioides spp. from environmental samples in Baja California: linking Valley Fever to soil and climate conditions. Fungal Ecology 5 : 177–190.
22. Brillhante RSN, Moreira RE Filho, Rocha MFG, Castelo-Branco DdSCM, Fechine MA, Lima RA, et al. (2012) Coccidioidomycosis in armadillo hunters from the state of Ceará, Brazil. Memórias do Instituto Oswaldo Cruz 107 : 813–815.
23. Cordeiro R A, Silva KRC, Brilhante RSN, Moura FBP, Duarte NFH, Marques FJF, et al. (2012) Coccidioides posadasii infection in bats, Brazil. Emerging Infectious Disease Journal 18 : 668. doi: 10.3201/eid1804.111641 22469192
24. Blair JE, Mendoza N, Force S, Chang YH, Grys TE (2013) Clinical specificity of the enzyme immunoassay test for coccidioidomycosis varies according to the reason for its performance. Clinical and Vaccine Immunology 20 : 95–98. doi: 10.1128/CVI.00531-12 23155124
25. Kuberski T, Herrig J, Pappagianis D (2010) False-positive IgM serology in coccidioidomycosis. Journal of Clinical Microbiology 48 : 2047–2049. doi: 10.1128/JCM.01843-09 20357210
26. Ampel NM (2007) The complex immunology of human coccidioidomycosis. Annals of the New York Academy of Sciences 1111 : 245–258. 17363432
27. Borchers AT, Gershwin ME (2010) The immune response in coccidioidomycosis. Autoimmunity Reviews 10 : 94–102. doi: 10.1016/j.autrev.2010.08.010 20728582
28. Wang H, LeBert V, Hung CY, Galles K, Saijo S, Lin X, et al. (2014) C-type lectin receptors differentially induce Th17 cells and vaccine immunity to the endemic mycosis of North America. Journal of Immunology 192 : 1107–1119. doi: 10.4049/jimmunol.1302314 24391211
29. Sampaio EP, Hsu AP, Pechacek J, Bax HI, Dias DL, Paulson ML, et al. (2013) Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. Journal of Allergy and Clinical Immunology 131 : 1624–1634. doi: 10.1016/j.jaci.2013.01.052 23541320
30. Vinh DC, Schwartz B, Hsu AP, Miranda DJ, Valdez PA, Fink D, et al. (2011) Interleukin-12 receptor β-1 deficiency predisposing to disseminated coccidioidomycosis. Clininal Infectious Disease 52: e99–e102. doi: 10.1093/cid/ciq215 21258095
31. Wheeler C, Lucas KD, Mohle-Boetani JC (2015) Rates and risk factors for coccidioidomycosis among prison inmates, California, USA, 2011. Emerging Infectious Diseases 21.
32. Frey CL, Drutz DJ (1986) Influence of fungal surface components on the interaction of Coccidioides immitis with polymorphonuclear neutrophils. Journal of Infectious Diseases 153 : 933–943. 3701107
33. Sun WK, Lu X, Li X, Sun QY, Su X, Song Y, et al. (2012) Dectin-1 is inducible and plays a crucial role in Aspergillus-induced innate immune responses in human bronchial epithelial cells. European Journal of Clinical Microbiology and Infectious Diseases 31 : 2755–2764. 22562430
34. Hussell T, Bell TJ (2014) Alveolar macrophages: plasticity in a tissue-specific context. Nature Reviews Immunology 14 : 81–93. doi: 10.1038/nri3600 24445666
35. Galgiani JN, Isenberg RA, Stevens DA (1978) Chemotaxigenic activity of extracts from the mycelial and spherule phases of Coccidioides immitis for human polymorphonuclear leukocytes. Infection and Immunity 21 : 862–865. 711340
36. Galgiani JN, Hayden R, Payne CM (1982) Leukocyte effects on the dimorphism of Coccidioides immitis. Journal of Infectious Diseases 146 : 56–63. 7086205
37. Drutz DJ, Huppert M (1983) Coccidioidomycosis: factors affecting the host-parasite interaction. Journal of Infectious Diseases 147 : 372–390. 6300253
38. Beaman L (1987) Fungicidal activation of murine macrophages by recombinant gamma interferon. Infection and Immunity 55 : 2951–2955. 3119493
39. Nesbit LA, Knox KS, Nguyen CT, Roesch J, Wheat LJ, Johnson SM, et al. (2013) Immunological characterization of bronchoalveolar lavage fluid in patients with acute pulmonary coccidioidomycosis. Journal of Infectious Diseases 208 : 857–863. doi: 10.1093/infdis/jit246 23737603
40. Hung CY, Jiménez-Alzate Mdel P, Gonzalez A, Wüthrich M, Klein BS, Cole GT (2014) Interleukin-1 receptor but not Toll-like receptor 2 is essential for MyD88-dependent Th17 immunity to Coccidioides infection. Infection and Immunity 82 : 2106–2114. doi: 10.1128/IAI.01579-13 24614655
41. Viriyakosol S, Jimenez Mdel P, Gurney MA, Ashbaugh ME, Fierer J (2013) Dectin-1 is required for resistance to coccidioidomycosis in mice. mBio 4: e00597–00512. doi: 10.1128/mBio.00597-12 23386437
42. Whiston E, Zhang Wise H, Sharpton TJ, Jui G, Cole GT, Taylor JW. (2012) Comparative transcriptomics of the saprobic and parasitic growth phases in Coccidioides spp. PloS One 7: e41034. doi: 10.1371/journal.pone.0041034 22911737
43. Wise HZ, Hung CY, Whiston E, Taylor JW, Cole GT (2013) Extracellular ammonia at sites of pulmonary infection with Coccidioides posadasii contributes to severity of the respiratory disease. Microbial Pathogens 59–60 : 19–28.
44. Cole GT, Hung CY, Sanderson SD, Hurtgen BJ, Wuthrich M, Klein BS, et al. (2013) Novel strategies to enhance vaccine immunity against coccidioidomycosis. PLoS Pathogens 9: e1003768. doi: 10.1371/journal.ppat.1003768 24367252
45. Munoz-Hernandez B, Palma-Cortes G, Cabello-Gutierrez C, Martinez-Rivera MA (2014) Parasitic polymorphism of Coccidioides spp. BMC Infectious Diseases 14 : 213. doi: 10.1186/1471-2334-14-213 24750998
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR SelectionČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



