-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
While T cells are required for protection against Mycobacterium tuberculosis infection, attempts to prevent tuberculosis by vaccines designed to elicit memory T cells have only been partially successful. Several vaccine candidates are in clinical trials, but progress has been slow because their ability to prevent disease must be empirically tested. There is little understanding of why certain antigens are targets of protective immunity. We have characterized an immunodominant CD8+ T cell response to the M. tuberculosis antigen TB10.4 (EsxH). CD8+ T cells specific for the TB10.44–11 epitope are primed early during infection and account for 30–50% of lung CD8+ T cells during chronic infection. Now we have used deep sequencing to characterize the TCR repertoire of TB10.44-11-specific CD8+ T cells in the lungs of infected mice. Interestingly, TB10.44-11-specific CD8+ T cells exhibit extreme clonal expansion of certain TCRβ with common structural features, most likely because of affinity selection. Affinity selection of T cells is more important when antigen presentation is limiting. Although the lung contains numerous bacteria during infection, antigen-presentation by infected APC may be limiting, mimicking a “low antigen” state. Thus, even T cells that have the potential to mediate protection may function inefficiently because of suboptimal T cell activation.
Published in the journal: Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection. PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004849
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004849Summary
While T cells are required for protection against Mycobacterium tuberculosis infection, attempts to prevent tuberculosis by vaccines designed to elicit memory T cells have only been partially successful. Several vaccine candidates are in clinical trials, but progress has been slow because their ability to prevent disease must be empirically tested. There is little understanding of why certain antigens are targets of protective immunity. We have characterized an immunodominant CD8+ T cell response to the M. tuberculosis antigen TB10.4 (EsxH). CD8+ T cells specific for the TB10.44–11 epitope are primed early during infection and account for 30–50% of lung CD8+ T cells during chronic infection. Now we have used deep sequencing to characterize the TCR repertoire of TB10.44-11-specific CD8+ T cells in the lungs of infected mice. Interestingly, TB10.44-11-specific CD8+ T cells exhibit extreme clonal expansion of certain TCRβ with common structural features, most likely because of affinity selection. Affinity selection of T cells is more important when antigen presentation is limiting. Although the lung contains numerous bacteria during infection, antigen-presentation by infected APC may be limiting, mimicking a “low antigen” state. Thus, even T cells that have the potential to mediate protection may function inefficiently because of suboptimal T cell activation.
Introduction
The adaptive immune system can generate 1014 unique TCRs, which provides the capacity to recognize an enormous universe of distinct antigens [1–4]. Despite our understanding of the genetic and structural basis for TCR diversity and antigen recognition, it remains challenging to predict the magnitude and diversity of T cell responses. The size of the T cell response to model antigens generally correlates with the abundance of antigen-specific T cells in the naïve repertoire (e.g., precursor frequency) [5–7]. Paradoxically, pathogen-specific T cell responses are often focused on a small number of the available antigenic epitopes and use a narrow TCR repertoire, a phenomenon termed “immunodominance”. Pathogens have numerous strategies to evade host immunity, hindering our ability to determine a priori how T cell diversity relates to antimicrobial immunity. Thus, the relationship between immunodominance and host defense during infection is incompletely understood. For pathogens that rapidly mutate, such as human immunodeficiency virus 1 (HIV-1), a diverse T cell response could benefit the host by efficiently detecting escape mutants, while a biased response could be detrimental. For slowly replicating pathogens that encode numerous antigens, the relation between diversity and protection is less clear.
The M. tuberculosis genome contains hundreds of epitopes that can potentially be recognized by murine and human CD8+ T cells [8]. The CD8+ T cell response against M. tuberculosis focuses on the TB10.4 protein (EsxH; Rv0288) in people as well as experimentally infected animals [8–13]. Following aerosol infection of C57BL/6 mice, 30–50% of the responding CD8+ T cells in the lungs recognize the Kb-restricted epitope TB10.44–11 (amino acid sequence IMYNYPAM), defining it as an immunodominant epitope [14–16]. Immunodominant T cell responses in patients with tuberculosis have been suggested to be both a correlate of protection and a marker of disease progression [17–20]. Elucidating how immunodominance arises and affects resistance to infection is crucial for developing successful vaccines, which usually target a limited number of antigens.
Here, we investigated the origin and protective capacity of immunodominant T cell responses following M. tuberculosis infection in both humans and mice. Extreme TCR bias, the presence of public TCRs, and strong selection of a complementarity determining region 3 (CDR3) β motif were shown by TCR sequencing of sorted tetramer+ cells from the lungs of infected mice. We discovered that TCR bias emerges soon after T cell priming in the lymph node (LN) and becomes more extreme during chronic infection. Cloning TB10.44-11-specific TCRs allowed us to develop retrogenic (Rg) mice to study immunodominant TCRs in vivo. Competition studies using TB10.44-11-specific Rg CD8+ T cells showed that small differences in T cell affinity lead to clonal dominance in vivo. Finally, TB10.44-11-specific CD8+ T cells transferred IFNγ-dependent protection against M. tuberculosis infection that required TAP1-dependent antigen presentation.
Results
Human CD8+ T cell expansions in lung granulomas
Deep sequencing of the TCRβ repertoire was performed on CD8+ T cells purified from 11 lung granulomas and one LN obtained from 5 patients undergoing lung resection for medically non-responsive tuberculosis (see Table 1, S1 Data). The CD8+ T cells in the granulomas were more clonal than the peripheral blood TCRβ repertoire of healthy individuals (Fig 1A and S2A Data). TCRβ expansions were detected in all lung samples (Fig 1B and S3 Data). Abundant clonotypes were detected in anatomically distinct granulomas from the same patient (Fig 1B). There was extensive overlap of the TCR sequences between granulomas from the same patient, although most sequences detected in each granuloma were unique to that lesion (Fig 1C). However, it was the shared sequences that were most abundant. For example, of 217 TCRβs common to all three granulomas from patient #23, 143 were the most abundant in each lesion (the top 10 are shown in Fig 1C and S2B Data). Thus, the same T cells clonotypes were abundant in different lesions from the same subject despite their varied pathology (see Table 1). Some TCRβs appeared to have undergone antigen-selection, as sequences with identical CDR3β amino acid sequences were encoded by distinct recombination events. For example, five distinct DNA recombination events led to the CDR3β sequence ‘CASSVDGGTEAFF’ and two occurred at a high frequency (1.16% and 0.9%, Fig 1D). Thus, CD8+ T cells in human granulomas undergo clonal expansion, and while there is considerable heterogeneity between distinct lesions, the most abundant clonotypes were shared between granulomas. As the antigen-specificity and the HLA restriction of these T cells were unknown, the inferences that we can make are limited. Furthermore, co-infection with HIV, present in four of the five subjects, could potentially confound the analysis, since HIV itself can alter the TCR repertoire of CD8+ T cells. Indeed, we cannot be certain whether these TCRs are specific for Mtb. Therefore, we next turned to an animal model of tuberculosis to address the origin and consequences of clonal CD8+ T cell expansions.
Tab. 1. Clinical characteristics of subjects. 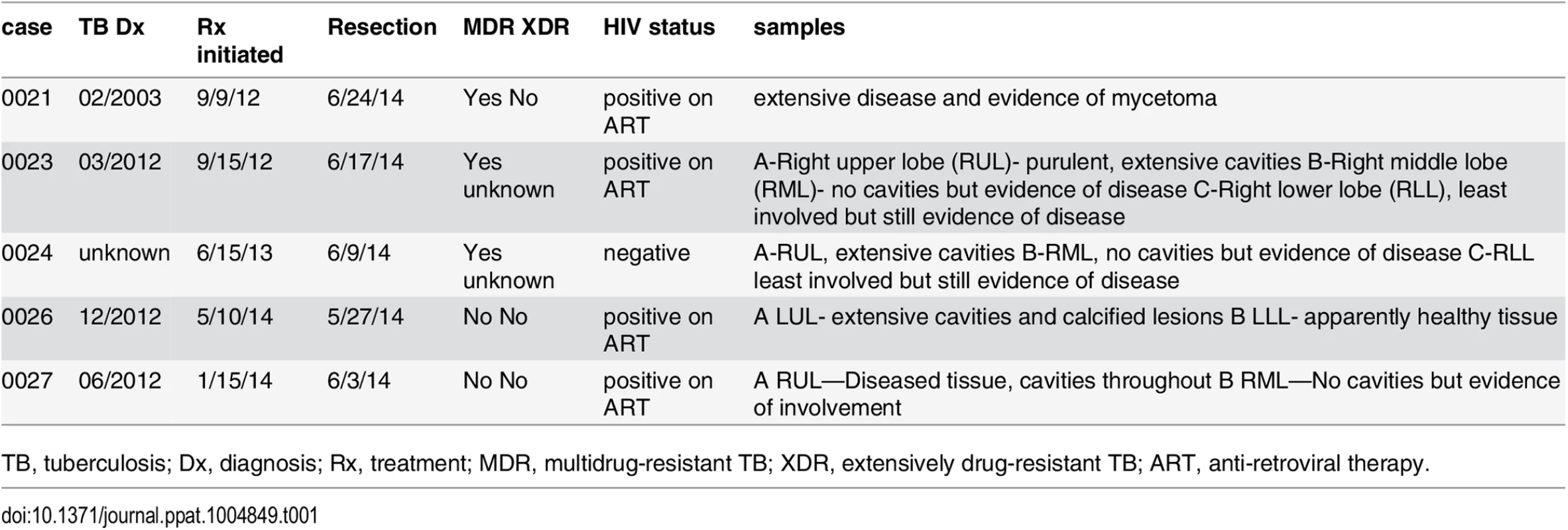
TB, tuberculosis; Dx, diagnosis; Rx, treatment; MDR, multidrug-resistant TB; XDR, extensively drug-resistant TB; ART, anti-retroviral therapy. Fig. 1. Human CD8+ T cells undergo clonal expansions in lung granulomas. 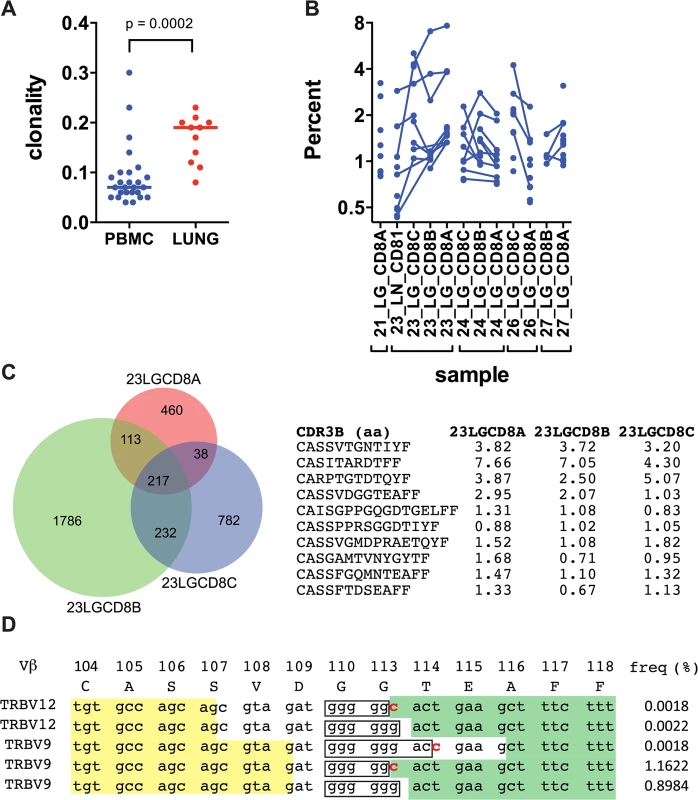
(a) Clonality of CD8+ TCR sequences obtained from pulmonary granulomas (n = 11) compared to PBMC from healthy donors (n = 26); p = 0.0002 by Mann-Whitney. (b) The frequency of the top ten clones in each sample. Clones shared between samples are connected with a line. (c) TCRVβ chains are shared among anatomically distinct granulomas from the same patient. A Venn diagram shows the overlap of unique TCRs from samples 23A, 23B, and 23C. The CDR3β amino acid sequences of the 10 most frequent clones that are shared by the 3 lesions are shown, along with their respective frequency in each lesion. (d) TCR selection suggested by distinct DNA sequences that encode the same CDR3β amino acid sequence. Sample 23LGCD8B contains five distinct recombination events encode the CDR3β amino acid sequence “CASSVDGGTEAFF.” Yellow shading, 3’ Vβ gene sequence; boxed sequence, D nucleotides; green shading, 5’ Jβ gene sequence; bold red letters indicate differences in the sequences. Other differences exist in the Vβ gene sequences. The respective frequency of each sequence is shown. The TB10.4-specific CD8+ T cell response is highly biased
The M. tuberculosis epitope TB10.44–11 elicits an immunodominant CD8+ T cell response in both people and mice [10,21]. To determine whether the number of distinct clonotypes among the responding T cells was limited or diverse, we sorted TB10.44-11-tetramer+CD8+ T cells from the lungs of six individual mice, infected with M. tuberculosis for nine weeks. Deep sequencing showed that the CD8+ T cell response to TB10.44–11 was significantly more clonal than the T cell repertoire from uninfected mice (Fig 2A). The TCRβ repertoire of each infected mouse was dominated by large expansions of two or three clones, although the dominant Vβ gene varied between individuals (Fig 2B). The dominance of TCRβ chains with identical CDR3β sequences indicated that the TB10.44-11-specific CD8+ T cell response was oligoclonal (Fig 2C).
Fig. 2. Clonal expansions of CD8+ T cells specific for the immunodominant antigen TB10.4. 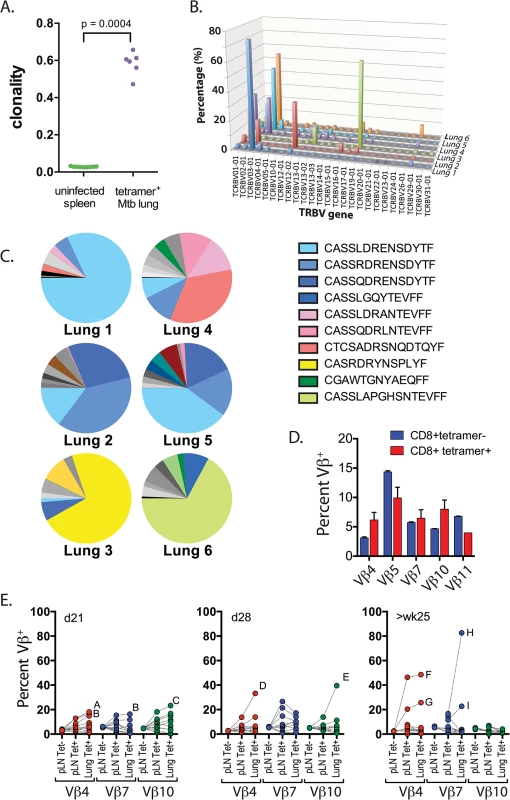
(a) Clonality of CD8+ TCR sequences obtained from infected lung granulomas (n = 6) compared to splenocytes from uninfected mice (n = 9); p = 0.0004 by Mann-Whitney. (b,c) Frequency of TCR Vβ families (b) and CDR3βs (c) of TB10.44-11-specific CD8+ T cells from the lungs of C57BL/6J mice infected with M. tuberculosis for 9 weeks. Data were obtained by Next-generation sequencing of tetramer-purified T cells from 6 individual mice. (d) Frequency of TCR Vβ4 (TRBV2), Vβ5 (TRBV12), Vβ7 (TRBV29), Vβ10 (TRBV4) and Vβ11 (TRBV16) families of TB10.44-11-tetramer+CD8+T (red) or TB10.44-11-tetramer-CD8+T (blue) cells from the pulmonary LN of C57BL/6J mice infected with M. tuberculosis at 21 days post infection. (e) Frequency of TCR Vβ4 (TRBV2), Vβ7 (TRBV29) and Vβ10 (TRBV4) families of CD8+ T cells from the pulmonary LN or lung of C57BL/6J mice infected with M. tuberculosis at 21 days (left panel), 28 days (middle panel) or >25 weeks (right panel) post infection. Dotted lines connect data from individual mice, some of which are labeled (A through I) to highlight mice with biased Vβ family usage. Data were obtained by flow cytometric analysis of TB10.44-11-tetramer+CD8+ T cells (Tet+) or TB10.44-11-tetramer-CD8+ T cells (Tet-) from three independent experiments, each with 4–10 mice per group. To determine whether TCR bias was established during priming in the LN or after T cell trafficking to the lungs, we used monoclonal antibodies (mAbs) specific for a subset of the known Vβ families (see Table 2). All 5 Vβ families were expressed by CD8+ T cells obtained from LNs of mice 21 days after infection, the majority of which are not specific for Mtb (Fig 2D). The distribution of these 5 Vβ families among tetramer+ cells in the LN was similar to the bulk CD8+ T cell population (Fig 2D). Starting on day 21 in the lung, and more dramatically by day 28 in the LN and lung, significant Vβ family bias was detected among TB10.44-11-specific CD8+ T cells. For example, Vβ4 (mouse ‘A’ and ‘B’), Vβ7 (mouse ‘B’), or Vβ10 (mouse ‘C’) dominated the TB10.44-11-specific CD8+ T cell response by day 21 post infection (Fig 2E). The dominant Vβ family was frequently expanded in both the LN and lung, suggesting that TCR bias developed early after T cell priming. Overtime, biases became more dramatic, suggesting ongoing preferential expansion of certain clones. The TB10.44-11-specific CD8+ T cells in one infected mouse (mouse ‘H’) were >80% Vβ7+ after 25 weeks of infection (Fig 2E and 2‘H’). Thus, after an initial priming of a broad repertoire in response to antigen, TCR bias among TB10.44-11-specific CD8+ T cells develops in the draining LN early after T cell priming and becomes established during the chronic phase of infection. The highly oligoclonal response and the dominance of CDR3β amino acid sequences suggest that the immunodominant T cell clonotypes undergo selection during infection.
Tab. 2. Anti-TCR antibodies and their specificity. 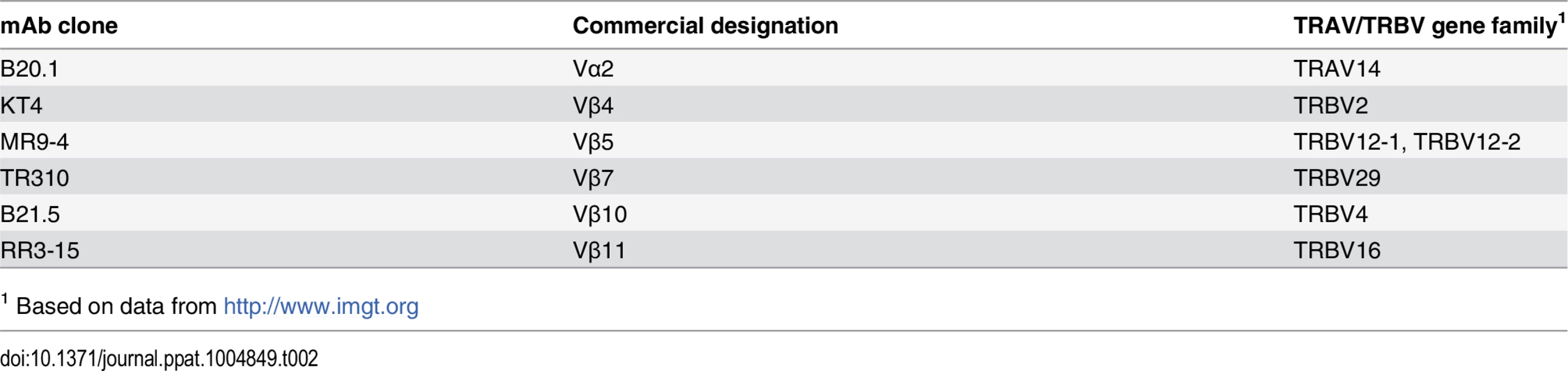
1 Based on data from http://www.imgt.org The paradox of high precursor frequency and extreme TCR bias
Two general mechanisms can explain how TCR bias develops during the TB10.44–11 response: 1) TB10.44-11-specific CD8+ T cells are drawn from a limited pool of naïve precursors; or 2) the naïve TB10.44-11-specific CD8+ T cells pool is diverse but competition leads to selection and bias. To discriminate between these possibilities, we measured the precursor frequency of TB10.44-11-specific CD8+ T cells in uninfected mice.
Using sequential tetramer staining and enrichment of antigen-specific T cells from the naïve repertoire [5,22], we determined that approximately 1 : 13,000 CD8+ T cells were specific for the TB10.44–11 epitope in the C57BL/6 naïve repertoire, or about 857 cells per mouse (Fig 3A and 3B). This precursor frequency is among the highest recorded for antigen-specific CD8+ T cells in the mouse [7].
Fig. 3. Precursor frequency of TB10.4-specific T cells. 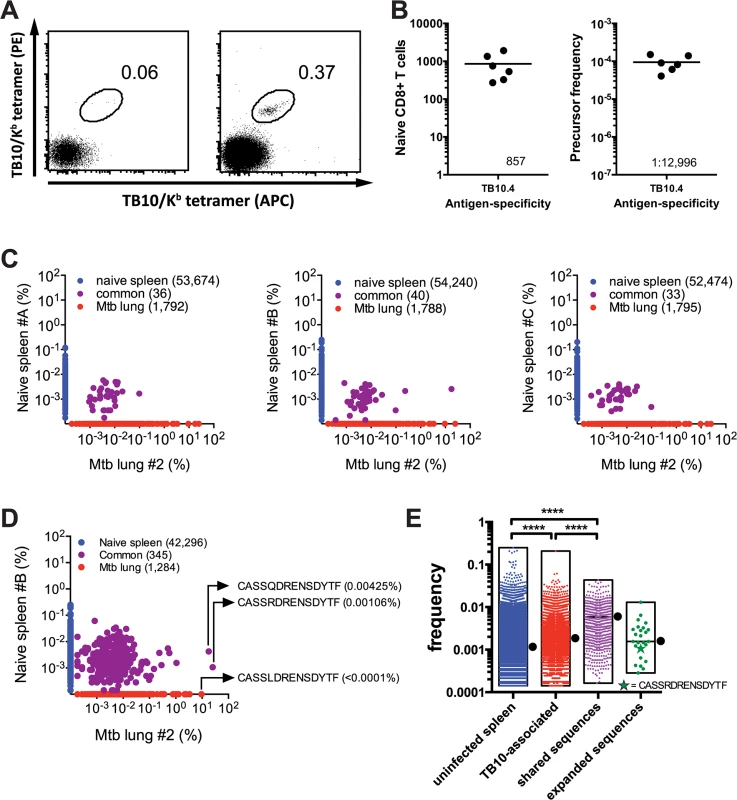
(a,b) Frequency of TB10.44-11-specific CD8+ T cells in naïve C57BL/6J mice. (a) Represented are dot plots of dual tetramer staining in CD8+ T cells in the unbound (left) or bound (right) fractions after immunomagnetic enrichment using tetramers. (b) Quantification of TB10.44-11-specific CD8+ T cells in naïve C57BL/6J mice, either as the total number (left panel) or as the frequency among CD8+ T cells (right panel). Data are representative from two experiments with 6–10 mice. (c,d,e) Frequency of TB10.44-11-associated TCRβ DNA (c) or amino acid (d,e) sequences in the T cell repertoire of uninfected C57BL/6J mice. (c) The unique TB10.44-11-associated TCRβ DNA sequences from lung#2 were compared pairwise to the TCRβ repertoire of three uninfected mice (spleen A-C). The frequency of unique TCRβ DNA sequences from uninfected mice are on the y-axes (blue); from M. tuberculosis infected lung on the x-axes (red); and sequences detected in both samples are colored purple. The number of unique sequences in each class is indicated in parentheses. (d) A pairwise comparison of unique TB10.44-11-associated CDR3β amino acid sequences from lung#2 and uninfected spleen B. (e) The frequency distribution of unique CDR3β amino acid sequences in the uninfected TCR repertoire. ‘Uninfected’ consists of all sequences from uninfected C57BL/6 mice. ‘TB10-specific’ are the TB10-specific sequences found in the uninfected splenic repertoire; ‘shared sequences’ are TB10-specific sequences detected in more than 2 infected mice; ‘expanded sequences’ are all “shared sequences” with frequency >1% in at least one infected mouse. Box represents minimum and maximum, and bar and dot is the median frequency. NS, not significant; ****, p < 0.0001, by one-way ANOVA with Kruskal-Wallis post test. The high frequency of TB10.44-11-specific CD8+ T cell precursors in C57BL/6 mice contrasts with the limited number of unique T cell clonotypes that comprise the post-infection TB10.44-11-specific CD8+ T cell repertoire. To estimate the frequency of TB10.44-11-specific TCRβ clones in the naïve repertoire, TB10.44-11-specific sequences from infected mice were used to interrogate data sets from three uninfected C57BL/6 mice (referred to below as spleen A, B, or C) each containing more than a million TCRβ sequences (Provided by David Hamm, Adaptive Biotechnologies reference data "Mus musculus TCR Beta from spleen", 2014). By performing a pairwise comparison, we identified TB10.44-11-specific TCRβ DNA sequences in the naïve T cell repertoire of uninfected mice (Fig 3C). For example, an abundant TCRβ from the lung of infected mouse #2 (17.8%) was present in spleen B (0.0026%); however, none of the abundant (>0.1%) TCRβs from the lung of infected mouse #2 were present in spleen A or C (Fig 3C). When the translated CDR3β amino acid sequences were used to query the naïve repertoire, the number of matches increased from 40 to 345; and several of the most highly represented CDR3βs were identified (Fig 3D). This raises the possibility that there are multiple DNA recombination events that can generate CDR3β regions capable of recognizing TB10.44–11 in the naïve repertoire. Importantly, the frequencies of TB10.44–11–associated TCRβs in the naïve repertoire were similar to the median frequency determined for the entire naïve T cell population (Fig 3E). For example, the median frequency of the clonotypes detected in naïve spleen #B was 0.0011%. The frequencies of “CASSQDRENSDYTF” and “CASSRDRENSDYTF” in the naïve spleen #B repertoire were 0.00425% and 0.00106%, respectively. Thus, the low frequency of these two CDR3β regions in the naïve TCR repertoire does not predict their massive clonal expansion after infection.
To make this analysis more quantitative, we determined the frequencies of all TB10.44-11-associated CDR3β sequences that could be identified in any of the three uninfected spleens (A, B, or C). Those sequences (“TB10-associated”, Fig 3E) had a slightly higher median frequency than the median of the entire splenic TCR repertoire (“uninfected spleen) (0.001845% vs. 0.001142%, P < 0.0001). We next focused on the subset of “TB10-associated” CDR3βs that were present in at least 2 of the 6 infected lungs, and defined these as “shared sequences” (Fig 3E). The median frequency of the “shared sequences” was 0.003614%, which was increased compared to “uninfected spleen” (P < 0.0001); this is in agreement with previous observations describing a higher frequency of public TCRs in the naïve repertoire [2]. Finally, the CDR3βs that we defined as “expanded sequences” (those with a frequency >1% among CD8+ T cells in the Mtb-infected lung and that account for 75% of the total TB10.44-11-associated sequences) had a frequency similar to “uninfected spleen” (0.001559%, not significant). Thus, TB10.44-11-associated sequences do not appear to be over-represented in the naïve repertoire compared to other sequences.
Interestingly, the individual frequency of highly represented sequences within an individual naive mouse varied by more than 10-fold, raising the possibility that the precursor frequency of individual CDR3β clonotypes could affect their representation in the post-immune repertoire (e.g., after infection). Interestingly, “CASSRDRENSDYTF,” which was one of the more successful CDR3βs as it was commonly detected during Mtb infection, was not present at a greater frequency than other TCRs in the naïve repertoire (Fig 3E).
Although T cells specific for the immunodominant epitope TB10.44–11 have a high precursor frequency in the naïve repertoire of C57BL/6 mice (Fig 3B), extreme TCR bias emerges during the CD8+ T cell response to TB10.44–11 (Fig 2b). While heterogeneity in the frequency of TB10.44-11-specific clonotypes found in the naïve repertoire could account for some of the bias (Fig 3E), the precursor frequency of individual clonotypes in the naïve repertoire does not appear to be the dominant factor influencing representation in the post-infection repertoire. We next evaluated the possibility that clonotypic dominance occurs because of clonal selection during infection.
Evidence for antigen-driven peripheral T cell selection
The CDR3 length of the unique TCRβ sequences from the infected and uninfected mice was similar with a median CDR3β length of 36–39 bases (Fig 4A). In contrast, 56% of the highly represented TCRs (e.g., frequency >1%) had a CDR3β length of 42. Analysis of the amino acid sequences with this CDR3β length (accounting for 36% of all TB10.44-11-specific sequences) revealed a strong consensus motif—“CASSxDReNsdytF” (Fig 4B). This motif was present in clonal expansions if different mice, and several distinct DNA recombination events generate the conserved aspartic acid (Asp, “D”) at CDR3β residue 6 (e.g., V-D recombination, germline encoded, N region addition; Fig 4C). Thus, we conclude that this residue is under strong selection.
Fig. 4. TB10.4-specific CD8+ T cells are selected during infection. 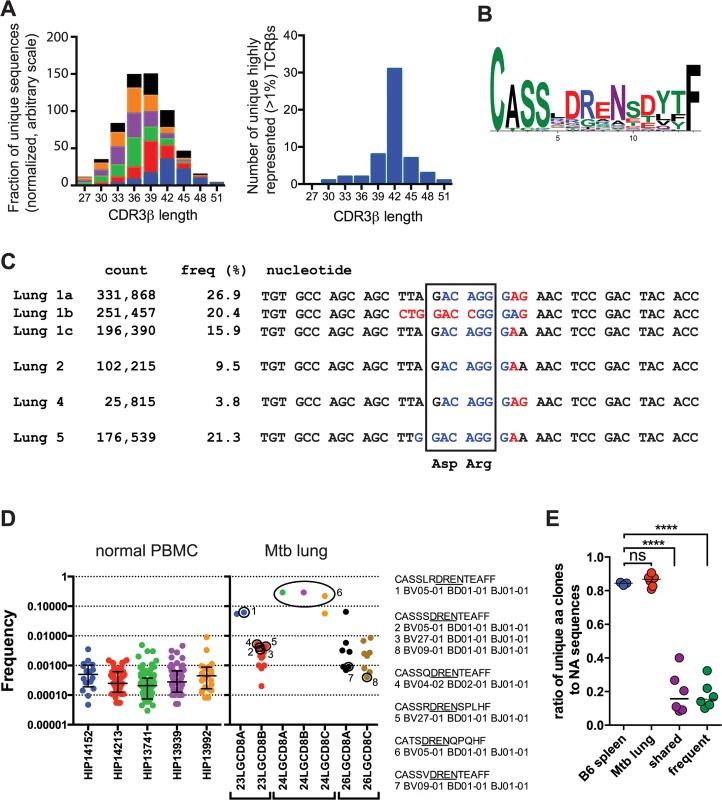
(a) Frequency distribution of CDR3β amino acid length of TB10.44-11-specific CD8+ T cells from the lungs of M. tuberculosis infected C57BL/6J mice. (b) Consensus analysis of the CDR3β amino acid sequence of TB10.44-11-specific CD8+ T cells with 14 amino acids in length. (c) VDJ DNA rearrangements for the public CDR3β CASSLDRENSDYTF found in four different C57BL/6J mice, showing Vβ (black), N (red), Dβ (blue), and Jβ (black) sequences. The count and frequency for each sequence in the respective lung is also shown. The box highlights the nucleotides that encode the conserved aspartic acid (Asp, “D”) and arginine (Arg, “R”) residues. (d) Frequency of human TCRs containing the “DREN” motif among normal PBMC or CD8+ T cells from TB patients. Each point represents a unique clonotype and their corresponding CDR3β amino acid sequences are shown for some. (e) Ratio of unique amino acid clones to nucleotide sequences in T cells from naïve and infected C57BL/6J mice. TCR sequences were analyzed from uninfected ‘B6 spleen’ (n = 3 mice); infected ‘Mtb lung’ (TB10.44-11-specific CD8+ T cells; n = 6 mice); or the following subsets of sequences: ‘frequent’ (>1% of the TB10-specific sequences) or ‘shared sequences’ (TB10.44-11-specific TCRs present in at least 2 mice). ****, p < 0.05 by one-way ANOVA and Holm-Sidak’s multiple comparison test. In addition to the selection of the conserved Asp, the over-representation of certain CDR3β regions in their entirety appeared to be the consequence of selection. In Mouse #1, 63% of the CDR3β sequences encoded the amino acid sequence “CASSLDRENSDYTF.” Remarkably, three distinct VDJ recombination events generated this CDR3β in Mouse #1 (Fig 4C). These data strongly suggest that these TCRs were selected. Not only were the TCRβ expansions extremely biased within each individual mouse, but some abundant sequences shared identical CDR3βs between mice, constituting so called “public” TCRs [2,23] (Fig 4C).
The “DREN” motif was also detected among TCRs from human lung granulomas in three of the five patients, and was expanded in patient #24 (Fig 4D). Two different patients share the motif “CASSxDRENTEAFF,” which is similar to the murine motif (Fig 4D). While the “DREN” motif was detected in the peripheral blood TCRβ repertoire of normal donors, those clonotypes had a 10-fold lower average frequency (Fig 4D and S4 Data). Some clonotypes found in the Mtb granulomas were 1000-fold enriched compared to the average “DREN” frequency in peripheral blood of normal donors (Fig 4D). These data are consistent with selection since distinct recombination events generate different TCRs with the “DREN” motif (Fig 4D). Finally, there is evidence for public TCRs as patients #23 and #26 have clones with the identical CDR3β sequence “CASSSDRENTEAFF”.
The extreme TCR bias (Fig 2C) and the identification of multiple VDJ recombination events encoding identical CDR3βs indicate that dominant T cell clonotypes undergo selective expansion during infection. To quantify selection during the response to M. tuberculosis, we calculated the ratio of unique amino acid sequences to unique nucleic acid sequences. For example, the same immunodominant CDR3β was encoded by three unique DNA sequences in mouse #1. Shared sequences (>2 mice) and abundant sequences (>1%) had lower median values (0.158 and 0.154, respectively) than the bulk population of TB10.44-11-specific CD8+ T cells (0.868) or T cells from uninfected mice (0.845), indicating strong selection at the level of the CDR3β amino acid sequence (p< 0.0001; Fig 4E). Thus, our TCR analysis indicates that clonotypic dominance occurs because of strong selection of certain CDR3β amino acid sequences.
Retrogenic mouse model for TB10.4-specific CD8+ T cells
To study the biology of TB10.44-11-specific CD8+ T cells and to delineate the mechanism(s) responsible for the development of TCR bias during M. tuberculosis infection, we developed retrogenic mice. To ensure that we could track the recombinant TCR-expressing CD8+ T cells, we used Vα2var mice, which have a single Vα gene (Vα2) but can still generate diversity through limited VαJα recombination [24]. Vα2var mice resisted tuberculosis and generated a dominant TB10.44-11-specific CD8+ T cell response, although it was more variable than in C57BL/6 mice (see S5 Data).
Single cell sorting of H2-Kb/TB10.44–11 tetramer+ cells from M. tuberculosis-infected Vα2var mice was followed by single cell PCR to determine the TCRα and TCRβ sequences. Three mice were analyzed and the TB10.44-11-specific CD8+ T cell response in each mouse was dominated by one or two TCR clonotypes (Fig 5A). Each identified dominant TCRβ paired with a single TCRα chain, supporting that these were true clonal expansions. Despite the constrained Vα2, the VαJα recombination site was remarkably diverse. In contrast, there were structural parallels between the CDR3β sequences from Vα2var (Fig 5B) and C57BL/6 mice (Figs 2 and 3). While the dominant TCRβs from the Vα2var mice used distinct Vβs and Jβs, there was enrichment of arginine (“R”) and aspartic acid (“D”) in the CDR3β (Fig 5B and 5C). To determine whether the enrichment of “R” or “D” was significant, we assessed their occurrence in the normal TCRβ repertoire. We queried the splenic TCRβ repertoire from three C57BL/6 mice representing over 1.1 million reads and ~53,000 unique sequences each (Fig 2A). The average frequency of “R”, “D”, or “RD” at CDR3β position 6, 7, or 6–7, was 10.1%, 7.5%, and 2.5% respectively, indicating “R”, “D”, and “RD” were significantly enriched among the clonally expanded TB10.44-11-specific CD8+ T cells (P < 0.0001; see S6 Data).
Fig. 5. A retrogenic mouse model for TB10-specific CD8+ T cells. 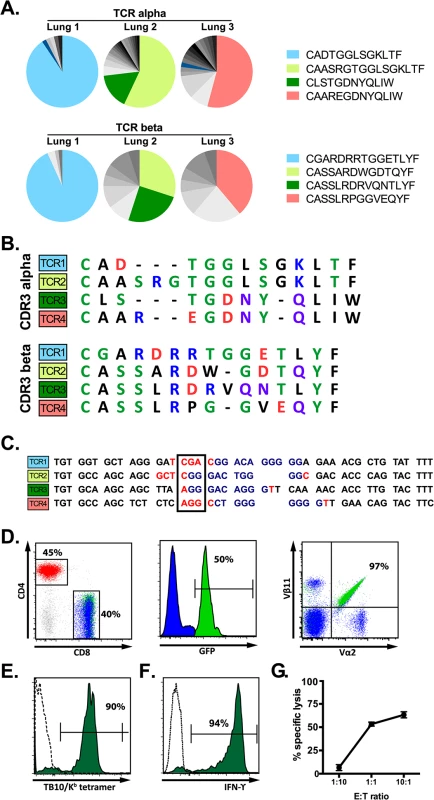
(a) Frequency and amino acid sequences of CDR3α (top panel) and CDR3β (bottom panel) regions of TB10.44-11-specific CD8+ T cells from the lung of individual Vα2var mice infected with M. tuberculosis. Data were obtained by single-cell sorting and sequencing of 60–200 cells per mouse (n = 3). (b) Sequences of CDR3α (top panel) and CDR3β (bottom panel) amino acid sequences of the dominant clones of TB10.44-11-specific CD8+ T cells expanded in the lung of Vα2var mice infected with M. tuberculosis. Dashes are gaps introduced for purpose of sequence alignment. (c) VDJ rearrangement (CDR3β) of the dominant TB10.44-11-specific CD8+ clonotypes from the lung of Vα2var mice infected with M. tuberculosis, displaying Vβ (black), N (red), Dβ (blue), and Jβ (black) sequences. Boxed codon is the conserved arginine (Arg, “R”). (d,e) Flow-cytometry analysis of T cells from uninfected retrogenic mice expressing TCR3. (d) Represented are a dot plot of CD4 vs CD8 staining of CD3+ T cells (left panel), histograms of GFP expression within CD8+ T cells (middle panel), and a dot plot of Vα2 vs. Vβ11 staining in CD8+ T cells (right panel). Red, CD4+ T cells; blue, are colored in CD8+GFP- T cells; green, CD8+GFP+ T cells. The numbers represent: the frequency of CD4+ vs. CD8+ T cells among gated CD3+ T cells (left); the frequency of CD3+CD8+ cells that express GFP (middle); and the frequency of CD3+CD8+GFP+ cells that are Vα2+Vβ11+ (right). (e) Tetramer staining of CD8+GFP- T cells (dashed line) and CD8+GFP+ T cells (filled green histogram). The numbers represent the frequency of CD3+CD8+GFP+ cells that are stained by the tetramer. (f) IFNγ production by lung T cells from TCR3 retrogenic mice infected with M. tuberculosis. Lung cells were stimulated with TB10.44-11-peptide and analyzed by intracellular cytokine staining and flow cytometry. Represented are CD8+GFP+ T cells stimulated by TB10.44-11-peptide (filled green histogram) or left unstimulated (dashed line). The numbers represent the frequency of CD3+CD8+GFP+ cells that produce IFNγ after peptide stimulation. (g) Specific lysis of TB10.44-11-peptide pulsed EL4 targets by TCR3 retrogenic T cells at different effector to target ratios. Data are representative of >10 (d, e), >5 (f), and 3 (g) independent experiments. The four dominant TCRs were cloned into retroviral vectors linked by the 2A sequence allowing the production of four different retrogenic mice (named Rg1 to Rg4, see S10 Data) that expressed greater numbers of CD8+ T cells specific for TB10.44–11 (Fig 5D). We confirmed that the correct TCRs were expressed based on expression of the expected Vα and Vβ chains by CD8+ T cells from the retrogenic mice (Fig 5D). The four TCRs cloned were shown to be specific for TB10.44–11 based on their binding to tetramers and activation of effector functions following stimulation with the TB10.44–11 peptide (Fig 5E–5G).
Priming and acquisition of effector function by naïve TB10.44-11-specific CD8+ T cells
Naïve (CD44loCD62Lhi) Rg3 CD8+ T cells were purified and transferred into congenically marked recipient mice infected with M. tuberculosis. Immediately after transfer (d6 or d7 after infection) very few Rg CD8+ T cells were detected (~100–200 cells per lung) and they were mostly naïve (Fig 6A). Rg CD8+ T cells began to acquire an activated phenotype (CD44hiCD62Llo) starting on d11 in the LN. Associated with their activation, the numbers of Rg3 CD8+ T cells in the LN dramatically increased during days 11–13 post-infection (Fig 6A). Subsequently, these Rg CD8+ T cells began to accumulate in the lung by day 13–15, and the Rg3 CD8+ T cell numbers continued to increase through day 18 (Fig 6A). The increase in cell numbers in the LN and lung correlated with proliferation, occurring first in the LN, then in the lung, and finally in the spleen (Fig 6B). Thus, priming of Rg3 CD8+ T cells tracks the kinetics that has been established for the IFNγ-response in intact mice and activation of transferred transgenic CD4+ T cells [25–28]. Following priming, the Rg3 CD8+ T cells rapidly acquired the ability to produce IFNγ in both the LN and lung (Fig 6C). Thus, Rg3 CD8+ T cells are primed in the LN and are subsequently recruited to the lung, where they express a variety of effector functions including the production of IFNγ, TNF, and granzyme B (see S7 Data), similar to endogenous TB10.44-11-specific CD8+ T cells.
Fig. 6. Retrogenic T cell priming and acquisition of effector functions. 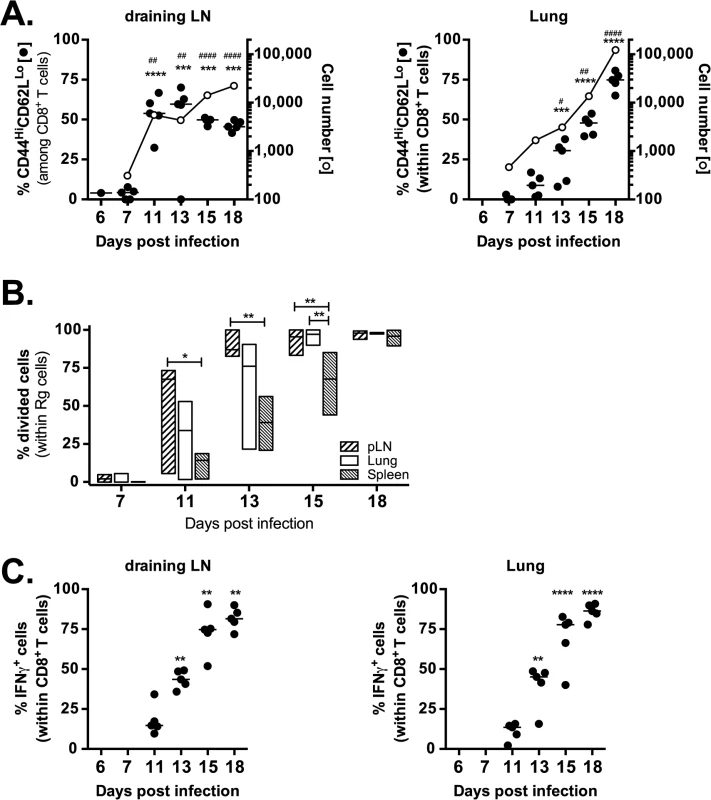
(a) Kinetic analysis of frequency (filled circles) and number (opened circles) of activated (CD44HiCD62LLo) Rg cells in the draining LN (left panel) and lung (right panel) following adoptive transfer into mice infected with M. tuberculosis. (b) Kinetic analysis of frequency of divided Rg cells in the draining LN, lung and spleen following adoptive transfer into mice infected with M. tuberculosis. (c) Kinetic analysis of frequency of IFNγ-producing Rg cells in the draining LN (left panel) and lung (right panel) following adoptive transfer into mice infected with M. tuberculosis. Data are representative from two (b) or three (a, c) independent experiments, each with 5 mice per group. (a,c) One way ANOVA with Dunnett’s post test to compare differences over time (vs. day 7 [a] or d11 [c]) time points. P<0.05 indicated by asterisks (phenotype or IFNγ) or hash marks (cell numbers). (b) One way ANOVA with Tukey’s post test to compare differences in proliferation between lung, LN and spleen; p<0.05 indicated by asterisks. TAP1 and IFNγ are required for protection mediated by CD8+ T cells
It is unknown whether the immunodominant CD8+ T cell response to TB10.4 is protective. Therefore, we activated Rg3 CD8+ T cells in vitro with TB10.44–11 peptide, IL-2 and IL-12 and after 60–72 hours, transferred them into sublethally irradiated mice and infected them with M. tuberculosis. We compared the protective capacity of Rg3 CD8+ T cells with ovalbumin-specific CD8+ T cells (e.g., OT-I cells).
When a large number of activated cells (e.g., 106) were transferred, both Rg3 and OT-I cells transferred considerable protection (Fig 7A). The ability of large numbers of OT-I cells to transfer protection has not been directly investigated, but may be due to their highly activated state and could involve the production of IFNγ, has shown previously for ovalbumin-specific CD4+ T cells [29]. As the number of transferred cells was titrated down, protection mediated by OT-I cells diminished (Fig 7A). In contrast, Rg3 cells continued to mediate significant protection even when as few as 10,000 cells were transferred (Fig 7A). Transfer of Rg4 T cells also conferred protection against M. tuberculosis challenge (see S8A Data).
Fig. 7. TAP1 and IFNγ are required for protection mediated by TB10-specific CD8+ T cells. 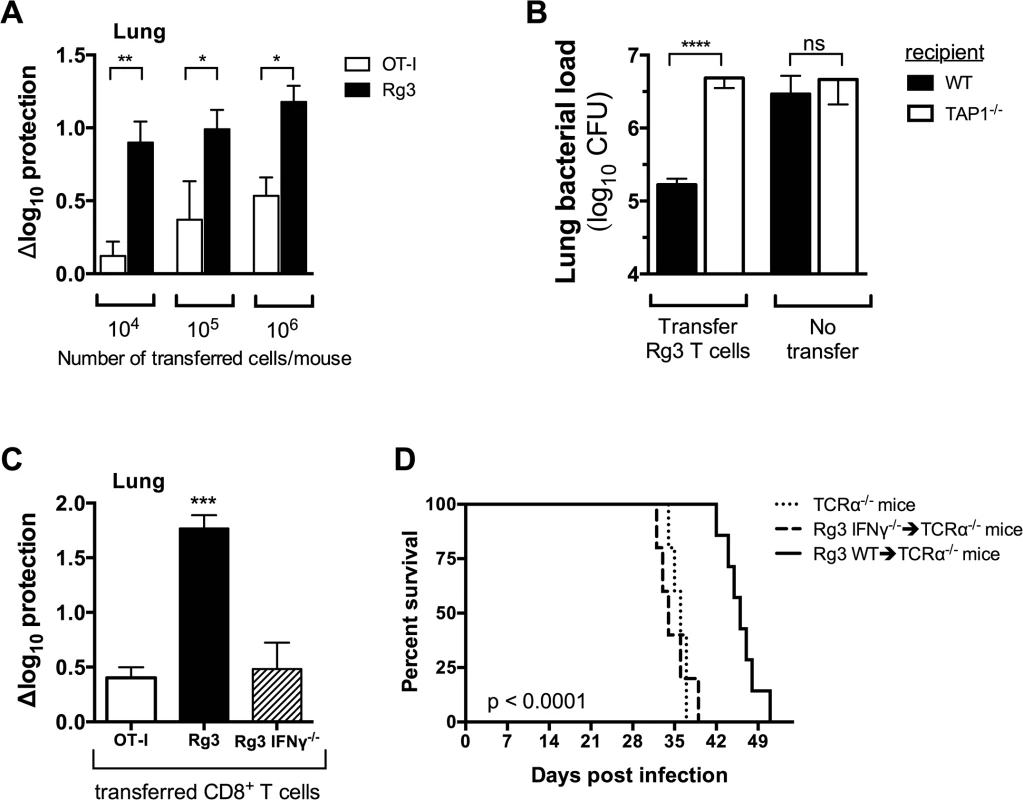
(a,b) Bacterial burden in the lung 21 days after adoptive transfer of in vitro stimulated Rg (a,b) or OT-I (a) CD8+ T cells into sub-lethally irradiated, M. tuberculosis-infected C57BL/6J (a,b) or TAP-1 deficient (b) recipients. (c) Bacterial burden in the lung 21 d after adoptive transfer of in vitro stimulated Rg, IFNγ-/- Rg, or OT-I CD8+ T cells into sub-lethally irradiated, M. tuberculosis-infected C57BL/6J recipients. (d) Survival curves after adoptive transfer of naïve Rg or IFNγ-/- Rg CD8+ T cells into M. tuberculosis-infected TCRα-/- recipients. Transfers used 106 cells/mouse in “b” and “c” or 105 cells/mouse in “d”. Statistical significance calculated using the method of the Log-rank (Mantel-Cox) test. Data are representative from two independent experiments, each with at least 5 mice per group. Other statistical testing done by one-way ANOVA and ad hoc post tests. NS, not significant; *, p < 0.05. To demonstrate that protection required TCR dependent recognition of antigen, we transferred activated Rg3 cells (106 cells/mouse) into sublethally irradiated WT or TAP1-/ - recipient mice. Rg3 effector CD8+ T cells were able to transfer protection to WT but not TAP1-/ - recipients (Fig 7B). As in vitro activation bypasses the need for in vivo priming, these results show that protection mediated by Rg3 CD8+ T cells requires recognition of antigens processed by the TAP-dependent class I MHC antigen-processing pathway.
We next determined which effector functions were required for protection. Rg3 mice were produced in an IFNγ-/- background and IFNγ-/- Rg3 CD8+ T cells compared to WT Rg3 CD8+ T cells for their ability to transfer protection. Under our experimental conditions, activated WT Rg3 CD8+ T cells (106 cells/mouse) transferred significantly more protection than OT-I or IFNγ-/- Rg CD8+ T cells (Fig 7C). These results show that transfer of protection by TB10.4-specific Rg CD8+ T cells requires IFNγ production (Fig 7C). Furthermore, naïve Rg3 CD8+ T cells (105 cells/mouse) prolonged the survival of M. tuberculosis-infected TCRα-/- mice in an IFNγ-dependent manner (Fig 7D). Collectively, these data show that TB10.4-specific CD8+ T cells can control M. tuberculosis infection and IFNγ production after recognition of antigen presented in vivo is required for protection.
Differences in TCR avidity can determine clonotypic dominance during infection
Two of the cloned TCRs (Rg3 and Rg4) differed in their binding to Kb/TB10.44–11 tetramers, revealing a difference in avidity (Fig 8A). No difference in the level of TCR level was detected (see S8B Data). Importantly, when transferred separately into intact mice, both Rg3 and Rg4 were primed, underwent expansion and trafficked to the lung with similar kinetics, and mediated protection (Figs 6 and 7 and see S8 Data). To investigate whether TCR avidity could affect immunodominance, we co-transferred naïve Rg cells expressing either TCR3 or TCR4 at a 1 : 1 ratio into congenic recipients and analyzed their relative abundance following infection. Following co-transfer into M. tuberculosis infected mice, Rg3 and Rg4 T cells initially maintained a 1 : 1 ratio and importantly, both Rg3 and Rg4 T cells were primed in the LN and increased in number (Fig 8B and 8C). However, by day 11 post infection, Rg4 T cells began to outnumber Rg3 T cells, and by day 14, Rg4 T cells outnumbered Rg3 T cells by a ratio of 10 : 1 in the LN (Fig 8B and 8C). In the lung, there was no change in the ratio or cell count until day 14, at which point Rg4 CD8+ T cells accounted for >90% of the Rg CD8+ T cells (Fig 8B). While the absolute number of both Rg3 and Rg4 CD8+ T cells increased in the lung by day 20, Rg4 CD8+ T cells outnumber Rg3 CD8+ T cells by 100 : 1 (Fig 8C). Although both Rg3 and Rg4 were able to expand and confer protection when transferred separately, Rg4 T cells dominated when in competition with Rg3 T cells. When co-transferred, both Rg3 and Rg4 CD8+ T cells were primed in the LN and proliferated; however, small differences in their affinity led to the establishment of clonotypic dominance by Rg4 CD8+ T cells during the T cell response to M. tuberculosis infection.
Fig. 8. Differences in TCR affinity can lead to clonotypic dominance during infection. 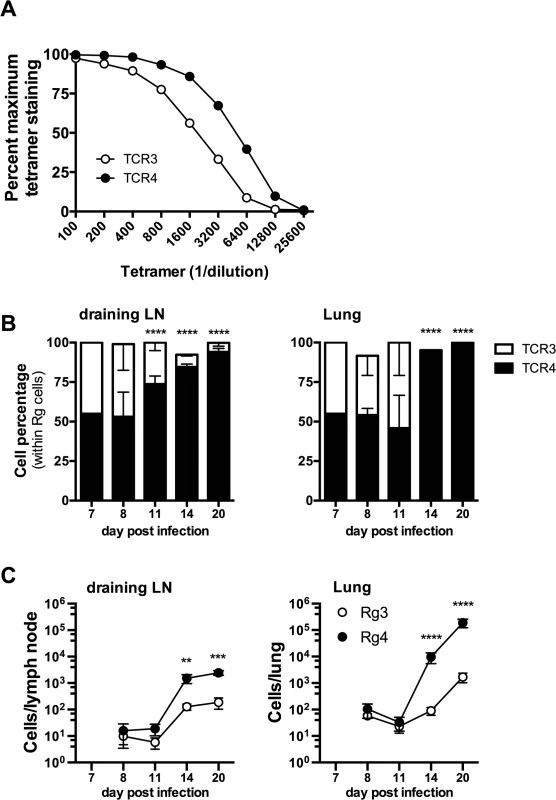
(a) Flow-cytometry analysis of affinity of Rg T cells from uninfected retrogenic mice expressing TCR3 (open circles) or TCR4 (filled circles), based on the frequency of tetramer staining of Rg cells across multiple tetramer concentrations. (b,c) Kinetic analysis of frequency (b) and number (c) of TCR3 (open symbols) or TCR4 (filled symbols) Rg cells in the draining LN (left panels) and lung (right panels) following adoptive co-transfer into mice infected with M. tuberculosis. Data are representative from two (b, c) or three (a) independent experiments, each with 5 mice per group. (b, c) Two way ANOVA with Holm-Sidak’s multiple comparison test; *, p < 0.05). Discussion
We report that extreme TCR bias develops during the polyclonal CD8+ T cell response to a single immunodominant epitope during tuberculosis in humans and in mice. In mice, preferential Vβ use by TB10.44-11-specific CD8+ T cells is detected in the LN within 3 weeks of infection, indicating that bias develops soon after T cell priming. With time, TCR clonality becomes more extreme. Why do a few clonotypes dominate the TB10.44-11-specific CD8+ T cells during M. tuberculosis infection?
TCR diversity arises by three principal mechanisms: 1) V, D, and J segments generate combinatorial diversity; 2) imprecise recombination and insertion of non-templated ‘N’ sequences at the VβD, DJβ and VαJα junctions; and 3) random assortment between TCRα and TCRβ chains [30,31]. By these mechanisms, people have the potential to generate >1014 unique TCRαβ receptors [1,32]. Thymic selection constrains the repertoire by ensuring that T cells that are unable to recognize MHC and the T cells that recognize self-antigens with high affinity are deleted. As a result, people have ~1010 T cells and each clonotype is represented by 10–500 T cells; therefore, the number of unique TCRs in any individual (~2.5 x 107) is far fewer than the number of potential TCRs [2,3]. Although sharing of TCRs between people is improbable, such ‘public’ TCRs are detected and may have special significance [23].
If the number of antigen-specific T cells in the naïve repertoire is limiting, clonotypic dominance could arise by a “founder” effect in which few T cells are primed and expand, leading to T cell populations of restricted diversity. A founder effect is unlikely to explain the TCR bias among TB10.44-11-specific CD8+ T cells because we detect ~900 naïve T cells in each C57BL/6 mouse, a precursor frequency that is among the highest recorded for antigen-specific CD8+ T cells in the mouse [7]. TB10.44-11-tetramer+ CD8+ T cells from the LNs of infected mice use many Vβ families, which also argues against a founder effect. Another possibility is that the frequency of each clonotype in the naïve repertoire is skewed and results in TCR bias after infection. We observe considerable heterogeneity in the relative abundance of naïve precursors with the potential to recognize the TB10.44–11. However, skewing in the naïve repertoire cannot solely explain the selection that we observe for TB10.44-11-specific CD8+ T cells following infection.
After infection, TB10.44-11-specific CD8+ T cells express a limited number of CDR3β sequences. Given the high precursor frequency, we predicted a priori that the response would be dominated by private clonotypes, i.e., TCRs unique to each individual. Instead, common CDR3β motifs are generated by independent VDJ recombination events, within the same mouse and among different individuals. The most frequently used and shared TCRβs are under selective pressure, determined using a modification of Warren’s method [33].
Developing retrogenic mice that over-produce CD8+ T cells specific for TB10.44–11 permitted us to perform competition experiments with naïve TB10.44-11-specific CD8+ T cells. A small difference in affinity significantly affects clonal representation and establishment of hierarchical dominance during infection. These data provide crucial experimental support for the idea of antigen-driven selection based on our TCR analysis. Although TCR affinity seems to be a major factor driving immunodominance, other factors can contribute. For example, the inflammatory environment and tissue-specific cues influence the fate of individual CD8+ T cells during infection [4], and these factors may contribute to the establishment of immunodominant T cell responses during tuberculosis.
Why is TB10.4 an immunodominant antigen? While there is a high precursor frequency, few of these T cell clones are significantly represented in the final immune response. The lack of a correlation between the precursor frequency and immunodominance in C57BL/6 and BALB/c mice [10] indicates that a high precursor frequency is not a prerequisite for immunodominance during chronic infection. TB10.4 belongs to a larger family of ESAT6-related proteins that are secreted by specialized type VII secretion systems, and many of the secreted proteins are immunodominant in different animal species and humans [34–36]. While the abundance of TB10.4 during infection is unknown, paucity of this antigen during T cell priming or limited antigen presentation in the infected lung could drive clonotypic dominance by selecting for higher affinity T cells.
Whether certain mycobacterial antigens that elicit immunodominant T cell responses act as “decoys” to distract the immune response from responding to subdominant epitopes that might be more important targets of protective immunity was raised by Baena and Porcelli [37]. The finding that portions of the mycobacterial genome that encode T cell epitopes was more evolutionarily conserved has fueled this idea and raised the possibility that T cell immunity could benefit M. tuberculosis, possibly by creating an inflammatory environment that facilitates transmission [38]. Work done by the Andersen group finds that cryptic epitopes of ESAT6 are minor components of natural immune response to M. tuberculosis but specific vaccination strategies that elicit CD4+ T cells specific for the subdominant epitopes generate more durable protection against tuberculosis [39–41].
Could TCR diversity (or bias) be a surrogate for the quality or effectiveness of T cell immunity? Spectratyping of peripheral blood T cells from tuberculosis patients reveals TCR skewing compared to healthy controls [20,42,43]. Extreme TCR bias (e.g., clonality) was noted primarily in the setting of severe clinical disease, raising the possibility that TCR bias is associated with disease progression [19,20]. Arguing against this interpretation is the presence of highly skewed TCR repertoires in lung granulomas from patients with latent tuberculosis [17]. In our cohort of patients, we also see the emergence of clonal T cell expansions in the lungs of patients with active disease, compared to the frequency of T cells in the peripheral blood of normal donors. Importantly, clonal expansions can be detected among peripheral blood CD4+ and CD8+ T cell in uninfected healthy individuals, with memory T cells being 50-fold less diverse than naive T cells [33]. While these data indicate that T cell expansions can occur independently of active infection, clonotypes expressing the CDR3β motif “DREN” were 1000-fold enriched in Mtb granulomas compared to their average frequency in the peripheral blood of normal donors. Thus, these expansions are several orders of magnitude greater than the expansions reported in uninfected individuals [33]. This may indicate that the "DREN" expansions are driven by Mtb infection. Furthermore, based on our murine data, we infer that T cell selection, possibly driven by affinity, is occurring. We are confident that we captured the majority of lung TB10.4-specific CD8+ T cells present in each individual mouse. In contrast, we observed considerable heterogeneity in the TCR repertoire obtained from distinct granulomas in each human subject. While this heterogeneity is not surprising based on the work on Flynn and Barry [44,45], it is important to recognize that TCR bias at the level of the granuloma may be driven by heterogeneity in bacteria and bacterial antigens, as well as the persistent immune response. Therefore, the links between TCR bias, functional capacity of T cells and protection during tuberculosis are still unclear, and longitudinal studies coupled with the functional study of antigen-specific T cells are needed to define what constitutes a protective T cell response against tuberculosis in both people and in experimental animal models, both in terms of TCR diversity and T cell function [46].
While antigen choice is a key part of vaccine development, predicting which immunogenic epitopes elicit memory responses that generate protective immunity during infection has not been straightforward. Some pathogens mutate to escape T cell surveillance; other pathogens avoid immune detection by sequestering their antigens. CD4+ and CD8+ T cells specific for TB10.4 are elicited following clinical tuberculosis infection. Based on the ability of TB10.4-specific CD4+ T cells to mediate protection in animal models, the TB10.4 antigen has been incorporated into subunit vaccines. Our data is the first to show that TB10.4-specific CD8+ T cells transfer protection and that protection requires antigen presentation by TAP1-dependent pathways. This implies that the TCR-mediated recognition of infected cells is a prerequisite for the antimicrobial activity of CD8+ T cells. Furthermore, IFNγ is a key mediator of bacterial control.
Why then did a vaccine designed to elicit TB10.4-specific CD8+ T cells fail to protect mice against M. tuberculosis [47]? We chose to measure protection in immunocompromised (e.g., sublethally irradiated) mice, as it is difficult to demonstrate CD8+ T cell mediated protection in mice with an intact CD4+ T cell compartment. Another factor that may impair the ability of TB10.4-specific CD8+ T cells to protect mice with an intact immune system is inefficient presentation of IMYNYPAM. Lindenstrom et al show that vaccination with native TB10.4 protein does not elicit TB10.4-specific CD8+ T cells because the amino acid sequence surrounding the epitope (e.g., IMYNYPAML) is inefficiently processed and presented. In contrast, a homologous protein (TB10.3, EsxR) contains a homologous sequence (e.g., IMYNYPAMM), which is more efficiently processed and presented. Less TB10.3 is produced by the bacterium than TB10.4, and because of this, Lindenstrom speculates that IMYNYPAM is not presented efficiently by infected macrophages. Our own data showing protection (Fig 7) used cells activated in vitro prior to adoptive transfer, bypassing the need for priming. TAP1-dependent protection mediated by these TB10.4-specific CD8+ T cells implies that infected cells in the lung present IMYNYPAM. However, the source of the antigen (e.g., TB10.3 vs. TB10.4) is uncertain. If IMYNYPAM-specific CD8+ T cells recognize the small amount of TB10.3 expressed by infected macrophages, then selecting CD8+ T cells with a high affinity for IMYNYPAM will be even more important for host resistance.
Our study took advantage of the well-characterized CD8+ T cell response to Mtb in mice, where responses to an epitope of TB10.4 elicit an immunodominant T cell response. This allowed us to track and purify antigen-specific CD8+ T cells during infection. We find that the TB10.4-specific CD8+ T cell response is characterized by extreme clonality despite originating from a high-frequency naïve precursor pool. We were able to show that structural features of the CDR3β region were important for epitope recognition, most likely because of clonal competition and affinity selection. Similarly, we found that human CD8+ T cells also undergo selection and clonal expansion. Although the clonal expansions we detected in humans were not as dramatic as in the mouse model, we believe that this is partially because we were unable to purify human antigen-specific CD8+ T cells, due to the lack of appropriate reagents. The ability to use tetramer-sorted cells allows one to analyze T cells that are all specific for a single epitope, which adds considerable power to the TCR analysis. Therefore, it is uncertain whether the extreme immunodominance we observe in the murine system will be found in humans, and this is the subject of active investigation. For example, greater T cell diversity is theoretically expected in humans, due to the contributions of HLA variability, but also empirically, as demonstrated by the large-scale studies to date that have generally found a more diverse T cell response in infected individuals [48,49]. However, this may also be due to technical barriers; while antigen-specific CD8+ T cell expansions are found during tuberculosis, they often appear to be unique to individuals and their private repertoires, which further complicates TCR analysis of antigen-specific T cells [48–50]. If this holds true, it could be difficult to fully exploit TCR analysis as a biomarker for following disease progression or treatment efficacy, although if expanded T cell clones from the lung are correlated with those that are present (and can be detected) in peripheral blood, algorithms might be developed that are independent of antigen-specificity.
Finally, based on the propensity of Mtb to drive clonal selection of CD8+ T cells, we infer that there is a paucity of antigen presentation in the infected lung. Such a state may arise because of inefficient cross-presentation of antigens by the class I MHC-processing pathway and could explain why CD8+ T cells have not proven to be as protective as CD4+ T cells. Similarly, if low levels of antigen presentation rapidly select for high affinity CD8+ T cells during infection, an effective vaccine will need to elicit similarly high affinity T cells, rather than large numbers of diverse T cells, if they are to be effective in controlling bacterial replication.
Materials and Methods
Ethics statement
The University of KwaZulu Natal (UKZN) Biomedical Research Ethics Committee (BREC) approved the study protocol, its associated informed consent documents and data collection tools. Written informed consent was obtained for all research subjects.
All animal experiments were performed in accordance with National and European Commission guidelines for the care and handling of laboratory animals. The studies were approved by the Institutional Animal Care and Use Committee at the Dana Farber Cancer Institute and the University of Massachusetts Medical School (Animal Welfare Assurance no. A3023-01 [DFCI] or A3306-01 [UMMS]), under Public Health Service assurance of Office of Laboratory Animal Welfare guidelines).
Human granuloma T cells
Lung tissue of approximately 3 cm3 was isolated from different areas of resected lungs, corresponding to the most diseased (A), intermediate (B), and healthiest tissue (C), typically corresponding the upper (A), lower (B) and middle lobe (C) (Table 1). The operating surgeon classified the tissue based on their experience and the pre-operative radiological data. Each sample was washed in multiple changes of HBSS and then diced into approximately 1 mm3 pieces, which were strained, re-suspended in 7mls of pre-warmed digestion media (R10 (RPMI supplemented with 10% FCS, 2 mM L-glutamate, 100 U/ml Penstrep), containing 0.5 mg/ml collagenase D (Roche) and 40 U/ml DNaseI (Roche), and transferred to GentleMACS C-tubes (Miltenyi) for mechanical digestion per manufacturers instructions. The resultant suspension was incubated for 60 min at 37 oC, subjected an additional mechanical digestion step. The resulting suspension was strained through a 70 μm cell strainer, washed twice in HBSS, stained and CD8+ T-cells sorted using the FACS ARIA system, gating on the live (nearIR, Invitrogen) singlet, CD45+, CD3+ and CD4- population. Cells were sorted directly into RLT buffer, and genomic DNA extracted using the DNeasy Minikit (Qiagen) as per manufactures instructions.
Mice
C57BL/6 (WT), CD45.1 (B6.SJL-PtprcaPepcb/BoyJ), CD90.1 (B6.PL-Thy1a/CyJ), OT-I (C57BL/6-Tg(TcrαTcrβ)1100Mjb/J), TCRα KO (B6.129S2-Tcrαtm1Mom/J), IFNγ KO (B6.129S7-Ifngtm1Ts/J) and TAP KO (B6.129S2-Tap1tm1Arp/J) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Vα2var mice [24] were bred at Jackson Laboratories (Bar Harbor, ME). Mice were 6 to 10 weeks old at the start of all experiments. Mice infected with M. tuberculosis were housed in a biosafety level 3 facility under specific pathogen-free conditions at DFCI or at UMMS.
Experimental infection and bacterial quantification
M. tuberculosis (Erdman strain) infection was performed via the aerosol route, and mice received 50–200 CFU/mouse. A bacterial aliquot was thawed, sonicated twice for 10 s in a cup horn sonicator, and then diluted in 0.9% NaCl–0.02% Tween 80. A 15 ml suspension of M. tuberculosis was loaded into a nebulizer (MiniHEART nebulizer; Vortran Medical Technologies) and mice were infected using a nose-only exposure unit (Intox Products). Alternatively, the bacterial aliquot was diluted in a final volume of 5ml, and mice were infected using a Glas-Col aerosol-generation device. At different times post-infection, mice were euthanized by carbon dioxide inhalation, organs were aseptically removed, individually homogenized and viable bacteria were enumerated by plating 10-fold serial dilutions of organ homogenates onto 7H11 agar plates. Plates were incubated at 37°C and M. tuberculosis colonies were counted after 21 d.
FACS analysis and cell sorting
Cell suspensions from lung, spleen and LNs were prepared by gentle disruption of the organs through a 70 μm nylon strainer (Fisher) or using the GentleMacs apparatus (Miltenyi Biotec, Germany) according to the manufacturer instructions. For lung preparations, tissue was digested for 30–60 min at 37 °C in 1 mg/mL collagenase (Sigma) prior to straining. Erythrocytes were lysed using a hemolytic solution (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM sodium EDTA pH 7.2) and, after washing, cells were resuspended in supplemented RPMI (10% heat inactivated FCS, 10 mM HEPES, 1 mM sodium pyruvate, 2 mM L-glutamine, 50 mg/ml streptomycin and 50 U/ml penicillin, all from Invitrogen) or MACS buffer (Miltenyi Biotec, Germany). Cells were enumerated in 4% trypan blue on a hemocytometer or using a MACSQuant flow cytometer (Miltenyi Biotec, Germany). Surface staining was performed with antibodies specific for mouse CD3 (clone 17A2), CD3ε (clone 145-2C11) CD4 (clone GK1.5), CD8 (clone 53–6.7), CD19 (clone 6D5), CD44 (clone IM7), CD62L (clone MEL-14), CD45.1 (clone A20), CD45.2 (clone 104), CD90.1 (clone OX-7), CD90.2 (clone 53–2.1), Vα2 (clone B20.1), Vβ4 (clone KT4), Vβ5 (clone MR9-4), Vβ7 (clone TR310), Vβ10 (clone B21.5), Vβ11 (clone RR3-15) (from Biolegend or BD Pharmingen, CA, USA). The specificity of the anti-Vα and-Vβ mAbs are shown in Table 2. The tetramers TB10.44–11-loaded H-2 Kb were obtained from the National Institutes of Health Tetramer Core Facility (Emory University Vaccine Center, Atlanta, GA, USA). All stainings were performed for 20 min on ice and, unless stated, cells were fixed before acquisition with 2% formaldehyde in PBS for 30–60 min. Cell analysis was performed on a FACS Canto (Becton Dickinson, NJ, USA) or on a MACSQuant flow cytometer (Miltenyi Biotec, Germany). Data were analyzed using FlowJo Software (Tree Star, OR, USA). For cell sorting, stained and non-PFA fixed cells were suspended in MACS buffer (Miltenyi Biotec, Germany) and deposited in collection tubes using a BD Canto flow cytometer (Becton Dickinson, NJ, USA). For both FACS analysis and cell sorting, single-lymphocyte events were gated by forward scatter versus height and side scatter for size and granularity.
Next generation sequencing
For TCRβ high-throughput sequencing, genomic DNA was purified from sorted cell populations and sequenced by Adaptive Biotechnologies Corp. (Seattle, WA) using the ImmunoSEQ assay (http://www.immunoseq.com) as previously described[51]. Data were analyzed using the ImmunoSEQ analyser toolset. Clonality is the entropy of the TCRB frequency distribution and is calculated as 1-(entropy/log2[# unique TCRs]). Here entropy, a measure of diversity within a complex data set, is also known as the Shannon-Wiener index, Shannon’s diversity index or Shannon’s entropy [52,53]. Thus “0” represents a diverse repertoire and “1” is a completely clonal repertoire.
Enumeration of naïve Ag-specific T cells
Analysis of the precursor frequency of naïve T cells was performed as previously described [54]. Briefly, the spleen and axillary, mesenteric, cervical, inguinal, popliteal, and salivary LN were harvested from individual mice, dispersed, and filtered through a 70-mm mesh and enumerated for total and CD8+ T cell composition. The cell suspension was then costained with identical PE - and APC-conjugated tetramers and then purified via anti - PE magnetic bead selection (Miltenyi Biotec, Germany). Positive and negative fractions were then surface stained with anti-MHC II, CD11b, CD19, and CD4 as a “dump” channel, and anti-CD8α, CD3 and CD44. Flow cytometry counting beads were added immediately before samples were collected by the cytometer to determine the fraction of tetramer+ events collected and used to determine the total number and frequency of tetramer+ cells in each animal. For analysis purposes, naïve cells were cells that were present in the bound fraction, costained with PE - and APC-conjugated tetramers, and did not express CD44.
Single cell sorting and single cell PCR
Live lymphocytes were sorted as TCRβ+CD8+Tet+CD19-CD4-CD11b-CD11c- as individual cells into wells of 96-well PCR plates containing 10 μl of reverse transcriptase buffer (50 mM Tris-HCl, 75 mM KCl, and 3 mM MgCl2), 2% Triton X-100, 500 μM dNTP with 1 μg BSA, 50 ng of oligo(dT) (12–18), 500 μM dTT, 50 μM of TCRα-specific primer, 50 μM of TCRβ-specific primer, 8 U of RNaseOUT, and 30 U of Moloney murine leukemia virus reverse transcriptase (Invitrogen Life Technologies). The plates were incubated for 90 min at 37°C, then heat inactivated for 20 min at 80°C. 2 μl of the cDNA were used for each of the nested PCRs for TCRα or TCRβ (see S9 Data for list of primers). The first round of each nested PCR amplification was performed by combining 2 μl of cDNA with 9 μl of Taq buffer (50 mM KCl, 10 mM Tris-HCl (pH 8.3), and 2.5 mM MgCl2), 500 μM dNTP, 0.3 U of Taq polymerase, 50 μM of TRACext - or TRBCext-specific primer for the constant region and an oligonucleotide mixture of 23 TRAVext or 19 TRBVext primers (each 50 μM final concentration). For the second round of the nested reaction, 2 μL of the first reaction were combined with 18 μL of Taq buffer (50 mM KCl, 10 mM Tris-HCl (pH 8.3), and 2.5 mM MgCl2), 500 μM dNTP, 0.6 U of Taq polymerase, 50 μM of TRACint - or TRBCint-specific primer for the constant region and an oligonucleotide mixture of 23 TRAVint or 19 TRBVint primers (each 50 μM final concentration). The PCR conditions for the first round of PCR were 94°C for 3 min followed by 35 cycles of 94°C for 20 s, 52°C for 45 s, and 72°C for 60 s, with a final extension at 72°C for 7 min. For the second round of PCR conditions were the same, but only for 26 PCR cycles. Contamination was monitored for all steps (sorting, reverse transcriptase, and PCR), by leaving 16 control wells empty per 96-well PCR plate sorted.
For TCR product sequencing, a total of 12 μl of the products from the second round of the nested PCR amplification was combined with 1.5 μl of 10X shrimp alkaline phosphatase reaction buffer (200 mM Tris-HCl (pH 8.0) and 100 mM MgCl2), 1 U of shrimp alkaline phosphatase (Amersham Biosciences), and 1 U of exonuclease I (New England Biolabs), and water to total 15 μl. The reaction was then heated to 37°C 30 min, 80°C 10 min, and cooled to 4°C, and the product was subjected to automated sequencing (Dana-Farber/Harvard Cancer Center High-Throughput Sequencing Core). The sequences of the four TCRs cloned are shown in S10 Data.
Generation of retrogenic mice
TCR retroviral constructs were generated as 2A-linked single open reading frames using PCR and cloned into a murine stem cell virus-based retroviral vector with a GFP marker as previously described [55,56]. Details of cloning strategies and primer sequences are available upon request (samuel.behar@umassmed.edu). Retroviral-mediated stem cell gene transfer was performed as previously described [55,56].
Intracellular cytokine staining
5×105 cells were plated in each well of a round bottom 96-well plate and incubated in the presence of TB10.44–11 peptide (10 μM; New England Peptide). Incubation in the presence of αCD3/αCD28 (1 μg/mL; BioLegend) or in the absence of stimuli were used as positive and negative controls, respectively. Cells were incubated for 1 h at 37° C, at which point Golgi Stop solution (BD Pharmingen, CA, USA) was added to each well for the remaining 4 h. Cells were collected after the 5 h stimulation and then surface stained with the antibodies described above, followed by intracellular staining for IFNγ (clone) using BD Permwash Kit (BD Pharmingen, CA, USA) as per manufacturer’s instructions.
In vitro CTL assay
In vitro cytotoxicity was determined using peptide - coated EL4 target cells differentially labeled with the cell proliferation dye efluor 450 (eBiosciences) as previously described [21]. Briefly, target cells were pulsed with 10 μM of TB10.4 peptide at 37°C for 1 h in complete medium or left unpulsed (as controls), followed by extensive washing. Target cell populations were then labeled with either 10 μM or 100 nM e450 dye in PBS for 20 min at room temperature, followed by extensive washing. Labeled populations were mixed at an equal cell ratio with effector retrogenic cells at 1 : 1:1 ratio in round bottom 96-well plates (100,000 cells/population, in triplicate). Plates were incubated for 4–12 h at 37°C, in the dark. After incubation, the cells were analyzed by flow cytometry, and the ratios of recovered GFP (retrogenic, effector) and e450-labeled target EL4 populations were determined.
Adoptive T cell transfer for priming and competition
Single cell suspensions of pools of spleens and LNs from naive retrogenic mice (6 to 10 wks post reconstitution) were prepared. CD8+ T cells were purified from each suspension using the CD8+ T cell isolation kit and magnetic separation (Miltenyi Biotec, Germany). After purification, cells were counted and transferred via the tail vein into congenically marked recipients (CD90.1), which had been infected 6–7 d earlier with virulent M. tuberculosis (Erdman) via the aerosol route. For priming experiments, 104 to 105 cells were transferred into each recipient. For competition experiments, cells were mixed at a 1 : 1 ratio (confirmed by FACS analysis prior to injection) and then transferred into each recipient.
Measurement of cell proliferation
For analysis of cell proliferation of retrogenic cells after adoptive transfer, bead-purified naïve Rg cells (see above) were labeled with 10 μM of the cell proliferation dye efluor 450 (eBiosciences) in PBS for 20 min at room temperature, followed by extensive washing.
In vitro activation of T cells
Single cell suspensions of pools of spleens and LNs from naive retrogenic or OT-I mice were prepared. CD8+ T cells were purified from each suspension using the CD8+ T cell isolation kit and magnetic separation (Miltenyi Biotec, Germany). After purification, cells were counted and mixed at a 1 : 1 ratio with peptide coated APCs in media containing 100U/mL of IL-2 and 10U/mL of IL-12. APCs used were red blood cell lysed splenocytes from naïve C57Bl6/j mice, pulsed with 10 μM of TB10.4 or SIINFEKL peptide at 37°C for 1 h in complete medium, followed by irradiation with 3200 Rads from a cesium-137 source and extensive washing. 2x106 cells were plated, 1 mL into each well of a 24 well plate. After 24 h, cells were fed with 1 mL of fresh media, containing 100U/mL of IL-2 and 10U/mL of IL-12. 48 h after the initial stimulation, cells were fed by removing 1 mL of culture media and addition of 1 mL of fresh media, containing 100U/mL of IL-2. 60–72 h after the initial stimulation, cells were extensively washed with complete media, and used for adoptive transfer experiments.
Adoptive T cell transfer for protection and survival
An adoptive transfer model was used to analyze the ability of T cells to mediate protection against pulmonary M. tuberculosis infection as previously described [21]. Briefly, C57BL/6 or TAP-ko mice were sublethally irradiated with 600 rad using a cesium-137 source. The next day, 104 to 106 bead-purified activated Rg T cells (or OT-1 cells, as controls) were transferred via the tail vein. Mice were infected with virulent M. tuberculosis (Erdman) via the aerosol route within 24 h of the adoptive T cell transfer. Three weeks after infection, the bacterial burden in the lung and spleen was determined. For survival experiments, naïve T cells (105 per mouse) were transferred into TCRα KO mice via the tail vein; following transfer, mice were infected with virulent M. tuberculosis (Erdman) via the aerosol route.
Statistical analysis
Population medians were used to compare TCR frequencies. Other data are represented as mean + SEM. For data with a verified for Gaussian distribution, a t-test was performed to compare two groups; otherwise, a Mann-Whitney U test was used. To compare more than 2 groups, one-way ANOVA, followed by Bonferroni post-hoc test was performed. Differences with a p<0.05 were considered significant and represented by *.
Supporting Information
Zdroje
1. Davis MM, Bjorkman PJ (1988) T-cell antigen receptor genes and T-cell recognition [published erratum appears in Nature 1988 Oct 20;335(6192):744]. Nature 334 : 395–402. 3043226
2. Venturi V, Price DA, Douek DC, Davenport MP (2008) The molecular basis for public T-cell responses? Nature Reviews Immunology 8 : 231–238. doi: 10.1038/nri2260 18301425
3. Jenkins MK, Chu HH, McLachlan JB, Moon JJ (2010) On the composition of the preimmune repertoire of T cells specific for Peptide-major histocompatibility complex ligands. Annual Review of Immunology 28 : 275–294. doi: 10.1146/annurev-immunol-030409-101253 20307209
4. Plumlee CR, Sheridan BS, Cicek BB, Lefrancois L (2013) Environmental cues dictate the fate of individual CD8+ T cells responding to infection. Immunity 39 : 347–356. doi: 10.1016/j.immuni.2013.07.014 23932571
5. Obar JJ, Khanna KM, Lefrancois L (2008) Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity 28 : 859–869. doi: 10.1016/j.immuni.2008.04.010 18499487
6. Kotturi MF, Scott I, Wolfe T, Peters B, Sidney J, et al. (2008) Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J Immunol 181 : 2124–2133. 18641351
7. Jenkins MK, Moon JJ (2012) The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol 188 : 4135–4140. doi: 10.4049/jimmunol.1102661 22517866
8. Blythe MJ, Zhang Q, Vaughan K, de CR Jr., Salimi N, et al. (2007) An analysis of the epitope knowledge related to Mycobacteria. ImmunomeRes 3 : 10. 18081934
9. Majlessi L, Rojas MJ, Brodin P, Leclerc C (2003) CD8+-T-cell responses of Mycobacterium-infected mice to a newly identified major histocompatibility complex class I-restricted epitope shared by proteins of the ESAT-6 family. Infection and Immunity 71 : 7173–7177. 14638811
10. Woodworth JS, Shin D, Volman M, Nunes-Alves C, Fortune SM, et al. (2011) Mycobacterium tuberculosis directs immunofocusing of CD8+ T cell responses despite vaccination. J Immunol 186 : 1627–1637. doi: 10.4049/jimmunol.1002911 21178003
11. Hoang TTKT, Nansen A, Roy S, Billeskov R, Aagaard C, et al. (2009) Distinct differences in the expansion and phenotype of TB10.4 specific CD8 and CD4 T cells after infection with Mycobacterium tuberculosis. PLoS ONE 4: e5928. doi: 10.1371/journal.pone.0005928 19529765
12. Axelsson-Robertson R, Weichold F, Sizemore D, Wulf M, Skeiky YAW, et al. (2010) Extensive major histocompatibility complex class I binding promiscuity for Mycobacterium tuberculosis TB10.4 peptides and immune dominance of human leucocyte antigen (HLA)-B*0702 and HLA-B*0801 alleles in TB10.4 CD8 T-cell responses. Immunology 129 : 496–505. doi: 10.1111/j.1365-2567.2009.03201.x 20002212
13. Axelsson-Robertson R, Loxton AG, Walzl G, Ehlers MM, Kock MM, et al. (2013) A broad profile of co-dominant epitopes shapes the peripheral Mycobacterium tuberculosis specific CD8+ T-cell immune response in South African patients with active tuberculosis. PLoS One 8: e58309. doi: 10.1371/journal.pone.0058309 23555576
14. Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM (2010) Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat Immunol 11 : 751–758. doi: 10.1038/ni.1904 20622882
15. Nobrega C, Nunes-Alves C, Cerqueira-Rodrigues B, Roque S, Barreira-Silva P, et al. (2013) T cells home to the thymus and control infection. J Immunol 190 : 1646–1658. doi: 10.4049/jimmunol.1202412 23315077
16. Billeskov R, Vingsbo-Lundberg C, Andersen P, Dietrich J (2007) Induction of CD8 T cells against a novel epitope in TB10.4: correlation with mycobacterial virulence and the presence of a functional region of difference-1. J Immunol 179 : 3973–3981. 17785835
17. Tully G, Kortsik C, Höhn H, Zehbe I, Hitzler WE, et al. (2005) Highly focused T cell responses in latent human pulmonary Mycobacterium tuberculosis infection. J Immunol 174 : 2174–2184. 15699149
18. Du G, Chen CY, Shen Y, Qiu L, Huang D, et al. (2010) TCR repertoire, clonal dominance, and pulmonary trafficking of mycobacterium-specific CD4+ and CD8+ T effector cells in immunity against tuberculosis. The Journal of Immunology 185 : 3940–3947. doi: 10.4049/jimmunol.1001222 20805423
19. Luo W, Su J, Zhang X-B, Yang Z, Zhou M-Q, et al. (2012) Limited T cell receptor repertoire diversity in tuberculosis patients correlates with clinical severity. PLoS ONE 7: e48117. doi: 10.1371/journal.pone.0048117 23110186
20. Jacobsen M, Detjen AK, Mueller H, Gutschmidt A, Leitner S, et al. (2007) Clonal expansion of CD8+ effector T cells in childhood tuberculosis. J Immunol 179 : 1331–1339. 17617626
21. Woodworth JS, Wu Y, Behar SM (2008) Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. JImmunol 181 : 8595–8603. 19050279
22. Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, et al. (2007) Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27 : 203–213. 17707129
23. Li H, Ye C, Ji G, Han J (2012) Determinants of public T cell responses. Cell research 22 : 33–42. doi: 10.1038/cr.2012.1 22212481
24. Correia-Neves M, Waltzinger C, Mathis D, Benoist C (2001) The shaping of the T cell repertoire. Immunity 14 : 21–32. 11163227
25. Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM (2002) Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infection and Immunity 70 : 4501–4509. 12117962
26. Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, et al. (2008) Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J ExpMed 205 : 105–115. 18158321
27. Gallegos AM, Pamer EG, Glickman MS (2008) Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J Exp Med 205 : 2359–2368. doi: 10.1084/jem.20080353 18779346
28. Reiley WW, Calayag MD, Wittmer ST, Huntington JL, Pearl JE, et al. (2008) ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. ProcNatlAcadSciUSA 105 : 10961–10966. doi: 10.1073/pnas.0801496105 18667699
29. Ji H, Liu Y, Zhang Y, Shen XD, Gao F, et al. (2014) T-cell immunoglobulin and mucin domain 4 (TIM-4) signaling in innate immune-mediated liver ischemia-reperfusion injury. Hepatology.
30. Lee J, Su EW, Zhu C, Hainline S, Phuah J, et al. (2011) Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways. Mol Cell Biol 31 : 3963–3974. doi: 10.1128/MCB.05297-11 21807895
31. Li H, Wu K, Tao K, Chen L, Zheng Q, et al. (2012) Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 56 : 1342–1351. doi: 10.1002/hep.25777 22505239
32. Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, et al. (2012) Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis 54 : 784–791. doi: 10.1093/cid/cir951 22267721
33. Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, et al. (2014) Diversity and clonal selection in the human T-cell repertoire. Proceedings of the National Academy of Sciences 111 : 13139–13144. doi: 10.1073/pnas.1409155111 25157137
34. Brodin P, Rosenkrands I, Andersen P, Cole ST, Brosch R (2004) ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol 12 : 500–508. 15488391
35. Majlessi L, Brodin P, Brosch R, Rojas MJ, Khun H, et al. (2005) Influence of ESAT-6 secretion system 1 (RD1) of Mycobacterium tuberculosis on the interaction between mycobacteria and the host immune system. The Journal of Immunology 174 : 3570–3579. 15749894
36. Woodworth JS, Fortune SM, Behar SM (2008) Bacterial protein secretion is required for priming of CD8+ T cells specific for the Mycobacterium tuberculosis antigen CFP10. Infect Immun 76 : 4199–4205. doi: 10.1128/IAI.00307-08 18591224
37. Baena A, Porcelli S (2009) Evasion and subversion of antigen presentation by Mycobacterium tuberculosis. Tissue Antigens 74 : 189–204. doi: 10.1111/j.1399-0039.2009.01301.x 19563525
38. Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, et al. (2010) Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. NatGenet 42 : 498–503. doi: 10.1038/ng.590 20495566
39. Lindenstrom T, Aagaard C, Christensen D, Agger EM, Andersen P (2014) High-frequency vaccine-induced CD8(+) T cells specific for an epitope naturally processed during infection with Mycobacterium tuberculosis do not confer protection. Eur J Immunol 44 : 1699–1709. doi: 10.1002/eji.201344358 24677089
40. Aagaard CS, Hoang TTKT, Vingsbo-Lundberg C, Dietrich J, Andersen P (2009) Quality and vaccine efficacy of CD4+ T cell responses directed to dominant and subdominant epitopes in ESAT-6 from Mycobacterium tuberculosis. J Immunol 183 : 2659–2668. doi: 10.4049/jimmunol.0900947 19620314
41. Woodworth JS, Aagaard CS, Hansen PR, Cassidy JP, Agger EM, et al. (2014) Protective CD4 T cells targeting cryptic epitopes of Mycobacterium tuberculosis resist infection-driven terminal differentiation. J Immunol 192 : 3247–3258. doi: 10.4049/jimmunol.1300283 24574499
42. Yang J, He J, Huang H, Ji Z, Wei L, et al. (2013) Molecular characterization of T cell receptor beta variable in the peripheral blood T cell repertoire in subjects with active tuberculosis or latent tuberculosis infection. BMC Infect Dis 13 : 423. doi: 10.1186/1471-2334-13-423 24010943
43. Yang J, Xu K, Zheng J, Wei L, Fan J, et al. (2013) Limited T cell receptor beta variable repertoire responses to ESAT-6 and CFP-10 in subjects infected with Mycobacterium tuberculosis. Tuberculosis (Edinb) 93 : 529–537. doi: 10.1016/j.tube.2013.05.007 23845455
44. Barry CE, Boshoff HI, Dartois V, Dick T, Ehrt S, et al. (2009) The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 7 : 845–855. doi: 10.1038/nrmicro2236 19855401
45. Lin PL, Rodgers M, Smith L, Bigbee M, Myers A, et al. (2009) Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun 77 : 4631–4642. doi: 10.1128/IAI.00592-09 19620341
46. Nunes-Alves C, Booty MG, Carpenter SM, Jayaraman P, Rothchild AC, et al. (2014) In search of a new paradigm for protective immunity to TB. Nat Rev Microbiol 12 : 289–299. doi: 10.1038/nrmicro3230 24590243
47. Gorman JV, Starbeck-Miller G, Pham NL, Traver GL, Rothman PB, et al. (2014) Tim-3 directly enhances CD8 T cell responses to acute Listeria monocytogenes infection. J Immunol 192 : 3133–3142. doi: 10.4049/jimmunol.1302290 24567532
48. Lewinsohn DA, Winata E, Swarbrick GM, Tanner KE, Cook MS, et al. (2007) Immunodominant Tuberculosis CD8 Antigens Preferentially Restricted by HLA-B. PLoS Pathogens 3: e127–1249. 17677004
49. Lewinsohn DM, Swarbrick GM, Cansler ME, Null MD, Rajaraman V, et al. (2013) Human Mycobacterium tuberculosis CD8 T Cell Antigens/Epitopes Identified by a Proteomic Peptide Library. PLoS ONE 8: e67016. 23805289
50. Lindestam Arlehamn CS, Gerasimova A, Mele F, Henderson R, Swann J, et al. (2013) Memory T Cells in Latent Mycobacterium tuberculosis Infection Are Directed against Three Antigenic Islands and Largely Contained in a CXCR3+CCR6+ Th1 Subset. PLoS Pathogens 9: e1003130. doi: 10.1371/journal.ppat.1003130 23358848
51. Lepore M, Kalinicenko A, Colone A, Paleja B, Singhal A, et al. (2014) Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRbeta repertoire. Nat Commun 5 : 3866. doi: 10.1038/ncomms4866 24832684
52. Stewart JJ, Lee CY, Ibrahim S, Watts P, Shlomchik M, et al. (1997) A Shannon entropy analysis of immunoglobulin and T cell receptor. Mol Immunol 34 : 1067–1082. 9519765
53. Sherwood AM, Emerson RO, Scherer D, Habermann N, Buck K, et al. (2013) Tumor-infiltrating lymphocytes in colorectal tumors display a diversity of T cell receptor sequences that differ from the T cells in adjacent mucosal tissue. Cancer Immunol Immunother 62 : 1453–1461. doi: 10.1007/s00262-013-1446-2 23771160
54. Moon JJ, Chu HH, Hataye J, Pagán AJ, Pepper M, et al. (2009) Tracking epitope-specific T cells. Nat Protoc 4 : 565–581. doi: 10.1038/nprot.2009.9 19373228
55. Holst J, Vignali KM, Burton AR, Vignali DAA (2006) Rapid analysis of T-cell selection in vivo using T cell-receptor retrogenic mice. Nat Methods 3 : 191–197. 16489336
56. Holst J, Szymczak-Workman AL, Vignali KM, Burton AR, Workman CJ, et al. (2006) Generation of T-cell receptor retrogenic mice. Nat Protoc 1 : 406–417. 17406263
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 5- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



