-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
Genome-wide gene expression profiles accumulate at an alarming rate, how to integrate these expression profiles generated by different laboratories to reverse engineer the cellular regulatory network has been a major challenge. To automatically infer gene regulatory pathways from genome-wide mRNA expression profiles before and after genetic perturbations, we introduced a new Bayesian network algorithm: Deletion Mutant Bayesian Network (DM_BN). We applied DM_BN to the expression profiles of 544 yeast single or double deletion mutants of transcription factors, chromatin remodeling machinery components, protein kinases and phosphatases in S. cerevisiae. The network inferred by this method identified causal regulatory and non-causal concurrent interactions among these regulators (genetically perturbed genes) that are strongly supported by the experimental evidence, and generated many new testable hypotheses. Compared to networks reconstructed by routine similarity measures or by alternative Bayesian network algorithms, the network inferred by DM_BN excels in both precision and recall. To facilitate its application in other systems, we packaged the algorithm into a user-friendly analysis tool that can be downloaded at http://www.picb.ac.cn/hanlab/DM_BN.html.
Published in the journal: Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants. PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003757
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003757Summary
Genome-wide gene expression profiles accumulate at an alarming rate, how to integrate these expression profiles generated by different laboratories to reverse engineer the cellular regulatory network has been a major challenge. To automatically infer gene regulatory pathways from genome-wide mRNA expression profiles before and after genetic perturbations, we introduced a new Bayesian network algorithm: Deletion Mutant Bayesian Network (DM_BN). We applied DM_BN to the expression profiles of 544 yeast single or double deletion mutants of transcription factors, chromatin remodeling machinery components, protein kinases and phosphatases in S. cerevisiae. The network inferred by this method identified causal regulatory and non-causal concurrent interactions among these regulators (genetically perturbed genes) that are strongly supported by the experimental evidence, and generated many new testable hypotheses. Compared to networks reconstructed by routine similarity measures or by alternative Bayesian network algorithms, the network inferred by DM_BN excels in both precision and recall. To facilitate its application in other systems, we packaged the algorithm into a user-friendly analysis tool that can be downloaded at http://www.picb.ac.cn/hanlab/DM_BN.html.
Introduction
The complex functions in eukaryotic cells are implemented through a highly organized regulatory network composed of concerted activities of many genes and gene products. Gene expression can be directly regulated by transcription factors (TFs) [1], the states of chromatin structures [2], [3] and small RNAs, and interactions among them [4]–[6]. In other words, the mRNA expression level of a gene is the output synthesized from the information of several input signals.
Gene knockout is a classic approach to studying gene functions and the collection of yeast knockout strains has enabled systematic genome-wide functional analysis [7]. Transcriptional profiles of mutant strains have been used as molecular phenotypes for functional analysis and genetic epistasis analysis [8], [9]. In addition, the expression profiles of single, double and triple deletion mutants of chromatin machinery components, protein kinases and phosphatases were used to analyze the functional overlaps among these proteins [3], [10]. Dion et al. constructed 15 mutants of lysines 5, 8, 12, and 16 to arginine in the histone H4 tail and characterized the resulting genome-wide gene expression changes [11].
Transcriptional regulatory networks in different cellular contexts have been constructed through the DNA microarray analysis of transcription factor deletion mutants and over expression strains in S. cerevisiae [1], [12] by directly linking the genetically perturbed transcription factors (TFs) with the genes that change expression in response to the perturbations. As none of the regulators works alone, probably more important than constructing such regulator-target networks is to understand how the regulators cooperate to form regulatory pathways to specifically regulate a transcriptional program or biological processes [3].
Here we use the transcriptional profiles of deletion mutants as the molecular phenotypes of the mutants to determine how the regulators interact genetically or cooperate functionally with each other to modulate gene expression. We propose a Bayesian network (BN) approach to reverse engineer regulator networks from these gene expression profiles. The approach excels previous methods such as context-dependent regulation and correlation coefficient analysis [12]–[14] in that it can easily integrate different datasets and infer causalities in the regulatory program. Nodes in the network are the genes deleted in the mutants and the algorithm greedily searches over all possible Bayesian network structures for the one that best summarizes the relationships among the global differential expression change profiles upon deleting these genes. Thus by exploring the relationships among the global differential gene expression profiles for the deletion mutant genes, we can obtain valuable causal or non-causal relationships among these regulatory deletion-mutant genes through the inferred BN structure.
Then, we used the above approach to analyze the global differential gene expression profiles of 544 single or double deletion mutants of transcription factors, chromatin machinery components, protein kinases and phosphatases in S. cerevisiae. The BN inferred identified with high precision and recall causal regulatory and non-causal interaction relationships among these regulators in different cellular contexts.
Results
Pair-wise similarity between expression profiles of regulator mutants
The deletion mutants of transcriptional regulators used in this study are nonessential genes in yeast under rich medium growth conditions, yeast extract peptone dextrose medium (YPD) or synthetic complete medium (SC). We compiled expression profiles of sequence-specific DNA binding transcription factors (STFs) deletion strains grown in SC and YPD mediums [12], [15]. We also collected the expression profiles for deletion mutants of protein kinases, phosphatases [10] and chromatin machinery components [3] (See Methods for more detailed data descriptions).
To confirm that regulators belonging to the same protein complex or regulatory pathway tend to share common targets [15], we used Jaccard similarity index (JI) to examine the similarities between targets profiles of the perturbed regulators (see Methods, Figure S1A, Table S1) and we observed that a STF is more likely to connect with another STF than with a general transcription regulator (GTFs e.g. chromatin modifiers and remodelers) whether the regulators are derived from the same data set or different data sets. Indeed, the percentage of known physical interactions or genetic interactions (downloaded form SGD) present among predicted gene pairs increases as the threshold of pair-wise Jaccard similarity index (JI) used in prediction increases (Figure S1B), suggesting that the similarities of gene expression profile changes after genetic perturbation of transcriptional regulators can be used to infer relationships among these regulators. However, JI is only a crude measure that is subject to different cutoffs and cannot infer directionality or causality of regulatory relationships. In contrast, Bayesian network is a solid statistical inference method that can infer directions or causality of regulatory relationships and is more appropriate for this task.
A perturbation based Bayesian network structure learning algorithm
A Bayesian network [16] is a directed probabilistic graphical model which represents conditional independency relationships between variables. The BN learning approach has been extensively used in previous works to analyze gene expression and other high throughput data sets [14], [17], [18]. Suppose that the expression of a deletion mutant gene (denoted by G) is fully determined by its three intermediate regulator genes (denoted by A, B, C), if the expression of genes A, B, C can be controlled precisely, we can find a specific expression configuration of A, B, C (e.g., A is up-regulated and B, C are down-regulated) so that the expression of G is as small as possible just like being deleted. As such, we can anticipate that the global differential gene expression profile of deleting G versus the wild type strain can be well predicted from the global differential expression profiles of deleting B, deleting C and over-expressing A, respectively. Although the datasets contain only genetic deletion strains, no over-expression strains, the global differential expression profile of the profile of over-expressing A is often opposite to that of deleting A, we can thus well predict the differential gene expression pattern of deleting G from the three differential gene expression profiles of deleting genes A, B and C, respectively. In general, if one gene is combinatorially regulated by a set of other genes, usually we can approximate its deletion-mutant differential expression ‘phenotype’ fairly well by the deletion-mutant differential expression ‘phenotypes’ of its regulator genes.
However, in deletion mutant experiments, it is typical that most genes have small expression changes in deletion mutant strains compared to their WT. For instance, 80% yeast genes have similar expressions to the WT strain in protein kinase or phosphatases deletions under the same growth condition [19]. Thus, the differential expression profiles of these regulators are sparse. The majority of ‘neutral’ gene expression changes (represented by ‘0's) in the differential expression profiles will artificially induce a high similarity between the deletion mutant genes (regulators) in classic BN learning methods.
To this end, we developed a new Bayesian network structure-learning algorithm called Deletion Mutant BN (DM_BN) (Figure 1), which is specifically designed for reverse engineering regulatory networks of deletion mutant genes from differential gene expression profiles in the corresponding deletion mutant strains. Note that, the input of this algorithm is a matrix of discrete values: 1, −1, 0, which denote the differential gene expression of the mutant strain versus the WT. Each column of the matrix records the differential gene expression profile for one deletion mutant gene. As described above, the training data for Bayesian network is skewed towards 0, it is not viable to exploit classical Bayesian network learning approaches based on discrete data [20]. Indeed, in our extensive comparison of the proposed DM_BN algorithm with state-of-the-art BN learning algorithms with three other scoring metrics [20]–[23], a well-known software package for BN learning [24] and two widely used non-Bayesian approaches to building regulatory networks [25], [26] on the yeast deletion mutant datasets, the significantly improved network inference quality fully confirmed the advantage of the DM_BN algorithm (See below).
Fig. 1. Overview of the Bayesian network learning algorithm DM_BN for reverse engineering regulatory networks from genetic perturbation data. 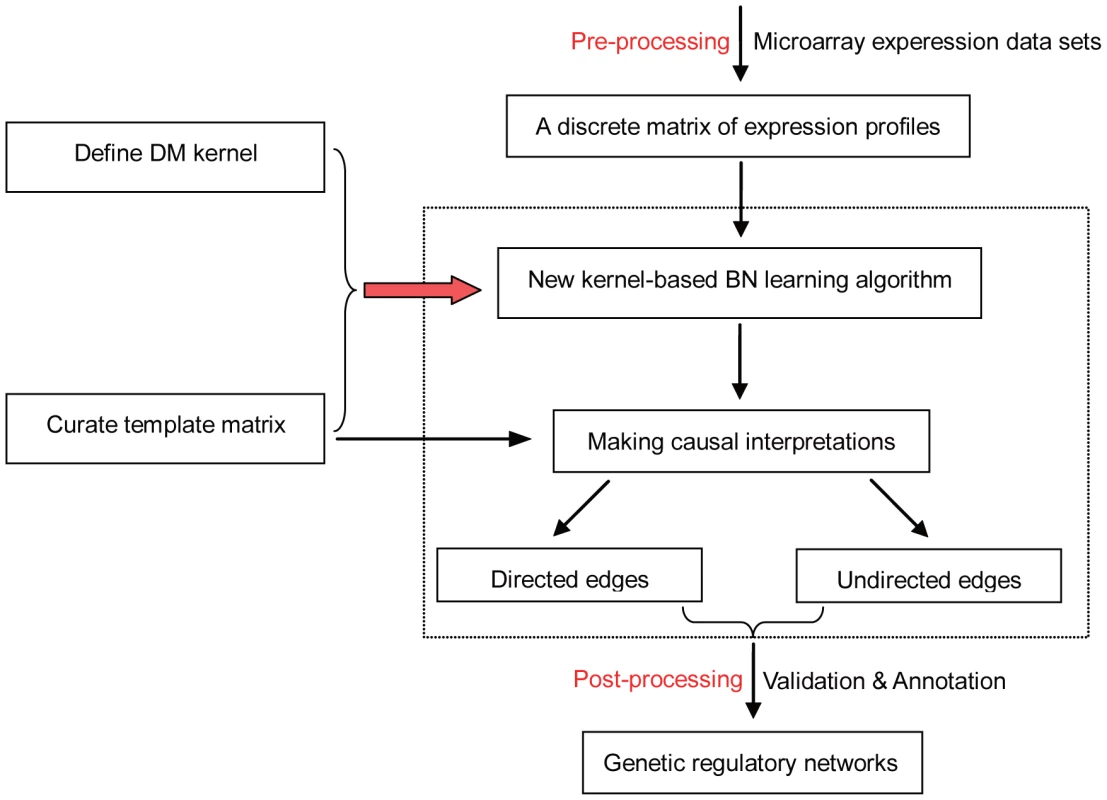
As an example, DM_BN takes as input deletion mutant gene expression profiles. The relative change of the mRNA expression levels of the deletion mutant strains versus the wild type (WT) is represented by 1 (significant up-regulation), −1 (significant down-regulation) and 0 (no significant change). The algorithm incorporate a new kernel to model the consistent gene expression changes upon perturbation and it employs a template of all potential regulator-regulator interactions to enable more accurate and much faster BN learning. After learning the BN structure, Meek's rule [28] is used to infer compelled (directed) and non-compelled (undirected) edges. The precision of the inferred network is then assessed by existing knowledge of protein complexes, regulatory relationships (MAPK signaling transduction pathways) and gene annotations. The main technical contribution of the DM_BN algorithm is to employ the kernel based approach to Bayesian network inference [27] and the introduction of a novel kernel for discrete data that is specifically designed for characterizing the deletion mutant data sets. Specifically, suppose and are two discrete variables which could take values 1, −1, 0, the trivial kernel for discrete data in [27] is defined as: , i.e., when ; and when . This is not viable for dealing with the deletion mutant data sets since the dominant value in such data is 0, the trivial kernel for discrete data will induce a large similarity output (1.0) for all most all gene pairs which are neither up-regulated nor down-regulated. To prevent this biased effects, we modified the trivial kernel to the DM kernel below:
The implication from the new DM kernel is clear: the differential expression changes of two genes in a deletion mutant experiment are considered similar (with kernel output 1.0) if they are either up-regulated or down-regulated simultaneously. The similarity between genes that are not responsive to the deletion mutant experiment is abandoned (with kernel output 0.0). In this way, only the information of the co-regulation activity is fed into the Bayesian network-learning algorithm (Methods).Another contribution of the DM_BN algorithm is the incorporation of the a priori knowledge from deletion mutant experiments into Bayesian network learning. For this purpose, we employ a network template to constrain the space of graph search in Bayesian network learning and to provide additional causal information in the learning and interpretation of Bayesian network structure. The basic idea of constructing the adjacency matrix of the network template (template matrix for short) is as follows: First, we start with an empty template matrix of zeros. Then, we define the list of target genes of a deletion mutant gene to be the genes whose mRNA levels either up - or down-regulated compared to the WT strain. If both deletion mutant genes A and B (with indices , respectively) are not in the target gene list of each other, the two genes do not seems to have a direct regulatory relationship, but they could cooperate to regulate other genes. So, if the target gene list of A and B overlap (i.e., at least one gene appear in both of the two target gene lists), the elements of the template matrix are set to 1, which means that either one of the two edges might appear in the final BN. Finally, if gene B appears in the target gene list of A, but A is not in the target gene list of B, we set , which means that could appear in the final BN while the reversed edge is forbidden. In rare occasions, when both A, B appear in the target gene list of each other, we set , since we do not know which direction of the interaction represents the dominant regulatory effect while the other represents the secondary feedback effect (Figure 1).
To identify potential causal interactions from Bayesian network structure, we have to determine whether the directionality of each edge in the network is reversible or not [28]. In this step, the template matrix again provides a priori causal information to guide the algorithm to disambiguate more edge directionalities. More details of the algorithm are presented in Methods.
Performance evaluation of BN structure inferences
To quantitatively compare the performance of the DM_BN learning algorithm with other approaches to infer regulatory networks, we curate a database of ground-truths protein-protein interactions, regulatory interactions, genetic/epistatic interactions and protein complexes from the KEGG and SGD databases. Here, methods being compared include alternative Bayesian network learning algorithms (the WinMine Toolkit [24], the BDeu scoring approach [22] with optimized prior [23] and the BIC scoring approach [20], [21] and non-Bayesian network approaches (the ARACNE [25] software, the Disruption Network [26] and the Jaccard similarity index (JI) approach). Details of these algorithms and the strategies used in the testing are described in Methods and Supplemental Note 1 (Text S1).
Basically, two key performance indicators are important for comparing the above algorithms: 1) Precision-recall curve, which quantifies the ability of an algorithm to correctly predict bona fide interactions between these regulators; 2) The precision of orientation, which measures the ability of an algorithm to predict correct directionality for each causal interaction. We first calculated the precision and recalls of all the predicted yeast regulator networks (Methods). In this computation, directionality is not considered in matching a predicted edge and a known interaction in the database, which is partly because we only have very limited knowledge about the causality of these ground-truth interactions. By plotting the corresponding precision-recall points (or point, if an algorithm predicts only one network) for each algorithm, we found that DM_BN algorithm outperforms all the alternative network construction approaches in both precision and recall (Figure 2, Table S2). In other words, regardless of causality, DM_BN algorithm has the highest precision of de novo network predictions over the whole range of recall rates.
Fig. 2. Precision and recall of networks inferred by DM_BN and by other network inference methods at various cutoffs. 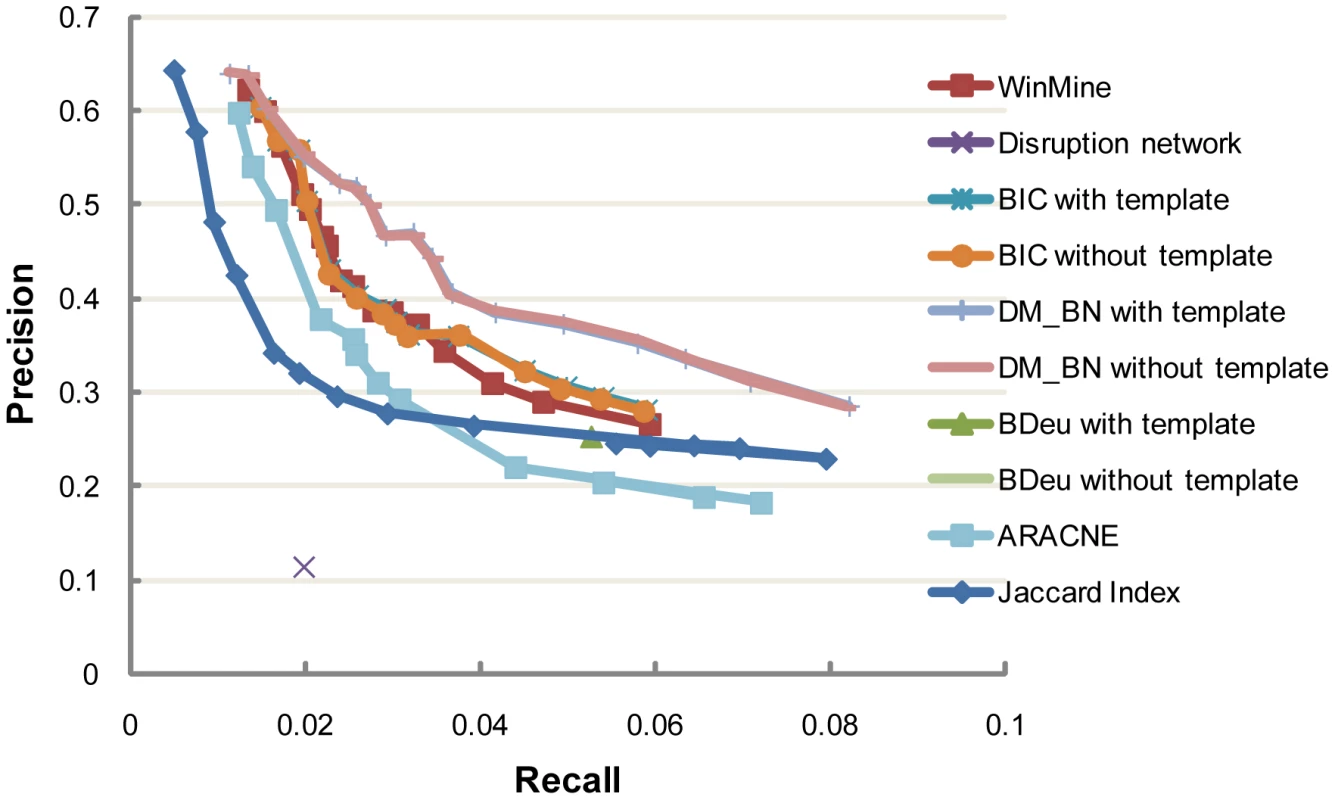
The colored lines show the precision and recall tradeoffs as a function of different values of Jaccard index threshold, mutual information threshold in ARACNE, kappa parameter in WinMine, weight of the penalty term in BIC and the parameter in DM_BN, respectively. The Disruption network approach and the BDeu metric do not have any tunable parameter and only one network can be predicted. We curated 5662 protein-protein interactions, regulatory interactions, genetic/epistatic interactions and protein complexes from the KEGG and SGD databases to evaluation the performance of different algorithms (See Table S2). A close examination of the BN inferred by the DM_BN algorithm suggested it indeed recapitulated many interactions in protein complexes or pathways. Specifically, the BN structure visualized in Figure 3 with precision 0.4704 and recall rate 0.0323 (Figure 2) includes both causal (represented by directed edges) and non-causal (by undirected edges) relationships among these regulators, which are known to take place in diverse biological processes to combinatorially regulate the expression of target genes. Moreover, we also computed the functional enrichment of these regulators based on their target genes (Methods). The result suggests that regulators that tightly interconnected in the BN more significantly share common functions than other regulator pairs (Figure 3, Table S3). The network learned by DM_BN algorithm further predicts how these regulators interact with each other in different cellular processes (Figure 3).
Fig. 3. The yeast regulatory network inferred from the deletion mutant gene expression profiles by DM_BN. 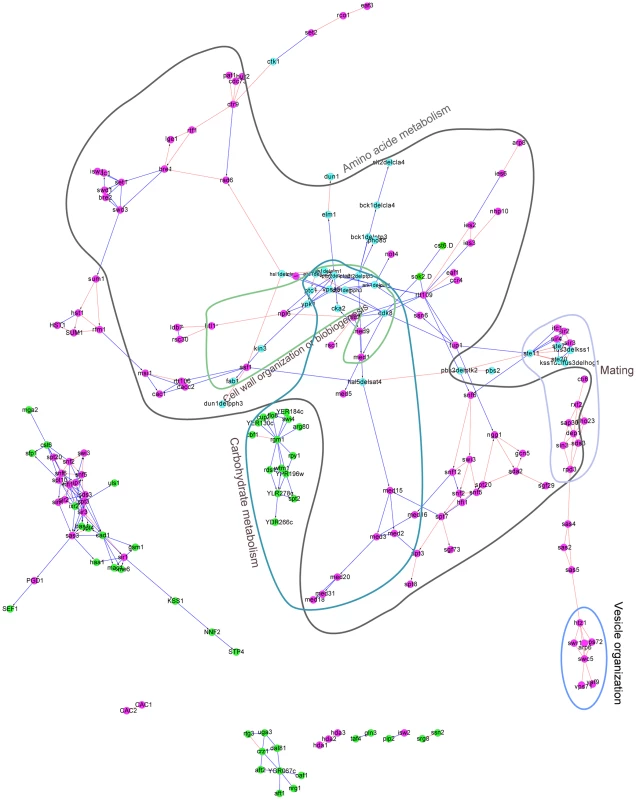
Each node in this network represents a single or doubly mutated regulator(s). The directionality of edges indicates predicted causal relationship between the regulators. Known regulator interactions (physical or epistatic interactions) were shown by red colored edges. Regulators involved in the same cellular process, such as amino acid metabolism, cell wall organization or biogenesis, are highlighted by circles with annotations. Purple nodes: chromatin machinery components (mostly general transcription factors); Cyan nodes: single or double mutants of protein kinases or phosphatases; Green nodes: sequence-specific DNA binding transcription factors. The parameter in the DM_BN algorithm was set to . For example, the predicted network module among subunits of the chromatin remodeling machinery complex (Figure 3, shown by purple nodes) has a high precision of 0.85217 (Table S4); the network module consisting of protein kinases Vps15, Ark1, Prk1, Cdk8, Cka2 and protein phosphatase Ptc1, Ptc2, Pph3, Ptp3 is involved in three interrelated cell processes: cell wall organization or biogenesis, amino acid metabolism and carbohydrate metabolism, which is consistent with biological knowledge; and the predicted network suggests that Rpd3 complex, Sir complex and Ste11 mediated MAPK kinase cascades pathway cooperate with each other in mating process (Figure 3, Table S3). We also observed that a STF is more likely to connect with another STF than with a GTF, which is similarly observed in the densely connected network inferred by the Jaccard index (JI) similarity measure (Figure S1A). Our results are also consistent with the E-MAP results, which are quantitative genetic interactions between phosphorylation related genes in S. cerevisiae [19]. For instance, it is known that histone variant H2A.Z (encoded by the Htz1 gene) exchange with histone H2A in nucleosomes through the SWR1 complex [29], [30] and that Htz1 displays positive genetic interactions with SWR1 (+3.5), Vps71 (+3.9) and Vps72 (+3.5) [19]. These interactions are all predicted by the network inferred by DM_BN (Figure 3). Indeed, the target sets of Htz1, SWR1, Vps71 and Vps72 deletion mutants have high similarity, with Jaccard indices (Methods) (Figure 3 (blue circle), Table S5). Moreover, the functional enrichment of the predicted SWR1 complex target gene sets for vesicle organization (Table S3) is consistent with the fact that SWR1 complex is required for vacuolar protein sorting [31]. More examples of the inferred pathway relationships are listed in Table S6, S7.
Prediction of causal relationships
Inferring correct directionalities for causal interactions or epistasis is an important aspect for regulatory network predictions. However, most non-Bayesian network algorithms are unable to do so. For example, the ARACNE [25] software and the Jaccard similarity index (JI) approach could only predict undirected interactions. Although the Disruption Network [26] could predict the direct causal relationships between deletion mutant genes and differentially expressed genes, such knowledge is derived from the deletion mutant experiments without performing causality inference. It is of special interest to see if an algorithm can make de novo predictions about causality among deletion mutant genes from the similarity of their genome-wide differential expression profiles. In principle, Bayesian network learning algorithms hold this promise and thus we compare the performance of the four BN learning algorithms (DM_BN, Winmine toolkit [24], the BDeu [22] and BIC scoring approaches [20], [21]) in predicting causal relationships.
Since the ground-truth causal relationships derived from existing databases for the 378 regulator genes is very limited, and also because we do not know the exact cellular contexts in which those causal relationships hold true, to quantify the performance in predicting causal relationships, we calculated the recall and the precision of all these network inference approaches except BDeu using the four MAPK (mitogen-activated protein kinase) cascades where clear causal relationships are well described among these kinases. The exclusion of BDeu here is simply because it does not have a tunable parameter to generate a relative sparse network that is comparable to the size of networks generated by the other three approaches. However, using a different evaluation approach, BDeu's causality prediction apparently does not perform as well as DM_BN and BIC (see below). Yeast contains at least four MAPK (mitogen-activated protein kinase) cascades that convert extracellular stimuli into intracellular signals during a variety of cellular processes, such as mating, cell wall remodeling and high osmolarity adaption [32]. We found that when all tools predicted roughly the same number of edges, the DM_BN algorithm with prior information can predict more interactions with correct orientations than other tools among kinases involved in the same signal transduction pathway (Table S8, S9, S10, S11).
To test whether the correct inference of edge direction is solely the result of applying a template, we examined the directionality of edges in BNs inferred by DM_BN without any template. At various parameters, 73.3–89.2% of the edges have the same direction as the regulator-DEG relationships identified in the deletion mutants experiments. These proportions are significantly higher than that expected by chance (random coin tossing p = 0.5, Binominal test p = 0.0625∼5.42e-07, Table 1). This indicates that the correct inference of edge directions by DM_BN is largely not attributed to using the template. However, the BN inferred by DM_BN with the template, did correct a small number edges incorrectly predicted when not using the template (1/34∼4/15 edges, Table 1). This is because the network template not only corrects edge orientation errors inconsistent, but also improves the global causal structure in the BN through the cascading interactions between edges. Therefore, a template is included in the actual implementation of the DM_BN algorithm as the default setting.
Tab. 1. Precision of edge orientations for networks inferred by DM_BN with/without template at different parameters. 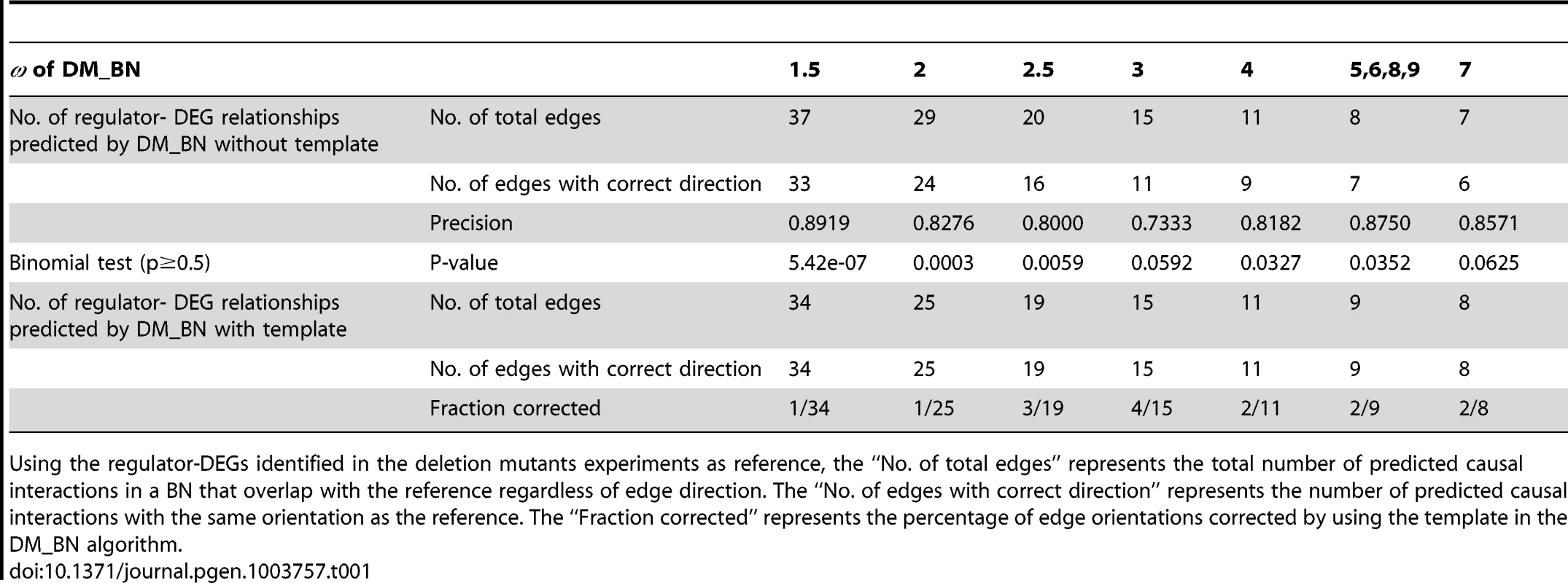
Using the regulator-DEGs identified in the deletion mutants experiments as reference, the “No. of total edges” represents the total number of predicted causal interactions in a BN that overlap with the reference regardless of edge direction. The “No. of edges with correct direction” represents the number of predicted causal interactions with the same orientation as the reference. The “Fraction corrected” represents the percentage of edge orientations corrected by using the template in the DM_BN algorithm. Using a similar approach, we also compared with other BN inference algorithms, the performance of DM_BN in de novo predicting causal relationships without using the a priori information encoded by the template. The DM_BN algorithm and the BIC scoring approach [20], [21] generally predict non-compelled directed edges remarkably more precise than the BDeu scoring method [22] or the WinMine toolkit [24] (Supplemental Note 2 and 3 in Text S1, Figure S2).
In particular, the causal relationships inferred by the DM_BN algorithm (with the network template) correctly recapitulated the linear cascade structure for regulators in the HOG signaling pathway involved in the osmotic stress response (Figure 4A). For instance, Ste11 MAPK kinase (MAPKKK) phosphorylates Pbs2 MAPK kinase (MAPKK). Then, the activated Pbs2 phosphorylates Hog1 in the MAPK kinase cascade pathway for osmostress adaptation [33].
Fig. 4. Causal relationships predicted by DM_BN. 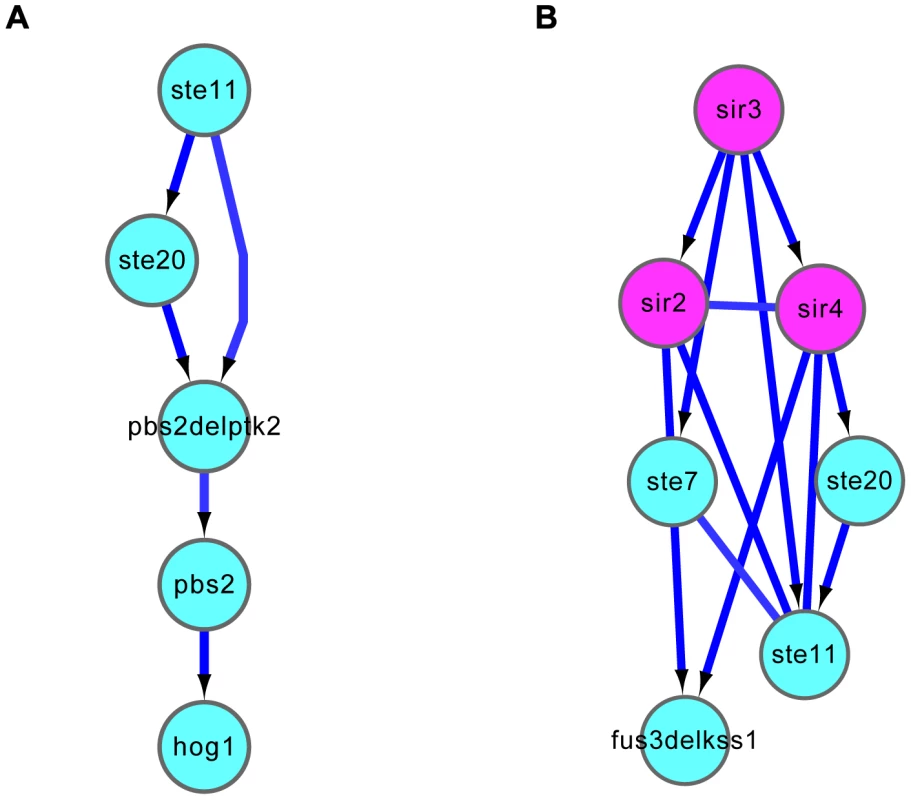
(A) Linear cascading relationships among regulators involved in HOG signaling pathway in response to osmotic stress were correctly inferred. (B) The predicted relationships between the SIR complex and the STE/FUS3/KSS MAPK signal transduction pathway are consistent with their roles in mating process. Purple nodes: components of the SIR complex; Cyan nodes: single or double mutants of protein kinases. The parameter in the DM_BN algorithm for generating these predictions was set to . See section “Novel regulator-regulator interactions predicted by DM_BN” for more details. In the mating process, DM_BN not only accurately grouped the SIR complex and the Ste11 mediated MAPK cascade pathways, but also correctly predicted the connectivity among components of the complex or the pathway (Figure 4B). The results correctly recovered the role of Ste7 and Ste11 protein kinases in two different MAPK Fus3 and Kss1 cascade pathways that controls mating, respectively [33], [34].
Novel regulator-regulator interactions predicted by DM_BN
The inferred causal relationships or non-causal interactions between these gene expression regulators not only confirmed known relationships, such as physical interactions and genetic epistasis relationships among these regulators, but also predicted many novel relationships that could be important in gene regulation.
For example, the DM_BN algorithm not only correctly predicted the connection between components in the SIR complex or in the MAPK pathway, but also predicted the dense connection between the SIR complex and Ste11-mediated MAPK cascades (Figure 4B, Table S3 and Table S12). Clustering of the expression profiles of the genes in these network modules shows that the genes up-regulated in the deletion mutants of Sir2, Sir3 and Sir4 (Figure 5A, right panel) are all within 10 kb to their nearest telomere. Meanwhile, the predicted functions of the genes down-regulated by all deletion mutants in Figure 5A are enriched for mating process (see also Table S3). All these findings are consistent with the knowledge that SIR complex plays roles in silencing at HML, HMR loci which carry unexpressed copies of mating-type genes and telomeres [35] and that SIR complex is comprised of two structural proteins Sir3 and Sir4, deletion of which will cause reduced mating rate at different levels [36]. The mRNA expression levels of Fus3 and Fus1 are very low in the deletion mutants of Sir2, Sir3 and Sir4 in all the three data sets (Figure 5A, left panel). However, the mRNA levels of other genes in the model are not changed compared to WT (except the expression levels of the deletion mutant genes themselves) (Figure 5A, left panel). From the causal, non-causal relationships predicted by DM_BN (Figure 4B) and the expression profiles of deletion mutants experiments (Figure 5A), we can infer a novel model implying that the Ste11 mediated MAPK cascades pathway may have overlapping functions with the SIR complex (Figure 5B). Thus, SIR complex could indirectly influence the mRNA expression of kinase Fus3, which is involved in the MAPK cascades pathway in mating process.
Fig. 5. Novel causal or non-causal relationships predicted by DM_BN and an inferred novel regulatory model involved in mating process. 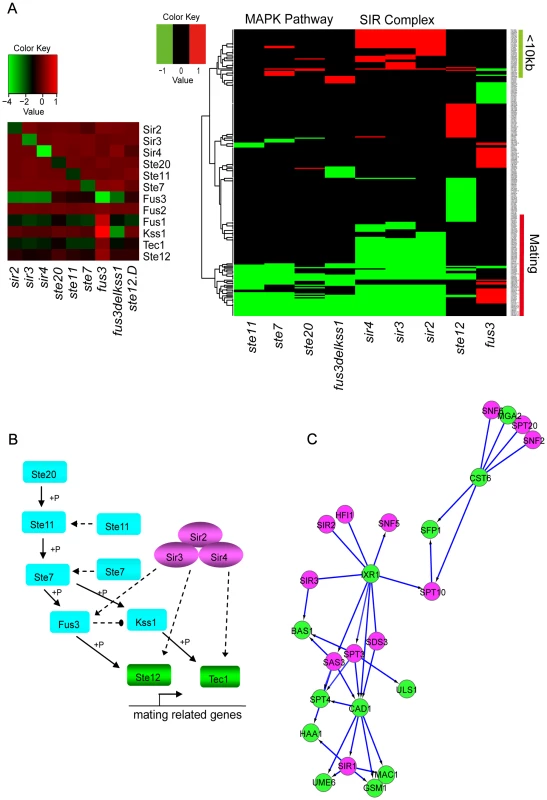
(A) Left panel: All genes that are significantly changed compared to the wild type upon deletion of subunits of the Sir Complex and kinases of Ste11-mediated MAPK cascading pathway. Right panel: Genes marked by green bar are <10 kb from the nearest telomeres, and genes marked by red bar are involved in mating process. (B) A novel predicted regulatory network that SIR complex and (Ste11-mediated) MAPK cascades pathway has functional overlap through kinase Fus3 (that were down regulated in the SIR complex) in mating. Known Ste11-mediated MAPK pathway is shown by Solid lines. Solid lines: known interactions, dashed lines: newly predicted interactions. (C) Novel causal relationships and undirected interactions predicted by DM_BN among STFs: Cst6, Sfp1, Bas1, Mac1, Gsm1, Ixr1, Haa1, Ume6 and Cad1, and some subunits of the SIR, SWI/SNF and SAGA complex, under parameter setting . Purple nodes: chromatin machinery components; Green nodes: sequence-specific DNA binding transcription factors. Although a STF is more likely to have a similar gene expression pattern with another STF than with a GTF generally (Figure 3A), the network suggests that STFs Cst6, Sfp1, Bas1, Mac1, Gsm1, Ixr1, haa1, Ume6 and Cad1 connect densely to GTFs (some subunits of SIR complex, SWI/SNF complex and SAGA complex) (Figure 5C). Clusters (Figure S3) of the expression profiles of these regulators revealed high similarities between the target profiles of the STFs, Sfp1 and Cst6, and the GTFs SWI/SNF complex and SAGA complex. Another example is the high similarity between the targets profiles of STFs: Ixr1, Cad1, Bas1 and Stp4; and GTFs: SIR complex, SAGA complex. Although no physical interactions or binding relationships between them have been reported in the literature, SAGA subunit Spt3 has been reported to have negative genetic interactions with Stp4 and Ixr1 [10]. These novel predictions by the DM_BN algorithm may serve as blueprints for further experimental explorations.
Discussion
Uncovering complex regulatory networks is an important and challenging task [1], [12], [19], [37]. Here, we introduced a new Bayesian network inference algorithm “DM_BN”, specifically designed to infer regulatory networks from gene expression profiles generated by gene perturbations, such as gene deletions. DM_BN can work with both small and large datasets and infer causal and non-causal relationships among the perturbed genes. To address the sparsity of gene expression changes in the perturbation experiments, we developed a kernel-based BN learning algorithm DM_BN, which is appropriate for modeling such gene expression data sets. Comparing with known biological interactions, both the recall and the precision of the network inferred by the proposed DM_BN algorithm are significantly higher than that inferred by WinMine and by the Jaccard Index (JI) similarity measure (Figure 2). The DM_BN network model not only successfully recapitulated known interactions among the yeast transcriptional regulators, but also predicted many novel interactions among these regulators and regulatory protein complexes, offering new insights into the yeast transcriptional regulatory network.
Our results show that the improved performance of the DM_BN algorithm can be mainly ascribed to the new kernel. Since an edge between two regulator genes is allowed in the network template if they share at least one target gene, the template matrix actually allowed all the possible interactions between these genes, hence has a very little predictive value by itself. However, the DM_BN algorithm still benefits from using the network template in two aspects. First, by eliminating all impossible edges, the template effectively reduced the search space to speed up the DM_BN algorithm. Second, by encoding the a priori regulator-target causal knowledge in deletion mutant experiments, the network template not only corrects edge orientations that are inconsistent with such information (Table 1), but also improves the global causal structure predicted by DM_BN through edge-edge interactions, as we demonstrated in the inference of MAPK pathways (Table S11).
Although the DM_BN approach has achieved big success in inferring yeast regulatory network from perturbation-based gene expression data sets, there are still a few limitations to its applications. For example, the mRNA expression levels of target genes are not fully representative of the activities and interactions of the regulators in modulating gene expression. This is because post-transcriptional changes and the regulators' context-specific transient activity were not measured in the experiments. Due to the intrinsic limitation of mRNA expression data, our method failed to identify certain relationships among the regulators under certain conditions, especially when the activity of the regulators is not screened in the microarray experiments. Nevertheless, these problems are not the fault of the proposed BN inference algorithm but rather inherent limitations of current experimental systems, which are expected to overcome by introducing other types of high-throughput datasets. In this sense, the application of the DM_BN algorithm is not limited to microarray expression profiles of genetic perturbations, it can actually be extended to work on many kinds of high-throughput data, such as epigenomic, transciptomic, proteomic data sets, and even quantitative phenotype data.
Methods
Data sets
All the gene expression profiles are downloaded from the Gene Expression Omnibus (GEO) database, including 269 transcription factors knockout strains grown in yeast extract peptone dextrose medium (YPD) [12], 150 deletion mutants of protein kinases and phosphatases [10], 165 mutants of chromatin machinery components [3] and 52 sequence-specific DNA binding transcription factors (STFs) deletion strains grown in synthetic complete medium (SC) [15]. Altogether the four data sets above contain gene expression profiles of 544 yeast deletion mutants. The series accession numbers of these data sets are GSE4654 [12], GSE25644 [10], GSE25909 [3] and GSE2324 [15].
Gene expression data analysis
The detailed DNA microarray normalization and statistical analysis procedures see described in Supplemental Methods (Text S1). After processing, the gene expression changes are represented by discrete values: 1 (significant up-regulation), −1 (significant down-regulation) and 0 (no significant expression change).
Inference of the optimal Bayesian network structure
We employ the kernel-based Bayesian network learning algorithm [27] with three modifications. First, we use the ‘DM kernel’ instead of the trivial kernel to handle the yeast deletion mutant datasets. Second, we use a template matrix to constrain the space of all possible Bayesian network structures. Details of the ‘DM kernel’ and the construction of the template matrix are described in the Results and will not be repeated here. Finally, we modified the BIC scoring function by increasing the weight of the complexity term for penalizing the Kernel Generalized Variance [38] measure. This is necessary for removing biological noise and increasing the precision and sparsity of the finally obtained network structure. Formally, the Bayesian network scoring function is modified as follows (cf. eqn. 4 in ref [27] for details):
Here, is the BIC score for node and its parents , and the overall score for a full Bayesian network is: . and are the Kernel Generalized Variance [38] for node sets and . is the multiplicative weight that we impose on the second term of the scoring function.With the DM kernel inside the KGV measure, the template matrix as a structural constraint and the modified scoring function, we can search for the Bayesian network structure that optimally fit the yeast deletion mutant datasets. Specifically, in each step, we consider 1) adding an edge that is consistent with the template; 2) deleting an edge from the current BN structure; 3) reversing the direction of an edge that will not violate the causal constraints embodied the template. In accordance with previous studies, we use the greedy ascent TABU search method [39] to find the ideal Bayesian network structure. Here, ‘TABU’ denotes a Meta searching strategy that prohibits the algorithm from ‘undoing’ a recent operation. It helps the search procedure from being getting stuck in the local optima regions [39]. Finally, we adopt an efficient dynamic graph acyclicity checking method [40] in the Bayesian network structure search, since the most computational intensive task in this study involves inferring a Bayesian network of up to ∼400 nodes, using the conventional static graph acyclicity checking method would be fairly slow.
Deriving causal knowledge from the Bayesian network structure
Interpreting the causalities in the Bayesian network structure is not a straight forward task. This is because there are equivalence classes of Bayesian network structures. All BNs in the equivalence class are semantically equivalent. They share the same set of skeletons (edge connections regardless of arrows), but differ in the directionalities of some edges [41]. As such, there are two types of edges in a Bayesian network: compelled edges, whose directionalities are fixed among BNs in the equivalence class and non-compelled edges, whose directionalities are not consistent in the equivalence class [42]. The authors also proposed an efficient algorithm to dissect a Bayesian network into compelled (directed) edges and non-compelled (undirected) edges (the results are collectively represented as a partially directed acyclic graph, a.k.a., PDAG) [42]. However, the approach is not well suited for our study because the template matrix contains much a priori causal information, which was not used by Chickering's algorithm [42].
To overcome this problem, we employ Meek's rules [28] to convert the Bayesian network structure into a PDAG. The merit of this algorithm is that prior causal knowledge could be fully exploited in making causal interpretations of the Bayesian network structure. As such, we extract asymmetries in the template matrix and impose those constraints in the causal interpretation algorithm [28]. In this way, the causal information conveyed in deletion mutant experiments is used maximally.
Jaccard index
Jaccard's similarity index [43] quantifies the similarity between two sets of elements. In this work, we use Jaccard index to evaluate the similarity between the deletion mutant expression profiles of two regulators, which is calculated as follows:
Here:: No. of common targets for regulator1 and regulator2.
: No. of genes whose expression significantly changed in the deletion mutant of regulator 1.
: No. of genes whose expression significantly changed in the deletion mutant of regulator 2.
Evaluating the Precision/Recall of the inferred network
We use known relationships between the 165 mutants in 30 chromatin modification complexes [3], ground-truths protein-protein interactions, regulatory interactions and genetic/epistatic interactions curated from the KEGG and SGD databases to evaluate the precision/recall for the predicted networks. For this purpose, we calculated the recall and the precision of a network using the formula below:
: the number of edges that are correctly predicted in the network (true positives, i.e., predicted edges that are also consistent with known protein-protein interactions).: the total number of known pair-wise interactions between nodes in the network (e.g., between subunits in known protein complexes).
: the number of edges in a predicted network.
In rare cases, a deletion-mutant regulator gene appears in more than one data set. Removing any copy of that gene will typically cause many of its interactors in that data set undetected. So, multiple copies of all these overlapping genes are retained in our analysis. In this scenario, no matter how many edges between two same genes from two different datasets are predicted in the network, we only count this interaction once. Moreover, in the analysis of causal relationships, if the directionalities of these edges are consistent, we retain the directionality of that interaction; otherwise, we treat this interaction as an undirected (non-compelled) edge.
Functional enrichment analysis
The significance of the functional enrichment of a gene list is computed by performing the hypergeometric test. In this work, all the GO annotation, phenotype and pathway data sets were downloaded from the SGD, KEGG database. The P-value is calculated as follows:
: The total number of genes with significant expression change in any deletion mutant experiment.: Number of genes in one particular functional category.
: Number of genes with significant expression change in the current deletion mutant experiment.
: Number of overlapped genes between the n genes and the S genes.
Strategies for systematic testing and evaluation of network-inference methods
To systematically compare the performance of the DM_BN algorithm with existing network-inference methods, we tested the ARACNE [25] software, the Disruption Network approach [26] and the Jaccard Index (JI) approach, which are not based on Bayesian networks. We also tested the WinMine Toolkit [24] and Bayesian network learning with two other scoring metrics (BIC [20], [21] and BDeu [22]) (Detailed description of each method is listed in Supplemental Note 1 in Text S1).
To ensure the comprehensiveness of the evaluation, for each method and whenever possible, we use a wide range of parameter settings to infer a number of networks to best reveal the tradeoffs between precision and recall. Specifically, for the DM_BN algorithm, we run it with a wide range of values for the parameter (See Methods “Inference of the optimal Bayesian network structure” for details) to infer a set of BNs with different number of edges. Similarly, for the WinMine toolkit [24], we run it at different values of the kappa parameter to infer BNs; for ARACNE [25], various mutual information thresholds are used to infer a set of regulatory networks; for the Jaccard index (JI) approach, a wide range of the JI similarity cutoffs are used for network construction and for the BIC score approach, different weights (like the parameter in DM_BN) are multiplied to the penalty term (, see Eqn. 42 in [20]) in the Bayesian information criterion to infer BNs. Note that we could only generate a single best network for the BDeu scoring approach [22] using the optimal ESS value [23] since there is no way to tune the precision-recall tradeoffs. Similarly, only a single network can be inferred for the disruption network approach [26]. This is because to determine whether a gene is significantly differentially expressed in a deletion mutant strain, we employed the default statistical tests and significance thresholds used in the original experimental study (See “Statistical analysis of expression profiles” in Supplemental Methods). It is not clear how to adjust the thresholds synchronously for the four data sets.
Supporting Information
Zdroje
1. YoungRA, LeeTI, RinaldiNJ, RobertF, OdomDT, et al. (2002) Transcriptional regulatory networks in Saccharomyces cerevisiae. Science 298 : 799–804.
2. KouzaridesT (2002) Histone methylation in transcriptional control. Current Opinion in Genetics & Development 12 : 198–209.
3. HolstegetFCP, LenstraTL, BenschopJJ, KimT, SchulzeJM, et al. (2011) The Specificity and Topology of Chromatin Interaction Pathways in Yeast. Molecular Cell 42 : 536–549.
4. ArndtGM, RaponiM (2003) Double-stranded RNA-mediated gene silencing in fission yeast. Nucleic Acids Research 31 : 4481–4489.
5. JacquierA (2009) The complex eukaryotic transcriptome: unexpected pervasive transcription and novel small RNAs. Nature Reviews Genetics 10 : 833–844.
6. MoazedD (2009) Small RNAs in transcriptional gene silencing and genome defence. Nature 457 : 413–420.
7. DavisRW, GiaeverG, ChuAM, NiL, ConnellyC, et al. (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418 : 387–391.
8. Van DriesscheN, DemsarJ, BoothEO, HillP, JuvanP, et al. (2005) Epistasis analysis with global transcriptional phenotypes. Nat Genet 37 : 471–477.
9. RobertsCJ, NelsonB, MartonMJ, StoughtonR, MeyerMR, et al. (2000) Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science 287 : 873–880.
10. HolstegeFCP, van WageningenS, KemmerenP, LijnzaadP, MargaritisT, et al. (2010) Functional Overlap and Regulatory Links Shape Genetic Interactions between Signaling Pathways. Cell 143 : 991–1004.
11. DionMF, AltschulerSJ, WuLF, RandoOJ (2005) Genomic characterization reveals a simple histone H4 acetylation code. Proceedings of the National Academy of Sciences of the United States of America 102 : 5501–5506.
12. IyerVR, HuZZ, KillionPJ (2007) Genetic reconstruction of a functional transcriptional regulatory network. Nature Genetics 39 : 683–687.
13. SegalE, ShapiraM, RegevA, Pe'erD, BotsteinD, et al. (2003) Module networks: identifying regulatory modules and their condition-specific regulators from gene expression data. Nature Genetics 34 : 166–176.
14. TavazoieS, BeerMA (2004) Predicting gene expression from sequence. Cell 117 : 185–198.
15. HughesTR, MartonMJ, JonesAR, RobertsCJ, StoughtonR, et al. (2000) Functional discovery via a compendium of expression profiles. Cell 102 : 109–126.
16. Pearl J (1988) Probabilistic Reasoning in Intelligent Systems. San Fransisco, CA: Morgan Kaufmann.
17. FriedmanN, LinialM, NachmanI, Pe'erD (2000) Using Bayesian networks to analyze expression data. Journal of Computational Biology 7 : 601–620.
18. ChangHH, RamoniMF (2009) Transcriptional network classifiers. BMC Bioinformatics 10: S1–S17.
19. ShokatKM, FiedlerD, BrabergH, MehtaM, ChechikG, et al. (2009) Functional Organization of the S-cerevisiae Phosphorylation Network. Cell 136 : 952–963.
20. Heckerman D (1999) A Tutorial on Learning with Bayesian Networks. In: Jordan M, editor. Learning in Graphical Models. Cambridge, MA: MIT Press.
21. SchwarzG (1978) Estimating the Dimension of a Model. The Annals of Statistics 6 : 461–464.
22. HeckermanD, GeigerD, ChickeringDM (1995) Learning Bayesian Networks: The Combination of Knowledge and Statistical Data. Machine Learning 20 : 197–243.
23. Steck H (2008) Learning the Bayesian Network Structure: Dirichlet Prior vs Data. In: McAllester DA, Myllymaki P, editors. UAI 2008, Proceedings of the 24th Conference in Uncertainty in Artificial Intelligence. Helsinki, Finland: AUAI Press. pp. 511–518.
24. ChickeringDM (2002) The WinMine Toolkit. Microsoft
25. MargolinAA, NemenmanI, BassoK, WigginsC, StolovitzkyG, et al. (2006) ARACNE: an algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. BMC Bioinformatics 7 Suppl 1: S7.
26. RungJ, SchlittT, BrazmaA, FreivaldsK, ViloJ (2002) Building and analysing genome-wide gene disruption networks. Bioinformatics 18 Suppl 2: S202–210.
27. BachFR, JordanMI (2002) Learning Graphical Models with Mercer Kernels. Advances in Neural Information Processing Systems 15 : 1009–1016.
28. MeekC (1995) Causal inference and causal explanation with background knowledge. Proceedings of the Eleventh Annual Conference on Uncertainty in Artificial Intelligence 403–410.
29. MizuguchiG, ShenXT, LandryJ, WuWH, SenS, et al. (2004) ATP-Driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303 : 343–348.
30. LukE, RanjanA, FitzGeraldPC, MizuguchiG, HuangY, et al. (2010) Stepwise Histone Replacement by SWR1 Requires Dual Activation with Histone H2A.Z and Canonical Nucleosome. Cell 143 : 725–736.
31. BonangelinoCJ, ChavezEM, BonifacinoJS (2002) Genomic screen for vacuolar protein sorting genes in Saccharomyces cerevisiae. Mol Biol Cell 13 : 2486–2501.
32. PosasF, TakekawaM, SaitoH (1998) Signal transduction by MAP kinase cascades in budding yeast. Curr Opin Microbiol 1 : 175–182.
33. SaitoH (2010) Regulation of cross-talk in yeast MAPK signaling pathways. Current Opinion in Microbiology 13 : 677–683.
34. ErredeB, GartnerA, ZhouZQ, NasmythK, AmmererG (1993) Map Kinase-Related Fus3 from Saccharomyces-Cerevisiae Is Activated by Ste7 Invitro. Nature 362 : 261–264.
35. MoazedD, KistlerA, AxelrodA, RineJ, JohnsonAD (1997) Silent information regulator protein complexes in Saccharomyces cerevisiae: A SIR2/SIR4 complex and evidence for a regulatory domain in SIR4 that inhibits its interaction with SIR3. Proceedings of the National Academy of Sciences of the United States of America 94 : 2186–2191.
36. KaeberleinM, McVeyM, GuarenteL (1999) The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes & Development 13 : 2570–2580.
37. ShilatifardA, LeeJS, SmithE (2010) The Language of Histone Crosstalk. Cell 142 : 682–685.
38. BachFR, JordanMI (2002) Kernel Independent Component Analysis. Journal of Machine Learning Research 3 : 1–48.
39. CvijovicD, KlinowskiJ (1995) Taboo search - an approach to the multiple minima problem. Science 267 : 664–666.
40. GiudiciP, CasteloR (2003) Improving Markov Chain Monte Carlo Model Search for Data Mining. Machine Learning 50 : 127–158.
41. Koller D, Friedman N (2009) Probabilistic Graphical Models: Principles and Techniques. MIT Press.
42. ChickeringDM (1995) A Transformational Characterization of Equivalent Bayesian Network Structures. Proceedings of the Eleventh Annual Conference on Uncertainty in Artificial Intelligence 87–98.
43. JaccardP (1901) Étude comparative de la distribution florale dans une portion des Alpes et des Jura. Bulletin de la Société Vaudoise des Sciences Naturelles 37 : 547–579.
Štítky
Genetika Reprodukčná medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy








