-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
Cyclic adenosine monophosphate (cAMP) has been implicated in the execution of diverse rhythmic behaviors, but how cAMP functions in neurons to generate behavioral outputs remains unclear. During the defecation motor program in C. elegans, a peptide released from the pacemaker (the intestine) rhythmically excites the GABAergic neurons that control enteric muscle contractions by activating a G protein-coupled receptor (GPCR) signaling pathway that is dependent on cAMP. Here, we show that the C. elegans PKA catalytic subunit, KIN-1, is the sole cAMP target in this pathway and that PKA is essential for enteric muscle contractions. Genetic analysis using cell-specific expression of dominant negative or constitutively active PKA transgenes reveals that knockdown of PKA activity in the GABAergic neurons blocks enteric muscle contractions, whereas constitutive PKA activation restores enteric muscle contractions to mutants defective in the peptidergic signaling pathway. Using real-time, in vivo calcium imaging, we find that PKA activity in the GABAergic neurons is essential for the generation of synaptic calcium transients that drive GABA release. In addition, constitutively active PKA increases the duration of calcium transients and causes ectopic calcium transients that can trigger out-of-phase enteric muscle contractions. Finally, we show that the voltage-gated calcium channels UNC-2 and EGL-19, but not CCA-1 function downstream of PKA to promote enteric muscle contractions and rhythmic calcium influx in the GABAergic neurons. Thus, our results suggest that PKA activates neurons during a rhythmic behavior by promoting presynaptic calcium influx through specific voltage-gated calcium channels.
Published in the journal: PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior. PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003831
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003831Summary
Cyclic adenosine monophosphate (cAMP) has been implicated in the execution of diverse rhythmic behaviors, but how cAMP functions in neurons to generate behavioral outputs remains unclear. During the defecation motor program in C. elegans, a peptide released from the pacemaker (the intestine) rhythmically excites the GABAergic neurons that control enteric muscle contractions by activating a G protein-coupled receptor (GPCR) signaling pathway that is dependent on cAMP. Here, we show that the C. elegans PKA catalytic subunit, KIN-1, is the sole cAMP target in this pathway and that PKA is essential for enteric muscle contractions. Genetic analysis using cell-specific expression of dominant negative or constitutively active PKA transgenes reveals that knockdown of PKA activity in the GABAergic neurons blocks enteric muscle contractions, whereas constitutive PKA activation restores enteric muscle contractions to mutants defective in the peptidergic signaling pathway. Using real-time, in vivo calcium imaging, we find that PKA activity in the GABAergic neurons is essential for the generation of synaptic calcium transients that drive GABA release. In addition, constitutively active PKA increases the duration of calcium transients and causes ectopic calcium transients that can trigger out-of-phase enteric muscle contractions. Finally, we show that the voltage-gated calcium channels UNC-2 and EGL-19, but not CCA-1 function downstream of PKA to promote enteric muscle contractions and rhythmic calcium influx in the GABAergic neurons. Thus, our results suggest that PKA activates neurons during a rhythmic behavior by promoting presynaptic calcium influx through specific voltage-gated calcium channels.
Introduction
Cyclic adenosine monophosphate (cAMP) is a potent second messenger that plays an important role in cellular responses to extracellular signals to regulate a wide array of biological processes. In the nervous system, cAMP has been implicated in controlling axon guidance, axonal regeneration, sensory function, learning and memory [1]–[4]. cAMP signaling is also critical for the execution of rhythmic physiological processes such as heart beating and circadian rhythm in a variety of organisms [5]–[8]. However, the mechanism by which cAMP controls rhythmic outputs remains unclear.
cAMP is synthesized by adenylyl cyclases (ACs), which are activated by G protein-coupled receptors (GPCRs) that are coupled to the heterotrimeric G protein α subunit, Gαs [9]. Work in a variety of cell types has shown that cAMP has three major molecular targets: cyclic nucleotide-gated (CNG) channels, exchange proteins directly activated by cAMP (Epac) and cAMP-dependent protein kinase (PKA) (Figure 1A and [9]). CNG channels are non-selective cation channels that are critical for the excitability of certain sensory neurons [10]. Epac proteins are guanine exchange factors for the small G protein Rap, and have been shown to regulate cardiac function and insulin secretion [11]. PKA is a conserved serine/threonine kinase that has been implicated in a wide array of biological processes, including cell growth, neural function, cell differentiation and metabolism [12]. In neurons and neurosecretory cells, PKA regulates the release of neurotransmitter and neuropeptides [13]. PKA activity has also been implicated in the execution of rhythmic behaviors, such as sleep and circadian locomotor activity in the fly [14], [15].
Fig. 1. PKA activity is essential for the Exp step. 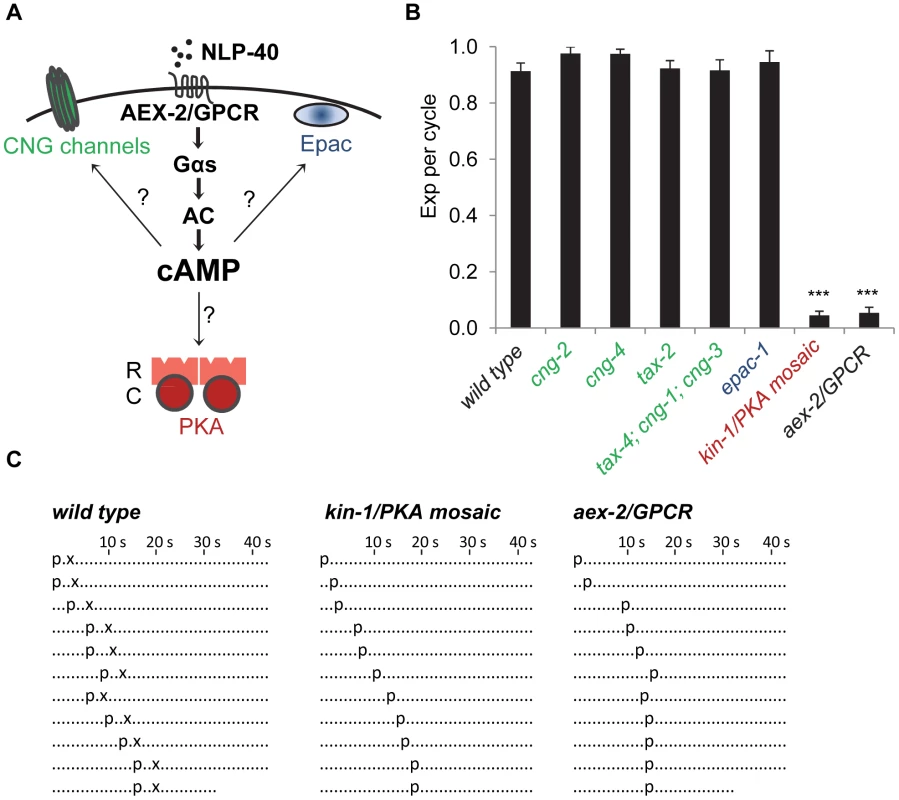
(A) Diagram of the NLP-40-AEX-2/GPCR peptidergic signaling pathway acting in the GABAergic neurons that controls rhythmic release of GABA during the enteric muscle contraction (Exp) step of the defecation motor program. The potential cAMP targets in this peptidergic pathway are indicated. The PKA holoenzyme is a tetramer with two regulatory subunits (R) and two catalytic subunits (C). (B) Quantification of the Exp step in young adult worms with the indicated genotypes. “Exp per cycle” is defined as the average of the ratio of Exp to pBoc. (C) Representative ethograms of ten consecutive defecation cycles of young adult worms with the indicated genotypes. The kin-1 mosaic represents constipated worms segregated from the strain DG3393: kin-1(ok338) I; tnEx109. Each dot represents 1 s. “p” stands for the pBoc step and “x” indicates the Exp step. aBoc is omitted. Means and standard errors are shown. Asterisks (***) indicate significant difference from wild type: p<0.005 in Student's t-test. PKA phosphorylates many substrates in excitable cells. For example, in cardiac muscles, PKA phosphorylates the ryanodine receptor and the L-type calcium channel to regulate heart beating [8], [16]. In neurons, several synaptic proteins, such as RIM-1α, synapsin and tomosyn are reported as PKA substrates that regulate neurotransmitter release [13], [17]. In addition, it has been shown that PKA can phosphorylate calcium channels in hippocampal neurons, which may account for PKA-dependent modulation of neurotransmitter release and gene expression [18]. However, it is unclear how PKA impacts the physiology of neurons to regulate rhythmic behavioral outputs.
The C. elegans defecation motor program is a simple rhythmic behavior that occurs about every 50 seconds [19]. Each cycle contains three sequential muscle contractions: the posterior body wall muscle contraction (pBoc), anterior body wall muscle contraction (aBoc), and enteric muscle contraction (Exp, which leads to the expulsion of the gut contents). The period of the defecation cycle is controlled by a pacemaker in the intestine, and the Exp step is initiated by the release of a neuropeptide-like protein NLP-40 from the intestine. NLP-40 instructs the excitation of a pair of GABAergic neurons (AVL and DVB), which in turn release the neurotransmitter GABA to trigger the Exp step [20]–[23]. NLP-40 activates the GPCR AEX-2 on the GABAergic neurons, which is coupled to the heterotrimeric G protein α subunit GSA-1/Gαs, leading to the activation of adenylyl cyclase and the production of cAMP [5], [23]. However, the molecular targets of cAMP in the GABAergic neurons are not known and how cAMP signaling impacts GABA release to mediate the Exp step is unclear.
In this study, we find that KIN-1, the C. elegans homolog of the PKA catalytic subunit, is essential for the rhythmic contraction of enteric muscles. By genetically manipulating the activity of PKA specifically in the GABAergic neurons, we establish that PKA is the downstream target of cAMP in the peptidergic signaling pathway that controls enteric muscle contraction. Furthermore, using in vivo calcium imaging, we find that PKA activates the DVB neuron by promoting calcium influx at presynaptic terminals, and that the voltage-gated calcium channels, UNC-2 and EGL-19, partially mediate PKA-dependent calcium influx. Thus, our results suggest that PKA signaling can control rhythmic behaviors by regulating presynaptic calcium entry.
Results
PKA regulates the Exp step
To identify cAMP effectors that mediate enteric muscle contraction, we first examined C. elegans mutant worms of putative cAMP targets for defects in the Exp step. The C. elegans genome encodes six CNG channels: cng-1, cng-2, cng-3, cng-4/che-6, tax-2 and tax-4 [24], some of which have well characterized roles in sensory transduction [10], whereas the functions of the other channels are largely unknown. Putative null or loss-of-function mutations in any of these CNGs caused no obvious defects in the defecation motor program period or in the execution of pBoc or Exp (Figure 1B and data not shown). The C. elegans genome encodes a single Epac homolog, epac-1. Putative null epac-1 mutants had no defects in the defecation motor program, including in the Exp step (Figure 1B and data not shown). Thus, CNG channels and Epac are unlikely to be the cAMP targets that control the execution of the Exp step.
The PKA catalytic subunit is encoded by a single gene, kin-1, in C. elegans. kin-1(ok338) loss-of-function mutants, which contain a 763 bp deletion in kin-1 that removes part of the catalytic domain, die during embryogenesis. The lethality of kin-1(ok338) animals can be partially rescued by mosaic expression of wild type kin-1 transgenes [25]. We found that a fraction of mosaic kin-1(ok338) animals that survive to adulthood had distended intestinal lumens indicative of constipation. These animals lacked the Exp step in nearly all defecation cycles but both execution of pBoc and cycle length were normal (Figure 1B and 1C and data not shown). The Exp defects seen in constipated kin-1 mosaic worms were almost identical to those of animals lacking nlp-40 or aex-2/GPCR, which are components of the peptidergic signaling pathway that activates the GABAergic neurons (Figure 1B and 1C and [23]). Taken together, we conclude that PKA activity is absolutely required for the generation of rhythmic enteric muscle contractions and that PKA is likely to be a major, if not the only, downstream target of cAMP during the Exp step of the defecation motor program.
PKA functions in GABAergic neurons to promote the Exp step
To determine whether PKA functions in GABAergic neurons to control the Exp step, we generated worms expressing dominant negative PKA transgenes specifically in these neurons. The PKA holoenzyme is a tetramer composed of two regulatory (R) subunits and two catalytic (C) subunits (Figure 1A). When cAMP levels are low, R subunits bind to and inhibit the activity of C subunits; when cAMP levels increase, two cAMP molecules bind to each of the R subunits at two distinct sites (site A and site B), leading to the dissociation of the PKA holoenzyme and activation of the C subunits [12]. It has been shown that a single amino acid substitution (G324D) in site B of the mouse regulatory subunit (RIα) abolishes cAMP binding and prevents the dissociation of the PKA holoenzyme in vitro [26]. Expression of this mutant R subunit in mice generates a dominant negative effect on PKA activity in vivo [27]. kin-2 encodes the sole R subunit in C. elegans, and KIN-2 shares 74% overall sequence similarity with mouse RIα, and 97% similarity in the cAMP binding sites (Figure S1). We mutated the corresponding Gly residue to Asp (G310D) in the B site of the KIN-2a isoform (referred to as PKA[DN], Figure 2A and S1) and expressed this construct specifically in GABAergic motor neurons (using the full length unc-47 promoter) [5], [23]. Two independently generated PKA[DN] transgenic lines (vjIs76 and vjIs77) displayed distended intestinal lumens, and dramatically reduced cycles in which Exp occurred (Figure 2B and 2D and data not shown). This phenotype was similar to, but not as severe as that of the mosaic kin-1 mutants, likely due to variable expression of the unc-47 promoter in DVB neurons (data not shown). Thus, PKA activity is required in GABAergic neurons to promote the Exp step.
Fig. 2. PKA functions in GABAergic neurons to regulate the Exp step. 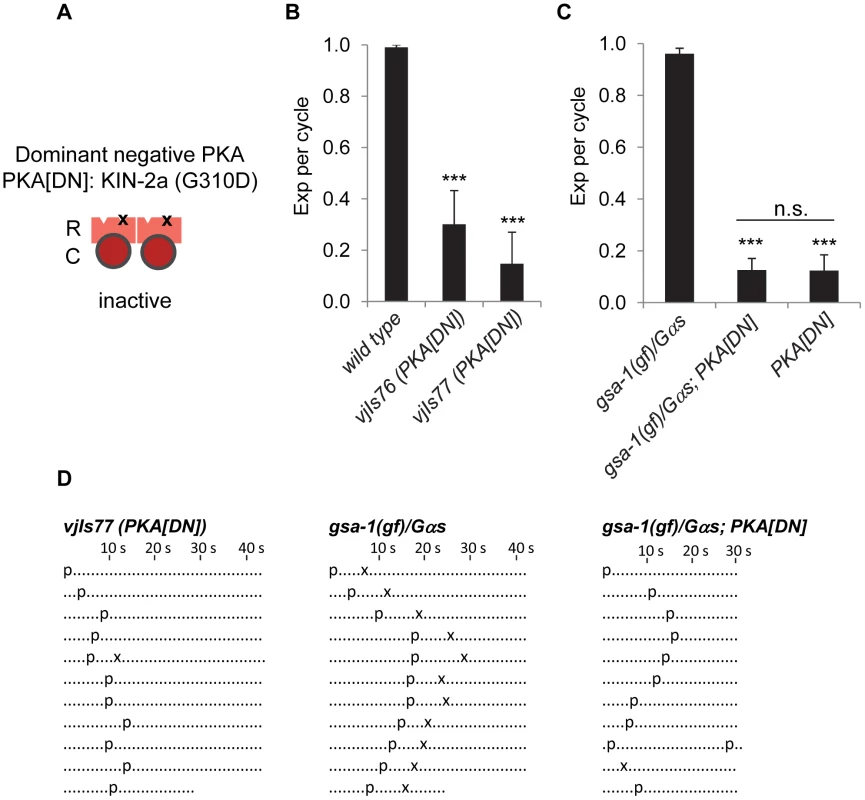
(A) Diagram showing the construction of dominant negative PKA (PKA[DN]). “R” and “C” indicate the PKA regulatory and catalytic subunit, respectively. “x” represents the substitution (G310D) in the site B of the regulatory subunit KIN-2a, which presumably blocks cAMP binding and prevents its dissociation with PKA catalytic subunit. (B) and (C) Quantification of the Exp step of young adults with the indicated genotypes. PKA[DN] denotes PKA dominant negative transgenic worms (vjIs76 and vjIs77) in which the mutated regulatory subunit kin-2a(G310D) was expressed specifically in GABAergic neurons using unc-47 full length promoter. gsa-1(gf) is a gain-of-function allele (ce81) of gsa-1/Gαs. (D) Representative ethograms of ten consecutive defecation cycles of young adult worms with the indicated genotypes. vjIs77 is used for PKA[DN] in (D). Each dot represents 1 s. “p” stands for the pBoc step and “x” indicates the Exp step. aBoc is omitted. Means and standard errors are shown. Asterisks (***) indicate significant difference from wild type in (B) and gas-1(gf)/Gas in (C): p<0.005 in Student's t-test. “n.s.” indicates no significant difference between indicated groups. PKA acts downstream of Gαs in the NLP-40-AEX-2/GPCR peptidergic signaling pathway
To determine whether PKA functions in the peptidergic signaling pathway activated in the GABAergic neurons that control the Exp step, we examined whether PKA[DN] transgenes could block Exp in animals in which this pathway is constitutively active. gsa-1/Gαs gain-of-function (gsa-1(gf)) mutations restore Exp to aex-2/GPCR mutants, consistent with a role for gsa-1/Gαs downstream of aex-2/GPCR [5]. However, gsa-1(gf) mutations failed to restore Exp to animals expressing PKA[DN] (Figure 2C and 2D). These data show that the effects of the AEX-2/GPCR peptidergic signaling pathway on Exp are dependent on PKA in the GABAergic neurons. For reasons that are unclear, the gsa-1(ce81); PKA[DN] double mutants had a slightly shorter cycle length compared with either single mutant (Figure 2D and data not shown).
To independently confirm that PKA functions in the AEX-2/GPCR peptidergic signaling pathway to regulate the Exp step, we generated a constitutively active kin-1/PKA variant (referred to as PKA[CA]) and examined whether PKA[CA] transgenes could bypass the requirement of nlp-40 or aex-2/GPCR. The His87 and Trp196 residues in the mouse PKA catalytic α subunit lie at the interface between the R and C subunits and are necessary for the interaction between them [28]. H87Q, W196R substitutions disrupt this interaction resulting in a constitutively active catalytic subunit that increases PKA activity in vivo [29], [30]. The C. elegans KIN-1a isoform shares 91% amino acid identity with the mouse PKA catalytic α subunit (Figure S2). We made the corresponding substitutions in the KIN-1a isoform (H96Q, W205R) (Figure 3A and S2), and generated two independent transgenic lines (vjIs102 and vjIs103) expressing KIN-1a(H96Q, W205R) specifically in GABAergic neurons (using the full length unc-47 promoter).
Fig. 3. Constitutively active PKA in GABAergic neurons partially bypasses the requirement of AEX-2/GPCR. 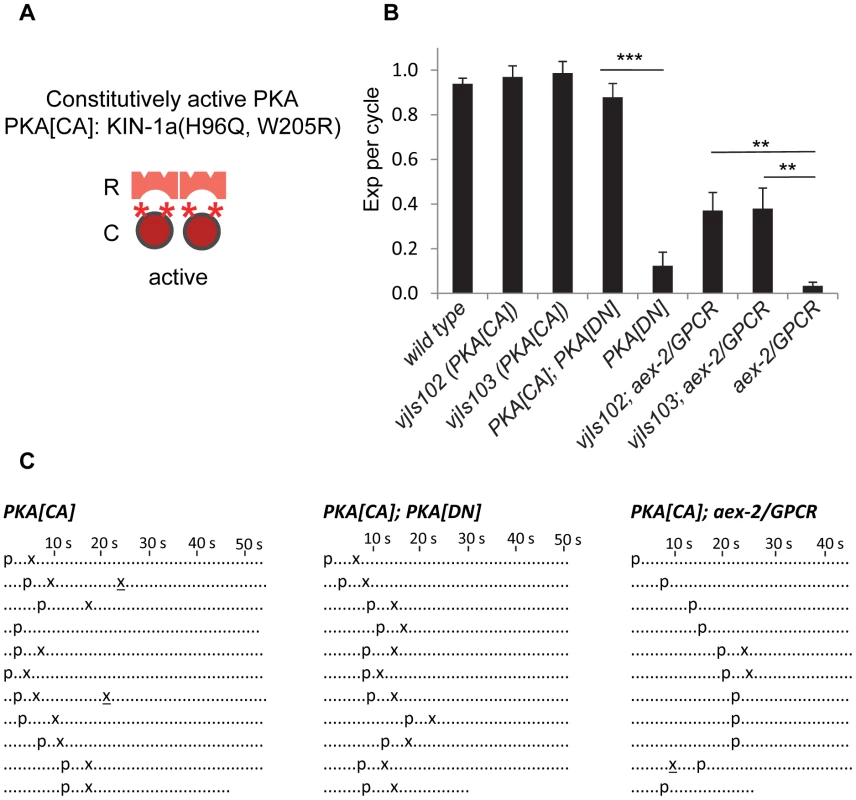
(A) Diagram showing the construction of the constitutively active PKA (PKA[CA]). “R” and “C” indicate the PKA regulatory and catalytic subunit, respectively. The two asterisks (*) represent the two mutations (H96Q, W205R) in the PKA catalytic subunit KIN-1a, which presumably disrupt its association with the regulatory subunit but do not affect its enzymatic activity. (B) Quantification of the Exp step of young adults with the indicated genotypes. PKA[CA] denotes PKA constitutively active transgenic worms (vjIs102 and vjIs103) expressing the mutated catalytic subunit kin-1a(H96Q, W205R) in the GABAergic neurons using the unc-47 full length promoter. vjIs103 and vjIs77 were used for the PKA[CA];PKA[DN] strain. (C) Representative ethograms of ten consecutive defecation cycles of young adult worms with the indicated genotypes. Each dot represents 1 s. “p” stands for the pBoc step and “x” indicates the Exp step. Ectopic Exp steps are indicated by “x”. Means and standard errors are shown. Asterisks indicate significant differences between indicated groups: ** P<0.01, *** P<0.005 in Student's t-test. Several pieces of evidence support the notion that PKA[CA] transgenes confer constitutive PKA activity. First, animals expressing PKA[CA] transgenes had grossly normal defecation motor programs (Figure 3B and 3C) but occasionally displayed “ectopic” Exp following normal Exp steps (4 out of 100 cycles in vjIs102 and 10 out of 100 cycles vjIs103. p = 0.11 and 0.03, respectively, n = 10, one tail t-test, compared to wild type) (Figure 3C). Ectopic Exp steps were also observed in kin-2(ce179) mutants (5 out of 100 cycles, p = 0.09, n = 10), which carry a mutation in the PKA regulatory subunit that is predicted to increase PKA catalytic activity [31]. Second, ectopic Exp steps are also observed in gsa-1/Gαs gain-of-function mutants, which mimic constitutive peptidergic activation of the pathway in GABAergic neurons [5]. Finally, PKA[CA] transgenes completely suppressed the Exp defects caused by PKA[DN] expression (Figure 3B and 3C), demonstrating that PKA[CA] is not inhibited by the PKA regulatory subunit.
We found that PKA[CA] transgenes partially restored Exp to aex-2/GPCR and nlp-40 mutants (Figure 3B, 3C, and S3). Similarly, kin-2 loss-of-function mutations restored Exp to nlp-40 mutants to a similar extent as PKA[CA] transgenes (Figure S3). Constitutively active acy-1/adenylyl cyclase transgenes in GABAergic neurons have also been reported to partially rescue the Exp defects of aex-2/GPCR mutants [5]. Taken together, these results are consistent with the idea that cAMP generated by the NLP-40-AEX-2/GPCR peptidergic signaling pathway in the GABAergic neurons activates PKA, which drives Exp by promoting GABA release.
PKA regulates rhythmic calcium influx in the DVB neuron
We previously found that during the defecation motor program, the axons of DVB neurons display robust, rapid calcium transients that peak just before each Exp step, suggesting that rhythmic presynaptic calcium influx in DVB drives rhythmic GABA release from DVB neurons. Using a genetically-encoded calcium indicator, GCaMP3 [32], we found that fluorescent spikes in DVB axons began about 3 seconds following the pBoc step, reached maximal intensity about 1 second later (immediately before each Exp step), and returned to baseline within 2 seconds (Figure 4A, 4B and 4C and Video S1 and [23]). In most cases, calcium transients appeared to initiate in the synaptic region of DVB neurons and often would spread along the axon to the cell body. In wild type animals the “normal” pattern of pBoc-calcium transient-Exp was highly reproducible, occurring 100% of the time (23 cycles, 11 animals, Figure 4C and 4D). In mutants lacking aex-2/GPCR, only about 9% of cycles adopted a normal pattern (4 out of 44 cycles, 11 animals), and the remaining 91% of cycles lacked both the calcium transients and Exp (40 out of 44 cycles, 11 animals, Figure 4C and 4D and Video S2). In contrast, in unc-25 mutants, which lack the GABA biosynthetic enzyme glutamic acid decarboxylase (GAD) [33], most of the cycles without Exp steps still produced a calcium spike (31 out of 33 cycles without Exp, 11 animals, Figure 4C and 4D and Video S3). These results show that the generation of calcium transients is dependent on the peptidergic signaling pathway but occurs independently of GABA release from DVB neurons, and are consistent with the idea that the calcium spikes in the synaptic region of DVB neurons drive the Exp step by triggering GABA release.
Fig. 4. PKA is necessary for calcium influx in DVB neurons. 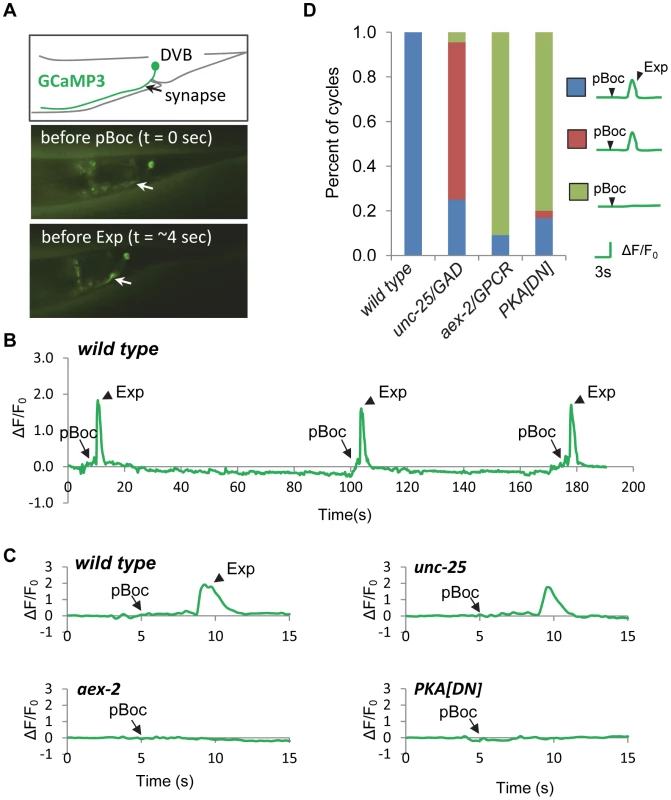
(A) Expression of the genetically-encoded calcium indicator, GCaMP3 in DVB neurons (vjIs58). Top: diagram of the DVB neuron in the tail region. Synapse means the neuromuscular junction where the DVB neuron innervates enteric muscles. Middle and Bottom: two snapshots from a real-time imaging video of wild type animals showing an increase in fluorescence in synaptic region of DVB neurons, as indicated by the white arrow, right before the Exp step. (B) A representative trace of the GCaMP3 fluorescence in the synaptic region of DVB neurons in wild type animals showing DVB neurons are rhythmically activated during three consecutive defecation cycles. Note that the cycle length is longer than 50 seconds, likely due to unc-13(s69) mutation, which was used to immobilize the worms for calcium imaging (See Materials and Methods for details). (C) Representative traces of the GCaMP3 fluorescence in the synaptic region of DVB neurons in worms with the indicated genotypes. The observed pBoc step and Exp step are indicated by arrows and arrowheads, respectively. (D) Classification of the different patterns of pBoc, fluorescent spikes in DVB and Exp in worms with the indicated genotypes. vjIs76 was used for the PKA[DN] strain. In (D), wild type: 23 cycles in 11 animals; unc-25: 44 cycles in 11 animals; aex-2: 44 cycles in 11 animals; PKA[DN]: 30 cycles in 12 animals. Our behavioral results show that PKA activity may be necessary and sufficient for GABA release from the GABAergic neurons. In principal, PKA could control GABA release directly by acting on the synaptic vesicle machinery at presynaptic terminals or indirectly by regulating the excitability of the GABAergic neurons. To distinguish between these two possibilities, we examined whether PKA[DN] or PKA[CA] transgenes impacted GCaMP3 fluorescence spikes in DVB neurons during the defecation motor program. PKA[DN] transgenes produced a calcium pattern that was similar to that observed in aex-2/GPCR mutants: 80% of cycles lacked both calcium transients and the Exp steps (31 cycles, 12 animals, Figure 4C and 4D and Video S4). On the other hand, PKA[CA] transgenes caused “ectopic” fluorescent transients in DVB neurons, which occurred once or twice in between cycles on average (22 regular calcium spikes and 26 ectopic calcium spikes observed during 24 cycles, 11 animals, Figure 5A, 5B and 5C and Video S6). These “ectopic” fluorescent transients were occasionally associated with an ectopic Exp (6 out of the 26 ectopic calcium spikes, 11 animals, see Discussion). The duration of both the “normal” and “ectopic” fluorescent transients in PKA[CA] transgenic animals was significantly longer compared to wild type controls (Figure 5D and Video S5 and S6). However, the amplitude of calcium transients was not significantly different (Figure 5E, wild type: ΔF/F0 = 1.83±0.17, n = 30; PKA[CA]: normal calcium transients ΔF/F0 = 2.37±0.22, n = 21, p = 0.06; ectopic calcium transients, ΔF/F0 = 2.08±0.22, n = 26, p = 0.39, two tail t-test, compared to wild type). Taken together, we conclude that PKA is essential for the generation of calcium transients in DVB neurons during the defecation cycle. In addition, PKA activity is sufficient to generate calcium transients and may positively regulate the duration but not the amplitude of calcium transients.
Fig. 5. Constitutively active PKA causes ectopic calcium spikes in DVB neurons and increases calcium spike duration. 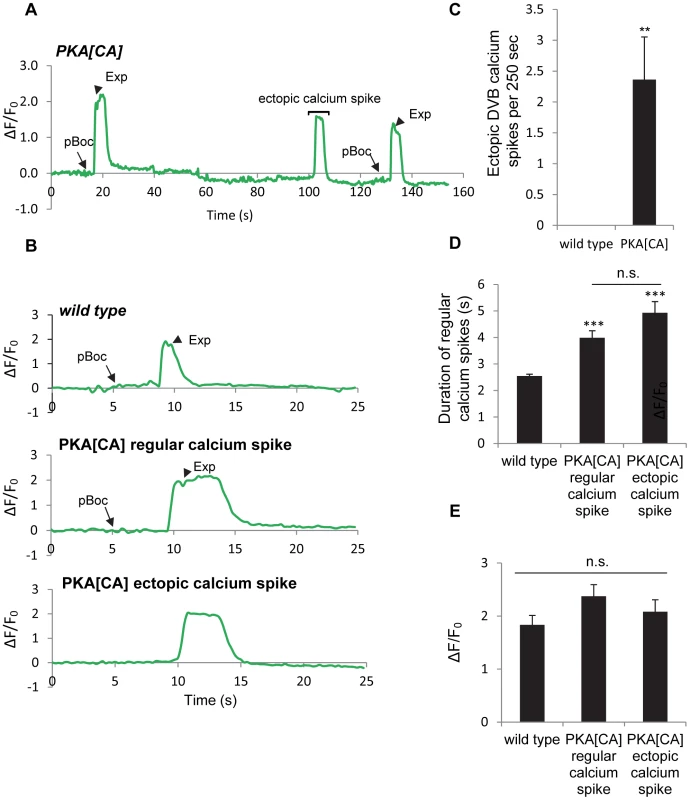
(A) A representative trace of the GCaMP3 fluorescence in the synaptic region of DVB neurons in transgenic worms expressing PKA[CA] (vjIs102) during two consecutive defecation cycles. An ectopic calcium spike in DVB neurons was observed, as indicated by the bracket. Note that the cycle length is longer than 50 seconds, likely due to unc-13(s69) mutation, which was used to immobilize the worms for calcium imaging (See Materials and Methods for details). (B) Representative traces of the GCaMP3 fluorescence in the synaptic region of DVB neurons in worms with the indicated genotypes. The observed pBoc step and Exp step are indicated by arrows and arrowheads, respectively. (C) Average frequency of ectopic calcium spikes in DVB neurons during a 250-second imaging period in wild type animals (0.0±0.00 events per 250 seconds, n = 10 animals) and vjIs102 (2.4±0.69 events per 250 seconds, n = 11 animals). (D) and (E) the duration and amplitude of regular DVB calcium spikes and ectopic calcium spikes in wild type and PKA[CA] (vjIs102) animals. Means and standard errors are shown. Asterisks indicate significant differences from wild type: ** P<0.01, *** P<0.005 in Student's t-test. “n.s.” indicates no significant difference between indicated groups. UNC-2/VGCC acts downstream of PKA and mediates calcium influx in the DVB neuron
Voltage-gated calcium channels (VGCCs) are critical for presynaptic calcium influx during regulated neurotransmitter release [34]. unc-2 encodes the α1 subunit of the C. elegans P/Q type VGCC. UNC-2 localizes to presynaptic terminals, and promotes calcium influx and neurotransmitter release [35], [36]. unc-2 has been reported to be expressed in the DVB neuron, and to regulate the Exp step [37]. Consistently, we observed the Exp step in only about 40% of the defecation cycles in unc-2(lj1) null mutants (Figure 6A). In unc-2 mutants expressing PKA[CA], the Exp step was observed in about 60% of cycles (Figure 6A). The incomplete restoration of the Exp step by PKA[CA] is consistent with the idea that UNC-2 functions downstream of or in parallel to PKA to regulate Exp.
Fig. 6. unc-2/VGCC functions downstream of PKA to mediate calcium influx in the GABAergic neurons. 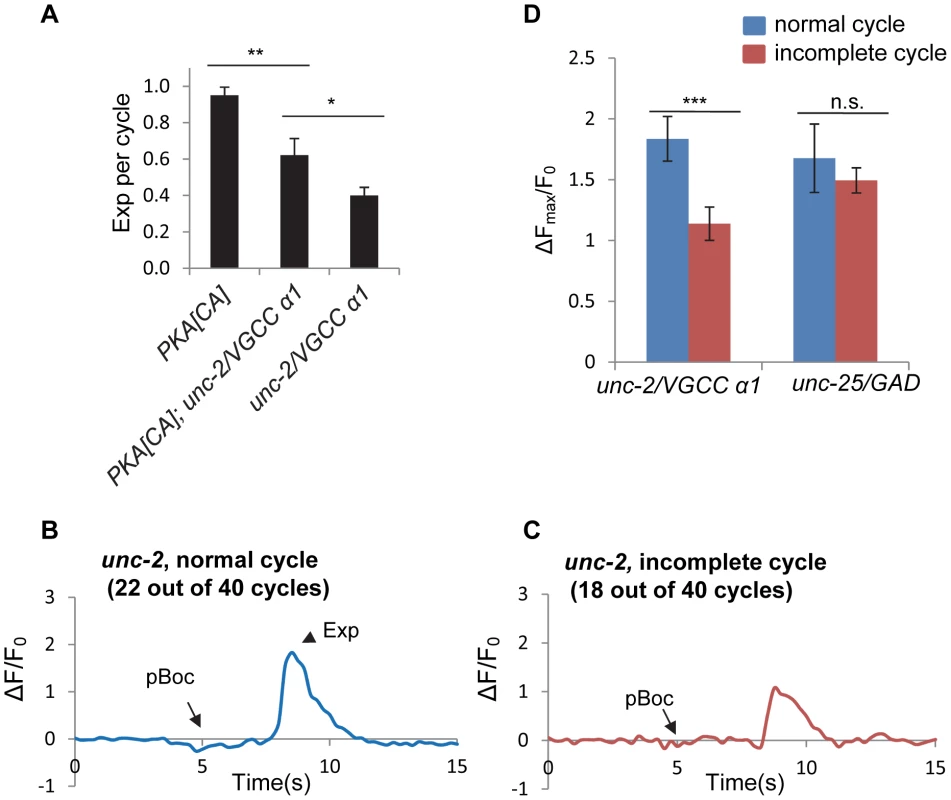
(A) Quantification of the Exp step of young adult worms with the indicated genotypes. vjIs103 was used for the PKA[CA] strain. (B) and (C) Representative trace of GCaMP3 fluorescence in the synaptic region of DVB neurons in unc-2 mutants in normal cycles, with the Exp step (B) and incomplete cycles, without the Exp step (C). (D) Comparison of the maximal change (ΔFmax/F0) of GCaMP3 fluorescence in the synaptic region of DVB neurons between the normal cycles and the incomplete cycles in unc-2 mutants and unc-25 mutants. vjIs58, the transgenic strain with GCaMP3 expressed in the DVB neuron, was used. Normal cycles: n = 22 in unc-2(lj1) mutants; n = 10 in unc-25(e156) mutants. Incomplete cycles: n = 18 in unc-2(lj1) mutants; n = 31 in unc-25(e156) mutants. Means and standard errors are shown. Asterisks indicate significant differences: * P<0.05, *** P<0.0005 in Student's t-test. To test whether the rhythmic calcium transients are mediated by UNC-2, we examined GCaMP3 fluorescence in synaptic regions of DVB neurons in unc-2 mutants. Surprisingly, fluorescent transients were observed in all cycles including the normal cycles that had the Exp steps and the incomplete cycles without the Exp steps (Figure 6B and 6C and Video S7 and S8). However, there was a significant reduction in the average amplitude of fluorescent transients in the incomplete cycles (that lacked Exp, n = 18) compared to the fluorescent transients in the normal cycles (those with Exp, n = 22) (Figure 6B, 6C and 6D). To determine if the reduction in fluorescence amplitude in incomplete cycles was a secondary effect of compromised Exp in these mutants, we examined fluorescence amplitudes in mutants lacking unc-25/GAD. We found no difference in the average amplitude between normal and incomplete cycles in unc-25/GAD mutants (n = 10 and 31 cycles, respectively, and Figure 6D). These results indicate that UNC-2 is not absolutely required for the generation of calcium transients but rather regulates the size of calcium transients, possibly allowing them to reach the threshold required for triggering Exp.
Other non voltage-gated calcium channels may also mediate calcium influx in the DVB neuron
Because unc-2 does not appear be to the only channel to mediate the calcium transients in DVB neurons for the Exp step, we next sought to identify other calcium channels that might function in this process. The C. elegans genome encodes two additional VGCC α1 subunits: egl-19, the L-type α1 subunit, and cca-1, the T-type α1 subunit [38], [39]. We found that loss-of-function cca-1/VGCC mutants had normal Exp steps, whereas loss-of-function egl-19/VGCC mutants displayed a modest reduction in the number of cycles with Exp (Figure 7A). Interestingly, in double mutants lacking both unc-2 and egl-19, the Exp step was nearly eliminated (Figure 7B). Furthermore, PKA[CA]; egl-19; unc-2 triple mutants had the same, severely reduced Exp frequency as the egl-19; unc-2 double mutants (Figure 7B). Thus, two VGCCs, egl-19 and unc-2, function downstream of PKA to control the Exp step.
Fig. 7. Other non voltage-gated calcium channels are required from calcium influx in the GABAergic neurons. 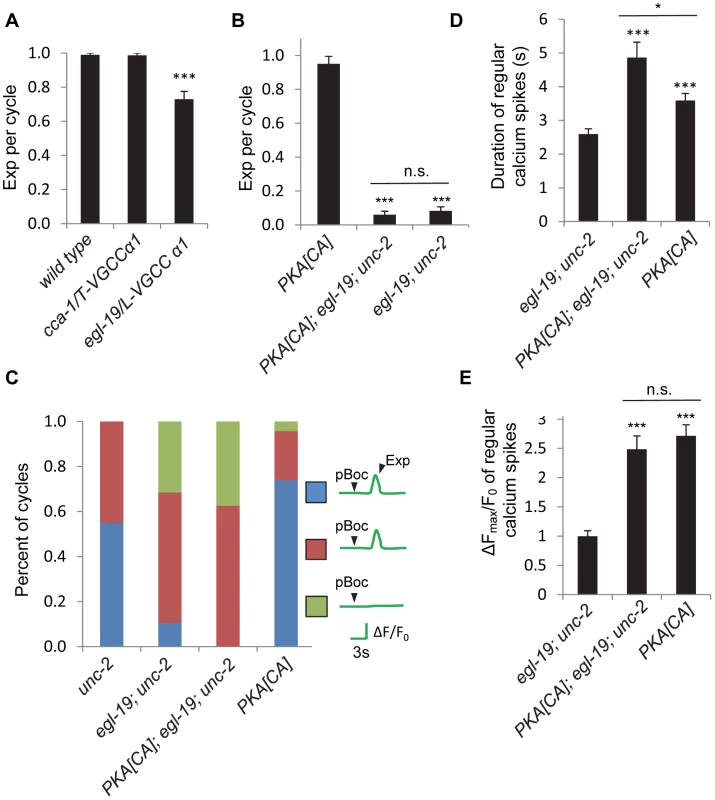
(A) and (B) Quantification of the Exp step of young adult worms with the indicated genotype types. (C) Classification of different patterns of pBoc, fluorescent spikes in DVB and Exp in worms with the indicated genotypes. unc-2: 40 cycles in 21 animals, egl-19; unc-2: 19 cycles in 11 animals, PKA[CA]; egl-19; unc-2: 24 cycles in 12 animals, PKA[CA]: 23 cycles in 12 animals. (D) and (E) Quantification of the duration and amplitude of regular DVB calcium spikes in worms with indicated genotypes. PKA[CA] represents transgenic worms with constitutively active PKA specifically expressed in GABAergic neurons (vjIs103 in (B) and vjIs102 in (C, D)). Note that in (C), (D) and (E), unc-2 and PKA[CA] strains, but not the egl-19; unc-2 and the PKA[CA]; egl-19; unc-2 strains contain the unc-13(s69) mutation for immobilization, as egl-19;unc-2 alone were almost completely paralyzed. vjIs58, the transgenic strain with GCaMP3 expressed in the DVB neuron, was used for the unc-2 strain; while vjIs64 was used for calcium imaging in other genotypes. Means and standard errors are shown. Asterisks indicate significant difference between indicated group and significant difference from wild type in (A), PKA[CA] in (B) and egl-19; unc-2 mutants in (D) and (E): * p<0.05; ***P<0.005 in Student's t-test. “n.s.” indicates no significant difference between indicated groups. To directly test whether egl-19/VGCC mediates calcium influx in DVB neurons, we next examined the effects of egl-19 mutations on GCaMP3 fluorescence in DVB neurons. We were unable to analyze calcium influx in egl-19 mutants due to an unexpected genetic interaction between egl-19 and unc-13(s69) (used to paralyze animals for imaging without stopping the defecation cycle) that suppressed the Exp defects of egl-19 mutants (see Materials and Methods). Instead, we examined calcium influx in egl-19; unc-2 double mutants (which were sufficiently paralyzed for imaging without the unc-13(s69) mutation). We found that the fraction of incomplete cycles increased to 80% (from 40% in unc-2 mutants, Figure 7C and Video S9). Finally, no calcium influx was observed at all in 35% of the incomplete cycles, a defect that was not observed in the unc-2 mutants alone (Figure 7C and Video S10). In egl-19; unc-2 double mutants expressing PKA[CA] transgenes, these defects in calcium influx were as severe as those observed in egl-19; unc-2 mutants (Figure 7C), suggesting that egl-19 and unc-2 function downstream of PKA to promote calcium influx into DVB.
egl-19 and unc-2 mutations were not able to block all effects of PKA[CA] transgenes, including the increased frequency of ectopic calcium transients and the increased duration of normal and ectopic calcium transients (Figures 7D and S4 and Video S11 and S12). In addition, the amplitude of regular calcium spikes of the incomplete cycles in egl-19; unc-2 mutants expressing PKA[CA] transgenic was similar to those in PKA[CA] transgenic animals, but significantly larger than those observe in egl-19; unc-2 double mutants (Figure 7E). Together these results suggest that the effects of egl-19 and unc-2 mutations on Exp can in part be attributed to their requirement for calcium influx in DVB neurons and that additional calcium channels must act downstream of PKA to mediate calcium influx in DVB.
Discussion
In this study, we identify PKA as the major downstream target of cAMP in the NLP-40-AEX-2/GPCR peptidergic signaling pathway that functions in the GABAergic neurons to regulate the Exp step during the defecation motor program in C. elegans. PKA controls rhythmic activation of the GABAergic neurons by promoting presynaptic calcium influx in these neurons. We find that the mechanism by which PKA functions to promote rhythmic calcium influx is partially dependent on the P/Q-type VGCC, UNC-2 and the L-type VGCC, EGL-19. These results suggest that PKA promotes rhythmic neurotransmitter release by controlling calcium influx in neurons during a rhythmic behavior.
PKA is essential for presynaptic calcium influx in DVB neurons
Our results suggest that PKA functions as an essential cue for calcium influx in neurons for their activation to control rhythmic behaviors, because both calcium spikes in DVB neurons and Exp steps are abolished in most of the defecation cycles in animals expressing PKA[DN] transgenes (Figure 2B, 2D, 4C and 4D). To our knowledge, this is the first in vivo example showing that PKA in neurons is absolutely required for the execution of a rhythmic behavior. In many preparations, PKA has been shown to modulate biological processes by regulating intracellular calcium concentration. One classic example is that PKA mediates the enhancement of calcium influx in cardiac myocytes to modulate the rate of heart beating in response to norepinephrine from the sympathetic nervous system [8]. In neurons, PKA also has a modulatory role in regulation of synaptic transmission and synaptic plasticity by phosphorylating several synaptic vesicle proteins that function downstream of calcium influx [13].
UNC-2 and EGL-19 mediate part of PKA-dependent calcium influx in DVB neurons
How does PKA control calcium influx in the GABAergic neurons? Our results suggest that PKA acts at least partially through UNC-2, the P/Q-type VGCC, and EGL-19, the L-type VGCC. unc-2 has been reported to be expressed in the DVB neuron and egl-19 is expressed in many neurons as well [37], [38], . Our behavioral and calcium imaging analysis of egl-19; unc-2 double mutants expressing PKA[CA] transgenes suggests that one or both of these channels must also function in enteric muscles to promote Exp since PKA[CA] can restore normal calcium spike amplitudes but it fails to restore the Exp step in egl-19; unc-2 double mutants (Figure 7B, 7C and 7E). Consistent with this idea, egl-19 has been reported to be expressed in muscles, including some of the enteric muscles [38]. Our data indicates that other non voltage-gated calcium channels must be also required for calcium influx in DVB neurons for the Exp step, since egl-19 and unc-2 mutations together could not completely block the calcium influx in DVB neurons or the effects of PKA[CA] transgenes on calcium influx (Figure 7C, 7D and 7E). The identification of these channels will further our understanding of the mechanism underlying PKA-dependent calcium influx in these neurons.
PKA may regulate calcium influx either by direct phosphorylation of UNC-2 and/or EGL-19 or by an indirect mechanism involving, for example, the regulation of membrane potential. Indeed, both mechanisms have been reported. The fight-or-flight response is controlled by PKA-dependent phosphorylation of the L-type calcium channels Cav1.1 and Cav1.2 which mediate calcium influx in skeletal and cardiac muscles, respectively, to enhance their contraction [8], [41]. EGL-19 does not have the homologous phosphorylation site. In pancreatic beta cells, PKA has been reported to phosphorylate ATP-sensitive potassium channels to regulate the membrane potential [42]. Interestingly, the egl-36/Shaw type potassium channel functions in DVB to regulate the Exp step [43], raising the possibility that it may be a target for PKA.
Our results also show that PKA[CA] transgenes increases the duration of calcium spikes in DVB neurons in both wild type and egl-19; unc-2 mutants, suggesting that PKA regulates calcium spike dynamics independently of egl-19 and unc-2. PKA might regulate the open time or the inactivation of other calcium channels or reduce the rate of calcium clearance from the synaptic region in DVB neurons. Consistent with our observation, injection of the PKA catalytic subunits in cells has been reported to increase the duration of calcium currents [44]–[46].
The activity of PKA in the GABAergic neurons during the defecation cycle
Our previous work indicates that NLP-40 functions as the timing signal from the intestine and it delivers the timing information to the GABAergic neurons by instructing their rhythmic activation [23]. In this study, we show that the downstream effector PKA is also instructive for the activation of the GABAergic neurons, because the PKA[CA] transgenes can elicit ectopic calcium spikes in between cycles. The observation that ectopic calcium spikes in DVB neurons do not occur in wild type animals suggests that endogenous PKA activity in these neurons must somehow be turned down rapidly following each Exp step. Thus, we propose a model in which rhythmic activation of PKA by the NLP-40-AEX-2/GPCR peptidergic pathway stimulates rhythmic calcium influx in the GABAergic neurons to drive the Exp step. It will be interesting to directly determine whether cAMP levels and/or PKA activity oscillate in DVB neurons and whether these oscillations correspond with the calcium oscillations in vivo. Several negative feedback mechanisms following PKA activation that have been reported in other preparations may also help establish rhythmic PKA activity in DVB neurons. These include the activation of by phosphodiesterases (PDEs) that break down cAMP, calcium-mediated inhibition of adenylyl cyclases and activation of specific phosphatases that counteract PKA activity [9], [47], [48]. PKA activity has been reported to be essential for the initiation of the cAMP-PKA-Ca2+ oscillation circuit in insulin-secreting MIN6 beta cells (Ni et al., 2010). Thus, the interplay between cAMP, PKA and calcium may be a general mechanism by which PKA generates oscillatory signaling circuits in neurons to control rhythmic behaviors.
Rhythmic PKA activation may be essential for the reliability of the Exp step during the defecation cycle, because PKA[CA] transgenes cannot fully restore Exp to animals that lack the NLP-40-AEX-2/GPCR peptidergic signaling pathway components (Figure 3B). Similarly, constitutive acy-1/adenylyl cyclase expression in the GABAergic neurons also only partially rescues the Exp defects of aex-2 mutants [5]. Interestingly, gsa-1(gf)/Gαs mutations almost fully rescue the Exp defects in aex-2/GPCR mutants [5]. Since GSA-1/Gαs functions upstream of both adenylyl cyclase and PKA, it is possible that gsa-1(gf) mutants retain the proper negative feedback mechanisms that allow PKA activity to remain rhythmic.
Rhythmic PKA activation may work together with other mechanisms to ensure the proper execution of the Exp step. Indeed, it has been postulated that a permissive signal may control the refractory period of enteric muscles by allowing Exp to occur only within a small window of time following the beginning of each cycle (the pBoc step) [5], [23]. We speculate that this permissive signal may be entrained to the pacemaker activity in the intestine, because most of the Exp steps observed in the aex-2 mutants expressing PKA[CA] did not happen at random times, but rather occurred a few seconds after the pBoc step. The absence of this permissive signal in between cycles may also explain why PKA[CA] elicits ectopic calcium spikes in DVB neurons more frequently than ectopic Exp steps. The identification of this permissive signal or the nature of the refractory period of enteric muscles would provide more comprehensive understanding on how the rhythmic Exp is reliably generated.
Using genetically-encoded PKA transgenes to dissect the function of PKA in vivo
In mammals, several genes encode different PKA regulatory and catalytic subunits, and each of these genes may have several different isoforms due to alternative splicing [12]. Although many PKA isoforms have been knocked out and knocked in in mice to dissect the role of PKA in vivo, compensation by remaining isoforms has complicated the interpretation of these studies [27], [49], [50]. Unlike mammals, C. elegans has a single gene (kin-2) for the PKA regulatory subunit and a single gene (kin-1) for the catalytic subunit, both of which share high similarities with their counterparts in higher mammals. Thus, C. elegans has the potential to be a good genetic model to dissect the physiological roles of PKA. However, studies on the contribution of PKA signaling in C. elegans have been limited since null mutants of either kin-1/catalytic subunit or kin-2/regulatory subunit are lethal. Various strategies using weak alleles of kin-2, pharmacological treatments with PKA inhibitors, and RNAi-mediated knockdown, have revealed roles for PKA in regulating neurotransmitter release, axon regeneration and behaviors [2], [31], [51]. However, non-specific effects of pharmacological PKA inhibitors and spreading of RNAi limit the utility of these approaches [52], [53]. The ability to manipulate PKA activity in a tissue specific manner using the PKA variants developed in this study represents a powerful approach for probing the function of PKA signaling in vivo.
Materials and Methods
Strains
Strains were maintained at 20°C on NGM plates with E. coli strain OP50 as food. The Bristol strain N2 was used as reference strain. The strains used in this study: OJ1672 cng-2(tm4267) IV, FX05036 cng-4/che-6 (tm5036) IV, OJ1748 tax-2(p671) I, KJ5562 tax-4(p678) III; cng-3(jh113) IV; cng-1(jh111) V, RB830 epac-1(ok655) III, DG3393 tnEx109; kin-1(ok338) I, OJ1540 aex-2(sa3) X, OJ1603 vjIs76 [Pttx-3::RFP, 40 ng/µl; Punc-47(FL):: kin-2a(G310D), 50 ng/µl] V, OJ1601 vjIs77 [Pttx-3::RFP, 40 ng/µl; Punc-47(FL):: kin-2a(G310D), 50 ng/µl] IV, KG421 gsa-1(ce81) I, OJ1908 gsa-1(ce81) I; vjIs77 IV, OJ1854 vjIs102 [Pmyo-2::NLS::GFP, 10 ng/µl; Punc-47(FL)::kin-1a(H96R, W205Q), 50 ng/µl] V, OJ1858 vjIs103 [Pmyo-2::NLS::GFP, 10 ng/µl; Punc-47(FL)::kin-1a(H96R, W205Q, 50 ng/µl)] I, OJ1896 vjIs103 I; vjIs77 IV, OJ1909 vjIs102 V; aex-2(sa3) X, OJ1910 vjIs103 I; aex-2(sa3) X, OJ680 unc-13(s69) I, OJ1213 vjIs58 [Pmyo-2::NLS::mCherry, 10 ng/µl; Punc-47(mini)::GCaMP3, 125 ng/µl] IV, OJ1443 unc-13(s69) I; vjIS58 IV, OJ1468 unc-13(s69) I; vjIS58 IV; aex-2(sa3) X, OJ1759 unc-13(s69) I; vjIs58 IV; vjIs76 V, OJ1859 unc-13(s69) I; unc-25(e156) III; vjIs58 IV, OJ1917 unc-13(s69) I; vjIs58 IV; vjIs102 V, OJ1526 unc-2(lj1) X; OJ1899 vjIs103 I; unc-2(lj1) X; OJ1919 unc-13(s69) I; vjIs58 IV; unc-2(lj1) X, JD21 cca-1(ad1650) X, OJ1911 egl-19(n582) IV, OJ1925 egl-19(n582) IV; unc-2(lj1) X, OJ1924 vjIs103 I; egl-19(n582) IV; unc-2(lj1) X, OJ1351 vjIs64 [Pmyo-2::NLS::mCherry, 10 ng/µl; Punc-47(mini)::GCaMP3, 125 ng/µl] II, OJ1905 unc-13(s69) I; vjIs64 II, OJ1918 vjIs64 II; egl-19(n582) IV; unc-2(lj1) X, OJ1923 unc-13(s69) I; vjIs64 II; vjIs102 V, OJ1949 vjIs64 II; egl-19(n582) IV; vjIs102 V; unc-2(lj1) X, OJ794 nlp-40(tm4085) I, OJ1524 kin-2(ce179) X, OJ1525 nlp-40(tm4085) I; kin-2(ce179) X, OJ1855 nlp-40(tm4085) I; vjIs102 V, OJ1856 nlp-40(tm4085) vjIs103 I.
cng-2(tm4267) mutants contain a 330 bp deletion and a single nucleotide (g) insertion (www.wormbase.org), which is predicted to generate a frame shift that truncates CNG-2 at the C-terminus. cng-2(tm4267) was originally reported to be sterile and lethal (Mitani Lab). However, we found that after outcross cng-2(tm4267) mutants were viable.
Behavioral assay for the defecation motor program
The defecation motor program was analyzed as previously described [19], [54]. Briefly, L4 stage hermaphrodites were transferred to a new plate. After about 20–24 hours, each worm was transferred to a fresh NMG plate and let to settle down for at least five minutes. Ten consecutive defecation cycles were scored for each worm using the Etho program software (http://depts.washington.edu/jtlab/software/otherSoftware.html) [54]. Only the pBoc and the Exp steps were scored, omitting the aBoc step. 8–10 worms were assayed for each genotype. “Exp per cycle” for each worm was calculated as the ratio of Exp over pBoc. The results were present as mean ± sem for each genotype. Unpaired two-tail Student's t-test with unequal variance was used to examine the significant difference between two different genotypes.
Molecular biology
The backbone of the plasmids constructed below was pPD49.26 (A. Fire).
Punc-47(FL), the 1444 bp full length unc-47 promoter, which was expressed in all GABAergic neurons in C. elegans [5], was amplified from N2 genomic DNA using 5′ primer: ccccccGCATGCatgttgtcatcacttcaaactt and 3′ primer ccccccGGATCCctgtaatgaaataaatgtgacgctg. The PCR product was partially digested with SphI and BamHI, and cloned into the MCSI of a derivative plasmid of pPD49.26.
Punc-47(mini), the 215 bp unc-47 mini promoter, which was only expressed in a subset of GABAergic neurons (4 RME, RIS, AVL and DVB neurons) [55], was amplified from N2 genomic DNA with 5′ primer ccccccGCATGCCTGCAGctttcggtttggagagtag and 3′ primer ccccccGGATCCctgtaatgaaataaatgtgacgctg. The PCR product was digested with SphI and BamHI, and cloned into the MCSI of a derivative plasmid of pPD49.26 (with AsiSI and NotI inserted between the NheI and KpnI in MCS II).
GCaMP3 (1353 bp), the genetically-encoded calcium indicator [32], was cloned from a plasmid with GCaMP3 sequence (a gift from Robert Chow, USC) by PCR with 5′ primer ccccccGCGATCGCAAAAatgggttctcatcatcatcatc and 3′ primer ccccccGCGGCCGCttacttcgctgtcatcatttg. The PCR product was digested with AsiSI and NotI, and was cloned into a derivative of pPD49.26 that had unc-47 mini promoter in MCS I and had AsiSI and NotI sites inserted between NheI and KpnI in MCS II to generate the plasmid pHW100: Punc-47(mini)::GCaMP3.
The wild type cDNA of kin-2a (1101 bp) was cloned from an N2 cDNA library (synthesized using NEB Protoscript RT-PCR kit) with 5′ primer ccccccGCTAGCAAAAatgtcgggtggaaacgaagag and 3′ primer ccccccGGTACCttaggtcatcagtttgacgtatgag. The Gly310 residue in KIN-2a was corresponding to the Gly324 residue in the site B of the mouse PKA regulatory subunit that will generate dominate negative PKA when it is mutated to Asp [26]. The kin-2a (G310D) variant was generated by two-step overlapping PCR using the following primers: pair 1 (5′ primer ccccccGCTAGCAAAAatgtcgggtggaaacgaagag and 3′ primer gaagaagagcgatttcGTcgaaatagtccgacattccaag and pair 2 (5′ primer cttggaatgtcggactatttcgACgaaatcgctcttcttc and 3′ primer ccccccGGTACCttaggtcatcagtttgacgtatgag).The final overlapping PCR product was digested with NheI and KpnI, and then cloned into the MCS II in a derivative plasmid from pPD49.26 that contained the unc-47 full length promoter in MCS I to generate the plasmid pHW154: Punc-47(FL)::kin-2a(G310D).
The wild type cDNA of kin-1a (1080 bp) was cloned from N2 cDNA library with 5′ primer ccccccGCTAGCAAAAatgctcaagtttctgaaacc and 3′ primer ccccccGGTACCttaaaactcggcaaactctttg. The His96 and Trp205 residues in KIN-1a are corresponding to the His87 and Trp196 in the mouse PKA catalytic subunits which would generate constitutively active PKA when they are mutated to Gln and Arg, respectively [30]. The KIN-1a (H96R, W205Q) variant was created by two-step overlapping PCR using the following primers: pair 1(5′ primer ccccccgctagcAAAAatgctcaagtttctgaaacc and 3′ primer gaatgcgcttttcgttcaacgtTtgctccacttgcttgagttttac), pair 2(5′ primer gtaaaactcaagcaagtggagcaAacgttgaacgaaaagcgcattc and 3′ primer tctggtgtgccgcacaatgtccTcgttcgtcctttgacacgtttc) and pair 3(5′ primer gaaacgtgtcaaaggacgaacgAggacattgtgcggcacaccaga and 3′ primer ccccccGGTACCttaaaactcggcaaactctttg).The final overlapping PCR product was digested with NheI and KpnI, was cloned into the MCS II in a derivative plasmid from pPD49.26 that contained the unc-47 full length promoter in MCS I to generate the plasmid pHW173: Punc-47(FL)::kin-1a(H96R, W205Q).
Sequencing was performed to confirm the mutations kin-2a(G310D) and kin-1a(H96R, W205Q) in the pHW154 and pHW173, respectively.
Generation of transgenic animals
Microinjection of expression plasmids into the gonad of C. elegans was performed to generate transgenic animals with extrachromosomal arrays, according to the standard procedure [56]. Generally, total DNA concentration of the injection solution was 100 ng/µl (using the plasmid pBluescript to fill up if needed). The extrachromosomal arrays were integrated into the genome to generate stable transgenic worms using UV irradiation. The integrated transgenic lines were outcrossed at least 6 times.
The plasmid pHW154 (Punc-47(FL)::kin-2a(G310D)) was injected at 50 ng/µl, together with the co-injection marker plasmid KP708 (Pttx-3::RFP) at 40 ng/µl to generate the array vjEx582 [Pttx-3::RFP, 40 ng/µl; Punc-47(FL):: kin-2a(G310D), 50 ng/µl]. This array was integrated into genome to generate the PKA[DN] transgenic strains (vjIs76 and vjIs77) with dominant negative PKA specifically expressed in GABAergic neurons.
The plasmid pHW173 (Punc-47(FL)::kin-1a(H96R, W205Q)) was injected at 50/µl, together with the co-injection maker KP1106 (Pmyo-2::NLS::GFP) at 10 ng/µl to generate the array vjEx709 [Pmyo-2::NLS::mCherry, 10 ng/µl; Punc-47(FL):: kin-1a(H96R, W205Q), 50 ng/µl]. This extrachomosomal array was integrated into genome to generate the PKA[CA] transgenic strains (vjIs102 and vjIs103) with constitutively active PKA specifically expressed in GABAergic neurons.
The plasmid pHW100 (Punc-47(mini)::GCaMP3) was injected at 125 ng/µl, together with co-injection marker plasmid KP1368 (Pmyo-2::NLS::mCherry) at 10 ng/µl to generate the array vjEx429 [Pmyo-2::NLS::mCherry,10 ng/µl; Punc-47(mini)::GCaMP3,125 ng/µl]. This array was integrated into genome to generate transgenic strains vjIs58 and vjIs64, which expressed GCaMP3 in AVL and DVB neurons.
In vivo calcium imaging
The calcium imaging experiment was performed as previously described [23]. We used two independent transgenic strains (vjIs58 and vjIs64) with AVL and DVB neurons expressing GCaMP3 to perform in vivo calcium imaging on DVB neurons. Both strains had normal Exp steps (data not shown). Although unc-13(s69) mutants had a longer cycle length (78.5±11.4 s, mean ±SD, n = 8), they were also most completely paralyzed and still had normal Exp steps [23]. Thus, unc-13(s69) mutation was included in all strains to immobilize animals for calcium imaging, except for those strains with elg-19(n582);unc-2(lj1) double mutations, because the elg-19(n582);unc-2(lj1) double mutants were almost completely paralyzed. Young adult worms were transferred to NGM-agarose plate seeded with the food OP50. These agarose plates were topped with cover slides and imaged under a Nikon eclipse 90i microscope equipped with a Nikon Plan Apo 40× oil objective (N.A. = 1.0), a standard GFP filter and a Photometrics Coolsnap ES2 camera. Only those worms kept continuous pumping and positioned laterally with the left side pointing to the objective were selected for imaging. Time lapse imaging was obtained using Metamorph 7.0 software (Universal Imaging). Each worm was recorded for 250 s at 4 frames per second (3×3 binning, exposure time ranging from 5 ms to 80 ms dependent on the baseline GCaMP3 fluorescence in DVB neuron in each individual worm). Unlike DVB, AVL neuron is located in the head, so we could not routinely perform the calcium imaging on AVL and observe the Exp step in the same field. However, during some experiments, we did observe that AVL fired at the same time as DVB in coiled worms in which we could see AVL and Exp in the same field.
The quantification of the GCaMP3 fluorescence intensity in the synaptic region of DVB neurons using Metamorph 7.0 software (Universal Imaging) was performed as previously described [23]. The synaptic region of DVB neurons was manually selected as region of interest (ROI) and the average of the GCaMP3 fluorescence intensity for each frame was recorded. Meanwhile, a similar area near the tail region was used as background fluorescence for each frame. The GCaMP3 fluorescence (F) was defined as the (ROI - background). The average of the GCaMP3 fluorescence in the first 10 frames was used as baseline fluorescence F0. For each frame, the change of the GCaMP3 was presented as ΔF/F0 = (F-F0)/F0. The duration of each calcium spike was defined as the time differences between the first frame in which the GCaMP3 fluorescence increased and the frame where the GCaMP3.0 fluorescence returned the baseline. Ectopic calcium spikes describe those calcium spikes that did not happen within 10 seconds after pBoc.
Supporting Information
Zdroje
1. SongHJ, MingGL, PooMM (1997) cAMP-induced switching in turning direction of nerve growth cones. Nature 388 : 275–279.
2. Ghosh-RoyA, WuZ, GoncharovA, JinY, ChisholmAD (2010) Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci 30 : 3175–3183.
3. PifferiS, BoccaccioA, MeniniA (2006) Cyclic nucleotide-gated ion channels in sensory transduction. FEBS Lett 580 : 2853–2859.
4. SilvaAJ, KoganJH, FranklandPW, KidaS (1998) CREB and memory. Annu Rev Neurosci 21 : 127–148.
5. MahoneyTR, LuoS, RoundEK, BraunerM, GottschalkA, et al. (2008) Intestinal signaling to GABAergic neurons regulates a rhythmic behavior in Caenorhabditis elegans. Proc Natl Acad Sci U S A 105 : 16350–16355.
6. ShaferOT, KimDJ, Dunbar-YaffeR, NikolaevVO, LohseMJ, et al. (2008) Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron 58 : 223–237.
7. LevineJD, CaseyCI, KalderonDD, JacksonFR (1994) Altered circadian pacemaker functions and cyclic AMP rhythms in the Drosophila learning mutant dunce. Neuron 13 : 967–974.
8. HellJW (2010) Beta-adrenergic regulation of the L-type Ca2+ channel Ca(V)1.2 by PKA rekindles excitement. Sci Signal 3: pe33.
9. Sassone-CorsiP (2012) The cyclic AMP pathway. Cold Spring Harb Perspect Biol 4: a011148.
10. KauppUB, SeifertR (2002) Cyclic nucleotide-gated ion channels. Physiol Rev 82 : 769–824.
11. GloerichM, BosJL (2010) Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50 : 355–375.
12. SkalheggBS, TaskenK (2000) Specificity in the cAMP/PKA signaling pathway. Differential expression,regulation, and subcellular localization of subunits of PKA. Front Biosci 5: D678–693.
13. SeinoS, ShibasakiT (2005) PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev 85 : 1303–1342.
14. MajercakJ, KalderonD, EderyI (1997) Drosophila melanogaster deficient in protein kinase A manifests behavior-specific arrhythmia but normal clock function. Mol Cell Biol 17 : 5915–5922.
15. JoinerWJ, CrockerA, WhiteBH, SehgalA (2006) Sleep in Drosophila is regulated by adult mushroom bodies. Nature 441 : 757–760.
16. ReikenS, LacampagneA, ZhouH, KheraniA, LehnartSE, et al. (2003) PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol 160 : 919–928.
17. BabaT, SakisakaT, MochidaS, TakaiY (2005) PKA-catalyzed phosphorylation of tomosyn and its implication in Ca2+-dependent exocytosis of neurotransmitter. J Cell Biol 170 : 1113–1125.
18. HellJW, YokoyamaCT, BreezeLJ, ChavkinC, CatterallWA (1995) Phosphorylation of presynaptic and postsynaptic calcium channels by cAMP-dependent protein kinase in hippocampal neurons. EMBO J 14 : 3036–3044.
19. ThomasJH (1990) Genetic analysis of defecation in Caenorhabditis elegans. Genetics 124 : 855–872.
20. BegAA, JorgensenEM (2003) EXP-1 is an excitatory GABA-gated cation channel. Nat Neurosci 6 : 1145–1152.
21. BranickyR, HekimiS (2006) What keeps C. elegans regular: the genetics of defecation. Trends Genet 22 : 571–579.
22. Dal SantoP, LoganMA, ChisholmAD, JorgensenEM (1999) The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell 98 : 757–767.
23. WangH, GirskisK, JanssenT, ChanJP, DasguptaK, et al. (2013) Neuropeptide secreted from a pacemaker activates neurons to control a rhythmic behavior. Curr Biol 23 : 746–754.
24. BargmannCI (1998) Neurobiology of the Caenorhabditis elegans genome. Science 282 : 2028–2033.
25. KimS, GovindanJA, TuZJ, GreensteinD (2012) SACY-1 DEAD-Box helicase links the somatic control of oocyte meiotic maturation to the sperm-to-oocyte switch and gamete maintenance in Caenorhabditis elegans. Genetics 192 : 905–928.
26. CorrellLA, WoodfordTA, CorbinJD, MellonPL, McKnightGS (1989) Functional characterization of cAMP-binding mutations in type I protein kinase. J Biol Chem 264 : 16672–16678.
27. WillisBS, NiswenderCM, SuT, AmieuxPS, McKnightGS (2011) Cell-type specific expression of a dominant negative PKA mutation in mice. PLoS One 6: e18772.
28. KimC, XuongNH, TaylorSS (2005) Crystal structure of a complex between the catalytic and regulatory (RIalpha) subunits of PKA. Science 307 : 690–696.
29. LiW, OhlmeyerJT, LaneME, KalderonD (1995) Function of protein kinase A in hedgehog signal transduction and Drosophila imaginal disc development. Cell 80 : 553–562.
30. OrellanaSA, McKnightGS (1992) Mutations in the catalytic subunit of cAMP-dependent protein kinase result in unregulated biological activity. Proc Natl Acad Sci U S A 89 : 4726–4730.
31. SchadeMA, ReynoldsNK, DollinsCM, MillerKG (2005) Mutations that rescue the paralysis of Caenorhabditis elegans ric-8 (synembryn) mutants activate the G alpha(s) pathway and define a third major branch of the synaptic signaling network. Genetics 169 : 631–649.
32. TianL, HiresSA, MaoT, HuberD, ChiappeME, et al. (2009) Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 6 : 875–881.
33. JinY, JorgensenE, HartwiegE, HorvitzHR (1999) The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J Neurosci 19 : 539–548.
34. CatterallWA (2011) Voltage-gated calcium channels. Cold Spring Harb Perspect Biol 3: a003947.
35. SahekiY, BargmannCI (2009) Presynaptic CaV2 calcium channel traffic requires CALF-1 and the alpha(2)delta subunit UNC-36. Nat Neurosci 12 : 1257–1265.
36. RichmondJE, WeimerRM, JorgensenEM (2001) An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature 412 : 338–341.
37. MathewsEA, GarciaE, SantiCM, MullenGP, ThackerC, et al. (2003) Critical residues of the Caenorhabditis elegans unc-2 voltage-gated calcium channel that affect behavioral and physiological properties. J Neurosci 23 : 6537–6545.
38. LeeRY, LobelL, HengartnerM, HorvitzHR, AveryL (1997) Mutations in the alpha1 subunit of an L-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J 16 : 6066–6076.
39. StegerKA, ShtondaBB, ThackerC, SnutchTP, AveryL (2005) The C. elegans T-type calcium channel CCA-1 boosts neuromuscular transmission. J Exp Biol 208 : 2191–2203.
40. Arellano-CarbajalF, Briseno-RoaL, CoutoA, CheungBH, LabouesseM, et al. (2011) Macoilin, a conserved nervous system-specific ER membrane protein that regulates neuronal excitability. PLoS Genet 7: e1001341.
41. EmrickMA, SadilekM, KonokiK, CatterallWA (2010) Beta-adrenergic-regulated phosphorylation of the skeletal muscle Ca(V)1.1 channel in the fight-or-flight response. Proc Natl Acad Sci U S A 107 : 18712–18717.
42. LightPE, Manning FoxJE, RiedelMJ, WheelerMB (2002) Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A - and ADP-dependent mechanism. Mol Endocrinol 16 : 2135–2144.
43. JohnstoneDB, WeiA, ButlerA, SalkoffL, ThomasJH (1997) Behavioral defects in C. elegans egl-36 mutants result from potassium channels shifted in voltage-dependence of activation. Neuron 19 : 151–164.
44. KaczmarekLK, JenningsKR, StrumwasserF, NairnAC, WalterU, et al. (1980) Microinjection of catalytic subunit of cyclic AMP-dependent protein kinase enhances calcium action potentials of bag cell neurons in cell culture. Proc Natl Acad Sci U S A 77 : 7487–7491.
45. CastellucciVF, KandelER, SchwartzJH, WilsonFD, NairnAC, et al. (1980) Intracellular injection of t he catalytic subunit of cyclic AMP-dependent protein kinase simulates facilitation of transmitter release underlying behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A 77 : 7492–7496.
46. OsterriederW, BrumG, HeschelerJ, TrautweinW, FlockerziV, et al. (1982) Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature 298 : 576–578.
47. WongW, ScottJD (2004) AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol 5 : 959–970.
48. GancedoJM (2013) Biological roles of cAMP: variations on a theme in the different kingdoms of life. Biol Rev Camb Philos Soc 88 : 645–68.
49. BrandonEP, IdzerdaRL, McKnightGS (1997) PKA isoforms, neural pathways, and behaviour: making the connection. Curr Opin Neurobiol 7 : 397–403.
50. NiswenderCM, WillisBS, WallenA, SweetIR, JettonTL, et al. (2005) Cre recombinase-dependent expression of a constitutively active mutant allele of the catalytic subunit of protein kinase A. Genesis 43 : 109–119.
51. MurrayP, CleggRA, ReesHH, FisherMJ (2008) siRNA-mediated knockdown of a splice variant of the PK-A catalytic subunit gene causes adult-onset paralysis in C. elegans. Gene 408 : 157–163.
52. MurrayAJ (2008) Pharmacological PKA inhibition: all may not be what it seems. Sci Signal 1: re4.
53. MayRC, PlasterkRH (2005) RNA interference spreading in C. elegans. Methods Enzymol 392 : 308–315.
54. LiuDW, ThomasJH (1994) Regulation of a periodic motor program in C. elegans. J Neurosci 14 : 1953–1962.
55. EastmanC, HorvitzHR, JinY (1999) Coordinated transcriptional regulation of the unc-25 glutamic acid decarboxylase and the unc-47 GABA vesicular transporter by the Caenorhabditis elegans UNC-30 homeodomain protein. J Neurosci 19 : 6225–6234.
56. MelloCC, KramerJM, StinchcombD, AmbrosV (1991) Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10 : 3959–3970.
Štítky
Genetika Reprodukčná medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



