-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
The pathogenic fungus Cryptococcus neoformans uses the Bwc1-Bwc2 photoreceptor complex to regulate mating in response to light, virulence and ultraviolet radiation tolerance. How the complex controls these functions is unclear. Here, we identify and characterize a gene in Cryptococcus, UVE1, whose mutation leads to a UV hypersensitive phenotype. The homologous gene in fission yeast Schizosaccharomyces pombe encodes an apurinic/apyrimidinic endonuclease acting in the UVDE-dependent excision repair (UVER) pathway. C. neoformans UVE1 complements a S. pombe uvde knockout strain. UVE1 is photoregulated in a Bwc1-dependent manner in Cryptococcus, and in Neurospora crassa and Phycomyces blakesleeanus that are species that represent two other major lineages in the fungi. Overexpression of UVE1 in bwc1 mutants rescues their UV sensitivity phenotype and gel mobility shift experiments show binding of Bwc2 to the UVE1 promoter, indicating that UVE1 is a direct downstream target for the Bwc1-Bwc2 complex. Uve1-GFP fusions localize to the mitochondria. Repair of UV-induced damage to the mitochondria is delayed in the uve1 mutant strain. Thus, in C. neoformans UVE1 is a key gene regulated in response to light that is responsible for tolerance to UV stress for protection of the mitochondrial genome.
Published in the journal: The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage. PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003769
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003769Summary
The pathogenic fungus Cryptococcus neoformans uses the Bwc1-Bwc2 photoreceptor complex to regulate mating in response to light, virulence and ultraviolet radiation tolerance. How the complex controls these functions is unclear. Here, we identify and characterize a gene in Cryptococcus, UVE1, whose mutation leads to a UV hypersensitive phenotype. The homologous gene in fission yeast Schizosaccharomyces pombe encodes an apurinic/apyrimidinic endonuclease acting in the UVDE-dependent excision repair (UVER) pathway. C. neoformans UVE1 complements a S. pombe uvde knockout strain. UVE1 is photoregulated in a Bwc1-dependent manner in Cryptococcus, and in Neurospora crassa and Phycomyces blakesleeanus that are species that represent two other major lineages in the fungi. Overexpression of UVE1 in bwc1 mutants rescues their UV sensitivity phenotype and gel mobility shift experiments show binding of Bwc2 to the UVE1 promoter, indicating that UVE1 is a direct downstream target for the Bwc1-Bwc2 complex. Uve1-GFP fusions localize to the mitochondria. Repair of UV-induced damage to the mitochondria is delayed in the uve1 mutant strain. Thus, in C. neoformans UVE1 is a key gene regulated in response to light that is responsible for tolerance to UV stress for protection of the mitochondrial genome.
Introduction
The ability to sense light provides well-known advantages to organisms, such as adapting to photosynthetic light sources in plants and for vision in animals, yet the benefits of light-sensing in non-photosynthetic and non-motile organisms are less established. The fungi contain a suite of potential photoreceptor proteins, with the White Collar complex (WCC) being found throughout the fungal kingdom, except for species in which the two genes encoding the complex were lost. Light influences different responses in different fungi, including phototropism, induction of pigmentation, asexual and sexual sporulation, changes is primary and secondary metabolism, and regulating the circadian clock: most of which are controlled, where established, by the WCC [1]–[3]. A major question is what advantage is provided in using light as an environmental signal to regulate these processes. One compelling hypothesis is that protecting DNA from damage provides a selective pressure, and that wavelengths in the visible spectrum are sensed to indicate the presence of deleterious ultraviolet radiation. However, a DNA repair system common to light-sensing fungi and that acts directly downstream of the WCC is unknown to date.
The White collar-1 and White collar-2 proteins interact to form a complex (WCC) capable of sensing blue and near UV light. Both proteins were originally characterized in the ascomycete fungus Neurospora crassa [4]–[7] where WC-1 acts as a photoreceptor. In N. crassa, WCC has roles to play both in light and dark environments. In the dark the WCC has a major function as a circadian clock component, via regulation of FRQ gene expression [8]–[10]. Light-dependent functions of WCC include conidiation, carotenoid production and mating [11], [12]. The photons and the signal are transduced via the chromophore flavin adenine dinucleotide (FAD) bound within an N-terminal specialized type of PAS domain (from the Per, Arnt, Sim proteins), named the LOV (light, oxygen and voltage) domain [13]. The two proteins interact using other PAS domains, to form the transcription factor complex that regulates transcription of target genes via their GATA-type zinc finger DNA-binding domains [4], [5], [7], [14].
The human pathogen Cryptococcus neoformans is a member of the phylum Basidiomycota, a distant relative to N. crassa. The fungus grows vegetatively as a budding yeast and during mating in a dikaryotic filamentous form. C. neoformans is divided into two varieties: var. grubii is the most prevalent in the clinic and var. neoformans is less common but was the most amenable to experimental methods until the discovery of an opposite mating partner for var. grubii about a decade ago [15]. C. neoformans primarily causes disease in immunocompromised people. The closest relative to C. neoformans is C. gattii, which is also a human pathogen but with a greater tendency to infect immunocompetent individuals [16]. Homologs of N. crassa wc-1 and wc-2 are present in Cryptococcus species, designated BWC1/CWC1 and BWC2/CWC2, and have been characterized in strains of both var. grubii and var. neoformans [17], [18]. The Bwc1-Bwc2 complex has three known functions in C. neoformans: it represses mating in the light, promotes virulence, and provides protection against UV light. The downstream targets of Bwc1-Bwc2 that control these functions remain to be elucidated.
Several genes have been identified from C. neoformans that are regulated by light. These include SXI1α and MFα1, found within the mating type locus and encoding a transcription factor and pre-pheromone protein required for sexual reproduction [17]. Their expression could explain the repression of mating by light. However, the regulation of these transcripts was compared after a long time exposure of 24 h in the light or constant darkness, such that this time point likely reflects indirect regulation by Bwc1-Bwc2. More recently a microarray experiment was carried out in C. neoformans to detect light-regulated transcripts with an hour of exposure to light. The HEM15 gene, encoding ferrochetalase that is the last step in the heme biosynthetic pathway, was identified as a gene under control of Bwc1-Bwc2 [19]. However, the phenotype of the knockout hem15 strain differs significantly from mutation of BWC1 or BWC2. For example, HEM15 is essential for viability, and yet the bwc1 and bwc2 mutants do not show any growth defects in the light or dark. Another light-regulated gene is CFT1, required for iron uptake and virulence [20], yet no iron-dependent phenotype of bwc1 and bwc2 mutants is known. Thus, while CFT1 and HEM15 may be targets of the Bwc1-Bwc2 complex, they likely play minimal roles in the physiological response of C. neoformans to light. Moreover these two genes are also under the control of other transcription factors in addition to WCC. As a transcription factor complex, it was puzzling that more light-regulated genes were not identified by microarray analysis that could potentially explain the phenotypes of deleting the WCC from C. neoformans.
The microarray results of C. neoformans contrast to ascomycete species. For instance, in N. crassa a microarray study in wild type, wc-1Δ, and wc-2Δ strains at different time periods identified 314 light-regulated genes, constituting 5.6% of the total detectable transcripts in the genome [11]. These genes were grouped into two broad categories, the early or late light responsive genes (ELRGs and LLRGs). Some of these ELRGs are involved in the synthesis of vitamins, photo-protective pigments, prosthetic groups and cofactors, cellular signaling, DNA processing, circadian rhythm and secondary metabolism. Many of the LLRGs are implicated in carbohydrate metabolism, fatty acid oxidation and free radical detoxification. In N. crassa there is also a link between the WCC and DNA repair, for example through the clock gene prd-4, which is a cell cycle checkpoint kinase 2 [21]–[23].
As the White Collar complex acts as an UV/blue light photoreceptor and mutation of the complex causes an increase in sensitivity to UV light, potential targets are hypothesized to be genes involved in repairing DNA damage for survival under UV stress. The UVE1 gene of C. neoformans was previously identified in a UV sensitive strain in a collection of insertional mutants [24]. The product of UVE1 is a homolog of an apurinic/apyrimidinic endonuclease that is best characterized in fission yeast Schizosaccharomyces pombe. In S. pombe the gene was called UVDE for UV damage endonuclease, and renamed uve1 for consistency with nomenclature [25], [26]. The mus-18/UVE-1 gene is the homolog characterized from N. crassa [27]. The protein removes UV-induced cyclobutane pyrimidine dimers and 6-4 photoproducts, acting in its own pathway termed the UVDE-dependent excision repair (UVER) pathway. UVDE recognizes single-stranded DNA nicks, apurinic/apyrimidinic sites, and nucleotide mismatches [26], [28]–[30], a suite of DNA lesions that also extends a possible role for UVDE in repairing the equivalent types of DNA damage caused by reactive oxygen species [31].
In S. pombe, UVDE is localized and functional in both the nucleus and mitochondria, and was suggested to act as a reserve mechanism for repairing UV-induced DNA damage in the mitochondria [32]. Homologs of UVE1 are present in a subset of species in the Archaea, Bacteria and Eukaryotes [25], [33]–[37], with one exception being humans where there is no homolog. A preliminary northern blot experiment suggested that UVE1 in C. neoformans var. neoformans is a light-regulated gene with two isoforms, triggering this investigation. We hypothesized that UVE1 is a downstream target of the WCC that functions in repairing UV-induced damage, and tested this hypothesis in the experiments described below.
Results
UVE1 is essential in C. neoformans for survival under UV stress
A T-DNA insertion mutant within the promoter of the UVE1 gene was identified previously [14]. Under UV stress conditions the mutant showed negligible survival as compared to the wild type (KN99α) and the unexposed strains (Figure 1A). Transformations derived from Agrobacterium T-DNA delivery can have phenotypes that are not due to the insertion of the T-DNA into the host genome. The UV sensitivity phenotype of the original mutant was verified by constructing UVE1 gene replacement strains for both C. n. var. neoformans and C. n. var. grubii. The knockout strains in both varieties had reduced survival after exposure to UV (Figure 1A,B). To confirm that the UV sensitivity phenotype was because of the absence of UVE1, an uve1Δ strain was complemented with a wild type copy of UVE1. The UVE1 complemented strain completely rescued the UV sensitivity phenotype (Figure 1). These results show that in C. neoformans the UVE1 gene is required for survival under UV stress.
Fig. 1. UVE1 of C. neoformans confers resistance to UV stress. 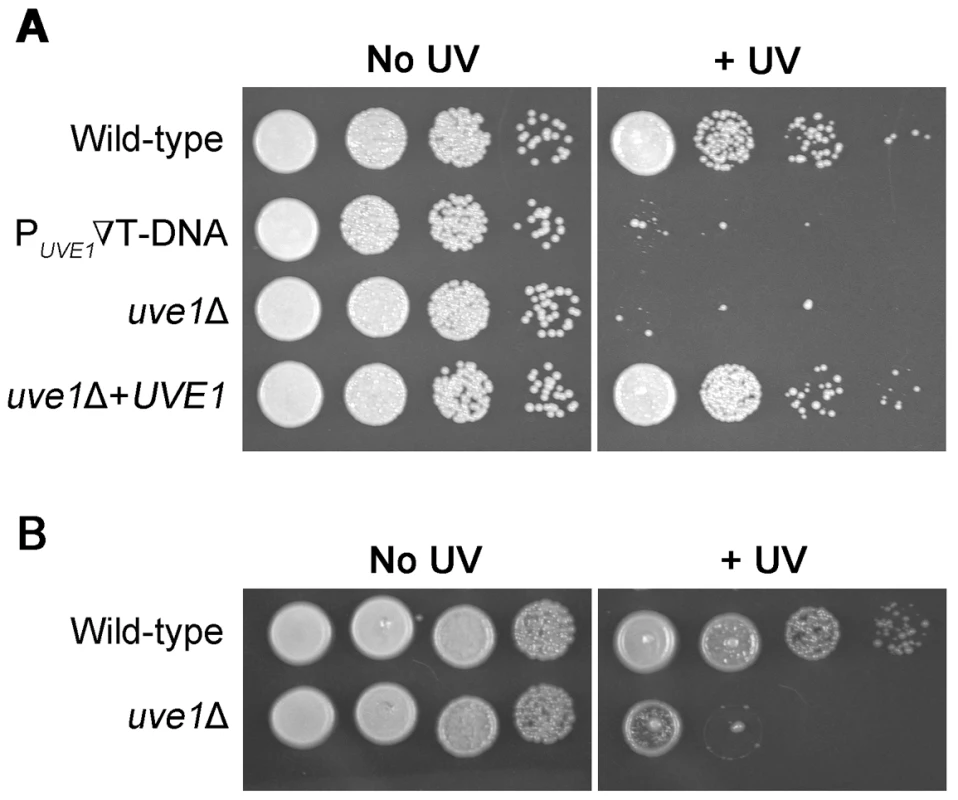
Ten-fold serial dilutions for different Cryptococcus strains grown at 30°C for 2 days. (A) Left panel untreated control and right panel treated with UV dose of 120 J/m2. Order of C. neoformans var. grubii strains from top to bottom KN99α, ST239E6, AI191, AI198. (B) Left panel untreated control and right panel treated with UV dose of 120 J/m2. Order of C. neoformans var. neoformans strains from top to bottom JEC21, AISVCN101. The responses of the uve1 mutant to stresses other than UV light were tested, showing no other phenotypes (Figure S1). The gene also plays no major role in the formation of mating filaments (Figure S2A, B). A prior analysis, using comparative growth in a pool of 48 strains in the mouse lung, suggested the UVE1 gene has no role in virulence [38]. To examine the uve1Δ strain in isolation, its virulence was tested in an insect model. While the bwc1Δ strain is less pathogenic than wild type in this model, the uve1Δ strain is equally virulent as wild type (Figure S2C). Thus, the only established function of Uve1 in C. neoformans is in response to UV stress.
UVE1 is a photoregulated gene in C. neoformans
Since UVE1 is required for surviving exposure to UV light, regulation of UVE1 was tested in response to light. Wild type strains of C. n. var. neoformans, C. n. var. grubii and C. gattii were grown in the dark, and one replicate provided a one hour exposure to white light. Expression of the UVE1 gene was examined by northern blot analysis on polyA RNA purified from these cultures. The UVE1 transcript levels were higher in light grown conditions for all wild type strains (Figure 2). Two transcripts were observed for var. neoformans strain JEC21, one longer (L, for light) induced specifically under light conditions and another shorter (D, dark) expressed in the dark. For var. grubii and C. gattii one isoform of UVE1 was expressed in response to light with negligible expression in dark grown conditions.
Fig. 2. UVE1 is a Bwc1-regulated gene in Cryptococcus. 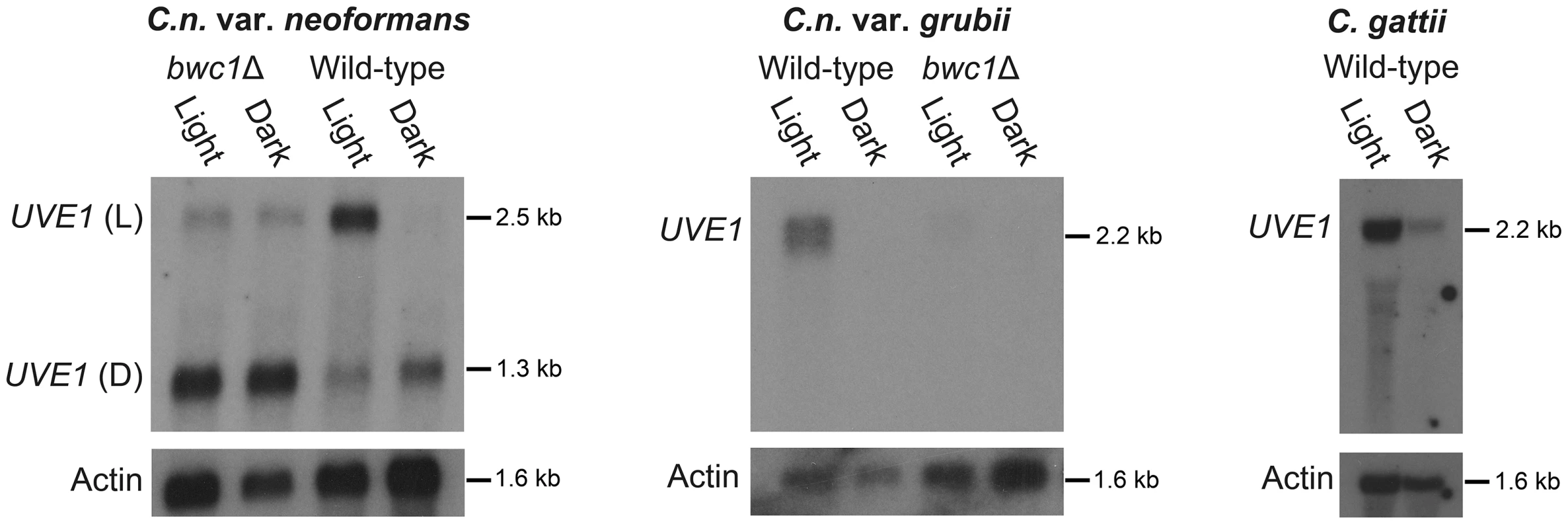
Northern blots of C. n. var. neoformans, C. n. var. grubii and C. gattii. From left to right, panel 1 is for JEC21 (WT) and AI5 (bwc1); the upper band is for UVE1 light (L) isoform and the lower band is for UVE1 dark (D) isoform. Panel 2 is for KN99α (WT) and AI81 (bwc1), panel 3 is for R265 (WT). All experiments were either 23 h dark+1 h light, (Light) or 24 h constant darkness (Dark). Blots were stripped and reprobed with actin as a loading control. The role for the Bwc1 photoreceptor in UVE1 induction by light was examined in the bwc1Δ deletion strains of C. neoformans. In the var. neoformans deletion there was loss of induction of the longer isoform by light, and residual expression of this isoform in both light and darkness. There was no observed effect of bwc1Δ on the shorter isoform in dark grown conditions. Dark isoforms expressed equally well in both light and dark conditions in some replicates, possibly reflecting the age of the culture or shading by other cells. In the var. grubii bwc1Δ mutant, the UVE1 transcript was barely detectable under either illumination regime (Figure 2). These analyses in the bwc1 mutant backgrounds indicate that UVE1 expression is controlled by the Bwc1-Bwc2 complex.
Rapid amplification of cDNA ends (RACE) was used in the var. neoformans strain to define the 5′ and 3′ ends of the two transcripts that were produced in the light and the dark. The UVE1 dark isoform is part of the UVE1 light isoform, with the dark isoform starting in the middle of the light isoform and both sharing a common 3′ end (GenBank accessions KF234405 and KF234406; Figure 3). Alignment of the Uve1 homologs from various fungi indicated that the dark isoform of C. neoformans is truncated and missing key residues in the active site of this protein (Figure 3; Figure S3).
Fig. 3. Two mRNA isoforms of UVE1 are produced in C. neoformans var. neoformans. 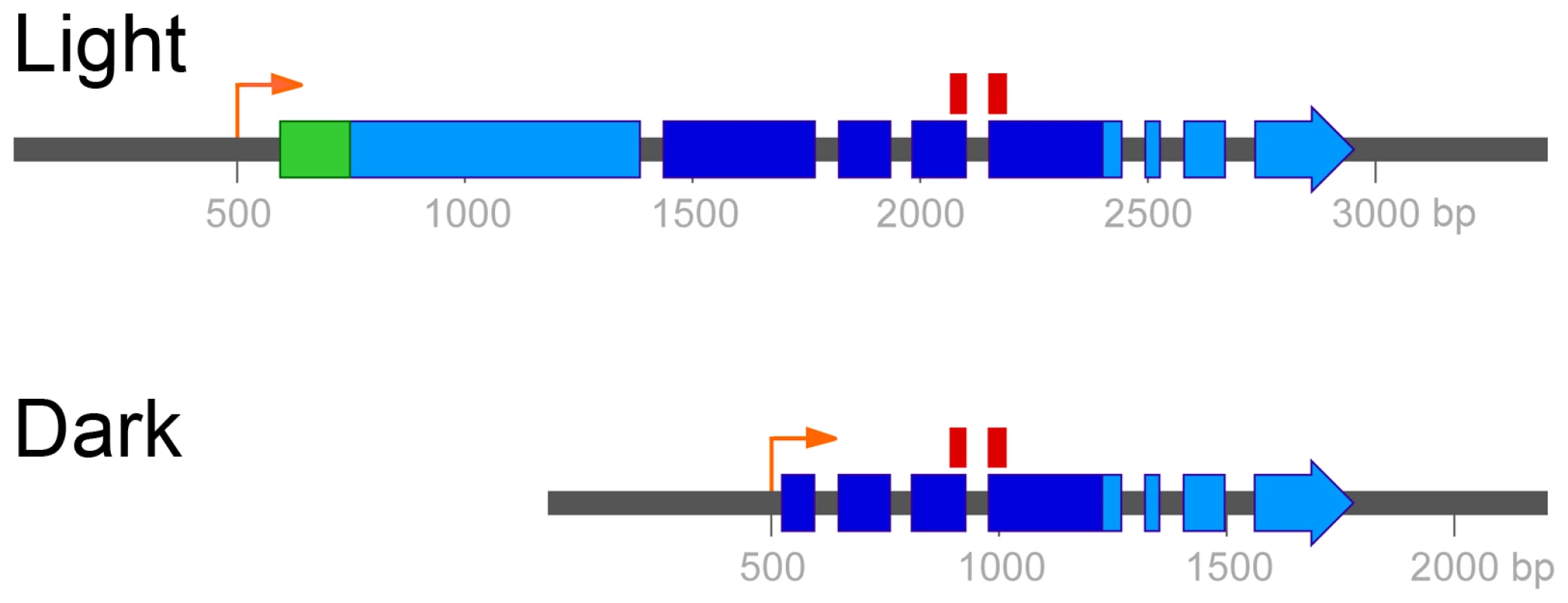
Boxes indicate coding regions, with the long light-induced isoform encoding a 660 amino acid residue protein and the shorter dark-expressed isoform encoding a 291 amino acid residue protein. Dark blue encompasses the pfam03851 domain that represents the conserved and active site of the endonuclease. The position of the 70 mer probe used in the C. neoformans microarray, which spans two exons common to both isoforms, is indicated above this region. The green region encodes the predicted mitochondrial localization signal. The orange arrows indicate the start of the UVE1 light and dark transcripts. The light-dependent regulation of UVE1 in wild type strains suggested that induction of the gene prior to UV exposure would correlate with increased UV resistance. The wild type, bwc1Δ and the complemented strains were grown in complete darkness overnight. One set was kept in the dark and the other set exposed to light for 2 hours, prior to treatment of both sets with UV light (Figure S4). All three strains grown in darkness showed similar levels of sensitivity to UV light. Exposure of the strains with the wild type copy of BWC1 to light before UV stress increased their resistance to UV, a property not seen for the bwc1Δ strain. Hence, light signaled by Bwc1 promotes UV resistance.
Two isoforms of UVE1 were observed in the var. neoformans strain, raising the possibility that differential transcript sizes may be a common feature for DNA repair genes in C. neoformans. To identify other genes involved in protecting the fungus against UV damage, a collection of 1200 defined knock out mutants in the var. grubii background [38] was screened for those sensitive to UV light. 13 strains were identified, including the uve1Δ strain in the collection (Figure S5A). For the corresponding genes, northern analysis were performed for var. neoformans and var. grubii cultured under light and dark conditions (Figure S5B). No altered size or induction in response to light was observed, as had been for the UVE1 gene.
Photoinduction of UVE1 homologs is conserved in fungi
To examine if UVE1 photoregulation is common among fungi, northern blot analysis of UVE1 was performed in two fungi, Neurospora crassa and Phycomyces blakesleeanus (Figure 4). The species are light-sensing models in the phylum Ascomycota and subphylum Mucoromycotina, respectively. In N. crassa the expression of UVE1 has already been reported in a microarray study of the light-induced genes [11]. For the N. crassa wild type strain we observed by northern blot analysis the induction of one transcript in light grown conditions and minimal expression of the UVE1 transcript in dark grown mycelia, confirming the previous microarray data. For the P. blakesleeanus wild type strain two transcripts were present in light grown conditions that were both absent in samples grown in the dark. Based on the expressed sequence tag information in the genome database, the 5′ end of the UVE1 homolog is shared between the two transcripts and the 3′ end differs, the reverse of the situation in C. neoformans var. neoformans. The longer isoform in P. blakesleeanus has a 3′ extension due to transcriptional read-through into the 3′ neighboring gene.
Fig. 4. Light regulation of UVE1 homologs through the White Collar complex is conserved in fungi. 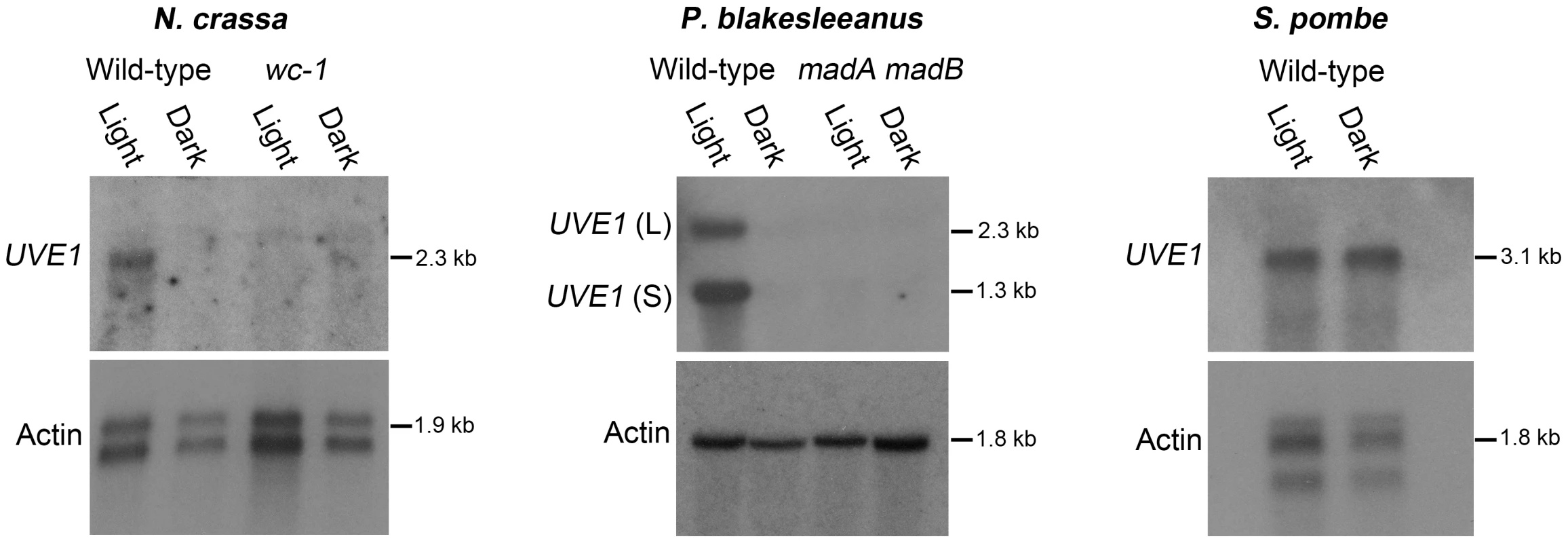
Northern blots for UVE1 homologs from N. crassa [FGSC 4200 (WT), FGSC 4398 (wc-1)], P. blakesleeanus [NRRL1555 (WT), L51 (madA madB)], and S. pombe [L972 (WT)]. All experiments were 1 h light exposure (Light) or constant darkness (Dark). Blots were stripped and reprobed with actin as a loading control. For clarity, the gene name UVE1 is used to refer to all homologs (mus-18 N. crassa; uvdE P. blakesleeanus; uve1 S. pombe). We also examined the transcript profile of UVE1 in a white collar-1 mutant (wc-1) of N. crassa and a madA-madB mutant of P. blakesleeanus (madA and madB are functional wc-1 and wc-2 homologs in this species [39]). We observed complete loss of light-dependent induction of UVE1 in these mutant strains of N. crassa and P. blakesleeanus. As an additional control to show that UVE1 induction was not an indirect effect of light on the media, UVE1 expression was examined in S. pombe, a “blind” species because it encodes no homologs of the WCC. We observed equal transcript levels of the S. pombe UVE1 homolog in cultures grown under light and dark conditions (Figure 4). These studies demonstrate that UVE1 is photoregulated among highly-diverged fungal species, and substantiates the WC-1 dependent light-induction of UVE1 in the fungal kingdom.
Cellular localization of C. neoformans Uve1 light and dark isoforms
The protein sequences predicted for the two isoforms in var. neoformans from RACE were examined by bioinformatic approaches for their subcellular localization. PSORT II and MitoProt analysis of UVE1 light and dark isoforms predicts the longer form to be most likely mitochondrial and no specific localization pattern for the dark isoform. To confirm these predictions, light and dark isoforms of UVE1 were fused to the N-terminal end of GFP and expressed in the uve1Δ strain AI191. To assess mitochondrial localization we used MitoTracker red, which specifically stains respiring mitochondria. Confocal fluorescence microscopy for the GFP-fused light form of Uve1 showed co-localization of Uve1-GFP with MitoTracker red, giving a yellow fluorescence in merged images (Figure 5A). No expression of Uve1-GFP light form was observed in the nucleus, confirmed by co-staining with Hoechst (Figure S6). For the dark isoform of Uve1-GFP, GFP localization was throughout the cell, but clearly excluded from the mitochondria (Figure 5B; Figure S6). Transformation of the UVE1-GFP constructs into a uve1Δ genetic background enabled a test of their functionality in complementing the UV sensitive phenotype. Expression of the light form of UVE1 rescued in part the UV sensitive phenotype of strain AI191; however, the dark isoform fused to GFP did not. These experiments suggest that the light isoform of UVE1 is localized solely to the mitochondria, and as such it protects the mitochondrial genome from lethal effects of UV-induced DNA damage in C. neoformans.
Fig. 5. Subcellular localization of Uve1 (L)-GFP and Uve1 (D)-GFP from <i>C. neoformans</i> var. <i>neoformans</i> in the vegetative yeast cells of <i>C. neoformans</i> var. <i>grubii</i> (A) Uve1 (L)-GFP localization and (B) Uve1 (D)-GFP localization. 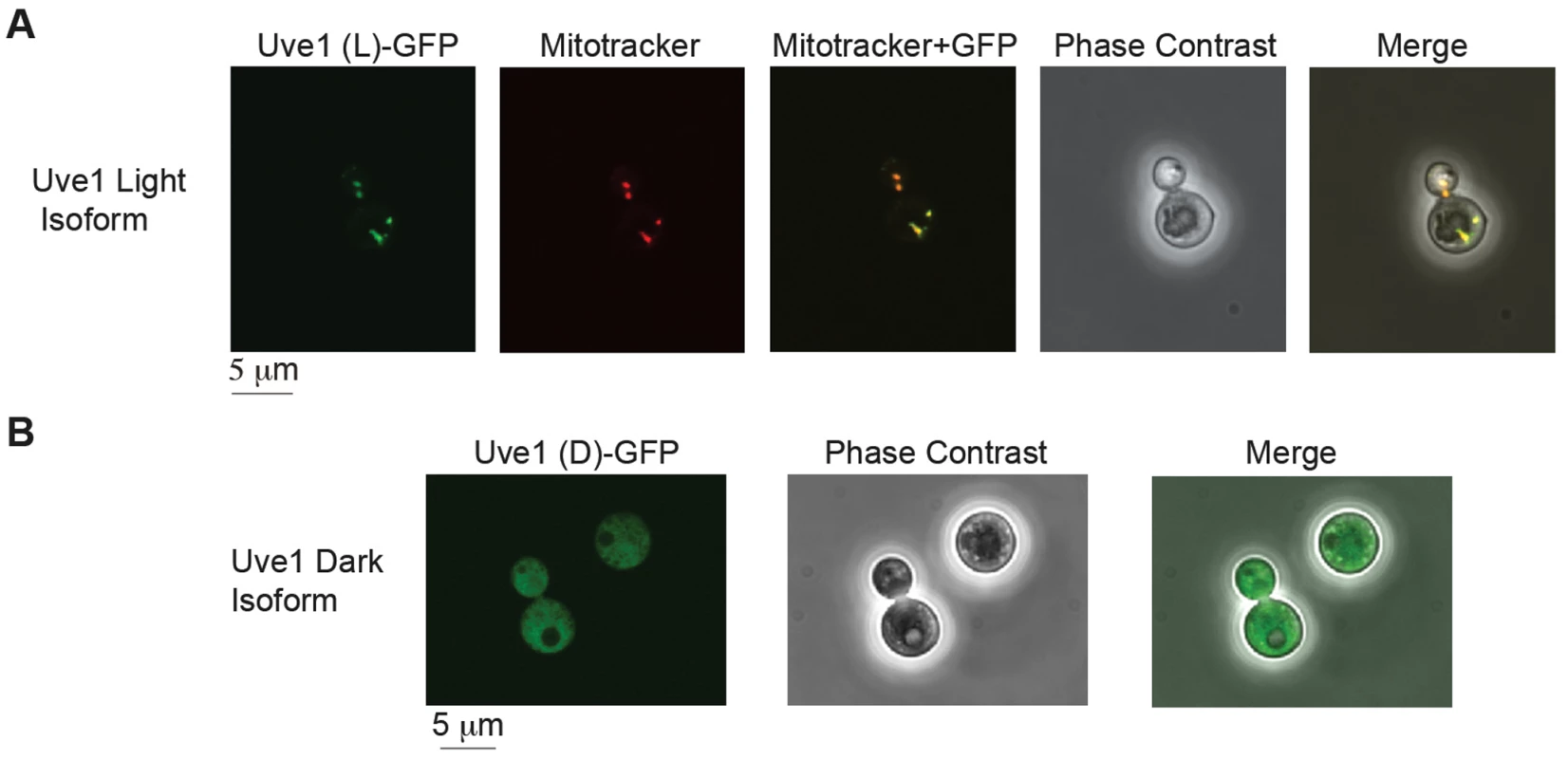
Functional conservation exists between Uve1 homologs of the basidiomycete C. neoformans and the ascomycete S. pombe
The function of Uve1 in the fungi at a biochemical level is best characterized in S. pombe [25], [29], [40], [41]. Alignment of the two homologs suggests that they are similar, sharing residues within the active site of the enzyme (Figure S3). To infer functional similarity, a cross-species complementation test was performed. An uve1Δ knockout was generated in S. pombe by replacing the gene via homologous recombination with the KanMX cassette that confers resistance to G-418. We then expressed in this S. pombe knockout strain the cDNA clones of light or dark isoforms of UVE1 from C. neoformans var. neoformans. The UV sensitivity of the S. pombe strains was tested (Figure 6A, B). The uve1 deletion strain was highly sensitive to UV irradiation, as was the control strain transformed with the empty vector. In the strain expressing the light isoform of UVE1, UV sensitivity was rescued as the strain survived UV doses equivalent to the wild type strain. For the uve1::KanMX+C. neoformans dark isoform, no rescue in the UV sensitive phenotype was observed. These observations suggest that the C. neoformans light isoform of UVE1 is functionally active and has the equivalent biochemical functions of Uvde from S. pombe required for repair of DNA damaged by UV exposure. It also suggests that the dark isoform of C. neoformans Uve1 may not have any function in conferring protection against UV.
Fig. 6. C. neoformans Uve1 is functionally similar to S. pombe UVDE. 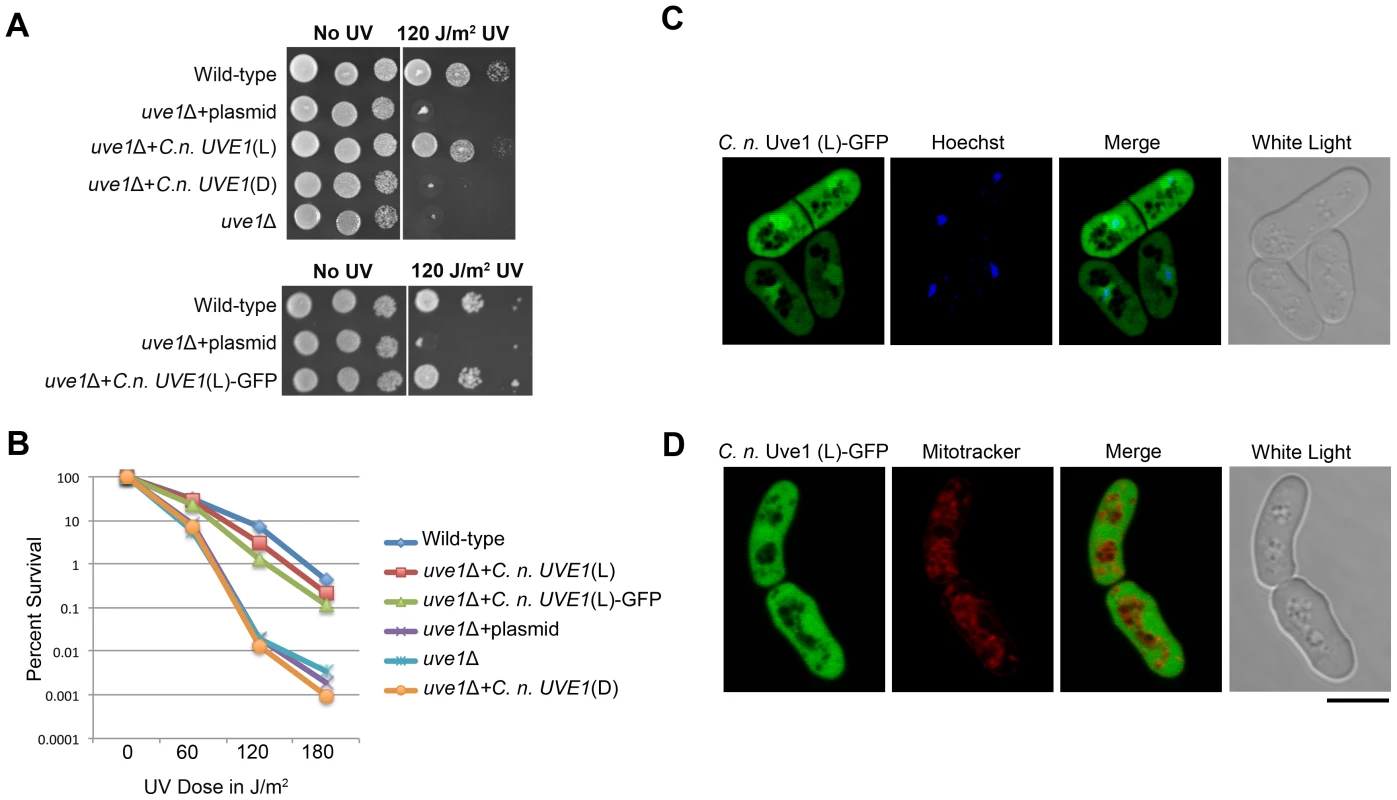
(A) The C. neoformans light (L) and dark (D) isoforms of UVE1 were expressed in an uve1 deletion strain of S. pombe. (A) Ten-fold serial dilutions for different strains of S. pombe grown at 30°C for 2 days. Left panel untreated control and right panel treated with UV dose of 120 J/m2. The strains used, from top to bottom are L972, AISVSP2, AISVSP4, AISVSP3 and AISVSP1. For the lower portion of the figure strains used are L972, AISVSP2, AISVSP15. (B) Graph of survival of strains L972, AISVSP4, AISVSP15, AISVSP2, AISVSP1 and AISVSP3 in response to UV stress of 0, 60, 120 and 180 J/m2. (C) Subcellular localization of C. neoformans Uve1 (L)-GFP compared to nuclei (Hoechst) in strain AISVSP1. (D) Localization of Uve1 (L)-GFP compared to mitochondria (MitoTracker). Scale bar = 10 µm. Cellular localization of C. neoformans Uve1 in S. pombe
C. n. var neoformans Uve1 was localized in S. pombe as a GFP fusion to see if the rescue of UV sensitivity phenotype in S. pombe is due to complementing mitochondrial or nuclear genome repair by Uve1 (Figure 6C, D). The Uve1-GFP construct was functional, complementing the UV sensitive phenotype of the S. pombe uve1 mutation (Figure 6A, B). The localization of Uve1 (L)-GFP is in part nuclear, as confirmed by co-localization with the nuclear Hoechst stain (Figure 6C). No localization in mitochondria was observed (Figure 6D). These results suggest that the Cryptococcus Uve1 protein, which seems to be important for mitochondrial DNA repair in C. neoformans, also plays a role in nuclear DNA repair in S. pombe. This observed localization pattern conforms to previous reports where Uve1 in S. pombe repairs nuclear DNA after UV stress, rather than mitochondrial DNA [32].
The C. neoformans uve1 mutant is impaired in its repair of mitochondrial DNA
If Uve1 localizes to mitochondria in C. neoformans, it is expected to play a role in mitochondrial DNA repair in consequence of DNA damage due to UV stress. As no nuclear localization was observed for Uve1, a negligible role of this endonuclease is expected in nuclear DNA damage repair. We performed a PCR-based DNA damage assay to assess the role of Uve1 in mitochondrial and nuclear DNA repair post-UV stress. The assay is based on the principle that damaged DNA impedes the progression of Taq polymerase on the template DNA in a PCR reaction [42]. Hence, there is an inverse relationship between the amount of DNA damage and PCR amplification products. Long template size increases the sensitivity of assay, as the longer the DNA the more chances of encountering damage (dimers). Small template amplification serves as a control, minimizing chances of encountering a damaged DNA strand, and is used for normalization of the amount of starting DNA or mitochondrial DNA copy number.
We compared DNA damage between the nuclear genome and mitochondrial genome of UV treated samples for both wild type and uve1Δ (Figure 7). After one hour there is delayed repair for both nuclear and mitochondrial genomes in the uve1Δ strain compared to wild type, probably reflecting retrograde signaling between the mitochondria and nucleus or that repair pathways of the nuclear genome can be ATP dependent [43]–[48]. The key observation is that in the uve1Δ strain there is lag in the mitochondrial repair. For later time points of 4 hour and 6 hours, the uve1Δ strain repair of mitochondrial genome is delayed as compared to wild type strain, which returned to normal (Figure 7). At 6 hours recovery, amplification of the mitochondrial genome in the wild type is as it was prior to DNA damage, but for uve1Δ the lesion damage still persists. However, the uve1Δ strain shows more efficient repair of lesions in the nuclear genome. For the 4 hour and the 6 hour time points, as the repair process of the mitochondrial genome initiates in uve1Δ by some unknown mitochondrial DNA repair enzymes, the repair of the nuclear genome is faster and comparable to wild type levels. These data implicate Uve1 in the efficient repair of UV-damaged mitochondrial DNA, with evidence for a complex interplay between mitochondrial and nuclear repair and the contributions of other repair pathways.
Fig. 7. Uve1 is required for efficient repair of mitochondrial DNA damage post UV stress. 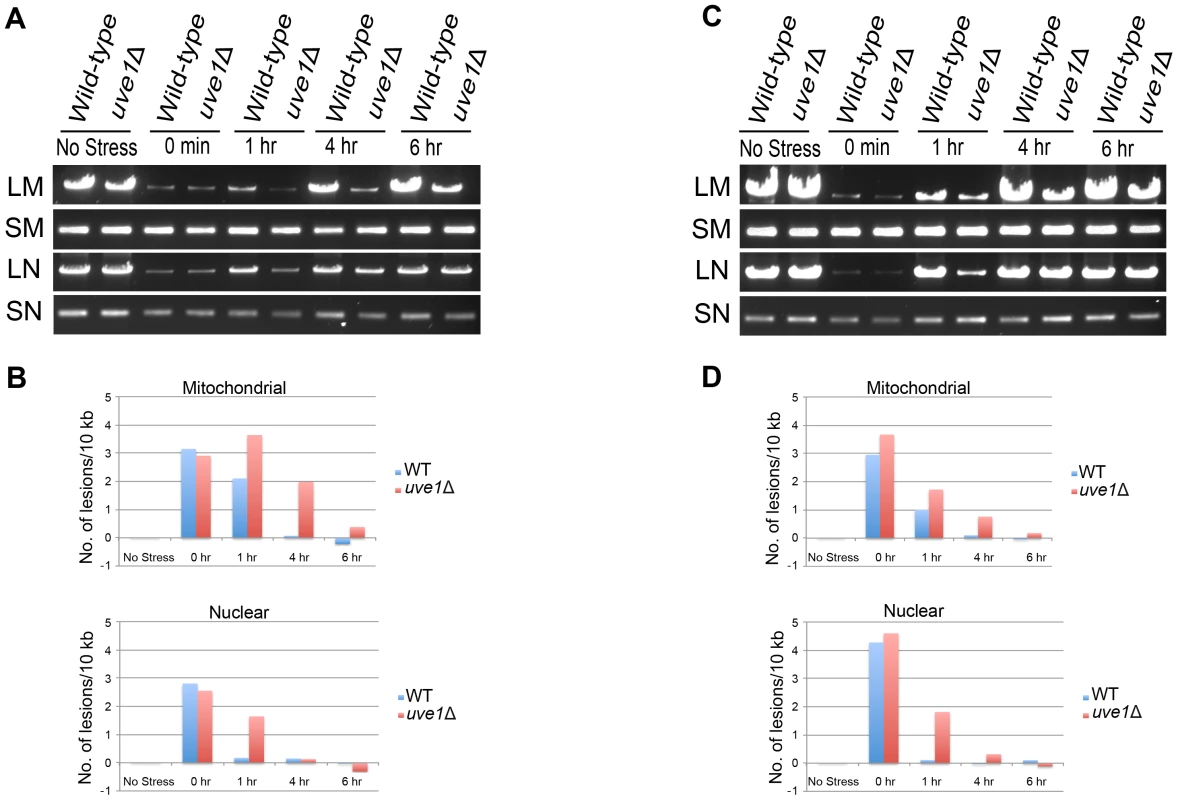
Two independent experiments were carried out on different days. (A, C) Agarose gels for the PCR amplification of mitochondrial and nuclear DNA on template DNA from undamaged control cells and template DNA from cells exposed to UV stress 50 J/m2. C. neoformans var. grubii strains KN99α (WT) and AI191 (uve1Δ) PCR amplification pattern for no stress, 0 hour, 1 hour, 4 hour and 6 hour post UV stress recovery. DNA amplification of long mitochondrial (LM) DNA, short mitochondrial (SM) DNA, long nuclear (LN) DNA and short nuclear (SN) DNA. (B, D) Graphical representations of DNA damage experiments in panels A and C, respectively. X-axis represents number of lesions/10 kb and Y-axis represents time in hours. Overexpression of Uve1 rescues the UV sensitive phenotype of bwc1 mutants
No induction of the UVE1 transcript encoding the functional form of the protein was observed under light conditions in bwc1Δ mutants (Figure 2). We examined if UVE1 is a direct target of the Bwc1-Bwc2 complex through two approaches. First, we tested if overexpression of Uve1 could rescue the UV hypersensitive phenotype of the bwc1Δ mutant in C. neoformans. Uve1 from var. neoformans was expressed under a galactose-inducible promoter (PGAL7) in the bwc1 deletion mutants of var. neoformans and var. grubii. The strains were grown overnight in media with glucose or galactose as the primary carbon source, serial diluted and plated, and UV sensitivity tests performed. Results from the var. neoformans strains are illustrated in Figure 8, and var. grubii in Figure S7. Uve1 overexpression rescued the UV sensitive phenotype of bwc1Δ as comparable to wild type when induced by galactose. In contrast, there was only slight rescue in strains grown in non-inducing glucose (Figure 8). For the bwc1Δ+PGAL7-UVE1 overexpression strains and wild type strains we performed northern analysis to check the levels of UVE1 induction (Figure S8). The levels of UVE1 transcripts were comparable between the galactose-induced PGAL7-UVE1 and the light-induced wild type strains. These results provide one piece of evidence for the Bwc1-Bwc2 complex directly controlling UV resistance in C. neoformans through regulation of the effector protein Uve1.
Fig. 8. UVE1 overexpression rescues the UV sensitive phenotype of bwc1Δ mutants in C. neoformans. 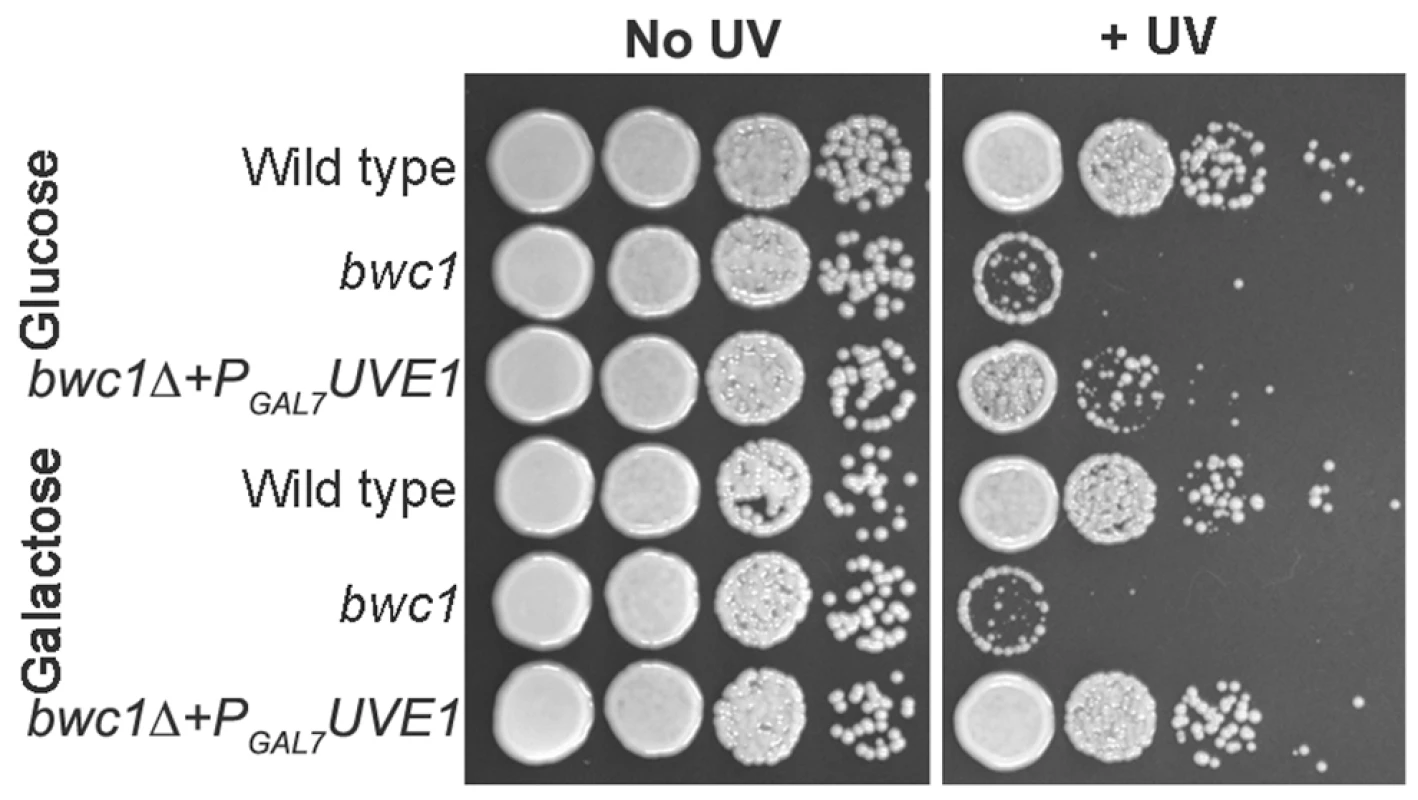
Ten-fold serial dilutions for different strains of C. neoformans var. neoformans grown at 30°C for 2 days. Left panel untreated control and right panel treated with UV dose of 120 J/m2. Top three strains (JEC21, AI5 and AISVCN53) grown overnight in 2% glucose and bottom three strains (JEC21, AI5 and AISVCN53) grown overnight in 2% galactose before inoculating onto YPD plates. Recombinant Bwc2 binds to the promoter of UVE1 of C. neoformans
The second piece of evidence that UVE1 is a direct target of Bwc1-Bwc2 comes from gel mobility shift assays. Bwc2 has a C-terminal GATA-type zinc finger whose binding target sites are not known in C. neoformans. We searched the promoter of UVE1 for putative Bwc2 binding sites based on those used by the WCC of N. crassa [11], [14], [49]. For instance, one light regulated element (LRE) found in the frq promoter is TCGATCCGCTCGATCCCCT, with the underlined nucleotides similar to a TCGATCTTCATCTCGATCTCCA sequence found in the promoter of C. neoformans UVE1. We amplified and radiolabeled the UVE1 promoter region with this site and performed gel mobility shift assays with recombinant Bwc2 (amino acids 26 to 383) expressed and purified from Escherichia coli (Figure 9A). A retardation in gel migration of the UVE1 promoter DNA was observed, that increased with higher Bwc2 concentration or by adding zinc which is expected for a zinc finger protein (Figure 9B). The nature of the higher mobility forms is unknown, but may represent aggregation of Bwc2 monomers. Control interactions using a non-specific DNA fragment confirmed the specificity of Bwc2 for the UVE1 promoter. These observations indicate that the UVE1 promoter is a direct target for Bwc2 binding.
Fig. 9. Bwc2 binds to the UVE1 promoter. 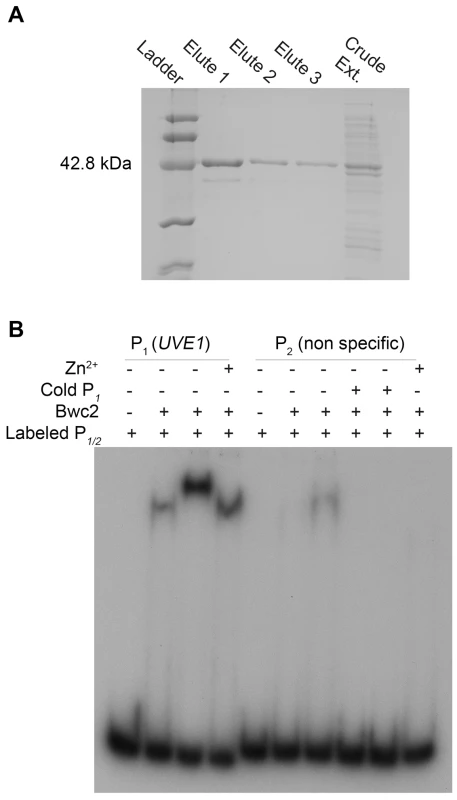
(A) SDS-PAGE gel for C. neoformans recombinant (His)6-Bwc2. Left to right, lane 1 is the low molecular weight Bio-Rad protein ladder, lane 2, 3, 4 are purified recombinant Bwc2 eluted fractions. Lane 5 is crude unpurified protein extract. (B) Gel mobility shift assay for a fragment of the JEC21 UVE1 promoter with purified Bwc2. Left to right, lane 1 contains just the UVE1 promoter P1 (PUVE1), lane 2, 3 and 4 contains P1+Bwc2 20 µg, 50 µg and 20 µg+Zn2+ respectively. Lane 5 is loaded with the non-specific radiolabeled probe P2. Lane 6, 7 are P1+Bwc2 20 µg and 50 µg. Lane 8, 9 are competition with the increasing amount of specific probe cold P1 to negate any binding observed in lane 7. Lane 10 is Bwc2 and P2 with Zn2+. Discussion
Light influences diverse aspects of fungal biology, presumably by acting on unique pathways in specific species. However, the potential for conserved regulation also exists, and this is predicted to reflect the original selective pressure(s) and current maintenance of light-sensing in extant fungi. Some responses to light in ascomycete fungi relate to protection against damage caused by light. For instance, expression of the DNA repair enzyme photolyase and genes for biosynthesis of carotenoid pigments are often induced by light. There are previous reports of links between light via its input in circadian rhythms with DNA repair in fungi, as well as in mice and humans. For instance PRD-4 is a checkpoint kinase 2 homolog in N. crassa that is regulated by the WCC and contributes to DNA repair [21]. Another DNA repair protein, XPA, has been shown to have circadian rhythm dependent oscillations in mouse brain [50], [51]. However, between 2.8–6% of genes are regulated at the transcript level in response to a light exposure in ascomycete species [11], [52], [53], yielding a long list of candidate genes for further analysis. In contrast, the basidiomycete C. neoformans may serve as a simpler model for understanding the evolution of light-sensing in fungi, because (a) it does not encode a photolyase gene and pigmentation is not induced by light, (b) few genes are induced in response to light at the transcript level [19], (c) there is no evidence of photoadaptation [17], a trait that influences the intensity of the response to light, and (d) the White collar complex contains only one protein with a zinc finger DNA binding domain [17], [18].
Here, we identify the UVE1 gene as a downstream target of the WCC in C. neoformans, and show that homologs are also light regulated in species that represent two other major branches in the fungal kingdom. We suggest that UVE1 acts as the key factor controlling the UV sensitive phenotype caused by mutating BWC1 or BWC2 in C. neoformans (Figure 1, Figure 10, Figure S4), and likely plays similar roles in other fungi to survive the deleterious effects of sunlight, which has UV wavelengths as an inevitable DNA damaging component.
Fig. 10. The involvement of Uve1 in the photosensory response of C. neoformans. 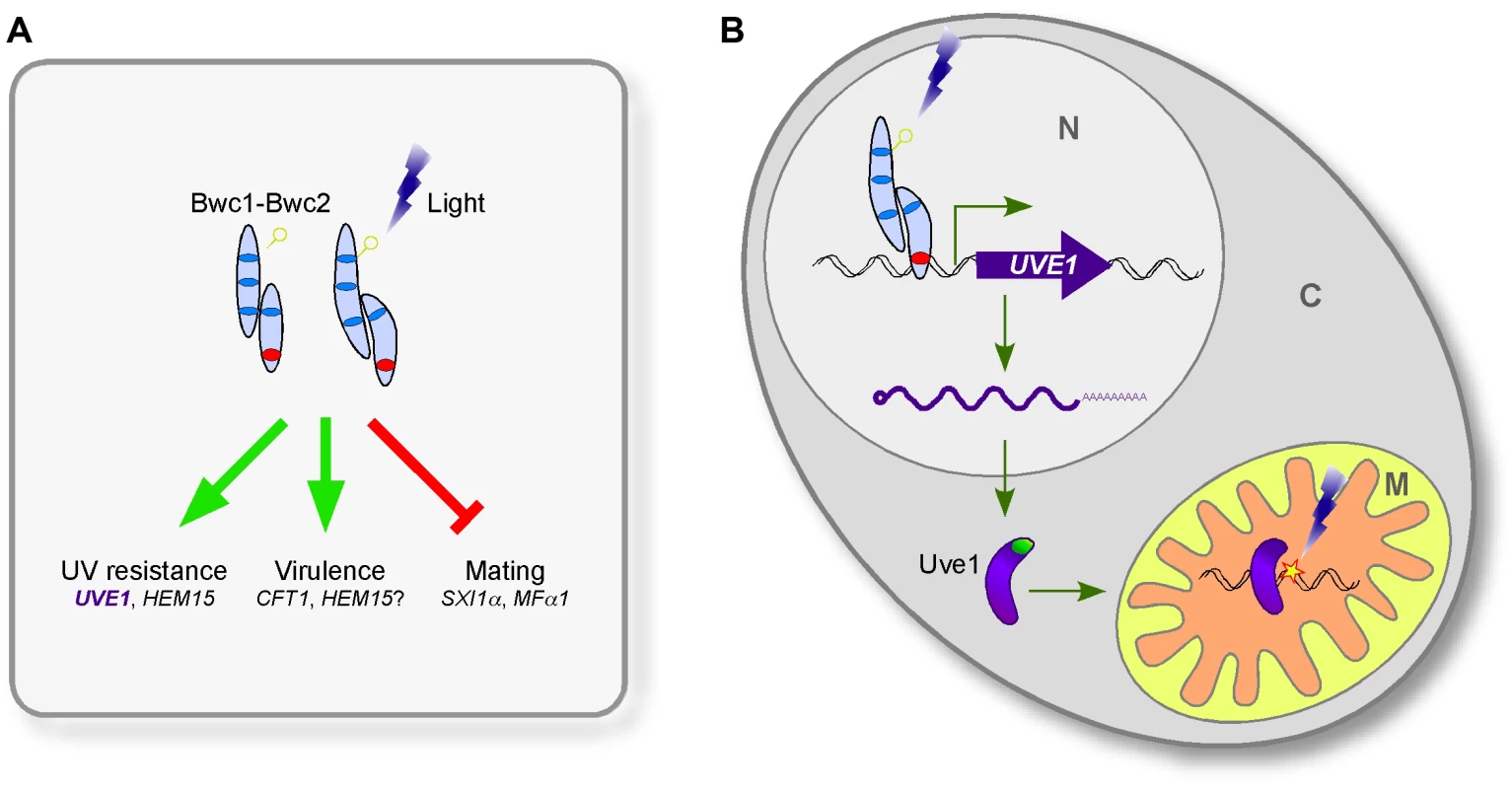
(A) Bwc1-Bwc2 influences three aspects of C. neoformans biology: mating, UV tolerance, and virulence. Uve1 impacts one of these three traits. (B) Uve1 endonuclease protects C. neoformans under UV radiation stress by protecting its mitochondrial genome. Photoreceptor Bwc1 senses blue/UV light, undergoes change in its flavin-binding domain, is activated, and forms a complex with Bwc2, a zinc finger transcription factor. Bwc2 with Bwc1 binds the UVE1 promoter to activate its transcription. Uve1 protein has a mitochondrial localization signal (green), and is transported to mitochondria. In mitochondria, on sensing the UV-induced DNA damage (star) Uve1 binds to damaged DNA and initiates repair by the UVDE DNA damage repair pathway. N nucleus; C cytoplasm; M mitochondria. Northern blot analysis and characterization of 5′ and 3′ ends of C. neoformans var. neoformans UVE1 showed two transcripts of differing size for the UVE1 gene. The functional complementation experiments (Figure 6) in S. pombe uve1Δ by the homologous UVE1 long isoform implies that the C. neoformans protein has similar DNA repair activities; such as against cyclobutane pyrimidine dimers, 6-4 photoproducts, apurinic/apyrimidinic sites, and stretches of single-stranded DNA nicks or gaps. Moreover the localization of C. neoformans Uve1-GFP in S. pombe is in part nuclear rather than mitochondrial (Figure 6). This explains the functional complementation of the UV stress tolerance phenotype in S. pombe uve1Δ strains, as in S. pombe the nuclear UVER pathway is attributed for survival under UV stress [32]. The UVE1 short isoform did not show any functional complementation. Bioinformatic analysis to identify the active domain of UVE1 (pfam03851) provides a possible explanation for the inactivity of the short isoform, because it does not encode the complete conserved region (Figure 3, Figure S3). Another possibility may be the absence of subcellular localization signals on the dark form, such that the protein is rendered inactive due to improper compartmentalization. Correct subcellular localization of proteins involved in DNA repair has been implicated in countering genotoxic stress [54], as improper localization can result in loss-of-function and may even lead to disease development in humans [55], [56].
The light isoform of Uve1 in Cryptococcus localizes to the mitochondria, as shown by Uve1-GFP fusion studies. C. neoformans is an obligate aerobe: it cannot survive loss of mitochondrial function from something like unrepaired DNA damage. Based on the following evidence: (a) no observed localization of Uve1 in nucleus (Figure S6); (b) localization of Uve1 to mitochondria (Figure 5); (c) strains with the UVE1 gene deleted exhibited reduced survival under UV stress and Uve1-GFP partially complements the UV sensitive phenotype of the uve1Δ strain; and (d) reduced mitochondrial DNA damage repair in uve1Δ strain comparatively to the wild type strain (Figure 7) suggest that Uve1 in Cryptococcus is required for protection of mitochondrial DNA for survival under UV stress. Overexpression of UVE1 in bwc1 mutants of either var. grubii or var. neoformans restores UV sensitivity to the wild type level (Figure 8), and recombinant Bwc2 physically binds to the promoter of UVE1 to cause a gel mobility shift (Figure 9B). These data further corroborate the hypothesis that UVE1 is a direct downstream target of Bwc1.
The light-induced genes in C. neoformans were previously sought by a whole genome microarray expression analysis in var. neoformans [19]. The UVE1 gene was not identified in that study although UVE1 transcript data are present for five of the six biological replicates, with an average 1.15 fold difference between dark and light treatment. By contrast, quantification by ImageJ of our northern blot data normalized to actin levels indicates that the longer isoform is upregulated about 16 fold in the light (Dataset S1). UVE1 remained undetected in microarray experiments because the 70-mer probe on the array is common to both light and dark transcript isoforms (Figure 3). The dark isoform must be under control of another transcription factor, using elements within the light isoform to drive transcription. These findings demonstrate one limitation of the microarray technique in comparison to more comprehensive transcript analysis techniques like tiling arrays or RNA-seq, or to conventional hybridization techniques like northern blotting that can detect alternative transcripts.
Two other phenotypes associated with mutation of BWC1 or BWC2 in C. neoformans are loss of the inhibition of mating by light and reduced virulence. To further verify the role of Uve1 in other BWC related phenotypes, we examined the role of Uve1 in C. neoformans mating by crossing uve1Δ strains of both mating types under light and dark conditions. We did not find any contribution of this gene in mating, as the uve1Δ strains behaved like wild type for repression of mating by light (Figure S2). Similarly, the phenotype of the uve1Δ mutant in animal studies does not phenocopy that of bwc1Δ or bwc2Δ. A large-scale analysis of virulence has been undertaken in C. neoformans, measuring competitive survival of strains in mouse lungs [38]. Both bwc1Δ and bwc2Δ strains showed reduced proliferation, consistent with their reported role in virulence [17]. In contrast, the uve1Δ mutant had no defect in this virulence assay. We corroborated these results in a wax moth larvae model of virulence (Figure S2). We also examined a possible role of UVE1 under oxidative stress based on the mild phenotype observed in S. pombe [31], but did not find any phenotypic difference in the uve1Δ strains compared to wild type. Thus, we postulate that Bwc1-Bwc2 modulates its function via more than one downstream target (Figure 10A); of which those for virulence and mating remain to be discovered in future studies. We propose a model for the relationship between light-sensing via the WCC and Uve1 function in the mitochondria in C. neoformans (Figure 10B). It is possible that this model applies also to other fungal species for the protection of mitochondrial, nuclear or both genomes under UV stress.
The White Collar complex is conserved across the fungal kingdom, with homologs present in the chytrids, Mucoromycotina, Glomeromycotina, Ascomycota and Basidiomycota. However the complex has been lost in some fungal lineages, like its absence from the Saccharomycotina [1], [2], [57]. One important question is whether any WCC downstream targets are conserved. Transcript comparisons by northern blot analysis in N. crassa and P. blakesleeanus, members of the Ascomycota and Mucoromycotina, demonstrate that UVE1 homologs are photo-regulated in at least one species in each of these fungal groups. The UVE1 homolog is also induced by light, as measured by microarray studies, in Aspergillus nidulans and N. crassa [11], [53]. Absence of regulation in bwc1 and madA-madB mutants in N. crassa and P. blakesleeanus further implicate White Collar-dependent regulation of UVE1. This suggests that in fungi that have White Collar, UVE1 is regulated in a light-dependent manner, and this regulation is lost or alternative regulation evolved in fungal species missing White Collar proteins. The presence of LOV domain containing flavin-binding photoreceptor proteins and UVE1 homologs in bacteria, like Bacillus subtilis [25], [58], warrant further examination if through convergent evolution the LOV domain type photoreceptors might be involved in regulation of UVE1 expression even more widely.
The repair of photo-damage by Uve1 is conserved in many fungi and important under UV stress, irrespective of the presence of base excision (BER) or nucleotide excision repair (NER) pathways. Repair of DNA damage from UV by Uve1 is faster in comparison to NER [40]; under ancient environments in which ultraviolet levels were higher than today Uve1 could have provided a selective advantage. We estimate from genome sequencing projects that 95% of the fungal genomes encoding WC-1 also encode a copy of UVE1. Many of the exceptions have homologs of photolyase present in their genome, which may play an equivalent role as Uve1, and can also repair mitochondrial DNA [59], [60].
In summary, light triggers a number of physiological and morphological changes in fungi. The advantages of using light as a signal that are conserved have remained unclear although there is increasing evidence for a role in protecting cells from damage. Here, we demonstrate that protection of DNA, including the mitochondrial genome, through photo-regulation of Uve1 provides a benefit that is present in fungi that are able to sense light through the White Collar Complex.
Materials and Methods
Generation of UVE1 knockouts and their complementation in C. neoformans
Gene knockout cassettes were constructed by fusion of around 1000 bp flanks 5′ and 3′ of the UVE1 gene with nourseothricin acetyltransferase (NAT) coding sequence for strain JEC21 (var. neoformans, serotype D) and neomycin phosphotransferase (NEO) coding sequence for strain KN99α (var. grubii, serotype A). Oligonucleotide primer sequences are listed in Table S1. To make JEC21 uve1Δ, 5′ and 3′ gene flanks were amplified by primer set AISV030/AISV034 and AISV032/AISV035, respectively, using JEC21 genomic DNA. To make KN99α uve1Δ, 5′ and 3′ gene flanks were amplified by primer set ai830/ai831 and ai832/ai833, respectively, using KN99α genomic DNA. The NAT and NEO ORFs were amplified by primer set ai290/ai006. Overlap PCR was performed to obtain 5′-UVE1-NAT-UVE1-3′ and 5′-UVE1-NEO-UVE1-3′ cassettes by mixing equimolar ratio of 5′-UVE1, NAT, UVE1-3′ for JEC21 and 5′-UVE1, NEO, UVE1-3′ for KN99α. Primers used to perform overlap PCR were AISV030/AISV032 and ai830/ai833 for JEC21 and KN99α, respectively. About 2 µg of 5′-UVE1-NAT-UVE1-3′ or 5′-UVE1-NEO-UVE1-3′ cassette was transformed into strains JEC21 and KN99α using biolistic delivery with a PDS 1000/He particle delivery system (Bio-Rad, Hercules, CA) [61]. Gene replacement was confirmed by PCR and Southern blots for the correct integration of the gene cassette. Strains and genotypes are provided in Table S2.
For complementation of UVE1 in serotype A, a wild type copy of UVE1 was amplified with primers ALID0001 and ALID0002 and cloned into the pCR2.1 TOPO plasmid. The insert was excised with BamHI-XhoI and subcloned into the BamHI-SalI site of pPZP-NATcc. The plasmid was transformed into strain AI191 (uve1::NEO) by biolistics, with positive transformants selected for growth on yeast extract-peptone-dextrose (YPD)+nourseothricin (100 µg/ml) plates. The plasmids used or constructed in this study are listed in Table S3.
The UV sensitivities of strains were tested by applying UV stress in an XL-1500 UV cross linker (Spectronics Corporation, Lincoln, NE). Unless otherwise stated, strains were exposed to the laboratory ambient light (400–800 LUX) during experiments.
Northern blot analysis of transcript levels of UVE1 homologs
Cultures were prepared for C. neoformans strains KN99α, AI81 (bwc1Δ), JEC21, AI5 (bwc1Δ), C. gattii (R265), S. pombe L972, N. crassa wild-type FGSC 4200, N. crassa (wc-1) FGSC 4398, P. blakesleeanus wild-type NRRL1555, and P. blakesleeanus (madA madB) mutant L51. All strains of each species were plated with equal optical density or numbers of spores in duplicates on 15 cm diameter petri dishes containing YPD, and kept in darkness. For N. crassa only, 50 ml liquid cultures were grown in 50 ml YPD medium. Cultures were grown for 23 h or 47 h depending on the growth kinetics of the species. On completion of 23 h or 47 h, one of the sets was exposed to cool white light (dual Sylvania 4100 K 32W bulbs) of 1600–2600 Lux for 1 h and the other set left in the dark. On completion of the 24 h or 48 h time periods both the light and dark cultures were scraped in the light or under safe red light (GBX LED safelight, Kodak, Rochester, NY). N. crassa cultures were harvested directly from the liquid medium. All cultures were pelleted, frozen using dry ice+ethanol, lyophilized and stored at −80°C.
Total RNA was isolated using Trizol reagent (Invitrogen, Grand Island, NY). To address the low transcript abundance of UVE1, total RNA was further purified for polyA mRNA isolation starting with 1 mg total RNA, using the PolyATract Kit (Promega, Madison, WI), except for P. blakesleeanus and C. gattii where 40 µg of total RNA were used. RNA samples were resolved on 1.4% agarose denaturing formaldehyde gels and blotted on to Zeta Probe membrane (Bio-Rad). Probes for northern analysis were amplified using specific primer sets (Table S4) and radiolabeled with [α-32P] dCTP (PerkinElmer, Waltham, MA) using the RediPrime II labeling kit (Amersham, Pittsburg, PA). The blots were stripped and re-probed with fragments of actin homologs as loading controls. Autoradiograms were scanned, and transcript levels were compared by ImageJ analysis (Dataset S1).
Subcellular localization of Uve1
The localization of the two isoforms of Uve1 within the cell was assessed by fusions to green fluorescent protein (GFP). C. n. var. neoformans strain JEC21 genomic DNA was used as the template to amplify the UVE1 light (L) isoform using primers AISV001/AISV003 and UVE1 dark (D) isoforms using primers AISV002/AISV003. The histone 3 promoter for Cryptococcus (PH3) and GFP-NAT were amplified from the pPZP-GFP-NATcc plasmid using ai255/AISV005 (overlap primer for L) or ai255/AISV006 (overlap primer for D) for PH3, and AISV004/ai256 for GFP-NAT. The overlap construct was amplified by mixing equimolar ratios of the three amplicons for the light and dark isoforms using primers M13F and M13R. About 2 µg of gel purified UVE1 L and D overlap constructs were transformed into strain AI191 by biolistics. Positives clones were selected by their growth on YPD+100 µg/ml nourseothricin plates and confirmed by PCR, DNA sequencing, western blotting for GFP, and fluorescence signal.
Strains AISVCN28 and AISVCN02 were used for localization of the Uve1 light and dark isoforms fused to GFP. Strains were stained with MitoTracker Red CMXRos (Invitrogen) at 3 nM, kept in the dark for 20 min, washed and suspended in phosphate buffered saline (PBS) and used for microscopy. Cells were imaged using Olympus confocal microscopes FLUOVIEW FV10i or FV300.
DNA damage assay
Cultures for C. neoformans strains KN99α (wild type) and AI191 (uve1Δ) were grown overnight in YPD and washed with distilled water. Cells were suspended in phosphate buffered saline to 4×104 cells/ml. For each strain totals of 150 ml cells were distributed in 30 ml aliquots for time points 0 min (after stress), 1 h, 4 h, 6 h and control (no stress). For each aliquot the cells were placed in 15 cm petri dishes and exposed to UV light (50 J/m2) using a UV cross linker. Immediately after the UV stress, cells were transferred to 50 ml tubes and kept on ice. Control and 0 min cells were pelleted and frozen in liquid nitrogen. For 1 h, 4 h and 6 h time points, cells were re-suspended in YPD and incubated at 30°C for these respective times, then centrifuged to pellet and snap frozen. All samples were lyophilized, and DNA was extracted by the CTAB buffer method [62].
The relative DNA damage to the mitochondrial and nuclear genomes were assessed using a PCR assay based on established methods [42]. Concentrations of DNA samples from each treatment were standardized by measuring them by spectrophotometry and making appropriate dilutions. Primers used for amplification of fragments of the mitochondrial genome were AISV87/AISV91 (11 Kb). PCR conditions for long mitochondrial PCR were 94°C 4 min, 23 cycles for 98°C 10 s, 68°C 15 min, and a final extension of 72°C 10 min using Ex Taq (Takara, Kyoto, Japan). For nuclear long amplification (8 kb) primers were AISV85/AISV95. Conditions for long nuclear PCR were 94°C 4 min, 23 cycles for 94°C 20 s, 58°C 20 s, 72°C 6 min and a final extension of 72°C 7 min. Short amplification primers for mitochondrial genome were AISV89/AISV99 and for the nuclear genome AISV85/AISV97 amplifying about 250 bp. PCR conditions for small mitochondrial and nuclear amplicons were 94°C 4 min, 23 cycles for 94°C 20 s, 55°C 20 s, 72°C 1 min, and a 72°C 7 min final extension. All PCR amplicons were resolved on agarose gels, and intensities were quantified using ImageJ software. DNA damage was compared by calculating relative amplification of large PCR fragments of the UV treated samples to that of the respective untreated controls using the method reported in reference [42], and adjusting for differences between nuclear and mitochondrial amplicon sizes.
Virulence assay in wax moth larvae
The Galleria mellonella virulence assay followed methods that were previously described [63]. Overnight cultures in YPD medium for strains KN99α, AI191 and AI181 were washed three times with PBS. Cells were suspended in PBS to 2×107 cells/ml. For each strain 11–12 larvae were injected with 5 µl of the cells, as well as the control PBS. Wax moth were incubated at 37°C and survival monitored daily.
Complementation tests with C. neoformans UVE1 isoforms in a S. pombe uve1 knockout strain
An S. pombe uve1 knockout strain was constructed to serve for the functional analysis of UVE1 isoforms from C. neoformans. For the construction of the gene knockout cassette, genomic DNA from S. pombe strain L972 was used to amplify around 320 bp 5′-uve1 and 300 bp 3′-uve1 fragments using primer pairs AISV007a/AISV009 and AISV010/AISV011, respectively. The KanMX fragment was amplified using primer set AISV007/AISV008 from plasmid pFA6a-GFP(S65T)-kanMX6. Overlap PCR was performed to generate the gene knockout cassette using primer set AISV007a/AISV011. Around 2 µg of the PCR construct were transformed into S. pombe (strain MM72-4A ura4-D18 h−) by lithium acetate transformation and cells plated on to YPD+100 µg/ml G-418. Gene knockouts were confirmed by PCR and Southern blotting. S. pombe uve1 knockout strain AISVSP1 was selected for C. neoformans UVE1 complementation studies.
UVE1 cDNA was reverse transcribed from RNA of C. neoformans strain JEC20 using Superscript III First strand Synthesis System (Invitrogen), as per company instructions (JEC20 is isogenic to JEC21, with a different MAT allele; [64]). The synthesized cDNA was amplified by site directed mutagenesis to abolish an NdeI site inconvenient for subcloning while conserving the encoded amino acid residue, and to introduce NdeI and BamHI restriction sites at the start and end of the UVE1 gene. Primers used for amplification of fragment 1 of the L and D form were AISV014/AISV013 and AISV015/AISV013, respectively. Primers used for amplification of fragment 2 were AISV012/AISV016. Overlap PCR was performed to amplify full L and D genes from fragment 1 and 2, using primers AISV014/AISV016 for UVE1 L form and AISV015/AISV016 for UVE1 D form. The NdeI and BamHI digested cassettes were ligated into the NdeI-BamHI site in the pREP42 vector enabling expression from an nmt promoter [39]. Positive clones were confirmed by sequencing. Plasmids containing UVE1 L and D isoforms were transformed into S. pombe strain AISVSP1 (ura4-D18 uve1::kanMX) by the lithium acetate method. Empty vector pREP42 was transformed into strain AISVP1 as a control. Positive S. pombe transformants were selected on minimal medium without uracil and were confirmed by PCR.
C. neoformans Uve1-GFP localization in S. pombe
C. neoformans var. neoformans Uve1 (L) C - terminal GFP localization in S. pombe was done by fusion of UVE1 (L) to GFP by overlap PCR. For fragment 1, UVE1 (L) was amplified from pREP42-UVE1 (L) using primers AISV014/AISV003 and the fragment 2, GFP, was derived from pPZP-GFP-NATcc using primers AISV004/AISV066. Overlap PCR joining fragments 1 and 2 was performed by primer set AISV014/AISV066. The overlap PCR product was cloned into pCR 2.1 TOPO, and transformed by heat shock into E. coli DH5α. Positive clones were selected and sequenced. A plasmid containing the desired Nde1-UVE1-GFP-BamHI overlap was digested with NdeI and BamHI. The Nde1-UVE1-GFP-BamHI digest was ligated with NdeI and BamHI digested pTN157. Plasmid pTN157-UVE1-GFP was transformed into S. pombe strain AISVSP1 (genotype ura4-D18 uve1::kanMX) by the lithium acetate method. Positive S. pombe transformants were selected on minimal medium without uracil and were confirmed by PCR. Transformants were examined for fluorescence signal and their UV resistant phenotype. Confocal microscopy was performed for strain AISVSP15. For both mitochondrial and nuclear staining, cells were grown in Edinburgh Minimal Medium (EMM). Mitochondrial staining was performed with MitoTracker Red CMXRos (Invitrogen) at a final concentration of 3 nM in water, kept in the dark for 20 min, washed and suspended in PBS and used for microscopy. Hoechst 33342 was used to stain the nucleus. Cells grown in EMM were washed and suspended in Hoechst (1 µg/ml in water) for 10 min. Cells were washed and suspended in PBS, and microscopy was performed.
UV survival experiments for S. pombe
Overnight cultures for strains L972, AISVSP1, AISVSP2, AISVSP3, AISVSP4 and AISVSP15 in YES media were subcultured the following day. Strains in exponential phase were dotted on YES medium in ten-fold serial dilutions. One set of plates was exposed to UV light (120 J/m2) using the UV cross linker, and another plate kept unexposed. Both UV treated and unexposed sets were incubated at 30°C for 2 days. For UV dose response experiments exponentially growing cells for strains at the same optical density were ten-fold serially diluted and equal volumes for all dilutions of the cells were plated on YES media. The control was kept unexposed to UV and others were exposed at UV doses of 60, 120 and 180 J/m2. All plates were incubated at 30°C for 3 days, and colony forming unit data analyzed for percentage survival.
UVE1 over-expression in C. neoformans bwc1 mutants
The UVE1 gene was over-expressed in bwc1 knockout backgrounds to assess if UVE1 can rescue the UV sensitive phenotype of bwc1 mutation. A fusion cassette of the JEC21 GAL7 promoter (PGAL7) and UVE1 gene was made by overlap PCR. The GAL7 promoter was amplified by primer set AISV025/AISV028 and UVE1 was amplified using primer set AISV027/AISV026 on JEC21 genomic DNA. The PGAL7-UVE1 fusion cassette was amplified by mixing GAL7 promoter and UVE1 PCRs in equimolar ratios by primer set AISV025/AISV026. The PGAL7-UVE1 fusion cassette was cloned in a TA vector (pCR 2.1 TOPO; Invitrogen), and transformed by heat shock in E. coli Top10 cells. Positive clones were selected and sequenced. A plasmid containing PGAL7 -UVE1 was digested with BamHI and XhoI. The fragment was ligated with pPZP-NEO Agrobacterium vector digested with the same enzymes, and transformed into Top10 cells. Positive clones were selected by their growth on kanamycin and verified by PCR and restriction digestion. The pPZP-NEO-PGAL7-UVE1 vector was transformed by electroporation into A. tumefaciens strain EHA105. Selected positive Agrobacterium transformants were co-cultured with C. neoformans strains AI5 and AI81. Positive transformants were selected for growth on YPD+G-418+cefotaxime plates. Transformants were cultured overnight in yeast nitrogen base (YNB) medium +2% galactose or 2% glucose, and 10-fold serial dilutions placed on YPD medium plates. Dotting was performed in duplicate and one set was exposed to UV radiation at 120 J/m2. Both sets were incubated at 30°C for 2 days.
Expression of recombinant Bwc2 and electrophoretic mobility shift assays
JEC21 RNA was reverse transcribed to make cDNA using the Superscript III First strand Synthesis System (Invitrogen). A fragment of BWC2 cDNA was amplified using primers AISV040/AISV041, containing BamHI and EcoRI restriction sites. The amplicon was digested with BamHI and EcoRI, and ligated into the pRSETA vector (Invitrogen) digested with the same enzymes. Top10 cells were transformed with the ligation product. An error free clone was identified by sequencing. The plasmid containing BWC2 was transformed into BL21(DE3)pLysS cells and selected on ampicillin+chloramphenicol SOB medium plates. Protein induction and expression using 1 mM IPTG was performed as described for the pRSET expression system by Invitrogen. The (Histidine)6-tagged Bwc2 protein was semi-purified using Pure Proteome Nickel Magnetic beads (Millipore Corporation, Billerica, MA) as per the manufacturer's instructions.
For electrophoretic mobility shift assays (EMSA), a 244 bp (P1) region 5′ of the start codon of UVE1 containing putative Bwc2 binding sites was amplified using primer set AISV019/AISV020. A control nonspecific DNA fragment (NS) of 278 bp was amplified from pRS426 vector using primers ALID1229/ALID1230. About 600 ng of the amplified fragment from UVE1 promoter region and nonspecific probe was radiolabeled with [γ-32P] dATP (PerkinElmer) using T4 polynucleotide kinase (New England Biolabs, Ipswich, MA). Labeling conditions were 1X T4 polynucleotide buffer, 5 µl 6000 Ci/mmol γ-32P ATP, 10 units T4 polynucleotide kinase in a 50 µl reaction mixture at 37°C for 30 min. The radiolabeled probes were purified using PCR purification columns (Qiagen, Germantown, MD). The composition of 5X EMSA binding buffer used was 20% w/v glycerol, 5 mM MgCl2, 250 mM NaCl, 2.5 mM EDTA pH 8, 10 mM DTT with or without 12 µM ZnSO4 (reference [65] with slight modifications). 20 to 50 µg of purified Bwc2 protein were pre-incubated with 1X EMSA binding buffer for 20 min at room temperature for all reactions. Total reaction mixture of 20 µl consisted of 1X EMSA binding buffer, 20 to 50 µg of purified protein, cold probe (if added), 1 µl of radiolabeled probe and 1X phosphate buffer, followed by 50 min incubation at room temperature. For competition reactions, 600 ng and 900 ng of the cold probes were included in the reaction mixture prior to addition of radiolabeled probes. At the end of 50 min incubation, samples were transferred to ice followed by addition of EMSA loading dye. Samples were loaded on 6% polyacrylamide Tris-borate-EDTA (TBE) gels (Invitrogen), and run at 130 V for 2 h in 1X TBE at 4°C. Autoradiography was performed by exposure to Gene Mate Blue ultra autoradiography films.
Supporting Information
Zdroje
1. Rodriguez-RomeroJ, HedtkeM, KastnerC, MüllerS, FischerR (2010) Fungi, hidden in soil or up in the air: light makes a difference. Annu Rev Microbiol 64 : 585–610.
2. IdnurmA, VermaS, CorrochanoLM (2010) A glimpse into the basis of vision in the kingdom Mycota. Fungal Genet Biol 47 : 881–892.
3. TischD, SchomollM (2010) Light regulation of metabolic pathways in fungi. Appl Microbiol Biotechnol 85 : 1259–1277.
4. BallarioP, VittoriosoP, MagrelliA, TaloraC, CabibboA, et al. (1996) White collar-1, a central regulator of blue light responses in Neurospora, is a zinc finger protein. EMBO J 15 : 1650–1657.
5. TaloraC, FranchiL, LindenH, BallarioP, MacinoG (1999) Role of a white collar-1-white collar-2 complex in blue-light signal transduction. EMBO J 18 : 4961–4968.
6. LiuY, HeQ, ChengP (2003) Photoreception in Neurospora: a tale of two White Collar proteins. Cell Mol Life Sci 60 : 2131–2138.
7. ChenCH, DunlapJC, LorosJJ (2010) Neurospora illuminates fungal photoreception. Fungal Genet Biol 47 : 922–929.
8. CrosthwaiteSK, DunlapJC, LorosJJ (1997) Neurospora wc-1 and wc-2: transcription, photoresponses, and the origins of circadian rhythmicity. Science 276 : 763–769.
9. DunlapJC, LorosJJ (2004) The Neurospora circadian system. J Biol Rhythms 19 : 414–424.
10. LiuY, Bell-PedersenD (2006) Circadian rhythms in Neurospora crassa and other filamentous fungi. Eukaryot Cell 5 : 1184–1193.
11. ChenCH, RingelbergCS, GrossRH, DunlapJC, LorosJJ (2009) Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J 28 : 1029–1042.
12. OlmedoM, Ruger-HerrerosC, CorrochanoLM (2010) Regulation by blue light of the fluffy gene encoding a major regulator of conidiation in Neurospora crassa. Genetics 184 : 651–658.
13. HeQ, ChengP, YangY, WangL, GardnerKH, et al. (2002) White collar-1, a DNA binding transcription factor and a light sensor. Science 297 : 840–843.
14. FroehlichAC, LiuY, LorosJJ, DunlapJC (2002) White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science 297 : 815–819.
15. LengelerKB, WangP, CoxGM, PerfectJR, HeitmanJ (2000) Identification of the MATa mating-type locus of Cryptococcus neoformans reveals a serotype A MATa strain thought to have been extinct. Proc Natl Acad Sci U S A 97 : 14455–14460.
16. Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A (2011) Cryptococcus From Human Pathogen To Model Yeast. Washington, DC: ASM Press.
17. IdnurmA, HeitmanJ (2005) Light controls growth and development via a conserved pathway in the fungal kingdom. PLoS Biol 3: e95.
18. LuYK, SunKH, ShenWC (2005) Blue light negatively regulates the sexual filamentation via the Cwc1 and Cwc2 proteins in Cryptococcus neoformans. Mol Microbiol 56 : 480–491.
19. IdnurmA, HeitmanJ (2010) Ferrochelatase is a conserved downstream target of the blue light-sensing White collar complex in fungi. Microbiology 156 : 2393–2407.
20. JungWH, ShamA, LianT, SinghA, KosmanDJ, et al. (2008) Iron source preference and regulation of iron uptake in Cryptococcus neoformans. PLoS Pathog 4: e45.
21. PregueiroAM, LiuQ, BakerCL, DunlapJC, LorosJJ (2006) The Neurospora checkpoint kinase 2: a regulatory link between the circadian and cell cycles. Science 313 : 644–649.
22. GamsbyJJ, LorosJJ, DunlapJC (2009) A phylogenetically conserved DNA damage response resets the circadian clock. J Biol Rhythms 24 : 193–202.
23. WakabayashiM, IshiiC, InoueH, TanakaS (2008) Genetic analysis of CHK1 and CHK2 homologues revealed a unique cross talk between ATM and ATR pathways in Neurospora crassa. DNA Repair 7 : 1951–1961.
24. IdnurmA, WaltonFJ, FloydA, ReedyJL, HeitmanJ (2009) Identification of ENA1 as a virulence gene of the human pathogenic fungus Cryptococcus neoformans through signature-tagged insertional mutagenesis. Eukaryot Cell 8 : 315–326.
25. TakaoM, YonemasuR, YamamotoK, YasuiA (1996) Characterization of a UV endonuclease gene from the fission yeast Schizosaccharomyces pombe and its bacterial homolog. Nucleic Acids Res 24 : 1267–1271.
26. KannoS, IwaiS, TakaoM, YasuiA (1999) Repair of apurinic/apyrimidinic sites by UV damage endonuclease; a repair protein for UV and oxidative damage. Nucleic Acids Res 27 : 3096–3103.
27. YajimaH, TakaoM, YasuhiraS, ZhaoJH, IshiiC, et al. (1995) A eukaryotic gene encoding an endonuclease that specifically repairs DNA damaged by ultraviolet light. EMBO J 14 : 2393–2399.
28. KaurB, FraserJLA, FreyerGA, DaveyS, DoetschPW (1999) A Uve1p-mediated mismatch repair pathway in Schizosaccharomyces pombe. Mol Cell Biol 19 : 4703–4710.
29. PaspalevaK, MoolenaarGF, GoosenN (2009) Damage recognition by UV damage endonuclease from Schizosaccharomyces pombe. DNA Repair 8 : 600–611.
30. KaurB, DoetschPW (2000) Ultraviolet damage endonuclease (Uve1p): a structure and strand-specific DNA endonuclease. Biochemistry 39 : 5788–5796.
31. FraserJLA, NeillE, DaveyS (2003) Fission yeast Uve1 and Apn2 function in distinct oxidative damage repair pathways in vivo. DNA Repair 2 : 1253–1267.
32. YasuhiraS, YasuiA (2000) Alternative excision repair pathway of UV-damaged DNA in Schizosaccharomyces pombe operates both in nucleus and in mitochondria. J Biol Chem 275 : 11824–11828.
33. EarlAM, RankinSK, KimKP, LamendolaON, BattistaJR (2002) Genetic evidence that the uvsE gene product of Deinococcus radiodurans R1 is a UV damage endonuclease. J Bacteriol 184 : 1003–1009.
34. GoosenN, MoolenaarGF (2008) Repair of UV damage in bacteria. DNA Repair 7 : 353–379.
35. MeulenbroekEM, PaspalevaK, ThomassenEAJ, AbrahamsJP, GoosenN, et al. (2009) Involvement of a carboxylated lysine in UV damage endonuclease. Protein Sci 18 : 549–558.
36. MeulenbroekEM, Peron CaneC, JalaI, IwaiS, MoolenaarGF, et al. (2013) UV damage endonuclease employs a novel dual-dinucleotide flipping mechanism to recognize different DNA lesions. Nucleic Acids Res 41 : 1363–1371.
37. PaspalevaK, ThomassenE, PannuNS, IwaiS, MoolenaarGF, et al. (2007) Crystal structure of the DNA repair enzyme ultraviolet damage endonuclease. Structure 15 : 1316–1324.
38. LiuOW, ChunCD, ChowED, ChenC, MadhaniHD, et al. (2008) Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell 135 : 174–188.
39. SanzC, Rodríguez-RomeroJ, IdnurmA, ChristieJM, HeitmanJ, et al. (2009) Phycomyces MADB interacts with MADA to form the primary photoreceptor complex for fungal phototropism. Proc Natl Acad Sci U S A 106 : 7095–7100.
40. YonemasuR, McCreadySJ, MurrayJM, OsmanF, TakaoM, et al. (1997) Characterization of the alternative excision repair pathway of UV-damaged DNA in Schizosaccharomyces pombe. Nucleic Acids Res 25 : 1553–1558.
41. BowmanKK, SidikK, SmithCA, TaylorJS, DoetschPW, et al. (1994) A new ATP-independent DNA endonuclease from Schizosaccharomyces pombe that recognizes cyclobutane pyrimidine dimers and 6-4 photoproducts. Nucleic Acids Res 22 : 3026–3032.
42. HunterSE, JungD, Di GiulioRT, MeyerJN (2010) The QPCR assay for analysis of mitochondrial DNA damage, repair, and relative copy number. Methods 51 : 444–451.
43. LiuZ, ButowRA (2006) Mitochondrial retrograde signaling. Annu Rev Genet 40 : 159–185.
44. Al-MehdiAB, PastukhVM, SwigerBM, ReedDJ, PatelMR, et al. (2012) Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci Signal 5: ra47.
45. UraK, ArakiM, SaekiH, MasutaniC, ItoT, et al. (2001) ATP-dependent chromatin remodeling facilitates nucleotide excision repair of UV-induced DNA lesions in synthetic dinucleosomes. EMBO J 20 : 2004–2014.
46. GilkersonR, BravoL, GarciaI, GaytanN, HerreraA, et al. (2013) The mitochondrial nucleoid: integrating mitochondrial DNA into cellular homeostasis. Cold Spring Harb Perspect Biol 5: a011080.
47. ZhangL, JonesK, GongF (2009) The molecular basis of chromatin dynamics during nucleotide excision repair. Biochem Cell Biol 87 : 265–272.
48. Owusu-AnsahE, YavariA, MandalS, BanerjeeU (2008) Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat Genet 40 : 356–361.
49. LambTM, GoldsmithCS, BennettL, FinchKE, Bell-PedersenD (2011) Direct transcriptional control of a p38 MAPK pathway by the circadian clock in Neurospora crassa. PLoS One 6: e27149.
50. KangTH, ReardonJT, KempM, SancarA (2009) Circadian oscillation of nucleotide excision repair in mammalian brain. Proc Natl Acad Sci U S A 106 : 2864–2867.
51. KangTH, Lindsey-BoltzLA, ReardonJT, SancarA (2010) Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc Natl Acad Sci U S A 107 : 4890–4895.
52. Rosales-SaavedraT, Esquivel-NaranjoEU, Casas-FloresS, Martínez-HernándezP, Ibarra-LacletteE, et al. (2006) Novel light-regulated genes in Trichoderma atroviride: a dissection by cDNA microarrays. Microbiology 152 : 3305–3317.
53. Ruger-HerrerosC, Rodríguez-RomeroJ, Fernández-BarrancoR, OlmedoM, FischerR, et al. (2011) Regulation of conidiation by light in Aspergillus nidulans. Genetics 188 : 809–822.
54. SwartzlanderDB, GriffithsLM, LeeJ, DegtyarevaNP, DoetschPW, et al. (2010) Regulation of base excision repair: Ntg1 nuclear and mitochondrial dynamic localization in response to genotoxic stress. Nucleic Acids Res 38 : 3963–3974.
55. CouthouisJ, HartMP, ShorterJ, DeJesus-HernandezM, ErionR, et al. (2011) A yeast functional screen predicts new candidate ALS disease genes. Proc Natl Acad Sci U S A 108 : 20881–20890.
56. HungMC, LinkW (2011) Protein localization in disease and therapy. J Cell Sci 124 : 3381–3392.
57. SalichosL, RokasA (2010) The diversity and evolution of circadian clock proteins in fungi. Mycologia 102 : 269–278.
58. LosiA, PolveriniE, QuestB, GärtnerW (2002) First evidence for phototropin-related blue-light receptors in prokaryotes. Biophys J 82 : 2627–2634.
59. YasuiA, YajimaH, KobayashiT, EkerAP, OikawaA (1992) Mitochondrial DNA repair by photolyase. Mutat Res 273 : 231–236.
60. GreenG, MacQuillanAM (1980) Photorepair of ultraviolet-induced petite mutational damage in Saccharomyces cerevisiae requires the product of the PHR1 gene. J Bacteriol 144 : 826–829.
61. ToffalettiDL, RudeTH, JohnstonSA, DurackDT, PerfectJR (1993) Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol 175 : 1405–1411.
62. PitkinJW, PanaccioneDG, WaltonJD (1996) A putative cyclic peptide efflux pump encoded by the TOXA gene of the plant-pathogenic fungus Cochliobolus carbonum. Microbiology 142 : 1557–1565.
63. MylonakisE, MorenoR, El KhouryJB, IdnurmA, HeitmanJ, et al. (2005) Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun 73 : 3842–3850.
64. Kwon-ChungKJ, EdmanJC, WickesBL (1992) Genetic association of mating types and virulence in Cryptococcus neoformans. Infect Immun 60 : 602–605.
65. HoldenNS, TaconCE (2011) Principles and problems of the electrophoretic mobility shift assay. J Pharmacol Toxicol Methods 63 : 7–14.
Štítky
Genetika Reprodukčná medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



