-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A Novel Highly Divergent Protein Family Identified from a Viviparous Insect by RNA-seq Analysis: A Potential Target for Tsetse Fly-Specific Abortifacients
Tsetse flies are the sole vector for African trypanosomes, causative agents of sleeping sickness in humans and nagana in cattle. Transcriptome and proteome analyses were utilized to examine the underlying mechanisms of tsetse lactation that occur during each reproductive cycle. These analyses revealed a dramatic shift to the synthesis of milk proteins during lactation and a novel milk-specific protein family. All members of this family were co-localized, shared sequence similarity and were expressed at 40× basal levels during milk secretion. Suppression of gene from this lactation-associated family impaired progeny development by reducing milk protein content and altering milk homeostasis. These novel genes represent an excellent target for tsetse-specific reproductive-based control mechanisms. In addition, the characterization of tsetse milk production revealed multiple factors that are functionally analogous between tsetse and mammalian lactation.
Published in the journal: A Novel Highly Divergent Protein Family Identified from a Viviparous Insect by RNA-seq Analysis: A Potential Target for Tsetse Fly-Specific Abortifacients. PLoS Genet 10(4): e32767. doi:10.1371/journal.pgen.1003874
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003874Summary
Tsetse flies are the sole vector for African trypanosomes, causative agents of sleeping sickness in humans and nagana in cattle. Transcriptome and proteome analyses were utilized to examine the underlying mechanisms of tsetse lactation that occur during each reproductive cycle. These analyses revealed a dramatic shift to the synthesis of milk proteins during lactation and a novel milk-specific protein family. All members of this family were co-localized, shared sequence similarity and were expressed at 40× basal levels during milk secretion. Suppression of gene from this lactation-associated family impaired progeny development by reducing milk protein content and altering milk homeostasis. These novel genes represent an excellent target for tsetse-specific reproductive-based control mechanisms. In addition, the characterization of tsetse milk production revealed multiple factors that are functionally analogous between tsetse and mammalian lactation.
Introduction
Tsetse reproductive biology is unusual among insects. Female tsetse give birth to a fully mature third instar larva (viviparity) after an extended intrauterine gestation. This reproductive strategy limits the capacity of tsetse mothers to only 8–10 offspring per lifetime [1]. To accommodate intrauterine larval development, the morphology and physiology of the female tsetse reproductive organs have undergone extensive modification. The reproductive tract has been expanded into a uterus to serve as a safe harbor for developing larvae. Ovarian development alternates between the right and left ovaries to produce a single oocyte during each gonotrophic cycle. The female accessory gland has been modified and expanded to provide milk that is secreted into the uterus and consumed by the developing larva [1]. The distinctive aspects of tsetse viviparity represent significant reproductive bottlenecks that could be exploited for population control. Furthermore, identification of factors specific to milk production could lead to development of novel tsetse-specific compounds that interfere with larval development and induce abortion (abortifacients) without impacting non-target insects.
The nutritional components of tsetse milk consist mainly of proteins and lipids emulsified in an aqueous base [2]. In total, 6–10 mg of nutrients (combined with 10 mg of water) are transferred to the larva in the milk during intrauterine development. Few studies have examined regulation of tsetse milk production, including an investigation of structural changes in the milk gland, radioisotope studies of nutrient movement within the mother during lactation, and direct examination of specific milk proteins [1], [3]–[9]. To date, six milk proteins have been characterized, including Transferrin [7], [10], a lipocalin (Milk Gland Protein1, MGP1 [6], [11]), two unknown milk proteins (MGP2–3; [12]), Acid Sphingomyelinase 1 (aSMase1; [9]) and Peptidoglycan Recognition Protein-LB (PGRP-LB, [13]). Furthermore, we recently showed that lipid metabolism is governed by the cooperative activity of insulin and juvenile hormone signaling pathways during the pregnancy cycle [14]. However, the full suite of proteins present in the milk and underlying mechanisms for their regulation during tsetse lactation and pregnancy have yet to be determined.
In this study, a satellite paper to our report on the whole genome sequence of the tsetse species Glossina morsitans morsitans [15], we used differential RNA-seq analyses to compare transcript abundance in females carrying an intrauterine larva (lactating) with females 24–48 hours after parturition (non-lactating or dry). The lactation period occurs during larvigenesis, while the dry period occurs over the course of oogenesis and embryogenesis [14]. In addition to transcriptome analysis, protein constituents of tsetse milk were identified through LC/MS/MS analyses of gut contents from nursing larvae. We describe the expression profile, the predicted structure based on an in silico approach, and the microsyntenic genomic organization of nine tsetse-specific milk proteins (MGP2–10) that we propose represent a highly divergent, novel protein family. siRNA-based knockdown analysis was employed to examine the functional roles of the MGP2–10 proteins during tsetse reproduction. Since MGP2–10 are tsetse-specific and have substantial influence over tsetse fecundity, we discuss their potential for exploitation in novel population reduction approaches. Lastly, we discuss our findings in light of milk secretions described from other lactating organisms.
Results
Identification of genes associated with lactation by transcriptome analyses
To understand the major products of lactation and factors that may be responsible for regulating their expression, we analyzed two RNA-seq libraries. The first library represents lactating females carrying an early third instar intrauterine larva, while the second represents dry females collected approximately 48 hours post-parturition, at which time they had an early embryo developing in the uterus and lactation has yet to commence. In total, over 42 million reads from each of the two libraries were recovered (Table 1). Overall read quality was high for both sample sets based on FastQC analysis. Removal of contaminating tsetse symbiont (Wigglesworthia, Sodalis and Wolbachia) specific sequences and cleanup resulted in a 4% reduction in the total number of sequences identified in lactating flies and a 5.1% reduction for dry flies, respectively (Table 1).
Tab. 1. Total read number generated by Illumina RNA-seq and following quality control measures including symbiont removal and elimination of low quality reads. 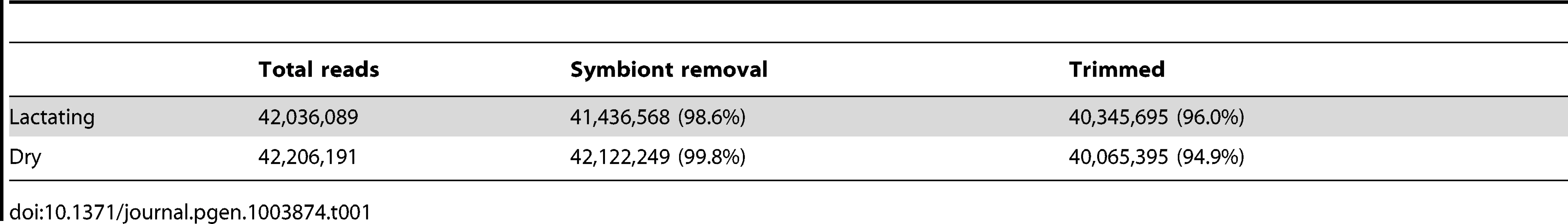
De novo assembly of the two datasets by Abyss [16], [17] and Trinity [18] generated 42,935 contigs that were subsequently identified according to multiple search parameters (Table S2). There were 34,674 contigs at least 200 bp in length, with the longest contig measuring 24,573 bp in size (Fig. S1). Distribution of reads per contig was comparable between the two datasets with the exception that there was a greater number of highly expressed genes in lactating flies (Fig. S2). Comparative analyses of contigs revealed that most were more highly expressed in dry flies compared to their lactating counterparts. A total of 297 contigs (2.1%) with at least 50 mapped transcriptome reads showed elevated expression in lactating versus dry flies (Fig. 1a; Table S2). Only 1311 were expressed at statistically different levels between the two datasets, with 48 contigs (4.2%) more highly expressed in lactating flies (Fig. 1b; Table S3). Classification of the lactation-expressed contigs based on specific metabolic and structural functions revealed enrichment for lipid metabolism, transport and storage, protein synthesis, secreted proteins, and those of unknown function (Fig. 2a).
Fig. 1. Fold changes in transcript expression for contigs based on RNA-seq analysis. 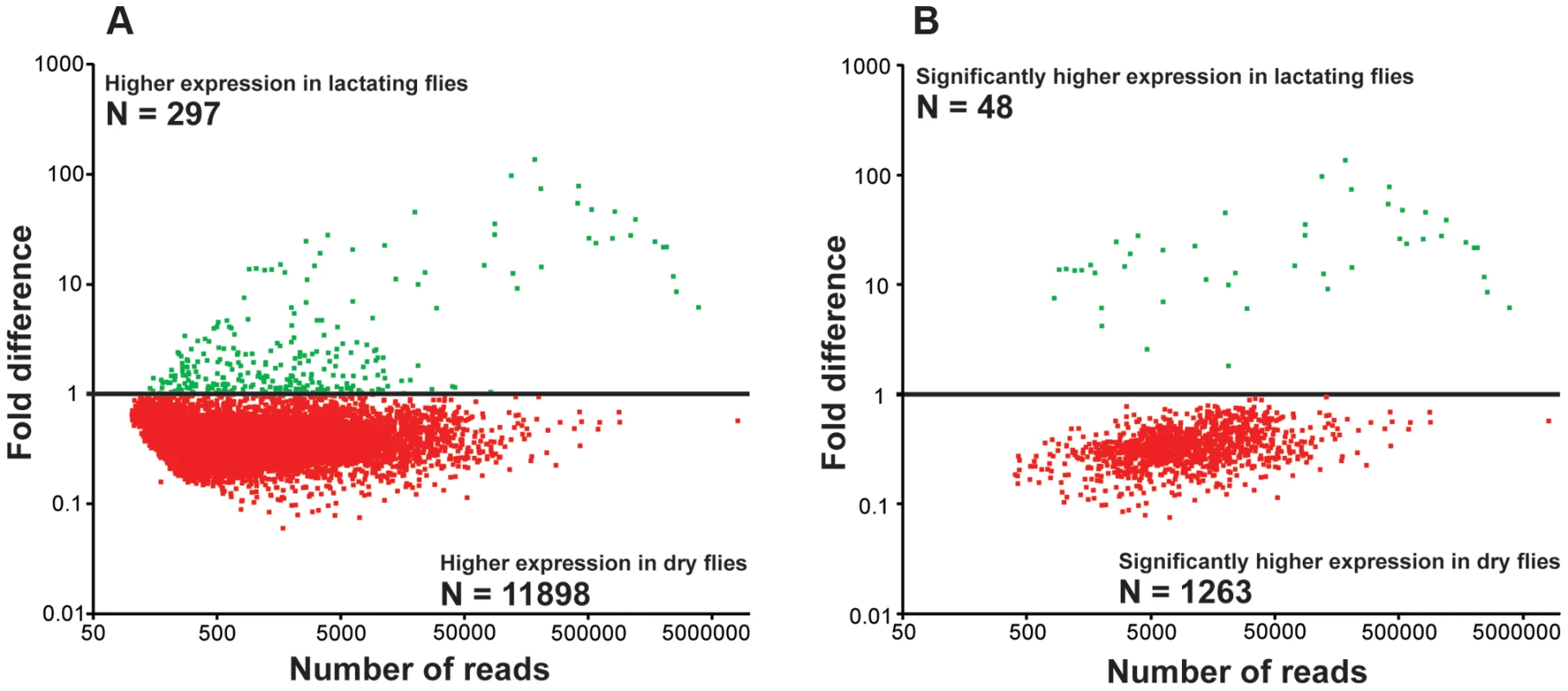
Green indicates expression higher in lactating flies and red indicates higher expression in dry flies. (A) Relative expression of each contig with at least 50 mapped reads. (B) Contigs with significantly different expression values from A (Kal's test with Bonferroni correction, P<0.05). Fig. 2. Gene ontology enrichment analysis. 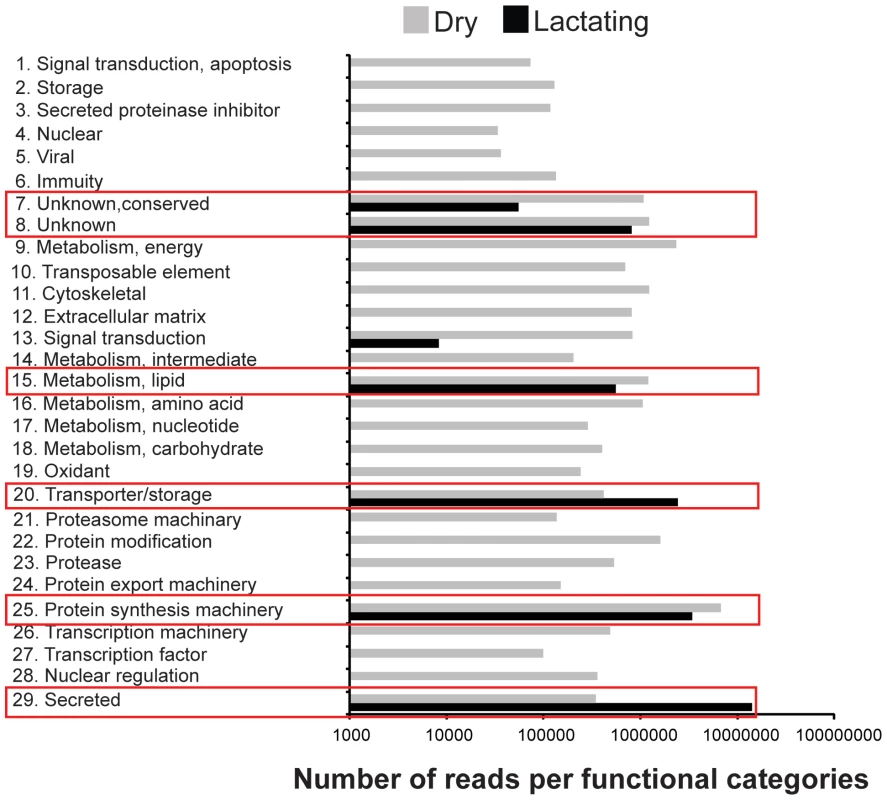
Reads in dry and lactating flies that mapped to genes with specific metabolic function. Examination of the most highly expressed contigs in lactating flies revealed known milk protein genes along with novel transcripts not previously associated with lactation. The known milk protein genes (mgp1, mgp2–3, tsf and asmase1) were expressed at least 10-fold higher in lactating versus dry flies (Fig. 3a–c; Table S2, S3). Of particular interest was the discovery of a group of seven new genes similar to the previously identified mgp2–3 genes that were upregulated during pregnancy (mgp4–10; Fig. 3b; Table S2). Transferrin and aSMase1, involved in iron transport and sphingolipid metabolism respectively, were the only other proteins that were highly expressed and showed increased transcript abundance in lactating flies (Fig. 3c; Table S2). Recently, the immunoregulatory Peptidoglycan Recognition Protein LB (PGRP-LB) was also detected in tsetse milk [13]. Based on this analysis, PGRP-LB expression patterns are different from those of other lactation associated-proteins, as its expression did not increase throughout pregnancy (Table S2). In addition to the genes described above, specific ribosomal RNAs were significantly elevated in lactating flies (Fig. 3d; Fig. S3), and may account for the overall increase in contigs coding for genes involved in protein synthesis (Table S2). Confirmation of transcript abundance during lactation was achieved by qPCR analysis of the mgp1–10, 28S, transferrin, pgrp-lb and asmase1 genes (Table 2; Text S1).
Fig. 3. Summary of specific genes that are differentially expressed in lactating compared to dry flies. 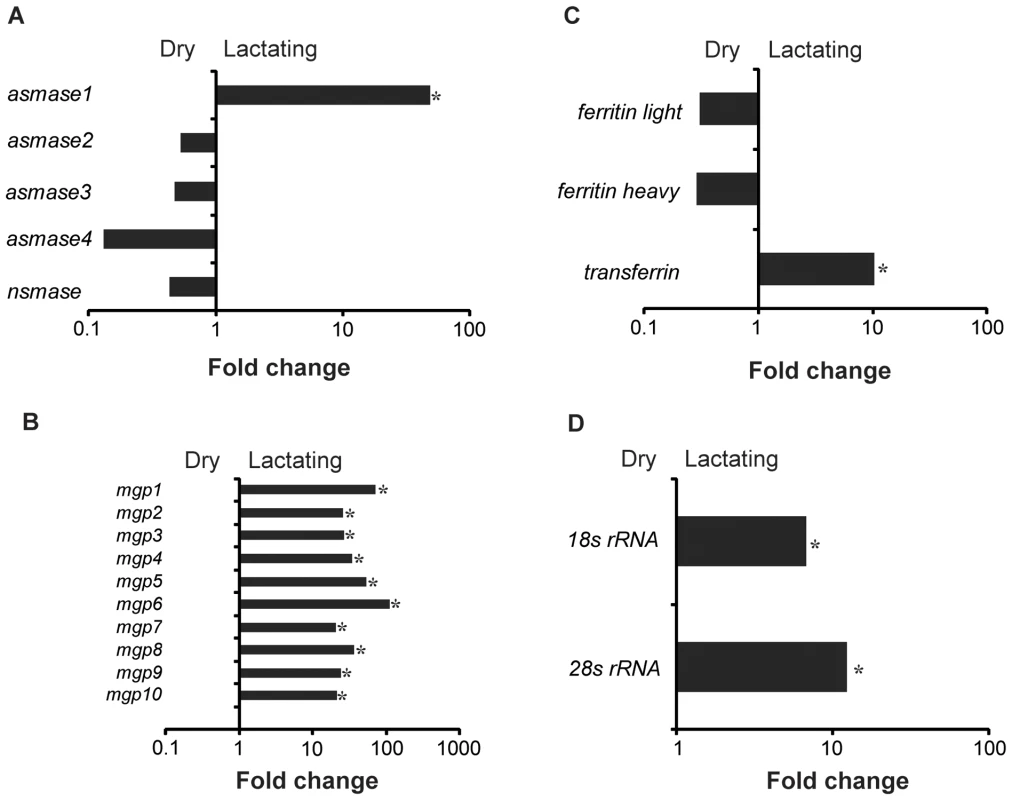
(A) Sphingomyelinase genes, asmase1–4 and nsmase. (B) Milk gland proteins genes, mgp1–10. (C) Iron-associated genes, non-hemecontaining ferritin, ferritin light, ferritin heavy and transferrin. (D) Ribosomal RNAs, 18S rRNA and 28S rRNA. *, significantly different expression values from B, Kal's test with Bonferroni correction, P<0.05. Tab. 2. Specific proteins documented in tsetse milk based by LC/MS/MS or expressed highly in the transcriptome study. 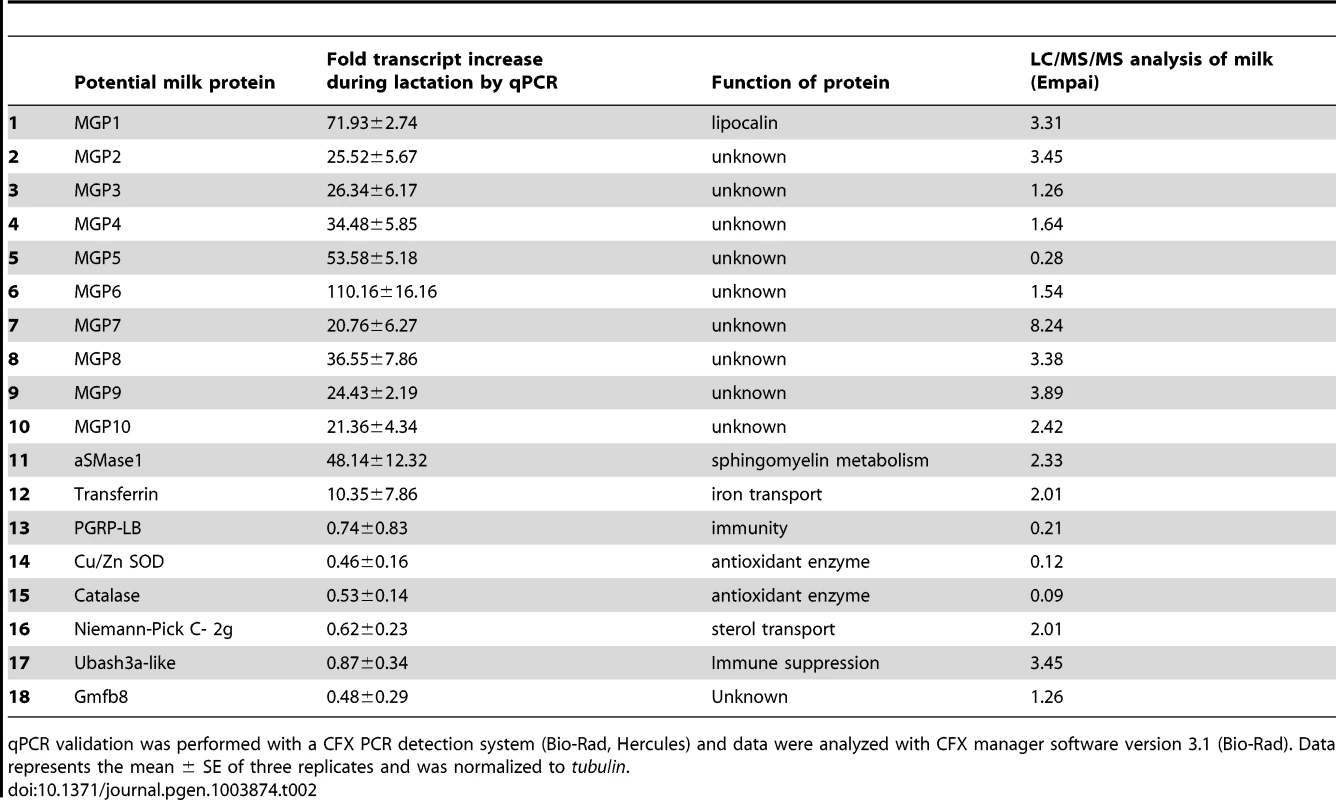
qPCR validation was performed with a CFX PCR detection system (Bio-Rad, Hercules) and data were analyzed with CFX manager software version 3.1 (Bio-Rad). Data represents the mean ± SE of three replicates and was normalized to tubulin. The majority of the contigs (94%) were more abundant in dry (non-lactating) compared to lactating flies (Fig. 1a; Table S2). Multiple gene families were highly expressed in dry flies (Fig. 2a, Table S2). Contigs encoding heat shock proteins and antioxidant enzymes were increased in dry flies, indicating that dry flies may be better suited than their lactating counterparts to respond to stress and environmental insult (Fig. S4). In particular, qPCR analysis validated the transcriptome data for Cu/Zn superoxide dismutase and catalase, which encode proteins that remove reactive oxygen species to prevent damage (Table 2). Lipid metabolism contigs were more abundant in dry flies with the exception of Brummer lipase, which was only two-fold higher than in lactating flies (Fig. S4; Table S2). Expression of tsetse yolk proteins, yolk protein 1–3 (yp1–3) was also higher in dry flies, reflecting the yolk protein synthesis that occurs between bouts of lactation (Fig. S4; Table S2). Contigs identified as trypsin showed decreased transcript abundance in lactating flies (Table S4). Given that the transcriptome analysis was from whole females, this finding likely correlates with lactating females' smaller bloodmeals that result from limitations on abdominal space imposed by developing intrauterine larva [5]. These results suggest that many processes in tsetse mothers are down regulated during lactation ( = higher in dry flies), when the female devotes energy and resources to synthesize milk-associated proteins to nourish the developing larva.
We conducted a secondary RNA-seq analysis after removing reads that mapped directly to the twelve most abundant lactation-specific genes (asmase1, mgp1–10 and transferrin). Removing these reads yielded only a 2.6% reduction in the dry fly dataset, but a drastic reduction of 47.2% was observed in the lactating fly dataset (Fig. 4a). This difference suggests that lactating flies invest over 47% of their total transcriptional activity toward producing the main protein constituents of tsetse milk (Fig. 4a). Each milk-specific gene accounted for 1.4–6.7% of the total read count in lactating flies, with the most reads mapping to mgp10 and transferrin (Fig. 4a). This removal resulted in a total of 2238 genes that were more highly expressed in lactating flies, but with only 151 that were significantly elevated relative to dry flies (Fig. 4b,c). Assignment by metabolic category resulted in a more balanced distribution of highly expressed contigs in lactating and dry flies (Fig. 5a). This second analysis revealed a few additional contigs whose expression increased during lactation; their expression was previously overshadowed by highly expressed milk-specific genes (Table S5; Table S6). These included dawdle (an activin signaling molecule), glyoxylate/hydroxypyruvate reductase (an enzyme that converts glycerate to hydroxypyruvate), choline-phosphate cytidylyltransferase (an enzyme in the Kennedy pathway that catalyzes choline phosphate to CDP-choline) and multiple ribosomal proteins (Fig. 5b, Tables S5, Table S6).
Fig. 4. Mapping of reads to lactation-specific genes and fold changes in transcript expression for contigs after removal of lactation-specific genes based on RNA-seq analysis. 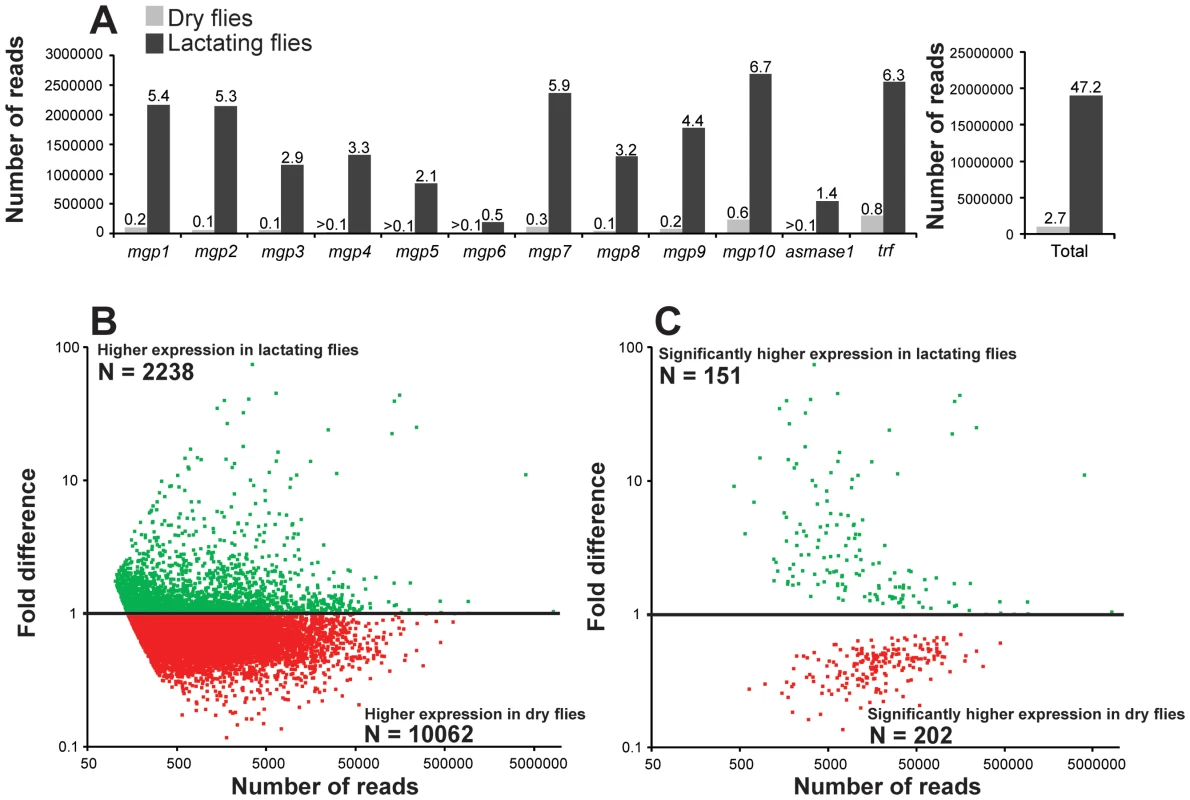
(A) Left, number of reads mapping to individual genes coding for the 12 milk-specific proteins. Numbers above columns are the percent of total sample that mapped to the specific gene. Right, Sum of reads from all 12 milk-specific proteins. Number above columns are percent of total samples. (B) Relative expression of each contig with at least 50 mapped reads after removal of Illuminia reads for milk-specific contigs. (C) Contigs with significantly different expression values from B, Kal's test with Bonferroni correction, P<0.05. Fig. 5. GO enrichment and genes identified following RNA-seq analysis after milk-specific gene removal. 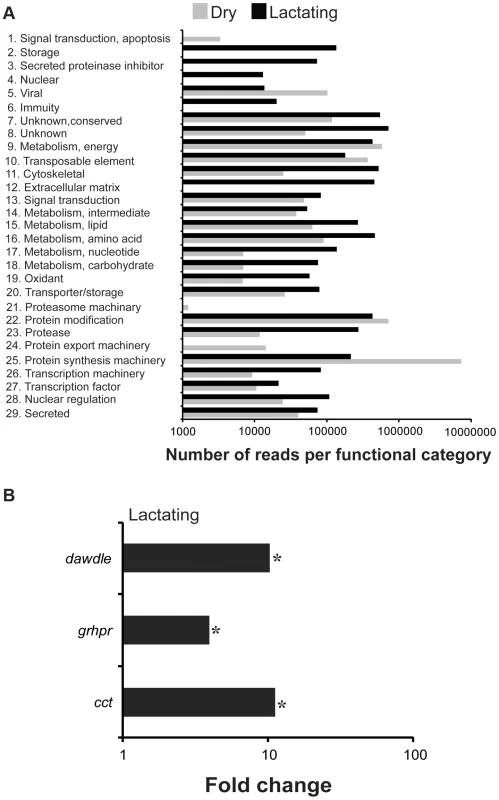
(A) Reads in dry and lactating flies that mapped to genes with specific metabolic function. *, indicating a significantly higher level in lactating flies based on chi-square test. (B) Select genes identified as increased during lactation following RNA-seq analysis after milk-specific gene removal. *, indicates significantly different between lactating and dry flies based on Kal's test followed by Bonferroni correction. Proteins present in tsetse milk as determined by proteome analysis
Using LC/MS/MS analyses on the gut contents of feeding larva, we identified 155 proteins that may be constituents of tsetse milk. Most of these proteins have a low exponentially modified protein abundance index (empai) value and are likely present in milk at low levels or may be products from the larval gut (Table S7). Most of the highly abundant proteins identified in tsetse milk were products of genes identified as highly expressed during lactation in our transcriptomics analysis, including MGP1–10, Transferrin and aSMase1 (Table 2). Previously, PGRP was documented in tsetse milk [13] and we confirmed the presence of this immune protein in the milk proteome (Table 2). In addition to the transcriptionally-abundant proteins, the milk proteome identified three other abundant proteins (empai >1.2; Table 2). These three proteins include a sterol binding protein (Niemann-Pick C-2g, NPC2G), Ubiquitin Associated and SH3 Domain Containing A (UBASH3A, a protein belonging to the T-cell ubiquitin ligand, TULA, family [19]), and a putative tsetse protein with unknown function (GmfB8). Transcript levels for NPC2G, GmfB8-like protein and UBASH3A were measured in the milk gland/fat body fraction during and after lactation and in the larval gut to confirm whether these are generated by the milk gland or if they are products of the gut (Fig. 6). Both npc2g and gmfb8-like protein expression were detected at high levels in the larval gut. Transcript level for UBASH3A was higher in the milk gland/fat body, showing an expression profile similar to PGRP-LB (Fig. 6), suggesting that this is likely a low abundance protein generated by the milk gland during lactation. These results provided further validation for our transcriptome-based identification of milk protein genes as actual secreted products in tsetse milk. In addition, we recovered potential milk proteins that are not under extensive transcriptional regulation during lactation.
Fig. 6. Validation of specific highly abundant proteins within the milk proteome. 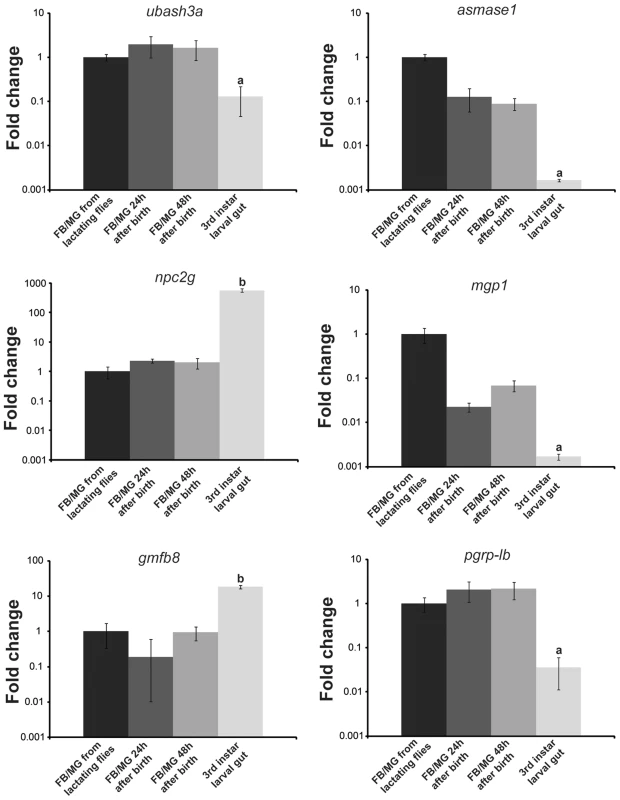
FB/MG, fat body/milk gland analyzed from lactating flies, 24 hours after birth and 48 h after birth along with 3rd instar larval gut. Transcript levels were determined by qPCR using a CFX PCR detection system (Bio-Rad, Hercules) and data were analyzed with CFX manager software version 3.1 (Bio-Rad). Data represents the mean ± SE of three replicates and was normalized to tubulin. a (lower) and b (higher), denotes significant difference by ANOVA with Tukey's test at P<0.01 in comparison to the other samples. Phylogenetic analysis, gene structure and predicted protein structure of novel milk proteins
BLASTx searches of the NCBI nucleotide collection failed to recover orthologous sequences to the MGP2–10 from any organism. Partial gene sequences encoding MGPs were identified from two other tsetse species, Glossina fuscipes fuscipes (MGP2, 5) and Glossina pallidipes (MGP3, 4), using RT-PCR with degenerate primers (Fig. S5, S6). Mining of sequence data from recent EST projects on the flesh fly, Sarcophaga crassipalpis [20], [21], revealed one sequence with marginal sequence similarity with the tsetse MGP2–10 (Fig. S5, S6). The average number of amino acids for MGP2–10 was 179.3 (range 170–191, Table 3) with a predicted molecular weight of 21.4 kD (range 20.4–22.4 kD; Table 3). The average isoelectric point for MGP2–10 was 6.2 (range 5.9–6.5; Table 3). The aliphatic index, or the relative volume of a protein occupied by aliphatic side chains (alanine, valine, isoleucine, and leucine) that is indicative of the stability of globular proteins [22], is moderate for MGP2–10, ranging from 64–86 (Table 2). There are no predicted glycosylation sites on MGP2–10, but there are at least four predicted phosphorylation sites for each MGP (Table 3). Amino acid alignments of MGP2–10 identified a conserved secretory peptide and three conserved regions with 68–100% and 12–100% nucleotide and amino acid similarity, respectively (Fig. 7a,b; Fig. S5). Phylogenetic analysis shows MGP2 and MGP4 as recently duplicated paralogs sharing 92% amino acid similarity (Fig. 8a; Fig. S6 a,b). Mapping of mgp2–10 coding sequences to genomic scaffolds revealed that these genes localize to a 40 kb microsyntenic region (Fig. 8a). The phylogeny for mgp2–10 splits these genes into two distinct groups, one consisting of mgp2,4,5,6,9,10 and the other of mgp3,7,8. When the phylogeny is mapped against the genome location of mgp2–10, mgp2,4,5,6,9,10 localized with a region surrounded by mgp3,7,8 (Fig. 8a). All mgp genes share a conserved exon-intron structure (Fig. 8a), despite showing varying levels of amino acid sequence similarity amongst them (Fig. 8b; Fig. S5). Our results indicate that MGP2–10 proteins are likely specific to Glossina, but it remains to be seen if evolutionarily-related sequences may exist in other closely-related viviparous genera (i.e. bat flies and sheep ked; data not available for these species). The sequence obtained from the flesh fly may represent either a class of proteins distinct from the tsetse MGP family, or could be highly divergent ancestral sequence to MGP2–10 genes found in tsetse.
Fig. 7. Selective pressure acting upon mgp gene family. 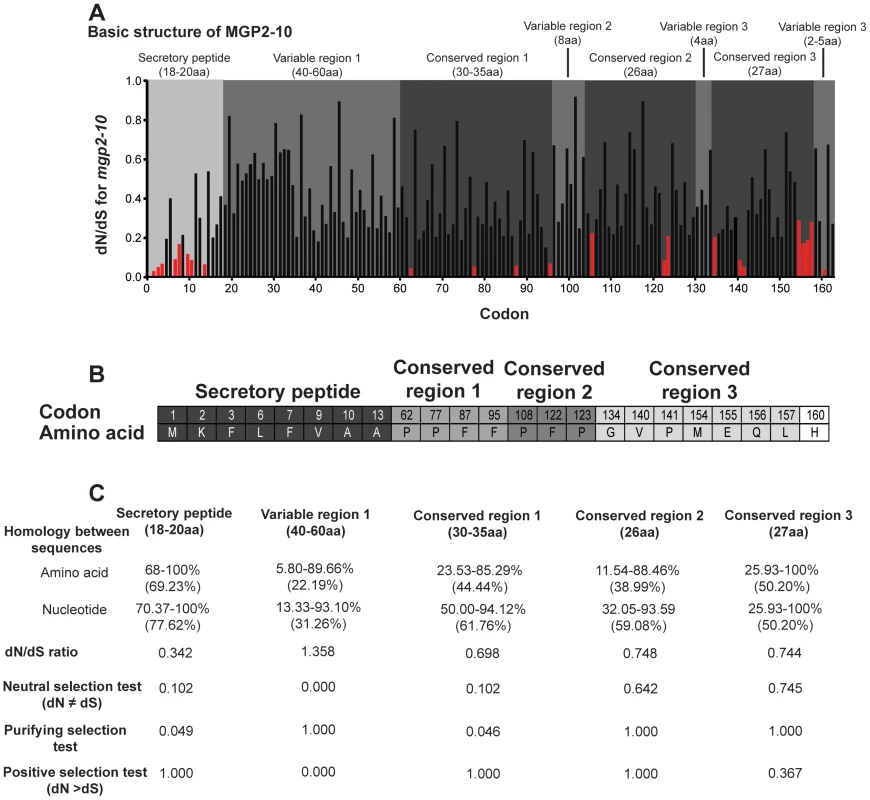
(A) Site-specific dN/dS analysis along a multiple alignment of MGP coding sequences. Residues identified as subject to negative selection under FEL analysis (posterior probability cutoff = 95) are indicated in red. Regions are described as conserved or variable according to visual examination of the multiple alignment and corroborated by dN/dS anslysis. (B) Specific codons and their corresponding amino acid sequence under negative selection. (C) Percent amino acid and nucleotide homology, average dN/dS ratio and selection tests for specific regions of MGP2–10. Positive, neutral and purifying test were conducted with codon-based Z-test in MEGA5 [25]. Fig. 8. Genome localization of Glossina morsitans and mgp2–10 phylogeny of genes for novel Glossina morsitans morsitans milk gland proteins. 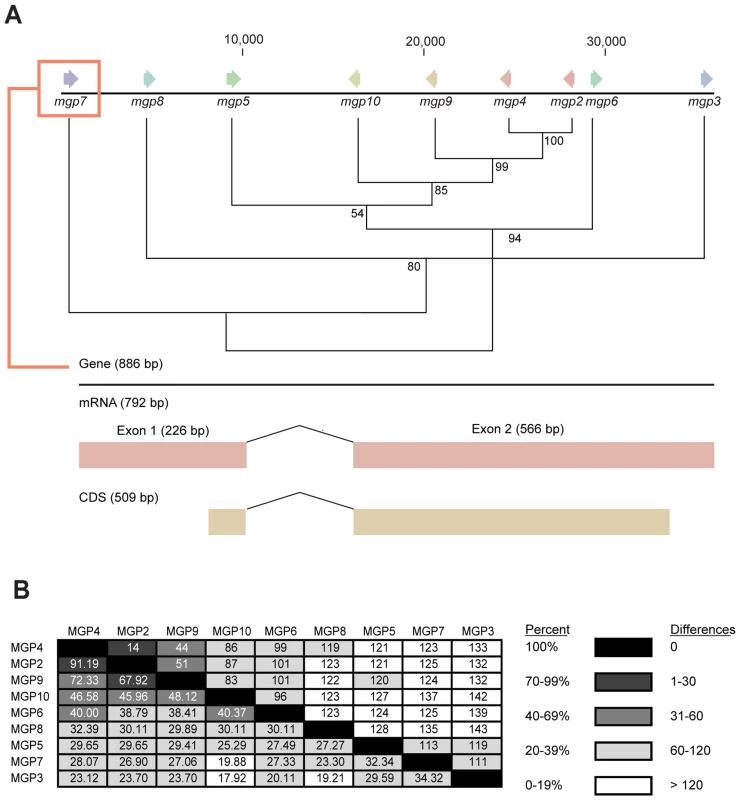
(A) Initial sequence alignment was completed using ClustalX [23], [124] and formatted with BioEdit [23]. Evolutionary analyses were conducted in MEGA4/5 [25], [26], [125] and displayed as a neighbor-joining tree. (B) Percent amino acid similarity (Bottom) and amino acid differences (Top) between MGP2–10. Tab. 3. Characteristics of novel milk gland proteins (MGP2–10). 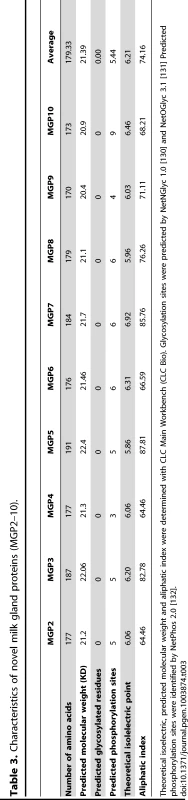
Theoretical isoelectric, predicted molecular weight and aliphatic index were determined with CLC Main Workbench (CLC Bio). Glycosylation sites were predicted by NetNGlyc 1.0 [130] and NetOGlyc 3.1 [131] Predicted phosphorylation sites were identified by NetPhos 2.0 [132]. Based on amino acid composition, the novel tsetse milk proteins provide all essential amino acids necessary for larval growth and development (Table S8). Protein structure predictions for the novel MGPs were generated by four individual programs. Structural predictions were ab initio as no homologous protein structures were available. The ab initio structure predictions from the four programs revealed that MGPs usually form multiple α-helices (6–10 per protein). However, no functional insights were provided by the I-TASSER program, since MGPs lack structural similarity to other characterized proteins (Fig. S7). Of particular interest, these proteins contain high percentage of hydrophobic amino acids (this study, [12]), including the hydrophobic secretory peptide that was identified in tsetse milk, indicating that this region is not always cleaved during secretion.
Examination of amino acid alignments identified several regions of moderate conservation across MGP2–10 from G. morsitans. To assess the relative selective pressures acting on these paralogous genes, we performed several computational analyses of nonsynonymous-to-synonymous substitution ratios (dN/dS) along the coding sequences for these genes. This type of analysis is usually conducted on orthologous genes in different species or on multiple alleles within a species, but utilization of this on paralogous genes could provide insight into regions critical to their function. When dN/dS substantially exceeds 1, evidence for positive selection ( = adaptive evolution) is inferred. In contrast, dN/dS = 1 implies neutral evolution, while dN/dS values closer to zero provide evidence for negative or purifying selection. Sequences were translated and multiple alignments were performed in ClustalX [23], followed by optimization in BioEdit [24] or MEGA 4/5 [25], [26]. We reverse-translated amino acid sequences to obtain codon alignments as input sequences for dN/dS analyses under both PARRIS [27] and FEL (Fixed Effects Likelihood; [28]) analyses in DataMonkey (www.DataMonkey.org; [29], [30]), a web-based implementation of the HyPhy algorithm [31]. PARRIS allows detection of positive selection across an entire coding sequence, while the FEL method is suitable for detecting positive or negative selection in a site-specific manner in small (10–15 sequences) datasets [28]. Under the PARRIS algorithm, we found no evidence for positive selection across the coding sequence of MGP2–10, suggesting that no residues in these proteins are targets of adaptive evolution. FEL analysis likewise showed no evidence for individual codons subject to positive selection. In contrast, while the preponderance of residues in MGP2–10 are apparently undergoing neutral evolution, FEL analysis indicates that the identified N-terminal secretory signal peptide is largely subject to purifying selection (Fig. 7a,b), suggesting that this region is indispensible for protein function or appropriate intra/intercellular transport. A minority of residues, largely dispersed throughout the C-terminal half of MGP2–10 are additionally subject to negative selection as evidenced by dN/dS ratios significantly less than 1 (p = 0.05, Fig. 7a,b). A role for amino acids under purifying selection outside of the secretory region is unknown. A majority of these conserved sites are proline (33.3%) and phenylalanine (26.6%) residues, suggesting these amino acids may be critical for MGP2–10 folding and/or function.
Previous examination of mammalian milk proteins revealed that discrete, specific sections of each gene are subject to neutral, negative or positive selection [32]. Using a similar MEGA-based analysis to specifically investigate MGP2–10 from G. morsitans, the secretory peptide and conserved region 1 appear to be largely under negative selection (Fig. 7b). The first variable region has a high dN/dS and is likely subject to neutral or positive selection (Fig. 7b), but additional MGP sequences need to be recovered from other Glossina sp. to more confidently determine site - or region-specific selective pressure across MGP coding sequences. Overall, these analyses indicate that only the secretory peptide and the first conserved region are likely subject to purifying selection, but additional analysis will be necessary once full-length MGP genes are recovered from other members of Glossina to establish regions of selection.
Novel milk proteins are specific to female reproduction
Using RT-PCR analysis to determine the tissue specificity of MGP expression, we found that expression of mgp2–10 is specific to the female fat body/milk gland (Fig. 9a). Temporal expression profiles obtained for these genes showed that mgp2–10 transcripts increase dramatically during larvigenesis and then rapidly decline within 24–48 h following parturition (Fig. 9b). This temporal and spatial expression profile is consistent with other characterized milk proteins, including mgp1 (Fig. 9a, [6]), asmase1 [9] and transferrin [7]. The temporal expression profiles for the MGP genes we identified from the two other tsetse species (gpmgp3,4 from G. pallidipes and gfmgp2,5 from G. fuscipes) were similar to those observed for mgp2–10 in G. morsitans. Transcript abundance was lower in teneral females and in females with developing intrauterine embryos, becoming progressively greater through larvigenesis (Fig. S8). In contrast, the MGP-like sequence discovered from the flesh fly, another brachyceran that exhibits larviposition but does not nourish the developing larva, was not expressed in this manner; we observed no differences in MGP-like gene expression between male and female flesh flies (nonpregnant vs. pregnant minus larval expression level; Fig. S8). Thus, even though a gene with moderate sequence similarity was identified in S. crassipalpis, its expression profile is incongruent with tsetse MGPs.
Fig. 9. Temporal and spatial expression of milk gland protein genes. 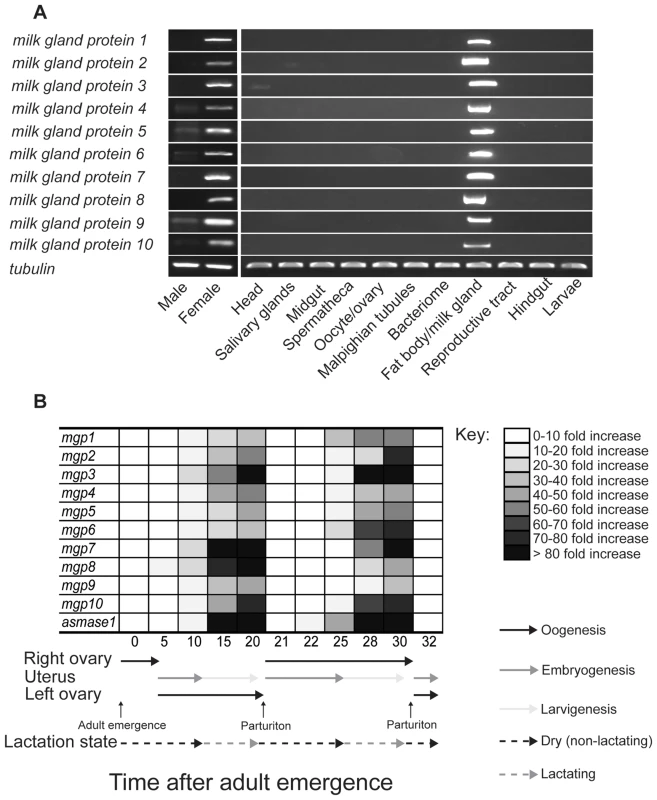
(A) Tissue specific RT-PCR. Data represents three replicates. ( B) Time course of mgp1–10 and asmase1 expression during the first two tsetse gonotrophic cycles. Transcript levels were determined by qPCR using a CFX PCR detection system (Bio-Rad, Hercules) and data were analyzed with CFX manager software version 3.1 (Bio-Rad). Data represents the mean ± SE of three replicates and was normalized to tubulin. MGP2–10 are critical components of tsetse milk
Injection of siRNAs targeting mgp5,7–9 significantly reduced corresponding target transcripts in lactating flies (Fig. 10a). Differences in the knockdown efficiency are likely due to the combined effects of technical variation and slight natural variation in the pregnancy cycle. Suppression of individual transcripts (even the highly expressed mgp7) had no effect on the number of pupae produced per female, the length of pregnancy, or the incidence of pupal emergence (Fig. 10b–d). This suggests functional redundancy among the multiple mgp paralogs, which are all expressed in a similar spatio-temporal manner during pregnancy. Simultaneous suppression of two MGPs (i.e. 5 and 7) reduced the number of pupae deposited per female by 10–15% and extended the duration of pregnancy by 2–3 d, but no difference was observed in the incidence of adult eclosion (Fig. 10b–d). When mgp5,7–9 were co-suppressed, fecundity was reduced by nearly 70% and in cases where mothers produced viable progeny, pregnancy was extended by 6–8 d (Fig. 10b–d). Together, these results suggest that the paralogous mgps share a critical function in tsetse reproduction.
Fig. 10. Phenotypes in lactating females following injection of MGP-specific siRNA. 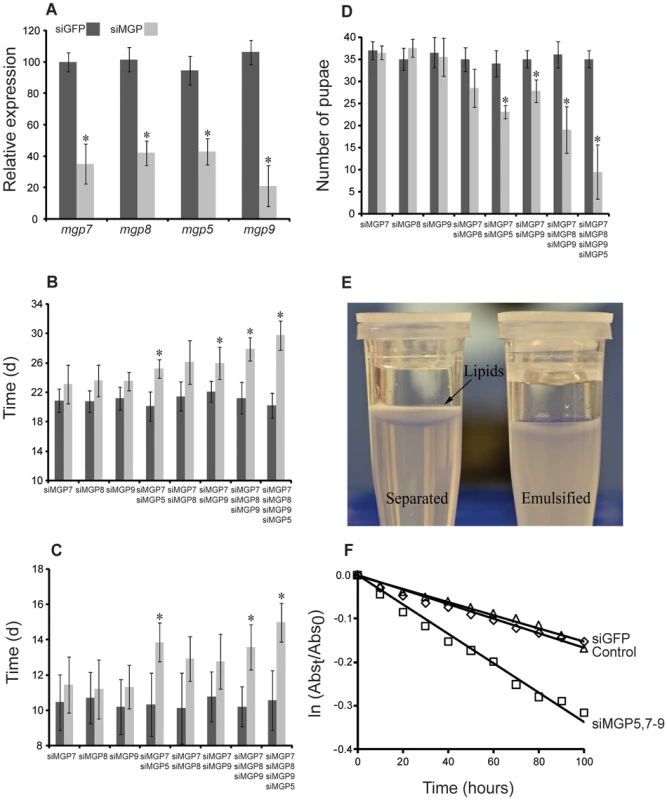
(A) Transcript levels determined by qPCR after siRNA injection, mean ± SE of three groups of 3 combined flies normalized to tubulin. (B) Duration of the 1st gonotrophic cycle after siRNA injection, mean ± SE of three groups of 30 flies. (C) Duration of the 2nd gonotrophic cycle after siRNA injection, mean ± SE of three groups of 30 flies. (D) Number of pupae deposited by 20 females over 40 d (Only those from the first two gonotrophic cycles were counted), mean ± SE of four groups of 20 flies. (E) Example of lipid separation from an unstable tsetse milk emulsion. (F) Rate of emulsion separation after MGP7–9 knockdown, mean ± SE of ten assays. *, denotes significant difference from siGFP-injected control following ANOVA with Tukey's test at P<0.01. Bradford assay of the milk protein content indicated that simultaneous knockdown of mgp5,7–9 reduced overall milk protein by nearly 22% (0.43±0.06 mg protein/5 µl milk) compared to siGFP treated controls (0.56±0.04 mg protein/5 µl milk). We hypothesized that these proteins may serve a function in maintaining milk lipid stability. To explore this possibility, we assessed the stability of milk emulsification after MGP knockdown utilizing an emulsion stabilization assay. Knockdown of mgp5,7–9 resulted in an increased rate of separation of the aqueous and lipid fractions of the milk by over two-fold (Fig. 10d,e). These findings suggest that the novel MGPs are not only an important amino acid/protein resource for the developing larva, but function to stabilize lipids within tsetse milk, allowing fat to remain homogenously distributed.
Discussion
Human African Trypanosomiasis (HAT; sleeping sickness) is a fatal disease that affects millions of people in sub-Saharan Africa. A disease caused by closely related parasites in animals, known as African Animal Trypanosomiasis (AAT; nagana), devastates agricultural and livestock production systems as well as land use in Africa. There are no prophylactic drugs that are affordable and highly efficacious, and simple tools for diagnosis or mammalian vaccines for control of HAT or AAT are lacking. Tsetse (Glossina sp.) are vectors of African trypanosomes and reduction of this insect population is a highly effective control strategy. The success of population reduction based control efforts largely is due to the low reproductive capacity of tsetse, which is viviparous yields few progeny per female over their lifespan.
Our main goals in this study were to provide an in-depth characterization of the tsetse lactation process, which is essential for intrauterine larval development and to discover novel biochemical, molecular or physiological targets for tsetse population control through reproductive suppression. Through transcriptomic analysis we identified twelve major tsetse milk proteins (MGP1–10, aSMase1 and Transferrin). Expression of these genes is tightly regulated through the transition between dry and lactating periods in order to optimize resource allocation for milk production. We also analyzed milk collected from larval guts using a proteomics approach both to characterize its composition and to verify secretion and transfer from mother to offspring of the nine milk proteins in addition to Transferrin, aSMASe1 and MGP1. Among the proteins identified as lactation products, MGP2–10 represent a group of secreted proteins unique to tsetse. Using a knockdown approach we showed that tandem suppression of multiple mgp variants resulted in substantial delays in parturition and up to a 70% reduction in fecundity. Likely, this impaired fecundity results both from a lack of protein resources and impaired lipid stabilization in the milk emulsion.
Transcriptome analysis reveals a shift in metabolism to milk production during larvigenesis
Several of our major findings from this transcriptome analysis included evidence for a substantial shift during tsetse milk production toward secreted proteins, genes involved in lipid metabolism, transporter genes, and specific genes coding for protein synthesis machinery. Structural studies have previously shown that there are extensive arrays of endoplasmic reticulum (ER) that develop in actively secreting milk gland cells, and then degenerate during an involution period following parturition [33]. A similar pattern of increased ER production in the milk gland was documented in another tsetse fly species, Glossina austeni [34] and in Melophagus ovinus, a viviparous sheep ked [35]. The rate of protein synthesis is elevated in the milk gland during periods of lactation, in accordance with an increase in ER [1], [36]–[38]. Increase of milk gland associated ER to allow for production of lactation-associated proteins is likely the reason for high abundance of transcripts for genes involved in protein synthesis during tsetse milk production. The products of mgp1–10 likely constitutes over 95% of the protein content in tsetse milk (this study, [6], [12]), accounting for the increase in contigs for secreted proteins. Transferrin and aSMase1 account for the high read abundance of contigs associated with transporters and lipid metabolism, respectively.
The extreme elevation of asmase1, mgp1–10 and transferrin transcript levels during lactation indicate that the expression of these 12 genes which constitutes less than 0.0005% of the contigs from the de novo library, represents over 45% of the RNA-seq library. In contrast, in dry flies, these same gene transcripts represent less than 2.6% of the library. A heavy investment in specific genes during reproduction is not uncommon [39]–[43], but in most studies the effect was measured directly within a specific organ, rather than the entire organism. As an example, the lactating wallaby invests over 50% of transcript abundance in the mammary gland to the production of protein bound for transfer in the milk [39]. In addition, most milk production transcripts, specifically those directly incorporated into milk, show drastic changes throughout lactation in mice and bovine within the mammary gland [44]–[46]. For tsetse, heavy transcript investment according to total Illumina read levels is based on the entire female fly rather than the isolated milk gland organ. This high investment is not surprising since at least 4–5 mg (20–25% of the total mass of the mother) of proteins are secreted by the milk gland during the 4–5 day period of lactation, representing 40–50% of the nutritional content of the milk [2]. Thus, female tsetse may be uniquely adapted to generate milk with its entire resources devoted to the transcription of milk proteins at the expense of other biological processes during lactation.
Increases in transferrin, asmase1 and mgp1 expression during lactation were expected since these proteins are recognized as major constituents of tsetse milk [7]. The role for Transferrin in tsetse milk has yet to be determined [7], though speculation suggests transferrin may serve as a source of iron as well as for immune development/protection [7]. Regarding other milk proteins, knockdown of asmase1 in lactating females reduces fecundity and severely impacts progeny fitness [9]. Biochemical studies have revealed that secreted aSMase1 is inactive and conversion to the biologically active form, which allows sphingomyelin digestion, occurs upon encountering the acidic conditions of the larval gut [9]. As a lipocalin, MGP1 likely carries a critical unknown hydrophobic ligand in the milk [6], and has been documented to be critical for tsetse fecundity [6].
Lipids, specifically triacylglycerides, constitute the other major nutritional components in tsetse milk [2], [47]. Recent studies have shown that Brummer (Bmm) lipase - and adipokinetic hormone (AKH)-mediated lipolysis are both critical for mobilizing lipids during tsetse reproduction [47]. Our transcriptome data indicate that only a single lipase, bmm, is increased during lactation, while expression of most other lipid metabolism genes are suppressed or expressed at levels equivalent to those seen in dry flies. Such minimal transcript variation is perhaps not surprising in light of recent studies on insect lipolysis, which reveal that most control occurs at the post-translational level, either through insulin signaling or through other factors that interact with the surface of the lipid droplet [48]–[51].
An argument for post-translational regulation is further supported by our recent study showing that insulin and juvenile hormone signaling both act to coordinate lipid metabolism in tsetse mothers through transcriptional regulation of select lipolytic/lipogenic genes including midway and bmm, while other such genes associated the lipid metabolism show little variation [14]. Further, a reasonable explanation for the lack of an observed increase in lipolysis genes is that these genes typically increase prior to lactation (late embryogenesis/early larvigenesis [14]), while our lactating sample was collected during the peak of lactation occurring during the last stages of larvigenesis. In addition, perilipin1 and perilipin2 transcripts are elevated in dry versus lactating flies and perhaps these proteins, which interact with lipid droplets, are necessary to accommodate the drastic increases in fat body volume that occurs during the involution periods that separate lactation cycles. In general, our results regarding expression of lipid metabolism genes support our previous studies that bmm-mediated lipolysis plays a critical role in regulating lipid homeostasis during pregnancy [14], [47].
Removal of reads mapping to the 12 abundant milk protein genes during RNA-seq analysis allowed for the identification of three additional genes that were enriched and highly expressed during milk production. Dawdle is an activin signaling molecule that has been linked to synaptic growth at the neuromuscular junction [52] and immunity [53] in Drosophila. As a member of transforming growth factor beta (TGF β) superfamily of growth factors, activin may be signaling growth of a specific tissue, possibly the milk gland, during lactation. In addition to the role in Drosophila, activin is key in regulating growth of the mammary gland during lactation in multiple mammals and has a critical role in breast cancer [54]–[56]. The increased levels of glyoxylate/hydroxypyruvate reductase, grhpr, may provide additional substrates to maintain homeostasis of proline, the main nutrient source in tsetse hemolymph [57]–[59], as milk production requires a massive amino acid investment [1]. Finally, expression of choline-phosphate cytidylyltransferase, cct, has been linked to changes in lipid droplet size [60], and this enzyme may be playing a role either in the fat body during the rapid lipolysis associated with tsetse lactation [47], or in the generation of fat globules for incorporation into the milk. Alternatively or in combination, CCT could be critical for the allocation of choline and choline-derivatives into milk during lactation. The provision of choline is essential for proper organismal growth and development [61], [62].
Our transcriptome data revealed that the majority of genes are expressed at higher levels in dry versus lactating flies. This difference is likely due to the fact that transcript levels for most genes are reduced in lactating flies at the expense of generating lactation-specific proteins. In dry flies, transcript elevation for genes associated with digestive processes likely corresponds to the increased bloodmeal size in flies not harboring an intrauterine larva [1]. Elevated transcripts for genes coding for heat shock proteins suggest that dry flies may be better suited for stress tolerance than their lactating counterparts. In addition, proteins involved in oocyte development are elevated in dry flies, likely since oocyte development is nearly complete before lactation begins [1]. Thus, the transcript profile diversity in dry flies is more robust, featuring a more global/representative expression of genes, compared to the rather specific gene set expressed in lactating flies.
Proteome validates highly expressed milk genes and identifies potential minor milk constituents
Recent studies focusing on MGP2 and MGP3 failed to verify their transfer to the nursing larvae since antisera were not available [12]. The proteomic analysis performed here confirms that these highly expressed genes synthesize milk proteins that are indeed transferred to the intrauterine larva. The proteomics data also confirm that Transferrin, MGP2–10, and aSMase1 are the primary protein components of the tsetse milk [6], [7], [9]. In addition our proteomic analysis also identified UBASH3A as a component of the tsetse milk. UBASH3A is a member of the TULA protein family and contains ubiquitin-associated (UBA) and Src-homology 3 (SH3) domains along with a histidine phosphatase domain [19], [63], [64]. A potent regulator of cellular function documented in most metazoan species [19], [64], UBASH3A is critical for regulation of T-cell proliferation and other aspects of the mammalian immune response, specifically for suppressing immune cell proliferation. The role of insect UBASH3A has not yet been determined but the presence of UBASH3A in tsetse milk suggests that it may play a role in modulating the immune system of the mother or progeny to allow intrauterine larval development. Along with potentially modulating mother-offspring immune relationship, tsetse's milk secretions also provide a route for the transmission of tsetse's microbial symbionts (Wigglesworthia and Sodalis, [65], [66]) and host immune responses may need to be regulated for symbiotic homeostasis. Our prior studies had shown that the presence of PGRP-LB in the milk is critical for symbiont transfer and overall offspring fitness [13] and the presence of UBASH3A may play a similar role in host-symbiont dynamics during the bacteria transfer within the milk. The ability to transfer symbionts to allow for maintenance of the microbiome in the offspring has been documented to be critical for tsetse immune maturation [67] and the development of the peritrophic matrix development [68]. Many other proteins were observed at lower levels; these low abundance proteins may be critical for larval development. Due to the recovery of milk from within the larval gut contents, we cannot rule out the possibility that these proteins could be products of the larval alimentary canal. Studies devoted to each low abundance peptide will be necessary to determine if it is a product of tsetse milk.
Identification of a highly divergent protein family as a critical component in the milk secretion
We identified seven new milk gland proteins, MGP4–10, that are similar to MGP2–3. MGP2–10 each contains a conserved secretory signal and multiple sites throughout three moderately-to-highly conserved regions with several residues under apparent strong purifying selection. Structural analysis of these MGPs failed to provide functional insights, but did reveal that these proteins are likely globular, consisting of multiple α-helices. Further study is necessary to conclusively determine the structures of these novel proteins. The coordinated high expression levels observed for mgp2–10 during lactation and reduced expression after parturition indicate that these proteins are under similar transcriptional regulation and that they may also serve as a source of proteins for larval nutrition [this study, 12]. Indeed, milk protein content was reduced by 20–25% when mgp5,7–9 transcripts were suppressed by 60–70%, suggesting that MGPs, based on total transcript abundance, likely account for 70–75% of the total protein content of tsetse milk. The MGP2–10 proteins also contain all amino acids, supporting the notion that they function as a complete protein resource for the developing larva. Furthermore, multiple phosphorylation sites associated with each protein suggests that MGP2–10 may also serve as a source of phosphate in tsetse milk. The lack of predicted glycosylation sites on MGP2–10 is not surprising since carbohydrate levels are extremely low in tsetse milk [2].
Previous studies have shown that low molecular weight proteins interact with lipids in tsetse milk [2]. This prompted us to investigate a potential role of MGPs for stabilization of milk-borne lipids. Here, we show that RNA interference of mgp7–9 results in acceleration of lipid separation from the aqueous phase of milk. This suggests that MGP7–9 (and likely the other MGPs) may represent the previously-documented, unidentified low molecular weight proteins associated with tsetse milk lipids [2]. MGP2–10 have a high proportion of hydrophobic amino acids [this study, 12], which may enable these proteins to interact with milk lipids. Thus, it appears that these newly-identified proteins are critical for maintenance of proper lipid/water dynamics in tsetse milk.
Similarities among MGP2–10 suggest that these proteins represent a highly divergent lactation-specific protein family from tsetse flies. These genes are localized to a single 40 kb chromosomal loci, have similar gene structures and their phylogeny correlates with their chromosomal organization indicating that mgp2–10 may have expanded by multiple gene duplication events from a common ancestor. It is possible that ancestral duplication events yielded two separate groups which may have been subsequently expanded as a result of unequal genetic crossing-over with the mgp2,4,5,6,9,10 being encoded on the antisense strand. Predicted three-dimensional structures between MGP2–10 is similar including multiple α-helices and a globular protein tertiary arrangement. mgp2–10 are under nearly identical transcriptional regulation showing increased expression during tsetse fly lactation and rapid decline during involution. These proteins also exhibit functional redundancy as a source of secreted amino acids in the milk and in sustaining lipid-protein homeostasis within the aqueous milk base. Although MGP2–10 have varying levels of amino acid similarities (18–91%), there are conserved regions they share outside of the secretory peptide. Specifically, 23 sites are under purifying selection (8 in the secretory peptide coding sequence and 15 dispersed throughout the remaining portions of the sequence), and these are likely critical to the functional role of MGP2–10 during tsetse lactation. Collectively, similarities between MGP2–10 indicate that these proteins constitute a novel family in tsetse similar to other highly divergent protein families, including caseins [69], [70], aquaporins/major intrinsic proteins [71], [72], odorant binding proteins [73], [74] and small heat shock proteins [75].
Comparative biology: Tsetse vs. mammalian lactation
Our previous work demonstrated that several mechanisms underlying tsetse lactation parallel characteristics of mammalian lactation. First, both systems have highly specialized lactating cells that cycle through periods of high productivity during lactation to low activity following involution [76], [77]. Second, there are multiple, functionally analogous proteins involved in tsetse and mammalian lactation [78], [79]. These proteins include a lipocalin (MGP1 vs. β-lactoglobulin [6], [38], [45], [80]), an iron-transfer protein (Transferrin vs. Lactoferrin [7], [10]), SMase in milk or the gut contents of feeding progeny [9], [81]–[83] and various immunity proteins (PGRP and UBASH3A vs. multiple mammalian immunity proteins, [this study], [ 45], [78], [84,85]. Third, the lipid content transferred to the developing offspring is similar during lactation in both systems. Fourth and finally, microbial symbionts are transferred from the mother to the developing offspring in both tsetse and mammals [66], [86]–[88]. There are however a few noteworthy differences between tsetse and mammalian lactation, such as the abundance of calcium transport proteins in mammalian not found in tsetse milk [this study], [76], [79,89]. This difference is unsurprising, since insects do not require large amounts of calcium for their chitin-based exoskeleton. In addition, tsetse milk contains a lower carbohydrate content than mammalian milk [76], [90], indicating that tsetse flies rely solely on lipids and protein for growth and development, rather than a combination of sugar/lipids/protein as in the mammalian case. Such reduced reliance on sugar is also unsurprising as tsetse flies have little to no detectable levels of glucose within their bodies and use proline as their circulating hemolymph resource, rather than a glucose-based substrate such as trehalose [1], [57].
Mammalian genomes contain no orthologous sequences to the nine novel tsetse MGPs. However, MGPs might function analogously to caseins in mammalian milk. Caseins are the major amino acid and calcium source for the mammalian neonate [65], [70], [91]. While MGPs do not carry calcium, they do, like caseins, represent a major amino acid resource in the milk [39], [46], [69], [92]. The presence of multiple phosphorlyation sites in MGPs suggests that this novel protein family may also act in tsetse milk as a source of phosphate as do caseins in mammalian milk [69], [70]. Furthermore, caseins are amphipathic molecules that form micelles, which interact directly with lipids both in vivo and in vitro [69], [93]. According to our results, MGPs likewise interact with lipids to promote stability of lipid emulsions in the aqueous tsetse milk. To determine if MGP2–10 have amphipathic structural properties like caseins, direct protein structural studies, rather than protein modeling, will be necessary. In addition, expansion of the casein and MGP gene families has occurred for both mammals and tsetse within specialized regions of their genomes. This indicates that expansion of these protein families (MGPs and caseins) is advantageous for provisioning the necessary nutrients in both tsetse and mammalian milk, respectively [this study], [ 69,92]. Finally, members of the MGP and casein families show substantial divergence in sequence similarity [this study], [69,78], which is a characteristic of proteins that are mainly nutritional components of milk. Proteins involved in mechanics of lactation, i.e. milk fat globule formation or have an enzymatic function, are typically more conserved within and between organisms [78]. These similarities further support the idea that MGPs perform an analogous role to mammalian caseins in tsetse milk.
Few studies have examined the effects of casein knockdown/knockout in mammals. In mice, knockout lines have been developed for α-, β - and κ-casein [94]–[96], and in goats there are naturally occurring deficiencies in α-casein [97]. Knockdown phenotypes differ dramatically, depending on the casein variant targeted. The knockout mutant for β-casein in mice [95] and null αS1-casein in goats [97] have no or minimal apparent effects on milk production, potentially due to increased expression of other casein genes to compensate for the loss of β-casein or αS1-casein, respectively. Offspring receiving milk from α-casein-null mothers experience delayed growth and life-long body size reduction, but only transient effects on physical and behavioral development [96]. The most drastic change is noted in κ-casein null mice, which fail to lactate [94]. Similar to suppression of caseins, knockdown of individual tsetse MGPs had only minimal effects on tsetse fecundity; more drastic changes occurred upon silencing multiple transcripts. In addition, a reduction in tsetse MGPs accelerated separation of lipid emulsions. Caseins likely interact similarly with lipids in mammalian milk to promote lipid emulsifications. Indeed, in addition to their biological roles, caseins have also been industrialized as emulsifying agents [98], [99]. This feature highlights the ability of these proteins to stabilize lipids present in the milk, as noted in tsetse. Proteomic studies examining mammalian milk fat globules have identified caseins, indicating that these proteins are associated with milk lipids [100]–[102]. Specifically, casein modification alters lipid composition and protein components of the milk fat globule in goats [103]. The analogous functions of MGP2–10 and caseins suggest roles for these proteins as a source of amino acids, as stabilizers of milk homogeneity, and as carriers of polyatomic ions (i.e. phosphate groups). These roles must be fulfilled by a specific abundant protein or protein family to satisfy nutritional requirements of an immature organism during periods of lactation.
Conclusions
This study provides the first complete examination of the mechanisms underlying tsetse fly lactation. In general, our results show that the majority of genes have lower expression during lactation with the exception of those directly involved in milk production. The combination of transcriptomic and proteomic analyses reveals there are 12 major milk gland proteins, which comprise ∼47% of the transcriptome of lactating flies, along with multiple minor protein constituents of tsetse milk. We have provided an overview of the combined results of this study (Fig. 11). Furthermore, we discovered a novel, tsetse-specific protein family, MGP2–10, that is expressed highly during lactation. Interference with expression of these proteins reduces tsetse fly fecundity, suggesting that this family of MGP genes may provide a target for development of tsetse-specific abortifacients. This study has also revealed that many of the underlying functional aspects of tsetse fly lactation are analogous to those of other lactating organisms. This example of convergent evolution suggests that tsetse flies could be used as an invertebrate model system to investigate the complex molecular and physiological aspects associated with obligate lactation.
Fig. 11. Summary of the results from our tsetse fly lactation study. 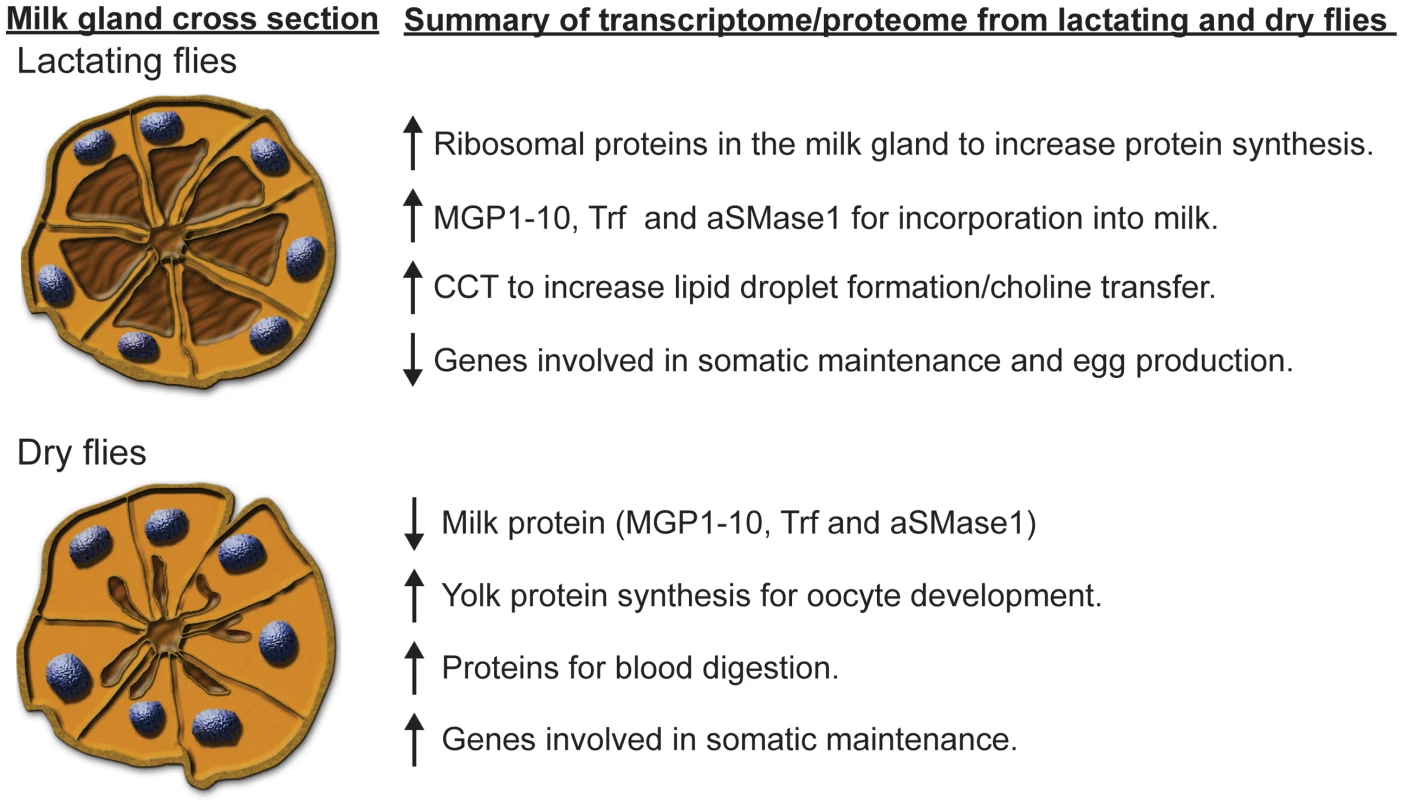
The cross section of the milk gland tubules was adapted from Yang et al. [12] and modified according to Ma et al. [33] to represent tubules in a lactating fly, characterized by secretory vacuoles full of milk and condensed nuclei, and in the milk gland of a dry fly, characterized by exhausted secretory vacuoles and expanded nuclei. Materials and Methods
Flies
Colonies of G. morsitans morsitans were reared at Yale University and the Institute of Zoology at the Slovak Academy of Sciences (SAS). The other two species (G. pallidipes and G. fuscipes) were reared at SAS. Flies were maintained on blood meals provided through an artificial feeding system at 48 h intervals [104]. Two groups of females were used for transcriptome analysis: the first group carried a third instar larva (lactating) while the second group was examined 48 h post parturition (dry or non-lactating). Developing progeny were removed from each female to ensure transcript changes were representative of differences between the mothers. For sex specific transcript analysis, males and females were collected 16–18 d after adult emergence. Tissue samples were collected from pregnant females (16–18 d after adult emergence) carrying third instar larvae 24 h after blood feeding. Samples for temporal expression analyses were collected according to progeny development status based on previous studies [9], [47]. Flesh flies, S. crassipalpis, acquired from Ohio State University were reared according to standard procedures [105].
RNA extraction and library preparation
Total RNA was extracted from individual flies or dissected tissues using Trizol reagent (Invitrogen, Carlsbad, CA, USA), following the recommended protocol. RNA was treated twice with the TURBO DNA Free kit (Ambion, Austin, TX, USA) to remove DNA, alcohol precipitated to remove residual salt, and further cleaned using the RNeasy kit (Qiagen, Maryland, USA). Total RNA (2–3 µg) was pooled from 10 flies extracted individually for each treatment. Sample quality and concentration was determined using a Bioanalyzer 2100 (Agilent, Palo Alto, CA, USA). Library construction was performed using standard protocols for Illumina mRNA-Seq sequencing by the W. M. Keck Foundation Microarray Resource at the Yale School of Medicine. Each single-end library was sequenced on one lane of the Genome Analyzer II platform (Illumina, San Diego, CA, USA).
Transcriptome analysis of tsetse lactation samples
To determine Illumina read quality, FastQC analysis was performed on the transcriptomes generated from dry and lactating flies. Due to the prevalence of tsetse symbiont sequences in the reads, a specific quality control step was included to reduce bacterial sequence reads using the known whole genome sequence data from Wigglesworthia [106], Wolbachia (unpublished) and Sodalis [107] determined from the same host species G. morsitans. Following symbiont specific sequence removal, remaining sequences were trimmed in CLC Genomics (CLC Bio) to remove ambiguous nucleotides. Contig libraries were constructed using Abyss [16], [17] followed by a secondary assembly with Trinity [18]. Functional annotation was accomplished using the BLASTx algorithm through comparison with sequences included in the NCBI protein database [108] as well as the KOG [109] and GO databases [110]. Conserved protein domains were detected using rpsBLAST [111] searches against the CDD, Pfam and Smart databases [112]. Predicted protein translations were submitted to SignalP to identify potential secretion products by screening for secretion signal motifs [113]. Additionally, contigs were compared to several proteomes obtained from Flybase [114] (D. melanogaster) and Vectorbase [115] (An. gambiae). Each read from each library was compared by BLASTn to the assembled coding sequences (CDS) using a word size of 25, m8 output and low complexity filter turned off. CDS coverage and CDS number of read “hits” from each library were computed from the BLAST output file. A hit was only considered significant if it had 97% or better identity to its target and no more than one gap. The same read could be mapped up to three different CDS to the extent that their BLAST scores were identical. Expression levels were determined using CLC Genomics Workbench (CLC bio, Cambridge, MA). Reads were mapped to our de novo assembly with an algorithm allowing only two mismatches and a maximum of 10 hits per read. RPKM was used as a measure of gene expression [116]. The proportion of read counts for each contig in relation to the total read counts in each sample was determined in order to calculate P-value differences in proportions by a Z-test following Bonferroni correction [117]. Fold change was determined as the ratio of RPKM of lactating flies vs. RPKM of dry flies. In addition to the analysis of the complete Illumina libraries, a secondary analysis was conducted featuring Illuminia libraries filtered to eliminate milk-specific contigs to reduce bias by these highly abundant proteins [116], [117]. Data from this study are available in Sequence Read Archive (SRA075330).
LC/MS/MS proteomic analysis of tsetse milk present in larval gut content
Pulled glass capillary tubes were used to collect milk samples by negative pressure from the guts of feeding third instar larvae, which were microscopically dissected from the uterus of pregnant females. Samples were stored in 1× protease inhibitor cocktail (Sigma-Aldrich). Proteins were precipitated with 10% trichloroacetic acid (Fisher Scientific) at 4°C overnight, collected by centrifugation (11,000×g, 30 minutes, 4°C) and washed two times with ice-cold acetone. Protein pellets were briefly dried and dissolved in 10 µl of protein pellet buffer (8M urea, 3M thiourea, and 1% dithiothreitol). Trypsin digestion was performed at 37°C for 12–16 h following dilution with distilled H2O to a final volume of 100 µl. Samples were stored at −80°C until analysis. Peptides were separated with a Waters nanoAcquity UPLC system (75 µm×150 mm BEH C18 eluted at 500 nl/min at 35°C) with Buffer A (100% water, 0.1% formic acid) and Buffer B (100% CH2CN, 0.075% formic acid). A linear gradient was established with 5% Buffer B, increasing to 50% Buffer B at 50 minutes and finally to 85% Buffer B at 51 minutes. MS/MS was acquired with an AB Sciex 5600 Triple Time-of-Flight mass spectrometer using 1 microscan followed by four MS/MS acquisitions. Neutral loss scans were obtained for 98.0, 49.0 and 32.7 amu. Seven separate 1 µl injections at an estimated 0.351 µg/µl concentration for a total of 2.457 µg on the column were used for analysis.
Mascot algorithm was used to analyze uninterrupted MS/MS spectra [118]. The Mascot Distiller program used MS/MS spectra to generate Mascot compatible files by combining sequential MS/MS scans from profile data that have the same precursor ion. Charge states of +2 and +3 were preferentially located with a signal-to-noise ratio of 1.2 or greater. A list of protein sequences was created and used in the BLASTx search against Trinity-assembled library from the pregnancy-specific analysis and positive matches were identified by tBLASTx against the NCBI and Swiss-Prot databases. Mascot scores were based on MOlecular Weight SEarch (MOWSE) relying on multiple matches of more than one peptide to the same predicted protein [119], [120]. The MOWSE based ions score is equal to (−10)*(Log10P), where P is the absolute probability that a match is random. Matches were considered significant when the probability of a random match fell below 5% (E value<0.05). Therefore, Mascot scores greater than 68 were above the significance threshold when searching the newly assembled library. Proteins were considered to be successfully identified when two or more peptides matched the same predicted protein and the Mascot score exceeded the significance threshold. The exponentially modified protein abundance index (empai) was employed to estimate levels of protein species based on the number of species detected compared to the number of possible peptides for specific protein [121], .
Sequence analysis for novel milk protein family
Chromosomal organization of genes and full length mRNA sequences for mgp2–10 were obtained by mapping Illumina high-throughput reads against G. m. morsitans genomic scaffolds in the CLC Genomics software package. Nucleotide and predicted protein sequences were aligned using PROMALS3D [123] and Clustal [124] and formatted with BioEdit [24]. Flesh fly, Sarcophaga crassipalpis, sequences were obtained from a previous EST project [20], [21]. Sequences of mgp2–10 from other tsetse species (G. pallidipes and G. fuscipes) were obtained from female cDNA by RT-PCR followed by cloning into T-vector plasmid (Invitrogen) and sequenced at the DNA Analysis Facility at Yale University (New Haven, CT). Pairwise phylogenetic tree construction and bootstrap analysis (10000 replicates) were performed using the MEGA4/5 sequence analysis suite [25], [125]. dN/dS analyses were performed using the FEL (Fixed Effects Likelihood [28]) and PARRIS [27] algorithms available via DataMonkey [29], [30], which is a web-based implementation of the HyPhy phylogenetic analysis program [31]. Sequences were translated, aligned, reverse translated and the stop codons removed in accordance with the requirements for sequence input to DataMonkey. Under the FEL method, posterior probabilities cutoffs were set at 95, which is equivalent to a p-value of 0.05 for the site-specific detection of codons under positive or negative selection. Analysis of specific regions of the MGP2–10 coding regions was conducted using MEGA5 according to previous milk protein studies [32] and individual regions were based upon protein coding regions with high or low levels of amino acid homology.
RNA isolation, RT-PCR and qPCR
For sex - and tissue-specific RT-PCR expression analyses, total RNA isolated from males and females and from dissected tissues was used as template for the Superscript III reverse transcriptase kit following the manufacturer's protocols (Invitrogen). Fat body and milk gland were analyzed as a combined samples since complete separation is nearly impossible due to the intricate association of these organs. PCR was performed with gene-specific primer pairs (Table S1) using the GoTaq DNA polymerase kit (Promega). The PCR amplification conditions were as follows: 95°C for 3 min, 35 cycles of 30 sec at 95°C, 52–56°C for 1 min, and 1 min at 70°C using a Bio-Rad DNA Engine Peltier Thermocycler (Hercules, CA).
For pregnancy-specific transcript abundance determination, qPCR analyses were performed using a CFX PCR detection system (Bio-Rad, Hercules). Data were analyzed with CFX manager software version 3.1 (Bio-Rad). Primer sequences used were the same as used in RT-PCR analyses (Table S1). Comparative Ct values for genes of interest were standardized by Ct values for the control gene (tubulin) relative to the average value for the control treatment or newly emerged flies, yielding the delta Ct value. All experiments were analyzed in triplicate and subject to ANOVA followed by Bonferroni correction and Dunnett's test.
RNA interference of MGP family
Short interfering RNAs (siRNA) consisting of two Duplex sequences (Table S1) were designed using Integrated DNA Technologies online software (IDT). Control siRNAs were designed against Green Fluorescent Protein (GFP; Table S1). Each oligo, designed to target a single mgp gene, was also compared to the reference RNA library/G. morsitans genome [14]) and the Trinity contigs library from this study to verify target specificity. The oligos for each strand of the siRNA were combined, and the concentration was determined spectrophotometrically followed by adjustment to 800–850 ng/µl per siRNA. Each female fly was injected with 2 µl siRNA solution into the thorax 8–10 d after adult emergence. Five days post-injection, gene expression levels were determined by qPCR and normalized to tubulin transcripts. For combined knockdown studies, siMGP constructs were mixed to yield a sample concentration of at least 600 ng/µl for each siRNA targeting a specific MGP transcript. Fecundity following MGP knockdown was assessed as previously described [9]. Finally, milk protein content was determined by Bradford assay (Bio-Rad) after extraction from the larval gut contents as described above.
Lipid emulsification assays
Emulsification assays were based on milk turbidity measurements. For this assay, milk was acquired from the guts of actively feeding larvae as before and diluted 10× prior to the assay. Samples were vortexed for 1 min at 10,000 rpm, and absorbance of the diluted emulsion was measured at 500 nm. Changes in absorbance were measured hourly for 10 h. Results were analyzed based on the slope of a regression, where ln (ABSt/ABS0) is plotted versus time based on the exponential model (ABSt = ABS0 e−kt). For this model, ABSt denotes absorbance at any time t, ABS0 is the initial absorbance, and k is the rate of absorbance decline in %/h.
Protein structure prediction
To generate structural models for MGP2–10, four web-based de novo protein modeling programs were consulted. QUARK is a recently developed ab initio assembly program that will first break proteins into small sequences, following which full-length sequence models are assembled using Monte Carlo simulations [126]. The I-TASSER program first develops a three-dimensional model and subsequently predicts function based on structural similarity with functionally defined proteins [127]. Phyre2 is a widely used protein homology/analogy recognition engine that can rapidly predict the structure of 250 residue proteins [128]. Finally, SPARKS-X is a program that performs well in comparison to other programs [129]. Each program was run under the default configuration and the resultant predicted protein structures were visualized using Discovery Studio 3.1 (Accelrys).
Supporting Information
Zdroje
1. TobeSS, LangleyPA (1978) Reproductive physiology of Glossina. Ann Rev Entomol 23 : 283–307.
2. CmelikSHW, BursellE, SlackE (1969) Composition of the gut contents of thrid-instar tsetse larvae (Glossina morsitans Westwood). Comp Biochem Physiol 29 : 447–453.
3. EjezieGC, DaveyKG (1976) Some effects of allatectomy in female tsetse, Glossina austeni. J Insect Physiol 22 : 1743–1749.
4. DenlingerDL (1975) Insect hormones as tsetse abortifacients. Nature 253 : 347–348.
5. DenlingerDL, MaW-C (1974) Dynamics of the pregnancy cycle in the tsetse Glossina morsitans. J Insect Physiol 20 : 1015–1026.
6. AttardoGM, GuzN, Strickler-DinglasanP, AksoyS (2006) Molecular aspects of viviparous reproductive biology of the tsetse fly (Glossina morsitans morsitans): Regulation of yolk and milk gland protein synthesis. J Insect Physiol 52 : 1128–1136.
7. GuzN, AttardoGM, WuY, AksoyS (2007) Molecular aspects of transferrin expression in the tsetse fly (Glossina morsitans morsitans). J Insect Physiol 53 : 715–723.
8. AttardoGM, RibeiroJMC, WuYN, BerrimanM, AksoyS (2010) Transcriptome analysis of reproductive tissue and intrauterine developmental stages of the tsetse fly (Glossina morsitans morsitans). BMC Genomics 11: .
9. BenoitJB, AttardoGM, MichalkovaV, TakacP, BohovaJ, et al. (2012) Sphingomyelinase activity in mother's milk is essential for juvenile development: a case from lactating tsetse flies. Biol Reprod 87 : 1–10.
10. Strickler-DinglasanPM, GuzN, AttardoG, AksoyS (2006) Molecular characterization of iron binding proteins from Glossina morsitans morsitans (Diptera : Glossinidae). Insect Biochem Mol Biol 36 : 921–933.
11. OsirEO, KotengoM, ChaudhuryMFB, OtienoLH (1991) Structural studies on the major milk gland protein of the tsetse fly, Glossina morsitans morsitans. Comp Biochem Physiol B-Biochem 99 : 803–809.
12. YangG, AttardoGM, LohsC, AksoyS (2010) Molecular characterization of two novel milk proteins in the tsetse fly (Glossina morsitans morsitans). Insect Mol Biol 19 : 253–262.
13. WangJ, AksoyS (2012) PGRP-LB is a maternally transmitted immune milk protein that influences symbiosis and parasitism in tsetse's offspring. Proc Natl Acad Sci USA 109 : 10552–10557.
14. BaumannA, BenoitJB, MichalkovaV, MirejiPO, AttardoGM, et al. (2012) Interactions between juvenile hormone and insulin signaling pathways regulate lipid levels during lactation and dry periods associated with tsetse fly pregnancy. Mol Cell Endocrinol 372 : 30–41.
15. International Glossina Genome Initiative (2013) Genome sequence of the tsetse fly (Glossina morsitans): vector of African trypanosomiasis. Science In preparation.
16. BirolI, JackmanSD, NielsenCB, QianJQ, VarholR, et al. (2009) De novo transcriptome assembly with ABySS. Bioinformatics 25 : 2872–2877.
17. RobertsonG, ScheinJ, ChiuR, CorbettR, FieldM, et al. (2010) De novo assembly and analysis of RNA-seq data. Nat Methods 7 : 909–912.
18. GrabherrMG, HaasBJ, YassourM, LevinJZ, ThompsonDA, et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29 : 644–652.
19. TsygankovAY (2009) TULA-family proteins: an odd couple. Cell Mol Life Sci 66 : 2949–2952.
20. RaglandGJ, DenlingerDL, HahnDA (2010) Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc Natl Acad Sci U S A 107 : 14909–14914.
21. HahnDA, RaglandGJ, ShoemakerDD, DenlingerDL (2009) Gene discovery using massively parallel pyrosequencing to develop ESTs for the flesh fly Sarcophaga crassipalpis. BMC Genomics 10 : 234.
22. IkaiA (1980) Thermostability and aliphatic index of globular proteins. J Biochem 88 : 1895–1898.
23. ThompsonJD, GibsonTJ, PlewniakF, JeanmouginF, HiggginsDG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nuc Acids Res 25 : 4876–4882.
24. HallTA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nuc Acids Sym Ser 41 : 95–98.
25. TamuraK, DudleyJ, NeiM, KumarS (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24 : 1596–1599.
26. TamuraK, PetersonD, PetersonN, StecherG, NeiM, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28 : 2731–2739.
27. SchefflerK, MartinDP, SeoigheC (2006) Robust inference of positive selection from recombining coding sequences. Bioinformatics 22 : 2493–2499.
28. PondSLK, FrostSDW (2005) Not so different after all: A comparison of methods for detecting amino acid sites under selection. Mol Biol Evol 22 : 1208–1222.
29. DelportW, PoonAFY, FrostSDW, PondSLK (2010) Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics 26 : 2455–2457.
30. PondSLK, FrostSDW (2005) Datamonkey: rapid detection of selective pressure on individual sites of codon alignments. Bioinformatics 21 : 2531–2533.
31. PondSLK, FrostSDW, MuseSV (2005) HyPhy: hypothesis testing using phylogenies. Bioinformatics 21 : 676–679.
32. PharoEA, De LeoAA, RenfreeMB, ThomsonPC, LefevreCM, et al. (2012) The mammary gland-specific marsupial ELP and eutherian CTI share a common ancestral gene. BMC Evol Biol 12 : 80.
33. MaWC, DenlingerDL, JarlforsU, SmithDS (1975) Structural modulations in the tsetse fly milk gland during a pregnancy cycle. Tissue & Cell 7 : 319–330.
34. Bonnanfant-JaisML (1974) Morphologie de la gland lactee d-une glossine, Glossina austeni Newst, au cours du cycle de gestation. I. Aspects ultrastructuraux en periode de gestation J Microscopy 19 : 265–284.
35. LenobleBJ, DenlingerDL (1982) The milk gland of the sheep ked, Melophagus ovinus - a comparison with Glossina. J Insect Physiol 28 : 165–167.
36. TobeSS, DaveyKG (1974) Autoradiographic study of protein synthesis in abdominal tissues of Glossina austeni. Tissue & Cell 6 : 255–268.
37. LangleyPA, BursellE (1980) Role of fat body and uterine gland in milk synthesis by adult female Glossina morsitans. Insect Biochem 10 : 11–17.
38. RiddifordLM, DhadiallaTS (1990) Protein synthesis by the milk gland and fat body of the tsetse fly, Glossina pallidipes. Insect Biochem 20 : 493–500.
39. LefevreCM, DigbyMR, WhitleyJC, StrahmY, NicholasKR (2007) Lactation transcriptomics in the Australian marsupial, Macropus eugenii: transcript sequencing and quantification. BMC Genomics 8 : 417.
40. MarinottiO, CalvoE, NguyenQK, DissanayakeS, RibeiroJM, et al. (2006) Genome-wide analysis of gene expression in adult Anopheles gambiae. Insect Mol Biol 15 : 1–12.
41. ParisiM, NuttallR, EdwardsP, MinorJ, NaimanD, et al. (2004) A survey of ovary-, testis-, and soma-biased gene expression in Drosophila melanogaster adults. Genome Biol 5: R40.
42. McGrawLA, ClarkAG, WolfnerMF (2008) Post-mating gene expression profiles of female Drosophila melanogaster in response to time and to four male accessory gland proteins. Genetics 179 : 1395–1408.
43. BionazM, PeriasamyK, Rodriguez-ZasSL, EvertsRE, LewinHA, et al. (2012) Old and new stories: revelations from functional analysis of the bovine mammary transcriptome during the lactation cycle. PLoS One 7: e33268.
44. LemayDG, NevilleMC, RudolphMC, PollardKS, GermanJB (2007) Gene regulatory networks in lactation: identification of global principles using bioinformatics. BMC Syst Biol 1 : 56.
45. PatelOV, CaseyT, DoverH, PlautK (2011) Homeorhetic adaptation to lactation: comparative transcriptome analysis of mammary, liver, and adipose tissue during the transition from pregnancy to lactation in rats. Funct Integr Genomics 11 : 193–202.
46. WickramasingheS, RinconG, Islas-TrejoA, MedranoJF (2012) Transcriptional profiling of bovine milk using RNA sequencing. BMC Genomics 13 : 45.
47. AttardoGM, BenoitJB, MichalkovaV, YangG, RollerL, et al. (2012) Analysis of lipolysis underlying lactation in the tsetse fly, Glossina morsitans. Insect Biochem Mol Biol 42 : 360–370.
48. WangB, MoyaN, NiessenS, HooverH, MihaylovaMM, et al. (2011) A hormone-dependent module regulating energy balance. Cell 145 : 596–606.
49. BellerM, BulankinaAV, HsiaoHH, UrlaubH, JackleH, et al. (2010) PERILIPIN-dependent control of lipid droplet structure and fat storage in Drosophila. Cell Metabolism 12 : 521–532.
50. BellerM, ThielK, ThulPJ, JackleH (2010) Lipid droplets: A dynamic organelle moves into focus. FEBS Letters 584 : 2176–2182.
51. TelemanAA (2010) Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem J 425 : 13–26.
52. EllisJE, ParkerL, ChoJ, AroraK (2010) Activin signaling functions upstream of Gbb to regulate synaptic growth at the Drosophila neuromuscular junction. Dev Biol 342 : 121–133.
53. ClarkRI, WoodcockKJ, GeissmannF, TrouilletC, DionneMS (2011) Multiple TGF-beta superfamily signals modulate the adult Drosophila immune response. Curr Biol 21 : 1672–1677.
54. BloiseE, CassaliGD, FerreiraMC, CiarmelaP, PetragliaF, et al. (2010) Activin-related proteins in bovine mammary gland: localization and differential expression during gestational development and differentiation. J Dairy Sci 93 : 4592–4601.
55. CiarmelaP, BloiseE, GrayPC, CarrarelliP, IslamMS, et al. (2011) Activin-A and myostatin response and steroid regulation in human myometrium: disruption of their signalling in uterine fibroid. J Clin Endocrinol Metab 96 : 755–765.
56. RobinsonGW, HennighausenL (1997) Inhibins and activins regulate mammary epithelial cell differentiation through mesenchymal-epithelial interactions. Development 124 : 2701–2708.
57. BursellE (1977) Synthesis of proline by the fat body of the tsetse fly (Glossina morsitans). metabolic pathways. Insect Biochem 7 : 427–434.
58. AttardoGM, Strickler-DinglasanP, PerkinSAH, CalerE, BonaldoMF, et al. (2006) Analysis of fat body transcriptome from the adult tsetse fly, Glossina morsitans morsitans. Insect Mol Biol 15 : 411–424.
59. PimleyRW, LangleyPA (1982) Hormone stimulated lipolysis and proline synthesis in the fat body of the adult tsetse fly, Glossina morsitans. J Insect Physiol 28 : 781–789.
60. KrahmerN, GuoY, WilflingF, HilgerM, LingrellS, et al. (2011) Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metabolism 14 : 504–515.
61. BlusztajnJK (1998) Choline, a vital amine. Science 281 : 794–795.
62. VanderzantES (1974) Development, significance, and application of artificial diets for insects. Ann Rev Entomol 19 : 139–160.
63. RigdenDJ (2008) The histidine phosphatase superfamily: structure and function. Biochem J 409 : 333–348.
64. TsygankovAY (2013) TULA-family proteins: a new class of cellular regulators. J Cell Physiol 228 : 43–49.
65. DenlingerDL, MaWC (1975) Maternal nutritive secretions as possible channels for vertical transmission of microorganisms in insects: the tsetse fly example. Ann NY Acad Sci 266 : 162–165.
66. AttardoGM, LohsC, HeddiA, AlamUH, YildirimS, et al. (2008) Analysis of milk gland structure and function in Glossina morsitans: milk protein production, symbiont populations and fecundity. J Insect Physiol 54 : 1236–1242.
67. WeissBL, MaltzM, AksoyS (2012) Obligate symbionts activate immune system development in the tsetse fly. J Immunol 188 : 3395–3403.
68. WeissBL, WangJ, MaltzMA, AksoyS (2013) Trypanosome infection establishment in the tsetse is influenced by microbiome-regulated host immune barrier. PLoS Pathogens 9: e1003318.
69. RijnkelsM (2002) Multispecies comparison of the casein gene loci and evolution of casein gene family. J Mammary Gland Biol Neoplasia 7 : 327–345.
70. GingerMR, GrigorMR (1999) Comparative aspects of milk caseins. Comp Biochem Physiol B 124 : 133–145.
71. CampbellEM, BallA, HopplerS, BowmanAS (2008) Invertebrate aquaporins: a review. J Comp Physiol B 178 : 935–955.
72. ZardoyaR (2005) Phylogeny and evolution of the major intrinsic protein family. Biol Cell 97 : 397–414.
73. GalindoK, SmithDP (2001) A large family of divergent Drosophila odorant-binding proteins expressed in gustatory and olfactory sensilla. Genetics 159 : 1059–1072.
74. GrahamLA, DaviesPL (2002) The odorant-binding proteins of Drosophila melanogaster: annotation and characterization of a divergent gene family. Gene 292 : 43–55.
75. CaspersGJ, LeunissenJA, de JongWW (1995) The expanding small heat-shock protein family, and structure predictions of the conserved “alpha-crystallin domain”. J Mol Evol 40 : 238–248.
76. McManamanJL, NevilleMC (2003) Mammary physiology and milk secretion. Adv Drug Del Rev 55 : 629–641.
77. NevilleMC, PiccianoMF (1997) Regulation of milk lipid secretion and composition. Ann Rev Nut 17 : 159–183.
78. LemayDG, LynnDJ, MartinWF, NevilleMC, CaseyTM, et al. (2009) The bovine lactation genome: insights into the evolution of mammalian milk. Genome Biol 10: R43.
79. O'DonnellR, HollandJW, DeethHC, AlewoodP (2004) Milk proteomics. Int Dairy J 14 : 1013–1023.
80. KontopidisG, HoltC, SawyerL (2004) Invited review: beta-lactoglobulin: binding properties, structure, and function. J Dairy Sci 87 : 785–796.
81. NybergL, FarooqiA, BlackbergL, DuanRD, NilssonA, et al. (1998) Digestion of ceramide by human milk bile salt-stimulated lipase. J Pediatr Gastroenterol Nutr 27 : 560–567.
82. DuanRD (2011) Physiological functions and clinical implications of sphingolipids in the gut. J Digest Dis 12 : 60–70.
83. DuanRD (2007) Sphingomyelinase and ceramidase in the intestinal tract. Eur J Lipid Sci Tech 109 : 987–993.
84. ClarksonRW, WaylandMT, LeeJ, FreemanT, WatsonCJ (2004) Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res 6: R92–109.
85. HettingaK, van ValenbergH, de VriesS, BoerenS, van HooijdonkT, et al. (2011) The host defense proteome of human and bovine milk. PLoS One 6: e19433.
86. DenlingerDL, MaWC (1975) Maternal nutritive secretions as possible channels for vertical transmission of microorganisms in insects: the tsetse fly example. Ann N Y Acad Sci 266 : 162–165.
87. Lara-VillosladaF, OlivaresM, SierraS, RodriguezJM, BozaJ, et al. (2007) Beneficial effects of probiotic bacteria isolated from breast milk. Br J Nutr 98 Suppl 1: S96–100.
88. MartinR, LangaS, ReviriegoC, JiminezE, MarinML, et al. (2003) Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr 143 : 754–758.
89. MangeA, BelletV, TuaillonE, de PerrePV, SolassolJ (2008) Comprehensive proteomic analysis of the human milk proteome: contribution of protein fractionation. J Chromat B-Anal Tech Biomed Life Sci 876 : 252–256.
90. ZivkovicAM, GermanJB, LebrillaCB, MillsDA (2011) Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci U S A 108 Suppl 1 : 4653–4658.
91. HollandJW, DeethHC, AlewoodPF (2004) Proteomic analysis of kappa-casein micro-heterogeneity. Proteomics 4 : 743–752.
92. LefevreCM, SharpJA, NicholasKR (2010) Evolution of lactation: ancient origin and extreme adaptations of the lactation system. Annu Rev Genomics Hum Genet 11 : 219–238.
93. DickinsonE (2006) Structure formation in casein-based gels, foams, and emulsions Colloids and Surfaces A: Physicochem. Eng Aspects 288 : 3–11.
94. ShekarPC, GoelS, RaniSDS, SarathiDP, AlexJL, et al. (2006) kappa-Casein-deficient mice fail to lactate. Proc Natl Acad Sci U S A 103 : 8000–8005.
95. KumarS, ClarkeAR, HooperML, HorneDS, LawAJR, et al. (1994) Milk composition and lactation of Beta casein deficient mice. Proc Natl Acad Sci U S A 91 : 11767–11767.
96. KolbAF, HuberRC, LillicoSG, CarlisleA, RobinsonCJ, et al. (2011) Milk lacking alpha-casein leads to permanent reduction in body size in mice. PLoS One 6.
97. ChanatE, MartinP, Ollivier-BousquetM (1999) alpha (S1)-casein is required for the efficient transport of beta - and kappa-casein from the endoplasmic reticulum to the Golgi apparatus of mammary epithelial cells. J Cell Sci 112 : 3399–3412.
98. RousseauD (2000) Fat crystals and emulsion stability - a review. Food Res Int 33 : 3–14.
99. AllenJC, WriedenWL (1982) Influence of milk proteins on lipid oxidation in aqueous emulsion .1. casein, whey protein and alpha lactalbumin. J Dairy Res 49 : 239–248.
100. FongBY, NorrisCS, MacGibbonAKH (2007) Protein and lipid composition of bovine milk-fat-globule membrane. Int Dairy J 17 : 275–288.
101. VanderghemC, BleckerC, DanthineS, DeroanneC, HaubrugeE, et al. (2008) Proteome analysis of the bovine milk fat globule: enhancement of membrane purification. Int Dairy J 18 : 885–893.
102. CharlwoodJ, HanrahanS, TyldesleyR, LangridgeJ, DwekM, et al. (2002) Use of proteomic methodology for the characterization of human milk fat globular membrane proteins. Anal Biochem 301 : 314–324.
103. CeboC, LopezC, HenryC, BeauvalletC, MenardO, et al. (2012) Goat alpha(s1)-casein genotype affects milk fat globule physicochemical properties and the composition of the milk fat globule membrane. J Dairy Sci 95 : 6215–6229.
104. MolooSK (1971) An artificial feeding technique for Glossina. Parasitology 63 : 507–512.
105. DenlingerDL (1972) Induction and termination of pupal diapause in Sarcophaga (Diptera: Sarcophagidae). Biol Bull 142 : 11–24.
106. RioRV, SymulaRE, WangJ, LohsC, WuYN, et al. (2012) Insight into the transmission biology and species-specific functional capabilities of tsetse (Diptera: Glossinidae) obligate symbiont Wigglesworthia. mBio 3.
107. TohH, WeissBL, PerkinSA, YamashitaA, OshimaK, et al. (2006) Massive genome erosion and functional adaptations provide insights into the symbiotic lifestyle of Sodalis glossinidius in the tsetse host. Genome Res 16 : 149–156.
108. AltschulSF, MaddenTL, SchafferAA, ZhangJ, ZhangZ, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 : 3389–3402.
109. TatusovRL, FedorovaND, JacksonJD, JacobsAR, KiryutinB, et al. (2003) The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4 : 41.
110. AshburnerM, BallCA, BlakeJA, BotsteinD, ButlerH, et al. (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25 : 25–29.
111. SchafferAA, AravindL, MaddenTL, ShavirinS, SpougeJL, et al. (2001) Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res 29 : 2994–3005.
112. BatemanA, BirneyE, DurbinR, EddySR, HoweKL, et al. (2000) The Pfam protein families database. Nucleic Acids Res 28 : 263–266.
113. NielsenH, EngelbrechtJ, BrunakS, von HeijneG (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10 : 1–6.
114. TweedieS, AshburnerM, FallsK, LeylandP, McQuiltonP, et al. (2009) FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res 37: D555–559.
115. LawsonD, ArensburgerP, AtkinsonP, BesanskyNJ, BruggnerRV, et al. (2007) VectorBase: a home for invertebrate vectors of human pathogens. Nucleic Acids Res 35: D503–505.
116. MortazaviA, WilliamsBA, McCueK, SchaefferL, WoldB (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5 : 621–628.
117. KalAJ, van ZonneveldAJ, BenesV, van den BergM, KoerkampMG, et al. (1999) Dynamics of gene expression revealed by comparison of serial analysis of gene expression transcript profiles from yeast grown on two different carbon sources. Mol Biol Cell 10 : 1859–1872.
118. HirosawaM, HoshidaM, IshikawaM, ToyaT (1993) MASCOT: multiple alignment system for protein sequences based on three-way dynamic programming. Comput Appl Biosci 9 : 161–167.
119. PappinDJ, HojrupP, BleasbyAJ (1993) Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol 3 : 327–332.
120. PerkinsDN, PappinDJ, CreasyDM, CottrellJS (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20 : 3551–3567.
121. IshihamaY, OdaY, TabataT, SatoT, NagasuT, et al. (2005) Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics 4 : 1265–1272.
122. ShinodaK, TomitaM, IshihamaY (2010) emPAI Calc–for the estimation of protein abundance from large-scale identification data by liquid chromatography-tandem mass spectrometry. Bioinformatics 26 : 576–577.
123. PeiJ, KimBH, GrishinNV (2008) PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res 36 : 2295–2300.
124. LarkinMA, BlackshieldsG, BrownNP, ChennaR, McGettiganPA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23 : 2947–2948.
125. KumarS, TamuraK, NeiM (2004) MEGA 3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5 : 150–163.
126. XuD, ZhangY (2012) Ab initio protein structure assembly using continuous structure fragments and optimized knowledge-based force field. Proteins 80 : 1715–1735.
127. RoyA, KucukuralA, ZhangY (2010) I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5 : 725–738.
128. KelleyLA, SternbergMJ (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4 : 363–371.
129. YangY, FaraggiE, ZhaoH, ZhouY (2011) Improving protein fold recognition and template-based modeling by employing probabilistic-based matching between predicted one-dimensional structural properties of query and corresponding native properties of templates. Bioinformatics 27 : 2076–2082.
130. BlomN, Sicheritz-PontenT, GuptaR, GammeltoftS, BrunakS (2004) Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4 : 1633–1649.
131. JuleniusK, MolgaardA, GuptaR, BrunakS (2005) Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology 15 : 153–164.
132. BlomN, GammeltoftS, BrunakS (1999) Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294 : 1351–1362.
Štítky
Genetika Reprodukčná medicína
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 4- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- The Challenges of Mitochondrial Replacement
- Concocting Cholinergy
- Genome-Wide Diet-Gene Interaction Analyses for Risk of Colorectal Cancer
- Statistical Power to Detect Genetic (Co)Variance of Complex Traits Using SNP Data in Unrelated Samples
- Mouse Pulmonary Adenoma Susceptibility 1 Locus Is an Expression QTL Modulating -4A
- Transcription-Associated R-Loop Formation across the Human CGG-Repeat Region
- Epigenetic Regulation by Heritable RNA
- Protein Quantitative Trait Loci Identify Novel Candidates Modulating Cellular Response to Chemotherapy
- Genome-Wide Profiling of Yeast DNA:RNA Hybrid Prone Sites with DRIP-Chip
- The Mechanism of Gene Targeting in Human Somatic Cells
- A LINE-1 Insertion in DLX6 Is Responsible for Cleft Palate and Mandibular Abnormalities in a Canine Model of Pierre Robin Sequence
- Interaction between Two Timing MicroRNAs Controls Trichome Distribution in
- DNA Glycosylases Involved in Base Excision Repair May Be Associated with Cancer Risk in and Mutation Carriers
- The Myc-Mondo/Mad Complexes Integrate Diverse Longevity Signals
- Evolutionarily Diverged Regulation of X-chromosomal Genes as a Primal Event in Mouse Reproductive Isolation
- Mutations in Conserved Residues of the microRNA Argonaute ALG-1 Identify Separable Functions in ALG-1 miRISC Loading and Target Repression
- Genetic Predisposition to In Situ and Invasive Lobular Carcinoma of the Breast
- Isl1 Directly Controls a Cholinergic Neuronal Identity in the Developing Forebrain and Spinal Cord by Forming Cell Type-Specific Complexes
- A Synthetic Community Approach Reveals Plant Genotypes Affecting the Phyllosphere Microbiota
- The Sequence-Specific Transcription Factor c-Jun Targets Cockayne Syndrome Protein B to Regulate Transcription and Chromatin Structure
- Determining the Control Circuitry of Redox Metabolism at the Genome-Scale
- DNA Repair Pathway Selection Caused by Defects in , , and Telomere Addition Generates Specific Chromosomal Rearrangement Signatures
- Methylome Diversification through Changes in DNA Methyltransferase Sequence Specificity
- Folliculin Regulates Ampk-Dependent Autophagy and Metabolic Stress Survival
- Fine Mapping of Dominant -Linked Incompatibility Alleles in Hybrids
- Unexpected Role of the Steroid-Deficiency Protein Ecdysoneless in Pre-mRNA Splicing
- Three Groups of Transposable Elements with Contrasting Copy Number Dynamics and Host Responses in the Maize ( ssp. ) Genome
- Sox5 Functions as a Fate Switch in Medaka Pigment Cell Development
- Synergistic Interactions between the Molecular and Neuronal Circadian Networks Drive Robust Behavioral Circadian Rhythms in
- Chromatin Landscapes of Retroviral and Transposon Integration Profiles
- Widespread Use of Non-productive Alternative Splice Sites in
- Ras GTPase-Like Protein MglA, a Controller of Bacterial Social-Motility in Myxobacteria, Has Evolved to Control Bacterial Predation by
- Cell Type-Specific Functions of Genes Revealed by Novel Adipocyte and Hepatocyte Circadian Clock Models
- Phenotype Ontologies and Cross-Species Analysis for Translational Research
- Embryogenesis Scales Uniformly across Temperature in Developmentally Diverse Species
- In Pursuit of the Gene: An Interview with James Schwartz
- Molecular Mechanisms of Hypoxic Responses via Unique Roles of Ras1, Cdc24 and Ptp3 in a Human Fungal Pathogen
- Analysis of the Genome and Transcriptome of var. Reveals Complex RNA Expression and Microevolution Leading to Virulence Attenuation
- Genotypic and Functional Impact of HIV-1 Adaptation to Its Host Population during the North American Epidemic
- RNA Editome in Rhesus Macaque Shaped by Purifying Selection
- Proper Actin Ring Formation and Septum Constriction Requires Coordinated Regulation of SIN and MOR Pathways through the Germinal Centre Kinase MST-1
- Interplay of the Serine/Threonine-Kinase StkP and the Paralogs DivIVA and GpsB in Pneumococcal Cell Elongation and Division
- A Quality Control Mechanism Coordinates Meiotic Prophase Events to Promote Crossover Assurance
- CNNM2 Mutations Cause Impaired Brain Development and Seizures in Patients with Hypomagnesemia
- The RNA-Binding Protein QKI Suppresses Cancer-Associated Aberrant Splicing
- Uncoupling Transcription from Covalent Histone Modification
- Rad51–Rad52 Mediated Maintenance of Centromeric Chromatin in
- FRA2A Is a CGG Repeat Expansion Associated with Silencing of
- A General Approach for Haplotype Phasing across the Full Spectrum of Relatedness
- A Novel Highly Divergent Protein Family Identified from a Viviparous Insect by RNA-seq Analysis: A Potential Target for Tsetse Fly-Specific Abortifacients
- A Central Role for in Regulation of Islet Function in Man
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Sequence-Specific Transcription Factor c-Jun Targets Cockayne Syndrome Protein B to Regulate Transcription and Chromatin Structure
- The Mechanism of Gene Targeting in Human Somatic Cells
- Genetic Predisposition to In Situ and Invasive Lobular Carcinoma of the Breast
- Widespread Use of Non-productive Alternative Splice Sites in
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



