-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
Steroid hormones play important roles in physiology and disease. These hormones are molecules produced and secreted by endocrine cells in the body and control sexual maturation, metabolism and reproduction. We found transcriptional regulators that underlie the specialized function of endocrine steroid-producing cells. In the steroid-producing cells of the fruit fly Drosophila, Ventral veins lacking (Vvl) and Knirps (Kni) turn on all the genes required for steroid production. When Vvl or Kni were inactivated in the cells where the hormone is made, the genes involved in steroid production were not activated. Because of the reduced steroid production, the juvenile larvae failed to develop and undergo maturation to adulthood. Inactivation of Vvl and Kni also reduces endocrine cell growth by disturbing their response to growth promoting signals. Genetic variations in humans with the loss of a homolog of Vvl have been associated with disorders caused by insufficient steroid production. Together with the fact that Vvl is highly expressed in the steroid-producing cells of Drosophila, this suggests that Vvl may be a conserved master regulator of steroid production. Our findings provide insight into the network of factors that control endocrine cell function and steroid hormone levels that could have implication for human diseases.
Published in the journal: Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps. PLoS Genet 10(6): e32767. doi:10.1371/journal.pgen.1004343
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004343Summary
Steroid hormones play important roles in physiology and disease. These hormones are molecules produced and secreted by endocrine cells in the body and control sexual maturation, metabolism and reproduction. We found transcriptional regulators that underlie the specialized function of endocrine steroid-producing cells. In the steroid-producing cells of the fruit fly Drosophila, Ventral veins lacking (Vvl) and Knirps (Kni) turn on all the genes required for steroid production. When Vvl or Kni were inactivated in the cells where the hormone is made, the genes involved in steroid production were not activated. Because of the reduced steroid production, the juvenile larvae failed to develop and undergo maturation to adulthood. Inactivation of Vvl and Kni also reduces endocrine cell growth by disturbing their response to growth promoting signals. Genetic variations in humans with the loss of a homolog of Vvl have been associated with disorders caused by insufficient steroid production. Together with the fact that Vvl is highly expressed in the steroid-producing cells of Drosophila, this suggests that Vvl may be a conserved master regulator of steroid production. Our findings provide insight into the network of factors that control endocrine cell function and steroid hormone levels that could have implication for human diseases.
Introduction
Steroid hormones have a conserved role in the regulation of developmental transitions, growth, metabolism and reproduction in animals [1]-[3]. Specialized endocrine tissues with cell-type specific complements of enzymes that form biochemical pathways mediate the biosynthesis of steroids. In Drosophila larvae, the steroid biosynthetic enzymes are expressed in the prothoracic gland (PG), the endocrine tissue of insects and the major source of the steroid hormone ecdysone. The production of ecdysone in the PG is regulated by a checkpoint control system in response to external and internal signals [2]. These checkpoints allow the endocrine system to assess growth and nutrient status before activating the biochemical pathway that increases the release of ecdysone, which triggers developmental progression.
Despite the importance of the coordinated expression in endocrine cells of the steroidogenic enzymes, the PG specific transcriptional regulatory networks that underlie steroidogenic cell function remain unknown. The steroidogenic function of the PG cells is defined by the restricted expression of the genes involved in ecdysone biosynthesis that mediate the conversion of cholesterol to ecdysone. The components of the ecdysone biosynthetic pathway include the Rieske-domain protein Neverland (Nvd) [4], [5], the short-chain dehydrogenase/reductase Shroud (Sro) [6] and the P450 enzymes Spook (Spo), Spookier (Spok), Phantom (Phm), Disembodied (Dib) and Shadow (Sad) [7]–[12] collectively referred to as the Halloween genes. Ecdysone produced by the PG is released into circulation and converted into the more active hormone, 20-hydroxyecdysone (20E), in peripheral tissues by the P450 enzyme, Shade (Shd) [13], [14].
The cell-type specific pattern and precise dynamics of the ecdysone titers suggest a tight transcriptional regulation of the biosynthetic enzymes in the PG. This is likely orchestrated by multiple transcription factors working in a network to achieve spatial and temporal control of steroid hormone production during development. The composition of this tissue-specific transcriptional regulation remains largely elusive, although some transcription factors are known to regulate ecdysone production in the PG [15]-[18]. The nuclear receptor DHR4 functions as a repressor of ecdysone biosynthesis in the PG and responds to prothoracicotropic hormone (PTTH) mediated activation of the mitogen-activated protein kinase (MAPK) pathway [17]. Loss of βFTZ-F1 in the PG has also been associated with reduced expression of phm and dib [18]. The zing-finger protein Without children (Woc) is required for ecdysone biosynthesis [19], although the pathway component regulated by Woc has not been identified. However, it is unclear if Woc, βFTZ-F1 and DHR4 bind directly to the regulatory regions that control expression of the ecdysone biosynthetic genes. In contrast, we recently showed that the transcription factor Broad (Br) regulates expression of the genes involved in ecdysone biosynthesis by direct binding to their promoters/enhancers [20]. Although these factors may be important for steroidogenic gene expression, other factors are likely required for the transcriptional regulatory network that defines the PG cell-specific expression of the ecdysone biosynthetic pathway components.
We recently characterized cis-regulatory elements required for the expression of phm and dib in the Drosophila PG [20], including a 69 bp promoter element located in the upstream phm region and a 86 bp region in the third intron of dib. These elements are important for the temporal up-regulation of phm and dib by Br isoform 4 (Br-Z4) that increases the ecdysteroidogenic capacity of the PG and allows the production of the high-level ecdysone pulse that triggers pupariation. To further characterize the tissue-specific regulation of the ecdysone biosynthetic pathway, we analyzed PG specific regulatory elements for the presence of transcription factor binding sites.
Here, we report a novel role for Ventral veins lacking (Vvl) and Knirps (Kni) in regulating ecdysteroidogenesis in Drosophila. The cis-regulatory elements responsible for PG specific expression of spok, phm and dib contain conserved Vvl and Kni binding sites. Expression of vvl is high in the PG compared to the whole animal, while kni expression is less PG-specific. Knock down of vvl and kni in the PG results in larval developmental arrest due to impaired ecdysone production. We show that Vvl and Kni specifically regulate expression of all the ecdysone biosynthetic enzymes through functionally important regulatory sites. Furthermore, we find that Molting defective (Mld) specifically regulates enzymes that catalyze early steps in the ecdysone biosynthetic pathway. Our study identifies Vvl as a PG cell-specific transcription factor that underlies steroidogenic cell function. We conclude that Vvl and Kni are involved in the transcriptional regulatory network of the PG that coordinates expression of biosynthetic enzymes required for ecdysone production during Drosophila development.
Results
Regulatory regions of ecdysone biosynthesis genes contain conserved binding sites for Vvl and Kni
We analyzed the phm and dib PG specific regulatory elements for transcription factor binding sites. Our in silico search revealed conserved binding sites for the POU-domain transcription factor Vvl and the nuclear receptor Kni in the phm promoter and dib enhancer (Fig. 1A and S1). Analysis of the phm promoter identified one conserved Vvl site and four Kni sites of which three are highly conserved, indicating that they are important regulatory sites. In support of this, mutations disrupting the Vvl site and one of the conserved Kni sites eliminate PG specific GFP reporter expression [20]. In contrast, mutations in the non-conserved Kni binding sites do not reduce PG expression. The third intron dib enhancer also contains one Vvl site and two Kni sites, both in regions that have been conserved. We also identified a 300 bp PG specific promoter for spok, encoding an enzyme that acts at an early step in the ecdysone biosynthetic pathway [9]. This element located −331 to −32 bp upstream of translation start drives specific PG reporter GFP expression. This spok promoter contains three Vvl and three Kni binding sites, although these sites are less conserved compared to the Vvl and Kni sites identified in the phm and dib regulatory elements. Expression of spok has previously been reported to require Molting defective (Mld), a nuclear zinc finger protein [9]. Since the DNA binding sequence motif for Mld has not yet been characterized, we were unable to examine potential Mld binding sites in the spok promoter.
Fig. 1. Vvl and Kni have binding sites in the promoters and enhancers of the ecdysone biosynthetic genes and are expressed in the PG. 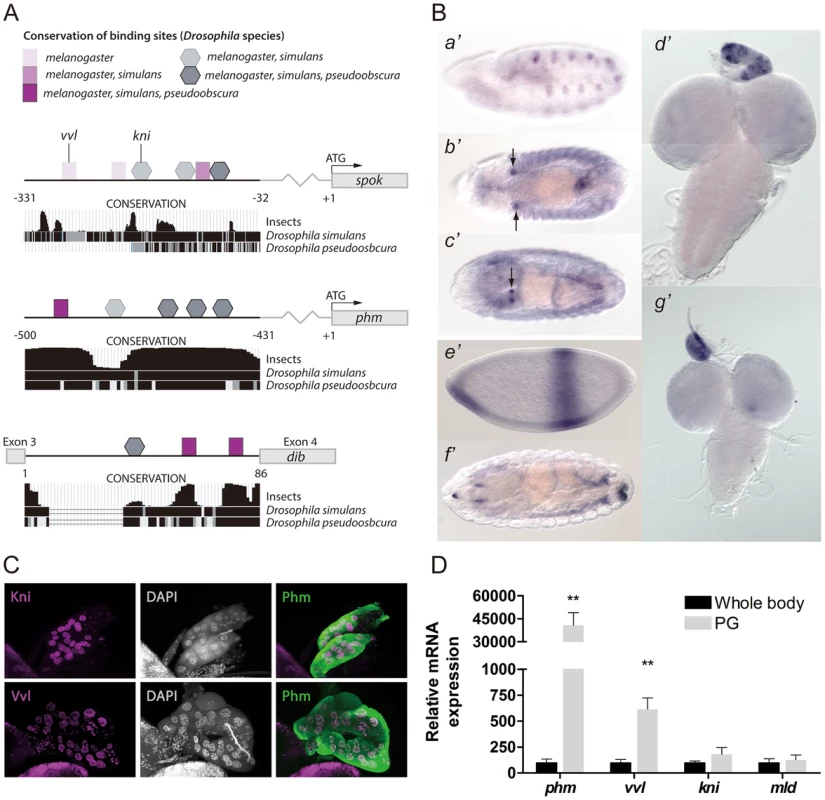
(A) An illustration showing binding sites in the PG specific cis-regulatory elements of spok and phm and dib. Binding sites are indicated by squares (Vvl) and pentagons (Kni) with shades indicating the conservation of the site between Drosophila species. Conservation tracks were obtained from the UCSC genome browser. (B) In situ hybridization of embryos and third instar larval brains and ring glands with antisense probes for vvl (a′–d′) or kni (e′–g′). (a′) Stage 11, shows vvl expression in the primordial cells of the trachea, while (b′) stage 13, (c′) stage 16 and (d′) L3 show strong vvl expression in the PG cells of the ring gland. (e′) stage 4, (f′) stage 16 and (g′) L3 show kni expression in the PG of L3 larvae, but not clearly in embryos. (C) Immunostaining of the PG from L3 larvae with antibodies against Kni and Vvl (magenta) and Phm (green). Co-localization with nuclei staining (DAPI: gray) indicates that Vvl and Kni are expressed in the nucleus of the PG cells. (D) Expression of phm, vvl, kni and mld measured by qPCR in tissue from whole body L3 larvae or dissected ring glands containing the PG of L3 larvae (n = 4). vvl is highly expressed in the ring gland compared to whole body, like phm, while the expression of kni and mld show a minor enrichment in the gland. Error bars indicate s.e.m. **P<0.01, versus whole body. Vvl and Kni are expressed in the prothoracic gland
The observation of Vvl and Kni binding sites in the promoter/enhancer of the steroidogenic enzymes prompted us to verify if these transcription factors are expressed in the PG. We performed in situ hybridization on third instar larvae and observed an intense staining of vvl mRNA in the PG (Fig. 1B). Moreover, strong embryonic vvl expression is seen in the primordium of the PG from stage 13. Importantly, the appearance of vvl in the PG precedes that of the biosynthetic genes which are expressed by stage 15 in the PG primordium [11], [12]. Although in situ expression of kni was undetectable in the PG of embryos, expression in the PG was observed at the L3 stage (Fig 1B). We also detected expression of vvl in nurse and follicle cells of adult female ovaries (Fig. S1).
Using specific antibodies, we also confirmed that Vvl and Kni are expressed in the PG and that these transcription factors localize in the nucleus (Fig. 1C). Although kni expression was not detected using in situ hybridization in the embryonic PG, expression of Kni was found in the PG at the L2 stage (Fig. S1). Next, we quantified vvl and kni expression in the ring gland (an organ with by far the most of its volume constituted by PG cells) compared to the whole body in order to see if these transcription factors are enriched in the PG (Fig. 1D). As a control, we measured phm expression, which indeed is highly expressed in the PG compared to the whole animal and mld, encoding a factor with a specific role in the PG, but with a broader expression pattern (Fig. 1D and S1) [12], [21]. Expression of vvl was highly enriched in the ring gland, like phm, while kni expression was less specific to this tissue similar to mld.
PG loss of vvl and kni result in developmental arrest due to failure in ecdysone production
Based on the potential regulatory role of Vvl and Kni, we next sought to determine if these transcription factors are required for PG expression of the genes involved in ecdysone biosynthesis. We used the PG specific phm-Gal4 (phm>) driver and observed that knock down of vvl in the PG using UAS-vvl-RNAi (vvl-RNAi) resulted in first instar (L1) arrest (Fig. 2A). Furthermore, RNAi mediated knock down of kni in the PG, by using phm> with a UAS-kni-RNAi (kni-RNAi), led to an L1 and second instar (L2) arrest phenotype. To exclude the contribution of off-target effects, we tested PG specific knock down of vvl and kni using other transgenic RNAi lines that target different regions of the vvl and kni mRNA and found that they produce similar phenotypes (Table S1). To support this, we also used the P0206-Gal4 (P0206>) driver that promotes weak expression in the PG cells [22]. When expression of vvl and kni was reduced using P0206>, development was arrested during later stages compared to when crossed with phm>. Knock down of mld in the PG with phm> driven UAS-mld-RNAi (mld-RNAi) also resulted in L1 arrest (Fig. 2A) consistent with mutant analysis [9], [21].
Fig. 2. Knock down of vvl, kni and mld in the PG results in developmental arrest and reduces ecdysteroid levels. 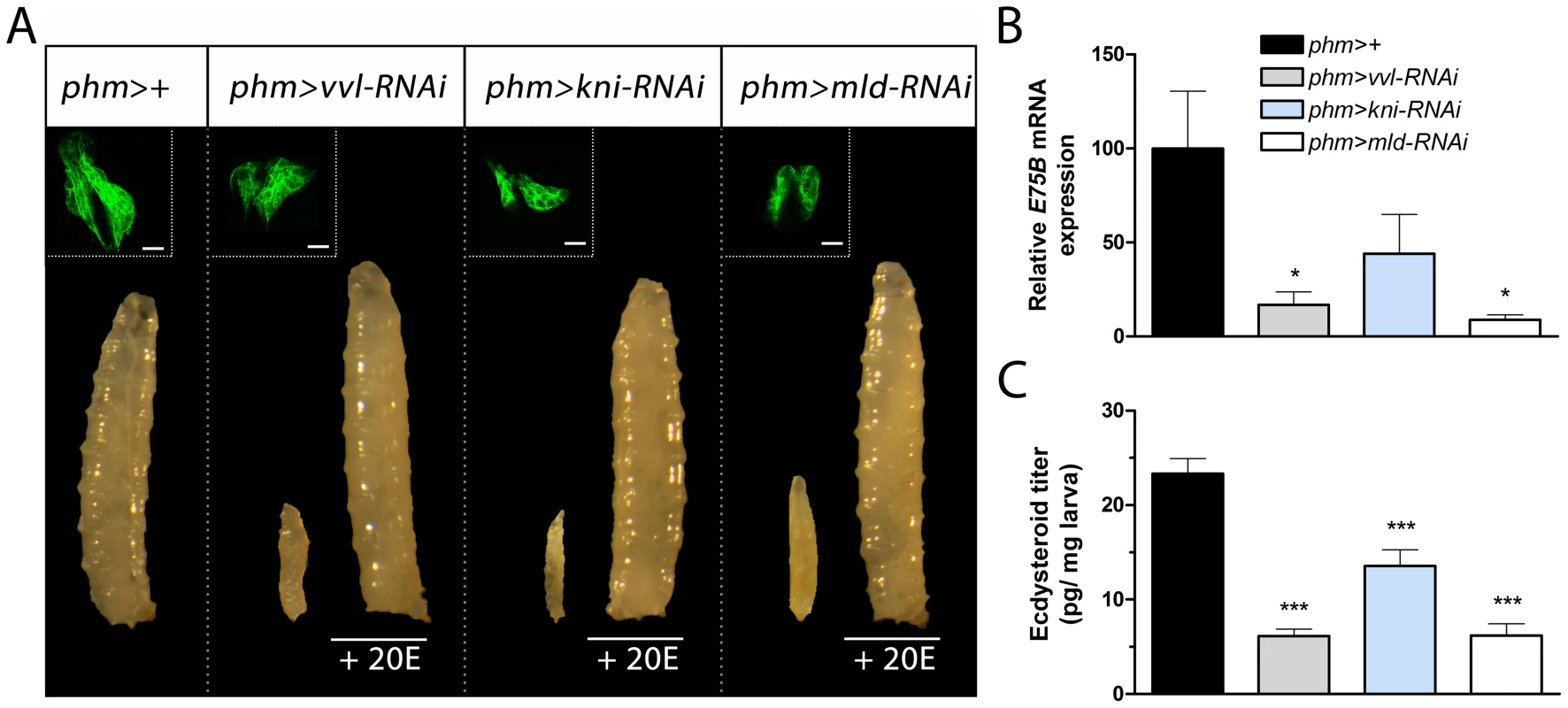
(A) RNAi mediated knock down of vvl, kni or mld in the PG using a PG specific driver (phm>) results in developmental L1 arrest for phm>vvl-RNAi and phm>mld-RNAi and L1 and L2 arrest for phm>kni-RNAi larvae. The morphology of the cells in the PG (GFP; green in the top left corner) is normal in phm>GFP,vvl-RNAi, phm>GFP,kni-RNAi and phm>GFP,mld-RNAi animals 36 hours AEL (scale bars, 20 µm). Supplying phm>vvl-RNAi, phm>kni-RNAi and phm>mld-RNAi larvae with 20-hydroxyecdysone (20E) rescues the developmental arrest. (B) Ecdysone levels, as measured by the ecdysone inducible gene E75B, is reduced in the mid-first instar (36 hours AEL) by knock down of vvl, kni or mld in the PG. (C) Ecdysteroid levels measured by ELISA confirm that L1 larvae with reduced expression of vvl, kni or mld in the PG have low levels of ecdysteroids 36 hours AEL compared to the control. Error bars indicate s.e.m. (n = 4). *P<0.05, ***P<0.001, versus the phm>+ control. If kni and vvl are involved in specifying the gland during embryonic development, reducing their expression may cause a lack of PG cell differentiation. We used a phm>GFP to label and examine the morphology of the PG in L1 larvae 36 hours after egg lay (AEL). PG cell number and morphology of L1 larvae with reduced expression of vvl, kni or mld in the PG were indistinguishable from the phm>+ control (Fig. 2A). This demonstrates that knock down of these factors does not compromise PG cell fate specification and survival. The developmental arrest indicates that loss of vvl and kni in the PG impair the cellular production of ecdysone. We therefore investigated the ecdysone levels in L1 larvae 36 hours AEL by measuring E75B mRNA expression in the whole animal, which has been used as a readout for ecdysone levels [20], [23]. Expression of E75B was significantly reduced in mid-first instar phm>vvl-RNAi and phm>mld-RNAi larvae compared to the control (Fig. 2B). This is consistent with the failure of phm>vvl-RNAi and phm>mld-RNAi larvae to molt to the L2 stage. A portion of larvae with knock down of kni in the PG undergoes the L1–L2 transition, suggesting that some of these animals can produce sufficient ecdysone for the L1–L2 molt. Consistent with this observation, knock down of kni in the PG did not lead to a significant reduction of E75B in the mid-first instar. To demonstrate that the observed phenotypes are a result of decreased ecdysone biosynthesis, we tested if ecdysone supplementation could rescue the developmental arrest. Indeed, animals with reduced expression of vvl, kni or mld in the PG were rescued by the addition of 20E to their food, showing that it is the lack of this hormone that is causing the arrest (Fig. 2A). To confirm this, we measured the ecdysteroid titer, which demonstrates that larvae with reduced expression of vvl, kni or mld in the PG have lower levels of ecdysteroids by the mid-first instar compared with the control (Fig. 2C). Taken together, these results indicate that the transcription factors Vvl and Kni, like Mld, are required for ecdysone biosynthesis in the endocrine cells of PG during early larval development.
Vvl and Kni are required for ecdysone production by regulating steroidogenic gene expression
We next investigated if Vvl and Kni regulate the expression of the genes involved in ecdysone biosynthesis. phm>vvl-RNAi and phm>kni-RNAi larvae showed reduction in the expression of phm, dib and sad by the mid-first instar 36 hours AEL compared to the control (Fig. 3A). Knock down of vvl also reduced expression of sro and spok, encoding enzymes believed to work in early steps in the pathway known as the black box [4], [5], [9]. However, expression of nvd, encoding a PG specific gene involved in the first step in the biosynthetic pathway, was not significantly reduced in the mid-first instar by knock down of vvl or kni in the PG. This further supports the notion that the PG is specified normally during embryogenesis. Previous studies have indicated that mld mutants have reduced ecdysone levels because of a specific lack of spok expression [9], [21]. Our knock down results involving mld-RNAi in the PG support that Mld is required specifically for the expression of spok, but not for the later acting products of phm, dib and sad.
Fig. 3. vvl, kni and mld are required for the expression of genes in the ecdysone biosynthetic pathway. 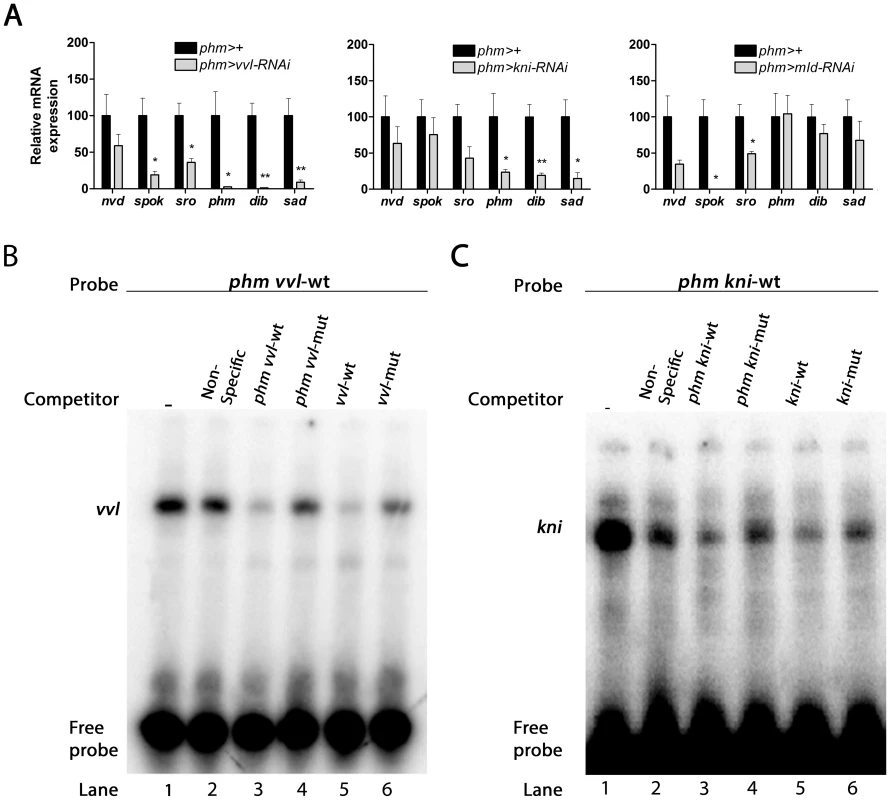
(A) Knock down of vvl, kni and mld in the PG reduces expression of genes in the steroidogenic pathway. vvl knock down results in a down-regulation of spok and sro, catalyzing early steps in the pathway, as well as a reduction of phm, dib and sad mediating the last three steps in the biosynthetic pathway. Knock down of kni results in down-regulation of phm, dib and sad, while knock down of mld causes a specific down-regulation of spok and a moderate reduction of sro. Expression was measured in mid-first instar larvae 36 hours AEL. Error bars indicate s.e.m. (n = 4). *P<0.05, **P<0.01, versus the phm>+ control. (B, C) Direct binding of Vvl or Kni to the regulatory sites in phm promoter indicated by electrophoretic mobility shift assay (EMSA). Nuclear extract was incubated with [γ32]ATP-labeled oligonucleotide sequences of phm promoter containing the vvl (B) or the kni sites (C) and resulted in shifted DNA-protein bands (lane 1). Competition assays were performed with unlabeled non-specific random oligonucleotide sequences (lane 2), the phm promoter containing the vvl or kni sites (lane 3), the phm promoter with mutated vvl or kni sites (lane 4), an oligonucleotide sequence with vvl or kni consensus motif sequence (lane 5), or with the consensus motif mutated (lane 6). The binding sites of these factors in the PG specific regulatory elements indicate that Vvl and Kni are involved in a transcriptional network necessary for co-expression of the biosynthetic enzymes. We therefore sought to establish if Vvl and Kni can bind directly to the PG specific regulatory elements by performing a DNA/protein binding assay. For this purpose, we performed electrophoretic mobility shift assays (EMSAs) with the conserved sites in the phm promoter since the functional importance of these sites has been confirmed [20]. Radiolabeled DNA oligonucleotide sequences that contained the conserved vvl or kni binding sites in the phm promoter required for PG expression (Fig. 1A) formed DNA/protein complexes with nuclear cell extract (Fig. 3B and C). These complexes were outcompeted by unlabeled oligonucleotide sequences containing consensus vvl or kni sites and by the unlabeled phm oligonucleotides containing the vvl or kni site, but not by the unlabeled phm oligonucleotides with mutated vvl or kni binding sites or by an unspecific oligonucleotide sequence. This finding demonstrates that the vvl and kni sites are required for formation of the DNA/protein complex and supports that Vvl and Kni regulate transcription of the genes involved in ecdysone biosynthesis by direct binding to their promoters and enhancers.
Vvl and kni are required to maintain expression of the biosynthetic genes during late larval development
The data indicate that Vvl and Kni are critical for the steroidogenic activity of the PG during early post-embryonic development. Later during larval development the up-regulation of ecdysone biosynthetic genes and the growth of the gland are required to produce the high-level ecdysone pulse that triggers metamorphosis. To investigate the role of Vvl and Kni during later stages of postembryonic development, we analyzed their expression in third instar (L3) larval ring glands from early (72 hours AEL), mid (96 hours AEL) and late (120 hours AEL) L3 larvae. In wild type larvae, expression of the steroidogenic genes showed no or little increase from the early to mid L3, but a dramatic up-regulation in the late L3 (Fig. 4A), coinciding with the high-level ecdysone peak that triggers pupariation 120 hours AEL [20]. While the expression of vvl showed only a minor increase during the L3 stage, a stronger up-regulation of kni and mld was observed. Compared to both vvl, kni and mld, expression of Br-Z4 was highly up-regulated in the late L3 consistent with its role in the temporal up-regulation of the biosynthetic genes important for the high-level ecdysone pulse 120 hours AEL that triggers pupariation [24]. Considering the tissue-specificity and that vvl expression in the PG shows little relation with the ecdysone titer, it seems likely that Vvl is important for the spatial control of ecdysone biosynthetic gene expression in the PG, but not for the temporal regulation during development. On the other hand, kni expression is less PG specific, but show more correlation with the ecdysone titer during L3.
Fig. 4. vvl, kni and mld have a specific role in regulating ecdysone biosynthesis in the L3 stage. 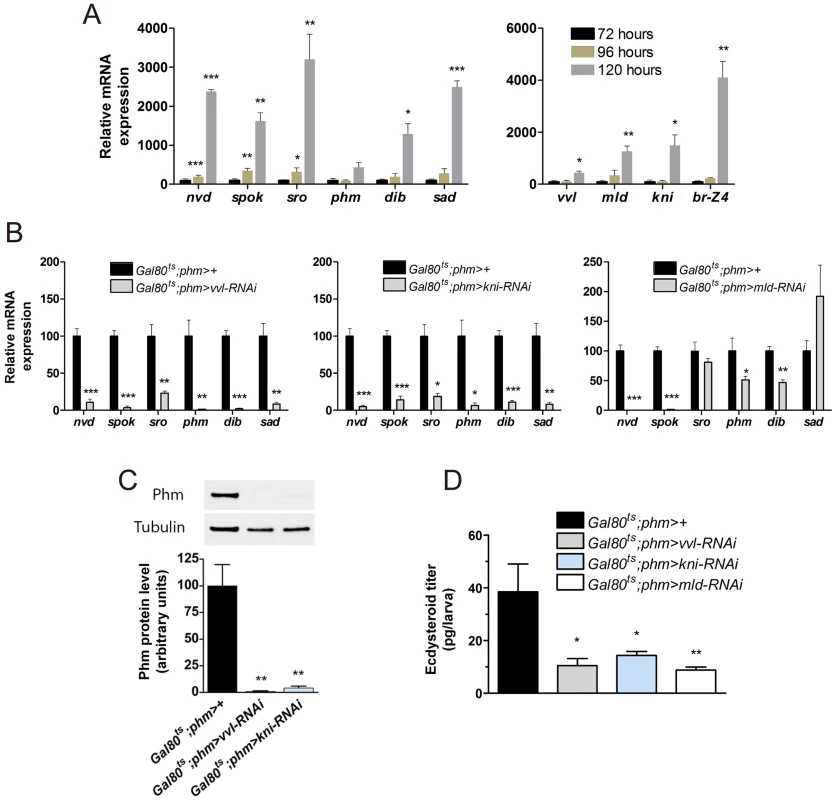
(A) Expression of steroidogenic genes in ring glands from wild type (phm-GFP) larvae increases little from early (72 hours AEL) to mid (96 hours AEL) third instar, but rises dramatically in the late (120 hours AEL) third instar. vvl expression exhibits a minor increase in the late third instar, while mld, kni and especially Br-Z4 show a strong increase (n = 4). (B) Expression in the ring gland from larvae with knock down of vvl, kni or mld during the L3 stage two days after temperature induced activation of the RNAi with the Gal80ts;phm> driver 96 hours AEL. Expression of all the steroidogenic genes were significantly reduced in animals with reduces vvl or kni expression. Knock down of mld results in a dramatic reduction in expression of nvd and spok that mediate two early steps in the biosynthesis of ecdysone (n = 4). (C) Quantified level of Phm protein in brain-ring gland complexes (BRGCs) from L3 larvae two days after temperature induced RNAi (96 hours AEL) normalized to Tubulin levels determined by immunoblotting (top panel) (n = 3). (D) Ecdysteroid levels determined by ELISA in L3 larvae with reduced PG expression of vvl, kni or mld two days after temperature induced activation of the RNAi effect (96 hours AEL) (n = 4). Error bars indicate s.e.m. *P<0.05, **P<0.01, ***P<0.001, versus the Gal80ts;phm>+ control. To determine whether Vvl and Kni are only required to set the initial expression of the biosynthetic enzymes during embryonic and early larval development or also to maintain PG expression during late larval stages, we used tub-Gal80ts;phm-Gal4 (Gal80ts;phm>) to conditionally induce the UAS-RNAi effect. Gal80ts;phm>vvl-RNAi and Gal80ts;phm>kni-RNAi larvae develop normally at 18°C and were shifted to 29°C to induce the RNAi at different times during larval stages. Development of most Gal80ts;phm>vvl-RNAi, Gal80ts;phm>kni-RNAi and Gal80ts;phm>mld-RNAi larvae was arrested in L3 when larvae were shifted to 29°C 120 hours AEL or earlier, while larvae that were shifted 144 hours AEL or later pupariated normally (Fig. S2). Because development is slowed down at 18°C, 120 hours AEL is corresponding to the late L2 stage under these conditions [25]. Thus, inducing RNAi in late L2 or earlier causes developmental arrest in L3, suggesting that it prevents the production of the high-level ecdysone pulse in late L3 that triggers metamorphosis.
Since expression of the biosynthetic genes detected in L1 larvae with reduced expression of vvl and kni was measured on RNA extracted from whole animals (Fig. 3A), and normalized to Rpl23 and Rpl32, ubiquitously expressed ribosomal housekeeping genes, it is possible that reduced PG cell growth could be responsible for the observed decrease in biosynthetic gene expression because of a reduced PG to whole animal size ratio. To exclude this possibility and to test whether Vvl and Kni are required to maintain PG specific expression of steroidogenic genes later during development, we analyzed expression in isolated ring glands from L3 larvae. We first confirmed the efficient reduction of vvl and kni mRNA levels compared to the control (Fig. S2). Additional analysis showed that expression of all of the steroidogenic enzymes was dramatically decreased in L3 ring glands in which vvl and kni were knocked down compared to the control (Fig. 4B). This demonstrates that the decreased expression of the ecdysone biosynthetic genes in vvl-RNAi and kni-RNAi animals is a consequence of a specific reduction in the transcription of steroidogenic genes, and not reduced glandular growth or a general reduction in transcription. Compared to vvl-RNAi and kni-RNAi, mld knock down had little or no influence on the transcription of the genes encoding enzymes acting in late steps in the biosynthetic pathway. However, spok and nvd levels were strongly reduced in the ring glands of mld-RNAi larvae compared to the control, suggesting that these are direct targets of mld regulation. This indicates that Mld is involved in the transcriptional regulation of the enzymes mediating early biosynthetic conversions of cholesterol. The observation that mld-RNAi also regulates nvd expression may explain why spok overexpression in the PG is insufficient to rescue mld mutants [9].
To examine the influence of vvl and kni knock down on the biosynthetic enzyme level, we measured Phm protein levels in brain-ring gland complexes (BRGCs) using immunoblotting analysis. Consistent with the reduced mRNA levels, these results show that Phm protein levels are dramatically reduced in vvl-RNAi and kni-RNAi larvae compared with the control (Fig. 4C). To reinforce that knock down of vvl, kni or mld in the PG impairs ecdysone biosynthesis, we also measured the ecdysteroid levels in L3 larvae. Ecdysteroid levels were reduced in L3 larvae where RNAi mediated knock down of vvl, kni or mld in the PG had been induced in the L2 stage (Fig. 4D).
The developmental arrest by PG inactivation of vvl and kni can be rescued by ecdysone and 20E
Taken together, the data suggest that the coordinated expression of steroidogenic enzymes in the PG requires Vvl and Kni function. To further corroborate our findings that Vvl and Kni are involved in co-regulating all components in the biosynthetic pathway, we examined whether supplementation of any 20E precursors to the larval growth medium was able to rescue the developmental arrest of vvl or kni RNAi larvae. When fed cholesterol, 7-dehydrocholesterol or 5β-ketodiol, most phm>vvl-RNAi and phm>kni-RNAi animals develop to small L2 larvae (Fig. 5A and B). Since phm>vvl-RNAi and phm>kni-RNAi arrest in L1 and L2 without supplementation, it appears that increasing the amount of substrate for ecdysone synthesis provides some compensation, but not complete rescue, when the pathway activity is reduced. Supporting this notion, providing intermediates further downstream in the pathway gradually increased rescue of phm>vvl-RNAi and phm>kni-RNAi larvae to the L3 stage. In particular, 20E and its precursor ecdysone efficiently rescue phm>vvl-RNAi and phm>kni-RNAi larvae to the L3 stage (Fig. 5B).
Fig. 5. Ecdysone and 20E efficiently rescue loss of vvl and kni in the PG. 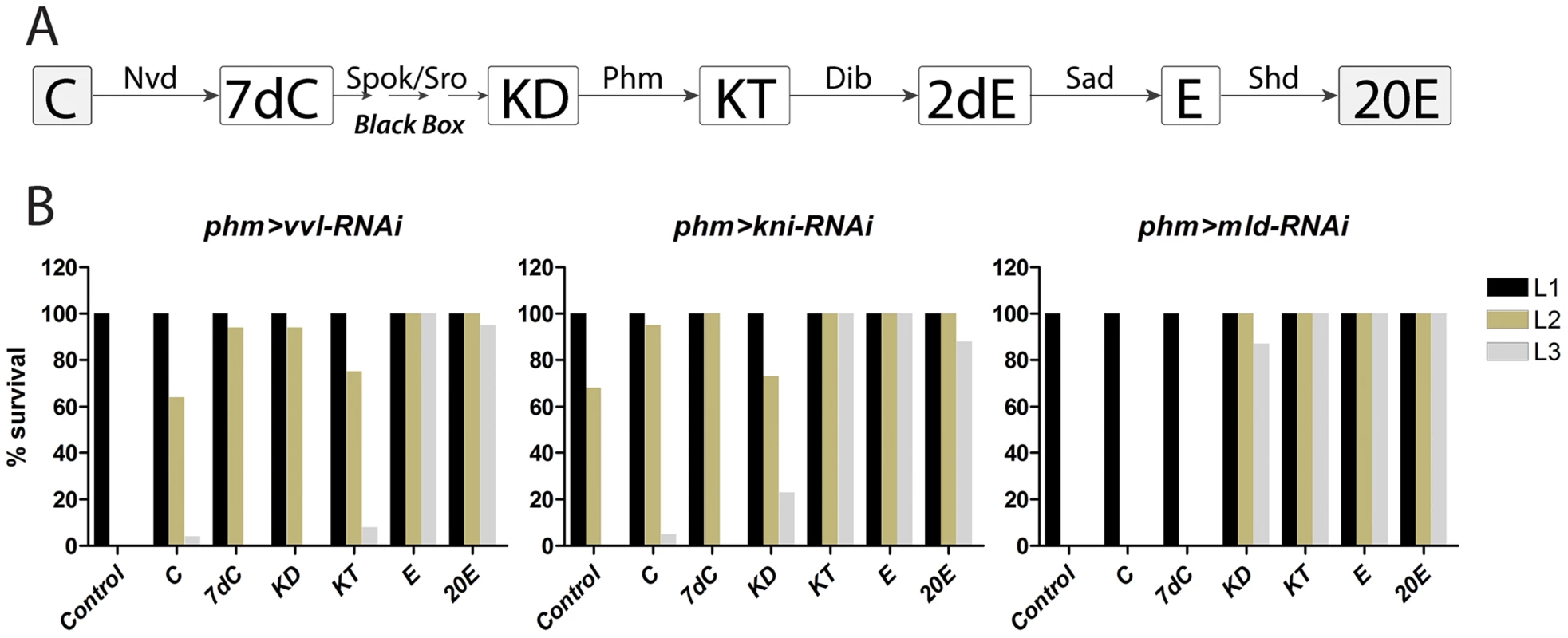
(A) Ecdysone biosynthetic scheme showing steps in the conversion of cholesterol to 20-hydroxyecdysone (20E). Note that ecdysone produced and released from the PG is converted to its active form 20E in peripheral tissues. (B) Percentage of larvae developing to the indicated stage. L1; first instar larvae, L2; second instar larvae, L3; third instar larvae. Resupplying precursors later in the pathway is gradually more efficient in rescuing arrest of larvae with reduced expression of vvl and kni in the PG. In contrast, only precursors downstream of the black box efficiently rescue mld-RNAi larvae, indicating that Mld regulates a gene product(s) involved in the reactions upstream of the 5β-ketodiol. C; cholesterol, 7dC; 7-dehydrocholesterol, KD; 5β-ketodiol, KT; 5β-ketotriol, 2dE; 2-deoxyecdysone, E; ecdysone, 20E; 20-hydroxyecdysone. We then tested whether increased availability of cholesterol substrate is sufficient to promote ecdysone biosynthesis. Indeed, supplementation with cholesterol increased E75E mRNA in wild type larvae and ecdysteroid levels in the control and in larvae with PG specific loss of vvl, kni or mld compared with animals grown on standard food (Fig. S3). Like rescue of the L1 arrest (Fig. 5B), cholesterol also provided minor rescue of the L3 developmental arrest observed when the RNAi effect was induced in the L2 stage (Table S2). Increasing cholesterol concentrations only provides minor rescue for loss of vvl and kni. In contrast, we confirmed that it provides complete compensation for loss of Niemann-Pick type C-1a (npc1a) (Fig. S3), which reduces substrate delivery for ecdysone biosynthesis [26], [27]. These results suggest that the hormone deficiency observed in vvl-RNAi and kni-RNAi larvae is a result of impaired ecdysone pathway activity and not compromised cholesterol substrate delivery, like in phm>npc1a-RNAi larvae. These findings overall indicate that silencing vvl or kni in the PG specifically inhibits synthesis of ecdysone by reducing the activity of the biosynthetic pathway.
Supplying the 5β-ketodiol and 5β-ketotriol, but not cholesterol or 7-dehydrocholesterol, rescued mld-RNAi larvae (Fig. 5B), consistent with Mld being required for expression of Nvd and Spok which mediate early steps in the pathway upstream of the 5β-ketodiol. We conclude, that Vvl and Kni are necessary for coordinating the tissue-specific expression of all steroidogenic genes in the endocrine cells of the PG, while Mld specifically regulates genes involved in early steps in the pathway responsible for the conversion of cholesterol to the 5β-ketodiol, an intermediate downstream of the black box reaction(s).
PTTH and insulin/TOR signaling in the PG is disturbed by loss of vvl and kni
Our data demonstrate that Vvl and Kni are specifically involved in transcriptional regulation of ecdysone biosynthetic components. However, when we analyzed the morphology of PG cells with reduced expression of vvl and kni, we found a mild decrease in PG cell size (Fig. 6A and B), indicating that knock down of these transcription factors also influence cellular growth. The major pathways that are thought to control PG cell growth are the PTTH and the insulin/TOR pathways [22], [28]–[32]. Therefore, we investigated the possibility that Vvl and Kni affect PG cell growth and ecdysone synthesis indirectly by interfering with PTTH and/or insulin/TOR signaling. The neuropeptide, PTTH promotes PG growth and ecdysone synthesis through activation of its receptor Torso, a receptor tyrosine kinase (RTK) expressed specifically in the PG [33]. Activation of the insulin receptor (InR), another RTK, in the PG also regulates cell growth and stimulates ecdysone synthesis in response to circulating insulin levels. Although crosstalk between systemic insulin mediated growth regulation and TOR signaling might occur, the TOR pathway cell-autonomously regulates growth in response to cellular nutrient levels [34]. We therefore investigated whether PTTH and insulin/TOR signaling in the gland is affected by knock down of vvl and kni. Analysis of torso transcript levels revealed that, while mld-RNAi larvae have normal torso mRNA levels, expression of the PTTH receptor is reduced in ring glands from L3 vvl-RNAi and kni-RNAi larvae (Fig. 6C). Consistent with down-regulation of the PTTH receptor, we found reduced levels of phosphorylated ERK, an indicator of MAPK activity and PTTH signaling [33], in BRGCs from vvl-RNAi and kni-RNAi larvae (Fig. S4). However, unlike the biosynthetic enzymes (Fig. 3), expression of torso was not reduced in L1 phm>vvl-RNAi larvae 36 hours AEL (Fig. S4), indicating that torso expression is initiated normally despite the loss of vvl in the PG. When examining the expression of the InR and components mediating insulin signaling, we found reduced expression in vvl-RNAi and kni-RNAi animals of 4EBP that encodes a negative growth regulator depressed by activation of the insulin pathway. Further, levels of akt, which encodes a serine/threonine kinase of the insulin signaling pathway [35], were increased, while levels of InR were decreased in vvl-RNAi larvae. Increased insulin signaling is generally associated with decreased expression of both 4EBP and InR [36], [37]. These results imply that loss of vvl and kni increases insulin signaling. The most likely explanation for increased insulin signaling in PG of animals with reduced vvl and kni expression is the low ecdysone levels, which cause a general increase of insulin release from the brain [29]. Thus, the disturbance of insulin signaling in the PG of vvl-RNAi and kni-RNAi animals seems unlikely to account for the PG cell growth reduction. However, we observed a strong transcriptional reduction of the S6 kinase (S6K), an important positive growth regulator downstream of TOR. This suggests that the combined reduction of both PTTH/Torso and TOR/S6K signaling in the PG contributes to the negative influence of vvl-RNAi and kni-RNAi on PG cell growth and ecdysone synthesis. Why does mld knock down not affect PG cell size negatively (Fig. 6A and B)? Since loss of mld does not affect torso expression (Fig. 6C), it is possible that disturbance of the TOR/S6K pathway alone is insufficient to impair growth, especially if this is combined with increased insulin signaling as indicated by the decreased InR and 4EBP mRNA levels in the ring glands of mld-RNAi larvae.
Fig. 6. vvl and kni knock down affects PG cell size and disturbs PTTH/Torso and insulin/TOR signaling. 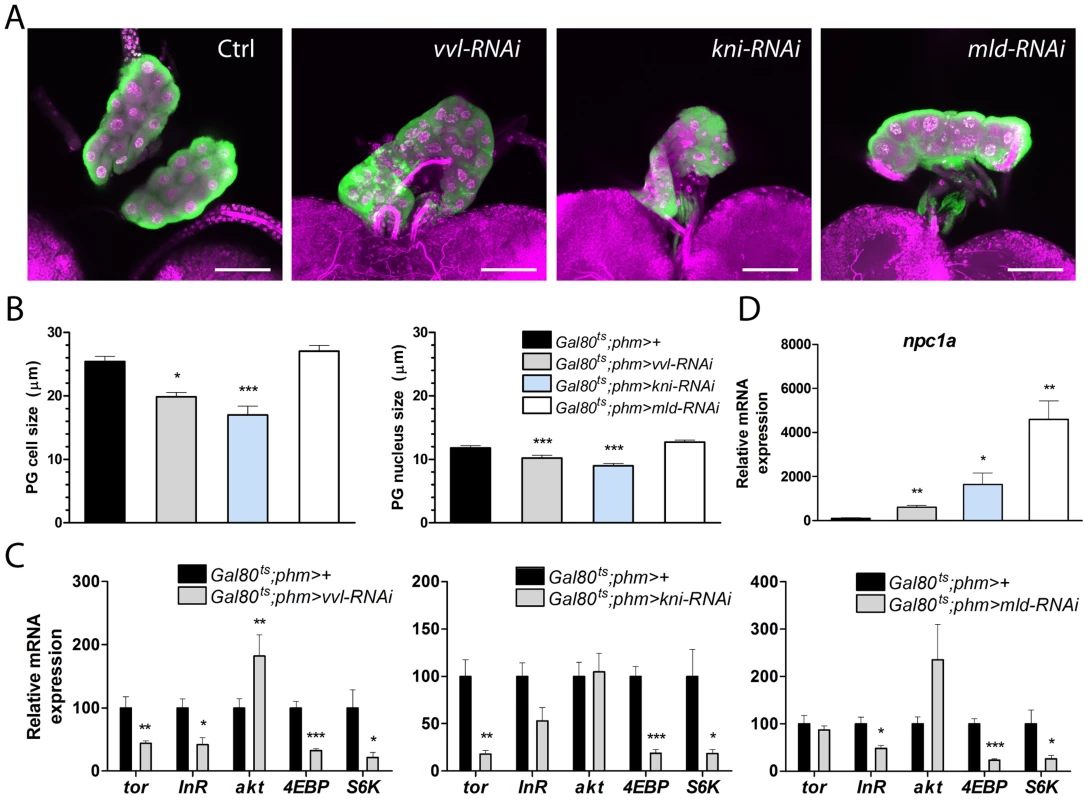
(A, B) PG (green) and nuclei (magenta: DAPI) show that the morphology of the gland is intact in late L3 larvae with reduced expression of vvl, kni, or mld in the PG. PG cell and nucleus size of L3 larvae (measured as the diameter) are reduced when vvl and kni are knocked down (n = 11). RNAi was induced using the Gal80ts;phm>96 hours AEL corresponding to the late L2 stage at 18°C. The PG was analyzed after two days at 29°C when control larvae are in the wandering stage. (C) Knock down of vvl and kni results in decreased expression of torso (tor), 4EBP and S6K in dissected ring glands containing the PG. Likewise, 4EBP and S6K are down regulated in ring glands of mld-RNAi animals that also exhibit reduced expression of the InR. Contrary, akt is increased in vvl-RNAi animals (n = 4). (D) Loss of vvl, kni or mld results in a strong increase of npc1a expression in the ring gland. In C and D, expression was measured in L3 larvae raised as described in A and B (n = 4). Error bars indicate s.e.m. *P<0.05, **P<0.01, ***P<0.001, versus the Gal80ts;phm>+ control. Knock down of vvl and kni affects Npc1a involved in cholesterol trafficking
Finally, we investigated whether loss of vvl and kni in the PG affects cholesterol substrate delivery for ecdysone synthesis. Surprisingly, we found that, whereas the biosynthetic genes show a strong decrease, npc1a exhibits a dramatic increase in the gland of vvl-RNAi, kni-RNAi and mld-RNAi larvae (Fig. 6D). This finding indicates that up-regulation of npc1a in the PG of vvl-RNAi, kni-RNAi and mld-RNAi larvae reflect a compensatory feedback regulation to maintain cholesterol homeostasis and/or increase substrate delivery to promote steroidogenesis. Down-regulation of biosynthetic activity in vvl-RNAi, kni-RNAi and mld-RNAi larvae reduces cholesterol flux through the ecdysone pathway and may lead to intracellular redistribution of cholesterol to maintain homeostasis through feedback regulation. We therefore explored the possibility that npc1a, which is required for normal cholesterol distribution and availability for steroid synthesis, is controlled by feedback regulation of cholesterol. Expression of npc1a is repressed by cholesterol in wild type larvae (Fig. S4), indicating that npc1a is feedback regulated. Recently, we showed that ecdysone biosynthesis is controlled by feedback circuits in the PG [20]. We therefore also examined whether ecdysone signaling in the gland is affected by knock down vvl, kni or mld. To test this, we measured mRNA levels of the ecdysone receptor (EcR) ring glands isolated from L3 larvae where vvl-RNAi, kni-RNAi or mld-RNAi had been induced in the PG during L2. Transcript levels of EcR were not affected in ring glands from vvl-RNAi and kni-RNAi larvae (Fig. S4), indicating that the responsiveness of the PG to ecdysone is not reduced. Taken together, these results suggest alterations of cholesterol uptake and trafficking in the PG when flow through the biosynthetic pathway is impaired.
Discussion
Drosophila developmental progression is dictated by tightly regulated ecdysone pulses released from the PG. Like any cell specialized for steroid biosynthesis, the PG expresses a set of enzymes that mediate steps in the conversion of cholesterol into steroids. The tissue-specific expression of these enzymes is key to the specialization of the cells that endows the PG with the competence to produce ecdysone. The transcriptional control mechanism underlying such regulation is likely orchestrated by a regulatory network of transcription factors. Here, we identify two transcription factors Vvl and Kni that are required for the expression of the biosynthetic enzymes in the ecdysone producing PG cells. Vvl is a POU domain transcription factor which has multiple important functions during Drosophila development. Mutations in vvl cause embryonic lethality with defects in the development of the trachea and the nervous system [38]–[41]. Moreover, Vvl is required for wing vein development and is involved in innate immunity by regulation of the expression of antimicrobial peptides [42], [43]. We show that Vvl is expressed in the PG during late embryogenesis and in the larval stages. One important characteristic of Vvl is that it maintains its own expression by autoregulation [44]. Once activated, Vvl maintains its expression and likely also the expression of the ecdysone biosynthetic genes in the PG. Knock down of vvl in the PG reduces the expression of all genes in the biosynthetic pathway, showing that Vvl is required for maintaining expression of all pathway components. Together with the high expression of Vvl in the gland, this suggests that Vvl is a master transcriptional regulator involved in specifying the genetic program that dictates PG cell identity including its tissue-specific expression of steroidogenic enzymes. It is interesting to note that human chromosome 6 deletions that affect POU3F2, a homolog of Vvl, have been associated with hypogonadotropic hypogonadism and adrenal insufficiency [45], [46], making it possible that Vvl is a conserved regulator of steroid biosynthesis.
The gap gene kni is known for its role in embryonic segmentation patterning and development of the trachea and wing vein [47]–[51] similar to vvl. Kni is a nuclear receptor with a zinc-finger motif that is unlikely to be ligand activated since it lacks a ligand-binding domain. Our data show that Kni is required for expression of the genes involved in ecdysone biosynthesis in the PG, suggesting that Kni functions as an activator in this situation. Although Kni is generally considered a short-range repressor [52], it is required to activate hairy expression in stripe 6 during embryogenesis [53]. Thus, Kni may act either as a repressor or as an activator in a context-dependent manner. In mammals, nuclear receptors are also key regulators of steroidogenic target genes encoding P450 enzymes [54]–[56].
Although Vvl and Kni specifically control genes in the steroidogenic pathway, other targets of these factors could also be important for ecdysone synthesis in the PG. During development the continuous growth of the PG cells and endoreplication of DNA is important to scale its hormone production to the capacity required for developmental progression. We found that both vvl-RNAi and kni-RNAi larvae have mildly reduced PG nuclei and cell size, which is likely to contribute to the reduced ecdysone levels in these animals. Kni has been shown to suppress endoreplication activity in the gut by regulating cell cycle genes [48]. This is in contrast to our observation indicating that loss of kni results in a reduction in the nuclei size, and hence, reduced polyploidy of the PG cells. Instead our results indicate that loss of vvl and kni reduces activity of PTTH/Torso and TOR/S6K signaling, two major pathways that promote growth and stimulate ecdysone biosynthesis [30], [31], [33], [57]. However, loss of vvl and kni had no effect on torso expression in the mid-first instar. This indicates that these factors are not required for the initial setting of torso expression, but for the maintenance of high torso expression during development. In tracheal cells, Vvl is required to maintain expression of the RTK breathless, but not for activating its initial expression [42], [58]. It is unclear how the transcription of the biosynthetic enzymes fluctuates during the low level ecdysone peaks in L1 and L2, before the induction of the steroidogenic pathway by PTTH stimulation [17]. Unlike PTTH/Torso, Vvl and Kni are required in the PG during L1 and L2 for the transition to the L3 stage, which suggests that Vvl and Kni are important for the proper transcription of the biosynthetic enzymes throughout larval development. Altogether, these data suggest that in addition to being required to initiate and maintain expression of the biosynthetic enzymes, Vvl and Kni play an indirect role important for ecdysone production by enabling PG cells to be competent to respond to PTTH and by regulating the TOR/S6K pathway. In contrast, Vvl and Kni are not required for normal expression of EcR in the gland, indicating that feedback regulation of ecdysone biosynthesis is not influenced by knock down of these factors [20]. In contrast to the transcription factor Br-Z4 involved in positive feedback regulation, which is strongly induced in the PG during late L3 to up-regulate expression of the biosynthetic pathway components, PG expression of vvl shows little relation with the high-level ecdysone peak that triggers pupariation. Taken together these data suggest that Vvl is required for maintaining PG specific expression (i.e. spatial control), while temporal regulation during development is controlled by other factors such as Br-Z4. Furthermore, our results confirm that Mld is required for PG expression of spok [9], but we also found that it controls Nvd, an enzyme that acts upstream of Spok in the biosynthetic pathway [4]. Thus, our data suggest that Mld is a specific regulator of the two early enzymes Nvd and Spok, while its function is not important for biosynthetic reactions that are downstream of Spok and the black box reaction(s) and the responsiveness of the PG to PTTH.
Our data show that Vvl and Kni are required in the PG during post-embryonic development to maintain PG specific expression of the ecdysone biosynthetic genes. During embryogenesis, vvl expression appears in the PG primordium by stage 13, after the embryonic ecdysone pulse (stage 8–12 [12]) that is required for morphogenesis and differentiation of the embryo. During early embryonic development where the PG primordium is not yet formed, the spatial expression patterns of kni and vvl (Fig. 1B) are different from the biosynthetic genes essential for the embryonic ecdysone pulse [8], [9], [11], [12], [59]. This suggests that Vvl and Kni regulate the biosynthetic genes in the PG, but not during early embryonic development. Consistent with this notion, vvl and kni mutants differentiate the embryonic cuticle [42], [60], unlike the ecdysone deficient mutants that are unable to produce the embryonic ecdysone peak [11], [59]. In adult females, the ovaries are believed to be the source of ecdysone consistent with expression of the ecdysone biosynthetic genes in the nurse and/or follicle cells [11]–[13]. In adult females, we find that vvl is expressed in both nurse and follicle cells, suggesting that Vvl may be involved in regulating expression of the ecdysone biosynthetic genes in the adult stage.
Interestingly, we observed that loss of vvl, kni or mld results in dramatic increase of npc1a expression in the PG. Npc1a is highly expressed in the PG where it is required for uptake and intracellular trafficking of cholesterol for steroidogeneis [26]. Larvae with loss of npc1a exhibit a punctuate pattern of sterol accumulation in the PG cells, indicating defects in cholesterol transport within the cells. Normally cholesterol is taken up as low density lipoproteins (LDLs) and trafficked within endosomes to the lysosomes where hydrolysis releases free cholesterol that is delivered to the plasma membrane and endoplasmic reticulum (ER) [61] where the first step in the conversion of cholesterol to ecdysone likely takes place. Why is npc1a up-regulated in the PG when ecdysone synthesis and pathway activity is impaired? It seems unlikely that Vvl, Kni and Mld are all involved in repression of npc1a. The block of flux through the biosynthetic pathway in the PG of vvl-RNAi, kni-RNAi and mld-RNAi animals may change intracellular cholesterol pools in the gland and affect feedback regulation to maintain cholesterol homeostasis. Our results indicate that npc1a is regulated by cholesterol suggesting that the up-regulation of npc1a may be part of a feedback regulatory response to changes in cellular cholesterol levels. This may indicate a compensatory mechanism to redistribute cholesterol by increasing storage of cholesterol esters and/or efflux to reduce free cholesterol levels when ecdysone biosynthesis is blocked. Moreover, npc1a is regulated by Br [27], a factor induced by EcR in the PG [20], implying that ncp1a may also be regulated by ecdysone feedback. Our study shows that cholesterol availability is an important parameter for ecdysone biosynthesis. Interactions between cholesterol and ecdysone feedback mechanisms may therefore be important for coordinating the supply cholesterol with the rate of steroidogenesis.
A key aspect of steroidogenesis is regulating the tissue-specific expression of the biosynthetic enzymes. We have shown here that the transcription factors, Vvl and Kni, are required for the coordinated expression of ecdysone biosynthetic genes in the PG. The transcriptional activation by Vvl and Kni is likely mediated by direct binding to cis-regulatory elements responsible for PG specific expression. This identifies an important new role for Vvl and Kni during post-embryonic development in the gene regulatory network of the steroid hormone producing cells in Drosophila.
Materials and Methods
Drosophila strains and husbandry
The following Drosophila strains were used in this study: w1118, UAS-vvl-RNAi (#110723), UAS-kni-RNAi (#2980), UAS-mld-RNAi (#101867) and UAS-npc1a-RNAi (#105405) from the Vienna Drosophila RNAi Center (VDRC); UAS-vvl-RNAi (#26228), UAS-kni-RNAi (#34705), tub-Gal80ts and UAS-CD8-GFP (UAS-GFP) from the Bloomington Drosophila Stock Center (BDSC); phm22-Gal4 (phm-Gal4) [9] and P0206-Gal4 [29]. A transgenic line phm-291-4B (phm-GFP) with a 69 bp phm promoter in a pH-stinger GFP reporter vector generated in [20] was used to collect ring glands by dissection for analyzing the development expression profile in the gland. Flies were raised on standard cornmeal food under a 12∶12 hour light:dark cycle. For experiments involving staged or timed larvae, flies were allowed to lay eggs at 25°C for 2–4 hours on apple juice agar plates supplemented with yeast paste in a humidified chamber. After 24 hours, 25 L1 larvae were collected and transferred to vials containing standard food. For experiments using tub-Gal80ts, eggs deposited at 25°C were immediately transferred to 18°C and 25 larvae were transferred to vials containing food 48 hours later. Images of phenotypes were captured with an Olympus SZX7 camera and analyzed using AxioVision software (Zeiss). Characterization of the PG-specific spok element was done as described [20] by generating transgenic animals with constructions of 5′-UTR spok elements in a pH-stinger GFP reporter vector.
Electrophoretic mobility shift assay (EMSA)
EMSA was carried out as previously described [20]. DNA oligonucleotide sequences (Table S3) were designed to cover Vvl and Kni binding sites in the phm promoter based on in silico analysis using Transfac and Jaspar databases. Oligos containing Vvl (Vvl-wt) or Kni (Kni-wt) consensus binding sites and oligos with mutations that disrupt the Vvl (Vvl-mut) or Kni (Kni-mut) binding sites were adapted from [62], [63]. The complementary oligonucleotides were annealed and labeled at the 5′-end labeling by [γ32P]ATP (Perkin Elmer) using T4 polynucleotide kinase (Fermentas) and purified using Microspin G-25 columns (GE Healthcare). The EMSA reaction was performed on ice by mixing Drosophila S2 cell nuclear extracts (Active Motif), dialysis buffer (25 mM Hepes pH 7.6, 40 nM KCl, 0.1 mM EDTA, 10% glycerol), gelshift buffer (25 mM Tris-HCl pH 7.5, 5 mM MgCl2, 60 mM KCl, 0.5 mM EDTA, 5% Ficoll 400, 2.5% glycerol, 1 mM DTT and protease inhibitors) and poly(dI-dC) (Invitrogen). The reaction mixture was supplemented and incubated with 25-50-fold molar excess of unlabeled competitor nucleotides before adding the radiolabeled probe. After incubation the mixture was supplemented with gelshift loading buffer and run on a 5% non-denaturing polyacrylamide gel and dried on a slab gel dryer (Savant) followed by exposure onto a phosporimager screen. The image was acquired using a Storm 840 scanner (Molecular Dynamics) and processed with ImageQuant software version 5.2.
Immunostaining
Tissue dissections were performed in PBS followed by fixation in 4% formaldehyde for 20 minutes at room temperature. For this study, the following primary antibodies were: mouse anti-GFP 1∶200 (Clontech, #632380); rabbit anti-Phm 1∶200 [18]; rat anti-Kni, 1∶1000 [64] and rat anti-Vvl 1∶1000 [65]. Tissues were incubated over night with primary antibodies at 4°C. Fluorescent conjugated secondary antibodies used were goat anti-mouse Alexa Fluor 488 (A11001, Invitrogen), goat anti-rabbit Alexa Fluor 555 (A21429, Invitrogen) and goat anti-rat Alexa Fluor 555 (A21434, Invitrogen). Secondary antibodies were diluted 1∶200 and incubated for two hours at room temperature. DAPI was used in 1∶500 for nuclei staining. Confocal images were captured using Zeiss LSM 710 laser scanning microscope and processed using ImageJ (NIH). Images of mid-first instar PG morphology were obtained by confocal imaging of live L1 larvae (36 hours AEL) mounted in 80% glycerol.
Feeding experiment with steroids and precursors
Preparation and synthesis of 3β,14α-Dihydroxy-5β-cholest-7-en-6-one (5β-ketodiol) and 3β,14α,25-Trihydroxy-5β-cholest-7-en-6-one (5β-ketotriol) were previously described [8]. For the steroid feeding rescue experiment, 30 mg of dry yeast was mixed with 57 µl H2O and 3 µl ethanol or supplemented with 3 µl of the following sterols dissolved in ethanol: 20E (Sigma; 450 µg), ecdysone (Sigma; 100 µg), cholesterol (Sigma; 45 µg), 7-dehydrocholesterol (Sigma; 200 µg), 5β-ketodiol (450 µg), or 5β-ketotriol (280 µg). Thirty larvae were transferred to the yeast paste on an apple juice agar plate and allowed to develop in a humid chamber at 25°C. The phenotype of the larvae was scored at day 5 prior to pupariation of w1118 control for rescue to the L3 stage. For other experiments with cholesterol supplementation of the food, standard cornmeal was supplied with cholesterol (Sigma) dissolved in ethanol to a final concentration of 40 µg/ml.
In situ hybridization
Digoxigenin (DIG)-labeled antisense RNA probes were synthesized using DIG RNA labeling mix (Roche) and T3 (Fermentas), T7 (Fermentas) or SP6 (Roche) RNA polymerase according to the manufacturer's instructions. For the kni probe, an EST clone GH19318 [66] was used as a templates. For the vvl probe, a portion of vvl gene was amplified by PCR with cDNA derived from w1118 larvae and the following primers: vvl_PA_CDS_F (5′-ATGGCCGCGACCTCGTACATGAC-3′) and vvl_PA_CDS_R (5′-CTAGTGGGCCGCCAACTGATGC-3′). For the mld probe, a portion of mld gene was amplified by PCR with the plasmid mld-pUAST [21]; a gift from S. M. Cohen and the following primers: mld_CDS_1_F (5′-ATGAGTGCCAACCGAAGAAGACG-3′) and mld_CDS_1_R (5′-CATCTGAGATTGGTCATGAGATTGTACTTGAGG-3′). PCR products containing the vvl and mld fragments were subcloned into SmaI-digested pBluescript II SK(-) and pCRII-Blunt-TOPO (Invitrogen), respectively, and then used as the templates for synthesizing RNA probes. Fixation, hybridization and detection were performed as previously described [8], [67].
Quantitative RT-PCR
For gene expression experiments using the whole animals, 30 L1 larvae or 4 L3 larvae were used for each replicate. For analysis of ring gland expression, 10–15 ring glands were dissected in PBS and directly transferred to RNA lysis buffer. RNA was extracted using the RNeasy mini kit (Qiagen) and DNase treated to avoid genomic DNA contamination according to the manufacturer's instructions. RNA was quantified using a NanoDrop (Thermo Scientific) and the integrity was assessed using agarose gel electrophoresis. Total RNA was used for cDNA synthesis with the SuperScript III First-Strand Synthesis kit (Invitrogen). Primers were designed using the Primer3 software [68] (Table S4). Relative gene expression was analyzed using a Mx3000P qPCR System (Agilent Technologies) with the QuantiTect SYBR Green PCR Kit (Qiagen) according to the manufacturer's instructions as described [10], [33], [69]. All reactions were subjected to 95°C for 10 min, followed by 45 cycles of 95°C for 15 sec, 60°C for 15 sec and 72°C for 15 sec. Dissociation curve analysis was applied to all reactions to ensure the presence of single specific PCR product. Non-reverse transcribed template controls and non-template controls were included to check for background and potential genomic contamination. No product was observed in these reactions. Efficiencies were calculated for each primer pair from standard curves generated from serial dilutions of a mix of cDNA from all control samples. PCR efficiencies were always close to 100%, which was therefore used as the standard in all calculations. Expression of target genes was normalized to reference gene, Rpl23 and Rpl32. We confirmed that these reference [32], [33], [70]–[74] are stably expressed across tissues and experimental conditions, by comparing Rpl23 and Rpl32 mRNA levels in cDNA synthesized from equal amounts of RNA extracted from different tissues and developmental stages (Fig. S2). Reference gene stability determined using qBASE Plus (Biogazelle NV, Zwijnaarde, Belgium) was within the recommended limits (M = 0.274 and CV = 0.095). For definition of these stability factors see [75].
Ecdysteroid measurements
For ecdysteroid measurements, ecdysteroids were extracted from whole animals as described [24]. Briefly, whole larvae were rinsed in water and stored at −80°C. Samples were homogenized in 0.5 ml methanol and the supernatant was collected following centrifugation at 14,000 g. The remaining tissue was re-extracted first in 0.5 ml methanol then in 0.5 ml ethanol. The pooled supernatants were evaporated using a SpeedVac and redissovled in ELISA buffer (1 M phosphate solution, 1% BSA, 4 M sodium chloride and 10 mM EDTA). ELISA was performed according to the manufacturer's instructions using a commercial ELISA kit (ACE Enzyme Immunoassay, Cayman Chemical) that detects ecdysone and 20-hydroxyecdysone with the same affinity [76]. Standard curves were generated using 20E (Sigma). Absorbance was measured at 405 nm on a plate reader, ELx80 (BioTek) using the Gen5 software (BioTek).
Western blotting
Four brain-ring gland complexes were dissected in cold PBS and transferred to 20 µl Laemmli Sample Buffer (Bio-Rad) supplemented with 2-mercaptoethanol. Samples were boiled for 5 minutes, centrifuged at 14,000 g and 10 µl supernatant were loaded on a 4–20% polyacrylamide gradient gel (Bio-Rad) followed by transfer onto a PVDF membrane (Millipore). Primary antibodies used were; mouse anti-α-tubulin, 1∶5,000 (T9026, Sigma Aldrich), rabbit anti-Phm, 1∶1,000 [18] and rabbit anti-phospho-ERK, 1∶1,000 (9101, Cell Signaling Technology). Secondary antibodies were goat anti-mouse IRDye 680RD, 1∶10,000 (926-68070, LI-COR) and goat anti-rabbit IRDye 800CW, 1∶10,000 (926–32211, LI-COR). The blot was scanned on an Odessey Fc (LI-COR) and the software, Image Studio for Odessey Fc, was used for image processing and protein quantification.
Statistics
The statistical differences between data sets were calculated using two-tailed Student's t-test and error bars represent standard error of the mean (s.e.m.).
Supporting Information
Zdroje
1. DanielsenET, MoellerME, RewitzKF (2013) Nutrient signaling and developmental timing of maturation. Curr Top Dev Biol 105 : 37–67.
2. RewitzKF, YamanakaN, O'ConnorMB (2013) Developmental checkpoints and feedback circuits time insect maturation. Curr Top Dev Biol 103 : 1–33.
3. TennessenJM, ThummelCS (2011) Coordinating growth and maturation - insights from Drosophila. Curr Biol 21: R750–757.
4. YoshiyamaT, NamikiT, MitaK, KataokaH, NiwaR (2006) Neverland is an evolutionally conserved Rieske-domain protein that is essential for ecdysone synthesis and insect growth. Development 133 : 2565–2574.
5. Yoshiyama-YanagawaT, EnyaS, Shimada-NiwaY, YaguchiS, HaramotoY, et al. (2011) The conserved Rieske oxygenase DAF-36/Neverland is a novel cholesterol-metabolizing enzyme. J Biol Chem 286 : 25756–25762.
6. NiwaR, NamikiT, ItoK, Shimada-NiwaY, KiuchiM, et al. (2010) Non-molting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the ‘Black Box’ of the ecdysteroid biosynthesis pathway. Development 137 : 1991–1999.
7. NamikiT, NiwaR, SakudohT, ShiraiK, TakeuchiH, et al. (2005) Cytochrome P450 CYP307A1/Spook: a regulator for ecdysone synthesis in insects. Biochem Biophys Res Commun 337 : 367–374.
8. NiwaR, MatsudaT, YoshiyamaT, NamikiT, MitaK, et al. (2004) CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J Biol Chem 279 : 35942–35949.
9. OnoH, RewitzKF, ShinodaT, ItoyamaK, PetrykA, et al. (2006) Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev Biol 298 : 555–570.
10. RewitzKF, RybczynskiR, WarrenJT, GilbertLI (2006) Identification, characterization and developmental expression of Halloween genes encoding P450 enzymes mediating ecdysone biosynthesis in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol 36 : 188–199.
11. WarrenJT, PetrykA, MarquesG, JarchoM, ParvyJP, et al. (2002) Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc Natl Acad Sci U S A 99 : 11043–11048.
12. WarrenJT, PetrykA, MarquesG, ParvyJP, ShinodaT, et al. (2004) Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem Mol Biol 34 : 991–1010.
13. PetrykA, WarrenJT, MarquesG, JarchoMP, GilbertLI, et al. (2003) Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci U S A 100 : 13773–13778.
14. RewitzKF, RybczynskiR, WarrenJT, GilbertLI (2006) Developmental expression of Manduca shade, the P450 mediating the final step in molting hormone synthesis. Mol Cell Endocrinol 247 : 166–174.
15. CaceresL, NecakovAS, SchwartzC, KimberS, RobertsIJ, et al. (2011) Nitric oxide coordinates metabolism, growth, and development via the nuclear receptor E75. Genes Dev 25 : 1476–1485.
16. DengH, KerppolaTK (2013) Regulation of Drosophila metamorphosis by xenobiotic response regulators. PLoS Genet 9: e1003263.
17. OuQ, MagicoA, King-JonesK (2011) Nuclear Receptor DHR4 Controls the Timing of Steroid Hormone Pulses During Drosophila Development. PLoS Biol 9: e1001160.
18. ParvyJP, BlaisC, BernardF, WarrenJT, PetrykA, et al. (2005) A role for betaFTZ-F1 in regulating ecdysteroid titers during post-embryonic development in Drosophila melanogaster. Dev Biol 282 : 84–94.
19. WarrenJT, WismarJ, SubrahmanyamB, GilbertLI (2001) Woc (without children) gene control of ecdysone biosynthesis in Drosophila melanogaster. Mol Cell Endocrinol 181 : 1–14.
20. MoellerME, DanielsenET, HerderR, O'ConnorMB, RewitzKF (2013) Dynamic feedback circuits function as a switch for shaping a maturation-inducing steroid pulse in Drosophila. Development 140 : 4730–4739.
21. NeubueserD, WarrenJT, GilbertLI, CohenSM (2005) molting defective is required for ecdysone biosynthesis. Dev Biol 280 : 362–372.
22. MirthC, TrumanJW, RiddifordLM (2005) The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr Biol 15 : 1796–1807.
23. ColombaniJ, AndersenDS, LeopoldP (2012) Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 336 : 582–585.
24. WarrenJT, YerushalmiY, ShimellMJ, O'ConnorMB, RestifoLL, et al. (2006) Discrete pulses of molting hormone, 20-hydroxyecdysone, during late larval development of Drosophila melanogaster: correlations with changes in gene activity. Dev Dyn 235 : 315–326.
25. HackneyJF, Zolali-MeybodiO, CherbasP (2012) Tissue damage disrupts developmental progression and ecdysteroid biosynthesis in Drosophila. PLoS ONE 7: e49105.
26. HuangX, SuyamaK, BuchananJ, ZhuAJ, ScottMP (2005) A Drosophila model of the Niemann-Pick type C lysosome storage disease: dnpc1a is required for molting and sterol homeostasis. Development 132 : 5115–5124.
27. XiangY, LiuZ, HuangX (2010) br regulates the expression of the ecdysone biosynthesis gene npc1. Dev Biol 344 : 800–808.
28. CaldwellPE, WalkiewiczM, SternM (2005) Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr Biol 15 : 1785–1795.
29. ColombaniJ, BianchiniL, LayalleS, PondevilleE, Dauphin-VillemantC, et al. (2005) Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310 : 667–670.
30. GhoshA, McBrayerZ, O'ConnorMB (2010) The Drosophila gap gene giant regulates ecdysone production through specification of the PTTH-producing neurons. Dev Biol 347 : 271–278.
31. LayalleS, ArquierN, LeopoldP (2008) The TOR pathway couples nutrition and developmental timing in Drosophila. Dev Cell 15 : 568–577.
32. McBrayerZ, OnoH, ShimellM, ParvyJP, BecksteadRB, et al. (2007) Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell 13 : 857–871.
33. RewitzKF, YamanakaN, GilbertLI, O'ConnorMB (2009) The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science 326 : 1403–1405.
34. WullschlegerS, LoewithR, HallMN (2006) TOR signaling in growth and metabolism. Cell 124 : 471–484.
35. MirthCK, RiddifordLM (2007) Size assessment and growth control: how adult size is determined in insects. Bioessays 29 : 344–355.
36. JungerMA, RintelenF, StockerH, WassermanJD, VeghM, et al. (2003) The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol 2 : 20.
37. PuigO, MarrMT, RuhfML, TjianR (2003) Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev 17 : 2006–2020.
38. AndersonMG, PerkinsGL, ChittickP, ShrigleyRJ, JohnsonWA (1995) drifter, a Drosophila POU-domain transcription factor, is required for correct differentiation and migration of tracheal cells and midline glia. Genes Dev 9 : 123–137.
39. CertelSJ, ThorS (2004) Specification of Drosophila motoneuron identity by the combinatorial action of POU and LIM-HD factors. Development 131 : 5429–5439.
40. InbalA, LevanonD, SalzbergA (2003) Multiple roles for u-turn/ventral veinless in the development of Drosophila PNS. Development 130 : 2467–2478.
41. CertelK, HudsonA, CarrollSB, JohnsonWA (2000) Restricted patterning of vestigial expression in Drosophila wing imaginal discs requires synergistic activation by both Mad and the drifter POU domain transcription factor. Development 127 : 3173–3183.
42. de CelisJF, LlimargasM, CasanovaJ (1995) Ventral veinless, the gene encoding the Cf1a transcription factor, links positional information and cell differentiation during embryonic and imaginal development in Drosophila melanogaster. Development 121 : 3405–3416.
43. JunellA, UvellH, DavisMM, Edlundh-RoseE, AntonssonA, et al. (2010) The POU transcription factor Drifter/Ventral veinless regulates expression of Drosophila immune defense genes. Mol Cell Biol 30 : 3672–3684.
44. CertelK, AndersonMG, ShrigleyRJ, JohnsonWA (1996) Distinct variant DNA-binding sites determine cell-specific autoregulated expression of the Drosophila POU domain transcription factor drifter in midline glia or trachea. Mol Cell Biol 16 : 1813–1823.
45. BonagliaMC, CicconeR, GimelliG, GimelliS, MarelliS, et al. (2008) Detailed phenotype-genotype study in five patients with chromosome 6q16 deletion: narrowing the critical region for Prader-Willi-like phenotype. Eur J Hum Genet 16 : 1443–1449.
46. IzumiK, HousamR, KapadiaC, StallingsVA, MedneL, et al. (2013) Endocrine phenotype of 6q16.1-q21 deletion involving SIM1 and Prader-Willi syndrome-like features. Am J Med Genet A 161 : 3137–3143.
47. NauberU, PankratzMJ, KienlinA, SeifertE, KlemmU, et al. (1988) Abdominal segmentation of the Drosophila embryo requires a hormone receptor-like protein encoded by the gap gene knirps. Nature 336 : 489–492.
48. FussB, MeissnerT, BauerR, LehmannC, EckardtF, et al. (2001) Control of endoreduplication domains in the Drosophila gut by the knirps and knirps-related genes. Mech Dev 100 : 15–23.
49. ChenCK, KuhnleinRP, EulenbergKG, VincentS, AffolterM, et al. (1998) The transcription factors KNIRPS and KNIRPS RELATED control cell migration and branch morphogenesis during Drosophila tracheal development. Development 125 : 4959–4968.
50. Gonzalez-GaitanM, RotheM, WimmerEA, TaubertH, JackleH (1994) Redundant functions of the genes knirps and knirps-related for the establishment of anterior Drosophila head structures. Proc Natl Acad Sci U S A 91 : 8567–8571.
51. LundeK, TrimbleJL, GuichardA, GussKA, NauberU, et al. (2003) Activation of the knirps locus links patterning to morphogenesis of the second wing vein in Drosophila. Development 130 : 235–248.
52. ArnostiDN, GrayS, BaroloS, ZhouJ, LevineM (1996) The gap protein knirps mediates both quenching and direct repression in the Drosophila embryo. EMBO J 15 : 3659–3666.
53. LangelandJA, AttaiSF, VorwerkK, CarrollSB (1994) Positioning adjacent pair-rule stripes in the posterior Drosophila embryo. Development 120 : 2945–2955.
54. ParkerKL, RiceDA, LalaDS, IkedaY, LuoX, et al. (2002) Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res 57 : 19–36.
55. ValP, Lefrancois-MartinezAM, VeyssiereG, MartinezA (2003) SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl Recept 1 : 8.
56. XuB, YangWH, GerinI, HuCD, HammerGD, et al. (2009) Dax-1 and steroid receptor RNA activator (SRA) function as transcriptional coactivators for steroidogenic factor 1 in steroidogenesis. Mol Cell Biol 29 : 1719–1734.
57. GibbensYY, WarrenJT, GilbertLI, O'ConnorMB (2011) Neuroendocrine regulation of Drosophila metamorphosis requires TGFbeta/Activin signaling. Development 138 : 2693–2703.
58. AndersonMG, CertelSJ, CertelK, LeeT, MontellDJ, et al. (1996) Function of the Drosophila POU domain transcription factor drifter as an upstream regulator of breathless receptor tyrosine kinase expression in developing trachea. Development 122 : 4169–4178.
59. ChavezVM, MarquesG, DelbecqueJP, KobayashiK, HollingsworthM, et al. (2000) The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development 127 : 4115–4126.
60. JurgensG, WieschausE, Nusslein-VolhardC, KludingH (1984) Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. II. Zygotic loci on the third chromosome. Rouxs Arch Dev Biol 193 : 283–295.
61. DuX, YangH (2013) Endosomal cholesterol trafficking: protein factors at a glance. Acta Biochim Biophys Sin (Shanghai) 45 : 11–17.
62. MillerSW, Avidor-ReissT, PolyanovskyA, PosakonyJW (2009) Complex interplay of three transcription factors in controlling the tormogen differentiation program of Drosophila mechanoreceptors. Dev Biol 329 : 386–399.
63. StruffiP, CoradoM, KaplanL, YuD, RushlowC, et al. (2011) Combinatorial activation and concentration-dependent repression of the Drosophila even skipped stripe 3+7 enhancer. Development 138 : 4291–4299.
64. DubuisJO, SamantaR, GregorT (2013) Accurate measurements of dynamics and reproducibility in small genetic networks. Mol Syst Biol 9 : 639.
65. HasegawaE, KitadaY, KaidoM, TakayamaR, AwasakiT, et al. (2011) Concentric zones, cell migration and neuronal circuits in the Drosophila visual center. Development 138 : 983–993.
66. StapletonM, LiaoG, BroksteinP, HongL, CarninciP, et al. (2002) The Drosophila gene collection: identification of putative full-length cDNAs for 70% of D. melanogaster genes. Genome Res 12 : 1294–1300.
67. LehmannR, TautzD (1994) In situ hybridization to RNA. Methods Cell Biol 44 : 575–598.
68. UntergasserA, CutcutacheI, KoressaarT, YeJ, FairclothBC, et al. (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res 40: e115.
69. RewitzKF, YamanakaN, O'ConnorMB (2010) Steroid hormone inactivation is required during the juvenile-adult transition in Drosophila. Dev Cell 19 : 895–902.
70. ChrostekE, MarialvaMS, EstevesSS, WeinertLA, MartinezJ, et al. (2013) Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet 9: e1003896.
71. EleftherianosI, WonS, ChtarbanovaS, SquibanB, OcorrK, et al. (2011) ATP-sensitive potassium channel (K(ATP))-dependent regulation of cardiotropic viral infections. Proc Natl Acad Sci U S A 108 : 12024–12029.
72. FabrowskiP, NecakovAS, MumbauerS, LoeserE, ReversiA, et al. (2013) Tubular endocytosis drives remodelling of the apical surface during epithelial morphogenesis in Drosophila. Nat Commun 4 : 2244.
73. FrostB, HembergM, LewisJ, FeanyMB (2014) Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci 17 : 357–366.
74. TalamilloA, HerbosoL, PironeL, PerezC, GonzalezM, et al. (2013) Scavenger receptors mediate the role of SUMO and Ftz-f1 in Drosophila steroidogenesis. PLoS Genet 9: e1003473.
75. VandesompeleJ, De PreterK, PattynF, PoppeB, Van RoyN, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034.
76. PorcheronP, FoucrierJ, GrosC, PradellesP, CassierP, et al. (1976) Radioimmunoassay of arthropod moulting hormone:beta-ecdysone antibodies production and 125 I-iodinated tracer preparation. FEBS Lett 61 : 159–162.
Štítky
Genetika Reprodukčná medicína
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 6- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Inflammation: Gone with Translation
- Recombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
- Caspase Inhibition in Select Olfactory Neurons Restores Innate Attraction Behavior in Aged
- Accurate, Model-Based Tuning of Synthetic Gene Expression Using Introns in
- A Novel Peptidoglycan Binding Protein Crucial for PBP1A-Mediated Cell Wall Biogenesis in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
- Introgression from Domestic Goat Generated Variation at the Major Histocompatibility Complex of Alpine Ibex
- Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in
- Coordination of Wing and Whole-Body Development at Developmental Milestones Ensures Robustness against Environmental and Physiological Perturbations
- Phenotypic Dissection of Bone Mineral Density Reveals Skeletal Site Specificity and Facilitates the Identification of Novel Loci in the Genetic Regulation of Bone Mass Attainment
- Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of -Factors in Two Independent Origins of C Photosynthesis
- Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity
- Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation
- Rosa26-GFP Direct Repeat (RaDR-GFP) Mice Reveal Tissue- and Age-Dependence of Homologous Recombination in Mammals
- Abnormal Type I Collagen Post-translational Modification and Crosslinking in a Cyclophilin B KO Mouse Model of Recessive Osteogenesis Imperfecta
- : Clonal Reinforcement Drives Evolution of a Simple Microbial Community
- Reviving the Dead: History and Reactivation of an Extinct L1
- Defective iA37 Modification of Mitochondrial and Cytosolic tRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate tRNA
- Early Back-to-Africa Migration into the Horn of Africa
- Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency
- Microbial Succession in the Gut: Directional Trends of Taxonomic and Functional Change in a Birth Cohort of Spanish Infants
- Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia
- Genetic Determinants of Long-Term Changes in Blood Lipid Concentrations: 10-Year Follow-Up of the GLACIER Study
- Palaeosymbiosis Revealed by Genomic Fossils of in a Strongyloidean Nematode
- Early Embryogenesis-Specific Expression of the Rice Transposon Enhances Amplification of the MITE
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
- Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
- N-WASP Is Required for Structural Integrity of the Blood-Testis Barrier
- The Transcription Factor TFII-I Promotes DNA Translesion Synthesis and Genomic Stability
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
- Digital Genotyping of Macrosatellites and Multicopy Genes Reveals Novel Biological Functions Associated with Copy Number Variation of Large Tandem Repeats
- ATRA-Induced Cellular Differentiation and CD38 Expression Inhibits Acquisition of BCR-ABL Mutations for CML Acquired Resistance
- The EJC Binding and Dissociating Activity of PYM Is Regulated in
- JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling
- Mouse Y-Linked and Are Expressed during the Male-Specific Interphase between Meiosis I and Meiosis II and Promote the 2 Meiotic Division
- Rasa3 Controls Megakaryocyte Rap1 Activation, Integrin Signaling and Differentiation into Proplatelet
- Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
- Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis
- The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
- The First Endogenous Herpesvirus, Identified in the Tarsier Genome, and Novel Sequences from Primate Rhadinoviruses and Lymphocryptoviruses
- Sequence of a Complete Chicken BG Haplotype Shows Dynamic Expansion and Contraction of Two Gene Lineages with Particular Expression Patterns
- Background Selection as Baseline for Nucleotide Variation across the Genome
- CPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
- The Effects of Codon Context on Translation Speed
- Glycogen Synthase Kinase (GSK) 3β Phosphorylates and Protects Nuclear Myosin 1c from Proteasome-Mediated Degradation to Activate rDNA Transcription in Early G1 Cells
- Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
- Muscle Structure Influences Utrophin Expression in Mice
- BLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
- Identification of Late Larval Stage Developmental Checkpoints in Regulated by Insulin/IGF and Steroid Hormone Signaling Pathways
- Transport of Magnesium by a Bacterial Nramp-Related Gene
- Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
- The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription
- The Rim15-Endosulfine-PP2A Signalling Module Regulates Entry into Gametogenesis and Quiescence Distinct Mechanisms in Budding Yeast
- Regulation of Hfq by the RNA CrcZ in Carbon Catabolite Repression
- Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions
- Positive Feedback of Expression Ensures Irreversible Meiotic Commitment in Budding Yeast
- Hecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
- Regulatory Mechanisms That Prevent Re-initiation of DNA Replication Can Be Locally Modulated at Origins by Nearby Sequence Elements
- Speciation and Introgression between and
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Early Back-to-Africa Migration into the Horn of Africa
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



