-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation in
For all eukaryotes, gamete formation is an essential aspect of sexual reproduction. Unlike in animals, where meiotic products directly become gametes, the germline in plants is established by two consecutive mitotic divisions after meiosis is completed. The first mitosis is asymmetric, forming a larger vegetative cell and a smaller generative cell. The smaller generative cell then divides to produce two sperm cells. Current knowledge indicates DUO1 (DUO POLLEN 1), a transcription factor, plays a key role in this process by controlling expression of other germline genes. But how DUO1 is activated in the generative cell is unknown. To better understand the mechanisms that govern sperm cell formation and activate DUO1 expression, we characterized, ARID1, encoding an ARID (AT-Rich Interacting Domain)-containing protein. We show that ARID1 is required for DUO1 activation and sperm cell formation in Arabidopsis. Furthermore, ARID1 physically associates with a histone deacetylase, facilitating the maintenance of histone acetylation between the vegetative nucleus and sperm nuclei. Thus, our study shows that a pollen-specific ARID protein plays an important role during sperm cell formation in a dual manner: as a transcription factor to activate DUO1 and as a potential component of the histone modification machinery to maintain epigenetic status in pollen.
Published in the journal: An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation in. PLoS Genet 10(7): e32767. doi:10.1371/journal.pgen.1004421
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004421Summary
For all eukaryotes, gamete formation is an essential aspect of sexual reproduction. Unlike in animals, where meiotic products directly become gametes, the germline in plants is established by two consecutive mitotic divisions after meiosis is completed. The first mitosis is asymmetric, forming a larger vegetative cell and a smaller generative cell. The smaller generative cell then divides to produce two sperm cells. Current knowledge indicates DUO1 (DUO POLLEN 1), a transcription factor, plays a key role in this process by controlling expression of other germline genes. But how DUO1 is activated in the generative cell is unknown. To better understand the mechanisms that govern sperm cell formation and activate DUO1 expression, we characterized, ARID1, encoding an ARID (AT-Rich Interacting Domain)-containing protein. We show that ARID1 is required for DUO1 activation and sperm cell formation in Arabidopsis. Furthermore, ARID1 physically associates with a histone deacetylase, facilitating the maintenance of histone acetylation between the vegetative nucleus and sperm nuclei. Thus, our study shows that a pollen-specific ARID protein plays an important role during sperm cell formation in a dual manner: as a transcription factor to activate DUO1 and as a potential component of the histone modification machinery to maintain epigenetic status in pollen.
Introduction
In contrast to animals, where meiotic products directly become gametes, the germline in plants is established by mitotic divisions after meiosis is completed. The male germline arises by an asymmetric mitotic division of each meiotic product. The resulting vegetative and generative cells of the bicellular pollen grain have distinct fates. The larger vegetative cell is arrested at the G1 phase of the cell cycle, while the smaller generative cell divides mitotically to produce the two male gametes or sperm cells [1]. The vegetative cell forms a pollen tube to convey the sperm cells to the female gametes. Several genes have been implicated in sperm cell formation [2]–[8]. Among these, DUO POLLEN1 (DUO1) encodes a male germ cell–specific R2R3 Myb transcription factor that is necessary for twin sperm cell formation [3].
Although many genes have been implicated in sperm cell formation, the molecular mechanism as to how the generative cell initiates the second mitosis and divides into two sperm cells remains unclear. By monitoring the expression of genes associated with germ cell specification, a previous study [9] demonstrated that DUO1 controls germline expression of a cyclin, CYCB1, and dictates correct differentiation of the male germline by promoting expression of MGH3 (MALE GAMETE-SPECIFIC HISTONE H3) [2], GCS1 (GENERATIVE CELL SPECIFIC 1) [5], and GEX2 (GAMETE EXPRESSED 2) [10]. Subsequently, they profiled DUO1 targets at a genome-wide level [11]. In contrast to the myriad DUO1 targets, how DUO1 transcription is regulated is less understood. Only microRNA159 (miR159) was found to inhibit DUO1 expression [12]. miR159 is greatly reduced but not absent at the bicellular stage [8], but DUO1 is gradually activated from the early bicellular stage to the middle bicellular stage [9], indicating that DUO1 activation is not due only to the decrease of miR159 at the bicellular stage and that other factors are required for DUO1 activation during sperm cell formation. However, such factors were unknown.
In animals, ARID (AT-Rich Interacting Domain) proteins exhibit a range of cellular functions, including participation in epigenetic regulation of gene expression during cell differentiation and development [13]. ARID is an ancient DNA-binding domain that is conserved throughout higher eukaryotes. In animals, ARID proteins are grouped in several subfamilies based on the presence of additional conserved domains [13]. In mice, ARID4A and ARID4B are required for male fertility [14], [15] and are associated with the histone deacetylase complex [16], [17]. JARID2, an important developmental regulator during cell cycle regulation [18], [19], is also a component of the Polycomb-repressive complex 2 (PRC2) either as an activator [20], [21] or a repressor [22], [23] in mammals. Thus, considering the importance of the cell cycle during sperm cell formation in plants and the increasing knowledge about the role of ARID domain-containing proteins both in gene regulation and germline development, we wanted to know whether plant ARID proteins might be involved in the regulation of sperm cell formation. The Arabidopsis genome encodes 10 ARID proteins that have been grouped into four subfamilies based on the presence of domains located at the C-terminus [24]. However, no function of these ARID proteins has been reported. In lotus, an ARID is required for early nodule development [24]. Here, we show that ARID1, an Arabidopsis pollen-specific ARID protein containing an ELM2 (EGL-27 and MTA1 homology) domain, is required for sperm cell formation, as plants with disrupted ARID1 function had an increased incidence of pollen with single sperm and exhibited reduced fertility. Furthermore, DUO1 expression was reduced in arid1 mutants and Chromatin Immunoprecipitation analysis and in vitro DNA binding assays demonstrated that ARID1 binds to the DUO1 promoter. Microscopic analysis showed that ARID1 is located in developmentally dynamic nuclear bodies during pollen development, and ARID1 obviously accumulated in the generative cell, which implies that the presence of ARID1 is correlated with the initiation of the second mitosis. We showed that ARID1 physically interacts with histone deacetylase 8 (HDA8) in vitro and in vivo, and an immunofluorescence assay showed that the histone acetylation signal expanded to the vegetative nucleus in arid1 pollen. Furthermore, an obviously reduced level of histone acetylation was observed at DUO1, whereas the level of histone methylation was not altered. These results imply that ARID1 is required for sperm cell formation by positively regulating DUO1 and for maintaining the levels of histone acetylation in pollen by associating with the histone modification machinery.
Results
ARID1 Is Required for Sperm Cell Formation in Arabidopsis
Microarray data [25], [26] and our RT-PCR results (Figure 1A) showed that one ARID, At2g46040, here named ARID1, was expressed in a pollen-specific manner. In addition to the ARID domain, ARID1 contains an ELM2 domain at the C-terminus. In animals, ELM2 domains mediate histone modifications by interacting with histone deacetylase [27], [28]. The combination of ARID and ELM2 domains in a single protein is plant-specific. Given the importance of cell cycle regulation by ARID proteins in animals [19], and that a mouse mutant in a gene encoding an ARID protein is male infertile [15], we suspected that disrupting ARID1 function might lead to disorganized cell divisions during pollen development. The arid1-1 mutant has a T-DNA insertion in the only intron of ARID1 (Figure S1A). The insertion did not abolish expression, as a truncated transcript upstream of the inserted location was detected (Figure S1A), suggesting that arid1-1 might be a weak allele. Plants homozygous for arid1-1 had short siliques and reduced seed set (Figure 1B and 1C). We identified homozygous plants by genotyping a F2 population of arid1-1 backcrossed with wild type plants. As only 10 of the 96 F2 plants were homozygous, we hypothesized that there might be a transmission problem. Reciprocal crosses with wild type plants showed that transmission through the female was normal, but was perturbed through the male (Table 1). To investigate whether the arid1-1 phenotype was caused by the T-DNA insertion, we constructed two ARID1 transgenes by engineering GFP or RFP tags at the C-terminus to a genomic fragment of ARID1, driven by its native promoter. ARID1-GFP or ARID1-RFP completely complemented the reduced seed set phenotype (Figure 1B and 1C). Because arid1-1 appeared to be a weak allele, we explored whether a more complete loss of arid1 function would have similar or more severe phenotypes. We therefore generated a binary construct expressing antisense ARID1 under the control of the native ARID1 promoter. The seed set of 48 independent transgenic lines was examined: 42 plants showed reduced seed set, ranging from 15% to 95% (Figure 1D). Transcript analysis of representative antisense lines confirmed that the phenotype of reduced seed set correlated with reduced transcript levels of ARID1 (Figure 1D). Because immature seeds from antisense lines with severely reduced seed set finally shriveled, we performed further analyses using arid1-1.
Fig. 1. Specific expression of ARID1 in pollen and disruption of ARID1 results in defective sperm cell formation. 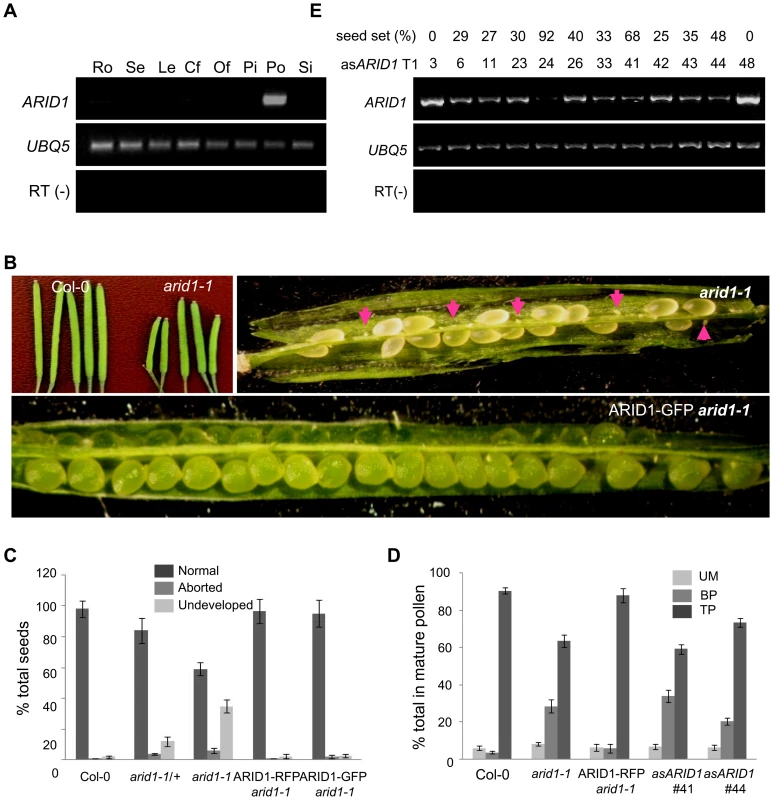
(A) Expression of ARID1 by RT-PCR. Ro, roots; Se, seedlings; Le, leaves; Cf, closed flower buds; Of, open flowers; Pi, unpollinated pistils; Po, mature pollen; Si, siliques. The RT (−) control PCR was performed with UBQ5 primers. (B) Representative siliques of WT and arid1-1 and complementation test. Undeveloped ovules are indicated with arrows. (C) Percentage of normal seeds (dark grey), aborted seeds (lighter grey), and undeveloped ovules (lightest grey) from self-pollinated plants are shown. Error bars represent standard deviation from the mean. (D) Seed set analysis in antisense ARID1 transgenic plants. Numbers in the bottom row represent individual T1 lines, and the corresponding numbers in the top row indicate the percentage of reduced seed set in each line. The gel shows the expression of ARID1 as assessed by RT-PCR analysis. (E) Distribution of unicellular microspores (UM, lightest grey), bicellular pollen (BP, lighter grey), and tricellular pollen (TP, dark grey) in mature anthers. At least 600 pollen grains were stained with DAPI and used for statistical analysis; Error bars represent standard deviation from the mean. Tab. 1. Reduced male transmission in <i>arid1-1</i> plants. 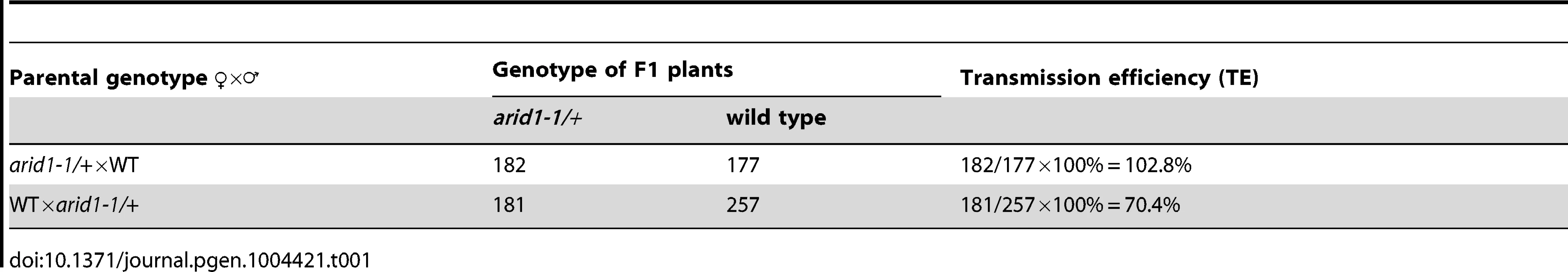
To dissect which developmental stage was defective in the arid1-1 mutant and antisense lines, we stained with DAPI to examine pollen development, used in vitro pollen germination assays to observe pollen germination and pollen tube growth, and performed ovule clearing analysis followed by DAB (Decolorized Aniline Blue) staining to evaluate pollen tube guidance. There were no detectable defects in pollen germination and pollen tube growth (Figure S1B) or pollen tube guidance (Figure S1C), but both the arid1-1 mutant and antisense lines showed increased incidence of single sperm-like cells in mature pollen (Figure 1E and Figure S1D). The stronger phenotypes observed in the antisense ARID1 transgenic plants suggest that the ARID domain present in truncated proteins in arid1-1 was partly functional. Because antisense ARID1 transgenic plants with strongly reduced or undetectable expression of ARID1 were sterile, we performed all further analyses in the arid1-1 mutant background. The defect in sperm cell formation was rescued when we introduced the ARID1-RFP construct into the arid1-1 mutant (Figure 1E). Taken together, our phenotypic analysis indicates that ARID1 is required for sperm cell formation.
ARID1 Positively Regulates DUO1 Expression during Sperm Cell Formation
The single sperm-like phenotype of arid1-1 was similar to the phenotypes of mutants such as duo1 [3], cdka1;1 [4], fbl17 [6], and duo3 [7]. We therefore used qPCR to examine whether the expression of these genes was disturbed in arid1-1. Of these, only DUO1 mRNA levels were reduced in arid1-1 (Figure 2A), suggesting that ARID1 positively regulates DUO1 at the transcriptional level, either directly or indirectly. DUO1 expression was also reduced in the antisense ARID1 plants with reduced seed set (Figure S2A). We also crossed a DUO1-RFP reporter into arid1-1 and saw that the DUO1-RFP signal was slightly reduced, in both bicellular pollen (Figure 2B, upper panels, yellow arrows) and mature pollen (Figure 2B, lower panels, yellow arrows). Since DUO1 is one of the targets of miR159 [12], we examined MIR159 expression in the arid1-1 mutant by qPCR, but found no change in miR159 levels (Figure S2B). As we recently showed that Anaphase Promoting Complex 8 (APC8) is involved in CYCB1 regulation at both the transcriptional level and protein degradation level [8], we crossed APC8-YFP and CYCB1-GFP into arid1-1. CYCB1 is mainly present during early pollen developmental stages but not in mature pollen [8], [9]. Overall CYCB1 expression in arid1-1 was not altered, as assessed by qPCR (Figure 2B). However, the weak accumulation of CYCB1 in the generative cell of wild type bicellular pollen was undetectable in arid1-1 (Figure 2C, red arrows), although no apparent change of CYCB1 accumulation in the vegetative cell was seen in arid1-1 (Figure 2C, white arrows). However, the APC8-YFP level was not affected in arid1-1 (Figure S2C). Similarly, we detected no effect on the expression of other known genes implicated in sperm cell function (Figure S2D), including HTR10 [2], GEX2 [10] and GEX1 [29]. Taken together, these results suggest that ARID1 might promote DUO1 expression directly, but independently, of miR159.
Fig. 2. Reduced DUO1 and CYCB1 expression in arid1-1. 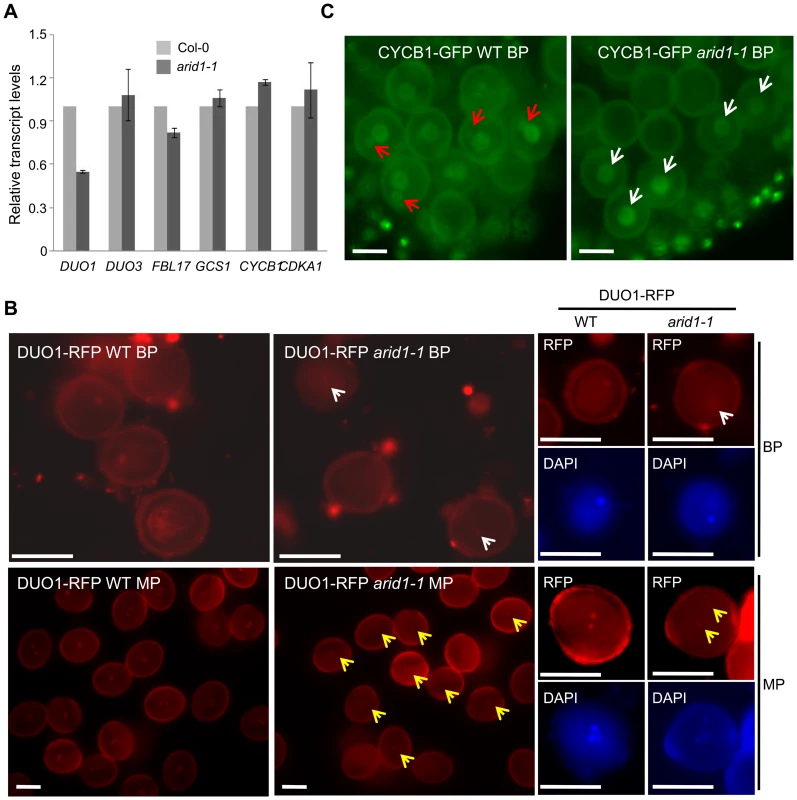
(A) Expression of sperm-specific genes in mature pollen of wild type and arid1-1. Error bars represent the SE from the mean of three biological replicates. (B) Expression of DUO1-RFP in wild type and arid1-1. Representative images for each genotype were acquired with the same exposure times. White and red arrows indicate reduced DUO1-RFP signal in bicellular pollen and mature pollen, respectively. Scale bar, 10 µm. (C) Expression of CYCB1-GFP in the bicellular pollen of wild type and arid1-1, respectively. The red arrows indicate visible GFP accumulation of CYCB1 in the generative nucleus of wild type pollen, and the white arrows indicate unchanged GFP signal in the vegetative nuclei of the arid1-1 pollen. Scale bar, 10 µm. ARID1 Binds to the DUO1 Promoter
To test how ARID1 affects DUO1 expression, we performed a ChIP assay to examine whether ARID1, as a transcription factor, binds DUO1 directly. We tested ARID1 occupancy at the genomic region of DUO1, including the ∼1.4 kb promoter region upstream of the ATG and the ∼300 bp UTR region downstream of the stop codon in ARID1-GFP transgenic plants (wild type plants as the negative control), using an antibody against GFP. We sub-divided the genomic region of DUO1 into ten subfragments of around 300 bp each (Figure 3A) and used EIF4A1 as a negative control. An obvious ARID1 occupancy over most of the DUO1 genomic region was detected in ARID1-GFP transgenic plants, with the peak of enrichment located between the ∼600–300 bp promoter region upstream of the ATG (Figure 3B, upper panel). In contrast, there was not much difference between wild type and ARID1-GFP plants for EIF4A1 enrichment (Figure 3B, upper panel), and enrichment from the no antibody control was negligible (Figure 3C, lower panel). To confirm the results from the ChIP assay, we performed an in vitro DNA binding assay. We expressed ARID1 with an in vitro transcription/translation system (Figure 3C, the band shown in the “input” lanes), and validated that ARID1 directly binds the DUO1 promoter region (Figure 3C). Therefore, both in vitro and in vivo evidence showed that ARID1 directly binds the DUO1 promoter. Together with the observation of reduced DUO1 expression in the arid1 mutant, we therefore conclude that ARID1 acts as an activator of DUO1, which is important for the initiation of the second mitosis for sperm cell formation.
Fig. 3. ARID1 binds to DUO1. 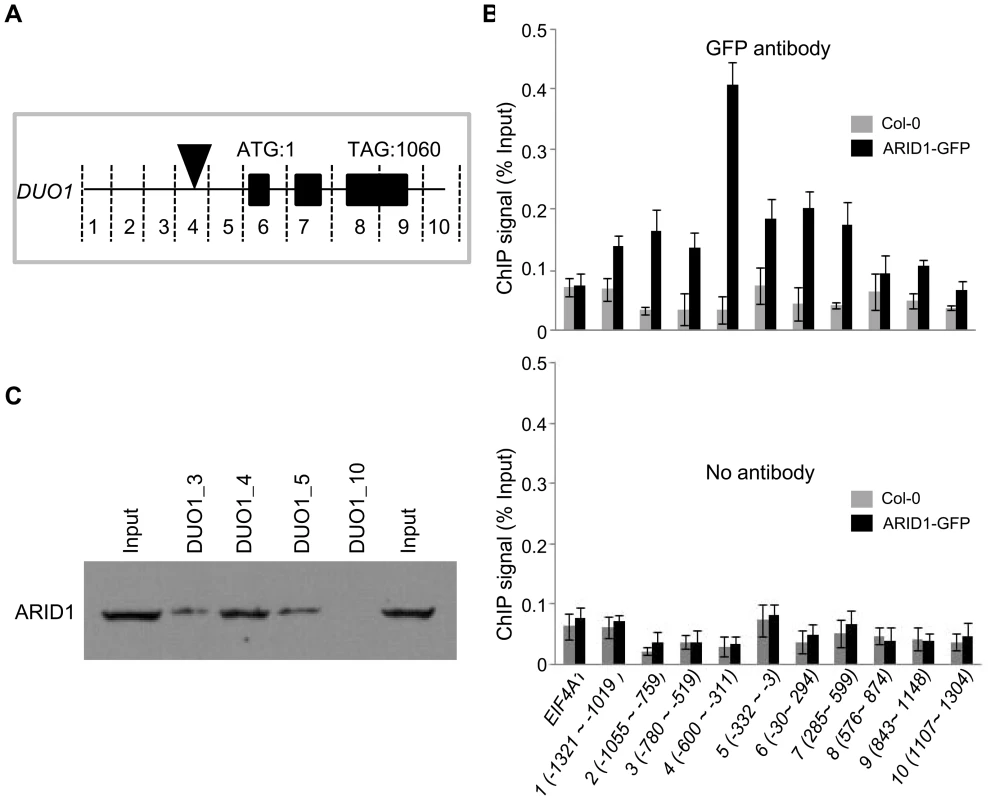
(A) Schematic of subfragments of DUO1 genomic DNA. The black rectangles represent exons. The black triangle indicates the region where ARID1 was most abundant. The position of the ATG was set to 1, and the fragments upstream or downstream were numbered; for example fragment “1” is −1321 to −1019 bp. (B) ChIP performed with wild type (gray bars) or ARID1-GFP (black bars) with GFP antibody (upper panel) and No antibody control (lower panel). EIF4A1 was used an internal negative control. The results were reproducible in two biological replicates. Error bars show SD calculated from three technical replicates. (C) DNA binding assay. Proteins were resolved by SDS-PAGE and then immunoblotted using anti-ARID1. The input lanes have 1/5 of the amount of ARID1 protein used in the DNA binding assay. The lanes numbered 3, 4, 5 and 10 (corresponding to regions mentioned in (A)) have 1/6 of the DNA-bound protein. ARID1 Locates in Nuclear Bodies and Is Developmentally Regulated during Sperm Cell Formation
Microarray analyses and our RT-PCR results indicated that ARID1 is pollen-specific. In order to substantiate the RT-PCR analyses, we constructed a promoter-reporter construct of proARID1:NLS-GFP. The GFP reporter was undetectable in root and shoot apical meristems but a weak GFP signal was detected in the vegetative nucleus in tricellular pollen (TP) (Figure S3A). Microarray analysis [26] indicated that ARID1 was more highly expressed (11 fold) in sperm than in pollen. Several genes with >7X higher expression in sperm than in pollen have been shown to be sperm-specific [2], [5], [10], [30]. We had therefore anticipated that ARID1 expression would be sperm-specific, so the ARID1 promoter:GFP reporter results were unexpected. We then examined the subcellular localization of pARID1:ARID1-GFP or -RFP fusion proteins in pollen. Microscopic analysis showed that ARID1-GFP localized to a single body in mature pollen (MP) (Figure S3B, white arrowheads). We occasionally (1%, n>500) observed a second ARID1-GFP body (Figure S3B, red arrowheads). To confirm the location of the ARID1-GFP body, we crossed HTR10-RFP, a sperm-specific marker [2] into ARID1-GFP transgenic plants. The ARID1-GFP body did not co-localize with HTR10 (Figure S3C, lower panel), but did co-localize with the vegetative nucleus in DAPI-stained mature pollen (Figure S3C, upper panel). We therefore concluded that ARID1-GFP was only present in the vegetative nucleus in mature pollen.
Considering that ARID1 can bind to the DUO1 promoter directly to promote DUO1 expression in both bicellular pollen and mature pollen (Figure 2, Figure 3, and Figure S2A), and DUO1 is specifically expressed from the early bicellular stage to mature pollen [3], we hypothesized that ARID1 might overlap with DUO1, in a cell type-specific pattern. We therefore examined the localization of ARID1 nuclear bodies at different developmental stages in ARID1-GFP and ARID1-RFP transgenic plants. To avoid interference due to the partial overlap of excitation wavelengths for DAPI and GFP, we mainly used ARID1-RFP transgenic plants for these observations. As in mature pollen (Figure S3B and S3C), the ARID1-RFP body was a single nuclear body in unicellular microspores (UM) (Figure 4A). Unexpectedly, increased numbers of nuclear bodies were observed in bicellular pollen (Figure S4). We categorized the ARID1-RFP nuclear bodies in bicellular pollen (BP) into four distinct patterns with similar incidences (Figure 4B): all foci within the vegetative nucleus; multiple foci in both the vegetative nucleus and the generative nucleus; several foci in the vegetative nucleus but a single dot in the generative nucleus; and much larger foci in the vegetative nucleus but a less intense single dot in the generative nucleus (yellow arrows indicate signals in the generative cell). Additional representative examples are shown in Figure S4C. Although the overall signal intensity in vegetative nuclei was much stronger than that in the generative cell, these images show that ARID1-RFP was in both the vegetative cell and generative cell at the bicellular stage, which is obviously different from the distribution in UM and MP. In TP, three patterns of ARID1-RFP bodies were identified (Figure 4C): most pollen (>60%) had a single ARID1-RFP body, as in mature pollen, but the rest had multiple foci in the vegetative nucleus or a single weakly fluorescent ARID1-RFP body in sperm nuclei (yellow arrows). Only a single nuclear body in the vegetative nucleus was observed in mature pollen (Figure S3B, S3C, and MP, Figure 4D). To further substantiate the biological significance of the presence of ARID1 in the generative cell, we constructed transgenic plants with ARID1 driven by LAT52 (LAT52:ARID1), a vegetative cell-specific promoter, and HTR10 (HTR10:ARID1), a generative cell and sperm cell-specific promoter, respectively. We observed reduced seed set (Figure S5A) and increased DUO1 expression (Figure S5B) in >10 independent T1 HTR10:ARID1 plants but not in LAT52:ARID1 plants, indicating that accumulation of ARID1 in the generative cell is biologically relevant during pollen development. Taken together, these data indicate that the subcellular distribution of ARID1-RFP nuclear bodies is variable during pollen development. The transient localization of ARID1-RFP in the generative nucleus is consistent with the idea that ARID1 might be required for initiation or progression of the second mitosis, by promoting DUO1 activation.
Fig. 4. ARID1 localization is developmentally dynamic during sperm cell formation. 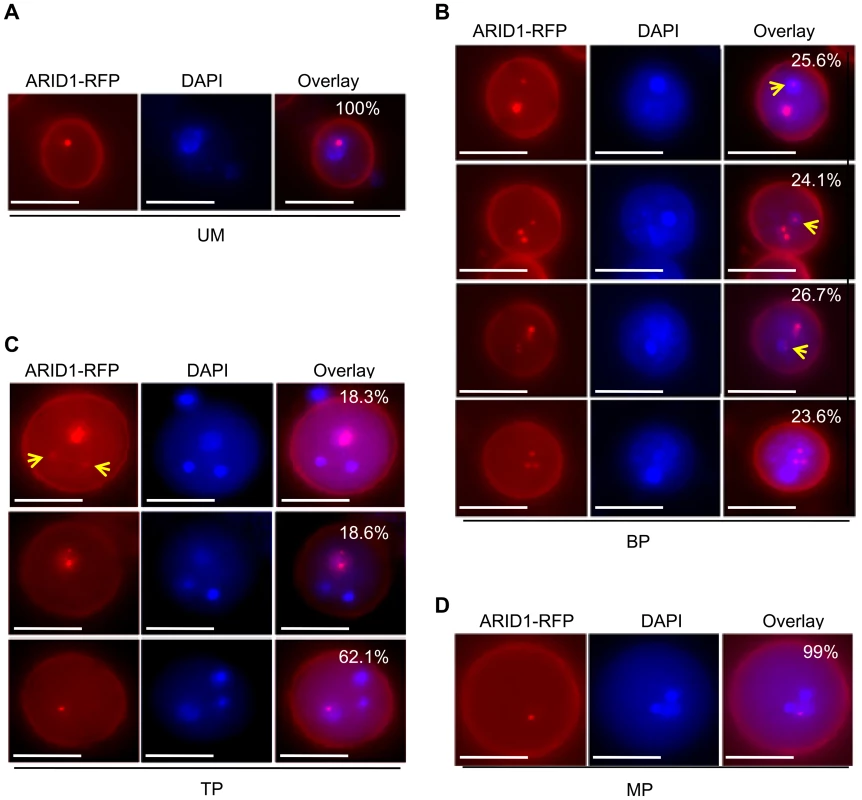
Representative images of unicellular microspores (UM) (A), bicellular pollen (BP) (B), tricellular pollen (TP) (C), and mature pollen (MP) (D) from ARID1-RFP transgenic plants. Yellow arrows indicate RFP signals in the generative nuclei and sperm cells. The percentages indicate incidence of each pattern. 600–800 pollen grains were analyzed for each stage. Scale bar, 10 µm. ARID1 Interacts with Histone Deacetylase 8
A human ELM2 domain-containing protein, MI-ER, directly interacts with Histone Deacetylase 1 (HDAC1) [27], [28]. To determine whether ARID1 might be associated with plant histone deacetylase complexes, we performed yeast two hybrid experiments. We detected an interaction (Figure 5A) between ARID1 and HDA8 (Histone Deacetylase 8), which is highly expressed in the vegetative nucleus but not in the generative nucleus or sperm cell nuclei (Figure S6A). Moreover, we found that the ELM2 domain of ARID1 was important for the interaction with HDA8 (Figure 5A), as was shown for an ELM2 protein with a histone deacetylase in human cells [28]. To confirm the yeast results, a recombinant HDA8-GST protein (Figure 5C) was used for pulldown assays, with GST as a negative control. ARID1-GFP was pulled down by HDA8-GST but not by GST (Figure 5B). To confirm this association in vivo, we performed Co-IP experiments and showed that HDA8-YFP was co-immunoprecipitated with antibodies that recognize the ARID1-Myc fusion protein (Figure 5D).
Fig. 5. ARID1 physically associates with Histone Deacetylase 8 (HDA8). 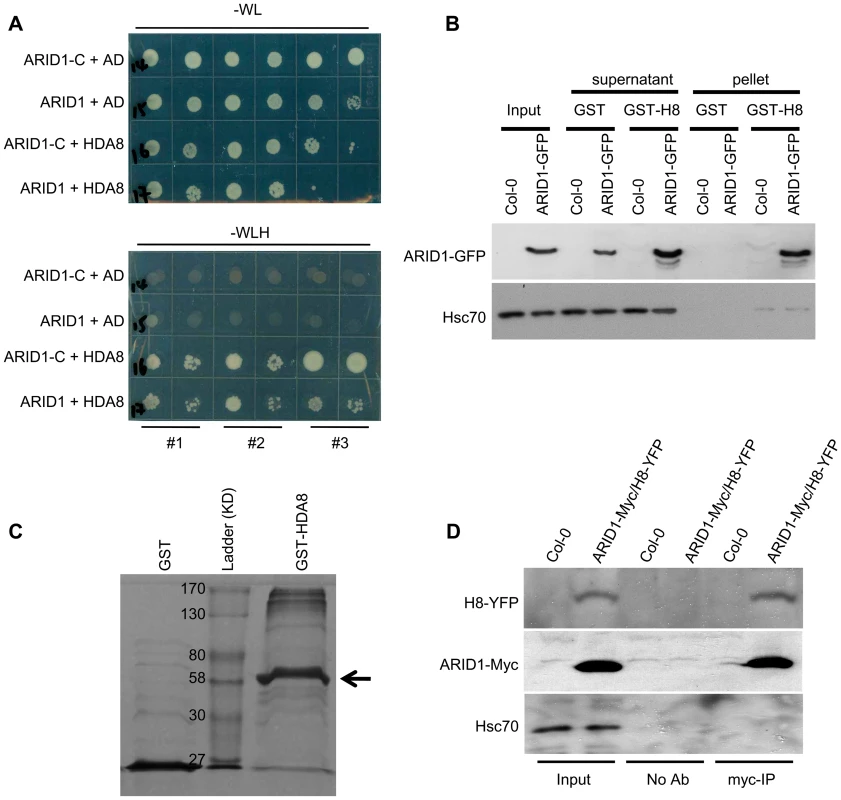
(A) Yeast cells co-expressing the indicated plasmids were grown on control (without Tryptophan and Leucine) or high stringency selection (without Tryptophan, Leucine, Histidine, and with 10 mM 3-AT) medium. ARID1, full length cDNA of ARID1; ARID1-C, the C-terminus-containing ELM2 domain of ARID1; HDA8, full length cDNA of HDA8. AD is pGAD10. The cultures from each of the indicated strains were diluted 100-fold and spotted. Three colonies were streaked for each pair. (B) ARID1 interacts with HDA8 by GST pulldown assay. Whole cell extracts from wild type or ARID1-GFP plants were applied onto GST and GST-HDA8 (abbreviated GST-H8) beads. GFP and Hsc70 (loading control) antibodies were used in immunoblotting. (C) Stained protein gel showing proteins used for the GST pulldown assay. 1/30 of the amount of each protein used in Figure 5A was resolved by SDS-PAGE. GST-H8, full length HDA8 fused to GST; GST alone was used as a control. GST-H8 is marked with an arrowhead. (D) ARID1 interacts with HDA8 by Co-IP. Inflorescences from ARID1-Myc; HDA8-YFP (abbreviated H8-YFP) doubly transgenic plants or from wild type were immunoprecipitated with Myc antibody. Myc, GFP and Hsc70 (loading control) antibodies were used for immunoblotting. Since ARID1 interacts with HDA8, we predicted that the in vivo histone acetylation level might be altered in the arid1-1 mutant. We therefore performed immunofluorescence with antibodies specific to H3K9 acetylation. In wild type pollen, the signal was only detected in the two sperm nuclei (Figure 6A, upper panel), but in the arid1-1 mutant, the immunofluorescence signal was also detected in the vegetative nucleus (Figure 6A, lower panel). The immunofluorescence signal with the Histone 3 antibody, used as a control, showed no difference between wild type and arid1-1 (Figure 6A). These results indicate that ARID1 is required to restrict histone acetylation to sperm cells.
Fig. 6. Altered histone acetylation in arid1-1. 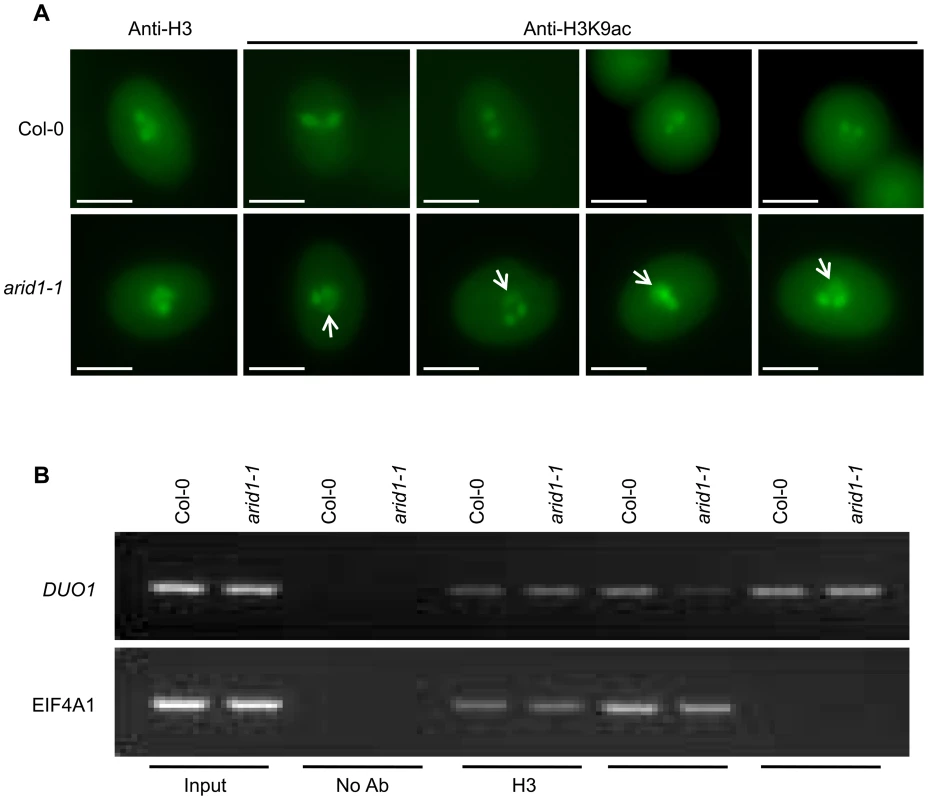
(A) Expanded pattern of histone acetylation signal in arid1-1 by immunofluorescence analysis. Mature pollen from wild type and arid1-1 plants was incubated with anti-H3 (control) and anti-H3K9ac antibodies. Representative examples for arid1-1 show histone acetylation in the vegetative nucleus (indicated by white arrowheads). 50–100 pollen grains for each genotype were examined. (B) Reduced histone acetylation at the DUO1 promoter in the arid1-1 mutant by ChIP. Inflorescences from wild type and arid1-1 were used for a ChIP assay with anti-H3, anti-H3K9ac, and anti-H3K4me3 antibodies. ChIP-DNA was used for PCR by amplifying 35 cycles for both EIF4A1 (negative control) and the DUO1_3 fragment, which bound ARID1. Similar results were obtained from three independent biological replicates; the results shown are from one replicate. For genes encoding proteins, increased expression is usually accompanied with increased active marks in euchromatin, that is histone H3 lysine 9 acetylation (H3K9Ac) and histone H3 lysine 4 trimethylation (H3K4me3). Antibodies to a constitutive histone (H3) reacted similarly with the control gene EIF4A1 and with DUO1, in both wild type and the arid1 mutant (Figure 6B). However, in the arid1 mutant the H3K9Ac level was obviously reduced at DUO1 but not at EIF4A1 (Figure 6B). Furthermore, in the arid1 mutant there was no detectable difference in the level of H3K4me3 at DUO1 or EIF4A1 (Figure 6B), suggesting that it is specifically histone acetylation that contributes to the reduced DUO1 expression. In addition, we detected slight de-repression of transposable elements (TEs) in the arid1-1 mutant (Figure S6B), accompanied with increased histone acetylation at these loci (Figure S6C), indicating that ARID1-mediated histone deacetylation activity also contributes to the silencing of TEs.
Discussion
Sperm cell formation is regulated by several genes [31]. Among these genes, DUO1 plays a key role in the initiation of the second mitosis and acts as a switch to turn on the expression of other germline-related genes [9]. No positive element has been reported to regulate DUO1 expression but, in addition to the negative regulation mediated by miR159 [12], a putative repressive GRSF (Germline-Restrictive Silencing Factor) binding site was noted in the DUO1 promoter [32]. However, mutagenesis of the putative GRSF binding site did not affect germline-specific expression of DUO1 [9] and the ∼150 bp proximal DUO1 promoter sequences that excluded the putative GRSF binding site were sufficient for germline-specific expression [9]. These results suggest that activation of the DUO1 promoter may depend on transcription factors that bind to the proximal region of the promoter and that are inherited and/or segregated during asymmetric division of the microspore.
Here we provide evidence for such a positive transcription factor, ARID1. ChIP analysis and a DNA binding assay showed that ARID1 directly binds to the ∼300–600 bp promoter region adjacent but distal to the 150 bp proximal region of the promoter (Figure 3). We surmise that the discrepancy from the observations in the previous study was based on whether or not the expression driven by the 150 bp proximal promoter was sperm cell-specific [9], but ignored the difference in expression level in the generative cell between the intact promoter and the 150 bp proximal promoter. Thus we suggest that ARID1 binding to the DUO1 promoter facilitates the activation of DUO1. To support the biological significance of this binding, we showed that disruption of ARID1 resulted in reduced DUO1 expression in germline cells (Figure 2). Although we did not observe decreased expression of three DUO1 direct targets (HTR10, GCS1, and GEX2), DUO1-mediated CYCB1 accumulation in the generative cell was affected in the arid1 mutant (Figure 2C). The unaffected expression of GCS1 and GEX2 in the arid1 mutant might be explained by redundancy with DUO3, since it also promotes expression of GCS1 and GEX2, but not of CYCB1 [7], and the expression of DUO3 was not affected in arid1 (Figure 2A). Together with the normal sperm cell formation in htr10, possibly due to redundancy with other HTR members, we suggest that defective sperm cell formation in arid1 results only from the disrupted function of the DUO1-CYCB1 module in germ cell division and not the function of DUO1-GCS1/GEX2 in germ cell specification, both of which contribute to severely defective sperm cell formation in duo1. Furthermore, that only the DUO1-CYCB1 module was disrupted, and not the DUO1-GCS1/GEX2/HTR10 module, possibly explains the much weaker phenotype in arid1-1, since specification of germ cells in arid1-1 might be maintained by the remnant DUO1 and/or DUO3-mediated activation of GCS1/GEX2/HTR10. We presume that DUO1-CYCB1-mediated generative cell division is prerequisite for sperm cell formation, and that unaffected DUO3 should partially suppress the phenotype of single sperm cell-like pollen in arid1, if DUO1 and/or DUO3-GCS1/GEX2/HTR10-mediated germ cell specification is parallel with DUO1-CYCB1-mediated germ cell division.
In addition, unlike the DUO1 expression pattern, ARID1 is initially expressed in the microspore nucleus and subsequently expanded or segregated into the generative nucleus during the first asymmetric division (Figure 4 and Figure S4), indicating that DUO1 activation is an active process mediated by ARID1 from the vegetative cell, and not passively accomplished due to the completion of the first asymmetric division. We propose a model (Figure 7) to explain how DUO1 could be coordinately and sequentially regulated by the negative regulator miR159 and by the positive regulator ARID1. In the vegetative cell, MIR159 is transcribed abundantly during the unicellular stage, and so might play a major role in blocking DUO1 expression. As pollen development proceeds, in spite of the gradually decreasing but still detectable repressive role of miR159 in bicellular pollen, ARID1, inherited from the microspore, partitions into the generative cell to bind to DUO1 and gradually promote DUO1 activation. We hypothesize that other factors together with AIRD1 are potentially involved in DUO1 activation, as duo1 is 100% penetrant and because the generative cell fails to divide in all duo1 pollen grains. In contrast, disruption of ARID1 only caused partial defects in sperm cell formation. Due to the absence of DUO1 accumulation in the vegetative nucleus of arid1-1, we hypothesize that unknown factors (other than ARID1-associated histone modification machinery) might take over the major role of miR159 restricting DUO1 expression in the vegetative cell nucleus in the weak arid1-1 mutant (Figure 2). In parallel, ARID1 might promote sperm cell formation by altering the epigenetic status in both the vegetative cell and the generative cell; ARID1 physically associates with histone deacetylases, which could affect expression of the unknown gene(s) involved in sperm cell formation. Our data showed that ARID1 is necessary for the balance of histone acetylation between the vegetative cell and sperm cells of mature pollen (Figure 6A), indicating that this characteristic could be carried over from bicellular pollen. Moreover, a recent study showed that increased histone acetylation in cultured microspores led to the switch from gametophytic division to sporophytic division [33], further indicating that histone acetylation is critical for cell cycle progression during sperm cell formation.
Fig. 7. Model for ARID1 function during sperm cell formation in Arabidopsis. 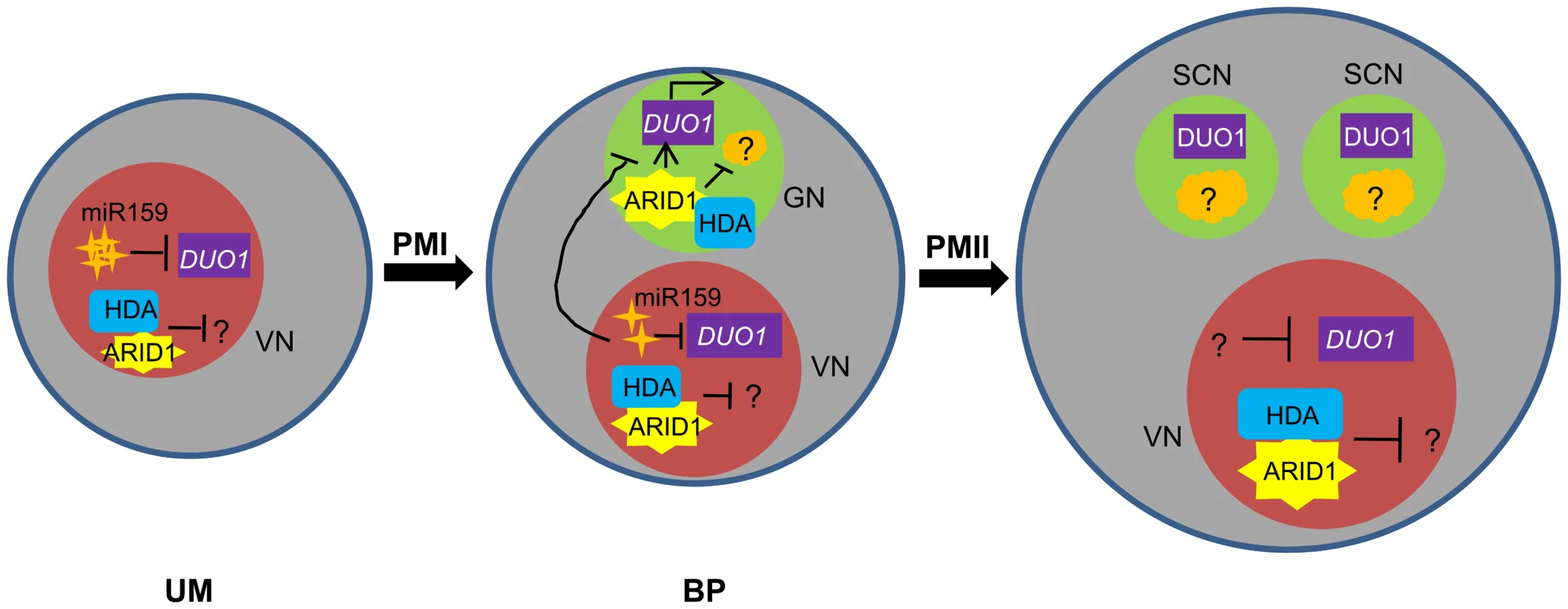
miR159 plays a major role in restricting DUO1 expression in the vegetative cell. As pollen development proceeds, miR159 abundance is gradually decreased and ARID1 expands its expression into the generative cell, possibly by responding to the decreased repressive role of miR159 in bicellular pollen. ARID1 then promotes DUO1 activation by directly binding to the DUO1 promoter, and thereby facilitates the initiation of sperm cell formation. On the other hand, ARID1 might repress expression of unknown negative regulators (orange) of cell cycle progression, by altering the epigenetic status of the generative cell. Once sperm cells are formed, ARID1 gradually decreases until it is eliminated from germline cells, so that DUO1 is steadily present and negative regulators possibly related to germline function start to be active, due to dissociation of ARID1 and histone deacetylase. Thus we hypothesize that the alteration of epigenetic status during sperm cell formation is correlated with a change in the subcellular localization of ARID1, which could facilitate cell cycle progression of the two consecutive mitoses. VN, vegetative nucleus; GN, generative cell; SC, sperm cells. Given the permanent presence of ARID1 in the vegetative cell and its short stay in the germline cell (Figure 4), we speculate that ARID1 might carry information that strengthens communication between two distinct cell types, facilitating initiation of the second mitosis or the process of sperm cell formation, by acting on related genes, in addition to the key regulator, DUO1. Our results indicated that ARID1-mediated DUO1 activation is important for sperm cell formation. It will be interesting to discover whether ARID1 regulates other genes and more broadly to deduce the functions of other plant ARID proteins, in order to understand the role of the conserved ARID domain. In animals, ARID proteins have been implicated in a variety of biological processes including embryonic development, cell lineage, gene regulation and cell cycle control. ARID1 is perhaps analogous to ARID4A and ARID4B in animals, which associate with the histone deacetylase complex [16],[17] and are involved in male fertility control by acting in the Retinoblastoma (RB) pathway [15]. ARID4A is a RB-binding protein, and it is well established that the RB pathway controls cell cycle progression in a variety of organisms [34], including plants [35], [36]. A plant homologue of RB, RBR (Rb-related), plays a pivotal role in male gametophyte patterning by regulating cell division and cell fate [37], as both the vegetative cell and generative cell over-proliferated in rbr mutants [37]. As this phenotype was not seen in the arid1 mutant or in arid1 antisense plants and as ARID1 does not contain a pRB-binding motif, LXCXE [16], it is unlikely that ARID1 binds RBR. However, there are 10 ARID proteins in Arabidopsis [24]and several other ARIDs are expressed in pollen [26], [38], so it is possible that other ARIDs are involved in the RB pathway to regulate sperm cell formation.
In addition to its transient presence in the germline cell, ARID1 is in the vegetative nucleus from the microspore to mature pollen stage (Figure 4). What does ARID1 do in the vegetative cell? There are two possibilities. First, ARID1, as a transcription factor, might regulate expression of unknown genes in the vegetative cell. This regulation perhaps includes transcriptional activation, such as for DUO1, and transcriptional repression. Together with the association of ARID1 with histone modification machinery, we suggest that ARID1 is required to regulate the chromatin environment around DUO1 so that it can achieve its maximal/optimal expression. Second, ARID1 in the vegetative cell might be required for maintaining the level of transposable element (TE) de-repression, as de-repression of many TEs occurs in the vegetative cell [39], although the biological significance of TE de-repression remains unclear. We demonstrated greater TE de-repression in arid1-1 pollen (Figure S6B), which was accompanied with increased histone acetylation at those de-repressed TE loci (Figure S6C). Therefore, we propose that the function of ARID1 in the vegetative nucleus is to modulate the overt de-repression of TEs by association with the histone modification machinery. A dual role for ARIDs, either as transcriptional activators or transcriptional repressors, has been reported in animals [21], [23]. Moreover, the ARID1 nuclear body (Figure 4) might be a processing center for ARID1-mediated histone modifications. We showed [40] that Cajal bodies, which are processing centers for RNA-directed DNA Methylation (RdDM), were similarly developmentally variable during sperm formation
Materials and Methods
Plant Materials
The arid1-1 T-DNA insertion mutant (SALK_047099) was obtained from the ABRC (www.arabidopsis.org). Seeds of HTR10-RFP and DUO1-RFP were kindly provided by Fred Berger and David Twell, respectively.
Plasmid Construction
For the construction of ARID1-GFP, ARID1-RFP, and ARID1-Myc plasmids, ARID1 was amplified from wild type (Columbia-0) genomic DNA with the primer pair ARID1F1 and ARIDR1, cloned into pENTR-D/TOPO, and then transferred into the plant expression destination vector pMDC107 to construct ARID1-GFP, into pMDC163 (GUS was replaced by a mRFP fusion tag) to construct ARID1-RFP, and into pEarleyGate303 to construct ARID1-Myc. For ARID1 promoter analysis, a 1.5-kb fragment upstream of the ATG was amplified using the primer pair ARID1F1/R4 and was subcloned into pENTR-D/TOPO and then transferred into the plant expression vector pGII-NLS3XGFP. For constructing the antisense ARID1 plasmid, cDNA was obtained using the primer pair ARID1F5/R5, cloned into pENTR-D/TOPO, then transferred into the plant expression vector pB7WG2 (digested with SacI and SpeI to replace the 35S promoter with the native ARID1 promoter and then digested with KpnI and ApaI to insert the LAT52-GFP cassette). To construct the LAT52:ARID1 and HTR10:ARID1 plasmids, cDNA was obtained using the primer pair ARID1F6/R1, cloned into pENTR-D/TOPO, then transferred into the plant expression vector pB7WG2 (digested with SacI and SpeI to replace the 35S promoter with the LAT52 promoter or the HTR10 promoter, respectively, then digested with KpnI and ApaI to insert the LAT52-GFP cassette). To construct ARID1 for the in vitro transcription and translation system, we inserted ARID1 full length cDNA, amplified with the primer pairs ARID1F2/R6 into the pCMVTNT vector (Promega). For yeast two hybrid experiments, cDNAs of ARID1 and ARID1-C were amplified from pollen RNA using RT-PCR and the primer pairs ARID1F2/R2 and ARID1F4/R2, then cloned into the pGBT9 yeast expression vector; the cDNA of HDA8 was amplified using the primer pair HDA8F1/R1, then cloned into the pGAD10 yeast expression vector. For GST pulldown experiments, the cDNA of HDA8 was amplified using primers HDA8F1/R1 and cloned into the pGEX-2TK vector. For the HDA8-YFP construct, HDA8 was amplified from wild type genomic DNA with the primer pair HDA8F2/R2, cloned into pENTR-D/TOPO, and then transferred into the plant expression destination vector pGWB40. Primer sequences are listed in Table S1.
Yeast-Two-Hybrid Experiments
The yeast two hybrid assays were performed according to the protocol available on the Clontech website, using strain AH109. Yeast transformation was performed using yeast transformation buffer (0.1 M LiAc, 40% PEG3350 in TE). Transformants were plated and selected on synthetic complete medium that lacked the specified amino acids. Positive colonies were inoculated and spotted with a 100-fold dilution onto synthetic complete medium lacking leucine, tryptophan, and histidine and containing 10 mM 3-amino-1,2,4-triazole. The plates were incubated for 2–4 d at 28°C for interaction analysis.
ChIP
ChIP was performed according to [8]. ARID1 occupancy at the DUO1 genomic region was determined by ChIP using a GFP antibody (Cat. 632460, Clontech, 1∶200) and inflorescences from wild type and ARID1-GFP transgenic plants, respectively. The occupancy of histone modification marks at DUO1 was determined by ChIP using Histone 3 antibody (Cat.06-755, Upstate, 1∶50), Histone Lysine 9 acetylation antibody (Cat. 17-10241, Upstate, 1∶200), and Histone Lysine 4 trimethylation antibody (Cat.ab8580, abCam, 1∶200) and inflorescences from wild type and the arid1-1 mutant, respectively. DNA present in the immunoprecipitates was quantified by qPCR or PCR relative to total input DNA. The results shown were consistent in two biological replicates. The primer sets used for the PCR are listed in Table S1.
DNA Binding Assays
DNA binding assays were performed as described [41] with the following modifications. Briefly, biotinylated DNA fragments corresponding to 3, 4, 5, and 10 (Figure 3) were generated by PCR using primer pairs DUO1_F3/R3, DUO1_F4/R4, DUO1_F5/R5, and DUO1_F10/R10, with labeling by 5′biotin at F3, F4, F5, and F10, respectively. Then 100 pmol of the biotinylated DNA fragments were incubated with 50 ul prewashed Streptavidin Agoraose Resin (Thermo, Cat.20349) in IP100 buffer (100 mM potassium glutamate, 50 mM Tris-HCl pH 7.6, 2 mM MgCl2, 0.5% NP40) for 2 h at room temperature with slight rotation, and washed five times in IP100 buffer. In parallel, ARID1 was subjected to TNT T7 in vitro transcription/translation with the TNT Coupled Wheat Germ Extract System (Promega, Cat.L4140). 25 ul freshly translated ARID1 protein was added to DNA-bound beads in the IP buffer plus complete protease inhibitor cocktail, and the mixture was rotated at 4°C for 2 h. Beads were washed eight times with IP100 buffer, then proteins were stripped off the beads by boiling with 2XSDS buffer and then subjected to SDS-PAGE. The ARID1 protein bound by biotinylated DNA was detected by immunoblotting with a 1∶200 dilution of anti-ARID1 (The peptide “SMVADEDAVDYSKT” was conjugated to KLH and used to raise rabbit polyclonal antibodies (GL Biochem)).
GST Pulldown
The pulldown assay was carried out as described previously [42]. GST and GST-HDA8 were expressed in E. coli BL21. Cells were disrupted by sonication and the proteins were purified by glutathione Sepharose 4B affinity chromatography. 600 µl of protein extract from inflorescences of ARID1-GFP plants was applied to the beads-protein mixture (30 µg total protein) and incubated for 2 h at 4°C on a rotating wheel. The beads were washed 5 times with IP lysis buffer. The bound (pellet) and unbound (supernatant) proteins were detected by immunoblotting with anti-GFP (Cat.632480, Clontech, 1∶2000 dilution) and anti-Hsc70 (Cat.SPA-818, Stressgen, 1∶10000 dilution) antibodies. 1/6 of the pellet fractions, and 1% and 0.1% of the supernatant fractions for anti-GFP and anti-Hsc70 IPs were used, respectively.
Co-Immunoprecipitation
The immunoprecipitation assay was carried out as described [42]. One gram of inflorescences from wild type or ARID1-Myc; HDA8-YFP doubly transgenic plants were ground in liquid nitrogen and homogenized in 2 ml of protein lysis buffer (50 mM Tris–HCl at pH 7.5, 150 mM NaCl, 0.2% NP-40, 2 mM DTT, 10% glycerol, complete protease inhibitor cocktail). The lysates were incubated at 4°C for 50 min on a rotating wheel and centrifuged at 16000 g to pellet debris, and then supernatants were pre-cleared for 20 min with protein G agarose beads. Equivalent lysate was mixed with Myc antibodies (Cat. SC-70463, Santa Cruz, 1∶200) pre-coupled to protein G agarose beads or to beads alone, respectively. After incubation for 2 h at 4°C, the immune complexes were washed with lysis buffer. Proteins from 1/6 of the “No Ab” (no antibody) IP and 1/6 of the anti-myc IP were analyzed by immunoblotting using anti-Myc, anti-GFP, and anti-Hsc70. Proteins from 1/100 of the input were used for the anti-Myc and anti-GFP blots, while proteins from 1/1000 of the input were used for the anti-Hsc70 blot.
Immunofluorescence Assay
Inflorescences were fixed for 30 minutes in methanol∶acetone (4∶1, v/v) at room temperature, and anthers were dissected to release tricellular pollen onto a slide covered with liquid pollen germination medium [43]. The slides were allowed to dry for about 20 min at room temperature and then were covered with a thin layer of agarose/gelatin/sucrose (0.94% low melting agarose/0.84% gelatin/0.3% w/v sucrose) for 10 minutes at 37°C. The slides were soaked in blocking buffer (PBS with 5% BSA) for 1 hour at 37°C, and then incubated with H3 antibody (1∶50 dilution, Cat. 06-755, Upstate) or H3K9ac antibody (1∶200 dilution, Cat. 1710241, Upstate), respectively, overnight at 4°C in a dark moist chamber. After washed with blocking buffer, pollen was incubated in blocking buffer containing Alexa Fluor 488 goat anti-rabbit antibody (1∶200 dilution, Cat.711-545-152, Jackson) for 6 h at room temperature. Slides were washed in PBS for five times, and observed with an Axiovert microscope under the GFP channel. Images were acquired using an AxioCamRM camera and AxioVision 4.8.1 software and processed using Adobe Photoshop CS2 (Adobe).
Supporting Information
Zdroje
1. McCormickS (2004) Control of male gametophyte development. Plant Cell 16 Suppl: S142–153.
2. OkadaT, EndoM, SinghMB, BhallaPL (2005) Analysis of the histone H3 gene family in Arabidopsis and identification of the male-gamete-specific variant AtMGH3. Plant J 44 : 557–568.
3. RotmanN, DurbarryA, WardleA, YangWC, ChaboudA, et al. (2005) A novel class of MYB factors controls sperm-cell formation in plants. Curr Biol 15 : 244–248.
4. IwakawaH, ShinmyoA, SekineM (2006) Arabidopsis CDKA;1, a cdc2 homologue, controls proliferation of generative cells in male gametogenesis. Plant J 45 : 819–831.
5. MoriT, KuroiwaH, HigashiyamaT, KuroiwaT (2006) GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol 8 : 64–71.
6. KimHJ, OhSA, BrownfieldL, HongSH, RyuH, et al. (2008) Control of plant germline proliferation by SCF(FBL17) degradation of cell cycle inhibitors. Nature 455 : 1134–1137.
7. BrownfieldL, HafidhS, DurbarryA, KhatabH, SidorovaA, et al. (2009) Arabidopsis DUO POLLEN3 is a key regulator of male germline development and embryogenesis. Plant Cell 21 : 1940–1956.
8. ZhengB, ChenX, McCormickS (2011) The anaphase-promoting complex is a dual integrator that regulates both MicroRNA-mediated transcriptional regulation of cyclin B1 and degradation of Cyclin B1 during Arabidopsis male gametophyte development. Plant Cell 23 : 1033–1046.
9. BrownfieldL, HafidhS, BorgM, SidorovaA, MoriT, et al. (2009) A plant germline-specific integrator of sperm specification and cell cycle progression. PLoS Genet 5: e1000430.
10. EngelML, Holmes-DavisR, McCormickS (2005) Green sperm. Identification of male gamete promoters in Arabidopsis. Plant Physiol 138 : 2124–2133.
11. BorgM, BrownfieldL, KhatabH, SidorovaA, LingayaM, et al. (2011) The R2R3 MYB transcription factor DUO1 activates a male germline-specific regulon essential for sperm cell differentiation in Arabidopsis. Plant Cell 23 : 534–549.
12. PalatnikJF, WollmannH, SchommerC, SchwabR, BoisbouvierJ, et al. (2007) Sequence and expression differences underlie functional specialization of Arabidopsis microRNAs miR159 and miR319. Dev Cell 13 : 115–125.
13. WilskerD, ProbstL, WainHM, MaltaisL, TuckerPW, et al. (2005) Nomenclature of the ARID family of DNA-binding proteins. Genomics 86 : 242–251.
14. OkadaY, ScottG, RayMK, MishinaY, ZhangY (2007) Histone demethylase JHDM2A is critical for Tnp1 and Prm1 transcription and spermatogenesis. Nature 450 : 119–123.
15. WuRC, JiangM, BeaudetAL, WuMY (2013) ARID4A and ARID4B regulate male fertility, a functional link to the AR and RB pathways. Proc Natl Acad Sci U S A 110 : 4616–4621.
16. LaiA, KennedyBK, BarbieDA, BertosNR, YangXJ, et al. (2001) RBP1 recruits the mSIN3-histone deacetylase complex to the pocket of retinoblastoma tumor suppressor family proteins found in limited discrete regions of the nucleus at growth arrest. Mol Cell Biol 21 : 2918–2932.
17. FleischerTC, YunUJ, AyerDE (2003) Identification and characterization of three new components of the mSin3A corepressor complex. Mol Cell Biol 23 : 3456–3467.
18. TakeuchiT, WatanabeY, Takano-ShimizuT, KondoS (2006) Roles of jumonji and jumonji family genes in chromatin regulation and development. Dev Dyn 235 : 2449–2459.
19. TakahashiM, KojimaM, NakajimaK, Suzuki-MigishimaR, TakeuchiT (2007) Functions of a jumonji-cyclin D1 pathway in the coordination of cell cycle exit and migration during neurogenesis in the mouse hindbrain. Dev Biol 303 : 549–560.
20. PengJC, ValouevA, SwigutT, ZhangJ, ZhaoY, et al. (2009) Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell 139 : 1290–1302.
21. ShenX, KimW, FujiwaraY, SimonMD, LiuY, et al. (2009) Jumonji modulates polycomb activity and self-renewal versus differentiation of stem cells. Cell 139 : 1303–1314.
22. PasiniD, CloosPA, WalfridssonJ, OlssonL, BukowskiJP, et al. (2010) JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature 464 : 306–310.
23. LiG, MargueronR, KuM, ChambonP, BernsteinBE, et al. (2010) Jarid2 and PRC2, partners in regulating gene expression. Genes Dev 24 : 368–380.
24. ZhuH, ChenT, ZhuM, FangQ, KangH, et al. (2008) A novel ARID DNA-binding protein interacts with SymRK and is expressed during early nodule development in Lotus japonicus. Plant Physiol 148 : 337–347.
25. HonysD, TwellD (2004) Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 5: R85.
26. BorgesF, GomesG, GardnerR, MorenoN, McCormickS, et al. (2008) Comparative transcriptomics of Arabidopsis sperm cells. Plant Physiol 148 : 1168–1181.
27. DingZ, GillespieLL, PaternoGD (2003) Human MI-ER1 alpha and beta function as transcriptional repressors by recruitment of histone deacetylase 1 to their conserved ELM2 domain. Mol Cell Biol 23 : 250–258.
28. WangL, CharrouxB, KerridgeS, TsaiCC (2008) Atrophin recruits HDAC1/2 and G9a to modify histone H3K9 and to determine cell fates. EMBO Rep 9 : 555–562.
29. Alandete-SaezM, RonM, LeiboffS, McCormickS (2011) Arabidopsis thaliana GEX1 has dual functions in gametophyte development and early embryogenesis. Plant J 68 : 620–632.
30. RonM, Alandete SaezM, Eshed WilliamsL, FletcherJC, McCormickS (2010) Proper regulation of a sperm-specific cis-nat-siRNA is essential for double fertilization in Arabidopsis. Genes Dev 24 : 1010–1021.
31. BorgM, TwellD (2010) Life after meiosis: patterning the angiosperm male gametophyte. Biochem Soc Trans 38 : 577–582.
32. HaerizadehF, SinghMB, BhallaPL (2006) Transcriptional repression distinguishes somatic from germ cell lineages in a plant. Science 313 : 496–499.
33. LiH, SorianoM, CordewenerJ, MuinoJM, RiksenT, et al. (2014) The histone deacetylase inhibitor trichostatin a promotes totipotency in the male gametophyte. Plant Cell 26 : 195–209.
34. HarbourJW, DeanDC (2000) The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev 14 : 2393–2409.
35. WildwaterM, CampilhoA, Perez-PerezJM, HeidstraR, BlilouI, et al. (2005) The RETINOBLASTOMA-RELATED gene regulates stem cell maintenance in Arabidopsis roots. Cell 123 : 1337–1349.
36. EbelC, MaricontiL, GruissemW (2004) Plant retinoblastoma homologues control nuclear proliferation in the female gametophyte. Nature 429 : 776–780.
37. ChenZ, HafidhS, PohSH, TwellD, BergerF (2009) Proliferation and cell fate establishment during Arabidopsis male gametogenesis depends on the Retinoblastoma protein. Proc Natl Acad Sci U S A 106 : 7257–7262.
38. LoraineAE, McCormickS, EstradaA, PatelK, QinP (2013) RNA-seq of Arabidopsis pollen uncovers novel transcription and alternative splicing. Plant Physiol 162 : 1092–1109.
39. SlotkinRK, VaughnM, BorgesF, TanurdzicM, BeckerJD, et al. (2009) Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136 : 461–472.
40. ScarpinR, SigautL, PietrasantaL, McCormickS, ZhengB, et al. (2013) Cajal Bodies Are Developmentally Regulated during Pollen Development and Pollen Tube Growth in Arabidopsis thaliana. Mol Plant 6 : 1355–1357.
41. VertG, ChoryJ (2006) Downstream nuclear events in brassinosteroid signalling. Nature 441 : 96–100.
42. LiCF, PontesO, El-ShamiM, HendersonIR, BernatavichuteYV, et al. (2006) An ARGONAUTE4-containing nuclear processing center colocalized with Cajal bodies in Arabidopsis thaliana. Cell 126 : 93–106.
43. BoavidaLC, McCormickS (2007) Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J 52 : 570–582.
Štítky
Genetika Reprodukčná medicína
Článek Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome EvolutionČlánek Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder PopulationČlánek Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among PancrustaceansČlánek Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery DiseaseČlánek Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 7- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Cuba: Exploring the History of Admixture and the Genetic Basis of Pigmentation Using Autosomal and Uniparental Markers
- Clonal Architecture of Secondary Acute Myeloid Leukemia Defined by Single-Cell Sequencing
- Mechanisms of Functional Variants That Impair Regulated Bicarbonate Permeation and Increase Risk for Pancreatitis but Not for Cystic Fibrosis
- Nucleosomes Shape DNA Polymorphism and Divergence
- Functional Diversification of Hsp40: Distinct J-Protein Functional Requirements for Two Prions Allow for Chaperone-Dependent Prion Selection
- Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome Evolution
- Activation of the Immune System by Combinations of Common Alleles
- Age-Associated Sperm DNA Methylation Alterations: Possible Implications in Offspring Disease Susceptibility
- Muscle-Specific SIRT1 Gain-of-Function Increases Slow-Twitch Fibers and Ameliorates Pathophysiology in a Mouse Model of Duchenne Muscular Dystrophy
- MDRL lncRNA Regulates the Processing of miR-484 Primary Transcript by Targeting miR-361
- Hypersensitivity of Primordial Germ Cells to Compromised Replication-Associated DNA Repair Involves ATM-p53-p21 Signaling
- Intrapopulation Genome Size Variation in Reflects Life History Variation and Plasticity
- SlmA Antagonism of FtsZ Assembly Employs a Two-pronged Mechanism like MinCD
- Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder Population
- Determinative Developmental Cell Lineages Are Robust to Cell Deaths
- DELLA Protein Degradation Is Controlled by a Type-One Protein Phosphatase, TOPP4
- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among Pancrustaceans
- UVB Induces a Genome-Wide Acting Negative Regulatory Mechanism That Operates at the Level of Transcription Initiation in Human Cells
- The Nesprin Family Member ANC-1 Regulates Synapse Formation and Axon Termination by Functioning in a Pathway with RPM-1 and β-Catenin
- Combinatorial Interactions Are Required for the Efficient Recruitment of Pho Repressive Complex (PhoRC) to Polycomb Response Elements
- Recombination in the Human Pseudoautosomal Region PAR1
- Microsatellite Interruptions Stabilize Primate Genomes and Exist as Population-Specific Single Nucleotide Polymorphisms within Individual Human Genomes
- An Intronic microRNA Links Rb/E2F and EGFR Signaling
- An Essential Nonredundant Role for Mycobacterial DnaK in Native Protein Folding
- Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery Disease
- The Genomic Landscape of the Ewing Sarcoma Family of Tumors Reveals Recurrent Mutation
- Evolution and Genetic Architecture of Chromatin Accessibility and Function in Yeast
- An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation in
- Stage-Dependent and Locus-Specific Role of Histone Demethylase Jumonji D3 (JMJD3) in the Embryonic Stages of Lung Development
- Genome Wide Association Identifies Common Variants at the Locus Influencing Plasma Cortisol and Corticosteroid Binding Globulin
- Regulation of Feto-Maternal Barrier by Matriptase- and PAR-2-Mediated Signaling Is Required for Placental Morphogenesis and Mouse Embryonic Survival
- Apomictic and Sexual Germline Development Differ with Respect to Cell Cycle, Transcriptional, Hormonal and Epigenetic Regulation
- Functional EF-Hands in Neuronal Calcium Sensor GCAP2 Determine Its Phosphorylation State and Subcellular Distribution , and Are Essential for Photoreceptor Cell Integrity
- Comparison of Methods to Account for Relatedness in Genome-Wide Association Studies with Family-Based Data
- Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
- Cis and Trans Effects of Human Genomic Variants on Gene Expression
- 8.2% of the Human Genome Is Constrained: Variation in Rates of Turnover across Functional Element Classes in the Human Lineage
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- A Loss of Function Screen of Identified Genome-Wide Association Study Loci Reveals New Genes Controlling Hematopoiesis
- Unraveling Genetic Modifiers in the Mouse Model of Absence Epilepsy
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
- The Coding and Noncoding Architecture of the Genome
- A Novel Locus Is Associated with Large Artery Atherosclerotic Stroke Using a Genome-Wide Age-at-Onset Informed Approach
- Brg1 Loss Attenuates Aberrant Wnt-Signalling and Prevents Wnt-Dependent Tumourigenesis in the Murine Small Intestine
- The PTK7-Related Transmembrane Proteins Off-track and Off-track 2 Are Co-receptors for Wnt2 Required for Male Fertility
- The Co-factor of LIM Domains (CLIM/LDB/NLI) Maintains Basal Mammary Epithelial Stem Cells and Promotes Breast Tumorigenesis
- Essential Genetic Interactors of Required for Spatial Sequestration and Asymmetrical Inheritance of Protein Aggregates
- Meiosis-Specific Cohesin Component, Is Essential for Maintaining Centromere Chromatid Cohesion, and Required for DNA Repair and Synapsis between Homologous Chromosomes
- Silencing Is Noisy: Population and Cell Level Noise in Telomere-Adjacent Genes Is Dependent on Telomere Position and Sir2
- The Two Cis-Acting Sites, and , Contribute to the Longitudinal Organisation of Chromosome I
- A Broadly Conserved G-Protein-Coupled Receptor Kinase Phosphorylation Mechanism Controls Smoothened Activity
- Requirements for Acute Burn and Chronic Surgical Wound Infection
- LIN-42, the PERIOD homolog, Negatively Regulates MicroRNA Transcription
- WAPL Is Essential for the Prophase Removal of Cohesin during Meiosis
- Expression in Planarian Neoblasts after Injury Controls Anterior Pole Regeneration
- Sox11 Is Required to Maintain Proper Levels of Hedgehog Signaling during Vertebrate Ocular Morphogenesis
- Accumulation of a Threonine Biosynthetic Intermediate Attenuates General Amino Acid Control by Accelerating Degradation of Gcn4 via Pho85 and Cdk8
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



