-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Comprehensive Identification of Single Nucleotide Polymorphisms Associated with Beta-lactam Resistance within Pneumococcal Mosaic Genes
Streptococcus pneumoniae is carried asymptomatically in the nasopharyngeal tract. However, it is capable of causing multiple diseases, including pneumonia, bacteraemia and meningitis, which are common causes of morbidity and mortality in young children. Antibiotic treatment has become more difficult, especially that involving the group of beta-lactam antibiotics where resistance has developed rapidly. The organism is known to be highly recombinogenic, and this allows variants conferring beta-lactam resistance to be readily introduced into the genome. Identification of the specific genetic determinants of beta-lactam resistance is essential to understand both the mechanism of resistance and the spread of resistant variants in the pneumococcal population. Here, we performed a genome-wide association study on 3,701 isolates collected from two different locations and identified candidate variants that may explain beta-lactam resistance as well as discriminating potential genetic hitchhiking variants from potential causative variants. We report 51 loci, containing 301 SNPs, that are associated with beta-lactam non-susceptibility. 71 out of 301 polymorphic changes result in amino acid alterations, 28 of which have been reported previously. Understanding the determinants of resistance at the single nucleotide level will be important for the future use of sequence data to predict resistance in the clinical setting.
Published in the journal: Comprehensive Identification of Single Nucleotide Polymorphisms Associated with Beta-lactam Resistance within Pneumococcal Mosaic Genes. PLoS Genet 10(8): e32767. doi:10.1371/journal.pgen.1004547
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004547Summary
Streptococcus pneumoniae is carried asymptomatically in the nasopharyngeal tract. However, it is capable of causing multiple diseases, including pneumonia, bacteraemia and meningitis, which are common causes of morbidity and mortality in young children. Antibiotic treatment has become more difficult, especially that involving the group of beta-lactam antibiotics where resistance has developed rapidly. The organism is known to be highly recombinogenic, and this allows variants conferring beta-lactam resistance to be readily introduced into the genome. Identification of the specific genetic determinants of beta-lactam resistance is essential to understand both the mechanism of resistance and the spread of resistant variants in the pneumococcal population. Here, we performed a genome-wide association study on 3,701 isolates collected from two different locations and identified candidate variants that may explain beta-lactam resistance as well as discriminating potential genetic hitchhiking variants from potential causative variants. We report 51 loci, containing 301 SNPs, that are associated with beta-lactam non-susceptibility. 71 out of 301 polymorphic changes result in amino acid alterations, 28 of which have been reported previously. Understanding the determinants of resistance at the single nucleotide level will be important for the future use of sequence data to predict resistance in the clinical setting.
Introduction
Recent research aimed at finding the genetic causes of beta-lactam resistance in Streptococcus pneumoniae has been focused on laboratory mutagenesis [1]–[4], sequence comparison [1], [5], [6], and identification of interspecies sequence transfer that promotes penicillin non-susceptibility [7]–[10]. Though these studies have increased our understanding, their resolution is limited, and a whole-genome systematic search in real population settings is still lacking. Indicative of this limited resolution is the frequent use of the term “mosaic genes” to describe pneumococcal resistance alleles [7]. Although recombinational mosaics are clearly identifiable as regions of several hundred nucleotides in resistance genes, it is likely that only a subset of the observed alterations are important in causing resistance. Genome wide association studies (GWAS) have been used to identify genetic loci associated with complex diseases ranging from cancer to mental illness in human populations [11]–[13]. While the method should, in theory, be applicable to bacterial populations, its use has been inhibited by significant difficulties. These are primarily due to the clonal population structure, and generally limited recombination within bacteria, which make the causal SNPs indistinguishable from other linked SNPs, effectively creating very large haplotype blocks [14], [15]. Attempts have been made to take this clonal structure into account in association analyses [16], [17], but strong linkage disequilibrium will always restrict the resolution of the approach. To overcome this, studies will require either populations with elevated recombination, a large diverse sample, or both, to make the statistical analysis robust.
The confounding effect from clonal population structure may be less problematic in highly recombinogenic bacteria. Homologous recombination brings genetic admixture into bacterial populations in a manner akin to sexual reproduction in humans, although it does not occur every generation, and only affects a small part of the genome in each occurrence. In S. pneumoniae, homologous recombination involves, on average, 2.3 kb of chromosomal DNA [18], about twice the size of an average pneumococcal gene, suggesting that large numbers of recombinational events must accumulate in order to break up linkage blocks smaller than this size. However, the recombination signals left after the action of natural selection are not uniformly distributed across the genome but are concentrated at particular loci, which are commonly known as recombination hotspots. These hotspots are coincident with genes involving the bacterial response to selection pressure, which includes host immune responses and antibiotic utilization, particularly beta-lactams [19], [20]. We hypothesized that the frequency of recombination at these sites would therefore be sufficient to allow the identification of causal SNPs associated with resistance to beta-lactams, given a large enough sample size. Continuing reduction in sequencing costs has allowed the scale of whole-genome bacterial population studies to increase [20], [21], and this should provide more robust statistical power for association analyses. The availability of multiple large bacterial population studies allows a replication of such association studies, providing stronger evidence for common causal SNPs as well as the potential to identify rarer causal SNPs, some of which might only be detected in unique population settings.
Here we conducted an association study using the pneumococcal populations from carriage cohorts in Maela refugee camp, Thailand [20], and Massachusetts, USA [21], two recent species-wide pneumococcal studies from which large numbers of whole genome sequences and phenotypes for beta-lactam susceptibility are available. Given the high recombination frequency in S. pneumoniae generally, the observed recombination hotspots covering antibiotic resistance genes, and the relatively large sample sizes of both studies, we hoped to overcome the challenges in performing bacterial association studies discussed above. Therefore this study aimed to more precisely identify the sets of variants associated with resistance, where they were located in the genome, and how they were distributed across the population.
Results
Identification of loci associated with beta-lactam non-susceptibility
We conducted an association study on whole genome SNPs and insertions or deletions (indels) to identify variants associated with beta-lactam non-susceptibility. Two rounds of analyses were performed separately using 3,085 genomes from pneumococcal strains collected from a carriage cohort in Maela [20], and 616 genomes from a carriage cohort from Massachusetts [21]. Based on the Clinical and Laboratory Standard Institute guidelines (CLSI, 2008), strains with penicillin minimum inhibitory concentration (MIC) ≤0.06 µg/ml were classified as susceptible; applying these cutoffs to our data gave 1,729 case (non-susceptible) and 1,951 control (susceptible) samples for our study (with 21 unknown). The Maela and Massachusetts populations comprise strains from multi-lineage backgrounds. Therefore, taking the population stratification into account is essential to separate the clonal population signals from true phenotypic associations. The population substructures utilized were those defined previously [20], [21], which in both cases were determined using a Bayesian clustering approach (see Methods). Based on this clustering information, the Cochran-Mantel-Haenszel (CMH) association statistic was employed to test for associations between beta-lactam non-susceptibility and specific variants, conditional on the population cluster. For each population, we screened for common alterations with minor allele frequency >0.01 and reported sites with a p value<0.01, incorporating a Bonferroni adjustment for multiple comparisons (Figure 1, Tables S1–S3).
Fig. 1. Summary of the genome-wide-association study conducted in two separate datasets. 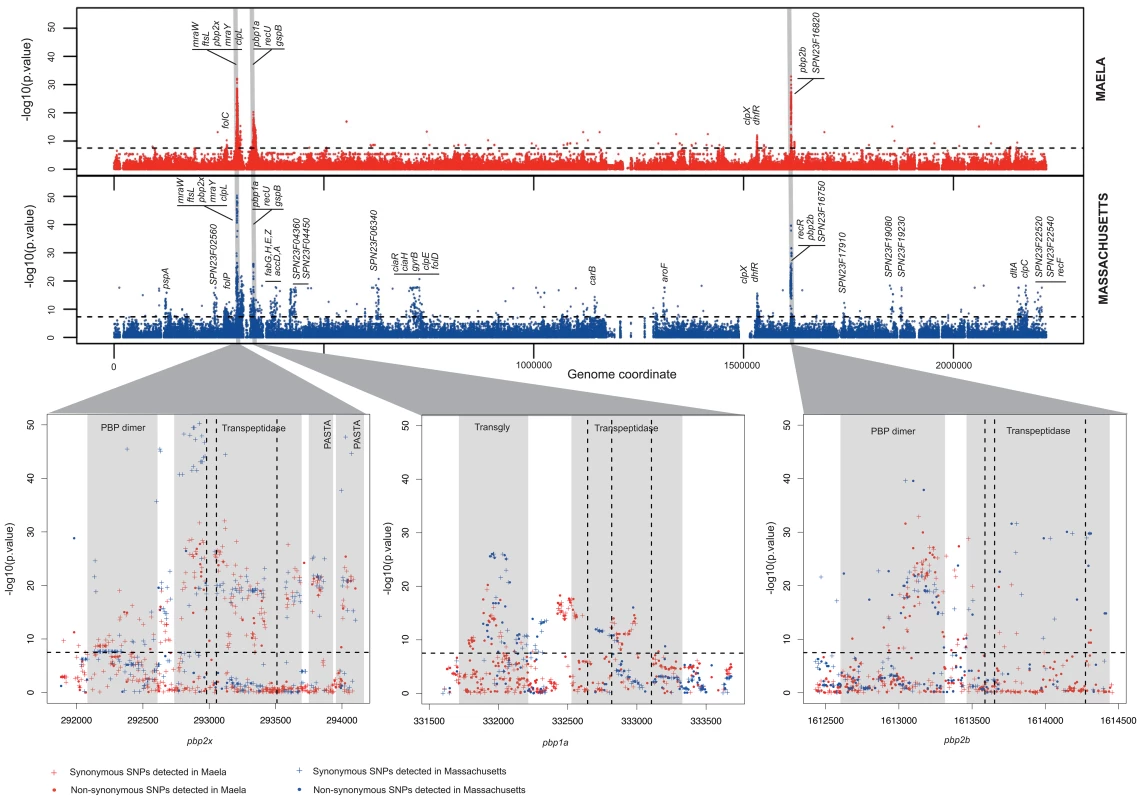
Manhattan plots summarize the association of whole-genome SNP variants with beta-lactam susceptibility in the Maela and Massachusetts data as well as particular gene regions which show strong associations. Top panel represents the statistical significance of association (y-axis) for each variant arranged in order on the genome (x-axis) in the Maela (red) and Massachusetts (blue) data. Horizontal dotted lines in both top and bottom panels indicate a significance cut-off after Bonferroni correction of p = 0.01. Genes with significant associations are annotated on top. Genes coding for penicillin binding proteins: pbp2x, pbp1a, and pbp2b, whose roles in beta-lactam resistance are well characterized, are highlighted in grey. Bottom panel expands the view of penicillin binding protein genes where most of the significant associations are detected: from left to right: pbp2x, pbp1a, and pbp2b. Protein domains identified within these genes are shaded in pale grey and labelled. The vertical dotted lines represent the active sites of the transpeptidase domain. Plus signs denote synonymous SNPs and dots denote non-synonymous SNPs. We found 858 and 1,721 SNPs associated with beta-lactam non-susceptibility in the Maela and Massachusetts populations respectively (Figure 2, Tables S2–S3). Among these, 301 SNPs were found to be associated with non-susceptibility in both populations. Considering that the two settings have different population structures that have evolved independently, these co-detected SNPs represent a set of candidates in which we can have more confidence. Rather counter-intuitively, more candidate SNPs were identified in the smaller dataset from Massachusetts than in the larger dataset from Maela. There are several potential explanations for this observation; one being that it is due to different linkage structure within the two datasets. Not all of the candidate SNPs will necessarily play a causative role; some may be tightly linked to causative SNPs, with insufficient recombination in the dataset to separate them (here called “hitchhiking” SNPs), and hence form part of the same haplotypes. To test this, we estimated the size of the haplotype blocks that harbor candidate SNPs in both the Maela and Massachusetts populations, using the criteria described in Gabriel et al. [22], [23] (see Methods). Haplotype block sizes detected in the Maela data are significantly smaller than the Massachusetts data (Mann Whitney test p value 6.53×10−9, Figure S1), indicating that many of the candidate SNPs detected in the Massachusetts data are potential hitchhikers, thereby generating some false positive results. The second potential explanation is due to the population stratification defined previously [20], [21]. As the clustering analyses were performed separately on each dataset, it is possible that the clustering information from the two datasets are not equivalent in their stringency, leading to a more strict control over population stratification in one population than the other. Nevertheless, 51 candidate loci, comprising a total of 301 discrete and linked SNPs, were co-detected in both the Maela and Massachusetts data; using these should provide a high-stringency data set that overcomes these population-specific effects. The co-detected loci include three intergenic SNPs, and 298 SNPs detected in coding sequences. The latter can be divided into 71 non-synonymous and 227 synonymous SNPs. Of these 51 loci, nine were single SNPs, and 42 were in linkage blocks of between two and 19 SNPs, of which 12 contained only a single non-synonymous SNP. Based on assembled sequences, we also investigated whether or not indels found in associated genes could contribute to the resistant phenotype. None of the identified indels showed significant association with resistance, after Bonferroni correction, at a p-value of <0.01.
Fig. 2. Summary of single nucleotide polymorphisms (SNPs) associated with beta-lactam non-susceptibility. 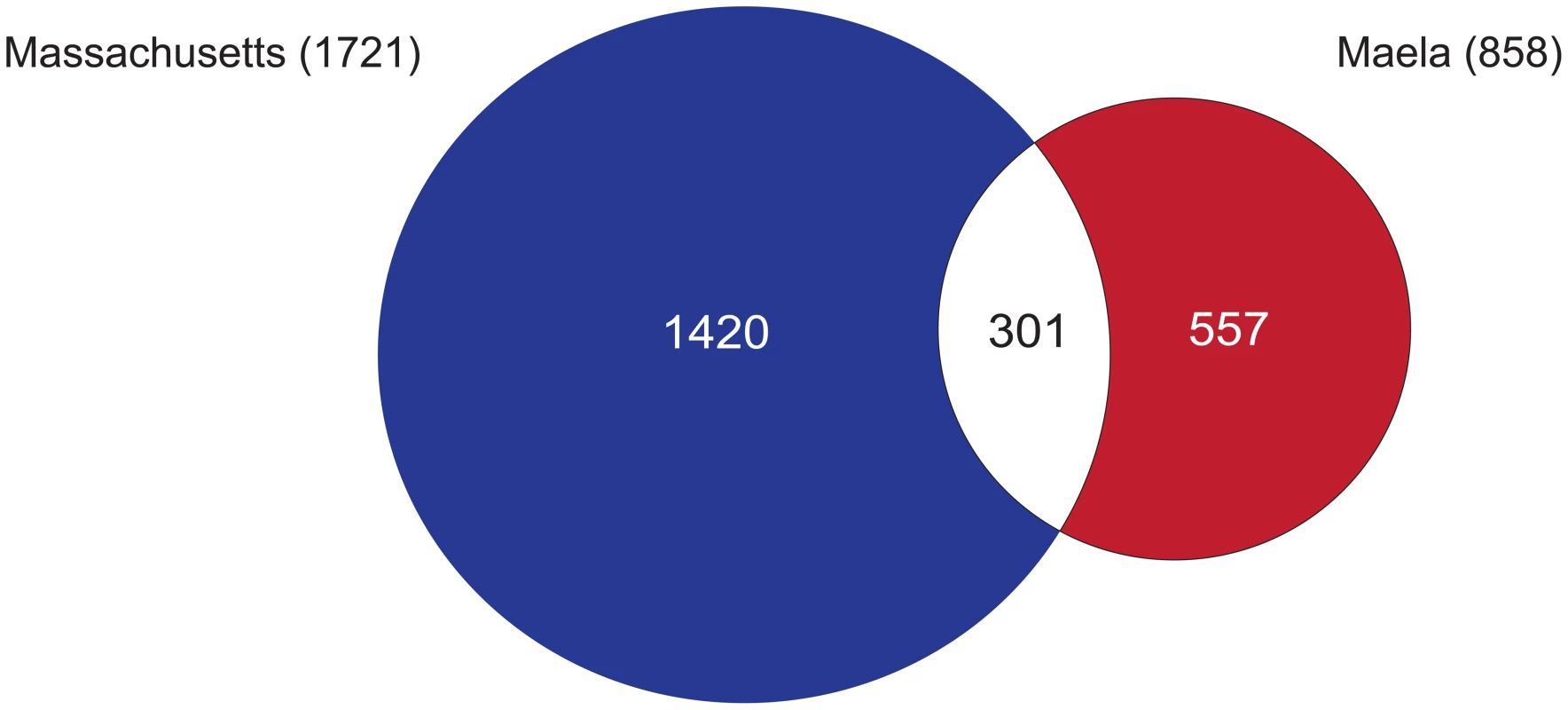
A Venn diagram summarises the number of SNPs reaching significance in each of the Maela and Massachusetts datasets, and those that are co-detected in both. To estimate how much of the phenotypic resistance in our samples could be explained by the identified SNPs, we performed cross-prediction tests using only the SNPs co-detected in both the Maela and Massachusetts association studies, tested back against each population separately. We found that close to 100% of the resistance in each population could be explained by all of the co-detected SNPs (Figure S2). Unlike human polygenic traits where each locus contributes only a small effect on the phenotype, each of these bacterial loci appears to have a much stronger effect, and indeed some have been shown experimentally to change the phenotype with only a single variant [1]. This can be demonstrated using odds ratios, which indicate the size of the effect of each associated SNP. While human GWAS studies report a median odds ratio of 1.33 per SNP [24], [25], our analysis gives a median odds ratio of 11.09 per SNP, indicating a stronger effect size. For both the Maela and Massachusetts populations, the percentage of resistance explained plateaued after the addition of approximately 10 loci in any order. This suggests that, at most, about 10 loci are required to make a susceptible strain non-susceptible and that multiple different combinations can achieve this. However, in each resistant isolate, combinations of more than ten loci are commonly detected, perhaps indicating that not all loci are involved in conferring resistance, but that some may play a compensatory role in reducing the fitness cost of resistance variants. In total, the co-detected variants are present in 100% and 98% of the Maela and Massachusetts resistant strains respectively, highlighting that a large proportion of possible resistance variants are captured in our study.
Biological relevance of candidate loci
For both population settings, loci found to be associated with beta-lactam non-susceptibility show higher enrichment in genes compared to intergenic regions than would be expected by chance (Fisher's Exact Test p-value<0.0001). Candidate loci are not randomly distributed across the whole genome, but clustered within certain genes (Figure 1). Co-detected loci in both datasets are localized in genes participating in the peptidoglycan biosynthesis pathway, including penicillin binding proteins (pbp2x, pbp1a, pbp2b) and two transferases required for cell wall biogenesis (mraW, mraY), the cell division pathway (ftsL, gpsB), heat shock protein and chaperones (clpL, clpX), the recombination pathway (recU) and a metabolic gene known to confer resistance to co-trimoxazole (dhfR). Some of these sites, particularly in the pbp genes, matched those previously reported to play an important role in beta-lactam resistance in the literature (Table S1). To our knowledge, out of 71 non-synonymous SNPs reported here, 43 SNPs are novel and potentially contribute to beta-lactam non-susceptibility in addition to those identified in previous studies.
Candidate loci in genes participating in the peptidoglycan biosynthesis pathway
Since most beta-lactam antibiotics work by inhibiting cell wall biosynthesis, it is not surprising to see significant associations between non-susceptible phenotypes and variants in genes participating in the peptidoglycan biosynthesis pathway, including pbp2x, pbp1a, pbp2b, mraW and mraY. Many single amino acid alterations in pbp2x, pbp1a and pbp2b have been previously demonstrated experimentally to increase pneumococcal resistance to beta-lactams. Mutations within or close to the active sites of the transpeptidase domain in penicillin binding proteins have been reported to be associated with penicillin resistance [26]–[28]. By interfering with the formation of a covalent complex between the active site serine and antibiotic molecules, these mutations help reduce the binding affinity of beta-lactam rings to the transpeptidase enzyme. This allows the pneumococci to form a functional cell wall, and thereby become non-susceptible. We observed many predicted loci co-localizing with or surrounding the transpeptidase active sites. These are recognized as three conserved amino acid motifs, SXXK, SXN and KT(S)G [1], [29] and are highlighted as vertical dotted lines in the bottom panel of Figure 1. Many known structurally characterized alterations in pbp genes have been rediscovered in our analysis, providing independent validation of some of our results. In pbp2x, we observed an association at T338A, which is located next to the active site 337. The side chain of T338 is required for hydrogen-bonding, and the T338A substitution results in a distortion of the active site [1]. In pbp1a, an alteration from TSQF to NTGY at position 574, which has been shown to have a lower acylation efficiency in vitro [1], [30], was also observed in this analysis. In addition to candidates known to confer structural changes that lead to resistance, we also observed association with E285Q in pbp1a which might contribute to a fitness compensation mechanism caused by resistance in pbp2b [31]. Other functional conformational changes, as well as variants that matched previous observations from sequence comparison, are tabulated in Table S1 [1], [2], [5], [6], [30]–[38]. Moreover, we observed substitutions outside pbp genes that could potentially affect antibiotic susceptibility, or represent compensatory mutations that interact epistatically with changes associated with resistance. These include the genes mraY and mraW, which encode transferases. Both function upstream of the pbp genes in the peptidoglycan biosynthesis pathway.
Candidate loci in genes outside the peptidoglycan biosynthesis pathway
The genome-wide screen provided us with an opportunity to identify associations outside the pbp genes and the peptidoglycan biosynthesis pathway, which are the direct target of beta-lactams. In both the Maela and Massachusetts datasets, nine independent loci comprising 31 SNPs were detected outside of these pathways. These include amino acid alterations in a major heatshock protein, clpL. Mutants lacking clpL have been previously reported to be more susceptible to penicillin [1]. The effect was attributed to the ability of clpL to interact with and stabilize the Pbp2x protein. In the Massachusetts data alone, we observed associations between resistance and ciaH, a histidine kinase sensor, and ciaR, its response regulator, consistent with previous studies reporting a large increase in resistance due to ciaH mutations. The mutations in the ciaH kinase sensor resulted in hyperactivation of the ciaR regulator, which in turn leads to increased beta-lactam resistance [1], [39]. Association signals from ftsL and gpsB genes were detected in both datasets. These two genes function in cell division and are essential for complete cell wall formation. Depletion of GpsB leads to cell deformation with a similar phenotype to that observed when the Pbp2x protein is inhibited by methicillin [40]. These identified candidates potentially interact with pbp genes, either directly or indirectly through regulation or participation in cell wall formation; however, experimental characterization will be required to explore the mechanisms of how these alterations influence beta-lactam susceptibility.
Candidate loci in genes conferring resistance to other antibiotics
Interestingly, strong discrete associations were also found in genes where specific variants are known to confer resistance to co-trimoxazole, an antibiotic targeting the bacterial DNA synthesis pathway [41]. We detected associations in dhfR (encoding dihydrofolate reductase) and folP (dihydropteroate synthase), which are required for folate synthesis and are essential for nucleotide biosynthesis. Given that beta-lactam and trimethoprim resistance arise from different mechanisms and that the dhfR and folP loci are not genetically linked to any other detected loci, it is curious as to why we observed these signals. A possible explanation could be the contemporaneous use of both beta-lactam and trimethoprim antibiotics in the host populations studied, which would drive co-selection for resistance to the two unrelated classes of antibiotics. In both the Maela and Massachusetts datasets, strains that are phenotypically resistant to beta-lactams are more likely to be phenotypically resistant to co-trimoxazole, suggesting that the two phenotypes did not occur independently (Fisher's exact test p-value<2.2×10−16, Table 1). Clinical records from Thailand listed beta-lactams and co-trimoxazole as the first and second most frequently prescribed antibiotics for upper respiratory infection treatments [42], indicating that co-selection pressures may have been possible if the two antibiotics were frequently used together.
Tab. 1. Co-occurrence of co-trimoxazole and beta-lactam resistance phenotypes. 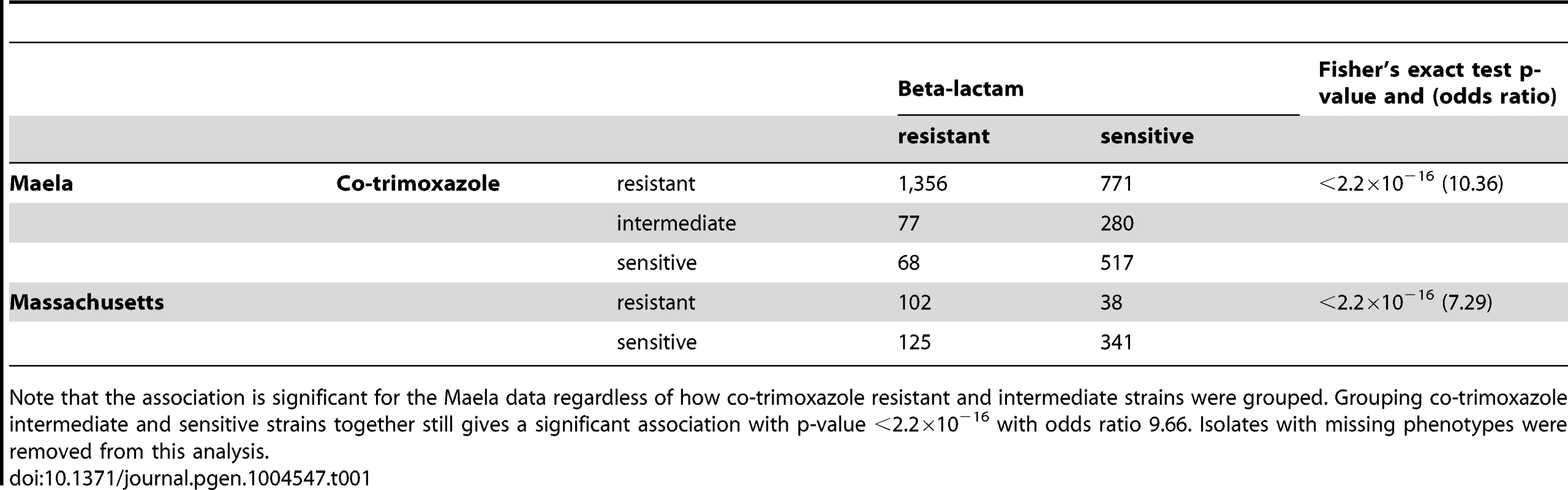
Note that the association is significant for the Maela data regardless of how co-trimoxazole resistant and intermediate strains were grouped. Grouping co-trimoxazole intermediate and sensitive strains together still gives a significant association with p-value <2.2×10−16 with odds ratio 9.66. Isolates with missing phenotypes were removed from this analysis. Beta-lactam specificity of resistance mutations
As some of the variants detected by our study are known to affect the binding affinity for beta-lactams, we looked to see whether the effect would be equivalent across all classes of beta-lactam antibiotics, or if resistance due to specific variants would be greater for certain classes of antibiotic. To test this, we replicated the analysis on the candidate loci using the continuous phenotype of the minimum inhibitory concentration (MIC) value for two classes of beta-lactam antibiotics; penicillins and cephalosporins (here represented by ceftriaxone). Penicillins and cephalosporins both possess a characteristic beta-lactam ring, but while the beta-lactam ring is fused to a 5-membered thiazolidine ring in penicillins, it is fused to a 6-membered dihydrothiazine ring in cephalosporins. The drugs also differ in side chains that differentiate their kinetic properties [43]. Figure 3 plots the differential association of each locus to the two beta-lactam antibiotics. Loci with stronger association towards penicillin are distributed along the positive y-axis, while those showing a stronger preference towards cephalosporins are distributed along negative y-axis. We found that loci do not contribute equally to different classes of beta-lactam antibiotics (Kruskal-Wallis rank sum test, p value<2.2×10−16), with a strong association of some loci towards resistance to either penicillins or cephalosporins.
Fig. 3. Specificity of association signals for co-detected candidate loci with different classes of beta-lactam antibiotics. 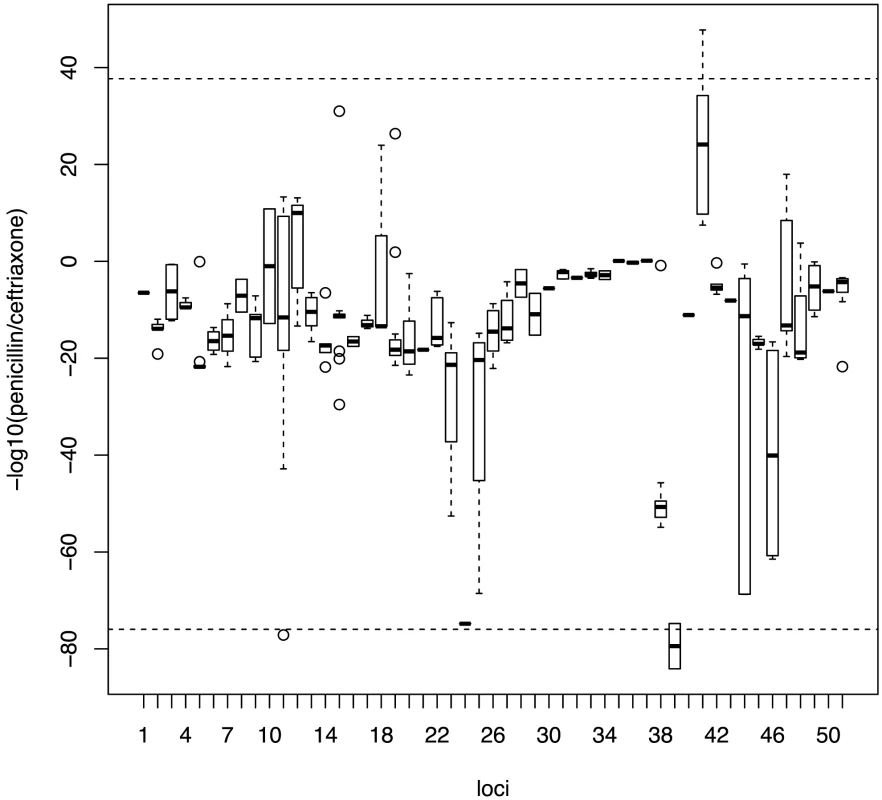
Bonferroni-adjusted p-values from associations with continuous phenotypes with each co-detected SNP were grouped into their linkage loci. Positive values on the y-axis show stronger association with penicillin resistance while negative values show stronger association with cephalosporin resistance. Horizontal dotted lines represent the 99th percentile. Distribution of candidate alleles in the Maela and Massachusetts populations
Given the known pneumococcal population structure in both the Maela and Massachusetts datasets [20], [21], we sought to explore and compare the prevalence of candidate beta-lactam resistance alleles identified as loci co-detected in the two populations. The two populations are composed of multiple pneumococcal lineages, many of which are present in one population but absent in the other. This difference in population structure has a large influence on the types of resistance alleles detected in each setting. Therefore, an unbiased comparison between the Maela and Massachusetts populations can only be made using the lineages common to both locations. PMEN-14, the globally dispersed multidrug resistant lineage, was detected in both populations (Maela: 2007–2010, Massachusetts 2001–2007), and thus allows a comparative view between the two datasets. PMEN-14 isolates from Maela and Massachusetts have significantly different beta-lactam resistance allelic profiles (Mann-Whitney U test, p value = 4.68×10−12).
Though the local beta-lactam resistance profiles are different, the pattern of their distribution across the Maela and Massachusetts pneumococci is similar. In both populations, the distribution of resistance alleles is not uniform (Figure 4). The multidrug resistant lineages PMEN-14 and PMEN-1, along with other vaccine target lineages appear to carry predicted resistance alleles at a higher frequency. This reflects the vaccine's design to target serotypes associated with antibiotic resistance [21], [44]. However, levels of beta-lactam resistance have generally remained stable post-vaccine introduction [21], [45], [46], which has resulted from the success of resistant non-vaccine lineages with a high frequency of resistance alleles (e.g. 35B in Massachusetts, NT in Maela; Figure 4) and serotype switching by resistant-vaccine type lineages such as PMEN-14 [21]. In pneumococcal populations dominated by NT lineages such as in Maela, the higher rate of recombination observed in these lineages [20], and the fact that they are not targeted by current vaccines, may allow them to act as both source and sink for resistance alleles, generating more combinations that are then seeded into the wider population.
Fig. 4. Frequency of putative resistance alleles from candidate loci in the Maela and Massachusetts populations. 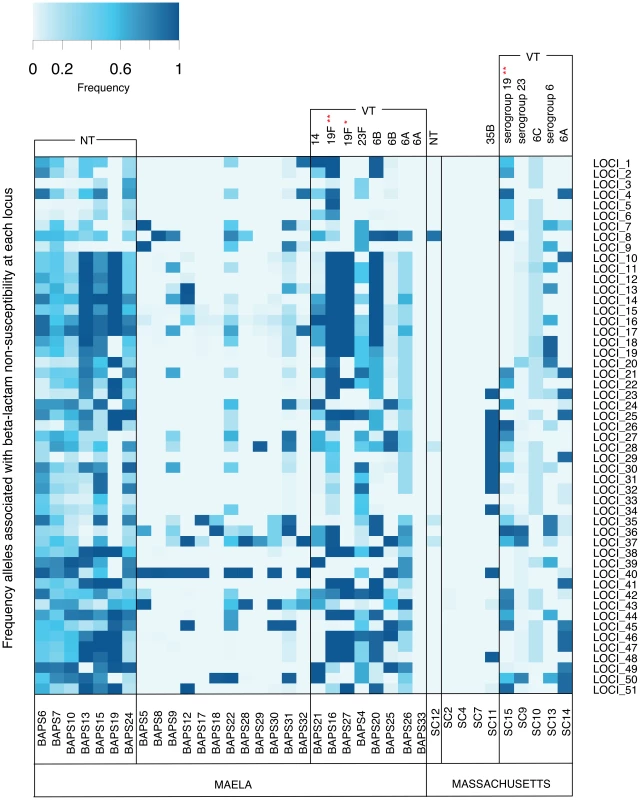
The resistant allele frequency at each locus (rows) in each population cluster (columns) is represented by different shades on a blue-grey scale. Vaccine and non-vaccine serotype status of each cluster are shown at the top of each column. Asterisks highlight two drug resistant global clones: * represents the PMEN1 lineage; ** represents the PMEN14 lineage. Note that mixed population clusters have been removed from the diagram. Discussion
The power of phenotype-genotype association studies in bacteria is limited by the clonal population structure and limited recombination within these organisms. One approach to overcoming this is to explicitly account for population structure in the analysis, and this was recently attempted for studies of host-association in Campylobacter [16] and Staphylococcus aureus [17], but the sensitivity of both was limited by a relatively small sample size. Our analysis used a S. pneumoniae data set of much larger size, enhancing statistical power in detecting associated variants. Our approach also took advantage of the higher level of recombination in the genes participating in the peptidoglycan biosynthesis pathway, some of which are known to be recombination hotspots [19], [20] significantly reducing the effect of long haplotype blocks within these important genes. Together, this enabled us to identify specific nucleotide variants underlying beta-lactam resistance in this organism, some of which were previously known, but many of which are novel.
Our analysis allows the refinement of the understanding of resistance beyond “mosaic genes” to identify likely causative variants, and shows that there are multiple loci which may contribute to resistance. We have also been able to show that, while some loci likely contribute universally to all beta-lactam resistance, some can demonstrate a stronger association with resistance to certain classes of antibiotics more than others.
We used this resistance variant dataset to examine the allele distribution within the sampled population. While specific lineages can vary between populations in the resistance loci present, a general finding was that a high frequency of resistance alleles could be found in both vaccine and non-vaccine lineages, a potential explanation for why vaccination has not reduced beta-lactam resistance within the population. Some non-vaccine target lineages with a high frequency of resistant alleles can act as both a source and a sink of resistance alleles within the population.
A limitation of our approach is that it is sensitive to recombination frequency and requires a non-clonal population and a large sample size. Although the recombination frequency of different bacteria is relatively fixed, current sequencing technologies do now allow very high sample sizes within bacterial populations, and this may increase the applicability of this approach in the future. The sensitivity of detection of association will also be enhanced by the occurrence of de-novo mutations conferring resistance (homoplasy; [19]), representing convergent evolution. However, this will only be possible using whole-genome sequencing, and these could not be detected by eukaryotic-style marker-SNP-based association studies.
Future use of whole-genome sequencing for antibiotic resistance/sensitivity prediction in clinical practice will rely on the ability to assign function to specific variants, rather than mosaic blocks, and this kind of study will be essential to enable these future applications. Nevertheless, results reported from this genome-wide association study are hypothesis-generating and will require further functional validation.
Materials and Methods
Phenotype and genotype data
The test populations represent the largest datasets for which whole genome sequences and antibiotic-resistance phenotypes are available - Maela [20] and Massachusetts [21]. Beta-lactam susceptibilities were determined in both datasets by disk diffusion following the CLSI 2008 guidelines [47]. Our analyses contained 1,501 non-susceptible, 1,568 susceptible and 16 unknown phenotypes in the Maela data; 228 non-susceptible, 383 susceptible and 5 unknown phenotypes from the Massachusetts data. The minimum inhibitory concentrations (MIC) of non-susceptible isolates were confirmed by the E-test method (bioMerieux, Marcy L'Etoile, France). Strain names and a full list of MICs from the Maela dataset [20] are given in Table S4. Strains and metadata for the Massachusetts dataset were given as supplementary data in ref [21].
Samples were previously sequenced as multiplexed libraries on Illumina Hiseq 2000 machines using 75-nt or 100 nt paired-end runs as described in [20], [21]. Short reads from both studies have previously been deposited in the European Nucleotide Archive under study number: Maela data - ERP000435, ERP000483, ERP000485, ERP000487, ERP000598 and ERP000599; and Massachusetts data as listed in Table S1 of [21]. Reads from both datasets were mapped onto a single reference genome, S. pneumoniae ATCC 700669 (European Molecular Laboratory (EMBL) accession FM211187) [48] using SMALT 0.5.7. (http://www.sanger.ac.uk/resources/software/smalt/). Bases were called from mapped sequences using the methods described in [49], resulting in 392,524 and 198,248 SNP calls from the Maela and Massachusetts data respectively. Genomes from Maela were de novo assembled using Velvet [50] with combinations of SSPACE [51], GapFiller [52], BWA [53] and Bowtie [54] as in [20] and genomes from Massachusetts were assembled with Velvet exclusively as described in [21]. Assembled sequences allowed variations from insertions and deletions (indels) to be incorporated for a deeper analysis at each locus.
Defining population structure
The Maela and Massachusetts populations represent species-wide data sets; they respectively consist of 65 and 46 different capsule types, and at least 277 and 154 known multilocus sequence types. The population substructures as determined in [20] [21] were used in this analysis. Briefly, whole genome-mapped sequences and concatenated core genome sequences were used in the Maela [20] and Massachusetts data [21], respectively, as input to the BAPS software [55]–[57]. BAPS was used to define the clonal population structure by estimating the structure based on non-reversible stochastic optimization. The method has successfully been applied to bacterial populations of several different species [58], [59]. Individual strains in Maela and Massachusetts data were first partitioned into clusters based on multiple runs of the estimation algorithm (Methods in [20][21]). This resulted in 33 and 16 initial clusters for the Maela and Massachusetts data, respectively. Due to the large sample size of the Maela dataset, BAPS was additionally run in a hierarchical manner. As described in [20], data from each of the primary clusters identified in the Maela data were re-analyzed to obtain secondary clusters within each primary cluster, and these were used to represent the population structure of Maela pneumococci.
Quality control
The haploid bacterial SNP information was treated as human mitochondrial sequence in PLINK v. 1.07 [60] and controlled for missing rate and allele frequency. We excluded variants with minor allele frequency <0.01, missingness by strain >0.1 and missingness by variants >0.1. For each site, the top two most common variants were parsed to the next analysis to reduce complexity in the test statistic.
Simulation on case-control association analysis
Intrinsic noise from genetic variation alone can lead to false positive signals. To estimate basal false positive rates and decide a suitable cut-off for each dataset, we separately ran 100 GWAS permutations with true genotypes but randomized binary phenotypes (Figure S3). None of the permutations of either the Maela or Massachusetts datasets achieved any significant association at p-value 0.01 with a Bonferroni correction for multiple testing, therefore validating a Bonferroni-adjusted cut-off at p-value 0.01 as our conservative threshold.
Case-control association analysis with real data
We first determined SNPs associated with beta-lactam resistance with binary phenotypes: susceptible or non-susceptible. However, the intrinsic clonal population structure of bacteria can result in high false positive rate in GWAS. The tests were thus performed conditioned on the population structure generated by BAPS in previous publications [20], [21] and controlled for genomic inflation factor. Based on known cluster information, the Cochran-Mantel-Haenszel (CMH) test for 2×2×K binary phenotype x variants | population cluster was employed with sites corrected for multiple testing using the Bonferroni correction at a p-value of 0.01. The application of the CMH test reduced the genomic inflation factor from 80.16 (mean chi-squared statistic = 68.99) to 2.56 (mean chi-squared statistic = 3.05) in the Maela data, and 13.18 (mean chi-squared statistic = 14.17) to 3.76 (mean chi-squared statistic = 4.73) in the Massachusetts data. The reductions in genomic inflation factor seen in both datasets suggest a decrease in false positive rates due to underlying population structure. However, the genomic inflation factors observed here are relatively high compared to those observed in human nuclear chromosome GWAS, suggesting that intrinsic clonal population structure is still an issue for bacterial association studies.
Testing the effect of cluster size of the population stratification
Genome wide association studies are sensitive to population stratification. While a stringent stratification helps reduce false positives, it potentially increases false negatives. Due to the size of the Maela dataset, we had available the (more relaxed) primary BAPS clusters, and the (more stringent) secondary BAPS clusters, and we therefore used these to investigate the effect of clustering size with respect to the number of discovered variants and false positive rate in our data. We separately repeated the Cochran-Mantel-Haenszel (CMH) test as described above using information on primary and secondary BAPS clusters as previously defined [20]. We detected greater numbers of variants with significant associations when stratified by primary clusters compared to secondary clusters (10,451 SNPs compared to 858 SNPs). Also, a higher false positive rate was observed in the analyses using the primary clusters than the secondary clusters (genomic inflation factor of 6.58 compared to 2.56). This result is consistent with what is expected, reflecting a trade-off between false positives and false negatives, and will be dependent on the sample size and underlying population structure.
Linkage analysis
A high genomic inflation factor indicated that some of the candidate alleles were influenced by population structure and were likely to be hitchhikers. We explicitly tested for linkage disequilibrium between candidate SNPs using Haploview version 4.2 [61]. The information was treated as male human X-chromosome to retain its haploidy. Haploview was devised for human genetics where linkages between distant sites are disrupted by crossing-over. Unlike human, bacterial recombination does not necessarily break long distance linkage. We therefore set Haploview to consider all pairwise comparisons under 2,200 kb, which is the size of the whole S. pneumoniae genome, thus incorporating all possible linkage predictions into our analysis. Using 95% confidence bounds as described in [22], a haplotype block was identified as a region with a low recombination rate (Figure S4). These linkage blocks were used to show the context of the predicted alleles and thus potential limitations of our study. We also compared physical linkage size (Figure S1) detected in the Maela and Massachusetts data. The smaller linkage blocks found in the Maela data suggest a higher likelihood of capturing recombination in the larger dataset and thus separating causative SNPs from hitchhiking SNPs.
Estimation of percent resistance in the population that can be explained by the candidate loci
We plotted the proportion of resistance in the population that could be explained by the co-detected loci in each of the test populations, using only combinations of variants observed in both the Maela and Massachusetts datasets (Figure S2). The order of loci added was permutated to accommodate all possible combinations.
Specificity to different classes of beta-lactams
To test whether or not there were variants conferring more specific resistance to certain classes of beta-lactam antibiotics, we repeated the analysis on co-detected candidate SNPs in both datasets and replaced the binary phenotypes with continuous phenotypes: penicillin MIC values and ceftriaxone MIC values. P-values calculated from penicillin MIC and ceftriaxone MIC for each SNP were grouped by the linkage structure.
Prevalence of candidate loci in the population
For each BAPS cluster in both the Maela and Massachusetts data, we calculated the mean prevalence of candidate loci by averaging the frequency of linked SNPs detected in each locus per cluster size.
All statistical analysis were performed in PLINK version 1.07 and R version 2.11.1. Graphical representations were created in R.
Supporting Information
Zdroje
1. HakenbeckR, BrucknerR, DenapaiteD, MaurerP (2012) Molecular mechanisms of beta-lactam resistance in Streptococcus pneumoniae. Future microbiology 7 : 395–410.
2. SmithAM, KlugmanKP (2003) Site-specific mutagenesis analysis of PBP 1A from a penicillin-cephalosporin-resistant pneumococcal isolate. Antimicrobial agents and chemotherapy 47 : 387–389.
3. ZerfassI, HakenbeckR, DenapaiteD (2009) An important site in PBP2x of penicillin-resistant clinical isolates of Streptococcus pneumoniae: mutational analysis of Thr338. Antimicrobial agents and chemotherapy 53 : 1107–1115.
4. CrisostomoMI, VollmerW, KharatAS, InhulsenS, GehreF, et al. (2006) Attenuation of penicillin resistance in a peptidoglycan O-acetyl transferase mutant of Streptococcus pneumoniae. Molecular microbiology 61 : 1497–1509.
5. SanbongiY, IdaT, IshikawaM, OsakiY, KataokaH, et al. (2004) Complete sequences of six penicillin-binding protein genes from 40 Streptococcus pneumoniae clinical isolates collected in Japan. Antimicrobial agents and chemotherapy 48 : 2244–2250.
6. HsiehYC, SuLH, HsuMH, ChiuCH (2013) Alterations of penicillin-binding proteins in pneumococci with stepwise increase in beta-lactam resistance. Pathogens and disease 67 : 84–88.
7. SauerbierJ, MaurerP, RiegerM, HakenbeckR (2012) Streptococcus pneumoniae R6 interspecies transformation: genetic analysis of penicillin resistance determinants and genome-wide recombination events. Molecular microbiology 86 : 692–706.
8. DingF, TangP, HsuMH, CuiP, HuS, et al. (2009) Genome evolution driven by host adaptations results in a more virulent and antimicrobial-resistant Streptococcus pneumoniae serotype 14. BMC genomics 10 : 158.
9. DowsonCG, HutchisonA, BranniganJA, GeorgeRC, HansmanD, et al. (1989) Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proceedings of the National Academy of Sciences of the United States of America 86 : 8842–8846.
10. ChiF, NolteO, BergmannC, IpM, HakenbeckR (2007) Crossing the barrier: evolution and spread of a major class of mosaic pbp2x in Streptococcus pneumoniae, S. mitis and S. oralis. International journal of medical microbiology : IJMM 297 : 503–512.
11. GoldsteinDB, AllenA, KeeblerJ, MarguliesEH, PetrouS, et al. (2013) Sequencing studies in human genetics: design and interpretation. Nature reviews Genetics 14 : 460–470.
12. SullivanPF, DalyMJ, O'DonovanM (2012) Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nature reviews Genetics 13 : 537–551.
13. PharoahPD, TsaiYY, RamusSJ, PhelanCM, GoodeEL, et al. (2013) GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nature genetics 45 : 362–370, 370e361–362.
14. FalushD, BowdenR (2006) Genome-wide association mapping in bacteria? Trends in microbiology 14 : 353–355.
15. VilhjalmssonBJ, NordborgM (2013) The nature of confounding in genome-wide association studies. Nature reviews Genetics 14 : 1–2.
16. SheppardSK, DidelotX, MericG, TorralboA, JolleyKA, et al. (2013) Genome-wide association study identifies vitamin B5 biosynthesis as a host specificity factor in Campylobacter. Proceedings of the National Academy of Sciences of the United States of America 110 : 11923–11927.
17. LaabeiM, ReckerM, RudkinJK, AldeljawiM, GulayZ, et al. (2014) Predicting the virulence of MRSA from its genome sequence. Genome Res 24 : 839–849.
18. CroucherNJ, HarrisSR, BarquistL, ParkhillJ, BentleySD (2012) A high-resolution view of genome-wide pneumococcal transformation. PLoS pathogens 8: e1002745.
19. CroucherNJ, HarrisSR, FraserC, QuailMA, BurtonJ, et al. (2011) Rapid pneumococcal evolution in response to clinical interventions. Science 331 : 430–434.
20. ChewapreechaC, HarrisSR, CroucherNJ, TurnerC, MarttinenP, et al. (2014) Dense genomic sampling identifies highways of pneumococcal recombination. Nat Genet 46 : 305–309.
21. CroucherNJ, FinkelsteinJA, PeltonSI, MitchellPK, LeeGM, et al. (2013) Population genomics of post-vaccine changes in pneumococcal epidemiology. Nat Genet 45 : 656–663.
22. GabrielSB, SchaffnerSF, NguyenH, MooreJM, RoyJ, et al. (2002) The structure of haplotype blocks in the human genome. Science 296 : 2225–2229.
23. WallJD, PritchardJK (2003) Haplotype blocks and linkage disequilibrium in the human genome. Nat Rev Genet 4 : 587–597.
24. ManolioTA (2010) Genomewide association studies and assessment of the risk of disease. N Engl J Med 363 : 166–176.
25. KuCS, LoyEY, PawitanY, ChiaKS (2010) The pursuit of genome-wide association studies: where are we now? J Hum Genet 55 : 195–206.
26. T GrebeRH (1996) Penicillin-binding proteins 2b and 2x of Streptococcus pneumoniae are primary resistance determinants for different classes of beta-lactam antibiotics. Antimicrob Agents Chemother Apr;40 (4) 829–834.
27. Granger DB-LG, TurgeonP, WeissK, RogerM (2005) Genetic analysis of pbp2x in clinical Streptococcus pneumoniae isolates in Quebec, Canada. J Antimicrob Chemother Jun 55(6): 832–839.
28. GrangerD B-LG, TurgeonP, WeissK, RogerM (2006) Molecular characteristics of pbp1a and pbp2b in clinical Streptococcus pneumoniae isolates in Quebec, Canada. J Antimicrob Chemother Jan 57 (1) 61–70.
29. NagaiK, DaviesTA, JacobsMR, AppelbaumPC (2002) Effects of amino acid alterations in penicillin-binding proteins (PBPs) 1a, 2b, and 2x on PBP affinities of penicillin, ampicillin, amoxicillin, cefditoren, cefuroxime, cefprozil, and cefaclor in 18 clinical isolates of penicillin-susceptible, -intermediate, and -resistant pneumococci. Antimicrobial agents and chemotherapy 46 : 1273–1280.
30. JobV, CarapitoR, VernetT, DessenA, ZapunA (2008) Common alterations in PBP1a from resistant Streptococcus pneumoniae decrease its reactivity toward beta-lactams: structural insights. The Journal of biological chemistry 283 : 4886–4894.
31. Albarracin OrioAG, PinasGE, CortesPR, CianMB, EcheniqueJ (2011) Compensatory evolution of pbp mutations restores the fitness cost imposed by beta-lactam resistance in Streptococcus pneumoniae. PLoS Pathog 7: e1002000.
32. Dessen AMN, GordonE, HopkinsJ, DidebergO (2001) Crystal structure of PBP2x from a highly penicillin-resistant Streptococcus pneumoniae clinical isolate: a mosaic framework containing 83 mutations. J Biol Chem Nov 30;276 (48) 45106–45112.
33. CarapitoRCL, VernetT, ZapunA (2006) Pneumococcal beta-lactam resistance due to a conformational change in penicillin-binding protein 2x. J Biol Chem Jan 20;281 (3) 1771–1777.
34. SmithAM, KlugmanKP (1998) Alterations in PBP 1A essential-for high-level penicillin resistance in Streptococcus pneumoniae. Antimicrobial agents and chemotherapy 42 : 1329–1333.
35. JobV, Di GuilmiAM, MartinL, VernetT, DidebergO, et al. (2003) Structural studies of the transpeptidase domain of PBP1a from Streptococcus pneumoniae. Acta crystallographica Section D, Biological crystallography 59 : 1067–1069.
36. SmithAM, KlugmanKP (2005) Amino acid mutations essential to production of an altered PBP 2X conferring high-level beta-lactam resistance in a clinical isolate of Streptococcus pneumoniae. Antimicrobial agents and chemotherapy 49 : 4622–4627.
37. OverwegK, BogaertD, SluijterM, de GrootR, HermansPW (2001) Molecular characteristics of penicillin-binding protein genes of penicillin-nonsusceptible Streptococcus pneumoniae isolated in the Netherlands. Microbial drug resistance 7 : 323–334.
38. GrebeT, HakenbeckR (1996) Penicillin-binding proteins 2b and 2x of Streptococcus pneumoniae are primary resistance determinants for different classes of beta-lactam antibiotics. Antimicrob Agents Chemother 40 : 829–834.
39. MullerM, MarxP, HakenbeckR, BrucknerR (2011) Effect of new alleles of the histidine kinase gene ciaH on the activity of the response regulator CiaR in Streptococcus pneumoniae R6. Microbiology 157 : 3104–3112.
40. LandAD, TsuiHC, KocaogluO, VellaSA, ShawSL, et al. (2013) Requirement of essential Pbp2x and GpsB for septal ring closure in Streptococcus pneumoniae D39. Mol Microbiol 90 : 939–955.
41. MaskellJP, SeftonAM, HallLM (2001) Multiple mutations modulate the function of dihydrofolate reductase in trimethoprim-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother 45 : 1104–1108.
42. ThamlikitkulV, ApisitwittayaW (2004) Implementation of clinical practice guidelines for upper respiratory infection in Thailand. Int J Infect Dis 8 : 47–51.
43. DePestelDD, BenningerMS, DanzigerL, LaPlanteKL, MayC, et al. (2008) Cephalosporin use in treatment of patients with penicillin allergies. Journal of the American Pharmacists Association : JAPhA 48 : 530–540.
44. DaganR (2009) Impact of pneumococcal conjugate vaccine on infections caused by antibiotic-resistant Streptococcus pneumoniae. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 15 (Suppl 3) 16–20.
45. HuangSS, PlattR, Rifas-ShimanSL, PeltonSI, GoldmannD, et al. (2005) Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics 116: e408–413.
46. HuangSS, HinrichsenVL, StevensonAE, Rifas-ShimanSL, KleinmanK, et al. (2009) Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics 124: e1–11.
47. CLSI (2008) Performace Standards for Antimicrobial Susceptibility Testing. CLSI document M100-S18 Wayne: Clinical and Laboratory Standard Institute.
48. CroucherNJ, WalkerD, RomeroP, LennardN, PatersonGK, et al. (2009) Role of conjugative elements in the evolution of the multidrug-resistant pandemic clone Streptococcus pneumoniaeSpain23F ST81. Journal of bacteriology 191 : 1480–1489.
49. HarrisSR, FeilEJ, HoldenMT, QuailMA, NickersonEK, et al. (2010) Evolution of MRSA during hospital transmission and intercontinental spread. Science 327 : 469–474.
50. ZerbinoDR, BirneyE (2008) Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome research 18 : 821–829.
51. BoetzerM, HenkelCV, JansenHJ, ButlerD, PirovanoW (2011) Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 27 : 578–579.
52. BoetzerM, PirovanoW (2012) Toward almost closed genomes with GapFiller. Genome biology 13: R56.
53. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
54. LangmeadB, TrapnellC, PopM, SalzbergSL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome biology 10: R25.
55. CoranderJ, WaldmannP, SillanpaaMJ (2003) Bayesian analysis of genetic differentiation between populations. Genetics 163 : 367–374.
56. CoranderJ, TangJ (2007) Bayesian analysis of population structure based on linked molecular information. Mathematical biosciences 205 : 19–31.
57. CoranderJ, MarttinenP, SirenJ, TangJ (2008) Enhanced Bayesian modelling in BAPS software for learning genetic structures of populations. BMC bioinformatics 9 : 539.
58. TopJ, WillemsRJ, van SchaikW, LeavisH, BontenM, et al. (2012) Restricted gene flow among hospital subpopulations of Enterococcus faecium. mBio 3: e00151–12.
59. ChengL, ConnorTR, SirenJ, AanensenDM, CoranderJ (2013) Hierarchical and spatially explicit clustering of DNA sequences with BAPS software. Molecular biology and evolution
60. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics 81 : 559–575.
61. BarrettJC, FryB, MallerJ, DalyMJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21 : 263–265.
Štítky
Genetika Reprodukčná medicína
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 8- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- DNA Double Strand Break Repair in Mitosis Is Suppressed by Phosphorylation of XRCC4
- Inference of Transposable Element Ancestry
- The Population Genetics of Evolutionary Rescue
- Retinoic Acid Activates Two Pathways Required for Meiosis in Mice
- Pooled Segregant Sequencing Reveals Genetic Determinants of Yeast Pseudohyphal Growth
- Comprehensive Identification of Single Nucleotide Polymorphisms Associated with Beta-lactam Resistance within Pneumococcal Mosaic Genes
- SMA-Causing Missense Mutations in Display a Wide Range of Phenotypes When Modeled in
- Branch Migration Prevents DNA Loss during Double-Strand Break Repair
- Transcriptome Sequencing from Diverse Human Populations Reveals Differentiated Regulatory Architecture
- Genetic Deletion of SEPT7 Reveals a Cell Type-Specific Role of Septins in Microtubule Destabilization for the Completion of Cytokinesis
- Tethering Sister Centromeres to Each Other Suggests the Spindle Checkpoint Detects Stretch within the Kinetochore
- Global Genetic Variations Predict Brain Response to Faces
- Demography and the Age of Rare Variants
- The Response to High CO Levels Requires the Neuropeptide Secretion Component HID-1 to Promote Pumping Inhibition
- Sp6 and Sp8 Transcription Factors Control AER Formation and Dorsal-Ventral Patterning in Limb Development
- The Groucho Co-repressor Is Primarily Recruited to Local Target Sites in Active Chromatin to Attenuate Transcription
- A Transposable Element Insertion Confers Xenobiotic Resistance in Drosophila
- The Genomic Architecture of Population Divergence between Subspecies of the European Rabbit
- Human Social Genomics
- Gene Expansion Shapes Genome Architecture in the Human Pathogen : An Evolutionary Genomics Analysis in the Ancient Terrestrial Mucorales (Mucoromycotina)
- Canonical Non-Homologous End Joining in Mitosis Induces Genome Instability and Is Suppressed by M-phase-Specific Phosphorylation of XRCC4
- Roles of Type 1A Topoisomerases in Genome Maintenance in
- The TRIM-NHL Protein LIN-41 Controls the Onset of Developmental Plasticity in
- Wnt-Mediated Repression via Bipartite DNA Recognition by TCF in the Hematopoietic System
- KDM6 Demethylase Independent Loss of Histone H3 Lysine 27 Trimethylation during Early Embryonic Development
- Integration of UPR and Oxidative Stress Signaling in the Control of Intestinal Stem Cell Proliferation
- miR171-Targeted Scarecrow-Like Proteins Bind to GT -Elements and Mediate Gibberellin-Regulated Chlorophyll Biosynthesis under Light Conditions
- Syndecan-1 Is Required to Maintain Intradermal Fat and Prevent Cold Stress
- LIN-3/EGF Promotes the Programmed Cell Death of Specific Cells in by Transcriptional Activation of the Pro-apoptotic Gene
- A System for Genome-Wide Histone Variant Dynamics In ES Cells Reveals Dynamic MacroH2A2 Replacement at Promoters
- Multiple Regulation of Rad51-Mediated Homologous Recombination by Fission Yeast Fbh1
- A Genome-Wide Association Study of the Maize Hypersensitive Defense Response Identifies Genes That Cluster in Related Pathways
- The RNA Helicases AtMTR4 and HEN2 Target Specific Subsets of Nuclear Transcripts for Degradation by the Nuclear Exosome in
- Asymmetric Division and Differential Gene Expression during a Bacterial Developmental Program Requires DivIVA
- A Model-Based Approach for Identifying Signatures of Ancient Balancing Selection in Genetic Data
- Chromatin Insulator Factors Involved in Long-Range DNA Interactions and Their Role in the Folding of the Drosophila Genome
- Conditional Inactivation of Upstream Binding Factor Reveals Its Epigenetic Functions and the Existence of a Somatic Nucleolar Precursor Body
- Evidence for Divisome Localization Mechanisms Independent of the Min System and SlmA in
- Patterns of Admixture and Population Structure in Native Populations of Northwest North America
- Response Regulator Heterodimer Formation Controls a Key Stage in S Development
- A Genetic Strategy to Measure Circulating Insulin Reveals Genes Regulating Insulin Production and Secretion
- EVA-1 Functions as an UNC-40 Co-receptor to Enhance Attraction to the MADD-4 Guidance Cue in
- Dysfunction of the CNS-Heart Axis in Mouse Models of Huntington's Disease
- An Otx/Nodal Regulatory Signature for Posterior Neural Development in Ascidians
- Phosphorylation of a Central Clock Transcription Factor Is Required for Thermal but Not Photic Entrainment
- Genome-Wide Patterns of Genetic Variation within and among Alternative Selective Regimes
- EF-P Dependent Pauses Integrate Proximal and Distal Signals during Translation
- Ku-Mediated Coupling of DNA Cleavage and Repair during Programmed Genome Rearrangements in the Ciliate
- Functional Specialization Among Members Of Knickkopf Family Of Proteins In Insect Cuticle Organization
- Playing RNase P Evolution: Swapping the RNA Catalyst for a Protein Reveals Functional Uniformity of Highly Divergent Enzyme Forms
- The Translational Regulators GCN-1 and ABCF-3 Act Together to Promote Apoptosis in
- Meta-Analysis of Genome-Wide Association Studies in African Americans Provides Insights into the Genetic Architecture of Type 2 Diabetes
- A -Regulatory Mutation of Causes Silky-Feather in Chickens
- VIB1, a Link between Glucose Signaling and Carbon Catabolite Repression, Is Essential for Plant Cell Wall Degradation by
- A Population Genetic Signal of Polygenic Adaptation
- A Conserved Dopamine-Cholecystokinin Signaling Pathway Shapes Context–Dependent Behavior
- The MAP Kinase p38 Is Part of Circadian Clock
- The Cohesin Subunit Rad21 Is Required for Synaptonemal Complex Maintenance, but Not Sister Chromatid Cohesion, during Drosophila Female Meiosis
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Meta-Analysis of Genome-Wide Association Studies in African Americans Provides Insights into the Genetic Architecture of Type 2 Diabetes
- KDM6 Demethylase Independent Loss of Histone H3 Lysine 27 Trimethylation during Early Embryonic Development
- The RNA Helicases AtMTR4 and HEN2 Target Specific Subsets of Nuclear Transcripts for Degradation by the Nuclear Exosome in
- EF-P Dependent Pauses Integrate Proximal and Distal Signals during Translation
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



