-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota
Tomato is one of the most important food crops worldwide, providing phytonutrients and color to our diet. Tomato yellow leaf curl virus (TYLCV) is one of the most devastating viruses of cultivated tomatoes and a key limiting factor to tomato production in major tomato-growing areas. The management of TYLCV is difficult because its whitefly vector populations can reach high numbers and relies heavily on the application of hazardous chemicals (insecticides). Breeding TYLCV-resistant tomato cultivars provides an attractive, environmentally friendly strategy to reduce yield losses caused by the virus. Considerable efforts have been invested in breeding TYLCV-resistant tomato cultivars. The discovery and utilization of genes controlling resistance can expedite the breeding process and highlight innate means to promote such resistance. Here we report the discovery of the gene controlling TYLCV resistance at the ty-5 locus. It was found that the messenger RNA surveillance factor Pelo is responsible for this resistance. Our discovery, together with the recent discovery of the gene controlling resistance at the Ty-1/Ty-3 locus, can lead to precision pyramiding of these two genes to enhance the spectrum of resistance of tomato plants to TYLCV, and possibly to other begomoviruses.
Published in the journal: A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota. PLoS Genet 11(10): e32767. doi:10.1371/journal.pgen.1005538
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005538Summary
Tomato is one of the most important food crops worldwide, providing phytonutrients and color to our diet. Tomato yellow leaf curl virus (TYLCV) is one of the most devastating viruses of cultivated tomatoes and a key limiting factor to tomato production in major tomato-growing areas. The management of TYLCV is difficult because its whitefly vector populations can reach high numbers and relies heavily on the application of hazardous chemicals (insecticides). Breeding TYLCV-resistant tomato cultivars provides an attractive, environmentally friendly strategy to reduce yield losses caused by the virus. Considerable efforts have been invested in breeding TYLCV-resistant tomato cultivars. The discovery and utilization of genes controlling resistance can expedite the breeding process and highlight innate means to promote such resistance. Here we report the discovery of the gene controlling TYLCV resistance at the ty-5 locus. It was found that the messenger RNA surveillance factor Pelo is responsible for this resistance. Our discovery, together with the recent discovery of the gene controlling resistance at the Ty-1/Ty-3 locus, can lead to precision pyramiding of these two genes to enhance the spectrum of resistance of tomato plants to TYLCV, and possibly to other begomoviruses.
Introduction
Tomato yellow leaf curl virus (TYLCV) is one of the most devastating viruses of cultivated tomatoes, Solanum (S.) lycopersicum. Although first identified in the eastern Mediterranean [1], it has spread into almost all tropical and subtropical regions [2, 3]. TYLCV induces severe cupping of apical leaves, yellowing, and stunting, resulting in considerable yield losses. TYLCV has become a major limiting factor to tomato production in major tomato-growing areas, including: China, Mexico, Florida and California [4, 5].
TYLCV is a monopartite begomovirus (family Geminiviridae) transmitted by the whitefly Bemisia tabaci (Gennadius). Population outbreaks of whiteflies are often associated with a high incidence of the disease [6]. The virus genome is composed of a single circular single-stranded DNA molecule of about 2,800 nucleotides. Management of TYLCV is difficult because its whitefly vector populations can reach enormous numbers. Therefore, breeding TYLCV-resistant tomato cultivars provides an attractive, environmentally sound, strategy to reduce yield losses inflicted by the virus [4, 7–9].
Considerable efforts have been invested in breeding TYLCV-resistant tomato cultivars [4]. As all cultivated tomato accessions are susceptible to the disease, wild tomato species were screened to identify, map and introgress resistance loci into S. lycopersicum. Among these are: Ty-2 that was introgressed from S. habrochaites, ty-5 presumably from S. peruvianum, and Ty-1, Ty-3 and Ty-4 from S. chilense accessions [10–17]. Because Ty-1 and Ty-3 are allelic [18, 19], the number of available genes conferring TYLCV resistance is quite limited. Recently, the gene responsible for TYLCV-resistance at the Ty-1/Ty-3 locus was identified and shown to code for an RNA-dependent RNA polymerase (RDR) [19]. Moreover, it was shown that Ty-1/Ty-3 confers resistance to TYLCV by increasing cytosine methylation of the viral genome, indicating that the resistance conferred by this locus acts through viral transcriptional gene silencing [20]. Thus far, no other gene conferring TYLCV resistance has been unambiguously identified.
Line TY172, carrying ty-5, is thought to be derived from four different wild tomato accessions, three of S. peruvianum: PI 126926, PI 126930, PI 390681, and one of S. Arcanum: LA0441 [21, 22]. The breeding procedure yielding TY172 was described before [17, 21].
TY172 is highly resistant to TYLCV: it shows no disease symptoms following infection and contains low levels of viral DNA [23]. TY172 exhibited the highest level of resistance in a field trial that compared fruit yield of various resistant accessions following TYLCV-inoculation [23]. It was also found that TY172, probably due to its high level of TYLCV-resistance, is a poor source for viral acquisition and transmission by whiteflies [24]. These characteristics emphasize the high potential of utilizing TY172 in breeding TYLCV-resistant tomato cultivars.
Classical segregation studies suggested that resistance in TY172 is controlled by three genes exerting a partially-dominant effect [21]. However, a study designed to map genes controlling TYLCV-resistance in TY172 showed that resistance is conferred by a previously unknown major recessive quantitative trait locus (QTL), termed ty-5, that maps to chromosome 4 and four minor OTLs [17].
Recently, a recessive resistance carried by the old commercial cultivar Tyking (Royal Sluis, The Netherlands) has been shown to co-localize with ty-5 [25]. The authors suggested that because one of the populations used by Anbinder et al. [17] also displayed a recessive gene action, the resistance in Tyking most likely corresponds to the resistance in TY172.
TYLCV-resistance inherited by TY172 at the ty-5 locus is highly associated with a gene encoding a NAC DOMAIN 1 protein (Nac1) [17]. Nac1 was previously implicated in the replication of another tomato-infecting begomovirus ToLCV, by interacting with its replication enhancer protein (REn) in cultivated tomato [26]. It was further shown that ToLCV induce Nac1 expression in infected susceptible cells, and that this up-regulation requires REn. Also, in a transient ToLCV replication system, over-expression of Nac1 resulted in a substantial increase in viral DNA accumulation. These results suggest that Nac1 plays an important role in replication-enhancement of ToLCV and possibly other begomoviruses, including TYLCV, in susceptible plants. Therefore, this gene, or more precisely its homolog in TY172, was initially referred to as a candidate gene reducing TYLCV replication and thus conferring resistance at the ty-5 locus [17].
The objective of this study was to fine-tune map ty-5. For this purpose, the associations between 27 polymorphic DNA markers spanning the ty-5 locus, including Nac1, and the resistance characteristics of individual plants inoculated with TYLCV in segregating populations were analyzed. These analyses, coupled with transgenic confirmation, identified the gene controlling resistance at the ty-5 locus as the tomato homolog of the messenger RNA surveillance factor Pelo.
Results
Fine-tune mapping and general characteristics of the ty-5 locus
To fine-tune map the introgression in the TYLCV-resistant line TY172, carrying the ty-5 gene, we have sequenced 800-to-900 bp fragments of its genome spanning the Nac1 gene region (first, approximately every 50 Kilo bp (Kbp), then every 10 Kbp and finally every 3 Kbp and at times even in smaller intervals). These sequencing results were compared to the sequence of the reference genome Heinz 1706, known to be susceptible to TYLCV (build SL2.40 in http://solgenomics.net/) in order to identify nucleotide polymorphisms. These polymorphisms were validated by sequencing M-82 as well. From these sequence comparisons a core set of 27 markers were generated and used throughout this study (S1 Table). Genomic DNA sequence comparisons between the resistant TY172 line and its susceptible counterparts did not yield significant insertions or deletions usually characterizing DNA sequences of species distantly related to the cultivated tomato, including S. peruvianum. In addition, no polymorphism was detected upstream of the Nac1 promoter gene-sequence beyond those displayed (S1 Fig). These results indicate that the control of TYLCV resistance is exerted either by Nac1, characterized by Tyrosine212-to-Cysteine substitution in TY172 (S2 Fig), or by a gene located downstream from its position on chromosome 4.
Nac1 is not the gene controlling TYLCV resistance at the ty-5 locus
To carry out the map-based analysis of ty-5, a series of 51 recombinant lines were generated. As this analysis progressed, it became evident that Nac1 is not the gene controlling TYLCV-resistance at the ty-5 locus. For example, a BC1F3 population fixed for the Nac1 allele originating from the susceptible line M-82 and segregating for markers 31 through 0.5 still displayed a strong association between the resistance and segregating markers at this range (Fig 1A). On the other hand, a BC2F3 population segregating solely for Nac1 did not display a significant association between the gene and disease symptoms (Fig 1B). These results delimit the ty-5 gene into approximately 351 Kb between Nac1 and the 5.8 marker (S1 Table).
Fig. 1. Association between DNA markers spanning the ty-5 locus and disease severity in representative segregating populations. 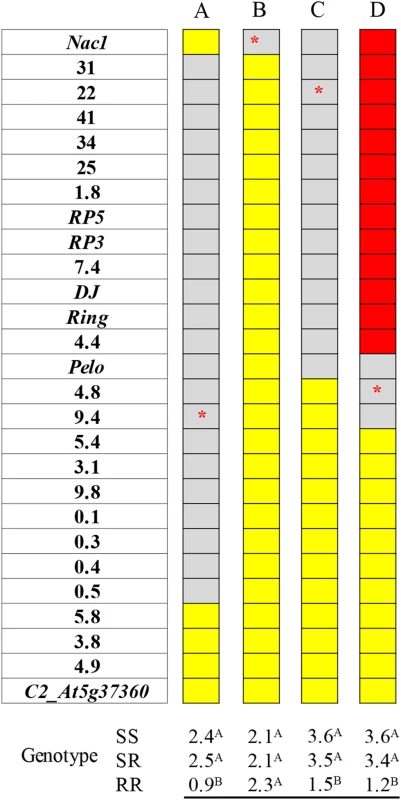
In the ruler presented to the left of each analysis: yellow-shaded markers are homozygous for the allele originated from the susceptible line M-82 (SS), red-shaded markers are homozygous for the allele originated from the resistant line TY172 (RR) and gray-shaded markers are heterozygous (SR); the analysis of variance presented at the bottom of each population was carried out with different markers: the marker in red asterisks is the one that was used as an independent variable in each analysis; different superscript letters above means indicate statistically significant difference, P<0.05, between genotypes for each population separately; A-D: four different representative segregating populations; DSI (disease severity index) was determined at 28 DPI (days post inoculation) and at 42 DPI, the DSI values presented is an average of both readings. Recombination frequency and map-based identification of the ty-5 gene
We have analyzed 5,662 plants from different segregating populations following a cross between the resistant TY172 and the susceptible M-82 line. 51 plants displaying recombination events among the polymorphic DNA markers were identified. These plants were allowed to self-pollinate to produce segregating populations that were again genotyped, inoculated and assayed for resistance (S3 Fig). These 51 recombinant plants enabled us to narrow down the resistant locus into a 23,250 bp fragment (in TY172), including the amplicons produced by markers 4.4 and 4.8, containing two genes: a Calcium dependent protein kinase (Cdpk2) [27] and the Pelo gene homolog [28] (S3 Fig). The identification of this region can be exemplified as follows:
A BC1F3 population segregating for the region flanked by Nac1 and marker 4.8 still displayed a strong association between the TYLCV-resistance trait and markers segregating at this range (Fig 1C). These results further delimit the ty-5 gene into a 268.2 Kb fragment between Nac1 and Pelo.
An F3 population (Fig 1D) that is: A. Fixed for the region originating from TY172 between Nac1 and marker 4.4; B. Fixed for the region originating from M-82 between marker 5.4 and marker C2_At5g37360; and C. Segregating for a region flanked by markers 4.4 and 5.4, continued to display a strong association between the TYLCV-resistance trait and the segregating markers (Fig 1D). These results (Fig 1), confirmed by 47 additional recombinant populations, 32 of which are presented in S3 Fig, demonstrate that the ty-5 gene resides between markers 4.4 and 4.8.
The region between markers 4.4 and 4.8 was sequenced in TY172 and M-82 and compared to the sequence of Heinz 1706. Seven single nucleotide polymorphisms (SNPs) were found in this region. Further sequencing of the polymorphic region between Pelo and marker 4.4 in selected recombinant plants of populations such as the one displayed in Fig 1D have shown that the three SNPs found in this region (between Pelo and marker 4.4, the region in which Cdpk2 is located) are unrelated to the resistance. This clearly demonstrated that Cdpk2 is not involved in the resistance, and enabled us to delimit the introgression into a polymorphic 2,386 bp region between Pelo and marker 4.8. The SNPs in this region were: a G1-to-A transition, single nucleotide insertion (A1565), and a T1961-to-A1962 transversion in the promoter region of the Pelo gene in TY172 (S4 Fig). In addition, a T47-to-G transversion in the first exon of Pelo was also observed (T2385-to-G2386 in S4 Fig). Additional sequencing of the polymorphic region between Pelo and marker 4.8 in selected recombinant plants obtained from populations such as the one displayed in Fig 1C enabled us to finally delimit the ty-5 gene into a 425 bp region containing two transversions. One transversion is the T1961-to-A1962 in the Pelo proximal promoter region and the other is the T47-to-G in the first exon of the gene (S4 Fig), resulting in a Valine16-to-Glycine substitution in TY172 (Fig 2). These results show that Pelo is the gene controlling TYLCV resistance at the ty-5 locus and that this resistance can be either attributed to its proximal promoter or its coding region. Noteworthy, other polymorphisms, but none of these SNPs, were found following sequencing of this region in DNA extracted from two-to-three plants each of PI 126926, PI 126930, PI 390681 and LA0441, the four accessions claimed to be the origin of ty-5 (demonstrated by their Pelo coding sequence in GenBank accession numbers KC567248 through KC567257). Moreover, screening of the 360 tomato genomes provided by Lin et al 2014 [29] showed no polymorphism at the two transversions mentioned above (the T1961-to-A1962 in the Pelo proximal promoter and the T47-to-G in the first exon of the gene).
Fig. 2. Amino-acid sequence of the Pelo gene in the resistant TY172 line compared to the susceptible line M-82. 
The substitution of Valine16 (susceptible lines) to a Glycine (resistant TY172 line) is highlighted with magenta; GenBank accession numbers for TY172 and M-82 are KC447285 and KC447286, respectively. Sequence analysis of a polymorphic region tightly linked to Pelo displayed complete identity between two plants of the old tomato hybrid Tyking genomic DNA with TY172 DNA (S4 Fig). Moreover, the amino-acids sequence of Pelo from Tyking was identical to its counterpart from TY172 (Fig 2). It is therefore highly likely that ty-5 in TY172 and Tyking originated from the same unknown source. Because to the best of our knowledge Tyking preceded TY172, we cannot exclude the possibility that ty-5 in TY172 was introgressed from Tyking. Nonetheless, the origin of ty-5 locus in Tyking is yet to be established.
Transcriptional analysis of Pelo
To test whether the SNP in the Pelo promoter-region is involved in the resistant phenotype, relative transcript level of the gene was compared between resistant and susceptible genotypes. The results show that relative transcript level of Pelo in the resistant line TY172 was not statistically different from the susceptible line M-82, whether the plants were infected with TYLCV or not (Table 1A). The relative transcript level of Pelo was also analyzed in another susceptible line used in this study, R13. The relative transcript level of Pelo in the resistant line TY172 was not statistically different from this susceptible line as well (Table 1B). These results suggest that the SNP in the Pelo promoter region is not associated with TYLCV resistance.
Tab. 1. Average relative transcript levels of the Pelo gene in resistant TY172 and susceptible M-82 and R13 plants. 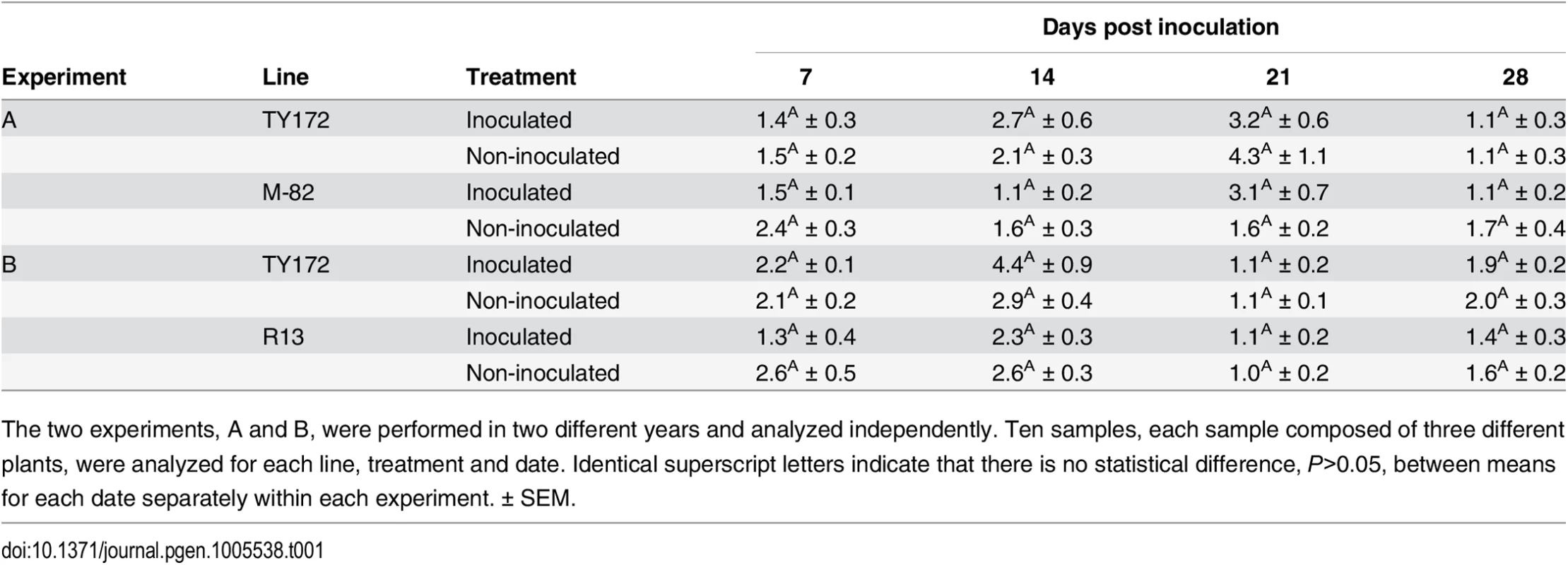
The two experiments, A and B, were performed in two different years and analyzed independently. Ten samples, each sample composed of three different plants, were analyzed for each line, treatment and date. Identical superscript letters indicate that there is no statistical difference, P>0.05, between means for each date separately within each experiment. ± SEM. Over-expression of Pelo
To confirm that indeed the SNP in the Pelo coding-region is responsible for the resistance phenotype, transgenic plants over-expressing each of the two Pelo alleles were developed. Two independent populations of transgenic TY172 plants segregating for pBINPelo-M-82 were analyzed (Table 2). Following inoculation with TYLCV, all transgenic plants displayed disease symptoms while their respective azygous controls were symptomless, similarly to inoculated non-transgenic TY172 plants. The transgenic plants showed an average disease severity index (DSI) values of 2.2 and 2.3 for TYT-10 and TYT-94, respectively, correlated with an approximately 3.5-fold higher average virus copy number (VCN) in transgenic plants as compared to their non-transgenic azygous controls (Table 2). The two transgenic TY172 lines were also assayed for transcript-expression levels of Pelo (Table 2). In both transgenic lines there was a significant increase in Pelo transcript level as compared to their non-transgenic azygous controls, a five-fold increase in the transgenic line TYT-10 (a T1 generation), and a 91-fold increase in the transgenic line TYT-94 (a T3 generation). These results clearly demonstrate that TY172 plants over-expressing the susceptible allele of Pelo are no longer resistant to TYLCV, although their DSI is lower than that expected from fully susceptible plants. A possible explanation can be the presence of the minor OTLs identified in TY172 [17].
Tab. 2. Effect of over-expressing the Pelo allele from M-82 plants in transgenic TY172 plants. 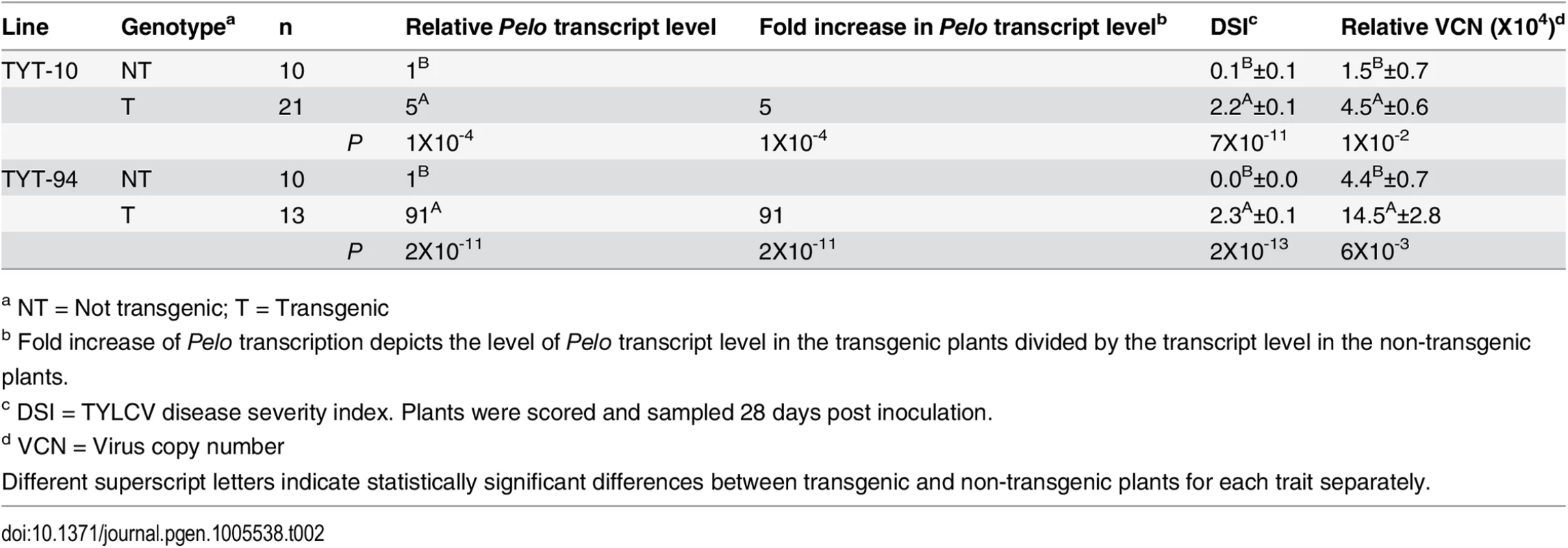
a NT = Not transgenic; T = Transgenic Three independent T1 populations of R13 plants segregating for pBINPelo-TY172 were also analyzed. While RNA level of the transgene was significantly increased in these lines (179, 229 and 173-fold in TYT-002, TYT-066 and TYT-103, respectively), no statistical difference was found in average DSI values between the transgenic and their azygous counterparts (Table 3). In all the three lines there was also no statistical difference in VCN between the transgenic plants and their azygous controls. These results show that over-expression of the resistant allele of Pelo does not affect the TYLCV-susceptible R13 plants, which is in agreement with the recessive nature of the ty-5 locus in segregating populations (Fig 1A, 1C and 1D).
Tab. 3. Effect of over-expressing the Pelo allele derived from TY172 plants in transgenic R13 plants. 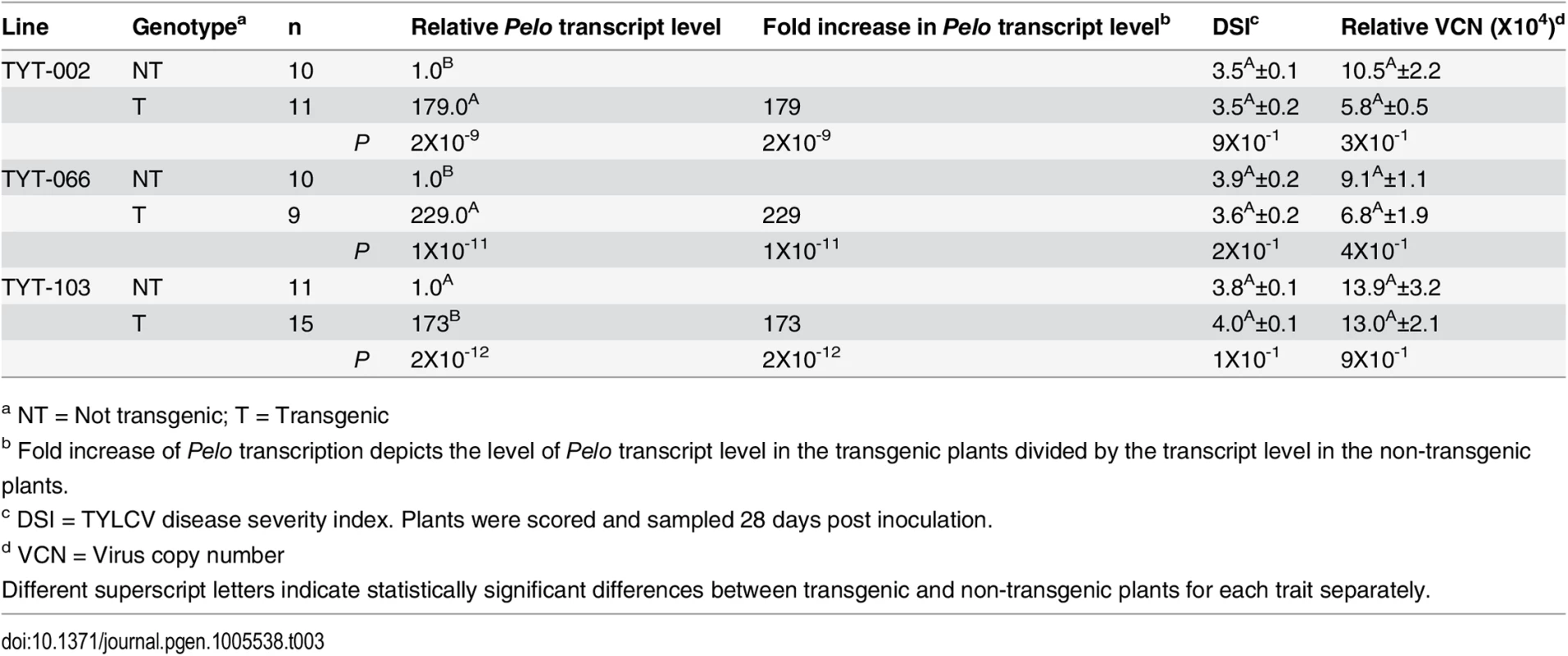
a NT = Not transgenic; T = Transgenic Silencing of Pelo
To further confirm that indeed the Pelo coding-region is responsible for the resistance phenotype, transgenic TYLCV-susceptible (R13) and resistant (TY172) plants harboring a Pelo silencing construct (pHannibal-Pelo) were developed. Three independent T1 populations of transgenic R13 plants segregating for the silencing construct pHannibal-Pelo were analyzed (Table 4). Following inoculation with TYLCV, all the transgenic plants showed no disease symptoms while their respective azygous controls showed severe disease symptoms (yellowing and cupping of the leaves, most clearly shown in the plant apex) (S5 Fig), similarly to inoculated R13 control plants (S6 Fig). These transgenic plants showed an average DSI values of practically 0, correlated with a 2.2-to-3.5 fold reduction in Pelo transcript level, and a 20-to-60 fold decrease in average VCN in transgenic plants as compared to their non-transgenic azygous controls (Table 4). However, when the two independent T1 populations of transgenic TY172 plants segregating for the silencing construct were analyzed there were no differences in disease severity between the transgenic plants and their respective azygous controls (Table 4 and S6 Fig). Both transgenic and azygous control alike showed no disease symptoms, with a DSI of 0, coupled with a six-to-nine fold reduction in Pelo transcript level in the transgenic plants as compared to their non-transgenic azygous controls (Table 4). Interestingly, despite the lack of difference in DSI, there was a significant difference in virus copy number: the transgenic plants showed a nine-to-tenfold decrease in VCN compared to their non-transgenic azygous control plants (Table 4).
Tab. 4. Effect of Pelo silencing in transgenic susceptible (R13) and resistant (TY172) plants. 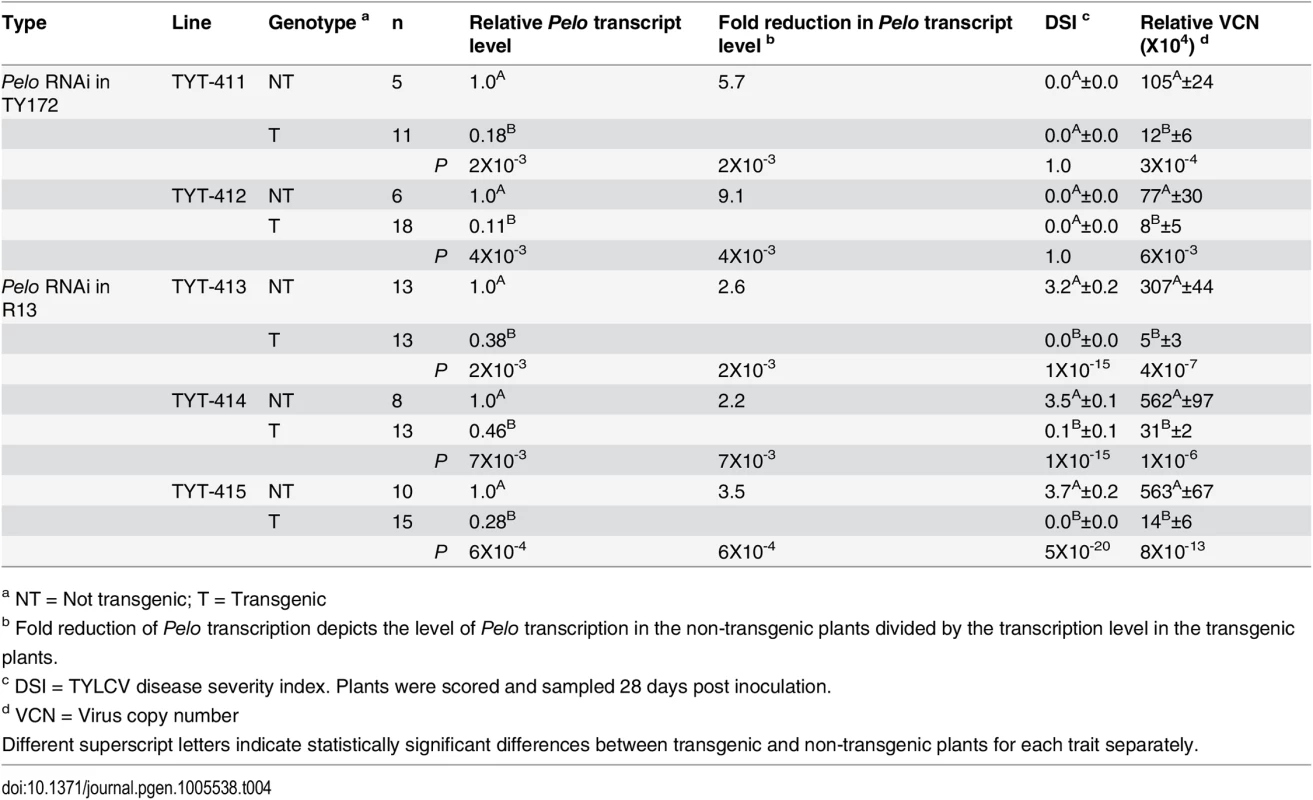
a NT = Not transgenic; T = Transgenic The effect of ty-5 in non-inoculated plants
A nearly isogenic BC4F3 segregating-population was developed by crossing TY172 (RR) as a maternal line and M-82 (SS) as a recurrent paternal line, using Pelo as the sole marker. Non-inoculated homozygous RR plants of this population displayed a significant reduction in fruit size, and a small insignificant reduction in total fruit yield and harvest index compared to the recurrent SS parent (Table 5). However, when compared to their homozygous SS counterparts the homozygous RR plants displayed a significant reduction of 23% in total yield and a 27% reduction in fruit size, suggesting that the presence of ty-5 may exerts a penalty.
Tab. 5. Yield components, per plant, of segregating non-inoculated BC4F3 plants in comparison to their recurrent parent M-82. 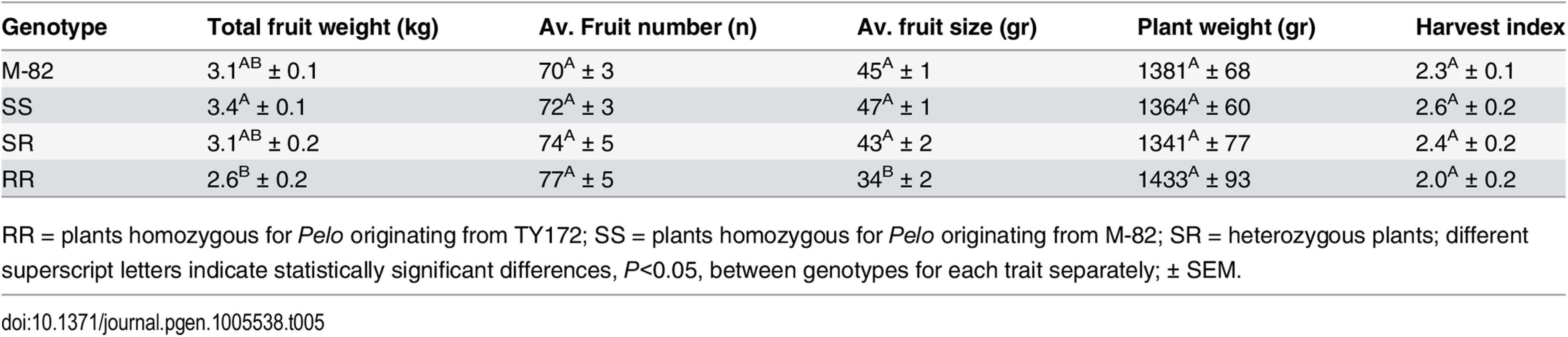
RR = plants homozygous for Pelo originating from TY172; SS = plants homozygous for Pelo originating from M-82; SR = heterozygous plants; different superscript letters indicate statistically significant differences, P<0.05, between genotypes for each trait separately; ± SEM. Discussion
TY172 is a tomato line expressing high level TYLCV-resistance. Based on a classical segregation study, it was suggested that the resistance is controlled by three genes that originated from S. peruvianum [21]. Subsequently, molecular mapping studies showed that TYLCV-resistance in TY172 is controlled by a major recessive QTL, termed ty-5, and four additional minor QTLs. The major QTL maps to chromosome 4 while the minor QTLs were mapped to chromosomes 1, 7, 9 and 11 [17].
This study was designed to identify the gene controlling TYLCV-resistance at the ty-5 locus. To accomplish this, a core set of 27 polymorphic DNA markers were designed. The use of 51 informative recombinant populations enabled us to exclude Nac1, a candidate gene suggested earlier [17], and delimits the ty-5 locus into a single gene encoding the tomato homolog of the messenger RNA surveillance factor Pelo. Two SNPs were identified: one in Pelo’s proximal promoter region and the other in its coding sequence. Our results further show that the relative transcript level of Pelo in the resistant TY172 plants was not statistically different from susceptible plants, either inoculated or not. It can therefore be suggested that the control of TYLCV-resistance is exerted by the SNP observed in the Pelo coding sequence, and not by the one observed in its proximal promoter region. These two SNPs and others observed upstream to their location could not be traced in sequences of two-to-three plants of each of the four S. peruvianum accessions claimed to be the origin of ty-5 [21]. This suggests that the Pelo allele residing in TY172 originated from a different source or that it represents a rare allele segregating in one or more of these four accessions. Noteworthy, Pelo and its upstream promoter sequence from the cultivar Tyking, carrying a recessive resistance co-localized with ty-5 [25], were found identical to TY172. Although we cannot exclude the possibility that the resistant Pelo allele originated from Tyking, we were not able to trace its origin in wild and other cultivated tomato accessions, including in those of the 360 tomato genomes provided by Lin et al 2014 [29].
Over-expression of the cultivated Pelo allele in TY172 resulted in increased viral titer and disease symptoms, while over-expression of its resistant counterpart in susceptible plants had no effect on TYLCV titer and disease severity. In addition, silencing of Pelo in susceptible plants rendered these transgenic plants highly resistant to TYLCV—the inoculated plants showed practically no disease symptoms, and a 20-to-60 fold reduction in virus titer compared to non-transgenic control plants. Finally, silencing Pelo in resistant TY172 plants had no effect on disease severity. Nonetheless, these transgenic plants had a nine-to-tenfold decrease in virus titer compared to their non-transgenic control plants. Taken together, these results confirm that Pelo controls the TYLCV-resistance at the ty-5 locus.
Pelo was recently implicated in the recycling phase of protein biosynthesis as part of eukaryotic and archaeal ribosome recycling complexes containing also an ABC-type ATPase (Abce1) [28]. Ribosome-driven protein biosynthesis has four phases: initiation, elongation, termination and recycling. Interestingly, recessive genes for resistance to plant viruses have been linked to components of the eukaryotic translation initiation complex. Translation initiation factors, particularly the eIF4E and eIF4G protein families, were found to be essential for RNA virus infections [30]. Here we show that a protein implicated in the latest phase of ribosome-driven protein biosynthesis controls the recessive resistance to TYLCV, a DNA virus.
Recessive resistance genes are more prevalent controlling resistance to viruses than resistance to fungal or bacterial pathogens which is primarily a dominant genetic trait [30]. Because typical plant viruses encode relatively few proteins, between four-to-ten, and need to recruit many different host components to complete their infection cycle, it was proposed that resistance genes would correspond to mutation or loss of host components required for a stage of the virus life cycle [31]. This fits well with the recessive nature attributed in this study to the Pelo allele variant residing in TY172.
Although many host factors are required for plant virus infections [32], analysis of recessive resistance identified in the natural diversity of crops has thus far only revealed a group of proteins linked to the translation machinery [30]. TYLCV-resistance controlled by the ty-5 gene Pelo is not an exception, but points to the ribosome recycling phase of protein synthesis, rather than to its initiation, as an intervening step promoting resistance.
Recycling of ribosomes for a new round of translation initiation is an essential part of protein synthesis. As recently summarized [28], Abce1, mentioned above, can dissociate ribosomes into subunits either after canonical termination by release factors (Rfs), or after recognition of stalled ribosomes by messenger RNA surveillance factors such as Pelo, an eukaryotic Rf1 (eRf1) paralog. Notably, Abce1 is able to physically interact with eRf1 and directly influence its function in stop-codon recognition and peptidyl-transfer RNA hydrolysis. Failure to accomplish or fully complete this task, anticipated in a recessive mutant, would most probably negatively affect viral as well as host-plant protein synthesis. This in turn may result in slower infection progression, but may also negatively impact aspects of host plant development or its horticultural performances [33]. A screen-house study, carried out in this study, demonstrate that uninfected nearly-isogenic BC4F3 plants, homozygous for the Pelo allele derived from TY172, displayed a significant reduction in fruit size, and a small but not significant reduction in total fruit yield and harvest index compared to the recurrent susceptible parent (Table 5). This suggests that the presence of ty-5 may exert a small penalty on yield. Whether these negative effects remain in more advanced BC generations is yet to be elucidated. Noteworthy, this discussion is limited to non-inoculated plants, to the determinate growth-habit characterizing TY172 and M-82 plants, and to a single genetic background (M-82), while excluding possible contribution that hybrid vigor may have towards increased yield in hybrid commercial plants carrying ty-5. It should be clarified, that under TYLCV-inoculation, ty-5 has a clear advantage over susceptible plants [17, 23].
In a recent forward genetic screen for Drosophila mutants that are resistant to Drosophila C virus (DCV), a virus resistant line was found which had a deficient expression of the Pelo gene [34]. The Pelo deficiency was found to limit the high level synthesis of the DCV capsid protein. It was suggested that the reduction in synthesis of viral capsid is due to the role of Pelo in dissociation of stalled 80S ribosomes and clearance of aberrant viral RNA and proteins [34]. It should be noted however, that DCV is an RNA virus, completely different from TYLCV, which is a ssDNA virus.
To the best of our knowledge, Pelo and its protein product have never been implicated in virus resistance in plants and thus offer an alternative route to obtain such resistance. Our suggestion that its effect may be expressed through its role in protein translation machinery is promising. However, a direct interaction of Pelo with proteins involved in viral replication cannot be excluded.
Materials and Methods
Plant material and experimental layout
5,662 segregating F2, F3, F4, BC1F1, BC1F2, BC1F3, BC1F4, BC1F5, BC2F2, BC2F3 and BC2F4 seedlings originating from a cross between TY172 and M-82 (LA3475), were inoculated with TYLCV and genotyped with polymorphic markers spanning the ty-5 locus. M-82 was chosen as the recurrent parent due to its determinate stature, facilitating working with large number of plants. The inoculation experiments were carried out in 8x16 sowing trays. Eight seedlings of the two parental lines were included in each tray while their F1 hybrid plants were included sporadically. Twenty-one days post inoculation (DPI), the seedlings were transplanted to the field or to 50-mesh screen-houses. Parental lines and their F1 hybrids were planted in a randomized block design, five or more plants in three blocks, while their segregating counterparts were planted at random. In the field plants were grown in rows, one m apart, allowing 50 cm space between plants, while in screen-houses in eight L pots, similarly arranged. Plants were grown using standard local cultural practices, including drip irrigation and fertilization.
The effect of the Pelo allele derived from TY172 on yield of non-inoculated segregating BC4F3 plants was estimated in a randomized-block screen-house experiment (three blocks, five plants per genotype per block). These BC4F3 plants, developed with M-82 as a recurrent parent and Pelo as the sole marker, were compared to M-82. Total fruit yield, fruit number, average fruit weight, plant weight and harvest index (fruit-yield/plant-weight ratio) were recorded for each plant.
Other lines and accessions used: 1. Heinz 1706—a TYLCV-susceptible determinate line obtained from the Tomato Genetics Resource Center (TGRC at http://tgrc.ucdavis.edu/). This line was initially used to sequence the tomato genome (SGN, http://solgenomics.net/); 2. R13—a TYLCV-susceptible indeterminate line (Hazera Genetics, Berurim, Israel); 3. LA1589, LA0441, PI 126926, PI 126930, and PI 390681—LA accessions were obtained from TGRC while PI accessions were obtained from the U.S. Department of Agriculture, Plant Genetic Resources Unit, Geneva, NY; 4. Seeds of the TYLCV-resistant hybrid Tyking were generously provided by Jaap Hoogstraten (Monsanto).
Whitefly maintenance, plant inoculation and disease scoring
Whitefly colonies (Bemisia tabaci, biotype B), reared on cotton plants (Gossypium hirsutum), were used for TYLCV (Genbank accession No. X15656) inoculation as described [23, 35]. Thereafter, plants were sprayed with imidacloprid (Bayer, Leverkusen, Germany) and held in an insect-proof greenhouse at 26–32°C. TYLCV-induced symptoms were evaluated for each plant separately at 28 and 42 DPI according to a disease severity index (DSI) of 0-to-4 as follows: (0) no visible symptoms, inoculated plants show same growth and development as non-inoculated plants; (1) very slight yellowing of leaflet margins on apical leaf; (2) some yellowing and curling of leaflet ends; (3) a wide range of leaf yellowing, curling and cupping, with reduction in size, yet plants continue to develop and (4) very severe plant stunting and yellowing, pronounced cupping and curling, plants stop growth [21, 24].
Genomic DNA extraction and polymorphism identification
Genomic DNA was extracted from individual plants according to [36]. A 4394 base-pair (bp) genomic region spanning the Nac1 gene sequence in M-82 was blasted against the tomato sequence database at the Solanaceae Genomics Network (SGN, http://solgenomics.net/) and found fully homologous to the 8,734,372–8,738,765 bp region of the scaffold DNA sequence file SL2.40sc03604, and 2,854,539–2,858,932 bp region of the tomato chromosome 4 sequence in the DNA sequence file SL2.40ch04 (version SL2.40). Sequences in SL2.40sc03604 and SL2.40ch04 were used to design polymerase chain reaction (PCR) primers expected to amplify 800-to-900 bp fragments of the tomato genome spanning the Nac1 gene region: first, approximately every 50 Kilo bp (Kbp), then every 10 Kbp and finally every 3 Kbp and at times less. Altogether, 257 such fragments were sequenced. Sequence analysis and locus-specific primer design were carried out with the DNAMAN sequence analysis software v4.1 (Lynnon BioSoft, Québec, Canada). DNA primers were purchased from Molecular Biology Center (Ness-Ziyyona, Israel).
The primers designed were used to PCR-amplify genomic DNA of TY172 and M-82 plants; amplification products were visualized by electrophoresis, extracted from the gel and directly sequenced according to [17]. These sequences were compared to the sequence of Heinz 1706 (http://solgenomics.net/) in order to detect single nucleotide polymorphisms (SNPs). These polymorphisms were further used to design polymorphic DNA markers, utilizing restriction endonucleases when necessary, that were analyzed for association with the resistance trait in segregating populations. Altogether, a core set of 27 polymorphic DNA markers, spanning the ty-5 locus, were utilized by PCR (S1 Table). Following amplification, PCR products were digested and visualized by electrophoresis according to [17].
Melting curve SNP genotyping for Pelo
We used a Melting curve SNP genotyping method (McSNP) with primers designated as McSNP F and R in S1 Table. Primers design was carried out by DYN R&D (Qesariyya, Israel). The McSNP genotyping reaction, described previously [37], was calibrated and carried out by DYN R&D using the LightCycler 480 instrument (Hoffmann-La Roche, Basel, Switzerland). Initial reaction conditions were: incubation at 95°C 10 min, followed by 50 cycles of 95°C 10 sec, a touch-down annealing 63°C→56°C 10 sec (-1.5°C per cycle) and 72°C 10 sec. Melting curve reaction conditions were: 95°C 1 min, 40°C 2 min, and 40–75°C degree, five acquisitions per degree. Results were analyzed using the LightCycler 480 SW 1.5 software (Hoffmann-La Roche), by DYN R&D utilizing its melt-curve analysis.
Silencing of Pelo
Construction of Pelo silencing vector: to silence Pelo, a pHannibal vector [38] expressing a sense and anti-sense fragment of the gene was constructed in two steps. In the first step, a 576 bp fragment of the Pelo gene (cDNA coordinates 560 to 1136) was amplified by PCR using the forward primer SPeloF-XhoI (5'-AGACTCGAGGACAATGTTCTACAGGCCTTTG-3'), containing a XhoI restriction site, and the reverse primer SPeloR-KpnI (5'-GACGGTACCCATCTCAAT GTCTTCCAGCTC-3'), containing a KpnI site. This fragment was cloned into the unique XhoI and KpnI sites present in the sense oriented arm of pHannibal. In the second step, the same 576 bp fragment of the gene was amplified by PCR performed with the forward primer SPeloF-XbaI (5'-ATCTAGAGACAATGTTCTACAGGCCTTTG-3'), containing a XbaI site, and the reverse primer SPeloR-ClaI (5'-CATCGATCATCTCAATGTC TTCCAGCTC-3'), containing a ClaI site. This fragment was cloned into the unique XbaI and ClaI sites present in the anti-sense oriented arm of pHannibal, thus creating pHannibal-Pelo.
To create a binary vector, pHannibal-Pelo was cloned under the cauliflower mosaic virus (35S) promoter and the nitric oxide synthase transcriptional terminator into the NotI site of the pBIN vector.
Transformations were carried out on cotyledon cuttings of TY172 and the susceptible R13 lines with Agrobacterium tumefaciens strain EHA105 as previously described [39]. R13 was chosen as the susceptible control due to its ease of transformation. Moreover, sequence analysis of Pelo in R13 revealed that it is identical to M-82.
Over-expression of Pelo
Pelo cDNA was cloned from both TY172 and M-82 plants, and inserted into a pBIN vector under the control of the 35S promoter. To create a pBIN expression vector, the cassette containing the 35S promoter, omega enhancer, and the NOS terminator was cloned into the HindIII-EcoRI sites of pBINPLUS.
To create the plasmids pBINPelo-M-82 and pBINPelo-TY172, the Pelo gene was PCR amplified using the primers PeloF (5’-CTAGGATCCatgaagattgttcgtagag-3’), containing a BamHI restriction endonuclease site, and PeloR (5’-CTAGCGGCCGCATCACATCTCAATGTCTTC-3'), containing a NotI site. The amplification product was restricted with both BamHI and NotI and cloned into the appropriate sites of the pBIN vector. Transformations were carried out as described above.
Characterization of transgenic plants
To validate incorporation of either the Pelo silencing construct or the over-expression constructs, DNA samples extracted from individual transformed plants served as templates in PCRs using a primer complementary to the 35S promoter (5’-CCTTCGCAAGACCCTTCCTCT-3’) and a primer complementary to 3’ of the Pelo gene sequence (5’-CTAGCGGCCGCATCACATCTCAATGTCTTC-3') for the silencing construct, while for the two Pelo transgenes a primer complementary to the Pelo gene sequence (5’-CTAGCGGCCGCATCACATCTCAATGTCTTC-3') was used. The PCRs were performed in a volume of 20 μl containing 15 ng of template DNA, 10 pmol of each primer, 0.2 mM of each dNTP, 2 mM MgCl2, 0.5 U of Taq DNA polymerase, and 1XPCR-buffer. The PCRs conditions were: 94°C 3 min, followed by 35 cycles of 94°C 30 s, 60°C 30 s, and 72°C 1 min. Final elongation was at 72°C 10 min. Amplification products were visualized by electrophoresis.
Detection of TYLCV DNA copy number in plants
TYLCV DNA copy-number in plants was determined using quantitative Real-Time PCR (qRT-PCR). Total DNA was extracted from plant apices. TYLCV primers were designed using the PRIMER 3 procedure (http://workbench.sdsc.edu/): TYRT2F = 5'-GCTGATCTGCCATCGATTTG-3' and TYRT2R = 5'-GGTTCTTCGACCTGGTATC-3' forming a 147 bp amplicon. The qRT-PCR was carried out on a Corbett Rotor-Gene 6000 (Qiagen, Düesseldorf, Germany) with the following profile: 40 cycles of 95°C 10 s, 60°C 15 s, and 72°C 20 s; qRT-PCR reactions (12 μl volume) included 3 μl of plant DNA, 6 μl of SYBR Fast Universal Readymix Kit (Kapa Biosystems, Boston, MA), and 0.125 μM of each primer.
DNA extracted from non-infected plants and water served as negative controls. Each qRT-PCR reaction was run in duplicate, with 5 replications per treatment. DNA of each sample was extracted from three different infected plants. For standard curve, PCR amplicon was cloned into pGEM-T Easy (Promega, Madison, WI). The plasmids were extracted using Plasmid Miniprep kit (Qiagen) and linearized by digesting with PstI. Gel-extracted fragments were quantified and used to create standard curves. Dilution series were performed by copy number following methods recommended by Applied Biosystems (Foster City, CA). Cycle threshold and copy number were determined using Corbett Rotor-Gene 6000 Series software. Amplification was followed by melt-curve analysis.
Pelo relative transcript levels
Pelo relative transcript levels were determined by qRT-PCR. Total RNA was extracted from tomato apex using TRI-reagent (Sigma-Aldrich, St. Louis, MO) and DNA contaminants were digested with TURBO DNA-free DNAase (Ambion, Austin, TX). The remaining RNA was used as template for cDNA synthesis using the Revertaid first strand cDNA synthesis Kit (Fermentas).
Pelo primers were designed using the Primer 3 procedure. Primers used for the qRT-PCR reaction of the Pelo transcript level were: PeloRTF = 5'-CCATGAGCGTCTGGCTATTC-3' and PeloRTR = 5'-GGAGACATGCATTGACGAGA-3', forming a 150 bp amplicon.
The qRT-PCRs (12 μl volume) were performed as outlined above, with the following profile: 40 cycles of 95°C 10 s, 58°C 15 s, and 72°C 20 s. 18S ribosomal RNA was used as reference utilizing the following primers: 18SF = 5'-GCGACGCATCATTCAAATTTC-3' and 18SR = 5'-TCCGGAATCGAACCCTAATTC-3'.
qRT-PCR analyses were performed using the Rotor-Gene Q detection system and data was collected and analyzed with the Rotor-Gene 6000 software version 1.7.28 (Qiagen). Relative abundance of Pelo transcripts were calculated by the formula: 2-(CT_Pelo-CT_18S), where CT represents the fractional cycle number at which the fluorescence crosses a fixed threshold (usually set on 0.1).
Statistical analyses
The association between DNA markers and DSI scores were evaluated by analyses of variance and chi-square (χ2) with the JMP statistical discovery software (SAS Institute, Cary, NC). An excellent agreement was found between χ2 and the respective analyses of variance; therefore, analyses of variance are presented throughout this manuscript.
Differences in the relative abundance of the Pelo transcripts between genotypes were analyzed, following transformation to their natural logarithm, by analyses of variance using JMP software. Differences among means were statistically evaluated based on Tukey-Kramer Honestly Significant Difference test [40].
Supporting Information
Zdroje
1. Cohen S, Harpaz I. Periodic rather than continual acquisition of a new tomato virus by its vector, the tobacco whitefly (Bemisia tabaci Gennadius). Entomol Exp Appl. 1964; 7 : 155–166.
2. Lefeuvre P, Martin DP, Harkins G, Lemey P, Gray AJA, Meredith S, et al. The spread of tomato yellow leaf curl virus from the Middle East to the world. PLoS Pathog. 2010; 6: e1001164. doi: 10.1371/journal.ppat.1001164 21060815
3. Navas-Castillo J, Fiallo-Olivé E, Sánchez-Campos S. Emerging virus diseases transmitted by whiteflies. Annu Rev Phytopathol. 2011; 49 : 219–248. doi: 10.1146/annurev-phyto-072910-095235 21568700
4. Lapidot M, Friedmann M. Breeding for resistance to whitefly-transmitted geminiviruses. Ann Appl Biol. 2002; 140 : 109–127.
5. Hanssen IM, Lapidot M, Thomma BPHJ. Emerging viral diseases of tomato crops. Mol Plant Microbe Interact. 2010; 23 : 539–548. doi: 10.1094/MPMI-23-5-0539 20367462
6. Moriones E, Navas-Castillo J. Tomato yellow leaf curl virus, an emerging virus complex causing epidemics worldwide. Virus Res. 2001; 71 : 123–134.
7. Morales FJ. Conventional breeding for resistance to Bemisia tabaci-transmitted geminiviruses. Crop Prot. 2001; 20 : 825–834.
8. Polston JE, Lapidot M. Management of Tomato yellow leaf curl virus: US and Israel perspectives. In: Czosnek H, ed. Tomato yellow leaf curl virus disease. Springer, the Netherlands; 2007. pp. 251–262.
9. Lapidot M, Legg JP, Wintermantel WM, Polston JE. Management of whitefly-transmitted viruses in open-field production systems. In: Loebenstein G, Katis N, editors. Advances in Virus Research. Burlington: Academic Press, 2014. Vol 90, pp. 147–206. doi: 10.1016/B978-0-12-801246-8.00003-2 25410102
10. Zamir D, Ekstein-Michelson I, Zakay Y, Navot N, Zeidan M, Sarfatti M, et al. Mapping and introgression of a tomato yellow leaf curl virus tolerance gene, TY-1. Theor Appl Genet. 1994; 88 : 141–146. doi: 10.1007/BF00225889 24185918
11. Hanson PM, Bernacchi D, Green SK, Tanksley SD, Venkataramappa M, Padmaja AS, et al. Mapping a wild tomato introgression associated with tomato yellow leaf curl virus resistance in a cultivated tomato line. J Am Soc Hortic Sci. 2000; 125 : 15–20.
12. Hanson PM, Green SK, Kuo G. Ty-2, a gene on chromosome 11 conditioning geminivirus resistance in tomato. Tomato Genet Coop Rep. 2006; 56 : 17–18.
13. Ji Y, Scott JW. Ty-3, a begomovirus resistance locus linked to Ty-1 on chromosome 6 of tomato. Tomato Genet Coop Rep. 2006; 56 : 22–25.
14. Ji Y, Schuster DJ, Scott JW. Ty-3, a begomovirus resistance locus near the Tomato yellow leaf curl virus resistance locus Ty-1 on chromosome 6 of tomato. Mol Breeding. 2007a; 20 : 271–284.
15. Ji Y, Scott JW, Hanson P, Graham E, Maxwell DP (2007b) Sources of resistance, inheritance, and location of genetic loci conferring resistance to members of the tomato-infecting begomoviruses. In: Czosnek H, ed. Tomato yellow leaf curl virus disease. Springer, the Netherlands; 2007b. pp. 343–362.
16. Ji Y, Scott JW, Schuster DJ, Maxwell DP. Molecular mapping of Ty-4, a new Tomato yellow leaf curl virus resistance locus on chromosome 3 of Tomato. J Am Soc Hortic Sci. 2009; 134 : 281–288.
17. Anbinder I, Reuveni M, Azari R, Paran I, Nahon S, Shlomo H, et al. Molecular dissection of Tomato leaf curl virus resistance in tomato line TY172 derived from Solanum peruvianum. Theor Appl Genet. 2009; 119 : 519–530. doi: 10.1007/s00122-009-1060-z 19455299
18. Verlaan MG, Szinay D, Hutton SF, de Jong H, Kormelink R, Visser RG, et al. Chromosomal rearrangements between tomato and Solanum chilense hamper mapping and breeding of the TYLCV resistance gene Ty-1. Plant J. 2011; 68 : 1093–1103. doi: 10.1111/j.1365-313X.2011.04762.x 21883550
19. Verlaan MG, Hutton SF, Ibrahem RM, Kormelink R, Visser RGF, Scott JW, et al. The Tomato Yellow Leaf Curl Virus Resistance Genes Ty-1 and Ty-3 Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases. PLoS Genet 2013; 9: e1003399. doi: 10.1371/journal.pgen.1003399 23555305
20. Butterbach P, Verlaan MG, Dullemans A, Lohuis D, Visser RGF, Bai Y, et al. Tomato yellow leaf curl virus resistance by Ty-1 involves increased cytosine methylation of viral genomes and is compromised by cucumber mosaic virus infection. PNAS 2014; 111 : 12942–12947. doi: 10.1073/pnas.1400894111 25136118
21. Friedmann M, Lapidot M, Cohen S, Pilowsky M. A novel source of resistance to tomato yellow leaf curl virus exhibiting a symptomless reaction to viral infection. J Am Soc Hortic Sci. 1998; 123 : 1004–1007.
22. Peralta IE, Knapp S, Spooner DM. New species of wild tomatoes (Solanum Section Lycopersicon: Solanaceae) from Northern Peru. Syst Bot. 2005; 30 : 424–434.
23. Lapidot M, Friedmann M, Lachman O, Yehezkel A, Nahon S, Cohen S, et al. Comparison of resistance level to tomato yellow leaf curl virus among commercial cultivars and breeding lines. Plant Dis. 1997; 81 : 1425–1428.
24. Lapidot M, Friedmann M, Pilowsky M, Ben Joseph R, Cohen S. Effect of host plant resistance to Tomato yellow leaf curl virus (TYLCV) on virus acquisition and transmission by its whitefly vector. Phytopathol. 2001; 91 : 1209–1213.
25. Hutton SF, Scott JW, Schuster DJ. Recessive resistance to Tomato yellow leaf curl virus from the tomato cultivar Tyking is located in the same region as Ty-5 on chromosome 4. HortScience 2012; 47 : 324–327.
26. Selth LA, Dogra SC, Rasheed MS, Healy H, Randles JW, Rezaian MA. A NAC domain protein interacts with tomato leaf curl virus replication accessory protein and enhances viral replication. Plant Cell. 2005; 17 : 311–325. 15608335
27. Kamiyoshihara Y, Iwata M, Fukaya T, Tatsuki M, Mori H. Turnover of LeACS2, a wound-inducible 1-aminocyclopropane-1-carboxylic acid synthase in tomato, is regulated by phosphorylation/dephosphorylation. Plant J. 2010; 64 : 140–150. doi: 10.1111/j.1365-313X.2010.04316.x 20659278
28. Becker T, Franckenberg S, Wickles S, Shoemaker CJ, Anger AM, Armache JP. et al. Structural basis of highly conserved ribosome recycling in eukaryotes and archaea. Nature 2012; 482 : 501–506. doi: 10.1038/nature10829 22358840
29. Lin T, Zhu G, Zhang J, Xu X, Yu Q, Zheng Z, et al. Genomic analyses provide insights into the history of tomato breeding. Nat Genet. 2014; 46 : 1220–1226. doi: 10.1038/ng.3117 25305757
30. Robaglia C, Caranta C. Translation initiation factors: a weak link in plant RNA virus infection. Trends Plant Sci. 2006; 11 : 40–45. 16343979
31. Fraser RSS. Genes for resistance to plant viruses. CRC Crit Rev Plant Sci. 1986; 3 : 257–294.
32. Kushner DB, Lindenbach BD, Grdzelishvili VZ, Noueiry AO, Paul SM, Ahlquist P. Systematic, genome-wide identification of host genes affecting replication of a positive-strand RNA virus. Proc Natl Acad Sci USA. 2003; 100 : 15764–15769. 14671320
33. Brown JK. Yield penalties of disease resistance in crops. Curr Opin Plant Biol. 2002; 5 : 339–344. 12179968
34. Wu X, He W-T, Tian S, Meng D, Li Y, Meng D, et al. Pelo is required for high efficiency viral replication. PLoS Pathog. 2014; 10(4): e1004034. doi: 10.1371/journal.ppat.1004034 24722736
35. Lapidot M. Screening for TYLCV-resistant plants using whitefly-mediated inoculation. In: Czosnek H, ed. Tomato yellow leaf curl virus disease. Springer, the Netherlands; 2007. pp. 329–342.
36. Fulton TM, Chunwongse J, Tanksley SD. Microprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Mol Biol Rep. 1995; 13 : 207–209.
37. Ye J, Parra EJ, Sosnoski DM, Hiester K, Underhill PA, Shriver MD. Melting curve SNP (McSNP) genotyping: a useful approach for diallelic genotyping in forensic science. J Forensic Sci. 2002; 47 : 593–600. 12051343
38. Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, et al. Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J. 2001; 27 : 581–590. 11576441
39. Azari R, Reuveni M, Evenor D, Nahon S, Shlomo H, Chen L, et al. Over-expression of UV-DAMAGED DNA BINDING PROTEIN 1 links plant development and phytonutrient accumulation in high pigment-1 tomato. J Exp Bot. 2010; 61 : 3627–3637. doi: 10.1093/jxb/erq176 20566564
40. Kramer CY (1956) Extension of multiple range tests to group means with unequal numbers of replications. Biometrics. 1956; 12 : 309–310.
Štítky
Genetika Reprodukčná medicína
Článek Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation DevelopmentČlánek A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in OvulationČlánek Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAsČlánek FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2Článek Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion inČlánek The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly SiteČlánek Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem CellsČlánek A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal ErythropoiesisČlánek Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation inČlánek Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in OocytesČlánek MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 10- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Gene-Regulatory Logic to Induce and Maintain a Developmental Compartment
- A Decad(e) of Reasons to Contribute to a PLOS Community-Run Journal
- DNA Methylation Landscapes of Human Fetal Development
- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development
- Transcriptional Derepression Uncovers Cryptic Higher-Order Genetic Interactions
- Silencing of X-Linked MicroRNAs by Meiotic Sex Chromosome Inactivation
- Virus Satellites Drive Viral Evolution and Ecology
- A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota
- Sequence to Medical Phenotypes: A Framework for Interpretation of Human Whole Genome DNA Sequence Data
- Your Data to Explore: An Interview with Anne Wojcicki
- Modulation of Ambient Temperature-Dependent Flowering in by Natural Variation of
- The Ciliopathy Protein CC2D2A Associates with NINL and Functions in RAB8-MICAL3-Regulated Vesicle Trafficking
- PPP2R5C Couples Hepatic Glucose and Lipid Homeostasis
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
- Intermediate Levels of CodY Activity Are Required for Derepression of the Branched-Chain Amino Acid Permease, BraB
- "Missing" G x E Variation Controls Flowering Time in
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Type IV Collagen Controls the Axogenesis of Cerebellar Granule Cells by Regulating Basement Membrane Integrity in Zebrafish
- Loss of a Conserved tRNA Anticodon Modification Perturbs Plant Immunity
- Genome-Wide Association Analysis of Adaptation Using Environmentally Predicted Traits
- Oriented Cell Division in the . Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1
- Disproportionate Contributions of Select Genomic Compartments and Cell Types to Genetic Risk for Coronary Artery Disease
- A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Ovulation
- The RNAPII-CTD Maintains Genome Integrity through Inhibition of Retrotransposon Gene Expression and Transposition
- Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAs
- Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector
- SCARN a Novel Class of SCAR Protein That Is Required for Root-Hair Infection during Legume Nodulation
- IBR5 Modulates Temperature-Dependent, R Protein CHS3-Mediated Defense Responses in
- NINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish
- Decay-Initiating Endoribonucleolytic Cleavage by RNase Y Is Kept under Tight Control via Sequence Preference and Sub-cellular Localisation
- Large-Scale Analysis of Kinase Signaling in Yeast Pseudohyphal Development Identifies Regulation of Ribonucleoprotein Granules
- FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2
- LINE-1 Mediated Insertion into (Protein of Centriole 1 A) Causes Growth Insufficiency and Male Infertility in Mice
- Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion in
- Genome-Scale Mapping of σ Reveals Widespread, Conserved Intragenic Binding
- Uncovering Hidden Layers of Cell Cycle Regulation through Integrative Multi-omic Analysis
- Functional Diversification of Motor Neuron-specific Enhancers during Evolution
- The GTP- and Phospholipid-Binding Protein TTD14 Regulates Trafficking of the TRPL Ion Channel in Photoreceptor Cells
- The Gyc76C Receptor Guanylyl Cyclase and the Foraging cGMP-Dependent Kinase Regulate Extracellular Matrix Organization and BMP Signaling in the Developing Wing of
- The Ty1 Retrotransposon Restriction Factor p22 Targets Gag
- Functional Impact and Evolution of a Novel Human Polymorphic Inversion That Disrupts a Gene and Creates a Fusion Transcript
- The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly Site
- The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study
- Parent-of-Origin Effects of the Gene on Adiposity in Young Adults
- Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem Cells
- Retinoic Acid Receptors Control Spermatogonia Cell-Fate and Induce Expression of the SALL4A Transcription Factor
- A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis
- Protein O-Glucosyltransferase 1 (POGLUT1) Promotes Mouse Gastrulation through Modification of the Apical Polarity Protein CRUMBS2
- KIF7 Controls the Proliferation of Cells of the Respiratory Airway through Distinct Microtubule Dependent Mechanisms
- Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation in
- Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in Oocytes
- Protein Homeostasis Imposes a Barrier on Functional Integration of Horizontally Transferred Genes in Bacteria
- A New Method for Detecting Associations with Rare Copy-Number Variants
- Histone H2AFX Links Meiotic Chromosome Asynapsis to Prophase I Oocyte Loss in Mammals
- The Genomic Aftermath of Hybridization in the Opportunistic Pathogen
- A Role for the Chaperone Complex BAG3-HSPB8 in Actin Dynamics, Spindle Orientation and Proper Chromosome Segregation during Mitosis
- Establishment of a Developmental Compartment Requires Interactions between Three Synergistic -regulatory Modules
- Regulation of Spore Formation by the SpoIIQ and SpoIIIA Proteins
- Association of the Long Non-coding RNA Steroid Receptor RNA Activator (SRA) with TrxG and PRC2 Complexes
- Alkaline Ceramidase 3 Deficiency Results in Purkinje Cell Degeneration and Cerebellar Ataxia Due to Dyshomeostasis of Sphingolipids in the Brain
- ACLY and ACC1 Regulate Hypoxia-Induced Apoptosis by Modulating ETV4 via α-ketoglutarate
- Quantitative Differences in Nuclear β-catenin and TCF Pattern Embryonic Cells in .
- HENMT1 and piRNA Stability Are Required for Adult Male Germ Cell Transposon Repression and to Define the Spermatogenic Program in the Mouse
- Axon Regeneration Is Regulated by Ets–C/EBP Transcription Complexes Generated by Activation of the cAMP/Ca Signaling Pathways
- A Phenomic Scan of the Norfolk Island Genetic Isolate Identifies a Major Pleiotropic Effect Locus Associated with Metabolic and Renal Disorder Markers
- The Roles of CDF2 in Transcriptional and Posttranscriptional Regulation of Primary MicroRNAs
- A Genetic Cascade of Modulates Nucleolar Size and rRNA Pool in
- Inter-population Differences in Retrogene Loss and Expression in Humans
- Cationic Peptides Facilitate Iron-induced Mutagenesis in Bacteria
- EP4 Receptor–Associated Protein in Macrophages Ameliorates Colitis and Colitis-Associated Tumorigenesis
- Fungal Infection Induces Sex-Specific Transcriptional Changes and Alters Sexual Dimorphism in the Dioecious Plant
- FLCN and AMPK Confer Resistance to Hyperosmotic Stress via Remodeling of Glycogen Stores
- MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
- Sex Bias and Maternal Contribution to Gene Expression Divergence in Blastoderm Embryos
- Transcriptional and Linkage Analyses Identify Loci that Mediate the Differential Macrophage Response to Inflammatory Stimuli and Infection
- Mre11 and Blm-Dependent Formation of ALT-Like Telomeres in Ku-Deficient
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- Identification of a Single Strand Origin of Replication in the Integrative and Conjugative Element ICE of
- The Type VI Secretion TssEFGK-VgrG Phage-Like Baseplate Is Recruited to the TssJLM Membrane Complex via Multiple Contacts and Serves As Assembly Platform for Tail Tube/Sheath Polymerization
- The Dynamic Genome and Transcriptome of the Human Fungal Pathogen and Close Relative
- Secondary Structure across the Bacterial Transcriptome Reveals Versatile Roles in mRNA Regulation and Function
- ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Regeneration
- Pelle Modulates dFoxO-Mediated Cell Death in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



