-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Sex Bias and Maternal Contribution to Gene Expression Divergence in Blastoderm Embryos
The early embryonic stage of development is unique as this stage begins under the genetic control of the mother and transitions to genetic control of the zygote. In order to investigate how these developmental specificities have shaped gene expression evolution, we studied the contribution of maternal gene expression to the pool of transcripts in the early embryo and how gene expression differs in early female and male embryos. We find extensive female-bias in gene expression, something not observed in adults. This is largely due to the delay in the establishment of a mechanism that balances gene expression from the differing number of X chromosomes in females and males. The maternal contribution to the transcript pool in the embryo is largely conserved over species, with some exceptions. However, there may also be some species-specific differences in the degradation rate of maternal transcripts, an often-overlooked contributor to variation in transcript level.
Published in the journal: Sex Bias and Maternal Contribution to Gene Expression Divergence in Blastoderm Embryos. PLoS Genet 11(10): e32767. doi:10.1371/journal.pgen.1005592
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005592Summary
The early embryonic stage of development is unique as this stage begins under the genetic control of the mother and transitions to genetic control of the zygote. In order to investigate how these developmental specificities have shaped gene expression evolution, we studied the contribution of maternal gene expression to the pool of transcripts in the early embryo and how gene expression differs in early female and male embryos. We find extensive female-bias in gene expression, something not observed in adults. This is largely due to the delay in the establishment of a mechanism that balances gene expression from the differing number of X chromosomes in females and males. The maternal contribution to the transcript pool in the embryo is largely conserved over species, with some exceptions. However, there may also be some species-specific differences in the degradation rate of maternal transcripts, an often-overlooked contributor to variation in transcript level.
Introduction
To investigate the role of gene expression in development and its evolution, gene expression must be examined at multiple developmental stages across populations or species. The early embryonic stages of development are of special interest because they are genetically controlled by both the mother and the zygote. Indeed in all animals, the earliest embryo is transcriptionally inactive and contains only maternally deposited mRNAs that are progressively eliminated while zygotic transcription takes over. In this study, we focus on two aspects of this handover of developmental control from the mother to the zygote: the activation of zygotic transcription and its regulation and the contribution of maternal mRNA content to embryonic gene expression. First in Drosophila, the early embryonic stage is characterized by higher transcript levels in females for many zygotic genes on the X chromosome, indicating incomplete dosage compensation before the canonical MSL-mediated X chromosomal dosage compensation mechanism of transcription is established [1,2]. Second, during the early phases of development, the proportion of maternal mRNAs in the embryo drops rapidly, due to a tightly co-regulated process whereby maternal RNAs are degraded as zygotic RNAs begin to be transcribed (referred to as the maternal to zygotic transition, reviewed in [3]). How these two factors, incomplete X chromosomal dosage compensation and maternal transcripts, contribute to the evolution of embryonic gene expression remains unclear.
Patterns of sex-specific gene expression variation and evolution have been the subject of considerable attention (see [4] for review). In Drosophila, there have been a number of genome-wide studies examining gene expression in female and male flies across species, in sex-specific and non sex-specific tissues [5,6], whole adult flies[7,8], and gonadectomized flies (primarily in D. melanogaster only [9,10]). These studies (and numerous others in other systems) have come to a number of common conclusions, for example, that there are generally more male-biased than female-biased genes [8], and that male-biased genes are fast evolving [5,7,8], as are genes expressed in sex-limited reproductive tissues [11].
Gene expression in embryos has largely not been a part of this discussion of evolution of sex-biased gene expression, as sex-specific transcriptomes of embryos have been previously out of reach. As embryonic development is critical to the fitness of the organism, and the selective pressures and developmental constraints of this stage differ, we hypothesized that some of the patterns observed in adults might differ in embryos. A factor that is of critical importance is the incompleteness of X chromosomal dosage compensation in the early embryo [1,2,12–14]. Additionally, many studies addressing sex-biased gene expression focus on sex-specific tissues as compared to somatic tissues. Many genes expressed in sex-specific tissues (particularly male-specific tissues) are also tissue-specific in expression. The blastoderm embryo has not yet formed tissue layers, and will not form sex-specific tissues until much later in development. Thus genes expressed in this stage, and gene expression changes, are more likely to be pleiotropic than those in sex-specific tissues later in development. However, there are also many genes at the blastoderm stage that are specific to this time of development, so they may be less pleiotropic over developmental time.
Regulation of gene expression in the Drosophila blastoderm embryo has long been the subject of fruitful and extensive study. During this stage, widespread activation of zygotic transcription and degradation of maternal transcripts takes place. A number of additional developmental processes also occur during this stage, including cellularization of the previously syncytial embryo, and important steps in both the segmentation of the embryo along the anterior-posterior axis and future development of tissue layers along the dorsal-ventral axis. Despite the considerable resources that have been employed to understand development at this stage, it remains difficult to study in a comparative context. Different species vary in the timing and the duration of developmental stages [15,16], and the tendency of particular species to lay unfertilized eggs [17] or withhold egg deposition until the egg is of an advanced stage [16] contribute to the difficulties of comparative study.
We previously developed a technique to sequence transcriptomes of single Drosophila embryos [1], which has facilitated comparative studies of transcript levels across species [2]. With the ability to examine and image single individuals, we are able to obtain samples of precisely the same developmental stage according to embryo morphology. This also avoids concerns of female egg withholding, and we are able to visually identify, and remove, unfertilized eggs. Single embryo methods have also made it possible to examine transcriptome-level data in individual female and male embryos [1,2], rather than the mixed-sex pools that are usually studied.
In order to determine the variation and evolutionary patterns of sex-specific gene expression across Drosophila species, we chose four species spanning the evolutionary history of the genus, Drosophila melanogaster, Drosophila yakuba, Drosophila pseudoobscura, and Drosophila virilis (Fig 1A). These species diverged from 5 to 40 million years ago [18], yet to a large degree, are conserved in their early embryonic gene expression [19].
Fig. 1. General changes of embryonic gene expression between four Drosophila species. 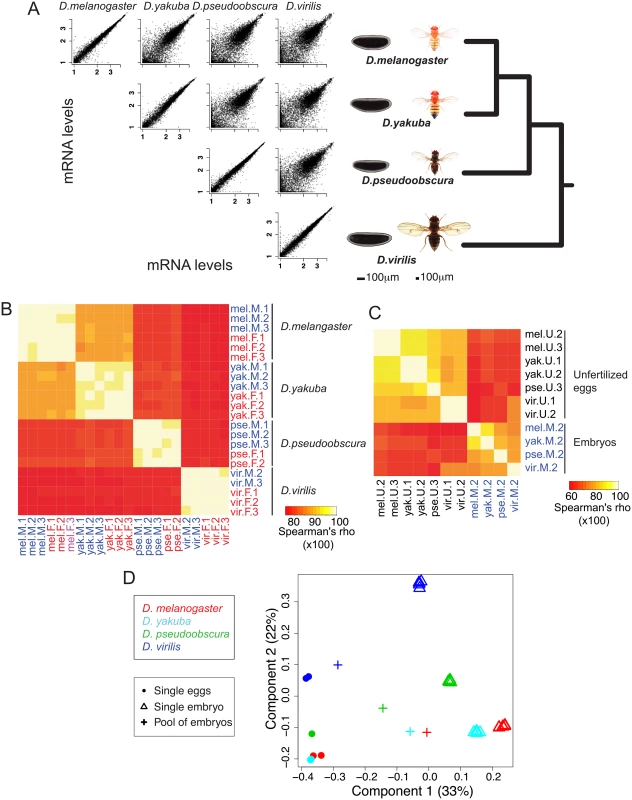
A. In this paper we compare the genome-wide mRNA levels in blastoderm embryos of four Drosophila species D. melanogaster, D. yakuba, D. pseudoobscura and D. virilis. Pairwise comparisons of mRNA levels between one male replicate in different species show that mRNA levels are overall highly conserved. As a comparison, scatter plots of two male replicates are shown for each species. B. Spearman correlations between mRNA levels of the 22 tightly staged embryos of our dataset (2–3 males/2–3 females per species). Correlations decrease with increasing phylogenetic distance. C. Spearman correlations between mRNA levels of the seven unfertilized eggs (1–2 per species) and a representative embryo sample from each species (“Male replicate 2”). Correlations are highest within eggs and embryos than between eggs and embryos. For B and C, the Spearman correlations are shown in percentages (so ranging from 0 to 100). D. Correspondence analysis (COA) of expression levels in the single eggs and embryos. Values for pools of embryos were projected to the COA. The proportion of the variance explained by the first components of the correspondence analysis is indicated in parentheses. Samples separate along first axis mainly according to developmental stage while they separate along second axis mainly according to species. Results
To characterize the sex-specific transcriptomes of individual blastoderm D. melanogaster, D. yakuba, D. pseudoobscura, and D. virilis embryos, we collected single embryos, imaged them to determine developmental stage, and extracted RNA and DNA for subsequent analysis (as in [1,2]). We chose the end of blastoderm stage (stage 5, mitotic cycle 14), when cellularization has been completed, but prior to gastrulation, as a precise timepoint observable from morphology, so that it would be a homologous timepoint across species. We used the extracted DNA in a PCR assay to determine the sex of the each embryo, using multiple Y-chromosome specific primers designed independently for each species (see Methods). We used RNA from individual female embryos (three replicates) and individual male embryos (three replicates) from each species (for a total of six libraries per species) to make libraries for RNA sequencing, without amplification of input RNA as previously described [1]. After sequencing, one female D. pseudoobscura embryo and one D. virilis male embryo were removed from analysis due to low data quality. In addition, we also harvested individual newly deposited unfertilized eggs (referred to hereafter simply as “eggs”, as opposed to the blastoderm stage individuals, referred to as “embryos”) from each species, and used them to construct single egg mRNA-Seq libraries (two libraries for D. melanogaster, D. yakuba and D. virilis and one library for D. pseudoobscura). Libraries were sequenced on an Illumina HiSeq 2000 DNA Sequencer.
We aligned reads to the D. melanogaster reference annotation (Flybase r5.39) or to D. yakuba, D. pseudoobscura or D. virilis annotations produced in a previous study [19] using Bowtie2 [20]. Transcript levels were inferred using eXpress [21] and gene levels were computed as the sum of corresponding transcript levels. Gene expression was normalized so that the 75th percentile levels of genes located on autosomes (Müller elements B, C, and E) was identical between samples, then log transformed (log10(normalized FPKM+1)). Additional normalization procedures were also performed (median, 95th percentile, TMM, quantile normalizations after eXpress as well as no further normalization other than the one performed by eXpress) and they gave essentially the same results (see the Methods section for further details). Below we focus on 5,985 genes that were expressed in at least one condition (egg, male or female embryo) in any species (normalized FPKM averaged over all replicates of a condition above 1 before log transformation) and for which we could establish a clear 1 : 1:1 : 1 orthology [19].
Pairwise plots of transcript levels show highly reproducible sampling within a species (plots along the diagonal in Fig 1A), with Spearman correlation coefficients of 0.98–0.99 (Fig 1B). There is also a high level of conservation of transcript levels in the blastoderm embryo across species. These correlations of transcript levels decrease with increasing evolutionary distance, for example, when comparing D. melanogaster to the other species, the highest correlation is with D. yakuba (median correlation of ~0.89), then with D. pseudoobscura (~0.81), and D. virilis (~0.78) (S1 Fig). Correlations for unfertilized eggs between these species are systematically slightly higher than that for embryos: again when comparing D. melanogaster eggs to the other species, median correlations are ~0.92 for D. yakuba, ~0.85 for D. pseudoobscura and ~0.83 for D. virilis (Fig 1C and S1 Fig). These higher correlations may imply more stringent developmental constraints on maternal transcript deposition (eggs), than early zygotic transcription (blastoderm embryos).
Our single embryo approach differs from most studies that have compared transcript levels genome-wide in pools of Drosophila embryos, typically collected over a two-hour window [22,23]. Pooling has theoretical disadvantages over a single embryo approach, because pooling combines mRNA content from embryos of various ages, which may bias subsequent analyses and obscure any meaningful comparative results. We had previously created an RNA-seq dataset obtained from pools of late-stage 4 to mid-stage 5 embryos in D. melanogaster, D. yakuba, D. pseudoobscura and D. virilis [19]. Having collected both the pooled [19] and individual egg/embryo (this study) datasets in the same four species, we compared them to more accurately determine the nature of the potential systematic “pooling” bias. We first compared gene levels between our single eggs and embryos through a correspondence analysis (COA, Fig 1D). As shown in Fig 1D, the correspondence analysis separates the samples by stage (first axis) and by species (second axis). We then projected on the COA expression data from pools of embryos spanning the end of stage 4 to mid-stage 5 that had been processed the same way as the single embryo/egg samples.
The pooled samples fall on the same position as single embryos and eggs along the second (species) axis but are intermediate between eggs and embryos on the developmental stage axis. In particular, the D. virilis pool sample resembles single egg samples more than other species (accordingly, levels from this sample are highly correlated with levels from eggs, Fig 1C), which is likely due to both higher proportion of undeveloped eggs in this species and differences in the distribution of stages within the collection window. Indeed, mRNA molecules were harvested from pools of embryos at any stage between late stage 4 and early stage 5, and not at a single time point. The distribution of time points within the collection window is likely to vary between species, and as a consequence the “averaged” levels from the sequenced mRNA pool do not represent the same “average” stages in the different species. In summary, pooling embryos of several developmental stages creates a confounding factor between developmental stage and species. Consequently, comparative analyses based on such pools of embryos may falsely attribute developmental differences as species differences and should be avoided as much as possible. In addition and unless specified, we averaged transcript levels over female embryo replicates, male embryo replicates, and egg replicates.
General patterns of sex-bias across species
We define a sex-biased gene as a gene with a higher transcript level in females compared with males or vice versa, as is used with studies of later stages of development when canonical dosage compensation is fully established on the X chromosome. As discussed in greater detail below, much of what we define as female-bias is likely due to lack of complete dosage compensation of the X chromosome (Muller A) at this stage of development. Our previous study showed that roughly half of zygotic genes on the X chromosome in D. melanogaster have equal transcript levels in females and males at this developmental stage, prior to the onset of MSL-mediated dosage compensation, and half are higher in females, suggesting no or partial dosage compensation [1]. However, with this study, we lack the allele-specific data necessary to validate whether transcripts are maternal or zygotic in origin, which is necessary for quantifying dosage compensation (maternal transcripts originate only from females, so no dosage imbalance exists for these templates). Thus, it is difficult to make assumptions about the expected compensation status or expression relative to dosage for genes at this point in development. We use the definition of sex-bias as having a higher transcript level in one sex, but make no assumption as to the mechanistic basis of this bias. This also permits comparison to the large body of work examining sex-specific transcript levels in later developmental stages.
Genome-wide, transcript levels are largely the same between female and male blastoderm embryos (Fig 1B). But sex chromosomes, and the incomplete nature of X chromosomal dosage compensation at this stage (prior to the onset of MSL-mediated dosage compensation, [1,2,12,13]), contribute to sex-specific expression differences. Generally, autosomal genes have similar, non-sex biased, transcript levels across species (Fig 2A–2C). In all species, at this period of developmental time, Müller A (the shared X chromosome) appears to have incomplete dosage compensation (Fig 2A). Genes from this shared sex chromosome are largely similarly female-biased across species (purple dots in Fig 2B). There is no sex-bias in maternal deposition of mRNAs, as these RNAs are deposited before the eggs are fertilized by X or Y bearing sperm. As around half of the transcripts at blastoderm stage are maternal in origin, the female-bias in genes at this time period presumably originates with zygotically expressed genes. The other striking pattern of sex-bias is on the neo-X chromosome in D. pseudoobscura, where Müller D has undergone chromosomal fusion with Müller A [24]. Müller D in D. pseudoobscura shows patterns of sex-bias similar to Müller A, with female-bias in transcript levels, indicating incomplete dosage compensation (Fig 2A–2C). This increase in female-bias is primarily due to a decrease in male expression in the D. pseudoobscura lineage (Fig 2D, Wilcoxon test p-value < 1.2 10−6 for all normalizations), which is consistent with females having two copies of Müller D as in the rest of the species where this element is an autosome, whereas D. pseudoobscura males only have a single copy.
Fig. 2. Gene expression on the neo-X (Müller D element) in D. pseudoobscura does not show complete dosage compensation. 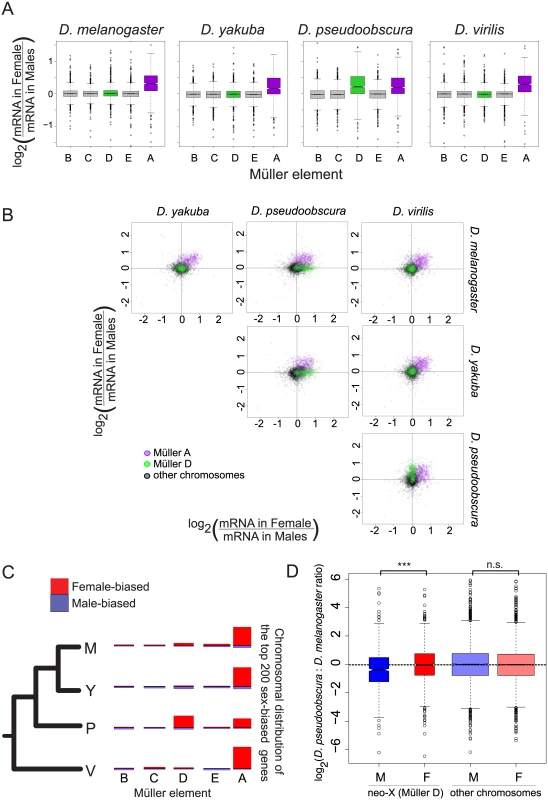
A. Gene expression on sex chromosomes shows partial dosage compensation in all species. The sex ratio (female/male) was calculated for each gene and compared between chromosomes. Genes from all autosomes show similar expression in both sexes (log2 ratio close to 0) while genes located on the Müller A element (purple) in all species and on the Müller D element (green) in D. pseudoobscura show a systematic bias towards higher ratio. B. Plot of pairwise sex ratio, the ratio of female transcripts to male transcripts, between species. The Müller A (purple) element is female-biased in all species, whereas the Müller D (green) is female-biased only in D. pseudoobscura. C. The top 200 most sex-biased genes are mostly female-biased, and are mostly located on sex chromosomes: the Müller A element in all species, and Müller D in D. pseudoobscura. Each chromosome was drawn with a length proportional to gene content. The height of red bars drawn above each chromosome and blue bars drawn below each chromosome represents the proportion of genes from this chromosome that are female-biased and male-biased, respectively. D. The decrease in sex ratio for genes located on the Müller D element in D. pseudoobscura is due to a decrease in male expression in D. pseudoobscura rather than an increase in females. We compared mRNA levels between D. pseudoobscura and D. melanogaster males and females for genes located either on the Müller D element or on other elements. Genes located on the Müller D element had similar mRNA levels in females but exhibited lower mRNA levels in D. pseudoobscura males compared to D. melanogaster males. ***: Wilcoxon test p-value < 0.001; n.s.: Wilcoxon test p-value > 0.05. Overall, there are more female-biased genes than male-biased genes, either proportionally of all genes with sex-bias (Fig 2C), or in total (S1 Table) at various cutoffs, for all species. This is in contrast to studies in adults, where only D. pseudoobscura was thought to have an overall higher level of female-biased genes than male-biased genes [8]. Presumably, our result differs due to incomplete dosage compensation at this stage, which leads to a greater proportion of female-biased genes. Similar to studies in adults [8], we observe that the magnitude of sex-bias is generally greater for male-biased genes (see S1 Table for various sex-bias cutoffs and Wilcoxon test p-values). While we fail to detect a general relationship between sex-bias and expression level, we do find that the most highly expressed genes on the X chromosomes (Müller A in all species, plus Müller D in D. pseudoobscura) are more likely to have the same transcript abundance in females and males (S2 Fig, species-specific Wilcoxon test p-values all < 2.5x10-9, comparing X chromosomal genes with a log10 transcript level of <2 to those >2). Most genes with high transcript levels at this stage are largely maternal in origin in D. melanogaster, which can potentially explain this result (the trend is likely to be similar in other species).
Species-specific divergence of sex-bias
In order to identify species-specific changes in sex-bias, we constructed rankings of sex-bias for all genes, within each species. The rank comparison of transcript levels between females and males of the same species provides a measurement largely unaffected by concerns of annotation or genome quality that can plague the analysis of quantitative mRNA levels between species. These rankings were used to investigate patterns of sex-bias across species, and across chromosomes. Female-bias and male-bias are then defined as being in the top or bottom percentiles of the sex rankings. Fig 3 presents results using the top and bottom 20% of sex-biased genes in each species, and qualitatively similar results for a range of cutoffs are presented in S2–S6 Tables. Genes reported as not sex-biased are in the middle 20% of the distribution (40–60%), and ranges of cutoffs for non-sex biased genes are also presented in S2–S6 Tables.
Fig. 3. Patterns of sex-bias over genes. 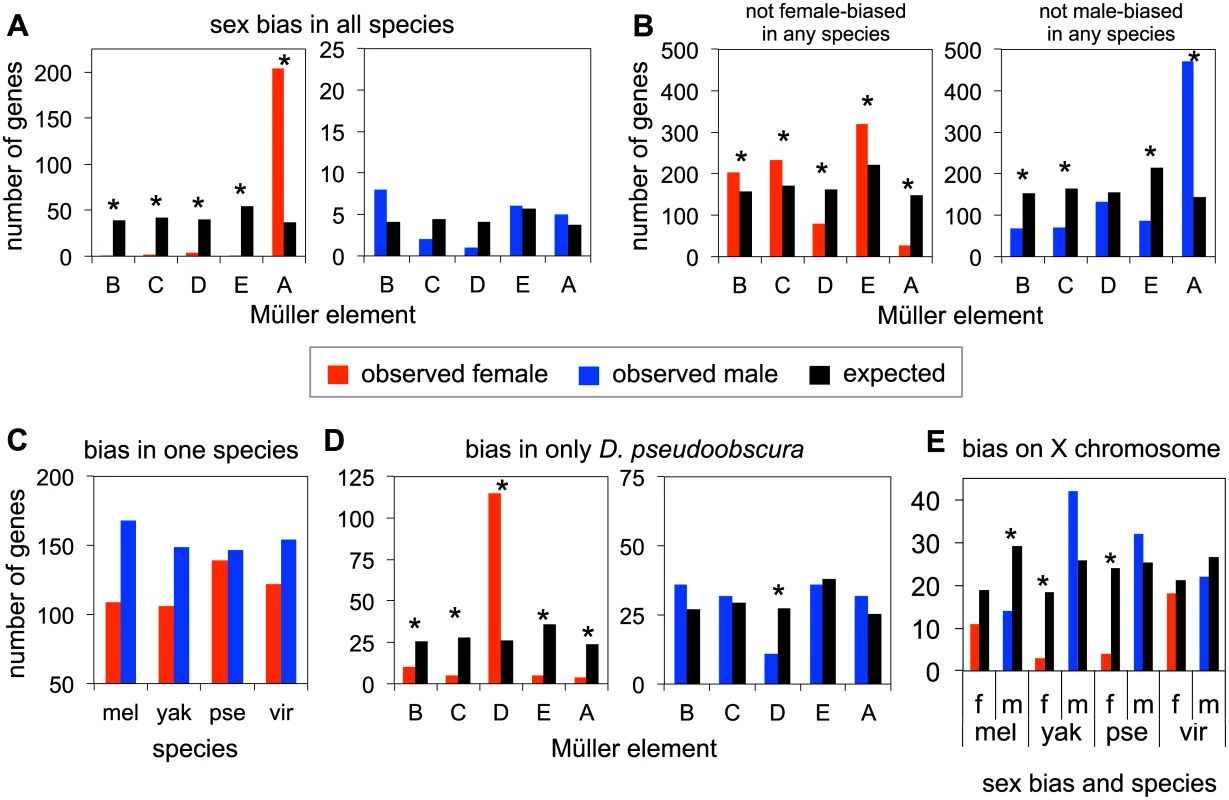
A. Numbers of genes that are female-biased (red) or male-biased (blue) in all four species across all chromosomes, as compared to the expected chromosomal distribution (black) proportional to number of genes on each chromosome. Note the difference in scale on the Y-axis between the female-bias and male-bias comparisons. B. Numbers of genes that are not female or male-biased in any of the four species, on each chromosome. C. Genes that are female or male-biased in only one species (and unbiased in the other three). D. Observed and expected chromosomal distributions of genes that are female or male-biased only in D. pseudoobscura. E. Genes that are female or male-biased on the X chromosome. Statistically significant results (p< 0.05) from chi-squared tests indicated with *, see S2–S5 Tables. There are a large number of genes that show female-bias across all four species, compared to the small number that show male-bias across all species (S2 Table). We next examined the chromosomal distribution of biased genes. The genes that show female-bias in all four species are highly enriched on Müller A, relative to an expected uniform chromosomal distribution, and this excess is highly significant (chi squared test, p = 2.06x10-31, Fig 3A and S2 Table). In contrast, the number of genes showing male-bias across all four species is quite small, and there is no significant pattern of chromosomal enrichment (Fig 3A and S2 Table). Thus embryos, like later developmental stages, have a largely feminized X chromosome, but show no evidence for demasculinization on this chromosome.
Next, we examined genes with no female-bias or no male-bias across all four species. The total number of genes across all four species showing no female-bias is remarkably similar to those showing no male-bias (S3 Table). Non-female biased genes are defined as those whose female to male transcript level ratios rank in the bottom 60% of the distribution, non-male biased genes are those whose female to male transcript level ratios rank in the top 60% (see S3 Table for other cutoffs). The similar number of genes having no female - or no male - bias in all four species is presumably a product of higher numbers of genes that are female-biased in all four species, and the higher number of genes that are male-biased in a single species. The chromosomal distribution of non-sex biased genes is largely the inverse of the patterns of sex-biased genes (Fig 3B). Genes showing no female-bias in any of the four species are underrepresented on Müller A (p = 1.28x10-22, S3 Table), and to a lesser extent, Müller D (p = 2.75x10-8, S3 Table), compared to the expected distribution. In contrast, genes that have no male-biased expression in any of the four species are highly overrepresented on Müller A (p = 3.09x10-64, S3 Table).
Analysis of genes that show a lineage-specific pattern of sex-bias is dominated by the female-bias (and lack of male-bias) of Müller D (XR) in D. pseudoobscura. To determine lineage specific genes, we used the sex-bias rankings in each species to determine those that were female or male-biased in one species, and unbiased in the other three species (see Methods, and S4 and S5 Tables for additional sex-bias and not sex-biased cutoffs). We find overall a higher number of genes with male-bias in only one species compared to genes that are female-biased in only one species. D. pseudoobscura has the highest level of lineage specific female-bias, and is the only species where the number of female-biased genes approaches male-biased genes (Fig 3C and S4 Table). Examining the observed chromosomal distribution of genes showing sex-bias only in D. pseudoobscura (Fig 3D and S5 Table), we observe a significant enrichment of female-biased genes on Müller D (chi squared test p = 9.26 x 10−15), compared to the expected distribution if sex-biased genes were randomly distributed across chromosomes. Conversely, we find a deficit of male-biased genes on Müller D (chi squared test, p = 0.01). This particular distribution of D. pseudoobscura-specific sex-biased genes is likely due to the chromosomal rearrangement that converted Müller D into a sex chromosome, broadly caused a decrease of gene expression in males for this chromosome, and thus an increase of female-biased genes and a decrease of male-biased genes.
Chromosomal distributions for lineage-specific sex-biased genes in the remaining species show no significant patterns, with the possible exception of Müller A (Fig 3E and S5 Table). However no systematic excess of species-specific sex-biased genes was detected on Müller A, whereas Müller A contains an excess of ancestrally female-biased genes. We observe both excesses and deficiencies of male-biased genes on Müller A, as well as deficiencies in female-biased genes, though only in some lineages. Though underrepresentation of female-biased genes and overrepresentation of male-biased genes in D. pseudoobscura on Müller A are driven by Müller D (i.e. male-biased genes are overrepresented on Müller A due to the avoidance of male-bias on Müller D), patterns in other species are potentially of interest. D. melanogaster has a deficiency of both female and male-biased genes on Müller A (Fig 3D), though only the male-biased genes met the threshold for statistical significance at almost all cutoffs (S5 Table). D. yakuba also has a deficiency of female-biased genes on Müller A (Fig 3D, chi sq. p = 0.002). Finally we found that species-specific female-bias (genes that have female-biased transcript levels in one species, but not the other three) is usually due to lower transcript abundance in males rather than higher transcript levels in females (S6 Table). Similarly, lineage-specific male-bias is primarily due to lower female transcript levels, though in some comparisons, male-bias is also observed to be due to an increase in male transcript level, with or without a change in female transcript level (see S6 Table for more detail).
Chromosome-specific divergence of mRNA levels
Gene expression linked to the X chromosome has been shown to evolve faster than for other chromosomes in Drosophila adults [25], and recently in embryos [26]. We examined our dataset for the same “faster-X” signal. We measured gene-wise expression divergence as the variance of mRNA levels for each gene along the Drosophila tree according a Brownian motion model (see Methods). We found no chromosome effect on phylogenetic variance of transcript abundance in females (Fig 4A, ANOVA p-value > 0.48) and a small and not significant chromosome effect in males (ANOVA p-value > 0.05), likely due to expression divergence of genes located on Müller element D in the D. pseudoobscura lineage (neo-X, Fig 4B). Chromosome effect was strongest for unfertilized eggs, in which genes located on Müller element A (X chromosome) showed a higher phylogenetic variance than genes located on other chromosomes (Fig 4C, ANOVA p-value < 0.05).
Fig. 4. Chromosomal distribution of inter-species gene expression divergence in females (A), males (B) and eggs (C). 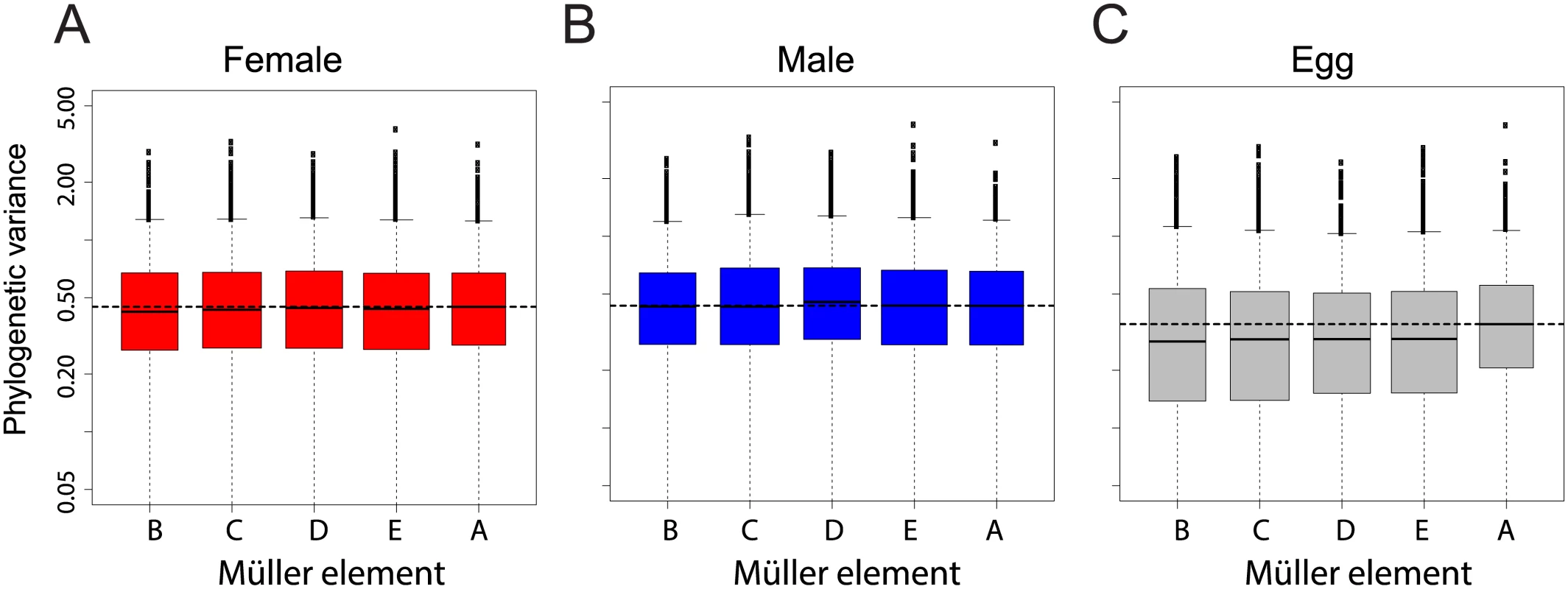
Divergence was measured as the variance of transcript levels along the Drosophila tree according a Brownian motion model. Horizontal dashed line represents the median variance on chromosome X. Chromosome had no effect on variance in females (ANOVA p-value > 0.48), it had a small and non-significant effect in males (ANOVA p-value>0.05), likely due to the higher variance for genes located on the neo-X. Chromosome had a significant effect on variance in eggs, likely due to the higher variance for genes located on the X (ANOVA p-value<0.05). We confirmed this result using an alternative method that considers the divergence of gene expression using Spearman's rank correlation coefficients between species across chromosomes (as in [25,26], a lower correlation is interpreted as higher divergence). In males and females, correlation was mostly similar between chromosomes and Müller A in particular did not show a lower trend (S3 Fig, blue and red boxes). Bootstrap support for a more divergent gene expression of Müller A compared to other chromosomes was low when comparing D. melanogaster, D. yakuba and D. virilis (~7 to 35%). It was higher for comparisons including D. pseudoobscura (~60–80%). Only in eggs did bootstrap support for divergence on the Müller A reach 95% (between ~45% and 99%, S3 Fig, grey). For all pairwise comparisons, bootstrap support for a more divergent gene expression of Müller A was systematically much higher in eggs than in embryos (with the extreme case of the D. melanogaster-D. yakuba comparison with a bootstrap support of 86% in eggs and ~8% in embryos, S3 Fig). Overall both methods gave similar results: gene expression on the X chromosome is faster than on other chromosomes for eggs only. We note that previous studies were based on expression levels from pooled samples of embryos collected at various time points during development. We reanalyzed this dataset and found that the faster-X effect was most pronounced for the earliest time points (S4 Fig and S5A Fig), that contain the highest proportion of maternally deposited transcripts (S5B Fig). It is thus likely that some of the reported embryonic faster-X evolution in embryos is occurring in maternally deposited transcripts (and thus in adult females) rather than in embryos. Other systematic bias that may account for the discrepancy between our results and others were tested and are described in the supplementary material. Overall, our dataset does not support the hypothesis of a faster-X effect on gene expression in Drosophila embryos but supports a faster-X effect in eggs.
Maternal contribution to transcript level divergence
All the early processes in embryonic development are controlled by maternally deposited mRNAs and proteins, and later, zygotic transcription is activated while maternal gene products degrade. The stage under examination in this study, the end of blastoderm stage, occurs after the first widespread activation of zygotic transcription. However, a large number of transcripts (~50% in D. melanogaster) are still maternal in origin. Any divergence we observed in transcript level between species could be due to differences in maternal transcripts or zygotic transcripts, and additionally, differences in zygotic transcripts could be due to changes in upstream maternal factors.
Because of the importance of maternal transcripts at this stage, we investigated the role of maternal genes in shaping divergence of transcript levels in embryos between species. From a previous experiment in D. melanogaster embryos including the blastoderm stage, using allele-specific mRNA-sequencing to distinguish maternal transcripts from zygotic transcripts [1], we established a list of genes that could be classified in three categories: genes whose transcripts were only deposited maternally, but not transcribed zygotically, genes with only zygotic transcription and no maternal contribution, and genes whose transcripts are both maternally deposited and zygotically transcribed. These classes are thereafter referred as “Mat”, “Zyg” or “Mat-Zyg”. We observed that maternal and zygotic genes (as predicted by [1]) segregate as expected in eggs and embryos: transcript levels for maternal genes are high in eggs, and transcripts levels for zygotic genes are largely absent in eggs and high in the embryo samples. Accordingly areas containing only “maternal” or “zygotic” genes are clearly visible on a 2D scatterplot comparing gene levels for egg versus embryo (Fig 5A). We used these expression values to learn the origin of a transcript among the three possible origins “Mat”, “Zyg” or “Mat-Zyg” using only our gene expression dataset. We randomly divided the 5408 D. melanogaster genes for which we knew the origin into a training set of 3606 (2/3) genes and a testing set of the remaining 1802 (1/3) genes. We then used the training set to learn a support vector machine (SVM) model from the egg and embryo expression values for each of the three classes of genes. The classification for each gene (“Mat”, “Zyg” or “Mat-Zyg”) was given a probability. The SVM accuracy was assessed based on the number of test genes that had been correctly or incorrectly classified with high probability (above 0.8, 0.8, and 0.7 for “Mat”, “Zyg” and “Mat-Zyg” genes respectively). We found that SVM classification was very accurate for maternal and zygotic genes and performed poorly for “Mat-Zyg” genes (S7 Fig). 96% of the maternal genes were correctly classified (1166 / 1211) and only eight maternal genes were classified as zygotic, all with low probability (none with high probability). 96% of the zygotic genes were correctly classified (308 / 320) and none were classified as maternal. Only 35% of the “Mat-Zyg” genes (93 / 268) were correctly classified. Consequently we mostly focused on predictions of maternal and zygotic genes for subsequent experiments and applied the SVM on our gene expression dataset to predict transcript origin for the other species.
Fig. 5. Evolution of the origin of an mRNA transcript in the embryo. 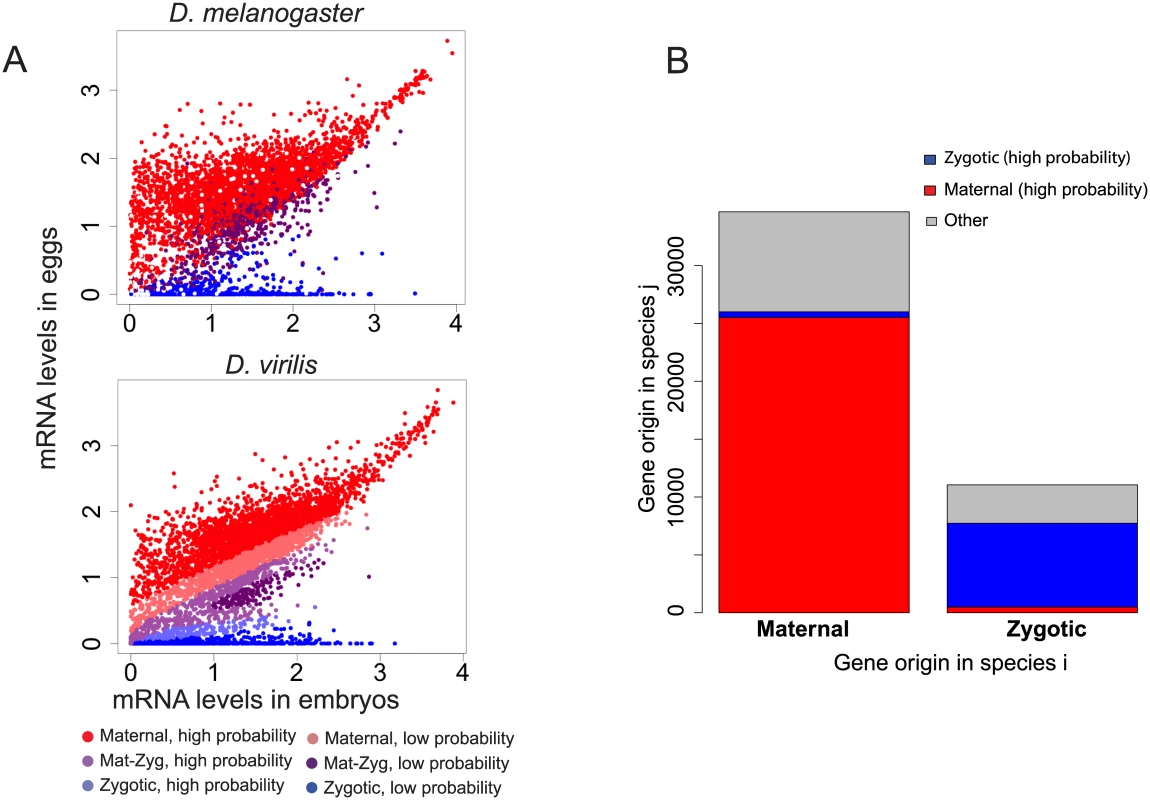
mRNAs in the blastoderm embryo can have two possible origins: they were either maternally deposited into the egg and persisted until this stage, or they were actively transcribed in the embryo. This means that genes can be classified in three categories: purely maternal genes (called “Mat”), purely zygotic genes (called “Zyg”), or genes for which some molecules were deposited and some were zygotically transcribed (called “Mat-Zyg”). A. This classification has been previously established in D. melanogaster [1]. mRNA levels tend to partition between the different classifications on a 2D plot comparing eggs and embryos. We learned an SVM on the D. melanogaster dataset and used it to predict gene classification in the other species. Statistics on the SVM are given in S7 Fig and show that maternal and zygotic genes can be well classified with high confidence, whereas mat-zyg genes are classified with poor success. Fig 5A shows the SVM results for D. virilis. Similar plots are given for D. yakuba and D. pseudoobscura in S8 Fig. Each classification was associated a confidence value, as “high” or “low”. B. We compared whether most genes have a conserved origin (“Mat <-> Mat”, “Mat-Zyg <-> Mat-Zyg” or “Zyg <-> Zyg”) or have switched origins between any two species. We only reported changes between maternal and zygotic categories classified by the SVM with high probabilities: “Mat <-> Zyg”. Other categories were merged into “other” (Mat-Zyg classifications and any SVM classifications associated with low probabilities). We found that most genes have a conserved origin, although a non-negligible fraction of genes have different origins (e.g. maternal in one species and zygotic in another). From the predictions of this model, we observe that most genes maintain their maternal or zygotic expression status across species. Genes that are maternal in one species almost always stay maternal in other species (Fig 5B). Similarly, genes that are categorized as having only zygotic expression at this stage primarily remain zygotic in other species (Fig 5B). Genes that remain in the same category (“Mat”, “Zyg”) across species are also more likely to retain similar transcript abundances across species than genes that switched from maternal to zygotic origin (or vice versa) (Fig 6A; Wilcoxon test p-value < 10−16).
Fig. 6. 
<b>A. Genes whose origin (“Mat <-> Zyg”) changes show higher species differences in embryonic mRNA levels compared to genes with conserved origin. B.</b> Divergence of mRNA levels between species is correlated with divergence of mRNA levels in the egg. We compared pairwise species differences of mRNA levels in the embryo and in the egg and found an overall positive correlation. We focused on the genes that had a conserved maternal (red), zygotic (blue) or Mat-Zyg (purple) origin in two species, or a change from maternal to zygotic (green). Grey points represent the complete dataset (all pairwise comparisons for the 5985 genes). <b>C/D.</b> Genes that have a divergent maternal or zygotic origin (“Mat <-> Zyg”) show highly divergent mRNA levels in the egg (<b>C</b>), which partially translates in the embryo (B). Points in green represent genes that are classified as maternal or zygotic in one species and have switched to zygotic or maternal in the other. Dark green was used for genes classified with high probabilities and light green was used for genes classified with low probabilities. <b>E.</b> Divergence of degradation rates correlates with embryonic expression in the embryo: an increase in mRNAs between species correlates with a decrease in degradation rates. We compared species differences in estimated degradation rates with species differences in mRNA levels in the embryo for the 667 genes that were predicted to have species-specific degradation rates according to a likelihood ratio test. Spearman correlation rho = 0.7, p. value < 2.2x10<sup>-16</sup>. Comparing species changes in transcript levels in blastoderm embryos and unfertilized eggs, we can explore the maternal contribution to the patterns we observe in the embryo. Changes in embryos correlate with changes in eggs for all genes (Spearman’s rho = 0.33), and notably for maternal genes (Spearman’s rho = 0.39 for genes categorized as maternal with high probability, red points labeled “Mat <-> Mat”, Fig 6B), indicating that the divergence in transcript level is partly maternal in origin. Changes in the embryo are unsurprisingly poorly predicted by changes in the egg for genes that stay zygotic across species (Fig 6B, blue dots). Changes for genes that stay “Mat-Zyg” across species have the highest correlation between embryos and unfertilized eggs (yellow points labeled “Mat-Zyg <-> Mat-Zyg”, Fig 6B, rho ~0.45, p-value < 2x10-19), suggesting that transcript levels may be under high levels of developmental constraint, as they manage to remain constant despite maternal degradation and zygotic transcription (although this category was poorly classified using the SVM). Genes that change from maternal deposition to zygotic transcription, and vice versa, across species harbor similar divergence of transcript abundances in unfertilized egg and embryo (Spearman’s rho = 0.39, green points labeled “Mat <-> Zyg” in Fig 6B). This latter correlation is probably driven by differences in what remains of maternal deposition in the embryo, although our dataset does not allow distinguishing this possibility. Interestingly, some of these genes that switch between maternal deposition and zygotic transcription have very different levels in the unfertilized egg (Fig 6C) but show much better conserved levels in the embryo (Fig 6D, green dots located along the diagonal). This suggests that despite a drastic change in maternal deposition in the egg, there has been conservation of mRNA levels for these genes in the embryo (and not in the egg).
Divergence of maternal mRNA deposition is a good but incomplete predictor of gene expression divergence in embryos (Fig 6B). Under the hypothesis that embryonic expression levels of maternal genes are solely dependent on the initial maternal deposition and subsequent degradation, our results suggest that degradation rates for maternal genes have diverged between D. melanogaster, D. yakuba, D. pseudoobscura and D. virilis. In order to test this hypothesis, we used the 1374 genes that are predicted as maternal with high probability in all four species (of note, this four-way conservation likely minimizes false positive "Mat" genes) and estimated their degradation rate according to the following formula, with r representing the degradation rate:
mRNA[embryo] and mRNA[egg] were obtained from the different abundance measurements of the embryo and egg replicates, respectively. The degradation rate r was estimated as the value that minimizes “mRNA[embryo]—mRNA[egg](1-r)“. We compared two models in which the degradation rate was either the same between species or species-specific by comparing degradation rates estimated either on each species separately or on the four species together. For about half of the genes (667/1374), the model of a species-specific degradation rate was more likely according to a likelihood ratio test and the corresponding degradation rates showed at least a twofold difference between any two species (this proportion varies from a third to half depending on normalization). Accordingly divergence of embryonic mRNA levels for these genes correlates with differences in predicted degradation rates (Fig 6E): an increase in degradation rates results in a decrease in embryonic mRNA levels (Spearman correlation of ~0.7). These differences in degradation rates poorly correlate with differences in mRNA content of the unfertilized egg samples (Spearman correlation of ~-0.15, S9 Fig).Discussion
The analysis we report here is the first evolutionary study of gene expression during early Drosophila development using sex-specific embryonic transcriptome profiling of species spanning the genus Drosophila. At this time, the transcriptional environment of the blastoderm embryo is unique as compared to later stages of development for two reasons. First, while some embryos carry two X chromosomes and others only one, canonical MSL-mediated X chromosomal dosage compensation is not yet established [1,2,12–14]. Second this is the period when developmental control is being handed off between the maternal and zygotic genomes [3]. This is all taking place at the same time that the embryo is undergoing developmental processes (such as formation of body axes) that are known to be dependent on concentration of proteins encoded by transcripts, and are absolutely critical to the formation of the animal.
Sex and the single embryo
The evolutionary processes that drive sex-biased gene expression are likely very different in adults as compared to early embryos. For this reason, it is interesting to compare the gene expression phenotypes in female and male embryos, relative to later developmental stages, to determine where the patterns of transcript abundance differ.
The most striking finding from our sex-specific data across species is the dominance of female-biased genes. This is in stark contrast to adults, where in almost all species, there are far more male-biased genes than female-biased genes [8]. While we observe female-biased genes to be more numerous, male-biased genes have a higher magnitude of sex-bias in embryos as well as in adults. These results are consistent with an incomplete X chromosomal dosage compensation, prior to the establishment of MSL-mediated dosage compensation. As we have shown in D. melanogaster, this incomplete dosage compensation causes large numbers of somewhat female-biased genes (generally, at the most 2x higher than male-biased genes), whereas the small number of genes we observe having male-bias are those whose transcript levels are being ramped up in order to establish MSL-mediated dosage compensation (such as the msl genes themselves), and thus are considerably more than 2x higher in males ([1], we observe this trend for msl-2 in all four species).
We observe an overrepresentation of female-biased genes on the shared X chromosome (Müller A) in the embryo to be common in all species, as has been repeatedly found for later developmental stages in Drosophila [6,7,27,28]. However, unlike these earlier studies [6,7,27,28], we do not observe a significant deficiency of male-biased genes of the shared X chromosome across species in the embryo. This pattern may be influenced by the incomplete nature of early zygotic dosage compensation, the small total number of male-biased genes at this stage, or other differences between embryo and later developmental stages. However, lineage-specific patterns of sex-bias are far less clear. In D. virilis, lineage-specific female or male-biased genes show no enrichment or paucity on the X chromosome (Müller A) while male-biased genes are observed to be both under-represented (D. melanogaster) and over-represented (D. yakuba) on the X chromosome. The basis for this confusing pattern is likely due to a combination of many factors, including the high number of female-biased genes shared amongst all four species.
The dominant signal we observe for genes showing species-specific sex-bias is the female-bias of D. pseudoobscura, consistent with studies in other developmental stages[8,29]. In the lineage leading to this species, a chromosomal fusion event formed a two-armed X chromosome, consisting of both Müller A and Müller D, whereas the other species have only the acrocentric Müller A element as an X chromosome. D. pseudoobscura has a large number of female-biased genes on Müller D, which are not female-biased in any other species, likely due to this chromosome being X linked, and compounded by the incomplete nature of dosage compensation during this time in development. This D. pseudoobscura-specific increase in female-bias transcript level on Müller D is due to a decrease in transcript levels in males compared to other species, consistent with the number of Müller D templates available. What is more remarkable is that this evolution of sex-bias by a decrease of transcript levels in one sex (rather than an increase in the other sex) is generalizable. We observe this pattern for most genes with lineage specific sex-bias, in both females and males, in all species. This would suggest that there may be a larger number of evolutionary paths to decreasing expression level, or perhaps many genes in the early embryo are transcribed at close to their maximal expression level, so that increasing transcript level would be difficult.
Lack of evidence for embryonic faster-X effect on transcript level
Another property of the embryonic transcriptome that may be quite distinct from the adult is the lack of a faster-X effect. Evolutionary theory holds that the X chromosome has the potential to play a disproportionately large role in divergence and speciation [30–32]. This increased level of divergence, termed the faster-X effect, has been found at the protein level by genomic studies, in some, but not all, lineages (see [31,33,34]). The faster-X effect has also been examined at the level of gene expression. Previous studies in Drosophila have demonstrated the higher divergence in gene expression on the X chromosome as compared to the autosomes[25,26]. The only other study to address this effect in embryonic gene expression did report higher divergence of transcript level on the X [26], but we did not replicate this result. Extensive probing into the dataset from this study and comparisons with ours (see results, Fig 4 and S3–S6 Figs), demonstrated that the differences in our findings were largely features of the data, rather than how it was processed or what subset of genes were compared. The primary difference in the methodology for gathering the samples was the use of pooled collection of embryos vs. our single embryo approach. In our data, we can only detect expression divergence on the X in the unfertilized eggs. It is likely that the pooled embryo samples used in [26] included some number of individuals from earlier stages (even eggs), which might explain at least to some extent why gene expression on the X chromosome appears more divergent in embryos collected in pools rather than as individuals.
Overall, our results demonstrate a faster-X effect for gene expression only in the earliest stages of embryogenesis. These stages of development are those dominated by maternally deposited transcripts, suggesting that the faster-X effect is observable in the mother rather than the embryo. Especially considering the high level of expression conservation between even distantly related species, the lack of a faster-X signal in our data may point to different selective pressures at this stage, such as high levels of developmental constraint on transcript levels in the early embryo.
Maternal contribution to blastoderm expression divergence
The blastoderm embryo transcriptome consists of transcripts both maternally deposited and zygotically transcribed[1,35]. Thus, the variation between species in the transcripts present at this developmental stage may be either maternal or zygotic in origin. This leads in turn to a set of fundamental but largely unaddressed questions about the conservation of gene expression in the mother or the zygote. For example, does the origin of a gene in a species (maternal or zygotic) inform about the origin of this gene in another species? Our model suggests that this is the case: a maternal gene in one species is likely to be a maternal gene in other species, and a gene transcribed zygotically in one species is likely to also be zygotically transcribed in other species. Unsurprisingly for maternal genes, differences of transcript level in the blastoderm embryo were already established in the egg and are thus maternal in origin. We do find that a small number of genes are likely to be maternal in one species and zygotic in another, or vice versa, and these genes are more divergent in their transcript level between species in the blastoderm embryo.
Cellular transcript content is the result of both transcript production and transcript degradation. The contribution of transcript degradation to transcript level “steady state” has been modeled in model systems [36–40], but has largely been neglected as a source of divergence between species. However, most studies have interpreted divergence of mRNA levels between species as divergence of transcription, neglecting the contribution of mRNA degradation (with the noticeable exception of yeast [41]). The earliest stages of development before zygotic transcription starts are the only time in the life of an animal when one can study mRNA degradation exclusively, because transcripts of maternal genes are degraded from the maternally deposited pool and not transcribed by the zygote yet. We used this property to estimate and compare divergence of degradation rates for maternal genes between species. We find evidence for general species-specific differences in modeled transcript degradation rates, which largely contributes to the difference in maternal transcript levels at the blastoderm stage. The functional consequences of these inferred changes in degradation rates remain unexplored.
Advantages of single embryo approaches
A number of the findings reported here were only made possible due to the use of single embryos in experiments. The sex-specific embryo data would have been considerably more difficult to collect by other methods. As we demonstrate here, this methodology has been especially fruitful when applied in comparative studies. The species we used here have different development times, and a tendency to lay different proportions of non-developing eggs. Collections of pools of embryos necessarily contain a distribution of developmental ages, and potentially can be “contaminated” by earlier or later stage embryos than the desired distribution. Both the stage distributions and levels of contamination are likely to vary between species. When different species contribute different numbers of non-developing eggs (often unfertilized eggs) to pooled samples, this adds considerable signal to inter-species comparisons (see Fig 1D), and confounds development differences with species differences. The ability to select individual embryos that have developed to the same (morphological) stage greatly improves accuracy and decreases development bias, which is critical in these cross-species studies.
Methods
Sample collection, library construction, sequencing
For single embryo collection, eggs were collected from 3–5 day old females of D. melanogaster (Oregon-R), D. yakuba (Tai18E2), D. pseudoobscura (MV2-25), and D. virilis (V46), dechorionated, and imaged under halocarbon oil to determine stage. Embryos were collected from a large number of mothers, so it is unlikely that multiple samples came from the same mother. Embryos were viewed on a Nikon Eclipse 80i light microscope, and were selected based on having completed cellularization but not yet having gastrulated. Embryos were then removed from the slide with a brush, cleaned of excess oil, placed into a drop of Trizol reagent (Ambion), and ruptured with a needle, then moved to a tube with more Trizol to be frozen at -80°C until extraction. RNA and DNA were extracted as in the manufacturer’s protocol, with the exception of extracting in an excess of reagent (1 mL was used) compared to expected mRNA and DNA concentration (see [1,2]).
Extracted DNA was subjected to whole genome amplification (Illustra GenomiPhi V2, GE Healthcare), and used for PCR assays to determine embryo sex. Primers were designed separately in each species, reactions multiplexed sets of primers to two different locations on the Y chromosome and a control locus not on the Y. Primer sets used can be found in S7 Table.
Extracted total RNA from single embryos was treated with TurboDNase (Ambion) prior to library construction. Embryo mRNA-Seq libraries were made for 3 replicate individual female embryos and three replicate male individuals per species. Egg mRNA-Seq libraries were constructed for three replicate individual eggs per species. mRNA-Seq libraries were constructed using TruSeq RNA sample preparation kits (Illumina), using standard protocols, and indexed to pool 12 samples (embryos or eggs) per lane. Library concentration was measured using the Qubit fluorometer (Life Technologies) and the qPCR-based Library Quantification kits (KAPA biosystems), and size was measured using the Bioanalyzer (Agilent). Libraries were sequenced on an Illumina HiSeq 2000 DNA Sequencer. Some samples were removed a posteriori because of lower quality (see Results) and we ended up using a total of 29 samples (22 embryo samples + 7 egg samples, listed in S8 Table).
Data availability
All reads are available at the NCBI GEO (accession number of the project: GSE68062; accession number of each sample listed in S8 Table). mRNA-seq reads on the pool datasets had previously been deposited in the NCBI GEO (accession numbers GSM1228784 and GSM1228785).
Data processing
We used the annotation version 5.39 for D. melanogaster and the annotation previously published for D. yakuba, D. pseudoobscura, and D. virilis [19].
We trimmed all sequenced tags so that their average quality was above 30 and mapped the tags to the transcripts using Bowtie2 [20] with command-line options ‘-a -N 1‘, thereby allowing all possible mappings. We then estimated transcript levels using eXpress [21]. Gene levels were obtained by summing levels over all transcripts of a gene. Gene levels were then normalized (see below), then log transformed (log10(normalized FPKM+1)). Orthology was assessed from [19].
Six normalization procedures were tested: (i) either no further normalization after eXpress, normalization after eXpress to the median (ii), to the 75th percentile (iii), to the 95th percentile (iv), TMM normalization (v) or quantile normalization (see [42] for review). The analyses presented in the main text were conducted on the 75th percentile normalization but the different normalizations essentially gave similar results. A few representative plots similar to the main figures are given in S10 and S11 Figs for the 6 normalizations. In addition, tables created from the different normalized datasets are available on GEO (accession number GSE68062).
Analysis of sex-bias
All data analysis was performed in R [43].
For Fig 2, sex ratios were calculated on non-log transformed expression levels. Genes were ranked according to the absolute log2(female:male) ratio, and the chromosomal distribution of the top 200 genes was assessed (Fig 2C).
For Fig 3, genes were ranked based on their female to male ratio (sex ratio). To avoid large sex ratios due to variance in transcript level for low abundance transcripts, we tested different transcript level cutoffs, and found that requiring the transcript be present in all four species at >0.33 FPKM on a log2 scale was sufficient. We define genes as being female-biased if their sex ratio was ranked in the top 20% (for other bias cutoffs see S1–S6 Tables) and as male-biased if their ratio was in the bottom 20%. Genes were defined as unbiased if their ratio fell within 10% of the median (i.e. the 40%-60% quantiles; see S1–S6 Tables for other cutoffs). Expected chromosomal distributions of genes for chi-squared tests were determined by the total number of genes identified as sex-biased, distributed based on the number of genes on each chromosome.
Measurements of gene-wise phylogenetic variance to test the “faster X” hypothesis
Gene-specific variance of expression was reconstructed according to a Brownian motion model [44]. Under a Brownian motion model, continuous characters evolve randomly along a phylogeny following a random walk. We used the R package ape (function ‘‘ace”) with the following parameters: model = "BM", method = "REML", type = "continuous" [45].
Comparison of chromosome-wise divergence of transcript levels between species
We approximated divergence of chromosome expression between species according to the correlation of gene expression for each chromosome. Per chromosome we measured Spearman correlation of transcript levels for all pairwise species combinations. We also generated 10,000 bootstrap replicates per chromosome of the pairwise Spearman correlations using the R function boot from the package boot.
We compared our dataset to the dataset from [23], which was obtained from the ArrayExpress database, using the accession number E-MTAB-404 [23,26]. Using this dataset that covers eight time windows during embryonic development, evidence for a faster X effect had been reported. We first verified that we indeed recovered the “faster X” effect in the microarray dataset [23] using our analysis pipeline. We found that expression was indeed more divergent on the X compared to other chromosomes, as published. We also noticed that the effect was most pronounced for early time points (S4 Fig).
Inter-species comparisons are done on datasets of orthologous genes. The method for pairing genes between species was done in very different ways in our study vs [23] (genome alignment in our case vs blast of microarray probes in the analysis by [23]). We thus checked whether differences between our dataset and the microarray dataset stems from differences in orthology assignment. We reassigned gene orthology in the [23] dataset by first identifying through BLAST which of our gene sequences matched the probes from the D. pseudoobscura and D. virilis microarrays. We removed any gene for which all probes assigned to one gene did not match the same gene from our annotations. Between species correspondence was based on [19]. We ended up with 1404 genes and then processed this dataset similarly to ours. We found that expression was still more divergent on the X chromosome after recalling orthologous genes in the microarray dataset (S5A Fig). Again, the effect was more pronounced for early time points.
Finally we found a correlation between overall chromosomal expression levels and expression divergence: the higher the expression in a chromosome, the higher the correlation between species (S6A Fig). The X chromosome systematically has a lower expression compared to the other chromosomes. This effect is much weaker with our dataset (S6B Fig). Overall however correlations between levels of expression and pairwise correlations between species on one hand and egg contamination in embryo pools on the other hand likely account for some but not all the discrepancy between both datasets.
SVM classification of gene origin
In order to predict the gene class (maternal, zygotic of maternal+zygotic) based on egg and embryonic expression, we learned an SVM using the D. melanogaster 5408 genes for which a maternal / zygotic / maternal+zygotic classification had previously been established by [1] using the function “svm” of the R package e1071[46] using the parameters kernel =“radial”, method =“C-classification”, gamma = 0.01, cost = 10, and otherwise default parameters. To select those parameters, the SVM was learned on a training set of 2/3 of the dataset (3606/5408) and tested on the remaining 1/3 (1802/5408). 100 replicates (with 100 different training and test sets) were performed and the parameters were selected to maximize the number of correctly classified genes, in particular to minimize the number of maternal genes predicted as zygotic (or vice versa). Of note, among the variety of parameters that we tested, the kernel and cost values showed the most variation in accuracy while other parameters were very robust. An example of the SVM performance using the chosen parameters is given S7 Fig. Once the parameters were selected, we relearned the SVM on the complete D. melanogaster dataset of 5408 genes and applied it to the other species. Genes were further categorized depending on the confidence of the classification: the classification was considered “highly confident” if the associated probability was above 0.8, 0.7 and 0.8 for maternal, zygotic and Mat-Zyg genes, respectively. As described in the result section and shown in S7 Fig, the resulting classification of maternal and zygotic genes was very accurate whereas classification of Mat-zyg genes was of poor quality.
Analysis of maternal contribution to blastoderm expression divergence
Degradation rates were estimated on a set of 1374 genes that had been classified as maternal with high probability in all four species according to the formula:
r being the degradation rate between eggs and embryos and epsilon following a normal distribution of mean 0 and variance the average variance of the dataset values. Non-log-transformed values were used for this analysis. All replicates were used (not the averaged values). r was estimated using the R function “optimize” as the value that minimizes “mRNA[embryo]—mRNA[egg](1-r)“. Two models were used and compared to optimize r for each gene: either r was common among the species, or it was species-specific. In other words, the optimization was either done on each species separately (species-specific degradation rate) or on the whole dataset together (species-common rate). The likelihood of both models was then calculated gene-wise using the above formula and the optimized r. Genes with species-specific degradation rates were selected according to a Likelihood ratio test with four degrees of freedom (Benjamini & Hochberg corrected p-value < 0.001) The list of genes with species-specific degradation rates was further refined by imposing a fold change > 2 between any two species.Supporting Information
Zdroje
1. Lott SE, Villalta JE, Schroth GP, Luo S, Tonkin LA, et al. (2011) Noncanonical compensation of zygotic X transcription in early Drosophila melanogaster development revealed through single-embryo RNA-seq. PLoS Biol 9: e1000590. doi: 10.1371/journal.pbio.1000590 21346796
2. Lott SE, Villalta JE, Zhou Q, Bachtrog D, Eisen MB (2014) Sex-specific embryonic gene expression in species with newly evolved sex chromosomes. PLoS Genet 10: e1004159. doi: 10.1371/journal.pgen.1004159 24550743
3. Tadros W, Lipshitz HD (2009) The maternal-to-zygotic transition: a play in two acts. Development 136 : 3033–3042. doi: 10.1242/dev.033183 19700615
4. Ellegren H, Parsch J (2007) The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet 8 : 689–698. 17680007
5. Haerty W, Jagadeeshan S, Kulathinal RJ, Wong A, Ravi Ram K, et al. (2007) Evolution in the fast lane: rapidly evolving sex-related genes in Drosophila. Genetics 177 : 1321–1335. 18039869
6. Meisel RP, Malone JH, Clark AG (2012) Disentangling the relationship between sex-biased gene expression and X-linkage. Genome Res 22 : 1255–1265. doi: 10.1101/gr.132100.111 22499666
7. Ranz JM, Castillo-Davis CI, Meiklejohn CD, Hartl DL (2003) Sex-dependent gene expression and evolution of the Drosophila transcriptome. Science 300 : 1742–1745. 12805547
8. Zhang Y, Sturgill D, Parisi M, Kumar S, Oliver B (2007) Constraint and turnover in sex-biased gene expression in the genus Drosophila. Nature 450 : 233–237. 17994089
9. Arbeitman MN, Fleming AA, Siegal ML, Null BH, Baker BS (2004) A genomic analysis of Drosophila somatic sexual differentiation and its regulation. Development 131 : 2007–2021. 15056610
10. Parisi M, Nuttall R, Edwards P, Minor J, Naiman D, et al. (2004) A survey of ovary-, testis-, and soma-biased gene expression in Drosophila melanogaster adults. Genome Biol 5: R40. 15186491
11. Meisel RP (2011) Towards a more nuanced understanding of the relationship between sex-biased gene expression and rates of protein-coding sequence evolution. Mol Biol Evol 28 : 1893–1900. doi: 10.1093/molbev/msr010 21239389
12. Franke A, Dernburg A, Bashaw GJ, Baker BS (1996) Evidence that MSL-mediated dosage compensation in Drosophila begins at blastoderm. Development 122 : 2751–2760. 8787749
13. Rastelli L, Richman R, Kuroda MI (1995) The dosage compensation regulators MLE, MSL-1 and MSL-2 are interdependent since early embryogenesis in Drosophila. Mech Dev 53 : 223–233. 8562424
14. Strukov YG, Sural TH, Kuroda MI, Sedat JW (2011) Evidence of activity-specific, radial organization of mitotic chromosomes in Drosophila. PLoS Biol 9: e1000574. doi: 10.1371/journal.pbio.1000574 21264350
15. Kuntz SG, Eisen MB (2014) Drosophila embryogenesis scales uniformly across temperature in developmentally diverse species. PLoS Genet 10: e1004293. doi: 10.1371/journal.pgen.1004293 24762628
16. Markow TA, Beall S, Matzkin LM (2009) Egg size, embryonic development time and ovoviviparity in Drosophila species. J Evol Biol 22 : 430–434. doi: 10.1111/j.1420-9101.2008.01649.x 19032497
17. Markow TA (2013) Parents without Partners: Drosophila as a Model for Understanding the Mechanisms and Evolution of Parthenogenesis. G3 (Bethesda).
18. Russo CA, Takezaki N, Nei M (1995) Molecular phylogeny and divergence times of drosophilid species. Mol Biol Evol 12 : 391–404. 7739381
19. Paris M, Kaplan T, Li XY, Villalta JE, Lott SE, et al. (2013) Extensive divergence of transcription factor binding in Drosophila embryos with highly conserved gene expression. PLoS Genet 9: e1003748. doi: 10.1371/journal.pgen.1003748 24068946
20. Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9 : 357–359. doi: 10.1038/nmeth.1923 22388286
21. Roberts A, Pachter L (2013) Streaming fragment assignment for real-time analysis of sequencing experiments. Nat Methods 10 : 71–73. doi: 10.1038/nmeth.2251 23160280
22. Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, et al. (2011) The developmental transcriptome of Drosophila melanogaster. Nature 471 : 473–479. doi: 10.1038/nature09715 21179090
23. Kalinka AT, Varga KM, Gerrard DT, Preibisch S, Corcoran DL, et al. (2010) Gene expression divergence recapitulates the developmental hourglass model. Nature 468 : 811–814. doi: 10.1038/nature09634 21150996
24. Kaiser VB, Bachtrog D (2010) Evolution of sex chromosomes in insects. Annu Rev Genet 44 : 91–112. doi: 10.1146/annurev-genet-102209-163600 21047257
25. Meisel RP, Malone JH, Clark AG (2012) Faster-X evolution of gene expression in Drosophila. PLoS Genet 8: e1003013. doi: 10.1371/journal.pgen.1003013 23071459
26. Kayserili MA, Gerrard DT, Tomancak P, Kalinka AT (2012) An excess of gene expression divergence on the X chromosome in Drosophila embryos: implications for the faster-X hypothesis. PLoS Genet 8: e1003200. doi: 10.1371/journal.pgen.1003200 23300473
27. Parisi M, Nuttall R, Naiman D, Bouffard G, Malley J, et al. (2003) Paucity of genes on the Drosophila X chromosome showing male-biased expression. Science 299 : 697–700. 12511656
28. Sturgill D, Zhang Y, Parisi M, Oliver B (2007) Demasculinization of X chromosomes in the Drosophila genus. Nature 450 : 238–241. 17994090
29. Assis R, Zhou Q, Bachtrog D (2012) Sex-biased transcriptome evolution in Drosophila. Genome Biol Evol 4 : 1189–1200. doi: 10.1093/gbe/evs093 23097318
30. Charlesworth B, Coyne JA, Barton NH (1987) The Relative Rates of Evolution of Sex Chromosomes and Autosomes. The American Naturalist 130 : 113–146.
31. Meisel RP, Connallon T (2013) The faster-X effect: integrating theory and data. Trends Genet 29 : 537–544. doi: 10.1016/j.tig.2013.05.009 23790324
32. Vicoso B, Charlesworth B (2006) Evolution on the X chromosome: unusual patterns and processes. Nat Rev Genet 7 : 645–653. 16847464
33. Presgraves DC (2008) Sex chromosomes and speciation in Drosophila. Trends Genet 24 : 336–343. doi: 10.1016/j.tig.2008.04.007 18514967
34. Avila V, Marion de Proce S, Campos JL, Borthwick H, Charlesworth B, et al. (2014) Faster-X effects in two Drosophila lineages. Genome Biol Evol 6 : 2968–2982. doi: 10.1093/gbe/evu229 25323954
35. De Renzis S, Elemento O, Tavazoie S, Wieschaus EF (2007) Unmasking activation of the zygotic genome using chromosomal deletions in the Drosophila embryo. PLoS Biol 5: e117. 17456005
36. Boettiger AN (2013) Analytic approaches to stochastic gene expression in multicellular systems. Biophys J 105 : 2629–2640. doi: 10.1016/j.bpj.2013.10.033 24359735
37. Bolouri H, Davidson EH (2003) Transcriptional regulatory cascades in development: initial rates, not steady state, determine network kinetics. Proc Natl Acad Sci U S A 100 : 9371–9376. 12883007
38. Cacace F, Paci P, Cusimano V, Germani A, Farina L (2012) Stochastic modeling of expression kinetics identifies messenger half-lives and reveals sequential waves of co-ordinated transcription and decay. PLoS Comput Biol 8: e1002772. doi: 10.1371/journal.pcbi.1002772 23144606
39. Elkon R, Zlotorynski E, Zeller KI, Agami R (2010) Major role for mRNA stability in shaping the kinetics of gene induction. BMC Genomics 11 : 259. doi: 10.1186/1471-2164-11-259 20409322
40. Manu, Surkova S, Spirov AV, Gursky VV, Janssens H, et al. (2009) Canalization of gene expression and domain shifts in the Drosophila blastoderm by dynamical attractors. PLoS Comput Biol 5: e1000303. doi: 10.1371/journal.pcbi.1000303 19282965
41. Dori-Bachash M, Shema E, Tirosh I (2011) Coupled evolution of transcription and mRNA degradation. PLoS Biol 9: e1001106. doi: 10.1371/journal.pbio.1001106 21811398
42. Dillies MA, Rau A, Aubert J, Hennequet-Antier C, Jeanmougin M, et al. (2013) A comprehensive evaluation of normalization methods for Illumina high-throughput RNA sequencing data analysis. Brief Bioinform 14 : 671–683. doi: 10.1093/bib/bbs046 22988256
43. R Core Team (2012) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
44. Felsenstein J (1973) Maximum-likelihood estimation of evolutionary trees from continuous characters. Am J Hum Genet 25 : 471–492. 4741844
45. Paradis E, Claude J, Strimmer K (2004) APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 20 : 289–290. 14734327
46. Meyer D, Dimitriadou E, Hornik K, Weingessel A, Leisch F (2014) e1071: Misc Functions of the Department of Statistics, TU Wien. R package version 1.6–4. ed.
47. Drosophila 12 Genomes C, Clark AG, Eisen MB, Smith DR, Bergman CM, et al. (2007) Evolution of genes and genomes on the Drosophila phylogeny. Nature 450 : 203–218. 17994087
48. Carvalho AB, Clark AG (2005) Y chromosome of D. pseudoobscura is not homologous to the ancestral Drosophila Y. Science 307 : 108–110. 15528405
Štítky
Genetika Reprodukčná medicína
Článek Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation DevelopmentČlánek A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor PelotaČlánek A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in OvulationČlánek Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAsČlánek FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2Článek Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion inČlánek The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly SiteČlánek Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem CellsČlánek A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal ErythropoiesisČlánek Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation inČlánek Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in OocytesČlánek MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 10- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Gene-Regulatory Logic to Induce and Maintain a Developmental Compartment
- A Decad(e) of Reasons to Contribute to a PLOS Community-Run Journal
- DNA Methylation Landscapes of Human Fetal Development
- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development
- Transcriptional Derepression Uncovers Cryptic Higher-Order Genetic Interactions
- Silencing of X-Linked MicroRNAs by Meiotic Sex Chromosome Inactivation
- Virus Satellites Drive Viral Evolution and Ecology
- A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota
- Sequence to Medical Phenotypes: A Framework for Interpretation of Human Whole Genome DNA Sequence Data
- Your Data to Explore: An Interview with Anne Wojcicki
- Modulation of Ambient Temperature-Dependent Flowering in by Natural Variation of
- The Ciliopathy Protein CC2D2A Associates with NINL and Functions in RAB8-MICAL3-Regulated Vesicle Trafficking
- PPP2R5C Couples Hepatic Glucose and Lipid Homeostasis
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
- Intermediate Levels of CodY Activity Are Required for Derepression of the Branched-Chain Amino Acid Permease, BraB
- "Missing" G x E Variation Controls Flowering Time in
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Type IV Collagen Controls the Axogenesis of Cerebellar Granule Cells by Regulating Basement Membrane Integrity in Zebrafish
- Loss of a Conserved tRNA Anticodon Modification Perturbs Plant Immunity
- Genome-Wide Association Analysis of Adaptation Using Environmentally Predicted Traits
- Oriented Cell Division in the . Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1
- Disproportionate Contributions of Select Genomic Compartments and Cell Types to Genetic Risk for Coronary Artery Disease
- A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Ovulation
- The RNAPII-CTD Maintains Genome Integrity through Inhibition of Retrotransposon Gene Expression and Transposition
- Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAs
- Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector
- SCARN a Novel Class of SCAR Protein That Is Required for Root-Hair Infection during Legume Nodulation
- IBR5 Modulates Temperature-Dependent, R Protein CHS3-Mediated Defense Responses in
- NINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish
- Decay-Initiating Endoribonucleolytic Cleavage by RNase Y Is Kept under Tight Control via Sequence Preference and Sub-cellular Localisation
- Large-Scale Analysis of Kinase Signaling in Yeast Pseudohyphal Development Identifies Regulation of Ribonucleoprotein Granules
- FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2
- LINE-1 Mediated Insertion into (Protein of Centriole 1 A) Causes Growth Insufficiency and Male Infertility in Mice
- Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion in
- Genome-Scale Mapping of σ Reveals Widespread, Conserved Intragenic Binding
- Uncovering Hidden Layers of Cell Cycle Regulation through Integrative Multi-omic Analysis
- Functional Diversification of Motor Neuron-specific Enhancers during Evolution
- The GTP- and Phospholipid-Binding Protein TTD14 Regulates Trafficking of the TRPL Ion Channel in Photoreceptor Cells
- The Gyc76C Receptor Guanylyl Cyclase and the Foraging cGMP-Dependent Kinase Regulate Extracellular Matrix Organization and BMP Signaling in the Developing Wing of
- The Ty1 Retrotransposon Restriction Factor p22 Targets Gag
- Functional Impact and Evolution of a Novel Human Polymorphic Inversion That Disrupts a Gene and Creates a Fusion Transcript
- The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly Site
- The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study
- Parent-of-Origin Effects of the Gene on Adiposity in Young Adults
- Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem Cells
- Retinoic Acid Receptors Control Spermatogonia Cell-Fate and Induce Expression of the SALL4A Transcription Factor
- A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis
- Protein O-Glucosyltransferase 1 (POGLUT1) Promotes Mouse Gastrulation through Modification of the Apical Polarity Protein CRUMBS2
- KIF7 Controls the Proliferation of Cells of the Respiratory Airway through Distinct Microtubule Dependent Mechanisms
- Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation in
- Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in Oocytes
- Protein Homeostasis Imposes a Barrier on Functional Integration of Horizontally Transferred Genes in Bacteria
- A New Method for Detecting Associations with Rare Copy-Number Variants
- Histone H2AFX Links Meiotic Chromosome Asynapsis to Prophase I Oocyte Loss in Mammals
- The Genomic Aftermath of Hybridization in the Opportunistic Pathogen
- A Role for the Chaperone Complex BAG3-HSPB8 in Actin Dynamics, Spindle Orientation and Proper Chromosome Segregation during Mitosis
- Establishment of a Developmental Compartment Requires Interactions between Three Synergistic -regulatory Modules
- Regulation of Spore Formation by the SpoIIQ and SpoIIIA Proteins
- Association of the Long Non-coding RNA Steroid Receptor RNA Activator (SRA) with TrxG and PRC2 Complexes
- Alkaline Ceramidase 3 Deficiency Results in Purkinje Cell Degeneration and Cerebellar Ataxia Due to Dyshomeostasis of Sphingolipids in the Brain
- ACLY and ACC1 Regulate Hypoxia-Induced Apoptosis by Modulating ETV4 via α-ketoglutarate
- Quantitative Differences in Nuclear β-catenin and TCF Pattern Embryonic Cells in .
- HENMT1 and piRNA Stability Are Required for Adult Male Germ Cell Transposon Repression and to Define the Spermatogenic Program in the Mouse
- Axon Regeneration Is Regulated by Ets–C/EBP Transcription Complexes Generated by Activation of the cAMP/Ca Signaling Pathways
- A Phenomic Scan of the Norfolk Island Genetic Isolate Identifies a Major Pleiotropic Effect Locus Associated with Metabolic and Renal Disorder Markers
- The Roles of CDF2 in Transcriptional and Posttranscriptional Regulation of Primary MicroRNAs
- A Genetic Cascade of Modulates Nucleolar Size and rRNA Pool in
- Inter-population Differences in Retrogene Loss and Expression in Humans
- Cationic Peptides Facilitate Iron-induced Mutagenesis in Bacteria
- EP4 Receptor–Associated Protein in Macrophages Ameliorates Colitis and Colitis-Associated Tumorigenesis
- Fungal Infection Induces Sex-Specific Transcriptional Changes and Alters Sexual Dimorphism in the Dioecious Plant
- FLCN and AMPK Confer Resistance to Hyperosmotic Stress via Remodeling of Glycogen Stores
- MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
- Sex Bias and Maternal Contribution to Gene Expression Divergence in Blastoderm Embryos
- Transcriptional and Linkage Analyses Identify Loci that Mediate the Differential Macrophage Response to Inflammatory Stimuli and Infection
- Mre11 and Blm-Dependent Formation of ALT-Like Telomeres in Ku-Deficient
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- Identification of a Single Strand Origin of Replication in the Integrative and Conjugative Element ICE of
- The Type VI Secretion TssEFGK-VgrG Phage-Like Baseplate Is Recruited to the TssJLM Membrane Complex via Multiple Contacts and Serves As Assembly Platform for Tail Tube/Sheath Polymerization
- The Dynamic Genome and Transcriptome of the Human Fungal Pathogen and Close Relative
- Secondary Structure across the Bacterial Transcriptome Reveals Versatile Roles in mRNA Regulation and Function
- ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Regeneration
- Pelle Modulates dFoxO-Mediated Cell Death in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy





