-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
While the influence of positive selection in diversifying animal venoms is widely recognized, the role of purifying selection that conserves the amino acid sequence of venom components such as peptide toxins has never been considered. In addition to unraveling the unique strategies of evolution of toxin gene families in centipedes and spiders, which are amongst the first terrestrial venomous lineages, we highlight the significant role of purifying selection in shaping the composition of animal venoms. Analysis of numerous toxin families, spanning the breadth of the animal kingdom, has revealed a striking contrast between the evolution of venom in ancient and evolutionarily young animal groups. Our findings enable the postulation of a new theory of venom evolution. The proposed ‘two-speed’ mode of evolution of venom captures the fascinating evolutionary history and the dynamics of this complex biochemical cocktail.
Published in the journal: The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals. PLoS Genet 11(10): e32767. doi:10.1371/journal.pgen.1005596
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005596Summary
While the influence of positive selection in diversifying animal venoms is widely recognized, the role of purifying selection that conserves the amino acid sequence of venom components such as peptide toxins has never been considered. In addition to unraveling the unique strategies of evolution of toxin gene families in centipedes and spiders, which are amongst the first terrestrial venomous lineages, we highlight the significant role of purifying selection in shaping the composition of animal venoms. Analysis of numerous toxin families, spanning the breadth of the animal kingdom, has revealed a striking contrast between the evolution of venom in ancient and evolutionarily young animal groups. Our findings enable the postulation of a new theory of venom evolution. The proposed ‘two-speed’ mode of evolution of venom captures the fascinating evolutionary history and the dynamics of this complex biochemical cocktail.
Introduction
Venom is an intriguing evolutionary innovation that is utilized by various animals for predation and/or defense. This complex biochemical cocktail is characterized by a myriad of organic and inorganic molecules, such as proteins, peptides, polyamines and salts that disrupt the normal physiology of the envenomed animal. Evolution of venom has been intensively investigated in more recently diverged lineages (for simplicity, we refer to them as ‘evolutionarily younger’ lineages), such as advanced snakes and cone snails, which originated ~54 [1] and ~33–50 [2, 3] million years ago (MA), respectively. Several venom-encoding genes in these animals have undergone extensive duplications [4, 5] and evolve rapidly under the influence of positive selection [6–10]. In contrast, the evolution of venom in most of the ancient lineages, such as cnidarians (corals, sea anemones, hydroids and jellyfish), coleoids (octopus, squids and cuttlefish), spiders and centipedes, remains understudied, if not completely overlooked. Perhaps the only exhaustively investigated ancient venomous clade are the scorpions, which originated in the Silurian about 430 MA [11, 12]. Moreover, certain potent toxins in species separated by considerable geographic and genetic distance can exhibit remarkable sequence conservation (Fig 1). Yet, research to date has solely focused on how positive selection has expanded the venom arsenal, while completely ignoring the role of negative (purifying) selection.
Fig. 1. Remarkable sequence conservation in distantly related toxins. 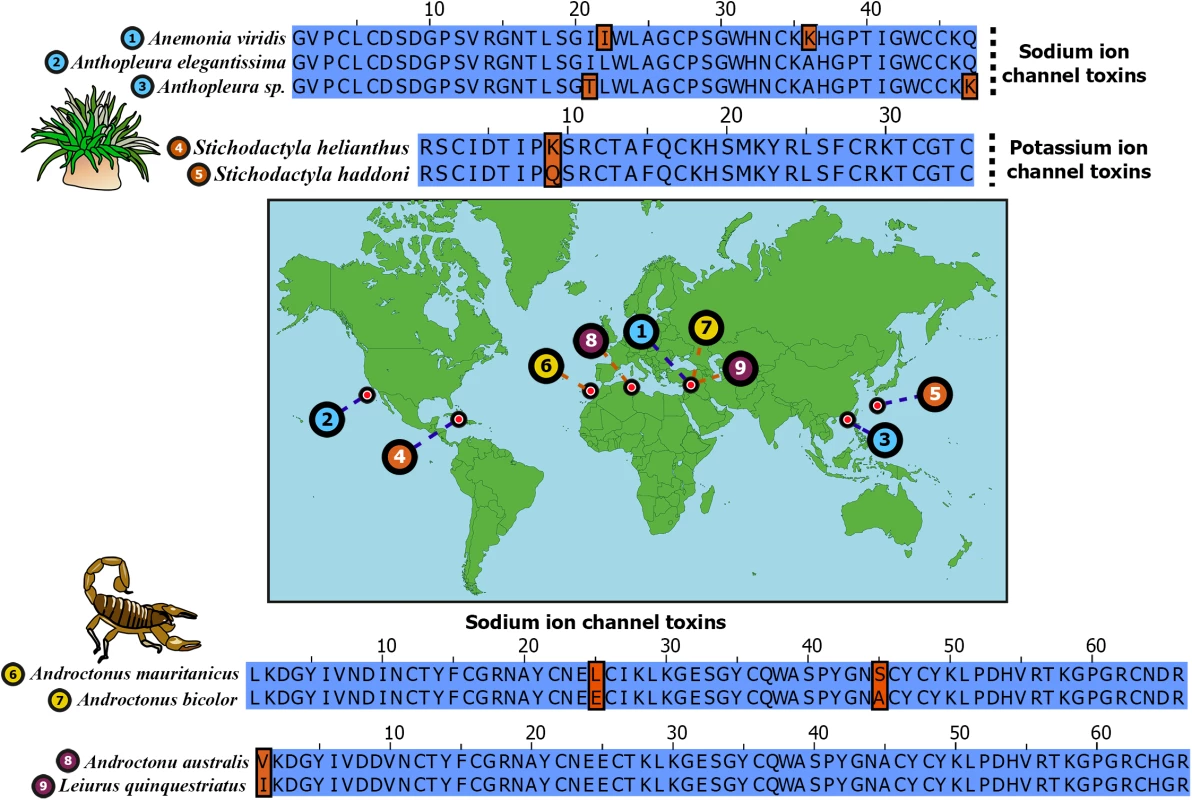
Sequence alignments of widely separated sea anemone and scorpion neurotoxins are depicted. Sampled locations of these toxins are indicated on the map. Identical positions in sequence alignments are shown in blue, while differing amino acids are shown in brown. Uniprot IDs of sequences are: 1) B1NWR0; 2) P01532; 3) P0C5F4; 4) P29187; 5) E2S062; 6) Q7YXD3; 7) D5HR48; 8) P01484 and 9) D5HR56. Phylum Cnidaria consists of animals such as sea anemones, jellyfish, corals and hyrdroids that originated in the Ediacaran Period, approximately 600 MA [13–15]. They are characterized by unique stinging organelles called nematocysts with which they inject venom. Cnidaria represents the oldest venomous lineage known and includes some of the most notorious animals, such as the sea wasp (Chironex fleckeri), a species of box jellyfish. Coleoids, which first appeared in the Early Devonian 380–390 MA [16], represent yet another neglected lineage of ancient venomous animals. Although the venomous nature of coleoids was established as early as 1888 [17], their venoms have received scant attention from toxinological research [17–20].
Centipedes are amongst the oldest living terrestrial venomous animals, with the fossil record extending back to ~420 MA [21]. All ~3,300 species of centipedes (class Chilopoda) described to date belong to five extant orders: Craterostigmomorpha, Geophilomorpha, Lithobiomorpha, Scolopendromorpha and Scutigeromorpha. They inject venom into victims via modified first pair of trunk limbs (forcipules) and use venom for predation and defense. Venoms of certain centipedes can cause excruciating pain, paresthesia, edema, necrosis [22, 23] and can be fatal to mammals as large as dogs [24]. Yet, only a handful of centipede toxins have been pharmacologically characterized to date. Similarly, despite their remarkable ability to target a diversity of ion channels, only toxins from certain medically significant species of spiders have been investigated to date [25]. Thus, the evolutionary history and phyletic distribution of venom from these aforementioned ancient lineages, which represent the first venomous animal groups, remain understudied [18, 26–28]. It should be noted that the divergence times of these lineages can be safely assumed to be equivalent to the time of origin of venom in those respective lineages, as all of the examined lineages are (i) venomous, (ii) do not share between them a common venomous ancestor, and/or (iii) for most of them the fossil data clearly indicates the presence of a venom delivery apparatus [29–36].
By examining a large number of nucleotide sequences from a diversity of species, we report for the first time the molecular evolutionary histories of a number of venom protein families in centipedes, spiders and Toxicofera (clade of venomous snakes and lizards) lizards. In contrast to the rapid evolution of venom in evolutionarily younger lineages, we report an unusually high conservation of venom in centipedes and spiders, despite their long evolutionary histories. Moreover, molecular evolutionary assessments of toxin-encoding genes distributed across the tree of life, has unraveled a surprisingly strong influence of negative selection on the venoms of ancient animals. Our findings reveal contrasting trajectories of venom evolution in ancient and evolutionarily young clades, and emphasize the significant roles of purifying and episodic selections in shaping animal venoms. Further, these results enabled the postulation of a new model of venom evolution that captures their evolutionary dynamics, and the rise and fall in evolutionary rates of animal venoms.
Results
Slow evolving centipede and spider venoms
Despite the fact that several centipede and spider toxins are capable of exhibiting a diverse array of pharmacological effects, their venoms remain poorly studied. To date, very few studies have examined the evolutionary mechanisms responsible for the diversification of toxins in centipedes [27, 28] and spiders [37–40]. Hence, we assessed the molecular evolutionary regimes of 17 and 10 gene families encoding toxins in the major lineages of centipedes and spiders, respectively. We computed the ratio of non-synonymous (dN) to synonymous (dS) substitutions, called omega (ω), where ω greater than, less than or equal to one is characteristic of positive, negative and neutral selection, respectively. A large proportion of centipede venoms are characterized by β-pore-forming toxins (β-PFT) that are similar to aerolysins and epsilon toxins from bacteria [28]. They are theorized to be responsible for myotoxic and edematogenic activities of centipede venoms [23, 28, 41]. β-PFT has undergone substantial gene duplication and diversification in centipedes [28]. In contrast to venom-encoding genes in evolutionarily younger lineages that continue experiencing positive selection when they diversify via recurrent duplication events, we find that β-PFTs are evolutionarily extremely constrained under negative selection, as indicated by ω smaller one (Table 1). Centipede venoms are also chiefly constituted by cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 (CAP) family members and toxins with low-density lipoprotein receptor Class A (LDLA) repeats [28]. While certain CAP proteins in the venoms of centipedes are characterized by trypsin inhibitory activities [42], the precise role of LDLAs remain unknown. We found that both these toxin classes have experienced a strong influence of purifying selection (Table 1).
Tab. 1. Molecular evolution of centipede PFT, CAP and LDLA venom proteins. 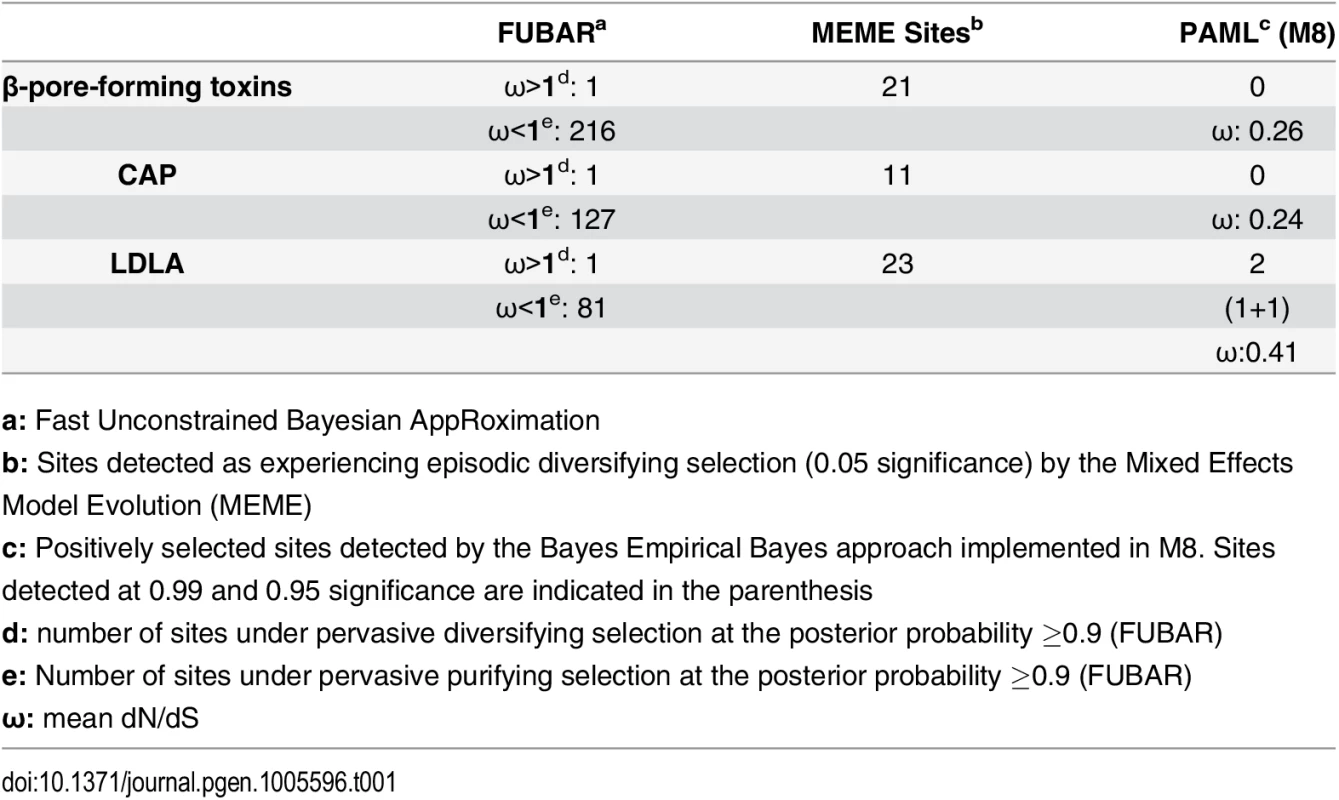
a: Fast Unconstrained Bayesian AppRoximation Scoloptoxin (SLPTX) is a family of cysteine-rich peptides found in the venoms of several centipedes, where different members exhibit a diversity of pharmacological activities [28]. SLPTX1 appears to be similar to insect peritrophic matrix proteins and has been theorized to be one of the most basally recruited toxins in centipedes [28]. Despite its long evolutionary history, this toxin exhibited lower-levels of sequence variations due to the influence of negative selection (Table 2). SLPTX10 and SLPTX15 families were reported to have undergone a functional radiation, where members exhibit neurotoxicity by targeting various voltage-gated ion channels: calcium (Cav), potassium (Kv) and sodium (Nav) ion channels [28]. Similarly, certain SLPTX11 family members are known for their anticoagulant and Kv channel inhibitory activities [28, 43]. We found that even these putatively potent toxins in centipedes were extremely well conserved under the influence of purifying selection (Table 2).
Tab. 2. Molecular evolution of scoloptoxins. 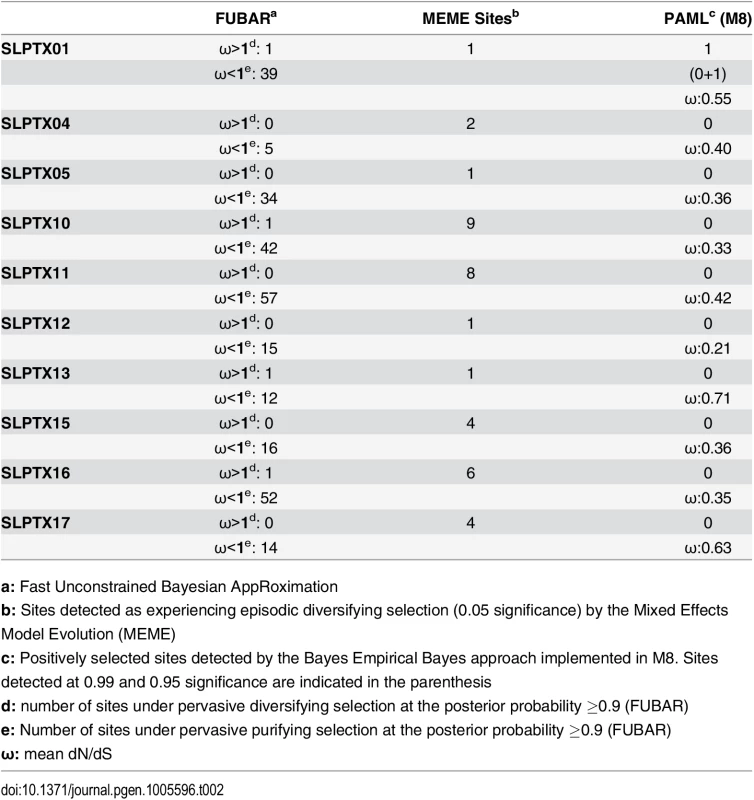
a: Fast Unconstrained Bayesian AppRoximation While SLPTX family 13 appears to have convergently adopted an inhibitory cysteine knot (ICK) scaffold, which is characteristic of various potent toxins from scorpions and spiders, SLPTX16 has adopted a Von Willebrand factor type C (VWC)-like domain. These peptides were highly conserved despite their putative role in prey envenoming and long evolutionary histories. Certain taxonomically restricted toxin families, called ‘novel families’ were recently reported in centipede venoms [28]. Only one (‘novel family 6’) amongst the four of these novel families examined was found to have evolved rapidly, while the rest were negatively selected (Table 3). Overall, centipede venom-encoding genes were found to have evolved under the heavy constraints of purifying selection (Fig 2A).
Fig. 2. Molecular evolution of venom in centipedes (A) and spiders (B). 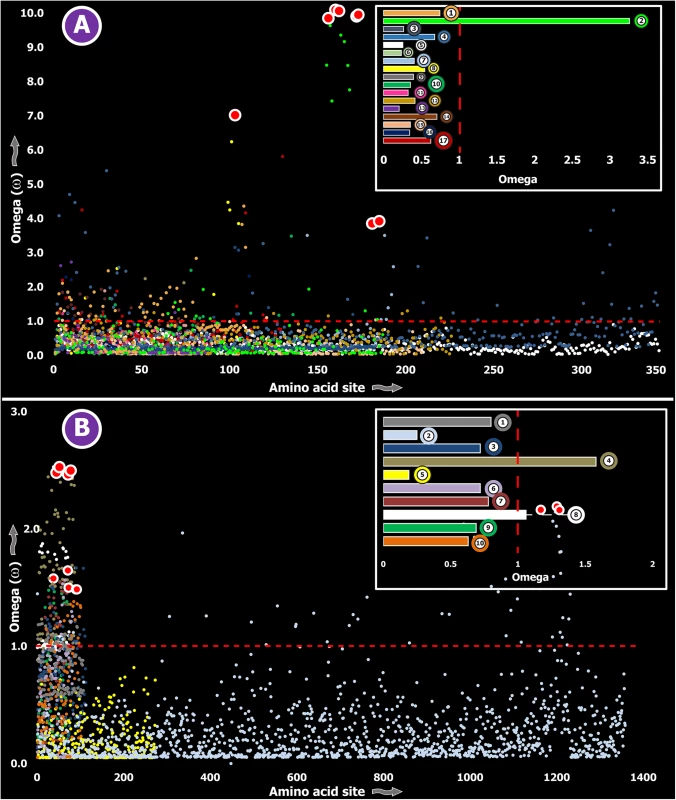
A plot of site-specific ω against amino acid positions for various centipede and spider venom-encoding genes is presented in panel A and B, respectively. Significantly detected positively selected sites (model 8; Bayes Empirical Bayes approach) are presented as large red circles. The red horizontal line represents the line of neutrality: points above and below this line indicate positive and negative selection, respectively. A corresponding bar plot is provided, which shows the computed ω value for the respective toxin class. Bar plot color code: Panel A 1) Novel family (NF) 8; 2) NF 6; 3) NF 4; 4) NF 1; 5) β-PFT; 6) CAP; 7) LDLA; 8) SLPTX 1; 9) SLPTX 4; 10) SLPTX 5; 11) SLPTX 10; 12) SLPTX 11; 13) SLPTX 12; 14) SLPTX 13; 15) SLPTX 15; 16) SLPTX 16; 17) SLPTX 17; Panel B 1) lycotoxins; 2) latrotoxins; 3) magi-1 family; 4) Kunitz toxins; 5) Sphingomyelinase D; 6) Huwentoxin-1 family; 7) κ/ω-hexatoxins; 8) κ-hexatoxins; 9) ω-hexatoxins; 10) Superfamily E ICKs. Tab. 3. Molecular evolution of centipede novel putative toxin families. 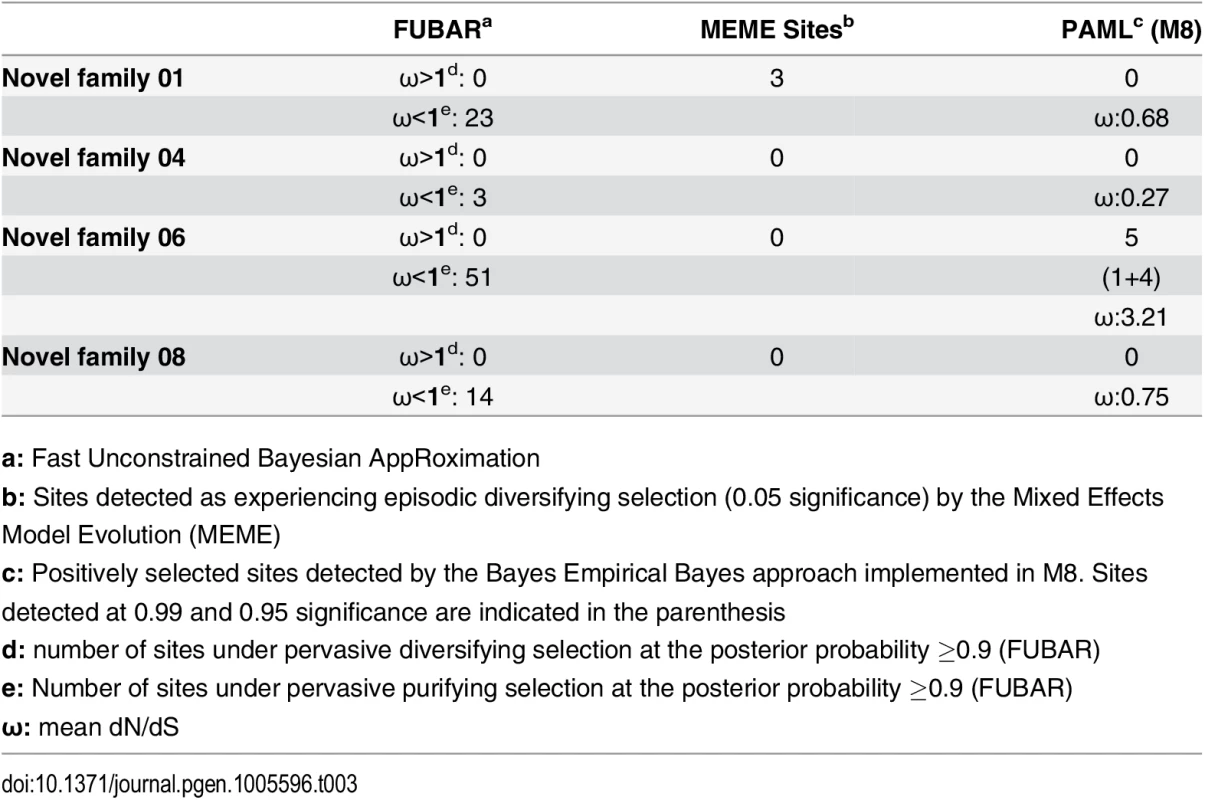
a: Fast Unconstrained Bayesian AppRoximation Spiders are known to have originated 416–359 MYA in the Devonian [44]. All spiders, with the exception of a few species, employ venom for predation. However, toxinological research to date has solely focused on characterizing venom from the medically significant species of spiders. Yet, venom from only 0.4% of the currently cataloged spider species have been characterized to date [25]. We determined the rate of evolution of several venom protein superfamilies in a diversity of spider lineages, such as the lethal latrotoxins secreted by widow spiders [Theridiidae: 223–180 MYA [45]]; Kunitz-type serine protease inhibitors and huwentoxins from tarantulas [Theraphosidae: 250–200 MYA [46]]; the magitoxin family from tarantulas and certain funnel-web spiders [Hexathelidae: 250–200 MYA [46]]; sphingomyelinase-D (SMase D) in the medically significant venoms of recluse spiders [Sicariidae: 145+ MYA [46]]; lycotoxin family [47] from wolf spiders [Lycosidae: ~120+ MYA [46]]; and super family E ICKs [48] secreted by tarantulas and brushed trapdoor spiders [Barychelidae: 250–200 MYA [46]]. These venom proteins are secreted in large amounts by the respective spider lineage and are known for a diversity of biochemical activities, such as insecticidal presynaptic neurotoxicity and the ability to stimulate neurotransmitter secretions [latrotoxins: [49, 50]], dermonecrotic properties [SMase D: [51]], Nav channel targeting capability–with some members additionally capable of targeting Cav channels [huwentoxin-1 family: [52, 53]], serine protease inhibition and the ability to block Kv channels [Kunitz toxins: [54]], insect Nav channel targeting [magi-1 family [55]], insect Cav channel targeting [ω-hexatoxins: [56]], insect Calcium activated potassium channel (KCa) targeting [κ-hexatoxins: [57]], and the Nav modulation and Cav blocking capabilities [Super Family E ICKs: [48]]. The computed ω values suggested a greater influence of purifying selection on nine out of ten toxin families examined, highlighting the slower evolution of spider venoms (S1 Table; Fig 2B).
Contrasting evolutionary regimes of venom in evolutionarily ancient and young clades
Computed ω values for the vast majority of venom-encoding genes in all ancient lineages examined in this study and in previous studies [18, 26, 38, 58], highlighted the significant role of negative selection, which was in stark contrast to those of evolutionarily younger lineages, such as the advanced snakes and cone snails [S1 Table; [6, 7, 59–62]]. We also evaluated the molecular evolution of venom families from Toxicofera lizards that originated ~166 MYA [63], and thus represent an intermediate state between ancient and recently originated lineages. Although, relative to advanced snakes, these lizards do not rely on venom for predation or defense to the same degree [6], the evolutionary rates of some of their largely secreted venom proteins exhibited rapid evolution as demonstrated by their high number of positively selected sites (Fig 3; S1 Table).
Fig. 3. Molecular evolution of venom in Toxicofera lizards. 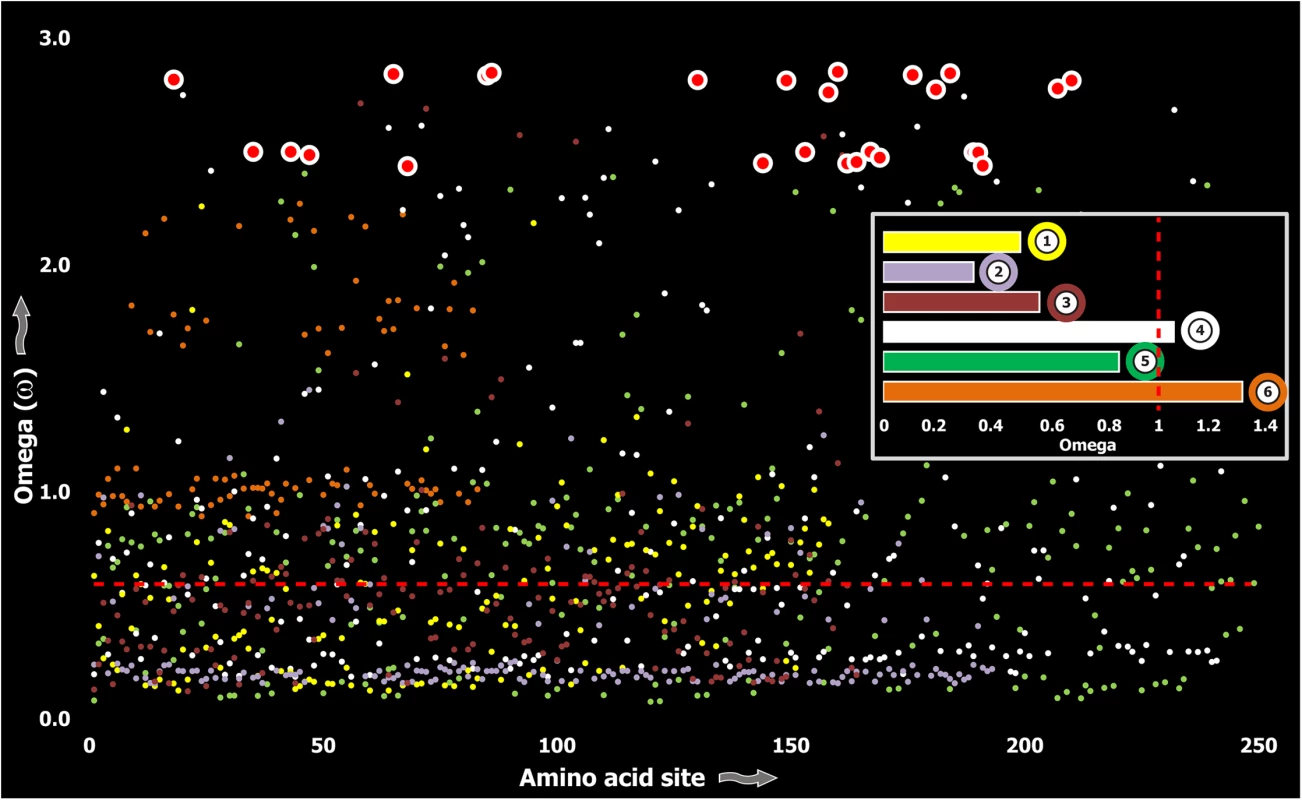
A plot of site-specific ω against amino acid positions for various Toxicofera lizard toxin types is presented. The red horizontal line represents the line of neutrality: points above and below this line indicate positive and negative selection, respectively. Bar plot color codes: 1) Phospholipase A2; 2) Nerve Growth Factors; 3) Natriuretic peptides and 4) CRiSPs; 5) Kallikreins; and 6) crotamines. Three (Kallikreins, CRiSPs and crotamines) among the six gene families examined exhibited an evidence for rapid evolution (ω>1 and/or more than 10 positively selected sites), while the remaining were found to be extremely well conserved (S1 Table). Further, we plotted site-wise ω against their respective amino acid position for each of the genes examined. Results indicated that a majority of sites in most venom proteins of ancient lineages evolved under the strong influence of negative selection (Figs 2–5).
Fig. 4. Molecular evolution of venom in cnidarians (A) and scorpions (B). 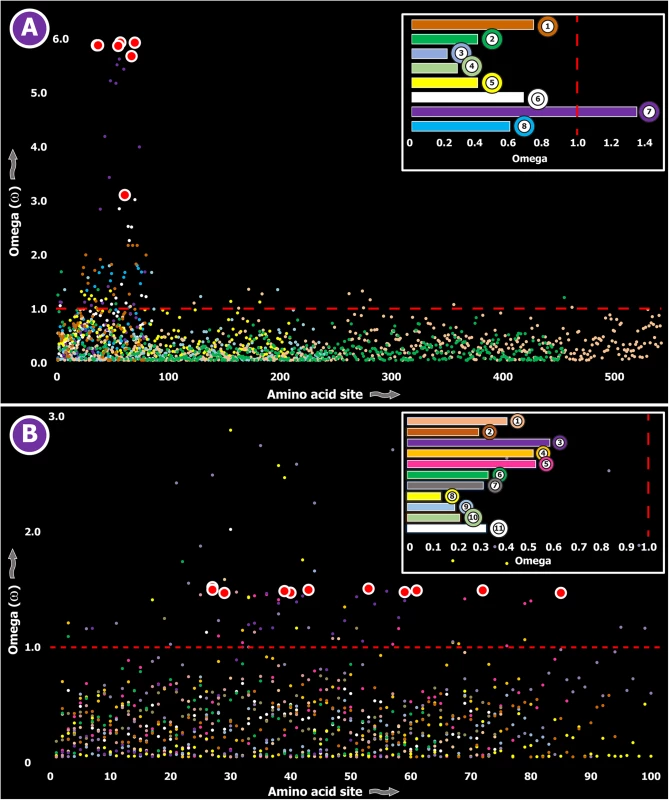
A plot of site-specific ω against amino acid positions for various cnidarian and scorpion venom-encoding genes is presented in panel A and B, respectively. Significantly detected positively selected sites (model 8; Bayes Empirical Bayes approach) are presented as large red circles. The red horizontal line represents the line of neutrality: points above and below this line indicate positive and negative selection, respectively. A corresponding bar plot is provided, which shows the computed ω value for the respective toxin class. Bar-plot color code: Panel A 1) SCRiPs; 2) JFTs; 3) Hydralysins; 4) Aerolysin-related toxins in sea anemone; 5) Actinoporins; 6) KTx Type 1; 7) KTx Type 3; 8) NaTx; Panel B 1) Short KTx; 2) Long KTx; 3) Chloride; 4) β-NaTx; 5) α-NaTx; 6) ICK; 7) DDH; 8) Glycine-rich toxins; 9) Bradykinin Potentiating Peptides; 10) Anionic; 11) Antimicrobial peptide toxins. Fig. 5. Molecular evolution of venom in coleoids. 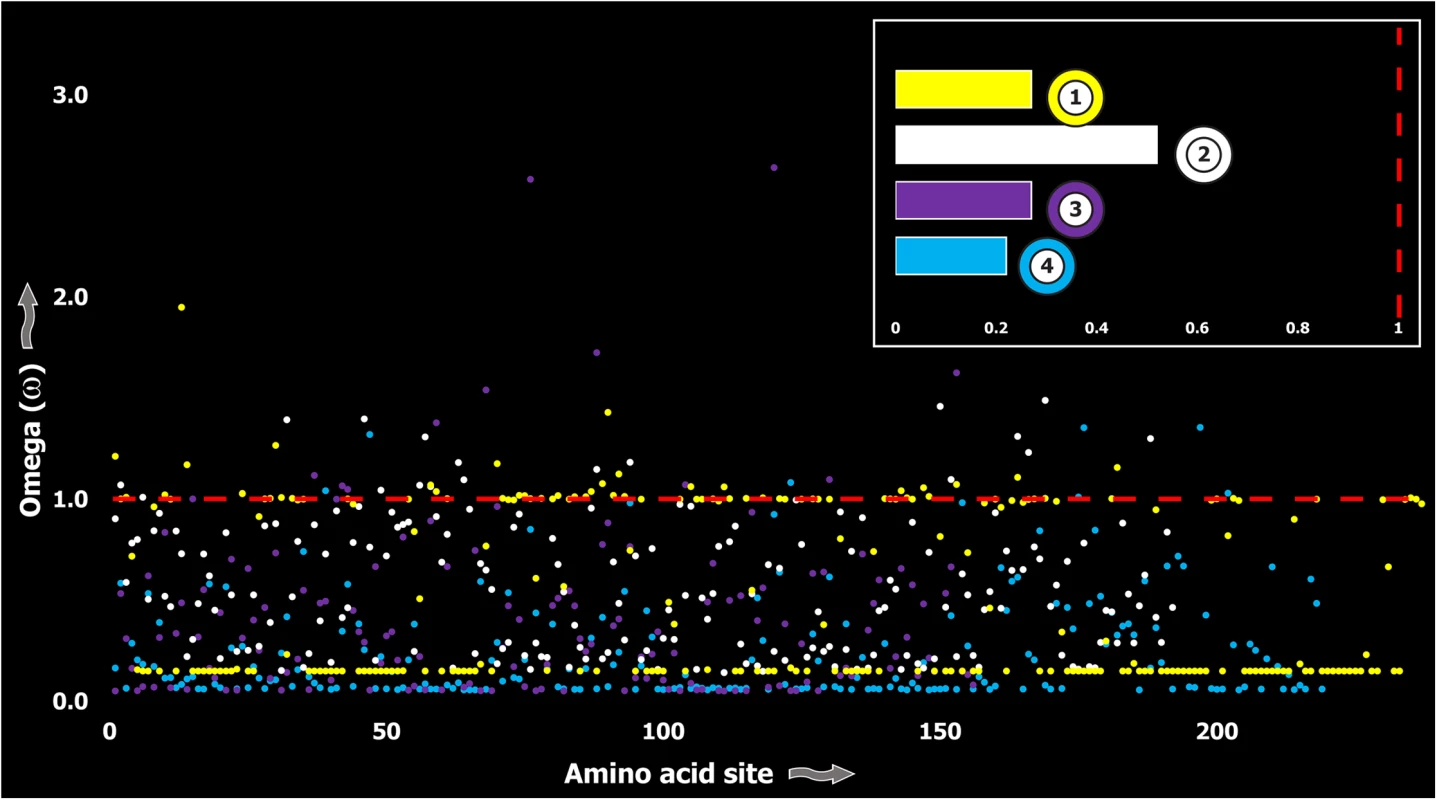
A plot of site-specific ω against amino acid positions for various coleoid toxin types is presented. The red horizontal line represents the line of neutrality: points above and below this line indicate positive and negative selection, respectively. Bar plot color codes: 1) Serine Protease; 2) PLA2; 3) Pacifestin and 4) CAP. In contrast, a large proportion of sites in toxins of evolutionarily young lineages rapidly mutated under the significant influence of positive Darwinian selection (Fig 6). Thus, a stark difference was found in the evolutionary regimes of ancient and evolutionarily young lineages (Fig 7).
Fig. 6. Molecular evolution of venom in advanced snakes (A) and cone snails (B). 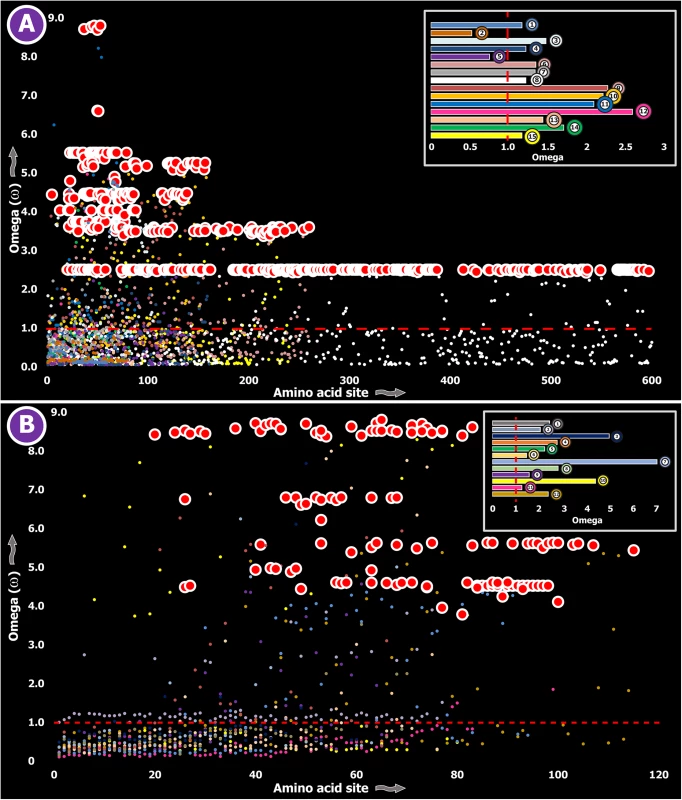
A plot of site-specific ω against amino acid positions for various advanced snake and cone snail venom-encoding genes is presented. Significantly detected positively selected sites (model 8; Bayes Empirical Bayes approach) are presented as large red circles. The red horizontal line represents the line of neutrality: points above and below this line indicate positive and negative selection, respectively. A corresponding bar plot is provided, which shows the computed ω value for the respective toxin class. Bar-plot color code: Panel A 1) β-defensins; 2) Cytotoxins; 3) PII-Disintegrins; 4) Group I PLA2s; 5) Group II PLA2s; 6) Kallikreins; 7) Psammophis SVMPs; 8) Advanced snake SVMPs; 9) Serine Proteases; 10) Lectins; 11) κ-3FTxs; 12) Type III α-neurotoxins; 13) Type II α-neurotoxins; Type I α-neurotoxins; and 14) CRISPs. Panel B Conus marmoreus—> 1) Superfamily M; 2) Superfamily I2; 3) Superfamily T; 4) Superfamily O2; C. geographus—> 5) Superfamily O1; 6) Superfamily O2; 7) Superfamily O1; 8) Superfamily M; 9) Superfamily A; 10) Conkunitzin; 11) Conantokin; 12) Con-ikot-ikot. Fig. 7. Schematic tree of life depicts the evolution of venom in animals, where blue, red and orange colored lines represent lineages that utilize venom for defense, predation or intraspecific needs, respectively. 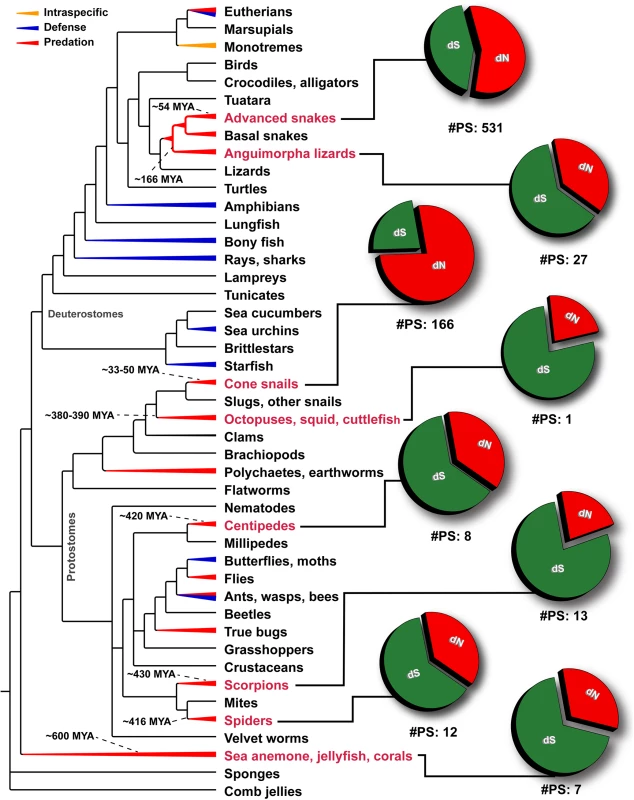
Divergence times of lineages examined in this study (labels indicated in red) have been indicated. Pie charts depict the proportion of synonymous (dS), non-synonymous (dN) mutations and the number of significantly detected positively selected sites (#PS) in the venom-encoding genes of the respective lineage. Depicted phylogenetic relationships are based on Ref. [13]. Using the mixed effects model of evolution (MEME) several sites that experienced short periods of diversifying selection were also identified in all the examined venomous clades, which indicated that certain sites in these toxin proteins undergo episodic adaptation (Tables 1–3; S1 Table). Considering the long evolutionary histories of these toxin types, we tested for nucleotide substitution saturation (see methods). These tests did not detect saturation in any of the examined datasets (S2 Table).
The influence of the length of a toxin on its omega
We performed regression analyses to evaluate the possibility of the length of the toxin determining its rate of evolution by plotting ω values for various toxin types against their respective lengths (S1 Fig). The coefficient of determination (r2) for toxin types in each of the examined lineages suggested an absence of correlation between the length of the toxin and its ω value, indicating that venom proteins have undergone rapid evolution or extreme sequence conservation irrespective of their size. While most conotoxins are of relatively shorter lengths, snake venom components such as three-finger toxins (3FTxs) and Snake Venom Metalloproteinases (SVMPs) are characterized by lengths of 80 and 600 amino acids, respectively. Despite such stark size differences, these toxins evolved rapidly. Similarly, several venom components in ancient lineages were characterized by a range of lengths. For example, most sea anemone and scorpion neurotoxins were of relatively shorter lengths (40–60 amino acids), while several pore-forming toxins were 450–550 amino acids long. Yet, these toxins were found to have evolved extremely slowly under the influence of purifying selection. Our results thus indicated that the stark differences in ω values for venom proteins of ancient and evolutionarily younger lineages did not result from the differences in size.
Discussion
The significance of purifying selection in the evolution venom
Animal venoms are assumed to rapidly diversify under the unabated influence of positive Darwinian selection. They have been theorized to undergo a chemical arms race with prey animals, where the evolution of venom resistance in prey and the invention of efficient toxins in the predatory venomous animal exert reciprocal selection pressure [64], as postulated in the Red Queen hypothesis of Van Valen [65]. While the influence of positive selection is widely recognized, the role of purifying selection in shaping animal venoms has rarely been considered. Investigation of a large number of toxin-encoding gene families in this study has revealed a significant influence of negative selection on venom. Whilst positive selection increases the diversity of venom proteins, purifying selection probably aids in preserving the potency of the venom by filtering out mutations that negatively affect toxin efficiency. However, rare mutations that increase the potency of the venom arsenal (e.g., evolution of novel biochemical activity or increased binding efficiency) are likely to be propagated and preserved in the population. In the absence of a conservatory evolutionary force, neutral or positive selection could modify key residues and result in the reduction of potency or, for worse, the complete loss of bioactivity, which could severely decrease the fitness of the animal. Thus, purifying selection pressure appears to be vital for sustaining the potency and, consequently, shaping the animal venom arsenal.
Certain toxins are more constrained than others
It has been recently demonstrated that PFTs in Cnidaria, which bind to cell membranes and punch holes, evolve under the heavy constraints of negative selection [26, 66]. The lack of variation in this group of toxins, which includes several unrelated toxin types (e.g., aerolysin-related toxins in sea anemones, independently recruited hydralysins in hydroids, actinoporins and jellyfish toxins), was theorized to be a result of their complex multi-subunit packaging [67] and their ability to attack highly conserved molecular targets, such as cell membranes [26]. Toxins that undergo oligomerization in other classes of animals have also been noted to evolve relatively slowly as a result of structural constraints like the need to conserve sites involved in subunit interaction. While most 3FTxs in snake venoms diversify rapidly, κ-3FTxs, which undergo dimerization, were found to accumulate relatively fewer variations [59]. Similarly, toxins that may function in a ‘non-specific’ manner may also experience negative selection. Here, non-specificity of action is defined as the ability to target regions in a structural/biochemical property dependent (e.g., surface electrostatic charge) and target motif independent manner. For example, cytotoxic 3FTxs and β-defensin toxins—two very potent snake venom proteins, induce cytotoxicity by non-specifically binding to negatively charged cell membranes using hydrophobicity [68] and positively charged molecular surface [69], respectively. As a result, unlike most snake venom components, these proteins remain evolutionarily constrained [59, 62]. Similarly, scorpion lipolytic toxins were also theorized to be evolutionarily constrained because of their non-specific mechanism of action [58]. We found that β-PFTs in centipede venoms, which are similar to the aerolysin-like toxins, evolve under the significant influence of negative selection (Table 1). The lack of variation in this group of toxins may suggest that they either undergo oligomerization like their aerolysin homologues in other lineages or the possibility that they may employ a non-specific mechanism of action. A plot of site-specific ω against their respective amino acid positions reveals the extreme conservation of such toxin types that employ unique strategies for causing toxicity in envenomed animals (S2 Fig). As it allows the targeting of a wide variety of animals, the strategy of exerting toxic action non-specifically or by targeting highly conserved molecular sites, appears to be advantageous and follows a contrastingly different evolutionary regime in comparison to toxins that specialize in attacking highly plastic molecular receptors.
The rise and fall of venom evolution
A comparison of evolutionary regimes of ancient and evolutionarily younger lineages suggests a fascinating strategy of venom evolution. When venomous animals venture into novel ecological niches, they encounter new types of prey and predatory animals. Consequently, in order to adapt and conquer niches, they would need to fine-tune venom proteins to efficiently target these new animals. Several sites detected as episodically adaptive—i.e., sites that experience short bursts of adaptive selection, in these ancient clades may be reflective of such shifts in ecology. We propose that these earlier periods in the evolutionary history of a venomous species are accompanied by the significant influence of diversifying selection on the venom arsenal, which would expand the range of target sites and/or result in the origination of novel biochemical activities. This is particularly advantageous, since novel toxins generated may facilitate the efficient and rapid incapacitation of newly encountered prey and predatory animals. The period of expansion is followed by longer periods of purification, where the significant influence of negative selection preserves the potency of the toxin. Whenever there is a major shift in ecology or environment, the aforementioned stages of evolution repeat. Thus, we propose that venom-encoding genes mostly employ a ‘two-speed’ mode of evolution, where episodic diversifying selection accompanies the earlier stages of ecological specialization (e.g., diet and range expansion), resulting in the rapid diversification of the venom arsenal, followed by a longer period of purification and fixation that ensure the sustainability of venom potency. The low sequence variation in venom-encoding genes of ancient clades could be reflective of such long periods of purification and fine-tuning. In contrast, advanced snakes and cone snails, being evolutionarily very young, could still be undergoing the period of expansion and, consequently, exhibit a pronounced signature of positive Darwinian selection.
However, it should be noted that the ‘two-speed’ model of evolution is likely applicable to venoms that serve predominantly predatory roles. Due to limited toxin sequence information from venoms that are employed for non-predatory functions (e.g., intraspecific competition in platypus, exclusively defensive roles in fishes, etc.), it remains to be seen whether they too follow our proposed evolutionary model.
To conclude, in addition to unraveling the evolutionary regimes of toxin families in centipedes and spiders, which are amongst the first terrestrial venomous lineages, our findings highlight the pivotal roles of purifying and episodic selections in shaping animal venoms. Our findings enabled the postulation of a new theory of venom evolution in the animal kingdom that emphasizes the dynamic nature of these complex biochemical cocktails.
Methods
Genome searches
Toxin homologues were identified in the recently published genome of the coastal European centipede Strigamia maritima [70] by querying amino acid sequences of each toxin type against all six reading frames using the tblastn tool [71].
Evolutionary analyses
Translated nucleotide sequences were aligned using MUSCLE 3.8 [72]. The best-fit model of nucleotide substitution for individual toxin datasets was determined according to the Akaike’s information criterion using jModeltest 2.1 [73] and model-averaged parameter estimates were used for the reconstruction of trees. Phylogenetic trees were built using PhyML 3.0 [74], where node support was evaluated with 1,000 bootstrapping replicates. Maximum-likelihood (ML) models [75] implemented in Codeml of the PAML package [76] were utilized to identify the influence of natural selection on toxin families[6]. As no a priori expectation exists, we compared likelihood values for a pair of models with different assumed ω distributions: M7 (β) versus M8 (β and ω) [77]. Only when the alternate model (M8) shows a better fit than the null model (M7) in the likelihood ratio test (LRT), are its results considered significant. LRT is estimated as twice the difference in ML values between the nested models, and is compared with the χ2 distribution with the appropriate degree of freedom—the difference in the number of parameters of the two models. Further, we used the Bayes empirical Bayes (BEB) approach [78] in M8 to detect amino acids under positive selection by calculating the posterior probability (PP) that a particular site belongs to a given selection class (neutral, conserved, or highly variable). Sites with PP ≥ 95% of belonging to the ‘‘ω > 1 class” are inferred to be positively selected. HyPhy’s [79] FUBAR approach [80] was used to detect sites evolving under pervasive diversifying and purifying selection pressures. MEME [81] was also employed to identify episodically diversifying sites. Sequence alignments used for selection assessments have been made available as a zipped file (S1 File; see S3 Table for accession list). Nucleotide substitution saturation was tested using DAMBE 5.5.9 [82] using the recommended protocol [83].
Supporting Information
Zdroje
1. Vidal N, Rage J, Couloux A, Hedges SB. Snakes (Serpentes) in The Timetree of Life, Hedges S. B. and Kumar S., Eds. Oxford University Press. 2009 : 390–7.
2. Olivera BM. E.E. Just Lecture, 1996. Conus venom peptides, receptor and ion channel targets, and drug design: 50 million years of neuropharmacology. Molecular biology of the cell. 1997;8(11):2101–9. 9362055; PubMed Central PMCID: PMC25694.
3. Duda TF Jr., Kohn AJ. Species-level phylogeography and evolutionary history of the hyperdiverse marine gastropod genus Conus. Mol Phylogenet Evol. 2005;34(2):257–72. doi: 10.1016/j.ympev.2004.09.012 15619440.
4. Chang D, Duda TF Jr. Extensive and continuous duplication facilitates rapid evolution and diversification of gene families. Mol Biol Evol. 2012;29(8):2019–29. doi: 10.1093/molbev/mss068 22337864.
5. Wong ES, Belov K. Venom evolution through gene duplications. Gene. 2012;496(1):1–7. Epub 2012/01/31. doi: 10.1016/j.gene.2012.01.009 22285376.
6. Sunagar K, Johnson WE, O'Brien SJ, Vasconcelos V, Antunes A. Evolution of CRISPs associated with toxicoferan-reptilian venom and mammalian reproduction. Mol Biol Evol. 2012;29(7):1807–22. doi: 10.1093/molbev/mss058 22319140.
7. Dutertre S, Jin AH, Vetter I, Hamilton B, Sunagar K, Lavergne V, et al. Evolution of separate predation - and defence-evoked venoms in carnivorous cone snails. Nature communications. 2014;5 : 3521. doi: 10.1038/ncomms4521 24662800; PubMed Central PMCID: PMC3973120.
8. Casewell NR, Wagstaff SC, Harrison RA, Renjifo C, Wuster W. Domain loss facilitates accelerated evolution and neofunctionalization of duplicate snake venom metalloproteinase toxin genes. Molecular biology and evolution. 2011;28(9):2637–49. doi: 10.1093/molbev/msr091 21478373.
9. Nakashima K, Ogawa T, Oda N, Hattori M, Sakaki Y, Kihara H, et al. Accelerated evolution of Trimeresurus flavoviridis venom gland phospholipase A2 isozymes. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(13):5964–8. Epub 1993/07/01. 8327468; PubMed Central PMCID: PMCPmc46847.
10. Duda TF Jr., Palumbi SR. Molecular genetics of ecological diversification: duplication and rapid evolution of toxin genes of the venomous gastropod Conus. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(12):6820–3. Epub 1999/06/09. 10359796; PubMed Central PMCID: PMCPmc21999.
11. Waddington J, Rudkin DM, Dunlop JA. A new mid-Silurian aquatic scorpion-one step closer to land? Biology letters. 2015;11(1). Epub 2015/01/16. doi: 10.1098/rsbl.2014.0815 25589484.
12. Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, Valdivia HH, Possani LD. Scorpion venom components that affect ion-channels function. Toxicon: official journal of the International Society on Toxinology. 2013;76(0):328–42. doi: http://dx.doi.org/10.1016/j.toxicon.2013.07.012
13. Erwin DH, Laflamme M, Tweedt SM, Sperling EA, Pisani D, Peterson KJ. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science. 2011;334(6059):1091–7. Epub 2011/11/26. doi: 10.1126/science.1206375 22116879.
14. Park E, Hwang DS, Lee JS, Song JI, Seo TK, Won YJ. Estimation of divergence times in cnidarian evolution based on mitochondrial protein-coding genes and the fossil record. Molecular phylogenetics and evolution. 2012;62(1):329–45. Epub 2011/11/02. doi: 10.1016/j.ympev.2011.10.008 22040765.
15. Menon LR, McIlroy D, Brasier MD. Evidence for Cnidaria-like behavior in ca. 560 Ma Ediacaran Aspidella. Geology. 2013. doi: 10.1130/g34424.1
16. Sturmer W. A small coleoid cephalopod with soft parts from the lower Devonian discovered using radiography. Nature. 1985;318(6041):53–5.
17. Lo Bianco S. Notizie biologiche riguardanti specialmente il periodo di maturita sessuale degli animali del Golfo di Napoli. Mitth Zool Stat Neapel. 1888;8 : 385–440.
18. Ruder T, Sunagar K, Undheim EA, Ali SA, Wai TC, Low DH, et al. Molecular phylogeny and evolution of the proteins encoded by coleoid (cuttlefish, octopus, and squid) posterior venom glands. Journal of molecular evolution. 2013;76(4):192–204. doi: 10.1007/s00239-013-9552-5 23456102.
19. Ghiretti F. Toxicity of octopus saliva against crustacea. Annals of the New York Academy of Sciences. 1960;90 : 726–41. 13704937
20. Ghiretti F. Cephalotoxin: the crab-paralysing agent of the posterior salivary glands of cephalopods. Nature. 1959;183 : 1192–3.
21. Edgecombe GD, Giribet G. Evolutionary biology of centipedes (Myriapoda: Chilopoda). Annual review of entomology. 2007;52 : 151–70. doi: 10.1146/annurev.ento.52.110405.091326 16872257.
22. Balit CR, Harvey MS, Waldock JM, Isbister GK. Prospective study of centipede bites in Australia. Journal of toxicology Clinical toxicology. 2004;42(1):41–8. Epub 2004/04/16. 15083935.
23. Malta MB, Lira MS, Soares SL, Rocha GC, Knysak I, Martins R, et al. Toxic activities of Brazilian centipede venoms. Toxicon: official journal of the International Society on Toxinology. 2008;52(2):255–63. doi: 10.1016/j.toxicon.2008.05.012 18586047.
24. McKeown K. Centipedes and centipede bites. The Australian Museum Magazine. 1930;(4):59–60.
25. King GF, Hardy MC. Spider-venom peptides: structure, pharmacology, and potential for control of insect pests. Annual review of entomology. 2013;58 : 475–96. doi: 10.1146/annurev-ento-120811-153650 23020618.
26. Jouiaei M, Sunagar K, Federman Gross A, Scheib H, Alewood PF, Moran Y, et al. Evolution of an ancient venom: recognition of a novel family of cnidarian toxins and the common evolutionary origin of sodium and potassium neurotoxins in sea anemone. Mol Biol Evol. 2015;32(6):1598–610. doi: 10.1093/molbev/msv050 25757852.
27. Undheim EA, Sunagar K, Hamilton BR, Jones A, Venter DJ, Fry BG, et al. Multifunctional warheads: diversification of the toxin arsenal of centipedes via novel multidomain transcripts. Journal of proteomics. 2014;102 : 1–10. doi: 10.1016/j.jprot.2014.02.024 24602922.
28. Undheim EA, Jones A, Clauser KR, Holland JW, Pineda SS, King GF, et al. Clawing through evolution: toxin diversification and convergence in the ancient lineage Chilopoda (centipedes). Molecular biology and evolution. 2014;31(8):2124–48. Epub 2014/05/23. doi: 10.1093/molbev/msu162 24847043; PubMed Central PMCID: PMCPmc4104317.
29. Anderson LI, Trewin NH. An Early Devonian arthropod fauna from the Windyfield cherts, Aberdeenshire, Scotland. Palaeontology. 2003;46 : 467–509. doi: 10.1111/1475-4983.00308 WOS:000183159900003.
30. Ozbek S, Balasubramanian PG, Holstein TW. Cnidocyst structure and the biomechanics of discharge. Toxicon: official journal of the International Society on Toxinology. 2009;54(8):1038–45. doi: 10.1016/j.toxicon.2009.03.006 19286000.
31. Kuhn-Nentwig L, Stocklin R, Nentwig W. Venom composition and strategies in spiders: Is everything possible? In Advances in Insect Physiology. Spider Physiology and Behaviour, ed. Casas J. Burlington, MA: Academic. 2011 : 1–86.
32. Gess RW. The earliest record of terrestrial animals in Gondwana: A scorpion from the Famennian (Late Devonian) Witpoort Formation of South Africa. African Invertebrates. 2013;54(2):373–9.
33. Foelix R, Erb B. Mesothelae have venom glands. Journal of Arachnology. 2010;38(3):596–8. doi: 10.1636/B10-30.1
34. Fry BG, Vidal N, Norman JA, Vonk FJ, Scheib H, Ramjan SF, et al. Early evolution of the venom system in lizards and snakes. Nature. 2006;439(7076):584–8. doi: 10.1038/nature04328 16292255.
35. Fry BG, Roelants K, Norman JA. Tentacles of venom: toxic protein convergence in the Kingdom Animalia. Journal of molecular evolution. 2009;68(4):311–21. doi: 10.1007/s00239-009-9223-8 19294452.
36. Olivera BM, Showers Corneli P, Watkins M, Fedosov A. Biodiversity of Cone Snails and Other Venomous Marine Gastropods: Evolutionary Success Through Neuropharmacology. Annual Review of Animal Biosciences. 2014;2(1):487–513. doi: 10.1146/annurev-animal-022513-114124 25384153.
37. Undheim EA, Sunagar K, Herzig V, Kely L, Low DH, Jackson TN, et al. A proteomics and transcriptomics investigation of the venom from the barychelid spider Trittame loki (brush-foot trapdoor). Toxins. 2013;5(12):2488–503. doi: 10.3390/toxins5122488 24351713; PubMed Central PMCID: PMC3873697.
38. Pineda SS, Sollod BL, Wilson D, Darling A, Sunagar K, Undheim EA, et al. Diversification of a single ancestral gene into a successful toxin superfamily in highly venomous Australian funnel-web spiders. BMC genomics. 2014;15 : 177. doi: 10.1186/1471-2164-15-177 24593665; PubMed Central PMCID: PMC4029134.
39. Binford GJ, Bodner MR, Cordes MH, Baldwin KL, Rynerson MR, Burns SN, et al. Molecular evolution, functional variation, and proposed nomenclature of the gene family that includes sphingomyelinase D in sicariid spider venoms. Molecular biology and evolution. 2009;26(3):547–66. doi: 10.1093/molbev/msn274 19042943; PubMed Central PMCID: PMC2767091.
40. Garb JE, Hayashi CY. Molecular evolution of alpha-latrotoxin, the exceptionally potent vertebrate neurotoxin in black widow spider venom. Molecular biology and evolution. 2013;30(5):999–1014. doi: 10.1093/molbev/mst011 23339183; PubMed Central PMCID: PMC3670729.
41. Undheim EA, King GF. On the venom system of centipedes (Chilopoda), a neglected group of venomous animals. Toxicon: official journal of the International Society on Toxinology. 2011;57(4):512–24. Epub 2011/01/25. doi: 10.1016/j.toxicon.2011.01.004 21255597.
42. Liu ZC, Zhang R, Zhao F, Chen ZM, Liu HW, Wang YJ, et al. Venomic and transcriptomic analysis of centipede Scolopendra subspinipes dehaani. Journal of proteome research. 2012;11(12):6197–212. doi: 10.1021/pr300881d 23148443.
43. Yang S, Liu Z, Xiao Y, Li Y, Rong M, Liang S, et al. Chemical punch packed in venoms makes centipedes excellent predators. Molecular & cellular proteomics: MCP. 2012;11(9):640–50. doi: 10.1074/mcp.M112.018853 22595790; PubMed Central PMCID: PMC3434766.
44. Selden PA, Shear WA, Bonamo PM. A spider and other arachnids from the Devonian of New York, and reinterpretations of Devonian Araneae. Palaeontology. 1991;34 : 241–81.
45. Ayoub NA, Hayashi CY. Spiders (Araneae) in The Timetree of Life, Hedges S. B. and Kumar S., Eds. Oxford University Press. 2009 : 255–9.
46. Penney D. Spider Research in the 21st Century: Trends and Perspectives: Siri Scientific Press; 2013.
47. Zhang Y, Chen J, Tang X, Wang F, Jiang L, Xiong X, et al. Transcriptome analysis of the venom glands of the Chinese wolf spider Lycosa singoriensis. Zoology. 2010;113(1):10–8. doi: 10.1016/j.zool.2009.04.001 19875276.
48. Chen J, Deng M, He Q, Meng E, Jiang L, Liao Z, et al. Molecular diversity and evolution of cystine knot toxins of the tarantula Chilobrachys jingzhao. Cellular and molecular life sciences: CMLS. 2008;65(15):2431–44. Epub 2008/06/27. doi: 10.1007/s00018-008-8135-x 18581053.
49. Kiyatkin NI, Dulubova IE, Chekhovskaya IA, Grishin EV. Cloning and structure of cDNA encoding alpha-latrotoxin from black widow spider venom. FEBS Lett. 1990;270(1–2):127–31. 1977615.
50. Ushkaryov YA, Rohou A, Sugita S. alpha-Latrotoxin and its receptors. Handb Exp Pharmacol. 2008;(184):171–206. Epub 2007/12/08. doi: 10.1007/978-3-540-74805-2_7 18064415; PubMed Central PMCID: PMCPMC2519134.
51. Swanson DL, Vetter RS. Loxoscelism. Clin Dermatol. 2006;24(3):213–21. doi: 10.1016/j.clindermatol.2005.11.006 16714202.
52. Liang SP, Chen XD, Shu Q, Zhang Y, Peng K. The presynaptic activity of huwentoxin-I, a neurotoxin from the venom of the chinese bird spider Selenocosmia huwena. Toxicon: official journal of the International Society on Toxinology. 2000;38(9):1237–46. Epub 2000/03/29. 10736477.
53. Herzig V, Wood DL, Newell F, Chaumeil PA, Kaas Q, Binford GJ, et al. ArachnoServer 2.0, an updated online resource for spider toxin sequences and structures. Nucleic Acids Res. 2011;39(Database issue):D653–7. doi: 10.1093/nar/gkq1058 21036864; PubMed Central PMCID: PMC3013666.
54. Yuan CH, He QY, Peng K, Diao JB, Jiang LP, Tang X, et al. Discovery of a distinct superfamily of Kunitz-type toxin (KTT) from tarantulas. PloS one. 2008;3(10):e3414. Epub 2008/10/17. doi: 10.1371/journal.pone.0003414 18923708; PubMed Central PMCID: PMCPMC2561067.
55. Corzo G, Gilles N, Satake H, Villegas E, Dai L, Nakajima T, et al. Distinct primary structures of the major peptide toxins from the venom of the spider Macrothele gigas that bind to sites 3 and 4 in the sodium channel. FEBS Lett. 2003;547(1–3):43–50. Epub 2003/07/16. 12860384.
56. Bloomquist JR. Mode of action of atracotoxin at central and peripheral synapses of insects. Invert Neurosci. 2003;5(1):45–50. Epub 2003/11/11. doi: 10.1007/s10158-003-0027-z 14608494.
57. Gunning SJ, Maggio F, Windley MJ, Valenzuela SM, King GF, Nicholson GM. The Janus-faced atracotoxins are specific blockers of invertebrate K(Ca) channels. The FEBS journal. 2008;275(16):4045–59. Epub 2008/07/16. doi: 10.1111/j.1742-4658.2008.06545.x 18625007.
58. Sunagar K, Undheim EA, Chan AH, Koludarov I, Munoz-Gomez SA, Antunes A, et al. Evolution stings: the origin and diversification of scorpion toxin peptide scaffolds. Toxins. 2013;5(12):2456–87. doi: 10.3390/toxins5122456 24351712; PubMed Central PMCID: PMC3873696.
59. Sunagar K, Jackson TN, Undheim EA, Ali SA, Antunes A, Fry BG. Three-fingered RAVERs: Rapid Accumulation of Variations in Exposed Residues of snake venom toxins. Toxins. 2013;5(11):2172–208. doi: 10.3390/toxins5112172 24253238; PubMed Central PMCID: PMC3847720.
60. Brust A, Sunagar K, Undheim EA, Vetter I, Yang DC, Casewell NR, et al. Differential evolution and neofunctionalization of snake venom metalloprotease domains. Molecular & cellular proteomics: MCP. 2013;12(3):651–63. doi: 10.1074/mcp.M112.023135 23242553; PubMed Central PMCID: PMC3591658.
61. Juarez P, Comas I, Gonzalez-Candelas F, Calvete JJ. Evolution of snake venom disintegrins by positive Darwinian selection. Molecular biology and evolution. 2008;25(11):2391–407. Epub 2008/08/15. doi: 10.1093/molbev/msn179 18701431.
62. Sunagar K, Undheim EA, Scheib H, Gren EC, Cochran C, Person CE, et al. Intraspecific venom variation in the medically significant Southern Pacific Rattlesnake (Crotalus oreganus helleri): biodiscovery, clinical and evolutionary implications. J Proteomics. 2014;99 : 68–83. doi: 10.1016/j.jprot.2014.01.013 24463169.
63. Hedges SB, Vidal N. Lizards, snakes, and amphisbaenians (Squamata) in The Timetree of Life, Hedges S. B. and Kumar S., Eds. Oxford University Press. 2009 : 383–9.
64. Casewell NR, Wuster W, Vonk FJ, Harrison RA, Fry BG. Complex cocktails: the evolutionary novelty of venoms. Trends in ecology & evolution. 2013;28(4):219–29. doi: 10.1016/j.tree.2012.10.020 23219381.
65. Van Valen L. A new evolutionary law. Evolutionary Theory. 1973;1 : 1–30.
66. Rachamim T, Morgenstern D, Aharonovich D, Brekhman V, Lotan T, Sher D. The Dynamically Evolving Nematocyst Content of an Anthozoan, a Scyphozoan, and a Hydrozoan. Molecular biology and evolution. 2014. doi: 10.1093/molbev/msu335 25518955.
67. Kristan K, Podlesek Z, Hojnik V, Gutierrez-Aguirre I, Guncar G, Turk D, et al. Pore formation by equinatoxin, a eukaryotic pore-forming toxin, requires a flexible N-terminal region and a stable beta-sandwich. The Journal of biological chemistry. 2004;279(45):46509–17. doi: 10.1074/jbc.M406193200 15322132.
68. Kumar TK, Jayaraman G, Lee CS, Arunkumar AI, Sivaraman T, Samuel D, et al. Snake venom cardiotoxins-structure, dynamics, function and folding. Journal of biomolecular structure & dynamics. 1997;15(3):431–63. Epub 1998/01/24. doi: 10.1080/07391102.1997.10508957 9439993.
69. Nascimento FD, Hayashi MA, Kerkis A, Oliveira V, Oliveira EB, Radis-Baptista G, et al. Crotamine mediates gene delivery into cells through the binding to heparan sulfate proteoglycans. The Journal of biological chemistry. 2007;282(29):21349–60. Epub 2007/05/11. doi: 10.1074/jbc.M604876200 17491023.
70. Chipman AD, Ferrier DE, Brena C, Qu J, Hughes DS, Schroder R, et al. The First Myriapod Genome Sequence Reveals Conservative Arthropod Gene Content and Genome Organisation in the Centipede Strigamia maritima. PLoS Biol. 2014;12(11):e1002005. doi: 10.1371/journal.pbio.1002005 25423365; PubMed Central PMCID: PMC4244043.
71. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of molecular biology. 1990;215(3):403–10. doi: 10.1016/S0022-2836(05)80360-2 2231712.
72. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic acids research. 2004;32(5):1792–7. doi: 10.1093/nar/gkh340 15034147; PubMed Central PMCID: PMC390337.
73. Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9(8):772. doi: 10.1038/nmeth.2109 22847109.
74. Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic biology. 2010;59(3):307–21. doi: 10.1093/sysbio/syq010 20525638.
75. Yang Z. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Molecular biology and evolution. 1998;15(5):568–73. 9580986.
76. Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Molecular biology and evolution. 2007;24(8):1586–91. doi: 10.1093/molbev/msm088 17483113.
77. Nielsen R, Yang Z. Likelihood models for detecting positively selected amino acid sites and applications to the HIV-1 envelope gene. Genetics. 1998;148(3):929–36. 9539414; PubMed Central PMCID: PMCPMC1460041.
78. Yang Z, Wong WS, Nielsen R. Bayes empirical bayes inference of amino acid sites under positive selection. Molecular biology and evolution. 2005;22(4):1107–18. doi: 10.1093/molbev/msi097 15689528.
79. Pond SL, Frost SD, Muse SV. HyPhy: hypothesis testing using phylogenies. Bioinformatics. 2005;21(5):676–9. doi: 10.1093/bioinformatics/bti079 15509596.
80. Murrell B, Moola S, Mabona A, Weighill T, Sheward D, Kosakovsky Pond SL, et al. FUBAR: a fast, unconstrained bayesian approximation for inferring selection. Molecular biology and evolution. 2013;30(5):1196–205. doi: 10.1093/molbev/mst030 23420840; PubMed Central PMCID: PMC3670733.
81. Murrell B, Wertheim JO, Moola S, Weighill T, Scheffler K, Kosakovsky Pond SL. Detecting individual sites subject to episodic diversifying selection. PLoS genetics. 2012;8(7):e1002764. doi: 10.1371/journal.pgen.1002764 22807683; PubMed Central PMCID: PMC3395634.
82. Xia X, Xie Z, Salemi M, Chen L, Wang Y. An index of substitution saturation and its application. Molecular phylogenetics and evolution. 2003;26(1):1–7. 12470932.
83. Xia X, Lemey P. Assessing substitution saturation with DAMBE in Philippe Lemey, Marco Salemi and Anne-Mieke Vandamme, eds. The Phylogenetic Handbook: A Practical Approach to DNA and Protein Phylogeny. 2nd edition Cambridge University Press. 2009 : 615–30.
Štítky
Genetika Reprodukčná medicína
Článek Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation DevelopmentČlánek A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor PelotaČlánek A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in OvulationČlánek Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAsČlánek FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2Článek Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion inČlánek The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly SiteČlánek Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem CellsČlánek A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal ErythropoiesisČlánek Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation inČlánek Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in OocytesČlánek MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 10- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Gene-Regulatory Logic to Induce and Maintain a Developmental Compartment
- A Decad(e) of Reasons to Contribute to a PLOS Community-Run Journal
- DNA Methylation Landscapes of Human Fetal Development
- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development
- Transcriptional Derepression Uncovers Cryptic Higher-Order Genetic Interactions
- Silencing of X-Linked MicroRNAs by Meiotic Sex Chromosome Inactivation
- Virus Satellites Drive Viral Evolution and Ecology
- A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota
- Sequence to Medical Phenotypes: A Framework for Interpretation of Human Whole Genome DNA Sequence Data
- Your Data to Explore: An Interview with Anne Wojcicki
- Modulation of Ambient Temperature-Dependent Flowering in by Natural Variation of
- The Ciliopathy Protein CC2D2A Associates with NINL and Functions in RAB8-MICAL3-Regulated Vesicle Trafficking
- PPP2R5C Couples Hepatic Glucose and Lipid Homeostasis
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
- Intermediate Levels of CodY Activity Are Required for Derepression of the Branched-Chain Amino Acid Permease, BraB
- "Missing" G x E Variation Controls Flowering Time in
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Type IV Collagen Controls the Axogenesis of Cerebellar Granule Cells by Regulating Basement Membrane Integrity in Zebrafish
- Loss of a Conserved tRNA Anticodon Modification Perturbs Plant Immunity
- Genome-Wide Association Analysis of Adaptation Using Environmentally Predicted Traits
- Oriented Cell Division in the . Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1
- Disproportionate Contributions of Select Genomic Compartments and Cell Types to Genetic Risk for Coronary Artery Disease
- A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Ovulation
- The RNAPII-CTD Maintains Genome Integrity through Inhibition of Retrotransposon Gene Expression and Transposition
- Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAs
- Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector
- SCARN a Novel Class of SCAR Protein That Is Required for Root-Hair Infection during Legume Nodulation
- IBR5 Modulates Temperature-Dependent, R Protein CHS3-Mediated Defense Responses in
- NINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish
- Decay-Initiating Endoribonucleolytic Cleavage by RNase Y Is Kept under Tight Control via Sequence Preference and Sub-cellular Localisation
- Large-Scale Analysis of Kinase Signaling in Yeast Pseudohyphal Development Identifies Regulation of Ribonucleoprotein Granules
- FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2
- LINE-1 Mediated Insertion into (Protein of Centriole 1 A) Causes Growth Insufficiency and Male Infertility in Mice
- Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion in
- Genome-Scale Mapping of σ Reveals Widespread, Conserved Intragenic Binding
- Uncovering Hidden Layers of Cell Cycle Regulation through Integrative Multi-omic Analysis
- Functional Diversification of Motor Neuron-specific Enhancers during Evolution
- The GTP- and Phospholipid-Binding Protein TTD14 Regulates Trafficking of the TRPL Ion Channel in Photoreceptor Cells
- The Gyc76C Receptor Guanylyl Cyclase and the Foraging cGMP-Dependent Kinase Regulate Extracellular Matrix Organization and BMP Signaling in the Developing Wing of
- The Ty1 Retrotransposon Restriction Factor p22 Targets Gag
- Functional Impact and Evolution of a Novel Human Polymorphic Inversion That Disrupts a Gene and Creates a Fusion Transcript
- The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly Site
- The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study
- Parent-of-Origin Effects of the Gene on Adiposity in Young Adults
- Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem Cells
- Retinoic Acid Receptors Control Spermatogonia Cell-Fate and Induce Expression of the SALL4A Transcription Factor
- A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis
- Protein O-Glucosyltransferase 1 (POGLUT1) Promotes Mouse Gastrulation through Modification of the Apical Polarity Protein CRUMBS2
- KIF7 Controls the Proliferation of Cells of the Respiratory Airway through Distinct Microtubule Dependent Mechanisms
- Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation in
- Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in Oocytes
- Protein Homeostasis Imposes a Barrier on Functional Integration of Horizontally Transferred Genes in Bacteria
- A New Method for Detecting Associations with Rare Copy-Number Variants
- Histone H2AFX Links Meiotic Chromosome Asynapsis to Prophase I Oocyte Loss in Mammals
- The Genomic Aftermath of Hybridization in the Opportunistic Pathogen
- A Role for the Chaperone Complex BAG3-HSPB8 in Actin Dynamics, Spindle Orientation and Proper Chromosome Segregation during Mitosis
- Establishment of a Developmental Compartment Requires Interactions between Three Synergistic -regulatory Modules
- Regulation of Spore Formation by the SpoIIQ and SpoIIIA Proteins
- Association of the Long Non-coding RNA Steroid Receptor RNA Activator (SRA) with TrxG and PRC2 Complexes
- Alkaline Ceramidase 3 Deficiency Results in Purkinje Cell Degeneration and Cerebellar Ataxia Due to Dyshomeostasis of Sphingolipids in the Brain
- ACLY and ACC1 Regulate Hypoxia-Induced Apoptosis by Modulating ETV4 via α-ketoglutarate
- Quantitative Differences in Nuclear β-catenin and TCF Pattern Embryonic Cells in .
- HENMT1 and piRNA Stability Are Required for Adult Male Germ Cell Transposon Repression and to Define the Spermatogenic Program in the Mouse
- Axon Regeneration Is Regulated by Ets–C/EBP Transcription Complexes Generated by Activation of the cAMP/Ca Signaling Pathways
- A Phenomic Scan of the Norfolk Island Genetic Isolate Identifies a Major Pleiotropic Effect Locus Associated with Metabolic and Renal Disorder Markers
- The Roles of CDF2 in Transcriptional and Posttranscriptional Regulation of Primary MicroRNAs
- A Genetic Cascade of Modulates Nucleolar Size and rRNA Pool in
- Inter-population Differences in Retrogene Loss and Expression in Humans
- Cationic Peptides Facilitate Iron-induced Mutagenesis in Bacteria
- EP4 Receptor–Associated Protein in Macrophages Ameliorates Colitis and Colitis-Associated Tumorigenesis
- Fungal Infection Induces Sex-Specific Transcriptional Changes and Alters Sexual Dimorphism in the Dioecious Plant
- FLCN and AMPK Confer Resistance to Hyperosmotic Stress via Remodeling of Glycogen Stores
- MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
- Sex Bias and Maternal Contribution to Gene Expression Divergence in Blastoderm Embryos
- Transcriptional and Linkage Analyses Identify Loci that Mediate the Differential Macrophage Response to Inflammatory Stimuli and Infection
- Mre11 and Blm-Dependent Formation of ALT-Like Telomeres in Ku-Deficient
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- Identification of a Single Strand Origin of Replication in the Integrative and Conjugative Element ICE of
- The Type VI Secretion TssEFGK-VgrG Phage-Like Baseplate Is Recruited to the TssJLM Membrane Complex via Multiple Contacts and Serves As Assembly Platform for Tail Tube/Sheath Polymerization
- The Dynamic Genome and Transcriptome of the Human Fungal Pathogen and Close Relative
- Secondary Structure across the Bacterial Transcriptome Reveals Versatile Roles in mRNA Regulation and Function
- ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Regeneration
- Pelle Modulates dFoxO-Mediated Cell Death in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



