-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation
Gene-gene interaction is assumed to be critical to the pathogenesis of human disease, but its contribution to human disease phenotype needs definitive documentation. Moreover, the underlying molecular mechanism for gene-gene interaction is unknown. Here we use atrial fibrillation (AF) as a model to demonstrate that gene-gene interaction plays an important role in disease pathogenesis. Only three of the ten AF loci identified by GWAS in European ancestry populations, including PITX2c, ZFHX3, and CAV1, were replicated in the Chinese population and thus selected for gene-gene interaction studies. We show that the PITX2c locus interacts with the ZHFX3 locus to increase the risk of AF. Because gene-gene interaction can generate synergistic effect that markedly increases risk of AF, we conclude that gene-gene interaction accounts for a significant portion of heritability of AF. Mechanistically, PITX2c positively regulates ZHFX3 via miR-1 and ZHFX3 positively regulates PITX2c, which generates a loop of cross-regulation of the two genes. Our study suggests that cyclic cross-regulation of gene expression is a molecular basis for gene-gene interaction involved in disease phenotype.
Published in the journal: Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation. PLoS Genet 11(8): e32767. doi:10.1371/journal.pgen.1005393
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005393Summary
Gene-gene interaction is assumed to be critical to the pathogenesis of human disease, but its contribution to human disease phenotype needs definitive documentation. Moreover, the underlying molecular mechanism for gene-gene interaction is unknown. Here we use atrial fibrillation (AF) as a model to demonstrate that gene-gene interaction plays an important role in disease pathogenesis. Only three of the ten AF loci identified by GWAS in European ancestry populations, including PITX2c, ZFHX3, and CAV1, were replicated in the Chinese population and thus selected for gene-gene interaction studies. We show that the PITX2c locus interacts with the ZHFX3 locus to increase the risk of AF. Because gene-gene interaction can generate synergistic effect that markedly increases risk of AF, we conclude that gene-gene interaction accounts for a significant portion of heritability of AF. Mechanistically, PITX2c positively regulates ZHFX3 via miR-1 and ZHFX3 positively regulates PITX2c, which generates a loop of cross-regulation of the two genes. Our study suggests that cyclic cross-regulation of gene expression is a molecular basis for gene-gene interaction involved in disease phenotype.
Introduction
Genome-wide association studies (GWAS) have been highly successful in identifying common genomic variants that are associated with complex human diseases or traits. However, these common variants have small effects, and in aggregate explain only a small fraction of heritability for most diseases or traits. The major portion of heritability remains missing, and this represents a major dilemma in complex trait genetics referred to as “missing heritability”. Gene-gene interaction has been proposed to be a contributor to the problem of missing heritability.
Gene-gene interaction has been long known to have an impact on an organism’s phenotype, for example, the color of a flower in plants and the color of a fly’s eye. However, it has been challenging to detect gene-gene interaction in human GWAS. Moreover, no gene interaction was functionally validated. Considering the potentially large number of gene-gene interaction, identification of true and casual interaction has been proven to be a daunting task. However, without doubt, studies of gene-gene interaction will contribute to the understanding of inheritance, particularly inheritance of important diseases and traits, and provide insights into the biological pathways and molecular mechanisms of disease pathogenesis.
Atrial fibrillation (AF) is the most common cardiac arrhythmia seen at the clinical setting and accounts for approximately one-third of hospitalizations for cardiac rhythm disturbances [1]. The prevalence of AF is 0.4%-1.0% in the general population, and increases with age, reaching 8% in people over 80 [1]. A similar prevalence rate of 0.77% was found for AF in the Chinese population [2]. AF accounts for 15% of all strokes, worsens heart failure, and independently increases the risk of stroke 5-fold and risk of cardiac death up to 1.9-fold [3]. Genetic factors play an important role in the pathogenesis of AF. The heritability of polygenic liability to AF has been estimated to be 0.62 [4].
To date, several major GWAS have been reported for common complex AF and identified variants in ten chromosomal loci that were associated with AF. The first GWAS for AF identified significant association between SNP rs2200733 near the PITX2c gene encoding paired-like homeodomain 2 transcript c on chromosome 4q25 and AF in several populations of European ancestry as well as one Hong Kong population [5]. Our group later reported that SNP rs2200733 confers a significant risk in the mainland Chinese Han population, too [6]. Then, two independent GWAS identified significant association between AF and SNPs rs2106261 [7] and rs7193343 [8], both of which are located in the ZFHX3 gene encoding zinc finger homeobox 3 on chromosome 16q22. We have found that rs2106261, but not rs7193343, showed significant association with AF in the Chinese Han population [9]. Later, a common variant in KCNN3 (encoding potassium intermediate/small conductance calcium-activated channel, subfamily N, member 3), rs13376333, was found to be associated with lone AF [10]. However, we found that rs13376333 did not show significant association with AF in the Chinese Han population [9]. Ellinor et al [11] identified six susceptibility loci for AF through meta-GWAS analysis. We have shown that only one SNP, rs3807989 at the CAV1 locus (encoding caveolin 1) among the six loci, were associated with AF in the Chinese Han population [12]. In this study, we studied the gene-gene interaction for three AF loci replicated in the Chinese population, i.e. SNP rs2106261 in ZFHX3, rs2200733 near PITX2c, and rs3807989 in CAV1. We provide strong genetic evidence that SNP rs2200733 near PITX2c and rs2106261 in ZFHX3 interact with each other, resulting in a synergistic effect that increases the odds ratios (ORs) to risk of AF. Most importantly, we also carried out a series of cellular and molecular studies to identify the molecular mechanisms underlying the gene–gene interaction. We found that PITX2c and ZFHX3 cross-regulate each other’s expression as well as expression of downstream genes such as NPPA (encoding atrial natriuretic factor or ANF), providing a novel molecular basis for their interaction at the molecular genetic level.
Results
Significant association between SNP rs2200733 near PITX2c on 4q25 and rs2106261 in ZFHX3 on 16q22 and AF in three independent populations
We previously reported that among the first three genetic loci for AF identified by GWAS in European ancestry populations, only rs2200733 at the PITX2c locus on 4q25 and rs2106261 in ZFHX3 on 16q22, but not rs13376333 in KCNN3, were replicated in the Chinese Han populations [6,9]. We, therefore, carried out a deeper study to determine whether there is gene-gene interaction between rs2200733 and rs2106261. We utilized a case control design which involves three independent populations. The initial association study was carried out with 569 AF patients and 1,996 non-AF control samples (referred to as the Discovery population). The positive findings in the Discovery population were validated in two independent replication populations. The first replication population consisted of 641 AF cases and 1,692 controls (referred to as Replication I population). The second replication population consisted of 810 cases and 1,627 controls (referred to as Replication II population). The clinical characteristics of the three study populations are shown in S1 Table.
We first examined the association of AF with each GWAS SNP individually. There was no deviation from the Hardy-Weinberg equilibrium for the two SNPs, rs2200733 and rs2106261 in the control groups of the three populations (S2 Table).
As shown in S3 Table, SNP rs2200733 showed highly significant association with AF in the Discovery population with a P value of 1.58×10-14 (OR = 1.70 (95% CI 1.48–1.94)) with the T allele as the risk allele. After adjusting for covariates of age and gender with multivariable logistical regression analysis, rs2200733 remained significantly associated with AF (Padj = 5.50×10-13, OR = 1.32 (95% CI 1.22–1.42)). SNP rs2200733 remained significant association with AF in Replication I population (Pobs = 1.27×10-11, OR = 1.57 (95% CI 1.38–1.79); Padj = 3.17×10-10, OR = 1.27 (95% CI 1.18–1.37)) and Replication II population (Pobs = 2.20×10-10, OR = 1.48 (95% CI 1.31–1.67); Padj = 7.84×10-10, OR = 1.22 (95% CI 1.14–1.29)). In the combined population, the association between SNP rs2200733 and AF was highly significant (Pobs = 2.83×10-33, OR = 1.57 (95% CI 1.46–1.69); Padj = 4.54×10-29, OR = 1.26 (95% CI 1.21–1.31)). In addition to analysis of allelic association, we also analyzed genotypic association assuming three different genetic models. As shown in S4 Table, highly significant genotypic associations were detected between SNP rs2200733 and AF in the Discovery population, Replication I population and Replication II population in an additive, dominant, or recessive model. In the combined cohort of the three populations, the genotypic associations between SNP rs2200733 and AF were also highly significant with Padj of 4.54×10-29 (OR = 1.61 (95% CI 1.48–1.75)), 1.82×10-23 (OR = 1.36 (95% CI 1.28–1.45)) and 4.69×10-16 (OR = 1.37 (95% CI 1.27–1.48)) under an additive, recessive and dominant model, respectively (S4 Table).
Similarly, SNP rs2106261 on 16q22 also showed significant allelic and genotypic association with AF in the Discovery population, Replication I population and Replication II population (S3 and S4 Tables, respectively). In the combined population, the allelic association between SNP rs2106261 and AF was highly significant (Pobs = 6.26×10-12, OR = 1.30 (95% CI 1.21–1.40); Padj = 3.03×10-12, OR = 1.16 (95% CI 1.11–1.21)) (S3 Table) with the A allele as the risk allele. Genotypic associations were also identified between rs2106261 and AF (Padj of 3.11×10-12 (OR = 1.33 (95% CI 1.23–1.45)), 1.02×10-12 (OR = 1.35 (95% CI 1.24–1.46)) and 1.42×10-6 (OR = 1.15 (95% CI 1.08–1.21)) under an additive, recessive and dominant model, respectively (S4 Table).
Gene-gene interaction between ZFHX3 variant rs2106261 and PITX2c variant rs2200733
To study the interaction between rs2106261 (G to A substitution, risk allele = A) and rs2200733 (C to T substitution, risk allele = T), we first defined the frequencies of nine possible two-locus genotypes (32 genotypes: GGCC, GGCT, GGTT, AGCC, AGCT, AGTT, AACC, AACT, AATT) in cases and controls of the three independent study populations. Then, we used the wild type non-risk GGCC genotype (non-risk homozygote for both loci) as baseline or reference, and estimated the OR for each of the eight other genotypes. As shown in Table 1 and Fig 1 and S1 Fig, compared with the GGCC non-risk reference genotype, the double risk homozygous genotype AATT showed a dramatically increased risk for AF with the highest ORs of 4.81 (95% CI 2.88–8.04) (Pobs = 3.83×10-10) and 6.64 (95% CI 3.64–12.11) (Padj = 6.38×10-10) before and after adjustment for covariates of age and gender, respectively, in the Discovery population. This interesting finding was replicated in two independent AF populations with ORs of 4.04 (95% CI 2.23–7.32) (Padj = 4.34×10-6, Replication I) and 5.70 (95% CI 3.34–9.71) (Padj = 1.58×10-10, Replication II). In the combined cohort of the three populations, AATT increased risk of AF with an OR of 5.36 (95% CI 3.87–7.43) (Padj = 8.00×10-24) (Table 1).
Tab. 1. ORs for 8 two-locus genotypes versus non-risk homozygous genotype GGCC as a reference for SNPs rs2200733 and rs2106261 in the Chinese Han populations. 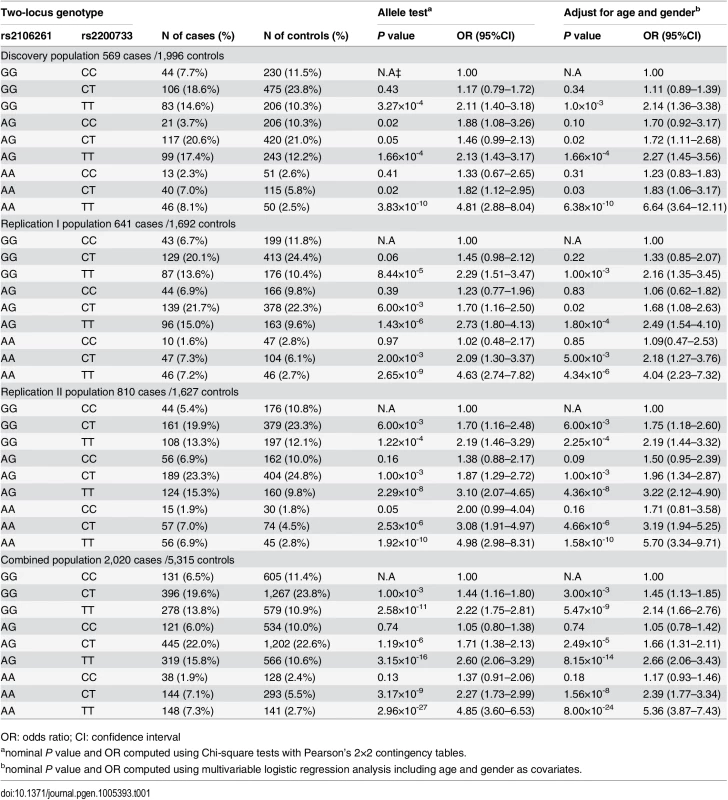
OR: odds ratio; CI: confidence interval Fig. 1. Odds ratios (ORs) for each two-locus genotype for GWAS SNPs rs2106261 in the ZFHX3 gene and rs2200733 close to the PITX2c gene involved in the pathogenesis of AF after adjustment for covariates. 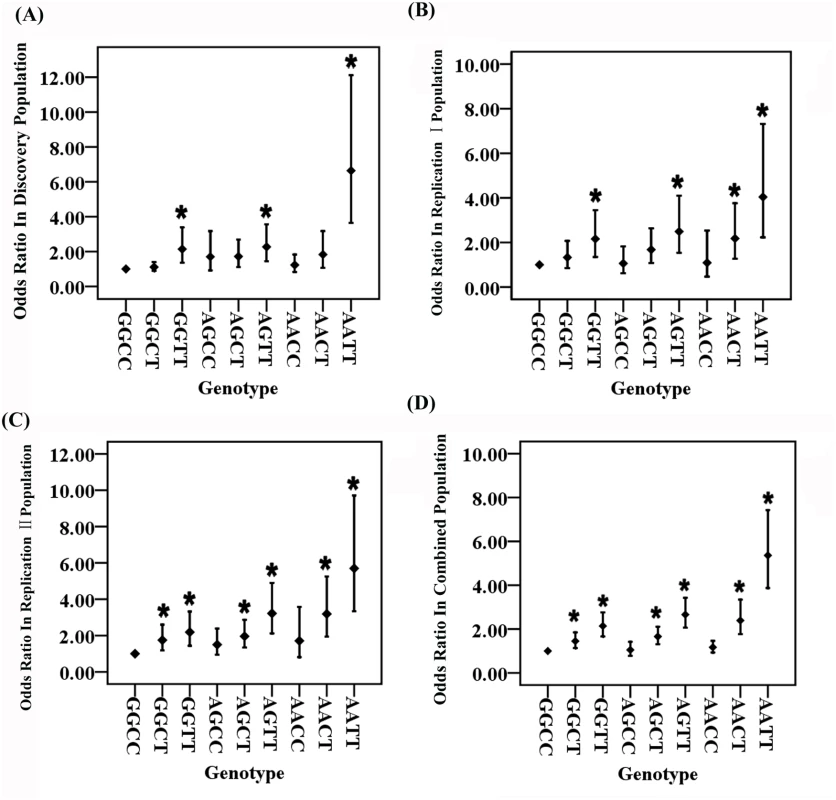
For two SNPs, there are a total of 9 genotypes. The wild type or non-risk GGCC genotype was used as the reference and ORs for other genotypes were estimated against the reference genotype using multivariable logistic regression analysis by including the age and gender as covariates. A. Analysis of ORs in the Discovery population. B. Analysis of ORs in the Replication I population. C. Analysis of ORs in the Replication II population. D. Analysis of ORs in the combined population with the Discovery, Replication I and Replication II cohorts. *P<0.01. The ORs among the different genotypes were compared for statistical significance using the Breslow-Day test (S5 Table). In all three independent populations as well as the combined population, ORs for genotype AATT (double risk homozygotes for both rs2106261 and rs2200733) were significantly higher than the ORs for each single-risk homozygotes (GGTT, homozygous risk genotype for rs2106261; AACC, homozygous risk genotype for rs2200733) (Table 1 and S5 Table). Moreover, the OR of 6.64 for double-risk homozygote AATT was higher than the combined ORs for the two single-risk homozygotes GGTT and AACC together (2.14+1.25 = 3.39) in the Discovery population (Table 1, Fig 1 and S1 Fig). Similar findings were observed in the Replication I population (4.34 vs. 3.25 (2.16+1.09)), the replication II population (5.70 vs. 3.90 (2.19+1.71)), or the combined cohort (5.36 vs. 3.31 (2.14+1.17)) (Table 1, Fig 1 and S1 Fig). These data provide genetic evidence for interaction between ZFHX3 variant rs2106261 and PITX2c variant rs2200733, which generates a synergistic effect that markedly increases the risk of AF.
Two other genotypes, GGTT and AGTT, significantly increased risk of AF compared to reference non-risk genotype GGCC, consistently in all three populations (Padj<0.006 after Bonferroni correction) (Table 1, Fig 1 and S1 Fig).
Molecular basis of gene-gene interaction: PITX2c positively regulates the expression of ZFHX3 via miR-1
To substantiate the novel finding of the genetic interaction between rs2106261 and rs2200733 as identified by the analyses above, we carried out functional studies to identify the underlying molecular mechanism of the interaction. The PITX2c gene near rs2200733 has been demonstrated to be an AF gene using mouse models and shown to regulate several genes in the atria [13–15]. Because PITX2c encodes a transcriptional factor, we hypothesized that PITX2c would regulate the expression of ZFHX3, generating a synergistic effect for gene-gene interaction. To test this hypothesis, we transfected HCT116 cells with a PITX2c-specific siRNA and a negative control siRNA (NC control) and then used real-time RT-PCR analysis to measure the expression level of ZFHX3. As shown in Fig 2, knockdown of PITX2c expression by siRNA significantly decreased the expression level of ZFHX3 (P = 4.00×10-3) (Fig 2A and 2C). In a parallel study, overexpression of a FLAG-tagged PITX2c protein by transfection of a p3×FLAG-PITX2c expression plasmid significantly increased the expression level of ZFHX3 (P = 0.01) (Fig 2B and 2C). These studies indicate that PITX2c positively regulates expression of ZFHX3.
Fig. 2. The PITX2c gene positively regulates expression of the ZFHX3 gene. HCT116 cells were transfected with siRNA specific for PITX2c or an expression plasmid for PITX2c and used for isolation of total RNA samples and real time RT-PCR analysis. 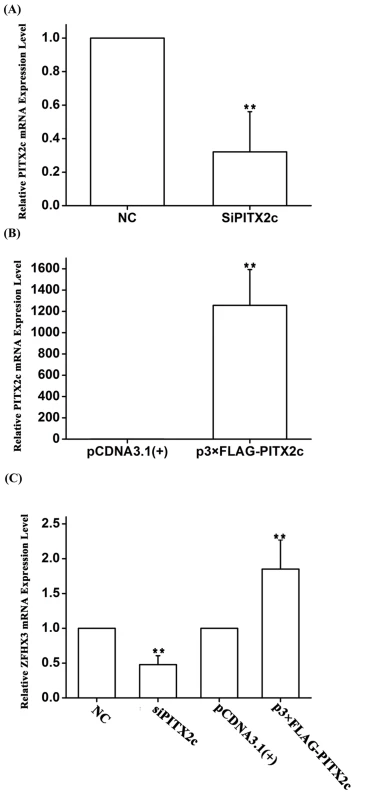
A. Real-time RT-PCR analysis for PITX2c. Transfection of siRNA for PITX2c successfully reduced expression of PITX2c. B. Real-time RT-PCR analysis for PITX2c. Transfection of an expression plasmid for PITX2c successfully increased expression of PITX2c. C. Real-time RT-PCR analysis for ZFHX3. Transfection of siRNA for PITX2c reduced expression of ZFHX3. Transfection of an expression plasmid for PITX2c successfully increased expression of ZFHX3. **P<0.01. To explore the molecular mechanism by which PITX2c regulates ZFHX3, we searched for a potential PITX2c binding site at the ZFHX3 promoter and regulatory region, but failed to find one. Because PITX2c was shown to negatively regulate the expression of miR-1 (microRNA 1–1) [15], we hypothesize that PITX2c may regulate expression of ZFHX3 through miR-1. To test this hypothesis, we transfected HCT116 cells with miR-1 mimics and control microRNA mimics and measured the expression level of ZFHX3. Both real-time RT-PCR analysis and Western blot analysis showed that miR-1 mimics significantly decreased expression of ZFHX3 at both mRNA (P = 4.00×10-4) and protein levels (P = 6.84×10-5), although the effect on the protein level was more robust (Fig 3A–3C). This interesting finding of down-regulation of ZFHX3 by miR-1 was confirmed in another cell line, SW620 at the ZFHX3 mRNA (P = 0.01) and protein levels (P = 4.89×10-4) (Fig 3D–3F). These results suggest that miR-1 negatively regulates expression of ZFHX3.
Fig. 3. Expression of ZFHX3 is negatively regulated by miR-1. 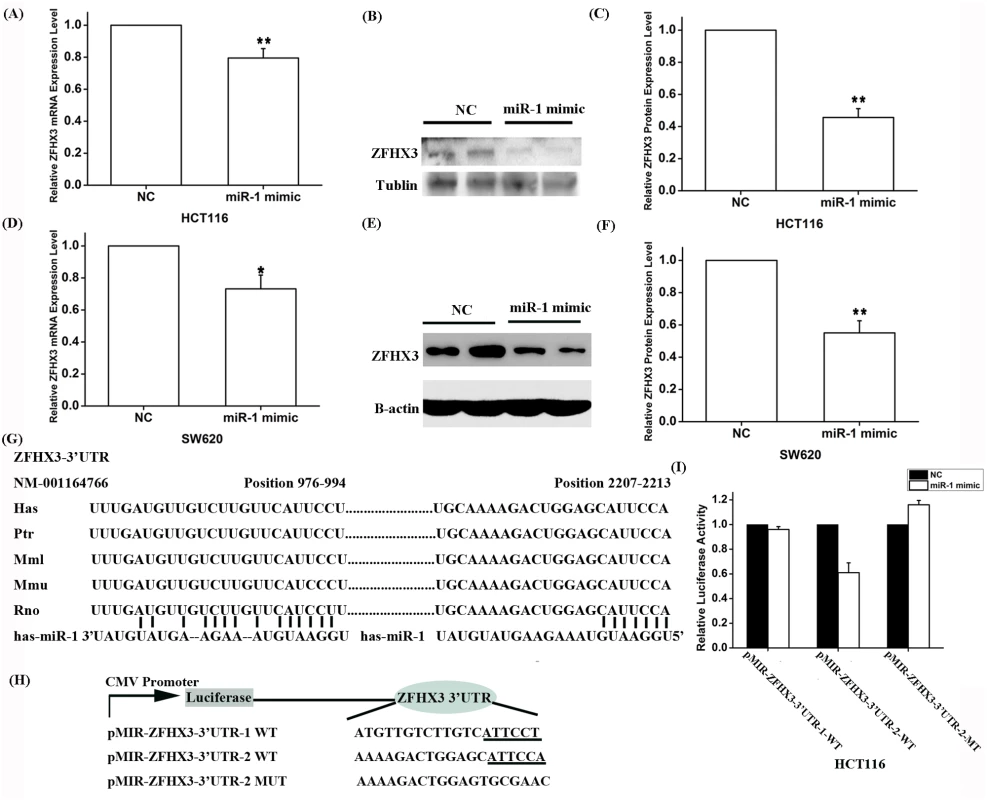
HCT116 (A-C) and SW620 (D-F) cells were transfected with miR-1 mimics and negative control mimics (NC) and used for isolation of RNA samples for real-time RT-PCR analysis or for isolation of protein extracts for Western blot analysis for the expression levels of ZFHX3 mRNA and protein. A. Real-time RT-PCR analysis revealed that the miR-1 mimics reduced the expression of ZFHX3 by 20% in HCT116 cells (P = 0.004). B, C. Western blot analysis revealed that the miR-1 mimics reduced the expression of the ZFHX3 protein by 54% in HCT116 cells (P = 6.84×10-5). D. Real-time RT-PCR analysis revealed that the miR-1 mimics reduced the expression of ZFHX3 by 27% in SW620 cells (P = 0.01). E, F. Western blot analysis revealed that the miR-1 mimics reduced the expression of the ZFHX3 protein by 45% in SW620 cells (P = 4.887×10-4). G. Identification of two putative miR-1 binding sites at the 3’-UTR of ZFHX3 by bioinformatic analysis and alignment of miR-1 binding sequences across species. H. A schematic diagram shows luciferase reporters containing the potential miR-1 binding site or the related mutated site. I. MiR-1 targets the second miR-1 binding site to regulate expression of ZFHX3. Luciferase assays revealed that compared to negative control mimics, miR-1 mimics significantly reduced luciferase activities from pMIR-ZFHX3–3’-UTR-2, but not from pMIR-ZFHX3-3’-UTR-1. *P<0.05; **P<0.01. To explore the molecular mechanism by which miR-1 regulates ZFHX3, we performed bioinformatic analysis by searching two databases, DIANA TOOLs and microRNA.org-Target and Expression, and found that the 3’-untranslated region (3’-UTR) of ZFHX3 contained two potential targeting sites for miR-1 (Fig 3G). We cloned each region containing a miR-1 binding site downstream of the firefly luciferase coding region in the pMIR-REPORT luciferase vector, resulting in luciferase reporters pMIR-ZFHX3-3’-UTR-1 (cloned genomic region: chr16 : 72819500 to 72820662) and pMIR-ZFHX3–3’-UTR-2 (cloned genomic region: chr16 : 72818241 to 72819390), respectively (Fig 3H). Each reporter was co-transfected with miR-1 mimics (100 nM) into HCT116 cells and luciferase assays were carried out. A schematic diagram shows luciferase reporters containing the potential miR-1 binding site or the related mutated site (Fig 3H). As shown in Fig 3I, miR-1 mimics significantly reduced luciferase activities from pMIR-ZFHX3–3’-UTR-2, but not that from pMIR-ZFHX3–3’-UTR-1. Mutation of the miR-1 binding site in pMIR-ZFHX3–3’-UTR-2 from CATTCCA to TGCGAAC abolished the miR-1-mediated reduction of the reporter luciferase activity (Fig 3I). These data suggest that miR-1 negatively regulates expression of ZFHX3 by targeting to the second binding site at the 3’-UTR of ZFHX3.
Molecular basis of gene-gene interaction: ZFHX3 positively regulates the expression of PITX2c
The AF SNP rs2106261 identified by GWAS is located within the ZFHX3 gene, therefore, we consider ZFHX3 as a strong candidate gene for AF at the chromosome 16q22 locus. As our genetic studies indicate a gene-gene interaction between PITX2c and ZFHX3, we hypothesized that ZFHX3 may regulate expression of PITX2c. Interestingly, knockdown of ZFHX3 expression by a specific siRNA significantly decreased expression of PITX2c about 2-fold (P = 5.00×10-3) (Fig 4A and 4C). Conversely, overexpression of ZFHX3 significantly increased expression of PITX2c by 2.96-fold (P = 2.00×10-3) (Fig 4B and 4D). Knockdown of ZFHX3 expression by siRNA reduced the transactivation activity from a reporter with a 1.5 kb DNA fragment upstream of the PITX2c transcriptional start site fused to the luciferase gene (PITX2c-PGL3) by 1.97-fold (P = 5.00×10-3) (Fig 4E).
Fig. 4. The ZFHX3 gene positively regulates expression of the PITX2c gene. 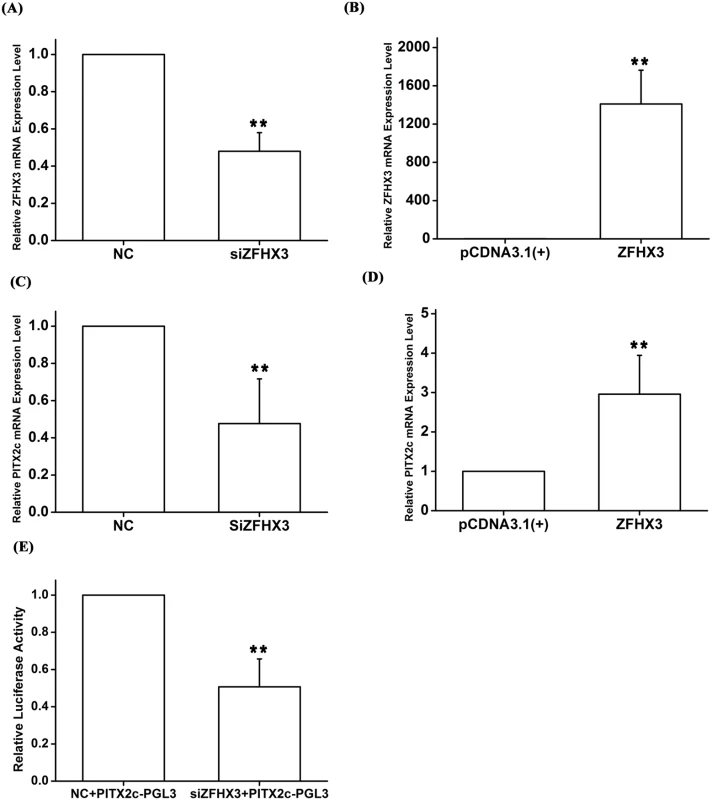
HCT116 cells were transfected with siRNA specific for ZFHX3 or an expression plasmid for ZFHX3 and used for isolation of total RNA samples, real time RT-PCR analysis and Luciferase assays. A. Real-time RT-PCR analysis for ZFHX3. Transfection of siRNA for ZFHX3 successfully reduced expression of ZFHX3. B. Real-time RT-PCR analysis for ZFHX3. Transfection of an expression plasmid for ZFHX3 successfully increased expression of ZFHX3. C. Real-time RT-PCR analysis for PITX2c. Transfection of siRNA for ZFHX3 reduced expression of PITX2c. D. Real-time RT-PCR analysis for PITX2c. Transfection of an expression plasmid for ZFHX3 successfully increased expression of PITX2c. E. Luciferase assays for the PITX2c promoter activity in cells transfected with a siRNA specific for ZFHX3 or a control scramble siRNA. *P<0.05; **P<0.01. Molecular basis of gene-gene interaction: Both PITX2c and ZFHX3 positively regulate the expression of NPPA
Several earlier studies showed that PITX2c regulates the expression of the NPPA gene encoding ANF (a cardiac protein hormone), but conflicting results on either positive regulation or negative regulation were obtained in different studies [13,15,16]. We tested the regulation of NPPA by PITX2c in HCT116 cells. As showed in Fig 5A, knockdown of PITX2c expression using siRNA significantly reduced expression of NPPA by 60% (P = 3.20×10-4). Overexpression of PITX2c by transfection of HCT116 cells with p3×FLAG-PITX2c significantly increased NPPA expression by 2.42 fold (P = 0.01) (Fig 5B).
Fig. 5. Both PITX2c and ZFHX3 positively regulate expression of the NPPA transcription. 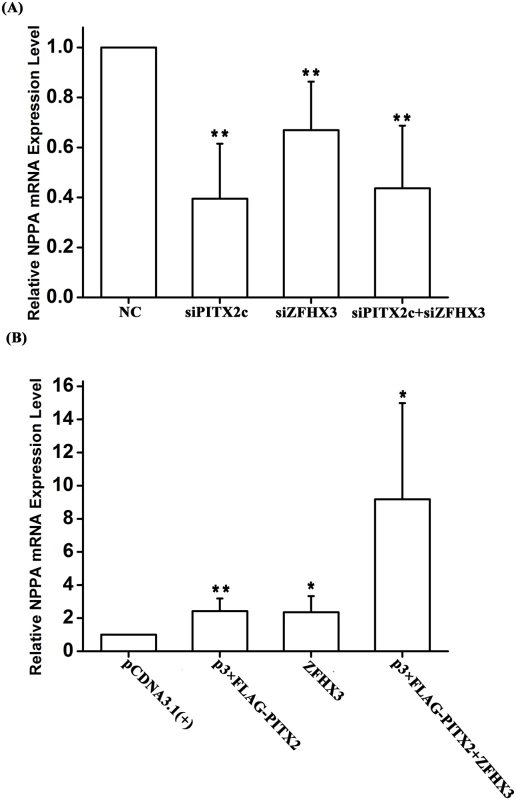
HCT116 cells were co-transfected with an expression plasmid for either PITX2c, ZFHX3 or both, or siRNA for either PITX2c, ZFHX3 or both and used for measurements of RT-PCR. A. Knockdown of PITX2c, ZFHX3 or both by siRNAs down-regulated NPPA expression. B. Overexpression of either PITX2c or ZFHX3 up-regulated NPPA expression. Co-expression of both PITX2c and ZFHX3 dramatically increased NPPA expression. *P<0.05; **P<0.01. Interestingly, we found that ZFHX3 also regulated NPPA expression. As shown in Fig 5A, knockdown of ZFHX3 expression by siRNA significantly decreased expression of NPPA (P = 4.00×10-3). Overexpression of ZFHX3 up-regulated NPPA expression 2.36 fold (P = 0.03) (Fig 5B).
Overexpression of both PITX2c and ZFHX3 dramatically increased expression of NPPA (P = 0.031) (Fig 5B). Knockdown of both PITX2c and ZFHX3 also reduced expression of NPPA (P = 1.00×10-3) (Fig 5A).
It was reported that PITX2c could also regulate expression of other downstream genes including NKX2.5 (encoding NK2 transcription factor related, locus 5), TBX5 (encoding T-box 5), KCNQ1 (encoding potassium voltage-gated channel, KQT-like subfamily, member 1), and SCN1B (encoding sodium channel, voltage-gated, type I, beta subunit) [13,15,17,18]. As shown in Fig 6, knockdown of the PITX2c expression by siRNA significantly increased expression of NKX2.5 by 3.10-fold, TBX5 by 2.32-fold, KCNQ1 by 1.55-fold, and SCN1B by 1.27-fold. Interestingly, knockdown of the ZFHX3 gene by siRNA also significantly increased expression of NKX2.5 by 3.45-fold and TBX5 by 3.23-fold, but decreased expression of SCN1B by 1.52-fold and did not affect expression of KCNQ1 (Fig 6). Co-transfection of both PITX2c siRNA and ZFHX3 siRNA also significantly reduced NKX2.5 by 2.91-fold, TBX5 by 2.42-fold, but did not affect expression of KCNQ1 or SCN1B (Fig 6).
Fig. 6. PITX2c and ZFHX3 regulate expression of NKX2.5, TBX5, KCNQ1 and SCN1B. 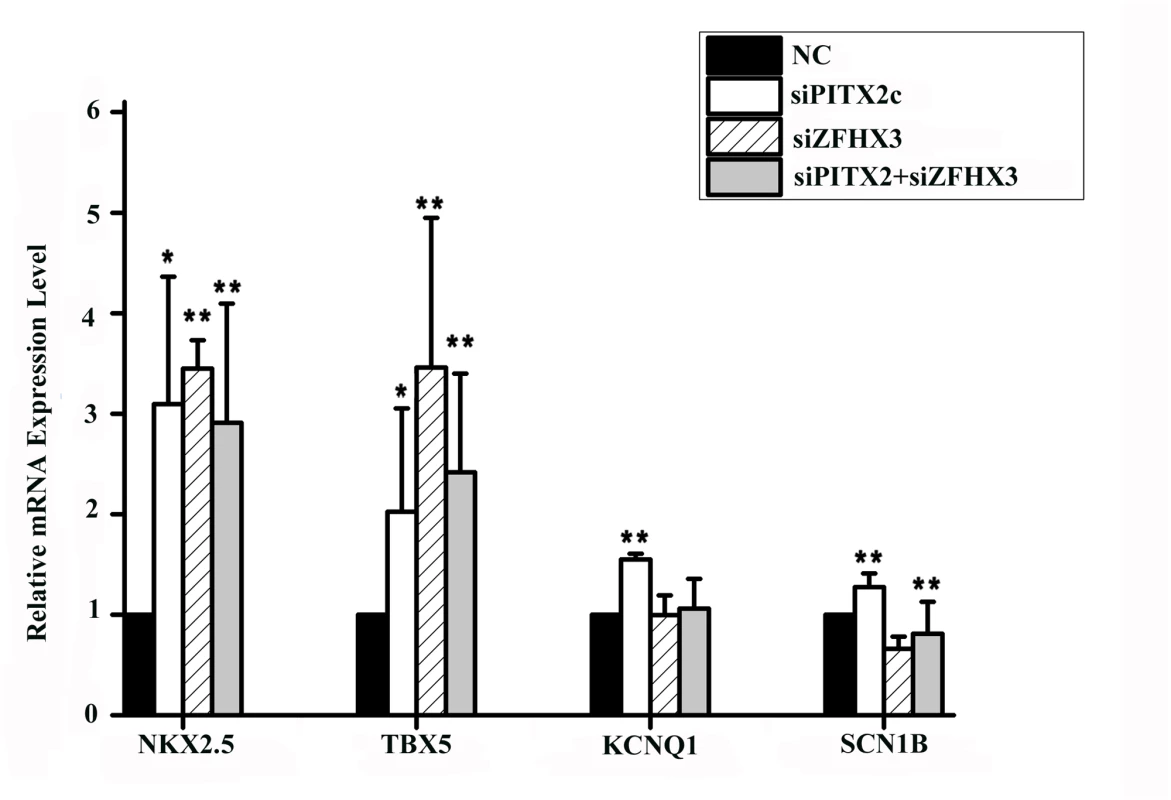
HCT116 cells were transfected with siRNA specific for PITX2c or ZFHX3 and used for isolation of total RNA samples and real-time RT-PCR analysis. Transfection of siRNA for PITX2c increased expression of NKX2.5, TBX5, KCNQ1 and SCN1B. Transfection of siRNA for ZFHX3 increased expression of NKX2.5 and TBX5,but decreased expression of SCN1B. ZFHX3 did not affect on the expression of KCNQ1. Transfection of siRNAs for both PITX2c and ZFHX3 increased expression of NKX2.5 and TBX5. No significant gene-gene interaction between ZFHX3 variant rs2106261 and CAV1 variant rs3807989 or between PITX2c variant rs2200733 CAV1 variant rs3807989
GWAS in European ancestry populations have identified ten genetic loci for AF [5,7,8,10,11]. We analyzed these loci in the Chinese Han population for their association with AF. We found that in addition to the ZFHX3 locus and the PITX2c locus reported previously [6,9], one other locus, rs3807989 in CAV1 encoding caveolin-1, also showed significant association with AF, whereas no significant association was identified for other loci [12]. Therefore, we also analyzed gene-gene interactions between rs2106261 and rs3807989 and between rs2200733 and rs3807989. The classical gene-gene analysis by comparing ORs for the nine two-locus genotypes did not reveal any significant synergistic effect between rs2106261 and rs3807989 (Table 2). The OR for the double risk homozygotes for both rs2106261 and rs3807989 (AAGG) was 1.25, which is smaller than the product of the ORs (1.18+1.15) for each single-risk homozygotes (AAAA, homozygous risk genotype for rs2106261; GGGG, homozygous risk genotype for rs3807989) (Table 2). These results suggest that there is no interaction between the ZFHX3 locus and the CAV1 locus for AF. Similarly, the OR for the double risk homozygotes for both rs2200733 and rs3807989 (TTGG) was 1.08, which is smaller than the product of the ORs (1.00+0.77) for each single-risk homozygotes (TTAA, homozygous risk genotype for rs2200733; CCGG, homozygous risk genotype for rs3807989) (Table 2). These results suggest that there is no interaction between the PITX2c locus and the CAV1 locus for AF.
Tab. 2. ORs for 8 two-locus genotypes versus non-risk homozygous genotype AAGG as a reference for SNPs rs2106261 and rs3807989, CCAA as a reference for SNPs rs2200733 and rs3807989 in the Chinese Han population. 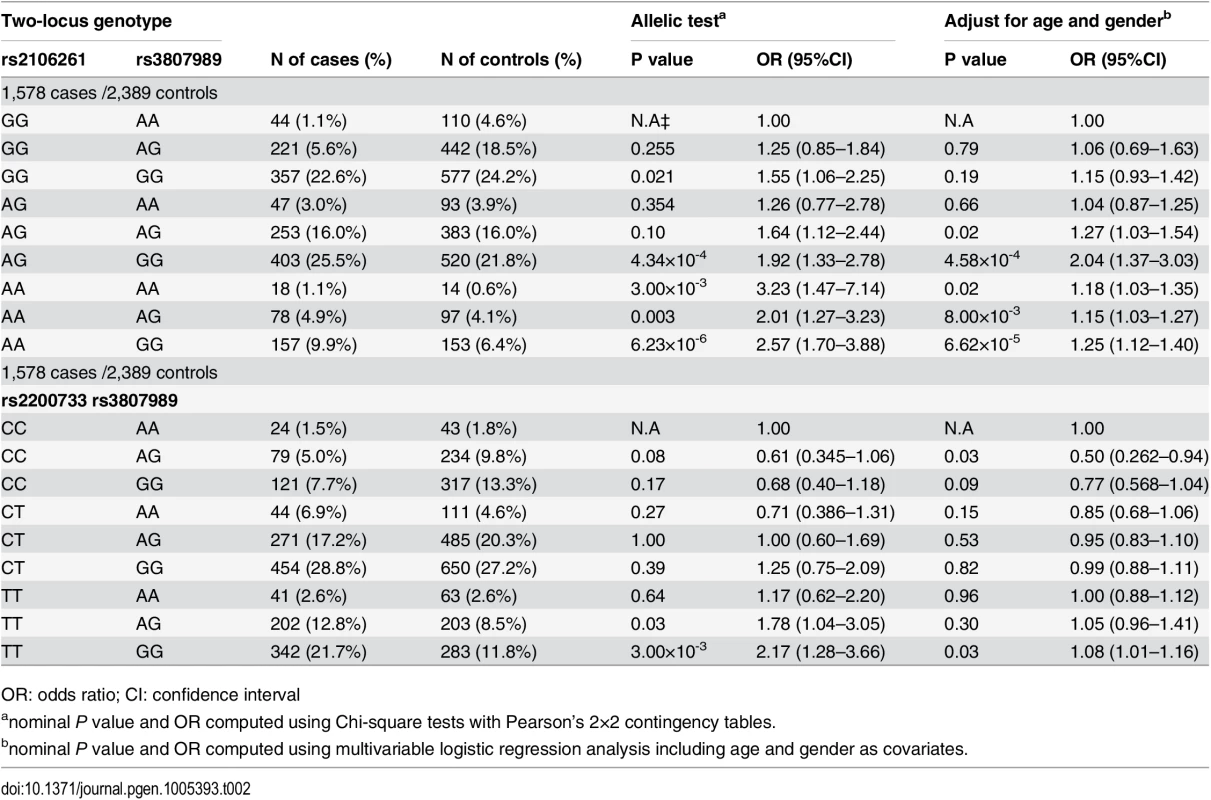
OR: odds ratio; CI: confidence interval Real-time RT-PCR analysis showed that knockdown of either ZFHX3 or PITX2c increased the expression level of CAV1 (Fig 7). Similar results were obtained with Western blot analysis (Fig 7). On the contrary, knockdown of CAV3 did not significantly affect the expression of ZFHX3 or PITX2c (Fig 8). Together, these data suggest that there is no cyclic cross-regulation between ZFHX3 and CAV1 or between PITX2c and CAV1.
Fig. 7. PITX2c and ZFHX3 negatively regulate expression of the CAV1 gene. 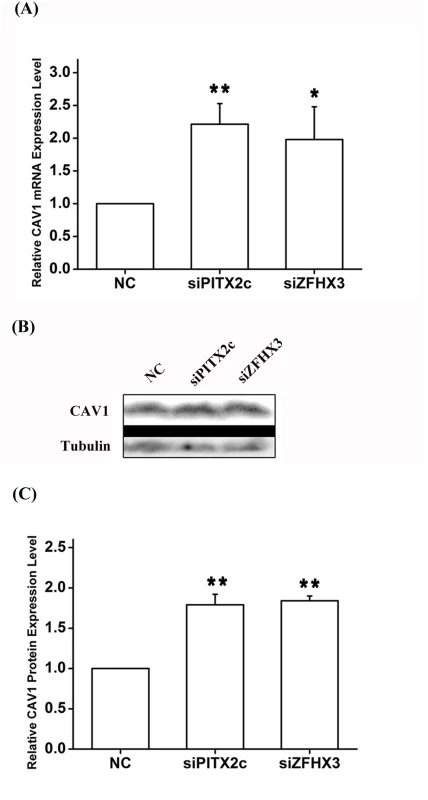
HCT116 cells were transfected with siRNA specific for PITX2c or siRNA specific for ZFHX3 and used for isolation of total RNA samples and real-time RT-PCR analysis. A. Real-time RT-PCR analysis for CAV1. Transfection of siRNA for PITX2c or siRNA for ZFHX3 successfully increased expression of CAV1. B, C. Western blot analysis revealed that PITX2c and ZFHX3 increased the expression of the CAV1 protein by 1.79-fold and 1.84-fold, respectively (P = 2.21×10-5, 2.00×10-7). **P<0.01; *<0.05. Fig. 8. The CAV1 gene does not affect expression of PITX2c or ZFHX3. 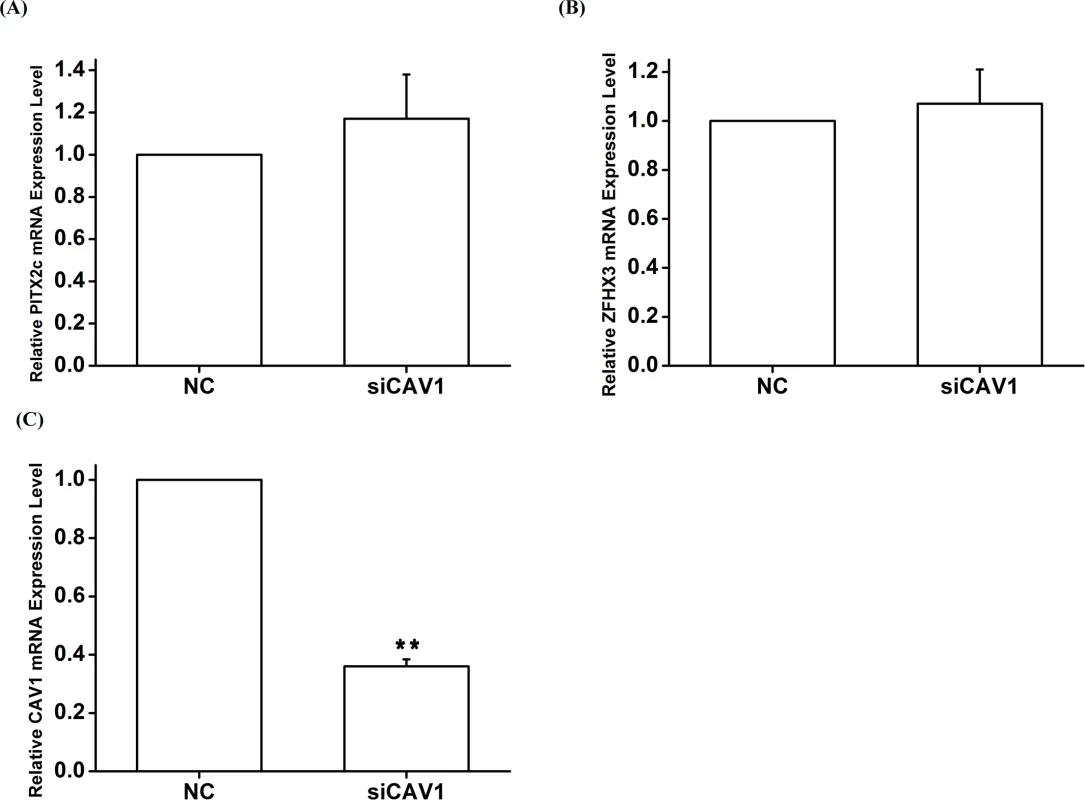
HCT116 cells were transfected with siRNA specific for CAV1 and used for isolation of total RNA samples and real-time RT-PCR analysis. A. Real-time RT-PCR analysis for PITX2. Transfection of siRNA for CAV1 did not significantly affect the expression of PITX2. B. Real-time RT-PCR analysis for ZFHX3. Transfection of siRNA for CAV1 did not significantly affect the expression of ZFHX3. C. Real-time RT-PCR analysis for CAV1. Transfection of siRNA for CAV1 successfully reduced expression of CAV1.**P<0.01; *<0.05. Analysis of gene-gene interactions by alternative gene-gene interaction programs
Many gene-gene programs have been developed in recent years, therefore, we also analyzed interaction among SNPs rs2200733/PITX2c, rs2106261/ZFHX3 and rs3807989/CAV1 using RERI and INTERSNP programs. RERI (relative excess risk due to interaction) analysis was developed to quantify the extent of synergistic effect by adopting a fundamental measure of additive interaction and relative excess risk due to interaction (RERI) [19]. Here we used this strategy to investigate interaction between rs2106261 and rs2200733 in terms of risk alleles A and T in the combined population. The RERI analysis can distinguish the additive effect from the synergistic effect [19]. A significant RERI value higher or lower than 0 is considered to demonstrate a synergistic effect, whereas a non-significant RERI value indicates an additive effect [19]. The results are shown in Table 3. First, we analyzed the synergistic effect when exposed to one copy of risk alleles at any one locus or both loci (H1). No significant synergistic effect was observed between rs2106261 and rs2200733 (RERI = 0.22 (95% CI -0.20–0.54), P = 0.13; RERI = 0.18 (95% CI -0.29–0.52), Padj = 0.22 after adjustment of covariates of age and gender). Second, we assessed the synergistic effect when exposed to two copies of risk alleles at any one locus or both loci (H2). A significant synergistic effect was detected between rs2106261 and rs2200733 with a RERI value of 2.26 (95% CI 1.06–3.73) (P<1.00×10-4; RERI = 2.87 (1.48–4.69), Padj<1.00×10-4 after adjustment of covariates of age and gender). Third, we assessed the synergistic effect when exposed to two copies of risk alleles at one locus and one copy of risk alleles at the other locus (H3). A significant synergistic effect was detected between rs2106261 and rs2200733 with a RERI value of 0.99 (95% CI 0.29–1.79) (P<1.00×10-4; RERI = 1.29 (95% CI 0.44–2.33), Padj<1.00×10-4 after adjustment of covariates of age and gender). These results provided statistical genetic evidence for the interaction between rs2106261 and rs2200733.
Tab. 3. Interaction between SNPs rs2106261 and rs2200733 in the Chinese Han population. 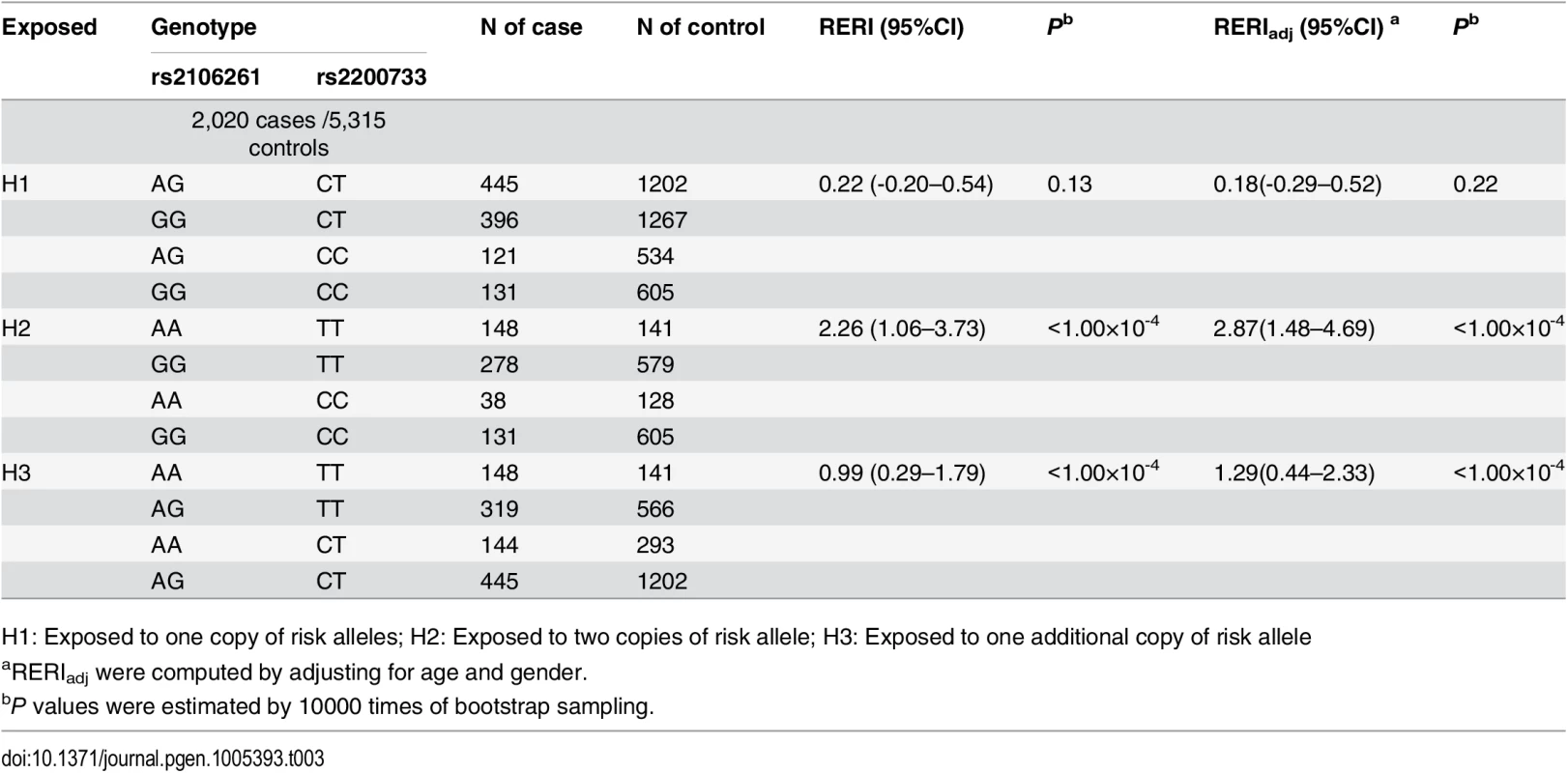
H1: Exposed to one copy of risk alleles; H2: Exposed to two copies of risk allele; H3: Exposed to one additional copy of risk allele The RERI analysis did not identify any significant interaction between ZFHX3 SNP rs2106261 and CAV1 variant rs3807989 (H1: RERI = 0.51 (95% CI -0.54–1.12), Padj = 0.19; H2: RERI = -0.52 (95% CI -5.64–1.41), Padj = 0.37; H3: RERI = 0.29 (95% CI -0.61–1.06), Padj = 0.36) (Table 4). The RERI analysis was also used to analyze the interaction between PITX2c variant rs2200733 and CAV1 variant rs3807989 (H1: RERI = 0.80 (95% CI -0.02–1.27), Padj = 0.11; H2: RERI = 1.37 (95% CI 0.24–2.71), Padj = 0.05; H3: RERI = 0.42 (95% CI -0.33–1.12), Padj = 0.08).
Tab. 4. Interaction analysis of rs2106261 vs. rs3807898 and rs2200733 vs. rs3807898 in the Chinese Han population. 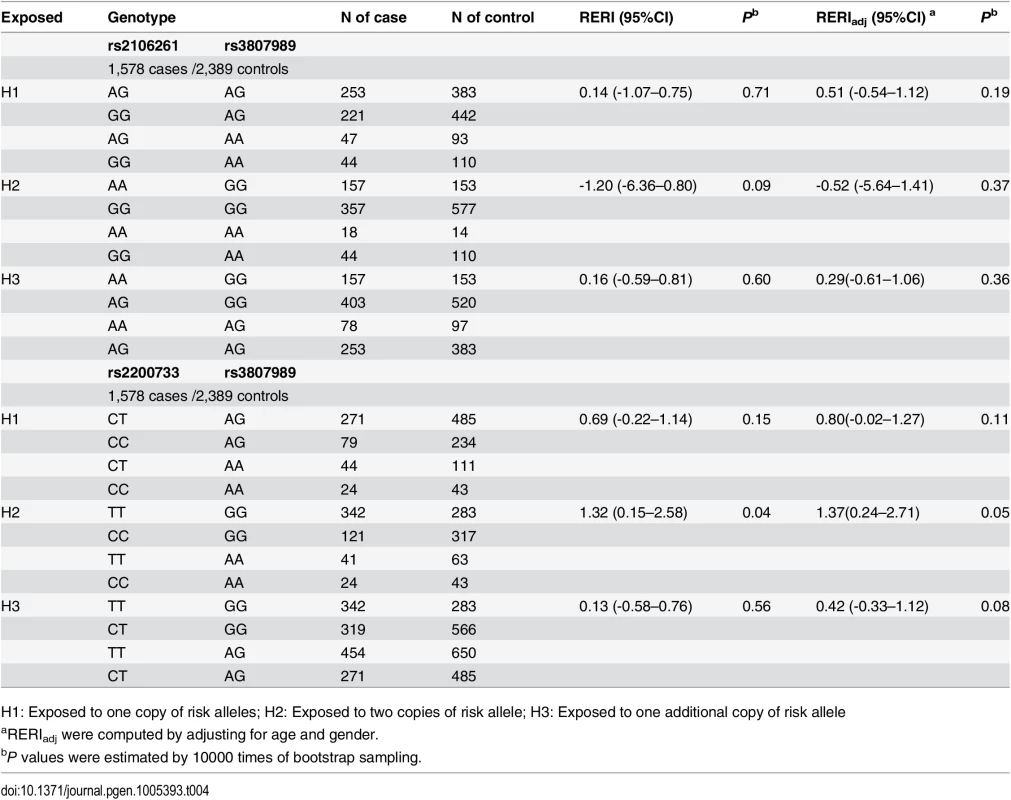
H1: Exposed to one copy of risk alleles; H2: Exposed to two copies of risk allele; H3: Exposed to one additional copy of risk allele We also analyzed gene-gene interaction using the INTERSNP program[20,21], which can analyze genotypic interactions under additive by additive, additive by dominant, dominant by additive and dominant by dominant terms. For rs2106261 and rs2200733, nominal significant interaction was found additive × additive after adjusting for age and gender (OR = 0.85, 95% CI: 0.74–0.97, Padj = 0.02), although the global test on all interaction terms were not significant (Table 5). After simplifying the model by removing dominant effects without significant loss of goodness-of-fit of the model (P = 0.11), the additive interaction on a multiplicative OR scale was also significant (OR = 0.85, 95% CI: 0.76–0.96, Padj = 0.01) (Table 5). A similar pattern was found for additive × additive interaction between rs2200733 and rs3807989 under models with dominant effects (OR = 1.40, 95% CI: 1.16–1.70, Padj = 1.00×10-3) and after removing dominant effects (OR = 1.25, 95% CI: 1.06–1.48, Padj = 7.00×10-3). No significant genotypic interaction was found for rs2106261 and rs3807989 under any model (Table 5).
Tab. 5. Genotypic interaction with logistic regression by INTERSNP developed by Cordell and Clayton [20,21]. ![Genotypic interaction with logistic regression by INTERSNP developed by Cordell and Clayton [<em class="ref">20</em>,<em class="ref">21</em>].](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image/6109b3450f5fd8c3e391e10f5299f840.png)
A: Additive; D: Dominant; OR: odds ratio; 95% CI: 95% confidence interval. Discussion
In this study, we show that gene-gene interaction plays an important role in generation of disease phenotype by identifying gene-gene interaction involved in the pathogenesis of a cardiac disorder, AF. We employed a multi-stage case control association design to compare the frequencies of all nine two-locus genotypes from GWAS SNPs rs2106261 in the ZFHX3 gene and rs2200733 close to the PITX2c gene. Our study involves a careful design with a Discovery population consisting of 569 cases and 1,996 controls, Replication I population with 641 cases and 1,692 controls, and Replication II population composed of 810 cases and 1,627 controls. The combined population has 2,020 cases and 5,315 controls, and is considered to represent a considerably large sample size in the modern population studies for AF. We consider this point as strength of this study. When SNP rs2106261 and rs2200733 were analyzed together, two-locus genotype AATT (double risk homozygote) showed the highest odds ratio (OR) of 6.64 (95% CI 3.64–12.11) (P = 6.38×10-10), 4.04 (95% CI 2.23–7.32) (P = 4.34×10-6), 5.70 (95% CI 3.34–9.71) (P = 1.58×10-10) and 5.36 (95% CI 3.87–7.43) (P = 8.00×10-24) in the Discovery, Replication I, II, and combined population, respectively, when compared to wild type non-risk genotype GGCC. The Breslow-Day test showed that the ORs for AATT were significantly higher than ORs for GGTT or AACC in all populations (P = 5.26×10-5 vs. GGTT and 2.94×10-22 vs. AACC in the combined population) and higher than the combined ORs for both GGTT and AACC (5.36 vs. 3.31 in the combined population). We also analyzed gene-gene interaction using the RERI analysis and identified synergistic effects between SNP rs2106261 and rs2200733 when exposed two copies of risk alleles at any one locus or both loci (H2) (P<1.00×10-4) or when exposed to two copies of risk alleles at one locus and one copy of risk alleles at the other locus (H3) (P<1.00×10-4) (Table 3). Analysis using the INTERSNP program revealed significant genotypic interaction between SNP rs2106261 and rs2200733 under an additive × additive model, but not under other models (Table 5). Overall, our studies establish that gene-gene interaction is involved in the pathogenesis of AF. Most importantly, our results suggest that gene-gene interaction accounts for heritability of human disease because it generates synergistic effects that markedly increase disease risk.
The present study identifies the interaction between two common GWAS loci for AF. Ritchie et al [22] previously found that the risk alleles of common variants rs2200733 and rs10033464 at the 4q25 PITX2c AF locus could predict whether carriers of rare mutations in SCN5A (encoding the cardiac sodium channel), NPPA, KCNA5 (encoding potassium voltage-gated channel, shaker-related subfamily, member 5), and NKX2.5 (encoding transcriptional factor NK2 homeobox 5) developed AF, suggesting potential interaction between common variants and rare mutation in familial AF. Moreover, Lubitz et al [23] studied AF risk signals within nine GWAS loci and found that there are at least four distinct AF susceptibility signals at the 4q25 AF locus upstream of PITX2c that may increase the risk of AF by 5-fold together.
Our cellular and molecular biological studies here on rs2200733/PITX2c and rs2106261/ZFHX3 identify a fundamental molecular mechanism underlying gene-gene interaction. SNP rs2200733 on 4q25 was the first genomic variant for AF identified by GWAS [5] and located 146 kb from the PITX2c gene encoding a paired-like homeodomain transcription factor 2 involved in the asymmetrical development of the heart and other organs [24–26]. Heterozygous knockout PITX2c mice developed atrial arrhythmias (atrial flutter, atrial tachycardia) upon programmed stimulation [13]. Kirchhof et al [14] showed that PITX2c is expressed in human and mouse left atria. Isolated hearts from heterozygous PITX2c knockout mice developed AF upon programmed stimulation and showed shortened action potential duration [14]. Chinchilla et al [15] showed that the expression level of PITX2c was decreased in AF patients and that atria-specific, but not ventricle-specific knockout of PITX2c, resulted in differences in action potential amplitude and increased expression of miR-1. Therefore, all evidence to date strongly suggests that PITX2c should be the causative gene for AF at the 4q25 locus. SNP rs2106261 is located within the ZFHX3 gene. ZFHX3 encodes a transcription factor [27] which contains four homeodomains and seventeen zinc fingers [28]. The ZFHX3 transcription factor appears to regulate myogenic [29] and neuronal differentiation [30]. Although the function of ZFHX3 in cardiac tissue is unknown, it is expressed in mouse hearts [31]. Here we show that PITX2c and ZFHX3 positively cross-regulates each other. PITX2c negatively regulates expression of miR-1, which negatively regulates expression of ZFHX3 by targeting a miR-1-binding site at the 3’-UTR, resulting in a positive regulation of ZFHX3 by PITX2c (Fig 9). Interestingly, ZFHX3 positively regulates expression of PITX2c. The net effect is a cyclic loop of cross-regulation between ZFHX3 and PITX2c (Fig 9). A cyclic loop of cross-regulation of two risk genes for a disease is expected to generate synergistic effects, which further increase disease risk, and therefore provides a novel molecular mechanism for gene-gene interaction. One important future direction is to determine whether this novel mechanism applies to other human disease and to plant and animal phenotypes in general.
Fig. 9. A schematic diagram showing the cyclic cross-regulation of <i>PITX2c</i> and <i>ZFHX3</i>, which underlies the interaction between <i>PITX2c</i> and <i>ZFHX3</i> involved in the pathogenesis of AF. 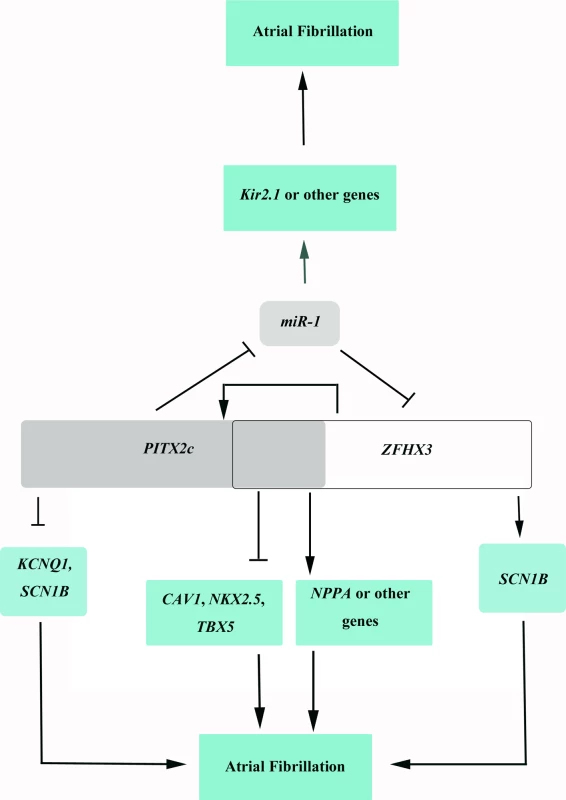
On the molecular level, how does the cyclic loop of cross-regulation between ZFHX3 and PITX2c generate interaction between the two genes and increase risk of AF? The expression level of miR-1 was shown to be reduced in human AF patients, which was correlated with up-regulation of potassium channel Kir2.1 and increased potassium current IK1 responsible for AF maintenance. PITX2c negatively regulates expression of miR-1, which increases IK1, resulting in AF. Decreased miR-1 increases expression of ZFHX3, which increases expression of PITX2c, further decreases expression of miR-1 and increases risk of AF (Fig 9). ZFHX3 increases expression of PITX2c, which decreases expression of miR-1 and increases risk of AF (Fig 9). PITX2c positively regulates expression of ZFHX3, which further increases expression of PITX2c, and leads to down-regulation of miR-1 expression and increased risk of AF (Fig 9). In addition to miR-1, PITX2c and ZFHX3 may regulate NPPA, TBX5, NKX2.5 or other downstream target genes to increase risk of AF (Fig 9). These results provide novel insights into the roles of gene-gene interaction in the pathogenesis of AF.
One other important insight from this study is that not all risk genes for AF interact each other. We have previously shown that genomic variants increase susceptibility of cardiovascular diseases in a population-specific manner. Although some variants increase disease risk in both European ancestry populations and Asian populations, but other variants show significant disease association only in Asian populations [9,11,32]. For AF, we found that among ten GWAS variants for AF identified in European ancestry populations, only three were associated with risk of AF in the Chinese population, including SNPs rs2106261 in the ZFHX3 gene, rs2200733 at the PITX2c locus, and rs3807989 in the CAV1 gene. Despite the robust gene-gene interaction identified for rs2106261/ZFHX3 gene and rs2200733/PITX2c, we did not identify any significant interaction between rs2106261/ZFHX3 and rs3807989/CAV1 with all three gene-gene interaction programs (Tables 2, 4 and 5). For interaction between rs2200733/PITX2c and rs3807989/CAV1, inconsistent results were obtained. Analysis for the OR for each multi-locus genotype and RERI analysis did not find gene-gene interaction between rs2200733 and rs3807989, whereas the INTERSNP program found significant interaction under a model of additive by additive. Future studies are needed to reconcile the differences between different programs developed for studying gene-gene interaction.
One limitation of the present study is that our statistical analysis was not adjusted for principal components to correct for possible stratification in Chinese samples due to a limited number of SNPs genotyped in the study populations. Genetic interaction may be especially susceptible to small degrees of population stratification, however, this may be unlikely given the replication of the finding in multiple populations.
In summary, we have found that gene-gene interaction can generate synergistic effects that markedly increase disease risk, therefore, accounting for a portion of heritability of human disease. Our identification of the gene-gene interaction between SNPs rs2106261 in the ZFHX3 gene and rs2200733 at the PITX2c locus provide significant insights into the pathogenesis of AF. We further show that PITX2c and ZFHX3 positively regulate each other at the molecular level, generating a loop of cross-regulation between PITX2c and ZFHX3. Our data provide an interesting molecular basis for some gene-gene interaction at the molecular genetic level.
Materials and Methods
Study subjects and preparation of genomic DNA samples
The subjects involved in the present study include AF patients and non-AF controls selected from the GeneID database [6,9,32–39]. All study subjects are of Han ethnic origin based on self-description. The study was approved by the Ethics Committee of Huazhong University of Science and Technology and the Ethics Committees from local hospitals, and consistent with the guideline in the Declaration of Helsinki. Written informed consent was obtained from the participants.
The diagnosis of AF was made by multiple experienced cardiologists and cardiac electrophysiologists using data from 12-lead surface electrocardiograms (ECGs) or Holter recordings. The ECG characteristics of AF include the absence of P waves, the presence of rapid oscillations or fibrillatory waves (F waves), and irregular R-R intervals [40–42]. The controls are healthy individuals who do not have AF at the time of physical examinations or from medical records.
Details of study subjects and GeneID and preparation of genomic DNA samples were described in S1 Text.
Genotyping of SNPs
Genotyping of SNPs was carried out using High-Resolution Melt (HRM) analysis as described previously by us [6,9,32–39]. HRM genotyping data were validated by direct sequencing analysis of 52 randomly selected study subjects. Primers for genotyping are listed in S6 Table. The HRM genotyping data matched the sequencing data.
Prediction of potential miR-1 binding sites
Details of bioinformatics prediction of miR-1 binding sites were described in S1 Text.
Plasmids, siRNAs, and microRNA mimics
Details of plasmids, siRNAs and microRNA mimics were described in S1 Text.
Cell culture and dual luciferase reporter assays
HCT116 and SW620 cells were cultured and transfected with plasmid DNA, siRNAs, and microRNA mimics using Lipofectamine 2000 and the Opti-MEM I reduced serum medium as described [43,44]. Luciferase activities were measured using the Dual-Glo luciferase assay kit (Gibco Life Technologies, Gaithersburg, MD, USA) as described previously by us [43,45]. Each experiment was performed in triplicate and repeated at least three times. Details of cell culture and luciferase assays were described in S1 Text.
Real-time PCR analysis
The expression levels of PITX2c, ZFHX3, NPPA, CAV1, NKX2.5, TBX5, KCNQ1, and SCN1B were measured using real-time RT-PCR analysis with SYBR green I mix as described by us previously [36,44] and described in detail in S1 Text. Primers for real-time RT - PCR analysis are listed in S6 Table.
Western blot analysis
Western blot analysis was carried out as described by us previously [43,44] and described in detail in S1 Text.
Statistical analysis
The genotyping data for all SNPs are included in S8–S13 Tables. The genotyping data from the control group for each SNP were first tested for the Hardy-Weinberg equilibrium using PLINK1.06 (http://pngu.mgh.harvard.edu). If a P value was >0.01, the genotyping data were considered to be in the Hardy-Weinberg equilibrium. Genotypic frequencies in controls were all in Hardy-Weinberg equilibrium (P>0.01). For case-control association analysis, we used Pearson’s 2×2 and 2×3 contingency table χ2 tests as implemented in PLINK1.06 (http://pngu.mgh.harvard.edu) to compute the P values for allelic and genotypic associations, respectively. The same PLINK1.06 program was used to estimate the odds ratio (OR) and 95% confidence interval (CI) for each association. In order to exclude confounding factors, multivariable logistic regression analysis was performed using SPSS 17.0 to adjust for gender and age.
For analysis of gene-gene interaction, SNP rs2106261 in ZFHX3 or SNP rs2200733 at the PITX2c locus each has two alleles (G vs. A for rs2106261; C vs. T for rs2200733). The two SNPs together generate nine different genotypes. We defined the homozygous, non-risk (or protective) two-locus genotype GGCC as the reference group, and then estimated the OR of AF for each of the other eight two-locus genotypes GGCT, GGTT, AGCC, AGCT, AGTT, AACC, AACT, and AATT in relation to the reference genotype. The Pearson’s 2×2 contingency table χ2 test was used to compute the nominal P values, ORs, and 95% CIs for each genotypic association using PLINK1.06. The Breslow-Day test was carried out to test whether the ORs between two different genotypes showed a statistically significant difference.
Gene-gene interaction was also measured by a relative excess risk due to interaction (RERI) analysis [19]. The RERI analysis analyzes was suggested to be more meaningful for disease prevention and intervention in public health [46], and advocated to be more biologically interpretable compared to that measured on the multiplicative scale [47]. A synergistic effect was defined as the extent of the combined effect of the exposures in excess of the sum of their individual effects [48]. We adopted a fundamental measure of RERI versus additive interaction to quantify the extent of synergistic effect in this study. The original form of RERI was defined as RERI = RR11-RR10-RR01+1, where subscript 11, 10 and 01 denote relative risks (RR) for doubly-exposed and individually-exposed to each risk factor when treating doubly-unexposed as a reference. When a RERI value equals to 0, it indicates a perfect additive model. Any significant deviation from 0 indicates a synergistic (+, positive values) or antagonistic (-, negative values) interaction. In a case-control study, RERI can be calculated by substituting ORs for RRs, yielding RERI = OR11-OR10-OR01+1. Although simply replacing RRs with ORs would induce an exaggeration problem for ORs [19,49], especially for a high prevalent disease, it is shown that RERI in terms of ORs is a good approximation of RERI in terms of RRs in a disease such as AF with a prevalence rate of 0.4%~0.8%) [49]. Under this circumstance, an OR is a good approximation of the RR. The statistical significance of RERI values in terms of ORs was addressed by the 95% confidence intervals based on the “MOVER” method, which utilizes the asymmetric intervals for ORs [19]. Since the SNPs are bi-allelic, it is of interest to explore if the interaction exists (1) when doubly-exposed to one copy of risk alleles (i.e. doubly heterozygous genotype) and (2) when doubly-exposed to two copies of risk alleles (i.e. double homozygous risk genotypes). In both scenarios, the doubly-unexposed is referred to as the homozygous non-risk genotype (e.g. GGCC). In addition, we tested the interaction when exposed one additional copy of risk alleles given being exposed to one copy of risk alleles. Note that the doubly-unexposed in this scenario is the doubly heterozygous genotype (e.g. AGCT). The P values were estimated by 10,000 times of bootstrap sampling. The P value of 0.05 or less than and the 95% CI of RERI through zero was considered to show statistical significance.
We also conducted a 4 degree of freedom test for genotypic interaction with logistic regression developed by Cordell and Clayton[20], which was implemented in the software INTERSNP[21] as Logistic Regression test #6. This model partitions the variance in AF risk into Additive and Dominant terms for each main effect, then into Additive by Additive, Additive by Dominant, Dominant by Additive and Dominant by Dominant terms. The test yielded ORs and 95% CI for each interaction term along with the global P values for the four terms. P values for individual terms were computed using Wald tests. We also used Logistic Regression test #5 in the INTERSNP program to test for additive interaction on a multiplicative ORs scale.
In molecular studies with quantitative data, a standard Student’s t-test was used to compare the means between two groups of variables. A P value of 0.05 or less was considered to show statistical significance.
Supporting Information
Zdroje
1. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, et al. (2001) Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285 : 2370–2375. 11343485
2. Hu D, Sun Y (2008) Epidemiology, risk factors for stroke, and management of atrial fibrillation in China. J Am Coll Cardiol 52 : 865–868. doi: 10.1016/j.jacc.2008.05.042 18755352
3. Fujii H, Kim JI, Yoshiya K, Nishi S, Fukagawa M (2011) Clinical characteristics and cardiovascular outcomes of hemodialysis patients with atrial fibrillation: a prospective follow-up study. Am J Nephrol 34 : 126–134. doi: 10.1159/000329118 21720157
4. Christophersen IE, Ravn LS, Budtz-Joergensen E, Skytthe A, Haunsoe S, et al. (2009) Familial aggregation of atrial fibrillation: a study in Danish twins. Circ Arrhythm Electrophysiol 2 : 378–383. doi: 10.1161/CIRCEP.108.786665 19808493
5. Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, et al. (2007) Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 448 : 353–357. 17603472
6. Shi L, Li C, Wang C, Xia Y, Wu G, et al. (2009) Assessment of association of rs2200733 on chromosome 4q25 with atrial fibrillation and ischemic stroke in a Chinese Han population. Hum Genet 126 : 843–849. doi: 10.1007/s00439-009-0737-3 19707791
7. Gudbjartsson DF, Holm H, Gretarsdottir S, Thorleifsson G, Walters GB, et al. (2009) A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet 41 : 876–878. doi: 10.1038/ng.417 19597491
8. Benjamin EJ, Rice KM, Arking DE, Pfeufer A, van Noord C, et al. (2009) Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet 41 : 879–881. doi: 10.1038/ng.416 19597492
9. Li C, Wang F, Yang Y, Fu F, Xu C, et al. (2011) Significant association of SNP rs2106261 in the ZFHX3 gene with atrial fibrillation in a Chinese Han GeneID population. Hum Genet 129 : 239–246. doi: 10.1007/s00439-010-0912-6 21107608
10. Ellinor PT, Lunetta KL, Glazer NL, Pfeufer A, Alonso A, et al. (2010) Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet 42 : 240–244. doi: 10.1038/ng.537 20173747
11. Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, et al. (2012) Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet 44 : 670–675. doi: 10.1038/ng.2261 22544366
12. Chen S, Wang C, Wang X, Xu C, Wu M, et al. (2015) Significant Association Between CAV1 Variant rs3807989 on 7p31 and Atrial Fibrillation in a Chinese Han Population. J Am Heart Assoc 4.
13. Wang J, Klysik E, Sood S, Johnson RL, Wehrens XH, et al. (2010) Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc Natl Acad Sci U S A 107 : 9753–9758. doi: 10.1073/pnas.0912585107 20457925
14. Kirchhof P, Kahr PC, Kaese S, Piccini I, Vokshi I, et al. (2011) PITX2c is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. Circ Cardiovasc Genet 4 : 123–133. doi: 10.1161/CIRCGENETICS.110.958058 21282332
15. Chinchilla A, Daimi H, Lozano-Velasco E, Dominguez JN, Caballero R, et al. (2011) PITX2 insufficiency leads to atrial electrical and structural remodeling linked to arrhythmogenesis. Circ Cardiovasc Genet 4 : 269–279. doi: 10.1161/CIRCGENETICS.110.958116 21511879
16. Ganga M, Espinoza HM, Cox CJ, Morton L, Hjalt TA, et al. (2003) PITX2 isoform-specific regulation of atrial natriuretic factor expression: synergism and repression with Nkx2.5. J Biol Chem 278 : 22437–22445. 12692125
17. Hilton T, Gross MK, Kioussi C (2010) Pitx2-dependent occupancy by histone deacetylases is associated with T-box gene regulation in mammalian abdominal tissue. J Biol Chem 285 : 11129–11142. doi: 10.1074/jbc.M109.087429 20129917
18. Tao Y, Zhang M, Li L, Bai Y, Zhou Y, et al. (2014) Pitx2, an atrial fibrillation predisposition gene, directly regulates ion transport and intercalated disc genes. Circ Cardiovasc Genet 7 : 23–32. doi: 10.1161/CIRCGENETICS.113.000259 24395921
19. Zou GY (2008) On the estimation of additive interaction by use of the four-by-two table and beyond. Am J Epidemiol 168 : 212–224. doi: 10.1093/aje/kwn104 18511428
20. Cordell HJ, Clayton DG (2002) A unified stepwise regression procedure for evaluating the relative effects of polymorphisms within a gene using case/control or family data: application to HLA in type 1 diabetes. Am J Hum Genet 70 : 124–141. 11719900
21. Herold C, Steffens M, Brockschmidt FF, Baur MP, Becker T (2009) INTERSNP: genome-wide interaction analysis guided by a priori information. Bioinformatics 25 : 3275–3281. doi: 10.1093/bioinformatics/btp596 19837719
22. Ritchie MD, Rowan S, Kucera G, Stubblefield T, Blair M, et al. (2012) Chromosome 4q25 variants are genetic modifiers of rare ion channel mutations associated with familial atrial fibrillation. J Am Coll Cardiol 60 : 1173–1181. doi: 10.1016/j.jacc.2012.04.030 22818067
23. Lubitz SA, Lunetta KL, Lin H, Arking DE, Trompet S, et al. (2014) Novel genetic markers associate with atrial fibrillation risk in Europeans and Japanese. J Am Coll Cardiol 63 : 1200–1210. doi: 10.1016/j.jacc.2013.12.015 24486271
24. Franco D, Campione M (2003) The role of Pitx2 during cardiac development. Linking left-right signaling and congenital heart diseases. Trends Cardiovasc Med 13 : 157–163. 12732450
25. Faucourt M, Houliston E, Besnardeau L, Kimelman D, Lepage T (2001) The pitx2 homeobox protein is required early for endoderm formation and nodal signaling. Dev Biol 229 : 287–306. 11203696
26. Mommersteeg MT, Hoogaars WM, Prall OW, de Gier-de Vries C, Wiese C, et al. (2007) Molecular pathway for the localized formation of the sinoatrial node. Circ Res 100 : 354–362. 17234970
27. Yasuda H, Mizuno A, Tamaoki T, Morinaga T (1994) ATBF1, a multiple-homeodomain zinc finger protein, selectively down-regulates AT-rich elements of the human alpha-fetoprotein gene. Mol Cell Biol 14 : 1395–1401. 7507206
28. Morinaga T, Yasuda H, Hashimoto T, Higashio K, Tamaoki T (1991) A human alpha-fetoprotein enhancer-binding protein, ATBF1, contains four homeodomains and seventeen zinc fingers. Mol Cell Biol 11 : 6041–6049. 1719379
29. Berry FB, Miura Y, Mihara K, Kaspar P, Sakata N, et al. (2001) Positive and negative regulation of myogenic differentiation of C2C12 cells by isoforms of the multiple homeodomain zinc finger transcription factor ATBF1. J Biol Chem 276 : 25057–25065. 11312261
30. Jung CG, Kim HJ, Kawaguchi M, Khanna KK, Hida H, et al. (2005) Homeotic factor ATBF1 induces the cell cycle arrest associated with neuronal differentiation. Development 132 : 5137–5145. 16251211
31. Ido A, Miura Y, Watanabe M, Sakai M, Inoue Y, et al. (1996) Cloning of the cDNA encoding the mouse ATBF1 transcription factor. Gene 168 : 227–231. 8654949
32. Wang F, Xu CQ, He Q, Cai JP, Li XC, et al. (2011) Genome-wide association identifies a susceptibility locus for coronary artery disease in the Chinese Han population. Nat Genet 43 : 345–349. doi: 10.1038/ng.783 21378986
33. Xu C, Wang F, Wang B, Li X, Li C, et al. (2010) Minor allele C of chromosome 1p32 single nucleotide polymorphism rs11206510 confers risk of ischemic stroke in the Chinese Han population. Stroke 41 : 1587–1592. doi: 10.1161/STROKEAHA.110.583096 20576952
34. Cheng X, Shi L, Nie S, Wang F, Li X, et al. (2011) The same chromosome 9p21.3 locus is associated with type 2 diabetes and coronary artery disease in a Chinese Han population. Diabetes 60 : 680–684. doi: 10.2337/db10-0185 21270277
35. Li X, Huang Y, Yin D, Wang D, Xu C, et al. (2013) Meta-analysis identifies robust association between SNP rs17465637 in MIA3 on chromosome 1q41 and coronary artery disease. Atherosclerosis 231 : 136–140. doi: 10.1016/j.atherosclerosis.2013.08.031 24125424
36. Xiong X, Xu C, Zhang Y, Li X, Wang B, et al. (2014) BRG1 variant rs1122608 on chromosome 19p13.2 confers protection against stroke and regulates expression of pre-mRNA-splicing factor SFRS3. Hum Genet 133 : 499–508. doi: 10.1007/s00439-013-1389-x 24190014
37. Bai Y, Nie S, Jiang G, Zhou Y, Zhou M, et al. (2014) Regulation of CARD8 expression by ANRIL and association of CARD8 single nucleotide polymorphism rs2043211 (p.C10X) with ischemic stroke. Stroke 45 : 383–388. doi: 10.1161/STROKEAHA.113.003393 24385277
38. Tu X, Nie S, Liao Y, Zhang H, Fan Q, et al. (2013) The IL-33-ST2L pathway is associated with coronary artery disease in a Chinese Han population. Am J Hum Genet 93 : 652–660. doi: 10.1016/j.ajhg.2013.08.009 24075188
39. Ren X, Xu C, Zhan C, Yang Y, Shi L, et al. (2010) Identification of NPPA variants associated with atrial fibrillation in a Chinese GeneID population. Clin Chim Acta 411 : 481–485. doi: 10.1016/j.cca.2009.12.019 20064500
40. Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, et al. (2006) ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 114: e257–354. 16908781
41. Oberti C, Wang L, Li L, Dong J, Rao S, et al. (2004) Genome-wide linkage scan identifies a novel genetic locus on chromosome 5p13 for neonatal atrial fibrillation associated with sudden death and variable cardiomyopathy. Circulation 110 : 3753–3759. 15596564
42. Zhang X, Chen S, Yoo S, Chakrabarti S, Zhang T, et al. (2008) Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell 135 : 1017–1027. doi: 10.1016/j.cell.2008.10.022 19070573
43. Zhou B, Ma R, Si W, Li S, Xu Y, et al. (2013) MicroRNA-503 targets FGF2 and VEGFA and inhibits tumor angiogenesis and growth. Cancer Lett 333 : 159–169. doi: 10.1016/j.canlet.2013.01.028 23352645
44. Xu Y, Zhou M, Wang J, Zhao Y, Li S, et al. (2014) Role of microRNA-27a in down-regulation of angiogenic factor AGGF1 under hypoxia associated with high-grade bladder urothelial carcinoma. Biochim Biophys Acta 1842 : 712–725. doi: 10.1016/j.bbadis.2014.01.007 24462738
45. Fan C, Liu M, Wang Q (2003) Functional analysis of TBX5 missense mutations associated with Holt-Oram syndrome. J Biol Chem 278 : 8780–8785. 12499378
46. Rothman KJ (1976) The estimation of synergy or antagonism. Am J Epidemiol 103 : 506–511. 1274952
47. Greenland S, Poole C (1988) Invariants and noninvariants in the concept of interdependent effects. Scand J Work Environ Health 14 : 125–129. 3387960
48. Wang X, Elston RC, Zhu X (2010) The meaning of interaction. Hum Hered 70 : 269–277. doi: 10.1159/000321967 21150212
49. Kalilani L, Atashili J (2006) Measuring additive interaction using odds ratios. Epidemiol Perspect Innov 3 : 5. 16620385
Štítky
Genetika Reprodukčná medicína
Článek Loss and Gain of Natural Killer Cell Receptor Function in an African Hunter-Gatherer PopulationČlánek Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2Článek Binding of Multiple Rap1 Proteins Stimulates Chromosome Breakage Induction during DNA ReplicationČlánek SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from DegradationČlánek Protein Composition of Infectious Spores Reveals Novel Sexual Development and Germination Factors inČlánek The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 RegulationČlánek Runx1 Transcription Factor Is Required for Myoblasts Proliferation during Muscle Regeneration
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 8- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Putting the Brakes on Huntington Disease in a Mouse Experimental Model
- Identification of Driving Fusion Genes and Genomic Landscape of Medullary Thyroid Cancer
- Evidence for Retromutagenesis as a Mechanism for Adaptive Mutation in
- TSPO, a Mitochondrial Outer Membrane Protein, Controls Ethanol-Related Behaviors in
- Evidence for Lysosome Depletion and Impaired Autophagic Clearance in Hereditary Spastic Paraplegia Type SPG11
- Loss and Gain of Natural Killer Cell Receptor Function in an African Hunter-Gatherer Population
- Trans-Reactivation: A New Epigenetic Phenomenon Underlying Transcriptional Reactivation of Silenced Genes
- Early Developmental and Evolutionary Origins of Gene Body DNA Methylation Patterns in Mammalian Placentas
- Strong Selective Sweeps on the X Chromosome in the Human-Chimpanzee Ancestor Explain Its Low Divergence
- Dominance of Deleterious Alleles Controls the Response to a Population Bottleneck
- Transient 1a Induction Defines the Wound Epidermis during Zebrafish Fin Regeneration
- Systems Genetics Reveals the Functional Context of PCOS Loci and Identifies Genetic and Molecular Mechanisms of Disease Heterogeneity
- A Genome Scale Screen for Mutants with Delayed Exit from Mitosis: Ire1-Independent Induction of Autophagy Integrates ER Homeostasis into Mitotic Lifespan
- Non-synonymous FGD3 Variant as Positional Candidate for Disproportional Tall Stature Accounting for a Carcass Weight QTL () and Skeletal Dysplasia in Japanese Black Cattle
- The Relationship between Gene Network Structure and Expression Variation among Individuals and Species
- Calmodulin Methyltransferase Is Required for Growth, Muscle Strength, Somatosensory Development and Brain Function
- The Wnt Frizzled Receptor MOM-5 Regulates the UNC-5 Netrin Receptor through Small GTPase-Dependent Signaling to Determine the Polarity of Migrating Cells
- Nbs1 ChIP-Seq Identifies Off-Target DNA Double-Strand Breaks Induced by AID in Activated Splenic B Cells
- CCNYL1, but Not CCNY, Cooperates with CDK16 to Regulate Spermatogenesis in Mouse
- Evidence for a Common Origin of Blacksmiths and Cultivators in the Ethiopian Ari within the Last 4500 Years: Lessons for Clustering-Based Inference
- Of Fighting Flies, Mice, and Men: Are Some of the Molecular and Neuronal Mechanisms of Aggression Universal in the Animal Kingdom?
- Hypoxia and Temperature Regulated Morphogenesis in
- The Homeodomain Iroquois Proteins Control Cell Cycle Progression and Regulate the Size of Developmental Fields
- Evolution and Design Governing Signal Precision and Amplification in a Bacterial Chemosensory Pathway
- Rac1 Regulates Endometrial Secretory Function to Control Placental Development
- Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2
- Functions as a Positive Regulator of Growth and Metabolism in
- The Nucleosome Acidic Patch Regulates the H2B K123 Monoubiquitylation Cascade and Transcription Elongation in
- Rhoptry Proteins ROP5 and ROP18 Are Major Murine Virulence Factors in Genetically Divergent South American Strains of
- Exon 7 Contributes to the Stable Localization of Xist RNA on the Inactive X-Chromosome
- Regulates Refractive Error and Myopia Development in Mice and Humans
- mTORC1 Prevents Preosteoblast Differentiation through the Notch Signaling Pathway
- Regulation of Gene Expression Patterns in Mosquito Reproduction
- Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation
- The Spalt Transcription Factors Generate the Transcriptional Landscape of the Wing Pouch Central Region
- Binding of Multiple Rap1 Proteins Stimulates Chromosome Breakage Induction during DNA Replication
- Functional Divergence in the Role of N-Linked Glycosylation in Smoothened Signaling
- YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers
- Coordinated Evolution of Influenza A Surface Proteins
- The Evolutionary Potential of Phenotypic Mutations
- Genome-Wide Association and Trans-ethnic Meta-Analysis for Advanced Diabetic Kidney Disease: Family Investigation of Nephropathy and Diabetes (FIND)
- New Routes to Phylogeography: A Bayesian Structured Coalescent Approximation
- SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from Degradation
- Satellite DNA Modulates Gene Expression in the Beetle after Heat Stress
- SHOEBOX Modulates Root Meristem Size in Rice through Dose-Dependent Effects of Gibberellins on Cell Elongation and Proliferation
- Reduced Crossover Interference and Increased ZMM-Independent Recombination in the Absence of Tel1/ATM
- Suppression of Somatic Expansion Delays the Onset of Pathophysiology in a Mouse Model of Huntington’s Disease
- Protein Composition of Infectious Spores Reveals Novel Sexual Development and Germination Factors in
- The Evolutionarily Conserved LIM Homeodomain Protein LIM-4/LHX6 Specifies the Terminal Identity of a Cholinergic and Peptidergic . Sensory/Inter/Motor Neuron-Type
- SmD1 Modulates the miRNA Pathway Independently of Its Pre-mRNA Splicing Function
- piRNAs Are Associated with Diverse Transgenerational Effects on Gene and Transposon Expression in a Hybrid Dysgenic Syndrome of .
- Retinoic Acid Signaling Regulates Differential Expression of the Tandemly-Duplicated Long Wavelength-Sensitive Cone Opsin Genes in Zebrafish
- The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 Regulation
- Genome-Wide Analysis of PAPS1-Dependent Polyadenylation Identifies Novel Roles for Functionally Specialized Poly(A) Polymerases in
- Runx1 Transcription Factor Is Required for Myoblasts Proliferation during Muscle Regeneration
- Regulation of Mutagenic DNA Polymerase V Activation in Space and Time
- Variability of Gene Expression Identifies Transcriptional Regulators of Early Human Embryonic Development
- The Drosophila Gene Interacts Genetically with and Shows Female-Specific Effects of Divergence
- Functional Activation of the Flagellar Type III Secretion Export Apparatus
- Retrohoming of a Mobile Group II Intron in Human Cells Suggests How Eukaryotes Limit Group II Intron Proliferation
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Exon 7 Contributes to the Stable Localization of Xist RNA on the Inactive X-Chromosome
- YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers
- SmD1 Modulates the miRNA Pathway Independently of Its Pre-mRNA Splicing Function
- Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



