-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
ARID1A Is Essential for Endometrial Function during Early Pregnancy
Endometriosis afflicts about 10% of women of reproductive age and is a major cause of pain and infertility. We showed attenuation of endometrial ARID1A in women with endometriosis as compared to women without endometriosis, and thus hypothesized that ARID1A plays an important role in ensuring normal fertility in the uterus. To test this hypothesis, we generated uterine-specific Arid1a knock-out mice, which were infertile due to defective implantation and decidualization. The mutant mice demonstrated increased endometrial epithelial proliferation with enhanced estrogen signaling and attenuation of epithelial PGR. Microarray and ChIP analysis revealed that Arid1a suppresses epithelial proliferation with PGR by regulating Klf15 expression. These data suggest that Arid1a plays an important role in steroid hormone signaling in endometrial function and dysfunction. Further investigation of ARID1A will be important for understanding altered endometrial function in infertility and endometriosis and in developing therapies for these disorders.
Published in the journal: ARID1A Is Essential for Endometrial Function during Early Pregnancy. PLoS Genet 11(9): e32767. doi:10.1371/journal.pgen.1005537
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005537Summary
Endometriosis afflicts about 10% of women of reproductive age and is a major cause of pain and infertility. We showed attenuation of endometrial ARID1A in women with endometriosis as compared to women without endometriosis, and thus hypothesized that ARID1A plays an important role in ensuring normal fertility in the uterus. To test this hypothesis, we generated uterine-specific Arid1a knock-out mice, which were infertile due to defective implantation and decidualization. The mutant mice demonstrated increased endometrial epithelial proliferation with enhanced estrogen signaling and attenuation of epithelial PGR. Microarray and ChIP analysis revealed that Arid1a suppresses epithelial proliferation with PGR by regulating Klf15 expression. These data suggest that Arid1a plays an important role in steroid hormone signaling in endometrial function and dysfunction. Further investigation of ARID1A will be important for understanding altered endometrial function in infertility and endometriosis and in developing therapies for these disorders.
Introduction
Endometriosis is one of the most significant diseases affecting females of reproductive-age and affects an estimated 5 million women in the United States. Endometriosis is defined as the presence of endometrium-like tissue outside of the uterine cavity. The incidence increases up to 50% in patients with infertility and up to 45% in patients with chronic pelvic pain [1,2]. Infertility and pregnancy loss are major public health concerns for reproductive-age women. Establishment of uterine receptivity by the sequential actions of estrogen (E2) and progesterone (P4) on uterine cells is critical for successful embryo apposition, attachment, implantation, and pregnancy maintenance. Lack of sufficient E2 and P4 action can result in infertility and pregnancy loss in humans [3,4] and mice [5]. One of the primary effects of E2 on the endometrium is stimulation of epithelial proliferation, while the primary effects of P4 are to inhibit epithelial proliferation and induce differentiation to an embryo receptive state [6,7]. Cellular E2 and P4 actions can occur directly on a specific cell type and indirectly via paracrine activity mediated by another cell type. P4 through its cognate receptor, the progesterone receptor (PGR), have important roles in the establishment and maintenance of pregnancy [7–10]. P4 attenuates E2 stimulated epithelial cell proliferation by epithelial PGR [11].
At the time of embryo implantation, the expression of PGR is promptly downregulated in the luminal epithelium in both humans and mice, and its expression is increased in stromal cells, anticipating the role of PGR in induction of decidualization [12]. Epithelial PGR acts to inhibit E2-induced epithelial proliferation. Epithelial PGR female mice are infertile due to embryo implantation defects indicating that epithelial PGR is essential for uterine function [11].
Endometriosis regression was found in some patients with endometriosis during pregnancy or who were exposed to progestin-based therapeutics [13,14]. However there are endometriosis patients who do not respond to treatment due to progesterone resistance. The molecular changes by P4 in the eutopic endometrium from women with endometriosis are either blunted or undetectable. P4 cannot inhibit E2-dependent growth of endometriosis[15]. The previous microarray studies of comparing women with and without endometriosis reported that many of the P4 target genes were altered at the time of implantation when P4 levels are highest [16,17]. P4 therapy also prevents the development of endometrial cancer associated with unopposed E2 by blocking E2 actions [18]. Expression of PGR was known as positively correlated with a good prognosis and responsiveness to progestin treatment [19]. However, more than 30% of patients with progestin treatment did not respond to progestin due to de novo or acquired progestin resistance [20–24]. The mechanism of progestin resistance is still unknown. Understanding the molecular mechanisms regulating E2 and P4 actions in the endometrium is critical in developing therapeutic approaches to alleviate this women’s health crisis.
Ovarian clear-cell and endometrioid carcinomas are associated with endometriosis through distinct but currently unknown mechanisms [25–27]. One of the possible mechanisms is linked to mutation of the AT-rich interactive domain 1A gene (ARID1A) [28]. ARID1A encodes BAF250a (ARID1A) protein which is one of the subunits in the switch/sucrose non-fermentable (SWI/SNF) chromatin remodeling complex [29]. ARID1A mutations leading to loss of the protein expression [30] have been found in 46% of ovarian clear-cell carcinomas and 30% of endometrioid ovarian carcinomas [28,31]. ARID1A is also critical for embryogenesis in mice and the maintenance of ES cell self-renewal, as well as lineage-specific differentiation of ES cells in vitro [32]. Embryos lacking one allele resulted in late embryonic lethality, complete loss of ARID1A led to developmental arrest around E6.5 without formation of a primitive streak and mesoderm. Ablation of ARID1A in mice ES cells led to altered cell morphology and proliferation [33]. However, little is known about the physiological or pathological effects of ARID1A expression in the endometrium.
We found that ARID1A levels are remarkably lower in endometrium from women with endometriosis compared to women without endometriosis. In an effort to overcome embryonic lethality of Arid1a knock-out mice, we have used conditional Arid1a knock-out mice in the uterus. In this study, we observed that the mutant mice are sterile due to increased epithelial cell proliferation which resulted in implantation defects. Our results suggest that Arid1a suppresses E2 signaling with PGR by modulating KLF15 expression indicating the critical role of Arid1a in the peri-implantation period.
Results
Attenuation of ARID1A in eutopic endometrial tissue from women with endometriosis
We examined the levels of ARID1A in endometrium from spontaneously cycling women using immunohistochemical analysis. We observed the most abundant levels of ARID1A protein throughout the menstrual cycle in women without endometriosis (S1 Fig). ARID1A proteins were strongly detected in the stromal and epithelial cells of endometrium from the proliferative phase and early, mid, and late secretory phases in women without endometriosis (n = 7 per stage). However, the levels of ARID1A were significantly lower in both the stromal and epithelial cells of endometrium from proliferative and secretory phase endometriosis patients (n = 28) compared to women without endometriosis (n = 28) (Fig 1).
Fig. 1. ARID1A loss in eutopic endometrial tissue from infertile women with endometriosis. 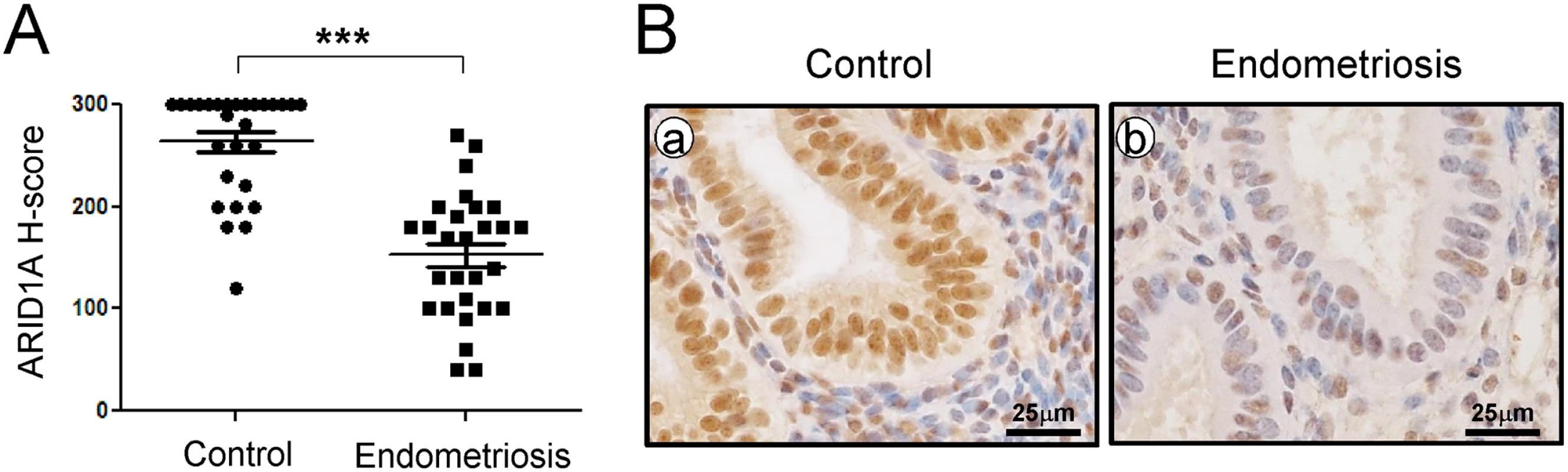
(A) The immunohistochemical histological score (H-score) of ARID1A proteins. The results represent the mean ± SEM. *** p<0.001. (B) Representative photomicrograph of immunohistochemical staining of ARID1A proteins in human endometrium with and without endometriosis. To determine whether ARID1A is expressed during pregnancy, we next examined the mRNA and protein levels of ARID1A in the uteri of wild-type mice during early pregnancy by real-time RT-PCR and immunohistochemical analysis (S2 Fig). The initiation of pregnancy was marked by the presence of the postcoital vaginal plug (0.5 dpc). The expression of Arid1a mRNA was strongly detected on 0.5 dpc, which consistently expressed until 6.5 dpc in the uterus. To further investigate the spatiotemporal expression profiles of ARID1A protein in the uterus during early pregnancy, we performed immunohistochemistry analysis during sequential time points. Consistent with the real-time PCR results, ARID1A proteins were also consistently strong in the nucleus of epithelial and stromal cells during early pregnancy. These data suggest that ARID1A may play an important role during early pregnancy.
Fertility defect of mice with ablation of Arid1a in the PGR-expressing cells
Arid1a knock-out mice resulted in embryo lethality [33]. Therefore, in order to investigate the role of Arid1a in the uterus, we generated a mouse model in which Arid1a gene expression is ablated specifically in the PGR-expressing cells (Pgrcre/+Arid1af/f; Arid1ad/d). ARID1A proteins were remarkably reduced in Arid1ad/d mice by western blot (S3A Fig). The uteri of Arid1af/f control mice showed abundant ARID1A proteins at the luminal epithelium, glandular epithelium and stroma, whereas this staining was absent in the Arid1ad/d mice (S3B Fig). These results confirm our successful ablation of Arid1a within the uterus of Arid1ad/d mice.
To investigate the impact of ablation of Arid1a on female fertility, female control (Arid1af/f) and Arid1ad/d mice were mated with wild-type male mice for 6 months. Arid1af/f mice (n = 9) had an average of 7.21± 0.29 pups/litter, whereas Arid1ad/d mice (n = 9) had no pups (S1 Table). These results revealed that Arid1ad/d mice were sterile. To test for an ovarian phenotype, female Arid1ad/d mice were examined for their ability to ovulate normally in response to a superovulatory regimen of gonadotropins [34]. Arid1ad/d mice yielded 19.86 ± 0.99 oocytes which did not differ significantly from Arid1af/f mice (19.50 ± 1.85) (S2 Table). Also, histological analysis of the Arid1ad/d ovary did not show any alterations in ovarian morphology Arid1ad/d mice showed normal development of corpora lutea (n = 5) (S4A Fig). The serum level of E2 and P4 were 4.40± 0.71 pg/ml and 11.57± 1.88 ng/ml, respectively in Arid1af/f mice, meanwhile 5.43± 0.50 pg/ml and 15.69± 1.96 ng/ml, respectively in Arid1ad/d mice. The serum level of E2 and P4 showed no significant statistical difference between the mice at 3.5 dpc (n = 3 per genotype) (S4B Fig). This result shows that ovarian morphology and functioning were not affected in the Arid1ad/d females suggesting that the fertility defect is primarily due to a uterine defect.
Implantation defect in Arid1ad/d mice
To determine the cause of infertility in Arid1ad/d mice, 8-week-old female Arid1af/f and Arid1ad/d mice were mated with intact wild-type male mice. Females were euthanized at 5.5 dpc of pregnancy, and the numbers of implantation sites were counted. Implantation sites were detected in the uterine horn of Arid1af/f mice, whereas there were no implantation sites in Arid1ad/d mice (Fig 2A). Histological analysis revealed that embryos could not attach to the uterine horn of Arid1ad/d mice while embryos were attached well in Arid1af/f mice and surrounded by decidualized cells (n = 5) (Fig 2B). To address a defect of embryo implantation in Arid1ad/d mice, mice were dissected at 4.5 dpc. Free-floating embryos (4.67 ± 1.33 per mouse) were found in the uterine horn of Arid1ad/d, whereas well attached embryos (5.50 ± 0.65 per mouse) were found in the uterine horn of Arid1af/f mice (n = 5) (Fig 2C). These results suggest that a failure of embryo attachment is one of the causes of the infertility observed in Arid1ad/d mice.
Fig. 2. A failure of implantation in Arid1ad/d mice. 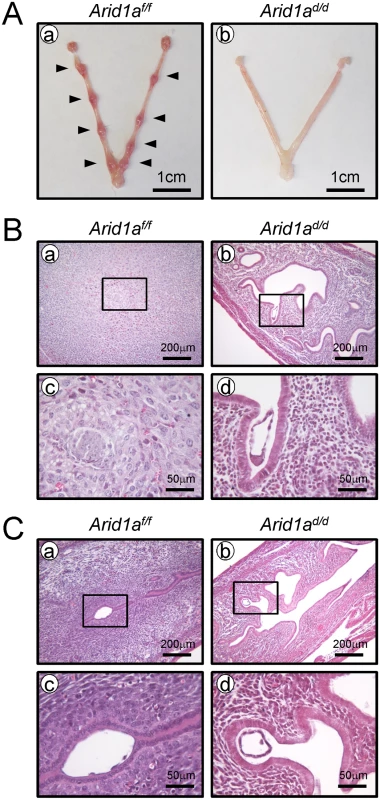
(A) Implantation sites were not detected in the uteri of Arid1ad/d mice (n = 5), compared with Arid1af/f mice at 5.5 dpc (n = 5). Arrow heads indicate implantation sites. (B) Histology of implantation site in Arid1af/f (a and c) and Arid1ad/d mice (b and d) at 5.5 dpc. (C) While well-attached embryos were found in Arid1af/f (a and c), free-floating embryos were found in the uterine cavity of Arid1ad/d mice at 4.5 dpc (b and d). Decidualization defect in Arid1ad/d mice
Embryo invasion transforms endometrial stromal cells into a decidual phenotype [35–37]. Patients with gynecological pathologies contributing to infertility, such as endometriosis, display markedly reduced decidualization and impaired uterine receptivity [38]. To access ARID1A function in stroma cells, we examined the levels of ARID1A in human primary endometrial stromal cells (hESCs) from patients with or without endometriosis by Western blot. All 6 hESCs from women without endometriosis showed strong expression of ARID1A in hESCs from women without endometriosis. Interestingly, 5 of 6 hESCs from women with endometriosis did not detect ARID1A protein (Fig 3A). This result suggests that ARID1A loss may cause an impaired decidualization in patients with endometriosis. Therefore, we next examined the role of Arid1a in decidualization.
Fig. 3. Arid1ad/d mice exhibited an altered decidualization. 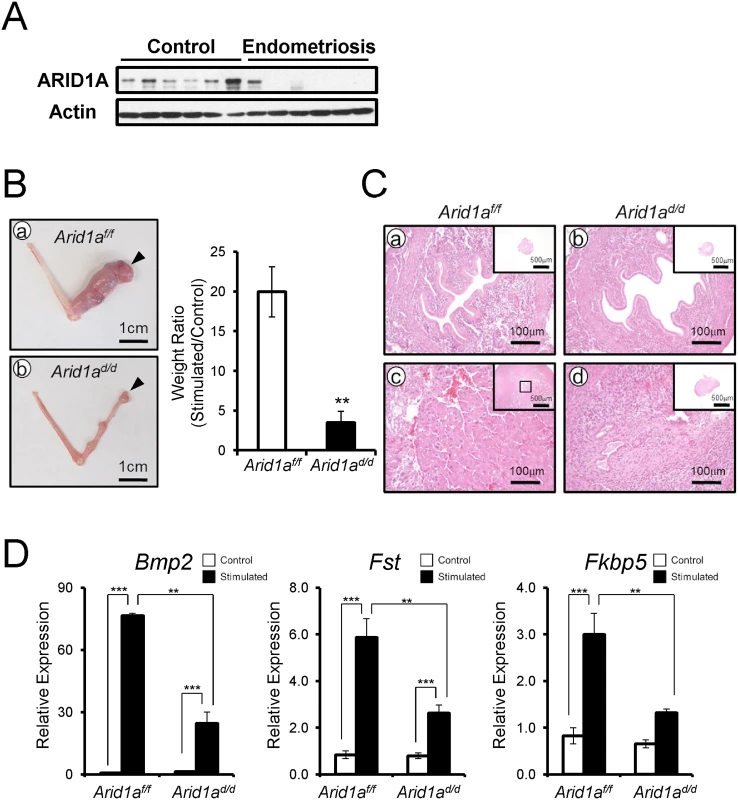
(A) The ARID1A expression were lower in infertile women with endometriosis (n = 6) compared to women without endometriosis (n = 6). (B) The decidualization is highly induced in Arid1af/f mice (a) but not Arid1ad/d mice. Arrow heads indicate stimulated horns. (b). The uterine weight ratio was significantly decreased in Arid1ad/d mice as compared to Arid1af/f (C) Histology of control and stimulated horn in Arid1af/f (a and c) and Arid1ad/d mice (b and d) at day 5, respectively (D) The expression of decidualization marker genes, Bmp2, Fst and Fkbp5 was measured in the uteri of control and stimulated horn. The results represent the mean ± SEM of three independent RNA sets. **, p < 0.01; ***, p < 0.001. We next examined the ability of Arid1ad/d mice to undergo decidualization after artificial hormonal induction. Ovariectomized Arid1af/f and Arid1ad/d mice were treated with E2+P4, and the uteri were mechanically stimulated to mimic the presence of an implanting embryo and to induce decidualization [34]. Control mice showed a decidual uterine horn that responded well to this artificial induction. However, Arid1ad/d mice exhibited a significant defect of decidual response. The weight ratio of stimulated to control horn was highly decreased in Arid1ad/d mice compared to Arid1af/f mice (Fig 3B). Histological analysis confirmed that well-developed decidual cells were detected in the decidual uterine horn of Arid1af/f mice, while differentiation of uterine stromal cells to decidual cells was not observed in the decidual uterine horn of Arid1ad/d mice (Fig 3C). In addition, the expression of known markers of decidualization, Bmp2, Fst, and Fkbp5, were significantly decreased in the decidual uterine horn of Arid1ad/d mice compared to the decidual uterine horn of Arid1af/f mice (Fig 3D). These data show that Arid1ad/d mice have a decidualization defect.
Aberrant activation of proliferation in the uterine epithelial cells of Arid1ad/d mice
In normal pregnant uteri, abundant proliferation was detected in epithelial cells and stromal cells at 2.5 dpc. Proliferation is markedly reduced in epithelial cells at 3.5 dpc for embryo attachment [39]. To determine whether a defect of embryo attachment is caused by an alteration in cell proliferation, we examined the expression of Ki67, a proliferative marker, at 3.5 dpc by immunohistochemistry. Ki67 immunohistochemistry showed that proliferation was highly increased in uterine epithelial cells of Arid1ad/d mice compared to Arid1af/f mice (Fig 4). These results suggest that abnormal epithelial proliferation in Arid1ad/d mice is one of the causes of the embryo attachment defect.
Fig. 4. The epithelial proliferation is highly increased in Arid1ad/d mice. 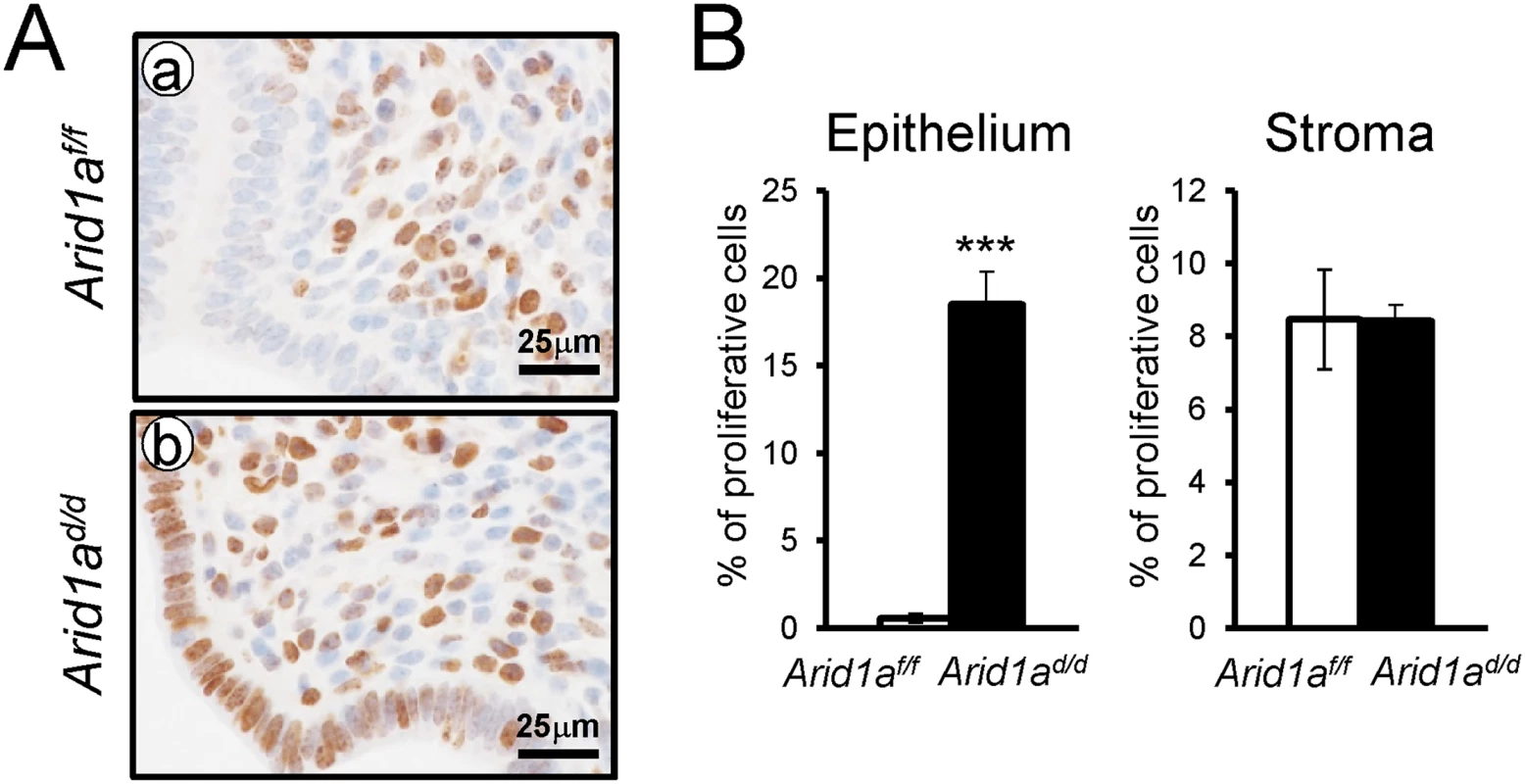
(A) Immunohistochemical analysis of Ki67 in Arid1af/f (a) and Arid1ad/d mice (b). (B) Quantification of Ki67 positive cells in epithelial and stroma cells. The results represent the mean ± SEM. ***, p < 0.001. Estrogen receptor activity is enhanced in the uterine epithelium of Arid1ad/d mice
E2 promotes epithelial cell proliferation in the uterus [6]. Since an increase of epithelial proliferation is observed in Arid1ad/d mice, we further investigated whether excess E2 signaling is caused by Arid1a ablation. To address excess E2 signaling, the expression of E2 responsive genes, C3, Clca3, Muc-1, and Ltf, were examined by real-time RT-PCR analysis. The expression of C3, Clca3, Muc-1, and Ltf were highly increased in Arid1ad/d mice compared to Arid1af/f mice (Fig 5A). An increase of phospho-ESR1, MUC1 and LTF protein expression was detected in the epithelium of the Arid1ad/d mice compared to the Arid1af/f mice, but ESR1 was not changed between the mice (Fig 5B). Arid1af/f mice had an average of 71.39± 2.58%, meanwhile Arid1ad/d mice had an average 69.02± 2.90% of positive stromal pESR1 cells. There are no significant differences. These results demonstrate that estrogen receptor activity is enhanced in the uterine epithelial cells of the Arid1ad/d mice.
Fig. 5. An increase of E2 signaling in Arid1ad/d mice. 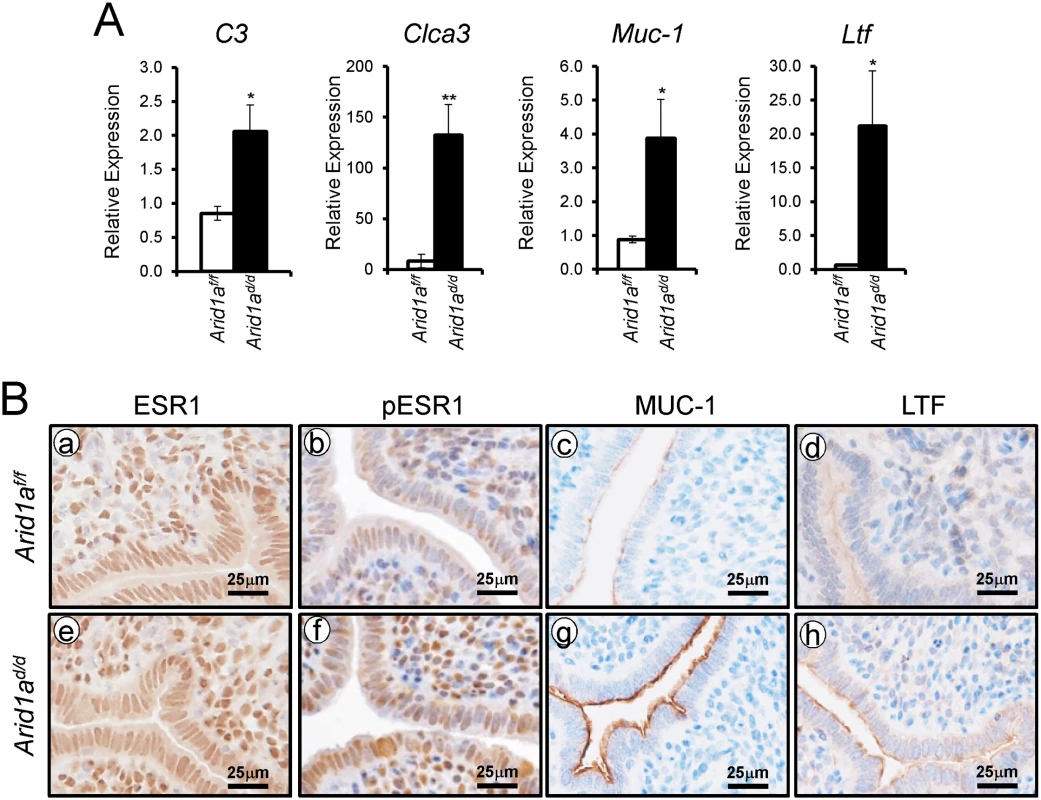
(A) Real-time RT-PCR analysis of C3, Clca3, Muc-1, and Ltf were performed on uteri of Arid1af/f and Arid1ad/d mice at 3.5 dpc. The results represent the mean ± SEM of six independent mouse sets. *, p < 0.05; **, p < 0.01. (B) Immunohistochemical analysis of ESR1 (a and e), pESR1 (b and f), MUC-1 (c and g) and LTF (d and h) in uteri of Arid1af/f and Arid1ad/d mice at 3.5 dpc. Epithelial PGR is reduced in the uteri of Arid1ad/d mice
Since excess E2 signaling is detected in the Arid1ad/d mice, we next investigated whether Arid1a ablation altered the expression of PGR. We performed PGR immunohistochemistry and real-time RT-PCR to assess the expression of PGR and its target genes in Arid1ad/d mice. Interestingly, epithelial PGR expression was highly reduced in Arid1ad/d mice compared to control mice (Fig 6A and 6B). The mRNA expression level of epithelial P4 target genes, Fst, Gata2, Areg, and Lrp2 were highly downregulated in Arid1ad/d mice. However the expression of Il13ra2 and Hand2 which are known as stromal P4-target genes were not changed (Fig 6C). These results suggest that Arid1a mediates estrogen activity by regulating epithelial PGR expression.
Fig. 6. Decreased epithelial PGR expression in Arid1ad/d mice. 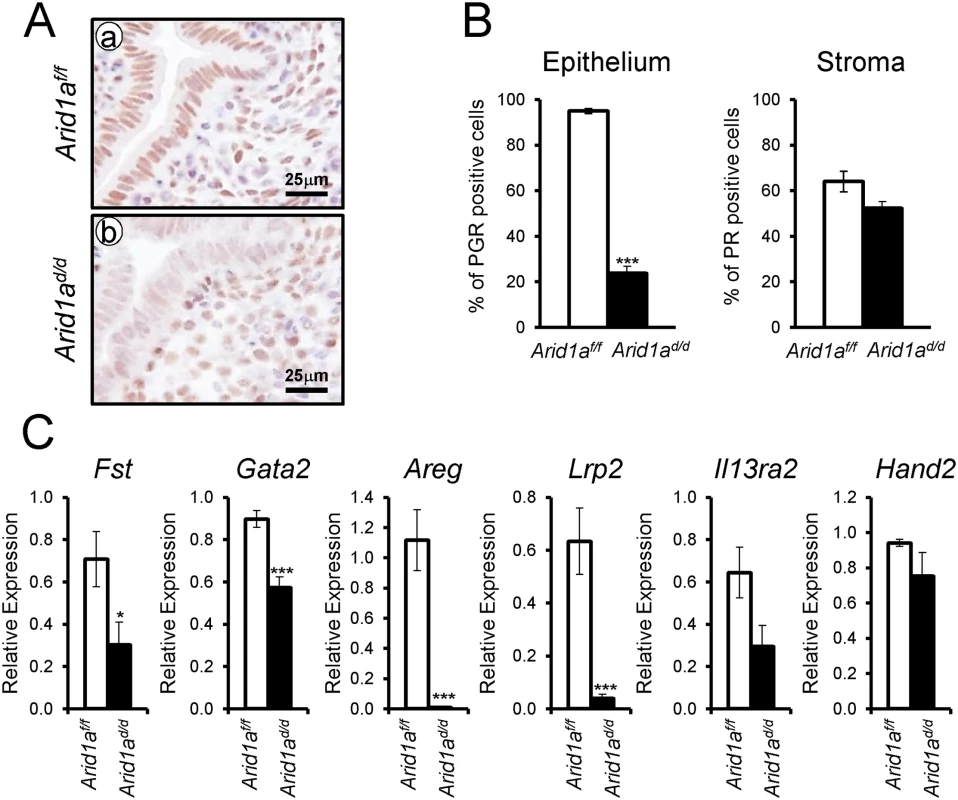
(A) Immunohistochemical analysis of PGR in Arid1af/f (a) and Arid1ad/d mice (b). (B) Quantification of PGR positive cells in epithelial and stroma cells. The results represent the mean ± SEM. ***, p < 0.001. (C) Real-time RT-PCR analysis of Fst, Gata2, Areg, Lrp2, Il13ra2, and Hand2 were performed on uteri of Arid1af/f and Arid1ad/d mice at 3.5 dpc. ARID1A suppresses E2 induced epithelial cell proliferation through KLF15
In order to identify the pathways that Arid1a regulates at implantation, we performed high density DNA microarray analysis on the uteri from Arid1af/f and Arid1ad/d mice at 3.5 dpc (GEO accession number: GSE72200). The microarray analysis showed that 1,358 were more highly expressed in Arid1ad/d mice and 1,198 genes were decreased by more than 1.5-fold. From the pathway analysis using Ingenuity Pathway Analysis (QIAGEN, Redwood City, CA), the altered pathways including cell-cycle control, DNA replication, and modification processes were identified (Table 1 and S3 Table). The results have been validated by qPCR analysis (Fig 7A). The immunohistochemistry results showed that the levels of MCM2 and MCM6 were increased in Arid1ad/d mice at 3.5 dpc (Fig 7B).
Tab. 1. Dysregulation of genes associated with cell cycle and DNA replication whose transcripts are up-regulated by <i>Arid1a</i> ablation. 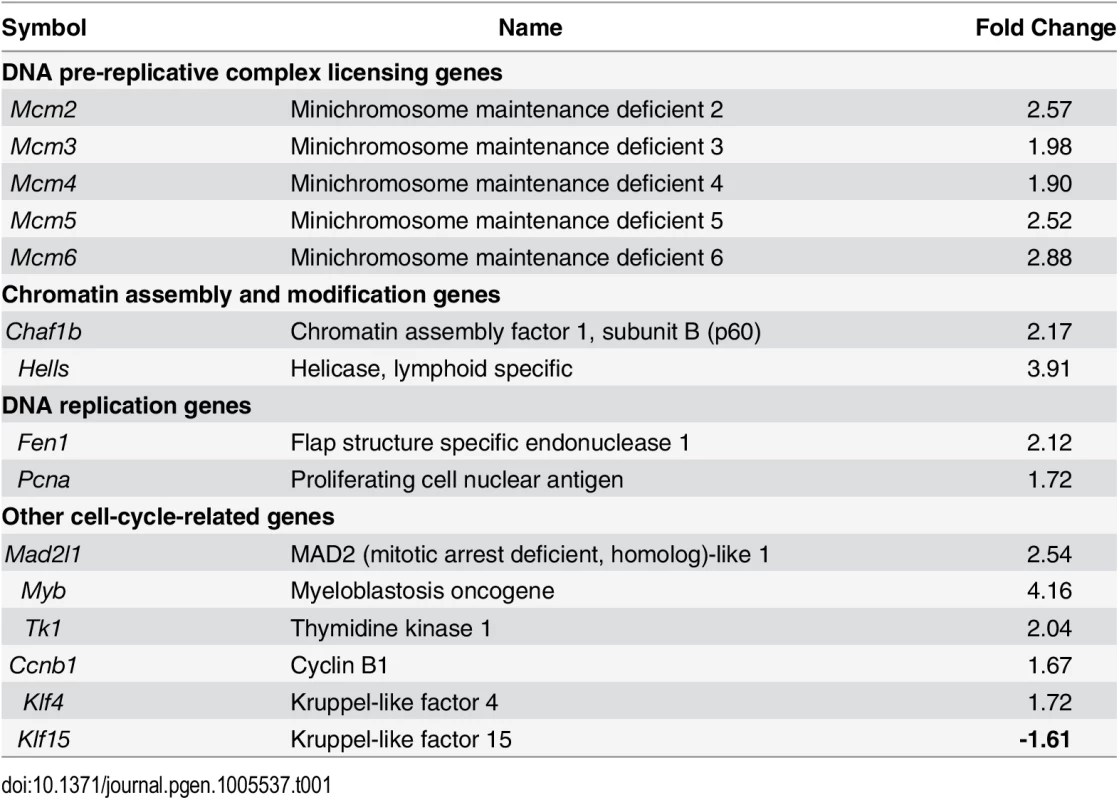
Fig. 7. The confirmation of dysregulated genes by Arid1a ablation. 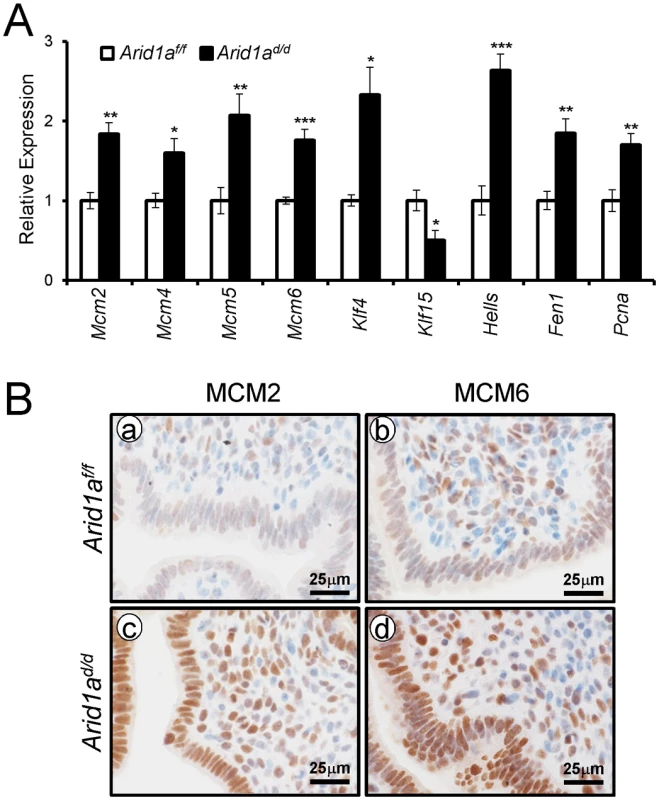
(A) The validation of microarray analysis by qPCR in Arid1af/f and Arid1ad/d mice at 3.5 dpc. The results represent the mean ± SEM. *, p < 0.05, **, p < 0.01, ***, p < 0.001. (B) Immunohistochemical analysis of MCM2 and MCM6 in the uteri of Arid1af/f (a and b) and Arid1ad/d (c and d) mice at 3.5 dpc. Two Kruppel-like factors (KLFs) have been implicated in E2 and P4 modulation of uterine proliferation [40,41]. Klf4 is increased by E2 and promotes DNA replication, whereas Klf15 is increased by P4 and inhibits growth via regulation of Mcm2 [41]. The down-regulation of PGR by ARID1A loss coincides with the down-regulation of Klf15 transcript abundance, which led to the hypothesis that ARID1A positively regulates Klf15 expression with PGR. To determine whether ARID1A and PGR bind to the putative Klf15 promoter, ChIP was performed on uterine chromatin from Arid1af/f and Arid1ad/d mice at 3.5 dpc. ChIP analysis exhibited that recruitment of PGR on HRE is significantly decreased by the absence of Arid1a indicating that klf15 is directly regulated by ARID1A and PGR (Fig 8A). We examined whether ARID1A physically interacts with PR-A or PR-B protein using immunoprecipitation analysis. We transfected with PGR constructs expressing either human PR-A or PR-B into Ishikawa cells. The lysates were then immunoprecipitated with anti-ARID1A antibodies, and then performed western blot analysis using anti-PGR antibodies. The immunoprecipitation results showed that ARID1A physically interacts with PR-A, not PR-B (Fig 8B). Next, we examined the protein levels of KLF4 and KLF15 to determine whether their dys-regulation might contribute to aberrant epithelial proliferation in Arid1ad/d mice. The expression of KLF4 was remarkably increased in Arid1ad/d mice compared to Arid1af/f mice while the expression of KLF15 was decreased in Arid1ad/d mice (Fig 8C). These results suggest that ARID1A regulates transcriptional activation of KLF15 through physical interaction with PR-A.
Fig. 8. ARID1A regulates epithelial proliferation via modulating KLF15 expression with PGR (A) ChIP assay performed with uterine chromatin isolated Arid1af/f and Arid1ad/d mice at 3.5 dpc using IgG, PGR, and ARID1A antibodies followed by qPCR. 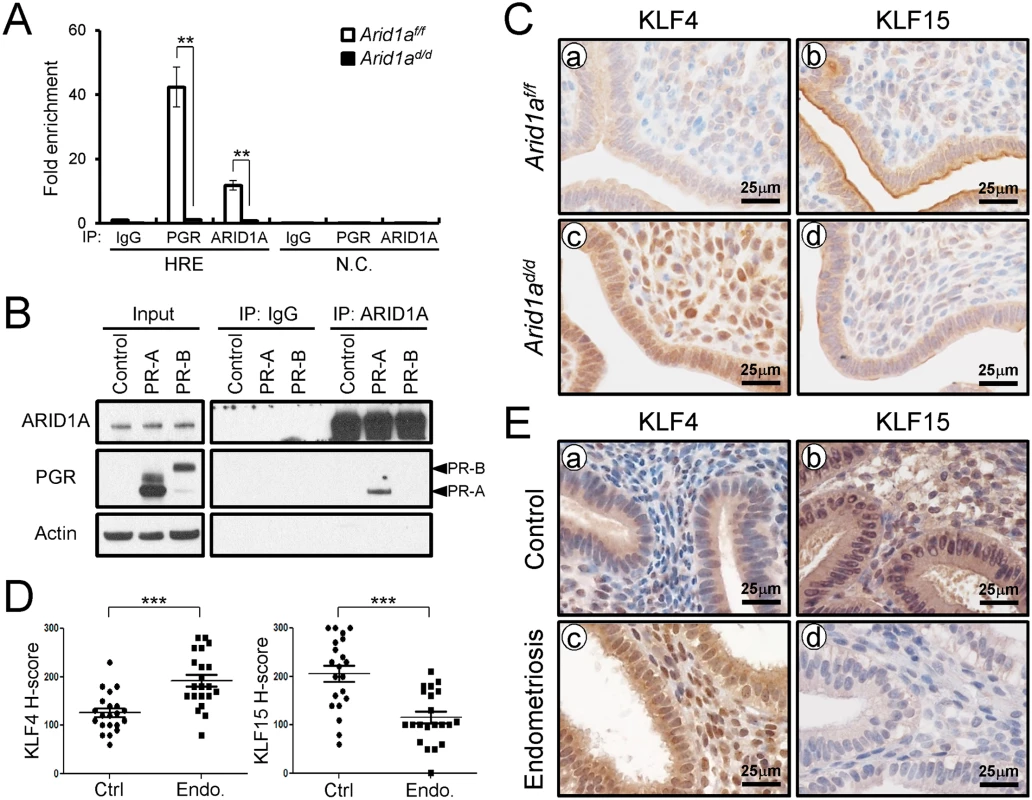
The results represent the mean ± SEM. **, p < 0.01. (B) Protein interaction between ARID1A and PGR was examined by immunoprecipitation and transient transfection in Ishikawa cells. (C) Immunohistochemical analysis of KLF4 and KLF15 were performed on uteri of Arid1af/f (a and b) and Arid1ad/d (c and d) mice at 3.5 dpc. (D) The immunohistochemical histological score (H-score) of KLF4 and KLF15 proteins (n = 21 per group at secretory phase). The results represent the mean ± SEM. *** p<0.001. (E) The level of KLF4 and KLF15 in human endometrium without (a and b) and with (c and d) endometriosis were performed by immunohistochemical analysis. To better understand the integration of ARID1A in endometriosis, immunohistochemistry analysis for KLF4 and KLF15 was performed with eutopic endometrium from secretory phase women with and without endometriosis (Fig 8D and 8E). As shown in the Arid1ad/d mice, eutopic endometrium from women with endometriosis showed increased KLF4 levels compared to control endometrium. The expression of KLF15 was very weak in eutopic endometrium from women with endometriosis, while its expression was strong in endometrial cells in control endometrium. These data suggest that KLF15 is a downstream mediator of the anti-proliferative action of P4 on E2-induced epithelial cell proliferation and ARID1A regulates KLF15 expression with PGR.
Discussion
Somatic ARID1A mutations are uniquely associated with endometriosis-related ovarian neoplasms [42–45]. ARID1A is located within chromosomal region 1p36, a region frequently deleted in a variety of human cancers [46,47]. Indeed, many studies have analyzed ARID1A expression in a variety of human cancers and demonstrated loss of ARID1A expression [43,48–50]. ARID1A was mutated in 46% of ovarian clear-cell carcinomas and 30% of endometrioid ovarian carcinomas [28,31]. Loss of ARID1A is also frequent in endometrial carcinoma [51–53]. Interestingly, Arid1ad/d mice showed aberrant active epithelial proliferation, but did not develop endometrial hyperplasia or cancer. Our results suggest that Arid1a loss alone is not enough to lead to the development of endometrial cancer.
Endometriosis is a common cause of infertility [54]. However, the roles of ARID1A in infertility and endometrial function have not been studied. In the present study, we report that ARID1A protein levels are significantly lower in the eutopic endometrium of women with endometriosis compared to women without endometriosis, and mice with conditional ablation of Arid1a in PGR positive cells (Arid1ad/d) were sterile. These results suggest a relationship between ARID1A loss and infertility. Since PGRCre mice show Cre recombinase activity in the pituitary, ovary, uterus and mammary glands, these mice may have infertility due to a defect of Arid1a in any of these tissues [55]. Arid1ad/d mice had normal ovarian function indicating that the conditional loss of Arid1a in the granulosa cells of the ovary did not influence ovarian function. Although our study does not rule out a pituitary defect, a failure of embryo attachment and decidualization in Arid1ad/d mice suggest that the fertility defect is primarily due to a uterine defect.
Receptivity in the mouse endometrium is dependent on ovarian steroid hormones. On 0.5 and 1.5 dpc, E2 promotes uterine epithelial cell proliferation and growth. On 2.5 dpc, P4 inhibits this epithelial proliferation, promoting receptivity, and inducing stromal cell proliferation [56]. We observed increased proliferation in the epithelium of Arid1ad/d mice at 3.5 dpc indicating enhanced epithelial E2 signaling. It is reported that enhanced epithelial E2 activity leads to implantation failure [57,58]. We also showed that conditional ablation of Arid1a results in elevated levels of phospho-ESR1, the active form of ESR1, and ESR1 target genes, C3, Clca3, Muc-1, and Ltf which plays an essential role in uterine receptivity and embryo attachment [59–61]. These results indicate that the attachment defect observed in Arid1ad/d mice is due to a failure of P4 to squelch E2 signaling in luminal epithelium as indicated by altered expression of MUC-1 and LTF which is tightly regulated at pre-implantation [62,63]. COUP-TF II mediates Bmp2 expression by controlling ESR1 activity in the murine uterus [58], and its expression is promoted by SWI/SNF in vascular endothelium [64]. Thus, we examined the expression of COUP-TF II in Arid1ad/d mice at 3.5 dpc by immunohistochemistry. COUP-TF II immunostaining were not different in uterine stroma cells of Arid1ad/d mice compared to Arid1af/f mice.
Previous studies have shown that PGR has an important role in inhibiting E2 induced epithelial proliferation [65,66]. A decrease of epithelial PGR is observed in Arid1ad/d mice resulting in down-regulated PGR target genes, Fst, Gata2, Areg, and Lrp2 [67]. Gata2, Areg, and Lrp2 localization is limited to the epithelium [68–70]. We examined the expression of Il13ra2 and Hand2 [71] which are known as stromal P4-target genes by RT-qPCR. These mRNA levels were not different between Arid1af/f and Arid1ad/d mice. These data suggest that Arid1a is mainly functional in the epithelial cells at the peri-implantation stage. However, ARID1A is expressed both in epithelium and stroma. It will be useful to ascertain its cell type specific role using epithelium cell specific knockout mouse models [72–74]. Stromal functions including proliferation and the expression of Hand2 and Il13ra2 are not altered at the peri-implantation stage of Arid1ad/d mice. However, its function in stroma cells may play an important role because the phenotypes of COUP-TFII [58] and Hand2 [71] knockout mice have a similar phenotype.
SWI/SNF complexes interact with several nuclear receptors, including glucocorticoid receptors, estrogen receptors and vitamin D3 receptors, to activate transcription of specific target genes [47,75]. Several studies have linked SWI/SNF and ARID1A to transcriptional regulation, particularly nuclear hormone-induced transcription and expression of cell-cycle regulators [76–78]. Our results suggest that ARID1A is pivotal to regulating transcription of PGR target genes to prepare receptivity in the uterus. Loss of ARID1A may have many effects on SWI/SNF complexes that lead to transcriptional dysfunction, including disruption of nucleosome sliding activity, assembly of variant SWI/SNF complexes, targeting to specific genomic loci, and/or recruitment of coactivator/corepressor activities. An impaired P4 response is seen in the endometrium of women with infertility and endometriosis [79–81]. However, molecular mechanisms of aberrant PGR function in uterine diseases remain uncertain. Although this study has not clearly addressed why the epithelium PGR was decreased in the Arid1a knockout uterus, our results show that ARID1A regulates PGR signaling to prepare receptivity in the uterus. ARID1A may regulate stability of PGR proteins. However, it is also possible that PGR is a target gene of ARID1A. Further investigation is required to elucidate the exact mechanism underlying a possible regulatory role of ARID1A in the regulation of steroid hormone signaling.
Interestingly, this Arid1ad/d phenotype is similar to Wnt7a-Cre PGRf/- mice, with epithelial-specific ablation of PGR. Wnt7a-Cre PGRf/- mice were infertile due to defects in embryo attachment, stromal cell decidualization, the inability to cease estrogen-induced epithelial cell proliferation, and the lack of P4 regulated expression of its epithelial target genes [11]. Stromal-epithelial cross talk is critical in pregnancy [11,82] and P4 achieves inhibition of E2-induced epithelial cell proliferation by coordinating stromal-epithelial cross-talk [7,9,10]. The Stromal functions including proliferation and the expression of Hand2 and Il13ra2 in Arid1ad/d mice are not altered at 3.5 dpc. These results suggest that Arid1a is mainly functional in the epithelial cells of the peri-implantation stage. An epithelium cell specific Arid1a knockout mouse model using Wnt7a-cre or Lactoferrin-iCre mouse [72–74] will be an invaluable approach to ascertain its cell type specific role. However, ARID1A is expressed both in epithelium and stroma. Its function in stroma cells may play an important role because the phenotypes of Hand2 [71] and COUP-TFII [58] knockout mice have similar phenotypes. Determining the role of Arid1a in stromal-epithelial cross talk will be critical in understanding the role of steroid hormone signaling and dysfunction associated with infertility and endometriosis.
To investigate the global impact on gene expression caused by the loss of Arid1a, we conducted microarrays at the peri-implantation stage and identified over 2,500 misregulated genes in the absence of Arid1a. Dr. Pollard’s group demonstrated that P4 blocks E2-induced DNA synthesis through the inhibition of replication licensing including MCM proteins [83,84]. There is a significant overlap in the list of genes associated with cell cycle and DNA replication between Dr. Pollard’s and our microarray results. In the uterine epithelium, E2 stimulates the expression of the MCMs while P4 inhibits the transcript abundance of MCM 2 to 6 [83,85]. The immunohistochemistry results showed aberrant overexpression of MCM2 and MCM6 in the epithelial cells of Arid1ad/d mice at the peri-implantation stage. A similar action can be ascribed to P4 and E2 in human endometrial epithelium as a loss of MCM proteins occurs in the secretory phase, and therefore P4 dominated this phase of the menstrual cycle [81,86]. However, aberrant overexpression of MCM2 and MCM6 may cause abnormal epithelial proliferation and early pregnancy loss. Despite the importance of this regulation in mice and humans, the molecular basis for the P4 and E2 regulation of DNA replication licensing is not understood. Our results demonstrate that ARID1A loss results in increased E2 sensitivity of the uterus in the presence of P4.
Kruppel-like factors (KLF) family play important roles in cellular proliferation, survival, differentiation, pluripotency, and epithelial-to-mesenchymal interactions [87]. The members of the KLF family are ubiquitously expressed in the uterus and have been increasingly implicated as critical co-regulators and integrators of steroid hormone actions [88]. The expression of KLF9 is lower in eutopic endometrium of women with endometriosis and endometrial KLF9 deficiency promotes endometriotic lesion establishment by the coincident deregulation of Notch-, Hedgehog-, and steroid receptor-regulated pathways [89–91]. However, the expression of Klf9 is not altered in Arid1ad/d mice.
Klf4 and Klf15 play a critical role in uterine proliferation by modulating E2 and P4 [40,41]. E2 induces Klf4 expression and promotes DNA replication, whereas Klf15 is induced by P4 and inhibits growth via regulation of Mcm2 [41]. Therefore, we focused on transcriptional regulation of Klf15 as an E2 regulated transcription factor. KLF15 binds to the MCM2 promoter in a P4 and E2 dependent fashion, which negatively regulates RNA Pol II association [41]. Klf15 expression suppresses E2 mediated MCM2 transcription. In vivo, Klf15 expression in the E2 exposed uterus mimics P4 action by inhibiting Mcm2 expression and epithelial cell DNA synthesis. ChIP analysis demonstrated that ARID1A and PGR directly bind to the PRE region of Klf15 promoter. Immunohistochemistry analysis showed an increase of KLF4 and a decrease of KLF15 expression in Arid1ad/d mice at the peri-implantation stage. These data establish Klf15 as a downstream mediator of the anti-proliferative action of P4 on E2-induced epithelial cell proliferation and ARID1A regulates E2-induced epithelial proliferation by modulating klf15 expression with PGR.
Following embryo attachment, the uterus again changes during a process known as decidualization whereby the epithelium undergoes apoptosis and the stroma proliferates and differentiates into a more epitheliod cell type [66]. We demonstrated that Arid1ad/d mice exhibited a defect of the decidual response. Bmp2 and Fkbp4 null females exhibited a defect of implantation and decidualization suggesting a critical role in decidualization [92–94]. Fst is a known Bmp2 target [95]. The decidualization markers, Bmp2, Fst, and Fkbp5, were significantly decreased in Arid1ad/d mice indicating that uterine specific ablation of Arid1a caused a significant decidualization defect.
In conclusion, ARID1A has a key role in implantation and decidualization, and that ARID1A expression is lost in endometriosis. Ablation of Arid1a affects epithelial proliferation in part via dysregulating KLF15 expression with PGR. Aberrant proliferative conditions of the human endometrium are common. Inappropriate proliferation of the uterus is one cause leading to endometriosis [96]. Determining the mechanism of Arid1a in uterine dysfunction associated with infertility and endometriosis will be critical to understanding both of these common uterine diseases for future therapy.
Materials and Methods
Ethics statement
The study has been approved by Institutional Review Committee of Michigan State University (IRB number: 07–712; r047700), Greenville Health System (IRB number: Pro00013885 and Pro00000993) and University of North Carolina (IRB number: 05–1757), and written informed consent was obtained from all participants. All protocols related to animals were overseen and approved by the Institutional Animal Care and Use Committee at Michigan State University (AUF number: 11/13-248-00). Animals were maintained in a designated animal care facility in accordance with Michigan State University’s institutional guidelines.
Human endometrium samples
The human endometrial samples were collected from Michigan State University’s Center for Women’s Health Research Female Reproductive Tract Biorepository, the Greenville Hospital System, and the University of North Carolina. Samples were collected as previously reported [97,98]. Briefly, to compare gene expression patterns of eutopic endometrium between those with and without endometriosis, 28 samples were collected from proliferative, early, mid, and late secretory phases (n = 7 per phase). For endometriosis eutopic endometrium, 28 samples were collected from proliferative, early, mid, and late secretory phases (n = 7 per phase). Endometrial biopsies were obtained at the time of surgery from regularly cycling women between the age of 18 and 45. The presence or absence of disease was confirmed during surgery. Women laparoscopically negative for this disease were placed into the control group, whereas women laparoscopically positive for this disease were placed in the endometriosis group. Use of an intrauterine device (IUD) or hormonal therapies in the 3 months preceding surgery was exclusionary for this study. Histologic dating of endometrial samples was done based on the criteria of Noyes [99] and confirmed by subsequent histo-pathological examination by an experienced Fertility specialist (B.A.L.).
Isolation of human primary endometrial stromal cells (hESCs) has been previously described [39]. hESCs were isolated from proliferative phase patients with or without endometriosis. Proteins were extracted using lysis buffer (150 mM NaCl, 0.125% Nonidet P-40 (vol/vol), 2.5 mM EDTA, and 10 mM Tris-HCl (pH 7.4) included with both a phosphatase inhibitor cocktail (Sigma Aldrich, St. Louis, MO) and a protease inhibitor cocktail (Roche, Indianapolis, IN). Twenty μg of protein lysates were electrophoresed via SDS-PAGE and were then transferred onto polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA). Western blot analysis was performed using anti-ARID1A (Abnova, Neihu District, Taipei City, Taiwan) and anti-Actin (Santa Cruz) antibodies.
Animals and tissue collection
Arid1a conditional knockout mice were generated by crossing Pgrcre/+ [55] with Arid1af/f [8] mice (Pgrcre/+Arid1af/f; Arid1ad/d). Pregnant uterine samples were obtained by mating Arid1af/f and Arid1ad/d female mice with C57BL/6 male mice with the morning of a vaginal plug being designated as 0.5 dpc. Mice were sacrificed at 3.5, 4.5 and 5.5 dpc and the number of implantation sites identified on 5.5 dpc. The level of progesterone and estrogen in serum were analyzed by the University of Virginia Center for Research in Reproduction Ligand Core. Uterine tissues were snap-frozen at the time of dissection and either stored at -80°C for RNA/protein extraction or fixed with 4% (vol/vol) paraformaldehyde for histology. For the fertility studies, adult female Arid1af/f and Arid1ad/d female mice were placed with wild-type male mice (n = 9). The mating cages were maintained for 6 months and the number of litters and pups born during that period was recorded. For ovulation and fertilization test, female mice (n = 3 per genotype) were superovulated by i.p. injection of 5 IU of PMSG (Fisher Sci.) followed 48 h later by 5 IU of hCG (Sigma-Aldrich) and mated with wild-type male mice. The following morning (0.5dpc), ovulated eggs were flushed from the oviducts on 1.5 dpc.
Induction of decidualization
The hormonally induced decidual response has been previously described [100]. Briefly, Arid1af/f and Arid1ad/d female mice at 6-weeks of age were ovariectomized (n = 3 per genotype). Two weeks post ovariectomy, Arid1af/f and Arid1ad/d were subjected to the following hormonal regimen: 100 ng of E2 per day for three days; two days rest; then, three daily injections of 1 mg of P4 + 6.7 ng of E2. Six hours following the third P4 and E2 injection, the left uterine horn was mechanically stimulated by scratching the full length of the anti-mesometrial side with a burred needle. The other horn was left unstimulated as a control. Daily injections of P4 (1 mg/mouse) + E2 (6.7 ng/mouse) were continued for five days to maximize the decidual response. Then, mice were sacrificed on day 5. The uteri were then excised, weighed and fixed in 4% paraformaldehyde for histological analysis.
Quantitative real-time PCR
RNA was extracted from the uterine tissues using the RNeasy total RNA isolation kit (Qiagen, Valencia, CA, USA). mRNA expression levels of decidual marker genes (Bmp2, Fst, and Fkbp5), Esr1 target genes (C3, Clca3, Muc-1, and Ltf) and Pgr target genes (Fst, Gata2, Areg, Lrp2, Il13ra2, and Hand2) were measured by real-time PCR TaqMan analysis using an Applied Biosystems StepOnePlus system according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA) and using pre-validated probes, primers, 18S RNA and Universal Master mix reagent purchased from Applied Biosystems (Applied Biosystems). Template cDNA was produced from 1 μg of total RNA using random hexamers and MMLV Reverse Transcriptase (Invitrogen Corp.). All real-time PCR was done by using three independent RNA sets. The mRNA quantities were normalized against 18S RNA using ABI rRNA control reagents.
Immunohistochemistry
Uterine sections from paraffin-embedded tissues were cut at 5 μm and mounted on silane-coated slides, deparaffinized, and rehydrated in a graded alcohol series before blocking with 10% normal goat serum in PBS (pH 7.5) and incubating with primary antibody diluted in 10% normal goat serum in PBS (pH 7.5) overnight at 4°C at the following dilutions: 1 : 500 for anti-ARID1A (Sc-98441, SantaCruz), 1 : 100 for anti-Ki67 (ab15580, Abcam), anti-ESR1 (DAKO Corp.), 1 : 100 for anti-phospho-ESR1 (Ab31477, Abcam), 1 : 1000 for MUC-1 (ab15481, Abcam), 1 : 2000 for LTF (07–682, Millipore), MA), 1 : 20000 for MCM2 (Sc-9839, SantaCruz), 1 : 20000 for MCM6 (Sc-9843, SantaCruz), 1 : 5000 for KLF4 (Sc-20691, SantaCruz), 1 : 5000 for FLK15 (ab2647, Abcam), and 1 : 1000 for anti-total PGR antibody (A0098, DAKO Corp.). On the following day, sections were washed in PBS and incubated with the appropriate species-specific HRP-conjugated secondary antibody (2 μg/ml; Vector Laboratories) for 1 hr at room temperature. Immunoreactivity was detected using the Vectastain Elite DAB kit (Vector Laboratories). A semiquantitative grading system (H-score) was used to compare the immunohistochemical staining intensities as previously described [101]. The number of PGR and Ki67-positive cells was counted in 200 epithelial cells and eight random fields of stromal cells.
Microarray analysis
Biotinylated cRNA were prepared according to the standard Affymetrix protocol from 500ng total RNA (Expression Analysis Technical Manual, 2001, Affymetrix). Following fragmentation, 15 ug of aRNA were hybridized for 16 hr at 45C on GeneChip Mouse Genome Array. GeneChips were washed and stained in the Affymetrix Fluidics Station 450. GeneChips were scanned using the Affymetrix GeneChip Scanner 3000 7G. The data were analyzed with RMA using Affymetrix default analysis settings and global scaling as the normalization method. The trimmed mean target intensity of each array was arbitrarily set to 100. The normalized, and log transformed intensity values were then analyzed using GeneSpring GX 12.6 (Agilent technologies, CA). Fold change filters included the requirement that the genes be present in at least 150% of controls for up-regulated genes and lower than 66% of controls for down-regulated genes. Hierarchical clustering data were clustered groups that behave similarly across experiments using GeneSpring GX 12.6 (Agilent technologies, CA). Clustering algorithm was Euclidean distance, average linkage.
Chromatinimmunoprecipitation (ChIP)
ChIP analysis was conducted by Active Motif (Carlsbad, CA, USA) using frozen mouse uteri of Arid1af/f and Arid1ad/d at 3.5 dpc. Uterine tissue samples (approximately 180 mg) were submersed in PBS containing protease inhibitors, cut into small pieces, and treated with fixation solution for 15 min at room temperature. Fixation was stopped by the addition of stop solution for 5 min. The tissue pieces were washed twice with PBS washing buffer, incubated with Chromatin Prep Buffer containing protease inhibitors and PMSF for 10 min on ice, homogenized by glass homogenizer for 30 strock, and finally spun down. Chromatin was isolated from disrupting the cells with a ChIP buffer containing protease inhibitors and PMSF. Lysates were sonicated using a Sonic Dismembrator FB120 (Fisher Scientific, Pittsburgh, PA, USA) to break chromatin into fragments with an average length of 0.5–1 kb. For each ChIP reaction, 100 μg of chromatin was immunoprecipitated by 4 μg of antibodies against PGR (sc7208; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and ARID1A (H00008289-M02; Abnova, Zhongli District, Taoyuan City 320, Taiwan). Following overnight incubation at 4°C, protein G agarose beads were added, and incubation at 4°C continued for another 3 hours. Immune complexes were washed five times with Wash Buffer AM1, eluted from the beads with Elution Buffer AM4 and subjected to RNase treatment and proteinase K treatment. Crosslinks were reversed by incubation for 30 min at 55°C and for 2 hours at 80°C. ChIP DNA was purified by DNA purification column. Purified DNA was used for real-time qPCR. Real-time qPCR was carried out in triplicate using SYBR Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA). The sequences of the primers used for HRE binding region [40,102] in Klf15 gene were 5’ - TAACCATCTGGGAAGTGGCT-3’ and 5’-GCCACTCTGGAACAGGATG-3’, and for negative control region in Klf15 gene were 5’-TCTCACTCGGGTGTGAAGCC-3’ and 5’-GTGGGAAGCGATGCACTTTG-3’ (S5 Fig). Immunoprecipitation with normal rabbit IgG was performed as a negative control. The resulting signals were normalized to input DNA.
Immunoprecipitation analysis
Ishikawa cells were cultured in DMEM/F12 medium (Gibco, Grand Island, NY) containing 10% fetal bovine serum (FBS; Gibco), and 1% penicillin streptomycin (Gibco) at 37°C under 5% CO2. The cells were transfected with the human PR-A and PR-B expression vectors using Lipofectamine 2000 (Invitrogen Corp.). The transfected cells were lysed by lysis buffer (150 mM NaCl, 0.125% Nonidet P-40 (vol/vol), 2.5 mM EDTA, and 10 mM Tris-HCl (pH 7.4)) included with both a phosphatase inhibitor cocktail (Sigma Aldrich, St. Louis, MO) and a protease inhibitor cocktail (Roche, Indianapolis, IN). Protein lysates were then immunoprecipitated with ARID1A antibodies (Abnova) with protein A-agarose (Pierce Biotechnology, Rockford, IL) and incubated overnight at 4°C. Immunocomplexes were washed 5 times with 1 ml of lysis buffer and were then subjected to western blot analysis using anti-PGR antibody (SantaCruz). The western blot analysis was performed as described previously [103].
Statistical analysis
For data with only two groups, the Student’s t test was used. For data containing more than two groups, one way ANOVA was used, followed by Tukey’s post hoc multiple range. All data are presented as means ± SEM. p < 0.05 was considered statistically significant. All statistical analyses were performed using the Instat package from GraphPad (San Diego, CA, USA).
Supporting Information
Zdroje
1. Eskenazi B, Warner ML (1997) Epidemiology of endometriosis. Obstetrics and gynecology clinics of North America 24 : 235–258. 9163765
2. Villa ML (1994) Endometriosis. The New England journal of medicine 330 : 70.
3. Csapo AI, Pulkkinen M (1978) Indispensability of the human corpus luteum in the maintenance of early pregnancy. Luteectomy evidence. Obstetrical & gynecological survey 33 : 69–81.
4. Prapas Y, Prapas N, Jones EE, Duleba AJ, Olive DL, et al. (1998) The window for embryo transfer in oocyte donation cycles depends on the duration of progesterone therapy. Hum Reprod 13 : 720–723. 9572441
5. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, et al. (1995) Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9 : 2266–2278. 7557380
6. Martin L, Finn CA, Trinder G (1973) Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. J Endocrinol 56 : 133–144. 4683406
7. Huet-Hudson YM, Andrews GK, Dey SK (1989) Cell type-specific localization of c-myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology 125 : 1683–1690. 2667965
8. Martin L, Das RM, Finn CA (1973) The inhibition by progesterone of uterine epithelial proliferation in the mouse. J Endocrinol 57 : 549–554. 4715727
9. Paria BC, Huet-Hudson YM, Dey SK (1993) Blastocyst's state of activity determines the "window" of implantation in the receptive mouse uterus. Proc Natl Acad Sci U S A 90 : 10159–10162. 8234270
10. Clarke CL, Sutherland RL (1990) Progestin regulation of cellular proliferation. Endocr Rev 11 : 266–301. 2114281
11. Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, et al. (2012) Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 26 : 1218–1227.
12. Tan J, Paria BC, Dey SK, Das SK (1999) Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology 140 : 5310–5321. 10537162
13. Kaunitz AM (1998) Injectable depot medroxyprogesterone acetate contraception: an update for U.S. clinicians. Int J Fertil Womens Med 43 : 73–83. 9609206
14. Olive DL, Lindheim SR, Pritts EA (2004) New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol 18 : 319–328. 15157645
15. Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, et al. (2006) Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol 248 : 94–103. 16406281
16. Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, et al. (2003) Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 144 : 2870–2881. 12810542
17. Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, et al. (2007) Gene Expression Analysis of Endometrium Reveals Progesterone Resistance and Candidate Susceptibility Genes in Women with Endometriosis. Endocrinology.
18. Jick SS (1993) Combined estrogen and progesterone use and endometrial cancer. Epidemiology 4 : 384. 8347751
19. Ehrlich CE, Young PC, Stehman FB, Sutton GP, Alford WM (1988) Steroid receptors and clinical outcome in patients with adenocarcinoma of the endometrium. Am J Obstet Gynecol 158 : 796–807. 2966586
20. Hahn HS, Yoon SG, Hong JS, Hong SR, Park SJ, et al. (2009) Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int J Gynecol Cancer 19 : 1068–1073. doi: 10.1111/IGC.0b013e3181aae1fb 19820370
21. Ramirez PT, Frumovitz M, Bodurka DC, Sun CC, Levenback C (2004) Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol 95 : 133–138. 15385122
22. Hoekstra AV, Kim JJ, Keh P, Schink JC (2008) Absence of progesterone receptors in a failed case of fertility-sparing treatment in early endometrial cancer: a case report. J Reprod Med 53 : 869–873. 19097521
23. Kim JJ, Chapman-Davis E (2010) Role of progesterone in endometrial cancer. Semin Reprod Med 28 : 81–90. doi: 10.1055/s-0029-1242998 20104432
24. Kaku T, Yoshikawa H, Tsuda H, Sakamoto A, Fukunaga M, et al. (2001) Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: central pathologic review and treatment outcome. Cancer Lett 167 : 39–48. 11323097
25. Ness RB (2003) Endometriosis and ovarian cancer: thoughts on shared pathophysiology. Am J Obstet Gynecol 189 : 280–294. 12861175
26. Vigano P, Somigliana E, Chiodo I, Abbiati A, Vercellini P (2006) Molecular mechanisms and biological plausibility underlying the malignant transformation of endometriosis: a critical analysis. Human reproduction update 12 : 77–89. 16172112
27. Bulun SE (2009) Endometriosis. The New England journal of medicine 360 : 268–279. doi: 10.1056/NEJMra0804690 19144942
28. Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, et al. (2010) ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med 363 : 1532–1543. doi: 10.1056/NEJMoa1008433 20942669
29. Van Rechem C, Boulay G, Leprince D (2009) HIC1 interacts with a specific subunit of SWI/SNF complexes, ARID1A/BAF250A. Biochemical and biophysical research communications 385 : 586–590. doi: 10.1016/j.bbrc.2009.05.115 19486893
30. Guan B, Wang TL, Shih Ie M (2011) ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer research 71 : 6718–6727. doi: 10.1158/0008-5472.CAN-11-1562 21900401
31. Jones S, Li M, Parsons DW, Zhang X, Wesseling J, et al. (2012) Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Human mutation 33 : 100–103. doi: 10.1002/humu.21633 22009941
32. Huang J, Zhao YL, Li Y, Fletcher JA, Xiao S (2007) Genomic and functional evidence for an ARID1A tumor suppressor role. Genes, chromosomes & cancer 46 : 745–750.
33. Gao X, Tate P, Hu P, Tjian R, Skarnes WC, et al. (2008) ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc Natl Acad Sci U S A 105 : 6656–6661. doi: 10.1073/pnas.0801802105 18448678
34. Lee JH, Kim TH, Oh SJ, Yoo JY, Akira S, et al. (2013) Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB journal: official publication of the Federation of American Societies for Experimental Biology.
35. Finn CA (1971) The biology of decidual cells. Advances in reproductive physiology 5 : 1–26. 4949999
36. Jayatilak PG, Glaser LA, Warshaw ML, Herz Z, Gruber JR, et al. (1984) Relationship between luteinizing hormone and decidual luteotropin in the maintenance of luteal steroidogenesis. Biology of reproduction 31 : 556–564. 6487695
37. Lala PK, Graham CH (1990) Mechanisms of trophoblast invasiveness and their control: the role of proteases and protease inhibitors. Cancer metastasis reviews 9 : 369–379. 2097085
38. Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ (2006) Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertility and sterility 85 : 564–572. 16500320
39. Lee CH, Kim TH, Lee JH, Oh SJ, Yoo JY, et al. (2013) Extracellular signal-regulated kinase 1/2 signaling pathway is required for endometrial decidualization in mice and human. PLoS One 8: e75282. doi: 10.1371/journal.pone.0075282 24086495
40. Hewitt SC, Li L, Grimm SA, Winuthayanon W, Hamilton KJ, et al. (2014) Novel DNA motif binding activity observed in vivo with an estrogen receptor alpha mutant mouse. Mol Endocrinol 28 : 899–911. doi: 10.1210/me.2014-1051 24713037
41. Ray S, Pollard JW (2012) KLF15 negatively regulates estrogen-induced epithelial cell proliferation by inhibition of DNA replication licensing. Proc Natl Acad Sci U S A 109: E1334–1343. doi: 10.1073/pnas.1118515109 22538816
42. Jones S, Wang TL, Kurman RJ, Nakayama K, Velculescu VE, et al. (2012) Low-grade serous carcinomas of the ovary contain very few point mutations. J Pathol 226 : 413–420. doi: 10.1002/path.3967 22102435
43. Guan B, Mao TL, Panuganti PK, Kuhn E, Kurman RJ, et al. (2011) Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. The American journal of surgical pathology 35 : 625–632. doi: 10.1097/PAS.0b013e318212782a 21412130
44. Jones S, Wang TL, Shih Ie M, Mao TL, Nakayama K, et al. (2010) Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 330 : 228–231. doi: 10.1126/science.1196333 20826764
45. Kuhn E, Wu RC, Guan B, Wu G, Zhang J, et al. (2012) Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. Journal of the National Cancer Institute 104 : 1503–1513. doi: 10.1093/jnci/djs345 22923510
46. Kuo KT, Mao TL, Chen X, Feng Y, Nakayama K, et al. (2010) DNA copy numbers profiles in affinity-purified ovarian clear cell carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research 16 : 1997–2008.
47. Wilson BG, Roberts CW (2011) SWI/SNF nucleosome remodellers and cancer. Nature reviews Cancer 11 : 481–492. doi: 10.1038/nrc3068 21654818
48. Lowery WJ, Schildkraut JM, Akushevich L, Bentley R, Marks JR, et al. (2012) Loss of ARID1A-associated protein expression is a frequent event in clear cell and endometrioid ovarian cancers. Int J Gynecol Cancer 22 : 9–14. doi: 10.1097/IGC.0b013e318231f140 22193641
49. Wang DD, Chen YB, Pan K, Wang W, Chen SP, et al. (2012) Decreased expression of the ARID1A gene is associated with poor prognosis in primary gastric cancer. PLoS One 7: e40364. doi: 10.1371/journal.pone.0040364 22808142
50. Samartzis EP, Noske A, Dedes KJ, Fink D, Imesch P (2013) ARID1A mutations and PI3K/AKT pathway alterations in endometriosis and endometriosis-associated ovarian carcinomas. Int J Mol Sci 14 : 18824–18849. doi: 10.3390/ijms140918824 24036443
51. Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, et al. (2013) Integrated genomic characterization of endometrial carcinoma. Nature 497 : 67–73. doi: 10.1038/nature12113 23636398
52. Rahman M, Nakayama K, Rahman MT, Katagiri H, Katagiri A, et al. (2013) Clinicopathologic analysis of loss of AT-rich interactive domain 1A expression in endometrial cancer. Human pathology 44 : 103–109. doi: 10.1016/j.humpath.2012.04.021 22939958
53. Wiegand KC, Lee AF, Al-Agha OM, Chow C, Kalloger SE, et al. (2011) Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol 224 : 328–333. doi: 10.1002/path.2911 21590771
54. Bulun SE (2009) Endometriosis. The New England journal of medicine 360 : 268–279. doi: 10.1056/NEJMra0804690 19144942
55. Soyal SM, Mukherjee A, Lee KY, Li J, Li H, et al. (2005) Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41 : 58–66. 15682389
56. Tibbetts TA, Mendoza-Meneses M, O'Malley BW, Conneely OM (1998) Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol Reprod 59 : 1143–1152. 9780321
57. Lee K, Jeong J, Kwak I, Yu CT, Lanske B, et al. (2006) Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nature genetics 38 : 1204–1209. 16951680
58. Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, et al. (2007) COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS genetics 3: e102. 17590085
59. Braga VM, Gendler SJ (1993) Modulation of Muc-1 mucin expression in the mouse uterus during the estrus cycle, early pregnancy and placentation. Journal of cell science 105 (Pt 2): 397–405. 7691839
60. Jeong JW, Lee KY, Lydon JP, DeMayo FJ (2006) Steroid hormone regulation of Clca3 expression in the murine uterus. The Journal of endocrinology 189 : 473–484. 16731779
61. Sundstrom SA, Komm BS, Ponce-de-Leon H, Yi Z, Teuscher C, et al. (1989) Estrogen regulation of tissue-specific expression of complement C3. The Journal of biological chemistry 264 : 16941–16947. 2674144
62. Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, et al. (1995) Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology 136 : 3639–3647. 7628404
63. Ward PP, Mendoza-Meneses M, Mulac-Jericevic B, Cunningham GA, Saucedo-Cardenas O, et al. (1999) Restricted spatiotemporal expression of lactoferrin during murine embryonic development. Endocrinology 140 : 1852–1860. 10098524
64. Davis RB, Curtis CD, Griffin CT (2013) BRG1 promotes COUP-TFII expression and venous specification during embryonic vascular development. Development 140 : 1272–1281. doi: 10.1242/dev.087379 23406903
65. Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ (2008) In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol 19 : 178–186. doi: 10.1016/j.semcdb.2007.12.001 18280760
66. Lee KY, DeMayo FJ (2004) Animal models of implantation. Reproduction 128 : 679–695. 15579585
67. Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, et al. (2005) Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 146 : 3490–3505. 15845616
68. Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, et al. (1995) Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol 9 : 691–705. 8592515
69. Rubel CA, Franco HL, Jeong JW, Lydon JP, DeMayo FJ (2012) GATA2 is expressed at critical times in the mouse uterus during pregnancy. Gene expression patterns: GEP 12 : 196–203. doi: 10.1016/j.gep.2012.03.004 22476030
70. Oh SJ, Kim TH, Lim JM, Jeong JW (2013) Progesterone induces expression of Lrp2 in the murine uterus. Biochemical and biophysical research communications 441 : 175–179. doi: 10.1016/j.bbrc.2013.10.037 24140060
71. Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, et al. (2011) The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 331 : 912–916. doi: 10.1126/science.1197454 21330545
72. Daikoku T, Ogawa Y, Terakawa J, Ogawa A, DeFalco T, et al. (2014) Lactoferrin-iCre: a new mouse line to study uterine epithelial gene function. Endocrinology 155 : 2718–2724. doi: 10.1210/en.2014-1265 24823394
73. Contreras CM, Akbay EA, Gallardo TD, Haynie JM, Sharma S, et al. (2010) Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Disease models & mechanisms 3 : 181–193.
74. Kim TH, Lee DK, Cho SN, Orvis GD, Behringer RR, et al. (2013) Critical tumor suppressor function mediated by epithelial Mig-6 in endometrial cancer. Cancer research 73 : 5090–5099. doi: 10.1158/0008-5472.CAN-13-0241 23811943
75. Trotter KW, Archer TK (2008) The BRG1 transcriptional coregulator. Nuclear receptor signaling 6: e004. doi: 10.1621/nrs.06004 18301784
76. Inoue H, Giannakopoulos S, Parkhurst CN, Matsumura T, Kono EA, et al. (2011) Target genes of the largest human SWI/SNF complex subunit control cell growth. The Biochemical journal 434 : 83–92. doi: 10.1042/BJ20101358 21118156
77. Nagl NG Jr., Wang X, Patsialou A, Van Scoy M, Moran E (2007) Distinct mammalian SWI/SNF chromatin remodeling complexes with opposing roles in cell-cycle control. The EMBO journal 26 : 752–763. 17255939
78. Nagl NG Jr., Zweitzig DR, Thimmapaya B, Beck GR Jr., Moran E (2006) The c-myc gene is a direct target of mammalian SWI/SNF-related complexes during differentiation-associated cell cycle arrest. Cancer research 66 : 1289–1293. 16452181
79. Tangen IL, Werner HM, Berg A, Halle MK, Kusonmano K, et al. (2014) Loss of progesterone receptor links to high proliferation and increases from primary to metastatic endometrial cancer lesions. European journal of cancer 50 : 3003–3010. doi: 10.1016/j.ejca.2014.09.003 25281525
80. Wetendorf M, DeMayo FJ (2014) Progesterone receptor signaling in the initiation of pregnancy and preservation of a healthy uterus. The International journal of developmental biology 58 : 95–106. doi: 10.1387/ijdb.140069mw 25023675
81. Lessey BA, Young SL (2014) Homeostasis imbalance in the endometrium of women with implantation defects: the role of estrogen and progesterone. Semin Reprod Med 32 : 365–375. doi: 10.1055/s-0034-1376355 24959818
82. Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS (2010) Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A 107 : 19272–19277. doi: 10.1073/pnas.1013226107 20974921
83. Pan H, Deng Y, Pollard JW (2006) Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc Natl Acad Sci U S A 103 : 14021–14026. 16966611
84. Tong W, Pollard JW (1999) Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E - and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol 19 : 2251–2264. 10022912
85. Pan H, Zhu L, Deng Y, Pollard JW (2006) Microarray analysis of uterine epithelial gene expression during the implantation window in the mouse. Endocrinology 147 : 4904–4916. 16794013
86. Niklaus AL, Aubuchon M, Zapantis G, Li P, Qian H, et al. (2007) Assessment of the proliferative status of epithelial cell types in the endometrium of young and menopausal transition women. Human reproduction 22 : 1778–1788. 17371803
87. Suske G, Bruford E, Philipsen S (2005) Mammalian SP/KLF transcription factors: bring in the family. Genomics 85 : 551–556. 15820306
88. Simmen RC, Heard ME, Simmen AM, Montales MT, Marji M, et al. (2015) The Kruppel-like factors in female reproductive system pathologies. Journal of molecular endocrinology 54: R89–R101. doi: 10.1530/JME-14-0310 25654975
89. Heard ME, Simmons CD, Simmen FA, Simmen RC (2014) Kruppel-like factor 9 deficiency in uterine endometrial cells promotes ectopic lesion establishment associated with activated notch and hedgehog signaling in a mouse model of endometriosis. Endocrinology 155 : 1532–1546. doi: 10.1210/en.2013-1947 24476135
90. Pabona JM, Simmen FA, Nikiforov MA, Zhuang D, Shankar K, et al. (2012) Kruppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis. The Journal of clinical endocrinology and metabolism 97: E376–392. doi: 10.1210/jc.2011-2562 22259059
91. Velarde MC, Geng Y, Eason RR, Simmen FA, Simmen RC (2005) Null mutation of Kruppel-like factor9/basic transcription element binding protein-1 alters peri-implantation uterine development in mice. Biology of reproduction 73 : 472–481. 15917344
92. Lee KY, Jeong JW, Wang J, Ma L, Martin JF, et al. (2007) Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol 27 : 5468–5478. 17515606
93. Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, et al. (2006) FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Molecular endocrinology 20 : 2682–2694. 16873445
94. Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, et al. (2005) Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A 102 : 14326–14331. 16176985
95. Kearns AE, Demay MB (2000) BMP-2 induces the expression of activin betaA and follistatin in vitro. Journal of cellular biochemistry 79 : 80–88. 10906757
96. Korhonen MO, Symons JP, Hyde BM, Rowan JP, Wilborn WH (1997) Histologic classification and pathologic findings for endometrial biopsy specimens obtained from 2964 perimenopausal and postmenopausal women undergoing screening for continuous hormones as replacement therapy (CHART 2 Study). American journal of obstetrics and gynecology 176 : 377–380. 9065185
97. Kim BG, Yoo JY, Kim TH, Shin JH, Langenheim JF, et al. (2015) Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod 30 : 1069–1078. doi: 10.1093/humrep/dev050 25750101
98. Yoo JY, Shin H, Kim TH, Choi WS, Ferguson SD, et al. (2014) CRISPLD2 is a target of progesterone receptor and its expression is decreased in women with endometriosis. PloS one 9: e100481. doi: 10.1371/journal.pone.0100481 24955763
99. Noyes RW, Hertig AT, Rock J (1975) Dating the endometrial biopsy. Am J Obstet Gynecol 122 : 262–263. 1155504
100. Finn CA, Martin L (1972) Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biology of reproduction 7 : 82–86. 5050152
101. Ishibashi H, Suzuki T, Suzuki S, Moriya T, Kaneko C, et al. (2003) Sex steroid hormone receptors in human thymoma. The Journal of clinical endocrinology and metabolism 88 : 2309–2317. 12727990
102. Mazur EC, Vasquez YM, Li X, Kommagani R, Jiang L, et al. (2015) Progesterone receptor transcriptome and cistrome in decidualized human endometrial stromal cells. Endocrinology 156 : 2239–2253. doi: 10.1210/en.2014-1566 25781565
103. Kim TH, Yoo JY, Kim HI, Gilbert J, Ku BJ, et al. (2014) Mig-6 suppresses endometrial cancer associated with Pten deficiency and ERK activation. Cancer Res 74 : 7371–7382. doi: 10.1158/0008-5472.CAN-14-0794 25377472
Štítky
Genetika Reprodukčná medicína
Článek The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis inČlánek Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Retraction: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Signaling from Within: Endocytic Trafficking of the Robo Receptor Is Required for Midline Axon Repulsion
- A Splice Region Variant in Lowers Non-high Density Lipoprotein Cholesterol and Protects against Coronary Artery Disease
- The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis in
- A NIMA-Related Kinase Suppresses the Flagellar Instability Associated with the Loss of Multiple Axonemal Structures
- Slit-Dependent Endocytic Trafficking of the Robo Receptor Is Required for Son of Sevenless Recruitment and Midline Axon Repulsion
- Expression of Concern: Protein Under-Wrapping Causes Dosage Sensitivity and Decreases Gene Duplicability
- Mutagenesis by AID: Being in the Right Place at the Right Time
- Identification of as a Genetic Modifier That Regulates the Global Orientation of Mammalian Hair Follicles
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- Evaluating the Performance of Fine-Mapping Strategies at Common Variant GWAS Loci
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- Differential Expression of Ecdysone Receptor Leads to Variation in Phenotypic Plasticity across Serial Homologs
- Receptor Polymorphism and Genomic Structure Interact to Shape Bitter Taste Perception
- Cognitive Function Related to the Gene Acquired from an LTR Retrotransposon in Eutherians
- Critical Function of γH2A in S-Phase
- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
- Integration of Genome-Wide SNP Data and Gene-Expression Profiles Reveals Six Novel Loci and Regulatory Mechanisms for Amino Acids and Acylcarnitines in Whole Blood
- A Genome-Wide Association Study of a Biomarker of Nicotine Metabolism
- Cell Cycle Regulates Nuclear Stability of AID and Determines the Cellular Response to AID
- A Genome-Wide Association Analysis Reveals Epistatic Cancellation of Additive Genetic Variance for Root Length in
- Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
- RAB-10-Dependent Membrane Transport Is Required for Dendrite Arborization
- Basolateral Endocytic Recycling Requires RAB-10 and AMPH-1 Mediated Recruitment of RAB-5 GAP TBC-2 to Endosomes
- Dynamic Contacts of U2, RES, Cwc25, Prp8 and Prp45 Proteins with the Pre-mRNA Branch-Site and 3' Splice Site during Catalytic Activation and Step 1 Catalysis in Yeast Spliceosomes
- ARID1A Is Essential for Endometrial Function during Early Pregnancy
- Predicting Carriers of Ongoing Selective Sweeps without Knowledge of the Favored Allele
- An Interaction between RRP6 and SU(VAR)3-9 Targets RRP6 to Heterochromatin and Contributes to Heterochromatin Maintenance in
- Photoreceptor Specificity in the Light-Induced and COP1-Mediated Rapid Degradation of the Repressor of Photomorphogenesis SPA2 in Arabidopsis
- Autophosphorylation of the Bacterial Tyrosine-Kinase CpsD Connects Capsule Synthesis with the Cell Cycle in
- Multimer Formation Explains Allelic Suppression of PRDM9 Recombination Hotspots
- Rescheduling Behavioral Subunits of a Fixed Action Pattern by Genetic Manipulation of Peptidergic Signaling
- A Gene Regulatory Program for Meiotic Prophase in the Fetal Ovary
- Cell-Autonomous Gβ Signaling Defines Neuron-Specific Steady State Serotonin Synthesis in
- Discovering Genetic Interactions in Large-Scale Association Studies by Stage-wise Likelihood Ratio Tests
- The RCC1 Family Protein TCF1 Regulates Freezing Tolerance and Cold Acclimation through Modulating Lignin Biosynthesis
- The AMPK, Snf1, Negatively Regulates the Hog1 MAPK Pathway in ER Stress Response
- The Parkinson’s Disease-Associated Protein Kinase LRRK2 Modulates Notch Signaling through the Endosomal Pathway
- Multicopy Single-Stranded DNA Directs Intestinal Colonization of Enteric Pathogens
- Recurrent Domestication by Lepidoptera of Genes from Their Parasites Mediated by Bracoviruses
- Three Different Pathways Prevent Chromosome Segregation in the Presence of DNA Damage or Replication Stress in Budding Yeast
- Identification of Four Mouse Diabetes Candidate Genes Altering β-Cell Proliferation
- The Intolerance of Regulatory Sequence to Genetic Variation Predicts Gene Dosage Sensitivity
- Synergistic and Dose-Controlled Regulation of Cellulase Gene Expression in
- Genome Sequence and Transcriptome Analyses of : Metabolic Tools for Enhanced Algal Fitness in the Prominent Order Prymnesiales (Haptophyceae)
- Ty3 Retrotransposon Hijacks Mating Yeast RNA Processing Bodies to Infect New Genomes
- FUS Interacts with HSP60 to Promote Mitochondrial Damage
- Point Mutations in Centromeric Histone Induce Post-zygotic Incompatibility and Uniparental Inheritance
- Genome-Wide Association Study with Targeted and Non-targeted NMR Metabolomics Identifies 15 Novel Loci of Urinary Human Metabolic Individuality
- Outer Hair Cell Lateral Wall Structure Constrains the Mobility of Plasma Membrane Proteins
- A Large-Scale Functional Analysis of Putative Target Genes of Mating-Type Loci Provides Insight into the Regulation of Sexual Development of the Cereal Pathogen
- A Genetic Selection for Mutants Reveals an Interaction between DNA Polymerase IV and the Replicative Polymerase That Is Required for Translesion Synthesis
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



