-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Genome-Wide Association Study with Targeted and Non-targeted NMR Metabolomics Identifies 15 Novel Loci of Urinary Human Metabolic Individuality
Human metabolism is influenced by genetic and environmental factors defining a person’s metabolic individuality. This individuality is linked to personal differences in the ability to react on metabolic challenges and in the susceptibility to specific diseases. By investigating how common variants in genetic regions (loci) affect individual blood metabolite levels, the substantial contribution of genetic inheritance to metabolic individuality has been demonstrated previously. Meanwhile, more than 150 loci influencing metabolic homeostasis in blood are known. Here we shift the focus to genetic variants that modulate urinary metabolite excretion, for which only 11 loci were reported so far. In the largest genetic study on urinary metabolites to date, we identified 15 additional loci. Most of the 26 loci also affect blood metabolite levels. This shows that the metabolic individuality seen in blood is also reflected in urine, which is expected when urine is regarded as “diluted blood”. Nonetheless, we also found loci that appear to primarily influence metabolite excretion. For instance, we identified genetic variants near a gene of a transporter that change the capability for renal re-absorption of the transporter’s substrate. Thus, our findings could help to elucidate molecular mechanisms influencing kidney function and the body’s detoxification capabilities.
Published in the journal: Genome-Wide Association Study with Targeted and Non-targeted NMR Metabolomics Identifies 15 Novel Loci of Urinary Human Metabolic Individuality. PLoS Genet 11(9): e32767. doi:10.1371/journal.pgen.1005487
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005487Summary
Human metabolism is influenced by genetic and environmental factors defining a person’s metabolic individuality. This individuality is linked to personal differences in the ability to react on metabolic challenges and in the susceptibility to specific diseases. By investigating how common variants in genetic regions (loci) affect individual blood metabolite levels, the substantial contribution of genetic inheritance to metabolic individuality has been demonstrated previously. Meanwhile, more than 150 loci influencing metabolic homeostasis in blood are known. Here we shift the focus to genetic variants that modulate urinary metabolite excretion, for which only 11 loci were reported so far. In the largest genetic study on urinary metabolites to date, we identified 15 additional loci. Most of the 26 loci also affect blood metabolite levels. This shows that the metabolic individuality seen in blood is also reflected in urine, which is expected when urine is regarded as “diluted blood”. Nonetheless, we also found loci that appear to primarily influence metabolite excretion. For instance, we identified genetic variants near a gene of a transporter that change the capability for renal re-absorption of the transporter’s substrate. Thus, our findings could help to elucidate molecular mechanisms influencing kidney function and the body’s detoxification capabilities.
Introduction
Genome-wide association studies with metabolic traits (mGWAS) investigate the relationship between genetic variance and metabolic phenotypes (metabotypes). In 2008, Gieger et al. presented the first mGWAS in serum of 284 individuals [1]. Since then, numerous mGWAS using different analytical platforms and ever larger study populations were published [2–8]. These studies discovered more than 150 genetic loci that associate with blood levels of more than 300 distinct metabolites. We refer to these loci as the genetically influenced metabotypes (GIMs), their ensemble defining the genetic part of human metabolic individuality. Many of the single nucleotide polymorphisms (SNPs) that associate with metabolic traits map to genetic regions coding for enzymes or metabolite transporters that are biochemically linked to the associated metabolites. Moreover, a large number of these GIMs have been previously linked to clinically relevant phenotypic traits. As intermediate traits on the pathways of many disorders, these GIMs have become valuable tools that allow unraveling disease mechanisms on the molecular level [9].
However, so far mGWAS have mostly been limited to studies of serum or plasma metabolite levels, thereby focusing on genetically influenced metabolic homeostasis in blood. Only a few studies investigated urine as a complementary body fluid enabling studies of kidney function and the detoxification capabilities of the human body. In 2011, we published the first mGWAS in urine [10] using proton nuclear magnetic resonance spectroscopy (1H NMR) to determine metabolite concentrations in urine of 862 male participants of the SHIP-0 cohort. We identified five genetic loci (SLC6A20, AGXT2, NAT2, HPD, and SLC7A9) that modulate urinary metabolite levels. While for this study metabolite concentrations were manually derived from the NMR spectra for a targeted set of metabolites, Nicholson et al. [5] directly used spectral features as abstract, non-targeted urinary metabolic traits in an mGWAS. Based on data for 211 participants of the MolTWIN and MolOBB studies, the authors identified SNPs at three loci (ALMS1/NAT8, AGXT2, and PYROXD2) that were associated with metabolic traits in urine. Two of these loci (ALMS1/NAT8 and PYROXD2) were replicated in an NMR-based mGWAS published by Montoliu et al. For that study, the authors analyzed non-targeted urinary traits from 265 subjects from the São Paolo metropolitan area [11]. Recently, Rueedi et al. [12] reported significant associations of NMR-derived non-targeted urinary traits in ten loci (ALMS1/NAT8, ACADL, AGXT2, NAT2, ABO, PYROXD2, ACADS, PSMD9, SLC7A9, and FUT2) using data from 835 participants of the CoLaus study, thus bringing the total number of reported urinary GIMs to eleven.
Here, we substantially extend our previous mGWAS with metabolic traits in urine, both in size and in scope. First, we metabolically characterize the urine samples of 3,861 male and female participants of the SHIP-0 study, thereby quadrupling the sample size when compared to previous studies. Second, we combine both targeted and non-targeted NMR-based metabolomics. In this way, we implement the approaches used in the studies by Nicholson et al., Montoliu et al., and Rueedi et al. alongside the targeted metabolomics approach used in our previous study. For an unbiased interpretation of our mGWAS results, we apply tools for evidence-based locus-to-gene mapping and automated assignment of metabolites to non-targeted NMR spectral features. Finally, besides determining the overlap of variants identified in our study with variants previously linked to clinical traits, we specifically investigate the overlap between variants influencing metabolic traits in both urine and blood.
Results
Our study is based on one-dimensional 1H NMR spectra of urine samples from 3,861 genotyped participants in the SHIP-0 cohort (see Methods). For the targeted metabolomics analysis, we manually quantified a set of 60 metabolites in these spectra (Fig 1A). For the non-targeted analysis, we used the same spectra and applied an automated processing algorithm to align the spectra and to perform dimensionality reduction [13]. In the subsequent analysis, we screened the targeted and the non-targeted metabolic traits as well as the pairwise ratios within each trait type for associations with genotyped and imputed variants in a two-step approach (Fig 1B). We identified a total of 23 genetic loci that display significant associations with targeted and/or non-targeted metabolic traits (Fig 2, Tables 1 and 2). All but one of the discovered loci replicated in data from the KORA F4 cohort (N = 1,691). For 15 loci, our study is, to the best of our knowledge, the first to report associations with urinary traits. For 7 of these 15 loci, associations have previously been reported with blood metabolites. Thus, 8 loci are entirely new (Fig 3). Finally, 11 of the 22 replicated loci host significantly associated variants that were previously associated with phenotypes of clinical relevance (Table 3).
Fig. 1. Study design. 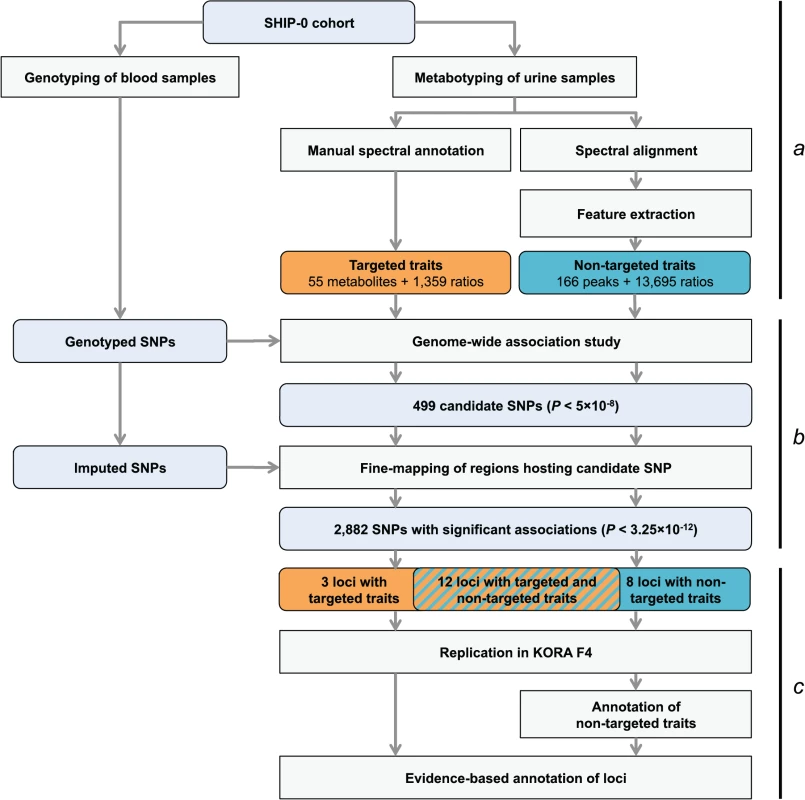
(a) Genotyping and metabotyping of 3,861 SHIP-0 study participants. One-dimensional 1H NMR spectra of the urine samples were recorded to derive targeted and non-targeted metabolic traits. (b) Two-staged mGWAS. First stage: genome-wide association tests using genotyped SNPs and 15,379 targeted and non-targeted traits. Second stage: fine mapping of regions with potentially significant associations using imputed SNPs. (c) Replication and interpretation. Genome-wide significantly associated SNPs were assigned to one of 23 distinct genetic loci. The loci and the significantly associated non-targeted traits were annotated using algorithmic approaches. 22 of the 23 loci could be replicated using genotype and metabotype data from 1,691 KORA F4 participants. Fig. 2. Manhattan plot of genetic associations to targeted and non-targeted traits. 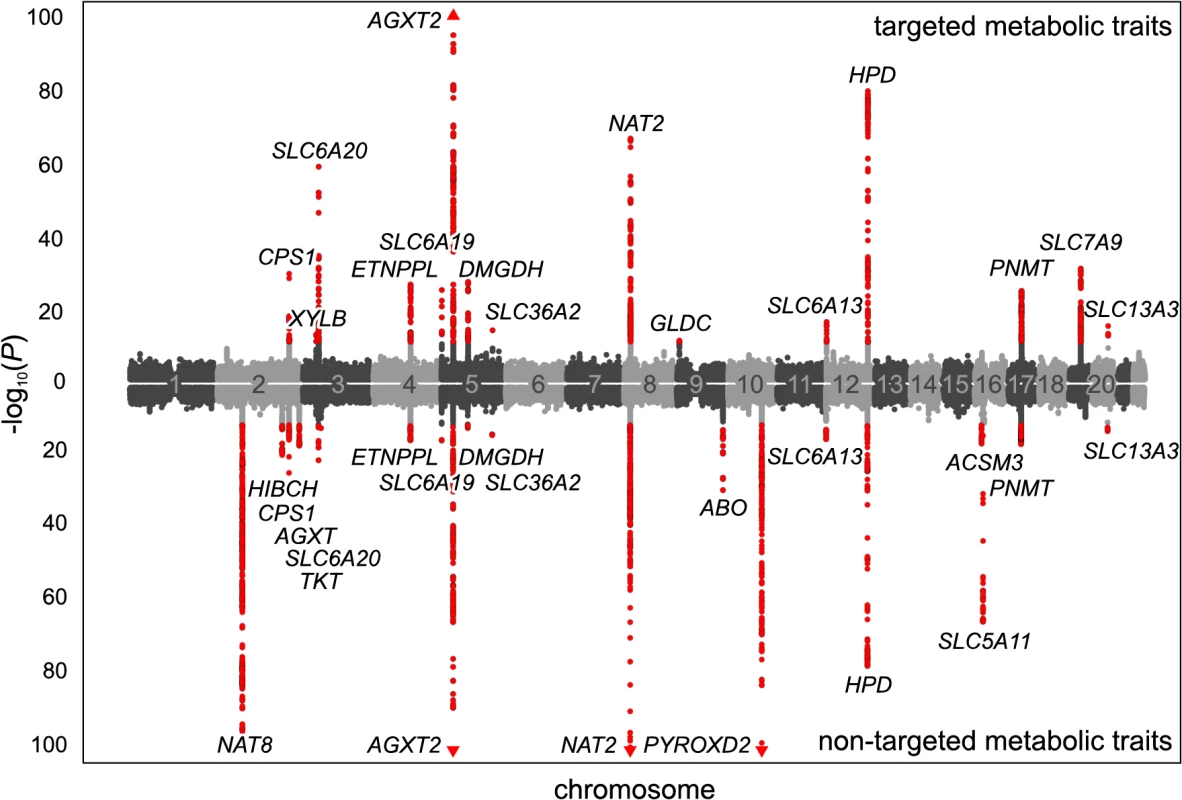
SNPs are plotted according to chromosomal location and the-log10 transformed P-value of the strongest association with targeted traits (top) and non-targeted traits (bottom). In case of associations with ratios, only associations with P-gain exceeding 15,180 (targeted metabolic traits) or 138,610 (non-targeted traits) were considered. Associations of genome-wide significance (P < 3.25×10−12) are plotted in red. Triangles indicate associations with P < 1.0×10−100. Significant associations within a physical distance of 1 Mb were assigned to a locus labeled after the most likely causative gene (as determined using an evidence-based approach for the identification of candidate genes; see Methods). Fig. 3. Loci with associated urinary metabolic traits and their overlap with previous mGWAS in blood and urine. 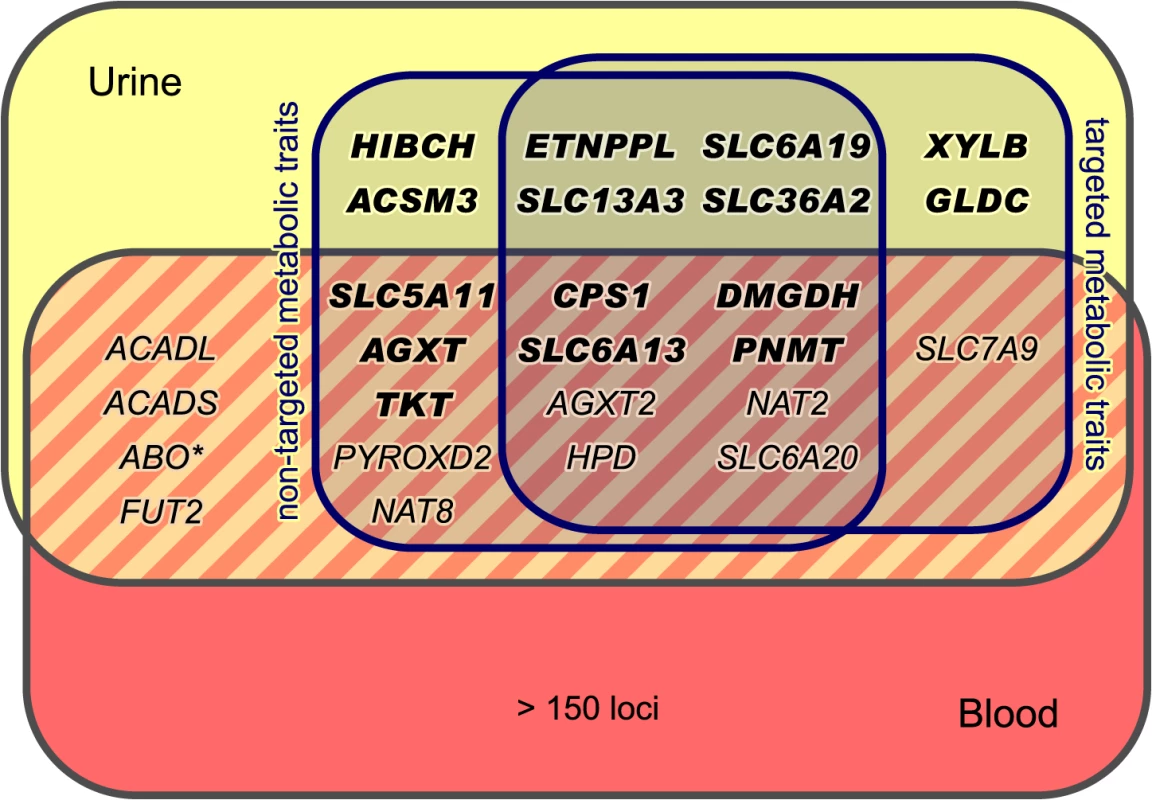
We identified and replicated genome-wide significant associations between metabolic traits and genetic variants in 22 genetic loci (named after the most likely causative gene). Three loci could only be identified using targeted metabolic traits, while 7 loci were exclusively discovered with non-targeted traits. 12 loci were identified using both targeted and non-targeted approaches. Loci with hitherto unknown associations with urinary metabolic traits are highlighted (totaling 15). We identified and replicated significant associations in 7 of the 11 loci that were reported in previous mGWAS in urine [5, 10–12]. We also discovered significant associations of the ABO locus (marked with an asterisk) with non-targeted traits, but this locus could not be replicated in KORA F4. When compared to previous mGWAS in blood, we find 14 loci that display associations with metabolic traits in both urine and blood [1, 4, 6–8, 14–24]. Tab. 1. Fifteen genetic loci as discovered in the SHIP-0 data set and their most significant associations to targeted metabolic traits. 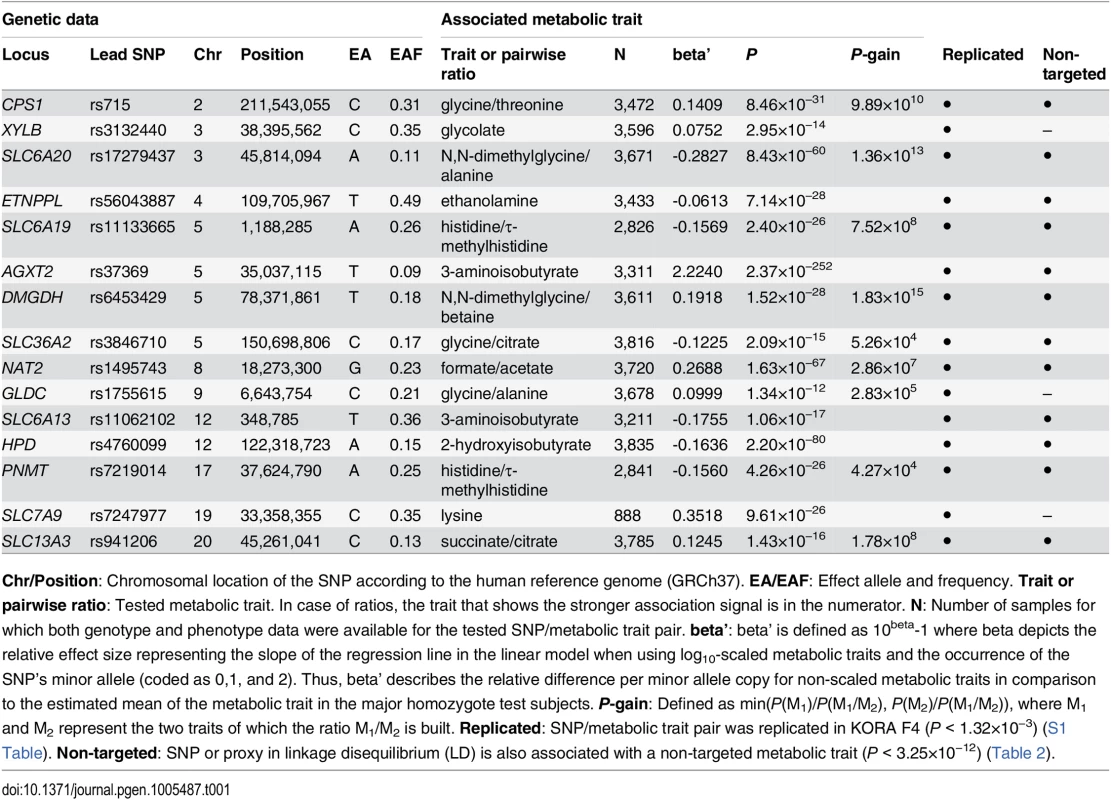
Chr/Position: Chromosomal location of the SNP according to the human reference genome (GRCh37). EA/EAF: Effect allele and frequency. Trait or pairwise ratio: Tested metabolic trait. In case of ratios, the trait that shows the stronger association signal is in the numerator. N: Number of samples for which both genotype and phenotype data were available for the tested SNP/metabolic trait pair. beta’: beta’ is defined as 10beta-1 where beta depicts the relative effect size representing the slope of the regression line in the linear model when using log10-scaled metabolic traits and the occurrence of the SNP’s minor allele (coded as 0,1, and 2). Thus, beta’ describes the relative difference per minor allele copy for non-scaled metabolic traits in comparison to the estimated mean of the metabolic trait in the major homozygote test subjects. P-gain: Defined as min(P(M1)/P(M1/M2), P(M2)/P(M1/M2)), where M1 and M2 represent the two traits of which the ratio M1/M2 is built. Replicated: SNP/metabolic trait pair was replicated in KORA F4 (P < 1.32×10−3) (S1 Table). Non-targeted: SNP or proxy in linkage disequilibrium (LD) is also associated with a non-targeted metabolic trait (P < 3.25×10−12) (Table 2). Tab. 2. Twenty genetic loci as discovered in the SHIP-0 data set and their most significant associations to non-targeted metabolic traits. 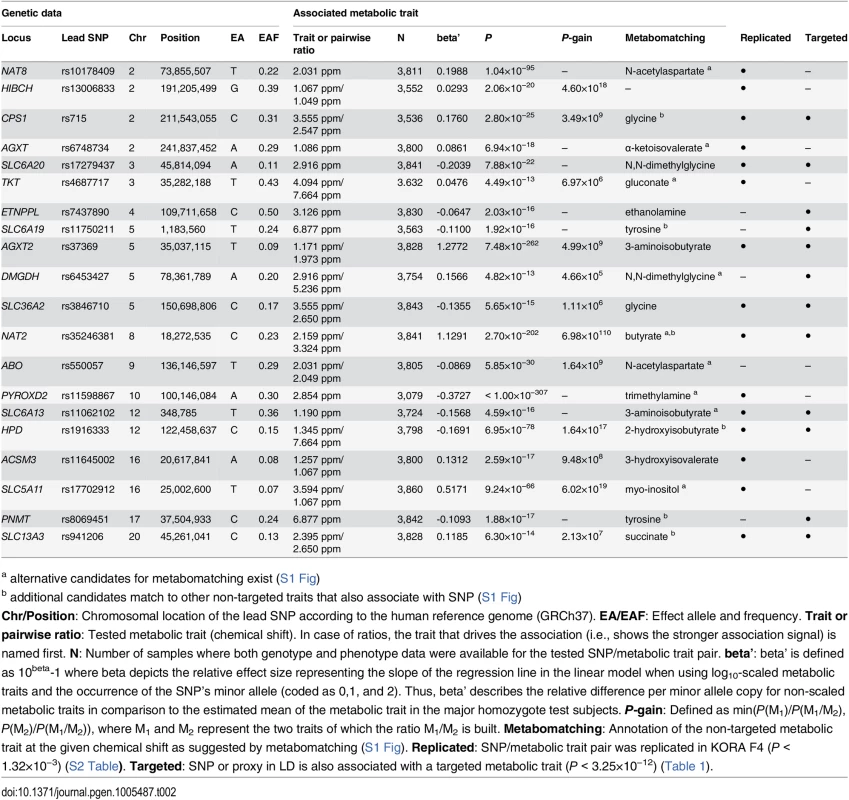
a alternative candidates for metabomatching exist (S1 Fig) Tab. 3. Twenty-two identified and replicated loci and their overlap with associations to metabolic traits and clinical phenotypes. 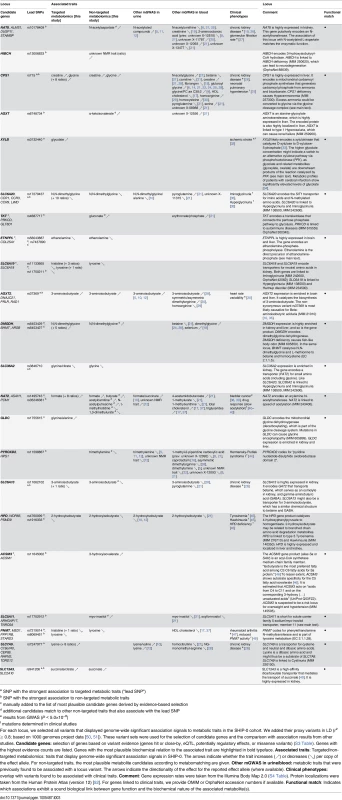
a SNP with the strongest association to targeted metabolic traits (“lead SNP”) mGWAS with targeted and non-targeted NMR features
For the targeted metabolomics analysis, the 1H NMR spectra were manually annotated to derive absolute metabolite concentrations (out of a panel of 60 compounds) for each sample. For the non-targeted analysis, the same spectra were automatically aligned and processed using the FOCUS package [13]. This resulted in NMR signal intensities at 166 distinct spectral positions (“chemical shifts”) per sample (see Methods). In previous mGWAS, we demonstrated the potential of testing pairwise ratios of metabolite concentrations to boost genetic association signals [1, 4, 6, 8, 10, 21, 53]. Recently, we showed that this approach can also be successfully applied to NMR-based mGWAS with non-targeted features [22]. Thus, we calculated the pairwise ratios of all metabolite concentrations with at least 300 valid data points over all samples (55×54/2 = 1,485 ratios of targeted traits) and NMR signal intensities (166×165/2 = 13,695 ratios of non-targeted traits), respectively (Fig 1A). Out of all 15,401 metabolic features (targeted and non-targeted traits and the ratios thereof), a total of 15,379 features with at least 300 valid data points were screened for genetic associations using 620,456 genotyped autosomal SNPs (Fig 1B). To this end, we computed age - and sex-adjusted linear models under the assumption of additive genetic effects for each SNP-metabolic trait pair. A total of 499 genotyped variants display associations with metabolic traits with P-values below 5×10−8.
Fine-mapping of chromosomal regions with associated variants
We used the 499 variants identified in the mGWAS to tag 54 distinct chromosomal regions at a window size of at least 2 Mb (centered to the tag SNPs). We then performed additional association studies using imputed variants (1000 genomes project imputation) in the tagged regions (Fig 1B). We considered associations with a P-value below the Bonferroni-adjusted significance threshold of α’ = 5×10−8/15,379 = 3.25×10−12 to be genome-wide significant. For ratio traits, we also required the P-gain to be greater than 1.52×104 for targeted traits and 1.38×105 for non-targeted traits (10 times the number of tested traits [53]). P-gain reflects the increase of association strength with the ratio trait when compared to the P-values that result from associations with the individual traits buildinging the ratio. A total of 2,882 genotyped or imputed SNPs display association signals below P < 3.25×10−12 and, in case of ratios, above the imposed P-gain threshold (Fig 2). All significantly associated SNPs within a physical distance of 1 Mb were assigned to one of 23 distinct genetic loci. Three loci display significant association signals only when imputed SNPs were used, and 8 loci show significant associations only when pairwise ratios of metabolic traits were considered. Twelve loci show significant associations in both targeted and non-targeted data sets. Three loci are only significantly associated with targeted traits (i.e., quantified metabolite concentrations or ratios thereof), whereas 8 loci are only significantly associated with non-targeted traits (spectral features or ratios thereof) (Fig 3). For each locus, we list the SNP that displays the strongest association signal (lead SNP) and its associated metabolic trait in Tables 1 and 2. In addition, we provide boxplots, regional association plots, and Q-Q plots for each locus in S2 Fig. The summary statistics for all association signals with P < 0.05 (P < 1×10−4 and P-gain ≥ 10 for associations with ratios) for each tested SNP can be downloaded from http://www.gwas.eu.
Systematic assignment of loci to genes and annotation of non-targeted metabolic traits
In general, the biological interpretation of association results from mGWAS requires the mapping of SNPs to candidate genes that are most likely causally linked to the observed changes in the metabotype. Furthermore, non-targeted metabolic traits that exhibit significant association signals have to be assigned to distinct metabolites. In our study, we implemented algorithmic approaches for both the locus-to-gene mapping and the assignment of non-targeted metabolic features.
As the first step in the candidate gene selection, we assigned the significantly associated SNPs to distinct loci using a physical distance threshold of 1Mb. Assigning variants within a locus to one of the covered genes based only on proximity or plausibility ignores haploblock structure and existing regulatory information for the SNPs such as expression quantitative trait loci (eQTL). To take such information into account and to achieve an unbiased selection of candidate genes, we collected evidence for each significantly associated SNP and its proxies in strong linkage disequilibrium (LD) using the SNiPA web server [51]. For each locus, we received a list of candidate genes that are linked to one or more associated variants (or a proxy in LD). Thereby, genes are linked via genomic proximity (i.e., if any of the variants is located within the candidate gene or is in close proximity), via eQTL associations (i.e., if any of the variants is associated with expression levels of the gene in a previous eQTL study), or via regulatory element association (i.e., if any of the variants is contained in a promoter/enhancer/repressor element that is associated with the gene). Moreover, missense variants or known pathogenic variants in the locus are considered to provide additional types of evidence for the linked genes. We finally assigned the locus to the gene with the strongest functional evidence (i.e., the gene showing the highest number of different types of evidences (max. 5) among the candidate genes; see Methods). In case of ambiguous assignments, the gene with the most plausible biological function was chosen. As an example, one locus contains a high number of SNPs with strong associations with non-targeted traits corresponding to N-acetylated compounds. These SNPs cover 12 different genes (see regional association plots in S2 Fig). The gene covered by the highest number of SNPs is ALMS1. However, there are 3 more genes in this locus with the same amount of functional evidence count as ALMS1 (S3 Table). One of these genes is NAT8, which encodes an N-acetyltransferase. Since there is a biologically meaningful link between the function of the NAT8 gene product and the associated metabolic traits, we annotated this locus with NAT8 as the most likely candidate gene. According to our evidence-based candidate genes assignment approach, the 23 loci map to the genes NAT8, HIBCH, CPS1, AGXT, XYLB, SLC6A20, TKT, ETNPPL, SLC6A19, AGXT2, DMGDH, SLC36A2, NAT2, ABO, GLDC, PYROXD2, SLC6A13, HPD, ACSM3, SLC5A11, PNMT, SLC7A9, and SLC13A3. S3 Table provides a complete list of candidate genes and the corresponding collected evidences.
For the identification of metabolites underlying non-targeted NMR traits, we used pseudo-spectra that display the strength of associations of a given SNP across the complete NMR spectrum [12, 22]. If the association is strong enough, these “association spectra” often exhibit a striking similarity to the reference NMR spectrum of the underlying metabolite(s). For the present study, we applied the “metabomatching” method introduced by Rueedi et al. [12] to perform an automated annotation of the association spectra for each genetic locus of interest. For 19 of the 20 loci that display significant associations with non-targeted traits, metabomatching suggests plausible metabolite candidates matching signals present in the association spectra (S1 Fig). For 10 of these 19 loci (CPS1, SLC6A20, ETNPPL, SLC6A19, AGXT2, SLC36A2, HPD, ACSM3, PNMT, and SLC13A3), the match between the association signal and the NMR spectrum of the candidate metabolite (as provided by the Urine Metabolome Database [54]) is strong and unique, which makes the assignment of a metabolite identity to a non-targeted trait unambiguous in these cases.
Replication
To replicate our findings, we used genotype data and urine samples from participants of the KORA F4 cohort (N = 1,691). From recorded 1H NMR spectra of the urine samples, we derived the targeted and non-targeted metabolic traits (metabolite concentrations, NMR spectral features, and the respective pairwise ratios) as for the discovery study. For 14 of the 15 new loci that show significant associations with targeted metabolic traits in the SHIP-0 data set, the top-ranking SNP/metabolic trait association replicates in KORA F4 (S1 Table). For the SLC7A9 locus, the association with lysine/valine does not replicate, possibly due to the difficulty in annotating lysine from the NMR spectra (> 75% missing values for lysine). However, the second-best, still genome-wide significant association of the tested SNP with valine replicates. For 15 of 20 loci that display significant association signals in the GWAS with non-targeted traits, we were able to replicate the best SNP/NMR trait association or, if this failed, the next, still significant follow-up association (S2 Table). The failure to replicate the remaining 5 loci might be due to the lower sample size in KORA, due to different fasting states of the subjects in the different cohorts, or due to a less perfect alignment of the NMR spectra, since we chose the same FOCUS parameters for aligning SHIP and KORA spectra instead of treating them separately. However, 4 of these 5 loci (ETNPPL, SLC6A19, DMGDH, PNMT) show also significant associations in the targeted SHIP-0 data set that replicate in the targeted KORA F4 data set (Table 1). Out of the 23 loci identified in the discovery study, ABO is the only locus that could not be replicated using either a targeted or a non-targeted metabolic trait in KORA F4, leaving 22 loci that display stable associations with metabolic traits in urine.
Overlap with previous mGWAS in urine and blood
We evaluated each identified and replicated locus in the light of previously reported associations with metabolic phenotypes and clinical traits. To this end, we selected all SNPs within a locus for which we found genome-wide significant associations with any urinary metabolic trait in the SHIP-0 cohort. Furthermore, we added all bi-allelic variants from the 1000 genomes project [50] (phase 1, version 3, European ancestry) that are in strong LD to these SNPs (r2 ≥ 0.8).
For 15 of the 22 loci, no associations with urinary metabolic traits were reported so far (HIBCH, CPS1, AGXT, XYLB, TKT, ETNPPL, SLC6A19, DMGDH, SLC36A2, GLDC, SLC6A13, ACSM3, SLC5A11, PNMT, and SLC13A3) (Fig 3, Table 3). The remaining 7 loci were already identified in our previous urine mGWAS (AGXT2, HPD, SLC7A9, SLC6A20, and NAT2) [10] or in the studies by Nicholson et al. [5] and Rueedi et al. [12] (NAT8 and PYROXD2). For all 7 loci, both trait association and direction of the observed effect are consistent with the results previously published.
We further compared our association results with those of published mGWAS with metabolic traits in blood (P < 5×10−8), including all studies listed in the NHGRI GWAS catalog [23] and other studies such as the mGWAS by Shin et al., which is based on metabolomics data from the KORA F4 and TwinsUK cohorts [1, 4, 6–8, 14–22, 24]. In total, 14 loci show significant associations with metabolic traits both in blood in one of these mGWAS and in urine in our study (Fig 3, Table 3). For 3 of these 14 loci (SLC6A20, PNMT, AGXT), we consider the associated metabolic traits in both media to be unrelated. In 5 cases (NAT2, NAT8, PYROXD2, SLC7A9, TKT), the genetic association analyses identified different, but related metabolites (i.e., the associated metabolites from urine and blood are either products/substrates of the locus’ candidate gene product, or are biochemically converted within another known enzymatic reaction, or belong to the same metabolite class). In 6 cases, the associations target the same metabolites in urine and blood (CPS1, AGXT2, DMGDH, SLC6A13, HPD, SLC5A11). For 5 of these 6 loci, the direction of the observed effect is the same, whereas for SLC5A11 (associated with myo-inositol), we observe an increase in urinary metabolite concentration per copy of the effect allele, as opposed to decreased levels reported in blood (Table 3). For this locus, we additionally investigated whether the effects seen in blood and urine are directly coupled. To this end, we made use of myo-inositol levels (normalized to circulating creatinine) measured through mass spectrometry (MS) in blood serum samples of the same KORA F4 participants [6] that form the replication cohort in this study. The ratio between the urinary myo-inositol (this study) and the serum myo-inositol levels shows an increase in association strength to the lead SNP in SLC5A11 (rs17702912) by seven orders of magnitude in comparison to the association of urinary myo-inositol alone (Purine < 1.95×10−24, Pblood < 1.50×10−4, Pratio < 2.43×10−31).
Overlap with disease-associated variants and risk genes
For 11 loci, our mGWAS identified significantly associated variants for which either the same variant or a proxy in strong LD was previously reported to be associated with clinical phenotypes according to data from the NHGRI GWAS catalog [23] (P < 5×10−8), OMIM variation, ClinVar [55], HGMD [56], or dbGaP [57]. Amongst others, these variants have been linked to chronic kidney disease (NAT8, CPS1, SLC6A13, and SLC7A9), pulmonary hypertension (CPS1), ischemic stroke (XYLB), Iminoglycinuria (SLC6A20), heart rate variability (AGXT2), Hawkinsuria (HPD), and pharmacogenomically relevant acetylation phenotypes (NAT2) (Table 3). In addition to these 11 loci with disease-associated variants, we found previously discovered connections between clinical traits and the assigned candidate gene for another 7 loci (HIBCH, AGXT, SLC6A19, DMGDH, SLC36A2, GLDC, ACSM3).
Discussion
In this study, we present the largest genome-wide association study with metabolic traits (mGWAS) in urine to date. In addition to quadrupling the sample size compared to previous mGWAS in urine, we analyzed both targeted traits (metabolite concentrations manually derived from NMR spectra) and non-targeted traits (NMR spectral features).
Fifteen new genetic loci linked to urinary metabolic traits
In total, we identified 23 genetic loci with significant associations between genetic variants and targeted or non-targeted metabolic traits in urine of SHIP-0 participants, 22 of which replicate in the independent KORA F4 cohort. To the best of our knowledge, 15 loci have not been linked to changes in the urine metabolome before. For the remaining 7 loci, our results are in line with the results from previous mGWAS in urine [5, 10, 12] regarding both the associated metabolic traits and the direction of the genetic effects (Table 3).
Targeted and non-targeted metabolomics are complementary
Though derived from the same NMR spectra, the list of GIMs identified with targeted traits and non-targeted traits partly differ. Of the 22 genetic loci reported in this study, only 12 loci were discovered in both targeted and non-targeted traits, whereas 7 loci show significant associations only with non-targeted traits, and 3 only with targeted traits (Fig 3 and Table 3).
For the data set used in the targeted analysis, the NMR spectra were manually annotated to identify and quantify the metabolites underlying the spectra. Involving human expert knowledge usually allows metabolite identification with very high confidence and yields more precise quantification, especially if signals of multiple metabolites overlap in the NMR spectrum. Furthermore, a manual annotation can to some extent compensate for different experimental and sample conditions, as alignment and pre-processing can be optimized for each spectrum individually. As an example, lysine exhibits characteristic signals in the NMR spectral region between δ = 1.68 and 1.76 ppm, which is often dominated by signals from a variety of additional metabolites, making the annotation of lysine a very difficult task. Thus, while lysine concentrations could be determined for 888 samples of the discovery cohort through manual quantification and yielded a significant genetic association at SLC7A9, the non-targeted approach did not capture any association signals for this locus.
However, a manual spectral annotation as performed in the targeted analysis is quite laborious, which limits the number of quantifiable metabolites in large studies. This leads to a bias towards a certain set of metabolites and, as a consequence, significant associations actually present in the NMR data might be missed. Also, a manual annotation in general bears some risk of annotator-induced bias [58]. As an automated method, the non-targeted analysis of spectra has the potential to overcome some of the limitations of targeted analyses. Here, the most prominent example is the PYROXD2 locus, where SNPs display exceptionally strong associations (P < 1.0×10−307) to the NMR signal intensities at δ = 2.854 ppm. We could not identify any significant associations within this locus using the targeted data. Thus, we assumed that our set of targeted traits did not cover the metabolite(s) corresponding to these signals. The challenge with genetically associated non-targeted traits lies in the lack of biochemical interpretability. To facilitate the assignment of non-targeted NMR traits to chemical compounds, we applied the metabomatching algorithm introduced by Rueedi et al. [12]. In case of PYROXD2, metabomatching suggests that the associated NMR signals correspond to trimethylamine. Thereby, the automated method replicates the findings of Nicholson et al. [5] where the authors manually annotated the associated signals based on expert knowledge. In case of our mGWAS with targeted traits, trimethylamine was not part of the metabolite panel and thus the association with PYROXD2 could only be discovered using non-targeted metabolic traits in combination with the automated metabomatching processing. Of course, automated annotation of non-targeted traits also has its limitations: the annotation through metabomatching relies on the association signals that genetic variants display over the NMR spectral range (“association spectra”) as well as on the existence of the relevant reference metabolite spectrum (see Methods and S1 Fig). In some cases, these association spectra are not meaningful enough to allow an unambiguous assignment of non-targeted features to metabolites, or they may be pointing to a metabolite not present in the reference set.
In summary, our study demonstrates that GWAS with NMR-determined metabolic traits can benefit from a combined application of both targeted and non-targeted metabolomics. Our results suggest that a targeted approach is better suited for the annotation of metabolites for which the corresponding NMR signals are in regions with a plethora of other signals as in some cases these signals cannot be resolved through non-targeted methods. Furthermore, genetic associations with targeted traits appear to be more robust, since 5 of the 12 loci that display associations with both targeted and non-targeted traits clearly display stronger association signals in the targeted data set (several orders of magnitude in case of the SLC6A20 locus; Tables 1 and 2). However, the non-targeted metabolic traits provide a less biased view on the metabolome, which in our case results in additional significantly associated genetic loci.
Functional metabolomics: from GIMs to testable hypotheses
Fifteen of the 22 identified and replicated loci show a plausible biochemical connection between functionally annotated genes and their associated metabolic traits (Table 3). This is similar to observations from previous mGWAS. For instance, Shin et al. reported biologically meaningful links between metabolites and genetic loci for 101 of 145 GIMs [21]. In case of genes with vague functional annotations, gene-metabolite associations from mGWAS provide testable hypotheses for further gene characterization. As an example, Suhre et al. experimentally confirmed the mGWAS driven hypothesis of SLC16A9 being a carnitine transporter [6]. Vice versa, with the help of mGWAS, the chemical structure of a non-targeted metabolic trait was elucidated through the function of the associated gene [8].
Another prominent finding of previous mGWAS is the overlap between disease relevant genetic variants and variants associated with metabolic traits. In the present study, we found 11 loci hosting variants that have previously been linked to clinical phenotypes. This includes associations with the estimated glomerular filtration rate (eGFR) and chronic kidney disease (CKD). Thus, the associated metabolites might, on the one hand, serve as intermediate traits for clinical endpoints. On the other hand, the associations might provide new insights regarding the involvement of specific metabolic pathways in pathomechanisms and the mediation of genetic risk loci through metabolic changes. For all 22 GIMs, we provide information on both the match of gene and metabolite function and the link to clinical traits in Table 3. In the following, we exemplify the value of our results for the characterization of gene functions in the light of clinical phenotypes.
As a first example, we identified significant associations of variants upstream of ETNPPL with ethanolamine. Interestingly, at the time when we received the first results from our association studies this gene was named AGXT2L1 and was assumed to encode an alanine-glyoxylate-aminotransferase. Based on this gene annotation, there was no obvious relation to the associated metabolite ethanolamine. In such cases, only dedicated experiments (similar to the one for the carnitine/SLC16A9 association mentioned above [6]) could validate the connection of ethanolamine to the gene product. Meanwhile, Veiga-da-Cunha et al. experimentally investigated the locus in an independent study and found that AGXT2L1 actually encodes an ethanolaminephosphate-phospholyase [59]. As a consequence, AGXT2L1 now carries the gene symbol ETNPPL. As ethanolamine is a direct precursor of ethanolaminephosphate via ethanolamine kinase (EC 2.7.1.28), our finding indeed matches the actual gene function. Besides the functional characterization of this locus, Veiga-da-Cunha et al. suggest that the ETNPPL-mediated degradation of ethanolaminephosphate balances the concentration of that metabolite in the central nervous system. They concluded that an altered ethanolaminephosphate homeostasis might contribute to mental disorders such as schizophrenia [59]. In line with this hypothesis, the ETNPPL expression rate in brain was previously found to be associated with schizophrenia [60]. ETNPPL is primarily expressed in brain and liver and the encoded protein is, amongst other tissues, highly localized in the cerebral cortex and the kidney (S4 Table and The Human Protein Atlas [52], http://www.proteinatlas.org/ENSG00000164089/tissue). Our results suggest that an excess of ethanolamine in urine could indicate alterations in ethanolaminephosphate homeostasis linked to a genetically reduced enzymatic activity of ETNPPL.
As a second example, we identified significant associations of genetic variants with 2-hydroxyisobutyrate (2-HIBA) in a locus comprising 9 different genes. According to our evidence-based locus to gene assignment, 4-hydroxyphenylpyruvate dioxygenase (HPD) is the most probable effector gene candidate. The association between 2-HIBA and this locus represents a well replicated finding: it was already identified in our previous NMR-based mGWAS in urine [10] and it has meanwhile also been discovered in an MS-based GWAS with blood metabolites [21]. Nonetheless, to the best of our knowledge, there is no obvious, known biological link between 2-HIBA and the HPD gene or any of the remaining 8 genes covered by this locus. In the literature, 2-HIBA is often referred to as a secondary metabolite that can be found in urine of humans and rats exposed to the volatile gasoline additives methyl-tert-butylether and ethyl-tert-butylether [61–63]. However, 2-HIBA has been identified by both MS - and NMR-based methods in almost all serum and urine samples of large human cohorts (e.g. ARIC [25], CoLaus [12], KORA [6, 21], SHIP [10], TasteSensomics [12], and TwinsUK [6, 21]) in relatively high concentrations (~ 40 μM in urine in this study), which suggests sources beyond gasoline for this metabolite (e.g. microbiota [64] or medication [65]). Interestingly, Dai et al. recently showed that 2-HIBA is an intermediate for the newly discovered but common 2-hydroxyisobutyrylation of lysine residues of histones [66], thus indicating an endogenous role of 2-HIBA. In this context, it is interesting to note that SETD1B is one of the genes within the identified locus on chromosome 12. SETD1B is a component of the methyltransferase complex that specifically methylates the lysine-4 residue of histone H3 [67]. This residue is amongst the 63 sites for 2-hydroxyisobutyrylation presented by Dai et al. [66]. Thus, one could speculate that in addition to its activity as a histone methylase, SETD1B may also be involved in the newly discovered process of histone hydroxyisobutyrylation, a hypothesis that may now be tested by dedicated experiments.
As a third example, we discuss the association of variants in XYLB with increased urinary glycolate levels. One of these variants, rs17118, causes an amino acid exchange in the XYLB gene product. XYLB encodes the enzyme xylulokinase [33], which catalyzes the phosphorylation of D-xylulose to D-xylulose-5-phosphate. In humans, the vast majority of D-xylulose is metabolized via xylulokinase [33, 68, 69]. However, there is an alternative metabolic pathway in which D-xylulose is metabolized by phosphofructokinase (PFK) (S3 Fig) [70]. Therein, one of the downstream products is glycolate. Thus, the genetic variants in XYLB might reduce the enzymatic activity of xylulokinase and thereby cause a shift towards the alternative pathway. Interestingly, the minor allele of rs17118 has been implicated in increased susceptibility for ischemic stroke [32]. Furthermore, Jung et al. found a significant association between elevated glycolate levels in plasma and cerebral infarction [34]. In the alternative pathway, glycolate is a precursor of oxalate, whose toxic effect has been demonstrated repeatedly [33, 71]. Very recently, Rao et al. postulated that circulating oxalate precipitate might be a potential mechanism for stroke [72]. In this context, the association between the SNP rs17118 and glycolate (identified in our study) suggests that the carriers of this variants have a higher risk of stroke (identified in [32]) possibly via increased levels of glycolate or oxalate through favoring the alternative D-xylulose degradation. Unfortunately, oxalate or any other metabolite in the two D-xylulose degradation pathways are not detected in our metabolomics analysis to further support our hypothesis.
Extending blood GIMs to urine
In total, 26 genetic loci that associate with urinary metabolic traits are known to date (22 identified or confirmed in this study plus 4 identified in previous studies [5, 11, 12], Fig 3). Of the 26 loci, only 8 loci lack corresponding SNP-metabolite associations in blood, and, based on current mGWAS, represent urine specific hits. All of these 8 loci were first reported in the present study. In case of the 14 loci with overlapping associations between blood and urine in our study (Table 3), 6 target the same metabolite in both media (CPS1, AGXT2, DMGDH, SLC6A13, HPD, SLC5A11). Interestingly, in all but one case (SLC5A11) the genetic effect has the same direction in both fluids, thus indicating that urine can be regarded as “diluted plasma” to some extent. For 5 of the 14 loci, we considered the associated metabolic traits in blood and urine to be biochemically related. Here, the metabolites are either products of the enzyme coded by the candidate gene (NAT8: N-acetylated compounds), or they are linked through an enzymatic reaction other than the reaction catalyzed by the candidate gene’s product (NAT2: 1,3-dimethylurate and 1-methylurate [73]; PYROXD2: trimethylamine and dimethylamine [EC 1.5.8.2]; SLC7A9: lysine and homocitrulline [EC 2.1.3.8]), or they belong to the same metabolite class (TKT: gluconate and erythronate are aldonates). The observed associations of related but different metabolites in blood and urine may be indicative either for biochemical conversions before excretion, or simply be a result of differences in the composition of the metabolite panels covered by the various mGWAS. In case of the remaining 3 loci, we find no direct biochemical or metabolic relationship between the metabolites in both media, since AGXT associates with an unknown compound in blood, PNMT associates with amino acids in urine and HDL cholesterol in blood, and SLC6A20 targets loosely related amino acid derivatives.
As an example for parallel effects in blood and urine, we identified an association between variants in the Carbamoyl-Phosphate Synthetase 1 (CPS1) gene and elevated urinary glycine levels. The strongest associated SNP rs715 was also identified in previous mGWAS with higher glycine concentrations in blood [14, 21, 24]. This variant has been highlighted previously as a putative regulator of CPS1 expression [74–76]. Furthermore, the second strongest glycine-associated SNP rs1047891 causes a non-synonymous mutation (Thr>Asn) in the C-terminal domain of the CPS1 polypeptide, which hosts the binding site for the allosteric activator N-acteyl-L-glutamate (NAG) [77]. Both SNPs are therefore potentially causative variants in this metabolic association. CPS1 is highly expressed in liver (S4 Table) and controls the first step in the urea cycle: ammonia is catalyzed to carbamoyl-phosphate, which in turn is the entry substrate of the urea cycle. CPS1 deficiency can lead to high ammonia levels in the body (Hyperammonemia, OMIM #237300) (S4 Fig). The association of the CPS1 variants with glycine can be explained by the conversion of excess ammonia to glycine via the glycine cleavage system [78, 79] and is thus biologically meaningful. The association between common variants in CPS1 and glycine might therefore be driven by mild forms of genetically induced Hyperammonemia. In this study, we could establish a link between genetic factors and a potential urinary marker for this condition.
SLC5A11 is the only locus where we observe an association with exactly the same metabolite in blood and urine but with reversed effects: while myo-inositol concentrations in urine increase per effect allele copy of the lead SNP, they decrease in serum (Table 3) [21]. The Solute Carrier Family 5 (Sodium/Inositol Cotransporter), Member 11 (SLC5A11) is a co-transporter of myo-inositol with sodium [80]. SLC5A11 was postulated to play a role in the regulation of serum myo-inositol concentrations [81], which was recently confirmed by an mGWAS in blood [21]. On the one hand, the influence of SLC5A11 on myo-inositol concentrations has been linked to apical transport and absorption in intestine [82]. On the other hand, SLC5A11 may be implicated in the re-absorption of myo-inositol in the proximal tubule of the kidney [83]. The opposite direction of the genetic influence in blood and urine as observed in our study suggests that SLC5A11 is actively involved in the re-absorption of myo-inositol. This assumption is further supported by the strong increase of the association strength when testing the ratio between urinary and serum myo-inositol. This could indicate that the reduced levels in blood are indeed caused by a reduced re-absorption rate in subjects that are homozygous regarding the effect allele.
As these examples demonstrate, mGWAS in urine extend our understanding of genetically influenced biochemical processes and can facilitate the knowledge transfer from blood to urine and vice versa. Currently, this transfer is limited by the comparatively low number of GIMs in urine (26) versus blood (>150). Further increasing the sample sizes of mGWAS in urine and the application of more sensitive MS-based metabolomics platforms as already used for blood mGWAS could compensate this bias.
Methods
Study samples
For this study, we used data from SHIP (Study of Health in Pomerania) and, for replication, from the KORA (Kooperative Gesundheitsforschung in der Region Augsburg) study. Both studies have been described extensively in the study design papers [84–86] and in our previous publications [4, 6, 10, 21].
SHIP is a longitudinal population study conducted in West-Pomerania, located in the northeastern part of Germany. 4,308 inhabitants in that region participated in the first phase “SHIP-0”. For the GWAS presented here, metabolically characterized urine samples and genotype data were jointly available for 3,861 study participants (1,960 female and 1,901 male, aged 20 to 81 years). KORA is a population study conducted in the municipal region of Augsburg in southern Germany. The KORA F4 cohort comprises 3,080 subjects. For the study presented here, both genotype and urine samples from 1,691 participants (865 female and 826 male, age 32 to 77) were available. In both studies, all participants have given written informed consent and the local ethics committees (SHIP: ethics committee of the University of Greifswald; KORA: ethics committee of the Bavarian Chamber of Physicians, Munich) have approved the studies.
Metabolomics data acquisition and processing
NMR measurements
In SHIP-0, non-fasting, spontaneous urine samples were collected from the study participants. In contrast, KORA F4 study participants were overnight-fasting prior to urine sample collection. All urine samples were stored at -80°C until the analysis. In preparation for the recording of NMR spectra, 75μl of phosphate buffer were added to 675μl of urine to set the pH to 7.0 (+/ - 0.35). The deuterated buffer contained 0.5 mM sodium trimethylsilylphosphate (TSP) to provide a reference substance for the annotation of the NMR spectra. One-dimensional 1H NMR spectra were recorded at the University of Greifswald, Germany using a Bruker DRX-400 spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany). Spectra were acquired at 300K and a frequency of 400.13 MHz using a standard one-dimensional NOESY-PRESAT pulse sequence with water peak suppression, as previously described in [10].
Targeted analysis
The Fourier-transformed and baseline-corrected NMR spectra were manually annotated by spectral pattern matching using the Chenomx Worksuite 7.0 by Chenomx, Inc. (Edmonton, Canada) to deduce absolute urinary metabolite concentrations. The panel of targeted metabolites comprises 60 compounds (including creatinine, which was used for normalization). In the discovery mGWAS, we used both metabolite concentrations (59) and pairwise ratios thereof (59 × 58/2 = 1,711) from the SHIP-0 data as phenotypic traits. Prior to analysis, individual metabolite concentrations were normalized to the annotated creatinine levels, and both concentrations and ratios were log10 transformed. Individual data points more than three times the standard deviation away from the mean were removed to avoid spurious associations. Finally, we considered only metabolic traits with at least 300 valid data points for the GWAS. In total, the final SHIP-0 data set comprises 1,518 targeted metabolic traits (55 metabolites and 1,463 ratios) that were tested for genetic associations.
Likewise, we processed the NMR spectra originating from the KORA F4 data set that was used for replication. Due to the smaller data set size, we lowered the burden for non-missing values to 100. The replication data set contains 53 metabolites and 1,359 pairwise ratios. It covers all metabolic traits that significantly associated in the discovery GWAS.
Non-targeted analysis
The same Fourier-transformed and baseline-corrected NMR spectra that were used for the targeted analysis were binned at a segment width of 0.0005 ppm. All signal intensities were normalized to the TSP reference peak. We used the FOCUS software (http://www.urr.cat/FOCUS) [13] for the subsequent processing of the NMR spectra. We excluded the spectral regions between δ = 4.6 and 5.0 ppm (water peak) and the regions below δ = 0.0 and above δ = 10.0 ppm, which do not contain any signals relevant for a metabolomics analysis. We performed the spectral alignment and feature extraction using the default parameters of FOCUS, resulting in a total of 166 NMR peaks in SHIP-0 and 217 peaks in KORA F4. As for the targeted data set, we computed pairwise ratios of the NMR peaks (SHIP-0 : 13,695; KORA F4 : 23,436). To compensate for dilution effects, we additionally normalized the signal intensities of individual NMR peaks to the annotated creatinine concentrations prior to analysis. All non-targeted traits (i.e., NMR peaks and peak ratios) were log10 transformed. In comparison to the targeted data set, we chose a less stringent removal of extreme values. Here, individual data points more than four standard deviations away from the mean were removed.
Genotype data
Both SHIP and KORA samples were genotyped using Affymetrix Human SNP Array 6.0 gene chips. SNPs were called using the Birdseed2 algorithm. In both data sets, the total genotyping rate was above 99%. 909,508 SNPs were genotyped in the SHIP-0 cohort, and 906,716 SNPs in the KORA F4 cohort. We excluded SNPs that violated the Hardy-Weinberg equilibrium (PHWE < 1.0×10−6, 8,623 in SHIP and 32,033 in KORA), or had a genotyping rate below 95% (57,160 in SHIP and 84,351 in KORA), or displayed minor allele frequencies (MAF) below 5% (227,967 in SHIP and 224,723 in KORA). After the exclusion, 620,456 autosomal SNPs remained in the SHIP-0 data set and 593,830 autosomal SNPs in the KORA F4 data set. Both SHIP and KORA genotypes were imputed in a two-stage process (pre-phasing followed by imputation). According to data from the 1000 genomes project (phase 1, March 2012 release [50]), we used SHAPEIT (v1.416) [87] for phasing in KORA F4 and IMPUTE (v2.2.2) [88] for imputation in KORA F4 and both phasing and imputation in SHIP-0. For the association analyses, we considered only imputed variants with a MAF ≥ 5%, PHWE ≥ 1.0×10−6, and an imputation quality score (IMPUTE info-score) ≥ 0.8.
Statistical analysis
Genome-wide association study
In both the SHIP-0 and KORA F4 cohorts, we used PLINK (v1.07) [89] to compute age - and sex corrected linear regression models under the assumption of an additive genetic model. For the discovery study based on the SHIP-0 data set, we carried out association tests for each genotyped autosomal SNP (filtered for the criteria described above) and all 15,379 targeted and non-targeted metabolic traits. In addition, we tested all imputed, quality-filtered SNPs within a physical distance of 1 Mb to each genotyped SNP that displayed an association signal of P < 5×10−8. This two-stage approach of testing imputed variants in selected candidate regions results in a drastically lowered computational burden when compared to association studies with imputed variants over the whole genomic range (0.9M vs. 15.9M tested SNPs). We considered associations to be genome-wide significant if the resulting P value was below the Bonferroni-adjusted significance threshold of 5×10−8/15,379 = 3.25×10−12. In case of associations with ratios, we imposed an additional significance criterion on the P-gain as suggested by Petersen et al. [53]. P-gain describes the observed increase in strength of association when compared to the association with the individual metabolic traits from which the ratio was computed (min(P(M1)/P(M1/M2), P(M2)/P(M1/M2))). A conservative Bonferroni-type lower limit on the P-gain at a significance level of 5% is given by ten times the number of tested ratio pairs. Thus, associations with ratios were considered to be significant if the P-gain exceeded 15,180 in case of targeted metabolic ratios and 138,610 in case of non-targeted ratios. The analysis of the PLINK output files was performed using in-house R (v3.0.2) code (available at http://www.gwas.eu).
Replication of association results
To replicate the significant associations discovered in the SHIP-0 data set, we used data from the independent KORA F4 cohort. For each genetic locus, we attempted to replicate the association of the SNP/metabolic trait pair that displayed the lowest P-value (S1 and S2 Tables). If an association did not replicate, we checked whether the tested SNP was significantly associated with other metabolic traits in SHIP-0. In that case, we tried to replicate these associations, beginning with the one that displayed the strongest association signal. To replicate associations with non-targeted traits, we selected the NMR feature with the minimum difference in chemical shift. In total, we attempted the replication of 38 SNP/metabolic trait associations. Thus, we considered associations with P < 0.05/38 = 1.32×10−3 to be successfully replicated in the KORA F4 cohort.
Genetic variant annotation and evidence-based locus to gene mapping
Annotation data for genetic variants as well as linkage disequilibrium (LD) data from the 1000 genomes project (phase 1 version 3, EUR panel [50]) were retrieved from SNiPA v1 (http://www.snipa.org) [51]. Full lists of association signals from the serum-based mGWAS [21] were obtained from the Metabolomics GWAS server (http://www.gwas.eu).
All genotyped and imputed SNPs that displayed genome-wide significant association signals (according to the aforementioned P-value and P-gain criteria) were assigned to distinct genetic regions (loci), based on a physical distance threshold of 1 Mb. Each of the resulting 23 genome-wide significant loci was then projected to candidate genes using an evidence-based procedure. To this end, we used the “block annotation” feature of SNiPA on the LD-extended (r2 ≥ 0.8) list of associated variants at each locus. This feature provides a condensed view of genes that are linked to any of the significantly associated variants or their LD-proxies via genomic proximity, eQTL association, or regulatory elements. Additionally, the block annotation highlights missense and pathogenic variants. Based on these data, we defined the following criteria to identify candidate genes: 1) Genomic proximity: genes that harbor or are in close proximity (<5kb) to any of the variants in the list. 2) eQTL association: genes where altered expression levels have been discovered to associate with any of the variants in the list. 3) Regulatory elements: potentially regulated genes that are associated with a promoter/enhancer/repressor element containing a variant of the list. Further evidence for potential involvement of a gene was assumed if 4) the variant list contains a missense variant for a protein product of this gene and 5) if an intragenic variant in the list is annotated as pathogenic in one of the phenotype databases contained in SNiPA. For each gene, we counted how many of the aforementioned criteria are met. Thus, the maximum evidence count for a candidate gene is five. If evidence-based gene selection was ambiguous, the gene with the most plausible biological function was chosen (S3 Table).
Metabomatching of non-targeted NMR traits
Metabomatching [12] is an automated annotation method that identifies metabolites likely to underlie an observed genetic association between a SNP and one or more non-targeted metabolic traits (www.unil.ch/cbg). It does so by comparing the association signal between a SNP and all non-targeted traits (pseudo-spectrum or association spectrum) to the 1H-NMR spectra of metabolites in a reference set, and assigning a score to each pseudo-spectrum to NMR spectrum match. The metabolites most likely to underlie the genetic association are the ones with the highest scores. We applied metabomatching to each SNP showing a significant association with an NMR feature or feature-ratio, using the 180 metabolites listed in the urine metabolome database [54] with an experimental NMR spectrum as reference set. The urine metabolome database is the subset of metabolites in the Human Metabolome Database (HMDB) [90] present in urine. Where indicated, 2-compound or effect direction-specific metabomatching was applied. Final candidates were manually identified, usually among the few top-ranked matches. Most likely candidates are used in the text; potential alternatives are listed, along with the metabomatching details, in S1 Fig.
Supporting Information
Zdroje
1. Gieger C, Geistlinger L, Altmaier E, Hrabě de Angelis M, Kronenberg F, Meitinger T, et al. Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genetics. 2008 Nov;4(11):e1000282. doi: 10.1371/journal.pgen.1000282 19043545
2. Hicks AA, Pramstaller PP, Johansson A, Vitart V, Rudan I, Ugocsai P, et al. Genetic determinants of circulating sphingolipid concentrations in European populations. PLoS Genetics. 2009 Oct;5(10):e1000672. doi: 10.1371/journal.pgen.1000672 19798445
3. Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, et al. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genetics. 2009 Jan;5(1):e1000338. doi: 10.1371/journal.pgen.1000338 19148276
4. Illig T, Gieger C, Zhai G, Römisch-Margl W, Wang-Sattler R, Prehn C, et al. A genome-wide perspective of genetic variation in human metabolism. Nature Genetics. 2010 Feb;42(2):137–41. doi: 10.1038/ng.507 20037589
5. Nicholson G, Rantalainen M, Li JV, Maher AD, Malmodin D, Ahmadi KR, et al. A genome-wide metabolic QTL analysis in Europeans implicates two loci shaped by recent positive selection. PLoS Genetics. 2011 Sep;7(9):e1002270. doi: 10.1371/journal.pgen.1002270 21931564
6. Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wägele B, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011 Sep 1;477(7362):54–60. doi: 10.1038/nature10354 21886157
7. Kettunen J, Tukiainen T, Sarin AP, Ortega-Alonso A, Tikkanen E, Lyytikäinen LP, et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nature Genetics. 2012 Mar;44(3):269–76. doi: 10.1038/ng.1073 22286219
8. Krumsiek J, Suhre K, Evans AM, Mitchell MW, Mohney RP, Milburn MV, et al. Mining the unknown: a systems approach to metabolite identification combining genetic and metabolic information. PLoS Genetics. 2012;8(10):e1003005. doi: 10.1371/journal.pgen.1003005 23093944
9. Suhre K, Gieger C. Genetic variation in metabolic phenotypes: study designs and applications. Nature Reviews Genetics. 2012 Nov;13(11):759–69. doi: 10.1038/nrg3314 23032255
10. Suhre K, Wallaschofski H, Raffler J, Friedrich N, Haring R, Michael K, et al. A genome-wide association study of metabolic traits in human urine. Nature Genetics. 2011 Jun;43(6):565–9. doi: 10.1038/ng.837 21572414
11. Montoliu I, Genick U, Ledda M, Collino S, Martin FP, le Coutre J, et al. Current status on genome-metabolome-wide associations: an opportunity in nutrition research. Genes & Nutrition. 2013 Jan;8(1):19–27.
12. Rueedi R, Ledda M, Nicholls AW, Salek RM, Marques-Vidal P, Morya E, et al. Genome-wide association study of metabolic traits reveals novel gene-metabolite-disease links. PLoS Genetics. 2014 Feb;10(2):e1004132. doi: 10.1371/journal.pgen.1004132 24586186
13. Alonso A, Rodríguez MA, Vinaixa M, Tortosa R, Correig X, Julià A, et al. Focus: a robust workflow for one-dimensional NMR spectral analysis. Analytical Chemistry. 2014 Jan 21;86(2):1160–9. doi: 10.1021/ac403110u 24354303
14. Xie W, Wood AR, Lyssenko V, Weedon MN, Knowles JW, Alkayyali S, et al. Genetic variants associated with glycine metabolism and their role in insulin sensitivity and type 2 diabetes. Diabetes. 2013 Jun;62(6):2141–50. doi: 10.2337/db12-0876 23378610
15. Sabater-Lleal M, Huang J, Chasman D, Naitza S, Dehghan A, Johnson AD, et al. Multiethnic meta-analysis of genome-wide association studies in >100 000 subjects identifies 23 fibrinogen-associated Loci but no strong evidence of a causal association between circulating fibrinogen and cardiovascular disease. Circulation. 2013 Sep 17;128(12):1310–24. doi: 10.1161/CIRCULATIONAHA.113.002251 23969696
16. Hong MG, Karlsson R, Magnusson PK, Lewis MR, Isaacs W, Zheng LS, et al. A genome-wide assessment of variability in human serum metabolism. Human Mutation. 2013 Mar;34(3):515–24. doi: 10.1002/humu.22267 23281178
17. Global Lipids Genetics C, Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, et al. Discovery and refinement of loci associated with lipid levels. Nature Genetics. 2013 Nov;45(11):1274–83. doi: 10.1038/ng.2797 24097068
18. Evans DM, Zhu G, Dy V, Heath AC, Madden PA, Kemp JP, et al. Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Human Molecular Genetics. 2013 Oct 1;22(19):3998–4006. doi: 10.1093/hmg/ddt239 23720494
19. Chambers JC, Zhang W, Lord GM, van der Harst P, Lawlor DA, Sehmi JS, et al. Genetic loci influencing kidney function and chronic kidney disease. Nature Genetics. 2010 May;42(5):373–5. doi: 10.1038/ng.566 20383145
20. Seppälä I, Kleber ME, Lyytikäinen LP, Hernesniemi JA, Mäkelä KM, Oksala N, et al. Genome-wide association study on dimethylarginines reveals novel AGXT2 variants associated with heart rate variability but not with overall mortality. European Heart Journal. 2014 Feb;35(8):524–31. doi: 10.1093/eurheartj/eht447 24159190
21. Shin S-Y, Fauman EB, Petersen A-K, Krumsiek J, Santos R, Huang J, et al. An atlas of genetic influences on human blood metabolites. Nature Genetics. 2014 Jun;46(6):543–50. doi: 10.1038/ng.2982 24816252
22. Raffler J, Rämisch-Margl W, Petersen AK, Pagel P, Blöchl F, Hengstenberg C, et al. Identification and MS-assisted interpretation of genetically influenced NMR signals in human plasma. Genome Medicine. 2013 Feb 15;5(2):13. doi: 10.1186/gm417 23414815
23. Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Research. 2014 Jan;42(Database issue):D1001–6. doi: 10.1093/nar/gkt1229 24316577
24. Demirkan A, Henneman P, Verhoeven A, Dharuri H, Amin N, van Klinken JB, et al. Insight in genome-wide association of metabolite quantitative traits by exome sequence analyses. PLoS Genetics. 2015 Jan;11(1):e1004835. doi: 10.1371/journal.pgen.1004835 25569235
25. Yu B, Zheng Y, Alexander D, Morrison AC, Coresh J, Boerwinkle E. Genetic Determinants Influencing Human Serum Metabolome among African Americans. PLoS Genetics. 2014 Mar;10(3):e1004212. doi: 10.1371/journal.pgen.1004212 24625756
26. Köttgen A, Pattaro C, Böger CA, Fuchsberger C, Olden M, Glazer NL, et al. New loci associated with kidney function and chronic kidney disease. Nature Genetics. 2010 May;42(5):376–84. doi: 10.1038/ng.568 20383146
27. Tin A, Colantuoni E, Boerwinkle E, Kottgen A, Franceschini N, Astor BC, et al. Using multiple measures for quantitative trait association analyses: application to estimated glomerular filtration rate. Journal of Human Genetics. 2013 Jul;58(7):461–6. doi: 10.1038/jhg.2013.23 23535967
28. Rhee EP, Ho JE, Chen MH, Shen D, Cheng S, Larson MG, et al. A genome-wide association study of the human metabolome in a community-based cohort. Cell Metabolism. 2013 Jul 2;18(1):130–43. doi: 10.1016/j.cmet.2013.06.013 23823483
29. Kleber ME, Seppälä I, Pilz S, Hoffmann MM, Tomaschitz A, Oksala N, et al. Genome-wide association study identifies 3 genomic loci significantly associated with serum levels of homoarginine: the AtheroRemo Consortium. Circulation Cardiovascular Genetics. 2013 Oct;6(5):505–13. doi: 10.1161/CIRCGENETICS.113.000108 24047826
30. Lange LA, Croteau-Chonka DC, Marvelle AF, Qin L, Gaulton KJ, Kuzawa CW, et al. Genome-wide association study of homocysteine levels in Filipinos provides evidence for CPS1 in women and a stronger MTHFR effect in young adults. Human Molecular Genetics. 2010 May 15;19(10):2050–8. doi: 10.1093/hmg/ddq062 20154341
31. Summar ML, Gainer JV, Pretorius M, Malave H, Harris S, Hall LD, et al. Relationship between carbamoyl-phosphate synthetase genotype and systemic vascular function. Hypertension. 2004 Feb;43(2):186–91. 14718356
32. Zhang Y, Tong Y, Zhang Y, Ding H, Zhang H, Geng Y, et al. Two Novel Susceptibility SNPs for Ischemic Stroke Using Exome Sequencing in Chinese Han Population. Molecular Neurobiology. 2014 Apr;49(2):852–62. doi: 10.1007/s12035-013-8561-0 24122314
33. Bunker RD, Bulloch EM, Dickson JM, Loomes KM, Baker EN. Structure and function of human xylulokinase, an enzyme with important roles in carbohydrate metabolism. The Journal of Biological Chemistry. 2013 Jan 18;288(3):1643–52. doi: 10.1074/jbc.M112.427997 23179721
34. Jung JY, Lee HS, Kang DG, Kim NS, Cha MH, Bang OS, et al. 1H-NMR-based metabolomics study of cerebral infarction. Stroke; a journal of cerebral circulation. 2011 May;42(5):1282–8. doi: 10.1161/STROKEAHA.110.598789 21474802
35. Bröer S, Bailey CG, Kowalczuk S, Ng C, Vanslambrouck JM, Rodgers H, et al. Iminoglycinuria and hyperglycinuria are discrete human phenotypes resulting from complex mutations in proline and glycine transporters. The Journal of Clinical Investigation. 2008 Dec;118(12):3881–92. doi: 10.1172/JCI36625 19033659
36. Kittel A, Müller F, König J, Mieth M, Sticht H, Zolk O, et al. Alanine-glyoxylate aminotransferase 2 (AGXT2) polymorphisms have considerable impact on methylarginine and beta-aminoisobutyrate metabolism in healthy volunteers. PloS ONE. 2014;9(2):e88544. doi: 10.1371/journal.pone.0088544 24586340
37. Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010 Aug 5;466(7307):707–13. doi: 10.1038/nature09270 20686565
38. Rothman N, Garcia-Closas M, Chatterjee N, Malats N, Wu X, Figueroa JD, et al. A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nature Genetics. 2010 Nov;42(11):978–84. doi: 10.1038/ng.687 20972438
39. Figueroa JD, Ye Y, Siddiq A, Garcia-Closas M, Chatterjee N, Prokunina-Olsson L, et al. Genome-wide association study identifies multiple loci associated with bladder cancer risk. Human Molecular Genetics. 2014 Mar 1;23(5):1387–98. doi: 10.1093/hmg/ddt519 24163127
40. Hein DW. Molecular genetics and function of NAT1 and NAT2: role in aromatic amine metabolism and carcinogenesis. Mutation Research. 2002 Sep 30;506–507 : 65–77. 12351146
41. Magalon H, Patin E, Austerlitz F, Hegay T, Aldashev A, Quintana-Murci L, et al. Population genetic diversity of the NAT2 gene supports a role of acetylation in human adaptation to farming in Central Asia. European Journal of Human Genetics: EJHG. 2008 Feb;16(2):243–51. 18043717
42. Patin E, Barreiro LB, Sabeti PC, Austerlitz F, Luca F, Sajantila A, et al. Deciphering the ancient and complex evolutionary history of human arylamine N-acetyltransferase genes. American Journal of Human Genetics. 2006 Mar;78(3):423–36. 16416399
43. Vatsis KP, Martell KJ, Weber WW. Diverse point mutations in the human gene for polymorphic N-acetyltransferase. Proceedings of the National Academy of Sciences of the United States of America. 1991 Jul 15;88(14):6333–7. 2068113
44. Gahl WA, Huizing M. Hermansky-Pudlak Syndrome. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, et al., editors. GeneReviews(R). Seattle (WA)1993.
45. Tomoeda K, Awata H, Matsuura T, Matsuda I, Ploechl E, Milovac T, et al. Mutations in the 4-hydroxyphenylpyruvic acid dioxygenase gene are responsible for tyrosinemia type III and hawkinsinuria. Molecular Genetics and Metabolism. 2000 Nov;71(3):506–10. 11073718
46. Fujino T, Takei YA, Sone H, Ioka RX, Kamataki A, Magoori K, et al. Molecular identification and characterization of two medium-chain acyl-CoA synthetases, MACS1 and the Sa gene product. The Journal of Biological Chemistry. 2001 Sep 21;276(38):35961–6. 11470804
47. Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014 Feb 20;506(7488):376–81. doi: 10.1038/nature12873 24390342
48. Rodríguez-Flores JL, Zhang K, Kang SW, Wen G, Ghosh S, Friese RS, et al. Conserved regulatory motifs at phenylethanolamine N-methyltransferase (PNMT) are disrupted by common functional genetic variation: an integrated computational/experimental approach. Mammalian Genome. 2010 Apr;21(3–4):195–204. doi: 10.1007/s00335-010-9253-y 20204374
49. Wang H, Fei YJ, Kekuda R, Yang-Feng TL, Devoe LD, Leibach FH, et al. Structure, function, and genomic organization of human Na(+)-dependent high-affinity dicarboxylate transporter. American journal of physiology Cell Physiology. 2000 May;278(5):C1019–30. 10794676
50. Genomes Project C, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012 Nov 1;491(7422):56–65. doi: 10.1038/nature11632 23128226
51. Arnold M, Raffler J, Pfeufer A, Suhre K, Kastenmüller G. SNiPA: an interactive, genetic variant-centered annotation browser. Bioinformatics. 2015 Apr 15;31(8):1334–6. doi: 10.1093/bioinformatics/btu779 25431330
52. Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015 Jan 23;347(6220):1260419. doi: 10.1126/science.1260419 25613900
53. Petersen AK, Krumsiek J, Wägele B, Theis FJ, Wichmann HE, Gieger C, et al. On the hypothesis-free testing of metabolite ratios in genome-wide and metabolome-wide association studies. BMC Bioinformatics. 2012;13 : 120. doi: 10.1186/1471-2105-13-120 22672667
54. Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, et al. The human urine metabolome. PloS ONE. 2013;8(9):e73076. doi: 10.1371/journal.pone.0073076 24023812
55. Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Research. 2014 Jan;42(Database issue):D980–5. doi: 10.1093/nar/gkt1113 24234437
56. Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Human Genetics. 2014 Jan;133(1):1–9. 24077912
57. Mailman MD, Feolo M, Jin Y, Kimura M, Tryka K, Bagoutdinov R, et al. The NCBI dbGaP database of genotypes and phenotypes. Nature Genetics. 2007 Oct;39(10):1181–6. 17898773
58. Larive CK, Barding GA Jr., Dinges MM. NMR spectroscopy for metabolomics and metabolic profiling. Analytical Chemistry. 2015 Jan 6;87(1):133–46. doi: 10.1021/ac504075g 25375201
59. Veiga-da-Cunha M, Hadi F, Balligand T, Stroobant V, Van Schaftingen E. Molecular identification of hydroxylysine kinase and of ammoniophospholyases acting on 5-phosphohydroxy-L-lysine and phosphoethanolamine. The Journal of Biological Chemistry. 2012 Mar 2;287(10):7246–55. doi: 10.1074/jbc.M111.323485 22241472
60. Shao L, Vawter MP. Shared gene expression alterations in schizophrenia and bipolar disorder. Biological Psychiatry. 2008 Jul 15;64(2):89–97. doi: 10.1016/j.biopsych.2007.11.010 18191109
61. Benson JM, Tibbetts BM, Barr EB. The uptake, distribution, metabolism, and excretion of methyl tertiary-butyl ether inhaled alone and in combination with gasoline vapor. Journal of Toxicology and Environmental Health Part A. 2003 Jun 13;66(11):1029–52. 12775515
62. Amberg A, Rosner E, Dekant W. Biotransformation and kinetics of excretion of methyl-tert-butyl ether in rats and humans. Toxicological Sciences. 1999 Sep;51(1):1–8. 10496672
63. McGregor D. Ethyl tertiary-butyl ether: a toxicological review. Critical Reviews in Toxicology. 2007 May;37(4):287–312. 17453936
64. Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2008 Feb 12;105(6):2117–22. doi: 10.1073/pnas.0712038105 18252821
65. Altmaier E, Fobo G, Heier M, Thorand B, Meisinger C, Römisch-Margl W, et al. Metabolomics approach reveals effects of antihypertensives and lipid-lowering drugs on the human metabolism. European Journal of Epidemiology. 2014 May;29(5):325–36. doi: 10.1007/s10654-014-9910-7 24816436
66. Dai L, Peng C, Montellier E, Lu Z, Chen Y, Ishii H, et al. Lysine 2-hydroxyisobutyrylation is a widely distributed active histone mark. Nature Chemical Biology. 2014 May;10(5):365–70. doi: 10.1038/nchembio.1497 24681537
67. Lee JH, Tate CM, You JS, Skalnik DG. Identification and characterization of the human Set1B histone H3-Lys4 methyltransferase complex. The Journal of Biological Chemistry. 2007 May 4;282(18):13419–28. 17355966
68. Bär A, Oesterhelt G. Conversion of [U-13C]xylitol and D-[U-13C]glucose into urinary [1,2-13C]glycollate and [1,2-13C]oxalate in man. International journal for vitamin and nutrition research Supplement = Internationale Zeitschrift fur Vitamin - und Ernahrungsforschung Supplement. 1985;28 : 119–33. 3938801
69. Conyers RA, Huber TW, Thomas DW, Rofe AM, Bais R, Edwards RG. A one-compartment model for calcium oxalate tissue deposition during xylitol infusions in humans. International journal for vitamin and nutrition research Supplement = Internationale Zeitschrift fur Vitamin - und Ernahrungsforschung Supplement. 1985;28 : 47–57. 3938803
70. Holmes RP, Assimos DG. Glyoxylate synthesis, and its modulation and influence on oxalate synthesis. The Journal of Urology. 1998 Nov;160(5):1617–24. 9783918
71. Conyers RA, Bais R, Rofe AM. The relation of clinical catastrophes, endogenous oxalate production, and urolithiasis. Clinical Chemistry. 1990 Oct;36(10):1717–30. 2208646
72. Rao NM, Yallapragada A, Winden KD, Saver J, Liebeskind DS. Stroke in primary hyperoxaluria type I. Journal of Neuroimaging. 2014 Jul-Aug;24(4):411–3. doi: 10.1111/jon.12020 23551880
73. Bayar C, Ozer I. A study on the route of 1-methylurate formation in theophylline metabolism. European Journal of Drug Metabolism and Pharmacokinetics. 1997 Oct-Dec;22(4):415–9. 9512943
74. Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nature Genetics. 2005 Jul;37(7):766–70. 15965474
75. Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007 Jun 29;129(7):1401–14. 17604727
76. Sewer A, Paul N, Landgraf P, Aravin A, Pfeffer S, Brownstein MJ, et al. Identification of clustered microRNAs using an ab initio prediction method. BMC Bioinformatics. 2005;6 : 267. 16274478
77. Pekkala S, Martinez AI, Barcelona B, Yefimenko I, Finckh U, Rubio V, et al. Understanding carbamoyl-phosphate synthetase I (CPS1) deficiency by using expression studies and structure-based analysis. Human Mutation. 2010 Jul;31(7):801–8. doi: 10.1002/humu.21272 20578160
78. Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Molecular and Cellular Biochemistry. 1973 Jun 27;1(2):169–87. 4585091
79. Kikuchi G, Motokawa Y, Yoshida T, Hiraga K. Glycine cleavage system: reaction mechanism, physiological significance, and hyperglycinemia. Proceedings of the Japan Academy Series B, Physical and Biological Sciences. 2008;84(7):246–63. 18941301
80. Roll P, Massacrier A, Pereira S, Robaglia-Schlupp A, Cau P, Szepetowski P. New human sodium/glucose cotransporter gene (KST1): identification, characterization, and mutation analysis in ICCA (infantile convulsions and choreoathetosis) and BFIC (benign familial infantile convulsions) families. Gene. 2002 Feb 20;285(1–2):141–8. 12039040
81. Groenen PM, Klootwijk R, Schijvenaars MM, Straatman H, Mariman EC, Franke B, et al. Spina bifida and genetic factors related to myo-inositol, glucose, and zinc. Molecular Genetics and Metabolism. 2004 Jun;82(2):154–61. 15172003
82. Aouameur R, Da Cal S, Bissonnette P, Coady MJ, Lapointe JY. SMIT2 mediates all myo-inositol uptake in apical membranes of rat small intestine. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2007 Dec;293(6):G1300–7. 17932225
83. Lahjouji K, Aouameur R, Bissonnette P, Coady MJ, Bichet DG, Lapointe JY. Expression and functionality of the Na+/myo-inositol cotransporter SMIT2 in rabbit kidney. Biochimica et Biophysica Acta. 2007 May;1768(5):1154–9. 17306760
84. John U, Greiner B, Hensel E, Lüdemann J, Piek M, Sauer S, et al. Study of Health In Pomerania (SHIP): a health examination survey in an east German region: objectives and design. Sozial - und Präventivmedizin. 2001;46(3):186–94. 11565448
85. Völzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, et al. Cohort profile: the study of health in Pomerania. International Journal of Epidemiology. 2011 Apr;40(2):294–307. doi: 10.1093/ije/dyp394 20167617
86. Holle R, Happich M, Löwel H, Wichmann HE, Group MKS. KORA—a research platform for population based health research. Gesundheitswesen. 2005 Aug;67 Suppl 1:S19–25. 16032513
87. Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nature Methods. 2012 Feb;9(2):179–81.
88. Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genetics. 2009 Jun;5(6):e1000529. doi: 10.1371/journal.pgen.1000529 19543373
89. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of Human Genetics. 2007 Sep;81(3):559–75. 17701901
90. Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Research. 2013 Jan;41(Database issue):D801–7. doi: 10.1093/nar/gks1065 23161693
91. Barngrover DA, Stevens HC, Dills WL Jr.D-Xylulose-1-phosphate: enzymatic assay and production in isolated rat hepatocytes. Biochemical and Biophysical Research Communications. 1981 Sep 16;102(1):75–80. 6458298
Štítky
Genetika Reprodukčná medicína
Článek The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis inČlánek Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Retraction: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Signaling from Within: Endocytic Trafficking of the Robo Receptor Is Required for Midline Axon Repulsion
- A Splice Region Variant in Lowers Non-high Density Lipoprotein Cholesterol and Protects against Coronary Artery Disease
- The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis in
- A NIMA-Related Kinase Suppresses the Flagellar Instability Associated with the Loss of Multiple Axonemal Structures
- Slit-Dependent Endocytic Trafficking of the Robo Receptor Is Required for Son of Sevenless Recruitment and Midline Axon Repulsion
- Expression of Concern: Protein Under-Wrapping Causes Dosage Sensitivity and Decreases Gene Duplicability
- Mutagenesis by AID: Being in the Right Place at the Right Time
- Identification of as a Genetic Modifier That Regulates the Global Orientation of Mammalian Hair Follicles
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- Evaluating the Performance of Fine-Mapping Strategies at Common Variant GWAS Loci
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- Differential Expression of Ecdysone Receptor Leads to Variation in Phenotypic Plasticity across Serial Homologs
- Receptor Polymorphism and Genomic Structure Interact to Shape Bitter Taste Perception
- Cognitive Function Related to the Gene Acquired from an LTR Retrotransposon in Eutherians
- Critical Function of γH2A in S-Phase
- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
- Integration of Genome-Wide SNP Data and Gene-Expression Profiles Reveals Six Novel Loci and Regulatory Mechanisms for Amino Acids and Acylcarnitines in Whole Blood
- A Genome-Wide Association Study of a Biomarker of Nicotine Metabolism
- Cell Cycle Regulates Nuclear Stability of AID and Determines the Cellular Response to AID
- A Genome-Wide Association Analysis Reveals Epistatic Cancellation of Additive Genetic Variance for Root Length in
- Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
- RAB-10-Dependent Membrane Transport Is Required for Dendrite Arborization
- Basolateral Endocytic Recycling Requires RAB-10 and AMPH-1 Mediated Recruitment of RAB-5 GAP TBC-2 to Endosomes
- Dynamic Contacts of U2, RES, Cwc25, Prp8 and Prp45 Proteins with the Pre-mRNA Branch-Site and 3' Splice Site during Catalytic Activation and Step 1 Catalysis in Yeast Spliceosomes
- ARID1A Is Essential for Endometrial Function during Early Pregnancy
- Predicting Carriers of Ongoing Selective Sweeps without Knowledge of the Favored Allele
- An Interaction between RRP6 and SU(VAR)3-9 Targets RRP6 to Heterochromatin and Contributes to Heterochromatin Maintenance in
- Photoreceptor Specificity in the Light-Induced and COP1-Mediated Rapid Degradation of the Repressor of Photomorphogenesis SPA2 in Arabidopsis
- Autophosphorylation of the Bacterial Tyrosine-Kinase CpsD Connects Capsule Synthesis with the Cell Cycle in
- Multimer Formation Explains Allelic Suppression of PRDM9 Recombination Hotspots
- Rescheduling Behavioral Subunits of a Fixed Action Pattern by Genetic Manipulation of Peptidergic Signaling
- A Gene Regulatory Program for Meiotic Prophase in the Fetal Ovary
- Cell-Autonomous Gβ Signaling Defines Neuron-Specific Steady State Serotonin Synthesis in
- Discovering Genetic Interactions in Large-Scale Association Studies by Stage-wise Likelihood Ratio Tests
- The RCC1 Family Protein TCF1 Regulates Freezing Tolerance and Cold Acclimation through Modulating Lignin Biosynthesis
- The AMPK, Snf1, Negatively Regulates the Hog1 MAPK Pathway in ER Stress Response
- The Parkinson’s Disease-Associated Protein Kinase LRRK2 Modulates Notch Signaling through the Endosomal Pathway
- Multicopy Single-Stranded DNA Directs Intestinal Colonization of Enteric Pathogens
- Recurrent Domestication by Lepidoptera of Genes from Their Parasites Mediated by Bracoviruses
- Three Different Pathways Prevent Chromosome Segregation in the Presence of DNA Damage or Replication Stress in Budding Yeast
- Identification of Four Mouse Diabetes Candidate Genes Altering β-Cell Proliferation
- The Intolerance of Regulatory Sequence to Genetic Variation Predicts Gene Dosage Sensitivity
- Synergistic and Dose-Controlled Regulation of Cellulase Gene Expression in
- Genome Sequence and Transcriptome Analyses of : Metabolic Tools for Enhanced Algal Fitness in the Prominent Order Prymnesiales (Haptophyceae)
- Ty3 Retrotransposon Hijacks Mating Yeast RNA Processing Bodies to Infect New Genomes
- FUS Interacts with HSP60 to Promote Mitochondrial Damage
- Point Mutations in Centromeric Histone Induce Post-zygotic Incompatibility and Uniparental Inheritance
- Genome-Wide Association Study with Targeted and Non-targeted NMR Metabolomics Identifies 15 Novel Loci of Urinary Human Metabolic Individuality
- Outer Hair Cell Lateral Wall Structure Constrains the Mobility of Plasma Membrane Proteins
- A Large-Scale Functional Analysis of Putative Target Genes of Mating-Type Loci Provides Insight into the Regulation of Sexual Development of the Cereal Pathogen
- A Genetic Selection for Mutants Reveals an Interaction between DNA Polymerase IV and the Replicative Polymerase That Is Required for Translesion Synthesis
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



