-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Multicopy Single-Stranded DNA Directs Intestinal Colonization of Enteric Pathogens
Multicopy single-stranded DNA (msDNA) is a unique molecule consisting of both an RNA and DNA portion. This molecule is produced by a reverse transcriptase and has no known natural function despite more than 30 years of study. We report that msDNA is important for both Salmonella Typhimurium and an enteropathogenic E. coli, two pathogens that cause diarrhea in susceptible hosts, to survive in the intestine. Using mutant strains incapable of producing msDNA, we show that msDNA is needed for Salmonella to grow in the absence of oxygen. Mutants grown in oxygen-deficient conditions have substantial changes in overall protein composition, including numerous proteins known to be important for anaerobic metabolism and growth in the intestine. Our findings link msDNA to the ability of Salmonella to thrive in an oxygen-deficient environment similar to the conditions inside the gut. We report that msDNA regulates the quantity of proteins, the first natural function attributed to this molecule. msDNA may represent a new class of regulatory molecules.
Published in the journal: Multicopy Single-Stranded DNA Directs Intestinal Colonization of Enteric Pathogens. PLoS Genet 11(9): e32767. doi:10.1371/journal.pgen.1005472
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005472Summary
Multicopy single-stranded DNA (msDNA) is a unique molecule consisting of both an RNA and DNA portion. This molecule is produced by a reverse transcriptase and has no known natural function despite more than 30 years of study. We report that msDNA is important for both Salmonella Typhimurium and an enteropathogenic E. coli, two pathogens that cause diarrhea in susceptible hosts, to survive in the intestine. Using mutant strains incapable of producing msDNA, we show that msDNA is needed for Salmonella to grow in the absence of oxygen. Mutants grown in oxygen-deficient conditions have substantial changes in overall protein composition, including numerous proteins known to be important for anaerobic metabolism and growth in the intestine. Our findings link msDNA to the ability of Salmonella to thrive in an oxygen-deficient environment similar to the conditions inside the gut. We report that msDNA regulates the quantity of proteins, the first natural function attributed to this molecule. msDNA may represent a new class of regulatory molecules.
Introduction
Retron reverse transcriptases (RT) in bacteria were first described in Myxococcus xanthus [1] and E. coli [2] in the 1980s and are now known to be widely distributed in the genomes of eubacteria and archaea (reviewed in [3]). All retrons contain three regions essential for production of msDNA: msr (RNA primer for reverse transcription), msd (template sequence), and a reverse transcriptase (RT). The retrons of pathogens, such as Salmonella Typhimurium (STm), may also encode an additional ORF of unknown function [4]. The product of the ‘retron’ is a small covalently linked RNA-DNA hybrid molecule called multicopy single-stranded DNA (msDNA) that is predicted to form complex secondary structures [5]. The predicted secondary structures of msDNA from enteric pathogens including STm, enteropathogenic E. coli and Vibrio spp. are similar [4] but the reverse transcriptase amino acid sequence from these enteric pathogens share little identity. The location of the retron as well as the number of retrons in each species varies. These observations suggest that retrons have been horizontally acquired by convergent evolution to function in a fashion that is specific to the biology of the host bacterium.
Although the molecular details of the production of msDNA have been heavily studied, no natural function has been attributed to this mysterious molecule despite 30 years of study (reviewed in [3]). A critical obstacle to elucidating the natural function of msDNA was the lack of any phenotype for mutants unable to make this molecule. We have shown that the retron reverse transcriptase encoded by STM3846 is essential for Salmonella Typhimurium (STm) to colonize the calf intestine [6], a natural model of enteric salmonellosis that recapitulates the earliest stages of human non-typhoidal Salmonella (NTS) infection. This was the first reported phenotype for a mutant lacking a retron reverse transcriptase.
NTS are major threats to global animal and human health, causing more than 90 million cases of gastroenteritis in people worldwide [7]. Human enteric salmonellosis is characterized by inflammatory diarrhea containing primarily neutrophils. To efficiently colonize the host, NTS use the type 3-secretion system 1 (T3SS-1) encoded on Salmonella Pathogenicity Island-1 (SPI-1) to invade the intestinal epithelium [8,9] and to promote the characteristic neutrophilic inflammatory response. The host inflammatory response gives Salmonella a competitive advantage over resident microflora. Within the intestinal lumen, the product of the neutrophilic oxidative burst generates tetrathionate from oxidation of thiosulfate [10]. Salmonella uses tetrathionate as a terminal electron acceptor within the anaerobic conditions of the intestinal lumen to gain a competitive advantage over resident microflora. Effectors of the TTSS-1 may directly activate epithelial production of inducible nitric oxide synthase (iNOS) thereby creating nitrate, an additional terminal electron acceptor [11]. The relative importance of nitrate during infection is illustrated by the fact that it is a powerful chemoattractant for Salmonella during anaerobiosis [12]. In addition, Salmonella uses host-derived nutrients such as ethanolamine [13] during intestinal inflammation. These strategies facilitate the growth of Salmonella in the complex microbial community of the intestine.
We used the enteric pathogen, Salmonella Typhimurium, to dissect the function of msDNA. In the work described here, we report that mutants lacking msDNA produced by the STM3846 reverse transcriptase are defective for colonization of the intestine using murine models of salmonellosis. This colonization defect is due, in part, to a growth defect for these mutants in anaerobic conditions. We show that mutants lacking msDNA have altered abundance of over 200 proteins in anaerobiosis, many of which are known to be required for growth in anaerobic conditions and for the pathogenesis of STm during enteric infection. Inappropriate abundance of proteins encoding alternate terminal electron acceptor reductases results in an inability of mutants lacking msDNA to utilize these compounds, inhibiting anaerobic growth in vitro. The mutants lacking msDNA can only utilize nitrate as an anaerobic terminal electron acceptor. Mutants lacking msDNA fail to colonize portions of the intestine lacking substantial neutrophilic inflammation, likely due to the ability to only utilize nitrate to support anaerobic growth. Finally, we report a similar defect in intestinal persistence for an enteropathogenic E. coli lacking its retron reverse transcriptase suggesting that msDNA is critical for enteric pathogens to thrive in the intestine of mammalian hosts. Thus, we report a role in regulating protein abundance for msDNA, the first reported natural function for any msDNA. msDNA may represent a new class of bacterial regulatory molecules.
Results
msDNA is critical for STm intestinal colonization
Retron reverse transcriptases, including the STM3846 reverse transcriptase of the St-85 retron, use msr to prime reverse transcription of the msd template sequence to produce msDNA [14] (Fig 1A). We generated a non-polar deletion of msd to establish that msDNA, and not some other potential product of the STM3846 RT, mediates STm colonization of the intestine. Neither the ΔSTM3846 mutant nor the Δmsd mutant produce msDNA and its production can be restored in both mutants by complementation in trans (Fig 1B). The additional ORF, STM3845, is dispensable for msDNA production.
Fig. 1. msDNA is produced by msd and STM3846 and is essential for Salmonella to colonize the intestine. 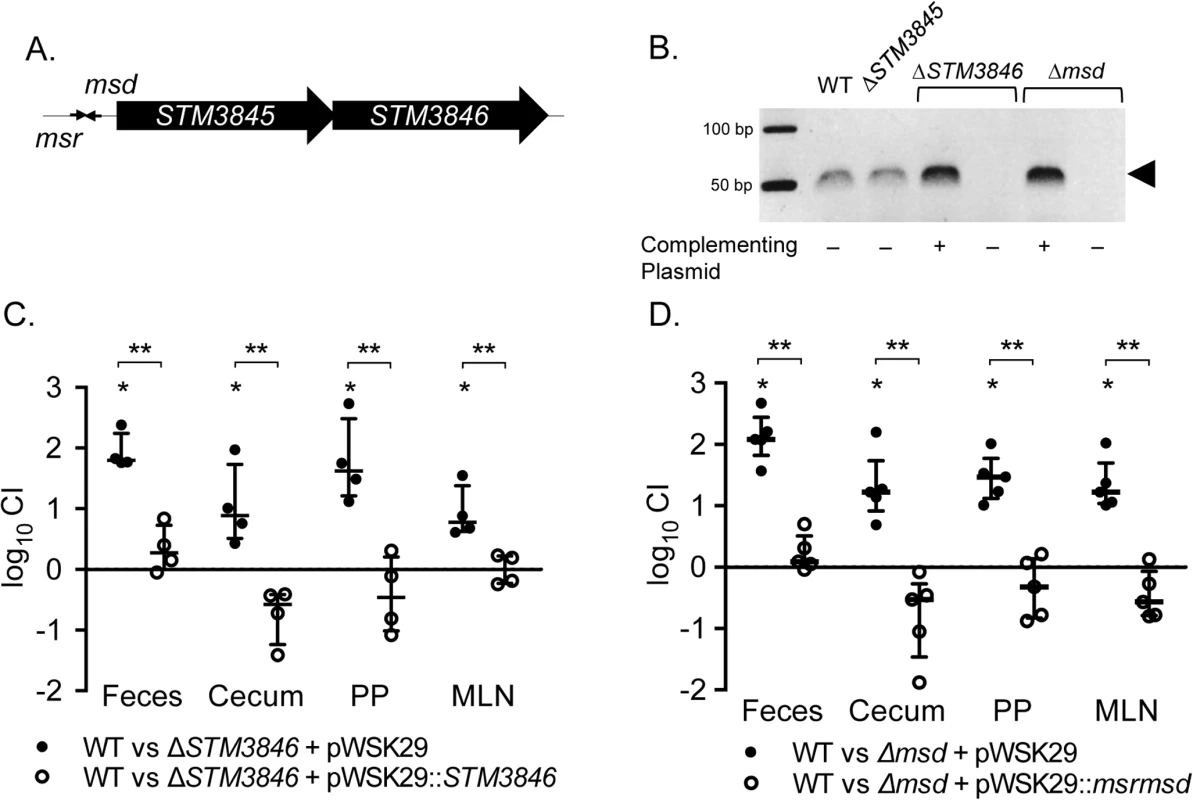
(A) Genomic context of the retron St-85 (adapted from [6]). (B) msDNA was isolated from late-exponential phase cultures normalized by OD600 and visualized on a native 12% polyacrylamide gel with in-gel ethidium bromide staining. msDNA is depicted by the arrowhead (WT—HA420; ΔSTM3845—JE143; ΔSTM3846 + pWSK29—JE122; ΔSTM3846 + pSTM3846—HA1446; Δmsd + pWSK29—JE144; Δmsd + pRetronpro—JE145). (C-D) C57Bl/6 mice were treated orally with 20 mg streptomycin and infected with an equivalent mixture of (C) WT (HA697) and ΔSTM3846 bearing empty vector (closed circles; n = 4; JE63) or WT and complemented ΔSTM3846 mutant (open circles; n = 4; JE65) 24-hours after antibiotic treatment or (D) WT and Δmsd bearing empty vector (closed circles; n = 5; JE156) or WT and complemented Δmsd mutant (open circles; n = 5; JE154). Feces were collected 24-hours after infection and mice were euthanized 96-hours post-infection to harvest cecum, Peyer’s patches (PP), and mesenteric lymph nodes (MLN). Organ homogenates were serially diluted and plated to enumerate CFU. Competitive index (CI) was determined by comparing the ratio of WT to mutant in the organ with that of the inoculum. Each data point represents data from a single animal with median and interquartile range indicated. Statistical significance was determined by a t-test with * P < 0.05 (WT vs mutant) and ** P < 0.05 (between infection groups). We used the murine colitis model [15], which responds to NTS infection with profound neutrophilic inflammation in the cecum, to dissect the function of the retron in intestinal colonization. We confirmed the requirement for STM3846 in colonization of the inflamed intestine in this model (Fig 1C). In addition, both the Δmsd and ΔSTM3846 mutants have indistinguishable phenotypes, suggesting that the effect of deletion of the RT is mediated by the msDNA itself. The ability of each of these mutants to colonize the intestine is rescued by complementation in trans (Fig 1C and 1D). In cell culture, only the Δmsd mutant invades epithelial cells at a level mildly reduced compared to the isogenic wild type (S1 Fig) suggesting that reduced tissue invasion is unlikely to be the cause of the phenotype that we observed during infection of animal models. Our findings definitively link msDNA to the ability of Salmonella to colonize the intestine.
msDNA and anaerobiosis
The intestine is a specialized and highly diverse niche. Oxygen tensions within the lumen decline from the stomach to the colon [16,17], and there is a gradient of increasing oxygen tension from the center of the lumen towards the epithelium [18]. Enteric pathogens must replicate in this hypoxic setting using both aerobic and anaerobic metabolic pathways [19,20] and express genes necessary for virulence in order to compete with resident microflora and colonize the host efficiently.
To determine whether the intestinal colonization defect of the STm msDNA mutants could be due to an inability to grow in oxygen limited conditions, we measured the growth of our mutants in the absence of oxygen, a condition where the retron is highly expressed [21]. Both mutants unable to produce msDNA have severe growth defects in rich media in anaerobic conditions (Fig 2A and 2B, S2 Fig), while the growth of these mutants in the presence of oxygen is similar to the isogenic WT in both rich and minimal media (Fig 2C–2F). The necessity for msDNA during anaerobic growth is consistent with the inability of msDNA-deficient mutants to efficiently colonize the intestine.
Fig. 2. Mutants lacking msDNA are defective for anaerobic growth. 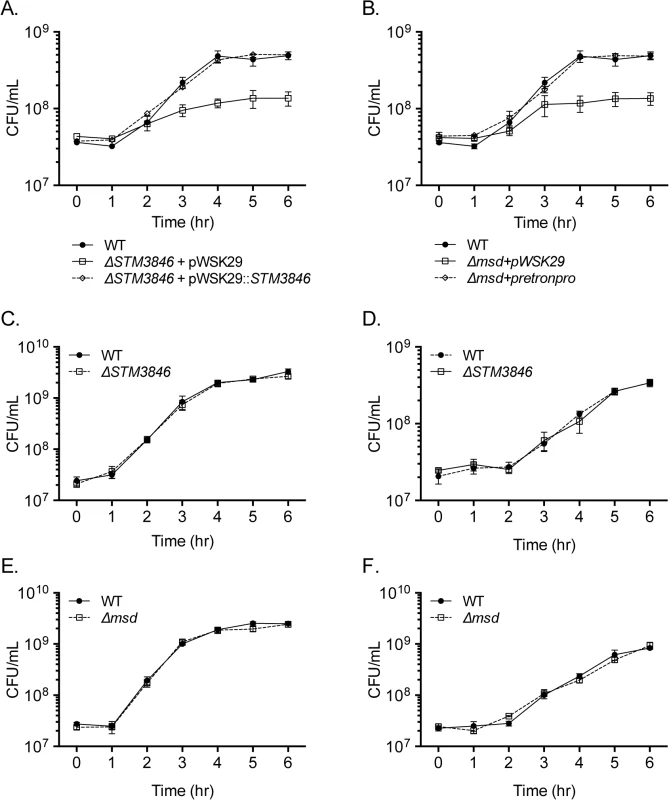
(A-B) Overnight cultures of WT (closed circles; HA420), ΔSTM3846 (A; JE122) or Δmsd (B; JE144) with empty vector (open squares), and complemented ΔSTM3846 (A; HA1446) or complemented Δmsd (B; JE145) mutant (open diamonds) were transferred into an anaerobic chamber, sub-cultured 1:100 into LB broth pre-incubated for 24 hours in anaerobic conditions, and incubated at 37°C. CFU were determined hourly. Data shown are mean +/- SEM of 3 independent experiments. (C-D) Aerobic growth of WT (closed circles; HA420) and ΔSTM3846 mutant (open squares; HA1444) in (C) LB and (D) M9 minimal media. (E-F) Aerobic growth of WT (closed circles; HA420) and Δmsd mutant (open squares; JE135) in (E) LB and (F) M9 minimal media. Each data point is the mean +/- SEM of three independent experiments. msDNA as a regulator
We hypothesized that msDNA might act as a trans regulator of gene expression for two reasons. First, small RNAs are well known to have regulatory properties through base pairing with DNA or mRNA transcripts [22]. Second, substantial over-expression of msDNA from one strain of E. coli in a heterologous strain lacking a retron resulted in small changes in the proteome [23]. To determine whether the msDNA produced by the St-85 retron might have regulatory properties, we evaluated the proteome of the WT and msDNA-deficient mutants (ΔSTM3846 and Δmsd) at late exponential phase, a time when the retron is expressed and msDNA is produced (Fig 1B and [24]), in both the presence and absence of oxygen. Of the 1504 total proteins identified, no significant differences in protein abundance between the WT and mutants in the presence of oxygen were detected (Fig 3 and S1 Table). This finding is consistent with previous findings that mutants lacking msDNA grow indistinguishably from the wild type organism in standard laboratory conditions (Fig 2C–2F). In addition, we noted that very few proteins differ in abundance between the ΔSTM3846 and Δmsd mutants, consistent with the hypothesis that the reverse transcriptase and msDNA operate in the same biological pathway.
Fig. 3. Proteins needed for anaerobic metabolism are dysregulated in mutants lacking msDNA. 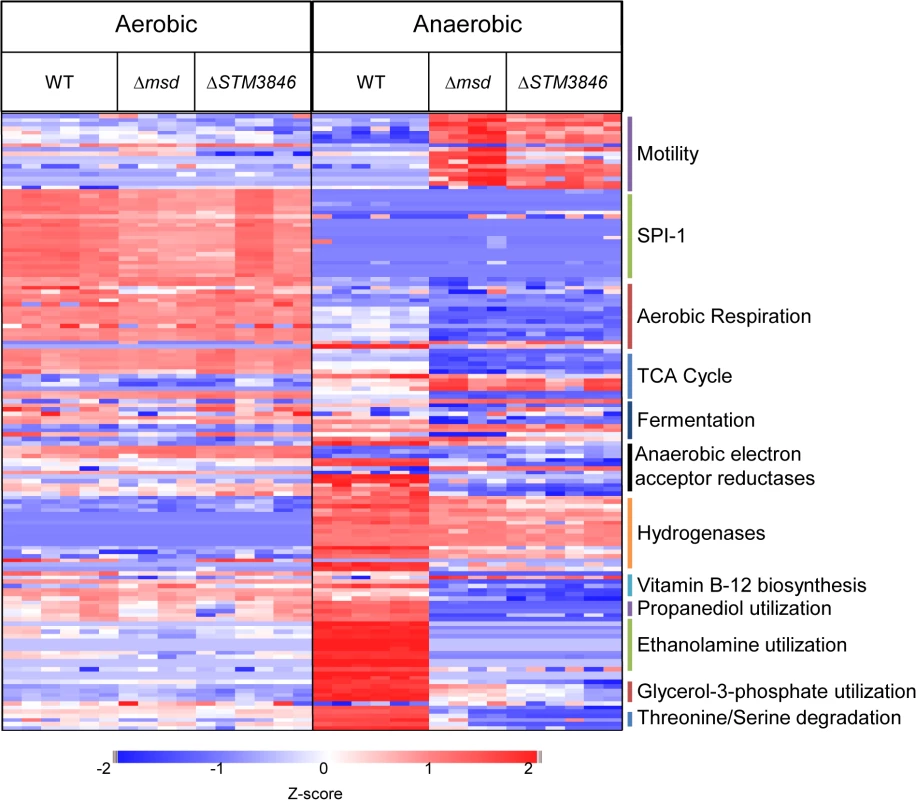
WT (HA420), Δmsd (JE135), and ΔSTM3846 (HA1444) were incubated either with agitation or statically in an anaerobic chamber for 4 hours. Proteins from bacterial cell pellets were digested and analyzed as described in Materials and Methods. Z-scores of 148 of 1504 total proteins identified from WT (n = 3) and Δmsd (n = 2) and ΔSTM3846 (n = 3) mutant cultures grown in both aerobic and anaerobic conditions. Functions of selected proteins are listed to the right of the heat map. In anaerobic conditions however, we identified 238 proteins that differed in abundance between the wild type and msDNA-deficient mutants (Fig 3 and S1 Table). Forty-three percent of proteins with reduced abundance in the mutant were involved in amino acid and carbohydrate transport/metabolism and energy production/conversion (Table 1). Twenty-five percent of all proteins of altered abundance did not belong to a functional grouping (Table 1). The abundance of proteins encoded on SPI-1 was unchanged in the absence of msDNA (Fig 3). Proteins necessary for motility were increased in abundance in anaerobically grown msDNA-deficient strains (Fig 3). However, this apparent increase did not result in a change in swimming motility of these strains in anaerobic conditions compared with the WT (S3 Fig). The abundance of numerous proteins known to be important for anaerobic growth and intestinal colonization was significantly reduced (Fig 3 and S1 Table), including proteins for 1,2 propanediol utilization [25], ethanolamine utilization [13], anaerobic sn-glycerol-3-phosphate utilization [26], anaerobic vitamin B12 biosynthesis [27], and serine/threonine degradation [28].
Tab. 1. Functional groupings of significantly dysregulated proteins in msDNA mutants. 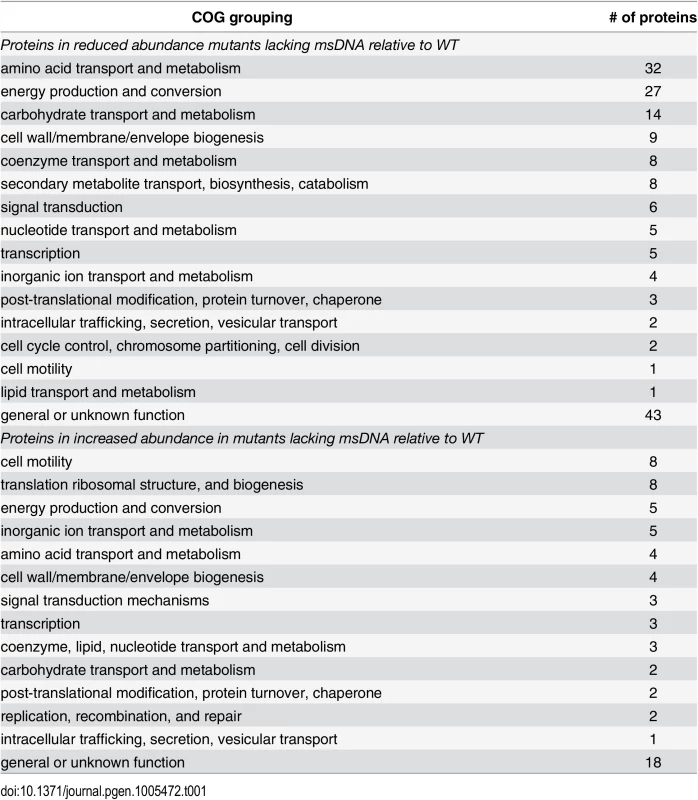
Proteins reduced in abundance, and proteins increased in abundance in msDNA mutants as determined by clusters of orthologous grouping [71]. Numerous proteins involved in reduction of anaerobic electron acceptors [29] were altered in abundance between msDNA mutants and wild type bacteria during anaerobic growth (Fig 3). Proteins important for the reduction of thiosulfate (PhsAB) and sulfide (AsrC) were of low abundance (Fig 4 [adapted from [30]] and S1 Table). In addition, proteins necessary for the reduction of DMSO (DmsA, STM4305.s) and fumarate (FrdA) were in low abundance in mutants lacking msDNA, although they did not meet our stringent criteria for statistical significance. Expression of genes necessary to utilize alternate electron acceptors is often induced by the presence of the electron acceptor [29] so the absence of a statistically significant reduction in some of these proteins is not surprising because these compounds were not present in the growth conditions we used. Interestingly, NapA, encoding the periplasmic nitrate reductase [29], was one of the proteins that was present in increased abundance in msDNA deficient mutants compared to the WT, and there was no change in the abundance of NarGH, one of the two other nitrate reductase complexes (Fig 4 and S1 Table). These data are consistent with the growth defect of our mutants in anaerobic conditions, and suggest that msDNA-deficient mutants have a severe dysregulation of proteins necessary for reduction of terminal electron acceptors needed during anaerobiosis.
Fig. 4. Anaerobic carbon metabolism is severely disrupted in msDNA-deficient mutants. 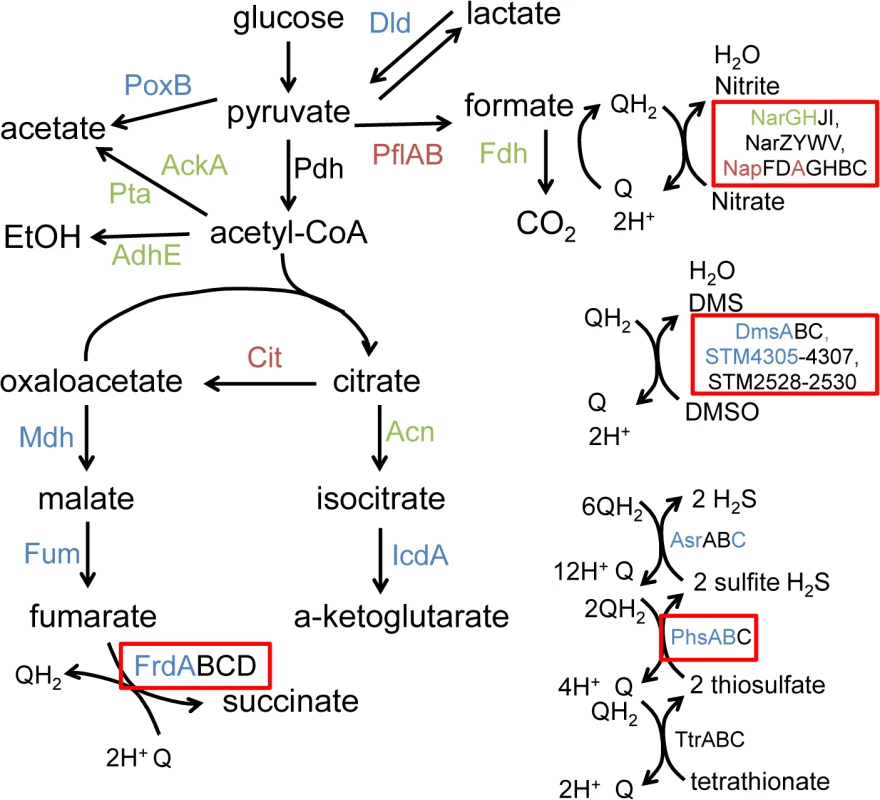
Proteins necessary for a given reaction are listed beside the reaction. In our proteomic analysis, proteins shown in blue were present in decreased in abundance in mutants unable to produce msDNA, those shown in red are present in increased in abundance in mutants unable to produce msDNA, and those in green are unchanged in abundance in mutants unable to produce msDNA. Proteins shown in black were not observed. Boxed proteins identify alternate electron acceptors used during anaerobic conditions. Figure adapted from [30]. Anaerobic terminal electron acceptor utilization in msDNA mutants
Our proteomic data predict that msDNA is critical for STm to produce proteins necessary for reduction of terminal electron acceptors critical for metabolism during anaerobic conditions. In order to confirm that the reduced abundance of anaerobic terminal electron acceptor reductases, as indicated by our proteomic data, has functional consequences, we tested the ability of the addition of various terminal electron acceptors to rescue anaerobic growth of the STM3846 mutant. We found that providing the alternate electron acceptors fumarate, DMSO, or thiosulfate to the culture media during anaerobic growth failed to restore growth of the strain lacking msDNA to WT levels (Figs 5B–5F, 6B and 6C). This finding makes sense, as our proteomic data suggest that the enzymes that transfer electrons to these terminal electron acceptors during anaerobic growth, thiosulfate reductase, sulfide reductase, fumarate reductase, and two DMSO reductases, are reduced in abundance in mutants that lack msDNA. However, the addition of nitrate to culture medium rescued the anaerobic growth of the reverse transcriptase mutant (Figs 5A and 6A). These data are consistent with our proteomic data showing that mutants lacking msDNA have adequate NarG and an increased amount of NapA allowing these strains to use nitrate as a terminal acceptor for electrons during anaerobic growth.
Fig. 5. Nitrate, but not fumarate, DMSO, or thiosulfate, rescues the anaerobic growth defect of the ΔSTM3846 mutant to WT levels. 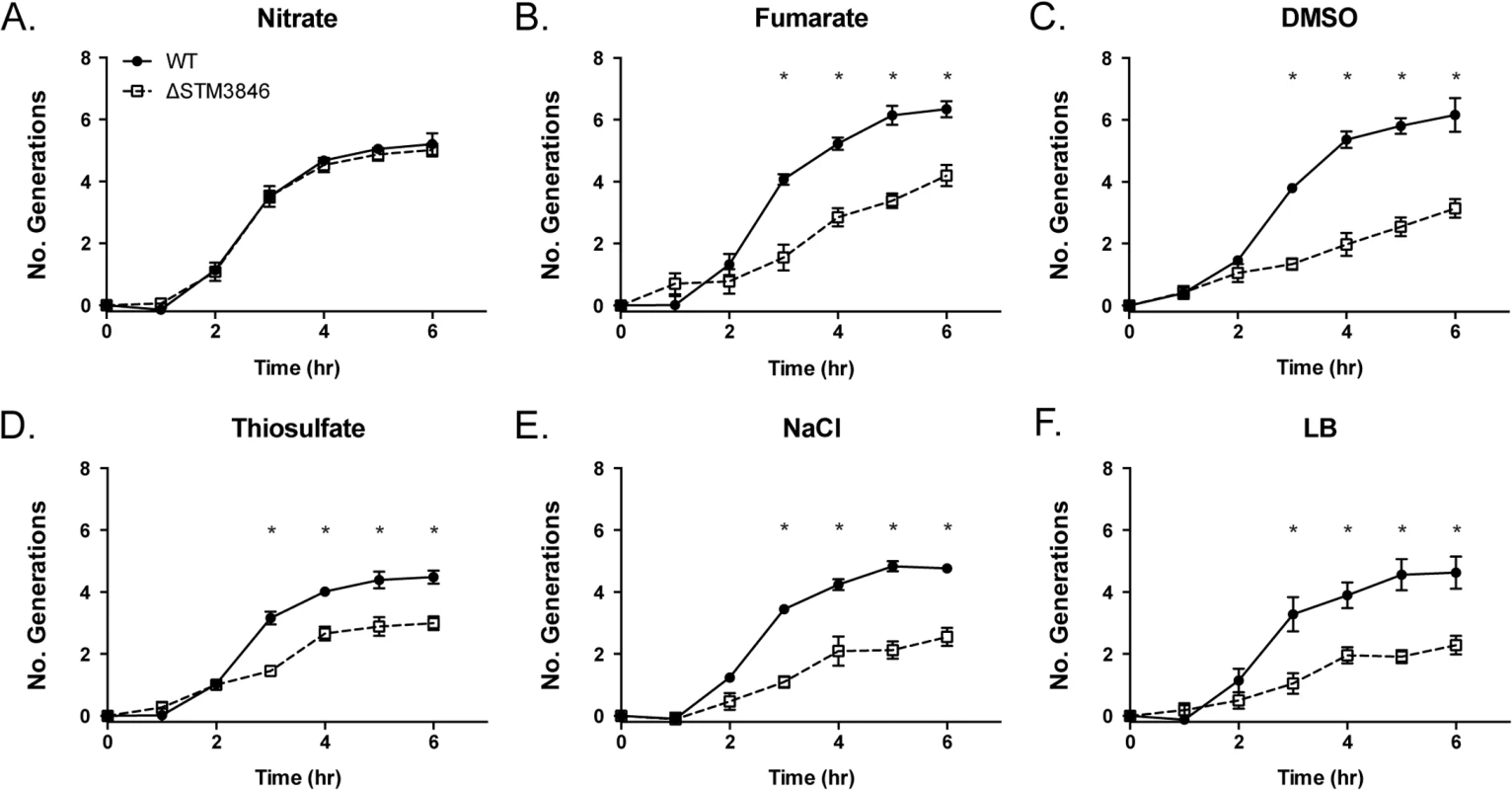
WT (closed circles; HA420) and the ΔSTM3846 mutant (open squares; HA1444) were grown anaerobically as described in Fig 2, in LB with added electron acceptor: (A) Added nitrate (40 mM), (B) Added fumarate (40 mM), (C) Added DMSO (1% v/v), (D) Added thiosulfate (40 mM) or control (E) NaCl (40 mM) or (F)—LB only)]. Data points represent the mean +/- SEM of four independent experiments. Statistical significance determined by t-test on log-transformed data with * P<0.05. Fig. 6. Growth defects of the ΔSTM3846 mutant in the presence of electron acceptors are linked to disruption of alternate electron acceptor reductases. 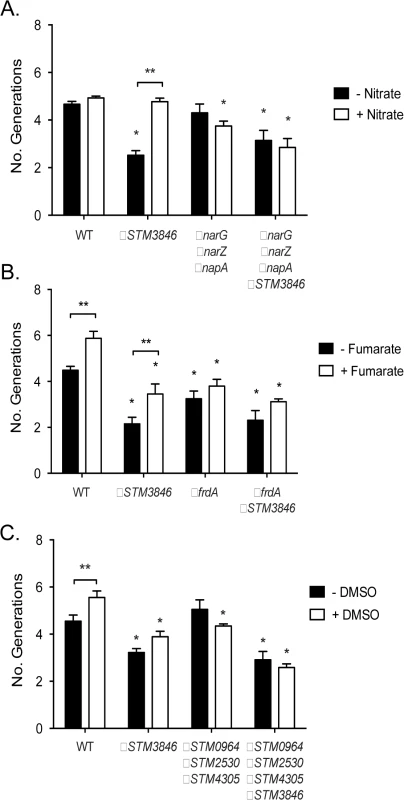
Bacterial cultures were grown anaerobically for 4 hours in the presence of alternate electron acceptors as indicated in Fig 5. (A) Anaerobic growth of the WT (HA420), a ΔSTM3846 mutant (HA1444), a mutant lacking all three nitrate reductases (ΔnarG ΔnarZ ΔnapA; CAL50), and a ΔnarG ΔnarZ ΔnapA ΔSTM3846 quadruple mutant (JE335) in the absence (black bars) or presence (white bars) of nitrate. (B) Anaerobic growth of the WT (HA420), a ΔSTM3846 mutant (JE142), a mutant lacking the fumarate reductase (ΔfrdA; JE309), and a ΔfrdA ΔSTM3846 double mutant (JE334) in the absence (black bars) or presence (white bars) of fumarate. (C). Anaerobic growth of the WT (HA420), a ΔSTM3846 mutant (HA1444), a mutant lacking all three putative DMSO reductases (ΔSTM0964 ΔSTM2530 ΔSTM4305; JE341), and a ΔSTM0964 ΔSTM2530 ΔSTM4305 ΔSTM3846 quadruple mutant (JE343) in the absence (black bars) or presence (white bars) of DMSO. Bars represent mean +/- SEM of at least three independent experiments. * Indicates significant difference between mutant and WT for that condition (P<0.05 by ANOVA). ** Indicates significant difference between conditions (P<0.05 by t-test). msDNA and colonization of the inflamed intestine
In the presence of an intact T3SS-1, NTS induce an inflammatory response that includes recruitment of luminal neutrophils and induction of inducible nitric oxide synthase as part of the inflammatory response [9,10,31], resulting in generation of tetrathionate and nitrate as available terminal electron acceptors in the inflamed intestine. To determine whether the colonization defects we observed were dependent on a functional T3SS-1 and host neutrophilic inflammatory response, we performed competitive infection experiments between the virulent WT and the ΔSTM3846 mutant both in the presence and absence of SPI-1 (Fig 7A). We observed that a ΔSTM3846 mutant colonizes the intestine poorly and associated organs. The modest colonization defect may be due to an inability to utilize carbon and amino acid sources within the inflamed intestine [13], or due to poor growth compared with WT prior to the host inflammatory response. Interestingly, the colonization defect of the ΔSTM3846 mutant in the mouse cecum was exacerbated in the absence of a functional T3SS-1, suggesting that a robust inflammatory response partially rescues mutants unable to produce msDNA (Fig 7A). Consistent with this finding both the small and large intestines, which lack appreciable neutrophilic inflammation (Fig 7C), are poorly colonized with the ΔSTM3846 mutant in mice inoculated with this strain alone (Fig 7B). In murine models that do not develop a neutrophilic infiltrate in the intestine in response to infection (murine typhoid model), the ΔSTM3846 mutant also colonizes poorly after oral infection (Fig 8A and 8B). Our results suggest that STM3846 is essential for STm to colonize the intestine, a defect that is partially rescued in the presence of a profound host inflammatory response, supporting the necessity for intact anaerobic metabolic pathways in intestinal colonization.
Fig. 7. The STM3846 reverse transcriptase mediates intestinal colonization of Salmonella Typhimurium. 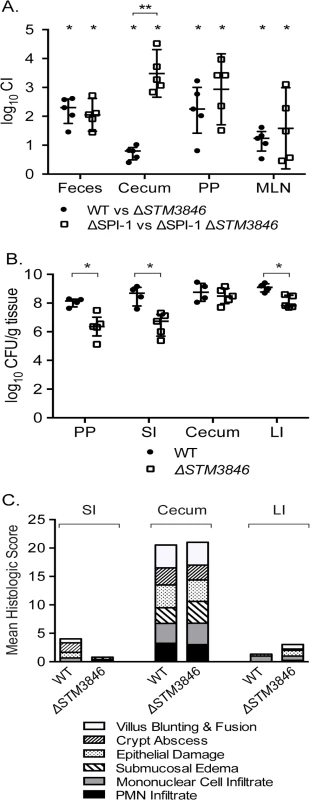
(A) Competitive index of WT (JE67) vs ΔSTM3846 (JE23; n = 5 mice; closed circles) and ΔSPI-1 (HA964) vs ΔSPI-1 ΔSTM3846 (JE25; n = 5; open squares) 4 days post-infection in the murine colitis model as described in Fig 1. (B) Organ colonization of mice infected with either WT (JE67; n = 4; closed circles) or ΔSTM3846 mutant (JE23; n = 5; open squares) in the murine colitis model. (C) Mean of histologic scores from indicated intestinal segments from mice in (B). Each point (in panels A, B) represents data from a single mouse with median and interquartile range indicated. Statistical significance determined as in Fig 1. PP—Peyer’s patches; SI—small intestine; LI—large intestine. Fig. 8. The ΔSTM3846 mutant colonizes the non-inflamed intestine poorly in the murine typhoid model. 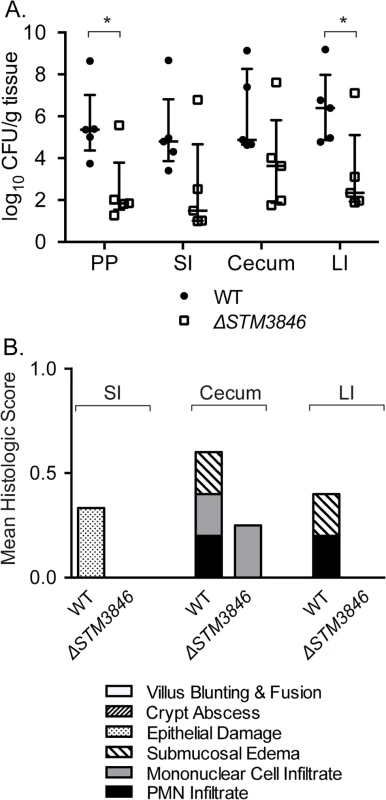
(A) Organ colonization of mice infected with either WT (JE67; closed circles; n = 5) or ΔSTM3846 mutant (JE23; open squares; n = 5). (B) Mean histologic score from mouse organs in (A). Each point represents a single mouse with median and interquartile range indicated. Statistical significance between WT and ΔSTM3846 mutant as determined by a t-test is indicated by * (P<0.05). msDNA in enteropathogenic E. coli
The msDNA of STm is similar in predicted secondary structure to msDNA of other enteric pathogens including enteropathogenic E. coli (EPEC; [4]), a close relative of STm. EPEC attaches to the epithelial surface causing characteristic attaching and effacing lesions and a malabsorptive diarrhea [32]. Despite the fact that the pathology caused by NTS and EPEC is distinct, both organisms colonize the intestine and cause diarrheal illness in susceptible hosts. We hypothesized that the RT of EPEC O127:H6, a serotype previously shown to produce msDNA [33], is necessary for this organism to colonize the gut. To test this hypothesis, we generated a non-polar deletion of the retron RT (ΔE2348C_3890) and performed competitive infections between this mutant and the WT. We found that an EPEC mutant lacking the RT fails to persist within the intestine of mice, both in the luminal contents and adherent to tissue (Fig 9). This defect was reversed by complementation in trans (S4 Fig). These data suggest that the importance of retron reverse transcriptases during intestinal infection is not restricted to salmonellae, and thus are likely to be more broadly applicable to enteric pathogens.
Fig. 9. Enteropathogenic E. coli is dependent on the retron reverse transcriptase to persist within the murine intestine. 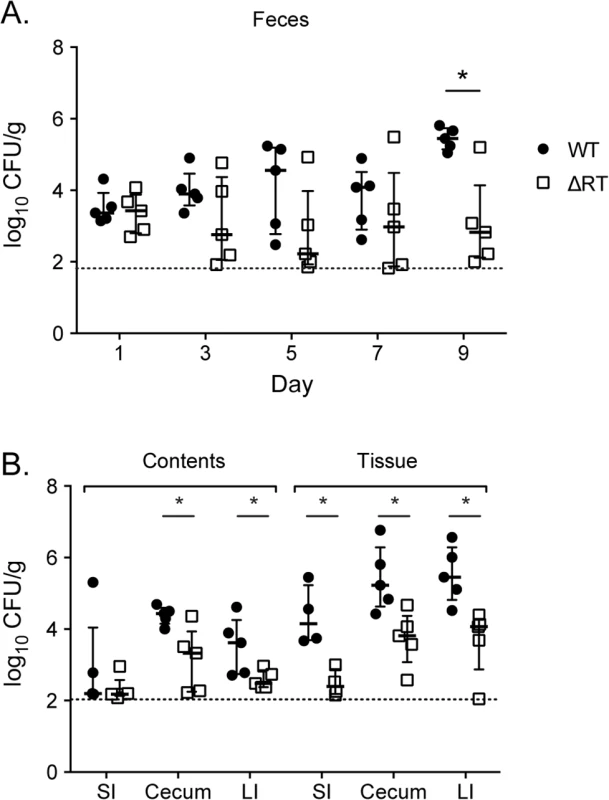
Five C57Bl/6 mice were infected with an equivalent mixture of EPEC O127:H6 (JE301) or the ΔRT mutant (JE304; ΔE2348C_3890) by gavage. Feces were collected every two days and mice euthanized at day 10 post-infection. Intestinal contents were removed and intestinal tissue washed in PBS then contents and tissue were homogenized, diluted, and plated separately. Organs were weighed and the abundance of WT and mutant determined per gram of tissue. (A) Fecal shedding of WT (closed circles) and ΔRT mutant (open squares) throughout infection and (B) in intestinal contents (luminal bacteria) and washed tissue (tissue adherent bacteria) after 10 days competitive infection. Competitive index and statistical significance were determined as for Fig 1 with * P < 0.05. The horizontal line represents the lower limit of bacterial detection. Discussion
The natural function of msDNA has remained elusive despite more than 30 years of study [1,2,5,34–38]. We describe the first phenotypes for any mutant lacking msDNA. Using the enteric pathogen S. Typhimurium, we show that msDNA produced by a retron reverse transcriptase is critical for efficient colonization of the mammalian intestine. In STm, msDNA is critical for the ability to grow in the absence of oxygen. Identification of these phenotypes creates the first opportunity for detailed studies of the molecular function of msDNA since the discovery of these unique molecules. We further showed that STm msDNA directs colonization of the intestine through regulation of the abundance of proteins necessary for central anaerobic metabolism. Thus, our data suggest that the natural function of msDNA may be to control protein abundance, the first natural function to be ascribed to any msDNA molecule.
In STm, msDNA is produced by the STM3846 reverse transcriptase using msd as a template sequence and msr as a primer. The msDNA from STm has a predicted 85-nucleotide DNA stem with no mismatched base pairs and a 4-nucleotide loop, and an RNA portion with two predicted smaller imperfect stem loop structures [4]. The RNA and DNA portions of msDNA are covalently joined by a unique 2’5’ phosphodiester linkage on a conserved guanine [39]. It is unclear whether the entire msr RNA sequence remains in the mature STm msDNA. Consistent with prior reports [34,39], we showed that both the RT and msd are requirements for production of msDNA. The intervening ORF, STM3845, is dispensable for msDNA production. This is perhaps not surprising as the presence of another ORF in addition to the RT in retrons is relatively rare, and appears to be more common on retrons borne by pathogens [4,40,41]. It has been suggested that the retron RT could produce a variety of different cDNA molecules if the sequence of the mRNA transcript is identical to that of the 5’ end of msr [42]. However, we observed similar defects in intestinal colonization and anaerobic growth of mutants lacking either the RT or msd. These data suggest that it is msDNA, and not some other potential product of the RT, that mediates intestinal colonization of STm.
Previous attempts to evaluate the function of msDNA have used artificial systems, failing to identify phenotypes for mutants lacking msDNA and to definitively identify the natural function of these molecules [23,43–47]. When an msDNA from one strain of E. coli with mismatched base pairs in the predicted DNA stem region is significantly overexpressed in a heterologous strain of E. coli lacking its own retron, the frequency of spontaneous mutation was increased due to sequestration of mismatch repair proteins [43,44,46]. Thus, the production of msDNA was thought to increase mutation frequency. However, no previous work has demonstrated that deletion of msDNA from a bacterium naturally producing msDNA decreases mutation frequency. We hypothesize that substantial over-expression of any mismatched DNA could increase mutation frequency by the same mechanism. Thus, this previous finding may not illuminate the true function of msDNA in the cell.
When mutants lacking the ability to make msDNA are grown without oxygen, 15% of all proteins we could identify were in altered abundance. However, no dysregulated proteins were identified during aerobic growth, consistent with the lack of identifiable phenotypes in the presence of oxygen. Our proteomic data were generated using cultures grown for the same duration of time under varying growth conditions. Some of the differences in protein abundance may result because wild type and mutant that cannot make msDNA grow differently during anaerobic conditions. However, our growth data suggest that the growth phase of the wild type and mutants unable to make msDNA are not dramatically different at the times we chose to collect samples for our analysis. Furthermore, the differences in protein abundance between the msDNA mutant and the wild type during anaerobic growth that we re-tested appear to be functionally significant. We show that the growth of mutants that cannot make msDNA, and that have reduced abundance of several alternate electron acceptor reductases needed during anaerobic growth, cannot be rescued by addition of the cognate alternate electrons. Furthermore, the msDNA mutant overproduces periplasmic nitrate reductase (NapA) and a wild type level of a second nitrate reductase (NarG). We show that these proteins and thus this pathway are functional, as the exogenous addition of the terminal electron acceptor nitrate rescues the anaerobic growth of mutants unable to make msDNA. Prior reports suggest that the DNA portion of msDNA can be engineered to act as a regulatory molecule by creating an antisense sequence in the DNA loop [45]. Our data suggest that the natural function of msDNA may be to act as a regulatory molecule although we have not yet identified specific regulatory targets.
There are two known master regulators of anaerobic metabolism in facultative anaerobes: fnr and arcA [48]. The transcriptional and protein profiles of anaerobically-grown Salmonella mutants deficient in fnr and arcA are established [49,50]. With few exceptions, the proteins of altered abundance in our proteomic data align poorly with genes regulated by either fnr or arcA. However, it is difficult to draw meaningful comparisons across our proteomic data and published transcriptional profiles of mutants grown in the absence of oxygen, because of protein profiles with transcript abundance are not directly comparable. Our data raise the possibility that regulation by msDNA may represent an additional pathway to regulate the abundance of proteins necessary for anaerobic metabolism. Further mechanistic study of the anaerobic regulation of gene and protein expression is critical to understanding the behavior of Salmonella in intestinal colonization.
Recent evidence suggests that Salmonella exploits the host inflammatory response to gain a competitive advantage in the intestinal lumen [10–13]. Reactive oxygen species produced by neutrophils oxidize thiosulfate to tetrathionate, a compound that Salmonella, but not resident microflora, uses as a terminal electron acceptor [10]. Epithelial-derived nitrate also contributes to the growth of Salmonella in the anaerobic conditions of the intestine by acting as a preferred electron acceptor in these conditions [31]. Some nutrients, such as ethanolamine, are used only during the neutrophilic inflammatory response [13]. We have shown that some of these processes in STm are altered in mutants unable to produce msDNA, along with many other proteins with less clearly defined roles in pathogenesis.
We also observed a defect in intestinal persistence of an EPEC mutant lacking its retron RT. Salmonella enterica and E. coli are close phylogenetic relatives and both cause diarrheal illness in susceptible hosts, but there are critical differences in the retron between organisms. The RT of EPEC O127:H6 is located in a different genomic context than the retron of STm and has a GC content of 51.8%, similar to the average GC content of 50.6% [51] suggesting that this gene was not acquired recently. This GC content in the EPEC retron is in contrast to the GC content of the retron of STm, 30.6% compared with the average GC content of 52.4% [4,52]. Unlike STm, the retron of EPEC O127:H6 lacks an additional ORF. The predicted secondary structures of msDNA from EPEC and STm are similar, however EPEC msDNA is predicted to have mismatched base pairs in the DNA stem [4]. Despite these differences, we report that EPEC mutants lacking the retron RT also have a phenotype during colonization of the intestine.
Critical differences also exist between the pathogenesis of EPEC and STm diarrheal diseases. In the intestine, Salmonella lives both in the lumen and invades the epithelium, replicating intracellularly and inducing a profound neutrophilic inflammatory diarrhea [53]. In contrast, EPEC attaches to the intestinal epithelium below the intestinal mucus in these regions of the gastrointestinal tract and remains extracellular [54]. Although our understanding of the molecular mechanism of the development of diarrhea during EPEC infection is incomplete, this infection causes a secretory diarrhea [32]. Thus, the mechanism of EPEC-induced diarrhea is substantially different than the inflammatory diarrhea caused by non-typhoidal salmonellae. Our data suggest that retron RTs are critical for colonization of the intestine by both of these pathogens, yet the phenotypes of these mutants in Salmonella versus EPEC during infection are different. While the role of retron reverse transcriptases and msDNA in intestinal colonization by enteric pathogens is likely to be ubiquitous, we hypothesize based both on our data and on the differences in diseases between these two organisms, that the processes regulated and the regulatory targets themselves are likely to be different.
We show that a natural function of msDNA is to regulate protein abundance, the first reported natural function of any msDNA molecule. STm mutants unable to make msDNA poorly colonize the murine intestine. This colonization defect is due to altered abundance of numerous proteins, including those necessary for central anaerobic metabolism, a process known to be necessary for the ability of STm to colonize the intestine of mammals. We observed that an EPEC mutant lacking its retron reverse transcriptase has a reduced ability to persist in the murine intestine, suggesting that the presence and function of msDNA may be broadly applicable to other enteric pathogens. Retrons are also widespread in non-pathogenic eubacteria (Reviewed in [3]) including most isolates of the environmental bacterium Myxococcus xanthus [35]. msDNA is present in high copy per cell [1], suggesting that the regulatory function of this molecule is critical for the lifestyle of the host bacterium. It is puzzling that this molecule appears to have a function under only certain conditions despite the fact that it is produced in abundance. One possible explanation for this phenomenon is that msDNA may sense environmental changes in order to regulate gene expression. This hybrid RNA-DNA molecule represents an exciting new class of bacterial regulatory molecules with broad application to the understanding of the lifestyles of pathogens and non-pathogens alike.
Materials and Methods
Bacterial strains
All bacterial strains, plasmids, and primers used for mutant construction are listed in S2 Table, S3 Table). All Salmonella strains are derivatives of ATCC 14028s. Enteropathogenic E. coli O127:H6 strain E2348/69 [55], a generous gift of M. Donnenberg, is the genetic background for all EPEC mutants described here. Mutants were constructed using a modification of the lambda-red recombination technique and antibiotic resistance cassettes removed as previously described [56,57] [58]. All Salmonella mutations were moved into a clean genetic background by P22 transduction [59]. Standard cloning protocols were used to generate complementing plasmids [60].
All bacterial cultures were grown at 37°C aerobically with vigorous agitation or standing in an anaerobic chamber with internal atmosphere of 5% H2, 5% CO2, and 90% N2 (Bactron I, ShelLab). For anaerobic growth experiments, bacteria were grown overnight aerobically then transferred into the anaerobic chamber and diluted 1 : 100 into media pre-equilibrated for at least 18 hours. Alternate electron acceptors (Sigma-Aldrich) sodium nitrate, sodium fumarate, sodium thiosulfate, and sodium tetrathionate were added to LB to a final concentration of 40mM. Sodium chloride (Sigma-Aldrich) at a final concentration of 40 mM served as a negative control. DMSO (Sigma-Aldrich) was added to LB to a final concentration of 0.1% (v/v). Bacteria were grown in Luria-Bertani (LB) broth or LB or MacConkey (Difco) agar supplemented with the following antibiotics as appropriate: kanamycin (50 mg/L), nalidixic acid (50 mg/L), carbenicillin (100 mg/L), streptomycin (100 mg/L), and chloramphenicol (20 mg/L).
All experiments were performed on at least three separate occasions. Bacterial generation number was calculated using the following equation: [log10(CFU final)—log10(CFU start)]/log10(2).
Mouse infections
Ethics Statement: This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Institutional Animal Care and Use Committees of Texas A&M University and North Carolina State University approved all animal experiments (protocol numbers 2012–084 and 2011–167 (TAMU) and 14–132-B (NCSU)). All experiments that utilized mice were performed using 8–12 week old female C57BL/6J mice (Jackson Laboratories). For competitive infection experiments, mice were infected by gavage with an equivalent ratio of WT and mutant bacteria. The competitive index was determined by dividing the ratio of WT to mutant bacteria in the selected organ by that ratio in the inoculum. For single infections, mice were infected with either WT or mutant bacteria. The harvested tissue was weighed, homogenized, and CFU was determined per gram of tissue collected.
Salmonella infections were performed as previously described [15]. For the murine colitis model, mice were administered 20 mg streptomycin in 75 μL sterile water by gavage. Twenty-four hours after treatment, mice were infected with approximately 108 CFU of Salmonella in 100 μL volume by gavage. Feces were collected 24 hours after infection. Mice were euthanized by carbon dioxide asphyxiation at 96 hours post-infection and organs harvested, homogenized, serially diluted, and plated on LB agar with appropriate antibiotics for enumeration of CFU. For the murine typhoid model, mice were treated with 75 μL sterile water by gavage. Mice were then infected and euthanized as above.
EPEC mouse infections were performed essentially as previously described [61]. Mice were infected with approximately 108 CFU in 100 μL volume by gavage. Feces were collected every other day for 9 days. Mice were euthanized 10 days post-infection. The aboral 5 cm of small intestine, the entire cecum, and the entire colon were collected. Intestinal contents were exposed through a longitudinal incision. The intestinal segment was placed into sterile PBS and vigorously agitated to remove intestinal contents. Intestinal tissue was washed in sterile PBS to remove remaining ingesta. Intestinal contents and tissue were homogenized separately, serially diluted, and plated on MacConkey agar and LB agar with appropriate antibiotics to enumerate CFU.
Histopathology
Samples from mouse ileum, cecum, and transverse colon were collected 96 hours post-infection and fixed in formalin. All tissues were routinely processed and stained with hematoxylin and eosin. All histologic analyses were performed by a veterinary pathologist blinded as to infection group. Tissues were scored (0–4) for each of the following parameters: polymorphonuclear cell (PMN) infiltration, mononuclear leukocyte infiltration, crypt abscess, submucosal edema, villus blunting, and epithelial damage as described [13,15,62,63].
msDNA isolation
msDNA was isolated from aerobic late log phase cultures normalized by OD600. Bacteria were lysed as for plasmid isolation (Qiagen Mini-prep) and msDNA isolated from the filtered fraction with subsequent ethanol precipitation. msDNA was visualized using a native polyacrylamide gel with in-gel ethidium bromide staining.
Invasion assays
Cell lines were purchased from American Type Culture Collection (ATCC) and used within 15 passages. HeLa cells (human cervical adenocarcinoma epithelial, ATCC CCL-2) were grown as recommended by ATCC. HeLa cells were seeded in 24-well plates at 5 x 104 cells/well approximately 24 h prior to infection.
Late-log phase cultures were prepared by inoculating 10 ml LB broth with 0.3 ml overnight shaking culture. Flasks were grown at 37°C with agitation for 3 hours. Bacteria were collected by centrifugation at 8000 x g for 90 seconds, resuspended in an equal volume of Hanks’ buffered saline solution (HBSS, Mediatech) and added directly to mammalian cells seeded in 24-well plates for 10 minutes. The multiplicity of infection was approximately 50. Non-internalized bacteria were removed by aspiration. Monolayers were washed three times in HBSS and were then incubated in growth media until 30 min post-infection. Thereafter, gentamicin was added at 50 μg/ml from 30–90 min p.i. to kill extracellular bacteria and reduced to 10 μg/ml from 90 min post-infection For enumeration of intracellular bacteria, monolayers were washed once in phosphate-buffered saline, and then solubilized in 0.2% sodium deoxycholate and serial dilutions were plated on LB agar.
Motility assays
Swimming motility was performed as previously described [64]. Swimming was assayed on plates containing 0.3% Difco Bacto Agar (LB agar base 25g/L). Plates were incubated either in open air or in the anaerobic chamber overnight prior to use for swimming assays. Overnight cultures of bacterial strains were grown at 37°C with agitation and cell numbers normalized by optical density. An aliquot of each normalized culture was transferred into the anaerobic chamber. The WT, ΔSTM3846, and Δmsd mutants (3 μl each) were spotted onto the same swimming agar plate and incubated at 37°C aerobically or anaerobically for 5 hours. The diameter of the cell spread was measured and compared with that of the WT on the same plate. Each assay was performed in triplicate on three independent occasions (anaerobic) or in four replicates on two independent occasions (aerobic).
Data analysis
Statistical analysis was performed using GraphPad Prism 6. All data were log transformed prior to analysis. Statistical significance was set at P < 0.05 and was determined using a t-test or ANOVA where indicated.
Proteomic analysis
Aerobic overnight cultures of the wild type and the ΔSTM3846 and Δmsd mutants were diluted 1 : 100 and incubated either aerobically or in an anaerobic chamber (Coy) for 4 hours on three independent occasions. Bacteria were pelleted and supernatants discarded. Cell pellets were resuspended in 100 mM NH4HCO3, pH 8.0 and lysed by vigorous vortexing in the presence of 0.1 mm silica/zirconia beads. Proteins were denatured and reduced with 8M urea and 5 mM dithiothrietol, respectively, for 30 minutes at 60°C. The proteins underwent enzymatic digestion for 3 hours at 37°C with 1/50 enzyme/protein (w/w) ratio of sequencing-grade trypsin. The resultant peptides were desalted for mass spectrometric (MS) analysis using C18 solid phase extraction cartridges (50 mg, 1 mL, Discovery, Supelco). The cartridges were activated with methanol, followed by equilibration with 0.1% TFA before loading the samples. The cartridges were then washed with 5% acetonitrile (ACN)/0.1% TFA and eluted with 80% ACN/0.1% TFA. Eluted peptides were concentrated in the vacuum centrifuge and diluted to a concentration of 0.5 mg/mL with water for the MS analysis.
Digested peptides were loaded into capillary columns (75 μm x 35 cm, Polymicro) packed with C18 beads (3 μm particles, Phenomenex) connected to a custom-made 4-column LC system [65]. The elution was performed using the following gradient: equilibration in 5% B solvent, 5–8% B over 2 min, 8–12% B over 18 min, 12–35% B over 50 min, 35–60% min over 27 min and 60–95% B over 3 min. (solvent A: 0.1% FA; solvent B: 90% ACN/0.1% FA) and flow rate of 300 nL/min. Eluting peptides were directly analyzed either on an Orbitrap (LTQ Orbitrap Velos, Thermo Scientific, San Jose, CA) mass spectrometer using chemically etched nanospray emitters [66]. Full scan mass spectra were collected at 400–2000 m/z range and the ten most intense ions were submitted to low-resolution CID fragmentation once (35% normalized collision energy), before being dynamically excluded for 60 seconds.
Tandem mass spectra were searched with MSFG+ against Salmonella enterica serovar Typhimurium 14028s and common contaminant sequences (downloaded from NCBI, all in forward and reversed orientations), using the following parameters: (i) partial tryptic digestion, (ii) 50 ppm parent mass tolerance, (iii) methionine oxidation as a variable modification. The peptides were filtered with a MSGF probability score [67] ≤ 1x10–9. Peak areas for each peptide were retrieved using the MultiAlign tool [68], and to ensure the quality of peptide-to-peak matching, the data was filtered with a Statistical Tools for AMT tag Confidence (STAC) score ≥ 0.7 and uniqueness probability ≥ 0.5 [69]. Additionally, proteins were required to have at least 2 peptides and at least one peptide with STAC ≥ 0.9. Peptide abundance values were log transformed and rolled-up into proteins using Qrollup tool, available in DAnTE [70]. Abundance values for each protein across all 32 conditions (WT, mutants, anaerobic, aerobic conditions, biological replicates, and technical replicates) were used to calculate a Z-score for each measurement where missing values were filled with 19.5. The Z-score transformation enables comparisons of trends across conditions and proteins to identify relevant abundance changes.
Supporting Information
Zdroje
1. Yee T, Furuichi T, Inouye S, Inouye M (1984) Multicopy single-stranded DNA isolated from a gram-negative bacterium, Myxococcus xanthus. Cell 38 : 203–209. 6088065
2. Lampson BC, Sun J, Hsu MY, Vallejo-Ramirez J, Inouye S, et al. (1989) Reverse transcriptase in a clinical strain of Escherichia coli: production of branched RNA-linked msDNA. Science 243 : 1033–1038. 2466332
3. Lampson BC, Inouye M, Inouye S (2005) Retrons, msDNA, and the bacterial genome. Cytogenet Genome Res 110 : 491–499. 16093702
4. Ahmed AM, Shimamoto T (2003) msDNA-St85, a multicopy single-stranded DNA isolated from Salmonella enterica serovar Typhimurium LT2 with the genomic analysis of its retron. FEMS Microbiol Lett 224 : 291–297. 12892895
5. Das R, Shimamoto T, Hosen SM, Arifuzzaman M (2011) Comparative Study of different msDNA (multicopy single-stranded DNA) structures and phylogenetic comparison of reverse transcriptases (RTs): evidence for vertical inheritance. Bioinformation 7 : 176–179. 22102774
6. Elfenbein JR, Endicott-Yazdani T, Porwollik S, Bogomolnaya LM, Cheng P, et al. (2013) Novel Determinants of Intestinal Colonization of Salmonella enterica Serotype Typhimurium Identified in Bovine Enteric Infection. Infect Immun 81 : 4311–4320. doi: 10.1128/IAI.00874-13 24019407
7. Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, et al. (2010) The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50 : 882–889. doi: 10.1086/650733 20158401
8. Tsolis RM, Adams LG, Ficht TA, Bäumler AJ (1999) Contribution of Salmonella typhimuriumVirulence Factors to Diarrheal Disease in Calves. Infect Immun 67 : 4879–4885. 10456944
9. Zhang S, Santos RL, Tsolis RM, Stender S, Hardt W-D, et al. (2002) The Salmonella enterica Serotype Typhimurium Effector Proteins SipA, SopA, SopB, SopD, and SopE2 Act in Concert To Induce Diarrhea in Calves. Infect Immun 70 : 3843–3855. 12065528
10. Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, et al. (2010) Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467 : 426–429. doi: 10.1038/nature09415 20864996
11. Lopez CA, Winter SE, Rivera-Chavez F, Xavier MN, Poon V, et al. (2012) Phage-mediated acquisition of a type III secreted effector protein boosts growth of salmonella by nitrate respiration. MBio 3. doi: 10.1128/mBio.00143-12 22691391
12. Rivera-Chavez F, Winter SE, Lopez CA, Xavier MN, Winter MG, et al. (2013) Salmonella uses energy taxis to benefit from intestinal inflammation. PLoS Pathog 9: e1003267. doi: 10.1371/journal.ppat.1003267 23637594
13. Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, et al. (2011) Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc Natl Acad Sci U S A 108 : 17480–17485. doi: 10.1073/pnas.1107857108 21969563
14. Lampson B, Inouye M, Inouye S (2001) The msDNAs of bacteria. Prog Nucleic Acid Res Mol Biol 67 : 65–91. 11525386
15. Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, et al. (2003) Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 71 : 2839–2858. 12704158
16. Hillman K, Whyte AL, Stewart CS (1993) Dissolved oxygen in the porcine gastrointestinal tract. Lett Appl Microbiol 16 : 299–302.
17. Rogers WP (1949) Aerobic metabolism in nematode parasites of the alimentary tract. Nature 163 : 879. 18130554
18. Crompton DW, Shrimpton DH, Silver IA (1965) Measurements of the oxygen tension in the lumen of the small intestine of the domestic duck. J Exp Biol 43 : 473–478. 5893422
19. Jones SA, Chowdhury FZ, Fabich AJ, Anderson A, Schreiner DM, et al. (2007) Respiration of Escherichia coli in the Mouse Intestine. Infect Immun 75 : 4891–4899. 17698572
20. Jones SA, Gibson T, Maltby RC, Chowdhury FZ, Stewart V, et al. (2011) Anaerobic Respiration of Escherichia coli in the Mouse Intestine. Infect Immun 79 : 4218–4226. doi: 10.1128/IAI.05395-11 21825069
21. Kroger C, Colgan A, Srikumar S, Handler K, Sivasankaran SK, et al. (2013) An infection-relevant transcriptomic compendium for Salmonella enterica Serovar Typhimurium. Cell Host Microbe 14 : 683–695. doi: 10.1016/j.chom.2013.11.010 24331466
22. Hebrard M, Kroger C, Srikumar S, Colgan A, Handler K, et al. (2012) sRNAs and the virulence of Salmonella enterica serovar Typhimurium. RNA Biol 9 : 437–445. doi: 10.4161/rna.20480 22546935
23. Jeong MA, Lim D (2004) A proteomic approach to study msDNA function in Escherichia coli. J Microbiol 42 : 200–204. 15459648
24. Rolfe MD, Rice CJ, Lucchini S, Pin C, Thompson A, et al. (2012) Lag Phase Is a Distinct Growth Phase That Prepares Bacteria for Exponential Growth and Involves Transient Metal Accumulation. J Bacteriol 194 : 686–701. doi: 10.1128/JB.06112-11 22139505
25. Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, et al. (2013) Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502 : 96–99. doi: 10.1038/nature12503 23995682
26. Iuchi S, Cole ST, Lin EC (1990) Multiple regulatory elements for the glpA operon encoding anaerobic glycerol-3-phosphate dehydrogenase and the glpD operon encoding aerobic glycerol-3-phosphate dehydrogenase in Escherichia coli: further characterization of respiratory control. J Bacteriol 172 : 179–184. 2403539
27. Moore SJ, Lawrence AD, Biedendieck R, Deery E, Frank S, et al. (2013) Elucidation of the anaerobic pathway for the corrin component of cobalamin (vitamin B12). Proceedings of the National Academy of Sciences 110 : 14906–14911.
28. Simanshu DK, Chittori S, Savithri HS, Murthy MR (2007) Structure and function of enzymes involved in the anaerobic degradation of L-threonine to propionate. J Biosci 32 : 1195–1206. 17954980
29. Unden G, Bongaerts J (1997) Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta 1320 : 217–234. 9230919
30. Trotter EW, Rolfe MD, Hounslow AM, Craven CJ, Williamson MP, et al. (2011) Reprogramming of Escherichia coli K-12 metabolism during the initial phase of transition from an anaerobic to a micro-aerobic environment. PLoS ONE 6: e25501. doi: 10.1371/journal.pone.0025501 21980479
31. Lopez CA, Winter SE, Rivera-Chávez F, Xavier MN, Poon V, et al. (2012) Phage-Mediated Acquisition of a Type III Secreted Effector Protein Boosts Growth of Salmonella by Nitrate Respiration. MBio 3. doi: 10.1128/mBio.00143-12 22691391
32. Vallance BA, Finlay BB (2000) Exploitation of host cells by enteropathogenic Escherichia coli. Proceedings of the National Academy of Sciences 97 : 8799–8806.
33. Lim D, Gomes TAT, Maas WK (1990) Distribution of msDNAs among serotypes of enteropathogenic Escherichia coli strains. Mol Microbiol 4 : 1711–1714. 1706455
34. Hsu MY, Eagle SG, Inouye M, Inouye S (1992) Cell-free synthesis of the branched RNA-linked msDNA from retron-Ec67 of Escherichia coli. Journal of Biological Chemistry 267 : 13823–13829. 1378431
35. Lampson BC, Inouye M, Inouye S (1991) Survey of multicopy single-stranded DNAs and reverse transcriptase genes among natural isolates of Myxococcus xanthus. J Bacteriol 173 : 5363–5370. 1715854
36. Lima TM, Lim D (1997) A novel retron that produces RNA-less msDNA in Escherichia coli using reverse transcriptase. Plasmid 38 : 25–33. 9281493
37. Pilousova L, Matiasovicova J, Sisak F, Havlickova H, Rychlik I (2005) Retron reverse transcriptase (rrtT) can be lost in multidrug resistant Salmonella enterica serovar Typhimurium DT 104 strains and influences virulence for mice. Vet Microbiol 111 : 191–197. 16289543
38. Sun J, Herzer PJ, Weinstein MP, Lampson BC, Inouye M, et al. (1989) Extensive diversity of branched-RNA-linked multicopy single-stranded DNAs in clinical strains of Escherichia coli. Proc Natl Acad Sci U S A 86 : 7208–7212. 2476815
39. Hsu MY, Inouye S, Inouye M (1989) Structural requirements of the RNA precursor for the biosynthesis of the branched RNA-linked multicopy single-stranded DNA of Myxococcus xanthus. Journal of Biological Chemistry 264 : 6214–6219. 2467910
40. Inouye K, Tanimoto S, Kamimoto M, Shimamoto T, Shimamoto T (2011) Two novel retron elements are replaced with retron-Vc95 in Vibrio cholerae. Microbiol Immunol 55 : 510–513. doi: 10.1111/j.1348-0421.2011.00342.x 21707739
41. Sun J, Inouye M, Inouye S (1991) Association of a retroelement with a P4-like cryptic prophage (retronphage phi R73) integrated into the selenocystyl tRNA gene of Escherichia coli. J Bacteriol 173 : 4171–4181. 1712012
42. Shimamoto T, Kawanishi H, Tsuchiya T, Inouye S, Inouye M (1998) In Vitro Synthesis of Multicopy Single-Stranded DNA, Using Separate Primer and Template RNAs, by Escherichia coli Reverse Transcriptase. J Bacteriol 180 : 2999–3002. 9603895
43. Maas WK, Wang C, Lima T, Hach A, Lim D (1996) Multicopy single-stranded DNA of Escherichia coli enhances mutation and recombination frequencies by titrating MutS protein. Mol Microbiol 19 : 505–509. 8830241
44. Maas WK, Wang C, Lima T, Zubay G, Lim D (1994) Multicopy single-stranded DNAs with mismatched base pairs are mutagenic in Escherichia coli. Mol Microbiol 14 : 437–441. 7885227
45. Mao J-R, Shimada M, Inouye S, Inouye M (1995) Gene Regulation by Antisense DNA Produced in Vivo. Journal of Biological Chemistry 270 : 19684–19687. 7544343
46. Mao JR, Inouye S, Inouye M (1996) Enhancement of frame-shift mutation by the overproduction of msDNA in Escherichia coli. FEMS Microbiol Lett 144 : 109–115. 8870259
47. Miyata S, Ohshima A, Inouye S, Inouye M (1992) In vivo production of a stable single-stranded cDNA in Saccharomyces cerevisiae by means of a bacterial retron. Proceedings of the National Academy of Sciences 89 : 5735–5739.
48. Unden G, Becker S, Bongaerts J, Holighaus G, Schirawski J, et al. (1995) O2-sensing and O2-dependent gene regulation in facultatively anaerobic bacteria. Arch Microbiol 164 : 81–90. 8588737
49. Evans MR, Fink RC, Vazquez-Torres A, Porwollik S, Jones-Carson J, et al. (2011) Analysis of the ArcA regulon in anaerobically grown Salmonella enterica sv. Typhimurium. BMC Microbiol 11 : 58. doi: 10.1186/1471-2180-11-58 21418628
50. Fink RC, Evans MR, Porwollik S, Vazquez-Torres A, Jones-Carson J, et al. (2007) FNR is a global regulator of virulence and anaerobic metabolism in Salmonella enterica serovar Typhimurium (ATCC 14028s). J Bacteriol 189 : 2262–2273. 17220229
51. Iguchi A, Thomson NR, Ogura Y, Saunders D, Ooka T, et al. (2009) Complete Genome Sequence and Comparative Genome Analysis of Enteropathogenic Escherichia coli O127:H6 Strain E2348/69. J Bacteriol 191 : 347–354. doi: 10.1128/JB.01238-08 18952797
52. McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, et al. (2001) Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413 : 852–856. 11677609
53. Santos RL, Raffatellu M, Bevins CL, Adams LG, Tukel C, et al. (2009) Life in the inflamed intestine, Salmonella style. Trends Microbiol 17 : 498–506. doi: 10.1016/j.tim.2009.08.008 19819699
54. Clements A, Young JC, Constantinou N, Frankel G (2012) Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes 3 : 71–87. doi: 10.4161/gmic.19182 22555463
55. Levine MM, Bergquist EJ, Nalin DR, Waterman DH, Hornick RB, et al. (1978) Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet 1 : 1119–1122. 77415
56. Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97 : 6640–6645. 10829079
57. Santiviago CA, Reynolds MM, Porwollik S, Choi SH, Long F, et al. (2009) Analysis of pools of targeted Salmonella deletion mutants identifies novel genes affecting fitness during competitive infection in mice. PLoS Pathog 5: e1000477. doi: 10.1371/journal.ppat.1000477 19578432
58. Porwollik S, Santiviago C, Cheng P, Long F, Desai PT, et al. (2014) Defined single-gene and multi-gene deletion mutant collections in Slamonella enterica sv. Typhimurium PLoS ONE Accepted. doi: 10.1371/journal.pone.0099820 25007190
59. Sternberg NL, Maurer R (1991) Bacteriophage-mediated generalized transduction in Escherichia coli and Salmonella typhimurium. In: Jeffrey HM, editor. Methods Enzymol: Academic Press. pp. 18–43. 1943777
60. Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory. x, 545 p. p.
61. Savkovic SD, Villanueva J, Turner JR, Matkowskyj KA, Hecht G (2005) Mouse model of enteropathogenic Escherichia coli infection. Infect Immun 73 : 1161–1170. 15664959
62. Day DW, Mandal BK, Morson BC (1978) The rectal biopsy appearances in Salmonella colitis. Histopathology 2 : 117–131. 669591
63. McGovern VJ, Slavutin LJ (1979) Pathology of salmonella colitis. Am J Surg Pathol 3 : 483–490. 534385
64. Bogomolnaya LM, Aldrich L, Ragoza Y, Talamantes M, Andrews KD, et al. (2014) Identification of novel factors involved in modulating motility of Salmonella enterica serotype typhimurium. PLoS One 9: e111513. doi: 10.1371/journal.pone.0111513 25369209
65. Livesay EA, Tang K, Taylor BK, Buschbach MA, Hopkins DF, et al. (2008) Fully automated four-column capillary LC-MS system for maximizing throughput in proteomic analyses. Anal Chem 80 : 294–302. 18044960
66. Kelly RT, Page JS, Luo Q, Moore RJ, Orton DJ, et al. (2006) Chemically etched open tubular and monolithic emitters for nanoelectrospray ionization mass spectrometry. Anal Chem 78 : 7796–7801. 17105173
67. Kim S, Gupta N, Pevzner PA (2008) Spectral probabilities and generating functions of tandem mass spectra: a strike against decoy databases. J Proteome Res 7 : 3354–3363. doi: 10.1021/pr8001244 18597511
68. LaMarche BL, Crowell KL, Jaitly N, Petyuk VA, Shah AR, et al. (2013) MultiAlign: a multiple LC-MS analysis tool for targeted omics analysis. BMC Bioinformatics 14 : 49. doi: 10.1186/1471-2105-14-49 23398735
69. Stanley JR, Adkins JN, Slysz GW, Monroe ME, Purvine SO, et al. (2011) A statistical method for assessing peptide identification confidence in accurate mass and time tag proteomics. Anal Chem 83 : 6135–6140. doi: 10.1021/ac2009806 21692516
70. Polpitiya AD, Qian WJ, Jaitly N, Petyuk VA, Adkins JN, et al. (2008) DAnTE: a statistical tool for quantitative analysis of-omics data. Bioinformatics 24 : 1556–1558. doi: 10.1093/bioinformatics/btn217 18453552
71. Tatusov RL, Koonin EV, Lipman DJ (1997) A genomic perspective on protein families. Science 278 : 631–637. 9381173
Štítky
Genetika Reprodukčná medicína
Článek The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis inČlánek Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2015 Číslo 9- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Retraction: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Signaling from Within: Endocytic Trafficking of the Robo Receptor Is Required for Midline Axon Repulsion
- A Splice Region Variant in Lowers Non-high Density Lipoprotein Cholesterol and Protects against Coronary Artery Disease
- The Chromatin Protein DUET/MMD1 Controls Expression of the Meiotic Gene during Male Meiosis in
- A NIMA-Related Kinase Suppresses the Flagellar Instability Associated with the Loss of Multiple Axonemal Structures
- Slit-Dependent Endocytic Trafficking of the Robo Receptor Is Required for Son of Sevenless Recruitment and Midline Axon Repulsion
- Expression of Concern: Protein Under-Wrapping Causes Dosage Sensitivity and Decreases Gene Duplicability
- Mutagenesis by AID: Being in the Right Place at the Right Time
- Identification of as a Genetic Modifier That Regulates the Global Orientation of Mammalian Hair Follicles
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- Evaluating the Performance of Fine-Mapping Strategies at Common Variant GWAS Loci
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- Differential Expression of Ecdysone Receptor Leads to Variation in Phenotypic Plasticity across Serial Homologs
- Receptor Polymorphism and Genomic Structure Interact to Shape Bitter Taste Perception
- Cognitive Function Related to the Gene Acquired from an LTR Retrotransposon in Eutherians
- Critical Function of γH2A in S-Phase
- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
- Integration of Genome-Wide SNP Data and Gene-Expression Profiles Reveals Six Novel Loci and Regulatory Mechanisms for Amino Acids and Acylcarnitines in Whole Blood
- A Genome-Wide Association Study of a Biomarker of Nicotine Metabolism
- Cell Cycle Regulates Nuclear Stability of AID and Determines the Cellular Response to AID
- A Genome-Wide Association Analysis Reveals Epistatic Cancellation of Additive Genetic Variance for Root Length in
- Tissue-Specific Gain of RTK Signalling Uncovers Selective Cell Vulnerability during Embryogenesis
- RAB-10-Dependent Membrane Transport Is Required for Dendrite Arborization
- Basolateral Endocytic Recycling Requires RAB-10 and AMPH-1 Mediated Recruitment of RAB-5 GAP TBC-2 to Endosomes
- Dynamic Contacts of U2, RES, Cwc25, Prp8 and Prp45 Proteins with the Pre-mRNA Branch-Site and 3' Splice Site during Catalytic Activation and Step 1 Catalysis in Yeast Spliceosomes
- ARID1A Is Essential for Endometrial Function during Early Pregnancy
- Predicting Carriers of Ongoing Selective Sweeps without Knowledge of the Favored Allele
- An Interaction between RRP6 and SU(VAR)3-9 Targets RRP6 to Heterochromatin and Contributes to Heterochromatin Maintenance in
- Photoreceptor Specificity in the Light-Induced and COP1-Mediated Rapid Degradation of the Repressor of Photomorphogenesis SPA2 in Arabidopsis
- Autophosphorylation of the Bacterial Tyrosine-Kinase CpsD Connects Capsule Synthesis with the Cell Cycle in
- Multimer Formation Explains Allelic Suppression of PRDM9 Recombination Hotspots
- Rescheduling Behavioral Subunits of a Fixed Action Pattern by Genetic Manipulation of Peptidergic Signaling
- A Gene Regulatory Program for Meiotic Prophase in the Fetal Ovary
- Cell-Autonomous Gβ Signaling Defines Neuron-Specific Steady State Serotonin Synthesis in
- Discovering Genetic Interactions in Large-Scale Association Studies by Stage-wise Likelihood Ratio Tests
- The RCC1 Family Protein TCF1 Regulates Freezing Tolerance and Cold Acclimation through Modulating Lignin Biosynthesis
- The AMPK, Snf1, Negatively Regulates the Hog1 MAPK Pathway in ER Stress Response
- The Parkinson’s Disease-Associated Protein Kinase LRRK2 Modulates Notch Signaling through the Endosomal Pathway
- Multicopy Single-Stranded DNA Directs Intestinal Colonization of Enteric Pathogens
- Recurrent Domestication by Lepidoptera of Genes from Their Parasites Mediated by Bracoviruses
- Three Different Pathways Prevent Chromosome Segregation in the Presence of DNA Damage or Replication Stress in Budding Yeast
- Identification of Four Mouse Diabetes Candidate Genes Altering β-Cell Proliferation
- The Intolerance of Regulatory Sequence to Genetic Variation Predicts Gene Dosage Sensitivity
- Synergistic and Dose-Controlled Regulation of Cellulase Gene Expression in
- Genome Sequence and Transcriptome Analyses of : Metabolic Tools for Enhanced Algal Fitness in the Prominent Order Prymnesiales (Haptophyceae)
- Ty3 Retrotransposon Hijacks Mating Yeast RNA Processing Bodies to Infect New Genomes
- FUS Interacts with HSP60 to Promote Mitochondrial Damage
- Point Mutations in Centromeric Histone Induce Post-zygotic Incompatibility and Uniparental Inheritance
- Genome-Wide Association Study with Targeted and Non-targeted NMR Metabolomics Identifies 15 Novel Loci of Urinary Human Metabolic Individuality
- Outer Hair Cell Lateral Wall Structure Constrains the Mobility of Plasma Membrane Proteins
- A Large-Scale Functional Analysis of Putative Target Genes of Mating-Type Loci Provides Insight into the Regulation of Sexual Development of the Cereal Pathogen
- A Genetic Selection for Mutants Reveals an Interaction between DNA Polymerase IV and the Replicative Polymerase That Is Required for Translesion Synthesis
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Arabidopsis AtPLC2 Is a Primary Phosphoinositide-Specific Phospholipase C in Phosphoinositide Metabolism and the Endoplasmic Reticulum Stress Response
- Bridges Meristem and Organ Primordia Boundaries through , , and during Flower Development in
- KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome
- XBP1-Independent UPR Pathways Suppress C/EBP-β Mediated Chondrocyte Differentiation in ER-Stress Related Skeletal Disease
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



