-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Cytomegalovirus microRNAs Facilitate Persistent Virus Infection in Salivary Glands
Micro (mi)RNAs are small non-coding RNAs that regulate the expression of their targets' messenger RNAs through both translational inhibition and regulation of target RNA stability. Recently, a number of viruses, particularly of the herpesvirus family, have been shown to express their own miRNAs to control both viral and cellular transcripts. Although some targets of viral miRNAs are known, their function in a physiologically relevant infection remains to be elucidated. As such, no in vivo phenotype of a viral miRNA knock-out mutant has been described so far. Here, we report on the first functional phenotype of a miRNA knock-out virus in vivo. During subacute infection of a mutant mouse cytomegalovirus lacking two viral miRNAs, virus production is selectively reduced in salivary glands, an organ essential for virus persistence and horizontal transmission. This phenotype depends on several parameters including viral load and mouse genetic background, and is abolished by combined but not single depletion of natural killer (NK) and CD4+ T cells. Together, our results point towards a miRNA-based immunoevasion mechanism important for long-term virus persistence.
Published in the journal: Cytomegalovirus microRNAs Facilitate Persistent Virus Infection in Salivary Glands. PLoS Pathog 6(10): e32767. doi:10.1371/journal.ppat.1001150
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001150Summary
Micro (mi)RNAs are small non-coding RNAs that regulate the expression of their targets' messenger RNAs through both translational inhibition and regulation of target RNA stability. Recently, a number of viruses, particularly of the herpesvirus family, have been shown to express their own miRNAs to control both viral and cellular transcripts. Although some targets of viral miRNAs are known, their function in a physiologically relevant infection remains to be elucidated. As such, no in vivo phenotype of a viral miRNA knock-out mutant has been described so far. Here, we report on the first functional phenotype of a miRNA knock-out virus in vivo. During subacute infection of a mutant mouse cytomegalovirus lacking two viral miRNAs, virus production is selectively reduced in salivary glands, an organ essential for virus persistence and horizontal transmission. This phenotype depends on several parameters including viral load and mouse genetic background, and is abolished by combined but not single depletion of natural killer (NK) and CD4+ T cells. Together, our results point towards a miRNA-based immunoevasion mechanism important for long-term virus persistence.
Introduction
The human cytomegalovirus (HCMV), a member of the β-herpesvirus family, is an important pathogen in immunocompromised patients and the leading cause of congenital birth defects with about 1/1,000 newborns affected [1]. After primary infection, herpesviruses establish a life-long latent infection, leaving the infected host at risk of subsequent reactivation and disease. During their co-evolution with their hosts, cytomegaloviruses have encountered a broad array of immune defense mechanisms, and have thus developed multiple strategies to counteract them (reviewed in [2]). Recently, both viral and cellular miRNAs have been identified as new players in the complex interaction between viruses and their hosts, providing interesting new candidates for targets of urgently needed antiviral drugs (for review see [3]). These small, ∼22 nucleotides long non-coding RNAs regulate the expression of their targets through both translational inhibition and regulation of target RNA stability (for review see [4]). While a single miRNA can regulate the expression of a large number of target genes, the extent of regulation of protein levels usually does not exceed two to three fold [5], [6]. Although a number of viruses (particularly of the herpesvirus family) have been shown to express miRNAs during productive infection and latency [3], the function of viral miRNAs in a physiologically relevant infection remains to be elucidated. Sullivan et al. showed that a miRNA mutant murine polyomavirus was not impaired during in vivo infection [7], and thus no phenotype of a viral miRNA knock-out mutant has been described so far. Here, we report on the first functional phenotype of a mouse cytomegalovirus (MCMV) lacking two miRNAs. During subacute infection, miRNA mutant virus production was selectively reduced in salivary glands, the major source of persistent CMV infection and virus spread from host-to-host [8], [9]. This phenotype depended on several parameters including viral load and mouse genetic background, and was abolished by combined depletion of natural killer (NK) and CD4+ T cells. Together, our results point towards a miRNA-based immunoevasion mechanism in an organ essential for long-term virus persistence and host-to-host transmission.
Results/Discussion
Generation of mutant viruses
During productive lytic infection a variety of virally encoded miRNAs are expressed by both HCMV (11 miRNAs) and MCMV (18 miRNAs), which accumulate to high levels throughout the course of infection [10], [11], [12], [13]. This was seen for a number of different cell types upon HCMV and MCMV infection. In MCMV infected fibroblasts, viral miRNAs constitute as much as two thirds of the total miRNA pool at three days post infection (dpi) as assessed by small RNA cloning [12]. At this time-point, two viral miRNAs, namely miR-M23-2 and miR-m21-1, together constituted ∼25% of the overall miRNAs in small RNA libraries. Both miRNAs belong to the m21/m22/M23 miRNA cluster and consist of two pairs of pre-miRNAs, which are expressed antisense to each other at the same genomic locus (Fig. 1A). Of note, this peculiar localization does not hinder their relative expression. We recently reported on an MCMV knock-out mutant lacking both of these viral miRNAs [11]. In this mutant (ΔmiR-M23-2), pre-miR-M23-2 was replaced by 276 nt of stuffer DNA resulting in the knock-out of both miR-M23-2 and miR-m21-1. We now constructed a second independent mutant (miR-M23-2-mut) by traceless mutagenesis [14] inserting 17 point mutations into the miRNA precursor sequence to disrupt hairpin formation and pre-miRNA processing (Fig. 1A). In addition, revertants were created for both viruses (ΔmiR-M23-2-rev and miR-M23-2-mut-rev) by fully restoring the native pre-miRNA locus. Expression of the respective miRNAs was measured by northern blot (Fig. 1B), confirming efficient knock-out and repair of miRNA expression in the mutant and revertant viruses, respectively. Expression of the neighboring genes and miRNAs of the m21/m22/M23 miRNA cluster were quantified by qPCR (for m21 and M23 mRNAs) (Fig. S1A, B) and northern blot (for mcmv-miR-M23-1-3p and miR-m22-1) (Fig. S1C). In both mutants and their respective revertants, no significant effect on expression levels of neighboring transcripts or miRNAs was observed. To demonstrate that miR-M23-2 is functional and can repress luciferase activity of a sensor target during lytic MCMV infection, we created a reporter construct expressing firefly luciferase containing a perfect match binding site for miR-M23-2 in its 3′UTR. Dual luciferase assays demonstrated that miR-M23-2 is functional and can repress luciferase activity of a sensor target during lytic MCMV infection with wild type (wt) MCMV and the revertant virus, but not with miR-M23-2-mut mutant virus (Fig. 1C).
Fig. 1. Obtention and characterization of miRNA mutant MCMV. 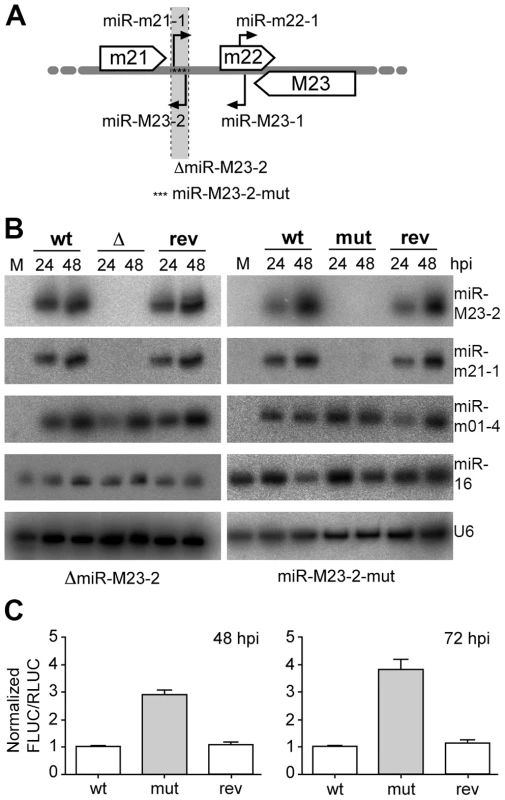
A. Schematic illustration of MCMV miRNAs expressed from the m21/m22/M23 MCMV miRNA cluster. Open arrows indicate the coding regions of the m21, m22 and M23 genes. Black arrows indicate pre-miRNAs and their orientation. The region deleted and replaced with stuffer DNA in the ΔmiR-M23-2 mutant MCMV is indicated (light grey and dotted lines), whereas asterisks indicate the point mutations in the miR-M23-2-mut mutant. B. Expression levels of miR-M23-2 and miR-m21-1 in NIH-3T3 fibroblasts infected at an MOI of 10 for 24 and 48 h with either wild type (wt) MCMV, MCMV-ΔmiR-M23-2 (Δ) and its revertant (rev) or wt MCMV, MCMV-miR-M23-2-mut (mut) and its corresponding revertant (rev) were determined by northern blot. The viral miRNA mcmv-miR-m01-4, the cellular miR-16 and the snRNA U6 were used as controls. M, mock. C. MiR-M23-2 is able to repress target gene expression late in infection. NIH-3T3 cells were infected either with wt MCMV, MCMV-miR-M23-2-mut or its revertant. 24 hpi cells were transfected with a dual luciferase reporter construct containing a perfect match for miR-M23-2 in the 3′-UTR of firefly luciferase (FLUC). Renilla luciferase (RLUC) activity was used as a transfection control. Luciferase activity was measured at 48 and 72 hpi. Both MCMV-ΔmiR-M23-2 and MCMV-miR-M23-2-mut are specifically attenuated in salivary glands during subacute infection
Neither of the two miRNA knock-out mutants showed any attenuation on murine embryonic fibroblasts in vitro ([11] and data not shown) indicating that the effect of miR-M23-2 mutation on the virus did not affect viral replication in general. It also confirmed that no second site mutation gravely impaired any essential genes of the mutant viruses. We thus started to infect C57BL/6 and BALB/c mice. While C57BL/6 mice are able to efficiently control acute MCMV infection, BALB/c mice are more susceptible. This is due to the activating NK cell receptor Ly49H, present in C57BL/6 but not BALB/c mice, which directly recognizes the MCMV m157 protein resulting in robust NK cell activation and enhanced virus control [15], [16]. Following three days of infection with either wt MCMV, ΔmiR-M23-2 or its revertant, no difference in virus titers were observed in lungs, spleen or kidney (Fig. S2). The lack of attenuation in any organ of both C57BL/6 and BALB/c at 3 dpi indicates that time might be an important factor to consider when investigating the function of viral miRNAs. As miRNAs are only able to repress de novo protein synthesis but have no effect on existing proteins, their effect depends on the decay of existing proteins unless their targets are significantly induced upon infection. Relevant levels of most viral miRNAs are probably not reached before 12 to 24 hours post infection (hpi), and it might take even longer until they are able to recruit significant amounts of their target RNAs to RISC complexes. As first viral progeny are released from infected cells as early as 24 hpi [17], viral miRNAs probably do not have sufficient time to regulate the majority of their targets during early stages of infection.
We therefore studied mice at 14 dpi. At this time, the immune system already controls the virus in most organs, except in lungs and salivary glands. Interestingly, infection with both mutants resulted in an attenuation of ∼100-fold in salivary glands of C57BL/6 mice. However, only minimal attenuation (<2-fold) was detectable in salivary glands of BALB/c mice (Fig. 2A and B). In contrast, all five viruses under study replicated to similar titers in lungs in both strains. The complete lack of any attenuation in lungs of C57BL/6 mice was surprising, but confirmed that all mice had been infected with an equal virus load. As this phenotype was comparable for both mutants and completely lifted by the two revertants, we can be confident that the knock-out of the two miRNAs was responsible for the observed attenuation. As a consequence, only the second set of mutants (MCMV-miR-M23-2-mut and its revertant) were used in further experiments. The lack of attenuation of the miRNA knock-out mutants at 3 dpi as well as the selective attenuation in C57BL/6 but not BALB/c mice at 14 dpi following intravenous (i.v.) infection, argues for a specific function of these two MCMV miRNAs in supporting persistent infection in salivary glands, and against an impairment of virus spread to this site. A few MCMV genes have been identified to be specifically required to maintain spread as well as persistent infection in salivary glands, but their mode of action has remained questionable [18].
Fig. 2. Phenotype of miRNA mutant and revertant MCMV in salivary glands and lungs. 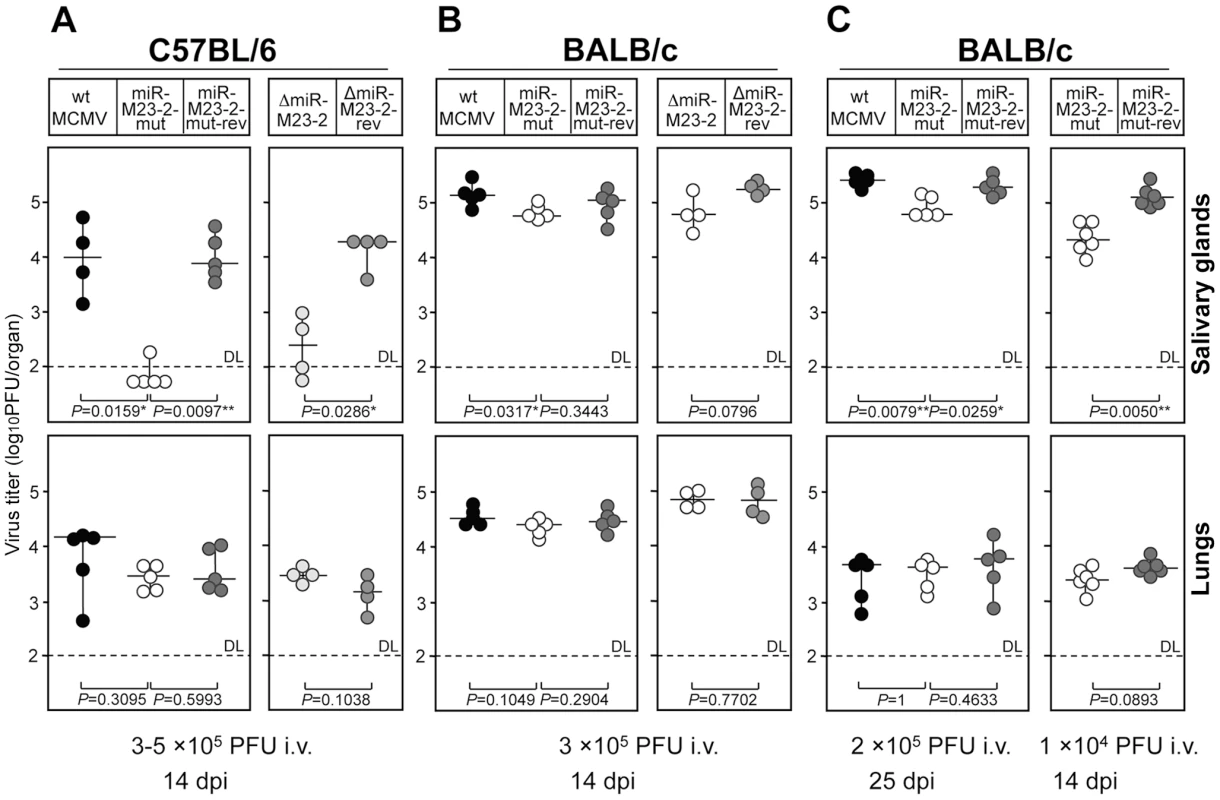
A. C57BL/6 mice were injected intravenously (i.v.) with 3×105 PFU (left panel) or 1×105 PFU (right panel) of indicated viruses. Virus titers in salivary glands and lungs were determined 14 days post infection. There were significant differences in virus titers in salivary glands between the groups of mice infected with miR-M23-2-mut and wt MCMV, miR-M23-2-mut and miR-M23-2-mut-rev, as well as between the groups of mice infected with ΔmiR-M23-2 and ΔmiR-M23-2-rev. B. BALB/c mice were injected i.v. with 3×105 PFU of indicated viruses. Virus titers in salivary glands and lungs were determined 14 dpi. C. BALB/c mice were injected i.v. with 2×105 PFU (left panel) or 1×104 PFU (right panel) of indicated viruses. Virus titers in salivary glands and lungs were determined 25 days (left panel) or 14 days (right panel) post infection. There were significant differences in virus titers in salivary glands between the groups of mice infected with miR-M23-2-mut and wt MCMV, miR-M23-2-mut and miR-M23-2-mut-rev (left panel), as well as between the groups of mice infected with miR-M23-2-mut and wt MCMV, and miR-M23-2-mut and miR-M23-2-mut-rev (right panel). Titers in organs of individual mice (circles) and median values (horizontal bars) are shown. DL = detection limit; * p<0.05; ** p<0.01. Level of attenuation is dependent on mouse strain and viral load
In order to test whether the miRNA knock-out mutant would require more time for attenuation to become apparent in BALB/c mice, we infected BALB/c mice for 25 days. While at 25 dpi virus titers in lungs had significantly dropped compared to 14 dpi, virus titers in salivary glands were very similar to those seen after 14 days. Only a very small, yet significant attenuation by ∼2-fold was observed in salivary glands (Fig. 2C), while no attenuation was observed in lungs. This clearly less pronounced attenuation of mutant virus in salivary glands in BALB/c mice could be the consequence of the inability of the host immune system to overcome high virus load in this strain, due to the less stringent innate immune control, as compared to C57BL/6 mice. Indeed, virus titers observed in salivary glands of BALB/c mice at both 14 and 25 dpi were significantly higher than in C57BL/6 mice. To test whether viral load in salivary glands had any effect on the observed phenotype, we repeated the infection of BALB/c mice with 20-fold less virus. Remarkably, virus dose reduction now resulted in a significantly greater attenuation (∼5 fold) of the mutant in salivary glands at 14 dpi (Fig. 2C). The attenuation of the mutant virus could also be seen in BALB/c mice infected intraperitoneally with 5×104 PFU (Fig. S3). To further characterize the effects of host genetics on the observed phenotype, we tested three other mouse strains with different susceptibility to MCMV infection (CBA/J, DBA/2 and 129/SvJ mice). In addition, we also included 129/SvJ.IFNγR−/− mice [19], which lack the IFNγ receptor and are thus even more susceptible to MCMV infection than their parental strain. Interestingly, miR-M23-2-mut was attenuated in salivary glands of all four strains at 14 dpi (Fig. 3). Despite very high viral load, attenuation was also observed in 129/SvJ.IFNγR−/− mice. Therefore, both host genetics and viral load are contributing factors involved in attenuation of the miRNA knock-out mutant in salivary glands.
Fig. 3. Attenuation of miRNA mutant MCMV in various mouse genetic backgrounds. 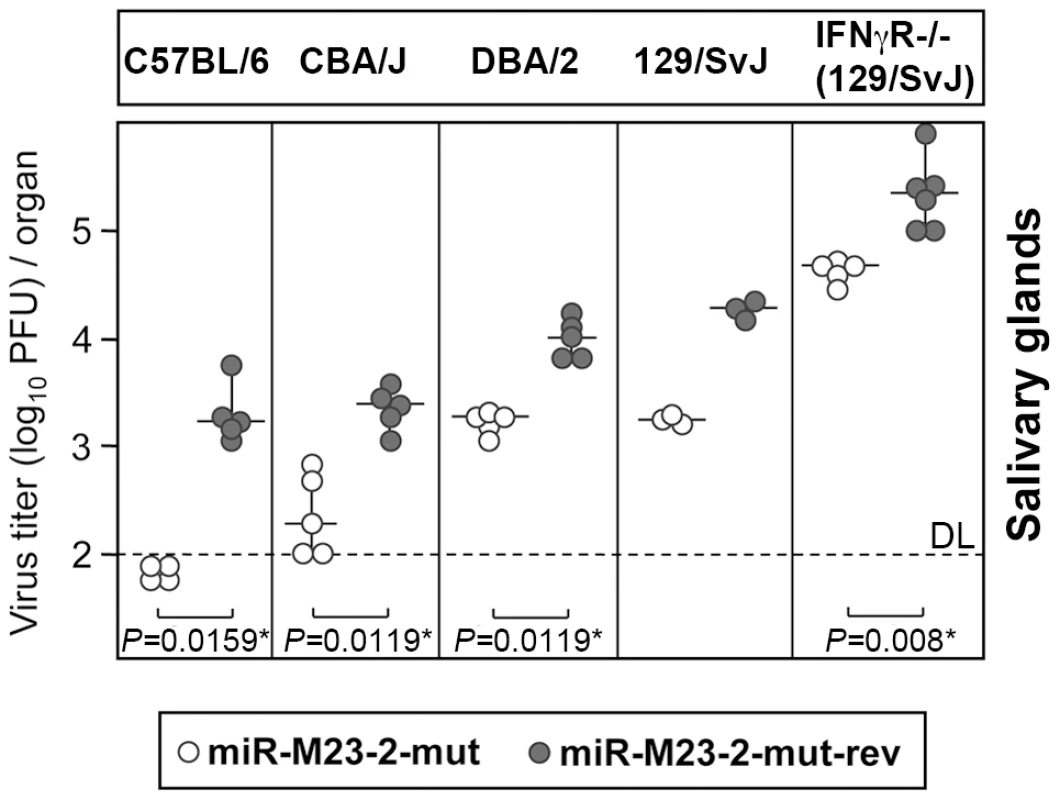
C57BL/6, CBA/J, DBA/2, 129/SvJ. and 129/SvJ IFNγR−/− mice were i.p. injected with 2×105 PFU of MCMV-miR-M23-2-mut or MCMV-miR-M23-2-mut-rev. Virus titers in salivary glands were determined 14 dpi. Titers in organs of individual mice (circles) and median values (horizontal bars) are shown. There were significant differences in virus titers in salivary glands between mice infected with MCMV-miR-M23-2-mut and MCMV-miR-M23-2-mut-rev for C57BL/6, CBA/J, DBA/2 and 129.SvJ IFNgR−/− mice. DL = detection limit; * p<0.05; ** p<0.01. Notably, control of MCMV in salivary glands is completely different than in any other tissue. The virus counteracts host defenses in this sentinel organ by means that are still not fully understood. Virus persistence in salivary gland tissues appears mainly to reflect the situation in acinar glandular epithelial cells. In these cells, MCMV replicates to high titers with a distinct morphogenesis and without causing gross tissue damage [20]. Infectious particles are stored and secreted from large cytoplasmic vacuoles, orientated towards excretion ducts, filled with high numbers (∼1,000) of virions [21]. Interestingly, for unknown reasons, virus isolated from salivary glands of naive mice at 14 dpi is also several fold more virulent than virus coming from any other organ or from cell culture. This gain in virulence is lost after a single round of replication in tissue culture [22]. As MCMV infection in salivary glands results in prolonged production of virus with only minimal cytopathic effects, viral miRNAs most likely gain more time to affect target protein levels in this setting. In contrast, severe tissue damage in lungs caused by prolonged and uncontrolled MCMV infection is the main cause of death in animals infected with a lethal dose of MCMV [23]. We speculate that the lack of attenuation in lungs after 14 and 25 days of infection may be explained by the dominance of lytic virus production over virus persistence. However, selective attenuation of the miRNA knock-out mutant in salivary glands may also reflect viral miRNAs targeting immune control mechanisms of particular importance in this organ or miRNA-mediated effects which, in other organs, are exerted by other viral genes.
Attenuation of MCMV-miR-M23-2-mut in salivary glands is reverted by combined but not single depletion of NK - and CD4-T-cells
In salivary glands MCMV infection is almost completely resistant to immunological control by CD8+ T cells. Only the concerted action of CD4+ T cells and cells with a natural killer (NK) cell-like phenotype finally results in termination of productive infection after many weeks or even months of infection [8], [9]. As such, CD4+ T cell deficient mice establish persistent infection for many months, which is restricted to salivary gland [21]. In addition, cytokine signaling is known to play an important role (reviewed in [18]). How the virus persists for a long time in salivary glands, in spite of fully primed immune control, remains an open question. In order to test whether these two MCMV miRNAs are involved in immunological control in salivary glands, we depleted both NK and CD4+ T cells in C57BL/6 mice. While the mutant virus was attenuated without NK - and T-cells depletion, combined depletion resulted in significantly higher virus titers (Fig. 4A). When we performed single depletion experiments for NK, CD4+ or CD8+ T cells in C57BL/6 mice, the depletion of NK and CD4+ T cells but not CD8+ T cells resulted in a ∼100-fold increase in virus titers (Fig. 4B). This is consistent with previously published data showing that CD4+ cells and NK cells control MCMV infection in salivary glands and prevent virus spread [8]. However, although depletion of NK cells or CD4+ T cells resulted in significant increase in titers of miR-M23-2-mut, the differences between equally depleted groups of mice infected with wt MCMV or miR-M23-2-mut-rev still remained significant.
Fig. 4. Reversion of the miRNA mutant phenotype by immune cells depletion. 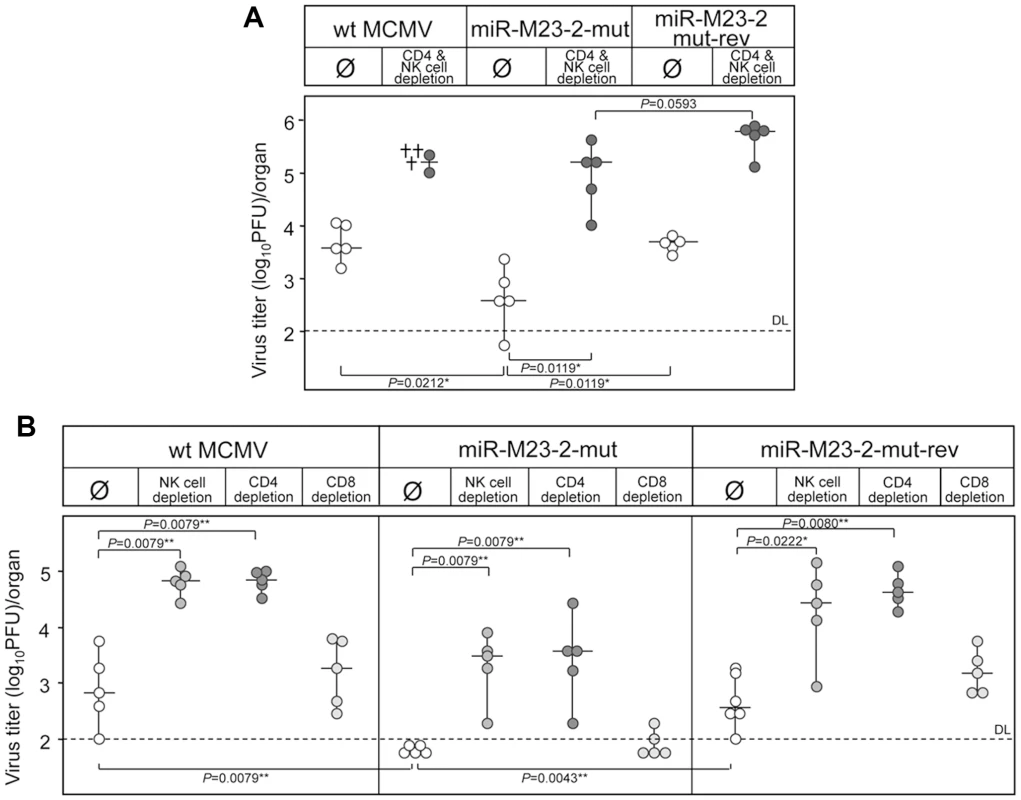
A. C57BL/6 mice depleted for both NK and CD4+ T-cells or mock treated were i.v. injected with 3×105 PFU of either wt MCMV, MCMV-miR-M23-2-mut or its revertant. Virus titers in salivary glands were determined 14 dpi. Differences in virus titers were significant between groups of undepleted mice infected with miR-M23-2-mut and wt MCMV, as well as with miR-M23-2-mut and miR-M23-2-mut-rev. Depletion of CD4+ T cells and NK cells resulted in significant increase in the titer of miR-M23-2-mut as well as miR-M23-2-mut-rev. Differences in virus titers between groups of depleted animals infected with miR-M23-2-mut and miR-M23-2-mut-rev were not significant. The crosses for the group of wt MCMV infected and CD4 and NK cell depleted group indicate animals that died a day before virus determination. B. C57BL/6 mice, undepleted or depleted of either CD4+ T cells, CD8+ T cells or NK-cells, were i.p. injected with 2×105 PFU of wt MCMV, MCMV-miR-M23-2-mut or its revertant. Virus titers in salivary glands were determined 14 dpi. Titers in organs of individual mice (circles) and median values (horizontal bars) are shown. DL = detection limit; * p<0.05; ** p<0.01. Salivary glands are a privileged site for prolonged CMV replication in spite of a fully primed immune response. Virus excretion in saliva is an important mechanism for host-to-host transmission of both murine and human cytomegaloviruses [1]. In mice, salivary glands also represent the first site to produce virus after reactivation [8]. In fact, MCMV was originally isolated from a salivary gland of a persistently infected mouse and was named salivary gland virus of the mouse [24]. Altogether, our results indicate that at least one of these two MCMV miRNAs supports persistent MCMV infection in salivary glands. We speculate that this particular function of viral miRNA has evolved to allow virus spread to new hosts via saliva. Interestingly, although the knock-out of both miRNAs resulted only in a very mild attenuation in BALB/c mice, attenuation was substantially increased by reducing the dose of infection. Dependence on viral load in BALB/c mice perhaps implicates cross-talk between infected cells and thus implies the involvement of cytokines and/or chemokines. The fact that only combined depletion of NK cells and CD4+ T cells abolished the attenuation may also argue for cytokine dependent attenuation. Attenuation was preserved even in IFNγR−/− mice, indicating that the mechanism is IFNγ independent. Upon severe immunosuppression, infection of connective tissue fibroblasts is also observed in salivary glands [21]. Therefore, we cannot exclude that insufficient immunological control following combined depletion of NK and CD4+ T cells simply masked the phenotype of viral miRNAs in persistently infected cells due to the contribution of lytic infection in surrounding cell populations like stromal fibroblasts.
Prediction of cellular targets of miR-M23-2
In order to get a hint about the possible mechanism behind the observed phenotype of our mutant virus, we predicted the potential cellular targets of miR-M23-2 and miR-m21-1 using the RepTar algorithm [25]. From these predictions, we extracted all genes relevant to the immune response, and found 166 and 200 putative targets for miR-m21-1 and miR-M23-2 respectively (Table S1). Among these predictions, 77 genes were predicted to be targeted by both miRNAs. The chemokine CXCL16 was among the top predicted targets with multiple putative binding sites for both miR-m21-1 and miR-M23-2 (Fig. S4). The predicted binding of miR-M23-2 was more favorable than that of miR-m21-1 in terms of free energy of pairing. CXCL16 is a recently discovered chemokine that is expressed in both soluble and transmembrane forms, ligates to CXCR6 chemokine receptor and guides migration of activated Th1 and Tc1 cells [26], as well as NK cells [27]. It is mainly expressed by dendritic cells and macrophages, but also by fibroblasts and endothelial and epithelial cells [28], [29]. We cloned the full length 3′UTR of CXCL16 in a luciferase reporter vector, and tested its regulation by both miR-M23-2, and miR-m21-1. While no measurable regulation was observed for miR-m21-1, miR-M23-2 readily regulated the CXCL16 reporter (Fig. S5A). In addition, using 2′-O-methylated antisense oligonucleotide, we confirmed that the CXCL16 reporter regulation by miR-M23-2 could be inhibited in a sequence specific manner (Fig. S5B). In order to test whether miR-M23-2 could also regulate the CXCL16 reporter in the context of virus infection, we transfected the CXCL16 luciferase reporter in cells infected either with the miR-M23-2-mut virus or its revertant. The repression of a luciferase reporter containing either a perfect match sensor, a mismatched sensor for miR-M23-2 or the 3′UTR of CXCL16, was only detectable in cells infected with the revertant, but not the mutant virus (Fig. S5C).
Both cellular and viral miRNAs are known to target a large number of different genes. As such, hcmv-miR-UL112-1 targets a number of viral transcripts including the major viral transactivator IE1 as well as the host's NK cell activating ligand MICB [25], [30], [31]. Knock-out of both miR-M23-2 and miR-m21-1 probably resulted in the loss of regulation of several genes. Therefore, although preliminary observations indicate that CXCL16 is a target of miR-M23-2 but not of miR-m21-1, it is very unlikely that its regulation is the sole reason for the attenuation of miR-M23-2/m21-1 mutant viruses. This concept is also supported by our findings that attenuation of the miR-M23-2/m21-1 mutant viruses revealed dependence on host genetics and viral load, indicating a multifactorial rather than a single mode of regulation. Indeed, our target predictions contained numerous other genes involved in innate immune response (Table S1). Although the exact mechanism by which miR-M23-2 and miR-m21-1 contribute to an efficient accumulation in salivary glands remains to be identified, we hypothesize that these viral miRNAs contribute to the generation of a microenvironment in this organ that is favorable for the virus to persist. Additional mechanisms such as the regulation of viral gene expression might also contribute to the observed phenotype. In this context, it is not surprising that lifting only one type of control, e.g. depletion of CD4+ T cells, was not sufficient to completely revert viral miRNA function.
Viral miRNAs have been implicated in the control of viral latency and reactivation although formal proof in vivo is still lacking [3]. Our data not only provide the first in vivo phenotype of viral miRNAs but also demonstrate that viral miRNAs are important in chronic infection in a site well known for its significance in host-to-host transmission.
Materials and Methods
Ethics statement
All of the protocols used for breeding of mice and different kinds of treatments were approved by the Ethical Committee of the Faculty of Medicine University of Rijeka and were performed in accordance with Croatian Law for the Protection of Laboratory Animals, which has been harmonized with the existing EU legislation (EC Directive 86/609/EEC).
Construction of mutant viruses
As pre-miR-M23-2 and pre-miR-m21-1 are located on opposing strands of the MCMV genome and are 98% complementary (the predicted 5′-end of pre-miR-m21-1 extends the 3′-end of pre-miR-M23-2 by one nucleotide), deletion of one miRNA always results in concordant knock-out of the other. To exclude unexpected effects on the genomic locus by the miRNA knock-out we constructed two independent mutants and revertants. The generation of the first mutant (MCMV-ΔmiR-M23-2) has been described [11]. The revertant virus was created by replacing the 276 bp insert with an expression cassette for galactokinase (GalK) and kanamycin resistance (Kn) in DH10B E.coli. The linear DNA fragment was generated by PCR on pGPS-GalK/Kn using primers H5-miR-M23-2-galK/Kn and H3-miR-M23-2-galk/Kn. Sequences of these and all other primers are provided in Table S2. After transferring the recombinant BAC to SW102 bacteria by electroporation, the wt pre-miR-M23-2 sequence was restored by traceless mutagenesis using a linear DNA fragment generated by PCR on pSM3fr using the PCR primers H5-miR-M23-rev and H3-miR-M23-rev [14]. The second mutant (MCMV-miR-M23-2-mut) was constructed solely in SW102 bacteria. First, the whole m21/m22/M23 locus of pSM3fr was replaced using homologous recombination by a GalK/Kn cassette amplified from pGPS-GalK/kn using primers H5-GalK/Kn-m22 and H3-GalK/Kn-m22. Next, the whole m21/m22/M23 miRNA cluster containing 4 miRNAs and part of the m22 gene was subcloned into pGPS1.1 (resulting in pGPS-m22) by PCR cloning using primers m22-for and m22-rev. To generate the mutant pre-miR-M23-2 template, 17 point mutations were inserted into the pre-miR-M23-2 within pGPS-m22 (resulting in pGPS-m22-miR-M23-2-mut) by oligonucleotide cloning with the two oligonucleotides pre-miR-M23-2-mut-s and pre-miR-M23-2-mut-as and an ApaL1 and PvuI digest. The m21/m22/M23 locus with the mutated pre-miR-M23-2 was excised from pGPS-m22-miR-M23-2-mut using EcoRV and inserted into pSM3fr-m22-galK/Kn by traceless mutagenesis resulting in the MCMV-M23-2-mut BAC. To generate the revertant, the GalK/Kn cassette was reinserted again as described above followed by reinsertion of the wt m21/m22/M23 miRNA cluster using the EcoRV fragment of pGPS-m22.
All viruses were reconstituted by transfecting the recombinant BACs into murine embryonic fibroblasts as described [23]. Virus titers were determined on MEFs by standard plaque assay [23].
Northern blot analysis
RNA was extracted using TRIzol and northern blotting was performed on 10 µg of total RNA as described before [11], [32]. Probes were 5′ 32P-radiolabelled oligodeoxynucleotides perfectly complementary to the miRNA sequence or to part of the U6 snRNA sequence. Blots were analyzed and quantified by phosphorimaging using a FLA5100 scanner from Fuji.
Quantitative PCR analysis of viral transcripts
Real time PCR analysis of m21 and M23 transcripts was performed as described before [12] using primers m21for and m21rev and M23for and M23rev respectively as indicated in Table S2.
Target prediction by RepTar algorithm
RepTar is a miRNA target prediction algorithm that is independent of evolutionary conservation considerations and is not limited to seed pairing sites. It is based on the finding that in some targets the miRNA binding site repeats in the 3′UTR. It identifies high scoring repetitive elements in each 3′UTR, matches them to the miRNA sequences and evaluates them as candidate miRNA binding sites. Based on the information gained from these repetitive sites, RepTar then searches for non-repetitive sites as well. The final set of Reptar predictions includes targets with conserved or non-conserved single or multiple binding sites of several types including: seed binding sites, seed wobble sites (seed sites that include G:U pairing in the seed), 3′ compensatory binding sites and full-match binding sites. The special properties of the algorithm make it advantageous for predicting targets of the less conserved viral miRNAs.
Dual luciferase experiments
The perfect match sensor for miR-M23-2 was obtained by annealing the oligonucleotides PM-M23-2-for and PM-M23-2-rev. The miR-M23-2 mismatch sensor was obtained by annealing the oligonucleotides MM-M23-2-for and MM-M23-2-rev. The 3′UTR of CXCL16 (position 16–1100) was PCR amplified from NIH-3T3 genomic DNA using primers CXCL16-for and CXCL16-rev. To each product, AttB1 and AttB2 sites were incorporated by PCR using primers AttB1-for and AttB2-rev (see Table S2). The resulting PCR products were then recombined sequentially in pDONR/Zeo and in psiCHECK-2 plasmids using Gateway technology (Invitrogen). NIH-3T3 cells were infected with wt MCMV, MCMV-miR-M23-2-mut as well as with MCMV-miR-M23-2-mut-rev at an MOI = 10 using centrifugal enhancement. 24 h post infection cells were transfected with psiCHECK-PM-M23-2 using the TransIT-3T3 Transfection kit (Mirus) following the manufacturer's instructions. At 48 and 72 h post infection luciferase activities were measured employing the Dual-Luciferase Reporter Assay (Promega).
For CXCL16 dual luciferase assays, constructs were co-transfected into NIH-3T3 cells with the indicated concentrations of miRNA mimic or Allstars negative control siRNA (Qiagen), using lipofectamine 2000 (Invitrogen) as per manufacturer's instructions. Luciferase activities were measured 48 h post transfection (Glomax, Promega). The inhibition of miRNA activity was performed by co-transfection of 100 nM 2′-O-methylated oligonucleotides specific for miR-M23-2 or for C. elegans miR-67 as a control. For CXCL16 luciferase assays in MCMV-infected cells, NIH-3T3 cells were infected with MCMV-miR-M23-2 mut or MCMV-miR-M23-2-mut-rev at an MOI = 10, using centrifugal enhancement. 24h post infection cells were transfected with psiCHECK-PM-M23-2, psiCHECK-MM-M23-2 or psiCHECK-CXCL16 using lipofectamine 2000 (Invitrogen) as per manufacturer's instructions. Luciferase activities were measured 48 h post transfection (Glomax, Promega).
Mice
BALB/c (H-2d), C57BL/6 (H-2b), CBA/J (H-2k), DBA/2 (H-2d), 129/SvJ (H-2b) and 129/SvJ IFNγR-/- (H-2b) mice were housed and bred under specific-pathogen-free conditions at the Central Animal Facility, Faculty of Medicine, University of Rijeka.
Infection conditions and detection of infectious MCMV in tissues, depletion of lymphocyte subsets, and statistical evaluation
Mice were injected either intraperitoneally or intravenously with indicated doses (1×104 PFU to 5×105 PFU) of tissue culture-grown wt MCMV or recombinant viruses in 0.5 ml of diluent. Organs were collected either 14 or 25 days after infection and virus titers were determined by a standard plaque-forming assay [33]. In vivo depletion of CD4+ and CD8+ T lymphocyte subsets and NK cells was performed by intraperitoneal injection of the mAbs to CD4 (YTS191.1), to CD8 (YTS 169.4) molecules [34] and to NK1.1 (PK136) [35]. Statistical significance of the difference between experimental groups was determined by the Mann-Whitney exact rank test.
Supporting Information
Zdroje
1. MocarskiES
ShenkT
PassRF
2007 Cytomegaloviruses.
KnipeDM
HowleyPM
GriffinDE
LambRA
MartinMA
Fields Virology. 5th ed Philadelphia Lippincott, Williams & Wilkins 2701 2772
2. ScalzoAA
CorbettAJ
RawlinsonWD
ScottGM
Degli-EspostiMA
2007 The interplay between host and viral factors in shaping the outcome of cytomegalovirus infection. Immunol Cell Biol 85 46 54
3. GottweinE
CullenBR
2008 Viral and cellular microRNAs as determinants of viral pathogenesis and immunity. Cell Host Microbe 3 375 387
4. AmbrosV
2004 The functions of animal microRNAs. Nature 431 350 355
5. BaekD
VillenJ
ShinC
CamargoFD
GygiSP
2008 The impact of microRNAs on protein output. Nature 455 64 71
6. SelbachM
SchwanhausserB
ThierfelderN
FangZ
KhaninR
2008 Widespread changes in protein synthesis induced by microRNAs. Nature 455 58 63
7. SullivanCS
SungCK
PackCD
GrundhoffA
LukacherAE
2009 Murine Polyomavirus encodes a microRNA that cleaves early RNA transcripts but is not essential for experimental infection. Virology 387 157 167
8. PolicB
HengelH
KrmpoticA
TrgovcichJ
PavicI
1998 Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med 188 1047 1054
9. PolicB
JonjicS
PavicI
CrnkovicI
ZoricaI
1996 Lack of MHC class I complex expression has no effect on spread and control of cytomegalovirus infection in vivo. J Gen Virol 77 Pt 2 217 225
10. BuckAH
Santoyo-LopezJ
RobertsonKA
KumarDS
ReczkoM
2007 Discrete clusters of virus-encoded microRNAs are associated with complementary strands of the genome and the 7.2-kilobase stable intron in murine cytomegalovirus. J Virol 81 13761 13770
11. DolkenL
PerotJ
CognatV
AliouaA
JohnM
2007 Mouse cytomegalovirus microRNAs dominate the cellular small RNA profile during lytic infection and show features of posttranscriptional regulation. J Virol 81 13771 13782
12. DunnW
TrangP
ZhongQ
YangE
van BelleC
2005 Human cytomegalovirus expresses novel microRNAs during productive viral infection. Cell Microbiol 7 1684 1695
13. GreyF
AntoniewiczA
AllenE
SaugstadJ
McSheaA
2005 Identification and characterization of human cytomegalovirus-encoded microRNAs. J Virol 79 12095 12099
14. WarmingS
CostantinoN
CourtDL
JenkinsNA
CopelandNG
2005 Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33 e36
15. AraseH
MocarskiES
CampbellAE
HillAB
LanierLL
2002 Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296 1323 1326
16. SmithHR
HeuselJW
MehtaIK
KimS
DornerBG
2002 Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci U S A 99 8826 8831
17. SacherT
PodlechJ
MohrCA
JordanS
RuzsicsZ
2008 The major virus-producing cell type during murine cytomegalovirus infection, the hepatocyte, is not the source of virus dissemination in the host. Cell Host Microbe 3 263 272
18. CampbellAE
CavanaughVJ
SlaterJS
2008 The salivary glands as a privileged site of cytomegalovirus immune evasion and persistence. Med Microbiol Immunol 197 205 213
19. HuangS
HendriksW
AlthageA
HemmiS
BluethmannH
1993 Immune response in mice that lack the interferon-gamma receptor. Science 259 1742 1745
20. LussierG
BerthiaumeL
PaymentP
1974 Electron microscopy of murine cytomegalovirus: development of the virus in vivo and in vitro. Arch Gesamte Virusforsch 46 269 280
21. JonjicS
MutterW
WeilandF
ReddehaseMJ
KoszinowskiUH
1989 Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med 169 1199 1212
22. OsbornJE
WalkerDL
1971 Virulence and Attenuation of Murine Cytomegalovirus. Infect Immun 3 228 236
23. ReddehaseMJ
WeilandF
MunchK
JonjicS
LuskeA
1985 Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol 55 264 273
24. SmithMG
1954 Propagation of salivary gland virus of the mouse in tissue cultures. Proc Soc Exp Biol Med 86 435 440
25. Stern-GinossarN
ElefantN
ZimmermannA
WolfDG
SalehN
2007 Host immune system gene targeting by a viral miRNA. Science 317 376 381
26. MatloubianM
DavidA
EngelS
RyanJE
CysterJG
2000 A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol 1 298 304
27. GeissmannF
CameronTO
SidobreS
ManlongatN
KronenbergM
2005 Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol 3 e113
28. AbelS
HundhausenC
MentleinR
SchulteA
BerkhoutTA
2004 The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol 172 6362 6372
29. DayC
PatelR
GuillenC
WardlawAJ
2009 The chemokine CXCL16 is highly and constitutively expressed by human bronchial epithelial cells. Exp Lung Res 35 272 283
30. GreyF
MeyersH
WhiteEA
SpectorDH
NelsonJ
2007 A human cytomegalovirus-encoded microRNA regulates expression of multiple viral genes involved in replication. PLoS Pathog 3 e163
31. Stern-GinossarN
SalehN
GoldbergMD
PrichardM
WolfDG
2009 Analysis of human cytomegalovirus-encoded microRNA activity during infection. J Virol 83 10684 10693
32. PfefferS
SewerA
Lagos-QuintanaM
SheridanR
SanderC
2005 Identification of microRNAs of the herpesvirus family. Nat Methods 2 269 276
33. BruneW
HengelH
KoszinowskiUH
2001 A mouse model for cytomegalovirus infection. Curr Protoc Immunol Chapter 19 Unit 19 17
34. CobboldSP
JayasuriyaA
NashA
ProsperoTD
WaldmannH
1984 Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature 312 548 551
35. KooGC
PeppardJR
1984 Establishment of monoclonal anti-Nk-1.1 antibody. Hybridoma 3 301 303
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek The Combined Effect of Environmental and Host Factors on the Emergence of Viral RNA RecombinantsČlánek Functional Interchangeability of Late Domains, Late Domain Cofactors and Ubiquitin in Viral BuddingČlánek Parvovirus Minute Virus of Mice Induces a DNA Damage Response That Facilitates Viral ReplicationČlánek Phenolglycolipid-1 Expressed by Engineered BCG Modulates Early Interaction with Human Phagocytes
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 10- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Social Media and Microbiology Education
- Antimicrobial Peptides: Primeval Molecules or Future Drugs?
- Phylodynamics and Human-Mediated Dispersal of a Zoonotic Virus
- Retroviral RNA Dimerization and Packaging: The What, How, When, Where, and Why
- Distinct Clones of Caused the Black Death
- Strain-Specific Differences in the Genetic Control of Two Closely Related Mycobacteria
- The Combined Effect of Environmental and Host Factors on the Emergence of Viral RNA Recombinants
- MHC Class I Bound to an Immunodominant Epitope Demonstrates Unconventional Presentation to T Cell Receptors
- Stimulates Immune Gene Expression and Inhibits Development in
- Crystal Structure of DotD: Insights into the Relationship between Type IVB and Type II/III Secretion Systems
- Cytomegalovirus microRNAs Facilitate Persistent Virus Infection in Salivary Glands
- Strategies to Avoid Killing by Human Neutrophils
- Transforming Growth Factor-β: Activation by Neuraminidase and Role in Highly Pathogenic H5N1 Influenza Pathogenesis
- Autoimmunity in Arabidopsis Is Mediated by Epigenetic Regulation of an Immune Receptor
- Functional Interchangeability of Late Domains, Late Domain Cofactors and Ubiquitin in Viral Budding
- Dengue Virus Ensures Its Fusion in Late Endosomes Using Compartment-Specific Lipids
- Nucleocapsid Promotes Localization of HIV-1 Gag to Uropods That Participate in Virological Synapses between T Cells
- Host Genetics and HIV-1: The Final Phase?
- Variations in the Hemagglutinin of the 2009 H1N1 Pandemic Virus: Potential for Strains with Altered Virulence Phenotype?
- High-Resolution Functional Mapping of the Venezuelan Equine Encephalitis Virus Genome by Insertional Mutagenesis and Massively Parallel Sequencing
- Viral Replication Rate Regulates Clinical Outcome and CD8 T Cell Responses during Highly Pathogenic H5N1 Influenza Virus Infection in Mice
- Calcineurin Inhibition at the Clinical Phase of Prion Disease Reduces Neurodegeneration, Improves Behavioral Alterations and Increases Animal Survival
- Parvovirus Minute Virus of Mice Induces a DNA Damage Response That Facilitates Viral Replication
- -Induced Inactivation of the Macrophage Transcription Factor AP-1 Is Mediated by the Parasite Metalloprotease GP63
- Epstein Barr Virus-Encoded EBNA1 Interference with MHC Class I Antigen Presentation Reveals a Close Correlation between mRNA Translation Initiation and Antigen Presentation
- Fidelity Variants of RNA Dependent RNA Polymerases Uncover an Indirect, Mutagenic Activity of Amiloride Compounds
- The Spread of Tomato Yellow Leaf Curl Virus from the Middle East to the World
- Concerted Action of Two Formins in Gliding Motility and Host Cell Invasion by
- Requirements for Receptor Engagement during Infection by Adenovirus Complexed with Blood Coagulation Factor X
- Release of Intracellular Calcium Stores Facilitates Coxsackievirus Entry into Polarized Endothelial Cells
- Gene Annotation and Drug Target Discovery in with a Tagged Transposon Mutant Collection
- Nuclear Export and Import of Human Hepatitis B Virus Capsid Protein and Particles
- Retention and Loss of RNA Interference Pathways in Trypanosomatid Protozoans
- Identification and Genome-Wide Prediction of DNA Binding Specificities for the ApiAP2 Family of Regulators from the Malaria Parasite
- Controlling Cellular P-TEFb Activity by the HIV-1 Transcriptional Transactivator Tat
- Direct Visualization of Peptide/MHC Complexes at the Surface and in the Intracellular Compartments of Cells Infected by
- Phenolglycolipid-1 Expressed by Engineered BCG Modulates Early Interaction with Human Phagocytes
- In Vitro and In Vivo Studies Identify Important Features of Dengue Virus pr-E Protein Interactions
- Inhibition of Nipah Virus Infection In Vivo: Targeting an Early Stage of Paramyxovirus Fusion Activation during Viral Entry
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Retroviral RNA Dimerization and Packaging: The What, How, When, Where, and Why
- Viral Replication Rate Regulates Clinical Outcome and CD8 T Cell Responses during Highly Pathogenic H5N1 Influenza Virus Infection in Mice
- Antimicrobial Peptides: Primeval Molecules or Future Drugs?
- Crystal Structure of DotD: Insights into the Relationship between Type IVB and Type II/III Secretion Systems
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



