-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Cell Wall Protein CwpV is
Antigenically Variable between Strains, but Exhibits Conserved
Aggregation-Promoting Function
Clostridium difficile is the main cause of antibiotic-associated
diarrhea, leading to significant morbidity and mortality and putting
considerable economic pressure on healthcare systems. Current knowledge of the
molecular basis of pathogenesis is limited primarily to the activities and
regulation of two major toxins. In contrast, little is known of mechanisms used
in colonization of the enteric system. C. difficile expresses a
proteinaceous array on its cell surface known as the S-layer, consisting
primarily of the major S-layer protein SlpA and a family of SlpA homologues, the
cell wall protein (CWP) family. CwpV is the largest member of this family and is
expressed in a phase variable manner. Here we show CwpV promotes C.
difficile aggregation, mediated by the C-terminal repetitive
domain. This domain varies markedly between strains; five distinct repeat types
were identified and were shown to be antigenically distinct. Other aspects of
CwpV are, however, conserved. All CwpV types are expressed in a phase variable
manner. Using targeted gene knock-out, we show that a single site-specific
recombinase RecV is required for CwpV phase variation. CwpV is
post-translationally cleaved at a conserved site leading to formation of a
complex of cleavage products. The highly conserved N-terminus anchors the CwpV
complex to the cell surface. Therefore CwpV function, regulation and processing
are highly conserved across C. difficile strains, whilst the
functional domain exists in at least five antigenically distinct forms. This
hints at a complex evolutionary history for CwpV.
Published in the journal: The Cell Wall Protein CwpV is Antigenically Variable between Strains, but Exhibits Conserved Aggregation-Promoting Function. PLoS Pathog 7(4): e32767. doi:10.1371/journal.ppat.1002024
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002024Summary
Clostridium difficile is the main cause of antibiotic-associated
diarrhea, leading to significant morbidity and mortality and putting
considerable economic pressure on healthcare systems. Current knowledge of the
molecular basis of pathogenesis is limited primarily to the activities and
regulation of two major toxins. In contrast, little is known of mechanisms used
in colonization of the enteric system. C. difficile expresses a
proteinaceous array on its cell surface known as the S-layer, consisting
primarily of the major S-layer protein SlpA and a family of SlpA homologues, the
cell wall protein (CWP) family. CwpV is the largest member of this family and is
expressed in a phase variable manner. Here we show CwpV promotes C.
difficile aggregation, mediated by the C-terminal repetitive
domain. This domain varies markedly between strains; five distinct repeat types
were identified and were shown to be antigenically distinct. Other aspects of
CwpV are, however, conserved. All CwpV types are expressed in a phase variable
manner. Using targeted gene knock-out, we show that a single site-specific
recombinase RecV is required for CwpV phase variation. CwpV is
post-translationally cleaved at a conserved site leading to formation of a
complex of cleavage products. The highly conserved N-terminus anchors the CwpV
complex to the cell surface. Therefore CwpV function, regulation and processing
are highly conserved across C. difficile strains, whilst the
functional domain exists in at least five antigenically distinct forms. This
hints at a complex evolutionary history for CwpV.Introduction
C. difficile is gram-positive spore forming anaerobe and a major cause of antibiotic-associated diarrhea [1]. C. difficile infection (CDI) often occurs in the nosocomial environment where infection management exerts significant economic pressure on healthcare systems. The strong association of CDI with antibiotic usage reflects disruption of the normal gut flora allowing effective colonization by C. difficile. Strains causing disease produce one or two related toxins, TcdA and TcdB, which modulate the activity of host cell Rho GTPases, destroying the integrity of the epithelial cell barrier and inducing a variety of effects on intestinal cells [2]. Using isogenic mutants in a hamster model of infection, recent studies have shown that toxin B plays an important role in pathogenesis but the situation is less clear for TcdA [3], [4]. C. difficile strains causing CDI however are remarkably diverse. Despite a high prevalence of certain strain types, for example NAP1/027 in North America and Europe around 2005–6, no particular type dominates and strain prevalences vary geographically and temporally [5].
Factors expressed on the bacterial cell surface are likely to contribute to host colonization via interactions with host tissue, the immune system and other bacterial cells. In common with a large number of bacterial species [6], C. difficile produces an S-layer, a paracrystalline proteinaceous array that completely coats the bacterial cell wall. The C. difficile S-layer is primarily composed of two proteins, the high molecular weight S-layer protein (HMW SLP) and the low molecular weight (LMW) SLP. These proteins are derived from the precursor SlpA [7] through cleavage mediated by the cysteine protease Cwp84 [8], [9]. The LMW and HMW SLPs form a stable non-covalently associated complex, which has been analysed by small-angle X-ray scattering [10]. The HMW SLP contains putative cell wall binding motifs (Pfam 04122) which are thought to mediate attachment to the underlying cell wall. The LMW SLP is highly immunogenic and variable between strains, with over 20 distinct sequences identified to date [11], [12], [13], [14].
C. difficile 630 contains 28 paralogs of the HMW SLP [15]. Known as the cell wall protein (CWP) family, all members contain two or three copies of the putative cell wall binding motif in addition to a second unique domain that may specify function. Transcriptomic and proteomic studies have shown several of these proteins to be expressed in vitro [16], [17]. Antibodies against some of these proteins are found in serum of infected patients [18] implying at least some of the CWPs are expressed in vivo during infection and accessible to the host immune system.
In C. difficile 630 [15], CwpV is the largest member of the CWP family and consists of three distinct domains: (1) an N-terminal region with putative cell wall binding activity, (2) a region of unknown function terminating in a serine-glycine-rich flexible linker; (3) nine repeats of 120 amino acids each. We recently described the phase variable expression of CwpV and proposed a mechanism of control involving a cwpV switch [19]. Briefly, between the cwpV promoter and open reading frame there is a 195 bp invertible region flanked by imperfect 21 bp inverted repeats. In the OFF orientation this sequence adopts a stem loop terminator, preventing formation of full-length transcripts while in the other orientation (ON), the terminator is absent so full length cwpV transcription proceeds and CwpV is expressed.
In this study we investigate the regulation, post-translational processing and function of CwpV across a diverse panel of C. difficile strains. We definitively identify the site-specific recombinase necessary for CwpV phase variation, and show that the cwpV switch orientation is the primary determinant of CwpV expression. We show that the CwpV N-terminal domain is responsible for cell wall anchoring and is well conserved across strains. In contrast, we find high sequence variation of the C-terminal repeat sequences across C. difficile strains with five antigenically distinct repeat types identified. Despite this variability, both post-translational processing and function of CwpV appear to be conserved across C. difficile strains. All types of CwpV exhibit an aggregation-promoting function, which is conferred by the variable C-terminal domain. The implications of these findings relating to the role of CwpV in C. difficile infection are discussed.
Results
The recombinase RecV controls expression of CwpV in C. difficile
Previously we identified the C. difficile recV gene as a strong candidate for the site-specific recombinase responsible for inversion of the cwpV DNA switch [19]. To definitively determine the role of recV in inversion of the cwpV switch, we constructed a recV knock-out in 630Δerm using ClosTron technology [20]. 630Δerm (wild-type) and four erythromycin resistant recV mutants, ΔrecV1-4, were confirmed by PCR (Figure 1). To establish the orientation of the cwpV switch in these ΔrecV strains, orientation-specific PCR was carried out [19] (Figure 1B). In wild-type clones, inversion of the cwpV switch was occurring as expected, shown by products with both primer pairs (Figure 1C i). All four ΔrecV clones only amplified an OFF product (Figure 1C ii) and were therefore designated ΔrecV OFF.
Fig. 1. Isolation of Δ<i>recV</i> mutants. A. 
A plasmid encoding recV (pRecV+) was introduced into C. difficile ΔrecV OFF by conjugation. The orientation of the cwpV switch in four ΔrecV(pRecV+) transconjugants was tested by PCR. In all four strains, cwpV switch inversion was observed indicating successful complementation of the switching defect (Figure 1C iii). RecV is a member of the phage integrase family of tyrosine recombinases and by alignment tyrosine-176 was identified as a putative catalytic residue. To confirm the key role of this residue, we constructed a Tyr-Phe mutant (pRecVY176F) which was introduced into ΔrecV OFF. All four ΔrecV(pRecVY176F) transconjugants only had the cwpV switch in the OFF orientation (Figure 1C iv). Therefore RecVY176F could not complement the ΔrecV OFF phenotype confirming the key role of tyrosine-176 in RecV - mediated inversion of the cwpV switch.
In order to isolate ΔrecV ON mutants, ΔrecV(pRecV+) was serially subcultured without thiamphenicol selection to allow loss of the pRecV+ plasmid. Thiamphenicol-sensitive colonies were tested for cwpV switch orientation and all were found to contain a single orientation, as the pRecV+ plasmid had been lost (data not shown). Four clones, from which only the ON orientation of the cwpV switch could be amplified, were isolated and designated ΔrecV ON (Figure 1C v). The lack of inversion in these strains confirmed the necessary role of RecV in cwpV switch inversion, and suggests that this is the only recombinase responsible for inversion.
During the plasmid-curing process we noted two distinct colony morphologies amongst the resulting thiamphenicol-sensitive colonies. Interestingly, these were associated with the two orientations of the cwpV switch. In Figure 1D it can be seen that ΔrecV OFF colonies have the same profuse, ruffled appearance as wild-type 630 colonies, whilst ΔrecV ON colonies are smaller and smoother-edged. The hypothesis that CwpV expression in a ΔrecV ON background causes a smaller colony phenotype was tested (see below).
CwpV is the major CWP on ΔrecV ON cells
The expression of CwpV in S-layer extracts of 630Δerm (WT), ΔrecV(pRecV+), ΔrecV(pRecVY176F), ΔrecV OFF and ΔrecV ON was analyzed by SDS-PAGE (Figure 2A). In WT and ΔrecV(pRecV+) extracts, the two cleavage products of CwpV, the ∼42 kDa (N-terminal fragment) and ∼116 kDa (repeat domain) can be seen. However, as CwpV is only expressed by the minority of ON cells, the overall level of expression in these cultures is low. No expression of CwpV is seen in ΔrecV(pRecVY176F) or ΔrecV OFF strains. Compared to WT cultures, ΔrecV ON strains express a high amount of CwpV. Densitometry analysis indicated that in ΔrecV ON strains, CwpV constituted 13.3% of the protein present in the S-layer (data not shown). These findings were confirmed by Western blot using anti-CwpVrptI (detects the ∼116 kDa CwpV repeat domain) and anti-CwpVNter (detects the ∼42 kDa N-terminal fragment) antibodies (Figures 2C). In all cases, expression of CwpV corresponded to cwpV switch orientation (Figure 2C).
Fig. 2. CwpV expression in Δ<i>recV</i> mutants. 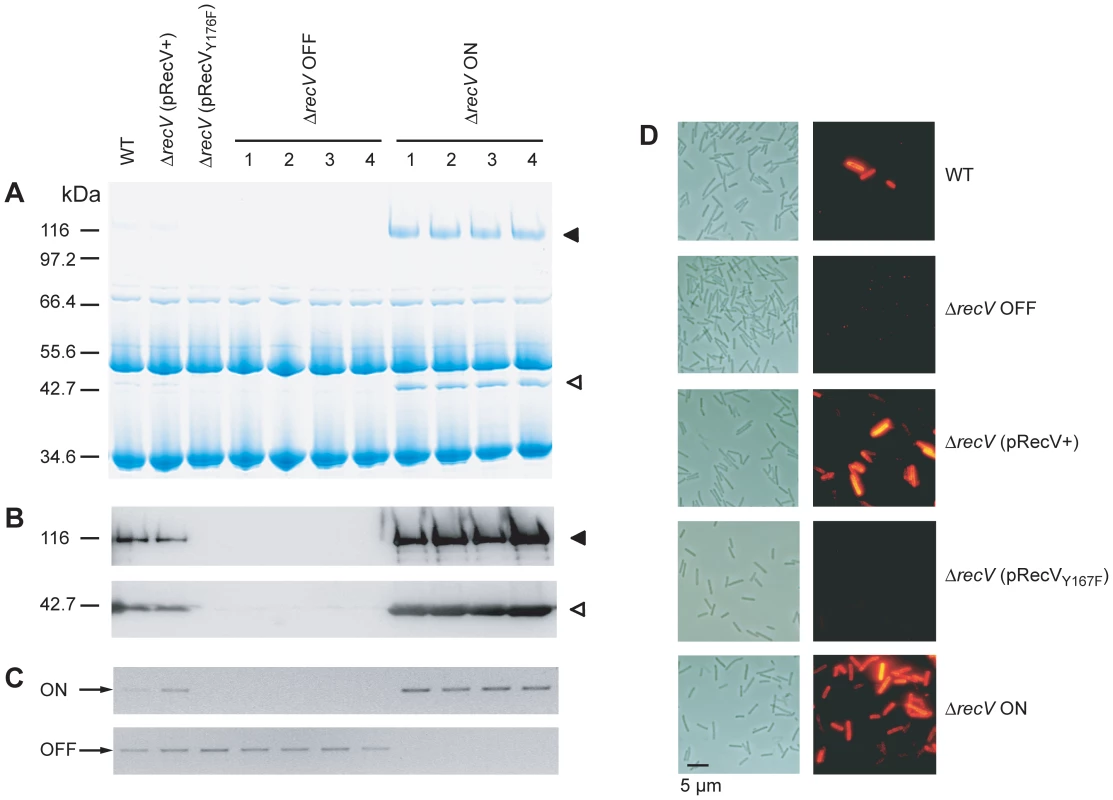
In order to assess CwpV expression at the individual cell level, C. difficile cultures were stained using CwpVrptI antibody and analyzed by fluorescence microscopy (Figure 2D). In the WT culture, ∼5% of cells are ON as previously reported [19]. In ΔrecV OFF, expression cannot be detected on any cell. In ΔrecV(pRecV+) cultures, phase variable expression of CwpV can be seen with approximately 15% of cells expressing CwpV. The higher proportion of CwpV-positive cells is probably due to the altered level of RecV expression from the multi-copy plasmid, pRecV+. In ΔrecV OFF(pRecVY176F) no CwpV expression can be detected, as expected due to a lack of switch inversion from OFF. In ΔrecV ON, CwpV expression can be detected in all cells. This suggests that cwpV switch orientation is the primary determinant of CwpV expression.
The CwpV C-terminal repeat region is variable across C. difficile strains
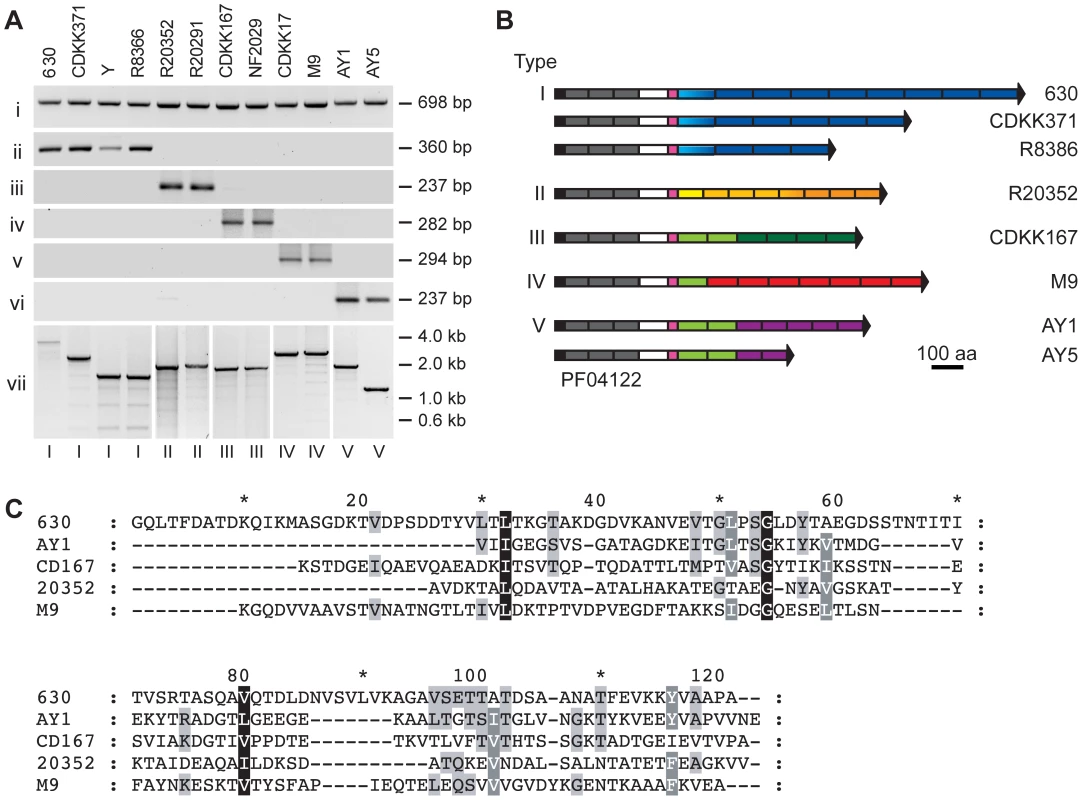
Given the clinical importance of C. difficile strain diversity we sought to determine the level of conservation of cwpV across a variety of strains. PCR analysis of a collection of C. difficile strains using primers specific for the 5′ region of cwpV revealed the presence of a common fragment in all strains (Figure 3Ai). However, amplification using primers specific for the 630 repeat region generated products only in a subset of strains (Figure 3A ii). We have previously shown that, in these strains, the cwpV gene is highly similar to that of 630, but differs in the number of C-terminal repeats [19] (Figure 1A, panel vii). CDKK371 contains six repeats and strains R8366 and Y each contain four repeats. The repeats, termed type I, are highly similar to each other in DNA and predicted amino acid sequence, both within any one strain and between strains. In each strain the first repeat is more divergent from the remaining repeated sequences, although it is still almost identical between strains.
We were unable to amplify the 3′ region of cwpV from the remaining strains studied using primers designed against the 630 type I repeats (Figure 3A ii), consistent with a comparative genomic microarray study using DNA probes from strain 630 which showed that, while the 5′ region of cwpV is present in each of 75 strains studied, the 3′ region was only detected in 17 strains [21]. PCR amplification of the 3′ end of the cwpV gene from two ribotype 027 strains, R20352 and R20291, was achieved using primers found to be conserved in the 5′ end of the R20352 cwpV gene and in CD0515, the gene located downstream of cwpV in 630. The amino acid sequence of R20352 CwpV was almost identical to 630 CwpV from the N-terminus to the serine/glycine-rich region. However, instead of containing repeats of 120 amino acids, these 027 strains contained eight 79 amino acid repeat regions. PCR analysis with primers specific for the R20352 CwpV repeats is shown (Figure 3A iii and vii). These repeats, designated type II, were completely distinct from type I repeats. Several 027 strains were analyzed and the repeats were virtually identical in sequence to one another with the exception of the first repeat, which was slightly divergent from the consensus but conserved between strains.
In other C. difficile strains, where the 5′ region of the gene was present but the 3′ region could not be amplified, genomic DNA was digested with AseI and then re-ligated to form circular fragments. Following inverse PCR, sequence analysis revealed cwpV from all strains to be practically identical to 630 cwpV extending to the serine/glycine-rich region. The 3′ region of cwpV from strains CDKK167 and NF2029 encode six repeats of 94 amino acids distinct in sequence from types I and II, and were designated type III. These repeats could be amplified using repeat-specific primers (Figure 3A iv and vii). Two toxin A-B+ strains, CDKK17 and M9, were found to have a fourth type of repeat sequence. The first repeat is very similar to the first type III repeat, whereas the remaining seven repeats of 98–100 amino acids are distinct from all other repeats and were designated type IV. Finally, non-toxic strains, AY1 and AY5, were found to have a combination of type III repeats and a further novel 79 amino acid repeat, designated type V. Primers designed against type IV and V repeats reflected the sequence analysis, shown in Figure 3A v–vii.
It should be emphasized that the five types of CwpV repeat are completely unrelated to one another. A diagrammatic representation of the eight unique CwpV protein architectures discovered so far is shown in Figure 3B. A multiple sequence alignment of the amino acid sequences of one repeat of each type is shown in Figure 3C, illustrating visually how unrelated the sequences are as no meaningful alignment can be made.
Having characterized these five repeat types, a larger collection of strains were analyzed by PCR to determine their repeat types (see Table 1). Our analysis of strains was not exhaustive, and it seems possible that further CwpV repeat types may be present amongst the considerable diversity of the C. difficile species. However, we focused on the characterization of these five known CwpV repeat types. For each type, a model strain was decided upon and used to represent that type for the rest of the study. We chose; for type I 630, for type II R20352, for type III CDKK167, for type IV M9 (which also contains one type III repeat) and for type V AY1 (which also contains two type III repeats). A full multiple sequence alignment of all the repeat sequences is shown in Figure S1.
Tab. 1. 
The types of repeats and their number were determined by Different CwpV repeat types are antigenically distinct
Given the completely different amino acid sequences for each repeat type we hypothesized that the repeats may be antigenically distinct. CwpV expression was therefore investigated using antibodies raised against recombinant CwpV domains. The CwpV domains used as antigens are indicated by black lines above the protein cartoons in Figure 4A. S-layer extracts were prepared for the representative strain of each type and were visualized by SDS-PAGE (Figure 4B). The significant variation in SlpA across these strains can be seen, as described previously [11]. Analysis by Western blotting with anti-CwpVNter antibody reveals a single band at ∼42 kDa in each strain (Figure 4C) as would be expected by the high level of conservation of the CwpV N-terminal domain across strains. This confirmed expression of CwpV and localization to the cell wall in all these strains.
Fig. 4. CwpV repeat types are antigenically distinct. 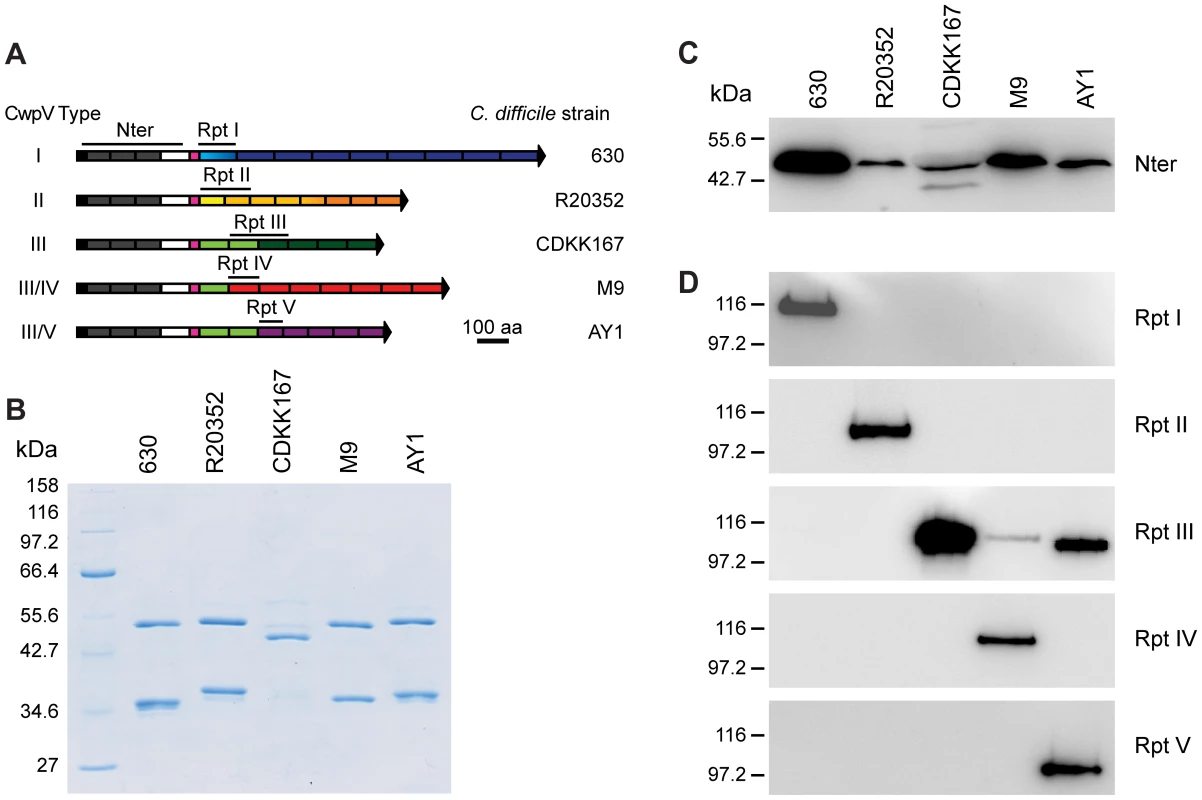
S-layer extracts were then analyzed using antibodies raised against the specific repeat types I–V. Antibodies against repeat types I, II, IV and V recognized only their cognate strains 630, R20352, M9 and AY1, respectively. (Figure 4D). Anti-CwpVrptIII detects CwpV repeats in CDKK167, M9 and AY1 which all contain at least one type III repeat. Detection of CwpV in strain M9 with anti-CwpVrptIII is weak, this may be due to the presence of only one type III repeat in this CwpV protein. These results demonstrate that, as expected based on distinct amino acid sequence, the CwpV repeat types are antigenically distinct.
Expression of all CwpV types is phase variable
Inversion of the cwpV switch has been detected in a wide variety of strains, but phase variable expression of CwpV has only been reported in strain 630 [19]. It therefore seemed possible that CwpV would be expressed in a phase variable manner in strains expressing all types of CwpV. Cultures of representative strains for each type were stained using the appropriate anti-CwpVrpt antibody for analysis by immunofluorescence microscopy (Figure 5). All strains expressed CwpV in a subset of cells, ranging from 0.1–10% of total cells, indicative of phase variable expression. The factors determining the proportion of CwpV-positive cells are yet to be determined, but it is clear that under standard laboratory culture conditions, a minority of cells express CwpV.
Fig. 5. Expression of all CwpV types is phase variable. 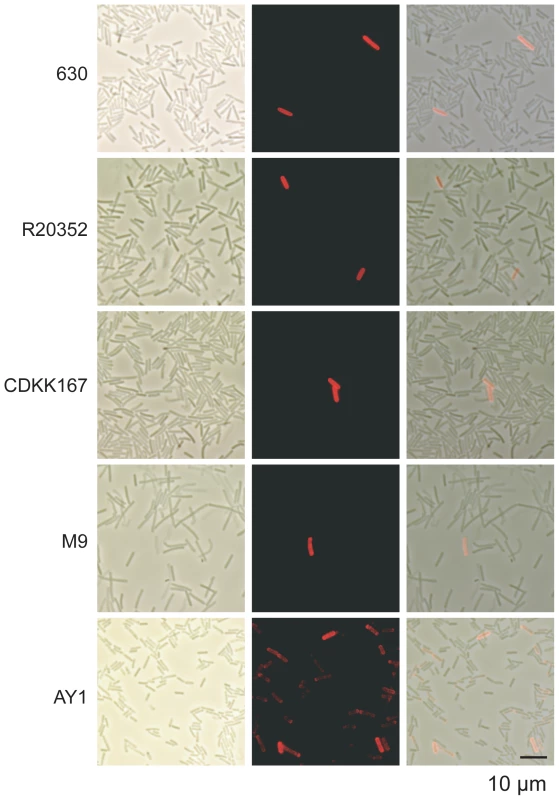
A conserved post-translational cleavage mechanism for all CwpV proteins
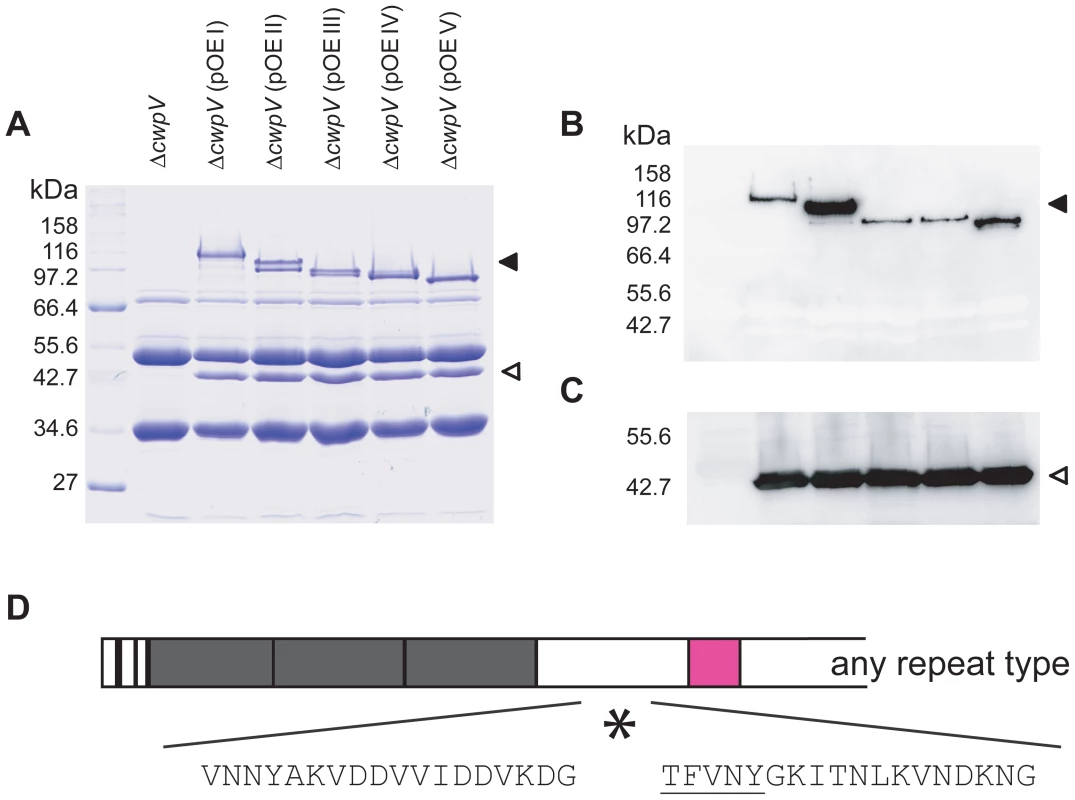
The five different CwpV proteins from 630, R20352, CDKK167, M9 and AY1 were modified to include a C-terminal Strep-Tag II and expressed in C. difficile 630ΔcwpV using a constitutive promoter. S-layer extracts were prepared from these strains, designated ΔcwpV(pOE I-V), and analyzed by SDS-PAGE (Figure 6A). A high level of CwpV expression can be seen for each CwpV protein, with CwpV constituting a significant proportion of the total S-layer extract. All five proteins are cleaved into the ∼42 kDa N-terminal fragment and C - terminal fragment of between 90 and 120 kDa, depending on their CwpV type. Western blotting using antibodies against the N-terminal domain detected the 42 kDa fragment in each extract and those against the Strep-Tag II detected the 90–120 kDa C-terminal CwpV fragment (Figure 6B). In order to determine the cleavage site for each CwpV protein, the N-terminal sequence of each strep-tagged C-terminal fragment was obtained. The first 5 amino acids of every C-terminal CwpV fragment was found to be TFVNY, unambiguously locating the cleavage site in the domain of CwpV between the N-terminal domain and the serine/glycine rich region, as depicted in Figure 6D. This domain is well-conserved between CwpV proteins of different types, and indicates that the CwpV post-translational cleavage mechanism is conserved across CwpV types. The protease responsible for SlpA cleavage has been identified as the surface expressed cysteine protease Cwp84 [8], [9] however it has been shown that Cwp84 is not required for CwpV cleavage [8]. We have analyzed expression of CwpV in strains containing knock-outs of either cwp84 or its paralog cwp13, and found that neither protease is required for cleavage of CwpV (data not shown).
All types of CwpV form a complex of the N-terminal and C-terminal fragments
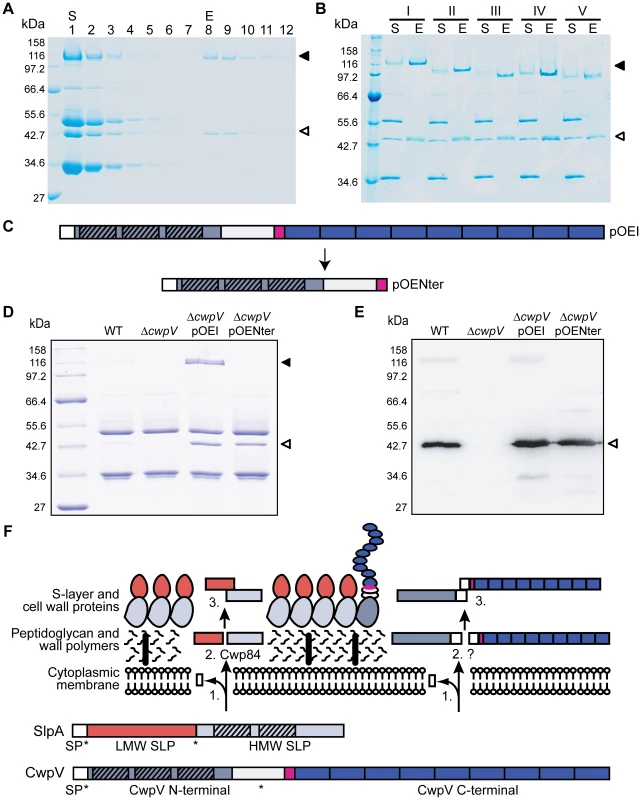
Post-translational cleavage of CwpV into two fragments is reminiscent of post-translational cleavage of SlpA into the HMW and LMW SLP fragments. In the case of SlpA these two fragments form a complex allowing the HMW SLP, containing the putative cell wall binding motifs, to anchor the LMW SLP to the underlying cell wall [10]. We therefore reasoned that CwpV cleavage may also be followed by complex formation to allow the N-terminal domain, containing putative cell wall binding motifs, to anchor the repetitive C-terminal fragment to the cell surface. To test this hypothesis, the C-terminal repeat region from strain ΔcwpV(pOEI) was purified by affinity chromatography using a StrepTactin resin. As shown in Figure 7A, the 42 kDa and 116 kDa fragments of CwpV were co-eluted despite the presence of the Strep-Tag II only in the larger fragment. This experiment was then repeated with S-layer extracts from ΔcwpV strains over-expressing CwpV types II–V. The complete S-layer extracts (S) and first elutions (E) are visualized by SDS-PAGE (Figure 7B). In all cases, both CwpV fragments co-eluted, clearly demonstrating complex formation. As this interaction is independent of repeat type, this strongly suggests that, as in SlpA, it is the highly conserved regions adjacent to the cleavage site that mediate complex formation.
The CwpV N-terminal fragment is responsible for cell wall anchoring
The role of the N-terminal fragment of CwpV containing putative cell binding motifs was then investigated. A truncated form of the 630 cwpV gene which lacks the whole repetitive sequence downstream of the serine/glycine region was created as depicted in Figure 7C. The plasmid encoding this protein, pOE Nter, was transferred to 630ΔcwpV to create ΔcwpV(pOENter). S-layer extracts from wild-type, ΔcwpV, ΔcwpV(pOE I) and ΔcwpV(pOENter) cultures were analysed by SDS-PAGE (Figure 7D). The ∼42 kDa N-terminal CwpV fragment is seen in all extracts other than ΔcwpV and its identity was confirmed by Western blot with anti-CwpVNter (Figure 7E). This suggests that the N-terminal fragment of CwpV alone is sufficient for stable association with the cell wall. The same amount of the N-terminal CwpV fragment is seen in the ΔcwpV(pOEI) and ΔcwpV(pOENter) S-layer extracts, showing that the truncated version of CwpV is equally well expressed on the cell surface as the full-length CwpV protein. We therefore named the N-terminal fragment of CwpV as the cell wall anchoring (CWA) fragment. This is the first reported experimental evidence that PF04122 motifs are responsible for cell wall anchoring.
A model for post-translational CwpV processing
The determination of the CwpV cleavage site, evidence of complex formation by the two CwpV cleavage products, and role of the N-terminal fragment in cell wall anchoring leads to an overall model for post-translational CwpV processing. The three steps involved are depicted in Figure 7F, and are compared to the steps known to occur for SlpA processing. The first step is export of the protein across the cell membrane and cleavage of the signal peptide. Once on the surface the protein is cleaved (step 2) by Cwp84 in the case of SlpA, or by an unknown mechanism in the case of CwpV. The third step is complex formation of the cleavage products, allowing the HMW SLP or the CwpV CWA fragment to anchor the complex to the cell surface. All three steps appear to be conserved amongst different CwpV types.
CwpV is an aggregation-promoting factor
As noted previously, ΔrecV ON strains showed a smaller, smoother-edged colony morphology than ΔrecV OFF or wild-type strains (see Figure 1D). We therefore investigated this phenomenon in greater detail. As shown in Figure 8A, wild-type and ΔcwpV strains exhibit the same disperse, ruffled colony morphology. Over-expression of the CwpV N-terminal CWA alone in ΔcwpV did not alter this colony morphology. However, over-expression of any of the five types of full-length CwpV caused the same smaller, smoother-edged colony morphology seen in ΔrecV ON mutants (Figures 1D and 8A). These results strongly suggest that it is the repetitive C-terminal domain of CwpV that mediates this phenotype. In order to investigate more closely the basis of this difference, the edges of colonies grown between glass and agar were visualized microscopically. Detail of the colony morphology at the cellular level can be seen in the 63× magnification images shown alongside the 1× images in Figure 8A. Wild-type, ΔcwpV and ΔcwpV(pOENter) colonies were seen to have diffuse edges, with directional protrusions of bacterial growth common at the colony edge. However, ΔcwpV(pOEI-V) exhibited denser, straighter colony boundaries, with rare if any directional protrusions. The observed macroscopic differences in colony morphology are therefore due to altered cellular organization within bacterial colonies.
Fig. 8. CwpV is an aggregation-promoting factor. 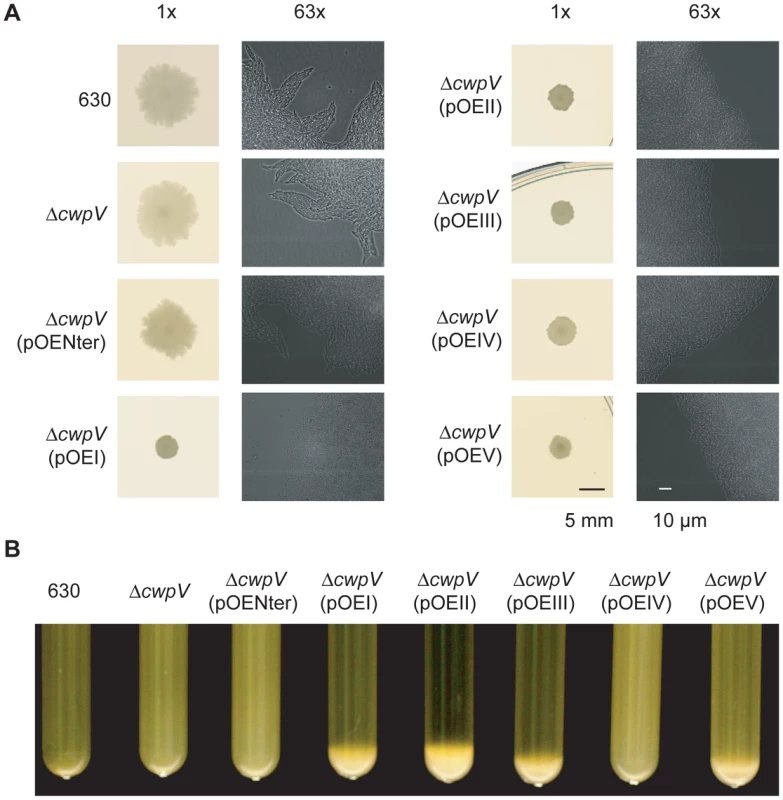
This altered cellular organization may result from CwpV-mediated auto-aggregation. Therefore the propensity of C. difficile to aggregate from suspension was assessed across the panel of strains. Over-expression of all CwpV types, except type IV, induced aggregation of C. difficile from a liquid suspension with a starting OD600 nm of 10 (Figure 8B). Aggregation was followed in real time, and could be observed in tubes where aggregation was occurring from as soon as 1 hour after suspension of bacteria (data not shown). It was found that only 630 CwpV (type I) induced aggregation of C. difficile from suspensions with an OD600 nm of 3, while 630 CwpV and R20352 CwpV (type II) induced aggregation from an OD600 nm of 6 (data not shown). An OD600 nm as high as 40 did not lead to type IV-mediated aggregation, nor aggregation of wild-type, ΔcwpV or ΔcwpV(pOENter) (data not shown). Therefore it appears that the ability to induce aggregation is highest for CwpV from 630, followed by R20352, then CDKK167 and AY1, with no observed propensity of M9 CwpV or the CwpV CWA fragment to induce aggregation of C. difficile from suspension.
Discussion
We previously described the phase-variable expression of CwpV, employing a novel transcriptional termination mechanism [19]. Here we show that CwpV can constitute a major cell wall protein and that it exhibits an auto-aggregation promoting function. Surprisingly, this function is mediated by the highly variable C-terminal repeat domain which we find in five entirely distinct sequence types. Auto-aggregation was analyzed at both the cellular level on solid media and within liquid culture. In vitro colonies are organized populations of bacteria and differences in colony morphology reflect the cellular organization of a population [22] that can translate to important differences affecting natural growth dynamics. Differences in the cellular organization of colonies was clearly demonstrated by microscopy (Figure 8A) with CwpV expression in all cells causing a denser, more randomly packed cellular organization than the diffuse, directional patterns of cellular packing seen at the edge of wild-type colonies. The CwpV-mediated aggregation of C. difficile cells from suspension strongly suggests that CwpV has a direct aggregation-promoting function rather than a direct effect on motility. In fact the motility of strains in soft agar was found to be similar, regardless of whether or not they expressed CwpV (see Figure S2 and Text S1).
Propensities of different CwpV types to cause aggregation varied, as observed by the different starting densities of suspensions required for aggregation, apart from type IV, which did not induce aggregation. This is likely to reflect different affinities of the different CwpV proteins for their ligand(s). The lack of an observable aggregation phenotype due to type IV repeats may be explained by a weaker affinity between this repeat type and its ligand, however the observed colony morphology phenotype does suggest that it carries out the same general function as the other types. Functional interactions between CwpV and motility factors, such as type IV pili and flagella, are worthy of further study. Indeed, the diffuse directional organization of wild-type colony edges seen in this study is reminiscent of twitching motility mediated by type IV pili in Clostridium perfringens [23]. C. difficile does have type IV pili clusters, which are conserved across strains [24]. Type IV pili may therefore be involved in the cellular organization of wild-type colonies grown on solid surfaces observed here. Auto-aggregative proteins have been shown to play roles in biofilm formation [25], [26], [27]. No studies on C. difficile biofilms have been reported, and in our experience C. difficile does not exhibit significant biofilm formation on abiotic surfaces (data not shown). However in infected mice large aggregates of C. difficile cells, described as exaggerated mats, were reported to be associated with regions of severe inflammation [28]. This description is indicative of a biofilm-like growth of C. difficile during infection. Multispecies biofilms in the human GI tract have been observed [29] and C. difficile is likely to require factors that promote appropriate intra - and inter-species interactions to colonize the gut. CwpV may play important roles in this process.
Aside from its aggregation-promoting function CwpV may have other functions. We investigated bacterial adhesion to Caco-2 cells, but no significant difference in adhesion was caused by CwpV expression (data not shown). Further work is required to assess whether CwpV expression affects colonization levels in vivo, and if so whether this is directly due to its aggregation-promoting activity. Understanding the roles of CwpV during in vivo infection is a priority and will be addressed using the isogenic mutants described in this study. Established animal models of infection focus more on the acute stage of C. difficile infection, rather than colonization [30]. However, recent developments of animal models better suited to studying the roles of C. difficile genes likely to be involved in colonization, such as CwpV, have been described [28], [31] that will facilitate our future investigation. The phase-variable expression of CwpV may be highly relevant to any in vivo role; its expression in a sub-set of cells as a contingency may be beneficial to the survival of the population as a whole.
The variation of CwpV repeat sequences uncovered in this study is extensive, with strains expressing one or two of five antigenically distinct types encoded by unrelated sequences. The lack of sequence homology between repeat types suggests acquisition by horizontal gene transfer. Any known mechanism of chromosomal sequence acquisition; homologous recombination, illegitimate recombination or additive integration could account for introduction of these sequences to the C. difficile chromosome [32]. However, without knowing the origin of these sequences, the mechanisms of transfer to C. difficile and incorporation into the genome are unclear. Human microbiome sequencing may shed light on the origins of CwpV sequences. It would seem that CwpV type exchange is occurring at a low frequency, as all strains tested from within one ribotype contain the same CwpV type. Current understanding of the phylogenetics and diversity of C. difficile strains is limited [21], [33]. However current data suggests a single clade is associated with one CwpV type; the HY (027) clade associates with type II CwpV, the A-B+ clade with type IV CwpV, the HA2 (078) and HA1 clades with type I CwpV. The locations of strains expressing type III and V CwpV within this phylogenetic tree of C. difficile are not yet known. As more C. difficile strains are sequenced and our understanding of evolutionary relationships between strains improves, it will become clearer as to when different CwpV types were acquired, and whether single or multiple independent acquisition events for each type have occurred. Host immune pressure is one possible selection that could promote CwpV variability, as one key difference between CwpV types is their antigenicity. Antigenic variability is also seen in the C. difficile surface proteins SlpA and Cwp66 [34]. Variation in surface proteins may also be involved in competition between different C. difficile strains or in resistance to bacteriophages.
Apart from the marked diversity in CwpV repeat sequences, other aspects of CwpV are remarkably conserved. Expression of all types of CwpV is phase variable suggesting that this complex regulatory mechanism, involving the conserved cwpV switch and the RecV recombinase, is important for optimal function of CwpV. Phase variation of bacterial surface proteins has been reported to be involved in numerous processes including immune evasion and colonization [35]. Given the sequence variability and aggregation-promoting function of CwpV, several explanations behind its phase variable regulation can be suggested. However, investigation into CwpV expression during in vivo infection is likely to shed most light on the importance of CwpV phase variability.
Conserved post-translational processing of CwpV and formation of a CWA-repeat domain complex again suggests that these processes are important for optimal CwpV expression and function. To date we only observe complex formation in cell wall proteins CwpV and SlpA, with all other CWPs studied (e.g. Cwp84, Cwp66, Cwp2) being presented on the surface without post-translational cleavage between the CWA and functional domains. An unidentified protease could mediate cleavage of CwpV, or it could undergo non-enzymatic auto-processing as reported for a number of protein families [36], [37]. Uncleaved SlpA is poorly tolerated on the cell wall, and is found in large quantities in the culture supernatant of a Δcwp84 mutant [9]. SlpA monomers once cleaved must interact on the cell surface to form a two-dimensional array. Expression of CwpV does not appear to disrupt the integrity of the S-layer and, by analogy, it seems likely that cleavage of CwpV is important for its stable incorporation into the S-layer. Given the high proportion of the total S-layer accounted for by CwpV in ΔrecV ON strains, it seems likely that CwpV interacts positively with other S-layer proteins to maintain the integrity of S-layer packing.
Our finding that the CwpV CWA domain is responsible for anchoring of the CwpV C-terminal domain to the underlying cell wall is the first direct evidence of the function of Pf04122 domains. These domains are also present in the HMW SLP of the C. difficile S-layer where the presumably mediate a similar role. They are also found in a large variety of Gram-positive bacteria and some Archaea, where they presumably fulfill a similar function of anchoring cell wall proteins to the underlying structure. This then adds to the diversity of mechanisms used by Gram-positive bacteria to anchor and display cell wall proteins [38], [39].
Phylogenetic studies [21] show that outbreak-causing strains of C. difficile have emerged independently from multiple lineages, indicating that virulent strains can emerge from across the diversity of the species, rather than being confined to a specific pathogenic lineage. This suggests that certain genetic elements common to all C. difficile strains underlie virulence. It is likely that the incidences of different lineages causing disease are dynamic, and we cannot predict which lineages may cause future outbreaks. Therefore understanding the common features of C. difficile as an ancient species is important, as in order for intervention strategies to be successful in the long-term they must target the full diversity of C. difficile strains, rather than specific lineages. CwpV appears to be a core component of C. difficile, including its phase variable regulation controlled by RecV, post-translational processing mechanism, and aggregation-promoting function. This highlights the importance of CwpV to C. difficile survival throughout evolution of this diverse species. Despite this conservation CwpV appears to have been under positive selection for antigenic variability. Further investigation into the roles of CwpV in the C. difficile life cycle may teach us a lot about what makes C. difficile a successful pathogen, and provide opportunities for new intervention strategies.
Materials and Methods
Bacterial strains and growth conditions
The C. difficile strains characterised for CwpV type in this study are described in Table 1. C. difficile laboratory-generated mutant and recombinant-plasmid containing strains used in this study are described in Table S3. C. difficile was routinely cultured on blood agar base II (Oxoid) supplemented with 7% horse blood (TCS Biosciences), brain-heart infusion (BHI) agar (Oxoid) or in BHI broth (Oxoid). Cultures were grown in an anaerobic cabinet (Don Whitley Scientific) at 37°C in an atmosphere of 10% CO2, 10% H2 and 80% N2. C. difficile strains containing recombinant plasmids were grown under thiamphenicol selection. Commercial chemically competent TOP10 E. coli cells (Invitrogen) were used for cloning and recombinant plasmid maintenance. Recombinant expression of CwpV fragments was carried out in Rosetta E. coli cells (Novagen). E. coli strains were routinely grown at 37°C on LB-agar plates or in LB liquid culture in the presence of selective antibiotics where appropriate to any transformed plasmid.
DNA manipulations
DNA manipulations were carried out according to standard techniques. C. difficile genomic DNA for use in cloning and PCR analysis was prepared as described previously [19]. Polymerase chain reactions (PCR) used KOD (Novagen), Expand Long-Template Polymerase (Roche Diagnostics) or Taq polymerase (Sigma) in accordance with the manufacturers' protocols using primers detailed in Table S1.
Production of recombinant plasmids
In order to produce a C. difficile 630Δerm knock out of recV, a retargeted ClosTron vector was designed using the intron design tool www.clostron.com. The top-scored target site in recV was selected (424/425s) and the intron targeting region required was commercially synthesized and cloned into pMTL007C-E2 (DNA 2.0) to generate a plasmid pLRP028. For complementation of the ΔrecV mutant, the recV gene from C. difficile 630 was amplified using primers NF1357 and NF1358 and cloned using SacI and BamHI into a C. difficile plasmid expression vector, based on the pMTL960 replicon [19], [40] to generate pCBR113 (pRecV+). Inverse PCR with primers NF1411 and NF1412, using pCBR113 as the template, was carried out to mutate the recV tyrosine176 to phenylalanine, producing pCBR115 (pRecVY176F+). To express CwpV fragments for use as antigens (Figure 4A) cwpV repeats from strains R20352 (type II), CDKK167 (type III), M9 (type IV) and AY1 (type V) were amplified by PCR using KOD polymerase (Novagen/Merck Chemicals Ltd) and primers described in Table S1. These fragments were directionally cloned using NcoI and XhoI into pET28a E. coli expression vector then transformed into TOP10 E. coli (Invitrogen) to generate plasmids pCBR069-072 described in Table S2. To clone the cwpV genes from strains of unknown genomic sequence, genomic DNA was digested overnight at 37°C with AseI (NEB) and then ligated overnight at 16°C using T4 ligase (NEB). Inverse PCR using primers NF401 and NF654 was carried out and amplification products were cloned into pCR4-TOPO (Invitrogen). pCBR044, containing the full-length cwpV gene from 630 under the control of the cwp2 promoter within the pMTL960 backbone [19], was modified to contain a 5′ XhoI site and strep-tag II (WSHPQFEK) encoding sequence by inverse PCR, generating the C. difficile strep-tagged 630 CwpV expression plasmid pCBR080 (pOEI). The cwpV genes from R20352, CDKK167, M9 and AY1 were amplified with the primers described in Table S1 and cloned in place of the 630 gene in pCBR080 to create plasmids pCBR105-107 and 109 (pOEII-V) (Table S2). Successful construction of recombinant plasmids was always confirmed by DNA sequencing (GATC Biotech).
Conjugation into C. difficile
Plasmids were transformed into E. coli CA434 and then conjugated into C. difficile as described previously [40] using thiamphenicol (30 mg ml−1) to select for pMTL960-based plasmids and cycloserine (250 mg ml−1) to counter-select for E. coli.
Purification of recombinant CwpV protein and generation of antibodies
Plasmids pCBR069-072 expressing repeat types II–V were transformed into E. coli Rosetta (Novagen/Merck Chemicals Ltd) and grown overnight at 37°C in Overnight Express media (Novagen/Merck Chemicals Ltd). Bacteria were collected, lysed using BugBuster (Novagen/Merck Chemicals Ltd) and the His-tagged fusion proteins purified by affinity chromatography using Ni-NTA agarose (Qiagen). Following purification, proteins were dialysed into 10 mM HEPES 150 mM NaCl. Antisera to the proteins were generated in rabbits using a commercial service (Covalab, Cambridge, UK). Anti-CwpVNter and anti-CwpVrptI were described previously [19].
S-layer extraction, SDS-PAGE and western immunoblotting
C. difficile cultures were grown overnight, harvested by centrifugation and S-layer extracts were prepared using low pH glycine incubation as described previously [7]. Proteins in the S-layer extracts were subjected to SDS-PAGE and western blotting according to standard protocols. All rabbit primary anti-CwpV antibodies (anti-CwpVNter, anti-CwpVrptI, anti-CwpVrptII, anti-CwpVrptIII, anti-CwpVrptIV and anti - CwpVrptV) were used at 1/5000 dilution, followed by anti-rabbit-HRP (Dako Cytomation) at 1/2000 dilution. Signal was detected using SuperSignal® West Pico Chemiluminescent Substrate (Pierce).
Immunocytochemistry
C. difficile cells from liquid culture were washed with PBS then fixed in 8% formaldehyde, which was quenched with 20 mM NH4Cl for 15 min prior to staining. Washed cell suspensions were re-suspended in 40 µl 1% BSA in PBS containing 1/20 dilutions of rabbit primary antibodies (anti-CwpVNter, anti-CwpVrptI, anti-CwpVrptII, anti-CwpVrptIII, anti-CwpVrptIV and anti - CwpVrptV) for 45 min. Bacteria were then washed and incubated with 1/40 anti-rabbit-rhodamine red-X (Jackson ImmunoResearch) in the dark for 45 min. Bacteria were washed with PBS and dH20 then allowed to air dry onto a microscope slide. Coverslips were mounted using ProLong gold anti-fade mounting reagent (Invitrogen) and allowed to set overnight. Bacteria were visualised using a Nikon Eclipse E600 microscope fitted with a Nikon DMX1200 camera.
N-terminal sequencing
S-layer extracts were separated on 10% SDS-PAGE gels and transferred to a PVDF membrane. The membrane was washed in water for 10 min, stained in 0.1% Coomassie Blue R in 50% MeOH for 5 min. After destaining in 50% MeOH, 10% acetic acid (3×5 min with rocking) and washing in water (3×5 min with rocking) the membrane was allowed to dry thoroughly. N-terminal sequencing by Edman degradation was then carried (PNAC Facility, Department of Biochemistry, University of Cambridge).
C. difficile S-layer extract streptactin binding/elution assay
StrepTactin resin (Novagen) was used according to manufacturers' instructions. Briefly, resin was washed with dH2O, equilibrated with wash buffer (150 mM NaCl, 100 mM Tris-HCl, 1 mM EDTA, pH 8.0) and mixed with S-layer extract. The mixture was incubated overnight at 4°C with rotation. The next day the resin was washed with wash buffer to remove non-specifically bound proteins. Elution buffer (150 mM NaCl, 100 mM Tris-HCl, 1 mM EDTA, 2.5 mM desthiobiotin, pH 8.0) was then used to elute strep-tagged proteins. All fractions were collected for analysis using SDS-PAGE and Coomassie staining.
Visualization of C. difficile colony morphology
For macroscopic visualization of C. difficile colony morphology strains were grown on BHI agar (Oxoid) for 5 days from an appropriate C. difficile inoculum such that isolated colonies grew on the plate. Plates were then scanned using a flat-bed scanner to record images of the colonies. For microscopic visualization of C. difficile colony morphology, cultures taken from a 24-hour old colony were inoculated directly on to glass-bottomed dishes (MatTek corporation). Anaerobic BHI 0.7% agar at 40°C was then carefully poured over the glass to cover the dish and allowed to set. C. difficile was grown between the glass and the agar for 3 days. Dishes were removed and images taken using a Zeiss Axiovert 200 widefield microscope at FILM, Imperial College London. Microscope images were analysed using Volocity 5.3.2 software (Improvision).
Supporting Information
Zdroje
1. McFarlandLV
2008
Antibiotic-associated diarrhea: epidemiology, trends and
treatment.
Future Microbiol
3
563
578
2. VothDEBallardJD
2005
Clostridium difficile toxins: mechanism of
action and role in disease.
Clin Microbiol Rev
18
247
263
3. KuehneSACartmanSTHeapJTKellyMLCockayneA
2010
The role of toxin A and toxin B in Clostridium
difficile infection.
Nature
467
711
713
4. LyrasDO'ConnorJRHowarthPMSambolSPCarterGP
2009
Toxin B is essential for virulence of Clostridium
difficile.
Nature
458
1176
1179
5. FreemanJBauerMPBainesSDCorverJFawleyWN
2010
The changing epidemiology of Clostridium
difficile infections.
Clin Microbiol Rev
23
529
549
6. SleytrUBEgelseerEMIlkNPumDSchusterB
2007
S-Layers as a basic building block in a molecular construction
kit.
FEBS J
274
323
334
7. CalabiEWardSWrenBPaxtonTPanicoM
2001
Molecular characterization of the surface layer proteins from
Clostridium difficile.
Mol Microbiol
40
1187
1199
8. KirbyJMAhernHRobertsAKKumarVFreemanZ
2009
Cwp84, a surface-associated cysteine protease, plays a role in
the maturation of the surface layer of Clostridium
difficile.
J Biol Chem
284
34666
34673
9. DangTHRiva LdeLFaganRPStorckEMHealWP
2010
Chemical probes of surface layer biogenesis in
Clostridium difficile.
ACS Chem Biol
5
279
285
10. FaganRPAlbesa-JoveDQaziOSvergunDIBrownKA
2009
Structural insights into the molecular organization of the
S-layer from Clostridium difficile.
Mol Microbiol
71
1308
1322
11. CalabiEFairweatherN
2002
Patterns of sequence conservation in the S-layer proteins and
related sequences in Clostridium difficile.
J Bacteriol
184
3886
3897
12. KatoHYokoyamaTArakawaY
2005
Typing by sequencing the slpA gene of
Clostridium difficile strains causing multiple
outbreaks in Japan.
J Med Microbiol
54
167
171
13. KarjalainenTSaumierNBarcM-CDelmeeMCollignonA
2002
Clostridium difficile genotyping based on
slpA variable region in S-layer gene sequence: an
alternative to serotyping.
J Clin Microbiol
40
2452
2458
14. EidhinDRyanADoyleRWalshJBKelleherD
2006
Sequence and phylogenetic analysis of the gene for surface layer
protein, slpA, from 14 PCR ribotypes of Clostridium
difficile.
J Med Microbiol
55
69
83
15. SebaihiaMWrenBWMullanyPFairweatherNFMintonN
2006
The multidrug-resistant human pathogen Clostridium
difficile has a highly mobile, mosaic genome.
Nat Genet
38
779
786
16. WrightAWaitRBegumSCrossettBNagyJ
2005
Proteomic analysis of cell surface proteins from
Clostridium difficile.
Proteomics
5
2443
2452
17. EmersonJEStablerRAWrenBWFairweatherNF
2008
Microarray analysis of the transcriptional responses of
Clostridium difficile to environmental and antibiotic
stress.
J Med Microbiol
57
757
764
18. WrightADrudyDKyneLK.BFairweatherNF
2008
Immunoreactive cell wall proteins of Clostridium
difficile identified by human sera.
J Med Microbiol
57
750
756
19. EmersonJEReynoldsCBFaganRPShawHAGouldingD
2009
A novel genetic switch controls phase variable expression of
CwpV, a Clostridium difficile cell wall
protein.
Mol Microbiol
74
541
556
20. HeapJTKuehneSAEhsaanMCartmanSTCooksleyCM
2010
The ClosTron: Mutagenesis in Clostridium refined and
streamlined.
J Microbiol Methods
80
49
21. StablerRAGerdingDNSongerJGDrudyDBrazierJS
2006
Comparative phylogenomics of Clostridium
difficile reveals clade specificity and microevolution of
hypervirulent strains.
J Bacteriol
188
7297
7305
22. ShapiroJA
1995
The significances of bacterial colony patterns.
Bioessays
17
597
607
23. VargaJJNguyenVO'BrienDKRodgersKWalkerRA
2006
Type IV pili-dependent gliding motility in the Gram-positive
pathogen Clostridium perfringens and other
clostridia.
Mol Microbiol
62
680
694
24. JanvilisriTScariaJThompsonADNicholsonALimbagoBM
2009
Microarray identification of Clostridium
difficile core components and divergent regions associated with
host origin.
J Bacteriol
191
3881
3891
25. KuboniwaMAmanoAHashinoEYamamotoYInabaH
2009
Distinct roles of long/short fimbriae and gingipains in homotypic
biofilm development by Porphyromonas
gingivalis.
BMC Microbiol
9
105
26. CorriganRMRigbyDHandleyPFosterTJ
2007
The role of Staphylococcus aureus surface
protein SasG in adherence and biofilm formation.
Microbiology
153
2435
2446
27. HuangYJLiaoHWWuCCPengHL
2009
MrkF is a component of type 3 fimbriae in Klebsiella
pneumoniae.
Res Microbiol
160
71
79
28. LawleyTDClareSWalkerAWGouldingDStablerRA
2009
Antibiotic treatment of Clostridium difficile
carrier mice triggers a supershedder state, spore-mediated transmission, and
severe disease in immunocompromised hosts.
Infect Immun
77
3661
3669
29. MacfarlaneSDillonJF
2007
Microbial biofilms in the human gastrointestinal
tract.
J Appl Microbiol
102
1187
1196
30. DouceGGouldingD
2010
Refinement of the hamster model of Clostridium
difficile disease.
Methods Mol Biol
646
215
227
31. SteeleJFengHParryNTziporiS
2009
Piglet models of acute or chronic Clostridium
difficile illness.
J Infect Dis
201
428
434
32. ThomasCMNielsenKM
2005
Mechanisms of, and barriers to, horizontal gene transfer between
bacteria.
Nat Rev Microbiol
3
711
721
33. HeMSebaihiaMLawleyTDStablerRADawsonLF
2010
Evolutionary dynamics of Clostridium difficile
over short and long time scales.
Proc Natl Acad Sci U S A
107
7527
7532
34. LemeeLBourgeoisIRuffinECollignonALemelandJF
2005
Multilocus sequence analysis and comparative evolution of
virulence-associated genes and housekeeping genes of Clostridium
difficile.
Microbiology
151
3171
3180
35. van der WoudeMW
2006
Re-examining the role and random nature of phase
variation.
FEMS Microbiol Lett
254
190
197
36. DautinNBarnardTJAndersonDEBernsteinHD
2007
Cleavage of a bacterial autotransporter by an evolutionarily
convergent autocatalytic mechanism.
EMBO J
26
1942
1952
37. BranniganJADodsonGDugglebyHJMoodyPCSmithJL
1995
A protein catalytic framework with an N-terminal nucleophile is
capable of self-activation.
Nature
378
416
419
38. SaraM
2001
Conserved anchoring mechanisms between crystalline cell surface
S-layer proteins and secondary cell wall polymers in Gram-positive
bacteria?
Trends Microbiol
9
47
49
39. MarraffiniLADeDentACSchneewindO
2006
Sortases and the art of anchoring proteins to the envelopes of
Gram-positive bacteria.
Microbiol Mol Biol Rev
70
192
221
40. PurdyDO'KeeffeTAElmoreMHerbertMMcLeodA
2002
Conjugative transfer of clostridial shuttle vectors from
Escherichia coli to Clostridium difficile through
circumvention of the restriction barrier.
Mol Microbiol
46
439
452
41. PopoffMRRubinEJGillDMBoquetP
1988
Actin-specific ADP-ribosyltransferase produced by a
Clostridium difficile strain.
Infect Immun
56
2299
2306
42. MerriganMSambolSJohnsonSGerdingDN
2003
Susceptibility of hamsters to human pathogenic
Clostridium difficile strain B1 following clindamycin,
ampicillin or ceftriaxone administration.
Anaerobe
9
91
95
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Innate Immune Sensing of DNA
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 4- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Low Diversity Variety Multilocus Sequence Types from Thailand Are Consistent with an Ancestral African Origin
- Genetic Assignment Methods for Gaining Insight into the Management of Infectious Disease by Understanding Pathogen, Vector, and Host Movement
- Innate Immune Sensing of DNA
- Engineered Resistance to Development in Transgenic
- -Mediated Detoxification of Reactive Oxygen Species Is Required for Full Virulence in the Rice Blast Fungus
- SLO-1-Channels of Parasitic Nematodes Reconstitute Locomotor Behaviour and Emodepside Sensitivity in Loss of Function Mutants
- Structure-Function Analysis of the LRIM1/APL1C Complex and its Interaction with Complement C3-Like Protein TEP1
- A Effector with Enhanced Inhibitory Activity on the NF-κB Pathway Activates the NLRP3/ASC/Caspase-1 Inflammasome in Macrophages
- The MARCH Family E3 Ubiquitin Ligase K5 Alters Monocyte Metabolism and Proliferation through Receptor Tyrosine Kinase Modulation
- is an Unstable Pathogen Showing Evidence of Significant Genomic Flux
- The Cell Wall Protein CwpV is Antigenically Variable between Strains, but Exhibits Conserved Aggregation-Promoting Function
- Engineering HIV-Resistant Human CD4+ T Cells with CXCR4-Specific Zinc-Finger Nucleases
- SUMO-Interacting Motifs of Human TRIM5α are Important for Antiviral Activity
- Completion of Hepatitis C Virus Replication Cycle in Heterokaryons Excludes Dominant Restrictions in Human Non-liver and Mouse Liver Cell Lines
- : Reservoir Hosts and Tracking the Emergence in Humans and Macaques
- On Being the Right Size: The Impact of Population Size and Stochastic Effects on the Evolution of Drug Resistance in Hospitals and the Community
- Bacterial and Host Determinants of MAL Activation upon EPEC Infection: The Roles of Tir, ABRA, and FLRT3
- Respiratory Syncytial Virus Interferon Antagonist NS1 Protein Suppresses and Skews the Human T Lymphocyte Response
- NF-κB Hyper-Activation by HTLV-1 Tax Induces Cellular Senescence, but Can Be Alleviated by the Viral Anti-Sense Protein HBZ
- Human Cytomegalovirus IE1 Protein Elicits a Type II Interferon-Like Host Cell Response That Depends on Activated STAT1 but Not Interferon-γ
- A New Model to Produce Infectious Hepatitis C Virus without the Replication Requirement
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- NF-κB Hyper-Activation by HTLV-1 Tax Induces Cellular Senescence, but Can Be Alleviated by the Viral Anti-Sense Protein HBZ
- Bacterial and Host Determinants of MAL Activation upon EPEC Infection: The Roles of Tir, ABRA, and FLRT3
- : Reservoir Hosts and Tracking the Emergence in Humans and Macaques
- On Being the Right Size: The Impact of Population Size and Stochastic Effects on the Evolution of Drug Resistance in Hospitals and the Community
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy