-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Multimeric Assembly of Host-Pathogen Adhesion Complexes Involved in Apicomplexan Invasion
article has not abstract
Published in the journal: Multimeric Assembly of Host-Pathogen Adhesion Complexes Involved in Apicomplexan Invasion. PLoS Pathog 10(6): e32767. doi:10.1371/journal.ppat.1004120
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004120Summary
article has not abstract
Apicomplexan parasites are the causative agents of diseases that include malaria, toxoplasmosis, and coccidiosis. These obligate intracellular parasites have evolved to use a conserved mechanism for host-cell invasion. The apicomplexan phylum is defined by the presence of micronemes and rhoptries, which are distinct organelles located at the apical end of the parasite. These organelles secrete molecules necessary for host-cell invasion [1]. Apicomplexan parasites can invade disparate cell types, including hepatocytes, erythrocytes, lymphocytes, macrophages, and cells lining the digestive tract. Unlike viruses and intracellular bacteria, apicomplexans actively invade host cells without relying on host uptake pathways. As such, host-cell sensing and subsequent invasion are driven entirely by the parasite in a dynamic and rapid process. Intracellular residence protects the parasite from immune attack and enables parasite replication prior to host-cell lysis and subsequent invasion of neighboring host cells.
The repertoire of ligand-receptor complexes utilized by parasites for entry into host cells is diverse. Some interactions occur through cell-specific receptors resulting in high-affinity interactions, while others occur through multiple lower-affinity interactions via surface moieties found on several cell types. Receptor-specific and general cell binding may explain host-cell tropism of different pathogens, although additional factors are important. There is growing evidence that multimeric assembly of parasite ligands and host surface molecules strengthens the host-pathogen interactions necessary for invasion. We discuss recent work that has advanced our knowledge of the assembly of adhesive complexes from two critical apicomplexan pathogens and highlight areas of research that require further investigation.
Concepts That Define Multimeric Assembly of Complexes
Affinity, avidity, and valency are necessary concepts to define receptor-ligand interactions. The strength of attachment for two binding partners is determined by the affinity of individual binding sites and the number of interacting binding sites (valency). Avidity is the accumulated strength of multiple affinities from multivalent binding sites. The avidity of a multivalent complex is typically far greater than the sum of the individual affinities because of synergism between independent sites: dissociation at one site will be compensated by a bound second site, leading to rapid reassociation at the first site. Parasite ligands have evolved to increase both affinity and valency, resulting in high avidity that is necessary to create strong interactions that anchor parasites to host cells. Further adhesion strengthening is achieved through increased local surface concentration of ligands resulting in multiple focused interactions. In this review, we highlight parasite protein ligands that have evolved diverse methods to form high-avidity complexes for invasion. Specific mechanisms include utilizing repeat units, tandem duplication of adhesive domains, and homo - or hetero-oligomerizing with multimeric host receptors upon engagement.
Plasmodium Sporozoite Motility and Invasion
Plasmodium falciparum sporozoites invade the cells of the mosquito salivary glands prior to injection into the human host. Once injected, sporozoites migrate through the dermis, enter capillaries, traverse Kupffer cells that form the endothelial lining of the liver, and finally invade hepatocytes. The best-characterized invasion complexes with roles during these processes are mediated by thrombospondin-related anonymous protein (TRAP) and circumsporozoite protein (CSP).
P. falciparum TRAP (PfTRAP) has a role in sporozoite gliding motility, salivary gland invasion, and sporozoite infectivity [2]. This adhesin is stored within micronemes and is released onto the cell surface at the anterior tip upon contact with a host cell. PfTRAP contains two adhesive domains: a von Willebrand factor type A (VWA) domain and a thrombospondin type-I repeat (TSR) domain. Attachment to host cells occurs through both the VWA domain, which is similar to the I-domains of integrins that are important for magnesium cation coordination, and the TSR domain that binds to abundantly expressed heparan sulphate proteoglycans (HSPGs) on the hepatocyte surface [3], [4]. Individually, each domain or repeat binds to its respective interacting molecule, and the overall avidity of binding is likely increased by the tandem clustering of multiple repeats and domains (Figure 1A).
Fig. 1. Multimeric assembly, clustered interactions, and molecular complexes between parasite ligands and host-cell receptors for invasion. 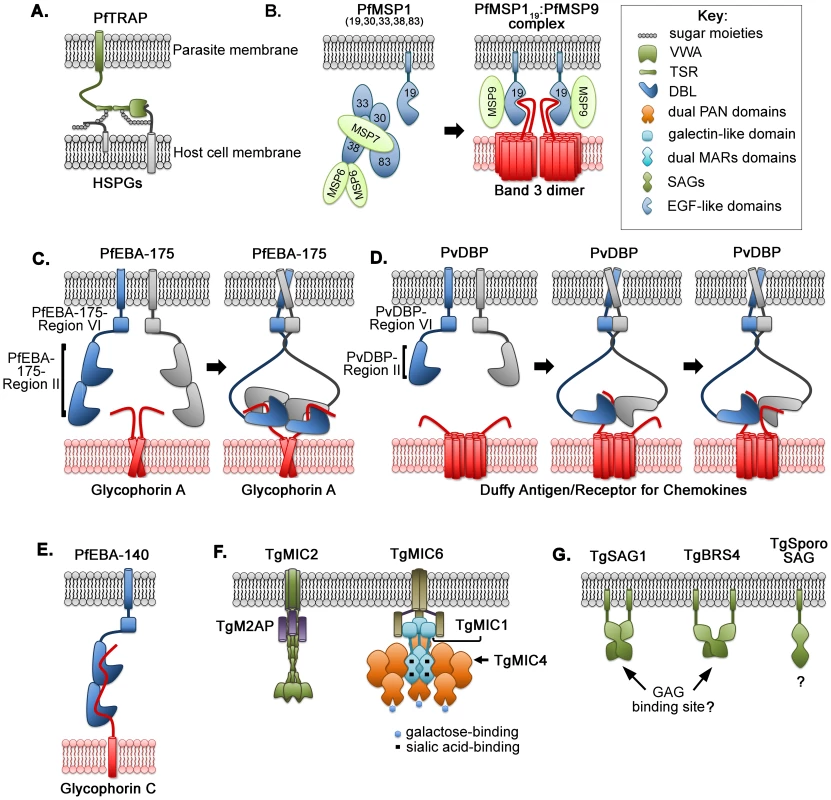
(A) PfTRAP engagement with heparan sulphate proteoglycans (HSPGs) on the hepatocyte surface; (B) proteolytic processing and shedding of PfMSP1 exposes the 19 kDa fragment (MSP119) that forms an invasion complex with MSP9 and the band 3 homodimer; (C) assembly of two PfEBA-175 monomers around dimeric glycophorin A of erythrocytes; (D) stepwise multimeric assembly of two PvDBP with two Duffy antigen/receptor for chemokines on reticulocyte surface; (E) monomeric interaction between PfEBA-140 and glycophorin C on erythrocytes; (F) proposed complexes of TgMIC2 and TgM2AP and of TgMIC1, TgMIC4, and TgMIC6 on the parasite surface; (G) variations in oligomeric states of GPI-anchored surface antigens (SAGs) create distinct interaction sites. P. falciparum CSP (PfCSP) is the most abundant antigen expressed on the surface of sporozoites and is the major antigen of a pre-erythrocytic malaria vaccine that confers limited protection [5]. PfCSP is anchored to the surface via a glycosylphosphatidylinositol (GPI) moiety and is crucial for sporozoite infection of hepatocytes [6]. PfCSP shares with PfTRAP the presence of TSR repeats [7]. The seven degenerate sulphatide binding motifs in the PfCSP TSR repeats bind the abundantly expressed HSPGs on host cells, resulting in high-avidity binding driven by the tandem duplication of individual repeats.
Plasmodium Merozoite Invasion of Red Blood Cells
The P. falciparum erythrocytic cycle begins with merozoite recognition and invasion of red blood cells (RBCs). Initial binding to the RBC is mediated by merozoite surface proteins (MSPs). The most abundant of the merozoite surface proteins is the complex of GPI-anchored MSP1 noncovalently attached to MSP6 and MSP7 [8]. MSP1 is proteolytically processed upon merozoite egress from a previously infected host cell. The multipartite MSP1 complex resides on the surface of the free merozoite and is shed at the time of RBC invasion to expose the C-terminal GPI-anchored MSP119 in complex with MSP9 for RBC entry. The MSP119/MSP9 multimer likely stabilizes and enhances the avidity of binding to the most abundant RBC membrane protein, the band 3 homodimer [9]. Engagement of band 3 is thought to be mediated by two epidermal growth factor (EGF)-like domains in MSP119 (Figure 1B).
The erythrocyte binding like (EBL) family has a defined role in recognition of and attachment to erythrocytes by engaging specific erythrocyte receptors [10]–[12]. EBL ligands are released from micronemes onto the apical surface of merozoites during invasion [13]. These proteins contain one or two conserved Duffy binding like (DBL) receptor-binding domains (Region II), a cysteine-rich domain (Region VI), and a transmembrane domain [14]. The EBL ligands in P. falciparum contain two DBL domains in Region II and include PfEBA-175, PfEBA-140/BAEBL, PfEBL-1, and PfEBA-181/JESEBL. Structural and biophysical studies have elucidated mechanisms of receptor engagement for members of this family.
The first member of the family to be structurally characterized was PfEBA-175 (Figure 1C). Two PfEBA-175 monomers dimerize around the glycosylated extracellular domains of glycophorin A dimers [15], [16], resulting in a high-avidity interaction [17], [18]. The sialylated glycans of glycophorin A are recognized by sialic acid-binding pockets created at the interface between Region II of each monomer [16]. The complex assembly requires both DBL domains of each monomer and is enhanced by additional regions of PfEBA-175 [17], [18].
In P. vivax, the Duffy-binding protein (PvDBP) contains a single DBL domain that binds to the Duffy antigen/receptor for chemokines (DARC) (Figure 1D), a nonsignaling G-protein-coupled receptor on reticulocytes [19]–[21]. Even though the DBL domain architectures of PvDBP and PfEBA-175 are different, these ligands have a similar mechanism of receptor engagement. PvDBP is monomeric in the absence of DARC, and DARC binding drives dimerization of PvDBP [22]. Examination of multimeric assembly in solution and capture of PvDBP∶DARC complexes by crystallography revealed the formation of a heterotrimer of two PvDBPs bound to one DARC, followed by a heterotetramer of two PvDBPs engaging two DARCs [23]. These complexes suggest stepwise assembly, which is likely to be cooperative, leading to a high-avidity PvDBP∶DARC interaction.
The two DBL domains of PfEBA-140 Region II independently bind to sialylated glycans of glycophorin C on erythrocytes [24]–[26]. While PfEBA-175 and PvDBP dimerize upon receptor engagement, PfEBA-140 may contact glycophorin C as a monomer (Figure 1E) [25], [26]. Additional studies are necessary to examine if multimeric assembly occurs upon receptor binding or if oligomerization is an important determinant of receptor specificity. PfEBA-140 Region II has also evolved novel glycan-binding pockets, distinct from those in PfEBA-175, and these do not require dimerization [25], [26].
Disruption of multimeric assembly is an effective method for antibody neutralization of parasite growth. An antibody that binds to the PfEBA-175 dimer interface and receptor-binding sites effectively disrupts binding to glycophorin A and blocks P. falciparum invasion [27]–[29]. Similarly, the residues at the dimer interface and DARC-binding groove are targeted by naturally acquired antibodies correlated with disruption of PvDBP binding [22], [23], [30]. These studies suggest that assembly of ligands around receptors leading to high-avidity interactions is an important determinant of receptor binding and that immune targeting of oligomeric interfaces in addition to receptor-binding pockets leads to protection.
Multimeric Micronemal Protein Complexes of Toxoplasma gondii
The microneme proteins (MICs) in Toxoplasma gondii preassemble in the endoplasmic reticulum and form complexes prior to transiting to the micronemes. The propensity to form oligomers with different combinations of partners likely allows the parasite to expand the receptor repertoire or fine-tune the specificity of receptor binding. To date, three major complexes have been identified and functionally characterized in T. gondii attachment to host cells. First, microneme protein 2 (TgMIC2), a member of the conserved TRAP family, is found in a heterohexameric complex with MIC2-associated protein (TgM2AP) (Figure 1F) and plays a fundamental role in gliding motility and host-cell attachment [31], [32]. Each TgMIC2 monomer binds one TgM2AP monomer via the TSR repeats in TgMIC2 [33]. Second, TgMIC8, which complexes with the lectin-like TgMIC3, is essential for rhoptry secretion and invasion [34]. Third, TgMIC6 forms a multimeric complex with two adhesins, TgMIC1 and TgMIC4, and contributes to invasion in vitro and virulence in vivo [35]–[37].
The TgMIC1∶4∶6 complex has been the most characterized structurally (Figure 1F). Although TgMIC1 was classified as a TRAP family member, structural studies of the N-terminal repeat units and C-terminal domain have revealed novel adhesion modules [36], [38]. The C-terminal galectin-like domain of TgMIC1 stabilizes the interaction with the EGF domains of TgMIC6, which in turn anchors the complex via a transmembrane domain [37], [38]. The N-terminus of TgMIC1 contains two micronemal adhesive repeats (MAR) that bind sialic acid [39]. TgMIC1 forms a disulfide-linked trimer, and each TgMIC1 monomer further engages a TgMIC4 monomer, creating a heterohexamer. The two tandem apple domains of TgMIC4 bind galactose-containing glycans [39]. The duplication of MAR repeats and apple domains, coupled with heterohexamerization, likely results in high avidity by increased valency for sialic acid and galactose.
Toxoplasma Surface Antigens
Surface antigen glycoproteins (SAGs) and SAG-related sequence proteins (SRS) are abundant and widely distributed GPI-anchored adhesins on the T. gondii surface at multiple stages of the life cycle [40]–[43]. They are optimally positioned for low-affinity, lateral interactions with the host-surface glycosaminoglycans, which act as receptors for Toxoplasma invasion [44], [45]. Crystal structures of SAGs revealed varying levels of dimerization: SAG1 forms a parallel homodimer with an extensive dimer interface [46], Bradyzoite-specific surface antigen (BRS4) exhibits a smaller dimer interface [47], while the SAG expressed in sporozoite stage (SporoSAG) is monomeric (Figure 1G) [48]. Variation in oligomeric state may impact receptor binding as the SAG1 and BRS4 dimers create basic pockets implicated in glycosaminoglycan engagement. The basic pocket is replaced by an acidic cap in SporoSAG, and the receptor moiety engaged is unclear. It is plausible that, like EBL-ligands, receptor binding induces or stabilizes dimerization of SAGs, although further structural studies in solution are necessary.
In summary, the organization of parasite ligands at the site of invasion is promoted by multivalent, high-avidity interactions with host-cell receptors and surface moieties. The strength of attachment can be further increased by clustering of adhesive complexes. This combination of clustered interactions and multimeric complexes not only ensures the parasite's successful entry into the host cell but also likely promotes evasion from the host's immune response by burying potentially protective antigenic epitopes. Increased avidity has been demonstrated for some but not all multivalent complexes, and future studies are necessary to clearly identify the effect of multimeric assembly on binding and avidity in cases in which this information is lacking. Assembly can also activate or enhance downstream signaling processes in other systems, and further studies are needed to decipher whether signaling is triggered by multimeric assembly during invasion. The structural determination of critical interfaces in ligand-receptor binding and the biochemical and biophysical elucidation of multimeric assembly mechanisms will provide novel perspectives on how the invasion process is manifested and regulated. This information will identify novel ways to block pathogen entry into host cells.
Zdroje
1. CarruthersVB, SibleyLD (1997) Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol 73 : 114–123.
2. WengelnikK, SpaccapeloR, NaitzaS, RobsonKJ, JanseCJ, et al. (1999) The A-domain and the thrombospondin-related motif of Plasmodium falciparum TRAP are implicated in the invasion process of mosquito salivary glands. EMBO J 18 : 5195–5204.
3. SultanAA, ThathyV, FrevertU, RobsonKJ, CrisantiA, et al. (1997) TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell 90 : 511–522.
4. SongG, KoksalAC, LuC, SpringerTA (2012) Shape change in the receptor for gliding motility in Plasmodium sporozoites. Proc Natl Acad Sci U S A 109 : 21420–21425.
5. AgnandjiST, LellB, SoulanoudjingarSS, FernandesJF, AbossoloBP, et al. (2011) First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 365 : 1863–1875.
6. RathoreD, SacciJB, de la VegaP, McCutchanTF (2002) Binding and invasion of liver cells by Plasmodium falciparum sporozoites. Essential involvement of the amino terminus of circumsporozoite protein. J Biol Chem 277 : 7092–7098.
7. DoudMB, KoksalAC, MiLZ, SongG, LuC, et al. (2012) Unexpected fold in the circumsporozoite protein target of malaria vaccines. Proc Natl Acad Sci U S A 109 : 7817–7822.
8. KauthCW, WoehlbierU, KernM, MekonnenZ, LutzR, et al. (2006) Interactions between merozoite surface proteins 1, 6, and 7 of the malaria parasite Plasmodium falciparum. J Biol Chem 281 : 31517–31527.
9. KariukiMM, LiX, YamodoI, ChishtiAH, OhSS (2005) Two Plasmodium falciparum merozoite proteins binding to erythrocyte band 3 form a direct complex. Biochem Biophys Res Commun 338 : 1690–1695.
10. CamusD, HadleyTJ (1985) A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230 : 553–556.
11. MayerDC, KanekoO, Hudson-TaylorDE, ReidME, MillerLH (2001) Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175. Proc Natl Acad Sci U S A 98 : 5222–5227.
12. GilbergerTW, ThompsonJK, TrigliaT, GoodRT, DuraisinghMT, et al. (2003) A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J Biol Chem 278 : 14480–14486.
13. SinghS, AlamMM, Pal-BhowmickI, BrzostowskiJA, ChitnisCE (2010) Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog 6: e1000746.
14. AdamsJH, SimBK, DolanSA, FangX, KaslowDC, et al. (1992) A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci U S A 89 : 7085–7089.
15. SimBK, ChitnisCE, WasniowskaK, HadleyTJ, MillerLH (1994) Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 264 : 1941–1944.
16. ToliaNH, EnemarkEJ, SimBK, Joshua-TorL (2005) Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell 122 : 183–193.
17. SalinasND, ToliaNH (2014) A quantitative assay for binding and inhibition of Plasmodium falciparum Erythrocyte Binding Antigen 175 reveals high affinity binding depends on both DBL domains. Protein Expr Purif 95 : 188–194.
18. WanaguruM, CrosnierC, JohnsonS, RaynerJC, WrightGJ (2013) Biochemical analysis of the Plasmodium falciparum erythrocyte-binding antigen-175 (EBA175)-glycophorin-A interaction: implications for vaccine design. J Biol Chem 288 : 32106–32117.
19. ChitnisCE, ChaudhuriA, HorukR, PogoAO, MillerLH (1996) The domain on the Duffy blood group antigen for binding Plasmodium vivax and P. knowlesi malarial parasites to erythrocytes. J Exp Med 184 : 1531–1536.
20. MillerLH, MasonSJ, DvorakJA, McGinnissMH, RothmanIK (1975) Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science 189 : 561–563.
21. MillerLH, MasonSJ, ClydeDF, McGinnissMH (1976) The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med 295 : 302–304.
22. BatchelorJD, ZahmJA, ToliaNH (2011) Dimerization of Plasmodium vivax DBP is induced upon receptor binding and drives recognition of DARC. Nat Struct Mol Biol 18 : 908–914.
23. BatchelorJD, MalpedeBM, OmattageNS, DeKosterGT, Henzler-WildmanKA, et al. (2014) Red blood cell invasion by Plasmodium vivax: structural basis for DBP engagement of DARC. PLoS Pathog 10: e1003869.
24. LoboCA, RodriguezM, ReidM, LustigmanS (2003) Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl). Blood 101 : 4628–4631.
25. MalpedeBM, LinDH, ToliaNH (2013) Molecular basis for sialic acid-dependent receptor recognition by the Plasmodium falciparum invasion protein erythrocyte-binding antigen-140/BAEBL. J Biol Chem 288 : 12406–12415.
26. LinDH, MalpedeBM, BatchelorJD, ToliaNH (2012) Crystal and solution structures of Plasmodium falciparum erythrocyte-binding antigen 140 reveal determinants of receptor specificity during erythrocyte invasion. J Biol Chem 287 : 36830–36836.
27. SimBK, NarumDL, ChattopadhyayR, AhumadaA, HaynesJD, et al. (2011) Delineation of stage specific expression of Plasmodium falciparum EBA-175 by biologically functional region II monoclonal antibodies. PLoS One 6: e18393.
28. ChenE, PaingMM, SalinasN, SimBK, ToliaNH (2013) Structural and functional basis for inhibition of erythrocyte invasion by antibodies that target Plasmodium falciparum EBA-175. PLoS Pathog 9: e1003390.
29. AmbroggioX, JiangL, AebigJ, ObiakorH, LukszoJ, et al. (2013) The epitope of monoclonal antibodies blocking erythrocyte invasion by Plasmodium falciparum map to the dimerization and receptor glycan binding sites of EBA-175. PLoS One 8: e56326.
30. ChootongP, NtumngiaFB, VanBuskirkKM, XainliJ, Cole-TobianJL, et al. (2010) Mapping epitopes of the Plasmodium vivax Duffy binding protein with naturally acquired inhibitory antibodies. Infect Immun 78 : 1089–1095.
31. HuynhMH, CarruthersVB (2006) Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS Pathog 2: e84.
32. JewettTJ, SibleyLD (2004) The toxoplasma proteins MIC2 and M2AP form a hexameric complex necessary for intracellular survival. J Biol Chem 279 : 9362–9369.
33. SongG, SpringerTA (2014) Structures of the Toxoplasma gliding motility adhesin. Proc Natl Acad Sci U S A 111 : 4862–4867.
34. KesslerH, Herm-GotzA, HeggeS, RauchM, Soldati-FavreD, et al. (2008) Microneme protein 8–a new essential invasion factor in Toxoplasma gondii. J Cell Sci 121 : 947–956.
35. CeredeO, DubremetzJF, SoeteM, DesleeD, VialH, et al. (2005) Synergistic role of micronemal proteins in Toxoplasma gondii virulence. J Exp Med 201 : 453–463.
36. BlumenscheinTM, FriedrichN, ChildsRA, SaourosS, CarpenterEP, et al. (2007) Atomic resolution insight into host cell recognition by Toxoplasma gondii. EMBO J 26 : 2808–2820.
37. SawmynadenK, SaourosS, FriedrichN, MarchantJ, SimpsonP, et al. (2008) Structural insights into microneme protein assembly reveal a new mode of EGF domain recognition. EMBO Rep 9 : 1149–1155.
38. SaourosS, Edwards-JonesB, ReissM, SawmynadenK, CotaE, et al. (2005) A novel galectin-like domain from Toxoplasma gondii micronemal protein 1 assists the folding, assembly, and transport of a cell adhesion complex. J Biol Chem 280 : 38583–38591.
39. MarchantJ, CowperB, LiuY, LaiL, PinzanC, et al. (2012) Galactose recognition by the apicomplexan parasite Toxoplasma gondii. J Biol Chem 287 : 16720–16733.
40. LekutisC, FergusonDJ, GriggME, CampsM, BoothroydJC (2001) Surface antigens of Toxoplasma gondii: variations on a theme. Int J Parasitol 31 : 1285–1292.
41. JungC, LeeCY, GriggME (2004) The SRS superfamily of Toxoplasma surface proteins. Int J Parasitol 34 : 285–296.
42. KasperLH, BradleyMS, PfefferkornER (1984) Identification of stage-specific sporozoite antigens of Toxoplasma gondii by monoclonal antibodies. J Immunol 132 : 443–449.
43. TomavoS, FortierB, SoeteM, AnselC, CamusD, et al. (1991) Characterization of bradyzoite-specific antigens of Toxoplasma gondii. Infect Immun 59 : 3750–3753.
44. CarruthersVB, HakanssonS, GiddingsOK, SibleyLD (2000) Toxoplasma gondii uses sulfated proteoglycans for substrate and host cell attachment. Infect Immun 68 : 4005–4011.
45. Ortega-BarriaE, BoothroydJC (1999) A Toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. J Biol Chem 274 : 1267–1276.
46. HeXL, GriggME, BoothroydJC, GarciaKC (2002) Structure of the immunodominant surface antigen from the Toxoplasma gondii SRS superfamily. Nat Struct Biol 9 : 606–611.
47. CrawfordJ, GrujicO, BruicE, CzjzekM, GriggME, et al. (2009) Structural characterization of the bradyzoite surface antigen (BSR4) from Toxoplasma gondii, a unique addition to the surface antigen glycoprotein 1-related superfamily. J Biol Chem 284 : 9192–9198.
48. CrawfordJ, LambE, WasmuthJ, GrujicO, GriggME, et al. (2010) Structural and functional characterization of SporoSAG: a SAG2-related surface antigen from Toxoplasma gondii. J Biol Chem 285 : 12063–12070.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA SplicingČlánek Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance GenesČlánek The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 6- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- BdlA, DipA and Induced Dispersion Contribute to Acute Virulence and Chronic Persistence of
- Morphotype Transition and Sexual Reproduction Are Genetically Associated in a Ubiquitous Environmental Pathogen
- A Nucleic-Acid Hydrolyzing Single Chain Antibody Confers Resistance to DNA Virus Infection in HeLa Cells and C57BL/6 Mice
- HopW1 from Disrupts the Actin Cytoskeleton to Promote Virulence in Arabidopsis
- Ly6C Monocytes Become Alternatively Activated Macrophages in Schistosome Granulomas with Help from CD4+ Cells
- Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA Splicing
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- Antibody Responses to : Role in Pathogenesis and Diagnosis of Encephalitis?
- Discovery of a Novel Compound with Anti-Venezuelan Equine Encephalitis Virus Activity That Targets the Nonstructural Protein 2
- Activation of Focal Adhesion Kinase by Suppresses Autophagy via an Akt/mTOR Signaling Pathway and Promotes Bacterial Survival in Macrophages
- Crossing the Interspecies Barrier: Opening the Door to Zoonotic Pathogens
- Catching Fire: , Macrophages, and Pyroptosis
- IscR Is Essential for Type III Secretion and Virulence
- Selective Chemical Inhibition of Quorum Sensing in Promotes Host Defense with Minimal Impact on Resistance
- The Glycosylated Rv1860 Protein of Inhibits Dendritic Cell Mediated TH1 and TH17 Polarization of T Cells and Abrogates Protective Immunity Conferred by BCG
- A Genome-Wide Tethering Screen Reveals Novel Potential Post-Transcriptional Regulators in
- Structural Insights into SraP-Mediated Adhesion to Host Cells
- Human IGF1 Regulates Midgut Oxidative Stress and Epithelial Homeostasis to Balance Lifespan and resistance in
- Cycling Empirical Antibiotic Therapy in Hospitals: Meta-Analysis and Models
- Rab11 Regulates Trafficking of -sialidase to the Plasma Membrane through the Contractile Vacuole Complex of
- Mitogen and Stress Activated Kinases Act Co-operatively with CREB during the Induction of Human Cytomegalovirus Immediate-Early Gene Expression from Latency
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- A Central Role for Carbon-Overflow Pathways in the Modulation of Bacterial Cell Death
- An Invertebrate Warburg Effect: A Shrimp Virus Achieves Successful Replication by Altering the Host Metabolome via the PI3K-Akt-mTOR Pathway
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
- A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees () Prevails after -Mediated, or , Transmission
- Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance Genes
- Ubiquitin-Mediated Response to Microsporidia and Virus Infection in
- Preclinical Detection of Variant CJD and BSE Prions in Blood
- Toll-Like Receptor 8 Agonist and Bacteria Trigger Potent Activation of Innate Immune Cells in Human Liver
- Progressive Proximal-to-Distal Reduction in Expression of the Tight Junction Complex in Colonic Epithelium of Virally-Suppressed HIV+ Individuals
- The Triggering Receptor Expressed on Myeloid Cells 2 Inhibits Complement Component 1q Effector Mechanisms and Exerts Detrimental Effects during Pneumococcal Pneumonia
- Differential Activation of Acid Sphingomyelinase and Ceramide Release Determines Invasiveness of into Brain Endothelial Cells
- Forward Genetic Screening Identifies a Small Molecule That Blocks Growth by Inhibiting Both Host- and Parasite-Encoded Kinases
- Defining Immune Engagement Thresholds for Control of Virus-Driven Lymphoproliferation
- Growth Factor and Th2 Cytokine Signaling Pathways Converge at STAT6 to Promote Arginase Expression in Progressive Experimental Visceral Leishmaniasis
- Multimeric Assembly of Host-Pathogen Adhesion Complexes Involved in Apicomplexan Invasion
- Biogenesis of Influenza A Virus Hemagglutinin Cross-Protective Stem Epitopes
- Adequate Th2-Type Response Associates with Restricted Bacterial Growth in Latent Mycobacterial Infection of Zebrafish
- Protective Efficacy of Passive Immunization with Monoclonal Antibodies in Animal Models of H5N1 Highly Pathogenic Avian Influenza Virus Infection
- Fructose-Asparagine Is a Primary Nutrient during Growth of in the Inflamed Intestine
- The Calcium-Dependent Protein Kinase 3 of Influences Basal Calcium Levels and Functions beyond Egress as Revealed by Quantitative Phosphoproteome Analysis
- A Translocated Effector Required for Dissemination from Derma to Blood Safeguards Migratory Host Cells from Damage by Co-translocated Effectors
- Functional Characterization of a Novel Family of Acetylcholine-Gated Chloride Channels in
- Both α2,3- and α2,6-Linked Sialic Acids on O-Linked Glycoproteins Act as Functional Receptors for Porcine Sapovirus
- The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
- MicroRNA-146a Provides Feedback Regulation of Lyme Arthritis but Not Carditis during Infection with
- Recombination in Enteroviruses Is a Biphasic Replicative Process Involving the Generation of Greater-than Genome Length ‘Imprecise’ Intermediates
- Cytoplasmic Viral RNA-Dependent RNA Polymerase Disrupts the Intracellular Splicing Machinery by Entering the Nucleus and Interfering with Prp8
- and Are Associated with Murine Susceptibility to Infection and Human Sepsis
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



