-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water Labeling
Microbial pathogens can adapt to changing conditions in their hosts by switching between different growth and physiological states. However, current methods for measuring microbial physiology in vivo are limited, hampering detailed dissection of host-pathogen interactions. Here we have used heavy water labeling to measure the growth rate and physiological state of Leishmania parasites in murine lesions. Based on the rate of in situ labeling of parasite DNA, RNA, protein, and lipids, we show that the growth rate of intracellular parasite stages is very slow, and that these stages enter a semi-quiescent state characterized by very low rates of RNA, protein, and membrane turnover. These changes in parasite growth and physiology are more pronounced than in in vitro differentiated parasites, suggesting that they are induced in part by the lesion environment. Despite their slow growth, the parasite burden in these lesions progressively increases as a result of low rates of parasite death and host cell turnover. We propose that these changes in Leishmania growth and physiology contribute to the development of a relatively benign tissue environment that is permissive for long term parasite expansion. This approach is suitable for studying the dynamics of other host-pathogen systems.
Published in the journal: Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water Labeling. PLoS Pathog 11(2): e32767. doi:10.1371/journal.ppat.1004683
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004683Summary
Microbial pathogens can adapt to changing conditions in their hosts by switching between different growth and physiological states. However, current methods for measuring microbial physiology in vivo are limited, hampering detailed dissection of host-pathogen interactions. Here we have used heavy water labeling to measure the growth rate and physiological state of Leishmania parasites in murine lesions. Based on the rate of in situ labeling of parasite DNA, RNA, protein, and lipids, we show that the growth rate of intracellular parasite stages is very slow, and that these stages enter a semi-quiescent state characterized by very low rates of RNA, protein, and membrane turnover. These changes in parasite growth and physiology are more pronounced than in in vitro differentiated parasites, suggesting that they are induced in part by the lesion environment. Despite their slow growth, the parasite burden in these lesions progressively increases as a result of low rates of parasite death and host cell turnover. We propose that these changes in Leishmania growth and physiology contribute to the development of a relatively benign tissue environment that is permissive for long term parasite expansion. This approach is suitable for studying the dynamics of other host-pathogen systems.
Introduction
A number of medically important bacterial, fungal and protozoan pathogens are associated with persistent chronic infections that can reactivate to cause acute disease long after initial infection [1–5]. With few exceptions [6], very little is known about the growth rate or physiological state of these pathogens during chronic stages of infection, reflecting limitations in current methods for measuring microbial growth in situ. This information is crucial for modeling host-pathogen dynamics and developing therapies that target these stages.
Leishmania spp are protozoan parasites that are associated with long-term chronic infections, as well as acute disease, ranging from self-healing cutaneous lesions to fatal visceral infections, in millions of people worldwide [7]. Infection is initiated by flagellated promastigote stages that are injected into the skin by a sandfly vector. Following their uptake by macrophages and other phagocytic cells, promastigotes differentiate to aflagellate amastigotes that proliferate in the phagolysosome compartment of these host cells [8,9]. A hallmark of all Leishmania infections is the formation of localized tissue lesions or granulomas composed primarily of infected and uninfected macrophages, at the site of the sandfly bite or in distal tissues such as the liver and spleen [10–12]. Depending on the Leishmania species involved and host genetics, lesion formation can be associated with immune control (but usually not eradication of the parasite) or parasite expansion and systemic infection. In murine models of infection, host resistance is associated with the development of a T-helper type 1 response, while lesion development occurs in susceptible animals that mount a T-helper type 2 response [13,14]. In contrast to our understanding of the host immune responses that underlie these different outcomes, very little is known about the growth rate or physiological state of Leishmania in these tissues. Transgenic parasite lines expressing luciferase or different fluorescent proteins have been developed and used to visualize parasite dynamics in vivo [15–18]. However, these approaches only provide a measure of net changes in parasite burden, which are determined by rates of parasite death and migration from infected tissues, as well as rates of replication. Furthermore, attempts to infer the physiological status of lesion amastigotes from transcriptomic and proteomic analyses have been hampered by the absence of conventional gene-specific transcriptional control in these parasites and the paucity of coordinated changes in the abundances of individual mRNA and proteins in different insect and mammalian-infective stages [19,20].
In this study we introduce the use of 2H2O labeling to measure Leishmania growth rate and metabolic activity in murine inflammatory lesions. In the presence of 2H2O, cells stably incorporate deuterium into a wide range of metabolites, which are subsequently incorporated into cellular macromolecules, providing a quantitative read-out of global rates of DNA replication, transcription, protein turnover and membrane lipid biosynthesis (S1 Fig., Fig. 1A)[21,22]. 2H2O is easily and safely administered to animals for periods of weeks to months and rapidly equilibrates across all tissues [23], making it suitable for measurement of slowly growing microbial populations in infected tissues. Using this approach, we show that amastigotes exhibit a constant, but very slow growth rate, in non-healing lesions and appear to enter into a distinct semi-quiescent metabolic state characterized by low rates of transcription and protein turnover. This quiescent state is distinct from that measured in non-dividing (insect) promastigote stages and may represent an adaptive response to a growth-restrictive intracellular microenvironment in granulomas. Using this approach we also identified parasite-specific metabolic pathways, such as polyunsaturated fatty acid biosynthesis that are up-regulated in situ. This approach has provided the first global analysis of the physiological state of the major mammalian-infective stage of Leishmania and is generally applicable to studying the in vivo growth and physiology of other microbial pathogens.
Fig. 1. Stage-specific changes in Leishmania growth rates. 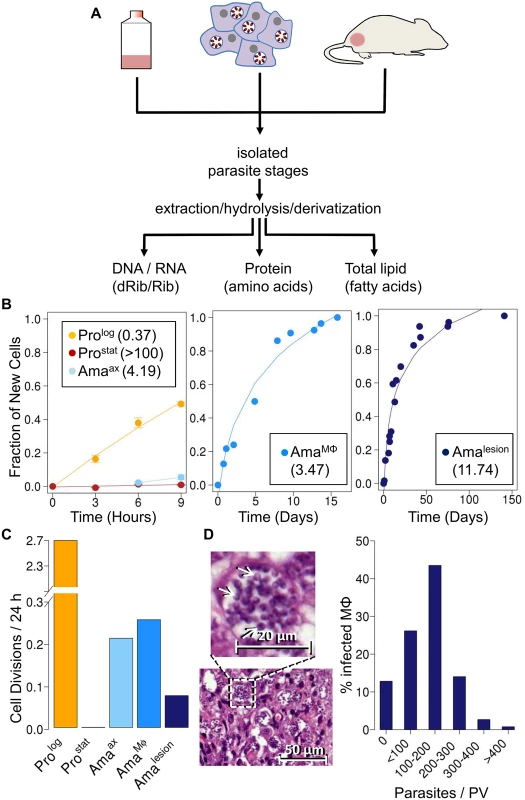
A. Schematic overview of 2H2O labeling protocol. Parasite stages were cultivated axenically in the presence 5% 2H2O, or isolated from infected macrophages or BALB/c lesion incubated or infused with 2H2O (final concentration 5%). Parasite stages were harvested at multiple time points and extracts containing total DNA/RNA, or total proteins and lipids generated from purified parasite fraction. Levels of deuterium enrichment in constituent dRib/Rib, amino acids and fatty acids were subsequently quantitated by GC-MS. B. Kinetics of 2H-labeling of DNA dRib in cultured promastigotes (Prolog, Prostat) and amastigotes (Amaaxenic), and in amastigotes isolated from macrophages (AmaMø) and murine lesions (Amalesion). The fraction of new cells (Y-axis) was calculated from the level of 2H-enrichment in dRib relative to maximum labeling observed in each parasite stage after long term (equilibrium) labeling. Calculated doubling times for each stage are shown in inset boxes. C. Comparative growth rates of different Leishmania stages, calculated from 2H-enrichment in dRib. D. Section of stained cutaneous lesion (with detail in insert) and calculated range of parasite numbers/phagolysosome. Abbreviations: dRib; deoxyribose, Rib; ribose. Results
Measurement of L. mexicana amastigote replication rates in vivo
As previously reported [24], cultivation of L. mexicana promastigotes stages in standard medium containing 5% 2H2O, leads to the deuterium-labeling of the deoxyribose (dRib) moiety of DNA (Fig. 1B). The labeling of dRib occurs as a result of gluconeogenesis, various sugar phosphate isomerization/epimerization reactions, the pentose phosphate pathway and ribonucleotide reductase (S1 Fig.), and the rate of incorporation of deuterium into promastigote DNA was growth-dependent. Specifically, the rate of labeling of exponentially growing promastigotes (Prolog) indicated a doubling time of 9 hours, concordant with cell counts, while no incorporation was observed in the DNA of non-dividing promastigotes (Prostat) (Fig. 1B). Significantly, labeling of the deoxyribose moiety of DNA was not affected by supplementation of the culture medium with ribose or a range of nucleosides and nucleotides to the culture medium (S2 Fig.), indicating that de novo synthesis of ribose/dRib is not affected by salvage pathways. Prostat were induced to differentiate to axenic amastigotes (Amaaxenic) by acidification of the medium and cultivation at elevated temperature. Following differentiation, Amaaxenic exhibited a doubling time of 4.2 days, substantially slower than Prolog (Fig. 1B, C). These data support the notion that amastigote differentiation is associated with activation of the parasite stress responses and a reduction in maximum growth rate, independent of exogenous nutrient levels [24].
Having shown that 2H2O labeling can be used to quantitate rates of parasite replication; we extended this approach to directly measure amastigote proliferation in murine tissue lesions. BALB/c mice were infected with L. mexicana parasites and subsequently labeled with 2H2O following the appearance of cutaneous lesions. A constant level of 5% 2H2O in the body water was established by providing mice with a bolus of 100% 2H2O and subsequent inclusion of 9% 2H2O in the drinking water for up to several months (S3 Fig.). Mice were culled at various time points and lesion amastigotes (Amalesion) isolated from infected tissues. Histological examination showed that these lesions primarily comprised heavily infected host cells (with parasites in large communal vacuoles) and no detectable necrosis (S4 Fig.). Amalesion purified from these tissues were free of intact host cells or nuclei, as determined by DAPI staining, and were further treated with DNAse to remove any extracellular host DNA released during tissue disruption (S5 Fig.). Contamination of parasite DNA with host DNA was estimated to be less than 20% as determined by direct quantitation of DNA (S5 Fig.). Deuterium enrichment in parasite DNA-dRib, increased with time, reaching a maximum 15% enrichment (EM1; excess molar fraction of M1) after about 40 days of labeling (Fig. 1B). Based on the rate of enrichment, Amalesion were found to have a remarkably constant doubling time of ~12 days, irrespective of the age of the lesion when the 2H2O labeling was initiated (ranging from 4 weeks to 4 months post-infection) (Fig. 1B,C). This growth rate is 32-fold slower than the maximum growth rate of promastigotes stages and approximately 4-fold slower than that measured for Amaaxenic (Fig. 1C), indicating that parasite growth in the granuloma microenvironment is highly constrained.
To investigate whether the slow growth of lesion amastigotes reflected growth-limiting conditions in the phagolysosome of infected macrophages, we measured amastigote replication in J774 macrophages. J774 macrophages were infected with L. mexicana promastigotes and internalized parasites allowed to differentiate to amastigotes, before cultures were incubated in the presence of 5% 2H2O. Infected macrophages were labeled for up to 20 days and amastigotes (AmaMø) isolated at various time points. Intracellular AmaMø were found to have a doubling time of 4 days (Fig. 1B), comparable to that of Amaaxenic and substantially faster than observed in Amalesion (Fig. 1B). These results suggest that the phagolysosomal compartment of non-activated macrophages is not growth limiting for amastigotes per se, and that additional factors in the lesion environment constrain parasite growth and/or induce a slow growth phenotype.
Quantitative analysis of hematoxylin and eosin (H&E) stained sections of BALB/c lesions harvested at day 85 post-infection showed that the majority of granuloma macrophages harbored between 100–200 amastigotes/phagolysosome (Fig. 1D). These values are in close agreement with parasite vacuole densities calculated assuming; (i) that each vacuole was established by a single parasite invasion event, (ii) a constant amastigote doubling time of 12 days and (iii) zero parasite death (120 parasites/vacuole). The presence of macrophages with fewer than 100 amastigotes/phagolysosome (28% of all infected macrophages) may reflect influx of uninfected macrophages that have been infected for a shorter period of time, or a subpopulation of slower growing parasites. On the other hand, the presence of small number of hyper-infected macrophages (up to 400 amastigotes/phagolysosome) may reflect a subpopulation of amastigotes with a faster growth rate and/or the uptake of multiple promastigotes/amastigotes during the initial infection. Overall, these analyses indicate that parasite and host cell turnover in lesions is minimal and that a significant majority of the granuloma host cells were infected very early after injection of parasites.
Measurement of RNA and protein turnover in lesion amastigotes
To further define the physiological state of Amalesion, we assessed the rate of incorporation of deuterium into the ribosyl moiety of RNA and proteinogenic amino acids in different parasite stages. Measurement of deuterium enrichment in the RNA ribosyl moiety primarily reflects ribosome biosynthesis, one of the most energy intensive processes in the cell [25] and is thus a measure of the metabolic state of a cell. As expected, Prolog exhibited the highest rate of RNA turnover, comparable to the rate observed for DNA synthesis (Fig. 2A). Appreciable levels of RNA turnover were also observed in Prostat, confirming that this non-dividing stage remains transcriptionally and metabolically active (Fig. 2A). Strikingly, all three amastigote stages (Amaaxenic, AmaMø and Amalesion) exhibited lower rates of RNA turnover than Prostat, providing direct evidence that amastigote differentiation is linked to a general shut-down of energy intensive processes (Fig. 2A-C).
Fig. 2. Rates of RNA turnover in cultured and intracellular Leishmania stages. 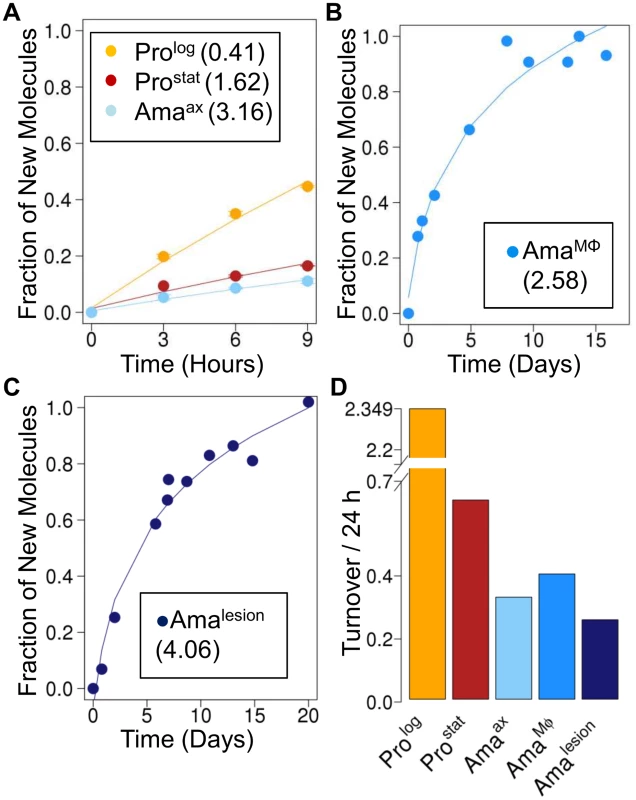
Kinetics of 2H-labeling of RNA ribose in (A) cultured parasite stages (Prolog, Prostat, Amaaxenic) (B) amastigotes isolated from infected J774 macrophages (AmaMø) and (C) amastigotes isolated from BALB/c lesions (Amalesion). The fraction of new molecules (Y-axis) was calculated from the level of 2H-enrichment in Rib relative to maximum labeling observed in each parasite stage after long term labeling. Inset boxes shows estimated RNA turnover (t1/2 in days) in each stage. D. Comparative rates of RNA turnover in different Leishmania developmental stages. The rate of protein synthesis/turnover provides another proxy for the metabolic state of a cell. As expected, deuterium was incorporated into a range of Leishmania proteinogenic amino acids via different transamination reactions and pathways of de novo biosynthesis [8,26] (S6A Fig.). While maximum levels of deuterium enrichment in different amino acids varied, with highest levels of enrichment in alanine (11% EM1) and glutamate (9% EM1), similar rates of protein turnover were calculated after normalization to maximum labeling, regardless of the amino acid used (S6B Fig.). Both dividing and non-dividing promastigote stages exhibited higher rates of protein synthesis than Amaaxenic and Amalesion (Fig. 3A-C). Interestingly, while the turnover of protein in Prolog and Amaaxenic stages occurred at approximately the same rate as DNA replication, protein turnover in Amalesion occurred at nearly twice the rate of DNA synthesis. Thus although Amalesion stages have the lowest absolute rates of protein turnover, the cellular proteome is turned over more times per cell division cycle than in other stages.
Fig. 3. Rates of protein turnover in cultured and intracellular Leishmania stages. 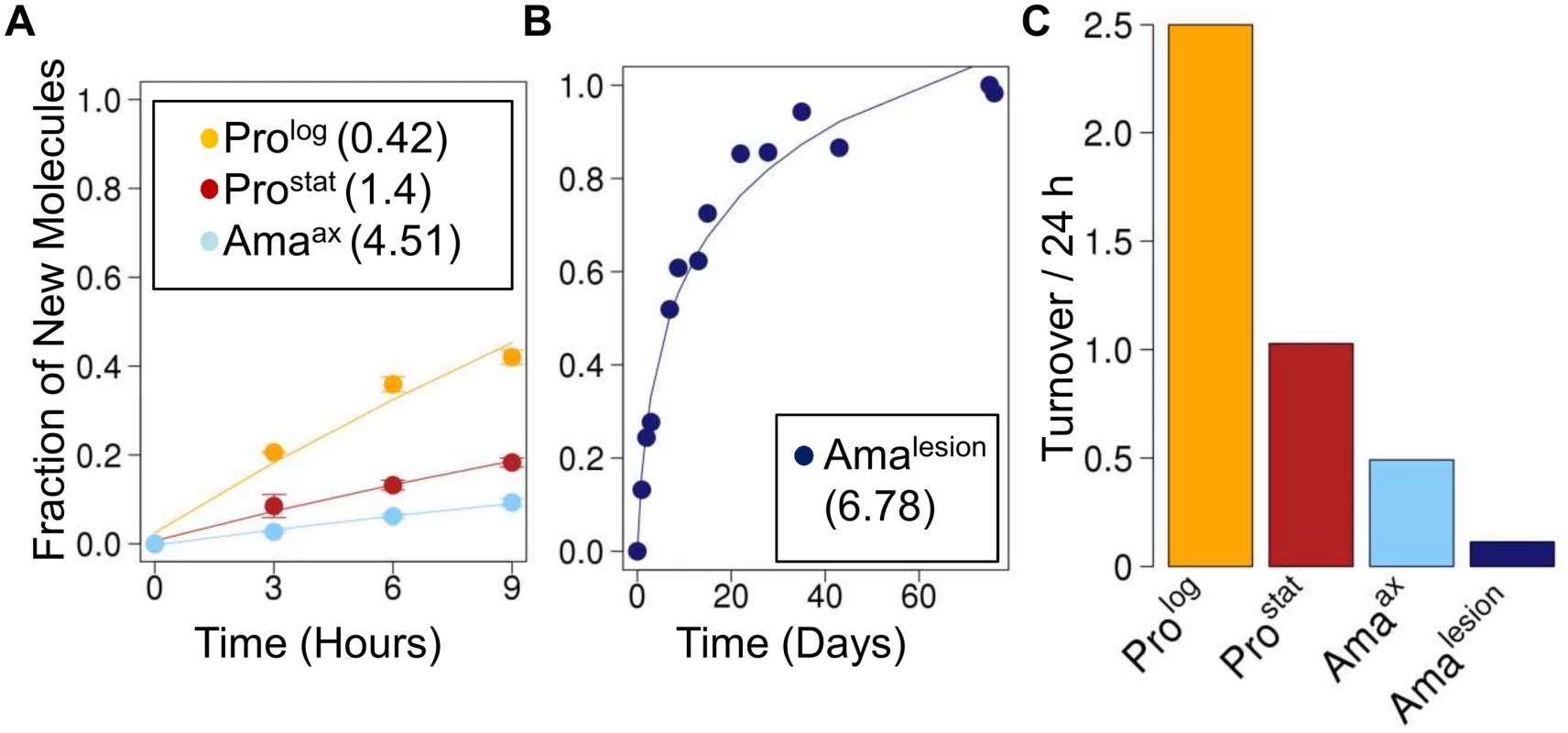
Parasite stages were 2H2O-labeled in culture or in situ in infected BALB/c mice and harvested at the indicated time points. Kinetics of 2H-labeling of proteinogenic alanine in (A) cultured parasite stages (Prolog, Prostat, Amaaxenic) and (B) amastigotes isolated from BALB/c lesion (Amalesion). The fraction of new molecules (Y-axis) was calculated from the level of 2H-enrichment in alanine relative to maximum labeling observed in each parasite stage after long term labeling. Inset boxes in A and B show turnover (t1/2) in days. C. Comparative rates of protein turnover in different Leishmania developmental stages. Note that similar estimates of protein turnover were obtained by measuring deuterium incorporation into other proteinogenic amino acids (S6 Fig.). Leishmania amastigotes also exhibit reduced membrane turnover in vivo, but are dependent on de novo synthesis of key fatty acids
The turnover of membrane phospholipids is intimately linked to cell division, organelle biogenesis and dynamic cellular functions, such as secretion and endocytosis. To investigate whether membrane turnover is reduced in Leishmania amastigotes we measured global rates of fatty acid turnover in different cultured and lesion-derived stages. Fatty acids are primarily incorporated into phospholipids, with little incorporation into other lipids (such as triacylglycerol), providing a direct measure of membrane biogenesis. As with proteinogenic amino acids, the extent to which 2H-is incorporated into different Leishmania fatty acids varies, depending on the extent to which they are generated via de novo synthesis or scavenged from the media or host (S7 Fig., S8 Fig.). However, when rates of labeling were normalized to maximum labeling, similar rates of turnover were obtained regardless of the fatty acid measured. As expected, Prolog exhibited fastest rates of fatty acid turnover, while both Prostat and Amaaxenic exhibited turnover rates that were comparable to, or slightly faster, than determined rates of protein turnover, respectively (Fig. 4). Strikingly, Amalesion exhibited very low rates of fatty acid turnover (t1/2 ~ 7.8 days, compared to 1.4 days in Amaaxenic), indicating a dramatic slow-down in global rates of membrane biogenesis in lesion stages.
Fig. 4. Stage-specific changes in fatty acid synthesis. 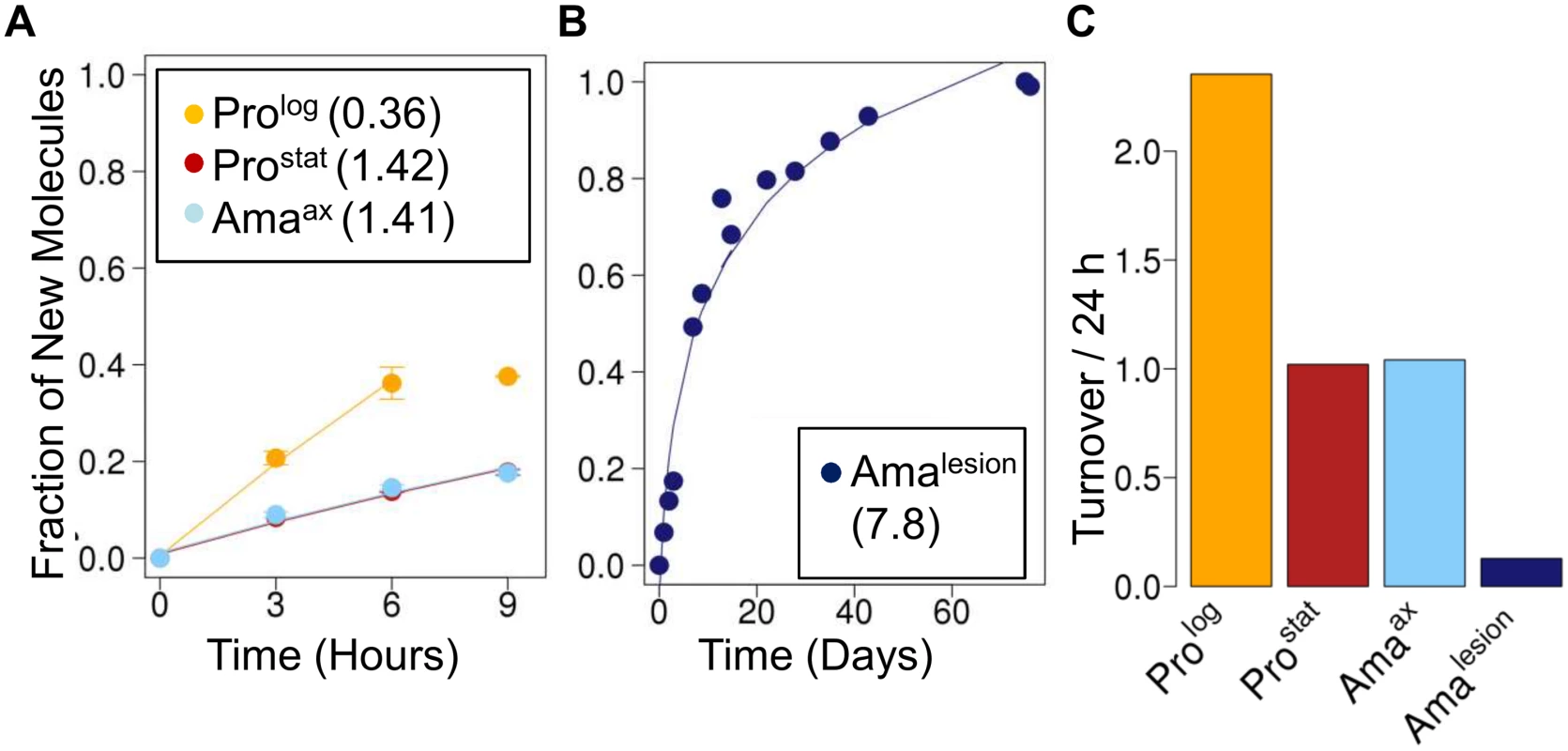
Parasite stages were 2H2O-labeled in culture or in situ in infected BALB/c mice and harvested at the indicated time points. Kinetics of 2H-labeling of the major fatty acid, C18:0 (stearic acid) in (A) cultured parasite stages (Prolog, Prostat, Amaaxenic) and (B) amastigotes isolated from BALB/c lesion (Amalesion). The fraction of new molecules (Y-axis) was calculated from the level of 2H-enrichment in stearate relative to maximum labeling observed in each parasite stage after long term labeling. Inset boxes show turnover (t1/2) in days. C. Comparative rates of stearic acid turnover in different Leishmania developmental stages. To further investigate whether the 2H-labeling of Amalesion fatty acids reflects de novo synthesis or uptake of 2H-labeled fatty acids from the host [8,27], we measured the maximum levels of 2H-enrichment in both parasite and host fatty acids derived from plasma or lymph nodes after a prolonged period of labeling (Fig. 5, S9 Fig.). The labeling of C14 : 0, C16 : 0 and C16 : 1 fatty acids was comparable in both Amalesion and serum samples, and somewhat lower than maximum levels of enrichment in Prolog, indicating that lesion amastigotes are largely dependent on salvage pathways for these fatty acids. In contrast, maximum 2H-enrichment in Amalesion C18 : 0, C18 : 1 and C18 : 2 was higher than in equivalent host fatty acids. This was particularly pronounced for parasite C18 : 1 (oleic acid) and C18 : 2 (γ-linoleic acid) which were 2-fold or >50-fold more highly labeled than corresponding host fatty acids. The absence of 2H-enrichment in plasma C18 : 2 is consistent with the absence of Δ12 oleic acid desaturase in animals, while the elevated levels of labeling of amastigote C18 : 1 and C18 : 2 indicate that these fatty acid are predominantly synthesized by the parasite. Significantly, the rate of turnover of γ-linoleic acid was very similar to that Amalesion DNA providing additional support for the notion that these stages have a slow replication rate of ~12 days in vivo. Amalesion also contained higher levels of very long chain, polyunsaturated fatty acids, than cultured promastigotes (S8, S9 Figs). These included C20 : 4 n-6 (arachidonic acid) and C22 : 6 n-3 which were labeled to the same extent as the equivalent host fatty acids (Fig. 5A). Because the additional 2H-enrichment in parasite γ-linoleic acid is not observed in these downstream fatty acids, they are most likely salvaged directly from the host cell (Fig. 5B). Collectively, these results show that fatty acid/membrane turnover is dramatically reduced in lesion amastigotes. Notwithstanding their reduced requirements, these stages appear to be dependent on both salvage and de novo synthesis for maintaining fatty acid levels. In particular, our data suggest that they are likely to be critically dependent on the de novo synthesis of C18 : 2, which is not synthesized by the host and appears to be depleted in the macrophage phagolysosome compartment.
Fig. 5. Lesion amastigotes utilize both salvage and de novo biosynthetic pathways to supply their fatty acid needs. 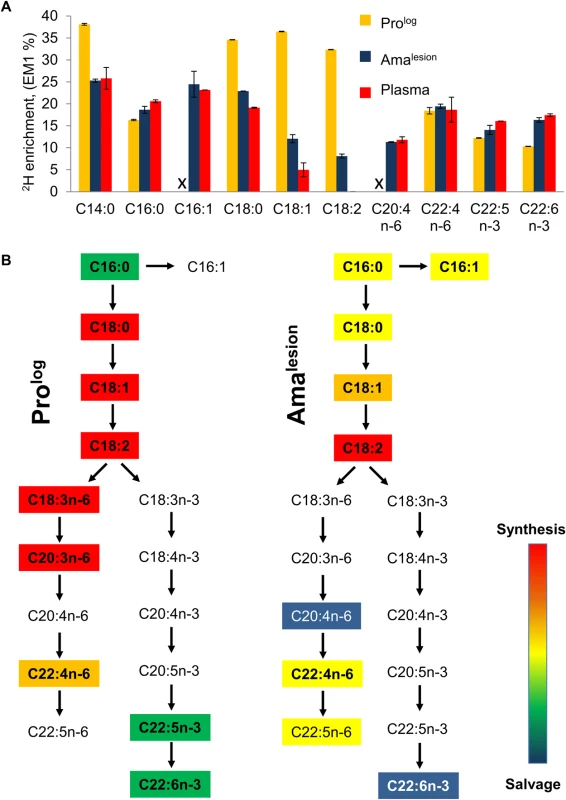
A. The maximum level of 2H-enrichment (EM1, %) in major cellular fatty acids of Prolog and Amalesion were determined after labeling for 7 days and >6 weeks, respectively. 2H-enrichment in the total plasma lipid of infected mice was also measured to determine the potential contribution of labeled host fatty acids to the parasite labeling. Note that while saturated and unsaturated C18 fatty acids are predominant fatty acids in both stages, the fatty acid composition of Amalesion differs from cultured promastigotes in containing elevated levels of C20:4 n-6, and polyunsaturated very long chain fatty acids (C22:4 n-6, C22:6 n-3) (S8 Fig.). The C:D nomenclature refers to overall chain length and number of double bonds in each fatty acid, respectively. n-3 and n-6 refers to the two major biosynthetic pathways involved in unsaturated fatty acid biosynthesis (where-3 and-6 refer to the position of double bond relative to the methyl carbon). B. Stage-specific differences in the levels of 2H-enrichment in fatty acid pools can be used to infer the contributions of de novo biosynthesis and salvage pathways. In particular, levels of 2H-enrichment in the major Amalesion C18 fatty acids, C18:1 (oleic acid) and C18:2 (linoleic acid), were appreciably higher than in host plasma, indicating that these stages are dependent on de novo biosynthesis. Conversely, the elevated levels of 2H-enrichment in C20:4 n-6 and C22:6n-3 compared to C18:1 precursor (comparable to plasma pools) indicate that these very long chain polyunsaturated fatty acids are primarily scavenged from the host cell. Discussion
Information on host-parasite dynamics within Leishmania-induced lesions is limited, reflecting the technical difficulty of measuring the growth rate or physiological state of parasites within these tissues. Estimates of parasite growth based on direct enumeration of parasite numbers or detection of transgenic parasite lines expressing luciferase or fluorescent proteins are limited in sensitivity and do not distinguish between dynamic changes in the rate of pathogen replication, death or migration out of infected tissues. These approaches also require the generation of transgenic parasite lines which may alter virulence phenotypes and in which expression of reporter proteins may vary. In this study we have utilized 2H2O labeling to explicitly measure the growth rate and other key physiological parameters of wild type L. mexicana amastigotes in non-healing cutaneous lesions. We show that lesion amastigotes replicate very slowly (doubling time ~12 days) throughout lesion development and appear to enter a distinct semi-quiescent state, characterized by low rates of transcription, protein turnover and membrane biogenesis. Significantly, the calculated rates of amastigote growth account for the increase in the total parasite burden in isolated lesions, as well as the mean parasite densities in the phagolysosomes of lesion macrophages, suggesting that parasite death and turnover in these lesions occurs to a minimal extent. These analyses also provide direct evidence that infected macrophages in L. mexicana-induced granulomas have a long life span. Specifically, based on the average parasite burden of infected macrophages (100–200 amastigotes/ phagolysosome) it is likely that a significant majority of infected host cells in the lesion had been infected very early in the infection and have sustained intracellular parasites for >12 weeks. While it is possible that some macrophage death and turnover could be masked by direct transfer of parasite-laden vacuoles from a ruptured macrophage to a naive recipient host cell [28], these findings are broadly consistent with a growing body of evidence suggesting that L. mexicana amastigotes repress a number of signaling pathways in macrophages, including those that activate apoptosis, autophagy or necrosis [29,30]. Overall, these findings suggest that L. mexicana-induced lesions are characterized by slow parasite growth and low macrophage turnover. We propose that the slow replication rate of intracellular amastigotes (triggered in part by intrinsic amastigote differentiation signals), minimizes overgrowth of the phagolysosome and contributes to the long life-time of infected macrophages. Slow growth may therefore be a key factor in generating a stable, permissive tissue niche within which the parasite burden can progressively expand.
A number of other bacterial, fungal and protozoan parasites induce granulomatous structures in their host, of which the most intensively studied are the pulmonary granulomas induced by Mycobacterium tuberculosis [31,32]. Our findings suggest that host-pathogen dynamics in the L. mexicana induced lesions differ substantially from those in M. tuberculosis granulomas in several respects. In particular, the M. tuberculosis-induced granulomas are generally characterized by high rates of macrophage infiltration and host cell death [31,32]. There is also increasing evidence that bacterial replication and turnover in these granulomas may be relatively high as a result of host-mediated bacterial killing and/or immune clearance [6,12,32,33]. This contrasts with the earlier view that M. tuberculosis bacilli have a low replication rate, and corresponding low death rates, leading to the observed plateauing in bacterial numbers during chronic infections [6,12,32,33]. These observations highlight marked differences in the way different pathogens adapt to, and potentially exploit the host’s attempt to wall off persistent infection with granulomas. Whether these differences are defined by intrinsic differences in pathogen growth rate remain to be determined.
While a number of microbial pathogens are thought to switch to a quiescent or semi-quiescent state during long-term chronic phases of infection, relatively little is known about the physiological/metabolic state of these stages [3,4]. Here we show that slowly replicating lesion amastigotes strongly repress energy-expensive processes such as transcription, protein synthesis and membrane lipid turnover. The down-regulation of these processes in amastigotes was more pronounced than in non-dividing promastigotes, highlighting the fact that metabolic quiescence is not necessarily linked to growth rate and that non-dividing stages can remain metabolically active. It was notable, that repression of RNA synthesis was less pronounced than for either protein or fatty acid biosynthesis. Leishmania lack transcription factors and gene-specific transcriptional control [19] and a higher basal level of RNA turnover may be needed for post-transcriptional regulation of gene expression. The strong repression of protein synthesis in Amalesion (10-fold and 5-fold lower than in Prostat or Amaaxenic, respectively) is consistent with recent studies demonstrating that promastigote to amastigote differentiation results in activation of the PERK kinase and phosphorylation of eIF2a, both of which regulate and repress protein translation and are required for amastigote virulence [34–36]. A general shut down in energy intensive processes, such as protein and fatty acid synthesis is also consistent with recent 13C-flux studies on isolated Amalesion, which identified a unique stringent metabolic response in these stages, characterized by reduced carbon utilization and more efficient mitochondrial catabolism of sugars and fatty acids [24]. Thus the physiological responses of amastigotes to the lesion microenvironment are complex and regulated at the level of DNA replication, transcription and protein synthesis, in addition to remodeling of central carbon metabolism.
Entry into this semi-quiescent state is likely to be triggered by a number of factors. Both Amaaxenic and AmaMø exhibited slow maximum rates of growth (doubling time 3.5–4 days) and RNA turnover, suggesting that entry into this state is a hardwired response to elevated temperature and/or low pH used to induce differentiation in vitro. These findings also suggest that the phagolysosome of non-activated J774 macrophages are not restrictive for amastigote growth. On the other hand, there is evidence that the growth of L. major amastigotes in cutaneous lesions is restricted by the chronic production of sub-lethal levels of nitrous oxide [18]. However, L. mexicana amastigotes reside within larger communal phagolysosome compartments and are intrinsically more resistant to macrophage microbicidal processes, including nitric oxide or reactive oxygen species [14,37]. They also appear to have evolved additional mechanisms for inhibiting macrophage activation and nitric oxide production [38]. The slow growth rate of L. mexicana amastigotes in lesions may therefore reflect both intrinsic parasite responses, as well as adaptive responses to other host microbicidal processes, nutrient deprivation and/or physical stresses in this niche [24].
The high parasite inoculum used in our studies is expected to lead to rapid recruitment of macrophages and early induction of lesion development [39]. In contrast, infection of mice with a low dose inoculum is associated with a significant delay in lesion formation that is preceded by an exponential increase in parasite numbers [40]. In the L. major—C57BL murine model, this silent expansive phase is associated with an increase in parasite burden consistent with an apparent parasite doubling time of ~2.3 days [40]. This growth rate is similar to the replication rate we observed for L. mexicana amastigotes in non-activated J774 macrophages, raising the possibility that Leishmania amastigotes may switch between different growth states during acute and long-term chronic phases of infection. The possibility that amastigotes exhibit a range of growth rates within lesion was also suggested by the detection of a small number (<20%) of hyper-infected macrophages with more than 200 parasites/macrophage. The existence of distinct amastigote growth/physiological states is analogous to the situation in the sandfly vector. Initial colonization of the mid-gut of this host is mediated by ‘procyclic’ promastigotes that are rapidly dividing and generally more sensitive to a variety of physiological and nutritional stresses. These stages transition though a number of physiological states before differentiating to non-replicating, metacyclic promastigotes (related to Prostat) in the foregut [41]. Metacyclic promastigotes are resistant to a number of stresses (including elevated temperature), suggesting that slow-growth represents a generalized response to elevated stresses in both the insect and mammalian hosts.
Many microbial pathogens acquire essential nutrients or metabolites via a combination of salvage pathways or de novo synthesis, leading to a level of redundancy that complicates efforts to identify and validate drug targets. While administration of 2H2O to infected mice results in metabolic labeling of both parasite and host lipids, analysis of the relative rates of labeling of parasite/host pools can be used to infer the contribution of salvage versus de novo biosynthetic pathway to parasite lipid homeostasis. In particular, we found that 2H-enrichment in Amalesion C18 fatty acids was substantially higher than in the equivalent host fatty acids, indicating that they are synthesize de novo by intracellular amastigotes. This was particularly striking for C18 : 2, which was labeled to a significant extent in Amalesion, but unlabeled in mouse plasma samples. The absence of labeling of plasma C18 : 2 was not surprising given that animals lack the Δ12 desaturase needed to synthesize linoleic acid and are dependent on uptake of this fatty acid in the diet. The high maximum labeling of C18 : 2 in Amalesion indicates that this fatty acid is limiting for parasite growth in the phagolysosome compartment and therefore that amastigotes may be dependent on their own Δ12 desaturase for intracellular growth [42,43]. On the other hand, Amalesion contained elevated levels of very long chain, polyunsaturated fatty acids, including C20 : 4 n-6 (arachidonic acid), C22 : 4 n-6 (adrenic acid), C22 : 5 n-6 (osbond acid) and C22 : 6 n-3 (cervonic acid) compared to promastigotes. These fatty acids were 2H-enriched to a higher level than parasite C18 precursors fatty acids and were labeled to the same extent as the equivalent host fatty acids, suggesting that they are derived primarily via salvage pathways. These findings add to accumulating evidence that Leishmania amastigotes acquire a range of lipids from the host cell [24,44,45] and suggest that salvage as well as de novo biosynthesis pathways are potential drug targets.
In summary, we show that 2H2O labeling can be used as a universal labeling procedure to measure microbial growth, physiology and metabolism in their animal hosts. This approach is well suited for studying the in vivo growth and metabolism of other microbial pathogens.
Materials and Methods
Cell culture
L. mexicana promastigotes (Prolog, Prostat) were cultured in RPMI 1640 medium supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS) at 27°C. Axenic amastigote (Amaaxenic) were generated by adjusting the pH of the medium of stationary phase promastigotes (day 5, ProStat) to pH 5.5 with 1 M HCl and addition of 10% FCS (20% v/v final), followed by incubation at 33°C for four days.
2H2O labeling
Cultivated parasites stages (Prolog, Prostat, Amaaxenic) were cultured in medium supplemented directly with phosphate buffered saline - 2H2O (99.9%, Cambridge Isotopes) to give a final concentration of 5% (v/v) 2H2O.
J774 macrophages (4 × 106) were grown overnight in RPMI 1640 medium supplemented with 10% (v/v) FCS, penicillin and streptomycin at 33°C [46], before being infected with L. mexicana Prostat at a MOI of 3. Non-internalized parasites were removed after 4 hr by washing macrophages twice with fresh RPMI medium. After 3 days, the medium of infected macrophages was replaced with fresh medium containing 5% 2H2O and cells harvested at various time points (ranging from 4 h and 16 days). For long term labeling experiments, the medium containing 5% 2H2O was replaced every 5 days.
BALB/c mice (6 week old) were infected with L. mexicana Prostat (106 in 50 μl PBS) near the base of the tail and lesion size monitored as previously described [46]. Infected mice were injected intra-peritoneally with 2H2O (99%, 35 μl/g body weight) containing 0.9% NaCl after the development of nascent lesions and serum 2H2O concentration subsequently maintained at 5% by supplementation of the drinking water with 9% 2H2O. 2H2O levels in the urine were routinely monitored as previously described [47]. To determine maximum labeling parasite metabolites (EM1), mice were labeled with 2H2O immediately after the infection and parasites harvested one month after the development of a granulomatous lesion.
Harvest of parasites
Cultured parasites stages were harvested with rapid metabolic quenching as previously described [48] and cell pellets (triplicate samples) were stored at -80°C prior to extraction. Infected J774 macrophages were metabolically quenched by chilling plates on ice and replacing the overlying culture medium with ice-chilled PBS. Infected macrophages were scraped from the plastic surface and lysed by repeated passage through a 25G needle (x10). After low speed centrifugation to remove host cell debris (60 g, 5 min, 4°C), amastigotes were recovered by sequential filtration through 5 μm and 3 μm pore filters and centrifugation (1500g, 10 min, 4°C) of the filtrate. The parasite pellet was washed three times with chilled PBS and any residual host cell DNA, removed by treatment of the pellet with 1000 U of DNAase in PBS with 5 mM MgCl2 for 2 h at 33°C.
Mice were culled humanely post-labeling (24 h to 150 days) and granulomatous lesions excised and immediately chilled in cold PBS. All subsequent procedures were carried out at <4°C. The isolated tissue mass was disrupted by passage through a cell strainer and host cells lyzed by passage through a 27 G syringe needle (x5). Intact host cells and host cell debris were removed by centrifugation (60 g, 10 min, 4°C) and released parasites harvested by centrifugation (1500 g, 10 min, 4°C). The pellet was washed three times with chilled PBS and the purity of the parasite extract confirmed by light microscopy. Samples for DNA analysis were treated with 1000 U of DNAase in PBS with 5 mM MgCl2 for 2 h at 33°C to remove any host cell DNA.
Histology
BALB/c lesions (85 day post-infection) were fixed with 10% paraformaldehyde in PBS, embedded in paraffin and tissue sections stained with hematoxylin and eosin (H&E) reagents.
Parasite extraction and sample preparation
Nucleic acids were extracted, hydrolyzed and dephosphorylated and released ribosyl and deoxyribosyl sugars derivitized as previously described, with some modifications [23,49,50]. In brief, nucleosides in 250μl H2O were incubated in HCl (0.01 M, 1.84 ml) and O-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine acetate (PFBHA, 25 mg/ml, 20 μl), at 90°C for 3 h. Oximes of ribose and deoxyribose were extracted in ethyl acetate/hexane mix (1 : 1 v/v) followed by pure ethyl acetate and the pooled organic phases were dried under nitrogen. Samples were silylated by sequential addition of ethyl acetate (20 μl) and N,O-bis(trimethylsilyl) trifluoroacetamide reagent (40 μl BSTFA + 1% TMCS, Thermo scientific) and incubation at 90°C for 1 h. The perfluorotritrimethylsilyl (PFtriTMS) derivatives of deoxyribose and ribose were analyzed by GC/MS in negative and positive chemical ionization mode (NCI, PCI) with methane as reagent gas. The fragments at m/z 530 and m/z 633 are abundant PCI fragments of deoxyribose and ribose, respectively, that correspond to the loss of CH4: [M+1–16]+.
All derivitized samples were analyzed by GC/MS using a DB5 capillary column (J&W Scientific, 30 m, 250 μm inner diameter, 0.25 μm film thickness), with a 10 m inert duraguard. The injector insert and GC/MS transfer line temperatures were 270 and 250°C, respectively. The oven temperature gradient was set to: 70°C (1 min); 70°C to 295°C at 12.5°C/min;295°C to 320°C at 25°C/min; 320°C for 2 min. All metabolites were identified based on GC-retention times and mass spectra of standards. The excess M1 fraction and the fraction of new cells/molecules were calculated as described [51] following the measurement of the M0 and the corresponding M+1 ion using selected ion monitoring (SIM).
Parental ions (M0) were as follows: deoxyribose: PCI = 530 m/z, NCI = 525 m/z; ribose: PCI = 618 m/z, NCI = 452 m/z; alanine: PCI = 318 m/z; aspartate: PCI = 476 m/z; glutamate: PCI = 490 m/z; stearate: EI: 298 m/z, PCI = 327 m/z; oleate: EI = 296, PCI = 325 m/z; linoleate: EI = 294 m/z, PCI = 323 m/z. The half-life (t½) was determined by plotting data points on a log time (days) scale, fitting a straight line to the data points and solving the equation y = m*x + c for y = 0.5. The t½ is given by exp(log days).
Total lipids were extracted in chloroform/methanol/water (1 : 2:0.8 v/v) as described previously [48]. The organic phase was dried in a centrifugal evaporator and resuspended in chloroform/methanol (2 : 1 v/v, 30 μl) and total fatty acids analyzed as their methylesters after addition of Meth-Prep II reagent (5 μl, Grace Davison, Alltech) and direct injection and analysis by GC/MS in electron impact (EI) mode and positive chemical ionization mode. The delipidated protein pellets were hydrolyzed in 6 M HCl (200 μl, 110°C, 18 hr) and insoluble material removed by centrifugation (16,100 g, 5 min, RT). The supernatant was dried under nitrogen and released amino acids converted to their TBDMS derivatives by addition of MTBSTFA-1% TBDMCS (30 μl) and pyridine (30 μl, 60°C, 30 min) prior to GC-MS analysis in positive chemical ionization mode as described above.
Enumeration of parasite numbers in lesion macrophages
BALB/c lesions (85 day post-infection) were fixed with 10% paraformaldehyde in PBS, embedded in paraffin and tissue sections stained with hematoxylin and eosin (H&E) reagents. Slides were imaged using a Zeiss Axioplan microscope and digital computer images were recorded with a Zeiss camera control unit and the corresponding dedicated software. The numbers of amastigotes was counted in 8 sections of 2 lesions evaluating >1200 phagolysosome in total. The number of parasites/vacuole was calculated assuming that (1) the phagolysosome compartment is a sphere, (2) that the average cross sectional diameter of the phagolysosome is equivalent to the largest vacuolar sections seen in the tissue sections and (3) that parasites are primarily arranged around the periphery of the phagolysosome at equal density. The average cross sectional diameter of the parasite occupied PVs was found to be 25.6 μm, while the average diameter of intracellular amastigotes was 2.5 μm.
To determine the maximum number parasites that fit in a vacuole section the ratio of the areas was calculated
and the maximum packing density of circles (PDmax2) was calculated to estimate that the maximum number of parasites in a vacuole section (Ptheor, max2) is Similarly, we determined the ratio of the volumes and used maximum packing density of spheres (PDmax3, Kepler conjecture) to determine that the maximum number of parasites in a vacuole (Ptheor, max3) is The number of parasites estimated to be present in the evaluated vacuoles (Pvac, estim) was then determined based on the number of parasites counted in a vacuole section (Pcount) as followsSupporting Information
Zdroje
1. Tischler AD, McKinney JD (2010) Contrasting persistence strategies in Salmonella and Mycobacterium. Curr Opin Microbiol 13 : 93–99. doi: 10.1016/j.mib.2009.12.007 20056478
2. Gengenbacher M, Kaufmann SHE (2012) Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol Rev 36 : 514–532. doi: 10.1111/j.1574-6976.2012.00331.x 22320122
3. Dembélé L, Franetich J-F, Lorthiois A, Gego A, Zeeman A-M, et al. (2014) Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat Med 20 : 307–312. doi: 10.1038/nm.3461 24509527
4. Rittershaus ESC, Baek S-H, Sassetti CM (2013) The normalcy of dormancy: common themes in microbial quiescence. Cell Host Microbe 13 : 643–51. doi: 10.1016/j.chom.2013.05.012 23768489
5. Srikanta D, Santiago-Tirado FH, Doering TL (2014) Cryptococcus neoformans: historical curiosity to modern pathogen. Yeast 31 : 47–60. doi: 10.1002/yea.2997 24375706
6. Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR (2009) A replication clock for Mycobacterium tuberculosis. Nat Med 15 : 211–214. doi: 10.1038/nm.1915 19182798
7. Murray HW, Berman JD, Davies CR, Saravia NG (2005) Advances in leishmaniasis. Lancet 366 : 1561–1577. 16257344
8. McConville MJ, Naderer T (2011) Metabolic pathways required for the intracellular survival of Leishmania. Annu Rev Microbiol 65 : 543–561. doi: 10.1146/annurev-micro-090110-102913 21721937
9. Kaye P, Scott P (2011) Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol 9 : 604–615. doi: 10.1038/nrmicro2608 21747391
10. Beattie L, Peltan A, Maroof A, Kirby A, Brown N, et al. (2010) Dynamic imaging of experimental Leishmania donovani-induced hepatic granulomas detects Kupffer cell-restricted antigen presentation to antigen-specific CD8 T cells. PLoS Pathog 6: e1000805. doi: 10.1371/journal.ppat.1000805 20300603
11. Moore JWJ, Moyo D, Beattie L, Andrews PS, Timmis J, Kaye PM (2013) Functional complexity of the Leishmania granuloma and the potential of in silico modeling. Front Immunol 4 : 35. doi: 10.3389/fimmu.2013.00035 23423646
12. Guirado E, Schlesinger LS. (2013) Modeling the Mycobacterium tuberculosis granuloma—the critical battlefield in host immunity and disease. Front Immunol 4 : 98. doi: 10.3389/fimmu.2013.00098 23626591
13. Sacks D, Noben-Trauth N (2002) The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol 2 : 845–858. 12415308
14. McMahon-Pratt D, Alexander J (2004) Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol Rev 201 : 206–24. 15361243
15. Lang T, Goyard S, Lebastard M, Milon G (2005) Bioluminescent Leishmania expressing luciferase for rapid and high throughput screening of drugs acting on amastigote-harbouring macrophages and for quantitative real-time monitoring of parasitism features in living mice. Cell Microbiol 7 : 383–392. 15679841
16. Romero I, Téllez J, Suárez Y, Cardona M, Figueroa R, et al. (2010) Viability and burden of Leishmania in extralesional sites during human dermal leishmaniasis. PLoS Negl Trop Dis 4: e819. doi: 10.1371/journal.pntd.0000819 20856851
17. Michel G, Ferrua B, Lang T, Maddugoda MP, Munro P, et al. (2011) Luciferase-expressing Leishmania infantum allows the monitoring of amastigote population size, in vivo, ex vivo and in vitro. PLoS Negl Trop Dis 5: e1323. doi: 10.1371/journal.pntd.0001323 21931877
18. Müller AJ, Aeschlimann S, Olekhnovitch R, Dacher M, Späth GF, Bousso P (2013) Photoconvertible pathogen labeling reveals nitric oxide control of Leishmania major infection in vivo via dampening of parasite metabolism. Cell Host Microbe. 14 : 460–467. doi: 10.1016/j.chom.2013.09.008 24139402
19. Kramer S (2012) Developmental regulation of gene expression in the absence of transcriptional control: The case of kinetoplastids. Mol Biochem Parasitol 181 : 61–72. doi: 10.1016/j.molbiopara.2011.10.002 22019385
20. Lynn MA, Marr AK, McMaster WR (2013) Differential quantitative proteomic profiling of Leishmania infantum and Leishmania mexicana density gradient separated membranous fractions. J Proteomics 82 : 179–192. doi: 10.1016/j.jprot.2013.02.010 23466312
21. Previs SF, Fatica R, Chandramouli V, Alexander JC, Brunengraber H, Landau BR (2004) Quantifying rates of protein synthesis in humans by use of 2H2O: application to patients with end-stage renal disease. Am J Physiol Endocrinol Metab 286: E665–672. 14693509
22. Neese RA, Misell LM, Turner S, Chu A, Kim J, et al. (2002) Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc Natl Acad Sci U S A 99 : 15345–15350. 12424339
23. Busch R, Neese RA, Awada M, Hayes GM, Hellerstein MK (2007) Measurement of cell proliferation by heavy water labeling. Nat Protoc 2 : 3045–3057. 18079703
24. Saunders EC, Ng WW, Kloehn J, Chambers JM, Ng M, McConville MJ (2014) Induction of a Stringent metabolic response in intracellular stages of Leishmania mexicana leads to increased dependence on mitochondrial metabolism. PLoS Pathog 10: e1003888. doi: 10.1371/journal.ppat.1003888 24465208
25. Warner JR (1999) The economics of ribosome biosynthesis in yeast. Trends Biochem Sci 24 : 437–440. 10542411
26. Busch R, Kim Y-K, Neese RA, Schade-Serin V, Collins M, et al. Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta 1760 : 730–744. 16567052
27. Ramakrishnan S, Serricchio M, Striepen B, Bütikofer P (2013) Lipid synthesis in protozoan parasites: a comparison between kinetoplastids and apicomplexans. Prog Lipid Res. 52 : 488–512. doi: 10.1016/j.plipres.2013.06.003 23827884
28. Real F, Florentino PTV, Reis LC, Ramos-Sanchez EM, Veras PS, et al. (2014) Cell-to-cell transfer of Leishmania amazonensis amastigotes is mediated by immunomodulatory LAMP-rich parasitophorous extrusions. Cell Microbiol 16 : 1549–1564 doi: 10.1111/cmi.12311 24824158
29. van Zandbergen G, Solbach W, Laskay T (2007) Apoptosis driven infection. Autoimmunity 40 : 349–52. 17516227
30. Donovan MJ, Maciuba BZ, Mahan CE, McDowell MA (2009) Leishmania infection inhibits cycloheximide-induced macrophage apoptosis in a strain-dependent manner. Exp Parasitol. 123 : 58–64. doi: 10.1016/j.exppara.2009.05.012 19500578
31. Petersen HJ, Smith AM (2013) The role of the innate immune system in granulomatous disorders. Front Immunol 4 : 120. doi: 10.3389/fimmu.2013.00120 23745122
32. Ramakrishnan L (2012) Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol 12 : 352–366. doi: 10.1038/nri3211 22517424
33. Davis JM, Ramakrishnan L (2009) The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136 : 37–49. doi: 10.1016/j.cell.2008.11.014 19135887
34. Lahav T, Sivam D, Volpin H, Ronen M, Tsigankov P, et al. (2010) Multiple levels of gene regulation mediate differentiation of the intracellular pathogen Leishmania. FASEB J 25 : 515–525. doi: 10.1096/fj.10-157529 20952481
35. Chow C, Cloutier S, Dumas C, Chou M-N, Papadopoulou B (2011) Promastigote to amastigote differentiation of Leishmania is markedly delayed in the absence of PERK eIF2alpha kinase-dependent eIF2alpha phosphorylation. Cell Microbiol 13 : 1059–1077. doi: 10.1111/j.1462-5822.2011.01602.x 21624030
36. Gosline SJC, Nascimento M, McCall L-I, Zilberstein D, Thomas DY, et al. (2011) Intracellular eukaryotic parasites have a distinct unfolded protein response. PLoS One 6: e19118. doi: 10.1371/journal.pone.0019118 21559456
37. Wilson J, Huynh C, Kennedy KA, Ward DM, Kaplan J, et al. (2008) Control of parasitophorous vacuole expansion by LYST/Beige restricts the intracellular growth of Leishmania amazonensis. PLoS Pathog 4: e1000179. doi: 10.1371/journal.ppat.1000179 18927622
38. Cortez M, Huynh C, Fernandes MC, Kennedy KA, Aderem A, Andrews NW (2011) Leishmania promotes its own virulence by inducing expression of the host immune inhibitory ligand CD200. Cell Host Microbe. 9 : 463–471. doi: 10.1016/j.chom.2011.04.014 21669395
39. Lira R, Doherty M, Modi G, Sacks D (2000) Evolution of lesion formation, parasitic load, immune response, and reservoir potential in C57BL/6 mice following high - and low-dose challenge with Leishmania major. Infect Immun 68 : 5176–5182. 10948141
40. Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D (2000) A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol 165 : 969–977. 10878373
41. Sacks D, Kamhawi S (2001) Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu. Rev. Microbiol 55 : 453–483. 11544364
42. Mukherjee S, Sen Santara S, Das S, Bose M, Roy J, Adak S (2012) NAD(P)H cytochrome b5 oxidoreductase deficiency in Leishmania major results in impaired linoleate synthesis followed by increased oxidative stress and cell death. J Biol Chem 287 : 34992–35003. doi: 10.1074/jbc.M112.389338 22923617
43. Alloatti A, Gupta S, Gualdrón-López M, Igoillo-Esteve M, Nguewa PA, et al. (2010) Genetic and chemical evaluation of Trypanosoma brucei oleate desaturase as a candidate drug target. PLoS One 5: e14239. doi: 10.1371/journal.pone.0014239 21151902
44. Winter G, Fuchs M, McConville MJ, Stierhof YD, Overath P (1994) Surface antigens of Leishmania mexicana amastigotes: characterization of glycoinositol phospholipids and a macrophage-derived glycosphingolipid. J Cell Sci 107 : 2471–2482. 7844164
45. Zhang O, Wilson MC, Xu W, Hsu F-F, Turk J, et al. (2009) Degradation of host sphingomyelin is essential for Leishmania virulence. PLoS Pathog 5: e1000692. doi: 10.1371/journal.ppat.1000692 20011126
46. Naderer T, Heng J, McConville MJ (2010) Evidence that intracellular stages of Leishmania major utilize amino sugars as a major carbon source. PLoS Pathog. 6: e1001245. doi: 10.1371/journal.ppat.1001245 21203480
47. Shah V, Herath K, Previs SF, Hubbard BK, Roddy TP (2010) Headspace analyses of acetone: a rapid method for measuring the 2H-labeling of water. Anal Biochem 404 : 235–237. doi: 10.1016/j.ab.2010.05.010 20488158
48. Saunders EC, Ng WW, Chamber JM, Ng M, Naderer T, et al. (2011) Isoptopomer profiling of Leishmania mexicana promastigotes reveals important roles for succinate fermentation and aspartate uptake in TCA cycle anaplerosis, glutamate synthesis and growth. J Biol Chem 286 : 27706–27717. doi: 10.1074/jbc.M110.213553 21636575
49. Rotureau B, Gego A, Carme B (2005) Trypanosomatid protozoa: a simplified DNA isolation procedure. Exp Parasitol. 111 : 207–209. 16139269
50. Wu M-Y, Chen B-G, Chang CD, Huang M-H, Wu T-G, et al. (2008) A novel derivatization approach for simultaneous determination of glyoxal, methylglyoxal, and 3-deoxyglucosone in plasma by gas chromatography-mass spectrometry. J Chromatogr A 1204 : 81–86. doi: 10.1016/j.chroma.2008.07.040 18692194
51. Neese RA, Siler SQ, Cesar D, Antelo F, Lee D, et al. (2001) Advances in the stable isotope-mass spectrometric measurement of DNA synthesis and cell proliferation. Anal Biochem 298 : 189–95. 11700973
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 2- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- 2014 Reviewer Thank You
- A Case for Two-Component Signaling Systems As Antifungal Drug Targets
- Prions—Not Your Immunologist’s Pathogen
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
- Livestock-Associated : The United States Experience
- The Neurotrophic Receptor Ntrk2 Directs Lymphoid Tissue Neovascularization during Infection
- The Intracellular Bacterium Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission
- CD200 Receptor Restriction of Myeloid Cell Responses Antagonizes Antiviral Immunity and Facilitates Cytomegalovirus Persistence within Mucosal Tissue
- Phage-mediated Dispersal of Biofilm and Distribution of Bacterial Virulence Genes Is Induced by Quorum Sensing
- CXCL9 Contributes to Antimicrobial Protection of the Gut during Infection Independent of Chemokine-Receptor Signaling
- Mitigation of Prion Infectivity and Conversion Capacity by a Simulated Natural Process—Repeated Cycles of Drying and Wetting
- Approaches Reveal a Key Role for DCs in CD4+ T Cell Activation and Parasite Clearance during the Acute Phase of Experimental Blood-Stage Malaria
- Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Invasion of Erythrocytes
- Crystal Structures of the Carboxyl cGMP Binding Domain of the cGMP-dependent Protein Kinase Reveal a Novel Capping Triad Crucial for Merozoite Egress
- Non-redundant and Redundant Roles of Cytomegalovirus gH/gL Complexes in Host Organ Entry and Intra-tissue Spread
- Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water Labeling
- A Working Model of How Noroviruses Infect the Intestine
- CD44 Plays a Functional Role in -induced Epithelial Cell Proliferation
- Novel Inhibitors of Cholesterol Degradation in Reveal How the Bacterium’s Metabolism Is Constrained by the Intracellular Environment
- G-Quadruplexes in Pathogens: A Common Route to Virulence Control?
- A Rho GDP Dissociation Inhibitor Produced by Apoptotic T-Cells Inhibits Growth of
- Manipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction and
- The RhoGAP SPIN6 Associates with SPL11 and OsRac1 and Negatively Regulates Programmed Cell Death and Innate Immunity in Rice
- Lymph-Node Resident CD8α Dendritic Cells Capture Antigens from Migratory Malaria Sporozoites and Induce CD8 T Cell Responses
- Coordinated Function of Cellular DEAD-Box Helicases in Suppression of Viral RNA Recombination and Maintenance of Viral Genome Integrity
- IL-33-Mediated Protection against Experimental Cerebral Malaria Is Linked to Induction of Type 2 Innate Lymphoid Cells, M2 Macrophages and Regulatory T Cells
- Evasion of Autophagy and Intracellular Killing by Human Myeloid Dendritic Cells Involves DC-SIGN-TLR2 Crosstalk
- CD8 T Cell Response Maturation Defined by Anentropic Specificity and Repertoire Depth Correlates with SIVΔnef-induced Protection
- Diverse Heterologous Primary Infections Radically Alter Immunodominance Hierarchies and Clinical Outcomes Following H7N9 Influenza Challenge in Mice
- Human Adenovirus 52 Uses Sialic Acid-containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells
- Super-Resolution Imaging of ESCRT-Proteins at HIV-1 Assembly Sites
- Disruption of an Membrane Protein Causes a Magnesium-dependent Cell Division Defect and Failure to Persist in Mice
- Recognition of Hyphae by Human Plasmacytoid Dendritic Cells Is Mediated by Dectin-2 and Results in Formation of Extracellular Traps
- Essential Domains of Invasins Utilized to Infect Mammalian Host Cells
- High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
- Yeast Prions: Proteins Templating Conformation and an Anti-prion System
- A Novel Mechanism of Bacterial Toxin Transfer within Host Blood Cell-Derived Microvesicles
- A Wild Strain Has Enhanced Epithelial Immunity to a Natural Microsporidian Parasite
- Control of Murine Cytomegalovirus Infection by γδ T Cells
- Dimorphism in Fungal Pathogens of Mammals, Plants, and Insects
- Recognition and Activation Domains Contribute to Allele-Specific Responses of an Arabidopsis NLR Receptor to an Oomycete Effector Protein
- Direct Binding of Retromer to Human Papillomavirus Type 16 Minor Capsid Protein L2 Mediates Endosome Exit during Viral Infection
- Characterization of the Mycobacterial Acyl-CoA Carboxylase Holo Complexes Reveals Their Functional Expansion into Amino Acid Catabolism
- Prion Infections and Anti-PrP Antibodies Trigger Converging Neurotoxic Pathways
- Evolution of Genome Size and Complexity in the
- Antibiotic Modulation of Capsular Exopolysaccharide and Virulence in
- IFNγ Signaling Endows DCs with the Capacity to Control Type I Inflammation during Parasitic Infection through Promoting T-bet+ Regulatory T Cells
- Identification of Effective Subdominant Anti-HIV-1 CD8+ T Cells Within Entire Post-infection and Post-vaccination Immune Responses
- Viral and Cellular Proteins Containing FGDF Motifs Bind G3BP to Block Stress Granule Formation
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Cytoplasmic Actin Is an Extracellular Insect Immune Factor which Is Secreted upon Immune Challenge and Mediates Phagocytosis and Direct Killing of Bacteria, and Is a Antagonist
- A Specific A/T Polymorphism in Western Tyrosine Phosphorylation B-Motifs Regulates CagA Epithelial Cell Interactions
- Within-host Competition Does Not Select for Virulence in Malaria Parasites; Studies with
- A Membrane-bound eIF2 Alpha Kinase Located in Endosomes Is Regulated by Heme and Controls Differentiation and ROS Levels in
- Cytosolic Access of : Critical Impact of Phagosomal Acidification Control and Demonstration of Occurrence
- Role of Pentraxin 3 in Shaping Arthritogenic Alphaviral Disease: From Enhanced Viral Replication to Immunomodulation
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- HITS-CLIP Analysis Uncovers a Link between the Kaposi’s Sarcoma-Associated Herpesvirus ORF57 Protein and Host Pre-mRNA Metabolism
- Molecular and Functional Analyses of a Maize Autoactive NB-LRR Protein Identify Precise Structural Requirements for Activity
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Control of Murine Cytomegalovirus Infection by γδ T Cells
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy












